CHAPTER 43 Spinal cord and spinal nerves: gross anatomy
This chapter deals with the gross anatomy of the structures which lie within the vertebral canal and its extensions through the intervertebral foramina, the spinal nerve or radicular (‘root’) canals. The spinal cord, its blood vessels and nerve roots lie within a meningeal sheath, the theca, which occupies the central zone of the vertebral canal and extends from the foramen magnum, where it is in continuity with the meningeal coverings of the brain, to the level of the second sacral vertebra in the adult. Distal to this level the dura extends as a fine cord, the filum terminale externum, which fuses with the posterior periosteum of the first coccygeal segment. Tubular prolongations of the dural sheath extend around the spinal roots and nerves into the lateral zones of the vertebral canal and out into the root canals, eventually fusing with the epineurium of the spinal nerves. Between the theca and the walls of the vertebral canal is the epidural (spinal extradural) space, which is loosely filled with fat, connective tissue containing small arteries and lymphatics, and an important venous plexus. Three-dimensional appreciation of the anatomy of the spinal theca and its surroundings is essential for the efficient management of spinal pain and of spinal injuries, tumours and infections. Equally significant clinically is the anatomy of the often precarious blood supply of the spinal cord and its associated structures. The increasing application and refinement of diagnostic imaging and endoscopic procedures lend a new importance to topographical detail here.
SPINAL CORD (MEDULLA)
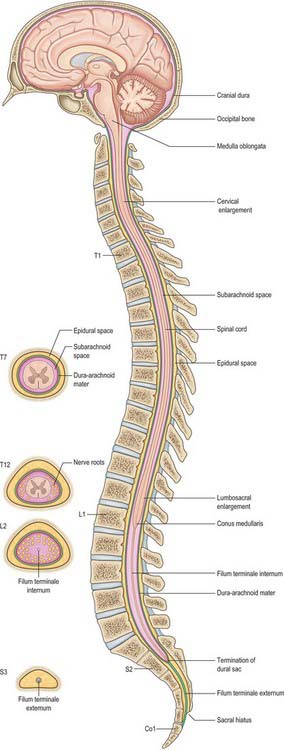
The spinal cord occupies the superior two-thirds of the vertebral canal (Fig. 18.1, Fig. 43.1). It is continuous cranially with the medulla oblongata, and narrows caudally to the conus medullaris, from whose apex a connective tissue filament, the filum terminale, descends to the dorsum of the first coccygeal vertebral segment. The cord extends from the upper border of the atlas to the junction between the first and second lumbar vertebrae: its average length in European males is 45 cm, its weight approximately 30 g. (For dimensional data consult Barson & Sands 1977.)
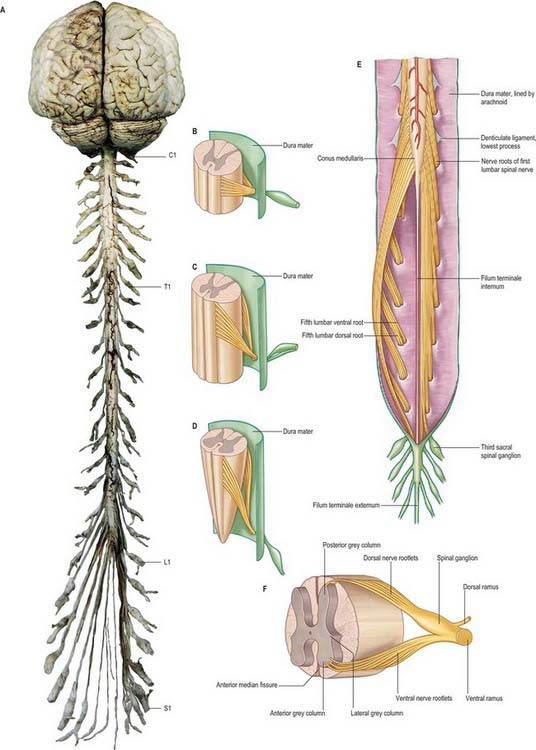
Fig. 43.1 A, Brain and spinal cord with attached spinal nerve roots and dorsal root ganglia, photographed from the dorsal aspect. Note the fusiform cervical and lumbar enlargements of the cord, and the changing obliquity of the spinal nerve roots as the cord is descended. The cauda equina is undisturbed on the right but has been spread out on the left to show its individual components. B–D, Formation of typical spinal nerve, ventral aspect. B, Cervical level; C, Thoracic level; D, Lumbar level. E, Lower end of spinal cord, filum terminale and cauda equina exposed from behind. The dura mater and the arachnoid have been opened and spread out. F, Spinal cord segment showing mode of formation of a typical spinal nerve and the gross relationships of the grey and white matter. (B–D, From Sobotta 2006.)
(Dissection by MCE Hutchinson, GKT School of Medicine; photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
During development, the vertebral column elongates more rapidly than the spinal cord, so that there is an increasing discrepancy between the anatomical level of spinal cord segments and their corresponding vertebrae. At stage 23, the vertebral column and spinal cord are the same length, and the cord ends at the last coccygeal vertebra: this arrangement continues until the third fetal month. At birth, the spinal cord terminates at the lower border of the second lumbar vertebra, and may sometimes reach the third lumbar vertebra. In the adult, the spinal cord is said to terminate at the level of the disc between the first and second lumbar vertebral bodies, which lies a little above the level of the elbow joint when the arm is by the side, and also lies approximately in the transpyloric plane (p. 1054). However, there is considerable variation in the level at which the spinal cord ends. It may end below this level in as many as 40% of subjects, or opposite the body of either the first or second lumbar vertebra: very occasionally it ends as high as the caudal third of twelfth thoracic or as low as the disc between the second and third lumbar vertebrae. Its position rises slightly in vertebral flexion, and there is some correlation with the length of the trunk, especially in females. The spinal cord varies in transverse width, gradually tapering craniocaudally, except at the levels of the enlargements. It is not cylindrical, being wider transversely at all levels, especially in the cervical segments.
The cervical enlargement is the source of the large spinal nerves that supply the upper limbs. It extends from the third cervical to the second thoracic segments, its maximum circumference (approximately 38 mm) is in the sixth cervical segment. (A spinal cord segment provides the attachment of the rootlets of a pair of spinal nerves.) The lumbar enlargement is the source of the large spinal nerves that supply the lower limbs, and extends from the first lumbar to the third sacral segments, the equivalent vertebral levels being the ninth to twelfth thoracic vertebrae. Its greatest circumference (approximately 35 mm) is near the lower part of the body of the twelfth thoracic vertebra, below which it rapidly dwindles into the conus medullaris.
Fissures and sulci extend along most of the external surface. An anterior median fissure and a posterior median sulcus and septum almost completely separate the cord into right and left halves, but they are joined by a commissural band of nervous tissue which contains a central canal.
The anterior median fissure extends along the whole ventral surface with an average depth of 3 mm, although it is deeper at caudal levels. It contains a reticulum of pia mater. Dorsal to it is the anterior white commissure. Perforating branches of the spinal vessels pass from the fissure to the commissure to supply the central spinal region. The posterior median sulcus is shallower, and from it a posterior median septum penetrates more than halfway into the cord, almost to the central canal. The septum varies in anteroposterior extent from 4 to 6 mm, and diminishes caudally as the canal becomes more dorsally placed and the cord contracts.
A posterolateral sulcus exists from 1.5 to 2.5 mm lateral to each side of the posterior median sulcus. Dorsal roots (strictly rootlets) of spinal nerves enter the cord along the sulcus. The white substance between the posterior median and posterolateral sulcus on each side is the posterior funiculus. In cervical and upper thoracic segments a longitudinal posterointermediate sulcus marks a septum dividing each posterior funiculus into two large tracts: the fasciculus gracilis (medial) and fasciculus cuneatus (lateral). Between the posterolateral sulcus and anterior median fissure is the anterolateral funiculus. This is subdivided into anterior and lateral funiculi by ventral spinal rootlets which pass through its substance to issue from the surface of the cord. The anterior funiculus is medial to, and includes, the emerging ventral rootlets, whilst the lateral funiculus lies between the roots and the posterolateral sulcus. In upper cervical segments, nerve rootlets emerge through each lateral funiculus to form the spinal accessory nerve which ascends in the vertebral canal lateral to the spinal cord and enters the posterior cranial fossa via the foramen magnum (Fig. 28.11).
The filum terminale, a filament of connective tissue approximately 20 cm long, descends from the apex of the conus medullaris. Its upper 15 cm, the filum terminale internum, is continued within extensions of the dural and arachnoid meninges and reaches the caudal border of the second sacral vertebra. Its final 5 cm, the filum terminale externum, fuses with the investing dura mater, and then descends to the dorsum of the first coccygeal vertebral segment. The filum is continuous above with the spinal pia mater. A few strands of nerve fibres which probably represent roots of rudimentary second and third coccygeal spinal nerves adhere to its upper part. The central canal is continued into the filum for 5–6 mm. A capacious part of the subarachnoid space surrounds the filum terminale internum, and is the site of election for access to the CSF (lumbar puncture).
DORSAL AND VENTRAL ROOTS
The paired dorsal and ventral roots of the spinal nerves are continuous with the spinal cord (Fig. 43.1F; see also p. 754). They cross the subarachnoid space and traverse the dura mater separately, uniting in or close to their intervertebral foramina to form the (mixed) spinal nerves. Since the spinal cord is shorter than the vertebral column, the more caudal spinal roots descend for varying distances around and beyond the cord to reach their corresponding foramina. In so doing they form a divergent sheaf of spinal nerve roots, the cauda equina, which is gathered round the filum terminale in the spinal theca, mostly distal to the apex of the cord.
Ventral spinal roots contain efferent somatic and, at some levels, preganglionic sympathetic, axons which extend from neuronal cell bodies in the ventral horns and intermediolateral columns respectively. There are also afferent nerve fibres in these roots. The rootlets comprising each ventral root emerge from the anterolateral sulcus in groups over an elongated vertical elliptical area (Fig. 43.1F). Dorsal spinal roots bear ovoid swellings, the spinal ganglia, one on each root proximal to its junction with a corresponding ventral root in an intervertebral foramen. Each root fans out into six to eight rootlets before entering the cord in a vertical row in the posterolateral sulcus. Dorsal roots are usually said to contain only afferent axons (both somatic and visceral) which are the central processes of unipolar neurones in the spinal root ganglia, but they may also contain a small number (3%) of efferent fibres and autonomic vasodilator fibres.
Each ganglionic neurone has a single short stem which divides into a medial (central) branch which enters the spinal cord via a dorsal root, and a lateral (peripheral) branch which passes peripherally to a sensory end organ. The central branch is an axon while the peripheral one is an elongated dendrite (but when traversing a peripheral nerve is, in general structural terms, indistinguishable from an axon). The region of spinal cord associated with the emergence of a pair of nerves is a spinal segment, but there is no actual surface indication of segmentation. Moreover, the deep neural sources or destinations of radicular fibres may lie far beyond the confines of the ‘segment’ so defined.
MENINGES
DURA MATER
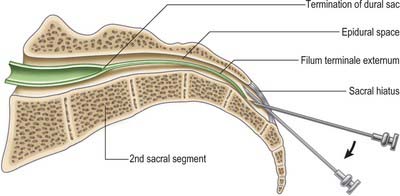
In some areas within the skull the dura mater can be distinguished from the endosteum, but at the base of the skull around the foramen magnum the two layers are fused and adherent to the bone. Distal to the foramen magnum, within the vertebral column, the dura is distinct from the tissues which line the vertebral canal, and separated from them by the epidural space (see below). The spinal dura mater forms a tube whose upper end is attached to the edge of the foramen magnum and to the posterior surfaces of the second and third cervical vertebral bodies, and also by fibrous bands to the posterior longitudinal ligament, especially towards the caudal end of the vertebral canal. The dural tube narrows at the lower border of the second sacral vertebra. It invests the thin spinal filum terminale, descends to the back of the coccyx, and blends with the periosteum.
Epidural space
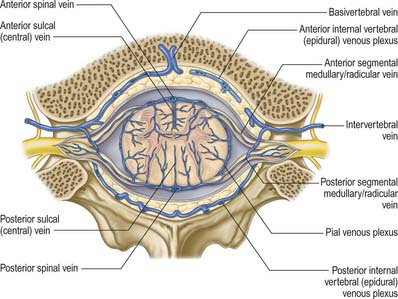
The epidural space lies between the spinal dura mater and the tissues which line the vertebral canal (Fig. 43.2). It is closed above by fusion of the spinal dura with the edge of the foramen magnum, and below by the posterior sacrococcygeal ligament which closes the sacral hiatus. It contains loosely packed connective tissue, fat, a venous plexus, small arterial branches, lymphatics and fine fibrous bands which connect the theca with the lining tissue of the vertebral canal. These bands, the meningovertebral ligaments, are best developed anteriorly and laterally. Similar bands tether the nerve root sheaths or ‘sleeves’ within their canals. There is also a midline attachment from the posterior spinal dura to the ligamentum nuchae at atlanto-occipital and atlanto-axial levels (Dean & Mitchell 2002). The venous plexus consists of longitudinally arranged chains of vessels, connected by circumdural venous ‘rings’. The anteriorly placed vessels receive the basivertebral veins.
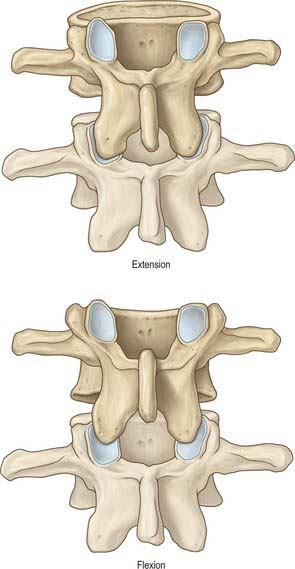
The shape of the space within each spinal segment is not uniform, though the segmental pattern is metamerically repeated. It is difficult to define the true shape of the ‘space’, because it changes with the introduction of fluid or as a result of preservation techniques. In the lumbar region, the dura mater is apposed to the walls of the vertebral canal anteriorly and attached by connective tissue in a manner that permits displacement of the dural sac during movement and venous engorgement. Adipose tissue is present posteriorly in recesses between the ligamentum flavum and the dura. The connective tissue extends for a short distance through the intervertebral foramina along the sheaths of the spinal nerves. Like the main thecal sac, the root sheaths are partially tethered to the walls of the foramina by fine meningovertebral ligaments.
Epidural injections
Contrast media and other fluids injected into the epidural space at the sacral level can spread up to the cranial base (p. 760). Local anaesthetics injected near the spinal nerves, just outside the intervertebral foramina, may spread up or down the epidural space to affect the adjacent spinal nerves or may pass to the opposite side. The paravertebral spaces of each side communicate via the epidural space, particularly at lumbar levels.
For a review of the morphology of the epidural space and a discussion of the nature of the lining layer of the vertebral canal, see Newell (1999).
Subdural space
The subdural space is a potential space in the normal spine because the arachnoid and dura are closely apposed (Haines et al 1993). It does not connect with the subarachnoid space, but continues for a short distance along the cranial and spinal nerves. Accidental subdural catheterization may occur during epidural injections. Injection of fluid into the subdural space may either damage the cord by direct toxic effects or by compression of the vasculature.
ARACHNOID MATER
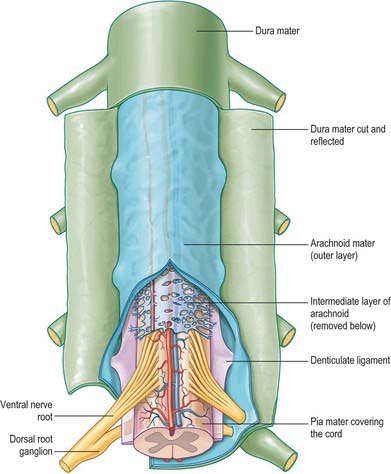
The spinal arachnoid mater, which surrounds the spinal cord, is continuous with the cranial arachnoid mater (Fig. 43.3). It is closely applied to the deep aspect of the dura mater. At sites where vessels and nerves enter or leave the subarachnoid space, the arachnoid mater is reflected on to the surface of these structures and forms a thin coating of leptomeningeal cells over the surface of both vessels and nerves. Thus a subarachnoid angle is formed as nerves pass through the dura into the intervertebral foramina. At this point, the layers of leptomeninges (arachnoid and pia) fuse and become continuous with the perineurium. The epineurium is in continuity with the dura. Such an arrangement seals the subarachnoid space so that particulate matter does not pass directly from the subarachnoid space into nerves. The existence of a pathway of lymphatic drainage from the CSF is controversial.
PIA MATER
The spinal pia mater (Fig. 43.3) closely invests the surface of the spinal cord and passes into the anterior median fissure. As in the cranial region, there is a subpial ‘space’, however over the surface of the spinal cord the subpial collagenous layer is thicker than in the cerebral region, and it is continuous with the collagenous core of the ligamentum denticulatum.
The ligamentum denticulatum is a flat, fibrous sheet which lies on each side of the spinal cord between the ventral and dorsal spinal roots. Its medial border is continuous with the subpial connective tissue of the cord and its lateral border forms a series of triangular processes, the apices of which are fixed at intervals to the dura mater. There are usually 21 processes on each side. The first crosses behind the vertebral artery where it is attached to the dura mater, and is separated by the artery from the first cervical ventral root. Its site of attachment to the dura mater is above the rim of the foramen magnum, just behind the hypoglossal nerve: the spinal accessory nerve ascends on its posterior aspect (Fig. 28.11). The last of the dentate ligaments lies between the exiting twelfth thoracic and first lumbar spinal nerves and is a narrow, oblique band which descends laterally from the conus medullaris. Changes in the form and position of the dentate ligaments during spinal movements have been demonstrated by cine-radiography.
Beyond the conus medullaris, the pia mater continues as a coating of the filum terminale.
INTERMEDIATE LAYER
In addition to the well-defined coats of arachnoid and pia mater, the cord is also surrounded by an extensive intermediate layer of leptomeninges. This layer is concentrated in the dorsal and ventral regions and forms a highly perforated, almost lace-like structure which is focally compacted to form the dorsal, dorsolateral and ventral ligaments of the spinal cord. Dorsally, the intermediate layer is adherent to the deep aspect of the arachnoid mater and forms a discontinuous series of dorsal ligaments which attach the spinal cord to the arachnoid. The dorsolateral ligaments are more delicate and fenestrated, and they extend from the dorsal roots to the parietal arachnoid. As the intermediate layer spreads laterally over the dorsal surface of the dorsal roots, it becomes increasingly perforated and eventually disappears. A similar arrangement is seen over the ventral aspect of the spinal cord, but the intermediate layer is less substantial.
The intermediate layer is structurally similar to the trabeculae which cross the cranial subarachnoid space, in that a collagenous core is coated by leptomeningeal cells. The intermediate layers of leptomeninges around the spinal cord may act as a baffle within the subarachnoid space to dampen waves of CSF in the spinal column. Inflammation within the spinal subarachnoid space may result in extensive fibrosis within the intermediate layer and the complications of chronic arachnoiditis.
COVERINGS AND RELATIONS OF THE SPINAL ROOTS AND NERVES IN THE RADICULAR CANAL
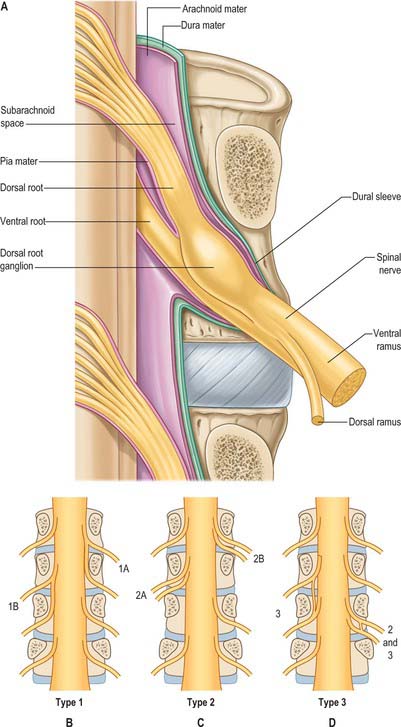
Tubular prolongations of the spinal dura mater, closely lined by the arachnoid, extend around the spinal roots and nerves as they pass through the lateral zone of the vertebral canal and through the intervertebral foramina (Fig. 43.3, Fig. 43.4A). These prolongations, the spinal nerve sheaths or root sheaths, gradually lengthen as the spinal roots become increasingly oblique. Each individual dorsal and ventral root runs in the subarachnoid space with its own covering of pia mater. Each root pierces the dura separately, taking a sleeve of arachnoid with it, before joining within the dural prolongation just distal to the spinal ganglion. The dural sheaths of the spinal nerves fuse with the epineurium, within or slightly beyond the intervertebral foramina. The arachnoid prolongations within the sheaths do not extend as far distally as their dural coverings, but the subarachnoid space and its contained CSF extend sufficiently distally to form a radiologically demonstrable root sleeve for each nerve. Shortening or obstruction of this sleeve seen on MRI indicates compression of the spinal nerve. At the cervical level, where the nerves are short and the vertebral movement is greatest, the dural sheaths are tethered to the periosteum of the adjacent transverse processes. In the lumbosacral region there is less tethering of the dura to the periosteum, though there may be an attachment posteriorly to the facet joint capsule.
CEREBROSPINAL FLUID (CSF)
The cerebrospinal fluid is described in detail on page 242. Although there is free communication between the spinal and cerebral subarachnoid spaces, the mode of circulation of the spinal CSF and the contribution that it makes to the overall circulation of CSF remains uncertain in man: CSF may be absorbed from the spinal subarachnoid space, and spinal arachnoid granulations and villi have been described (Kiddo et al 1976).
SPINAL NERVES
In those body segments which largely retain a metameric (segmental) structure, e.g. the thoracic region, spinal nerves show a common plan (Fig. 43.5). The dorsal, epaxial, ramus passes back lateral to the articular processes of the vertebrae and divides into medial and lateral branches which penetrate the deeper muscles of the back: both branches innervate the adjacent muscles and supply a band of skin from the posterior median line to the lateral border of the scapula (Fig. 43.6). The ventral, hypaxial, ramus is connected to a corresponding sympathetic ganglion by white and grey rami communicantes. It innervates the prevertebral muscles and curves round in the body wall to supply the lateral muscles of the trunk. Near the midaxillary line it gives off a lateral branch which pierces the muscles and divides into anterior and posterior cutaneous branches. The main nerve advances in the body wall, where it supplies the ventral muscles and terminates in branches to the skin.
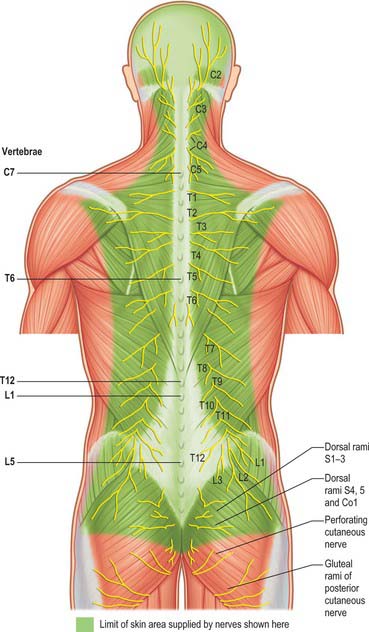
Fig. 43.6 Cutaneous distribution of the dorsal rami of the spinal nerves. The nerves are shown lying on the superficial muscles. The nerves are numbered on the right side; the spines of the seventh cervical, sixth and twelfth thoracic, and first and fifth lumbar vertebrae are labelled in on the left side.
Spinal nerves are united ventral and dorsal spinal roots, attached in series to the sides of the spinal cord. The term spinal nerve strictly applies only to the short segment after union of the roots and before branching occurs. This segment, the spinal nerve proper, lies in the intervertebral foramen: it is sometimes mistakenly called the ‘nerve root’. There are 31 pairs of spinal nerves: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, 1 coccygeal. The abbreviations C, T, L, S and Co, with appropriate numerals, are commonly applied to individual nerves. The peripheral nerves emerge through the intervertebral foramina. At thoracic, lumbar, sacral and coccygeal levels the numbered nerve exits the vertebral canal by passing below the pedicle of the corresponding vertebra, e.g. L4 nerve exits the intervertebral foramen between L4 and L5. However, in the cervical region, nerves C1–7 pass above their corresponding vertebrae. C1 leaves the vertebral canal between the occipital bone and atlas and hence is often termed the suboccipital nerve. The last pair of cervical nerves does not have a correspondingly numbered vertebra and C8 passes between the seventh cervical and first thoracic vertebrae. Each nerve is continuous with the spinal cord by ventral and dorsal roots; the latter each bears a spinal ganglion (‘dorsal root ganglion’).
SPINAL ROOTS AND GANGLIA
Ventral (anterior) roots
Ventral roots contain axons of neurones in the anterior and lateral spinal grey columns. Each emerges as a series of rootlets in two or three irregular rows in an area approximately 3 mm in horizontal width.
Dorsal (posterior) roots
Dorsal roots contain centripetal processes of neurones sited in the spinal ganglia. Each consists of medial and lateral fascicles which both diverge into rootlets which enter the spinal cord along the posterolateral sulcus. The rootlets of adjacent dorsal roots are often connected by oblique filaments, especially in the lower cervical and lumbosacral regions.
Little is known of the detail of the regions of entry and emergence of afferent and efferent rootlets in humans, but these zones of transition between the central and peripheral nervous systems have been extensively described in rodents (Fraher 2000) (see also p. 63).
Appearance and orientation of roots at each spinal level
The size and direction of spinal nerve roots vary. The upper four cervical roots are small, the lower four are large. Cervical dorsal roots have a thickness ratio to the ventral roots of 3 : 1, which is greater than in other regions. The first dorsal root is an exception, being smaller than the ventral and it is occasionally absent. The conventional view is that the first and second cervical spinal roots are short, running almost horizontally to their exits from the vertebral canal, and that from the third to the eighth cervical levels the roots slope obliquely down. Obliquity and length increase successively, although the distance between spinal attachment and vertebral exit never exceeds the height of one vertebra. An alternative view (Kubik & Müntener 1969) states that upper cervical roots descend, the fifth is horizontal, the sixth to eighth ascend, the first two thoracic roots are horizontal, the next three ascend, the sixth is horizontal and the rest descend. This view is based on the observation that the cervicothoracic part of the spinal cord grows more in length than other parts.
Thoracic roots, except the first, are small, and the dorsal root only slightly exceeds the ventral in thickness. They increase successively in length. In the lower thoracic region, the roots descend in contact with the spinal cord for at least two vertebrae before emerging from the vertebral canal.
Lower lumbar and upper sacral roots are the largest, and their rootlets are the most numerous. Coccygeal roots are the smallest. Kubik & Müntener (1969) confirm that lumbar, sacral and coccygeal roots descend with increasing obliquity to their exits. The spinal cord ends near the lower border of the first lumbar vertebra, and so the lengths of successive roots rapidly increase: the consequent collection of roots is the cauda equina (Fig. 43.1A). The largest roots, and hence the largest spinal nerves, are continuous with the spinal cervical and lumbar enlargements and innervate the upper and lower limbs.
Spinal ganglia (dorsal root ganglia)
Spinal ganglia are large groups of neurones on the dorsal spinal roots. Each is oval and reddish; its size is related to that of its root. A ganglion is bifid medially where the two fascicles of the dorsal root emerge to enter the cord. Ganglia are usually sited in the intervertebral foramina, immediately lateral to the perforation of the dura mater by the roots (Fig. 43.1B). However, the first cervical ganglion lies on the vertebral arch of the atlas, the second lies behind the lateral atlantoaxial joint, the sacral lie inside the vertebral canal, and the coccygeal ganglion usually lies within the dura mater. The first cervical ganglia may be absent. Small aberrant ganglia sometimes occur on the upper cervical dorsal roots between the spinal ganglia and the cord.
SPINAL NERVES PROPER
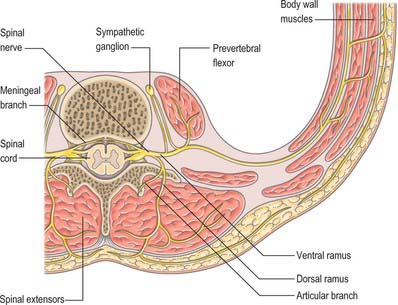
Immediately distal to the spinal ganglia, ventral and dorsal roots unite to form spinal nerves (see Fig. 43.5, Fig. 15.15). These very soon divide into dorsal and ventral rami, both of which receive fibres from both roots. At all levels above the sacral, this division occurs within the intervertebral foramen. Division of the sacral spinal nerves occurs within the sacral vertebral canal, and the dorsal and ventral rami exit separately through posterior and anterior sacral foramina at each level. Spinal nerves trifurcate at some cervical and thoracic levels, in which case the third branch is called a ramus intermedius. At or distal to its origin each ventral ramus gives off recurrent meningeal (sinuvertebral) branches and receives a grey ramus communicans from the corresponding sympathetic ganglion. The thoracic and first and second lumbar ventral rami each contributes a white ramus communicans to the corresponding sympathetic ganglia. The second, third and fourth sacral nerves also supply visceral branches, unconnected with sympathetic ganglia, which carry a parasympathetic outflow direct to the pelvic plexuses.
Cervical spinal nerves enlarge from the first to the sixth nerve. The seventh and eighth cervical and the first thoracic nerve are similar in size to the sixth cervical nerve. The remaining thoracic nerves are relatively small. Lumbar nerves are large, increasing in size from the first to the fifth. The first sacral is the largest spinal nerve, thereafter the sacral nerves decrease in size. The coccygeal nerves are the smallest spinal nerves. The size of the spinal nerve and its associated structures within the intervertebral foramen is not in direct relation to the size of the foramen. At lumbar levels, though L5 is the largest nerve, its foramen is smaller than those of L1–4, which renders this nerve particularly liable to compression.
In the radicular (‘root’) canal and intervertebral foramen, the spinal nerve is related to the spinal artery of that level and its radicular branch, and to a small plexus of veins. At the outer end of the foramen the nerve may lie above or below transforaminal ligaments.
Meningeal nerves
Recurrent meningeal (or sinuvertebral) nerves (Fig. 43.7) occur at all vertebral levels. They are mixed sensory and sympathetic nerves, represented by numerous fine filaments amongst which one, or two to four, larger trunks may be evident. At cervical levels the autonomic roots arise from the grey rami that form the vertebral nerve (p. 461). At thoracic and lumbar levels, each nerve is formed by a somatic root from the ventral ramus and by an autonomic root from the grey ramus communicans of that segment. Each nerve pursues a recurrent course through the intervertebral foramen, passing ventral to the spinal nerve, to enter the vertebral canal, where it divides into ascending, descending, and transverse branches. These branches communicate with corresponding branches from the segments above and below, and from the opposite side, forming arcades along the floor of the vertebral canal. Meningeal branches of the arcades form a plexus on the ventral surface of the dural sac and nerve root sleeves which attenuates laterally; the posterior paramedian dura is devoid of nerve endings. Skeletal branches are distributed to the posterior longitudinal ligament, the periosteum of the vertebral bodies, and to the posterior and posterolateral aspects of the intervertebral discs. Vascular branches accompany the veins and arteries of the vertebral canal and those of the vertebral bodies. The upper three cervical meningeal nerves ascend through the foramen magnum into the posterior cranial fossa, where they innervate the dura mater that covers the clivus. En route, they innervate the median atlanto-axial joint and its ligaments.
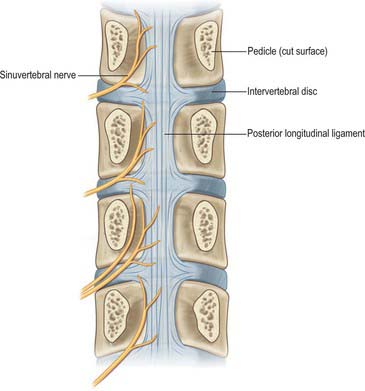
Fig. 43.7 The course and skeletal distribution of the lumbar sinuvertebral nerves. Each nerve supplies the intervertebral disc at its level of entry into the vertebral canal, the disc above, and the intervening posterior longitudinal ligament. In about one-third of cases, the nerve at a particular level may be represented by more than one filament.
Functional components of spinal nerves
A typical spinal nerve contains somatic efferent fibres and somatic and visceral afferent fibres. Some, but not all, spinal nerves also contain preganglionic autonomic fibres.
Somatic components
Somatic efferent fibres innervate skeletal muscles and are axons of α, β and γ neurones in the spinal ventral grey column. Somatic afferent fibres convey impulses into the CNS from receptors in the skin, subcutaneous tissue, muscles, tendons, fasciae and joints: they are peripheral processes of unipolar neurones in the spinal ganglia.
Visceral components
Preganglionic visceral efferent sympathetic fibres are axons of neurones in the spinal intermediolateral grey column throughout the thoracic and upper two or three lumbar segments: they join the sympathetic trunk via corresponding white rami communicantes and synapse with postganglionic neurones which are distributed to smooth muscle, myocardium or exocrine glands. The preganglionic visceral efferent parasympathetic fibres are axons of neurones in the spinal lateral grey column of the second to fourth sacral segments: they leave the ventral rami of corresponding sacral nerves and synapse in pelvic ganglia. The postganglionic axons are distributed mainly to smooth muscle or glands in the walls of the pelvic viscera. Visceral afferent fibres have cell bodies in the spinal ganglia. Their peripheral processes pass through white rami communicantes and, without synapsing, through one or more sympathetic ganglia to end in the walls of the viscera. Some visceral afferent fibres may enter the spinal cord in the ventral roots.
Central processes of ganglionic unipolar neurones enter the spinal cord by dorsal roots and synapse on somatic or sympathetic efferent neurones, usually through interneurones, completing reflex paths. Alternatively, they may synapse with other neurones in the spinal or brain stem grey matter which give origin to a variety of ascending tracts.
VARIATIONS OF SPINAL ROOTS AND NERVES
The courses of spinal roots and nerves in relation to the thecal sac and vertebral and radicular canals may be aberrant. An individual intervertebral foramen may contain a duplicated sheath, nerve and roots, which will then be absent at an adjacent level. Abnormal communications between roots may occur within the vertebral canal. These anomalies have been described and classified for the lumbosacral spine by Neidre & Macnab (1983) (Fig. 43.4).
RAMI OF THE SPINAL NERVES
Ventral (anterior primary) rami supply the limbs and the anterolateral aspects of the trunk, and in general are larger than the dorsal rami. Thoracic ventral rami run independently and retain a largely segmental distribution. Cervical, lumbar and sacral ventral rami connect near their origins to form plexuses. Dorsal rami do not join these plexuses. The ventral rami are described in the appropriate regional Sections.
Dorsal (posterior primary) rami of spinal nerves are usually smaller than the ventral rami and are directed posteriorly. Retaining a segmental distribution, all, except for the first cervical, fourth and fifth sacral and the coccygeal, divide into medial and lateral branches which supply the muscles and skin of the posterior regions of the neck and trunk (Fig. 43.6).
Cervical dorsal spinal rami
Each cervical spinal dorsal ramus, except the first, divides into medial and lateral branches which all innervate muscles. In general only medial branches of the second to fourth, and usually the fifth, supply the skin. Except for the first and second, each dorsal ramus passes posteriorly around an articular pillar as far as the root of the transverse process medial to a posterior intertransverse muscle, which it supplies.
First cervical dorsal ramus (suboccipital nerve)
The first cervical dorsal ramus, the suboccipital nerve, is larger than the ventral (Fig. 42.59). It emerges superior to the posterior arch of the atlas and inferior to the vertebral artery and enters the suboccipital triangle to supply rectus capitis posterior major and minor, obliquus capitis superior and inferior, and semispinalis capitis. A filament from the branch to the inferior oblique joins the second dorsal ramus. The suboccipital nerve occasionally has a cutaneous branch which accompanies the occipital artery to the scalp, and connects with the greater and lesser occipital nerves. It may also communicate with the accessory nerve.
Second cervical dorsal ramus
The second cervical dorsal ramus is slightly larger than the ventral and all the other cervical dorsal rami (Fig. 42.59, Fig. 29.15). It runs between the lamina of the axis and the inferior oblique, below which it divides into a large medial and smaller lateral branch. The dorsal ramus or its medial branch receives communicating branches from the first cervical dorsal ramus which pass both through and around inferior oblique. A descending communicating branch crosses the C2–3 facet joint to reach the third cervical dorsal ramus.
Greater occipital nerve
The medial branch, termed the greater occipital nerve, passes transversely across inferior oblique deep to semispinalis capitis, which it innervates. Near the origin of inferior oblique, it turns rostrally along rectus capitis posterior major, where it receives a communicating branch from the third occipital nerve before turning dorsally to pierce semispinalis capitis. It emerges onto the scalp by passing above an aponeurotic sling between trapezius and sternocleidomastoid near their occipital attachments It ascends with the occipital artery, divides into branches which connect with the third occipital and lesser occipital nerves, and which supply the back of the auricle and the skin of the scalp as far forward as the coronal suture. The lateral branch of the second cervical dorsal ramus supplies longissimus capitis, semispinalis capitis and splenius capitis, and sends a communicating branch to the lateral branch of the third cervical dorsal ramus.
Greater occipital neuralgia
Greater occipital neuralgia is a syndrome of pain and paraesthesiae felt in the distribution of the greater occipital nerve. The pathology lies at the dorsal root ganglion. A similar syndrome may be caused by upper facet joint arthritis involving the second cervical root.
Third cervical dorsal ramus
The third cervical dorsal ramus is intermediate in size between the second and fourth. It courses back round the articular pillar of the third cervical vertebra, medial to the posterior intertransverse muscle, and divides into medial and lateral branches. The lateral branch passes dorsally across the surface of semispinalis capitis to supply it and the overlying longissimus capitis and splenius capitis. It receives a communicating branch from the lateral branch of the second cervical dorsal ramus, and sends a similar branch to the lateral branch of the fourth cervical dorsal ramus. The deep medial branch curves dorsally and medially around the waist of the articular pillar before ending in multifidus. It sends an articular branch to the C3–4 facet joint, and may also send a branch into semispinalis capitis. The superficial medial branch, the third occipital nerve, curves around the lateral and dorsal surfaces of the C2–3 facet joint, which it supplies. It continues transversely, deep to semispinalis, which it supplies, and sends a communicating branch to the greater occipital nerve. Just above the second cervical spinal process, the third occipital nerve turns dorsally to pierce semispinalis capitis, splenius capitis and trapezius, and becomes cutaneous over a small area immediately below the superior nuchal line. Communicating branches join the cutaneous branches of the greater and lesser occipital nerves.
Dorsal rami of the lower five cervical nerves
The dorsal rami of the lower five cervical nerves curve back round the vertebral articular pillars and divide into lateral and medial branches. The lateral branches supply longissimus cervicis, splenius cervicis and iliocostalis cervicis. The medial branches curve around the lateral and dorsal surfaces of the articular pillar of their segment, deep to semispinalis capitis. They send articular branches to the facet joints above and below their course before entering and supplying multifidus. Terminal branches reach interspinalis at each segment. Cutaneous branches arise consistently from the fourth, commonly from the fifth, sixth and eighth, but not usually from the seventh medial branches. These branches pass across multifidus or through semispinalis cervicis and turn dorsally around the medial border of semispinalis capitis, to pierce splenius cervicis and trapezius and reach the skin.
Thoracic dorsal spinal rami
At each segmental level, the thoracic dorsal rami pass dorsally through an aperture bounded by the transverse processes superiorly and inferiorly, the facet joint medially, and the superior costotransverse ligament laterally. Thereafter, each dorsal ramus follows a prolonged course laterally, between the anterior lamella of the superior costotransverse ligament anteriorly, and the costolamellar ligament and the posterior lamella of the superior costotransverse ligament posteriorly. In this narrow space, the dorsal ramus divides into lateral and medial branches. The lateral branches continue laterally as far as the costotransverse joint, above which they turn dorsally and inferiorly to supply the levatores costarum, and enter the longissimus thoracis and iliocostalis muscles. Lateral branches from the upper six thoracic dorsal rami remain intramuscular. Those from the lower six levels emerge from iliocostalis lumborum to pierce serratus posterior inferior and latissimus dorsi in line with the costal angles. They descend across as many as four ribs before becoming superficial. The twelfth thoracic lateral branch sends a filament medially along the iliac crest, then passes down to the anterior gluteal skin. Some upper thoracic lateral branches may become cutaneous.
Each medial branch hooks dorsally around the lateral margin of the posterior lamella of the superior costotransverse ligament, lying a variable distance above the tip of the transverse process. At the first to fourth, and at the ninth and tenth thoracic levels, the medial branch cross the superior tip of the transverse process to turn inferiorly and medially across the posterior surface of the transverse process, lying between the attachment sites of multifidus medially and semispinalis laterally. Each nerve continues this oblique course to supply the multifidus and semispinalis. At the fifth to eighth thoracic levels, the medial branches assume a course parallel to that of other levels, but do not reach the transverse process, instead passing superior and dorsal to the plane of the transverse processes. At the eleventh and twelfth levels, in keeping with the relatively short transverse processes at these levels, the medial branches run close to the superior articular processes. Each medial branch supplies articular branches to the facet joints above and below its course. Medial branches of the upper six thoracic dorsal rami furnish cutaneous branches that pierce trapezius and the rhomboids to reach the skin near the spinous processes. Cutaneous branches may occasionally arise from the medial branches of the lower six thoracic dorsal rami.
Lumbar dorsal spinal rami
After leaving its spinal nerve, each of the first to fourth lumbar dorsal rami passes through an aperture in the dorsal leaf of the intertransverse ligament just above a transverse process (Fig. 43.8). Each dorsal ramus supplies the overlying medial intertransverse muscle before dividing into lateral and medial branches. The lateral branches supply the longissimus and iliocostalis components of the lumbar erector spine. The upper three lumbar lateral branches emerge from the lateral border of iliocostalis lumborum and pierce the aponeurosis of latissimus dorsi to become cutaneous, They cross the iliac crest to reach the gluteal skin, some reach as far as the level of the greater trochanter. Medial branches cross the junction of the superior articular process and transverse process and hook medially between the mammillary and accessory processes, deep to the mammillo-accessory ligament. Medial to the ligament, each medial branch sends articular branches to the facet joints above and below its course, before entering multifidus, which it supplies. Terminal branches reach interspinalis at each segment.
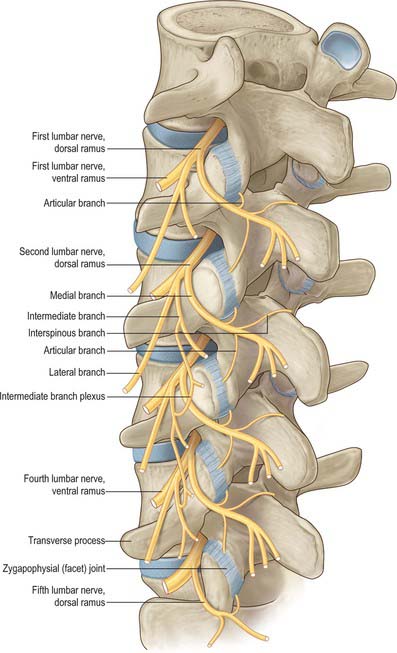
Fig. 43.8 A left posterolateral view of the lumbar spine showing the branches of the lumbar dorsal rami.
The fifth dorsal ramus has a longer course than other dorsal rami. It arches over the ala of the sacrum, lying against the root of the first sacral superior articular process. Opposite the base of the lumbosacral joint it sends a branch into the lowest fibres of longissimus thoracis, and a communicating branch to the first sacral dorsal ramus, before terminating as a medial branch that hooks around the lumbosacral joint to end in multifidus.
Sacral dorsal spinal rami
Sacral dorsal rami are small, diminishing in size inferiorly, and other than the fifth, all emerge though the dorsal sacral foramina. The upper three are covered at their exit by multifidus, and divide into medial and lateral branches. Medial branches are small and end in multifidus. Lateral branches join together and with branches of the last lumbar and fourth sacral dorsal rami to form loops dorsal to the sacrum. Branches from these loops run dorsal to the sacrotuberous ligament and form a second series of loops under gluteus maximus. From these, two or three gluteal branches pierce gluteus maximus (along a line from the posterior superior iliac spine to the coccygeal apex) to supply the posterior gluteal skin.
The dorsal rami of the fourth and fifth sacral nerves are small and lie caudal to multifidus. They unite with each other and with the coccygeal dorsal ramus to form loops dorsal to the sacrum: filaments from these supply the skin over the coccyx.
VASCULAR SUPPLY OF SPINAL CORD, ROOTS AND NERVES
ARTERIES
The spinal cord, its roots and nerves are supplied with blood by both longitudinal and segmental vessels (Fig. 43.9). Three major longitudinal vessels, a single anterior and two posterior spinal arteries (each of which is sometimes doubled to pass on either side of the dorsal rootlets), originate intracranially from the vertebral artery and terminate in a plexus around the conus medullaris. The anterior spinal artery forms from the fused anterior spinal branches of the vertebral artery, and descends in the anterior median fissure of the cord. Each posterior spinal artery originates either directly from the ipsilateral vertebral artery or from its posterior inferior cerebellar branch, and descends in a posterolateral sulcus of the cord. The segmental arteries are derived in craniocaudal sequence from spinal branches of the vertebral, deep cervical, intercostal and lumbar arteries. These vessels enter the vertebral canal through the intervertebral foramina and anastomose with branches of the longitudinal vessels to form a pial plexus on the surface of the cord. The segmental spinal arteries send anterior and posterior radicular branches to the spinal cord along the ventral and dorsal roots. Most anterior radicular arteries are small, and end in the ventral nerve roots or in the pial plexus of the cord. The small posterior radicular arteries also supply the dorsal root ganglia: branches enter at both ganglionic poles to be distributed around ganglion cells and nerve fibres. (See also Crock 1996.)
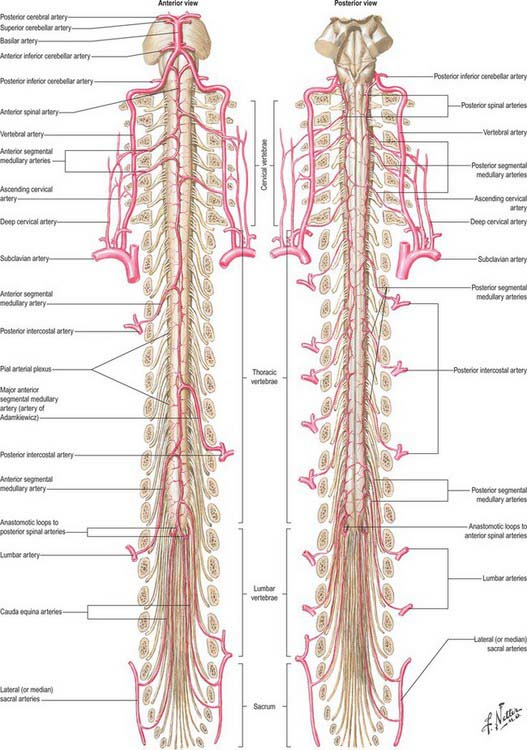
Fig. 43.9 Arteries of the spinal cord.
(Reprinted from Netter Anatomy Illustration Collection, © Elsevier Inc. All Rights Reserved.)
Segmental medullary feeder arteries
Some radicular arteries, mainly situated in the lower cervical, lower thoracic and upper lumbar regions, are large enough to reach the anterior median sulcus where they divide into slender ascending and large descending branches. These are the anterior medullary feeder arteries (Dommisse 1975). They anastomose with the anterior spinal arteries to form a single or partly double longitudinal vessel of uneven calibre along the anterior median sulcus. The largest anterior medullary feeder, the great anterior segmental medullary artery of Adamkiewicz, varies in level, arising from a spinal branch of either one of the lower posterior intercostal arteries (T9–11), or of the subcostal artery (T12), or less frequently of the upper lumbar arteries (L1 and L2). It most often arises on the left side (Carmichael & Gloviczki 1999). Reaching the spinal cord, it sends a branch to the anterior spinal artery below and another to anastomose with the ramus of the posterior spinal artery which lies anterior to the dorsal roots. It may be the main supply to the lower two-thirds of the cord. Central branches of the anterior spinal artery enter the anterior median fissure, and then turn right or left to supply the ventral grey column, the base of the dorsal grey column, including the dorsal nucleus, and the adjacent white matter (Fig. 43.9).
Each posterior spinal artery contributes to a pair of longitudinal anastomotic channels, anterior and posterior to the dorsal spinal roots. These are reinforced by posterior medullary feeders from the posterior radicular arteries. The latter are variable in number and size, but smaller, more numerous and more evenly distributed than the anterior medullary feeders. The anterior channel is joined by a ramus from the descending branch of the great anterior segmental medullary artery of Adamkiewicz. In all longitudinal spinal arteries the width of the lumen is uneven, and complete interruptions may occur. At the conus medullaris they communicate by anastomotic loops. Anastomoses other than those between the pial or peripheral spinal arterial branches may be important, e.g. a posterior spinal series of anastomoses between rami of the dorsal divisions of segmental arteries near the spinous processes.
Intramedullary arteries
The central branches of the anterior spinal artery supply about two-thirds of the cross-sectional area of the cord. The rest of the dorsal grey and white columns and peripheral parts of the lateral and ventral white columns are supplied by numerous small radial vessels which branch from posterior spinal arteries and the pial plexus. In a microangiographic study of the human cervical spinal cord, up to six anterior, and eight posterior, radicular spinal arteries were described, and up to eight central branches arose from each centimetre of the anterior spinal artery (Turnbull et al 1966).
Spinal cord ischaemia
The spinal cord can rely neither for its transverse nor for its longitudinal blood supply entirely on the longitudinal arteries. The anterior longitudinal artery and the intramedullary arteries are functional endarteries, although overlap of territories of supply has been described. Damage to the anterior longitudinal artery can result in loss of function of the anterior two-thirds of the cord. The longitudinal arteries cannot supply the whole length of the cord, and the input of the segmental medullary feeder vessels is essential. This is especially true of the artery of Adamkiewicz (great anterior segmental medullary artery), which may effectively carry the major supply for the lower cord. The midthoracic cord, distant from the main anterior medullary feeders, is particularly liable to become ischaemic after periods of hypotension.
VEINS
The venous drainage of the spinal cord (Fig. 43.11) follows a similar pattern to that of its arterial supply (Gillilan 1970). Intramedullary veins within the substance of the cord drain into a plexus of surface veins, the coronal plexus. There are six tortuous longitudinal channels within this plexus, one in each of the anterior and posterior median fissures, and four others which run on either side of the ventral and dorsal nerve roots. Only the anterior median vein, which drains the central grey matter, is consistently complete. These vessels connect freely and drain superiorly into the cerebellar veins and cranial sinuses, and segmentally mainly into medullary veins. The segmental veins drain into the intervertebral veins and thence into the external vertebral venous plexuses, the caval and azygos systems.
Segmental veins
Anterior and posterior medullary veins run along some of the ventral and dorsal roots. They are larger than radicular veins, and drain the cord but not the roots themselves. Like the medullary feeder arteries, they are largest in the cervical and lumbar regions of the cord, but do not necessarily occur in the same segments as the medullary feeders. Anterior and posterior great medullary veins may arise in the lower thoracic or upper lumbar cord segments. There are 8–14 anterior medullary veins; posterior medullary veins are more numerous.
Very small anterior and posterior radicular veins occur in most spinal segments, accompanying and draining the ventral and dorsal roots and some of the cord at the points of entry and exit of the rootlets: they usually drain into the intervertebral veins.
THE EFFECTS OF INJURY
SPINAL CORD INJURY AND VERTEBRAL COLUMN INJURY
In the assessment of a patient with spinal injury and neurological damage, it is important to remember that the level of cord and root injury will not coincide with that of the skeletal damage to the vertebral column.
In estimating the vertebral levels of cord segments in the adult, a useful approximation is that in the cervical region the tip of a vertebral spinous process corresponds to the succeeding cord segment (i.e. the sixth cervical spine is opposite the seventh spinal segment); at upper thoracic levels the tip of a vertebral spine corresponds to the cord two segments lower (i.e. the fourth spine is level with the sixth segment), and in the lower thoracic region there is a difference of three segments (i.e. the tenth thoracic spine is level with the first lumbar segment). The eleventh thoracic spine overlies the third lumbar segment and the twelfth is opposite the first sacral segment. In making this estimate by palpation of the vertebral spines, the relationship of the individual spines to their vertebral bodies should be remembered (p. 745).
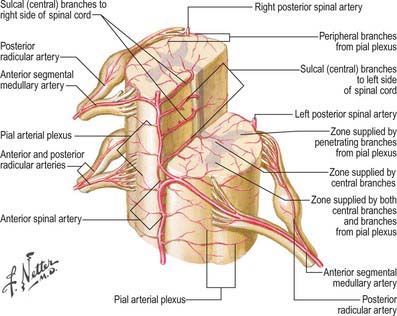
Fig. 43.10 Arterial disposition within the spinal cord.
(Reprinted from Netter Anatomy Illustration Collection, © Elsevier Inc. All Rights Reserved.)
Complete division above the fourth cervical segment causes respiratory failure because of the loss of activity in the phrenic and intercostal nerves. Lesions between C5 and T1 paralyse all four limbs (quadriplegia), the effects in the upper limbs varying with the site of injury: at the fifth cervical segment paralysis is complete; at the sixth, each arm is positioned in abduction and lateral rotation, with the elbow flexed and the forearm supinated, due to unopposed activity in the deltoid, supraspinatus, rhomboid and brachial flexors (all supplied by the fifth cervical spinal nerves). In lower cervical lesions upper limb paralysis is less marked. Lesions of the first thoracic segment paralyse small muscles in the hand and damage the sympathetic outflow to the head and neck, resulting in contraction of the pupil, recession of the eyeball, narrowing of the palpebral fissure and loss of sweating in the face and neck (Horner’s syndrome). However, sensation is retained in areas innervated by segments above the lesion, thus cutaneous sensation is retained in the neck and chest down to the second intercostal space, because this area is innervated by the supraclavicular nerves (C3 and C4). At thoracic levels, division of the cord paralyses the trunk below the segmental level of the lesion, and both lower limbs (paraplegia). The first sacral neural segment is approximately level with the thoracolumbar vertebral junction: injury, which commonly occurs here, paralyses the urinary bladder, the rectum and muscles supplied by the sacral segments, and cutaneous sensibility is lost in the perineum, buttocks, the back of the thighs and the legs and soles of the feet. The roots of lumbar nerves descending to join the cauda equina may be damaged at this level, causing complete paralysis of both lower limbs. Lesions below the first lumbar vertebra may divide or damage the cauda equina, but severe nerve damage is uncommon and is usually confined to the spinal roots at the level of the trauma. Neurological symptoms may also occur as a result of interference with the spinal blood supply, particularly in the lower thoracic and upper lumbar segments.
Spinal cord injury without radiological abnormality: ‘SCIWORA’
The spinal cord may be damaged without radiological evidence of skeletal injury in some injuries to the vertebral column. This is particularly liable to occur if the vertebral canal is abnormally narrowed, usually by osteoarthritic changes. In the elderly patient there may in addition be occlusive arterial disease, directly compromising an already precarious blood supply to the cord. This type of injury not uncommonly occurs in hyperextension injuries of the cervical spine in this age group. The cause of the damage may be direct injury to neural tissue by osteophytes or by an infolded ligamentum flavum, or direct or indirect injury to the vasculature of the cord. For cervical spinal injury, several cord syndromes have been described, relating the clinical picture to the anatomy of the neurological lesion within the cord. The commonest of these is central cord syndrome, which usually results from hyperextension injury to an osteoarthritic neck, in which the major injury is to the central grey matter. This gives a greater motor loss in the upper than in the lower limbs, with variable sensory loss. In anterior cord syndrome, which may occur in flexion–compression injuries of the neck, the damage occurs in the area of supply of the anterior spinal artery, sparing the posterior columns: the motor loss is usually proportionately greater in the lower than in the upper limbs, while sensory loss is less of a problem.
LESIONS OF THE SPINAL ROOTS, NERVES AND GANGLIA
Spinal roots, nerves and ganglia may be damaged in the vertebral and root canals and at the intervertebral foramina. Neurofibromas may occur on the roots and nerves in the root canals. As they enlarge these tumours become dumb-bell shaped, with both an intra- and an extra-spinal component in continuity: the clinical picture may thus include paradoxical features as the asymmetrical space-occupying lesion grows. Root compression usually presents acutely with pain which may be severe. The pain, paraesthesiae and numbness occur in a dermatomal distribution, and it may be difficult to demonstrate sensory loss on the trunk, because of dermatomal overlap. Severe traction injuries of the upper limbs may cause avulsion of spinal roots from the cord in the cervical region.
The anatomical basis for pain of spinal origin
In the diagnosis and description of pain of spinal origin it is particularly important to distinguish between radicular pain and somatic referred pain.
Radicular pain is caused by disorders that affect a spinal nerve or its dorsal root ganglion. This type of pain characteristically radiates, along a narrow band, into the area of the limb (or trunk wall) that the spinal nerve supplies, and is typically (although not always) lancinating in quality, not unlike an electric shock.
Referred pain is pain felt in the distribution of a nerve that is not the nerve that innervates the source of pain. Typically these nerves are derived from the same spinal cord segment. Referred pain is felt deeply, is typically aching in quality, and is felt across a broad area; although it may radiate into a limb (or around the trunk wall), it is typically constant in location. Patients may find it hard to define the outer boundaries of this pain, but they can clearly identify the centre of its distribution. Referred pain can be subdivided into somatic referred pain and visceral referred pain, according to its origin. Visceral referred pain is caused by disorders in viscera. In some instances, the referral can be quite remote from the viscus, as in cardiac pain that radiates into the neck. Somatic referred pain is caused by disorder of muscle and skeletal structures. Examples include lumbar spinal pain referred to the gluteal region, thigh, or leg; cervical spinal pain referred to the upper limb girdle; and cervical or thoracic spinal pain referred to the chest wall, where it can mimic cardiac pain. Often the source of somatic referred pain cannot be determined by clinical examination, and requires invasive tests to pinpoint its location.
LESIONS OF THE CONUS AND CAUDA EQUINA
Lesions of the conus and cauda equina, e.g. tumours, cause bilateral deficit, often with pain in the back extending into the sacral segments and to the legs. Loss of bladder and erectile function can be early features. There are lower motor neurone signs in the legs with fasciculation and muscle atrophy. Sensory loss usually involves the perineal or ‘saddle area’ as well as involving other lumbar and sacral dermatomes. There may be congenital abnormalities, e.g. spina bifida, lipomata or dystematomyelia, and the conus may extend below the lower border of L1, often with a tethered filum terminale. Extramedullary lesions include prolapsed intervertebral discs. A midline (central) disc protrusion in the lumbar region may present with involvement of only the sacral segments.
CLINICAL PROCEDURES
ACCESS TO CEREBROSPINAL FLUID
The safest approach to the cerebrospinal fluid (CSF) is to enter the lumbar cistern of the subarachnoid space in the midline, well below the level at which the spinal cord normally terminates (see above). The fine needle employed is unlikely to damage the mobile nerve roots of the cauda equina. This procedure is called lumbar puncture. It is also possible to access the CSF by midline puncture of the cerebello-medullary cistern (cisterna magna) (Ch. 16): this is cisternal puncture.
Lumbar puncture: adult
Lumbar puncture in the adult may be performed with the patient either sitting or lying on the side on a firm flat surface. In each position, the lumbar spine must be flexed as far as possible, in order to separate the vertebral spines maximally and expose the ligamentum flavum in the interlaminar window (Fig. 43.12). A line is then taken between the highest points of the iliac crests: this line intersects the vertebral column just above the palpable spine of L4. With the spines now identified, the skin is anaesthetized and a needle is inserted between the spines of L3 and L4 (or L4 and L5). Exact identification of the level by palpation is difficult (Broadbent et al 2000). The soft tissues which the needle will ultimately traverse should also be anaesthetized, though care should be taken lest the injection of an excessive amount of local anaesthetic compromises appreciation of the structures being traversed. These include the subcutaneous fat, and supraspinous and interspinous ligaments down to the ligamentum flavum itself. The lumbar puncture needle may then be inserted in the midline or just to one side, and angled in the horizontal and sagittal planes sufficiently to pierce the ligamentum flavum in or very near the midline. There is then a slight loss of resistance as the needle enters the epidural space, and careful advancement will next pierce the dura and arachnoid to release CSF.
Lumbar puncture: neonate and infant
At full term (40 weeks) the spinal cord usually terminates somewhat lower than the adult level, sometimes reaching the body of L3. The supracristal plane intersects the vertebral column slightly higher (L3–4). By the second postnatal month the level of cord termination has usually reached its permanent position level with the body of the first lumbar vertebra. The lower end of the subarachnoid spine is found at sacral levels 1 or 2. These differences must be borne in mind when identifying the landmarks before undertaking lumbar puncture in the neonate and infant.
A lumbar puncture is performed by placing the baby in a position, either lying or ‘sitting’, which gives maximum convex curvature to the lumbar spine. A needle with trocar is inserted into the back between the spines of the third and fourth lumbar vertebrae and into the subarachnoid space below the level of the conus medullaris. The space between L3 and L4 is approximately level with the iliac crests and it is usual to insert the needle and trocar into the intervertebral space immediately above or below the iliac crests.
Cisternal puncture
In cisternal puncture, the cisterna magna (Ch. 16) is entered by midline puncture through the posterior atlanto-occipital membrane. Further details of this difficult specialist technique are beyond the scope of this book.
ACCESS TO THE EPIDURAL SPACE
The epidural space lies between the spinal dura and the wall of the vertebral canal. It contains epidural fat and a venous plexus. Access to this space, usually in the lumbar region, is required for the administration of anaesthetic and analgesic drugs, and for endoscopy. The caudal route is used mainly for analgesic injections.
Thoracic and cervical epidurals
It is possible to access the epidural space at thoracic and cervical levels, but the specialist techniques required are outside the scope of this book. The principles are the same as those for lumbar epidurals, but the special anatomy of the vertebral spines at the other levels requires the angle of approach to be modified.
Lumbar epidural
For access to the lumbar epidural space, the approach is as for lumbar puncture. The intention in epidural injection is to avoid dural puncture, so it is best to enter the epidural space in the midline posteriorly, where the depth of the space is greatest. Techniques for entering the epidural space rely on the appreciation of loss of resistance to injection of the chosen medium (usually air or saline) as the space is entered. This loss of resistance results from the fact that the pressure in the epidural space is subatmospheric. There is very little distance between the ligamentum flavum and the underlying dura on either side of the median plane.
Caudal epidural
The route of access to the caudal epidural space is via the sacral hiatus. The space is therefore entered below the level of termination of the dural sac, i.e. the subarachnoid space and its contained CSF, which usually extends to the level of the second sacral segment. Occasionally the dural sac ends as high as the fifth lumbar vertebra, and very rarely it may extend to the third part of the sacrum, in which case it is occasionally possible to enter the subarachnoid space inadvertently during the course of a sacral nerve block.
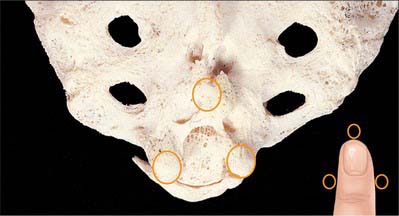
With the patient in the lateral position or lying prone over a pelvic pillow, the sacral hiatus is identified by palpation of the sacral cornua (Fig. 43.13). These are felt at the upper end of the natal cleft approximately 5 cm above the tip of the coccyx. Alternatively, the sacral hiatus may be identified by constructing an equilateral triangle based on a line joining the posterior superior iliac spines: the inferior apex of this triangle overlies the hiatus. After local anaesthetic infiltration, a needle is introduced at 45° to the skin, to penetrate the posterior sacrococcygeal ligament and enter the sacral canal. Once the canal is entered, the hub of the needle is lowered so that the needle may pass along the canal (Fig. 43.2, Fig. 43.14). If the needle is angled too obliquely it will strike bone; if it is placed too superficially it will lie outside the canal. The latter malposition can be confirmed by careful injection of air while palpating the skin over the lower sacrum.
Barson AJ, Sands J. Regional and segmental characteristics of the human adult spinal cord. J Anat. 1977;123:797-803.
Bogduk N. Clinical Anatomy of the L Spine and Sacrum, 4th edn. Edinburgh: Elsevier Churchill Livingstone, 2005.
Broadbent CR, Maxwell WE, Ferrie R, Wilson DJ, Gawne-Cain M, Russell R. Ability of anaesthetists to identify a marked lumbar interspace. Anaesthesia. 2000;55:1122-1126.
Carmichael SW, Gloviczki P. Anatomy of the blood supply to the spinal cord: the artery of Adamkiewicz revisited. Perspect Vasc Surg. 1999;12:113-122.
Crock HV. An Atlas of Vascular Anatomy of the Skeleton and Spinal Cord. London: Martin Dunitz, 1996.
Dean NA, Mitchell BS. Anatomic relation between the nuchal ligament (ligamentum nuchae) and the spinal dura mater in the craniocervical region. Clin Anat. 2002;15:182-185.
Dommisse GF. The Arteries and Veins of the Human Spinal Cord From Birth. Edinburgh: Churchill Livingstone, 1975.
Fraher JP. The transitional zone and CNS regeneration. J Anat. 2000;196:137-158.
Gillilan LA. Veins of the spinal cord. Anatomic details; suggested clinical applications. Neurology. 1970;20:860-868.
Haines DE, Harkey HL, Al-Mefty O. The ‘subdural’ space: a new look at an outdated concept. Neurosurgery. 1993;32:111-120.
Proposes the view that the subdural ‘space’ is a pathological cleavage plane rather than a normal anatomical element..
Kiddo DK, Gomez DG, Pavese AM, Potts DG. Human spinal arachnoid granulations. Neuroradiol. 1976;11:221-228.
Kubik S, Müntener M. Zur Topographie der spinalen Nervenwurzeln. II Der Einfuss des Wachstums des Duralsackes, sowie der Krümmagen und der Bewegungen der spinalen Nervenwurzeln. Acta Anat. 1969;74:149-168.
An alternative view of the obliquity of the cervicothoracic spinal nerve roots based on observations of differential cord growth..
Neidre A, Macnab I. Anomalies of the lumbosacral nerve roots. Spine. 1983;8:294-299.
Newell RLM. The spinal epidural space. Clin Anat. 1999;12:375-379.
Turnbull IM, Brieg A, Hassler O. Blood supply of cervical spinal cord in man. A microangiographic cadaver study. J Neurosurg. 1966;24:951-965.