Chapter 5 Third Week of Development
Rapid development of the embryo from the embryonic disc is characterized by the following:
The third week of embryonic development occurs during the week of the first missed menstrual period, that is, the fifth week after the onset of the last normal menstrual period. Cessation of menstruation is usually an indication that conception has occurred. Approximately 3 weeks after conception, a normal pregnancy can be detected with ultrasonography (Fig. 5-1).
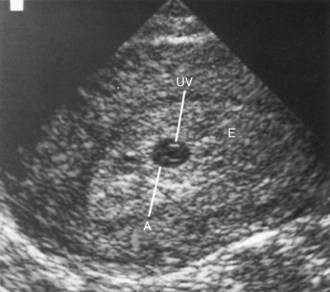
Figure 5–1 Endovaginal ultrasonogram of a conceptus 3 weeks after conception implanted in the posterior endometrium, showing the umbilical vesicle. The endometrium completely surrounds the conceptus. A, Amnion; UV, umbilical sac; E, endometrium.
(Courtesy E. A. Lyons, MD, Professor of Radiology, and Obstetrics and Gynecology, and Anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
Gastrulation: Formation of Germ Layers
Gastrulation is the process by which the bilaminar embryonic disc (Fig. 5-2A to H) is converted into a trilaminar embryonic disc. Each of the three germ layers (ectoderm, endoderm, and mesoderm) of the embryonic disc gives rise to specific tissues and organs (see Fig. 6-4). Gastrulation is the beginning of morphogenesis (development of the form and structure of various organs and parts of the body). This process begins with the formation of the primitive streak (see Fig. 5-2B and C).
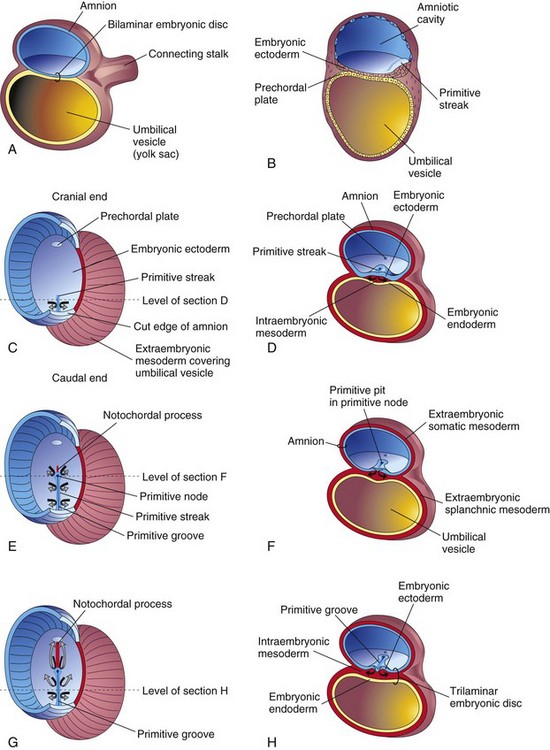
Figure 5–2 Formation of the trilaminar embryonic disc (days 15 to 16). The arrows indicate invagination and migration of the mesenchymal cells between the ectoderm and the endoderm. C, E, and G, Dorsal views of the embryonic disc early in the third week, exposed by removal of the amnion. B, D, F, and H, Transverse sections through the embryonic disc at the levels indicated.
Primitive Streak
At the beginning of the third week, a thickened linear band of epiblast, the primitive streak, appears caudally in the median plane of the dorsal aspect of the embryonic disc (Fig. 5-2B). The primitive streak results from proliferation and migration of cells of the epiblast to the median plane of the embryonic disc (Fig. 5-2D). As the primitive streak elongates by the addition of cells to its caudal end, its cranial end proliferates to form the primitive node (Fig. 5-2E and F). Concurrently, a narrow primitive groove develops in the primitive streak that ends in a small depression in the primitive node, the primitive pit (Fig. 5-2F). As soon as the primitive streak appears, it is possible to identify the embryo’s craniocaudal axis (cranial and caudal ends), dorsal and ventral surfaces, and right and left sides. Shortly after the primitive streak appears, cells leave its deep surface and form mesoblast, a loose network of embryonic connective tissue known as mesenchyme (Figs. 5-2H and 5-3B and C) that forms the supporting tissues of the embryo.
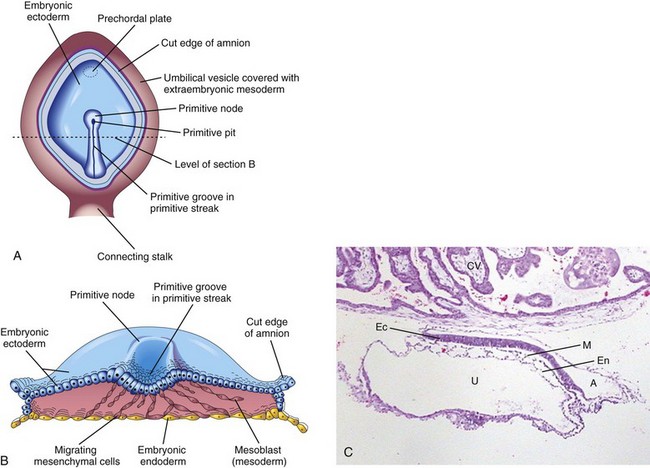
Figure 5–3 A, Dorsal view of a 16-day embryo. The amnion has been removed to expose the embryonic disc. B, Illustration of the cranial half of the embryonic disc during the third week. The disc has been cut transversely to show the migration of mesenchymal cells from the primitive streak to form the mesoblast that soon organizes to form the intraembryonic mesoderm. C, Sagittal section of a trilaminar embryo showing ectoderm (Ec), mesoderm (M), and endoderm (En). Also visible are the amniotic sac (A), the umbilical vesicle (U), and chorionic villi (CV).
(C, Courtesy Dr. E. Uthman, Houston/Richmond, Texas.)
Under the influence of various embryonic growth factors, including BMP signaling, epiblast cells migrate through the primitive groove to become endoderm and mesoderm. Mesenchymal cells have the potential to proliferate and differentiate into diverse types of cells, such as fibroblasts, chondroblasts, and osteoblasts. Recent studies indicate that signaling molecules (nodal factors) of the transforming growth factor β (TGF-β) superfamily induce the formation of mesoderm.
The primitive streak actively forms mesoderm until the early part of the fourth week; thereafter, its production slows down. The streak diminishes in relative size and becomes an insignificant structure in the sacrococcygeal region of the embryo (Fig. 5-4A to D).
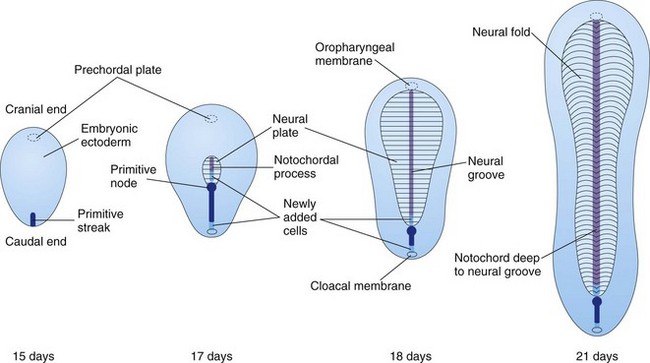
Figure 5–4 Dorsal views of the embryonic disc, showing how it lengthens and changes shape during the third week. The primitive streak lengthens by the addition of cells at its caudal end; the notochordal process lengthens by the migration of cells from the primitive node. At the end of the third week, the notochordal process is transformed into the notochord.
Notochordal Process and Notochord
Some mesenchymal cells migrate cranially from the primitive node and pit, forming a median cellular cord, the notochordal process (Figs. 5-2 G, 5-4B to D, and 5-5A to C). This process soon acquires a lumen, the notochordal canal (Fig. 5-5C and D). The notochordal process grows cranially between the ectoderm and endoderm until it reaches the prechordal plate, a small, circular area of cells that is an important organizer of the head region (Fig. 5-2C). The rod-like notochordal process can extend no farther because the prechordal plate is firmly attached to the overlying ectoderm. Fused layers of ectoderm and endoderm form the oropharyngeal membrane (Fig. 5-6C) located at the future site of the oral cavity (mouth).
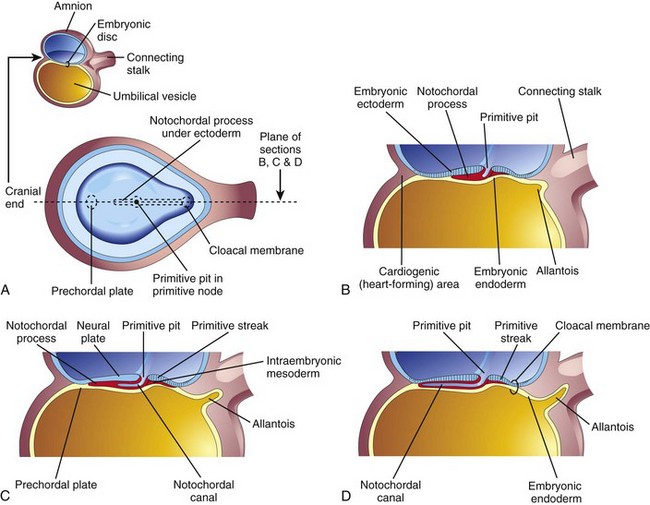
Figure 5–5 Illustrations of the development of the notochordal process. The small sketch at the upper left is for orientation. A, Dorsal view of the embryonic disc (at approximately 16 days), exposed by removal of the amnion. The notochordal process is shown as if it were visible through the embryonic ectoderm. B, C, and D, Median sections, at the same plane as shown in A, illustrating successive stages in the development of the notochordal process and canal. The stages shown in C and D occur at approximately 18 days.
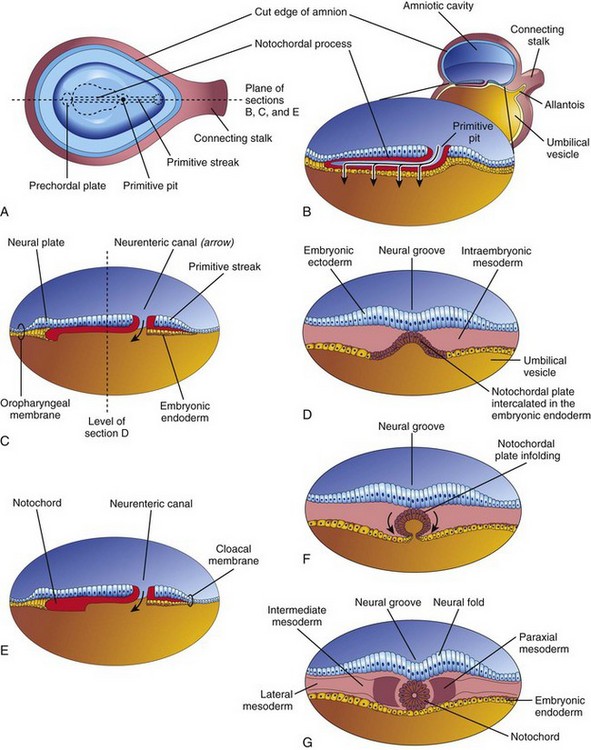
Figure 5–6 Development of the notochord by transformation of the notochordal process. A, Dorsal view of the embryonic disc (at approximately 18 days), exposed by removing the amnion. B, Three-dimensional median section of the embryo. C and E, Similar sections of slightly older embryos. D, F, and G, Transverse sections of the trilaminar embryonic disc shown in C and E.
Some mesenchymal cells from the primitive streak and the notochordal process migrate laterally and cranially between the ectoderm and endoderm until they reach the margins of the embryonic disc. These mesenchymal cells are continuous with the extraembryonic mesoderm that covers the amnion and the umbilical vesicle (Fig. 5-2D and F). Some cells from the primitive streak migrate cranially on each side of the notochordal process and around the prechordal plate. They meet cranially to form the cardiogenic mesoderm in the cardiogenic area, where the primordium of the heart begins to develop at the end of the third week. Caudal to the primitive streak there is a circular area—the cloacal membrane—that indicates the future site of the anus (Fig. 5-6D).
The notochord is a cellular rod that:
• Further defines the axis of the embryo and gives it some rigidity
• Serves as the basis for the development of the axial skeleton (such as the bones of the head and vertebral column)
The vertebral column forms around the notochord, which extends from the oropharyngeal membrane to the primitive node. The notochord degenerates and disappears as the bodies of the vertebrae form, but parts of it persist as the nucleus pulposus of each intervertebral disc. The notochord functions as the primary inductor in the early embryo. It induces the overlying embryonic ectoderm to thicken and form the neural plate (see Figs. 5-4B and C and 5-6A to C), the primordium of the central nervous system.
Allantois
The allantois appears on approximately day 16 as a small, sausage-shaped diverticulum (outpouching) that extends from the caudal wall of the umbilical vesicle into the connecting stalk (see Fig. 5-5B, C, and D). The allantois is involved with early blood formation and is associated with the urinary bladder as well. The blood vessels of the allantois become the umbilical arteries and veins.
Neurulation: Formation of the Neural Tube
The processes involved in the formation of the neural plate and neural folds and closure of these folds to form the neural tube constitute neurulation. These processes are completed by the end of the fourth week.
Neural Plate and Neural Tube
As the notochord develops, it induces the embryonic ectoderm over it to thicken and form an elongated plate of thickened neuroepithelial cells called the neural plate (Fig. 5-5C). The ectoderm of the neural plate (neuroectoderm) gives rise to the central nervous system and other structures such as the retina. At first, the elongated neural plate corresponds precisely in length to the underlying notochord. It appears cranial to the primitive node and dorsal to the notochord and the mesoderm adjacent to it (Fig. 5-4B). As the notochord elongates, the neural plate broadens and eventually extends cranially as far as the oropharyngeal membrane (Fig. 5-4C).
On approximately day 18, the neural plate invaginates along its central axis to form a median longitudinal neural groove that has neural folds on each side (see Fig. 5-6F and G). The neural folds are particularly prominent at the cranial end of the embryo and are the first signs of brain development (Fig. 5-7C). By the end of the third week, the neural folds have begun to move together and fuse, converting the neural plate into a neural tube (Figs. 5-7F and 5-8). Neural tube formation is a complex cellular and multifactorial process involving genes and extrinsic and mechanical factors.
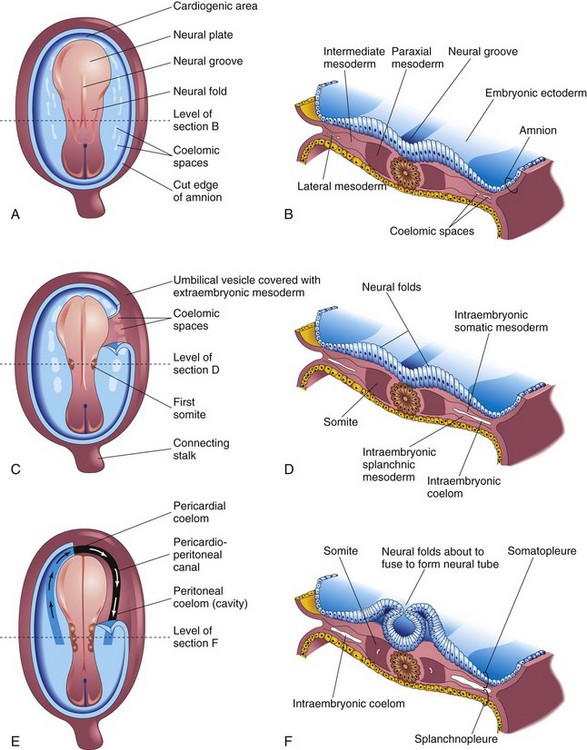
Figure 5–7 Illustrations of embryos 19 to 21 days old, illustrating the development of the somites and the intraembryonic coelom. A, C, and E, Dorsal view of the embryo, exposed by removal of the amnion. B, D, and F, Transverse sections through the embryonic disc at the levels shown. A, A presomite embryo of approximately 18 days. C, An embryo of approximately 20 days, showing the first pair of somites. A portion of the somatopleure on the right has been removed to show the isolated coelomic spaces in the lateral mesoderm. E, A three-somite embryo (approximately 21 days old), showing the horseshoe-shaped intraembryonic coelom, exposed on the right by removal of part of the somatopleure.
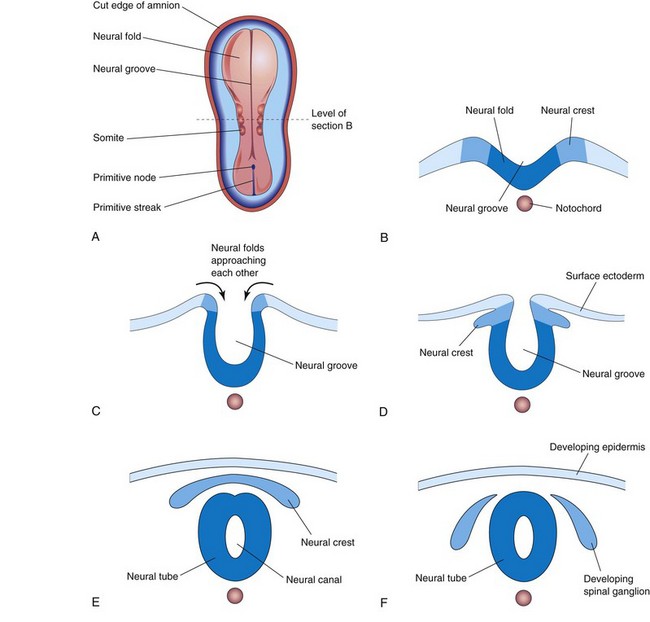
Figure 5–8 Diagrammatic transverse sections through progressively older embryos, illustrating the formation of the neural groove, neural tube, and neural crest up to the end of the fourth week.
The neural tube soon separates from the surface ectoderm (Fig. 5-8E). The free edges of the ectoderm fuse so that this layer becomes continuous over the neural tube and the back of the embryo. Subsequently, the surface ectoderm differentiates into the epidermis of the skin. Neurulation is completed during the fourth week (see Chapter 6).
Neural Crest Formation
As the neural folds fuse to form the neural tube, some neuroectodermal cells lying along the crest of each neural fold lose their epithelial affinities and attachments to neighboring cells (Fig. 5-8A to C). As the neural tube separates from the surface ectoderm, these neural crest cells migrate dorsolaterally on each side of the neural tube. They form a flattened irregular mass, the neural crest, between the neural tube and the overlying surface ectoderm (Fig. 5-8D and E). The neural crest soon separates into right and left parts that migrate in a wave to the dorsolateral aspects of the neural tube (see Fig. 5-8F). Neural crest cells also migrate widely within the mesenchyme. Neural crest cells differentiate into various cell types (see Fig. 6-4), including the spinal ganglia and the ganglia of the autonomic nervous system. The ganglia of cranial nerves V, VII, IX, and X are partially derived from neural crest cells. Neural crest cells also form the sheaths of the peripheral nerves and the pia mater and arachnoid mater.
Development of Somites
As the notochord and the neural tube form, the intraembryonic mesoderm on each side of them proliferates to form a thick, longitudinal column of paraxial mesoderm (see Figs. 5-6G and 5-7B). Each column is continuous laterally with the intermediate mesoderm, which gradually thins into a layer of lateral mesoderm. The lateral mesoderm is continuous with the extraembryonic mesoderm that covers the umbilical vesicle and amnion (see Fig. 4-3B). Toward the end of the third week, the paraxial mesoderm differentiates and begins to divide into paired cuboidal bodies, somites, on each side of the developing neural tube (Fig. 5-7C and E). The somites form distinct surface elevations on the embryo and appear somewhat triangular on transverse section (Fig. 5-7D and F). Because the somites are so prominent during the fourth and fifth weeks, they are used as one of the criteria for determining an embryo’s age (see Chapter 6 and Table 6-1).
The first pair of somites appears at the end of the third week (see Fig. 5-7C) near the cranial end of the notochord. Subsequent pairs form in a craniocaudal sequence. Somites give rise to most of the axial skeleton and the associated musculature, as well as to the adjacent dermis of the skin.
Somite formation from the paraxial mesoderm is preceded by expression of the forkhead transcription factors Fox C1 and C2. The craniocaudal segmental pattern of the somites is regulated by the Delta-Notch (Delta 1 and Notch 1) signaling pathway. A molecular oscillator, or clock, has been proposed as the mechanism responsible for the orderly sequencing of the somites.
Development of Intraembryonic Coelom
The intraembryonic coelom (body cavity) first appears as small, isolated, coelomic spaces in the lateral mesoderm and cardiogenic (heart-forming) mesoderm (Fig. 5-6A to D). These spaces coalesce to form a single, horseshoe-shaped cavity—the intraembryonic coelom (see Fig. 5-7E and F). The coelom divides the lateral mesoderm into two layers:
• A somatic, or parietal (somatopleure), layer that is continuous with the extraembryonic mesoderm that covers the amnion
• A splanchnic, or visceral (splancnopleure), layer that is continuous with the extraembryonic mesoderm that covers the umbilical vesicle
The somatopleure and the overlying embryonic ectoderm form the body wall (Fig. 5-7F), whereas the splancnopleure and the underlying embryonic endoderm form the wall of the gut. During the second month, the intraembryonic coelom is divided into three body cavities: the pericardial cavity, the pleural cavities, and the peritoneal cavity (see Chapter 9).
Early Development of Cardiovascular System
At the end of the second week, embryonic nutrition is obtained from the maternal blood by diffusion through the chorion, extraembryonic coelom, and umbilical vesicle. The early formation of the cardiovascular system correlates with the urgent need for transportation of oxygen and nourishment to the embryo from the maternal circulation through the chorion.
At the beginning of the third week, blood vessel formation, or vasculogenesis, begins in the extraembryonic mesoderm of the umbilical vesicle and connecting stalk. Vasculogenesis begins in the chorion (Fig. 5-9A and B). Blood vessels develop approximately 2 days later. At the end of the third week, a primordial uteroplacental circulation has developed (Fig. 5-10).
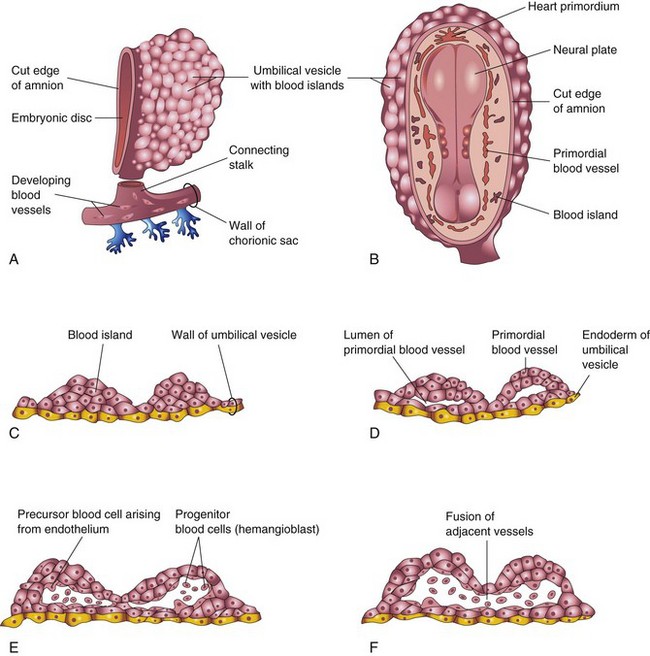
Figure 5–9 Successive stages in the development of blood and blood vessels. A, The umbilical vesicle (yolk sac) and a portion of the chorionic sac (at approximately 18 days). B, Dorsal view of the embryo exposed by removing the amnion. C to F, Sections of blood islands, showing progressive stages in the development of blood and blood vessels.
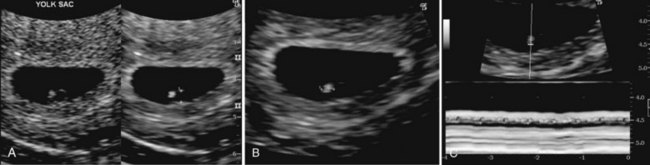
Figure 5–10 Endovaginal scan of a 4-week embryo. A, 2-mm secondary umbilical vesicle (calipers), B, Bright (echogenic) 2.4-mm, 4-week embryo (calipers). C, Cardiac activity of 116 beats/minute demonstrated with motion mode. Calipers used to encompass two beats.
(Courtesy E. A. Lyons, M.D., Professor of Radiology, and Obstetrics and Gynecology, and Anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
Vasculogenesis and Angiogenesis
Blood vessel formation in the embryo and the extraembryonic membranes during the third week may be summarized as follows (Fig. 5-9C to F):
• Mesenchymal cells differentiate into endothelial cell precursors, or angioblasts (vessel-forming cells), that aggregate to form isolated angiogenic cell clusters known as blood islands (Fig. 5-9B and C).
• Small cavities appear within the blood islands by the confluence of intercellular clefts.
• Angioblasts flatten to form endothelial cells that arrange themselves around the cavities in the blood islands to form the primordial endothelium.
• These endothelium-lined cavities soon fuse to form networks of endothelial channels.
• Vessels sprout by endothelial budding into adjacent nonvascularized areas and fuse with other vessels.
Blood cells develop from specialized endothelial cells of vessels (hemangioblasts) on the umbilical vesicle and allantois at the end of the third week (see Fig. 5-9E and F). Blood formation (hematogenesis) does not begin within the embryo until the fifth week. This process occurs first in various parts of the embryonic mesenchyme, chiefly the liver, and later, in the spleen, bone marrow, and lymph nodes. Fetal and adult erythrocytes are also derived from hematopoietic progenitor cells (hemangioblasts). The mesenchymal cells that surround the primordial endothelial blood vessels differentiate into muscular and connective tissue elements of the vessels.
The heart and great vessels form from mesenchymal cells in the heart primordium, or cardiogenic area (see Fig. 5-7A and 5-9B). Paired, endothelium-lined channels—endocardial heart tubes—develop during the third week and fuse to form a primordial heart tube. The tubular heart joins with blood vessels in the embryo, connecting stalk, chorion, and umbilical vesicle to form a primordial cardiovascular system (Fig. 5-11C). By the end of the third week, blood is flowing, and the heart begins to beat on day 21 or 22. Thus, the cardiovascular system is the first organ system to reach a primitive functional state. The embryonic heartbeat can be detected by Doppler ultrasonography during the fourth week, approximately 6 weeks after the last normal menstrual period (Fig. 5-10).
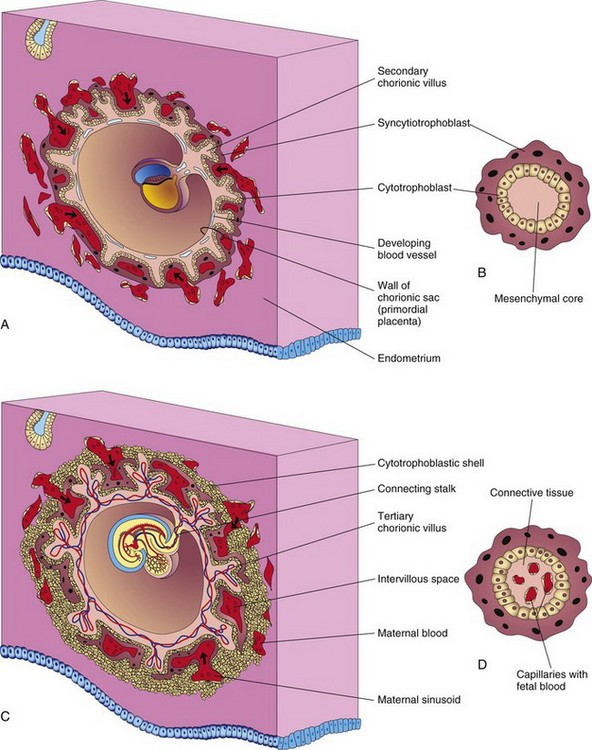
Figure 5–11 Illustrations of the development of the secondary chorionic villi into the tertiary chorionic villi. A, Sagittal section of an embryo (at approximately 16 days). B, Section of a secondary chorionic villus. C, Section of an embryo (at approximately 21 days). D, Section of a tertiary chorionic villus. By the end of the third week, a primordial uteroplacental circulation has developed.
Development of Chorionic Villi
Shortly after the primary chorionic villi are formed at the end of the second week, they begin to branch. Early in the third week, mesenchyme grows into the primary villi, forming a core of loose mesenchymal (connective) tissue (see Fig. 5-11A and B). The villi at this stage—secondary chorionic villi—cover the entire surface of the chorionic sac. Mesenchymal cells in the villi soon differentiate into both capillaries and blood cells (see Fig. 5-11 C and D). When capillaries are present, the villi are called tertiary chorionic villi. The capillaries in the chorionic villi fuse to form arteriocapillary networks that soon become connected with the embryonic heart through vessels that differentiate from the mesenchyme of the chorion and connecting stalk. By the end of the third week, embryonic blood begins to flow slowly through the capillaries in the chorionic villi. Oxygen and nutrients in the maternal blood in the intervillous space diffuse through the walls of the villi and enter the embryo’s blood (Fig. 5-11C). Carbon dioxide and waste products diffuse from blood in the fetal capillaries through the wall of the villi into the maternal blood. Concurrently, cytotrophoblastic cells of the chorionic villi proliferate and extend through the syncytiotrophoblast to form a cytotrophoblastic shell, which gradually surrounds the chorionic sac and attaches it to the endometrium (see Fig. 5-11C).
Villi that attach to the maternal tissues through the cytotrophoblastic shell are called stem chorionic villi (anchoring villi). The villi that grow from the sides of the stem villi are called branch chorionic villi (terminal villi). It is through the walls of the branch villi that the main exchange of material between the blood of the mother and the embryo takes place. The branch villi are bathed in continually changing maternal blood in the intervillous space.
Sacrococcygeal Teratoma
Remnants of the primitive streak may persist and give rise to a large tumor known as a sacrococcygeal teratoma (Fig. 5-12). Because it is derived from pluripotent primitive streak cells, the tumor contains tissues derived from all three germ layers in incomplete stages of differentiation. Sacrococcygeal teratomas are the most common tumors in newborn infants and have an incidence of approximately 1 in 27,000 neonates. These tumors are usually surgically excised promptly, and the prognosis is good.
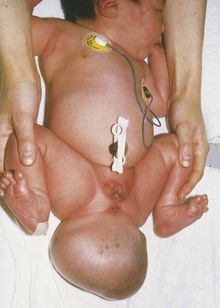
Figure 5–12 A female infant with a large sacrococcygeal teratoma that developed from remnants of the primitive streak.
(Courtesy A. E. Chudley, M.D., Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital, University of Manitoba, Winnipeg, Manitoba, Canada.)
Abnormal Neurulation
Disturbance of neurulation may result in severe abnormalities of the brain and spinal cord (see Chapter 16). Neural tube defects are among the most common congenital anomalies. Meroencephaly (anencephaly), or partial absence of the brain, is the most severe defect. Available evidence suggests that the primary disturbance affects the neuroectoderm. Failure of the neural folds to fuse and form the neural tube in the brain region results in meroencephaly, and in the lumbar region, spina bifida cystica (see Fig. 16-9).
Abnormal Growth of Trophoblast
Sometimes the embryo dies and the chorionic villi do not complete their development; that is, they do not become vascularized to form tertiary villi. These degenerating villi may form cystic swellings, called hydatidiform moles. These moles exhibit variable degrees of trophoblastic proliferation and produce excessive amounts of human chorionic gonadotropin. In 3% to 5% of such cases, these moles develop into malignant trophoblastic lesions, called choriocarcinomas. These tumors invariably metastasize (spread) by way of the blood to various sites, such as the lungs, vagina, liver, bone, intestine, and brain.
Clinically Oriented Questions
1. Can drugs and other agents cause congenital anomalies of the embryo if they are present in the mother’s blood during the third week? If so, what organs would be most susceptible?
2. Are there increased risks for the embryo associated with pregnancies in women older than 40 years of age? If so, what are they?