Rodent and Rabbit Anesthesia
After completion of this chapter, the reader will be able to:
• Summarize the common problems that may arise when anesthetizing rodents and rabbits.
• List the preanesthetic and anesthetic agents suitable for use in these species.
• Describe the technique of endotracheal intubation in rabbits.
• Describe the problems that can arise when monitoring anesthesia in rodents and rabbits.
• State aspects of intraoperative care that are of particular importance when anesthetizing rodents and rabbits.
• Describe how to cope with common anesthetic emergencies in rodents and rabbits.
• Describe the most common problems associated with postanesthetic care of rodents and rabbits.
• List the analgesics that can be used in rodents and rabbits.
Anesthesia of small mammals (rabbits, guinea pigs, rats, mice, gerbils, and hamsters) is a specialized branch of veterinary anesthesia, but the general principles of good anesthetic practice provide basic guidance. The main difficulties encountered when anesthetizing these animals result from the following situations:
• Lack of familiarity with the species
• A failure to appreciate the poor health status of some patients
Once these problems are appreciated, anesthesia of small mammals and other exotic species should be as successful as anesthesia of dogs and cats.
PATIENT EVALUATION
To anesthetize small mammals safely and effectively, it is important to perform a clinical examination and obtain a case history. Although the information required is similar to that needed for more familiar species, many of these small animals are owned by children, and accurate information may not always be obtainable. Even when an adult or older child is caring for the animal, it may be difficult to be certain that the animal is eating and drinking normally, because many of these species are fed ad lib.
It should be recalled that the life span of these small mammals is considerably shorter than that of dogs and cats. Geriatric animals present a greater risk when anesthetized; a hamster, for example, will be nearing the end of its natural life when aged only 18 to 24 months. Some basic biologic data are given in Table 11-1.
TABLE 11-1
Biologic Data for Small Mammals
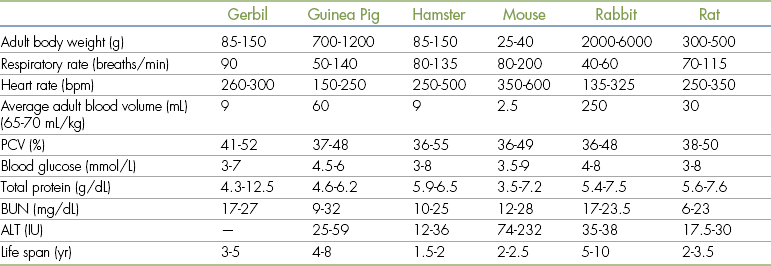
ALT, Alanine aminotransferase; BUN, blood urea nitrogen; IU, international units; PCV, packed cell volume.
For a physical examination to be performed, any animal must be safely and humanely handled and restrained. Handling is easier if small rodents are brought to the veterinary clinic in a small container, although they should not be left in a cardboard box for a prolonged period because they can easily gnaw through the container and escape. Rabbits can usually be transported in a small transport box, and cat-sized carriers are suitable. Because rabbits are a prey species and cats are one of their predators, it is not surprising that placing a rabbit in a transport box that has been previously used for cats can be extremely stressful; this should be avoided. Similarly, it is advisable for the technician to wash the hands and preferably wear a fresh gown or coat before examining and handling these species if he or she has previously been working with dogs and cats.
Before the animal is handled, it should be observed undisturbed so that its normal behavior and respiratory pattern and rate can be noted.
Handling and Restraint
Mice are best picked up by the base of the tail and lifted clear of their transport box. They can then be allowed to rest on the operator’s forearm and their external appearance assessed. To restrain them for administration of injectable anesthetics or other drugs, allow mice to rest on a rough surface (e.g., a towel or the bars of their cage). They can then be grasped by the skin overlying the shoulders and lifted clear. The tail can be gripped between the operator’s fingers as shown in Figure 11-1. Subcutaneous administration of medication is made into the skin overlying the shoulders and can be carried out single-handedly. An assistant should administer intraperitoneal and intramuscular injections while the operator restrains the animal as shown in Figures 11-2 and 11-3. Restraining the mouse by its scruff can interfere with respiration. This causes no problems in healthy animals, but care should be taken if the animal is showing signs of respiratory disease. Young mice can be extremely active and may jump out of their transport box as soon as the lid is removed; handling these agile young animals requires fast reactions.
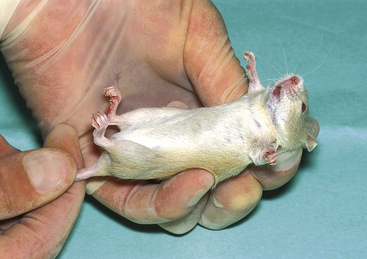
FIGURE 11-1 Restraint of a mouse. Mice can be restrained by the skin overlying the shoulders, with the tail held between the operator’s fingers.
Rat
Most pet rats are friendly and easy to handle. They should be picked up around the shoulders and lifted clear of the transport box. They can then be allowed to rest on the handler’s forearm and be gently restrained by the tail or around the shoulders. If the animal resents handling (which it may if it is in pain—for example, if it has arthritis), it can be picked up by the base of the tail as mice are. It can then be placed on a rough surface and grasped around the shoulders. When holding a rat in this way, the operator can avoid being bitten by positioning the thumb under the mandible as shown in Figure 11-4. It is important not to grasp the animal’s chest too firmly because this can interfere with respiratory movements, causing the animal to panic and struggle. Although subcutaneous injections can be given into the scruff while the animal is also being restrained, it is usually easier to obtain the assistance of a colleague. Intramuscular and intraperitoneal injections are given in the same way as in the mouse, but an assistant is needed for these procedures (Figures 11-5 and 11-6).
FIGURE 11-4 Restraint of a rat. Note that the thumb is positioned below the mandible to prevent biting. The chest is held gently to avoid interfering with respiration.
Hamster
Hamsters vary considerably in their temperament, and care should be taken when handling them. This species is normally active at night and asleep during the day, and if necessary they should be gently awakened before being handled. Most animals can be cupped in the operator’s hands, as shown in Figure 11-7, and an external examination carried out. If it is necessary to immobilize the animal, it should be covered by the operator’s hand with the skin overlying the shoulders and back grasped firmly (Figure 11-8). It is important to grasp sufficient skin; otherwise, the animal can turn in the operator’s grasp and may bite (Figure 11-9). An assistant can make intramuscular, intraperitoneal, or subcutaneous injections into the same sites as in the rat and mouse. Hamsters should not be allowed to run unrestrained on the consulting room table because they appear to lack depth perception and may fall to the floor and injure themselves.
FIGURE 11-7 Gentle restraint of a hamster. Restraint of a hamster for clinical examination by cupping in the operator’s hands.
Gerbil
Gerbils are very active and can easily escape from their transport container unless quickly immobilized. Preventing escape is best achieved by the operator covering the animal with a hand and grasping around the animal’s shoulders with the thumb positioned under the mandible to prevent biting. With the animal immobilized in this way, an assistant can administer subcutaneous injections into the flank and intramuscular and intraperitoneal injections in the same site as for other small rodents. Gerbils can also be immobilized by grasping the base of the tail, but the skin of the tail is delicate and easily damaged.
Guinea Pig
On initial examination a guinea pig may be completely immobile, but when attempts are made to restrain it, the animal can become very agitated and run around its transport box at high speed. It should be immobilized by grasping it swiftly and firmly around the shoulders. It can then be lifted clear of the transport container, and the operator’s other hand can be used to support its hindquarters (Figure 11-10). With the animal restrained in this way an assistant can administer subcutaneous injections into the flank and intramuscular and intraperitoneal injections into the same site as with other rodents. If drugs are to be given by the subcutaneous route, an alternative approach is placing the restrainer’s hands on either side of the guinea pig’s body to immobilize the animal on the examination table. An assistant can then inject into the skin overlying the shoulders.
Rabbit
Rabbits vary considerably in body weight, ranging from dwarf breeds weighing as little as 400 g up to giant breeds that can weigh 10 kg. Most domestic rabbits weigh 2 to 5 kg and are relatively easy to restrain, but care must be taken because they are easily frightened. When attempting to escape, they may kick out with their back legs. This can injure the person attempting to handle them and may also result in serious injury to the rabbit (e.g., fracture of the lumbar vertebrae). It is therefore important to provide support to the animal’s back at all times and never to leave the animal unrestrained on the consulting room table.
Rabbits should be grasped by the skin overlying the shoulders and lifted clear of the transport container. As the rabbit is lifted, the operator’s other hand should be positioned under the animal’s abdomen to support its body weight as shown in Figure 11-11. The rabbit can then be placed on the examination table. The animal should not be released until its feet are in firm contact with the table surface. It can then be restrained by gently holding the skin over the shoulders. Rabbits should never be picked up by the ears because these are delicate structures.

FIGURE 11-11 Lifting a rabbit. When a rabbit is lifted out of its transport box or cage, the skin overlying the shoulders should be grasped firmly and the abdomen supported. The operator’s forearms are used to provide support to the animal’s back.
An assistant can make intramuscular injections into the quadriceps or into the lumbar muscles while the operator restrains the animal by placing hands and arms along either side of its body. Intravenous injection is most easily carried out into the marginal ear veins. The skin of the ears is sensitive, and animals will often jerk in response to venipuncture. To avoid a jerk and to prevent discomfort, the skin overlying the vein can be desensitized using a local anesthetic cream (e.g., EMLA, AstraZeneca). The cream is applied thickly over the vein and covered with a waterproof dressing (e.g., plastic food wrap) and a protective adhesive bandage. The cream is left in place for approximately 45 minutes and then removed and the ear wiped clean. This provides full skin thickness anesthesia for at least an hour. This technique is particularly useful for placing “over-the-needle” catheters. As an alternative to the ear veins, the cephalic veins on the forelegs can also be used, or the lateral saphenous veins on the hindleg. These vessels are fragile, and it is easy to produce a hematoma, even when venipuncture has been carried out successfully on the initial attempt.
If an assistant is unavailable, rabbits can be securely restrained by wrapping them in a towel, lab coat, or surgical gown, as shown in Figure 11-12. Provided it is wrapped securely, the animal will remain immobile, and it is usually possible to carry out venipuncture successfully with the marginal ear veins.
Physical Examination of Small Mammals
As mentioned earlier, the animal should first be observed undisturbed in its transport box if possible and can then be restrained as previously described for more detailed examination. The animal’s respiratory rate and pattern can be assessed and its heart rate recorded either by palpating the heartbeat or by using a stethoscope. Although normal rates are given in Table 11-1, these will rarely be observed in patients because most will show a marked increase in heart and respiratory rates owing to the stress of examination. Rabbits, for example, frequently have respiratory rates in excess of 250 breaths/min during routine clinical examination. The type of examination that can be carried out is limited by the size of the animal being examined, but in rabbits it is possible to auscultate and percuss the chest as in cats.
TABLE 11-2
Volumes of Fluid Administration for Adult Small Mammals∗

∗All fluids should be warmed to body temperature before administration.
In all species, the following are of particular importance:
• Discharges from the eyes and nose may indicate the presence of respiratory disease. Rats are commonly seen with a black or reddish brown discharge around their eyes or nose. This is a buildup of porphyrin secretions, which when wiped with a damp swab will appear bright red. This can lead owners to report that their animal has been bleeding from its eyes or nose. These secretions are a nonspecific response to stress or illnesses such as chronic respiratory disease.
• Labored or noisy respiration is also indicative of respiratory disease.
• Soiling of the perineum can indicate gastrointestinal disturbances.
• An unkempt or “starey” appearance of the coat is a general sign of ill health in small mammals.
• Loss of skin tone in response to dehydration is more difficult to detect in small mammals than in dogs and cats. If loss of elasticity is noted, it usually indicates that more than 10% of body weight has been lost as fluid. When small mammals are markedly dehydrated, the eyes become sunken. This is commonly seen in rabbits and small mammals that are anesthetized for treatment of dental disease. Because the disease may have been present for some time, the animal may have had a prolonged period of reduced food and water intake. It is essential that these animals receive supportive fluid therapy before anesthesia.
• Palpation of the regions overlying the back and pelvis is helpful in assessing body condition. If the prominences of the vertebrae and of the pelvis are easily palpable, it is likely that the animal has lost a considerable amount of body fat.
• It is difficult to examine the mucous membranes in small rodents, but in the rabbit both the gingiva and conjunctiva can be inspected easily. They should have a normal reddish coloration, and the capillary refill time should be under 1 second. As with dogs and cats, abnormal coloration of the mucous membranes may indicate underlying disease.
Diagnostic Tests
Preanesthetic blood tests are rarely undertaken in small rodents but may be of value in some circumstances (e.g., in rabbits with suspected hepatic lipidosis). Urine samples are easily obtained from small rodents because these species frequently urinate when handled. Diabetes mellitus is relatively common in Chinese hamsters and is also seen occasionally in rabbits and guinea pigs. In these latter species it is frequently asymptomatic.
Radiography may be required before some surgical procedures. For example, radiography of the skull is helpful in assessing underlying dental problems before flushing the tear ducts to correct blockage. Radiography is also indicated before removal of suspected uterine adenocarcinoma in rabbits to identify secondary tumors in the lungs.
PREANESTHETIC PATIENT CARE
Withholding Food before Anesthesia
Small rodents and rabbits do not vomit, and there is generally no reason to withhold food or water before anesthesia. Withholding food from small rodents for prolonged periods can be detrimental because it can predispose to hypoglycemia. Withholding food from rabbits and guinea pigs can also trigger digestive disturbances that can result in enterotoxemia, which can be fatal. One exception to the no-fasting rule is if the planned operation involves the stomach, in which case a 3- to 4-hour fasting period will reduce the volume of digesta.
Successful recovery from an operation and anesthesia in these species is critically dependent on reestablishing a normal feeding pattern. It is therefore strongly recommended that food be available up until 1 to 2 hours before anesthesia and provided again as soon as the animal has recovered. The anesthetist should be aware that many of these animals are nocturnal and will not feed during the day. Postoperative pain and discomfort can also decrease appetite in the period after the operation.
Correction of Preexisting Problems
If animals are in poor condition, every attempt should be made to commence supportive therapy before anesthesia. One common problem is dehydration. Unfortunately the small body size of these animals makes administration of fluids difficult. In rabbits the marginal ear veins and cephalic veins can be used, but in rodents the small size of the veins does not allow easy intravenous catheterization. One alternative is to administer fluids by the subcutaneous or intraperitoneal route, although subcutaneous administration is unlikely to be effective if dehydration is severe. The intraosseous route can also be used and can be a valuable means of providing prolonged fluid therapy in rabbits, guinea pigs, and rats.
Calculation of fluid volume and administration rates is done according to body weight. Small mammals require higher maintenance rates than dogs and cats (100 mL/kg every 24 hours). All of the commonly used fluids used in small animal practice can be administered to rodents and rabbits. Suggested volumes for administration are listed in Table 11-2.
PREANESTHETIC AGENTS
Although the general principles governing the use of preanesthetic agents (see Chapter 3) apply in small mammals, these agents are less frequently used than in dogs and cats. This is primarily because of the methods of anesthesia that are used in small mammals. Because many anesthetic protocols include a combination of anesthetic agents to be given by subcutaneous, intraperitoneal, or intramuscular injection, there is often little advantage in giving a sedative agent before this. If anesthesia is to be induced with an anesthetic chamber, prior sedation is rarely needed except in rabbits. However, preanesthetic agents should be used in the following circumstances:
• Preanesthetics can be used to reduce salivation associated with some anesthetics (e.g., ketamine) and to reduce bronchial secretions, particularly in animals with preexisting respiratory disease. Atropine is frequently used for this purpose, but in rabbits it is often relatively ineffective, because many animals have high levels of atropinase. It is therefore advisable to use glycopyrrolate in rabbits.
• Opioid analgesics may be given 30 to 45 minutes before induction of anesthesia. This reduces the concentration of volatile anesthetic needed to maintain anesthesia and provides preemptive analgesia (see Chapter 7).
• Sedatives or tranquilizers should be given to rabbits before induction of anesthesia with volatile agents (see detailed discussion later in this section).
All of the agents that are commonly used for preanesthetic medication in dogs and cats can be used in small mammals. Their properties and side effects are very similar, but some vary in their actions. Suggested dose rates and effects are listed in Table 11-3.
TABLE 11-3
Preanesthetic Agents for Use in Small Mammals
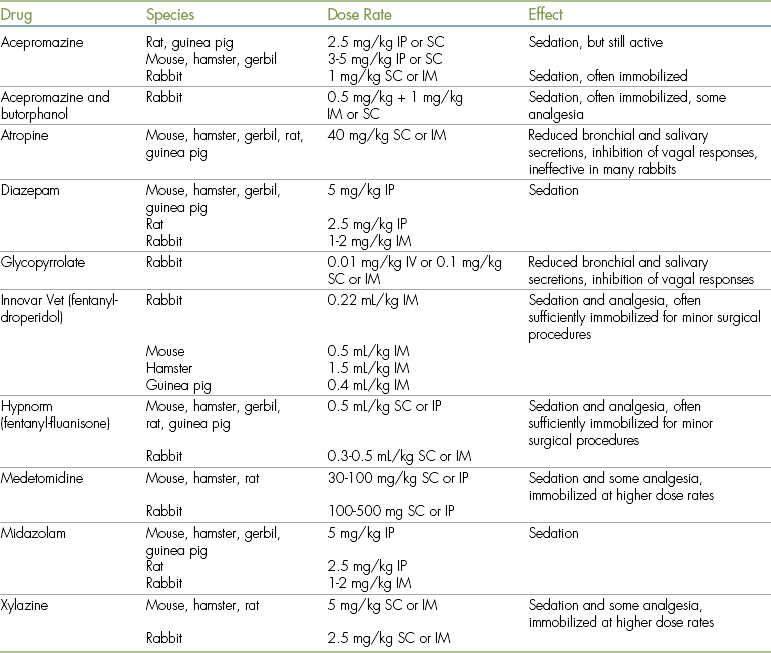
IM, Intramuscular; IP, intraperitoneal; IV, intravenous; SC, subcutaneous.
Anticholinergics
Both atropine and glycopyrrolate can be used in small mammals with the same indications as in dogs and cats. As mentioned earlier, glycopyrrolate is preferred to atropine for use in rabbits because the effect of atropine is less predictable in this species.
Phenothiazines
Phenothiazines such as acepromazine can be used to sedate small mammals. When used in rodents, acepromazine will sedate the animal but will not immobilize it. In rabbits, acepromazine has excellent sedative effects and will often provide sufficient restraint for procedures such as radiography.
Benzodiazepines
Both diazepam and midazolam have marked sedative effects in rodents and rabbits, unlike their effects in dogs and cats. They can be administered by intraperitoneal, intramuscular, or intravenous injection and are often used in combination with other agents to produce balanced anesthesia. The sedative properties, although pronounced, are not usually sufficient to immobilize an animal for a minor procedure such as radiography.
Alpha2-Adrenoreceptor Agonists
Xylazine, medetomidine, and dexmedetomidine can be used to produce sedation with some analgesia in small mammals. At higher dose rates the effects can be sufficient to immobilize some animals. This effect is most reliable in the rabbit, and medetomidine or dexmedetomidine can be used to provide sedation and restraint for radiography in this species. One side effect of medetomidine and dexmedetomidine, vomiting (which is often seen in dogs and cats), does not occur in small mammals because these animals do not vomit. The other side effects of these agents, such as hyperglycemia, diuresis, and respiratory and cardiovascular system depression, do occur. A major advantage of these sedatives is that their action can be reversed by administration of specific antagonists. Both yohimbine and atipamezole have been used for this purpose in small mammals. Atipamezole is preferable because it has fewer side effects. It can be given through the subcutaneous, intraperitoneal, intramuscular, and intravenous routes. Absorption after subcutaneous injection is rapid; the drug generally acts within 5 to 10 minutes. Dose rates of 0.5 to 1.0 mg/kg are required, depending on the dose of medetomidine that has been administered.
Opioids
The use of opioids in the preanesthetic period to provide preemptive analgesia is discussed in Chapter 7. More commonly, opioids are used in small mammals in combination with sedative agents to provide chemical restraint and analgesia for minor procedures such as suturing superficial wounds and draining abscesses. In North America a commercially prepared mixture of fentanyl and droperidol (Innovar Vet) was formerly available for this purpose, and a similar mixture of fentanyl and fluanisone (Hypnorm) is still available in Europe. A mixture of acepromazine and butorphanol is useful for getting blood samples from rabbits because it provides some sedation and analgesia and dilates the ear veins.
GENERAL ANESTHESIA
Induction Techniques and Agents
Although techniques similar to those used for anesthetic induction in dogs and cats can be used in small mammals, practical considerations limit the use of the intravenous route except in rabbits. A wide range of different anesthetic agents can be used in these species, and suggested dose rates are given in Table 11-4. Formulas for anesthetic mixtures used in small mammals are given in Box 11-1.
TABLE 11-4
Anesthetic and Related Drugs for Use in Small Mammals∗
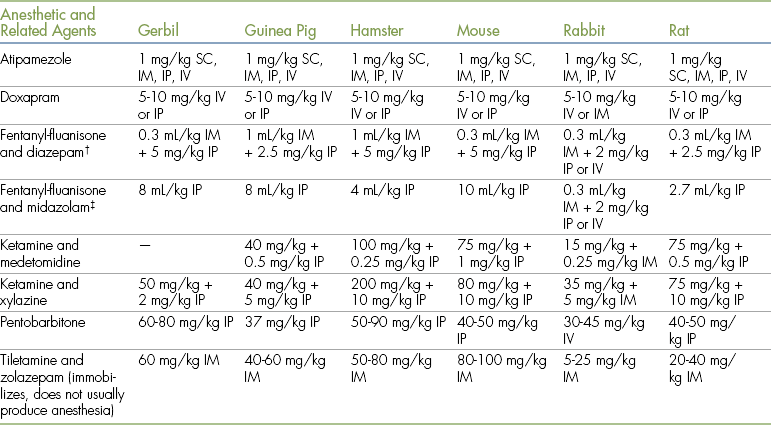
IM, Intramuscular; IP, intraperitoneal; IV, intravenous; SC, subcutaneous.
∗Note that there may be considerable variation among strains, and these dose rates should be taken as a general guide only.
†These drugs cannot be mixed together and must be given separately.
‡Doses are milliliters of a combination of fentanyl/fluanisone and midazolam, prepared as 2 mL water for injection plus 1 mL of 5 mg/mL midazolam and 1 mL of “Hypnorm” (Janssen, fentanyl-fluanisone).
In rabbits, the subcutaneous and intramuscular routes are often used, although intravenous injection of short-acting agents is also possible in some animals. For small mammals, intraperitoneal injection is a simple and relatively painless injection route for induction agents. The intraperitoneal route appears to be less painful than intramuscular injection, although the technique is less familiar. The technique is similar in most small rodents, in which an assistant extends the right hindlimb and injects the anesthetic into the middle of the right posterior quadrant of the abdomen. This technique avoids the bladder, which lies in the midline just in front of the pelvis. Use of the right side of the abdomen also avoids the cecum, which is large and thin-walled in rodents.
Although the technique for intraperitoneal injection is simple to carry out, administration of anesthetics by this route has important practical implications. If an anesthetic is given intravenously, the dose that is administered can be titrated to provide the required effect in that particular animal. It is therefore relatively simple to adjust the dose to account for individual, breed, and strain variation, and overdosing or underdosing is easy to avoid. When anesthetics are given intraperitoneally (or by subcutaneous or intramuscular injection), a calculated dose is given, and there is no opportunity to adjust it to suit the requirements of the particular animal. As large variations in response to anesthetics have been noted in small rodents, it is advisable to select an anesthetic regimen that has a wide safety margin (preferably one that is completely or partially reversible) if injection routes other than intravenous are used.
A further problem associated with use of the intraperitoneal or intramuscular route is that relatively high dose rates are required compared with those that are needed when drugs are given intravenously. One consequence is that recovery times tend to be prolonged, which is particularly undesirable in small mammals because of the high risk of hypothermia.
Cyclohexamine Agents
When used alone, ketamine has limited effect in small mammals, even at high doses. In rodents it barely immobilizes the animal and does not provide sufficient analgesia for even superficial surgical procedures such as suturing of skin wounds. In rabbits, use of ketamine alone provides restraint, but the degree of analgesia is insufficient for surgery. Ketamine-acepromazine and ketamine-diazepam or ketamine-midazolam produces surgical anesthesia in some rabbits, but these combinations generally produce only light anesthesia in small rodents. Ketamine is most effective when combined with an alpha2-agonist such as medetomidine or xylazine, because these agents have analgesic activity. Ketamine with medetomidine or xylazine produces surgical anesthesia in most rodents and rabbits, but the effects of these agents are less uniform in guinea pigs, and some animals may not be at a sufficient depth of anesthesia for an operation to be carried out humanely. Although relatively little information is available at present concerning the use of dexmedetomidine in small mammals, experience in other species indicates it will have equivalent effects to medetomidine. As in other species, the dose required is approximately 50% of the medetomidine dose. Because ketamine has limited effects when used alone in small mammals, reversal of xylazine, medetomidine, or dexmedetomidine will considerably reduce the length of the recovery period. However, because the analgesic effects are also reversed, another analgesic should be administered to provide postoperative pain relief.
Tiletamine in combination with zolazepam (Zoletil, Telazol) produces light to medium planes of anesthesia in small rodents. It offers little advantage in comparison with ketamine combined with diazepam or midazolam, and it produces less analgesia than ketamine in combination with xylazine or medetomidine.
Neuroleptanalgesics
As mentioned earlier, the combinations of fentanyl-droperidol and fentanyl-fluanisone provide restraint and analgesia in small mammals. Fentanyl and fluanisone also can be combined with a benzodiazepine to provide surgical anesthesia. The addition of midazolam or diazepam provides muscle relaxation and increases the depth of anesthesia. Recovery can be enhanced by reversal of fentanyl with a mixed agonist-antagonist opioid such as butorphanol or nalbuphine. This reverses the respiratory depression and some of the sedation caused by the fentanyl component of the anesthetic mixture but continues to provide postoperative analgesia. Although antagonists of benzodiazepine (e.g., flumazenil) can be administered to speed recovery, their duration of action is short, and resedation may occur.
The effects of fentanyl and droperidol together with benzodiazepines are less predictable, and this mixture is best avoided in small mammals.
Barbiturates
Although pentobarbital has been widely used for anesthesia of small mammals, it has a very narrow safety margin and produces severe cardiovascular and respiratory depression. Recovery from pentobarbital is prolonged and can be associated with involuntary excitement. For these reasons its use is best avoided. Thiopental and methohexital can be given by intravenous injection in rabbits to produce a short period of anesthesia, and they have effects similar to those seen in dogs and cats.
Propofol
Propofol produces short periods of surgical anesthesia in small rodents, but, because it must be given by intravenous injection, it is rarely used in these species. Propofol can be used in rabbits to provide a short period of light anesthesia, sufficient for induction of anesthesia followed by endotracheal intubation and maintenance of anesthesia with gas anesthetics. If high doses of propofol are given to rabbits in an attempt to produce a surgical plane of anesthesia, respiratory arrest often occurs. When propofol is administered to rabbits, it should be injected slowly (e.g., over 1 to 2 minutes for an induction dose in a 3-kg rabbit). When administered in this way, it rarely causes significant respiratory depression.
Alphaxalone
The steroid anesthetic alphaxalone can be used to induce anesthesia in rabbits when given by intravenous injection. This anesthetic causes minimal cardiovascular depression, and recovery after its use is relatively rapid.
Inhalation Anesthetics
Induction of anesthesia with inhalation agents is probably the safest and most effective means of providing anesthesia in small rodents. Although mask induction is possible, it is usually most convenient to induce anesthesia in an anesthetic chamber. Suitable chambers can be purchased commercially or can be constructed from clear plastic containers. The size of the chamber should be such that it can be filled rapidly with anesthetic vapor from the anesthetic machine. This will ensure that induction of anesthesia is rapid and smooth with a brief period of involuntary excitement. Anesthetic vapors are denser than air, so the chamber should be filled from the bottom and excess anesthetic gases removed from the top. A suitable design is shown in Figure 11-13.
Isoflurane, desflurane, and sevoflurane can all be used safely in small rodents. The concentrations required for induction and maintenance are similar to those used in dogs and cats. Provided the anesthetic chamber is filled rapidly, induction is generally complete in 2 to 3 minutes. Recovery is also rapid, with rodents recovering their righting reflex within 5 to 10 minutes after 20 to 30 minutes of anesthesia. After a further 10 to 15 minutes they will appear to be fully recovered. As in dogs and cats, induction of anesthesia and recovery are rapid with isoflurane and even more rapid with desflurane and sevoflurane.
Inhalation anesthetics should be delivered with a precision vaporizer. Induction of anesthesia in a chamber in which liquid anesthetic is placed on a gauze pad is extremely dangerous because high concentrations (>20%) of anesthetic vapor are produced.
After induction of anesthesia, the animal can be removed from the chamber and brief (<30 seconds) procedures carried out. For longer procedures it is usually more convenient to maintain anesthesia by placing a face mask on the animal. Suitable masks can be either purchased commercially or constructed from plastic syringes. As with dogs and cats it is important that waste anesthetic gases be scavenged effectively, and this is most easily achieved by using a commercial apparatus designed for this purpose.
The use of gas anesthetics in rabbits can be difficult because they frequently hold their breath when exposed to these agents. Breath-holding can be prolonged and is sometimes associated with marked bradycardia. If a mask is used, animals appear to resent the procedure and may struggle violently. If placed in an anesthetic chamber, they attempt to avoid inhaling the anesthetic and may make violent attempts to escape. It is therefore preferable to administer preanesthetic medication (e.g., acepromazine, diazepam, midazolam, or medetomidine) before inducing anesthesia with a face mask or chamber. After this medication has taken effect, a mask can be used to administer 100% oxygen for 1 to 2 minutes before introducing the induction agent. The animal may still hold its breath but is unlikely to struggle. If breath-holding occurs, the mask should be briefly removed and replaced when the animal commences breathing again. An alternative approach is to administer a short-acting induction agent such as propofol and maintain anesthesia with an inhalation agent.
Summary of Recommended Techniques
Because of the ease of control of depth of anesthesia, the simple and convenient method of induction, and the rapid recovery, inhalation agents are often the anesthesia method of choice in small mammals. If injectable anesthetics are preferred, ketamine with an alpha2-agonist, or fentanyl-fluanisone with a benzodiazepine are the combinations of choice. If an injectable anesthetic combination has been administered and the desired depth of anesthesia is not reached, it is possible to administer an additional drug to deepen anesthesia. However, it is often preferable to deepen anesthesia with a low concentration of an inhalation agent or alternatively to provide local analgesia by infiltrating the surgical site with local anesthetic. These techniques are also useful when dealing with high-risk patients. In these circumstances a low dose of an injectable anesthetic combination can be given to provide a light plane of anesthesia, and inhalation anesthetics or local anesthetics can be used to provide surgical anesthesia.
Intubation and Maintenance of Anesthesia
The apparatus used to anesthetize small rodents and rabbits is similar to that used in dogs and cats, but the size of the patient limits some of the equipment that can be used. Generally, anesthetic gases are delivered with a face mask; however, in rabbits endotracheal intubation is a relatively simple technique to perform and is recommended as a routine procedure. Endotracheal intubation can be carried out by visualizing the larynx with a laryngoscope and blade created for this purpose (such as a Wisconsin or Flecknell blade) or a canine otoscope. Alternatively, a blind technique can be used. These procedures are outlined in detail in Procedure 11-1, p. 316.
Uncuffed endotracheal tubes are preferred. A typical 3-kg rabbit requires a tube with a 3- to 3.5-mm diameter. Very small rabbits (<800 g) need tubes with a diameter of less than 2.5 mm, which can be purchased from specialist suppliers. A rabbit’s airway can also be maintained using a laryngeal mask. These are simple to use in this species and allow some control of respiration. As an alternative to intubation, a nasal catheter can be passed and positioned in the back of the pharynx. This allows oxygen supplementation during oral surgery but does not enable ventilation to be assisted effectively. Nasal catheters can also be used in small rodents to deliver oxygen or anesthetic gases (Figure 11-14). This is particularly useful when carrying out dental procedures. Waste anesthetic gases can be removed by placing an extract tube close to the animal’s nose, and the potential problem reduced by using appropriately low fresh gas flows.
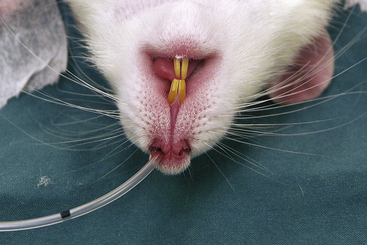
FIGURE 11-14 Nasal catheter being used to deliver oxygen to a rat. The catheter is an infant nasogastric feeding tube, but any suitably sized soft catheter can be used.
An anesthetic machine with an out-of-circuit precision vaporizer should be used. Open, non-rebreathing systems are preferred to closed-circuit systems because they offer less resistance and have less equipment dead space. Examples of suitable non-rebreathing systems include the Bain circuit and Ayres T-piece. With smaller rabbits, it is advisable to use low–dead space pediatric connectors to attach the endotracheal tube to the breathing circuit. Low–dead space T-piece systems designed for use in human beings are also useful for rabbits (Figure 11-15). Fresh gas flow rates are calculated in the same way as for dogs and cats (see Box 4-3).
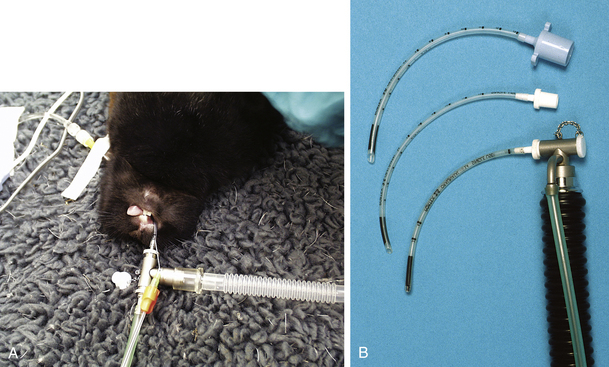
FIGURE 11-15 A, Low–dead space pediatric T-piece and endotracheal connector suitable for anesthetic delivery in a rabbit. B, Endotracheal tube connectors. Top, Standard connector; middle, pediatric connector; bottom, pediatric connector and T-piece.
Monitoring
Depth of Anesthesia: Before a surgical or other painful procedure is started, it is essential to ensure that the animal is at an appropriate depth of anesthesia. The most reliable method in rodents is to assess the pedal withdrawal reflex (discussed in Chapter 5) or tail pinch reflex. To assess the tail pinch, the operator firmly pinches the tip of the tail with fingernails. It is important to pinch hard enough to produce a painful stimulus, but not so hard as to damage the tail. If the animal is too lightly anesthetized for surgery, it will flick its tail and may vocalize. The tail pinch response is usually lost at light to medium planes of anesthesia, and this is followed by a loss of the pedal withdrawal response at medium to deep planes of anesthesia. Most surgical procedures can be carried out when the pedal withdrawal reflex is absent or barely detectable. In rabbits and guinea pigs the ear pinch reflex can also be used to measure anesthetic depth.
Ocular reflexes are not as useful in small mammals as in the dog and cat. With most anesthetic regimens, the position of the eye remains fixed in rodents, and the palpebral (blink) reflex may still be present at surgical planes of anesthesia. In rabbits, there is considerable variation in loss of ocular reflexes; however, at deep planes of anesthesia the eye may rotate and protrude. Because cardiac arrest may occur shortly after the animal reaches such a deep plane of anesthesia, this appearance indicates that supportive measures should be initiated immediately and administration of anesthetic should be reduced or terminated.
Heart Rate and Rhythm: The small size of rodents and rabbits and their rapid heart rate can make it difficult to monitor heart rate and rhythm, and it is not usually possible to palpate a peripheral pulse. Auscultation of the chest wall is possible in rabbits and guinea pigs but difficult in smaller rodents. An esophageal stethoscope can be used in rabbits, and the heartbeat can be detected by palpating the chest wall in all species. However, because the heart rate often exceeds 250 beats per minute (bpm) in many of these animals, it is not possible to accurately assess the heart rate. Problems can also arise when an electrocardiograph is used, because many instruments have an upper heart rate limit of 250 or 300 bpm and may also be unable to detect the low-amplitude signals generated in small rodents.
Capillary Refill Time: The small size of rodents usually prevents use of capillary refill time for assessment of peripheral perfusion, although it is possible to assess this in rabbits. The color of the mucous membranes can give some indication of problems associated with blood loss, cyanosis, and poor peripheral perfusion. In addition to inspection of the gingiva, the color of light reflected in the eyes can be used to detect cyanosis or pallor caused by blood loss in albino animals.
Blood Loss: Because these animals are small, total blood volume is small—approximately 70 mL/kg of body weight. A 100-g hamster will have a total blood volume of only 7 mL. As in dogs and cats, loss of more than 15% (approximately 1 mL in this example) can lead to signs of circulatory failure. It is therefore critically important to monitor blood loss by carefully weighing swabs and assessing other losses at the surgical site.
Arterial Blood Pressure: Blood pressure can be monitored in rabbits either with a catheter placed in the central ear artery or noninvasively with an oscillometric technique. The pressure cuff should be placed either on the forelimb, just proximal to the elbow, or on the hindlimb, proximal to the stifle (Figure 11-16). The success of this technique depends on both the size of the rabbit and the particular monitor. As an alternative, a Doppler probe can be placed over a suitable artery, and, if the probe is combined with use of a blood pressure cuff and sphygmomanometer, an estimate of arterial pressure can be obtained (see Chapter 5).
Respiratory Rate and Depth: The pattern and depth of respiration can be monitored by observing the chest movements, although this becomes difficult once surgical drapes have been placed. Because of the small size of these animals, there is usually no reservoir bag in the anesthetic circuit, and respiration cannot be monitored by bag movement. It is helpful to use an electronic monitor, but as with the electrocardiograph, the small size of the animal and rapid respiratory rate can make some monitors ineffective.
Although the pattern and rate of respiration change during anesthesia, they can both vary greatly depending on the anesthetic regimen used. Becoming familiar with one or two regularly used regimens allows changes to be interpreted more reliably. In general, once anesthesia has been induced, respiratory rate decreases markedly, especially because most of these animals will show tachypnea before induction. Typical respiratory rates during anesthesia are 50 to 100 breaths/min for small rodents and 30 to 60 breaths/min for rabbits. A reduction to less than 50% of the estimated normal respiratory rate (see Table 11-1) should give cause for concern. As in dogs and cats, it is more common to see gradual changes in rate, rather than a sudden reduction. For this reason, it is helpful to keep a written anesthetic record when assessing the state of the animal during anesthesia.
Pulse Oximetry: Pulse oximeters can be used to monitor both the adequacy of oxygenation and the heart rate, but not all instruments function well in small rodents. The high heart rates may exceed the upper limits of the monitor, and the low signal strength means the signal may not be detectable. A monitor with an upper limit of at least 350 bpm is needed, and it is useful to have a variety of different probe designs. A reliable signal can usually be obtained by placing the probe across the hind foot in small rodents or across a toe in larger rabbits, but the tail, tongue, and ear are also useful in some animals (Figures 11-16 and 11-17).
Capnography: Side-stream capnographs can be used to monitor respiratory function in small animals, although the volume of gas sampled may be very large in relation to the animal’s tidal volume. Mainstream capnographs introduce too much equipment dead space into the anesthetic breathing circuit and are not recommended in these species, but low–dead space, human pediatric versions can be used successfully in rabbits.
Thermoregulation: It is critically important to monitor and maintain body temperature during anesthesia and in the postoperative period. Because of their small body size, rodents and rabbits have an increased ratio of surface area to body weight, which may lead to rapid cooling during anesthesia. Heat loss can be much more rapid than in dogs and cats. For example, the rectal temperature in a mouse can fall 5° to 6° C (9° to 11° F) in 5 to 10 minutes after induction of anesthesia. The following procedures help avoid hypothermia:
• Monitor rectal temperatures with an electronic thermometer rather than a glass clinical thermometer. Glass clinical thermometers can indicate a minimum temperature of only 35° C, and the animal may be colder than this when the first measurement is made.
• Adopt good standards of asepsis, but keep the area of fur that is shaved at the surgical site to a minimum, and use the minimum quantity of skin disinfectant.
• Place the animal on a warming pad as soon as it has lost consciousness, and provide additional insulation if needed.
• Always warm fluids to body temperature before administration.
• Continue measures to prevent heat loss in the recovery period (see the following section).
POSTOPERATIVE CARE
The provision of appropriate postoperative care is critical to the successful outcome of anesthesia and surgery in small mammals. Supportive measures to maintain body temperature must be continued, and a quiet, warm, secure environment should be provided. Because heat loss can occur relatively rapidly, an appropriate recovery environment should be set up before commencement of anesthesia and an operation. The animal can then be transferred to the recovery area immediately after completion of the operation. While the animal is immobile and unconscious, an environmental temperature of approximately 35° C (95° F) should be maintained. This can be lowered to 26° to 28° C (79° to 81° F) as the animal recovers. Warm and comfortable bedding must be provided. Synthetic sheepskin is ideal, but if this is unavailable, shredded paper or tissues can be used. Sawdust is unsuitable because it tends to crust around the nose, eyes, and mouth. Good-quality hay should be provided to guinea pigs and rabbits once they have recovered their righting reflex. This type of bedding allows the animal to surround itself with insulating material, which provides both warmth and a sense of security and encourages early feeding. Small mammals of other species should also be encouraged to eat soon after recovery and should be given their preferred foods.
Animals should also be provided with water, but care must be taken that they do not spill water bowls, because the animal will lose heat rapidly if it becomes wet. The animal may also fail to drink from an unfamiliar water container, and when a case history is obtained before anesthesia it is important to find out what type of container the animal is accustomed to using. In most circumstances, it is advisable to administer warmed (37° C or 98.6° F) subcutaneous or intraperitoneal dextrose-saline (4% dextrose, 0.15% saline) at the end of the operation to provide some fluid supplementation in the immediate postoperative period.
ANESTHETIC EMERGENCIES
Changes in the depth and pattern of respiration usually precede respiratory arrest. Careful monitoring of respiratory function will usually allow corrective measures to be taken before an emergency arises. If the animal has been intubated, respiration can be assisted by delivery of 100% oxygen from the anesthetic machine. As with larger species, it is important to check that the endotracheal tube is properly positioned and has not become disconnected from the breathing circuit or obstructed. If the animal has not been intubated, respiration can be assisted by extending the head and neck and gently compressing the chest. Attempts to assist ventilation with a face mask are usually unsuccessful. In small rodents, a soft piece of rubber tubing can be placed over the nose and mouth and the lungs inflated by gently blowing down the tube (Figure 11-18). Respiration can also be stimulated by administration of doxapram, but this drug has a relatively short duration of action (approximately 10 minutes), and repeated doses may be needed. Efforts should be made to determine the cause of the respiratory depression and to initiate corrective measures.
Circulatory Failure
Treatment of circulatory failure and cardiac arrest is similar to that in dogs and cats, but the small size of these animals causes some practical problems. Fluid therapy is difficult because of the small size of the superficial vessels, although it is possible to place over-the-needle catheters in the tail vein of rats and the medial tarsal vein in guinea pigs. In rabbits, intravenous access is much easier, and catheters can be placed in the marginal ear veins or cephalic veins. The jugular vein is relatively mobile in the rabbit and is more difficult to locate and catheterize than in the dog and cat.
Loss of blood can be treated by transfusion from a donor animal. Fortunately, problems of incompatibility are rare on initial transfusion; however, it is likely to be more difficult to locate a suitable donor than when dealing with dogs and cats. As an alternative, a plasma volume expander such as dextran or hetastarch can be administered. All of the commonly available products can be administered safely to small mammals, providing appropriate allowance is made for their smaller circulating volumes. In smaller species in which intravenous access is not practical, intraperitoneal or subcutaneous administration of warmed electrolyte solutions can slowly replace fluid deficits or blood loss but will be of minimal benefit if rapid hemorrhage is occurring. As discussed earlier, preventing problems by minimizing hemorrhage through meticulous surgical technique is important.
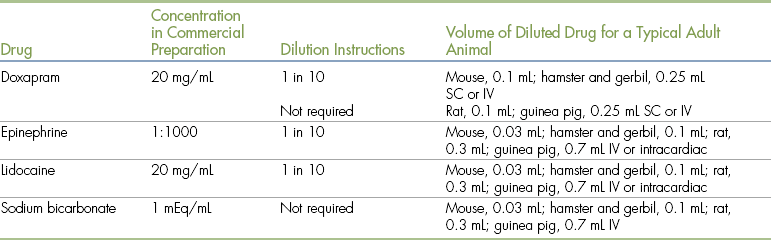
If cardiac arrest occurs, external cardiac massage and emergency drugs such as epinephrine can be used to try to resuscitate the animal (see Chapter 12). One significant problem is the practical difficulty of rapidly calculating drug dose rates when an emergency occurs. It is much simpler to use a list of dose rates and volumes, expressed as the dose volumes for a typical adult animal of each species. This will help avoid errors and speed therapy (Table 11-5).
POSTOPERATIVE ANALGESIA
As discussed in Chapter 7, the use of analgesics in veterinary practice has become more widespread in recent years. Although most dogs and cats now receive at least some perioperative analgesia, these drugs are often not used as frequently in small mammals. This is probably a result of a number of factors, including poor ability to recognize pain in these small animals and a lack of knowledge about the safety and efficacy of analgesic agents. However, it is critically important to provide postoperative analgesia to these patients, because most small mammals will fail to eat or drink if they are experiencing postoperative pain.
Pain Assessment in Small Mammals
Pain assessment in dogs and cats is not always easy, but most veterinarians and veterinary technicians are relatively familiar with the normal behavior of these species. The normal behavior and general appearance of small rodents and rabbits are often less well appreciated, and as a result the signs associated with pain may be overlooked. In addition, several species of small mammals are nocturnal and may not be active when observed during normal working hours. They may also remain immobile in the presence of an observer if they perceive the observer as a threat. It is therefore not always easy to use behavior and changes in posture to assess pain. However, it is important to overcome these difficulties so that pain can be prevented or controlled effectively in these small animals.
As in dogs and cats, an initial assessment of the animal should be made without disturbing the animal. The animal’s appearance and posture may be abnormal, and it may appear hunched. Its coat may be unkempt and ruffled because of a lack of grooming and the presence of piloerection. Rats may have a blackish discharge around their eyes and nose because of a buildup of secretions from their harderian glands. It is uncertain whether this buildup of material results from reduced grooming or whether it is a response to stress, but it is a valuable indicator that the animal is not healthy and may be in pain. While it is being observed, the animal may demonstrate normal inquisitive behavior and explore its environment, but as mentioned earlier, if it remains motionless, this may be because it feels threatened rather than because it is in pain. If the animal has positioned itself in the back of its cage or pen or has hidden in bedding, this can be a sign of fear but may also indicate pain.
When encouraged to move, the animal may have an abnormal gait or posture and may show uncharacteristic signs of aggression. Rats, mice, and gerbils will usually rear when investigating what has disturbed them, and the absence of this behavior may indicate pain. When handled, rather than attempting to evade capture, animals in pain may be apathetic or may be aggressive and bite the handler. When it is examined, the animal may respond to manipulation or palpation of a painful area by vocalizing or trying to bite. Confusingly, some small mammals such as guinea pigs will also vocalize loudly when not in pain and may respond to any manipulation by tensing their muscles and remaining immobile. Similar immobility can also be seen in rabbits. Abdominal pain in rabbits, rats, and mice often produces characteristic behaviors involving contraction of the abdominal muscles, pressing of the abdomen to the floor, and, in rabbits and rats, arching of the back.
Rabbits and small mammals may stop eating and drinking when experiencing pain. This can be difficult to detect if food is provided ad lib, but the subsequent loss in body weight can easily be monitored. This is one of the easiest ways of following a small mammal’s progress after surgery or during treatment of any disease condition. The inappetence caused by pain is a serious problem in small mammals, because failure to drink can rapidly lead to significant dehydration and lack of food intake can predispose to the development of hypoglycemia in small rodents. In rabbits, guinea pigs, and chinchillas, disturbances in food intake can lead to the development of life-threatening gastrointestinal disturbances.
As experience is gained in observing the normal behavior patterns of small mammals, abnormalities will be detected with greater confidence. Although relatively specific signs of pain such as guarding of an injured area may be seen, many of the signs are relatively nonspecific and can also occur in response to nonpainful conditions. It is therefore important to consider the appearance of the animal in relation to its case history and other clinical findings. Although it is highly desirable to try to assess pain in each individual patient so that appropriate analgesia therapy can be administered, it is not unreasonable to accept that some analgesic treatment will be needed after every surgical procedure.
Analgesic Agents
None of the analgesics that are currently marketed for use in dogs and cats provide any product information regarding their use in small mammals. It is worth noting, however, that all of these products were originally tested for safety and efficacy in small rodents. This information provides reassurance that the drugs can be used safely to provide effective pain relief in these species. There is less information available on the use of analgesics in rabbits and guinea pigs, but extensive clinical experience indicates that most analgesics can be used safely in these animals. Suggested dose rates are listed in Table 11-6. The options for pain management are similar to those available for dogs and cats, but the small size of the animal may limit the use of techniques such as epidural administration of drugs or use of fentanyl patches.
TABLE 11-6
Analgesic Agents for Use in Small Mammals∗
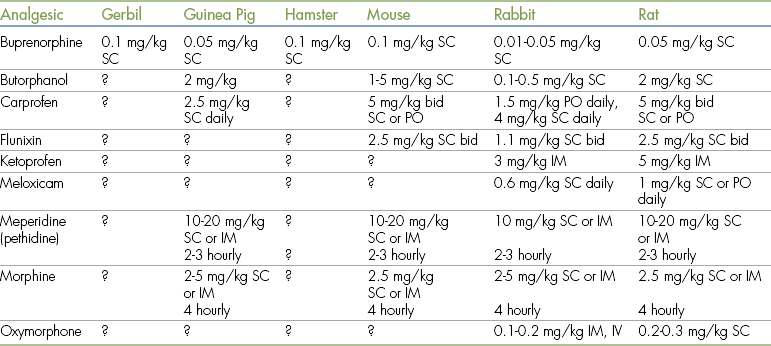
IM, Intramuscular; IV, intravenous; PO, by mouth; SC, subcutaneous.
∗These are only suggestions based on clinical experience and the limited published data that are available. Dose rates should be adjusted depending on the clinical response of the animal. A “?” indicates that there is insufficient information to make a firm recommendation of an appropriate dose.
Data modified from Flecknell PA, Waterman-Pearson AE, eds: Pain management in animals, London, 2001, Harcourt International.
All of the morphine-like drugs that can be used in dogs and cats can also be used in small mammals but often have a shorter duration of action in these species. Meperidine lasts for only 30 to 60 minutes in rodents, for example. Buprenorphine, which has a duration of action of approximately 6 to 12 hours in small mammals, is often preferred. Some authorities question whether partial agonists such as buprenorphine are potent enough to control severe pain; however, clinical experience suggests that this analgesic is effective after most surgical procedures in small rodents and rabbits. Buprenorphine may cause behavioral abnormalities in small rodents (such as eating sawdust bedding in rats). However, this side effect appears rare, and most animals appear to benefit from the use of buprenorphine after surgery. If other opioid analgesics are to be used, repeated administration is likely to be needed to provide effective pain relief. One useful means of avoiding the need for frequent injections of drug is to combine administration of an opioid with the use of a nonsteroidal antiinflammatory drug (NSAID) (see the following section).
Nonsteroidal Antiinflammatory Drugs
NSAIDS (particularly the more potent NSAIDs such as carprofen, ketoprofen, and meloxicam) can provide very effective pain relief in small mammals. Considerable basic information is available from pharmaceutical companies concerning the safety and efficacy of these analgesics in small rodents. Although there have been no reports of adverse reactions to these drugs in small mammals, it seems advisable to adopt the same precautions as those in dogs and cats (see Chapter 7). Prolonged use (more than a few days) should be avoided when possible, although clinical experience suggests that meloxicam can be used for extended periods in rabbits to control dental pain. Because of the risks of renal toxicity should hypotension occur during anesthesia, only carprofen or meloxicam should be administered preoperatively. A significant advantage of the use of NSAIDs is that they appear to have a prolonged duration of action in small mammals. A single dose of carprofen, ketoprofen, or meloxicam may provide analgesia for 12 to 24 hours. Meloxicam has the additional advantage of being available in some countries as a palatable liquid preparation. This makes it easier for owners to continue analgesic administration if needed.
Local Anesthetics
Local anesthetics can be used to provide postoperative analgesia. As in dogs and cats, they can be infiltrated around surgical wounds or administered as specific nerve blocks. Although the safety of these agents in small mammals is similar to that in dogs and cats, it is relatively easy to inadvertently overdose rodents because of their small size. Care must be taken to calculate the dose accurately (maximum recommended doses in rodents: lidocaine 10 mg/kg, bupivacaine 4 mg/kg; maximum recommended dose in rabbits: same as for cats, see Chapter 7). It is important to note that the duration of action of some local anesthetics may be shorter in rodents than in larger species, such as dogs. As mentioned earlier, the application of topical agents such as EMLA cream provides analgesia for venipuncture or placement of intravenous catheters.
Chronic Pain
Small mammals may have a range of chronic conditions that can cause pain. Rats may have arthritis; guinea pigs, chinchillas, and rabbits may have dental disease; and neoplasia is common in many species. NSAIDs can often be used successfully to control the pain associated with these conditions, although it is necessary to carefully monitor the patient for side effects, especially during long-term use of these agents.
Administration of Analgesics
Intravenous administration of analgesics is difficult in rodents but relatively straightforward in rabbits. Because of the small muscle mass in rodents, subcutaneous administration is preferred. Oral administration can be difficult and may require firm physical restraint that can exacerbate pain from surgical wounds. Provided the animal is eating, analgesics can be added to highly palatable food items (e.g., doughnuts for rats and mice). Analgesics for rats or mice can also be incorporated into fruit or meat-flavored gelatin. Commercial flavored gelatin should be prepared with half of the recommended quantity of water, and after the mixture is allowed to cool, a measured quantity of analgesic is added. After the gelatin has set, it can be cut into cubes of an appropriate weight. The remaining gelatin should be labeled and stored in a refrigerator, taking note of any legal requirements concerning storage of controlled drugs. Commercial gelatin mixed with beef extract may also be used, as can the commercial spread Nutella.
As in dogs and cats, preemptive administration of analgesics may provide more effective pain relief than postoperative use and may reduce the amount of anesthetic required for the surgical procedure. If volatile anesthetics are being used, opioids can be administered safely before the operation, and the concentration of anesthetic delivered can be reduced as necessary to maintain a safe level of anesthesia. For example, when buprenorphine is administered preoperatively to rodents, the concentration of isoflurane needed to provide surgical anesthesia can be reduced by approximately 25% to 50%. If injectable agents are used, preemptive analgesia is more difficult, because injectable anesthetics are often given as a single dose by the intraperitoneal or subcutaneous route and cannot be titrated according to their effect in the individual animal. Because the commonly used opioids will potentiate the actions of injectable anesthetic agents, their preoperative administration could lead to inadvertent anesthetic overdose. Because of the limited information available concerning the degree of interaction between injectable anesthetics and opioids, it is safer in this case to administer opioid analgesics at the end of the operation in small rodents, as the depth of anesthesia is becoming lighter.
Not all NSAIDs can be safely used preoperatively, because of potential adverse effects such as renal hypotension and prolonged bleeding times. Carprofen and meloxicam can both apparently be safely used for preemptive analgesia.
Although the use of analgesic drugs remains the most important technique for reducing postoperative pain, pain medication must be integrated into the overall plan for perioperative care. Animals must be provided with a postoperative recovery area appropriate to their particular needs. For example, it is stressful for a small mammal to recover in the same room as its predators (e.g., cats), and the resulting change in the animal’s behavior pattern could mask signs of pain.
REVIEW QUESTIONS
1. Glycopyrrolate should be used in rabbits, instead of atropine, because:
a. Atropine is highly toxic in rabbits
b. Many rabbits have high levels of atropinase, so atropine is relatively ineffective
2. Pulse oximeters can be used in small mammals, but they may not be reliable because:
a. The heart rate of the animal may exceed the upper range of the instrument
b. The hemoglobin absorption characteristics are different in rodents and dogs and cats
c. Pulse oximeters do not function on animals that have dark fur
3. The position of the eye cannot be used to assess the depth of anesthesia in rodents because:
a. The eye is too small to assess its position accurately
b. The position of the eye does not change during anesthesia
c. The eye rotates downward in very light planes of anesthesia
4. An advantage of using medetomidine combined with ketamine for anesthesia of rodents and rabbits is that:
a. It is readily absorbed from body fat
b. It can be given by mouth to produce anesthesia
c. It promotes gut motility and so reduces the occurrence of postoperative inappetence
d. It can be partially reversed using atipamezole, allowing faster recovery
5. An adult mouse weighing 40 g will have a blood volume of approximately:
6. When fluids such as lactated Ringer’s solution are given to small mammals, they should be:
a. Used at about 4° C so that they are rapidly absorbed
b. Administered orally, because it is not possible to use any other route
c. Warmed to body temperature before administration, to avoid causing hypothermia
d. Given only postoperatively, to avoid overloading the circulation
7. Anesthetic breathing circuits for use in small rabbits should:
a. Be constructed of only plastic components because rabbits are allergic to latex
b. Have low equipment dead space
8. When small rodents are anesthetized with injectable anesthetics:
a. It is not necessary to administer oxygen, because most anesthetics stimulate respiration
b. Oxygen should be administered, because most anesthetics depress respiration
c. Carbon dioxide should be included in the fresh gas mixture to stimulate respiration
d. Nitrous oxide should always be used; otherwise the depth of anesthesia will be insufficient for surgery
9. Postoperative analgesic should be given to rodents and rabbits to alleviate pain, but:
a. NSAIDs cannot be used because they cause gastric ulceration at normal therapeutic doses in these species
b. Opioids (narcotics) cause severe respiratory depression and so must never be used
c. Opioids must be given with care if a neuroleptanalgesic mixture has been used for anesthesia
d. Local anesthetics cannot be used because they produce cardiac arrest even at low doses in these species
10. If postoperative pain is not alleviated in rabbits, then: