Chapter 19 Manifestations and Management of Disease in Foals
MATURITY
GESTATIONAL PERIOD
In contrast to most other domesticated species, in mares the duration of the gestational period is highly variable. The gestational age is typically calculated from the day of insemination to the day of parturition, a value that may overestimate the true gestational period by as much as 7 days. The mean gestational length in the thoroughbred is consistently reported to be 340 to 342 days,1-5 but the range of normal gestational ages is wide, with an estimated 95% confidence interval of 327 to 357 days.1 The mean duration of pregnancy also appears to be relatively consistent across breeds: Friesians, 3326 or 3387 days; Arabians, 332 days8; Dutch Freiberger mares, 336 days9; draught breeds, 343 days7; Haflinger ponies, 341 days; Fjord ponies, 342 days; Shetland ponies, 337 days7; and ponies in England, 3335 or 325 days.10 Several factors appear to determine the length of gestation in mares. Colts on average have a longer gestation than fillies, with a reported difference of 1.5 to 2.5 days.2-46 Colts are also heavier, have a heavier placenta, and take longer to stand.4 The time of conception within the breeding season affects the duration of the gestational period. Mares that conceive early in the breeding season have longer pregnancies than those bred toward the end of season. This difference may be as great as 10 days.1,2 An influence of mare, sire, and dam’s sire on gestational length was recently reported in Friesian mares6 and an effect of sire was reported in Freibergers.9 A study of thoroughbreds concluded that dam, in addition to foal gender and month of conception, but not sire, had a significant effect on the duration of pregnancy.2 The age or parity of the mare does not appear to have an influence,2,3 but maternal age has been correlated with decreasing foal birthweights. Twinning is also an important cause of shortened gestational periods and in utero growth retardation.11
The terminology associated with birth maturity in most species is straightforward but is difficult in horses because of the variability in normal gestational length. Retrospective studies report wide variability in gestational age but unfortunately do not report physical characteristics or foal survival. Gestational ranges of 305 to 365 days, 315 to 387 days, and 286 to 370 days have been described.2,3,12 The term “premature” in other domesticated animals and in human beings refers to the birth of an infant or animal after a gestational period shorter than normal. A premature human infant is now defined as one who is delivered at least 21 days before the mean pregnancy duration of 266 days. The use of a gestational age to classify equine prematurity has been described, with the most commonly used definition being a foal born before 320 days of gestation.5 This definition was based on significantly lower birthweights and poor outcomes of foals born before 320 days.13 It could also be argued that foals born outside the lower 95% confidence interval of the normal gestational period would best fit the classification of premature; this would be less than 325 days in thoroughbreds, using data extrapolated from Hintz and others.3 It is clear that any precise classification of prematurity based solely on estimated gestational age would falsely classify a small number of appropriately mature animals. There are similar difficulties in classifying animals that have experienced a longer than normal gestational period. Again, using the upper confidence interval limits from thoroughbreds, foals born after 356 days could be regarded as postterm. A distinction should be made between postterm and postmature, the latter describing a condition of increased neonatal morbidity as a consequence of failing placental function.
Dysmaturity is a term commonly used to describe foals that have experienced some degree of intrauterine growth retardation (IUGR). Such foals typically demonstrate some signs of physical immaturity, such as a low birthweight. Dysmature foals can have shortened, normal, or prolonged gestation lengths. Other terms used to classify foals with incomplete maturation include viable and nonviable13 and ready and unready for birth.14 In a review of terminology, Koterba suggested the terms viable and nonviable were inappropriate because outcomes of premature foals are heavily influenced by access to facilities and the value of the animal.13 The concept of readiness for birth was used to categorize foal outcomes based primarily on the degree of maturation of the fetal hypothalamus-pituitary-adrenal (HPA) axis. Although this plays a critical role in determining postpartum survival, other factors, including the degree of physical maturation and the consequences of an adverse intrauterine environment, are also relevant in determining the ultimate outcome.13 Premature maturation of the HPA axis often takes place at an inappropriate developmental stage for some body systems, causing asynchrony of organ maturation and postnatal problems.15 Extending the concept of readiness for birth, Rossdale introduced the term twilight foals to describe those foals with accelerated but incomplete maturation of the HPA axis at the time of birth.5,16
The physical characteristics associated with prematurity include a low birthweight and small body size, a short and shiny haircoat, a prominent rounded head, periarticular laxity, and droopy ears. Foals typically have moderate flexor laxity with elevation of the toe, but some have contracture of the fetlock. Muscle development is usually poor. Most demonstrate generalized weakness and hypotonia and have difficulty in standing. Severely premature foals may have lids naturally sutured closed and little hair covering their bodies. Many have difficulty in maintaining body temperature, blood pressure (BP), and blood glucose.
Dysmature foals commonly experience some degree of IUGR. This is usually reflected by the birth of a foal that is small for its gestational age. The average relative weight of the term foal to its dam is approximately 10%. Postmature foals usually have an acceptable birthweight with a large frame but poor muscle development. This gives the foal a lanky appearance. In contrast to premature animals, fetlock contracture is common, although laxity can be present. Consistent with their prolonged gestation, postterm or postmature foals often have erupted incisors and a long haircoat. In term foals the central incisors typically erupt during the first 5 to 7 days of postnatal life.
CAUSES OF PREMATURE DELIVERY
The pregnant uterus is highly responsive to contractile agents such as oxytocin and prostaglandins throughout gestation. Consequently, one of the most important causes of premature birth and perinatal morbidity and mortality is the induction of labor with exogenous oxytocin or prostaglandins. The adverse consequences of premature induction of parturition were identified in a study in which parturition was induced either before 300 days’ gestation or between 300 and 320 days’ gestation.17 The overall survival rate was only 5%, with the youngest surviving animal delivered after 318 days’ gestation. Other surviving foals were all delivered after 320 days’ gestation. The decision to prematurely terminate a pregnancy may be made deliberately in the “normal” mare, or the termination may be necessary because of significant maternal disease. The latter frequently involves delivery of a compromised and often premature foal by cesarean section. Chemical induction of parturition sometimes occurs when late pregnancy intestinal problems are misinterpreted as ineffective labor. Premature birth can occur as a sequela to placental problems, including placental infection, edema, and/or detachment (premature placental separation). Placental insufficiency as a result of twinning is another cause of IUGR.
The consumption by pregnant mares of tall fescue pasture infected with Neotyphodium coenophialum leads to range of abnormal signs including prolongation of gestation, perinatal mortality, and agalactia.18 The large skeletal frame of the postmature foal predisposes mares to dystocia. The delay in parturition may be caused by toxin-induced interference with fetal corticotropin-releasing hormone (CRH) and delay in maturation of the HPA axis. Foals born to mares grazing endophyte-infected fescue pasture have normal thyroxine and reverse T3 but reduced triiodothyronine levels compared with control foals.19 This is also consistent with failure of cortisol-induced maturation of thyroid function. A syndrome of congenital hypothyroidism has been reported in foals in Western Canada.20 Signs include prolonged gestation, dysmaturity, and a range of musculoskeletal abnormalities including flexural deformities, delayed ossification, and mandibular prognathism. The specific cause has not been determined, although consumption of diets that contain nitrate or are deficient in iodine is suspected.21
MATURATION OF THE FETAL HYPOTHALAMIC-PITUITARY-ADRENAL AXIS
The maturation of several organ systems coincides with changes in the fetal HPA axis.15 Fetal cortisol is critical for organ maturation, but if the fetus is exposed too early in gestation or to too large a quantity IUGR may occur. The fetus is protected from cortisol during much of gestation. The type 2 isoform of the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD) converts excess biologically active cortisol into inactive cortisone in the placenta, thereby reducing the exposure of the fetus. The postnatal adrenal gland, under the influence of ACTH from the pituitary, can readily synthesize cortisol from cholesterol and pregnenolone (P5). Several important enzymes are required for this conversion, including 3β-HSD, P450scc, and P450C17. These enzymes are either inhibited or deficient during most of pregnancy, again protecting the developing fetus from excessive cortisol. Consequently during the majority of gestation the major products of steroidogenesis are progesterone and the 5α-reduced progestagens and not cortisol.22 Foals, like other species studied, undergo enhanced adrenal activity before birth. This is reflected by high plasma cortisol and ACTH concentrations in term newborn foal plasma in the first hours after birth.23 There is also a substantial change in the amount and localization within the adrenal gland of 3β-HSD, P450scc, and P450C17 around the time of birth.24
The trigger(s) for the process that results in fetal cortisol production, organ maturation, and birth are not known. Data from sheep indicate that upregulation of CRH messenger ribonucleic acid (mRNA) in the fetal hypothalamus and proopiomelanocortin in the fetal pituitary is a key initiating event.15,25 At the same time there is upregulation of adrenocorticotropic hormone (ACTH) receptors and key steroidogenic enzymes in the fetal adrenal glands. The consequence is a progressive increase in circulating ACTH and cortisol in the fetus. The rise in fetal cortisol has a direct effect on the placenta to increase prostaglandin H synthase 2, leading to secretion of prostaglandins such as prostaglandin E2 (PGE2).15,25,26 Prostaglandins further stimulate the fetal HPA axis, stimulate placental 11β-HSD-1 (which favors the production of cortisol from cortisone), and also facilitate the conversion of estrogen from pregnenolone. It is not known if these events occur in the pregnant mare, but they do appear to be consistent across most species studied.
An important difference between equids and other species is the timing of these events before parturition.15 In pregnant ewes, maturation of the HPA axis occurs during final 20 days of a 150-day gestation. In contrast, the production of significant fetal cortisol appears to occur during the final 48 to 72 hours of pregnancy in mares.27 Several important maturational events appear to be tightly associated with the prepartum increase in ACTH and cortisol.22 These include changes in red blood cell (RBC) and white blood cell (WBC) parameters, most notably a large increase in the neutrophil-to-lymphocyte ratio (N:L).27,28 Hepatic and renal glucose-6-phosphatase, a key enzyme of gluconeogenesis, also increases sharply around the time of birth,29 coinciding with increases in hepatic and skeletal muscle glycogen stores.
The prepartum rise in plasma cortisol likely induces deiodination of the outer ring of T4 to produce the biologically active triiodothyronine (T3).30 Adequate levels of T3 are required for a number of biologic functions including postnatal thermogenesis. Normal term foals have very high levels of thyroid hormones, including T3, at the time of birth.31 These levels decline over the initial weeks or months of postnatal life. A relationship between circulating T3 levels and cortisol was reported in premature, dysmature, and mature foals,16 and the increase in T3 appears to be dependent on maturation of the HPA axis.32 Both cortisol and T3 are critical for lung maturation, particularly the normal postpartum reabsorption of lung liquid.32
ACCELERATED MATURATION OF THE FETAL HYPOTHALAMIC-PITUITARY-ADRENAL AXIS
Several factors can induce premature maturation of the fetal HPA axis. Hypoxemia is a potent stimulator of the axis in sheep, with rises in fetal ACTH and cortisol.33 The HPA axis can also be manipulated using exogenous glucocorticoids; betamethasone is commonly administered to women in danger of preterm birth in order to hasten HPA maturation and therefore improve the chances of postnatal survival. Poor nutrition before and after conception in sheep produces a shortened gestational period and hastened maturation of the fetal HPA axis.25 Placental and/or fetal infection also can accelerate maturation of the HPA axis. The cytokines induced by infection increase prostaglandin synthesis and decrease metabolism. Prostaglandins exert a range of actions in addition to promoting cortisol production.
The stimuli associated with precocious HPA axis maturation in foals are not well described. The exception is infection of fetal membranes, in which foals are often delivered preterm with laboratory findings consistent with axis maturation. Spatial and nutritional deprivation resulting from a thoroughbred foal placed in a pony uterus using embryo transfer also leads to premature maturation of the fetal adrenal.10,34
Unfortunately, many late-term maternal diseases do not appear to have a significant effect on foal maturity. Hypoxemia associated with anesthesia and colic surgery in pregnant mares during the final 60 days of pregnancy results in a high rate of preterm delivery of compromised foals that did not survive.35 It is likely that the insult in these cases was so severe that the interval between surgery and delivery was inadequate for maturation of the axis to occur. Another important consideration in determining outcomes would be the effect of hypoxia and/or ischemia on other fetal organ systems.
TREATMENT OF THE AT-RISK LATE PREGNANT MARE
Unfortunately, the administration of corticosteroids to the pregnant mare appears to have little effect on maturation of the fetal HPA axis, at least when doses considered safe are used.5 Direct injection of ACTH1–24 to the fetus results in increased fetal cortisol, but the effect is dependent on gestational age, with maximal responses occurring at around day 313 and with no measurable benefit when administered before day 295.27 Direct administration of CRH, ACTH, or betamethasone to the fetus using ultrasound-guided intramuscular (IM) injection results in increased maternal progestagen levels consistent with maturation of the fetal adrenal gland.36,37 The procedure itself can lead to abortion in a small number of mares. Exogenous ACTH1–24 administered to late pregnant pony mares had an impact on both gestational length and fetal maturation.38 Depot ACTH1–24 given to mares at 300, 301, and 302 days of gestation produced a shortened gestational length and lower birthweight but evidence of HPA axis maturation. A confounding effect in this study was the time of conception, with the most significant findings observed in mares bred later in the breeding season.
It is preferable to maintain the fetus in utero to ensure not only adequate HPA axis maturation but also effective ossification and maturation of other body systems. Consequently, the primary focus of therapy for a mare with placental infection is to eliminate the pathogenic organisms, reduce inflammation, and maintain the pregnancy. The specific management of placentitis is not the focus of this chapter, but treatment may involve broad-spectrum antibiotics, nonsteroidal antiinflammatory drugs, pentoxifylline, β2-adrenoreceptor agonists, and altrenogest. The efficacy of altrenogest use in mares with placentitis has been questioned.22
The termination of postterm pregnancies is a difficult decision for practitioners, particularly with the emotional response that is common in many owners. Given the wide variation in gestational range it is almost always in the best interests of the foal to let the pregnancy continue. If facilities are available then rectal and transabdominal ultrasound assessment of the fetus and chorioallantois should be made, looking carefully for thickening or detachment of the fetal membranes. Ideally any induction of parturition should be based on appropriate changes in physical characteristics of the mare and in milk electrolytes. Mares grazing endophyte-infected tall fescue can be medicated with dopamine receptor antagonists, such as domperidone.18
LABORATORY ASSESSMENT
The laboratory data will indirectly reflect the degree of HPA axis maturation. Premature or dysmature foals that fail to survive often have minimal cortisol secretion in the face of adequate endogenous ACTH. Furthermore, the change in plasma cortisol in response to exogenous ACTH1–24 (0.125 mg IM) is inconsistent and usually inadequate.23 The foal with incomplete adrenal maturation will have low total white cell and neutrophil counts and an N:L that is characteristically less than 1:1.28 It is important to determine if sepsis is present as neutropenia is also a common feature of this condition. Evidence of shifting toward immature cell types and neutrophil toxicity should indicate primary sepsis or prematurity or dysmaturity complicated by sepsis. Premature foals that fail to improve their total WBC and neutrophil counts over the initial 24 to 48 hours of treatment have an even poorer prognosis for survival. Changes in red cell indices have also been reported in nonstressed preterm foals.28 Most notable is an elevated mean corpuscular volume in preterm foals. An elevated plasma fibrinogen concentration is considered to be a good prognostic factor in premature foals as it often reflects prepartum exposure to bacterial infection. Induced or spontaneously delivered term foals have a significantly higher plasma glucose concentration than premature foals.39 Plasma creatinine levels are often elevated in newly born preterm or dysmature foals as a result of placental dysfunction. This increase is independent of foal renal function. Measurement of low cortisol levels coupled with increased progestagens would provide further evidence that effective maturation of the HPA axis has not occurred in the foal before birth.40
ESTABLISH A PROGNOSIS
The prognosis for survival of a prematurely delivered foal is dependent on a range of factors including the gestational age, the reasons for delivery, complications associated with delivery, available resources (facilities and expertise), and financial limitations of the owner. Survival of very premature foals (280 to 300 days) would typically require a history of chronic in utero stress with resultant precocious maturation of the HPA axis and critical organ systems. The majority of such foals would still require a lengthy and costly period of hospitalization and experience a range of complications, some of which could be life-threatening. Foals delivered prematurely as a consequence of chemical induction of parturition without evidence of chronic in utero stress or via cesarean section typically have a high mortality rate even when delivered close to calculated due dates. Foals delivered under these circumstances before 300 days will almost certainly die, irrespective of available resources.
A complete blood count (CBC) and fibrinogen estimation are key factors in determining short-term prognosis. A normal or elevated neutrophil count, N:L, or total WBC count is a positive indicator for survival, as these values typically reflect maturation of the HPA axis. In a survey of 135 neonates admitted to the University of Florida with a gestational age of ≤320 days, short-term survival was in part predicted by total WBC count, neutrophil count, lymphocyte count, and the N:L at presentation.41 The N:L of surviving premature foals (12.5:1) was well above that reported for both normal term foals (2.5:1) and nonsurviving premature foals. Many of the surviving animals were exposed to confirmed or suspected placental infection. Outcome was not affected by gestational age (surviving foals 311 days and nonsurvivors 307 days). These data confirm that in utero stress, with hastened maturation of the HPA axis, is a good prognostic factor for survival in foals that are delivered preterm. In many foals the greater the neutrophil count the better the outlook, at least in terms of short-term survival. A high plasma fibrinogen concentration is also considered to be a positive factor. Reevaluation of the white cell indices on day 2 for appropriate increases also supports a favorable prognosis.
A history of placental infection appears to be a positive factor when predicting survival in preterm foals. One obvious downside is that many of these foals are born with aspiration pneumonia (a result of in utero aspiration of contaminated amniotic fluid) and/or systemic sepsis. This, coupled with the fact that many foals have an impaired immune system, warrants the use of broad-spectrum antimicrobial therapy.
Consideration should also be given to the long-term outcomes of preterm foals. These animals are at risk for significant and permanent musculoskeletal problems as a result of bone and ligamentous immaturity. Foals that survive the neonatal period are smaller than their peers, and this difference will often remain noticeable when they are weanlings and yearlings. The differences may be less obvious at 2 years and older. Other common complications, such as pneumonia, will further reduce the growth rate in the first 6 months of life. There is nothing to indicate that premature fillies will experience fertility problems as adults.
CLINICAL PROGRESSION
The clinical progression usually reflects the degree of endocrinologic maturity, additional perinatal stresses, and the extent of physical maturity. Typically, foals born prematurely but chronically exposed to an appropriate in utero stress such as placental infection will appear weak and depressed in the immediate postpartum period. Some will require resuscitation. After a longer than normal period of postural adaptation they will usually manage to stand but will often require assistance. Suckle reflex and appetite may be reduced or absent, and many will need to be fed initially via nasogastric tube. They will frequently have trouble maintaining their body temperature and blood glucose levels. After the initial 24-hour period many of these foals demonstrate improvement both in physical strength and mentation. Their appetite for milk will often exceed that of a healthy term foal. Foals with inadequate maturation of the HPA axis will frequently require immediate resuscitation. They may mimic the clinical progression of in utero stressed premature foals until 12 to 18 hours of age. This initial period after delivery can be deceptive, with many foals showing degrees of improvement, which often promote owner optimism. The rise of hormones accompanying delivery may lead to improvement in alertness and strength. However, after this period a range of progressive abnormalities develop. These include systemic weakness, depression, seizures, respiratory failure, and intolerance to feeding. Cardiovascular collapse may ensue, the first sign of which is a reduction in the intensity of peripheral pulses, followed by a reduction in urine flow, development of subcutaneous edema, and deteriorating neurologic function. Poor tissue perfusion leads to lactate accumulation and a mixed metabolic and respiratory acidosis. Death will certainly occur without aggressive support, and even with high-level intensive care the mortality rates are very high.
TREATMENT OF THE PREMATURE OR DYSMATURE FOAL
It is critical to perform a thorough physical examination, as problems of altered maturity can involve many organ systems. Successful outcomes are dependent not only on careful management of identified problems but also in predicting the problems that may arise in the hours, days, or weeks to come. Most premature and dysmature foals experience some degree of pulmonary insufficiency. Factors that predispose these foals to respiratory problems include structural and functional immaturity, a naïve and potentially immature immune system, altered pulmonary vascular reactivity, a highly compliant rib cage, and a propensity for prolonged or persistent recumbency. Final maturation of the respiratory system appears to be highly dependent on a functional HPA system. Arterial blood gas (ABG) analysis is an important tool in the assessment of respiratory function, and the lower arterial oxygen concentration in term newborn foals is further decreased in dysmature or premature foals. Extrapulmonary shunts account for more than 30% of the cardiac output, in contrast to <10% in normal full-term foals.42 Ventilation-perfusion mismatching also occurs because of a poorly reactive pulmonary vasculature and dependent atelectasis. Deficiency of lung surfactant is not likely to play a primary role in the respiratory dysfunction in most premature or dysmature foals, as it is usually fully developed in most foals by 300 days, but it could be delayed until after 340 days in some foals.43 The most severe form of respiratory failure is neonatal respiratory distress syndrome (RDS), a disease characterized by progressive respiratory failure, severe hypoxemia and hypercapnia, coma, and death. A diffuse severe alveolar pattern is a classic radiographic finding. Intervention would ideally involve mechanical ventilation, bovine or synthetic surfactant, and glucocorticoids; however, outcomes are extremely poor, irrespective of the level of care. Fortunately RDS is relatively uncommon; most premature foals will, however, demonstrate a less severe manifestation of lung dysfunction characterized by reduced ventilation capacity, tachypnea, hypoxemia, and varying levels of hypercapnia. These foals are very susceptible to dependent lung atelectasis from recumbency. Most foals will benefit from supplemental intranasal oxygen with initial flow rates of 5 L/min recommended. Adjustment in flow rate is dictated by positive changes in ABG analyses or improvement in ventilation rate and depth. It is important to avoid prolonged periods of lateral recumbency in order to minimize the impact of atelectasis. If the foal is unable to stand, then placement in sternal recumbency is recommended. This is made easier by use of a specially constructed V-pad.
Failure of the cardiovascular system is common in foals with partial or incomplete maturation of the HPA axis. Management is challenging in part because of inconsistent responses to standard inotrope and vasoreactive therapy. Successful treatment is reliant on early detection of reduced perfusion. This is reflected clinically by cool extremities, the presence of limb and ventral edema, and darkening of the mucous membranes with prolongation of the capillary refill time. As failure ensues, peripheral pulses will become difficult to palpate, blood pH will fall, and there will be increases in plasma lactate and anion gap. Indirect (or direct) measurement of mean BP along with determination of blood lactate will help guide therapy. An initial approach to the treatment of failing perfusion may involve intravenous plasma followed if necessary by dopamine (3 to 5 μg/kg/min) and/or dobutamine infusion (5 to 20 μg/kg/min). The volume and type of fluids given should be carefully monitored, as fluid overload and hypernatremia are common. Urine output should be appropriate for the volume of fluids administered, and anuria or oliguria should be treated aggressively. This may include low-dose dopamine or fenoldopam infusion, furosemide boluses or infusion, or mannitol infusion. Establishment of urine flow is critical in terms of survival.
Signs of gastrointestinal (GI) tract dysfunction are rarely evident on initial assessment of premature or dysmature foals; however, most will not tolerate aggressive force-feeding. These foals commonly develop intestinal stasis with reduced fecal passage, gas accumulation, and gastric distention. The combination of prolonged asphyxia and prematurity is also a risk factor for the development of necrotizing enterocolitis (NEC). Feeding should be restricted to very small volumes (e.g., 10 to 20 mL hourly) until the foal appears to be systemically stable. Concurrent parenteral nutrition (PN) is indicated in order to prevent loss of body weight. Foals should be monitored closely for signs of GI dysfunction irrespective of feeding volume or frequency. Such monitoring includes assessing fecal passage, monitoring for changes in abdominal size (assessed using a measuring tape), testing for gastric reflux if a nasogastric tube is in place, and frequently assessing with transabdominal ultrasound.
Premature and dysmature foals are susceptible to hypothermia. Thermogenic mechanisms develop late in gestation and are related to circulating T3 levels. As discussed previously, thyroid hormone generation is closely tied to maturation of the HPA axis. Consequently problems with thermogenesis are exacerbated in preterm foals with incomplete adrenal function. Body temperature needs careful management, as rapid warming may result in peripheral vasodilatation and possible cardiovascular collapse. Initially the foal should be covered by blankets and removed from any drafts. Intravenous and oral fluids should be warmed before use. Once the foal begins to demonstrate vigor, heat lamps and circulating warm-water blankets can be used.
Premature and dysmature foals often have inadequate gluconeogenic enzyme activity and limited glycogen stores at the time of birth. Consequently most will have difficulty maintaining a normal blood glucose concentration. This is managed acutely by infusion of 10 mL/kg of a 10% dextrose solution over several minutes, followed by a constant infusion at about 6 mg/kg/min (approximately 200 mL/hour of a 5% dextrose solution to a 30-kg foal). Blood glucose should be monitored regularly to avoid hyperglycemia. Some foals with persistent hyperglycemia benefit from insulin supplementation.
Skeletal maturity is assessed by radiographing a carpus and a tarsus for evidence of incomplete ossification (Fig. 19-1). Accelerated ossification does not appear to be a feature of foals born prematurely after exposure to chronic in utero stress. Incomplete ossification coupled with periarticular laxity predisposes the premature or dysmature foal to long-term skeletal problems. Foals with incomplete ossification and more than 30% reduction of the central and/or third tarsal bones with pinching or fragmentation of the dorsal aspects of affected bones commonly develop degenerative joint disease and have a guarded prognosis for future athletic performance. Restriction of exercise is recommended in order to minimize collapse of developing carpal or tarsal bones, but forced recumbency may predispose the foal to or exacerbate pulmonary disease. Furthermore, normal load bearing encourages ossification. Periarticular laxity predisposes the premature foal to angular limb deformities that facilitate abnormal load bearing and increase the risk of cuboidal bone crush injury of the carpus or hock. Splinting and attention to hoof care are recommended if angular limb deviation develops. In most cases flexural deformities and laxities improve over time. Dorsal splints are recommended for flexural deformities involving the fetlock, and heel extensions are helpful to foals with flexural laxity.
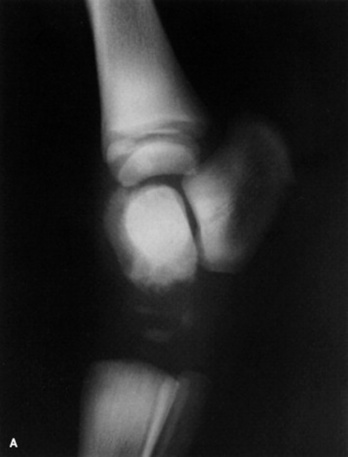
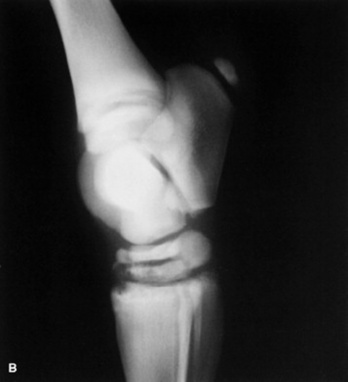
Fig. 19-1 A, Lateral tarsus of a 1-day-old, 305-day gestational age colt. Note the lack of ossification of the small tarsal bones. B, Lateral tarsus of the same foal as in A, at 3 weeks of age, showing irregular ossification. Without the initial radiograph, increasing ossification could have been confused with bone lysis and osteomyelitis. The foal is reported to be sound at 6 months of age.
There are several reasons why colostral transfer of maternal immunoglobulin may not occur in premature foals. Mares may have lactated prematurely or not at all, and -the foal may not be able to suck. It is crucial to ensure that the premature neonate receive ample amounts of high-quality colostrum (>20 mL/kg) in the first 6 hours after birth, although the intestinal tract may not be capable of efficient colostral uptake or may not tolerate large volumes of liquid. Consequently, plasma transfusion is often used even in foals less than 18 to 24 hours of age. A serum immunoglobulin G (IgG) level should be measured to confirm successful transfer of immunity (>800 mg/dL).
The use of glucocorticoid therapy in the management of prematurity is controversial. Dexamethasone has been used in human medicine because of its potency, but it is associated with adverse side effects including hypertension, hyperglycemia, and catabolism.44 Hydrocortisone has a shorter half-life and lower biologic activity and is as effective for improving lung function in preterm human infants without the side effects of dexamethasone.
WEAKNESS AND/OR DEPRESSION
When confronted with a neonate with the primary complaint of weakness with or without accompanying depression, a number of differential diagnoses must be ruled out (see Box 20-1). The gestational and postnatal age of the neonate should be established. If weakness has been present since birth, then in utero acquired bacterial or viral infections, birth asphyxia and trauma, chronic placental problems, and congenital anomalies should be placed higher on the list of differential diagnoses. Lethargy and loss of suckle are often the first signs of neonatal illness. A full udder on the dam accompanies poor nursing behavior in the neonate. If the neonate is depressed and has injected mucous membranes and hyperemic coronary bands, then sepsis is the primary differential diagnosis and the most life-threatening. If the neonate is relatively bright but is becoming a “dishrag,” consider peripartum hypoxia and early signs of hypoxic-ischemic encephalopathy. If the newborn shows signs of physical immaturity such as tendon laxity and silky hair coat, then weakness may be a result of progressive fatigue, hypothermia, hypoxia, and/or hypoglycemia. Unfortunately, many weak foals begin to fade as a result of multiple problems. Glycogen branching enzyme deficiency in certain quarter horse and Paint lineages is associated with a range of abnormal signs that could include persistent recumbency.45
If weakness is present without accompanying depression, then several other differential diagnoses should be considered. Neuromuscular diseases include botulism, white muscle disease, and congenital myopathies. Botulism is an infection acquired via the GI tract. Consequently, signs appear in neonates that are usually 10 days of age or older. Although most cases of nutritional myodegeneration (NMD) occur during the first year of life among rapidly growing large animal neonates, an in utero form of NMD may occur, resulting in clinical signs in affected foals soon after birth. If weakness is detected in one or more limbs immediately after birth, peripheral nerve and muscle damage associated with birth trauma should be ruled out. Foals with rupture of the gastrocnemius muscle will be unable to rise or stand unsupported.46
It should be determined whether any drugs or anesthetics were administered to the dam before or at the time of delivery, as many agents cross the placenta and can exert depressive and other adverse effects on the fetus. For example, one study reported that phenylbutazone administered to normal pregnant mares crossed the placenta and resulted in substantial concentrations of phenylbutazone and its active metabolite oxyphenbutazone. Although clinical signs of phenylbutazone toxicity were not noted in the foals postnatally,47 adverse effects are possible, particularly if other problems are present. Drug-induced neonatal depression is particularly important after cesarean deliveries. Maternally administered anesthetics and analgesics can suppress respiration and heart rate in the newborn. In horses, both xylazine and detomidine cause maternal and fetal bradycardia and reduced cardiac output.48,49 These effects cause a reduction in placental perfusion and fetal oxygenation. If the newborn shows depression associated with maternal administration of these drugs, yohimbine can be given as an antagonist. Weakly basic drugs, when given to the mare, tend to concentrate in the fetus. Diazepam is an example of such a drug that crosses the placenta rapidly and accumulates in the fetal circulation, resulting in lethargy, hypotonia, and hypothermia in the neonate after delivery. Flumazenil has been used to reverse the sedative effects of benzodiazepines. Maternal systemic illnesses of various types may also result in a weak newborn.
Many neonatal disorders are associated with severe electrolyte and metabolic derangements. Weakness is a common clinical manifestation of hypoglycemia, metabolic acidosis, hyponatremia, hypernatremia, and hyperkalemia. Such abnormalities may occur before or at the time of birth, and laboratory assessment of the weak newborn is essential for accurate diagnosis. Young foals with hypocalcemia can have stiff gait, muscular tremor, tachycardia, sweating, muscular tremor, and recumbency.50 Profound weakness associated with metabolic acidosis is commonly observed in foals with diarrhea. Correction of the acidosis by intravenous administration of bicarbonate usually produces rapid improvement.
A number of congenital bacterial, fungal, and viral infections that cause abortions and stillbirths may also result in the birth of a live, weak neonate. Clinical manifestations of fetal infections depend on the age of the fetus and virulence and trophism of the infecting agent (see individual diseases).
Generally, weakness secondary to uroperitoneum, renal, and liver failure, postnatally acquired infections, and neonatal isoerythrolysis (NI) is not expected to appear during the first 24 hours of age. Rather, foals with NI are usually presented between 24 and 72 hours of age, foals with uroperitoneum at 2 to 5 days of age or older, and neonates with postnatally acquired infections most commonly at 2 to 5 days of age or older.
NMD associated with selenium and/or vitamin E deficiency may produce localized (dysphagia) or generalized paresis.
Paraplegia and tetraplegia are commonly associated with spinal cord compression. Compression of the spinal cord in neonates most commonly results from vertebral body malformations, osteomyelitis, or fractures. Most malformations involve the occipital condyles of the skull and the first two cervical vertebrae (OAAM). Generally, vertebral body malformations occur sporadically; genetic, nutritional, and environmental factors have been implicated. Osteomyelitis and vertebral body abscess may be a sequela to bacteremia after neonatal sepsis or pneumonia. Rhodococcus equi vertebral osteomyelitis with or without associated pulmonary infection has been reported in foals.51 Leukocytosis and hyperfibrinogenemia are commonly observed in neonates with vertebral body abscesses. In most instances vertebral abscesses do not infiltrate the pachymeninges so the cerebrospinal fluid (CSF) either is normal or has a mild elevation of protein and/or a mild pleocytosis.
A complete neurologic examination is an important component of the workup of the weak neonate. In particular, it should be noted if the weakness is accompanied by signs of depression and diffuse cerebral disease. Limb reflexes should be tested to establish whether components of the spinal reflex pathways are involved in the disease process (sensory nerve, lower motor neuron, neuromuscular junction, muscle). For example, foals with severe spinal cord hemorrhage may have relatively normal mentation, but spinal reflexes may be greatly diminished and profound weakness may be present. Animals with other types of spinal cord disease (e.g., trauma, vertebral malformations) may also show weakness and ataxia yet appear clinically to have normal cerebral function. Virtually any severe systemic disease such as generalized infection can cause both profound depression and weakness in a neonate without the presence of actual brain pathology. Primary neurologic disease in neonates is rare; commonly, neurologic dysfunction is associated with multisystemic disease. A thorough comprehensive physical examination and workup are required to define a problem list and formulate an appropriate management plan. A CBC, blood cultures, and assessment of immunoglobulin status provide an indication of the likelihood of sepsis. Hypoxia and metabolic acidosis are ruled out by assessing ABG status, and electrolyte disturbances and hypoglycemia are evaluated by measuring serum electrolytes and blood glucose concentration. Collection of CSF to assess the central nervous system (CNS) is usually performed when disease in other organ systems that may account for the altered mental state has been ruled out and no improvement in the patient’s condition is observed after correction of electrolyte, blood gas, and metabolic derangements.
SEIZURES
IDENTIFICATION OF NEONATAL SEIZURE ACTIVITY
Seizures may be generalized or partial, depending on the part of the cerebral cortex affected by abnormal electrical activity. Involuntary muscle activity, opisthotonos, paddling, and extensor rigidity are signs associated with a generalized convulsion. In the neonate, more subtle neurologic signs may also be associated with seizure activity. In the human infant, particularly the premature infant, the neuromuscular system is not fully developed at birth and is therefore unable to fully express the abnormal electrical activity in cerebral neurons. Abnormal breathing patterns, lip smacking, chomping, rapid eye movements, small limb movements, and tremor may be the only signs indicating seizure activity in the human infant. Similar signs in the abnormal neonatal foal have also been attributed to seizure activity.52
In the large animal neonate, several conditions should be distinguished from seizure activity. Bizarre movements associated with rapid eye movement (REM) sleep, particularly prominent in the premature foal, are frequently confused with seizure activity by the inexperienced observer. Signs can be very similar and include rapid eye movements, rhythmic paddling of the limbs, and chomping. The two conditions can be distinguished by attempting to arouse the animal; if activity is associated with REM sleep, the animal should be easily aroused to full consciousness. A foal that is simply resisting restraint in lateral recumbency may also appear to be having a seizure, and violent paddling of the limbs and occasionally opisthotonos are noted. If confusion exists as to the cause of the activity, the animal is encouraged to stand, and its behavior is then evaluated. Finally, in the foal the cataplexy-narcolepsy syndrome may be confused with convulsions. This “fainting foal syndrome” was first described in 1924 in three Suffolk foals that showed signs within a few hours after birth,53 and a familial occurrence was recently reported in miniature horse foals.54 Any exciting stimulus, including petting and restraint, can trigger the attacks, in which affected foals suddenly appear to be asleep, with flaccid limbs yet open eyes.
Once seizure activity is identified, the cause of the seizures should be identified, if possible. A complete history is obtained, including a detailed description of the delivery process, and complete physical and neurologic examinations are performed. Any signs of trauma, infection, or congenital malformations should be noted. Evaluation of hematologic data and IgG status, combined with historical and physical examination parameters, results in an assessment of the likelihood of sepsis. Blood glucose and serum electrolyte concentrations should be determined promptly. A chemistry panel, blood gas analysis, bacterial cultures of blood and other body fluids, and possibly CSF analysis and skull radiographs, complete the database in most cases.
Before attempting to collect CSF (see Chapter 35), the benefit of the information likely to be obtained must be weighed against the small risk to the patient and the inconvenience of having to analyze the sample within 30 minutes of collection. In the large animal neonate, as in the adult, either the atlantooccipital or lumbosacral site may be used. Depending on the state of consciousness of the neonate, local anesthesia with manual restraint or light sedation, or general anesthesia may be required to obtain the fluid. For collection of fluid from the atlantooccipital site, a 20-gauge, 1½-inch needle with a clear hub may be used. A change in resistance is felt when the needle penetrates the dural membranes, and CSF appears in the plastic hub as soon as the subarachnoid space is entered. Approximately 5 to 10 mL of fluid may be removed safely from foals.52
Urinary reagent strips can be used to rapidly obtain general information on the fluid. If blood is detected, the sample should be spun down after the cytologic examination. RBCs contaminating the sample will settle, and the supernatant should be colorless. If hemorrhage occurred before the procedure, the sample remains xanthochromic (yellow). Glucose should be present in “trace” or “+” amounts in the normal sample. Negative values in the adult suggest severe meningitis but in the neonate may also be caused by profound hypoglycemia. The total protein level is increased in neonatal foal CSF compared with the level in the CSF of the adult horse, averaging 1.38± 0.5g/L (138±50 mg/dL) during the first 40 hours after delivery,55 and slight xanthochromia is often present. Immaturity of the blood-brain barrier is postulated as one reason for the difference in CSF protein between adult and neonatal animals.
Vascular accidents in the neonate are tentatively diagnosed on the basis of a xanthochromic sample, elevated total protein levels, increased numbers of erythrocytes, and microscopic identification of erythrophagocytosis (best). CSF analysis is most useful in determining the presence of septic meningitis. Elevation of the total protein level (>150 mg/dL) and neutrophil count in addition to a positive Gram stain and bacterial culture results in a straightforward diagnosis of bacterial meningitis, and the prognosis is considered poor for the animal.56 However, infection in the CNS can be difficult to detect until the process becomes generalized, and the lack of positive cultures and Gram stain does not rule out CNS infection. An elevated albumin quotient suggests increased blood-brain permeability and can be seen in both hypoxic-ischemic brain injury and meningitis, but an elevated IgG index indicates increased intrathecal IgG production and is more compatible with a diagnosis of meningitis.57
Ultrasonography and computed tomography (CT) are important procedures for evaluating anatomic causes of seizures (hemorrhage, infarct, malformations) in the human infant. Because the fontanelles are usually closed in the large animal neonate, ultrasound imaging is of limited or no use. Premortem diagnosis of agenesis of the corpus callosum and associated malformations was made using CT scanning in a foal that had an abnormally shaped head and seizures refractory to anticonvulsant therapy.57 Identification of the specific abnormalities early in the clinical course allowed the owners to make a more informed decision regarding the treatment of the foal, and the clinicians to acquire valuable information regarding the prognosis associated with a specific malformation in the horse.
TREATMENT OF SEIZURES
Generalized seizures should be controlled immediately. Diazepam is often the initial drug chosen for seizure control because of its rapid effect. A dose of 5 to 20 mg for a 45-kg neonate is slowly administered, and its effect monitored. In some individuals one dose controls the seizure, and repeat seizures are not observed, whereas in others, multiple doses at frequent intervals may be necessary. In these animals other longer-acting anticonvulsants are often required.
Phenobarbital acts by raising the seizure threshold, and its peak effect is seen at approximately 30 minutes. An initial dose of 10 to 20 mg/kg diluted in saline and given intravenously (IV) over 15 minutes has been used successfully to control seizures in clinical patients. This initial dose is followed by a maintenance dosage of 10 mg/kg IV every 12 hours. Oral tablets may also be used. The major side effect of phenobarbital in foals has been mild sedation and ataxia. Interactions between phenobarbital and other drugs usually involve induction of the hepatic microsomal enzyme system. Weaning from anticonvulsant therapy should be gradual to avoid recurrence of seizure activity.
Phenytoin has also been used for seizure control in the newborn foal. The initial dose is 5 to 10 mg/kg IV followed by 1 to 5 mg/kg every 2 to 4 hours. This dosage resulted in effective seizure control in several foals unresponsive to both diazepam and phenobarbital, but it also appeared to cause marked depression in some patients. Little is known about the pharmacokinetics of the drug in the large animal neonate.
Pentobarbital anesthesia has also been used to control seizures, but its use has been associated with marked respiratory depression, hypotension, hypothermia, and prolonged anesthesia. Xylazine is also a potent sedative in the foal, but its side effects have also included markedly depressed cardiovascular and respiratory function and prolonged recovery in abnormal foals. Neither pentobarbital nor xylazine is recommended for seizure control in the foal unless no other agents are available.
CONDITIONS ASSOCIATED WITH SEIZURES
CNS dysfunction in asphyxiated large animal neonates is discussed in Chapter 16. Disorders of sodium can also cause seizures in young foals; these are discussed in detail in Chapters 22 and 44.
Meningitis
Although bacterial meningitis may occur as a primary entity, it more commonly is a result of generalized sepsis in neonates with failure of passive transfer (FPT). Agents that cause meningitis are the same as those that cause septicemia, most commonly bacteria such as Escherichia coli, Enterobacter species, Salmonella species, and Streptococcus species. Because clinical signs of meningitis are easily confused with hypoxic ischemic encephalopathy (HIE) and septicemia without localization in the CNS, diagnosis depends on CSF analysis (see Chapter 35). Treatment recommendations for treating bacterial CNS infections may be found in Chapter 35. Although there is one report on the successful treatment of two neonatal foals with suspected meningitis using third-generation cephalosporins,58 in many cases, once the diagnosis is made the infectious process is often well advanced both in the brain and in other tissues, resulting in a poor outcome.
RESPIRATORY DISTRESS
The transition from the fluid-filled lung of the fetus to an organ that is responsible for efficient gas exchange is both rapid and complicated. The process can be complicated by a number of factors, including prematurity or dysmaturity, aspiration of meconium or milk, and bacterial, viral, or fungal infection. A highly compliant chest wall, an inefficient immune system, and failure to derive adequate antibody from colostrum (partial or total FPT) are additive factors that predispose the neonate to respiratory problems.
The detection of respiratory disease in the newborn foal can be difficult. Thoracic auscultation can be highly misleading. Minute ventilation (frequency × tidal volume) is increased in the healthy neonate, resulting in easily heard bronchovesicular sounds. There is no need to accentuate breath sounds with rebreathing techniques. During the first few hours after birth, fluid can normally be auscultated throughout both lung fields and within the trachea. End-inspiratory crackles are commonly heard over the dependent lung during and shortly after rising from lateral recumbency. This is presumably because of simple atelectasis. Foals with respiratory disease will frequently have abnormal lung sounds, such as crackles and wheezes, but neonates with even severe pulmonary disease will occasionally have little detectable abnormality during auscultation. Clinical signs that are often associated with pulmonary tract disease in older foals and adult horses are frequently lacking in the sick neonatal foal. Fetal foals develop and mature in a relatively hypoxic environment within the uterus and therefore are more likely to tolerate postnatal hypoxemia than older foals or adults. Cough is also uncommon, likely owing to a postnatal delay in maturation of irritant receptors within airway and delayed onset of the laryngopharyngeal cough reflex. This is clinically relevant in that aspiration of milk into the lower airway associated with force-feeding can go undetected for several days. Of additional importance is that the respiratory rate and rhythm frequently do not accurately reflect arterial concentrations of oxygen or carbon dioxide. This is particularly relevant in foals that are showing signs suggestive of asphyxial injury, where rising arterial CO2 concentrations occur in response to hypoventilation and fail to cause an increase in minute ventilation. In these foals the primary drive for ventilation is arterial O2 rather than CO2.
In the absence of ABG data or radiographic information, the clinician must rely on vague signs, such as restlessness and agitation, increased respiratory rate, or respiratory distress. Historical information may also aid in diagnosis. This should include an estimation of gestational age, recognition of any maternal problems (e.g., fever, dystocia, placentitis, or prepartum vaginal discharge), the presence or absence of meconium staining of amniotic fluid, and an assessment of colostral quality and quantity. Failure to make an early identification of pulmonary disease often results in unfavorable outcome, with chronic pneumonia resulting. Malformations, inflammation, or other abnormalities of the upper respiratory tract can cause clinical signs of respiratory distress, stridor, and dysphagia and result in lower respiratory tract problems as well. Several nonrespiratory conditions also cause clinical signs that mimic respiratory disease.
The ideal diagnostic tools for investigation of neonatal respiratory disease include ABG analysis and thoracic radiography. ABG analysis is the most sensitive clinical tool used to assess lung function. The sample is usually collected from the dorsal metatarsal artery, easily palpated in most foals on the lateral aspect of the third metatarsal bone. Alternative sites include the brachial artery, located at the level of the medial collateral ligament of the elbow joint, and the carotid artery, but hematoma formation is a common sequel to aspiration from the latter site. The sample will remain useful for up to 90 minutes in a capped plastic syringe at room temperature.
Interpretation of the ABG sample involves consideration of the amount of struggling and position of the foal during sample collection. Normal ABG values for neonates of different postnatal and gestational ages are presented in Table 19-1. Lateral recumbency can reduce the PaO2 by as much as 30 mm Hg. The sample needs to be handled appropriately, paying strict attention to avoidance of air contamination, which will artificially increase the PaO2 and decrease PaCO2. The inspired oxygen concentration must also be considered when analyzing ABG values. With supplemental oxygen, PaO2 is increased variably, depending on the inspired oxygen concentration (FiO2), the amount of pathology present (particularly the extent of right-to-left shunting), the respiratory rate and tidal volume of the foal, and whether the oxygen is delivered by nasal insufflation. A flow rate of 10 L/min, delivered by nasal insufflation, increased the PaO2 to 298 ± 69 mm Hg in the normal, term newborn foal59; this flow rate was thought to approximate an FiO2 of 1.60 In the induced premature foal the PaO2 increased only to 111 ± 35 mm Hg.59 If the respiratory rate of a foal is rapid and shallow, the supplemental oxygen will be “diluted” by room air because of the large quantity of room air entering the upper respiratory tract, and the concentration of alveolar oxygen will probably be much less than 100%. The two most common respiratory-derived ABG derangements include hypoxemia with normocapnia or hypocapnia and hypoxemia with hypercapnia. It is important to distinguish acute from chronic hypercapnia. Acute hypercapnia is associated with a more substantial drop in blood pH and may lead to circulatory collapse and coma, particularly if accompanied by acute hypoxemia. Chronic exposure to elevated CO2 permits adaptation and more subtle clinical effects. The change in pH is less dramatic, primarily because of enhanced bicarbonate reabsorption in the proximal tubules of the kidney. This effect begins within 6 to 12 hours of exposure to increased concentrations of CO2 and is maximal by 3 to 4 days. Hypercapnia can be exacerbated by fever or the administration of carbohydrates or bicarbonate. The latter is often clinically relevant and highlights the danger of giving large amounts of sodium bicarbonate to foals with pulmonary disease.
Interpretation of blood gas values of venous blood (see Table 19-1) can be very deceptive and should be restricted to evaluation of metabolic conditions (e.g., metabolic acidosis) and not pulmonary gas exchange. To avoid problems associated with regional blood sampling, peripheral venous blood -should be taken from a free-flowing jugular vein, because the metabolic status of the head is usually stable. To obtain a sample representative of the whole body, mixed venous blood is drawn from the right atrium. Determination of mixed venous blood oxygen saturation is a good test for assessing the overall adequacy of oxygen delivery to tissues because it reflects the balance between oxygen delivery and oxygen use.
Several factors need to be considered when evaluating foal thoracic films. Thoracic radiographs are routinely taken only in the standing or recumbent lateral position in foals, with dorsoventral positioning reserved for the anesthetized or very depressed foal. Thus interpretation can be limited because of positioning limitations. If the neonate has been in lateral recumbency for extended periods of time, atelectasis may result in diffuse or localized interstitial infiltrates that usually resolve once lung reexpansion occurs. It can be very difficult to accurately distinguish bacterial pneumonia from atelectasis and pulmonary edema on the basis of radiographic appearance alone. In these cases additional diagnostic aids (cultures, hematology, necropsy) should be used in conjunction with radiology to reach an accurate diagnosis. A false overinterpretation of disease is common because of motion artifact, caused by a combination of long exposure times, poor patient compliance, and high spontaneous ventilation rates. When the radiographic appearance of the lung fields is evaluated, the type of infiltrate (interstitial, nodular, alveolar, mixed), severity, and location (diffuse, perihilar, cranioventral, craniodorsal, caudodorsal, caudoventral) should be noted. Other soft-tissue structures (including the heart, vessels, and diaphragm) and bones (ribs, vertebrae, long bones) should also be evaluated.
Serial thoracic radiographs are useful in monitoring the progress of a respiratory condition. Radiographic changes may either follow or precede changes in clinical condition, and major changes can occur surprisingly rapidly (Fig. 19-2). Clinical signs of pneumonia frequently resolve much earlier than chest radiographs and hemograms return to normal. Unfortunately, both ABG analysis and radiography are difficult to perform in field situations.
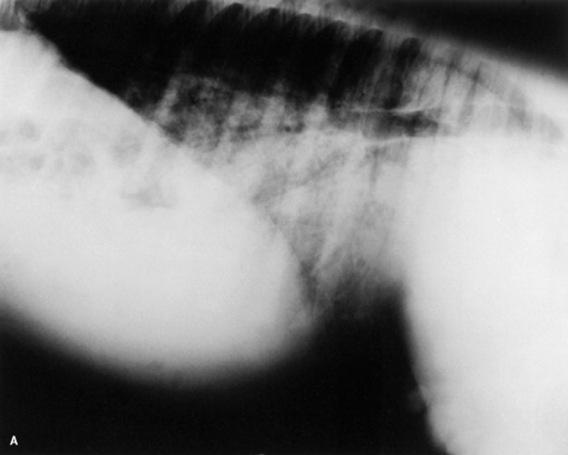
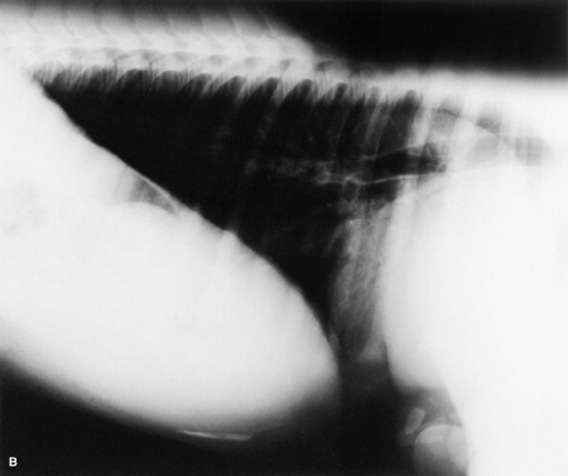
Fig. 19-2 A, Standing lateral chest radiograph of a 7-day-old thoroughbred filly with severe angular limb deformities that experienced an acute onset of severe respiratory distress and cyanosis after a walk outside the stall. Intubation and 100% oxygen administration raised the PaO2 to only 48 mm Hg. Severe pulmonary interstitial disease is present in the caudoventral lung fields, and the tentative diagnosis was bacterial pneumonia. No modifications were made in the treatment regimen (the same antibiotics being given for a wound were continued), and over the following 24 hours the filly clinically improved. B, Repeat radiographs taken 3 days after the first ones revealed marked resolution of the infiltrates. The diagnosis remains open, but pulmonary edema was suspected.
Ultrasonographic evaluation of the foal’s thorax can yield useful information in a variety of disease processes, including pleural effusion, such as hemothorax or pleuritis, bronchopneumonia, or abscessation. It is also the preferred method for diagnosing rib fracture or dislocation and congenital heart disease and thus is often a useful technique to differentiate cardiac and pulmonary causes of hypoxemia.61
SPECIFIC RESPIRATORY CONDITIONS
Upper Respiratory Tract Disorders
Upper respiratory tract disorders are relatively uncommon in neonates. Conditions affecting pharyngeal and laryngeal function are important, as they predispose to aspiration pneumonia. Dyspneic neonates also have difficulty nursing and are subsequently likely to become malnourished. Congenital defects of the upper respiratory tract include collapsed trachea, stenotic nares, choanal atresia, epiglottal cyst, and guttural pouch tympany (foal). There have also been recent reports of dorsal displacement of the soft palate (DDSP) as a cause of acute dyspnea, stridor, and dysphagia in neonatal foals.62,63 Endoscopic examination of the upper airways of these foals revealed that the dorsally displaced soft palate was edematous, flaccid, and redundant. To varying degrees, flaccidity and swelling of other pharyngeal and laryngeal structures (e.g., arytenoid cartilages, epiglottis, or palatopharyngeal arch) were also noted.63 Both medical63 and surgical62 treatment of the condition have been suggested. In one study, medical management with antiinflammatory drugs, enteral feeding via nasogastric tube, and broad-spectrum antibiotics (for the coexisting aspiration pneumonia) resulted in dramatic and permanent resolution of the problems within 2 to 4 days. The cause of these abnormalities remains unknown at this time but may involve primary pharyngeal and palatal muscular laxity.63
Impaired pharyngeal and laryngeal function may result from physical deformation or neuromuscular disorders. Pharyngeal and laryngeal injuries are often associated with improper application or use of damaged feeding tubes and oral medication equipment. Compression of the larynx by a retropharyngeal abscess or mass tends to cause inspiratory dyspnea; aspiration pneumonia is a common sequela. Partial occlusion of the upper airway induces turbulent airflow and subsequently mucosal edema. Placement of a tracheostomy tube provides an alternate, sometimes lifesaving, airway and rests the inflamed mucosa.
NMD, hyperkalemic periodic paralysis, and botulism may induce laryngeal paresis. Dysphagia and subsequent aspiration pneumonia are common sequelae of pharyngeal and laryngeal dysfunction associated with NMD and botulism. Exercise- and excitement-induced respiratory stridor has been described in foals with hyperkalemic periodic paralysis.64
Collapsed trachea is a rare congenital or acquired condition. Clinical signs include an intermittent honking cough, stridor, and dyspnea with mild exercise. There is no stenosis of the trachea; rather, a dynamic dorsoventral collapse during inspiration. The caudal cervical and cranial thoracic sections of the trachea in the area of the thoracic inlet are most frequently affected. Acquired tracheal collapse is commonly associated with fractured ribs and compression of the trachea at the thoracic inlet by the subsequent bony callus.
Diagnosis of most upper airway disorders can usually be made with a combination of radiography and endoscopy. A 7-mm outside diameter (OD) endoscope is usually small enough to pass through the ventral meatus of horse and pony foals that weight over 30 lb. An integral part of the diagnostic approach to the neonate with suspected upper airway obstruction is assessment of the lungs for aspiration pneumonia. If the primary upper respiratory problem is not corrected and normal nursing is allowed, the pneumonic process will likely persist and become chronic.
Respiratory Infection
Bacterial infection of the lower respiratory tract most commonly occurs during or shortly after birth but can also take place before parturition through aspiration of contaminated amniotic fluid. This may take place in mares with bacterial placentitis. In the newborn foal, pneumonia can result from direct aspiration or inhalation of bacteria or from the hematogenous spread of organisms in foals that are bacteremic. The most common bacterial organisms that have been associated with pulmonary disease in foals are identical to those that cause systemic sepsis. The most common isolates include E. coli, Klebsiella pneumoniae, Pasturella species, Actinobacillus species, and Streptococci species. Less common isolates include, but are not limited to, Salmonella species, Enterobacter species, Pseudomonas species, Serratia marcescens, Staphylococcus species, and Yersinia pseudotuberculosis.
The diagnosis of pneumonia involves identification of the causative organism. Isolation of bacteria can be attempted from blood culture or from culture of amniotic fluid or placental tissue if in utero infection is suspected. Lower airway culture can be difficult, as a tracheal aspiration can be dangerous in a compromised neonate. An alternative method involves passage of a guarded swab through a nasotracheal tube into the lower airway. The tip of the nasotracheal tube can also be cultured if it has been present in the airway for a prolonged period. A CBC and measurement of an acute phase protein, such as fibrinogen, may support a diagnosis of infection but will not be helpful in localization of infection to the respiratory tract. The treatment of bacterial lung disease involves a combination of respiratory support techniques and antibiotic therapy. The neonatal foal readily develops dependent atelectasis in lateral recumbency. Consequently, positioning in sternal rather than lateral recumbency results in improved ventilatory capacity and higher arterial oxygen tension. Broad-spectrum antibiotic therapy should be commenced as soon as lung disease is suspected. A good choice is a β-lactam antibiotic, such as penicillin or ampicillin, combined with an aminoglycoside. The emergence of E. coli resistance to gentamicin in certain regions may limit its future use. The third-generation cephalosporins, such as ceftiofur, ceftazidime, ceftriaxone, and cefotaxime, have distinct advantages over aminoglycosides in the treatment of bacterial pneumonia. They have superior penetration into the lung, and effective tissue concentrations are easily achieved by intravenous or intramuscular routes. Because premature discontinuation of antibiotic therapy has resulted in relapse in a number of cases, repeat radiographs and hematology (complete blood cell count and plasma fibrinogen) are highly recommended before discontinuation of antibiotic therapy. A minimum course of therapy of 3 to 4 weeks’ duration is not unusual in cases of severe pneumonia. Premature foals with pneumonia should be monitored particularly closely for the development of bacterial pneumonia resistant to the antibiotics being used.
Several viruses have been documented as causes of pneumonia in the neonatal foal. These include equine herpesvirus type 1 (EHV-1) and type 4 (EHV-4), equine influenza, equine viral arteritis virus, and adenovirus. Of these, EHV-1 is the most common. Herpesviral pneumonia is frequently fatal, even in the face of aggressive supportive therapies such as mechanical ventilation. The antiviral drug acyclovir has been used. The difficulty is establishing a diagnosis early in the course of treatment. Several factors appear common to EHV-1–infected foals, but none should be considered pathognomonic. These include leukopenia with neutropenia and lymphopenia, and depletion of the myeloid cell lines on cytologic examination of bone marrow aspirates. The presence of dilated retinal vessels and a red discoloration to the optic disc on fundic examination has also been suggested as a common antemortem finding. Infection with adenovirus can be a problem in any immunocompromised foal, especially Arabian foals with severe combined immunodeficiency (SCID) syndrome.
In utero infection with Histoplasma capsulatum can result in placentitis, abortion, or birth of an infected foal with multiple organ disease, including granulomatous pneumonia. An antemortem diagnosis can be difficult to establish but is aided by tracheal aspirate and bronchoalveolar lavage when characteristic yeastlike organisms (3 to 5 μm in diameter) are seen within macrophages. Neonatal and maternal serum should be positive for anti-Histoplasma antibodies using an agar gel immunodiffusion test. The disease has been successfully treated in adults using amphotericin B, but reports of neonatal survival are lacking. Infection with Candida species (especially Candida albicans) is an infrequent complication in foals with chronic bacterial infection. Lengthy antimicrobial use is an apparent risk factor for infection, and many cases begin with oral candidiasis. The diagnosis is based on a history that often includes persistent low-grade fever, worsening respiratory disease or the development of synovitis, and isolation of the organism through blood culture. Successful treatment of neonatal candidiasis has been achieved with ketoconazole, amphotericin B, or fluconazole.
Meconium Aspiration Syndrome
In utero asphyxia or umbilical cord occlusion can result in fetal passage of meconium into amniotic fluid. Hypoxia induces a redistribution of blood flow away from less vital organs, including the gastrointestinal tract, resulting in mesenteric vasoconstriction and secondary intestinal ischemia. Transient hyperperistalsis and anal sphincter relaxation occur, thereby allowing passage of meconium. Meconium aspiration may occur before, during, or immediately after delivery as a result of fetal gasping. Meconium can produce a variety of clinical signs including mechanical airway obstruction (ball-valve effect) and regional air trapping, chemical pneumonitis and alveolitis, alveolar edema, and displacement of surfactant by free fatty acids in meconium, leading to decreased lung compliance, small airway obstruction, and focal atelectasis.65-67 These events lead to increased pulmonary vascular and airway resistance and ventilation-perfusion mismatching. Meconium may also enhance the growth of bacterial species within the respiratory tract, resulting in secondary bacterial pneumonia. It may be difficult to differentiate meconium aspiration from bacterial pneumonia, especially if the birth was unattended. Occasionally, chronic placentitis is associated with both bacterial pneumonia and meconium aspiration.
If meconium has been aspirated into the pharynx, then gentle suctioning of the nasal and oral cavities is recommended. The ideal time to suction the airways is while the animal is still in the birth canal, before it has taken its first breath. If the foal shows signs of meconium aspiration below the vocal cords, nasotracheal intubation and careful, aseptic suctioning are recommended. Intranasal oxygen should be administered during suctioning. ABG analysis dictates what long-term respiratory and metabolic support is necessary. Mild to moderate hypoxemia can be treated with humidified intranasal oxygen (2 to 10 L/min). Severe hypoxemia with accompanying hypercapnia requires positive pressure ventilation (PPV) and is associated within increased mortality. If surfactant displacement and secondary atelectasis is contributing to hypoxemia, continuous positive airway pressure (CPAP) alone may improve oxygenation while avoiding any unnecessary increase in peak airway pressure. Exogenous surfactant administration has been advocated to treat the surfactant dysfunction, although efficacy data are lacking. Intravenous dimethyl sulfoxide (DMSO) (0.5 to 1 gm/kg) administered as a 10% solution may help reduce alveolar and interstitial edema. Systemic antibiotic therapy is recommended to prevent secondary bacterial pneumonia. Good airway hygiene and coupage are crucial.
A diagnosis of meconium aspiration is based on a history of meconium-contaminated amniotic fluid and a meconium-stained newborn. Radiographs typically show a ventrocranial distribution of pulmonary infiltrate characteristic of aspiration. Clear, brownish fluid may drip from the nose.
Milk Aspiration
Aspiration of milk into the lower airway may occur as a complication of a wide range of conditions. Most foals that aspirate milk also demonstrate nasal regurgitation of milk. Unfortunately the decreased sensitivity of the upper and lower airway to foreign material may make diagnosis of milk aspiration difficult. Aspiration can occur in foals with cleft palate, persistent DDSP, botulism, HIE, or generalized weakness resulting from sepsis or prematurity. Iatrogenic contamination of the airway can occur when bottle-feeding is forced or if the foal is too weak or sleepy to receive feeding. Substantial and sometimes fatal pneumonia can result from inappropriate placement of a nasogastric tube.
The diagnosis of milk aspiration is supported by historical data (nasal regurgitation of milk), physical examination findings (abnormal lung and tracheal sounds), and laboratory data (inflammatory leukogram, elevated fibrinogen, hypoxemia). Radiographic examination commonly reveals a heavy, perihilar, and/or ventrally located interstitial density with or without air bronchograms.
The treatment of milk aspiration involves long-term, broad-spectrum antimicrobial therapy and prevention of further contamination of the airway. The underlying cause should be pursued diagnostically and treated. This may necessitate the use of further diagnostic tests, including endoscopy and plain and contrast radiography. Enteral feeding through a nasogastric or esophagostomy tube is indicated until the underlying problem has been resolved. Persistent or intermittent DDSP in the neonate frequently resolves over time, but this may take weeks to months.
Pneumothorax68 and Hemothorax
Pneumothorax is usually an iatrogenic sequela of PPV of diseased lungs, but it may occur spontaneously or as a result of birth trauma or from ruptured bullae within the lung parenchyma. During mechanical ventilation uneven alveolar ventilation leads to alveolar rupture and dissection of air into the interstitium. The air moves along bronchioles and other lung structures to pleural surfaces, forming blebs. This air may rupture into the pleural space. The condition should be strongly suspected if the respiratory condition suddenly worsens while an animal is being ventilated. Clinical signs may include respiratory distress, shift of cardiac point of maximum impulse, cyanosis, and hypotension. Although auscultation may reveal decreased breath sounds, it may be misleading because of the wide referral of breath sounds. Percussion is usually fairly unremarkable, unless the condition is very severe. Radiographs are indicated to confirm the diagnosis, but, if radiology is unavailable or the animal is very distressed, a direct needle aspiration is diagnostic and therapeutic.
Pneumothorax may be treated conservatively if no distress is associated with the air leak and the condition appears stable. Stress should be minimized. Chest tube insertion is indicated in human infants with continuing air leak, if underlying pulmonary disease is causing respiratory distress, and in those patients receiving mechanical ventilation. A trocar catheter is sterilely introduced into the chest cavity, and the catheter is secured, with the suture material crisscrossed tightly around the catheter. Suction is applied at −15 cm H2O after confirmation of chest tube position by chest radiograph. Suction is discontinued when the tube has drained no air for 24 to 48 hours and when extrapulmonary air has been resolved radiographically for 24 to 48 hours. The tube may then be placed under a water seal for an additional 24 hours, and if no air accumulates the tube may be removed.
Hemothorax is occasionally noted in the large animal neonate. It has occurred secondary to unstable fractured ribs, with puncture of the lung parenchyma resulting in hemorrhage into the pleural space. Occasionally hemothorax may remain undiagnosed until clinical signs of anemia, hypovolemia, or shock appear in the young animal.
Idiopathic or Transient Tachypnea in the Neonate
A syndrome observed in Clydesdale, thoroughbred, and Arabian neonatal foals has been the combination of fever and tachypnea. The condition appears to be more frequent during hot, humid weather conditions. The pathogenesis of the condition is unknown, but it is speculated that it results from a transient problem in central or peripheral control of thermoregulation and/or respiratory rate and pattern.
Affected foals are usually of normal gestation and experience a normal birth. Most display normal activity for a variable period after birth, with a sudden onset of clinical signs. Occasionally a foal may show mild signs of CNS derangement (e.g., lack of affinity for the mare, wandering). There are usually no signs of pulmonary abnormalities as assessed by thoracic radiographs or ABG analysis. Body temperature is variable among foals, ranging from 102° F to 108° F (39° C to 42.2° C). A generally poor response to antipyretics has been noted. The respiratory rate and breathing pattern often resemble panting (respiratory rate >80 breaths/min). The condition usually resolves spontaneously within a few days to weeks.
Before idiopathic tachypnea is diagnosed, it is extremely important to rule out a pneumonic process or other pulmonary abnormality, other forms of infection, metabolic acidosis, and other causes of an increased respiratory rate. Hematology, chest radiographs, and arterial blood gases should be within normal limits, and bacterial cultures should be negative.
Treatment is directed at controlling the body temperature; body clipping, alcohol baths, and maintenance of a cool environment are the most effective methods. If infection cannot be entirely ruled out, antibiotic therapy should be used.
Treatment of Respiratory Distress
It is beyond the scope of this book to provide detailed information on the respiratory support of the large animal neonate, and the reader is referred to other articles and texts for additional information on mechanical ventilation and other topics.69-71 Oxygen therapy is extremely useful in the treatment of the large animal neonate with respiratory disease. The decision as to when to institute oxygen therapy is somewhat subjective and is based both on clinical signs and on blood gas analysis. Increased respiratory rate, labored respiration, increased intercostal and abdominal muscle activity, and restlessness are considered indications for a trial of oxygen therapy. A PaO2 <55 to 60 mm Hg in lateral recumbency is considered an objective indication for oxygen therapy, although many foals with a PaO2 of 50 to 55 mm Hg on room air that were recovering from pneumonia apparently did well and displayed no signs of hypoxia. If blood gas analysis is not available, clinical signs indicating a favorable response to oxygen therapy include a decrease in effort of breathing, decrease in respiratory rate, and a more comfortable-appearing animal. An absence of response may indicate a nonrespiratory origin of the clinical signs, severe lung pathology, a cardiac malformation resulting in right-to-left shunting of blood, or inadequate inspired oxygen concentration.
The inspired oxygen concentration is most easily increased by nasal insufflation using a bias flow of humidified oxygen. Although an oxygen-delivery mask can be used, its presence interferes with nursing or feeding, and it may be poorly tolerated by the alert foal. Depending on the severity of disease and size of the individual, oxygen is initially delivered at a flow rate of about 5 L/min, and the response is noted. The catheter tip should be advanced into the nasopharynx, and the opposite end should be secured to the nostril using tape or sutures in active foals. The actual oxygen concentration delivered to the alveoli depends on several factors, including the position of the tube and the depth and rate of breathing. Oxygen therapy should be directed at maintaining a PaO2 of 80 to 100 mm Hg, and the flow rate should be adjusted according to blood gas results. Oxygen therapy should be on a continuous basis, and weaning from support should be done gradually. Transtracheal oxygen delivery may beneficial in larger foals, hypoxemic neonatal foals that have a very rapid, shallow breathing pattern, and foals with severe pulmonary disease that are unresponsive to nasal insufflation.72 A percutaneous catheter system is placed using local anesthetic and is secured to the skin. The distal location of the catheter bypasses a substantial volume of dead space, and probably results in a higher alveolar oxygen concentration. One advantage of this method of oxygen delivery has been the ability to provide long-term oxygen therapy to unrestrained foals.72
Unfortunately, oxygen therapy is not effective in correcting hypoventilation, and if hypercapnia is progressive and accompanied by signs of increasing respiratory distress, some type of mechanical ventilatory support is usually indicated. This decision to provide mechanical ventilation must take into account several considerations, including the worth of the individual, the commitment of the owners, the facility and manpower availability, and the type of disease process present.
Regardless of the level of respiratory support provided, the importance of meticulous respiratory supportive technique cannot be overemphasized. Maintenance in sternal position, frequent turning from side to side, regular coupage, and use of proper suction technique are all very important components of respiratory support.
DISTENDED AND/OR PAINFUL ABDOMEN
APPROACH TO DIAGNOSIS
The large animal neonate with a painful or distended abdomen can present a diagnostic challenge to the clinician. Medical and surgical causes of colic and GI disease in the foal include ileus and bowel distention associated with peritonitis, hypoxic gut damage and metabolic disturbances, enteritis caused by dietary changes, viral infections and bacterial pathogens, gastroduodenal ulcer disease (GDUD), impaction associated with ascarid infections, intussusception, thromboembolic disease, small intestinal volvulus, colon torsion, uroperitoneum, strangulating abdominal hernias, and congenital GI lesions. The clinical challenge is to distinguish medical from surgical lesions to permit rapid and appropriate therapy. Abdominal surgery in young foals, particularly neonates, is associated with increased morbidity and mortality and a higher incidence of intraabdominal adhesion formation when compared with surgery in mature horses.73 Medical causes of GI disease such as enteritis and peritonitis carry an increased risk of generalized sepsis and death if the patient’s cardiovascular status and metabolic parameters are not monitored and stabilized in a timely manner.
Box 19-1 lists some of the more common conditions associated with the acute abdomen in large animal neonates. Physical examination findings can be very similar between neonates requiring surgical intervention and those with only an infectious problem, such as enteritis. If abdominal distention is present, every effort should be made to identify its cause. Because the neonatal foal is considerably smaller than the adult, some of the diagnostic techniques routinely used in the adult (rectal palpation, assessment of shape of abdomen) are of limited value in assessing the acute abdomen in the neonate. Bilateral, tympanitic distention of the paralumbar fossae is suggestive of generalized ileus or large bowel obstruction (e.g., meconium impaction). Other diagnostic aids including abdominal radiographs, transabdominal ultrasonography, abdominal ballottement, and transcutaneous abdominal palpation are not practical in the adult but are useful diagnostic tools in the newborn foal. Abdominal ultrasound is a critical diagnostic procedure in horses of all ages.
The approach to the neonate with a painful or distended abdomen should include a complete history, including any abnormalities noted during the perinatal period, the type and dose of any analgesics previously administered, and -whether there is history of diarrhea in other foals or horses on the farm. The age of the foal helps determine the risk of certain conditions. Young foals less than 2 weeks of age are more likely to experience colic caused by meconium retention, peritonitis associated with generalized sepsis, hypoxic gut damage, uroperitoneum, and congenital deformities including lethal white syndrome (e.g., mesenteric aganglionosis), inguinal and scrotal hernias, and atresia of the anus or colon.74-76 We have also seen increasing numbers of young foals with clostridial enteritis that are presented for treatment for colic. Older foals are more likely to suffer from intussusceptions, enteritis, gastroduodenal ulceration, and thromboembolic disease.74,75
The neonate’s age at the onset of abdominal distress also may provide diagnostic clues. For example, foals with meconium impaction or congenital GI malformations such as atresia coli tend to be presented for treatment during the first 12 to 36 hours of age, whereas foals with uncomplicated ruptured urinary tracts are usually presented at about 3 days, when the abdomen is visibly distended. The character, quantity, and frequency of defecation and urination should be determined. Surgical GI lesions such as intussusception and large colon displacement have occurred secondary to enteritis. On the other hand, in the early stages, enteritis alone can cause severe abdominal distention or severe pain, in the absence of diarrhea. NEC and clostridial enteritis can be particularly painful conditions. Most foals with ruptured urinary bladders display abnormalities in urination, but in some cases normal micturition has been noted. Reduction in urine output from a neonate with a distended abdomen is not pathognomonic for uroperitoneum. Urine volume is typically reduced as a result of dehydration secondary to a variety of abnormalities, including GI disease.77 The colic associated with uroperitoneum is not usually severe.
Assessment of the degree of pain being exhibited is an important part of the examination of the neonate with a distended abdomen. Foals more commonly show signs of abdominal discomfort than do calves. In a retrospective study of foals undergoing exploratory celiotomy, uncontrollable pain and severe abdominal distention were the primary reasons the animals were taken to surgery. Sever abdominal pain can also occur in foals with non-surgical lesions, such as sever enteritis, making the decision for surgical exploration difficult in some cases.78 However, persistent tachycardia in a neonate with a heart rate in excess of 150 beats per minute (bpm) despite administration of analgesics and in the absence of fever is suggestive of a surgical GI lesion.
The degree of compromise to the cardiovascular and pulmonary systems should be assessed. A neonate with an abdominal crisis is often in need of immediate stabilization because of shock secondary to endotoxemia or hypovolemia. Exploratory celiotomy in neonates that receive inadequate presurgical supportive therapy is associated with a number of complications, including poor tolerance to anesthesia. The degree of respiratory compromise secondary to the abdominal problem should also be considered, particularly if the animal is a surgical candidate. For example, foals with long-standing uroperitoneum may have pleural effusion and pulmonary abnormalities as well as serum electrolyte abnormalities, all of which may predispose to anesthetic problems (hypoxemia, hypercapnia, cardiac arrhythmias).
It is very important to establish the likelihood of generalized or localized infection such as enteritis. Generalized sepsis can interfere with the function of many organ systems, including the GI tract. The first signs of enteritis are often severe abdominal distention and colic, with diarrhea becoming apparent a few hours to days later (Fig. 19-3); the severity of these signs may warrant surgical exploration of the abdomen. Leukopenia may be observed in foals with septicemia, enteritis, peritonitis, and surgical GI lesions. An unexplained metabolic acidosis may also indicate impending enteritis.
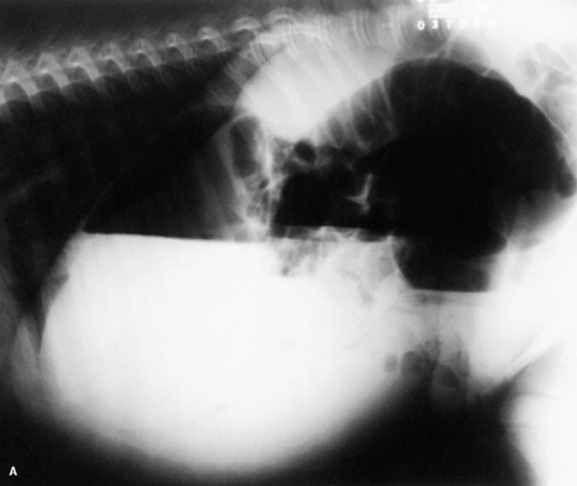
Fig. 19-3 A, Abdominal radiograph (standing) of a 48-hour-old foal that was presented with meconium impaction and a distended abdomen. The foal also had a metabolic acidosis, hypoglycemia, and leukopenia. Note the gaseous distention of the large intestines, which was suggestive of ileus and possibly obstruction. B, Standing abdominal radiograph of the same foal 24 hours later after passage of diarrhea. Following removal of the meconium, profuse bloody diarrhea was observed, suggesting enteritis. With passage of the diarrhea, gas distention resolved.
Additional information on the physical examination of the abdomen and GI tract can be found in Chapter 17. It is difficult to distinguish fluid accumulation in the large colon from accumulation in the peritoneal cavity using physical examination alone, and additional diagnostic procedures are usually required to distinguish the two (see later). In general, nasogastric intubation in the neonatal foal does not seem to be as useful a diagnostic technique as it is in the adult horse. Gastric reflux can be difficult to obtain, even if the stomach appears markedly distended on radiographs, and a moderate amount may be obtained in cases of ileus. If large volumes of reflux are obtained, however, obstructive disease (e.g., of the pylorus and small intestine) is considered more likely.78
Neonates with enteritis, uroperitoneum, and other abdominal problems can have markedly deranged serum electrolyte concentrations (hyperkalemia, hyponatremia, and metabolic acidosis are typical). Failure to recognize the severity of these abnormalities or adequately treat them can result in the death of the patient.
Abdominal radiographs can be very helpful in identifying segments of the intestinal tract that are distended, fluid in the peritoneal cavity, and the composition of ingesta in the GI tract (e.g., sand, meconium) (Figs. 19-4 and 19-5). A good knowledge of normal radiographic anatomy of the intestinal tract is important for accurate interpretation (Fig. 19-6). Adequate radiographs are obtained in foals up to 250 kg if available radiograph equipment includes a grid, rare earth screens, and sufficient milliampere-second (mAs) (5 to 28) and kilovolt peak (kVp) (75 to 95) levels. With experience in viewing normal and abnormal abdominal radiographs, the likelihood of an obstructive lesion versus simple ileus can be established in some but not all cases. The presence of erectile, distended loops of small intestine is most consistent with a diagnosis of obstructive disease. Intramural gas is suggestive of NEC. It can be very difficult to differentiate large colon torsion or displacement from simple gas and fluid distention secondary to ileus. Contrast radiography can help to define the location and nature of GI problems such as duodenal stricture and abnormalities of the small colon or rectum. Further details on the radiographic diagnosis of abdominal disorders are contained in other articles. 78a,78b,78c,78d
Fig. 19-4 Sand accumulation in the ventral colon of a 1-month-old foal with chronic diarrhea and intermittent colic.
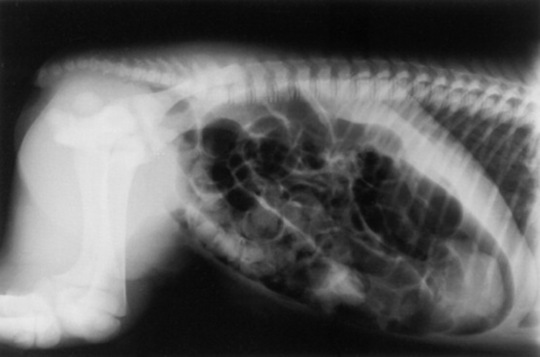
Fig. 19-5 Abdominal radiograph (lateral recumbency) of a 7-hour-old miniature horse foal with atresia ani, showing meconium packed into a gas-distended large colon. The extent of the atresia proximally from the anus is not visible.
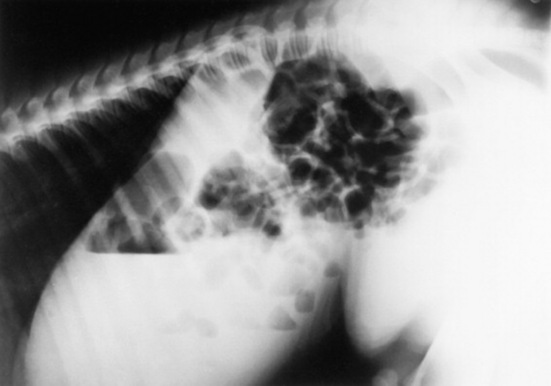
Fig. 19-6 Normal standing abdominal radiograph in a neonatal foal. Note the prominent fluid line in the stomach and the presence of gas in various portions of the tract.
Abdominal ultrasonography can be of value in diagnosing certain conditions that may be contributing to a distended or painful abdomen, including fluid-distended small and large intestine, ascarid impaction, intussusception, colonic impaction, uroperitoneum, and abnormalities of the umbilical vessels and urachus. Ultrasonography also permits characterization of small intestinal motility, distention, and bowel wall thickness. Healthy foals have flaccid, fluid-filled loops of small intestine. The presence of rounded, distended loops of small intestine is suggestive of an ileus, enteritis, or possible small bowel obstructive disease. The location, amount, character, and echogenicity of free peritoneal fluid can also be determined (Fig 19-7).61,79,80 A large accumulation of peritoneal fluid with increased echogenicity (or fibrin) is suggestive of peritonitis, whereas an excessive volume of hypoechogenic peritoneal fluid is suggestive of uroperitoneum.
Fig. 19-7 Ultrasound view of the caudal abdomen of a 24-hour-old premature foal with a torn urachus, showing (from top to bottom) the ventral abdominal wall, free abdominal fluid (black), the urinary bladder (oval structure to the right), and small intestinal loops (cross-sectional view).
In our opinion, except in a couple of specific conditions, peritoneal taps are of limited value in diagnosis of the acute abdomen in the neonate. Extreme caution should be exercised to avoid perforating the bowel while attempting to acquire a sample of peritoneal fluid, particularly if intestinal distention is present. The intestine of the neonate is easily ripped by inadvertent perforation with a needle or teat cannula, even if the neonate is well restrained. It is probably safest to perform the procedure using ultrasonography to image the fluid pocket and the needle position. Foals with uroperitoneum are the ideal candidates for abdominocentesis. Clear yellow, urine-like peritoneal fluid is easily and safely obtained in these patients because the excessive volume of peritoneal fluid allows the abdominal viscera to float well above the ventral floor of the abdomen.
Normal peritoneal fluid is similar to that in the adult, except that the normal WBC count is lower (1500 cells/μl or less).81 Cytologic examination should also be performed to determine the cell types present and to detect the presence of bacteria and toxic neutrophils (suggesting peritonitis) and fecal material (suggesting either inadvertent gut tap or bowel rupture). The total protein and WBC count may be elevated in a variety of conditions. These include severe enteritis; urachal, umbilical, or severe bladder infection; and primary peritonitis, in addition to conditions in which there is ischemic bowel requiring surgical resection. On the other hand, as in the adult, normal peritoneal fluid has accompanied a number of surgical intestinal lesions, such as large colon displacement. Therefore peritoneal fluid analysis is of limited value in distinguishing the surgical patient but can be useful in the diagnosis of peritonitis. In addition, peritoneal fluid analysis usually results in a straightforward diagnosis of uroperitoneum. In virtually all cases of uroperitoneum observed to date, peritoneal creatinine level was greater than the serum level (usually >2:1). Also, the acquisition of free-flowing blood from the abdominal tap usually allows a diagnosis of hemoperitoneum.
Endoscopy of the upper and lower GI tract of the foal can be performed if appropriately sized equipment is available (8 to 10 mm OD, 180 to 250 cm long).77 The esophagus and stomach can be examined for erosion, ulceration, perforation, and other abnormalities. Suspected impactions or malformations of the rectum and small colon can also be examined.74
In summary, accurate identification of foals with acute abdominal problems requiring surgery can be very difficult even with the use of ancillary diagnostic procedures, and mistakes are commonly made because there are no clear-cut and consistent differences between medical and surgical cases. Findings suggestive of the need for surgical exploration include severe and unrelenting pain and persistent tachycardia.
SPECIFIC CONDITIONS
Meconium Impaction
Meconium impaction is the most common cause of colic in the newborn foal. This condition is more common in colts because of the narrow pelvic canal. Many foals show some degree of straining and discomfort while passing meconium, but in most instances it is passed uneventfully by 24 to 48 hours of age. The meconium most commonly becomes impacted in the rectum or small colon. The clinical signs associated with meconium impaction in the otherwise normal foal include repeated attempts to defecate, straining with the back arched, swishing of the tail, and restlessness. Nursing stimulates defecation through an oral-anal reflex, so signs of discomfort may appear shortly after each milk meal. If left untreated, meconium impactions lead to varying degrees of abdominal distention. The foal’s abdomen becomes gas distended, with tympany detected over the paralumbar fossa. Digital examination often reveals a rectum packed with hard fecal balls. Occasionally, the impaction is located more proximally (large or small colon) and radiography or ultrasonography is required for diagnosis.
Low doses of analgesics such as dipyrone (10 to 22 mg/kg IV), flunixin meglumine (0.25 to 1 mg/kg IV), and butorphanol (0.01 to 0.1 mg/kg IV) may be required to prevent self-trauma during colicky episodes. Xylazine may exacerbate gut stasis and can cause respiratory depression, and it should be used with caution in newborn foals. A gravity enema with mild soap and warm water or a commercial Fleet enema usually results in prompt evacuation of the meconium. Refractory meconium impactions may respond to acetylcysteine retention enemas.82 The supplies and procedure for a retention enema are as follows: Mix together 150 mL water, 6 g of acetylcysteine powder, and 20 g of sodium bicarbonate (baking soda). Insert a well-lubricated 12 or 14 French, cuffed Foley urinary catheter into the rectum and inflate the cuff. Slowly infuse 120 to 180 mL of the retention enema solution. Plug the end of the catheter. Tape the catheter loosely to the foal’s tail. Leave in place a minimum of 15 minutes, then deflate the cuff and remove the catheter. This procedure can be repeated several times. Care must be taken to avoid traumatizing the rectal mucosa by stiff tubing or multiple enemas with harsh detergents. Clinical signs associated with meconium impaction in the compromised foal may be absent. In asphyxiated or premature individuals that are receiving little or no enteral feeding, meconium may remain in the large colon for days, gradually forming into hard concretions that are diagnosed by palpation or radiographs or at postmortem examination. In these cases the routine administration of an enema is often ineffective in mobilizing the impaction because it is high in the large colon. Additional therapy includes intravenous fluids, oral fluids, and laxatives (60 to 120 mL of mineral oil with ½ to 1 oz of psyllium, 60 to 120 mL of milk of magnesia). If the gas distention becomes severe, transcutaneous bowel trocarization can be pursued. We avoid the use of Dioctyl sodium sulfosuccinate (DSS) as an oral cathartic because it can cause excessive irritation, resulting in diarrhea and colic. Analgesics may also be helpful in controlling the neonate’s discomfort and in reducing the risk of self-trauma. Although most meconium impactions can be successfully treated with aggressive medical therapy, those few foals that are refractory to treatment or display uncontrollable pain are candidates for surgical intervention.
Uroperitoneum
Uroperitoneum is a relatively common cause of abdominal distention and depression in the neonatal foal. The condition predominates in males but may occur in females as well. Uroperitoneum may be congenital or acquired. The congenital form occurs as a result of failure of the dorsal wall of the bladder to close during development.83 The most common cause of uroperitoneum is a ruptured urinary bladder, but other sites in the urinary tract may also leak, including the ureters, urachus, and urethra. Most cases of ruptured bladders are presumed to occur during parturition because of external pressure on a distended bladder. This form occurs most commonly in colts. Uroperitoneum can also occur secondary to ischemic necrosis or infection of the urinary bladder or urachus in the compromised foal.78 Critically ill, recumbent foals may rupture their bladders while being lifted and turned with a full bladder or as a result of chronic overdistention associated with the generalized disease state. Foals with botulism may also rupture their bladders secondary to bladder atony and chronic overdistention. Older foals of either sex may experience bladder rupture secondary to focal infection of the umbilical arteries and/or urachus, or ischemic necrosis of the apex of the bladder.
Clinical signs of uroperitoneum are rarely noticed before 48 to 72 hours of age, particularly if the foal is not being watched closely. The first signs may be urinary incontinence or frequent attempts to urinate, with only small amounts voided. Sometimes, particularly in those animals that experience rupture sometime after birth, there is a history of a period of normal urination, which at some point stopped or became abnormal. Loss of suckle, mild colic, and increasing abdominal distention are usually accompanied by worsening depression and increasing heart and respiratory rate. If the condition is allowed to persist, foals become increasingly weak and dyspneic and may be presented in cardiovascular collapse. Fillies with ruptured ureters have been reported to have a characteristic protruding perineum, presumably as a result of retroperitoneal accumulation of fluid.84
Laboratory findings commonly associated with uroperitoneum are elevated serum creatinine and blood urea nitrogen (BUN), hyperkalemia, hyponatremia, hypochloremia, and metabolic acidosis. These changes are probably a result of the normal diet of the foal (milk being relatively high in potassium [25 mEq/L] and low in sodium [12 mEq/L]) and the third spacing of urine in the peritoneal cavity. With urine potassium concentration relatively higher than serum levels, and with urine sodium concentration lower than serum levels, the net effect of partial equilibration of serum with peritoneal fluid across a semipermeable membrane is hyponatremia and hyperkalemia, along with an inability to excrete the waste products of metabolism. Hyperkalemia may be severe enough to induce potentially fatal bradyarrhythmias. In hospitalized foals that developed uroperitoneum as a secondary complication, these typical electrolyte abnormalities are not consistently observed. Because most of those foals were receiving replacement intravenous fluids (high in sodium, low in potassium) and very little milk, it was theorized that intake has a great influence on the electrolyte abnormalities associated with uroperitoneum.85 On the other hand, the electrolyte abnormalities typically associated with uroperitoneum are not pathognomonic for that disorder. Foals with renal failure, blocked urethra, white muscle disease, and enteritis have shown the same electrolyte changes.
A diagnosis of uroperitoneum often can be made quickly using transabdominal ultrasound and a 5- or 7.5-MHz transducer to visualize large volumes of free, nonechogenic fluid within the abdomen and a small, irregularly shaped, collapsed bladder. Abdominocentesis usually produces a free flow of peritoneal fluid that has a low cell count, low specific gravity, and at least twice the creatinine concentration of peripheral blood. If the creatinine is the same in both serum and peritoneal fluid, other explanations for the clinical signs should be investigated. The WBC count, total protein, and cytology of the fluid should also be determined. Most uncomplicated cases of ruptured bladders have fairly normal values for peritoneal fluid. In some cases, however, an increased WBC count and total protein and the presence of bacteria may suggest peritonitis. This may be a result of the urine in the abdomen, but more commonly there is a primary ongoing infectious problem (necrotic urachus or bladder, enteritis), and the prognosis becomes worse. If laboratory facilities are not available, new methylene blue can be injected into the bladder using a urinary catheter, and a few minutes later a sample of peritoneal fluid should have a blue discoloration if a ruptured bladder is present. However, this technique may not allow detection of other causes of uroperitoneum such as a ruptured ureter or distal urachus. Positive contrast cystography using a 10% solution of water-soluble media may be helpful in detecting the location of the urinary tract leakage. The ability to obtain urine on catheterization of the urinary bladder does not rule out uroperitoneum. Hematology and blood cultures should be performed to detect primary or secondary sepsis.
Treatment of uroperitoneum is surgical repair. However, the foal with uroperitoneum should not be rushed to surgery without first carefully stabilizing it. Serum electrolytes and blood gases should be run to determine the extent of hyperkalemia, hyponatremia, and acidosis present. Although the total amount of water in the body is usually grossly increased by the peritoneal accumulation of urine, effective circulating volume may be drastically reduced. If the eyeballs are sunken and the pulse quality and capillary refill time are poor, aggressive fluid therapy is indicated to support the circulation. This is best performed by concurrently removing as much fluid as possible from the abdomen with a teat cannula, 14G catheter, or peritoneal dialysis catheter to avoid worsening fluid overload and respiratory distress. The fluids of choice to treat the typical electrolyte alterations associated with uroperitoneum are saline, dextrose, and possibly sodium bicarbonate solutions, depending on the degree of acidosis present. In most instances continuous dextrose infusion is effective in decreasing the serum potassium level to an acceptable level, but values should be rechecked before anesthesia is induced. Insulin and dextrose may also be used to treat hyperkalemia, but the patient must be monitored for hypoglycemia. One suggested regimen is regular insulin at 0.1 to 0.2 U/kg subcutaneously (SC) or IV accompanied by a continuous intravenous dextrose infusion (4 to 8 mg/kg/min). Some individuals also have pleural fluid accumulation and atelectasis secondary to the abdominal distention, so oxygenation and ventilation during and after surgery should be closely monitored. Broad-spectrum antibiotics should be started immediately after samples are taken for culture if infection is suspected.
The prognosis for uncomplicated ruptured urinary bladders is usually good (>80% survival), provided the animal is stabilized before anesthesia. The presence of concurrent septicemia carries with it a considerably poorer prognosis.85 In one retrospective study among foals with uroperitoneum, 100% of those foals with a negative sepsis score lived and only 57% of foals with a positive sepsis score survived.85
Gas or Fluid Accumulation in the Gastrointestinal Tract: Ileus
Abdominal distention and colic secondary to excessive gas and/or fluid accumulation in all or a portion of the GI tract are common complications in the compromised neonate undergoing intensive care. The exact mechanisms responsible for the presumably altered GI motility are not well defined. Ileus is associated with the absence of intestinal sounds, abdominal distention, and intolerance of oral feeds characterized by gastric reflux. Auscultation of reduced GI borborygmi does not always correlate with the degree of intestinal compromise and decreased motility. Transabdominal ultrasonography helps identify absence of intestinal motility and the location and degree of intestinal distention. Ileus and the attending abdominal distention can cause severe colic and can induce respiratory distress in a weak or premature foal with preexisting pulmonary compromise.
Metabolic and infectious causes of ileus in the foal include hypokalemia, hypocalcemia, hypoxic-ischemic bowel injury, bowel obstruction, peritonitis, enterocolitis, and endotoxemia. Hypokalemia is associated with anorexia, diarrhea, and renal loss. Hypocalcemia is associated with prematurity, decreased dietary intake, excessive bicarbonate administration, diuretic therapy, and those conditions such as asphyxia, toxemia, and sepsis that stimulate release of cortisol and catecholamines. Peripartum hypoxia results in a preferential decrease in blood flow to the gut and kidneys. Poor perfusion of the intestines leads to varying degrees of mucosal damage and decreased motility. Severely damaged bowel requires a period of gut rest to allow healing to occur before oral feeds are resumed. Premature resumption of enteral feeding is associated with colic, maldigestion, diarrhea (often bloody), and translocation of intraluminal bacteria across damaged bowel wall into the bloodstream. Some of the more common causes of bowel distention in the neonate include meconium retention, intussusception, ascarid impaction, and small intestinal volvulus. Peritonitis may be associated with intraabdominal abscessation, severe enteritis or GDUD, and generalized septicemia. The most common causes of enteritis in foals include rotavirus, Clostridia species, Salmonella species, and dietary changes. Endotoxemia is usually part of generalized septicemia. Chronic bowel distention, regardless of the cause, further impedes return of normal gut motility. In the foal, aerophagia, particularly in the struggling or hypoxic neonate, often results in gas distention that is not easily removed through a nasogastric tube, because gas tends to move quickly through the GI tract (Fig. 19-8). Abdominal distention is also commonly observed during mechanical ventilation in the foal and as a result of overfeeding in the calf. Foals with botulism are often intolerant of enteral feeding, probably because of altered GI motility. Use of certain milk replacers can result in bloat, colic, and diarrhea, even in the apparently healthy orphan neonate. Discontinuation of or a decrease in the amount of enteral feeding and, if possible, increased activity of the patient usually result in resolution of the problem.
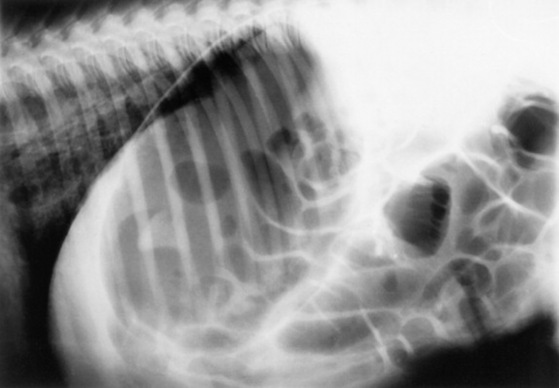
Fig. 19-8 Marked gastric and generalized small intestinal gas distention in a septic, collapsed, 3-day-old thoroughbred colt. There is no evidence of obstruction. The patient was treated with supportive therapy and recovered.
Abdominal radiographs reveal gas-distended loops of small or large intestine and may identify bowel obstruction. Sonographic examination permits evaluation of bowel wall thickness, peritoneal fluid volume and echogenicity, gut patency, intramural gas accumulation, location and degree of intestinal distention, and presence or absence of motility. An abdominal sonogram should be performed to rule out the presence of an intussusception or other obstructive lesion before any prokinetic therapy is initiated.
Management of ileus includes nasogastric decompression, cessation or reduced volume and frequency of enteral feeds if gastric reflux is present, parenteral alimentation if enteral feeding cannot be maintained at a rate of at least 10% of body weight per day, enema administration to relieve distal meconium or fecal retention, correction of any underlying electrolyte abnormalities, exercise for ambulatory foals, and judicious use of prokinetic agents. The gut -atrophies without enteral feeding. Glutamine and butyrate are essential fuels for the small and large bowel respectively and have been added to enteral formulas for people and some oral fluid replacement formulations for animals to help maintain and restore enterocyte health.86 Prokinetic drugs should not be used when bowel obstruction or compromised bowel integrity is suspected. Prokinetic agents that have been used include metoclopramide, bethanechol, and erythromycin. There have been anecdotal reports of small intestinal intussusception after prokinetic use in neonatal foals.
Surgical Gastrointestinal Lesions
Most types of displacement, torsion, volvulus, and entrapments that occur in the adult horse may also occur in the neonatal foal, although probably at a lower frequency (Fig. 19-9). Large colon displacement, intussusception, and small intestinal volvulus have also been observed secondary to enteritis and colitis.
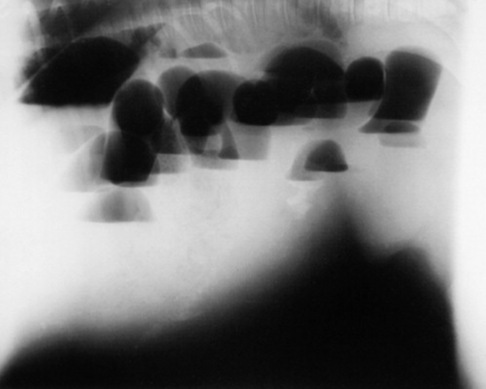
Fig. 19-9 Standing abdominal radiograph of a 3-week-old foal that was presented with signs of severe pain. Note the multiple, erectile loops of distended small intestine with fluid lines. Obstructive disease was suspected. On surgical exploration of the abdomen, obstructing adhesions secondary to a previous surgery were found.
Surgical correction of congenital GI defects may be attempted. Atresia ani, atresia recti, and atresia coli have been well documented in the foal, and intestinal aganglionosis has been observed in association with recessive lethal white foals, which are usually the products of mating between two overo paint horses.76 Acute colic, progressive abdominal distention, and lack of meconium staining after repeated enemas have been the most common findings in newborn foals with atresia coli. Barium enemas may be of use in identifying foals with a short small colon but may also be misleading.87 Surgical exploration of the abdomen offers definitive diagnosis of the severity of the malformation and the possibility of correction, but the owner should be informed before surgery of the high frequency of inoperable lesions and the high failure rate after reattachment.87 Before any surgery is contemplated, a thorough physical examination should be performed to identify any other congenital malformations. Surgical correction of atresia ani is often successful, particularly if the atresia is limited only to a persistent membrane blocking the anus and the anal sphincter is normal. The prognosis for atresia coli is guarded. Poor intestinal motility, technical difficulties of attaching bowel segments that are so different in size, anastomosis breakdown, and peritonitis after surgery are common complications.87 An inguinal hernia is another congenital lesion occasionally requiring surgical intervention. Such hernias occur in colts and may be caused by compression during parturition. Most congenital inguinal hernias are handled conservatively because the condition is often self-limiting by the time the foal is 3 to 6 months old. Treatment includes daily manual reduction of the hernia and frequent observation to detect possible bowel strangulation. Indications for surgical intervention in foals with congenital hernias include rupture of the common vaginal tunic, persistent colic, severe edema of the prepuce and scrotum, and trauma to the skin overlying the hernial sac. Surgical hernias are difficult to reduce manually, and loops of intestines are often palpable in the subcutaneous tissues of the scrotum and medial thigh.88 Unilateral castration is usually performed on the affected side.
Other GI lesions in foals that may require an exploratory laparotomy include intussusception, small or large intestinal volvulus, and mechanical obstruction (e.g., food or ascarid impaction, phytobezoar, fecalith). Intussusceptions are reported in young horses less than 3 years of age. An intussusception is formed when one segment of intestine and its mesentery invaginates into the lumen of the adjacent bowel immediately aboral to it. The invaginated segment is called the intussusceptum, and the enveloping segment is called the intussuscipiens. Small intestinal intussusceptions can involve the jejunum, ileum, or ileocecal junction. Other sites of obstruction include cecocecal and cecocolic junctions. Intussusceptions are most common in foals less than 6 weeks old.89
Causes of intussusception include segmental motility differences (e.g., a hypermotile section of bowel adjacent to an atonic segment of bowel) and local changes in the bowel wall (e.g., abscessation). Causes of altered peristalsis include -enteritis, heavy ascarid infestation, mesenteric arteritis, and sudden dietary changes.90 Changes in the bowel wall have included granulomas, papillomas, and intramural leiomyoma. Anoplocephala perfoliata has been associated with ileocecal intussusceptions. Clinical signs include varying degrees of discomfort depending on the site of obstruction and its duration. Abdominal pain can be severe but is often low grade and intermittent, accompanied by decreased manure production. Ultrasonography is a useful diagnostic aid. Sonographically the cross-sectional view of the intussusception reveals a target-like pattern with a thick hypoechoic rim. The outer rim is created by severe edema of the entering and returning bowel walls of the intussusceptum.
Treatment involves surgical exploration. Early cases can be manually reduced, followed by surgical resection. Because of the ileum’s tenuous blood supply and the inaccessibility of the ileocecal junction, intussusceptions involving the ileum are usually treated with a side-to-side jejunostomy or ileocecostomy. Ileoileal intussusceptions have been reported to have a better prognosis than jejunal or ileocecal intussusception. Foals that have multiple sites of intussusception have a poor prognosis.
Volvulus may involve the small or large intestines. Small intestinal volvulus is the most common, especially among foals between 2 and 4 months of age. Signs include abdominal distention, gastric reflux, persistent tachycardia, severe pain, and sonographic evidence of uniform, severe bowel distention with bowel wall edema (>3 to 4 mm) and absence of motility. As expected, survival is poorer following correction of strangulating versus nonstrangulating lesions. Strangulating lesions of the small intestines are associated with poor survival compared with large intestinal lesions and have a higher incidence of fatal complications. Parasitic migration and abrupt dietary changes are among conditions thought to predispose to volvulus development.
Surgical colic in foals carries a poorer long-term prognosis than in adult horses. One study91 examined the survival rate among 67 foals <150 days of age that underwent colic surgery. The most common lesions requiring a celiotomy were small colon impaction, large colon impaction, jejunal volvulus, and ascarid impaction. A poor prognosis was associated with strangulating lesions. Foals less than 14 days of age experienced more early postoperative complications and suffered poor long-term survival because of adhesion formation. Only 25% of foals less than 14 days of age survived in the short term compared with a survival rate of 71% in foals older than 14 days of age.
Another study92 examined the outcome among 119 young horses less than 1 year of age that underwent exploratory celiotomy. Among all foals the most common cause for surgery was small intestinal strangulation. Uroperitoneum and meconium impaction were the most common conditions in neonatal foals, and intussusception and enteritis were more common among older foals. Significant elevations in packed cell volume (PCV) (37% to 54%), heart rate (80 to 134 bpm), nucleated cell counts, total protein in peritoneal fluid (3.1 to 32.8 × 103/μL, 2.9 to 4.9 g/L), and rectal temperature (38.2° C to 39.2° C) were observed in nonsurvivors compared with survivors. Nonsurvivors had significantly decreased serum bicarbonate, chloride, sodium, and venous pH values. Thirty-three percent of foals that survived surgery had evidence of intraabdominal adhesions.
Necrotizing Enterocolitis
NEC has been described in equine neonates.93 It is a syndrome of acute intestinal necrosis.94 In human infants, prematurity is the single greatest risk factor, with only a small percentage of affected infants being full term. The causes of NEC are not well defined, but predisposing factors include ischemic hypoxic gut injury, presence of intraluminal bacteria, and enteral feeding. After GI ischemia, mucosal cell metabolism diminishes and the protective mucous layer is lost. This allows enzymes to break down the mucosal barrier, and intraluminal bacteria can then invade and multiply within the bowel wall. Enteral feeding provides substrate for the bacteria. Pneumatosis intestinalis develops, and the bowel frequently ruptures. Abdominal signs include abdominal distention, tenderness, ileus, and ascites. The condition may appear as a fulminant, rapidly progressive disease or progress at a much slower pace.94,95 One affected equine neonate was premature and was undergoing treatment for respiratory distress when the abdominal crisis occurred. Another was a term foal that had experienced a prolonged delivery and was presented at 24 hours of age because of weakness and abdominal pain. Abdominal distention and abdominal pain, followed by ventral colon rupture, were noted in both foals.93
Clinical signs associated with varying degrees of hypoxic, ischemic gut injury include ileus, gastric reflux, colic, lethargy, abdominal distention, and diarrhea. Reflux and feces may be positive for blood. Generalized sepsis often accompanies NEC. NEC should be distinguished from intestinal ileus secondary to other neonatal diseases, other surgical GI lesions, bacterial or viral enterocolitis, and intolerance to a milk diet. Although no single laboratory test is specific for NEC, the abdominal radiograph often reveals pneumatosis cystoides intestinalis, bowel wall edema, and an abnormal gas pattern consistent with ileus. Ultrasonography may reveal intramural gas accumulation. If intestinal perforation has occurred, pneumoperitoneum and septic peritonitis may also be noted.93,94 Intestinal perforation is associated with a poor prognosis.
Gastrointestinal Ulceration
GDUD in the older suckling foal is covered in greater detail in Chapter 32. GI ulceration has also been associated with a number of neonatal diseases, including asphyxiation, enteritis, and septicemia.96 The most significant form of the disease in this age group is cardiac gland disease—ulceration in the cardiac gland mucosa immediately beneath the margo plicatus. Ulceration presumably occurs in response to altered perfusion and may result in perforation and fatal peritonitis. In endoscopic surveys of neonatal foals in the United States, England, and Ireland, approximately one half of foals less than 3 months of age had evidence of gastric squamous mucosal ulceration.97,98 The prevalence of lesions was greatest in 2- to 9-day-old foals and 30- to 59-day-old foals. In the young foals, typical lesions were golden-colored crusts in the squamous mucosa next to the margo plicatus along the greater curvature in association with diffuse ulceration and erosion and, commonly, squamous epithelial desquamation.98 In one of these studies, foals that had a previous disorder (e.g., diarrhea, illness, transport) were more likely to have a glandular mucosal lesion than those that had not (9% vs 4%).98
The clinical signs of bruxism, excessive salivation, and colic that are commonly associated with GDUD in the 1- to 4-month-old foal are rarely observed in the neonatal foal. Frequently, gastric perforation is the first indication of the problem. Perforated and bleeding gastric ulcers have been diagnosed as early as 24 hours of age. There is also a high incidence of abomasal ulceration in calves, but rarely do they become symptomatic unless perforation occurs. Currently the cause and pathophysiology of the condition in the neonatal period is not known. Because of the difficulty of diagnosing the condition without specialized endoscopic equipment and the often catastrophic consequences of subsequent perforation or pyloric or duodenal stricture formation, antiulcer medications are often used prophylactically in the compromised neonatal foal. Foals at highest risk for ulcers are sick neonates that are not recumbent and foals with hypoxic gut damage, enterocolitis, and painful orthopedic conditions. Foals that are chronically recumbent frequently maintain a high pH that may a result of ileus and enterogastric reflux.99 In normal foals the mean hourly baseline gastric pH ranged from 3.2 to 3.7. Milk intake had a dramatic but transient alkalinizing effect on pH. H2 blockers including ranitidine and omeprazole significantly raised gastric pH. Ranitidine administered IV at 2 mg/kg or orally at 6.6 mg/kg significantly raised gastric pH.
Initial therapy includes the use of H2 blockers (cimetidine 15 to 20 mg/kg orally [PO] q6h; ranitidine 6 to 8 mg/kg PO q8h), cytoprotective agents (sucralfate 20 mg/kg PO q6h), and a new class of drugs, proton pump inhibitors (omeprazole 4 mg/kg PO q24h). Omeprazole is highly effective and has the advantage of once-daily administration. Treatment regimens should continue for a minimum of 21 to 28 days. In acute stages some foals receive additional pain relief from over-the-counter antacid solutions (10 to 20 mL q3-4h). Some foals are in too much pain to nurse and benefit from withholding enteral feeds temporarily and maintaining them on a brief course of parenteral alimentation using a mixture of amino acids, lipids, dextrose, electrolytes, and vitamins. Currently recommended dosages for antiulcer medications are listed in Chapter 32.
Hemoperitoneum
Hemoperitoneum is a relatively uncommon cause of abdominal distention in the large animal neonate. The structures most commonly responsible for the hemorrhage are the umbilical vessels and the liver or spleen when ruptured secondary to trauma. Occasionally other structures such as a ruptured granulosa cell tumor may bleed.100 Depending on the cause and severity of the hemorrhage, clinical signs relating to hypovolemia and anemia may be mild or severe and may appear shortly after birth or in the older foal. Diagnosis of hemoperitoneum is based on the retrieval of free-flowing blood on peritoneal tap and the detection of free fluid in the abdomen. Ultrasound examination may be of benefit in detecting the source of the bleeding. Of critical importance, regardless of the source of the hemorrhage, is prevention of hypovolemic shock, and intensive patient monitoring and intervention are often indicated. Whole blood replacement may be necessary. If an internally bleeding animal with an unstable cardiovascular system is rushed to surgery without prior stabilization, profound shock may occur, and a poor outcome usually results.
DIARRHEA IN NEONATAL FOALS
Diarrhea occurs commonly in foals of all ages and represents one of the most common medical conditions requiring veterinary intervention. The approach to diagnosis can be difficult, and establishment of a definitive diagnosis in a field setting is uncommon. The veterinarian must therefore consider a number of factors in order to construct a list of most likely causes. These factors include the age of the affected animal, the numbers of foals affected, the volume and character of the feces, and the duration of signs. Some limited diagnostic tests can be used to rule in or out many of the common causes of foal diarrhea.
In some foals, diarrheal diseases are associated with signs of colic. The abdominal pain can be severe and can mimic that seen in foals with strangulating intestinal lesions, making case management difficult. Colicky signs frequently precede the onset of diarrhea. Passage of a nasogastric tube is as important in colicky foals as it is in adults. Foals with intestinal ileus or inflammatory diseases of the small intestine commonly produce large volumes of gastric fluid on reflux. The control of pain is required in order to reduce the chances of injury and to facilitate evaluation. Intramuscular butorphanol can be very helpful in providing pain relief in foals with colitis. α2-Adrenergic agonists should be used with caution in neonates because of their depressive effect on the cardiopulmonary system. If they are to be used, then start with lower doses or consider combining with butorphanol. Nonsteroidal antiinflammatory drugs should be avoided until a diagnosis is established. Neonatal foals generally tolerate flunixin well but require a slightly larger dose rate but reduced frequency when compared with older animals.101,102
There are many causes of diarrhea in young foals, including bacteria and bacterial toxins, viruses, nutritional factors, parasites, and antibiotic use. Normal physiologic adaptive processes can also produce diarrhea in most foals.
DIFFERENTIAL DIAGNOSES
Bacteria
E. coli is the most important mediator of systemic sepsis in newborn foals but is not a common primary cause of diarrhea in this age group. Some reports implicate an association between E. coli and diarrhea in foals. Enterotoxigenic E. coli was isolated from a 3-day-old diarrheic foal from Virginia.103 The isolate was of the 0101 serotype, was heat labile-like—toxin positive, but negative for heat stabile. Earlier inoculation studies indicated that F4 (formerly K88)-positive E. coli was not likely to cause diarrhea in foals, although it may have a synergistic role in foals infected with other potential pathogens, such as rotavirus.104,105
Intestinal disease mediated by clostridial toxins occurs in foals worldwide. The toxins are most commonly derived from biotypes A and C of Clostridium perfringens or from Clostridium difficile. Classic intestinal clostridiosis of foals is caused by C. perfringens biotype C and is characterized by colic, rapid dehydration, cardiovascular collapse, and hemorrhagic diarrhea. The disease occurs most commonly in foals less than 10 days of age, and often less than 36 hours of age. It is associated with a high mortality, and outcomes are rarely influenced by treatment.106 Death may occur rapidly, and occasionally it occurs before any diarrhea has been passed. Biotype C produces both alpha- and beta-toxins, along with variable amounts of enterotoxin. Cases can occur sporadically or as outbreaks and on some farms occur annually, presumably associated with carriage in specific mares. Biotype A of C. perfringens has become well recognized as a specific cause of foal diarrhea over the past decade. Biotype A produces alpha-toxin and enterotoxin and is associated with a slightly lower mortality rate than biotype C. Affected foals are more likely to respond to directed or supportive care.106 The development of clinical signs is rapid, and diarrhea may or may not contain blood; in ‘our experience passage of bloody diarrhea is common but usually very transient. Reported risk factors for C. perfringens diarrhea in neonatal foals in Colorado include breed (stock horse type); birth on dirt, sand, or gravel; housing in stalls or on dry lots during the first 3 days of life; and maternal feeding practices. Feeding a low-grain diet prepartum was associated with a decreased risk of neonatal disease.107 Hematologic findings are consistent with toxemia. This includes hemoconcentration; an initial leukopenia, characterized by a neutropenia with a left shift to immature forms and toxicity; and a lymphopenia. In chronic cases a rebound leukocytosis and hyperfibrinogenemia may develop. Altered coagulation may be evident clinically through prolonged bleeding or spontaneous hemorrhage or through a propensity to develop thrombosis.
A definitive diagnosis of C. perfringens diarrhea is rarely established in practice. The diagnosis is usually based on signalment, clinical features, and outcome, rather than on detection of specific clostridial toxins. Positive fecal culture is strongly supportive, noting that healthy foals may shed low numbers of C. perfringens. Identification of toxin is ideal but is limited because of availability of appropriate commercial assays. Fecal enterotoxin detection assays are available but lack sensitivity, particularly with biotype C isolates. Biotyping of C. perfringens isolates after culture can be achieved using polymerase chain reaction (PCR) analysis for toxin gene sequences. This may be helpful in increasing the accuracy of the diagnosis but again falls short in establishing a definitive cause. Fecal Gram stain is easy to perform and may support an early clinical suspicion of disease if there are abundant numbers of large gram-positive organisms or spores present.
Clostridium difficile can produce an identical clinical syndrome to C. perfringens, although it appears to be more variable with respect to fecal blood. It also occurs as sporadic cases or as clusters or outbreaks. It is important to recognize that clostridial infection can produce a severe inflammatory syndrome that is restricted to the small intestine and may not cause diarrhea. Affected foals can present with signs that mimic strangulating small bowel disease including severe abdominal pain, gastric reflux, and sanguineous peritoneal fluid. Two principal toxins can be liberated from C. difficile, an enterotoxin (toxin A) and a cytotoxin (toxin B). Both nontoxigenic and toxigenic strains exist and can be differentiated only after culture using molecular techniques. Consequently, commercial toxin tests (available for both toxin A and toxin B) are recommended in addition to fecal culture to establish a diagnosis. As with C. perfringens cases, a fecal Gram stain can also increase suspicion. Hematologic changes associated with C. difficile infection can mimic those associated with C. perfringens disease.
Most cases of clostridial enterocolitis require aggressive medical intervention, irrespective of clostridial type. Antimicrobial therapy should be both specific and broad spectrum. Targeted antimicrobial treatment typically includes both metronidazole and penicillin. The majority of affected foals require, at minimum, intravenous crystalloid solutions; some also benefit from plasma or synthetic colloids and inotrope and vasopressor therapy. Additional therapies include C. perfringens biotype C and D antitoxin, di-tri-octahedral smectite clay, and lactase enzyme replacement.108,109 The use of C. perfringens antitoxin and toxoid is off-label and not without some risk. Pretreatment with antihistamines before antitoxin has been suggested.108 Prevention of clostridial enterocolitis centers on hygienic housing practices and avoidance of overfeeding of late pregnant mares. Affected foals and their mares should be isolated and strict protocols instituted to limit cross-contamination. Administration of C. perfringens type C and D toxoid to pregnant mares has been used with anecdotal success on farms with recurrent problems with C. perfringens biotype C. The use of prophylactic metronidazole is highly controversial but understandable on properties with a high disease prevalence. Some isolates of C. difficile are reportedly resistant to metronidazole,108 and although this appears to be dependent on geographic location, the widespread prophylactic use could promote resistance. Prophylactic use of probiotic preparations has also been commonly recommended but is difficult to justify on the basis of limited and conflicting efficacy data.110,111
Bacteroides fragilis is a gram-negative anaerobic rod and occurs in both enterotoxigenic and nonenterotoxigenic forms. Enterotoxigenic strains of B. fragilis have been incriminated with diarrhea in several species including lambs, calves, pigs, humans, and foals.112 Enterotoxigenic B. fragilis was isolated from young foals (aged 2 to 60 days) with diarrhea.113 Half of these foals had other potential pathogens detected, including Salmonella or rotavirus. In a study reviewing 20 isolates of B. fragilis from hospitalized foals with diarrhea, only four had the enterotoxin gene.112 The most common isolate from foal feces was a nonenterotoxigenic strain, casting doubt as to clinical relevance.
Enterococcus (group D Streptococcus) durans has also been implicated as a cause of diarrhea in several species including foals.104 Similarly, Aeromonas hydrophila was isolated more commonly from foals with diarrhea than from control animals, suggesting a potential role in foal diarrhea.114
R. equi is an important cause of pulmonary disease in foals. Although abdominal involvement appears common, the relevance of R. equi as a significant primary enteric pathogen in terms of number of animals affected is likely small. An intestinal syndrome has been directly attributed to R. equi that involves necrosis of the small intestinal Peyer’s patches and multifocal thickening and necrosis of the cecum and large intestine.115 The changes include multiple areas of intestinal ulceration with a thin covering of fibrin and neutrophils. There is also an associated mesenteric lymphadenopathy. The diagnosis of R. equi ulcerative colitis is difficult for a variety of reasons. In contrast to Salmonella infection, in which recovery of the organism from the feces has significance, the recovery of R. equi is common in asymptomatic animals. Up to 100% of foals older than 2 weeks of age may shed large numbers of the bacteria (>104 colony-forming units [cfu] per gram of feces).116
Salmonella infection of neonatal foals is also associated with high mortality. As with clostridial infection, cases can occur sporadically or as part of an outbreak. Mares provide the most important source of Salmonella to newborn foals, but it is rare that both mare and foal develop clinical disease. Clinical signs can become apparent by 24 hours of age but are more common in older foals. The severity of signs is related to virulence of the serotype involved, inoculation dose, and level of host immunity. In contrast to adult infections, detectable bacteremia occurs commonly in affected foals. Consequently foals that may survive the initial intestinal or systemic disease remain at risk for secondary complications that include osteomyelitis, synovitis, meningitis, uveitis, hepatitis, or pyelonephritis. In some foals these complications may not become clinically apparent until days or weeks after resolution of enteric disease. Therefore appropriate and sustained broad-spectrum antibiotic therapy is important in foals known to be affected. The selection of antibiotic should be based on known sensitivity patterns, understanding that in vitro sensitivity may not accurately translate into clinical efficacy because of the intracellular location of Salmonella. For example, the limited distribution of aminoglycosides can lead to therapeutic failure despite often promising in vitro sensitivity.
Viruses
Rotavirus is generally considered to be the most common cause of infectious diarrhea in foals. There are seven known groups of rotavirus (A through G), and many different serotypes within each group. Group A is the primary cause of rotaviral diarrhea in foals, with G3 being the most common serotype. Rotaviruses have the ability to change their surface proteins over time, and this rearrangement of gene segments takes place during co-infections with other strains. This leaves the possibility for many variations of the virus.
Transmission may be direct from animal to animal or indirect through fomites. Disease occurs after a short incubation period. Experimentally, this period may be as brief as 48 hours.117 Rotavirus replicates within the intestine and invades the lining of the proximal small intestine, causing villous cell death and a resultant loss of absorptive area. Diarrhea may result from several mechanisms: (1) a loss of absorptive capacity coupled with a decrease in lactase production can lead to an osmotic load of undigested lactose delivered to an immature hindgut; (2) a compensatory crypt cell proliferation may cause an increase in intestinal secretion; and (3) the virus produces an enterotoxin that causes or contributes to the development of diarrhea. The putative viral enterotoxin and cytotoxin, NSP4, is a nonstructural glycoprotein of rotavirus that is released from virus-infected enterocytes.118 NSP4 is a noncompetitive inhibitor of the Na-glucose symporter and also enhances intestinal chloride secretion.
Disease can be seen between 2 and 160 days of age but is most common in foals less than 60 days old. Indeed, most clinical infections probably occur between 5 and 35 days of age. The presence and severity of diarrhea are highly dependent on the degree of hindgut maturation. Consequently, infection in foals less than 2 weeks of age may result in life-threatening watery diarrhea, whereas infected older foals may have minimal or no diarrhea because of effective colonic compensation of osmotic and fluid loads. Diarrhea when present is often watery but is nonfetid in odor.
There is serologic evidence that broodmares may have an important role in propagation of the virus within a herd. Infections can occur as isolated cases or as outbreaks following periods of overcrowding and stress. Shedding after infection is usually complete by 10 days after the cessation of clinical signs but may occur intermittently for up to 9 months.119 The virus can persist in the environment for up to 9 months, and disinfection usually necessitates the use of substituted phenolic compounds.
The diagnosis of rotaviral diarrhea is based on an appropriate signalment, clinical signs, and detection of the virus in feces. Tests include electron microscopy and commercial immunoassays (latex agglutination or enzyme-linked immunosorbent assay [ELISA]). Virus is shed in large concentration early on during infection.120 Central to the management of affected foals is maintenance of hydration through enteral and/or intravenous fluid therapy. Bismuth subsalicylate is commonly administered to foals with diarrhea, irrespective of the underlying cause.
Prevention involves good hygiene and reduction in crowding. Vaccination of mares during pregnancy has yielded variable results in terms of efficacy.121,122 Some evidence suggests that vaccination may at a minimum delay the onset of disease, thereby reducing both the severity and duration of diarrhea. Limited data from Japan indicate a potential benefit of bovine colostral immunoglobulin powder in the prevention of rotaviral diarrhea.123 A commercial egg-protein—derived supplement has also been used in foals to reduce the prevalence of neonatal diarrhea, but controlled research data are lacking.
Coronavirus can also cause diarrhea in foals during the neonatal period, although it appears to be very uncommon.124-127 The few reports of clinical disease indicate that disease caused by coronavirus can be very severe. Antemortem diagnosis can be made using electron microscopy, serology, or commercial fecal-capture ELISA. As with rotaviral infection, shedding is greatest in the early stages of disease. Adenovirus is unlikely to play an important role in foal diarrhea, with the exception of foals with severe, combined immunodeficiency syndrome.128
Parasites
Strongyloides westeri is a common parasite of foals, with early infection of the foal occurring through mare’s milk. Experimental studies have indicated that higher numbers of infective larvae than are found in milk are needed to produce diarrhea.129 In addition, foals with high egg counts (prepatent period 8 to 14 days) are often asymptomatic. S. westeri is susceptible to a variety of anthelmintics, including ivermectin.130 Eimeria leuckarti is commonly found in feces of foals from approximately 30 to 125 days of age.131 It is unlikely to be a cause of diarrhea in foals.132
Cryptosporidium parvum is not considered to be an important pathogen of foals in terms of numbers of animals diseased. Based on epidemiologic studies the parasite appears to be concentrated in some breeding operations.133 Foals diseased with Cryptosporidium shed enormous numbers of infective oocysts into the environment. The parasite is considered to be coccidian-like but differs from coccidia in terms of size (4 to 6 μm diameter compared with 23 to 34 μm for other coccidia), host specificity (not host specific), pathogenesis (invades only epithelium), and drug sensitivity (resistant to many drugs). Infection is by the fecal-oral route. The oocysts can survive in the soil or water for months. They do not require a period of sporulation outside of the host to become infective. In a study of asymptomatic foals in Ohio and Kentucky, it was reported that between 15% and 31% of foals were shedding Cryptosporidium and that shedding began between 4 and 19 weeks of age and persisted no more than 14 weeks.134 All shedding had ceased by weaning and was not identified in adult horses. Each generation of the Cryptosporidium lifecycle is brief, with maturation occurring in as little as 12 hours. Consequently, the prepatent period is very short, approximately 72 to 96 hours. Although not determined in foals, a heavily infected calf can shed approximately 50 billion oocysts within a 7-day period.
The diagnosis is usually made through microscopic examination of the feces. Acid-fast or Ziehl-Neelsen stains are required to detect oocysts. Immunofluorescence assays and flow cytometry techniques have also been described. C. parvum infection may be seen with concurrent enteric or systemic infections. The disease is generally self-limiting in immunocompetent animals. Historically the pharmacologic control of Cryptosporidium has been difficult, but paromomycin, nitazoxanide, or azithromycin may be efficacious.
Giardia may be found in normal foals, with infection rates reported to be 17% to 35%. Giardia is present in all age groups, and it is believed foals acquire infection from nursing mares. Concurrent infection with Cryptosporidium and Giardia may be observed. Disease should be suspected if large numbers of parasites are seen on fecal analysis. Affected animals should respond within a few days to treatment with metronidazole; failure to respond should alert to other pathogens.
Nutrition
Nutritional causes of diarrhea include overingestion of milk (as might occur when the mare and foal are separated and rejoined) or overfeeding orphaned or sick foals. Overwhelming the ability of the small intestine to digest and absorb results in presentation of milk to the colon, where it is fermented and produces osmotically active sugars and acids.135 In a controlled study of foals less than 5 days of age, an elemental isotonic diet (Osmolyte) produced diarrhea in healthy foals when fed as the sole source of nutrition. Older foals fed a similar diet apparently did not develop diarrhea.* Caution should be exercised in using elemental diets designed for humans in the foal without adequate prior testing of tolerance to the diet. Orphan foals and foals fed commercial mare milk replacer may experience diarrhea associated with these diets. Foals that are fed raw cow’s milk frequently experience diarrhea and failure to thrive. Cow milk replacer uncommonly causes diarrhea but remains a less than ideal replacement. In contrast, foals fed goat’s milk grow well and rarely develop diarrhea, although they may develop a metabolic alkalosis of minimal to no clinical significance.
Transient lactase deficiency has been proposed in foals.137-139 An oral lactose tolerance test is conducted by a 4-hour fast and administration of 1 g/kg of body weight in a 20% solution of α-lactose powder and observation of an increase of plasma glucose of 35 mg/dL by 90 minutes.139 It has been postulated that agents such as rotavirus that damage epithelial cells may cause prolongation of the diarrhea because of temporary lactase deficiency (lactase is produced in mucosal cells). Lactase and cellulobiase are present at birth and decline after 4 months of age.140
Foal Heat Diarrhea
Diarrhea developing during days 5 to 14 of life has been termed “foal heat diarrhea” because of the time relationship to the occurrence of postfoaling estrus in the mare. Diarrhea has developed in foals in this age group that have been raised separated from the dam on a consistent diet and isolated from pathogens, so it does not appear to be causally related to estrus. There is no demonstrable change in the composition of mare’s milk during this time period.141 S. westeri has been investigated and is not the causative agent of foal heat diarrhea.129 The most likely cause of foal heat diarrhea is the establishment of normal flora in the hindgut. Foal heat diarrhea is typically preceded by coprophagy 2 to 3 days before the onset of diarrhea.142 Classic foal heat diarrheas are mild and require no specific therapy. Continued diarrhea, fever, or depression with signs of reduced sucking activity on the mare should raise concern about other causative agents and should prompt appropriate diagnostic testing and treatment.
Clinical Pathology and Diagnostic Imaging
In addition to the fecal tests described under specific causes, tests for signs of systemic involvement are indicated in foals with frequency and amounts of diarrhea that may produce dehydration or in foals with fever and depression and/or loss of suck reflex. CBCs may indicate toxemia or systemic sepsis with neutropenia and shifting to immature forms. Computation of a sepsis score is indicated in foals with diarrhea that are less than 7 days of age. Assessment of renal function through BUN, creatinine, and urinalysis may reveal renal azotemia of prerenal or renal origin and warrant prolonged fluid therapy. Assessment of electrolyte and acid-base balance is warranted in neonatal diarrheas, because hyponatremia caused by losses in the GI tract and renal compromise can be significant. Prolonged diarrhea can lead to significant metabolic acidosis, requiring fluid and bicarbonate replacement (see p. 355). Fecal culture and determination of fecal leukocytes and occult blood are additional diagnostic tests that may indicate a more severe disease condition. Fecal leukocytes are common in diseases caused by enteroinvasive pathogens, such as Salmonella. Ultrasound and radiography are also helpful at differentiating enteritis or colitis from other causes of colic or abdominal distention.
Treatment and Prognosis
The three main components of therapy for diarrhea in the neonatal foal consist of (1) fluid therapy (either oral or intravenous), (2) intestinal protectants and adsorbents, and (3) antibiotics if indicated to treat suspected bacteremia or clostridiosis. Sodium-containing isotonic intravenous fluids are an important component of diarrhea therapy in the compromised neonate. Potassium is lost in severe diarrhea and, if hyperkalemia is not present, should be supplemented by adding 15 to 20 mEq/L of KCl to fluids. Foals that are not nursing normally may have hypoglycemia and may need glucose-containing fluids. Acid-base correction by volume expansion and replacement of bicarbonate can be lifesaving and blood pH and calculated bicarbonate concentrations should be monitored frequently when significant intestinal fluid losses occur.
In general terms, milk should not be withheld from foals with diarrhea. The clear exceptions are foals with colic and those with bloody diarrhea. The foal can be muzzled for 8 to 12 hours while the mare is milked out, and the foal provided oral fluids through stomach tube or a bottle if a suck reflex is present. Although labels of electrolyte replacers do not always specify “for use in foals,” many preparations used in calves (see Table 20-9) have been used successfully in foals. Most of these preparations provide insufficient energy and should be used for short intervals of no more than 24 to 36 hours unless PN of some type is provided to maintain blood glucose levels.
Intestinal protectants may be all that are required in uncomplicated cases or may be used in conjunction with other therapies. Bismuth subsalicylate, kaolin or pectin, and activated charcoal have been used for this purpose. Suggested advantages of bismuth subsalicylate90 are its neutralization of bacterial toxins and antisecretory effect through its local antiprostaglandin activity.143,144
Systemic antibiotics should be used in the neonate with diarrhea that may be septicemic or may have compromised immunity. Blood cultures (see Chapter 18) should be obtained before initiation of antimicrobial therapy. Antibiotics with a spectrum against gram-negative and gram-positive organisms should be used. In general terms, renal toxicity associated with aminoglycoside use is uncommon in clinical practice. The clear exception are foals that are dehydrated, most commonly because of ongoing losses through diarrhea. Consequently the monitoring of renal function is indicated when potentially nephrotoxic drugs are used.
Plasma therapy for hypoproteinemia associated with FPT or protein-losing enteropathy is useful to maintain plasma oncotic pressure and expected protein binding of medications. Diarrheic foals with albumin levels below 2 g/dL or total plasma protein levels less than 4.2 g/dL may benefit from plasma therapy.
Prevention and Control
Prevention is best accomplished by minimizing density of populations of horses, separating of age groups, providing appropriate sanitation and hygiene (see Chapter 46), and obtaining adequate colostrum of good quality (see Chapter 53).
LAMENESS AND RELUCTANCE TO WALK
INFECTIOUS LAMENESS
Etiology
Septic arthritis, septic physitis, and osteomyelitis as a complication of or sequel to bacteremia produce lameness and reluctance to move. Terminology to describe this condition has included “joint-ill,” “navel-ill,” septic physitis, septic polyarthritis, and septic epiphysitis.145 Blood-borne bacteria from a previous illness or concurrent with an active nidus of infection produce infection in synovial membranes, growth plates, or periarticular bone. Sources of infection include primary bacteremia with or without FPT146 (see Chapter 53), pneumonia, umbilical infection, enteritis, and extension of local infection from penetrating wounds. Thirty eight of 140 foals with confirmed septic arthritis demonstrated evidence of umbilical disease. In foals, bacteria that produce septic arthritis include the causative agents of systemic sepsis: E. coli, Klebsiella species, Actinobacillus equuli, Salmonella species, R. equi, and Streptococcus species.146 A review of 78 foals with septic arthritis ranked the frequency of isolation of bacterial agents. Gram-negative bacteria were cultured from 51 of 78 foals. In addition, the probability of susceptibility of these isolates to various antibiotics was determined. Blood culture produced more bacterial isolates, although joint aspiration yielded bacteria in 69% of foals sampled.147 Bacterial species cultured from blood and synovial fluid were identical in 16 of 88 foals. Negative cultures occurred in 31% of foals with later confirmed septic arthritis.147 Gram-negative bacteria were more common in younger foals, and gram-positive infections became increasingly more common with advancing age of the foal.147
Clinical Signs and Differential Diagnoses
Signs may be extremely variable. Sudden onset of lameness in one leg in an apparently healthy neonate with or without joint distention, pain, or edema may be noted. Other presentations may be observed, consisting of sudden onset of lameness with systemic signs of illness or evidence of multiple joint distention, pain, and edema in a neonate with obvious illness and a diagnosis of septicemia. Prematurity or FPT should raise the index of suspicion. The chief differential diagnosis is trauma; lameness is often attributed by the owner to the dam’s stepping on the newborn. Any neonate less than 45 days of age with sudden onset of lameness should be considered infected until proven otherwise.
Clinical Pathology and Radiology
Diagnosis may be obvious, with signs of septicemia, a positive sepsis score, and a swollen, hot, and painful joint. Peripheral leukocyte counts, rectal temperature, level of alertness, and appetite may be normal with localized infections. Joint aspiration may reveal normal synovial fluid if the infection is in the early stages of synovial membrane inflammation or physeal or bone involvement.145 Synovial fluid with greater than 10,000 WBCs/μL and greater than 70% neutrophils indicates that infection is likely.146 Cytology and Gram stain of the synovial fluid may further aid diagnosis. Thin, turbid, or brown synovial fluid with increased leukocytes is considered evidence of infection.145 Culture of synovial fluid is often negative, even when infection is present. Improved recovery of bacteria may be obtained with thioglycolate broth or brain-heart broth with an agar slant and sodium polyanethole sulfonate (SPS) to prevent clotting and inhibit aminoglycoside and trimethoprim antibiotics.148 Normal synovial fluid does not rule out septic physitis or osteomyelitis.145 Careful examination of high-quality radiographs is important for the detection of bone lysis in cases of osseous infection. Initial radiographs may be normal because the degree of damage is often not detectable for 10 to 14 days after initial infection occurs.145 Radiographic features of septic arthritis include soft-tissue swelling, widening or collapse of the joint space, osteoporosis, and osteosclerosis. Repeat radiographs taken at 3- to 7-day intervals are valuable for assessing the degree of damage if lameness persists.145 Ultrasound may be used to confirm involvement of the joint and rule out periarticular or tenosynovial infection to avoid iatrogenic contamination of the joint during arthrocentesis. Joint distention and hyperechogenic fragments in the synovial fluid are suggestive of septic arthritis. Normal synovial fluid is anechoic. Bone scans or CT may facilitate early detection.
Pathophysiology
Hematogenous spread of bacteria resulting in bone infection may follow a variety of pathways.145 A nidus of infection may develop at the junction of cartilage and subchondral bone. The low pressure, slow flow, and reduced oxygen pressure of the blood supply at cartilage-bone junctions may predispose to establishment of infection in these areas. Level of immunity and degree of maturation of bone of the foal may be additional predisposing factors.145 Destruction of the epiphysis and extension of infection into the joint may be primary in some cases, rather than starting as a primary synovial membrane infection that spreads to the epiphysis and physis. A classification has been proposed to reflect the various pathogeneses of infection (see Box 19-2). Infection of the small bones of the tarsus has been reported to be more common than that of the carpus,149 and the metaphysis of ribs and vertebral bodies may be involved.146 Synovitis produces severe inflammation and depletion of the cartilage matrix and collagen framework, which can cause irreversible damage.145,146 Eburnation of cartilage leads to exposure of subchondral bone and extension of infection in bone.145
Treatment
Aims of treatment are to remove the infectious agent, protect and minimize cartilage damage, and minimize secondary osteoarthrosis. If partial or complete FPT has occurred, an additional aim is to provide immunoglobulins through plasma transfusion (volume dependent on IgG concentration, but 2 or more liters IV may be required).145 Treatment described for bacteremia should be used promptly. Umbilical ultrasound should be performed, because many infections may be not be visible externally. Systemic antibiotics provide adequate levels of antibiotic in normal and inflamed joints.145,150 Antibiotics or antibiotic combinations with both gram-negative and gram-positive spectrum should be used initially, with selection modified by culture results. Ampicillin or a first-generation cephalosporin in conjunction with amikacin or gentamicin has had a good probability of antimicrobial sensitivity.147 The third-generation cephalosporin ceftiofur may be used as monotherapy. Antimicrobials (antibiotics) should be continued for at least 3 weeks. Joint lavage with 1 to 3 L of balanced polyionic buffered (pH-adjusted) fluids helps to remove bacteria and inflammatory mediators that damage cartilage and improves outcome.147 An infected joint is a medical emergency, and treatment should be carried out immediately after clinical diagnosis of probable sepsis, even before all cultures and clinical pathology test results are back. Arthrotomy or arthroscopic lavage for joints with fibrin and debris has been advocated and appears effective.148,151 Delivery of antibiotics to chronically infected tissues via regional limb perfusion or intraosseous infusion has been described.152 Confinement and limb immobilization may decrease pain, inflammation, and cartilage damage. Short-term use of nonsteroidal antiinflammatory drugs such as flunixin or phenylbutazone at prescribed dosages may be indicated, although the risk that these agents will induce gastric ulceration in foals is well described.
Prognosis
Duration, extent of bone involvement, and degree of damage affect the prognosis. A recent study determined that 79% of foals with septic arthritis had IgG levels less than 8 g/L (800 mg/dL). Infection of multiple joints, delay in onset of treatment, and presence of concurrent bony lesions on radiographs were associated with a poorer prognosis.147 In one study, 67% of foals with septic arthritis were discharged when treatment was initiated within 24 hours of the onset of clinical signs.147 Better outcomes were observed when treatment included joint lavage. Of foals treated for septic arthritis, 26% went on to perform their intended function.
If FPT has occurred and multiple joints are involved, the prognosis is poor because, in foals with FPT and lameness, multiorgan involvement is common.146 Initial detection should warrant a guarded prognosis. The long duration of treatment and sometimes costly multiple therapeutic modalities should be discussed with the owner at the outset. Reevaluation of the patient at regular intervals is indicated. Recurrence after cessation of therapy sometimes occurs.
NONINFECTIOUS LAMENESS
Rhabdomyolysis in foals deficient in vitamin E and selenium may be precipitated by stress such as septicemia. Affected foals are reluctant to move, paretic, and occasionally dysphagic. Pelvic limb muscles are often palpably firm. Elevated serum creatinine kinase and electrolyte disturbances of hyponatremia and hyperkalemia may be observed.153
In foals, rupture of the common digital extensor tendon may be present at birth or may develop within a few days of life. Rupture occurs within the carpal synovial sheath and produces swelling over the dorsolateral surface of the carpus at the level of the intercarpal and carpometacarpal joints. Palpation of the fibers reveals tearing of the tendon. Splinting of the limb for 3 to 4 weeks usually results in healing.154
Contracture of joints or tendons of the limbs produces difficulty in movement and predisposes to FPT by impeding the ability to adequately nurse. Degree of contracture varies from mild to severe and may be associated with scoliosis and/or torticollis.155 Foals with congenital contracture of tendons of the front limbs may spontaneously rupture the common digital extensor tendon. Conservative therapy consisting of splinting the front limbs to induce tendon laxity may be helpful.
Developmental Causes
Incomplete ossification of the cuboidal bones (see Fig. 19-1) of the carpus and tarsus of newborn foals is considered to be related to the development of flexural and angular deformities of the foal.156 Twins, premature foals, foals that are small for gestational age, and foals with in utero acquired infection are likely to have incomplete ossification of cuboidal bones at birth.156,157 Radiographic analysis and grading of the degree of ossification have been suggested for these foals.157 Foals with substantially reduced ossification may damage bone with limb loading. Limited exercise may be prudent until ossification begins to increase radiographically, which can be within 7 days. Hypothyroidism (see Chapter 41) has been identified in foals with angular limb deformities, contracted tendons, and tarsal bone collapse.158,159
PATENT URACHUS, OMPHALITIS, AND OTHER UMBILICAL ABNORMALITIES
PATENT URACHUS
Patent urachus is a persistence after birth of the tubular connection between the bladder and umbilicus. The urachus drains the bladder into the allantoic sac during gestation. Urine flow should gradually change, with some urine entering the amniotic sac through the urethra in later gestation. At birth, with umbilical cord rupture the urachus should be closed, and urine should be voided through the urethra. Foals with a patent urachus may dribble urine from the urachus during or after urination or may simply have a constantly wet umbilical stump.
Etiology
Various causes have been suggested for failure of the urachus to close and completely involute. Early severance or ligation of the umbilical cord, inflammation, infection, and excessive physical handling of the neonate have been implicated.160 Rather than being the original cause for hospital admission, patent urachus develops as a complication of hospitalization in a significant percentage of foals in neonatal intensive care. Weakness of abdominal musculature may contribute to the problem in sick foals.
Clinical Signs and Differential Diagnosis
Differential diagnoses include concurrent infection of the navel (omphalophlebitis). Ultrasound may assist the diagnosis and determine the involvement of umbilical arteries or vein.161 Moist hairs around the umbilicus and visualization of fluid coming from the navel are diagnostic. Ultrasound examination of the internal structures of the umbilicus is strongly recommended.
Clinical Pathology
Identification of concurrent infection is essential. A complete physical examination should be performed. If abnormalities are noted, serum IgG, CBC, and urinalysis are helpful for detecting susceptibility to infection and presence of systemic or urinary tract infection.
Pathophysiology
Congenital patent urachus caused by excessive torsion on the umbilical cord in utero occurs in 6% of normal foals.162 The obstruction of the urachus caused by the torsion causes retention of urine in the bladder and overdistends the proximal urachus, which interferes with normal involution.162 Infection of umbilical structures or the urachus itself may result in inflammation and failure to completely involute. In a review of 16 cases of umbilical cord infections in foals, 13 of the foals had patent urachus.163 The majority of these foals had acquired patent urachus after birth, with the youngest age of onset being 3 days and the mean age of onset being 12 days. Excessive manipulation and improper lifting of the foal’s abdomen in the presence of high urethral sphincter tone may force urine within the bladder out into the involuting urachus. In our experience, farms have experienced outbreaks of patent urachus when procedures (such as tests for FPT) have been implemented that require handling of foals in the first 12 to 24 hours of life. A similar cause may be responsible for the increased incidence of patent urachus in hand-reared calves.
Treatment and Prognosis
Therapy consists of either conservative management through monitoring or medical treatment for infection and cauterization of the urachus with iodine, phenol, or silver nitrate sticks applied into the urachus. Persistence of urine dribbling despite cauterization, the detection of involvement of other umbilical structures through ultrasound, and a rent in the urachus that produces subcutaneous swelling are indications for surgery. Not all foals that have persistent patent urachus have an infected umbilicus. Use of general anesthesia and removal of the entire urachus to the tip of the bladder are performed in foals with an infected or enlarged urachus. Associated arteries and veins should be ligated and removed if they are infected or necrotic. Merely ligating the exterior stump can trap organisms and cause infection. In our neonatal unit the majority of patients with acquired patent urachus respond to conservative therapy. Late-onset patent urachus (5 days of age) may be more refractory to conservative therapy.163 Complications are uncommon but may include bladder necrosis and uroperitoneum caused by extension of infection and inflammation of the urachus.
Prevention
Allowing the umbilical cord to rupture without ligation or the careful use of specific umbilical clamps after birth has been suggested to decrease the incidence of patent urachus. Minimum handling of neonates and careful restraint may prevent pressure buildup in the bladder and subsequent patent urachus.
OMPHALITIS AND OMPHALOPHLEBITIS
Definition and Etiology
Omphalitis is inflammation of umbilical structures that may include the umbilical arteries, umbilical vein, urachus, or tissues immediately surrounding the umbilicus. The umbilicus consists of three types of structures and undergoes functional and anatomic changes at birth. Two umbilical arteries connect internal iliac arteries to the placenta. These later regress and become the round ligaments of the bladder. One umbilical vein connecting the placenta to the liver and porta cava regresses to become the round ligament of the liver within the falciform ligament. The urachus connects the fetal bladder to the allantoic cavity.
Umbilical abscess or infection of any of the three components of the umbilicus may produce local infection or be a source of septicemia. The source of infection is most commonly the external environment, coupled with FPT. Omphalophlebitis may extend the length of the umbilical vein into the liver and result in liver abscessation.
Clinical Signs and Differential Diagnosis
When the umbilicus is enlarged and draining purulent material, infection is easily noted. In other cases the umbilicus may be dry and larger in diameter than expected. In addition, neonates may have a completely normal-appearing, dry external navel and be severely ill from infection of the urachus, umbilical arteries, or vein. In a neonate with sepsis without external signs of infection, involvement of the umbilicus can be difficult to determine. The presence of pain on palpation of the umbilicus indicates inflammation. Ultrasound aids in the detection of involvement of the urachus or arteries and vein.161 The umbilical area of neonates less than 20 days of age with fever of unknown origin should be scanned. Hematoma developing after umbilical rupture may produce distention of the umbilical stump shortly after birth.
Overt signs of infection include heat, swelling, purulent discharge, or pain. Concurrent signs of systemic infection such as joint infection, pneumonia, diarrhea, meningitis, or uveitis may be noted. Infection in more than one umbilical vessel in the neonate is common, and urachal involvement is frequent. Umbilical abscessation that is walled off and does not involve deeper structures is a less severe problem and may be treated with drainage without surgical removal of the entire umbilicus. The depth of involvement may be determined by standing behind the neonate and pressing the hands together above the umbilicus to detect internal masses and painful areas.
Diagnostic Methods
In addition to detection of overt umbilical inflammation as described, ultrasonography may aid in evaluating a normal-appearing navel.164 The umbilical vein, arteries, and urachus may be imaged in the newborn (see Chapter 18). The umbilical arteries leave the umbilical stalk and course on the outer edges of the urachus in a parallel fashion.164 In the foal the urachus connects the apex of the bladder with the umbilicus and is located along the midline immediately adjacent to the body wall. Persistent dilation of the umbilical vein or arteries with a hypoechoic-to-echogenic fluid is seen with infection. If ultrasound is performed by a skilled ultrasonographer familiar with normal ultrasonographic findings of the umbilicus, there is an excellent correlation between surgical and ultrasound findings.164
Treatment and Prognosis
Early treatment with antibiotics and supportive care as described for the septicemic foal (see Chapter 18) may allow resolution before development of abscessation and distention of the urachus or the umbilical arteries and vein. Established infection, which may occur within 24 hours, may necessitate surgical removal of involved structures in addition to medical therapy.163 When omphalophlebitis extends into the liver, the umbilical vein may be marsupialized to facilitate drainage and flushing. The prognosis is very good when adequate passive transfer of colostral immunoglobulins has occurred and when joints or other structures are not involved. Sequelae such as renal abscessation, joint or bone infection, peritonitis, and other complications described for septicemia may develop if therapy is started too late or discontinued prematurely.
ANEMIA AND ICTERUS
Definition and Etiology
Anemia in the neonate should be interpreted in the context of the realization that normal hematologic values of the neonate may vary from those of the adult. In foals, values of hemoglobin and PCV are similar to those in adult horses but decrease during the first weeks and months of life to below those in adults.165 Foals have low iron stores during the first 5 months of life; this is reflected in decreased serum ferritin concentration, increased serum total iron binding capacity, and decreasing mean corpuscular hemoglobin concentration.166 Microcyte production is observed rapidly after birth.166 Absolute RBC and total blood volume decrease at between 2 days and 2 weeks of age and then progressively increase.167
In addition to frank blood loss from an injury, diseases causing anemia in the neonate include NI, non-NI immune-mediated hemolytic anemia, blood loss caused by gastric ulcer or ovarian cyst rupture producing hemoperitoneum, anemia of chronic disease associated with localized infections, piroplasmosis, and equine infectious anemia.
Clinical Signs and Differential Diagnosis
Rapidly developing anemias such as those associated with NI produce signs of weakness, pale or jaundiced mucous membranes, fever, and depression. Hemoperitoneum produces weakness and pale mucous membranes. Suspected drug-induced, immune-mediated hemolytic anemia and thrombocytopenia have been reported in the foal.168 Intestinal parasitism does not normally lead to anemia during the neonatal period. Chronic localized infection may produce anemia of chronic disease.
Clinical Pathology
Intravascular hemolysis may produce hemoglobinuria and hemoglobinemia. Icterus develops when the ability of the liver to conjugate bilirubin is exceeded. Mainly, indirect bilirubin is elevated. Anisocytosis is observed in responsive anemias. Nonspecific stimulation of bone marrow may produce a leukocytosis.
Therapy
Determination of the nature of the anemia may allow specific treatment. NI is discussed in Chapter 53. Drug-induced or autoimmune anemias may be treated with corticosteroids (0.05 to 0.1 mg of dexamethasone per kilogram twice a day IM or IV). Blood transfusion after cross-match may be indicated when anemia develops rapidly or PCV drops below 14%. Massive red cell destruction may trigger disseminated intravascular coagulation, and the actual cause of death may be a result of activation of the clotting system by RBC destruction and reticuloendothelial system removal.169 Associated conditions such as metabolic acidosis and hypoglycemia should be corrected. Anemia of chronic disease requires correction of the primary disease condition.
FEVER
Definition and Etiology
Fever (rectal temperature >38.9° C [102° F]) as a clinical sign must be interpreted differently in neonates than in the adult because of the variations of anticipated response to systemic illness, temperature regulation control differences, and susceptibility to environmental changes in temperature. In foals with septicemia, fever (> 38.9° C [102° F]) was present in fewer than 30% of cases, and hypothermia was noted in approximately 20%.56 Consequently fever is considered an unreliable clinical sign for determination of sepsis in neonates. Older foals with localized infection such as in joints or bone are more likely to have fever.
Differential Diagnosis
The chief differential diagnoses for fever in neonates are fever caused by infections with viral or bacterial pathogens, seizures with subsequent generation of heat by muscular overactivity, pyrogens generated from hemolysis in NI, and environmentally induced hyperthermia. The condition of transient tachypnea of the newborn may produce significantly elevated temperatures in warm environments.170 Neonatal foals have a rapid respiratory rate that appears to be an attempt at heat loss through a panting type of mechanism. Extreme care must be used in attributing the pyrexia and fever to transient tachypnea syndrome alone by ruling out the presence of infection through physical examination, chest radiographs, blood gases, and computation of a sepsis score. Pathophysiology is similar to that in the adult and is discussed thoroughly in Chapter 4.
Treatment and Prognosis
Although a conservative approach to fever in older animals may be appropriate, the presence of a fever in a neonate warrants rigorous diagnostic evaluation and aggressive therapeutic intervention. The immaturity of the neonatal immune system, the high fatality rate, and the frequency of devastating sequelae to bacterial infection warrant a complete examination of the neonate.
Because fever may be beneficial to the animal, the need to administer antipyretics to the febrile neonate is controversial. Body temperatures lower than 40.8° C (105.4° F) are not considered detrimental unless they are associated with heat stroke or seizures,171 in which case cooling and antipyretics are indicated. Because many antipyretics are antiprostaglandins that can cause deleterious GI and renal effects, these agents should be used judiciously. Dipyrone is often used in neonates in our clinic for antipyretic response because it lacks most of the adverse GI side effects found with most nonsteroidal antiinflammatory drugs. Correction of the initiating cause and maintenance of fluid balance are also important. Prognosis depends on immunoglobulin status and stage of disease when treatment is initiated. Significant fatality rates occur in neonates with bacterial infections. Transient tachypnea has an excellent prognosis, with neonates becoming normothermic within 2 to 3 weeks of age. Clipping a long and thick haircoat, using fans in stalls, and removing the animal from direct sunlight may reduce heat stress to the neonate.
CYANOSIS
Definition and Etiology
Cyanosis is the purple-blue coloration observed on mucous membranes or skin caused by reduced or poorly oxygenated hemoglobin in blood.172 Causes for this condition include congenital heart disease, respiratory impairment, or any circulatory condition that produces a right-to-left shunt. The degree of cyanosis depends on the arterial oxygen saturation, hemoglobin concentration, pH, peripheral circulation, and temperature of the neonate.172 Shock and hypothermia are important causes of peripheral cyanosis (see Box 20-4).
Pathophysiology
The affinity of hemoglobin for oxygen is reflected in the standard oxyhemoglobin dissociation curve. This curve is similar for neonates but is affected by the amount of 2,3-diphosphoglycerate (DPG) in the erythrocyte. The foal does not have a fetal hemoglobin but has decreased amounts of 2,3-DPG, which causes oxygen to bind more tightly to hemoglobin and thus to be released in lesser amounts to the tissues.173 Severe hypothermia and acidosis cause the oxygen dissociation curve to shift to the right and therefore contribute to tissue hypoxia. Cyanosis can be either central or peripheral.172 Peripheral cyanosis results from increased peripheral extraction of oxygen from normally saturated blood or a significant decrease in the perfusion to an extremity.172 In the neonate, causes include septic shock and severe hypothermia. Central cyanosis is more common in neonates and is related to congenital heart disease that causes right-to-left shunting or severe respiratory conditions that result in hypoxia. Paroxysmal atrial fibrillation in three foals with cyanosis shortly after birth is described.174
Treatment
Examination and clinical pathologic evaluation for metabolic causes of cyanosis, hypothermia, and cardiac abnormalities should be conducted. History, medication use, auscultation, thoracic radiographs, and arterial blood gases are useful in determining the degree of the respiratory component of cyanosis. Therapy for respiratory causes is discussed in the sections on respiratory distress. Electrocardiography and echocardiography may be required for identification of cardiac anomalies. Circulatory compromise caused by hypothermia, hypoglycemia, and shock requires aggressive fluid therapy, respiratory support, and environmental temperature correction.
OLIGURIA AND STRANGURIA
Definition and Etiology
In the neonate, urination is usually observed within 6 to 10 hours after birth. Frequency of voiding is every few hours. Urine volume produced in the foal is approximately 148 mL/kg/day.175 Urine specific gravity is low (1.001 to 1.012) because of the high water content of milk. Specific gravity readings of 1.018 to 1.025 are approaching maximum in concentrated urine.176
The major causes of slow or painful discharge of urine (stranguria) in neonatal foals are ruptured bladder, bacterial cystitis, urachitis, and reduced urine production (oliguria) resulting from reduced renal perfusion. Pollakiuria, dysuria, and cystitis are complications occasionally observed with urachal abscesses.176
Pathophysiology
Ruptured bladder (see also Chapter 34) occurs most frequently in male foals and is believed to be caused by occlusion of the male urethra during birth, a full bladder, and great pressures during birth when the mare pushes to expel the fetus. Inadequate pressure flow to the kidney producing oliguria may be caused by congenital cardiac anomalies, asphyxia, sepsis, diarrhea, or endotoxemia. Straining from cystitis can be severe and can mimic meconium impaction. Infection of the bladder may be associated with urachal infection. Inappropriate antidiuretic hormone (ADH) secretion occurs in stressed human infants, resulting in decreased urine production and electrolyte abnormalities. During periods of reduced glomerular filtration rate (GFR), drugs excreted by the kidney may accumulate, resulting in toxicity. Inability to excrete a water load associated with excessive fluid therapy may result in fluid accumulation and pulmonary or generalized edema. Uroperitoneum may result in stranguria or oliguria. Postrenal obstruction syndromes are rare in the neonate. Ectopic ulcers have been reported in foals177 as a cause of incontinence and hydronephrosis. A syndrome of apparent pain on attempting the first urination is observed in some male foals; urinary bladder catheterization for 1 to 3 days may resolve the problem.
Clinical Signs
A carefully obtained history of events of the birth and neonatal period is important. Observation of defecation, posture during urination, frequency, and estimated amounts of urination should be noted. Excessive stretching of the front legs, dorsoventral flexion of the back, and colic may be observed with uroperitoneum. Detection of oliguria requires careful observation or catheterization of the bladder to determine presence of urine and amount of urine production. Free catch of urine and examination for WBCs aid in diagnosis of cystitis.
Azotemic neonates with oliguria have signs of depression, dehydration, poor pulse quality, prolonged capillary refill, reduced jugular distensibility, and retracted eyeballs. Elevated levels of serum urea and creatinine may be observed with uroperitoneum, often with concurrent electrolyte abnormalities of hyponatremia, hyperkalemia, and hypochloremia. If the presence of uroperitoneum is suspected, abdominocentesis should be performed. The fluid can be analyzed for potassium and creatinine and compared with serum values. Urea rapidly equilibrates between abdominal fluid and serum. Peritoneal fluid creatinine levels will be 1.8 to 2 times those of serum with uroperitoneum. A syndrome of high creatinine in newborn foals associated with maternal conditions or events of birth and without renal disease has been reported. Serial creatinine determinations reveal a gradual decline toward normal values over several days. Consequently a single serum creatinine determination should not be used to determine prerenal, renal, or postrenal uremia in the foal.
Treatment
Administration of balanced electrolyte solutions such as lactated Ringer’s solution or saline and determination of urine production are important. Specific electrolyte and acid-base disturbances should be corrected slowly. Prolonged ischemia of the kidneys may result in permanent renal parenchymal damage. Lack of urine production after restoration of fluid balance should be an indication for diuretic therapy with furosemide (0.5 to 2 mg/kg IV) or osmotic diuresis with mannitol (0.25 to 0.5 mg/kg IV over 20 minutes and repeated in 4 hours if no response occurs). If adequate urine production has not developed, administration of dobutamine (2 to 10 g/kg/min) or dopamine (2 to 5 μg/kg/min) may be attempted. Treatment of concurrent sepsis, endotoxemia, hypoproteinemia, respiratory distress, or other abnormality should be attempted. If adequate urine flow is not produced, maintenance levels of fluids should be administered to prevent fluid overload. Body weight determinations three to four times a day help to prevent overhydration by detecting fluid accumulation. Urinalysis and clearance calculations may add further insight to the origin and degree of primary renal involvement. Progressive development of uremia and generalized edema is associated with a poor prognosis. When oliguria is present, serial BUN and creatinine determinations should be performed and urine production monitored. Recumbent neonates may be catheterized and urine production quantitated.
HEART MURMUR
Definition and Etiology
Heart murmurs in the neonate may be heard normally before physiologic closing of the ductus arteriosus during the first 1 to 5 days of life. Other causes of murmurs include congenital anomalies, severe anemia, and infectious valvular disease.
Clinical Signs and Differential Diagnosis
Physical examination for other signs of heart disease helps determine the severity of the murmur. Jugular pulse, weak or irregular arterial pulse, and palpable thrill indicate a serious condition. Signs of weakness, cyanosis, and tachypnea are indications of poor cardiac performance. Timing and location of the heart murmur should be determined. The electrocardiogram (ECG) may reveal atrial or ventricular enlargement. Thoracic radiography may aid in determining heart size and in detecting pulmonary edema or distended pulmonary vessels. Echocardiography may reveal atrial or ventricular enlargement, thickened ventricular walls, anomalous orientations of outflow tracts, or ventricular septal defects (VSDs).178
Patent ductus arteriosus (PDA) produces a continuous murmur localized over the left heart base.179 The diastolic component may not be heard with auscultation over other parts of the heart. As pulmonary hypertension develops, the murmur is shortened to a holosystolic type with normal arterial pulse. Large shunting of blood produces a bounding arterial pulse caused by wide fluctuations of systolic and diastolic pressures.179 The ECG is normal unless atrial enlargement is present and increasing QRS amplitudes are observed.178 Radiographs may reveal an enlarged heart with increased vascularity caused by left-to-right shunting of blood. Echocardiography may reveal an increased left atrial and left ventricular diastolic dimension or volume and hyperdynamic septal and left ventricular wall systolic motion (depending on the degree of right-to-left shunt).178 Catheterization and angiography may further delineate the degree of shunting.180 Recent studies have indicated the ductus architecture changes days before birth, which prepares the ductus for closure. Triggering factors for closure include increased blood oxygenation and lower pressures resulting from vasodilation of pulmonary vasculature at birth.
VSD produces a large, harsh, holosystolic murmur that is loudest on the right cranial region of the thorax and is softer over the left heart base.181 The ECG may be normal or may show increased amplitude of the QRS, with larger shunts and alterations in chamber size. Radiography may reveal heart size increase, left atrial enlargement, and dilated pulmonary vasculature.178 Two-dimensional echocardiography may show aortic and septal discontinuity.178 Injection of saline bubbles into the left ventricle and observation of bubbles in the right atrium or ventricle document a left-to-right shunting of blood.178 Tetralogy of Fallot or other types of complex malformations often produce loud murmurs and are associated with cyanosis, weakness, fatigue, and stunted growth.182 Tetralogy of Fallot produces a systolic ejection murmur heard at the left heart base.178 Electrocardiography may reveal negative QRS complexes in leads I, II, and aVF, suggesting right ventricular hypertrophy.178 Echocardiography may reveal a thickened right ventricular wall, septal echo dropout in the area of the VSD, rightward displacement of the aortic root, and an abnormal pulmonary outflow region.178 Saline injection into the jugular vein demonstrates right-to-left flow from the right ventricle to the left ventricle or the aorta.
Treatment
PDA has been treated by chemical closure using indomethacin in human neonates, but it has not been used in veterinary medicine.183 Other global antiprostaglandins, including flunixin meglumine, have been used in attempts to assist with chemical closure of the ductus arteriosus in foals. The efficacy of this procedure has not been determined. Minimal fluid administration is also suggested to be of assistance. VSDs may pose few problems if the degree of shunting is small. Other complex cardiac anomalies producing murmurs may be treated symptomatically for a short time, but the long-term prognosis is extremely poor.