Mixed Odontogenic Tumors
The group of mixed odontogenic tumors, composed of proliferating odontogenic epithelium in a cellular ectomesenchyme resembling the dental papilla, poses problems in classification. Some of these lesions show varying degrees of inductive effect by the epithelium on the mesenchyme, leading to the formation of varying amounts of enamel and dentin. Some of these lesions (the common odontomas) are clearly nonneoplastic developmental anomalies; others appear to be true neoplasms. The nature of others is uncertain.
In some instances, the histopathologic findings alone cannot distinguish between the neoplastic lesions and the developmental anomalies. Clinical and radiographic features often are of considerable assistance in making this distinction.
AMELOBLASTIC FIBROMA
The ameloblastic fibroma is considered to be a true mixed tumor in which the epithelial and mesenchymal tissues are both neoplastic. It is an uncommon tumor, but the data regarding its frequency are difficult to evaluate because (particularly in earlier reports) some lesions that were diagnosed as ameloblastic fibroma may actually have represented the early developing stage of an odontoma.
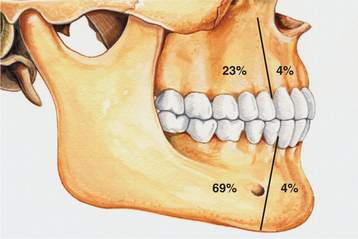
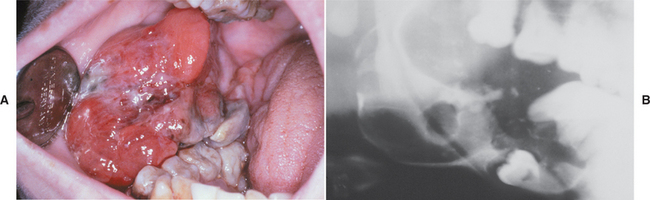
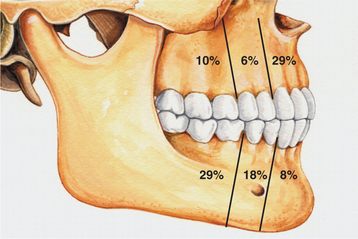
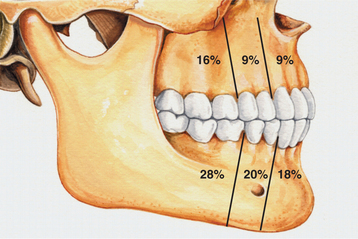
CLINICAL AND RADIOGRAPHIC FEATURES: Ameloblastic fibromas tend to occur in younger patients; most lesions are diagnosed in the first two decades of life. This lesion, however, is occasionally encountered in middle-aged patients. The tumor is slightly more common in males than in females. Small ameloblastic fibromas are asymptomatic; larger tumors are associated with swelling of the jaws. The posterior mandible is the most common site; about 70% of all cases are located in this area (Fig. 15-98). Convincing examples of this tumor arising within the gingival soft tissue have only recently been described, but this appears to represent a rare phenomenon.
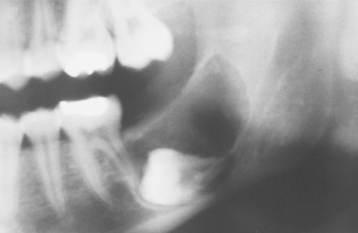
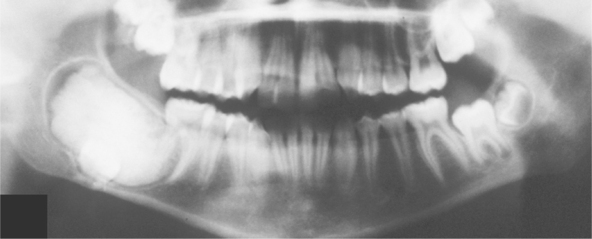
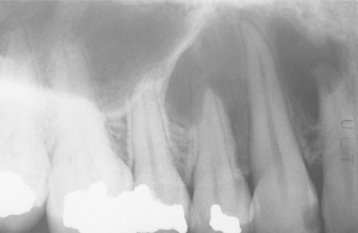
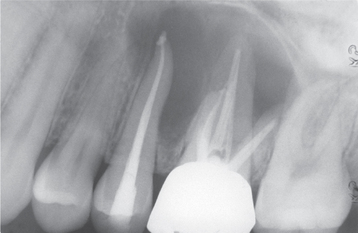
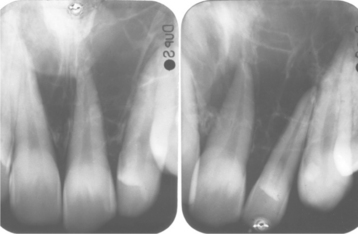
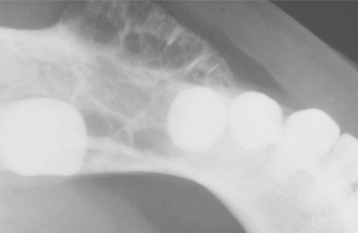
Radiographically, either a unilocular or multilocular radiolucent lesion is seen, with the smaller lesions tending to be unilocular. The radiographic margins tend to be well defined, and they may be sclerotic. An unerupted tooth is associated with the lesion in about 75% of cases (Fig. 15-99). The ameloblastic fibroma may grow to a large size, and cases that involve a considerable portion of the body and ascending ramus of the mandible have been reported.
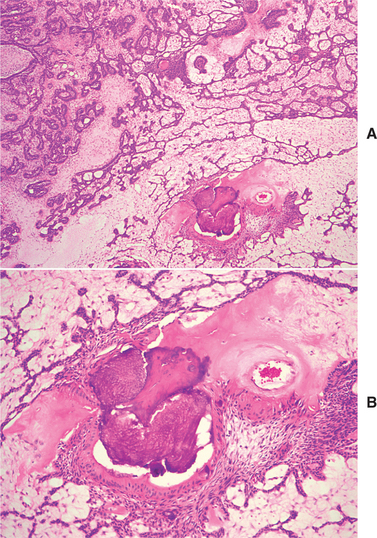
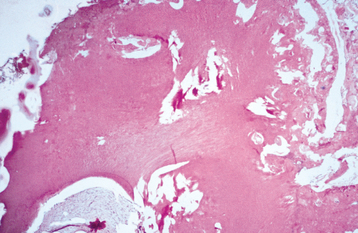
HISTOPATHOLOGIC FEATURES: The ameloblastic fibroma appears as a solid, soft tissue mass with a smooth outer surface. A definite capsule may or may not be present. Microscopically, the tumor is composed of a cell-rich mesenchymal tissue resembling the primitive dental papilla admixed with proliferating odontogenic epithelium. The latter may have one of two patterns, both of which are usually present in any given case. The most common epithelial pattern consists of long, narrow cords of odontogenic epithelium, often in an anastomosing arrangement. These cords are usually only two cells in thickness and are composed of cuboidal or columnar cells (Fig. 15-100). In the other pattern, the epithelial cells form small, discrete islands that resemble the follicular stage of the developing enamel organ. These show peripheral columnar cells, which surround a mass of loosely arranged epithelial cells that resemble stellate reticulum. In contrast to the follicular type of ameloblastoma, these follicular islands in the ameloblastic fibroma seldom demonstrate microcyst formation.
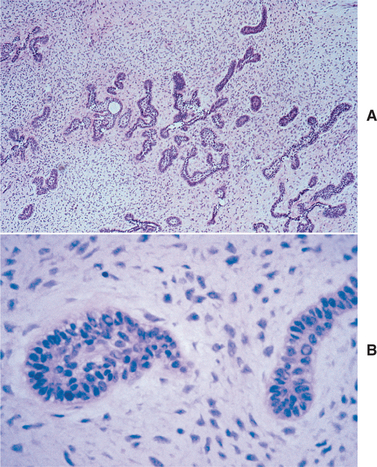
Fig. 15-100 Ameloblastic fibroma. A, Long, narrow cords of odontogenic epithelium supported by richly cellular, primitive connective tissue. B, Basophilic epithelial islands with peripheral nuclear palisading.
The mesenchymal portion of the ameloblastic fibroma consists of plump stellate and ovoid cells in a loose matrix, which closely resembles the developing dental papilla. Collagen formation is generally inconspicuous. Juxtaepithelial hyalinization of the mesenchymal portion of the tumor is sometimes seen, and occasionally diffuse areas of hyalinized acellular lesional tissue are evident.
In recent years, a few examples of ameloblastic fibroma occurring in conjunction with calcifying odontogenic cyst have been reported.
TREATMENT AND PROGNOSIS: The proper management of ameloblastic fibroma has been an ongoing topic of debate. Although initially it was believed that the ameloblastic fibroma was an innocuous lesion that seldom recurred after simple local excision or curettage, subsequent reports seemed to indicate a substantial risk of recurrence after conservative therapy. The highest recurrence rate (43.5%) was recorded in a series of cases from the Armed Forces Institute of Pathology, and it could be argued that this was a biased sample of larger lesions that were inherently more difficult to manage. In other series of cases, from 0% to 18% of ameloblastic fibromas were reported to recur after conservative removal and an adequate follow-up period. Based on these data, recent recommendations have emphasized conservative initial therapy for ameloblastic fibroma. More aggressive surgical excision should probably be reserved for recurrent lesions. Approximately 45% of the cases of the rare ameloblastic fibrosarcoma develop in the setting of a recurrent ameloblastic fibroma.
AMELOBLASTIC FIBRO-ODONTOMA
The ameloblastic fibro-odontoma is defined as a tumor with the general features of an ameloblastic fibroma but that also contains enamel and dentin. Some investigators believe that the ameloblastic fibro-odontoma is only a stage in the development of an odontoma and do not consider it to be a separate entity. Certainly the histopathologic features of a developing odontoma may overlap somewhat with ameloblastic fibro-odontoma. There are well-documented examples, however, of this tumor exhibiting progressive growth and causing considerable deformity and bone destruction. Such lesions appear to be true neoplasms. However, distinguishing between a developing odontoma and an ameloblastic fibro-odontoma may be difficult based on histopathologic grounds alone.
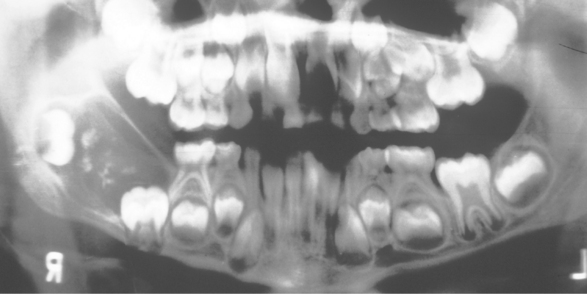
CLINICAL AND RADIOGRAPHIC FEATURES: The ameloblastic fibro-odontoma is usually encountered in children with an average age of 10 years. It is rarely encountered in adults. Like the ameloblastic fibroma, ameloblastic fibro-odontomas occur more frequently in the posterior regions of the jaws (Fig. 15-101). There is no significant sex predilection. The lesion is commonly asymptomatic and is discovered when radiographs are taken to determine the reason for failure of a tooth to erupt. Large examples may be associated with a painless swelling of the affected bone.
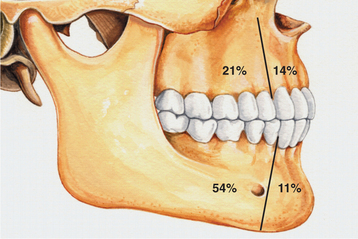
Fig. 15-101 Ameloblastic fibro-odontoma. Relative distribution of ameloblastic fibro-odontoma in the jaws.
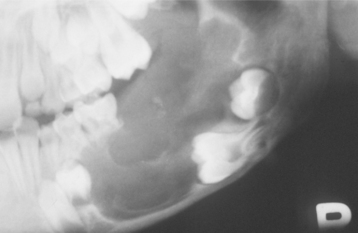
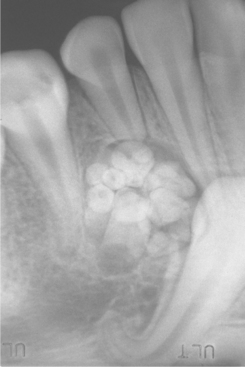
Radiographically, the tumor shows a well-circumscribed unilocular or, rarely, multilocular radiolucent defect that contains a variable amount of calcified material with the radiodensity of tooth structure. The calcified material within the lesion may appear as multiple, small radiopacities or as a solid conglomerate mass (Fig. 15-102). In most instances, an unerupted tooth is present at the margin of the lesion, or the crown of the unerupted tooth may be included within the defect. Some ameloblastic fibro-odontomas contain only a minimal amount of calcifying enamel and dentin matrix and appear radiographically as radiolucent lesions (Fig. 15-103). These cannot be differentiated from the wide variety of unilocular radiolucencies that may involve the jaws. At the other extreme, some ameloblastic fibro-odontomas appear as largely calcified masses with only a narrow rim of radiolucency about the periphery of the lesion.
HISTOPATHOLOGIC FEATURES: The soft tissue component of the ameloblastic fibro-odontoma is microscopically identical to the ameloblastic fibroma and has narrow cords and small islands of odontogenic epithelium in a loose primitive-appearing connective tissue that resembles the dental papilla. The calcifying element consists of foci of enamel and dentin matrix formation in close relationship to the epithelial structures (Fig. 15-104). The more calcified lesions show mature dental structures in the form of rudimentary small teeth or conglomerate masses of enamel and dentin. Some researchers have designated a similar tumor in which the calcifying component consists only of dentin matrix and dentinoid material as ameloblastic fibro-dentinoma. It is questionable whether this lesion represents a separate entity, and it is probably best considered as only a variant of the ameloblastic fibro-odontoma.
TREATMENT AND PROGNOSIS: A patient with an ameloblastic fibro-odontoma is generally treated by conservative curettage, and the lesion usually separates easily from its bony bed. The tumor is well circumscribed and does not invade the surrounding bone.
The prognosis is excellent, and recurrence after conservative removal is unusual. Development of an ameloblastic fibrosarcoma after curettage of an ameloblastic fibro-odontoma has been reported, but this is exceedingly rare.
AMELOBLASTIC FIBROSARCOMA (AMELOBLASTIC SARCOMA)
The rare ameloblastic fibrosarcoma is considered to be the malignant counterpart of the ameloblastic fibroma, and slightly more than 60 cases have been documented in the literature. Interestingly, in most cases, only the mesenchymal portion of the lesion shows features of malignancy; the epithelial component remains rather bland. These tumors may apparently arise de novo, although in approximately half of known cases, the malignant lesion represents a recurrence of a tumor previously diagnosed as an ameloblastic fibroma or an ameloblastic fibro-odontoma.
CLINICAL AND RADIOGRAPHIC FEATURES: Ameloblastic fibrosarcomas occur about 1.5 times as often in males as in females. The lesion tends to occur in younger patients (mean reported age, 27.5 years). Although either the maxilla or the mandible may be involved, about 80% of cases have occurred in the mandible. Pain and swelling associated with rapid clinical growth are the common complaints.
Radiographically, the ameloblastic fibrosarcoma shows an ill-defined destructive radiolucent lesion that suggests a malignant process (Fig. 15-105).
HISTOPATHOLOGIC FEATURES: Ameloblastic fibrosarcomas contain an epithelial component similar to that seen in the ameloblastic fibroma, although it is frequently less prominent than that present in the typical ameloblastic fibroma. The epithelial component appears histopathologically benign and does not demonstrate any cytologic atypia. The mesenchymal portion of the tumor, however, is highly cellular and shows hyperchromatic and often-bizarre pleomorphic cells (Fig. 15-106). Mitoses are usually prominent. In some cases with multiple recurrences, the epithelial component becomes progressively less conspicuous so that the tumor eventually shows only a poorly differentiated fibrosarcoma.
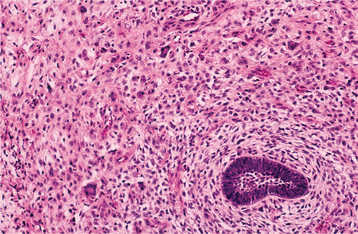
Fig. 15-106 Ameloblastic fibrosarcoma. The cellular mesenchymal tissue shows hyperchromatism and atypical cells. A small island of ameloblastic epithelium is present.
In a few instances, dysplastic dentin or small amounts of enamel may be formed. Some have called such lesions ameloblastic dentinosarcomas or ameloblastic fibro-odontosarcomas. This additional subclassification, however, appears unnecessary. Another rare event that actually may be overrepresented in the literature is concurrent malignant transformation of both the epithelial and mesenchymal elements of an ameloblastic fibroma, resulting in an ameloblastic carcinosarcoma.
TREATMENT AND PROGNOSIS: Once the diagnosis of ameloblastic fibrosarcoma has been confirmed, radical surgical excision appears to be the treatment of choice. Curettage or local excision is usually followed by rapid local recurrence. The tumor is locally aggressive and infiltrates adjacent bone and soft tissues.
The long-term prognosis is difficult to ascertain because of the few reported cases with adequate follow-up, with the best estimates suggesting that 20% of these patients will succumb to their tumor. Most deaths have resulted from uncontrolled local disease, and metastatic tumor has been documented in only 3 of 53 evaluable cases.
ODONTOAMELOBLASTOMA
The odontoameloblastoma is an extremely rare odontogenic tumor that contains an ameloblastomatous component and odontoma-like elements. Fewer than 20 cases have been reported with sufficient documentation to support this diagnosis. This tumor was formerly called ameloblastic odontoma and was confused with the more common (though still relatively rare) lesion currently designated as ameloblastic fibro-odontoma. Because the clinical behavior of these two tumors is quite different, they should be distinguished from one another. This neoplasm is also frequently confused with an odontoma that is in its early stages of development.
CLINICAL AND RADIOGRAPHIC FEATURES: Because of the rarity of odontoameloblastomas, little reliable information is available. The lesion appears to occur more often in younger patients, and either jaw can be affected. Pain, delayed eruption of teeth, and expansion of the affected bone may be noted.
Radiographically, the tumor shows a radiolucent, destructive process that contains calcified structures. These have the radiodensity of tooth structure and may resemble miniature teeth or occur as larger masses of calcified material similar to a complex odontoma.
HISTOPATHOLOGIC FEATURES: The histopathologic features of the odontoameloblastoma are complex. The proliferating epithelial portion of the tumor has features of an ameloblastoma, most often of the plexiform or follicular pattern. The ameloblastic component is intermingled with immature or more mature dental tissue in the form of developing rudimentary teeth, which is similar to the appearance of a compound odontoma, or conglomerate masses of enamel, dentin, and cementum, as seen in a complex odontoma.
TREATMENT AND PROGNOSIS: Multiple recurrences of odontoameloblastomas have been reported after local curettage, and it appears that this tumor has the same biologic potential as the ameloblastoma. It is probably wise to treat a patient with this lesion in the same manner as one with an ameloblastoma. However, there are no valid data on the long-term prognosis.
ODONTOMA
Odontomas are the most common types of odontogenic tumors. Their prevalence exceeds that of all other odontogenic tumors combined. Odontomas are considered to be developmental anomalies (hamartomas), rather than true neoplasms. When fully developed, odontomas consist chiefly of enamel and dentin, with variable amounts of pulp and cementum. In their earlier developmental stages, varying amounts of proliferating odontogenic epithelium and mesenchyme are present.
Odontomas are further subdivided into compound and complex types. The compound odontoma is composed of multiple, small toothlike structures. The complex odontoma consists of a conglomerate mass of enamel and dentin, which bears no anatomic resemblance to a tooth. In most series, compound odontomas are more frequently diagnosed than complex, and it is possible that some compound odontomas are not submitted for microscopic examination because the clinician is comfortable with the clinical and radiographic diagnosis. Occasionally, these lesions may show features of both compound and complex odontoma.
CLINICAL AND RADIOGRAPHIC FEATURES: Most odontomas are detected during the first two decades of life, and the mean age at the time of diagnosis is 14 years. The majority of these lesions are completely asymptomatic, being discovered on a routine radiographic examination or when films are taken to determine the reason for failure of a tooth to erupt. Odontomas are typically relatively small and seldom exceed the size of a tooth in the area where they are located. However, large odontomas up to 6 cm or more in diameter are occasionally seen. These large odontomas can cause expansion of the jaw.
Odontomas occur somewhat more frequently in the maxilla than in the mandible. Although compound and complex odontomas may be found in any site, the compound type is more often seen in the anterior maxilla; complex odontomas occur more often in the molar regions of either jaw. Occasionally, an odontoma will develop completely within the gingival soft tissues.
Radiographically, the compound odontoma appears as a collection of toothlike structures of varying size and shape surrounded by a narrow radiolucent zone (Figs. 15-107 and 15-108). The complex odontoma appears as a calcified mass with the radiodensity of tooth structure, which is also surrounded by a narrow radiolucent rim. An unerupted tooth is frequently associated with the odontoma, and the odontoma prevents eruption of the tooth (Fig. 15-109). Some small odontomas are present between the roots of erupted teeth and are not associated with disturbance in eruption. The radiographic findings are usually diagnostic, and the compound odontoma is seldom confused with any other lesion. A developing odontoma may show little evidence of calcification and appear as a circumscribed radiolucent lesion. A complex odontoma, however, may be radiographically confused with an osteoma or some other highly calcified bone lesion.
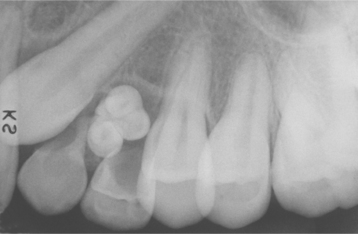
Fig. 15-107 Compound odontoma. A small cluster of toothlike structures is preventing the eruption of the maxillary canine. (Courtesy of Dr. Robert J. Powers.)
HISTOPATHOLOGIC FEATURES: The compound odontoma consists of multiple structures resembling small, single-rooted teeth, contained in a loose fibrous matrix (Fig. 15-110). The mature enamel caps of the toothlike structures are lost during decalcification for preparation of the microscopic section, but varying amounts of enamel matrix are often present. Pulp tissue may be seen in the coronal and root portions of the toothlike structures. In patients with developing odontomas, structures that resemble tooth germs are present.
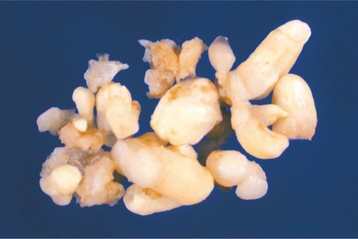
Fig. 15-110 Compound odontoma. Surgical specimen consisting of more than 20 malformed toothlike structures.
Complex odontomas consist largely of mature tubular dentin. This dentin encloses clefts or hollow circular structures that contained the mature enamel that was removed during decalcification. The spaces may contain small amounts of enamel matrix or immature enamel (Fig. 15-111). Small islands of eosinophilic-staining epithelial ghost cells are present in about 20% of complex odontomas. These may represent remnants of odontogenic epithelium that have undergone keratinization and cell death from the local anoxia. A thin layer of cementum is often present about the periphery of the mass. Occasionally, a dentigerous cyst may arise from the epithelial lining of the fibrous capsule of a complex odontoma.
Tumors of Odontogenic Ectomesenchyme
The central odontogenic fibroma is an uncommon and somewhat controversial lesion. Approximately 70 examples have been reported. Formerly, some oral and maxillofacial pathologists designated solid fibrous masses that were almost always associated with the crown of an unerupted tooth as odontogenic fibromas. Most oral and maxillofacial pathologists today consider such lesions to represent only hyperplastic dental follicles, and these should not be considered to be neoplasms.
CLINICAL AND RADIOGRAPHIC FEATURES: Odontogenic fibromas have been reported in patients whose ages ranged from 4 to 80 years (mean age, 40 years). Of those cases reported in the literature, a 2.2:1.0 female-to-male ratio has been noted, indicating a strong female predilection. About 45% of reported cases have occurred in the maxilla; most maxillary lesions are located anterior to the first molar tooth (Fig. 15-112). In the mandible, however, about half of the tumors are located posterior to the first molar. One third of odontogenic fibromas are associated with an unerupted tooth. Smaller odontogenic fibromas are usually completely asymptomatic; larger lesions may be associated with localized bony expansion or loosening of teeth.
Radiographically, smaller odontogenic fibromas tend to be well-defined, unilocular, radiolucent lesions often associated with the periradicular area of erupted teeth (Fig. 15-113). Larger lesions tend to be multi-locular radiolucencies. Many lesions have a sclerotic border. Root resorption of associated teeth is common, and lesions located between the teeth often cause root divergence. Approximately 12% of central odontogenic fibromas will exhibit radiopaque flecks within the lesion.
HISTOPATHOLOGIC FEATURES: Lesions reported as central odontogenic fibroma have shown considerable histopathologic diversity; this has led some authors to describe two separate types, although this concept has been questioned. The so-called simple odontogenic fibroma is composed of stellate fibroblasts, often arranged in a whorled pattern with fine collagen fibrils and considerable ground substance (Fig. 15-114). Small foci of odontogenic epithelial rests may or may not be present. Occasional foci of dystrophic calcification may be seen. Some investigators have suggested that this lesion actually belongs with the spectrum of odontogenic myxoma and should be designated as a myxofibroma. A more collagenized odontogenic fibroma needs to be differentiated from a desmoplastic fibroma, which is a more aggressive lesion. The desmoplastic fibroma, however, does not have an epithelial component.
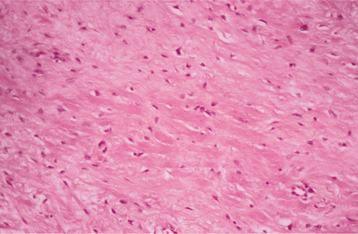
Fig. 15-114 Odontogenic fibroma (simple type). Scattered fibroblasts within a collagenous background. No epithelial rests were found on multiple sections from this tumor.
The central odontogenic fibroma, World Health Organization (WHO) type, has a more complex pattern, which often consists of a fairly cellular fibrous connective tissue with collagen fibers arranged in interlacing bundles. Odontogenic epithelium in the form of long strands or isolated nests is present throughout the lesion and may be a prominent component (Fig. 15-115). The fibrous component may vary from myxoid to densely hyalinized. Calcifications composed of cementum-like material or dentinoid are present in some cases.
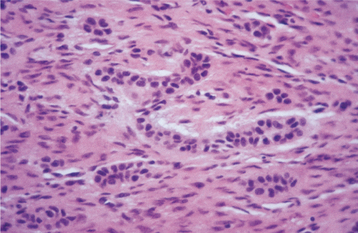
Fig. 15-115 Odontogenic fibroma (World Health Organization [WHO] type). A cellular fibroblastic lesion containing narrow cords of odontogenic epithelium.
Twelve examples of central odontogenic fibroma associated with a giant cell granuloma–like component have been reported since 1992 (Fig. 15-116). It seems unlikely that this process represents a “collision” tumor with synchronous occurrence of an odontogenic fibroma and a giant cell granuloma. Several of these lesions have recurred, and the recurrences typically exhibit both components. Whether the odontogenic fibroma somehow induced a giant cell response in these patients or whether this is a distinct biphasic lesion remains to be clarified.
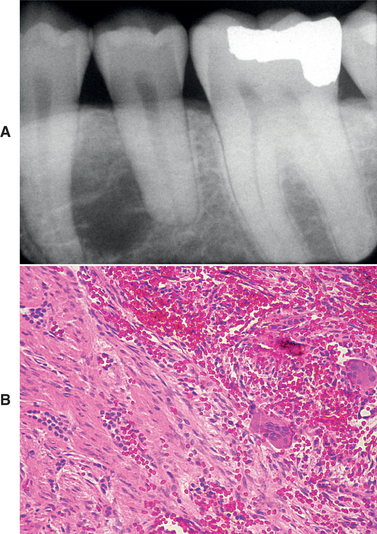
Fig. 15-116 Odontogenic fibroma (WHO type) with associated giant cell granuloma. A, Unilocular radiolucency between the left mandibular bicuspids. B, Microscopic examination reveals two distinct patterns. On the left, one can see cords of odontogenic epithelium within a fibrous background, consistent with odontogenic fibroma (WHO type). Typical features of central giant cell granuloma are present on the right side of the field.
TREATMENT AND PROGNOSIS: Odontogenic fibromas are usually treated by enucleation and vigorous curettage. Although the tumor does not have a definite capsule, it appears to have a limited growth potential, particularly in the anterior regions of the jaws. A few recurrences have been documented, but the prognosis is very good.
PERIPHERAL ODONTOGENIC FIBROMA
The relatively uncommon peripheral odontogenic fibroma is considered to represent the soft tissue counterpart of the central (intraosseous) odontogenic fibroma. In the past, some authors have designated clinically and histopathologically similar lesions as odontogenic epithelial hamartoma or as peripheral fibroameloblastic dentinoma. It is likely that all of these terms refer to the same lesion, and peripheral odontogenic fibroma seems to be the most appropriate designation. A few series of this lesion have been reported in the past 2 decades, bringing the total num-ber of cases in the literature to approximately 175.
CLINICAL AND RADIOGRAPHIC FEATURES: The peripheral odontogenic fibroma appears as a firm, slow-growing, and usually sessile gingival mass covered by normal-appearing mucosa (Fig. 15-117). Rarely, multifocal or diffuse lesions have been described. Clinically, the peripheral odontogenic fibroma cannot be distinguished from the much more common fibrous gingival lesions (see Chapter 12). The lesion is most often encountered on the facial gingiva of the mandible. Most lesions are between 0.5 and 1.5 cm in diameter, and they infrequently cause displacement of the teeth. Peripheral odontogenic fibromas have been recorded in patients across a wide age range, with most identified from the second to the seventh decades of life.
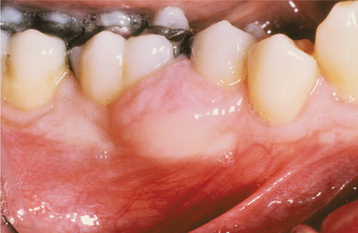
Fig. 15-117 Peripheral odontogenic fibroma. This sessile gingival mass cannot be clinically distinguished from the common peripheral ossifying fibroma. (Courtesy of Dr. Jerry Stovall.)
Radiographic studies demonstrate a soft tissue mass, which in some cases has shown areas of calcification. The lesion, however, does not involve the underlying bone.
HISTOPATHOLOGIC FEATURES: The peripheral odontogenic fibroma shows similar histopathologic features to the central odontogenic fibroma (WHO type). The tumor consists of interwoven fascicles of cellular fibrous connective tissue, which may be interspersed with areas of less cellular, myxoid connective tissue. A granular cell change has been rarely identified in the connective tissue component, and giant cell granuloma–like areas have been described in a few lesions. Islands or strands of odontogenic epithelium are scattered throughout the connective tissue. These may be prominent or scarce. The epithelial cells may show vacuolization. Dysplastic dentin, amorphous ovoid cementum-like calcifications, and trabeculae of osteoid may also be present.
GRANULAR CELL ODONTOGENIC TUMOR (GRANULAR CELL ODONTOGENIC FIBROMA)
The rare granular cell odontogenic tumor was initially reported as “granular cell ameloblastic fibroma.” Subsequently, it was designated as granular cell odontogenic fibroma, but the noncommittal term granular cell odontogenic tumor is probably more appropriate, given the controversial nature of the lesion. Approximately 30 cases of this unusual tumor have been reported.
CLINICAL AND RADIOGRAPHIC FEATURES: Patients with granular cell odontogenic tumors have all been adults at the time of diagnosis, with more than half being older than 40 years of age. More than 70% of the cases have developed in women. The tumor occurs primarily in the mandible and most often in the premolar and molar region. Some lesions are completely asymptomatic; others present as a painless, localized expansion of the affected area. A few cases of granular cell odontogenic tumor have been described in the gingival soft tissues as well.
Radiographically, the lesion appears as a well-demarcated radiolucency, which may be unilocular or multilocular and occasionally shows small calcifications (Fig. 15-118).
HISTOPATHOLOGIC FEATURES: The granular cell odontogenic tumor is composed of large eosinophilic granular cells, which closely resemble the granular cells seen in the soft tissue granular cell tumor (see page 536) or the granular cells seen in the granular cell variant of the ameloblastoma (see page 709). Narrow cords or small islands of odontogenic epithelium are scattered among the granular cells (Fig. 15-119). Small cementum-like or dystrophic calcifications associated with the granular cells have been seen in some lesions.
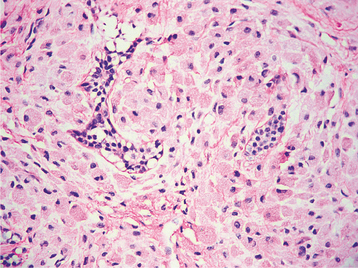
Fig. 15-119 Granular cell odontogenic tumor. Sheet of large granular mesenchymal cells with small nests of odontogenic epithelium.
The nature of the granular cells is controversial. Ultrastructural studies reveal the features of mesenchymal cells, and bodies consistent with lysosomal structures have been identified within the lesional cell cytoplasm. Immunohistochemically, the granular cells in the granular cell odontogenic tumor do not react with antibodies directed against S-100 protein, in contrast to the positive S-100 reactivity of the granular cell tumor.
TREATMENT AND PROGNOSIS: The granular cell odontogenic fibroma appears to be completely benign in the overwhelming majority of instances and responds well to curettage. Only one recurrence has been documented, and a solitary example of a malignant central granular cell odontogenic fibroma has been reported.
ODONTOGENIC MYXOMA
Myxomas of the jaws are believed to arise from odontogenic ectomesenchyme. They bear a close microscopic resemblance to the mesenchymal portion of a developing tooth. Formerly, some investigators made a distinction between odontogenic myxomas (derived from odontogenic mesenchyme) and osteogenic myxomas (presumably derived from primitive bone tissue). However, most authorities in orthopedic pathologic practice do not accept that myxomas occur in the extragnathic skeleton, and all myxomas of the jaws are currently considered to be of odontogenic origin.
CLINICAL AND RADIOGRAPHIC FEATURES: Myxomas are predominantly found in young adults but may occur across a wide age group. The average age for patients with myxomas is 25 to 30 years. There is no sex predilection. The tumor may be found in almost any area of the jaws, and the mandible is involved more commonly than the maxilla (Fig. 15-120). Smaller lesions may be asymptomatic and are discovered only during a radiographic examination. Larger lesions are often associated with a painless expansion of the involved bone. In some instances, clinical growth of the tumor may be rapid; this is probably related to the accumulation of myxoid ground substance in the tumor.
Radiographically, the myxoma appears as a unilocular or multilocular radiolucency that may displace or cause resorption of teeth in the area of the tumor (Fig. 15-121). The margins of the radiolucency are often irregular or scalloped. The radiolucent defect may contain thin, wispy trabeculae of residual bone, which are often arranged at right angles to one another (Fig. 15-122). Large myxomas of the mandible may show a “soap bubble” radiolucent pattern, which is indistinguishable from that seen in ameloblastomas (Fig. 15-123).
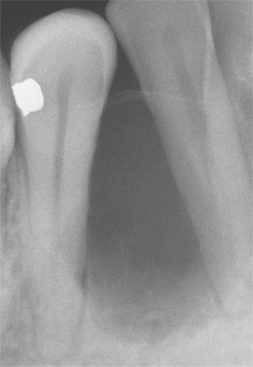
Fig. 15-121 Odontogenic myxoma. Unilocular radiolucency between the right mandibular lateral incisor and cuspid.
HISTOPATHOLOGIC FEATURES: At the time of surgery or gross examination of the specimen, the gelatinous, loose structure of the myxoma is obvious (Fig. 15-124). Microscopically, the tumor is composed of haphazardly arranged stellate, spindle-shaped, and round cells in an abundant, loose myxoid stroma that contains only a few collagen fibrils (Fig. 15-125). Histochemical study shows that the ground substance is composed of glycosaminoglycans, chiefly hyaluronic acid and chondroitin sulfate. Immunohistochemically, the myxoma cells show diffuse immunoreactivity with antibodies directed against vimentin, with focal reactivity for muscle-specific actin. Small islands of inactive-appearing odontogenic epithelial rests may be scattered throughout the myxoid ground substance. These epithelial rests are not required for the diagnosis and are not obvious in most cases. In some patients, the tumor may have a greater tendency to form collagen fibers; such lesions are sometimes designated as fibromyxomas or myxofibromas. There is no evidence that the more collagenized variants deserve separate consideration, although some investigators have suggested that these may represent part of a spectrum that includes the central odontogenic fibroma at the other endpoint.
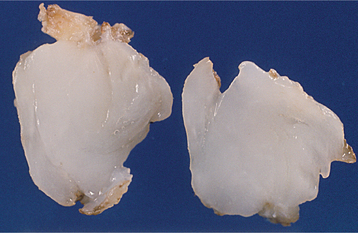
Fig. 15-124 Odontogenic myxoma. Gross specimen of case shown in Fig. 15-121, demonstrating a white gelatinous mass.

Fig. 15-125 Odontogenic myxoma. A loose, myxomatous tumor can be seen filling the marrow spaces between the bony trabeculae. The inset shows stellate-shaped cells and fine collagen fibrils.
A myxoma may be microscopically confused with other myxoid jaw neoplasms, such as the rare chondromyxoid fibroma (see page 657) or the myxoid neurofibroma (see page 528). Chondromyxoid fibroma should have areas of cartilaginous differentiation, whereas myxoid neurofibromas tend to have scattered lesional cells that are positive for antibodies directed against S-100 protein. Myxoid change in an enlarged dental follicle or the dental papilla of a developing tooth may be microscopically similar to a myxoma. Evaluation of the clinical and radiographic features, however, will prevent overdiagnosis of these lesions as myxomas.
TREATMENT AND PROGNOSIS: Small myxomas are generally treated by curettage, but careful periodic reevaluation is necessary for at least 5 years. For larger lesions, more extensive resection may be required because myxomas are not encapsulated and tend to infiltrate the surrounding bone. Complete removal of a large tumor by curettage is often difficult to accomplish, and lesions of the posterior maxilla, in particular, should be treated more aggressively in most instances. Recurrence rates from various studies average approximately 25%. In spite of local recurrences, the overall prognosis is good, and metastases do not occur.
In rare cases the myxoma microscopically shows marked cellularity and cellular atypism. Some have designated these lesions as myxosarcomas or malignant odontogenic myxoma. They appear to have a more aggressive local course than do the usual myxomas. Death because of involvement of vital structures by the tumor has been described, but distant metastases have not been reported.
CEMENTOBLASTOMA (TRUE CEMENTOMA)
Many oral and maxillofacial pathologists consider the cementoblastoma to represent an odontogenic tumor. However, other pathologists have pointed out that the histopathologic features of cementoblastomas of the jaws are identical to those of a bone tumor, osteoblastoma, seen both in the jaws and extragnathic skeleton. Cementoblastomas are discussed in Chapter 14 (see page 655).
BIBLIOGRAPHY
Odontogenic Cysts and Tumors—General References and Classification
Barnes L, Eveson JW, Reichart P, et al, eds. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon, France: IARC Press, 2005.
Jones, AV, Craig, GT, Franklin, CD. Range and demographics of odontogenic cysts diagnosed in a UK population over a 30-year period. J Oral Pathol Med. 2006;35:500–507.
Kramer, IRH, Pindborg, JJ, Shear, M. Histological typing of odontogenic tumors, ed 2. New York: Springer-Verlag, 1992.
Kramer, IRH, Pindborg, JJ, Shear, M. The World Health Organization histological typing of odontogenic tumours: introducing the second edition. Eur J Cancer B Oral Oncol. 1993;29B:169–171.
Kreidler, JF, Raubenheimer, EJ, van Heerden, WFP. A retrospective analysis of 367 cystic lesions of the jaw—the Ulm experience. J Craniomaxillofac Surg. 1993;21:339–341.
Nakamura, T, Ishida, J, Nakano, Y, et al. A study of cysts in the oral region: cysts of the jaws. J Nihon Univ Sch Dent. 1995;37:33–40.
Neville, BW, Damm, DD, Allen, CM. Odontogenic cysts and tumors. In: Gnepp D, ed. Diagnostic surgical pathology of the head and neck. Philadelphia: WB Saunders, 2001.
Philipsen, HP, Reichart, PA. The development and fate of epithelial residues after completion of the human odontogenesis with special reference to the origins of epithelial odontogenic neoplasms, hamartomas and cysts. Oral Biosci Med. 2004;1:171–179.
Shear, M. Developmental odontogenic cysts. An update. J Oral Pathol Med. 1994;23:1–11.
Shear, M, Speight, P. Cysts of the oral and maxillofacial regions, ed 4. Oxford: Blackwell, 2007.
Ackermann, G, Cohen, MA, Altini, M. The paradental cyst: a clinicopathologic study of 50 cases. Oral Surg Oral Med Oral Pathol. 1987;64:308–312.
Adelsperger, J, Campbell, JH, Coates, DB, et al. Early soft tissue pathosis associated with impacted third molars without pericoronal radiolucency. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:402–406.
Benn, A, Altini, M. Dentigerous cysts of inflammatory origin: a clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:203–209.
Clauser, C, Zuccati, G, Barone, R, et al. Simplified surgical-orthodontic treatment of a dentigerous cyst. J Clin Orthod. 1994;28:103–106.
Craig, GT. The paradental cyst: a specific inflammatory odontogenic cyst. Br Dent J. 1976;141:9–14.
Curran, AE, Damm, DD, Drummond, JF. Pathologically significant pericoronal lesions in adults: histopathologic evaluation. J Oral Maxillofac Surg. 2002;60:613–617.
Daley, TD, Wysocki, GP. The small dentigerous cyst: a diagnostic dilemma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:77–81.
Delbem, AC, Cunha, RF, Afonso, RL, et al. Dentigerous cysts in primary dentition: report of 2 cases. Pediatr Dent. 2006;28:269–272.
Gorlin, RJ. Potentialities of oral epithelium manifest by mandibular dentigerous cysts. Oral Surg Oral Med Oral Pathol. 1957;10:271–284.
Kusukawa, J, Irie, K, Morimatsu, M, et al. Dentigerous cyst associated with a deciduous tooth: a case report. Oral Surg Oral Med Oral Pathol. 1992;73:415–418.
Lustmann, L, Bodner, L. Dentigerous cysts associated with supernumerary teeth. Int J Oral Maxillofac Surg. 1988;17:100–102.
Motamedi, MHK, Talesh, KT. Management of extensive dentigerous cysts. Br Dent J. 2005;198:203–206.
Takeda, Y, Oikawa, Y, Furuya, I, et al. Mucous and ciliated cell metaplasia in epithelial linings of odontogenic inflammatory and developmental cysts. J Oral Sci. 2005;47:77–81.
Ziccardi, VB, Eggleston, TI, Schneider, RE. Using fenestration technique to treat a large dentigerous cyst. J Am Dent Assoc. 1997;128:201–205.
Aguiló, L, Cibrián, R, Bagán, JV, et al. Eruption cysts: retrospective clinical study of 36 cases. ASDC J Dent Child. 1998;65:102–106.
Bodner, L, Goldstein, J, Sarnat, H. Eruption cysts: a clinical report of 24 new cases. J Clin Pediatr Dent. 2004;28:183–186.
Clark, CA. A survey of eruption cysts in the newborn. Oral Surg Oral Med Oral Pathol. 1962;15:917.
Kuczek, A, Beikler, T, Herbst, H, et al. Eruption cyst formation associated with cyclosporin A: a case report. J Clin Periodontol. 2003;30:462–466.
Seward, MH. Eruption cyst: an analysis of its clinical features. J Oral Surg. 1973;31:31–35.
Brannon, RB. The odontogenic keratocyst—a clinicopathologic study of 312 cases. Part I: Clinical features. Oral Surg Oral Med Oral Pathol. 1976;42:54–72.
Robinson, HBG. Classification of cysts of the jaws. Am J Orthod Oral Surg. 1945;31:370–375.
Ahlfors, E, Larsson, A, Sjögren, S. The odontogenic keratocyst: a benign cystic tumor? J Oral Maxillofac Surg. 1984;42:10–19.
Ali, M, Baughman, RA. Maxillary odontogenic keratocyst: a common and serious clinical misdiagnosis. J Am Dent Assoc. 2003;134:877–883.
Agaram, NP, Collins, BM, Barnes, L, et al. Molecular analysis to demonstrate that odontogenic keratocysts are neoplastic. Arch Pathol Lab Med. 2004;128:313–317.
Barnes L, Eveson JW, Reichart P, et al, eds. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon, France: IARC Press, 2005.
Bataineh, AB, Al Qudah, MA. Treatment of mandibular odontogenic keratocysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1988;86:42–47.
Blanas, N, Freund, B, Schwartz, M, et al. Systematic review of the treatment and prognosis of the odontogenic keratocyst. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1988;90:553–558.
Brannon, RB. The odontogenic keratocyst—a clinicopathologic study of 312 cases. Part I: Clinical features. Oral Surg Oral Med Oral Pathol. 1976;42:54–72.
Brannon, RB. The odontogenic keratocyst—a clinicopathologic study of 312 cases. Part II: Histologic features. Oral Surg Oral Med Oral Pathol. 1977;43:233–255.
Brøndum, N, Jensen, VJ. Recurrence of keratocysts and decompression treatment: a long-term follow-up of forty-four cases. Oral Surg Oral Med Oral Pathol. 1991;72:265–269.
Chehade, A, Daley, TD, Wysocki, GP, et al. Peripheral odontogenic keratocyst. Oral Surg Oral Med Oral Pathol. 1994;77:494–497.
Chi, AC, Owings, JR, Muller, S. Peripheral odontogenic keratocyst: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:71–78.
Fornatora, ML, Reich, RF, Chotkowski, G, et al. Odontogenic keratocyst with mural cartilaginous metaplasia: a case report and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:430–434.
Forssell, K, Forssell, H, Kahnberg, K-E. Recurrence of keratocysts: a long-term follow-up study. Int J Oral Maxillofac Surg. 1988;17:25–28.
Garlock, JA, Pringle, GA, Hicks, ML. The odontogenic keratocyst: a potential endodontic misdiagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1988;85:452–456.
Henley, J, Summerlin, D-J, Tomich, C, et al. Molecular evidence supporting the neoplastic nature of odontogenic keratocyst: a laser capture microdissection study of 15 cases. Histopathology. 2005;47:582–586.
Ide, F, Shimoyama, T, Horie, N. Peripheral odontogenic keratocyst: a report of 2 cases. J Periodontol. 2002;73:1079–1081.
Jackson, IT, Potparic, Z, Fasching, M, et al. Penetration of the skull base by dissecting keratocyst. J Craniomaxillofac Surg. 1993;21:319–325.
Kakarantza-Angelopoulou, E, Nicolatou, O. Odontogenic keratocysts: clinicopathologic study of 87 cases. J Oral Maxillofac Surg. 1990;48:593–599.
Kolokythas, A, Fernandes, RP, Pazoki, A, et al. Odontogenic keratocyst: to decompress or not to decompress? A comparative study of decompression and enucleation versus resection/peripheral ostectomy. J Oral Maxillofac Surg. 2007;65:640–644.
Kratochvil, FJ, Brannon, RB. Cartilage in the walls of odontogenic keratocysts. J Oral Pathol Med. 1993;22:282–285.
Levanat, S, Kappler, R, Hemmerlein, B, et al. Analysis of the PTCH1 signaling pathway in ovarian dermoids. Int J Mol Med. 2004;14:793–799.
Makowski, GJ, McGuff, S, van Sickels, JE. Squamous cell carcinoma in a maxillary odontogenic keratocyst. J Oral Maxillofac Surg. 2001;59:76–80.
Meiselman, F. Surgical management of the odontogenic keratocyst: conservative approach. J Oral Maxillofac Surg. 1994;52:960–963.
Morgan, TA, Burton, CC, Qian, F. A retrospective review of treatment of the odontogenic keratocyst. J Oral Maxillofac Surg. 2005;63:635–639.
Myoung, H, Hong, S-P, Hong, S-D, et al. Odontogenic keratocyst: review of 256 cases for recurrence and clinicopathologic parameters. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:328–333.
Neville, BW, Damm, DD, Brock, TR. Odontogenic keratocysts of the midline maxillary region. J Oral Maxillofac Surg. 1997;55:340–344.
Pogrel, MA, Jordan, RCK. Marsupialization as a definitive treatment for the odontogenic keratocyst. J Oral Maxillofac Surg. 2004;62:651–655.
Preston, RD, Narayana, N. Peripheral odontogenic keratocyst. J Periodontol. 2005;76:2312–2315.
Rodu, B, Tate, AL, Martinez, MG. The implications of inflammation in odontogenic keratocysts. J Oral Pathol. 1987;16:518–521.
Shear, M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 1. Clinical and early experimental evidence of aggressive behavior. Oral Oncol. 2002;38:219–226.
Shear, M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 2. Proliferation and genetic studies. Oral Oncol. 2002;38:323–331.
Shear, M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 3. Immunocytochemistry of cytokeratin and other epithelial cell markers. Oral Oncol. 2002;38:407–415.
Voorsmit, RACA. The incredible keratocyst: a new approach to treatment. Dtsch Zahnarztl Z. 1985;40:641–644.
Williams, TP, Connor, FA, Jr. Surgical management of the odontogenic keratocyst: aggressive approach. J Oral Maxillofac Surg. 1994;52:964–966.
Yamazaki, M, Cheng, J, Nomura, T, et al. Maxillary odontogenic keratocyst with respiratory epithelium: a case report. J Oral Pathol Med. 2003;32:496–498.
Yoon, JH, Kim, SG, Lee, SH, et al. Simultaneous occurrence of an odontogenic keratocyst and giant cell granuloma-like lesion in the mandible. Int J Oral Maxillofac Surg. 2004;33:615–617.
Orthokeratinized Odontogenic Cysts
Crowley, TE, Kaugars, GE, Gunsolley, JC. Odontogenic keratocysts: a clinical and histologic comparison of the parakeratin and orthokeratin variants. J Oral Maxillofac Surg. 1992;50:22–26.
Li, T-J, Kitano, M, Chen, X-M, et al. Orthokeratinized odontogenic cyst: a clinicopathological and immunocytochemical study of 15 cases. Histopathology. 1998;32:242–251.
Siar, CH, Ng, KH. Orthokeratinised odontogenic keratocysts in Malaysians. Br J Oral Maxillofac Surg. 1988;26:215–220.
Vuhahula, E, Nikai, H, Ijuhin, N, et al. Jaw cysts with orthokeratinization: analysis of 12 cases. J Oral Pathol Med. 1993;22:35–40.
Wright, JM. The odontogenic keratocyst: orthokeratinized variant. Oral Surg Oral Med Oral Pathol. 1981;51:609–618.
Nevoid Basal Cell Carcinoma Syndrome
Cohen, MM, Jr. Nevoid basal cell carcinoma syndrome: molecular biology and new hypotheses. Int J Oral Maxillofac Surg. 1999;28:216–223.
Evans, DGR, Ladusans, EJ, Rimmer, S, et al. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet. 1993;30:460–464.
Goldstein, AM, Pastakia, B, DeGiovanna, JJ, et al. Clinical findings in two African-American families with the nevoid basal cell carcinoma syndrome (NBCC). Am J Med Genet. 1994;50:272–281.
Gorlin, RJ. Nevoid basal cell carcinoma (Gorlin) syndrome. Genet Med. 2004;6:530–539.
Gorlin, RJ, Goltz, R. Multiple nevoid basal cell epithelioma, jaw cysts and bifid rib syndrome. N Engl J Med. 1960;262:908–914.
Kimonis, VE, Goldstein, AM, Pastakia, B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299–308.
Lo Muzio, L, Nocini, P, Bucci, P, et al. Early diagnosis of nevoid basal cell carcinoma syndrome. J Am Dent Assoc. 1999;130:669–674.
Lo Muzio, L, Staibano, S, Pannone, G, et al. Expression of cell cycle and apoptosis-related proteins in sporadic odontogenic keratocysts and odontogenic keratocysts associated with the nevoid basal cell carcinoma syndrome. J Dent Res. 1999;78:1345–1353.
Manfredi, M, Vescovi, P, Bonanini, M, et al. Nevoid basal cell carcinoma syndrome: a review of the literature. Int J Oral Maxillofac Surg. 2004;33:117–124.
Shanley, S, Ratcliffe, J, Hockey, A, et al. Nevoid basal cell carcinoma syndrome: review of 118 affected individuals. Am J Med Genet. 1994;50:282–290.
Woolgar, JA, Rippin, JW, Browne, RM. A comparative histologic study of odontogenic keratocysts in basal cell nevus syndrome and non-syndrome patients. J Oral Pathol. 1987;16:75–80.
Woolgar, JA, Rippin, JW, Browne, RM. The odontogenic keratocyst and its occurrence in the nevoid basal cell carcinoma syndrome. Oral Surg Oral Med Oral Pathol. 1987;64:727–730.
Cataldo, E, Berkman, M. Cysts of the oral mucosa in newborns. Am J Dis Child. 1968;116:44–48.
Donley, CL, Nelson, LP. Comparison of palatal and alveolar cysts of the newborn in premature and full term infants. Pediatr Dent. 2000;22:321–324.
Fromm, A. Epstein’s pearls, Bohn’s nodules and inclusion cysts of the oral cavity. J Dent Child. 1967;34:275–287.
Jorgenson, RJ, Shapiro, SD, Salinas, CF, et al. Intraoral findings and anomalies in neonates. Pediatrics. 1982;69:577–582.
Paula, JDR, Dezan, CC, Frossard, WTG, et al. Oral and facial inclusion cysts in newborns. J Clin Pediatr Dent. 2006;31:127–129.
Bell, RC, Chauvin, PJ, Tyler, MT. Gingival cyst of the adult: a review and report of eight cases. J Can Dent Assoc. 1997;63:533–535.
Breault, LG, Billman, MA, Lewis, DM. Report of a gingival “surgical cyst” developing secondarily to a subepithelial connective tissue graft. J Periodontol. 1997;68:392–395.
Buchner, A, Hansen, LS. The histomorphologic spectrum of the gingival cyst in the adult. Oral Surg Oral Med Oral Pathol. 1979;48:532–539.
Cairo, F, Rotundo, R, Ficarra, G. A rare lesion of the periodontium: the gingival cyst of the adult—a report of three cases. Int J Periodontics Restorative Dent. 2002;22:79–83.
Cunha, KG, Carvalho Neto, LG, Saraiva, FM, et al. Gingival cyst of the adult: a case report. Gen Dent. 2005;53:215–216.
Nxumalo, TN, Shear, M. Gingival cyst in adults. J Oral Pathol Med. 1992;21:309–313.
Altini, M, Shear, M. The lateral periodontal cyst: an update. J Oral Pathol Med. 1992;21:245–250.
Baker, RD, D’Onofrio, ED, Corio, RL. Squamous-cell carcinoma arising in a lateral periodontal cyst. Oral Surg Oral Med Oral Pathol. 1979;47:495–499.
Carter, LC, Carney, YL, Perez-Pudlewski, D. Lateral periodontal cyst: multifactorial analysis of a previously unreported series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:210–216.
Cohen, D, Neville, B, Damm, D, et al. The lateral periodontal cyst: a report of 37 cases. J Periodontol. 1984;55:230–234.
Fantasia, JE. Lateral periodontal cyst: an analysis of forty-six cases. Oral Surg Oral Med Oral Pathol. 1979;48:237–243.
Greer, RO, Johnson, M. Botryoid odontogenic cyst: clinicopathologic analysis of ten cases with three recurrences. J Oral Maxillofac Surg. 1988;46:574–579.
Gurol, M, Burkes, EJ, Jr., Jacoway, J. Botryoid odontogenic cyst: analysis of 33 cases. J Periodontol. 1995;66:1069–1073.
Kerezoudis, NP, Donta-Bakoyianni, C, Siskos, G. The lateral periodontal cyst: aetiology, clinical significance and diagnosis. Endod Dent Traumatol. 2000;16:144–150.
Ramer, M, Valauri, D. Multicystic lateral periodontal cyst and botryoid odontogenic cyst: multifactorial analysis of previously unreported series and review of literature. N Y State Dent J. 2005;71:47–51.
Rasmusson, LG, Magnusson, BC, Borrman, H. The lateral periodontal cyst: a histopathological and radiographic study of 32 cases. Br J Oral Maxillofac Surg. 1991;29:54–57.
Wysocki, GP, Brannon, RB, Gardner, DG, et al. Histogenesis of the lateral periodontal cyst and the gingival cyst of the adult. Oral Surg Oral Med Oral Pathol. 1980;50:327–334.
Barnes L, Eveson JW, Reichart P, et al, eds. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon, France: IARC Press, 2005.
Buchner, A. The central (intraosseous) calcifying odontogenic cyst: an analysis of 215 cases. J Oral Maxillofac Surg. 1991;49:330–339.
Buchner, A, Merrell, PW, Hansen, LS, et al. Peripheral (extraosseous) calcifying odontogenic cyst. Oral Surg Oral Med Oral Pathol. 1991;72:65–70.
Daniels, JSM. Recurrent calcifying odontogenic cyst involving the maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:660–664.
Ellis, GL. Odontogenic ghost cell tumor. Semin Diagn Pathol. 1999;16:288–292.
Ellis, GL, Shmookler, BM. Aggressive (malignant?) epithelial odontogenic ghost cell tumor. Oral Surg Oral Med Oral Pathol. 1986;61:471–478.
Fregnani, ER, Pires, FR, Quezada, RD, et al. Calcifying odontogenic cyst: clinicopathological features and immunohistochemical profile of 10 cases. J Oral Pathol Med. 2003;32:163–170.
Goldenberg, D, Sciubba, J, Tufano, RP. Odontogenic ghost cell carcinoma. Head Neck. 2004;26:378–381.
Gorlin, RJ, Pindborg, JJ, Clausen, FP, et al. The calcifying odontogenic cyst—a possible analogue to the cutaneous calcifying epithelioma of Malherbe: an analysis of fifteen cases. Oral Surg. 1962;15:1235–1243.
Hong, SP, Ellis, GL, Hartman, KS. Calcifying odontogenic cyst: a review of ninety-two cases with reevaluation of their nature as cysts or neoplasms, the nature of the ghost cells and subclassification. Oral Surg Oral Med Oral Pathol. 1991;72:56–64.
Iida, S, Fukuda, Y, Ueda, T, et al. Calcifying odontogenic cyst: radiologic findings in 11 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:356–362.
Johnson, A, III., Fletcher, M, Gold, L, et al. Calcifying odontogenic cyst: a clinicopathologic study of 57 cases with immunohistochemical evaluation for cytokeratin. J Oral Maxillofac Surg. 1997;55:679–683.
Li, T-J, Yu, S-F. Clinicopathologic spectrum of the so-called calcifying odontogenic cysts: a study of 21 intraosseous cases with reconsideration of the terminology and classification. Am J Surg Pathol. 2003;27:372–384.
Lu, Y, Mock, D, Takata, T, et al. Odontogenic ghost cell carcinoma: report of four new cases and review of the literature. J Oral Pathol Med. 1999;28:323–329.
Praetorius, F, Hjørting-Hansen, E, Gorlin, RJ, et al. Calcifying odontogenic cyst: range, variations and neoplastic potential. Acta Odontol Scand. 1981;39:227–240.
Tajima, Y, Yokose, S, Sakamoto, E, et al. Ameloblastoma arising in calcifying odontogenic cyst. Oral Surg Oral Med Oral Pathol. 1992;74:776–779.
Toida, M. So-called calcifying odontogenic cyst: review and discussion on the terminology and classification. J Oral Pathol Med. 1998;27:49–52.
Yoshida, M, Kumamoto, H, Ooya, K, et al. Histopathological and immunohistochemical analysis of calcifying odontogenic cysts. J Oral Pathol Med. 2001;30:582–588.
Gardner, DG, Kessler, HP, Morency, R, et al. The glandular odontogenic cyst: an apparent entity. J Oral Pathol. 1988;17:359–366.
High, AS, Main, DMG, Khoo, SP, et al. The polymorphous odontogenic cyst. J Oral Pathol Med. 1996;25:25–31.
Hussain, K, Edmondson, HD, Browne, RM. Glandular odontogenic cysts: diagnosis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:593–602.
Kaplan, I, Gal, G, Anavi, Y, et al. Glandular odontogenic cyst: treatment and recurrence. J Oral Maxillofac Surg. 2005;63:435–441.
Koppang, HS, Johannessen, S, Haugen, LK, et al. Glandular odontogenic cyst (sialo-odontogenic cyst): report of two cases and literature review of 45 previously reported cases. J Oral Pathol Med. 1998;27:455–462.
Magnusson, B, Göransson, L, Ödesjö, B, et al. Glandular odontogenic cyst: report of seven cases. Dentomaxillofac Radiol. 1997;26:26–31.
Manor, R, Anavi, Y, Kaplan, I, et al. Radiological features of glandular odontogenic cyst. Dentomaxillofac Radiol. 2003;32:73–79.
Noffke, C, Raubenheimer, EJ. The glandular odontogenic cyst: clinical and radiological features; review of the literature and report of nine cases. Dentomaxillofac Radiol. 2002;31:333–338.
Qin, X-N, Li, J-R, Chen, X-M, et al. The glandular odontogenic cyst: clinicopathologic features and treatment of 14 cases. J Oral Maxillofac Surg. 2005;63:694–699.
Shen, J, Fan, M, Chen, X, et al. Glandular odontogenic cyst in China: report of 12 cases and immunohistochemical study. J Oral Pathol Med. 2006;35:175–182.
Tran, P-T, Cunningham, CJ, Baughman, RA. Glandular odontogenic cyst. J Endod. 2004;30:182–184.
Colgan, CM, Henry, J, Napier, SS, et al. Paradental cysts: a role for food impaction in the pathogenesis? A review of cases from Northern Ireland. Br J Oral Maxillofac Surg. 2002;40:163–168.
Craig, GT. The paradental cyst: a specific inflammatory odontogenic cyst. Br Dent J. 1976;141:9–14.
David, LA, Sàndor, GKB, Stoneman, DW. The buccal bifurcation cyst: is non-surgical treatment an option? J Can Dent Assoc. 1998;64:712–716.
Fowler, CB, Brannon, RB. The paradental cyst: a clinicopathologic study of six new cases and review of the literature. J Oral Maxillofac Surg. 1989;47:243–248.
Pompura, JR, Sàndor, GKB, Stoneman, DW. The buccal bifurcation cyst: a prospective study of treatment outcomes in 44 sites. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:215–221.
Shohat, I, Buchner, A, Taicher, S. Mandibular buccal bifurcation cyst: enucleation without extraction. Int J Oral Maxillofac Surg. 2003;32:610–613.
Stoneman, DW, Worth, HM. The mandibular infected buccal cyst-molar area. Dent Radiogr Photogr. 1983;56:1–14.
Carcinoma Arising in Odontogenic Cysts
Cavalcanti, MGP, Veltrini, VC, Ruprecht, A, et al. Squamous-cell carcinoma arising from an odontogenic cyst—the importance of computed tomography in the diagnosis of malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:365–368.
Chaisuparat, R, Coletti, D, Kolokythas, A, et al. Primary intraosseous odontogenic carcinoma arising in an odontogenic cyst or de novo: a clinicopathologic study of six new cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:196–202.
Gardner, AF. The odontogenic cyst as a potential carcinoma: a clinicopathologic appraisal. J Am Dent Assoc. 1969;78:746–755.
Hennis, HL, Stewart, WC, Neville, B, et al. Carcinoma arising in an odontogenic keratocyst with orbital invasion. Doc Ophthalmol. 1991;77:73–79.
Makowski, GJ, McGuff, S, van Sickels, JE. Squamous cell carcinoma in a maxillary odontogenic keratocyst. J Oral Maxillofac Surg. 2001;59:76–80.
Scheer, M, Koch, AM, Drebber, U, et al. Primary intraosseous carcinoma of the jaws arising from an odontogenic cyst—a case report. J Craniomaxillofac Surg. 2004;32:166–169.
Stoelinga, PJ, Bronkhorst, FB. The incidence, multiple presentation and recurrence of aggressive cysts of the jaws. J Craniomaxillofac Surg. 1988;16:184–195.
Swinson, BD, Jerjes, W, Thomas, GJ. Squamous cell carcinoma arising in a residual odontogenic cyst: case report. J Oral Maxillofac Surg. 2005;63:1231–1233.
van der Waal, I, Rauhamaa, R, van der Kwast, WAM, et al. Squamous cell carcinoma arising in the lining of odontogenic cysts: report of 5 cases. Int J Oral Surg. 1985;14:146–152.
Waldron, CA, Mustoe, TA. Primary intraosseous carcinoma of the mandible with probable origin in an odontogenic cyst. Oral Surg Oral Med Oral Pathol. 1989;67:716–724.
Yasuoka, T, Yonemoto, K, Kato, Y, et al. Squamous cell carcinoma arising in a dentigerous cyst. J Oral Maxillofac Surg. 2000;58:900–905.
Yoshida, H, Onizawa, K, Yusa, H. Squamous cell carcinoma arising in association with an orthokeratinized odontogenic keratocyst. Report of a case. J Oral Maxillofac Surg. 1996;54:647–651.
Buchner, A, Merrell, PW, Carpenter, WM. Relative frequency of central odontogenic tumors: a study of 1,088 cases from northern California and comparison to studies from other parts of the world. J Oral Maxillofac Surg. 2006;64:1343–1352.
Carlson, ER, Marx, RE. The ameloblastoma: primary, curative surgical management. J Oral Maxillofac Surg. 2006;64:484–494.
Eversole, LR, Leider, AS, Hansen, LS. Ameloblastomas with pronounced desmoplasia. J Oral Maxillofac Surg. 1984;42:735–740.
Gardner, DG. Some current concepts on the pathology of ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:660–669.
Gardner, DG. Critique of the 1995 review by Reichart et al of the biologic profile of 3677 ameloblastomas. Oral Oncol. 1999;35:443–449.
Gardner, DG, Pecak, AMJ. The treatment of ameloblastoma based on pathologic and anatomic principles. Cancer. 1980;46:2514–2519.
Ghandhi, D, Ayoub, AF, Pogrel, MA, et al. Ameloblastoma: a surgeon’s dilemma. J Oral Maxillofac Surg. 2006;64:1010–1014.
Gortzak, RA, Latief, BS, Lekkas, C, et al. Growth characteristics of large mandibular ameloblastomas: report of 5 cases with implications for the approach to surgery. Int J Oral Maxillofac Surg. 2006;35:691–695.
Hirota, M, Aoki, S, Kawabe, R, et al. Desmoplastic ameloblastoma featuring basal cell ameloblastoma: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:160–164.
Hong, J, Yun, P-Y, Chung, I-H, et al. Long-term follow up on recurrence of 305 ameloblastoma cases. Int J Oral Maxillofac Surg. 2007;36:283–288.
Keszler, A, Paparella, ML, Dominguez, FV. Desmoplastic and non-desmoplastic ameloblastoma: a comparative clinicopathological analysis. Oral Dis. 1996;2:228–231.
Kishino, M, Murakami, S, Fukuda, Y, et al. Pathology of the desmoplastic ameloblastoma. J Oral Pathol Med. 2001;30:35–40.
Leibovitch, I, Schwarcz, RM, Modjtahedi, S, et al. Orbital inva-sion by recurrent maxillary ameloblastoma. Ophthalmology. 2006;113:1227–1230.
Leider, AS, Eversole, LR, Barkin, ME. Cystic ameloblastoma: a clinicopathologic analysis. Oral Surg Oral Med Oral Pathol. 1985;60:624–630.
Martins, WD, Fávaro, DM. Recurrence of an ameloblastoma in an autogenous iliac bone graft. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:657–659.
Melrose, RJ. Benign epithelial odontogenic tumors. Semin Diagn Pathol. 1999;16:271–287.
Miller, RS, Biddinger, PW, Marciani, RD, et al. Simultaneously occurring ameloblastoma of the maxilla and mandible: case report. Otolaryngol Head Neck Surg. 2004;131:324–326.
Philipsen, HP, Ormiston, IW, Reichart, PA. The desmo- and osteoplastic ameloblastoma: histologic variant or clinicopathologic entity? Int J Oral Maxillofac Surg. 1992;21:352–357.
Philipsen, HP, Reichart, PA, Takata, T. Desmoplastic ameloblastoma (including “hybrid” lesion of ameloblastoma): biological profile based on 100 cases from the literature and own files. Oral Oncol. 2001;37:455–460.
Philipsen, HP, Reichart, PA. Classification of odontogenic tumours: a historical review. J Oral Pathol Med. 2006;35:525–529.
Raubenheimer, EJ, van Heerden, WFP, Noffke, CEE. Infrequent clinicopathological findings in 108 ameloblastomas. J Oral Pathol Med. 1995;24:227–232.
Reichart, PA, Philipsen, HP, Sonner, S. Ameloblastoma: biological profile of 3677 cases. Oral Oncol. 1995;31B:86–99.
Richard, BM, Thyveetil, M, Sharif, H, et al. Ameloblastoma with stromal multinucleated giant cells. Histopathology. 1994;25:497–499.
Sachs, SA. Surgical excision with peripheral ostectomy: a definitive, yet conservative, approach to the surgical management of ameloblastoma. J Oral Maxillofac Surg. 2006;64:476–483.
Said-Al-Naief, NAH, Lumerman, H, Ramer, M, et al. Keratoameloblastoma of the maxilla: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:535–539.
Sampson, DE, Pogrel, MA. Management of mandibular ameloblastoma: the clinical basis for a treatment algorithm. J Oral Maxillofac Surg. 1999;57:1074–1077.
Schafer, DR, Thompson, LDR, Smith, BC, et al. Primary ameloblastoma of the sinonasal tract. Cancer. 1998;82:667–674.
Small, IA, Waldron, CA. Ameloblastoma of the jaws. Oral Surg Oral Med Oral Pathol. 1955;8:281–297.
Takata, T, Miyauchi, M, Ito, H, et al. Clinical and histopathological analyses of desmoplastic ameloblastoma. Pathol Res Pract. 1999;195:669–675.
Takata, T, Miyauchi, M, Ogawa, I, et al. Immunoexpression of transforming growth factor b in desmoplastic ameloblastoma. Virchows Arch. 2000;436:319–323.
Waldron, CA, El-Mofty, S. A histopathologic study of 116 ameloblastomas with special reference to the desmoplastic variant. Oral Surg Oral Med Oral Pathol. 1987;63:441–451.
Ackerman, GL, Altini, M, Shear, M. The unicystic ameloblastoma: a clinicopathologic study of 57 cases. J Oral Pathol. 1988;17:541–546.
Cunha, EM, Fernandes, AV, Versiani, MA, et al. Unicystic ameloblastoma: a possible pitfall in periapical diagnosis. Int Endod J. 2005;38:334–340.
Gardner, DG, Corio, RL. The relationship of plexiform unicystic ameloblastoma to conventional ameloblastoma. Oral Surg Oral Med Oral Pathol. 1983;56:54–60.
Lau, SL, Samman, N. Recurrence related to treatment modalities of unicystic ameloblastoma: a systematic review. Int J Oral Maxillofac Surg. 2006;35:681–690.
Li, T-J, Browne, RM, Matthews, JB. Expression of proliferating cell nuclear antigen (PCNA) and Ki-67 unicystic ameloblastoma. Histopathology. 1995;26:219–228.
Philipsen, HP, Reichart, PA. Unicystic ameloblastoma: a review of 193 cases from the literature. Oral Oncol. 1998;34:317–325.
Robinson, L, Martinez, MG. Unicystic ameloblastoma: a prognostically distinct entity. Cancer. 1977;40:2278–2285.
Vickers, RA, Gorlin, RJ. Ameloblastoma: delineation of early histopathologic features of neoplasia. Cancer. 1970;26:699–710.
Peripheral (Extraosseous) Ameloblastoma
Baden, E, Doyle, JL, Petriella, V. Malignant transformation of peripheral ameloblastoma. Oral Surg Oral Med Oral Pathol. 1993;75:214–219.
Buchner, A, Merrell, PW, Carpenter, WM. Relative frequency of peripheral odontogenic tumors: a study of 45 new cases and comparison with studies from the literature. J Oral Pathol Med. 2006;35:385–391.
Gardner, DG. Peripheral ameloblastoma: a study of 21 cases including 5 reported as basal cell carcinoma of the gingiva. Cancer. 1977;39:1625–1633.
Ide, F, Kusama, K. Difficulty in predicting biological behavior of peripheral ameloblastoma. Oral Oncol. 2004;40:651–652.
Ide, F, Kusama, K, Tanaka, A, et al. Peripheral ameloblastoma is not a hamartoma but rather more of a neoplasm. Oral Oncol. 2002;38:318–320.
LeCorn, DW, Bhattacharyya, I, Vertucci, FJ. Peripheral ameloblastoma: a case report and review of the literature. J Endod. 2006;32:152–154.
Martelli-Júnior, H, Souza, LN, Santos, LA, et al. Peripheral ameloblastoma: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:e31–e33.
Philipsen, HP, Reichart, PA, Nikai, H, et al. Peripheral ameloblastoma: biological profile based on 160 cases from the literature. Oral Oncol. 2001;37:17–27.
Woo, SB, Smith-Williams, JE, Sciubba, JJ, et al. Peripheral ameloblastoma of the buccal mucosa: case report and review of the English language literature. Oral Surg Oral Med Oral Pathol. 1987;63:78–84.
Yamanishi, T, Ando, S, Aikawa, T, et al. A case of extragingival peripheral ameloblastoma in the buccal mucosa. J Oral Pathol Med. 2007;36:184–186.
Malignant Ameloblastoma and Ameloblastic Carcinoma
Akrish, S, Buchner, JA, Shoshani, Y, et al. Ameloblastic carcinoma: report of a new case, literature review, and comparison to ameloblastoma. J Oral Maxillofac Surg. 2007;65:777–783.
Corio, RL, Goldblatt, LI, Edwards, PA, et al. Ameloblastic carcinoma: a clinicopathologic assessment of eight cases. Oral Surg Oral Med Oral Pathol. 1987;64:570–576.
Eliasson, A, Moser, RJ, III., Tenholder, MF. Diagnosis and treatment of metastatic ameloblastoma. South Med J. 1989;82:1165–1168.
Eversole, LR. Malignant epithelial odontogenic tumors. Semin Diagn Pathol. 1999;16:317–324.
Goldenberg, D, Sciubba, J, Koch, W, et al. Malignant odontogenic tumors: a 22-year experience. Laryngoscope. 2004;114:1770–1774.
Hall, JM, Weathers, DR, Unni, KK. Ameloblastic carcinoma: an analysis of 14 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:799–807.
Lau, SK, Tideman, H, Wu, PC. Ameloblastic carcinoma of the jaws: a report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:78–81.
Okada, H, Davies, JE, Yamamoto, H. Malignant ameloblastoma: a case study and review. J Oral Maxillofac Surg. 1999;57:725–730.
Simko, EJ, Brannon, RB, Eibling, DE. Ameloblastic carcinoma of the mandible. Head Neck. 1998;20:654–659.
Slootweg, PJ, Müller, H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol. 1984;57:168–176.
Suomalainen, A, Hietanen, J, Robinson, S, et al. Ameloblastic carcinoma of the mandible resembling odontogenic cyst in a panoramic radiograph. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:638–642.
Üzüm, N, Akyol, G, Asal, K, et al. Ameloblastic carcinoma containing melanocyte and melanin pigment in the mandible: a case report and review of the literature. J Oral Pathol Med. 2005;34:618–620.
August, M, Faquin, W, Troulis, M, et al. Clear cell odontogenic carcinoma: evaluation of reported cases. J Oral Maxillofac Surg. 2003;61:580–586.
Bang, G, Koppang, HS, Hansen, LS, et al. Clear cell odontogenic carcinoma: report of three cases and lymph node metastasis. J Oral Pathol Med. 1989;18:113–118.
Brandwein, M, Said-Al-Naief, N, Gordon, R, et al. Clear cell odontogenic carcinoma: report of a case and analysis of the literature. Arch Otolaryngol Head Neck Surg. 2002;128:1089–1095.
Ebert, CS, Dubin, MG, Hart, CF, et al. Clear cell odontogenic carcinoma: a comprehensive analysis of treatment strategies. Head Neck. 2005;27:536–542.
Eversole, LR. On the differential diagnosis of clear cell tumours of the head and neck. Eur J Cancer B Oral Oncol. 1993;29B:173–179.
Fan, J, Kubota, E, Imamura, H, et al. Clear cell odontogenic carcinoma: a case report with massive invasion of neighboring organs and lymph node metastasis. Oral Surg Oral Med Oral Pathol. 1992;74:768–775.
Hansen, LS, Eversole, LR, Green, TL, et al. Clear cell odontogenic tumor—a new histologic variant with aggressive potential. Head Neck Surg. 1985;8:115–123.
Kumamoto, H, Kawamura, H, Ooya, K. Clear cell odontogenic tumor in the mandible: report of a case with an immunohistochemical study of epithelial cell markers. Pathol Int. 1998;48:618–622.
Kumamoto, H, Yamazaki, S, Sato, A, et al. Clear cell odontogenic tumor in the mandible: report of a case with duct-like appearances and dentinoid induction. J Oral Pathol Med. 2000;29:43–47.
Waldron, CA, Small, IA, Silverman, H. Clear cell ameloblastoma—an odontogenic carcinoma. J Oral Maxillofac Surg. 1985;43:709–717.
Yamamoto, H, Inui, M, Mori, A, et al. Clear cell odontogenic carcinoma. A case report and literature review of odontogenic tumors with clear cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:86–89.
Courtney, RM, Kerr, DA. The odontogenic adenomatoid tumor: a comprehensive review of 21 cases. Oral Surg Oral Med Oral Pathol. 1975;39:424–435.
Damm, DD, White, DK, Drummond, JF, et al. Combined epithelial odontogenic tumor: adenomatoid odontogenic tumor and calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol. 1983;55:487–496.
Giansanti, JS, Someren, A, Waldron, CA. Odontogenic adenomatoid tumor (adenoameloblastoma). Oral Surg Oral Med Oral Pathol. 1970;30:69–86.
Leon, JE, Mata, GM, Fregnani, ER, et al. Clinicopathological and immunohistochemical study of 39 cases of adenomatoid odontogenic tumour: a multicentric study. Oral Oncol. 2005;41:835–842.
Miyake, M, Nagahata, S, Nishihara, J, et al. Combined adenomatoid odontogenic tumor and calcifying epithelial odontogenic tumor: report of a case and ultrastructural study. J Oral Maxillofac Surg. 1996;54:788–793.
Philipsen, HP, Reichart, PA. Adenomatoid odontogenic tumour: facts and figures. Oral Oncol. 1999;35:125–131.
Philipsen, HP, Srisuwan, T, Reichart, PA. Adenomatoid odontogenic tumor mimicking a periapical (radicular) cyst: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:246–248.
Poulson, TC, Greer, RO. Adenomatoid odontogenic tumor: clinicopathologic and ultrastructural concepts. J Oral Maxillofac Surg. 1983;41:818–824.
Calcifying Epithelial Odontogenic Tumor
Anavi, Y, Kaplan, I, Citir, M, et al. Clear-cell variant of calcifying epithelial odontogenic tumor: clinical and radiographic characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:332–339.
Aviel-Ronen, S, Liokumovich, P, Rahima, D, et al. The amyloid deposit in calcifying epithelial odontogenic tumor is immunoreactive for cytokeratins. Arch Pathol Lab Med. 2000;124:872–876.
Basu, MK, Matthews, JB, Sear, AJ, et al. Calcifying epithelial odontogenic tumour: a case showing features of malignancy. J Oral Pathol. 1984;13:310–319.
Bridle, C, Visram, K, Piper, K, et al. Maxillary calcifying epithelial odontogenic (Pindborg) tumor presenting with abnormal eye signs: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e12–e15.
Cheng, Y-SL, Wright, JM, Walstad, WR, et al. Calcifying epithelial odontogenic tumor showing microscopic features of potential malignant behavior. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:287–295.
Dantas da Silveira, EJ, Gordón-Núñez, MA, Guerra Seabra, FR, et al. Peripheral calcifying epithelial odontogenic tumor associated with generalized drug-induced gingival growth: a case report. J Oral Maxillofac Surg. 2007;65:341–345.
Franklin, CD, Pindborg, JJ. The calcifying epithelial odontogenic tumor: a review and analysis of 113 cases. Oral Surg Oral Med Oral Pathol. 1976;42:753–765.
Germanier, Y, Bornstein, MM, Stauffer, E, et al. Calcifying epithelial odontogenic (Pindborg) tumor of the mandible with clear cell component treated by conservative surgery: report of a case. J Oral Maxillofac Surg. 2005;63:1377–1382.
Gopalakrishnan, R, Simonton, S, Rohrer, MD, et al. Cystic variant of calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:773–777.
Houston, GD, Fowler, CB. Extraosseous calcifying epithelial odontogenic tumor: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:577–583.
Kaplan, I, Buchner, A, Calderon, S, et al. Radiological and clinical features of calcifying epithelial odontogenic tumour. Dentomaxillofac Radiol. 2001;30:22–28.
Kawano, K, Ono, K, Yada, N, et al. Malignant calcifying epithelial odontogenic tumor of the mandible: report of a case with pulmonary metastasis showing remarkable response to platinum derivatives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:76–81.
Kumamoto, H, Sato, I, Tateno, H, et al. Clear cell variant of calcifying epithelial odontogenic tumor (CEOT) in the maxilla: report of a case with immunohistochemical and ultrastructural investigations. J Oral Pathol Med. 1999;28:187–191.
Philipsen, HP, Reichart, PA. Calcifying epithelial odontogenic tumour: biological profile based on 181 cases from the literature. Oral Oncol. 2000;36:17–26.
Pindborg, JJ. A calcifying epithelial odontogenic tumor. Cancer. 1958;11:838–843.
Seim, P, Regezi, JA, O’Ryan, F. Hybrid ameloblastoma and calcifying epithelial odontogenic tumor: case report. J Oral Maxillofac Surg. 2005;63:852–855.
Solomon, A, Murphy, CL, Weaver, K, et al. Calcifying epithelial odontogenic (Pindborg) tumor-associated amyloid consists of a novel human protein. J Lab Clin Med. 2003;142:348–355.
Veness, MJ, Morgan, G, Collins, AP, et al. Calcifying epithelial odontogenic (Pindborg) tumor with malignant transformation and metastatic spread. Head Neck. 2001;23:692–696.
Baden, E, Doyle, J, Mesa, M, et al. Squamous odontogenic tumor: report of three cases including the first extraosseous case. Oral Surg Oral Med Oral Pathol. 1993;75:733–738.
Goldblatt, LI, Brannon, RB, Ellis, GL. Squamous odontogenic tumor: report of five cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1982;54:187–196.
Haghighat, K, Kalmar, JR, Mariotti, AJ. Squamous odontogenic tumor: diagnosis and management. J Periodontol. 2002;73:653–656.
Ide, F, Shimoyama, T, Horie, N, et al. Intraosseous squamous cell carcinoma arising in association with a squamous odontogenic tumour of the mandible. Oral Oncol. 1999;35:431–434.
Kim, K, Mintz, SM, Stevens, J. Squamous odontogenic tumor causing erosion of the lingual cortical plate in the mandible: a report of 2 cases. J Oral Maxillofac Surg. 2007;65:1227–1231.
Krithika, C, Vardhan, BG, Saraswathy, K, et al. Radiolucency in the anterior maxilla associated with an impacted tooth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:164–168.
Kusama, K, Kawashima, A, Nagai, H, et al. Squamous odontogenic tumor of the maxilla: report of a case. J Oral Sci. 1998;40:119–122.
Leider, AS, Jonker, A, Cook, HE. Multicentric familial squamous odontogenic tumor. Oral Surg Oral Med Oral Pathol. 1989;68:175–181.
Mills, WP, Davilla, MA, Beattenmuller, EA, et al. Squamous odontogenic tumor: report of a case with lesions in three quadrants. Oral Surg Oral Med Oral Pathol. 1986;61:557–563.
Philipsen, HP, Reichart, PA. Squamous odontogenic tumor (SOT): a benign neoplasm of the periodontium. A review of 36 reported cases. J Clin Periodontol. 1996;23:922–926.
Pullon, PA, Shafer, WG, Elzay, RP, et al. Squamous odontogenic tumor: report of six cases of a previously undescribed lesion. Oral Surg Oral Med Oral Pathol. 1975;40:616–630.
Wright, JM. Squamous odontogenic tumor-like proliferations in odontogenic cysts. Oral Surg Oral Med Oral Pathol. 1979;47:354–358.
Gardner, DG. The mixed odontogenic tumors. Oral Surg Oral Med Oral Pathol. 1984;58:166–168.
Hansen, LS, Ficarra, G. Mixed odontogenic tumors: an analysis of 23 new cases. Head Neck Surg. 1988;10:330–343.
Philipsen, HP, Reichart, PA. Classification of odontogenic tumors: a historical review. J Oral Pathol Med. 2006;35:525–529.
Philipsen, HP, Reichart, PA, Praetorius, F. Mixed odontogenic tumours and odontomas. Considerations on interrelationship. Review of the literature and presentation of 134 new cases of odontomas. Oral Oncol. 1997;33:86–99.
Slootweg, PJ. An analysis of the interrelationship of the mixed odontogenic tumors—ameloblastic fibroma, ameloblastic fibro-odontoma and the odontomas. Oral Surg Oral Med Oral Pathol. 1981;51:266–276.
Tomich, CE. Benign mixed odontogenic tumors. Semin Diagn Pathol. 1999;16:308–316.
Chen, Y, Li, T-J, Gao, Y, et al. Ameloblastic fibroma and related lesions: a clinicopathologic study with reference to their nature and interrelationship. J Oral Pathol Med. 2005;34:588–595.
Cohen, DM, Bhattacharyya, I. Ameloblastic fibroma, ameloblastic fibro-odontoma, and odontoma. Oral Maxillofac Surg Clin North Am. 2004;16:375–384.
Dallera, P, Bertoni, F, Marchetti, C, et al. Ameloblastic fibroma: a follow-up of six cases. Int J Oral Maxillofac Surg. 1996;25:199–202.
Darling, MR, Daley, TD. Peripheral ameloblastic fibroma. J Oral Pathol Med. 2006;35:190–192.
Lin, C-C, Chen, C-H, Lin, L-M, et al. Calcifying odontogenic cyst with ameloblastic fibroma: report of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:451–460.
Mosby, EL, Russell, D, Noren, S, et al. Ameloblastic fibroma in a 7-week-old infant: a case report and review of the literature. J Oral Maxillofac Surg. 1998;56:368–372.
Sawyer, DR, Nwoku, AL, Mosadomi, A. Recurrent ameloblastic fibroma: report of 2 cases. Oral Surg Oral Med Oral Pathol. 1982;53:19–24.
Takeda, Y. Ameloblastic fibroma and related lesions: current pathologic concept. Oral Oncol. 1999;35:535–540.
Trodahl, JN. Ameloblastic fibroma: a survey of cases from the Armed Forces Institute of Pathology. Oral Surg Oral Med Oral Pathol. 1972;33:547–558.
Yoon, JH, Kim, HJ, Yook, JI, et al. Hybrid odontogenic tumor of calcifying odontogenic cyst and ameloblastic fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:80–84.
Zallen, RD, Preskar, MH, McClary, SA. Ameloblastic fibroma. J Oral Maxillofac Surg. 1982;40:513–517.
Chang, H, Shimizu, MS, Precious, DS. Ameloblastic fibro-odontoma: a case report. J Can Dent Assoc. 2002;68:243–246.
Cohen, DM, Bhattacharyya, I. Ameloblastic fibroma, ameloblastic fibro-odontoma, and odontoma. Oral Maxillofac Surg Clin North Am. 2004;16:375–384.
Dhanuthai, K, Kongin, K. Ameloblastic fibro-odontoma: a case report. J Clin Pediatr Dent. 2004;29:75–78.
Favia, GF, Di Alberti, L, Scarano, A, et al. Ameloblastic fibro-odontoma: report of two cases. Oral Oncol. 1997;33:444–446.
Furst, I, Pharoah, M, Phillips, J. Recurrence of an ameloblastic fibro-odontoma in a 9-year-old boy. J Oral Maxillofac Surg. 1999;57:620–623.
Howell, RM, Burkes, EJ. Malignant transformation of ameloblastic fibro-odontoma to ameloblastic fibrosarcoma. Oral Surg Oral Med Oral Pathol. 1977;43:391–401.
Miller, AS, Lopez, CF, Pullon, PA, et al. Ameloblastic fibro-odontoma. Oral Surg Oral Med Oral Pathol. 1976;41:354–365.
Sekine, J, Kitamura, A, Ueno, K, et al. Cell kinetics in mandibular ameloblastic fibro-odontoma evaluated by bromodeoxyuridine and proliferating cell nuclear antigen immunohistochemistry: case report. Br J Oral Maxillofac Surg. 1996;34:450–453.
Slootweg, PJ. Epithelio-mesenchymal morphology in ameloblastic fibro-odontoma: a light and electron microscopic study. J Oral Pathol. 1980;9:29–40.
Van Wyk, CW, Van der Vyver, PC. Ameloblastic fibroma with dentinoid formation/immature dentinoma: a microscopic and ultrastructural study of the epithelial-connective tissue interface. J Oral Pathol. 1983;12:37–46.
Altini, M, Thompson, SH, Lownie, JF, et al. Ameloblastic sarcoma of the mandible. J Oral Maxillofac Surg. 1985;43:789–794.
Carlos-Bregni, R, Mosqueda-Taylor, A, Meneses-Garcia, A. Ameloblastic fibrosarcoma of the mandible: report of two cases and review of the literature. J Oral Pathol Med. 2001;30:316–320.
Chomette, G, Auriol, M, Guilbert, F, et al. Ameloblastic fibrosarcoma of the jaws—report of three cases. Pathol Res Pract. 1983;178:40–47.
DeLair, D, Bejarano, PA, Peleg, M, et al. Ameloblastic carcinosarcoma of the mandible arising in ameloblastic fibroma: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:516–520.
Kobayashi, K, Murakami, R, Fujii, T, et al. Malignant transformation of ameloblastic fibroma to ameloblastic fibrosarcoma: case report and review of the literature. J Craniomaxillofac Surg. 2005;33:352–355.
Kunkel, M, Ghalibafian, M, Radner, H, et al. Ameloblastic fibrosarcoma or odontogenic carcinosarcoma: a matter of classification? Oral Oncol. 2004;40:444–449.
Leider, AS, Nelson, JF, Trodahl, JN. Ameloblastic fibrosarcoma of the jaws. Oral Surg Oral Med Oral Pathol. 1972;33:559–569.
Muller, S, Parker, DC, Kapadia, SB, et al. Ameloblastic fibrosarcoma of the jaws: a clinicopathologic and DNA analysis of five cases and review of the literature with discussion of its relationship to ameloblastic fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:469–477.
Slater, LJ. Odontogenic sarcoma and carcinosarcoma. Semin Diagn Pathol. 1999;16:325–332.
Williams, MD, Hanna, EY, El-Naggar, AK. Anaplastic ameloblastic fibrosarcoma arising from recurrent ameloblastic fibroma: restricted molecular abnormalities of certain genes to the malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:72–75.
Wood, RM, Markle, TL, Barker, BF, et al. Ameloblastic fibrosarcoma. Oral Surg Oral Med Oral Pathol. 1988;66:74–77.
Mosqueda-Taylor, A, Carlos-Bregni, R, Ramírez-Amador, V, et al. Odontoameloblastoma. Clinico-pathologic study of three cases and critical review of the literature. Oral Oncol. 2002;38:800–805.
Gupta, DS, Gupta, MK. Odontoameloblastoma. J Oral Maxillofac Surg. 1986;44:146–148.
Kaugars, GE. Ameloblastic odontoma (odonto-ameloblastoma). Oral Surg Oral Med Oral Pathol. 1991;71:371–373.
LaBriola, JD, Steiner, M, Bernstein, ML, et al. Odontoameloblastoma. J Oral Surg. 1980;38:139–143.
Ashkenazi, M, Greenberg, BP, Chodik, G, et al. Postoperative prognosis of unerupted teeth after removal of supernumerary teeth or odontomas. Am J Orthod Dentofacial Orthop. 2007;131:614–619.
Budnick, SD. Compound and complex odontomas. Oral Surg Oral Med Oral Pathol. 1976;42:501–506.
Cohen, DM, Bhattacharyya, I. Ameloblastic fibroma, ameloblastic fibro-odontoma, and odontoma. Oral Maxillofac Surg Clin North Am. 2004;16:375–384.
Ide, F, Shimoyama, T, Horie, N. Gingival peripheral odontoma in an adult: case report. J Periodontol. 2000;71:830–832.
Kaugars, GE, Miller, ME, Abbey, LM. Odontomas. Oral Surg Oral Med Oral Pathol. 1989;67:172–176.
Miki, Y, Oda, Y, Iwaya, N, et al. Clinicopathological studies of odontoma in 47 patients. J Oral Sci. 1999;41:173–176.
Owens, BM, Schuman, NJ, Mincer, HH, et al. Dental odontomas: a retrospective study of 104 cases. J Clin Pediatr Dent. 1997;21:261–264.
Sapp, JP, Gardner, DG. An ultrastructural study of the calcifications in calcifying odontogenic cysts and odontomas. Oral Surg Oral Med Oral Pathol. 1977;44:754–766.
Sedano, HO, Pindborg, JJ. Ghost cell epithelium in odontomas. J Oral Pathol. 1975;4:27–30.
Tomizawa, M, Otsuka, Y, Noda, T. Clinical observations of odontomas in Japanese children: 39 cases including one recurrent case. Int J Paediatr Dent. 2005;15:37–43.
Allen, CM, Hammond, HL, Stimson, PG. Central odontogenic fibroma WHO type: a report of 3 cases with an unusual associated giant cell reaction. Oral Surg Oral Med Oral Pathol. 1992;73:62–66.
Daniels, JSM. Central odontogenic fibroma of the mandible: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:295–300.
Dunlap, CL. Odontogenic fibroma. Semin Diagn Pathol. 1999;16:293–296.
Gardner, DG. The central odontogenic fibroma: an attempt at clarification. Oral Surg Oral Med Oral Pathol. 1980;50:425–432.
Gardner, DG. Central odontogenic fibroma current concepts. J Oral Pathol Med. 1996;25:556–561.
Handlers, JP, Abrams, AM, Melrose, RJ, et al. Central odontogenic fibroma: clinicopathologic features of 19 cases and review of the literature. J Oral Maxillofac Surg. 1991;49:46–54.
Ide, F, Sakashita, H, Kusama, K. Ameloblastomatoid, central odontogenic fibroma: an epithelium-rich variant. J Oral Pathol Med. 2002;31:612–614.
Kaffe, I, Buchner, A. Radiologic features of central odontogenic fibroma. Oral Surg Oral Med Oral Pathol. 1994;78:811–818.
Mosqueda Taylor, A, Bermudez Flores, V, Diaz Franco, MA. Combined central odontogenic fibroma and giant cell granuloma-like lesion of the mandible: report of a case and review of the literature. J Oral Maxillofac Surg. 1999;57:1258–1262.
Odell, EW, Lombardi, T, Barrett, AW, et al. Hybrid central giant cell granuloma and central odontogenic fibroma-like lesions of the jaws. Histopathology. 1997;30:165–171.
Ramer, M, Buonocore, P, Krost, B. Central odontogenic fibroma—report of a case and review of the literature. Periodontal Clin Investig. 2002;24:27–30.
Slootweg, PJ, Müller, H. Central fibroma of the jaw: odontogenic or desmoplastic. Oral Surg Oral Med Oral Pathol. 1983;56:61–70.
Peripheral Odontogenic Fibroma
Baden, E, Moskow, BS, Moskow, R. Odontogenic epithelial hamartoma. J Oral Surg. 1968;26:702–714.
Buchner, A. Peripheral odontogenic fibroma. J Craniomaxillofac Surg. 1989;17:134–138.
Buchner, A, Ficarra, G, Hansen, LS. Peripheral odontogenic fibroma. Oral Surg Oral Med Oral Pathol. 1987;64:432–438.
Buchner, A, Merrell, PW, Carpenter, WM. Relative frequency of peripheral odontogenic tumors: a study of 45 new cases and comparison with studies from the literature. J Oral Pathol Med. 2006;35:385–391.
Daley, TD, Wysocki, GP. Peripheral odontogenic fibroma. Oral Surg Oral Med Oral Pathol. 1994;78:329–336.
Ficarra, G, Sapp, JP, Eversole, LR. Multiple peripheral odontogenic fibroma, World Health Organization type, and central giant cell granuloma: a case report of an unusual association. J Oral Maxillofac Surg. 1993;51:325–328.
Ide, F, Obara, K, Mishima, K, et al. Peripheral odontogenic tumor: a clinicopathologic study of 30 cases. General features and hamartomatous lesions. J Oral Pathol Med. 2005;34:552–557.
Martelli-Junior, H, Mesquita, RA, de Paula, AM, et al. Peripheral odontogenic fibroma (WHO type) of the newborn: a case report. Int J Paediatr Dent. 2006;16:376–379.
Siar, CH, Ng, KH. Clinicopathological study of peripheral odontogenic fibromas (WHO-type) in Malaysians (1967-95). Br J Oral Maxillofac Surg. 2000;38:19–22.
Slabben, H, Altini, M. Peripheral odontogenic fibroma: a clinicopathologic study. Oral Surg Oral Med Oral Pathol. 1991;72:86–90.
Weber, A, van Heerden, WF, Ligthelm, AJ, et al. Diffuse peripheral odontogenic fibroma: report of 3 cases. J Oral Pathol Med. 1992;21:82–84.
Granular Cell Odontogenic Tumor
Brannon, RB, Goode, RK, Eversole, LR, et al. The central granular cell odontogenic tumor: report of 5 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:614–621.
Gesek, DJ, Adrian, JC, Reid, EN. Central granular cell odontogenic tumor: a case report including light microscopy, immunohistochemistry, and literature review. J Oral Maxillofac Surg. 1995;53:945–949.
Meer, S, Altini, M, Coleman, H, et al. Central granular cell odontogenic tumor: immunohistochemistry and ultrastructure. Am J Otolaryngol. 2004;25:73–78.
Orsini Machado de Sousa, S, Soares de Araújo, N, Maia Melhado, R, et al. Central odontogenic granular cell tumor: immunohistochemical study of two cases. J Oral Maxillofac Surg. 1998;56:787–791.
Piattelli, A, Rubini, C, Goteri, G, et al. Central granular cell odontogenic tumour: report of the first malignant case and review of the literature. Oral Oncol. 2003;39:78–82.
Rinaggio, J, Cleveland, D, Koshy, R, et al. Peripheral granular cell odontogenic fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:676–679.
Vincent, SD, Hammond, HL, Ellis, GL, et al. Central granular cell odontogenic fibroma. Oral Surg Oral Med Oral Pathol. 1987;63:715–721.
Waldron, CA, Thompson, CW, Conner, WA. Granular cell ameloblastic fibroma. Oral Surg Oral Med Oral Pathol. 1963;16:1202–1213.
White, DK, Chen, S-Y, Hartman, KS, et al. Central granular cell tumor of the jaws (the so-called granular cell ameloblastic fibroma). Oral Surg Oral Med Oral Pathol. 1978;45:396–405.
Yih, W-Y, Thompson, C, Meshul, CK, et al. Central odontogenic granular cell tumor of the jaw: report of case and immunohistochemical and electron microscopic study. J Oral Maxillofac Surg. 1995;53:453–459.
Barker, BF. Odontogenic myxoma. Semin Diagn Pathol. 1999;16:297–301.
Barros, RE, Domingnez, PV, Cabrini, RL. Myxoma of the jaws. Oral Surg Oral Med Oral Pathol. 1969;27:225–236.
Chiodo, AA, Strumas, N, Gilbert, RW, et al. Management of odontogenic myxoma of the maxilla. Otolaryngol Head Neck Surg. 1997;117:S73–S76.
Goldblatt, LI. Ultrastructural study of an odontogenic myxoma. Oral Surg Oral Med Oral Pathol. 1976;42:206–220.
Gosh, BC, Huvos, AG, Gerald, FP, et al. Myxoma of the jaw bones. Cancer. 1973;31:237–240.
Kaffe, I, Naor, H, Buchner, A. Clinical and radiological features of odontogenic myxoma of the jaws. Dentomaxillofac Radiol. 1997;26:299–303.
Lamberg, MA, Calonius, BP, Makinen, JE, et al. A case of malignant myxoma (myxosarcoma) of the maxilla. Scan J Dent Res. 1984;92:352–357.
Li, T-J, Sun, L-S, Luo, H-Y. Odontogenic myxomas: a clinicopathologic study of 25 cases. Arch Pathol Lab Med. 2006;130:1799–1806.
Lo Muzio, L, Nocini, PF, Favia, G, et al. Odontogenic myxoma of the jaws: a clinical, radiologic, immunohistochemical, and ultrastructural study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:426–433.
Noffke, CEE, Raubenheimer, EJ, Chabikuli, NJ, et al. Odontogenic myxoma: review of the literature and report of 30 cases from South Africa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:101–109.
Pahl, S, Henn, W, Binger, T, et al. Malignant odontogenic myxoma of the maxilla: case with cytogenetic confirmation. J Laryngol Otol. 2000;114:533–535.
Peltola, J, Magnusson, B, Happonen, R-P, et al. Odontogenic myxoma—a radiographic study of 21 tumours. Br J Oral Maxillofac Surg. 1994;32:298–302.
Schmidt-Westhausen, A, Becker, J, Schuppan, D, et al. Odontogenic myxoma—characterisation of the extracellular matrix (ECM) of the tumour stroma. Eur J Cancer B Oral Oncol. 1994;30B:377–380.
Simon, ENM, Merkx, MAW, Vuhahula, E, et al. Odontogenic myxomas: a clinicopathologic study of 33 cases. Int J Oral Maxillofac Surg. 2004;33:333–337.
White, DK, Chen, SY, Mohnae, AM, et al. Odontogenic myxoma: a clinical and ultrastructural study. Oral Surg Oral Med Oral Pathol. 1975;39:901–917.
Zhao, M, Lu, Y, Takata, T, et al. Immunohistochemical and histochemical characterisation of the mucosubstances of odontogenic myxoma: histogenesis and differential diagnosis. Pathol Res Pract. 1999;195:391–397.
Zimmerman, DC, Dahlin, DC. Myxomatous tumors of the jaws. Oral Surg Oral Med Oral Pathol. 1958;11:1069–1080.
*Dr. Charles A. Waldron wrote the original version of this chapter in the first edition of this book.