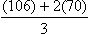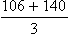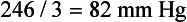Anatomy and Physiology of Pregnancy
• Determine obstetric history by using the five- and two-digit systems.
• Describe the various types of pregnancy tests including the timing of tests and interpretation of results.
• Explain the expected maternal anatomic and physiologic adaptations to pregnancy for each body system.
• Differentiate among presumptive, probable, and positive signs of pregnancy.
• Compare normal adult laboratory values with values for pregnant women.
• Compare the characteristics of the abdomen, the vulva, and the cervix of the nullipara and the multipara.
• Identify the maternal hormones produced during pregnancy, their target organs, and their major effects on pregnancy.
The goal of maternity care is a healthy pregnancy with a physically safe and emotionally satisfying outcome for mother, infant, and family. Consistent health supervision and surveillance are of utmost importance in achieving this outcome. However, many maternal adaptations are unfamiliar to pregnant women and their families. Helping the pregnant woman recognize the relation between her physical status and the plan for her care assists her in making decisions and encourages her to participate in her own care.
Gravidity and Parity
An understanding of the following terms used to describe pregnancy and the pregnant woman is essential to the study of maternity care (Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010):
• gravida: a woman who is pregnant
• multigravida: a woman who has had two or more pregnancies
• multipara: a woman who has completed two or more pregnancies to 20 or more weeks of gestation
• nulligravida: a woman who has never been pregnant
• nullipara: a woman who has not completed a pregnancy with a fetus or fetuses who have reached 20 weeks of gestation
• parity: the number of pregnancies in which the fetus or fetuses have reached 20 weeks of gestation when they are born, not the number of fetuses (e.g., twins) born. Whether the fetus is born alive or is stillborn (fetus who shows no signs of life at birth) does not affect parity
• postdate or postterm: a pregnancy that goes beyond 42 weeks of gestation
• preterm: a pregnancy that has reached 20 weeks of gestation but ends before completion of 37 weeks of gestation
• primigravida: a woman who is pregnant for the first time
• primipara: a woman who has completed one pregnancy with a fetus or fetuses who have reached 20 weeks of gestation
• term: a pregnancy from the completion of 37 weeks of gestation to the end of week 42 of gestation
• viability: capacity to live outside the uterus; there are no clear limits of gestational age or weight. Infants born at 22 to 25 weeks of gestation are considered to be on the threshold of viability and are especially vulnerable to brain injury if they survive. Survival rates for infants with birth weights of less than 500 g is approximately 45%.
Gravidity and parity information is obtained during history-taking interviews. Obtaining and documenting this information accurately is important in making a plan of care for the pregnant woman.
Two commonly used systems of summarizing the obstetric history are discussed here. Gravidity and parity is described with only two digits: the first digit represents the number of pregnancies the woman has had, including the present one; and parity is the number of pregnancies that have reached 20 or more weeks of gestation before the birth. For example, if the woman had twins at 36 weeks with her first pregnancy, parity would still be counted as one birth (gravida [G]1, para [P]1) (Cunningham et al., 2010). If the woman becomes pregnant a second time, she would still be G2P1 until she gives birth at 38 weeks when she would then become G2P2.
Another system that is commonly used consists of five digits separated with hyphens. This system provides more specific information about the woman’s obstetric history, although it may not provide accurate information about parity because it provides information about births and not pregnancies reaching 20 weeks of gestation (Beebe, 2005). The first digit represents gravidity; the second digit represents the total number of term births; the third indicates the number of preterm births; the fourth identifies the number of abortions (miscarriage or elective termination of pregnancy); and the fifth is the number of children currently living. The acronym GTPAL (gravidity, term, preterm, abortions, living children) may be helpful in remembering this system of notation. For example, if a woman pregnant only once gives birth at week 34 and the infant survives, the abbreviation that represents this information is 1-0-1-0-1. During her next pregnancy, the abbreviation is 2-0-1-0-1. Additional examples are given in Table 13-1.
Pregnancy Tests
Early detection of pregnancy allows early initiation of care. Human chorionic gonadotropin (hCG) is the earliest biochemical marker for pregnancy, and pregnancy tests are based on the recognition of hCG or a beta (β) subunit of hCG. Production of β-hCG begins as early as the day of implantation and can be detected as early as 7 to 10 days after conception (Blackburn, 2007). The level of hCG increases until it peaks at about 60 to 70 days of gestation and then declines until about 80 days of pregnancy. It remains stable until about 30 weeks and then gradually increases until term. Higher than normal levels of hCG may indicate abnormal gestation (e.g., a fetus with Down syndrome) or multiple gestation; an abnormally slow increase or a decrease in hCG levels may indicate ectopic pregnancy or impending miscarriage (Burton, Sibley, & Jauniaux, 2007; Stewart, 2007).
Serum and urine pregnancy tests are performed in clinics, offices, women’s health centers, and laboratory settings, and urine pregnancy tests may be performed at home. Both serum and urine tests can provide accurate results. A 7- to 10-ml sample of venous blood is collected for serum testing. Most urine tests require a first-voided morning urine specimen because it contains levels of hCG approximately the same as those in serum. Random urine samples usually have lower levels. Urine tests are less expensive and provide more immediate results than do serum tests (Stewart, 2007).
Many different pregnancy tests are available (Fig. 13-1). The wide variety of tests precludes discussion of each; however, several categories of tests are described here. The nurse should read the manufacturer’s directions for the test to be used and determine if the woman understands the directions. A study by Wallace and associates (2009) reported that instructions for most home pregnancy tests do not meet the criteria recommended for compliance with the guidelines for use of plain language and that most instructions were written at a seventh grade level or above.

FIG. 13-1 Many pregnancy test products are available over the counter. (Courtesy Dee Lowdermilk, Chapel Hill, NC.)
Radioimmunoassay (RIA) pregnancy tests for the beta subunit of hCG in serum or urine samples use radioactively labeled markers and are usually performed in a laboratory. These tests are accurate with low hCG levels (5 milli-International Units/ml) and can confirm pregnancy before the first menstrual period. Results are available within a few hours (Stewart, 2007).
Radioreceptor assay (RRA) is a serum test that measures the ability of a blood sample to inhibit the binding of radiolabeled hCG to receptors. The test is 90% to 95% accurate from 6 to 8 days after conception (Pagana & Pagana, 2009).
Enzyme-linked immunosorbent assay (ELISA) testing is the most popular method of testing for pregnancy. It uses a specific monoclonal antibody (anti-hCG) with enzymes to bond with hCG in urine. As an office or home procedure, it requires minimal time and offers results in less than 5 minutes. A positive test result is indicated by a simple color-change reaction. Depending on the specific test, levels of hCG as low as 25 milli-International Units/ml can be detected as early as 7 days after conception (Stewart, 2007).
ELISA technology is the basis for most over-the-counter home pregnancy tests. With these one-step tests, the woman usually applies urine to a strip and reads the results. The test kits come with directions for collection of the specimen, the testing procedure, and reading of the results. A positive test result is indicated by a simple color change reaction or a digital reading. Most manufacturers of the kits provide a toll-free telephone number to call if users have concerns and questions about test procedures or results (see Teaching for Self-Management box). The most common error in home pregnancy tests is performing the test too early in pregnancy (Stewart, 2007).
Interpreting the results of pregnancy tests requires some judgment. The type of pregnancy test and its degree of sensitivity (ability to detect low levels of a substance) and specificity (ability to discern the absence of a substance) must be considered in conjunction with the woman’s history. This includes the date of her last menstrual period (LMP), her usual cycle length, and results of previous pregnancy tests. It is important to know if the woman is a substance abuser and what medications she is taking, because medications such as anticonvulsants and tranquilizers can cause false-positive results, whereas diuretics and promethazine can cause false-negative results (Pagana & Pagana, 2009). Improper collection of the specimen, hormone-producing tumors, and laboratory errors also can cause false results.
Depending on the specific test, levels of hCG as low as 6.3 milli-International Units/ml can be detected as early as the first day of a missed menstrual period as reported by Cole and coworkers (2005). These researchers found that most of the over-the-counter pregnancy tests in the study were less sensitive (25 to 100 milli-International Units/ml) and detected only a small percentage of pregnancies on the first day of a missed period even though most products claimed to be 99% accurate. Tomlinson and colleagues (2008) found that digital readings of low hCG levels (i.e., 25 milli-International Units/ml) were more accurately interpreted by consumers than nondigital tests.
Women who use a home pregnancy test should be advised about the variations in accuracy reporting and to use caution when interpreting results. Whenever there is any question, further evaluation or retesting is appropriate.
Adaptations to Pregnancy
Maternal physiologic adaptations are attributed to the hormones of pregnancy and to mechanical pressures arising from the enlarging uterus and other tissues. These adaptations protect the woman’s normal physiologic functioning, meet the metabolic demands pregnancy imposes on her body, and provide a nurturing environment for fetal development and growth. Although pregnancy is a normal phenomenon, problems can occur.
Signs of Pregnancy
Some of the physiologic adaptations are recognized as signs and symptoms of pregnancy. Three commonly used categories of signs and symptoms of pregnancy are presumptive (those changes noticed by the woman—e.g., amenorrhea, fatigue, nausea and vomiting, breast changes); probable (those changes observed by an examiner—e.g., Hegar sign, ballottement, pregnancy tests); and positive (those signs that are attributable only to the presence of the fetus—e.g., hearing fetal heart tones, visualization of the fetus, and palpating fetal movements). Table 13-2 summarizes these signs of pregnancy in relation to when they might occur and other causes for their occurrence.
Reproductive System and Breasts
Changes in Size, Shape, and Position: The phenomenal uterine growth in the first trimester is stimulated by high levels of estrogen and progesterone. Early uterine enlargement results from increased vascularity and dilation of blood vessels, hyperplasia (production of new muscle fibers and fibroelastic tissue) and hypertrophy (enlargement of preexisting muscle fibers and fibroelastic tissue), and development of the decidua. By 7 weeks of gestation, the uterus is the size of a large hen’s egg; by 10 weeks of gestation, it is the size of an orange (twice its nonpregnant size); and by 12 weeks of gestation, it is the size of a grapefruit. After the third month, uterine enlargement is primarily the result of mechanical pressure of the growing fetus.
As the uterus enlarges it also changes in shape and position. At conception the uterus is shaped like an upside-down pear. During the second trimester, as the muscular walls strengthen and become more elastic, the uterus becomes spherical or globular. Later, as the fetus lengthens, the uterus becomes larger and more ovoid and rises out of the pelvis into the abdominal cavity.
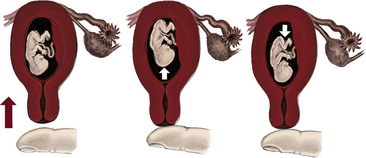
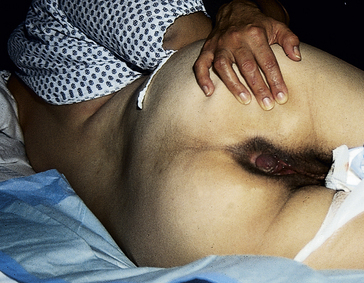
The pregnancy may “show” after the fourteenth week, although this depends to some degree on the woman’s height and weight. Abdominal enlargement may be less apparent in the nullipara with good abdominal muscle tone (Fig. 13-2). Posture also influences the type and degree of abdominal enlargement that occurs. In normal pregnancies, the uterus enlarges at a predictable rate. As the uterus grows it may be palpated above the symphysis pubis some time between the twelfth and fourteenth weeks of pregnancy (Fig. 13-3). The uterus rises gradually to the level of the umbilicus at 22 to 24 weeks of gestation and nearly reaches the xiphoid process at term. Between weeks 38 and 40, fundal height drops as the fetus begins to descend and engage in the pelvis (lightening) (see Fig. 13-3, dashed line). Generally, lightening occurs in the nullipara about 2 weeks before the onset of labor and at the start of labor in the multipara.
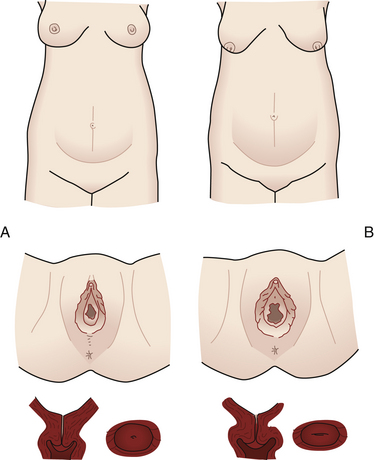
FIG. 13-2 Comparison of abdomen, vulva, and cervix in nullipara (A), and multipara (B), at the same stage of pregnancy.
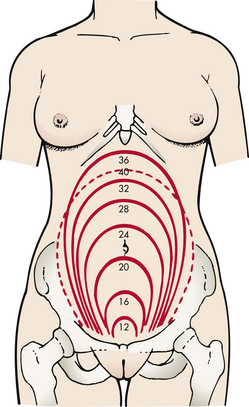
FIG. 13-3 Height of fundus by weeks of normal gestation with a single fetus. Dashed line, height after lightening. (From Seidel, H., Ball, J., Dains, J., Flynn, J., Solomon, B., & Stewart, R. [2011]. Mosby’s guide to physical examination [7th ed.]. St. Louis: Mosby.)
Uterine enlargement is determined by measuring fundal height, a measurement commonly used to estimate the duration of pregnancy. However, variation in the position of the fundus or the fetus, variations in the amount of amniotic fluid present, the presence of more than one fetus, maternal obesity, and variation in examiner techniques can reduce the accuracy of this estimation of the duration of pregnancy (see Chapter 15).
The uterus normally rotates to the right as it elevates, probably because of the presence of the rectosigmoid colon on the left side, but the extensive hypertrophy (enlargement) of the round ligaments keeps the uterus in the midline. Eventually the growing uterus touches the anterior abdominal wall and displaces the intestines to either side of the abdomen (Fig. 13-4). Whenever a pregnant woman is standing, most of her uterus rests against the anterior abdominal wall, and this contributes to altering her center of gravity.
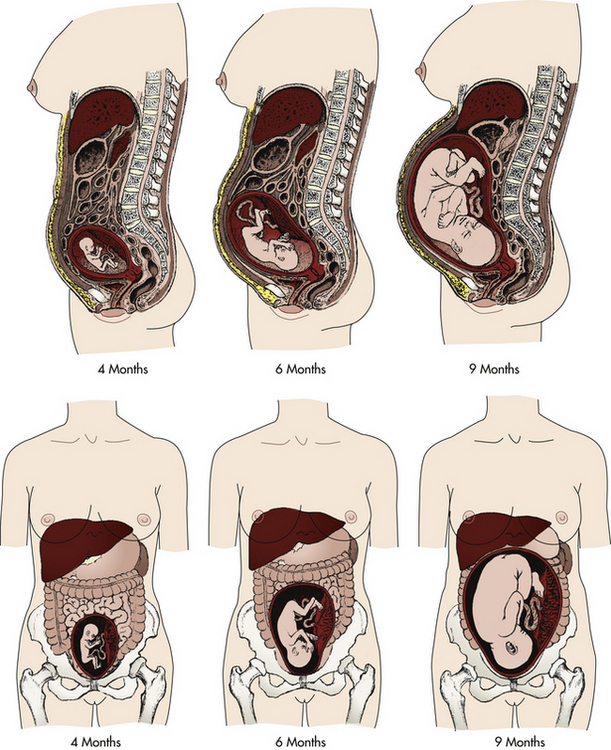
FIG. 13-4 Displacement of internal abdominal structures and diaphragm by the enlarging uterus at 4, 6, and 9 months of gestation.
At approximately 6 weeks of gestation, softening and compressibility of the lower uterine segment (the uterine isthmus) occur (Hegar sign) (Fig. 13-5). This results in exaggerated uterine anteflexion during the first 3 months of pregnancy. In this position the uterine fundus presses on the urinary bladder, causing the woman to have urinary frequency.
Changes in Contractility: Soon after the fourth month of pregnancy, uterine contractions can be felt through the abdominal wall. These contractions are referred to as the Braxton Hicks sign. Braxton Hicks contractions are irregular and painless and occur intermittently throughout pregnancy. These contractions facilitate uterine blood flow through the intervillous spaces of the placenta and thereby promote oxygen delivery to the fetus. Although Braxton Hicks contractions are not painful, some women complain that they are annoying. After the twenty-eighth week these contractions become much more definite, but they usually cease with walking or exercise. Braxton Hicks contractions can be mistaken for true labor; however, they do not increase in intensity or frequency or cause cervical dilation.
Uteroplacental Blood Flow: Placental perfusion depends on the maternal blood flow to the uterus. Blood flow increases rapidly as the uterine size increases. Although uterine blood flow increases 20-fold, the fetoplacental unit grows more rapidly. Consequently, more oxygen is extracted from the uterine blood during the latter part of pregnancy (Cunningham et al., 2010). In a normal term pregnancy, one sixth of the total maternal blood volume is within the uterine vascular system. The rate of blood flow through the uterus ranges from 450 to 650 ml/min at term, and oxygen consumption of the gravid uterus increases to meet fetal needs. A low maternal arterial pressure, contractions of the uterus, and maternal supine position are three factors known to decrease blood flow. Estrogen stimulation may increase uterine blood flow. Doppler ultrasound examination can be used to measure uterine blood flow velocity, especially in pregnancies at risk because of conditions associated with decreased placental perfusion such as hypertension, intrauterine growth restriction, diabetes mellitus, and multiple gestation (Blackburn, 2007). By using an ultrasound device or a fetal stethoscope, the health care provider may hear the uterine souffle (sound made by blood in the uterine arteries that is synchronous with the maternal pulse) or the funic souffle (sound made by blood rushing through the umbilical vessels and synchronous with the fetal heart rate).
Cervical Changes: A softening of the cervical tip called Goodell sign can be observed at approximately the beginning of the sixth week in a normal, unscarred cervix. This sign is brought about by increased vascularity, slight hypertrophy, and hyperplasia (increase in number of cells) of the muscle and its collagen-rich connective tissue, which becomes loose, edematous, highly elastic, and increased in volume. The glands near the external os proliferate beneath the stratified squamous epithelium, giving the cervix the velvety appearance characteristic of pregnancy. Friability (tissue is easily damaged) is increased can cause slight bleeding after coitus with deep penetration or after vaginal examination. Pregnancy also can cause the squamocolumnar junction, the site for obtaining cells for cervical cancer screening, to be located away from the cervix. Because of all these changes, evaluation of abnormal Papanicolaou (Pap) tests during pregnancy can be complicated. However, careful assessment of all pregnant women is important, because about 3% of all cervical cancers are diagnosed during pregnancy (Copeland & Landon, 2007).
The cervix of the nullipara is rounded. Lacerations of the cervix almost always occur during the birth process. With or without lacerations, however, after childbirth the cervix becomes more oval in the horizontal plane, and the external os appears as a transverse slit (see Fig. 13-2).
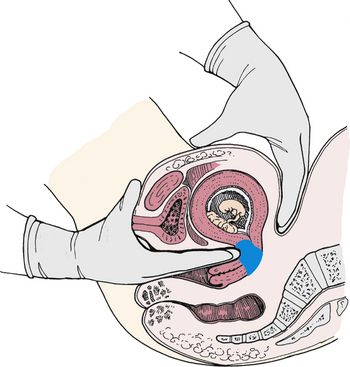
Changes Related to the Presence of the Fetus: Passive movement of the unengaged fetus is called ballottement and can be identified generally between the sixteenth and eighteenth weeks. Ballottement is a technique of palpating a floating structure by bouncing it gently and feeling it rebound. In the technique used to palpate the fetus, the examiner places a finger in the vagina and taps gently upward, causing the fetus to rise. The fetus then sinks, and a gentle tap is felt on the finger (Fig. 13-6).
The first recognition of fetal movements, or “feeling life,” by the multiparous woman may occur as early as the fourteenth to sixteenth weeks. The nulliparous woman may not notice these sensations until the eighteenth week or later. Quickening is commonly described as a flutter and is difficult to distinguish from peristalsis. Fetal movements gradually increase in intensity and frequency. The week when quickening occurs provides a tentative clue in dating the duration of gestation.
Vagina and Vulva
Pregnancy hormones prepare the vagina for stretching during labor and birth by causing the vaginal mucosa to thicken, the connective tissue to loosen, the smooth muscle to hypertrophy, and the vaginal vault to lengthen. Increased vascularity results in a violet-bluish vaginal mucosa and cervix. The deepened color, termed the Chadwick sign, may be evident as early as the sixth week but is easily noted at the eighth week of pregnancy (Blackburn, 2007).
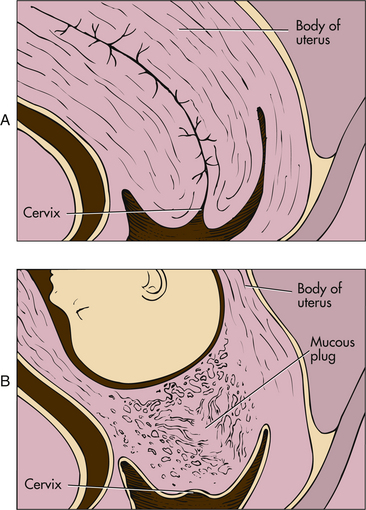
Leukorrhea is a white or slightly gray mucoid discharge with a faint musty odor. This copious mucoid fluid occurs in response to cervical stimulation by estrogen and progesterone. The fluid is whitish because of the presence of many exfoliated vaginal epithelial cells caused by the hyperplasia of normal pregnancy. This vaginal discharge is never pruritic or blood stained. Because of the progesterone effect, ferning usually does not occur in the dried cervical mucus smear as it would in a smear of amniotic fluid. Instead a beaded or cellular crystallizing pattern is seen in the dried mucus (Cunningham et al., 2010). The mucus fills the endocervical canal, resulting in the formation of the mucous plug (operculum) (Fig. 13-7). The operculum acts as a barrier against bacterial invasion during pregnancy.
During pregnancy the pH of vaginal secretions is more acidic than normal (ranging from approximately 3.5 to 6 [normal 4 to 5]) because of increased production of lactic acid (Cunningham et al., 2010). Although this acidic environment provides more protection from some organisms, the pregnant woman is more vulnerable to other infections, especially yeast infections because the glycogen-rich environment is more susceptible to Candida albicans (Duff, Sweet, & Edwards, 2009).
The increased vascularity of the vagina and other pelvic viscera results in a marked increase in sensitivity. The increased sensitivity may lead to a high degree of sexual interest and arousal, especially during the second trimester of pregnancy. The increased congestion plus the relaxed walls of the blood vessels and the heavy uterus may result in edema and varicosities of the vulva. The edema and varicosities usually resolve during the postpartum period.
External structures of the perineum are enlarged during pregnancy because of an increase in vasculature, hypertrophy of the perineal body, and deposition of fat (Fig. 13-8). The labia majora of the nullipara approximate and obscure the vaginal introitus; those of the parous woman separate and gape after childbirth and perineal or vaginal injury. Figure 13-2 compares the perineum of the nullipara and the multipara.
Breasts
Fullness, heightened sensitivity, tingling, and heaviness of the breasts occur in the early weeks of gestation in response to increased levels of estrogen and progesterone. Breast sensitivity varies from mild tingling to sharp pain. Nipples and areolae become more pigmented, secondary pinkish areolae develop, extending beyond the primary areolae, and nipples become more erectile. Hypertrophy of the sebaceous (oil) glands embedded in the primary areolae, called Montgomery tubercles (see Fig. 4-6), may be seen around the nipples. These sebaceous glands may have a protective role in that they keep the nipples lubricated for breastfeeding.
The richer blood supply causes the vessels beneath the skin to dilate. Once barely noticeable, the blood vessels become visible, often appearing in an intertwining blue network beneath the surface of the skin. Venous congestion in the breasts is more obvious in primigravidas. Striae gravidarum may appear at the outer aspects of the breasts.
During the second and third trimesters, growth of the mammary glands accounts for the progressive breast enlargement. The high levels of luteal and placental hormones in pregnancy promote proliferation of the lactiferous ducts and lobule-alveolar tissue, so that palpation of the breasts reveals a generalized, coarse nodularity. Glandular tissue displaces connective tissue, and as a result, the tissue becomes softer and looser.
Although development of the mammary glands is functionally complete by midpregnancy, lactation is inhibited until a decrease in estrogen level occurs after the birth. A thin, clear, viscous secretory material (precolostrum) can be found in the acini cells by the third month of gestation. Colostrum, the creamy white-to-yellowish-to-orange premilk fluid, may be expressed from the nipples as early as 16 weeks of gestation (Blackburn, 2007). See Chapter 25 for discussion of lactation.
General Body Systems
Maternal adjustments to pregnancy involve extensive changes in the cardiovascular system, both anatomic and physiologic. Cardiovascular adaptations protect the woman’s normal physiologic functioning, meet the metabolic demands pregnancy imposes on her body, and provide for fetal developmental and growth needs.
Slight cardiac hypertrophy (enlargement) is probably secondary to the increased blood volume and cardiac output that occurs. The heart returns to its normal size after childbirth. As the diaphragm is displaced upward by the enlarging uterus, the heart is elevated upward and rotated forward to the left (Fig. 13-9). The apical impulse, a point of maximal intensity (PMI), is shifted upward and laterally about 1 to 1.5 cm. The degree of shift depends on the duration of pregnancy and the size and position of the uterus.
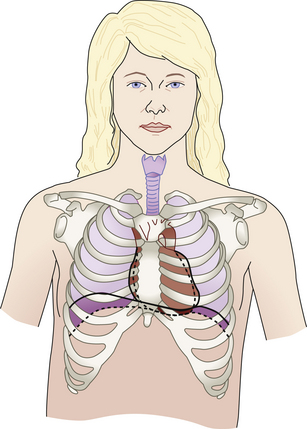
FIG. 13-9 Changes in position of heart, lungs, and thoracic cage in pregnancy. Broken line, nonpregnant; solid line, change that occurs in pregnancy.
The changes in heart size and position and increases in blood volume and cardiac output contribute to auscultatory changes common in pregnancy. There is more audible splitting of S1 and S2, and S3 may be readily heard after 20 weeks of gestation. In addition, systolic and diastolic murmurs may be heard over the pulmonic area in some women. These are transient and disappear shortly after the woman gives birth (Cunningham et al., 2010).
Between 14 and 20 weeks of gestation, the pulse increases about 10 to 15 beats/min, which then persists to term. Palpitations may occur. In twin gestations the maternal heart rate increases significantly in the third trimester (Blackburn, 2007).
The cardiac rhythm may be disturbed. The pregnant woman may experience sinus arrhythmia, premature atrial contractions, and premature ventricular systole. In the healthy woman with no underlying heart disease, no therapy is needed; however, women with preexisting heart disease will need close medical and obstetric supervision during pregnancy (see Chapter 30).
Blood Pressure: Arterial blood pressure (brachial artery) is affected by age, activity level, presence of health problems, and circadian rhythm. Other factors include use of alcohol, smoking, and pain. Additional factors must be considered during pregnancy. These factors include maternal anxiety, maternal position, and size and type of blood pressure apparatus (Pickering, Hall, Appel, Falkner, Graves, Hill, et al., 2005).
Maternal anxiety can elevate readings. If an elevated reading is found, the woman is given time to rest, and the reading is repeated.
Maternal position affects readings. Brachial blood pressure is higher when the woman is sitting than when she is lying in the lateral recumbent position. The position of the arm can also make a difference in the measurement. If the arm is above the heart, the reading will be lower than the accurate reading; if held below the heart, the reading will be higher. Therefore at each prenatal visit the reading should be obtained in the same arm and with the woman in the same position with her back and arm supported and with her upper arm at the level of the right atrium (Monga, 2009; Pickering et al., 2005; Sibai, 2007). The position and arm used should be recorded along with the reading (Box 13-1).
The proper-size cuff is absolutely necessary for accurate readings. Too small a cuff yields a false high reading; too large a cuff yields a false low reading (Pickering et al., 2005).
Caution also should be used when comparing auscultatory and oscillatory blood pressure readings, because discrepancies can occur. Automated monitors may give inaccurate readings in women with hypertensive conditions (Gordon, 2007).
Systolic blood pressure usually remains the same as the prepregnancy level but may decrease slightly as pregnancy advances. Diastolic blood pressure begins to decrease in the first trimester, continues to drop until 24 to 32 weeks, then gradually increases and returns to prepregnancy levels by term (Blackburn, 2007).
Calculating the mean arterial pressure (MAP) (mean of the blood pressure in the arterial circulation) can increase the diagnostic value of the findings. Normal MAP readings in the nonpregnant woman are 86.4 mm Hg ± 7.5 mm Hg. MAP readings for a pregnant woman are slightly higher (Gordon, 2007). One way to calculate MAP is illustrated in Box 13-2.
Some degree of compression of the vena cava occurs in all women who lie flat on their backs during the second half of pregnancy (see Fig. 19-5). Some women experience a decrease in their systolic blood pressure of more than 30 mm Hg. After 4 to 5 minutes a reflex bradycardia is noted, cardiac output is reduced by half, and the woman feels faint. This condition is referred to as supine hypotensive syndrome (Cunningham et al., 2010).
Compression of the iliac veins and inferior vena cava by the uterus causes increased venous pressure and reduced blood flow in the legs (except when the woman is in the lateral position). These alterations contribute to the dependent edema, varicose veins in the legs and vulva, and hemorrhoids that develop in the latter part of term pregnancy (Fig. 13-10).
Blood Volume and Composition: The degree of blood volume expansion varies considerably. Blood volume increases by approximately 1500 ml, or 40% to 45% above nonpregnancy levels (Cunningham et al., 2010). This increase consists of 1000 ml plasma plus 450 ml red blood cells (RBCs). The blood volume starts to increase at about the tenth to twelfth week, peaks at about the thirty-second to thirty-fourth week, and then decreases slightly at the fortieth week. The increase in volume of a multiple gestation is greater than that for a pregnancy with a single fetus (Blackburn, 2007). Increased volume is a protective mechanism. It is essential for meeting the blood volume needs of the hypertrophied vascular system of the enlarged uterus, for adequately hydrating fetal and maternal tissues when the woman assumes an erect or supine position, and for providing a fluid reserve to compensate for blood loss during birth and the puerperium. Peripheral vasodilation maintains a normal blood pressure despite the increased blood volume in pregnancy.
During pregnancy there is an accelerated production of RBCs (normal, 4.2 to 5.4 million/mm3). The percentage of increase depends on the amount of iron available. The RBC mass increases by about 20% to 30% (Blackburn, 2007).
Because the plasma increase exceeds the increase in RBC production, a decrease occurs in normal hemoglobin values (12 to 16 g/dl blood [non-pregnant]) and hematocrit values (37% to 47% [non-pregnant]). This state of hemodilution is termed physiologic anemia. The decrease is more noticeable during the second trimester than at other times, when rapid expansion of blood volume takes place faster than RBC production. A hemoglobin value that drops below 11 g/dl should be considered abnormal and often is due to iron deficiency anemia (Samuels, 2007).
The total white cell count increases during the second trimester and peaks during the third trimester. This increase is primarily in the granulocytes; the lymphocyte count stays approximately the same throughout pregnancy. Table 13-3 lists the normal laboratory values during pregnancy.
TABLE 13-3
LABORATORY VALUES FOR PREGNANT AND NONPREGNANT WOMEN
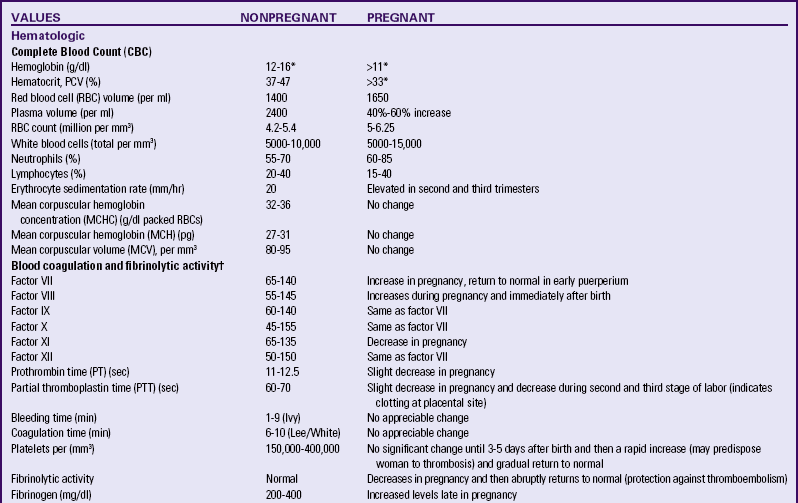
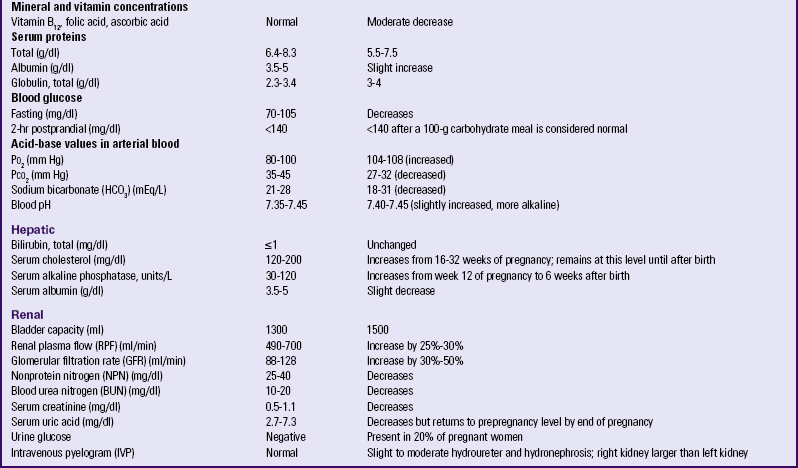
ng, Nanogram; pg, picogram; PCV, packed cell volume.
∗At sea level. Permanent residents of higher altitudes (e.g., Denver) require higher levels of hemoglobin.
†Pregnancy represents a hypercoagulable state.
Source: Blackburn, S. (2007). Maternal, fetal, & neonatal physiology: A clinical perspective (3rd ed.). St. Louis: Saunders; Gordon, M. (2007). Maternal physiology. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone; Pagana, K., & Pagana, T. (2009). Mosby’s diagnostic and laboratory test reference (9th ed.). St. Louis: Mosby.
Cardiac Output: Cardiac output increases from 30% to 50% over the nonpregnant rate by the thirty-second week of pregnancy; it declines to about a 20% increase at 40 weeks of gestation. This elevated cardiac output is caused by an increase in stroke volume and heart rate and occurs in response to increased tissue demands for oxygen (Monga, 2009). Cardiac output in late pregnancy is appreciably higher when the woman is in the lateral recumbent position than when she is supine. In the supine position the large, heavy uterus often impedes venous return to the heart and affects blood pressure. Cardiac output increases with any exertion, such as labor and birth. Table 13-4 summarizes cardiovascular changes in pregnancy.
TABLE 13-4
CARDIOVASCULAR CHANGES IN PREGNANCY
| Heart rate | Increases 10-15 beats/min |
| Blood pressure | Systolic: slight or no decrease from prepregnancy levels |
| Diastolic: slight decrease to midpregnancy (24-32 weeks) and gradually returns to prepregnancy levels by the end of pregnancy | |
| Blood volume | Increases by 1500 ml or 40%-50% above prepregnancy level |
| Red blood cell mass | Increases 18% |
| Hemoglobin | Decreases |
| Hematocrit | Decreases |
| White blood cell count | Increases in second and third trimesters |
| Cardiac output | Increases 30%-50% |
Source: Gordon, M. (2007). Maternal physiology. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone.
Circulation and Coagulation Times: The circulation time decreases slightly by week 32. It returns to near normal close to term. There is a greater tendency for blood to coagulate (clot) during pregnancy because of increases in various clotting factors (factors VII, VIII, IX, X, and fibrinogen). This, combined with the fact that fibrinolytic activity (the splitting up or the dissolving of a clot) is depressed during pregnancy and the postpartum period, provides a protective function to decrease the chance of bleeding but also makes the woman more vulnerable to thrombosis, especially after cesarean birth.
Respiratory System
Structural and ventilatory adaptations occur during pregnancy to provide for maternal and fetal needs. Maternal oxygen requirements increase in response to the acceleration in the metabolic rate and the need to add to the tissue mass in the uterus and breasts. In addition, the fetus requires oxygen and a way to eliminate carbon dioxide.
Elevated levels of estrogen cause the ligaments of the rib cage to relax, permitting increased chest expansion (see Fig. 13-9). The transverse diameter of the thoracic cage increases by about 2 cm, and the circumference increases by 6 cm (Cunningham et al., 2010). The costal angle increases, and the lower rib cage appears to flare out. The chest may not return to its prepregnant state after birth (Seidel, Ball, Dains, Flynn, Solomon, & Stewart, 2011).
The diaphragm is displaced by as much as 4 cm during pregnancy. As pregnancy advances, thoracic (costal) breathing replaces abdominal breathing, and it becomes less possible for the diaphragm to descend with inspiration. Thoracic breathing is accomplished primarily by the diaphragm rather than by the costal muscles (Blackburn, 2007).
The upper respiratory tract becomes more vascular in response to elevated levels of estrogen. As the capillaries become engorged, edema and hyperemia develop within the nose, pharynx, larynx, trachea, and bronchi. This congestion within the tissues of the respiratory tract gives rise to several conditions commonly seen during pregnancy, including nasal and sinus stuffiness, epistaxis (nosebleed), changes in the voice, and a marked inflammatory response that can develop into a mild upper respiratory infection (Gordon, 2007).
Increased vascularity of the upper respiratory tract also can cause the tympanic membranes and eustachian tubes to swell, giving rise to symptoms of impaired hearing, earaches, or a sense of fullness in the ears.
Pulmonary Function: Respiratory changes in pregnancy are related to the elevation of the diaphragm and to chest wall changes. Changes in the respiratory center result in a lowered threshold for carbon dioxide. The actions of progesterone and estrogen are presumed responsible for the increased sensitivity of the respiratory center to carbon dioxide. (Table 13-5 summarizes respiratory changes in pregnancy.) Although pulmonary function is not impaired by pregnancy, diseases of the respiratory tract may be more serious during this time (Cunningham et al., 2010). One important factor responsible for this circumstance may be the increased oxygen requirement
TABLE 13-5
RESPIRATORY CHANGES IN PREGNANCY
| Respiratory rate | Unchanged or slightly increased |
| Tidal volume | Increased 30%-40% |
| Vital capacity | Unchanged |
| Inspiratory capacity | Increased |
| Expiratory volume | Decreased |
| Total lung capacity | Unchanged to slightly decreased |
| Oxygen consumption | Increased 20%-40% |
Source: Gordon, M. (2007). Maternal physiology. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone.
Basal Metabolic Rate: The basal metabolic rate (BMR) increases during pregnancy. This increase varies considerably depending on the prepregnancy nutritional status of the woman and fetal growth (Blackburn, 2007). The BMR returns to nonpregnant levels by 5 to 6 days after birth. The elevation in BMR during pregnancy reflects increased oxygen demands of the uterine-placental-fetal unit and greater oxygen consumption because of increased maternal cardiac work. Peripheral vasodilation and acceleration of sweat gland activity help dissipate the excess heat resulting from the increased BMR during pregnancy. Pregnant women experience heat intolerance, which is annoying to some women. Lassitude and fatigability after only slight exertion are experienced by many women in early pregnancy. These feelings, along with a greater need for sleep, may persist and may be caused in part by the increased metabolic activity.
Acid-Base Balance: By about the tenth week of pregnancy, there is a decrease of about 5 mm Hg in the partial pressure of carbon dioxide (Pco2). Progesterone may be responsible for increasing the sensitivity of the respiratory center receptors, so that tidal volume is increased, Pco2 decreases, the base excess (HCO3 or bicarbonate) decreases, and pH increases slightly. These alterations in acid-base balance indicate that pregnancy is a state of compensatory respiratory alkalosis (Gordon, 2007). These changes also facilitate the transport of CO2 from the fetus and O2 release from the mother to the fetus (see Table 13-3).
Renal System
The kidneys are responsible for maintaining electrolyte and acid-base balance, regulating extracellular fluid volume, excreting waste products, and conserving essential nutrients.
Anatomic Changes: Changes in renal structure during pregnancy result from hormonal activity (estrogen and progesterone), pressure from an enlarging uterus, and an increase in blood volume. As early as the tenth week of pregnancy, the renal pelves and the ureters dilate. Dilation of the ureters is more pronounced above the pelvic brim, in part because they are compressed between the uterus and the pelvic brim. In most women, the ureters below the pelvic brim are of normal size. The smooth-muscle walls of the ureters undergo hyperplasia, hypertrophy, and muscle tone relaxation. The ureters elongate, become tortuous, and form single or double curves. In the latter part of pregnancy, the renal pelvis and ureter are dilated more on the right side than on the left because the heavy uterus is displaced to the right by the sigmoid colon.
Because of these changes, a larger volume of urine is held in the pelves and ureters, and urine flow rate is slowed. The resulting urinary stasis or stagnation has the following consequences:
• A lag occurs between the time urine is formed and when it reaches the bladder. Therefore clearance test results may reflect substances contained in glomerular filtrate several hours before.
• Stagnated urine is an excellent medium for the growth of microorganisms. In addition, the urine of pregnant women contains more nutrients, including glucose, thereby increasing the pH (making the urine more alkaline). This makes pregnant women more susceptible to urinary tract infection.
Bladder irritability, nocturia, and urinary frequency and urgency (without dysuria) are commonly reported in early pregnancy. Near term, bladder symptoms may return, especially after lightening occurs.
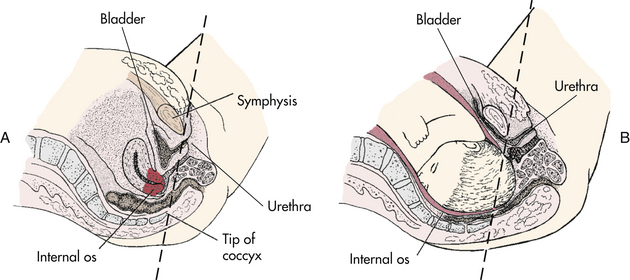
Urinary frequency results initially from increased bladder sensitivity and later from compression of the bladder (see Fig. 13-8). In the second trimester, the bladder is pulled up out of the true pelvis into the abdomen. The urethra lengthens to 7.5 cm as the bladder is displaced upward. The pelvic congestion that occurs in pregnancy is reflected in hyperemia of the bladder and urethra. This increased vascularity causes the bladder mucosa to be traumatized and bleed easily. Bladder tone may decrease, which increases the bladder capacity to 1500 ml. At the same time, the bladder is compressed by the enlarging uterus, resulting in the urge to void even if the bladder contains only a small amount of urine.
Functional Changes: In normal pregnancy, renal function is altered considerably. Glomerular filtration rate (GFR) and renal plasma flow (RPF) increase early in pregnancy (Monga, 2009). These changes are caused by pregnancy hormones, an increase in blood volume, the woman’s posture, physical activity, and nutritional intake. The woman’s kidneys must manage the increased metabolic and circulatory demands of the maternal body and the excretion of fetal waste products. Renal function is most efficient when the woman lies in the lateral recumbent position and least efficient when the woman assumes a supine position. A side-lying position increases renal perfusion, which increases urinary output and decreases edema. When the pregnant woman is lying supine, the heavy uterus compresses the vena cava and the aorta, and cardiac output decreases. As a result, blood flow to the brain and heart is continued at the expense of other organs, including the kidneys and uterus.
Fluid and Electrolyte Balance: Selective renal tubular reabsorption maintains sodium and water balance regardless of changes in dietary intake and losses through sweat, vomitus, or diarrhea. From 500 to 900 mEq of sodium is normally retained during pregnancy to meet fetal needs. To prevent excessive sodium depletion, the maternal kidneys undergo a significant adaptation by increasing tubular reabsorption. Because of the need for increased maternal intravascular and extracellular fluid volume, additional sodium is needed to expand fluid volume and to maintain an isotonic state. As efficient as the renal system is, it can be overstressed by excessive dietary sodium intake or restriction or by use of diuretics. Severe hypovolemia and reduced placental perfusion are two consequences of using diuretics during pregnancy.
The capacity of the kidneys to excrete water during the early weeks of pregnancy is more efficient than it is later in pregnancy. As a result, some women feel thirsty in early pregnancy because of the greater amount of water loss. The pooling of fluid in the legs in the latter part of pregnancy decreases renal blood flow and GFR. This pooling of blood in the lower legs is sometimes referred to as physiologic edema or dependent edema and requires no treatment. The normal diuretic response to the water load is triggered when the woman lies down, preferably on her side, and the pooled fluid reenters general circulation.
Normally the kidney reabsorbs almost all of the glucose and other nutrients from the plasma filtrate. In pregnant women, however, tubular reabsorption of glucose is impaired, so that glucosuria occurs at varying times and to varying degrees. Normal values range from 0 to 20 mg/dl, meaning that during any day, the urine is sometimes positive and sometimes negative for glucose. In nonpregnant women, blood glucose levels must be at 160 to 180 mg/dl before glucose is “spilled” into the urine (not reabsorbed). During pregnancy, glucosuria (glycosuria) occurs when maternal glucose levels are lower than 160 mg/dl. Why glucose, as well as other nutrients such as amino acids, is wasted during pregnancy is not understood, nor has the exact mechanism been discovered. Although glucosuria may be found in normal pregnancies (2+ levels may be seen with increased anxiety states), the possibility of pregestational or gestational diabetes mellitus must be kept in mind.
Proteinuria usually does not occur in normal pregnancy except during labor or after birth (Cunningham et al., 2010). However, the increased amount of amino acids that must be filtered may exceed the capacity of the renal tubules to absorb it; thus small amounts of protein are then lost in the urine. The amount of protein excreted is not an indication of the severity of renal disease, nor does an increase in protein excretion in a pregnant woman with known renal disease necessarily indicate a progression in her disease. However, a pregnant woman with hypertension and proteinuria must be carefully evaluated because she may be at greater risk for an adverse pregnancy outcome (Gordon, 2007) (see Table 13-3).
Integumentary System
Alterations in hormonal balance and mechanical stretching are responsible for several changes in the integumentary system during pregnancy. Hyperpigmentation is stimulated by the anterior pituitary hormone melanotropin, which is increased during pregnancy. Darkening of the nipples, the areolae, the axillae, and the vulva occurs about the sixteenth week of gestation. Facial melasma, also called chloasma or mask of pregnancy, is a blotchy, brownish hyperpigmentation of the skin over the cheeks, nose, and forehead, especially in dark-complexioned pregnant women. Chloasma appears in 50% to 70% of pregnant women, beginning after the sixteenth week and increasing gradually until term. The sun intensifies this pigmentation in susceptible women. Chloasma caused by normal pregnancy usually fades after birth.
The linea nigra (Fig. 13-11) is a pigmented line extending from the symphysis pubis to the top of the fundus in the midline; this line is known as the linea alba before hormone-induced pigmentation. In primigravidas the extension of the linea nigra, beginning in the third month, keeps pace with the rising height of the fundus; in multigravidas, the entire line often appears earlier than the third month. Not all pregnant women develop linea nigra, and some women notice hair growth along the line with or without the change in pigmentation.
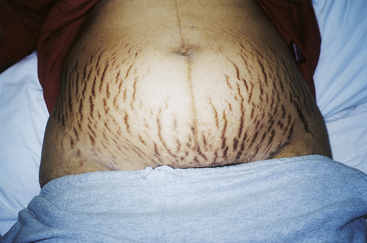
FIG. 13-11 Striae gravidarum and linea nigra in a dark-skinned woman. (Courtesy Shannon Perry, Phoenix, AZ.)
Striae gravidarum, or stretch marks (seen over lower abdomen in Fig. 13-11), which appear in 50% to 90% of pregnant women during the second half of pregnancy, may be caused by action of adrenocorticosteroids. Striae reflect separation within the underlying connective (collagen) tissue of the skin. These slightly depressed streaks tend to occur over areas of maximal stretch (i.e., abdomen, thighs, and breasts). The stretching sometimes causes a sensation that resembles itching. The tendency to develop striae may be familial. After birth they usually fade, although they never disappear completely. Color of striae varies depending on the pregnant woman’s skin color. The striae appear pinkish on a woman with light skin and are lighter than surrounding skin in dark-skinned women. In the multipara, in addition to the striae of the present pregnancy, glistening silvery lines (in light-skinned women) or purplish lines (in dark-skinned women) are commonly seen. These represent the scars of striae from previous pregnancies.
Angiomas are commonly referred to as vascular spiders. They are tiny, star-shaped or branched, slightly raised and pulsating end-arterioles usually found on the neck, thorax, face, and arms. They occur as a result of elevated levels of circulating estrogen. The spiders are bluish in color and do not blanch with pressure. Vascular spiders appear during the second to the fifth month of pregnancy in almost 65% of Caucasian women and 10% of African-American women. The spiders usually disappear after birth (Blackburn, 2007).
Pinkish-red, diffuse mottling or well-defined blotches are seen over the palmar surfaces of the hands in about 60% of Caucasian women and 35% of African-American women during pregnancy (Blackburn, 2007). These color changes, called palmar erythema, are related primarily to increased estrogen levels.
Some dermatologic conditions have been identified as unique to pregnancy or as having an increased incidence during pregnancy. Mild pruritus (pruritus gravidarum) is a relatively common dermatologic symptom in pregnancy. The problem usually resolves in the postpartum period (Papoutsis & Kroumpouzos, 2007). Systemic diseases can also cause pruritus, but these causes are uncommon or rare (Cappell, 2007). Preexisting skin diseases may complicate pregnancy or be improved. (See Chapter 30 for further discussion.)
Gum hypertrophy may occur. An epulis (gingival granuloma gravidarum) is a red, raised nodule on the gums that bleeds easily. This lesion may develop around the third month and usually continues to enlarge as pregnancy progresses. It is usually managed by avoiding trauma to the gums (e.g., using a soft toothbrush). An epulis usually regresses spontaneously after birth.
Nail growth may be accelerated. Some women may notice thinning and softening of the nails. Oily skin and acne vulgaris may occur during pregnancy. For some women, the skin clears and looks radiant. Hirsutism, the excessive growth of hair or growth of hair in unusual places, is commonly reported. An increase in fine hair growth may occur but tends to disappear after pregnancy; however, growth of coarse or bristly hair does not usually disappear. The rate of scalp hair loss slows during pregnancy, while increased hair loss may be noted in the postpartum period.
Increased blood supply to the skin leads to increased perspiration. Women feel hotter during pregnancy, a condition possibly related to a progesterone-induced increase in body temperature and the increased BMR.
Musculoskeletal System
The gradually changing body and increasing weight of the pregnant woman cause noticeable alterations in her posture (Fig. 13-12) and the way she walks. The great abdominal distention that gives the pelvis a forward tilt, decreased abdominal muscle tone, and increased weight bearing require a realignment of the spinal curvature late in pregnancy. The woman’s center of gravity shifts forward. An increase in the normal lumbosacral curve (lordosis) develops, and a compensatory curvature in the cervicodorsal region (exaggerated anterior flexion of the head) develops to help her maintain her balance. Aching, numbness, and weakness of the upper extremities may result. Large breasts and a stoop-shouldered stance will further accentuate the lumbar and dorsal curves. Walking is more difficult, and the waddling gait of the pregnant woman, called “the proud walk of pregnancy” by Shakespeare, is commonly seen. The ligamentous and muscular structures of the middle and lower spine may be severely stressed. These and related changes often cause musculoskeletal discomfort, especially in older women or those with a back disorder or a faulty sense of balance.
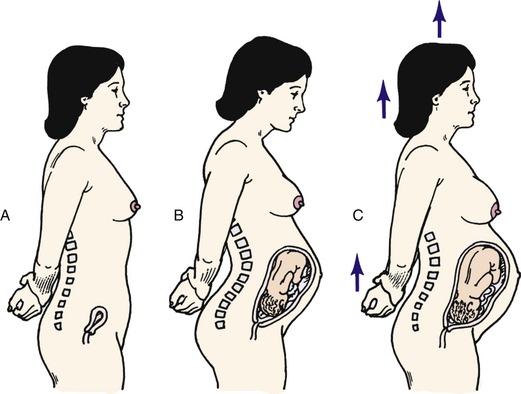
FIG. 13-12 Postural changes during pregnancy. A, Nonpregnant posture. B, Incorrect posture during pregnancy. C, Correct posture during pregnancy.
Slight relaxation and increased mobility of the pelvic joints are normal during pregnancy. These adaptations permit enlargement of pelvic dimensions to facilitate labor and birth. The degree of relaxation varies, but considerable separation of the symphysis pubis and the instability of the sacroiliac joints may cause pain and difficulty in walking. Obesity and multifetal pregnancy tend to increase the pelvic instability. Peripheral joint laxity also increases as pregnancy progresses, but the cause is not known (Murray & Hassall, 2009).
The muscles of the abdominal wall stretch and ultimately lose some tone. During the third trimester, the rectus abdominis muscles may separate (Fig. 13-13), allowing abdominal contents to protrude at the midline. The umbilicus flattens or protrudes. After birth, the muscles gradually regain tone; however, separation of the muscles (diastasis recti abdominis) may persist.
Neurologic System
Little is known regarding specific alterations in function of the neurologic system during pregnancy, aside from hypothalamic-pituitary neurohormonal changes. Specific physiologic alterations resulting from pregnancy may cause the following neurologic or neuromuscular symptoms:
• Compression of pelvic nerves or vascular stasis caused by enlargement of the uterus may result in sensory changes in the legs.
• Dorsolumbar lordosis may cause pain because of traction on nerves or compression of nerve roots.
• Edema involving the peripheral nerves may result in carpal tunnel syndrome during the last trimester (Samuels & Niebyl, 2007). The syndrome is characterized by paresthesia (abnormal sensation such as burning or tingling) and pain in the hand, radiating to the elbow. The sensations are caused by edema that compresses the median nerve beneath the carpal ligament of the wrist. Smoking and alcohol consumption can impair the microcirculation and may worsen the symptoms. The dominant hand is usually affected most, although as many as 80% of women experience symptoms in both hands. Symptoms usually regress after pregnancy. In some cases, surgical treatment may be necessary (Samuels & Niebyl).
• Acroesthesia (numbness and tingling of the hands) is caused by the stoop-shouldered stance (see Fig. 13-12, B) assumed by some women during pregnancy. The condition is associated with traction on segments of the brachial plexus.
• Tension headache is common when anxiety or uncertainty complicates pregnancy. However, vision problems, sinusitis, or migraine also may be responsible for headaches.
• “Light-headedness,” faintness, and even syncope (fainting) are common during early pregnancy. Vasomotor instability, postural hypotension, or hypoglycemia may be responsible.
• Hypocalcemia can cause neuromuscular problems such as muscle cramps or tetany.
Gastrointestinal System
Appetite: During pregnancy, the woman’s appetite and food intake fluctuate. Early in pregnancy, some women have nausea with or without vomiting (morning sickness), possibly in response to increasing levels of hCG and altered carbohydrate metabolism (Gordon, 2007). Morning sickness or nausea and vomiting of pregnancy (NVP) appears at about 4 to 6 weeks of gestation and usually subsides by the end of the third month (first trimester) of pregnancy (see Chapter 15). Severity varies from mild distaste for certain foods to more severe vomiting. The condition may be triggered by the sight or odor of various foods. By the end of the second trimester, the appetite increases in response to increasing metabolic needs. Rarely does NVP have harmful effects on the embryo, fetus, or the woman. Whenever the vomiting is severe or persists beyond the first trimester, or when it is accompanied by fever, pain, or weight loss, further evaluation is necessary, and medical intervention is likely (see Chapter 29).
Women also may have changes in their sense of taste, leading to cravings and changes in dietary intake. Some women have nonfood cravings (called pica), such as for ice, clay, and laundry starch. Usually the subjects of these cravings, if consumed in moderation, are not harmful to the pregnancy if the woman has adequate nutrition with appropriate weight gain (Gordon, 2007). See Chapter 14 for a discussion of nutrition in pregnancy.
Mouth: The gums become hyperemic, spongy, and swollen during pregnancy. They tend to bleed easily because the increasing levels of estrogen cause selective increased vascularity and connective tissue proliferation (a nonspecific gingivitis). Epulis (discussed in the section on the integumentary system) may develop at the gumline. Some pregnant women complain of ptyalism (excessive salivation), which may be caused by the decrease in unconscious swallowing by the woman when nauseated or from stimulation of salivary glands by eating starch (Cunningham et al., 2010).
Esophagus, Stomach, and Intestines: Herniation of the upper portion of the stomach (hiatal hernia) occurs after the seventh or eighth month of pregnancy in about 15% to 20% of pregnant women. This condition results from upward displacement of the stomach, which causes the hiatus of the diaphragm to widen. It occurs more often in multiparas and older or obese women.
Increased estrogen production causes decreased secretion of hydrochloric acid; therefore peptic ulcer formation or flare-up of existing peptic ulcers is uncommon during pregnancy and symptoms may improve (Gordon, 2007).
Increased progesterone production causes decreased tone and motility of smooth muscles, resulting in esophageal regurgitation, slower emptying time of the stomach, and reverse peristalsis. As a result, the woman may experience “acid indigestion” or heartburn (pyrosis) beginning as early as the first trimester and intensifying through the third trimester.
Iron is absorbed more readily in the small intestine in response to increased needs during pregnancy. Even when the woman is deficient in iron, it will continue to be absorbed in sufficient amounts for the fetus to have a normal hemoglobin level.
Increased progesterone (causing loss of muscle tone and decreased peristalsis) results in an increase in water absorption from the colon and may cause constipation. Constipation also may result from hypoperistalsis (sluggishness of the bowel), food choices, lack of fluids, iron supplementation, decreased activity level, abdominal distention by the pregnant uterus, and displacement and compression of the intestines. If the pregnant woman has hemorrhoids (see Fig. 13-10) and is constipated, the hemorrhoids may become everted or may bleed during straining at stool.
Gallbladder and Liver: The gallbladder is quite often distended because of its decreased muscle tone during pregnancy. Increased emptying time and thickening of bile caused by prolonged retention are typical changes. These features, together with slight hypercholesterolemia from increased progesterone levels, may account for the development of gallstones during pregnancy.
Hepatic function is difficult to appraise during pregnancy; however, only minor changes in liver function develop. Occasionally, intrahepatic cholestasis (retention and accumulation of bile in the liver, caused by factors within the liver) occurs late in pregnancy in response to placental steroids and may result in pruritus gravidarum (severe itching) with or without jaundice (Cappell, 2007) (see Chapter 30).
Abdominal Discomfort: Intraabdominal alterations that can cause discomfort include pelvic heaviness or pressure, round ligament tension, flatulence, distention and bowel cramping, and uterine contractions. In addition to displacement of intestines, pressure from the expanding uterus causes an increase in venous pressure in the pelvic organs. Although most abdominal discomfort is a consequence of normal maternal alterations, the health care provider must be constantly alert to the possibility of disorders such as bowel obstruction or an inflammatory process.
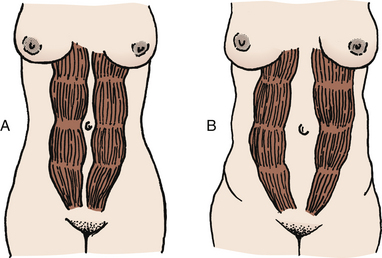
Appendicitis may be difficult to diagnose in pregnancy because the appendix is displaced upward and laterally, high and to the right, away from McBurney’s point (Fig. 13-14).
Endocrine System
Profound endocrine changes are essential for pregnancy maintenance, normal fetal growth, and postpartum recovery.
Pituitary and Placental Hormones: During pregnancy, the elevated levels of estrogen and progesterone (produced first by the corpus luteum in the ovary until about 14 weeks of gestation and then by the placenta) suppress secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by the anterior pituitary. The maturation of a follicle and ovulation do not occur. Although the majority of women have amenorrhea (absence of menses), at least 20% have some slight, painless spotting during early gestation. Implantation bleeding and bleeding after intercourse related to cervical friability can occur. Most of the women experiencing slight gestational bleeding continue to term and have normal infants; however, all instances of bleeding should be reported and evaluated.
After implantation, the fertilized ovum and the chorionic villi produce hCG, which maintains the production by the corpus luteum of estrogen and progesterone until the placenta takes over production (Burton et al., 2007).
Progesterone is essential for maintaining pregnancy by relaxing smooth muscles, resulting in decreased uterine contractility and prevention of miscarriage. Progesterone and estrogen cause fat to deposit in subcutaneous tissues over the maternal abdomen, back, and upper thighs. This fat serves as an energy reserve for both pregnancy and lactation. Estrogen also promotes the enlargement of the genitals, the uterus, and the breasts and increases vascularity, causing vasodilation. Estrogen causes relaxation of pelvic ligaments and joints. It also alters metabolism of nutrients by interfering with folic acid metabolism, increasing the level of total body proteins, and promoting retention of sodium and water by kidney tubules. Estrogen may decrease secretion of hydrochloric acid and pepsin, which may be responsible for digestive upsets such as nausea.
Serum prolactin produced by the anterior pituitary begins to increase early in the first trimester and increases progressively to term. It is responsible for initial lactation; however, the high levels of estrogen and progesterone inhibit lactation by blocking the binding of prolactin to breast tissue until after the birth (Gordon, 2007).
Oxytocin is produced by the posterior pituitary in increasing amounts as the fetus matures. This hormone can stimulate uterine contractions during pregnancy, but high levels of progesterone prevent contractions until near term. Oxytocin also stimulates the let-down or milk-ejection reflex after the birth in response to the infant’s sucking at the mother’s breast and during sex play if the mother’s nipples are stimulated .
Human chorionic somatomammotropin (hCS), previously called human placental lactogen, is produced by the placenta and has been suggested to act as a growth hormone and contribute to breast development. It also may decrease the maternal metabolism of glucose and increase the amount of fatty acids for metabolic needs; however, its function is poorly understood (Burton et al., 2007).
Thyroid Gland: During pregnancy, gland activity and hormone production increase. The increased activity is reflected in a moderate enlargement of the thyroid gland caused by hyperplasia of the glandular tissue and increased vascularity (Cunningham et al., 2010). Thyroxine-binding globulin (TBG) increases as a result of increased estrogen levels. This increase begins at about 20 weeks of gestation. The level of total (free and bound) thyroxine (T4) increases between 6 and 9 weeks of gestation and plateaus at 18 weeks of gestation. Free thyroxine (T4) and free triiodothyronine (T3) return to nonpregnant levels after the first trimester. Despite these changes in hormone production, hyperthyroidism usually does not develop in the pregnant woman (Cunningham et al.).
Parathyroid Gland: Parathyroid hormone controls calcium and magnesium metabolism. Pregnancy induces a slight hyperparathyroidism, a reflection of increased fetal requirements for calcium and vitamin D. The peak level of parathyroid hormone occurs between 15 and 35 weeks of gestation, when the needs for growth of the fetal skeleton are greatest. Levels return to normal after birth.
Pancreas: The fetus requires significant amounts of glucose for its growth and development. To meet its need for fuel, the fetus not only depletes the store of maternal glucose but also decreases the mother’s ability to synthesize glucose by siphoning off her amino acids. Maternal blood glucose levels decrease. Maternal insulin does not cross the placenta to the fetus. As a result, in early pregnancy the pancreas decreases its production of insulin.
As pregnancy continues, the placenta grows and produces progressively greater amounts of hormones (i.e., hCS, estrogen, and progesterone). Cortisol production by the adrenals also increases. Estrogen, progesterone, hCS, and cortisol collectively decrease the mother’s ability to use insulin. Cortisol stimulates increased production of insulin but also increases the mother’s peripheral resistance to insulin (i.e., the tissues cannot use the insulin). Decreasing the mother’s ability to use her own insulin is a protective mechanism that ensures an ample supply of glucose for the needs of the fetoplacental unit. The result is an added demand for insulin by the mother that continues to increase at a steady rate until term. The normal beta cells of the islets of Langerhans in the pancreas can meet this demand for insulin.
Adrenal Glands: The adrenal glands change little during pregnancy. Secretion of aldosterone is increased, resulting in reabsorption of excess sodium from the renal tubules. Cortisol levels also are increased (Blackburn, 2007).
KEY POINTS
• The biochemical, physiologic, and anatomic adaptations that occur during pregnancy are profound and revert to the nonpregnant state after birth and lactation.
• Maternal adaptations are attributed to the hormones of pregnancy and to mechanical pressures exerted by the enlarging uterus and other tissues.
• ELISA testing, with monoclonal antibody technology, is the most popular method of pregnancy testing and is the basis for most over-the-counter home pregnancy tests.
• Presumptive, probable, and positive signs of pregnancy aid in the diagnosis of pregnancy; only positive signs (identification of a fetal heartbeat, verification of fetal movements, and visualization of the fetus) can establish the diagnosis of pregnancy.
• Adaptations to pregnancy protect the woman’s normal physiologic functioning, meet the metabolic demands pregnancy imposes, and provide for fetal development and growth needs.
• Although the pH of the pregnant woman’s vaginal secretions is more acidic, she is more vulnerable to some vaginal infections, especially yeast infections.
• Increased vascularity and sensitivity of the vagina and other pelvic viscera may lead to a high degree of sexual interest and arousal.
• Some adaptations to pregnancy result in discomforts such as fatigue, urinary frequency, nausea, and breast sensitivity.
• As pregnancy progresses, balance and coordination are affected by changes in the woman’s joints and her center of gravity.
![]() Audio Chapter Summaries Access an audio summary of these Key Points on
Audio Chapter Summaries Access an audio summary of these Key Points on ![]()