Chapter 29 Fertilization, embryonic, fetal and placental development
After reading this chapter, you will be able to:
Introduction
This chapter will build on Chapters 24, 25 and 27. The luteal phase of the fertile cycle initiates very distinct trajectories for mother and conceptus (see website). Following the luteinizing hormone/follicle-stimulating hormone (LH/FSH) surge, hormonal signals from the corpus luteum induce extensive adaptations in maternal renal, cardiovascular, respiratory, immunological, metabolic, endometrial and mammary systems. When fertilization occurs, these accelerate in response to signals from the conceptus and the endometrium. Before and after implantation, paracrine ‘cross-talk’ between conceptus and the endometrium prepares for attachment, implantation, differentiation of the trophoblast and development of the conceptus. At the same time, neuroendocrine signals are relayed from conceptus to the pituitary–ovarian and pituitary–thyroid regulatory axes; the peripheral immune system and maternal brain. These extend the functional lifespan of the corpus luteum; induce adaptations in maternal thyroid, stress and immune systems; and alter maternal sleep–wake cycles and food preferences, to accommodate the fertile cycle.
Following successful maternal recognition of the presence of the conceptus, adaptations that began following ovulation are enhanced and others specific to the fertile cycle are initiated. Maternal adaptations meet the distinct requirements of the conceptus during the first trimester and prepare for very different conditions needed to sustain fetal and neonatal life. Beginning with fertilization, this chapter focuses on the regulation of implantation, trophoblast infiltration, unique features of the gestational sac and formation of key organ systems during the first trimester. It will then examine fetoplacental interactions, including the fetoplacental circulation and endocrine unit; functional dynamics of amniotic fluid, lung formation and development in preparation for extrauterine life.
Fertilization
Spontaneous fertilization requires synchronized maturation and transport of the cumulus–oocyte complex (COC) and thousands of spermatozoa, within a tight timeframe. Once the COC is picked up by fimbriae of the fallopian tube, the oocyte has an estimated lifespan of 6–24 hours and spermatozoa 24–48 hours following arrival in the vaginal cavity (Johnson 2007:176). Both cells rapidly undergo a series of developmental and maturational processes, ensuring that only one of the 2–4 million spermatozoa that arrive in the vagina during sexual intercourse acquires the capacity to fuse with the oocyte membrane and release the sperm nucleus into the oocyte cytoplasm (Evans 2002, Kaji & Kudo 2004, Talbot et al 2003).
Following ovulation, the cumulus cell mass remains metabolically coupled to the oocyte through gap junctions. These somatic cells undertake a number of complex activities, including secretion of growth factors and antioxidants for the enclosed oocyte, facilitate oocyte travel and pickup and activate the capitation processes, all of which enable effective and successful fertilization (see website).
The fallopian tubes
The fallopian tubes have critical functions following ovulation. The inner linings and secretions facilitate bi-directional transport of oocyte and spermatozoa; create favourable conditions for oocyte maturation, sperm storage, capacitation, fertilization and successive cleavage of the fertilized egg; and aid its transport towards the designated implantation site in the uterus (Leese et al 2001, Shafik et al 2005) (Fig. 29.1).

Figure 29.1 Female reproductive tract.
(Reproduced with permission from Carlson 1994:15; Fig. 1-13.)
Anatomically part of the uterus, inner layers of the fallopian tubes are continuous with those in the cavity. They are composed of an internal mucosa of ciliated and secretory epithelium sensitive to changes in pituitary gonadotrophins and ovarian steroids, across the cycle (Casan et al 2000, Lei et al 1993). Intermediate layers of smooth muscle contain blood, lymph vessels, and steroid-sensitive adrenergic neurons that enable coordinated tubular motility, for the journey of the blastocyst to the uterus (Habayeb et al 2008; Wang et al 2004, 2006).
Around ovulation, smooth muscles display characteristic movements that bring the infundibulum, or distal portion of the tube, into apposition with the ovary containing the dominant follicle, by a change in orientation of muscles surrounding the ovarian fimbriae (Hunter 1988, Zervomanolakis et al 2009). One fimbria – slightly longer than the rest – reaches out to the tubal pole of the ovary and involutes, in synchrony with ovulation, to pick up the COC from the peritoneal cavity, into the enlarged trumpet-shaped infundibulum, lined with a very dense layer of ciliated secretory epithelium.
Oocyte and blastocyst transport within the tube is facilitated by:
Following fertilization, the blastocyst enters the isthmus, which has thick mucosal folds and the greatest concentration of muscle fibres. The diameter increases from 1–2 mm at the uterotubal junction, to more than 1 cm at its distal end, with a lumen ranging from 1 to 100 mm.
The interstitial portion is continuous with the uterine cavity and characterized by a marked increase in ciliated cells, and alterations in the shape of secretory cells. Muscles at the uterotubal junction are formed from four bundles. These hormonally sensitive interlacing spiral fibres allow strong constriction and relaxation of the interstitial portion of the tube. This regulates sperm transport and storage, and movement of the blastocyst towards the uterine cavity (Hunter 1988, Wildt et al 1998).
Cyclical changes
Across the ovarian cycle, the tubular mucosa undergoes cyclical alterations similar to the endometrial lining of the uterus. In the first half of the cycle, secretory and ciliated cells become larger under the influence of oestrogens. Around ovulation, ciliated cells become broader and lower while secretory cells become more distended with fluid. Following ovulation, microscopic holes appear in secretory cell membranes, which coalesce to release secretions accumulated during the first half of the cycle (Hunter 1988).
Higher rates of ciliary movements within the tube ipsilateral to the dominant follicle are regulated by increased blood flow, higher temperature and higher concentrations of ovarian steroids, compared to the contralateral tube (Zervomanola et al 2009). Around ovulation, beating of the dense concentration of cilia in the fimbriated portion is closely synchronized, propelling the COC into the ampulla. During this period, cilia in the ampulla also beat in the direction of the isthmus, suggesting that they further propel the COC towards the site of fertilization, taking the newly fertilized egg and surrounding cells from the ampulla and aiding subsequent movements of the zygote towards endometrial implantation (Hunter 1988).
The tubular environment is highly responsive to changing metabolic needs of the oocyte and blastocyst, through rhythms in secretion of growth factors, antioxidant enzymes and other regulatory molecules, and the supply of chemical and nutritive factors within the fallopian tubes following ovulation (Lapointe et al 2005). The oocyte and blastocyst are exposed to rhythmic secretion of growth regulatory molecules, and precisely timed modulations occur in oxygen tension, glucose concentrations, electrolytes and macromolecules, in different parts of the tube. The composition of tubular fluid complements the low rate of metabolite consumption in the oocyte and newly fertilized egg (Fleming et al 2004, Leese 1995, 2002, Leese et al 2001).
During their journey through the fallopian tube, the oocyte and blastocyst are composed of avascularized cells that undergo maturational changes and a highly regulated series of cleavage divisions, while utilizing exogenous sources of nutrients and growth factors.
Both demonstrate optimal functioning in an alkaline environment, with low concentrations of glucose and oxygen and plentiful supplies of albumin and growth factors (Leese 1995). The nutritive environment of the tubular lumen is regulated by hormonally induced secretions of the tubular mucosa and metabolic activities of the cumulus cell mass which surrounds the oocyte and blastocyst until implantation (Leese et al 2001) (Fig. 29.2).
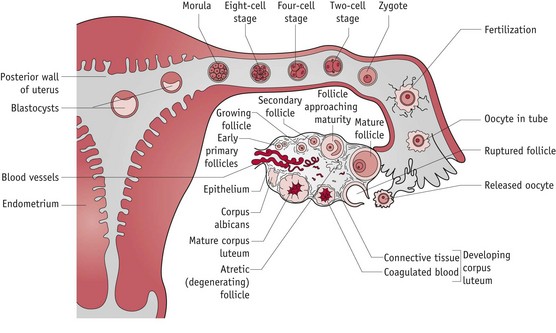
Figure 29.2 The cumulus–oocyte complex and the corpus luteum following ovulation.
(Reproduced with permission from Blackburn 2007:74.)
Sperm transport
Spermatozoa enter the genital tract in approximately 3–4 mL of seminal fluid which buffers the acidity of vaginal secretions. Over 99% are immediately lost by leakage from the vagina. Those remaining spend variable times in the cervix, showing differential states of motility and rates of transport through the uterus which may increase the chances of fertilization. This reservoir of spermatozoa in the cervix are actively transported in successive waves of peristalsis to a second sperm reservoir site in the isthmus, and finally to the fertilization site, at the isthmic–ampullary junction (Bahat et al 2003, Kunz et al 1998, Wildt et al 1998, Zervomanolakis et al 2009).
Cyclical changes in the vaginal canal around ovulation provide a protected environment for ejaculated sperm. This is mirrored by cyclical changes in cervical and vaginal tissues, including relaxation and widening of the cervix, and an increase in the quantity of cervical mucus, which demonstrates a characteristic ‘stretchiness’ that facilitates sperm transport (Drobnis & Overstreet 1992).
Sperm capacitation
During their active transport through the genital tract, spermatozoa undergo a final series of maturational changes before a small number are ready for fertilization. The first of these is capacitation, caused by interactions between spermatozoa and secretions of the cervix, uterus and fallopian tube during their journey to the site of fertilization. Capacitation is when the composition of the cell membrane undergoes removal of most glycoprotein molecules added during ejaculation. Capitation seems to induce hyperactive motility, providing increased thrusting power and alterations in cell surface properties enabling entry to the zona pellucida surrounding the oocyte cell membrane (Alberts et al 2002, Drobnis & Overstreet 1992). Sperm also acquire:
Fusion of oocyte and spermatozoon
The final set of morphological transformations in spermatozoa are stimulated by binding to the zona pellucida. During this critical process, the acrosome swells and its membranes fuse with the overlying plasma membrane (see Fig. 29.3). Initially, attachment is very loose, involving a number of spermatozoa. Firmer binding follows as an oocyte-binding protein on the sperm head region is recognized by sperm receptors on the zona pellucida. The inner plasma membrane at the apical end of the spermatozoa fuses with the outer membrane of the acrosome and forms a series of membrane-bound vesicles. Proteolytic enzymes released by the acrosome reaction digest sections of the zona pellucida surrounding the sperm head. Subsequent movement through the zona occurs very rapidly, creating immediate access to the oocyte membrane. Only spermatozoa that have undergone this acrosomal exocytosis can fuse with the oocyte. Usually, the spermatozoon that makes first contact with the oocyte proceeds to fertilization. In the process of fusion, the plasma membrane of the sperm head is enveloped by microvilli on the oocyte surface (Johnson 2007, Tosti & Boni 2004).
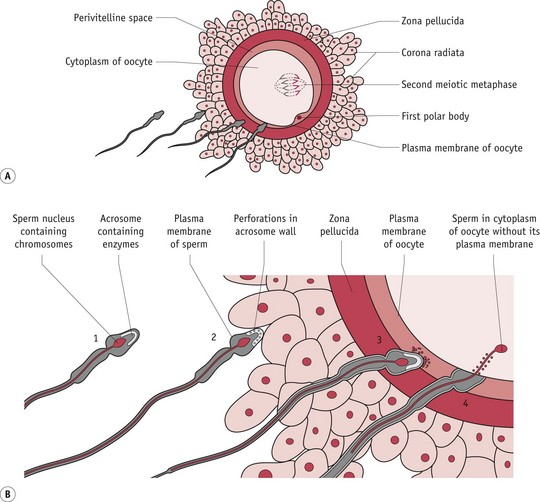
Figure 29.3 Fertilization and cortical reaction. After capacitation, the plasma membrane of the sperm and the outer membrane of the acrosome fuse and the membranes break down, releasing enzymes that allow the sperm to penetrate the corona radiata. Sperm digest their way through the zona pellucida via enzymes associated with the inner acrosomal membrane. Sperm are engulfed by the oocyte plasma membrane. Cortical granules are released when the sperm cell contacts the membrane. These granules cause other sperm in contact with the membrane to detach.
(Reproduced with permission from Moore & Persaud 2008:30; Fig. 2-14A,B.)
Immediately after oocyte and spermatozoon fusion, the cortical reaction occurs. This is a series of ionic changes occurring in the oocyte cytoplasm, and cortical granules formed following ovulation bind to the oocyte membrane, thus releasing their content into the space between the surface of the oocyte and the surrounding zona pellucida. The vesicles contain enzymes that modify the structure of the plasma membrane blocking the entry of further spermatozoa.
From zygote to …
The zygote is the newly formed cell containing maternal and paternal chromosomes and measures about 0.15 mm in diameter (FitzGerald & FitzGerald 1994:12). Within 2–3 hours of fertilization, the zygote proceeds with the final phase of meiosis that was halted immediately following fertilization, transmitting one set of chromosomes to the next generation. The remaining set is discarded to a second polar body, on the periphery, which later undergoes apoptosis, along with the first one formed at ovulation (Fig. 29.3). Female chromosomes then divide mitotically, yielding one haploid set within the main body of the cytoplasm.
The cytoplasmic content of the sperm cell membrane combines with that of the oocyte and over the next 2–3 hours the sperm nuclear membrane gradually breaks down. Between 4 and 7 hours after cell fusion, two sets of haploid chromosomes are formed into male and female pronuclei, as each becomes surrounded by distinct membranes, in opposite poles of the cell. During this period, chromosomes synthesize DNA in preparation for the first mitotic division. As chromosomal content increases, the pronuclear membranes break down, bringing together two sets of male and female chromosomes. These events form the diploid complement of a new individual and the cell immediately proceeds with a first mitotic division, passing its genetic material to two daughter cells and forming the two-cell conceptus (Downs 2008) (Fig. 29.4).

Figure 29.4 Various stages of cleavage and formation of the blastocyst.
(Reproduced with permission from Moore & Persaud 2008: 36; Fig. 2-18A–F.)
… morula
Successive rounds of cleavages occur at approximately 12-hour intervals until 8–16 increasingly smaller cells are formed within the zona pellucida and remaining fragments of the cumulus cell mass. When cell cleavage has produced 8–16 cells, the conceptus changes its morphology and undergoes compaction to become a morula (resembling a mulberry) (Fig. 29.4). This process maximizes contiguity between adjacent cell membranes (see website).
Glucose is consumed in increasing quantities and the morula utilizes a large number of growth factors for replication (Leppens-Luisier et al 2001), including epidermal growth factor (EGF), insulin-like growth factors (IGFs), and hypoxia-inducible factors (HIFs) (Leese 1995). All blastomeres show intense staining for gonadotrophin-releasing hormone (GnRH), in possible readiness to stimulate human chorionic gonadotrophin (hCG) release just before implantation (Casan et al 1999, Kikkawa et al 2002). During this phase of formation, the morula remains within the zona pellucida. This smooth outer covering provides an overall structure preventing premature adhesion of the blastomeres to the wall of the fallopian tube and providing an immunological barrier between maternal tissue and the genetically distinct cells of the morula (Johnson 2007).
… to blastocyst
Over 24 hours, the morula continues cell cleavage to 16–32 cells. Between these two points, outer cells commit to trophoblast lineage, while the inner cell mass (ICM) retains the capacity for pluripotency. Expression of critical genes for blastocyst formation commences in the 16-cell morula (Armant 2005). Outer cells begin to pump fluid internally to form a fluid-filled cavity called the blastocoele. Nudged to one side by this fluid, the ICM expresses gap junctions, allowing transfer of ions and small molecules from one cell to the next (Downs 2008). Meanwhile, the outer layer underlying the zona pellucida differentiates into flattened trophectoderm cells that combine with the zona to protect the ICM from destruction by maternal immune cells and signals the endometrium to initiate adhesion (Schultz 1998).
The fertilized egg has now become a blastocyst (Fig. 29.4). The trophoectoderm expresses mRNA for leptin and both outer and inner cells express mRNA, protein and receptors for GnRH, and the cannabinoid receptor (CB1) (Battista et al 2008, Casan et al 1999).
Composed of 34–64 cells, the blastocyst is programmed to prepare for the embryonic phase of formation, beginning as a free-living organism immersed in uterine secretions actively accumulated by the trophoblasts and utilized as metabolic substrates, while exchange of oxygen and carbon dioxide occurs by diffusion (Burton et al 2002, Leese 1995). Over the next 3 days, paracrine cross-talk between the blastocyst and endometrium coordinates blastocyst activation with differentiation of the endometrium to a receptive state, from 7–9 days following ovulation (Fitzgerald et al 2008, Simon et al 1997).
The corpus luteum
During the fertile cycle, the functional lifespan of the corpus luteum extends from 14 to around 280 days, but the endocrine–paracrine regulation is not well understood, particularly after the first 6 weeks of pregnancy (Handschuh et al 2007, Muyan & Boime 1997, Oon & Johnson 2000).
Research suggests that the close connections between uterine and ovarian arteriovenous systems deliver hCG and other regulatory factors from the endometrium and blastocyst, to the corpus luteum, from the early luteal phase of fertile cycles (see website).
Relaxin
Following the LH/FSH surges, large luteal cells (LLCs) and small luteal cells (SLCs) develop the capacity to secrete peptide and steroid hormones, in equal amounts. Relaxin, a peptide hormone of the insulin-like growth factor family, is a major hormone of the corpus luteum of pregnancy. Beginning during the luteal phase, under the stimulatory influence of LH/hCG, relaxin secretion peaks at 10 weeks’ gestation, decreases by around 20% and is present in maternal plasma, at stable concentrations, for the remainder of pregnancy (Bell et al 1987). Ovarian relaxin:
Hormonal signals
Within a week of fertilization, intact hCG (see website) appears in the maternal circulation and rapidly increases in the first 4 weeks after implantation, reaching peak levels of more than 100,000 mIU/mL around 9–10 weeks’ gestation, before falling rapidly to levels under 50,000 mIU/mL from around 18–20 weeks, until the end of pregnancy (Chard et al 1995, Kosaka et al 2002). In contrast, α-hCG levels increase progressively until term (Nagy et al 1994). At present, the exact mechanisms regulating intact hCG secretion are not well understood. Placental GnRH and leptin regulate secretion for the first 8 weeks of pregnancy (Islami et al 2003). Thereafter, hCG secretion as well as uterine and placental expression of LH/hCG receptors seem to be maintained by an autoregulatory mechanism (Cameo et al 2004, Kikkawa et al 2002). As pregnancy progresses, rising levels of fetal adrenal steroids seem to inhibit placental hCG (Tsakiri et al 2002).
hCG appears to have a fundamental role in regulating the establishment, development and maintenance of human pregnancy and the onset of labour (Ambrus & Rao 1994, Handschuh et al 2007, Ticconi et al 2007). Before attachment, trophectoderm cells of the blastocyst secrete hCG (Lopata et al 1997). Following attachment, hCG is released from mitotically active mononuclear cytotrophoblast cells, multinuclear syncytiotrophoblasts and extravillous cytotrophoblasts that differentiate from the trophoblast following implantation (Handschuh et al 2007, Muyan & Boime 1997). This early pregnancy rise in hCG secretion by the syncytiotrophoblast, extraplacental chorion and endometrial lining of the uterus and fallopian tubes constitutes the first neuroendocrine signal that induces maternal recognition of the fertile cycle (Handschuh et al 2007).
Preparation for implantation
In readiness for implantation, endometrial glands accumulate glycogen, proteins, a variety of growth factors, sugars and lipid droplets, and secretory activity peaks at around 6 days after ovulation (Burton et al 2002, 2007). Some secretions supply the conceptus with essential nutritional and regulatory molecules until around 9 weeks’ gestation, while growth factors stimulate proliferation of cytotrophoblast cells following implantation and secretion of hCG and human placental lactogen (hPL) by the syncytiotrophoblast from 6 to 12 weeks (Burton et al 2007, Hustin & Franchimont 1992, Jauniaux et al 2003).
Ovarian progesterone stimulates increased expression of two potent angiogenic factors: angiogenin in stromal cells and vascular endothelial growth factor (VEGF) in stromal cells and neutrophils associated with microvessel walls (Gargett et al 2001, Ma et al 2001). By the mid-secretory phase, a subepithelial capillary plexus has formed into a complex network of vessels (see Web Fig. 29.1) and newly regrown arterioles become increasingly spiral as they lengthen more rapidly than the endometrium thickens (Gargett et al 2001, Starkey 1993, Strauss & Coutifaris 1999).
Pre-decidualization
During the secretory phase, stromal cells release a growing number of new matrix proteins together with surface expression of their receptors, including laminin, fibronectin, collagen and integrins, while the earlier cross-linking collagen fibrils are degraded. This process of matrix remodelling creates a thicker, looser and more soluble structure for trophoblast infiltration (see website).
Blastocyst–endometrial communication
When fertilization occurs, the designated attachment site undergoes extensive synchronized changes transforming it from a usual state of active rejection of blastocyst attachment, to a brief state of receptivity, forming a ‘window of implantation’ commencing 7 days after ovulation (Fitzgerald et al 2008, Hustin 1992, Hustin & Franchimont 1992, Lessey 2000). Some changes are regulated by paracrine cross-talk with the free floating blastocyst, while others are activated by attachment.
As the blastocyst hatches from the zona pellucida, around 6 days after fertilization, trophoblast cells surrounding the ICM make initial contact with the endometrium, by close apposition of trophoblast plasma membranes with the apical membranes of surface epithelial cells, followed by rapid proliferation and formation of junctional complexes with the surface epithelium. Apical membranes of epithelial cells display a variety of progesterone- and oestrogen-induced changes that facilitate cell recognition and interaction with the trophectoderm. These include a progressive shortening of the microvilli, creating a flatter surface, reduced thickness of the normally dense coating of glycoproteins, and inhibition of gap junctions, facilitating adhesion (Fride 2008, Hustin & Franchimont 1992, Johnson 2007, Lessey 2000).
Adhesion and attachment
Around the designated site of implantation, the luminal epithelium expresses calcitonin and the blastocyst-dependent heparin-binding epidermal growth factor (HB-EGF). As the zona pellucida is shed, direct communication between cell adhesion molecules and their receptors on trophoblast and surface epithelium begins the dynamically balanced processes of attachment, implantation and trophoblast replication (Fitzgerald et al 2008) (see website).
Implantation – endometrial response
The surface epithelium, uterine glands and decidua show distinct responses to implantation. As the tiny blastocyst lodges in the crypt of the endometrial folds and localized oedema moulds a chamber around the trophoblast surface, epithelial cells above the newly created site multiply rapidly to form a complete cover for the embedded blastocyst enveloped in a growing mantle of differentiating trophoblast cells, by approximately 9 days following fertilization (Armant 2005). More extensive hormone-induced changes occur within the underlying decidua, producing a range of matrix proteins to form a loose lattice-type network allowing free passage of water, ions and large molecules to the trophoblast cells.
Like epithelial glands, decidual cells synthesize and release specific glycoproteins. One of these has been identified as a growth factor-binding protein that may participate in regulating the pace and extent of trophoblast implantation (Hustin & Franchimont 1992). Decidual cells that release relaxin have also been found to contain prolactin, which is regulated by a number of factors from adjoining cells in the decidua, placenta and membranes. Activities of decidual prolactin include regulation of glandular secretions, expression of adhesion and proteolytic molecules, and modulation of specific aspects of the immune response (Gubbay et al 2002, Jabbour & Critchley 2001) (see website).
Implantation – myometrium
Within the myometrium, hCG and progesterone induce cellular enlargement and depress the excitability of uterine muscle by decreasing the uptake of cellular free calcium, blocking the ability of oestrogens to stimulate α-adrenergic receptors and downregulating myometrial gap junctions (Ambrus & Rao 1994, Phillips et al 2005, Ticconi et al 2007). Rising levels of progesterone act centrally to depress oxytocin neuronal activity, which is likely to cause a decline in uterine peristalsis during implantation (Rodway & Rao 1995).
Formation of the cytotrophoblast shell and gestational SAC
Once the blastocyst has embedded in the decidua, rapidly proliferating trophoblast cells follow three distinct pathways, dividing into weakly proliferating villous cytotrophoblasts (vCTBs) that subsequently fuse to form a syncytium of terminally differentiated multinucleated syncytiotrophoblasts (STs) while the stem cell population of vCTBs form a monolayer directly beneath the STs to renew them. Some of these responses include processes that reduce rejection of the growing embryo (see website).
During the first trimester, STs actively take up secretions from the surrounding endometrial glands and contribute to gaseous and waste exchange (Ellery et al 2009). Throughout pregnancy, they are the major source of hormones, receptors, growth factors and regulatory enzymes (Burton et al 2007, Ferretti et al 2007, Habayeb et al 2008, Islami et al 2003, Jauniaux & Gulbis 2000, Tarrade et al 2001). These include hCG, human placental growth hormone (hPGH), prolactin, atrial natriuretic peptide (ANP), leptin, oestrogens, progesterone, and a growing list of regulatory glycoproteins including GnRH, corticotrophin-releasing hormone (CRH) and thyrotrophin-releasing hormone (TRH) (Cootauco et al 2008, Douglas 2010, Ferretti et al 2007, Pasqualini 2005).
As trophoblast differentiation proceeds, the ICM first subdivides into primary endoderm and ectoderm cell groups, both of which contribute to embryonic and extra-embryonic tissues (see Fig. 29.5). During the second week of life, regulatory cells within the ectoderm establish a defining organizational structure called the primitive streak, which establishes polarity of the body axis and consequently positions all embryonic organs and extra-embryonic compartments, while the endoderm group form a major part of the yolk sac, which performs the functions of a mature placenta, during the first trimester (Downs 2008, Jones 1997).
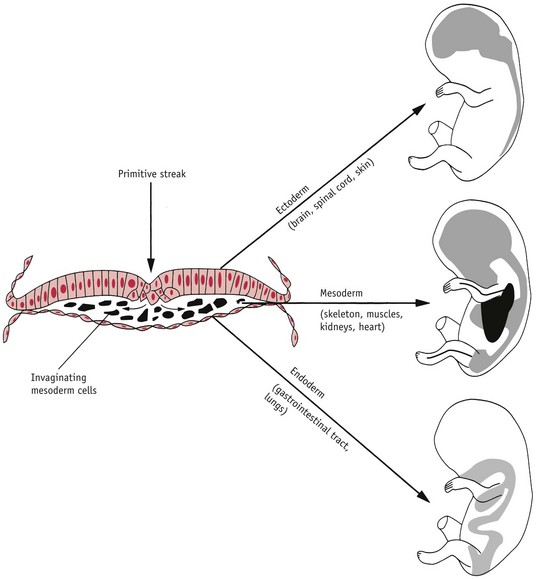
Figure 29.5 The trilaminar disc illustrating endoderm, ectoderm and mesoderm.
(Reproduced with permission from Dunstan 1990:214.)
Over the last 10 years, major advances have been made in identifying the unique biology of this period of gestation (see website).
While the cytotrophoblast shell acts as an effective barrier to maternal concentrations of oxygen, the expanding syncytiotrophoblastic mantle erodes the epithelium of surrounding endometrial glands, releasing their secretions into the extracellular matrix (Burton et al 2007). Glandular secretions enter channels forming around the cytotrophoblast shell. This activity is evident from 17 days post conception and only begins to decline when embryonic formation is complete, at the end of the first trimester (Burton et al 2007, Jauniaux & Gulbis 2000).
Histiotrophic nutrition
During the first 8–10 weeks of pregnancy, the secretory pattern of epithelial glandular cells extends that of the luteal phase, with continued glycogen secretion and a rapid rise in a number of glycoproteins (Burton et al 2001, 2007; Muller-Schottle et al 1999). These include glycodelin A, relaxin, hCG, α-tocopherol transfer protein, a powerful antioxidant, and MUC-1, a large progesterone-dependent glycoprotein (Burton et al 2002, 2007, Jauniaux et al 2004). Synthesis of glycodelin A parallels the profile of hCG in the maternal circulation during the first trimester. This glycoprotein regulates endometrial receptivity, has potent immunosuppressive activity, and is phagocytosed by the ST for recycling in anabolic pathways within the extra-embryonic compartment. MUC-1 is also taken up by the syncytiotrophoblast membrane and provides a rich and energy-free source of amino acids for its synthetic requirements (Burton et al 2002, Seppala et al 2002, Tseng et al 1999). The transfer pathway for these molecules is histiotrophic (extracellular) rather than haemotrophic (vascular) which characterizes the fetal phase of gestation (Burton et al 2002) (Fig. 29.6).
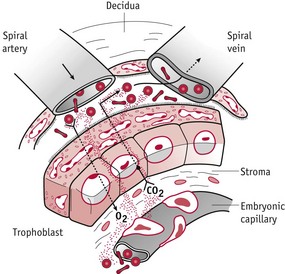
Figure 29.6 Diagram showing uteroplacental circulation and the trophoblast barrier.
(Reproduced from Jauniaux & Gulbis 1997: 229; Fig. 13.3, with kind permission of OUP. www.oup.com)
Conditions for embryogenesis and early placental formation
Recent studies suggest that the optimal environment for embryonic and early placental formation includes a reduced oxygen tension of between 2.5% and 5% (see website). Embryonic haemoglobin, which lasts for the first 8 weeks of gestation, combines with oxygen at very low tension found in interstitial fluids. Until the middle of the second month of gestation, all embryonic erythrocytes are nucleated; hence, blood viscosity remains very high and the mean radius of the emerging villous vascular system is very low (Jauniaux et al 2003). The conceptus therefore derives external nutritional support histiotrophically, from secretory products of the decidua and uterine glands. These secretory products are initially phagocytosed by the trophectoderm of the blastocyst, then by vCTBs and the endoderm of the yolk sac (Burton et al 2001, 2002).
Developments within the endometrium fully complement this activity (see Web Fig. 29.1). From the luteal phase of the cycle, glandular cells secrete rapidly increasing amounts of glycoproteins promoting cell growth and organ differentiation. The underlying decidua undergoes considerable biochemical and structural adaptations, forming an array of matrix proteins and differentiated secretory cells that provide:
Formation of extra-embryonic fluid compartments
As implantation of the blastocyst proceeds during the first 2 weeks after ovulation, the extra-embryonic mesoderm lining the cytotrophoblast shell progressively increases, and contains isolated spaces by 12 days after fertilization. At the same time, the ICM becomes a bilaminar disk, composed of high columnar cells (primary ectoderm) on the dorsal surface and a layer of differentiated cuboidal cells on the ventral surface, adjacent to the blastocyst cavity (primary endoderm). Over the next few days, a wave of new endodermal cells migrate from the primary endoderm, to line the blastocyst cavity and form the primary yolk sac or exocoelomic cavity during the fourth week of gestation. The complex fluid is derived from an ultrafiltration of maternal serum through villous stromal channels and from the secondary yolk sac, which forms at the beginning of the fourth week of gestation. Containing high concentrations of amino acids, regulatory proteins, vitamins and hormones, this fluid acts as a reservoir of molecules, prior to their use by the yolk sac during the first 8–10 weeks of pregnancy (Jauniaux & Gulbis 2000, Jauniaux et al 1994).
Once the primary yolk sac forms, a thick acellular material called the extra-embryonic mesoderm is secreted between the exocoelomic membrane and the cytotrophoblast. Over the next couple of days, this tissue divides to form a second chorionic cavity, between the primary yolk sac and the cytotrophoblast. An inner layer of cytotrophoblast cells delaminate to form amniogenic cells. Some differentiate into amnioblasts and organize into a specialized, semi-permeable membrane composed of a single layer of cuboidal epithelial cells on a loose connective tissue matrix (Jauniaux & Gulbis 2000). Formation of the primordial amniotic cavity seems to arise by cavitation of the primary ectoderm, which opens and then reforms to create a complete membrane around the amniotic cavity containing the emerging embryo.
Fluid formed within the amniotic cavity is largely secreted by the emerging embryo, containing much lower concentrations of all molecules and trace elements than fluid in the exocoelomic cavity (Jauniaux & Gulbis 2000). This indicates that the amniotic membrane separating the two compartments is not permeable to large molecules, and most glandular and trophoblast proteins are probably absorbed by the embryo through the secondary yolk sac (Jauniaux & Gulbis 2000, Jauniaux et al 1993, 1994). As illustrated in Figure 29.7, during the first 8 weeks of embryonic formation, the amniotic cavity is dwarfed by the larger and highly dynamic exocoelomic cavity containing the free-floating yolk sac, which directly nourishes and regulates the emerging embryo until around 10 weeks’ gestation (Jauniaux & Gulbis 1997, Jones 1997).
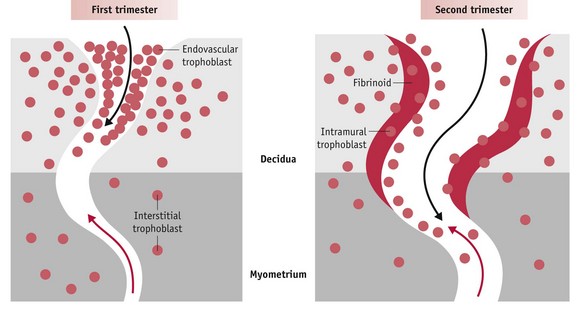
Figure 29.7 Diagram illustrating endovascular trophoblast migration into decidual segments of spiral arteries during the first trimester (left) and interstitial migration into the myometrial segments from 12-14 weeks. Red arrow: direction of maternal blood flow; black arrow: direction of endovascular trophoblast migration.
(Reproduced with permission form Pijnenborg et al 2006:948: Fig. 7.)
The secondary yolk sac
Around 12 days following fertilization, the primary yolk sac breaks up into a number of smaller vesicles while the endoderm beneath the embryonic disk grows out to form the secondary yolk sac. The secondary yolk sac grows rapidly, becoming larger than the amniotic cavity by the fifth week of gestation (Jones 1997). With successive foldings of the emerging embryo during the dynamic process of gastrulation, between 3 and 6 weeks’ gestation, the neck of the secondary yolk sac is constricted to form the yolk stalk, which connects the definitive yolk sac to the primitive gut.
Until it begins to degenerate at around 9 weeks’ gestation, the secondary yolk sac consists of three distinct layers:
Blood cells and capillaries develop within the centre of the chorionic villi. At approximately 19 days, the two sets of vessels establish contact, creating the beginnings of a vascular connection between the embryonic compartment and the early placenta (Carlson 1994:72). Blood cell formation in the secondary yolk sac continues until haematopoiesis begins in the embryonic liver and spleen at around 8 weeks’ gestation (Jauniaux & Moscoso 1992).
Formation of key organ systems
At present, there are well-characterized anatomical descriptions of sequential periods of human embryo formation and fragmentary findings on regulators of individual systems (Calvo et al 2002, Moore & Persaud 2008, Williams 2008). (See website for more information.)
At 2–4 weeks’ gestation
precursors of the thyroid, renal, adrenal and gonadal organs begin to form. Experimental findings indicate that distinct progenitor cells of the anterior pituitary gland appear well before the gland takes shape, around 7 weeks’ gestation (Moore & Persaud 2008, Simmons et al 1990, Tsakiri et al 2002). Five distinct cell types are defined by the trophic hormones produced; prolactin and growth hormone (GH) from lactotrophs and somatotrophs; thyroid-stimulating hormone (TSH) from thyrotrophs; luteinizing hormone (LH) and follicle stimulating hormone (FSH) from gonadotrophs; and proopiomelanocortin (POMC), which gives rise to adrenocorticotrophic hormone (ACTH), from corticotrophs.
At 7.5 weeks’ gestation
prolactin receptors are present in a wide variety of emerging organs, notably mesenchymal cells surrounding the developing adrenal cortex, kidneys, lungs, pancreas, duodenum, as well as cardiac and skeletal myocytes (Freemark et al 1997). These and other findings indicate that prolactin released from the pituitary has a key role in cell proliferation and differentiation that begins from the end of the first trimester. Cellular distribution of expression of prolactin receptors alters as its functions change from inducing cell proliferation, to hormone synthesis and organ maturation, as pregnancy advances (Freemark 1999, Symonds et al 1998).
At 8 weeks’ gestation
thyroid hormone receptor proteins are present in the brain, and thyroxine (T4) of maternal origin is present in coelomic fluid 2 weeks earlier (Iskaros et al 2000). Current evidence on the relative concentrations of maternal thyroid hormone in maternal serum and embryonic fluid compartments shows that transfer during early pregnancy is tightly regulated by a significant rise in the presence of enzymes in the decidua and gestational sac that metabolize T4 and T3 to inactive compounds (Calvo et al 2002, Liggins 1994). The emerging embryo is exposed to rigidly regulated levels of maternal thyroid hormones to regulate neuronal proliferation and migration of neurons in different parts of the brain (Williams 2008).
The urogenital systems and adrenal cortex are formed from mesoderm, one of three definitive germ layers derived from the primary ectoderm (Downs 2008, Kempna & Fluck 2008). A longitudinal elevation of mesoderm – the urogenital ridge – forms on either side of the dorsal aorta. The part giving rise to the renal system is the nephrogenic cord, while the adrenal cortex and bipotential gonads are initially part of the adrenogonadal primordium, between the urogenital ridge and dorsal mesentery, until they become distinct organs at around 4 weeks’ gestation (Goto et al 2006, Moore & Persaud 2008). During the fifth week of gestation, precursors of adrenal cells form cords that migrate and accumulate at the cranial end of the mesonephros – the first set of kidneys – where they condense to form the first outline of the adrenal glands (Mesiano & Jaffe 1999). Large spherical primordial sex cells appear in a restricted area of the endoderm and yolk sac stalk and are subsequently carried along the hind gut to the gonadal ridge where they become incorporated in the primary sex cords (Jauniaux & Moscoso 1992, Jones 1997).
By 6–7 weeks
adrenal and renal systems begin to function before the genital system. Two distinct zones have differentiated, a small outer definitive zone and an inner temporary or fetal zone that forms 80–90% of the cortex (Goto et al 2006, Tsakiri et al 2002).
From 7 to 12 weeks’ gestation
cortisol is synthesized in the transitional zone, which is located at the interface between the definitive and fetal zones. Cortisol exerts negative feedback regulation on ACTH release from the emerging anterior pituitary gland, which is critical to prevent excess ACTH from stimulating adrenal hyperplasia, resulting in increased synthesis of precursors for androgen synthesis (Goto et al 2006, Kempna & Fluck 2008). This early release of cortisol protects the emerging female embryo from the virilizing effects of androgens and facilitates normal female sexual differentiation of the external genitalia at 7–12 weeks’ gestation (Goto et al 2006; Mesiano & Jaffe 1997, 1999).
By 9 weeks’ gestation
the definitive kidneys have begun to excrete urine into the surrounding fluid and the onset of glomerular filtration is reflected in the marked rise in protein composition of amniotic fluid between the first and second trimester (Calvo et al 2002, Moore & Persaud 2008). Throughout fetal life, waste products are not processed by the kidneys but are transferred from amniotic fluid into maternal blood for elimination by the maternal kidneys (Moore & Persaud 2008).
While these and other organ systems unfold during the first 9–10 weeks of gestation, placental formation remains within the boundaries of the decidua.
The deciduochorial placenta
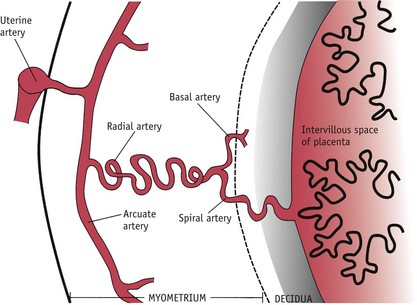
During formation of anchoring villi, proliferating extravillous cytotrophoblasts (evCTBs) extend from the syncytium, forming columns of cells that enter decidual blood vessels. As this process continues, the intervillous space begins to open, exposing the migratory extravillous trophoblast cells to a physiological increase in oxygen tension, from 9 weeks’ gestation. This environment alters the expression of specific transcription factors that triggers the expression of an invasive population of migratory evCTBs (Caniggia et al 2000). In addition, evCTBs also express receptors for hPGH which has recently been found to increase the invasive potential of evCTBs in culture (Lacroix et al 2005). Further differentiation of the extravillous cells to a more invasive phenotype allows them to enter and remodel the spiral arteries, to create the low-resistance vascular system that is essential for fetal growth (Caniggia & Winter 2002, Caniggia et al 2000, Hustin 1992).
During the first 12 weeks, extravillous trophoblast migration into the spiral arteries occurs primarily within the decidual segments. First the distal tips of these blood vessels are plugged with evCTBs that extend from the trophoblastic shell or the proliferating tips of the emerging villi. Sheets of these endovascular trophoblasts migrate along the capillary walls, against maternal blood flow, and accumulate within the lumen of the spiral arteries (see Fig. 29.8). During the subsequent process of vascular infiltration, cells strip away sections of the endothelium and burrow beneath this layer, to replace elastic tissue and smooth muscle with cytotrophoblast cells that appear to surround themselves with large quantities of fibrinoid material. Later, surface endothelial cells grow over the new underlying tissues. Throughout, the convoluted walls of the spiral arteries are converted into tubes of fibrinoid material with no elastic tissue or smooth muscle fibres. This results in terminal coils of the spiral arteries reaching 2–3 mm in diameter and underlying segments undergo a generalized non-uniform dilation as pregnancy advances and lose the capacity to respond to the vasomotor influences of continued autonomic innervation (Burton et al 2009, Douglas 2010, Hustin et al 1988, Pijnenborg et al 2006).
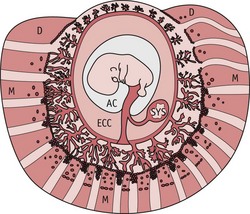
Figure 29.8 Diagram of a gestational sac at 8–9 weeks’ gestation, showing the myometrium (M), decidua (D), placenta (P), and exocoelomic cavity (EEC), the largest space inside the gestational sac from 5–9 weeks’ gestation. Arrows indicate uteroplacental blood circulation beginning in the periphery of the placenta.
(Reproduced with permission from Burton et al 2006:203; Fig. 8.)
During the first 10 weeks of pregnancy, ultrasound studies suggest that decidual blood vessels do not reach the intervillous space. While small amounts of plasma percolate through the plugs from these low-pressure vessels, chorionic villous sampling has rarely demonstrated the presence of maternal blood. As illustrated in Figure 29.6, current evidence suggests that during the first 9–10 weeks, the intervillous space is not immediately connected with the maternal circulation and is not yet bathed by maternal blood (Hustin & Schaaps 1987). As illustrated in Figure 29.9, estimates of uterine blood flow also support this evidence. In non-pregnant women, uterine blood flow is approximately 45 mL/min, rising by around 10 mL/min during the first trimester. In contrast, much larger increases occur during the second and third trimesters, to reach over 750 mL/min by the end of pregnancy (Burton et al 2009, de Swiet 1991:51).
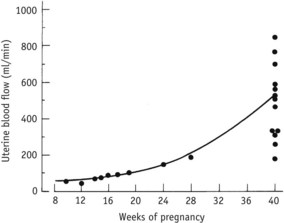
Figure 29.9 Uterine blood flow in pregnancy.
(Reproduced with permission from Chamberlain and Broughton Pipkin 1998:51.)
From embryo to fetus
From 12 weeks onwards, trophoblast infiltration extends into myometrial segments of many spiral arteries (Pijnenborg et al 2006). Evidence suggests that this infiltration largely occurs through the endometrial stroma to enter the vessel walls from the outside (Burton et al 2009). As in decidual segments, this activity replaces muscular and elastic tissue with fibrinoid material that converts them into widened, funnel-like tubes with no capacity to respond to the vasomotor influences of continued autonomic innervation (Caniggia et al 2000). At the same time, the trophoblast shell becomes thinner and more irregular as fetal growth enlarges its internal volume (Jauniaux et al 2003). An increasing number of extravillous cells become distinct from the shell surface and these gradually open up low-pressure flow of maternal blood within the intervillous space. Experimental evidence suggests that the velocity of blood flow from the spiral arterioles to the intervillous space is similar to an actively flowing brook entering a reed-filled marsh (Burton et al 2009, Ramsey et al 1976) (Fig. 29.10).
This direct communication between maternal blood and placental villi coincides with the onset of rapid growth of the fetus and placenta. During this phase, the roughly formed organ systems undergo progressive differentiation and rapid growth that requires large increases in uteroplacental size and blood volume (Mark et al 2006). Structural alterations to all segments of uterine blood vessels create conditions for the emergence of an expanding low-pressure system that optimizes gas and nutritional exchange across the placental interface (Burton et al 2009).
Growth of the amniotic compartment
Between 7 and 12 weeks’ gestation, the production of amniotic fluid increases from 3 to 30 mL (Jauniaux & Gulbis 2000). This causes the amnion to swell, until it takes over the chorionic space, bringing amnion and chorion in contact for the first time and enclosing the embryo, except for the umbilical area, within a single cavity. As the sac subsequently grows into the endometrial cavity, this portion of the chorion is gradually compressed against the decidua capsularis – the area of decidual lining surrounding the site of implantation. With continued growth, blood supply is reduced and these villi slowly degenerate, although trophoblast cells between them remain viable for the remainder of the pregnancy. This portion of the chorion (chorion laeve) forms an interface between the amnion and areas of decidua not occupied by the definitive placenta (Fig. 29.11).
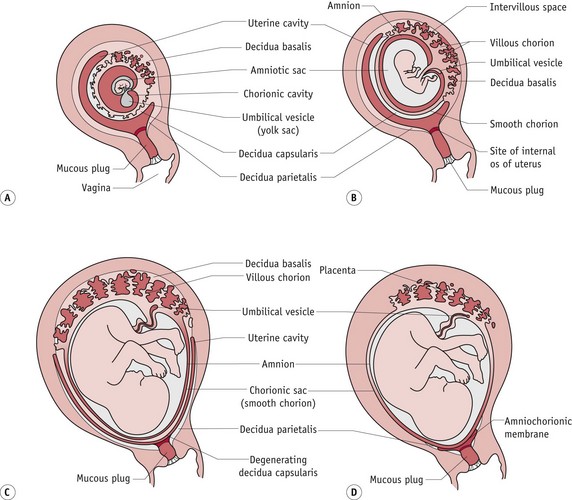
Figure 29.11 Diagram illustrating changes within compartments of the gestational sac and the changing relations of membranes to the decidua from 12 to 22 weeks’ gestation.
(Reproduced with permission from Moore and Persaud 2008:112; Fig. 7-1D–F.)
The chorion laeve has the following features:
The definitive placenta
As villi of the chorion laeve disappear, those attached to the decidua basalis rapidly develop to form the mature placenta. The cytotrophoblast shell extends laterally and penetrates deeper into maternal tissue between the anchoring villi, and increasingly complex branching villi extend in the intervillous space. Each stem villus forms the centre of the villous tree. Fetal arterioles carrying poorly oxygenated blood enter the villi and break up into an extensive arteriocapillary–venous network. The 60–70 branching villi that make up the mature placenta provide a large surface area for gaseous and metabolic exchange between fetal blood within the villi and maternal blood circulating slowly around the external surface from the intervillous space (Sheppard & Bonnar 1989) (see Fig. 29.12). (See Reflective activity Web 29.1).
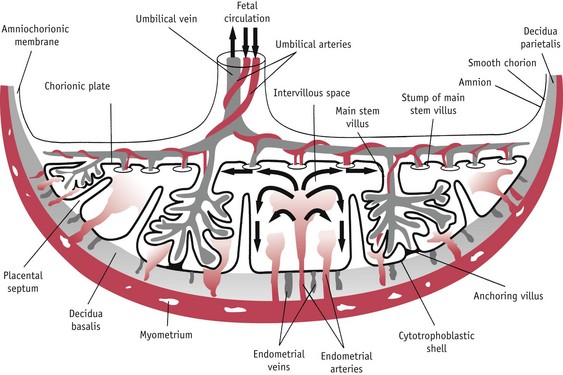
Figure 29.12 Schematic drawing of a transverse section through a full-term placenta showing the fetoplacental and maternal–placental circulation.
(Reproduced with permission Moore & Persaud 2008:116; Fig. 7-7.)
Fetal oxygen requirements
While intraplacental and fetal concentrations of oxygen increase significantly from the end of the first trimester, a PO2 gradient of 13.3 mmHg persists between placenta and decidua at 16 weeks’ gestation and PO2, O2 saturation and O2 content gradients are present between fetal blood and placenta, and between placental and underlying maternal tissues (Jauniaux et al 2003). Low fetal blood PO2, and O2 saturation have also been found in the third trimester (Jauniaux et al 2001). The highest oxygen content is in the umbilical vein, which is 30–35 mmHg and 80–90% saturated. By the time blood reaches the left atrium, O2 content has fallen to 26–28 mmHg and lower concentrations supply the lungs and lower body (Blackburn 2007). With homeostatic functions performed by maternal and placental systems, the fetus is therefore fully adapted to an aqueous environment with significantly lower oxygen concentrations than those required by maternal and placental tissues.
Fetal regulation of amniotic fluid volume
Until about 20 weeks, the dynamics of amniotic fluid volume are thought to involve a significant transmembrane pathway from maternal blood via the placenta and movement of fluid and other molecules across the fetal skin, offering no impediment to the flow of fluid into the amniotic sac. From 17 to 25 weeks’ gestation, this pattern of flow diminishes, as the fetal skin begins to keratinize. During the second half of pregnancy, the fetal kidneys, lungs, gastrointestinal system and circulation make a growing contribution to the volume and circulation of amniotic fluid.
Volume increases from approximately 350–450 mL at 20 weeks, to 700–1000 mL at 36–39 weeks, and then begins to decline (Moore & Persaud 2008). Estimates of daily volumes near term suggest that fetal urine contributes 500 mL, lung fluid 300–400 mL, and 400 mL is removed by fetal swallowing (Moore & Persaud 2008). The fluid passes into the fetal circulation and waste products cross the placental membrane and enter maternal blood in the intervillous space. Through these pathways the water content of amniotic fluid is changed approximately every 3 hours (Moore & Persaud 2008).
Mechanisms regulating amniotic fluid volume are poorly understood. During the second half of pregnancy, amniotic fluid is hypo-osmolar compared to maternal and fetal plasma. This osmotic gradient would be expected to drive water from the amniotic cavity into the maternal and fetal circulations via the umbilical cord, placenta and membranes. However, the precise volumes that move through these pathways remain to be confirmed (Brace 1995, Gilbert et al 1991). (See website.)
Functions of amniotic fluid
This dynamic volume of fluid provides:
Feto-placental circulation
The fetus relies on the placenta for respiration, nutrition and excretion, and fetal blood circulates throughout the placenta to realize these requirements. Fetal circulation differs from adult circulation in that blood is oxygenated in the placenta and not in the lungs. This system requires:
The intrauterine circulation takes shape during early embryonic formation: umbilical veins bring oxygenated blood to the primitive heart from the chorion frondosum; vitelline veins return blood from the yolk sac; and cardinal veins return blood from the rest of the body. Blood enters the heart via the sinus venous and flows through a single atrium and ventricle. When the ventricle contracts, blood is pumped through the bulbus cordis, passes into the dorsal aorta and eventually returns to deliver waste products to the chorion (Moore & Persaud 2008).
During fetal life, the vascular system becomes more extensive as it parallels the increasing size and complexity of individual organs and tissues. Oxygenated blood returns from a greatly enlarged placenta, via the umbilical vein. Approximately 50% of this blood enters a hepatic microcirculation and later joins the inferior vena cava via the hepatic veins. The remaining blood passes directly to the inferior vena cava, through a shunt called the ductus venosus. Blood flow through this vascular bypass is thought to be regulated by a physiological sphincter that responds to changing volume in the umbilical vein and helps to protect the fetus from erratic fluctuations in blood pressure (Walker 1993).
In addition to well-oxygenated blood from the placenta, the inferior vena cava receives less-oxygenated blood from the abdomen, pelvis and lower limbs, representing more than 66% of total venous return. Before entering the heart, the inferior vena cava bifurcates into two channels: the foramen ovale links it to the left atrium and a small inlet links it to the right atrium. In the left atrium, the foramen ovale ends in a one-way valve that only permits blood flow from right to left. Flow patterns in the right atrium allow 50% of oxygenated blood returning from the placenta to be shunted to the left atrium. This right-to-left flow through the foramen ovale is maintained by the larger quantity and greater speed of blood flow from the inferior vena cava on the right, compared to that entering the left atrium via the pulmonary veins from the lungs. During fetal life, lung tissue extracts oxygen from the low circulating blood volume entering from the right ventricle and returns poorly oxygenated blood to the left atrium. Low venous return from the lungs is actively maintained by the exposure of pulmonary vessels to blood PO2, which keeps them in a state of hypoxic vasoconstriction (Moore & Persaud 2008, Walker 1993).
A small volume of well-oxygenated blood from the inferior vena cava enters the right atrium. This is mixed with poorly oxygenated blood returning from the head via the superior vena cava, which mainly enters the right ventricle through the tricuspid valve. From the right ventricle, only 10% of blood enters the lungs via pulmonary arteries. The remaining 90% is diverted into the descending aorta via a muscular artery called the ductus arteriosus, which connects the main pulmonary artery to the aorta. Throughout fetal life, patency and therefore relaxation of this muscular artery is actively maintained by the low fetal PO2, high circulating levels of PGE2 and local release of PGI2 (Amash et al 2009). As a result of this shunt, most blood leaving the right ventricle perfuses the lower body and the placenta (Moore & Persaud 2008, Walker 1993).
In the left atrium, the pulmonary component combines with a much larger volume of more highly oxygenated blood from the inferior vena cava. From the left atrium, blood passes into the left ventricle. A small amount of this blood supplies the heart, two-thirds leaves via the ascending aorta to perfuse the upper part of the body with highly oxygenated blood, while the remaining one-third flows through the aortic isthmus to the descending aorta and then to the lower body, and placenta (Walker 1993). Two hypogastric arteries convey deoxygenated blood to the placenta.
Shunting of blood on the venous side by the foramen ovale and on the arterial side by the ductus arteriosus are structural devices that enable the blood to bypass the lungs and be directed to the placenta. This large volume of poorly oxygenated blood returning to the placenta flows much more rapidly than the more highly oxygenated blood supplying the upper body. The rate of flow tends to be most rapid in the descending aorta. From this vessel, blood is directly pumped into the umbilical arteries and returns for gaseous exchange in the placenta (Walker 1993) (Fig. 29.13).
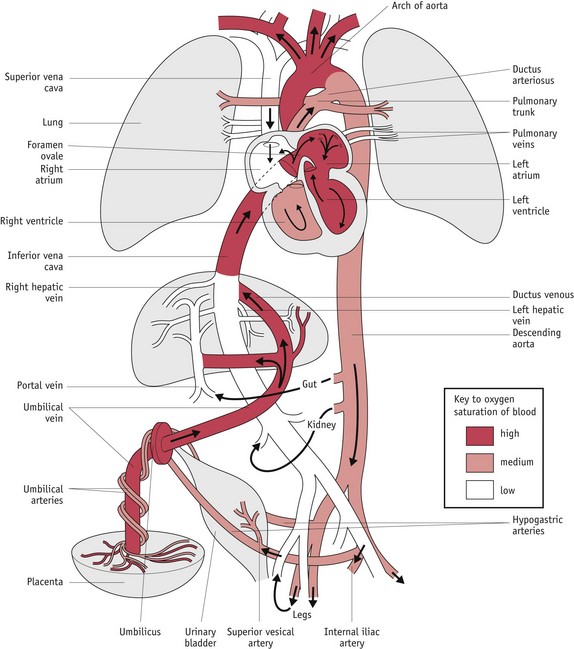
Figure 29.13 Fetal circulation: colours indicate oxygen saturation of the blood and the arrows show the course of the blood from the placenta to the heart.
(Reproduced with permission from Moore & Persaud 2008:328; Fig. 13-46.)
During intrauterine life, the fetal–placental circulation operates as a single unit, providing a low-resistance, high-capacity reservoir in the vascular bed of the placenta, maintained by the absence of valves in the umbilical veins. From animal experiments, total fetal–placental blood volume is estimated at 100–120 mL/kg body weight, with 80–90 mL/kg representing fetal blood volume at any given time, while the remainder is contained within the umbilical–placental circulation. This additional capacity helps to protect the fetal circulation from fluctuations in blood pressure and blood flow distribution at a time when autoregulation of regional blood flow is not fully developed.
Lung formation
Around 24 days following conception, a pouch-like laryngotracheal diverticulum arises, as a small ventral protrusion from an area of the foregut, close to that which becomes the stomach (Blackburn 2007). Once formed, the foregut elongates, separating the emerging stomach from the section that gives rise to the lungs. From an initial protrusion, the lungs take shape through a series of bifurcations beginning with the emergence of right and left lobular buds corresponding in number to those in the mature organ.
Between 5 and 26 weeks’ gestation, these branch a further 16 times, to generate the respiratory trees. All bronchial airways are formed by 16 weeks and further growth proceeds by elongation and widening of existing airways. Towards the end of this phase, each terminal bronchiole divides into two or more respiratory bronchioles, while surrounding mesodermal tissues become highly vascularized.
From 26 weeks onwards, respiratory bronchioles continually subdivide to produce approximately 20–70 million primitive alveoli that are vascularized by a dense network of capillaries (Moore & Persaud 2008) (Figs 29.14 and 29.15).
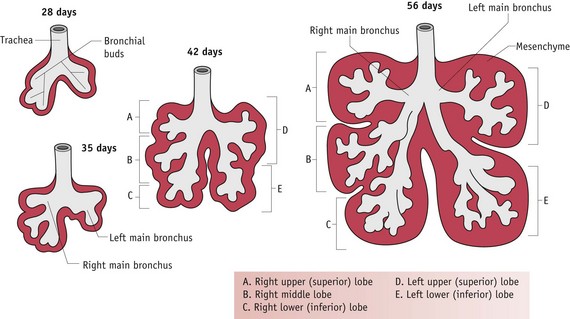
Figure 29.14 Successive stages in development of the bronchial buds, bronchi and lungs.
(Reproduced with permission from Moore & Persaud 2008:203; Fig. 10-8.)
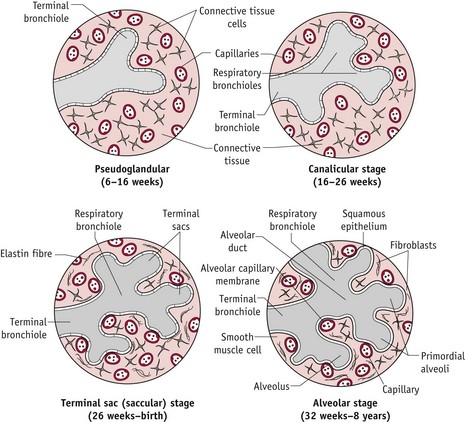
Figure 29.15 Diagrammatic sketches of histologic sections illustrating stages of lung development from 6 weeks to 8 years.
(Reproduced with permission from Moore & Persaud 2008:204; Fig. 10-9B–D.)
During fetal life, these potential air spaces are filled with liquid actively secreted by the pulmonary epithelium from the surrounding circulation. Experiments have found that the volume of liquid increases from 4–6 mL/kg body weight at mid-gestation, to more than 20 mL/kg body weight near term. This liquid expansion of potential air space maintains a small distending pressure in the lumen that approximates functional residual capacity of the aerated lung in the newborn. Lung fluid also assists in the formation of surfactant-producing epithelial cells that can be identified from approximately 24 weeks’ gestation.
There are a successive series of development, from changes within the lining of the bronchioles, to prepare the lungs for extrauterine breathing and gaseous exchange. From 17 weeks, the cuboidal epithelial cells provide energy and act as precursors for the development of surfactant. Surfactant is crucial in reducing surface tension of the terminal sacs and stabilizing the membrane, which prevents lung collapsing following the onset of respiration (see Chapter 45 and see website for more information).
Intermittent breathing movements occur from approximately 11 weeks’ gestation and from 24 weeks the pattern of this activity is integrated with circadian rhythms in heart rate, temperature, body movements and sleep that seem to be mediated by maternal melatonin which freely crosses the placenta (Tamura et al 2008). These episodic breathing movements in utero stimulate the development of lung tissue and respiratory muscles and some experimental evidence suggests that they may increase cardiac output and blood flow to vital organs including the heart, brain and placenta. Rhythmic breathing movements are regulated by brainstem adrenergic respiratory neural networks, and adrenaline from the adrenal medualla (see website).
Hormonal regulation of lung development and maturation
Differentiation of type II pneumocytes and production of surfactant are closely associated with increasing levels of different hormones within the fetal circulation, particularly cortisol, oestrogens, adrenaline, hPL, prolactin, triiodothyronine (T3) and placental CRH (see website).
Central role of the adrenal cortex and placenta
Steroidogenic enzymes are present in the emerging adrenal cortex from 6–7 weeks’ gestation. Synthesis of the main hormones, dehydroepiandrosterone (DHEA) and its sulphate (DHEA-S), and pregnenolone sulphate, begins from 6 weeks’ gestation, while cortisol synthesis occurs between 7 and 12 weeks’ gestation (Goto et al 2006, Kempna & Fluck 2008, Tsakiri et al 2002).
From 8–10 weeks’ onwards, DHEA-S and pregnenolone sulphate are increasingly utilized by the placenta, as essential substrates for formation of oestrogens and progesterone ( Pasqualini 2005). Transfer of maternal glucocorticoids to the fetus is regulated by a placental glucocortical ‘barrier’.
Regulatory mechanisms are essential to stimulate normal placental and fetal growth and protect rapidly growing fetal organs from excess glucocorticoid exposure (Wyrwoll et al 2009). This also enables the preparation of intrauterine tissues and fetal organs for the transition from pregnancy to labour (see website).
Development of the adrenal cortex
The significance of the adrenal glands is indicated by their spectacular growth and secretory capacity during intrauterine life. Cells forming the cortex appear at 3–4 weeks’ gestation and enlarge rapidly to equal the size of the kidneys by the end of the first trimester. By 6–8 weeks’ gestation, the cortex forms two distinct zones: a large inner fetal zone, an outer definitive zone that forms a thin subcapsular rim, and an indistinct transitional area between the two (Goto et al 2006, Tsakiri et al 2002). During the second trimester, the adrenals enlarge in direct proportion to the increase in total body weight. At this time, their relative weight is 35 times the adult value. In the third trimester, gland weights continue to increase but at a slower rate than the rest of the body. At term, the fetal adrenals are similar in size to those in the adult (Pearson Murphy & Branchaud 1994, Seron-Ferre & Jaffe 1981) (see website).
Adrenal growth seems to be largely regulated by hCG, ACTH, oestradiol, prolactin, melatonin and growth factors derived from within the glands, along with those from other fetal organs, including the placenta, liver and kidneys (Freemark et al 1997, Tsakiri et al 2002). Until the latter part of pregnancy, the active steroid-producing fetal zone is predominantly involved in synthesizing DHEA-S, for the placenta (Pepe & Albrecht 1990). During the last 3 months of pregnancy, maternal melatonin stimulates adrenal weight gain and synthesis of DHEA-S, while at the same time negatively regulates the process of maturation by inhibiting ACTH- and CRH-induced production of cortisol.
Adrenal medulla
In contrast to the cortex, the chromaffin cells are formed from adjacent sympathetic ganglia that are derived from the neural crest. Unlike the cortex, this part of the gland does not become a discrete structure during fetal life. During this period, small islands of chromaffin cells are scattered throughout the cortex and larger and much more active islets form independently of the medulla, along the outside of the aorta (Mesiano & Jaffe 1997). Immature chromaffin cells have been observed from approximately 8 weeks and significant concentrations of noradrenaline have been found from 15 weeks’ gestation. No degeneration has been found to occur in these cells until the postnatal period (Phillippe 1983).
Within the adrenal gland, small groups of cells containing measurable amounts of noradrenaline have been observed at 9 weeks but their content remains low until the third trimester. While noradrenaline remains predominant in the fetal response to stress, the rising capacity for cortisol production near term produces a sharp increase in the capacity of chromaffin cells to produce adrenaline. The proportion of adrenaline increases progressively from approximately 28 weeks to term, when it constitutes around 50% of total catecholamine content of the gland. As measured in amniotic fluid, catecholamine metabolites increase between the second and third trimester and plasma catecholamine concentrations rise progressively during the course of labour. These changes mediate a range of cardiovascular, metabolic and respiratory responses to labour and birth (Lagercrantz & Marcus 1992, Lagercrantz & Slotkin 1986, Phillippe 1983).
Conclusion
Conditions required for implantation and formation of the distinct biological environments for embryonic and fetal formation and growth begin during the luteal phase of the cycle. The presence of the conceptus is established by hormonal communications which accelerate adaptations in central and peripheral maternal systems.
The midwife can use this knowledge to identify distinct changes that coincide with embryo formation and fetoplacental development and enhance the woman’s understanding of what is unfolding to support information and advice provided.