CHAPTER 30 Oral cavity
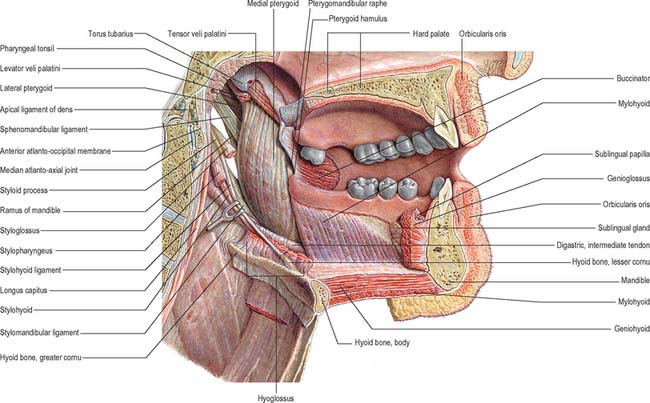
The mouth or oral cavity extends from the lips and cheeks externally to the anterior pillars of the fauces internally, where it continues into the oropharynx (Fig. 30.1). The mouth can be subdivided into the vestibule external to the teeth and the oral cavity proper internal to the teeth. The palate forms the roof of the mouth and separates the oral and nasal cavities. The floor of the mouth is formed by the mylohyoid muscles and is occupied mainly by the tongue. The lateral walls of the mouth are defined by the cheeks and retromolar regions. Three pairs of major salivary glands (parotid, submandibular and sublingual) and numerous minor salivary glands (labial, buccal, palatal, lingual) open into the mouth. The muscles in the oral cavity are associated with the lips, cheeks, floor of the mouth and tongue. The muscles of the lips and cheeks are described with the face in Chapter 29. The muscles of the soft palate are described with the pharynx in Chapter 33.
The mouth is concerned primarily with the ingestion and mastication of food, which is mainly the function of the teeth. The mouth is also associated with phonation and ventilation.
CHEEKS
The external features of the cheeks are described in Chapter 29. Internally, the mucosa of the cheek is tightly adherent to buccinator and is thus stretched when the mouth is opened and wrinkled when closed. Ectopic sebaceous glands may be evident as yellow patches (Fordyce’s spots). Their numbers increase in puberty and in later life.
Few structural landmarks are visible. The parotid duct drains into the cheek opposite the maxillary second molar tooth at a small parotid papilla. A hyperkeratinized line (the linea alba) may be seen at a position related to the occlusal plane of the teeth. In the retromolar region, a fold of mucosa containing the pterygomandibular raphe extends from the upper to the lower alveolus. The entrance to the pterygomandibular space (which contains the lingual and inferior alveolar nerves) lies lateral to this fold and medial to the ridge produced by the anterior border of the ramus of the mandible: this is the site for injection for an inferior alveolar nerve block, commonly used to anaesthetize the ipsilateral lower teeth and gums.
Vascular supply and innervation
The cheek receives its arterial blood supply principally from the buccal branch of the maxillary artery, and is innervated by cutaneous branches of the maxillary division of the trigeminal nerve, via the zygomaticofacial and infraorbital nerves, and by the buccal branch of the mandibular division of the trigeminal nerve.
LIPS
The external features of the lips are described in Chapter 29. The central part of the lips contains orbicularis oris. Internally, the labial mucosa is smooth and shiny and shows small elevations caused by underlying mucous glands.
The position and activity of the lips are important in controlling the degree of protrusion of the incisors. With normal (competent) lips, the tips of the maxillary incisors lie below the upper border of the lower lip, and this arrangement helps to maintain the ‘normal’ inclination of the incisors. When the lips are incompetent, the maxillary incisors may not be so controlled and the lower lip may even lie behind them, thus producing an exaggerated proclination of these teeth. A tight, or overactive, lip musculature may be associated with retroclined maxillary incisors. The lips are kept moist both by tongue deposition of saliva and by numerous minor salivary glands within them. These glands are liable to trauma by the teeth, particularly in the lower lip: this can produce a mucocele as a result of either extravasation of saliva into the submucosal tissues or retention of saliva within the gland or its duct.
Vascular supply and innervation
The lips are mainly supplied by the superior and inferior labial branches of the facial artery. The upper lip is innervated by superior labial branches of the infraorbital nerve and the lower lip is innervated by the mental branch of the mandibular division of the trigeminal.
ORAL VESTIBULE
The oral vestibule is a slit-like space between the lips or cheeks on one side and the teeth on the other. When the teeth occlude, the vestibule is a closed space that only communicates with the oral cavity proper in the retromolar regions behind the last molar tooth on each side. Where the mucosa that covers the alveolus of the jaw is reflected onto the lips and cheeks, a trough or sulcus is formed which is called the fornix vestibuli. A variable number of sickle-shaped folds containing loose connective tissue run across the fornix vestibuli. In the midline these are the upper and lower labial frena (or frenula). Other folds may traverse the fornix near the canines or premolars. The folds in the lower fornix are said to be more pronounced than those in the upper fornix (Fig. 30.2).
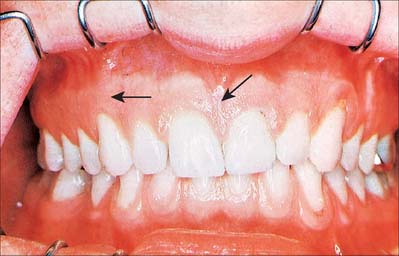
Fig. 30.2 Anterior view of the dentition in centric occlusion, with the lips retracted. Note the pale pink, stippled gingivae and the red, shiny, smooth alveolar mucosa. The degree of overbite is rather pronounced and the gingiva and its epithelial attachment have receded onto the root of the upper left canine. Note frena (arrows).
The upper labial frenulum is normally attached well below the alveolar crest. A large frenulum with an attachment near or on the crest may be associated with a midline gap (diastema) between the maxillary first incisors. This can be corrected by simple surgical removal of the frenulum (frenulectomy), because it contains no structures of clinical importance. Prominent frena may compromise the stability of dentures.
ORAL MUCOSA
The oral mucosa is continuous with the skin at the labial margins (vermilion border) and with the pharyngeal mucosa at the oropharyngeal isthmus. It varies in structure, function and appearance in different regions of the oral cavity and is traditionally divided into lining, masticatory and specialized mucosae.
LINING MUCOSA
The lining mucosa is red in colour, and covers the soft palate, ventral surface of the tongue, floor of the mouth, alveolar processes excluding the gingivae, and the internal surfaces of the lips and cheeks. It has a non-keratinized stratified squamous epithelium which overlies a loosely fibrous lamina propria, and the submucosa contains some fat deposits and collections of minor mucous salivary glands. The oral mucosa covering the alveolar bone – which supports the roots of the teeth – and the necks (cervical region) of the teeth is divided into two main components. That portion lining the lower part of the alveolus is loosely attached to the periosteum via a diffuse submucosa and is termed the alveolar mucosa. It is delineated from the masticatory gingival mucosa, which covers the upper part of the alveolar bone and the necks of the teeth, by a well-defined junction, the mucogingival junction. The alveolar mucosa appears dark red; the gingival appears pale pink. These colour differences relate to differences in the type of keratinization and the proximity to the surface of underlying small blood vessels which may sometimes be seen coursing beneath the alveolar mucosa.
MASTICATORY MUCOSA AND THE GINGIVAE
Masticatory mucosa, i.e. mucosa that is subjected to masticatory stress, is bound firmly to underlying bone or to the necks of the teeth, and forms a mucoperiosteum in the gingivae and palatine raphe. Gingival, palatal and dorsal lingual mucosae are keratinized or parakeratinized.
The gingivae may be further subdivided into the attached gingivae and the free gingivae. Attached gingivae are firmly bound to the periosteum of the alveolus and to the teeth, whereas free gingivae, which constitute approximately a 1 mm margin of the gingivae, lie unattached around the cervical region of each tooth. The free gingival groove between the free and attached gingivae corresponds roughly to the floor of the gingival sulcus which separates the inner surface of the attached gingivae from the enamel. The interdental papilla is that part of the gingivae which fills the space between adjacent teeth. The surface of the attached gingivae is characteristically stippled, although there is considerable interindividual variation in the degree of stippling, and variation according to age, sex and the health of the gingivae. The free gingivae are not stippled. A mucogingival line delineates the attached gingivae on the lingual surface of the lower jaw from the alveolar mucosa towards the floor of the mouth. There is no corresponding obvious division between the attached gingivae and the remainder of the palatal mucosa because this whole surface is orthokeratinized masticatory mucosa, which is pink.
A submucosa is absent from the gingivae and the midline palatine raphe, but is present over the rest of the hard palate. Posterolaterally it is thick where it contains mucous salivary glands and the greater palatine nerves and vessels, and it is anchored to the periosteum of the maxillae and palatine bones by collagenous septa.
Vascular supply and lymphatic drainage
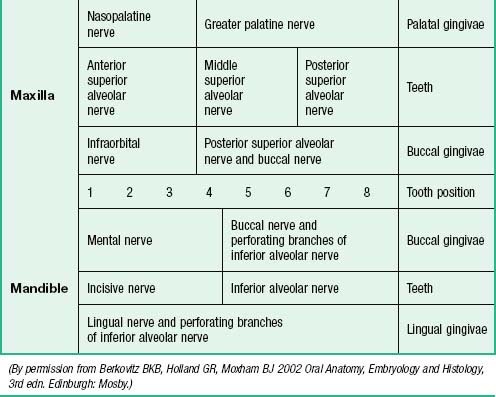
The gingival tissues derive their blood supply from the maxillary and lingual arteries. The buccal gingivae around the maxillary cheek teeth are supplied by gingival and perforating branches from the posterior superior alveolar artery and by the buccal branch of the maxillary artery. The labial gingivae of anterior teeth are supplied by labial branches of the infraorbital artery and by perforating branches of the anterior superior alveolar artery. The palatal gingivae are supplied primarily by branches of the greater palatine artery.
The buccal gingivae associated with the mandibular cheek teeth are supplied by the buccal branch of the maxillary artery and by perforating branches from the inferior alveolar artery. The labial gingivae around the anterior teeth are supplied by the mental artery and by perforating branches of the incisive artery. The lingual gingivae are supplied by perforating branches from the inferior alveolar artery and by its lingual branch, and by the main lingual artery, a branch of the external carotid artery.
No accurate description is available concerning the venous drainage of the gingivae, although it may be assumed that buccal, lingual, greater palatine and nasopalatine veins are involved. These veins run into the pterygoid plexuses (apart from the lingual veins, which may pass directly into the internal jugular veins).
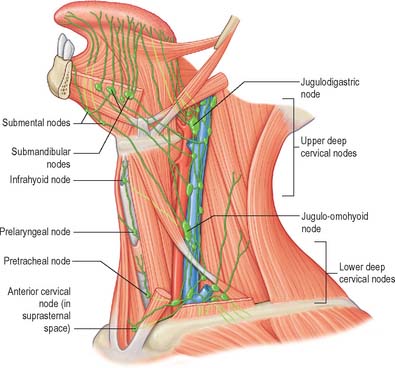
The lymph vessels of the labial and buccal gingivae of the maxillary and mandibular teeth unite to drain into the submandibular nodes, though in the labial region of the mandibular incisors they may drain into the submental lymph nodes. The lingual and palatal gingivae drain into the jugulodigastric group of nodes, either directly or indirectly through the submandibular nodes.
Innervation
The nerves supplying the gingivae in the upper jaw come from the maxillary nerve via its greater palatine, nasopalatine, and anterior, middle and posterior superior alveolar branches (see Table 30.2). Surgical division of the nasopalatine nerve, for example during the removal of an ectopic canine tooth, causes no obvious sensory deficit in the anterior part of the palate, which suggests that the territory of the greater palatine nerve reaches as far forwards as the gingivae lingual to the incisor teeth or the nerve has large regenerative potential. The mandibular nerve innervates the gingivae in the lower jaw by its inferior alveolar, lingual and buccal branches.
SPECIALIZED ORAL MUCOSA
The specialized mucosa covering the anterior two-thirds of the dorsum of the tongue is described on page 507.
OROPHARYNGEAL ISTHMUS
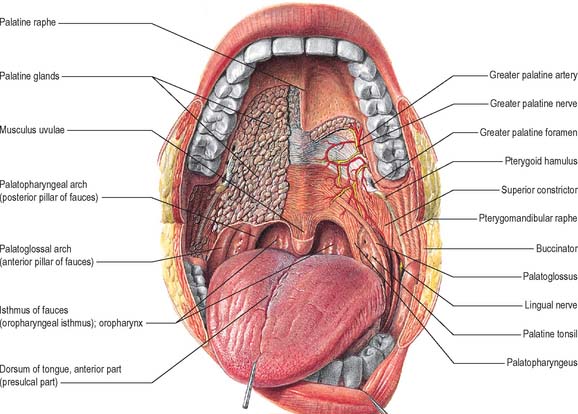
The oropharyngeal isthmus lies between the soft palate and the dorsum of the tongue, and is bounded on both sides by the palatoglossal arches. Each palatoglossal arch runs downwards, laterally and forwards, from the soft palate to the side of the tongue and consists of palatoglossus and its covering mucous membrane (Fig. 30.3). The approximation of the arches shuts off the mouth from the oropharynx, and is essential to deglutition (see Ch. 33).
FLOOR OF THE MOUTH
The floor of the mouth is a small horseshoe-shaped region situated beneath the movable part of the tongue and above the muscular diaphragm formed by the mylohyoid muscles (Fig. 30.4, see Fig. 30.7). A fold of tissue, the lingual frenulum, extends onto the inferior surface of the tongue from near the base of the tongue. It occasionally extends across the floor of the mouth to be attached onto the mandibular alveolus, known colloquially as a ‘tongue tie’; historically, this has been removed to aid speech, but the evidence for this is scanty. The submandibular salivary ducts open into the mouth at the sublingual papilla (caruncle), which is a large centrally positioned protuberance at the base of the tongue.
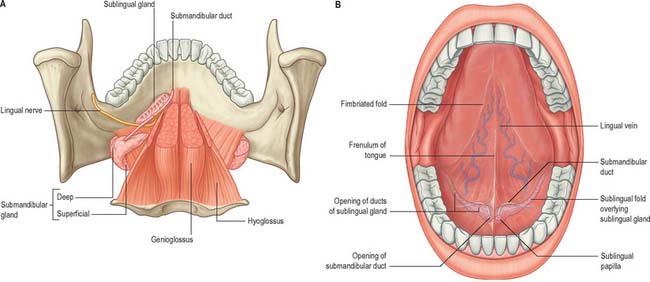
Fig. 30.4 Floor of the mouth. A, Viewed from above when the bulk of the tongue musculature has been removed. Note the relationships between the lingual nerve and the submandibular duct, and between the submandibular and sublingual salivary glands. B, The ventral surface of the tongue visible when the tip of the tongue is turned upwards.
(From Drake, Vogl and Mitchell 2005.)
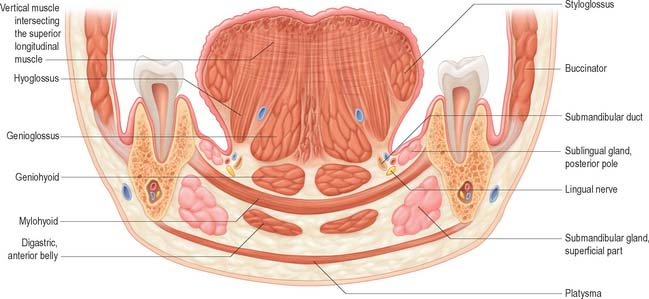
Fig. 30.7 Coronal section through the tongue, the mouth and the body of the mandible opposite the first molar tooth.
The sublingual folds lie on either side of the sublingual papilla and cover the underlying submandibular ducts and sublingual salivary glands. The blood supply of the floor of the mouth is described with the blood supply of the tongue (see p. 505). The main muscle forming the floor of the mouth is mylohyoid, with geniohyoid lying immediately above it.
Mylohyoid
Mylohyoid lies superior to the anterior belly of digastric and, with its contralateral fellow, forms a muscular floor for the oral cavity. It is a flat, triangular sheet attached to the whole length of the mylohyoid line of the mandible (Fig. 30.4A, see Fig. 30.7). The mylohyoid line is of variable length, sometimes ending before the lower third molar (wisdom) tooth. The posterior fibres of mylohyoid pass medially and slightly downwards to the front of the body of the hyoid bone near its lower border. The middle and anterior fibres from each side decussate in a median fibrous raphe that stretches from the symphysis menti to the hyoid bone. The median raphe is sometimes absent, in which case the two muscles form a continuous sheet, or it may be fused with the anterior belly of digastric. In about one-third of subjects there is a hiatus in the muscle through which a process of the sublingual gland protrudes.
The inferior (external) surface is related to platysma, the anterior belly of digastric, the superficial part of the submandibular gland, the facial and submental vessels, and the mylohyoid vessels and nerve. The superior (internal) surface is related to geniohyoid, part of hyoglossus and styloglossus, the hypoglossal and lingual nerves, the submandibular ganglion, the sublingual gland, the deep part of the submandibular gland and its duct, the lingual and sublingual vessels, and, posteriorly, the mucous membrane of the mouth.
Mylohyoid receives its arterial supply from the sublingual branch of the lingual artery, the maxillary artery, via the mylohyoid branch of the inferior alveolar artery, and the submental branch of the facial artery.
Geniohyoid
Geniohyoid is a narrow muscle which lies above the medial part of mylohyoid (see Figs 30.6, 30.10). It arises from the inferior mental spine (genial tubercle) on the back of the symphysis menti, and runs backwards and slightly downwards to attach to the anterior surface of the body of the hyoid bone. The paired muscles are contiguous and may occasionally fuse with each other or with genioglossus.
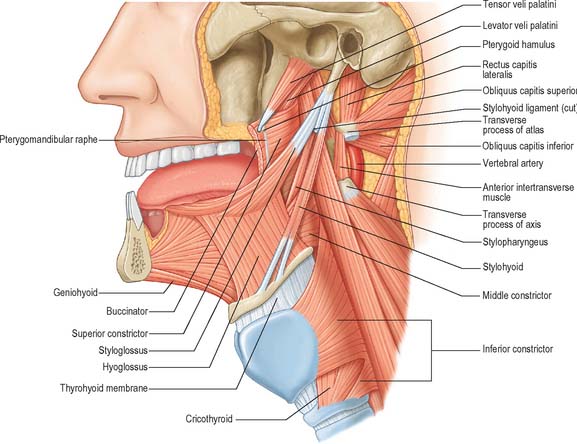
Fig. 30.6 Muscles of the tongue and pharynx. Palatoglossus is not shown here, but is depicted in Fig. 33.4.
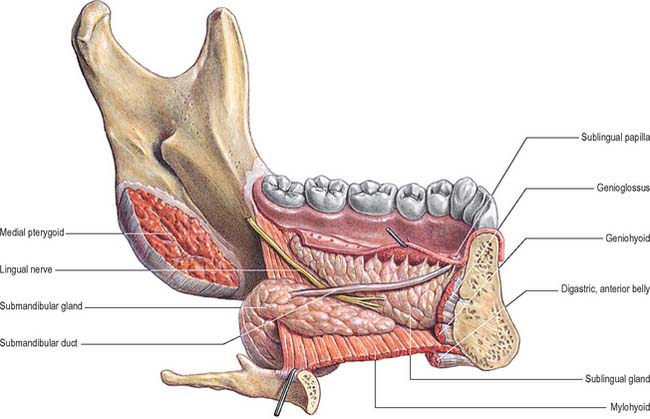
Fig. 30.10 Lingual nerve, submandibular duct, and submandibular and sublingual salivary glands in the floor of the mouth. Lingual aspect, left side.
(From Sobotta 2006.)
PALATE
The palate forms the roof of the mouth and is divisible into two regions, namely the hard palate in front and soft palate behind.
HARD PALATE
The hard palate is formed by the palatine processes of the maxillae and the horizontal plates of the palatine bones (see Fig. 30.14A). The hard palate is bounded in front and at the sides by the tooth-bearing alveolus of the upper jaw and is continuous posteriorly with the soft palate. It is covered by a thick mucosa bound tightly to the underlying periosteum. In its more lateral regions it also possesses a submucosa containing the main neurovascular bundle. The mucosa is covered by keratinized stratified squamous epithelium which shows regional variations and may be ortho- or parakeratinized.
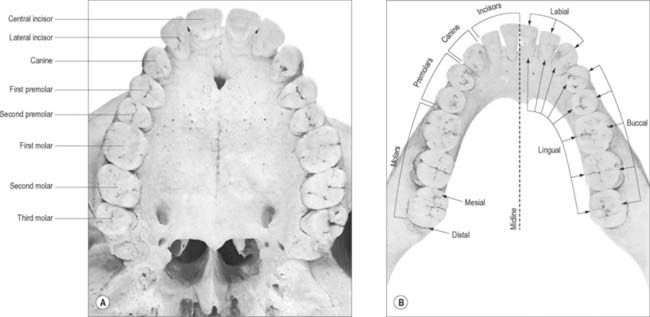
Fig. 30.14 The permanent teeth: occlusal aspect. A Upper dental arch. B, Lower dental arch. The terminology employed for the identification of teeth according to their location is shown in the lower jaw: the same terminology is used to describe the teeth in the upper jaw.
The periphery of the hard palate consists of gingivae. A narrow ridge, the palatine raphe, devoid of submucosa, runs anteroposteriorly in the midline. An oval prominence, the incisive papilla, lies at the anterior extremity of the raphe. It covers the incisive fossa at the oral opening of the incisive canal and also marks the position of the fetal nasopalatine canal. Irregular transverse ridges or rugae, each containing a core of dense connective tissue, radiate outwards from the palatine raphe in the anterior half of the hard palate: their pattern is unique.
The submucosa in the posterior half of the hard palate contains minor mucous-type salivary glands (Fig. 30.3). They secrete via numerous small ducts which often drain into a larger duct that opens bilaterally at the paired palatine foveae. These depressions, sometimes a few millimetres deep, flank the midline raphe at the posterior border of the hard palate. They provide a useful landmark for the extent of an upper denture; if not observed during construction of the denture they cause the denture to become unstable when the soft palate moves during deglutition and mastication. The upper surface of the hard palate is the floor of the nasal cavity and is covered by ciliated respiratory epithelium.
Vascular supply and lymphatic drainage of the hard palate
The palate derives its blood supply principally from the greater palatine artery, a branch of the third part of the maxillary artery. The greater palatine artery descends with its accompanying nerve in the palatine canal, where it gives off two or three lesser palatine arteries which are transmitted through lesser palatine canals to supply the soft palate and tonsil, and anastomose with the ascending palatine branch of the facial artery. The greater palatine artery emerges onto the oral surface of the palate at the greater palatine foramen adjacent to the second maxillary molar and runs in a curved groove near the alveolar border of the hard palate to the incisive canal. It ascends this canal and anastomoses with septal branches of the nasopalatine artery to supply the gingivae, palatine glands and mucous membrane.
The veins of the hard palate accompany the arteries and drain largely to the pterygoid plexus.
Innervation of the hard palate
The sensory nerves of the hard palate are the greater palatine and nasopalatine branches of the maxillary nerve, which all pass through the pterygopalatine ganglion. The greater palatine nerve descends through the greater palatine canal, emerges on the hard palate from the greater palatine foramen, runs forwards in a groove on the inferior surface of the bony palate almost to the incisor teeth and supplies the gums and the mucosa and glands of the hard palate (Fig. 30.3). It also communicates with the terminal filaments of the nasopalatine nerve. As it leaves the greater palatine canal, it supplies palatine branches to both surfaces of the soft palate. The lesser (middle and posterior) palatine nerves, which are much smaller, descend through the greater palatine canal and emerge through the lesser palatine foramina in the tubercle of the palatine bone to supply the uvula, tonsil and soft palate. The nasopalatine nerves enter the palate at the incisive foramen and are branches of the maxillary nerve which pass through the pterygopalatine ganglion to supply the anterior part of the hard palate behind the incisor teeth.
Fibres conveying taste impulses from the palate probably pass via the palatine nerves to the pterygopalatine ganglion, and travel through it without synapsing to join the nerve of the pterygoid canal and the greater petrosal nerve to the facial ganglion, where their cell bodies are situated. The central processes of these neurones traverse the sensory root of the facial nerve (nervus intermedius) to pass to the gustatory nucleus in the nucleus of the tractus solitarius. Parasympathetic postganglionic secretomotor fibres from the pterygopalatine ganglion run with the nerves to supply the palatine mucous glands.
TONGUE
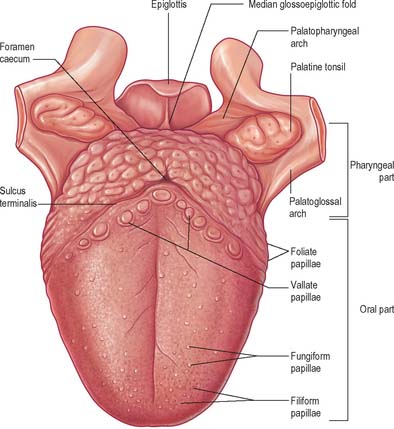
The tongue is a highly muscular organ of deglutition, taste and speech. It is partly oral and partly pharyngeal in position, and is attached by its muscles to the hyoid bone, mandible, styloid processes, soft palate and the pharyngeal wall. It has a root, an apex, a curved dorsum and an inferior surface. Its mucosa is normally pink and moist, and is attached closely to the underlying muscles. The dorsal mucosa is covered by numerous papillae, some of which bear taste buds. Intrinsic muscle fibres are arranged in a complex interlacing pattern of longitudinal, transverse, vertical and horizontal fasciculi and this allows great mobility. Fasciculi are separated by a variable amount of adipose tissue which increases posteriorly. The root of the tongue is attached to the hyoid bone and mandible, and between them it is in contact inferiorly with geniohyoid and mylohyoid. The dorsum (posterosuperior surface) is generally convex in all directions at rest. It is divided by a V-shaped sulcus terminalis into an anterior, oral (presulcal) part which faces upwards, and a posterior, pharyngeal (postsulcal) part which faces posteriorly. The anterior part forms about two-thirds of the length of the tongue. The two limbs of the sulcus terminalis run anterolaterally to the palatoglossal arches from a median depression, the foramen caecum, which marks the site of the upper end of the embryonic thyroid diverticulum (thyroglossal duct). The oral and pharyngeal parts of the tongue differ in their mucosa, innervation and developmental origins.
ORAL (PRESULCAL) PART
The presulcal part of the tongue is located in the floor of the oral cavity. It has an apex touching the incisor teeth, a margin in contact with the gums and teeth, and a superior surface (dorsum) related to the hard and soft palates. On each side, in front of the palatoglossal arch, there are four or five vertical folds, the foliate papillae, which represent vestiges of larger papillae found in many other mammals. The dorsal mucosa has a longitudinal median sulcus and is covered by filiform, fungiform and circumvallate papillae (Fig. 30.5). The mucosa on the inferior (ventral) surface is smooth, purplish and reflected onto the oral floor and gums: it is connected to the oral floor anteriorly by the lingual frenulum. The deep lingual vein, which is visible, lies lateral to the frenulum on either side. The plica fimbriata (fimbriated fold), a fringed mucosal ridge directed anteromedially towards the apex of the tongue, lies lateral to the vein. This part of the tongue develops from the lingual swellings of the mandibular arch and from the tuberculum impar, and this embryological derivation explains its sensory innervation.
PHARYNGEAL (POSTSULCAL) PART
The postsulcal part of the tongue constitutes its base and lies posterior to the palatoglossal arches. Although it forms the anterior wall of the oropharynx, it is described here for convenience. Its mucosa is reflected laterally onto the palatine tonsils and pharyngeal wall, and posteriorly onto the epiglottis by a median and two lateral glossoepiglottic folds which surround two depressions or valleculae. The pharyngeal part of the tongue is devoid of papillae, and exhibits low elevations. There are underlying lymphoid nodules which are embedded in the submucosa and collectively termed the lingual tonsil. The ducts of small seromucous glands open on the apices of these elevations. The postsulcal part of the tongue develops from the hypobranchial eminence. On the rare occasions that the thyroid gland fails to migrate away from the tongue during development, it remains in the postsulcal part of the tongue as a functioning lingual thyroid gland.
MUSCLES OF THE TONGUE
The tongue is divided by a median fibrous septum, attached to the body of the hyoid bone. There are extrinsic and intrinsic muscles in each half, the former extending outside the tongue and moving it bodily, the latter wholly within it and altering its shape. The extrinsic musculature consists of four pairs of muscles, namely genioglossus, hyoglossus, styloglossus (and chondroglossus) and palatoglossus.
Genioglossus
Genioglossus is triangular in sagittal section, lying near and parallel to the midline. It arises from a short tendon attached to the superior genial tubercle behind the mandibular symphysis, above the origin of geniohyoid. From this point it fans out backwards and upwards (Fig. 30.6). The inferior fibres of genioglossus are attached by a thin aponeurosis to the upper anterior surface of the hyoid body near the midline (a few fasciculi passing between hyoglossus and chondroglossus to blend with the middle constrictor of the pharynx). Intermediate fibres pass backwards into the posterior part of the tongue, and superior fibres ascend forwards to enter the whole length of the ventral surface of the tongue from root to apex, intermingling with the intrinsic muscles. The muscles of opposite sides are separated posteriorly by the lingual septum. Anteriorly they are variably blended by decussation of fasciculi across the midline. The attachment of the genioglossi to the genial tubercles prevents the tongue from sinking back and obstructing respiration, therefore anaesthetists pull the mandible forward to obtain the full benefit of this connection.
Hyoglossus
Hyoglossus is thin and quadrilateral, and arises from the whole length of the greater cornu and the front of the body of the hyoid bone (Fig. 30.6). It passes vertically up to enter the side of the tongue between styloglossus laterally and the inferior longitudinal muscle medially. Fibres arising from the body of the hyoid overlap those from the greater cornu.
Hyoglossus is related at its superficial surface to the digastric tendon, stylohyoid, styloglossus and mylohyoid, the lingual nerve and submandibular ganglion, the sublingual gland, the deep part of the submandibular gland and duct, the hypoglossal nerve and the deep lingual vein. By its deep surface it is related to the stylohyoid ligament, genioglossus, the middle constrictor and the inferior longitudinal muscle of the tongue, and the glossopharyngeal nerve. Posteroinferiorly it is separated from the middle constrictor by the lingual artery. This part of the muscle is in the lateral wall of the pharynx, below the palatine tonsil. Passing deep to the posterior border of hyoglossus are, in descending order: the glossopharyngeal nerve, stylohyoid ligament and lingual artery.
Chondroglossus
Sometimes described as a part of hyoglossus, this muscle is separated from it by some fibres of genioglossus, which pass to the side of the pharynx. It is about 2 cm long, and arises from the medial side and base of the lesser cornu and the adjoining part of the body of the hyoid. It ascends to merge into the intrinsic musculature between hyoglossus and genioglossus. A small slip occasionally springs from the cartilago triticea and enters the tongue with the posterior fibres of hyoglossus.
Styloglossus
Styloglossus is the shortest and smallest of the three styloid muscles (Fig. 30.6). It arises from the anterolateral aspect of the styloid process near its apex, and from the styloid end of the stylomandibular ligament. Passing downwards and forwards, it divides at the side of the tongue into a longitudinal part, which enters the tongue dorsolaterally to blend with the inferior longitudinal muscle in front of hyoglossus, and an oblique part, overlapping hyoglossus and decussating with it.
Stylohyoid ligament
The stylohyoid ligament is a fibrous cord which extends from the tip of the styloid process to the lesser cornu of the hyoid bone (Fig. 30.6). It gives attachment to some fibres of styloglossus and the middle constrictor of the pharynx and is closely related to the lateral wall of the oropharynx. Below it is overlapped by hyoglossus. The ligament is derived embryologically from the second branchial arch. It may be partially calcified.
Palatoglossus
Palatoglossus is closely associated with the soft palate in function and innervation, and is described with the other palatal muscles (see Ch. 33).
Intrinsic muscles
The intrinsic muscles are the bilateral superior and inferior longitudinal, the transverse and the vertical (Fig. 30.7).
Superior longitudinal
The superior longitudinal muscle constitutes a thin stratum of oblique and longitudinal fibres lying beneath the mucosa of the dorsum of the tongue. It extends forwards from the submucous fibrous tissue near the epiglottis and from the median lingual septum to the lingual margins. Some fibres are inserted into the mucous membrane.
Inferior longitudinal
The inferior longitudinal muscle is a narrow band of muscle close to the inferior lingual surface between genioglossus and hyoglossus. It extends from the root of the tongue to the apex. Some of its posterior fibres are connected to the body of the hyoid bone. Anteriorly it blends with styloglossus.
Transverse
The transverse muscles pass laterally from the median fibrous septum to the submucous fibrous tissue at the lingual margin, blending with palatopharyngeus.
Vertical
The vertical muscles extend from the dorsal to the ventral aspects of the tongue in the anterior borders.
The intrinsic muscles alter the shape of the tongue. Thus, contraction of the superior and inferior longitudinal muscles tend to shorten the tongue, but the former also turns the apex and sides upwards to make the dorsum concave, while the latter pulls the apex down to make the dorsum convex. The transverse muscle narrows and elongates the tongue, while the vertical muscle makes it flatter and wider. Acting alone or in pairs and in endless combination, the intrinsic muscles give the tongue precise and highly varied mobility, important not only in alimentary function but also in speech.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE OF THE TONGUE
Lingual artery
The tongue and the floor of the mouth are supplied chiefly by the lingual artery, which arises from the anterior surface of the external carotid artery. It passes between hyoglossus and the middle constrictor of the pharynx to reach the floor of the mouth accompanied by the lingual veins and the glossopharyngeal nerve. At the anterior border of hyoglossus, the lingual artery bends sharply upwards (Fig. 30.8). It is covered by the mucosa of the tongue and lies between genioglossus medially and the inferior longitudinal muscle laterally. Near the tip of the tongue, it anastomoses with its contralateral fellow; this contribution is important in maintaining the blood supply to the tongue in any surgical resection of the tongue. The branches of the lingual artery form a rich anastomotic network, which supplies the musculature of the tongue, and a very dense submucosal plexus. Named branches of the lingual artery in the floor of the mouth are the dorsal lingual, sublingual and deep lingual arteries.
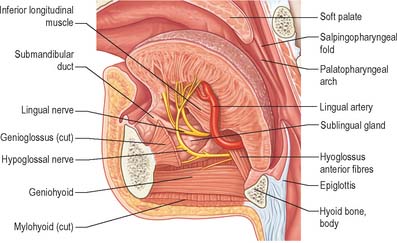
Fig. 30.8 Left half of the tongue, viewed from the medial side, showing the lingual artery and ramifications of the hypoglossal and lingual nerves.
Dorsal lingual arteries
The dorsal lingual arteries are usually two or three small vessels. They arise medial to hyoglossus and ascend to the posterior part of the dorsum of the tongue. The vessels supply its mucous membrane, and the palatoglossal arch, tonsil, soft palate and epiglottis. They anastomose with their contralateral fellows.
Sublingual artery
The sublingual artery arises at the anterior margin of hyoglossus. It passes forward between genioglossus and mylohyoid to the sublingual gland, and supplies the gland, mylohyoid and the buccal and gingival mucous membranes. One branch pierces mylohyoid and joins the submental branches of the facial artery. Another branch courses through the mandibular gingivae to anastomose with its contralateral fellow. A single artery arises from this anastomosis and enters a small foramen (lingual foramen) on the mandible, situated in the midline on the posterior aspect of the symphysis immediately above the genial tubercles.
Deep lingual artery
The deep lingual artery is the terminal part of the lingual artery and is found on the inferior surface of the tongue near the lingual frenulum.
In addition to the lingual artery, the tonsillar and ascending palatine branches of the facial and ascending pharyngeal arteries also supply tissue in the root of the tongue. In the region of the valleculae, epiglottic branches of the superior laryngeal artery anastomose with the inferior dorsal branches of the lingual artery.
Lingual veins
The lingual veins are formed from the union of the dorsal lingual and deep lingual veins and the vena comitans of the hypoglossal nerve. The veins draining the tongue follow two routes. Dorsal lingual veins drain the dorsum and sides of the tongue, join the lingual veins accompanying the lingual artery between hyoglossus and genioglossus, and empty into the internal jugular vein near the greater cornu of the hyoid bone. The deep lingual vein begins near the tip of the tongue and runs back just beneath the mucous membrane on the inferior surface of the tongue. It joins a sublingual vein from the sublingual salivary gland near the anterior border of hyoglossus and forms the vena comitans nervi hypoglossi, which run back with the hypoglossal nerve between mylohyoid and hyoglossus to join the facial, internal jugular or lingual vein. The lingual veins usually join the facial and retromandibular veins (anterior division) to form the common facial vein, which drains into the internal jugular vein.
Lymphatic drainage
The mucosa of the pharyngeal part of the dorsal surface of the tongue contains many lymphoid follicles aggregated into dome-shaped groups, the lingual tonsils. Each group is arranged around a central deep crypt, or invagination, which opens onto the surface epithelium. The ducts of mucous glands open into the bases of the crypts. Small isolated follicles also occur beneath the lingual mucosa. The lymphatic drainage of the tongue can be divided into three main regions, marginal, central and dorsal. The anterior region of the tongue drains into marginal and central vessels, and the posterior part of the tongue behind the circumvallate papillae drains into the dorsal lymph vessels. The more central regions may drain bilaterally, and this must be borne in mind when planning to remove malignant tumours of the tongue that are approaching the midline. If the tumour has a propensity for lymphatic spread, both cervical chains may be involved.
Marginal vessels
Marginal vessels from the apex of the tongue and the lingual frenulum area descend under the mucosa to widely distributed nodes. Some vessels pierce mylohyoid as it contacts the mandibular periosteum to enter either the submental or anterior or middle submandibular nodes, or else to pass anterior to the hyoid bone to the jugulo-omohyoid node. Vessels arising in the plexus on one side may cross under the frenulum to end in contralateral nodes. Efferent vessels of median submental nodes pass bilaterally. Some vessels pass inferior to the sublingual gland and accompany the companion vein of the hypoglossal nerve to end in jugulodigastric nodes. One vessel often descends further to reach the jugulo-omohyoid node, and passes either superficial or deep to the intermediate tendon of digastric.
Vessels from the lateral margin of the tongue cross the sublingual gland, pierce mylohyoid and end in the submandibular nodes. Others end in the jugulodigastric or jugulo-omohyoid nodes. Vessels from the posterior part of the lingual margin traverse the pharyngeal wall to the jugulodigastric lymph nodes (Fig. 30.9).
Central vessels
The regions of the lingual surface draining into the marginal or central vessels are not distinct. Central lymphatic vessels ascend between the fibres of the two genioglossi; most pass between the muscles and diverge to the right or left to follow the lingual veins to the deep cervical nodes, especially the jugulodigastric and jugulo-omohyoid nodes. Some pierce mylohyoid to enter the submandibular nodes.
Dorsal vessels
Vessels draining the postsulcal region and the circumvallate papillae run posteroinferiorly. Those near the median plane may pass bilaterally. They turn laterally, join the marginal vessels and all pierce the pharyngeal wall, passing around the external carotid arteries to reach the jugulodigastric and jugulo-omohyoid lymph nodes. One vessel may descend posterior to the hyoid bone, perforating the thyrohyoid membrane to end in the jugulo-omohyoid node.
INNERVATION OF THE TONGUE
The muscles of the tongue, with the exception of palatoglossus, are supplied by the hypoglossal nerve. Palatoglossus is supplied via the pharyngeal plexus (see Ch. 33). The pathways for proprioception associated with the tongue musculature are unknown, but presumably may involve the lingual, glossopharyngeal or hypoglossal nerves, and the cervical spinal nerves which communicate with the hypoglossal nerve.
The sensory innervation of the tongue reflects its embryological development: the anterior two-thirds is derived from first-arch mesenchyme and the posterior one-third from third-arch mesenchyme. The nerve of general sensation to the anterior two-thirds is the lingual nerve, which also carries taste sensation derived from the chorda tympani branch of the facial nerve. The nerve supplying both general and taste sensation to the posterior one-third is the glossopharyngeal nerve. An additional area in the region of the valleculae is supplied by the internal laryngeal branch of the vagus nerve.
Lingual nerve
The lingual nerve is sensory to the mucosa of the floor of the mouth, mandibular lingual gingivae and mucosa of the presulcal part of the tongue (excluding the circumvallate papillae). It also carries postganglionic parasympathetic fibres from the submandibular ganglion to the sublingual and anterior lingual glands.
The lingual nerve arises from the posterior trunk of the mandibular nerve in the infratemporal fossa (see Fig. 31.13A) where it is joined by the chorda tympani branch of the facial nerve and often by a branch of the inferior alveolar nerve. It then passes below the mandibular attachment of the superior pharyngeal constrictor and pterygomandibular raphe, closely applied to the periosteum of the medial surface of the mandible, until it lies opposite the distal (posterior) root of the third molar tooth, where it is covered only by the gingival mucoperiosteum. At this point it can contact the lingual cortical plate and may be at the level of, or higher than, the alveolar bone crest. It next passes medial to the mandibular attachment of mylohyoid, which carries it progressively away from the mandible, and separates it from the alveolar bone covering the mesial root of the third molar tooth, and then passes downward and forward on the deep surface of mylohyoid (i.e. the surface nearer the mucosa covering the floor of the mouth), crossing the lingual sulcus beneath the mucosa. In this position it lies on the deep portion of the submandibular gland which bulges over the top of the posterior border of mylohyoid. It passes below the submandibular duct which crosses it from medial to lateral, and curves upward, forward and medially to enter the tongue (Figs 30.8, 30.10). Within the tongue the lingual nerve lies first on styloglossus and then the lateral surface of hyoglossus and genioglossus, before dividing into terminal branches that supply the overlying lingual mucosa. The lingual nerve is connected to the submandibular ganglion (see Fig. 31.15) by two or three branches, and also forms connecting loops with twigs of the hypoglossal nerve at the anterior margin of hyoglossus.
The lingual nerve is at risk during surgical removal of (impacted) lower third molars, and, after such operations, up to 0.8% of patients may develop lingual sensory disturbance, which may persist in 0.3% (Robinson & Smith 1996). The nerve is also at risk during operations to remove the submandibular salivary gland, because the duct must be dissected from the lingual nerve, and because its connection to the submandibular ganglion pulls it into the operating field.
Glossopharyngeal nerve
The glossopharyngeal nerve is distributed to the posterior one-third of the tongue and the circumvallate papillae. It communicates with the lingual nerve. The course of the glossopharyngeal nerve in the neck is described in Chapter 28.
Hypoglossal nerve
The course of the hypoglossal nerve in the neck is described in Chapter 28. After crossing the loop of the lingual artery a little above the tip of the greater cornu of the hyoid, it inclines upwards and forwards on hyoglossus, passing deep to stylohyoid, the tendon of digastric and the posterior border of mylohyoid. Between mylohyoid and hyoglossus the hypoglossal nerve lies below the deep part of the submandibular gland, the submandibular duct and the lingual nerve, with which it communicates. It then passes onto the lateral aspect of genioglossus, continuing forwards in its substance as far as the tip of the tongue (Fig. 30.8). It distributes fibres to styloglossus, hyoglossus and genioglossus and to the intrinsic muscles of the tongue. If the nerve suffers either iatrogenic or pathological damage, the tongue, on protrusion, will deviate towards the unaffected side and there may be wasting of the affected side.
Special sensory innervation of the tongue
The sense of taste is dependent on scattered groups of sensory cells, the taste buds, which occur in the oral cavity and pharynx and are particularly plentiful on the lingual papillae of the dorsal lingual mucosa.
Dorsal lingual mucosa
The dorsal mucosa is somewhat thicker than the ventral and lateral mucosae, is directly adherent to underlying muscular tissue with no discernible submucosa, and is covered by numerous papillae. The dorsal epithelium consists of a superficial stratified squamous epithelium, which varies from non-keratinized stratified squamous epithelium posteriorly, to fully keratinized epithelium overlying the filiform papillae more anteriorly. These features probably reflect the fact that the apex of the tongue is subject to greater dehydration than the posterior and ventral parts and is subject to more abrasion during mastication. The underlying lamina propria is a dense fibrous connective tissue, with numerous elastic fibres, and is continuous with similar tissue extending between the lingual muscle fasciculi. It contains numerous vessels and nerves from which the papillae are supplied, and also large lymph plexuses and lingual glands.
Lingual papillae
Lingual papillae are projections of the mucosa covering the dorsal surface of the tongue (Fig. 30.5). They are limited to the presulcal part of the tongue, produce its characteristic roughness and increase the area of contact between the tongue and the contents of the mouth. There are four principal types, named filiform, fungiform, foliate and circumvallate papillae, and all except the filiform papillae bear taste buds. Papillae are more visible in the living when the tongue is dry.
Filiform papillae
Filiform papillae are minute, conical or cylindrical projections which cover most of the presulcal dorsal area, and are arranged in diagonal rows that extend anterolaterally, parallel with the sulcus terminalis, except at the lingual apex, where they are transverse. They have irregular cores of connective tissue and their epithelium, which is keratinized, may split into whitish fine secondary processes (Fig. 30.11). They appear to function to increase the friction between the tongue and food, and facilitate the movement of particles by the tongue within the oral cavity.
Fungiform papillae
Fungiform papillae occur mainly on the lingual margin but also irregularly on the dorsal surface, where they may occasionally be numerous (Fig. 30.11). They differ from filiform papillae because they are larger, rounded and deep red in colour, this last reflecting their thin, non-keratinized epithelium and highly vascular connective tissue core. Each usually bears one or more taste buds on its apical surface.
Foliate papillae
Foliate papillae lie bilaterally in two zones at the sides of the tongue near the sulcus terminalis, each formed by a series of red, leaf-like mucosal ridges, covered by a non-keratinized epithelium. They bear numerous taste buds.
Circumvallate papillae
Circumvallate papillae are large cylindrical structures, varying in number from 8 to 12, which form a V-shaped row immediately in front of the sulcus terminalis on the dorsal surface of the tongue. Each papilla, 1–2 mm in diameter, is surrounded by a slight circular mucosal elevation (vallum or wall) which is separated from the papilla by a circular sulcus (Figs 30.12, 30.13). The papilla is narrower at its base than its apex and the entire structure is generally covered with non-keratinized stratified squamous epithelium. Numerous taste buds are scattered in both walls of the sulcus, and small serous glands (of von Ebner) open into the sulcal base.
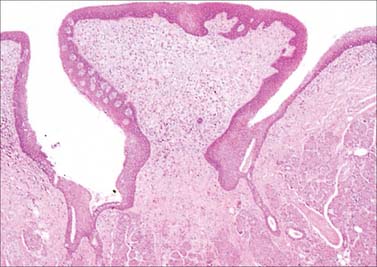
Fig. 30.12 Section through a circumvallate papilla. Serous glands (of von Ebner) empty via ducts into the base of the trench and numerous taste buds are contained within the stratified epithelium of the papillary wall (pale structures on the inner wall of the cleft, left side).
(By permission from Young B, Heath JW 2000 Wheater’s Functional Histology. Edinburgh: Churchill Livingstone.)
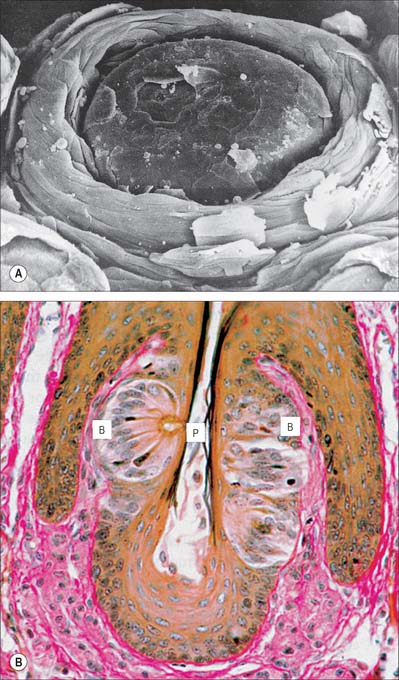
Fig. 30.13 Circumvallate papilla. A, Scanning electron micrograph showing a circumvallate papilla surrounded by a trench. B, Section of circumvallate papilla showing pale barrel-shaped taste buds (B) in its walls. P, apical pore.
(A, By courtesy of S Franey and by permission from Berkovitz BKB, Holland GR, Moxham BJ 2002 Oral Anatomy, Embryology and Histology, 3rd edn. Edinburgh: Mosby; B, by permission from Dr JB Kerr, Monash University, from Kerr JB 1999 Atlas of Functional Histology. London: Mosby.)
Taste buds
Taste buds are microscopic barrel-shaped epithelial structures which contain chemosensory cells in synaptic contact with the terminals of gustatory nerves. They are numerous on all types of lingual papillae (except filiform papillae), particularly on their lateral aspects. Taste buds are not restricted to the papillae, and are scattered over almost the entire dorsal and lateral surfaces of the tongue and, rarely, on the epiglottis and lingual aspect of the soft palate. Each taste bud is linked by synapses at its base to one of three cranial nerves which carry taste, i.e. the facial, glossopharyngeal or vagus. They share some physiological features with neurones, for example action potential generation and synaptic transmission, and are therefore often referred to as paraneurones.
There is considerable individual variation in the distribution of taste buds in humans. They are most abundant on the posterior parts of the tongue, especially around the walls of the circumvallate papillae and their surrounding sulci, where there is an average of 250 taste buds for each of the 8–12 papillae. Over 1000 taste buds are distributed over the sides of the tongue, particularly over the more posterior folds of the two foliate papillae, whereas they are rare, and sometimes even absent, on fungiform papillae (≤3 per papilla). Taste buds have been described on the fetal epiglottis and soft palate but most disappear from these sites during postnatal development.
Microstructure of taste buds
Each taste bud is a barrel-shaped cluster of 50–150 fusiform cells which lies within an oval cavity in the epithelium and converges apically on a gustatory pore, a 2 μm wide opening on the mucosal surface. The whole structure is about 70 μm in height by 40 μm across and is separated by a basal lamina from the underlying lamina propria. A small fasciculus of afferent nerve fibres penetrates the basal lamina and spirals around the sensory cells. Chemical substances dissolved in the oral saliva diffuse through the gustatory pores of the taste buds to reach the taste receptor cell membranes, where they cause membrane depolarization.
Innervation of taste buds
Individual nerve fibres branch to give a complex distribution of taste bud innervation. Each fibre may have many terminals, which may spread to innervate widely separated taste buds or may innervate more than one sensory cell in each bud. Conversely, individual buds may receive the terminals of several different nerve fibres. These convergent and divergent patterns of innervation may be of considerable functional importance.
The gustatory nerve for the anterior part of the tongue, excluding the circumvallate papillae, is the chorda tympani, which travels via the lingual nerve. In most individuals, taste fibres run in the chorda tympani to cell bodies in the facial ganglion, but occasionally they diverge to the otic ganglion, which they reach via the greater petrosal nerve. Taste buds in the inferior surface of the soft palate are supplied mainly by the facial nerve, through the greater petrosal nerve, pterygopalatine ganglion and lesser palatine nerve: they may also be supplied by the glossopharyngeal nerve. Taste buds in the circumvallate papillae, postsulcal part of the tongue and in the palatoglossal arches and the oropharynx are innervated by the glossopharyngeal nerve, and those in the extreme pharyngeal part of the tongue and epiglottis receive fibres from the internal laryngeal branch of the vagus.
Each taste bud receives two distinct classes of fibre: one branches in the periphery of the bud to form a perigemmal plexus, the other forms an intragemmal plexus within the bud itself which innervates the bases of the receptor cells. The perigemmal fibres contain various neuropeptides including calcitonin gene-related peptide (CGRP) and substance P, and appear to represent free sensory endings. Intragemmal fibres branch within the taste bud and each forms a series of synapses.
Central connections
On entering the brainstem, gustatory afferents constitute the tractus solitarius and terminate in the rostral third of the nucleus solitarius of the medulla (see Ch. 19).
Taste discrimination
Gustatory receptors detect four main categories of taste sensation, classified as salty, sweet, sour and bitter; other taste qualities have been suggested, including metallic and umami (Japanese: taste typified by monosodium glutamate). Although it is commonly stated that particular areas of the tongue are specialized to detect these different tastes, evidence indicates that all areas of the tongue are responsive to all taste stimuli. Each afferent nerve fibre is connected to widely separated taste buds and may respond to several different chemical stimuli. Some respond to all four classic categories, others to fewer or only one. Within a particular class of tastes, receptors are also differentially sensitive to a wide range of similar chemicals. Moreover, taste buds alone are able to detect only a rather restricted range of chemical substances in aqueous solution. It is difficult to separate the perceptions of taste and smell, because the oral and nasal cavities are continuous. Indeed, much of what is perceived as taste is the result of airborne odorants from the oral cavity which pass through the nasopharynx to the olfactory area above it.
Perceived sensations of taste are the results of the processing (presumably central) of a complex pattern of responses from particular areas of the tongue.
Autonomic innervation of the tongue
The parasympathetic innervation of the various glands of the tongue is from the chorda tympani branch of the facial nerve which synapses in the submandibular ganglion: postganglionic branches are distributed to the lingual mucosa via the lingual nerve. The postganglionic sympathetic supply to lingual glands and vessels arises from the carotid plexus and enters the tongue through plexuses around the lingual arteries. Isolated nerve cells, perhaps postganglionic parasympathetic neurones, have been reported in the postsulcal region: presumably they innervate glandular tissue and vascular smooth muscle.
TEETH
INTRODUCTION AND TERMINOLOGY
Humans have two generations of teeth: the deciduous (primary) dentition and the permanent (secondary) dentition. The first deciduous teeth erupt into the mouth at about 6 months after birth and all of the deciduous teeth have erupted by 3 years of age. The first permanent molar erupts at or around 6 years, and thence the deciduous teeth are exfoliated one by one to be replaced by their permanent successors. A complete permanent dentition is present when the third molars erupt at or around the age of 18–21 years. In the complete deciduous dentition there are 20 teeth, five in each jaw quadrant. In the complete permanent dentition there are 32 teeth, eight in each jaw quadrant.
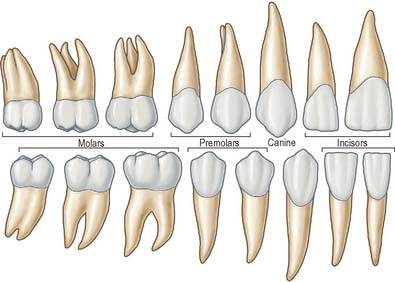
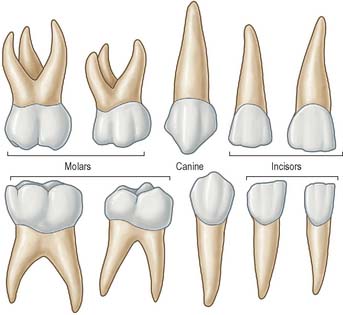
There are three basic tooth forms in both dentitions: incisiform, caniniform and molariform. Incisiform teeth (incisors) are cutting teeth, and have thin, blade-like crowns. Caniniform teeth (canines) are piercing or tearing teeth, and have a single, stout, pointed, cone-shaped crown. Molariform teeth (molars and premolars) are grinding teeth and possess a number of cusps on an otherwise flattened biting surface. Premolars are bicuspid teeth that are restricted to the permanent dentition and replace the deciduous molars.
The tooth-bearing region of the jaws can be divided into four quadrants, the right and left maxillary and mandibular quadrants. A tooth may thus be identified according to the quadrant in which it is located (e.g. a right maxillary tooth or a left mandibular tooth). In both the deciduous and permanent dentitions, the incisors may be distinguished according to their relationship to the midline. Thus, the incisor nearest the midline is the central (first) incisor and the incisor that is more laterally positioned is termed the lateral (second) incisor. The permanent premolars and the permanent and deciduous molars can also be distinguished according to their mesiodistal relationships. The molar most mesially positioned is designated the first molar, and the one behind it is the second molar. In the permanent dentition, the tooth most distally positioned is the third molar. The mesial premolar is the first premolar, and the premolar behind it is the second premolar.
The terminology used to indicate tooth surfaces is shown in Fig. 30.14B. The aspect of teeth adjacent to the lips or cheeks is termed labial or buccal, that adjacent to the tongue being lingual (or palatal in the maxilla). Labial and lingual surfaces of an incisor meet medially at a mesial surface and laterally at a distal surface, terms which are also used to describe the equivalent surfaces of premolar and molar (postcanine) teeth. On account of the curvature of the dental arch, mesial surfaces of postcanine teeth are directed anteriorly and distal surfaces are directed posteriorly. Thus, the point of contact between the central incisors is the datum point for mesial and distal. The biting or occlusal surfaces of postcanine teeth are tuberculated by cusps which are separated by fissures forming a pattern characteristic of each tooth. The biting surface of an incisor is the incisal edge.
TOOTH MORPHOLOGY
There are two incisors, a central and a lateral, in each half jaw or quadrant (Figs 30.14, 30.15). In labial view, the crowns are trapezoid, the maxillary incisors (particularly the central) are larger than the mandibular. The biting or incisal edges initially have three tubercles or mamelons, which are rapidly removed by wear. In mesial or distal view, their labial profiles are convex while their lingual surfaces are concavo-convex (the convexity near the cervical margin is caused by a low ridge or cingulum, which is prominent only on upper incisors). The roots of incisors are single and rounded in maxillary teeth, but flattened mesiodistally in mandibular teeth. The upper lateral incisor may be congenitally absent or may have a reduced form (peg-shaped lateral incisor).
Behind each lateral incisor is a canine tooth with a single cusp (hence the American term cuspid) instead of an incisal edge. The maxillary canine is stouter and more pointed than the mandibular canine whose cusp tip is inclined lingually. The canine root, which is the longest of any tooth, produces a bulge (canine eminence) on the alveolar bone externally, particularly in the upper jaw. Although canines usually have single roots, those of the lower jaw may sometimes be bifid.
Distal to the canines are two premolars, each with a buccal and lingual cusp (hence the term bicuspid). The occlusal surfaces of the maxillary premolars are oval (the long axis is buccopalatal) and a mesiodistal fissure separates the two cusps. In buccal view, premolars resemble the canines but are smaller. The maxillary first premolar can have two roots (one buccal, one palatal) but may have one, and very rarely three, roots (two buccal and one palatal) and this makes the tooth more likely to fracture on removal. The maxillary second premolar usually has one root. The occlusal surfaces of the mandibular premolars are more circular or squarer than those of the upper premolars. The buccal cusp of the mandibular first premolar towers above a diminutive lingual cusp to which it is connected by a ridge separating the mesial and distal occlusal pits. In the mandibular second premolar a mesiodistal fissure usually separates a buccal from two smaller lingual cusps. Each lower premolar has one root, but very rarely the root of the first is bifid. Lower second premolars fail to develop in about 2% of individuals.
Posterior to the premolars are three molars whose size decreases distally. Each has a large rhomboid (upper jaw) or rectangular (lower jaw) occlusal surface with four or five cusps. The maxillary first molar has a cusp at each corner of its occlusal surface and the mesiopalatal cusp is connected to the distobuccal by an oblique ridge. A smaller cusplet or tubercle (cusplet of Carabelli) usually appears on the mesiopalatal cusp (most commonly in Caucasian races). The tooth has three widely separated roots, two buccal, of which the mesiobuccal is larger and broader and the distobuccal is rounder and smaller, and one large palatal: their proximity to the maxillary air sinus is thought to be the reason first molar roots are wide apart and second and third molar roots are converged. The smaller maxillary second molar has a reduced or occasionally absent distopalatal cusp. Its three roots show varying degrees of fusion. The maxillary third molar, the smallest, is very variable in form. It usually has three cusps (the distopalatal being absent) and commonly the three roots are fused.
The mandibular first molar has three buccal and two lingual cusps on its rectangular occlusal surface, the smallest cusp being distal. The cusps of this tooth are all separated by fissures. It has two widely separated roots, one mesial and one distal. The smaller mandibular second molar is like the first, but has only four cusps (it lacks the distal cusp of the first molar) and its two roots are closer together. The mandibular third molar is smaller still and, like the upper third molar, is variable in form. Its crown may resemble that of the lower first or second molar and its roots are frequently fused. As it erupts anterosuperiorly, the third molar is often impacted against the second molar, producing food packing which subsequently causes inflammation. The maxillary third molar erupts posteroinferiorly and is rarely impacted. One or more third molars (upper or lower) fail to develop in up to 30% of individuals.
Impacted mandibular third molars
In many subjects there is a disproportion between the size of the teeth and the size of the jaws such that there is insufficient space for all the teeth to erupt. As the third mandibular molar teeth (the wisdom teeth) are the last to erupt, they are often impeded in their eruption and either become impacted or remain unerupted deeply within the jaw bone. If the tooth is completely covered by bone and mucosa, it is very unlikely to cause any symptoms, and the subject remains unaware of its presence unless the tooth is seen on a routine dental radiograph. Very rarely, the surrounding dental follicle may undergo cystic degeneration which can ‘hollow out’ the jaw, usually the mandible, to a considerable degree. The developing cyst may displace the tooth as it expands and the tooth may end up as far away as the condylar neck or coronoid process.
More commonly, the erupting wisdom tooth erupts partially before impacting against the distal aspect of the second molar. When this occurs, symptoms are common due to recurrent soft tissue inflammation and infection around the partially erupted tooth. This condition is known as pericoronitis and if the infecting organism is virulent, the infection may rapidly spread into the adjacent tissue spaces as described elsewhere. The tooth only merits surgical removal if the patient suffers a severe bout or multiple bouts of pericoronitis. Surgery is not immediately indicated because it is associated with a degree of morbidity: the lingual and inferior alveolar nerves, which are often in close proximity to the tooth, may be damaged during its removal. The lingual nerve passes across the surface of the periosteum lingually to the lower wisdom tooth, separated from the tooth only by a cortical plate of bone no thicker than an egg shell. Damage to this nerve results in altered sensation to the ipsilateral side of the tongue. The root apices of the impacted tooth often lie immediately above the mandibular canal, and removal of the tooth can result in damage to the underlying neurovascular bundle, causing altered sensation in its dermatome. Maxillary third molars are only rarely impacted.
Deciduous teeth
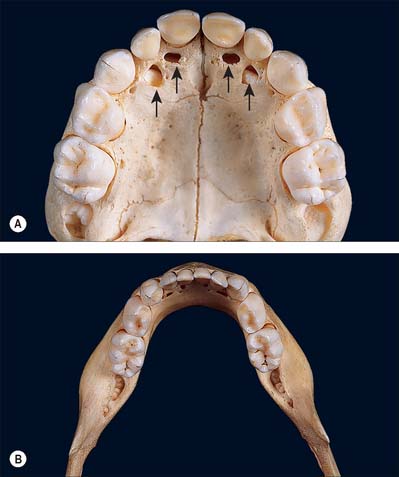
The incisors, canine and premolars of the permanent dentition replace two deciduous incisors, a deciduous canine and two deciduous molars in each jaw quadrant (Figs 30.16, 30.17). The deciduous incisors and canine are shaped like their successors but are smaller and whiter and become extremely worn in older children. The deciduous second molars resemble the first permanent molars rather than their successors, the premolars. The upper first deciduous molar has a triangular occlusal surface (its rounded ‘apex’ is palatal) and a fissure separates a double buccal cusp from the palatal cusp. The lower first deciduous molar is long and narrow; its two buccal cusps are separated from its two lingual cusps by a zigzagging mesiodistal fissure. Both deciduous molars have large buccal protuberances on their mesial aspect. Upper deciduous molars have three roots (fusion of the palatal and distobuccal root is commonplace), and lower deciduous molars have two roots. These roots diverge more than those of permanent teeth because each developing premolar tooth crown is accommodated directly under the crown of its deciduous predecessor. The roots of deciduous teeth are progressively resorbed by osteoclast-like cells (odontoclasts) prior to being shed.
Eruption of teeth
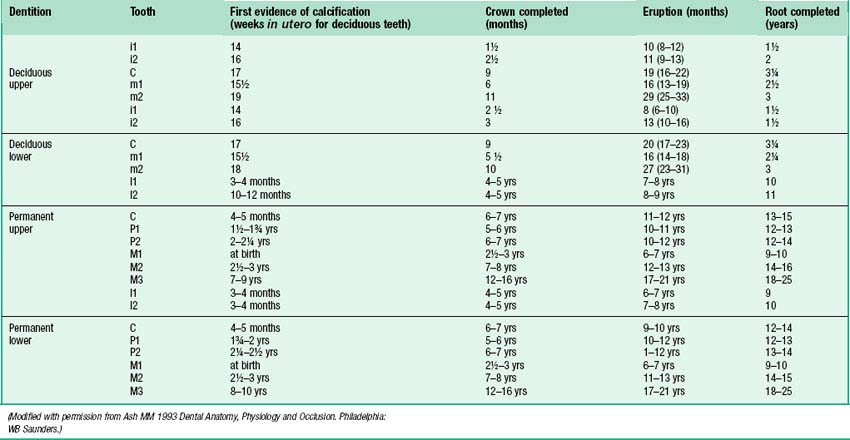
Information on the sequence of development and eruption of teeth into the oral cavity (Fig. 30.18) is important in clinical practice and also in forensic medicine and archaeology. The tabulated data provided in Table 30.2 are largely based on European-derived populations and there is evidence of ethnic variation. When a permanent tooth erupts, about two-thirds of the root is formed and it takes about another 3 years for the root to be completed. For deciduous teeth, root completion is more rapid (Table 30.1). The developmental stages of initial calcification and crown completion are less affected by environmental influences than is eruption, the timing of which may be modified by several factors such as early tooth loss and severe malnutrition.
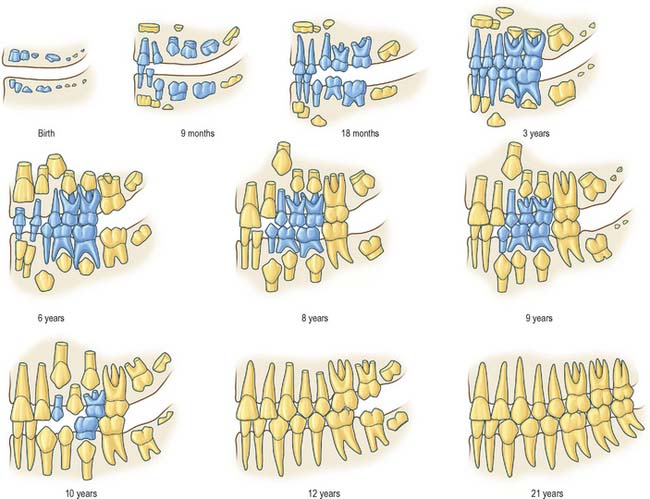
Fig. 30.18 Development of the deciduous (blue) and permanent (yellow) teeth.
(Modified with permission from Schour I, Massler M 1941 The development of the human dentition. J Am Dent Assoc 28: 1153–1160.)
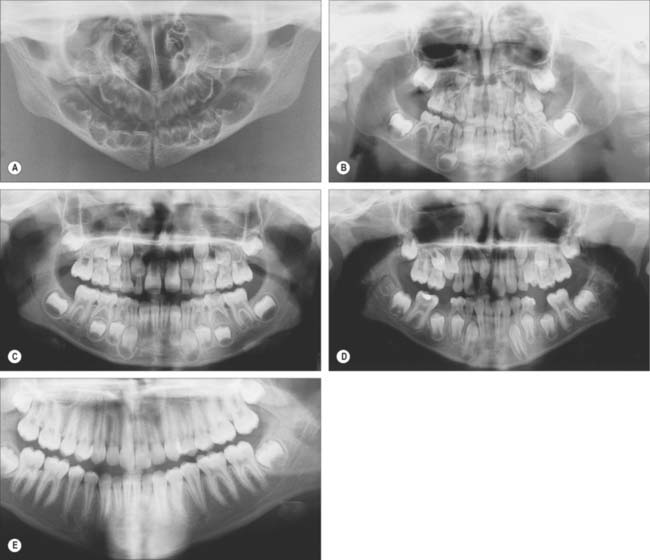
Fig. 30.19 shows the panoramic appearance of the dentition seen with orthopantomograms at the time of birth, 2½, 6½, 10 and 16 years of age.
Dental alignment and occlusion
It is possible to bring the jaws together so that the teeth meet or occlude in many positions. When opposing occlusal surfaces meet with maximal ‘intercuspation’ (i.e. maximum contact), the teeth are said to be in centric occlusion. In this position the lower teeth are normally opposed symmetrically and lingually with respect to the upper. Some important features of centric occlusion in a normal (idealized) dentition may be noted. Each lower postcanine tooth is slightly in front of its upper equivalent and the lower canine occludes in front of the upper. Buccal cusps of the lower postcanine teeth lie between the buccal and palatal cusps of the upper teeth. Thus, the lower postcanine teeth are slightly lingual and mesial to their upper equivalents. Lower incisors bite against the palatal surfaces of upper incisors, the latter normally obscuring about one-third of the crowns of the lower. This vertical overlap of incisors in centric occlusion is the overbite. The extent to which upper incisors are anterior to lowers is termed the overjet. In the most habitual jaw position, the resting posture, the teeth are slightly apart, the gap between them being the freeway space or interocclusal clearance. During mastication, especially with lateral jaw movements, the food is comminuted, which facilitates the early stages of digestion.
The ideal occlusion is a rather subjective concept. If there is an ideal occlusion, it can only presently be defined in broad functional terms. Therefore, the occlusion can be considered ‘ideal’ when the teeth are aligned such that the masticatory loads are within physiological range and act through the long axes of as many teeth in the arch as possible; mastication involves alternating bilateral jaw movements (and not habitual, unilateral biting preferences as a result of adaptation to occlusal interference); lateral jaw movements occur without undue mechanical interference; in the rest position of the jaw, the gap between teeth (the freeway space) is correct for the individual concerned; the tooth alignment is aesthetically pleasing to its possessor.
Variations from the ideal occlusion may be termed malocclusions (although these could be regarded as normal, since they are more commonly found in the population: 75% of the population in the USA has some degree of occlusal ‘disharmony’). However, the majority of malocclusions should be regarded as anatomical variations rather than abnormalities, as they are rarely involved in masticatory dysfunction or pain, although they may be aesthetically displeasing.
Variations in tooth number, size and form
The incidence of variation in number and form, which is often related to race, is rare in deciduous teeth but not uncommon in the permanent dentition. One or more teeth may fail to develop, a condition known as hypodontia. Conversely, additional or supernumerary teeth may form, producing hyperdontia. The third permanent molar is the most frequently missing tooth: in one study, one or more third molars failed to form in 32% of Chinese, 24% of English Caucasians and 2.5% of West Africans. In declining order of incidence, other missing teeth are maxillary lateral incisors, maxillary or mandibular second premolars, mandibular central incisors and maxillary first premolars.
Hyperdontia affects the maxillary arch much more commonly than the mandibular dentition. The extra teeth are usually situated on the palatal aspect of the permanent incisors or distal to the molars. More rarely, additional premolars develop. Supernumerary teeth in the incisor region can often impede the eruption of the permanent teeth and indeed this is often the first indication of their presence. A supernumerary tooth situated between the central incisors is known as a mesiodens. Teeth may be unusually large (macrodontia) or small (microdontia). For example, the crowns of maxillary central incisors may be abnormally wide mesiodistally; in contrast, a common variant of the maxillary lateral incisor has a small, peg-shaped crown. Epidemiological studies reveal that hyperdontia tends to be associated with macrodontia and hypodontia with microdontia, the most severely affected individuals representing the extremes of a continuum of variation. Together with family studies, this indicates that the causation is multifactorial, combining polygenic and environmental influences.
Some variations in the form of teeth, being characteristic of race, are of anthropological and forensic interest. Mongoloid dentitions tend to have shovel-shaped maxillary incisors with enlarged palatal marginal ridges. The additional cusp of Carabelli is commonly found on the mesiopalatal aspect of maxillary first permanent or second deciduous molars in Caucasian but rarely in Mongoloid dentitions. In African races, the mandibular second permanent molar often has five rather than four cusps.
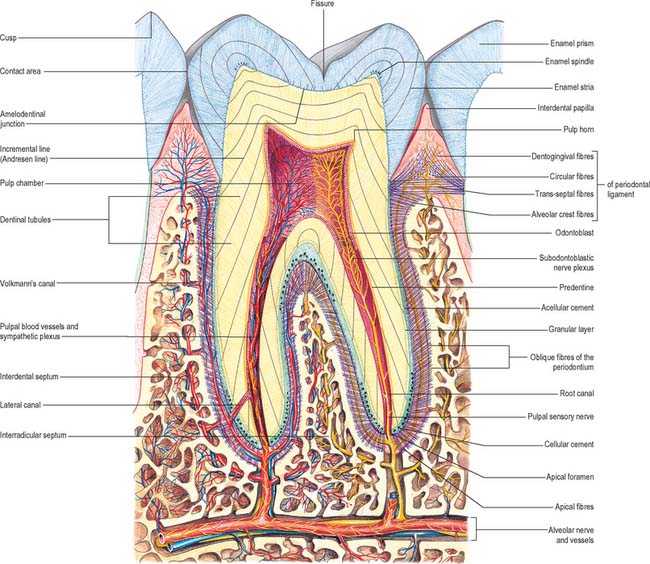
General arrangement of dental tissues
A tooth consists of a crown covered by very hard translucent enamel and a root covered by yellowish bone-like cementum (Fig. 30.20A). These meet at the neck or cervical margin. A longitudinal ground section (Fig. 30.20B) reveals that the body of a tooth is mostly dentine (ivory) with an enamel covering up to about 2 mm thick, while the cementum is much thinner. The dentine surrounds a central pulp cavity, expanded at its coronal end into a pulp chamber and narrowed in the root as a pulp canal, opening at or near its tip by an apical foramen, occasionally multiple. The pulp is a connective tissue, continuous with the periodontal ligament via the apical foramen. It contains vessels for the support of the dentine and sensory nerves.
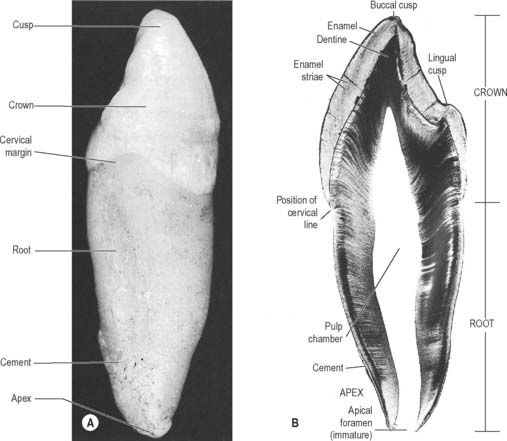
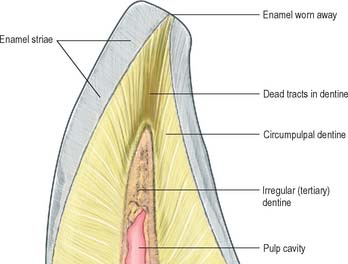
Fig. 30.20 The principal parts of a tooth. A, An extracted upper right canine tooth viewed from its mesial aspect. The root is covered by cement (partially removed), and the curved cervical margin is convex towards the cusp of the tooth. B, A ground section of a young (permanent) lower first premolar tooth sectioned in the buccolingual longitudinal plane, photographed with transmitted light. The enamel striae are incremental lines of enamel growth (compare with Fig. 30.24). Within the dentine the lines of the dentinal tubules are visible, forming S-shaped curves in the apical region but straighter in the root.
The root is surrounded by alveolar bone, its cementum separated from the osseous socket (alveolus) by the connective tissue of the periodontal ligament, approximately 0.2 mm thick (Fig. 30.21). Coarse bundles of collagen fibres, embedded at one end in cementum, cross the periodontal ligament to enter the osseous alveolar wall, these bundles of collagen being termed Sharpey’s fibres. Near the cervical margin, the tooth, periodontal ligament and adjacent bone are covered by the gingiva. On its internal surface the gingiva is attached to the tooth surface by the junctional epithelium, a zone of profound clinical importance because just above it is a slight recess, the gingival sulcus. As the sulcus is not necessarily self-cleansing, dental plaque may accumulate in it and this predisposes to periodontal disease.
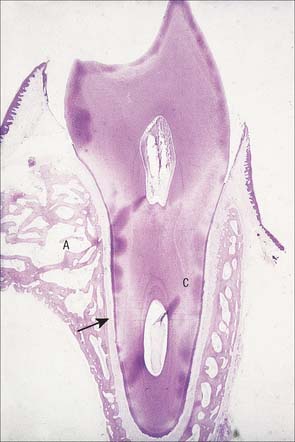
Fig. 30.21 Demineralized section of a tooth with its root attached to the surrounding bone by the periodontal ligament. A, alveolar bone; C, root of tooth lined by cementum; arrow indicates periodontal space.
(By courtesy of Dr D Lunt.)
Enamel
Enamel is an extremely hard and rigid material which covers the crowns of teeth. It is a heavily mineralized epithelial cell secretion, containing 95–96% by weight crystalline apatites (88% by volume) and less than 1% organic matrix. The organic matrix comprises mainly unique enamel proteins, amelogenins and non-amelogenins such as enamelins, tuftelins. Although comprising a very small percentage of the weight and volume of enamel, the organic matrix permeates the whole of enamel. As its formative cells are lost from the surface during tooth eruption, enamel is incapable of further growth. Repair is limited to the remineralization of minute carious lesions.
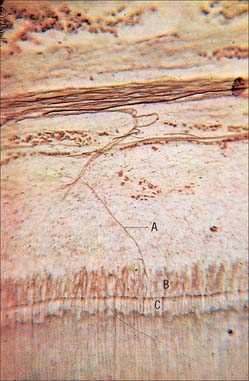
Enamel reaches a maximum thickness of 2.5 mm over cusps and thins at the cervical margins. It is composed of closely packed enamel prisms or rods. In longitudinal section, enamel prisms extend from close to the enamel–dentine junction to within 20 μm of the surface, where they are generally replaced by prismless (non-prismatic, aprismatic) enamel (Fig. 30.22A). In cross-section the prisms are mainly horseshoe-shaped and are arranged in rows that are staggered such that the tails of the prisms in one row lie between the heads of the prism in the row above (prism pattern 3) (Fig. 30.22B) and the tails are directed rootwards. The appearance of prism boundaries results from sudden changes in crystallite orientation. Prisms have a diameter of approximately 5 μm, and are packed with flattened hexagonal hydroxyapatite crystals, far larger than those found in collagen-based mineralized tissues.
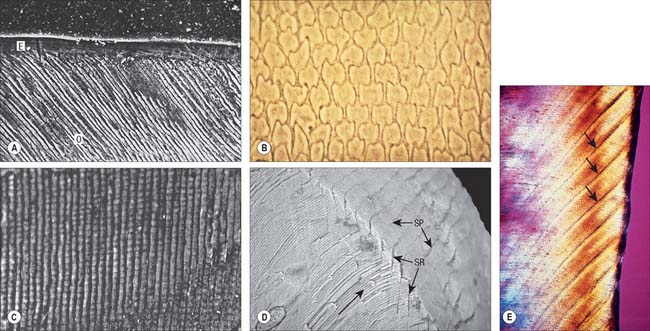
Fig. 30.22 Enamel. A, Scanning electron micrograph of acid-etched outer enamel (O) showing enamel prisms, approximately 5 μm wide. A layer of prismless enamel (E) is evident on the surface. B, Ground cross-section showing cross-sectional keyhole (fish scale) appearance of enamel prisms (pattern 3). C, Ground longitudinal section viewed with phase contrast showing prisms (vertical lines) and cross-striations (horizontal lines). D, Low-power scanning electron micrograph illustrating the relationship of enamel prism direction (long arrow), striae of Retzius (SR) and surface perikymata (SP). E, Ground longitudinal section showing enamel striae (arrows). Viewed between crossed polarizing filters.
(A, by courtesy of Professor D Whittaker; B, by permission from Berkovitz BKB, Holland GR, Moxham BJ 2002 Oral Anatomy, Embryology and Histology, 3rd edn. Edinburgh: Mosby; C, by permission from Berkovitz BKB, Holland GR, Moxham BJ 2002 Oral Anatomy, Embryology and Histology, 3rd edn. Edinburgh: Mosby; D, by permission from: Kelley J, Smith TM. Age at first molar emergence in early Miocene Afropithecus turkanensis and life-history evolution in the Hominoidea. Journal of Human Evolution 2003; 44:307–329; E, by courtesy of Dr AD Beynon)
Two types of incremental lines are visible in enamel, short-term and long-term. At intervals that average 4 μm – with a range of 2–3 μm at the enamel dentine junction and 4–6 μm in outer enamel – along its length, each prism is crossed by a line, probably reflecting diurnal swelling and shrinking in diameter during its growth. This short-term daily growth line is known as a cross-striation (Fig. 30.22C). The longer-term incremental lines pass from the enamel–dentine junction obliquely to the surface, where they end in shallow furrows, perikymata, visible on newly erupted teeth (Fig. 30.22D). Each line, known as an enamel stria of Retzius, represents a period that ranges between 6 and 12 days of enamel growth with a modal value of 9 days. This is known as an individual’s periodicity and it is constant in all teeth from the same individual but varies considerably between individuals (Reid & Dean 2006) (Fig. 30.22E). A prominent striation, the neonatal line, is formed in teeth whose mineralization spans birth. Neonatal lines are present in the enamel and dentine of teeth mineralizing at the time of birth (all the deciduous teeth and the first permanent molars; see Fig. 30.14) and are therefore of forensic importance, indicating that an infant has survived for a few days after birth. They reflect a disturbance in mineralization during the first few days after birth. Other accentuated lines can be observed throughout enamel growth; these are generally reflections in disturbances in ameloblast secretion caused by illness or nutritional deficiencies. In serious cases these disturbances will manifest as hypoplasia (enamel thinning on the tooth surface).
Dentine
Dentine is a yellowish avascular tissue which forms the bulk of a tooth. It is a tough and compliant composite material, with a mineral content of 70% dry weight (largely crystalline hydroxyapatite with some calcium carbonate) and 20% organic matrix (type I collagen, glycosaminoglycans and phosphoproteins). Its conspicuous feature is the regular pattern of microscopic dentinal tubules, 3 μm in diameter, which extend from the pulpal surface to the enamel–dentine junction. The tubules show lateral and terminal branching near the enamel–dentine junction (Fig. 30.23A) and may project a short distance into the enamel (enamel spindles). Each tubule encloses a single cytoplasmic process of an odontoblast whose cell body lies in a pseudostratified layer which lines the pulpal surface. Processes are believed to extend the full thickness of dentine in newly erupted teeth, but in older teeth they may be partly withdrawn and occupy only the pulpal third, while the outer regions contain probably only extracellular fluid. The diameter of the dentine tubule is narrowed by deposition of peritubular dentine. This is different from normal dentine (intertubular dentine) because it is more highly mineralized and lacks a collagenous matrix. Peritubular dentine can therefore be identified by microradiography (Fig. 30.23B). In time, it may completely fill the tubule, a process which gives rise to translucent dentine and which commences in the apical region of the root.
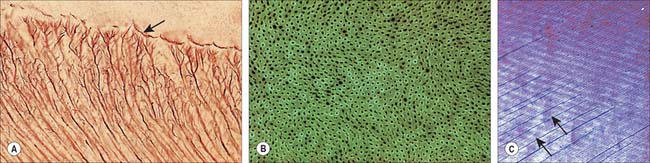
Fig. 30.23 Dentine. A, Ground longitudinal section showing branching of dentine tubules near the enamel–dentine junction (arrow). B, Microradiograph of transversely sectioned dentinal tubules surrounded by a more radiopaque and therefore more mineralized zone of peritubular dentine. C, Ground longitudinal section viewed in polarized light showing alternate light and dark bands representing long-period incremental lines (Andresen lines). The bands are approximately orientated at right angles to the direction of the dentinal tubules (arrows).
(By permission from Berkovitz BKB, Holland GR, Moxham BJ 2002 Oral Anatomy, Embryology and Histology, 3rd edn. Edinburgh: Mosby.)
The outermost zone (10–20 μm) of dentine differs in the crown and the root. In the crown it is referred to as mantle dentine and differs in the orientation of its collagen fibres. In the root, the peripheral zone presents a granular layer – with less overall mineral – beyond which is a hyaline layer which lacks a tubular structure and may function to produce a good bond between the cementum and dentine.
Dentine is formed slowly throughout life, and so there is always an unmineralized zone of predentine at the surface of the mineralized dentine, adjacent to the odontoblast layer at the periphery of the pulp. Biochemical changes within the mineralizing matrix mean that preentine stains differently to the matrix of the mineralized dentine. The predentine–dentine border is generally scalloped, because dentine mineralizes both linearly and as microscopic spherical aggregates of crystals (calcospherites). Dentine, like enamel, is deposited incrementally, and exhibits both short- and long-term incremental lines. Long-term lines are known as Andresen lines and in the mid-axial region are approximately 20 μm apart: they represent increments of about 6–12 days (Fig. 30.23C). Daily incremental lines (von Ebner lines) in the mid-axial plane are typically 4 μm apart; they are considerably closer together (1–2 μm apart) at the enamel–dentine junction and in root dentine. Where mineralization spans birth (i.e. all deciduous teeth and usually the first permanent molars) a neonatal line is formed in dentine similar to that which is seen in enamel, and it signals the abrupt change in both environment and nutrition which occurs at birth. As with enamel, disturbances in growth of dentine will exhibit as accentuated lines, commonly called contour lines of Owen.
Primary dentine formation proceeds at a steady but declining rate as first the crown and then the root is completed. A slow and intermittent deposition of dentine (regular secondary dentine) continues throughout life and further reduces the size of the pulp chamber; it is distinguished from primary dentine by the reduction and haphazard arrangement of dentinal tubules within it. The presence of the odontoblast process means that dentine is a vital tissue. It responds to adverse external stimuli – such as rapidly advancing caries, excessive wear or tooth breakage – by forming poorly mineralized dead tracts, in which the odontoblasts of the affected region die and the tubules remain empty (tertiary dentine). A dead tract may be sealed from the pulp by a thin zone of sclerosed dentine and the deposition of irregular (tertiary) dentine by newly differentiated pulp cells (Fig. 30.24).
Dental pulp
Dental pulp provides the nutritive support for the synthetic activity of the odontoblast layer. It is a well-vascularized, loose connective tissue, enclosed by dentine and continuous with the periodontal ligament via apical and accessory foramina. Several thin-walled arterioles enter by the apical foramen and run longitudinally within the pulp to an extensive subodontoblastic plexus. Blood flow rate per unit volume of tissue is greater in the pulp than in other oral tissues, and tissue fluid pressures within the pulp appear to be unusually high.
As well as typical connective tissue cells, pulp uniquely contains the cell bodies of odontoblasts whose long processes occupy the dentinal tubules. Pulp also has dendritic antigen-presenting cells. Approximately 60% of pulpal collagen is type I, and the bulk of the remainder is type III. As dentine deposition increases with age, the pulp recedes until the whole of the crown may be removed without accessing the pulp.
Dental pulp is extensively innervated by unmyelinated postganglionic vasoconstrictor sympathetic nerve fibres from the superior cervical ganglion, which enter with the arterioles, and by myelinated (Aδ) and unmyelinated (C) sensory nerve fibres from the trigeminal ganglion, which traverse the pulp longitudinally and ramify as a plexus (Raschkow’s plexus) beneath the odontoblast layer (Figs 30.25, 30.26). Here, any myelinated nerve fibres lose their myelin sheaths and continue into the odontoblast layer, and some enter the dentinal tubules, especially the region beneath the cusps. Stimulation of dentine, whether by thermal, mechanical or osmotic means, evokes a pain response. Pulp nerves release numerous neuropeptides.
Cementum
Cementum is a bone-like tissue which covers the dental roots, and is 50% by weight mineralized (mainly hydroxyapatite crystals). However, unlike bone, cementum is avascular and lacks nerves. The cementum generally overlaps the enamel slightly, although it may meet it end on. Occasionally, the two tissues may fail to meet, in which case dentine is exposed in the mouth. If the exposed dentinal tubules remain patent, then the teeth may be sensitive to stimuli such as cold water. In older teeth, the root may become exposed in the mouth as a consequence of occlusal drift and gingival recession, and cementum is often abraded away by incorrect tooth brushing and dentine exposed.
Like bone, cementum is perforated by Sharpey’s fibres, which represent the attachment bundles of collagen fibres in the periodontal ligament (extrinsic fibres). New layers of cementum, which are deposited incrementally throughout life to compensate for tooth movements, incorporate new Sharpey’s fibres. Incremental lines are irregularly spaced. The first cement to be formed is thin (up to 200 μm), acellular and contains only extrinsic fibres. Cementum formed later towards the root apex is produced more rapidly and contains cementocytes lying in lacunae joined by canaliculi. The latter are mainly directed towards their source of nutrients from the periodontal ligament. This cementum contains both extrinsic fibres derived from the periodontal ligament and intrinsic fibres of cementoblastic origin which lie parallel to the surface. Varying arrangements of layering between cellular and acellular cement occur. With increasing age, cellular cement may reach a thickness of a millimetre or more around the apices and at the branching of the roots, where it compensates for the loss of enamel by attrition. Cementum is not remodelled but small areas of resorption with evidence of repair may be seen.
Forensic anatomy of teeth
In forensic medicine, dental evidence is valuable in identification of individuals, especially following mass disasters; estimation of age at death of skeletonized remains; and establishing guilt in cases of criminal injury by biting.
If teeth have been restored, extracted or replaced by a denture, an individual will have a virtually unique dentition which may have been recorded by the dentist in the form of charts, radiographs or plaster casts. Teeth are the most indestructible bodily structures and can provide identification when trauma or fire has rendered the face unrecognizable. Moreover, the chronology of crown development, eruption and root formation can be used to estimate age until the third molar is completed at about 21 years. The method is even applicable to the fetus because the weight of mineralized tissue in teeth is closely related to age from about 22 weeks’ gestation until birth. The time taken for a crown to form can be calculated from ground sections with considerable accuracy by counting the number of daily cross-striations from the neonatal line. For permanent teeth, the time taken for the crown to form can be calculated by counting the number of the enamel striae and multiplying by the individual’s periodicity. The age of adult teeth can be estimated from factors such as wear of the crown, reduction in size of the pulp and increase in thickness of cement in the apical half of the root. However, probably the most useful single characteristic is the amount of translucent dentine in the root, which is proportional to age. Such estimations are within 5–7 years of the chronological age and likely to be closer to the true age than those derived from skeletal changes.
Periodontal ligament
The principal functions of the periodontal ligament are to support the teeth, generate the force of tooth eruption and provide sensory information about tooth position and forces to facilitate reflex jaw activity. The periodontal ligament is a dense fibrous connective tissue 0.2 mm wide which contains cells associated with the development and maintenance of alveolar bone (osteoblasts and osteoclasts) and of cementum (cementoblasts and odontoclasts). It also contains a network of epithelial cells (epithelial cell rests of Malassez) which are embryological remnants of an epithelial root sheath. They have no evident function but may give rise to dental cysts.
The majority of collagen fibres of the periodontal ligament are arranged as variously oriented dense fibre bundles that connect alveolar bone and cementum and which may help to resist movement in specific directions (Fig. 30.27). About 80% of the collagen in the periodontal ligament is type I, most of the remainder is type III. The rate of turnover of collagen is probably the highest of any site in the body, for reasons which are as yet unclear. A very small volume of fibres are oxytalan fibres.
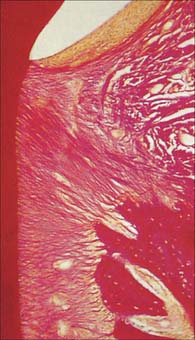
Fig. 30.27 Decalcified longitudinal section of a tooth showing groups of periodontal ligament fibres (the alveolar crest fibres and the horizontal fibres) in the region of the alveolar crest. van Gieson stain.
(By permission from Berkovitz BKB, Holland GR, Moxham BJ 2002 Oral Anatomy, Embryology and Histology, 3rd edn. Edinburgh: Mosby.)
The periodontal ligament has a rich nerve and blood supply. The nerves are both autonomic (for the vasculature) and sensory (for pain and proprioception). The majority of proprioceptive nerve endings appear to be Ruffini-like endings. The blood vessels tend to lie towards the bone side of the periodontal ligament and the capillaries are fenestrated. Tissue fluid pressures appear to be high.
Alveolar bone
That part of the maxilla or mandible which supports and protects the teeth is known as alveolar bone. An arbitrary boundary at the level of the root apices of the teeth separates the alveolar processes from the body of the mandible or the maxilla (Fig. 30.28). Like bone in other sites, alveolar bone functions as a mineralized supporting tissue, gives attachment to muscles, provides a framework for bone marrow and acts as a reservoir for ions, especially calcium. It is dependent on the presence of teeth for its development and maintenance, and requires functional stimuli to maintain bone mass. Where teeth are congenitally absent, as for example in anodontia, it is poorly developed, and it atrophies after tooth extraction, which can cause difficulties in the prosthodontic rehabilitation of patients.
Fig. 30.28 Anterior part of the left side of the mandible, with superficial bone removed on the buccal side to show roots of a number of teeth, some of which have also been sectioned vertically. Note the cortical plate of compact bone lining the sockets of the teeth (the lamina dura of radiographs: see Fig. 30.30), and the flat table of bone surmounting the interdental bone septa. In this specimen the mandibular canal is widely separated from the roots of the teeth, a variable condition.
The alveolar tooth-bearing portion of the jaws consists of outer and inner alveolar plates. The individual sockets are separated by plates of bone termed the interdental septa, while the roots of multi-rooted teeth are divided by interradicular septa. The compact layer of bone which lines the tooth socket has been called either the cribriform plate, on account of its content of vascular (Volkmann’s) canals which pass from the alveolar bone into the periodontal ligament, or bundle bone, because numerous bundles of Sharpey’s fibres pass into it from the periodontal ligament (Fig. 30.29).
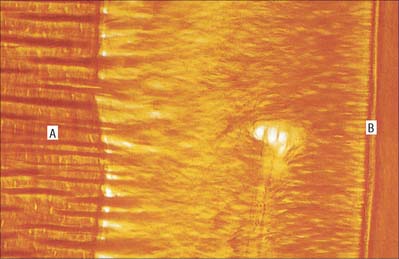
Fig. 30.29 Decalcified section of a root of a tooth showing Sharpey’s fibres from the periodontal ligament entering alveolar bone (A). The Sharpey’s fibres in bone are seen to be thicker, but less numerous, than those entering the cementum (B) on the tooth surface. van Gieson stain.
(By permission from Berkovitz BKB, Holland GR, Moxham BJ 2002 Oral Anatomy, Embryology and Histology, 3rd edn. Edinburgh: Mosby.)
In clinical radiographs, the bone lining the alveolus commonly appears as a continuous dense white line about 0.5–1 mm thick, the lamina dura (Fig. 30.30). However, this appearance gives a misleading impression of the density of alveolar bone: the X-ray beam passes tangentially through the socket wall, and so the radiopacity of the lamina dura is an indication of the quantity of bone the beam has passed through, rather than the degree of mineralization of the bone. Superimposition also obscures the Volkmann’s canals. Chronic infections of the dental pulp spread into the periodontal ligament, which leads to resorption of the lamina dura around the root apex. The presence of a continuous lamina dura around the apex of a tooth therefore usually indicates a healthy apical region (except in acute infections where resorption of bone has not yet begun).
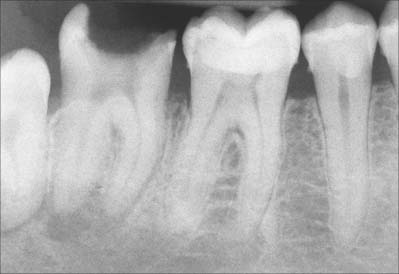
Fig. 30.30 Bite-wing radiograph of teeth and surrounding bone. Note the different radiopacities of enamel and dentine. In a healthy tooth, such as the first molar illustrated here, the lamina dura is complete and appears as a radiopaque line. In the case of the adjacent second molar tooth in which the bulk of the crown has been lost due to dental caries, an abscess has formed at the base of the tooth and as a result the lamina dura has lost its continuity.
(By courtesy of Ms Nadine White.)
On the labial and buccal aspects of upper teeth, the two cortical plates usually fuse, and there is very little trabecular bone between them, except where the buccal bone thickens over the molar teeth near the root of the zygomatic arch. It is easier and more convenient to extract upper and lower teeth by widening the tooth socket in a buccal direction due to its thinness. Anteriorly in the lower jaw, labial and lingual plates are thin, but in the molar region the buccal plate is thickened as the external oblique line. Near the lower third molar, the lingual bone is much thinner than the buccal and it is important to realize that the lingual nerve is easily damaged by poor technique.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE OF THE TEETH AND SUPPORTING STRUCTURES
The main arteries to the teeth and their supporting structures are derived from the maxillary artery, one of the two terminal branches of the external carotid artery. The upper teeth are supplied by branches from the superior alveolar arteries and the lower teeth by branches from the inferior alveolar arteries.
Superior alveolar arteries
The upper jaw is supplied by posterior, middle and anterior superior alveolar (dental) arteries. The posterior superior alveolar artery usually arises from the third part of the maxillary artery in the pterygopalatine fossa. It descends on the infratemporal surface of the maxilla, and divides to give branches that enter the alveolar canals to supply molar and premolar teeth, adjacent bone and the maxillary sinus, and other branches that continue over the alveolar process to supply the gingivae. The middle and anterior superior alveolar arteries are branches from the infraorbital artery.
The infraorbital artery often arises with the posterior superior alveolar artery. It enters the orbit posteriorly through the inferior orbital fissure and runs in the infraorbital groove and canal with the infraorbital nerve. When the small middle superior alveolar artery is present, it runs down the lateral wall of the maxillary sinus and forms anastomotic arcades with the anterior and posterior vessels, terminating near the canine tooth. The anterior superior alveolar artery curves through the canalis sinuosus to supply the upper incisor and canine teeth and the mucous membrane in the maxillary sinus. The canalis sinuosus swerves laterally from the infraorbital canal and inferomedially below it in the wall of the maxillary sinus, following the rim of the anterior nasal aperture, between the alveoli of canine and incisor teeth and the nasal cavity. It ends near the nasal septum where its terminal branch emerges. The canal may be up to 55 mm long.
Inferior alveolar artery
The inferior alveolar (dental) artery, a branch of the maxillary artery, descends in the infratemporal fossa posterior to the inferior alveolar nerve. Here, it lies between bone laterally and the sphenomandibular ligament medially. Before entering the mandibular foramen it gives off a mylohyoid branch, which pierces the sphenomandibular ligament to descend with the mylohyoid nerve in its groove on the inner surface of the ramus of the mandible. The mylohyoid artery ramifies superficially on the muscle and anastomoses with the submental branch of the facial artery. The inferior alveolar artery then traverses the mandibular canal with the inferior alveolar nerve to supply the mandibular molars and premolars and divides into the incisive and mental branches near the first premolar.
The incisive branch continues below the incisor teeth (which it supplies) to the midline, where it anastomoses with its fellow, although few anastomotic vessels cross the midline. In the canal the arteries supply the mandible, tooth sockets and teeth via branches which enter the minute hole at the apex of each root to supply the pulp. The mental artery leaves the mental foramen and supplies the chin and anastomoses with the submental and inferior labial arteries. Near its origin the inferior alveolar artery has a lingual branch, which descends with the lingual nerve to supply the lingual mucous membrane. The pattern of branching of the inferior alveolar artery reflects that of the nerves of the same name.
Arterial supply of periodontal ligaments
The periodontal ligaments supporting the teeth are supplied by dental branches of alveolar arteries. One branch enters the alveolus apically and sends two or three small rami into the dental pulp through the apical foramen, and other rami into the periodontal ligament. Interdental arteries ascend in the interdental septa, sending branches at right angles into the periodontal ligament, and terminate by communicating with gingival vessels that also supply the cervical part of the ligament. The periodontal ligament therefore receives its blood from three sources: from the apical region; ascending interdental arteries; and descending vessels from the gingivae. These vessels anastomose with each other, which means that when the pulp of a tooth is removed during endodontic treatment, the attachment tissues of the tooth remain vital.
Venous drainage of the teeth
Veins accompanying the superior alveolar arteries drain the upper jaw and teeth anteriorly into the facial vein, or posteriorly into the pterygoid venous plexus. Veins from the lower jaw and teeth collect either into larger vessels in the interdental septa or into plexuses around the root apices and thence into several inferior alveolar veins. Some of these veins drain through the mental foramen to the facial vein; others drain via the mandibular foramen to the pterygoid venous plexus.
Lymphatic drainage of the teeth
The lymph vessels from the teeth usually run directly into the ipsilateral submandibular lymph nodes. Lymph from the mandibular incisors, however, drains into the submental lymph nodes. Occasionally, lymph from the molars may pass directly into the jugulodigastric group of nodes.
INNERVATION OF THE TEETH
The regional innervation of the teeth and gingivae is shown in Table 30.2. The teeth in the upper jaw are supplied by the superior alveolar nerves, while those in the lower jaw are supplied by the inferior alveolar nerve.
Superior alveolar nerves
The teeth in the upper jaw are supplied by the three superior alveolar (dental) nerves which arise from the maxillary nerve in the pterygopalatine fossa or in the infraorbital groove and canal. The posterior superior alveolar (dental) nerve leaves the maxillary nerve in the pterygopalatine fossa and runs anteroinferiorly to pierce the infratemporal surface of the maxilla, at which point it is possible to perform a local anaesthetic block which will anaesthetize the pulps of the premolar and molar ipsilateral teeth, and then descends under the mucosa of the maxillary sinus. After supplying the lining of the sinus the nerve divides into small branches which link up as the molar part of the superior alveolar plexus, supplying twigs to the molar teeth. It also supplies a branch to the upper gingivae and the adjoining part of the cheek.
The middle superior alveolar (dental) nerve arises from the infraorbital nerve as it runs in the infraorbital groove, and runs downwards and forwards in the lateral wall of the maxillary sinus. It ends in small branches which link up with the superior dental plexus, supplying small rami to the upper premolar teeth. This nerve is variable, and it may be duplicated or triplicated or absent.
The anterior superior alveolar (dental) nerve leaves the lateral side of the infraorbital nerve near the midpoint of its canal and traverses the canalis sinuosus in the anterior wall of the maxillary sinus. It curves first under the infraorbital foramen, then passes medially towards the nose and finally turns downwards and divides into branches to supply the incisor and canine teeth. It assists in the formation of the superior dental plexus and it gives off a nasal branch, which passes through a minute canal in the lateral wall of the inferior meatus to supply the mucous membrane of the anterior area of the lateral wall as high as the opening of the maxillary sinus, and the floor of the nasal cavity. It communicates with the nasal branches of the pterygopalatine ganglion and finally emerges near the root of the anterior nasal spine to supply the adjoining part of the nasal septum.
Inferior alveolar (dental) nerve
The course of the inferior alveolar nerve in the infratemporal fossa is described in Chapter 31. Just before entering the mandibular canal the inferior alveolar nerve gives off a small mylohyoid branch which pierces the sphenomandibular ligament and enters a shallow groove on the medial surface of the mandible, following a course roughly parallel to its parent nerve. It passes below the origin of mylohyoid to lie on the superficial surface of the muscle, between it and the anterior belly of digastric, both of which it supplies. It gives a few filaments to supply the skin over the point of the chin.
In the mandibular canal, the inferior alveolar nerve runs downward and forward, generally below the apices of the teeth until below the first and second premolars where it divides into terminal incisive and mental branches. The incisive branch continues forward in a bony canal or in a plexiform arrangement, giving off branches to the first premolar, canine and incisor teeth, and the associated labial gingivae. The lower central incisor teeth receive a bilateral innervation, fibres probably crossing the midline within the periosteum to re-enter the bone via numerous canals in the labial cortical plate.
The mental nerve passes upward, backward and outward to emerge from the mandible via the mental foramen between and just below the apices of the premolar teeth. It immediately divides into three branches, two of which pass upward and forward to form an incisor plexus labial to the teeth, supplying the gingiva (and probably the periosteum). From this plexus and the dental branches, fibres turn downwards and then lingually to emerge on the lingual surface of the mandible on the posterior aspect of the symphysis or opposite the premolar teeth, probably communicating with the lingual or mylohyoid nerve. The third branch of the mental nerve passes through the intermingled fibres of depressor anguli oris and platysma to supply the skin of the lower lip and chin. Branches of the mental nerve also communicate with terminal filaments of the mandibular branch of the facial nerve.
Variations in the fascicular organization of the inferior alveolar nerve are clinically important when extracting impacted third molars. The nerve may appear as a single bundle lying a few millimetres below the roots of the teeth, or it may lie much lower and almost reach the lower border of the bone, so that it gives off a variable number of large rami which pass anterosuperiorly towards the roots before dividing to supply the teeth and interdental septa. Only rarely is it plexiform. The nerve may lie on the lingual or buccal side of the mandible (slightly more commonly on the buccal side). Even when the third molar tooth is in a normal position, the nerve may be so intimately related to it that it grooves its root. Exceptionally the nerve may be similarly related to the second molar.
Nerves may pass from the substance of temporalis to enter the mandible through the retromolar fossa or the condylar head, where they communicate with branches of the inferior alveolar nerve. Foramina occur in some 10% of retromolar fossae and infiltration in this region can abolish the sensation which occasionally remains after an inferior alveolar nerve block. Similarly, branches from the buccal, mylohyoid and lingual nerves may enter the mandible and provide additional routes of sensory transmission from the teeth. Thus, even when the inferior alveolar nerve has been anaesthetized correctly, pain may still be experienced by a patient when undergoing dental cavity preparation.
Pain sensation in teeth
The teeth are supplied by nociceptors that generate pain sensation of a very high order. The mechanism underlying this sensitivity is of considerable clinical significance and is controversial. Currently, the most widely accepted view is that fluid movements through the dentine tubules stimulate nerve endings at the periphery of the dental pulp (hydrodynamic hypothesis).
Local analgesia
It is technically possible to achieve profound regional anaesthesia by depositing local anaesthetic solution adjacent to the trigeminal nerve trunks or their branches within the infratemporal fossa. These injections can either be performed transorally – posterior superior alveolar nerve block, maxillary nerve block, inferior alveolar nerve block, lingual nerve block and mandibular nerve block – or more rarely by an external route through the skin of the face – maxillary nerve block, inferior alveolar nerve block and mandibular nerve block.
In the case of the mandible, the anterior teeth can be anaesthetized by simple diffusion techniques as the bone is relatively thin. However, this is not adequate for the cheek teeth due to the increased thickness of the bone. In this case, the inferior alveolar nerve has to be anaesthetized before it enters the inferior alveolar canal. The needle has to be placed within the pterygomandibular space to achieve a successful inferior alveolar nerve block. The lingual nerve is also usually blocked, as it lies close to the inferior alveolar nerve. Because of the other structures within the infratemporal fossa, it is vitally important that the operator has a detailed knowledge of the anatomy in this region to understand, and therefore try to avoid, the complications that may arise. Any damage to blood vessels in the infratemporal fossa, generally the pterygoid venous plexus, can lead to haematoma formation. In extreme cases, bleeding can track through the inferior orbital fissure resulting in a retrobulbar haematoma, which can result in loss of visual acuity or blindness. Intravascular injection of local anaesthetic solution (which usually contains adrenaline [epinephrine]) can have profound systemic effects and for this reason an aspirating syringe is always used to check that the needle has not entered a vessel prior to injection (vessels in this area that theoretically may be entered include the maxillary and internal carotid arteries). If the needle is placed too medially, it may enter medial pterygoid, while if directed too laterally, it may penetrate temporalis. In either case, there will be lack of anaesthesia followed later by trismus. If the needle is placed too deeply, anaesthetic solution may cause a temporary facial nerve palsy due to loss of conduction from the facial nerve in the region of the parotid gland.
SALIVARY GLANDS
Salivary glands are compound, tubuloacinar exocrine glands whose ducts open into the oral cavity. They secrete saliva, a fluid which lubricates food to assist deglutition, moistens the buccal mucosa, which is important for speech, and provides an aqueous solvent necessary for taste and a fluid seal for sucking and suckling. They also secrete digestive enzymes, e.g. salivary amylase, and antimicrobial agents, e.g. immunoglobulin A (IgA), lysozyme and lactoferrin, into saliva. Conditions where there is a significant decrease in the production of saliva (xerostomia) may result in periodontal inflammation and dental caries. An illustration of the position of the major salivary glands and their ducts is shown in Fig. 30.31.
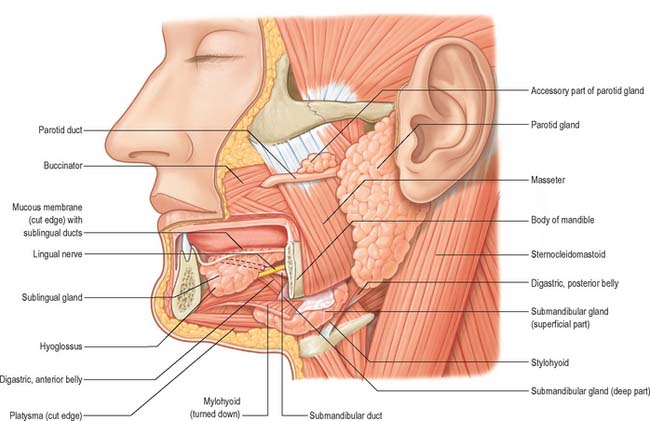
Fig. 30.31 The salivary glands of the left side. The cranial region of the superficial part of the submandibular gland has been excised and mylohyoid has been cut and turned down to expose a portion of the deep part of the gland.
The major salivary glands are the paired parotid, submandibular and sublingual glands. In addition, there are numerous minor salivary glands scattered throughout the oral mucosa and submucosa.
Approximately 0.5 L of saliva is secreted per day. Salivary flow rates are typically 0.3 mL/min when unstimulated, and rise to 1.5–2 mL/min when stimulated. Flow rate is negligible during sleep. In the unstimulated state, the parotid gland contributes 20%, the submandibular gland 65%, and the sublingual and minor salivary glands 15% of the daily output of saliva. When stimulated, the parotid contribution rises to 50%.
PAROTID GLAND
The parotid gland is the largest salivary gland. It is almost entirely serous. The parotid duct runs through the cheek and drains into the mouth opposite the maxillary second permanent molar tooth. The parotid gland is situated in front of the external ear and is described in detail in relation to the face (see Ch. 29).
SUBMANDIBULAR SALIVARY GLAND
The submandibular gland is irregular in shape and about the size of a walnut. It consists of a larger superficial and a smaller deep part, continuous with each other around the posterior border of mylohyoid. It is a seromucous (but predominantly serous) gland.
Superficial part of the submandibular gland
The superficial part of the gland is situated in the digastric triangle where it reaches forward to the anterior belly of digastric and back to the stylomandibular ligament, by which it is separated from the parotid gland. Above, it extends medial to the body of the mandible. Below, it usually overlaps the intermediate tendon of digastric and the insertion of stylohyoid. This part of the submandibular gland presents inferior, lateral and medial surfaces, and is partially enclosed between two layers of deep cervical fascia that extend from the greater cornu of the hyoid bone. The superficial layer is attached to the lower border of the mandible and covers the inferior surface of the gland. The deep layer is attached to the mylohyoid line on the medial surface of the mandible and covers the medial surface of the gland.
The inferior surface, covered by skin, platysma and deep fascia, is crossed by the facial vein and the cervical branch of the facial nerve. Near the mandible the submandibular lymph nodes are in contact with the gland and some may be embedded within it.
The lateral surface is related to the submandibular fossa on the medial surface of the body of the mandible and the mandibular attachment of medial pterygoid. The facial artery grooves its posterosuperior part, lies at first deep to the gland and then emerges between its lateral surface and the mandibular attachment of the medial pterygoid to reach the lower border of the mandible.
The medial surface is related anteriorly to mylohyoid, from which it is separated by the mylohyoid nerve and vessels and branches of the submental vessels. More posteriorly, it is related to styloglossus, the stylohyoid ligament and the glossopharyngeal nerve, which separate it from the pharynx. In its intermediate part the medial surface is related to hyoglossus, from which it is separated by styloglossus, the lingual nerve, submandibular ganglion, hypoglossal nerve and deep lingual vein (sequentially from above down). Below, the medial surface is related to the stylohyoid muscle and the posterior belly of digastric.
Deep part of the submandibular gland
The deep part of the gland extends forwards to the posterior end of the sublingual gland. It lies between mylohyoid inferolaterally, hyoglossus and styloglossus medially, the lingual nerve superiorly, and the hypoglossal nerve and deep lingual vein inferiorly (Fig. 30.10).
Vascular supply and lymphatic drainage
The arteries supplying the gland are branches of the facial and lingual arteries. The lymph vessels drain into the deep cervical group of lymph nodes (particularly the jugulo-omohyoid node), interrupted by the submandibular nodes.
Innervation
The secretomotor supply to the submandibular gland is derived from the submandibular ganglion. This is a small, fusiform body which lies on the upper part of hyoglossus. There are additional ganglion cells in the hilum of the gland. Like the ciliary, pterygopalatine and otic ganglia, the submandibular is a peripheral parasympathetic ganglion. It is superior to the deep part of the submandibular gland and inferior to the lingual nerve, and is suspended from the latter by anterior and posterior filaments. Though related to the lingual nerve, the ganglion is connected functionally with the facial nerve and its chorda tympani branch.
As with the other cranial parasympathetic ganglia, there are three roots associated with the submandibular ganglion. The motor, parasympathetic root is the posterior filament which connects it to the lingual nerve. This conveys preganglionic fibres from the superior salivatory nucleus which travel in the facial, chorda tympani and lingual nerves to the ganglion, where they synapse. The postganglionic fibres are secretomotor to the submandibular and sublingual salivary glands. Some fibres may also reach the parotid gland. The sympathetic root is derived from the plexus on the facial artery. It consists of postganglionic fibres from the superior cervical ganglion which traverse the submandibular ganglion without synapsing. They are vasomotor to the blood vessels of the submandibular and sublingual glands. Five or six branches from the ganglion supply the submandibular gland and its duct. Other fibres pass through the anterior filament that connects the submandibular gland to the lingual nerve and are carried to the sublingual and anterior lingual glands. Sensory fibres are derived from the lingual nerve.
Submandibular duct
The submandibular duct is usually 5 cm long and has a thinner wall than the parotid duct. It begins from numerous tributaries in the superficial part of the gland and emerges from the medial surface of this part of the gland behind the posterior border of mylohyoid. It traverses the deep part of the gland, and then passes at first up and slightly back for approximately 5 mm, this sharp bend over the free edge of mylohyoid being known as the genu of the duct. It then runs forwards between mylohyoid and hyoglossus passing between the sublingual gland and genioglossus to open in the floor of the mouth on the summit of the sublingual papilla at the side of the frenulum of the tongue (Figs 30.4, 30.10). It lies between the lingual and hypoglossal nerves on hyoglossus, but, at the anterior border of the muscle, it is crossed laterally by the lingual nerve, terminal branches of which ascend on its medial side. As the duct traverses the deep part of the gland it receives small tributaries draining this part of the gland. It has been suggested previously that the genu of the duct predisposes to the stasis of saliva and thereby encourages salivary stone (sialolith) formation, but this is somewhat controversial and largely unproven.
Like the parotid gland, the duct system of the submandibular gland can be visualized by sialography (Fig. 30.32).
SUBLINGUAL SALIVARY GLAND
The sublingual gland is the smallest of the main salivary glands: each gland is narrow, flat, shaped like an almond, and weighs approximately 4 g. The sublingual gland lies on mylohyoid, and is covered by the mucosa of the floor of the mouth, which is raised as a sublingual fold (Fig. 30.4). The anterior end of the contralateral sublingual gland lies in front, and the deep part of the submandibular gland lies behind. The mandible above the anterior part of the mylohyoid line, the sublingual fossa, is lateral, and genioglossus is medial, separated from the gland by the lingual nerve and submandibular duct.
The sublingual glands are seromucous, but predominantly mucous.
Vascular supply, innervation and lymphatic drainage
The arterial supply is from the sublingual branch of the lingual artery and the submental branch of the facial artery. Innervation is via the submandibular ganglion. Lymphatic drainage is to the submental nodes.
Sublingual ducts
The sublingual gland has 8–20 excretory ducts (Fig. 30.10). Smaller sublingual ducts open, usually separately, from the posterior part of the gland onto the summit of the sublingual fold (a few sometimes open into the submandibular duct). Small rami from the anterior part of the gland sometimes form a major sublingual duct (Bartholin’s duct), which opens with, or near to, the orifice of the submandibular duct. This duct may be visualized occasionally in a submandibular sialogram (Fig. 30.32).
Ranula
If the ducts draining any salivary gland become obstructed, the gland itself is at risk of developing a retention cyst where the retained secretions dilate the gland itself rather like a balloon. This phenomenon is seen mostly in the minor salivary glands which line the lips and oral cavity, where it is known as a mucocele. Trauma such as persistent lip biting results in scarring of the overlying oral mucosa and obstruction of the small drainage duct. When trauma occurs in the floor of the mouth and obstructs the drainage duct/s of the sublingual gland, the resulting retention cyst is known as a ranula. (Ranula is the Latin name for a frog and is used here because the tense cystic swelling is said to resemble the throat of a croaking frog.)
A ranula usually presents as a large tense bluish swelling anteriorly in the floor of the mouth just to one side of the midline, which often displaces the tongue. Occasionally the developing retention cyst herniates through a midline dehiscence where the two mylohyoid muscles fail to meet in the midline anteriorly, and then the ranula may present as a submental swelling or as a combined submental and floor of mouth swelling, a ‘plunging’ ranula. The treatment of a ranula is excision of the sublingual gland responsible.
MINOR SALIVARY GLANDS
The minor salivary glands of the mouth include the labial, buccal, palatoglossal, palatal and lingual glands. The labial and buccal glands contain both mucous and serous elements. The palatoglossal glands are mucous glands and are located around the pharyngeal isthmus. The palatal glands are mucous glands and occur in both the soft and hard palates. The anterior and posterior lingual glands are mainly mucous. The anterior glands are embedded within muscle near the ventral surface of the tongue and open by means of four or five ducts near the lingual frenulum and the posterior glands are located in the root of the tongue. The deep posterior lingual glands are predominantly serous. Serous glands (of von Ebner) occur around the circumvallate papillae, their secretion is watery, and they probably assist in gustation by spreading taste stimuli over the taste buds and then washing them away.
MICROSTRUCTURE
Salivary glands have numerous lobes composed of smaller lobules separated by dense connective tissue which is continuous with the capsule of the gland, and contains excretory (collecting) ducts, blood vessels, lymph vessels, nerve fibres and small ganglia. Each lobule has a single duct, whose branches terminate at dilated secretory ‘endpieces’, which are tubular or acinar in shape (Fig. 30.33). Their primary secretion is modified as it flows through intercalated, striated and excretory ducts into one or more main ducts which discharge saliva into the oral cavity. They contain a variable amount of intralobular adipose tissue: adipocytes are particularly numerous in the parotid gland.
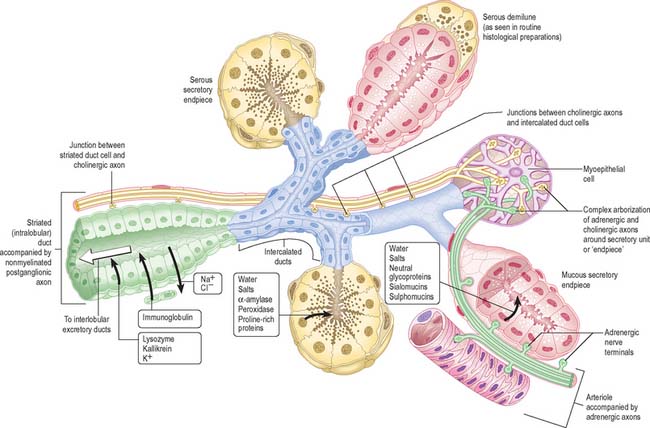
Fig. 30.33 Architecture of a generalized salivary gland. Solid black arrows indicate the direction of transport of salivary components and the open white arrow the direction of salivary flow. The innervation of the ducts, secretory units and arterioles is shown.
The secretory endpieces of the human parotid gland are almost exclusively serous acini (Fig. 30.34A): mucous tubules or acini are rare. In the submandibular gland, secretory units are predominantly serous acini, with some mucous tubules and acini (Fig. 30.34B). Mucous tubules are often associated with groups of serous cells at their blind ends, appearing as crescent-shaped serous demilunes in routine histological preparations. However, this appears to be a fixation artefact, as tissue prepared by rapid freezing methods lacks serous demilunes and the serous secretory cells align with mucous cells around a common lumen. In the sublingual gland, mucous tubules and acini predominate (Fig. 30.34C), but serous cells also occur, as acini or as serous demilunes.
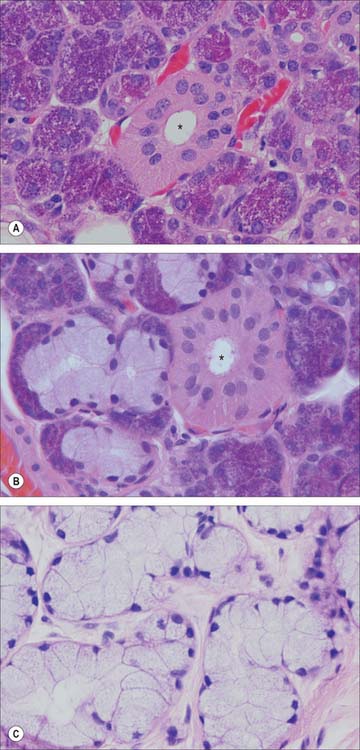
Fig. 30.34 The microstructure of the salivary glands. A, Parotid gland; B, mixed secretory units of the submandibular gland; C, mucous acini in the sublingual gland. Asterisks indicate the lumen of a striated duct.
(By courtesy of Mr Peter Helliwell and Dr Joseph Mathew, Department of Histopathology, Royal Cornwall Hospitals Trust, UK.)
Serous cells are approximately pyramidal in shape. Their nuclei vary in shape and position, but are more rounded and situated less basally than in mucous cells. Apically, the cytoplasm is filled by proteinaceous secretory (zymogen) granules with high amylase activity. Additionally, serous cells secrete kallikrein, lactoferrin and lysozyme, an antibacterial enzyme whose synthesis has been localized in particular to the serous demilunes of the submandibular and sublingual glands, and which is important in the defence against oral pathogens. In the human parotid and submandibular glands, zymogen granules also show a positive periodic acid–Schiff staining reaction, which indicates the presence of polysaccharides, and some texts refer to these cells as seromucous. Mucous cells are cylindrical and have flattened, basal nuclei. Their apical cytoplasm is typically packed with large, pale-staining and electron-translucent secretory droplets.
Ducts
Intercalated, striated (both intralobular) and extralobular collecting ducts lead consecutively from the secretory endpieces. The lining cells of intercalated ducts are flat nearest the secretory endpiece, but become cuboidal. Intercalated ducts function primarily as a conduit for saliva but, together with the striated ducts, may also modify its content of electrolytes and secrete IgA. Striated ducts are lined by a low columnar epithelium and are so called because their lining cells have characteristic basal striations. The latter are regions of highly infolded basal plasma membrane, between which lie columns of vertically aligned mitochondria. The nuclei are consequently displaced by the basal striations from a typical ductal basal position to a central or even apical location (Fig. 30.34). Infolding of the basal plasma membrane, and local abundance of mitochondria, are typical features of epithelial cells which actively transport electrolytes. Here, the cells transport potassium and bicarbonate into saliva: they produce a hypotonic saliva by reabsorbing sodium and chloride ions in excess of water. Striated ducts modify electrolyte composition and secrete IgA, lysozyme and kallikrein. IgA is produced by subepithelial plasma cells and transported transcytotically across the epithelial barrier to be secreted, once it has been dimerized by epithelial secretory component, into the saliva. This is also a function of serous acinar cells and other secretory epithelia, notably the lactating breast (see Ch. 54). The intralobular ductal system of the sublingual gland is less well developed than that of the parotid and submandibular glands.
Collecting ducts are metabolically relatively inert conduits which run within interlobular connective tissue septa in the glands. They transport saliva to the main duct which opens onto the mucosal surface of the buccal cavity. The lining epithelium of collecting ducts varies. It may be pseudostratified columnar, stratified cuboidal or columnar in the larger ducts, and has a distinct basal layer. It becomes a stratified squamous epithelium near the buccal orifice.
Myoepithelial cells
Myoepithelial cells (Fig. 30.33) are contractile cells (see Ch. 2) associated with secretory endpieces and with much of the ductal system. They lie between the basal lamina and the epithelial cells proper. They extend numerous cytoplasmic processes around serous acini and are often termed basket cells. Myoepithelial cells associated with ducts are more fusiform in shape, and are aligned along the length of the duct. Their cytoplasm contains abundant actin microfilaments which mediate contraction under the control of both sympathetic and parasympathetic stimulation. The outflow of saliva is thus accelerated through reduction in the luminal volume of secretory endpieces and ducts, contributing to the secretory pressure.
CONTROL OF SALIVARY GLAND ACTIVITY
The observed wide and rapid variation in the composition, quantity and rate of salivary secretion in response to various stimuli suggests an elaborate control mechanism. Secretion may be continuous, but at a low resting level, and may also occur spontaneously. It is mainly a response to the drying of the oral and pharyngeal mucosae. A rapid increase can be superimposed on the resting level, e.g. during mastication or when stimulated by the autonomic innervation. The controlled variation in the activity of the many types of salivary effector cells (serous, seromucous and mucous secretory cells, myoepithelial cells, epithelial cells of all the ductal elements and the smooth muscle of local blood vessels) affects the quantity and quality of saliva. There is no clear evidence that circulating hormones evoke secretion directly at physiological levels, but they may alter the response of glandular cells to neural stimuli.
The control of salivation depends on reflex nerve impulses. The afferent inputs to the reflex arc pass to brainstem salivatory centres, especially from taste and mechanoreceptors in the mouth. A variety of other sensory modalities in and around the mouth are also involved, e.g. smell, for certain aspects of submandibular secretion in man. The afferent input is integrated centrally by the salivatory centres, which are themselves influenced by higher centres. The latter may provide facilitatory or inhibitory influences, which presumably explains why the mouth becomes dry under stress. The efferent drive to the glands passes via parasympathetic and sympathetic outputs from the centres. Relatively little is known about the connections of the preganglionic parasympathetic neurones in the salivatory centres, and virtually nothing is known about either the central location of the sympathetic preganglionic neurones, or the output pathways. No peripheral inhibitory mechanisms exist in the glands.
The typical pattern of innervation is shown in Fig. 30.33, but details vary in different glands, and with age. Only the more constant features are illustrated and described here. Cholinergic nerves often accompany ducts and arborize freely around secretory endpieces, but adrenergic nerves usually enter glands along arteries and ramify with them. The main secretomotor nerves are predominantly non-myelinated axons: the few myelinated axons that have been seen are presumably either preganglionic efferent or visceral afferent. Within the glands the nerve fibres intermingle, such that cholinergic and adrenergic axons often lie in adjacent invaginations of one Schwann cell. Secretion and vasoconstriction are mediated via separate sympathetic axons. A single parasympathetic axon may, through serial en passant terminals, induce vasodilatation, secretion and myoepithelial contraction.
Secretory endpieces are usually the most densely innervated structures in the gland: individual cells often have both cholinergic and adrenergic innervation. Secretion of water and electrolytes, which creates the foundation for the volume of saliva secreted, is the outcome of a complex set of processes which is largely induced by parasympathetic impulses. Secretion of protein is an ongoing constitutive process wherever it occurs. The regulated exocytosis of pre-packaged proteins, which is the principal source of protein secretion into saliva, depends on the relative levels of activity in sympathetic and parasympathetic fibres.
The ductal elements of salivary glands can markedly modify the composition of saliva. They are less densely innervated than secretory endpieces but their activity is also under neural control. Adrenal aldosterone promotes resorption of sodium and release of potassium into saliva by striated ductal cells, as it does in kidney tubules. Myoepithelial contraction is stimulated mainly by adrenergic innervation, but there may be an additional role for cholinergic axons.
TISSUE SPACES AROUND THE JAWS
The dissemination of infection in soft tissues is influenced by the natural barriers presented by bone, muscle and fascia. However, the tissue spaces around the jaws are primarily defined by muscles, principally mylohyoid, buccinator, masseter, medial pterygoid, superior constrictor and orbicularis oris (Fig. 30.35). None of these ‘spaces’ is actually empty and they should merely be regarded as potential spaces that are normally occupied by loose connective tissue. It is only when inflammatory products such as hyaluronase destroy the loose connective tissue that a definable space is produced.
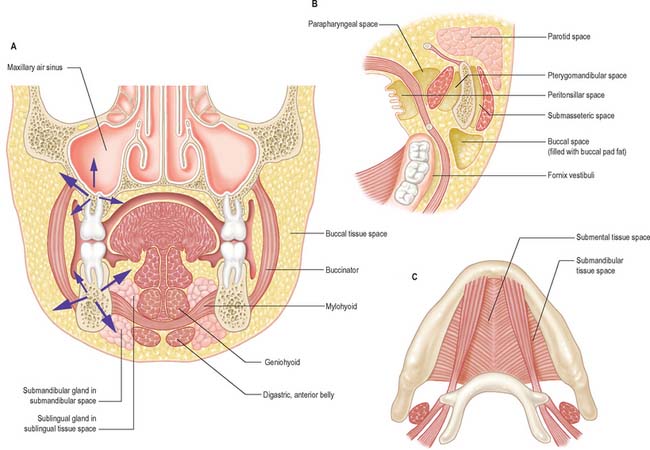
Fig. 30.35 Potential tissue spaces around the jaws. A, Coronal section showing the sublingual and submandibular spaces in the floor of the mouth and the possible routes for the spread of infections from periapical dental abscesses (left). B, Horizontal section through the mandibular molar region showing the associated tissue spaces. C, Inferior view of the floor of the mouth (suprahyoid region of the neck) showing the position of the submandibular and sublingual tissue spaces.
(B and C, By permission from Berkovitz BKB, Moxham BJ 2002 Head and Neck Anatomy. London: Martin Dunitz.)
The spaces are paired except for the submental, sublingual and palatal spaces.
POTENTIAL TISSUE SPACES AROUND THE LOWER JAW
The important potential tissue spaces of the lower jaw are the submental, submandibular, sublingual, buccal, submasseteric, parotid, pterygomandibular, peritonsillar, parapharyngeal and retropharyngeal spaces (Fig. 30.35).
The submental and submandibular spaces are located below the inferior border of the mandible beneath mylohyoid, in the suprahyoid region of the neck. The submental space lies beneath the chin in the midline, between the mylohyoid muscles and the investing layer of deep cervical fascia. It is bounded laterally by the two anterior bellies of the digastric muscles. The submental space communicates posteriorly with the two submandibular spaces. The submandibular space is situated between the anterior and posterior bellies of the digastric muscle and communicates with the sublingual space around the posterior free border of mylohyoid. The sublingual space lies in the floor of the mouth, above the mylohyoid muscles, and is continuous across the midline: it communicates with the submandibular spaces over the posterior free borders of the mylohyoid muscles, helped by the submandibular gland’s slight extension over this edge.
The remaining tissue spaces are illustrated in Fig. 30.35B. The buccal space is located in the cheek, on the lateral side of buccinator. The submasseteric spaces are a series of spaces between the lateral surface of the ramus of the mandible and masseter: they are formed because the fibres of masseter have multiple insertions onto most of the lateral surface of the ramus. The pterygomandibular space lies between the medial surface of the ramus of the mandible and medial pterygoid, and the parotid space lies behind the ramus of the mandible, in and around the parotid gland. The parapharyngeal space is bounded by the superior constrictor of the pharynx and the medial surface of medial pterygoid. It is restricted to the infratemporal region of the head and the suprahyoid region of the neck and communicates with the retropharyngeal space, which itself extends into the retrovisceral space in the lower part of the neck (the tissue spaces of the neck are described in Chapter 28, and of the pharynx in Chapter 33). The peritonsillar space lies around the palatine tonsil between the pillars of the fauces, and is part of the intrapharyngeal space. It is bounded by the medial surface of the superior constrictor of the pharynx and its mucosa.
POTENTIAL TISSUE SPACES AROUND THE UPPER JAW
The tissue spaces of the upper jaw are usually associated with spread of infection from the teeth. They are the canine (infraorbital), palatal and infratemporal spaces. The canine (infraorbital) space associated with the canine fossa lies between the levator labii superioris and zygomaticus muscles. The palatal space is not truly a tissue space in the hard palate, as the mucosa there is firmly bound to the periosteum. However, inflammation can strip away some of this periosteum to produce a well-circumscribed abscess. The infratemporal space is the upper extremity of the pterygomandibular space. It is closely related to the maxillary tuberosity and therefore the upper molars.
DENTAL ABSCESS
Abscesses developing in relation to the apices of roots ultimately penetrate the surrounding bone where it is thinnest. The position of the resultant swelling in the soft tissues is largely determined by the relationship between muscle attachments and the sinus (the path taken by the infected material) in the bone. Thus, in the lower incisor region, because the labial bone is thin, abscesses generally appear as a swelling in the labial sulcus, above the attachment of mentalis. The abscess may open below mentalis, when it will point beneath the chin. If an abscess from a lower postcanine tooth opens below the attachment of buccinator, the swelling is in the neck; if it opens above, the swelling is in the buccal sulcus. If an abscess opens lingually above mylohyoid, the swelling is in the lingual sulcus; if it is below, the swelling is in the neck. Third molar abscesses tend to track into the neck rather than the mouth, because mylohyoid ascends posteriorly and the termination of its attachment is variable, sometimes terminating before the position of the third molar, thereby allowing relatively unhindered access into the tissue spaces of the neck.
Apart from canine teeth, which have long roots, abscesses on upper teeth usually open buccally below, rather than above, the attachment of buccinator. Because its root apex is occasionally curved towards the palate, abscesses of the upper lateral incisors may track into the palatal submucosa. Abscesses of upper canines often open facially just below the orbit. Here, the swelling may obstruct drainage in the angular part of the facial vein which has no valves, and it is therefore possible for infected material to travel via the angular and ophthalmic veins into the cavernous sinus, potentially causing a cavernous sinus thrombosis. Abscesses on the palatal roots of upper molars usually open on the palate.
The superficial lamina of deep cervical fascia opposes the spread of abscesses towards the surface, and pus beneath it tends to migrate laterally. If the pus is in the anterior triangle, it may find its way into the mediastinum, anterior to the pretracheal lamina, but because the fascia here is so thin, it more often approaches the surface and ‘points’ above the sternum. Pus behind the prevertebral lamina may extend laterally and point in the posterior triangle, or it may perforate the lamina and the buccopharyngeal fascia to bulge into the pharynx as a retropharyngeal abscess.
Upper second premolars and first and second molars are related to the maxillary sinus. When this is large, the root apices of these teeth may be separated from its cavity solely by the lining mucosa. Sinus infections may stimulate the nerves entering the teeth, simulating toothache in the ipsilateral premolars and molars; this phenomenon is known as referred pain. Upper first premolars and third molars may be closely related to the maxillary sinus. With loss of teeth, alveolar bone is extensively resorbed. Thus, in the edentulous mandible, the mental nerve, originally inferior to premolar roots, may lie near the crest of the bone. In the edentulous maxilla, the sinus may enlarge to approach the oral surface of the bone. Both of these facts are of importance when planning surgery in this area. Occasional bony prominences, termed the torus mandibularis, torus maxillaris and torus palatinus, may lie lingual to the lower premolars or molars, or the upper molars. They lie in the midline of the palate and may need surgical removal before satisfactory dentures can be fitted.
Severe systemic infections during the time the teeth are developing may lead to faults in enamel, which are visible as horizontal lines (cf. Harris’s growth lines).