Chapter 72The Suspensory Apparatus
Anatomy and Pathophysiology
The suspensory ligament (SL) is correctly called the third interosseous muscle, but it is referred to as the SL throughout this text. The anatomy in the forelimbs and hindlimbs is similar, and separate mention of the hindlimb is made only when substantial differences exist.
The SL can be divided into three separate regions that are subject to injury: the proximal part, the body, and the branches. For clinical purposes, in the forelimb the proximal part extends from 4 to 12 cm distal to the accessory carpal bone, and in the hindlimb, from 2 to 10 cm distal to the tarsometatarsal joint. The distal sesamoidean ligaments are considered individually.
In the forelimb the SL originates from two differently shaped lobes that rapidly fuse. In the hindlimb this division is less obvious. The SL contains a variable amount of muscular tissue (2% to 11%), which tends to be bilaterally symmetrical.1 In the forelimb the SL originates from the palmar carpal ligament and the proximal aspect of the third metacarpal bone (McIII), whereas in the hindlimb it originates principally from the proximoplantar aspect of the third metatarsal bone (MtIII). There is an accessory ligament of the SL that extends proximally and originates on the plantar aspect of the fourth tarsal bone. The SL in the forelimb is approximately rectangular in cross-section, but it is more rounded in the hindlimb. There are also fibers that arise from the axial aspect of the fourth metacarpal bone (McIV).
The body of the SL descends between the second metacarpal or metatarsal bone (McII, MtII) and the McIV or the fourth metatarsal bone (MtIV) and divides into two branches at a variable site in the midmetacarpal (midmetatarsal) region. The level of division is usually bilaterally symmetrical. Each branch inserts on the abaxial surface of the corresponding proximal sesamoid bone (PSB). Each branch detaches a thin extensor branch dorsodistally that courses obliquely across the pastern to join the dorsal digital extensor tendon just above the proximal interphalangeal joint. Each extensor branch also blends with the corresponding collateral sesamoidean ligament.
The distal sesamoidean ligaments are the functional continuation of the SL in the digit and consist of the straight sesamoidean ligament, oblique sesamoidean ligaments, cruciate sesamoidean ligaments, and short sesamoidean ligaments. All attach proximally to the base of the PSBs and the proximal scutum. The straight sesamoidean ligament is a flat trapezoidal band that inserts via the scutum medium onto the proximal aspect of the middle phalanx. The oblique sesamoidean ligaments are triangular structures that converge to insert on the palmar aspect of the proximal phalanx. The cruciate sesamoidean ligaments form the palmar wall of the distal palmar synovial recess of the metacarpophalangeal joint. They consist of two thin layers of tissue that cross each other and insert on the proximal palmar tuberosities of the proximal phalanx. The short sesamoidean ligaments insert on the proximal palmar aspect of the proximal phalanx and are difficult to separate from the dorsal aspect of the oblique sesamoidean ligaments.
The palmar ligament of the fetlock, also referred to as the intersesamoidean ligament, is a thick collagen structure that completely covers the palmar and axial surfaces of the PSBs and is strongly attached to them. Together with the PSBs, the palmar ligament forms the proximal scutum. The concave palmar surface of the proximal scutum provides a smooth surface over which the digital flexor tendons glide.
In the forelimb the SL is innervated by the palmar metacarpal nerves, derived from the lateral palmar nerve, which receives fibers from the ulnar and median nerves.2 The hindlimb SL is innervated by the plantar metatarsal nerves, branches from the deep branch of the lateral plantar nerve, which is derived from the tibial nerve. The proximal aspect of the SL is closely related to the distal palmar outpouchings of the carpometacarpal joint in the forelimb3 and the plantar outpouchings of the tarsometatarsal joint in the hindlimb.4
The principal function of the SL is to prevent excessive extension of the fetlock joint.5 During weight bearing the relative tension in the SL and digital flexor tendons regulates the stresses applied to different aspects of the McIII. When a limb is fully load bearing, the distal parts of the SL branches are apposed closely to the abaxial aspects of the metacarpal condyles and then move to the palmar aspect as the fetlock drops. During hyperextension the PSBs move distally and dorsally, so the branches of the SL act as articular surfaces to balance the position of the McIII. If the limb is loaded asymmetrically, so that torque is on the fetlock, the SL branches contribute to joint stability on the side opposite compression of the joint.
Some evidence exists that training increases the strength of the SL; the mean absolute load to failure in a single load-to-failure compression test was significantly higher in horses that were in race training compared with those that were confined to box or paddock rest.6 In the trained group failure was most likely to be by fracture of a PSB, whereas in the untrained group the SL failed. However, when six 2-year-old Thoroughbred (TB) fillies underwent an 18-month controlled exercise program including galloping and were compared with six fillies that were restricted to walking exercise, no differences in the collagen fibril mass-average diameter in the body of the SL were found.7 Mass-average diameter is correlated with ligament strength.
Proximal Suspensory Desmitis in the Forelimb
Proximal suspensory desmitis (PSD) is a common injury in the forelimbs8-12 of athletic horses and may occur unilaterally or bilaterally. Some confusion has occurred about what constitutes PSD, and many clinicians have used this term for lameness that is worse with the affected limb on the outside of a circle and that is alleviated by analgesia of the proximal palmar metacarpal region but in which radiological and ultrasonographic findings have been negative. In our experience this case scenario is relatively unusual. Ultrasonographic abnormalities of the proximal SL are usually detectable, and the absence of detectable structural abnormality should alert the veterinarian to search for an alternative diagnosis. However, in a small proportion of horses abnormalities of the SL that were not apparent using ultrasonography have been identified using magnetic resonance imaging (MRI).13,14
PSD usually results in sudden-onset lameness, which can be remarkably transient, resolving within 24 hours unless the horse is worked hard. In horses with more chronic desmitis, lameness may be persistent. Lameness varies from mild to moderate and is rarely severe, unless the lesion is extensive. Lameness in Standardbred (STB) racehorses may be apparent only at high speeds. Bilateral PSD may result in loss of action rather than overt lameness, which occurs more commonly in flat racehorses than other sports horses, probably because of failure to recognize earlier, subtle unilateral lameness. However, dressage horses and event horses may be similarly affected. In a jumping horse the rider’s complaint may be of failure of a horse to land with a specific forelimb leading. While ground reaction force is greater in the nonlead forelimb when landing from a fence, the lead forelimb has greater extension of the fetlock, resulting in potentially greater stress on the suspensory apparatus. So with SL injury the horse may be reluctant to land with the lame forelimb leading. Lameness is usually worse on soft ground, especially with the affected limb on the outside of a circle, and when subtle may be more easily felt by a rider than seen by an observer. In some horses lameness is never detectable either in hand or on the lunge and is only apparent when the horse is ridden. Lameness may not be apparent at the working trot but may be detectable at the medium or extended trot. Presumably this reflects increased stress on the SL because of increased extension of the fetlock at extended trot compared with working trot. Recognition of these features in the history may be important, because acute lameness often resolves rapidly, and working the horse hard to reproduce lameness, with the inherent risk of worsening the injury, may be undesirable. Lameness is often transiently accentuated by distal limb flexion. We believe that this is because the suspensory apparatus is relaxed during flexion and undergoes sudden stretching when the limb is loaded.
In the acute phase slight edema in the proximal metacarpal region, localized heat, and distention of the medial palmar vein may occur, but these features may be transient or absent. Pressure applied to the SL against the palmar aspect of the McIII or forced extension and protraction of the limb may elicit pain, but the absence of pain does not preclude the presence of PSD.
The feet should be evaluated carefully, because frequently foot imbalance is a predisposing factor. Back-at-the-knee and tied-in below the knee conformations also may be predisposing factors.
PSD is a common compensatory injury; therefore the whole horse should be evaluated to ensure that other causes of lameness are not missed. There is a relationship between front foot pain and PSD. PSD can occur in forelimbs and hindlimbs simultaneously.
Diagnostic Analgesic Techniques
If PSD is suspected, perineural analgesia of the lateral palmar nerve using lateral3 or medial15 approaches (2 mL mepivacaine) or the medial and lateral palmar metacarpal nerves (2 mL per site) is indicated (see Chapter 10). This should result in substantial improvement in, or alleviation of, lameness within 10 minutes, assuming PSD is the only cause of lameness. However, neither technique is necessarily specific. Blockade of the lateral palmar nerve also has the potential to alleviate pain associated with a lateral source of pain in the more distal aspect of the limb (e.g., a splint). The risks of influencing middle carpal joint pain are less than with the subcarpal approach, but with the lateral approach local anesthetic solution may diffuse and improve lameness associated with the middle carpal joint16 or with the carpal canal. Perineural analgesia of the palmar metacarpal nerves may alleviate pain associated with the middle carpal or carpometacarpal joints because of local diffusion or inadvertent deposition of local anesthetic solution into the distopalmar outpouchings of the carpometacarpal joint capsule. There is no right or wrong method, but it is important to be aware of the limitations of whichever technique is used. One author (SJD) usually blocks the medial and lateral palmar metacarpal nerves using a lateral approach, with the limb non–weight bearing. In a difficult, potentially dangerous horse that strikes out, the lateral palmar nerve is blocked with the limb bearing weight. It is easy to hit the lateral palmar nerve using the medial approach, causing the horse sudden severe pain; therefore this technique is rarely used. A false-negative result may be achieved because of inadvertent injection into the carpal sheath or failure of the local anesthetic solution to diffuse proximally to the most proximal extent of a lesion. Although the SL receives innervation from fibers from the median and ulnar nerves, perineural analgesia of the ulnar nerve usually resolves or substantially improves lameness associated with PSD. However, in a minority of horses perineural analgesia of the median and ulnar nerves is required to abolish lameness completely.
Intraarticular analgesia of the middle carpal joint may result in partial improvement or complete alleviation of pain associated with the proximal aspect of the SL in some horses (15 [60%] of 25 horses).16 Using a dorsal approach to the middle carpal joint rather than a palmarolateral approach should theoretically reduce the risks of diffusion of local anesthetic solution to the proximal aspect of the SL and palmar metacarpal nerves; however, in practice the difference seems minor. Comparison of the relative responses to middle carpal analgesia (6 mL mepivacaine; assessed 10 minutes after injection) and perineural analgesia of the lateral palmar nerve or the palmar metacarpal nerves is potentially useful but can be highly misleading. Generally a horse with lameness caused by PSD responds better to perineural analgesia than intraarticular analgesia, but this is not universal. Similarly, primary middle carpal joint pain usually is improved best by intraarticular analgesia, but this is not always the case. Middle carpal joint pain and PSD may occur concurrently, especially in STB and TB racehorses. The clinician should evaluate the response to these diagnostic analgesic techniques in light of the following:
Perineural analgesia of the palmar nerves at the level of the base of the PSBs often results in lameness because of PSD appearing worse. We believe that this reflects some loss of proprioceptive function of the distal aspect of the limb so that the horse is less able to protect the painful SL. Perineural analgesia of the palmar nerves (at midmetacarpal level) and palmar metacarpal nerves (distal to the button of the McII and the McIV) (four-point or low palmar block) often results in some improvement in pain associated with PSD, possibly because of proximal diffusion of local anesthetic solution via lymphatic vessels or along fascial planes. However, a recent contrast study indicated that this may not occur.17 Perhaps improvement may be due to pain extending further distally in the SL than the site of detectable injury.
More than one source of pain may be contributing to lameness. PSD and concurrent foot pain occur commonly. Hindlimb lameness also may be present, especially in the contralateral hindlimb, so it is important to assess and to reevaluate the whole horse.
Differential Diagnosis
PSD should be differentiated from middle carpal joint pain, being aware that especially in young TB racehorses and STB racehorses lesions may occur in both locations simultaneously. Osteoarthritis of the carpometacarpal joint occasionally occurs (see page 417). Horses with pain associated with palmar cortical fatigue fractures or stress reactions of the McIII11,18-20 respond similarly to diagnostic analgesic techniques; however, in horses with fracture, lameness tends to be more severe and worse on firm ground and often deteriorates the farther the horse trots (see page 413). Avulsion fractures of the McIII at the origin of the SL (see page 417) occur less frequently and tend to be associated with more persistent and severe lameness.1,21 Pain associated with the carpal sheath or carpal retinaculum also should be considered (see Chapter 75). Perineural analgesia of the deep branch of the lateral palmar nerve or the palmar metacarpal nerves alone should not alleviate pain associated with the deep digital flexor tendon (DDFT) or its accessory ligament (ALDDFT), the superficial digital flexor tendon (SDFT), or the fetlock region, without simultaneous blockade of the palmar nerves. However, horses with proximal lesions of the SDFT or ALDDFT may show partial improvement in lameness.
Diagnostic Ultrasonography
Diagnostic ultrasonography is essential for accurate diagnosis of PSD. The limb should be evaluated in transverse and longitudinal planes, and careful comparison should be made with the contralateral limb. High-quality images are required, because lesions can be subtle and easily missed if the gain controls are too high or if the transducer is not focused on the SL. Artifacts are readily created if the transducer is not in complete contact with the limb. The contours of the proximal palmar aspect of the metacarpal region can make obtaining longitudinal images difficult, especially because the proximal palmar aspect of the McIII slopes backward. Therefore creating hypoechoic artifacts at the enthesis of the SL on the McIII is easy. Cross-sectional area measurements may be extremely valuable, especially in horses with acute PSD, because enlargement of the ligament may be the only detectable ultrasonographic abnormality. Bear in mind that muscular tissue appears less echogenic than does ligamentous tissue and that proximally the SL originates in two halves. The entire cross-section of the SL cannot be seen from a palmar approach, and marginal lesions may be missed unless oblique images are also obtained. A convex transducer or virtual convex transducer allows a greater proportion of the proximal abaxial aspects of the SL to be evaluated in transverse images compared with a linear transducer. Previous injuries to the SL may not resolve fully to restore normal, uniform echogenicity. Be aware that poor diagnostic analgesic technique may result in aspiration of air, which creates artifacts. This usually resolves in 24 hours. A thin band of the SL passes proximally from the enthesis on the McIII to blend with the palmar carpal fascia. The anechogenic space seen dorsal to the SL at this level is fluid in the palmar recess of the carpometacarpal joint and should not be mistaken for a lesion (Figure 72-1).
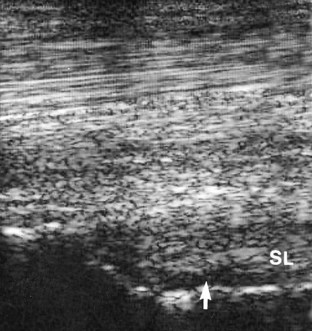
Fig. 72-1 Longitudinal (proximal is to the left) ultrasonographic image of the proximal metacarpal region. The thin echogenic band from the suspensory ligament (SL) passes proximally from the enthesis of the suspensory ligament on the third metacarpal bone to blend with the palmar fascia. Dorsal to the suspensory ligament is anechogenic fluid within the palmar recess of the carpometacarpal joint capsule (arrow).
Abnormalities associated with PSD include the following22 (Figures 72-2 and 72-3):
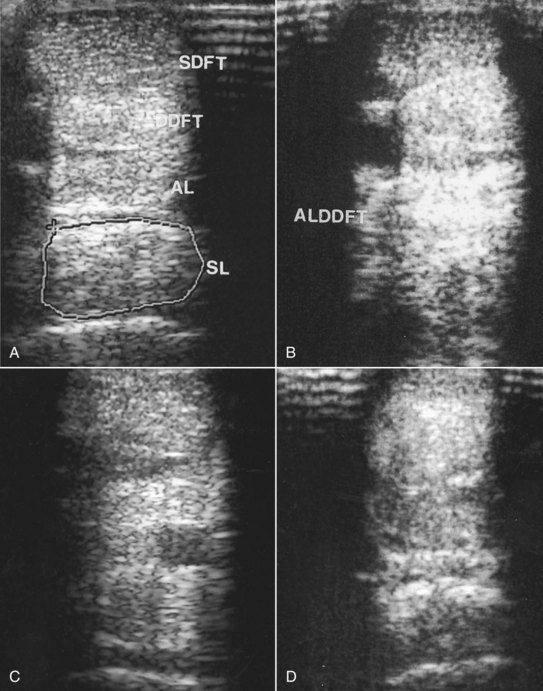
Fig. 72-2 A, Transverse ultrasonographic image of the metacarpal region of a 3-year-old Thoroughbred, with mild forelimb lameness, at 9 cm distal to the accessory carpal bone. The suspensory ligament (SL) shows a slight overall reduction in echogenicity compatible with proximal suspensory desmitis. The ligament is enlarged (cross-sectional area 1.39 cm2 compared with 1.25 cm2 in the contralateral limb). The lesion extended less than 1 cm proximodistally. B, Transverse ultrasonographic image of the metacarpal region at 8 cm distal to the accessory carpal bone of the right forelimb of a 7-year-old medium-level dressage horse. The entire cross-sectional area of the SL is reduced in echogenicity. The lesion extended 1.5 cm proximodistally. Note also the hyperechoic appearance of the accessory ligament of the deep digital flexor tendon (ALDDFT). C, Transverse ultrasonographic image of the proximal metacarpal region of a 6-year-old event horse with acute-onset right forelimb lameness. There is a hypoechoic lesion in the medial aspect of the SL that extends less than 1 cm proximodistally. Medial is left. D, Transverse ultrasonographic image of the right forelimb of a 7-year-old dressage horse with bilateral forelimb lameness. The palmar aspect of the SL is slightly increased in echogenicity, but the dorsal aspect is diffusely hypoechogenic.
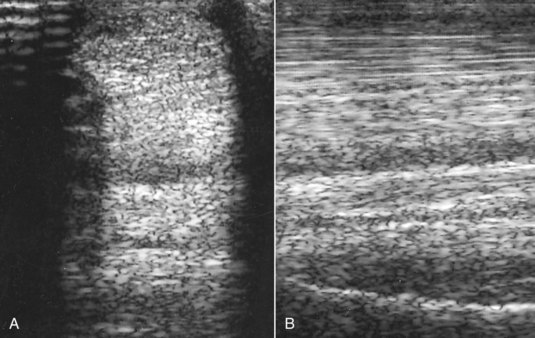
Fig. 72-3 A, Transverse ultrasonographic image of the proximal metacarpal region at 8 cm distal to the accessory carpal bone of the left forelimb of a Grand Prix show jumper. The horse had developed acute severe lameness immediately after completing a jumping round 2 weeks previously. The dorsal half of the suspensory ligament is diffusely hypoechogenic. B, Longitudinal ultrasonographic image of the proximal metacarpal region of the same horse. Proximal is to the left. The dorsal aspect of the suspensory ligament is diffusely hypoechogenic.
In a horse with bilateral PSD an obvious lesion may be detectable in the lamer limb, but abnormalities may be much more subtle and occasionally not apparent in the less lame limb. In a 3-year-old TB that sustains PSD at 2 years of age, mild lameness may be recurrent, and discerning any structural abnormality other than enlargement of the SL may not be possible.
The degree of ultrasonographic abnormality (cross-sectional area involved and proximodistal extent of the lesion) usually reflects the severity of the lameness. In horses with acute PSD the ultrasonographic abnormalities may be subtle, although if lameness is unilateral, slight enlargement of cross-sectional area may be detectable. Care should be taken to compare measurements in the contralateral limb at the same distance distal to the accessory carpal bone. Ultrasonographic abnormalities may worsen over the next 10 to 14 days, and reevaluation may be useful to confirm the diagnosis.
In horses with an avulsion fracture of the McIII at the origin of the SL, the fracture fragment is usually readily identifiable and generally is associated with only a focal lesion in the SL itself, usually restricted to the dorsal aspect (see page 417).
Radiography and Radiology
Because of the potential nonspecificity of local analgesic techniques I recommend radiographic examination of the carpus and proximal metacarpal region using at least flexed lateromedial, dorsolateral-palmaromedial oblique, dorsomedial-palmarolateral oblique, and dorsopalmar images. In racehorses, endurance, and event horses a skyline image of the third carpal bone may also be required. Usually no detectable radiological abnormalities of the McIII occur in horses with acute PSD. With chronic PSD, a generalized area of increased opacity of the proximal aspect of the McIII may be seen in dorsopalmar images. This increased radiopacity should be differentiated from that associated with a palmar cortical fatigue fracture,23 which is invariably medial. A focal linear region of increased opacity may reflect the presence of an entheseous spike. In a lateromedial image subcortical endosteal reaction in the proximal palmar aspect of the McIII may be apparent. These secondary bony changes (endosteal and entheseous new bone) in a forelimb reflect chronicity of the injury and are associated with a more guarded prognosis.
Nuclear Scintigraphy
Reports in the literature about the usefulness of nuclear scintigraphy for diagnosing PSD are confusing because of failure to correlate scintigraphic findings with ultrasonographic and radiological findings and because avulsion fractures of the McIII were not considered separately.24,25 Nuclear scintigraphy is generally unnecessary for diagnosing PSD, provided that good-quality ultrasonographic images are obtained, but may give additional information about associated bone turnover at the origin of the SL. Pool and bone phase images may be negative. Abnormal radiopharmaceutical uptake in the pool phase may actually reflect early bone uptake. Increased uptake of 99mTc-methylene diphosphonate was identified in the proximal palmar aspect of the McIII in approximately 6% of 40 horses with ultrasonographic evidence of PSD.26 Therefore negative scintigraphic images do not preclude the presence of PSD. The presence of increased radiopharmaceutical uptake (IRU) reflecting enthesis injury is usually associated with more severe lameness that takes longer to resolve. IRU associated with either an avulsion fracture of the McIII at the insertion of the SL or a stress fracture is likely to be more intense. IRU in the bone phase, seen in the absence of ultrasonographic and radiological abnormalities, is more likely to reflect a primary pathological condition of bone.
Magnetic Resonance Imaging
MRI is usually not necessary for the diagnosis of PSD but should be considered if no ultrasonographic abnormality can be identified.13,14 Interpretation is confounded by the presence of variable amounts of muscle and adipose tissue in the center of each lobe of the SL, which has high signal intensity.27,28 Increased signal intensity in the surrounding ligamentous tissue reflects injury and may be accompanied by enlargement of the SL and sometimes adhesions to the McII, McIII, and McIV. However, it is important to recognize the normal fibers of the SL, which originate from the axial aspect of the McIV extending up to 7 cm distal to the carpometacarpal joint.28 MRI may be more sensitive than ultrasonography for identification of entheseous new bone and endosteal reaction at the ligament’s origin. MRI is the most accurate method of detection of syndesmopathy between the McIII and either the McII or the McIV, which is an unusual but important differential diagnosis. It is also valuable for the detection of primary bone trauma of the McIII.
Computed Tomography
Computed tomography (CT) or contrast-enhanced CT can also be useful for the diagnosis of PSD and entheseous new bone.
Treatment
Most horses with acute forelimb PSD respond well to box rest and controlled walking exercise for 3 months.29,30 Uncontrolled turnout is contraindicated. Attention to correct foot balance is important. Elevation of the heel is contraindicated because it increases load on the SL. A premature resumption of work usually results in recurrent injury. Approximately 90% of horses resume full athletic function without recurrent injury.1 In the early period after return to work circumstances that create hyperextension of the fetlock should be avoided. For example, dressage horses should avoid medium and extended trot. Attention should also be paid to the footing on which the horse works. Horses with chronic PSD may require more prolonged rehabilitation, and in a small proportion lameness is persistent. If low-grade residual lameness persists after 3 to 6 months but serial ultrasonographic examinations reveal no change in echogenicity, then introduction of trotting exercise sometimes stimulates further repair and resolution of lameness. Some TB racehorses with chronic lesions have been able to be maintained in training with judicious use of phenylbutazone, without clinically significant deterioration of the lesion. No fatalities associated with PSD occurred in 630 TB racehorses examined post mortem because of musculoskeletal injuries.29 Some STB racehorses with acute lesions have been managed by slightly reducing the training schedule, administering local injections of corticosteroids and hyaluronan, using symptomatic antiinflammatory therapy (local icing, liniments such as dimethyl sulfoxide [DMSO] and corticosteroid paints, and phenylbutazone as necessary), and shortening the toes and increasing hoof angle. Although a few treated horses were able to race successfully, prognosis after rest was better.29
Extracorporeal shock wave treatment or radial pressure wave therapy (three treatments at 2-week intervals) was successful in some horses with chronic lesions that had failed to respond to conservative management.31,32 Ten (50%) of 20 horses with chronic forelimb PSD with lameness of greater than 3 months’ duration were in full work 6 months after treatment.32 Repeated long-term treatments are sometimes required. Local infiltration with 4 to 6 mL of 2% iodine in almond oil has been used successfully.8
In some horses the lesions disappear completely ultrasonographically. In others echogenicity may increase, but uniform echogenicity is never restored. Rest should be continued until the ultrasonographic appearance remains stable.
Surgical splitting of the SL has been used in some horses with PSD with successful results in some horses; however, the results have been unpredictable.14,33 There are currently no long-term, peer-reviewed studies of the results of injection of pigs’ urinary bladder matrix, mesenchymal stem cells, or other biological agents such as platelet-rich plasma, although anecdotally successful results have been reported. In sports horses with chronic injuries that have failed to respond to conservative management, one author (SJD) has successfully used either mesenchymal stem cells or desmoplasty combined with injection of platelet-rich plasma.
Proximal Suspensory Desmitis in the Hindlimb
PSD in the hindlimb results in either an insidious or a sudden-onset lameness that may be mild or severe. Some horses show poor performance rather than a recognized lameness. In contrast to the forelimb, lameness may persist and remain severe, despite restriction to box rest. Such persistence probably is caused by a compartment-like syndrome and pressure on the adjacent plantar metatarsal nerves.34,35 In view of the chronicity of some lesions when first identified and the finding of secondary radiological changes in sound horses, some lesions likely exist subclinically or are associated with a low-grade lameness that goes unrecognized. The incidence of bilateral lesions is higher than in forelimbs.
PSD in the hindlimb occurs in horses in all athletic disciplines and of all ages and is a particular problem in dressage horses.36 There is an association between straight hock conformation and hyperextension of the metatarsophalangeal joint and hindlimb PSD (Figure 72-4). Such conformational abnormalities were identified in nine (21%) of 42 horses with hindlimb PSD but in only four (8%) of 50 horses examined consecutively with hindlimb lameness unrelated to the suspensory apparatus.37![]() Straight hock conformation may predispose to PSD or develop secondarily; hyperextension of the metatarsophalangeal joint may develop as a sequela to PSD, probably as the result of progressive degeneration of the SL. A long-toe and low-heel conformation also may be a predisposing factor, especially if associated with abnormal orientation of the distal phalanx, with the plantar aspect lower than the toe.29
Straight hock conformation may predispose to PSD or develop secondarily; hyperextension of the metatarsophalangeal joint may develop as a sequela to PSD, probably as the result of progressive degeneration of the SL. A long-toe and low-heel conformation also may be a predisposing factor, especially if associated with abnormal orientation of the distal phalanx, with the plantar aspect lower than the toe.29

Fig. 72-4 The hindlimbs of a 7-year-old show jumper with proximal suspensory desmitis of the right hindlimb. Note the relatively straight hock conformation and the sloping pasterns associated with hyperextension of the hind fetlocks. The horse also has rather low heels and long toes.
PSD in the hindlimb in a STB racehorse is common and usually results in an abnormal gait at high speeds, which may or may not be apparent at the trot. Unilateral left hindlimb lameness may manifest as the horse drifting to the right shaft and being on the left line and vice versa.
Horses with acute hindlimb PSD may have localized heat and swelling and pain on pressure applied to the SL, but frequently no localizing clinical features are apparent.
Lameness is often characterized by a reduced height of arc of foot flight, with or without intermittent catching of the toe. There may be reduced extension of the metatarsophalangeal joint unless the functional integrity of the SL is compromised. The cranial phase of the stride may be shortened. Lameness may be accentuated by proximal or distal limb flexion. Bilateral lesions may result in poor hindlimb action rather than obvious hindlimb lameness. Performance horses may not be overtly lame in hand, but when ridden show reduced hindlimb impulsion, difficulties in transitions, stiffness, resistant behavior, evasions such as bolting, difficulties in turning or stopping, reluctance to perform certain dressage movements, or reduced power when jumping. Lameness may be more obvious on a circle on the lunge, but unlike in forelimb PSD the lameness is not necessarily worse with the lamer limb on the outside. Approximately 50% of horses with hindlimb PSD are lamer with the affected limb on the outside or inside of a circle. As with many horses with hindlimb lameness, lameness is often more obvious when the horse is ridden, especially when the rider sits on the diagonal of the lame or lamer limb.
Diagnostic Analgesic Techniques
Perineural analgesia of the plantar nerves (midmetatarsal level) and plantar metatarsal nerves may result in slight improvement in lameness because of proximal diffusion of the local anesthetic solution or distal extension of pain in the SL, or concomitant injury of a SL branch. Lameness usually is improved substantially by perineural analgesia of the medial and lateral plantar metatarsal nerves (2 mL of mepivacaine 2% per site) or the deep branch of the lateral plantar nerve distal to the tarsus, but lameness may not be alleviated fully. Improvement is usually seen within 10 minutes of injection. Critical evaluation of the degree of improvement is considered essential if considering treatment by neurectomy of the deep branch of the lateral plantar nerve. If there is a component of entheseous pain, this may result in only partial improvement in lameness, which is further improved by deep infiltration of local anesthetic solution toward the plantar aspect of the MtIII. PSD may occur together with pain associated with the tarsometatarsal joint, and if lameness is improved but not abolished by subtarsal analgesia, addition of intraarticular analgesia of the tarsometatarsal joint may abolish the lameness. False-negative results also may be obtained because of inadvertent injection into the tarsal sheath or the tarsometatarsal joint capsule. Subtarsal analgesia can influence tarsometatarsal joint pain, and occasionally (two [8%] of 24 horses34), intraarticular analgesia of the tarsometatarsal (TMT) joint alleviates pain associated with PSD. It is also important to recognize that improvement in lameness after perineural analgesia of the deep branch of the lateral plantar nerve is not synonymous with PSD. There may be other causes of pain, improvement of which results in improvement in lameness. However, an improvement in lameness of 85% or more is usually associated with PSD. Nonetheless, I recommend comparing the response with the effect of intraarticular analgesia of the TMT joint. Perineural analgesia of the tibial nerve alone alleviates pain associated with PSD without substantially influencing tarsal pain. However, the tibial nerve is large; therefore 20 minutes may pass before analgesia is achieved effectively. In horses with bilateral hindlimb stiffness or lack of hindlimb impulsion, best improvement may be seen after perineural analgesia of the deep branch of the lateral plantar nerve performed bilaterally simultaneously. Some horses have secondary sacroiliac joint region pain, and the rider may complain of persistent lack of hindlimb power unless this region is also blocked.
Diagnostic Ultrasonography
High-quality ultrasonographic images are essential for accurate diagnosis, but are not always possible to achieve because of the shape of the limb, position of blood vessels, and the presence of other artifacts. The proximal aspect of the SL should be examined from the plantaromedial aspect of the metatarsal region to evaluate as much of the cross-sectional area as possible. Large vessels plantarolateral to the SL may result in broad linear anechoic artifacts within the SL (Figure 72-5). In large Warmblood horses in particular the SL is situated deeply, and the ultrasound transducer must be focused accordingly. In transverse and longitudinal images the most proximal part of the SL in a normal horse may appear slightly less echogenic than the DDFT (Figure 72-6). Detection of subtle abnormalities requires careful comparison with the contralateral limb, bearing in mind that lesions may be bilateral, and measurement of cross-sectional area or dorsoplantar thickness. Cross-sectional area measurements underestimate real size because the ligament cannot be imaged in its entirety proximally. The SL should be imaged routinely in transverse and longitudinal planes. The shape of the proximoplantar aspect of the metatarsal region influences how easy it is to obtain high-quality images. Use of a standoff pad can provide a larger contact area and thereby enhance image quality in some horses. Acquisition of transverse images using either a convex array or a virtual convex transducer may permit evaluation of more of the mediolateral extent of the SL than using a linear transducer. It is also important to recognize that injury may be localized to either the medial or the lateral aspect of the SL.
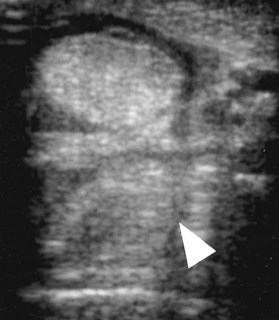
Fig. 72-5 Transverse ultrasonographic image of the proximal metatarsal region of a horse with proximal suspensory desmitis. Medial is to the left. The narrow anechogenic band through the suspensory ligament (arrowhead) is an artifact caused by the overlying plantar vessel. The suspensory ligament has a diffuse reduction in echogenicity.
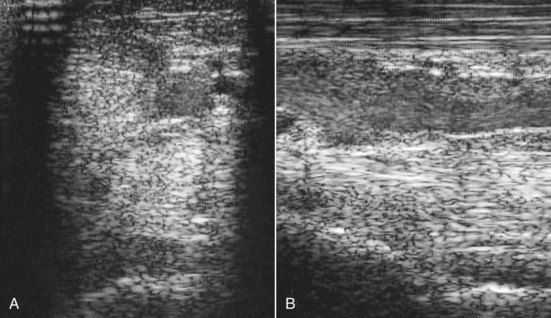
Fig. 72-6 A, Transverse ultrasonographic image of the proximal metatarsal region of a normal horse, 4 cm distal to the tarsometatarsal joint. Medial is to the left. The suspensory ligament is similar in echogenicity to the deep digital flexor tendon. B, Longitudinal ultrasonographic image of the proximal metatarsal region of a normal horse. Proximal is to the left.
In hindlimb PSD focal anechogenic areas are relatively unusual, except in the STB racehorse. More commonly the SL is enlarged, with poor demarcation of its borders and a diffuse reduction in echogenicity of part or all of the cross-sectional area of the ligament (see Figure 72-5) (Figure 72-7). Fibrosis or ectopic mineralization occurs more often in hindlimbs than in forelimbs. An irregular contour of the plantar aspect of the MtIII may reflect enthesophyte formation. In some horses, especially those with abnormal conformation, the lesions may progress despite box rest.
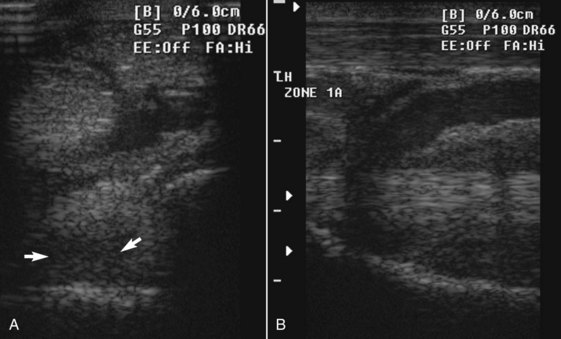
Fig. 72-7 Transverse (A) ultrasonographic image at 4 cm distal to the tarsometatarsal joint and longitudinal image (B) of the left, proximal metatarsal region of an 11-year-old show jumper with left hindlimb lameness caused by proximal suspensory desmitis. The damaged suspensory ligament is diffusely hypoechogenic dorsomedially (arrows) with diffuse loss of fiber pattern in the longitudinal image. The overlying blood vessel is dilated.
Occasionally there are concurrent injuries of the SL branches, especially in horses with abnormal conformation, and consideration should be given to routine evaluation of the SL in its entirety.
Radiography and Radiology
Distal hock joint pain and PSD may coexist, so it is recommended that a comprehensive radiographic examination of the hock and proximal metatarsal region is performed using four standard images. Diagnosis should never be based on radiology alone, because some sound horses have some increased radiopacity of the proximal aspect of the MtIII (see Figure 43-2). In horses with chronic active PSD this tends to be more extensive. In the dorsoplantar image, increased opacity of the proximal aspect of the MtIII is often more obvious laterally. In a lateromedial image subcortical increased radiopacity and alteration of the trabecular pattern of the proximoplantar aspect of the MtIII may be caused by endosteal new bone, extending up to 4 cm proximodistally (Figure 72-8). The plantar cortex may itself be thickened, and in addition enthesophyte formation may be visible on the plantar aspect. However, in some horses with acute injury no radiological abnormality is detectable. Radiology is also important to identify osteoarthritis of the distal hock joints, which may be contributing to pain and lameness.
Nuclear Scintigraphy
Nuclear scintigraphy is not a sensitive means of detecting PSD in hindlimbs.26 Pool phase images have been found to be positive in only 12% of 46 horses with ultrasonographic evidence of PSD. In bone phase images, IRU in the proximoplantar aspect of the MtIII was found in 12% of 82 horses (Figure 72-9). IRU was associated with more severe ultrasonographic lesions. IRU in the proximoplantar aspect of the MtIII, with no detectable ultrasonographic abnormality of the SL and no radiological change associated with enthesopathy, may be associated with a primary pathological condition of bone, such as bone trauma or entheseous reaction at the origin of the SL, which has been demonstrated using MRI.
Magnetic Resonance Imaging and Computed Tomography
Lesions of the proximal aspect of the SL that were not evident on ultrasonography have been identified with MRI13,14,38,39 or computed tomography. MRI has also identified adhesions to the MtII and to the MtIV (Figure 72-10, A) and endosteal and entheseous reactions at the ligament’s origin (Figure 72-10, B).
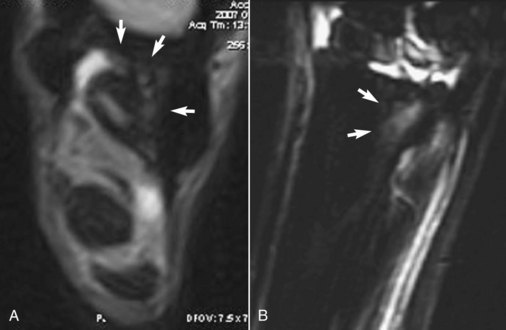
Fig. 72-10 A, Transverse T2* gradient echo low-field magnetic resonance image of the proximal metatarsal region of a 6-year-old show jumper. Lateral is to the right. There are adhesions between the suspensory ligament and the axial aspect of the fourth metatarsal bone and the plantar aspect of the third metatarsal bone (MtIII) (arrows). B, Sagittal short tau inversion recovery (STIR) magnetic resonance image of the left hindlimb of a 3-year-old Thoroughbred flat racehorse with mild architectural abnormalities of the suspensory ligament origin detected ultrasonographically. The horse exhibited severe lameness. There is diffuse increased signal intensity in the proximoplantar aspect of the MtIII at the origin of the suspensory ligament.
Differential Diagnosis
PSD should be differentiated from pain associated with the tarsometatarsal joint, an avulsion fracture of the MtIII at the origin of the SL, primary stress reactions in the MtIII, bone trauma, and syndesmopathy between the MtIII and either the MtII or the MtIII.
A recent study compared the results of ultrasonography and MRI in 46 limbs of 38 horses in which lameness was improved at least 60% by perineural analgesia of the deep branch of the lateral plantar nerve. Using MRI as the gold standard, 21 limbs had PSD, including nine with osseous abnormalities of the MtIII; four horses had osseous injury at the origin of the SL as the only abnormality.39 However, in nine limbs there were injuries unrelated to the SL, including OA of the distal tarsal joints, enthesopathy of an intertarsal ligament, and osseous pathology of the central or fourth tarsal bones or the MtIII in a variety of locations. No diagnosis was reached for the cause of lameness in 12 limbs. It was suggested that neuropathy of the deep branch of the lateral plantar nerve could possibly be the source of pain in these 12 horses. Ultrasonography had a moderate sensitivity (0.77) and low sensitivity (0.33) for diagnosis of PSD with MRI as the gold standard, with both false-positive and false-negative results.
Treatment
The prognosis for horses with PSD in the hindlimb with conservative management generally has been poor. Only six (14%) of 42 horses seen in a referral practice were able to resume full work without detectable lameness for at least 1 year; all six had been lame for less than 5 weeks.37 All these horses showed substantial improvement in clinical signs within 3 months of the onset of lameness. Two additional horses resumed full work but developed lameness in another limb. Seven horses improved greatly and were able to work, despite persistent mild lameness. Twenty-four horses (57%) had persistent or recurrent lameness. Results from a first opinion practice also were disappointing, with only 10 (59%) of 17 horses resuming work.1
Horses with acute (less than 4 to 6 weeks’ duration) hindlimb PSD respond reasonably well to local infiltration with corticosteroids, aimed at reducing inflammation and therefore swelling and thus minimizing the risk of developing a compartment syndrome (see the following discussion). Foot imbalance is corrected and egg bar shoes are used to reduce extension of the fetlock. Horses with chronic PSD have a guarded prognosis regardless of the treatment. Lameness often tends to persist unchanged, even after prolonged box rest, which is unusual for a primary soft tissue lesion. In some horses lesions are progressive. Local infiltration with corticosteroids, polysulfated glycosaminoglycans, hyaluronan, or homeopathic drugs such as Actovegin and Traumeel has given disappointing results.
In some horses an initial improvement in lameness is seen after box rest and controlled walking exercise for 2 to 3 months, and then no further improvement is seen. Increasing the exercise despite the lameness sometimes results in further improvement. Some horses have worked satisfactorily while being treated with phenylbutazone, without apparent deterioration of clinical signs or ultrasonographic abnormalities. This indicates that in some horses the SL is able to withstand load and the horse can work satisfactorily provided that pain is controlled.
Extracorporeal shock wave therapy or radial pressure wave therapy appears to be helpful in some horses.32 Eighteen of 30 horses with PSD in forelimbs or hindlimbs were in work 6 months after treatment. Eighteen (40%) of 45 horses with unilateral or bilateral chronic (lameness of greater than 3 months’ duration) hindlimb PSD were in full work 6 months after treatment32; however, a small proportion of these horses have experienced recurrent lameness subsequently. Horses with mild or moderate ultrasonographic lesions responded better than those with severe lesions. Injection of 2% iodine in almond oil resulted in 12 (55%) of 22 horses returning to work.29
Tibial neurectomy performed in eight horses enabled six to return to full athletic function (show jumping and horse trials) for at least 2 years after surgery, with no postoperative complications.14 Neurectomy of the deep branch of the lateral plantar nerve has been combined with incising the thin fascial covering of the plantar aspect of the SL and was successful in 79% of 200 horses,40 91% of 84 horses,41 and 62.5% of 16 horses.35 It has also been combined with osteostixis.40,42 A further 70% of 120 horses treated by one author (SJD) have returned to full athletic function after neurectomy of the deep branch of the lateral plantar nerve, but two horses experienced deterioration because of progressive lesions in the SL.14 Both of these horses had preexisting excessively straight hock conformation and/or hyperextension of the fetlock of the lame limb. This has also been reported elsewhere,43 and these conformational abnormalities are considered to be contraindications for surgery.
Postoperative management depends on the severity of the initial injury. Improvement in ultrasonographic appearance is generally seen by 2 months postoperatively, but this may in part reflect neurogenic muscle atrophy. Although some horses may successfully return to work at this stage, horses with severe injuries and those with osseous pain may need a considerably longer rehabilitation period. The presence of IRU associated with PSD usually indicates that a prolonged convalescence will be required. Neurectomy may result in atrophy of the muscle component of the SL, which could potentially influence its function.44 The SL is closely related anatomically to the distal hock joints by its accessory ligament. Horses with low-grade residual lameness may respond to intraarticular medication of the distal hock joints.
Fasciotomy of the deep plantar metatarsal fascia has been successful in some horses.14 Injection of about 30 mL of bone marrow also is claimed to be successful, especially if combined with fasciotomy, allowing 85% of horses to return to the former level of function14 (see also Chapter 73). Injection of platelet-rich plasma or mesenchymal stem cells has also been combined with fasciotomy, but without neurectomy and anecdotally is successful.
Gross Pathological and Histopathological Findings
Postmortem examinations have been performed on both hindlimbs of eight horses, six with unilateral lameness and two with bilateral lameness.22,34 Abnormalities of the SLs were confined to the lame limbs. The SLs were grossly enlarged, with thickening of surrounding fascia and periligamentous tissues, especially on the plantar aspect. Histological changes in the SL included hypercellularity and acellular areas; hemosiderin deposition; fibrosis; hyalinization of collagen; an increased number of fibrous septae, some with blood vessels; neovascularization; and chondroid metaplasia. Although chondroid metaplasia was seen at the ligament-bone interface in lame and sound limbs, intraligamentous chondroid metaplasia was seen only in the lame limbs. These histological abnormalities are consistent with degenerative rather than inflammatory changes.
Evidence of compression of adjacent peripheral nerves was found in the lame limb of five horses.22,34 Abnormalities of the plantar metatarsal nerves included thickening of the perineurium, perineural fibrosis, reduction or absence of nerve fibers, and Renaut bodies. Similar abnormalities have been identified in the deep branch of the lateral plantar nerve bilaterally in 11 bilaterally lame horses and unilaterally in the lame limb of five unilaterally lame horses.35
These changes support the theory that PSD in the hindlimb results in a compartment syndrome and some pain may possibly be neuropathic in origin.
Avulsion Fractures of the Third Metacarpal or Metatarsal Bone at the Origin of the Suspensory Ligament
Avulsion fractures of the third metacarpal or metatarsal bone at the region of the SL are discussed on page 417.
Suspensory Desmitis: Body Lesions
Desmitis of the body of the SL is principally an injury of horses that race, STBs and TBs (flat racehorses and jumpers). In TBs in Europe the incidence is much higher in horses that race over fences (National Hunt Racing and point to pointing; see Chapter 112) compared with flat racehorses. Although PSD and desmitis of the medial and/or lateral branches of the SL are relatively common injuries in event horses, show jumpers, dressage horses, and endurance horses, body lesions are recognized less frequently, except as associated with an exostosis of the McII or the MtIV (the splint bones) or as a sequela to a branch injury. Injuries occur in forelimbs and hindlimbs, and in STBs several limbs may be affected concurrently, whereas in TBs lesions generally are restricted to the forelimb.29,45 There is frequently a poor correlation between the extent of the lesions and the degree of lameness. Performance, however, can be compromised despite lack of evidence of overt lameness. In STB racehorses lameness may be observed or tolerated while athletic function continues. This continued exercise may result in progressive injury or injury to other tendonous or ligamentous structures.
Soreness on palpation of the SL is not synonymous with structural damage. Event horses frequently have sore SLs for several days after fast work or competition, but rarely does this appear to be associated with a clinically significant problem. Soreness does not appear to be a warning sign of impending desmitis. Upper-level show jumpers may have a sore forelimb SL the day after competition, which often highlights overload caused by lameness in another limb.
Clinical Signs
The clinical signs associated with suspensory body desmitis vary in presence and degree and include the following:
Diagnosis
Diagnosis is based on clinical signs and ultrasonographic assessment. Diagnostic analgesic techniques rarely are required unless another contributory cause of lameness or recurrence of previous desmitis is suspected. In a horse with previous desmitis a previously enlarged SL may have no detectable change in size, shape, or reaction to palpation. Perineural analgesia of the palmar metacarpal nerves proximal to the site of the suspect lesion should remove associated lameness.
The body of the SL of a normal horse is not always uniform in echogenicity because of variable amounts of muscle tissue in the ligament and variations in the level of bifurcation of the SL between horses. This can make detection of subtle lesions difficult. The maximum injury zone is frequently at the region of the bifurcation of the SL, which may appear hypoechogenic in normal horses (Figure 72-11). Careful comparison with the contralateral limb may help if clinical signs are unilateral. Ultrasonographic abnormalities associated with active desmitis include the following:
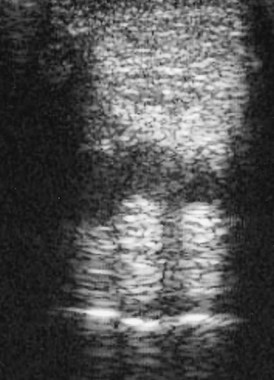
Fig. 72-11 Transverse ultrasonographic image of a normal forelimb suspensory ligament at the level of the beginning of the bifurcation, 18 cm distal to the accessory carpal bone. The central, less echogenic region should not be mistaken for a lesion.
Enlargement may be the only detectable abnormality. Careful comparison with the contralateral limb can be helpful. Reference can be made to studies of sizes in normal horses of similar breed, but caution is urged because notable differences do exist between U.S. and European TBs. The extent of ultrasonographic abnormality is not always correlated with the severity of clinical signs. A reasonable correlation exists with the severity of the ultrasonographic grade of the injury and the prognosis; however, a diffuse reduction in echogenicity of the cross-sectional area is also associated with a poor prognosis. Lesions may extend into the branches, which should also be examined by ultrasonography.
Many lesions persist long term, although focal lesions in STBs may fill in. In TBs a readily detectable defect often persists, despite some increase in echogenicity and slight reduction in size. This can limit the value of serial ultrasonographic evaluations to determine when a horse might be able to withstand work. Determining objectively to what extent healing has taken place and the strength of the repair tissue often is not possible. This may explain the high rate of recurrence of body lesions in STBs and TBs. Racing careers of STBs can usually be extended, whereas managing TBs is much more difficult, because lameness in TBs rarely stabilizes.29 Horses with slight lesions may be responsive to symptomatic treatment, and a horse may train and race well; however, the lesion is likely to be progressive. The risk of catastrophic breakdown of the suspensory apparatus must always be considered in a TB racehorse. In STB racehorses rest for about 3 months may confer no extra advantage to symptomatic treatment and continued training30 with respect to racing performance. Lesions are usually progressive, and periodic ultrasonographic monitoring is recommended.
There is a high rate of reinjury to the body of the SL of the same or the contralateral limb or to a branch of the same or the contralateral limb subsequent to desmitis of the body of the SL.29
Radiographic examination of the McII and the McIV is indicated if clinical examination suggests abnormal modeling or a fracture, because surgical amputation may be indicated29 (see page 421).
Management
Treatment is aimed at reducing inflammation by the administration of systemic nonsteroidal antiinflammatory drugs (NSAIDs), local or systemic corticosteroids, hydrotherapy, and controlled exercise. Cryotherapy is used by some clinicians to provide pain relief, but a real risk of progressive deterioration of the lesion exists (see Chapter 89).
Progress is monitored clinically and by serial ultrasonographic examinations. Ideally there should be progressive reduction in cross-sectional area, improvement in echogenicity and fiber alignment, no restrictive periligamentous fibrosis, and reasonable stability in all of these features as exercise is increased. In STBs handwalking exercise is advocated for the first 4 weeks.29 Provided that some improvement in the ultrasonographic appearance of the ligament occurs and the horse is sound when jogged in hand, the horse is harnessed and walked in the jog cart for another 4 weeks. Flat shoes are preferred. The horse is reassessed by ultrasonography after 8 weeks, and if further filling in of the defect occurs, short slow jogging is reintroduced and gradually increased. A third ultrasonographic examination is performed after 8 weeks of jogging, and provided that the lesion is uniformly echogenic to the remainder of the SL and has a linear arrangement of fibers, normal jogging and speed work are resumed. Although this method is successful in some STBs, in others a longer period of convalescence is required.
Continued medical therapy includes daily hydrotherapy—for example, whirlpool boots, stable and exercise bandages, massage with emollient oils, leg sweats, and leg tighteners with or without DMSO. The aim is to stimulate circulation, reduce inflammation, and provide pain relief. The work program should be adapted so that days of light work are interspersed with hard work. Swimming is a useful method of maintaining cardiovascular fitness but should not be used alone, because the musculoskeletal system requires regular stimulation to avoid other injuries.
Intralesional treatment with β-aminopropionitrile fumarate has been used in STB and TB racehorses with moderate to severe injuries of the SL body30; however, a licensed product is no longer available. Eleven of 18 horses successfully returned to racing, although some dropped in class. A recent in vitro study compared the potential for various blood-based biological products to induce SL regeneration and indicated that bone marrow aspirates were potentially superior to platelet-rich plasma.46 However, nine of nine STB racehorses treated by intralesional injection of platelet-rich plasma returned to racing, with similar performance to that of control horses for 2 years after treatment.47 It is important to note, however, that STBs have a much higher tolerance of SL injuries than TBs.
Surgical splitting of the body of the SL usually is not advocated in STBs or TBs.29 However, occasionally, large chronic lesions with associated McII or McIV fractures are split, combined with surgical removal of the fracture piece in STB and, less commonly, TB racehorses. Pin firing combined with rest may be of benefit in some horses with chronic desmitis29 (see Chapter 88).
Suspensory Desmitis Associated with an Exostosis on the Second or Fourth Metacarpal or Metatarsal Bone (Splint)
Suspensory desmitis associated with exostosis of the McII (MtII) or the McIV (MtIV) is discussed on page 419 (Figure 72-12).
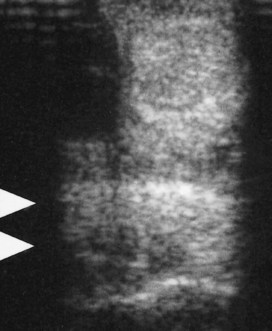
Fig. 72-12 Transverse ultrasonographic image of the midmetacarpal region of a 7-year-old show jumper with intermittent left forelimb lameness associated with localized enlargement of the axial aspect of the second metacarpal bone (a splint). Slight pain could be elicited by pressure. Separating the axial border of the bone from the suspensory ligament by palpation was not possible. Lameness was alleviated by local infiltration of local anesthetic solution. Echogenic material (arrowheads) extends from the medial aspect of the suspensory ligament, which was slightly hypoechoic dorsomedially. Surgical exploration revealed a loose spicule of bone adjacent to the splint and a granulomatous reaction between it and the suspensory ligament. A focal defect was found in the medial border of the suspensory ligament.
Suspensory Desmitis Associated with Fracture of the Distal Third of the Second or Fourth Metacarpal or Metatarsal Bone
Suspensory desmitis associated with fracture of the distal third of the McII (MtII) or the McIV (MtIV) is discussed on page 421.
Suspensory Desmitis: Branch Lesions
Desmitis of the medial and/or lateral branches of the SL in forelimbs and hindlimbs is a relatively common injury in all types of sports horses. Usually only a single branch is affected in a single limb, although both branches may be affected, especially in hindlimbs. Foot imbalance often is recognized in affected horses and may be a predisposing factor. Some horses, particularly event horses, have acute-onset distention of the metacarpophalangeal joint capsule concurrent with SL branch desmitis. Detection of radiological abnormalities (modeling or fracture of the distal aspect of the McII or the McIV or a PSB) in some horses at the time of recognition of an acute, first-time injury suggests a subclinical preexisting problem. As with PSD, there is some evidence that some suspensory branch injuries in hindlimbs may be degenerative in origin.48
Clinical Signs
The clinical signs depend on the degree of damage and chronicity of the lesion(s) and include localized heat and swelling. Swelling often is caused by enlargement of the branch, together with periligamentous edema or fibrosis. In some hindlimb injuries swelling may be predominantly because of periligamentous fibrosis. Associated distention of the metacarpophalangeal or metatarsophalangeal joint capsule may occur because of the subsynovial location of the axial aspect of the distal third of the ligament.49 In hindlimbs sometimes considerable distention of the digital flexor tendon sheath (DFTS) occurs, making assessing the SL branches by palpation difficult. Mild swelling may also easily be overlooked unless a standardized method of palpation is used. A normal SL is uniform in dorsopalmar (dorsoplantar) width throughout its length. With the limb bearing full weight, place the thumbs of both hands on the dorsal and palmar (plantar) aspect of the SL body and follow the ligament distally. The thumbs should remain equidistant unless there is enlargement of the SL. The medial and lateral branches should both be assessed routinely. Pain usually is elicited by direct pressure applied to the injured branch or by passive flexion of the fetlock. Lameness varies, may be absent, usually is proportional to the degree of damage, and is related inversely to the duration of injury. A notable exception is in the hindlimbs of older dressage horses, in which occasionally both branches sustain damage, and strong adhesions develop between them. These horses experience persistent, severe lameness. Occasionally, younger horses from any discipline develop acute-onset, severe, and persistent hindlimb lameness associated with progressive stretching of a single branch and the development of periligamentous fibrosis. There is associated hyperextension of the fetlock.
Diagnosis
Diagnosis is based on clinical signs and ultrasonographic examination. Diagnostic analgesic techniques usually are required only if more than one lesion is suspected as the cause of lameness. In horses with acute branch injury with concurrent distention of the metacarpophalangeal joint capsule, we suggest that the horse be reevaluated after 2 to 3 weeks. If joint capsule distention and pain on manipulation persist, then the joint should be blocked intraarticularly.
The entire SL should be examined by ultrasonography, because lesions may extend beyond areas that are palpably abnormal. Ultrasonographic abnormalities include the following (Figures 72-13 to 72-15):
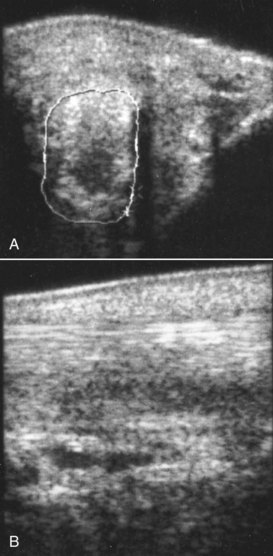
Fig. 72-13 A, Transverse ultrasonographic image of the medial branch of the suspensory ligament of the right forelimb of a 14-year-old Grand Prix dressage horse. The suspensory ligament branch is enlarged (cross-sectional area 2.02 cm2) and has a large, almost anechoic lesion. The echogenic material subcutaneously also contributed to the soft tissue swelling. B, Longitudinal ultrasonographic image of the medial branch of the suspensory ligament of the right forelimb of the same horse. Proximal is to the left. There is a marked loss of fiber pattern within the suspensory ligament. Note also the subcutaneous echogenic material.
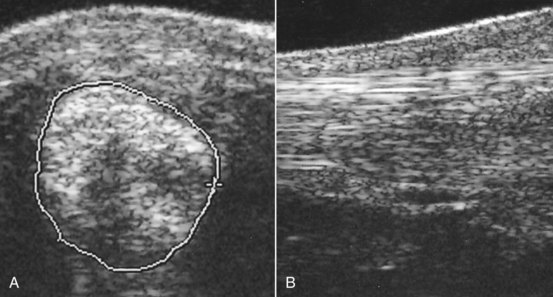
Fig. 72-14 A, Transverse ultrasonographic image of the lateral branch of the suspensory ligament of the left hindlimb of a 7-year-old intermediate-level event horse with lameness of 2 weeks’ duration. The suspensory branch is considerably enlarged (cross-sectional area 2.0 cm2). Its margins are poorly defined, and there is marked loss of echogenicity. B, Longitudinal ultrasonographic image of the lateral branch of the suspensory ligament of the same horse. Proximal is to the left. The ligament is hypoechoic, with loss of fiber pattern.
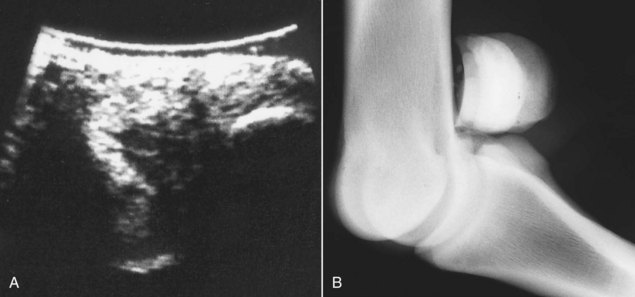
Fig. 72-15 A, Transverse ultrasonographic image of the lateral branch of the suspensory ligament, close to its insertion on the lateral proximal sesamoid bone, of the right hindlimb of a 9-year-old advanced event horse with acute-onset lameness. The ligament has a large, almost anechoic defect. B, Flexed, slightly oblique lateromedial radiographic image of the right hind fetlock of the same horse. The lateral proximal sesamoid bone is positioned slightly more dorsally. Several small osseous opacities are seen dorsal to it. These were removed arthroscopically, and torn fibers at the suspensory ligament branch insertion were debrided.
The branches should be examined in transverse and longitudinal planes. Lesions restricted to the insertion are sometimes detectable only in longitudinal images. Sometimes horses show slight localized swelling and heat in the region of a SL branch, with a subtle alteration in gait, in which no ultrasonographic abnormality of the SL can be identified. The swelling appears to be principally periligamentous. Some of these horses can be maintained safely in work, whereas in others clinical signs deteriorate with the development of a branch lesion. Recurrent lameness may also result from tearing of periligamentous fibrous tissue. Occasionally, concurrent lesions of the oblique sesamoidean ligaments occur.
Radiological examination of the ipsilateral splint bone and PSB should be performed. Abnormalities may include the following:
Fractures influence the treatment and prognosis. However, radiological evidence of sesamoiditis is not well correlated with the outcome, although primary sesamoiditis unassociated with SL desmitis can cause recurrent lameness (see Chapter 36). Occasionally lesions cannot be identified using ultrasonography but are recognized using MRI.
Management
Treatment depends on the occupation of the horse, the breed, and the severity of the clinical signs and ultrasonographic abnormalities. There are no large-scale, peer-reviewed reports of the success of management of SL branch injuries in either racehorses or sports horses. Horses with acute central core lesions may be treated by splitting (desmoplasty), with horses returning to work within 9 months. Horses with more peripheral or poorly demarcated lesions are not suitable for splitting, but their injuries can be managed conservatively by appropriate trimming and shoeing, box rest, and a controlled increasing exercise program. Intralesional injection of β-aminopropionitrile fumarate (five 7-mg injections on alternate days) combined with a controlled increasing work program over 6 to 9 months has been successful.30 There are anecdotal reports of the success of mesenchymal stem cell treatment or intralesional injection of platelet-rich plasma or bone marrow. Our experience to date is that the results are rather unpredictable, although some excellent outcomes have been achieved.
Dressage horses and show jumpers with minor lesions and only subtle ultrasonographic abnormalities have been managed successfully by appropriate trimming and shoeing with egg bar shoes, combined with modification of the training program for 6 to 8 weeks. Some horses can be managed successfully and maintained in work by aggressive local therapy (e.g., use of whirlpool boots, laser or ultrasound therapy, leg sweats, and cold therapy). Continued work in an event horse usually results in substantial progression of the lesion. In a TB racehorse, continued training in the face of SL branch injury runs the risk of an acute breakdown injury of the suspensory apparatus. In STB racehorses symptomatic treatment is common, with NSAIDs as required, daily icing, topical application of a DMSO-cortisone liniment, and modification of the training schedule to light jogging or swimming between races. Lesions may remain stable or progress slowly and usually ultimately compromise performance substantially. Management is more difficult in horses that race at a high level, in which training cannot be reduced enough or the speed of the race results in overload of the compromised structure. Horses seem to do less well on a slower track surface, with a deep cushion, compared with a fast track surface.
Ultrasonographically, lesions are often slow to resolve and some persist long term (longer than 18 months). Some horses are able to resume full work after a convalescent period of at least 9 months, despite the persistence of a lesion, although the incidence of recurrent injury is high. If a lesion persists as viewed by ultrasonography, knowing when to recommend resumption of work is difficult. Generally the horse should have been rested at least 6 months, and no appreciable change should be found in the ultrasonographic appearance of the SL branch between two examinations 3 months apart. With increased exercise the ligament should remain stable in size and echogenicity.
Arthroscopic examination of the palmar (plantar) pouch of the metacarpophalangeal (metatarsophalangeal) joint may be indicated if there is persistent synovial distention associated with an axial lesion of the SL branch.49 Intrasynovial torn fibers can be debrided and, occasionally, small avulsion fractures removed. Thirteen of 18 horses (72%) with an axial branch injury that involved the fetlock joint returned to full athletic function for between 5 months and 6 years.49
Horses with forelimb or hindlimb suspensory branch lesions with periligamentous fibrosis, or echogenic material extending between the two branches (seen more often in hindlimbs) have a poor prognosis. This echogenic material represents firm fibrous adhesions between the branches. Periligamentous fibrosis impedes mobility of the SL branch and is often associated with a disproportionately severe lameness compared with lesions of the branch itself. There are anecdotal reports of surgical debridement, but the results have been disappointing. An abnormally low position of the PSBs is also a poor prognostic indicator, reflecting degeneration and stretching of the suspensory apparatus.
Proximal Sesamoid Bone Fracture Associated with a Branch Injury
Proximal sesamoid fractures associated with SL branch injury are discussed on page 404.
Avulsion Fracture of a Proximal Sesamoid Bone at the Insertion of the Palmar Annular Ligament
Avulsion injuries of the palmar annular ligament (PAL) at its insertion on a PSB are not common29,50 and must be differentiated carefully from abaxial PSB fractures (see Chapter 36, page 405). Injuries occur in forelimbs and hindlimbs. Horses with acute injury show lameness associated with diffuse soft tissue swelling palmar to a PSB.29 Swelling does not involve the ipsilateral SL branch unless that branch has been damaged concurrently. There may also be mild distention of the fetlock joint capsule and the DFTS. It may be possible to elicit pain by firm pressure applied to the abaxial aspect of the PSB. In horses with more chronic injury no localizing clinical signs may be apparent. Radiological examination may reveal a punched-out lesion of the PSB (Figure 72-16) or a small detached fragment of bone (Figure 72-17), the origin of which cannot be determined. Ultrasonographic examination helps to determine the origin of the fragment. The PAL should be examined from the palmar aspect of the limb and then followed around to its insertion. An osseous echogenic body may be seen attached to the PAL close to the PSB. Treatment by surgical removal of the fragment or conservative management has resulted in return to full athletic function.29,50
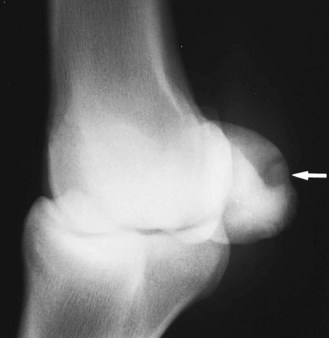
Fig. 72-16 Dorsolateral-palmaromedial oblique radiographic image of the right front fetlock of a 7-year-old advanced event horse. A punched-out radiopacity is visible on the palmar aspect of the bone (arrow). This is an avulsion injury at the insertion of the palmar annular ligament. Note also the new bone on the palmar distal aspect of the bone.
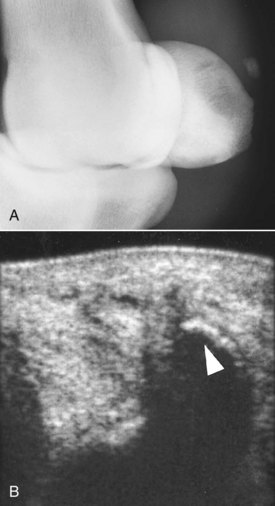
Fig. 72-17 A, Dorsomedial-palmarolateral oblique radiographic image of the right front fetlock of a 9-year-old Grand Prix show jumper with acute-onset lameness associated with soft tissue swelling on the medial aspect of the fetlock, palmar to the medial branch of the suspensory ligament. There was also slight edematous swelling around the distal aspect of the medial branch of the suspensory ligament. There is an osseous fragment palmar to the proximal sesamoid bones, but its origin could not be determined radiologically. B, Transverse ultrasonographic image of the palmaromedial aspect of the fetlock. A thick hyperechoic line represents the osseous fragment (arrow) that was an avulsion of the insertion of the palmar annular ligament.
Damage of the Palmar (Plantar) Ligament of the Fetlock (Intersesamoidean Ligament)
Focal Tears in the Body of the Intersesamoidean Ligament
Focal tears in the body of the intersesamoidean ligament are an unusual cause of lameness and can be diagnosed definitively only by arthroscopic evaluation of the palmar (plantar) pouch of the metacarpophalangeal (metatarsophalangeal) joint.29 Lameness is usually acute in onset and moderate to severe. Lameness improves with rest but often persists. There may be effusion in the metacarpophalangeal (metatarsophalangeal) joint. Lameness is improved by intraarticular analgesia. Usually no identifiable radiological abnormalities occur. Small focal tears are extremely difficult to identify using ultrasonography. Nuclear scintigraphic examination of a small number of horses with small focal tears not involving the insertion of the ligament has revealed slight generalized IRU in the fetlock region but not localized to the PSBs. Horses with focal ligament tears have a poor response to surgical debridement and prolonged rest, with or without intraarticular medication, and the prognosis for return to athletic function is guarded.
Degeneration or Partial Rupture of the Intersesamoidean Ligament
Degenerative lesions and partial rupture of the intersesamoidean ligament have been seen only in older, mature athletic horses and are relatively rare.51 The condition has been recognized in forelimbs and hindlimbs and may occur as a single injury or with other soft tissue or bony lesions.
Lameness is acute in onset and moderate to severe. Mild distention of the metacarpophalangeal (metatarsophalangeal) joint capsule or the DFTS may occur. Lameness may be improved by intraarticular analgesia, but the response to perineural analgesia of the palmar (plantar) and palmar (plantar) metacarpal (metatarsal) nerves is often better.
Diagnosis is based on ultrasonography, and a number of abnormalities have been identified:
Careful examination of the digital flexor tendons, the PAL, and the distal sesamoidean ligaments should be performed to identify any concurrent injuries.
Radiological examination occasionally may reveal osteolytic lesions on the axial aspect of the PSBs (see the following discussion).
The prognosis for return to athletic function is extremely guarded.29
Insertional Injury of the Intersesamoidean Ligament
Aseptic necrosis of the axial aspect of the PSBs has been described as a cause of lameness in forelimbs and hindlimbs.49,50 The condition has been characterized by the development of radiolucent zones in the axial aspect of one or both PSBs (Figure 72-18), lesions that have also been seen with infection (see the following discussion). Histological examination of two horses indicated that these lesions reflect trauma of the insertion of the intersesamoidean ligament.29 Insertional lesions of the palmar ligament of the fetlock also may occur without radiological change and have been identified arthroscopically (Figure 72-19).
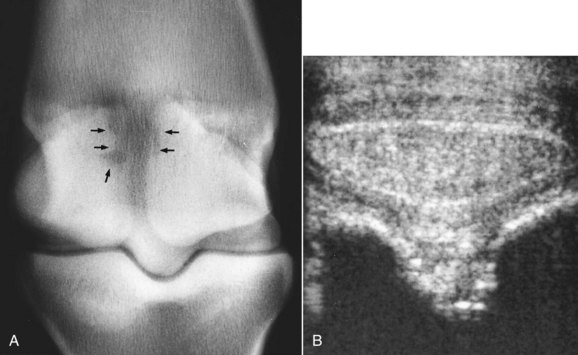
Fig. 72-18 A, Dorsoplantar radiographic image of the right hind fetlock of a 6-year-old Thoroughbred with severe lameness associated with moderate distention of the metatarsophalangeal joint capsule and mild distention of the digital flexor tendon sheath. Medial is to the left. The axial borders of the proximal sesamoid bones are poorly defined proximally, with radiolucent areas in the bone (arrows). Histological examination confirmed traumatic insertional injuries of the palmar ligament of the fetlock. B, Transverse ultrasonographic image of the plantar aspect of the fetlock of the same horse. Medial is to the left. Note the irregularity of the outline of the proximal sesamoid bones and the rather heterogeneous echogenicity of the intersesamoidean ligament.
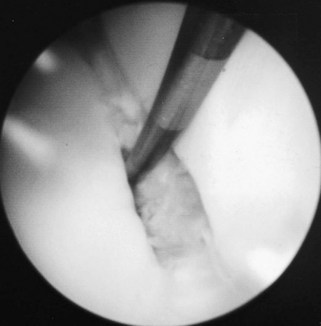
Fig. 72-19 Arthroscopic picture of focal tear in the palmar ligament of the fetlock at its insertion on the medial proximal sesamoid bone.
(Courtesy I.M. Wright, Newmarket, Suffolk, England.)
In contrast to horses with associated infection, usually no substantial soft tissue swelling occurs in the fetlock region, although distention of the metacarpophalangeal (metatarsophalangeal) joint capsule, the DFTS, or both may occur. In some horses, eliciting pain by palpation may be difficult. Lameness may vary from moderate to severe and is often worse with the horse walking in a circle compared with walking in a straight line.
Perineural analgesia by a four-point block usually results in much better improvement in lameness than intraarticular analgesia. In the acute stage radiological examination findings may be negative. Ultrasonographic evaluation may be unrewarding, but in some horses an irregular outline of one of the PSBs may be seen, with reduced echogenicity of the intersesamoidean ligament (see Figure 72-18).
Nuclear scintigraphic examination usually reveals focal, intense IRU in one or both PSBs, in contrast to the pattern of uptake that has been seen with focal lesions in the body of the intersesamoidean ligament (see the previous discussion). IRU localized to a PSB also may be caused by an incomplete fracture or subchondral trauma. Sequential radiological examination may reveal the development of lucent zones on the axial aspect of the proximal sesamoid bones.
These lesions may be associated with long-term lameness, although surgical debridement has resulted in return to former athletic function in some horses.52,53 Arthroscopic evaluation usually reveals a defect in the palmar ligament (see Figure 72-19), through which a probe can be inserted into the bony defect. Occasionally the palmar ligament is intact and must be incised to permit access to the osseous defect. Defects usually are more pronounced in one PSB, but they can involve both PSBs. Damaged palmar ligament and bone are debrided. Occasionally, to approach lesions not involving the articular surface, it may be necessary to create a small apical fracture to gain access to the bony defect.
Infection of the Axial Aspect of the Proximal Sesamoid Bones
Aseptic necrosis51,52 and osteomyelitis54 of the PSBs have been described, but in one author’s experience (SJD) aseptic necrosis occurs more commonly. Aseptic necrosis is probably an inappropriate term, because the Editors believe that lesions develop secondary to trauma of the insertion of the intersesamoidean ligament (see the previous discussion). Infection is not necessarily associated with a known route of infection, but several horses with infection have been seen after puncture wounds in the pastern region or cellulitis29 (Figure 72-20).
Infection of the PSBs may occur in forelimbs and hindlimbs and is characterized by radiolucent zones involving the axial margin of the medial or lateral PSB, or both. Pain may be elicited by pressure over the PSBs. Fetlock flexion usually is resented. Distention of the fetlock joint capsule and the DFTS and surrounding soft tissue swelling are variable features. Synoviocentesis may reveal evidence of infection in adjacent structures. In some horses a penetrating wound can be identified in the palmar aspect of the fetlock or pastern. Lameness varies from moderate to severe.
High-resolution dorsal 15- to 20-degree proximal-palmarodistal (plantarodistal) oblique radiographic images, with adequate penetration, are essential for diagnosis. If the radiographs are underexposed, lesions will be missed. Ultrasonography is useful for confirming the diagnosis and for identifying other concurrent lesions (see Figure 72-20), such as extensive tenosynovitis of the DFTS, deposition of echogenic material around the affected PSB, reduced echogenicity of the intersesamoidean ligament, and lesions of the digital flexor tendons or adhesions between them.1

Fig. 72-20 A, Dorsopalmar radiographic image of the right metacarpophalangeal joint of an 8-year-old Thoroughbred with massive swelling of the metacarpal region, restricted flexibility of the fetlock, and distention of the digital flexor tendon sheath. There are large radiolucent zones involving the distal axial borders of the proximal sesamoid bones. B, Transverse ultrasonographic image of the distal aspect of the metacarpal region of the same horse. Note the asymmetrical appearance of the proximal sesamoid bones and the difference in distance between them and the dorsal border of the deep digital flexor tendon (DDFT). C, Transverse ultrasonographic image of the distal metacarpal region of the same horse. Note the abnormal amount of fluid within the digital flexor tendon sheath and the echogenic material around the DDFT. D, Palmar oblique ultrasonographic image. Note the subcutaneous echogenic material and the distended vessels. These lesions were associated with infection.
The prognosis with conservative or surgical management is extremely guarded.
Straight Sesamoidean Desmitis
Desmitis of the straight sesamoidean ligament is an unusual cause of lameness that usually occurs in a forelimb, but it has been recognized in hindlimbs.55-58 One of the authors (SJD) has recognized it most commonly in event horses and show jumpers. The condition may occur alone, or together with injury to either an oblique sesamoidean ligament or other soft tissue lesions in the digit. Lameness is sudden in onset, usually with no detectable swelling in the acute phase. Some swelling may develop subsequently, but this can be difficult to detect if the lesion is far proximal. Concurrent effusion may occur in the DFTS, and palpation on the palmar midline of the pastern region may elicit pain, but not invariably so. Lameness may be severe acutely and then rapidly improving but persisting. Lameness is alleviated by palmar nerve blocks at the level of the base of the PSBs. Diagnosis is based on ultrasonography or MRI. Ultrasonographic abnormalities include the following (Figure 72-21):
Fig. 72-21 Transverse ultrasonographic images of the left (on the left) and right proximal pastern regions of an advanced event horse with acute-onset, severe right forelimb lameness after completion of a cross-country course. The lameness improved greatly within 24 hours. Flexion of the right front fetlock elicited pain, but no swelling or pain on direct palpation could be detected. Lameness was alleviated by perineural analgesia of the palmar nerves at the level of the fetlock joint. The right straight sesamoidean ligament is enlarged (cross-sectional area 1.46 cm2) compared with the left (1.35 cm2), resulting in loss of space between it and the deep digital flexor tendon. The ligament is also hypoechogenic.
Care should be taken not to misinterpret the normal hypoechoic area in the most distal part of the ligament as a lesion. This represents the cartilaginous scutum at the insertion.
Lesions may occur localized to the insertion, either unilaterally or bilaterally, and alone or in conjunction with other causes of palmar foot pain, such as navicular disease.
There is often no palpable abnormality unless the injury is severe. Lameness is removed by perineural analgesia of the palmar nerves at the base of the PSBs. However, improvement may be seen after palmar digital nerve blocks if these are performed in the midpastern region or because of proximal diffusion from a more distal injection site. Although some lesions can be identified ultrasonographically, the shape of the pastern influences the ease with which these structures can be imaged accurately. Recent experience with MRI suggests that these injuries may occur more frequently than previously recognized. MRI seems more sensitive than ultrasonography for the detection of these injuries.58,57 Improvement in lameness may also be seen after intrathecal analgesia of the DFTS, presumably because of local diffusion of local anesthetic solution.
Occasionally, concurrent lesions of the straight and oblique sesamoidean ligaments occur and have a more guarded prognosis.
Treatment has consisted of box rest, corrective trimming and shoeing, and controlled walking exercise, with periodic reevaluation with ultrasonography. Intralesional injections of bone marrow, platelet-rich plasma, or mesenchymal stem cells could be considered, although limited experience to date has not yielded good results. Usually, progressive resolution of lameness occurs over 6 to 8 weeks, with gradual improvement in the ultrasonographic appearance of the ligament over 6 months. However, the prognosis for return to full athletic function at a high level of competition is guarded because the incidence of recurrent injury is high. Severe lesions have shown poor capacity to heal, with persistent lameness.
Loss of tension (relaxation) of the straight sesamoidean ligament may occur if the SL is ruptured, resulting in instability or luxation of the proximal interphalangeal joint (palmar or plantar subluxation). Ultrasonographic abnormalities include the following:
Oblique (Middle) Sesamoidean Desmitis
Desmitis of one or both oblique sesamoidean ligament(s) occurs in forelimbs and hindlimbs, but it is a more common source of forelimb lameness. Lameness is usually acute in onset and moderate to severe. In the acute stage often no localizing clinical signs are apparent, but in the following 7 to 10 days swelling may develop in the pastern region, and pain may be elicited by firm palpation. However, lesions may occur without detectable palpable abnormalities, alone or with another cause of lameness. Severe lesions may be associated with dorsal subluxation of the proximal interphalangeal joint, characterized clinically by an abnormal convex contour on the dorsal aspect of the pastern at the level of the joint. With chronic lesions dorsal extension of the joint margins of the proximal interphalangeal joint may be seen radiologically. Lameness, which is often worst on a soft surface, is usually alleviated by palmar (abaxial sesamoid) nerve blocks. One should note that tendonitis of the medial or lateral branch of the SDFT is a more common injury in the pastern, has a similar clinical presentation, and is more likely to be associated with detectable soft tissue swelling. Lesions of an oblique sesamoidean ligament may be localized to the origin immediately distal to the PSB, and pain associated with such lesions may be abolished by intraarticular analgesia of the fetlock joint, reflecting the close anatomical relationship between the palmar (plantar) distal outpouchings of the fetlock joint capsule. Intrathecal analgesia of the DFTS may also result in resolution of lameness, presumably because of local diffusion of local anesthetic solution.
One also must recognize that enthesophyte formation on the palmar aspect of the proximal phalanx at the site of insertion of the oblique sesamoidean ligaments is a common incidental radiological abnormality frequently unassociated with clinical signs and often is not associated with any detectable ultrasonographic structural abnormality of the ligament. Well-circumscribed, smoothly marginated osseous bodies, possibly old avulsion fractures from the base of a PSB, are common radiological and ultrasonographic observations in mature horses in which the oblique sesamoidean ligament appears to be structurally normal.
Ultrasonographic abnormalities of the oblique sesamoidean ligament are often identified concurrently with some other cause of lameness—for example, osteoarthritis of the proximal interphalangeal joint or desmitis of a branch of the SL.
Ultrasonographic abnormalities include one or more of the following (Figures 72-22 and 72-23):
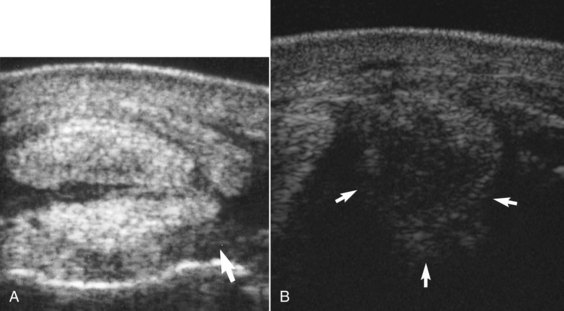
Fig. 72-22 A, Transverse ultrasonographic image of a 7-year-old Thoroughbred with left forelimb lameness of 10 days’ duration. Medial is to the left. The lateral oblique sesamoidean ligament is diffusely hypoechoic (arrow). B, Transverse ultrasonographic image of a 12-year-old Warmblood with left hindlimb lameness of 6 days’ duration obtained immediately distal to the lateral proximal sesamoid bone. The oblique sesamoidean ligament is enlarged and diffusely hypoechogenic (arrows).
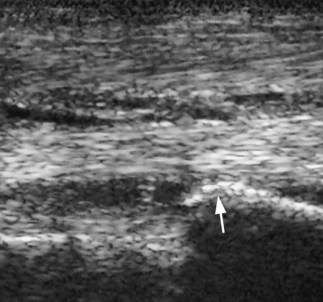
Fig. 72-23 Longitudinal ultrasonographic image of the right front pastern of a 7-year-old pleasure riding horse with moderate lameness. Proximal is to the left. A large enthesophyte (arrow) is visible on the palmar aspect of the proximal phalanx. The region of insertion of the oblique sesamoidean ligament is reduced markedly in echogenicity. The horse also had osteoarthritis of the proximal interphalangeal joint.
Each ligament should be evaluated carefully in transverse and longitudinal planes. Occasionally lesions occur together with injuries to other soft tissues of the pastern, so all structures should be evaluated systematically. Some lesions are not detectable with ultrasonography but are identified using MRI.57,58
Horses with a primary injury of the distal sesamoidean ligaments have a high incidence of recurrent injury if returned to full athletic function after conservative management. The effects of surgical splitting or of biological agents such as platelet-rich plasma or mesenchymal stem cells remain unproven. However, horses with primary lameness from another cause may have an enlarged oblique sesamoidean ligament of normal echogenicity, apparently unassociated with clinical signs.
Progressive Atraumatic Breakdown of the Suspensory Ligaments
Progressive degenerative changes in hindlimb SLs have been seen in a small number of horses that originally showed PSD and had straight hock conformation. These horses developed hyperextension of the hind fetlock joints (Figure 72-24). The condition has been characterized by a diffuse decrease in echogenicity of the proximal aspect of the SLs, which becomes progressively more extensive distally. A similar clinical condition has been recognized in older horses, especially broodmares, with progressive hyperextension (dorsiflexion) of the hind fetlock joints, which may result in abrasion of the plantar aspect of the fetlocks. Progressive hyperextension of the hind fetlock joints also has been seen in middle-aged performance horses with apparent lengthening of branches of the SL and periligamentous fibrous reaction. Some breeds, including the Peruvian Paso59-61 and Andalusian horses, seem particularly at risk and may show evidence of the condition in all four limbs. The condition is generally bilateral and is usually associated with a deterioration in hindlimb gait rather than an overt lameness, although Peruvian Pasos may be more severely affected. Secondary lateral luxation of the SDFT from the point of hock occurs occasionally, probably associated with the straight angle of the hock.

Fig. 72-24 A, The hindlimbs of a 14-year-old Thoroughbred gelding with hyperextension of the hind fetlocks and soft tissue enlargement around the distal aspect of the branches of the suspensory ligaments. The superficial digital flexor tendon of the right hindlimb was subluxated laterally. B, The distal aspect of the suspensory ligament branches of the same horse. Considerable fibrous tissue surrounds the branches. The ligaments were discolored substantially, with no normal-appearing ligamentous tissue.
Histologically the lesions are characterized by chondroid metaplasia, associated proteoglycan deposition, and acellular and fibrillated collagen fibers and merger of adjacent fascicles of fibrillated collagen to form megafascicles. Granulation tissue in interfascicular septae makes abortive attempts at repair.
The cause of these conditions is not known. They are probably complex disorders involving genetic, molecular, conformational, and biomechanical factors. It has been suggested that in the Peruvian Paso the condition is associated with a systemic disorder characterized by proteoglycan accumulation61; however, this conclusion has been refuted.62 The SLs and other tissues from affected Peruvian Pasos and unaffected STBs and Quarter Horses were examined using Safranin-O staining for detection of proteoglycan. Proteoglycan deposition was not unique to the affected Peruvian Pasos, being present in the nuchal ligament, heart, muscle, and other tissues, with similar or greater amounts in the control horses. However, greater amounts were detected in the SLs of affected horses compared with control horses. It was concluded that cartilage metaplasia and associated proteoglycan deposition in affected SLs were the response to injury rather than the cause.
The condition is generally progressive. Flat shoes with plantar extensions help to lift the fetlock and may prevent secondary trauma, but the prognosis for proper athletic function is guarded.
Traumatic Disruption of the Suspensory Apparatus
Traumatic disruption of the suspensory apparatus is discussed on page 969.