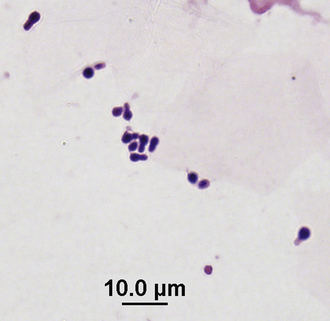
Clinical Microbiology
When you have completed this chapter, you will be able to:
1 Describe methods for collection and handling of samples for microbiology testing.
2 List the steps of the Gram stain procedure.
3 Describe the procedure for inoculation of agar plates, broth, and slant tube media and explain proper incubation methods for bacterial cultures.
4 List types of culture media commonly used for primary isolation of microorganisms and explain the specific use of each.
5 List and describe the characteristics of bacterial colonies that are evaluated on primary isolates.
6 Describe common staining procedures and biochemical tests used for identification of bacteria.
7 Describe the procedures for performing blood and urine cultures.
8 List common bacterial species encountered in small and large animal practices.
9 Describe the procedure for performing antimicrobial susceptibility testing.
10 List the common fungal organisms encountered in veterinary practice and describe the procedures for sample collection and identification of these organisms.
11 Describe the general principles of serologic analysis for bacterial and viral antigens.
12 Define nosocomial infection and explain methods for control of nosocomial infections.
DIAGNOSTIC METHODS
The choice of methods for examining a specimen in the microbiology laboratory depends on the type of specimen and the pathogen sought. Traditionally, microbiologists have attempted to isolate agents in various types of culture systems and then use various identification schemes to characterize them. This is still the most frequently used method in bacteriology. However, there are times when the organism may be difficult to cultivate or may not be viable in the specimen presented to the laboratory. In these cases, demonstration of specific microbial antigens or nucleic acid in the specimen may be more rapid and cost effective. Immunohistochemical assays that have been introduced into veterinary diagnostics include enzyme immunoassays, latex particle agglutination, and protein A coagglutination procedures. In some diseases, such as botulism and mycotoxicoses, establishing the presence of a microbial toxin is necessary, rather than identifying the organism that produces it. Sometimes, a specific immunologic response by the patient to an infectious agent can establish the diagnosis. Serum can be tested for the presence of specific antibodies, or skin tests can be performed. Another diagnostic method is direct examination of exudates and tissue biopsy specimens. Some microorganisms present such unique morphologic characteristics, host inflammatory responses, and lesions that a preliminary diagnosis can be established without the need for further laboratory testing.
Recent developments in biotechnology are providing new methods for direct detection of infectious agents. Direct nucleic acid hybridization probe and gene amplification protocols have tremendous potential for detecting microbial pathogens. These procedures are highly specific and can be extremely sensitive. Because they detect the genes (or portions of genes) of organisms and can differentiate closely related organisms based on the presence of a unique genetic sequence, the identified strains are frequently described as genotypes. DNA probe assays are particularly well suited for in situ hybridization in tissue in which the location and distribution of the organisms must be determined, identification of slow-growing or difficult-to-isolate organisms, and for identification of toxicogenic strains of bacteria that cannot be differentiated from nontoxicogenic strains through the use of conventional methods. Nucleic acid amplification assays use primers and polymerase chain reaction (PCR) to provide specificity and sensitivity to detect as few as one organism or 1 to 10 copies of the specific gene sequence. Because of this exquisite sensitivity, specimen collection and handling procedures are critical. Cross-contamination of samples with as little as a single copy of a microbial gene carried on gloves, laboratory bench tops, or aerosolized droplets may result in false-positive test results.
Ultimately, the goal of these molecular techniques is the direct determination of identities and antimicrobial susceptibility patterns of microorganisms in clinical specimens. As the technology for nucleic acid amplification currently stands, application of the procedure is limited to large referral laboratories and research laboratories. Partial or full automation and improved technology will begin to reduce costs and increase access to these assays. Despite their sensitivity, molecular detection procedures will not totally replace conventional culture and serologic procedures because the results of nucleic acid amplification procedures and the results of culture or serology mean different things. Nucleic acid amplification procedures are used to determine whether DNA or RNA from a particular organism is present in the specimen; they reveal nothing about the viability of the organism (because they can detect DNA from dead organisms) or whether the organism is involved in an infectious process. Culture, on the other hand, clearly demonstrates the viability of the organism, whereas a rise in titer of antibody to a specific organism strongly suggests infection.
COLLECTION OF SPECIMENS
A tremendous diversity of microbial agents, specimen sources, and samples are routinely considered in the microbiology laboratory. Specimen selection, collection, and transport requirements may also vary significantly, depending on the agent to be detected and the assay to be performed. Therefore it is important that technicians be alert to the potential of receiving and implementing specific instructions about specimen collection and handling for each patient rather than anticipating generic procedures.
PROPER SPECIMEN COLLECTION
The goal of specimen collection is to obtain a sample from the patient that is representative of the disease process. Therefore the culture specimen must be from the actual infection site (Figure 18-1). It must be collected with a minimum of contamination from adjacent tissues or secretions. Material swabbed from superficial body surfaces (skin or mucous membranes) will usually yield a mixed growth of bacteria, often making it difficult to identify a significant pathogen. Culture specimens recovered from body orifices and draining tracts are frequently contaminated with normal flora. The most useful specimens are those aspirated from normally sterile, closed body compartments after the surface has been aseptically prepared.
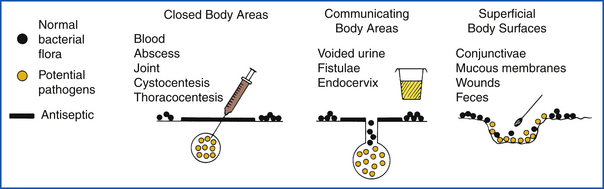
FIGURE 18-1 Methods used to collect bacterial culture specimens and probable sources of contamination.
Optimal times and sites for specimen collection must be observed. Infections by some viruses and mycoplasmas are acute processes that are followed by secondary invasion by opportunistic bacteria; therefore sampling must be performed early in the course of a disease. When viruses and bacteria localize in specific tissues, collection should target such sites. Specimens obtained at necropsy for culture should be collected as soon as possible after the death of the animal (see Chapter 39).
Whenever possible, culture specimens should be obtained before the administration of antimicrobials, especially if the suspected pathogen may be susceptible to the antimicrobial or the antimicrobial may be concentrated at the site of infection. However, the administration of antimicrobials does not necessarily preclude the usefulness of cultures. The antimicrobial drug may be diluted to an ineffective level in culture medium, thereby allowing the pathogen to grow. Antimicrobial-resistant or superinfecting bacteria may still be recovered. In addition, the effectiveness of therapy can be evaluated by determining the relative numbers of bacteria present.
An adequate quantity of material should be obtained for complete examination. Aliquots of body fluids (>1 ml), exudates, or pieces of tissue (>3 cm3) are always more useful than a swab. Smears can be prepared for direct examination, and multiple culture media can be inoculated when adequate material is submitted. Quantitative results can also be obtained if needed.
Appropriate collection devices and specimen handling must be used to ensure optimal survival and recovery of significant microorganisms (Figure 18-2). Sterile swabs are acceptable for transferring most samples from the patient to culture media. If the culture medium is not immediately inoculated, the swab must be placed in a swab transport system (CultureSwab, BD Diagnostic Systems; Copan Transport Swabs, Copan Diagnostics, Inc.) or into a transport medium. Transport media are designed to maintain optimal conditions for survival of the suspected pathogen without allowing overgrowth by contaminating saprophytes. Semisolid transport media, such as Amies transport medium with charcoal for aerobic bacteria (growth in the presence of oxygen) or the Port-A-Cul Anaerobic Transport System (BD Diagnostic Systems) for anaerobic bacteria (requires absence of oxygen), can preserve specimens on swabs for several days. Swabs should not be placed in nutritive broths before inoculation of isolation media because an insignificant nonpathogen may overgrow and prevent recovery of the pathogen. Specimens can be collected in various sterile containers that do not contain preservatives or anticoagulants for transport. If tissues are collected for culture, each piece must be packaged separately in a leak-proof, sterile container.
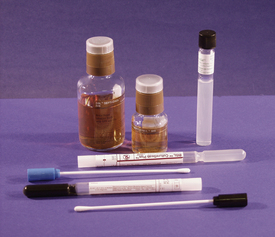
FIGURE 18-2 Culturette swab transport systems with transport media (black medium is Amies transport medium with charcoal), Port-A-Cul Anaerobic Transport Tube (BD Diagnostic Systems), and blood culture bottles. Swabs are used to collect culture inoculum and placed into transport systems or tube of medium for preservation of the viability of bacteria during transportation to the laboratory for culture. Blood culture bottles are inoculated with blood to prevent coagulation and contamination during transport to the laboratory for incubation.
Each culture specimen container must be properly labeled. Identification of the patient by name, species, case number, or owner, as appropriate, should be legibly indicated. If more than one veterinarian works in the practice, the one in charge of the case should be identified so that questions about history and preliminary reports can be communicated efficiently. The source of the specimen should also be included on the label. As discussed later, the source of the specimen will be a significant factor in deciding how to set up the culture, which bacteria to identify, and how to interpret the results. If the culture specimen is to be sent to a referral laboratory, additional clinical history should be included. Results of previous culture attempts, other laboratory tests, and antimicrobial treatments should be reported, as well as the major clinical manifestations and duration of illness, so that laboratory personnel will be able to recognize and identify significant findings.
SPECIAL COLLECTION AND HANDLING PROCEDURES
Some groups of microorganisms require special collection and handling for optimal isolation. Anaerobic bacteria must be kept away from oxygen. Often, a sterile syringe with a fine-gauge needle (22- to 23-gauge) is the best collection device for aspirating exudates from an infected site. The specimen can be transported to the laboratory in the syringe: if air is expressed, the needle is removed to prevent injuries, and the syringe is capped to prevent leakage. Otherwise, the specimen should be transferred to an appropriate anaerobic transport device. Survival and subsequent isolation of anaerobes are enhanced by keeping them in the reduced microenvironment in which they are found. Therefore, as stated previously, exudate and pieces of tissue are better specimens than swabs. If a swab is collected, it must be placed in an appropriate anaerobic transport device. Handling a specimen as if it contains anaerobes will not jeopardize the viability of aerobic bacteria. Exudates, biopsy material, and tissue should be submitted as quickly as possible to the microbiology laboratory.
For attempts to isolate fungi and mycobacteria, swabs are usually not the best specimens. These agents tend to cause chronic infections, often with small numbers of organisms present. Too few organisms may be present on a swab, or in the case of mycobacteria, they may adhere to the swab, and culture results will be negative.
The more fastidious groups of microorganisms (e.g., Mycoplasma, Chlamydia, and Rickettsia and viruses) require special selective transport media. These media are usually formulated to contain antimicrobials that will inhibit the growth of other microorganisms while preserving the viability of the desired agent. Specific transport media and instructions for proper use should be obtained from a referral laboratory that is capable of providing the desired culture service.
PROCESSING SPECIMENS
Processing of specimens should be performed in a dedicated laboratory work area to reduce the risk of transmission of infectious agents to patients and staff. Laboratory work should not be performed in areas such as hallways where personnel could accidently become contaminated while walking by. Care also must be taken to avoid cross-contamination of specimens by aerosol or transfer of agents on the laboratory bench surface. Unnecessary traffic and excessive air currents can easily contribute to the creation of aerosols, cross-contamination, and environmental contamination of culture media, thus resulting in spurious culture results.
Each specimen received in the microbiology laboratory should be carefully and individually evaluated, with consideration given to anatomic source and condition of the specimen, animal family of the patient, clinical history, and special requests from the veterinarian. Each pathogen has a preferred habitat in which it will grow and specific mechanisms for causing disease. Therefore, for a particular manifestation of disease, there will be a limited number of agents that should be considered as likely pathogens. Table 18-1 lists the most common bacterial species associated with infections of various sites in animals. If the technician can focus the search for pathogens on these most likely agents, results will often be obtained much more rapidly and with less expense.
CONDITION OF THE SPECIMEN
If there is evidence that the specimen has become grossly contaminated or dried out, if it is of insufficient quantity, if there has been excessive delay in receipt, or if any other evidence of mishandling is present, an attempt should be made to obtain a second sample. Specimens should be processed the same day they are collected, or they should be kept refrigerated if a delay is anticipated.
DIRECT MICROSCOPIC EXAMINATION
Direct microscopic examination of exudates, impression smears from tissues, or infected body fluids is the most important laboratory procedure that can be used for microbiologic diagnosis. It provides immediate information on the types and numbers of microorganisms present and the type of host cellular inflammatory response. The likelihood of infection can be determined, as can the probable type of agent (i.e., virus, bacterium, or fungus), which in turn determines the nature of the diagnostic assays needed. The most likely pathogen (or predominant organism) may tentatively be identified. This information may be used to provide guidance in selection of optimal culture conditions and as the basis for the interpretation of the significance of subsequent culture results. In some cases it may be all the information the veterinarian needs.
In many situations, application of Gram stain is the procedure of choice because it allows differentiation of gram-positive and gram-negative bacteria. However, some bacteria do not stain well with Gram stain. Gram-negative bacteria may not be well differentiated from the background in exudates and tissue impression smears.
Other tissue stains (i.e., Giemsa and Wright stains or methylene blue wet mounts) may be more useful for detecting all microorganisms present in the smear. Although these stains are more efficient in demonstrating the presence and morphology of bacteria, they do not provide differentiation of gram-positive and gram-negative bacteria. Careful direct examination may be sufficient for diagnosis without cultures, or it can narrow the diagnostic likelihood to a few bacterial species. This information helps in the selection of optimal culture conditions for identification of suspected pathogens.
Gram Stain Procedure and Interpretation
The technique for preparing a gram-stained slide is as follows:
1. Prepare a thin smear from tissue exudates or bacterial suspension on a clean slide and allow smear to air dry.
2. Fix material to the slide so that it does not wash off during the staining procedure by passing the slide, right side up, through a flame three or four times.
3. Flood smear with crystal violet solution, and let stand for 1 minute.
4. Wash smear briefly with tap water.
5. Flood smear with Gram iodine solution, and let stand for 1 minute.
6. Wash with tap water, and decolorize until solvent flows colorlessly from the slide. This usually requires 5 to 10 seconds.
7. Wash briefly with tap water, and flood the slide with safranin counterstain for 30 to 60 seconds.
8. Wash briefly with tap water, blot and air dry, and examine.
The stained smear is best examined by using the 100× (oil immersion) objective of the microscope. Gram-positive bacteria retain the crystal violet iodine complex and appear dark blue or purple. Gram-negative bacteria lose the primary complex, take up the secondary dye safranin, and appear red. Fungi (yeasts) appear gram-positive. Inflammatory cells appear gram-negative, and epithelial cells may appear gram-positive or gram-negative, depending on the thickness of the smear. Backgrounds usually appear gram-negative but may appear gram-positive if they are thick and inadequately washed. Fibrin, mucus, and erythrocytes often stain gram-negative and may mask detection of gram-negative bacteria.
BACTERIAL ISOLATION AND IDENTIFICATION PROCEDURES
The equipment and supplies required for the performance of basic diagnostic bacteriology tests depend on the scope of services to be provided. Some of the more common items are as follows: binocular microscope, incubator, anaerobic culture system, staining reagents or kits, specimen collection devices, swabs, transport media, isolation and identification media (Table 18-2), packaged identification systems (Boxes 18-1 and 18-2), and miscellaneous instruments, supplies, and appropriate reagents for the diagnostic procedures to be performed.
TABLE 18-2
Bacteriologic Plate and Tube Media for the Practitioner’s Laboratory
| Purpose and Inoculation | Reactions and Interpretations |
| Blood Agar Plate (Trypticase Soy Agar With 5% Sheep Blood) | |
| Primary isolation medium for all specimens in which pathogenic bacteria are suspected. Always streak for colony isolation. | Observe growth rates, colony morphologic characteristics, hemolysis. Test selected colonies for Gram reaction, catalase, and oxidase. Inoculate differential tests and antimicrobial susceptibility tests from well-isolated colonies. |
| MacConkey Agar | |
| A primary isolation and differential plating medium for selection and recovery of Enterobacteriaceae and related gram-negative bacteria. Inoculate by streaking for colony isolation. | Growth is usually gram-negative. Pink to red colonies (with increased redness of the medium) are lactose fermenters (e.g., species of Escherichia, Klebsiella, and Enterobacter). Colorless colonies (often with a slight change of the medium to yellow) are nonlactose fermenters. |
| Hektoen Enteric Agar | |
| A direct plating medium for fecal specimens that is highly selective for Salmonella. Inoculate by streaking for colony isolation. | Disaccharide fermenters are moderately inhibited and produce bright orange to yellow to salmon to pink colonies. Salmonella colonies are blue-green, typically with back centers from hydrogen sulfide. Proteus colonies may resemble Salmonella. |
| Selenite Broth or Tetrathionate Broth | |
| Enrichment broth for the selective enhancement of growth by Salmonella from specimens containing heavy concentrations of mixed bacteria, such as feces. Inoculate relatively heavily, and incubate 18-24 hr. | Subculture to MacConkey agar and Hektoen enteric agar for isolation of Salmonella. |
| Triple Sugar Iron (TSI) Agar Slant | |
| A differential medium for detection of carbohydrate (glucose, lactose, sucrose) fermentation and production of hydrogen sulfide. Inoculate by the butt once with an inoculating needle and by streaking the slant. Incubate with a loose cap. | Yellow color change indicates acidification caused by carbohydrate fermentation. In the butt, glucose stabbing fermentation is detected; in the slant, lactose and sucrose fermentation is detected (includes glucose fermentation as an intermediate product). Red color change indicates alkalinization caused by lack of carbohydrate fermentation. Black color indicates hydrogen sulfide production. Results are recorded as slant/butt; A = acid (yellow), K = alkaline (red), or NC = no change. |
| Christensen’s Urea Agar Slant | |
| A differential medium for detection of urease production by an organism. Inoculate by streaking heavily over the slant. | Urease-positive bacteria produce a pink-red color change in the slant and sometimes throughout the butt. Urease-negative bacteria allow the medium to remain the original yellow color. |
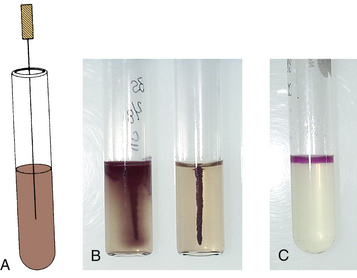
| Motility Media∗ | |
| A test medium for determining if an organism is motile or nonmotile. Inoculate by stabbing the center of the tube with an inoculating needle. Incubate at 35° C for most organisms; incubate at room temperature if Listeria is suspected. | Motile organisms migrate from the stab line, flaring out to cause turbidity in the medium. Nonmotile organisms grow only along the stab line; the surrounding medium remains clear. |
| Indole Test Media∗ | |
| A test medium for detecting the ability of bacteria to produce indole as one of the degradation products of tryptophan metabolism. Inoculate, incubate 24-48 hr, then add Kovac’s reagent to detect indole. | Development of a red color at the interface of the reagent and the broth within seconds after adding the reagent indicates a positive test result. |
∗Combination media that provide for several tests in the same tube, such as SIM (sulfide-indole-motility), MIO (motility indole ornithine), or MIL (motility indole lysine), can be purchased.
The most expensive item is a good-quality binocular light microscope with a 100× oil immersion objective. Dark-field and phase-contrast options are useful but not essential. Small countertop incubators are available. Important characteristics of a quality incubator include (1) insulated walls to maintain a constant temperature; (2) an adequate seal to maintain a humid atmosphere; (3) a capacity for plates, tubes, and candle jars; (4) a thermometer to check the temperature, which should not fluctuate more than ±2o C; and (5) an adjustable, thermostatically controlled heating element.
CULTURE MEDIA
Several formulations of media are needed in the bacteriology laboratory for isolation of various microbial agents and for identification of these microorganisms. Both dehydrated and prepared media are readily available today. It is usually much more convenient for small laboratories to purchase prepared media than to prepare their own. In addition, the quality of purchased media will be much more consistent, and these media will usually be quality tested before they are distributed. There are numerous distributors of prepared media throughout the United States. A few national and regional distributors are listed in Box 18-2. Names and addresses of other suppliers can be obtained from local hospitals and by searching the World Wide Web. Some microbiology supply distributors have a full line of prepared plates and tubes of media available, and ancillary biochemical reagents, stains, and miscellaneous supplies.
Purpose of Specific Media
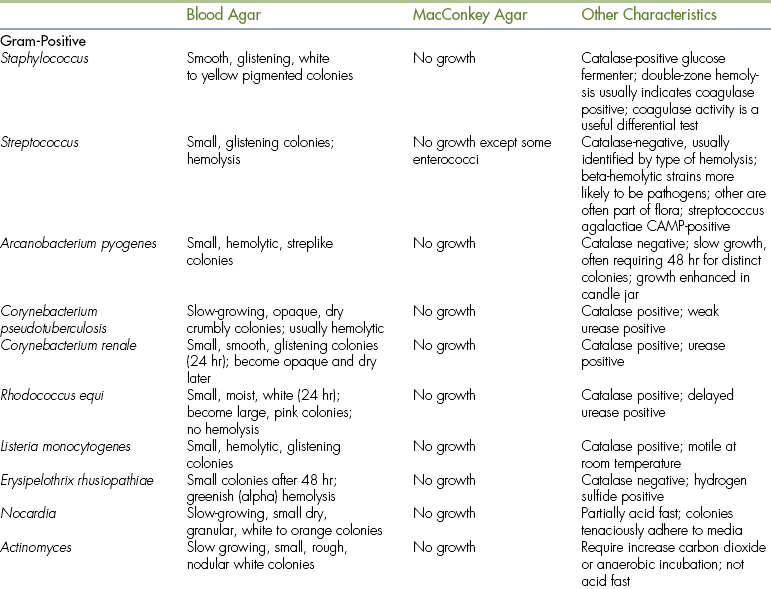
Solid media in plates are used for primary isolation of bacteria from clinical specimens. This type of medium allows distribution of the specimen in such a way that isolated colonies develop, each representing a single bacterial cell. Some primary isolation media contain inhibitory ingredients that allow them to be selective for specific groups of bacteria. MacConkey agar is selective for bacteria that can grow in the presence of bile salts, which is similar to the environment found in the intestines. A differential medium contains an indicator system that can distinguish different bacteria, even though both types may grow. The lactose-fermenting ability of bacteria on MacConkey agar is a differential reaction. Table 18-2 lists some of the more commonly used culture media, the indicated use of the media, and selective and differential characteristics.
Inoculation of Media
Before media are inoculated, each tube or plate should be labeled with a distinct identification and the date of inoculation. Plates should be labeled on the bottom with a waterproof marker. The swabs used for collection of most clinical specimens can be used for direct inoculation of primary isolation media. In the laboratory, a sterile swab can also be used to transfer inoculum material from liquid and tissue specimens to isolation media. The same swab can be used for inoculation of several media if the least inhibitory medium is inoculated first and the most inhibitory medium is inoculated last; for example, blood agar can be inoculated first, and then MacConkey agar can be inoculated.
Between one fourth and one third of the surface of the agar plates should be inoculated with the specimen. The inoculum is then progressively diluted across the agar by successive steps of streaking with a bacteriologic loop (Figure 18-3). There are several different streaking technique modifications, and any method that yields isolated colonies is satisfactory. With the practice of a light touch to avoid tearing the agar and experience in anticipating the amount of bacterial growth that will occur, slight modifications can be made in technique from one specimen to the next to achieve the best isolation of colonies.
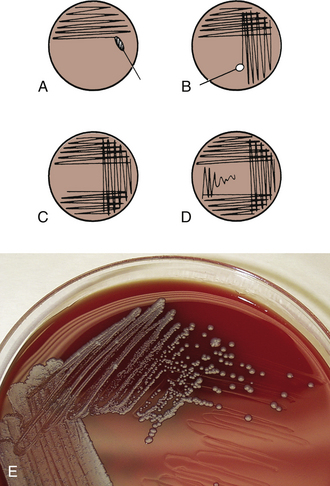
FIGURE 18-3 Plate inoculation and streaking method for isolation of bacterial colonies. A, Inoculate with swab, covering one fourth to one third of plate. B, Streak lightly, overlapping previous area. C, Flame loop, allow it to cool, and streak next area. D, Repeat as in C. The photo illustrates well isolated colonies.
Media dispensed in tubes may be a broth or semisolid agar, or media may be poured as a slant. Broth media can be inoculated with a loop or an inoculating wire by touching the side of the tube just below the surface of the medium. Depending on the purpose of the slant medium, it may require inoculation by stabbing the deep (or butt) portion of the agar (e.g., triple sugar iron [TSI] slants); the slant surface is then streaked from bottom to top (Figure 18-4). When a semisolid medium for motility testing is inoculated (Figure 18-5), it is important for the inoculating wire to be inserted and removed along the same tract within the medium.
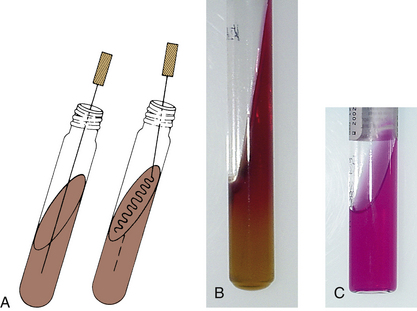
FIGURE 18-4 Inoculation procedure for agar tube media. A, Inoculation of agar slant and butt, such as triple sugar iron (TSI). The inoculation needle is first stabbed into the butt and then removed and streaked over the agar slant surface in a back-and-forth motion. B, Alkaline slant and acid butt reaction (K/A) in TSI. C, Positive urease reaction after slant inoculation.
INCUBATION CONDITIONS
Inoculated plates are incubated in an inverted position to prevent condensation of water on the lid. If water drops to the agar surface, it can mix the bacterial growth rather than allowing it to develop as isolated colonies. If tube media have screw tops, they should be left loose during incubation.
Cultures should be placed in incubation at an optimal temperature as quickly as possible. The majority of cultures for isolation of pathogenic bacteria are incubated at 35° C. Although optimal growth may occur at other temperatures, in most cases, alternate temperatures are more important for differentiation of bacteria than for primary isolation.
Atmosphere
Most common pathogenic bacteria are aerobes or facultative anaerobes and will grow well in an atmosphere of room air. However, oxygen is toxic to obligate anaerobic bacteria, requiring that a special culture container from which all oxygen has been removed be used for incubation. Two excellent anaerobic systems for the small laboratory are the BioBag Type A environmental chamber and the BBL GasPak anaerobic system (BD Diagnostic Systems, Sparks, MD). Each system consists of a hydrogen generator, a catalyst to facilitate the depletion of oxygen from the atmosphere by combining with the hydrogen, and a sealable container to hold these components and the culture plates. Certain bacteria—such as Campylobacter, Brucella, Haemophilus, and Mycoplasma—have specialized atmospheric and nutritional requirements so that specimens to be cultured for these agents are best forwarded to reference laboratories.
Time
All inoculated plates should be examined after 15 to 24 hours of incubation (overnight). Most cultures will have sufficient growth for evaluation and identification at this time. Culture specimens that contained bacteria on direct microscopic examination but yield negative results after this time or specimens that may be expected to contain slow-growing bacteria should be incubated for up to 3 days before a final negative report is issued. Incubation of primary isolation plates beyond 3 days is rarely indicated unless there is reason to suspect the presence of an unusually slow-growing pathogen.
ROUTINE CULTURE SYSTEM
The majority of specimens for culture in the veterinary microbiology laboratory can be processed in a routine manner with a minimum of media. The approach presented in this section is not represented as a comprehensive culture system that will successfully isolate and identify all potentially pathogenic bacteria; rather, it is meant as a basic guideline for the veterinary technician who has the opportunity to provide a diagnostic bacteriology service within a private veterinary practice. The system is designed to be cost effective when used for routine aerobic cultures, which will usually account for 80% to 90% of culture requests. Often, the veterinarian’s immediate objective is for the laboratory to characterize the isolate sufficiently to guide antimicrobial selection or to perform an antimicrobial susceptibility test rather than to pursue definitive identification. The challenge for the technician is to discern when it is better to refer a specimen to another laboratory for more sophisticated diagnostic evaluation.
Primary Isolation Media
Blood agar, containing 5% sheep blood, is the most widely used primary isolation medium because of its ability to support growth of most pathogenic bacteria. It is also a standard medium used extensively for describing colony morphologic characteristics and hemolytic patterns. MacConkey agar is also commonly used as a primary isolation medium. Although use of MacConkey agar is not always essential, it often provides significant information about bacteria and may provide presumptive identification, or at least group classification, of the isolate. If MacConkey agar is inoculated as a primary isolation medium, rather than used as a differential medium for subcultures, the identification process is often moved forward by 1 day.
In many laboratories, it is customary to include an enrichment broth as part of the primary isolation medium. One of the most common broth media used for this purpose is thioglycolate. This medium can support growth of many anaerobic or facultative anaerobic bacteria that might not be recovered on primary plates incubated aerobically.
Primary growth in a broth medium is frequently difficult to interpret. It must always be compared with a direct microscopic examination because contaminating bacteria from the environment or indigenous flora may overgrow a pathogen in the specimen. Specimens should never be cultured solely in a broth medium for primary isolation. Further discussion of the interpretation of broth subcultures is presented later.
When specific pathogens are sought in specimens, modifications of the basic culture setup can be incorporated into the laboratory routines. Procedures that may enhance the likelihood of recovering specific pathogens are discussed later in this chapter.
Preliminary Evaluation of Cultures
Efficient evaluation of primary cultures requires considerable skill, which is acquired through experience in the microbiology laboratory. Decisions that must be made about isolated bacteria include their possible significance as pathogens, which bacteria require further identification, and what additional tests are needed. As the veterinary technician gains experience in the laboratory and becomes acquainted with common bacterial pathogens, these decisions will become less challenging. Clinically useful results usually only require that identification of bacteria is usually carried to the presumptive level by a few key characteristics rather than to a definitive identification. Only isolates considered to be clinically significant need to be identified. Identification of bacterial growth that results from environmental contamination or indigenous microflora is wasted effort.
From the initial examination of primary cultures, considerable information can be obtained to help distinguish which bacteria should be characterized in further detail. The important characteristics of primary cultures to be noted include (1) the number of different types of bacteria isolated, (2) the relative number of each type, (3) the colony morphologic characteristics of the various isolates, and (4) the changes in the media surrounding the colonies. While making the preliminary evaluation of primary cultures, the technician must keep in mind the source of the specimen. If it was obtained from a normally sterile body site (e.g., joint fluid) and was properly handled, any growth is likely to be significant. If the specimen is from a site normally colonized by microflora (e.g., intestinal tract), interpretation becomes much more difficult. In general, if there is scant aerobic growth of three or more bacteria, the result probably reflects normal flora. Most bacterial infections, other than mixed anaerobic infections, are usually caused by only one or two agents. When a specimen from an infectious process is carefully collected, growth of a single organism in nearly pure culture will often be observed. Therefore the most abundant colony type is usually the most important.
Some general guidelines for selection of significant isolates can be derived from colony morphologic characteristics, although exceptions will always occur. Usually circular, smooth, raised or convex, opaque to gray colonies with an entire edge are more likely to be significant. Large, rough, granular, irregular, spreading, or heavily pigmented colonies
are likely to be insignificant unless large numbers are recovered in nearly pure culture.
Changes in the media should be carefully noted. Hemolysis in blood agar is often a good indication of a possible pathogen. Sometimes the hemolytic pattern provides adequate identification (Figure 18-6), such as the double zone of hemolysis produced by many coagulase-positive isolates of Staphylococcus spp. Pigment production can be an important characteristic to note on primary cultures. The differential features of MacConkey agar (i.e., ability to grow, lactose fermentation) are important bits of information that can aid in the identification of an isolate. Odors produced by bacteria are difficult to describe adequately but, after experience is gained, become another useful identifying characteristic.
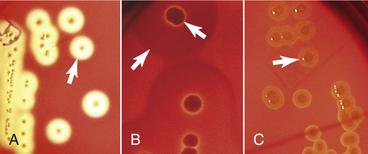
FIGURE 18-6 Types of hemolysis observed in blood agar plates: A, Complete hemolysis also named β-hemolysis if organism is Streptococcus. B, Double-zone hemolysis as produced by Staphylococcus intermedius. C, Alpha-hemolysis produced by some strains of Streptococcus.
The novice microbiologist may be required to rely on several differential tests for the identification of isolates. As experience is gained and confidence develops, more isolates will be recognized on the primary plates. Knowledge of the more common bacterial species to expect from a specimen (see Table 18-1) will provide a differential list of bacteria to consider so that it is not necessary to face each culture as a complete unknown.
Recording, Interpreting, and Reporting Results
Although it is impossible to devise rigid rules that provide for adequate processing of all specimens, some routines are helpful for observing and recording results of cultures. A laboratory worksheet should be developed for recording all observations. These records should contain sufficient detail so that anyone who works in the laboratory can take over and complete the culture without a special briefing. A worksheet that provides adequate room for a flow chart type of illustration of culture processing and observation is easy to follow (Figure 18-7). These work records may become part of the medical record, so care should be taken to ensure that they are complete and accurate (see Chapter 5).
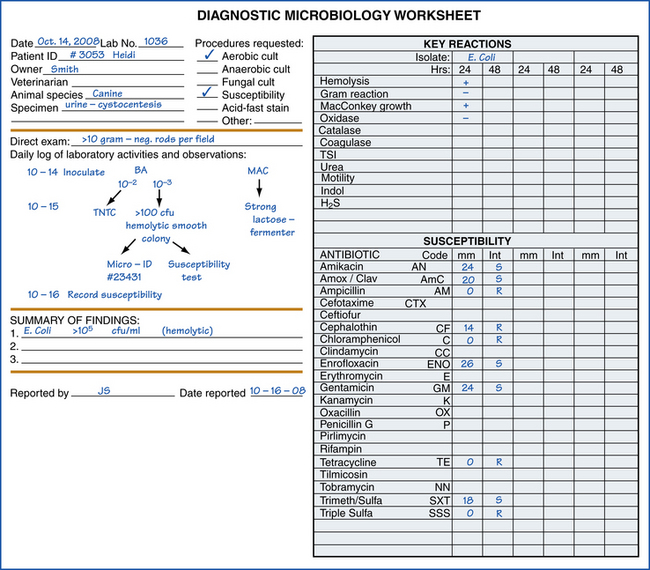
FIGURE 18-7 Example of a laboratory worksheet for recording results of various laboratory procedures, including microbial identification and susceptibility tests.
As an aid to interpreting culture results, the relative abundance of growth of each type of colony should be recorded. A convenient system of recording is a scale of 1+ to 4+, in which each step on the scale represents the number of quadrants of the primary culture plate in which the colony is growing. For example, if the only colonies are in the initial streak lines in which the specimen was inoculated on the plate, growth would be rated 1+. If growth is so abundant that colonies are found in the fourth quadrant (the final streak lines), growth is rated 4+. Any bacterium isolated from broth subculture, but not on primary inoculated plates, is rated 1+, regardless of the abundance of growth on the subculture plate. Bacterial cultures should not be evaluated empirically as positive or negative because this semiquantitative method helps the clinician to interpret the significance of the results. Specimens from most acute bacterial infections that have not been treated with antimicrobials will yield 3+ to 4+ growth. However, because of poor collection technique, mishandling the specimen, presampling antimicrobial therapy, or chronic infections, a smaller number of bacteria may be recovered. The clinician must decide whether these smaller numbers of bacteria are significant. If the culture is from a normally sterile body site, these culture results are often significant.
Indigenous Flora
Specimens cultured from sites populated with indigenous bacterial flora (often described as normal flora) are more difficult to interpret. Usually these cultures are insignificant if they result in scant growth, especially if it is a mixture of bacteria. To avoid wasting time precisely identifying the microflora, the technician should become familiar with the organisms normally found at various body sites (Table 18-3). Many of these bacteria are potential pathogens. If they are identified because of common recognition and are specifically reported while other, less familiar bacteria are overlooked, the report may mislead the clinician by implying undue significance.
Reporting results of cultures from sites with indigenous flora can be a perplexing problem. Often, it is better to specify which specific pathogens have been excluded by careful cultural examination, such as “no Salmonella isolated.” Between the extremes of trying to identify everything and reporting “normal flora,” the technician and clinician must agree regarding the most useful information expected from a given specimen. Perhaps certain potential pathogens that may be considered significant for the specimen should be carefully sought. In other situations a predominant bacterium can be identified or groups of organisms reported (e.g., coliforms, diphtheroids).
Identification Procedures
Identification of clinically significant bacteria is best accomplished by means of a few rapid tests that can presumptively differentiate organisms. To one who is experienced, such characteristics as colony morphology, hemolysis, growth on MacConkey agar, and odor may be adequate for presumptive identification. Often, additional differential tests are needed for more precise identification. Figure 18-8 presents a useful approach to identification of unknown isolates when needed.

FIGURE 18-8 General flow chart for identification of common aerobic veterinary bacterial pathogens. TSI, Triple sugar iron; SIM, sulfide-indole-motility.
Gram Reaction: The first differential characteristic to be considered is the reaction to Gram stain. Staining with Gram stain can be performed on thin smears of bacteria from a single colony (see Gram Stain Procedure and Interpretation). Potassium hydroxide, 3%, may be used as an alternate and more rapid test for Gram reaction of isolated colonies. A small drop of 3% potassium hydroxide (no larger than a colony) is dispensed on a slide, and a colony of bacteria is picked from the blood agar plate with a bacteriologic loop and mixed into the 3% potassium hydroxide. The loop is slowly and gently lifted at 5-second intervals to see whether a viscous gel is sticking to the loop. The formation of any sticky strand that can be lifted with the loop indicates a gram-negative bacterium. The reaction should appear within 20 to 30 seconds. Gram-positive organisms will diffusely mix in the 3% potassium hydroxide. Cellular morphologic characteristics of the gram-positive bacteria are important differential characteristics that require careful examination of a stained smear.
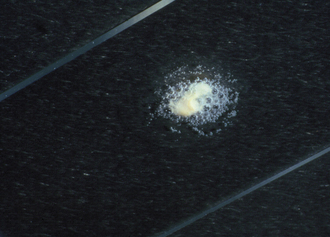
Catalase Test: Catalase activity is an important and rapid test for differentiating Staphylococcus from Streptococcus spp. and Erysipelothrix spp. and Arcanobacterium pyogenes from other small gram-positive rods. Hydrogen peroxide (3%) is the only reagent needed and can be readily purchased from any drugstore. It should be stored in a dark bottle in the refrigerator. The slide catalase test is performed by picking bacteria from the center of a colony with a needle or loop and smearing the bacteria on a clean, dry slide. A drop of hydrogen peroxide is added over the bacteria and immediately observed for bubbles of oxygen that will be released if catalase is present (Figure 18-9). Lack of bubbles is a negative test result. The order of the test procedure must not be reversed, or false-positive results may be obtained. If any blood agar is introduced into the test, it can also cause a false-positive result.
Oxidase Test: Cytochrome oxidase activity should be determined for all gram-negative bacteria except strong lactose fermenters, which will be negative. Commercial cytochrome oxidase test reagents are readily available (Figure 18-10). The reaction is supposed to be clearly visible within a few seconds, but with some reagents, the reaction may be delayed for up to 2 minutes for Pasteurella and Actinobacillus spp. A heavy inoculum must be used for accurate testing. A wooden stick or platinum loop should be used to pick colonies for testing because trace amounts of iron from other loops can cause false-positive results.
Presumptive Identification: When Gram reaction, cellular morphologic characteristics, and catalase and oxidase results have been determined, the bacteria can be tentatively grouped, and differential tests can be selected as indicated in Figure 18-8 for identification.
Isolates of Streptococcus are usually characterized by the type of hemolysis (see Figure 18-6) they produce. β-hemolytic Streptococcus isolates are usually considered to be potential pathogens. α-Hemolytic and nonhemolytic Streptococcus isolates usually originate from normal flora of skin and mucous membranes and are not considered significant unless they are obtained from normally sterile sites.
Isolates of Staphylococcus should be differentiated from those of Micrococcus (Table 18-4), which are considered to be nonpathogenic. Glucose-fermenting ability, determined in TSI agar slants, can be used for differentiation of these genera. If a double zone of hemolysis (see Figure 18-6) is observed on the blood agar plate, the bacterium can be identified as a coagulase-positive Staphylococcus without need for further testing. All other Staphylococcus isolates should be tested for coagulase activity because coagulase activity correlates with pathogenicity. Speciation of coagulase-positive and coagulase-negative Staphylococcus spp. may be attempted in special cases, if desired, by using a range of tests or commercial identification kits.
TABLE 18-4
Differentiation of Gram-Positive, Catalase-Positive Cocci
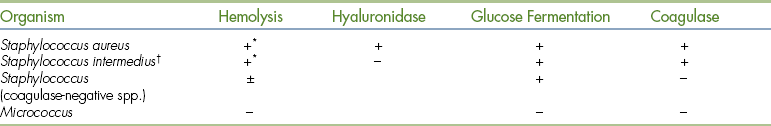
∗Double zones of complete and incomplete hemolysis are frequently observed.
†S. intermedius is positive for pyrrolidonyl arylamidase activity when PYR disks are used; this quickly differentiates it from S. aureus.
The small gram-positive rods can be differentiated by inoculating TSI, urea, and sulfide-indole-motility (SIM) medium. The results of these tests, and colony morphology and catalase activity, can identify the isolate (Table 18-5). Individual characteristics of the important pathogens in this group will be discussed later.
TABLE 18-5
Differentiation of Small, Non−Spore-Forming Gram-Positive Rods
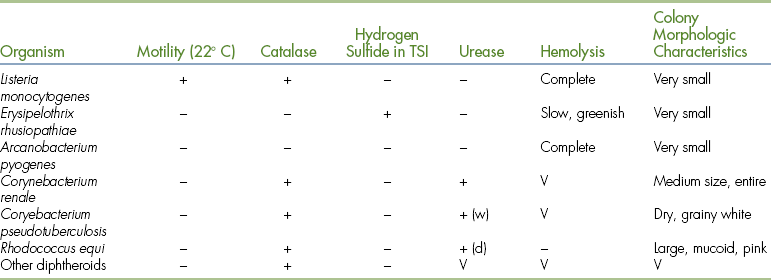
d, Delayed, may require up to 2 weeks; TSI, triple sugar iron (agar); V, variable results; w,weak.
Most gram-negative, oxidase-negative bacteria are members of the Enterobacteriaceae family. These bacteria are reactive in biochemical tests and can be identified by one of several different systems. The most rapid and economical methods for differentiating the Enterobacteriaceae family members are the commercially available packaged multitest systems. These systems are discussed later. A few other organisms that are oxidase-negative may be isolated infrequently. The most common reason for nonenteric oxidase-negative results is a false-negative oxidase test result. When such results are suspected, further differentiation of oxidase-negative bacteria, as shown in Table 18-6, is necessary.
TABLE 18-6
Differentiation of Gram-Negative, Oxidase-Negative Bacteria
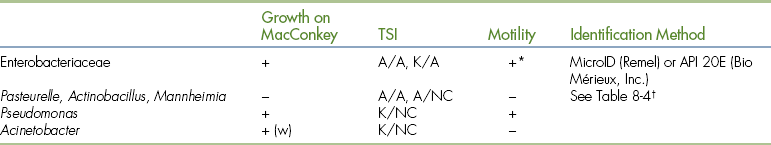
A, Acid; K, alkaline; NC, no change; TSI, total sugar iron (agar); w, weak.
∗Klebsiella is a nonmotile.
†Negative oxidase results are caused by very weak reactions.
The most frequently isolated oxidase-positive, gram-negative bacteria of veterinary importance can be differentiated by using three tubes of media (TSI, urea, SIM) as shown in Table 18-7.
TABLE 18-7
Differentiation of Gram-Negative, Oxidase-Positive Bacteria
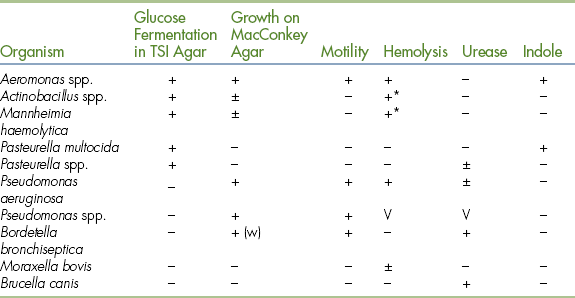
TSI, Total sugar iron; V, variable; w, weak.
∗Hemolysis under the colony.
Definitive Identification: The identification procedures discussed in this chapter are presumptive methods. Definitive identification of some isolates may require extensive testing. The cost of such identification in time, media, and specialized techniques is usually not justifiable in a small practice laboratory. Unusual isolates should be forwarded to a referral laboratory for further identification. The isolate should be subcultured to an agar slant medium that does not contain a fermentable carbohydrate, or it should be heavily inoculated onto a swab. The swab can be transported in a transport medium, such as Amies transport medium. Do not attempt to ship agar plates. Invariably, they become contaminated and overgrown, dehydrated, or broken.
Commercial Identification Kits: Commercial development of kit systems for identification of bacteria has been one of the most important advances in clinical bacteriology. These systems provide a cost-effective method for identification of bacteria in low-volume laboratories. Most kits consist of a number of test compartments arranged in a compact unit. The systems generally involve the use of microtechnique tests in various types of media systems. They may include compartments of solid agar, dehydrated broth, substrate or reagent disks, and supplementary conventional tests. All compartments are inoculated with organisms from an isolated colony or colonies. After the specified period of incubation and the addition of required reagents, the results are recorded as positive or negative for each test. For many of the systems, these reactions have variously weighted values so that the positive results will produce a unique profile number for each combination of positive and negative results. Most systems provide profile directories or registers for identification of the isolate most likely to produce the set of observed reactions.
The low-volume laboratory may find these systems to be more cost effective than attempting to maintain a large inventory of conventional media. They have a reasonable shelf life (6 to 18 months) and require minimum storage space because of the compact construction. Accuracy is better than that achieved with conventional media in most small laboratories because most reactions are easy to interpret and results can be decoded more rapidly compared with sorting through conventional identification tables. Finally, depending on the specific system, most bacteria can be identified within 4 to 24 hours after isolation.
The manufacturer’s directions and precautions must be carefully observed or misidentification will occur. If the system is limited to oxidase-negative enteric bacteria, only those organisms should be inoculated. Other organisms can still yield a profile number, which will result in an incorrect identification. Problems can also arise from inoculation with an older culture, improper concentration of inoculum, or mixed cultures. As experience is gained, accuracy will be increased.
When one of these systems is selected, factors to consider include the ease of inoculation, manipulations required to add reagents, the availability of interpretive charts or numeric coding devices, and the database used in development of profile registers. Often, it is difficult to discover whether significant numbers of veterinary pathogens are included in the databases for there to be a reasonable probability of correct identification of unique veterinary pathogens. The most beneficial use of these systems is the identification of members of the Enterobacteriaceae family (see Box 18-1). All enteric identification systems have essentially the same degree of accuracy and reliability of performance. The systems that seem to have gained widest acceptance in veterinary bacteriology include API 20E (bioMérieux, Inc., Hazelwood, MO), MicroID (Remel, Lenexa, KS), and Enterotube II (BD Diagnostic Systems, Sparks, MD). They provide excellent results.
Several packaged kit systems are marketed for identification of bacteria other than Enterobacteriaceae (see Box 18-1). Although these systems may provide more definitive identifications of some organisms, they have limited usefulness in small veterinary laboratories. Presumptive identification methods outlined in this chapter are frequently adequate.
The identification kits for yeast and anaerobes are useful for large-volume laboratories, but usually, the need for them in the small laboratory is not adequate to be cost effective.
SPECIAL CULTURE PROCEDURES
The detection of viable bacteria in an animal’s blood has considerable diagnostic and prognostic importance. Blood cultures are indicated for fever of unknown origin; suspected bacteremia associated with endocarditis, arthritis, or meningitis; and neonatal septicemias. Blood cultures should be obtained from dogs that have antibodies to Brucella canis to aid in confirmation of the diagnosis.
Special care must be taken to prevent contamination of blood cultures with skin microflora. The venipuncture site should be decontaminated by using surgical scrubbing procedures (see Chapter 28) and should not be palpated after preparation unless a sterile glove is used. Blood can be obtained by using a syringe and needle or a closed-vacuum bottle system. Often the concentration of bacteria in blood is too low to detect by direct inoculation of plate media. Therefore inoculation of commercially available blood culture media bottles is recommended. Ideally, a sample of 5 to 10 ml of blood should be obtained for culture. Blood samples in anticoagulants, such as heparin and ethylenediaminetetraacetic acid (EDTA), are not acceptable for culture because of the poor survival of some bacteria in the presence of these anticoagulants.
Blood culture bottles should be incubated at 35° C to 37° C for at least 7 days and examined daily for macroscopic evidence of growth. Positive cultures can be recognized by one or more of the following characteristics: turbidity, gas bubbles, fluffy or compact colonies, and hemolysis of the blood. When growth is observed, gram-stained smears and subcultures on plate media should be prepared for examination and identification of the organism. Negative-appearing blood culture broths should be subcultured in blinded fashion before being discarded and reported as negative. Blood cultures in which attempts to isolate Brucella spp. have been made should be incubated for 2 to 4 weeks before being discarded as negative.
Urine Cultures
Urine is an excellent growth medium for many bacteria because it contains electrolytes, water-soluble vitamins, residual amounts of glucose, and various nitrogenous compounds. Therefore careful attention must be given to proper collection and handling of urine for culture, or a small and insignificant number of bacteria can rapidly multiply to significant numbers. Urine specimens for culturing can be collected in three ways: free catch, catheterization, or cystocentesis (see Chapter 28). The distal urethra and genitalia are colonized with microflora that contaminate free-catch and catheterization specimens. If the skin has been adequately prepared for cystocentesis and the needle does not come in contact with any abdominal organ other than the bladder, any bacteria isolated from the specimen should be significant. Cultures should be set up within 2 hours of collection to reduce overgrowth with insignificant bacteria that may contaminate urine specimens. If cultures cannot be established within 2 hours, the sample must be refrigerated to slow the bacterial growth. Refrigeration begins to fail after 18 to 24 hours. Therefore the best method for identifying urinary tract infections is to establish cultures as soon as possible.
The use of blood agar and MacConkey agar as selective and differential isolation media is recommended for the culture of all urine specimens. There is no need for broth medium for enrichment. The bacteriologic examination of urine specimens collected by methods other than cystocentesis should provide an estimate of the number of microorganisms per milliliter of urine as an aid to interpreting the results. This can be accomplished by inoculating the blood agar plate with a standard dilution loop calibrated to deliver approximately 0.001 ml (Figure 18-11). Each colony that grows represents 103 organisms/ml in the specimen; therefore the number of colonies is multiplied by 1000 to obtain the concentration of organisms in the specimen. The number of bacteria can also be estimated through direct microscopic examination of a gram-stained smear of uncentrifuged urine. If one or more bacteria per oil immersion field are observed, usually more than 105 organisms/ml should be present in cultures. If more than two types of bacteria are isolated, a second specimen should be collected and cultured to distinguish a mixed infection from contamination or mishandling of the specimen. Bacterial counts can be low because of improper handling of the specimen, dilution from forced fluid therapy, or cystocentesis samples from patients with urethritis that has not become established as a concomitant cystitis.
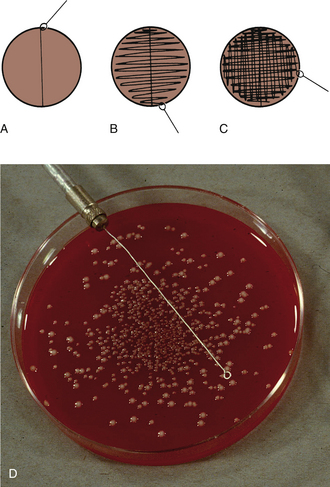
FIGURE 18-11 Procedure for inoculating media for semiquantitative bacterial colony counts when culturing urine. A, Primary inoculation with calibrated loop. B, Streak at right angles to primary inoculation. C, Streak at right angles to previous streak. D, Photo illustrates a plate with >100 colonies resulting from inoculation with a 1 μl loop indicating >105 bacteria/ml of urine.
COMMON BACTERIAL SPECIES
The bacterial pathogens frequently associated with many infectious processes are listed in Table 18-1. Some of the colony morphologic, growth, and identifying characteristics of these bacteria are listed in Table 18-8. Additional details are given in the following discussion of special isolation and identification techniques. Clinically important characteristics are noted.
GRAM-POSITIVE COCCI
Staphylococcus spp. are catalase-positive cocci that occur in grapelike clusters. They are frequently isolated from pyogenic lesions, such as wounds, dermatitis, otitis, mastitis, cystitis, and osteomyelitis. They are usually divided into coagulase-positive and coagulase-negative groups. The coagulase-positive species, S. aureus and S. intermedius, are more important pathogens, and the others are usually considered to be less pathogenic. One of the most important identifying characteristics that should be noted is the development of a double zone of hemolysis (an inner zone of complete hemolysis and a second zone of incomplete hemolysis, see Figure 18-6). This is a common identifying characteristic of most coagulase-positive isolates from animals. Mannitol fermentation is not a reliable correlate of coagulase activity in staphylococcal isolates from animals. Because of a high incidence of acquired antimicrobial resistance, these organisms should be tested for antimicrobial susceptibility.
Streptococcus Species
Streptococcus spp. are catalase-negative cocci that occur singly, in pairs, or in short chains. Chain formation is more easily demonstrated in broth cultures. Streptococcus is the most common bacterial pathogen of the horse and can be found to cause pyogenic infections and mastitis in all species of animals. However, each species tends to be rather host specific. Therefore the streptococcal pathogens of humans rarely cause infections in animals, and animals are usually not reservoirs of human pathogens. Some species cause specific diseases. Streptococcus equi ssp. equi is the cause of strangles in horses. Streptococcus agalactiae is an important cause of bovine mastitis. It can be identified by the CAMP test (Figure 18-12). Definitive biochemical and serologic (Lancefield typing) testing is usually not clinically important. For clinical interpretation, it is important to evaluate the hemolysis produced on blood agar (see Figure 18-6). β-hemolysis (complete clearing) usually correlates well with potential pathogenicity; α-hemolysis (incomplete greenish discoloring) and γ-hemolysis (nonhemolytic) are usually indications of normal flora of skin and mucous membranes. However, when isolated in nearly pure culture from normally sterile body sites, these organisms can be considered to be clinically significant. Susceptibility to antimicrobials is usually predictable, which means antimicrobial susceptibility testing may be an unnecessary expense.
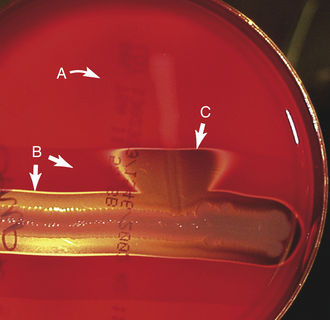
FIGURE 18-12 CAMP test for Streptococcus agalactiae. The isolate (A) to be tested is inoculated perpendicular to a stock strain of double-zone hemolytic Staphylococcus (B), producing a synergistic triangle of hemolysis (C) as a positive CAMP test.
The enteric group D streptococci have been renamed as Enterococcus spp. Urinary tract infections are the most common presentation of these organisms; and Enterococcus spp. occasionally infect wounds and cause bacteremia. They are emerging as significant nosocomial agents and are particularly troublesome because they are likely to be resistant to many antimicrobials.
ANAEROBIC COCCI
Most anaerobic cocci belong to the genus Peptostreptococcus. When isolated, these agents are usually associated with mixed anaerobic infections.
GRAM-POSITIVE RODS
Bacillus spp. are common contaminants isolated in the laboratory. They are ubiquitous in soil, water, air, and dust. They are large spore-forming rods that usually grow as large, rough, granular, or spreading colonies. They are usually hemolytic. Occasionally, strains of Bacillus will be isolated that react as if they are gram-negative and oxidase-positive. However, they can be identified by the presence of spores in stained smears. Bacillus anthracis (the agent that causes anthrax) is the important pathogenic species. It is extremely virulent for humans. Do not attempt to culture it.
Clostridium spp. are large, spore-forming anaerobic rods. The pathogenic species are noted for their potent toxins and extensive destruction of tissue. Infections may be accompanied by an accumulation of gas (emphysema) in the tissues. Laboratory diagnosis of the toxic diseases (tetanus, botulism, enterotoxemia) and differentiation of the infectious diseases (blackleg, malignant edema, bacillary hemoglobinuria, etc.) require the assistance of reference diagnostic laboratories. Often a gram-stained smear is useful for ruling out clostridial disease or indicating it as a possibility. Clostridium perfringens is occasionally isolated from deep wounds with extensive tissue necrosis, such as compound fractures. The bacterium requires an anaerobic atmosphere for growth and frequently produces a double zone of hemolysis.
C. perfringens is also associated with enteritis and diarrhea in dogs. In some cases, the presence of enterotoxigenic strains of C. perfringens can be presumptively identified in fecal smears by evaluating the smears for the presence of increased bacterial spores because sporulation is associated with the release of enterotoxin. Spores appear as unstained, small, oval structures and are usually surrounded by a halo of stained bacterial cellular debris unless a specific spore stain is applied (Figure 18-13).
Small Rods
Corynebacterium spp. are small, club-shaped rods that tend to occur in palisades or in an angular arrangement because of their “snapping” division. Colonies are usually quite small at 24 hours, but continue to enlarge and vary greatly by species. Most species are catalase-positive. Arcanobacterium pyogenes (previously called Actinomyces pyogenes) produces a small pinpoint colony, hemolysis, and a catalase-negative reaction. Cellular morphologic characteristics must be evaluated carefully to differentiate it from Streptococcus spp. It is the most common pyogenic agent in ruminants. Rhodococcus equi is a cause of pneumonia and abscesses in foals. Morphologically, individual cells are coccobacillary and larger than other Corynebacterium organisms. Corynebacterium pseudotuberculosis causes chronic abscesses in goats and sheep, and it has recently reemerged in the western United States as the cause of abscesses in horses known as pigeon breast disease. The Corynebacterium renale group causes pyelonephritis and cystitis in cows. There are many other Corynebacterium spp. that are nonpathogenic commensals of the skin; they are frequently referred to collectively as diphtheroids.
Listeria monocytogenes is a small, non−spore-forming rod that is catalase-positive. It is the only small gram-positive rod that is motile at room temperature. It is an infrequent cause of abortion in large animals and septicemia in young animals. In ruminants, it causes an encephalitis known as circling disease. The bacteria localize in the pons and medulla (brainstem). Cultures from other parts of the brain may be negative. Isolation may require specific selective and enrichment techniques available in reference laboratories.
Erysipelothrix rhusiopathiae is a pleomorphic rod that is usually slender and small. The colony is small, and an incomplete, greenish hemolysis (alpha like) is produced. The cellular morphologic characteristics must be carefully evaluated to differentiate it from Streptococcus spp. because both are catalase-negative. A definitive characteristic that differentiates it from other gram-positive rods is the production of hydrogen sulfide. E. rhusiopathiae is most commonly encountered as a cause of septicemic or arthritic disease of pigs, but it is occasionally a cause of endocarditis in dogs.
Filamentous Rods
The Actinomycetaceae family contains several clinically important bacteria that are distinguished by forming branching, filamentous gram-positive rods. Most Actinomyces spp. are anaerobic bacteria that may tolerate low levels of oxygen. Therefore some species can be isolated in a candle jar, but the most efficient isolation can be achieved with an anaerobic system. Actinomyces spp. colonies are slow to develop, requiring up to 5 days, and are usually raised and irregular in shape. When isolated, they are usually recovered from pyogranulomatous lesions of soft tissue, pyothoraces, or osteomyelitis. Nocardia spp. are partially acid-fast, which means a modified staining procedure must be used to differentiate them from Actinomyces spp. In place of the acid-alcohol decolorizer, only an acid decolorizer is used to demonstrate acid-fastness. Nocardia spp. are aerobic bacteria with colonies usually appearing after 2 to 5 days of incubation. The colonies are rough and have a dry, granular texture. They adhere tenaciously to the media. Nocardia spp. are occasionally isolated from pyothoraces and wounds. They may be serious mastitis pathogens in some dairy herds. Dermatophilus congolensis is another branching, filamentous bacterium. It often has a beaded appearance with transverse and longitudinal divisions. It is an uncommon cause of skin infections of horses and ruminants. The organism can be demonstrated in smears of pus from under the elevated scabs containing tufts of hair. Streptomyces spp. are aerobic, filamentous bacteria that are not acid-fast. They are abundant in soil and may be isolated as contaminants.
Anaerobes
Anaerobic, gram-positive, non−spore-forming rods belong to the genera Bifidobacterium, Eubacterium, and Propionibacterium. If definitive identification of these organisms is needed, culture specimens should be sent to a reference diagnostic laboratory. They are usually isolated in mixed cultures from pyogenic lesions.
ACID-FAST BACTERIA
Mycobacteria are mostly small, short rods but are occasionally pleomorphic. They stain poorly with Gram stain but are acid-fast. These bacteria are rarely isolated in veterinary practice laboratories because special procedures and media are usually required. However, preparation of an acid-fast stained impression smear can be a useful diagnostic procedure for making a presumptive diagnosis of mycobacterial infection. Positive findings are significant; however, negative findings have limited predictive value. Mycobacterium avium ssp. paratuberculosis may be demonstrated in acid-fast stained smears prepared from intestinal mucosa or mesenteric lymph nodes of ruminants. Mycobacterium avium infection of birds can frequently be confirmed by examination of acid-fast smears prepared from the liver or intestinal mucosa. Occasionally, abundant acid-fast organisms can be demonstrated in the feces.
Isolation of the zoonotic agents of tuberculosis, Mycobacterium bovis and Mycobacterium tuberculosis, should not be attempted in a practice laboratory. Infrequently, a rapid-growing Mycobacterium sp. may be isolated from a case of bovine mastitis. The colonies will usually appear after 3 to 5 days of incubation. These organisms should be forwarded to a reference laboratory for definitive identification.
GRAM-NEGATIVE BACTERIA
The Enterobacteriaceae family of bacteria is the largest group of potential pathogens and the most frequently isolated bacteria. The normal habitat of these organisms is the digestive tract and soil; therefore they will usually grow on MacConkey agar and are frequently insignificant contaminants of specimens. They are small gram-negative rods, with some pleomorphism. Some of the common identifying characteristics include oxidase negativity, glucose fermentation, and motility (except Klebsiella spp.). Genus and species identification requires numerous biochemical tests, and serotyping and genotyping are frequently necessary to identify pathogenic strains. Acquired antimicrobial resistance from R-factors (plasmids) is common in this family of bacteria, making antimicrobial susceptibility testing a necessary clinical evaluation of isolates.
Most non-Enterobacteriaceae, gram-negative bacteria are oxidase-positive, and growth on MacConkey agar is variable.
Coliforms
Escherichia coli can frequently be presumptively identified by the strong lactose fermentation reaction it produces on MacConkey agar. Strains causing tissue infections and cystitis are frequently hemolytic. E. coli is frequently associated with diarrhea in neonates (especially pigs, calves, and lambs). The pathogenic strains causing diarrhea are best identified by genotyping and other specialized laboratory testing, such as use of the Escherichia coli Antigen Test (K99 Pilitest, VMRD, Inc., Pullman, WA). However, presumptive evidence of E. coli involvement in diarrhea (scours) can be obtained by gram-staining a smear taken from small intestinal mucosa shortly after the death of the animal. If a large number (>25) of gram-negative rods are observed in each oil immersion field, it is a strong indication that E. coli is a cause of diarrhea. Klebsiella spp. and Enterobacter spp. are occasionally involved in infections of the respiratory and urinary tracts and in mastitis. They are becoming more important in veterinary medicine as superinfecting agents after antimicrobial therapy.
Salmonella Species
Salmonella spp. can cause diarrhea and septicemia in all animals and in humans. When feces are to be cultured, selective and enrichment media should be used to increase the probability of successful isolation of Salmonella. Hektoen enteric agar and selenite enrichment broth (see Table 18-2) are recommended (brilliant green agar and XLD agar are also commonly used selective media). The enrichment broth should be subcultured to both MacConkey and Hektoen enteric agar. Nonlactose-fermenting colonies can rapidly be screened with Salmonella polyvalent O antiserum to identify them. For the purpose of defining the epidemiology of salmonellosis outbreaks, the isolates should be forwarded to a reference laboratory for serotyping.
Proteus Species
Proteus spp. are frequently isolated as specimen contaminants or secondary invaders. They are important pathogens of the urinary tract. Related genera of bacteria that do not swarm on blood agar are Morganella and Providencia, and they can be readily identified by using kits. The swarming Proteus spp. sometimes interfere with isolation of other organisms. This problem can be solved by using phenylethyl alcohol (PEA) blood agar plates. Proteus and other gram-negative organisms will be inhibited, providing easier isolation of gram-positive organisms.
Other Enteric Organisms
There are many other members of the Enterobacteriaceae family—including Serratia, Citrobacter, Edwardsiella, Enterobacter, Pantoea, and Hafnia spp.—which are infrequently isolated. Careful clinical evaluation is necessary to determine their significance. Often a repeated culture helps confirm the significance of isolation.
Aeromonas Species
Aeromonas spp. are oxidase-positive rods that grow on MacConkey agar. They are commonly found in soil, water, and sewage and frequently infect aquatic animals. They are infrequently a cause of septicemia in terrestrial animals.
Actinobacillus
Actinobacillus spp. are oxidase-positive, small rods that usually grow on MacConkey agar. The colony morphologic characteristics are similar to those of Pasteurella spp. Actinobacillus equuli is the most frequently isolated species. It produces a sticky colony. It is frequently the cause of septicemic infections in foals. It can be isolated from most horses as part of the mucosal flora, but is generally only an opportunistic pathogen in older horses.
Pasteurella Species
Pasteurella spp. are usually associated with respiratory tract infections in most animals. In cats, they are frequently recovered from abscesses. They are small, oxidase-positive coccobacilli. Pasteurella multocida produces a characteristic musty odor. Identification can be aided by noting the typically weak glucose fermentation reaction in a TSI tube. Pasteurella spp. tend to be nonreactive in most commercial identification kit systems and may be misidentified. Hemolytic strains, previously known as P. haemolytica, have been renamed Mannheimia haemolytica for bovine respiratory isolates, and some ovine strains are now called P. trehalosi. Antimicrobial resistance is a growing problem in isolates from food animals, indicating a need to perform susceptibility tests.
Haemophilus Species
Haemophilus spp. are often part of the normal flora of mucous membranes. A few species are important pathogens, usually of the respiratory system. They are small coccobacilli that require specially enriched media for growth. They may grow as satellite colonies around Staphylococcus spp. on blood agar. In addition to the nutritional growth requirements, an increased concentration of carbon dioxide is necessary. These bacteria are susceptible to antibiotics and environmental stress factors, such as drying; therefore specimens must be collected and handled carefully or isolation will be unsuccessful.
Pseudomonas Species
Pseudomonas spp. are common soil and water bacteria. They are usually considered to be opportunistic pathogens of wounds and otitis. Infrequently, they are isolated from the respiratory and urinary tracts. There are many species, but Pseudomonas aeruginosa is the most common pathogen. It produces water-soluble yellow-green pigments that diffuse into the medium, and it has a distinctive odor that aids in recognition. Most isolates are quite resistant to antimicrobials and should routinely be tested for susceptibility.
Bordetella bronchiseptica is a small coccobacillus that is frequently recovered from respiratory tract infections of dogs and is emerging as an important respiratory pathogen of cats. It is associated with atrophic rhinitis in pigs and is infrequently isolated from respiratory tract infections of other animals. Colonies are slow to develop and may only be pinpointed after 48 hours. Growth occurs on MacConkey agar. It is oxidase-positive, urease-positive (often within 4 hours), and citrate-positive.
Brucella Species
Brucella spp. are small coccobacilli that are usually associated with reproductive failure: abortion and infertility. Some species require increased carbon dioxide for growth; however, Brucella canis can be isolated in an aerobic atmosphere. Growth is slow, often requiring 3 to 7 days for colonies to be detectable. Suspected Brucella isolates should be sent to a reference laboratory for definitive identification because of the regulatory and zoonotic importance of these agents.
Other Gram-Negative Rods
Many gram-negative bacteria have limited or undetermined clinical importance. Included are bacteria such as Moraxella, Acinetobacter, and Branhamella spp. and related pleomorphic coccobacilli. These organisms are commonly found as part of the flora of mucous membranes and are usually secondary, opportunistic pathogens. They are relatively nonreactive in most conventional biochemical tests. Thus identification is usually difficult, even for reference diagnostic laboratories.
Anaerobes
The gram-negative anaerobes (Bacteroides, Porphyromonas, Prevotella, Fusobacterium spp.) are frequently involved in mixed infections in abscesses and necrotic tissue. Porphyromonas spp. have recently been associated with canine periodontal disease. The obligate anaerobes are normally found in the digestive tract, so infections resulting from contamination of tissues with mucous membrane flora or intestinal contents frequently contain these organisms. If obligate anaerobes are isolated, evaluation of the cellular morphologic characteristics provides adequate clinical information. Species identification is rarely important. Taxonomic advances have resulted in the reclassification of some former Bacteroides spp. into the genera Dichelobacter, Porphyromonas, and Prevotella.
SPIROCHETES AND CURVED BACTERIA
Leptospira spp. cause febrile infections, often followed by abortion and infertility. These spirochetes are difficult to isolate and usually die within a few hours while being transported to a laboratory. Dark-field examination of urine may aid in establishing a diagnosis. Most diagnoses are made by serologic testing or PCR assay.
Borrelia burgdorferi is a tick-transmitted spirochete that causes Lyme disease in humans and arthritis and lameness in dogs. Canine borreliosis may be accompanied by high rectal temperature and lymphadenopathy. Detection of serum antibodies to B. burgdorferi is the diagnostic test of choice in dogs. Isolation of Borrelia by culture is difficult and often nonproductive. Borreliosis is of importance in the United States in dogs and other animals only within areas infested by ticks carrying this agent.
Brachyspira hyodysenteriae is a spirochete that causes dysentery in pigs. Cultural isolation is beyond the capability of most laboratories. Diagnosis of this infection may be made by examining smears of colonic mucosa for numerous large spirochetes.
Campylobacter spp. cause two different types of disease conditions. One group contains important reproductive pathogens, causing abortion and infertility. Because of special needs for enrichment and selective media and a microaerophilic atmosphere, specimens for isolation of Campylobacter spp. should be sent to veterinary diagnostic laboratories specially equipped for Campylobacter culture. The second group includes important zoonotic enteric pathogens. Most public health and hospital laboratories are equipped to isolate this group. Campylobacter spp. are curved gram-negative rods. They can be recognized by dark-field or phase-contrast microscopy by their darting motility.
Helicobacter spp. are helical or curved gram-negative bacteria that colonize the gastric mucosa of humans, dogs, and cats and the intestinal tracts of some rodents, birds, and swine. Some species have been associated with gastritis and peptic ulceration, whereas other species are considered to be nonpathogenic flora of the gastric mucosa of animals. They can be detected and identified in histologic sections, by culture in reference laboratories, and by association with strong urease activity in gastric mucus.
MYCOPLASMA SPECIES
Mycoplasma spp. are small bacteria that lack cell walls and, as a result, are not easily stained and observed in exudates. Arthritis and pneumonia are the most common mycoplasmal diseases. The role of Mycoplasma spp. in urogenital infections is not well characterized. Occasionally, strains can be isolated on blood agar plates inoculated with urine from dogs with cystitis. Special media and techniques are required for isolation and identification of most Mycoplasma spp. Therefore arrangements should be made with a reference laboratory for Mycoplasma transport media and specimen shipping instructions.
The agent of feline infectious anemia (formerly Haemobartonella felis) has recently been renamed Mycoplasma haemofelis. Examination of a stained blood smear frequently results in identification of clinical cases. Molecular detection assays are also available.
OTHER FASTIDIOUS BACTERIA
Diagnosis of several fastidious bacterial infections is best accomplished by using molecular detection systems specific for gene sequences of nucleic acids or serology. Some of the agents most amenable to molecular detection include Bartonella, Rickettsia, Neorickettsia, Chlamydophila, Chlamydia, Ehrlichia, Mycoplasma, and Mycobacterium spp.
ANTIMICROBIAL SUSCEPTIBILITY TESTING
One of the most important functions of the clinical microbiology laboratory is to provide information that can assist in the selection of appropriate therapy for infectious diseases. All antimicrobial agents have limitations in their spectra of activity. Therefore a universal antimicrobial for all infections is not available. Some organisms are intrinsically resistant to an antimicrobial, whereas others acquire resistance. The most common mechanism for acquired resistance is the acquisition of extrachromosomal pieces of DNA, such as plasmids (R-factors) and bacteriophages. As a result, the bacteria are able to produce enzymes that modify or inactivate the antimicrobial, enable the cell to resist accumulation of the drug, or alter target sites and reduce the activity of the drug. Because the acquired resistance traits are not static, the antimicrobial susceptibility pattern (antibiogram) is not predictable for many organisms. Therefore susceptibility tests are necessary.
INDICATIONS FOR SUSCEPTIBILITY TESTING
Susceptibility testing is indicated for most rapidly growing, aerobic and facultative anaerobic, clinically significant bacteria. Testing should be avoided for isolates representing normal flora and for those bacteria with predictable susceptibility to the antimicrobial of choice. Gram-positive bacteria other than Staphylococcus spp. have rather predictable antibiograms; therefore routine testing is not needed. However, susceptibility testing may be indicated if the antimicrobial of choice cannot be safely and economically administered to the patient. Unpredictable resistance patterns are frequently observed with the gram-negative bacteria, thus requiring testing. Most slow-growing and anaerobic bacteria have rather predictable antibiograms, so testing is not necessary. If acquired resistance is found to be a problem in these organisms, special methods will be necessary for testing them.
In most cases the veterinarian will have started antimicrobial therapy before the laboratory results are available. When the test results become available, therapy can be altered or modified to provide safe, effective, least-cost therapy. In some situations the culture specimen will be from a moribund or dead animal. Susceptibility testing may still be important because it can establish patterns of antimicrobial susceptibility for the organism when encountered in other animals in the herd or region.
SUSCEPTIBILITY TEST METHODS
The simplest type of susceptibility test is one that determines the presence of an enzyme that can inactivate an antimicrobial. Penicillin resistance in Staphylococcus spp. is acquired by gaining the ability to produce β-lactamase, an enzyme that inactivates most penicillin derivatives. Sensitive and rapid tests, such as Cefinase (BD Diagnostic Systems), are available for detection of this enzyme. If the test result is negative, penicillin or a penicillin derivative is usually the drug of choice, and no further testing is needed. If the isolate is producing β-lactamase, further antimicrobial susceptibility testing will be needed to select an alternative therapy. A β-lactamase test can be a useful part of a mastitis culture procedure to rapidly evaluate the appropriateness of penicillin therapy because penicillin is one of the most frequently administered antimicrobials.
In most cases, tests for antimicrobial-inactivating enzymes are not available. Therefore most routine susceptibility tests measure the degree of susceptibility of the isolate to each of several antimicrobials. The broth dilution susceptibility test system is the most precise method and is considered the reference method. This test is performed by introducing a standardized inoculum of an organism into a series of tubes (or wells in a microculture plate) containing serial dilutions of an antimicrobial in medium (Figure 18-14). The lowest concentration of antimicrobial that macroscopically inhibits growth of the organism is the minimal inhibitory concentration (MIC). The MIC of an antimicrobial for a given isolate represents the degree of susceptibility to the drug. If the antimicrobial is going to be used in therapy, the MIC must be achieved at the site of infection to effectively inhibit bacterial growth.
FIGURE 18-14 Broth dilution susceptibility test. The organism grew in broth containing antibiotic in the amounts of 0.5, 1, and 2 μg/ml, but growth was inhibited in the tube containing 4 μg/ml. Therefore the minimal inhibitory concentration is 4 μg/ml.
The most commonly used method of antimicrobial susceptibility testing in small laboratories is the agar diffusion test in which antimicrobial-impregnated paper disks are applied to the surface of agar that has been streaked with a standardized inoculum. As the antimicrobial is absorbed from the disk into the agar, it begins diffusing in a radial pattern (Figure 18-15). As the antimicrobial diffuses, it becomes more dilute, thereby creating a gradient effect of decreasing concentrations. The bacterial inoculum on the agar begins to grow in all areas except the places in which the antimicrobial concentration exceeds the MIC of the isolate. Zones of inhibition of growth can be observed around the disks. In carefully controlled studies, the diameters of the zones of inhibition have been correlated with MIC values. The results of the diffusion test can then be semiquantitatively interpreted, usually as susceptible, intermediate, or resistant.
FIGURE 18-15 Antibiotic diffusion susceptibility test. A, As antibiotic diffuses from the disk, the concentration of antibiotic is highest near the disk and logarithmically diluted as it diffuses radially into a larger area. At some point, the antibiotic is diluted below the minimal inhibitory concentration for the test organism, which allows the organism to grow. B, The resulting zones of inhibition are measured and interpreted with the use of Table 18-9.
The diffusion test is easy to set up, but it requires careful attention to detail to ensure that the results are accurate. Mueller-Hinton agar has been selected as the standard culture medium so that the composition of the agar can be more uniformly controlled. However, this medium will not support growth of some fastidious pathogens, such as Streptococcus, Listeria, Corynebacterium, Erysipelothrix, and Pasteurella spp. and some other gram-negative bacteria. For these bacteria, serum or blood enrichment is necessary. Therefore it may be more practical to use blood agar plates for susceptibility tests in low-volume laboratories. Results are usually comparable to those obtained with Mueller-Hinton agar; however, false-resistant results will often be obtained on the blood agar during testing of trimethoprim and sulfonamide activity. Fresh plates with the proper depth of agar must be used to avoid altering the kinetics of antimicrobial diffusion in a shallow or dehydrated plate.
Inoculum density should be standardized to prevent significant variations in zone sizes and misinterpretations. Susceptibility tests should always be performed with a pure culture of bacteria. Bacteria in mixed cultures can inhibit growth of slower-growing or fastidious organisms. Therefore if mixed cultures are tested, antimicrobial resistance of a pathogen may not be detected. Direct susceptibility testing of clinical specimens is discouraged, and, if performed, the results should always be verified by testing isolates in pure culture.
Standard antimicrobial disks should be purchased rather than attempting to prepare them from therapeutic drug solutions. It is important to make certain that the disks contain the same amount of antimicrobial as is listed in the interpretation chart (Table 18-9). Otherwise the results will not correlate with the desired MIC values. All cartridges of disks not in current use should be stored in a −20° C freezer; those currently in use should be kept in the refrigerator to prevent deterioration of the antimicrobials.
TABLE 18-9
Zone Diameter (Measured in Millimeters) Interpretive Standards for Susceptibility Tests
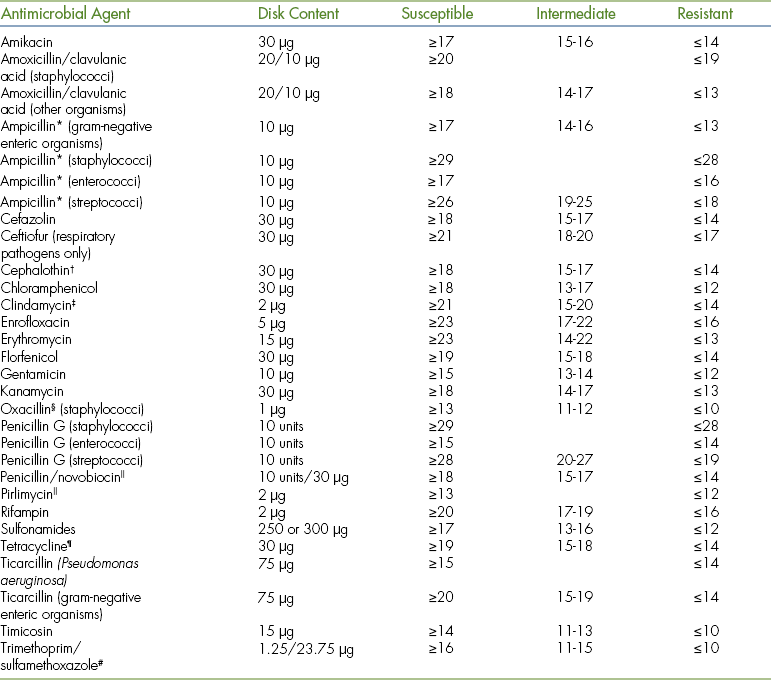
Ampicillin is used to test for susceptibility ot amoxicillin and hetacillin.
∗Modified from National Committee for Clinical Laboratory Standards document M31-A2, Table 2, pp. 55-59, 2002.
†Cephalothin is used to test all first-generation cephalosporins, such as cephapirin and cefadroxil. Cefazolin should be tested separately with the gram-negative enteric organisms.
‡Clindamycin is used to test for susceptibility to clindamycin and lincomycin.
§Oxacillin is used to test for susceptibility to methicillin, nafcillin, and cloxacillin.
 Available as an infusion product for treatment of bovine mastitis during lactation.
Available as an infusion product for treatment of bovine mastitis during lactation.
¶Tetracycline is used to test for susceptibility to chlortetracycline, oxytetracycline, minocycline, and doxycycline.
#Trimethoprim/sulfamethoxazole is used to test for succeptibility to trimethoprim/sulfadiazine and ormetoprim/sulfadimethoxine.
DIFFUSION TEST PROCEDURE
Select four or five well-isolated colonies of the same morphologic type from an agar plate culture. Touch the top of each colony with a wire loop, and transfer the growth to a tube containing 0.5 to 1 ml of saline solution or broth. The turbidity of the bacterial suspension should be equivalent to a MacFarland No. 0.5 standard, which is just turbid enough that a slight change in optical density of the tube is macroscopically visible. Within 15 minutes after preparing the inoculum suspension, dip a sterile nontoxic cotton swab into the suspension, and rotate the swab several times with firm pressure on the inside wall of the tube to remove excess inoculum from the swab. Then inoculate the agar plate by streaking the swab over the entire agar surface. Repeat the streaking procedure two or more times, rotating the plate approximately 60 degrees each time to ensure an even distribution of inoculum.
Test Procedure
Place the appropriate antimicrobial-impregnated disks, selected from the list in Table 18-9, on the surface of the agar. Note that some disks serve as class disks for a group of related antimicrobials, thereby reducing the need for testing each drug individually. The disks should be distributed evenly on the surface of the agar so that they are no closer than 24 mm from center to center. This is best accomplished with a dispensing apparatus. Using a sterile forceps or needle tip, gently press each disk to the agar to ensure complete contact. Because some of the drug begins to diffuse immediately, a disk should not be moved once it has come in contact with the agar. Finally, invert the plates, and place them in the incubator. The inoculated test plate is incubated in an aerobic atmosphere at 35° C for 18 hours.
Measuring Zones of Inhibition
After 16 to 18 hours of incubation of a properly inoculated plate, zones of inhibition around the disks should be uniformly circular with a uniformly confluent or almost completely confluent lawn of growth between zones. If only isolated colonies grow, the inoculum was too light, and the test should be repeated. The zone diameters should be carefully measured, including the diameter of the disk, and recorded to the nearest millimeter. The endpoint should be taken as the area showing no obvious visible growth (not including faint growth of any colonies that can be detected only with difficulty at the edge of the zone of inhibited growth). Large colonies growing within a clear zone of inhibition should be subcultured, reidentified, and retested. Strains of Proteus mirabilis and Proteus vulgaris may swarm into areas of inhibited growth around certain antimicrobials. The zones of inhibition are usually clearly outlined, and the veil of swarming growth is ignored. With the sulfonamides, organisms may grow through several generations before they are inhibited. Slight growth (80% or more inhibition) with sulfonamides is therefore disregarded, and the margin of heavy growth is measured to determine the zone diameter.
Results
Interpret the sizes of the zones of inhibition by referring to Table 18-9, and report results for the organism as susceptible, intermediate, or resistant to each antimicrobial.
INTERPRETATION AND LIMITATIONS
Antimicrobial susceptibility is not an all-or-none phenomenon. Instead, bacteria have a degree of susceptibility as defined by the MIC value. Therefore interpretation of diffusion test results as “zone or no zone” is unacceptable. Small zones may represent organisms that can tolerate higher levels of the antimicrobial (high MIC) than can be achieved at the site of infection. The measured diameter of the inhibition zone must be compared with the standards in Table 18-9 to determine whether the degree of susceptibility is comparable to the therapeutic level of the antimicrobial. The classification of “susceptible versus resistant” is a practical simplification of the various susceptibilities of organisms in terms of expected clinical response to standard dose therapy.
Although the diffusion test has been accepted as a standard test and is used in most veterinary microbiology laboratories, some limitations should be kept in mind. This test system is not applicable to slow-growing isolates or for use in special atmospheres. In many cases the interpretative criteria (see Table 18-9) are based on assumptions derived from knowledge of pharmacodynamics of antimicrobials in humans and efficacy in treating human pathogens. Dosages, absorption, and distribution of antimicrobials may be significantly different in the various species of animals. Levels of drug in tissues may significantly differ from levels in serum, such as low levels in cerebrospinal fluid. From the chart, a test result may be interpreted as susceptible, but the drug may not be able to penetrate to the site of infection. Conversely, ampicillin, for example, is concentrated several fold in the urine and may exceed the MIC value for an organism that has a small zone of inhibition. Therefore susceptibility test results are not absolute rules for antimicrobial therapy. They should be used as guidelines in selecting therapy in addition to clinical judgment and knowledge of the pharmacokinetics and pharmacodynamics of the antimicrobials.
Some veterinary microbiology laboratories are using microdilution tests to determine the MIC of clinical isolates. The MIC value can be compared with the levels of drug that can be obtained in the animal for final interpretation.
Intermediate indicates infection caused by a strain with antimicrobial agent MICs that approach usually attainable blood and tissue levels for which response rates may be lower than for susceptible isolates. This category implies clinical applicability in body sites in which the drugs are physiologically concentrated (e.g., quinolones and lactams in urine) or when a high dose of drug can be used (e.g., lactams).
Resistant strains are not inhibited by the usually achievable systemic concentrations of the antimicrobial when normal dosage schedules are used, and/or they may have MICs that fall within the range in which specific microbial resistance mechanisms are likely and clinical efficacy has not been reliable in treatment studies.
MYCOLOGY
The fungal agents that technicians will most likely be expected to identify in a clinical laboratory are dermatophytes and some yeasts. Dermatophytes can be readily cultured and identified in local laboratories. The invasive systemic mycoses are usually encountered less frequently and require specialized laboratory facilities and procedures for identification.
DERMATOPHYTES
The dermatophytes are keratinophilic (keratin-seeking) fungi that invade hair, nails, and the superficial layers of the skin but not living tissue. They may cause chronic, mild inflammation rather than intense inflammation. Lesions are usually characterized by spreading areas of pruritus and accumulating crusty debris. Lesions can be single or multifocal, and hair loss is variable. Because of the peripherally expanding nature of the lesion, it is also referred to as ringworm. Lesions can be notably different in various species of animals, from a dry, minimally inflamed lesion without hair loss on cats to a large, wartlike crusty lesion on ruminants.
Specimen Collection
Representative bits of hair, scale, or crust should be collected from the area of suspected dermatophyte lesions. Care must be exercised to prevent heavy contamination with saprophytic fungi or bacteria, which can overgrow the culture of the desired pathogen. If the lesion is likely to be contaminated, it should be cleansed gently with 70% alcohol before samples are collected. Various dermatophytes are best recovered from unique parts of the lesion, and so samples of scale, crust, and hair should be selected. Pluck broken, frayed, or distorted stubs of hair within the lesion area. Do not cut off hair to use as a specimen for culture. Brush-sampling is the preferred method for obtaining a dermatophyte culture specimen from asymptomatic cats. Use a sterilized (or new) toothbrush to vigorously brush suspected lesions or brush the entire animal for 2 to 3 minutes as if grooming. Then lightly press the brush against the surface of the culture medium several times for inoculation. Avoid pressing too firmly because the agar may tear and subsurface inoculum will not grow well. Crush and separate large pieces of debris when inoculating media. To culture nails suspected of having dermatophytic invasion, make fine shavings with a scalpel. Scatter the specimen over the entire surface of the culture medium. Press the hair and scale onto the agar, but do not bury them in the medium. If samples are not placed directly on culture media, they should be placed in a clean, dry envelope. Do not seal them in a tube or place in transport media. When moisture is allowed to accumulate, bacteria and yeast may overgrow.
Direct Examination
All specimens for fungal culture should be evaluated by direct microscopic examination. Direct mounts can be prepared by mixing a small portion of the material in two or three drops of 10% potassium hydroxide on a microscope slide. Addition of black India ink to the potassium hydroxide solution will facilitate observation of fungal elements in the specimen. Add a coverslip over the wet mount, and examine for the presence of delicate hyphae in skin scales or for the accumulation of spores on the surface of an infected hair (ectothrix).
Culture Procedure
Sabouraud dextrose agar is the standard medium for isolation of fungi and can be used for the successful isolation of dermatophytes. Selective media, such as Mycosel (BD Diagnostic Systems, Sparks, MD), are modified with antibiotics to inhibit bacteria and saprophytic fungi. A selective and differential medium (DTM [dermatophyte test medium]) is the most convenient medium available (Synbiotics Corp., San Diego, CA; BactiLab, Inc., Mountain View, CA). The medium contains a phenol red indicator, which turns red as a dermatophyte grows and produces alkaline metabolic products. Occasionally, dermatophytes do not sporulate as well on DTM as on Sabouraud medium, which can hinder identification. This problem can be overcome by using a supplemental medium, such as Rapid Sporulation Medium (BactiLab, Inc., Mountain View, CA), to enhance sporulation and identification of dermatophytes.
After the agar is inoculated, the cap should be replaced but left loose so that air exchange can occur. The culture is allowed to incubate at room temperature (22° C to 25° C). Placement on an open shelf or counter allows daily observation for up to 2 weeks for growth and color change in the medium. Dermatophytes are identified on the basis of both their gross colony characteristics and microscopic morphologic characteristics. Rate of growth, texture, pattern of growth, color of the colony, and pigmentation of the reverse of the colony should be noted. Most dermatophyte colonies are white or light shades of apricot, yellow, or cream to tan. Darkly colored brown or black fungi are likely to be contaminants. The dermatophytes rapidly change the color of the DTM agar to red, even before a colony is apparent. The red color may appear as early as 3 to 5 days after inoculation and rapidly spreads to most of the agar. Nonpathogenic fungi that grow on the medium do not produce an early color change, although the medium may become red after it is heavily overgrown.
Definitive identification of a dermatophyte and speciation require microscopic examination of wet tape mounts prepared in lactophenol cotton blue stain. The slide is examined for microconidia, macroconidia, hypha structures, and other identifying characteristics. The distinguishing morphologic features of the common dermatophytes are illustrated in most clinical microbiology textbooks.
SYSTEMIC MYCOSES
The three most important systemic mycoses are coccidioidomycosis, histoplasmosis, and blastomycosis. They are serious zoonotic agents; therefore the small laboratory should not attempt to isolate them in culture systems. All culture work must be carried out in an approved biohazard safety hood. The small laboratory is limited to direct microscopic examination of clinical material. Stained smears and wet mounts are useful diagnostic tools. The size and structural characteristics of these agents in the tissue or yeast phase can serve as specific identifying criteria. If cultures are desired, the clinical material should be inoculated onto isolation media, and the inoculated tubes should be shipped to a reference laboratory. Delays in inoculation of isolation media will result in loss of viability and overgrowth of the sample by contaminating bacteria.
Sporotrichosis is a chronic infection characterized by nodular lesions of the skin or subcutaneous tissues. Sporothrix schenckii usually gains entrance by traumatic implantation into the tissue. Therefore there is little danger of contagion except from cats that frequently harbor large numbers of yeast cells in lesions. The agent can be observed in direct examinations of tissue and exudates or isolated and identified by routine methods.
YEASTS
There are only a few clinical situations in which yeasts are significant veterinary pathogens. In general, animals seem to be much more resistant to yeast infections than humans. If yeasts are suspected, a direct smear of exudate should be stained for microscopic examination. The best approach to the isolation of yeast is to inoculate blood agar and Sabouraud dextrose agar. The blood agar is incubated at 35° C, and the Sabouraud agar at room temperature. Media should be held at least 2 weeks before discarding them as negative. Therefore agar slants in tubes are preferable to plates because they do not dehydrate as rapidly. Culture and identifying methods for the most common pathogenic yeasts are described.
Malassezia pachydermatis
Malassezia pachydermatis is frequently found in cases of external otitis and is emerging as a cause of seborrheic and hypersensitivity reactions associated with dermatitis. It is readily observed in smears of exudate stained with Gram stain as an oval, bottle-shaped, monopolar budding yeast (Figure 18-16). Isolation in cultures can be difficult, but is best attempted by inoculating Sabouraud dextrose agar and incubating it at 35° C in a carbon dioxide incubator.
Cryptococcus neoformans
In direct smears, Cryptococcus neoformans may be presumptively identified by its abundant capsular material. Negative staining with India ink provides a black background that outlines the clear capsule for easier observation. It can be isolated on Sabouraud dextrose agar or blood agar. Cryptococcus neoformans can be differentiated from other nonpathogenic yeasts because it will grow at 35° C to 37° C and is urease-positive. The urease test is performed by inoculating the same urea agar slant that is used for differentiating bacteria.
Candida albicans
Candida albicans is a frequently encountered opportunistic fungal pathogen. Infections usually involve mucous membranes. In direct microscopic examinations of wet mounts, unicellular budding yeasts without a capsule are observed. Limited hypha development may also be observed. Candida albicans is readily isolated on Sabouraud dextrose agar or blood agar. Definitive identification can be made by demonstration of germ tube (pseudohypha) development after 3 to 4 hours of incubation in rabbit serum.
VIROLOGY
Laboratory diagnosis of viral diseases depends on the examination of appropriate specimens for evidence of viral infection and then attempting to correlate infection with disease. Because of the nature of viruses and the special laboratory procedures required, most clinical laboratories perform only limited viral diagnostic procedures. Viruses are obligate intracellular parasites. Therefore they are best recovered from living tissue. Isolation of viruses in dead tissues is reduced in direct proportion to the length of time since death and the extent of autolysis.
VIRUS ISOLATION
Isolation and identification of viruses depend on the inoculation of susceptible living cells for cultivation of the virus. The major methods of providing these living cells include monolayer cell cultures, embryonated hen eggs, and laboratory animal inoculation. These techniques require special laboratory facilities and up to 2 weeks for recovery of a virus. Special care must be taken to ensure that a viable virus is delivered to the laboratory for isolation attempts. Therefore specimens should be collected early in the course of infection when viruses are most numerous. At death, virus numbers in the tissues are usually reduced, and extensive postmortem bacterial growth is often present. The presence of bacteria in the sample can be damaging to the cells that are being used as recovery hosts for the virus. Therefore specimens should be carefully collected, refrigerated, and promptly delivered to the diagnostic laboratory. Special arrangements should be made with laboratory personnel so that they can be prepared to process the sample when it arrives. Transport media containing antibiotics and virus-stabilizing agents are often available from viral diagnostic laboratories.
MICROSCOPIC EVALUATION
In some cases, viral infection can be identified by microscopic examination of infected tissues for the presence of pathognomonic changes or of body fluids for the presence of viral particles. Some viral infections produce distinct changes in host cells, such as the intranuclear inclusions of infectious canine hepatitis, which can provide a definitive diagnosis. Electron microscopic examination of body fluids and washings allows the direct visualization of viral particles. This procedure is often used for diagnosis of respiratory and enteric viral infections because it is rapid and can detect mixed viral infections. The procedure does not require viable viruses, as long as they have retained their structural integrity. Direct electron microscopic examination is limited to cell-free viruses, such as those found in body fluids, rather than examination of infected tissue. Most diagnostic laboratories perform negative-contrast staining; therefore virus identification is limited to morphologic identification. If immunoelectron microscopy procedures are available, the type of virus within a group can be identified.
ANTIGEN DETECTION
Antigen detection methods are the most frequently used viral diagnostic procedures. Advantages of antigen detection compared with viral isolation include rapid results, less expense, less technically demanding procedures, and less dependence on the presence of viable virus in the sample because most viral antigens remain intact after death of the virus. The most common methods of antigen detection are immunohistochemical staining, hemagglutination, and solid-phase immunoassays. Hemagglutination assays are not easily standardized for use in clinical laboratories.
Immunohistochemical Staining
Examination of selected clinical specimens by using a specific antibody labeled with a marker as a probe to identify viral antigen is a rapid and highly reliable diagnostic method. Markers on the antibody can include fluorescent compounds, enzymes, or colloidal gold. The two most important limitations of these procedures are the need for specific antibodies to the viral antigens and a system for detecting the marker. For immunofluorescence, a microscope with an ultraviolet (UV) light source is required.
In the immunoperoxidase method assay, the specific antibody is labeled with an enzyme (usually horseradish peroxidase) instead of a fluorescent label. Attachment of the antibody to tissue sections or smears is detected by using a chromogenic substrate that is deposited at the site of the enzyme-antibody attachment and produces a slide similar to other differential stains. The slide can then be examined by light microscopy. Several of these assays have been developed by using monoclonal antibodies to detect viral antigens in tissue, including examination of formalin-fixed specimens.
Solid-Phase Immunoassays
The use of enzyme immunoassays is based on the excellent ability of this method to be adapted to kits that meet practical needs, such as minimizing reagent cost, reducing technician time required to perform the assay, and simplifying the test protocol. These test systems are frequently referred to by the acronym ELISA (enzyme-linked immunosorbent assay). As a result, several kit systems are available for the detection of antigens from viruses and other infectious agents (Figure 18-17) and for detection of antibodies specific for infectious agents. Three typical configurations of ELISAs are illustrated in Figure 18-18. At present, there are several different solid phases commonly used in these assays. The most common solid phase for multiple tests is the microtiter well, but for individual clinical tests, dipstick, immunofiltration, immunomigration, and immunochromatographic formats are more efficient. Immunoassay diagnostic kit manufacturers usually offer technical assistance to kit users. If you have questions about a protocol or test result or are experiencing difficulty in conducting a test after carefully reading and following the manufacturer’s instructions, call the company’s technical services department and ask for assistance. Most companies provide a toll-free telephone number to facilitate this and will welcome an opportunity to assist you in using their products.
FIGURE 18-17 Solo Step CH (Heska Corp.) lateral flow immunoassay cassette for the detection of antigens produced by canine heartworms in serum. A positive test result has developed in the left cassette, and a negative test result is shown in the cassette on the right.
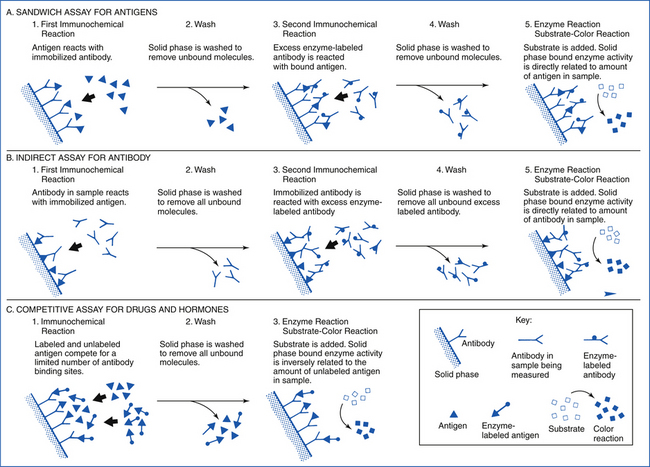
FIGURE 18-18 Enzyme immunoassay configurations and major steps in assay procedures. (Courtesy E.S. Bean and E.T. Maggio, San Diego.)
Assays that have been successfully developed for detection of viral antigen include feline leukemia virus (FeLV) in blood (Assure FeLV and Witness FeLV, Synbiotics Corp.; SNAP FeLV Antigen Test, IDEXX Laboratories, Inc., Westbrook, ME), FeLV in saliva (Assure FeLV), canine parvovirus in feces (Assure Parvo, Synbiotics Corp., San Diego, CA; SNAP Parvo Antigen Test, IDEXX Laboratories, Inc., Westbrook, ME), and influenza virus in respiratory tract specimens (Directigen Flu A, BD Diagnostic Systems, Sparks, MD). Kits are also available to detect bacterial antigens (Escherichia coli Antigen Test [K99 Pilitest], VMRD, Inc., Pullman, WA) and heartworm antigens (Solo Step CH, Heska Corp., Loveland, CO; SNAP Heartworm Antigen Test, IDEXX Laboratories, Inc., Westbrook, ME; Witness HW, Synbiotics Corp., San Diego, CA).
SEROLOGY
Serologic testing is an important tool in the diagnosis of infectious diseases. Serology is used extensively in the diagnosis of viral infections and in disease surveillance programs. Serologic tests for detecting a specific antibody have been developed for nearly every infectious agent. However, this indirect approach to diagnosing infection on the basis of a host immune response after exposure to an infectious agent has limitations. Serologic tests may vary in their sensitivity and specificity because of the type of test, immunogenicity and cross-reactivity of the antigen, and biologic variation of immune responses by individual animals. Nevertheless, serologic tests often remain the best diagnostic test available. In veterinary medicine, serologic tests are often required by regulatory agencies to prove an animal is not infected or a carrier of a particular infectious agent.
ANTIBODY RESPONSE TO INFECTION
The chronology of exposure to an infectious agent and subsequent development of an antibody response are illustrated in Figure 18-19. After exposure, there is a variable period of incubation, followed by clinical illness. During the time of clinical illness, the animal may be febrile, and this is when the greatest number of microorganisms are present. Therefore it is more likely to transmit the infectious agent, and the best samples can be obtained for recovery of the agent at this time. After a variable period from onset of clinical signs (usually after 5 to 10 days), the animal begins to produce antibodies to the agent. Continued production of antibodies after the animal is no longer ill, referred to as the convalescent phase, will cause the titer (serum antibody level) to rise for 1 month or longer.
INTERPRETATION OF SEROLOGIC TEST RESULTS
The presence of antibodies to a particular organism in an animal serum sample is not always a simple and absolute diagnosis of current or recent clinical illness caused by the organism. Sources of antibody in the serum of an animal include convalescent antibody after clinical disease or persistent infection, antibody response caused by exposure to an organism without clinical disease occurring, active immune response to vaccination, and passive transfer of maternal antibodies to the neonate. Therefore detection of antibodies in a single serum sample often has limited importance unless the finding can be correlated with other clinical indications of the disease. In most situations, it is necessary to collect two samples—the first one early in the course of the illness (acute) and the second one (convalescent) at a later time—to demonstrate a change in antibody titer that would indicate recent antigenic stimulation. The change must be at least two dilution increments (usually fourfold), such as an increase from 1:4 to 1:16 or greater, to be considered diagnostically significant. This change in titer is known as a seroconversion.
The presence of antibody in serum may be reported quantitatively as a titer or qualitatively as positive or negative. Qualitative tests have less diagnostic value than those tests that report titers, unless the result is negative, in which case the test can exclude some agents from the differential diagnosis unless the serum was collected early in the course of a disease. Most qualitative tests, such as immunodiffusion for equine infectious anemia (Coggins test) and bovine leukosis, are surveillance tests to identify animals that have been exposed and are possible carriers. Occasionally, a single, high-titered serum can aid in establishing a diagnosis, but it always leaves some question of whether the titer increased in association with clinical disease or is a stable, convalescent high titer.
When an animal is exposed to an infectious agent, the first antibodies produced are usually of the immunoglobulin (Ig) M class, with later antibody production being IgG. Some tests are designed to differentiate these antibody class responses, which can be helpful in confirming a diagnosis. If the antibody response to a virus is of the IgM type, the animal has recently been exposed. If the response is mostly IgG, it was probably exposed several weeks to months previously. Often IgM antibodies are less specific than IgG and may cross-react, resulting in false-positive test results. The test can be modified to exclude IgM reactions to prevent this from happening.
Serologic tests are frequently relied on as the only diagnostic procedures for abortion cases. Results are often difficult to interpret for two reasons. First, by the time abortion occurs because of infection of the fetus and its subsequent death, the dam is already in the convalescent phase of antibody production, so a seroconversion cannot be demonstrated. Second, the stress of abortion or other clinical illness may trigger reactivation of a latent viral infection. This provides an antigenic stimulus to the animal’s immune system and increased antibody production. However, it is specific for the latent viral infection, not the current clinical illness.
COLLECTION OF SERUM
Technicians will frequently be responsible for collecting and handling serum samples. Improper methods can reduce the value of test results. The timing of serum collection is important (see Figure 18-19). The first sample should be collected as soon as possible after the animal begins to show signs of clinical illness. If the sample is not collected within the first 5 to 7 days, antibody titers might have already risen. The convalescent sample should be collected at least 10 days after the acute sample. Generally, 14 to 21 days between samples is recommended, but in young animals with a less efficient immune response, up to 4 to 6 weeks may be necessary to demonstrate a seroconversion. The technical procedures of serologic tests are difficult to duplicate exactly; therefore results of tests performed on different days or in different laboratories should not be compared to demonstrate a seroconversion.
Blood should be collected aseptically by venipuncture (see Chapter 28) with a new needle and syringe or evacuated clot tube (Vacutainer tubes and SST Sterile Serum Separation Tubes, BD). Do not use recycled, washed needles, syringes, or tubes. Residual detergent may cause hemolysis or may be toxic to cell cultures used in the test systems. Blood should be allowed to stand at room temperature until a clot has formed; the serum should then be removed from the clot and placed in a new, sterile tube. It may be necessary to separate the serum from the clot by centrifugation to prevent transferring cellular components of the blood. Anticoagulants should not be used to collect plasma because they can be toxic in some test systems. Avoid freezing the whole blood because that will cause hemolysis. The transfer of serum from the original blood tube must be performed aseptically. Contaminating microorganisms can grow rapidly in serum and alter the immunoglobulin molecules. Therefore serum samples (separated from the clot and cells) should be refrigerated until testing is commenced, if within 72 hours. For longer periods of storage, serum may be preserved in a freezer (−20° C). Frozen serum samples should be packaged and shipped with adequate insulation and ice to prevent thawing before arrival at the laboratory. If a second sample will be collected, the first sample should be held until both can be sent to the laboratory together for valid paired testing.
For shipment, the tubes of serum must be carefully labeled and packed so that they will not leak or break. When environmental temperatures are high, refrigerant and insulating materials should be used to preserve samples during transport.
SEROLOGIC TEST PROCEDURES
Only a few serologic tests have been standardized and packaged for efficient use in veterinary practice laboratories. When kits are used, it is important that the directions be followed carefully because modification of any part of the procedure can cause spurious results. A positive (or known titer) serum and a negative serum should be kept on hand (if not already included in the kit) and included in the test each time it is performed to verify accuracy of the results. Serology (antibody-detecting) kits are available for feline immunodeficiency virus (SNAP FIV Antibody/FeLV Antigen Test, IDEXX Laboratories, Inc., Loveland, CO), canine brucellosis (D-Tec CB, Synbiotics Corp., San Diego, CA), paratuberculosis (Mycobacterium paratuberculosis Antibody Test, ImmuCell Corp., Portland, ME), feline heartworm (Solo Step FH, Heska Corp., Loveland, CO), and canine borreliosis and ehrlichiosis (Canine SNAP 3Dx Test, IDEXX Laboratories, Inc., Westbrook, ME). The most frequent mistakes made in performance of serologic tests include the use of dirty or contaminated equipment, failure to adhere to instructions (especially incubation times), and unfamiliarity with interpreting results.
CLINICAL IMMUNOLOGY
PURPOSE OF EVALUATING IMMUNE SYSTEM FUNCTION
As the function and complexity of the immune system have been elucidated, an increasing need for laboratory diagnosis of dysfunction of the immune system has been recognized. Clinical immunologic laboratory support has become a well-established part of the diagnostic services and patient care procedures in human medicine. Similar assays are becoming available in veterinary medicine, but few tests are readily available for use in practice laboratories.
Several types of diagnostic problems present the need for laboratory evaluation of possible immunologic disorders. These disorders can be classified into four types: allergies, autoimmune diseases, immunodeficiencies, and immunoproliferative diseases. In young animals, disorders of the immune system may be observed as developmental defects (sometimes inherited) or may be caused by a failure of passive transfer of maternal antibodies. However, immune function abnormalities can be observed in animals of all ages because of effects of aging, various drugs, or environmental exposure to immunomodulating toxins. A few simple procedures will be discussed in this section. The Recommended Reading list should be consulted for detailed and theoretic discussions of other immune system function assays.
LABORATORY TESTS FOR IMMUNOLOGIC DISORDERS
Allergies are usually diagnosed by physical examination, history, and response to intradermal inoculation of test antigens.
Autoimmune disorders can be diagnosed more efficiently by evaluating lesions obtained by biopsy. Morphologic evaluation, combined with various immunohistochemical staining procedures, provides the most definitive diagnosis. It is best to consult referral laboratories to learn which specimens they are able to analyze and how the sample should be submitted.
Immunoproliferative diseases may result in the production of abnormal amounts or unusual types of immunoglobulin proteins, referred to as gammopathies. The techniques of electrophoresis (usually on cellulose acetate) and immunoelectrophoresis are the laboratory tests most frequently used to diagnose these disorders. Abnormalities of routine laboratory tests, such as total protein in serum or urine and albumin/globulin (A/G) ratios, frequently indicate a need for these specialized tests. Because of the infrequent demand for these tests and the cost of electrophoresis equipment, most practices request these services from referral laboratories.
Immunodeficiency or immunosuppressive disorders are the most frequently encountered immune system dysfunctions. Most assays of immune cell function require specialized equipment and procedures that are usually available in only a few reference laboratories. Some cellular function assays require submission of viable cells for evaluation. Recently developed assays are used to detect and quantify receptors on the surface of cells, such as CD18 deficiency in Holstein calves. Assays are being developed in research laboratories for genetic analysis of lymphocytes to detect both immunodeficiency and immunoproliferative dysfunctions. Determination of immunoglobulin levels, as an indication of B-lymphocyte function or passive transfer status, is a readily available laboratory test that will be discussed.
FAILURE OF PASSIVE TRANSFER
The newborns of most domestic animals depend on absorption of maternal antibodies from colostrum for protection from infectious diseases. Failure of the neonatal animal to obtain and absorb adequate colostral immunoglobulins is frequently associated with increased morbidity and mortality from bacteremia and common neonatal diseases. Determination of the passive transfer status of foals and calves is an important evaluation that can modify patient care. Although total serum protein levels can indicate relative levels of immunoglobulins, this indirect measurement is subject to considerable variability. The reference method for quantitating serum immunoglobulins is the radial immunodiffusion (RID) test. The RID test consists of agar containing antisera specific for a particular antigen. In this case, the antigen is a particular immunoglobulin class, such as IgG. Each test requires that quantitated standards be tested at the same time for comparison. Therefore if single samples are being tested, the cost per sample will be more, and it might be more cost effective to send samples to a referral laboratory. (Commercially produced RID kits for canine, feline, bovine, and equine immunoglobulins are available from VMRD, Inc.)
Passive transfer status of neonates can be evaluated rapidly and inexpensively in the practice laboratory. Field test kits that quickly assay plasma or blood concentrations of IgG are available for detecting failure of passive transfer in calves, foals, and llamas (VMRD, Inc., Midland BioProducts Corp., and IDEXX Laboratories, Inc.).
NOSOCOMIAL INFECTIONS
An infection that results from exposure to an infectious agent while the patient is in the hospital is considered to be nosocomial (hospital acquired). The nosocomial infection may become clinically apparent during hospitalization or after discharge from the hospital. Infections that are incubating at the time of admission are defined as community acquired, even though they become clinically apparent only during hospitalization. In veterinary practices, in addition to nosocomial infections of patients, zoonotic infections transmitted to the staff and clients can be considered part of the biosafety problem.
The incidence of nosocomial infections in veterinary hospitals is not well documented, but it is probably similar to the incidence in human hospitals, which ranges from 3% to 5% of hospitalized patients. The incidence is known to vary with the size and type of hospital and the sophistication of infection control programs. The highest incidence rates are observed in large referral or teaching institutions. The most important institutional risk factors appear to be an increased number of personnel having contact with the patient and an increased mean number of hospital days per patient. Therefore these infections are becoming an increasingly significant problem in teaching hospitals and large group practices in which intensive medical and surgical care is available through a large staff. These institutions also tend to care for patients with more critical and chronic diseases. Because these patients have increased susceptibility to opportunistic infection, the occurrence of a nosocomial infection does not necessarily indicate negligence by the hospital staff.
The stressed condition of hospitalized animals often makes them more susceptible to infections than the general population. Factors that predispose an individual animal to nosocomial infection may include extremes of age (old age or neonatal period); debilitating disease; diagnostic or medical procedures, such as urethral catheterization or immunosuppressive therapy (corticosteroids or cytotoxic drugs); long periods of hospitalization; antimicrobial therapy; presence of other infections; and presence of surgical hardware and drains. For some of the infectious diseases, such as canine distemper, the immunization status of the patient will determine its susceptibility.
Many nosocomial infections are caused by opportunistic microorganisms that infrequently cause infections in healthy animals. However, when the high-risk patient (increased susceptibility) is exposed, the agent can cause disease. Other highly virulent organisms, such as canine parvovirus and Salmonella spp., may cause disease in otherwise healthy patients. The greatest impact on the incidence of nosocomial infections can be made by understanding the sources of exposure and spread of these infectious agents. Microorganisms enter the hospital in or on people, animals, inanimate objects, air currents, and occasionally insects. Within the hospital, they are maintained in or on a variety of reservoirs, including patients with infections, healthy carriers, inanimate surfaces, solutions, food, staff, and insects. From these reservoirs, the potential pathogens may be disseminated by contact or by air to hospital personnel and patients.
The most important vehicles for the spread of nosocomial agents are the hands of hospital personnel. Therefore proper and frequent hand washing is the most important strategy for reducing the rate of nosocomial and zoonotic infections.
AGENTS OF NOSOCOMIAL INFECTIONS
Bacteria are the most frequent infectious agents involved in nosocomial infections, but viruses, fungi, and protozoa can also be involved. The commonly involved bacteria tend to be somewhat environmentally resistant, and the increasing use of antibiotic therapy has led to an increased level of antibiotic resistance by nosocomial agents. In the presence of limited antibiotic use, penicillin-susceptible, gram-positive cocci of the genera Streptococcus and Staphylococcus are the most common agents. With increased antibiotic use, penicillin-resistant Staphylococcus is frequently detected. Currently, the major problems are with multiple antibiotic-resistant, gram-negative bacilli, such as Escherichia coli and Salmonella, Klebsiella, Enterobacter, Serratia, and Pseudomonas spp. Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci are beginning to emerge as the next wave of serious nosocomial agents. Colonization (growth and establishment) of the body surfaces of the patient by these nosocomial bacterial pathogens is usually a prerequisite to infection. Therefore the patient becomes its own major reservoir of these agents once the organisms are transferred to it during hospitalization. Common reservoir sites are the lower intestinal tract and the naso-oropharyngeal area. Antimicrobial chemotherapy is the most important predisposing factor that allows the patient to become colonized because the antimicrobial suppresses normal flora and selects for resistant organisms. The most frequent locations of nosocomial bacterial infections are the urinary and respiratory systems and surgical wounds. Occasionally, infections become bacteremic. Clostridial enterocolitis in dogs has been identified as a nosocomial problem in several large teaching hospitals.
Viral infections are the second most frequent group of nosocomial infections in hospitalized patients, but are probably the most important nosocomial infections of outpatients. This is because some of these agents are easily transmitted and are highly infectious to susceptible but otherwise healthy animals. Diseases in this group include canine distemper, canine parvovirus, feline panleukopenia, and respiratory viral diseases of all animals (feline viral rhinotracheitis, equine influenza, infectious bovine rhinotracheitis, canine tracheobronchitis, etc.).
Other viral diseases that are not as contagious can be transmitted to susceptible patients at a veterinary hospital if adequate preventive measures are not followed. The resulting disease would be classified as a nosocomial infection. Examples include transmission of viruses of feline leukemia and equine infectious anemia by blood transfusions.
Fungi have rarely been recognized as nosocomial agents in veterinary medicine. As the awareness level of this problem increases, no doubt more fungal infections will be identified, especially with improved intensive care of immunocompromised patients. Yeasts, such as Candida albicans, have occasionally been identified. The dermatophytes do not cause life-threatening infections and are usually overlooked, but they can also be transmitted as nosocomial agents to both patients and hospital staff.
Infection of animals by protozoan pathogens can be acquired in the veterinary hospital. Cryptosporidium spp. are relatively resistant to disinfectants and have been the cause of nosocomial enteritis. If litter pans are not properly cleaned, other animals and hospital staff could be exposed to toxoplasmosis. Hemotropic parasites (Mycoplasma haemofelis, Anaplasma, Ehrlichia, and Babesia) can be transmitted to other patients by blood transfusions or surgical instruments that have not been adequately washed and disinfected.
RECOGNITION AND CONTROL OF NOSOCOMIAL INFECTIONS
Technicians frequently have the opportunity to be the first persons to recognize a nosocomial infection problem by taking note of an unusual number of isolations of a single pathogen or the appearance of an unusual antibiogram. Excellent diagnostic microbiology laboratory support for accurate identification and antimicrobial susceptibility testing of infectious agents is an essential tool for defining the scope of the nosocomial infection problem.
Measures that can help reduce or control nosocomial infections include sterilization of equipment and supplies, aseptic treatment techniques, isolation practices, judicious use of antimicrobial drugs, diligent hand washing between examining patients, disposal of trash, and establishment of sound housekeeping protocols. These protocols should provide for adequate cleaning, disinfection, and maintenance of patient-care equipment and environmental surfaces, such as cages, tables, floors, and walls.
The control measures that would be necessary to prevent all nosocomial infections are impractical and not economically feasible. Hospitals contain patients with increased susceptibility to infection, and short of total isolation in a controlled environment, few measures are biologically guaranteed. The risk for each patient of acquiring an infection must be individually evaluated. If the risk is sufficiently great, reverse isolation procedures may be indicated to prevent the patient from being exposed to potential pathogens. If active or passive immunizing products are available, their use should be encouraged. Routine immunization programs can effectively prevent many of the viral infections that have been discussed.
ANTISEPTICS, DISINFECTANTS, AND STERILIZATION
The effective use of antiseptics, disinfectants, and sterilization procedures is an important factor in preventing nosocomial infections. Microorganisms vary widely in their susceptibility to germicidal treatments. Bacterial endospores are the most resistant type. In descending order of relative resistance after bacterial spores are mycobacteria, fungal spores, nonenveloped viruses, vegetative fungi, enveloped viruses, and vegetative bacterial cells. The differences in chemical resistance of various vegetative bacteria are relatively minor, except for the mycobacteria, which are relatively resistant to many disinfectants. Other factors that may have a significant effect on the results of disinfection are concentration of the chemical, length of exposure to the chemical, amount of organic matter (soil, blood, feces) present, type and condition (porosity, cracks, etc.) of the material to be disinfected, ambient temperature, and the nature and number of contaminating microorganisms. Good physical cleaning will allow better penetration of crevices and porous material. Generally, the higher the concentration of the chemical agent or the longer a process is continued, the greater its effectiveness. For temperature-based procedures, increasing temperatures will usually increase efficacy.
Veterinary practices and hospitals should select disinfectants that are registered by the U.S. Environmental Protection Agency (EPA) and labeled as one-step cleaner-disinfectants for use in hospitals. The label should indicate that these products are effective in hard water up to 400 ppm hardness and in the presence of 5% serum. Most nonporous surfaces can be efficiently cleaned and disinfected with the newer combinations of twin-chain quaternary ammonium compounds (C8/C10 dimethylammonium chloride) and alkyldimethylbenzylammonium chloride. Product labels must always be consulted for proper mixing and diluting instructions and intended applications. Chemical incompatibilities may occur if products are mixed. Therefore do not attempt to combine germicides or alter treatment procedures from the manufacturer’s specifications.
BIOLOGIC SAFETY
Potential hazards in the veterinary hospital may be associated with infectious or chemical materials, physical facilities, and animal handling. Management should develop a comprehensive safety program that includes consideration of these dangers and preparedness for fire, accidents, and other disasters. This discussion will deal primarily with biologic hazards related to infectious agents in the laboratory and hospital.
Each individual has responsibility for protecting himself or herself and others from accidental infection. Laboratory coats should be worn to prevent contamination of street clothes and dissemination of pathogens to homes and families. Disposable examination gloves should be worn when handling heavily contaminated materials. Good hand-washing procedures should become a habit in the laboratory—between procedures if there is a chance of contamination and always before leaving the laboratory. Mouth pipetting should be prohibited in laboratories handling infectious material. Automatic or bulb pipetting devices should be used. Syringes and needles are poor substitutes for pipettes because they tend to favor creation of aerosols that may be inhaled. There is also the inherent danger of self-inoculation when handling syringes and needles. Self-inoculation must be guarded against, both in the laboratory and when inoculating animals. Centrifuge accidents, which may produce infectious aerosols, should be prevented by selecting compatible tubes, performing proper balancing, and not exceeding recommended centrifugal forces.
Good housekeeping procedures that will maintain a neat, uncluttered work area should be adopted. Eating, drinking, and smoking should not be allowed in work areas, even during break periods when there is no laboratory activity.
Immunization of personnel is recommended when they are at increased risk of infection. A minimal prophylactic immunization for all personnel employed in veterinary hospitals and laboratories should include rabies vaccine and tetanus toxoid. Other immunization products may be recommended in areas in which there is an unusually high risk of exposure to a particular infectious agent.
Primary containment equipment and laboratory design features are important factors in biologic safety. Directional airflow should be from clean areas to areas of contamination and should then be exhausted from the building without recirculation. Small veterinary laboratories and hospitals usually cannot justify the cost of biologic safety cabinets for diagnostic procedures. However, some infectious agents are of sufficient hazard that they must be handled only in laboratories with special design features, including biohazard cabinets. Zoonotic pathogens that small laboratories should not attempt to isolate include the agents of anthrax, brucellosis, plague, tuberculosis, tularemia, and systemic mycoses.
The clinical laboratory has a responsibility to decontaminate potentially infectious materials and wastes before they are discarded. Many states have adopted statutes and regulations that stipulate how hazardous waste materials must be handled. Clinical veterinary laboratories are required to comply with these rules and EPA and U.S. Occupational Safety and Health Administration (OSHA) requirements (see Chapter 6). All diagnostic specimens (swabs), inoculated media, viable cultures, glassware, instruments, and equipment should be considered to be contaminated. Decontamination methods should be applied before waste materials are discarded or reusable products are cleansed. The most practical decontamination procedure for most infectious wastes is use of the steam autoclave. Other methods include physical procedures (incineration, boiling, irradiation) and chemical agents (phenolics, hypochlorites, formaldehyde).
1. Anonymous. Difco & BBL manual. Sparks, Md: BD Diagnostic Systems; 2007.
2. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; Approved Standard, ed 2. Wayne, Pa: Clinical and Laboratory Standards Institute; 2002. CLSI Document M31-A2,
3. Detrick, B., et al. Manual of molecular and clinical laboratory immunology,, ed 7. Washington, DC: American Society for Microbiology; 2006.
4. Greene, C.E. Infectious diseases of the dog and cat, ed 3. Philadelphia: Saunders Elsevier; 2006.
5. Hirsch, D.C., et al. Veterinary microbiology,. Ames, Iowa: Blackwell Pub; 2004.
6. Larone, D.H. Medically important fungi: a guide to identification,, ed 4. Washington, DC: American Society for Microbiology; 2002.
7. Murray, P.R., et al. Manual of clinical microbiology,, ed 9. Washington, DC: American Society for Microbiology; 2007.
8. Research Committee of the National Mastitis Council. Laboratory handbook on bovine mastitis. Madison, Wis: National Mastitis Council; 1999.
9. Winn, W.C., et al. Color atlas and textbook of diagnostic microbiology, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2006.
 TECHNICIAN NOTE
TECHNICIAN NOTE