Chapter 39 Diseases of the Eye
OPHTHALMIC HISTORY AND EXAMINATION
Before pursuing a detailed ophthalmic history, it is imperative to document the species, breed, age, gender, coat color, and use of the animal(s) to be examined and to obtain a general medical history. Because ophthalmic diseases of large animals may be genetic, an awareness of breed-related ocular abnormalities is also important.
The primary complaints of the owner regarding the animal’s eye(s) or vision may generally be categorized into one of the following areas of concern:
Additional reasons for obtaining a thorough ophthalmic history and performing a detailed ocular examination are to follow up on a preexisting or previously treated eye condition or to examine the eyes as part of a prepurchase examination. Examinations for inherited eye diseases in horses may be performed by board-certified veterinary ophthalmologists and registration forms submitted to the Equine Eye Registration Foundation.1
OPHTHALMIC HISTORY
A series of questions should be directed to the owner or responsible person regarding the signs observed, the duration and clinical course of the condition, the animal’s ability to function in its normal environment, the existence of previous eye problems, and whether related animals or other animals on the premises have been affected. Potential causes for an ophthalmic or visual problem, including any possible relationship to neurologic or iatrogenic (e.g., drug-induced) disease, toxin exposure, or systemic illness, should be explored. To ensure that the necessary questions are asked in a reasonable sequence, a history form is suggested (Fig. 39-1).
OPHTHALMIC EXAMINATION PROCEDURES
General Inspection
It is optimal to observe the animal’s activities and movements in its normal environment. Before restraining the animal, the examiner should study the animal’s unencumbered movements, posture, coordination, and head carriage. During this initial inspection, the animal’s vision and its response to visual stimuli should also be observed.
As the animal is approached, closer inspection reveals whether facial and ocular symmetry and normal eye movements are present. Signs of ocular pain (i.e., blepharospasm, apparent photophobia, or epiphora) are noted, as well as size and position of the globes and the presence of ocular or nasal discharge, opacities, or masses.
Restraint
Adequate restraint is an essential prerequisite to performing a detailed ophthalmic examination in large animals. Manual restraint of small ruminants and neonates is usually adequate. For most cattle, restraint with a chute, head catch, and halter is essential; restraining the horse with a halter in stocks is recommended. However, chemical restraint may be necessary in cattle and is almost always needed for horses before a thorough examination can be performed. This may consist of a combination of injectable sedative (e.g., xylazine or detomidine for horses), with or without an injectable analgesic (e.g., butorphanol for horses), with auriculopalpebral (and occasionally frontal) nerve blocks using a local anesthetic agent such as 2% lidocaine. A neuroophthalmic assessment, including menace responses and palpebral/pupillary light reflexes, should be done before administration of sedatives, analgesics, or local anesthetics.
Neuroophthalmic Assessment
An evaluation of the integrity of cranial nerves associated with normal ocular function is conducted (see Chapter 8). This includes a rapid assessment aimed at determining the animal’s ability to do the following:
Instruments and Materials
After a general inspection, restraint, and neuroophthalmic assessment, a detailed ophthalmic examination is performed. A few basic instruments and materials facilitate an efficient and thorough examination. These include a focused light source (a 3.5-V halogen rechargeable light source with a Finoff transilluminator is preferred), a direct ophthalmoscope, magnifying loupes, and thumb forceps (blunt-tipped forceps with shallow serrations are recommended). Sterile fluorescein dye strips, tear test strips, culture swabs, physiologic saline solution (flushing solution), topical anesthetic (0.5% proparacaine), and mydriatic solution (1% tropicamide) are often also necessary. For irrigating nasolacrimal ducts, polyethylene tubing (5 French) should be available.
Detailed Examination
For recording results of the ophthalmic examination, use of a standard form is recommended (Fig. 39-2). The detailed examination begins with palpation of the boundaries of orbit for irregularities, asymmetry, masses, or fractures. Next, the globe is retropulsed to assess for increased resistance (indicating a space-occupying mass) and to inspect the anterior aspect of the nictitating membrane (“third eyelid”). Retropulsion should not be done if the cornea is compromised by a deep ulcer or laceration.
Fig 39-2 Use of an ophthalmologic examination form allows the clinician to perform a complete, systematic ocular examination.
At this point, the examiner determines if ocular cultures or tear measurements are desired, because these procedures must be completed before further manipulations are performed and before topical pharmacologic agents are instilled.2 Depending on the clinical signs, severity of ocular disease, and species being examined, viral, bacterial (e.g., Chlamydia, Mycoplasma), or fungal cultures may be indicated. Sterile swabs moistened with saline and appropriate enrichment broth or transport media are applied to the tissue to be cultured (usually cornea or conjunctiva). The moistened tip is placed in direct contact with the tissue surface and the swab rotated by spinning the end of the stem with the fingertips.
The rest of the ophthalmic examination is performed in a darkened area, initiated by directing a focused light through each pupil to establish the presence of a fundus reflex (light reflected from back of eye that normally fills pupil space). By evaluating the fundus reflex in each eye, the examiner may compare pupil sizes, characterize pupillary light reflexes, and assess clarity of the ocular media.
Examination of ocular structures should be performed in a set pattern (i.e., anterior to posterior).3-6 Use of an ophthalmic examination form is helpful in systematically guiding the clinician through the examination and providing a record of examination findings (see Fig. 39-2).
The eyelids are inspected for integrity, position, and movement. Each lid is digitally everted for inspection of the margins, meibomian gland openings, and palpebral conjunctiva. Paresis, malposition (entropion, ectropion), defects, masses, inflammation (swelling, ulceration, exudates), alopecia, foreign bodies, and abnormal lashes are noted.
The nictitating membranes are examined for normal position, integrity of surfaces and margin, degree of pigmentation, and the presence of follicles or masses. To inspect for foreign bodies possibly concealed by the third eyelid, topical anesthetic solution (0.5% proparacaine) is instilled repeatedly onto the ocular surface. Two drops every 20 to 30 seconds for four applications is generally adequate. After topical anesthesia is applied, the nictitating membrane is grasped and manipulated with blunt-tipped, slightly serrated thumb forceps, and both sides are examined for foreign bodies.
Normally the conjunctiva appears moist, glistening, and semitransparent. Signs of conjunctivitis are chemosis (conjunctival edema), hyperemia, and ocular discharge. Color changes of the conjunctiva usually accompany anemia (blanched, pale) or icterus (yellow, amber). Chemosis may indicate severe hypoproteinemia. Conjunctival lesions noted include focal swellings, follicles, adhesions, or masses. The sclera underlying the bulbar conjunctiva is inspected for color, contour, swellings, masses, pigmented areas, or surface irregularities.
The avascular cornea should be smoothly contoured and transparent with a moist, reflective surface. The cornea is examined for irregularities and opacities and for the presence of blood vessels and melanin. Corneal edema appears as a hazy blue corneal opacity and should be characterized as localized or diffuse. With severe corneal edema, the epithelial surface may bulge and bullae (vesicles) may be noted. With corneal suppuration and necrosis, the cornea becomes more densely opaque and acquires a beige, green, or milky appearance. Infectious keratitis is characterized by suppuration and necrosis. Corneal abscesses occur as focal areas of suppuration within the stroma underlying a nonulcerated cornea.
Corneal opacities may also result from focal or diffuse scarring, areas of corneal degeneration or dystrophy, or stretching of Descemet’s membrane from previous elevation of intraocular pressure or previous trauma. Inflammatory products clustered on the corneal endothelium (keratic precipitates) appear as multiple beige or brown foci, usually on the ventral aspect of the corneal endothelial surface. This finding indicates the presence of anterior uveitis.
Lacrimal system examination entails evaluation of both secretory and excretory components. Normal secretions result in a moist, glistening ocular surface. Although not typically performed in large animals, tear test strips may be used to quantify the volume of aqueous tear secretion (see Ancillary Diagnostic Procedures). To examine the excretory components, the upper and lower puncta and nasal openings of the nasolacrimal system are identified. Any overflow of tears onto the face (epiphora) is noted. Causes of increased ocular secretions (e.g., frictional irritants, foreign bodies, corneal ulcers, ocular inflammation) must be ruled out. Causes for stimulation of lacrimal secretions must be differentiated from causes of outflow occlusion, such as congenital atresia and acquired obstruction of the nasolacrimal system.
Fluorescein dye instillation determines if corneal ulceration is present and aids in assessment of nasolacrimal system patency. Passage of dye from the nasal opening of the nasolacrimal duct within 5 minutes confirms patency. Retrograde irrigation of the nasolacrimal duct by inserting a length of 5-Fr flexible tubing into the nasal punctum and flushing with physiologic saline solution may be necessary to differentiate insufficient drainage from excessive secretions.
Intraocular examination begins with evaluation of the clarity and depth of the anterior chamber. Opacities within the anterior chamber include inflammatory products (cells and fibrin), proteins (flare), red blood cells (hyphema), or white blood cells (hypopyon). Suspended or clustered inflammatory materials in the anterior chamber indicate intraocular inflammation (anterior uveitis). Besides the presence of exudates, loss of anterior chamber transparency may result from lens luxation, anterior synechia, or intraocular masses (neoplasia or foreign bodies). Loss of normal anterior chamber depth may result from flattening of the cornea, leakage of aqueous humor, staphyloma formation (protrusion of uvea through the cornea), iris bombé (forward bulging of the iris caused by iris-lens adhesions), or forward displacement of the lens. Increased depth of the anterior chamber may be caused by a protruding cornea (keratoconus) or posterior displacement of the iris or lens.
The iris is inspected for altered contour, pigmentation, mobility, neovascularization, pupil size and shape, and the presence of iridal masses (including the normal granula iridica). Transillumination of iris masses allows differentiation of solid iridal masses (e.g., melanoma) from iris cysts. In animals with lightly colored or spotted hair coats, multicolored irides should be recognized as normal variants. Although uncommon, congenital iris thinning (hypoplasia) may be noted as dark, flat, or translucent areas. The lens-iris interface is best evaluated when the pupil is dilated. Iris membranes, adhesions, or strands should be characterized as congenital (persistent pupillary membranes) or acquired (synechiae or remnants of iris atrophy).
Pupillary openings are evaluated for size, shape, symmetry, movements, and opacities. Direct and indirect (consensual) pupillary light reflexes are assessed. The examiner must recall that pupillary light reflexes are not a test of vision (i.e., abnormal responses may be observed in visual animals, and normal reflexes may occur in nonvisual animals). Pupillary abnormalities that should be noted are inequality in size (anisocoria), abnormal movements (hippus), abnormal location (corectopia), or abnormal shape (dyscoria).
Opacities of the pupil usually result from loss of lens transparency or from presence of intraocular exudates. Obscuration of the pupil space may occur with severe miosis (from acute anterior uveitis), condensation of anterior chamber exudates, or synechiae formation (from chronic uveitis). In animals with normal pupillary light reflexes, complete examination of the lens and structures posterior to the lens (i.e., vitreous and fundus) may be achieved only after dilation with a mydriatic agent such as 1% tropicamide, which usually occurs 20 to 30 minutes after instillation.
Using a focused light, the lens is inspected for a smooth, transparent, convex anterior capsule and normal position (no part of the equator should be visible). When evaluating for lens opacities (cataracts), the examiner should direct the focused light through the axial part of the lens to establish the presence of a fundus reflex. Cataracts may be classified according to the extent to which a fundus reflex occurs; a partial reflex indicates an incomplete cataract, whereas an absent reflex indicates a complete cataract). Focal cataractous changes are observed as dark areas seen within the area of reflected light.
An ophthalmoscope must be used to examine the vitreous and fundus. Using the monocular direct ophthalmoscope, the vitreous should be in focus with a dioptric setting between +6 to +1, and the fundus is usually in focus between +1 and −2 diopters. The vitreous is examined for congenital remnants (retained hyaloid structures) and opacities, including degenerative materials or exudates.
Examination of the fundus begins with identifying the optic disc (papilla) and studying its size and shape. The shape, location, and vascular pattern of the optic disc and the appearance of the fundus vary considerably among species. In ruminants the optic disc margin typically appears irregular and fluffy, indicating myelination of axons entering the optic disc. However, it tends to be horizontally elliptical or kidney shaped and located in the tapetal portion of the fundus.4 An optic disc with extensive myelination may be elevated above the surface of the fundus (sometimes called pseudopapilledema). In ruminants the major retinal arterioles are large and are accompanied by venules that anastomose on the surface of the optic disc. The dorsal arteriole and venule usually intertwine as they course away from the disc over the midtapetum (Fig. 39-3). By contrast, the equine fundus is characterized by a large, pink or salmon-colored, horizontally elliptical or oval disc located in the nontapetum5 (Fig. 39-4). In equidae, multiple small retinal blood vessels extend radially from the margin of the disc, and no anastomotic venules are visible over the optic disc.
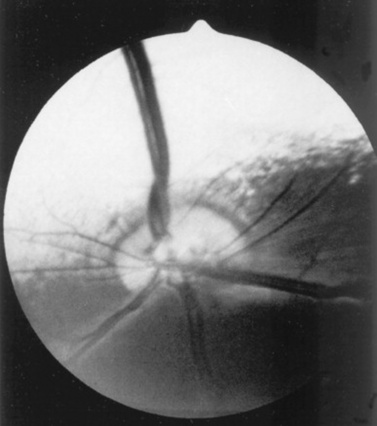
Fig 39-3 Normal ruminant fundus characterized by a large, kidney-shaped, myelinated optic disc. Note that the large retinal arteriole and venule intertwine as they course dorsally.
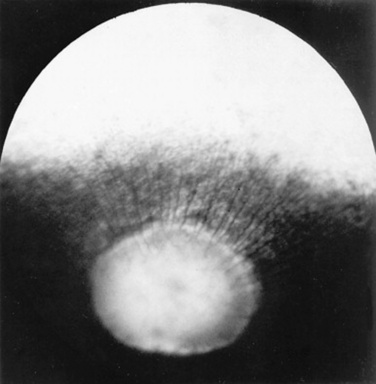
Fig 39-4 Normal equine fundus characterized by a large horizontally elliptical optic disc with numerous small retinal vessels entering (arterioles) and exiting (venules) the margin of the optic disc. Note that the optic disc lies in the nontapetal portion of the fundus.
In both ruminants and horses the fibrous tapetum is penetrated by choroidal capillaries; thus the fundus in these species is typified by dark, stippled foci termed stars of Winslow. Coloration of the tapeta of large animals also varies considerably and may range from gold to bluish green. In animals with heterochromia irides, areas of the fundi may characteristically be devoid of pigmentation and may lack a tapetum. These areas may appear orange or red because of direct visualization of the choroidal vasculature.
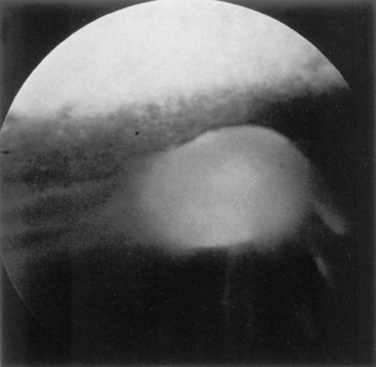
Abnormalities of the optic disc include hypoplasia (micropapilla), elevation (papilledema), depression (cupping), degeneration (atrophy; Fig. 39-5), and vascular changes (e.g., congestion, attenuation, hemorrhage). The tapetal fundus is evaluated for clarity, coloration, pigmentation (Fig. 39-6), and integrity of the retinal vessels (Fig. 39-7). The nontapetal fundus is evaluated for uniformity of pigmentation. Both tapetal and nontapetal areas are assessed for retinal elevations or separations (Fig. 39-8), hemorrhages, degenerations, disorganization (dysplasia), or scleral defects (colobomas).
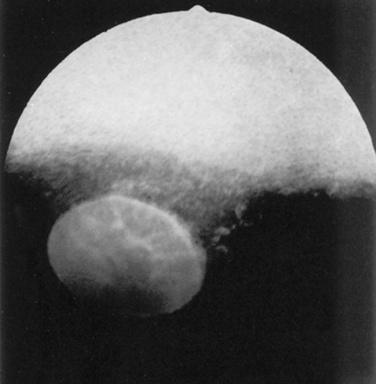
Fig 39-5 Optic nerve atrophy (equine). The margin of the disc is quite distinct because of myelin loss, which is characteristic of optic nerve atrophy. Note absence of retinal blood vessels.
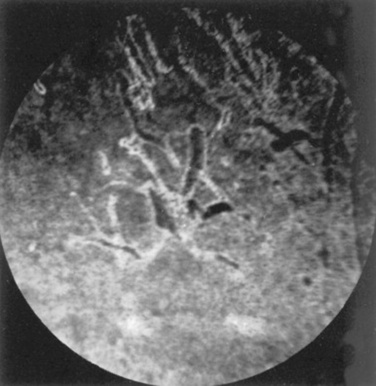
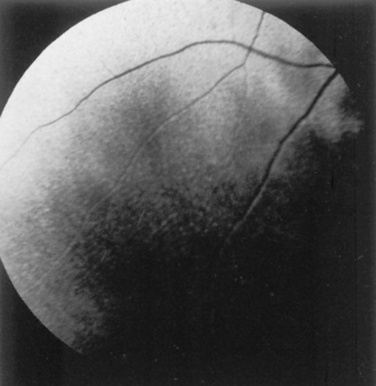
Fig 39-6 Pigmentary changes after traumatic chorioretinopathy. Irregular linear areas of hypopigmentation and hyperpigmentation are present in the tapetal fundus of a horse after ocular trauma. Pigmentary changes reflect retinal pigment epithelial disturbance from previous hemorrhage and edema.
Courtesy Dr. K.N. Gelatt.
ANCILLARY DIAGNOSTIC PROCEDURES
Several additional procedures may form important supplements to the complete ophthalmic examination. Although some ancillary procedures require specialized equipment and expertise, many may be performed in general practice.
Fluorescein and rose bengal are ocular surface stains most often used as aids in diagnosing conjunctival and corneal diseases. The tip of a sterile dye-strip is moistened with saline or eye-irrigating solution, and a drop of the stain is instilled onto the eye. Fluorescein is a water-soluble dye used to detect exposed corneal stroma resulting from an epithelial defect (erosion), stromal ulceration, or descemetocele. The pattern of fluorescein staining for a descemetocele is characterized by a donut-shaped area of positive fluorescence, with the perimeter retaining stain and the center or deepest area (Descemet’s membrane) not retaining stain. Fluorescein may also be used to evaluate the patency of nasolacrimal ducts because an open duct allows the transmission of stain, which may be observed exiting the duct system at the nasal orifice. Rose bengal is retained by devitalized surface cells and is therefore useful in detection of subtle abnormalities such as hyperplastic or desquamating cells associated with ocular surface drying, herpetic infection, or squamous cell carcinoma.
Although tear deficiencies are uncommon in large animals, tear test strips may be beneficial to quantify aqueous tear production in selected cases. A sterile filter paper strip (40 × 5 mm with notched end) is inserted into the lower conjunctival fornix. In large animals it is sufficient to measure the amount of wetting in 30 seconds (≥20 mm is normal).
Cytologic evaluation of ocular surface scrapings or intraocular aspirates may differentiate between inflammatory and neoplastic diseases or in some cases may provide a definitive diagnosis. Orbital aspirates may be diagnostic in cases of exophthalmos caused by neoplasia (e.g., lymphosarcoma). Immunofluorescent testing of cytologic specimens may confirm viral (e.g., infectious bovine rhinotracheitis) or chlamydial infections.
Bacterial cultures taken from the ocular surface or from ocular aspirates, with subsequent antimicrobial susceptibility testing, may be necessary for definitive diagnosis and appropriate treatment of ocular infections. The diagnostic laboratory performing ocular cultures may offer suggestions on culture procedures, including preferred transport media and handling of samples. It is especially important to consult with the laboratory in advance when anticipating culturing for fungi, Mycoplasma, Chlamydia, or viral agents.
Tonometry, a means of measuring the intraocular pressure (IOP), is useful in diagnosing glaucoma (elevated IOP) and uveitis (low IOP) and in assessing response to therapy for these conditions. Digital tonometry (gently indenting the globe through the upper eyelid) provides a general evaluation of IOP, at best characterizing the globe hypotensive, normotensive, or hypertensive. Digital tonometry should not be done if the cornea is compromised by a deep ulcer or laceration. By contrast, applanation tonometry using the Tonopen provides accurate and reproducible IOP readings in large animals and is routinely performed at referral institutions or specialty practices. Schiøtz tonometry is not applicable to large domestic species.
Biomicroscopy, using a portable handheld slit lamp, is useful for identifying the location and nature of anterior ocular opacities. Focal irritants (e.g., ectopic cilia, small foreign bodies) may only be visible with the magnification provided using biomicroscopy. In addition, subtle opacities of the lens and anterior vitreous may only be detected with the use of a slit-lamp biomicroscope.
Funduscopic examination may be performed relatively quickly and easily using the technique of indirect ophthalmoscopy. Monocular indirect ophthalmoscopy is performed using a handheld light source and a separate 20- or 28-diopter focusing lens. Binocular indirect ophthalmoscopy uses a light source mounted on a head band with an incorporated prism and binocular viewing apertures. Binocular indirect ophthalmoscopy provides a stereoscopic, panoramic view of the fundus and is routinely performed by veterinary ophthalmologists. The light sources used for indirect ophthalmoscopy may be adjusted to relatively high intensities and therefore enhance visualization of the fundus through partially opacified or hazy ocular media.
Other ancillary diagnostic procedures include electroretinography, visual-evoked potentials, and imaging procedures (radiography, ultrasonography, computed tomography). Although plain skull radiographs and some contrast studies (e.g., dacryocystorhinography) may be performed in a general practice setting, the remaining procedures require techniques and equipment usually available only at referral centers.
SIGNS OF OCULAR DISEASE
The five major signs of eye disease are as follows:
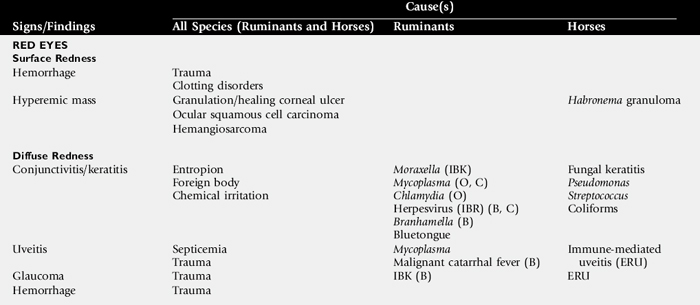
Although any one of these signs alone may be the most obvious evidence of ocular disease, they frequently occur in various combinations. This section provides a general description of the signs and examples of ocular diseases in which a particular sign predominates. Table 39-1 summarizes common signs of ocular disease in large animals.
OCULAR OR PERIOCULAR ASYMMETRY
Ocular or periocular asymmetry results from unilateral changes in anatomy of the orbit, orbital contents, globe, eyelids, or pupils. Such changes often involve reduction or increase in volume of a certain tissue. Reduction in tissue volume occurs with congenital hypoplasia, cicatricial shrinkage, atrophy, or dehydration. Increase in tissue volume may involve the whole globe (buphthalmos) or be characterized by irregular enlargement, as seen with inflammatory or neoplastic lesions involving the globe, orbit, or lids. Asymmetry may also result from neurologic dysfunction. Common examples include reduced palpebral fissure size (secondary to facial nerve paralysis), strabismus, third eyelid protrusion, and anisocoria (see Chapter 8). This section describes a method of approaching the eye examination and of categorizing lesions noted. It is not the intent to describe in detail each of the diseases that may be noted; these are covered in other sections of this chapter.
Forward displacement of the eye (exophthalmos) is often associated with a space-occupying orbital lesion or, less often, a congenitally shallow, underdeveloped orbit. Posterior malposition of the globe (enophthalmos) may result from active globe retraction caused by pain or from loss of supporting retrobulbar soft tissues. Congenital strabismus is a developmental abnormality that results in ocular asymmetry and is typically seen in Jersey, Shorthorn, and Holstein cattle.7
Unequal globe size can also account for ocular asymmetry. A congenitally small globe (microphthalmia) occurs as a genetic defect in cattle and horses.7,8 Microphthalmia is frequently accompanied by multiple ocular anomalies and sometimes is associated with multiple organ involvement. Acquired variations in ocular size usually result from fibrosis and shrinking (phthisis bulbi) secondary to chronic uveitis, or stretching of the globe (megaloglobus, buphthalmos) because of glaucoma.
Asymmetry of the upper or lower lid may occur as a result of entropion, ectropion, blepharitis, conjunctivitis, or facial nerve paralysis (ptosis). Nictitating membrane (third eyelid) protrusion is frequently seen secondary to active retraction of the globe in response to ocular pain, enophthalmos caused by loss of orbital contents (as seen with marked dehydration or malnutrition), presence of third eyelid masses, orbital space-occupying masses, or neurologic disorders (e.g., Horner’s syndrome, tetanus). Pupillary asymmetry, or anisocoria, may occur for a variety of reasons, including Horner’s syndrome, intraocular diseases (uveitis, glaucoma, unilateral retinal lesions), diseases involving the optic nerve or brainstem, and previous use of pharmacologic agents such as atropine that alter iris smooth muscle function.
The presence of an ocular mass may be the primary cause of ocular asymmetry. Ocular surface neoplasms are relatively common in horses and cattle. Ocular squamous cell carcinomas usually arise from nonpigmented tissues of the nictitating membrane, the lateral limbal region, or the eyelid margin. They may appear as irregularly raised surface masses or, less often, as smooth, vascularized lesions that invade the globe. Ulceration, exudation, and mucopurulent ocular discharge are frequent concurrent findings (see Ocular Neoplasia). Periocular sarcoids are also common in horses and appear as firm, raised, nonulcerative lesions.9 Other ocular tumors, such as adenomas, adenocarcinomas, angiomas, angiosarcomas, mastocytomas, and melanomas, occur in large domestic animals but are relatively uncommon. Dermoids and orbital cysts are congenital masses involving the eye or orbit. Other nonneoplastic ocular masses seen in large animals include firm parasitic and foreign body granulomas and soft, fluctuant subconjunctival swelling characteristic of prolapsed periorbital fat. Ocular and orbital pseudotumors have also been described in the horse.10
OCULAR COLOR CHANGE
Changes in the color of the ocular or periocular tissues or the presence of opacities in the clear ocular media (cornea, aqueous humor, lens, or vitreous) are important features of ocular disease. Such changes must be differentiated from normal congenital differences in ocular pigmentation. Developmental color dilution or absence of ocular pigmentation results in light or multicolored irides (heterochromia iridis). When this occurs unilaterally, the resulting appearance may be striking. Examples of abnormal coloration include hyperemia of conjunctival (superficial) or episcleral (deep) blood vessels associated with ocular inflammation (see Red Eyes, Table 39-1), hemorrhage secondary to trauma or coagulopathies, pallor of the conjunctiva, which reflects severe anemia, and yellowing of the sclera and sometimes iris, indicating icterus.
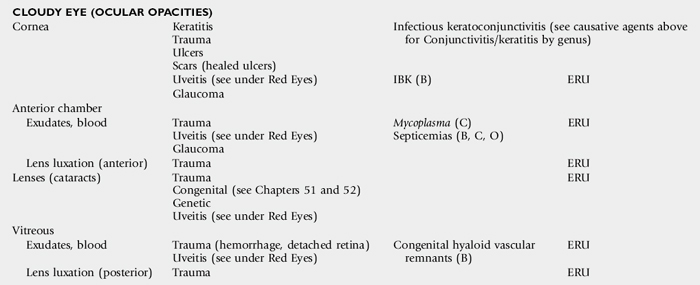
Opacities of the ocular media may occur either as surface (corneal) or intraocular (anterior chamber, lens, or vitreous) phenomena. Sources of corneal opacification include melanosis (“pigmentation”) secondary to chronic exposure, grayish scars from previous episodes of ulcerative keratitis, neovascularization secondary to chronic inflammation, and bluish discoloration caused by corneal edema. These color changes frequently occur in various combinations in more severe keratitis, especially those of infectious origin such as chronic keratoconjunctivitis caused by Chlamydia species, Mycoplasma species, or Moraxella bovis. Cataracts are perhaps the most obvious cause of intraocular opacities in large animals. However, the presence of exudates within the aqueous humor or vitreous, congenital vascular remnants in the vitreous, or retinal detachment may also account for intraocular opacities (see Table 39-1).
OCULAR DISCHARGE
Ocular discharges are characterized as serous (“epiphora”), mucoid (catarrhal), purulent, or hemorrhagic (sanguineous). The type of discharge may be used to aid in determination of the severity and chronicity of the eye disease. For example, serous discharge generally indicates milder forms of eye disease, whereas mucopurulent or hemorrhagic discharge indicates more serious disorders. A notable exception to this generalization is equine recurrent uveitis (ERU), which is a serious and potentially blinding disease but is usually associated with serous discharge (see Immune-Mediated Ocular Diseases). The nature of ocular discharge tends to change as the disease progresses or improves. This is most notable in inflammatory or infectious ocular diseases. Initially, the discharge is predominantly serous; however, it tends to become mucopurulent with chronicity (see Table 39-1).
Epiphora describes facial wetting and results from overflow of tears over the eyelid margin. This may result from excessive secretion of tears or from obstruction of the nasolacrimal system. In large animals, reflex lacrimation with an associated overabundance of tears is the typical response to ocular inflammation (e.g., conjunctivitis, keratitis, uveitis). When epiphora is noted, careful digital and visual examination for foreign bodies within the conjunctival fornix or under the third eyelid is indicated. Epiphora is generally one of the earliest signs of conjunctivitis, ulcerative keratitis, or anterior uveitis. In cattle with keratoconjunctivitis caused by Moraxella bovis, epiphora is present several days before visible corneal ulceration occurs11 (see Infectious Bovine Keratoconjunctivitis).
Developmental defects or malformations of the nasolacrimal duct system (e.g., imperforate puncta) may account for ineffectual outflow of tears in neonates. In these cases the presence of epiphora may be misinterpreted as an overproduction of tears. Previously undiagnosed congenital defects may also be the cause of persistent ocular discharge in adult animals. Acquired obstructions of the nasolacrimal ducts may result from infections, foreign bodies, facial trauma, nasal tumors, or sinusitis that involve the duct system. When nasolacrimal obstruction is present, the nature of the ocular discharge depends on the chronicity of the lesion and the presence or absence of infection within the nasolacrimal system. Whether congenital or acquired, simple nonseptic obstructions are characterized by epiphora. Occlusions with concurrent sepsis result in mucopurulent discharge from the eye or nostril on the affected side. Excessive mucus production is a feature of follicular conjunctivitis, possibly as a result of the rubbing of elevated lymphoid follicles on apposing conjunctival surfaces. Lymphoid follicles are noted in subacute or chronic forms of chlamydial conjunctivitis in sheep and with Onchocerca larval migration in horses. Mucoid ocular discharge may be observed concurrently with epiphora in acute ocular surface infections caused by viral or chlamydial agents. Excessive, tenacious mucus may also result from inadequate secretion of the aqueous component of tears (i.e., keratoconjunctivitis sicca). Although keratoconjunctivitis sicca is not diagnosed as commonly in large animals as it is in dogs, it has been reported in horses, usually as a complication of guttural pouch pathology.12
Purulent to mucopurulent material is the characteristic ocular discharge when bacterial organisms, including Mycoplasma species, are the primary cause of, or secondary contaminants in, ocular disease. Bacterial conjunctivitis occurs frequently in large domestic species and manifests as red eyes with copious amounts of mucopurulent ocular exudate. Ocular foreign bodies and surface masses (e.g., squamous cell tumors) typically have associated bacterial infections. Mucopurulent discharge in the absence of ocular inflammation suggests infection of the nasolacrimal sac (dacryocystitis) or ducts, with reflux of exudate from the lacrimal puncta.
Sanguineous of hemorrhagic discharge most often occurs after blunt or penetrating trauma to the eye (see Ocular Trauma). Foreign body penetration may damage the eyelid, conjunctiva, or globe, resulting in bleeding onto the ocular surface. Corneal ulcers may rupture and result in uveal prolapse and subsequent hemorrhage on the ocular surface. Ulcerative conjunctivitis from abrasion or infection may result in bleeding into the tear fluids. Similarly, ocular surface tumors may become ulcerative and cause bloody ocular discharge. Whenever blood is noted on the surface of the eye, it is imperative that a thorough ophthalmic examination be performed to determine the cause and to evaluate integrity of the globe.
OCULAR PAIN
Blepharospasm, epiphora, apparent photophobia, and periocular hyperesthesia are signs of ocular pain. Animals with severe ocular pain usually resist manipulation of the eyelids or any form of ocular examination by persistently jerking the head away from the examiner and by closing the eyelids tightly. In cases of persistent ocular inflammation, discomfort and pruritus may be manifested by rubbing and self-trauma to ocular or periocular structures.
Ocular pain may result from blunt or penetrating trauma. Corneal ulceration and uveitis are painful sequelae to ocular trauma. Limbal (scleral) ruptures from blunt injury or penetrating lacerations of the fibrous tunic may result in uveal prolapse (staphyloma), which is extremely painful. Periocular trauma may cause eyelid swelling or paresis with exposure and drying of ocular surface tissues, resulting in painful ulcerative keratitis. Inflammatory diseases of nontraumatic origin (e.g., infectious keratoconjunctivitis, ERU) may also cause severe ocular pain in an affected animal. Other causes of ocular pain include frictional irritation, resulting from entropion, trichiasis, distichia, or ectopic cilia, or direct irritation of the ocular surface by foreign material. Foreign bodies causing ocular irritation in large animals are typically plant materials such as seeds, hay stems, straw, twigs, bark, or thorns, although particles of sand or soil can also cause severe ocular irritation. Nonembedded particulate matter is usually entrapped by mucus and washed out of the eye by reflex tearing; therefore it typically results only in transient discomfort. By contrast, embedded foreign material (i.e., between ocular surface layers or within ocular tissues) causes persistent ocular pain.
BLINDNESS
Visual deficits in large animals manifest in a variety of ways. Obvious signs include bumping into objects in the path of locomotion and being unable to respond to visual stimuli such as light or hand motions. Other signs of blindness are reliance on stationary objects, such as fences, railings, or other animals, to maneuver within the environment. Behavioral changes include reluctance to move or to venture into unfamiliar areas. The nonvisual animal is frequently found standing isolated from the group. Searching nystagmus is also seen in some animals with congenital blindness.
Nonvisual animals attempt to compensate for loss of vision with their other senses, resulting in behaviors that seem peculiar. For example, as an apparent overcompensation for visual deficits, the blind animal may raise its head extremely high with the ears erect at the slightest auditory stimuli. A similarly dramatic response to olfactory stimuli may be noted in affected animals when snorting or intensive sniffing associated with nervousness and maximum neck extension is observed. Frequently, blind animals will show exaggerated elevation of the limbs while walking. This must be differentiated from true hypermetria (see Chapter 8). Partial loss of vision may be difficult to determine, and detection depends on observing more subtle behavioral changes such as slight head cocking or tilting, difficulty maneuvering in dim light, or shying and startling from objects on one side or objects present in some specific part of the visual field. Animals may effectively compensate for congenital blindness or slow diminution of vision, particularly when they remain with other unaffected animals in a familiar environment. Visual disturbance may not be apparent until an affected animal is isolated or moved to an unfamiliar area.
There are numerous causes of blindness in large animals, including those that involve only the visual system and some that involve other nervous system tissues or are multisystemic (Box 39-1). A functional approach to blindness involves anatomically classifying the cause as one of the following:
 Failure of the central nervous system (CNS), including the optic nerve, to transmit or assimilate the visual stimuli appropriately.
Failure of the central nervous system (CNS), including the optic nerve, to transmit or assimilate the visual stimuli appropriately.Box 39-1 Lesions and Diseases Causing Visual Deficits or Blindness
OBSTRUCTION OF THE OCULAR MEDIA*
* See Cloudy Eye (Ocular Opacities) in Table 39-1.
Assessment of the pupillary light reflexes (PLRs) aids in the localization of the lesion. Animals with lesions involving the retina, optic nerves, optic chiasm, or optic tracts generally do not have a normal PLR, whereas those with more central (“higher”) lesions involving the lateral geniculate bodies, optic radiations, or occipital (visual) cortex are likely to exhibit a normal PLR (see Chapter 8).
CAUSES OF TRAUMA
Because the eye is anatomically prominent in horses and food animals, it is prone to blunt and sharp trauma, which can range in severity from a mild abrasion caused by a conjunctival foreign body to a severe corneal laceration with globe rupture and orbital bone fracture. Ocular injuries may result from a variety of causes, including foreign materials such as soil, sand, or stones, which may be thrown into the eye during running or by the wind; trauma from disciplinary action; scratches by vegetable matter such as hay, weed stems, tree limbs, or thorns; exposure to chemical irritants; and sudden, violent head movements during training, working, or grooming. Other sources of ocular injury include stanchion, stall or trailer latches, hooks, protruding nails, bucket handles, fencing materials, and other animals, particularly horned ruminants.
Recumbent neonates and animals with CNS disease or severe illnesses that cause depressed mentation often suffer eye injuries from abrasion by debris or bedding materials such as sand, straw, hay, or wood shavings. Such injuries may be prevented by protecting the eye from trauma through the use of a padded hood or soft mats under the head, by keeping the cornea well lubricated in dehydrated animals with reduced blinking frequency, and by administering sedation to prevent thrashing. Following ocular trauma, opportunistic or pathogenic organisms may become established in the wound bed and may cause superficial or deep corneal infections.13 Ocular flora native to the conjunctiva include potential pathogens that may cause severe infections.14
OCULAR EXAMINATION IN CASES OF HEAD TRAUMA
The goals of examination are to determine the degree of ocular trauma and to offer a prognosis for recovery of vision and preservation of the eye. The history should elicit information as to the cause and duration of the injury, previous ocular and systemic disease or therapy, and a description of any recent sedation, anesthesia, or therapy.
Blunt or sharp facial trauma frequently results in damage to the orbit and globe, including fractures or soft tissue injuries of the orbit, corneal abrasions and edema, hyphema, traumatic uveitis, lens luxation, traumatic cataract, vitreal hemorrhage, retinal tear or detachment, corneal or scleral rupture, or proptosis. Therefore, the orbit and globe and vision should be examined as thoroughly as possible when evaluating a patient with facial trauma.
Ophthalmic examination can be performed only after adequate restraint of the head (see previous discussion). Intravenous (IV) sedation, sensory and motor nerve blocks, topical anesthesia, and the use of a halter, twitch (in horses), or nose tongs (in cattle) often are necessary for adequate examination of the traumatized eye.
TRAUMA TO THE ORBIT
Orbital injuries in domestic animals frequently include fractures of the orbital rim and zygomatic arch and damage to the supraorbital process of the frontal bone.15 Fractures of the orbit may be identified by palpation, conventional or digital radiography, computed tomography (CT), or magnetic resonance imaging (MRI). Contusions or lacerations of orbital soft tissues and temporary or permanent neurologic dysfunction also may be present.
Radiographic examination of the bony orbit in large animals is technically difficult and often unrewarding with conventional radiography. Standard lateral and dorsoventral views require powerful radiographic equipment for penetration of bony structures in horses and cattle.16 Large fractures may be identified, but distinct delineation of the bony orbit is difficult because of overlying sinus and nasal structures. An oblique view of the frontal bone is often the most helpful projection.16 This view may be taken with a portable machine because only minimal radiographic penetration of skull structures is required. Outlining the orbit and surrounding bony structures allows identification of fractures, osteomyelitis, with or without bony sequestra, and soft tissue abnormalities, including swelling and radiopaque foreign bodies. If a periorbital sinus is involved in the fracture, subcutaneous emphysema may be present. The use of digital radiology may allow enhanced visualization of the bony structures of the orbit, especially when only subtle changes are present.17 Digital radiography systems used in conjunction with portable equipment may provide adequate detail in standard as well as oblique projections. CT and MRI are available at many referral centers. Detail of the bony and soft tissue structures is greatly enhanced by the cross-sectional views acquired by CT, as are the structures of the calvarium, sinuses, and teeth.17 MRI is also very beneficial in delineating soft tissue abnormalities within the orbit and sinuses.17-19 Immediate evaluation for orbital fractures should include careful examination of the globe. In cases with substantial swelling, ice packs applied to the fracture site may reduce swelling. Systemic antiinflammatory therapy (e.g., flunixin meglumine, ketoprofen, phenylbutazone) may also be given. Systemic antibiotics should be used if sinus involvement is suspected. If the fracture fragments are only minimally displaced, surgical intervention may not be required. Surgical repair should be considered if fragment displacement or entrapment of extraocular muscles has occurred or could occur.16,20 Trauma sufficient to cause fractures may also result in neurologic dysfunction and immobility of the eyelids.
If eyelid movement is impaired, the globe must be adequately protected and lubricated until neurologic function returns. If the globe is only minimally exposed, a sterile ophthalmic lubricant may be used at least three or four times daily. In more severe cases, a nictitating membrane flap or temporary partial tarsorrhaphy may be required. If neurologic dysfunction is permanent and results in significant exposure conjunctivitis or keratitis, partial or complete permanent tarsorrhaphy should be performed.16 Enucleation or enucleation with the placement of an orbital prosthesis may be required for severely affected eyes.
Scleral rupture is another potential consequence of blunt trauma to the orbit and globe. A recent study found that the most consistent clinical sign of scleral rupture in horses is eyelid and conjunctival swelling.21 Other clinical features of this condition are hyphema, subconjunctival hemorrhage, and a collapsed anterior chamber. Ultrasonographic findings include poorly defined scleral margins and echoic/hyperechoic material in the anterior and posterior chambers and in the vitreous.21
Traumatic puncture wounds of the eyelids and conjunctiva may result in orbital cellulitis and exophthalmos in food animals and horses. The onset of swelling may be sudden. Pyrexia and leukocytosis may be present. If a retrobulbar abscess occurs, temporomandibular movement causes extreme pain; the animal may have the eyes partially closed, be off feed, and stand with the neck extended. The client may observe a relatively sudden onset of exophthalmos, eyelid swelling, severe chemosis, and exposure keratitis.16,22 Therapy should consist of systemic and topical antibiotics. Ophthalmic ointments and lubricants should be used to provide protection from desiccation of the exposed cornea and conjunctiva. With time, the cellulitis may organize into a discrete abscess that can be located by palpation or ultrasound. The wound or abscess should be debrided or drained to facilitate healing, especially if a well-organized abscess is present.
Although traumatic proptosis is uncommon, it may occur in horses and food animals. The prognosis for return of vision is guarded to poor, depending on the extent of the damage to the optic nerve and retina. If the globe is ruptured or the extraocular muscles are avulsed, the eye should be enucleated. If the extent of the damage cannot be evaluated initially, the globe should be repositioned, a temporary tarsorrhaphy performed, and the globe reevaluated after 7 to 10 days of topical therapy. Treatment should include topical broad-spectrum antibiotics and atropine. With severe fractures or serious ocular damage, consultation or referral to the appropriate specialist is advised.
TRAUMA TO THE EYELID
Eyelid trauma is frequently accompanied by injuries to other ocular structures. Careful ocular examination should be part of the evaluation of animals with eyelid trauma.23 Injuries may range from swelling (blepharedema) or orbital cellulitis to extensive lacerations and avulsion. Blepharedema may be accompanied by hemorrhage and usually resolves quickly without therapy; however, recovery may be hastened by the use of ice packs and systemically administered ketoprofen or flunixin meglumine.
Horses are particularly prone to eyelid lacerations because of the prominence of the eye and their tendency toward sudden head movements when startled. Lacerations may be divided into those without eyelid margin involvement, those with eyelid margin involvement, and avulsions of part or all of an eyelid. For all types of eyelid injury, several basic principles should be followed. Lacerations should be treated promptly to avoid distortion from excessive swelling, infection, scarring, and loss of function. Lacerated or displaced tissue should not be excised. It is impossible to replace the mucocutaneous junction of the eyelid margin. If eyelid margin is sacrificed, the risk of scar formation and secondary corneal damage is high. The laceration should be thoroughly and carefully flushed with sterile saline and explored to remove all foreign material. In acute trauma, cold compresses may assist in decreasing swelling. In suturing an eyelid laceration, it is essential to preserve the eyelid margin; therefore, eyelid lacerations should be repaired with minimal debridement. In cases of long-standing or infected lacerations, the wound should be packed with an antibiotic dressing for 24 to 48 hours before surgical repair.24 In cases of avulsion of part or all of the eyelid, a variety of blepharoplastic procedures may be performed to help restore functional eyelid margin.23-25
For a more detailed description of the principles of surgical repair of the eyelids, an ophthalmic surgical text is recommended.23,24,26
Postoperative care of all eyelid lacerations should include standard wound hygiene, application of fly repellent and topical ophthalmic antibiotics, and prevention of self-trauma. In contaminated wounds, systemic antibiotic therapy is indicated for 5 to 7 days. Tetanus prophylaxis should be administered.
Improper repair of eyelid lacerations can lead to abnormal function and secondary problems, including chronic epiphora and associated dermatitis, exposure keratitis, ulcerative keratitis, cicatricial entropion or ectropion, conjunctivitis, and pigmentary keratitis.
TRAUMA TO THE NICTITATING MEMBRANE
Lacerations involving the nictitating membrane (third eyelid) should be repaired to avoid irritation and damage to the cornea. This appears to be more important in horses than in ruminants. The margin should be realigned as precisely as possible, and the lacerated conjunctiva should be repaired with absorbable, small suture material such as 5-0 to 7-0 polyglactin.* Topical ophthalmic antibiotics should be used three to six times daily for 7 to 10 days. The entire nictitating membrane should be excised only if it is irreparably damaged.
TRAUMA TO THE CONJUNCTIVA
Conjunctival lacerations result in swelling (chemosis) and hemorrhage. The cornea and sclera should be examined carefully for evidence of lacerations or perforation. If the globe is excessively soft on digital palpation, the anterior chamber is shallow or flat, or hyphema or subconjunctival hemorrhage is present, a concurrent scleral laceration is probable.12,21
Chemosis and hemorrhage frequently resolve without therapy. However, if the chemosis is severe enough to cause exposure and drying of tissues, topical sterile ophthalmic lubricants or antibiotic ointments are indicated to prevent secondary irritation. Conjunctival lacerations rarely require closure unless they are extensive. Subconjunctival hemorrhage sustained during parturition is common in foals and calves and requires no therapy, although topical ocular lubricants or antibiotic ointments are often prescribed.
TRAUMA TO THE CORNEA
Corneal injuries in horses and food animals include blunt compressive trauma, foreign body penetration, ulcerative keratitis, and lacerations. Corneal perforation often results in iris, or iris and ciliary body, prolapse. Therapy is dictated by the type and extent of corneal injury, the complications encountered, the intended use and economic value of the animal, and other financial considerations.
Blunt Trauma to the Cornea
Blunt trauma to the globe from lead shanks, whips, and other objects can result in corneal endothelial injury and subsequent edema. Signs of traumatic uveitis also may accompany such an injury. The corneal edema that results from blunt trauma to the globe may be focal, linear, or diffuse. Therapy for blunt trauma includes a topical hypertonic (5%) saline solution or ointment two to four times a day to decrease corneal edema.27 A linear keratopathy, characterized by a nonedematous, deep, striate, refractile opacity in the cornea, may represent a focal thinning or break in Descemet’s membrane that has resulted from blunt trauma to the cornea. This type of lesion must be distinguished from Haab’s striae, which are linear breaks in Descemet’s membrane that result from elevated IOP in glaucoma.
Corneal Foreign Bodies
Plant matter embedded in the epithelium or superficial stroma is the most frequently encountered corneal foreign body. Foreign bodies usually are easily removed with a moistened, cotton-tipped applicator or ophthalmic forceps. Sedation, motor or sensory nerve blocks, and topical anesthesia facilitate removal. Culture and sensitivity tests and cytologic examination of corneal samples are recommended before therapy is initiated. The cornea is stained with fluorescein dye to evaluate the extent of corneal ulceration. While awaiting laboratory results, medical therapy should include a topical ophthalmic antibiotic (bacitracin-neomycin-polymyxin, gentamicin, or tobramycin three or six times daily) and atropine (as needed). The prognosis is guarded until the cornea heals; the eye should be reevaluated in 24 to 48 hours.
Complications of corneal foreign bodies include bacterial and fungal infection, corneal perforation, and severe corneal scars that may restrict vision. Subpalpebral lavage systems are used in severe injuries when frequent, prolonged therapy is needed or when treating intractable animals (see later under Bacterial Keratitis in Horses).28
Corneal Ulcers
Corneal ulcers in horses are usually initiated by trauma and should be considered contaminated by bacteria or fungi until proved otherwise. Trauma may play a lesser role in food animals in which primary infectious etiologies are more common (see Infectious Bovine Keratoconjunctivitis). The conjunctival fornices and eyelids should be carefully examined for foreign material. Diagnosis is based on cytologic examination, culture and sensitivity testing of corneal samples, and fluorescein staining of the cornea. Material for bacterial and fungal culture is collected from the ulcer with sterile rayon-tipped swabs. Cotton swabs are less satisfactory because cotton exhibits some antimicrobial properties. The eyelid margins and skin should be avoided, and better culture results are obtained if the swab is moistened with a sterile solution (sterile water or saline) before specimen collection. The most reliable results are obtained if the swabs are placed in a transport medium at the time of collection.* The use of a culture tube with saline in an enclosed, breakable ampule is also helpful.29 The ampule is crushed, and fluid is allowed to moisten the swab before specimen collection. Immediately after collection, the swab is replaced in the tube or inoculated onto standard bacterial and fungal agar plates, or blood agar and thioglycolate broth.
Corneal scrapings for microscopic examination are collected with the use of topical anesthesia. The eyelids are retracted, and the margin of the corneal ulcer is gently rubbed with a small brush, cytology spatula, or the blunt, handle end of a Bard-Parker scalpel blade until a small amount of cellular material is collected. This material is transferred to two to six clean glass slides, spread over a 1-cm (0.4-inch) area and allowed to air-dry. One smear is stained with Diff-Quik; one with Wright-Giemsa, periodic acid—Schiff (PAS), or GMS for fungal hyphae; one with Gram stain; and possibly one with new methylene blue.
Therapy of corneal ulcers is based on the removal of the cause if it is still present, control or prevention of infection with topical antimicrobials, use of topical atropine for relief of painful ciliary body spasm and prevention of synechia, and, in horses, the systemic use of nonsteroidal antiinflammatory drugs (NSAIDs) such as flunixin meglumine, ketoprofen, or phenylbutazone. Some ophthalmologists also like to sterilize the base of the ulcer by application of povidone-iodine solution (Betadine) diluted 50:50 with sterile saline or collyrium. The initial choice of topical antibiotic should be based on the results of cytologic evaluation and later modified, if necessary, according to the results of culture and sensitivity testing. One study showed that oxytetracycline combined with polymyxin B had in vitro efficacy against isolates from infectious keratitis that was comparable to gentamicin and superior to chloramphenicol.30 If cytology demonstrates fungal hyphae, initial therapy with a topical antifungal agent should be instituted immediately. One study demonstrated that natamycin,* miconazole, itraconazole, and ketoconazole are superior to fluconazole† based on the results of in vitro susceptibility testing.31 Voriconazole is a newer antifungal drug that may be compounded for use in veterinary ophthalmology. Subpalpebral lavage systems greatly facilitate the delivery of topical medication to the equine eye (see Bovine Keratitis in Horses).28,32
Surgical intervention should be considered in cases of deep corneal ulceration and especially when Descemet’s membrane is exposed. Surgical procedures most often used for corneal ulceration include conjunctival pedicle flap, keratoplasty, and tarsorrhaphy.12,33-35 Ophthalmic tissue adhesives and soft contact lenses may also be used as nonsurgical therapy for deep corneal ulcers. Perforating ulcers with iris prolapse and mixed bacterial and fungal keratitis or ulcers present greater than 2 weeks usually have a poor visual outcome.36
Corneal Lacerations
Corneal lacerations may be caused by sharp, protruding objects or projectiles. Corneal lacerations can occur with or without scleral laceration and, if they are nonperforating, may be treated as corneal ulcers. By contrast, perforating corneal lacerations must be repaired surgically. Preoperative preparation includes tetanus prophylaxis (horses and goats), systemic antibiotics, and sample collection for corneal culture and sensitivity. Ocular ultrasonography is very useful in determining the integrity of intraocular structures. However, care must be exercised during ocular examination, ultrasonography, and during surgery and anesthesia (especially induction and recovery) because extrusion of the intraocular contents may occur if excessive pressure is exerted on the globe or if the eyelids are forced open. In some cases, complete examination of the globe should be delayed until the animal is anesthetized.
General anesthesia, adequate magnification, proper instrumentation, appropriate suture material and needles, and adequate postoperative care are necessary for successful repair of a corneal laceration. Postoperative therapy must include topical antibiotics and mydriatics/cycloplegics, along with systemic antiinflammatory agents.34,37 The prognosis for recovery of vision and preservation of the globe generally is guarded. Complications that may occur after repair of corneal lacerations include phthisis bulbi, corneal fibrosis, synechia formation, blindness, retinal detachment, cataract formation, uveitis, endophthalmitis, bacterial or mycotic keratitis, and wound dehiscence with subsequent iris prolapse.
The prognosis after surgical repair of corneal or corneoscleral lacerations is best when the animal is presented immediately with a small wound in which the cornea or sclera is sealed and the anterior chamber has re-formed. Minimal hyphema, clear intraocular media, a clearly visible fundus, and laceration length of less than 15 mm are additional findings that indicate a favorable prognosis.33,36 In horses the success rate when only the cornea is involved is about 70% for recovery of vision and 90% for a cosmetically acceptable globe.33 With corneoscleral lacerations the prognosis is much worse. In our experience the success rate in such cases is 20% for recovery of vision and 70% for a cosmetically acceptable globe. Most phthisical globes are not considered cosmetically acceptable. Therefore, enucleation should be considered initially with severe corneoscleral lacerations because the prognosis is poor for return of vision and guarded for preservation of the globe. Several surgical procedures have been described in horses to provide a cosmetic appearance to the globe and orbit, including placement of an intraocular silicone prosthesis.38-40
TRAUMA TO THE UVEAL TRACT
Trauma to the globe can damage the iris, ciliary body, and choroid. The resulting inflammatory response may range from very mild with rapid recovery to loss of vision and chronic discomfort. Signs of inflammation of the iris and ciliary body include blepharospasm, epiphora, miosis, aqueous flare, corneal edema, fibrin in the anterior chamber, hyphema, hypopyon, low IOP, and synechia formation. Concurrent damage to the corneal epithelium may be present and must be evaluated with fluorescein dye. Damage to the choroid may also affect the retina and lead to retinal detachment or degeneration.
Traumatic uveitis is treated in a manner similar to uveitis of other etiologies. Therapy is directed toward dilating the pupil to prevent synechia formation, cycloplegia to prevent painful ciliary spasm, and controlling the intraocular inflammatory response. Topical atropine is instilled to maintain mydriasis and provide cycloplegia. Topical corticosteroids and prostaglandin inhibitors such as 0.1% diclofenac* are used to decrease inflammation of the anterior segment.
The topical corticosteroid of choice is 1% prednisolone acetate or 0.1% dexamethasone.16,41,42 Subconjunctival injection of corticosteroids may also be quite beneficial in controlling inflammation. However, the use of topical and subconjunctival corticosteroids must be avoided in the presence of corneal ulceration or abrasion. Systemic medication should include flunixin meglumine, ketoprofen, phenylbutazone, or oral corticosteroids. In horses, prednisolone is given by mouth at 0.5 to 2 mg/kg for 7 to 21 days. Care must be taken to avoid secondary complications from systemic corticosteroids.
Trauma-induced hyphema usually has a good prognosis if the blood has clotted and fills less than half the anterior chamber. Stall rest, topical 1% atropine, topical corticosteroids, and systemic antiinflammatory therapy should be instituted to control the associated uveitis. If a penetrating wound is suspected, topical and systemic antibiotic therapy should be included with periodic fluorescein staining. Surgical intervention to remove large blood clots is rarely indicated because it may result in additional bleeding or may worsen the uveitis. Dilute tissue plasminogen activator* (tPA, 25 to 50 μg) may be injected into the anterior chamber to disrupt or lyse intraocular hemorrhage and fibrin and to aid in resolution of synechiae. For maximum effectiveness, tPA should be used within 24 to 72 hours of clot formation.43,44 Systemic tPA for use in humans is diluted for intraocular use but is expensive. However, diluting the drug and repackaging it into sterile vials that are stored at −70° C and then thawed for injection has made the cost more reasonable for veterinary use.
Chronic uveitis after ocular trauma may be associated with lens damage or the introduction of infectious or foreign agents into the eye and carries a poor prognosis. If the lens is ruptured, its surgical removal is advocated. Ocular perforation is a frequent cause of panophthalmitis in food animals and horses. Although therapy consists of systemic and topical antibiotics, as well as tetanus prophylaxis in susceptible species, panophthalmitis usually necessitates eventual enucleation.
TRAUMA TO THE LENS
Blunt or sharp trauma to the eye may damage the lens by causing lens opacity (cataract), rupturing the lens capsule, or less often may cause a shift in position (subluxation or luxation). A luxated lens should be removed if it causes obstructive glaucoma or chronic corneal edema from endothelial contact, or if it becomes cataractous and reduces vision.
Release of lens protein into the eye after lens capsule rupture may induce severe granulomatous uveitis. In such cases the eye should be treated vigorously for lens-induced uveitis with topical atropine and topical and systemic antiinflammatory drugs. If the globe has been penetrated, topical and systemic antibiotics are indicated. The prognosis for preservation of vision is poor. Lens removal is often required to control the inflammatory response.
Cataracts (lens opacities) associated with ocular trauma may occur acutely or develop weeks after the initial injury. The opacity may be only focal and not appreciably affect vision, or it may be complete and cause a visual deficit. If the remainder of the eye is normal, surgical removal of the cataractous lens may improve vision.45 Removal of cataracts secondary to uveitis is not recommended currently; however, with the advent of new surgical techniques, procedures such as combined vitrectomy (possibly to remove the immunologic stimulus in recurrent uveitis) and lensectomy may become more commonplace.46
TRAUMA INVOLVING THE VITREOUS
Trauma to the eye may result in hemorrhage into the vitreous or release of inflammatory products that cause vitreal degeneration. Either circumstance can result in vitreal syneresis (liquefaction), formation of vitreal traction bands, and subsequent retinal detachment. Symptomatic treatment of inflammation is generally adequate; however, vitrectomy may be beneficial in the management of severe vitreal hemorrhage.16 Foreign material that becomes entrapped in the vitreous may be an inciting factor for endophthalmitis. If infectious endophthalmitis is suspected, diagnostic paracentesis of the vitreous or anterior chamber should be performed to obtain samples for bacterial and fungal culture and for sensitivity and cytologic examination. After the samples are obtained, but before removing the needle from the globe, 200 μg gentamicin and 2.2 mg cefazolin, or 200 μg gentamicin and 1 mg vancomycin, should be injected into the vitreous.47 Further therapy should be based on culture and sensitivity (C&S) results, as well as cytologic findings. If a foreign body is identified, removal and vitrectomy may be beneficial, but the prognosis for successful treatment is poor.
TRAUMA TO THE RETINA
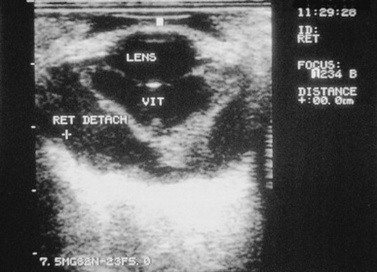
Retinal tears, hemorrhage, edema, and detachment may be caused by trauma.48,49 In cases of opaque ocular media, retinal separation may be diagnosed by ocular ultrasonography (Fig. 39-9). Retinal degeneration may follow ocular trauma. Retinal hemorrhage and edema should be treated with systemic corticosteroids. With current technology, surgical repair of retinal tears, lacerations, or detachments in food animals and horses may be feasible in selected cases.
TRAUMA TO THE OPTIC NERVE
The pathogenesis of damage to the optic nerve is not well understood. Shearing forces at the optic foramen from displacement of the brain after severe head trauma (Fig. 39-10), direct contusion or avulsion of the optic nerve, or loss of blood supply to the nerve and subarachnoid hemorrhage probably all have roles in optic nerve injury.50 Early examination may reveal only a dilated pupil that may be partially or completely unresponsive to light. Later, changes may include optic nerve atrophy (Fig. 39-11) and peripapillary retinal pigmentation changes. Therapy with systemic antiinflammatory drugs may be of benefit, but severe damage is usually irreversible.
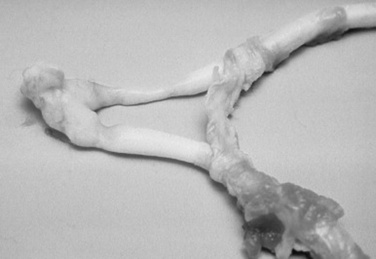
Fig 39-10 Optic nerves and chiasm of a foal that was blind as a result of head trauma. Note the constrictions of the optic nerves caused by necrosis and degeneration.
Courtesy Dr. C.L. Martin.
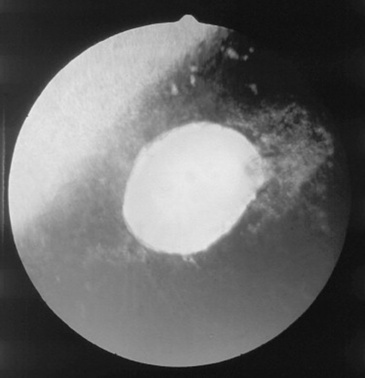
Fig 39-11 Appearance of the optic nerve 3 months after head trauma in a horse. Note the pale optic disc and peripapillary retinal degeneration.
Courtesy Dr. C.L. Martin.
Traumatic optic nerve atrophy is usually characterized by sudden onset of unilateral or bilateral blindness; dilated, fixed pupils; and a lack of menace response. In horses the traumatic episode is frequently characterized by damage to the poll from rearing over backward and striking the back of the head, from rearing up and hitting a ceiling beam, or from blunt trauma (blows) to the side or front of the face. The animal usually stands without loss of consciousness, and the injury is not considered serious by the owner at the time. Initially, blindness with a normal-appearing ocular fundus is observed.
Within 3 to 4 weeks after the trauma, examination of the fundus reveals a pale optic disc (see Fig. 39-11). Later, loss of peripapillary retinal vessels is usually evident. The optic disc often appears depressed, with increased prominence of the lamina cribrosa. Confirmation of optic nerve or optic tract lesions causing blindness may be made by the absence of a direct pupillary light reflex with a normal electroretinogram. In some cases the pathologic lesion is a rupture of the nerve axons from stretching forces produced by movement of the brain.50 Chiasmal hemorrhage and fractures of the basisphenoid bone may be observed at necropsy. Therapy with systemic corticosteroids (dexamethasone, 1 mg/kg) and IV dimethyl sulfoxide (DMSO) has generally not been successful,50 although the lack of response to medical therapy appears related to the severity of the injury.
A segmental optic nerve atrophy involving one to three quadrants of the optic disc occurs in horses. The appearance is characterized by pallor, loss of normal vasculature, and increased prominence of the lamina cribrosa in the affected quadrants. The etiology is unknown; however, traumatic injury is suspected in most cases. Response to medical therapy is poor.
CHEMICAL INJURY
Ocular irritation caused by insecticides and disinfectants inadvertently applied to the eye is relatively common in the farm or ranch setting. Chemical burns to the cornea and adnexa may have serious consequences and may warrant a poor prognosis for salvage of the eye. Alkali burns are more severe than acid burns. Corneal burns from acids tend to be sharply demarcated and nonprogressive, whereas alkali burns cause progressive coagulation, melting, and sloughing of the corneal stroma.16 Chemically induced melting of the cornea must be differentiated from bacterial and fungal infections. Treatment for a suspected or known chemical burn should include lavage of the affected area with copious amounts (500 to 2000 mL) of sterile saline solution. Tap water may be used by the owner until veterinary assistance is available. It may be necessary to sedate the animal. No attempt should be made to neutralize the substance because this may cause precipitation within the cornea. The damage should then be evaluated with the aid of local nerve blocks.
Treatment should include appropriate topical antimicrobials, atropine, and a collagenase inhibitor such as autologous serum or possibly acetylcysteine,* which inhibits collagenases and metalloproteinases by binding calcium. The commercially available preparations contain 10% and 20% acetylcysteine and should be diluted to 5% and 10% to avoid epithelial toxicity. Acetylcysteine lasts only about 4 days once opened and should be refrigerated. Hourly application of serum or acetylcysteine may be needed. Systemic antiinflammatory drugs should be used to control secondary uveitis. Therapeutic soft contact lenses have been used to protect the corneal stroma in patients with extensive corneal ulcerations.
THERMAL INJURY
Facial burns secondary to barn or stable fires may damage the eyelids, conjunctiva, and cornea. Thermal injuries also may cause anterior uveitis and exfoliation of the lens capsule. Therapy for minor burns to the eyelids is directed toward keeping the injured area moist with antibiotic dressings and protecting the cornea if eyelid dysfunction occurs. Treatment for injury to the conjunctiva or cornea should include topical antibiotic and systemic antiinflammatory drugs in horses. Full-thickness eyelid burns may require grafting procedures to protect the cornea and to minimize scarring.24,26 Third eyelid or conjunctival flaps may be required to protect the cornea until eyelid function returns.
INFECTIOUS OCULAR DISEASES
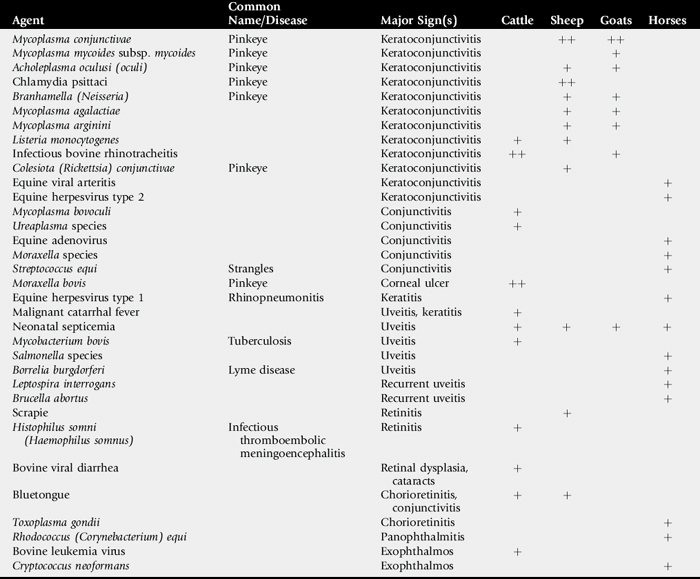
This section describes the major infectious ophthalmic diseases of large animals (Table 39-2), concluding with a discussion of infectious bovine keratoconjunctivitis (IBK), or “pinkeye,” the most common ocular disease of cattle.
MYCOPLASMAL KERATOCONJUNCTIVITIS IN GOATS AND SHEEP
Definition and Etiology
Mycoplasma conjunctivae has been frequently isolated throughout the world from epidemics of keratoconjunctivitis, respiratory disease, and arthritis in goats and sheep.51-54Mycoplasma mycoides subsp. mycoides has been isolated from an epidemic of mastitis, arthritis, and keratoconjunctivitis in goats.55Acholeplasma oculusi (oculi) has been isolated from sheep and goats in epidemics of keratoconjunctivitis.56,57Mycoplasma agalactiae and Mycoplasma arginini have also been described as causing keratoconjunctivitis and systemic disease.58
Clinical Signs and Differential Diagnoses
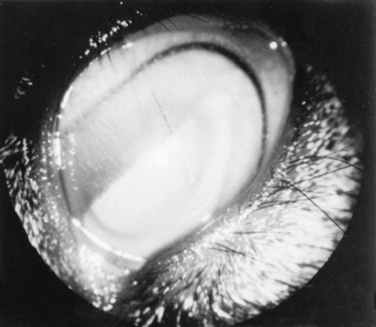
Clinical signs of mycoplasmal keratoconjunctivitis include epiphora, conjunctival hyperemia, and occasionally follicular conjunctivitis. In experimental conjunctival inoculation with M. conjunctivae, clinical signs began on day 2 and lasted for 5 weeks.59 Later in the disease, keratitis with corneal neovascularization (Fig. 39-12) and occasionally anterior uveitis can be seen.51,60 One case of choroiditis and hyalitis has been described.61 Signs can be seen in individuals, as well as in herd or flock outbreaks. The disease is usually unilateral but can be bilateral. Differential diagnoses include other infectious causes of keratoconjunctivitis, such as Chlamydia species (sheep), Branhamella (Neisseria) species, aerobic bacteria, parasites, and infectious bovine rhinotracheitis (goats), as well as noninfectious causes such as trauma.
Clinical Pathology
In conjunctival scrapings taken early in the disease, many neutrophils are seen; later, lymphocytes predominate. Plasma cells and necrotic epithelial cells are also seen.62 Organisms can be seen occasionally in epithelial cell cytoplasm as coccobacillary or varied forms.61 Pigment granules can be mistaken for organisms.56,62,63
Mycoplasmal organisms can be cultured and identified from conjunctival swabs; serum antibody titers can be measured,64,65 or polymerase chain reaction (PCR) can be used to identify M. conjunctivae in conjunctival smears.66,67 Egwu and Faull68 describe rising serum and lacrimal antibody titers in sheep topically inoculated with M. conjunctivae. However, Trotter et al.52 report low serum titers to M. conjunctivae in normal animals and no rise in titer in animals inoculated with M. conjunctivae subconjunctivally that subsequently developed signs of disease.
Epidemiology
Mycoplasmal infections apparently are transmitted directly from animal to animal, as evidenced by herd or flock outbreaks. The presence of carrier animals is postulated, and M. conjunctivae can be cultured from unaffected animals.69,70 Animals can become reinfected. Keratoconjunctivitis can be induced in sheep with topical inoculation of M. conjunctivae.68,71,72 Clinical signs were identical to natural outbreaks and spread to uninoculated sheep. The organism can be cultured from eyes long after clinical signs abate.59 See Chapter 38 for more details on M. mycoides.
Treatment and Prognosis
In most animals, mycoplasmal keratoconjunctivitis associated with M. conjunctivae is transient. Affected animals usually recover spontaneously in 10 days, although some animals seem to have recurring episodes that last for several weeks. In a controlled clinical trial, one dose (20 mg/kg) of long-acting oxytetracycline was given to experimentally inoculated lambs. This treatment seemed to hasten the cessation of clinical signs, although the results were not analyzed statistically.73 The treatment did not, however, eliminate the M. conjunctivae infection. Other drugs recommended for the ocular disease include topical oxytetracycline or oxytetracycline and polymyxin B.51 Subconjunctival oxytetracycline is not currently recommended because it may cause a severe inflammatory reaction. In vitro antibiotic testing of M. conjunctivae shows that tylosin, oxytetracycline, streptomycin, and chlortetracycline are suitable for treatment.74
Prevention and Control
Introduction of new animals into a herd or flock has been implicated in starting an outbreak of keratoconjunctivitis. Therefore, isolation and, if necessary, treatment of new animals are important before contact with the herd. No other specific recommendations have been made for prevention and control of M. conjunctivae. See Chapter 38 for control of M. mycoides subsp. mycoides.
CHLAMYDIAL KERATOCONJUNCTIVITIS IN SHEEP
Definition and Etiology
Chlamydial agents have been isolated from outbreaks of keratoconjunctivitis in sheep flocks. The agent was originally described as a strain of Chlamydia psittaci. The agent is now called Chlamydophila pecorum, which can also cause abortion (see Chapter 43) and polyarthritis (see Chapter 38) in lambs.75,76
Clinical Signs and Differential Diagnoses
Early clinical signs consist of epiphora, chemosis, and conjunctival hyperemia. Later in the disease, follicle formation in the conjunctiva becomes prominent. Still later, corneal neovascularization may be seen. Most cases are bilateral and symmetric.77,78 In some flock outbreaks of keratoconjunctivitis, outbreaks of polyarthritis are also noted. Most lambs that develop chlamydial polyarthritis will also develop conjunctivitis.77,78 Differentials include other infectious causes of keratoconjunctivitis, such as Mycoplasma and Branhamella (Neisseria) species, aerobic bacteria, and parasites, as well as noninfectious causes such as trauma.
Clinical Pathology
Early in the disease, conjunctival smears show numerous neutrophils and some lymphocytes. Later, there are more neutrophils and fewer mononuclear cells. Cytoplasmic chlamydial inclusions are occasionally seen in up to a third of the eyes scraped and can be definitively identified by fluorescent antibody staining.75 Conjunctival epithelial cells are often necrotic. Chlamydial organisms can be cultured from conjunctival scrapings and from blood taken from sheep with polyarthritis and conjunctivitis.77-79 In one study, titers to chlamydial antibodies were found at 1:16 or higher in a number of the affected lambs, although titers on normal lambs were not reported.78 Polymerase chain reaction (PCR) can also be used to identify the organism.
Epidemiology
Chlamydial organisms are apparently transmitted by direct contact, as evidenced by flock outbreaks. Chlamydial organisms caused conjunctivitis in five lambs inoculated topically.80 An uninoculated lamb housed with the five lambs also developed conjunctivitis. Lambs subsequently developed follicular conjunctivitis.79 In another study, chlamydial organisms were injected intraarticularly, intravenously, and intramuscularly and caused polyarthritis and conjunctivitis.79
Treatment and Prognosis
In uncomplicated cases the disease is self-limiting, and eyes are normal within 2 to 3 weeks.78 The same treatments indicated for Mycoplasma mycoides subsp. mycoides (systemic oxytetracycline and, when possible, a topical tetracycline ophthalmic preparation) are also effective in treating chlamydial conjunctivitis/polyarthritis of sheep.
BRANHAMELLA (NEISSERIA) OVIS KERATOCONJUNCTIVITIS IN SHEEP AND GOATS
Branhamella ovis is a gram-negative diplococcus similar to Moraxella species. This agent has been cultured from sheep and goats with keratoconjunctivitis;81,82 however, it can also be cultured from normal eyes.83 B. ovis has also been cultured from cattle with serous conjunctivitis and rarely keratitis.84 Clinical signs are usually mild and include epiphora and conjunctival hyperemia.
In one outbreak, neutrophils and gram-negative coccobacilli were seen on conjunctival scrapings. B. ovis was cultured from the initial outbreak and was then instilled into the conjunctival sac of goats with or without ultraviolet radiation. The experimentally infected goats developed epiphora and conjunctivitis, but no keratitis.85 In another study, B. ovis instilled into the conjunctival sac of lambs induced reddened conjunctiva and follicle formation. B. ovis could be cultured from these eyes for up to 20 days after inoculation.86
In one outbreak, animals were treated with parenteral tylosin and topical neomycin, polymyxin B, and a corticosteroid, and all 10 recovered.81 Bankemper et al.85 used subconjunctival penicillin to treat affected goats.
SCRAPIE-ASSOCIATED RETINOPATHY IN SHEEP AND GOATS
The scrapie agent causes a degenerative CNS disease in sheep and less often in goats. Barnett and Palmer87 described two sheep with scrapie that also had multifocal hyperreflective areas in the tapetum, histologically seen as small areas of retina raised by an accumulation of eosinophilic material between photoreceptors and retinal pigment epithelium. The eosinophilic material was characterized as a complex lipid. It was not shown that scrapie had caused the lesions.87 Subsequently, using a monoclonal antibody, a goat with natural scrapie was found to have the scrapie prion protein in the retina without any microscopic retinal lesions.88 A sheep experimentally infected with scrapie was also found to have prion proteins in the retina,89 as was a sheep experimentally infected with the prion responsible for bovine spongiform encephalopathy (BSE).90 Prion proteins can also be found in the nictitating membrane,90,91 which may permit the antemortem diagnosis of scrapie. (See Chapter 35 for more information on scrapie.)
BLUETONGUE-INDUCED RETINAL DYSPLASIA IN SHEEP AND CATTLE
Bluetongue is a disease of ruminants caused by an arbovirus that is transmitted by Culicoides gnats. Clinical signs include fever and vasculitis that leads to oral lesions, lameness, swollen face, pulmonary edema, and death (see Chapter 32). In pregnant ewes vaccinated with modified live virus (MLV) on day 40 of gestation, fetuses developed cerebral anomalies.92 These anomalies have also been described in clinical cases in which ewes had been vaccinated with MLV at about 5 or 6 weeks of gestation. In addition, when the fetus was vaccinated with MLV vaccine between days 50 and 75 of gestation, lesions of retinitis and choroiditis were noted that appeared centered around retinal vessels. In some eyes, inflammatory lesions produced persistent areas of retinal dysplasia.93
LISTERIA MONOCYTOGENES IN SHEEP, CATTLE, AND HORSES
Listeriosis in ruminants is manifested mainly as either an encephalitis or as a septicemia in neonates, or as a reproductive problem manifested as abortions. Ocular signs with the neural form include facial paralysis and ptosis, often unilateral, on the side of the central lesion; medial strabismus, often on the ipsilateral side because of involvement of the abducens nucleus; nystagmus; and amaurosis.94 Uveitis with hypopyon has been described in chronic cases.95
Listeria monocytogenes has been cultured from conjunctival smears taken from sheep and cattle with keratoconjunctivitis.96 In most of the sheep, Branhamella (Neisseria) ovis was also cultured. Clinical signs included conjunctival hyperemia, epiphora, photophobia, and corneal opacification. Treatment with topical chlortetracycline was curative. Walker and Morgan97 provide a description of two experimental sheep that developed unilateral anterior uveitis. L. monocytogenes was cultured from the conjunctiva of each animal. Both animals recovered after treatment with parenteral ampicillin and topical antibiotics. L. monocytogenes has been cultured from the conjunctiva of three cows with keratitis (one with keratitis and uveitis) and from a corneal scraping of one horse with keratitis.98 In another case report, L. monocytogenes was cultured from the cornea of a horse with chronic keratitis.99 Diagnosis is usually achieved by isolation of L. monocytogenes from tissues at necropsy. Early treatment with broad-spectrum antibiotics may be effective in some cases.
INFECTIOUS BOVINE RHINOTRACHEITIS KERATOCONJUNCTIVITIS IN GOATS
Goats are susceptible to infectious bovine rhinotracheitis (IBR) virus, which in some cases may result in ocular disease. In one goat with ocular signs, conjunctivitis and keratitis with keratoconus were seen 5 days after the onset of severe respiratory illness. IBR virus was isolated from ocular and nasal discharge.100
COLESIOTA (RICKETTSIA) KERATOCONJUNCTIVITIS IN SHEEP
Colesiota (or Rickettsia) conjunctivae has been described as the cause of infectious keratoconjunctivitis in sheep, but documentation is sparse. The organism has not been cultured; only identified on conjunctival scrapings. Clinical signs include epiphora, conjunctival hyperemia, and corneal neovascularization.101 Several authors have since suspected that this organism is the same as Chlamydia psittaci (now known as Chlamydophila pecorum).102,103
INFECTIOUS BOVINE RHINOTRACHEITIS CONJUNCTIVITIS
Etiology
IBR is a herpesvirus that may involve the respiratory or reproductive tracts, nervous system, or conjunctiva or may cause widespread systemic disease (see also Chapter 31). Conjunctivitis is the most common ocular manifestation of the disease, and it may occur as an isolated clinical entity or with involvement of other body systems.104-106
Clinical Signs and Differential Diagnoses
Although conjunctivitis is frequently bilateral, it can be unilateral. Ocular discharge; initially serous and later becoming mucopurulent, is usually seen without blepharospasm. Chemosis may be severe, especially by 1 week after infection. Both the palpebral and the bulbar conjunctiva are injected, and petechial hemorrhages may occur. Multiple white plaques 0.2 to 0.5 mm in diameter may develop on the palpebral and, to a lesser extent, the bulbar conjunctival surfaces at 1 to 2 weeks after onset of clinical signs. These may coalesce later in the disease (5 to 9 days). Corneal vascularization and perilimbal edema and opacification occur in severe cases. Iridocyclitis (seen as miosis) may occasionally occur in severe cases.
Corneal changes of IBR are differentiated from those of infectious bovine keratoconjunctivitis (IBK) caused by Moraxella bovis by their peripheral rather than central distribution and lack of corneal ulceration in IBR, unless IBR and IBK occur concurrently in the same eye (see later IBK section).Corneal vascularization and opacification in malignant catarrhal fever accompany marked signs of anterior uveitis107 and other signs of generalized vasculitis.
Although ocular disease may occur as an isolated entity, ocular signs may be found in animals with upper respiratory tract signs, including rhinitis and dyspnea. Affected animals may be pyrexic, and a fall in milk yield may occur. Abortion in pregnant animals may occur following ocular manifestations of the disease.
Diagnostic Procedures
IBR can be recovered from infected eyes during the first 7 to 9 days of the disease but infrequently thereafter. Swabs may be taken for viral isolation in cell culture, which is probably the most reliable means of making a definitive diagnosis. Fluorescent antibody techniques may be used on conjunctival scrapings, and serology may be helpful if blood samples can be collected during the acute and convalescent stages of the disease. PCR is also being used. Histopathology to detect intranuclear inclusions is not likely to allow reliable diagnosis of the disease.107,108
Pathophysiology
Specific strains of the virus usually cause only one form of the disease (e.g., ocular form) in a herd. Ocular infection results in lymphoid hyperplasia, visible as white plaques. On histology, these are composed of plasma cells and lymphocytes in the conjunctival stroma and subepithelial area. Mild conjunctival epithelial ulceration may occur. During the recovery phase of the disease, diphtheritic membranes secondary to conjunctival necrosis develop on the conjunctival surface.
Treatment and Prognosis
Recovery from the conjunctival form of the disease is spontaneous within 10 to 20 days. In certain situations, palliative treatment may be helpful. This is achieved by cleaning the ocular discharge from the lids and applying a topical broad-spectrum antibiotic to prevent secondary bacterial infection. Treatment of the conjunctival form of the disease with topical antiherpetic agents has not been studied and would rarely be practical or cost-effective.
Prevention and Control
Vaccination of susceptible animals is the most effective means to prevent and control the disease. IBR vaccination programs are discussed in Chapter 48.
MALIGNANT CATARRHAL FEVER KERATOCONJUNCTIVITIS
Malignant catarrhal fever (MCF) is a sporadic disease characterized by fever, lymphadenopathy, and generalized vasculitis resulting in inflammation of the mucosal membranes of the mouth, nose, and eye; the skin; and the gastrointestinal (GI) and nervous systems, with variable but usually high mortality. The African form is caused by alcelaphine herpesvirus type 1. The North American form is caused by a similar virus, ovine herpesvirus.109,110
Various forms of the disease are described on the basis of clinical signs (see Chapter 32). Ocular involvement is seen in the acute “head and eye” form, which is the most common presentation of the disease. Ocular signs include photophobia, epiphora, episcleral injection and scleritis, severe conjunctivitis, keratitis (corneal opacification caused by edema and vascularization appearing perilimbally), anterior uveitis, and exophthalmia. Less significantly, and difficult to diagnose clinically, retinal vasculitis may develop. Bullous keratopathy may develop as a result of edema in the anterior cornea, with subsequent rupture of bullae to form painful corneal erosions.111 The absence of central corneal ulceration distinguishes the disease from IBK, and the severity of the ocular lesions is worse than would be expected in IBR, bovine viral diarrhea/mucosal disease (BVD/MD), or bluetongue. Differentiating this disease from Rinderpest could be clinically difficult in areas where both are endemic in cattle.
Serology and PCR are being used to confirm the clinical diagnosis.112,113 The ocular lesions are those of a nonsuppurative uveitis and vasculitis. The lesions involve the conjunctiva, cornea, anterior uvea, and retinal blood vessels; the choroid is rarely involved. Serofibrinous and cellular infiltrates develop in the uvea and retina as a result of vasculitis and thrombosis. Perivascular cuffing and optic neuritis may be detected histologically.114-116
Prognosis for the eyes and the animal’s recovery is poor.111 Most importantly, in endemic areas, cattle should be kept away from sheep, which may act as a reservoir for the disease, and affected animals should be isolated.
BOVINE MYCOPLASMAL CONJUNCTIVITIS
Mycoplasma bovoculi and Ureaplasma species have been isolated from cattle with conjunctivitis and IBK. Inoculation of normal calves with M. bovoculi or Ureaplasma isolates produced conjunctivitis characterized by serous discharge and localized to diffuse conjunctival hyperemia. Experimentally induced conjunctivitis ran a course of over 1 month. Cases can be confirmed by culture or PCR performed on conjunctival swabs.117 Mycoplasmal infection may predispose the animals to development of IBK from Moraxella bovis118,119 (see later IBK section). Therefore, although treatment of mycoplasmal conjunctivitis per se may not be warranted, it may be advisable in areas where IBK is endemic. Topical oxytetracycline ointment applied three times daily or intramuscular (IM) injection of long-acting oxytetracycline is recommended.
Other mycoplasmal organisms (e.g., M. arginini) can be isolated from cows’ conjunctiva but are not thought to cause the disease. M. bovis was found using PCR performed on normal conjunctival samples,117 but it was not thought to cause conjunctivitis. M. bovigenitalium, M. bovirhinis, and M. bovoculi were isolated from members of two cattle herds with conjunctivitis and bronchopneumonia.120 However, when viral cultures were performed, IBR was isolated in the same individuals. The mycoplasmal organisms may have contributed to the disease in these herds. In another cattle herd with outbreak of respiratory disease, keratoconjunctivitis followed, and M. bovis, M. bovirhinis, and M. bovoculi were isolated in various combinations from affected calves.121
HISTOPHILUS SOMNI CONJUNCTIVITIS AND RETINITIS
Thromboembolic meningoencephalitis (TEME) is a fatal septicemia caused by infection with Histophilus somni (see Chapter 35). Calves and young adult cattle can be affected, but the disease most often occurs in feedlot cattle less than 1 year of age. The organism is capable of damaging vascular endothelial cells and activating blood clotting. Therefore, most of the ocular histologic signs are referable to thrombosis of retinal vessels.
Although conjunctivitis may be seen, the main ocular findings are in the fundus. Retinal hemorrhages and exudates may be focal or diffuse. Retinal infiltrates may elevate the retina and involve the vitreous. Retinal edema, hemorrhage and necrosis, vascular thrombosis, and infiltration of the retina and vitreous with neutrophils are seen histologically. Eosinophilic cytoid bodies (swollen axons) are seen in the nerve fiber layer of the retina. Retinal detachments may result from retina edema. Later in the disease, areas of chorioretinitis result in chorioretinal scars. The anterior segment is less involved in this disease than in MCF, in which keratitis and anterior uveitis are usual.122,123
BOVINE VIRAL DIARRHEA—INDUCED RETINAL DYSPLASIA, CATARACTS, MICROPHTHALMIA, OPTIC NEURITIS, AND LEUKOCORIA
The causative agent of bovine viral diarrhea (BVD) is a pestivirus (part of the togavirus group; genus Pestivirus, family Togaviridae) that, in congenitally affected animals, causes retinal inflammation and necrosis. Cattle infected between days 75 and 150 of gestation may produce calves with cerebellar hypoplasia or ocular lesions. Calves with ocular signs may be blind, and nystagmus may be present. Pupillary light reflexes may or may not be absent. Other abnormalities may include microphthalmia, cataract, leukocoria (either as a result of cataract or dense white inflammatory infiltrate in the anterior vitreous; Fig. 39-13), retinal hemorrhages, chorioretinitis, retinal dysplasia or folds, retinal detachment, or optic neuritis or atrophy.124-127 The optic disc may appear atrophic, and areas of tapetal color change and hyperreflectivity may be seen, with retinal vascular attenuation. In some cases, inflammatory debris may persist in the vitreous after birth, precluding adequate fundic examination. Congenital cataracts also occur in this disease, and although the pathophysiology is unknown, they probably develop secondary to the intraocular inflammation and necrosis. Cataracts mainly involve the lens cortex.
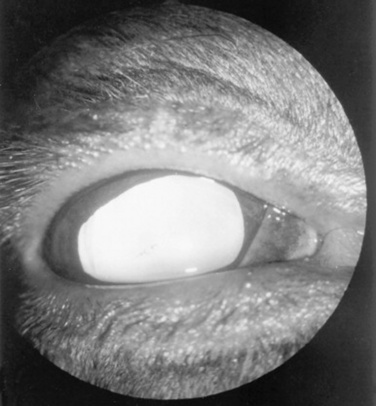
Fig 39-13 Leukocoria (white pupil) in a Simmental calf caused by inflammatory debris in the vitreous and on the posterior capsule of the lens after intrauterine infection with bovine viral diarrhea.
Transplacental infection may occur when the dam is infected during pregnancy. The severity of the disease is related to fetal age at the time of intrauterine infection. Severe fetal disease and often fetal death occur in cattle infected between 100 and 200 days of gestation. Infected fetuses can also survive and become persistently infected with the virus. In these cattle, viral antigens can be found in neurons of the retina and CNS in the absence of clinical signs.128 Ocular discharges have been reported in cases of acute or chronic cases of BVD, although the significance of these observations is uncertain.127,129
Serum samples collected from affected calves before ingestion of colostrum can be submitted for serologic assessment using the serum-virus neutralization test, or virus isolation can be attempted from buffy coat cells of a whole-blood sample collected into EDTA. (See Chapter 32.)
BLUETONGUE CONJUNCTIVITIS
Conjunctivitis and mucopurulent ocular discharge may be seen in cattle chronically infected with bluetongue virus. Although topical antibiotics could be applied to reduce secondary bacterial infection and reduce the ocular discharge, this would rarely be necessary.130
BOVINE LEUKOSIS AS A CAUSE OF EXOPHTHALMOS
Lymphosarcoma in adult cattle is usually caused by bovine leukemia virus, although noninfectious sporadic cases are reported in young animals. Lymphosarcoma may result in unilateral or bilateral progressive exophthalmos.131,132 This is the most common orbital neoplasm in cattle. If undiagnosed, exposure keratitis and chemosis develop. Intraocular involvement can occur, although it is less common than orbital neoplasia. Generalized lymphadenopathy or other signs of generalized lymphosarcoma usually accompany the orbital form. Specific serologic tests will confirm the diagnosis. Enucleation or exenteration is rarely indicated because of the poor prognosis for affected animals. Differential considerations for progressive exophthalmos include frontal or maxillary sinusitis or nasal neoplasia, actinomycosis, and actinobacillosis.
OCULAR MANIFESTATIONS OF TUBERCULOSIS
Tuberculosis caused by Mycobacterium bovis may cause granulomatous lesions in the eye of affected cattle. The uveal tract (iris, ciliary body, or choroid) is initially affected, with later expansion of granulomas into other ocular structures. Uveitis, keratitis, and chorioretinitis with retinal detachment are seen clinically.133
OCULAR MANIFESTATIONS OF NEONATAL SEPTICEMIA
Neonatal septicemia in calves, foals, lambs, and kids may occur in the first few weeks after birth and may arise from umbilical infection or oral intake of bacteria (see Chapter 18). Septicemia is especially common in colostrum-deprived neonates. Secondary meningitis, polyarthritis, uveitis, and chorioretinitis may develop. Ocular signs include miosis, aqueous flare with fibrin deposition in the anterior chamber, hypopyon or hyphema, and in severe cases, panophthalmitis. Bacteria involved include Escherichia coli, Streptococcus species, Pasteurella species, Salmonella species, Rhodococcus equi, Corynebacterium pyogenes, and Klebsiella species. The incidence of infection with different bacteria varies among the domestic species. Therapy should include systemic antibacterial agents (based, when possible, on sensitivity testing) and treatment for uveitis. Prognosis for cases treated early is still guarded.134-137
BACTERIAL KERATITIS IN HORSES
Definition and Etiology
Bacterial keratitis occurs when a traumatic corneal ulcer becomes infected with opportunistic bacteria; no bacteria are known to initiate ulcers in horses. The most devastating clinical manifestations are associated with Pseudomonas aeruginosa and Streptococcus equi subsp. zooepidemicus. Additional bacteria isolated from infected corneas include nutritionally variant streptococci,138 Staphylococcus species, E. coli, Acinetobacter species, Clostridium species,139Corynebacterium species, and others.140,141
Clinical Signs and Differential Diagnoses
Whether infected or not, corneal ulcers cause signs of pain (blepharospasm, epiphora, apparent photophobia). The conjunctiva is hyperemic, and the ulcerated area of the cornea retains fluorescein stain. A deep ulcer (resembling a crater in the corneal stroma) should be assumed to be infected. Superficial ulcers, on the other hand, are usually not infected, and the cornea maintains its normal curvature in the ulcerated area. Other signs of an infected ulcer are rapid progression and white or yellowish opacity of the cornea (signifying corneal stromal influx of neutrophils and bacterial colonization). An ulcer that is rapidly becoming wider or deeper or a “melting” ulcer, in which corneal stroma liquefies (Fig. 39-14), is highly suggestive of a bacterial infection. Fungal keratitis can appear similar but usually has a more insidious, or at least a slower, course. However, a cornea with fungal keratitis can become secondarily infected with bacteria. Bacterial keratitis can also present as a stromal abscess.142 In these cases a cellular infiltrate is seen in the corneal stroma over which the initially damaged epithelium has healed. Therefore, such lesions do not stain with fluorescein, and many topical antibiotics cannot reach the site of infection.
Clinical Pathology
A diagnosis of a bacterial keratitis is made using Gram-stained corneal scrapings. Stained scrapings usually show many bacteria (some intracellular) and many neutrophils (some degenerate). Bacterial organisms are definitively identified after culture of the ulcerated cornea.
Pathophysiology
Bacterial keratitis is the result of pathogenic or opportunistic organisms colonizing a damaged cornea. The cornea is most likely to be damaged by mechanical trauma, but chemical damage is seen occasionally. Many different types of bacteria can be cultured from a normal eye, including Corynebacterium, Streptococcus, Staphylococcus, Bacillus, and rarely Pseudomonas species or other gram-negative bacteria.143,144 Damage to the epithelium enables bacteria to adhere to the exposed corneal stroma and begin replicating.145 Some bacteria such as Pseudomonas elaborate collagenases and other proteoglycanolytic enzymes,146 which results in corneal melting. Proteases and collagenases liberated by white blood cells and possibly corneal epithelial and stromal cells also contribute to the melting process. In this way, what begins as a small wound to the cornea can progress to a corneal perforation within 24 to 48 hours.
Treatment and Prognosis
Prognosis is guarded for any corneal ulcer that is rapidly progressing or melting. These ulcers can easily progress to corneal perforation and loss of the eye despite timely, appropriate treatment. On the other hand, with vigorous therapy some eyes with infected ulcers can be saved, leading to a visual eye, although usually a permanently scarred cornea.
In any ulcer in which a bacterial component is suspected, C&S of the ulcer and a corneal scraping for Gram staining and cytology should be taken. The horse is restrained or sedated, a palpebral block performed, topical anesthetic applied to the cornea, and a culture of the ulcer taken with a moistened swab. The ulcer margins are then scraped with a Kimura spatula or the blunt, handle end of a scalpel blade.
In cases of equine bacterial keratitis, including stromal abscesses, the therapeutic goals are to eliminate the bacteria, prevent or slow melting if present, and treat the concurrent uveitis. Because this requires very frequent applications of numerous medications to a horse with a painful and often fragile eye, a subpalpebral lavage system is usually required.
PLACEMENT OF SUBPALPEBRAL LAVAGE SYSTEM
The following equipment is necessary:
The horse should be tranquilized and/or twitched. The auriculopalpebral nerve is blocked over the zygomatic arch to paralyze the orbicularis oculi muscle and reduce spontaneous eyelid movement. The supraorbital (frontal) nerve is blocked at its exit from the supraorbital process to anesthetize the upper eyelid (Fig. 39-16). Topical anesthetic (proparacaine) is then applied to the eye by directing a gentle stream into the upper conjunctival fornix with a small syringe with a broken-off 25-gauge needle.
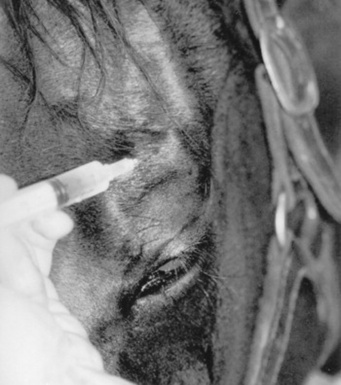
Fig 39-16 Frontal (supraorbital) nerve block in horse. A 25-gauge needle is inserted into the supraorbital foramen, and 5.0 mL of lidocaine is injected alongside the nerve.
The blunt end of the 12-gauge needle is used to probe the lateral conjunctival fornix to establish the placement of the needle. The needle then is reversed and pushed through the eyelid in a lateral direction using a pair of needle holders as resistance (Fig. 39-17). The tip of the needle enters the dorsalmost aspect of the fornix and exits near the orbital rim (Fig. 39-18). After the needle is pushed through the skin (Fig. 39-19), the Silastic Mila tubing is pushed through the needle, starting at the blunt end. When the tubing appears at the sharp end of the needle, the needle and tubing are pulled through the eyelid (Fig 39-20). The tube is then pulled up until the footplate fits snugly up into the dorsal conjunctival fornix (Fig. 39-21). During the manipulations, care must be taken to ensure that the needle or the hands of the operator do not push against the cornea.
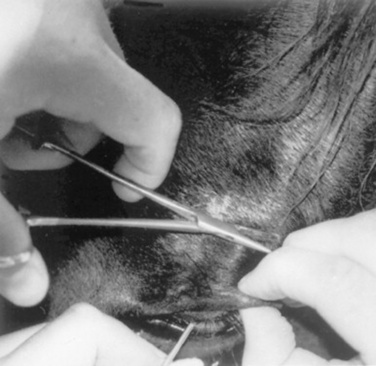
Fig 39-17 The 12-gauge needle is pushed through the eyelid with open needle holders used as resistance and to keep the eyelid skin from tenting.
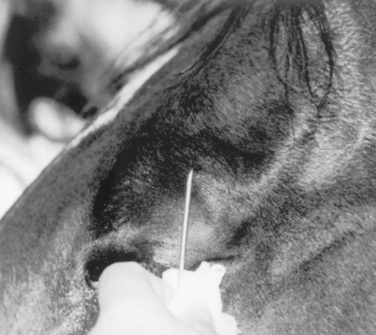
Fig 39-18 Preparing to push the 12-gauge needle with a gauze sponge before placing a palpebral lavage system.
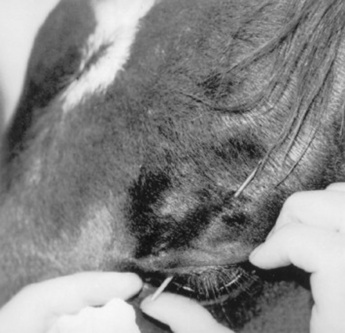
Fig 39-19 Correct orientation of the 12-gauge needle as it is advanced through the upper lid from deep in the dorsal conjunctival fornix to exit near the orbital rim. Needle pushed through the eyelid laterally. Note that the needle was inserted 45 degrees to the eyelid margin. The lavage system is now threaded through the needle and the needle removed.
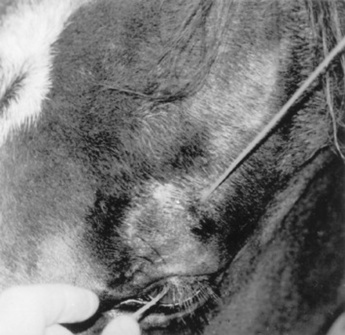
Fig 39-20 The 12-gauge needle is withdrawn dorsally from the upper eyelid and the lavage tubing pulled through until the footplate is snug in the dorsal fornix.
Tape is used to secure the tubing to the upper eyelid, and sutures secure the tape to the skin (Fig. 39-22). Alternatively, the green-plastic securing device in the pack can be sutured near the exit hole, securing the tubing without putting tape on the tubing. Additional tape and skin sutures can be placed several centimeters from the first tape, or tubing can be braided through the forelock. The Silastic tubing is then run down the neck through several braids to keep it secure. A catheter is run into this distal end of the tubing, an injection port is fixed to the end of the catheter, and the catheter and tubing are taped to an applicator stick to prevent kinking. The stick is then taped to a braid of the mane.
Fig 39-22 Subpalpebral lavage system secured in final position with tape “wings” sutured to the skin.
Poorly placed lavage tubes or tubes that slip ventrally can rapidly produce a corneal ulcer. A displaced tube footplate can also cause topical medications to leak into the subcutaneous tissues, rapidly leading to a swollen and inflamed eyelid. Therefore the tube position should be checked at least once daily by gentle dorsal traction on the exposed tubing to ensure it has not slipped into the conjunctival cul-de-sac. Excess traction will pull the footplate through the conjunctiva and into the eyelid itself. Most horses tolerate this system well, and we have kept the tubing in place for up to 6 weeks. Occasionally, horses try to rub their heads and can damage the tubing; neck cradles or protective eye cups can be used in such horses.
MEDICAL/SURGICAL APPROACHES AND GOALS
In a cornea with rapidly developing keratitis or melting ulcer, the clinician should always suspect the presence of Pseudomonas organisms, although other organisms such as Streptococcus have also been described. Treatment should be started with an appropriate antibiotic used every 1 to 2 hours. Fluoroquinolones such as ciprofloxacin (Ciloxan, Alcon) and ofloxacin (Ocuflox, Allergan)147 are good choices for treatment of infected ulcers. They are not necessary for bacterial prophylaxis in uninfected ulcers. If gram-positive organisms are seen on the corneal scraping, ticarcillin, cefazolin (Ancef), penicillin, or ampicillin can be used.148,149 Cefazolin is mixed to a concentration of 55 mg/mL,150 ampicillin to 10 mg/mL,148 and penicillin G to 100,000 U/mL. If the ulcer continues to worsen, antibiotics should be changed on the basis of the initial sensitivity results, and corneas should always be recultured in these cases because organisms can become resistant to the first antibiotic used. For resistant organisms, sensitivity testing that gives minimum inhibitory concentration (MIC) may be very useful because higher drug concentrations are more readily and safely attainable in the cornea than in the systemic circulation.
Melting of the cornea can be treated with collagenase and protease inhibitors. Experimentally, these drugs were not always effective in reducing melting.151 However, some ophthalmologists believe that these drugs are efficacious. Certainly the main goal of therapy should be to kill the microorganism that is elaborating the enzymes. Autologous serum, EDTA, or acetylcysteine can be used topically, as can systemic doxycycline (10 mg/kg orally twice daily),137 for their antiprotease and anticollagenase activity.
Surgical therapy can also be used and may improve prognosis. Conjunctival pedicle grafts can be used to bring a blood supply and subconjunctival fibroblasts to deep corneal ulcers and possibly slow progression and aid stromal reconstruction149 while still allowing medication to reach the site of the ulcer. A conjunctival graft allows observation of the cornea so that treatment can be changed as corneal health improves or if the cornea continues to deteriorate. A nictitating membrane “flap” should not be used in rapidly progressing or deep ulcers because it does not allow topical medication or observation of the cornea. In the presence of a third eyelid flap, topically applied medications fail to reach the cornea, worsening of the ulcer goes unnoticed, reculturing cannot be performed, and appropriate changes in therapy cannot be instituted.
A corneoconjunctival transposition using autologous tissue or a corneal graft (penetrating keratoplasty) using donor cornea is an excellent treatment to repair very deep ulcers, descemetoceles, and perforations if melting has ceased and the ulcers are sterile.140,151,152 Other substances that can be substituted for cornea are porcine small intestinal submucosa,153 equine amniotic membrane,154 or bovine pericardium (Dura Guard, Synovis). Placement of any graft requires microsurgical instruments, techniques, training, and experience. Highly specialized techniques, such as keratectomy with a conjunctival graft, penetrating keratoplasty, posterior lamellar keratoplasty, or deep lamellar endothelial keratoplasty, are necessary to treat stromal abscesses.155-158
The initial desired response to appropriate therapy of a malacic ulcer is simply cessation of worsening of the ulcer. That is, the ulcer does not appear to be healing or shrinking but also is not becoming larger or deeper. This suggests that bacteria have been killed and tissue destruction has halted. Epithelium will then begin to grow down the sides of the ulcer, covering the stroma or Descemet’s membrane, and blood vessels will slowly begin growing into the cornea from the limbus. Once reepithelialization is complete, new infection with microorganisms is unlikely. If surgery has not been performed, however, the area that had been ulcerated will be (sometimes markedly) thinner than the surrounding stroma. Thickening of this area will occur by very slow reconstruction of stromal collagen by corneal fibroblasts (“keratocytes”) or when corneal blood vessels fill the old ulcer bed. This can take weeks to months, and until this happens, the cornea is susceptible to traumatic rupture.
Another goal of therapy for an infected ulcer is inhibition of “reflex uveitis” associated with any corneal irritation and mediated by the trigeminal nerve. Topical atropine is used for this purpose because it decreases pain associated with ciliary body spasm and maintains pupil dilation, which reduces the chance of posterior synechia formation. Atropine should be applied to effect (i.e., until reduced pain or pupil dilation noted). This may be as frequently as every 1 or 2 hours initially but may soon be limited to once daily or once every other day. Because topical atropine is absorbed into the systemic circulation and is associated with altered GI motility, borborygmi and signs of colic should be assessed frequently. Topical or systemic NSAIDs also can be used to decrease corneal and uveal inflammation. However, these drugs do slow corneal neovascularization to some extent, and topical NSAIDs (as with corticosteroids) are contraindicated in infected ulcers.159,160 Topical corticosteroids are sometimes recommended to reduce corneal vascularization and minimize scar formation once epithelialization is complete. However, corneal vascularization is a critical means by which corneal stroma (and strength) is re-formed, and topical corticosteroids hasten regression of granulation tissue, suggesting that this approach may not be wise. In fact, no evidence indicates that steroid administration decreases the final size of the scar, and steroids may compromise healing and predispose to corneal rupture.
FUNGAL KERATITIS IN HORSES
DEFINITION AND ETIOLOGY
Fungal keratitis (or keratomycosis) occurs when an ulcerated cornea becomes infected with a mycotic organism. As with bacteria, no fungi are known to initiate corneal ulcers. The most common genera isolated in cases of equine fungal keratitis are Aspergillus and Fusarium species, but Cylindrocarpon destructans and Phycomycetes, Penicillium, Paecilomyces, Candida, Mucor, Alternaria, and species of other genera have been cultured.161-165 Horses seem to be unusually susceptible to fungal keratitis compared with the other domestic species.
Clinical Signs and Differential Diagnoses
Fungal keratitis has various manifestations.166 A common presentation is a corneal ulcer, often with a history of chronicity. Typical history is a nonhealing or worsening ulcer despite antibiotic and antiinflammatory therapy. The eye is painful; the conjunctiva is hyperemic; and blepharospasm, epiphora, and apparent photophobia are also present. Corneal edema and cellular infiltrates surround the ulcer. Sometimes the cellular infiltrates can be very dense and appear as a white or yellow area throughout the corneal ulcer. The cellular infiltrate can be deep in the cornea and in some cases on the endothelial surface of the cornea, or even protruding into the anterior chamber, because fungus has a predilection for Descemet’s membrane in the horse cornea. Corneal neovascularization is usually seen (Fig. 39-23). Signs of secondary uveitis can be severe. If ulcerated, fluorescein will stain the cornea. In some cases of fungal keratitis, however, the epithelium heals over the fungal infection, forming a stromal abscess, and the cornea will not stain with fluorescein.
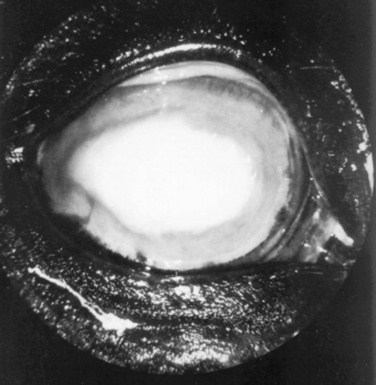
Fig 39-23 Right eye of horse with fungal keratitis. Note corneal neovascularization and dense cellular infiltrates in the cornea.
Fungal keratitis can also present as chronic, mild corneal disease. Small, multifocal, superficial opacities can be seen. In some cases there are small focal areas of fluorescein stain retention; sometimes there is no uptake of stain. Horses usually appear mildly painful with some epiphora; usually there is neither corneal neovascularization nor uveitis.
In all cases of fungal keratitis, differential diagnoses include other causes of corneal ulceration, such as bacteria (which may be coincident).
Clinical Pathology
A diagnosis of superficial fungal keratitis is made when fungal hyphae or yeast are seen on cytology or cultured from corneal scrapings (Fig. 39-24). Because fungi have a predilection for the deeper stroma and Descemet’s membrane, a diagnosis of deep keratitis may require a full-thickness corneal biopsy from which the fungus is identified using culture or histopathology. Unfortunately, this predilection means some diagnoses of fungal keratitis are made only after enucleation.167 If culture of an ulcer is positive when scrapings or biopsy do not show hyphae, the possibility of a commensal organism or incidental surface contaminant must be considered; especially if only one or two colony-forming units are cultured. Because treatment is prolonged and expensive, a definitive diagnosis of fungal colonization ideally should be made before treatment is initiated. The diagnostic utility of PCR for fungal keratitis is currently under investigation.
Pathophysiology
Fungal hyphae do not colonize intact cornea. Damage to epithelium is necessary for pathogenic or opportunistic fungi to begin growth in the corneal stroma. This usually results from a traumatic incident that may or may not be noticed by the owner. The use of antibiotics and corticosteroids alters normal flora and decreases the normal immune response, which may encourage fungal growth. Fungi implicated in keratomycoses are usually present in the horse’s environment and can be cultured from more than 90% of normal horse eyes, with Aspergillus species being the most common isolate.161 There is one report in the literature of keratomycosis caused by Candida albicans secondary to disseminated candidiasis.168
Treatment and Prognosis
Treatment consists of eliminating the fungus from the cornea and controlling secondary bacterial invasion and melting, along with reflex uveitis (if present), as for bacterial keratitis. Antifungal agents are required, and the course of treatment usually lasts for a number of weeks. Subpalpebral lavage systems are usually essential for delivering these drugs. Healing is usually not complete until corneal neovascularization has reached the infected area, except when the fungus has reached Descemet’s membrane, and in these cases blood vessel growth does not always stop fungal growth. Medical therapy is most effective for treating superficial disease. For deep disease, surgical therapy can also be used with the medical therapy. Deep lamellar and penetrating keratoplasties probably carry the best prognosis,150,155,167,169 although superficial keratectomies with a conjunctival graft can also be used for more superficial lesions.
A number of antifungal agents are available. Miconazole, used as the undiluted IV preparation, was once the drug of choice for many ophthalmologists. Unfortunately, it is no longer available commercially in this formulation, although it can be compounded. Natamycin (Natacyn) is manufactured as an ophthalmic suspension and is the drug of choice for treating fungal keratitis in humans. However, natamycin does not penetrate intact epithelium well, and some Fusarium species may be resistant to this drug.170 Fluconazole (Diflucan) is now often recommended as a replacement for miconazole. However, one study reported that fluconazole had lower in vitro activity than a number of other antifungal drugs.171 The IV preparation is a 2 mg/mL solution, which is used undiluted as a topical preparation. Miconazole and fluconazole do penetrate intact corneal epithelium when given topically, but drug concentrations in the stroma are higher if epithelium is absent.172 Fluconazole can also be used subconjunctivally, intracamerally, or intravitreally. Ophthalmologists are also beginning to use fluconazole systemically to treat deep corneal fungal disease because this drug does reach adequate concentrations in the aqueous humor, which may be appropriate for deep stromal or endothelial infections.173 No toxicity studies have been performed on the horse, but anecdotal evidence does not suggest toxicities, and fluconazole has been associated with resolution of deep disease.
Compounded formulations of itraconazole/DMSO ointment have also been used for equine keratomycosis. High concentrations of itraconazole can be achieved in the corneal stroma with this preparation, even when the overlying epithelium is intact, and it has been used successfully in clinical cases.174 In contrast to fluconazole, systemically administered itraconazole does not penetrate normal equine eyes.175
Other drugs have been suggested as treatment for fungal keratitis. Voriconazole administered either topically or systemically does penetrate normal equine eyes and reaches concentrations that could be therapeutic.176 Miconazole dermatologic or vaginal creams seem to work for mild, superficial disease; however, no clinical trials describing their efficacy and safety for ocular use have been reported. Amphotericin B has good antifungal properties but can be very irritating and is probably not the drug of choice. In vitro research shows that silver sulfadiazine is fungicidal against isolates from equine patients with keratomycosis.170 Unfortunately, in vitro results do not necessarily correlate with clinical results in patients with keratomycosis. Corneal penetration of the drug, the horse’s immune system, and the site and severity of the fungal infection all play important roles in choice of drug.170
In addition to the antifungal agent selected, topical mydriatic/cycloplegic agents such as atropine should be used to combat the accompanying uveitis, and topical antibiotics are indicated to prevent superinfection with bacteria. Systemic NSAIDs should be used for the secondary uveitis, especially at the beginning of treatment, when fungal death can exacerbate the uveitis. However, NSAIDs probably slow neovascularization of the cornea, and treatment with these drugs should be decreased as the uveitis is controlled.177
Prognosis is guarded to poor in many cases of fungal keratitis. Usually the best possible outcome is a visual eye with some degree of residual corneal scarring and possibly synechiae. Because no evidence suggests that corticosteroids decrease the eventual size of the scar, and because they will promote the presence of residual hyphae, the use of corticosteroids is not recommended in a healing fungal ulcer. Complications of fungal keratitis include perforation of the ulcer with loss of the eye, superinfection with bacteria, and phthisis bulbi.
UVEITIS ASSOCIATED WITH LEPTOSPIROSIS IN HORSES AND COWS
Leptospirosis is caused by a filamentous bacterium known as a spirochete. Disease is seen in most domestic animals as well as humans. Various serovars of Leptospira interrogans sensu stricto, L. kirschneri, and L. santarosai have been shown to affect various organs, such as kidneys, liver, spleen, muscles, CNS, and eyes and have been associated with abortions.178 These organisms primarily cause a vasculitis and endotheliitis in these organs.179
Because leptospiral organisms cause vasculitis, it is reasonable to assume that uveitis might be present in an acute infection, and this has been reported in horses during the acute phase of leptospirosis.180 Uveitis has also been seen experimentally in a calf during acute disease.181 However, the role of leptospires in uveitis seen weeks to months after the acute disease remains much more controversial. Evidence for such a role is most complete in the horse, in which leptospiral uveitis and its role in equine recurrent uveitis (ERU) or “periodic ophthalmia” have been described.180,182,183 Uveitis in these horses was bilateral or unilateral and frequently was recurrent, leading to loss of or decrease in vision.182 Uveitis was not seen until 18 to 24 months after the acute outbreak of leptospirosis. Treatment with systemic antibiotics did not seem to affect the uveitis. In this study, serum titers to L. interrogans serovar pomona often remained high for at least 6 years.182 In another study, uveitis was seen in 22 eyes of 18 ponies experimentally infected with serovar pomona. The earliest sign of uveitis was seen at 1 year after inoculation. Anterior uveitis with cataract formation and posterior synechiae were also seen; as were recurrences.183
Recently, leptospiral organisms have been identified using PCR184 or by culture184-186 in the eyes of horses with ERU. A study in California using a PCR assay for Leptospira showed that 30 of 55 eyes (21 of 30 horses) with ERU had detectable Leptospira DNA in their aqueous humor.184 Interestingly, in other studies, treatment with systemic antibiotics did not decrease the inflammation.187-189 In Western Europe a total of 618 vitreous and/or aqueous samples were taken from the eyes of 501 horses with either active ERU or a history of ERU. Leptospires were isolated from 199 (32.2%) of the samples. Most belonged to serogroup grippotyphosa, with the rest in serogroup australis, sejroe, pomona, or javanica.185 In contrast, 36 samples of vitreous from 21 normal horses did not grow leptospires.186 Although leptospires are involved in ERU in many horses with this syndrome, controlling or treating this disease remains a problem. The best means of symptomatic control of ERU at this time appears to be the use of ocular bioerodible cyclosporin A implants.190 These are placed intravitreally or, more recently, suprachoroidally and were investigated for this drugs ability to control immune-mediated inflammation elsewhere in the body and within the eye. More recently, however, cyclosporine has been shown to be toxic to L. interrogans in vitro at the same concentration that is achieved in uveal tissue with a suprachoroidal implant.190 In one study a leptospiral vaccine in horses with ERU was not recommended.191 (See also Equine Recurrent Uveitis.)
OCULAR MANIFESTATIONS OF EQUINE ADENOVIRUS
Equine adenovirus is a DNA virus that causes bronchopneumonia in foals, especially if they are immunodeficient. Mucopurulent nasal and ocular discharge accompanies the respiratory system disease. Histologically, swelling and necrosis of conjunctival cells with intranuclear inclusions are seen, with accumulation of neutrophils in the lumina and adventitia of uveal blood vessels.192
OCULAR MANIFESTATIONS OF SALMONELLOSIS IN HORSES
Salmonella species cause one of the more common and serious bacterial enteritides in foals and adult horses. It is often accompanied by septicemia in foals. Anterior uveitis and hypopyon have been seen in animals with salmonellosis, and Salmonella species can sometimes be cultured from these eyes.193
MORAXELLA CONJUNCTIVITIS IN HORSES
Two reports have described a Moraxella species recovered from several horses in herd outbreaks of conjunctivitis, ocular discharge, and erosions of eyelid epithelium at the canthi.194,195 The organism was similar but not identical to M. bovis, and the disease was reproduced experimentally in horses by instillation of the organism into the conjunctival sac. Huntington et al.195 described successful treatment of the lesions with chloramphenicol ointment, whereas Hughes and Pugh194 described the lesions as healing spontaneously.
OCULAR MANIFESTATIONS OF EQUINE VIRAL ARTERITIS
Equine viral arteritis is a rare disease caused by an RNA virus classified as Arterividae. Most animals are subclinically infected, but ocular and nasal discharges; palpebral, periorbital, limb, or ventral edema; skin rash; pyrexia; rhinitis; leukopenia; abortions; and neonatal death are seen.196,197 Corneal opacity and apparent photophobia have also been described.198 The virus characteristically causes a panvasculitis.
OCULAR MANIFESTATIONS OF RHODOCOCCUS (CORYNEBACTERIUM) EQUI IN HORSES
Rhodococcus equi is a gram-positive coccobacillus that causes bronchopneumonia in young foals. One report of R. equi from the eye of a foal with bilateral panophthalmitis and pneumonia can be found.199 Clinically, the foal had bilateral miosis and hypopyon.
OCULAR MANIFESTATIONS OF BORRELIOSIS IN HORSES
Borrelia burgdorferi, the agent of Lyme disease, has been most often described as causing polyarthritis in horses, cows, and dogs. However, one case of apparent ocular disease has been reported in a pony infected with B. burgdorferi.200 Unilateral anterior and posterior uveitis was noted, and spirochetes were found in the anterior chamber. Other clinical signs included arthritis and synovitis of both carpal joints.
OCULAR MANIFESTATIONS OF CRYPTOCOCCOSIS AND HISTOPLASMOSIS IN HORSES
Exophthalmia and blindness caused by Cryptococcus neoformans have been described in a horse.201 The frontal sinus and retrobulbar area were involved with a fungal granuloma, but the eye itself was normal. The chorioretinitis seen in other species associated with C. neoformans has not been described in the horse. Cryptococcus albidus has been cultured from the cornea of a horse with chronic keratitis.202 Organisms consistent with Histoplasma spp. were seen on cytology of a corneal scraping taken from a horse with chronic keratitis.203 The horse was successfully treated with topical fluconazole.
OCULAR MANIFESTATIONS OF EQUINE HERPESVIRUS TYPE 2 (EHV-2)
Equine herpesvirus serotype 2 (EHV-2) has been isolated from eyes in herd outbreaks of keratoconjunctivitis in horses.204,205 Clinical signs in one outbreak included apparent photophobia, epiphora, corneal neovascularization, corneal color change, and pinpoint ulcerations; eyes healed within 2 weeks.204 In the other outbreak, conjunctivitis, and multifocal superficial corneal opacities were seen; eyes healed within 2 weeks on topical idoxuridine.205 Experimental inoculation of EHV-2 intranasally in two ponies pretreated with dexamethasone caused conjunctivitis, as well as lymphadenopathy and coughing.206 Conjunctiva from both ponies was positive for virus by PCR 6 months after inoculation. EHV-2 can also be isolated from the blood of normal horses,207 and positive PCR results can be obtained from normal eyes as well as eyes with keratoconjunctivitis, making diagnosis difficult.
Miller et al.208 confirmed EHV-2 by fluorescent antibody staining after isolating virus from the cornea of a thoroughbred mare with multiple superficial punctate corneal lesions. The keratitis was successfully treated with topical 1% trifluridine ophthalmic solution.*
OCULAR MANIFESTATIONS OF STRANGLES (STREPTOCOCCUS EQUI SUBSP. EQUI)
Strangles is a respiratory infection caused by Streptococcus equi subsp. equi that can have an accompanying ocular discharge.209 Chorioretinal depigmentation was noted in the nontapetal fundus of several horses in one group clinically diagnosed with strangles. Because these lesions repigmented with time, it was suggested that they were caused by embolism to the choroid during bacteremia.210 One case of panophthalmitis caused by S. equi in a horse has been described.211 Ten days after a bout of strangles, this horse developed anterior uveitis, which progressed to corneal stromal abscesses and panophthalmitis. S. equi was cultured from the eye at enucleation.
OCULAR MANIFESTATIONS OF EQUINE HERPESVIRUS TYPE 1
Equine herpesvirus serotype 1 (EHV-1) is a cause of rhinopneumonitis in horses. It has been cultured from cases of superficial punctate keratitis in the horse; the significance of these isolations is unknown.212
Six foals were experimentally inoculated intranasally with EHV-1.213 All developed typical mild signs of upper respiratory infection. One foal developed vision problems 1 month after inoculation. Bilateral chorioretinitis was diagnosed. On necropsy, chorioretinal degeneration with mononuclear cell infiltration in some areas was seen, as well as demyelination of the optic nerve and mononuclear cell infiltrate in parts of the CNS.213 These findings are not surprising in that EHV-1 causes vasculitis and CNS disease in horses.214
OCULAR MANIFESTATIONS OF BRUCELLOSIS IN HORSES
Brucella abortus has been suggested as a cause for ERU,215 although serum agglutination titers for B. abortus in normal horses and horses with ERU are similar.216
OCULAR MANIFESTATIONS OF MYCOBACTERIUM AVIUM IN HORSES
A case of anterior uveitis and bilateral chorioretinitis with retinal detachments has been described in a horse from Denmark. Acid-fast organisms were seen in both eyes as well as in numerous other organs. Mycobacterium avium was cultured from these organs.217
INFECTIOUS BOVINE KERATOCONJUNCTIVITIS
Infectious bovine keratoconjunctivitis (IBK), or “pinkeye,” is the most common ocular disease of cattle and has been identified in cattle populations worldwide. The clinical signs of IBK include corneal ulceration, corneal edema, photophobia, blepharospasm, and lacrimation. In less severely affected animals, recovery with or without permanent corneal scarring occurs. In the most severely affected animals, corneal rupture and lens/iris prolapse may occur, causing permanent blindness. IBK is most common in calves, typically affecting one eye, although both eyes may be affected. Estimates of annual incidence of IBK are 5% of all beef cattle, with more than 50% of herds affected.218 Epizootics occur, with case-attack rates approaching 90% to 100% of yearling cattle.219,220 Cattle of all breeds may be affected; however, a higher incidence is reported in Herefords221 and a lower incidence in Brahmans and cattle with more pigmentation at the ocular margins.222,223
Economic Impact
Cattle with IBK have reduced weight gain that results in economic losses to producers. Postweaning 205-day weights of bulls and heifers with IBK showed losses of 36 and 40 pounds (16 and 18 kg), respectively, over unaffected herdmates.224 In 1169 pasture-raised calves over a 4-year period, the average weight reduction was 11 pounds (5 kg) for calves with IBK in one eye and 35 pounds (15.75 kg) for calves with IBK in both eyes.225 A recent study of more than 45,000 health records of weaned calves demonstrated almost 20-pound (9-kg) lighter weaning weights in calves diagnosed with IBK versus healthy calves.221 The economic losses from reduced weight gains along with treatment-associated expenses account for annual losses in the United States estimated at $150 million in 1976.226 It is likely that current losses greatly exceed this estimate.
Etiology and Epidemiology
Moraxella bovis is the only organism for which Koch’s postulates have been fulfilled with respect to IBK.227 However, other viruses and bacteria have also been associated with IBK. Infectious bovine rhinotracheitis (IBR) virus228,229 and Mycoplasma species230 are probably the most important and likely act as risk factors for IBK by enhancing opportunities for corneal injury231-233 and increasing ocular and nasal discharge, which may facilitate transmission of M. bovis.
Other potentially pathogenic bacteria have been isolated from the eyes of cattle with IBK. In 1966, hemolytic gram-negative cocci were isolated from calves with severe keratitis and corneal ulceration.234 In Australia, Neisseria species were isolated in 24 of 25 outbreaks of IBK; M. bovis was identified in only two of these outbreaks.235 Neisseria (Branhamella) catarrhalis was reported in almost 45% of IBK cases, whereas M. bovis was isolated from only 28% of IBK cases.236 M. bovis and Neisseria species were also cultured from normal eyes of cattle.237,238 M. ovis and Neisseria ovis were reported from cattle in Israel with IBK.239,240 During summer 2002 in northern California, the majority of bacterial isolates from IBK-affected calves were hemolytic gram-negative cocci.241 N. ovis experimentally inoculated into the eyes of calves did not cause lesions typical of IBK, despite previous corneal irradiation.242M. bovis has been reported in mule deer with keratoconjunctivitis, although experimental inoculation of M. bovis isolates into eyes of mule deer fawns did not result in disease.243 Recently, hemolytic and cytolytic activity from culture filtrates of M. ovis isolated from cattle with IBK has been described, suggesting a possible role for bacteria other than M. bovis in IBK pathogenesis.244
In addition to bacterial infection, other risk factors for IBK include flies, solar irradiation, and mechanical trauma from plant awns. M. bovis survives up to 3 days on the external surface245 and 2 days in the gut246 of face flies (Musca autumnalis). IBK was experimentally induced in cattle that were exposed to face flies that had fed on M. bovis cultures.247 Insecticide-impregnated ear tags or back/face rubbers to reduce fly populations have proved effective in reducing IBK in cattle populations.248 An association between solar irradiation and IBK is also documented.249-251 Corneas of calves exposed to ultraviolet (UV) irradiation incur corneal epithelial cell degeneration 252 that predisposes eyes to establishment of M. bovis and IBK. An experimental model for IBK has been developed in which calves are exposed once daily for 2 or 3 days to UV irradiation using sunlamps held 24 inches (60 cm) from the corneal surface; after the last exposure, animals are infected by instillation of M. bovis into the eye.253-255 Plant awns, by causing mechanical corneal damage, can predispose eyes to infection with M. bovis and IBK.
Hemolytic strains of M. bovis are considered pathogenic, whereas nonhemolytic, nonpathogenic M. bovis can be isolated from normal cattle,219,256-258 from cattle exhibiting conjunctivitis,259 and simultaneously with hemolytic M. bovis from cattle with IBK.258 Outbreaks of IBK typically occur annually during summer months, and such outbreaks may be caused in part by cattle harboring M. bovis subclinically.258,260 Shifting between hemolytic and nonhemolytic phenotypes of M. bovis has also been described.261
Pathophysiology of Moraxella bovis
M. bovis produces many hydrolytic enzymes, including C4 esterase, C8 esterase-lipase, C14 lipase, phosphoamidase, phosphatase, leucine and valine aminopeptidases, and gelatinase.262 Of these, however, only two proteins have been linked to pathogenicity: pili and cytotoxin. Pilin proteins of M. bovis are of the N-methylphenylalanine type (type 4 pili263-265) and enable bacteria to adhere to the corneal epithelium and colonize the surface of the cornea.266-268
The M. bovis cytotoxin (cytolysin/hemolysin) is a pore-forming protein that is also considered necessary for pathogenesis. Broth supernatants of hemolytic, but not nonhemolytic, strains of M. bovis will cause lysis of bovine erythrocytes, neutrophils, lymphoma cells, and corneal epithelial cells in vitro.269-272 The lytic activity of M. bovis cytotoxin occurs through calcium-dependent formation of transmembrane pores in target cell membranes.273 Ocular lesions induced by a purified hemolytic and cytolytic fraction of M. bovis are identical to the ocular lesions observed in naturally occurring IBK; extracts from nonhemolytic M. bovis do not result in corneal lesions.274
An association between the M. bovis cytotoxin and the RTX (repeats in the structural toxin) family of bacterial exoproteins followed the discovery that M. bovis cytotoxin induces the formation of pores in target cell membranes.273 It was subsequently shown that an approximately 110-kilodalton protein in concentrated culture supernatants from cytolytic M. bovis cultures could be recognized by a monoclonal antibody to HlyA, an RTX toxin of uropathogenic Escherichia coli.275 The presence of RTX toxins has been reported in a numerous animal pathogens, including Mannheimia (Pasteurella) haemolytica, the agent of shipping fever pleuropneumonia in cattle;276 Actinobacillus pleuropneumoniae, an agent of swine pleuropneumonia;277 Pasteurella aerogenes, an abortifacient in swine and other mammals;278 and Actinobacillus equuli, associated with foal septicemia.279 Enterohemorrhagic E. coli O157:H7 also harbors a plasmid-encoded RTX toxin.280
The best-characterized RTX toxin is HlyA of uropathogenic E. coli. The gene encoding this toxin is assigned the abbreviation hlyA and is contained within a four-gene operon organized 5′-C-A-B-D-3′. The product of the RTX A gene is a structural toxin that must be activated by the RTX C gene product to become hemolytic.281-283 The activation occurs through fatty acylation of conserved lysine residues.284,285 After activation the toxin is secreted by membrane transport proteins encoded by the B and D genes and a third protein, TolC.286,287 The regulation of transcription through the RTX operon in E. coli is a complex process and involves the protein RfaH and JUMPStart DNA sequences.288 As with E. coli hemolysin, the M. bovis cytotoxin gene (mbxA) is contained within an operon that encodes activation and export proteins; this operon is called the mbx operon of M. bovis and is absent in nonhemolytic M. bovis.289 The mbx operon defines a pathogenicity island, and acquisition/loss of mbx genes may explain the ability of M. bovis to change from the hemolytic to nonhemolytic phenotype.290
Immunity to Moraxella bovis
Secretory IgA is the major immunoglobulin found in normal bovine lacrimal secretions.291 During experimentally induced IBK, tear IgG1 and IgG2 concentrations increase.292 Early studies suggested that calves with more severe IBK had higher lacrimal IgA titers to crude M. bovis antigen preparations than calves with less severe IBK.293 A subsequent study identified a predominant tear IgG response to a crude, whole-cell M. bovis antigen in calves with naturally occurring IBK and concluded that M. bovis—specific antibodies in lacrimal secretions did not prevent IBK in calves.294 Enzyme-linked immunosorbent assay (ELISA) has been used to quantify nonspecific M. bovis antigens in tears and has revealed that IgA titers are higher than IgG titers.295 A study on a small number of calves then suggested that both lacrimal (secretory IgA) and humoral (IgG) antibodies against M. bovis whole-cell antigen conferred resistance against IBK, versus a humoral IgG antibody response alone.296 It was concluded that serum antibodies against M. bovis may account for a reduction in the length and severity of clinical signs associated with IBK. Unfortunately, none of these studies adequately controlled for total antibody isotypes present in serum or ocular secretions. In addition, crude M. bovis antigen preparations were used in these assays.
In other diseases associated with RTX toxin—producing pathogens, the protective role of immunity against RTX toxins is not well understood. In one study a Mannheimia (Pasteurella) haemolytica leukotoxin subunit vaccine was not protective;297 however, calves infected with a mutant strain of M. haemolytica that secreted inactive leukotoxin had reduced lung lesion scores but similar clinical severity scores as calves receiving wild-type M. haemolytica.298 The introduction of genes encoding the hemolysin (HlyA) into other E. coli strains increased virulence,299 although an HlyA-deficient mutant was still pathogenic.300 Pigs infected with nonhemolytic Actinobacillus pleuropneumoniae developed lung lesions that were similar to lesions in pigs infected with a virulent strain, and it was concluded that the hemolysin of A. pleuropneumoniae serotype 2 was not essential for disease.301 However, another study found that pigs vaccinated with A. pleuropneumoniae serotype 1 hemolysin were protected after challenge.302,303 These studies underscore the fact that immunity to diseases caused by RTX toxin—producing bacteria is complex and may not depend on an antibody response to an RTX toxin alone.
Experimental Vaccination
Early studies that reported reduced M. bovis infection rates and decreased occurrence of IBK after reexposure to M. bovis indicated that vaccination against IBK might prevent disease.250 Subsequent work showed that calves vaccinated intramuscularly with live M. bovis had less severe IBK after challenge.304 Formalin-killed M. bovis was also reported to be as effective as live cultures in preventing experimentally induced IBK305; under field conditions, however, a formalin-killed autogenous bacterin was not efficacious.306
In an effort to identify other candidate M. bovis vaccine antigens, researchers began to examine the use of component or subunit vaccines to prevent IBK. In early studies, M. bovis pilin antigens were found to protect calves from homologous challenge.307 Purified M. bovis ribosomes were not protective.308 Bacterin-containing pili plus corneal-degrading enzymes were protective in field trials, and protection was correlated with the corneal-degrading enzyme level in the vaccine.309 In that study, however, the exact composition of corneal-degrading enzymes was not reported. In a later study, two commercial M. bovis pilus-based vaccines were not protective for calves in a heterologous challenge model.310 Although pilin is immunogenic, there is marked antigenic diversity between different pilin types because of the presence of two structural pilin genes and variability in the amino acid composition of the pilin molecule caused by inversions within pilin genes.267,268,311,312 Limited antigenic cross-reactivity was reported between heterologous pili,313,314 and emergence of novel pilus types can precipitate IBK outbreaks.315 Such antigenic variability is believed to reduce the overall efficacy of pilin-based vaccines. Nevertheless, more recent work has demonstrated conserved epitopes across different pilin types,316,317 suggesting a possible future role for conserved pilin antigens in vaccines against IBK.
Unlike pili, the M. bovis cytotoxin seems to be more conserved across different M. bovis strains. IBK-affected cattle were shown to develop systemic immune responses to cytotoxin318-321 and antihemolysin antibodies to one M. bovis strain, neutralized hemolysin from 33 different strains of M. bovis.321 A partly purified cytotoxin vaccine also protected calves against IBK after challenge with heterologous M. bovis.318 These reports suggest a role for a cytotoxin-based vaccine to prevent IBK from multiple M. bovis strains, which could therefore be superior to traditional pilus-based vaccines.
Methods to purify partly the M. bovis cytotoxin have now been published,322 and its efficacy in a vaccine to prevent IBK has been demonstrated.323 The labor-intensive process of preparing native cytotoxin has also led to the investigation of recombinant M. bovis cytotoxin vaccines to prevent IBK. In one field trial, calves vaccinated with the recombinant carboxy terminus of M. bovis cytotoxin had a lower cumulative proportion of ulcerated eyes compared with saline and adjuvant control calves.324
Parenteral routes of vaccination have been tested most often, although aerosol vaccination also has been found to be effective against IBK.325
Treatment and Prevention
IBK prevention hinges on minimizing risk factors for disease, reducing infection of the ocular surface with M. bovis through antimicrobial use, and vaccination. To reduce fly populations, insecticide-impregnated ear tags and topical insecticides with back and face rubbers are employed. Clipping mature grasses before cattle are turned out may also help minimize risks associated with direct mechanical corneal injury from plant awns.
Moraxella bovis is susceptible to numerous approved antimicrobials. These include penicillin administered subconjunctivally,326 oxytetracycline parenterally or orally (20 mg/kg once or twice followed by 2 g/calf/day for 10 days),327-329 florfenicol intramuscularly (two 20-mg/kg injections 48 hours apart) or subcutaneously (40 mg/kg once),330,331 ceftiofur crystalline-free acid subcutaneously (6.6 mg ceftiofur equivalents/kg once) in the posterior aspect of the pinna,241 and tulathromycin (2.5 mg/kg) subcutaneously.332
For vaccination against IBK, most producers use commercially available bacterins. Many different vaccines are available but are not universally effective, as previously discussed. Anecdotal evidence suggests that autogenous vaccines against non—M. bovis isolates of gram-negative cocci from IBK-affected eyes may provide an alternative prevention strategy to conventional commercial M. bovis vaccines when such vaccines are ineffective.
IMMUNE-MEDIATED OCULAR DISEASES
Except for the conjunctiva, the eye has no lymphatic drainage. Access of antigens to potentially reactive lymphoid tissue is also restricted by the avascularity of the cornea and the presence of selective blood-ocular barriers. As unlikely as immunologically mediated abnormalities might seem under these circumstances, immune-related inflammation remains a leading cause of blindness in the horse. Reports of immune-mediated ocular disease in ruminants are rare.
Ocular Immunology
The conjunctiva represents an extension of the mucosal immune system. A variety of immune cells can be found in conjunctival tissue, including intraepithelial CD8+ lymphocytes and mast cells, as well as aggregates of CD4+ T-helper (Th) and B lymphocytes, CD1+ dendritic cells and macrophages, and within the substantia propria.333 The conjunctiva processes ocular surface antigens with the help of regional lymph nodes. Antigen presentation is likely preceded by local tissue damage and release of inflammatory mediators that recruit inflammatory cells to the site. Under the influence of cytokines such as interferon alpha (IFN-α), tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-12 released by local and recruited cells, tissue dendritic cells process and carry host and pathogen molecules to regional lymph nodes to activate CD4+ Th cells. Stimulated lymphocytes migrate back to the conjunctiva (T cells) and lacrimal gland (B cells), where they participate in cell-mediated reactions and local production of IgA and to a lesser degree IgG, respectively.333-335
Because the globe itself is without lymphatic drainage, intraocular antigens must be processed at a distant site. These antigens pass into the systemic circulation, preferentially stimulating the spleen as well as the bone marrow and other distant lymphoid organs. After 5 to 7 days, sensitized lymphocytes migrate back to the eye and localize within the uvea and limbal conjunctiva. As with those on the ocular surface, these immunologically competent cells are capable of antibody production and can participate in cell-mediated reactions. Further exposure to the same antigen can provoke an anamnestic response, endowing the uveal and limbal tissues with behavior comparable to that of a regional lymph node.336
The intraocular immune response varies from that of a classic immune reaction, presumably to limit excessive inflammation within the eye. Anterior chamber—associated immune deviation (ACAID) is characterized by preferential stimulation of T suppressor cells that inhibit development of both CD4+ and B cells necessary in delayed-type hypersensitivity reactions and production of complement-fixing isotypes of antibody, respectively.333,337 Cells in the uvea and corneal endothelium also express Fas ligand (FasL), which limits inflammation through apoptosis of activated leukocytes entering the eye.333
Each of the four major types of immunologic reactions occurs within the eye.338 Type I (immediate) hypersensitivity is usually manifest as conjunctivitis, an acute local inflammatory reaction that follows IgE-mediated release of histamine, proinflammatory cytokines and chemotactic mediators from tissue mast cells, and synthesis of cytokines, leukotrienes, prostaglandins, thromboxane, platelet-activating factor (PAF), and kinins.339 These mediators of the IgE/mast cell inflammatory pathway increase vascular permeability, constrict smooth muscle, dilate blood vessels, and activate leukocyte chemotaxis and the complement cascade. Allergic reactions of the lids and conjunctiva undoubtedly occur in all domestic species.
Type II (cytotoxic/cytolytic) hypersensitivity is an antibody-mediated cytolytic reaction in which the antigen is a cell surface or basement membrane component. Three basic effector pathways lead to cell destruction: (1) opsonization, with increased efficiency of phagocytic destruction; (2) antibody-dependent cellular cytotoxicity, inducing the release of enzymes capable of destroying cells and digesting basement membranes; and (3) lysis of immunoglobulin-bearing cells.340 Conjunctival damage resulting from autoantibodies directed against epithelial basement membranes is described in equine ocular pemphigoid.
Type III (immune complex) hypersensitivity may share similar effector mechanisms with those described for cytolytic reactions, but antigen locale accounts for dissimilar disease manifestations in these two pathways.339 This immune complex reaction may explain the clinical signs of pemphigus foliaceus341 and the intraocular inflammation observed in horses after influenza vaccination or contact with infected animals.342 Antibody-mediated cell destruction has also been implicated in uveitis.338
Type IV (cell-mediated/delayed) hypersensitivity is an important factor in contact allergy of the lids and conjunctiva and may also play a role in ocular toxoplasmosis. The tissue destruction associated with herpetic keratoconjunctivitis in the horse has been attributed to a cell-mediated response.338 Increasing evidence implicates delayed hypersensitivity in the pathogenesis of equine recurrent uveitis.343,344 The reaction requires an initial antigen exposure that results in sensitization of antigen-specific T lymphocytes. Reintroduction of antigen induces interleukin production, with subsequent T-cell activation, proliferation, and cytokine production. Once activated by cytokines, recruited leukocytes display increased activity to many antigens.345
ALLERGIC BLEPHAROCONJUNCTIVITIS
In humans, several forms of allergic conjunctivitis are mediated by immunoglobulin E (IgE), and histamine (H2) receptors have been discovered on the human ocular surface.346 The ubiquitous presence of H2 receptors in domestic animals implies a similar distribution334 and the potential for immediate hypersensitivity reactions of the eyelids and conjunctiva.
Affected animals demonstrate acute swelling of the eyelids and conjunctiva, accompanied by serous ocular discharge, mild conjunctival hyperemia, and pruritus. If the stimulus persists, multiple subconjunctival aggregates of lymphocytes appear as tiny, semitransparent follicles within the conjunctival cul-de-sac. In contrast to bacterial conjunctivitis, crusting and purulent discharge are not typical of allergic conjunctivitis.
Diagnosis of allergic blepharoconjunctivitis is often presumptive, based only on careful and thorough elimination of all other causes of lid and conjunctival swelling. Trauma, orbital inflammation, neoplasia, mechanical irritants, conjunctival parasites, and other infectious agents (both ocular and systemic) should be considered. In support of an allergic etiology, conjunctival cytology may reveal eosinophils in response to mast cell degranulation.
The offending allergen may be difficult to identify. Insect stings and toxic plants (e.g., nettle) are possible causes, as are molds and pollens. Allergic conjunctivitis was described in 17 of 187 cows pastured adjacent to a field of blossoming cotton.347 A group of Angus-Holstein cattle demonstrated excessive lacrimation and ocular pruritus associated with familial allergic rhinitis. Several inhaled allergens have been incriminated, including capeweed, clover, dock, lucerne, pepper tree, paspalum, wattle, ryegrass, sorrel, and fungal extracts.348 New feeds and certain drugs (e.g., oxytetracycline, penicillin, sulfas) may produce generalized urticaria, with accompanying eyelid and conjunctival edema.349 Similar findings have been reported in cattle with milk allergy. Agents directly inducing mast cell degranulation through osmotic or charge interactions include hypertonic saline, nonsteroidal antiinflammatory drugs (NSAIDs), thiopental, opiates, neuromuscular blocking agents, mannitol, radiocontrast agents, polymyxin B, and vancomycin.350 Occasionally, allergic conjunctivitis may be associated with a topical medication such as neomycin. Clinical signs exacerbate with continued application and diminish when the medication is discontinued.
Ocular signs subside with removal of the offending allergen, but this is often impractical. Individual animals may be treated with a topical ophthalmic corticosteroid preparation such as 0.05% dexamethasone ointment* to hasten resolution of swelling and redness. An agent with antiprostaglandin activity such as oral or parenteral flunixin meglumine† (0.5 mg/kg every 12 hours) may be of benefit in the horse. Signs associated with urticaria respond to a decreasing regimen of oral prednisone or prednisolone, initiated at a dosage of 1 mg/kg once daily in the nonpregnant animal.351 Single parenteral doses of short-acting corticosteroids, epinephrine, or antihistamines have also been used with reported success in food animals.348
OCULAR MANIFESTATIONS OF IMMUNE-MEDIATED DERMATOSES
Pemphigus refers to a group of chronic blistering diseases that affect healthy skin and mucous membrane. Although these disorders are of presumed autoimmune origin, their exact pathogenesis is unknown. Direct immunofluorescence reveals intercellular deposition of immunoglobulin G (IgG) and complement within the epidermis. Circulating autoantibodies can be demonstrated in humans.352 Pemphigus foliaceus and bullous pemphigoid have been described in the horse.353-356
Ocular manifestations may include ulceration or crusting of the periocular skin and erosions of the conjunctiva. Chemosis and hyperemia are likely; secondary corneal disease may follow that of the mucous membranes. Diagnosis is based on clinical findings, cytology, histopathology, and positive immunofluorescence of affected skin. See the discussion of immune-mediated dermatologic disorders for therapeutic recommendations and prognosis.
Other immune-mediated diseases, such as systemic lupus erythematosus (SLE), occur infrequently in the horse but can affect the eyelids. In addition to skin lesions, vasculitis may be manifest by hemorrhages in mucous membranes, including the conjunctiva.353,357
EOSINOPHILIC KERATOCONJUNCTIVITIS
Eosinophilic keratoconjunctivitis is an uncommon disorder of horses characterized clinically by corneal ulceration and plaque formation in one or both eyes.358-361 Its name is derived from the predominance of eosinophils found in cytologic samples. The specific cause of this disorder is still unknown.
Clinical Signs
Clinical signs may be unilateral or bilateral and include nonspecific signs of blepharospasm, ocular discharge, and conjunctival hyperemia. Perilimbal corneal ulcers appear as raised, white corneal plaques because of adherent caseous exudates, often accompanied by corneal edema and superficial vascularization.
Diagnosis
Differential diagnoses include mycotic keratitis, onchocercal keratoconjunctivitis, neoplasia, foreign body granuloma, traumatic keratitis, and calcific degeneration. Definitive diagnosis is based on clinical signs and cytologic findings. Eosinophils and segmented neutrophils predominate in corneal scrapings, with fewer mast cells, plasma cells, and lymphocytes. Light microscopy of corneal tissue reveals coalescing foci of degenerated collagen fibers in the corneal plaques.
Pathophysiology
The exact cause of the disorder is unknown. The finding of eosinophils in equine ocular surface disease is usually attributed to parasitic infection by Onchocerca or Habronema species,362 although neither has been identified in the reported cases to date. One proposed mechanism for eosinophilic keratoconjunctivitis is an allergic or inflammatory response to long-term use of ivermectin as an anthelmintic, triggering the complement cascade and cellular chemotaxis in patients with ocular onchocerciasis.359 Similarities to vernal keratoconjunctivitis in humans suggest that eosinophil-granule major basic protein may play a significant role in the equine disease, inhibiting corneal epithelial migration and protein synthesis and promoting collagen degeneration.363 Eosinophil-derived collagenase also has been reported to degrade type I collagen, the predominant collagen in cornea.358
Treatment and Prognosis
Treatment consists of topically applied 0.05% dexamethasone and prophylactic topical antibacterial ointments every 6 hours until clinical signs resolve. Lesions remodel with minimal corneal scarring, but mean duration of treatment in one series of patients was 64 days (range, 45 to 105 days).359 Use of ophthalmic NSAID preparations may increase the severity of clinical signs of eosinophilic keratoconjunctivitis in horses because of potentiation of leukotrienes, the primary promoter of eosinophilic inflammation.358 Excision of the corneal plaques by superficial keratectomy appears to enhance healing, attributable to removal of the eosinophil-granule major basic protein.358,359
IMMUNE-MEDIATED KERATITIS
Nonulcerative Keratouveitis
Nonulcerative keratouveitis is an uncommon corneal disease in the horse characterized by a pink, vascularized and somewhat localized stromal infiltrate of predominantly lymphocytes near the limbus.364,365 The overlying epithelium is intact, so fluorescein dye is not retained by the cornea. Accompanying anterior uveitis is typically severe and unremitting, with blepharospasm, epiphora, conjunctival hyperemia, corneal edema, miosis, aqueous flare, and hypopyon. The primary differential diagnosis is a corneal stromal abscess; other considerations include fungal keratitis, eosinophilic keratoconjunctivitis, onchocerciasis, and neoplasia.
The pathogenesis is presumably immune mediated, based on histopathology and response to therapy.364 Systemic leptospirosis was incriminated etiologically in a 2-year-old thoroughbred filly with a unilateral corneal infiltrate of lymphocytes and macrophages and a choroidal infiltrate of lymphocytes, eosinophils, and basophils that was also positive to IgG and C3.366 Brooks et al.364 speculated that autoimmunity against corneal antigens may play a role.
Before beginning therapy, it is essential to rule out stromal abscessation and fungal keratitis, in which the prescribed antiinflammatory regimen would be contraindicated. Treatment consists of a potentially lifelong regimen of a potent topical corticosteroid that will penetrate through intact corneal epithelium (1% prednisolone acetate or 0.1% dexamethasone) every 4 to 6 hours, 1% cyclosporine every 12 hours, and/or an NSAID such as flurbiprofen every 8 hours.364,367,368 Topical 1% atropine is administered to effect to minimize ciliary spasm and limit synechiae. A systemic NSAID such as flunixin meglumine is administered every 12 to 24 hours. Clinical signs typically recur if medication is discontinued. Prognosis is poor, without expectation of cure. Chronic inflammation may result in phthisis bulbi or unremitting pain that necessitates enucleation.369
Nonulcerative keratitis
Chronic, nonulcerative corneal opacities without signs of overt discomfort or intraocular inflammation have been described.370,371 In a report of 19 horses age 5 to 11 years diagnosed with nonulcerative keratitis, 11 horses had clinical signs for more than 12 months before referral.370 The disorder comprises three distinct clinical entities, classified as superficial, midstromal, or endothelial, based on the location of the corneal pathology. Superficial keratitis is characterized by a superficial, white to yellow infiltrate with diffuse, mild to moderate vascularization. Midstromal lesions are typically more diffuse, with denser cellular and vascular components. A deep cellular infiltrate with mild corneal vascularization and variable degrees of diffuse corneal edema are typical of endothelial keratitis. Intraocular inflammation is not a feature of the disorder, regardless of lesion location. Differential diagnoses include stromal abscess, eosinophilic keratoconjunctivitis, bullous keratopathy, and nonulcerative keratouveitis.
An immune-mediated pathogenesis is theorized, based on histopathologic characteristics and response to therapy. Regardless of lesion depth, histopathology reveals a predominantly lymphocytic-plasmacytic infiltrate, with stromal fibrosis and vascularization. Three of five horses were seropositive for Leptospira in one study.370 Presumably the immune system is reacting to a self-antigen or antigens of a foreign protein or infectious agent within the cornea. Even though infectious agents have not been documented in this particular disorder, immunologic cross-reaction with self-antigens (i.e. molecular mimicry) may occur, as described with leptospiral organisms or their DNA in the equine cornea.372,373
Lesions of superficial stromal keratitis can be controlled with long-term topical dexamethasone and/or cyclosporine applied every 12 to 24 hours.370,371 Lesions in 4 of 11 horses treated by superficial keratectomy and conjunctival grafting resolved without the need for ongoing medication, suggesting that surgical removal of the inciting antigen will stop the inflammatory process. A similar chronic therapeutic regimen is used in midstromal keratitis; Matthews371 states that topical corticosteroids are less effective than cyclosporine in the deeper stromal disorder. Keratectomy followed by a conjunctival graft was curative in one horse.370 Response to antiinflammatory agents varies in patients with endothelial keratitis, resulting from differences in pathogenesis. Only two of four horses were controlled with constant topical dexamethasone and cyclosporine in Gilger et al.’s report.370 In contrast, Matthews371 described complete resolution of endothelial keratitis with topical 1% dexamethasone applied every 6 hours for 3 to 7 days. Although retention of vision is likely in most cases of nonulcerative keratitis, long-term or even lifelong therapy is required to control the corneal disease.
EQUINE RECURRENT UVEITIS (PERIODIC OPHTHALMIA, “MOON BLINDNESS”)
Definition and Etiology
Equine recurrent uveitis (ERU) is distinguished by a pattern of intraocular inflammation in which recurring episodes of acute uveitis are separated by periods of clinical quiescence. Inflammation of the iris and ciliary body (anterior uveitis or iridocyclitis) predominates in the early stages; repeated episodes damage the cornea, lens, vitreous, retina, and optic nerve. A more insidious form of ERU characterized by persistent, low-grade inflammation occurs in the Appaloosa and draft breeds of horse.
Equine recurrent uveitis is a leading cause of blindness in the horse and mule. Although the exact prevalence is unknown, estimates as high as 10% to 25% have been reported .374,375 The financial impact on the equine industry is estimated at 100 to 250 million U.S. dollars annually as a result of the effects on performance and the costs of veterinary care.376
Despite extensive clinical research, the specific cause of ERU is still unknown. The pathogenesis is immune mediated, and characterization of T-lymphocyte populations in affected horses documents a delayed hypersensitivity reaction as the basic immunologic mechanism underlying the recurrent inflammatory episodes.344 Identification of the triggering antigen has proved more elusive, suggesting the disease does not result from the persistence of or repeated exposure to a single antigen, but rather to a variety of circulating antigens or native ocular antigens. Leptospira interrogans serovar pomona is the most frequently incriminated infectious pathogen,375 but diversification of T-cell responses to a particular antigen or group of antigens over time may result in evolution of the immune response to encompass endogenous ocular self-antigens.341,377
Clinical Signs
The ocular lesions observed in ERU vary, depending on the severity and duration of the disease.369,374,376,378,379 ERU can occur at any age, but the initial uveitis episode frequently occurs in horses 4 to 8 years of age. Acute episodes are painful, characterized by blepharospasm and excessive tearing. Affected eyes are often described by owners as “red and/or cloudy” because of changes in the conjunctiva, cornea, anterior chamber, or vitreous. Dilation of subconjunctival vessels near the limbus, termed “ciliary flush,” may intensify the generalized conjunctival hyperemia. As corneal endothelial function decreases, diffuse corneal edema gives the eye a bluish white appearance. The cornea may also exhibit peripheral, circumferential vascularization, cellular precipitates on its inner (endothelial) surface, and linear stromal opacities.
Increased uveal vessel permeability causes the aqueous humor to appear cloudy after influx of plasma proteins (flare), inflammatory cells (hypopyon), erythrocytes (hyphema), or fibrin into the anterior chamber (Fig. 39-25). The iris often appears edematous and lackluster or “muddy.” A change in iris color may be noted in breeds with lightly colored eyes, changing from blue to green in response to uveal edema, vascular congestion, and cellular infiltration. Prostaglandins and other inflammatory mediators cause pupillary constriction, favoring the formation of adhesions between the iris and lens (posterior synechiae) that distort the pupillary shape. Even without adhesions, the inflamed iris responds poorly to mydriatic agents. Intraocular pressure (IOP) is usually decreased because of diminished aqueous production by the inflamed ciliary body, but intermittent IOP elevations can occur.380 The ciliary body can also deposit cellular exudates within the anterior vitreous, creating an opacity within the pupillary space that may be mistaken for cataract.
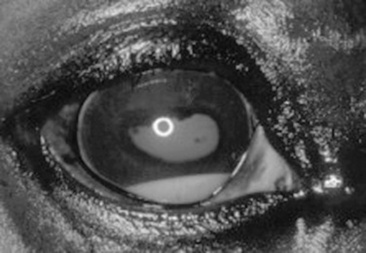
Fig 39-25 Active uveitis characterized by hypopyon, pupillary irregularities secondary to posterior synechiae, melanin adherent to the anterior lens capsule, and a dull tapetal reflection.
Active chorioretinitis causes dullness and loss of detail in affected tissues. Retinal detachment may follow choroidal exudation. Multifocal depigmented or hyperpigmented foci on either side of the optic disc are the inactive sequelae of chorioretinitis (“chorioretinal scars”), commonly referred to as peripapillary “butterfly” lesions (Fig. 39-26).
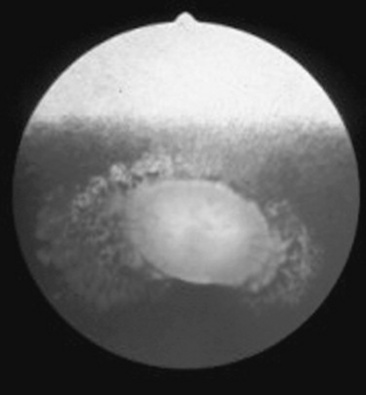
Fig 39-26 Fundus adjacent to optic disc takes on mottled appearance as a result of pigment migration after previous chorioretinal inflammation in recurrent uveitis. Because of their shape, such lesions are sometimes referred to as “butterfly lesions.”
Intraocular damage increases each time inflammation recurs. Permanent corneal opacity caused by edema results if the corneal endothelium is severely compromised. Chronic recurrent uveitis is characterized by widespread posterior synechiae, iris depigmentation or hyperpigmentation, and iris atrophy. The anterior chamber may appear shallow if aqueous trapped in the posterior chamber by extensive iris-to-lens adhesions causes the iris to balloon forward (iris bombé). Most lens changes occur weeks or months after uveitis begins. Abnormalities may range from pigment flecks on the anterior lens capsule (Fig. 39-27) to dense cataracts. Lens luxation often follows degeneration of the lens zonules and vitreous. Retinal detachment may also follow vitreous liquefaction or may result from traction by fibrous tissue bands within the vitreous. If retinal degeneration is substantial, the optic disc atrophies (Fig. 39-28). Permanent hypotony is followed by shrinkage of the globe (phthisis bulbi). Conversely, chronic uveitis may result in secondary glaucoma. The combination of these acute and chronic ocular lesions determines the degree of vision loss in the affected animal.
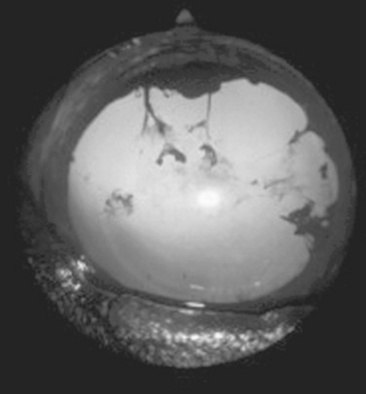
Fig 39-27 Prior episodes of anterior uveitis are indicated by pigmented remnants on the anterior lens capsule following adhesions of iris to lens (posterior synechiae). The pupil is pharmacologically dilated.
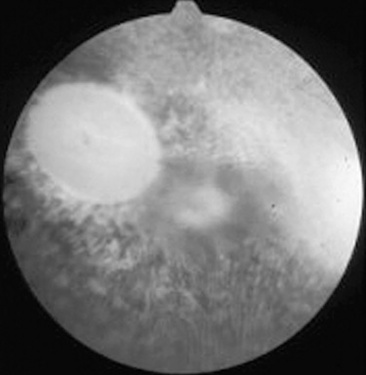
Fig 39-28 Disc pallor and nontapetal depigmentation accompany a dark-gray pre-retinal exudate in a patient with chronic active recurrent uveitis. This patient had poor vision.
Equine recurrent uveitis may be accompanied by transient and variable inflammation of the pineal gland.381-384 Similar pineal inflammation has been reported in experimentally induced recurrent uveitis in laboratory animals.
Diagnosis
Not every case of uveitis in the horse qualifies as ERU. Diagnosis is based on a chronic, recurrent history of intraocular inflammation and the presence of characteristic ocular lesions. In cases in which the history is unknown but recurrent disease is suspected, at least three of the following indicators of previous inflammation should be observed before a presumptive diagnosis of ERU is made: corneal edema or vascularization, synechiae, iris atrophy or color change, lens capsular pigmentation, cataract, lens luxation/subluxation, vitreous opacities or traction bands, retinal detachment, or peripapillary scarring.374 Other causes of a red and painful eye that can mimic acute ERU include conjunctivitis, corneal ulceration, corneal stromal abscessation, keratouveitis, and glaucoma.
Serologic testing of paired serum samples for Leptospira species or other infectious agents incriminated as causes of equine uveitis (see Table 39-2) may contribute to a diagnosis of ERU, but negative titers neither exclude the diagnosis nor eliminate leptospirosis as a contributing factor to the disease.385,386 Acute leptospiral infections are characterized by high-titer seroreactivity to at least one serovar by the eighth day;387 the titer usually falls with time, but seroreactivity may persist for many years. Some authors suggest that a leptospiral titer of 1:400 or higher is of clinical importance, particularly for Leptospira interrogans serovar pomona.374 A higher titer in the aqueous than in the serum is indicative of intraocular antibody production and further supports a leptospiral cause for the uveitis.388 Onchocerca microfilariae may be identified in conjunctival biopsies, although detection of live microfilariae does not necessarily indicate a causal relationship.389,390 Equine leukocyte antigen (ELA) typing may help determine susceptibility to ERU.391
Histopathologic lesions of the ciliary body considered pathognomonic for ERU include the presence of a thick, noncellular hyaline membrane adherent to the nonpigmented epithelium (NPE) and the presence of eosinophilic linear cytoplasmic inclusion bodies within the NPE.392,393 Clusters of lymphocytes and plasma cells also accumulate in the posterior iris, ciliary body, near the ora ciliaris retinae, within the choroid, and near the optic nerve head.376,394 Dense bundles of fibrils coupled with necrotic cells and mononuclear inflammatory cell infiltrates characterize the changes within the vitreous.395
Pathophysiology
Breed has been established as a risk factor for ERU. In a 1988 retrospective study of more than 16,000 equine patients at Cornell University, researchers determined that the Appaloosa had a significantly higher risk of developing uveitis than did thoroughbreds.396 A subsequent New York field study substantiated the breed predilection, concluding that the Appaloosa was 8.3 times more likely to develop uveitis than all other breeds combined.375 Within the Appaloosa breed, those with overall light hair coats and focal dark spots are more likely to develop ERU than horses with a dark, basic coat pattern and a light “blanket” over the rump.376 Trotters and warmbloods were overrepresented in a report of 130 ERU-affected horses in Germany.397 Of 669 mares included in a serologic study of leptospirosis, significantly fewer positive titers were found in thoroughbreds and standardbreds.398
In humans, an immunogenetic predisposition to certain types of uveitis has been linked to the major histocompatibility complex (MHC), a closely aligned cluster of genes designated the HLA region (for human leukocyte antigen), located on a single chromosome.399 Similar genetic loci occur in the horse, and the gene products are referred to as equine leukocyte antigens (ELAs). Products of these genes are glycoproteins found either on most nucleated cells (class I antigens) or restricted to accessory cells such as monocytes or macrophages (class II antigens). The immunoregulatory role of the MHC is especially important in discriminating self-peptides from those of nonself origin. Recent studies have shown an increased risk of uveitis linked with the MHC class I haplotype ELA-A9 in a group of German warmblood horses.391 As proposed in studies of human HLA-associated uveitis, cross-reactivity between self-antigens and ELA cell surface peptides could explain an inadvertent immunologic attack on normal cells.400,401
Experimental findings in ERU patients indicate that a T-cell—mediated autoimmune mechanism underlies the recurrent episodes of inflammation. T lymphocytes are the predominant cell type to infiltrate the anterior uvea,343,394 choroid,402 and vitreous403 of horses with ERU, and affected horses demonstrate cell-mediated immunity to retinal autoantigens and peptides.403-405 B lymphocytes have been reported primarily in retinas from horses seroreactive for L. interrogans serovar pomona, suggesting that leptospira-associated ocular inflammation may be a distinct subset of equine uveitis.406 Equine ciliary body epithelium may play a role in recruitment and activation of leukocytes through expression of a chemotactic cytokine (chemokine),407 although in the normal ocular microenvironment, ciliary body pigment epithelium suppresses T-cell activation by direct cell contact and the action of unidentified molecular mediators.408 Analysis of mRNA collected from horses with uveitis demonstrates elevated levels of IL-2 and IFN-γ, indicating a Th1 response in the disease process.409 In the absence of bacteria or viruses, this Th1 response by CD4+ uveal T-lymphocytes suggests a delayed-type hypersensitivity (DTH) reaction to self-antigens or sequestered antigens in the uveal tract.344,410 In contrast to the ocular T-lymphocyte population, systemic lymphocytes of ERU-affected horses do not exhibit a Th1 response.344 The expression of a deviant MHC class II antigen on resident ocular cells (e.g., Müller, retinal pigment epithelial) suggests that aberrant immune regulation may also play a role in ERU.343,411
Aqueous and vitreous immunoglobulin levels have been used to characterize immunologic responses within ERU-affected eyes. Using radioimmunoassay, an early study found that aqueous levels of IgG, IgM, and IgA were 50% to 120% greater in diseased eyes than in normal controls, but that the IgG/albumin ratio suggested leakage of protein through an impaired blood-aqueous barrier rather than intraocular antibody synthesis.412 Subsequent reports support local ocular antibody production but disagree on the dominant immunoglobulin in ERU-affected eyes. Wagner et al.413 found selectively increased IgA levels in the vitreous of affected horses; Eule et al.414 reported substantial IgM titers in 79.6% of ERU samples. In contrast to the intraocular immunoglobulins, there are no significant differences in serum immunoglobulin concentrations between healthy and ERU-affected horses.413,414
Both exogenous and endogenous antigens have been proposed as stimuli for these basic immunologic responses. One theory suggests that an infectious agent such as L. interrogans (or another, perhaps noninfectious, exogenous antigen) causes the initial iridocyclitis. Sensitized immunocompetent cells enter the uvea during this first inflammatory episode, imparting immunologic memory that is specific for the inciting antigen. Subsequent challenge of these cells by the immunogen causes recurrence of the inflammatory reaction.338,415 However, the premise of an infectious agent that exclusively induces and maintains ERU through a classic anamnestic response does not fully account for the disorder’s clinical course and response to therapy.
The role of leptospiral infection in ERU has been studied extensively in recent years. All major serogroups of L. interrogans have been identified in the horse and implicated as initiating factors in ERU.375,386,416-419 In a report of 130 ERU cases in Germany, 58.8% demonstrated positive titers to leptospirosis, an incidence 7 to 10 times higher than the control population.397 Anti-Leptospira antibodies have been found in the serum, tears, aqueous humor, and vitreous of infected horses.386,417,420-422 Wollanke et al.422 reported positive serum antibody titers to Leptospira serovars in 25% (24/97) of normal-eyed horses and 22% (50/227) of ERU-affected horses, but only the ERU-affected animals had positive antibody titers in the vitreous.422 Leptospiral organisms have been cultured from the aqueous and vitreous of ERU-affected horses.385,386,419,423,424 Horses seropositive to L. interrogans serovar pomona are reportedly 13.2 times more likely to have signs of uveitis than seronegative horses.375 Ocular signs during the acute infection are subtle or absent, but overt ocular inflammation develops months to years later.425-428 Risk factors for equine leptospirosis include rodent and wildlife exposure, proximity to ponds and rivers, a dense equine population on site, and increasing age.398,429,430
Although direct Leptospira-mediated injury to the eye cannot be ruled out in the pathogenesis of ERU, a growing body of evidence instead links leptospiral infection with autoimmune responses to ocular tissue components. Complement-binding anti-Leptospira antibodies capable of cross-reacting with equine corneal tissue and lens have been found in the tear film and aqueous humor of horses with leptospirosis.420,421,431 These antibodies bind corneal epithelial cells, activating complement and initiating tissue damage, a mechanism replicated in tissue culture.432 A leptospiral protein epitope that shares antigenic determinants with the equine cornea and lens has been found in bacterial homogenates,372 and a DNA fragment of several serovars of L. interrogans was determined to encode a 90-kilodalton protein that cross-reacts with equine corneal proteins.373,433 Novel leptospiral lipoproteins, identified as LruA and LruB, stimulate local intraocular IgA and IgG production and also cross-react with equine ciliary body, lens, and retina.434 Immunohistopathologic examination has also demonstrated leptospiral cross-reactivity with iris pigment epithelium and retina from horses with ERU.406,435 This antigenic relationship between Leptospira species and equine ocular tissues supports the concept of molecular mimicry as a contributing factor in ERU; exposure to exogenous antigens that share molecular structural sequences with equine self-antigens initiates an autoimmune response.342
Toxoplasmosis, brucellosis, salmonellosis, streptococcal hypersensitivity, Escherichia coli, Rhodococcus equi, borreliosis,436,437 intestinal strongyles, and onchocerciasis have also been implicated as causes of ERU, with no consistency in culture or serology results in affected horses.369,375 Viruses suspected of a role in ERU include equine influenza virus, equine herpesvirus (EHV-1, EHV-4),438 equine arteritis virus, and possibly equine infectious anemia.369,374,376 More recent studies on vitreous and serum samples from affected horses question the role of Borrelia burgdorferi, Borna disease virus, and Toxoplasma in ERU.439,440
Both humoral and cell-mediated hypersensitivities have been implicated in the lesions of ocular onchocerciasis. Immunoelectrophoretic studies have demonstrated an influx of IgG and complement (C3) into the tears of affected horses in response to larval death.441 The resulting chemotaxis of mast cells, eosinophils, and lymphocytes perpetuates the inflammatory response and facilitates destruction of the parasite. Human patients with ocular onchocerciasis demonstrate conjunctival infiltration by CD3+ T lymphocytes and increased expression of class II MHC antigens in conjunctiva and iris,442 as well as deficiencies in suppressor T-cell function that may interfere with the normal regulation of antibody function.443
In addition to the role of infectious agents in the pathogenesis of ERU, autoimmunity may occur when a normally sequestered component is exposed to lymphoid cells or when the antigenicity of a component increases as a result of a structural alteration.444 Several endogenous ocular proteins, including retinal soluble antigen (S-antigen, or S-Ag), interphotoreceptor retinoid-binding protein (IRBP), and uveal melanin-associated proteins are known to induce uveitis in various animal models.445-448 Clinical studies also implicate these potent autoantigens in the pathogenesis of some forms of human uveitis.449-451 An autoimmune phenomenon in response to damaged uveal tissue has been proposed in the pathogenesis of ERU.452 The isolation of S-antigen in the horse and the subsequent finding of anti-S antibodies in the aqueous humor and vitreous of horses with uveitis support the theory that this species is similarly capable of local production of antibodies to normally sequestered autoantigens.403-405453 Experimental uveitis with features similar to spontaneous ERU has also been induced in horses after injection of IRBP in complete Freund’s adjuvant.402 Autoantibodies to S-Ag and IRBP were found in 72% of vitreous specimens from horses with uveitis.403 A more recent equine study concluded retinal S-Ag is a weaker autoantigen than IRBP; T and B cells were activated after immunization with S-Ag, but only one of five horses developed uveitis or demonstrated inflammatory cell infiltration of the uveal tract.454 Because the retina and NPE of the ciliary body originate from neuroectoderm, it is even possible that ciliary body damage may release an S-like antigen or another uveitogenic substance.455 Evidence suggests that response to S antigen is predominantly T-cell dependent.456
Verma et al.434 proposed a link between leptospiral cross-reactivity and the release of other ocular autoantigens, based on strong IgG and IgA responses to LruA and LruB lipoproteins in uveitic eyes but not in companion sera. The early phase of ERU may involve production of non-complement-fixing antibody and non-DTH T lymphocytes specific for LruA and LruB. The antibodies and cells react with the leptospiral lipoproteins, initiating a process that ultimately liberates IRBP and other ocular autoantigens.
The concept of “epitope spreading” has been offered as an explanation for the relapsing character of ERU.457 The theory proposes that after destruction of an initial target, the immune response spreads from the first autoantigenic determinant to others not previously recognized by the immune system.458,459 Active uveitis subsides as regulatory cells suppress the inflammation, recurring as the immune response shifts to an epitope of the same autoantigen (intramolecular spreading) or a completely different autoantigen (intermolecular spreading). A recent 22-month study of peripheral T-cell reactions in eight horses with spontaneous ERU demonstrated intramolecular shifts to different S-Ag—derived (6/8) or IRBP-derived (5/8) epitopes and intermolecular shifts in all horses, spreading from IRBP-derived to S-Ag—derived peptides (5/8), or vice versa (3/8).457 A shift of the immune reaction could be correlated to new uveitic episodes in 10 of 14 relapses that occurred during the observation period. The confounding factor in this theory is the shifts in immune response observed during quiescent stages, perhaps to minor uveitogenic epitopes that fail to result in overt inflammation, or as part of an unknown regulatory or protective function of these T-cell clones.
Regardless of etiology, the ocular inflammatory process may attract other reactive lymphocytes to the eye. During primary uveitis, only 10% of the ocular immunoglobulin-secreting cells are specific for the inciting antigen. The remaining cells produce antibodies against immunogens that may not have entered the eye, but with which the host had previous contact. As a consequence, the eye may develop recurrent inflammation after systemic exposure to any one of multiple antigens. It is therefore conceivable that subsequent episodes of uveitis may differ etiologically, creating a perplexing clinical picture.460
Treatment
ACTIVE INFLAMMATION
Reduction of intraocular inflammation is the primary therapeutic objective in acute uveitis. Preservation of vision depends on successful management at this stage, when sight-threatening sequelae are minimal. If a specific cause for the uveitis is identified, it is also targeted pharmacologically. In most cases, symptomatic therapy combines corticosteroids, NSAIDs, and mydriatic/cycloplegic agents. Nonspecific suppression of T-lymphocyte activation with cyclosporine implants461 and surgical removal of T cells and potentially organisms from the eye by core vitrectomy462 are recent innovations aimed at preventing recurrence of disease.
No therapy is indicated in nonpainful eyes with lesions of chronic end-stage uveitis. Those eyes that remain painful or do not respond to therapy are candidates for enucleation or evisceration, followed by silicone prosthesis implantation in the orbit or sclera, respectively.
Corticosteroids
The severity of the uveitis dictates the routes and frequency of corticosteroid administration. Although topical therapy is most often used, efficacy is limited by the agents’ relatively short contact time with the eye. Therefore, topical corticosteroids must be applied three or four times daily, even in eyes with mild clinical signs. In more severe uveitis, topical preparations should be applied every 2 to 4 hours or combined with other routes of therapy. A subpalpebral lavage system should be considered when such frequent application is indicated (see under Bacterial Keratitis in Horses).
Either ophthalmic solution or ophthalmic ointment is acceptable for topical use in the horse. Prednisolone acetate* has excellent intraocular penetration and is considered the drug of choice. Potent dexamethasone preparations (Maxidex)† are also effective. In general, therapy should be continued for at least 2 weeks after clinical signs have resolved. Ideally, that assessment includes an objective IOP measurement to ensure resolution of ciliary body inflammation and dysfunction.
The subconjunctival injection of a repository corticosteroid preparation is an alternative or supplement to frequent topical therapy. Triamcinolone acetonide‡ is effective for 1 to 3 weeks when injected in a 0.5- to 1.0-mL volume (20 to 40 mg) beneath the superior bulbar conjunctiva. Subconjunctival methylprednisolone acetate§ has comparable antiinflammatory effect but is more likely to cause granuloma formation at the injection site. Duration of effect of either drug depends on the severity of the uveitis. Nonocular use of either drug has been linked to equine laminitis.
Evaluation of inflamed eyes should always include topical application of fluorescein dye to rule out ulcerative keratitis. This precaution is especially critical when considering the use of subconjunctival corticosteroids that deliver prolonged and irreversible effects. Topical and subconjunctival corticosteroids are contraindicated in the presence of corneal ulcers because they delay healing, potentiate the destructive effects of endogenous and microbial enzymes, and predispose the cornea to secondary infection.
NONSTEROIDAL ANTIINFLAMMATORY DRUGS
Parenteral corticosteroids may be used when topical and subconjunctival agents are ineffective in controlling inflammation, but NSAIDs are usually preferred in such cases. Use of these antiprostaglandin agents counteracts an important mediator of intraocular inflammation, minimizing the role of parenteral steroids in uveitis therapy and the attendant risk of laminitis. Flunixin meglumine (Banamine) is the NSAID of choice for the eye, administered at a dose of 0.5mg/kg intravenously or intramuscularly twice daily for 5 days, then 0.25 to 0.5mg/kg orally once or twice daily. Oral phenylbutazone* at 4.4 mg/kg twice daily can be used in cases of mild uveitis or in animals requiring chronic low-dose oral prophylaxis for recurrent disease. Dosage requirements for aspirin make it less practical in acute cases, but prolonged oral administration of 15 mg/kg twice daily has been used to avert relapses. Frequency of NSAID administration should be reduced as clinical response occurs, because antiprostaglandins have been linked to gastrointestinal ulceration and renal dysfunction with high doses or chronic use.
Topical ophthalmic NSAIDs are generally more costly than and not as potent as corticosteroids if used alone. However, an additive antiinflammatory effect can be seen when topical NSAIDs are used in conjunction with topical corticosteroids in horses with acute or resistant uveitis. Although generally considered a safe alternative to topical corticosteroids in the presence of corneal ulceration, topical NSAIDs have been implicated in the development of melting corneal ulcers in humans. Available generic solutions include 0.03% flurbiprofen sodium† and 0.1% diclofenac sodium.‡ Dosage frequency is empirical, with intervals ranging from 6 to 12 hours.
MYDRIATIC/CYCLOPLEGIC AGENTS
A parasympatholytic mydriatic/cycloplegic agent must be used if equine uveitis is to be managed successfully. By dilating the pupil and decreasing iris-to-lens contact, the chance of posterior synechia formation—and secondary glaucoma—is reduced. An adequately dilated pupil may also promote vision during the acute episode. Ciliary spasm is relieved, making the horse more comfortable, and the iridociliary vessels return to a more normal state of permeability, with normalization of aqueous humor constituents.
Topical application of 1% atropine solution or ointment is indicated two to four times daily until the pupil dilates. The ultimate goal is to maintain mydriasis with the least frequent application possible, keeping in mind the resistance of the inflamed iris and ciliary body to the effects of atropine. Horses on an intensive parasympatholytic regimen should be strictly monitored for signs of reduced gut motility and colic because systemic effects occur with frequent topical atropine administration.463 Pupillary dilation may persist for 4 weeks or more after cessation of therapy.
If mydriasis is slow or incomplete, 10% phenylephrine hydrochloride solution may be used topically in conjunction with atropine.464 Although a study in the horse suggests that phenylephrine is ineffective when combined with a parasympatholytic agent, investigators did not rule out a possible benefit if dosage or duration of therapy was increased.465 However, frequently applied phenylephrine has been associated with the development of corneal ulcers, corneal endothelial toxicity with secondary corneal edema, and increased uveal exudation, so response to the drug should be carefully monitored.
ANTIBIOTICS
Because current evidence suggests an immune rather than an infectious basis for recurrent uveitis, antibiotics have assumed a secondary role in ERU management. Topical antibiotic preparations may discourage opportunistic bacteria during intensive corticosteroid therapy; however, few will cross the intact cornea and reach therapeutic levels in the anterior chamber or uveal tract. In horses with positive leptospiral titers in serum or ocular fluids, systemic antibacterial therapy with oral doxycycline (10 to 20 mg/kg twice daily for 4 weeks) may minimize recurrences of uveitis.466
OTHER THERAPIES
If leptospiral infection has been well documented in a group of horses with uveitis, periodic vaccination against the disease may be considered. However, although vaccination significantly increased the interval to recurrence (median, 126 days) compared with nonvaccinated controls (median, 86 days), the practice failed to slow the progression of disease in a group of 41 ERU-affected horses.467 Currently, no commercial bacterin is approved for use in the horse.
An intracameral injection (25 μg/0.1 mL) of tissue plaminogen activator (tPA) can be used to accelerate fibrinolysis and clear hypopyon in the anterior chamber of horses with severe uveitis. However, tPA should be avoided in eyes with evidence of hemorrhage in the previous 48 hours.
The precise role of Onchocerca species in the pathogenesis of ERU is not yet determined, and considerable controversy exists regarding the necessity or benefit of microfilaricidal therapy in cases of ocular onchocerciasis (see later under Ocular Parasites).
Acupuncture and homeopathic remedies such as poultices of chamomile and oral methylsulfonylmethane (MSM) have been used in the treatment of ERU, but efficacy of these unconventional modalities is unknown.
Prevention of Disease Recurrence
CYCLOSPORINE
Cyclosporin A (CsA) is a noncytotoxic, immunosuppressive drug that blocks the transcription of IL-2 and decreases T-cell responsiveness during the initiation of inflammation.468,469 These properties could block the nonspecific activation of T cells in recurrent episodes of ERU. With its poor inherent antiinflammatory properties, cyclosporine is likely to be more effective in preventing recurrences than treating active inflammation. Unfortunately, topical application of cyclosporine fails to achieve effective intraocular levels in horses and other species.470 However, reports of sustained intraocular levels of CsA in ocular tissues471,472 and resolution of clinical signs in experimental uveitis after implantation of a CsA-impregnated device into the vitreous of rabbit eyes473 set the stage for implantation of a similar device into the anterior vitreous of horses with experimental uveitis. The CsA-containing implant significantly decreased the duration and severity of inflammation, cellular infiltration, tissue destruction, protein concentrations, and the level of transcription of proinflammatory cytokines in the experimental group.461 The intravitreal device also prevented recurrences in 81% of horses with spontaneous ERU, but overall success was limited by complications from intraocular hemorrhage, cataract progression, and retinal detachment.474 Because of significant risk of postoperative complications in the face of concurrent inflammation, a horse with active uveitis is not a suitable candidate for cyclosporine implantation until the inflammation is adequately controlled by conventional means.376 Intravitreal delivery devices containing other immunosuppressive agents such as tacrolimus are also effective in suppressing inflammation after intravitreal implantation in rabbits with experimental uveitis.475
To minimize ocular morbidity related to implantation, ongoing studies of cyclosporine have focused on development and evaluation of a deep scleral delivery device.476 Superficial episcleral implantation failed to achieve substantial intraocular levels of CsA or control inflammatory episodes in ERU-affected horses. Therefore, effects of a bioerodible implant infused with CsA and inserted into the suprachoroidal space beneath a partial-thickness scleral flap 1 cm posterior to the dorsolateral limbus were studied. Initial reports of the suprachoroidal device are encouraging. High concentrations of CsA were achieved in the equine ciliary body, choroid, retina, and optic nerve. In horses with severe ERU, only 15% of eyes were blind a mean of 14.2 months after implantation. In contrast, 90% of patients with severe ERU treated conventionally are blind within 1 year.376 In vitro studies of CsA also documented a direct inhibitory effect on Leptospira growth at concentrations achievable within the uveal tissues after deep scleral implantation. Duration of CsA delivery with current devices is approximately 24 months.
CORE VITRECTOMY
Pars plana vitrectomy has been used to remove fibrin, inflammatory cells, and debris trapped in the vitreous; improve vision; and delay progression of clinical signs in affected horses.462,477,478 Proponents theorize that removal of T cells or infectious organisms such as Leptospira may reduce adverse interactions between the vitreous and the uveal tract, thereby reducing the recurrence of ERU. The technique appears more beneficial in European warmbloods with ERU than in Appaloosas with ERU in the United States. In one German study, recurrence of ERU was prevented in 85% (29/34) of treated eyes followed for 5 months to 5 years, but 45% of horses developed significant cataract formation.462 A study of vitrectomy performed in the United States was also complicated by postoperative cataract formation and progressive loss of vision, despite some decrease in recurrence of uveitis.479 More recent reports of vitrectomy performed on more than 1200 eyes at the University of Munich described no further recurrences of ERU in 98% of patients.480 Cataract formation and retinal detachment were reportedly rare in this group of animals. Investigators explained their success on the basis of improved patient selection and surgical expertise.
Prognosis
The long-term prognosis for vision in horses with recurrent uveitis is poor, although statistics of actual rates of vision loss are limited. Dwyer’s 11-year study of ERU-affected horses reported that 56% of the 160 study animals experienced blindness in one or both eyes.376 Appaloosas and Leptospira-seropositive horses were at increased risk for blindness over the course of the study. All seropositive Appaloosas (100%) lost vision in at least one eye; 50% were completely blind. Of seronegative Appaloosas, 72% lost vision in one or both eyes; 29% were totally blind. Seropositive horses of other breeds lost vision in one or both eyes 51% of the time, with total blindness in only 17%. Seronegative, non-Appaloosas had the best prognosis, with 34% losing vision in one or both eyes and total blindness in only 6%.
BOVINE-SPECIFIC OPHTHALMIA
A recurrent uveitis of cattle has been described and compared to that of the horse.481,482 As with ERU, its definitive etiology is unknown, although a viral infection was originally suggested. Clinical signs include conjunctival hyperemia, corneal edema and vascularization, inflammatory cells and hemorrhage within the anterior chamber and uveal tract, and retinal and choroidal edema and hemorrhage. The disorder is uncommon and does not share the notoriety of its equine counterpart. Therapy is directed at reducing inflammation, as described for the horse.
OCULAR PARASITES
Parasites are an often overlooked cause of ocular disease in large animals. Many ocular parasitic diseases can threaten vision and can reduce the economic value of the animal through decreased function, decreased production, or both. The mechanisms by which parasites damage ocular tissues are extremely varied and range from direct tissue effects of aberrant parasite migration to complex immunopathologic responses to parasitic antigens. This section reviews the major ocular parasites of large animals, with reference to the ocular tissue of primary importance. Parasitic eyelid diseases are discussed in Chapter 40.