Structural Disorders and Neoplasms of the Reproductive System
• Describe the various structural disorders of the uterus and vagina.
• Discuss the pathophysiology of selected benign and malignant neoplasms of the female reproductive tract.
• Compare the common medical and surgical therapies for selected benign gynecologic conditions.
• Explain diagnostic procedures in client-centered terms.
• Examine the emotional effects of benign and malignant neoplasms.
• Develop a nursing care plan for a woman with endometrial cancer who has had a hysterectomy.
• Differentiate treatments for preinvasive and invasive conditions.
• Identify critical elements for teaching clients with selected benign or malignant neoplasms.
• Investigate health-promoting behaviors that reduce cancer risk.
• Assess the effects of and treatments for malignant neoplasms during pregnancy.
• Discuss the development and sequelae of gestational trophoblastic neoplasia.
Women are at risk for structural disorders and neoplastic diseases of the reproductive system from the age of menarche through menopause and the older years. Problems may include structural disorders of the uterus and vagina related to pelvic relaxation and urinary incontinence and chronic pain related to vulvodynia. Benign neoplasms of the reproductive organs, such as fibroids and cysts, and malignant neoplasms of the reproductive system also may occur. Benign tumors usually do not endanger life, tend to grow slowly, and are not invasive. Malignant tumors (cancers) grow rapidly in a disorganized manner and invade surrounding tissues. The development of structural disorders and benign or malignant neoplasms can have far-reaching effects for the woman and her family. Beyond the obvious physiologic alterations, the woman also experiences threats to her self-concept and her ability to cope. A woman’s concept of herself as a sexual being can be affected by the condition and its treatments. A woman’s family also is challenged in the way it responds to her diagnosis. When cancer occurs with pregnancy, it adds to the complexity of physical and emotional responses to childbearing.
Nurses have important roles in teaching women about early detection and treatment and in providing supportive care to women and their families. This chapter presents information that will assist the nurse in assessing and identifying problems associated with structural problems or benign or malignant reproductive neoplasms. Nursing care concepts related to early detection, treatment methods, and education are included.
Structural Disorders of the Uterus and Vagina
Alterations in pelvic support include uterine displacement and prolapse, cystoceles and rectoceles, urinary incontinence, and genital fistulas. Research by Wu and colleagues (2009) suggests that the prevalence of these disorders will increase by as much as 55% in the United States between the years 2010 and 2050 as the numbers of older women increase.
Uterine Displacement and Prolapse
The round ligaments normally hold the uterus in anteversion, and the uterosacral ligaments pull the cervix backward and upward (see Fig. 4-3). Uterine displacement is a variation of this normal placement. The most common type of displacement is posterior displacement, or retroversion, in which the uterus is tilted posteriorly, and the cervix rotates anteriorly. Other variations include retroflexion and anteflexion (Fig. 11-1).
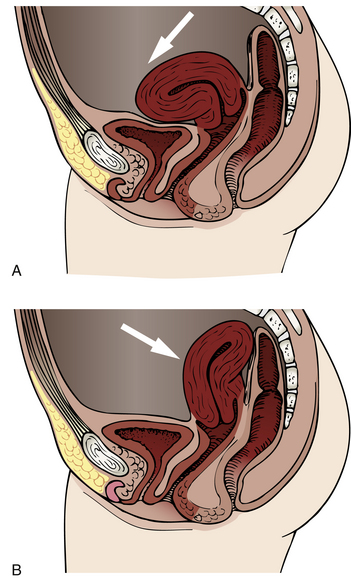
FIG. 11-1 Types of uterine displacement. A, Anterior displacement. B, Retroversion (backward displacement of the uterus).
By 2 months postpartum, the ligaments should return to normal length, but in about one third of women, the uterus remains retroverted. This condition is rarely symptomatic, but conception may be difficult because the cervix points toward the anterior vaginal wall and away from the posterior fornix, where seminal fluid pools after coitus. If symptoms occur, they may include pelvic and low back pain, dyspareunia, and exaggeration of premenstrual symptoms.
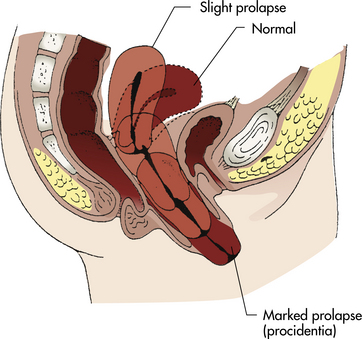
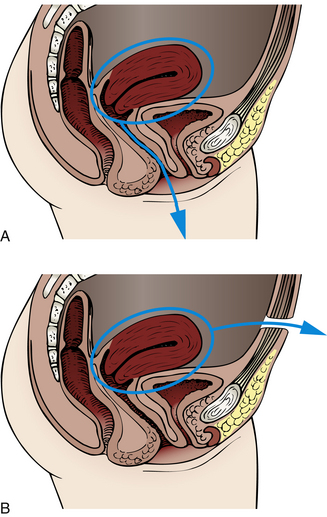
Uterine prolapse is a more serious type of displacement. The degree of prolapse can vary from mild to complete. In complete prolapse, the cervix and body of the uterus protrude through the vagina, and the vagina is inverted (Fig. 11-2).
Uterine displacement and prolapse can be caused by congenital or acquired weakness of the pelvic support structures (often called pelvic relaxation). In many cases, problems can be a delayed but direct result of childbearing. Although extensive damage may be noted and repaired shortly after birth, symptoms related to pelvic relaxation most often appear during the perimenopausal period, when the effects of ovarian hormones on pelvic tissues are lost, and atrophic changes begin. Pelvic trauma, stress and strain, and the aging process also are contributing factors. Other causes of pelvic relaxation include reproductive surgery and pelvic radiation.
Clinical Manifestations: Symptoms of pelvic relaxation generally relate to the structure involved: urethra, bladder, uterus, vagina, cul-de-sac, or rectum. The most common complaints are pulling and dragging sensations, pressure, protrusions, fatigue, and low backache. Symptoms may be worse after prolonged standing or deep penile penetration during intercourse. Urinary incontinence can be present.
Cystocele and Rectocele
Cystocele and rectocele almost always accompany uterine prolapse, causing the uterus to sag even farther backward and downward into the vagina. Cystocele (Fig. 11-3, A) is the protrusion of the bladder downward into the vagina that develops when supporting structures in the vesicovaginal septum are injured. Anterior wall relaxation gradually develops over time as a result of congenital defects of support structures, childbearing, obesity, or advanced age. When the woman stands, the weakened anterior vaginal wall cannot support the weight of the urine in the bladder; the vesicovaginal septum is forced downward, the bladder is stretched, and its capacity is increased. With time the cystocele enlarges until it protrudes into the vagina. Complete emptying of the bladder is difficult because the cystocele sags below the bladder neck. Rectocele is the herniation of the anterior rectal wall through the relaxed or ruptured vaginal fascia and rectovaginal septum; it appears as a large bulge that may be seen through the relaxed introitus (see Fig. 11-3, B).
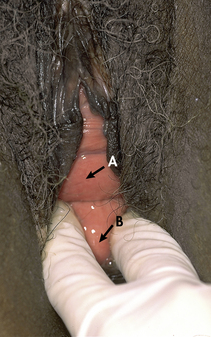
FIG. 11-3 A, Cystocele. B, Rectocele. (From Seidel, H., Ball, J., Dains, J., Flynn, J., Solomon, B., & Stewart, R. [2011]. Mosby’s guide to physical examination [7th ed.]. St. Louis: Mosby.)
Clinical Manifestations: Cystoceles and rectoceles often are asymptomatic. If symptoms of cystocele are present, they include complaints of a bearing-down sensation or that “something is in my vagina.” Other symptoms include urinary frequency, retention, and/or incontinence, and possible recurrent cystitis and urinary tract infections (UTIs). Pelvic examination will reveal a bulging of the anterior wall of the vagina when the woman is asked to bear down. Unless the bladder neck and urethra are damaged, urinary continence is unaffected. Women with large cystoceles complain of having to push upward on the sagging anterior vaginal wall to be able to void.
Rectoceles may be small and produce few symptoms, but some are so large that they protrude outside of the vagina when the woman stands. Symptoms are absent when the woman is lying down. A rectocele causes a disturbance in bowel function, the sensation of bearing down, or the sensation that the pelvic organs are falling out. With a very large rectocele it may be difficult to have a bowel movement. Each time the woman strains during bowel evacuation, the feces are forced against the thinned rectovaginal wall, stretching it more. Some women facilitate evacuation by applying digital pressure vaginally to hold up the rectal pouch.
Urinary Incontinence
Urinary incontinence (UI) affects young and middle-aged women, with the prevalence increasing as the woman ages. Although nulliparous women can have UI, the incidence is higher in women who have given birth and increases with parity. Women who are overweight and those who have had a hysterectomy are also at increased risk (Sung & Hampton, 2009). There are conflicting data about ethnicity and race as contributing factors (Waetjen, Laio, Johnson, Sampselle, Sternfield, Harlow, & Gold, 2007). Conditions that disturb urinary control include stress incontinence due to sudden increases in intraabdominal pressure (such as that due to sneezing or coughing); urge incontinence, caused by disorders of the bladder and urethra, such as urethritis and urethral stricture, trigonitis, and cystitis; neuropathies, such as multiple sclerosis, diabetic neuritis, and pathologic conditions of the spinal cord; and congenital and acquired urinary tract abnormalities. Research also suggests that a significant number of women have undiagnosed urinary incontinence (Wallner, Porten, Meenan, O’Keefe Rosetti, Calhoun, Sarma, & Clemens, 2009).
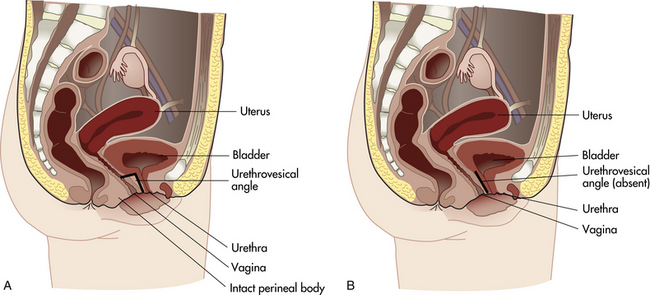
Stress incontinence may follow injury to bladder neck structures. A sphincter mechanism at the bladder neck compresses the upper urethra, pulls it upward behind the symphysis, and forms an acute angle at the junction of the posterior urethral wall and the base of the bladder (urethrovesical angle) (Fig. 11-4). To empty the bladder, the sphincter complex relaxes, and the trigone contracts to open the internal urethral orifice and pull the contracting bladder wall upward, forcing urine out. The angle between the urethra and the base of the bladder is lost or increased if the supporting pubococcygeus muscle is injured; this change, coupled with urethrocele, causes incontinence. Urine spurts out when the woman is asked to bear down or cough in the lithotomy position.
Genital Fistulas
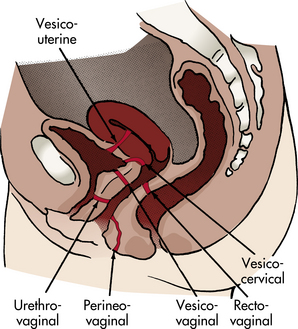
Genital fistulas are perforations between genital tract organs. Most occur between the bladder and the genital tract (e.g., vesicovaginal); between the urethra and the vagina (urethrovaginal); and between the rectum or sigmoid colon and the vagina (rectovaginal) (Fig. 11-5). Genital fistulas also may be a result of a congenital anomaly, gynecologic surgery, obstetric trauma, cancer, radiation therapy, gynecologic trauma, or infection (e.g., in the episiotomy).
Collaborative Care
Assessment for problems related to structural disorders of the uterus and vagina focuses primarily on the genitourinary tract, the reproductive organs, bowel elimination, and psychosocial and sexual factors. A complete health history, a physical examination, and laboratory tests are done to support the appropriate medical diagnosis. The nurse must assess the woman’s knowledge of the disorder, its management, and possible prognosis. Possible nursing diagnoses for structural problems of the uterus and vagina include the following:
• Deficient knowledge related to:
• Constipation or diarrhea related to:
• Ineffective coping related to:
• Interrupted family processes related to:
• lack of skill in self-care procedures
• lack of understanding of the reasons for the need to comply with therapy
• Social isolation, spiritual distress, disturbed body image, or chronic low self-esteem related to:
The health care team works together to treat the disorders related to alterations in pelvic support and to assist the woman in management of her symptoms. In general, nurses working with these women can provide information and self-care education to prevent problems before they occur, to manage or reduce symptoms and promote comfort and hygiene if symptoms are already present, and to recognize when further intervention is needed. This information can be part of all postpartum discharge teaching or can be provided at postpartum follow-up visits in clinics or physician/nurse-midwife offices, during postpartum home visits, or during gynecologic health examinations. In addition, information on how to prevent or recognize problems can be a topic for workshops for women or health fairs in community settings.
Besides providing information about prevention, nurses participate in a team effort to prepare the woman for surgery and self-care after discharge. Preoperative teaching involves the primary nurse, operating room nurse, surgeon, and anesthesia provider. Postoperatively, a nurse in the health promotion setting may be most aware of the woman’s living circumstances, physical limitations, and social problems and therefore may be best suited to coordinate continuity of care after discharge.
Interventions for specific problems depend on the problem and the severity of the symptoms. If discomfort related to uterine displacement is a problem, several interventions can be implemented. Kegel exercises (see p. 91) can be performed several times daily to increase muscle strength. A knee-chest position performed for a few minutes several times a day can correct a mildly retroverted uterus. A fitted pessary to support the uterus and hold it in the correct position (Fig. 11-6) may be inserted in the vagina. Usually a pessary is used only for a short time because it can lead to pressure necrosis and vaginitis. Good hygiene is important; some women can be taught to remove the pessary at night, cleanse it, and replace it in the morning. If the pessary is always left in place, regular douching with commercially prepared solutions or weak white vinegar solutions (1 tablespoon to 1 quart [liter] of water) to remove increased secretions and keep the vaginal pH at 4 to 4.5 is suggested. After a period of treatment, most women are free of symptoms and do not require the pessary. Surgical correction is rarely indicated.
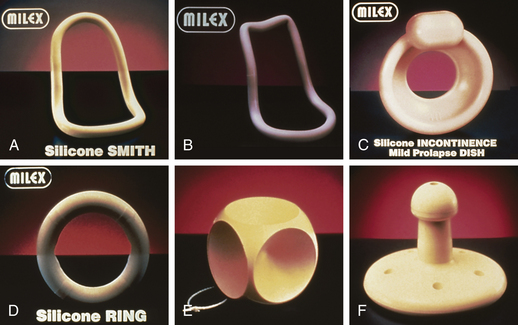
FIG. 11-6 Examples of pessaries. A, Smith. B, Hodge without support. C, Incontinence dish without support. D, Ring without support. E, Cube. F, Gellhorn. (Courtesy Milex Products, Inc., a division of CooperSurgical, Trumbull, CT.)
Treatment for uterine prolapse depends on the degree of prolapse. Pessaries may be useful in mild prolapse. Estrogen therapy also may be used in the older woman to improve tissue tone. If these conservative treatments do not correct the problem, or if there is a significant degree of prolapse, abdominal or vaginal hysterectomy (see later discussion) is usually recommended (Lentz, 2007).
Mild to moderate urinary infections can be significantly decreased or relieved in many women by bladder training and pelvic muscle (Kegel) exercises (Bersuk, 2007; Dumoulin & Hay-Smith, 2010). Other management strategies include pelvic flow support devices (i.e., pessaries), vaginal estrogen therapy, serotonin-norepinephrine reuptake inhibitors, electrical stimulation, insertion of an artificial urethral sphincter, and surgery (e.g., anterior repair) (Tarnay & Bhatia, 2010).
Nursing care for women with urinary incontinence includes assessment for depression that can result from decreased quality of life and functional status. Women also may need guidance about changes in lifestyle (e.g., losing weight) and education about pelvic muscle exercises (Peterson, 2008; Sung, West, Hernandez, Wheeler, Myers, Subak, et al., 2009).
Treatment for a cystocele includes use of a vaginal pessary or surgical repair. Pessaries may not be effective. Anterior repair (colporrhaphy) is the usual surgical procedure and is commonly performed for large, symptomatic cystoceles. This involves a surgical shortening of pelvic muscles to provide better support for the bladder. An anterior repair is often combined with a vaginal hysterectomy. Kegel exercises may be beneficial for symptoms of urinary and fecal incontinence (Lentz, 2007).
Small rectoceles may not require treatment. The woman with mild symptoms may derive relief from a high-fiber diet and adequate fluid intake, stool softeners, or mild laxatives. Vaginal pessaries usually are not effective. Large rectoceles that are causing significant symptoms are usually repaired surgically. A posterior repair (colporrhaphy) is the usual procedure. This surgery is performed vaginally and involves shortening the pelvic muscles to provide better support for the rectum (Lentz, 2007). Anterior and posterior repairs may be performed at the same time and with a vaginal hysterectomy.
Management of genital fistulas depends on the location. Surgical repair is the usual treatment; however, it may not be successful.
Nursing care of the woman with a cystocele, rectocele, or fistula requires great sensitivity, because the woman’s reactions are often intense. She may become withdrawn or hostile because of embarrassment caused by odors and soiling of her clothing that are beyond her control. She may have concerns about engaging in sexual activities because her partner is repelled by these problems. The nurse can tactfully suggest hygiene practices that reduce odor. Commercial deodorizing douches are available, or noncommercial solutions, such as chlorine solution (1 teaspoon of household chlorine bleach to 1 quart of water) may be used. The chlorine solution also is useful for external perineal irrigation. Sitz baths and thorough washing of the genitals with unscented, mild soap and warm water help. Sparse dusting with deodorizing powders can be useful. If a rectovaginal fistula is present, enemas given before leaving the house may provide temporary relief from oozing of fecal material until corrective surgery is performed. Irritated skin and tissues may benefit from use of the heat lamp or application of an emollient ointment. Hygienic care is time consuming and may need to be repeated frequently throughout the day; protective pads or pants may have to be worn. All of these activities can be demoralizing to the woman and frustrating to her and her family. If surgical repair is performed, nursing care focuses on preventing infection and helping the woman avoid putting stress on the surgical site.
Benign Neoplasms
Benign neoplasms include a variety of nonmalignant cysts and tumors of the ovaries, the uterus, the vulva, and other organs of the reproductive system.
Ovarian Cysts
Functional ovarian cysts (Fig. 11-7) are dependent on hormonal influences associated with the menstrual cycle. These cysts may be classified as follicular cysts, corpus luteum cysts, theca-lutein cysts, endometrial cysts, and polycystic ovary syndrome. Other benign ovarian neoplasms include dermoid cysts and ovarian fibromas.
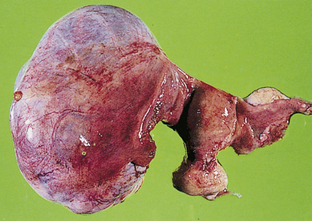
FIG. 11-7 Ovarian cyst. (From Seidel, H., Ball, J., Dains, J., Flynn, J., Solomon, B., & Stewart, R. [2011]. Mosby’s guide to physical examination [7th ed.]. St. Louis: Mosby.)
Follicular Cysts
Follicular cysts develop most commonly in normal ovaries of young women as a result of the mature graafian follicle failing to rupture, or when an immature follicle does not resorb fluid after ovulation. A cyst is usually asymptomatic unless it ruptures, in which case it causes severe pelvic pain. If the cyst does not rupture, it usually shrinks after two or three menstrual cycles.
Corpus Luteum Cysts
Corpus luteum cysts occur after ovulation and are possibly caused by an increased secretion of progesterone that results in an increase of fluid in the corpus luteum. Clinical manifestations associated with a corpus luteum cyst include pain, tenderness over the ovary, delayed menses, and irregular or prolonged menstrual flow. A rupture can cause intraperitoneal hemorrhage. Corpus luteum cysts usually disappear without treatment within one or two menstrual cycles.
Theca-Lutein Cysts
Theca-lutein cysts are uncommon and in up to 50% of cases are associated with hydatidiform mole (see Chapter 28). Theca-lutein cysts develop as a result of prolonged stimulation of the ovaries by human chorionic gonadotropin (hCG). They also may occur if the woman has taken ovulation induction drugs; if she is pregnant and a large placenta is present, such as in the presence of a multiple gestation; or if the woman has diabetes (Katz, 2007). The cysts are almost always bilateral. A feeling of pelvic fullness may be noted by the woman if the ovary is enlarged, but most women are asymptomatic.
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) occurs when an endocrine imbalance results in high levels of estrogen, testosterone, and luteinizing hormone (LH) and decreased secretion of follicle-stimulating hormone. This syndrome is associated with a variety of problems in the hypothalamic-pituitary-ovarian axis and with androgen-producing tumors. The condition can be transmitted as an X-linked dominant or autosomal dominant trait (Stein-Leventhal syndrome). Multiple follicular cysts develop on one or both ovaries and produce excess estrogen. The ovaries often double in size. Clinical manifestations include obesity, hirsutism (excessive hair growth), irregular menses or amenorrhea, and infertility. Impaired glucose tolerance and hyperinsulinemia occur in about 40% of women with PCOS (Lobo, 2007). Affected women are at high risk for developing type 2 diabetes mellitus and possibly cardiovascular diseases (Benson, Hahn, Tan, Janssen, Schedlowski, & Elsenbruch, 2010). PCOS is often diagnosed in adolescence when menstrual irregularities and other symptoms appear (Lobo).
Collaborative Care
A variety of interventions may be implemented for the woman with a functional cyst. If expectant management is the treatment, the woman is advised to keep appointments for pelvic examinations to monitor the changes in size of the cyst (enlarging or shrinking). Pharmacologic interventions such as analgesics may be prescribed for pain management. Oral contraceptives may be ordered for several months to suppress ovulation for functional cysts. Large cysts (greater than 8 cm) or cysts that do not shrink may be removed surgically (cystectomy). Corpus luteum cysts are treated similarly. Theca-lutein cysts are usually managed conservatively (they usually regress) or by removal of the hydatidiform mole (Katz, 2007).
Nursing care focuses on educating the woman regarding treatment options as well as pain management with analgesics or comfort measures such as heat to the abdomen or relaxation techniques. If surgery is performed, the nurse provides preoperative and postoperative care. Discharge teaching includes signs of infection, postoperative incision care, the possibility of recurrence, and advice regarding follow-up appointments.
The treatment for PCOS depends on what symptoms are of greatest concern to the woman. Lifestyle modifications (e.g., losing weight) and management of presenting symptoms such as infertility, irregular menses, and hirsutism are the focus. Oral contraceptives (OCs) are the usual treatment for irregular menses, if pregnancy is not desired, because they inhibit LH and decrease testosterone levels. OCs can also lessen acne to some degree. Gonadotropin-releasing hormone (GnRH) analogs may be used to treat hirsutism if oral contraceptives do not improve this condition. If pregnancy is desired, ovulation-inducing medications are given (Lobo, 2007). Metformin and other insulin medications for type 2 diabetes also are used to lower insulin, testosterone, and glucose levels, which in turn can reduce acne, hirsutism, abdominal obesity, amenorrhea, and other symptoms in women with PCOS (Lobo).
Nurses can provide information and counseling for women with PCOS. Information may be needed about the syndrome or about its long-term effects on the woman’s health. Research has shown that women report symptoms of psychologic distress including depression, anxiety, and social fears (Benson et al., 2010). Women may need to discuss their feelings about the physical manifestations of PCOS and may need emotional support if they have self-image problems related to the symptoms. Teaching about lifestyle modifications such as exercise and diet may be needed as well as education about the medications that are prescribed. Information about finding a support group or information on the Internet may be useful.
Other Benign Ovarian Cysts and Neoplasms
Two other ovarian neoplasms are dermoid cysts and ovarian fibromas. Dermoid cysts are germ cell tumors, usually occurring in childhood. These cysts contain substances such as hair, teeth, sebaceous secretions, and bones. Unless the cyst is large enough to put pressure on other organs, it is usually asymptomatic. Dermoid cysts may develop bilaterally and are often attached to the ovary. Treatment is usually surgical removal.
Ovarian fibromas are solid ovarian neoplasms developing from connective tissue and most often occurring after menopause. Fibromas range in size from small nodules to large masses weighing more than 23 kg. Most fibromas are unilateral. They are usually asymptomatic, but if large enough, they may cause ascites, feelings of pelvic pressure, or abdominal enlargement. Treatment is usually surgical removal.
Nursing care of women who have surgery for the removal of dermoid cysts and ovarian fibromas is similar to that described for functional ovarian cysts.
Uterine Polyps
Uterine polyps may be endometrial or cervical in origin. They are tumors that are on pedicles (stalks) arising from the mucosa (Fig. 11-8). The etiology is unknown, although they may develop in response to hormonal stimulus or be the result of inflammation. Polyps are the most common benign lesions of the cervix and endometrium that occur during the reproductive years. These polyps may be single or multiple. Endocervical polyps are most common in multiparous women older than 40 years. The woman may be asymptomatic or she may have premenstrual or postmenstrual bleeding or postcoital bleeding (Nelson & Gambone, 2010).
Collaborative Care
Clinical management of endometrial polyps is by surgical removal. Cervical polyps are usually removed in an office or clinic procedure without anesthesia. The polyp is grasped with a clamp and twisted or cut off. All polyps should be sent for pathologic examination. Endometrial sampling (which may require local anesthesia) should be done to determine if other pathologic conditions are present (Katz, 2007).
Nursing care includes preparing the woman for what to expect during the removal procedure and encouraging relaxation and breathing exercises and providing support during the procedure. After the procedure the woman is advised to avoid using tampons, sexual intercourse, and douching for up to 1 week or until the site is healed. She is taught how to identify signs of infection and to notify her health care provider if she experiences heavy bleeding (more than one pad in 1 hour).
Leiomyomas
Leiomyomas, also known as fibroid tumors, fibromas, myomas, or fibromyomas, are slow-growing benign tumors arising from the muscle tissue of the uterus (Nelson & Gambone, 2010). They are the most common benign tumors of the reproductive system, occurring most often after age 50 years. They tend to occur more often in African-American women and women who have never been pregnant (Nelson & Gambone). Fibroids also occur more often in women who are overweight (Katz, 2007). They rarely become malignant. Because their growth is influenced by ovarian hormones, these benign tumors can become quite large when the woman is pregnant or taking hormone therapy. They often spontaneously shrink after menopause when circulating ovarian hormones are diminished (Katz; Nelson & Gambone).
Clinical Manifestations and Diagnosis
The cause of leiomyomas remains unknown, although genetic factors may be involved in their development. Most of the tumors are found in the body of the uterus. Leiomyomas are classified according to the location in the uterine wall. Subserous leiomyomas develop beneath the peritoneal surface of the uterus and appear as small or large masses that protrude from the outer uterine surface (Fig. 11-9, A). Intramural leiomyomas are tumors that develop within the wall of the uterus (see Fig. 11-9, B). Submucosal leiomyomas are the least common tumors, but often cause the most symptoms. These tumors develop in the endometrium and protrude into the uterine cavity (see Fig. 11-9, C). Leiomyomas can develop in the cervix and on the broad ligaments (see Fig. 11-9, D). They can grow on pedicles or stalks (see Fig. 11-9, E). Occasionally these break off the pedicle and attach to other tissues (become parasitic).
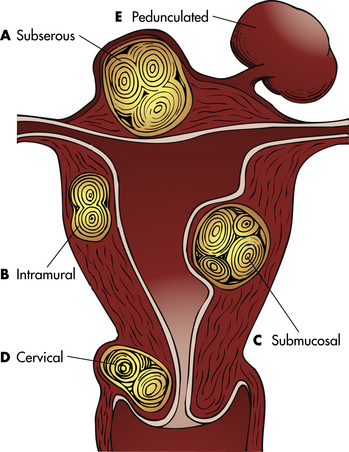
FIG. 11-9 Types of leiomyomas. A, Subserous. B, Intramural. C, Submucosal. D, Cervical. E, Pedunculated.
Most women are asymptomatic; abnormal uterine bleeding is the most common symptom of fibroids. If the tumor is very large, pelvic circulation may be compromised, and surrounding viscera may be displaced. A woman may complain of backache, low abdominal pressure, constipation, urinary incontinence, or dysmenorrhea (painful menstruation). Nausea and vomiting may occur if the tumor is obstructing the intestines. The woman also may notice an abdominal mass if the tumor is large. Anemia can occur if the woman has excessive bleeding. Pedunculated tumors can twist and become necrotic, causing pain.
The tumors appear to be influenced by the presence of estrogen. Fibroids can affect implantation and maintenance of pregnancy. During pregnancy, the tumors may produce complications such as preterm labor, miscarriage, or dystocia (difficult labor). The severity of the symptoms seems to be directly related to the size and location of the tumors.
Care Management
Knowledge of the medical-surgical management of leiomyomas is essential in planning nursing care. The knowledge enables the nurse to work collaboratively with other health care providers and to meet the woman’s informational and emotional needs. Clinical management for benign tumors of the uterus depends on the severity of the symptoms, the age of the woman, and her desire to preserve childbearing potential (see Nursing Process box: Woman with a Leiomyoma).
Medical Management
Medications: If symptoms are mild, regular checkups may suffice to observe for growth or changes in size. Nonsteroidal antiinflammatory drugs (NSAIDs) may be prescribed for pain; oral contraceptives inhibit ovulation and may relieve symptoms; and GnRH agonists such as leuprolide acetate (Lupron, Synarel) may be prescribed to reduce the size of the leiomyoma. Other medications used include medroxyprogesterone acetate (Depo-Provera), danazol (Danocrine), mifepristone (Mifeprex), and selective estrogen receptor modulators (SERMs) (e.g., raloxifene) (Katz, 2007; Nelson & Gambone, 2010; Wu, Chen, & Xie, 2007). The ideal medical therapy for treating fibroids has not been found, and research in this area continues (Sankaran & Manyonda, 2008).
The woman who prefers medical treatment will need information about the various medications, their actions and side
effects, and routes of administration. A woman who is receiving GnRH agonists to decrease the size of the fibroid must understand that regrowth will occur after the treatment is stopped. She also must know that a small loss in bone mass and changes in lipid levels can occur; therefore, long-term use is not recommended. Adding raloxifene to GnRH administration has been effective in preventing these effects in some premenopausal women (Nelson & Gambone, 2010; Sankaran & Manyonda, 2008). Amenorrhea may occur; however, women who wish to avoid pregnancy should use a nonhormonal or barrier method of contraception. A discussion of administration methods for GnRH agonists, including subcutaneous and intramuscular injections, intranasal administration, and subcutaneous implantation, will assist the woman in making a decision about her preferred method of administration (see Medication Guide, p. 205).
Uterine Artery Embolization: Uterine artery embolization (UAE) is a treatment during which polyvinyl alcohol (PVA) pellets are injected into selected blood vessels to block the blood supply to the fibroid and cause shrinkage and resolution of symptoms (Katz, 2007). The procedure is done under local anesthesia and conscious sedation and can be done as an outpatient procedure, although some women will have the procedure in the hospital setting and remain overnight or be discharged within 4 to 6 hours (Katz; Pisco, Bilhim, Duarte, & Santos, 2009). An incision is made into the groin, and a catheter is threaded into the femoral artery to the uterine artery. An arteriogram identifies the vessels supplying the fibroid. Most fibroids are reduced in size by 50% within 3 months. Temporary amenorrhea or early menopause can occur in some women. Although symptom improvement occurs for most women, data are lacking about the effects on future fertility and pregnancy outcomes. Long-term effects of the procedure are unknown (Katz).
Preoperative teaching includes advising the woman not to drink alcohol or smoke and not to take aspirin or anticoagulant medications 24 hours before the procedure. If the procedure is done on an outpatient basis, the woman will usually need to take acid-suppressing medications, NSAIDs, and antihistaminic drugs as well as laxatives beginning the day before the procedure (Pisco et al., 2009). The woman is told to expect cramping during injection of the PVA pellets. Explanations about what to expect postoperatively include pelvic pain, fever, malaise, and nausea and vomiting that may be caused by acute fibroid degeneration. Pain can be controlled with NSAIDs or narcotic analgesics if needed. Postoperative nursing assessments include checking for bleeding in the groin, taking vital signs, assessing pain level, and checking the pedal pulse and neurovascular condition of the affected leg (Hiller, Miller, & Stavas, 2005). Discharge teaching includes signs of possible complications and when to notify the physician, self-care instructions, and follow-up advice (see Teaching for Self-Management box: Care after Uterine Artery Embolization).
Surgical Management
In addition to the surgical options of hysterectomy and myomectomy, other techniques have been developed to treat leiomyomas. These include laparoscopic techniques; hysteroscopic techniques; myolysis by heat, cold, and laser; and magnetic resonance–guided focused ultrasound surgery. Not all of these
techniques are suitable for every woman nor are all of them universally available to women (Istre, 2008).
Laser Surgery: Laser surgery or electrocauterization can be used to destroy small fibroids through a laparoscopic (abdominal) or hysteroscopic (vaginal) approach. Hysteroscopic uterine ablation (vaporization of tissues) can be performed under local or general anesthesia, usually as an outpatient procedure. Medical therapy using GnRH agonists to control bleeding temporarily and to suppress endometrial tissue may be given for 8 to 12 weeks before surgery. Although the uterus remains in place, the vaporization process can cause scarring and adhesions in the uterine cavity, affecting future fertility. Thus this procedure is for women who wish to retain their uterus but no longer desire childbearing potential (Nelson & Gambone, 2010). Risks of the procedure include uterine perforation, cervical injury, and fluid overload (caused by the leaking into blood vessels of fluid used to expand the uterus during surgery). The woman may experience postoperative cramping and a slight vaginal discharge for a few days. Before discharge the following information is given:
• Analgesics or NSAIDs can be used for pain relief as needed.
• Normal activities can be resumed within several days.
• Vaginal discharge is to be expected for 4 to 6 weeks.
• Use of tampons or vaginal intercourse should be avoided for 2 weeks.
• The next menstrual period may be irregular.
• The woman should be reminded about the effects of ablation on her fertility, if appropriate.
• The physician should be called if the woman has heavy bleeding or signs of infection.
Myomectomy: If the tumor is near the outer wall of the uterus, the uterine size is no larger than at 12 to 14 weeks of gestation, and symptoms are significant, myomectomy (removal of the tumor) may be performed (Katz, 2007). Myomectomy can be performed through a laparoscopic or abdominal incision approach or a vaginal (hysteroscopic) approach. Myomectomy leaves the uterine muscle walls relatively intact, thereby preserving the uterus and allowing the possibility of future pregnancies (Agdi & Tulandi, 2008). It is usually performed in the proliferative phase of the menstrual cycle to avoid interrupting a possible pregnancy. GnRH therapy may be given before surgery to reduce the size of the fibroid. Fibroids can recur after myomectomy; further treatment may be needed (Nelson & Gambone, 2010).
Hysterectomy: Hysterectomy (removal of the entire uterus) is the treatment of choice if bleeding is severe or if the fibroid is obstructing normal function of other organs. An abdominal or vaginal surgical approach depends on the size and location of the tumors. For example, abdominal hysterectomy is usually performed for leiomyomas larger than a uterus would be at 12 to 14 weeks of gestation or for multiple leiomyomas. The uterus is removed through either a vertical or transverse incision. In some circumstances the cervix is not removed. Vaginal approaches can be used for smaller tumors. In both abdominal and vaginal approaches, the uterus is removed from the supporting ligaments (broad, round, and uterosacral). These ligaments are then attached to the vaginal cuff, allowing maintenance of normal depth of the vagina (Fig. 11-10). Alternatives to these procedures are the laparoscopic assisted vaginal hysterectomy (LAVH) and the laparoscopic assisted supracervical hysterectomy (LASH). LAVH converts an abdominal procedure to a vaginal one by using a laparoscope in the abdomen to assist with removal of the uterus. LASH allows the cervix to remain. Both are associated with a quicker recovery and fewer postoperative complications (Mueller, Renner, Haeberle, Lermann, Oppelt, Beckemann, & Thiel, 2009; Nieboer, Johnson, Lethaby, Tavender, Curr, Garry, et al., 2009).
Preoperative Care: Assessments needed before surgery include the woman’s knowledge of treatment options, her desire for future fertility if she is premenopausal, the benefits and risks of each procedure, preoperative and postoperative procedures (Boxes 11-1 and 11-2), and the recovery process (Askew, 2009). If the woman demonstrates understanding of this information, she can make an informed decision about treatment and feel a sense of control over the surgical experience. Resources on helping women to make decisions about treatment can be found at the website for the Fibroid Treatment Collective at www.fibroid.org.
Psychologic assessment is essential, particularly for a woman who is scheduled for a hysterectomy. Areas to be explored include the significance of the loss of the uterus for the woman, misconceptions about effects of surgery, and adequacy of her support system. Women who have not completed their childbearing, who believe that their self-concept is related to having a uterus (to be a complete woman), who feel that sexual functioning is related to having a uterus, or who have too little or too much anxiety about the surgery may be at risk for postoperative emotional reactions (Leppert, Legro, & Kjerulff, 2007). Yen and associates (2008) found that postoperatively most women reported positive feelings about femininity and their body image, less anxiety and depression, but still reported a worsening of sexual functioning.
Postoperative Care: Postoperative assessments and care after myomectomy and abdominal hysterectomy are similar to those for other abdominal surgery (Box 11-3). Assessments specific to abdominal and vaginal hysterectomy include assessment for vaginal bleeding (one perineal pad saturated in less than 1 hour is excessive), urinary retention (especially after vaginal hysterectomy), perineal pain after vaginal hysterectomy, and psychologic assessments (e.g, depression) (Leppert et al., 2007; Yen, Chen, Long, Chang, Yen, & Ko, 2008).
Discharge Planning and Teaching: Discharge planning and teaching are similar for myomectomy and hysterectomy (see Teaching for Self-Management box: Care After Myomectomy or Hysterectomy). Myomectomy and vaginal hysterectomy may be performed in an ambulatory setting, and women may be discharged the evening of the surgery. Women who have an abdominal hysterectomy may have a 1- to 2-day stay in the hospital before being discharged.
If a hysterectomy was performed, the woman is reminded that she will experience cessation of menses. If the woman is premenopausal, she will not experience menopause at this time unless her ovaries also were removed. In this case there will be no reason for her to consider hormone replacement therapy. If the ovaries are removed, the woman will need the most current information on the risks and benefits of hormone replacement therapy (see Chapter 6). Other symptoms she may experience include pain, sleep disturbance, fatigue, anxiety, and depression.
Vaginal intercourse may be uncomfortable at first, especially after vaginal procedures. Use of water-soluble lubricants, relaxation exercises, and positions that control penile penetration may be beneficial (Katz, 2007). Women can be assured that this discomfort will decrease over time.
The schedule for follow-up care depends on the procedure performed, but usually a postoperative visit is scheduled within a week. Vaginal screening with cytology/Papanicolaou (Pap) test after total hysterectomy for a nonmalignant reason is not recommended (American Cancer Society [ACS], 2010a); however, vaginal cancer can occur after hysterectomy and health care providers may continue to recommend Pap screening to assess for vaginal cancer (Slomovitz & Coleman, 2007).
Vulvar Problems
Bartholin cysts are the most common benign lesions of the vulva. They arise from obstruction of the Bartholin duct, which causes it to enlarge. Small cysts often are asymptomatic; however, large cysts or infected cysts cause symptoms such as vulvar pain, dyspareunia (painful intercourse), and a feeling of a mass in the vulvar area (Eckert & Lentz, 2007).
Collaborative Care: If the woman is asymptomatic no treatment is necessary. If the cyst is symptomatic or infected, surgical incision and drainage may provide temporary relief. Cysts tend to recur; therefore, a permanent opening for drainage may be recommended. This procedure is called marsupialization and is the formation of a new duct opening for drainage (Eckert & Lentz, 2007).
Nursing care after surgery includes teaching the woman about pain-relief measures such as sitz baths, heat lamps to the perineum, and use of analgesics. The woman is taught to assess the incision site for signs of healing and infection and to take antibiotics, if prescribed, for prevention of infection.
Vulvodynia
Vulvar pain is a common gynecologic problem. Vulvodynia, also called vulvar pain syndrome or vulvovestibulitis, is reportedly experienced by 4% to 27% of women. The incidence is thought to be the same for women of all races and ethnicities (Kingdon, 2009).
Vulvodynia is a complex condition thought to be a chronic pain disorder of the vulvar area. The term vulvodynia is used if pain is present with no visible abnormality or no identified neurologic diagnosis (Katz, 2007). Pain can be described as provoked (e.g., by inserting a tampon or having vaginal intercourse) or unprovoked and localized to the vestibule or generalized over the vulvar area (Kingdon, 2009).
Etiology has not been established although psychologic and biologic theories have been proposed. The most common theory is that vulvodynia is caused by a chronic neuropathic pain syndrome. Inflammation may also be a causative factor and continues to be investigated. (Zolnoun, Hartmann, Lamvu, As-Saine, Maixner, & Steege, 2006). Past sexual and emotional experiences and personality traits are being investigated as causes because they can influence the perception and interpretation of nerve impulses (Kingdon, 2009).
A feature of neuropathic pain is allodynia, which is a painful sensation that is from something not supposed to be painful. It commonly occurs in women with vulvodynia. Several triggers that reportedly cause allodynia in the vulvar area include use of oral contraceptives; presence of candidiasis or human papillomavirus; wearing tight-fitting underwear and pants, especially synthetic materials; being a victim of childhood sexual abuse; and using chemical irritants such as scented detergents, soaps, and bubble baths (Arnold, Bachmann, Rosen, & Rhoads, 2007; Goldstein & Burrows, 2008; Harlow, Vitonis, & Stewart, 2008). However, with all these triggers, research evidence is conflicting and the need for scientific evidence is ongoing.
Collaborative Care: Assessment of a woman with possible vulvodynia includes a health history including a mental health history, specifically inquiring about anxiety or depression. A thorough pain assessment is essential. Questions about provoking and palliative factors, the quality of pain, radiation of the pain, strength of the pain and the timing of pain occurrence are included. A history may elicit complaints about burning, stinging or irritation in the vulvar area, and reports of how the woman feels her symptoms affect her physical activities and ability for sexual intimacy.
A thorough pelvic examination is recommended to rule out other causes of pain such as infection or trauma. The vulva should be inspected for erythema, ulcerations, and hyperpigmentation (Goldstein & Burrows, 2008; Katz, 2007). A cotton swab is used to identify areas of pain on pressure to confirm presence of allodynia. A systematic assessment (e.g., using positions of the face of a clock) is suggested, and ratings of pain should be rated as mild, moderate, or severe. Reed (2006) suggests the indentation of the swab be about 5 cm. A speculum examination (a pediatric size is recommended) is used to examine the vagina for redness, erosions, and dryness. A swab of vaginal secretions is obtained and can be tested for yeast, increased white blood cells, and pH. Cultures for Candida and bacteria can be obtained. A bimanual examination may be performed (Katz; Reed).
Management strategies are individualized to the woman. Often a series of therapies or a combination of therapies will be implemented to find the best treatment. Currently there is little evidence to support one therapy over another. Oral medications include gabapentin and tricyclic antidepressants (Harris, Horowitz, & Bordiga, 2007; Katz, 2007; Reed, Caron, Gorenflo, & Haefner, 2006). Topical therapies include the use of lidocaine 5% ointment that can be applied nightly or prophylactically (i.e., before sexual intercourse) (Katz, 2007).
Other therapeutic measures that have been tried and are reportedly helpful for symptoms include pelvic floor exercises, biofeedback, vaginal dilator training, hypnosis, and cognitive-behavioral therapy (Hartmann, Strauhal, & Nelson, 2007; Munday, Buchan, Ravenhill, Wiggs, & Brooks, 2007; Katz, 2007; Pukall, Kandyba, Amsel, Khalifé, & Binik, 2007). ![]()
Hygienic measures suggested for women with vulvodynia include wearing white cotton underwear, using 100% cotton menstrual pads, using soaps and detergents for sensitive skin, avoiding wearing tight clothing over the vulvar area, avoiding lubricants that contain propylene glycol, and using natural oils such as olive oil for lubricants (Kingdon, 2009).
Surgery is usually not recommended until other measures have proven to be ineffective. The surgical procedure is a vestibulectomy, a difficult procedure that removes the vestibule and hymen and has a high rate of complications (Katz, 2007). Research by Bergeron, Khalife, Glazer, & Binik (2008) found this procedure to be no more effective than less invasive measure such as biofeedback.
Client information about vulvodynia including how to locate support groups is available on various websites including:
• International Society for the Study of Vulvovaginal Disease: www.issvd.org
• National Vulvodynia Association: www.nva.org
• Vulvar Pain Society: www.vulvarpainsociety.org
Nurses can recommend these websites to women who want more information about vulvodynia. Nurses also need to keep current on the latest research so that care can be evidence based.
Malignant Neoplasms
Malignant neoplasms of the reproductive system include cancers of the endometrium, the cervix, the ovary, the vulva, the vagina, and the uterine tubes. In 2010 an estimated 83,750 women in the United States were diagnosed with a gynecologic cancer; an estimated 27,710 died (ACS, 2010a). Overweight and obesity are associated with increased risk for developing many cancers, including cancers of the endometrium, ovary, and cervix. Evidence also suggests that being overweight increases the risk for cancer recurrence and decreases the likelihood of survival for these cancers (ACS).
Cancer of the Endometrium
Endometrial cancer is the most common malignancy of the reproductive system (ACS, 2010a). It is most commonly seen in perimenopausal and postmenopausal women between ages 50 and 65. Certain risk factors have been associated with the development of endometrial cancer, including obesity, nulliparity, infertility, late onset of menopause, diabetes mellitus, hypertension, PCOS, and family history of ovarian or breast disease (ACS; Creasman, 2007a). There appears to be an increase in risk for endometrial cancer in families with hereditary nonpolyposis colorectal cancer (HNPCC). Hormone imbalance, however, seems to be the most significant risk factor (American College of Obstetricians and Gynecologists [ACOG], 2008). Numerous studies have correlated the use of exogenous estrogens (unopposed stimulation, i.e., absence of progesterone) in postmenopausal women with an increased incidence of uterine cancer. Tamoxifen taken by women for breast cancer also has been related to a slight increase in endometrial cancer (Creasman). Pregnancy and use of low-dose oral contraceptive pills appear to offer some protection (ACS). The incidence of endometrial cancer among Caucasian women is higher than that among African-American and Hispanic women; however, the mortality rates are more than one and one half times higher in African-American women (Ries, Melbert, Krapcho, Stinchcomb, Howlader, Horner, et al., 2008).
Endometrial cancer is slow growing and for that reason has a good prognosis if diagnosed at a localized stage. Most endometrial cancers are adenocarcinomas that develop from endometrial hyperplasia. The tumor usually develops in the fundus of the uterus and can spread directly to the myometrium and cervix, as well as to other reproductive organs. Metastasis (spread of cancer from its original site) is through the lymphatic system in the pelvis and through the blood to the liver, the lungs, and the brain.
Care Management
Assessment and Nursing Diagnoses
Assessment includes a history of physical symptoms. The cardinal sign of endometrial cancer is abnormal uterine bleeding (e.g., postmenopausal bleeding and premenopausal recurrent metrorrhagia). Thirty percent of postmenopausal bleeding is caused by carcinoma. Late signs include a mucosanguineous vaginal discharge, low back pain, or low pelvic pain. A pelvic examination may reveal the presence of a uterine enlargement or mass.
Histologic examination is used for diagnosis. A Pap smear of cellular material obtained by aspiration of the endocervix will identify only one third to one half of cases. Fractional curettage or endometrial biopsy yields the most accurate results. Fractional curettage involves scraping the endocervix and endometrium for histologic evaluation to determine the grade of neoplasm and its stage (extent). Perforation of the uterus is a possible complication of this procedure. Endometrial biopsy will identify about 90% of cases (Creasman, 2007a; Hacker, 2010a). It is usually done on an outpatient basis under local anesthesia. A suction-type curette is used to remove tissue for sampling. It is recommended that women at risk for HNPCC have an annual biopsy beginning at age 35 years (ACS, 2010a). Other diagnostic tests that may be useful include hysteroscopy (examination of the uterus through an endoscope) and vaginal ultrasonography. Tests to determine the spread of cancer include liver function tests, renal function tests, chest x-ray, intravenous pyelography (IVP), barium enema, computed tomography (CT), magnetic resonance imaging (MRI), bone scans, and biopsy of suggestive tissues. The International Federation of Gynecology and Obstetrics (FIGO) classification system is used to describe the stages of endometrial carcinoma (Table 11-1).
TABLE 11-1
FIGO CLASSIFICATION OF ENDOMETRIAL CARCINOMA∗
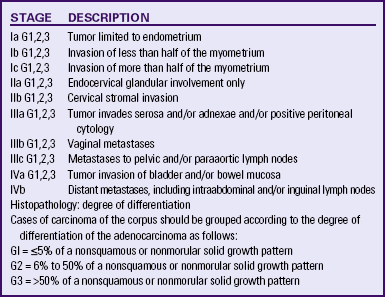
∗Approved by FIGO, October 1988, Rio de Janeiro.
Source: Creasman, W. (2007a). Adenocarcinoma of the uterus. In P. DiSaia & W. Creasman (Eds.), Clinical gynecologic oncology (7th ed.). St. Louis: Mosby.
Possible nursing diagnoses that would apply to a woman with endometrial cancer include the following:
• Deficient knowledge related to:
• Decisional conflict related to:
• Impaired skin integrity related to:
• Acute or chronic pain related to:
Expected Outcomes of Care
Planning for care of the woman with endometrial cancer depends on the stage of cancer and the treatment selected. Examples of expected outcomes are that the woman will do the following:
• Demonstrate understanding of her diagnosis of endometrial cancer, the treatments available, and her prognosis.
• Make informed decisions about treatment options.
• Describe a decrease in anxiety and fear.
• Report that pain is reduced or manageable.
• Experience no skin breakdown or infection related to treatment.
• State that she understands the effects of cancer and treatment on her body image and that her concerns are reduced.
• Report that she and her partner expect to be able to resume mutually satisfying sexual relations after treatment.
Plan of Care and Interventions
Therapeutic Management: Collaborative efforts from various health disciplines are needed to work with the woman with endometrial cancer. All must have an understanding of the treatments that may be used.
For stage I adenocarcinoma of the endometrium limited to the uterus, total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO) is the usual treatment (Creasman, 2007a). Radiation use in stage I continues to be studied; it can reduce the risk of recurrence, but evidence does not demonstrate improved survival rates or reduce metastasis to distant sites. It can be used when the woman is a poor surgical risk (Lu & Slomovitz, 2007). A radical hysterectomy (abdominal hysterectomy with wide excision of parametrial tissue laterally and uterosacral ligaments posteriorly), BSO, and pelvic node dissection usually are performed for stage II endometrial cancer. If nodes are positive or if there is extensive uterine disease or metastasis outside the uterus, external pelvic radiation (see p. 257) is usually done postoperatively. Internal radiation therapy or brachytherapy (placement of an applicator loaded with a radiation source into the uterine cavity) (see p. 258) also may be used before surgery or combined with external radiation (Lu & Slomovitz). Treatment of advanced stages is individualized but usually includes a TAH-BSO plus chemotherapy or radiation, or both (Hacker, 2010a).
Chemotherapy is used to treat advanced and recurrent disease, although no effective treatment regimen has been established (Lu & Slomovitz, 2007). Agents that have been somewhat effective include cisplatin, doxorubicin, carboplatin, cyclophosphamide, 5-fluorouracil, and paclitaxel (Lu & Slomovitz). Chemotherapy may cause hair loss, anemia, and bone marrow depression, as well as other side effects (Table 11-2).
TABLE 11-2
Common Side Effects of Chemotherapy Agents Used for Gynecologic Cancers∗
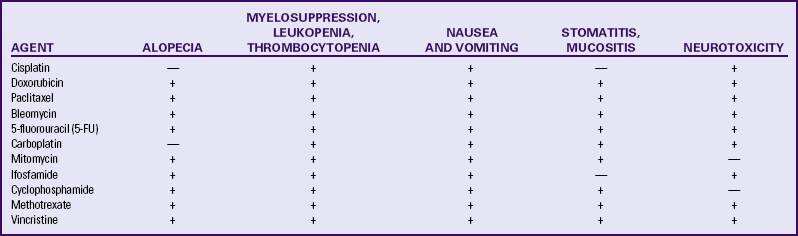
∗Incidence and seriousness of side effects may be dose related.
Sources: Chu, C., & Rubin, S. (2007). Basic principles of chemotherapy. In P. DiSaia & W. Creasman (Eds.), Clinical gynecologic oncology (7th ed.). St. Louis: Mosby; Facts and Comparisons. (2009). Drug facts and comparisons. St Louis: Wolters Kluwer; Kunos, C., & Waggoner, S. (2007). Principles of radiation therapy and chemotherapy in gynecologic cancer. In V. Katz, G., Lentz, R. Lobo, & D. Gershenson (Eds.), Comprehensive gynecology (5th ed.). Philadelphia: Mosby.
Progestational therapy—use of medroxyprogesterone (Depo-Provera) and megestrol (Megace)—may be effective for recurrent cancers, especially those that are estrogen receptor positive. These drugs usually do not cause acute side effects. Tamoxifen and raloxifene (see Medication Guide on pp. 226-227) are antiestrogens that have shown some effectiveness against recurrent endometrial cancer (Creasman, 2007a; Lu & Slomovitz, 2007).
Nursing Management: Nursing care is individualized to the woman and her specific situation and diagnosis. Interventions for the woman having surgery are directed by assessment of her perception of the anticipated surgery, her knowledge of what to expect after surgery, and any preoperative special procedures, such as cleansing enemas or douches. In today’s practice of short hospital stays even for radical surgery, many of these preoperative procedures are performed at home before admission, so assessment of understanding becomes a critical nursing
action (see Cultural Considerations box). Nursing care for the woman having a TAH-BSO will be similar to that care for a woman having a hysterectomy for leiomyoma described earlier. The following section focuses on care of the woman having a radical hysterectomy.
Preoperative Care: The nurse working with the woman preparing for a radical hysterectomy and pelvic node dissection should explain any preoperative procedures to be done (see Box 11-2). Additional teaching is needed for the woman having a radical hysterectomy regarding possible postsurgical events (e.g., a suprapubic drain often remains in place for several days to a week).
Postoperative Care: Assessment of vital signs usually follows a postanesthesia protocol, gradually decreasing in frequency to two to four times a day. Intravenous fluids are maintained at a rate rapid enough to maintain hydration and electrolyte balance and are usually discontinued when the woman is taking oral fluids well and has no elevated temperature. A regular diet is resumed as tolerated. Intake and output are monitored. The Foley catheter is usually removed the morning after surgery and the first few voidings are measured.
The woman should turn and take deep breaths with assistance as needed. Breath sounds are assessed, and any deviations from normal are reported immediately. The most significant single cause of morbidity and prolonged hospitalization after major procedures is respiratory complications. Anesthesia and surgery alter breathing patterns and ability to cough. Atelectasis, pneumonia, and pulmonary embolus may occur.
To promote venous return and prevent deep vein thrombosis, the woman may wear antiembolic stockings or wear pneumonic pressure devices (i.e., boots) while she is in bed (Chard, 2010). Leg exercises and early ambulation are beneficial. Most women are encouraged to get out of bed the evening of or the day after surgery. Assistance in getting up and walking may be needed.
Hemorrhage is always a possible complication after surgery. The wound drainage tube is emptied as needed or every 4 hours, and the amount and character of drainage are recorded. Drainage from any tube is assessed for bleeding. Vaginal drainage, if any, should be serosanguineous. Hematuria is noted and recorded. The primary health care provider is kept apprised of any deviations from normal expectations.
Paralytic ileus may occur after surgery in which the intestines have been manipulated. Use of a nasogastric tube, limiting oral fluids, and early ambulation all support the return of gastrointestinal function. An enema or suppository may bring relief of flatus and stimulate the return of bowel function. Oral laxatives should not be given until lower bowel function has returned.
Narcotic analgesics and NSAIDs are used for postoperative pain. Patient-controlled analgesia pumps are commonly used to deliver the narcotic medications (Lowdermilk, 2008). Nursing measures such as massages, repositioning, and emotional support are all helpful adjuncts to pharmacologic control of discomfort.
Because the in-hospital convalescent period is generally short, close observation by the nurse and attention to detail are critical. Nursing actions appropriate to this period include monitoring for urinary retention after the catheter is removed, monitoring the woman’s appetite and diet, monitoring bowel function, and encouraging progressive ambulation and self-care.
Discharge Planning and Teaching: Discharge planning and teaching are done throughout the preoperative and postoperative phases and culminate during the convalescent phase. Discharge teaching topics for the woman with a radical hysterectomy are similar to those that can be found in the Teaching for Self-Management box: Care After Myomectomy or Hysterectomy (see also Nursing Care Plan: Hysterectomy for Endometrial Cancer).
Care for the woman who has had external or internal radiation therapy is the same as that described for the woman with cervical cancer (see later discussion).
Nursing care for the woman undergoing chemotherapy will depend on the type of drug given. If alopecia is likely, the nurse can suggest wigs, scarves, or other kinds of head coverings. If the therapy affects the appetite or causes gastrointestinal side effects, suggestions such as those in Box 11-4 may be useful.
After discharge the woman may require continued nursing care or monitoring of her physical status or advice for management of effects of treatment or the cancer. The family is likely to have to provide much of the woman’s care. Nurses must identify what families see as their greatest need so that interventions are planned that best use the family’s resources.
Psychologic care for the woman with endometrial cancer is essential. A women needs to be able to discuss her concerns about having cancer and the potential for recurrence. She may have fears of death; permanent disfigurement and change in functioning; altered feelings of self as a woman; and concerns regarding her femininity, sexuality, and loss of reproductive capacity. She may have questions arising from things she has heard about posthysterectomy changes, radiation therapy, or chemotherapy. Significant others should be encouraged to express their questions and concerns as well. The woman and her significant others may benefit from a referral to a community cancer support group (see the ACS website, www.cancer.org).
Evaluation
The nursing care of a woman with endometrial cancer is evaluated by using the expected outcomes and measurable criteria to ascertain the degree to which the outcomes were met.
Incidence and Etiology
Cancer of the ovary is the second most frequently occurring reproductive cancer and causes more deaths than any other female genital tract cancer (ACS, 2010a). Because the symptoms of this type of cancer are vague and definitive screening tests do not exist, ovarian cancer is often diagnosed in an advanced stage. The 5-year survival rate for cancer diagnosed at a localized stage is about 94%; however, only about 15% of all ovarian cancers are found at this stage. For advanced stages, the rate is about 28% (ACS). Malignant neoplasia of the ovaries occurs at all ages, including in infants and children. However, cancer of the ovary is seen primarily in women older than age 50 with the greatest number of cases found in women ages 60 to 64 years (Copeland, 2007).
Major histologic cell types occur in different age-groups, with malignant germ cell tumors most common in women between 20 and 40 years of age and epithelial cancers occurring in the perimenopausal age-groups. The spread of ovarian cancer is by
direct extension to adjacent organs, but distal spread can occur through lymphatic spread to the liver and the lungs.
The cause of ovarian cancer is unknown; however, a number of risk factors have been identified. These factors include nulliparity, infertility, previous breast cancer, family history of ovarian or breast cancer, and history of HNPCC. Inherited BRCA1 and BRCA2 mutations increase the risk, but 90% of women do not have inherited ovarian cancer (Coleman & Gershenson, 2007; Copeland, 2007). Women of North American or northern European descent have the highest incidence of ovarian cancers. Pregnancy and use of oral contraceptives seem to have some protective benefits against ovarian cancer, whereas use of postmenopausal estrogen may increase the risk (ACS, 2010a). Genital exposure to talc, a diet high in fat, lactose intolerance, and use of fertility drugs have been suggested as risk factors, but research findings are inconclusive (Berek, 2010; Coleman & Gershenson; Copeland).
Clinical Manifestations and Diagnosis
Ovarian cancer has been called a silent disease because early warning symptoms that would send a woman to her health care provider are absent (e.g., no bleeding or other discharge and no pain). Abdominal bloating, noticeable increase in abdominal girth, pelvic or abdominal pain, difficulty eating or feeling full quickly, and urinary urgency or frequency have been identified as the four most common symptoms of early ovarian cancer (Goff, Mandel, Drescher, Urban, Gough, Schurman, et al., 2007). The increase in abdominal girth (caused by ovarian enlargement or ascites) is usually attributed to an increase in weight or a shift in weight that is seen commonly in women entering their middle years. An ovary enlarged 5 cm or more than normal that is found during routine examination requires careful diagnostic workup. Pelvic pain, anemia, and general weakness and malnutrition are signs of late-stage disease.
Early diagnosis of ovarian cancer is uncommon. Attempts at early detection have not proven to be reliable. Taking a family history is important because it may reveal cancer of the uterus or breast. Transvaginal ultrasound, CA-125 antigen (a tumor-associated antigen) testing, and frequent pelvic examinations have all been used without a great deal of success because these tests do not have high levels of sensitivity and specificity (Fields & Chevlen, 2006). Research continues on the use of proteomics (study of proteins in blood) to identify ovarian cancer in its earlier stages (ACS, 2010a; Cesario, 2010). Emerging technology includes tumor cell profiling and nanotechnology (use of microchips to sense biomarkers that are unique to a specific cancer).
Transvaginal ultrasound and CA-125 screening currently are not recommended for routine screening in the general population but are recommended for women who are at high risk (e.g., BRCA1 mutation carriers) (ACS, 2010a). Routine pelvic examination continues to be the only practical screening method for detecting early disease, even though few cancers are detected in women without symptoms. Any ovarian enlargement should be considered highly suggestive and needs further evaluation by laparoscopy or laparotomy. Responsibility for diagnosis rests with the pathologist. The size of the tumor is not indicative of the severity of disease. Clinical staging is done surgically and gives direction to treatment and prognosis (Copeland, 2007).
Therapeutic Management
Treatment is dictated by the stage of the disease at the time of initial diagnosis. Surgical removal of as much of the tumor as possible is the first step in therapy. This may involve just the removal of one ovary and tube or the radical excision of the uterus, ovaries, tubes, and omentum. Cytoreductive surgery (the debulking of the poorly vascularized larger tumors) also is done. The smaller the volume of tumor remaining, the better the response to adjuvant therapy. Because about three fourths of women are in stage II, III, or IV disease at the time of diagnosis, surgical cure is not possible; therefore, after tumor reduction surgery is performed, women with epithelial cell carcinoma will receive chemotherapy.
A combination of antineoplastic drugs such as paclitaxel and cisplatin or carboplatin and paclitaxel is recommended for most women with advanced disease. Women being treated with chemotherapy are followed up closely with laboratory and radiologic tests and CA-125 levels to monitor their response to the therapy (Copeland, 2007).
Second-look surgery is a technique used to determine the response of the disease to chemotherapy and to determine whether treatment should be continued; however, this procedure usually is not done unless it is part of a research protocol (Copeland, 2007).
Radiation has been used to treat early-stage disease, and some women have had long-term survival after debulking surgery followed by radiation therapy. It has also been used as a palliative measure in advanced disease (Coleman & Gershenson, 2007).
Nursing Implications
Lockwood-Rayermann, Donovan, Rambo, and Kuo (2009) reported on data analyzed from a survey conducted by the National Ovarian Cancer Coalition. These researchers concluded that awareness of the symptoms of and risk factors for ovarian cancer are low in the general population. Therefore, nurses need to be involved in raising the awareness of these risks and symptoms with the public and with women who are seeking care in health care settings such as clinics and physician offices (Cesario, 2010).
Goff and associates (2007) developed a symptom index to be used in identifying women at risk for ovarian cancer who might benefit from early screening. The index includes asking about symptoms (pelvic/abdominal pain, urinary urgency/frequency, increased abdominal size/bloating, and difficulty eating/feeling full) and the frequency and duration of the symptoms. The index is considered positive if any of the symptoms occurred more than 12 times per month and had been present for less than 1 year. Nursing can incorporate asking about these symptoms when women are seen for annual examinations or other gynecologic health visits and encouraging women to keep a symptom diary (Cesario, 2010).
The woman diagnosed with ovarian cancer has concerns similar to those described for the woman with endometrial and cervical cancer. Nursing interventions for the woman having surgery, chemotherapy, or external radiation therapy are described in other sections of this chapter.
Women with advanced ovarian cancer have a significant rate of recurrence. Follow-up for 5 years must be intensive. When a cure or remission cannot be achieved, palliative measures are initiated that alleviate symptoms of the progressing disease and provide comfort and maximal function. As the disease progresses, nutritional support, including enteral feedings and parenteral hyperalimentation, may be needed because of the effects on the gastrointestinal tract of both the disease and the treatments. The goal of nursing care is assisting the woman to maintain quality of life and to remain at home with her family as much as possible.
Because the period between a focus on cure and a focus on palliation is often prolonged, the woman with ovarian cancer is apt to experience most of the grief stages described by Kübler-Ross and to need support and encouragement through each stage. After diagnosis the woman often experiences denial and then anger. As treatment begins, she may “bargain” for a cure. If treatment is successful and death is forestalled by remission or cure, the process of adjustment to dying ceases, and the woman again focuses on life and its challenges. When treatment fails to secure a cure or remission ends, the woman must turn again to the task of adjustment (see Legal Tip).
Family and friends also have diverse feelings. When grieving is prolonged, as it often is when the woman has cancer, the stress can be enormous and can interfere with other interpersonal relationships. If the woman is hospitalized, the environment may further intrude on relationships, limiting privacy and access to the woman and hindering opportunities for caring gestures. The nurse can assist the woman and her family to share their feelings with each other and help them to develop a support network. Referral to a cancer support group may be useful (see National Ovarian Cancer Coalition, www.ovarian.org).
Cancer of the Cervix
Cancer of the cervix is the third most common reproductive cancer. The accessible location of the cervix to both cell and tissue study and direct examination have led to a refinement of diagnostic techniques, contributing to improved diagnosis and management of these disorders. The incidence of invasive cancer has decreased over the last 30 years, reducing mortality rates. However, the incidence of preinvasive cancer has increased, and more women in their 20s and 30s are being diagnosed with preinvasive cervical lesions (ACS, 2010a).
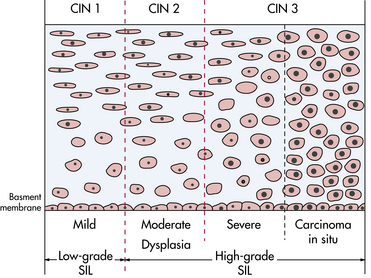
Cancer of the cervix begins as neoplastic changes in the cervical epithelium. Terms that have been used to describe these epithelial changes or preinvasive lesions include dysplasia and cervical intraepithelial neoplasia (CIN); CIN is the term currently used. CIN 1 refers to abnormal cellular proliferation in the lower one third of the epithelium; this change tends to be self-limiting and generally regresses to normal. CIN 2 involves the lower two thirds of the epithelium and may progress to carcinoma in situ. CIN 3 involves the full thickness of the epithelium and often progresses to carcinoma in situ. Carcinoma in situ (CIS) is diagnosed when the full thickness of epithelium is replaced with abnormal cells (Creasman, 2007b) (Fig. 11-11). Terms used to describe neoplastic changes in abnormal cervical cytology reports are low-grade and high-grade squamous intraepithelial lesions (SILs); however, CIN continues to be a common term used in clinical practice.
Preinvasive lesions are limited to the cervix and usually originate in the squamocolumnar junction or transformation zone (Fig. 11-12). Intensive study of the cervix and the cellular changes that take place has shown that most cervical tumors have a gradual onset rather than an explosive one. Preinvasive conditions may exist for years before the development of invasive disease. These preinvasive conditions are highly treatable in many cases.
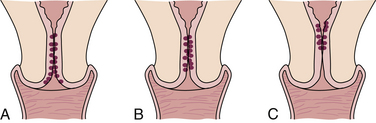
FIG. 11-12 Location of squamocolumnar junction according to age. The location where the endocervical glands meet the squamous epithelium becomes progressively higher with age. A, Puberty. B, Reproductive years. C, Postmenopausal.
Invasive carcinoma is the diagnosis when abnormal cells penetrate the basement membrane and invade the stroma. There are two types of invasive carcinoma of the cervix: microinvasive and invasive. Microinvasive carcinoma is defined as one or more lesions that penetrate no more than 3 mm into the stroma below the basement membrane with no areas of lymphatic or vascular invasion (Creasman, 2007b). Invasive carcinoma describes invasion that goes beyond these parameters. The staging of invasive carcinoma extends from stage 0 (CIS) to stage IVb (distant metastasis or disease outside the true pelvis). A number of substages within each stage also exist. Clinical stages for cancer of the cervix are shown in Table 11-3.
TABLE 11-3
FIGO Classification of Cervical Carcinoma
| STAGE | DESCRIPTION |
| 0 | Carcinoma in situ, intraepithelial carcinoma |
| I | The carcinoma is strictly confined to the cervix (extension to the corpus should be disregarded) |
| Ia | Invasive cancer identified only microscopically; all gross lesions, even with superficial invasion, are stage Ib cancers. Invasion is limited to measured stromal invasion with maximum depth of 5 mm and no wider than 7 mm |
| Ia1 | Measured invasion of stroma 3 mm in depth and no wider than 7 mm |
| Ia2 | Measured invasion of stroma >3 mm and 5 mm in depth and no wider than 7 mm. |
| The depth of invasion should not be >5 mm taken from the base of the epithelium, surface or glandular, from which it originates. Vascular space involvement, venous or lymphatic, should not alter the staging | |
| Ib | Clinical lesions confined to the cervix or preclinical lesions greater than stage Ia |
| Ib1 | Clinical lesions 4 cm or less |
| Ib2 | Clinical lesions >4 cm |
| II | Involvement of the vagina but not the lower third, or infiltration of the parametria but not out to the side wall |
| IIa | Involvement of the vagina, but no evidence of parametrial involvement |
| IIb | Infiltration of the parametria, but not out to the side wall |
| III | Involvement of the lower third of the vagina or extension to the pelvic side wall. All cases with a hydronephrosis or nonfunctioning kidney should be included, unless they are known to be attributable to other cause |
| IIIa | Involvement of the lower third of the vagina but not out to the pelvic side wall if the parametria are involved |
| IIIb | Extension onto the pelvic side wall and/or hydronephrosis or nonfunctional kidney |
| IV | Extension outside the reproductive tract |
| IVa | Involvement of the mucosa of the bladder or the rectum |
| IVb | Distant metastasis or disease outside the true pelvis |
Source: Monk, B., & Tewari, K. (2007). Invasive cervical cancer. In P. DiSaia & W. Creasman (Eds.), Clinical gynecologic oncology (7th ed.). St. Louis: Mosby.
Approximately 90% of cervical malignancies are squamous cell carcinomas; 10% are adenocarcinomas. Squamous cell carcinomas can spread by direct extension to the vaginal mucosa, the pelvic wall, the bowels, and the bladder. Metastasis usually occurs in the pelvis, but it can occur to the lungs and the brain through the lymphatic system.
The average age range for the occurrence of cervical cancer is 40 to 50 years; however, preinvasive conditions may exist for 10 to 15 years before the development of an invasive carcinoma. About 70% to 80% of cervical cancers are caused by human papillomavirus (HPV) (Creasman, 2007b). A strong link has been established between HPV types 16 and 18 and cervical neoplasia. Eighteen other types have been associated with genital tract infections and also may be associated with CIN (ACOG, 2008; Creasman). Other sexually transmitted infections that are identified as risk factors are herpes simplex virus 2 and possibly cytomegalovirus (Creasman). Risk factors include early age at first coitus (younger than 20 years); multiple sexual partners (more than two); a sexual partner with a history of multiple sexual partners; high parity, and belonging to a lower socioeconomic group. Potential factors include long-term use of oral contraceptives, cigarette smoking, and intrauterine exposure to diethylstilbestrol (DES) (ACS, 2010a; Creasman; Monk & Tewari, 2007). Low levels of beta-carotene, vitamin C, and folate are being investigated as potential risk factors (Monk & Tewari).
The incidence of cervical cancer in the United States is highest in Hispanic women and lowest in Native-American women, whereas the highest mortality occurs in African-American women (ACS, 2010a). Factors that may influence cervical screening behaviors for these groups include lack of a health promotion or disease prevention perspective, lack of knowledge about Pap tests and availability of services, financial barriers, and failure of health care providers to recommend screening (Giarratano, Bustamante-Forest, & Carter, 2005). There also is a high rate of CIN in human immunodeficiency virus–positive women, suggesting that altered immune status is a risk factor (Creasman, 2007b).
Clinical Manifestations and Diagnosis
Preinvasive cancer of the cervix is often asymptomatic. Abnormal bleeding, especially postcoital bleeding, is the classic symptom of invasive cancer. Other late symptoms include rectal bleeding, hematuria, back pain, leg pain, and anemia. Diagnosis includes taking a history that includes menstrual and sexual activity information, particularly sexually transmitted infections and abnormal bleeding episodes (Creasman, 2007b). A pelvic examination usually is normal except in late-stage cancer.
The single most reliable method to detect preinvasive cancer is the Pap test, which can detect 90% of early cervical changes. The U.S. Preventive Services Task Force (USPSTF) and the ACS recommend Pap tests to begin about 3 years after a woman becomes sexually active but not later than age 21. Annual screening is recommended to age 30 (with conventional Pap test; every 2 years if liquid-based Pap tests). After age 30 and three negative Pap tests, screening may be done every 2 to 3 years in consultation with the health care provider. Women ages 65 to 70 with no abnormal tests in the previous 10 years may choose to stop screenings. Women who have had total hysterectomies for benign disease can choose to stop having Pap tests (ACS, 2010a; USPSTF, 2009). Women in high risk categories should have more frequent Pap tests.
Pap test results in the past have been recorded by using several different classification systems. The reporting system most often used today is the Bethesda system, one that reports on gynecologic cytology as well as histology of cervical lesions (Box 11-5). Changes secondary to inflammation, treatment (e.g., radiation), and contraceptive devices can be reported, as well as changes caused by infections. Epithelial cell abnormalities are described in three categories: atypias, or atypical squamous cells (ASC); low-grade squamous intraepithelial lesions (LSILs); and high-grade squamous intraepithelial lesions (HSILs).
Several options for follow-up of a finding of ASC of undetermined significance (ASC-US) are suggested. These include immediate colposcopy, repeating cytology at 6 months and 12 months, or HPV testing and referral for colposcopy if test is positive. If the initial cytology test was obtained by a liquid-based method, HPV testing is preferred instead of follow-up with repeated cytology (ACOG, 2008). A finding of LSIL may include HPV and CIN 1. Colposcopy is recommended for evaluation of LSIL except in adolescents; teens can be followed with cytology tests at 6 and 12 months. HPV deoxyribonucleic acid (DNA) testing should not be used (ACOG). HSILs include lesions described as CIN 2, CIN 3, and carcinoma in situ (CIS). Follow-up for a report of HSIL includes colposcopy or loop electrosurgical excision (ACOG).
Colposcopy is the examination of the cervix with a stereoscopic binocular microscope that magnifies the view of the cervix. Usually a solution of 3% acetic acid is applied to the cervix for better visualization of the epithelium and to identify areas for biopsy. Colposcopy is not an invasive procedure and is usually well tolerated by the woman. However, the woman who is scheduled for colposcopy because of an abnormal Pap test may be anxious about the procedure and may need explanations or written information about what to expect during the procedure.
Biopsy is the removal of cervical tissue for study, and several techniques can be used. An endocervical curettage is an effective diagnostic tool in about 90% of cases. It can be performed as an outpatient procedure with little or no anesthesia. It may be uncomfortable, and interventions to help the woman relax and cope with the pain may be needed.
Conization and loop electrosurgical excision procedure (LEEP) (see later discussion) can be done as outpatient procedures, although neither is usually performed unless the biopsy is positive or the results of the colposcopy are unsatisfactory. Conization involves removal of a cone of tissue from the exocervix and endocervix (Fig. 11-13). It can be a cold knife procedure, a laser excision, or an electrosurgical excision (see later discussion). There are two advantages to a cone biopsy. It can be used (1) to establish the diagnosis and (2) to effect a cure. If CIS is diagnosed, and if the woman wishes to retain her childbearing capacity, conization removes the abnormal tissue; further treatment (e.g., hysterectomy) is unnecessary. The woman is monitored with Pap tests and colposcopy when indicated.
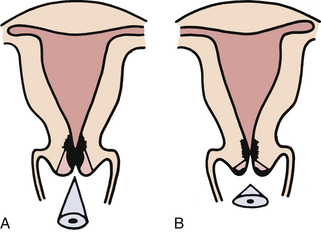
FIG. 11-13 A, Cone biopsy for endocervical disease. Limits of lesion were not seen colposcopically. B, Cone biopsy for cervical intraepithelial neoplasia of the exocervix. Limits of lesion were identified colposcopically. (Source: Creasman, W. (2007). Preinvasive disease of the cervix. In P. DiSaia & W. Creasman (Eds.), Clinical gynecologic oncology (7th ed.). St. Louis: Mosby.
If invasive cancer is diagnosed, other diagnostic tests can assess the extent of spread (see earlier discussion under endometrial cancer). Once the extent of the cancer is known, treatment begins.
Care Management
For the woman diagnosed with invasive carcinoma of the cervix, pretherapy assessment includes physical, psychologic, and educational components, regardless of whether surgery or radiation is the method of treatment. Physical assessment includes a review of current medications because medications for other medical problems may have to be continued. Skin is assessed to identify potential pressure points; respiratory and gastrointestinal status and state of nutrition are important factors to assess. Urinalysis and complete blood count also are commonly performed. An electrocardiogram and a chest x-ray examination may be done if use of a general anesthetic is anticipated for surgery or placement of internal applicators.
Psychologic assessment is important because frequently these women are emotionally distressed about the diagnosis and anticipated treatment (i.e., fear of being radioactive and fear of surgery and pain) and fear that family or significant others will become distant.
Educational assessment involves identifying the woman’s current knowledge base regarding the diagnosis and proposed therapeutic regimen.
Nursing diagnoses for the woman having surgery for cervical cancer are similar to those identified for the woman having a hysterectomy for endometrial cancer (see Nursing Care Plan, p. 249). Nursing diagnoses for a woman having external or internal radiation therapy are listed in the Nursing Process box: Woman Having Radiation Therapy).
Medical and Surgical Management
Once a diagnosis has been identified, a course of treatment is planned. For preinvasive lesions, several techniques are used. As stated, because many preinvasive conditions are detected in
younger women who may wish to continue childbearing, treatment is geared toward eradicating abnormal cells while attempting to preserve the structure of the cervix. The techniques currently available for preinvasive lesions are cryotherapy, laser therapy, and LEEP, all of which have comparable success rates in treating CIN (ACOG, 2008).
Treatment for invasive cancer includes surgery, radiation therapy, and chemotherapy. Once the cancer is staged, treatment is begun. Microinvasive cancer is usually treated with conization, but a hysterectomy is often done if childbearing is not desired. The choice of treatment for early-stage invasive cancer is by either hysterectomy or chemoradiation therapy (Monk & Tewari, 2007). A radical hysterectomy is performed if the cancer has extended beyond the cervix but not to the pelvic wall. Locally advanced stages of cervical cancer usually are treated with radiation therapy, both external and internal, and chemotherapy. Late stages are usually treated with radiation and chemotherapy. Five-year survival rates are more than 97% when the cancer is localized (ACS, 2010a). Cisplatin is the most commonly used chemotherapy agent.
Cryosurgery: Cryosurgery uses a freezing technique that freezes abnormal cells, and when sloughing occurs, normal tissue is regenerated. Side effects occurring after treatment are usually few and not serious. A profuse watery discharge can
persist for 2 to 4 weeks. Follow-up examination and a Pap test are scheduled in 4 to 6 months. Endocervical cells are thought to regenerate, leaving a normal cervical canal in most instances. Spotting and cervical stenosis are rare complications. Surveillance with frequent Pap tests and colposcopic examination must continue indefinitely after this type of conservative therapy. Persistent abnormal cells require reevaluation, and plans are made for repeated cryosurgery or other therapy.
Laser Ablation: Laser ablation uses a laser mounted on a colposcope that allows precise direction of a beam of light (heat) to remove diseased tissue. An endometrial sampling is recommended before ablation to avoid removal of unrecognized invasive cancer. For treatment of the cervix (relatively insensitive tissue), the woman may need no anesthesia. Some women complain of a burning or cramping sensation that is tolerable for most women. The cervix treated with CO2 laser will show epithelial regrowth beginning by 2 days afterward. The site is usually healed in 4 to 6 weeks. The original architecture of the cervix is preserved, and the squamocolumnar junction remains visible; however, there can be more damage to normal tissues than with other treatments. Women usually have less vaginal discharge than with cryosurgery, but can have more discomfort after the procedure (Noller, 2007).
Electrosurgical Excision: The LEEP is a standard treatment for cervical intraepithelial neoplasia in the United States. This procedure uses a wire loop electrode that can excise and cauterize with minimal tissue damage (Fig. 11-14). Healing is rapid, and there is only a mild discharge afterward. Possible complications include bleeding, cervical stenosis, infertility, and loss of cervical mucus (ACOG, 2008).
Radical Hysterectomy: Radical hysterectomy involves removal of the uterus, the tubes, the ovaries, the upper third of the vagina, the entire uterosacral and uterovesical ligaments, and all of the parametrium on each side, along with pelvic node dissection encompassing the four major pelvic lymph node chains: ureteral, obturator, hypogastric, and iliac. Dissection serves to preserve the bladder, the rectum, and the ureters while removing as much of the remaining tissue of the pelvis as is feasible. Women with positive pelvic nodes usually receive postoperative whole pelvis irradiation (Monk & Tewari, 2007).
Nursing Management: Nursing care for the woman having a radical hysterectomy was discussed in the previous section on endometrial cancer (p. 248).
Radiation Therapy: Radiation may be delivered by internal radium applications to the cervix or external radiation therapy that includes lymphatics of the pelvic side wall. In preparation for radiation therapy the woman must maintain good nutritional status and a high-protein, high-vitamin, and high-calorie diet. Anemia, if present, should be corrected before initiating radiotherapy.
External radiation therapy and internal radiation therapy are given in various combinations for the best results and are tailored to each woman and her particular lesion. For example, external radiation may be given first to treat regional pelvic nodes and to shrink the tumor. External irradiation is usually an outpatient procedure given 5 days a week for 4 to 6 weeks. Internal radiation therapy consists of one or two intracavitary treatments at least 2 weeks apart (Monk & Tewari, 2007).
External irradiation is provided by megavoltage machines such as cobalt, and supervoltage machines such as linear accelerators and betatron, all of which have the distinct advantage of providing a more homogeneous dose to the pelvis. Before treatment begins, a localization procedure is done to determine the best way to deliver the treatments. Markings or small tattoos are placed on the body to make sure the woman is positioned correctly to get the treatment (Workman, 2010).
For internal radiation therapy, the woman may be treated in the hospital or in a special outpatient unit. If treatment is done in the hospital, the woman is taken to the operating room, and while she is under general or spinal anesthesia, a specially designed applicator is placed into her vagina and cervix. X-rays are taken to make sure the applicator is correctly placed. The woman is returned to her room, where the radioactive source is placed into the applicator (Fig. 11-15). The source remains in place from 12 hours to 3 days. If treatment is in the outpatient setting, the applicator is inserted into the uterus in a treatment room; use of high-dose implants shortens the treatment time and is being used more frequently than low-dose implants because no hospital stay is required (Monk & Tewari, 2007; Workman, 2010).
In advanced carcinoma of the cervix, conventional intracavitary applicators are not applicable. Interstitial therapy uses a template to guide the transperineal insertion of a group of 18-gauge hollow steel needles into the lesion (Fig. 11-16). After the needles are placed, the iridium wires are inserted when the woman is returned to her room.
Nursing Management: Nursing actions for external and internal radiation differ, so they are discussed separately.
External Therapy: Before external radiation therapy the woman’s anxiety may be so high that information given by the radiologist may not be processed. The nurse should reinforce or fill in gaps, especially related to the following: the equipment, which is similar to that used for x-ray examination except larger; the hyperbaric oxygen chamber, which may be used to increase cellular oxygen and thus make tumor cells more radiosensitive; the radiotherapist, who will be behind a shield, but still close by and in communication with her; the position she will be put in and asked to maintain for some minutes; and the therapy, which is painless.
During the course of the therapy, the woman is counseled regarding maintaining general good health. To maintain good skin care the woman is taught to assess her skin often; avoid soaps, ointments, cosmetics, and deodorants if the axilla is being irradiated because these may contain metals that would alter the dose she receives and could lead to skin breakdown; wear loose clothing over the area and cotton underwear (or no underwear); use an air mattress or cover the mattress with foam pads or sheepskin; avoid exposing the irradiated areas to temperature extremes (e.g., hot tubs); and especially avoid removing the markings made by the radiologist. If her skin becomes red or itchy she can treat it with remedies recommended by the radiologist (e.g., aloe vera lotion, Aquaphor, or warm sitz baths). To treat skin that is broken or desquamating, the woman is shown how to use remedies prescribed by the radiologist (e.g., irrigation with warm water, application of antibiotic or lanolin ointment, exposure to air, and application of a loose dressing). The use of adhesive (or any) tape directly on the target area of skin should be avoided (Workman, 2010).
To maintain good nutrition the woman is reminded to keep a daily record of weight; use high-protein supplements; eat small, attractive, appetizing meals that are more bland than spicy; and keep the environment light, airy, clean, and quiet (especially before and after meals). A dietitian consult may be needed to help the woman and her family plan to meet the woman’s nutritional needs. If the woman is ill enough to be hospitalized, she may need total parenteral nutrition or tube feedings. Nausea interferes with adequate intake; therefore, the woman may take antiemetics, as necessary. High daily fluid intake (2 to 3 L) should be suggested if not contraindicated. To increase her comfort, minimize infection, and promote adequate food intake, the woman is encouraged to perform frequent oral hygiene. Box 11-4 provides other suggestions for nutritional problems associated with radiation treatment.
The nurse explains, as necessary, the need for routine blood studies to monitor white blood cell count (to determine degree of immunosuppression). The woman and her family will need information about neutropenia, thrombocytopenia, and anemia and precautions to be taken. Because the woman is more vulnerable to infection, she is reminded of general measures to avoid infection (e.g., practice good hygiene, avoid people with infection, avoid large crowds, keep environment clean).
After the radiation treatment is completed, the woman needs information for self-care (see the Teaching for Self-Management box: Care After External Radiation Therapy). She should also be informed that side effects of the treatment, especially fatigue and altered taste sensations, can continue for weeks after the therapy is completed.
Internal Therapy: Internal radiation therapy may require hospitalization or may be done in a special outpatient unit. Radiation safety officers determine the precautions to be observed in each situation. This discussion focuses on treatment in the hospital setting, but similar precautions would be used in the outpatient setting. Printed instruction sheets are usually available, stating precautions to be followed for each type of radiation substance used. A precaution sign is placed on the door of the woman’s room.
Nurses must protect themselves from overexposure to radiation. Precautions include the following (Workman, 2010):
• Careful isolation techniques: wearing gloves while handling bodily fluids and observing good handwashing technique. These behaviors reflect knowledge that alpha and beta rays cannot pass through skin but may be in body fluids and excrement.
• Careful planning of nursing activity to limit time (to 30 minutes or less per 8 hours) spent in proximity to the woman to avoid exposure to gamma rays, which can penetrate several inches of lead.
Exposure to radiation is controlled in three ways: distance, time, and shielding (with lead). For the woman with sealed radiotherapy, a movable lead screen can be placed between the area in which the therapeutic applicator is located and the personnel. The lead screen also is used to protect visitors from radiation. Increasing the distance from the source also decreases exposure (Workman, 2010).
Familiarity with applicators is a must for all nurses working with people receiving radiotherapy so that if a “strange object” is found in the linen or on the floor, it is not touched. Today most hospital protocols include having a lead container and forceps in the room for use if a radioactive implant is dislodged.
The woman is prepared for insertion with the following care, which is accompanied by an explanation for each activity. To reduce the need for an enema or attention to bowel elimination for a few days, the gastrointestinal tract is usually prepared by using low-residue diet, enemas, and sometimes bowel sedation. The vaginal vault is usually prepared with an antiseptic douche, such as povidone-iodine.
An indwelling urinary catheter is inserted, as ordered, to prevent bladder distention that could dislodge the applicator. Food and fluids are withheld for a specified time before the procedure in anticipation of the use of general anesthesia. Preoperative medications may be ordered for the morning of the procedure. Deep-breathing exercises, range of motion (ROM) exercises, and positioning are all demonstrated before the procedure to minimize the effects of immobilization afterward. An intravenous (IV) solution will probably be started before the procedure, and IV therapy may be continued if nausea prevents good intake of oral fluids. The woman is assured that pain will be managed.
Explanations about restricted visitation of personnel and visitors also are given in the preinsertion phase. Women are often encouraged to bring reading materials to the hospital to combat the boredom that isolation imposes on them. In addition many units are equipped with television, CD, and video/DVD players.
The applicator is inserted into the woman’s vaginal vault during surgery and after the usual postanesthesia recovery care, the woman is returned to her room, where the applicators are loaded with the radioactive substance.
A lead shield is placed next to the bed in line with the woman’s pelvic area to protect the caregivers and visitors. Vital signs are monitored every 4 hours. Active ROM and deep-breathing exercises are encouraged every 2 hours; the woman is positioned on her back and may not be permitted to turn from side to side, although log-rolling may be done occasionally to relieve back pressure. The head of the bed may or may not be elevated slightly.
The woman’s diet is changed from clear liquid to low residue, as ordered. Many individuals have difficulty eating while lying flat or even if the bed is elevated slightly. The nurse arranges the food so that it is easy to reach. Finger foods or liquids are generally more manageable. Parenteral or oral fluids are given, up to 3 L daily.
The urinary catheter remains in the bladder while the implant is in place. However, no perineal or catheter care is given. Intake and output are measured. The woman is given a partial bath, washing only above her waist. Massage is restricted to her shoulders and neck. Linen is changed only as absolutely necessary. Any linen or equipment used is retained in the room until therapy is complete to prevent loss of an applicator or seed. If vaginal or rectal bleeding or hematuria occurs, the physician is notified immediately.
Emotional support is provided by planning to be with the woman for short periods; encouraging her to verbalize concerns and needs; and encouraging family members, clergy, or others to visit for short periods daily or to communicate by telephone. Pregnant women and children are not permitted to visit.
Many women undergoing internal radiation treatment are given medication to prevent complications and to promote comfort during the procedure. Such medications might include antibiotics to prevent bladder infections, heparin injections to prevent thrombophlebitis, sedatives for relaxation, antiemetics for nausea, and narcotics for pain. The woman is considered radioactive during the time the internal sources are in place (Workman, 2010).
After the radium is removed the Foley catheter is removed, and the woman is assisted in getting out of bed the first time. She is usually discharged the same day. Discharge teaching can be found in the Teaching for Self-Management box: Care After Internal Radiation Therapy. The woman and her family are reassured that she is not radioactive after the treatment.
Posttreatment complications range from those arising from immobilization, such as thrombophlebitis, pulmonary embolism, and pneumonia, to those arising from the treatment itself, such as hemorrhage, skin reactions (rashes or inflammation), diarrhea, cramping, dysuria, and vaginal stenosis. The woman is assessed for any of these complications before discharge.
The woman may experience altered patterns of sexuality related to treatment side effects. A decrease in vaginal secretions and sensation may occur, as well as vaginal stenosis. These can contribute to decreased sexual desire, because pain and discomfort during intercourse can affect the desire to resume sexual activities. The nurse can initiate a discussion with the woman and her partner, offer information about the effects of radiation on the ability to have sexual intercourse, and offer suggestions for specific problems, such as using a water-based lubricant for vaginal dryness and using a vaginal dilator as directed by her health care provider. If necessary, the couple can be referred to other resources.
Complications of Radiation Therapy: Morbidity as a direct result from properly conducted therapy is usually minimal. Some of the morbidity seen may be caused by the uncontrolled tumor and not by the therapy. Acute treatment complications occurring during or shortly after therapy include irritation of the rectum, the small bowel, and the bladder; reactions in the skinfolds; and mild bone marrow suppression. Dysuria and frequency may occur. Late complications, although not common, include genital fistulas and necrosis (Yashar, 2007).
Recurrent and Advanced Cancer of the Cervix
Approximately one third of women with invasive cervical cancer will have recurrent or persistent disease after therapy. The 5-year survival rate is approximately 17% (Monk & Tewari, 2007). Irradiation of metastatic areas is commonly successful in providing local control and symptomatic relief. Irradiation for recurrent disease may be considered for women who were initially treated with surgery. Further radiation may not be effective for those women who were initially treated with radiation.
Pelvic Exenteration: The woman who has recurrence only within the pelvis may be considered for pelvic exenteration if a cure is thought to be possible. A total exenteration involves removal of the perineum, the pelvic floor, the levator muscles, and all reproductive organs. Additionally, pelvic lymph nodes, rectum, sigmoid colon, urinary bladder, and distal ureters are removed, and a colostomy and ileal conduit are constructed (Monk & Tewari, 2007) (Fig. 11-17, A). In select cases the procedure can be modified to either an anterior or a posterior exenteration. In anterior pelvic exenteration all of the previously mentioned pelvic viscera are removed except the rectosigmoid, which is preserved. Urine is rerouted through an ileal conduit (see Fig. 11-17, B). In the posterior pelvic exenteration procedure, all pelvic viscera with the exception of the bladder are removed. The feces are rerouted through a colostomy (see Fig. 11-17, C). A neovagina (new vagina) may be constructed.
FIG. 11-17 Pelvic exenteration procedures. A, Anterior exenteration. B, Posterior exenteration. C, Total exenteration.
Women are carefully selected for this procedure; 5-year survival rates range from 20% to 62% (Monk & Tewari, 2007). Many of the complications that follow this surgery are those that follow any form of major surgery, for example, pulmonary embolism, pulmonary edema, myocardial infarction, and cerebrovascular accident. These complications are seen immediately after surgery. Infection originating in the pelvic cavity usually occurs later, if it occurs.
Nursing Management: Nursing care of the woman having a pelvic exenteration depends on what is removed. General preoperative considerations include assessments similar to those for a woman having a radical hysterectomy. Additionally, a thorough sexual assessment is needed because of the drastic changes involved. The woman needs information about the construction of a neovagina if that is an option. She will need to be assessed for stoma site selection and information about management of colostomy or ileal conduit if appropriate. Extensive preoperative bowel preparation is needed before surgery. Pain management is discussed, as is what to expect postoperatively (e.g., nasogastric tubes, arterial catheters). Significant others should be included in preoperative discussions when possible, because their postoperative support is essential.
Postoperative care usually begins in an intensive care unit until the woman’s condition is stable. She is monitored for signs of complications, including shock, hemorrhage, pulmonary embolus and other pulmonary complications, fluid and electrolyte imbalance, and urinary complications (Monk & Tewari, 2007). Nursing care continues after the woman is stabilized and moved back to her room. Wound care consists of irrigation with half-strength normal saline, followed by drying of the area with either a hair dryer on cool setting or a heat lamp placed at least 12 inches from the perineal area. The woman is taught how to care for her colostomy or ileal conduit when she is able to begin self-care. Assessment for psychologic reactions is important. The woman will probably experience a grief reaction over her mutilated body. She may become depressed during the long convalescence.
The woman may be discharged to a long-term care facility or to her home. She will need assistance in her physical care for at least 6 months. Teaching needed for home care includes colostomy or ureterostomy care; dietary needs for healing; perineal care, including use of perineal pads to protect clothing from discharge; ROM exercises and physical activities permitted by her health care provider; and signs of complication, especially infection and bowel obstruction.
Because the woman will have sexual disruption and possibly be unable to have vaginal intercourse (if the vagina is not reconstructed), counseling about sexual activity is needed. Usually even with a vaginal reconstruction, vaginal intercourse is not advised until healing has taken place, usually in 12 to 18 months. Women with neovaginas may complain of decreased vaginal sensations or chronic discharge, or that the vagina is too short or too long. Women with colostomies or ureterostomies may worry about leakage or odors during sexual activities or may be concerned about their change in appearance. They may need counseling about alternative activities for sexual expression for themselves and their partners. The woman and her partner may need referral for further sexual counseling.
Chemotherapy: Chemotherapy may be used in advanced cancer of the cervix to reduce tumor size before surgery or as adjuvant therapy for poor-prognosis tumors. In general, no long-term benefits are derived with chemotherapy, although chemotherapy concurrent with radiation therapy can improve survival (Monk & Tewari, 2007). Cisplatin is the most effective; other chemotherapeutic agents used singly or in combination include 5-fluorouracil, carboplatin, cyclophosphamide, ifosfamide, methotrexate, mitomycin C, bleomycin, paclitaxel, and hydroxyurea (Chu & Rubin, 2007).
Cancer of the Vulva
Vulvar carcinoma accounts for about 4% of all female genital malignancies and is the fourth most commonly occurring gynecologic cancer. It appears most frequently in older women in their middle 60s to 70s; DNA testing of these women often shows mutation of the p53 tumor suppressor gene (ACS, 2010b). Vulvar cancers in older women do not appear to be etiologically related to HPV infection. The incidence of vulvar cancer, specifically vulvar intraepithelial neoplasia (VIN), is increasing in younger women. Almost 20% of vulvar cancers occur in women younger than 50 years of age, and most women are in their twenties. HPV infection is thought to be responsible for most of these cancers (ACS). Women who have a history of genital warts (condylomata acuminata) and who smoke have an increased risk of developing VIN (ACS).
By far the majority (90%) of vulvar carcinoma is squamous cell; other vulvar neoplasms are attributed to Paget’s disease, adenocarcinoma of Bartholin glands, fibrosarcoma and melanoma, and basal cell carcinoma. VIN is the first neoplastic change, progressing over time to cancer in situ (CIS) and then to invasive cancer. Metastasis is by direct extension and lymphatic spread (Hacker, 2010b).
Prognosis depends on the size of the lesion and the tumor grade at the time of diagnosis. Fifty percent of women have symptoms for 2 to 16 months before seeking treatment. Fortunately, vulvar cancer grows slowly, extends slowly, and metastasizes fairly late. Even with a pattern of delayed diagnosis, survival rates are approximately 96% for all stages if nodes are negative. Survival rates drop to 66%, however, if lymph node metastasis has occurred (Stehman, 2007).
Clinical Manifestations and Diagnosis
Itching is the most common symptom of VIN; a lump or lesion is more common with invasive cancer (Hacker, 2010b). The most common site for vulvar lesions is on the labia majora. The vulvar lesion is usually asymptomatic until it is 1 to 2 cm in diameter. When it is symptomatic, women may complain of vulvar pruritus, burning, or pain. Necrosis and infection of the lesion result in ulceration with bleeding or watery discharge.
VINs are usually multifocal in young women. Unifocal lesions are associated with invasive cancer and are more common in older women. Initially, growth is superficial but later extends into the urethra, the vagina, and the anus. In approximately 50% of late cases, superficial inguinal and femoral lymph nodes become involved (Stehman, 2007).
Simple biopsy with histologic evaluation reveals the diagnosis. The areas of pathologic involvement are identified by staining the vulva with toluidine blue (1%), allowing an absorption time of 3 to 5 minutes, and then washing with acetic acid (2% to 3%); abnormal tissue retains the dye. Biopsy is necessary to rule out such conditions as sexually transmitted infections (e.g., chancroid, granuloma inguinale, syphilis), basal cell carcinoma, and CIS. In situ malignancies are initially small, red, white, or pigmented friable papules. In Paget’s disease the lesions are red, moist, and elevated. Melanomas appear as bluish-black, pigmented, or papillary lesions. Melanomas metastasize through the bloodstream and lymphatics (Hacker, 2010b).
Collaborative Care
Therapeutic Management: Treatment varies, depending on the extent of the disease. Laser surgery, cryosurgery, or electrosurgical excision may be used to treat VIN. A disadvantage to these treatments is that healing is slow, and the treated area is painful. A local wide excision may be performed for localized lesions. Recurrence can occur after these treatments, so follow-up is important (Frumovitz & Bodurka, 2007).
Several types of vulvectomy procedures are used for CIS and invasive cancer. A skinning vulvectomy involves removal of the superficial vulvar skin; it is rarely performed.
A simple vulvectomy involves removal of all of the vulva (external genital organs including the mons pubis, labia majora and minora, and possibly the clitoris). The clitoris usually can be saved if cancer is not present.
For invasive disease, a partial or complete radical vulvectomy is performed. A partial vulvectomy is the removal of part of the vulva and deep tissues; a complete vulvectomy includes the removal of the whole vulva, deep tissues, and the clitoris (Fig. 11-18).
FIG. 11-18 Radical vulvectomy. Note dotted lines denoting vulvectomy incision and inguinal groin incisions.
Skin grafts may be done if a large area of skin is removed during the vulvectomy; however, most surgeries can be closed without grafts. If grafts are needed, a surgeon who performs reconstruction surgery may be consulted (ACS, 2010b).
The inguinal nodes may be removed through an inguinal (groin) node dissection. Usually only lymph nodes on the same side as the cancer are removed; however, nodes on both sides may be removed if the cancer is in the middle (see Fig. 11-18). Swelling of the leg often is a problem after this surgery.
A sentinel node biopsy may be done instead of the inguinal node dissection. This procedure involves injecting blue dye or radioactive material into the tumor site. A scan is performed to identify the sentinel (first) node to pick up the dye or radioactive material. The node is removed for microscopic study. If cancer cells are found, the rest of the lymph nodes will be removed, but if cancer is not found, further lymph node removal is not done. This procedure continues to be studied for use with vulvar cancers (ACS, 2010b).
External radiation therapy can be used to shrink tumors before surgery, but it is not the primary treatment. Postoperative external radiation therapy can be used for women who are at risk for recurrence. Radiation treatment causes dermatitis and ulceration that are uncomfortable for the woman.
Chemotherapy has not been very effective as a treatment except for the topical application of 5-fluorouracil for VIN or CIS. This treatment is painful and not used often. Chemotherapy continues to be investigated in combination with radiation as an adjunct to surgery in advanced cancer of the vulva (ACS, 2010b).
Nursing Management: Nursing care for the woman with vulvar cancer is similar to that for other gynecologic malignancies. A history of symptoms and a physical examination as well as an assessment of the woman’s understanding of the surgical procedure and her emotional state should be done. Nursing diagnoses for women with vulvar cancer are similar to those for other gynecologic cancers; possible nursing diagnoses specific to problems with vulvar cancer treatment include the following:
Interventions for the woman treated with laser therapy include applying topical steroids to the area, administering sitz baths and drying the area with a hair dryer, applying local anesthetics, or giving oral pain medication as needed. Women need to be informed that pain may get worse 3 to 4 days after the treatment.
Because there can be recurrences, information about vulvar self-examination and the need for follow-up with a health care provider is reinforced. Information about community support groups may be helpful, although a study by Likes and coworkers (2008) found that women reported increased anxiety after having contact with Internet support groups.
A woman undergoing radical vulvectomy requires some special nursing actions in addition to the routine postoperative care given (see Teaching for Self-Management box: Care After Radical Vulvectomy). Additional nursing actions focused on the prevention of infection include the following:
• Irrigate the surgical site with half-strength normal saline or other recommended solution after each elimination.
• Dry the area thoroughly by using a hair dryer on cool setting or a heat lamp.
• Use a bed cradle or other means to lift the bed covers and allow air to circulate around the wound.
• Give stool softeners to decrease straining and disruption of the suture line.
• Note any change in color of the surgical site.
• Note any drainage or foul odor and, if present, notify primary health care provider.
The woman is at high risk for sexual dysfunction related to the effects of the surgery. For example, she may have concerns that her partner will be repulsed by the scarring and loss of the vulva. She also may have concerns about reaching orgasm and vaginal numbness or painful vaginal penetration. Nursing actions that focus on minimizing these risks include the following:
• Encouraging verbalization of feelings
• Providing privacy for discussion
• Encouraging open communication between the woman and her partner
• Discussing when sexual activity can be safely resumed
• Discussing alternative methods to achieve sexual satisfaction
• Providing information about use of vaginal dilators and water-soluble lubricants for painful vaginal intercourse
Cancer of the Vagina
Vaginal carcinomas account for less than 2% of gynecologic malignancies, with a peak incidence between 50 and 70 years of age. Most lesions are squamous cell carcinomas, and are secondary rather than primary carcinomas of the vagina. Vaginal intraepithelial neoplasia (VAIN) is uncommon, and clear-cell adenocarcinoma is even rarer. It is found primarily in young women (ages 15 to 30 years) and is related to intrauterine exposure to DES. Sarcoma botryoides (embryonal rhabdomyosarcoma) occurs in infants and children (Dotters & Katz, 2007).
The etiology is unknown, but vaginal cancer may be caused by chronic vaginal irritation, vaginal trauma, and genital viruses (e.g., HPV). Women with VAIN often have had cancer or currently have cancer of another part of the genital tract (Slomovitz & Coleman, 2007). Vaginal lesions, usually seen in the upper one third of the vagina, often extend into the bladder and the rectum in late stages. Metastasis can occur early because of the rich lymphatic drainage in the vaginal area.
Some women with vaginal cancer are asymptomatic. Diagnosis often comes after an abnormal Pap test. Symptoms that have been associated with vaginal cancer include bleeding after coitus or examination, dyspareunia, and watery discharge. Bladder involvement results in urinary frequency or urgency; rectal extension causes painful defecation. A pelvic examination may reveal a single lesion, although multiple lesions are common (Hacker, 2010b).
Colposcopy examination and biopsy of Schiller-stained areas disclose the diagnosis. Therapy for vaginal cancer is directed by the extent of the lesion and the age and condition of the woman. Local excision is the preferred therapy for localized lesions. Topical application of 5-fluorouracil cream has been used with varying results. Laser surgery may be used to treat VAIN. Radical hysterectomy and removal of the upper vagina with dissection of the pelvic nodes or internal and external radiation are options for invasive cancer. Radiation therapy is the usual treatment of choice (Dotters & Katz, 2007). If a vaginectomy is performed, sexual function will be lost without reconstructive surgery. Chemotherapy has not been effective in treatment of vaginal cancer, although studies are ongoing on the effectiveness of chemotherapy in combination with radiation. In early-stage cancer, 5-year survival rates are greater than 80%, with stage II survival rates in the 50% range (Hacker, 2010b).
Nursing care for the woman with vaginal cancer is similar to that for other gynecologic cancers. Sexual counseling or referral may be needed.
Cancer of the Uterine Tubes
Primary carcinoma of the uterine (fallopian) tube (usually the distal one third) is one of the rarest cancers of the female genital tract (less than 1%), with a peak incidence between ages 50 and 60 years. The cause is unknown. Most women are asymptomatic in the early stages of tubal cancer. Vaginal bleeding is the most common symptom of tubal cancer, but clear vaginal discharge and lower abdominal pain also occur frequently. An enlarging unilateral pelvic mass or ascites may occur and is often misdiagnosed as ovarian carcinoma or endometrial carcinoma. Differential diagnosis of tubal cancer is usually made postoperatively. Because uterine tube cancer is so rare, there is no established management. Current therapy guidelines parallel those established for ovarian carcinoma; therefore, tumor-reducing surgery such as a total abdominal hysterectomy with BSO, omentectomy (removal of connective tissue covering the organs), and lymph node sampling are performed (Sunde, Kaplan, & Rose, 2007). Postoperative therapy consists of chemotherapy with cisplatin or other platinum-based drugs combined with paclitaxel, sometimes followed by second-look surgery to determine whether further treatment is needed. Radiation therapy is sometimes used if the disease is limited to the tube, ovary, and uterus, although reports of its effectiveness vary. Overall 5-year survival rates for all stages is 69%; 5-year survival rates for stages I and II are approximately 80% (Sunde et al.).
Nursing care for the woman with uterine tube cancer is similar to that of the woman with ovarian cancer.
Cancer and Pregnancy
Cancer occurs with relative infrequency during the reproductive years. Approximately 1 of every 1000 pregnant women will have cancer (Cohn, Ramaswamy, & Blum, 2009). These malignancies may be responsible for up to one third of maternal deaths. Although all forms of neoplasms have been documented in conjunction with pregnancy, the most frequently occurring types are breast cancer, cervical cancer, melanomas, ovarian cancer, leukemia and lymphomas, tubal cancers, and thyroid cancers. Bone, colorectal, vulvar, uterine, and vaginal cancers are rarely diagnosed during pregnancy. When pregnancy and cancer coincide, therapeutic issues are complex, and intense reactions occur in the woman, her family, and the health care team. Women are confronted with issues such as continuing or terminating the pregnancy. The selection and timing of therapies such as chemotherapy, radiation, and surgery are all affected by the pregnancy. Add to this the conflicting feelings the woman has (i.e., the joy of pregnancy versus the fear and anxiety associated with cancer), and the task of providing comprehensive care for the woman and her family presents a formidable challenge to the health care team. A brief discussion of the most frequent types of cancers that occur during pregnancy and the current therapies associated with them follows.
Cancer of the Breast
Approximately 1% to 2% of women are pregnant or lactating at the time of diagnosis of cancer of the breast (Tewari, 2007). Breast cancer complicates about 1 in 3000 pregnancies. The survival rate for women who are diagnosed with breast cancer while pregnant may be as low as 15% to 20% because the disease is generally in the advanced stages when first diagnosed (Tewari). Diagnosis is often delayed because breast engorgement may obscure the mass from palpation, and increased density of the tissue makes mammographic visualization more difficult. In addition, increased vascularity and lymphatic drainage in the breast of a pregnant woman may increase the speed of metastasis. Treatment is the same as for the nonpregnant woman, although surgery is usually the treatment of choice for breast cancer in pregnancy (Tewari). If an invasive tumor is found, it must be determined whether the tissue is positive or negative for estrogen receptors (ERs). ER-negative tumors spread more rapidly than ER-positive tumors and are more common in pregnancy.
Maternal-fetal management involves consideration of the gestational age of the fetus, the extent of disease, the tumor growth potential, and the proposed treatment. Termination of the pregnancy in early stages of the disease appears to have no effect on survival. There is little evidence to suggest that pregnancy affects the malignant process. Therapeutic abortion may become an issue in the presence of advanced disease and may be deemed necessary to achieve effective palliation. Lumpectomy or partial mastectomy is the most commonly used surgical procedure, but radical mastectomy is tolerated well in these women. For advanced disease in the second or third trimester, alkylating agents, 5-fluorouracil, doxorubicin, and vincristine are relatively safe for the fetus. Radiation therapy is avoided if at all possible until after the birth, because even with careful shielding the fetus may still receive sufficient radiation to produce detrimental effects (Tewari, 2007).
There is no agreement about whether a postpartum woman with breast cancer should breastfeed, although many surgeons recommend formula feeding. There are theoretic concerns that if one of the oncogenes for breast cancer is a virus, as many have postulated, the remaining breast may be contaminated, and the virus may be passed to the newborn, possibly acting as a latent inducer of breast carcinoma. Another reason is that lactation increases vascularity in the remaining breast, which may contain a neoplasm as well (Tewari, 2007).
Breastfeeding after lumpectomy is possible, but the site of the incision may interrupt the milk ducts or prevent the nipple from extending during feeding. Breastfeeding is contraindicated if the woman is receiving chemotherapy. Women receiving radiation will have diminished ability to lactate in the irradiated breast (Copeland & Landon, 2007; Tewari, 2007).
Pregnancy incidence after mastectomy is influenced by many factors, including prior treatment and duration of survival. About 7% of women will have one or more pregnancies within the first 5 years after mastectomy. In general, women with good prognoses (e.g., no positive nodes) are likely to be counseled to wait at least 2 years before attempting pregnancy (Cohn et al., 2009).
Cancer of the Cervix
The incidence of cervical cancer concurrent with pregnancy is reported to be 3% or about 1 in 2200 pregnancies, making it the most common reproductive tract cancer associated with pregnancy (Copeland & Landon, 2007). Birth can be accomplished by either the vaginal or the cesarean route; however, there is some concern regarding vaginal birth in the presence of invasive disease because the risk of hemorrhage and metastatic seeding from local trauma may be increased. The outcome for the woman with cervical cancer is roughly the same as that for the nonpregnant woman (Tewari, 2007).
Cervical abnormalities are diagnosed during pregnancy with an abnormal Pap test. If the report suggests that the pregnant woman has a squamous intraepithelial lesion, a colposcopy, possibly with directed biopsy, is done. If invasive disease is not found, treatment is delayed until after the woman gives birth. Colposcopy is often repeated every 6 to 8 weeks until the birth and again postpartum. Conization is not advised during pregnancy unless necessary to rule out invasive cancer because it is associated with bleeding, miscarriage, and preterm birth (Tewari, 2007).
The therapy of invasive carcinoma of the cervix during pregnancy is affected by many factors. The stage of the disease and the trimester in which the cancer is diagnosed are important. Equally important are the beliefs and desires of the woman and her family in terms of initiating therapy that can interrupt the pregnancy, as opposed to postponing the therapy until fetal viability is achieved. If the woman chooses not to continue the pregnancy, external radiation to the pelvis is done. Miscarriage usually occurs, and then internal radiation is done. If miscarriage does not occur, a modified radical hysterectomy may be performed. If the woman desires to continue the pregnancy, treatment of early-stage invasive cervical cancer can be delayed until fetal viability is reached, without harmful effects on the woman. Cesarean birth is usually performed, followed by radiation therapy (Copeland & Landon, 2007; Tewari, 2007).
Leukemia
The average age for pregnant women with acute leukemia is 28 years; incidence during pregnancy is about 1 in 75,000 (Cohn et al., 2009). Pregnancy seems to have no specific effect on the course of the disease, except that vigorous therapy is detrimental to early gestation. Preterm labor and postpartum hemorrhage are associated with acute leukemia (Tewari, 2007). Acute myelocytic leukemia (60% of cases) has a more fulminant course and requires immediate therapy; in the presence of chronic myelocytic leukemia, therapy can be delayed somewhat. Some pregnant women with the chronic form of the disease who had chemotherapy and radiation therapy directed at the spleen have given birth to apparently healthy infants. The decision to terminate the pregnancy rests with the woman and her family; however, prompt, aggressive therapy is always advisable if remission is to be achieved. Decisions may be influenced by the aggressiveness of the disease.
Hodgkin’s Disease
Hodgkin’s disease is a malignant lymphoma that affects many younger people and complicates about one in 6000 pregnancies. Younger women (younger than 40 years) have a better prognosis (Tewari, 2007).
Pregnancy appears to have no effect on the disease and vice versa, other than those effects resulting from therapy. Radiation therapy of the nodes and multiagent chemotherapy result in about a 90% cure rate. Unless gestation is well into the third trimester, delay in initiating therapy should be minimal, which brings up the dilemma of therapeutic abortion. Radiation therapy to diseased areas above the diaphragm can be initiated during the third trimester with proper shielding of the fetus (Cohn et al., 2009). Chemotherapy is strongly contraindicated during the first trimester but certain agents (antitumor antibiotics and antimicrotubule agents) appear safe to use in the second and third trimesters. Termination of the pregnancy during the course of the disease is not definitely indicated, although treatment decisions are easier (Tewari, 2007).
Melanoma
Malignant melanoma may be one of the rare cancers that can be affected by pregnancy. This is suggested by reports in which pregnancy has been shown to induce or exacerbate a melanoma. These suggestions are based on changes that occur naturally during pregnancy and include hyperpigmentation, an increase in melanocyte-stimulating hormone (MSH), and increased production of estrogen. ERs have been identified in about half of all melanomas (Tewari, 2007).
Although pregnancy has been implicated in the more rapid metastases to regional lymph nodes, stage for stage there does not seem to be a significant difference in the survival of pregnant and nonpregnant women. As a result, most authorities recommend that women who have histories of malignant melanoma delay pregnancy for 2 to 3 years after surgical excision, because this is the period of highest risk for recurrence (Copeland & Landon, 2007).
Diagnosis is established by biopsy. Therapy consists of radical local excision. For most other malignancies, the placenta is unexplainably resistant to invasion by maternal cancer. Although melanoma accounts for few cases of malignant disease during pregnancy, it is the most common cancer to metastasize to the placenta (Tewari, 2007).
Thyroid Cancer
The incidence of thyroid cancer in pregnancy is not established. Normally the thyroid gland enlarges during pregnancy, and an asymptomatic nodular mass is a common finding. Diagnosis is usually by cytologic testing of fine-needle aspirate. Thyroid suppression is the preferred treatment during pregnancy for a benign lesion. For a papillary or follicular malignancy found in the first or second trimester, the woman is advised to have a thyroidectomy in the second trimester followed by thyroid suppression (Tewari, 2007). If a tumor is found in the third trimester, surgery can be delayed until after the birth. With other thyroid malignancies, treatment is individualized based on the wishes of the woman and her family (Tewari).
Colon Cancer
The incidence of colon cancer in pregnancy is approximately 1 in 13,000 (Copeland & Landon, 2007). The signs and symptoms such as constipation, hemorrhoids, and backache are often attributed to pregnancy, resulting in diagnosis at a more advanced stage. Colonoscopy and biopsy are usually not done in pregnancy because these procedures can cause placental abruption and fetal injury from maternal hypoxia or hypotension.
Management of the cancer is based on the weeks of gestation and tumor stage. In a woman who is less than 20 weeks of gestation, surgery may be performed to remove the tumor. If she is more than 20 weeks of gestation, surgery may be delayed until after the birth. Chemotherapy and radiation are usually not used in pregnancy for colon cancer but may be used after the birth (Cohn et al.; Copeland & Landon).
Other Gynecologic Cancers
Cancer of the Vulva: The diagnosis of preinvasive (VIN) disease during pregnancy is becoming more common with the increase in the diagnosis of CIS of the vulva (Tewari, 2007). Therapy is postponed until the postpartum period. If invasive disease (a rare occurrence) is diagnosed during the first trimester, vulvectomy with bilateral groin dissection may be done after the fourteenth week. When it is diagnosed in the third trimester, local wide excision is done, deferring definitive surgery until after birth. Pregnancy does not alter the course of the disease. Vaginal birth can be attempted if the surgical wounds are healed (Tewari).
After radical vulvectomy and bilateral inguinal node dissection, a woman who becomes pregnant again can carry the pregnancy to term and give birth vaginally. If vaginal stenosis is present and could impede birth, cesarean birth may be more appropriate (Tewari, 2007).
Cancer of the Vagina: Except for clear-cell adenocarcinoma of DES-exposed women, cancer of the vagina is rare. If clear-cell adenocarcinoma of the cervix and vagina or sarcoma is found in the upper vagina, the preferred surgery is radical hysterectomy, upper vaginectomy, and bilateral pelvic lymphadenectomy, followed by chemotherapy. Radiation is usually not advocated during pregnancy. Pregnancy does not seem to affect the course of the disease or the prognosis.
Cancer of the Uterus: Endometrial carcinoma during pregnancy is very rare. Diagnosis was usually an incidental finding after therapeutic abortion or surgery, and the lesions were minimally invasive or noninvasive. Recommended therapy is TAH-BSO and adjuvant radiotherapy (Tewari, 2007).
Cancer of the Uterine Tube: With a peak incidence between 50 and 55 years of age, concurrent pregnancy is only a remote possibility. Should it occur, the recommended therapy (TAH-BSO with postoperative radiotherapy or chemotherapy) is the same as that for the nonpregnant woman. Removal of the uterine tube is an alternative treatment (Tewari, 2007).
Cancer of the Ovary: Cancer of the ovary is the second most frequent reproductive cancer that occurs with pregnancy. Still, ovarian malignancy is relatively rare, being reported to occur in approximately 1 in 18,000 (Cohn et al., 2009).
Ovarian masses occur frequently during pregnancy. Because corpus luteum cysts account for a high percentage of these masses and because 99% of these resolve by the fourteenth week, any mass smaller than 5 cm may simply be observed until the end of the first trimester. Any mass larger than 5 cm, one that is growing, or one that does not resolve after the fourteenth week warrants further investigation. Abdominal palpation and ultrasound are the diagnostic tools of choice during pregnancy. However, in many cases, laparotomy is necessary to confirm the diagnosis. Laparotomy after 18 weeks of gestation has negligible fetal wastage associated with the procedure and is therefore considered safe (Tewari, 2007).
An ovarian tumor may be first diagnosed at birth or after birth because the enlarged uterus obscured its presence. Definitive diagnosis is needed before treatment is selected. For stage I tumors, treatment includes conservative surgery (unilateral oophorectomy and salpingectomy) and use of chemotherapy. If diagnosis occurs in the second or third trimester, treatment choices are difficult to make. They include interrupting the pregnancy and starting chemotherapy immediately, preserving pregnancy and starting chemotherapy with the fetus in utero (controversial), or delaying chemotherapy until the fetus is more mature and early scheduled birth is a low risk to the fetus (Tewari, 2007).
Cancer Therapy and Pregnancy
Decisions about the type and timing of therapy for cancer in the pregnant woman evoke moral and philosophic dilemmas, as well as complex medical judgments and intense emotional responses.
Ethical Considerations: When a pregnant woman has cancer and her survival is contingent on treatment that will harm the fetus, the health care team must work with the woman and her significant others to make decisions about how to proceed with her care. If a one-client model of ethical decision making is used, the risk-benefit analysis is applied to the maternal-fetal unit. The pregnant woman decides what is best for her and the fetus. The woman may accept or refuse treatment. If a two-client model is used for decision making, more weight is given to fetal well-being, but the pregnant woman cannot be forced to accept harm to herself for the sake of the fetus. Thus she could elect to accept treatment.
The fetus is at risk with either chemotherapy or radiation therapy. The effect of cancer therapy on the fetus can include death, miscarriage, teratogenesis, alteration in growth and development, alterations in function, and genetic mutation. The long-term effects on the fetus are unknown. These theoretic dangers must be weighed against the potential detrimental effects to the mother if treatment is withheld (Gilbert, 2011).
Timing of Therapy: Timing of therapy also is an important issue to discuss. Because most cancer therapy (except surgery) is geared toward having a differential and noxious effect on rapidly growing tissue, the fetus is most at risk during the first trimester, when organogenesis and rapid tissue growth occur. Surgery offers the least potential risk to the fetus; however, the risk of miscarriage and preterm labor may be increased.
Chemotherapy is avoided in the first trimester if at all possible. Although use of most chemotherapeutic agents has had isolated reports of fetal abnormalities, data on the agents used after the first trimester have recorded surprisingly few fetal abnormalities. The placenta may act as a barrier against the chemotherapeutic agents; therefore, although risk still exists, the judicious use of chemotherapy after the first trimester can result in live births with few congenital abnormalities. Acute drug toxicities may occur if treatment has occurred just before birth. Breastfeeding by women who are taking chemotherapeutic drugs is not recommended because most of these drugs may be excreted in breast milk (Copeland & Landon, 2007; Tewari, 2007).
Radiation therapy presents its own set of issues. During embryonic development, tissues are extremely radiosensitive. If cells are genetically altered or killed during this time, the child either will fail to survive or will be deformed. From a radiologic stance, there are three significant periods in embryonic development (Tewari, 2007):
1. Preimplantation: If irradiation does not destroy the fertilized egg, it probably does not affect it significantly.
2. Critical period of organogenesis: During this period, especially between days 18 and 38, the organism is most vulnerable; microcephaly, anencephaly, eye damage, growth restriction, spina bifida, and foot damage may occur.
3. After day 40: Large doses may still cause observable malformation and damage to the central nervous system.
Pregnancy After Cancer Treatment: If cancer therapy has not included the removal of the uterus, ovaries, or uterine (fallopian) tubes, there is a possibility that the woman may still be able to become pregnant. Although a woman’s menstrual cycle may have resumed, pregnancy may be difficult to achieve. Therapy that has affected the pituitary or thyroid gland may make conception difficult. Radiation appears to have the most deleterious effects on the endocrine system. The use of chemotherapy may result in temporary or permanent sterility, depending on the drug, the dose, and the length of time since the therapy was completed. Rates of ovarian failure are increased with pelvic irradiation (Tewari, 2007).
Of growing concern is the increase in the number of childhood and adolescent cancer survivors. Long-term effects of therapy on fertility, including incidence of congenital anomalies, are not well known. Counseling issues to be discussed with the pregnant woman after cancer treatment include the risk of recurrence and the likely sites of recurrence, how the prior cancer treatment can affect fertility or reproductive outcome, and if a future pregnancy will adversely affect a tumor that is estrogen-receptor positive (Copeland & Landon, 2007).
For recovery from the disease and treatment to be complete, a delay of at least 2 years from the end of therapy to conception often is advised (Tewari, 2007). Before conception, a woman who has had cancer should have a complete physical examination to rule out complications that may place her or a fetus in jeopardy. Cardiac, pulmonary, hematologic, neurologic, renal, or gonadal function can be impaired. The woman and the potential father (if partnered) may be referred for reproductive and genetic counseling as well.
Gestational Trophoblastic Disease
Gestational trophoblastic disease (GTD) is a term that encompasses a spectrum of disorders arising from the placental trophoblast. It includes hydatidiform mole (see Chapter 28), invasive mole, and choriocarcinoma. Gestational trophoblastic neoplasia (GTN) refers to persistent trophoblastic tissue that is presumed to be malignant (Soper & Creasman, 2007).
Box 11-6 describes the clinical classifications of GTN. For several reasons, GTN is recognized as the most curable gynecologic malignancy. There is a sensitive marker produced by the tumor (hCG); the tumor is extremely sensitive to various chemotherapeutic agents; high risk factors in the disease process can be identified, allowing individualized therapy; and the aggressive use of multiple treatment methods is possible.
Malignant disease follows normal pregnancy in about 30% to 50% of cases and hydatidiform mole in about 25% of cases. Miscarriage or ectopic pregnancy or another gestational event precedes about 25% of cases (Soper & Creasman, 2007). Metastasis occurs most often in the lungs, the vagina, the liver, and the brain.
Continued bleeding after evacuation of a hydatidiform mole is usually the most suggestive symptom of GTN. Other clinical signs include abdominal pain and uterine and ovarian enlargement. Signs of metastasis include pulmonary symptoms (e.g., dyspnea, cough). The diagnosis is usually confirmed by increasing or plateauing hCG levels after evacuation of a molar pregnancy. Once diagnosis is confirmed, other clinical studies (e.g., CT scan of lungs and brain, chest x-ray, pelvic ultrasound, and liver scan) are done to determine the extent of the disease (Soper & Creasman, 2007).
For women who wish to preserve their fertility and who have low risk nonmetastatic or low risk metastatic GTN, single-agent chemotherapy is chosen. Methotrexate has been the treatment of choice for years. High-dose methotrexate followed by folinic acid “rescue” within 24 hours also has shown excellent results and causes fewer toxic effects (Soper & Creasman, 2007). Dactinomycin also has been used with equally good results and is used for women with liver or renal disease, both of which are contraindications for methotrexate. Hysterectomy with adjuvant chemotherapy is often the choice of treatment for nonmetastatic tumors in women who have completed their childbearing (Soper & Creasman).
Women who have metastasis are classified as having either a good or poor prognosis, depending on the absence or presence of brain or liver metastasis, unsuccessful prior chemotherapy, symptoms lasting longer than 4 months, and serum β-hCG levels greater than 40,000 milli-International Units/ml. Treatment progresses from single-agent chemotherapy in the good-prognosis metastatic GTN to multiple-agent chemotherapy and multiple methods of treatment for the poor-prognosis group. Cure rates for the good-prognosis group are almost as positive as for those with nonmetastatic disease, both approaching 100% (Soper & Creasman, 2007).
Therapy is continued until negative hCG levels are obtained. After successful chemotherapy follow-up by serum hCG levels varies. One schedule is to obtain levels every 2 weeks for 3 months, every month for up to a year after completing therapy and every 6 to 12 months up to 3 to 5 years (Kavanagh & Gershenson 2007; Soper & Creasman, 2007). Physical examinations are done at least yearly as are chest radiographs if indicated. Contraception is needed until the woman has been in remission for 6 months to 1 year (Kavanagh & Gershenson; Soper & Creasman). Oral contraceptives are preferred, but barrier methods are acceptable if oral contraceptives are contraindicated; intrauterine devices (IUDs) are contraindicated (Gilbert, 2011). During a subsequent pregnancy, pelvic ultrasonography is recommended because the woman is at higher risk (1% to 2%) to develop another molar pregnancy. Serum hCG levels should be obtained 6 weeks after the birth (Kavanagh & Gershenson).
KEY POINTS
• Gynecologic disorders diminish the quality of life for affected women and their families.
• Structural disorders of the uterus and vagina related to pelvic relaxation and urinary incontinence can be a delayed result of childbearing, but they can be seen in young or childless women.
• Bladder training and pelvic muscle exercises can significantly decrease or relieve mild to moderate urinary incontinence.
• The development of neoplasms, whether benign or malignant, can have a significant physical and emotional effect on a woman and her family.
• Abnormal uterine bleeding is the most common symptom of leiomyomas or fibroid tumors.
• Various alternatives to hysterectomy exist for structural and benign disorders of the uterus; women need to be informed about the risks and benefits to make an informed decision about treatment.
• Endometrial cancer is the most common reproductive system malignancy.
• Hysterectomy is the usual treatment for early-stage endometrial cancer.
• Human papillomavirus infection is the primary cause of cervical cancer and is linked to vulvar cancer in women younger than 40 years of age.
• The squamocolumnar junction is an important landmark identified with neoplastic changes of the cervix.
• Preinvasive cancer of the cervix may be treated with techniques such as electrosurgical excision, cryotherapy, and laser therapy to save the structure of the cervix, particularly in women who desire to retain childbearing ability.
• External and internal radiation therapy in combination is as successful as surgery in treating early stages of cancer of the cervix.
• A Pap test will detect approximately 90% of early cervical dysplasias.
• Cancer of the ovary causes more deaths than any other female genital tract cancer.
• Nurses can control their exposure to radiation by increasing the distance from the radiation source, by limiting the time of exposure, and by using lead shielding.
• Cancer is relatively infrequent during pregnancy, occurring about once in every 1000 pregnancies.
• Radiation or chemotherapy treatment of the pregnant woman who has cancer places the fetus at risk for death, miscarriage, teratogenesis, and alterations in growth and development.
• Gestational trophoblastic neoplasms are highly curable but require close monitoring of hCG levels after treatment.
![]() Audio Chapter Summaries Access an audio summary of these Key Points on
Audio Chapter Summaries Access an audio summary of these Key Points on ![]()