OTHER FOOT DISORDERS
Osteochondrosis/osteochondritis
Osteochondrosis is the generic term used to describe a group of syndromes that share the common pathology of idiopathic bone disease (Ekman & Carlson 1998). The pathogenesis of osteochondritis is unclear, but all presentations are characterised by an interruption of normal enchondral ossification, together with a greater or lesser degree of focal death of the local trabeculated bone (Caselli et al 1998). A number of examples of osteochondritis affecting specific bone sites are named eponymously to the physician who first reported the disease. The onset of osteochondritis generally occurs during childhood, especially during times of rapid growth, but the full effects of the resultant joint and bone damage may not become apparent, and problematic, until adulthood. Osteochondritis may give rise to focal bone deformation during the healing phase of the disease, where the forces imposed by body mass impinge on the areas of abnormal ossification or diseased bone, causing a change in the local bone architecture. The altered shape of the involved bone sites may give rise to pathologies later in life.
Classification
Osteochondritis may be classified in relation to the anatomical location of the enchondral ossification defect (Griffin 1994):
Alternatively, osteochondritis may be classified by the effects brought about by local forces on the area of diseased enchondral bone:
Aetiology
The true aetiology of the various presentations of osteochondritis is unknown, but they have been linked to hereditary factors, local trauma, nutritional factors and local ischaemia within the affected area of bone (Ekman & Carlson 1998, Walsh & Dorgan 1988).
Diagnosis
Plain radiographs are usually used to diagnose osteochondritis, but minor or early-stage lesions may be overlooked as they are difficult to visualise on a plain radiograph. Lesions are identified readily using bone scan, computerised axial tomography scan and magnetic resonance imaging (Bohndorf 1998).
Differential diagnosis
The differential diagnosis for all the osteochondritides can include osteomyelitis, bone tumours and fractures (Caselli et al 1998).
Treatment
The treatment of all presentations of osteochondritis focuses on rest and immobilisation to allow the affected bone to heal with no or only minimal distortion, together with the use of painkillers (such as NSAIDs), as necessary, during the acute or early presentations of the disease.
Rest and immobilisation of the affected bone is essential:
Early initiation of treatment is especially important where the involved osteochondritic bone forms part of a joint, in order to reduce the likelihood of later osteoarthritic changes, pain and dysfunction of the affected joint.
The format, intensity and duration of the regimen of rest is tailored to the presenting problem, and can be achieved by a variety of means, such as rigid splinting, soft splinting and strapping, and other means to reduce weight bearing, and traction and compressive forces on the involved bone.
CASE STUDY 4.3 PRESENTATION IN AN ADULT
OSTEOCHONDRITIS DISSICANS:
A 54-year-old woman presented with a 10-month history of pain in the right foot. She worked as a radiography assistant in a local hospital. She recalled that on 16 December of the previous year, whilst walking along a corridor from the x-ray department to one of the wards to deliver a patient’s radiograph, she experienced a sudden and severe shooting pain in the right forefoot. The pain located to the base of the second toe, and continued throughout that day, and she was unable to walk without limping heavily on the right foot. That night, she applied ice to the foot, and self-medicated with ibuprofen. By the next day, the pain had not reduced at all. There was some mild local swelling in the area of the right second metatarsophalangeal joint (MTPJ). The patient reported that a radiograph, taken within 24 hours of the incident, failed to show any bone lesion. It was assumed by the GP that she had suffered some form of foot strain. The swelling cleared within 3 weeks, and the severe pain gradually subsided over the next 3 months, although the right second toe continued to be very painful on movement at the second MTPJ.
She was referred to the podiatrist in September of the following year. On examination, it was noted that her feet showed the typical forefoot deformities of moderate hallux limitus, with 45° of available passive dorsiflexion at the first MTPJ. Passive sagittal plane movement of the right second toe was much reduced, with crepitus and pain at the right second MTPJ. There was a palpable dorsiplantar thickening of the right forefoot in the area of the second MTPJ. Plain radiographs showed marked degenerative changes at the head of the right second metatarsal and the base of the associated proximal phalanx, with flattening of the metatarsal dome, osteophytosis at the head of the right second metatarsal and base of the associated proximal phalanx, and local bone sclerosis.
On the basis of the history and the September radiograph the patient was diagnosed as showing arthritic degeneration of the right second MTPJ. It was presumed that the patient may have suffered an osteochondritis dissicans the previous December, secondary to an overload phenomenon imposed by the loss of movement at the first MTPJ, and possibly in association with a degree of age-related osteoporosis. The bone lesion at the second metatarsal head did not show up on the radiograph taken within 24 hours of the original incident, as the radiograph was taken at too early a stage in the pathology for it to be visible on plain radiography. Subsequent degenerative changes had developed over the ensuing months, causing osteoarthritis at the right second MTPJ.
The right forefoot problem was treated by conservative therapy, initially with defective clinical plantar padding, then with a bespoke cushioned orthotic and the use of a rocker-soled shoe. This reduced her forefoot pain to what the patient felt was an acceptable level. As the patient did not wish to contemplate forefoot surgery, she was not referred to an orthopaedic or podiatric surgeon.
Freiberg’s disease (Freiberg’s infraction)
This form of osteochondritis affects the metatarsal head(s). In four out of five cases the patient is a girl aged between 12 and 15 years (Griffin 1994, Manusov et al 1996b).
Pathology
Freiberg’s disease may present uni- or bilaterally, as a focus of ischaemia and bone necrosis within the head of a metatarsal, leading to collapse of both the articular surface and the underlying area of subchrondral bone – the so-called eggshell fracture. The head of the second metatarsal is affected in almost 70% of cases, and the third metatarsal head in almost 30% of cases, but any of the metatarsal heads may be subject to infraction (Griffin 1994). The predominance of osteochondritis affecting the second metatarsal head is thought to be due to the greater length of the second metatarsal in relation to the first and third metatarsals, and its resultant susceptibility to local trauma during the gait cycle, especially at toe-off. It is thought that the blood supply to the epiphyseal plate is interrupted by repeated microtraumata, causing an area of avascular necrosis within the metatarsal head (Manusov et al 1996b).
Clinical picture
The patient presents with increasing pain and associated swelling, and possible bruising on the dorsum of the foot, overlying the affected metatarsal head. Gait is affected and the patient may limp (Caselli et al 1998). The pain worsens on weight bearing and with activity. The range of motion at the affected MTPJ is decreased, particularly when active and/or weight-bearing dorsiflexion of the toes is attempted. Pain and crepitus is likely to be elicited on passive movement of the affected joint.
Diagnosis and differential diagnosis
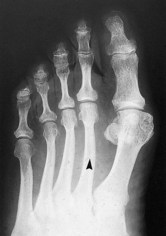
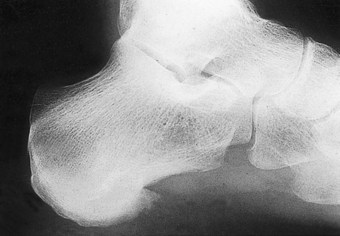
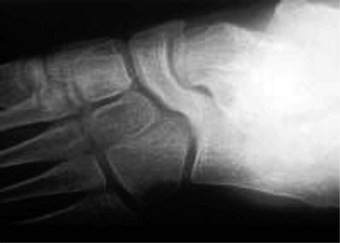
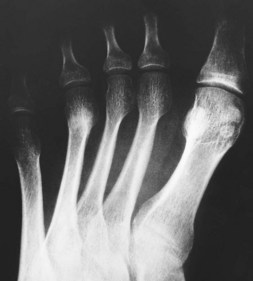
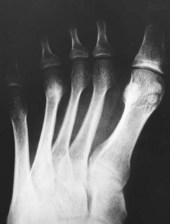
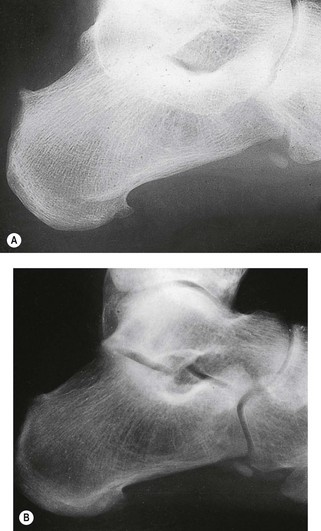
In the early stages of the disease plain radiographs may show little apparent bone involvement. In the later stages, as healing progresses, new bone growth in the affected area can be seen. The classical radiographic presentation in the adult who has undergone an episode of Freiberg’s infraction as a teenager, or an untreated Freiberg’s disease in a younger person, is of a flattened metatarsal head, with associated signs of osteoarthritic changes, such as bone sclerosis and osteophyte formation, occurring at the metatarsal head (Fig. 4.15). The base of the associated proximal phalanx may also show degenerative changes. The differential diagnoses of an acute presentation in a young person should exclude a march fracture, rheumatoid arthritis, intermetatarsal bursitis and other overuse injuries that may arise in relation to sports or dancing.
Treatment
The principal focus of the treatment of Freiberg’s disease is to reduce or eliminate weight-bearing forces to the affected bone area during the 6 weeks of the acute phase of the disease. This is achieved by imposing a regimen of non-weight bearing on the affected foot, with rest and painkillers as necessary. Non-weight bearing during the acute phase of the disease is necessary to ensure that retrograde pressure from the base of the proximal phalanx on the affected metatarsal head at toe-off is minimised, allowing bone healing to proceed without loss of the normal architecture of the affected metatarsal head. Non-weight bearing on the affected foot is achieved by the use of crutches, together with soft splintage of the foot, or rigid splintage of the lower limb and foot.
Freiberg’s disease may cause permanent alteration to or loss of the normal dome shape of the affected metatarsal head, even in cases where the disease has been identified in its early stages. The long-term treatment of these cases includes:
CASE STUDY 4.4 ADOLESCENT PRESENTATION
FREIBERG’S INFRACTION:
A 12-year-old girl presented with a history of recent pain in her left forefoot. She related the onset of her symptoms to a sponsored walk that she had completed 3 weeks previously. She was otherwise in good health, with no marked foot deformity or swelling. She had not experienced any similar event in the past.
On examination the focus of pain was located to the second metatarsophalangeal joint (MTPJ) and the pain was exacerbated by both passive and active extension of the second toe, although there was no crepitus on movement of the second MTPJ. She was unable to stand on tiptoe on the left foot. The differential diagnosis included Freiberg’s infraction of the head of the second metatarsal or stress fracture of the second metatarsal. Plain radiographs of the left foot excluded a stress fracture (as there was no indication of bone callous formation, which should have showed 3 weeks after the onset of symptoms) but the radiograph did not point to a definite diagnosis of Freiberg’s disease.
The patient was treated symptomatically with orthoses designed to reduce plantar pressures at the painful second MTPJ area. These reduced her pain to a tolerable level. She was advised to rest the foot, and to wear stiff-soled shoes, to reduce movement at the MTPJs. Her condition was monitored. At 6 months after her initial visit a second radiographic examination was requested. The second radiograph showed pathological changes at the left second metatarsal head. The normal domed shape had been lost, and the head of the second metatarsal was flattened and ‘squared off’, and local bone sclerosis was noted.
After 18 months of palliative care the patient’s symptoms subsided. There is an increased risk of this patient developing premature osteoarthritis at the left second MTPJ in later life.
Kohler’s disease
This form of osteochondritis affects the ossification centre of the navicular bone. Classically, Kohler’s disease of the navicular affects young boys aged between 2 and 9 years. There is a male/female ratio of 4 : 1.
Pathology
It is thought that repetitive minor trauma to the navicular bone causes patchy ossification (Manusov et al 1996b).
Clinical picture
The patient presents with, or the parent notes, pain and possibly swelling in the medial–plantar area of the instep, with focal tenderness in the area of the navicular. Small children may become reluctant to run around or play as normal.
Diagnosis and differential diagnosis
Initially, little shows on radiography, but radiographs from patients who have had Kohler’s disease of the navicular in earlier childhood typically show anteroposterior narrowing (wafering) of the navicular, with increased bone density and loss of normal trabeculation in later life. In this age group, there are few other disease processes that give rise to these presenting features.
Treatment
Some cases of Kohler’s disease are self-resolving. Others require the use of soft splintage, such as semicompressed felt valgus filler pads, or supportive orthoses and appropriate footwear to maintain the architecture of the longitudinal arch of the foot during healing. Paracetamol (e.g. Calpol™) may be used to control pain in young children. Surgery is not usually indicated.
Osteochondritis dissicans of the talus
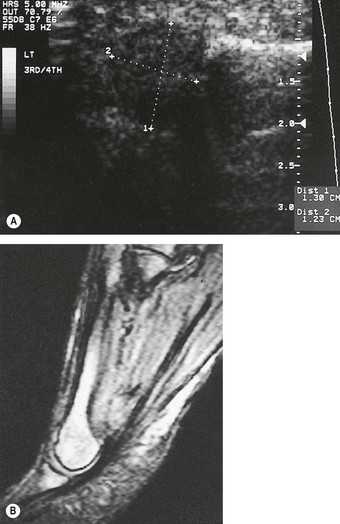
Osteochondritis dissicans of the talus more commonly affects the talar head, although it may also affect the trochlear surface. The incidence is reported as 1 in 5000 (Griffin 1994), and it is more common amongst sports people such as skaters, runners and gymnasts (Manusov et al 1996a). There is often a history of recent trauma or severe inversion ankle sprain (Kaeding & Whitehead 1998), or the patient may recall only a relatively minor injury, such as slipping or stumbling.
Pathology
The bone lesion is classified in four stages of presentation:
Clinical picture
The patient presents with a painful, swollen ankle. The location of the pain and the swelling can aid the diagnosis of the exact site of the bone lesion. For example, osteochondritis dissicans of the talar head gives rise to focal pain and swelling in the medial area of the midtarsal joint, whereas osteochondritis dissicans of the trochlear surface of the talus will cause pain and swelling that relates to the anterior aspect of the ankle. However, if a loose body has formed within the ankle or talonavicular joints, the site of the pain and swelling is far less constant, and the patient describes episodes of acute focal pain and swelling that change location from day to day as the fragment of bone moves around within the joint cavity. Patients often report that the ankle ‘locks’ or ‘catches’, and that the ankle and subtalar joint area is unstable on weight bearing.
Diagnosis and differential diagnosis
Radiographs may not identify the damaged area, especially in the early stages. Special views may need to be requested to visualise all aspects of the affected bone. Computerised tomography scans and magnetic resonance imaging are much more sensitive than radiography in the early diagnosis of osteochondritis dissicans of the ankle–subtalar–midtarsal joint complex.
Treatment
The treatment of stages I and II includes rest and immobilisation for 6 weeks. This is achieved by the use of a below-knee plaster of Paris cast, an Aircast® boot, or an ankle–foot orthosis, together with the use of crutches to aid in walking. As healing progresses, the use of a firm walking boot, rather than a normal shoe, is of benefit, as it restricts movement of the rearfoot complex, yet allows relatively normal ambulation.
Stages III and IV may require surgical removal of the loose bone fragment, via arthroscopy, or internal fixation to reattach the detached bone fragment.
Sever’s disease
Sever’s disease is perhaps the most common cause of heel pain in children, aged between 7 and 12 years (Griffin 1994, Manusov et al 1996a).
Pathology
Heel pain in Sever’s disease occurs as the result of an overuse syndrome, and is not a true avascular necrosis. Although it is commonly referred to as an osteochondritis, it is more accurately classified as a traction apophysitis.
The calcaneus has two centres of ossification: the primary centre, which is within the body of the calcaneum; and the secondary centre, which is within the posterior area of the bone. These centres of ossification unite when the bone attains maturity (becomes fully ossified) at about 12 years of age. Traction forces from the Achilles tendon on immature bone can induce epiphyseal distraction, (between the body and the posterior leaflet of the calcaneus) due to its insertion onto the middle third of the posterior surface of the calcaneum. Repetitive contractions of the muscles of the posterior compartment (gastrocnemius and soleus) may also predispose to microfractures at the calcaneal epiphyseal plate. A similar pathology is noted at the insertion of the patellar tendon into the tibial tubercle in Osgood Schlatter’s disease (Madden & Mellion 1996).
Clinical picture
The condition may be unilateral or bilateral. The patient presents with pain and tenderness that localises to the posterior aspect of the calcaneus. Pain is maximal after vigorous or impact exercise, such as running or gymnastics. There may be an associated warmth and oedema at the posterior heel area, and wearing shoes with a close heel counter aggravates symptoms. It affects boys more often than girls, and onset may link with growth spurts, especially in physically active or overweight children (Madden & Mellion 1996). Many patients will also show biomechanical compensation for structural foot anomalies that tend to reduce shock absorption at heel strike and expose the heel to abnormal ground reaction forces (Madden & Mellion 1996).
Diagnosis and differential diagnoses
Diagnosis in the early stages of the disease is based on the history and the presenting symptoms. Diagnostic signs include: increased pain on passive dorsiflexion of the foot at the ankle, and exacerbation of symptoms when standing on tiptoe (a positive Sever’s sign) (Madden & Mellion 1996). Radiographs of the calcaneus appear normal in the early stages of the disease process. As the disorder progresses, the apophyseal area shows sclerosis and fragmentation, although this finding is not necessarily diagnostic as this picture can also been seen in asymptomatic patients (Kaeding & Whitehead 1998).
The differential diagnosis should exclude a duck-bill fracture of the posterior leaflet of the calcaneum, Achilles tendon pathologies and deep retrocalcaneal bursitis.
Treatment
Many authors believe Sever’s disease to be self-limiting, as it tends to resolve spontaneously when calcaneal ossification is completed, usually at about 12 years of age. However, the use of physical therapies such as PRICE (pain control, rest, ice, compression and elevation) is beneficial in the acute phase of the disease. When symptoms are severe, a non-weight-bearing regimen, such as a below-knee cast for 6 weeks, or an Aircast® boot, and crutches, together with a course of NSAIDs is indicated. When the presenting symptoms are mild, the use of a figure-of-eight bandage to the affected rearfoot with a heel raise in the shoe is useful.
A heel raise should continue to be used after the acute phase has passed, in order to reduce the pull of the Achilles tendon on the posterior surface of the calcaneum. This will also minimise further trauma to the epiphyseal plate during the healing phase. Night splints, such as those used for the treatment of plantar fasciitis (Powell et al 1998) are also of benefit. After healing is complete, a regimen of rehabilitation exercises to stretch the Achilles tendon will be needed to prevent or reduce a tendency to ankle equinus, and a programme of stretching exercises and ice massage should continue to be used after strenuous activity. The patient should undergo a full biomechanical evaluation, and orthoses should be prescribed to correct any noted faults. The routine use of well-fitting trainers with good shock absorption should be recommended.
Iselin’s disease
Iselin’s disease is a traction apophysitis that affects the base of the fifth metatarsal, at the insertion of peroneus brevis tendon (Lehman et al 1986). The proximal apophysis of the fifth metatarsal usually does not ossify fully until approximately 16 years of age. Overuse of the peroneus brevis tendon prior to full ossification causes inflammation, swelling and tenderness, bruising and pain at the dorsolateral area at the base of the fifth metatarsal (the styloid process). Diagnosis is confirmed by the presenting symptoms, together with increased local pain when the foot is everted against resistance. The differential diagnosis should exclude a stress fracture of the styloid process, a Jones fracture or an avulsion fracture of the styloid process. Symptoms usually respond to a short period of rest (Griffin 1994). In less responsive cases, the foot should be strapped into eversion, to minimise the pull of the peroneus brevis tendon, until the symptoms subside.