Chapter 40 Diseases of the Skin
PEMPHIGUS FOLIACEUS
Definition and Etiology
The term pemphigus is derived from the Greek word for “blister” and is used to describe a group of autoimmune vesiculobullous disorders characterized histologically by intraepidermal acantholysis and immunologically by intercellular deposition of immunoglobulin. Pemphigus foliaceus is the most common of this group of autoimmune diseases; in large animals it has been reported in horses,1,2 goats,3,4 and a donkey.5 In small animals, pemphigus foliaceus has been putatively associated with drugs,6 but this has not been identified in large animals. The factors precipitating the development of pemphigus foliaceus in large animals are unknown. The clinical lesions recognized in horses and goats are primarily scaling and crusting.
Pathophysiology
Pemphigus foliaceus is characterized by the production of autoantibodies. In humans and dogs, these are directed against transmembrane proteins (desmoglein 1 in humans and a minority of dogs). The pemphigus autoantibody binds to the transmembrane protein, resulting in the release or activation of one or more proteolytic enzymes. These enzymes destroy the attachments between adjoining epidermal cells. The result is acantholysis; the epidermal cells assume a rounded shape and separate from one another, leading to the formation of intraepidermal clefts and vesicles.7
Clinical Signs
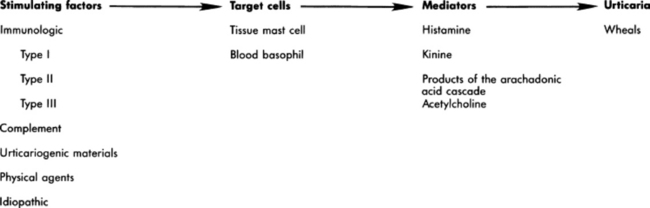
Pemphigus foliaceus is characterized clinically as a generalized exfoliative dermatitis (Fig. 40-1). Ventral or peripheral limb edema and crusts are the most common clinical signs.1 In one recent study, no age, breed, or gender predilection was noted, although (80%) of affected horses first exhibited signs between September and February.1 In the horse, lesions are usually first noted on the head, limbs, or ventrum. Initial lesions also may be associated with fever, depression, or rarely urticaria. The disease usually progresses to involve the entire body over days to weeks. The primary lesion is a pustule, but these are fragile and transient lesions. Pustules rupture soon after formation, resulting in erosions, epidermal collarettes (rings of exfoliating superficial epidermis), scale, and crust. The lesions may or may not be associated with pruritus or pain.1,2*
In the goat, pemphigus foliaceus also presents as a generalized exfoliative dermatitis. In the limited number of cases described, lesions were initially noted on the limbs, perineal region, and ventrum. The lesions consisted of crusting and scaling resulting from rupture of vesicles and bullae. Pruritus and malaise appear to be variable findings.3
Diagnosis
Diagnosis of pemphigus foliaceus in large animals is typically based on biopsy of lesions submitted for routine histopathology. Characteristic histologic findings include intragranular to subcorneal cleft and vesicle formation associated with acantholysis. Both follicular and surface epithelia are frequently involved. Neutrophils tend to predominate in the inflammatory infiltrate, although eosinophils may also be present.1-3 Because certain strains of Trichophyton species of dermatophytes may also cause acantholysis, any histology suggestive of pemphigus foliaceus must be stained for fungi.8 Direct immunofluorescence or immunohistochemistry as an aid in the diagnosis of pemphigus foliaceus in horses and goats has also been reported but is somewhat limited to research and academic institutions.3,7,9 Indirect immunofluorescence testing (for pemphigus antibodies in serum) is reported to be unreliable for the diagnosis of pemphigus foliaceus in horses.7,10
Therapy
Treatment is corticosteroids at immunosuppressive doses, such as prednisolone at 1 mg/kg every 24 hours (q12h) or dexamethasone at 0.08 to 0.1 mg/kg q24h, then tapering. Oral prednisolone is preferred to prednisone because some horses are unable to metabolize prednisone into the active metabolite of prednisolone.11 Injectable gold (Solganal, Schering) was also used successfully, but this product is no longer available. There are anecdotal reports of benefits using another gold salt, aurothiomalate (Myochrysine, Rhône-Poulenc Rorer), 1 mg/kg intramuscularly (IM) every 7 days. Gold salts take 1 to 3 months to reach effectiveness, when dosage frequency can be tapered to every 14 to 30 days. Adverse reactions of gold salts, although rare in the horse, include thrombocytopenia and glomerulonephropathy.
There are also reports of azathioprine (1 to 3 mg/kg q24-48h) being used for various autoimmune skin diseases in horses.12,13 A potential side effect is thrombocytopenia because horses have low levels of the enzyme thiopurine methyltransferase (TPMT),14 which is responsible for the metabolization of azathioprine in other species, including humans. However, I have administered azathioprine (1 to 3 mg/kg for 1month, then q48h) to eight healthy horses with no deleterious effects.15 Azathioprine is used as a steroid-sparing drug with corticosteroids, eventually to decrease the steroid needed. Approximate cost in a 500-kg horse for daily azathioprine is $300/month.
Goats have also been treated successfully with corticosteroids (dexamethasone, prednisolone) and aurothioglucose.3,4,9 Dosages approximate those for the horse.
Prognosis
The response to treatment in equine pemphigus foliaceus varies from patient to patient. Many horses require lifelong administration of medication to control the clinical signs; others may be gradually weaned from medication without further relapse. In one study in which follow-up information was available for 13 horses, four were euthanized because of complications from the disease or its treatment. The reported cases of caprine pemphigus foliaceus are insufficient in number to establish a prognosis reliably.
BULLOUS PEMPHIGOID
Bullous pemphigoid is an autoimmune, vesiculobullous, and ulcerative disorder that affects the cutaneous basement membrane zone (BMZ). It has been rarely noted in horses.7,16 Initiating triggers for the disease in the horse are unknown.
The pathophysiology of bullous pemphigoid in horses is assumed to be similar to that described in other mammals. Complement-activating anti-BMZ antibodies bind to a glycoprotein antigen in the lamina lucida of the BMZ. In horses, this has been shown to be bullous pemphigoid antigen II (also called collagen XVII). Complement activation results in degranulation of mast cells and chemotaxis of neutrophils and eosinophils. Eosinophils release tissue-destructive enzymes with resultant injury to the BMZ, loss of dermoepidermal adherence, and subsequent blister formation.7
Equine bullous pemphigoid is characterized clinically by painful, crusted, or ulcerative lesions of the skin (face and axillae), mucous membranes, and mucocutaneous junctions. Bullae are rare.7,16 Ulceration may involve the gastrointestinal (GI) tract. The diagnosis is based on histopathologic and, when available, immunofluorescent findings. Treatment is the same as for pemphigus foliaceus; the few cases reported have not had favorable outcomes, but I have seen a case that initially responded well to corticosteroids.
ATOPIC DERMATITIS
Definition and Etiology
Atopic dermatitis may be defined as an abnormal immunologic response to environmental allergens, such as pollens, barn dust, and molds. It is increasingly being recognized as a cause of pruritus in horses. The disease may be seasonal or nonseasonal, depending on the allergen(s) involved. Age, breed, and gender predilections have not been extensively reported. A familial predisposition may be present.17
The presumed etiology is a type I (immediate) hypersensitivity response, mediated by immunoglobulin E (IgE). Although evidence indicates that atopic horses do produce allergen-specific IgE,18 etiology is largely extrapolated from other species. The IgE is presumed to be directed against specific allergens. When that allergen is bound to two or more IgE antibodies on the surface of a mast cell, the mast cell releases granules containing various substances that cause erythema, vascular leaking, and pruritus.
Clinical Signs
Pruritus, often affecting the face, distal legs, or trunk, is the most common clinical sign. Alopecia, erythema, urticaria, and papules may all be present. Urticarial lesions may be severe but nonpruritic. Horses may have a secondary pyoderma, typified by excess scaling, small epidermal collarettes, or encrusted papules (“miliary dermatitis”). Diagnosis of atopic dermatitis is based on clinical signs and the exclusion of other diagnoses, especially parasite (Culicoides) allergy.
Diagnosis
Diagnosis is based on clinical signs and exclusion of other pruritic skin disease. Confirmation to formulate allergen-specific immunotherapy (ASIT, “hyposensitization”) is based on either intradermal testing (IDT) or serum allergy tests. The IDT involves a series of intradermal injections of aqueous allergen extracts along with a positive (histamine) and negative (saline) control. The injections are usually performed over the lateral cervical or thoracic region. The injection sites are then observed for 30 minutes to 24 to 48 hours for evidence of wheal formation associated with the injection site. A positive reaction does not necessarily mean that the horse’s clinical signs are caused by the reacting allergen, but rather that the horse has antibodies to the allergen that, on intradermal exposure, trigger those clinical signs. False-negative IDT reactions may occur, the most important cause of which is the use of corticosteroids, antihistamines, or phenothiazine tranquilizers before testing.
Thus, although horses with atopic dermatitis generally have a higher incidence of positive reactions than healthy horses, the diagnosis (as in other species) cannot be solely made on the basis of the IDT or serologic test alone; rather, these tests should be interpreted in light of the history of the disease; for example, a horse with seasonal signs is more likely to have an allergic response to seasonal allergens (pollens in summer, barn dust in winter). This interpretation thus will help the clinician determine which allergens might be relevant in hyposensitization, should the owners choose that treatment.18-21
There is continuing controversy in the horse (and other domestic species) in regard to IDT versus the serologic tests available. These tests look for the allergen-specific IgE in the animal’s blood.22 I have used serologic tests as the basis for determining the allergens to be used for hyposensitization when the owner did not want the horse shaved for the IDT or the horse was receiving antihistamines. Preferentially, IDT and/or serologic testing is performed on horses with atopic dermatitis and with owners who are interested in pursuing hyposensitization. It should be remembered that in regard to food allergy, neither serologic testing nor IDT likely has any relation to reality. Clinical research is ongoing to determine the most important allergens, their testing dilutions, and effective control substances.23,24
Therapy
Corticosteroid treatment is usually effective in the control of pruritus or urticaria resulting from atopic dermatitis. The usual oral medication used is prednisolone (1 mg/kg q24h), although dexamethasone (0.05 mg/kg q 24h) may also be used. The injectable dexamethasone solution may be used orally, although the clinician should remember that the bioavailability is 60% to 70% of the injectable route.
Corticosteroids in horses may cause various adverse effects, including steroid hepatopathy, laminitis, and iatrogenic hyperadrenocorticism. Therefore, other modalities of treatment may be used, such as the antihistamines hydroxyzine pamoate (200 to 400 mg/500 kg q12h), or cetirizine (0.2 mg/kg q12h), doxepin (a tricyclic antidepressant with antihistaminic effects; 300 to 600 mg/500 kg q12h), or diethylcarbamazine syrup (6 to 12 mg/kg q24h). Hydroxyzine, cetirizine, and doxepin may cause either drowsiness or nervousness, although these adverse effects are uncommon. Some clinicians have noted improvement when an essential fatty acid (EFA) product is added to the feed; Platinum Performance has been used successfully in some atopic horses as an adjunctive treatment.*
In general, hyposensitization injections for any manifestation of atopic dermatitis in the horse should be evaluated for efficacy for at least 12 months. The veterinarian should maintain consistent communication with the client to monitor the progress of treatment and encourage the owner to continue with the injections for the full year. If hyposensitization is successful, it is thought that, as in other domestic species, most horses will need to be maintained on the injections for life, although sometimes at a reduced frequency (one to three times monthly). Approximately 70% of the owners of atopic horses at the Veterinary Teaching Hospital, School of Veterinary Medicine, University of California, Davis (UCD), that have had IDT or serologic testing have elected to try hyposensitization. In general, I find that approximately 60% to 70% of atopic horses improve with hyposensitization25; other researchers have reported even better results, but in a noncontrolled study.26
URTICARIA
Definition and Etiology
Urticaria is characterized by transient focal swellings in the skin or mucous membranes called wheals, which represent localized areas of dermal edema. Angioedema is essentially identical but involves the subcutaneous tissues. The swelling of angioedema is diffuse, often involving the entire face and neck of the animal.
Urticaria is more frequently recognized in the horse than in ruminants. Allergic urticaria is usually caused by atopic dermatitis (environmental allergens such as pollens),17,20,21,27 drug eruptions (especially antibiotics and nonsteroidal antiinflammatory drugs), contact allergies, and food allergies (rarely).7
Physical urticarias are less common and involve a nonimmunologic pathogenesis. The three most important categories include mechanically induced, such as dermatographism, essentially a “pressure” urticaria; cold urticaria; and exercise-induced urticaria. Miscellaneous diseases that can cause urticaria include dermatophytosis (initial lesions),28 pemphigus foliaceus, “stress” (sometimes seen in racehorses immediately before a race), and vasculitis (Box 40-1). Urticaria is a recognized manifestation of milk allergy in cattle.29
Pathophysiology
The wheals result from vasodilation and transudation of fluid from capillaries and small blood vessels. Both immunologic and nonimmunologic factors can trigger the release of mediators from mast cells and blood basophils that will ultimately produce the characteristic wheals (Fig. 40-2). The most frequently cited immunologic mechanism of urticaria is a type I hypersensitivity (IgE). Non-IgE-dependent, immune-mediated urticaria may be induced by either type II (cytotoxic, involving antibody and complement) or type III (immune complex) hypersensitivity reactions. Complement-activated urticaria may involve either immunologic or nonimmunologic mechanisms and may occur through either the classic or the alternative pathway. Urticariogenic materials can induce wheal formation without involving immunologic mechanisms by being ingested, injected, or contacting the animal. Physical agents, including mechanical injury, thermal changes, and solar radiation, may induce urticaria. An example of mechanically induced urticaria is dermatographism (whealing after blunt scratch injury to the skin). Local or generalized exposure to heat or cold may induce urticarial lesions in certain individuals.
Clinical Signs and Differential Diagnosis
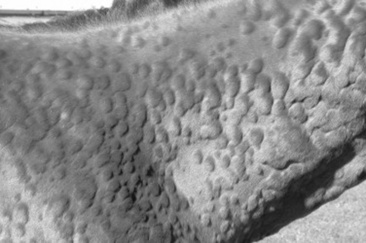
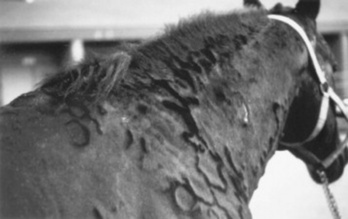
Wheals range from 1 to 10 cm (0.4 to 4 inches) in diameter and tend to involve the cervical and craniolateral thorax (Fig. 40-3). The animal may or may not be pruritic. Alopecia is not usually a feature of urticaria. The most important factor in distinguishing urticaria from other nodular diseases is that the individual lesions pit with pressure. This is easily demonstrated in the early stages of wheal formation, when the lesions consist primarily of dermal edema. In the later stages, when there is cellular infiltration into the dermis, the pitting is less apparent.
Diagnosis
Urticaria is usually a clinical diagnosis; the diagnostic dilemma is to determine the cause of the urticarial eruption. A skin biopsy not only will lend support to the clinical diagnosis of urticaria, but also may show evidence of pemphigus foliaceus, dermatophytosis, or vasculitis, Initiation of ectoparasite control, IDT or serologic tests for atopic dermatitis, and feed trials may all be used to determine the underlying disease.27
MILK ALLERGY
The principal cutaneous manifestation of milk allergy is urticaria and is usually seen in cows during the drying-off period. Increased intramammary pressure presumably causes milk proteins to gain access to the circulation, where they induce a type I hypersensitivity reaction.30 The disorder is believed to be hereditary and familial, with cattle of the Channel Island breeds demonstrating increased susceptibility.
The urticarial reaction can be localized or generalized. Other clinical signs that may be noted include muscle tremors, respiratory distress, restlessness, ataxia, dullness, and even maniacal behavior. Diagnosis is made by observing an edematous swelling at the site of an intradermal injection of the cow’s milk or the milk protein casein diluted 1:1000, in combination with the appropriate clinical signs.30,31
Treatment involves the use of antihistamines early in the course of the disease. Prevention requires avoiding milk retention. An affected cow is likely to suffer recurrences of milk allergy, so culling is usually recommended.
ERYTHEMA MULTIFORME
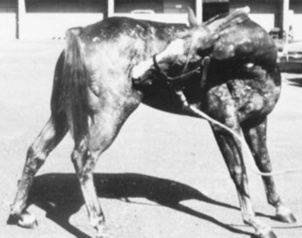
Erythema multiforme (EM) has been recognized clinically in the horse and in one bull (Fig. 40-4). This is an immunologic reaction in the skin, and programmed keratinocyte cell death (apoptosis) is the prominent change seen on biopsy. The keratinocytes may be killed specifically by killer lymphocytes. The many possible etiologic factors include infectious diseases, drugs, systemic disease, and neoplasia. In the horse, drugs are probably the most frequent inducers of EM, although in many cases an underlying disease is undiagnosed.32,33 In one bull the EM may have been caused by a pyelonephritis found on necropsy.34 Clinical lesions are characterized by macules, papules, urticarial lesions, or vesicular bullous lesions. Individual lesions may expand peripherally, leading to the formation of target-like lesions. Scaling and crusting are usually not a feature of equine EM, unless the disease is characterized by erosions or ulcers. Individual lesions can persist for several days, unlike urticarial lesions. Pruritus and pain are variable but usually are not seen. Lesions may occur in association with or after an infection or drug administration. The late Dr. A.A. Stannard of UCD had theorized that reticulated and hyperesthetic leukotrichia may also represent a type of EM in the horse.33 Interestingly, a similar disease, toxic epidermal necrolysis, has rarely been reported in cattle.34a
Differential diagnoses include urticaria, amyloidosis, and other nodular papular diseases. The diagnosis is made on history, physical examination, and skin biopsies. The histologic changes are distinctive and often include a lichenoid pattern with keratinocyte apoptosis. Vesicular lesions may be present and can include more confluent areas of keratinocyte destruction, with massive spongiosis and subepidermal and intraepidermal edema.
Treatment should be directed toward the underlying cause, if one can be found. A drug eruption should be high on the list, and searching the history for recent drug administration is important. Although some cases of EM are self-limiting and may resolve within 1 to 3 months,32 corticosteroid treatment as for pemphigus foliaceus may be tried. In my experience, reticulated leukotrichia does not resolve spontaneously and does not respond to corticosteroids.
VASCULITIS
Vasculitis is a histopathologic term that implies the presence of inflammatory changes in the walls of blood vessels, and it is associated with a broad spectrum of disorders. Cutaneous vasculitis is recognized in horses and is most often seen as a feature of drug reactions, urticaria, photoactivated/leukocytoclastic vascultitis, or purpura hemorrhagica. Affected vessels may be limited to the skin or may involve other organs, resulting in systemic disease. The cutaneous lesions are characterized by purpura, necrosis, and ulceration, most often affecting the head and extremities (Fig. 40-5).
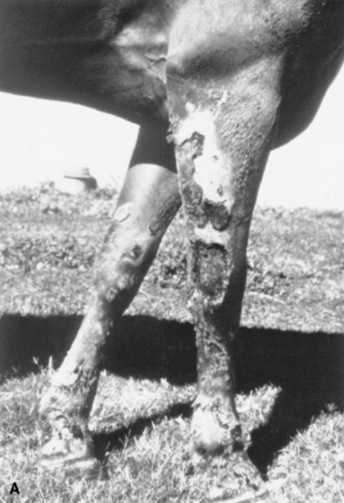
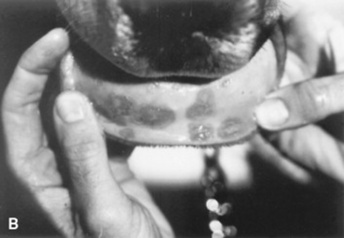
Fig 40-5 Cutaneous vasculitis in a horse. A, Vasculitis involving the forelimbs. B, Vasculitis involving the mucous membranes of the inner lip.
Pastern leukocytoclastic vasculitis (photoaggravated vasculitis) seems to be more common (if poorly understood) in California and the western United States as a clinical entity. It generally affects mature horses and produces lesions confined to the lower extremities that lack pigment. Lesions are multiple and well demarcated, with the medial and lateral aspects of the pastern the most common sites. Initially, erythema, oozing, crusting, erosions, and ulcerations develop, followed by edema of the affected limb(s). Chronic cases may develop a rough or “warty” surface. The pathogenesis is uncertain; an immune complex etiology has been suggested, and lesions being restricted to nonpigmented areas suggests a role for ultraviolet (UV) radiation. Drug reactions may be a potential cause.33 A recent report also implicated Staphylococcus intermedius.35
The differential diagnosis is photosensitization, particularly that caused by contact. The diagnosis is confirmed by skin biopsy, which demonstrates leukocytoclastic vasculitis with vessel wall necrosis and thrombosis involving the small vessels in the superficial dermis. These changes may be difficult to demonstrate.
Treatment involves corticosteroids at relatively high doses (prednisolone at 1 mg/kg q12h or dexamethasone at 0.08 to 0.2 mg/kg q24h) for 2 weeks, then tapered over the next 4 to 6 weeks. Reducing UV light exposure is helpful, either by bandaging affected legs or stabling inside during daylight hours, or both. In some cases, topical corticosteroids (e.g., betamethasone valerate cream 0.1% or triamcinolone spray 0.015%) may enable the horse to be weaned off systemic corticosteroids. I have also used pentoxifylline (8 to 10 mg/kg q12h) as an adjunct treatment in one case, to good effect. Another alternative (but more expensive) is 0.1% tacrolimus ointment q24h (Protopic, Astellas Pharma US). This disease does not usually recur, which downplays sunlight’s role in its initiation. These horses are prone to secondary bacterial infections, and a month of antibiotic treatment (usually trimethoprim-sulfa) is often indicated if the horse has fever or if the legs are extensively swollen.
Cutaneous vasculitis seemingly is rare in ruminants; one report in calves describes lesions on the forelegs and ear tips occurring within a month on one farm. Histology showed a fibrinous-necrotic or leukocytoclastic vasculitis. Glucocorticoid (prednisolone) therapy was effective.36
Vasculitis and purpura hemorrhagica are discussed in more detail in Chapter 37.
DRUG ERUPTION
A drug eruption is a cutaneous reaction to any agent that enters the circulation by ingestion, injection, inhalation, or percutaneous absorption. Drug eruptions may or may not be associated with systemic signs. Many drug eruptions are thought to be immunologically mediated hypersensitivity reactions, although on occasion they may occur with the initial administration of a drug and therefore without any prior sensitizing exposure characteristic of an immunologic reaction. Drug eruptions may also occur after years of repeated asymptomatic exposure to a drug, although this is probably less common than formerly supposed. Any medication can cause a drug eruption, but the compounds most frequently incriminated include antibacterial agents (especially semisynthetic penicillins and the sulfas), phenothiazine tranquilizers, nonsteroidal antiinflammatory drugs (NSAIDs) and antipyretics (especially phenylbutazone), local anesthetics, and anticonvulsants. In general, the more recent a drug has been given, the more likely it may be the cause of a skin disease; the clinician should try to determine a temporal association between administration of the drug and the skin disease.
Because drug eruptions can result in a wide variety of cutaneous manifestations, they must be considered in the differential diagnosis of all skin disorders. Certain clinical symptoms are more often associated with drug eruptions. Urticaria and angioedema, diffuse erythema, papular rashes, intense pruritus that is poorly responsive to corticosteroids (Fig. 40-6), sharply demarcated ulcers secondary to vasculitides, vesicular and bullous eruptions, and photosensitization should arouse clinical suspicion of a drug eruption. Typically, cutaneous lesions are noted 24 to 48 hours after drug administration, although there may be a longer lag interval. The eruption usually subsides within 24 to 48 hours after exposure ceases, although lesions may persist up to 6 months after the offending agent is eliminated.33
A diagnosis of drug eruption is based on clinical suspicion associated with an incriminating history of drug administration and by ruling out other possible causes. In a suspected case, all medications should be discontinued. If lifesaving medications are being administered, a chemically unrelated compound with similar pharmacologic effects should be substituted. Administration of corticosteroids may provide some relief, but drug eruptions are variably responsive to corticosteroids. Although development of a cutaneous reaction after readministration of the suspected agent would support a diagnosis of drug eruption, readministration is not advisable because it can be fatal. Future exposure to any implicated compounds and chemically related substances should be avoided.
CONTACT DERMATITIS
Definition and Etiology
Contact dermatitis is recognized in both horses and ruminants and can be subdivided into irritant and allergic contact dermatitis. Irritant contact dermatitis occurs more often and is defined as a cutaneous reaction to an irritating concentration of an offending agent. The substance chemically damages the skin without immunologic mediation. The reaction may occur after a single contact with a strong irritant or after repeated contacts with a milder irritant. Allergic contact dermatitis represents a cutaneous reaction in a sensitized animal to a nonirritating concentration of the offending agent. Tissue damage is immunologically mediated by delayed-type hypersensitivity (type IV); thus prior exposure is required to sensitize the skin to the material eliciting the dermatitis.7,29 It may be difficult to differentiate between the two types of contact dermatitis, and it may not be clinically important. In my experience the vast majority of contact dermatitis cases are iatrogenic, caused by topical products placed on the skin by veterinarians or owners.
Clinical Signs
The clinical lesions associated with allergic and irritant contact dermatitis are very similar. Predisposed areas include the muzzle, the extremities, and the areas contacted by tack. Early lesions include erythema, edema, and vesiculation, which progress to erosions, ulcerations, and crusting and ultimately to lichenification and hyperpigmentation. A gravity-induced drip pattern may be evident when the irritant is a liquid.37
Diagnosis
Patch testing is possible but usually impractical. Provocative exposure is the most useful test for diagnosis of contact dermatitis, although it does not reliably distinguish between allergic and irritant contact dermatitis. Provocative exposure requires avoiding contact with all suspected agents for 7 to 10 days to permit clearing of the skin lesions. The patient is then reexposed to these agents on an individual basis at 7- to 10-day intervals while being observed for recurrence of the dermatitis. When a positive reaction is observed, challenge with the suspected agent should be repeated to confirm the diagnosis. The process is time-consuming, requiring patience and cooperation from the owner.
Therapy
An animal with a suspected or confirmed diagnosis of acute or irritant contact dermatitis should be placed in an environment where there is negligible chance of exposure to agents that might have produced the dermatitis. Symptomatic treatment pending spontaneous resolution includes gently washing the affected regions with water. Pentoxifylline at the dose noted earlier for vasculitis may be helpful in horses.
DERMATOPHILOSIS (STREPTOTHRICOSIS, RAIN SCALD, LUMPY WOOL, STRAWBERRY FOOT ROT)
Definition and Etiology
Dermatophilosis is caused by an actinomycete bacterium, Dermatophilus congolensis. The organism is a gram-positive, non-acid-fast, branching, filamentous, aerobic bacteria that divides longitudinally and then transversely to form parallel rows of coccoid zoospores. Dermatophilosis affects horses, cattle, sheep, and goats, as well as a wide host range of other mammals.38-41
Three conditions must be present for Dermatophilus to manifest itself: a carrier animal, moisture, and skin abrasions. Chronically affected animals are the primary source of infection; however, they become a serious source of infection only when their lesions are moistened, which results in the release of zoospores, the infective stage of the organism. Mechanical transmission of the disease occurs by both biting and nonbiting flies, ticks, and possibly fomites. Because normal healthy skin is quite impervious to infection with D. congolensis, some predisposing factor that results in decreased resistance of the skin is necessary for infection to occur, especially prolonged wetting of the skin by rain. The organism has only recently been isolated from the environment of affected animals. Although the distribution is worldwide, the frequency of occurrence of dermatophilosis varies with geographic location. There is no apparent age, breed, or gender predilection.
Clinical Signs
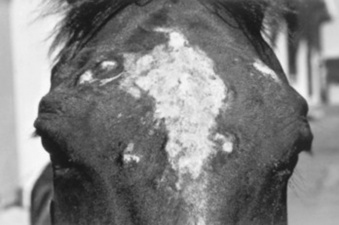
Dermatophilosis is usually seen during the fall and winter months, with the dorsal surface of the animal most often affected (Fig. 40-7). In horses, this association with the wetter months of the year has led to the term “rain scald.” Occasionally the lesions involve the lower extremities when animals are kept in wet pastures (“dew poisoning”) or if horses are left in the stall while the stall is cleaned with high-pressure water hoses. In the early stages of disease the lesions can be felt easier than they can be seen. Thick crusts can be palpated under the hair coat. Removing the crusts and attached hair exposes a pink, moist skin surface, with both the removed hair and the exposed skin assuming a paintbrush shape. The undersurface of the crusts are usually concave, with the roots of the hairs protruding. In cattle and goats the older term “cutaneous streptothricosis” is sometimes used. In sheep the condition is referred to as “lumpy wool” or “mycotic dermatitis” when wooled areas are affected and “strawberry foot rot” when the distal extremities are involved.
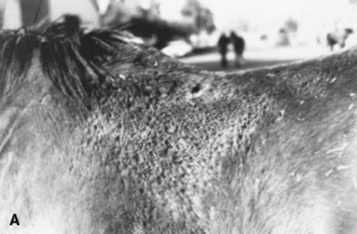

Fig 40-7 Dermatophilosis (Dermatophilus congolensis). A, Horse with suppurative crusts and matted hair on the back. B, Scaling and crusting over withers and neck of a bovine.
Body areas predisposed to infection include those that are most susceptible to maceration and trauma. The distal extremities, muzzle, and entire length of the dorsum are frequently the initial sites of infection. Nonpigmented skin is also reported to be more susceptible to infection. Horses develop lesions in areas rubbed by tack, and cattle often have involvement of the udder and scrotum. Goat kids reportedly have a tendency to develop lesions on the pinnae and under the surface of the tail. Sheep may develop a form of dermatophilosis that begins as an encrusted, proliferative dermatitis at the coronet region; removal of the external crusts reveals a pink-red granulation bed that resembles the surface of a strawberry. Lesions can extend from the hoof to the hock, and with loss of the crusts, ulceration occurs. Under appropriate conditions, dermatophilosis can become generalized.
Diagnosis
Diagnosis is made by demonstrating the “railroad track” cocci on impression smears. A portion of one of the crusts should be minced and mixed with a few drops of sterile water on a glass slide, Gram stained, and examined microscopically. Alternatively, bacterial culture or histopathology may be used for diagnosis, particularly in chronic cases. A thick crust composed of alternating layers of parakeratotic stratum corneum, dried serum, and degenerating neutrophils is the most characteristic change. A superficial folliculitis may be a prominent feature of the disease.38 In Gram-stained sections the branching, filamentous organisms can be observed in the crusts and in the follicles.
Therapy
The animal should be removed from the wet environment, if possible. Careful removal of possibly painful crusts, washing with iodophors or lime sulfur, and administration of antibiotics (for horses, 22,000 mg/kg procaine penicillin G intramuscularly twice daily, or 30 mg/kg trimethoprim-sulfa orally twice daily) for 7 to 10 days.41 Long-acting oxytetracycline given as a single 20-mg/kg intramuscular dose has been reported to be effective for bovine and ovine dermatophilosis. The crusts are important in contagion, so these should be disposed of rather than brushed to the ground.
FOLLICULITIS/FURUNCULOSIS AND IMPETIGO
Definition and Etiology
Bacterial folliculitis (superficial pyoderma) is defined as inflammation of the hair follicles secondary to a bacterial infection. Furunculosis is the term used to describe a follicular infection that breaks through the follicular wall (“boil”). Bacterial infection that causes subcorneal pustules but does not involve the hair follicles is called impetigo. Folliculitis and furunculosis are often seen in horses28,38,41 and goats40 but are an uncommon problem in cattle and sheep. Impetigo is relatively common in cattle and goats.
Bacterial folliculitis is usually caused by a coagulase-positive Staphylococcus species. Both S. aureus and S. intermedius have been isolated.28,42 In one study in horses, S. aureus accounted for twice as many isolates as S. intermedius; the same study isolated some strains of S. hyicus as well.43 Many isolates are resistant to penicillin G.43 Occurrence of pyoderma has been linked to poor nutrition and husbandry in some cases.44
Clinical Signs
Clinical signs of staphylococcal pyoderma are most often crusts, usually in a circular pattern suggestive of dermatophytosis (this may be the reason that equine pyoderma is underdiagnosed), epidermal collarettes (circular skin lesions with an exfoliative border as seen in dogs with superficial pyoderma), or encrusted papules similar to the miliary dermatitis reaction pattern in cats.28 These infections tend to vary in intensity of pruritus. Histology often shows folliculitis and furunculosis, but bacterial colonies are not always seen. A truncal form of bacterial folliculitis (contagious acne, contagious pustular dermatitis, Canadian horsepox) is often associated with poor grooming and trauma from tack and saddle, warm wet weather, and heavy work. It is painful and interferes with working and riding. It is usually caused by a coagulase-positive Staphylococcus species but may also result from Corynebacterium pseudotuberculosis45 (more often a cause of deep pyoderma, as discussed later). In horses, folliculitis often develops in the saddle and lumbar region, particularly in the summer. The affected area initially may be swollen and sensitive, followed by formation of follicular papules and pustules. These may become confluent or may rupture, forming plaques and crusts. Deep pyoderma followed by ulceration may develop over large areas of the body, especially the neck, sides of the thorax, inner surface of the thighs, and prepuce.
A pastern bacterial infection (pastern folliculitis) is often seen. Again, the causative agent is usually a coagulase-positive Staphylococcus species. As with most “primary pyodermas,” the mechanism by which the organism gains its foothold is unknown; it is not contagion or poor sanitary conditions. The lesions are usually limited to the posterior aspect of the pastern and fetlock regions; one or more limbs may be involved. The initial lesions consist of papules and pustules. If left untreated, the lesions coalesce and may produce large areas of ulceration and suppuration, which may be quite painful. The disease is usually not associated with systemic signs, and the general health of the horse is not affected.
A relatively uncommon nodular disease termed botryomycosis mimics actinomycosis or a deep fungal infection but is most often caused by Staphylococcus species in the horse. These may require surgical excision as well as long-term antibiotics.
Public Health Considerations
In a 2000 study, methicillin-resistant, coagulase-negative staphylococcal species were cultured from healthy horses in Japan; the authors concluded, “These organisms must be considered a potential threat to horses and veterinarians who care for them.”46 In a 2006 study from the Netherlands, methicillin-resistant coagulase-negative staphylococci were found frequently.47 The organism was usually Staphylococcus sciuri, as opposed to S. epidermidis, which was found in the humans in close contact with these horses. No methicillin-resistant Staphylococcus aureus (MRSA) was found in healthy horses.
In contrast, a single strain of MRSA was isolated from both humans (13%) and horses (4.7%) on horse farms in Canada and New York state.48 In looking at horses admitted to a university teaching hospital (Ontario Veterinary College), MRSA was isolated from 120 (5.3%) of 2283 horses. Of these 120 horses, 50.8% were positive at admission, and clinical infections attributable to MRSA were present or developed in 14 animals. Horses colonized at admission were more likely to develop clinical MRSA infection. Administration of ceftiofur or aminoglycosides during hospitalization was the only risk factor associated with nosocomial MRSA colonization. Another strain of MRSA was isolated from a small number of horses at the Veterinary University, Vienna, Austria.49
Of most concern is the finding of humans reporting skin lesions after contact with a community MRSA-positive affected foal, despite short-term contact with standard protective barriers. The isolates from the foal were indistinguishable from those affecting humans.50
Therapy
The antibiotic usually used for bacterial skin infections in the horse is trimethoprim-sulfamethoxazole (TMS) orally (PO, 30 mg/kg q12h for 2 to 6 weeks, longer for deep infections).7,28 Interestingly, dosing intervals for intravenous (IV) administration of TMS in horses may not be appropriate for use in donkeys or mules. Donkeys eliminate the drugs rapidly compared with horses.51 In cases of staphylococcal resistance to TMS, enrofloxacin or doxycycline may be used. Doxycycline is less expensive, but is associated with a higher incidence of colic. Dosage is usually 10mg/kg q12h. Off-label use of oral enrofloxacin formulations for poultry, ruminants, or swine has been suggested; a dose for the poultry formulation has been suggested as 7.5 mg/kg PO once daily. The formulation for poultry may be used; the dose is 7.5 mg/kg PO once daily. Use of enrofloxacin in young horses (<2 years old) should be avoided because of concerns about damage to the articular cartilage.52 A recent report of the usage of an oral gel formulation of enrofloxacin (100 mg/mL of gel) showed good clinical efficacy for infections in several organs; however, almost one-third of the horses had some diarrhea, and 10% had oral lesions.53 The authors believed that this latter side effect could be overcome with a tap water rinse of the oral cavity after administration. Interestingly, enrofloxacin binds to melanin in equine hair, although the clinical implication is unknown.54 Ceftiofur sodium 2.2 mg/kg, q12-24h, IM or IV, may also be used for pyoderma in horses, although its usefulness over a long period is limited by its parenteral route. In one report of 15 horses, vancomycin was used, alone or in combination with an aminoglycoside, to treat MRSA and enterococcal infections. The average vancomycin dosage was 7.5 mg/kg q8h IV over 30 minutes. The antibiotic, alone or in combination with an aminoglycoside, was safe and effective. Because of the problems with emerging resistance, the authors recommended vancomycin use in horses be limited to cases in which culture and susceptibility indicate effectiveness and no reasonable alternative treatment exists.55
For localized lesions, mupirocin ointment 2% or silver sulfadiazine cream (Silvadene) may be effective. As shampoos, ethyl lactate (Etiderm, VIRBAC, Fort Worth, Texas) or chlorhexidine (2% to 4%) are helpful.
Pyoderma in ruminants has not been studied as much as in horses. In goats, lesions (often pustules that progress to crusts) usually begin on the udder and spread to the abdomen, thigh, perineum, and face. Pruritus is variable. Stress from parturition is also an important predisposing factor in goats. In sheep a generalized, staphylococcal, scalded skin—like disease has been reported in lambs.56 In addition, staphylococcal infections in association with Psoroptes ovis infestation have been noted.57,58
In cattle, furunculosis caused by C. pseudotuberculosis manifests as a locally extensive, raised, raw lesion that is frequently hemorrhagic. The lesions are almost constantly covered with a serosanguineous exudate, and palpation of the lesions usually elicits a pain response. Treatment is similar to treatment of dermatophilosis, taking into consideration culture and susceptibility results, as well as withdrawal times for food animals.
EQUINE STAPHYLOCOCCAL CELLULITIS
Cellulitis is a severe, deep, suppurative process in which a poorly defined area of infection tends to dissect through tissue planes. Although many organisms are capable of producing cellulitis, staphylococcal cellulitis has been recognized as a specific disease entity in thoroughbred racehorses.59 Coagulase-positive staphylococci have been isolated from all reported cases, and when speciated, the isolates were classified as Staphylococcus aureus.60 Other studies of pathogenic Staphylococcus species from horses have suggested that S. aureus and S. intermedius are the major cutaneous pathogens,60,61 with no significant difference in their susceptibility patterns.60 In a recent retrospective study of limb cellulitis in 44 horses, coagulase-positive Staphylococcus spp were cultured from 33 of 36 horses from which specimens were obtained. Horses that were febrile at admission or that developed laminitis were significantly less likely to survive.61a
The cause of staphylococcal cellulitis is uncertain. Management practices may be associated with the condition because bathing, grooming devices, and handlers can be a source of the organism.61
The initial symptom is acute swelling and lameness that involves one or more limbs. Lesions progress rapidly by dissection along tissue planes. The overlying skin becomes devitalized and frequently sloughs. Accompanying systemic signs include increased rectal temperature and heart rate. Laminitis of either the affected or the contralateral limbs, osteomyelitis, and bacteremia are possible sequelae.59 Diagnosis is based on the results of bacterial culture and biopsies for routine histopathology.
Treatment must be aggressive and initiated early in the course of the disease. Pending the results of bacterial culture and sensitivity, treatment should include broad-spectrum antibiotics such as potassium penicillin G and gentamicin sulfate or TMS. To decrease edema, promote weight bearing, and reduce the chance of laminitis, therapy should include NSAIDs, hydrotherapy, and support wraps.59 The prognosis for complete recovery is guarded.
EQUINE CORYNEBACTERIUM PSEUDOTUBERCULOSIS CELLULITIS
Unlike equine staphylococcal cellulitis, solitary or multiple abscesses or nodules with many draining tracts that progress to diffuse cellulitis are often caused by Corynebacterium pseudotuberculosis; when it affects the pectoral region, it is termed “pigeon fever” in the United States. This type of deep Corynebacterium infection may occur where caseous lymphadenitis is common in sheep, although proximity to sheep is not a requirement, and it also may be seen seasonally when insect population and activity are maximal. Insect vectors seem probable, especially stable, horn, and house flies.62 The draining nodules or abscesses are especially common in the pectoral region and occasionally affect the face, neck, axilla, groin, and limbs; they begin deep and enlarge, often with much edema, and rupture in 1 to 4 weeks, discharging viscid, creamy pus, a major source of contamination. Abscesses most often rupture externally.
Diagnosis is by clinical signs, with the infection readily identified by bacterial culture of aspirate samples from abscesses. The synergistic hemolysis inhibition test is useful for diagnosis of internal abscesses but is unreliable for external abscesses.63
Treatment depends on location. For example, if the abscess is in the axilla and thus is painful on movement or prevents locomotion, establishment of drainage is important, and antibiotics are indicated. The antibiotics most often used are procaine penicillin (20,000 to 50,000 IU/kg/day) with rifampin (3 to 5 mg/kg PO); alternatively, TMS (30 mg/kg q12h) may be used.64 Treatment with TMS and rifampin concurrently may lead to a greater incidence of colitis and should be avoided. If the decision is made to use antibiotics but drainage cannot be easily established (e.g., axillary abscess, with owner unwilling to allow veterinarian to use trocar and drain), the antibiotics must be used for a minimum of 1 month. If the abscess is solitary and not causing pain or fever, antibiotics are usually not necessary, but bringing the abscess to a head with hot packs or heat-inducing agents (ichthymol) is important. Once an abscess has drained, gentle cleaning with tamed iodines or chlorhexidine is indicated.
PAPILLOMATOUS DIGITAL DERMATITIS (DIGITAL DERMATITIS, FOOT WARTS, HEEL WARTS, HAIRY FOOT WARTS, MORTELLARO’s DISEASE, STRAWBERRY HEEL WARTS)
Papillomatous digital dermatitis (PDD) of cattle is an infectious, contagious dermatitis of the digital skin of cattle. It is primarily a disease of housed dairy cattle. PDD was first described in Italy in 1974 and in New York in 1980. During the early 1990s the reported first observations of PDD increased dramatically in the United States, and this disease is a major cause of lameness in dairy cattle in many countries.65,66 Financial losses are from reduced milk production, reduced reproductive efficiency, costs of treatment, and premature culling. European and U.S. studies estimate the cost per case to be approximately $130. Prevalence in endemically infected herds is typically 10% to 60%.
Clinical Signs
About 80% of PDD lesions occur on the plantar aspect of the hindfoot, immediately proximal to the heel bulbs and adjacent to or extending into the interdigital space.65 Less common sites for lesions are the plantar aspect of a forefoot and the dorsal aspect of any foot. Multiple lesions may exist on a single animal, even on a single foot, but lesions are confined to the digital skin and have not been reported to occur above the level of the dewclaws.65
The gross appearance of the lesions and the predilection for hindlimbs and skin-horn junctions, especially those bordering the heel bulbs and interdigital space, distinguish this disease from other bovine dermatitides. Lesions are most often 2 to 6 cm (0.8 to 2.4 inches) across at their greatest dimension, are circular or oval, and have clearly demarcated, raised borders.65,67 Lesion borders are often surrounded by hairs that are two to three times normal length. Lesion surfaces may have filiform papillae varying in length from 1 mm to 3 cm (1.2 inches) and 0.5 to 1 mm in diameter, thus the name “hairy foot warts.” Lesions lacking the filiform papillae (“hairs”) may have a granular surface. Lesions vary in color and appearance. Washed surfaces are generally very painful and either red and granular or composites of white, yellow, gray, brown, or black papillary areas with red, granular areas interspersed.65 The lesions bleed easily if traumatized. When PDD lesions develop in the interdigital space, they frequently occur on a preexisting fibroma.
The lesions slowly enlarge and become raised (papillomatous) masses 2 to 6 cm in diameter that are red, gray, or black and oval, spherical, or U shaped. Proliferative or papillomatous lesions are seen less often in Europe than in the United States and are less common on dairies with a consistent treatment and control program.
A foul odor may be present and appears to be caused by secondary bacterial growth in the exudate covering the PDD lesion. Swelling of the pastern and fetlock regions is not present in uncomplicated cases. Lameness is a herd characteristic on dairies where PDD has a high prevalence but is an inconsistent finding on individual infected cattle and is not consistently related to lesion size or maturity.68 If PDD lesions remain untreated, the claws of feet with plantar or palmar lesions may develop a clubbed appearance because the cow prefers to bear weight on (and wear down) the toes.65
Differential Diagnoses
Differential diagnoses for an individual case of PDD include interdigital necrobacillosis (foot rot, “foul in the foot,” or interdigital phlegmon), interdigital hyperplasia (corns or interdigital fibroma), interdigital dermatitis, and traumatic injury with granulation tissue. For herd problems of lameness localized to the digit, differential diagnoses should include PDD, interdigital necrobacillosis, interdigital dermatitis, laminitis, excessive sole wear from caustic or abrasive flooring, and improper claw trimming. If signs of polysystemic disease exist, coronitis from viral diseases (e.g., bovine viral diarrhea) should also be considered.
Epidemiology
Once introduced into a herd, PDD spreads rapidly within adult cows, often affecting the majority of adults within the first year of infection.65 When established in a herd, lameness is most often seen in first- and second-lactation cows.65,67 Lesions are typically present in a proportion of older cows, but lameness is less apparent than in the younger cows. Bulls and yearling heifers may also be affected but usually comprise a small fraction of clinical cases. In California the disease appears to be most severe during the spring and summer months,65 whereas in Europe it is most severe in the winter months. Free-stall herds are more often affected than stanchion (tie-stall) herds, probably because of better foot hygiene in the stanchions. Cattle confined to pasture are rarely affected,69 but the disease is emerging in some South American countries with wet pastures during part of the year. PDD is almost exclusively seen in dairy cattle, with Holsteins more likely to be affected than Jersey or dual-purpose breeds.69,70 Eradication of PDD from an endemically infected herd is unlikely.
Retrospective epidemiologic studies indicated that two risk factors are significant in high-prevalence herds: muddy or wet conditions and purchasing replacement cattle from off premises.69,71,72 Other risk factors identified were the use of outside hoof trimmers and not washing hoof-trimming equipment between cows.72
Attempts to transmit the disease experimentally were successful when scrapings from active lesions were applied to the feet of calves subjected to constant moisture and low oxygen tension. Constant moisture and low oxygen tension are present on confinement dairies if manure management and hygiene are not adequate. Poor free-stall or bedding area management will exacerbate the problem by forcing cows to stand in manure slurry for longer periods and will not allow the feet of cattle to dry out periodically.
Pathogenesis
Numerous obligate anaerobic organisms have been associated with PDD.73 Spirochetes from the genus Treponema have been identified most consistently.74-78 During experimental transmission studies, Treponema species are the first organisms to appear, comprise the bulk of the colonizing bacterial mat found on active lesions, and are the organisms that invade the epidermis and dermis.76 These spirochetes also produce a humoral response in cows with active lesions.65,78 PDD lesions show gross and histologic similarities to viral papillomas; however, bovine papillomavirus has not been found.65
Current evidence indicates that PDD is multifactorial, involving environmental, microbial, host, and management factors. Factors such as rough flooring; poor drainage; accumulation of feces and urine on floors; dirty, wet, or uncomfortable bedding areas; and overcrowding have an adverse effect on digital skin health and could increase the risk of PDD. The mode of transmission between cows and between herds is currently unclear, although one study found concurrent spirochetal infection of feet and colon in cattle.79 Clinically and subclinically affected cows and fomites such as foot-trimming instruments, livestock trailers, and farm equipment may be sources of infection for naive herds.
A California study found that antibodies against two antigenically distinct spirochetes were increased on dairies with PDD compared with PDD-free dairies.78 Also, cattle with PDD on a high-prevalence dairy were much more likely to have antibodies to the spirochetes than were cattle without lesions on the same dairy.78 There was no cross-reactivity between the two spirochetes or to other spirochetal diseases of cattle.78 The concentration of clinical disease in the younger animals of an endemically infected herd suggests that some degree of immunity may develop in older cows. Nonetheless, chronic and recurrent cases in otherwise healthy adult cattle have been reported, and immunity to PDD, if it does develop, may be incomplete or temporary. One study found high recurrence or new lesions 7 to 12 weeks after successful treatment.65 Spontaneous regression of lesions and resolution of lameness have also been observed but appear to be rare.
Pathologic Findings
Histopathologic criteria to establish a diagnosis of PDD are as follows65:
If performed, biopsies should be full-thickness 6-mm punch biopsies, washed off with sterile saline, and placed in buffered formalin. Histopathology is helpful to confirm a diagnosis of PDD but is not necessary because lesions have such a characteristic appearance and location.
Preliminary results of a biopsy study on treatment and recurrence of PDD indicate that the gross visual and histopathologic diagnoses were in agreement for active lesions before treatment. Histopathology on day 28 after treatment with lincomycin or oxytetracycline, however, found a high percentage (55%) of lesions that visually appeared to be healed but still had microscopic evidence of infection.80 It could not be determined if lesions were incompletely healed or recurrent infections.
Treatment and Prevention
The most common treatments for PDD involve the use of topical antibiotics.68,81 There are limited reports of nonantibiotic products being efficacious, although research and development continue. Currently, no antibiotics are labeled for treatment of PDD in the United States; therefore, adherence to extralabel drug use regulations is imperative.
The most common treatment for PDD in the United States is topical oxytetracycline or lincomycin used as a spray or applied with a bandage. For topical spray treatments, oxytetracycline or lincomycin are mixed with deionized or distilled water in a 2- to 4-L agricultural sprayer (25 g/L oxytetracycline, 8 g/L lincomycin) and applied directly to the heels of PDD-affected cattle once daily for 5 to 10 days. Recurrence is sufficiently common that topical treatments need to be repeated every 45 to 60 days on affected cattle to control the disease. No antibiotic residue violations have been reported resulting from topical application of antibiotics.82 Efficacy from parenteral antibiotics has been inconsistent.
Footbaths are often used on dairies to control PDD and other infectious causes of lameness, although most footbath products have not been rigorously evaluated.68 Products used include antibiotics, copper or zinc sulfate (5%), formalin (5%), and various proprietary products. On large dairies, footbaths may be more effective at controlling PDD when the disease is at a low prevalence (<10%). When the disease has a high prevalence, individual topical treatment is probably more efficacious at reducing the prevalence. The efficacy of footbaths is reduced if feces and debris are allowed to accumulate in the treatment solution. This problem can be limited by placing two footbaths in tandem, with the first containing water or a mild detergent solution for cleaning the feet and the second containing the antiseptic solution. The footbath solution should be changed every 150 to 300 cow passages, depending on soiling. Footbaths should be a minimum of 8 feet long and 2 to 3 feet wide, with a depth of 5 to 6 inches so that cows have to place all four feet in the solution as they walk through. The baths should be covered to limit dilution by rain and should be located in an exit alley off of the milking parlor to avoid splash contamination of the teat ends before milking. Additional footbaths can be placed in other locations to treat bulls, dry cows, and heifers. Treatments should occur every 2 to 7 days depending on response on the individual dairy. Laws regarding the use and disposal of certain chemicals, as well as where on the dairy such medications can be used, often vary among locales. Consultation with local regulatory agencies is prudent before initiation of a control program on a dairy.
The efficacious use of footbaths on dairies signals major husbandry problems on dairies because the environmental and hygiene problems are often not addressed.83 Improvement of stall, corral, and alley hygiene; provision of dry and comfortable bedding; reduction of stocking rate; and improved ventilation to allow drying of stalls and alleys may result in reduction of the incidence or severity of clinical cases. Claw-trimming equipment, mobile tilt tables, and livestock trailers should be thoroughly cleaned and disinfected to prevent potential transmission of the agent(s) of PDD between dairies.
A Treponema bacterin was developed, tested, and licensed for use in the United States. A blind field study where half the lactating cows were vaccinated and half received a placebo vaccine (no Treponema) did not find a prophylactic or therapeutic effect of the vaccine.84
INTERDIGITAL DERMATITIS
Interdigital dermatitis (ID) is acute or chronic inflammation of the interdigital skin that usually does not cause lameness. Inflammation is confined to the epidermis. Diffuse epidermal erosion in the interdigital cleft may be seen in early cases. More chronic cases show hyperkeratosis, which creates a roughened appearance to the interdigital skin and dorsal and palmar commissural skin folds. A malodorous, gray, serous exudate may be present, and there is mild sensitivity to pressure. This condition is frequently accompanied by cracks in the heel (heel horn erosion), with potential underrunning of the heel horn, and some researchers consider ID and heel horn erosion to be the same disease (IDHE). When severe, IDHE will cause lameness.
Dichelobacter nodosus and Fusobacterium necrophorum may be primary or contributory pathogens for ID. Several investigators have speculated that ID and PDD are forms of the same disease complex. Both share several histologic characteristics, including spirochetal involvement, and both can be successfully treated and prevented with the same topical antibiotics or footbaths. However, ID can persist on dairies that practice regular foot bathing because the causative organisms may survive within deep heel cracks that are not permeated by footbath solutions. During claw trimming, heel cracks must be trimmed away to allow for exposure.
Interdigital dermatitis differs from interdigital necrobacillosis (foot rot), in which infection extends into the dermis, leading to fissure formation, infection of deeper structures, and cellulitis of the pastern and fetlock regions.
PAPILLOMAS (WARTS, FIBROPAPILLOMAS)
Definition and Etiology
Cutaneous papillomas or warts occur (in decreasing order of frequency) in cattle, horses, goats, and sheep.85-88 The growths usually occur in young animals,88 as well as on teats of mature cattle and goats, and are white, tan, or gray, firm protruding masses with a dry, horny surface. They vary in size from 1 to 500 mm and may be single or multiple. Species-specific papillomaviruses (subgroup of papovavirus) are responsible for causing warts. It is now accepted that parts of the bovine papillomavirus (BPV) genome are found in naturally occurring equine sarcoids.89 There are at least 10 BPV strains, designated BPV-1 through BPV-10 (Table 40-1),90 and there are probably more. Only one strain of virus is currently recognized in horses, goats, and sheep, but little research on papillomas has been done in these species, and two horse and two goat strains are likely.
Clinical Signs
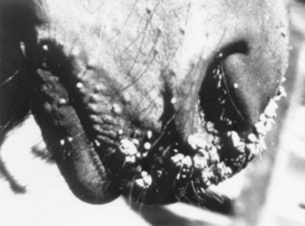
Warts are usually small, benign growths that appear on young animals under 2 years of age, persist for 3 to 12 months, and then spontaneously regress without causing clinical signs (other than a blemish). In cattle, warts on the teats, penis, or interdigital skin91 or in the alimentary tract may produce clinical signs of pain or occlusion. Warts usually develop in the ears of young cattle after tagging or tattooing, especially if instruments are not disinfected between individuals; these are not age related. Teat warts also predispose to environmental mastitis. Occasionally, individuals with (presumed) defective cellular immunity may develop multiple, extremely large warts that may result in weight loss.
In horses, warts on the face are rather common but rarely cause a significant problem (Fig. 40-8). The previously reported congenital wart in young horses has now been more properly termed a hamartomatous lesion (epidermal nevus) and has been shown not to be virus induced.92
Warts are relatively rare in goats. They occur mainly on teats and may spread throughout the herd. A 1936 report described a herd outbreak involving the head, neck, shoulders, and forelegs in milking goats.93 Does without adequate pigmentation on the udder develop persistent mammary gland warts that may undergo transformation to become squamous cell carcinomas.94 There are probably at least two different strains of goat papilloma virus (head/neck and mammary).
Warts are rare in sheep but may occur on the face or legs. Differential diagnosis in sheep and goats includes contagious ecthyma (orf), ulcerative dermatosis, dermatophilosis, and sheeppox and goatpox.
Treatment and Control
Small warts can be crushed, pinched off, or surgically removed. Cryosurgery can be used on larger warts. Many regress spontaneously within a few months, even without treatment.
When show animals are involved or when animals have multiple large papillomas, tissue can be removed and made into crude autogenous vaccine (2 mL intradermally three times weekly) by homogenizing, grinding, freeze-thawing twice, filtering, and killing virus with 0.5% formalin. Autogenous wart vaccines are variable in their efficacy, as are commercial vaccines.95-97 The latter rarely seem to result in effective regression of existing warts, but may prevent the development of new lesions if the same BPV strain is involved. Autogenous vaccines are capable of preventing new lesions caused by the same BPV strain in a herd. There is no indication that cattle vaccines have any efficacy in other species. No wart vaccines for horses, sheep, or goats are currently marketed.
Because the viruses can be directly or fomite transmitted, prevention involves isolation, preventing animals from rubbing on each other, and not sharing halters, brushes, and other equipment. Dipping of dehorning, tagging, and tattooing instruments in a viricidal solution between animals will also slow spread of the virus.
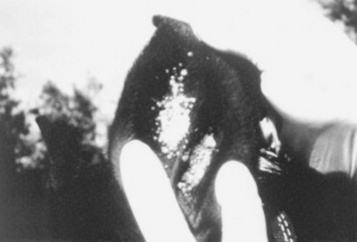
AURAL PLAQUES
Aural plaques may be a form of viral papilloma that often affects the inner pinna (Fig. 40-9). Nonpruritic, these plaques may also occur on the genitalia and mammary glands. The color varies from pink to grayish-white. Plaques do not resolve spontaneously, as do the “classic” papillomas seen in young horses. Biopsy or “shaving off” aural papillomas may stimulate reduction or resolution of the masses, although in some horses, this may only be temporary (6 to 12 months). This has been theorized to be due to the release of “papilloma antigens” into the blood stream during the surgical procedure, prompting an immune response against the tumor. Imiquimod (Aldara, 3M, Minneapolis), a nonsteroidal local immune response—modifier cream, has been helpful in some cases, used three times weekly, every other week, for 2 to 4 months. Owners should wear gloves and should be forewarned that there is frequently an impressive inflammatory reaction to the cream in the initial weeks of treatment.
A papillomavirus has been demonstrated in aural plaques on electron microscopy and with immunohistochemical techniques.98
PSEUDOCOWPOX
Pseudocowpox is a common parapoxvirus of cattle related to the viruses of contagious ecthyma (soremouth) of sheep and goats and bovine papular stomatitis (see Chapter 32 for these diseases). All three parapoxviruses may cause nodular lesions on humans. The lesions of pseudocowpox are usually confined to the teats of cattle, and the disease is common worldwide. Cyclic waves of reinfection occur in a herd, where it causes minor teat lesions characterized initially by a small papule 2 to 3 mm in diameter, followed by crusting and circular spread of the lesion. Approximately 10 days later, the 15- to 20-mm lesion appears as a ring or horseshoe-shaped scab.99 Lesions occasionally involve the udder, medial thighs, or scrotum. Deep ulceration is rare. There are no systemic signs of illness.29 The major problem associated with the teat lesions is an increased incidence of mastitis. The most important and common differential diagnoses are bovine herpes mammillitis and viral papillomas. Rare viruses involving the teat include vaccinia and cowpox.99 Cowpox is a rare disease of cattle in Europe that causes ulcers and may also produce lesions in humans. Vesiculation is rare in pseudocowpox, in contrast to bovine herpes mammillitis, vaccinia, and cowpox.29
BOVINE HERPES MAMMILLITIS (BOVINE HERPESVIRUS, BOVINE ULCERATIVE MAMMILLITIS)
Bovine mammillitis teat lesions are caused by bovine herpesvirus type 2 (BHV-2), which is widely disseminated in most cattle populations.100,101 The disease may be epidemic or endemic. The virus may also cause oral lesions, udder lesions, or generalized skin disease in cattle. The teat lesions start as swollen, tender, edematous teats. Vesicles may appear in some lesions, whereas others ulcerate almost immediately. The teats become painful, and ulcers require 3 to 10 weeks to heal.100,101 Mastitis is increased because the scabs on the teats are laden with bacteria. Diagnosis may be confirmed by isolation of the virus, the BHV-2 serum neutralization tester, or histologic demonstration of herpesvirus particles.100,101 Therapy consists of segregation of affected animals from the rest of the herd, and affected cows should be milked last. Milkers should wash hands between cows. In severe cases, secondary infection may be controlled by topical antibiotic creams or parenteral antibiotics, with proper residue avoidance precautions in place.
SHEEPPOX AND GOATPOX
Sheeppox and goatpox are caused by capripoxviruses.102 Both occur in Africa, Asia, and the Middle East; goatpox also occurs in parts of Europe and the United States. The two diseases are clinically similar, although sheeppox has the most severe systemic signs of the animal pox diseases. However, recent studies have showed that the viruses are phylogenetically distinct and can be differentiated by molecular tools.103 The diseases affect all ages, causing pyrexia, anorexia, conjunctivitis, rhinitis, and skin lesions. Prophylaxis using attenuated vaccines is the preferred control measure because the immunity is long-lasting.102,103 Morbidity is high. Mortality may reach 80% with sheeppox but usually is low with goatpox. Humans may develop skin lesions from goatpox.
DERMATOPHYTOSIS (RINGWORM)
Dermatophytosis refers to infections of the keratinized tissues of the skin (stratum corneum layer of epidermis, hair, claws, hoof, and horns) by Microsporum and Trichophyton species. Dermatophytosis is relatively common in the horse and in cattle, less so in goats, and rare in sheep.
Etiology and Pathogenesis
The most common equine dermatophyte species isolated from horses are Trichophyton equinum, Microsporum equinum, Trichophyton mentagrophytes, and Trichophyton verrucosum.7,28,104 T. verrucosum infections in humans caused by transmission from horses have been reported.105 In ruminants the majority of dermatophytosis lesions are caused by T. verrucosum and to a lesser extent by T. mentagrophytes. The transmission of the disease is usually from animal by direct contact or indirectly through fomites such as grooming instruments, tack, housing, fencing, or feed bunks. The incubation period may range from 1 to 6 weeks.
Several factors may influence the susceptibility of an animal to dermatophyte infection. Age is probably most important, with younger animals more susceptible to infection. The susceptibility of young animals is most likely related to lack of prior exposure/infection and thus no immunity, as well as crowding of young animals and conditions that decrease resistance to infection (e.g., poor nutrition). Environmental factors such as warmth and humidity also may play a role. Calves kept indoors or exposed to foggy weather with little or no sunlight have an increased incidence.
Under normal circumstances, dermatophytes only invade fully keratinized, nonliving tissues.7 This results in weakened hair shafts, leading to alopecia.
In many cases, dermatophytosis is theorized to be a self-limiting disease, with the duration of infection ranging from 1 to 4 months. The spontaneous regression is at least partly related to the development of immunity, of which cell-mediated immunity is the more important. The immunity that develops is probably not complete, and its duration is unknown.
Clinical Signs
The lesions are often circular and usually appear first on the face or in the axillary/girth area and may spread over the trunk, rump, neck, head, and limbs (Fig. 40-10). Occasionally in the horse, dermatophytoses may be limited to the pastern region. Rarely, dermatophytes may be a cause of coronary band disease in horses. The mane and tail are rarely involved. Cattle and goats are frequently affected around the eyes and face (Fig. 40-11).
Lesions may be superficial or deep. Superficial infections are much more common and manifest with the development of thick crusts or more generally a diffuse moth-eaten appearance with desquamation and alopecia, sometimes in a ring pattern. A small crust may form over the follicle, and the hair is lost. Occasionally the initial lesions may be urticarial, progressing to multifocal, sharply demarcated areas of alopecia and scaling. The degree of pruritus varies but is usually mild or absent. Erythema is usually absent or obscured by pigmented skin. The lesions in cattle are usually characterized by excessive crusting, taking on an almost wartlike appearance.
Dermatophytosis must be considered in the differential diagnosis of any dermatoses characterized by multifocal alopecia or scaling and crusting. For a solitary lesion in horses, the occult sarcoid is the primary differential diagnosis. The two most important differential diagnoses of more extensive lesions are dermatophilosis and pemphigus foliaceus.
Diagnosis
Direct microscopic examination of infected hairs is of value, but in general the most common and reliable method of diagnosing dermatophytosis is fungal culture (see Chapter 11). Broken hairs at the periphery of lesions are most satisfactory for this purpose. Large crusts and areas of separation should be avoided. The use of specialized indicator media is preferred. On occasion, dermatophytosis is diagnosed histopathologically. Interestingly, Trichophyton species occasionally may cause acantholysis, mimicking pemphigus foliaceus on histopathology.106
Therapy
Although 50% captan (2 tablespoons powder in 1 gallon water) has been recommended in the past and is certainly safe for tack, its effectiveness has been questioned. Lime Sulfur (LymDyp, IVX Animal Health, St. Joseph, MO), 1 cup to 1 gallon of water, and bleach 1:10 with water are both effective, but messy, odiferous, and staining. Miconazole shampoos are becoming more widely used and may be as effective. In Europe and Canada an enilconazole rinse (Imaveral, Jaansen), approved for horses and cattle, is highly effective.
Systemic treatment is occasionally needed. The efficacy and proper dose of griseofulvin in horses has not been thoroughly researched. However, a dosage of 100 mg/kg daily for 7 to 10 days has been advocated and has been used with good success on a small number of horses by the author. Griseofulvin is a teratogen and should not be used in pregnant mares. Alternatively, 20% sodium iodine (NaI) may be given IV (250 mL/500-kg horse once or twice every 7 days) but also is contraindicated in pregnant mares because it may cause abortion.28
Vaccinating cattle against T. verrucosum has long been successful in Eastern Europe and Scandinavia. Effective control of ringworm in cattle has been achieved in regions implementing systematic vaccination.107 Vaccination against T. equinum in horses may reduce the incidence of new infections and protect a high percentage (>80%) of vaccinates from infection. These data are based on results with an inactivated vaccine containing both conidia and mycelial elements.108 In cattle, newer vaccines consisting of recombinant protein and deoxyribonucleic acid (DNA) derived from heat-shock protein 60 of T. mentagrophytes have shown success experimentally.109 Such vaccines are not yet available in the United States.
SPOROTRICHOSIS
Sporotrichosis is caused by the yeast Sporothrix schenckii, which is most common in vegetation. The yeast becomes pathogenic in animals as a result of its dimorphic ability to convert from a yeastlike form at (tissue) temperatures between 35° C and 37° C (95° F and 98.6° F) to a mycelial phase (with branching, septate hyphae) at environmental or laboratory temperatures of 25° to 30° C (77° to 86° F).110 The disease has been reported in many species of domestic animals.
The initial lesions are nodules, which frequently ulcerate, and the disease may progress to a lymphatic-cording disease. Rarely, the fungus will eventually spread to the lungs. Diagnosis is made by demonstrating the organism on histopathology, immunofluorescent antibody testing on affected tissues, impression smears, and culture.111 This is a zoonosis, so care should be taken in handling suspected samples.
Successful therapy with a number of different systemic iodine preparations (NaI, KI) has been reported. The organic iodides have proved to be superior in efficacy to the inorganic iodides in the treatment of equine sporotrichosis, with ethylenediaminedihydroiodide* being the drug of choice. This product is in the form of a feed additive and can be mixed with a small amount of grain and administered at 1 to 2 mg/kg of the active ingredient once to twice daily for the first week, then 0.5 to 1.0 mg/kg once daily for the remainder of the treatment. In general, lesions will begin to regress during the first month of treatment, and therapy should be continued for at least 1 month beyond the complete resolution of all cutaneous nodules and the healing of any ulcerated lesions. Discontinuing therapy prematurely will invariably result in an unnecessary relapse of the disease. During treatment the horse should be closely observed for any evidence of iodide toxicity (iodism), which includes excess scaling and alopecia, a serous ocular or nasal discharge, excess salivation, anorexia, depression, coughing, nervousness, or cardiovascular abnormalities. Should any of these signs develop, the treatment should be discontinued for 1 week, then resumed at three-quarters the dosage at which the iodism was noted. In most cases the treatment is subsequently well tolerated.112 Although both itraconazole and terbinafine have been shown to be effective in vitro against the organism isolated from a horse, I am unaware of any clinical reports in this species.113
PHAEOHYPHOMYCOSIS
Phaeohyphomycosis is actually caused by a number of ubiquitous fungi that are either saprophytes or plant pathogens. These include Alternaria, Dreschlera, Cladophialophora, Cladosporium, Phialophora, and Stemphylium species. The correct terminology currently depends on the histologic appearance of the organism, as follows114:
Cutaneous lesions arise from either trauma to the skin or disseminated disease. Animals with disseminated disease probably have a deficient immune system. Similar to the other deep mycoses, these lesions present as expanding nodules and draining tracts. Diagnosis is by biopsy or occasionally cytology. Histopathology shows foamy or epithelioid macrophages, and special stains will show the organisms. Veterinarians and their staff should be careful in handling material from infected animals, because the inadvertent inoculation of these organisms can cause human infections.
Treatment has generally included surgical excision or amputation if practical. Potassium iodide (PI), as for sporotrichosis, and (if the owners can afford it) fluconazole at 5 mg/kg may be effective.
ZYGOMYCOSIS
Similar to phaeohyphomycosis, zygomycosis is caused by a number of related fungal species that are ubiquitous saprophytes. Gastrointestinal and respiratory tract involvement is possible. Cutaneous lesions are thought to be caused by wound penetration. The disease typically occurs in tropical and subtropical areas. The two orders of Zygomycetes that cause disease are Mucorales, including the genera Rhizopus and Mucor, and Entomophthorales, including the genera Conidiobolus and Basidiobolus. Most cutaneous reports are in horses, although ruminants may have other organ systems affected.
The skin disease is ulcerative, nodular, and generally found on the trunk and neck. Generally, only one large nodule is present. Diagnosis is by histopathology and culture; a serum agar immunoprecipitation test has been useful in diagnosing conidiobolomycosis. Surgical removal, if possible, is preferred for treatment. Medical options include the iodides (not effective against the order Mucorales) and possibly the azoles or amphotericin B.7
PYTHIOSIS
Not a true yeast, but rather a protista, Pythium insidiosum is considered to be the causative agent of “swamp cancer,” also known as “Florida horse leech,” bursattee, and kunker. This organism is found in tropical and subtropical areas worldwide. The pythiosis lesion occurs most often on the limbs, abdomen, neck, and lips and consists of dense granulation tissue containing masses of yellow-gray necrotic tissue, which sometimes are calcified and often are present as cores in fistulae and are removable intact. Such masses are known as “leeches” or “kunkers.” The granuloma ulcerates and extends peripherally and may reach a very large size in a short time; the overlying and adjacent skin is destroyed by both the inflammatory reaction and the self-mutilation by the horse. Important differential diagnoses are systemic fungal infections, habronemiasis (“summer sore”), and neoplasia. Although most reports in large animals are in horses, cattle and sheep may also be affected.115,116
Histopathologic examination of affected tissue reveals pyogranulomatous inflammation directly surrounding the organism. Isolation of organisms from the lesions is necessary for further identification and study, but histologic demonstration of the protozoa within tissues that are reacting to its presence is critical in establishing the causal relationship in an individual lesion. In obtaining culture, the kunkers are preferred to the actual tissue. For samples that cannot be processed immediately, acceptable handling techniques include storage at room temperature for up to 3 days, refrigeration for up to 5 days, shipping on cold packs, and storage in antibiotic solution, each combined with subsequent inoculation on selective media.117 Recent advances in ELISA or molecular techniques offer better potential for organism detection and identification.118 Wide surgical excision combined with immunotherapy has the best chance of success.118,119
PEDICULOSIS
Lice are obligatory ectoparasites that are generally host specific. Adults and nymphs are seldom able to live more than a few days away from their host. Large domestic animals suffer from infestation with several species of lice that belong to the orders Mallophaga, the biting lice, and Anoplura, the sucking lice (Table 40-2).120
Table 40-2 Lice Associated with Large Domestic Animals
| Host | Mallophaga (Biting) | Anoplura (Sucking) |
|---|---|---|
| Horse | Bovicola (Damalinia) equiWerneckiella equi equi | Haematopinus asini |
| Cattle | Bovicola (Damalinia) bovis | Haematopinus eurysternus |
| Haematopinus quadripertusus | ||
| Haematopinus tuberculatus | ||
| Linognathus vituli | ||
| Solenopotes capillatus | ||
| Goats | Bovicola (Damalinia) caprae | Linognathus stenopsis |
| Bovicola (Damalinia) limbatus | Linognathus africanus | |
| Bovicola (Damalinia) crassipes | Linognathus vituli | |
| Sheep | Bovicola (Damalinia) ovis | Linognathus pedalis |
| Bovicola (Damalinia) capre | Linognathus ovillusLinognathus africanus |
Clinical infestations are most apparent during the winter months and reflect efficient louse reproduction during the late fall and that summertime temperatures on body areas exposed to sunlight are too high for lice. Apparent “carrier” animals within a herd maintain populations during the “off” season and serve as a source for reinfestation of the herd during the fall.120 The hallmark of infestation is pruritus, and many clinical changes result from self-trauma. The neck and tail are typically affected first,121 but infestation and clinical signs may become generalized. The coat becomes dry and scaly. Patchy alopecia and crusted ulcerations result from excoriation. A heavily infested animal may become anemic. Significant hide damage occurs as a result of excoriation, and hairballs may accumulate in the gastrointestinal (GI) tract from self-grooming. Reduced productivity and weight loss result from decreased feed intake associated with restlessness. Diagnosis is usually by examination; a hand lens is useful (e.g., otoscope without cone). Interestingly, when a horse is exercised and becomes warm and sweaty, the lice will climb out toward the tip of the hair and are easier to find.122
Several treatment approaches exist. Ivermectin at 200 μg/kg every 14 days for two applications is effective for sucking lice but not biting lice. Application of an appropriate topical insecticide to infested animals and to all contact animals at 2-week intervals for two or three treatments is usually curative for both types of lice. Repetition of treatment is necessary to break the louse life cycle because eggs are not killed by insecticides and will hatch despite therapy. Effective topical agents include pyrethroids, permethrins, selenium sulfide, imidacloprid, phoxim, and fipronil.122 Appropriate attention should be paid to withdrawal times in food animals. Cleaning of fomites such as blankets, brushes, and rope halters is probably indicated, although lice do not live long off the host.
TROMBICULIDIASIS
Trombiculidiasis is caused by the larval stages of mites commonly known as “harvest mites” or “chiggers.” The most common species affecting large animals are Eutrombicula alfreddugesi and Neotrombicula autumnalis. The adults and nymphs are free living. The larvae feed on mammalian hosts, secreting substances in their saliva that hydrolyze the epidermis and allow extraction of tissue fluids. The individual larval stage is short, but the infestation may be ongoing with the acquisition of new larvae from the environment. In the Northern Hemisphere, E. alfreddugesi is active from late spring through early fall, whereas N. autumnalis is more common in the late summer to midfall. The mites may be found in wooded areas, grass, and hay.122,123
The clinical signs of trombiculidiasis are crusts and papules, especially on the face, neck, and extremities. Pruritus is variable.123 Diagnosis may be made early in the course of infestation by careful inspection (e.g., hand lens) and finding the minute, red-orange larvae in the center of a papule. The six-legged larvae have round bodies and may be identified specifically on skin scrapings or acetate tape preparations.122 Although the larvae only remain on the animal host for several days, clinical signs may persist longer. Thus, trombiculidiasis should be suspected even in the absence of mites in an animal with appropriate signs in the summer and fall.
The disorder is in theory self-limiting because of the short time of attachment of the parasite. However, if the pruritus is severe, corticosteroid administration is indicated.122 Various products have been used on the horse to eliminate the larvae; 5% lime sulfur, fipronil, and permethrin are generally the safer parasiticides to use.
MANGE
Psoroptic Mange
Psoroptic mange has been recognized in horses, cattle, sheep, and goats (and rabbits). The host specificity of Psoroptes equi, P. ovis, P. natalensis, and P. cuniculi is controversial; two recent reports state that the mites are all genetically homogenous with little or no host specificity,122,124 whereas another report documents an inability to transfer Psoroptes ovis to goats.125 The mites do not affect people. Psoroptic mange is common in cattle but has been eradicated from horses and sheep in the United States; it is still a concern for sheep production in the United Kingdom and elsewhere in the world. The mites have a 2-week life cycle on their host but can live away from the host for up to 3 weeks. Psoroptes mites live on the surface of the epidermis and do not burrow. Symptomatic infestation tends to be more prevalent during the cooler months.
The hallmark of infestation in all species is pruritus. In cattle, crusted papular lesions are typically first apparent on the withers but then generalize, resulting in weight loss, secondary infections, and decreased production.126 Horses develop papules, crusts, and alopecia most often at the base of the mane, tail, ears, and intermandibular area, with subsequent spreading to the trunk.122 An otitis externa may be present, seemingly with Psoroptes cuniculi.122 In sheep, typical lesions include papules and crusts in wooled areas, and secondary infection with Staphylococcus aureus may occur.127 The intense pruritus can be debilitating. Certain sheep breeds (e.g., merino) are more susceptible to infestation than others.125 The immune response in sheep has been extensively studied, with early innate and longer-term adaptive cutaneous immunoinflammatory responses as well as mite antigen—directed IgE being reported.126 Goats usually have lesions restricted to the ears, although infestation and symptoms may spread to the neck and body.29
Diagnosis is based on demonstrating mites in skin scrapings and ear swabs from affected animals. Psoroptic mites are recognized by their round bodies and long, segmented pedicles.29
A number of topical insecticides have been recommended for treatment and should be applied to all affected and contact animals according to the manufacturer’s guidelines. These include deltamethrin, coumaphos, diazinon, malathion, toxaphene, and lime sulfur.29,123,128 The contaminated environment should also be treated. Ivermectin, doramectin, and moxidectin have been used for Psoroptes species in large animals at 0.2 mg/kg subcutaneously (SC),123,129,130 but treatment does not eliminate live mites from all animals. It has been recommended that P. ovis–infested cattle be isolated for a minimum of 14 days after treatment to prevent transmission to susceptible contact cattle.130 Psoroptes-infested ears in horses should not be treated topically because of the resulting discomfort; reliance is on systemic treatment. Psoroptic mange is a reportable disease in the United States.
Chorioptic Mange
Chorioptic mange, also known as leg mange, is common in cattle and sheep, uncommon in goats, and seen with variable frequency in horses depending on geographic area. Draft horses may be more susceptible than other breeds. Chorioptes species are relatively host specific and include C. bovis, C. texanus, C. ovis, C. equi, and C. capre.29,123,131 The mites do not penetrate the epidermis123 and do not affect humans.130 They have a life cycle that spans 2 to 3 weeks and can live off the host for only a few days.29
This disease is variably pruritic, more so in ruminants than horses, and less so than infestations with Psoroptes or Sarcoptes mites. Lesions consisting of papules, erythema, scaling, crusting, ulceration, and alopecia result from self-trauma. In cattle the lower aspects of the hindlimbs, the perineum (particularly the perianal fossa), tail, and scrotum are usually affected. Sheep typically demonstrate involvement of the lower limbs and scrotum. Goats have involvement of the lower limbs. Horses typically have lesions on the pasterns, although the ventral abdomen may become involved in severe cases. In all species, infestation can become generalized.122,123,130
Mites are often numerous and readily demonstrated with skin scrapings. Cattle may be asymptomatic.130 Chorioptic mange will respond to treatments described for psoroptic mange, although ivermectin and related compounds are more effective in ruminants than horses; in horses, lime sulfur or fipronil applied once weekly for at least 1 month is recommended.122,132,133 A recent study in horses showed efficacy using a single dose of moxidectin 2% oral gel (Equest, Fort Dodge, IA) at the manufacturer's recommended therapeutic dose of 0.4 mg/kg body weight.133a Chorioptic mange is a reportable disease in the United States.
Sarcoptic Mange
Sarcoptic mange is an uncommon, contagious disease of horses, cattle, sheep, and goats. The etiologic agent is Sarcoptes scabiei; several subspecies are relatively host specific but can be transmitted to humans. The mite burrows into the epidermis, where the egg is deposited, and its life cycle is complete in 10 to 17 days. Transmission is usually by direct contact, but the mite has a variable survival time off the host; therefore, environmental and fomite transmission is possible.122,123
The clinical signs are all referable to the severe pruritus caused by the mite. Lesions include papules, scaling, crusting, ulceration, and alopecia. The head (especially the ears) and neck are usually the initial areas of involvement, although lesions become generalized.29,122,123 Horses in areas frequented by infected wild foxes may have lesions affecting the legs and the ventral abdomen.122 Dairy cattle may have an associated udder cleft dermatitis.134 Sheep tend to have initial involvement of the nonwool areas. The ears should always be scraped for mites; the mite is identified by its rounded body, terminal anus, short legs, and long unsegmented pedicles.29,122,123
Because mites may be present only in small numbers, negative skin scrapings do not rule out the disease. Diagnosis should be based on clinical suspicion and response to therapy.7,29,122,123
Topical acaricides have been recommended for treatment and are applied to all affected and contact animals at 10- to 14-day intervals for four to six treatments. These include lindane, coumaphos, diazinon, malathion, toxaphene, and lime sulfur.29,122,123 Ivermectin, 0.2 mg/kg at 2-week intervals for two to four injections (ruminants) or orally (horses), has been shown to be effective, as has injectable moxidectin and topical doramectin. The contaminated environment should also be treated. It is recommended that the state regulatory agency for livestock disease control be consulted for methods of treatment and products to use because sarcoptic mange is a reportable disease.
Demodectic Mange
Demodectic mange is a rare disorder, although it has been recognized in all the large domestic species. The mites live in the hair follicles and in the sebaceous and sweat glands and are host specific. They are not contagious between members of the same species, but Demodex species are presumably transmitted from mother to offspring during the first few days of life by direct contact with the dam. Little else is known regarding the life cycle of the mite.122,123 Two species of Demodex are recognized in the horse.7,122 Demodex caballi is a normal inhabitant of the pilosebaceous apparatus of the eyelids and muzzle and may be found in skin scrapings of these areas on horses in the absence of skin lesions. Demodex equi inhabits the pilosebaceous apparatus of the remainder of the body and is the species found on horses that has been associated with disease. The species found on cattle, sheep, and goats are Demodex bovis, D. ovis, and D. caprae, respectively.29,122,123,130
Clinical signs are variable, depending on the species affected. The disease is quite rare in horses, which may develop alopecia of the head and trunk; I have seen one case of concurrent demodicosis and chorioptic mange on the legs of a draft horse. Underlying pituitary dysfunction of the pars intermedia has been seen in some horses.122 Pruritus and secondary pyoderma are variable. Goats and cattle usually develop nodular lesions that involve the face, shoulder, and neck.135,136 In goats the nodular contents are white and caseous; microscopic examination for mites differentiates the lesions from Corynebacterium pseudotuberculosis.136 Sheep tend to develop periocular nodular lesions.
Diagnosis is based on recognizing mites with microscopic examination of skin scrapings or exudates obtained from nodular lesions. The mites are elongated and have short, stubby legs.29,122,123
No treatment has proved to be consistently effective in large animals, although relatively few case reports exist in the literature. Amitraz has not been successful in the treatment of caprine demodicosis.136 Horses sprayed with 0.025% amitraz developed somnolence, depression, ataxia, muscular weakness, and progressive large intestinal impaction, suggesting that amitraz is contraindicated in equids.137 Daily ivermectin has been suggested as a successful treatment for equine demodicosis, but dosages have not been codified. Oral ivermectin (0.67 mg/kg once weekly for 12 weeks) and pour-on eprinomectin (0.5 mg/kg) each successfully treated one goat with generalized demodicosis.138
CULICOIDES HYPERSENSITIVITY
The females of various Culicoides fly species (“no-see-ums,” “punkies”) may feed either on the dorsal or the ventral surface of the horse, affecting the mane, saddle, and rump or causing a ventral midline dermatitis in a diffuse pattern. Alopecia, papules, crusts, and erythema may all be present (Fig. 40-12). The insects induce a hypersensitivity response through salivary antigens; this condition is termed Culicoides hypersensitivity (Queensland itch, sweet itch) and is recognized in various areas of the world. Evidence indicates a hereditary predisposition to develop the hypersensitivity. Histopathology of lesions frequently reflects a hypersensitivity response.139 Although many species of Culicoides exist, the two most commonly suspected of causing the allergic response are C. variipennis and C. nubeculosus.
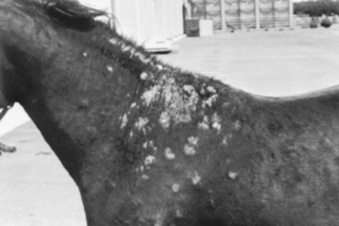
Fig 40-12 Severe Culicoides hypersensitivity in a horse, showing typical distribution of withers, shoulders, and head (not visible).
Recent studies have attempted to define the nature of the hypersensitivity response. One study found serum antibodies to Culicoides salivary glands in both healthy horses exposed to Culicoides bites and in horses with insect “dermal” hypersensitivity.140 In contrast, no antibodies were detected in serum from native Icelandic horses which had not been exposed to Culicoides. Anti—salivary gland immunoglobulin G (IgG) antibodies were detected in both groups of horses, whereas IgE antibodies were only detected in clinically affected horses.140 A more recent study compared two groups of affected Icelandic horses: those born in Iceland (no previous exposure to Culicoides) and transported to Europe, where they were then exposed to the flies, and those born in mainland Europe. The former had IgE and IgG antibodies to a greater number of the insects’ salivary proteins.141 Another study found significantly more IgE-bearing cells in the dermis and epidermis of acute and chronic lesions than in skin biopsies from healthy horses.142 Further evidence for a hypersensitivity response was demonstrated in a study in which intradermal injection of a Culicoides antigen extract induced T lymphocyte and eosinophil accumulation in the skin of affected horses.143 Other studies have supported the immunologic nature of this disease.144-148
The hallmark of disease is pruritus. Although no gender predisposition has been noted, Icelandic horses may have a higher incidence of allergic reactions to the Culicoides insects than other breeds. The disease is uncommon in horses under 1 year of age, with an onset usually between 2 and 4 years. Culicoides hypersensitivity may be seasonal, at least during the first few years of life, in temperate climates. Diagnosis is primarily by clinical signs. Culicoides antigens (commercially available in the United States by Greer, Lenoir, NC) seem useful in both diagnosis and treatment,149,150 but this needs to be documented in large case studies.
Therapy is aimed at insect control, especially the following:
VENTRAL MIDLINE DERMATITIS OF HORSES
This is a reaction pattern in horses to ectoparasites, and not necessarily specific for any one arthropod. Culicoides hypersensitivity, Onchocerca cervicalis infestation, and black flies (Simuliidae) may cause generalized ventral midline dermatitis (VMD), whereas horn flies (Haematobia irritans) cause a focal dermatitis. Severe chorioptic mange may also involve the ventral midline. In all cases, the lesions have variable alopecia, pruritus, and crusts. Treatment is based on eliminating or repelling the causative organism.
OTHER FLYING INSECTS
Stable flies (Stomoxys calcitrans), black flies (Simuliidae), horn flies (Haematobia irritans), horse flies (Tabanus and Hybomitra species), deer flies (Chrysops species), house flies (Musca domestica), face flies (Musca autumnalis), and mosquitos are commonly associated with irritant and allergic skin disease, as well as being vectors of many parasites. They must be controlled by limiting breeding areas and by repellents; 2% permethrins are often used. It is important to remember the relationship of these various flies to the environments they favor or require for breeding and when they feed, as follows:
| Horse flies, deer flies | Vegetation and water | Daytime feeders |
| Horn flies | Cattle | Daytime feeders |
| Stable flies | Manure/decaying bedding | Daytime feeders |
| Black flies | Running water | Morning and evening |
| Culicoides | Standing water, manure/decaying vegetation | Twilight to dawn |
| Mosquitoes | Water | Dusk to 2 hours after sunset |
SCREWWORM INFESTATION
Screwworm flies cause primary myiasis; the species of importance in the Americas is Cochliomyia hominivorax (Callitroga americana). The fly was formerly found throughout the American tropics and subtropics from the southern United States to northern Chile.151,152 It is no longer found in the United States or Mexico as a result of state, federal, and international eradication efforts.153
The adult fly is about three times the size of a house fly, with a metallic bluish or blue-green color.151,153 Females are attracted to fresh wounds (castration, dehorning, branding and shearing sites), abraded body orifices, areas soiled by discharges or excretions, and navels of newborns. They lay batches of 150 to 500 white eggs on the margin of damaged tissue in rows that overlap like shingles. Eggs hatch within 24 hours, and the larvae begin to feed in a head-downward position. Larvae are obligate parasites that require living tissue as feedstuff. They cannot develop in carrion. Larval development continues for 4 to 10 days, and at the time of their maturation, they may have created a cavity 10 to 12 cm (4 to 5 inches) in diameter.152 Mature larvae are about 2 cm in length, pink in color, pointed anteriorly, and blunt posteriorly. The larvae then drop to the soil and pupate. The life cycle averages about 21 days and is favored by hot, humid weather.
Screwworms do not have a dormant stage in their life cycle and cannot overwinter in cold climates.151 Thus the susceptible stage of development is the pupae, which will not survive soil temperatures below 15° C (59° F). The feeding larvae burrow deeply, creating a cavernous lesion characterized by liquefaction necrosis, profuse brownish exudate, and an objectionable odor. The syndrome is self-perpetuating in that wounds infested by screwworm larvae become increasingly attractive to gravid females. The end result is often death of the host as a result of secondary bacterial infection, toxemia, and fluid loss.152
Treatment of wounds requires clipping and cleansing, as well as destroying all larvae. Dressings containing larvicides and antiseptics should be applied. Preparations containing lindane or organophosphates have been used in an ointment or gel base. The treatment is repeated twice weekly. When large numbers of animals are affected, a 0.25% solution of coumaphos, chlorfenvinphos, or fenchlorphos may be applied to herds with a power sprayer. Calves may become ill from the spray; thus applications should be restricted to the ventral abdomen.152 More recently, the curative efficacy of fipronil 1% against C. hominivorax larvae infestation in castration wounds was 100%.154 Even more impressive, doramectin as a prophylactic treatment was 100% effective in prevention of C. hominivorax infestations.155
Control of screwworm flies in the United States has been achieved largely through the release of sterile males, because of the single mating tendency of the female fly. The eradication program has proceeded through Mexico south to the twenty-first-degree parallel.151 Screwworm myiasis is a reportable disease in the United States, and suspect larvae should be preserved in 70% alcohol for positive identification.156
BLOW FLY STRIKE (FLEECEWORMS, WOOLMAGGOTS, SECONDARY SCREWWORMS)
Blow flies are found throughout the Western Hemisphere. The species of importance include Cochliomyia macellaria (secondary screwworm), Phaenicia sericata (green bottle fly), and Phormia regina (black blow fly). The flies tend to be most common in the warmer regions, with the exception of P. regina, which is widespread throughout the cooler parts of North America and Europe.151 Blow flies cause serious loss of sheep and wool in many countries.153 Female flies are attracted to decaying animal matter, such as wounds infested by primary screwworms, infected sores, carcasses, and fleeces that are dampened with feces, urine, or bloody fluids.151 They lay eggs in batches of up to 300, which hatch within hours. The larvae feed on necrotic tissue but may invade healthy tissue. Larvae develop for 3 to 5 days and, when fully mature, are white and 6 to 12 mm in length. The larvae then drop to the ground for pupation. As soil temperatures fall, larvae fail to pupate and may overwinter until the following spring. The entire life cycle is generally complete in 2 to 4 weeks under ideal conditions of warm temperatures and high humidity.152
Unlike primary screwworms that feed in pocket-like aggregations, secondary screwworms tend to be dispersed throughout the infested tissue. In wool infestation the larvae may remain on the skin surface, feeding on the decomposing wool, or may penetrate the skin through small abrasions. The most common site of involvement is the breech. Infested tissue attracts more ovipositing females, and thus the syndrome is perpetuated.151 Affected sheep are restless and do not feed. They move with their heads close to the ground, bite or kick at their wounds, and continually wriggle their tails. Affected wool is moist and brown with an obvious odor. Animals may become systemically ill and die.156
Treatment of individual wounds is as described for screwworms. Control involves management practices that decrease the incidence of wounds or skin irritations. Sheep are often clipped below the tail and between the hindlimbs (“crutched”), where wool is likely to become saturated with urine or feces. Castration, shearing, docking, and lambing are avoided during the summer season.151
CUTANEOUS ONCHOCERCIASIS
Definition and Etiology
Cutaneous onchocerciasis in horses is a common filarial dermatitis with a worldwide distribution. Its incidence in the United States has decreased dramatically since the introduction of ivermectin for routine deworming. In a recent report from South America, Onchocerca cervicalis microfilariae were detected in midventral skin biopsy samples in 215 (17.9%) of 1200 horses examined, and the adult worms were recovered from 200 (16.6%) ligamentum nuchae from the same animals.157
The disease is caused by the microfilaria of O. cervicalis and is seen primarily in adult horses.158,159 Adults typically are found coiled in the funicular part of the ligamentum nuchae, where they produce calcified nodules. Viviparous females may live for up to 5 years and produce large numbers of microfilariae that migrate through connective tissues to the superficial layers of the dermis. Preferential areas of microfilarial localization include the ventral midline, lower eyelid, and lateral limbus of the eye.158 The infection is transmitted by Culicoides, which act as an intermediate host. The larvae are ingested by the vector and undergo development into the third-stage larvae (L3) in approximately 2 weeks. The L3 larvae enter the animal host through lesions created by the feeding vector.33 Many horses are infected with the parasite without demonstrating clinical disease.33,158
Pathophysiology
Pathogenesis is believed to involve a hypersensitivity reaction to antigens released by dying microfilariae.33,158 This theory has support because not all infected horses demonstrate disease, neither the presence nor the severity of the dermatitis is correlated with the number of organisms present, and treatment with filaricides often causes a temporary exacerbation of clinical signs.
Clinical Signs
Clinical signs occur most often in older horses and can include both ocular and cutaneous lesions.33,158 Ocular lesions include uveitis, conjunctivitis, keratitis, and depigmentation of the lateral limbus.160 Cutaneous lesions include diffuse or patchy alopecia, erythema, and scaling. Focal cutaneous depigmentation is common. Lesions tend to occur in regions where microfilariae are typically present in highest concentrations, such as the ventral midline, face (Fig. 40-13), base of the mane, anteromedial proximal forelimbs, and anterior pectoral region. An inflammatory, alopecic, or hyperpigmented area in the center of the forehead is highly suggestive of the disorder.33 The dermatitis is generally reported as being nonseasonal and nonpruritic.33,161
Diagnosis and Histopathology
Because many normal horses have microfilariae without disease, the finding of microfilariae does not prove that they are the cause of the cutaneous lesions. In addition, because microfilariae tend to “nest” in the dermis, there is a tremendous difference in the number of microfilariae recovered from adjacent skin samples.158,162 Thus, although the absence of microfilariae makes cutaneous onchocerciasis unlikely, it does not definitively exclude it as a diagnosis. Differential diagnoses should include ventral midline dermatitis caused by the horn fly Haematobia irritans, hypersensitivity reaction to Culicoides, dermatophytosis, and infestation with mange mites.
Ultimately, diagnosis should be based on history, supportive clinical findings, exclusion of other differential diagnoses, and most importantly, response to treatment.
A positive Onchocerca microfilarial saline preparation demonstrates slender, delicate microfilariae that are approximately 8 × 220 μm. Typical histopathologic changes include an eosinophilic and lymphocytic perivascular dermatitis, a finding that is nonspecific and common to many other equine parasitic dermatoses (see Chapter 11). Aggregations of microfilariae may be found in the superficial dermis or perifollicular region.33,158,162
Therapy
Ivermectin or moxidectin are the treatments of choice and are administered at 0.2 mg/kg PO.163,164 Most horses improve within 2 to 3 weeks. Minor adverse reactions, including fever and swelling of the periorbital, facial, and ventral midline regions, may occur in up to 25% of infected horses treated with ivermectin; moxidectin did not cause posttreatment dermal reactions in one study.164 Severe reactions may benefit from treatment with corticosteroids, but most reactions resolve within 24 to 72 hours. Because there is no effective adulticide, recurrence may be noted as soon as 2 months after therapy. Most animals remain free of clinical signs for 6 to 12 months.161,163 Re-treatment is recommended at 4-month intervals.
In cattle, onchocerciasis has been caused by the microfilariae of Onchocerca gutturosa and Onchocerca lienalis and in Africa, Onchocerca ochengi.165,166 Flies of Simuliidae are the vectors. The disease causes nodules and crust on the skin, including the teats; adult worms surrounded by neutrophils were detected free in the teat canal.166 Ivermectin and moxidectin are effective treatments.165
STEPHANOFILARIASIS
Cutaneous stephanofilariasis is a filarial dermatitis of cattle. The disease is caused by organisms of the genus Stephanofilaria and has been observed in cattle in many countries.167,168 Within the United States the disease is most prevalent in the western and southwestern regions, where it is caused by Stephanofilaria stilesi.156,159,169 Adults and microfilariae inhabit the epidermis. The parasite is transmitted by the female horn fly (Haematobia irritans) when it feeds on lesions on the ventral midline.159 The microfilariae are ingested and develop to infective L3 larvae in 2 to 3 weeks. The L3 stage is introduced into the animal host on subsequent feedings.169
Clinical signs of cutaneous stephanofilariasis include a ventral midline dermatitis initially associated with a papular eruption. The scrotum may also be affected.170 The lesions progress to nodules, alopecia, and crusted ulcers. There may be mild pruritus.152 Diagnosis is based on history and appropriate clinical signs, excluding differential diagnosis and demonstration of the nematode. Biopsies may be taken as described for equine onchocerciasis, or the crust can be removed from an acute lesion and deep skin scrapings performed to recover the parasite. The tissues should be minced in isotonic saline and then examined microscopically. Multiple skin scrapings may be required to demonstrate the parasite. Either adults or microfilariae may be found. The adult male is about 3 mm in length and the adult female 6 mm in length.167 Microfilariae are 50 μm in length.167 Characteristic histopathologic findings include a perivascular dermatitis associated with an eosinophilia. Microfilariae may be noted in the dermis, and adults in epithelial-lined cysts at the base of the hair follicles, although both may be difficult to find.167
There is no approved treatment for stephanofilariasis, although approved topical organophosphates have been suggested.159 Some authors have not recommended treatment because clinical signs are relatively mild in many animals.169 Cattle with zebu ancestry may be more resistant.168
HYPODERMA (WARBLES)
Definition and Etiology
Infestation with the larvae of Hypoderma species is a common and serious economic problem in cattle and is recognized sporadically in horses that are pastured near cattle. Occurrences have also been reported in sheep, goats, and humans.171 Warble flies are present in the Northern Hemisphere between 25 and 60 degrees latitude in more than 50 countries of North America, Europe, Africa, and Asia. Hypoderma species are not established south of the equator.152,171 The U.S. cattle industry loses millions of dollars annually because of cattle grubs.
Two species of Hypoderma parasitize cattle: H. bovis and H. lineatum. The latter prefers warmer climates and is the only Hypoderma species present in the southern United States. Each of the species occurs in the northern United States and in Canada.152 H. lineatum and H. bovis have very similar life cycles, but the stages of H. lineatum tend to occur 3 to 8 weeks earlier than those of H. bovis. Timing of the life cycle also varies with local geographic and climatic factors.152,171
The adult flies are beelike in appearance, covered with dense chin bristles (hair), 12 to 188 mm in length, and have nonfunctional mouthparts.172 They have a short lifespan, only 1 week. Adult flies are active in the spring to early summer, with H. lineatum appearing 3 to 4 weeks before H. bovis. Female H. lineatum flies deposit eggs on the hairs of the legs or lower body, whereas H. bovis tends to deposit eggs on the rump or upper parts of the hindlimbs. Total egg production by a single female has been estimated to range from 500 to 800.172 The eggs hatch in 3 to 7 days, after which the larvae penetrate the skin and begin their migration through the connective tissues. H. lineatum larvae migrate to the subcutaneous connective tissues of the esophageal wall, whereas H. bovis larvae migrate toward the spinal canal. The first-stage (L1) larvae remain in this location for 2 to 4 months, during autumn and early winter. Between January and February the larvae begin a final migration through the connective tissues to the subdermal tissue of the back of the host, where they form a breathing hole through the skin. Cysts (warbles) develop around L1 larvae. Within the cyst the larvae undergo two molts over a 4- to 6-week period. The L3 larvae (grub) emerge from the breathing pore, fall to the ground, and pupate. Adult flies emerge from the pupae in 1 to 3 months, and emergent adults are ready to mate almost immediately.172,156 The life cycle is complete in 1 year. Most larvae fail to reach normal size and complete their life cycle in the horse.171,172
Clinical signs
CATTLE
During the spring and summer when adult flies are active, “fly worry” may be a serious problem. Cattle exhibit a stampeding behavior called “gadding” when chased by ovipositing females, even though the flies do not bite or sting. This fear reaction results in self-injury and decreased feeding and milk production.172 Diagnosis of warble flies in goats by ELISA has been reported, but it is not known whether this is applicable to cattle.173
Clinical lesions are not usually observed in association with migration of L1 larvae unless the larvae die along the migratory path.171 Lesions that have been associated with infestation of L1 larvae of H. bovis include fat necrosis and inflammation of the connective tissues surrounding the spinal canal. Secondary periostitis, osteomyelitis, and rarely paralysis or other nervous disorders may occur. Similarly, infestation with L1 larvae of H. lineatum in the submucosa of the esophagus may cause inflammation and edema in the surrounding tissues. Swallowing and eructation may be hindered, resulting in bloat and subsequent respiratory failure.171
Lesions most often observed with Hypoderma infestation are attributed to L3 larvae. Warbles occur along the back from the shoulders to the tailhead and from the dorsal midline to a point about one-third the distance down the sides. The lesions may be firm to fluctuant, raised, and painful to the touch. They measure approximately 3 cm in diameter and contain a breathing pore that usually exudes a yellowish serum. Excisional biopsy of a nodular lesion reveals the larvae within a cystlike structure that contains yellow fluid. The surrounding tissue is necrotic. Secondary infection of the cysts can result in large, suppurating abscesses. The number of warbles in an infested animal may range from 1 to 300. Infestations are most serious in younger animals and become progressively less severe with age. Emergence of the grub results in healing, although carcasses retain evidence of infestation and are devalued at slaughter.152,171
HORSES
Most horses have only one or two grubs, but heavy infestation is present in rare cases. Lesions associated with L3 larvae include small, nodular swellings that develop dorsally and frequently in the region of the withers. Most lesions have a breathing pore.156 Differential diagnosis is most often nodular collagenolytic granuloma (nodular necrobiosis), but mastocytoma, sterile nodular panniculitis, and amyloidosis should also be considered. Posterior paralysis associated with involvement of the spinal cord has been reported in both horses and cattle.171
The season, location of the lesion(s), and presence of a breathing pore are usually diagnostic. Larvae can be recovered by enlarging the breathing pore with a scalpel and extracting the grub.
Therapy
When small numbers of cattle are affected with just a few grubs, manual removal of the grubs is possible by simply enlarging the breathing pore. This is usually the preferred treatment in horses as well, if only one or two lesions are present. Care must be taken to remove the larvae in their entirety because breaking the larvae and rupturing the cyst during removal can result in a severe systemic reaction.152,171
Systemic insecticides are the only means of eliminating the migrating larvae. Organophosphates or the macrocyclic lactones (doramectin, eprinomectin, ivermectin, moxidectin) have been used for this purpose and are applied on the midline of the back.171,174 Administration of insecticides should be timed to provide treatment in early autumn after all eggs have hatched and larvae are in the early stages of their connective tissue migration. Third-stage larvae are less susceptible to insecticides, and destruction of larvae in later stages of migration increases the risk of serious secondary illness. It is advisable not to administer systemic insecticides later than 8 to 12 weeks before the anticipated first appearance of grubs along the back.152,171 In the northern United States and Canada, treatment of cattle with systemic insecticides is not recommended from October 1 to March 1 because this is when H. bovis larvae are located in the epidural space and H. lineatum larvae reside in the esophagus.152