INHALATION ANESTHETICS
As mentioned in Chapter 1, the birth of modern anesthesia can be traced back to the mid 1800s, when inhalant anesthetics were first used clinically (diethyl ether in 1842, nitrous oxide in 1844, and chloroform in 1847). Before that time surgery was performed under extreme and less than ideal conditions. Surgeons often had to work at breakneck speed while the conscious patients were restrained by attendants. Needless to say, surgery at that time involved fear, risk, and pain. Indeed, the introduction of inhalant agents marked one of the most significant advances of medical science by giving surgeons the ability to perform surgery safely and humanely.
The first inhalant anesthetics are no longer used because they have been gradually replaced by the halogenated agents, which continue to be among the safest and most commonly used anesthetics. In fact, their use is so commonplace for such a wide variety of routine veterinary procedures that it is difficult to imagine caring for patients without them.
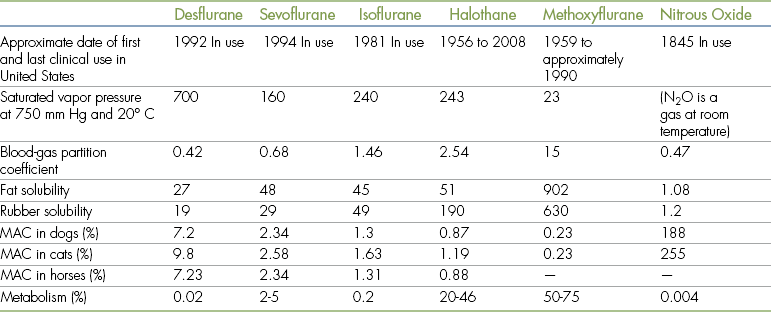
The inhalation anesthetics in common use at the present time are the halogenated compounds isoflurane and sevoflurane. Desflurane and nitrous oxide are occasionally used in some practices and academic and research settings. Enflurane is not used in veterinary patients owing to problematic adverse effects. Halothane has recently become unavailable in the United States, and methoxyflurane has not been available for two decades. However, as a result of many years of extensive use, methoxyflurane and halothane remain useful points of comparison for the currently used agents isoflurane and sevoflurane. Many other inhalation agents that were used in the past (including diethyl ether, chloroform, divinyl ether, and trichloroethylene) are now of historical interest only.
Diethyl Ether
Diethyl ether was for many years the most widely used anesthetic because of several desirable characteristics. Animals anesthetized with this agent maintained relatively stable cardiac output, heart rhythm, respirations, and blood pressure. Ether also produced good muscle relaxation and analgesia. Despite these advantages, ether had significant drawbacks that resulted in its eventual replacement by other agents. Ether was very irritating to tracheal and bronchial mucosa, resulting in increased salivation, mucous secretions, and increased risk of laryngospasm and airway blockage. Induction and recovery from ether anesthesia were often prolonged, and postoperative nausea and vomiting were common. In addition, ether is flammable and explosive and requires an explosion-proof refrigerator for safe storage. Devastating operating room fires sometimes resulted from the use of ether in conjunction with oxygen. Ether is of interest to the modern anesthetist because in the early 1900s Dr. Guedel described the classic stages and planes of anesthesia by observing physiological responses to this agent.
Halogenated Organic Compounds
Isoflurane and sevoflurane, the most commonly used inhalation agents in veterinary practice, are classified as halogenated organic compounds. Other agents in this class include desflurane, halothane, methoxyflurane, and enflurane. Halogenated agents are liquids at room temperature (although the boiling point of desflurane is near room temperature). They are stored inside the vaporizer of the anesthetic machine and evaporate in the oxygen that flows through the vaporizer, with the exception of desflurane, which requires a special injection-type vaporizer. The resulting mixture of oxygen and anesthetic is delivered to the patient through a breathing circuit (discussed in detail in Chapter 4).
Mode of Action and Pharmacology
The mechanism of action of halogenated anesthetics on the CNS is not fully understood, although it has been suggested that these anesthetics inhibit nerve cell function in the brain and spinal cord.
The uptake, distribution, and elimination of these agents are very different from those of injectable agents. A basic understanding of these differences is necessary if the agents are to be used effectively and safely. What follows is a summary of the movement of these drugs within the body.
Within the anesthetic machine, liquid anesthetic is vaporized, mixed with oxygen, and delivered to the patient by mask or endotracheal tube. The anesthetic travels via the air passages to the lungs, where it diffuses across the alveolar cell membranes and enters the bloodstream. The rate of diffusion is controlled by the concentration gradient between the alveolus and the bloodstream, as well as the lipid solubility of the drug. During the induction period, the concentration of the agent in the alveolus is high, and the concentration in the blood is low. This creates a steep concentration gradient, and diffusion of anesthetic from the alveolus into the blood is rapid during this period.
As with injectable agents, inhalation agents are carried to the body tissues in the blood. Consequently, tissues with greater blood flow (brain, heart, kidney) are more quickly saturated with anesthetic than tissues with lesser blood flow such as skeletal muscle and fat. Because of their relatively high lipid solubility, most inhalation agents readily leave the circulation and enter the brain, inducing anesthesia. The depth of anesthesia is determined by the partial pressure of the anesthetic agent in the brain. This in turn is related to the partial pressure of anesthetic in the blood and alveoli. Anesthesia is maintained as long as sufficient quantities of inhalation agent are delivered to the alveoli so that the blood, alveolar, and brain concentrations are maintained.
When the concentration of the inhalation agent administered is reduced or discontinued by adjustment of the anesthetic machine vaporizer, the amount of anesthetic in the alveolus is reduced. Because the blood level is still high, the concentration gradient now favors the diffusion of anesthetic from the blood into the alveoli. The blood levels of the anesthetic are quickly reduced, provided the animal continues to breathe and eliminate anesthetic from the alveoli. The anesthetist can hasten the elimination of anesthetic by periodically bagging the animal with 100% oxygen. This removes anesthetic from the alveoli and reestablishes a steep concentration gradient between the alveoli and the blood. As the concentration of the anesthetic in the blood falls, the agent leaves the brain and the patient wakes up.
Isoflurane, sevoflurane, and desflurane undergo minimal liver metabolism because they are eliminated from the body chiefly through the lungs. Some of the older halogenated compounds (in particular, methoxyflurane) have very high lipid solubility and accumulate in body fat. Unlike isoflurane, sevoflurane, and desflurane, older agents rely to a significant degree on liver metabolism and renal excretion for their complete elimination from the body.
Effects on Major Organ Systems
Although halogenated inhalation agents vary somewhat in their effects, the following characteristics are common to all (see Tables 3-1 to 3-4).
Adverse Effects
• Animals with head trauma or brain tumors may develop dangerously increased intracranial pressure when anesthetized with inhalation agents, especially if carbon dioxide levels in the blood are allowed to increase. However, the currently used halogenated anesthetics are considered safe for animals with a history of epilepsy.
Cardiovascular System
• Because inhalation anesthetics may decrease blood pressure, they have a potential to decrease renal blood flow. This can be clinically significant in animals with preexisting renal disease or in animals receiving nephrotoxic drugs such as gentamicin or nonsteroidal antiinflammatory drugs (NSAIDs) (see Chapter 7).
Respiratory System
• Hypoventilation is a possible adverse effect of all inhalation agents. Hypoventilation predisposes the animal to carbon dioxide retention and respiratory acidosis.
Despite this list of potential adverse effects, halogenated anesthetics are considered safe for most patients relative to other anesthetics. However, safety depends to a large degree on the care with which these agents are administered and the vigilance of the anesthetist in monitoring their effect on the patient.
Physical and Chemical Properties
Inhalant anesthetics differ considerably in their anesthetic effects, in part because of differences in their physical and chemical properties. The properties of chief importance to the anesthetist include vapor pressure, partition coefficient, minimum alveolar concentration (MAC), and rubber solubility. These agents also vary in their pharmacologic properties, including their effects on the cardiovascular, respiratory, and other vital systems. The physical properties and pharmacology of commonly used inhalation anesthetic agents are summarized in Table 3-6.
Vapor Pressure
When a liquid is in a closed container, some of the molecules evaporate from the liquid to form a gas. With time the number of molecules leaving the liquid equals the number of molecules reentering the liquid—a state called equilibrium. Vapor pressure is the amount of pressure exerted by the gaseous form of a substance when the gas and liquid states are in equilibrium. In other words, vapor pressure of an inhalation anesthetic is a measure of its tendency to evaporate. Vapor pressure is both agent- and temperature-dependent and is commonly measured at 20° C (68° F), which is considered to be room temperature. Vapor pressure is significant to the way these agents are used because it determines how readily the anesthetic liquid evaporates in the anesthetic machine vaporizer.
Agents with a high vapor pressure, such as isoflurane, sevoflurane, desflurane, and halothane, are described as volatile because they evaporate readily. For example, the maximum useful level of isoflurane in the fresh gas delivered to a patient is 5%. But because isoflurane evaporates so readily, if not controlled it can reach a concentration of over 30%—a level that would cause a fatal anesthetic overdose. This is why volatile agents must be delivered from a precision vaporizer, which precisely controls the amount of anesthetic delivered and therefore allows them to be used safely.
For this reason, most precision vaporizers intended for use with isoflurane allow a maximum concentration of 5%, a level sufficient for clinical use. Volatile agents generally cannot be used in nonprecision vaporizers because they do not adequately control delivery of the agent, and there is an increased risk of overdose. Isoflurane is an exception, however, as there is a nonprecision vaporizer called the Stevens Vaporizer that is intended for use with this agent. Close monitoring of the patient and anesthetic machine by a skilled anesthetist is required, however, if a nonprecision vaporizer is used (see Chapter 4).
Some agents, such as the discontinued agent methoxyflurane, have relatively low vapor pressure and do not require the use of a precision vaporizer. At 20° C the maximum methoxyflurane concentration attainable in the anesthetic circuit is 4%, a safe level for this agent. Consequently a nonprecision vaporizer, such as a glass jar with a wick (see Figure 4-26), is adequate for vaporizing methoxyflurane. Although precision vaporizers are available for use with methoxyflurane, they are not required to deliver this agent.
All precision vaporizers are designed to deliver one specific halogenated agent (e.g., isoflurane, sevoflurane, or desflurane) because they are designed and calibrated for the vapor pressure unique to that agent. The vapor pressures of isoflurane and halothane are so similar, however, that a recently serviced halothane vaporizer should deliver isoflurane at a concentration within 10% of the dial setting. For this reason, when isoflurane was first introduced it was sometimes used extra-label in halothane vaporizers. Now that isoflurane vaporizers are widely available, this practice is discouraged because of potential liability resulting from either extra-label use or from confusion of or inadvertent mixing of isoflurane and halothane.
Although it is unacceptable to combine agents in the same vaporizer, it is acceptable to switch from one anesthetic to another during the course of surgery if the patient demonstrates an adverse reaction to an anesthetic. In this case, separate vaporizers must be available for each anesthetic, and either the vaporizers must be connected in series or the inlet and outlet hoses must be rapidly changed from one vaporizer to the other when it is time to make the change.
Partition Coefficient
Many of the physiologic effects of inhalant anesthetics can be explained by their solubility characteristics in various substances such as air within the alveoli, blood, lipid, and tissue. Solubility is usually expressed as a partition coefficient, which is a ratio of the concentration of an agent in two substances.
The blood-gas partition coefficient is a ratio of the concentration of an inhalation agent in the blood and in the alveolar gas. It is therefore a measure of the solubility of an inhalant anesthetic in blood as compared with alveolar gas. A blood-gas partition coefficient of 0.5 (a low coefficient) indicates that an anesthetic is half as soluble in the blood as it is in the alveolar gas. So at equilibrium, two thirds of the anesthetic are in the alveolar gas and one third is in the blood. In contrast, a blood-gas partition coefficient of 2 (a high coefficient) indicates that the anesthetic is twice as soluble in the blood as in the alveolar gas. In this case, at equilibrium, one third is in the alveolar gas and two thirds are in the blood. So an anesthetic with a low blood-gas partition coefficient is less soluble in blood than an anesthetic with a high blood-gas partition coefficient.
This is of importance to the anesthetist because the blood-gas partition coefficient indicates the speed of induction and recovery one should expect for a given inhalant anesthetic. The lower the blood-gas partition coefficient for an inhalant anesthetic, the faster the expected induction and recovery. This is because the relative concentration of the agent in the alveoli will remain high, creating a wide concentration gradient between the alveolar gas and the blood. So when the agent enters or leaves the blood, it does so at a rapid rate. An example of an agent with a low blood-gas partition coefficient is sevoflurane, an anesthetic with very rapid induction and recovery characteristics.
In contrast, an agent with a high partition coefficient is highly soluble in the blood and tissues. Because the anesthetic is readily absorbed into the blood and tissues (called the sponge effect), high levels of the anesthetic do not build up within the alveoli. This low concentration gradient causes the agent to enter the blood slowly and gradually. As a result, agents with high partition coefficients induce anesthesia less rapidly than do agents with low partition coefficients. Similarly, agents with high partition coefficients are slow to leave tissues, especially fat, and this gradual release results in a slow recovery. Methoxyflurane is an example of an agent with a high partition coefficient and, as expected, demonstrates relatively slow induction and recovery rates.
The blood-gas partition coefficient of an inhalant agent strongly influences the clinical use of the agent in the following ways:
• Induction. Agents with a low blood-gas partition coefficient (isoflurane, sevoflurane, desflurane, and halothane) may be used for mask and chamber inductions, because inductions are rapid enough to induce the patient safely and in a reasonable length of time. Methoxyflurane, an agent with a high partition coefficient, cannot be used this way.
• Maintenance. Agents with low blood-gas partition coefficients also have the advantage of allowing a rapid patient response to changes in anesthetic concentration during anesthesia. Patients anesthetized with isoflurane or sevoflurane may respond within a few minutes to changes in the vaporizer setting. If an agent with a higher partition coefficient is used (such as methoxyflurane), the anesthetist will observe a slower patient response to changes in the vaporizer setting.
• Recovery. Patients anesthetized with agents with low blood-gas partition coefficients have a relatively fast recovery time. Patients anesthetized with sevoflurane or isoflurane are often fully awake within a relatively short time after the vaporizer is turned off. Patients anesthetized with methoxyflurane often sleep quietly for 30 to 60 minutes after anesthesia.
Minimum Alveolar Concentration
The MAC of an anesthetic agent is the lowest concentration at which 50% of patients show no response to a painful stimulus (e.g., a hemostatic forceps applied to the base of the tail or creation of a surgical incision). For example, isoflurane has a MAC of 1.3% in the dog. This means that if 10 dogs were anesthetized with isoflurane delivered at a setting of 1.3%, five of the dogs would respond to a painful stimulus and five would not. Thus the MAC is used to determine the average setting that must be used to produce surgical anesthesia and is a measure of the potency of the agent. An agent with a low MAC is a more potent anesthetic than an agent with a high MAC. For example, halothane, which has a lower MAC than isoflurane, is more potent than isoflurane. Therefore a higher concentration of isoflurane will be necessary to maintain a similar anesthetic depth.
For any inhalation anesthetic, a vaporizer setting of approximately 1 × MAC will maintain light surgical anesthesia, 1.5 × MAC will maintain moderate surgical anesthesia, and 2 × MAC will maintain deep surgical anesthesia in most patients. These figures are useful only as a rough guide: MAC varies with the age, metabolic activity, and body temperature of the patient. Factors such as disease, pregnancy, obesity, and use of other agents may also alter the potency of an anesthetic agent in a given patient. The anesthetist should also be aware that the response to an anesthetic depends on the concentration of the anesthetic in the patient’s brain, which is not necessarily the same as that indicated by the anesthetic machine vaporizer dial setting, particularly early in the induction period (see Chapter 4).
For example, a concentration of approximately 2% isoflurane therefore can be expected to maintain moderate surgical anesthesia in most dogs. Halothane has a slightly lower MAC (0.87%), and a vaporizer setting between 1% and 1.5% is often adequate to maintain anesthesia. Sevoflurane has a high MAC (approximately 2.4), and the maintenance level can be expected to be close to 3.5%. This is only a rough guideline, and the anesthetist must, of course, monitor each animal’s response to the anesthetic to determine the optimum setting for that individual.
Use of Halogenated Organic Compounds
Isoflurane, a halogenated organic compound, is currently the most commonly used inhalant agent for induction and maintenance of general anesthesia in North America. Isoflurane is approved for use only in dogs and horses, although it has gained widespread use in a wide variety of species including exotic and zoo animals and in recent years has gradually replaced its predecessor halothane largely because of fewer cardiovascular adverse effects seen with this agent.
Physical and chemical properties.: Isoflurane has a relatively high vapor pressure (240 mm Hg), and therefore a precision vaporizer is normally used to deliver this agent.
The blood-gas partition coefficient of isoflurane is extremely low (1.46). This, combined with the relatively low tissue solubility of this agent, results in extremely rapid induction and recovery. Isoflurane is therefore better suited to mask or chamber induction than are slower-acting agents such as methoxyflurane and halothane. Unfortunately, some animals appear to be irritated by isoflurane vapors and resist mask induction. Recovery from anesthesia is also rapid, and the anesthetist must refrain from turning off the anesthetic machine vaporizer until the end of surgery because return of consciousness may commence as rapidly as a few minutes after isoflurane is discontinued. Because of the low partition coefficient, the anesthetist can change the patient’s depth of anesthesia rapidly during the course of anesthesia. An animal under anesthesia that appears too deep or too light usually responds rapidly (within a few to several minutes) after adjustment of the vaporizer dial.
The MAC of isoflurane is 1.3% to 1.63% in the common domestic species. This means that anesthesia is maintained in most patients at a concentration of 1.5% to 2.5%. The rubber solubility of isoflurane is low, and there is little absorption of this anesthetic into rubber components of the anesthetic machine and breathing circuit. Isoflurane is stable at room temperature, and no preservative is necessary. This is an advantage because, unlike with halothane, no preservative residue accumulates in isoflurane vaporizers. However, like all vaporizers, isoflurane vaporizers still require periodic maintenance and calibration.
• When used at normal anesthetic levels, isoflurane maintains cardiac output close to that of preanesthetic levels. It causes some depression of myocardial cells, has little effect on heart rate, and does not sensitize the heart muscle to epinephrine-induced arrhythmias. Of the volatile anesthetics commonly used in veterinary anesthesia, isoflurane is considered to have the fewest adverse cardiovascular effects and is therefore considered to be the inhalation agent of choice for patients with cardiac disease. Vasodilation and decreased blood pressure may be observed, however, particularly at deeper levels of anesthesia.
• Isoflurane depresses the respiratory system. In patients anesthetized with isoflurane, the respiratory rate decreases over time. A concentration of two to three times the MAC causes respiratory arrest in common domestic species.
• Isoflurane maintains cerebral blood flow and is considered a good anesthetic for animals with head trauma or brain tumors, provided the concentration is 1% or less.
• Nearly all of the isoflurane administered to a patient is eliminated through the lungs once the vaporizer is turned off. Isoflurane has low fat solubility; consequently, little retention in body fat stores, little hepatic metabolism (0.2 %), and very little renal excretion of metabolites occur. For this reason isoflurane is well suited to animals with liver or kidney disease. Isoflurane is also a good anesthetic for use in neonatal and geriatric animals, in which hepatic metabolism and renal excretion mechanisms may be less efficient than in the healthy adult animal.
• Isoflurane induces adequate to good muscle relaxation.
• Isoflurane has little or no analgesic effect in the postanesthetic period. This, combined with the rapid recoveries seen with this agent, may lead to pain and excitement during recovery unless the animal is treated with analgesics.
• When exposed to desiccated CO2 absorbent, isoflurane can produce carbon monoxide, which has over 200 times greater affinity for hemoglobin binding sites than oxygen. Carbon monoxide displaces oxygen from the binding sites, causing hypoxemia. Animals with carbon monoxide poisoning may be asymptomatic, but cherry red blood and mucous membranes suggest carbon monoxide exposure, which must be treated promptly.
Sevoflurane
Sevoflurane is the second most commonly used inhalant anesthetic for induction and maintenance of general anesthesia (isoflurane being the most commonly used agent). Chemically, it is a halogenated organic compound closely related to isoflurane and shares many of the same characteristics. Sevoflurane is labeled for use in dogs but has been used in a wide variety of species including exotic and zoo animals.
Physical and chemical properties.: The vapor pressure of sevoflurane, although somewhat lower than that of isoflurane, is relatively high (160 mm Hg). Therefore a precision vaporizer is required to deliver this agent.
The blood-gas partition coefficient is even lower than isoflurane’s, allowing even more rapid inductions and recoveries. Observed time to intubation has been reported as 5 to 7 minutes after mask induction (compared with 6 to 8 minutes for isoflurane). Mask induction with sevoflurane is typically associated with less struggling than with isoflurane because this agent is nonirritating and has a more pleasant odor than isoflurane. Because of these characteristics, sevoflurane is the agent best suited to mask and chamber inductions. The high controllability of anesthetic depth associated with sevoflurane has made this agent popular in equine anesthesia, despite its relatively high cost (currently, approximately 10 times that of isoflurane).
The MAC of sevoflurane is 2.34% to 2.58% in common domestic species. Sevoflurane is therefore a less potent agent than isoflurane, and higher concentrations are required to induce and maintain anesthesia. A concentration of 4% to 6% is required for mask induction (compared with 3% to 5% for isoflurane), and 2.5% to 4% is the normal maintenance range (compared with 1.5% to 2.5% for isoflurane).
Sevoflurane can react with the potassium hydroxide (KOH) or sodium hydroxide (NaOH) in desiccated carbon dioxide absorbents to produce a chemical (compound A) that can cause renal damage in rats. This effect is most pronounced in closed-circle systems, in low-flow systems, and at high sevoflurane concentrations. Renal damage has not been reported in dogs or cats anesthetized with sevoflurane, and the potential for nephrotoxicity appears to be low in these species.
Effects and adverse effects.: The effects of sevoflurane on major organ systems are similar to those of isoflurane.
• Some myocardial depression, vasodilation, and dose-related hypotension are seen with this agent. Like isoflurane, sevoflurane does not sensitize the heart to epinephrine-induced arrhythmias.
• Sevoflurane may depress respiration slightly more than isoflurane. Apnea lasting at least 30 seconds during surgical anesthesia has been reported.
• Like isoflurane, sevoflurane is primarily eliminated by the lungs (with 2% to 5% biotransformed in the liver).
• Sevoflurane does not significantly increase cerebral blood flow and can be used for anesthesia of patients with head trauma or brain tumors.
• Sevoflurane induces adequate muscle relaxation.
• Paddling, excitement, and muscle fasciculations have been reported, primarily during the recovery period.
• Sevoflurane has no analgesic effect at subhypnotic doses. As with isoflurane, an analgesic agent must be administered after any painful procedure, before the patient wakes up.
Desflurane
Desflurane is a halogenated organic inhalant anesthetic closely related to isoflurane. It can be used for induction and maintenance of anesthesia, although its expense and some adverse effects currently preclude common use in veterinary patients.
Physical and chemical properties.: Desflurane has the lowest blood-gas partition coefficient (0.42%) of any of the commonly used agents and therefore produces inductions and recoveries that are approximately twice as fast as those of isoflurane (sometimes referred to as “one-breath anesthesia” because it can seem as if a patient is anesthetized or wakes up after taking one breath).
The vapor pressure of desflurane is extremely high (700 mm Hg), and the boiling point is near room temperature (at 23.5° C). Because of these properties, this agent requires a special, electronic, heated vaporizer that keeps the agent under pressure to prevent it from boiling off. The high cost of desflurane and the vaporizer is a significant factor limiting the use of this agent in veterinary medicine.
This agent is the least potent of any of the halogenated agents used in veterinary patients, as evidenced by a MAC of 7.2% to 9.8% in common domestic species. A concentration of 10% to 15% is required for mask induction (compared with 3% to 5% for isoflurane), and 8% to 12% is needed for maintenance (compared with 1.5% to 2.5% for isoflurane).
Like isoflurane, very little of desflurane is metabolized by the liver (0.02%).
Effects and adverse effects.: Desflurane vapors are very pungent and may induce coughing and breath-holding. This makes mask induction with this agent difficult, unless the patient is premedicated. The effects on the nervous, cardiovascular, and respiratory systems are similar to those of isoflurane with the following exception: desflurane is reported to cause transient increases in heart rate and blood pressure in humans. This phenomenon (called sympathetic storm) has not been reported in domestic animals. Desflurane has the greatest tendency of any of the agents to cause the production of carbon monoxide when passed through dry carbon dioxide absorbent.
Other Halogenated Agents
Halothane.: Halothane (Fluothane), first introduced in 1956, was until relatively recently one of the most commonly used inhalation agents in veterinary anesthesia. In recent years it has gradually been replaced by isoflurane and sevoflurane.
Physical and chemical properties: Many of the physical and chemical properties of halothane are very similar to those of isoflurane. Like isoflurane, halothane has a relatively high vapor pressure (243 mm Hg) and therefore normally requires a precision vaporizer for its safe use. Halothane delivered through a nonprecision vaporizer may readily reach a concentration over 30%, which dangerously exceeds the normal concentration required for anesthesia (0.5% to 4%). Special techniques are required for use of halothane in a nonprecision vaporizer.
Halothane has a moderately low partition coefficient (2.54), producing inductions and recoveries that, although longer than those of isoflurane, are sufficiently rapid to allow the patient to be induced by mask. Mask induction results in unconsciousness and stage III anesthesia within about 10 minutes. Recovery time from anesthesia varies with length of anesthesia, patient condition, and the concurrent use of other agents; however, animals generally assume sternal recumbency in less than 1 hour after the anesthetic is discontinued. Because of halothane’s moderate lipid solubility, a portion of the anesthetic is retained within body fat stores rather than being eliminated by the lungs during recovery. About 20% to 46% of halothane is subsequently metabolized by the liver and excreted by the kidneys.
Halothane has a very low MAC (0.87 to 1.19) and so is more potent than any of the commonly used agents. A concentration of 2% to 4% (up to 10% in large animals) is required for mask induction (compared with 3% to 5% for isoflurane), and 0.5% to 1.5% (1% to 2% in large animals) is the normal maintenance range (compared with 1.5% to 2.5% for isoflurane).
Halothane has moderate rubber solubility. This is of concern to the anesthetist because rubber hoses, reservoir bags, and other anesthetic machine parts may absorb halothane during the course of anesthesia. Release of the agent from these machine parts may delay patient recovery after the vaporizer has been turned off.
Halothane is somewhat unstable and for commercial use is mixed with the preservative thymol. The presence of a preservative may cause a buildup of residue within the vaporizer, turning the liquid in the vaporizer yellow. The residue may cause moving parts in some vaporizers to stick, so periodic cleaning and recalibration are recommended.
Effects and adverse effects: Halothane is a relatively safe agent for veterinary use; however, it does have the following effects on organ function:
• Halothane sensitizes the heart to the action of catecholamines (such as epinephrine) and thus may induce arrhythmias. Arrhythmias may be treated by increasing oxygen flow and ensuring that anesthetic depth is adequate. If this does not alleviate the arrhythmia, the patient may be given IV lidocaine or switched to another anesthetic, if available.
• Halothane is a myocardial depressant, decreasing myocardial contraction and cardiac output. This effect is dose-dependent (that is, the higher the concentration, the greater the effect).
• Halothane causes vasodilatation. This, along with heart muscle depression, causes a fall in blood pressure that is roughly proportional to anesthetic depth. For this reason, IV fluid support is recommended in hypovolemic or hypotensive patients.
• Halothane is a respiratory depressant, and respiratory rate and tidal volume usually fall if anesthesia is prolonged. Respiratory arrest may occur at high concentrations. Like all inhalant anesthetics, halothane readily crosses the placenta and may depress respiration in the neonates.
• From 20% to 46% of halothane is metabolized; therefore the potential for liver damage exists.
• Halothane may increase cerebral blood flow, which may lead to increased intracranial pressure in patients with head trauma or brain tumors.
• Halothane use is associated with malignant hyperthermia, a rare but often fatal disorder of thermoregulation that occurs in genetically predisposed animals. Affected animals show increased temperature, muscle rigidity, and cardiac arrhythmias and may die. Treatment consists of removal from halothane, cooling, and administration of oxygen and specific drugs such as dantrolene.
Methoxyflurane.: Although no longer commercially available in North America, methoxyflurane was used extensively for many years, and because of unique physical properties, remains a good point of comparison when learning about currently used agents such as isoflurane and sevoflurane.
The vapor pressure of methoxyflurane is significantly lower (23 mm Hg) than that of isoflurane (240 mm Hg) or sevoflurane (160 mm Hg), and as a result methoxyflurane can be delivered using a nonprecision vaporizer, whereas currently used agents must be delivered from a precision vaporizer.
The blood-gas partition coefficient of methoxyflurane (15) is considerably higher than that of isoflurane (1.46), as is the lipid solubility. These two factors combine to produce slow inductions and recoveries. For this reason, unlike currently used agents, this agent is not useful for mask or chamber inductions.
The MAC of methoxyflurane is considerably lower (0.23%) than that of the other volatile inhalation anesthetics, making this agent more potent than currently used halogenated agents.
Methoxyflurane has a much higher rubber solubility (630) than isoflurane (49) and therefore readily dissolves in reservoir bags, hoses, and rubber endotracheal tubes. This may lead to deterioration of these products unless they are rinsed out immediately after use. This characteristic may also result in considerable release of methoxyflurane gas into the anesthetic circuit even after the vaporizer has been turned off. This is not a significant problem with currently used agents.
Because of its high lipid solubility, methoxyflurane is retained in body fat stores so that approximately 50% to 75% is metabolized and excreted by the liver and kidney. Fluoride ions and other potentially toxic metabolites are produced as a result of hepatic metabolism, and the presence within the kidney of these toxic metabolites may lead to renal damage. The currently used agents isoflurane and sevoflurane are almost entirely (>95%) eliminated by the lungs; consequently liver and kidney damage are not of major concern.
Enflurane.: Enflurane, a volatile gaseous anesthetic used in human medicine, has not found wide acceptance in veterinary anesthesia because of adverse effects. Induction and recovery are relatively rapid and smooth, with minimal effects on heart rate and minimal sensitization of the myocardium to epinephrine. However, enflurane causes profound respiratory depression, and spontaneous ventilation is poor. In the dog, enflurane also induces significant muscle hyperactivity, and seizure-like muscle spasms may result.
Nitrous Oxide
Nitrous oxide, introduced as an anesthetic more than 150 years ago, is still used in human anesthesia and, to a much lesser extent, in veterinary anesthesia. Unlike other inhalation anesthetics, nitrous oxide is a gas at room temperature, is stored in blue compressed gas cylinders, and does not require a vaporizer. Like oxygen, it is administered with a flow meter, and it is mixed in concentrations of 40% to 67% with oxygen before being delivered to the patient.
When used with other agents, nitrous oxide (N2O) speeds induction and recovery and provides additional analgesia. Nitrous oxide also reduces the MAC (and therefore the vaporizer setting) of other anesthetics by 20% to 30%. This reduces the risk of adverse effects on the cardiovascular, pulmonary, and other systems.
The advantages of nitrous oxide were significant when older agents such as methoxyflurane and halothane were in use. The advantages are much less important since the advent of newer agents such as isoflurane and sevoflurane, however, because of the rapid inductions and recoveries characteristic of these agents and because of the availability of a wide variety of effective injectable analgesics. For these reasons, nitrous oxide is now seldom used in general practice.
There are unique characteristics of nitrous oxide, including adverse effects and other cautions, with which the anesthetist must be familiar if using this agent. The reader is directed to Appendix B for detailed information about its use.
CENTRAL NERVOUS SYSTEM AND RESPIRATORY STIMULANTS
Most anesthetic agents and many adjuncts are respiratory system depressants, so respiratory depression is commonly seen during anesthesia. This complication is usually managed by precise control of anesthetic depth and, if needed, manual or mechanical-assisted or mechanical-controlled ventilation. In emergency situations, severe respiratory depression associated with opioids and alpha2-agonists can be treated with reversal agents, but the beneficial effects of the corresponding agonist, including sedation, analgesia, and hypnosis, will also be lost. When complete reversal is not desirable or when agents that cannot be reversed are used, the anesthetist may need to use other methods to manage respiratory depression. The pharmacologic agent most commonly used for this purpose is doxapram.
Doxapram
Doxapram is a noncontrolled injectable analeptic agent used in small animals to stimulate respirations and speed awakening during recovery or in emergency situations. It is also commonly used to stimulate respirations in neonates after dystocia or cesarean section. It is most commonly administered intravenously to adult animals. In neonates it is often administered by placing a few drops under the tongue (sublingual administration), although it can be given subcutaneously or in the umbilical vein.
Mode of Action and Pharmacology
Although the mode of action is not completely known, doxapram stimulates the CNS, including the respiratory centers in the brain stem.
Effects on Major Organ Systems
Within 2 minutes of IV injection, doxapram will temporarily increase respiratory rate and depth.
Adverse Effects
Although doxapram has a relatively wide margin of safety, it may cause hyperventilation, hypertension, and arrhythmias in some patients. It must not be used in patients with a history of seizures because the drug lowers the seizure threshold. Doxapram must be used only in the presence of adequate oxygen levels in the brain, otherwise CNS damage may result. Several other cautions are detailed in pharmacology references.
Use of Doxapram
After an initial injection, if CNS and respiratory stimulation is inadequate, a second injection can be given 15 to 20 minutes later. The dose of doxapram varies widely depending on the situation. For instance, in small animals the dose is much lower when used to reverse respiratory depression from inhalant agents (1.1 mg/kg IV) than when used to reverse respiratory depression from barbiturates (5.5 to 11 mg/kg IV).
Because this agent is supplied as a 20-mg/mL solution, and there are approximately 20 drops in 1 mL, 1 drop of the solution contains approximately 1 mg of doxapram. For neonatal puppies, 1 to 5 drops can be dripped under the tongue (1 to 2 drops for kittens), depending on the size and degree of depression, or 1 to 5 mg (1 to 2 mg for kittens) can be given subcutaneously to stimulate respirations.
KEY POINTS
1. Preanesthetic agents reduce the required dose of general anesthetics, minimize adverse effects, ease induction and recovery, provide muscle relaxation, and reduce patient stress and discomfort.
2. Although not anesthetics, the anticholinergics atropine and glycopyrrolate are used to prevent bradycardia, bronchoconstriction, excessive salivation, and other parasympathetic effects. These agents must be used sparingly, and with caution, as they can produce serious adverse effects.
3. Tranquilizing agents include phenothiazines, benzodiazepines, and alpha-2-agonists. Phenothiazines have a wide margin of safety but may cause hypotension in some patients. Benzodiazepines have a calming effect on geriatric and debilitated animals and are excellent for prevention and treatment of seizures. Alpha2-agonists are potent sedatives and produce excellent muscle relaxation but may cause serious cardiovascular and respiratory complications in some patients.
4. Opioid agonists, partial agonists, and agonist-antagonists may be used as preanesthetic agents, analgesics, and (in combination with tranquilizers) neuroleptanalgesics and induction agents. Many of the best analgesics available are in this group. With few exceptions, their use is subject to government regulation regarding purchase, handling, and dispensing.
5. Opioids, alpha2-agonists, and benzodiazepines have corresponding reversal agents that can be used to sequentially sedate or anesthetize and then wake patients when a procedure or surgery is completed.
6. Although commonly used injectable and inhalation anesthetics have a relatively good safety profile, they have the potential to produce significant cardiovascular, respiratory, and thermoregulatory system depression.
7. Injectable anesthetics include propofol, dissociatives (ketamine and tiletamine), barbiturates (thiopental and methohexital), neuroleptanalgesic combinations, and etomidate.
8. Barbiturates are divided into classes based on duration of action. The ultra–short-acting agents thiopental and methohexital are used for anesthetic induction (and maintenance in the case of methohexital), the short-acting agent pentobarbital is used to stop seizures and for laboratory animal anesthesia, and the long-acting agent phenobarbital is used for seizure control. These classes differ in their lipid solubility, duration of effect, and distribution within the body.
9. Barbiturates show unusual potency in patients that are acidotic, hypoproteinemic, or hypotensive. They may cause prolonged recoveries in sighthounds.
10. Most intravenous (IV) anesthetics are administered by titration (or “to effect”) to achieve the minimum effective dose.
11. Injectable anesthetics are eliminated by redistribution, liver metabolism, and renal excretion, whereas commonly used inhalation anesthetics are eliminated primarily by exhalation from the lungs.
12. Dissociatives such as ketamine and tiletamine produce a state of dissociative anesthesia characterized by intact reflexes, central nervous system (CNS) excitement, apneustic respiration, tachycardia, and intact or increased muscle tone. Concurrent use of a tranquilizer is recommended to promote muscle relaxation and to prevent excitement during recovery.
13. Neuroleptanalgesia is a profound hypnotic state produced by the simultaneous administration of an opioid and a tranquilizer. These agents provide relatively safe induction in debilitated patients.
14. Propofol, methohexital, and etomidate are induction agents that can be given by repeat injection to maintain anesthesia.
15. The inhalation agents in common use are isoflurane and sevoflurane. Both agents are administered by means of an anesthetic machine and either a mask or an endotracheal tube.
16. Inhalation anesthetic agents vary in their blood-gas partition coefficient, vapor pressure, and minimum alveolar concentration (MAC). These properties affect the speed of induction and recovery, the type of vaporizer that should be used, and the vaporizer setting that is required for anesthetic induction and maintenance.
17. All inhalation anesthetics may cause respiratory depression and decrease cardiac output and blood pressure. In addition, halothane may potentiate cardiac arrhythmias. Of the commonly used agents, isoflurane and sevoflurane are considered to have the greatest margin of safety and the shortest induction and recovery times.
18. Reversal agents and analeptics may be given after anesthesia to hasten anesthetic recovery. Doxapram is a nonspecific respiratory stimulant that may accelerate arousal from barbiturate or inhalation anesthesia.
REVIEW QUESTIONS
1. A neuroleptanalgesic is a combination of:
a. An opioid and an anticholinergic
b. An anticholinergic and a tranquilizer
2. Most preanesthetics will not cross the placental barrier.
3. It is recommended that atropine not be given to an animal that has tachycardia.
4. Anticholinergic drugs such as atropine block the release of acetylcholine at the:
a. Muscarinic receptors of the parasympathetic system
b. Nicotinic receptors of the parasympathetic system
5. High doses of opioids can cause bradycardia and respiratory depression.
6. Severe bradycardia caused by dexmedetomidine is best treated with the following drug:
7. Opioids may be reversed with:
8. Which of the following drugs will precipitate out when mixed with other drugs or solutions?
9. Etomidate is particularly well suited for induction of dogs with which of the following problems?
10. Which of the following is an example of a dissociative anesthetic?
11. One of the disadvantages of the drug methohexital is that animals that are anesthetized with it may demonstrate excitement during recovery.
12. Compared with methoxyflurane, isoflurane is considered to have a:
13. An anesthetic agent that has a low blood-gas partition coefficient will result in __________ induction and recovery time.
14. Which of the following has the lowest blood-gas partition coefficient?
15. As a rough guideline, to safely maintain a surgical plane of anesthesia, the vaporizer should be set at about:
16. Propofol sometimes causes transient apnea. To avoid this, the anesthetist should:
17. One problem frequently associated with recovery from tiletamine-zolazepam in dogs is:
For the following questions, more than one answer may be correct.
18. The concentration of barbiturate entering the brain is affected by a variety of factors such as:
19. Effects that are commonly seen after administration of a dissociative include:
20. Adverse effects common with isoflurane include:
22. Factors that may affect the speed of anesthetic induction with a volatile gaseous anesthetic include:
23. Which of the following are alpha2-agonists?