Chapter 44 The preterm baby and the small baby
Introduction
For the purpose of classification, management and research studies, newborn babies are considered according to their gestation, their birthweight relative to their gestation (centiles) and their actual birthweight. The midwife’s role centres on the prevention of prematurity, on preparing parents for identifying risk factors antenatally, and, in the event of preterm birth, on working with the team to support parents in the neonatal period. Please see website for more in-depth information.
Gestation
Low gestational age at birth is a principal factor associated with perinatal mortality. Babies born very preterm are at particular risk of sensory, cognitive and motor dysfunction (Cooke 2005, Marlowe et al 2005). Data are now available to monitor trends, and inform care for preterm births. In 2006 in England and Wales, 7.6% of live births were preterm, 88% were born at term, and 4% were born post term, with corresponding infant mortality rates of 41.0, 1.9, and 1.5 deaths per 1000 live births (ONS 2009). Almost two-thirds of all infant deaths occurred to babies born preterm. Infant mortality was highest at the very low gestational ages.
Antenatally, gestation is estimated from the date of the last menstrual period and the woman’s normal cycle, clinical examination and early ultrasound scan measurements and uterine growth (see Chs 32 and 33).
Scoring systems estimate the neonatal gestation following delivery and neonatal units will use one, or an adaptation of one or two of the common ones. Improved accuracy of antenatal ultrasound scans has led to less reliance on these scales. The Dubowitz scale (Dubowitz et al 1970) (see Fig. 44.1) is the most widely used in the UK, and providing the baby is well and examined within a few hours of delivery, is accurate to within 2 weeks. It involves scoring the baby on neurological states as well as external criteria but may be inappropriate for use with sick or ventilated neonates.
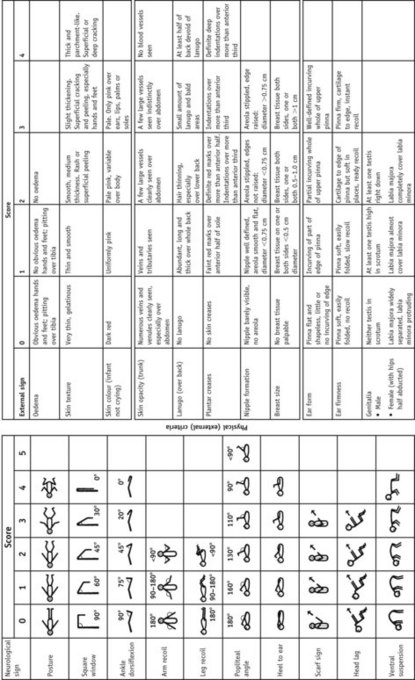
Figure 44.1 The Dubowitz score: graph for reading gestational age from total score.
(From Dubowitz et al 1970 with permission.)
The Ballard score (an adaptation of the Dubowitz score) is a newer system (Fig. 44.2).
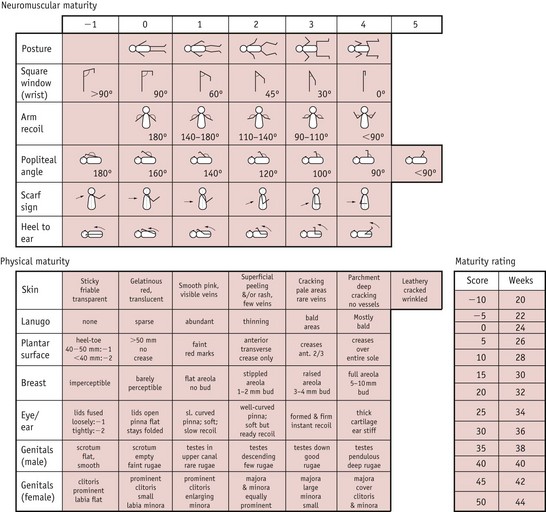
Figure 44.2 The Ballard score. Each of the clinical and neurological features is assessed and scored. The gestational age is determined by comparing the total score with the maturity rating grid.
(From Johnston et al 2003.)
These scoring systems require careful examination of the baby, looking at characteristics of appearance, reflexes and behaviour, providing an indication of whether the baby is small or premature.
Centiles
Using an appropriate centile chart, the weight, head circumference and length of the baby is plotted against gestation and an assessment made of the growth. The centile chart forms an important part in providing a dynamic growth record and a link to the neonatal management (see Ch. 41).
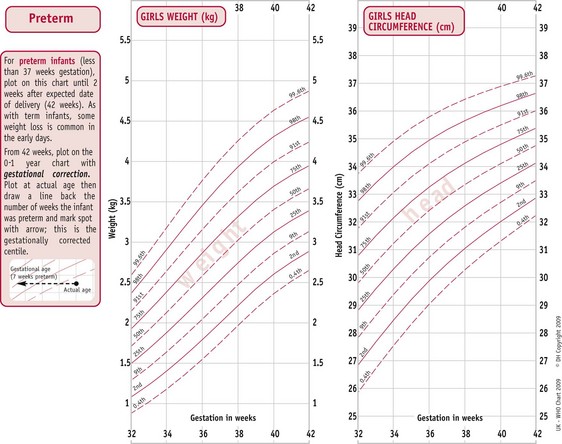
The UK World Health Organization (WHO) growth charts (2009) are based on measurements collected by the WHO in six different countries (Fig. 44.3) (see website). These describe optimal rather than average growth and set breastfeeding as the norm, illustrating how all healthy children are expected to grow.
Figure 44.3 WHO growth charts, preterm infants. A. Weight for preterm infants less than 37 weeks (female). B. Girls’ head circumference.
(By kind permission of RCPCH/WHO/Department of Health 2009.)
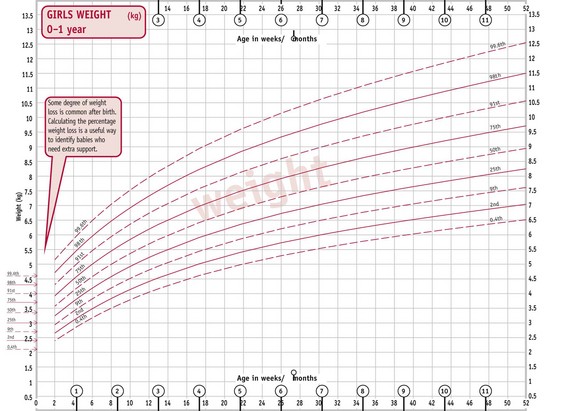
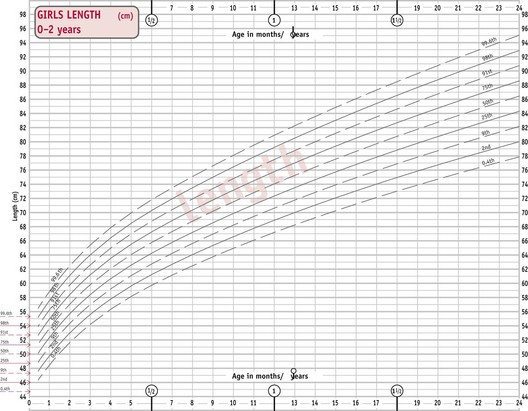
C. Girls’ weight 0–1 year. D. Girls’ length 0–2 years.
(From http://www.rcpch.ac.uk/Research/UK-WHO-Growth-Charts, accessed 8/6/2010. Reproduced with permission.)
These classifications rely on accurate assessment of gestational age at delivery.
Birthweight
Babies may be grouped according to their birthweight – especially useful when the gestation is unknown. Many studies relate to birthweight rather than gestation as it is a better predictor of outcome. The WHO definition of low birthweight (WHO 1977) is internationally adopted, with further subdivisions as shown below:
Rapid recent developments in neonatology have resulted in the survival of LBW infants above 1500 g becoming commonplace.The distribution of births by gestational age varies considerably by birthweight. This has a signifiant effect on mortality and morbidity (ONS 2009) (see website).
Preterm and SGA babies are considered separately in this chapter, although there is overlap in causes, management and complications. A baby may be preterm, SGA and LBW, and needs consideration from all perspectives.
The preterm baby
Causes
Infants born prematurely are at high risk of complications in the neonatal period and for later neurodevelopmental problems (Blackburn 2007). The causes of, and effective intervention strategies to prevent, preterm onset of labour have been researched. Specific events leading to onset of preterm labour and delivery are still uncertain and are likely to be a series or combination of maternal and fetal factors, or unexplained, rather than a single event, and include:
Some of these problems can be addressed antenatally. A crucial part of the midwife’s role is in education of women about normal pregnancy features and what should be reported to the healthcare team.
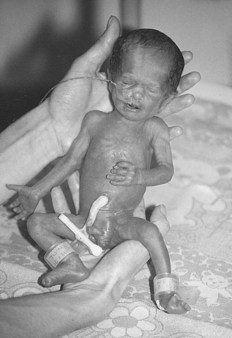
Characteristics (Fig. 44.4)
Preterm babies are immature and not ready to adapt to extrauterine life. Their appearance depends on their maturity, but generally:
Management
The preterm baby is unable to perform many physiological functions adequately due to immaturity. Neonatal care aims to compensate for these deficiencies until the infant is able to cope unaided. Accurate assessment of the baby’s condition at birth and prompt resuscitation are essential. Subsequent meticulous care and close observation are required to detect even small departures from normal physiological function, which can lead to serious complications.
Labour and delivery
Antenatal factors that influence fetal outcome include gestational age; steroid administration; predicted fetal weight; multiple pregnancy; sex; sepsis; presence and severity of any pathology; fetal growth restriction with abnormal Doppler flow studies; and fetal anomaly.
Preterm labour often progresses rapidly. If preterm labour is anticipated, birth should be planned in a maternity unit with the appropriate level of neonatal intensive care facilities. Transfer to another hospital is increasingly likely within a managed clinical network and should be discussed if clinically appropriate. Written information, including this possibility, should be given to all parents at booking.
All resuscitation equipment must be checked and fully functional prior to the birth. An experienced paediatrician and midwife or neonatal nurse must be present at delivery to ensure immediate and expert resuscitation. Thermal care is vital at this point, as cold stress can increase mortality and morbidity in preterm infants (see Ch. 42). The radiant heater should be turned on prior to delivery. The room temperature should be increased to 26°C.
Infants over 30 weeks’ gestation should be dried thoroughly and wrapped in a warm towel, and a hat applied. Infants under 30 weeks’ gestation should not be dried but should have their bodies placed immediately in a plastic occlusive wrap. This should not be covered with a towel as the radiant heater needs to be directly above the baby (UK Resuscitation Council 2008). This allows visualization of the chest and ease of access whilst preventing evaporative heat loss and skin damage.
Usually, preterm infants are small and fragile and are born requiring stabilization rather than active resuscitation. Some units administer surfactant prophylactically on the resuscitaire if infants require intubation under 30 weeks’ gestation. Other babies are given surfactant if they require ventilation and a diagnosis of respiratory distress syndrome is confirmed.
Common problems
Respiratory
The most common problems for preterm babies are respiratory disorders (discussed more fully in Chapter 45). The more preterm the baby, the more structurally and physiologically immature the respiratory system.
Respiratory distress syndrome
The incidence of respiratory distress syndrome (RDS) is inversely related to the gestational age of the baby. RDS is a developmental deficiency in surfactant synthesis accompanied by lung immaturity and hypoperfusion. Surfactant is usually produced in larger quantities between 32 and 35 weeks’ gestation and RDS is therefore rarely seen in infants beyond this gestation. Surfactant is produced by type II pneumocytes; it assists in the reduction of the surface tension of the lung and prevents complete alveolar collapse on expiration. Surfactant synthesis is reduced with hypoxaemia and acidaemia and the asphyxiated preterm infant may make such weak respiratory effort that he cannot release what little surfactant he has from the pneumocytes. The work of breathing is increased and the baby quickly becomes exhausted. The baby tries to compensate by increasing the respiratory rate and pressures. In term or more mature preterm infants, an expiratory grunt may be audible in the baby’s attempt to maintain lung volume. Intercostal and substernal recession can be quite marked – almost pulling to the backbone. There is a characteristic ‘ground glass’ appearance on chest X-ray with an air bronchogram as the air-filled major airways stand out.
Onset is before 4 hours, and the pattern of the disease gets worse over the first 24–36 hours, then stabilizes and gradually improves. Treatment is aimed at support and active intervention. Support includes maintaining oxygenation, ventilation, a normal pH and adequate perfusion, tissue oxygenation and hydration. Active intervention includes administration of exogenous surfactant and use of continuous positive airways pressure (CPAP) or other modes of ventilatory support if required.
Apnoeas
Many preterm babies have apnoeas associated with their prematurity and require constant monitoring (Finer et al 2006). Parents need to understand the reasons for this, appreciating they resolve with maturity. Airway position is essential when nursing preterm infants, to reduce the potential for apnoeas caused by mechanical obstruction of the airway. Apnoea monitors should be removed several days prior to discharge so that parents gain confidence and do not become reliant on them. Caffeine is given as a stimulant until these apnoeas of prematurity resolve (Darnell et al 2006).
Oxygen therapy
Oxygen has been used more than any other drug in the treatment of preterm infants in the past 60 years. Oxygen therapy should provide adequate tissue oxygenation without creating oxygen toxicity. Excessive and fluctuating quantities is one factor in the aetiology of retinopathy of prematurity (Campbell 1951, Tin & Gupta 2007), though clinicians are still unsure how much oxygen these babies actually need, or how much is safe to give. Further major, multi-sited research is underway in Canada (see website).
Temperature control
Although brown fat is utilized during cold stress, VLB and preterm infants often have little or no brown fat stores to maintain the core temperature. The high surface area of preterm infants in relation to their size, and their thin, porous skin, facilitate rapid heat loss through evaporation.
Radiant heaters may exacerbate the problem by increasing the evaporative heat loss and their use is inappropriate for very small babies, who should be nursed in a humidified incubator for the first week of life.
At home, following an emergency or unexpected preterm birth, if no resuscitation is required, the midwife can place the baby on the mother’s abdomen or chest for skin-to-skin contact, once dried. The baby’s head should be covered with the mother and baby well wrapped in dry blankets. The aim is to maintain body temperature in the thermoneutral range, at which energy requirements are reduced to a minimum and oxygen consumption is less (see Ch. 42).
Hypoglycaemia
This is a common problem for preterm babies in the first 48–72 hours. Stores of brown fat, white fat and glycogen are too small to maintain blood sugar level when energy requirements are particularly high. Because of the heat loss from their large surface area, increased effort of breathing that accompanies respiratory difficulties, and greater rate of growth, more energy is expended for their weight than in their term counterparts. Asphyxia, sepsis, ischaemia and hypothermia all aggravate this. Preterm infants have immature liver function, with reduced availability of liver enzymes responsible for gluconeogenesis and glycogenolysis, and are less able to produce alternative substrates, such as ketone bodies (Hume et al 2005).
Blood sugar levels should be checked every 4–6 hours during the first 48–72 hours after birth using a glucometer. Controversy still surrounds the clinical definition and significance of hypoglycaemia, but it is generally accepted that the neonatal brain can be damaged by hypoglycaemia, whether symptomatic or not (Schwarz et al 2003). Current recommendations are to maintain serum blood glucose levels above 2.6 mmol/L (Cornblath et al 2000).
Jaundice
Physiological jaundice is exacerbated in preterm babies as the liver is immature and therefore conjugation of bilirubin is further delayed (see Ch. 46).
The severity of jaundice may be caused by the delayed passage of meconium in preterm babies, particularly if enteral feeds are not commenced for several days, or with respiratory distress syndrome. This delay can contribute to hyperbilirubinaemia as the bilirubin in meconium may be reabsorbed.
Red blood cell lifespan is related to gestational age and may be only 35–50 days in the ELBW infant who may have ongoing low-grade haemolysis. Another contributing factor is the low levels of serum albumin in ELBW infants, which may limit extracellular binding and transport of bilirubin when concentrations are high (Cashore 2000).
Patent ductus arteriosus (PDA)
The ductus arteriosus fails to close in some very preterm and low-birthweight babies, partly because of immaturity and partly because the chemical conditions are not suitable in babies who are hypoxic and acidotic. Generally, in healthy preterm infants above 30 weeks’ gestation, the ductus arteriosus has closed functionally by 4 days. Only 11% of these infants have a PDA, compared to 65% of infants less than 30 weeks’ gestation with severe respiratory distress (Clyman 2004).
There are generally three physiological characteristics of a PDA in a preterm infant:
The presence of a PDA results in interstitial oedema and decreased pulmonary compliance due to pulmonary oedema. Prolonged dependence on ventilatory support increases the risk of chronic lung disease (see website).
Nutrition
The rate of growth in preterm babies is greater than that in their mature counterparts, thus they require a greater energy intake. To achieve a rate of growth similar to that in utero, the preterm baby needs 540–600 kJ/kg/day, equating to approximately 180–200 mL/kg/day of breast milk or standard formula milk. This may be achieved by giving smaller volumes of low-birthweight formula milk, or by adding calorific supplements to breast milk. Larger babies will gain weight on smaller volumes but may also need over 200 mL/kg/day.
Wherever possible, a mother’s own fresh expressed breast milk is preferred, as it will be tailor-made to her baby’s requirements, whatever the gestation (Blackburn 2007) (see Ch. 43).
The mother should be encouraged to breastfeed, if possible, or start expressing milk, within a few hours of delivery, with a minimum of six times every 24 hours. The sooner this is initiated, the greater the chance of establishing breastfeeding successfully. Midwives should teach all mothers how to express milk by hand and pump and how to store breast milk safely.
Many mothers find breastfeeding a preterm baby rewarding but time-consuming and tiring, and need to be advised that stress and fatigue can adversely affect milk production. Advice should be given on ways to increase their supply should it diminish, including sufficient rest, using hand and machine expression, and an adequate diet (see Ch. 43).
Low-birthweight formula milks have a constitution that provides the baby with more energy, protein, vitamins and minerals per millilitre than term formula milks or breast milk. These have long-chain polyunsaturated fatty acids (LCPFA) added to them. These have been identified in human milk, term and preterm, as being important for normal development of the brain, retina and neural tissue in particular.
Feeding methods
The method of feeding depends on the size, maturity and condition of the baby. Sucking is seen in the fetus as early as 13 weeks’ gestation (Hafstrom & Kjellmer 2000), although suck and swallow coordination is not efficient until nearer term. Well babies of any size or gestation showing signs of sucking may be tried with breast, cup or bottle feeds (see Ch. 43). Careful supervision is necessary to ensure that the fluid intake is adequate and that the baby is not becoming too tired by prolonged attempts at feeding.
Immature babies have an increased risk of aspiration and must be closely monitored. They tire easily and may benefit from a regimen of mixed breast/cup/bottle and tube feeds initially, until they can cope with more. Non-nutritive sucking during gastric feeding has been shown to facilitate the development of sucking behaviour (Pinelli & Symington 2007); putting the baby to the breast at these times can be beneficial to the mother and baby and for the establishment of lactation.
Intravenous feeding
Ventilated babies may be fed by the intravenous route initially, due to risks of:
If ventilation or the condition of the baby does not allow oral feeds for a prolonged period, total parenteral nutrition is commenced, providing the baby with individually tailored nutrients and calories based on serum electrolyte results.
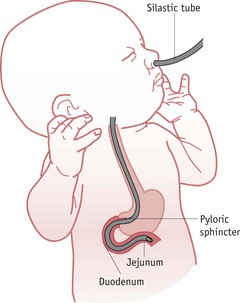
Naso/orojejunal feeding
Rarely, VLBW babies on long-term ventilation or those suffering from severe gastro-oesophageal reflux may be fed via a transpyloric or jejunal tube, which places milk directly into the bowel (Fig. 44.5), minimizing respiratory embarrassment by keeping the stomach empty. Once the baby’s condition has improved, naso/orogastric feeding is commenced.
Nutritional supplements
Preterm babies have unique nutritional requirements due to inadequate stores at birth, and immaturity causing delay in developments, such as red blood cell production. They require additional supplementation of certain nutrients, including protein, vitamins, iron and minerals, to achieve optimal growth and development. Current Department of Health and NHS recommendations are that all infants should receive daily vitamin supplements, such as children’s vitamin drops (vitamins A, C and D), from the age of 1 month to 5 years. These are especially important for preterm babies as they have small stores of fat-soluble vitamins and are at risk of vitamin deficiency (see website).
Parent–baby relationship
Parents must have an opportunity to see and hold their baby, however briefly, before transfer to the neonatal unit. The paediatrician must see the parents later to provide an account of their baby’s condition, care and prognosis. A mother may feel quite bereft in a postnatal ward, especially when surrounded by other mothers who have their babies beside them. It is common for parents to display signs of grief at this time, as they mourn the healthy baby they expected and fear getting attached to the small or ill baby they actually have. There must be good communication between the neonatal unit and the postnatal ward staff to ensure the family have the support they need.
Parents and siblings are encouraged to become involved in their baby’s care as much as possible, from the earliest days, even if the baby is being ventilated. Very few babies are too sick to be cuddled for a short while or gently held by parents, although minimal handling techniques are generally applied to their care.
Many preterm babies are in hospital for several weeks or months before they are ready to be discharged home. During this time, the parents participate in an increasing amount of their baby’s care and will gradually gain confidence. Before discharge, it is helpful for the mother to stay in hospital to care for her baby day and night, on the neonatal unit or in a transitional care ward.
Complications of prematurity
Several complications occur more commonly in the preterm than in the mature baby, relative to the degree of immaturity of the various systems or nerve centres controlling them. Extreme prematurity is a major cause of perinatal death, thus it is important to effectively reduce, identify and treat complications.
Chronic lung disease (CLD)
Chronic lung disease (previously known as bronchopulmonary dysplasia) most commonly occurs in preterm and low-birthweight babies and is defined clinically as the sustained need for oxygen supplementation after 4 weeks of age when oxygen has been required since birth. Babies with CLD will often require long-term care and follow-up.
Features of CLD include the following:
Rates of CLD vary considerably amongst NICUs, and with different treatment regimens (see website).
Infection
A preterm infant is more vulnerable to infections owing to:
In order to protect preterm babies as much as possible, the following guidelines should be followed:
Hypocalcaemia
Early hypocalcaemia (low serum calcium levels) may occur within the first 72 hours of life in preterm babies, infants of diabetic mothers and in those suffering from asphyxia, respiratory distress syndrome or sepsis. Asphyxia results in the excretion of high levels of calcitonin from the parathyroid glands. This reduces calcium mobilization from the bones. Vitamin D, required for parathyroid hormone action on bones and the gut, is also deficient in the preterm baby. Supplementation may be necessary.
Hypoxic ischaemic encephalopathy (HIE) (birth asphyxia)
The preterm infant has little energy reserve should there be any interruption to the oxygen supply, and in this event the heart will not continue pumping for long. Cardiac glycogen stores allow the heart to continue in cases of asphyxia. Continued cardiac function is required to remove the accumulated lactic acid from the brain. Since glycogen stores are reduced, the capacity of the preterm baby to withstand asphyxia is reduced.
Clinical presentation of the baby with HIE varies with severity. Six aspects of clinical presentation are assessed and may be predictive of outcome:
Treatment is aimed at minimizing further cerebral damage, alleviating symptoms and early detection of any complications, such as cerebral haemorrhage or hydrocephalus. Drugs are given to maintain cerebral perfusion and blood pressure, to reduce cerebral oedema and control seizures.
Investigations to assess the severity of damage include ultrasound, CT scanning, MRI scanning and EEG recording. Prognosis depends on the severity of the insult, but may result in severe neurological damage. Until recently, no treatment has shown to be effective for preventing brain damage (see website).
Cerebral haemorrhage and associated lesions
The incidence of neonatal cerebral haemorrhages in preterm and VLBW babies has declined in the past two decades, partly due to the use of prophylactic antenatal steroids. Cranial ultrasound scanning (see Fig. 44.6) is routinely performed on all preterm babies. Small bleeds are commonly found within a few hours of birth, even after what seemed an easy delivery, and are classified depending on site and severity.
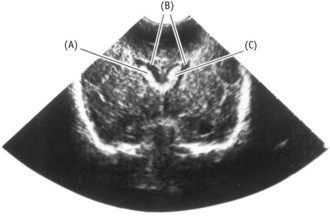
Figure 44.6 Coronal cranial ultrasound showing intraventricular haemorrhages (B) in dilated lateral ventricles (A).
(From Kelnar et al 2007.)
Major haemorrhages may cause ischaemic changes in the white matter around the ventricles, resulting in periventricular cystic leucomalacia (PVL) which is cyst formation with major neurological implications. Whilst the rate of periventricular haemorrhage (PVH) has reduced, the incidence of these ischaemic lesions appears to have increased, with implications for the morbidity of preterm and VLBW infants.
Close monitoring of fetal and neonatal wellbeing during labour and in the NNU is crucial in identifying and managing problems and maintaining stability, as is gentle handling and good pain management (see website).
Certain drugs can reduce the rate of cerebrospinal fluid production, thereby reducing hydrocephalus. Regular lumbar punctures may be performed to relieve excess pressure, but if the problem persists, the surgical insertion of ventricular shunts may be necessary, to drain the fluid into the abdomen.
Anaemia
Anaemia is common in preterm babies. The shorter intrauterine period prevents the accumulation of an adequate iron store and the immature gastrointestinal system does not easily digest iron supplements. The underactive bone marrow is unable to keep up sufficient red blood cell production to match the rapid rate of growth and increase in circulation. Ill babies require frequent blood tests and may have had much blood removed for sampling. Blood transfusions are often necessary, although some babies make good progress in spite of a low haemoglobin level.
Vitamin K deficiency bleeding (VKDB)
Newborn infants have reduced levels of the vitamin K-dependent clotting factors, including prothrombin and factors VII, IX and X. This is a consequence of poor transport of vitamin K across the placenta as well as a lack of intestinal colonization by bacteria that normally synthesize vitamin K. There is substantially more vitamin K in formula milk than breast milk.
VKDB is classified according to the timing of onset:
Early VKDB is rare and is seen in babies of mothers taking certain drugs, such as vitamin K-antagonist anticoagulants (warfarin), some antituberculosis medications (isoniazid) and some anticonvulsants (phenobarbital or phenytoin). At booking, the midwife should ask about any medicines the mother is taking and refer her to the obstetrician/physician to review these. Alternative medication may reduce the neonatal risk.
Classic VKDB is preventable by the recommended schedule of vitamin K 0.5–1 mg intramuscularly at birth (Puckett & Offringa 2000).
Late VKDB occurs almost exclusively in breastfed babies and is more likely to present as intraventricular or pulmonary bleeding, rather than gastrointestinal, with an associated high morbidity rate of up to 33% (Bor et al 2000). The cause is multifactorial, often related to hepatic disease or malabsorption syndrome. Repeated transfusions of blood, plasma and/or clotting factors are given and vitamin K is administered, in an attempt to treat the condition successfully.
Babies in the high-risk group include:
Midwives need to discuss individual risk factors with parents and obtain informed consent for vitamin K administration (see website).
Retinopathy of prematurity (ROP)
ROP is one of the few causes of childhood visual impairment that is largely preventable. Many extremely preterm babies will develop some degree of ROP, although in the majority this never progresses beyond mild disease which resolves spontaneously.
Preventive care involves reducing the incidence of preterm and low-birthweight babies and continuing with high-quality, controlled neonatal intensive care (Campbell 1951) (see website).
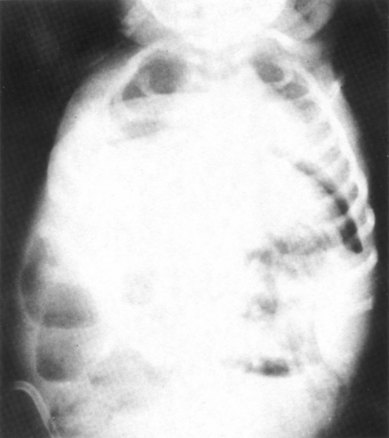
Necrotizing enterocolitis (NEC)
Necrotizing enterocolitis is an inflammatory disease of the bowel normally associated with septicaemia, thought to result from the proliferation of bacteria in the bowel which then penetrate the wall at points where ischaemic damage has occured. Oedema, ulceration and haemorrhages of the bowel wall are found, which may progress to perforation or peritonitis.
The condition typically develops in preterm and low-birthweight babies who have been ill with asphyxia, respiratory distress, hypoglycaemia, hypothermia or cardiovascular disease. Predisposing factors include: variations in bowel perfusion associated with exchange transfusion; hypotension; patent ductus arteriosus; polycythaemia (when the blood is too thick to flow readily); and thrombosis or spasm of the mesenteric vessels from an umbilical catheter.
The problem usually manifests within a few days of starting milk feeds, as it is then that colonization of the intestine is more likely.
The diagnosis is confirmed by the appearance of the bowel on radiography (Fig. 44.7) (see website).
Breast milk does afford some protection against NEC (Dai & Walker 1999), and midwives should promote breastfeeding for all babies, as it is particularly important for sick, low-birthweight and preterm babies.
The neonatal intensive care unit (NICU) environment
The NICU is a busy, bright, harsh and noisy environment that could not be further from the circumstances experienced in utero. One of the aims of neonatal care is to achieve similar rates of growth and development of the baby to those that would have been attained in utero, and practitioners need to be aware of the impact of surroundings, interventions, care and treatments undertaken for the neonate’s wellbeing.
Developmental care programmes are practised in many units, considering control of external stimuli, clustering of nursing activities and positioning of the neonates, particularly the preterm. Application of the synactive theory of infant development (Als et al 1994) through serial observations of the baby is helpful in identifying the baby’s areas of success at coping and vulnerability. It is important to communicate these strengths and vulnerabilities to the parents and identify strategies to support the baby in the NICU.
Interventions that are used to minimize the stress encountered by these infants and individualize the care-giving according to the infant’s tolerance include:
These programmes appear to demonstrate some benefits to preterm babies in terms of short-term growth, decreased respiratory support, decreased length of hospital stay and improved neurological outcomes at 2 years corrected age (Symington & Pinelli 2001). However, many studies into developmental care have small sample sizes and include multiple interventions, making interpretation difficult.
Small-for-gestational-age (SGA) babies
Incidence
One-third of all babies of low birthweight fall into this category and the majority of SGA babies are mature, having a gestational age of 37 weeks or more.
Causes
It is not uncommon for preterm babies to also be SGA and many of the predisposing factors are the same in both situations:
Characteristics (Fig. 44.8)
Babies who are SGA are categorized into two groups according to whether they are affected by asymmetrical or symmetrical growth restriction.
Asymmetrical growth restriction
Growth is normal until about the third trimester of pregnancy, when complications such as pre-eclampsia develop. This adversely affects placental function, leading to reduced growth resulting from malnutrition. This is a relatively late phenomenon and the degree of growth restriction depends on the severity of the causative condition. Head circumference and length are within normal limits for the gestational age of the baby, but birthweight is low in proportion to head circumference when plotted on a centile chart.
Symmetrical growth restriction
The main underlying causes are early intrauterine infections, such as cytomegalovirus, rubella or toxoplasmosis, maternal substance abuse, such as fetal alcohol syndrome, and other drugs taken early in the pregnancy. These have a toxic or teratogenic effect on the placenta, with fetal growth affected from the time these substances were introduced in the pregnancy. Some chromosomal anomalies and malformations also result in symmetrical SGA babies. The appearance of the baby is similar to that described above, but the head circumference is in proportion to the overall size and weight. The prognosis for these babies is poorer than those asymmetrically grown, since they have been compromised for a much longer period.
Management
A detailed booking history antenatally is essential, as this may identify risk factors associated with intrauterine growth restriction (IUGR). Careful assessment of uterine size and growth is important to enable early detection of a slow or reducing rate of growth of the fetus, possibly indicating the need for a more detailed assessment of fetal wellbeing.
A great deal of the care required by SGA babies during the first 48 hours following birth, centres around prevention and early recognition of any possible complications. In most cases, these babies can be cared for in normal postnatal wards with their mothers and do not need admission to a neonatal intensive or special care unit. Transitional care wards are an ideal place to care for those babies with minor problems, such as poor temperature control.
Labour and delivery
The growth-restricted fetus is chronically hypoxic and consequently tolerates the stresses of labour and delivery poorly, as the blood supply to the placenta is further interrupted during each contraction. The midwife should anticipate the possibility of fetal distress and perinatal asphyxia. Close fetal monitoring and observation of the liquor for meconium during labour are imperative. A paediatrician should be present at the birth if the baby is known to be significantly small or compromised. Expert and urgent resuscitation is vital, if required, particularly if there is meconium-stained liquor. This will prevent further hypoxia and respiratory complications that may result in long-term neurological damage.
SGA babies have only a thin layer of subcutaneous fat and a relatively large surface area, thus lose heat very rapidly. The room temperature must be raised prior to delivery and the baby dried and wrapped in warm blankets quickly following birth. Since 80% of heat loss occurs through the head, a hat should be placed on the baby once the head is dry.
If the baby is well enough, early feeding is important to counter hypoglycaemia resulting from insufficient energy stores in these babies.
Complications
Hypoxic ischaemic encephalopathy (HIE) (birth asphyxia)
This condition is mainly avoidable in countries with good antenatal care. The midwife must facilitate close and careful monitoring during labour and early intervention to expedite delivery in the event of fetal distress. Even modern methods of assessing fetal wellbeing are relatively insensitive and the midwife needs to be vigilant. SGA babies have limited reserves to cope with perinatal asphyxia and are at high risk. Mild symptoms of HIE may resolve after a few days, with little or no residual cerebral damage. More severe injuries may result in fits and cerebral palsy.
Meconium aspiration syndrome
SGA babies tolerate perinatal stress and hypoxia poorly. Hypoxia causes relaxation of the anal sphincter, allowing meconium to be passed into the liquor. Asphyxiated fetuses gasp in utero and will inhale the liquor and meconium into the bronchial tree, and then, with the first breath at delivery, further into the trachea. This clogs the lungs and commonly leads to pneumonitis, pneumothoraces or a secondary bacterial pneumonia.
Skilled resuscitation at delivery is vital. If the baby does not breathe, the nares and oropharynx should be suctioned prior to bag and mask resuscitation. If examination with a laryngoscope (by a skilled practitioner) shows meconium in the larynx, the baby should be intubated and the trachea suctioned through the endotracheal tube. If the baby does breathe spontaneously and is vigorous, suction and intubation are unlikely to be helpful. Close observation of the baby is imperative for the first 24 hours following meconium staining of the liquor, to identify early signs of any respiratory problems or infection (see website).
Hypothermia
Growth-restricted babies have a deficit of subcutaneous and brown fat. Hypothermia is a risk because of the relatively high surface area to bodyweight ratio. It is critical that the baby is dried and wrapped quickly following birth and the axillary temperature monitored carefully in the first 48 hours. The baby may be nursed in an incubator next to the mother in the postnatal or transitional care ward, or cared for with skin-to-skin contact with the mother to stabilize the temperature.
Hypoglycaemia
This is a common problem for SGA babies – in most cases prevented with early and regular feeding. These babies particularly benefit from breastfeeding, as they are also at a greater risk of neonatal necrotizing enterocolitis. Low-birthweight formula milks are widely used, with the advantage of being more energy-dense than regular formula milk.
SGA babies have small livers and small glycogen stores, and their energy reserves are used during labour, particularly if prolonged or difficult. Asphyxia and hypothermia will exacerbate the problem of hypoglycaemia. Frequent recordings of the blood glucose level are important within the first 48 hours, at least 4-hourly until they are stable and maintained above 2.6 mmol/L.
Polycythaemia
Polycythaemia occurs when the venous packed cell volume is 0.65 L/L (65%) and is a consequence of chronic intrauterine hypoxia. To improve the oxygen-carrying capacity of the blood, the haemoglobin level may rise to more than 20 g/dL. This can result in jaundice and posibly cerebral irritation. Treatment may require exchange plasma transfusion and additional fluids (see website).
Poor feeding
Asymmetrically growth-restricted babies tend to feed eagerly and thrive from birth. Symmetrically growth-restricted babies, however, who have been starved for a prolonged period in utero, often continue the slow rate of growth postnatally and may remain small, although a degree of catch-up growth is often evident.
Substance abuse
Babies born to substance-abusing mothers are often growth restricted, particularly following the prolonged use of heroin, alcohol and nicotine (Smith et al 2006). It is often very difficult to obtain an honest and accurate history of drugs taken during pregnancy from the woman. These babies, as well as being at risk of withdrawal symptoms and future developmental problems, are often poor at withstanding labour and may suffer perinatal hypoxia, and associated complications.
The baby of a known narcotic-abusing mother is not given neonatal naloxone during resuscitation, as this initiates rapid withdrawal symptoms as any narcotics in the baby’s circulation are rapidly broken down.
Implementing a scoring system for the frequency and severity of withdrawal symptoms, such as hyperactivity, irritability, high-pitched cry, sneezing and poor feeding, provides a framework for levels of treatment and interventions. These include nursing in quiet darkened surroundings, swaddling, and possibly medication.
Follow-up care for small and preterm babies
The paediatrician usually follows up preterm and SGA babies after discharge from hospital to assess progress, development, and general condition. Midwives, health visitors and general practitioners see the infant more frequently and play a crucial role in monitoring for and recognizing associated complications, or deviations from normal development. Symmetrically growth-restricted and neurologically damaged babies need particularly close follow-up for several years in paediatric outpatient clinics.
Hearing must be carefully checked and most neonatal units perform hearing tests routinely for preterm, sick and SGA babies. Babies are at particular risk of hearing impairment if they have experienced hypoxia, sepsis or hyperbilirubinaemia or have received certain drugs (such as gentamicin or furosemide).
Some areas now employ specialized NICU liaison nurses or midwives to monitor these small babies in the community, offering advice and support to the parents and other health professionals. This develops continuity of advice and prevents readmission to hospital through early recognition and treatment of minor problems.
Outcomes
Low-birthweight babies have an increased risk of:
With recent advances and developments in neonatal care, babies above 32 weeks’ gestation and above 1500 g now have a much greater chance of intact survival than previously, and in a small study, one-third of babies born at 23 weeks’ gestation survived to be discharged from hospital, but none were free from substantial morbidity (see website).
Ethical issues
Complex and difficult ethical issues arise in the care of very preterm and VLBW babies, especially when complications substantially increasing the risk of long-term handicap arise, or if chromosomal abnormalities or major congenital malformations are present (see website).
During the period of care, difficult decisions arise when the baby is surviving solely because of the supportive care being given, yet the risk of handicap is known to be extremely high. Professionals and parents then need time to discuss the situation openly. Space is required to reflect on the possible consequences of continuing full intensive care as long as it is required, or of withdrawing such care to allow the baby to die in peace and dignity. This is one of the most agonizing and difficult decisions both parents and professionals have to face.
Cultural factors and personal values and beliefs are deeply challenged at times like this and will influence the decisions made. Sometimes, parents appreciate the opportunity to discuss the situation with a minister of religion, or with a counsellor who is not directly involved in the care of their baby. Such help can be invaluable during this extremely stressful period (see Ch. 70).
Conclusion
Many mothers feel extremely guilty when they give birth to a preterm or low-birthweight baby, often blaming themselves for actions taken or omitted during the pregnancy. Midwives must offer as much support as possible during these times.
Individuals react differently to stress and midwives and neonatal staff need to learn to recognize the signs in parents and develop appropriate skills to enable families to cope during this difficult time. They must also recognize the signs of stress in themselves and in colleagues. Opportunities to share and discuss problems can be of immense benefit to all concerned. Effective personal coping strategies are therefore essential for those working with families with preterm, sick and VLBW babies.
Midwives are a key part of the team providing care to these small babies and their parents, and need to work closely with their colleagues to ensure a seamless, sensitive and high-quality service both initially and on a long-term basis.
Als H, Lawhon G, Duffy FH, et al. Individualized developmental care for the very low birthweight preterm infant. Medical and neurofunctional effects. Journal of the American Medical Association. 1994;272(11):853-859.
Blackburn ST. Maternal, fetal and neonatal physiology: a clinical perspective. Philadelphia: WB Saunders; 2007.
Bor O, Akgun N, Yakut A, et al. Late haemorrhagic disease of the newborn. Pediatrics International. 2000;42(1):64-66.
Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia: a clinical approach. Medical Journal of Australia. 1951;ii:48-50.
Cashore D. Bilirubin and jaundice in the micropremie. Clinics in Perinatology. 2000;27:171-179.
Clyman RI. Mechanisms facilitating closure of the ductus arteriosus. In Polin RA, Fox WW, Abman SH, editors: Fetal and neonatal physiology, ed 3, Philadelphia: Saunders, 2004.
Cooke RWI. Perinatal and postnatal factors in very preterm infants and subsequent cognitive and motor abilities. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2005;90(1):F60-F63.
Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding the definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105(5):1141-1145.
Dai D, Walker WA. Protective nutrients and bacterial colonization in the immature human gut. Advances in Pediatrics. 1999;46:353-382.
Darnell RA, Ariagno RL, Kinney HC. The late preterm infant and the control of breathing, sleep and brainstem development: a review. Clinics In Perinatology. 2006;33(4):883-914.
Dubowitz LMS, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. Journal of Pediatrics. 1970;77(1):1-10.
Finer N, Higgins R, Kattwinkel J, et al. Summary proceedings from the Apnoea of Prematurity Group. Pediatrics. 2006;117(3 Pt 2):S47-S51.
Hafstrom M, Kjellmer I. Non-nutritive sucking in the healthy pre-term infant. Early Human Development. 2000;60(1):13-24.
Hume R, Burchell A, Williams FL, et al. Glucose homeostasis in the newborn. Early Human Development. 2005;81(1):95-101.
Johnston PGB, Flood K, Spinks K. The newborn child, ed 9. Edinburgh: Churchill Livingstone; 2003.
Kelnar CJH, Harvey D, Simpson C. The sick newborn baby, ed 4. London: Baillière Tindall; 2007.
McElrath TF, Robinson JN, Ecker JL, et al. Neonatal outcome of infants born at 23 weeks’ gestation. Obstetrics and Gynecology. 2001;97(1):49-52.
Marlowe N, Wolke D, Bracewell M, et al. Neurologic and developmental disability at six years of age after extremely preterm birth. New England Journal of Medicine. 2005;352(1):9-19.
Office for National Statistics (ONS). Gestation – specific infant mortality by social and biological factors, England and Wales 2006. (website) www.ons.gov.uk, 2009.
Pinelli J, Symington A: Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants, Cochrane Database of Systematic Reviews (4):CD001071, 2007.
Puckett RM, Offringa M: Prophylactic vitamin K for vitamin K deficiency bleeding in neonates, Cochrane Database of Systematic Reviews (4):CD002776, 2000.
Rich-Edwards JW, Stampfer MJ, Manson JE, et al. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. British Medical Journal. 1997;31(7105):396-400.
Royal College of Paediatrics and Child Health (RCPCH). UK-WHO growth charts – early years. http://www.rcpch.ac.uk/Research/UK-WHO-Growth-Charts. Access Nov 2010
Schwarz R, Cornblath M, Kalhan SC. Hypoglycaemia in the neonate. In: Kendall Stevenson D, Sunshine P, Benitz W, editors. Fetal and neonatal brain injury: mechanisms, management and the risks of practice. Cambridge: Cambridge University Press; 2003:553-570.
Smith L, LaGasse L, Derauf C, et al. The Infant Development, Environment and Lifestyle study: effects of prenatal methamphetamine exposure; polydrug exposure and poverty on intrauterine growth. Pediatrics. 2006;118(3):1149-1156.
Symington A, Pinelli J: Developmental care for promoting development and preventing morbidity in preterm infants, Cochrane Database of Systematic Reviews (4):CD001814, 2001.
Tin W, Gupta S. Optimum oxygen therapy in preterm babies. Archives of Diseases in Childhood. Fetal and Neonatal Edition. 2007;92:F143-F147.
UK Resuscitation Council. Resuscitation at birth. London: Resuscitation Council UK; 2008.
Vadillo-Ortega F, Estrada-Gutierrez G. Role of matrix proteins in premature labour. International Journal of Obstetrics and Gynaecology. 2005;112(Suppl 1):19-22.
World Health Organization (WHO). Manual of international statistical classification of diseases, injuries and causes of death, Vol. I. Geneva: WHO, 1977.