Antepartum Hemorrhagic Disorders
• Differentiate among causes of early pregnancy bleeding, including miscarriage, ectopic pregnancy, premature dilation of the cervix, and hydatidiform mole.
• Discuss signs and symptoms, possible complications, and management of miscarriage, ectopic pregnancy, premature dilation of the cervix, and hydatidiform mole.
• Compare and contrast placenta previa and abruptio placentae (placental abruption) in relation to signs and symptoms, complications, and management.
• Discuss the diagnosis and management of disseminated intravascular coagulation.
• Examine the role of the nurse in the health care team approach to the treatment of bleeding disorders.
Bleeding in pregnancy may jeopardize maternal and fetal well-being. Maternal blood loss decreases oxygen-carrying capacity, which places the woman at increased risk for hypovolemia, anemia, infection, and preterm labor, and adversely affects oxygen delivery to the fetus. Fetal risks from maternal hemorrhage include blood loss or anemia, hypoxemia, hypoxia, anoxia, and preterm birth. Hemorrhagic disorders in pregnancy are medical emergencies. The incidence and type of bleeding vary by trimester. Ruptured ectopic pregnancy and abruptio placentae (placental abruption) have the highest incidence of maternal mortality. Prompt assessment and intervention by the health care team is essential to save the lives of both the woman and her fetus.
Early Pregnancy Bleeding
Bleeding during early pregnancy is alarming to the woman and of concern to health care providers. The common bleeding disorders of early pregnancy include miscarriage (spontaneous abortion), premature dilation of the cervix (incompetent cervix), ectopic pregnancy, and hydatidiform mole (molar pregnancy).
Miscarriage (Spontaneous Abortion)
A pregnancy that ends as a result of natural causes before 20 weeks of gestation is defined as a miscarriage (spontaneous abortion). This 20-week marker is considered to be the point of viability, when a fetus may survive in an extrauterine environment. A fetal weight less than 500 g also may be used to define an abortion (Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010). The term miscarriage is used throughout this discussion because this term is more appropriate than abortion to use with clients. Abortion may be perceived as an insensitive term to use with families who are grieving a pregnancy loss. Therapeutic or elective induced abortion is discussed in Chapter 8.
Incidence and Etiology
Approximately 10% to 15% of all clinically recognized pregnancies end in miscarriage (Simpson & Jauniaux, 2007). The majority—greater than 80% of miscarriages—are early pregnancy losses, occurring before 12 weeks of gestation (Cunningham et al., 2010). Of all clinically recognized pregnancy losses, at least 50% result from chromosomal abnormalities (Cunningham et al.; Simpson & Jauniaux). Other possible causes of early miscarriage include endocrine imbalance (as in women who have luteal phase defects, hypothyroidism, or insulin-dependent diabetes mellitus with high blood glucose levels in the first trimester), immunologic factors (e.g., antiphospholipid antibodies), systemic disorders (e.g., lupus erythematosus), and genetic factors. Infections are not a common cause of early miscarriage (Cunningham et al.), but there is an increased risk for a spontaneous abortion with varicella infection in the first trimester (Gilbert, 2011).
A late miscarriage, sometimes called a second-trimester loss, occurs between 12 and 20 weeks of gestation. It usually results from maternal causes, such as advancing maternal age and parity, premature dilation of the cervix and other anomalies of the reproductive tract, inadequate nutrition, tobacco, alcohol, and caffeine use (Cunningham et al., 2010), obesity, and stressful life events (Gilbert, 2011). Little can be done to prevent genetically caused pregnancy loss, but correction of maternal disorders, a healthy lifestyle, adequate early prenatal care, and treatment of pregnancy complications can do much to prevent other causes of miscarriage.
Types
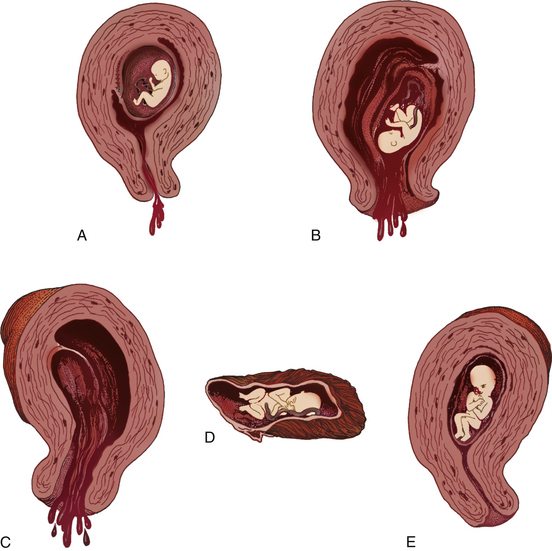
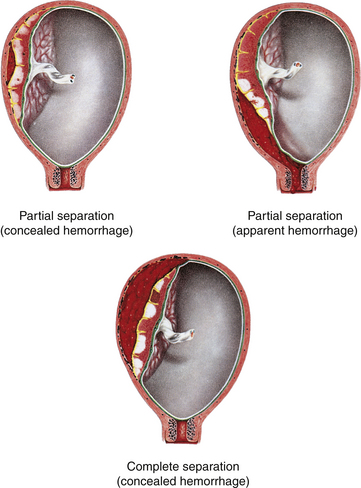
The types of miscarriage include threatened, inevitable, incomplete, complete, and missed. All types of miscarriage can recur in subsequent pregnancies. All types except the threatened miscarriage can lead to infection (Fig. 28-1).
Clinical Manifestations
Signs and symptoms of miscarriage depend on the duration of pregnancy. The presence of uterine bleeding, uterine contractions, or abdominal pain is an ominous sign during early pregnancy and must be considered a threatened miscarriage until proven otherwise.
If miscarriage occurs before the sixth week of pregnancy, the woman may report what she believes is a heavy menstrual flow. Miscarriage that occurs between weeks 6 and 12 of pregnancy causes moderate discomfort and blood loss. After week 12, miscarriage is typified by severe pain, similar to that of labor, because the fetus must be expelled. Diagnosis of the type of miscarriage is based on the signs and symptoms present (Table 28-1).
TABLE 28-1
ASSESSING MISCARRIAGE AND THE USUAL MANAGEMENT
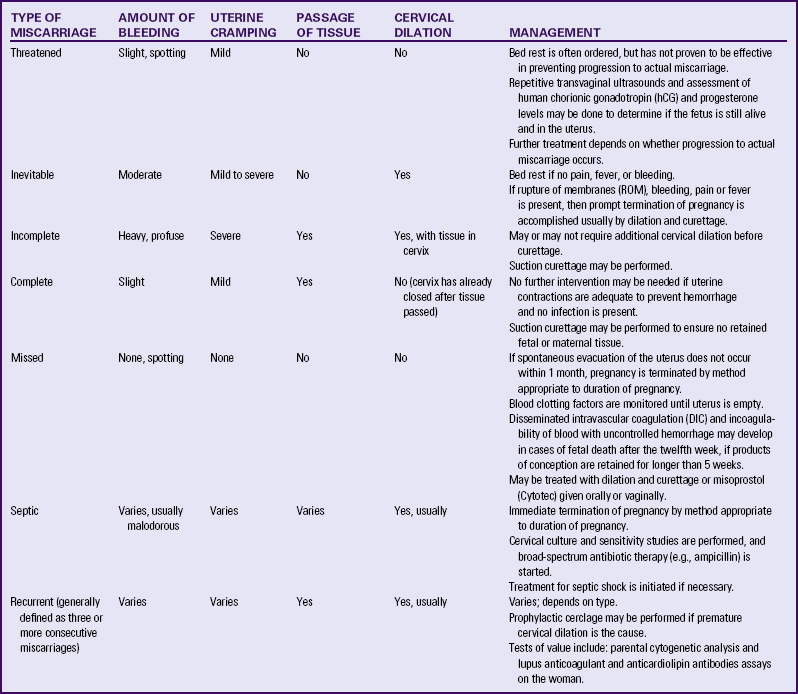
Source: Cunningham, F., Leveno, K., Bloom, S., Hauth, J., Rouse, D., & Spong, C. (2010), Williams obstetrics (23rd ed.). New York: McGraw-Hill; Gilbert, E. (2011). Manual of high risk pregnancy & delivery (5th ed.). St. Louis: Mosby .
Symptoms of a threatened miscarriage (see Fig. 28-1, A) include spotting of blood but with the cervical os closed. Mild uterine cramping may be present.
Inevitable (see Fig. 28-1, B) and incomplete (see Fig. 28-1, C) miscarriages involve a moderate to heavy amount of bleeding with an open cervical os. Tissue may be present with the bleeding. Mild to severe uterine cramping may be present. An inevitable miscarriage is often accompanied by rupture of membranes (ROM) and cervical dilation. Passage of the products of conception will occur. An incomplete miscarriage involves the expulsion of the fetus with retention of the placenta.
In a complete miscarriage (see Fig. 28-1, D), the cervix has already closed after all fetal tissue was expelled. Slight bleeding may occur and mild uterine cramping may be present, as well.
The term missed miscarriage (see Fig. 28-1, E) refers to a pregnancy in which the fetus has died, but the products of conception are retained in utero for up to several weeks. It may be diagnosed by ultrasonic examination after the uterus stops increasing in size or even decreases in size. There may be no bleeding or cramping, and the cervical os remains closed.
Recurrent early (habitual) miscarriage is three or more spontaneous pregnancy losses before 20 weeks of gestation. The causes of recurrent miscarriage are the same as those discussed earlier in this section. Another possible cause of recurrent pregnancy loss is parental chromosomal abnormalities. The evaluation of couples experiencing recurrent pregnancy loss usually includes karyotyping of both partners and evaluating the woman’s uterine cavity and testing for antiphospholipid antibody syndrome. No cause can be identified in approximately half of all couples who experience recurrent pregnancy loss. However, 60% to 70% of these couples will go on to have a successful pregnancy with no treatment (Cunningham et al., 2010).
Miscarriages can become septic, although this is uncommon. Symptoms of a septic miscarriage include fever and abdominal tenderness. Vaginal bleeding, which may be slight to heavy, is usually malodorous.
Management
Initial Care: Management depends on the classification of the miscarriage and on signs and symptoms (see Table 28-1). Traditionally, threatened miscarriages have been managed expectantly with supportive care. However, there are no proven
effective therapies for this condition. Bed rest, although often prescribed, does not prevent progression to actual miscarriage. Repetitive transvaginal ultrasounds and measurement of human chorionic gonadotropin (hCG) and progesterone levels may be performed to determine if the fetus is alive and within the uterus (Cunningham et al., 2010).
Follow-up treatment depends on whether the threatened miscarriage progresses to actual miscarriage or symptoms subside and the pregnancy remains intact (see the Nursing Process box: Miscarriage). If bleeding and infection do not occur, expectant management is a reasonable option. In approximately half of all threatened miscarriages managed in this way, the pregnancy continues (Cunningham et al., 2010).
Once the cervix begins to dilate the pregnancy cannot continue and miscarriage becomes inevitable. If all the products of conception are passed, no surgical intervention is necessary. If heavy bleeding, excessive cramping, or infection is present, however, the remaining embryonic, fetal or placental tissue must be removed from the uterus, usually by suction curettage. In women who are clinically stable, expectant management to allow spontaneous resolution of an incomplete miscarriage is another treatment option (Cunningham et al., 2010; Gilbert, 2011).
Most missed miscarriages eventually end spontaneously. Women may be offered expectant management at the time the pregnancy loss is diagnosed. Expectant management results in eventual spontaneous miscarriage in 16% to 76% of cases (Gilbert, 2011).
Medical management is another treatment option if bleeding and infection are not present. Prostaglandin medications (e.g., misoprostol [Cytotec]) may be given orally or vaginally and is usually effective in completing the miscarriage within 7 days (Cunningham et al., 2010). If medical management is chosen, nursing care is similar to the care for any woman whose labor is being induced (see Chapter 33). Special care may be needed for management of side effects of prostaglandin, such as nausea, vomiting, and diarrhea. If the products of conception are not passed completely, the woman may be prepared for manual or surgical evacuation of the uterus.
A third management option, and one that is often chosen, is dilation and curettage (D&C), a surgical procedure in which the cervix is dilated and a suction curette is inserted to scrape the uterine walls and remove uterine contents (Cunningham et al., 2010). Before a surgical procedure is performed, a full history should be obtained and general and pelvic examinations conducted. General preoperative and postoperative care is appropriate for the woman requiring surgical intervention for miscarriage. Analgesics and anesthesia that are appropriate to the procedure are used. The nurse reinforces explanations, answers any questions or concerns, and prepares the woman for surgery.
After evacuation of the uterus, oxytocin is often given to prevent hemorrhage. For excessive bleeding after the miscarriage, ergot products such as ergonovine (Methergine) or a prostaglandin derivative such as methylcarboprost tromethamine (Hemabate) may be given to contract the uterus. (See the Medication Guide: Drugs Used to Manage Postpartum Hemorrhage, on p. 82 in Chapter 34). Antibiotics are given as necessary. Analgesics, such as antiprostaglandin agents (e.g., nonsteroidal antiinflammatory drugs [NSAIDS]), may decrease discomfort from cramping. Transfusion therapy may be required for shock or anemia. The woman who is Rh negative and is not isoimmunized is given Rho(D) immune globulin (Cunningham et al., 2010).
Psychosocial aspects of care focus on what the pregnancy loss means to the woman and her family. Grief from perinatal loss is complex and unique to each individual. Explanations are provided regarding the nature of the miscarriage, expected procedures, and possible future implications for childbearing.
As with other fetal or neonatal losses, the woman should be offered the option of seeing the products of conception. She may also want to know what the hospital does with the products of conception or whether she needs to make a decision about final disposition of fetal remains.
Follow-up Care at Home: The woman will likely be discharged home within a few hours after a D&C or as soon as
her vital signs are stable, vaginal bleeding remains minimal, and she has recovered from anesthesia. Discharge teaching emphasizes the need for rest. If significant blood loss has occurred, iron supplementation may be ordered. Teaching includes information about normal physical findings, such as cramping, type and amount of bleeding, resumption of sexual activity, and family planning (see the Teaching for Self-Management box). Frequently the woman and her partner want to know when she may become pregnant again. Discuss with them the importance of completely resolving the loss before attempting another pregnancy (Gilbert, 2011). Follow-up care should assess the woman’s physical and emotional recovery. Referrals to local support groups should be provided as needed. Share Pregnancy and Infant Loss Support, Inc. (www.nationalshare.org) is an excellent online resource for families that have experienced an early pregnancy loss.
Follow-up telephone calls after a loss are important. The woman may appreciate a telephone call on what would have been her due date. These calls provide opportunities for the woman to ask questions, seek advice, and receive information to help process her grief.
Recurrent Premature Dilation of the Cervix (Incompetent Cervix)
One cause of late miscarriage is recurrent premature dilation of the cervix (incompetent cervix), which has traditionally been defined as passive and painless dilation of the cervix during the second trimester. This definition assumes an all-or-nothing role for the cervix: it is either competent or incompetent. Current thinking is that cervical competence is variable and exists as a continuum that is determined in part by cervical length. Other related causative factors include composition of the cervical tissue and the individual circumstances associated with the pregnancy in terms of maternal stress and lifestyle. Iams (2009) refers to this condition as cervical insufficiency.
Etiology
Etiologic factors include a history of previous cervical trauma such as lacerations during childbirth, excessive cervical dilation for curettage or biopsy, or ingestion of diethylstilbestrol (DES) by the woman’s mother while pregnant with the woman. Because DES has not been used since the early 1970s, however, this risk factor should soon be of only historic interest. Multiple gestation alone does not produce reduced cervical competency or justify prophylactic cervical cerclage (Ludmir & Owen, 2007). Other causes are a congenitally short cervix and cervical or uterine anomalies.
Diagnosis
Reduced cervical competence is a clinical diagnosis, based on history. Short labors, recurring loss of the pregnancy at progressively earlier gestational ages, advanced cervical dilation at the time of first presentation for care, and a history of prior cervical surgery or trauma suggest reduced cervical competence (Iams, 2009). Ultrasound examination during pregnancy is used to diagnose this condition objectively. A short cervix (less than 25 mm) is indicative of reduced cervical competence. Often the short cervix is accompanied by cervical funneling (beaking) or effacement of the internal cervical os (Cunningham et al., 2010; Iams; Ludmir & Owen, 2007).
Management
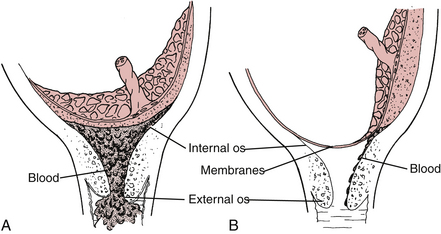
Medical management consists of bed rest, pessaries, antibiotics, antiinflammatory drugs, and progesterone supplementation (Iams, 2009). Surgical management, with placement of a cervical cerclage, may be chosen instead. During pregnancy the McDonald technique is often the procedure of choice. In this procedure suture is placed around the cervix beneath the mucosa to constrict the internal os of the cervix (Fig. 28-2) (Cunningham et al., 2010). A cerclage may be placed prophylactically or as a rescue procedure once the cervix has been found to be effaced or dilated (Cunningham et al.; Gilbert, 2011).
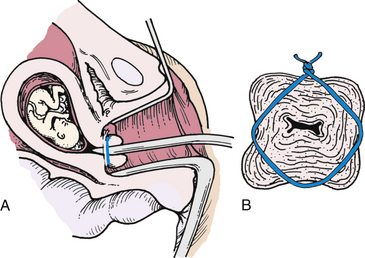
FIG. 28-2 A, Cerclage correction of premature dilation of the cervical os. B, Cross-sectional view of closed internal os.
A prophylactic cerclage is usually placed at 11 to 15 weeks of gestation. The cerclage is electively removed (usually an office or a clinic procedure) when the woman reaches 37 weeks of gestation, or it may be left in place until spontaneous labor begins. Occasionally the cerclage is left in place and a cesarean birth performed. The best treatment for reduced cervical competence is uncertain at this time. Research results indicate![]() that selective cerclage placement during pregnancy based on repeated ultrasound examination of the cervix may produce pregnancy outcomes that are just as good as those obtained after prophylactic cerclage placement. Ultrasound surveillance begins at 15 to 16 weeks of gestation. Cerclage placement is offered if the cervical length falls to less than 20 to 25 mm before 23 to 24 weeks (Iams, 2009). Risks of the procedure include premature rupture of membranes (PROM), preterm labor, and chorioamnionitis. Although no consensus has been reached, 24 weeks is often used as the upper gestational age limit for cerclage placement (Iams).
that selective cerclage placement during pregnancy based on repeated ultrasound examination of the cervix may produce pregnancy outcomes that are just as good as those obtained after prophylactic cerclage placement. Ultrasound surveillance begins at 15 to 16 weeks of gestation. Cerclage placement is offered if the cervical length falls to less than 20 to 25 mm before 23 to 24 weeks (Iams, 2009). Risks of the procedure include premature rupture of membranes (PROM), preterm labor, and chorioamnionitis. Although no consensus has been reached, 24 weeks is often used as the upper gestational age limit for cerclage placement (Iams).
The nurse assesses the woman’s feelings about her pregnancy and her understanding of reduced cervical competence. Evaluating the woman’s support systems is also important. Because the diagnosis of reduced cervical competence is usually not made until the woman has lost one or more pregnancies, she may feel guilty or to blame for this impending loss. Assessing for previous reactions to stresses and appropriateness of coping responses is therefore important. The woman needs the support of her health care providers, as well as that of her family.
Follow-up Care at Home
The woman will likely be on bed rest for a least a few days immediately following cerclage placement. She will also probably be advised to avoid sexual intercourse until after a postoperative check. Thereafter, decisions about physical activity and intercourse are individualized, based on the status of the woman’s cervix, as determined by digital and ultrasound examination (Ludmir & Owen, 2007). The woman must understand the importance of initial activity restriction at home and the need for close observation and supervision. Tocolytic medications may be prescribed to prevent uterine contractions and further dilation of the cervix. If so, the woman must be instructed on the expected response and possible side effects. Additional instruction includes the need to watch for and report signs of preterm labor, ROM, and infection. Finally, the woman should know the signs that would warrant an immediate return to the hospital, including strong contractions less than 5 minutes apart, ROM, severe perineal pressure, and an urge to push. If management is unsuccessful and the fetus is born before viability, appropriate grief support should be provided. If the fetus is born prematurely, appropriate anticipatory guidance and support will be necessary.
Ectopic Pregnancy
An ectopic pregnancy is one in which the fertilized ovum is implanted outside the uterine cavity (Fig. 28-3). Two percent of all first-trimester pregnancies in the United States are ectopic, and these account for 9% of all pregnancy-related maternal deaths. Women are less likely to have a successful subsequent pregnancy after an ectopic pregnancy (Cunningham et al., 2010; Gilbert, 2011). Ectopic pregnancy is also a leading cause of infertility.
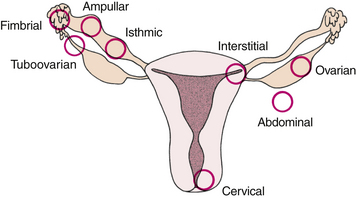
FIG. 28-3 Sites of implantation of ectopic pregnancies. Order of frequency of occurrence is ampulla, isthmus, interstitium, fimbria, tuboovarian ligament, ovary, abdominal cavity, and cervix (external os).
Ectopic pregnancies are often called tubal pregnancies because approximately 95% are located in the uterine tube (Cunningham et al., 2010). Although they are much less common, ectopic pregnancies can also occur in the abdominal cavity, on an ovary, or on the cervix. Of all tubal ectopic pregnancies, more than half (approximately 55%) are located in the ampulla, or largest portion of the tube (Gilbert, 2011).
The reported incidence of ectopic pregnancy rose through 1990 in the United States. Since then, because more cases are managed medically, reliable data on the actual number of ectopic pregnancies have not been available (Cunningham et al., 2010). Some of the increased incidence is likely because of improved diagnostic techniques, such as more sensitive β-hCG measurement and transvaginal ultrasound, resulting in the identification of more cases. Other causes for the rise include an increased incidence of sexually transmitted infections, tubal infection and damage, popularity of contraceptive methods that predispose failures to be ectopic (e.g., the intrauterine device [IUD]), use of tubal sterilization methods that increase the chance of ectopic pregnancy, increased use of assisted reproductive techniques, and increased use of tubal surgery (Cunningham et al.; Gilbert, 2011).
Ectopic pregnancy is classified according to site of implantation (e.g., tubal, ovarian, or abdominal). The uterus is the only organ capable of containing and sustaining a term pregnancy. Only approximately 5% of abdominal pregnancies reach viability. Surgery to remove the embryo or fetus is usually performed as soon as an abdominal pregnancy is identified, however, because of the high risk for hemorrhage at any time during the pregnancy (Cunningham et al., 2010; Gilbert, 2011). The chance of fetal survival in an abdominal pregnancy depends on gestational age at birth. The risk for fetal deformity in an abdominal pregnancy is high as a result of pressure deformities caused by oligohydramnios. The most common problems include facial or cranial asymmetry, various joint deformities, limb deficiency, and central nervous system anomalies (Fig. 28-4) (Cunningham et al.; Gilbert).
Clinical Manifestations
Most cases of ectopic (tubal) pregnancy are diagnosed before rupture based on the three most classic symptoms: (1) abdominal pain, (2) delayed menses, and (3) abnormal vaginal bleeding (spotting) that occurs approximately 6 to 8 weeks after the last normal menstrual period (Gilbert, 2011). Abdominal pain occurs in almost every case. It usually begins as a dull, lower quadrant pain on one side. The discomfort can progress from a dull pain to a colicky pain when the tube stretches, to sharp, stabbing pain (Cunningham et al., 2010; Gilbert). It progresses to a diffuse, constant, severe pain that is generalized throughout the lower abdomen (Gilbert). Up to 90% of women with an ectopic pregnancy report a period that is delayed 1 to 2 weeks or is lighter than usual, or an irregular period. Mild to moderate dark red or brown intermittent vaginal bleeding occurs in up to 80% of women (Gilbert).
If the ectopic pregnancy is not diagnosed until after rupture has occurred, referred shoulder pain may be present in addition to generalized, one-sided, or deep lower quadrant acute abdominal pain. Referred shoulder pain results from diaphragmatic irritation caused by blood in the peritoneal cavity. The woman may exhibit signs of shock, such as faintness and dizziness, related to the amount of bleeding in the abdominal cavity and not necessarily related to obvious vaginal bleeding. An ecchymotic blueness around the umbilicus (Cullen sign), indicating hematoperitoneum, may also develop in an undiagnosed, ruptured intraabdominal ectopic pregnancy.
Tubal Pregnancy Management
The differential diagnosis of ectopic pregnancy involves consideration of numerous disorders that share many signs and symptoms. Many of these women come to the emergency department experiencing first-trimester bleeding or pain. Miscarriage, ruptured corpus luteum cyst, appendicitis, salpingitis, ovarian cysts, torsion of the ovary, and urinary tract infection are possible diagnoses. The key to early detection of ectopic pregnancy is having a high index of suspicion for this condition. Every woman with abdominal pain, vaginal spotting or bleeding, and a positive pregnancy test should undergo screening for ectopic pregnancy.
The most important screening tools for ectopic pregnancy are quantitative β-hCG levels and transvaginal ultrasound examination. When β-hCG levels are greater than 1500 to 2000 milli-International Units/ml, a normal intrauterine pregnancy should be visible on transvaginal ultrasound. Therefore, if β-hCG levels are greater than 1500 milli-International Units/ml but no intrauterine pregnancy is seen on transvaginal ultrasound, an ectopic pregnancy is very likely. β-hCG levels will probably be redrawn every 48 hours to determine if the pregnancy is viable. A transvaginal ultrasound may also be repeated to determine if the pregnancy is inside the uterus. Sometimes the location of an ectopic pregnancy will be visible on transvaginal ultrasound (Cunningham et al., 2010; Gilbert, 2011).
Another laboratory test that can be ordered to decide if the pregnancy is developing normally is a progesterone level. A progesterone level greater than 25 ng/ml almost always rules out the presence of an ectopic pregnancy. However, a progesterone level less than 5 ng/ml suggests either an ectopic pregnancy or an abnormal intrauterine pregnancy (Cunningham et al., 2010).
The woman should also be assessed for the presence of active bleeding, which is associated with tubal rupture. If internal bleeding is present, assessment may reveal vertigo, shoulder pain, hypotension, and tachycardia. A vaginal examination should be performed only once, and then with great caution. Approximately 20% of women with a tubal pregnancy have a palpable mass on examination. Rupturing the mass is possible during a bimanual examination, thus a gentle touch is critical.
Medical Management: Medical management involves giving methotrexate to dissolve the tubal pregnancy. Methotrexate is an antimetabolite and folic acid antagonist that destroys rapidly dividing cells. The woman must be hemodynamically stable to be eligible for medical management. The best results following methotrexate therapy are usually obtained if the mass is unruptured and measures less than 3.5 cm in diameter by ultrasound, if no fetal cardiac activity is noted on ultrasound, and if the serum β-hCG level is less than 5000 milli-International Units/L (Cunningham et al., 2010). To be a candidate for medical management, the woman must also be willing to comply with posttreatment lifestyle restrictions and monitoring. Methotrexate therapy avoids surgery and is a safe, effective, and cost-effective way of managing many cases of tubal pregnancy. The woman is informed of how the medication works, possible side effects, whom to call if she has concerns or if problems develop, and the importance of follow-up care (Box 28-1).
Surgical Management: Surgical management depends on the location and cause of the ectopic pregnancy, the extent of tissue involvement, and the woman’s desires regarding future fertility. One option is removal of the entire tube (salpingectomy). If the tube has not ruptured and the woman desires future fertility, salpingostomy may be performed instead. In this procedure an incision is made over the pregnancy site in the tube and the products of conception are gently and very carefully removed. The incision is not sutured but left to close by secondary intention instead, given that this method results in less scarring.
If surgery is planned, general preoperative and postoperative care is appropriate for the woman with an ectopic pregnancy. Before surgery, vital signs (pulse, respirations, and blood pressure [BP]) are assessed every 15 minutes or as needed, according to the severity of the bleeding and the woman’s condition. Preoperative laboratory tests include determination of blood type and Rh factor, complete blood cell count, and serum quantitative β-hCG level. Ultrasonography is used to confirm an extrauterine pregnancy. Blood replacement may be necessary. The nurse verifies the woman’s Rh and antibody status and administers Rho(D) immune globulin postoperatively if appropriate.
Follow-up Care: The woman and her family should be encouraged to share their feelings and concerns related to the loss. Future fertility should be discussed. A contraceptive method should be used for at least three menstrual cycles to allow time for the woman’s body to heal (Gilbert, 2011). Every woman who has been diagnosed with an ectopic pregnancy should be instructed to contact her health care provider as soon as she suspects that she might be pregnant because of the increased risk for recurrent ectopic pregnancy. These women may need referral to grief or infertility support groups. In addition to the loss of the current pregnancy, they are faced with the possibility of future pregnancy losses or infertility.
Hydatidiform Mole (Molar Pregnancy)
Hydatidiform mole (molar pregnancy) is a benign proliferative growth of the placental trophoblast in which the chorionic villi develop into edematous, cystic, avascular transparent vesicles that hang in a grapelike cluster. Hydatidiform mole is a gestational trophoblastic disease. Gestational trophoblastic disease (GTD) is a group of pregnancy-related trophoblastic proliferative disorders without a viable fetus. In addition to hydatidiform mole, GTD includes invasive mole, gestational choriocarcinoma, placental site trophoblastic tumor, and gestational trophoblastic neoplasia (GTN) (American College of Obstetricians and Gynecologists [ACOG], 2004). (See Chapter 11 for a discussion of GTN.)
Incidence and Etiology
Hydatidiform mole occurs in 1 in 1000 pregnancies in the United States (Cohn, Ramaswamy, & Blum, 2009). The cause is unknown, although it may be related to an ovular defect or a nutritional deficiency. Women at increased risk for hydatidiform mole formation are those who have had ovulation stimulation with clomiphene (Clomid) and those who are in their early teens or older than 40 years of age. Other risk factors include history of miscarriage and nutritional factors (e.g., deficient intake of carotene and animal fats) (Bess & Wood, 2006; Cohn et al.).
Types
A hydatidiform mole may be further categorized as a complete or partial mole. The complete mole results from fertilization of an egg in which the nucleus has been lost or inactivated (Fig. 28-5, A). The nucleus of a sperm (23, X) duplicates itself (resulting in the diploid number 46,XX) because the ovum has no genetic material or the material is inactive. It is also possible for an “empty” egg to be fertilized by two normal sperm, thereby producing either a 46,XX or 46,XY karyotype. The mole resembles a bunch of white grapes (see Fig. 28-5, B). The hydropic (fluid-filled) vesicles grow rapidly, causing the uterus to be larger than expected for the duration of the pregnancy. Usually the complete mole contains no fetus, placenta, amniotic membranes, or fluid (Fig. 28-6). Maternal blood has no placenta to receive it; therefore, hemorrhage into the uterine cavity and vaginal bleeding occur. Approximately 15% to 20% of women with a complete mole have evidence of persistent GTD (Cunningham et al., 2010).
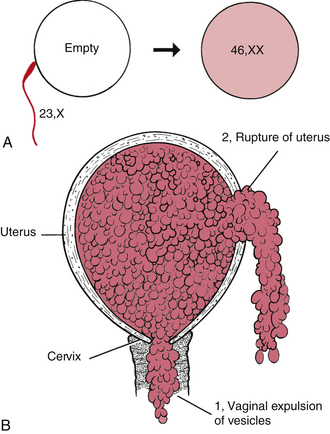
FIG. 28-5 A, Chromosomal origin of complete mole. Single sperm (color) fertilizes an “empty” ovum. Reduplication of sperm’s 23, X set gives completely homozygous diploid 46,XX. B, Uterine rupture with hydatidiform mole. 1, Vaginal expulsion of mole through cervix. 2, Rupture of uterus and spillage of mole into peritoneal cavity (rare).
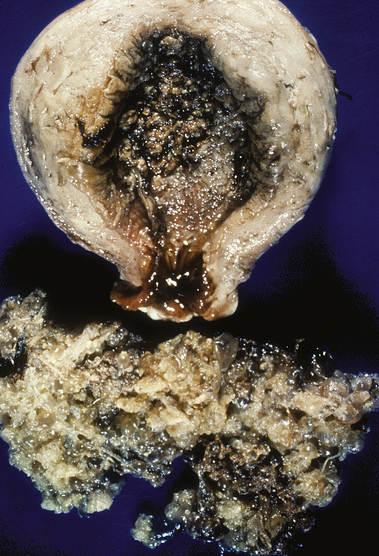
FIG. 28-6 Gross specimen in a woman treated for complete hydatidiform mole with primary hysterectomy. (Courtesy John Soper, M.D.) (From DiSaia P., & Creasman W. [2007]: Clinical gynecologic oncology [7th ed.]. Philadelphia: Mosby.)
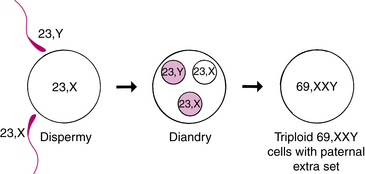
For a partial mole, chromosomal studies often show a karyotype of 69,XXY; 69,XXX; or rarely 69,XYY. This arrangement occurs as a result of two sperm fertilizing an apparently normal ovum (Fig. 28-7). Partial moles often have embryonic or fetal parts and an amniotic sac. Congenital anomalies are usually present. The risk of persistent GTD is much less than with a complete mole. If persistent GTD does occur, it is usually not a choriocarcinoma (Cunningham et al., 2010).
Clinical Manifestations
In the early stages the clinical manifestations of a complete hydatidiform mole cannot be distinguished from those of normal pregnancy. Later, vaginal bleeding occurs in almost 95% of cases. The vaginal discharge may be dark brown (resembling prune juice) or bright red and either scant or profuse. It may continue for only a few days or intermittently for weeks. Early in pregnancy the uterus in approximately one half of affected women is significantly larger than expected from menstrual dates. The percentage of women with an excessively enlarged uterus increases as length of time since the last menstrual period increases. Approximately 25% of affected women have a uterus smaller than would be expected from menstrual dates.
Anemia from blood loss, excessive nausea and vomiting (hyperemesis gravidarum), and abdominal cramps caused by uterine distention are relatively common findings. Women may also pass vesicles, which are frequently avascular edematous villi, from the uterus. Preeclampsia occurs in approximately 70% of women with large, rapidly growing hydatidiform moles and occurs earlier than usual in the pregnancy. If preeclampsia is diagnosed before 24 weeks of gestation, hydatidiform mole should be suspected and ruled out. Hyperthyroidism is another serious complication of hydatidiform mole. Usually treatment of the hydatidiform mole restores thyroid function to normal. Partial moles cause few of these symptoms and may be mistaken for an incomplete or missed miscarriage (Cohn et al., 2009; Nader, 2009; Roberts & Funai, 2009).
Diagnosis
Transvaginal ultrasound and serum hCG levels are used for diagnosis. Transvaginal ultrasound is the most accurate tool for diagnosing a hydatidiform mole. A characteristic pattern of multiple diffuse intrauterine masses, often called a snowstorm pattern, is seen in place of, or along with, an embryo or a fetus. The trophoblastic tissue secretes the hCG hormone. In a molar pregnancy, hCG levels are persistently high or rising beyond 10 to 12 weeks of gestation, the time they would begin to decline in a normal pregnancy (Gilbert, 2011).
Management
Although most moles abort spontaneously, suction curettage offers a safe, rapid, and effective method of evacuating a hydatidiform mole if necessary (Cunningham et al., 2010; Gilbert, 2011). Induction of labor with oxytocic agents or prostaglandin is not recommended because of the increased risk of embolization of trophoblastic tissue. Post-evacuation administration of Rho(D) immune globulin to women who are Rh negative is necessary to prevent isoimmunization (Gilbert, 2011).
The nurse provides the woman and her family with information about the disease process, the necessity for a long course of follow-up, and the possible consequences of the disease. The nurse also helps the woman and her family cope with the pregnancy loss and recognize that the pregnancy was not normal. In addition, the woman and her family are encouraged to express their feelings, and information is provided about local support groups or counseling resources as needed. Internet resources such as Share: Pregnancy and Infant Loss Support, Inc., at www.nationalshare.org and the International Society for the Study of Trophoblastic Disease at www.isstd.org may also be useful. Explanations about the importance of postponing a subsequent pregnancy and contraceptive counseling are provided to emphasize the need for consistent and reliable use of the method chosen.
Follow-up Care
Follow-up care includes frequent physical and pelvic examinations along with weekly measurements of the β-hCG level until the level decreases to normal and remains normal for 3 consecutive weeks. Monthly measurements are then taken for 6 months. The follow-up assessment period usually continues for a year. During that time, a rising β-hCG level and an enlarging uterus may indicate GTD (Gilbert, 2011).
Late Pregnancy Bleeding
The major causes of bleeding in late pregnancy are placenta previa and premature separation of the placenta (abruptio placentae or placental abruption). Rapid assessment for and diagnosis of the cause of bleeding are essential to reduce maternal and perinatal morbidity and mortality (Table 28-2).
TABLE 28-2
SUMMARY OF FINDINGS: ABRUPTIO PLACENTAE AND PLACENTA PREVIA
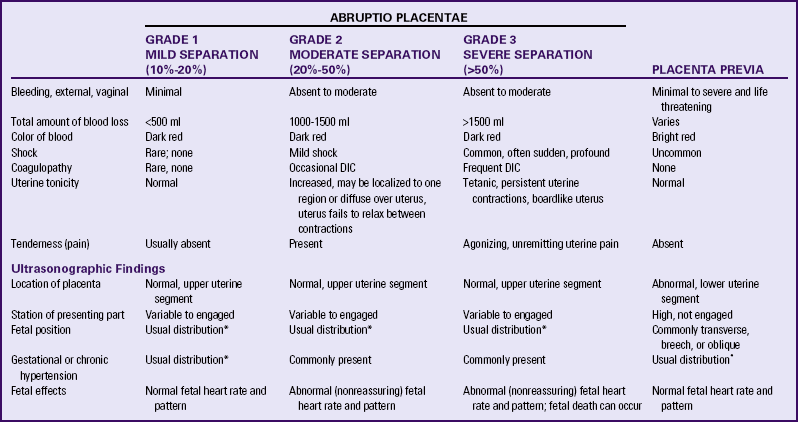
DIC, Disseminated intravascular coagulation.
*Usual refers to the expected variations of incidence seen when there is no concurrent problem.
Placenta Previa
Because of advances in ultrasonography, especially transvaginal ultrasound, and an increased understanding of the changing relationship between the placenta and the internal cervical os as pregnancy progresses, definitions and classifications of placental previa have changed. In placenta previa the placenta is implanted in the lower uterine segment such that it completely or partially covers the cervix or is close enough to the cervix to cause bleeding when the cervix dilates or the lower uterine segment effaces (Fig. 28-8) (Hull & Resnik, 2009). When transvaginal ultrasound is used, the placenta is classified as a complete placenta previa if it totally covers the internal cervical os. In a marginal placenta previa the edge of the placenta is seen on transvaginal ultrasound to be 2.5 cm or closer to the internal cervical os. When the exact relationship of the placenta to the internal cervical os has not been determined or in the case of apparent placenta previa in the second trimester, the term low-lying placenta is used (Hull & Resnik).
Incidence and Etiology
Placenta previa affects approximately 1 in 200 pregnancies at term. Some evidence suggests that the incidence of placenta previa is increasing, perhaps as a result of the increasing cesarean birth rate. In addition to a history of previous cesarean birth, other risk factors for placenta previa include advanced maternal age (more than 35 to 40 years of age), multiparity, history of prior suction curettage, and smoking (Hull & Resnik, 2009). Cigarette smoking leads to a decrease in uteroplacental oxygenation and thus a need for increased placental surface area. Placenta previa is more likely to occur in women with multiple gestations because of the larger placental area associated with these pregnancies. Women who had placenta previa in a previous pregnancy are more likely than others to develop the problem in a subsequent pregnancy, perhaps as a result of a genetic predisposition. Previous cesarean birth and curettage in the past for miscarriage or induced abortion are risk factors for placenta previa because both result in endometrial damage and uterine scarring (Francois & Foley, 2007; Hull & Resnik).
Clinical Manifestations
Placenta previa is typically characterized by painless bright red vaginal bleeding during the second or third trimester. In the past, placenta previa was usually diagnosed after an episode of bleeding. Currently, however, most cases are diagnosed by ultrasound before significant vaginal bleeding occurs. This bleeding is associated with the disruption of placental blood vessels that occurs with stretching and thinning of the lower uterine segment (Francois & Foley, 2007). The initial bleeding is usually a small amount and stops as clots form. It can recur, however, at any time (Gilbert, 2011).
Vital signs may be normal, even with heavy blood loss, because a pregnant woman can lose up to 40% of her blood volume without showing signs of shock. Clinical presentation and decreasing urinary output may be better indicators of acute blood loss than vital signs alone. The fetal heart rate (FHR) is normal (reassuring) unless a major detachment of the placenta occurs.
Abdominal examination usually reveals a soft, relaxed, nontender uterus with normal tone. The presenting part of the fetus usually remains high because the placenta occupies the lower uterine segment. Thus the fundal height is often greater than expected for gestational age. Because of the abnormally located placenta, fetal malpresentation (breech and transverse or oblique lie) is common.
Maternal and Fetal Outcomes
The major maternal complication associated with placenta previa is hemorrhage. Another serious complication is development of an abnormal placental attachment (e.g., placenta accreta, increta, or percreta) (see Chapter 34). If excessive bleeding cannot be controlled, hysterectomy may be necessary (Cunningham et al., 2010; Hull & Resnik, 2009). Because most women with placenta previa give birth by cesarean, surgery-related trauma to structures adjacent to the uterus and anesthesia complications are also possible. In addition, blood transfusion reactions, anemia, thrombophlebitis, and infection may occur.
The greatest risk of fetal death is caused by preterm birth. Other fetal risks include stillbirth, malpresentation and fetal anemia. Intrauterine growth restriction (IUGR) has also been associated with placenta previa. This association can be related to poor placental exchange (Gilbert, 2011). One study found an increased incidence of fetal anomalies in pregnancies complicated by placenta previa (Cunningham et al., 2010).
Diagnosis
All women with painless vaginal bleeding after 20 weeks of gestation should be assumed to have a placenta previa until proven otherwise. A transabdominal ultrasound examination should be performed initially followed by a transvaginal scan, unless the transabdominal ultrasound clearly shows that the placenta is not located in the lower uterine segment. A transvaginal ultrasound is better than a transabdominal scan for accurately determining placental location (Hull & Resnik, 2009). If ultrasonographic scanning reveals a normally implanted placenta, a speculum examination may be performed to rule out local causes of bleeding (e.g., cervicitis, polyps, carcinoma of the cervix), and a coagulation profile is obtained to rule out other causes of bleeding.
Management
Once placenta previa has been diagnosed, a management plan is developed. The woman will be managed either expectantly or actively, depending on the gestational age, amount of bleeding, and fetal condition (see the Nursing Process box: Placenta Previa).
Expectant Management: Expectant management (observation and bed rest) is implemented if the fetus is at less than 36 weeks of gestation and has a normal (reassuring) FHR tracing, the bleeding is mild (<250 ml) and stops, and the woman is not in labor. The purpose of expectant management is to allow the fetus time to mature (Gilbert, 2011). The woman will initially be hospitalized in a labor and birth unit for continuous FHR and contraction monitoring. Large-bore intravenous (IV) access should be initiated immediately. Initial laboratory tests include hemoglobin, hematocrit, platelet count, and coagulation studies. A “type and screen” blood sample should be maintained at all times in the hospital’s transfusion services department to allow for immediate crossmatch of blood component therapy if necessary. If the woman is at less than 34 weeks of gestation, antenatal corticosteroids should be administered (Francois & Foley, 2007; Gilbert).
If the bleeding stops, the woman will most likely be placed on bed rest with bathroom privileges and limited activity (able to use the bathroom, shower, and move around her hospital room for 15 to 30 minutes at a time, four times a day). No vaginal or rectal examinations are performed, and the woman is placed on “pelvic rest” (nothing in the vagina). Ultrasonographic examinations may be performed every 2 to 3 weeks. Fetal surveillance may include a nonstress test (NST) or biophysical profile
(BPP) once or twice weekly. Bleeding is assessed by checking the amount of bleeding on perineal pads, bed pads, and linens. Serial laboratory values are evaluated for decreasing hemoglobin and hematocrit levels and changes in coagulation values. The woman should also be monitored for signs of preterm labor. Magnesium sulfate can be given for tocolysis if uterine contractions are identified (Francois & Foley, 2007; Gilbert, 2011).
The woman with placenta previa should always be considered a potential emergency because massive blood loss with resulting hypovolemic shock can occur quickly if bleeding resumes. The possibility always exists that she will require an emergency cesarean for birth. Placenta previa in a preterm gestation may be an indication for transfer to a tertiary-care perinatal center, given that a neonatal intensive care unit may be necessary for care of the preterm infant. Also, because many community hospitals are not prepared to perform emergency surgery 24 hours per day, 7 days per week, transfer to a tertiary-care center may be necessary to ensure constant access to cesarean birth.
Home Care: Sometimes women with placenta previa are discharged from the hospital before giving birth to be managed at home. The woman’s condition should be stable, and she should have experienced no vaginal bleeding for at least 48 hours before discharge (Hull & Resnik, 2009). A candidate for home care must meet other strict criteria as well. She should be willing and able to comply with activity restrictions (bed rest with bathroom privileges and pelvic rest), have access to a telephone, close supervision by family or friends in the home, and constant access to transportation. If bleeding resumes, she will need to return to the hospital immediately. She must also be able to keep all appointments for fetal testing, laboratory assessments, and prenatal care. Visits by a perinatal home care nurse may be arranged.
If hospitalization or home care with activity restriction is prolonged, the woman may have concerns about her work- or family-related responsibilities or may become bored with inactivity. She should be encouraged to participate in her own care and decisions about care as much as possible. Provision of diversionary activities or encouragement to participate in activities she enjoys and can perform during bed rest is needed. Participation in a support group made up of other women on bed rest while hospitalized or online if at home may be a helpful coping mechanism. (See the Teaching for Self-Management box: Coping with Activity Restriction on page 663 in Chapter 27.)
Active Management: If the woman is at or beyond 36 weeks of gestation or bleeding is excessive or persistent, immediate cesarean birth is indicated (Hull & Resnik, 2009). Expectant management will be terminated as soon as the fetus is mature, if excessive bleeding develops, active labor begins, or any other obstetric reason to terminate the pregnancy (e.g., chorioamnionitis) develops (Gilbert, 2011). Cesarean birth is indicated in all women with ultrasound evidence of placenta previa. An asymptomatic woman whose placenta lies more than 2 cm from the cervical os, however, can labor safely (Francois & Foley, 2007; Hull & Resnik).
If cesarean birth is planned, the nurse continuously assesses maternal and fetal status while preparing the woman for surgery. Maternal vital signs are assessed frequently for decreasing BP, increasing pulse rate, changes in level of consciousness, and oliguria. Fetal assessment is maintained by continuous electronic fetal monitoring (EFM) to assess for signs of hypoxia.
Blood loss may not cease with the birth of the infant. The large vascular channels in the lower uterine segment may continue to bleed because of that segment’s diminished muscle content. The natural mechanism to control bleeding so characteristic of the upper part of the uterus—the interlacing muscle bundles, the “living ligature” contracting around open vessels—is absent in the lower part of the uterus. Postpartum hemorrhage may therefore occur even if the fundus is contracted firmly (see chapter 34).
Emotional support for the woman and her family is extremely important. The actively bleeding woman is concerned not only for her own well-being but also for the well-being of her fetus. All procedures should be explained, and a support person should be present. The woman should be encouraged to express her concerns and feelings. If the woman and her support person or family desire pastoral support, the nurse can notify the hospital chaplain service or provide information about other supportive resources.
Premature Separation of Placenta (Abruptio Placentae [Placental Abruption])
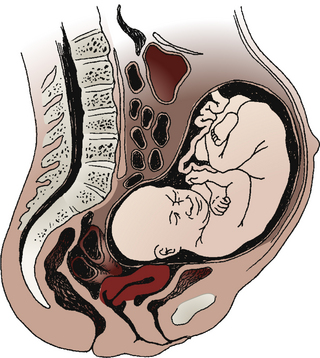
Premature separation of the placenta, or abruptio placentae, is the detachment of part or all of a normally implanted placenta from the uterus (Fig. 28-9). Separation occurs in the area of the decidua basalis after 20 weeks of gestation and before the birth of the infant.
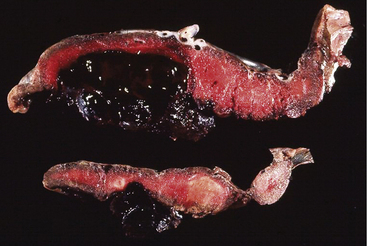
FIG. 28-9 Abruptio placentae. Premature separation of normally implanted placenta. A large retroplacental clot is present. (From Creasy, R., Resnik, R., Iams, J., Lockwood C., & Moore, T. [2009]. Creasy & Resnik’s maternal-fetal medicine: Principles and practice [6th ed.]. Philadelphia: Saunders.)
Incidence and Etiology
Premature separation of the placenta is a serious complication that accounts for significant maternal and fetal morbidity and mortality. Approximately 1 in 75 to 1 in 226 pregnancies is complicated by placental abruption. The range in incidence likely reflects both variable criteria for diagnosis and an increased recognition of milder forms of abruption. Approximately one third of all antepartum bleeding is caused by placental abruption (Francois & Foley, 2007).
Maternal hypertension, whether chronic or pregnancy related, is the most consistently identified risk factor for abruption. Cocaine use is also a risk factor because it causes vascular disruption in the placental bed. Blunt external abdominal trauma, most often the result of motor vehicle accidents (MVAs)
or maternal battering, is another frequent cause of placental abruption (Cunningham et al., 2010; Francois & Foley, 2007). Other risk factors include cigarette smoking, a history of abruption in a previous pregnancy, preterm PROM, and the presence of inherited or acquired thrombophilias (e.g., factor V Leiden mutation or protein S deficiency) (Cunningham et al.; Hull & Resnik, 2009; Paidas & Hossain, 2009). Abruption is more likely to occur in twin gestations than in singletons (Francois & Foley). Women who have had two previous abruptions have a recurrence risk of 25% in the next pregnancy (Hull & Resnik).
Classification
The most common classification of placental abruption is according to type and severity. This classification system is summarized in Table 28-2.
Clinical Manifestations
The separation may be partial or complete, or only the margin of the placenta may be involved. Bleeding from the placental site may dissect (separate) the membranes from the decidua basalis and flow out through the vagina (70% to 80%), it may remain concealed (retroplacental hemorrhage) (10% to 20%), or both (Fig. 28-10) (Francois & Foley, 2007; Gilbert, 2011). Clinical symptoms vary with degree of separation (see Table 28-2). If cesarean birth is performed, blood clots may be noted on entry into the uterus. A blood clot often will be attached to the posterior surface of the placenta (referred to as a retroplacental clot) (see Fig 28-9).
Classic symptoms of placental abruption include vaginal bleeding, abdominal pain, and uterine tenderness and contractions (Cunningham et al., 2010; Hull & Resnik, 2009). Bleeding may result in maternal hypovolemia (i.e., shock, oliguria, anuria) and coagulopathy. Mild to severe uterine hypertonicity is present. Pain is mild to severe and localized over one region of the uterus or diffuse over the uterus with a boardlike abdomen.
Extensive myometrial bleeding damages the uterine muscle. If blood accumulates between the separated placenta and the uterine wall, it may produce a couvelaire uterus. The uterus appears purple or blue, rather than its usual “bubble gum pink” color, and contractility is lost. Shock may occur and is out of proportion to blood loss. Laboratory findings include a positive Apt test result (blood in the amniotic fluid); a decrease in hemoglobin and hematocrit levels, which may appear later; and a decrease in coagulation factor levels. Clotting defects (e.g., disseminated intravascular coagulation) are present in approximately 40% of women who develop a large abruption (Francois & Foley, 2007). A Kleihauer-Betke (KB) test may be ordered to determine the presence of fetal-to-maternal bleeding (transplacental hemorrhage), although this test appears to have no value in the general workup of women with abruption. The KB test may be useful to guide Rho(D) immune globulin therapy in Rh-negative women who have had an abruption (Hull & Resnik, 2009).
Maternal and Fetal Outcomes
The mother’s prognosis depends on the extent of placental detachment, overall blood loss, degree of coagulopathy present and time between placental detachment and birth. Maternal complications are associated with the abruption or its treatment. Hemorrhage, hypovolemic shock, hypofibrinogenemia, and thrombocytopenia are associated with severe abruption. Renal failure and pituitary necrosis may result from ischemia. In rare cases, women who are Rh negative can become sensitized if fetal-to-maternal hemorrhage occurs and the fetal blood type is Rh positive.
Placental abruption is associated with a perinatal mortality rate of 20% to 30%. If more than 50% of the placenta is involved, fetal death is likely to occur. Other fetal and neonatal risks include IUGR and preterm birth (Francois & Foley, 2007; Hull & Resnik, 2009). Risks for neurologic defects, cerebral palsy, and death from sudden infant death syndrome are also increased in newborns following placental abruption (Cunningham et al., 2010; Francois & Foley).
Diagnosis
Placental abruption is primarily a clinical diagnosis. Although ultrasound can be used to rule out placenta previa, it cannot detect all cases of abruption. A retroplacental mass may be detected with ultrasonographic examination, but negative findings do not rule out a life-threatening abruption. In fact, at least 50% of abruptions cannot be identified on ultrasound (Hull & Resnik, 2009). Hypofibrinogenemia and evidence of DIC support the diagnosis, but many women with placental abruption do not develop coagulopathy. The diagnosis of abruption is confirmed after birth by visual inspection of the placenta. Adherent clot on the maternal surface of the placenta and depression of the underlying placental surface are usually present (see Fig. 28-9) (Francois & Foley, 2007; Gilbert, 2011).
Placental abruption should be highly suspected in the woman who experiences a sudden onset of intense, usually localized, uterine pain, with or without vaginal bleeding. Initial assessment is much the same as for placenta previa. Physical examination usually reveals abdominal pain, uterine tenderness, and contractions. The fundal height may be measured over time because an increasing fundal height indicates concealed bleeding. Approximately 60% of live fetuses exhibit abnormal (nonreassuring) FHR patterns, and elevated uterine resting tone may also be noted on the monitor tracing (Francois & Foley, 2007). Coagulopathy, as evidenced by abnormal clotting studies (fibrinogen, platelet count, partial thromboplastin time [PTT], fibrin split products), may be present if a large or complete abruption has occurred.
Management
Expectant Management: Management depends on the severity of blood loss and fetal maturity and status. If the fetus is less than 34 weeks of gestation and both the woman and fetus are stable, expectant management can be implemented. The woman is monitored closely because the abruption may extend at any time. The fetus will be regularly assessed for evidence of appropriate growth, because there is risk for IUGR. In addition, assessments of fetal well-being (e.g., NST and BPP) are performed regularly. See Chapter 26 for further discussion of these tests. Corticosteroids will be given to accelerate fetal lung maturity (Hull & Resnik, 2009).
Active management: Immediate birth is the management of choice if the fetus is at term gestation or if the bleeding is moderate to severe and the mother or fetus is in jeopardy. At least one large-bore (16- to 18-gauge) IV line should be started. Maternal vital signs are monitored frequently to observe for signs of declining hemodynamic status, such as increasing pulse rate and decreasing BP. Serial laboratory studies include hematocrit or hemoglobin determinations and clotting studies. Continuous EFM is mandatory. An indwelling catheter is inserted for continuous assessment of urine output, an excellent indirect measure of maternal organ perfusion. Blood and fluid volume replacement may be necessary, along with administering blood products to correct any coagulation defects.
Vaginal birth is usually feasible and is desirable, especially in cases of fetal death. Labor induction or augmentation may be initiated so long as the mother and fetus are closely monitored for any evidence of compromise. Cesarean birth should be reserved for cases of fetal distress or other obstetric complications. Cesarean birth should not be attempted when the women has severe and uncorrected coagulopathy because it can result in uncontrollable bleeding (Francois & Foley, 2007).
Nursing care of women experiencing moderate to severe abruption is demanding because it requires constant close monitoring of the maternal and fetal condition. Information about, placental abruptio, including the cause, treatment, and expected outcome, is given to the woman and her family. Emotional support is also extremely important because the woman and her family may be experiencing fetal loss in addition to the woman’s critical illness.
Cord Insertion and Placental Variations
Velamentous insertion of the cord (vasa previa) is a rare placental anomaly associated with placenta previa and multiple gestation. The cord vessels begin to branch at the membranes and then course onto the placenta (Fig. 28-11). ROM or traction on the cord may tear one or more of the fetal vessels. As a result the fetus may rapidly bleed to death. Battledore (marginal) insertion of the cord (Fig. 28-12, A) increases the risk of fetal hemorrhage, especially after marginal separation of the placenta.
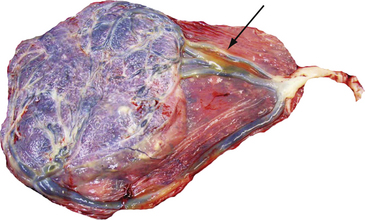
FIG. 28-11 Vasa previa (velamentous insertion of cord). Arrow shows velamentous cord insertion in the placenta. (From Creasy, R., Resnik, R., Iams, J., Lockwood, C., & Moore, T. [2009]. Creasy & Resnik’s maternal-fetal medicine: Principles and practice [6th ed.]. Philadelphia: Saunders.)
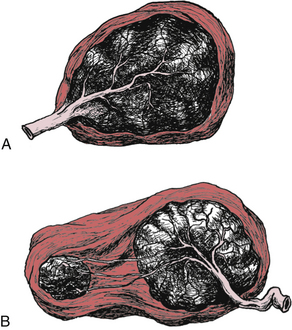
FIG. 28-12 Cord insertion and placental variations A, Battledore placenta. B, Placenta succenturiate.
In rare instances the placenta may be divided into two or more separate lobes, resulting in succenturiate placenta (see Fig. 28-12, B). Each lobe has a distinct circulation. The vessels collect at the periphery, and the main trunks eventually unite to form the vessels of the cord. Blood vessels joining the lobes may be supported only by the fetal membranes and are therefore in danger of tearing during labor, birth, or expulsion of the placenta. During placental expulsion, one or more of the separate lobes may remain attached to the decidua basalis, preventing uterine contraction and increasing the risk of postpartum hemorrhage.
Clotting Disorders in Pregnancy
Normally a delicate balance (homeostasis) exists between the opposing hemostatic and fibrinolytic systems. The hemostatic system stops the flow of blood from injured vessels, first by a platelet plug, which is followed by the formation of a fibrin clot. The coagulation process involves an interaction of the coagulation factors that constantly circulate in the bloodstream in which each factor sequentially activates the factor next in line, the “cascade effect” sequence. The fibrinolytic system is the process through which the fibrin clot is split into fibrinolytic degradation products and circulation is restored.
Clotting Problems
Disseminated Intravascular Coagulation: Disseminated vascular coagulation (DIC), or consumptive coagulopathy, is a pathologic form of clotting that is diffuse and consumes large amounts of clotting factors, causing widespread external bleeding, internal bleeding, or both, and clotting (Cunningham et al., 2010). DIC is never a primary diagnosis. Instead it results from some problem that triggered the clotting cascade, either extrinsically, by the release of large amounts of tissue thromboplastin, or intrinsically, by widespread damage to vascular integrity.
In the obstetric population, DIC is most often triggered by the release of large amounts of tissue thromboplastin, which occurs in placental abruption (the most common cause of severe consumptive coagulopathy in obstetrics) and in the retained dead fetus syndrome and the anaphylactoid syndrome of pregnancy (amniotic fluid embolus). Severe preeclampsia, HELLP syndrome, and gram-negative sepsis are examples of conditions that can trigger DIC because of widespread damage to vascular integrity (Cunningham et al., 2010; Gilbert, 2011). DIC is an overactivation of the clotting cascade and the fibrinolytic system, resulting in depletion of platelets and clotting factors, which results in the formation of multiple fibrin clots throughout the body’s vasculature, even in the microcirculation. Blood cells are destroyed as they pass through these fibrin choked vessels. Thus DIC results in a clinical picture of clotting, bleeding, and ischemia (Cunningham et al.). Clinical manifestations and laboratory test results are summarized in Box 28-2.
Management: Medical management in all cases of DIC involves correction of the underlying cause (e.g., removal of the dead fetus, treatment of existing infection or of preeclampsia or eclampsia, or removal of an abrupted placenta). Volume replacement, blood component therapy, optimization of oxygenation and perfusion status, and continued reassessment of laboratory parameters are the usual forms of treatment. Vitamin K administration and recombinant activated factor VIIa may be considered as additional therapies (Francois & Foley, 2007).
Nursing interventions include assessment for signs of bleeding (see Box 28-2) and signs of complications from the administration of blood and blood products, administering fluid or blood replacement as ordered, cardiac and hemodynamic monitoring, and protecting the woman from injury. Because renal failure is one consequence of DIC, urinary output is closely monitored by using an indwelling catheter. Urinary output must be maintained at more than 30 ml/hr (Gilbert, 2011). Vital signs are assessed frequently. If DIC develops before birth, continuous electronic fetal monitoring is necessary. The woman should be maintained in a side-lying tilt to maximize blood flow to the uterus. Oxygen may be administered through a nonrebreather face mask at 8 to 10 L/min or per hospital protocol or physician order. DIC usually is “cured” with the birth and as coagulation abnormalities resolve.
The woman and her family will be anxious and concerned about her condition and prognosis. The nurse offers explanations about care and provides emotional support to the woman and her family through this critical time.
KEY POINTS
• Blood loss during pregnancy should always be regarded as a warning sign until the cause is determined.
• Some miscarriages occur for unknown reasons, but fetal or placental maldevelopment and maternal factors account for many others.
• The type of miscarriage and signs and symptoms direct care management.
• Recurrent premature dilation of the cervix (incompetent cervix) may be treated with a cervical cerclage; the woman is instructed on recognizing the warning signs of preterm labor, ROM, and infection.
• Ectopic pregnancy is a significant cause of maternal morbidity and mortality, even in developed countries.
• Both complete and partial hydatidiform moles can progress to become gestational trophoblastic neoplasias, one of several types of gestational trophoblastic disease.
• Premature separation of the placenta (placental abruption) and placenta previa are differentiated by type of bleeding, uterine tonicity, and presence or absence of pain.
• Management of late-pregnancy bleeding requires immediate evaluation; care is based on gestational age, amount of bleeding, and fetal condition.
• Disseminated intravascular coagulation is a pathologic form of clotting that causes widespread bleeding and clotting. It is never a primary diagnosis, but always results from some problem that triggered the clotting cascade.