Hypertensive Disorders in Pregnancy
• Describe the characteristics of gestational hypertension, preeclampsia, eclampsia, and chronic hypertension.
• Identify the maternal-fetal complications associated with hypertensive disorders in pregnancy.
• List risk factors for preeclampsia.
• Describe the pathophysiologic mechanisms of preeclampsia and eclampsia.
• Explain how the abnormal laboratory values present in HELLP syndrome are produced by the pathophysiology that occurs with severe preeclampsia.
• Differentiate the antepartum, intrapartum, and postpartum management of the woman with mild or severe preeclampsia and mild or severe gestational hypertension.
• Describe appropriate nursing actions during and after an eclamptic seizure.
• Discuss the preconception, antepartum, intrapartum, and postpartum management of the woman with chronic hypertension.
Gestational hypertensive disorders develop during pregnancy, labor, or after birth. These disorders include gestational hypertension, preeclampsia, and eclampsia. Chronic hypertensive disorders precede pregnancy or develop before 20 weeks of gestation. Women with chronic hypertension can also develop superimposed preeclampsia or eclampsia. The classification, pathophysiologic changes, assessment and management of hypertensive disorders of pregnancy are discussed in this chapter with a primary focus on preeclampsia. The care of women with hypertensive disorders during the perinatal period requires a collaborative effort, including early detection, thorough assessment, and timely intervention.
Significance and Incidence
Hypertensive disorders complicate 5% to 10% of all pregnancies and are a common medical complication during pregnancy. The incidence varies among hospitals, regions, and countries (Gilbert, 2011; Sibai, 2007). In the United States, the rate of pregnancy-associated hypertension for all ages and ethnic groups has increased by approximately 1% each year since 2000, reaching the rate of 39.1 per 1000 live births in 2006. The annual rate of increase for chronic hypertension has risen at an even faster pace, from 2% per year in the 1990s to 6% annually since 2000, reaching the rate of 10.8 in 2006. The rate of chronic hypertension in mothers ages 40 and older is nearly ten times higher than for those younger than age 20 (30.4 compared with 3.9 per 1000 live births) (Martin, Hamilton, Sutton, Ventura, Menacker, Kirmeyer, & Mathews, 2009).
Morbidity and Mortality
Hypertensive disorders contribute significantly to maternal and infant morbidity and mortality worldwide (Sibai, 2007). Maternal complications associated with hypertensive disorders include placental abruption (abruptio placentae), acute respiratory distress syndrome (ARDS), stroke, cerebral hemorrhage, hepatic or renal failure, disseminated intravascular coagulation (DIC), and pulmonary edema. Most perinatal complications are related to placental insufficiency which causes intrauterine growth restriction (IUGR), prematurity associated with indicated preterm birth, hypoxia/acidosis, or placental abruption (Gilbert, 2011).
Pregnancy-associated hypertension accounts for 10% to 15% of maternal deaths worldwide (Askie, Duley, Henderson-Smart, & Stewart, 2007). Preeclampsia is the second leading cause of maternal mortality in the United States (Hawfield & Freedman, 2009). The majority of maternal deaths result from complications of hepatic rupture, placental abruption, or eclampsia (Roberts & Funai, 2009).
Classification
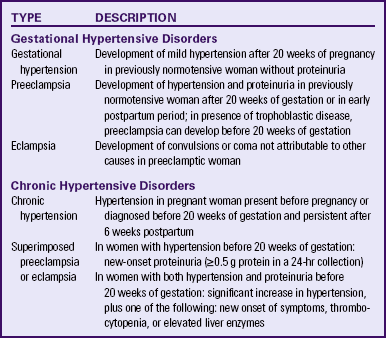
The classification of hypertensive disorders in pregnancy is confusing because standard definitions are not used consistently by all health care providers. The classification system most commonly used in the United States is based on reports from the American College of Obstetricians and Gynecologists (ACOG) (2002) and the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy (Working Group) (2000). This classification system is summarized in Table 27-1.
TABLE 27-1
CLASSIFICATION OF HYPERTENSIVE STATES OF PREGNANCY
Sources: American College of Obstetricians and Gynecologists (ACOG). (2002). Diagnosis and management of preeclampsia and eclampsia. ACOG Practice Bulletin No. 33. Washington DC: ACOG; Sibai, B. (2007). Hypertension. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone.
Gestational Hypertension
Gestational hypertension is the onset of hypertension without proteinuria after week 20 of pregnancy (ACOG, 2002; Working Group, 2000). Hypertension is defined as a systolic blood pressure (BP) greater than 140 mm Hg or a diastolic BP greater than 90 mm Hg. The hypertension should be recorded on at least two separate occasions at least 4 to 6 hours apart and within a 1-week period (ACOG, 2002; Sibai, 2007; Working Group, 2000).
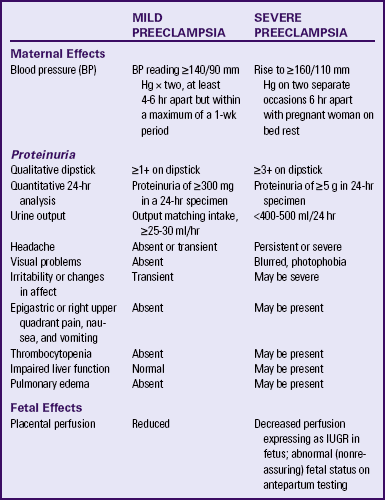
Gestational hypertension is the most frequent cause of hypertension during pregnancy, with an incidence of 6% to 17% in primigravidas and 2% to 4% in multiparous women. It occurs much more frequently in women with multifetal pregnancies (Sibai, 2007). While gestational hypertension can occur at any time after 20 weeks of pregnancy, it usually develops at or after 37 weeks of gestation. Women with gestational hypertension have no evidence of preexisting hypertension, and their BPs return to normal levels within 6 weeks after giving birth. Gestational hypertension is further classified as either mild or severe. The definitions of mild and severe gestational hypertension are the same as the definitions for blood pressure readings for mild and severe preeclampsia (Table 27-2). Women with mild gestational hypertension usually have good pregnancy outcomes. Some women who are initially thought to have gestational hypertension will eventually be diagnosed with chronic hypertension instead. Others will go on to develop proteinuria, thereby changing their diagnosis to preeclampsia. Women who are diagnosed with gestational hypertension before 35 weeks of gestation are more likely to progress to preeclampsia than women whose onset of hypertension occurs closer to term (Sibai).
TABLE 27-2
DIFFERENTIATION BETWEEN MILD AND SEVERE PREECLAMPSIA
FHR, Fetal heart rate; IUGR, intrauterine growth restriction.
Sources: American College of Obstetricians and Gynecologists (ACOG). (2002). Diagnosis and management of preeclampsia and eclampsia. ACOG Practice Bulletin No. 33. Washington, DC: ACOG; Sibai, B. (2007). Hypertension. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone.
Preeclampsia
Preeclampsia is a pregnancy-specific condition in which hypertension and proteinuria develop after 20 weeks of gestation in a previously normotensive woman. A significant contributor to maternal and perinatal morbidity and mortality, preeclampsia complicates approximately 3% to 7% of all pregnancies (American Academy of Pediatrics [AAP] & ACOG, 2007). Preeclampsia is a vasospastic, systemic disorder and is usually categorized as mild or severe for purposes of management (ACOG, 2002; Working Group, 2000). Table 27-2 lists criteria for mild and severe preeclampsia, and Table 27-3 gives common laboratory changes that occur in mild and severe preeclampsia.
TABLE 27-3
COMMON LABORATORY CHANGES IN PREECLAMPSIA
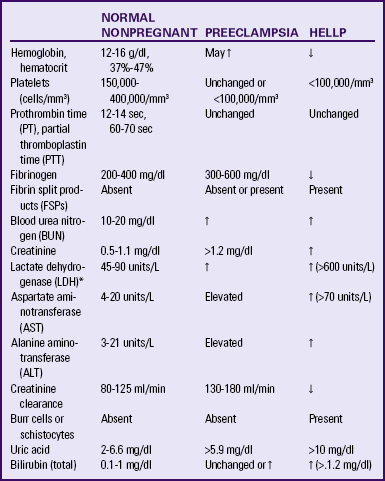
*LDH values differ according to the test or assays being performed.
Sources: American College of Obstetricians and Gynecologists (ACOG). (2002). Diagnosis and management of preeclampsia and eclampsia. ACOG Practice Bulletin No. 33. Washington, DC: AGOG; Cunningham, F., Leveno, K., Bloom, S., Hauth, J., Rouse, D., & Spong, C. (Eds.). (2010). Williams obstetrics (23rd ed.). New York: McGraw-Hill; Dildy, G. (2004). Complications of preeclampsia. In G. Dildy, M. Belfort, G. Saade, J. Phelan, G. Hankins, & S. Clark (Eds.), Critical care obstetrics (4th ed.). Malden, MA: Blackwell Science; Sibai, B. (2007). Hypertension. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone.
Eclampsia
Eclampsia is the onset of seizure activity or coma in a woman with preeclampsia, with no history of preexisting pathology, which can result in seizure activity (Roberts & Funai, 2009; Sibai, 2007). Eclamptic seizures can occur before, during, or after birth. Approximately one third of eclamptic seizures occur after birth, almost always within the first 48 hours postpartum (Roberts & Funai).
Chronic Hypertension
Chronic hypertension is defined as hypertension that is present before the pregnancy or develops before 20 weeks of gestation (Roberts & Funai, 2009). Hypertension initially diagnosed during pregnancy that persists longer than 6 weeks postpartum is also classified as chronic hypertension (Sibai, 2007). Other authorities believe that a diagnosis of chronic hypertension can be made only if the BP has not returned to normal levels by 12 weeks after birth (Roberts & Funai). Most women with mild chronic hypertension experience uncomplicated pregnancies. However, those with severe chronic hypertension have an increased risk of perinatal mortality (Gilbert, 2011).
Chronic Hypertension with Superimposed Preeclampsia
Women with chronic hypertension may develop superimposed preeclampsia, which increases the morbidity for mother and fetus. A diagnosis of chronic hypertension with superimposed preeclampsia is made with the following findings (Sibai, 2007):
Preeclampsia
Preeclampsia is a condition unique to human pregnancy. Signs and symptoms develop only during pregnancy and disappear soon after birth of the fetus and placenta. Common risk factors associated with the development of preeclampsia are listed in Box 27-1. Preeclampsia occurs most often with primigravid women or multiparous women with a new partner. Age distribution remains U-shaped; women younger than 19 years and older than 40 years have the highest rates of occurrence (Gilbert, 2011).
The cause of preeclampsia is unknown. Many theories have been suggested to explain the etiology of preeclampsia. Current theories that are still being considered include abnormal trophoblast invasion, coagulation abnormalities, vascular endothelial damage, cardiovascular maladaptation, and dietary deficiencies or excesses. Immunologic factors and genetic predisposition may also play important roles (Sibai, 2007).
Pathophysiology
Preeclampsia can progress along a continuum from mild to severe preeclampsia to eclampsia. Current thought is that the pathologic changes that occur in the woman with preeclampsia are caused by disruptions in placental perfusion and endothelial cell dysfunction (Gilbert, 2011; Peters, 2008). These changes are present long before the clinical diagnosis of preeclampsia is made (Roberts & Funai, 2009). Normally in pregnancy the spiral arteries in the uterus widen from thick-walled muscular vessels to thinner, saclike vessels with much larger diameters. This change increases the capacity of the vessels, allowing them to handle the increased blood volume of pregnancy. Because this vascular remodeling does not occur or only partially develops in women with preeclampsia, decreased placental perfusion and hypoxia result (Peters). Placental ischemia is thought to cause endothelial cell dysfunction by stimulating the release of a substance that is toxic to endothelial cells. This anomaly causes generalized vasospasm, which results in poor tissue perfusion in all organ systems, increased peripheral resistance and BP, and increased endothelial cell permeability, leading to intravascular protein and fluid loss and ultimately to less plasma volume. The main pathogenic factor is not an increase in BP but poor perfusion as a result of vasospasm and reduced plasma volume (Fig. 27-1) (Gilbert; Peters; Roberts & Funai). Figure 27-2 demonstrates how endothelial cell dysfunction causes many of the common signs and symptoms of preeclampsia.
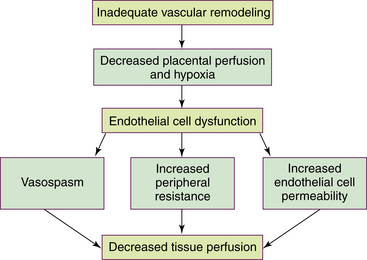
FIG. 27-1 Etiology of preeclampsia: disruptions in placental perfusion and endothelial cell dysfunction
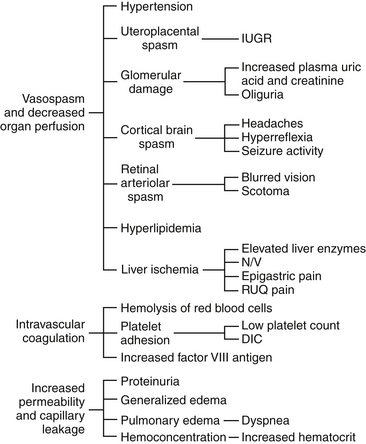
FIG. 27-2 Consequences of endothelial cell dysfunction. DIC, Disseminated vascular coagulation; IUGR, intrauterine growth restriction, N/V, nausea/vomiting; RUQ, right upper quadrant. (From Gilbert, E. [2011]. Manual of high risk pregnancy & delivery [5th ed.]. St. Louis: Mosby.)
Reduced kidney perfusion decreases the glomerular filtration rate and can lead to degenerative glomerular changes and oliguria. Pathologic changes in the endothelial cells of the glomeruli (glomerular endotheliosis) are uniquely characteristic of preeclampsia. Protein, primarily albumin, is lost in the urine. Uric acid clearance is decreased. Serum uric acid levels, however, increase. Sodium and water are retained. Acute tubular necrosis and renal failure may occur (Gilbert, 2011; Peters, 2008; Roberts & Funai, 2009).
Plasma colloid osmotic pressure decreases as serum albumin levels decrease. Intravascular volume is reduced as fluid moves out of the intravascular compartment, resulting in hemoconcentration, increased blood viscosity, and tissue edema. The hematocrit value increases as fluid leaves the intravascular space. Arteriolar vasospasm can lead to endothelial damage and increased capillary permeability, predisposing the woman to pulmonary edema (see Fig. 27-2) (Gilbert, 2011; Roberts & Funai, 2009).
Decreased liver perfusion can lead to impaired liver function and elevated liver enzyme levels. If hepatic edema and subcapsular hemorrhage develop, the woman may complain of epigastric or right upper quadrant pain. Hemorrhagic necrosis in the liver can result in a subcapsular hematoma, which is a rare occurrence (Gilbert, 2011). Rupture of a subcapsular hematoma is a life-threatening complication and a surgical emergency (see Fig. 27-2).
Neurologic complications associated with preeclampsia include cerebral edema and hemorrhage and increased central nervous system (CNS) irritability. CNS irritability manifests as headaches, hyperreflexia, positive ankle clonus, and seizures. Arteriolar vasospasms and decreased blood flow to the retina can lead to visual disturbances such as scotoma (dim vision or blind or dark spots in the visual field) and blurred or double vision (Gilbert, 2011; Roberts & Funai, 2009).
Decreased placental perfusion contributes significantly to restriction of fetal growth and the increased incidence of placental abruption, premature birth, and early degenerative aging of the placenta. The rate of fetal complications is directly related to the severity of the disease (Peters, 2008; Sibai, 2007).
Hellp Syndrome
HELLP syndrome is a laboratory diagnosis for a variant of severe preeclampsia that involves hepatic dysfunction, characterized by hemolysis (H), elevated liver enzymes (EL), and low platelet count (LP). It is not a separate illness. No consensus has been reached, however, regarding which laboratory tests should be used to diagnose HELLP syndrome or what values should be considered abnormal (Sibai, 2007). Table 27-3 lists laboratory changes that occur in HELLP syndrome.
The pathophysiologic changes of HELLP syndrome occur as a result of arteriolar vasospasm, endothelial cell dysfunction with fibrin deposits, and adherence of platelets in blood vessels. Red blood cells are damaged as they pass through narrowed blood vessels and become hemolyzed, resulting in a decreased red blood cell and platelet count, as well as hyperbilirubinemia. Endothelial damage and fibrin deposits in the liver lead to impaired liver function and can cause hemorrhagic necrosis. Liver enzymes are elevated when hepatic tissue is damaged (Gilbert, 2011)
HELLP syndrome usually develops in the third trimester of pregnancy, or within 48 hours after birth. The clinical presentation of HELLP syndrome is often nonspecific. Most women with the disorder report a history of malaise, influenza-like symptoms, epigastric or right upper quadrant abdominal pain, nausea, vomiting, and headaches. A small number of women may exhibit symptoms related to thrombocytopenia, such as bruising or hematuria (Peters, 2008; Sibai, 2007).
Because no agreement has been reached regarding the diagnostic criteria for HELLP syndrome, its reported incidence varies. It has been reported to occur in anywhere from 5% to 20% of women with preeclampsia (Emery, 2005; Gilbert, 2011). HELLP syndrome appears to occur more frequently
in Caucasian women than women of other races. A diagnosis of HELLP syndrome is associated with an increased risk for adverse perinatal outcomes, including pulmonary edema, acute renal failure, DIC, placental abruption, liver hemorrhage or failure, ARDS, sepsis, and stroke (Sibai, 2007). Perinatal mortality rates range from 7.4% to 20.4% with a maternal mortality of approximately 1% (Sibai). The rate of preterm birth in women with HELLP syndrome is approximately 70%, with 15% of these occurring before 28 weeks of gestation. Most of the perinatal deaths occur before 28 weeks of gestation, in association with placental abruption or severe IUGR (Sibai).
Care Management
Identifying and Preventing Preeclampsia
Numerous clinical trials have examined various interventions to prevent preeclampsia such as low-dose aspirin, antioxidants, calcium, magnesium, zinc, restricted protein or sodium intake,
and fish oil supplements. None of these interventions demonstrated a significant benefit in preventing or reducing the severity of preeclampsia (Sibai, 2007). ![]()
No reliable test has been developed that can be used as a routine screening tool for predicting preeclampsia (Peters, 2008). Several studies found that women with high levels of two proteins in their blood (soluble endoglin and fms-like tyrosine kinase 1) were more likely to develop preeclampsia. These proteins reduce levels of placental growth factor (PIGF) (Hellwig, 2007). Another study found a correlation between preeclampsia and very low levels of 25-hydroxyvitamin D (Ravin, 2008). ![]()
Although research offers future promise, much work remains before a screening test for preeclampsia is available for widespread clinical use. Nurses should be aware of what strategies are being studied and use the most valid results, so that they can counsel pregnant women about interventions that are evidence based. Meanwhile, the best preeclampsia prevention methods include early prenatal care for the identification of women at risk and early detection of the disease.
Health Assessment and Screening
Health assessment and screening begins with a systemic evaluation, which includes history taking, physical examination, and laboratory testing. (See the Nursing Process box for more details.)
Interview: The woman’s personal health profile is reviewed during the first prenatal visit. (See Chapter 15 for additional information.) The risk factors associated with the development of preeclampsia are also evaluated during the interview see Box 27-1).(
Physical Examination: Accurate measurement of BP is essential in the early detection of hypertensive disorders. Personnel caring for pregnant women need to be consistent in taking and recording BP measurements in a standardized manner (see Box 13-1).
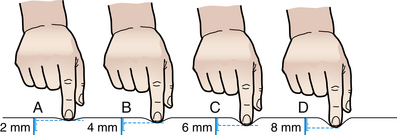
Assessment for edema is another component of the physical examination, although the presence of edema is no longer included in the definition of preeclampsia. Edema is assessed for distribution, degree, and pitting. Dependent edema is edema of the lowest or most dependent parts of the body, where hydrostatic pressure is greatest. If a pregnant woman is ambulatory, the edema may first be evident in the feet and ankles. If she is confined to bed, the edema is more likely to occur in the sacral region. Pitting edema leaves a small depression or pit after finger pressure is applied to the swollen area. The pit, which is caused by movement of fluid to adjacent tissue away from the point of pressure, normally disappears within 10 to 30 seconds. Although the amount of edema is difficult to quantify, the method shown in Figure 27-3 may be used to record relative degrees of edema formation.
Deep tendon reflexes (DTRs) reflect the balance between the cerebral cortex and spinal cord. They are evaluated as a baseline and to detect any changes. The biceps and patellar reflexes are assessed and the findings recorded (Fig. 27-4 and Table 27-4). To elicit the biceps reflex, the examiner strikes a downward blow over the thumb, which is situated over the biceps tendon (see Fig. 27-4, A). Normal response is flexion of the arm at the elbow, described as a 2+ response. The patellar reflex is elicited with the woman’s legs hanging freely over the end of the examining table or with the woman lying on her side with the knee slightly flexed (see Fig. 27-4, D). The patellar tendon (inferior to the patella) is tapped with a percussion hammer. Normal response is the extension or kicking out of the leg.
TABLE 27-4
ASSESSING DEEP TENDON REFLEXES
| GRADE | DEEP TENDON REFLEX RESPONSE |
| 0 | No response |
| 1+ | Sluggish or diminished |
| 2+ | Active or expected response |
| 3+ | More brisk than expected, slightly hyperactive |
| 4+ | Brisk, hyperactive, with intermittent or transient clonus |
Source: Seidel, H., Ball, J., Dains, J., Flynn, J., Solomon, B., & Stewart, R. (2011). Mosby’s guide to physical examination (7th ed.). St. Louis: Mosby.
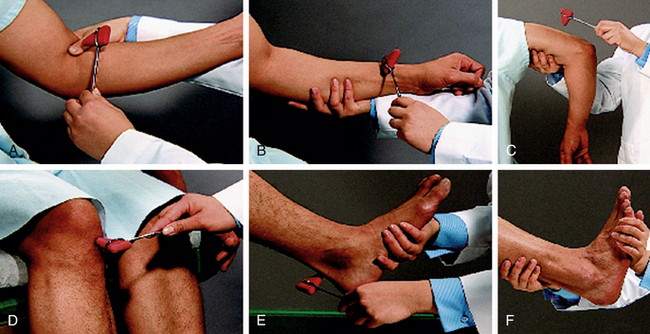
FIG. 27-4 Location of tendons for evaluation of deep tendon reflexes. A, Biceps. B, Brachioradial. C, Triceps. D, Patellar. E, Achilles, F, Evaluation of ankle clonus. (From Seidel, H., Ball, J., Dains, J., Flynn, J., Solomon, B., & Stewart, R. [2011]. Mosby’s guide to physical examination [7th ed.]. St. Louis: Mosby.)
To assess for hyperactive reflexes (clonus) at the ankle joint, the examiner supports the leg with the knee flexed (see Fig. 27-4, F). With one hand the examiner sharply dorsiflexes the foot, maintains the position for a moment, and then releases the foot. Normal (negative clonus) response is elicited when no rhythmic oscillations (jerks) are felt while the foot is held in dorsiflexion. When the foot is released, no oscillations are seen as the foot drops to the plantar-flexed position. Abnormal (positive clonus) response is recognized by rhythmic oscillations of one or more “beats” felt when the foot is in dorsiflexion and seen as the foot drops to the plantar-flexed position.
The presence of proteinuria is determined from dipstick testing on a clean-catch or a catheterized urine specimen or evaluation of a 24-hour urine collection. Proteinuria is defined as a concentration at or greater than 30 mg/dl (≥1+ on dipstick measurement) in at least two random urine specimens collected at least 6 hours apart. In a 24-hour specimen, proteinuria is defined as a concentration at or greater than 300 mg/24 hours. A diagnosis of severe preeclampsia requires a concentration of ≥5 g protein in a 24-hour urine collection or a value of ≥3+ on dipstick (ACOG, 2002; Sibai, 2007). Because a 24-hour collection to measure the quantity of protein and creatinine clearance is more reflective of true renal status, it is preferred over dipstick testing. Alkaline, concentrated, or dilute urine can yield a false reading. Urine contaminated with bacteria, blood, and amniotic fluid also can yield a false positive for proteinuria (Gilbert, 2011; Peters 2008).
During the examination the woman is evaluated for signs and symptoms of severe preeclampsia such as severe headaches (usually frontal), epigastric pain (heartburn), right upper quadrant abdominal pain, or visual disturbances such as scotoma, photophobia, or double vision. The signs and symptoms of mild versus severe preeclampsia are summarized in Table 27-2.
Mild Gestational Hypertension and Mild Preeclampsia
The goals of therapy for women with mild gestational hypertension and mild preeclampsia are to ensure maternal safety and to have the woman give birth to a healthy newborn as close to term as possible. At or near term the plan of care for a woman with mild gestational hypertension or mild preeclampsia is most likely to be the induction of labor, preceded, if necessary, by cervical ripening. When mild gestational hypertension or mild preeclampsia is suspected earlier in gestation the woman should be hospitalized for several days for a thorough evaluation of maternal-fetal status. After the evaluation is completed, a multidisciplinary plan of care is developed with the woman and her family. Immediate birth may not be in the best interest of the fetus. Women with mild gestational hypertension or mild preeclampsia that are less than 36 weeks of gestation may be discharged with close maternal and fetal surveillance (expectant management) (Barton & Sibai, 2008; Gilbert, 2011).
Home Care: Women with mild gestational hypertension and mild preeclampsia can be safely managed at home, provided they have frequent maternal and fetal evaluation. Criteria for home health care include BP less than 150/100; proteinuria less than 500 mg per day; normal platelet count, liver enzymes, and creatinine levels; normal (reassuring) fetal status; and no signs or symptoms of severe preeclampsia (Barton & Sibai, 2008; Gilbert, 2011). Successful home care requires the woman to be well educated about preeclampsia and highly motivated to follow the plan of care. All teaching should include the woman and her family, and time must be allowed for them to absorb information, ask questions, and voice concerns. Methods for enhancing learning include visual aids, videotapes or DVDs, handouts, and demonstrations with return demonstrations. Furthermore, the effects of illness, language, age, cultural beliefs, and support systems must be considered (see the Nursing Process box).
Maternal and Fetal Assessment: Maternal assessment includes measurement of hematocrit, platelet count, liver function tests, and a 24-hour urine protein assessment once each week. In addition, women with mild gestational hypertension or mild preeclampsia are usually seen twice weekly for the evaluation of BP and urine protein by dipstick. Women may also be asked to take their BP and perform urine dipstick testing each day (see the Teaching for Self-Management box: Assessing and Reporting Clinical Signs of Preeclampsia). Fetal evaluation usually includes daily fetal movement counts and nonstress testing or a biophysical profile once or twice weekly until birth. (See Chapter 26 for more information on fetal assessment tests.) Ultrasound evaluation of amniotic fluid volume and determination of estimated fetal weight are performed at the time mild preeclampsia is diagnosed and serially thereafter, depending on findings (Sibai, 2007).
Activity Restriction: Complete or partial bed rest for the duration of the pregnancy is still frequently recommended. However, no evidence has been found that this practice improves pregnancy outcome. ![]() Moreover, prolonged bed rest is known to increase the risk of thrombophlebitis (Sibai, 2007). Other adverse physiologic outcomes related to complete bed rest include cardiovascular deconditioning; diuresis with accompanying fluid, electrolyte, and weight loss; muscle atrophy; and psychologic stress. These changes begin on the first day of bed rest and continue for the duration of therapy. Therefore, restricted activity, rather than complete bed rest, is recommended (Sibai).
Moreover, prolonged bed rest is known to increase the risk of thrombophlebitis (Sibai, 2007). Other adverse physiologic outcomes related to complete bed rest include cardiovascular deconditioning; diuresis with accompanying fluid, electrolyte, and weight loss; muscle atrophy; and psychologic stress. These changes begin on the first day of bed rest and continue for the duration of therapy. Therefore, restricted activity, rather than complete bed rest, is recommended (Sibai).
Women with mild preeclampsia generally feel reasonably well; boredom from activity restriction is therefore common. Diversionary activities, visits from friends, telephone conversations, and creation of a comfortable and convenient environment are ways to cope with the boredom. Gentle exercise (e.g., range of motion exercises, stretching, Kegel exercises, pelvic tilts) is important in maintaining muscle tone, blood flow, regularity of bowel function, and a sense of well-being (see the Teaching for Self-Management box: Coping with Activity Restriction).
A high risk pregnancy can be very stressful for the woman and her family. Family stressors include separation from family members when hospitalized, need for activity restriction, financial concerns, ability to manage the household, family activities, and child care. The family will need to use coping mechanisms and support systems to help them through this crisis. An excellent web-based support group for pregnant women on bed rest is Sidelines (www. sidelines.org) (Gilbert, 2011). Relaxation techniques can also help to reduce stress and prepare the woman for labor and birth.
Diet: Women with mild preeclampsia may have a regular diet with adequate protein (60 to 70 g), calcium (1200 mg), 400 mcg of folic acid, and adequate zinc and sodium (2 to 6 g). Adequate fluid intake (six to eight 8-ounce glasses of water per day) is encouraged to enhance renal perfusion and bowel function (Gilbert, 2011). (See the Teaching for Self-Management box: Diet for Preeclampsia.)
Severe Gestational Hypertension and Severe Preeclampsia
Women with severe gestational hypertension are at greater risk for pregnancy complications than are women with mild preeclampsia. Therefore, women with severe gestational hypertension should be managed as if they have severe preeclampsia. Women diagnosed with severe gestational hypertension or severe preeclampsia should be hospitalized immediately for a thorough evaluation of maternal-fetal status (Sibai, 2007; Sibai & Barton, 2008). Maternal assessments include monitoring of BP, urine output, cerebral status, and the presence of epigastric pain, abdominal tenderness, signs of labor, or placental abruption. Laboratory evaluation includes a platelet count, liver enzymes, and serum creatinine (see Table 27-3). Fetal assessment consists of continuous fetal heart rate (FHR) monitoring, a biophysical profile, and ultrasound assessment of fetal growth and amniotic fluid volume (Sibai).
A multidisciplinary plan of care is developed with the woman and her family. The goals of care management are to ensure maternal safety, assess the degree of maternal and fetal risk, formulate a plan for giving birth, and prevent eclampsia and other serious complications. If the pregnancy is 34 weeks of gestation or greater, birth might be accomplished promptly, either by
cesarean or after labor induction. By 34 weeks of gestation, the risks of continuing the pregnancy are considered greater than the risks of preterm birth (Sibai, 2007; Sibai & Barton, 2008).
If the pregnancy is less than 34 weeks of gestation, the plan includes pharmacologic therapy to prevent seizures and control BP, and continuous maternal-fetal surveillance for indicators of worsening condition (Sibai, 2007; Sibai & Barton, 2008). Corticosteroids (betamethasone) will be ordered to enhance fetal lung maturation. The dose is 12.5 mg intramuscularly (IM), repeated in 24 hours. Optimal benefit begins 24 hours after the first dose is administered and lasts for 7 days (Gilbert, 2011).
Women who are less than 34 weeks of gestation can be monitored closely and allowed to continue the pregnancy, if their BP is adequately controlled and fetal testing is normal (reassuring). The woman should be hospitalized in a tertiary-care center that is able to provide both maternal and neonatal intensive care. Most women managed with close observation will develop a maternal or fetal indication for giving birth within 2 weeks. Immediate birth is indicated (regardless of the gestational age) if signs of fetal stress, placental abruption, HELLP syndrome, oliguria, pulmonary edema, eclampsia, or uncontrolled high blood pressure develop (Sibai, 2007; Sibai & Barton, 2008).
Intrapartum Care: Intrapartum nursing care is directed toward the early identification of FHR abnormalities and the prevention of maternal complications. Continuous FHR and uterine contraction monitoring are initiated and the woman should be assessed for signs of placental abruption such as hypertonic contractions or vaginal bleeding. Other maternal assessments include review of the central nervous, cardiovascular, pulmonary, and renal systems. Vital signs and assessments are performed as ordered and per hospital policy (see the Nursing Care Plan). Client and family education and
supportive measures are also initiated (Gilbert, 2011; Simpson & Creehan, 2008).
The woman with severe preeclampsia is maintained on bed rest with the side rails up in a quiet, darkened environment. Emergency drugs, oxygen, and suction equipment should be checked and readily available (Box 27-2). In order to reduce the risk of pulmonary edema, total intravenous (IV) and oral fluids should not exceed 125 ml/hr. Intensive hemodynamic monitoring is not a routine standard of care and is indicated only in the presence of pulmonary edema or oliguria unresponsive to fluid challenge or severe hypertension unresponsive to medications. A pulmonary artery (Swan-Ganz) catheter can be inserted to evaluate central venous and pulmonary artery pressures (Gilbert, 2011; Simpson & Creehan, 2008) (see Chapter 31).
Magnesium Sulfate: Magnesium sulfate is the drug of choice in the prevention and treatment of seizure activity caused by severe preeclampsia or eclampsia. Magnesium sulfate is almost always administered intravenously as a secondary infusion (piggyback) by a volumetric infusion pump. Per protocol or physician’s order, an initial loading dose of 4 to 6 g of magnesium sulfate is infused over 15 to 30 minutes. This dose is followed by a maintenance dose of magnesium sulfate that is diluted in an IV solution (e.g., 40 g of magnesium sulfate in 1000 ml of lactated Ringer’s solution [1 g = 25 ml]) and administered by an infusion pump at 2 g/hr. This dose should maintain a therapeutic serum magnesium level of 4 to 7 mEq/l. After the loading dose, transient lowering of the arterial BP may occur secondary to relaxation of smooth muscle (Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010; Gilbert, 2011; Sibai, 2007).
Magnesium sulfate is rarely given intramuscularly because the absorption rate cannot be controlled, injections are painful, and tissue necrosis may occur. However, the IM route may be used with some women who are being transported to a tertiary-care center. The IM dose is 4 to 5 g given in each buttock, a total of 10 g (local anesthetic can be added to the solution to reduce injection pain), and can be repeated at 4-hour intervals. The Z-track technique should be used for the deep IM injection, followed by gentle massage at the site.
Magnesium sulfate interferes with the release of acetylcholine at the synapses, decreasing neuromuscular irritability, depressing cardiac conduction, and decreasing CNS irritability. Because magnesium is excreted in the urine, accurate recordings of maternal urine output must be obtained. If renal function declines, all of the magnesium sulfate will not be adequately excreted resulting in magnesium toxicity. Expected side effects of magnesium sulfate are a feeling of warmth, flushing, diaphoresis, and burning at the IV site. Symptoms of mild toxicity include lethargy, muscle weakness, decreased or absent DTRs, double vision, and slurred speech. Increasing toxicity may be indicated by maternal hypotension, bradycardia, bradypnea, and cardiac arrest (Gilbert, 2011). Blood can be drawn to precisely determine the serum magnesium level if mild or severe toxicity is suspected (Box 27-3).
Magnesium sulfate does not seem to affect the FHR in a healthy term fetus. Doses of magnesium sulfate that prevent maternal seizures have been determined to be safe for the fetus. Neonatal serum magnesium levels approximate the levels of the mother (Roberts & Funai, 2009). Findings from several research studies suggest that magnesium sulfate administration during labor may provide a protective effect against the development of cerebral palsy in preterm very low birthweight infants (Cunningham et al., 2010). ![]()
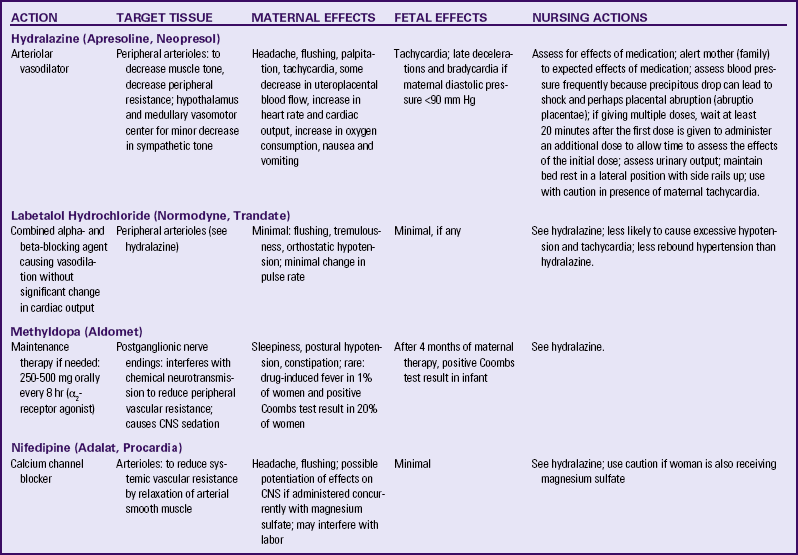
Antihypertensive Medications: Antihypertensive medications are indicated when the systolic BP exceeds 160 mm Hg, or the diastolic BP exceeds 110 mm Hg. Maternal risks associated with severe hypertension include left ventricular failure and cerebral hemorrhage. In order to maintain uteroplacental perfusion, antihypertensive therapy must not decrease the arterial pressure too much or too rapidly. Hydralazine, labetalol, and nifedipine are effective drugs for treating hypertension intrapartum. They may also be used during pregnancy or in the postpartum period for BP control (Cunningham et al., 2010; Gilbert, 2011; Sibai, 2007). Table 27-5 compares antihypertensive agents used to treat hypertension in pregnancy.
Postpartum Care: Throughout the postpartum period the woman will need careful assessment of her vital signs, intake and output, DTRs, and level of consciousness. The magnesium sulfate infusion is continued after birth for seizure prophylaxis as ordered, usually for 12 to 24 hours. Assessments for effects and side effects continue until the medication is discontinued. Given that magnesium sulfate potentiates the action of narcotics, CNS depressants, and calcium channel blockers, these drugs must be administered with caution.
The symptoms of preeclampsia or eclampsia usually resolve within 48 hours after birth. However, approximately 30% of cases of eclampsia and HELLP syndrome occur postpartum. The nurse should regularly assess the woman for any symptoms of preeclampsia such as headaches, visual disturbances, or epigastric pain. Clinical signs that demonstrate resolution of preeclampsia include diuresis and decreased edema (Barton & Sibai, 2008; Gilbert, 2011).
The preeclamptic woman is unable to tolerate excessive postpartum blood loss because of hemoconcentration. Oxytocin or prostaglandin products are used to control bleeding. Ergot products (e.g., Ergotrate, Methergine) are contraindicated because they increase BP.
Severe preeclampsia and HELLP syndrome contribute to small-for-gestational age infants and premature birth (Peters, 2008; Sibai, 2007). Nursing care that facilitates bonding and attachment includes providing the family with photographs of the infant, keeping the family informed of the infant’s status, encouraging the father to visit the neonatal intensive care unit (NICU) and taking the woman to the NICU by wheelchair after her condition has stabilized (Gilbert, 2011). Postpartum recovery may be prolonged as a result of the physiologic consequences of prolonged activity restriction. The nurse should accompany the woman when she ambulates and assess for weakness, dizziness, shortness of breath, and muscle soreness.
Hypertension may persist for days or weeks after birth. Women with severe gestational hypertension or severe preeclampsia are frequently discharged from the hospital on an antihypertensive medication such as labetalol or nifedipine. If this is the case, the BP needs to be checked frequently either at home or at the health care provider’s office. Often BP returns to normal within a few weeks after birth and antihypertensive medications can be discontinued.
Future Health Care: The woman with preeclampsia has a sevenfold increased risk of developing preeclampsia or eclampsia in a future pregnancy. She also has an increased risk of adverse perinatal outcomes such as preterm labor and birth, fetal growth restriction, placental abruption, and fetal death. Care management during a future pregnancy is directed toward increased maternal surveillance and frequency of prenatal visits, close monitoring for signs of severe hypertension and preeclampsia, serial ultrasound evaluation for fetal growth and amniotic fluid volume, and home blood pressure monitoring (Barton & Sibai, 2008).
Women with preeclampsia (especially early onset and severe preeclampsia) have an increased risk of chronic hypertension and cardiovascular disease later in life. The postpartum period provides an excellent opportunity to educate women about lifestyle changes that may decrease their risk for developing future health problems (Gilbert, 2011; Sibai, 2007).
Eclampsia
Eclampsia is usually preceded by premonitory signs and symptoms, including persistent headache, blurred vision, severe epigastric or right upper quadrant abdominal pain, and altered mental status. However, convulsions can appear suddenly and without warning in a seemingly stable woman with only minimal BP elevations (Sibai, 2007). The convulsions that occur in eclampsia are frightening to observe. Tonic contraction of all body muscles (seen as arms flexed, hands clenched, legs inverted) precedes the tonic-clonic convulsion. During this stage muscles alternately relax and contract. Respirations are halted and then begin again with long, deep, stertorous inhalations. Hypotension follows, and muscular twitching, disorientation, and amnesia persist for a while after the convulsion.
Immediate Care
Nursing actions during a convulsion are directed toward ensuring a patent airway and client safety (see the Emergency box.)
It is important to note the time of onset and duration of the seizure. Call for help but do not leave the bedside. Make certain that the side rails on the bed are raised; pad them with a folded blanket or pillow if possible. Women with eclampsia have been known to sustain fractures from falling out of bed during the seizure. Immediately after the convulsion, lower the head of the bed and turn the woman onto her side. This helps prevent aspiration of vomitus (Gilbert, 2011).
Nursing actions after a convulsion are directed toward maternal stabilization. First assess the status of the woman’s airway, breathing, and pulse. Suction secretions from her glottis to clear the airway, insert an oral airway, and administer oxygen at 10 L/min by nonrebreather face mask. If an IV infusion is not in place, insert one with an 18-gauge needle. If an IV line was in place before the seizure, it may have infiltrated and will need to be restarted immediately. As soon as IV access is obtained, administer magnesium sulfate as ordered (Gilbert, 2011).
If eclampsia develops after initiating magnesium sulfate therapy, additional magnesium sulfate or another anticonvulsant (e.g., diazepam [Valium]) may be administered. Fetal and neonatal effects of diazepam include decreased (absent or minimal) FHR variability, neonatal hypotonia, decreased respirations, and depressed sucking reflex. However, with adequate blood magnesium levels, the eclamptic woman will rarely continue to have seizures (Chan & Winkle, 2006, Sibai, 2007).
A rapid assessment of uterine activity, cervical status, and fetal status is performed after the convulsion. During a convulsion the uterus becomes hypercontractile and hypertonic. As a result, the membranes may have ruptured or the cervix may have dilated rapidly, and birth may be imminent. The fetal heart rate tracing may demonstrate bradycardia, late decelerations, minimal baseline variability, or any combination. These findings usually resolve within a few minutes after the convulsion ends and the woman’s hypoxia is corrected (Sibai, 2007). Assist the woman with hygiene and a change of linens and gown because she may have been incontinent of urine or stool during the convulsion.
Laboratory tests to evaluate liver enzymes and platelet count are ordered to assess for HELLP syndrome. Other tests include determination of electrolyte levels and clotting profile for DIC (see Table 27-3). Blood is typed and crossmatched for administration of packed red blood cells as needed.
After stabilization of the woman and fetus, a decision will be made regarding timing and method of birth. Eclampsia alone is not an indication for immediate cesarean birth. The route of birth (induction of labor versus cesarean birth) depends on maternal and fetal condition, fetal gestational age, and the cervical Bishop score. Regional anesthesia is not recommended for eclamptic women with coagulopathy or a platelet count less than 50,000/mm3 (Sibai, 2007).
Chronic Hypertension
Chronic hypertension affects approximately 4% to 5% of all pregnancies (Gilbert, 2011). Approximately 90% of women with chronic hypertension have primary or essential hypertension. In the remaining 10%, the hypertension is secondary to a medical condition such as renal or collagen disease (Sibai, 2007). Chronic hypertension in pregnancy is associated with an increased incidence of placental abruption, superimposed preeclampsia, and increased perinatal mortality (three- or fourfold). Fetal effects include fetal growth restriction and preterm birth (Cunningham et al., 2010).
Ideally the management of chronic hypertension in pregnancy begins before conception. An evaluation is performed to assess the cause and severity of the hypertension, and the presence of any target organ damage (e.g., heart, eye, and kidney). Moreover, the woman should be encouraged to make lifestyle changes prior to conception such as smoking and alcohol cessation, participating in aerobic exercise, and losing weight if indicated. A diet that includes a maximum of 2.4 g sodium per day is recommended (Gilbert, 2011). These lifestyle modifications should continue throughout the pregnancy.
Based on the history and physical findings, women with chronic hypertension are classified as either high or low risk for pregnancy complications. Women who are high risk are managed with antihypertensive medication and frequent assessments of maternal and fetal well-being. Methyldopa (Aldomet) is most often recommended for treating chronic hypertension in pregnancy. However, because it is rarely used for treating chronic hypertension in nonpregnant women, it may not be practical to switch medications because of pregnancy. Labetalol and nifedipine are other antihypertensive medications used during pregnancy (see Table 27-5). Women with low risk chronic hypertension may not require any antihypertensive medication at all during pregnancy (Cunningham et al., 2010; Sibai, 2007).
Women who are high risk are monitored closely, and the method and timing of the birth are dependent on the maternal and fetal status. After giving birth the woman should be monitored closely for complications such as pulmonary edema, renal failure, heart failure, and encephalopathy. Women with chronic hypertension can breastfeed if they desire. All antihypertensive medications are present to some degree in breast milk. Levels of methyldopa in breast milk appear to be low and are considered safe. Labetalol also has a low concentration in breast milk. Little is known about the transfer of calcium channel blockers, such as nifedipine, in breast milk, but no apparent side effects have been noted in infants (Sibai, 2007).
There is a need for improved prenatal screening, prevention, and treatment strategies to reduce the incidence and severity of hypertensive disorders in pregnancy and improve the health of women long term (Wagner, Barac, & Garovic, 2007).
KEY POINTS
• Hypertensive disorders during pregnancy are a leading cause of maternal and infant morbidity and mortality worldwide.
• The cause of preeclampsia is unknown. No reliable test has yet been developed that can be used as a routine screening tool for predicting preeclampsia.
• Preeclampsia is a multisystem disease. The pathophysiologic changes associated with preeclampsia are present long before clinical manifestations such as hypertension become evident.
• HELLP syndrome is a variant of severe preeclampsia, not a separate illness.
• Once preeclampsia becomes clinically evident, therapeutic interventions may slow the progression of the disease, allowing the pregnancy to continue, but the underlying pathology continues.
• Magnesium sulfate, the anticonvulsant of choice for preventing or controlling eclamptic seizures, requires careful monitoring of reflexes, respirations, and urinary output. The antidote, calcium gluconate or calcium chloride, should be on the unit.
• Nursing actions during a convulsion are directed toward ensuring a patent airway and client safety.
• Complications associated with chronic hypertension in pregnancy include placental abruption, superimposed preeclampsia, fetal growth restriction, and increased perinatal mortality.
• Women with preeclampsia have an increased risk of adverse perinatal outcomes in a future pregnancy and are at risk of developing chronic hypertension and cardiovascular disease later in life.
![]() Audio Chapter Summaries Access an audio summary of the Key Points on
Audio Chapter Summaries Access an audio summary of the Key Points on ![]()