Chapter 128Lameness in Foals
Like all horses, foals are prone to injury and lameness; however, considering the immature and fragile musculoskeletal system, the incidence of serious injury is surprisingly low. Exposure of a foal’s naive immune system to the array of pathogens places the foal at high risk of infectious causes of lameness. Degree of lameness in foals ranges from subtle and nearly imperceptible to non–weight bearing. Although no published studies document the incidence of lameness in foals, the economic impact on the horse industry is substantial when factors such as cost of diagnosis and treatment, long-term morbidity and mortality, and the effect on athletic potential are considered. Economic and emotional losses may be substantial for horse owners and breeders, enough so that they may abandon the horse industry. Most lameness is self-limiting, however, and in many instances early recognition and prompt treatment makes a difference in costly medical bills and improves the long-term soundness or survival. It is imperative that veterinarians learn to recognize the early clinical signs of these serious disorders and to initiate prompt treatment. Clients should be made aware of the potential danger of a wait-and-see approach and a delay in treatment.
Evaluation of a Lame Foal
Foals often resist handling and limb manipulation, making assessing responses difficult and potentially confusing. It is imperative that the veterinarian is patient when evaluating foals. The basics of lameness localization are the same as in adult horses. The stance and any obvious swelling or sensitive areas on palpation should be noted. It is important to palpate the contralateral limb to gauge the foal’s response to manipulation of that limb. Hoof and limb temperature (hot or cold) and digital pulse amplitude should be evaluated. Detailed assessment with hoof testers is invaluable in differentiating causes of hoof lameness. Any aberration in hoof development (e.g., club foot, deformed foot, unbalanced feet, or mismatched sizes of feet) should be noted. Postural or conformational changes, including sudden angular limb deviation, limb laxity, or contraction, may result from altered load bearing associated with lamemess or from structural changes from injury. Careful palpation of all structures of the skeletal system, including all palpable surfaces of joints, bones, digital flexor tendons, and ligaments, with the foal in a standing position and with each limb flexed is important. Swelling should be differentiated as intraarticular, periarticular, or both.
Gait deficits in foals manifest like those in adult horses in that lameness is expressed as reduced time or weight on the lame limb.1 The phase of the stride in which the gait is altered is the result of reducing loading of the limb during the painful portion of locomotion. Foals are more likely to show lameness in dramatic fashion by carrying a limb (i.e., not bearing weight) or exaggerating efforts to reduce load on the limb. It is important to observe the foal at a walk to discern subtle differences in stride and foot placement that may be missed at a trot.
Routine ancillary diagnostic tests used in foal lameness evaluation include diagnostic analgesia; radiographic, ultrasonographic, and nuclear scintigraphic examinations; and arthrocentesis and aspiration of other fluid pockets.1-4 Diagnostic analgesia should be a routine component of lameness localization in foals just as in mature horses, unless a fracture is suspected. Any areas of suspect pathological bone conditions should be evaluated radiologically. Weekly follow-up radiological evaluation may be required to recognize bony changes. Soft tissue evaluation is aided by ultrasonography, especially when the veterinarian is looking for fluid pockets or alterations in soft tissue architecture. Nuclear scintigraphy has limited applications for skeletal evaluation in foals because normal radiopharmaceutical uptake in the physes can confuse or mask subtle abnormalities.
Noninfectious Causes of Lameness
Noninfectious causes of lameness include external and internal traumatic injuries and developmental or metabolic musculoskeletal diseases. Lameness also may result as a sequela to vascular disorders or may be immune-mediated secondary to an infectious problem.
Lameness resulting from external trauma varies with the area involved and the severity of the injury. Essentially any portion of the skeletal system is prone to injury. Traumatic injuries resulting in lameness are common but fortunately self-limiting in most instances. Boisterous activity, coupled with a hazardous environment, places a foal at an increased risk of sustaining injury compared with an adult horse. Trauma from other horses (a mare kicking a foal or collision injuries with other foals) is a common cause of lameness. Impact with stationary objects while a foal is being chased may result in serious injury. Internal trauma includes overexertion injuries, such as a foal running excessively with a mare or being chased, resulting in fracture of the proximal sesamoid bones (PSBs), or rupture of the suspensory apparatus or other soft tissue structures (Figure 128-1). Common fractures in foals include those involving the long bones, physes, PSBs, carpal and tarsal cuboidal bones, and the distal phalanx.1,2,5,6

Fig. 128-1 Typical crouched stance of a foal with rupture of the left gastrocnemius muscle subsequent to overextension while chasing a mare. The foal is unable to extend the limb or support full weight on it. Note the excessive flexion of the hock.
Fractures of Long Bones
Clinical evaluation may be diagnostic in foals with lameness of traumatic origin. Unstable fractures or severe soft tissue injuries cause obvious, immediate clinical signs. If a fracture is suspected, radiographs are necessary to confirm the diagnosis and determine the severity of the injury (Figure 128-2).
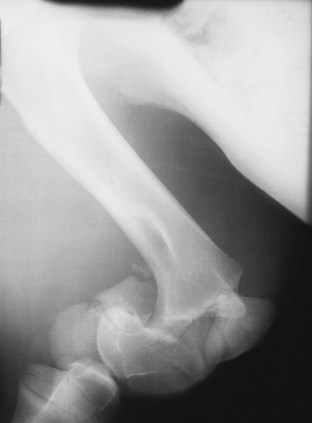
Fig. 128-2 Slightly oblique lateromedial radiographic image of the femur of a foal. There is an unstable, displaced fracture of the distal aspect of the femur, the result of trauma. This is a Salter-Harris type II fracture.
Fractures of long bones most often result from external trauma such as a kick or having the limb pinned beneath an object while rolling. A limb misloaded while rearing may result in fracture of the tibia, femur, or pelvis. If a limb is unstable distal to the femur or humerus because of a fracture, the limb should be immobilized before transport. Surgical approaches and repair techniques for individual fracture types are described elsewhere.
Prognosis for a foal after fracture depends on anatomical location of the fracture; the complexity, orientation, and degree of soft tissue injury around the fracture; and whether the fracture is open. Secondary problems associated with prolonged lameness or confinement and immobilization often dictate the degree of long-term soundness. These problems include excessive laxity of the metacarpophalangeal or metatarsophalangeal joint, varus deviation, or deformity of the foot, such as an underslung or crushed heel and overgrowth of the toe. Mechanical laminitis is not as common as in adult horses, but it does occur. Contracture or excessive laxity of the fractured limb associated with disuse may prevent future soundness.
Fractures of the radius, ulna, and tibia are generally more amenable to surgical repair than are femoral and humeral fractures. If economics do not allow surgery, conservative management of foals with femoral and humeral fractures by stall confinement is a feasible alternative to surgical intervention. Teaching the foal to support its weight on a straw bale or butt bar may alleviate load on the contralateral limb for hindlimb fractures. Complications encountered after surgical repair of femoral or humeral fractures, such as implant failure or infection, are usually fatal. Without postoperative complications, however, a foal has a greater chance of soundness for light athletic use with surgical management. Fractures of the radius and tibia require surgical stabilization if any displacement of the fracture is present. Most foals have a fair to good prognosis for light to medium athletic use or for breeding purposes. Foals with fractures of the ulna that have any displacement should undergo surgery, but those with nondisplaced fissure fractures do not require surgical fixation. Foals with nondisplaced fractures of the ulna should have stall confinement for a minimum of 8 to 10 weeks and undergo radiological monitoring every other day for the first 2 weeks and then weekly to ensure displacement does not occur. Foals with displaced, unstable fractures of the metacarpal and metatarsal bones generally have a guarded to poor prognosis for future athletic use, with or without surgery. Foals with simple, stable fractures of the metacarpal and metatarsal bones not requiring surgery have a good prognosis.
Fractures of the Pelvis
Pelvic fractures in foals are common and usually results from a foal misloading a hindlimb when rearing or from flipping over and landing on one side. Gait and degree of lameness depend on the portion of the pelvis involved and the amount of trauma to the surrounding tissue. Common sites of injury include the tuber coxae, tuber ischium, acetabulum, and the shaft of the ilium. Deep palpation of the area may elicit pain.
The characteristics of the lameness may reflect the site of injury. A foal with a fracture of a tuber ischium is reluctant to advance the ipsilateral hindlimb and has a shortened cranial phase and prolonged caudal phase of the stride. As with many pelvic injuries, the tail often is elevated and held to the opposite side, and the foal may carry the limb when running but bear full weight while walking. Conversely, foals with fractures of a tuber coxae have a shortened caudal phase of the stride, and the injured tuber coxae is often lower than the contralateral side.
Treatment for foals with pelvic fractures includes confinement for 8 to 12 weeks followed by a gradual return to controlled exercise. Improvement in the degree of lameness should be seen within 2 to 3 weeks, depending on the region of the pelvis injured. Foals with acetabular injuries may require a longer duration of confinement before improvement is seen. If the foal is fully weight bearing, controlled walking for short periods after 3 weeks can be started if the foal is tractable.
Prognosis for foals with pelvic fracture not involving the acetabulum is fair to good for light use but guarded for competitive athletics. Some horses are able to race following healing of pelvic fractures. Most foals with pelvic fractures involving the acetabulum have a poor prognosis for an athletic future; however, they generally survive to make breeding animals. In the Editors’ experience, the prognosis for athletic function is not invariably poor because, in contrast to adult horses, foals have a remarkable capacity for bone remodeling, and even some severe articular fractures can heal satisfactorily, allowing normal athletic function.
Fractures of the Physes
Physeal fractures have been categorized and defined using the Salter-Harris classification scheme.2-7 A type I fracture occurs through the physis and involves only the zone of hypertrophied chondrocytes; the adjacent epiphysis and the metaphysis are not involved. A type II fracture occurs across the physis and extends into a portion of the metaphysis. A type III fracture involves the physis and epiphysis and extends into the joint. A type IV fracture includes involvement of the physis, epiphysis, and metaphysis. A compression injury to the physis is referred to as a type V fracture. This classification scheme is applicable to pressure physes that contribute to the longitudinal growth of bone and are subject to compressive forces. Traction physes, such as the olecranon growth plate, are subject to tensile forces and are not included in this classification scheme.
Physeal fractures are common because physeal bone is weak compared with diaphyseal bone. Diagnosis of physeal injuries is made on clinical and radiological findings (Figure 128-3). Clinical signs include varying degree of lameness, pain on palpation of the injured area, swelling, and possibly instability of the limb or angular deviation of the limb distal to the fracture. The primary differential diagnosis for a stable physeal fracture is infectious physitis.
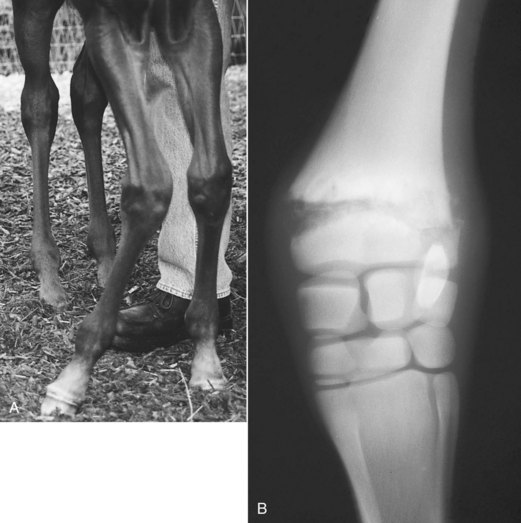
Fig. 128-3 A, Marked carpus valgus deformity of the right forelimb as a result of a distal, radial physeal fracture. Note also the slight varus deformity of the left carpus. B, Dorsopalmar radiographic image of the right carpus. Lateral is to the right. There is a displaced Salter-Harris type III fracture of the distal radial physis. Note the widening of the physis medially, resulting in the valgus deformity.
Surgical repair is necessary in foals with displaced, unstable physeal fractures. The major difficulty in surgical stabilization is the short section of epiphysis available for purchase. This shortcoming generally is overcome with the use of a form of T-plate, cross pins, transfixation pins, or tension-band wiring. If adequate reduction and stability are achieved, the prognosis for future soundness is good.
Cuboidal Bone Injury
Injury to the cuboidal bones of the tarsus or carpus often is associated with failure or delay of endochondral ossification in premature or dysmature neonates.5,6 Crushing and malformation of the soft cartilaginous precursors of the cuboidal bones result in osteoarthritis and varying degrees of lameness (Figure 128-4). Outwardly the limb may appear normal or have an angular deviation, or, if a hindlimb is involved, it may be sickle hocked or curbed. Attempts at prevention of this condition are more effective than treatment after its onset, although these are limited in effectiveness. Prolonged stall confinement of premature neonates is recommended until radiological signs of ossification of the cuboidal bones occur. Ossification generally requires 4 to 8 weeks but may vary. Foals affected with crushing of cuboidal bones rarely make competitive athletes, although most are suitable for light use, breeding, or as comfortable pasture animals. Those with milder forms of compression and modeling or tapering of the third and central tarsal bones may make competitive athletes.
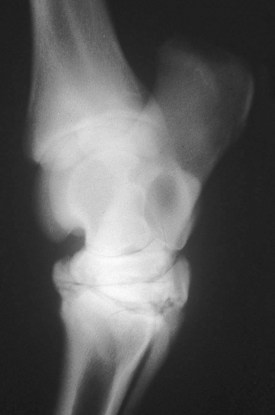
Fig. 128-4 Slightly oblique lateromedial radiographic image of a hock of a mature horse with crushing of the third tarsal bone and secondary osteoarthritis of the centrodistal and tarsometatarsal joints, the result of incomplete ossification of the third tarsal bone as a neonate. Note the wedge shape of the third tarsal bone and the abnormal dorsal contour.
Fractures of the Distal Phalanx
Nonarticular fractures of the palmar processes of the distal phalanx occur with high frequency in Thoroughbred foals (Figure 128-5). Other types of fractures of the distal phalanx occur but are less common. The forelimbs frequently are involved, and the lateral palmar process is more commonly fractured; bilateral and biaxial fractures occur occasionally. In the hindlimb, medial and lateral plantar process fractures occur evenly. Palmar/plantar process fractures may be detected radiologically as incidental findings and may not necessarily cause lameness.
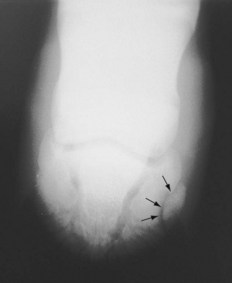
Fig. 128-5 Dorsoproximal-palmarodistal oblique radiographic image of a foot of a foal. Lateral is to the right. There is a nonarticular fracture of the lateral palmar process of the distal phalanx (arrows).
Salient clinical findings associated with a fracture of a palmar/plantar process of the distal phalanx include acute onset of lameness, which may be intermittent in nature, and lameness that is almost always more noticeable on sharp turns. Foals consistently have pain when hoof testers are applied across the heel. Atypically a response may be noticed with pressure at the toe. When diffuse pain is noted during hoof tester evaluation, along with an increased temperature of the foot, subsolar abscessation should be considered.
Treatment includes confinement to a small area with soft, uniform footing for 4 to 6 weeks. Lameness should improve in the first week. External coaptation, such as a foot cast or glue-on shoe, is contraindicated and may potentiate development of club foot conformation. Follow-up radiographs are not mandatory unless lameness persists or recurs. The prognosis is good. Differential diagnosis in foals suspect of fracture of the distal phalanx includes subsolar bruising, subsolar abscessation, infectious osteitis of the distal phalanx, and infectious arthritis of the distal interphalangeal (DIP) joint.
Fractures of the Proximal Sesamoid Bones
Fractures of the PSBs are common in young foals. Fractures usually are confined to one limb, but several limbs may be involved. Uniaxial (one) or biaxial (both PSBs) fractures in a single limb can be found. PSB fractures occur most commonly in foals between 2 weeks and 2 months of age. Fractures most often involve the base or apex of the PSB (Figure 128-6). Midbody PSB fractures occur less commonly. Hindlimb PSB fractures are less common, but they generally occur in conjunction with PSB fractures in a forelimb.
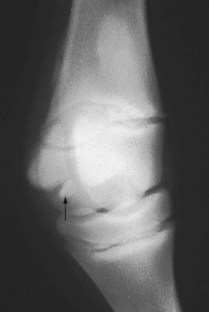
Fig. 128-6 Dorsolateral-palmaromedial oblique radiographic image of a metacarpophalangeal joint of a foal. There is a wedge-shaped basilar fracture (arrow) of the lateral proximal sesamoid bone.
Lameness varies from moderate to severe and is exaggerated on turns. Often increased digital pulse amplitude is apparent, and palpation of the involved bone elicits a painful response. Depending on the fracture type and location, the fetlock joint capsule may be distended or regional swelling may occur over the fractured PSB. Diagnosis is confirmed radiologically.
Conservative management is recommended and involves confinement to a small area for 6 to 8 weeks. If swelling is noticed, a distal limb bandage should be applied. Surgical repair is not beneficial in foals because of the lack of substance of the PSBs. Prognosis depends on the type of fracture, number of PSBs involved, and the number of limbs involved. Most PSBs have an elongated appearance (megasesamoid) after fractures heal, but foals may be suitable for athletic use if healing is complete (Figure 128-7). The prognosis for athletic use is worse if both PSBs in one limb are affected, or if fractures occur in more than one limb, compared with a uniaxial fracture in a single limb.
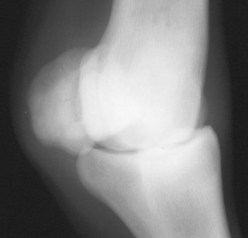
Fig. 128-7 Dorsolateral-palmaromedial oblique radiographic image of the metacarpophalangeal joint seen in Figure 128-6, obtained when the horse was a yearling. The basilar fracture of the lateral proximal sesamoid bone has healed, resulting in an elongated, distorted appearance of the bone.
Developmental Orthopedic Disease
Developmental orthopedic disease (DOD) is an uncommon cause of lameness in foals less than 2 to 4 months old.3,8 However, DOD is a common skeletal disease in weanlings and yearlings and generally is considered self-limiting. DOD is complex and includes acquired flexural deformity, osteochondrosis, physeal dysplasia (physitis) and resulting angular limb deformity, and cervical vertebral malformation. The pathogenesis, clinical manifestations, and management are discussed in Chapters 54, 57, 59, and 60.
Diagnosis of osteochondrosis is based on clinical evaluation and radiological findings.3,8 Clinical findings include synovial distention and varying degrees of lameness in the acute stage. Most young foals (<2 months old) afflicted with osteochondrosis have several joints or bones involved. These foals are subsequently lamer than older foals and usually have severe osteochondrosis. The cause often is not found because individual foals usually are involved, although heredity likely plays a primary role. Radiological findings may initially include osteochondral fragmentation or erosive changes in subchondral bone, or may be normal. Therefore it is important to plan on serial radiological studies to monitor the progression of changes.
Conservative management for young foals with osteochondrosis almost always is recommended and includes rest, antiinflammatory drugs, systemic chondroprotective drugs, and occasionally joint lavage and injection with hyaluronan. If a free fragment is evident, surgical retrieval may be beneficial; however, one should keep in mind that surgery for osteochondrosis is an elective procedure that should be performed at a time optimal for the foal. If a nondisplaced fragment occurs in a weanling or younger age foal, surgery may be delayed until the yearling period and monitored radiologically (Figure 128-8). Time will allow maturation of the underlying bone so minimal debridement and curettage will be necessary. In many instances the extra time allows these fragments to remineralize, and surgical removal is not necessary. In a longitudinal study of 43 Dutch Warmblood foals, osteochondrosis lesions were found at the cranial aspect of the intermediate ridge of the tibia in 68% at 1 month of age, but at 11 months of age abnormalities were detected in only 18%.9 Lesions in the lateral trochlear ridge of the femur developed later, with an incidence of 20% at 5 months of age, but by 11 months of age abnormalities were detected in only 3%.
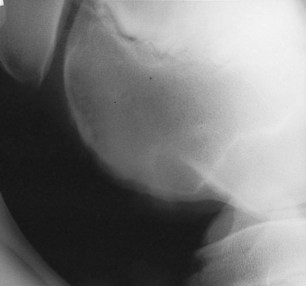
Fig. 128-8 Lateromedial radiographic image of a stifle. There is irregularity in the outline of the cortex of the lateral trochlear ridge of the femur with subtle alteration of the subchondral bone opacity. This is the result of osteochondrosis. There are no displaced fragments; therefore surgery as a weanling is not required, although the foal should be monitored both clinically and radiologically.
Vascular Thrombosis
Vascular thrombosis is an unusual cause of lameness in foals. Usually a central focus provides emboli, the most common being a vegetative lesion on a heart valve. Clinical signs include a variable degree of lameness involving one or more limbs. The limb may feel cold during periods of lameness but normal at other times. Response to diagnostic analgesia is inconsistent. Ultrasonographic examination of the heart usually reveals a lesion on a heart valve. Although any valve may be involved, most commonly the aortic or mitral valves are affected. Bacterial blood cultures should be obtained, especially during febrile episodes, if present.
Treatment consists of long-term antimicrobial drugs, based on bacterial cultures if available, and optionally rheological agents such as pentoxifylline. Follow-up ultrasonographic examination is important to determine the effectiveness and duration of treatment. The prognosis is guarded and depends on the degree of ischemia in the distal extremity.
Traumatic Nerve Injury
Collision injuries involving the proximal aspect of the forelimb of foals may result in injury to the radial nerve or components of the brachial plexus. Most commonly a foal has a dropped elbow, is unable to advance the limb, and drags the limb during ambulation. Differential diagnosis includes fracture of the humerus, the scapula, or a cranially located rib, and radiological evaluation should be performed to differentiate these conditions. The condition may be transient, lasting only a few hours if attributable to neuropraxia, or may be permanent.
Treatment for nerve injury consists of antiinflammatory drugs, including corticosteroids and nonsteroidal drugs, and dimethyl sulfoxide. Supportive care, such as physical manipulation of the limb and splinting, may aid in preventing flexural limb deformity. Acupuncture may be beneficial in some foals with nerve injury. In general, if no improvement is seen within 3 to 5 days of treatment, only 25% of foals recover.
Miscellaneous Soft Tissue Injury
Muscle tears are common in young Thoroughbreds. Longitudinal rents, 3 to 5 cm in length, in the biceps femoris, superficial gluteal muscles, or vastus lateralis are most often seen. Commonly there is a fluctuant fluid pocket in the tear, which resolves over several weeks as the edges of the tear undergo fibrosis. Lameness is usually mild to moderate and transient in nature, lasting for several days. Less common soft tissue injuries include traumatic rupture of the suspensory apparatus or rupture of the gastrocnemius muscle, and foals with these injuries have generally had a poor prognosis for long-term athletic function. However, in a recent report 23 (82%) of 28 foals with rupture of the gastrocnemius muscle survived to discharge from the hospital, and 13 (81%) of 16 horses that reached 2 years of age trained or raced.10
Infectious Causes of Lameness
Determining the location and cause of lameness in foals attributable to infection is crucial in deciding a course of therapy and assessing prognosis. As a general rule, assuming that all foal lameness is infectious in origin until proved otherwise is safest. In this instance, prompt appropriate treatment uniformly results in a more favorable outcome than if treatment is delayed.11
The clinical complex of infectious arthritis, tenosynovitis, infectious osteitis, or osteomyelitis occurs in foals commonly,11-16 especially in foals less than 4 months of age but occasionally in older foals. The different forms of infection can be discussed together because the pathogenesis is common. Bacteria may gain entry through the umbilicus, respiratory tract, or gastrointestinal tract, although direct penetration of a synovial structure from external trauma may result in synovial infection. Hematogenous dissemination of bacteria allows localization into metaphyseal, physeal, or epiphyseal cartilage and is believed to be associated with the rich vascular network composed of metaphyseal loops, sinusoidal veins, and epiphyseal and transphyseal vessels that supply the end of the long bones and synovium (Figure 128-9).2,13 The sluggish blood flow and vascular stasis of nutrient vessels approaching a cartilage interface allows bacteria to proliferate and colonize. Bacterial colonization incites an acute inflammatory response associated with kinin, complement, and coagulation system activation, eventually leading to cartilage destruction. Vasoactive amines and prostaglandins acting as chemotactic agents result in rapid accumulation of inflammatory cells. Inflammatory products released from synoviocytes, fibroblasts, chondrocytes, neutrophils, and monocytes result in the degradation of articular cartilage proteoglycan, collagen, and hyaluronan. Mediators involved include collagenases, elastases, lysosomal enzymes, plasmin, prostaglandins, metalloproteinases, monokines, and free radicals. Extrinsic mediators include interleukins, bacterial lipopolysaccharides, and physical forces placed on the damaged cartilage, all of which further compound collagen degradation. Further compromise of cartilage occurs because of poor nutrition of the chondrocytes, caused primarily by fibrin clot formation over the articular surface, and by thrombosis in the synovial vasculature. The primary mediators of infectious arthritis and eventual cartilage destruction are toxic metabolites released from chondrocytes. Cartilage matrix degradation and proteoglycan loss may occur within 2 days and collagen loss by 9 days after bacterial inoculation.2,17,18
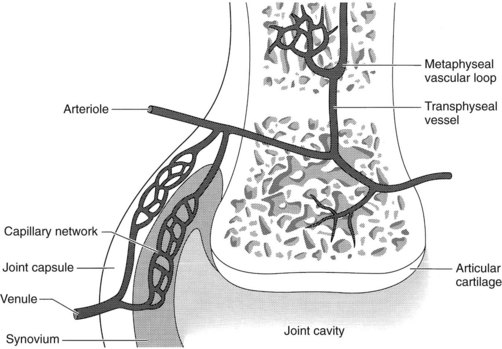
Fig. 128-9 Vascular supply to long bone and articular soft tissue. Blood-borne bacteria may colonize at the metaphyseal vascular loop and disseminate to the physis, epiphysis, or synovial membrane.
(Modified from Kobluk CN, Ames TR, Geor RJ, editors: The horse: diseases & clinical management, Philadelphia, 1995, Saunders.)
No explanation is apparent for disease localization in the epiphysis, metaphysis, physis, or joint. However, certain joints do appear to be affected more frequently than others. For example, the stifle, hock, metacarpophalangeal, and metatarsophalangeal joints are involved more often than the carpus or pastern. Infectious arthritis and osteomyelitis of hematogenous origin have been classified into five types based on location of the structure involved (Figure 128-10).13,19
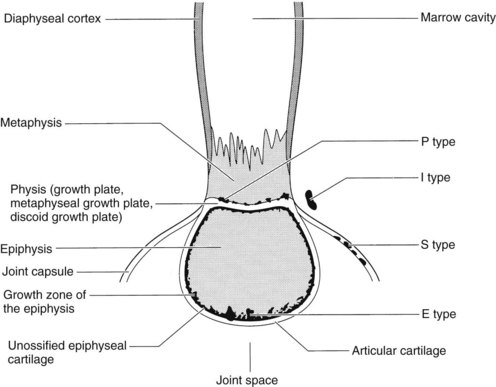
Fig. 128-10 Morphology of the long bone and types of S, E, P, and I infections. P type, Physeal-type infectious arthritis; I type, bacterial invasion into a physis or joint from a periarticular abscess; S type, infectious synovitis; E type, epiphyseal-type infectious arthritis.
(Modified from Kobluk CN, Ames TR, Geor RJ, editors: The horse: diseases & clinical management, Philadelphia, 1995, Saunders.)
Infectious synovitis (S type) affects foals usually within the first 10 days of life. Several joints are usually involved, and few, if any, radiological changes are seen. Predominant clinical signs include periarticular soft tissue swelling, effusion, and lameness. Arthrocentesis reveals an elevation in nucleated cell count (usually >50,000/mcL), and degenerate neutrophils predominate.
Epiphyseal-type (E type) infectious arthritis involves the joint and adjacent epiphysis. Foals are generally several weeks of age or older. One or more joints may be infected, and other systemic illnesses may occur concomitantly. Radiological evidence of epiphyseal involvement commonly is seen.
Physeal type (P type) may occur in foals from 1 week to 4 months of age. Degree of soft tissue swelling and lameness may vary, and generally only one site is involved. Sympathetic effusion eventually may occur in a nearby joint (articular structure in close proximity). Lesions may be seen radiologically in the metaphysis, physis, or epiphysis (Figure 128-11). Pathological fracture may result because of weakening of the involved structure.
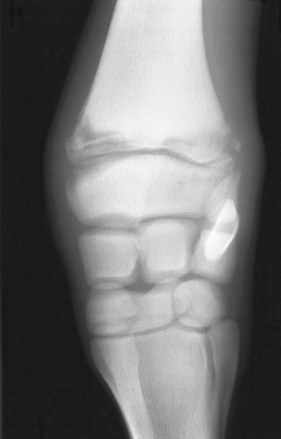
Fig. 128-11 Dorsopalmar radiographic image of a carpus of a foal with P-type osteomyelitis of the distal radial physis. Lateral is to the right. Note the soft tissue swelling and the extensive radiolucent areas in the physis, metaphysis, and epiphysis. Infection has caused fragmentation of the lateral aspect of the metaphysis.
Small tarsal or carpal cuboidal bone (T type) osteomyelitis may result in collapse of the central or third tarsal bones (Figure 128-12). Several joints commonly are affected.
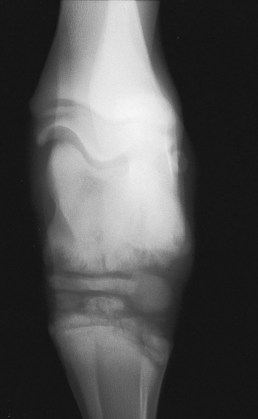
Fig. 128-12 Dorsoplantar radiographic image of a hock of a young foal with type T osteomyelitis involving the cuboidal bones of the tarsus. Lateral is to the right. Note the mottled opacity of the central and third tarsal bones and the proximolateral aspect of the third and fourth metatarsal bones. These extensive lesions warrant a grave prognosis.
Invasion into a physis or joint from a periarticular soft tissue abscess is called I-type infection. Most commonly joints of the upper part of the limb, such as the hip or stifle, are involved. If soft tissue abscessation is detected early, infectious arthritis often can be avoided.
Most commonly foals with osteomyelitis have acute-onset lameness, diffuse regional swelling, heat, and detectable pain of the involved area. Fever is generally present before and at the onset of lameness but may go undetected. Degree of lameness depends on what bone is involved, the time frame, and stage of detection. Owners often erroneously assume lameness results from trauma because signs occur acutely. A common assumption is that the mare stepped on the foal (the Editors).
Diagnosis of complex infection is based on physical examination findings and the results of arthrocentesis (if intrasynovial involvement occurs) and radiographic examination.2,11,13,15,16 Foals with infectious osteitis or early osteomyelitis may have no or subtle radiological changes, but within 1 to 2 weeks radiological changes consist of erosive, radiolucent changes surrounded by increased radiopacity and eventual proliferation of new bone. Therefore it is important to perform follow-up radiology to monitor the progression of the disease.
Other imaging modalities are of limited value. Sensitivity of nuclear scintigraphy alone is questionable in young foals because of the high metabolic activity of developing bone.10 Labeled white cell imaging using nuclear medicine techniques is useful but adds expense and radiation exposure and has not gained widespread acceptance in private practice.
Ultrasonographic examination is most helpful in identifying soft tissue injury or inflammatory processes in the upper limb. Abscessation of the soft tissue adjacent to the infected bone is a common finding in foals with physeal infection. Using ultrasonographic guidance, abscesses can be aspirated for culture and antimicrobial susceptibility testing and opened, drained, and lavaged.
Arthrocentesis is the key in diagnosing infectious arthritis, but a negative culture does not rule out infection.4,11-15,20 Joint fluid should be obtained in ethylenediaminetetraacetic acid tubes to evaluate the differential and total nucleated white blood cell count and protein concentration and for cytological evaluation. Anaerobic and aerobic culture and antimicrobial susceptibility tests should be performed. Using an antimicrobial-removal device may be of benefit to potentiate a positive culture if the foal is receiving antimicrobial drugs. Blood cultures should be collected.
In foals and horses suspected of having infectious arthritis, synovial fluid cultures are positive 64% of the time.12,14,17,18,20 In my practice, of 158 synovial samples submitted in 1 year, there were only 64 (40%) positive isolates. Reasons for low positive culture results include previous administration of antimicrobial agents, partial success of the immune system, the intrinsic bacteriocidal properties of infected synovial fluid, poor storage, or no bacteria present in the synovial fluid.
If there is radiological evidence of osteomyelitis well away from a joint, a sample of bone and debris may be obtained using a Michelle trephine. An alternative technique is to use a 2.5- or 3.2-mm drill bit and collect the shavings for culture and antimicrobial susceptibility testing.11 This tract then may be used for intraosseous infusion of an antimicrobial agent.
The results of hematological testing can be confusing. Complete blood count and fibrinogen level should be assessed, but results are often within normal limits initially because infection is localized. Serial hemograms should be performed in this instance. Any elevation in the white blood cell count or fibrinogen level in a lame foal with fever should be assumed to reflect bone or joint infection until proved otherwise. A normal hemogram does not rule out infectious arthritis or osteomyelitis. Foals less than 1 month of age should have immunoglobulin G levels checked to evaluate for failure of passive transfer.
Treatment of foals with infectious arthritis consists of joint lavage with sterile polyionic fluids through large-bore needles or by using an arthroscope. If a large amount of fibrin and cellular debris is in the synovial fluid, arthrotomy and lavage with or without primary closure of the joint may be indicated. Joint lavage may be repeated every 1 to 3 days depending on the clinical response. A long-term closed suction drainage system may be used as well.11 Broad-spectrum systemic antimicrobial drugs should be started immediately and adjusted accordingly based on the clinical progression and culture and antimicrobial susceptibility testing.
Other treatments for joint and bone infection include local intraosseous injection or regional hyperperfusion with antimicrobial agents. To perform intraosseous injection, an 18- or 20-gauge needle is inserted into the physis or adjacent bone. An aminoglycoside such as amikacin (250 to 500 mg) may be used. Alternatively, a cannulated intraosseous infusion needle (Sussmane-Raszynski intraosseous infusion needle, Cook Incorporated, Bloomington, Indiana, United States) may be inserted and maintained for repeat use (Figure 128-13). To perform regional hyperperfusion, a site over the lateral digital neurovascular bundle is prepared aseptically, and a rubber Esmarch tourniquet is placed in the proximal metacarpal region. A 23-gauge butterfly catheter is inserted in the digital vein. An antimicrobial solution (500 mg of amikacin diluted in 10 mL of sterile water) is infused into the digital vein. The tourniquet is removed after 15 minutes, and a lower-limb bandage is applied. The procedure may be repeated as necessary. Regional digital hyperperfusion is especially useful in treating foals with infectious osteitis of the distal phalanx and infectious arthritis or osteomyelitis of the distal aspect of a limb. I have not encountered complications, such as distal limb ischemia, from tourniquet application.
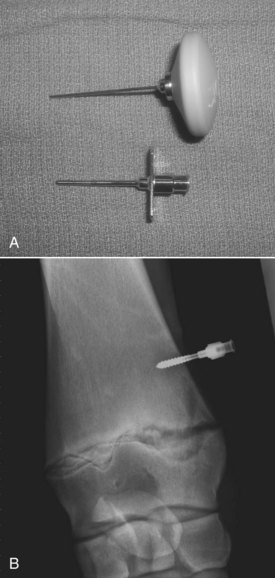
Fig. 128-13 A, Sussmane-Raszynski intraosseous infusion needle (Cook Incorporated, Bloomington, Indiana, United States). B, Dorsopalmar radiographic image showing an intraosseous infusion needle inserted in the distal radius of a weanling to manage P-type osteomyelitis of the distal medial radial physis.
Foals with infectious arthritis should be allowed to rest for a minimum of 3 to 4 weeks to prevent further traumatic cartilage damage.2 Systemic administration of chondroprotective agents or intraarticular administration of hyaluronan may be of benefit.
Severe erosive lesions of the physis and metaphysis may result in pathological fracture and collapse of the bony column, resulting in an unstable fracture or severe angular deviation. Surgery may be indicated, but prognosis is poor for soundness if this occurs.
The prognosis for foals with infectious arthritis and osteomyelitis depends on the antimicrobial susceptibility of the organism and how early effective treatment is instituted.12-20 In general, foals treated early for articular infections have a good prognosis for full recovery. Foals with articular infection with subchondral bone involvement have a poor prognosis, and the clinical course of disease and treatment are prolonged. Foals with focal bony lesions, involving only the physis without bone instability, have a good prognosis. Those with a pathological fracture or severe angular limb deformities have a poor prognosis and may be candidates for humane destruction.
Immune-Mediated Synovitis
Immune-mediated synovitis is a sequela to a primary inflammatory focus such as pneumonia, umbilical remnant infection, or a peripheral abscess.11,21 Rhodococcus equi pneumonia is the most common primary focus of infection. The syndrome results from the deposition of immune complexes in the synovial lining and complement activation, resulting in synovitis. The condition usually involves more than one synovial structure. Foals usually have a stiff gait resulting from the synovial distention, and severe lameness is unusual.
The primary rule out for immune-mediated synovitis is early infectious polyarthritis, causing only moderate lameness and mild synovial fluid distention. Cytological evaluation of joint fluid in foals with immune-mediated synovitis commonly reveals nucleated cell counts of less than 20,000 nucleated cells/mcL. The cells are well-preserved neutrophils and large mononuclear cells. Foals with infectious arthritis generally have nucleated cell counts greater than 50,000 nucleated cells/mcL, and neutrophils are degenerate.
Therapy for foals with immune-mediated synovitis is based on identifying and resolving the underlying disease. Synovitis is self-limiting once the offending cause is removed. Systemic chondroprotective agents may be of benefit to the health of the joint because prolonged inflammation may cause cartilage damage.
Infection of the Digit
Infection of the digit is a frequent cause of severe lameness in foals. Infection may be contained to the subsolar or hoof wall regions, or it may involve the distal phalanx or the DIP joint. It is critical to differentiate between these sites as soon as possible. Infection at all sites causes increased intensity of the digital pulse amplitudes and, at some stage, increased temperature of the hoof capsule. With subsolar or wall abscessation, pressure applied with hoof testers usually produces a painful response along the toe and sole, unless overlying horn is separated from the sensitive tissue caused by accumulation of purulent material. Radiology in these foals reveals gas accumulation in the involved area. If the distal phalanx is infected, radiolucency or sequestration may be evident. Diffuse swelling at the coronary band usually means involvement of the DIP joint, and arthrocentesis should be performed in an area remote from the swelling.
Subsolar abscesses should be drained early to prevent involvement of deeper tissues. To facilitate drainage, the foot can be soaked in hot water and bandaged with an Animalintex pack (3M Animal Care Products, St. Paul, Minnesota, United States). Both procedures soften the horn. Surgical curettage of the distal phalanx and lavage of the infected bone should be performed in foals with sequestration. A sterile, antiseptic bandage should be maintained after surgery for a minimum of 3 to 4 weeks or until a healthy keratin covering has grown over the exposed bone. Ancillary treatment includes regional digital hyperinfusion using a dilute antimicrobial solution. A good prognosis is warranted in foals with infection of the distal phalanx if the disease is detected and treated early. If more than 25% of the distal phalanx is involved, prognosis for future soundness worsens. Recurrence of infection after initial curettage requires additional surgery. Because of the limited surface area and porous nature of the distal phalanx in the foal, recurrence worsens the prognosis considerably.