CHAPTER 40 Shock
CHAPTER 40 Shock
ETIOLOGY AND EPIDEMIOLOGY
Shock is the inability to provide sufficient perfusion of oxygenated blood and substrate to tissues to maintain organ function. Oxygen delivery is directly related to the arterial oxygen content (oxygen saturation and hemoglobin concentration) and to cardiac output (stroke volume and heart rate). Changes in metabolic needs are met primarily by adjustments in cardiac output. Stroke volume is related to myocardial end-diastolic fiber length (preload), myocardial contractility (inotropy), and resistance of blood ejection from the ventricle (afterload) (see Chapter 138). In a young infant whose myocardium possesses relatively less contractile tissue, increased demand for cardiac output is met primarily by a neurally mediated increase in heart rate. In older children and adolescents, cardiac output is most efficiently augmented by increasing stroke volume through neurohormonally mediated changes in vascular tone, resulting in increased venous return to the heart (increased preload), decreased arterial resistance (decreased afterload), and increased myocardial contractility.
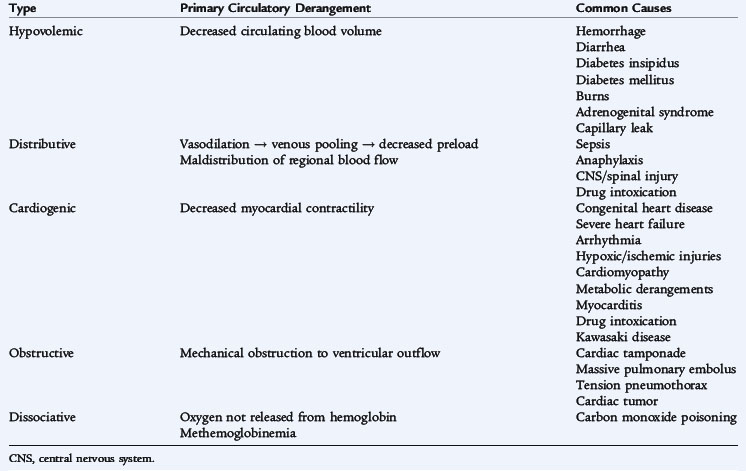
HYPOVOLEMIC SHOCK
Acute hypovolemia is the most common cause of shock in children. It results from loss of fluid from the intravascular space secondary to inadequate intake or excessive losses (vomiting and diarrhea, blood loss, capillary leak syndromes, or pathologic renal fluid losses) (Table 40-1). Reduced blood volume decreases preload, stroke volume, and cardiac output. Hypovolemic shock results in increased sympathoadrenal activity, producing an increased heart rate and enhanced myocardial contractility. Neurohormonally mediated constriction of the arterioles and capacitance vessels maintains blood pressure, augments venous return to the heart to improve preload, and redistributes blood flow from nonvital to vital organs. If hypovolemic shock remains untreated, the increased heart rate may impair coronary blood flow and ventricular filling, while elevated systemic vascular resistance increases myocardial oxygen consumption, resulting in worsening myocardial function. Ultimately, intense systemic vasoconstriction and hypovolemia produce tissue ischemia, impairing cell metabolism and releasing potent vasoactive mediators from injured cells. Cytokines and other vasoactive peptides can change myocardial contractility and vascular tone and promote release of other inflammatory mediators that increase capillary permeability and impair organ function further.
DISTRIBUTIVE SHOCK
Abnormalities in the distribution of blood flow may result in profound inadequacies in tissue perfusion, even in the presence of a normal or high cardiac output. This maldistribution of flow usually results from abnormalities in vascular tone. Septic shock is the most common type of distributive shock in children. Other causes include anaphylaxis, neurologic injury, and drug-related causes (see Table 40-1).
Distributive shock may present with the systemic inflammatory response syndrome (SIRS), defined as two or more of the following: temperature greater than 38° C or less than 36° C; heart rate greater than 90 beats/min or more than two standard deviations above normal for age; tachypnea; or white blood count greater than 12,000 cells/mm3, less than 4000 cells/mm3, or greater than 10% immature forms.
CARDIOGENIC SHOCK
Cardiogenic shock is caused by an abnormality in myocardial function and is expressed as depressed myocardial contractility and cardiac output with poor tissue perfusion. Compensatory mechanisms may contribute to the progression of shock by depressing cardiac function further. Neurohormonal vasoconstrictor responses increase afterload and add to the work of the failing ventricle. Tachycardia may impair coronary blood flow, which decreases myocardial oxygen delivery. Increased central blood volume caused by sodium and water retention and by incomplete emptying of the ventricles during systole results in elevated left ventricular volume and pressure, which impair subendocardial blood flow. As compensatory mechanisms are overcome, the failing left ventricle produces increased ventricular end-diastolic volume and pressure, which leads to increased left atrial pressure, resulting in pulmonary edema. This sequence also contributes to right ventricular failure because of increased pulmonary artery pressure and increased right ventricular afterload.
Primary cardiogenic shock may occur in children who have congenital heart disease. Cardiogenic shock also may occur in previously healthy children secondary to viral myocarditis, dysrhythmias, or toxic or metabolic abnormalities or after hypoxic-ischemic injury (see Chapters 141, 142, 145, and 147, as well as Table 40-1).
OBSTRUCTIVE SHOCK
Obstructive shock results from mechanical obstruction of ventricular outflow. Causes include congenital lesions such as coarctation of the aorta, interrupted aortic arch, and severe aortic valvular stenosis, along with acquired diseases (e.g., hypertrophic cardiomyopathy) (see Table 40-1). For neonates presenting in shock, obstructive lesions must be considered.
DISSOCIATIVE SHOCK
Dissociative shock refers to conditions in which tissue perfusion is normal, but cells are unable to use oxygen because the hemoglobin has an abnormal affinity for oxygen, preventing its release to the tissues (see Table 40-1).
CLINICAL MANIFESTATIONS
All forms of shock produce evidence that tissue perfusion and oxygenation are insufficient (increased heart rate, abnormal blood pressure, alterations of peripheral pulses). The etiology of shock may alter the initial presentation of these signs and symptoms.
Hypovolemic Shock
Hypovolemic shock is distinguished from other causes of shock by history and the absence of signs of heart failure or sepsis. In addition to the signs of sympathoadrenal activity (tachycardia, vasoconstriction), clinical manifestations include signs of dehydration (dry mucous membranes, decreased urine output) or blood loss (pallor). Recovery depends on the degree of hypovolemia, the patient’s preexisting status, and rapid diagnosis and treatment. The prognosis is good, with a low mortality (<10%) in uncomplicated cases.
Distributive Shock
Patients with distributive shock usually have tachycardia and alterations of peripheral perfusion. In early stages, when cytokine release results in vasodilation, pulses may be bounding, and vital organ function may be maintained (an alert patient, with rapid capillary refill and some urine output in warm shock). As the disease progresses untreated, extremities become cool and mottled with a delayed capillary refill time. At this stage, the patient has hypotension and vasoconstriction. If the etiology of distributive shock is sepsis, the patient often has fever, lethargy, petechiae, or purpura, and he or she may have an identifiable source of infection.
Cardiogenic Shock
Cardiogenic shock results when the myocardium is unable to supply the cardiac output required to support tissue perfusion and organ function. Because of this self-perpetuating cycle, heart failure progressing to death may be rapid. Patients with cardiogenic shock have tachycardia and tachypnea. The liver is usually enlarged, a gallop is often present, and jugular venous distention may be noted. Because renal blood flow is poor, sodium and water are retained, resulting in oliguria and peripheral edema.
LABORATORY AND IMAGING STUDIES
Shock requires immediate resuscitation before obtaining laboratory or diagnostic studies. Following initial stabilization (including glucose administration if hypoglycemia is present), the type of shock dictates the necessary laboratory studies. All patients with shock may benefit from determination of a baseline arterial blood gas and blood lactate level to assess the impairment of tissue oxygenation. Measurement of mixed venous oxygen saturation aids in the assessment of the adequacy of oxygen delivery. In contrast to other forms of shock, patients with sepsis often have high mixed venous saturation values because of impairment of mitochondrial function and inability of tissues to extract oxygen. A complete blood count can potentially assess intravascular blood volume after equilibration following a hemorrhage. Electrolyte measurements in patients with hypovolemic shock may identify abnormalities from losses. Patients presenting in distributive shock require appropriate bacterial and viral cultures to identify a cause of infection. If cardiogenic or obstructive shock is suspected, an echocardiogram assists with the diagnosis and, in the case of tamponade, assists with placement of a pericardial drain to relieve the fluid. Patients with dissociative shock require detection of the causative agent (carbon monoxide, methemoglobin). The management of shock also requires monitoring of arterial blood gases for oxygenation, ventilation (CO2), and acidosis, and frequently assessing the levels of serum electrolytes, calcium, magnesium, phosphorus, and blood urea nitrogen (BUN).
TREATMENT
General Principles
The key to therapy is the recognition of shock in its early, partially compensated state, when many of the hemodynamic and metabolic alterations may be reversible. Initial therapy for shock follows the ABCs of resuscitation. Later therapy can then be directed at the underlying cause. Therapy should minimize cardiopulmonary work, while ensuring cardiac output, blood pressure, and gas exchange. Intubation, combined with mechanical ventilation with oxygen supplementation, improves oxygenation and decreases or eliminates the work of breathing but may impede venous return if distending airway pressures (positive end-expiratory pressure [PEEP] or peak inspiratory pressure) are excessive (see Chapter 61). Blood pressure support is crucial because the vasodilation in sepsis may reduce perfusion despite supranormal cardiac output.
Monitoring a child in shock requires maintaining access to the arterial and central venous circulation to record pressure measurements, perform blood sampling, and measure systemic blood pressure continuously. These measurements facilitate the estimation of preload and afterload. Regional monitoring with near infrared spectroscopy may allow early, noninvasive detection of alterations in perfusion.
Organ-Directed Therapeutics
Fluid Resuscitation
Alterations in preload have a dramatic effect on cardiac output. In hypovolemic and distributive shock, decreased preload significantly impairs cardiac output. In these cases, early and aggressive fluid resuscitation is important and greatly affects outcome. In cardiogenic shock, an elevated preload contributes to pulmonary edema.
Selection of fluids for resuscitation and ongoing use is dictated by clinical circumstances. Crystalloid volume expanders generally are recommended as initial choices because they are effective and inexpensive. Most acutely ill children with signs of shock may safely receive, and usually benefit greatly from, a 20-mL/kg bolus of an isotonic crystalloid over 5 to 15 minutes. This dose may be repeated until a response is noted. Colloids contain larger molecules that may stay in the intravascular space longer than crystalloid solutions and exert oncotic pressure, drawing fluid out of the tissues into the vascular compartment. However, long-term risks of colloids may exceed benefits. Care must be exercised in treating cardiogenic shock with volume expansion because the ventricular filling pressures may rise without improvement of the cardiac performance. Carefully monitoring cardiac output or central venous pressure guides safe volume replacement.
Cardiovascular Support
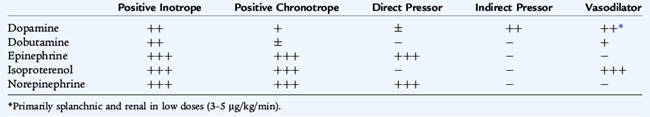
In an effort to improve cardiac output after volume resuscitation or when further volume replacement may be dangerous, a variety of inotropic and vasodilator drugs may be useful (Table 40-2). Therapy is directed first at increasing myocardial contractility, then at decreasing left ventricular afterload. The hemodynamic status of the patient dictates the choice of the agent.
Therapy usually is initiated with dopamine at 3 to 15 μg/kg/min. In children who fail to respond to several increments of an initial pressor, a more potent cardiotonic agent, such as epinephrine or norepinephrine, may be indicated. In addition to improving contractility, certain catecholamines cause an increase in systemic vascular resistance. The addition of a vasodilator drug may improve cardiac performance by decreasing the resistance against which the heart must pump (afterload). Afterload reduction may be achieved with dobutamine, milrinone, amrinone, nitroprusside, nitroglycerin, and angiotensin-converting enzyme inhibitors. The use of these drugs may be particularly important in late shock, when vasoconstriction is prominent.
Respiratory Support
The lung is a target organ for inflammatory mediators in shock and SIRS. Respiratory failure may develop rapidly and become progressive. Intervention requires endotracheal intubation and mechanical ventilation accompanied by the use of supplemental oxygen and PEEP. Care must be taken with the process of intubation, because a child with compensated shock may suddenly decompensate on administration of sedative medications that reduce systemic vascular resistance. Severe cardiopulmonary failure may be managed with inhaled nitric oxide and, if necessary, extracorporeal membrane oxygenation (see Chapter 61).
Renal Salvage
Poor cardiac output accompanied by decreased renal blood flow may cause prerenal azotemia and oliguria/anuria. Severe hypotension may produce acute tubular necrosis and acute renal failure. Prerenal azotemia is corrected when blood volume deficits are replaced or myocardial contractility is improved, but acute tubular necrosis does not improve immediately when shock is corrected. Prerenal azotemia is associated with a serum BUN-to-creatinine ratio of greater than 10:1 and a urine sodium level less than 20 mEq/L; acute tubular necrosis has a BUN-to-creatinine ratio of 10:1 or less and a urine sodium level between 40 and 60 mEq/L (see Chapter 165). Aggressive fluid replacement is often necessary to improve oliguria associated with prerenal azotemia. Because the management of shock requires administering large volumes of fluid, maintaining urine output greatly facilitates patient management.
Prevention of acute tubular necrosis and the subsequent complications associated with acute renal failure (hyperkalemia, acidosis, hypocalcemia, edema) is important. The use of pharmacologic agents to augment urine output is indicated when the intravascular volume has been replaced. The use of loop diuretics, such as furosemide, or combinations of a loop diuretic and a thiazide agent may enhance urine output. Infusion of low-dose dopamine, which produces renal artery vasodilation, also may improve urine output. Nevertheless, if hyperkalemia, refractory acidosis, hypervolemia, or altered mental status associated with uremia occurs, dialysis or hemofiltration should be initiated.
COMPLICATIONS
Shock results in impairment of tissue perfusion and oxygenation and activation of inflammation and cytokine pathways. The major complication of shock is multiple organ system failure, defined as the dysfunction of more than one organ, including respiratory failure, renal failure, liver dysfunction, coagulation abnormalities, or cerebral dysfunction. Patients with shock and multiple organ failure have a higher mortality rate and, for survivors, a longer hospital stay.
PROGNOSIS
Early recognition and goal-directed intervention in patients with shock improve survival. Mortality rates for septic shock in children have decreased from greater than 90% in the 1960s to 8% to 9%. Specific mortality rates for other forms of shock are not readily available. However, delays in treatment of hypotension increase the incidence of multiple organ failure and mortality. Goal-directed therapy focused on maintaining mixed venous oxygen saturation may improve survival.
PREVENTION
Prevention strategies for shock are focused, for the most part, on shock associated with sepsis and hypovolemia. Some forms of septic shock can be prevented through use of immunizations (Haemophilus influenzae type b, meningococcal, pneumococcal vaccines). Decreasing the risk of sepsis in a critically ill patient requires adherence to strict handwashing, isolation practices, and minimizing the duration of indwelling catheters. Measures to decrease pediatric trauma do much to minimize hemorrhage-induced shock.
 SEE
SEE