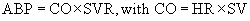Anesthetic Problems and Emergencies
After completion of this chapter, the reader will be able to:
• List the most common reasons why anesthetic emergencies occur, including problems arising from human error, equipment failure, and the adverse effects of anesthetic agents.
• Explain how anesthesia of geriatric and pediatric patients differs from anesthesia of healthy adult dogs and cats.
• Describe the problems involved in anesthetizing each of the following: brachycephalic dogs; sighthounds; obese animals; and patients affected by trauma or cardiovascular, respiratory, hepatic, or renal disease.
• Describe the role of the veterinary technician in responding to anesthetic emergencies.
• Explain the importance of oxygen supplementation in the trauma patient.
• List various ways of administering oxygen
• List the most common causes of the following anesthetic problems: inadequate anesthetic depth, excessive anesthetic depth, pale mucous membranes, prolonged capillary refill time, dyspnea, tachypnea, bradycardia, tachycardia, and cardiac arrhythmias.
• Describe the appropriate response to common emergencies, including dyspnea, respiratory arrest, and cardiac arrest.
• List the most common problems that may arise in the recovery period and the appropriate action that can be taken to prevent or treat these problems.
General anesthesia poses little risk to most patients when performed by capable personnel using an anesthetic protocol appropriate for the animal. Emergencies are uncommon, and the overwhelming majority of patients recover from anesthesia with no lasting ill effects. After successfully anesthetizing hundreds of patients, it is easy for the technician to be lulled into a false sense of security. However, it is vitally important that the anesthetist remember that every anesthetic procedure has the potential to cause the death of the animal. The anesthetist must remain watchful for problems that may arise in even the most routine anesthetic procedure.
A survey of British veterinary clinics revealed a mortality rate of one death for every 870 anesthetic procedures in healthy dogs and one death for every 552 procedures in healthy cats. The same study found a mortality rate of 1 in 30 patients where systemic disease was present. An American study of 3239 cases found the incidence of anesthetic complications to be 12% in dogs and 10.5% in cats, with a mortality rate of 0.43% (4.3 per 1000) in both dogs and cats.∗ A Canadian study of 16,000 anesthetized animals found the incidence of cardiac arrest to be approximately 1 in 900 patients. Of the dogs with anesthetic complications, bulldogs, Pekingese, and other brachycephalic breeds; Weimaraners; and Jack Russell terriers were disproportionately represented. Emergency anesthesia was associated with a much greater risk than elective anesthesia.
This chapter describes problems that may arise during anesthesia, ranging from minor (such as maintaining appropriate anesthetic depth) to major (including respiratory arrest and cardiac arrest). Appropriate responses to various anesthetic emergencies are presented, and the reasons the anesthetic problems may arise (and procedures for their prevention) are emphasized. The challenges associated with anesthesia of patients with special problems such as heart disease or brachycephalic conformation are also discussed.
REASONS THAT ANESTHETIC PROBLEMS AND EMERGENCIES ARISE
Although an awareness of the correct response to an anesthetic emergency is essential, it is even more important to understand why emergencies arise and how they may be prevented. Most anesthetic problems and emergencies are the result of one or more of the following factors: (1) human error, (2) equipment failure, (3) adverse effects of anesthetic agents, and (4) patient-related factors.
Human Errors That May Lead to Anesthetic Problems and Emergencies
Human error is, unfortunately, a contributing cause in some anesthetic deaths. Human errors commonly encountered in veterinary practice include the following:
• Failure to obtain an adequate history or perform an adequate physical examination on the patient
• Inadequate experience with the anesthetic machine or anesthetic agents being used
• Failure to devote sufficient time or attention to the anesthetized patient
• Failure to recognize and respond to early signs of patient difficulty
Failure to Obtain an Adequate History or to Perform a Physical Examination
Ideally, every patient scheduled for anesthesia should have a complete physical examination, and a thorough history should be obtained. In practice, this is not always possible. Animals are sometimes dropped off at the veterinary clinic by owners who are in a hurry and reluctant to stop and answer questions. Animals may be brought in by neighbors or friends of the owner, or by other persons unfamiliar with the animal’s history. The receptionist or other person admitting the animal to the hospital may fail to ask important questions or may not transmit the information to the anesthetist or veterinarian. The physical examination is sometimes cursory or omitted entirely. The net result is that significant information may be overlooked. For example, the anesthetist may be unaware that a patient has not been fasted or that an animal scheduled for surgery is dehydrated as a result of vomiting and diarrhea. An anesthetic protocol that is safe for a healthy patient could be inappropriate for these animals, and an anesthetic problem or even death of the patient could result.
Lack of Familiarity with the Anesthetic Machine or Anesthetic Agents
It is the responsibility of the veterinarian, and in some states or provinces a requirement of the Veterinary Association, to ensure that his or her personnel are sufficiently trained and knowledgeable to competently perform all required procedures. Although unskilled personnel working under a veterinarian’s direct supervision may assist with some aspects of an anesthetic procedure, skilled tasks, such as induction of anesthesia and monitoring of anesthetized patients, must be assigned only to personnel (veterinarians or technicians) who have sufficient training, knowledge, and experience to recognize abnormalities and danger signals and to respond appropriately.
Incorrect Administration of Drugs
Many anesthetic agents have a narrow margin of safety between therapeutic and toxic doses. The incorrect administration of drugs may have serious or even fatal consequences, and may arise from any of the following:
• Failure to weigh the patient and calculate an accurate dose.
• Mathematical errors (particularly decimal errors, which can result in an error of 10 times or 100 times in the amount of drug given).
• Use of the wrong medication (e.g., calculating a dose of atropine and drawing up acepromazine instead).
• Use of the wrong concentration of a medication. This is a common problem with drugs that are available in several different concentrations (e.g., atropine and acepromazine). Obviously, the concentration used in calculating the dose must be the same as that drawn up into the syringe.
• Administration of anesthetics by the incorrect route (e.g., administration of an intramuscular dose of ketamine by the intravenous [IV] route).
• Confusion between syringes drawn up for two different patients. This involves either a failure to label the syringes or a failure to read the labels correctly.
Personnel Who Are Preoccupied or in a Hurry
Although efficiency is desirable in any anesthetic procedure, it is not necessary or advisable that the anesthetist feel hurried. A technician who is feeling rushed is more likely to make mistakes such as injecting barbiturates perivascularly or inserting an endotracheal tube into the esophagus. Unfortunately, it is common for the technician working in a busy practice to feel pressured and distracted. The technician responsible for anesthesia may be simultaneously called on to restrain patients for examination or procedures, answer the phone, perform laboratory tests, take radiographs, discharge animals, clean soiled kennels, and carry out other similar tasks. However, when an animal is anesthetized, the technician’s top priority must be monitoring that patient, because failure to satisfactorily perform this duty may result in the death of the animal.
The technician usually does not have the luxury of being constantly by the animal’s side throughout the procedure. In most work situations, periodic absences are necessary. However, the anesthetist should return to check the patient at least once every 5 minutes, or more frequently if the patient’s status requires close monitoring. If necessary, other tasks must be temporarily set aside to allow the anesthetist to return to the patient.
Fatigue
Veterinarians and technicians often become fatigued, particularly at the end of a busy day. Anesthetic emergencies may arise when personnel are tired and less alert than normal, possibly because minor problems are not detected and corrected at an early stage. If possible, surgeries that are lengthy or difficult should be scheduled early in the day.
Inattentiveness
One of the most serious human errors in anesthesia is the failure to monitor and recognize danger signals. It is obviously better for the patient—and easier for the anesthetist—to detect and address anesthetic problems early, rather than late. For example, when anesthetic depth is excessive, an animal may show a gradually decreasing respiratory rate, from 10 to 12 breaths/min in a dog or cat to fewer than 8 breaths/min (at which point the anesthetist should consider adjusting the vaporizer to a lower setting); then to 4 breaths/min (at which point the vaporizer should be turned off and the animal bagged with oxygen); then from 4 breaths/min to 0 (at which point cardiac arrest may quickly follow if these changes are not recognized and managed).
The anesthetist’s attitude toward patient care is a key factor in the safety of anesthesia. The conscientious anesthetist will monitor the animal often to ensure that the patient is not in trouble. A brief check of the patient’s pulse rate, respiratory rate, and capillary refill time takes less than 1 minute and gives the anesthetist a good assessment of the patient’s status. The best attitude is one of low-level anxiety that is relieved only when a quick examination of the patient reveals that all vital signs and depth indicators are within acceptable limits.
Equipment Issues That May Lead to Anesthetic Problems and Emergencies
Equipment failure is an uncommon cause of anesthetic emergencies, but it does occur. In many cases, failure of the anesthetic machine is, in fact, caused by a failure of the operator to maintain and monitor the machine properly. The importance of a preanesthetic check of the anesthetic machine, as described in Chapter 4, cannot be overemphasized.
The following equipment problems are occasionally encountered in routine anesthesia.
Carbon Dioxide Absorbent Exhaustion
Patients on a rebreathing system rely on the carbon dioxide absorbent to remove expired CO2 from the circuit, preventing inhalation of excessive levels of this toxic gas. If CO2 is not removed from the circuit, the patient will experience hypercapnia (elevated blood CO2). Signs of this disorder include tachypnea (rapid respiration), tachycardia, and cardiac arrhythmias. Examination of the CO2 absorbent may reveal an obvious color change, if exhausted, although this does not always occur.
Empty Oxygen Tank
Failure to deliver oxygen to the patient is one of the most serious and yet one of the most easily preventable mistakes that an anesthetist can make. Before starting an anesthetic procedure, the anesthetist must ensure that the tank contains sufficient oxygen for the duration of the surgery. (For information on calculating the amount of oxygen present in a tank, refer to p. 108.) During the procedure, the oxygen tank pressure and flow meter should be checked every 5 minutes during anesthesia. The anesthetist must ensure that either oxygen or room air is continuously provided to the patient. At the end of a procedure, the patient should be disconnected from the machine before the oxygen flow meter is turned off.
It is important to be able to recognize when the machine is no longer delivering oxygen to the patient. If the oxygen flow meter reads zero, the patient is not receiving any oxygen, regardless of the oxygen tank pressure. Occasionally when the oxygen tank is nearly empty, the oxygen tank pressure gauge reads zero but the flow meter indicates some oxygen flow. Even though the tank is still delivering a small amount of oxygen, loss of oxygen pressure is imminent and the tank must be changed immediately.
The anesthetist must be aware of the proper response when oxygen delivery to the patient is stopped, whether because of machine malfunction or an empty tank. If the oxygen flow stops (i.e., the flow meter reads zero despite the efforts of the anesthetist to establish flow) and the patient is on a non-rebreathing system, the anesthetist should immediately disconnect the hose from the endotracheal tube, allowing the patient to breathe room air until oxygen delivery is reestablished. (If a circle system with a full reservoir bag is in use, the patient can remain connected for a short period of time.)
Misassembly of the Anesthetic Machine
It is essential that the person handling the anesthetic machine be familiar with every connection, hose, dial, and component of the machine. Before using an unfamiliar machine, the anesthetist should take a few minutes to examine it carefully for the location of the controls and to understand the direction and path of gas flow within the machine. Every time a connection, such as a Bain circuit, is added or removed, the anesthetist must trace the flow of gas, ensuring that the correct pattern of flow is maintained and that all connections are secure. Failure to do so can result in the patient not receiving anesthetic gases or rebreathing expired CO2.
Endotracheal Tube Problems
Although the endotracheal tube is, strictly speaking, not a part of the anesthetic machine, it is a critical component of the anesthetic delivery system and is subject to many problems. Endotracheal tubes may become blocked during anesthesia, cutting off the flow of anesthetic gas and oxygen to the patient. Blockages may be the result of twisting or kinking of the tube; accumulation of material such as blood, mucus, or saliva within the tube; or inappropriate positioning of the tube (as may occur when the neck is flexed). The endotracheal tube should be premeasured from the incisor teeth to the mid-neck, and it should be advanced no further than the position of the carina. If the tube is accidentally advanced into a bronchus, the patient may become hypoxic and hypercapnic.
Endotracheal tube blockage (if complete) results in a cessation of oxygen flow to the patient and retention of carbon dioxide. The patient may become dyspneic and may develop cardiac arrhythmias. Eventually, respiratory arrest may occur. The anesthetist usually becomes aware of the problem by observing the patient’s exaggerated breathing pattern or by noting that the reservoir bag no longer inflates and deflates with the patient’s respirations. If a problem is suspected, the anesthetist should quickly check the endotracheal tube function in two ways:
1. Attempt to bag the patient and observe if the chest rises. If the endotracheal tube is blocked, no chest movement will be seen, and there will be considerable resistance to the passage of air into the patient.
2. Disconnect the animal from the machine. With the endotracheal tube still in place, feel for air coming out of the tube when the patient’s chest is compressed. If no air movement is felt, a blockage may be present. In this case, the tube should be removed and another endotracheal tube or mask used to deliver oxygen to the patient. If blood, mucus, or similar material is causing the obstruction, suction with a 20-mL syringe and a feeding tube cut to the length of the endotracheal tube may be helpful.
Vaporizer Problems
Each vaporizer is designed for a specific agent. A potentially disastrous problem can arise if the wrong anesthetic is put into a vaporizer. Each anesthetic liquid has its own vapor pressure (the amount of anesthetic that vaporizes at 20° C), and anesthetic vaporizers are calibrated on the basis of a particular anesthetic being used in the vaporizer with a particular vapor pressure. If an anesthetic is put into the incorrect vaporizer, it is possible that when the vaporizer is set at 1% a higher or lower concentration will be delivered because the anesthetic has a different vapor pressure than the anesthetic for which the vaporizer was designed. This leads to the patient’s anesthetic depth becoming unexpectedly deep or light. Other problems involving vaporizers are as follows:
• Vaporizers should not be tipped. Tipping may lead to leakage of anesthetic into the oxygen bypass channel, resulting in higher concentrations of anesthetic reaching the patient and therefore potential overdose.
• Occasionally, a vaporizer dial may stick or become jammed. If the dial cannot be adjusted, the patient should be transferred to another machine.
• Anesthetic machines equipped with two or more vaporizers in series should be monitored carefully to ensure that both vaporizers are not turned on at the same time.
• Vaporizers should not be overfilled. If too much anesthetic is put into the vaporizer, it should be drained until the fluid level is at, or below, the indicator line.
Pop-off Valve Problems
Occasionally, an anesthetist inadvertently leaves the pop-off valve in a closed position. If the pop-off valve is closed and the oxygen flow rate is greater than the patient’s oxygen requirement, pressure within the circuit will rapidly rise. This can happen with use of a closed system in which the oxygen flow rate has inadvertently been set higher than the metabolic oxygen consumption (approximately 10 mL/kg/min). As pressure rises in the circuit, the reservoir bag will expand, as will the patient’s lungs. This prevents exhalation and also decreases the venous return to the heart. This in turn may decrease cardiac output, cause blood pressure to fall rapidly, and can lead to death within a short time unless recognized and corrected.
To detect the problem at an early stage, the anesthetist should frequently monitor the reservoir bag size and attempt to maintain it at no more than two-thirds full of gas during anesthesia. The reservoir bag size is easily adjusted by changing the oxygen flow rate or by opening and closing the pop-off valve.
Adverse Effects of Anesthetic Agents
Each injectable or inhalation agent has the potential to harm a patient and, in some cases, cause death. Several strategies are used to reduce this potential:
• The anesthetic protocol must be chosen to reflect the special needs of the patient. For example, acepromazine is a poor preanesthetic for patients with low blood pressure because this agent may cause vasodilation, further decreasing the blood pressure. Similarly, halothane, although rarely used now, is not the preferred inhalation agent for patients with cardiac arrhythmias because it may cause arrhythmias to worsen. Isoflurane, the common replacement for halothane, may cause a significant drop in blood pressure, and therefore blood pressure monitoring should be performed on the patient. Animals that are fearful or excited may have increased levels of epinephrine circulating throughout their bodies. Drugs such as the alpha2-agonists (e.g., xylazine) should be avoided, as they will augment the arrhythmogenic effects of epinephrine. In each case the veterinarian might choose to use an alternative agent.
• The anesthetist must be familiar with the side effects and contraindications associated with each of the preanesthetic and general anesthetic agents used in the hospital. For example, the anesthetist who administers an alpha2-agonist should be aware of its potential to cause bradycardia, cardiac arrhythmias, vomiting, bloating, and respiratory depression.
• Multidrug use to achieve balanced anesthesia can be safer than anesthesia with a single drug, provided that the doses of the individual drugs are appropriately reduced. For example, the concentration of isoflurane needed to anesthetize an animal is significantly reduced if the animal is premedicated with acepromazine and butorphanol, as compared with an animal that is not premedicated. If the same isoflurane concentration were used in both situations, the multidrug regimen could be dangerous for the patient.
A detailed description of the pharmacology and physiologic effects of preanesthetic and general anesthetic agents is given in Chapter 3.
Patient Factors That May Lead to Anesthetic Problems and Emergencies
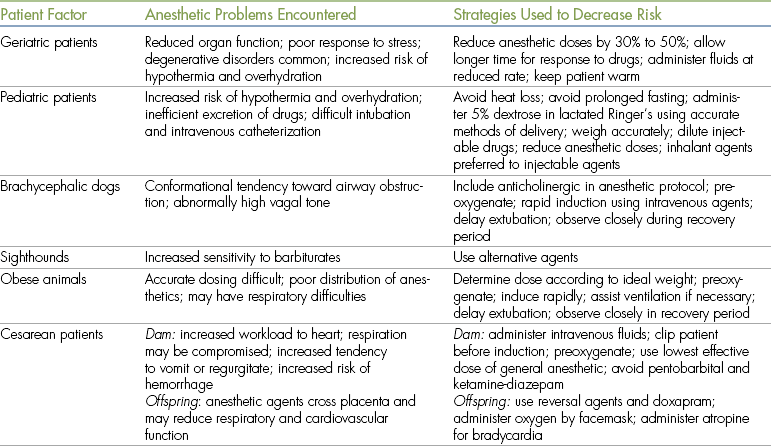
Animals presented for anesthesia may have systemic abnormalities that considerably increase anesthetic risk. Patients with a preoperative status of class P4 or class P5 are particularly difficult to anesthetize successfully. Challenging patients routinely encountered in veterinary practice include geriatric animals, neonates, brachycephalic animals, sighthounds, and obese animals. Cesarean delivery of puppies or kittens also places unique demands on the anesthetist because the response of both the dam and the offspring to anesthetic agents must be considered. Animals that have experienced recent trauma may be presented for emergency surgery, and the anesthetist must be prepared to deal with shock, respiratory difficulties, and cardiac arrhythmias in these patients. Animals with cardiac problems such as heartworm disease or congestive heart failure may require anesthesia for diagnostic or therapeutic procedures. Similarly, animals may require anesthesia despite the presence of renal or hepatic disease. Although a detailed discussion of the anesthetic challenges posed by these and other patients is beyond the scope of this book, it is desirable that the technician be familiar with some of the special problems encountered when anesthetizing these animals. These are summarized in Table 12-1.
Geriatric Patients
A geriatric patient is one that has reached 75% of the average life expectancy for that species and breed. In these patients the functions of critical organs such as the heart, lungs, kidneys, and liver are reduced in comparison with the healthy, young patient. Geriatric animals have less functional reserve than do younger animals, and a relatively poor response to stress. Often they are less able to adequately maintain their state of hydration than younger patients. In addition, geriatric animals are often affected by degenerative disorders such as diabetes mellitus, cancer, congestive heart failure secondary to mitral valve insufficiency, and chronic renal disease, all of which are of concern to the anesthetist. Because of the high incidence of health problems in these animals, the importance of a thorough history and physical examination cannot be overemphasized. Preoperative tests such as a blood chemistry panel, urinalysis, chest radiographs, and an electrocardiogram (ECG) may be advisable for these patients.
Geriatric animals typically have reduced anesthetic requirements, and doses of anesthetic agents are often decreased by one half to one third compared with doses for healthy, young patients. In the case of barbiturates, dose requirements may be as little as one twentieth of the normal dose. Response to drugs is slower, and the technician should allow more time for IV injections to take effect. Recovery from anesthesia also may be prolonged in geriatric animals, partly because of decreased renal and hepatic function (and hence, decreased ability to excrete drugs). Geriatric patients also have a tendency to develop hypothermia because they have a reduced ability to regulate body temperature.
The use of IV fluids is generally advocated in geriatric patients because they have less tolerance for hypotension and often have reduced kidney function. Geriatric animals are, however, at increased risk for developing overhydration, so IV fluids should be given with care.
Pediatric Patients
A veterinary patient under 3 months of age is generally considered to be at increased risk when anesthetized, compared with a mature animal. When working with these patients, the anesthetist must be aware of special considerations during the preanesthetic, anesthetic, and recovery periods.
Preoperative fasting of the pediatric patient may not be advisable because hypoglycemia and dehydration can occur after even a short period of fasting. Oral fluids are usually allowed up to 1 hour before induction. To prevent hypoglycemia during surgery, many veterinarians use 5% dextrose in lactated Ringer’s for IV fluid therapy of anesthetized pediatric patients. (This can be formulated by adding 100 mL of 50% dextrose to 1 L of lactated Ringer’s solution.) The fluid administration rate should not exceed 5 mL/kg/hr unless shock or dehydration is present, because these animals are prone to overhydration if fluid administration is rapid. The use of a syringe driver or pediatric microdrip administration set with a delivery rate of 60 drops/mL and a burette is helpful in preventing inadvertent overinfusion of fluid.
For calculation of drug doses, an accurate weight must be obtained. For animals weighing less than 5 kg, a pediatric or gram scale gives more reliable weights than does a conventional scale. Injectable agents may require dilution because otherwise the dose may be too small to measure or administer accurately. The dose of injectable anesthetics given to pediatric animals is often one half to two thirds of the dose given to mature animals because very young animals have less plasma protein binding of drugs and lack an efficient mechanism to metabolize drugs within the liver. Injectable anesthetic agents that require liver metabolism for inactivation (e.g., thiopental and pentobarbital) can be expected to have a prolonged effect in puppies or kittens less than 8 weeks of age and should be avoided. Renal function is also inefficient compared with the adult animal, and excretion of drugs by this route may be slow.
Many veterinarians prefer to anesthetize pediatric patients with inhalant agents (particularly isoflurane) because administration and elimination of these agents is accomplished through the respiratory tract and patient recovery tends to be rapid.
Certain anesthetic procedures such as intubation and IV catheterization can be more challenging in pediatric patients because of their small body size. The larynx may be difficult to see, and use of a laryngoscope may be required. It is often necessary to cut endotracheal tubes short to avoid bronchial intubation.
Apart from obvious differences in size, the monitoring of pediatric patients is similar to that of adults. The anesthetist should be particularly watchful for bradycardia, which is associated with poor cardiac output in anesthetized animals under 4 weeks of age. Alpha2-agonists may cause significant bradycardia and should be avoided in these patients. Premedication with atropine may not be effective because response to atropine is unpredictable in patients younger than 14 days old.
Pediatric patients are prone to hypothermia because of their lack of subcutaneous fat, their relatively large body surface area, and their reduced ability to shiver. Particular care should be taken to avoid heat loss during surgery. This is accomplished through the use of warmed IV fluids and circulating warm water heating pads or Bair Huggers. It is also essential to make sure that all air is removed from IV lines to avoid the risk of air embolism.
Brachycephalic Dogs
Technicians are often called on to anesthetize brachycephalic dogs such as the English bulldog, pug, Boston terrier, and Pekingese. Because of their conformation, these animals may have one or more anatomic characteristics that impede air exchange, including very small nasal openings, an elongated soft palate, and a small-diameter trachea. Any anesthetic agent that depresses respiration or reduces muscle tone in the pharyngeal and laryngeal area will cause increased respiratory difficulty in these animals. In some cases this may be fatal, particularly if the animal is not intubated and an open airway cannot be maintained. These problems are most evident in animals undergoing surgery to correct conformation defects in the pharyngeal region (e.g., soft palate resection), because postoperative swelling or hemorrhage may occur, increasing the risk of respiratory difficulty.
In addition to respiratory problems, many brachycephalic animals have abnormally high parasympathetic tone, which may cause bradycardia. Use of atropine or glycopyrrolate in these patients is helpful for increasing heart rates before surgery.
The induction period is particularly difficult for brachycephalic dogs. If possible, the anesthetist should preoxygenate brachycephalic patients for 5 minutes before induction. This is done by gently restraining the animal and administering oxygen through a facemask. This procedure helps maintain adequate blood oxygen levels and gives the animal an extra margin of safety during the induction period that follows. Induction should be rapid in order to gain control over the airway, and for this reason IV induction agents are generally preferred over mask induction. Agents that are rapidly metabolized (e.g., propofol, ketamine-diazepam, and methohexital) are preferred. The dog must be adequately anesthetized to allow rapid and efficient intubation. Difficulties may be encountered because of the large amount of redundant tissue in the pharynx. This reduces visibility of the laryngeal opening, and the use of a laryngoscope is helpful in these patients. The anesthetist may find that the endotracheal tube that fits the trachea is smaller than expected, considering the size and weight of the dog.
Anesthesia usually can be safely maintained through the use of an inhalation anesthetic. With the help of an endotracheal tube, breathing during anesthesia may, in fact, be superior to that of the normal awake brachycephalic animal. Agents that allow rapid recovery (particularly isoflurane or sevoflurane) are preferred because dyspnea is common in these dogs during the early recovery period.
After surgery the patient should be observed closely until it is extubated and breathing well. Vigilance is necessary well into the recovery period because patients may develop airway obstructions even after attempting to stand. The endotracheal tube should be left in place as long as possible because the animal will maintain an open airway as long as the tube is in place. It is possible to give a low dose of morphine or hydromorphone just before turning off the vaporizer. This will allow the endotracheal tube to be left in longer, as these drugs suppress the cough reflex. Oxygen should be delivered until the patient is extubated. Once the endotracheal tube has been removed, the animal’s head and neck should be extended, and the animal should be watched closely for dyspnea and cyanosis. If dyspnea is seen, the mouth should be kept open with a mouth gag and the tongue pulled forward. Administration of oxygen by mask or even reinduction (with ketamine-diazepam, propofol, or other IV induction agent) and reintubation may occasionally be necessary. It is advisable to have supplemental oxygen and supplies for reintubation (i.e., a laryngoscope, new endotracheal tube, and the appropriate dose of an inducing agent) readily available in the recovery area in case dyspnea occurs after extubation.
Excitement and stress should be minimized as much as possible in the recovery period, especially if airway surgery was carried out. Some patients may require mild tranquilization or the use of opioid analgesics to reduce the rapid respirations that can worsen laryngeal swelling. Corticosteroids are also helpful in some patients.
Sighthounds
Several canine breeds (including the greyhound, saluki, Afghan hound, whippet, and Russian wolfhound) show increased sensitivity to anesthetic agents, particularly thiobarbiturates such as thiopental. The reason for this increased sensitivity is not entirely understood, but it may involve a lack of body fat for redistribution of the drug and inefficient hepatic metabolism of many drugs. Fortunately, many induction agents (including diazepam and ketamine, methohexital, propofol, isoflurane, and sevoflurane) can be safely used as alternatives to thiobarbiturates in these animals.
Obese Animals
Some patients presented for anesthesia have a high percentage of body fat. Because the blood supply to fat is relatively poor, anesthetics are not efficiently distributed to fat stores. Obese dogs, therefore, require lower doses of drugs on a per kilogram basis than do normal dogs. It is advisable to decrease the dose of preanesthetic and anesthetic agents so that the dose is determined according to a weight halfway between the normal breed weight and the actual weight.
Obese animals also may have some degree of respiratory difficulty, further complicating the anesthetic process. Dogs that show respiratory difficulties should receive oxygen by facemask for 5 minutes before induction. They may also require the use of induction techniques similar to those used in brachycephalic dogs and may require ventilatory support during maintenance of anesthesia (see Chapter 6).
Obese dogs and toy breeds often exhibit rapid shallow respirations during anesthesia. This breathing pattern may result in hypercapnia. The anesthetist who observes persistent rapid and shallow respirations should assume control over respiration by bagging the patient with oxygen and inhalant anesthetic, once every 5 seconds, until increased anesthetic depth and slower respirations are observed. The anesthetist can also slow down the respiratory rate by administering opioids such as hydromorphone or oxymorphone, especially if the elevated rate is a result of surgical stimulation, although occasionally opioids may cause panting.
Cesarean Section
The parturient patient faces risks that must be dealt with by the veterinary team. These include the following:
• Aspiration of vomitus because of a partially full stomach
• Decreased lung capacity because of a diaphragm that is pushed cranially from a distended uterus
• Increased cardiac workload because of advanced pregnancy
• Physiologic anemia because of increased plasma volume without a corresponding increase in the number of red blood cells (this is accentuated as the number of fetuses increases)
• Poor regulation of blood pressure
• Decreased anesthetic requirements because of the effect of progesterone and its metabolites on gamma-aminobutyric acid (GABA) receptors
Essentially, all anesthetic drugs administered to the pregnant patient (with the exception of neuromuscular blocking agents and local anesthetics) will readily cross the placenta and affect the newborn. Although it is essential that the patient receive adequate anesthetic agent to provide immobilization and analgesia for the surgery, it is advisable to use minimal doses of those agents that depress respiration in the puppies or kittens.
Hemorrhage from the uterus is a common complication of cesarean surgery, and even nonhemorrhaging patients have an increased risk of shock. Therefore, an IV catheter should be placed and fluids should be administered intraoperatively to all cesarean patients.
It is helpful to do as much preparation of the cesarean patient preoperatively as possible, thereby reducing the anesthesia time. If possible, clipping and preparation should be initiated before induction. Whether the patient is awake or anesthetized, it is advisable that patient clipping and surgical preparation be done as much as possible with the patient gently restrained in left lateral recumbency rather than in dorsal recumbency. The latter position may cause the heavy uterus to compress the vena cava, decreasing venous return to the heart.
Various anesthetic techniques are used for cesarean surgeries, depending on the preference of the veterinarian:
• Epidural analgesia combined with a tranquilizer or neuroleptanalgesia is popular because this technique, once mastered, provides inexpensive but effective anesthesia with minimal depression of the patient or the neonates. IV fluids and oxygen should be administered in conjunction with epidural analgesia, and blood pressure should be monitored.
• General anesthesia using a variety of injectable and inhalant agents is also commonly used, with anesthetics given at the lowest effective dose to maintain anesthesia without unnecessarily depressing pediatric respiration. Because of the dam’s increased sensitivity to medications, the dose of inhalant anesthetic required is often reduced by up to 40%. Propofol and ketamine are commonly used.
• Preoxygenation is helpful, regardless of the anesthetic protocol.
• Opioid agents are favored by some veterinarians for cesarean anesthesia because they are reversible in both the dam and the neonates through the use of naloxone or another reversal agent.
• Use of diazepam should be avoided because this agent is poorly metabolized by pediatric animals.
In the cesarean patient the stomach empties more slowly, and the queen or bitch is more likely to eat small meals frequently, increasing the risk of food within the stomach. As time permits, the patient should be given appropriate premedication with an agent that can decrease vomiting, such as an anticholinergic. Induction should allow for quick intubation, and therefore the IV route is preferred over mask induction. During the postanesthetic period, the veterinary technician should ensure that the patient has a good swallowing reflex before extubation is performed. The technician should ensure that the patient is in a sternal position, to reduce the risk of any aspiration of vomitus. If the patient does vomit after extubation, the patient must immediately have its head lowered below the rest of the body, and suction of the oropharynx should commence immediately. Once the oropharyngeal area is clear, the patient should be placed back in sternal recumbency and may require a short period of oxygenation. The patient should be monitored for the next 24 to 48 hours for pneumonia (signs of which include pyrexia, dyspnea, hyperpnea, and depression), and the veterinarian may prescribe a course of antibiotics.
The distended uterus of the parturient patient causes lung capacity, functional residual volume, and tidal volume (VT) to decrease. In the parturient patient there is also an increased demand for oxygen. With such patients the need for supplemental oxygen before and during induction becomes important to reduce the risk of oxygen desaturation should intubation become difficult. Induction should be done as quickly as possible; mask induction is not advisable.
Hypotension is also a potential problem for the cesarean patient. It is important that large bore catheters be used, and in some situations, such as dogs over 20 kg, it may be advantageous to have two catheters in place to ensure that rapid volume infusion is possible. Drugs chosen should help maintain blood pressure or at least not cause significant variation from normotension.
Because of physiologic anemia, the veterinary team must work with a patient that has a decreased ability to get oxygen to the necessary parts of the body. Oxygen delivery may be further compromised by an inability to control blood pressure as noted earlier. Such patients must be preoxygenated before and during the induction procedure to reduce the chance of hypoxemia and anaerobic glycolysis. Either or both of these situations can result in arrhythmias or acid-base imbalance.
Puppies or kittens delivered by cesarean section often show signs of reduced respiratory and cardiovascular function when first delivered. If respiration appears inadequate or if cyanosis is present, oxygen should be administered by facemask. If necessary, the newborn animal can be intubated with a 16- or 18-gauge IV catheter and gently bagged with oxygen every 5 seconds. Aspiration of fluid from the mouth and nose with an eyedropper or bulb syringe may also be useful. The use of reversal agents and doxapram (1 to 2 drops delivered under the tongue or 0.1 to 0.2 mL injected into the root of the tongue) is common. If bradycardia is present, a drop of dilute atropine (0.25 mg/mL) can be administered under the tongue or injected into the tongue. Gentle cardiac massage and oxygen may also be helpful.
The newborn should be allowed to nurse as soon as the mother appears to be recovered from anesthesia (or, with supervision, during the recovery period). The dam may be disoriented and should be closely watched to ensure the safety of the newborn puppies or kittens. Anesthetic agents excreted in the milk appear to have little effect on nursing or viability of the neonates. Postoperative analgesics such as butorphanol or buprenorphine assist the mother’s recovery.
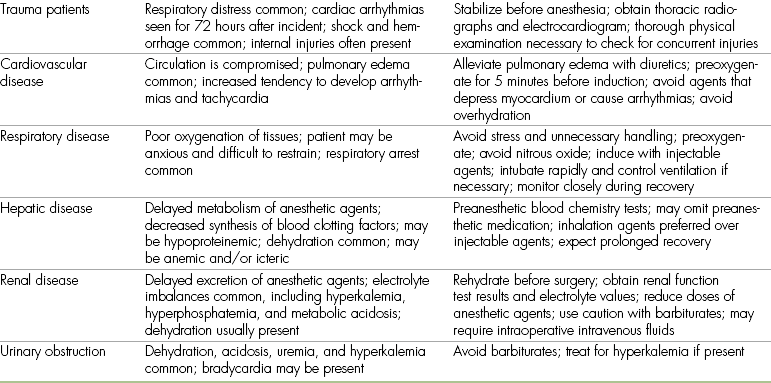
Trauma Patients
Animals that have recently undergone trauma, such as being hit by a car, may have numerous ailments that greatly increase anesthetic risk. Respiratory difficulties are common and may be the result of pneumothorax, pulmonary contusions, hemorrhage, or diaphragmatic hernia. Any one of these decreases the VT of the patient and therefore can cause a decrease in oxygenation. Lack of adequate oxygen exchange will lead to hypoxemia, which will lead to myocardial hypoxia and therefore arrhythmias, cell death, and acid-base imbalances. Increased CO2 levels caused by lack of proper ventilation will also lead to acid-base imbalances and arrhythmias. Loss of blood or sequestration of fluid will result in changes in blood pressure, which must be corrected before anesthesia. Fluid sequestration can result from such situations as burns, where serum (fluid) oozes from the blood vascular system into the burn site. This causes a decrease in circulating volume and therefore a change in blood pressure.
Anemia may be present in the traumatized patient as a result of loss of blood directly or sequestration of blood into the trauma site. It has been shown in human medicine that a pelvic fracture can sequester as much as 40% of the circulating blood volume.
Details of these various issues are further described in this chapter.
Changes in Blood Pressure: Any change in cardiac output or vascular tone will have an effect on blood pressure. The veterinary technician is reminded that depth of anesthesia will affect both of these parameters. Therefore depth of anesthesia should always be considered in the anesthetized patient when blood pressure decreases. Inadequate blood pressure decreases tissue perfusion. This will result in tissue hypoxia or anoxia leading to tissue glycolysis via the anaerobic method, with the production of lactic acid and therefore an acid-base imbalance. It is therefore important that blood pressure be properly monitored. Either the Doppler or oscillometric method can be used, along with adequate digital palpation of the pulse in an extremity as well as core arteries (lingual or femoral). A poor or weak pulse along with slow capillary refill (>2 seconds) is suggestive of hypotension, and mean arterial blood pressure of less than 60 mm Hg indicates inadequate tissue perfusion. Once it has been determined that the blood pressure is abnormal, the veterinary technician should alert the veterinarian in charge (VIC).
Hypotension may be addressed by crystalloid fluid administration at rates of 10 to 20 mL/kg/hr. In the short term, it may be necessary to give small fluid boluses of 10 to 20 mL/kg over 15 minutes for cats (approximately 1 mL/kg/min) and 20 to 40 mL/kg over 15 minutes for dogs (approximately 2 mL/kg/min) to help improve blood pressure quickly. One must remember that most crystalloids will be gone from the intravascular fluid space in less than 2 hours. Fluid overload, especially in the cat, should always be closely monitored. During fluid administration the technician should monitor the heart rate, blood pressure, mucous membrane color, and capillary refill time. Signs of fluid overload in the awake patient may include the following:
Most of these clinical signs are not evident in the anaesthetized patient, and so it becomes prudent to auscultate the lungs in all four quadrants if fluids are being administered rapidly (Figure 12-1).
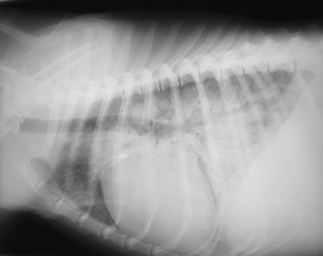
FIGURE 12-1 Pulmonary edema secondary to fluid overload in a cat. (From Battaglia AM: Small animal emergency and critical care for veterinary technicians, ed 2, St Louis, 2007, Elsevier.)
Colloids are often beneficial if blood pressure cannot be maintained, especially if the plasma protein is less than 3.5 g/dL. As colloids are large macromolecules, they remain within the vascular system for a longer period of time than crystalloids. Colloids will increase the colloidal osmotic pressure and hence help stabilize blood pressure before and/or during anesthesia. Colloids include the following:
Colloids are given in much smaller volumes than crystalloids and should always be given in concert with crystalloids. Results of colloid administration include increased cardiac output and blood pressure. Doses for colloids vary depending on the type that is used, but in general, for dogs one uses 10 to 20 mL/kg, and for cats 5 to 10 mL/kg.
If drugs are required to stabilize blood pressure, the veterinarian may choose one or more of various medications such as dopamine (1 to 5 mcg/kg/min) if the heart rate is low or dobutamine (1 to 5 mcg/kg/min) for the patient with normal heart rate but decreased blood pressure.
Respiratory Problems in the Trauma Patient: The trauma patient may have a compromised respiratory system owing to chest trauma. Direct trauma to the chest wall may cause an inability for the bellows system of lung inflation to work resulting from air or fluid entering the chest cavity, or fluid or blood entering the lung parenchyma. In the former situation the lung tissue collapses owing to the lack of negative pressure in the chest cavity, and in the latter the alveoli are unable to exchange CO2 and O2 because of the presence of fluid in the alveoli. It is imperative that any air or fluid within the chest cavity be removed before the anesthetic procedure via a chest tap. Fluid within the lung parenchyma will need to be removed with a diuretic provided that there is an absence of lung contusions. During these procedures the animal should be receiving oxygen by one of the following methods.
Flow-by oxygen: Flow-by oxygen involves using an oxygen source with a pressure-reducing valve and an oxygen line, which is held in front of the patient’s nose. Alternatively the technician may choose to use a breathing circuit (preferably one that has not had anesthetic gases pass through it recently) directed at the patient’s nose. In either case it is important that the oxygen hose or circuit not be held too close to the patient, as this may cause some distress. Flow rates of 50 to 100 mL/kg/min will provide inspired oxygen levels of 40% to 50%.
Nasal catheters: The nasal catheter technique involves insertion of either a nasal cannula, as is used in human medicine, or a nasal catheter (Figure 12-2). Before insertion of either a cannula or a nasal catheter, a topical lubricant should be applied to the tube. A nasal catheter is measured from the nasal meatus to the angle of the mandible, or medial canthus of the eye. The diameter of a cannula that can be inserted is limited by the size of the nasal meatus. The diameter of a catheter is limited by the size of the nasal meatus as well as the nasal passage, which in a cat is a maximum of approximately 4 to 5 French units and in a dog is a maximum of 10 to 12 French units. Oxygen flow rates for this technique are approximately 10 mL/kg/min.
Oxygen collars: It is possible to supply oxygen with the use of an Elizabethan collar and clear plastic wrap placed across the lower two thirds to three quarters of the opening, and an oxygen tube entering at the base of the neck (Figure 12-3). As oxygen is heavier than room air, it will remain in the lower portion of the E-collar. The upper uncovered portion allows for the escape of heat and CO2.
Thoracocentesis: Thoracocentesis (or chest tap) may be required to remove air (pneumothorax), blood, or other fluid (pleural effusion) from the chest cavity. This will normally be done by a veterinarian, but the veterinary technician should be familiar with the equipment and procedure. If there is an external wound in the chest wall, then a temporary bandage should be securely placed over the defect to stop additional air from entering the chest cavity until it can be surgically repaired. If time permits, the lateral chest wall from the seventh to ninth intercostal spaces (caudal to the point of the elbow) should be shaved and aseptically prepared (approximately a 4 cm × 4 cm area). The animal will probably be most comfortable in a sternal or standing position during this procedure. For trauma to the lung parenchyma to be reduced, a 20- to 22-gauge, 1- to 1½-inch IV catheter should be used, although some practitioners prefer a winged infusion set. A three-way stopcock and large syringe are also required.
If air is present in the chest, the catheter will be inserted dorsal to the costochondral junction. If fluid is present within the chest cavity the catheter will need to be inserted below the costochondral junction. The catheter is inserted at the seventh to ninth intercostal space, keeping the needle or catheter toward the cranial aspect of the rib. The artery, vein, and nerve pass along the caudal aspect of each rib and should be avoided. The catheter or needle will pass through the various tissue layers fairly readily, until it encounters the pleura of the chest cavity. Extra force is usually required to enter into the chest cavity at this point, and a “pop” may be felt as the catheter or needle passes into the chest cavity. At this time the stylette of the catheter is removed and a three-way stopcock and syringe (at least 6 to 60 mL) are attached to the catheter (syringe size will be determined by the VIC, as will the type of procedure being done—collection of a sample versus emergency thoracocentesis to remove fluid or air). The needle or catheter should now be directed so it is parallel to the chest wall, to reduce the risk of trauma to the lung parenchyma as the patient breathes. The fluid or air should now be quickly withdrawn from the chest cavity, using the three-way stopcock to expel any fluid or air that accumulates in the syringe. The total volume and character of the fluid or air that is removed from the chest cavity should be noted. Some of the fluid should be kept for analysis. Once the animal shows clinical signs that it can breathe more comfortably, then the anesthetic procedure may be started if required. Oxygen support should be available during the induction phase (i.e., facemask) and intubation, which should proceed quickly. The ability to support ventilation should also be an option if required.
Cardiac arrhythmias are common in the first 12 to 72 hours after chest trauma. The veterinarian may request an ECG as part of the preanesthetic workup because cardiac arrhythmias may be seen as long as 3 days after chest trauma. Shock is also common in animals that have undergone significant trauma, particularly if hemorrhage has been severe. Serious internal injuries such as fractures and herniated or ruptured organs may pose further difficulties for the veterinarian and anesthetist.
Very few trauma patients require anesthesia immediately after the accident, and as a general rule it is wise to stabilize these animals before anesthesia. Delaying anesthesia offers two advantages: (1) it allows time for a thorough workup to assess the extent of the injuries, and (2) it provides some time to stabilize the animal’s condition (which reduces anesthetic risk). The patient should be closely monitored for signs of dyspnea, cardiac arrhythmias, or altered mentation. It is advisable to obtain thoracic radiographs before anesthesia for repair of internal injuries (such as fractures) resulting from trauma. Studies have shown that one third of patients with traumatic forelimb, hindlimb, or pelvic injuries have concurrent thoracic injuries that could jeopardize the safety of anesthesia. It is obviously advisable to identify and treat a disorder such as pneumothorax before anesthetizing an animal for the repair of a fractured femur. Fortunately, many thoracic injuries improve with cage rest, and if anesthesia can be delayed for 24 to 72 hours after the traumatic incident, the anesthetist usually encounters fewer problems.
Cardiovascular Disease
Common cardiovascular disorders in patients scheduled for anesthesia include anemia, shock, cardiomyopathy (primary, or secondary to hyperthyroidism), and congestive heart disease (secondary to mitral valve insufficiency). In endemic areas, heartworm disease is also common.
Hypoxia, hypercapnia, electrolyte imbalance, hypothermia, vagal stimulation, and anesthetic overdose can all lead to cardiac dysfunction. Preanesthetic evaluation and laboratory evaluation can help rule out many of these abnormalities, or at least alert the veterinary anesthetist of potential problems. Evaluation of heart rate and synchrony with the pulse is also an important aspect of cardiovascular evaluation.
The most common cardiac problem seen by the veterinary anesthetist is bradycardia. This is commonly caused by various drugs, either preanesthetics or general anesthetics, which are used as part of the anesthetic protocol. It is important for the veterinary technician to know the normal ranges for the heart rate for various species (please refer to Table 5-2). Heart rates below these values will result in decreased cardiac output and therefore decreased perfusion and blood pressure. The following equation is one of which every veterinary technician should be aware:
• ABP: Arterial blood pressure
• SVR: Systemic vascular resistance (the degree of systemic arterial dilation or constriction)
• SV: Stroke volume (the volume of blood the heart pumps out with each contraction; influenced by the volume of venous blood returning to the heart and the contractile force of the heart muscle)
Anesthetic drugs may decrease not only heart rate but also force of contraction, and may decrease blood return to the heart (also known as preload). The decision to treat bradycardia will ultimately be the choice of the veterinarian, but the veterinary technician should be ready to give the appropriate dose of anticholinergic such as atropine or glycopyrrolate, although the former has a quicker onset of action (1 to 2 minutes intravenously) than the latter (up to 15 minutes). It should be remembered that anticholinergics might be selected by the attending veterinarian for treating bradycardia caused by increased vagal tone. However, many veterinary anesthesiologists no longer recommend the routine use of anticholinergics, and certainly these drugs do not work well for other types of bradyarrhythmias. Drugs that may cause an increase in parasympathetic tone include the alpha2-agonists (medetomidine or dexmedetomidine, xylazine), the opioids, and occasionally the phenothiazines. When bradycardia is caused by anesthetic drugs that do not increase vagal tone, it is best treated with catecholamine drugs such as dopamine (1 to 5 mcg/kg/min). If the heart rate has dropped to 50% of normal value and/or blood pressure has dropped to dangerously low levels, then epinephrine becomes a drug of choice (0.01 mcg/kg).
Alternatively, reassessing the depth of anesthesia and turning the patient anesthetic level down, if appropriate, might be all that is needed. The temperature of the patient should also be assessed, as this can be another reason for bradycardia.
Arrhythmias may result from a variety of physiologic causes including the following:
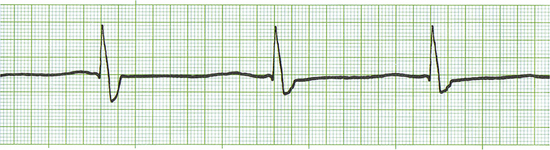
Clinically it may be difficult to discern on physical examination if a patient is having arrhythmias. However, the veterinary technician can detect some arrhythmias by auscultating the heart while palpating the pulse and checking for a lack of coordination between the two, which is often called “dropped beats.” If dropped beats are discernible, an ECG should be obtained to determine what type of arrhythmia is present and what precautions may need to be taken. The only real way to determine the presence of arrhythmias is with an ECG recording.
As a veterinary paraprofessional there are certain arrhythmias that may require immediate attention by the veterinarian. It is therefore important for the veterinary technician to recognize the normal ECG so that he or she can advise the veterinarian that there may be some abnormality present. Following are some of the arrhythmias that may be encountered in trauma patients or anesthetized patients:
• Premature ventricular contractions
• First- and second-degree heart block (which is normal in the equine species)
See Figures 5-6 to 5-15 for examples of commonly encountered arrhythmias.
The veterinarian must be informed immediately about any of the listed arrhythmias because each could be life-threatening. Various drugs such as beta blockers or lidocaine may be indicated in tachyarrhythmias, and atropine may be indicated to treat atrioventricular (AV) heart block. The veterinarian will decide what is the most appropriate drug to use.
Tachyarrhythmias may be treated either with lidocaine in dogs or with beta blockers or calcium channel blockers in cats, as lidocaine tends to be more toxic in the cat. It may be necessary to treat with a constant rate infusion (CRI) if the arrhythmia continues, as lidocaine has a very short half-life. However, a variety of different causes of and specific treatments for tachyarrhythmias exist, and discussion of them is beyond the scope of this book. On a short-term basis while drugs are being prepared, it may be necessary to slow the heart down by applying pressure to the eyeballs or massaging the carotid sinus. Either will increase parasympathetic stimulation.
Many animals with heart disease have concurrent pulmonary disease, particularly pulmonary edema, which further complicates anesthesia. Diuretics such as furosemide may be helpful in alleviating pulmonary edema before anesthesia.
As with patients that have undergone recent trauma, before initiating anesthesia it is generally advisable to stabilize the patient’s condition by treating cardiovascular and respiratory disease to alleviate the signs as much as possible. Preoxygenation using a facemask or oxygen chamber for 5 minutes immediately before induction is also extremely helpful in reducing anesthetic risk in animals with cardiovascular or respiratory difficulties.
The veterinarian and anesthetist should ensure that anesthetic agents that depress the myocardium or that exacerbate arrhythmias (e.g., alpha2-agonists and halothane) are avoided as much as possible in these animals. Opioid agents, diazepam, and isoflurane or sevoflurane offer the advantage of relative lack of toxicity to the heart.
When anesthetizing animals with cardiovascular problems, the anesthetist should be aware of the increased risk of overhydration through excessive or rapid administration of IV fluids. Even the standard infusion rate of 10 mL/kg/hr may be dangerous for these animals. Therefore it is advisable to frequently monitor these patients for signs of pulmonary edema such as ocular or nasal discharge, increased lung sounds, and increased respiratory rate. Central venous pressure monitoring, if available, is useful for detection of overhydration.
Respiratory Disease
Of all the animals to undergo anesthesia, those with respiratory problems are perhaps the most challenging for the anesthetist. Examples of these patients include animals with pleural effusion (i.e., free fluid present in the chest cavity), diaphragmatic hernia, pneumothorax, pulmonary contusions resulting from trauma, pneumonia, tracheal collapse, and pulmonary edema. Poor oxygenation is often present in these animals, and many show signs of tachypnea, dyspnea, and cyanosis. If possible, anesthesia should be delayed until respiratory function has improved. If surgery is absolutely required (e.g., to place a chest tube), local analgesia and gentle manual restraint may be preferable to general anesthesia. Administration of oxygen via previously described techniques is helpful for patients with respiratory compromise. Nitrous oxide should be avoided in patients with respiratory distress because the administration of 100% oxygen is usually necessary to maintain adequate oxygenation.
Before anesthesia, it is important to thoroughly evaluate the animal and, if possible, to find the cause of the respiratory distress. Patients that are presented for anesthesia should be assessed as to their ability to move air into and out of the lungs. Such patients as brachycephalics or those with collapsing trachea can be compromised with regard to their ability to adequately move air even when they are awake. These patients should be preoxygenated during the preanesthetic period and may also need assisted ventilation during the anesthetic procedure.
An animal under anesthesia will frequently have a reduced VT and decreased respiratory rate. Even when standard anesthetic protocols are used, it is common for the VT to be decreased by 25% to 40% and for the respiratory rate to drop to 8 to 12 breaths/min. During this time there can be an increase in the CO2 levels, which can result in acid-base imbalances. Such changes may also lead to hypoxemia unless the patient is breathing 100% oxygen. The technician must remember that the inspired air is divided into two portions. Dead space ventilation (VD) is a relatively constant portion of inspired air that fills the upper airways and bronchi. This portion of ventilation is not involved in gas exchange. Some drugs such as anticholinergics may increase dead space. Alveolar ventilation (VA) is the inspired air that fills the alveoli, where oxygen and CO2 are exchanged at the capillary level. Thus the total or tidal volume of air taken in (VT) is the sum of the dead space air plus alveolar air. VD will always be the first air that is inspired, but because VD is a relatively constant volume and cannot be changed, any decrease in VT will result in a decrease in alveolar gas exchange.
One can then see how levels of CO2 may increase and levels of O2 may decrease. These changes can come about from either decreased ventilation or apnea. Generally drugs are not used to improve the ventilatory rate or volume, because drugs such as doxapram will result in only a period of increased ventilatory rate followed by apnea. There will also be a corresponding increase in oxygen demand by the central nervous system with this drug. The technician should properly assess the depth of anesthesia and lighten it if possible. If this is not possible, the technician should provide intermittent ventilation at a volume of 12 to 15 mL/kg per breath. During the anesthetic protocol it is also important that the anesthetist remember to sigh the patient every 5 to 10 minutes to reduce these issues of hypoventilation, atelectasis, and/or apnea.
Patients that are presented for anesthesia should also be assessed as to their oxygen-carrying capabilities by evaluating the packed cell volume (PCV) and the oxygen saturation with a pulse oximeter or a blood gas analysis machine such as the i-STAT if either of these monitors is available. Patients that have a perceived or real decrease in PCV (hematocrit) or an oxygen saturation below 92% should receive oxygen supplementation before the anesthetic protocol for a minimum of 5 minutes. This will ensure saturated oxygen levels within the blood vascular system and the tissues, thus reducing the chance of arrhythmias or hypoxic events.
Respiratory Problems during Anesthesia: In spite of all of the precautions taken it is possible for the veterinary anesthetist to have respiratory problems with patients. Clinical signs may include dyspnea or cyanosis. When assessing these patients, the anesthetist should determine the following:
• The patient’s respiratory character (short and shallow, or deep and exaggerated) and volume
• The depth of anesthesia (not too light or too deep)
• Whether the abnormal breathing is associated with pain (inadequate depth of anesthesia or pain control)
• Whether the endotracheal tube is placed correctly—not too far in (bronchial intubation) or out of the trachea
• Whether the endotracheal tube is partly occluded with mucus or blood
• The oxygen saturation with pulse oximetry or blood gas analysis
The cause of the respiratory dysfunction will determine what should be done. However, intermittent positive pressure ventilation (IPPV; see Chapter 6) is usually performed (with the vaporizer turned down) while the cause of the respiratory dysfunction is determined. The technician must ensure that adequate pressures are being achieved during IPPV (15 to 20 cm H2O in small animal patients and 35 to 40 cm H2O in large animal patients) to allow for adequate inflation of the alveoli. Occasionally it may be necessary to reintubate the patient to rule out a partial blockage or misplacement of the endotracheal tube.
Radiographs and thoracocentesis are particularly helpful. Thoracocentesis not only is useful in diagnosis, but also may be therapeutic if large volumes of air or fluid can be removed from the chest.
Diaphragmatic Hernia: One of the most common procedures requiring anesthesia of an animal in respiratory distress is surgical repair of a diaphragmatic hernia. When preparing to anesthetize these patients, as with all patients showing signs of dyspnea, it is advisable to preoxygenate for 5 to 10 minutes before surgery. Head-down positions should be avoided before and during anesthesia because they may result in further movement of abdominal contents into the thorax. If possible, an induction method that allows rapid intubation (i.e., use of an injectable agent) is preferred over mask induction. After induction, some patients may show signs of respiratory depression and even respiratory arrest, and the anesthetist must be prepared to intubate rapidly and assist or control ventilation. Nitrous oxide should not be used because it can cause diffusion of the gas into the displaced stomach and intestines and further distend these organs. Ventilatory assistance must be provided by manual “bagging” of the patient, or a ventilator may be used. The animal should be closely observed for cyanosis. Pulse oximetry, capnography, and arterial blood gas determination are helpful aids for assessing ventilation. Blood gas levels can be evaluated with many blood machines such as the Heska i-STAT hand-held analyzer or the IRMA TRUpoint portable analyzer (Figure 12-4).
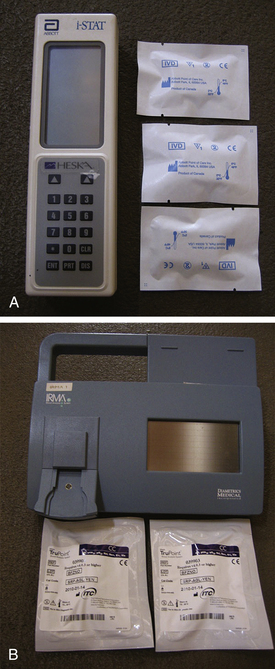
FIGURE 12-4 Blood gas machines. A, Heska i-STAT hand-held analyzer. B, IRMA TRUpoint portable analyzer.
These patients require close observation during the recovery period. Administration of oxygen may be necessary if signs of respiratory distress are seen. Pneumothorax is common after chest surgery, and affected patients may require chest tube placement or removal of air from the pleural space using a syringe and needle.
Hepatic Disease
Animals with liver disease are subject to increased anesthetic risk because of the central role that organ plays in drug metabolism, synthesis of blood clotting factors and other serum proteins, and carbohydrate metabolism. Some animals with liver disease are hypoproteinemic, which may lead to increased potency of barbiturate agents. Patients with chronic liver failure are also commonly dehydrated, thin, and icteric and may be anemic.
Preanesthetic medication should be given with care or omitted from the protocol because most of these agents require hepatic metabolism before they can be excreted. Acepromazine in particular may have long-lasting effects in patients with compromised hepatic function. Agents that can be reversed are preferred. Use of ketamine and diazepam should also be avoided. Induction and maintenance of anesthesia is best achieved using isoflurane or propofol, which require little or no hepatic function for elimination.
Renal Disease
The kidneys are the organs most involved in maintaining the volume and electrolyte composition of body fluids. This helps explain why animals with renal disease are often dehydrated and may have severe electrolyte and acid-base imbalances, including metabolic acidosis and hyperkalemia. General anesthesia may be particularly stressful for these patients because renal blood flow is decreased during anesthesia and renal function may be further compromised, particularly if the animal is hypotensive. Use of injectable nonsteroidal antiinflammatory agents such as ketoprofen during anesthesia may reduce renal perfusion even further.
Renal function tests such as urine specific gravity, blood urea nitrogen (BUN), and creatinine may be useful in obtaining an accurate picture of renal function. Preoperative water deprivation may not be advisable in some patients with renal disease because dehydration can occur rapidly after withdrawal of oral fluids. Water should be offered up to 1 hour before premedication. The patient with renal disease should be rehydrated as much as possible before surgery, and electrolyte problems should be identified and addressed. Administration of IV fluids is often continued throughout the anesthetic and postanesthetic periods until the animal is fully hydrated and able to drink unassisted.
Many preanesthetic and anesthetic agents and their metabolites are eliminated from the body by renal excretion. For this reason, animals with compromised renal function may show prolonged recovery after anesthesia if conventional doses are used. It is prudent to reduce doses of anesthetic drugs (including acepromazine, xylazine, diazepam, ketamine, and barbiturates) in these patients. Barbiturates, in particular, have increased potency in acidotic and uremic animals and should be used with great caution in patients with renal disease. Inhalation agents (particularly isoflurane) have some advantages over injectable agents, although halothane can produce fluoride ions, which are damaging to the kidneys.
Animals with urinary blockages (including male cats with urethral obstructions caused by struvite or calcium oxalate crystals) pose similar problems to the anesthetist. Many of these cats are depressed, dehydrated, uremic, acidotic, and hyperkalemic. Hyperkalemic animals are at particular risk for cardiac arrest. Hyperkalemia may sometimes be suspected during auscultation because bradycardia is often seen if plasma potassium levels exceed 6 mEq/L. Some blood chemistry analyzers are capable of measuring electrolytes quickly and easily. Treatment of hyperkalemia may require the use of sodium bicarbonate, 10% calcium gluconate, and/or dextrose and should be done only with close supervision and guidance from the veterinarian. Conditions stressful to the animal should be avoided as much as possible because the release of epinephrine from the adrenal glands may potentiate cardiac arrhythmias.
The administration of inhalation agents (particularly isoflurane) to cats with urinary blockages may be less hazardous than the use of injectable drugs because minimal renal excretion is required for patient recovery. Propofol or ketamine-diazepam may be used intravenously with caution and at reduced doses, provided normal kidney function is present. Cats with obstructions showing extreme depression may not require general anesthesia, particularly if a local anesthetic such as lidocaine gel is administered as part of the urethral catheterization procedure.
RESPONSE TO ANESTHETIC PROBLEMS AND EMERGENCIES
Despite every precaution, the veterinary technician is likely to encounter anesthetic problems and emergencies throughout the course of a career. The nature of the technician’s response may mean the difference between life and death for the anesthetized patient.
Role of the Veterinary Technician in Emergency Care
Ideally, emergency response is a team effort involving the veterinarian, technician, and other hospital staff. Normally the veterinarian acts as the team leader, directing the staff in emergency procedures. However, the veterinarian may be performing surgery on the patient when an anesthetic emergency arises and therefore may have other pressing concerns. The technician must be prepared to take an active role in resuscitating his or her patient and not rely solely on the already-busy veterinarian. Constant communication between the veterinarian and the technician is obviously important under these circumstances.
It is a good idea to conduct periodic “dress rehearsals” or mock resuscitations in which all staff members participate. Everyone in the hospital should be familiar with the location of the crash kit and IV fluids. Procedures such as warming towels in a clothes dryer, making up hot water bottles, and drawing up drugs into a syringe can be readily taught to hospital staff, which, once the staff has mastered these tasks, will free the veterinarian and technician to perform more demanding tasks.
Occasionally an emergency arises when the veterinarian is absent from the hospital or unavailable to assist. For example, seizures may occur in the postoperative recovery period. Most provincial and state regulations allow the technician to undertake emergency care if the veterinarian is absent. To protect the veterinarian and technician from liability, however, it is advisable to discuss in advance the procedures that the veterinarian will authorize the technician to do in an emergency. It is helpful to have written instructions available in the form of an emergency protocol authorized by the veterinarian.
General Approach to Emergencies
It cannot be assumed that every anesthetic emergency should be treated in the same way. For example, the veterinarian and animal owner may elect not to resuscitate a severely ill or debilitated animal that undergoes cardiac arrest during anesthesia. Cost considerations may influence treatment in some cases, because emergency care is labor-intensive, and treatment costs may be considerable. Most veterinarians, however, will not stop to consider cost if the emergency arises during a routine surgery, such as a spay, and will do everything possible to revive the animal.
When responding to an emergency, the technician should bear in mind the principles of emergency care listed in Procedure 12-1, p. 345.
Emergency Situations That May Arise during Anesthesia
Although anesthetic emergencies are by their nature unpredictable, certain problems occur with some frequency. The following situations will be addressed in detail:
• Animals that will not stay anesthetized
• Animals that are too deeply anesthetized
• Prolonged capillary refill time
Animals That Will Not Stay Anesthetized
Occasionally, the anesthetist will have difficulty in maintaining a patient at sufficient anesthetic depth. Often the veterinarian becomes aware of the problem when the patient shows signs of movement in response to surgical stimulation. If depth appears inadequate, the anesthetist should check the following:
• Has the vaporizer been turned off, or is the setting too low to maintain an adequate depth of anesthesia?
• Does the vaporizer contain anesthetic?
• Is the endotracheal tube in the trachea? This can be easily determined by checking to see if the reservoir bag expands and contracts as the animal breathes. If so, the endotracheal tube is in the trachea. Movement of the reservoir bag also tells the anesthetist that the endotracheal tube is connected to the Y-piece and that the tube is not blocked. Other procedures used to determine the location and patency of the endotracheal tube include palpation of the neck, and compression of the reservoir bag to see if the chest expands.
• Is air leaking around the endotracheal tube? If so, the patient is probably inspiring some room air, which dilutes the anesthetic gas entering the lungs. Air leakage can be detected by closing the pop-off valve, inflating the reservoir bag, and gently pressing on the bag while listening for the sound of air escaping from the animal’s mouth. A soft hiss of escaping air is acceptable at a pressure manometer reading of 20 cm H2O, but a large gush of exiting air should alert the anesthetist that either the endotracheal tube is too small or the cuff is not sufficiently inflated. If this is the case, the cuff can be further inflated or the pharyngeal area can be packed with damp gauze.
• Is the patient apneic? This is most commonly seen immediately after the intubated animal is connected to the machine, particularly if propofol or thiopental was used for induction. Prolonged apnea may lead to arousal from anesthesia because adequate quantities of vaporized anesthetic will not enter the lungs or the bloodstream. If arousal appears imminent, it may be necessary to periodically bag the animal with a mixture of oxygen and anesthetic until anesthetic depth is adequate.
• Are the patient’s respirations too shallow to draw sufficient anesthetic into the lungs? Rapid, shallow respiration, commonly seen in toy dogs and obese animals, may be associated with insufficient anesthetic depth. The anesthetist should assist ventilation by bagging these patients (with the vaporizer on) every 5 to 10 seconds.
• Is the anesthetic machine assembled correctly, and are all connections tight? Occasionally, hoses become detached from the machine or the endotracheal tube, in which case the patient will not receive any anesthetic from the machine.
• Is the oxygen flow rate adequate to vaporize the anesthetic? For most precision vaporizers, a minimum flow rate of at least 500 mL/min is necessary for accurate delivery of anesthetic. Very high oxygen flow rates or excessive use of the oxygen flush valve may also result in unpredictable vaporization of anesthetic.
• Is the anesthetic machine functioning correctly? Repeated episodes of awakening during anesthesia may indicate poor vaporizer function. If a halothane or isoflurane vaporizer setting of 3% to 4% seems necessary to maintain anesthesia in many patients, cleaning and recalibration of the vaporizer are probably necessary.
• Is the exaggerated respiratory movement actually an agonal (near death) phenomenon, indicating dangerous anesthetic depth rather than a light plane?
If none of these reasons can explain the patient’s arousal, the anesthetist should consult with the veterinarian. It may be necessary to increase the vaporizer setting, administer an analgesic, or switch to a different anesthetic in order to achieve the desired anesthetic depth.
Animals That Are Too Deeply Anesthetized
An animal that is too deeply anesthetized will usually show the following signs:
• A respiratory rate of 6 breaths/min or fewer; shallow respirations, or dyspnea.
• Pale or cyanotic mucous membranes.
• Capillary refill time greater than 2 seconds.
• Weak pulse; systolic blood pressure less than 80 mm Hg (indirect measurement).
• Cardiac arrhythmias may be present; irregular QRS complexes (ventricular complexes) on the ECG, or abnormal complexes such as ventricular premature complexes (VPCs) may be present.
• Cold extremities; body temperature is often less than 35° C.
• Absent reflexes, including palpebral and corneal reflexes.
The anesthetist should use judgment in interpreting the signs listed. The presence of one or two signs may not indicate excessive depth, provided the other signs are absent. Vital signs also vary depending on the preanesthetic and general anesthetics used (e.g., atropine may affect heart rate and pupil dilation). The more parameters that the anesthetist considers, the more accurate the depth assessment is likely to be.
There are several reasons why anesthetic depth may be excessive. In most cases the vaporizer setting is too high for the patient being anesthetized or, in the case of injectable agents, too high a dose has been given. Occasionally the animal may have a preexisting problem such as shock or anemia that increases susceptibility to anesthetic overdose (Procedure 12-2, pp. 345).
Pale Mucous Membranes
Pale mucous membranes may arise from several causes. Some patients have preexisting anemia secondary to diseases such as feline leukemia, hemolytic anemia, bleeding disorders, neoplasia, or chronic renal disease. In other cases blood loss may have occurred during surgery. Some anesthetic agents (particularly inhalation agents, propofol, and acepromazine) cause vasodilation and decrease blood pressure, resulting in poor perfusion of capillary beds and pale mucous membranes in some animals. Hypothermia or pain can also reduce blood supply to the tissues and can cause pale mucous membranes.
If pale mucous membranes are observed during surgery, follow Procedure 12-3, p. 345.
Prolonged Capillary Refill Time
The observation of a capillary refill time greater than 2 seconds suggests that blood pressure is inadequate to perfuse superficial tissues. The presence of hypotension should be suspected in any animal with a slow capillary refill time. Hypotension was the most common anesthetic complication found in one study of dogs and cats (Gaynor and colleagues, 1999). Hypotension may be present before the induction of anesthesia, as in the case of animals undergoing emergency surgery after trauma. Hypotension or shock also may develop secondary to blood loss during surgery or may occur in patients that are at a very deep plane of anesthesia. Acepromazine and the inhalation agents may also cause hypotension in susceptible patients. Follow Procedure 12-4, p. 345, if a prolonged capillary refill time is observed. The treatment for shock in the anesthetized patient is similar to that in the conscious patient and should be performed under the supervision of a veterinarian (Procedure 12-5, p. 346).
Dyspnea and/or Cyanosis
Any patient showing dyspnea or cyanosis during the administration of an anesthetic should be immediately brought to the veterinarian’s attention. The presence of dyspnea indicates that the animal is unable to obtain sufficient oxygen or remove adequate CO2 with normal respiratory movements. Cyanosis indicates that tissue oxygenation is inadequate. Dyspnea and cyanosis often are seen together and if not managed quickly may be followed by respiratory arrest, in which respiratory efforts cease and the amount of oxygen available to the tissues rapidly declines.
The most common sources of respiratory distress during anesthesia are as follows:
• The animal is unable to obtain oxygen from the anesthetic machine because the oxygen supply has run out, the flow meter has been turned off, or the anesthetic circuit or endotracheal tube is blocked.
• The animal is unable to breathe normally because of airway obstruction or respiratory pathology. Causes of airway obstruction include endotracheal tube blockage, excessive flexion of the head and neck, laryngospasm, bronchoconstriction, aspiration of stomach contents after vomiting or regurgitation, and brachycephalic conformation. Common causes of respiratory pathology include pneumothorax, pulmonary edema, diaphragmatic hernia, and pleural effusion. Use of heavy surgical drapes or constricting bandages also may impair normal respiration.
• The animal is too deeply anesthetized, to the point that respiration and other vital functions are adversely affected.
Respiratory problems are life-threatening and should be addressed as discussed in Procedure 12-6, p. 346.
Tachypnea
Tachypnea, or rapid respirations, must be differentiated from dyspnea, in which respiratory distress is present. Tachypnea may arise at any time during anesthesia and may be disconcerting to the anesthetist. It is particularly common during procedures using opioids. Tachypnea also may be seen if anesthetic depth is inadequate, in which case it is often accompanied by tachycardia and spontaneous movement. Paradoxically, tachypnea also may occur in deep anesthesia as a response to low blood oxygen and high blood carbon dioxide levels. Tachypnea is also seen in hyperthermic patients, including animals with malignant hyperthermia.
If tachypnea is seen, follow Procedure 12-7, p. 346.
Abnormalities in Cardiac Rate and Rhythm
Tachycardia, bradycardia, and cardiac arrhythmias are commonly seen in anesthetized patients.
Tachycardia is present if the heart rate during stage III anesthesia is greater than 140 bpm for a large dog, 160 bpm for a small dog, 200 for a cat, 60 for a horse, or 100 for a cow. It may result from the administration of drugs such as atropine, ketamine, or epinephrine. Tachycardia may also be a preexisting condition in animals with hyperthyroidism, shock, congestive heart failure, and other conditions. An elevation in heart rate is also a common response to surgical stimulation, although it does not necessarily indicate insufficient anesthetic depth unless accompanied by rapid respiration, spontaneous movement, or active reflexes.
Not all cases of tachycardia require treatment (e.g., those in patients with otherwise normal cardiac function), but the anesthetist should notify the veterinarian before assuming that tachycardia is not significant. It is also important to check the vaporizer setting and anesthetic depth and adjust them if necessary.
Bradycardia can be defined as a heart rate less than 60 to 70 bpm in a dog, <100 bpm in a cat, <25 bpm in a horse or <40 bpm in a cow. Bradycardia may be secondary to the administration of alpha2-agonists, or opioids, particularly if anticholinergics were not administered preoperatively. Bradycardia also may result from increased activity of the vagus nerve in response to endotracheal intubation, ocular surgery, or handling of the viscera by the surgeon. Bradycardia may also occur if the animal is very deeply anesthetized, and when seen in this context it is a warning that respiratory and cardiac arrest may be imminent. Other causes of bradycardia include hypertension, hyperkalemia, hypothermia, and hypoxia.
Not all cases of bradycardia require treatment. If capillary refill, pulse oximeter readings, blood pressure, and pulse strength appear normal, tissue perfusion may be adequate and treatment may be unnecessary. The veterinarian should be consulted and should direct treatment. If anesthetic depth is excessive, the vaporizer setting should be adjusted, and bagging with 100% oxygen (empty the breathing bag into the scavenging system and refill it with pure oxygen) may be helpful. Bradycardia as a result of the administration of drugs or because of excessive vagal stimulation may be treated with anticholinergics, reversal agents, or a change in the anesthetic protocol.
The term cardiac arrhythmia (or cardiac dysrhythmia) refers to any one of a number of electrocardiographic abnormalities, including “dropped beats,” VPCs arising spontaneously from individual heart muscle cells, and sustained episodes of tachycardia. These abnormalities are most easily detected through the use of an ECG. The alert technician also may note that a pulse deficit is present in animals with some types of arrhythmias.
Cardiac arrhythmias commonly arise in animals given arrhythmogenic drugs such as barbiturates, alpha2-agonists, and halothane. Such arrhythmias are often of short duration and well tolerated in young, healthy patients but may be a significant problem in animals with preexisting heart disease and in geriatric patients. Arrhythmias are particularly common during induction and light anesthesia. They may also be the result of respiratory depression and subsequent hypoxia. Other causes of cardiac arrhythmias include preexisting heart disease, electrolyte and acid-base disturbances, gastric volvulus, thoracic surgery, endotracheal intubation, and hypercapnia. Hypercapnia may arise from poor anesthetic technique, including exhaustion of the CO2 absorber crystals or inadequate oxygen flow rates. Cardiac arrhythmias should be treated in consultation with the veterinarian (Procedure 12-8, p. 346).
Respiratory Arrest
Respiratory arrest is the cessation of respiratory efforts by the patient. It may lead to cardiac arrest and is therefore potentially fatal. Not all cases of respiratory arrest require immediate action by the anesthetist. Respiratory efforts may temporarily cease after the IV injection of ketamine, barbiturates, propofol, and other respiratory depressants. Minimal respiratory efforts may also be seen after a period of prolonged bagging with oxygen and in this case reflect low blood carbon dioxide and high blood oxygen levels. Whether respiratory arrest is the result of drug administration or excessive bagging with oxygen, the anesthetist must be sure that other vital signs, particularly heart rate and mucous membrane color, are normal. If the heartbeat is regular, the heart rate is greater than the minimum acceptable rate (see Table 5-2), the pulse is strong, and mucous membranes are pink, the patient does not usually require immediate treatment for respiratory arrest. Pulse oximeter readings are helpful in indicating if the patient’s oxygenation status is adequate (i.e., a reading greater than 95% usually indicates that bagging is not necessary). To be safe, occasional “breaths” of oxygen (one every 30 seconds) can be delivered to the patient during this period to prevent hypoxia. However, premature bagging with oxygen may extend the period of apnea by removing carbon dioxide from the blood, which is a stimulus for the patient to resume breathing. The anesthetist who suspects that respiratory efforts have temporarily ceased because of administration of drugs or ventilation with oxygen should closely monitor the patient’s heart rate and mucous membrane color for 1 to 2 minutes before assuming that a serious condition exists. If spontaneous respiration does not resume within this time, the veterinarian should be consulted, and it may be advisable to begin bagging the patient with oxygen.
True respiratory arrest is much more serious and requires immediate attention. This condition may arise because of anesthetic overdose, cessation of oxygen flow, or preexisting respiratory disease such as pneumothorax or diaphragmatic hernia. Affected animals may show warning signs such as dyspnea and/or cyanosis before respiratory arrest occurs. Other vital signs, such as heart rate, capillary refill time, pulse strength, and pupil size, are often abnormal. Pulse oximetry values rapidly fall below 90%. The treatment of respiratory arrest involves the steps shown in Procedure 12-9, p. 347.
Bagging should continue until the heart rate, mucous membrane color, and pulse oximeter values have been restored to normal. Once this is achieved, the anesthetist should discontinue bagging for 15 to 30 seconds and closely observe the patient for respiratory efforts. If none are seen, bagging should resume. On occasion, the anesthetist may be faced with a patient in respiratory arrest in the absence of an anesthetic machine. It is possible to substitute an Ambu bag (Figure 12-5) or even institute mouth-to–endotracheal tube or mouth-to-muzzle resuscitation in these cases.
Cardiac Arrest
Cardiac arrest may occur at any time during anesthesia. In most cases the anesthetist receives some warning that arrest is imminent, in the form of a short period in which cyanosis, dyspnea or respiratory arrest, and prolonged capillary refill are evident, often accompanied by an arrhythmia. If cardiac arrest appears imminent, the anesthetist should immediately alert the veterinarian and the hospital staff while continuing to monitor the heart by auscultation, by palpation of the chest, or through the use of an ECG. A patient experiencing cardiac arrest rapidly develops the following signs:
• No heartbeat can be auscultated or palpated, and normal QRS complexes are absent from the ECG tracing.
• There is no palpable arterial pulse, and blood pressure readings (if available) are 25 mm Hg or less.
• Mucous membranes are gray or cyanotic, and capillary refill may be prolonged.
• Pupils are widely dilated with no response to light, and corneal reflex is absent.
• Respiration is absent except for intermittent, abrupt gasps (agonal breaths).
Coordinated action by all hospital staff members is essential to reverse cardiac arrest by performing cardiopulmonary cerebrovascular resuscitation (CPCR). Once arrest occurs, permanent brain damage may result if oxygen delivery to the brain is not reestablished within 4 minutes, by either cardiopulmonary resuscitation (CPR) or restoration of cardiac function. Ideally, at least five staff members should participate in the resuscitative efforts as follows: one person performs chest compressions, the second person bags the animal, the third assesses the pulse during compressions and checks the pulse or ECG when compressions are temporarily suspended, the fourth draws up and administers drugs on the veterinarian’s orders, and the fifth maintains a record of patient status and resuscitative treatments. The minimum number of people required is three, with compressions performed by one person, tasks two and five by a second person, and tasks three and four by a third.
The essential steps in performing CPCR have traditionally been summarized by the mnemonic ABCDE, which stands for airway, breathing, circulation, drugs, ECG. Of these, the single most important one is circulation. Current evidence suggests that initiating steps to generate circulation of blood should be done first, particularly if the patient arrests under anesthesia and is correctly intubated, thus CABDE.
Circulation: Cardiac compressions should be initiated immediately, as they have been shown to be the single most important factor in successful return of spontaneous circulation (ROSC). The animal should be turned on its right side with its feet toward the person doing compressions and the head tilted down, if possible.
• Cardiac compressions for a large dog, a foal, or a small ruminant: A firm object such as a book, sandbag, or rolled-up towel should be placed under the animal’s chest just behind the elbow. The heel of the compressor’s hand should compress the chest against this object, with the pressure applied at the point where the chest is widest. Both hands should be used to compress the chest. The chest wall should be allowed to bounce back rapidly after each compression.
• Cardiac compressions for a medium-sized dog: One hand should be placed under the chest and the other hand at the fifth intercostal space, just over the heart itself (Figure 12-6). The chest is then compressed between the two hands.
FIGURE 12-6 Correct location for cardiac compressions for a medium-sized dog or a cat. In the case of dogs undergoing a laparotomy at the time of arrest, the surgeon may immediately initiate internal compressions by opening the diaphragm, entering the thorax, and compressing the heart.
• Cardiac compressions for a cat or small dog: Compression may be done by using the thumb to compress the chest against the fingers of the same hand.
• Note: Attempting to perform compressions in adult cattle and horses does not produce effective circulation. CPCR is limited to drug administration and ventilation in these patients.
The rate of compressions should be one to two times per second to generate a heart rate of approximately 100 bpm. The chest should be compressed by approximately one third of the diameter of the chest wall. The aim of the compressions is to manually force blood through the heart and, ultimately, to the tissues. It is believed that compressions also may assist circulation by increasing pressure in the chest, indirectly inducing blood flow. Each compression should result in a palpable femoral pulse, which should be periodically monitored by another staff member, if possible. If a pulse is not detected and the mucous membrane color does not improve, the method of compression should be adjusted by changing the rate or intensity, by repositioning the patient, or by assigning the compression task to another staff member. A Doppler probe placed on the cornea may also be useful in detecting pulses from effective compressions.
If two people are administering CPR, one person should bag every 10 to 12 seconds while the other compresses the chest. Bagging and compressions should be delivered simultaneously. In the case of a technician working alone, compressions should be given priority—as long as the patient has a patent airway or endotracheal tube, the compressions will result in some gas exchange. Once CPR is initiated, it should not be discontinued for longer than 3 to 5 seconds at a time.
If external cardiac compressions are not effective, as shown by failure to achieve a palpable pulse or pink mucous membranes within 2 minutes, internal compressions may be attempted. In the case of dogs weighing over 20 kg, some authorities suggest that internal compressions should be initiated immediately after cardiac arrest is identified. Investigators have shown that external chest compression in dogs weighing more than 20 kg results in less than 30% of normal cardiac output, whereas internal massage results in outputs of up to 70% of normal. There is understandable reluctance on the part of many veterinarians and technicians to enter the chest to perform internal massage; however, controlled studies have demonstrated that the success rate for resuscitation of large dogs is much greater if internal cardiac massage is performed.
For performance of internal compressions, the lateral thorax is quickly clipped and rinsed with alcohol, a self-adhering sterile drape is applied to the prepared area, and a skin incision is made between the seventh and eighth ribs, using a scalpel. The incision is extended through the muscle until the chest cavity is encountered. Care should be taken to avoid incising lung tissue, which lies immediately below the pleura. The operator’s hand is inserted between the ribs (the use of a retractor may be necessary to separate the ribs adequately). The heart is grasped, and gentle but firm pressure is applied to the ventricles at a rate of 100 times per minute. If resuscitation efforts are successful, a palpable heartbeat may return within seconds or minutes. Surgical closure and antibiotic therapy are essential after reestablishment of cardiac function by internal compression.
When doing compressions, it is necessary to stop periodically to determine whether the heart has resumed spontaneous contractions. This is easy to ascertain by palpation when doing internal compressions, but more difficult with external compression methods. Spontaneous contractions can be detected by discontinuing external compression and either palpating for a spontaneous pulse or observing an ECG for QRS complexes. Auscultation may also be useful. The presence of increasing ETco2 concentrations on a capnograph also indicates ROSC.
If spontaneous contractions are not observed, external or internal compressions can be resumed, although after 15 minutes they are unlikely to be successful in establishing a heartbeat. Use of a defibrillator is helpful in some situations but should be authorized and directly supervised by a veterinarian.
If spontaneous contractions are observed, cardiac compressions should be discontinued, although bagging must be maintained until spontaneous breathing is established, which may require up to several hours. The anesthetist should periodically check the capillary refill time, mucous membrane color, and heart rate and should discontinue bagging only if these vital signs appear normal. If mucous membrane color deteriorates or if spontaneous respiration does not occur within 1 minute after bagging has been discontinued, bagging should be resumed.
Airway and Breathing: If the animal is intubated and connected to an anesthetic machine, one staff member should note the time of arrest and immediately initiate respiratory support by turning off the vaporizer and nitrous oxide flow and bagging the animal with 100% oxygen at the rate of one breath every 10 to 12 seconds. Mask administration of oxygen is inadequate; if an endotracheal tube is not present, it is essential that the animal be intubated immediately. In addition, the anesthetist must ensure that the patient’s chest rises slightly during bagging, indicating that the airway is not blocked or the tube is not placed in the esophagus.
Drugs: Drugs are commonly administered to aid recovery. In all cases, the veterinarian, if present, should authorize the dosage, route, and nature of drugs to be administered.
If an IV catheter is present, the drugs are normally given through it, followed by IV fluids at a dosage of 20 mL/kg (cats) or 40 mL/kg (dogs) as rapidly as possible. Caution should be used when administering fluids to patients in cardiac arrest because overhydration and pulmonary edema are common sequelae. If IV access is difficult, drugs may be given by injection into the base of the tongue or by intratracheal administration. Intratracheal administration may involve injection of the drug directly into the tracheal lumen, or the drug may be administered by means of a urinary catheter passed through the endotracheal tube. For intratracheal administration, the dose of emergency drug given should be twice the recommended IV dose.
Intracardiac injections should be avoided if possible because injections by this route require the interruption of cardiac compressions and have some potential to damage the myocardium or lacerate coronary blood vessels. As a last resort drugs may be injected into the left ventricle.
Agents that are commonly administered to assist with ROSC are epinephrine, vasopressin, atropine, dopamine, and dobutamine. The current recommended doses of emergency drugs are given in Tables 12-2 and 12-3.
Epinephrine is the drug most commonly used for initial treatment of cardiac arrest. The currently recommended dose is 1 mL/10 kg—thus 0.5 mL for cats, 1 mL for small dogs, 2 mL for medium-sized dogs, and 3 mL for large dogs, all drawn up from a 1:1000 solution. Epinephrine is administered every 3 to 5 minutes.
Vasopressin may be used in place of epinephrine or alternated with doses of epinephrine. The recommended dose is 0.4 mL/10 kg. Like epinephrine, vasopressin is administered every 3 to 5 minutes.
Atropine is frequently indicated with anesthesia-related cardiac arrest in order to decrease parasympathetic tone and can be administered every 15 to 20 minutes.
Dopamine or dobutamine infusions are also advised by many authorities because these drugs increase the force and rate of cardiac contractions.
Bicarbonate administration is no longer recommended unless the animal is hyperkalemic.
Calcium injections are also no longer advocated, except in hyperkalemic or severely hypocalcemic animals.
Electrocardiogram: Placement of ECG leads can give the CPCR team information regarding the underlying “rhythm” of the patient. Compressions should not be halted in order to place leads. It is imperative that alcohol not be used to wet lead-patient contacts if a defibrillator is available, as it could result in an explosion. Compressions should be stopped for only a few seconds to allow the ECG to be evaluated. One of three rhythms may be observed:
• Asystole: no electrical activity (“flatline”)
• Ventricular fibrillation: coarse vertical zigzag lines that do not resemble normal complexes
• Pulseless electrical activity: organized electrical activity that frequently resembles normal or near-normal complexes
Asystole, in which the heart has no electrical or mechanical activity, is most likely to respond to the CPCR technique described earlier (compressions, ventilation, drugs).
Ventricular fibrillation represents disorganized muscular activity of the heart (see Figure 5-15). During open-chest CPCR the heart muscle “writhes” and has the appearance of a bag of worms. This type of cardiac arrest is best treated by electrical defibrillation (Procedure 12-10, p. 347).
Pulseless electrical activity (also known as electromechanical dissociation [EMD]) is the hardest situation to treat. Patients with this type of cardiac arrest may respond to standard CPCR (Figure 12-7).
Aftercare: After ROSC the patient requires intensive monitoring of cardiovascular and respiratory function, as well as an assessment of brain function. It is very common for patients to experience another cardiac arrest within 24 hours of successful CPCR. Monitoring may include blood pressure (indirect or direct), blood gases, pulse oximetry, continuous ECG, and capnography. Decisions regarding treatment with fluids and infusions are often made on a minute-to-minute basis as patient status changes rapidly in one direction or the other. In an ideal situation, cardiovascular, ventilatory, and neurologic status will improve, infusions will be decreased and finally discontinued, and the patient will be discharged. Drugs commonly used in the postresuscitation period include lidocaine to treat ventricular arrhythmias, inotropes (dobutamine, dopamine), fluids, sodium bicarbonate, corticosteroids, furosemide, and mannitol.
Unfortunately, many patients that sustain cardiac arrest cannot be revived. In the case of those patients in which cardiac function is reestablished, conditions such as pulmonary edema and cerebral edema may occur. Cerebral edema is manifested by seizures, failure to return to consciousness, and temporary or permanent neurologic damage.
Problems That May Arise in the Recovery Period
Regurgitation during Anesthesia and Postanesthesia Vomiting
Regurgitation is a passive phenomenon that may occur even during deep anesthesia. In a regurgitating animal, stomach contents exit through the cardiac sphincter, move up the esophagus, and enter the pharynx, nasopharynx, and oral cavity. Once in the pharynx, stomach contents may be aspirated into the respiratory tract. Regurgitation is most common in animals placed in a head-down position during surgery, because this causes increased pressure on the stomach, and in ruminants. Unlike vomiting, regurgitation is not accompanied by retching or other outward signs, and in fact the only sign apparent to the anesthetist may be a small amount of fluid draining from the animal’s mouth or nose. Treatment of regurgitation involves immediate intubation (if a cuffed endotracheal tube is not already present) and removal of as much regurgitated material as possible through suction.
Vomiting during or after anesthesia is a relatively common phenomenon, particularly in brachycephalic dogs. Unlike regurgitation, vomiting is an active phenomenon, often accompanied by retching. Vomiting usually occurs as the animal is losing consciousness during induction or as it is returning to consciousness during anesthetic recovery. Vomiting is potentially most dangerous if the animal is unconscious and the airway is not protected with an endotracheal tube. In this situation, the vomitus may be easily aspirated into the trachea. Aspiration of vomitus may cause immediate signs of dyspnea and cyanosis as a result of airway obstruction and bronchospasm. If the patient survives this episode, signs of aspiration pneumonia (including fever, increased respiratory rate, and increased lung sounds) may appear over the next 24 to 48 hours. It is imperative, therefore, that the anesthetist prevents the accumulation of vomitus within the oral cavity of the unconscious patient and the subsequent aspiration of the material into the air passages.
To do this, an endotracheal tube should be immediately inserted if time and level of consciousness of the patient allow. If this cannot be achieved, the animal’s head should immediately be placed at a lower level than the rest of its body (e.g., over the edge of the surgery table). This helps prevent passive flow of liquid material into the trachea. When the vomiting stops, it may be necessary to manually clean the oral cavity, using suction if available. If respiratory arrest occurs because of airway blockage, the animal should be intubated and bagged with oxygen.
Unconscious animals have a low risk of aspiration if a cuffed endotracheal tube is already in place during the vomiting episode. It is for this reason that the endotracheal tube is customarily left in place until the patient regains the swallowing reflex and is close to consciousness. If vomiting is seen in an unconscious animal that has a cuffed endotracheal tube in place, the anesthetist should ensure that the cuff of the tube is inflated and position the animal’s head lower than the rest of its body to prevent accumulation of vomitus within the oral cavity.
Occasionally, an animal may regurgitate after esophageal intubation. If this occurs the tube should be left in place to direct the contents away from the pharynx. Another endotracheal tube should be placed in the trachea, if possible, while the first tube is still in place. Fortunately, most vomiting episodes occur after the animal has regained consciousness and the ability to swallow. It is not usually necessary to intubate conscious animals during a vomiting episode; however, the anesthetist should ensure that the head is kept extended and as low as possible.
Occasionally the technician may be called on to anesthetize an animal that has not been fasted before induction. These patients may be at risk of vomiting and/or regurgitation during induction, maintenance, and recovery. The anesthetist can help avoid problems by ensuring that rapid induction and intubation techniques are used. For this reason, an injectable agent is preferred over masking in these patients. A cuffed endotracheal tube with adequate diameter is essential. If possible, head-down positions should be avoided during surgery to prevent excessive pressure on the stomach. The anesthetist should also ensure that suction is readily available in case of regurgitation or vomiting. Use of antiemetic drugs such as metoclopramide may be helpful in some cases.
Postanesthesia Seizures and Excitement
Seizures are occasionally seen in animals recovering from anesthesia. Seizures may be caused by the administration of ketamine, by diagnostic procedures such as myelography, or by patient disorders such as epilepsy or hypoglycemia.
It is important that the anesthetist differentiate between seizures and excitement during recovery. Excitement usually occurs after barbiturate anesthesia (particularly after the use of pentobarbital to treat status epilepticus) and most often appears as spontaneous paddling of the limbs and occasionally as vocalization. Geriatric animals may also vocalize and appear confused after anesthesia. Usually, treatment is unnecessary other than the calm reassurance of the patient. Sedatives can be helpful, especially if the patient did not receive a sedative in the preanesthetic period. Occasionally, excitement or dysphoria may be seen after the administration of high doses of opioids to animals that have not been tranquilized (particularly cats). Treatment with naloxone to partially reverse the opioid or a tranquilizer to reduce the dysphoric effects may be helpful in these animals. Excitement is rarely observed in animals that receive opioids for moderate or severe pain.
In contrast, seizures appear as spontaneous twitching or uncontrolled movements of the head, neck, and limbs and are often triggered by a stimulus such as sound or touch. Animals given ketamine may show stiff forelimbs, opisthotonus, and exaggerated responses to touch or noise.
Animals undergoing postoperative excitement or seizures should be brought to the veterinarian’s attention. Elimination of stimuli such as light, sound, and touch may be adequate to resolve the episode. Adequate postoperative analgesia should be provided. If seizures are present, many animals respond well to administration of IV or rectal diazepam at a rate of 0.2 to 0.4 mg/kg. If diazepam is not effective or is unavailable, the animal may be anesthetized with propofol in sufficient quantity to induce sedation and eliminate seizures.
Animals manifesting seizures or excitement during recovery require surveillance and nursing care to prevent self-injury. In the case of cats recovering from ketamine anesthesia, it may be necessary to trim the front claws or to bandage the paws to prevent the animal from scratching its face.
All animals experiencing seizures should be monitored for hyperthermia and cyanosis. Hyperthermia can be treated by the application of cool wet towels. Cyanosis should be treated by the administration of oxygen by facemask or by endotracheal tube (if unconscious).
Dyspnea during the Recovery Period
Dyspnea resulting from upper airway obstruction is the most common cause of death in the postanesthetic period. Dyspnea in cats is usually caused by laryngospasm, whereas dyspnea in dogs is most commonly associated with breed-related (e.g., brachycephalic) obstruction of the entrance to the trachea.
Laryngospasm is a condition in which the cartilages in the laryngeal area become so tightly closed that air is unable to enter the trachea. This condition commonly arises in cats because of this species’ extremely active laryngeal reflex. In some recovering cats, the removal of the endotracheal tube initiates reflex closure of the airway. This reflex is normally useful to the cat in that it prevents the aspiration of food or water into the larynx in the conscious animal; however, in the unconscious animal it may well result in complete airway blockage. Laryngeal edema may result from repeated attempts to intubate during light anesthesia. Clinically, this resembles laryngospasm.
Cats undergoing laryngospasm or laryngeal edema may breathe with an audible stertor or wheeze. They typically show exaggerated thoracic movements, gasping, and upward movement of the head during inspiration. If conscious, the animal usually appears anxious or excited. Laryngospasm must be differentiated from growling, which is common in cats recovering from anesthesia. In the case of growling, the noises are particularly evident on expiration, whereas in laryngospasm the respiration is labored and the noise is most evident during inspiration.
If a cat shows signs of laryngospasm during recovery from anesthesia, the anesthetist should check the mucous membrane color and pulse oximeter readings (if available). If the cat’s mucous membranes appear pink and the Sao2 is greater than 90%, the obstruction is likely partial rather than complete. In this case, the situation may resolve without treatment, although administration of oxygen by facemask may be helpful, provided it does not stress the cat. It may be helpful to extend the neck and hold the tongue rostrally. If cyanosis is present or Sao2 readings are less than 90%, and the cat is losing consciousness—and if these signs are not alleviated by the administration of oxygen by facemask—the animal must be intubated. If intubation is impossible, the veterinarian may elect to perform a tracheotomy to reestablish airflow. The animal should be kept anesthetized for 2 hours and given furosemide and corticosteroids to reduce swelling. The cat can be extubated once the cords appear less rounded and swollen. Nasal oxygen is useful during extubation.
Laryngospasm is easier to prevent than to treat. When cats are being anesthetized, gentle intubation technique is essential to avoid unnecessary laryngeal trauma. Use of lidocaine spray and/or gel is also helpful during intubation. Early extubation is recommended in cats so that the tube is removed before the laryngeal reflex returns.
Dyspnea in brachycephalic breeds of dogs usually occurs because the airway is obstructed by the soft palate or by other redundant tissue in the pharynx. However, there are many other potential causes of obstruction, including foreign objects such as blood clots, gauze sponges, or even extracted teeth. Animals that have undergone surgery of the pharynx or larynx often undergo postoperative tissue swelling that may lead to airway obstruction.
However it arises, airway obstruction will usually not become evident until after the endotracheal tube has been removed. Strategies to prevent and treat postoperative dyspnea in brachycephalic dogs are outlined on p. 325.
A patient that requires supplemental oxygen during the recovery period can have it administered by one of four routes: facemask, nasal cannula, E-collar, or oxygen cage or tent, all as previously described in this chapter. As a general rule, the flow rate should be at least 100 mL/kg/min. If possible, oxygen being delivered to an awake animal should be humidified (e.g., by directing the flow of oxygen through a bottle of distilled water before delivery to the patient).
Prolonged Recovery from Anesthesia
Animals experiencing prolonged recovery from anesthesia should be examined by the veterinarian. There are many possible reasons why a patient may be slow to recover, including impaired renal or hepatic function, hypothermia, individual susceptibility to a particular anesthetic, breed variation (particularly sighthounds), or the presence of a disorder such as shock or hemorrhage. Excessive anesthetic depth or prolonged anesthesia may also result in delayed recovery. Use of certain agents (including intramuscular ketamine, or repeated injections of barbiturates) may be associated with prolonged recovery even in healthy animals. In the cat it is known that hypothermia in itself can cause significant delay in recovery even if all other organs are functioning properly.
Recovery may be hastened in several ways (Procedure 12-11, p. 347).
KEY POINTS
1. Although anesthetic complications are uncommon, the technician must be able to anticipate and respond to emergencies in an efficient and knowledgeable fashion.
2. Human error may result in anesthetic problems. Such errors may include the failure to obtain an adequate history or perform a physical examination, errors related to a lack of familiarity with the anesthetic machine or drugs used, the incorrect administration of drugs, and errors related to fatigue, inattentiveness, or distraction.
3. Examples of equipment failure or operator carelessness include carbon dioxide absorber exhaustion, failure to deliver sufficient oxygen to the patient, misassembly of the anesthetic machine, failure of the vaporizer or pop-off valve, or endotracheal tube problems.
4. Anesthetic agents may cause problems during anesthesia, and the anesthetic protocol must be chosen to reflect the special needs of each patient. The anesthetist must be familiar with the adverse side effects associated with the use of each agent in the anesthetic protocol.
5. Some patients are at increased risk for anesthetic complications because of preexisting factors such as old age, organ failure, recent trauma, or breed-related conformation.
6. Geriatric patients have less reserve than younger patients and have reduced anesthetic requirements. Pediatric patients also require reduced doses of injectable agents and are prone to hypothermia and hypoglycemia.
7. Brachycephalic dogs have anatomic characteristics that make respiration difficult, particularly during the recovery period. Preoxygenation before induction, rapid induction and intubation, and close monitoring during recovery are essential.
8. Thiobarbiturates should be used with extreme care in sighthounds. Alternative agents are preferable in most situations.
9. Obese animals should receive anesthetic doses according to their ideal body weight.
10. Pregnant animals presented for cesarean section are at increased anesthetic risk. Various anesthetic techniques (including epidural anesthesia, balanced anesthesia, and neuroleptanalgesia) are sometimes used as alternatives to inhalation anesthesia in these patients. Almost all anesthetic agents may cause depression of fetal respiration and/or circulation, and the use of reversing agents may be advisable.
11. If possible, patients that have undergone recent trauma should be stabilized and thoroughly evaluated before anesthesia.
12. Animals with cardiovascular or respiratory disease may require special anesthetic techniques such as preoxygenation and manual control of ventilation.
13. Hepatic or renal disease may delay excretion of injectable agents, and prolonged recovery times may be seen.
14. Emergency care is ideally a team effort involving all hospital personnel. It is helpful to have preauthorized emergency protocols and periodic “dress rehearsals.”
15. It may be difficult to maintain adequate anesthetic depth in some patients. Incorrect placement of the endotracheal tube, incorrect vaporizer setting, inadequate endotracheal tube size, and many other factors may contribute to this problem.
16. Excessive anesthetic depth may result from excessive administration of anesthetic agents or from preexisting patient problems. It may be necessary to bag the patient with 100% oxygen to achieve a lighter plane of anesthesia.
17. Pale mucous membranes may be the result of anemia, hemorrhage, or poor perfusion. Prolonged capillary refill time suggests that hypotension (or, if severe, shock) is present.
18. Cyanosis is a critical emergency and arises because of insufficient delivery of oxygen to the tissues. It may result from a machine problem, airway or endotracheal tube blockage, or respiratory difficulties resulting from excessive depth, pneumothorax, or respiratory disease. Oxygen delivery to the patient must be reestablished through masking, intubation, or tracheostomy.
19. Abnormalities in cardiac rate and rhythm may result from the administration of anesthetic agents, electrolyte abnormalities, hypercapnia, hypoxia, and many other factors.
20. Respiratory arrest that is accompanied by cyanosis and/or bradycardia is an emergency and must be treated by ventilation with 100% oxygen.
21. Cardiac arrest should be treated according to the principles of ABCD: establish a patent airway, bag the patient with 100% oxygen, initiate internal or external cardiac massage, and administer epinephrine and other drugs. The current trend for an intubated patient is to start with cardiac massage, thus following a CABD approach.
22. Regurgitation and/or vomiting may be dangerous in the anesthetized animal because of the danger of airway obstruction and aspiration pneumonia.
23. Postanesthesia seizures may be treated by eliminating external stimuli and administering diazepam.
24. Dyspnea caused by laryngospasm or brachycephalic airway obstruction may be treated by administration of oxygen by mask, reintubation of the patient, or tracheostomy.
25. Animals experiencing prolonged recovery from anesthesia require close observation and nursing care. An effort should be made to determine the reason for delayed arousal of each patient.
REVIEW QUESTIONS
1. When an animal scheduled for a surgical procedure is brought in by a neighbor who is in a hurry, the best thing to do is:
a. Instruct the receptionist to have the neighbor sign the consent form
b. Ask the neighbor to take the animal back home
c. Ask the neighbor some quick questions about the animal
d. Have the neighbor sign the consent form and ensure that the owner is called before the procedure is initiated
2. In preparation for an anesthetic procedure, you have drawn up a syringe of barbiturate and an identical syringe of saline. You are then called to the examination room to assist the veterinarian. About 10 minutes later you return to prepare the animal for induction. With the IV catheter in place, you are just about to inject some saline into the animal when you realize that you are not sure if the syringe contains saline. The best thing to do would be to:
a. Inject a small amount of the solution and see what effect it has
b. Discard both syringes and start over
c. Ask the person who was holding the animal which syringe had saline in it
d. Discard both syringes, label some new syringes, and start over
3. You are about to use the anesthetic machine and notice that although the flow meter is working, the pressure gauge on the oxygen tank reads close to zero. The best thing to do would be to:
a. Assume that the pressure gauge may be faulty and wait and see if the flow meter stops working
c. Call the repair person to have the pressure gauge checked
d. Use low-flow anesthesia techniques and ignore the pressure gauge reading
4. While monitoring a patient on an anesthetic machine, you realize that the oxygen tank has become empty. The best thing to do would be to:
a. Disconnect the patient from the circuit, put on a new oxygen tank, and then reconnect the patient to the circuit
b. Remove the circuit from the patient to allow it to breathe room air for the remainder of the procedure
5. If the pop-off valve is inadvertently left shut, it will:
a. Stop the oxygen flow from entering the circuit
b. Convert the circuit to low-flow anesthesia
6. You look at the oxygen tank and note that 1000 psi of pressure is left in the tank, but the flow meter now reads zero and you cannot obtain a flow by twisting the knobs. The best thing to do would be to assume:
a. The oxygen tank pressure gauge is malfunctioning and you need to recheck the flow meter
b. The oxygen pressure is adequate and the flow meter is simply not registering the flow
c. The animal is not getting oxygen, and you need to remove the animal from the circuit until a new machine is found or the problem is corrected
7. A geriatric patient is considered to be one that:
a. Is greater than 10 years old
b. Is greater than 15 years old
8. Brain damage may occur when there is inadequate oxygenation of the tissues for longer than ___ minutes.
9. When a technician is performing CPR alone, the ratio of cardiac compressions to ventilation should be:
10. To ensure that the benefit an animal obtains from CPR is not lost, one should not discontinue cardiac compressions for longer than:
For the following questions, more than one answer may be correct.
12. One may suspect that the endotracheal tube is malfunctioning even if it is in the trachea because:
a. Compression of the reservoir bag does not result in the raising of the chest
c. The animal cannot be kept at an adequate plane of anesthesia
13. One may suspect that the pop-off valve has been closed or that it is malfunctioning if the:
14. Administration of the normal rate of fluids (10 mL/kg/hr) during an anesthetic procedure may result in overhydration in the:
15. Brachycephalic dogs may be at increased anesthetic risk because of their:
16. To decrease the anesthetic risk associated with a brachycephalic dog, the anesthetist may elect to:
a. Use atropine as part of the anesthetic protocol
b. Preoxygenate the animal before giving any anesthetic
c. Use an injectable anesthetic to hasten induction rather than masking
17. Animals that undergo cesarean section are at increased risk during anesthesia because of:
a. Decreased respiratory function
b. Increased chance of aspiration vomitus
18. Anesthetic agents or drugs that one may want to avoid in the animal with cardiovascular disease include:
19. An animal that has liver dysfunction may be hypoproteinemic and therefore requires ____ for induction compared with that needed for a normal dog.
20. Too light a plane of anesthesia may be the result of:
a. A flow rate that is too low
b. An incorrect vaporizer setting
21. Tachypnea may result from:
22. When the blood pressure drops the veterinarian may ask the technician to infuse a colloid. Which of the following is not a colloid?
23. Laryngospasm is more common in the dog than in the cat.
24. Treatment of bradycardia can always be reversed with an anticholinergic.