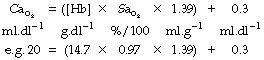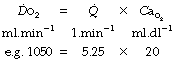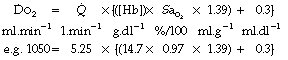Chapter 25 Anaemia
 Anaemia has little effect on pulmonary gas exchange but decreases oxygen carriage in the arterial blood in direct proportion to the reduction in haemoglobin concentration.
Anaemia has little effect on pulmonary gas exchange but decreases oxygen carriage in the arterial blood in direct proportion to the reduction in haemoglobin concentration.Anaemia is a widespread pathophysiological disorder that interferes with oxygen transport to the tissues. In developed countries it has a varied aetiology, including iron deficiency, chronic haemorrhage, end-stage renal failure or depletion of vitamin B12. However, in the Third World it is endemic, major factors including malnutrition and infestation with various parasites such as hookworm and bilharzia. In many countries, haemoglobin concentrations within the range 6–10 g.dl−1 are regarded as normal.
Anaemia per se has no major direct effects on pulmonary function. Arterial Po2 and saturation should remain within the normal range in uncomplicated anaemia, and the crucial effect is on the arterial oxygen content and therefore oxygen delivery. Important compensatory changes are increases in cardiac output, greater oxygen extraction from the arterial blood and to a lesser extent the small rightward displacement of the oxyhaemoglobin dissociation curve. However, there are limits to these adaptations, which define the minimal tolerable haemoglobin concentration, and also the exercise limits attainable at various levels of severity of anaemia.
Physiological aspects of blood transfusion and blood substitutes are discussed on page 197.
Pulmonary Function
Gas Exchange
Alveolar Po2 is determined by dry barometric pressure, inspired oxygen concentration and the ratio of oxygen consumption to alveolar ventilation (page 139). Assuming that the first two are unchanged, and there being good evidence that the latter two factors are unaffected in the resting state by anaemia down to a haemoglobin concentration of at least 5 g.dl−1 (see below), then there is no reason why alveolar Po2 or Pco2 should be affected by uncomplicated anaemia down to this degree of severity.
The increased cardiac output (see below) will cause a small reduction in pulmonary capillary transit time which, together with the reduced mass of haemoglobin in the pulmonary capillaries, causes a modest decrease in diffusing capacity (page 152). However, such is the reserve in the capacity of pulmonary capillary blood to reach equilibrium with the alveolar gas (see Figure 9.2) that it is highly unlikely that this would have any measurable effect on the alveolar/end-pulmonary capillary Po2 gradient, which in the normal subject is believed to be of the order of only 10−6 mmHg. Thus pulmonary end-capillary Po2 should also be normal in anaemia.
Continuing down the cascade of oxygen partial pressures from ambient air to the site of use in the tissues, the next step is the gradient in Po2 between pulmonary end-capillary blood and mixed arterial blood. The Po2 gradient at this stage is caused by shunting and the perfusion of relatively underventilated alveoli. There is no evidence that these factors are altered in anaemia, and arterial Po2 should therefore be normal. Because the peripheral chemoreceptors are stimulated by reduction in arterial Po2 and not arterial oxygen content (page 71), then there should be no stimulation of respiration unless the degree of hypoxia is sufficient to cause anaerobic metabolism and lactacidosis.
The Haemoglobin Dissociation Curve
It is well established that red blood cell 2,3-diphosphoglycerate levels are increased in anaemia (page 194), typical changes being from a normal value of 5 mmol.l−1 to 7 mmol.l−1 at a haemoglobin concentration of 6 g.dl−1.1 This results in an increase in P50 from 3.6 to 4.0 kPa (27 to 30 mmHg). This rightward shift of the dissociation curve would have a negligible effect on arterial saturation, which has indeed been reported to be normal in anaemia. The rightward shift will, however, increase the Po2 at which oxygen is unloaded in the tissues, mitigating to a small extent the effects of reduction in oxygen delivery so far as tissue Po2 is concerned.
Arterial Oxygen Content
Although the arterial oxygen saturation usually remains normal in anaemia, the oxygen content of the arterial blood will be reduced in approximate proportion to the decrease in haemoglobin concentration. Arterial oxygen content can be expressed as follows:
where Cao2 is arterial oxygen content, [Hb] is haemoglobin concentration, Sao2 is arterial oxygen saturation, 1.39 is the combining power of haemoglobin with oxygen (page 189) and 0.3 is dissolved oxygen at normal arterial Po2.
Oxygen Delivery
The important concept of oxygen delivery (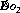 ) is considered in detail on page 202. It is defined as the product of cardiac output (
) is considered in detail on page 202. It is defined as the product of cardiac output (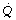 ) and
) and 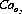 .
.
(the right-hand side is multiplied by a scaling factor of 10 to account for the differing units of volume).
Combining equations (1) and (2):
(the right-hand side is again multiplied by a scaling factor of 10).
Normal values give an oxygen delivery of approximately 1000 ml.min−1, which is about four times the normal resting oxygen consumption of 250 ml.min−1. Extraction of oxygen from the arterial blood is thus 25% and this accords with an arterial saturation of 97% and mixed venous saturation of 72%.
If the small quantity of dissolved oxygen (0.3 ml.dl−1) is ignored, then oxygen delivery is seen to be proportional to the product of cardiac output, haemoglobin concentration and arterial oxygen saturation. There is, of course, negligible scope for any compensatory increase in saturation in a patient with uncomplicated anaemia at sea level.
Effect of Anaemia on Cardiac Output
Equation (3) shows that, if other factors remain the same, a reduction in haemoglobin concentration will result in a proportionate reduction in oxygen delivery. Thus a haemoglobin concentration of 7.5 g.dl−1, with unchanged cardiac output, would halve delivery to give a resting value of 500 ml.min−1, which would be approaching the likely critical value. However, patients with quite severe anaemia usually show little evidence of hypoxia at rest and, furthermore, achieve surprisingly good levels of exercise. Because arterial saturation cannot be increased, full compensation can be achieved only by a reciprocal relationship between cardiac output and haemoglobin concentration. Thus, if haemoglobin concentration is halved, maintenance of normal delivery will require a doubling of cardiac output. Full compensation may not occur, but fortunately a reduction in haemoglobin concentration is usually accompanied by some increase in cardiac output.
Acute anaemia. Early studies of cardiac output and anaemia involved measurement of cardiovascular parameters in patients before and after treatment for uncomplicated anaemia.2 Cardiac output was significantly greater before the patients’ haemoglobin concentration increased from 5.9 to 10.9 g.dl−1. There was, however, a negative correlation between age and cardiac index in the anaemic state, reflecting the relative inability of the older patient to compensate. More recent studies have involved deliberately reducing the haemoglobin concentration isovolaemically in volunteers and patients.3-6 One of these studies reduced the haemoglobin concentration from 13.1 to 5.0 g.dl−1, and the effects of this on the cardiovascular system are shown in Figure 25.1.4 In these healthy volunteers the predictable linear relationship between cardiac index and haemoglobin concentration can easily be seen (Figure 25.1B). The increase in cardiac output seen in response to acute anaemia is much less in anaesthetised patients.5
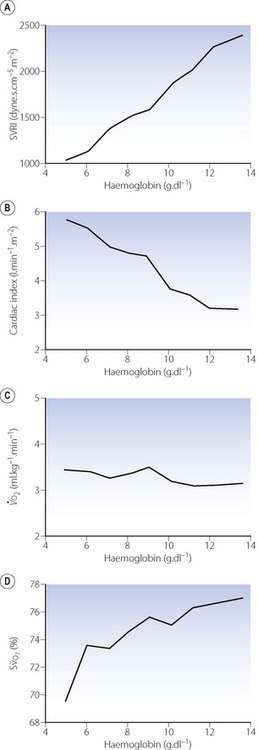
Fig. 25.1 Cardiovascular changes in response to acute isovolaemic reduction of mean haemoglobin concentration from 13.1 to 5.0 g.dl−1. (A) Systemic vascular resistance index (SVRI) falls in direct proportion to Hb concentration as blood viscosity decreases; (B) Cardiac index doubles when Hb has fallen to 5.0 g.dl−1 in these healthy volunteers; (C) Oxygen delivery (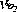 ) remains constant due to the increase in cardiac output exactly matching the decrease in arterial oxygen content; (D) Mixed venous oxygen saturation (
) remains constant due to the increase in cardiac output exactly matching the decrease in arterial oxygen content; (D) Mixed venous oxygen saturation (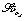 ) falls as the tissues extract more oxygen.
) falls as the tissues extract more oxygen.
(After reference 4.)
The mechanism underlying the increase in cardiac output is not clear, but is due to increases in both stroke volume and heart rate.4 Likely explanations for these changes include reduced cardiac afterload due to lowered blood viscosity (Figure 25.1A), and increased preload due to greater venous return secondary to increased tone in capacitance vessels.7
Chronic anaemia. In one study of isovolaemic reduction of haemoglobin concentration, down to a mean value of 10 g.dl−1, the anaemia was then maintained at the same level for 14 days.3 Immediately after induction of anaemia there was a marked increase in cardiac output (55%), but this decreased to only 14% above control levels after 14 days.
The Influence of Cardiac Output on Oxygen Delivery
Following the acute reduction of haemoglobin concentration in healthy subjects,3,4 cardiac output increased sufficiently to maintain normal or near-normal oxygen delivery (Figure 25.1C). However, in sustained anaemia, the increase in cardiac output (only 14%) is insufficient to maintain oxygen delivery, which decreases to 25% below control values. Similarly, in a study of anaemic patients,2 oxygen delivery was reduced in proportion to the degree of anaemia.
Without an increase in cardiac output, it is likely that a haemoglobin concentration of 6–8 g.dl−1 would be the minimal level compatible with life. It is clear that the ability of the cardiovascular system to respond to anaemia with an increase in cardiac output is an essential aspect of accommodation to anaemia, and this is less effective in anaesthetised patients, the elderly or other subjects with reduced cardiac reserve.
Relationship between oxygen delivery and consumption. The relationship between oxygen delivery and consumption has been considered on page 202 et seq. When oxygen delivery is reduced, for whatever reason, oxygen consumption is at first maintained at its normal value, but with increasing oxygen extraction and therefore decreasing mixed venous saturation (Figure 25.1D). Below a ‘critical’ value for oxygen delivery, oxygen consumption decreases as a function of delivery, and is usually accompanied by evidence of hypoxia, such as increased lactate in peripheral blood. Values for critical oxygen delivery depend upon the pathophysiological state of the patient and vary from one condition to another.
It has not been clearly established what is the critical level of oxygen delivery in uncomplicated anaemia in humans. Studies of acutely induced anaemia have found no evidence of tissue hypoxia, though in one study, at a haemoglobin concentration of 5 g.dl−1, oxygen consumption was reduced in spite of oxygen delivery being well maintained. In volunteers maintained at a haemoglobin concentration of 10 g.dl−1 for 14 days, oxygen delivery decreased from about 1200 to 900 ml.min−1 while oxygen consumption remained virtually unchanged.3 Similarly, a study of treated anaemic patients found no increase in oxygen consumption when haemoglobin concentration was increased from mean value of 6 to 11 g.dl−1.2 Thus these patients with long-term anaemia seemed to have all remained above the critical value for oxygen delivery down to haemoglobin values of about 6 g.dl−1.
Anaemia and Exercise
Maintenance of constant oxygen consumption in the face of reduced delivery can only be achieved at the expense of a reduction in mixed venous saturation, as a result of increased extraction of oxygen from the arterial blood. This has been clearly demonstrated in both acute (Figure 25.1D) and sustained anaemia.3 A reduction in the oxygen content of mixed venous blood curtails the ability of the anaemic patient to encroach on a useful reserve of oxygen, which is an important response to exercise. Reduction of haemoglobin to 10 g.dl−1 resulted in a curtailment of oxygen consumption attained at maximal exercise from the control values of 3.01 l.min−1 (normalised to 70 kg body weight) down to 2.53 l.min−1 in the acute stage, and 2.15 l.min−1 after 14 days of sustained anaemia (Figure 25.2).3 The increase in cardiac output required for the same increase in oxygen consumption was greater in the anaemic state, and cardiac output at maximal oxygen consumption was slightly less than under control conditions. Maximal exercise in the anaemic state resulted in a reduction of mixed venous oxygen saturation to the exceptionally low value of 12%, compared with control values of 23% during maximal exercise with a normal haemoglobin concentration.
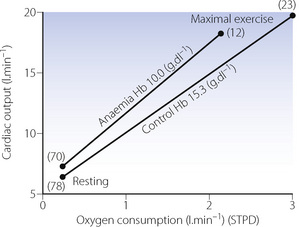
Fig. 25.2 Cardiac output as a function of oxygen consumption during rest and maximal exercise under control and isovolaemic anaemic conditions. Numbers in parentheses indicate mean mixed venous oxygen saturation.
(Redrawn from reference 3 on the assumption that mean weight of the subjects was 70 kg, by permission of the author, and the Editors and publishers of Journal of Applied Physiology.)
Brisk walking on level ground normally requires an oxygen consumption of about 1 l.min−1 and a cardiac output of about 10 l.min−1. At a haemoglobin level of 5 g.dl−1, this would require a cardiac output of about 20 l.min−1 to permit an oxygen consumption of 1 l.min−1 with a satisfactory residual level of mixed venous oxygen saturation. It will be clear that, at this degree of anaemia, cardiac function is a critical factor determining the mobility of a patient.
Exercise tolerance may be limited by either respiratory or circulatory capacity. In uncomplicated anaemia, there is no reason to implicate respiratory limitation, and exercise tolerance is therefore, to a first approximation, governed by the remaining factors in the oxygen delivery equation (3) (above). On the assumption that the maximal sustainable cardiac output is only marginally affected by anaemia, it is to be expected that exercise tolerance will be reduced in direct proportion to the haemoglobin concentration. Available evidence supports this hypothesis (Figure 25.3).
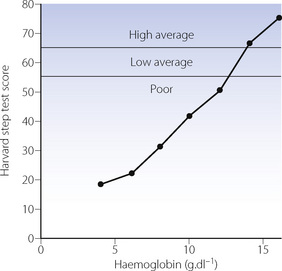
Fig. 25.3 Relationship between capacity for exercise and haemoglobin concentration.
(After reference 8 by permission of the authors, and the Editor and publishers of Clinics in Haematology.)
Using haemoglobin to enhance athletic performance. The corollary of the preceding description is the question of improving athletic performance by increasing haemoglobin concentration above the normal range. This used to be achieved by removal of blood for replacement of red cells after a few weeks when the subject has already partially restored his haemoglobin concentration, a procedure known as blood doping. The same effect is now much more conveniently achieved by the administration of erythropoietin. Studies of trained athletes in this area are notoriously difficult, and it is easy to confuse the effects of changes in blood volume and haemoglobin concentration. Furthermore, blood doping involves the subject continuing his training after removal of blood while he is anaemic. This may well make his training more effective, as is the case when training is undertaken at altitude. In the pioneer study of Ekblom et al in 1972,9 it was reported that, following reinfusion of blood (resulting in an increase in haemoglobin concentration from 13.2 to 14.9 g.dl−1), maximal oxygen consumption was increased from 4.40 to 4.79 l.min−1, and time to exhaustion during uphill treadmill running was extended from 5.43 to 6.67 minutes. These findings were challenged in subsequent studies, but confirmed in a well-controlled study of highly trained runners,10 in which a mean haemoglobin concentration of 16.7 g.dl−1 was attained with significant increases in maximal oxygen uptake from 4.85 to 5.10 l.min−1. Differences of this magnitude are critically important in the arena of modern athletic competition.
What is the Optimal Haemoglobin Concentration in the Clinical Setting?11
Evolution has resulted in a haemoglobin concentration of 13–16 g.dl−1 presumably for sound biological reasons, and this value must represent the best compromise between oxygen carriage, cardiac output and blood viscosity. However, blood transfusion has always been, and currently remains, a hazardous procedure and a haemoglobin concentration of over 10 g.dl−1 was for many years regarded as acceptable. At this level, cardiac output increases are modest and though exercise tolerance may be reduced this is unlikely to trouble the patient. There is evidence that lower values will be acceptable in some circumstances. Jehovah’s Witnesses, whose religious beliefs prevent them from consenting to blood transfusion, frequently undergo major surgery and survival is reported following haemoglobin values of less than 3 g.dl−1, albeit with substantial cardiovascular and respiratory support. Studies of these patients12 indicate that perioperative death is uncommon if haemoglobin concentration remains above 5 g.dl−1. There is also a suggestion that low haemoglobin values may actually be beneficial, with lowered blood viscosity improving blood flow through diseased vessels and so increasing tissue oxygenation, though evidence for a clinically relevant effect is lacking. A target haemoglobin concentration of 10 g.dl−1 may therefore be too conservative in fit healthy patients, or those with chronic anaemia,13 and a haemoglobin level of 7 g.dl−1 is probably acceptable in these groups.11 This view was confirmed in a randomised controlled study of intensive care patients in whom haemoglobin values of 7–9 g.dl−1 were associated with improved outcome compared with those in whom haemoglobin was maintained at over 10 g.dl−1.14
The organ that limits the acceptable degree of anaemia is the heart, where oxygen extraction is normally in excess of 50%. Increased oxygen extraction as a compensatory mechanism is therefore limited and coronary blood flow must increase to facilitate the greater oxygen requirement of a raised cardiac output. Thus any patient with ischaemic heart disease will be considerably less tolerant of anaemia than those with normal coronary arteries, as shown in the study of intensive care patients already described.14 For these patients, particularly in the postoperative period when cardiac output is elevated to the same extent as during moderate exercise, the optimal haemoglobin may be as high as 12.8 g.dl−1.15
Chronic renal failure leads to a lack of renal erythropoietin release and severe symptomatic anaemia results, with patients commonly having haemoglobin levels of less than 8 g.dl−1. The availability of recombinant human erythropoietin has allowed partial correction of anaemia in many patients, leading to a substantial improvement in quality of life for most. There is, however, debate about the optimal target haemoglobin concentration to aim for.16 There is good evidence that the chronic severe anaemia associated with renal disease commonly leads to cardiac complications.17 Unfortunately, there is also some evidence that correction of haemoglobin to normal values is associated with increased cardiac complications in these patients, and a value of around 12 g.dl−1 seems to be the safest compromise.16
References
1. Torrance J, Jacobs P, Restrepo A, Eschbach J, Lenfant C, Finch CA. Intraerythrocytic adaptation to anemia. N Engl J Med.. 1970;283:165-169.
2. Duke M, Abelmann WH. The hemodynamic response to chronic anemia. Circulation. 1969;39:503-515.
3. Woodson RD, Wills RE, Lenfant C. Effect of acute and established anemia on O2 transport at rest, submaximal and maximal work. J Appl Physiol.. 1978;44:36-43.
4. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217-221.
*5. Ickx BE, Rigolet M, Van der Linden PJ. Cardiovascular and metabolic response to acute normovolemic anemia. Anesthesiology. 2000;93:1011-1016.
6. Leung J, Weiskopf R, Feiner J, et al. Electrocardiographic ST-segment changes during acute, severe isovolemic hemodilution in humans. Anesthesiology. 2000;93:1004-1010.
7. Chapler CK, Cain SM. The physiologic reserve in oxygen carrying capacity: studies in experimental haemodilution. Can J Physiol Pharmacol.. 1986;64:7-12.
8. Viteri FE, Torun B. Anaemia and physical work capacity. Clin Hematol.. 1974;3:609-626.
9. Ekblom B, Goldbarg AN, Gullbring B. Response to exercise after blood loss and reinfusion. J Appl Physiol.. 1972;33:175-180.
10. Buick FJ, Gledhill N, Froese AB, Spriet L, Meyers EC. Effect of induced erythrocythemia on aerobic work capacity. J Appl Physiol.. 1980;48:636-642.
11. Wedgwood JJ, Thomas JG. Peri-operative haemoglobin: an overview of current opinion regarding the acceptable level of haemoglobin in the peri-operative period. Eur J Anaesthesiol.. 1996;13:316-324.
12. Viele MK, Weiskopf RB. What can we learn about the need for transfusion from patients who refuse blood? The experience with Jehovah’s Witnesses. Transfusion. 1994;34:396-401.
13. Welch HG, Meehan KR, Goodnough LT. Prudent strategies for elective red blood cell transfusion. Ann Intern Med.. 1992;116:393-402.
*14. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med.. 1999;340:409-417.
15. Kettler D. ‘Permissive anaemia’ compared with blood transfusion in patients with cardiac disease: another point of view. Curr Opin Anaesthesiol.. 1994;7:908-918.
16. Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369:381-388.
17. Schunkert H, Hense H-W. A heart price to pay for anaemia. Nephrol Dial Transplant. 2001;16:445-448.