Chapter 28 Infertility and assisted conception
After reading this chapter, you will be able to:
Introduction
Midwives will frequently care for couples that have received assistance in achieving their pregnancy. It is important to understand the processes they have to go through to become pregnant, which can contribute to the attitudes and anxieties demonstrated by individuals and create greater challenges for the midwifery team. Most couples will have received their fertility treatment in the private sector and occasionally the transition into normal NHS care may itself be stressful. This chapter provides a background on the issues related to infertility and the factors contributing to the patients’ approach to their pregnancy. It is useful for midwives to establish links with their local fertility services to provide support and information on patients to ease the transition into their care. Improved networking between fertility nurses and midwives would complement care provided in both areas. Awareness of the range of treatment options and the side-effects from treatment provides insight into both the physical and emotional condition of couples requiring midwifery care.
Multiple births are a key issue of concern in both areas of practice and it is essential that couples understand the risks and the care required when pregnant. Singleton pregnancies with the prevention of twin and triplet pregnancies are the ultimate goal of all fertility clinics, requiring appropriate management and monitoring of treatment. Many nurses and midwives have specialized in fertility, providing care and extending their role to perform ultrasound scanning, intrauterine insemination, embryo transfer and implications counselling (Barber 2002). Where possible, it is important for midwives and nurses to normalize pregnancies for couples, enabling them to enjoy their much-desired pregnancy.
Technological advances within the field of reproduction have increased public awareness of infertility and demand for related services. Approximately one in six couples will experience problems conceiving a child (Hull et al 1985, Templeton et al 1990) and will seek assistance to achieve a pregnancy. Since the birth of the world’s first ‘test-tube baby’ over 30 years ago, over one million babies have been born in the UK from in vitro fertilization (IVF). Research has highlighted the stigma, psychological morbidity and long-term implications caused by the experience of infertility on couples (Kerr et al 1999), and these factors impact on successful and unsuccessful couples. Over 95% of service provision exists within the private sector, thus providing a financial hurdle that couples must overcome prior to commencing their treatment.
Human fertilisation and embryology authority (HFEA)
The HFEA was created by the 1990 Act to license and monitor clinics performing various fertility treatments (including IVF, donated egg/sperm/embryo procedures, or research on embryos). All licensed clinics must have a delegated ‘person responsible’ who ensures that the conditions of the licence are carried out. Several forms of treatment are not controlled by the HFEA but may still potentially create similar problems to licensed treatments, including funding, ovarian hyperstimulation and multiple births. The HFEA carries out annual inspections to enable clinics to renew their licences, which include reviewing the welfare of any potential child, clinical and laboratory standards, protocols for practice in all areas and the safety of patients and their families.
As well as a Code of Practice and information to staff and patients, the HFEA maintains a formal register of information regarding specific donors, donor treatments and children born from these treatments (HFEA 2009). (For more information, see the website).
Causes of infertility
See Table 28.1.
Table 28.1 Main causes of infertility
| Incidence | |
|---|---|
| Anovulation | 26% |
| Endometriosis | 3% |
| Tubal damage | 13% |
| Unexplained | 30% |
| Male factor | 30% |
Anovulation
Anovulation may be diagnosed by the GP and may be corrected by drug therapy to initiate ovulation with an anti-oestrogen, such as clomifene – a common treatment for polycystic ovary syndrome (PCOS). Anovulation must be investigated as it may be caused by pathology such as hyperprolactinaemia, which could be corrected and prevent the woman undergoing fertility treatments.
Elevated levels of prolactin inhibit the normal hormonal feedback loop that initiates ovulation. If serum prolactin is elevated to 1000 mU/L, the test should be repeated. This could be caused by stress or from a prolactin-secreting pituitary adenoma or macroadenomas diagnosed with MRI. Treatment includes bromocriptine or cabergoline, which reduce the elevated levels of prolactin and restore normal endocrine activity and facilitate ovulation.
Anovulation can be divided into primary and secondary amenorrhoea – these are primarily caused by pituitary tumours, pituitary ablation, Kallmann syndrome and cancer treatments (see Box 28.1).
Polycystic ovary syndrome (PCOS)
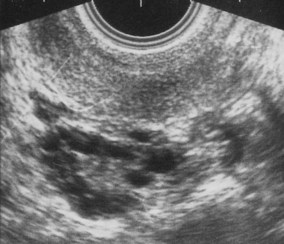
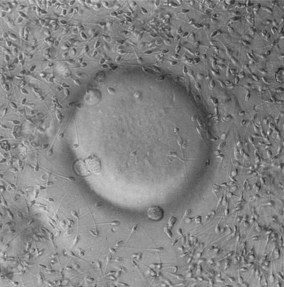
This syndrome is frequently detected in women undergoing investigations for anovulation (Kousta et al 1999). It is characterized by cystic ovaries with more than 10 cysts, 2–8 mm in diameter, distributed around and through an echodense, thickened stroma (Fig. 28.1).
Endocrine features include a raised serum level of luteinizing hormone (LH) and/or testosterone, causing symptoms of acne, hirsutism (hyperandrogenism), oligoamenorrhoea and obesity (Box 28.2). The hypersecretion of LH is associated with menstrual irregularity and infertility. Obesity leads to hypersecretion of insulin, stimulating ovarian secretion of androgens with increased risk of the development of type 2 diabetes (Kousta et al 2000). Anovulation is also associated with endometrial hyperplasia due to increased oestrogen production unopposed by progesterone (Balen & Jacobs 1997). Women with a body mass index (BMI) >28 kg/m2 and <20 kg/m2 will have decreased fertility. There is an associated deficiency in gonadotrophin production with excessive weight loss, due to diminished production of gonadotrophin-releasing hormone (GnRH).
Ovarian failure
Ovarian failure can happen at any age. If prior to puberty, it is commonly associated with chromosomal abnormality, such as Turner syndrome (45X) (see Ch. 26), or sterility resulting from radiotherapy or chemotherapy for childhood malignancy. Ovarian failure linked with raised gonadotrophins and cessation of periods prior to the age of 40 is associated with autoimmune failure, infection, previous surgery and cancer treatments. There is also a suggested link with familial forms of fragile X (Balen & Jacobs 1997).
Endometriosis
This is caused when endometrial tissue is located outside the uterus, around the pelvis. This may be noted at laparoscopy as blue/black pigmentation (old lesions), red vasculated lesions (active lesions) and white non-pigmented papules (just activating) (Gould 2003). Retrograde menstruation is thought to be the most common cause of endometriosis but altered immune function is also thought to be associated with the condition. It causes pelvic pain, dyspareunia, dysmenorrhea and infertility. Pelvic adhesions, especially around the ovaries and tubes, with cystic lesions on the ovaries, called endometriomas, are common. Symptoms are linked with the menstrual cycle, age and hormonal therapy; treatments include drugs that interfere with the cycle. One group are GnRH agonists, which cause pituitary desensitization, inducing amenorrhoea; another are inhibitors of gonadotrophin secretion, such as danazol, which also have androgenic effects that cause unpleasant side-effects, including hot flushes, acne, oily skin, hirsutism, reduced libido, weight gain, nausea and headaches. Both groups of drug temporarily stop menses and reduce levels of antiendometrial autoantibodies (Balen & Jacobs 1997).
Tubal factors
Tubal damage is commonly associated with pelvic inflammatory disease (PID), ectopic pregnancy, sterilization and adhesions. Increases in sexually transmitted diseases increase the risk of PID and tubal damage. Chlamydia is the most frequently reported infection and is often asymptomatic, which increases the risk of cross-contamination and failure to treat (Byrd 1993). Adhesions commonly result following pelvic infection and subsequently create further problems, including distortion and/or blockage of the fallopian tubes; development of hydrosalpinx; impaired tubal motility and movement of the oocyte; and ovarian adhesion against the pelvic sidewall, which may interfere with the movement of the oocyte into the fimbria of the fallopian tube (Dechaud & Hedon 2000), increasing the risk of ectopic pregnancy (see Ch. 54 and website).
Unexplained infertility
Unexplained infertility is the inability to conceive after 1 year without any identified causative factors. Approximately 40–65% of couples in this category will conceive spontaneously within 3 years (Balen & Jacobs 1997). Age has a direct effect on the duration of time to try to conceive naturally prior to commencing fertility treatment.
Treatment options consist of improving fertility initially for the woman with drugs to enhance ovulation. Also it is possible to improve sperm function by inseminating prepared sperm into the uterus (intrauterine insemination [IUI]).
Male infertility
Male factor infertility contributes to 30% of couples seeking treatment. A decline in semen quality over the last few decades has been suggested, though the evidence remains inconclusive with little scientific knowledge of the aetiology (Shakkebaek & Keiding 1994). A full and comprehensive history of each case is an essential element in the assessment of male fertility and should include:
Causes of infertility include:
Tests should include the following:
Table 28.2 WHO criteria for a normal sperm count
| Volume | 2 mL or more |
| pH | 7.2 or more |
| Count | ≥20 × 106/mL (azoospermia is diagnosed when no sperm is found in the ejaculate and oligospermia is diagnosed when the concentration of sperm is vastly reduced) |
| Motility | 50% or more with forward progression, or 25% or more with rapid progression (within 60 minutes of ejaculation) (abnormal = asthenozoospermia) |
| Morphology | The 1999 edition of the WHO manual does not define normal ranges for morphology but notes that data from IVF programmes suggest that as sperm morphology falls below 15% normal forms (teratozoospermia), the fertilization rate decreases |
| MAR test (Antisperm antibodies) | <50% of motile sperm with adherent particles |
| Immunobead test (Antisperm antibodies) | <50% of motile sperm with adherent beads |
Source: WHO 1999
Various solutions to these problems are available and micromanipulation techniques, such as intracytoplasmic sperm injection (ICSI), have helped to overcome many male infertility problems (see website). Previously, steroids were administered to decrease the male immune response and thus improve chances of fertilization, but now are rarely used. Environmental factors such as pesticides, alcohol, cigarettes and drug abuse can reduce male fertility and a decrease in consumption of recreational toxins may sometimes improve semen parameters.
Female infertility – treatment and management
Ovulation induction
There are two types of drug regimen for ovulation induction – the most basic of fertility treatments.
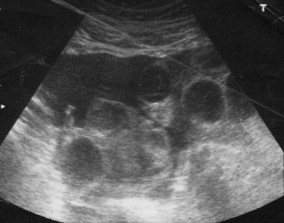
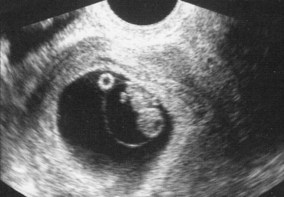
OHSS occurs if too many follicles are stimulated during a treatment cycle of ovulation induction (OI), intrauterine insemination (IUI) and in vitro fertilization (IVF). As many follicles are stimulated, especially in the case of PCO and PCOS, this causes abdominal ascites, pleural and pericardial effusions, discomfort, nausea, vomiting, difficulty breathing, electrolyte imbalance leading to dehydration, and an increased risk of deep vein thrombosis. Ultrasound examination reveals enlargement of the ovaries to a diameter greater than 5 cm (Fig. 28.2).
Donor insemination (DI)
This treatment is appropriate for couples with azoospermia, paternal genetic abnormalities or those unable to afford IVF and ICSI, with national success rates of 9.6% per cycle (Thornton 2000). DI is carried out during the woman’s own natural cycle or with superovulation. Monitoring with transvaginal ultrasound is undertaken to identify one leading follicle prior to ovulation and insemination. When more than two leading follicles are stimulated with superovulation, the cycle should be cancelled due to the risk of multiple pregnancy.
Intrauterine insemination (IUI)
IUI involves monitored superovulation and insemination of prepared sperm 35 hours post administration of human chorionic gonadotrophin (hCG) to initiate ovulation. The semen may be prepared for insemination using one of a variety of techniques, including sperm swim up and gradient density procedures (see website). This treatment is appropriate for slightly suboptimal sperm parameters, unexplained infertility with normal semen parameters and factors such as female age. It does not provide information on potential problems associated with fertilization and has lower success rates than does IVF.
Gamete intrafallopian tube transfer (GIFT)
GIFT has generally been superseded by IVF, though is still offered by some clinics. GIFT involves superovulation, following which the oocytes are removed via laparoscopy and a prepared sperm sample is deposited into the fallopian tube to facilitate fertilization. Consequently, this is not suitable for women with tubal damage and it yields no information on the possible fertilization problems.
IVF
Louise Brown was born in 1978 following pioneering work by Steptoe and Edwards, and since that time IVF has enabled many thousands of couples to achieve a much-desired child. The technique combines superovulation, transvaginal ultrasound-guided oocyte retrieval, insemination of oocyte with sperm in the laboratory, fertilization and replacement of embryos.
Debate surrounds the number of embryos to be replaced in the uterus but many clinics in the UK routinely replace two embryos, achieving similar pregnancy rates to three-embryo replacements.
Current IVF treatments use daily injections of drugs (gonadotrophins) to induce the development of multiple follicles in the ovaries. The oocytes mature within these follicles, are collected and are fertilized in the laboratory.
Immature eggs from unstimulated ovaries can also be collected, matured in the laboratory for 24–48 hours and, once mature, fertilized prior to embryo transfer. Hence, oocyte maturation happens in the laboratory rather than the body.
Drug management (Table 28.3)
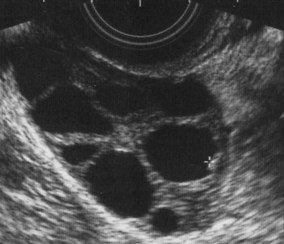
Superovulation is achieved with the administration of FSH injections – a purified preparation delivered subcutaneously via autoinjector (dosage from 50 to 350 IU), which recruits a cohort of follicles and promotes development and maturation (Fig. 28.3). To ensure maturation of the follicles and oocytes, administration of hCG is required 35 hours prior to oocyte retrieval.
To establish appropriate management of superovulation and avoid premature ovulation due to the LH surge, many units incorporate GnRH agonists or antagonists to prevent ovulation (administered either subcutaneously or nasally). These bind to GnRH receptors on the pituitary gonadotrophins and desensitize the pituitary. The antagonists lead to immediate suppression and are used for a much shorter time, and initiate a flare response which causes a withdrawal bleed in the woman.
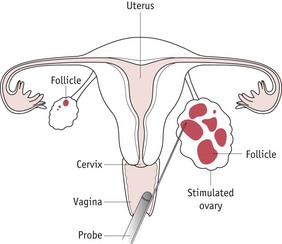
Oocyte collection
This is undertaken as an outpatient procedure using transvaginal ultrasound (Fig. 28.4). Intravenous sedation reduces levels of pain and anxiety and partners can accompany the women through the procedure. Follicles are aspirated using gentle suction via a preset vacuum pump into small tubes, which are identified by the embryologist. A microscope is required to identify the cumulus/oocyte mass. Follicles are frequently reinflated with culture medium to encourage recovery of oocytes. Retrieved oocytes are placed in the incubator with appropriate labelling of the dishes and compartment in the incubator and are double witnessed to ensure correct safety procedures.
Sperm preparation
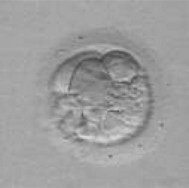
Sperm samples may be collected either prior to oocyte retrieval or following the procedure, but should be prepared within 30 minutes of production. Sperm preparation follows the same procedure as described in the IUI treatment. Semen samples may be frozen and subsequently defrosted for preparation prior to insemination. After insemination, the spermatozoa and oocytes (Fig. 28.5) are cultured overnight, then, approximately 16–18 hours later, are assessed for signs of fertilization, and the number and grade of polar bodies extruded from the oocyte. These criteria can also aid in the detection of abnormal fertilization. The appearance of two pronuclei – one each from the sperm and oocyte – signifies normal fertilization. Occasionally, more than two pronuclei are detected, indicating abnormal fertilization, and these embryos should not be transferred.
Fertilization
Fertilization commences when the sperm binds to the zona pellucida of the oocyte (for more information, see website and Ch. 29).
Embryo grading
Embryos are closely monitored for quality and potential ability to implant and create a pregnancy. Embryo grading is based on visual morphological criteria; it cannot rule out the possibility of a genetic abnormality within the embryo and therefore does not guarantee the selection of a viable embryo for replacement. Preimplantation genetic diagnosis (PGD) is the only way to identify a potential genetic anomaly, including: chromosomal, X-linked, autosomal recessive and dominant, and mitochondrial abnormalities (ESHRE PGD Consortium Steering Committee 2000).
For more information on embryo grading, see website.
Fragmentation
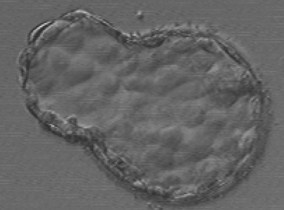
The causes of fragmentation within the embryo (Fig. 28.7) are unknown and have been linked with poor culture conditions and blastomere loss through apoptosis, possibly from chromosomal abnormalities. Whatever the underlying pathology, fragmentation is clearly associated with decreased implantation (Scott 2002). The process of fragmentation has been identified as early as the two-cell stage and continues to develop throughout cleavage.
Embryo transfer
Embryo transfer takes place approximately 48 hours after oocyte recovery. The embryo normally contains several blastomeres at this stage, ranging from two to six in number (Fig. 28.6). Embryos may be cultured for 5 days until they have reached blastocyst stage before returning them into the uterus, but there is no evidence to prove that blastocyst replacement provides better pregnancy rates than do the day-2 transfers.
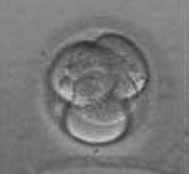
(Courtesy of Dr Susan Pickering, Senior Lecturer, Division of Women’s Health, Kings College, London.)
A speculum is inserted into the vagina, and the cervix is wiped with a dry swab to remove excess mucus. The embryos are placed into a fine plastic catheter and passed, sometimes by ultrasound guidance, through the cervix into the endometrium (Fig. 28.8). If the cervix is convoluted or a tight internal os is encountered, the malleable outer sheath of the catheter is adapted to pass through the obstruction. After embryo replacement, the woman is placed on luteal support to prevent a fall in progesterone levels, which could directly affect the function of the endometrium at this crucial time during potential implantation. Commonly, progesterone pessaries are administered twice daily until the pregnancy test is performed 14 days post embryo transfer. After IVF treatment, the ovaries contain multiple corpora lutea, which remain enlarged for the subsequent few weeks; these may contribute to symptoms of bloating and discomfort and the woman should be advised of the symptoms.
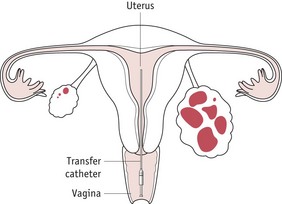
(Courtesy of Janet Currie, Sister, Oxford Fertility Unit, Level 4, Women’s Centre, Headington, Oxford.)
Blastocyst transfer is another treatment option for couples undergoing IVF treatment. During normal physiological fertilization the sperm fertilizes the oocyte in the fallopian tube, which moves down the tract until it reaches the endometrium around day 5 post fertilization. Blastocyst development has been difficult to achieve in vitro due to inadequate culture media. As technology has developed, improved sequential media have resulted in higher rates of blastocyst development. Embryos are usually replaced on day 2 or 3, which does not correlate with implantation in vivo. As some embryos will not reach blastocyst stage but look completely normal at the day 2–3 stage, it has been suggested that blastocyst transfer enables the embryologist to assess the quality of the embryo for longer and in greater detail, allowing a better choice of embryos with increased implantation potential (Fig. 28.9). New technology associated with blastocyst transfer has improved success rates and centres are beginning to replace single blastocysts to maintain good pregnancy rates and decrease the multiple pregnancy rate. Blastocysts can be frozen to ensure patients can maximize their potential chance of success from a fresh cycle. Vitrification – a new technique of ultra-rapid freezing – allows the embryo to be chilled to liquid nitrogen temperatures (−196°C) in a fraction of a second (Mukaida et al 2006).
In vitro maturation (IVM)
Women who have polycystic ovaries are most at risk of developing OHSS when undergoing fertility treatment, and in vitro maturation (IVM), which does not require fertility drugs, has been licensed in the UK. Without the superovulation of the ovaries there is no risk of the syndrome developing. This technique is established internationally and in 2007 the first babies (twins) were born in the UK.
IVM is appropriate in women with infertility requiring assisted conception and the success rate is known to be significantly related to the number of immature oocytes retrieved, and can be predicted by the antral follicle count (AFC). IVM may be particularly beneficial to women who have an AFC of more than 20. Women with PCOS or with ultrasonographic evidence of polycystic ovaries who are ovulating may be particularly amenable to IVM.
Standard IVF involves a lengthy process of pituitary downregulation with GnRH analogues followed by stimulation by gonadotrophins. This process is time consuming, the drugs involved are known to have side-effects, and the typical cost of drug treatment per cycle is currently around £600–1200. The only drug used in a cycle of IVM is a single injection of hCG 35 hours prior to oocyte collection. IVM has been demonstrated to be an effective treatment for infertile women with polycystic ovaries, although IVF is currently the gold standard treatment for infertility. Approximately 25–50% of women attending IVF clinics have PCO or PCOS (Jurema & Nogueira 2006).
There are many women presenting for fertility treatment for whom both treatments may be appropriate. It is essential they have reliable information on which to base their choice of treatment. No randomized controlled trials currently exist that compare IVF and IVM, although there has been a case-control study comparing such (see Table 28.4).
Table 28.4 Comparison of IVF and IVM
| IVM | IVF | |
| New | Well established | |
| Fertilization rate* | 77% | 78% |
| Cleavage rate* | 95% | 94% |
| Risk of ovarian hyperstimulation syndrome (OHSS) | Reduced | High risk |
| OHSS rate* | 0% | 12.1% |
| Treatment cycles | Shorter | |
| Treatment | hCG injection only | Daily injections required to stimulate ovaries |
| Oocytes | Fewer collected and fertilized | |
| Implantation, clinical pregnancy and live birth rates | Reduced rate – not significant | |
| Pregnancy rate | Difficult to predict at present | Good success rate |
* Data from a case–control study
There were no cases of OHSS in the IVM group. Severe OHSS occurs in around 1 in a 100 standard IVF cycles and usually requires admission to hospital for a few days. Severe OHSS is even more common in women who have polycystic ovaries on ultrasound scan or who have PCOS.
Advantages of IVM treatment compared with IVF
Studies from Scandinavian IVM programmes, in which only one or two embryos are transferred, report pregnancy rates in the region of 15–25% per cycle, though this is higher (≈30%) for women less than 36 years of age. In the region of 400 babies have been born worldwide from IVM treatment. Recent studies examining the health of these children have been reassuring, showing no increase in rates of abnormality. However, it must be recognized that the number of babies born is still relatively limited (Jurema & Nogueira 2006).
Factors affecting success in IVM treatment
One of the main factors affecting the success rate of IVF treatment, and possibly IVM, is the age of the woman.
The more eggs collected, the more embryos are produced, and greater the choice of embryos for transfer. The total number of resting follicles measured during a routine ultrasound scan is known as the antral follicle count (AFC) (see website) – shown to be an important predictor of success with IVM. On average, immature eggs are retrieved from half of the antral follicles present. However, as with IVF, there is always the risk that no eggs will be retrieved even when follicles are present or that those that are will not mature, fertilize or produce transfer embryos.
Cryopreservation
The cryostorage of semen, an essential service provided by most assisted conception units, is offered to patients who are or have:
The freezing/thaw procedure, however, will damage the cells and may reduce pre-freeze motility.
Embryo freezing can be performed provided that enough suitable spare embryos are available post embryo transfer. Embryos may be frozen at the pronuclear or the cleavage stage (two to eight cells); and must be good quality (grades 1, 2 and 3 or A, B and C) with less than 20% cytoplasmic fragmentation (Dale & Elder 1997) for the best chance of survival following the freeze/thaw procedure. It has been suggested that uneven blastomeres and large amounts of fragmentation could inhibit survival potential.
Cryopreservation of oocytes provides a treatment option for women prior to cancer treatment that may cause temporary or permanent sterility. Unfortunately, success rates from the use of frozen eggs is very low and only one baby has been successfully born from this technique in the UK.
Egg donation
In the UK, egg donation is maintained altruistically, primarily by anonymous donors, though occasionally donation from a family member or friend may be offered. This treatment can be used by women following the onset of premature menopause; genetic abnormality; or multiple failed IVF attempts.
Due to the national shortage of donated eggs, the National Gamete Donation Trust was established to promote altruistic donation. Many fertility units have long waiting lists for donors and have introduced alternative options, such as egg sharing. This treatment provides reduced-cost IVF treatment to couples willing to donate half of their eggs to another recipient couple. This creates ethical debate and the HFEA have produced guidelines for good practice and further review the appropriateness of the treatment for couples (HFEA 2009). Any couple who donate eggs have to undergo thorough counselling, detailed history taking, and investigations including karyotyping, blood grouping, screening for infections such as hepatitis A, B and C, HIV and syphilis, and cystic fibrosis screening. To donate eggs, the donor undergoes a full IVF cycle, which is time consuming and risky. Those with pre-existing disease and familial cancers are normally dissuaded from donation. Contraindications would include any uncertainty from partners.
Some patients travel overseas to obtain donated eggs, creating further problems for UK professionals as other countries do not have regulatory bodies, such as the HFEA, so there are no restrictions on the number of embryos that can be replaced. This has led to an increase in multiple births in the UK, which has serious consequences for all involved.
Surrogacy
Surrogacy involves a couple commissioning a woman to act as a host for their own genetic embryo and is a solution for women without a uterus or for whom a pregnancy may be contraindicated. It is illegal in the UK to pay a surrogate, but they may have their expenses paid. In English law, the woman who gives birth to the baby is the legal mother of the child, irrespective of the genetic origin of the child. The genetic parents therefore have to apply to adopt the child from the birth mother and this may be problematic should she change her mind about giving up her baby.
Outcome from IVF treatments
Outcome studies have closely monitored the births and development of children conceived from the techniques. Factors contributing to adverse outcome associated with assisted reproductive technology (ART) include maternal age, medical indications for infertility, paternal age and multiple pregnancies. The ICSI procedure has raised several issues as the process can potentially use sperm carrying genetic abnormalities, structural defects introduced by mechanical or biochemical damage when introducing foreign material into the oocyte and bypassing the natural selection process of fertilization (Kurinczuk 2003). Genetic screening is offered to couples requiring ICSI with poor sperm parameters (see website).
Multiple births create further problems with morbidity and mortality of children conceived from ART (Koivurova et al 2002). Women who have IVF are 20 times more likely to have a multiple pregnancy. Approximately 24% of all IVF births are multiple, which means that 40% of IVF babies are twins or triplets (One at a Time 2009). Twins and triplets have increased risks of cerebral palsy:
Petterson et al (1993) suggested that 1 in 10 pregnant women with twins and 1 in 5 pregnant women with triplets, whatever the mode of conception, who reach 20 weeks’ gestation, will experience at least one of the following: a child with cerebral palsy, an infant death, stillbirth.
One of the treatment options now offered to couples to alleviate the situation is multifetal pregnancy reduction – frequently performed in the United States. Data suggest that surviving twins from the procedure have an eightfold increase of cerebral palsy and an eightfold increased risk of periventricular leukomalacia (Geva et al 1998). The preferred option in the UK is to replace fewer embryos (either two or one) to reduce the incidence of multiple births (Hazekamp et al 2000) (Fig. 28.10).
The health of the mother may also be affected. Of mothers pregnant with twins, 20% experience hypertension, 30% will develop pre-eclampsia, and there is a 12% risk of developing gestational diabetes, and this increases the likelihood of longer periods of hospitalization during pregnancy together with a negative impact upon the family (see Ch. 59).
Stress and infertility
Infertility may have a great impact on an individual’s physical and psychological wellbeing (Hammarberg 2003, Kerr et al 1999, Pfeffer & Woollett 1983). The process of IVF is invasive, time consuming, and involves intimate procedures including vaginal ultrasound scanning, transvaginal ultrasound oocyte recovery, embryo transfer, administering injections and producing a sperm sample. Couples frequently feel stigmatized and embarrassed by their infertility, and can experience a growing sense of isolation, creating stress and anxiety in their daily lives. Success rates are low, so many couples experience multiple episodes of grief and loss leading to depression. Emotions described by couples include loss of self-esteem, mourning, threat, guilt, marital problems and also health problems (Guerra et al 1998).
The costs incurred by IVF treatment are a major stress factor for couples undergoing treatment. The government has initiated a review of fertility treatments by the National Institute of Clinical Excellence (NICE) that will establish national standards for fertility treatment and end the ‘postcode lottery’ that currently exists. According to the latest draft, three attempts with fresh embryos and three attempts with frozen provide the best chance of achieving a pregnancy. One in six couples experience problems with fertility in Britain, and there are approximately 27,000 IVF attempts every year; 80% of cycles take place in private practice. If the cycles were provided by the NHS, it would cost an estimated £400m and there are concerns how the income would be generated (BBC News 2003).
Reflective activity 28.2
How would you, as a midwife, help a couple normalize their pregnancy and childbirth experience after fertility treatment?
Counselling is an integral part of the process of fertility treatment, whatever stage a couple may be undergoing, and this is provided throughout the programme independently by licensed units. Mothers who conceive by IVF have higher anxiety levels related to the survival and normality of the unborn babies, damage caused by childbirth and separation from babies after birth, compared with matched controls (McMahon et al 1997). Midwife support during pregnancy is crucial to these couples, who may feel more vulnerable than parents who have conceived naturally. It is important for couples to normalize the pregnancy after the intensity of the fertility treatment, and this may be challenging for the team caring for the couples in primary, secondary and tertiary care.
Understanding the causes of infertility and the treatment processes are important so the midwife can perceive the degree of stress and the financial burden of assisted conception. Couples who experience difficulty with conception have to deal with the stress, frustration and stigma associated with being infertile. Many undergo treatments that have a low level of success that are not available on the NHS. The whole experience may damage them and their relationships and many will not achieve a long-desired pregnancy. It is important for midwives to understand the processes couples have undergone to achieve their pregnancy.
Key Points
Balen A, Jacobs H. Infertility in practice. New York: Churchill Livingstone; 1997.
Barber D. The extended role of the fertility nurse – practical realities. Human Fertility. 2002;5(1):13-16.
BBC News. ‘More IVF’ on the NHS. (website) http://news.bbc.co.uk, 2003.
Byrd C. Chlamydia trachomatis genital infections. The West Virginia Medical Journal. 1993;89(8):331-333.
Child T, Adul-Juli A, Huleki B, Tan SL. In vitro maturation of oocytes from unstimulated ovaries, normal ovaries, polycystic ovaries and women with polycystic ovarian syndrome. Fertility & Sterility. 2001;76:936-942.
Dale B, Elder K. In vitro fertilization. Cambridge: Cambridge University Press; 1997.
Dechaud H, Hedon B. What effect does hydrosalpinx have on assisted reproduction? The role of salpingectomy remains controversial. Human Reproduction. 2000;15(2):234-235.
ESHRE PGD Consortium Steering Committee. ESHRE Preimplantation Genetic Diagnosis (PGD) Consortium: data collection II (May 2000). Human Reproduction. 2000;15(12):2673-2683.
Geva E, Lerner-Geva L, Stavorosky Z, et al. Multifetal pregnancy reduction: a possible risk factor for periventricular leukomalacia in premature newborns. Fertility and Sterility. 1998;69(5):845-850.
Gould D. Women’s health – endometriosis. Nursing Standard. 2003;17(27):47-53.
Guerra D, Llobera A, Veiga A, et al. Psychiatric morbidity in couples attending a fertility service. Human Reproduction. 1998;13(6):1733-1736.
Hammarberg K. Stress in assisted reproductive technology: implications for nursing practice. Human Fertility. 2003;6(1):30-33.
Hazekamp J, Bergh C, Wennerholm U, et al. Avoiding multiple pregnancies in ART. Human Reproduction. 2000;15(6):1217-1219.
Human Fertilisation and Embryology Authority (HFEA). Human Fertilisation and Embryology Authority Code of Practice, ed 5. London: HFEA; 2001.
Human Fertilisation and Embryology Authority (HFEA). Code of Practice and Guidelines. (website) http://guide.hfea.gov.uk/guide/, 2009.
Hull M, Glazener C, Kelly N, et al. Population study of causes, treatment and outcome of infertility. British Medical Journal. 1985;291(6510):1693-1697.
Jurema M, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertility and Sterility. 2006;86(5):1277-1289.
Kerr J, Brown C, Balen A. The experience of couples who have infertility treatment in the United Kingdom; results of a survey performed in 1997. Human Reproduction. 1999;14(4):934-938.
Koivurova S, Hartikainen A, Gissler M, et al. Neonatal outcome and congenital malformations in children born after in-vitro fertilization. Human Reproduction. 2002;17(5):1391-1398.
Kousta E, White D, Cela E, et al. The prevalence of polycystic ovaries in women with infertility. Human Reproduction. 1999;14(11):2720-2723.
Kousta E, Cela E, Lawrence N, et al. The prevalence of polycystic ovaries in women with a history of gestational diabetes mellitus. Clinical Endocrinology. 2000;53(4):501-507.
Kurinczuk J. From theory to reality – just what are the data telling us about ICSI offspring health and future fertility and should we be concerned. Human Reproduction. 2003;18(5):925-931.
McMahon C, Ungerer J, Beaurepaire J, et al. Anxiety during pregnancy and fetal attachment after in-vitro fertilization. Human Reproduction. 1997;12(1):176-182.
Mukaida T, Oka T, Goto K, et al. Artificial shrinkage of blastocoels using either a micro needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Human Reproduction. 2006;21(12):3246-3252.
One at a Time. www.oneatatime.org.uk/126.htm, 2009.
Petterson B, Nelson K, Watson L, et al. Twins, triplets, and cerebral palsy in births in Western Australia in the 1980’s. British Medical Journal. 1993;307(6914):1239-1243.
Pfeffer N, Woollet A. The experience of infertility. London: Virago; 1983.
Royal College of Obstetricians and Gynaecologists (RCOG). Long-term consequences of polycystic ovary syndrome – Guideline No. 33. London: RCOG; 2003.
Scott L. Embryological strategies for overcoming recurrent assisted reproductive technology treatment failure. Human Fertility. 2002;5(4):206-214.
Shakkebaek K, Keiding N. Changes in semen in the testis. British Medical Journal. 1994;309(6965):1316-1317.
Snick H, Snick T, Evers J, et al. The spontaneous pregnancy prognosis in untreated subfertile couples: the Walcheren primary care study. Human Reproduction. 1997;12(7):1582-1588.
Templeton A, Fraser C, Thompson B. The epidemiology of infertility in Aberdeen. British Medical Journal. 1990;301(6744):148-152.
Thornton S. Infertility in men. Update Postgraduate Centre Series – Infertility. Amsterdam: Excerpta Medica; 2000.
World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction, ed 4. Cambridge: Cambridge University Press; 1999.