Chapter 96Shock Wave Therapy
Historical Perspective
Early studies in people in which shock waves (SWs) were used to disintegrate ureteral stones resulted in radiologically evident remodeling of the pelvis.1 These findings sparked the initial studies investigating the use of SWs in orthopedic applications. These studies and anecdotal clinical use resulted in the initial five standard indications used in human medicine: (1) calcifying tendonitis of the shoulder (tendinosis calcarea), (2) tennis elbow (lateral epicondylitis), (3) golfer’s elbow (medial epicondylitis), (4) heel spurs (plantar fasciitis), and (5) pseudarthrosis. Focused shock wave therapy in horses started in Germany in 1996, and applications were primarily based on those from human medicine.2 Because of positive experiences treating people with insertional desmopathies, the first equine application was the use of shock wave therapy (SWT) in horses with proximal suspensory desmitis. Initial clinical responses were positive; therefore SWT was attempted in numerous other equine conditions, including navicular syndrome and distal hock joint pain.3,4 Initially, the probe was positioned behind the navicular bone from the heel, but when ultrasonographic imaging showed that the distal sesamoidean impar ligament could be seen through the frog, this location was then used to administer SWT to the navicular bone and associated structures.
The first equipment was large, requiring water cycling and degassing, and use was limited to horses under general anesthesia. The development of more affordable, portable, and durable equipment led to an initial expansion of its use and applications. However, as further knowledge was gained in equine medicine and surgery, the use of SWT has contracted somewhat, and attention is now paid to applications that consistently provide good clinical outcomes.
Shock Wave Generators
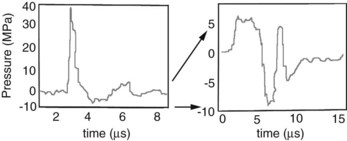
Two distinctly different pressure waves have been loosely described as SWT, SWs and radial pressure waves (RPWs). True SWs are pressure waves that meet specific physical parameters, including a rapid rise time (within nanoseconds), high peak pressures, and a gradual decrease in pressure over a few milliseconds, often with a negative pressure component (Figure 96-1 and Table 96-1).5 SWs occur naturally associated with lightning, planes breaking the sound barrier, or explosions. Electricity is used as a driving energy source to generate SWs used for medical purposes. RPWs are generated by pneumatically powered sources that drive a metal rod to strike a plate in contact with the skin surface. RPWs have slower rise times and lower peak pressures than SWs. Differences between SWs and RPWs are important because they may not affect tissue similarly; consequently, the type and equipment used are critical when evaluating the effect on tissues and efficacy of treatment. Editors’ note: However, published results in the horse have to date shown little difference in efficacy. A comprehensive review of SWs and RPWs, along with a description of equipment currently available, was recently published.6
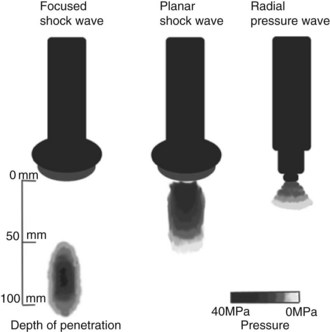
Fig. 96-1 The schematic demonstrates the three types of pressure waves described. Focused shock waves concentrate the pressure at a focal point that can be centered up to approximately 75 mm deep. Planar shock waves are not focused and have a more diffuse pattern with less penetration. Radial pressure wave generators have a lower maximum pressure and deposit the maximum pressure on the surface.
(Reprinted with permission from Robinson EN, Sprayberry KA: Current therapy in equine medicine, ed 6, St. Louis, 2009, Saunders.)
TABLE 96-1 Comparison of Shock Waves and Radial Pressure Waves
| SHOCK WAVES | RADIAL PRESSURE WAVES | |
|---|---|---|
 |
||
| Rise time | 5-10 ns | 50 µs |
| Focusing | Yes | No* |
| Maximum pressure location | At focal point | On surface |
| Energy loss | Minimal through fluid/tissue | Loss proportional to the square of the distance |
| Peak pressure | ≈100 MPa | ≈10 MPa |
| Energy flux density | 0-3 mJ/mm2 | 0-0.3 mJ/mm2 |
* Includes “focused” end piece.
Reprinted with permission from Robinson EN, Sprayberry KA: Current therapy in equine medicine, ed 6, St. Louis, 2009, Saunders.
Dose Dependence
There is a dose effect of SWT that is a combination of the energy flux density (EFD) and the number of pulses. The EFD (mJ/mm2) is the amount of energy (millijoules) in the focal area (mm2). There is a lower limit of EFD that must be reached for SWT to be effective and an upper level over which EFD is high enough to create damage at any number of pulses. This range depends on species, tissue being treated, waveform, and generator.
It is difficult to transpose doses from other species to horses. When 1000 pulses at an EFD of 0.28 mJ/mm2 was applied to rabbit Achilles tendons, there was a useful inflammatory reaction; but there was inflammation and tendon necrosis at 0.6 mJ/mm2.7 However, EFDs of 0.6 mJ/mm2 or more are used in horses without complications. The minimal dose to achieve the desired effect is not known, and we may not need to use such high levels. There is a current trend to use low EFDs for a number of applications. One thousand pulses at 0.18 mJ/mm2 stimulated neovascularization of the tendon-bone junction in dogs.8 A dose of 200 pulses at 0.12 mJ/mm2 was better than 500 pulses in an Achilles tendonitis model in rats.9 At this time, the ideal energy levels and pulse numbers remain empirical in most equine applications.
The frequency (pulses/sec) of the pulse application appears unimportant, although SWT using lower frequencies takes longer. From a biological standpoint, frequency of all equine SWT applications is relatively slow, and tissue heating or other untoward biological effects seen at high rates do not occur, although white hair occasionally develops at treatment sites.
Applications
Bone
Early studies of SWs on bones yielded variable results and were confusing. Very-high-energy treatment of rodent and rabbit bones resulted in physical disruption of the bone and periosteum, which led to the thought that bone remodeling in response to SWT was the result of physical damage.10,11 In other studies using more appropriate tissue and in different species, bone remodeling occurred without physical disruption. The ability for SWT to stimulate bone healing resulted in an interest to use SWs for the management of nonunion fractures. In one study, nonunion fractures were created in 10 dogs using segmental radial osteotomies.12 Five dogs were treated with 4000 SWs at 0.54 mJ/mm2, and five served as untreated controls. All treated dogs had osseous union by 12 weeks compared with only one untreated control dog.12 The first widespread musculoskeletal use of SWs in people was for management of nonunion fractures. Clinical trials have consistently demonstrated bone healing following SWT in delayed union fractures and nonunion fractures. Wang reported 80% success13; Schaden, 75% success14; and Rompe, 72% success—results that were remarkably consistent.15
The mechanisms of SW-induced osteogenic stimulation are currently being investigated. There were increased osteoprogenitor colony-forming units from the marrow of rat femora 24 hours after treatment with 500 SWs at 0.16 mJ/mm2.16 A dose response was seen with the highest supernatant concentrations of transforming growth factor–β1 (TGF-β1) after 500 SWs; lower numbers of pulses were ineffective, and higher numbers were inhibitory. The same treatment protocol (500 SWs at 0.16 mJ/mm2) was administered to rats with segmental bone defects and resulted in increased expression of TGF-β1 and vascular endothelial growth factor–A (VEGF-A) mRNA and increased osteoprogenitor cells.17 In both studies, it appeared that SWT stimulated osteogenesis without physical disruption of the bone. Subsequent studies have shown that SWT increases concentrations of VEGF and bone morphogenic protein–2, as well as neovascularization and ultimately an increase in load to failure in a rabbit femoral fracture model.18
The application of SWT to bone was the initial musculoskeletal application in people and horses. The applications and outcomes in human medicine have grown and are positive. The applications of SWs in horses have not been pursued as aggressively as may have been expected. One initial concern was the potential for the creation of microfractures that could weaken the bone after treatment, a theory that has some support. The application of 9000 pulses of RPWs at 0.175 mJ/mm2 or 9000 SWs at 0.15 mJ/mm2 to cadaver third metacarpal bones (McIIIs) from racehorses that suffered catastrophic injuries of the contralateral limb caused a small but significant increase in microcrack density, and RPWs increased microcrack length.16 In dorsal cortical bone specimens of the McIII 2000 pulses of SWs or RPWs subjected to pulse increments of either 500-SWs (0.15 mJ/mm2) or RPWs (0.16 mJ/mm2), there were no detectable changes in modulus of elasticity.17 Histologically, there were no increases in microfractures demonstrated with either SWs or RPWs.17 Microfractures did not develop in an in vivo study in which 1000 pulses at a high EFD (1.8 mJ/mm2) were used.
In an in vivo equine pilot study that used 2000 SWs at 0.89 mJ/mm2, two treated McIIIs had 30% more activated osteons than the contralateral untreated control McIII.18 SWs were compared with periosteal irritation (stimulated by scarifying the periosteum with a needle) in the third metatarsal bone (MtIII) of four horses. The SW-treated MtIIIs had 56% more double-labeled osteons than those with only periosteal irritation, and the osteonal response was located at both the endosteal and the periosteal surfaces. This preliminary finding demonstrated that SWs exert an effect on bone that is different from simple periosteal scarification. Scintigraphic and histological examination of the fourth metatarsal bone after 2000 pulses at 0.15 mJ/mm2 showed increased radiopharmaceutical uptake in the treatment area and an increased number of osteoblasts.19
Twenty-two Thoroughbred (TB) racehorses with dorsal metacarpal disease (see Chapter 102) manifest as pain on palpation, a thickened dorsal cortex of the McIII, and longitudinal radiolucent lines that were unresponsive to conventional therapy were treated with RPWs.20 Three treatments at 2-week intervals were administered, and a modified training program was instituted. The mean time to the first race was 4.5 months. Similar results are seen with focused SWT. I treat horses with periostitis with focused SWT (0.14 mJ/mm2, 800 pulses), give 1 week of walking, and then resume training for 2 weeks; the cycle is repeated two more times. When horses are treated early in the disease process, most can continue in training without an extended rest period.
Horses with stress fractures of the tibia, humerus, and the McIII are candidates for management with SWT. Without controlled studies, it is difficult to compare SWT with healing in horses with nontreated fractures or in those receiving osteostixis. It is my impression that TB racehorses with dorsal cortical fractures of the McIII can return to training in a similar period as those that have received osteostixis. Six of 10 horses with McIII stress fractures were pain free and/or showed radiological evidence of healing by 90 days.21 Two horses took more than 120 days, and two were retired for other reasons. In 20 horses with single oblique dorsal cortical McIII stress fractures treated with RPWs, the mean time from the last treatment to the first race was 5 months.20
Horses with fractures of the second and fourth metacarpal (metatarsal) bones and exostoses are frequently treated with SWT. Focused SWT was used in four horses with closed fractures and in three with open, infected, comminuted fractures of the proximal aspect of the splint bones.22 Horses with infected fractures were first treated locally and systemically with antibiotics, local wound curettage, and ostectomy of small loose fragments. SWT began when signs of infection were resolved and the skin was healed. The horses were treated with three treatments at 10- to 14-day intervals. They were administered 600 to 900 pulses, depending on the size of the lesion, at an EFD of 0.15 mJ/mm2. Radiographs were obtained after the third treatment and 4 weeks later. Handwalking began after the second or third treatment. In horses without an infection, training began 10 to 12 weeks after fracture, whereas in those with infected fractures, training began 13 to 16 weeks after fracture. All the horses were sound and returned to normal use. There were no horses that did not undergo SWT for comparison.
Horses with fractures of the distal phalanx that can be approached with SWT through the frog are good candidates for treatment, but efficacy is anecdotal. Because it is not possible to get the SWs to penetrate through the hoof wall or the sole, fractures must be near the center of the distal phalanx and accessible through the frog. Some horses have shown positive outcomes.
There are only limited reports of using SWT in horses with long bone fractures and arthrodesis stabilized with internal fixation. Expense is a consideration, but access to apply SWT is a major limiting factor. SWT cannot be used effectively on horses with external coaptation such as a cast. SWT improved callus formation in the immediate postoperative period in dogs with bilateral 3-mm osteotomy gaps in the midtibia stabilized with a plate and treated with 2000 pulses at 0.18 mJ/mm2.23 At 12 weeks there was more radiologically visible callus and more histologically evident cortical bone formation than in control untreated dogs.23 Early treatment of fractures may be beneficial in the horse.
Tendons and Ligaments
In vivo studies of tendon and ligament injuries have provided support for the use of SWT. Rabbits with collagenase-induced patellar tendonopathy were treated twice with 1500 pulses at 0.29 mJ/mm2.24 Tendons were harvested 4 and 16 weeks after SWT. SW-treated tendons had a 7% and 10% increase in strength at 4 and 16 weeks, respectively, compared with untreated controls. There were increased hydroxyproline concentrations in treated tendons and more blastlike tenocytes after 4 weeks. When 500 SWs at 0.12 mJ/mm2 were applied to long digital tendon grafts placed to stabilize surgically disrupted anterior cruciate ligaments in rabbits, the treated bone–tendon interface had more trabecular bone ingrowth than controls.25 The contact between the bone and tendon was improved, and subsequently the strength of the graft was increased 24 weeks postoperatively. Growth factors known to affect tendon repair, including TGF-β1 and insulin-like growth factor I, were increased after SWT in rats.9
Tissue explants were used to evaluate the effect of SWs on healthy pony tendons and ligaments.26 Six hundred pulses at 0.14 mJ/mm2 increased glycosaminoglycan (GAG) and protein synthesis in explants harvested 3 hours after treatment. However, by 6 weeks there was a decrease in GAG and collagen synthesis.26 In a similar study, samples harvested at 3 hours after treatment revealed histological findings of disorganization of matrix structure and increase in degraded collagen.27 Gene expression of type I collagen and matrix metalloproteinase 1 was increased at 6 weeks after SWT.27 In these studies the degraded collagen and disorganization of the collagen at 3 hours after treatment warrant some concern about a possible detrimental effect of SWT. However, these studies were done in normal pony tendon and ligament, and one would expect these findings to already be present in injured soft tissues.
The most common application and the greatest amount of SW research in the horse have related to tendons and ligaments.
Suspensory Desmitis
To study the effects of SWT in horses, ultrasonography was used to monitor healing in two studies of suspensory ligaments after collagenase-induced desmitis.28,29 In both studies, ultrasonographic measurements showed the defects healed faster in the SW-treated groups. Different outcomes were evaluated histologically in each study, but a positive effect of SWT was found in both studies. In forelimbs treated with SWT, there was increased metachromasia consistent with increased production of extracellular matrix in the SWT group.28 In hindlimbs there were more newly formed small collagen fibrils in the SWT group.29 The exact mechanisms by which these outcomes occur are not fully understood and it is unknown if the differences between treated and controls would result in a stronger outcome or less recurrence of injury in horses with suspensory desmitis. Immunocytochemical evaluation of suspensory ligaments treated by SWT showed increased TGF-β1 after treatment.29
Clinical response to SWT is consistently better in forelimbs than in hindlimbs. In a retrospective clinical study of horses with forelimb proximal suspensory desmitis (PSD) managed with 2000 pulses at 0.15 mJ/mm2, 61.8% of horses with desmitis were in full work by 6 months, and 55.9% were still working at 1 year.30 Editors’ note: However, it must be borne in mind that many horses with forelimb PSD respond well to conservative management of rest alone (see Chapter 72). Of horses with hindlimb suspensory desmitis, 40.9% were in full work at 6 months, but only 18.2% were in full work at 1 year.30 Results using RPW therapy in horses with forelimb or hindlimb lameness of at least 3 months’ duration before treatment associated with PSD were remarkably similar.31,32 Response was related to lesion severity determined ultrasonographically. At a recent equine practitioners’ meeting,* the consensus on outcome was similar to these reports. Many practitioners do not consider horses with hindlimb suspensory desmitis to be good candidates for SWT alone. A combination of SWT and plantar fasciotomy to relieve alleged pressure on the proximal aspect of the suspensory ligament and to stimulate healing of the defect may provide a better prognosis. Horses with body and branch desmitis are also commonly treated and thought to have acceptable outcomes; however, there have been no controlled clinical trials, and information is anecdotal.
Superficial Digital Flexor Tendonitis
There are two studies that have evaluated the short-term effect of SWT on collagenase-induced superficial digital flexor (SDF) tendonitis in the midmetacarpal region.33,34 Ultrasonographically, the treated and control groups were similar throughout the studies. Histologically, the treated tendons were more “mature,” indicating that the healing process was occurring at a faster rate. Treated tendons had more parallel collagen fibers than controls in one study, and treated tendons had increased neovascularization in the other study. In both studies there was an initial decrease in inflammation associated with SWT, but in neither study was tendon strength evaluated. A notable finding after SWT of tendonitis is the rapid improvement in clinical appearance. The peritendonous inflammation that results in the “bowed” appearance decreases quickly after SWT. One must be careful not to interpret that as tendon healing. The echogenicity and fiber alignment of the tendon injury must be monitored with ultrasonography.
Additional Applications
Osteoarthritis
Osteoarthritis (OA) of the distal hock joints was one of the first applications of SWs in the United States. In an early study of 74 horses with “bone spavin” refractory to medical therapy given SWT using a single high energy (0.89 mJ/mm2) treatment of 2000 pulses, 59 (80%) horses improved by 90 days.4 There are horses with OA of numerous joints that anecdotally have improved after SWT.35 However, responses are mixed. In some horses, lameness decreases for a few months after SWT, whereas in others there is no change. I cannot find a logical explanation. SWT was evaluated experimentally in an osteochondral fragment–exercise model of OA of the middle carpal joint (see Chapter 84).36 In treated horses, there was a decrease in total protein concentration in synovial fluid and less lameness on day 28 compared with controls.36
Navicular Syndrome
Clinical signs in horses with navicular syndrome may improve after SWT.37,38 In 16 horses with long-term follow-up examination treated with 1000 pulses through the frog and 1000 pulses from between the heel bulbs under general anesthesia at 0.89 mJ/mm2, masked video analysis from before and 6 months after treatment showed 56% of horses improved at least 1 lameness grade.37 Owners reported 69% of the horses returned to the previous level of activity. There was no comparative control group. In another study, all 26 horses treated under general anesthesia with 3000 pulses at 0.56 mJ/mm2 were free of lameness at 6 months.38
Navicular syndrome is complex, and as diagnostic techniques have improved, we now know that pain can originate from the deep digital flexor tendon, navicular bursa, the collateral sesamoidean or distal sesamoidean impar ligaments, the navicular bone itself, or other structures. SWT is indicated for horses that do not adequately respond to medical therapy and corrective shoeing. However, it must be recognized that navicular syndrome is a progressive disease, and SWT is not a cure.
Collateral Ligament Injury of the Distal Interphalangeal Joint
There is a recent report of the use of SWT or RPW therapy for the treatment of horses with collateral ligament injuries of the distal interphalangeal joint. There was no significant difference in outcome for horses managed by box rest and controlled exercise alone or combined with SWT or RPW therapy.39
Back Pain
SWT has been used for numerous causes of back pain, including impinging dorsal spinous processes, OA of articular facet joints, and soft tissue injury. There are two approaches to therapy for back pain. First is the treatment of specific sites of bony pathology identified by radiography, scintigraphy, and ultrasonography. Both sides of the back are treated with 35- and 80-mm probes (designed to treat equine backs; Equitron, Sanuwave Inc., Marietta, Georgia, United States) at 0.15 mJ/mm2. Most horses improve after one or two treatments. A dose of 50 pulses/cm of bone “sclerosis” was suggested.23 Second, horses that have back pain without specific skeletal pathology may respond to treatment of the muscle with SWT.2,40 The 35- and 80-mm probes are moved along the dorsum of the horse. When the horse shows a painful reaction or muscle fasciculation, 80 to 100 pulses are applied at the site. There are anecdotal reports of SWT used for treating sacroiliac disease; an 80-mm probe may reach the dorsal sacroiliac ligaments but is unlikely to reach the sacroiliac joints per se.
Some horses with OA of the cervical articular facets (see Chapter 53) respond well to SWT, and others have a limited response. It is important to identify the specific facet joint to be treated and focus the SWs on the facet similarly to an injection into the joint. Two to three treatments at 2- to 3-week intervals are performed. If response is acceptable, then the treatment is repeated as needed.
Therapy Planning
When considering the application of SWs, obviously an accurate diagnosis is imperative. Next one needs to consider whether one can and should focus SWs on the pathology. SWs will not penetrate the hoof wall. Bone can be treated; however, 70% of the energy is lost in the bone. Therefore little energy is available on the opposite side of the bone. Most focused generators have a series of standoffs that adjust the depth of penetration. Any site that can be seen ultrasonographically can be treated with SWs, and ultrasonography can be used to measure the required depth if necessary. RPWs are different from the more powerful focused SWs in that the energy of the wave decreases proportional to the square of the depth; thus the depth of penetration is limited.
A three-dimensional treatment plan should be used to apply SWs to the full width and depth of the injured area.23 For example, to treat a horse with PSD, the entire width and depth of the proximal aspect of the suspensory ligament are treated by using two probes, one with a 20-mm and the other with a 35-mm focal distance (and a commonly used generator; Equitron, Sanuwave Inc.). From the palmar midline, both probes are used to administer SWT to the axial aspect of the suspensory ligament, and then the 20-mm probe is used from oblique angles just palmar and axial to both splint bones to treat the remaining abaxial aspects of the ligament. The probes are slowly moved around the treatment area when applying SWT. This should expose the entire proximal suspensory ligament and the palmar/plantar aspects of the third metacarpal or metatarsal bones to the SWs.
SWs will completely reflect at an air interface, and it is important to avoid air interfaces during treatments near the thorax and abdomen. The pressure wave will be reflected and can cause hemorrhage at the interface. It is important to have the generator in good contact with the skin surface. Typically, the hair is clipped with a No. 40 blade. To obliterate the air interface found between the probe and skin, ultrasound coupling gel is the best agent to use, but water, mineral oil, and petroleum jelly are all suitable. It is best to slowly move the probe in the direction of the clipped hair, and then move back up and down again, with care taken to recoat the area with the gel. The convex radial generators may get air bubbles in the concavity, making coupling difficult.41
Combination therapies are frequently used to treat soft tissue injuries. Platelet-rich plasma and pig’s urinary bladder matrix (ACellVet, Webster Veterinary Supply, Sterling, Massachusetts, United States) injected intralesionally in combination with SWT may result in some additive effects. My personal experience is favorable when I use a combination of ACellVet in horses with SDF tendonitis at the time of the first of three SWTs. The direct effect of SWs on stem cells in vivo is unknown. For this reason, when used together, I suggest SWT should precede the injection of stem cells, and the next SWT should be delayed for 3 weeks. Editor’s Note: Since the effect of SWs on stem cells is unknown and given that stem cell therapy is still unproven and rather expensive, it may be best to select either SWT or stem cell therapy rather than combine the modalities.
Potential Complications
Very few complications are seen with SWT. There are dose-related effects, and it is possible to overtreat an area. With most commonly available devices, there are general guidelines to assist the veterinarian to calculate the appropriate dose. Transient swelling at the treatment site is occasionally noted and is more commonly seen when using RPW devices. White hairs may develop at the treatment site. As noted above, air- or gas-filled structures should be avoided. What is the effect of SWs on a physis? Some say it stimulates growth, whereas others say it inhibits growth. Studies in rabbits showed SWs can lead to premature closure of a physis; however, this work has not been repeated in horses.42
Analgesia
Analgesia has been a welfare concern since the beginning of SWT in the horse. There are concerns that analgesia after treatment may result in worsening of an injury when a horse returns to work. In vivo evaluation of skin sensation after both focused SWT and RPW therapy showed some analgesia for 48 hours after treatment.43 There was no analgesia of the nerve field after the application of SWs and RPWs directly to the palmar digital nerve; thus it was concluded that analgesia was a local effect. In horses with unilateral navicular syndrome and OA where analgesia was measured as a change in increase in peak vertical force after treatment, analgesia from SWT was identified that peaked at 48 hours after application.44 In a similar study using horses with unilateral navicular syndrome and RPWs, no analgesia was found.45 The RPWs may not reach the depth sufficient to affect pain sensation from the palmar foot structures. Many racing and horse show jurisdictions have specific rules about SWT, and they should be consulted.