Odontogenic Cysts and Tumors*
Orthokeratinized Odontogenic Cyst
Nevoid Basal Cell Carcinoma Syndrome
Gingival (Alveolar) Cyst of the Newborn
Carcinoma Arising in Odontogenic Cysts
TUMORS OF ODONTOGENIC EPITHELIUM
Malignant Ameloblastoma and Ameloblastic Carcinoma
Clear Cell Odontogenic Carcinoma
Calcifying Epithelial Odontogenic Tumor
TUMORS OF ODONTOGENIC ECTOMESENCHYME
Peripheral Odontogenic Fibroma
Odontogenic cysts and tumors constitute an important aspect of oral and maxillofacial pathology. Odontogenic cysts are encountered relatively commonly in dental practice. Odontogenic tumors, by contrast, are uncommon lesions. Even in the specialized oral and maxillofacial pathology laboratory, fewer than 1% of all specimens received are odontogenic tumors.
Odontogenic Cysts
With rare exceptions, epithelium-lined cysts in bone are seen only in the jaws. Other than a few cysts that may result from the inclusion of epithelium along embryonic lines of fusion, most jaw cysts are lined by epithelium that is derived from odontogenic epithelium. These are referred to as odontogenic cysts. (Nonodontogenic jaw cysts are discussed in Chapter 1.)
Odontogenic cysts are subclassified as developmental or inflammatory in origin. The inciting factors that initiate the formation of developmental cysts are unknown, but these lesions do not appear to be the result of an inflammatory reaction. Inflammatory cysts are the result of inflammation. Box 15-1 presents categories of odontogenic cysts modified from the 2005 World Health Organization (WHO) classification. (The periapical cyst is discussed in Chapter 3.)
DENTIGEROUS CYST (FOLLICULAR CYST)
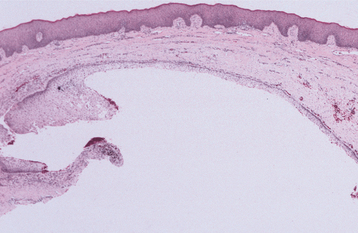
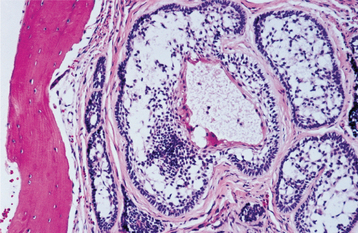
The dentigerous cyst is defined as a cyst that originates by the separation of the follicle from around the crown of an unerupted tooth. This is the most common type of developmental odontogenic cyst, making up about 20% of all epithelium-lined cysts of the jaws. The dentigerous cyst encloses the crown of an unerupted tooth and is attached to the tooth at the cemento-enamel junction (Fig. 15-1). The pathogenesis of this cyst is uncertain, but apparently it develops by accumulation of fluid between the reduced enamel epithelium and the tooth crown.
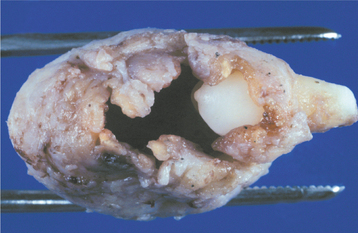
Fig. 15-1 Dentigerous cyst. Gross specimen of a dentigerous cyst involving a maxillary canine tooth. The cyst has been cut open to show the cyst-to-crown relationship.
Although most dentigerous cysts are considered to be developmental in origin, there are some examples that appear to have an inflammatory pathogenesis. For example, it has been suggested that, on occasion, a dentigerous cyst may develop around the crown of an unerupted permanent tooth as a result of periapical inflammation from an overlying primary tooth. Another scenario involves a partially erupted mandibular third molar that develops an inflamed cystlike lesion along the distal or buccal aspect. Although many such lesions probably are due to inflammation associated with recurrent pericoronitis, these lesions are usually diagnosed as examples of dentigerous cyst, especially because it is impossible to determine histopathologically whether the inflammatory component is primary or secondary in nature. The term paradental cyst sometimes has been applied to these lesions, but the use of this term in the literature is confusing because it also has been used to describe examples of what is known as the buccal bifurcation cyst.
CLINICAL AND RADIOGRAPHIC FEATURES: Although dentigerous cysts may occur in association with any unerupted tooth, most often they involve mandibular third molars. Other relatively frequent sites include maxillary canines, maxillary third molars, and mandibular second premolars. Dentigerous cysts rarely involve unerupted deciduous teeth. Occasionally, they are associated with supernumerary teeth or odontomas.
Although dentigerous cysts may be encountered in patients across a wide age range, they are discovered most frequently in patients between 10 and 30 years of age. There is a slight male predilection, and the prevalence is higher for whites than for blacks. Small dentigerous cysts are usually completely asymptomatic and are discovered only on a routine radiographic examination or when films are taken to determine the reason for the failure of a tooth to erupt. Dentigerous cysts can grow to a considerable size, and large cysts may be associated with a painless expansion of the bone in the involved area. Extensive lesions may result in facial asymmetry. Large dentigerous cysts are uncommon, and most lesions that are considered to be large dentigerous cysts on radiographic examination will prove to be odontogenic keratocysts or ameloblastomas. Dentigerous cysts may become infected and be associated with pain and swelling. Such infections may arise in a dentigerous cyst that is associated with a partially erupted tooth or by extension from a peri-apical or periodontal lesion that affects an adjacent tooth.
Radiographically, the dentigerous cyst typically shows a unilocular radiolucent area that is associated with the crown of an unerupted tooth. The radiolucency usually has a well-defined and often sclerotic border, but an infected cyst may show ill-defined borders. A large dentigerous cyst may give the impression of a multilocular process because of the persistence of bone trabeculae within the radiolucency. Dentigerous cysts, however, are grossly and histopathologically unilocular processes and probably never are truly multilocular lesions.
The cyst-to-crown relationship shows several radiographic variations. In the central variety, which is the most common, the cyst surrounds the crown of the tooth and the crown projects into the cyst (Fig. 15-2). The lateral variety is usually associated with mesioangular impacted mandibular third molars that are partially erupted. The cyst grows laterally along the root surface and partially surrounds the crown (Fig. 15-3). In the circumferential variant, the cyst surrounds the crown and extends for some distance along the root so that a significant portion of the root appears to lie within the cyst (Fig. 15-4). Rarely, a third molar may be displaced to the lower border of the mandible or higher up into the ascending ramus. Maxillary anterior teeth may be displaced into the floor of the nose, and other maxillary teeth may be moved through the maxillary sinus to the floor of the orbit. Dentigerous cysts may displace the involved tooth for a considerable distance. Root resorption of adjacent erupted teeth can occur.
Fig. 15-2 Dentigerous cyst. Central type showing the crown projecting into the cystic cavity. (Courtesy of Dr. Stephen E. Irwin.)
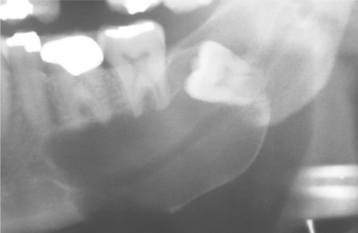
Fig. 15-3 Dentigerous cyst. Lateral variety showing a large cyst along the mesial root of the unerupted molar. This cyst exhibited mucous cell prosoplasia. (Courtesy of Dr. John R. Cramer.)
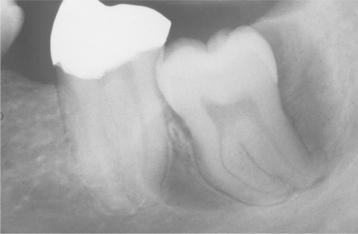
Fig. 15-4 Dentigerous cyst. Circumferential variety showing cyst extension along the mesial and distal roots of the unerupted tooth. (Courtesy of Dr. Richard Marks.)
Radiographic distinction between a small dentigerous cyst and an enlarged follicle about the crown of an unerupted tooth is difficult and may be largely an academic exercise (Fig. 15-5). For the lesion to be considered a dentigerous cyst, some investigators believe that the radiolucent space surrounding the tooth crown should be at least 3 to 4 mm in diameter. Radiographic findings are not diagnostic for a dentigerous cyst, however, because odontogenic keratocysts, unilocular ameloblastomas, and many other odontogenic and nonodontogenic tumors may have radiographic features that are essentially identical to those of a dentigerous cyst.
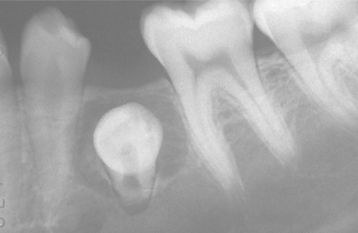
Fig. 15-5 Dentigerous cyst or enlarged follicle. Radiolucent lesion involving the crown of an unerupted mandibular premolar. Distinction between a dentigerous cyst and an enlarged follicle for a lesion of this size by radiographic and even histopathologic means is difficult, if not impossible. (Courtesy of Dr. Wally Austelle.)
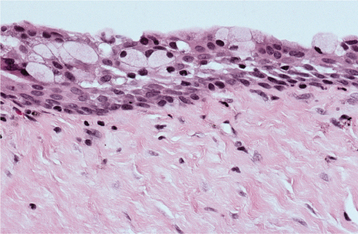
HISTOPATHOLOGIC FEATURES: The histopathologic features of dentigerous cysts vary, depending on whether the cyst is inflamed or not inflamed. In the noninflamed dentigerous cyst, the fibrous connective tissue wall is loosely arranged and contains considerable glycosaminoglycan ground substance. Small islands or cords of inactive-appearing odontogenic epithelial rests may be present in the fibrous wall. Occasionally these rests may be numerous, and at times pathologists who are not familiar with oral lesions have misinterpreted this finding as ameloblastoma. The epithelial lining consists of two to four layers of flattened nonkeratinizing cells, and the epithelium and connective tissue interface is flat (Fig. 15-6).
Fig. 15-6 Dentigerous cyst. This noninflamed dentigerous cyst shows a thin, nonkeratinized epithelial lining.
In the fairly common inflamed dentigerous cyst, the fibrous wall is more collagenized, with a variable infiltration of chronic inflammatory cells. The epithelial lining may show varying amounts of hyperplasia with the development of rete ridges and more definite squamous features (Fig. 15-7). A keratinized surface is sometimes seen, but these changes must be differentiated from those observed in the odontogenic keratocyst. Focal areas of mucous cells may be found in the epithelial lining of dentigerous cysts (Fig. 15-8). Rarely, ciliated columnar cells are present. Small nests of sebaceous cells rarely may be noted within the fibrous cyst wall. These mucous, ciliated, and sebaceous elements are believed to represent the multipotentiality of the odontogenic epithelial lining in a dentigerous cyst.
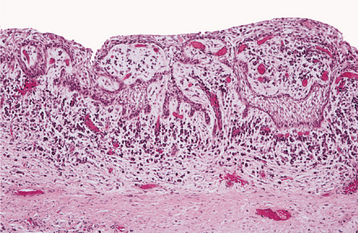
Fig. 15-7 Dentigerous cyst. This inflamed dentigerous cyst shows a thicker epithelial lining with hyperplastic rete ridges. The fibrous cyst capsule shows a diffuse chronic inflammatory infiltrate.
Gross examination of the wall of a dentigerous cyst may reveal one or several areas of nodular thickening on the luminal surface. These areas must be examined microscopically to rule out the presence of early neoplastic change.
Because a thin layer of reduced enamel epithelium normally lines the dental follicle surrounding the crown of an unerupted tooth, it can be difficult to distinguish a small dentigerous cyst from simply a normal or enlarged dental follicle based on microscopic features alone. Again, this distinction often represents largely an academic exercise; the most important consideration is ensuring that the lesion does not represent a more significant pathologic process (e.g., odontogenic keratocyst or ameloblastoma).
TREATMENT AND PROGNOSIS: The usual treatment for a dentigerous cyst is careful enucleation of the cyst together with removal of the unerupted tooth. If eruption of the involved tooth is considered feasible, then the tooth may be left in place after partial removal of the cyst wall. Patients may need orthodontic treatment to assist eruption. Large dentigerous cysts also may be treated by marsupialization. This permits decompression of the cyst, with a resulting reduction in the size of the bone defect. The cyst can then be excised at a later date, with a less extensive surgical procedure.
The prognosis for most dentigerous cysts is excellent, and recurrence seldom is noted after complete removal of the cyst. However, several potential complications must be considered. Much has been written about the possibility that the lining of a dentigerous cyst might undergo neoplastic transformation to an ameloblastoma. Although undoubtedly this can occur, the frequency of such neoplastic transformation is low. Rarely, a squamous cell carcinoma may arise in the lining of a dentigerous cyst (see page 700). It is likely that some intraosseous mucoepidermoid carcinomas (see page 490) develop from mucous cells in the lining of a dentigerous cyst.
ERUPTION CYST (ERUPTION HEMATOMA)
The eruption cyst is the soft tissue analogue of the dentigerous cyst. The cyst develops as a result of separation of the dental follicle from around the crown of an erupting tooth that is within the soft tissues overlying the alveolar bone. One example of eruption cysts developing in a child who was taking cyclosporin A has been described. Presumably the cysts developed because of collagen deposition in the gingival connective tissue that resulted in a thicker, less penetrable, pericoronal roof.
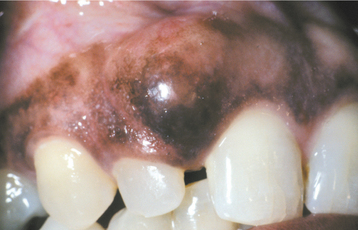
CLINICAL FEATURES: The eruption cyst appears as a soft, often translucent swelling in the gingival mucosa overlying the crown of an erupting deciduous or permanent tooth. Most examples are seen in children younger than age 10. Although the cyst may occur with any erupting tooth, the lesion is most commonly associated with the deciduous mandibular central incisors, the first permanent molars, and the deciduous maxillary incisors. Surface trauma may result in a considerable amount of blood in the cystic fluid, which imparts a blue to purple-brown color. Such lesions sometimes are referred to as eruption hematomas (Fig. 15-9).
HISTOPATHOLOGIC FEATURES: Intact eruption cysts seldom are submitted to the oral and maxillofacial pathology laboratory, and most examples consist of the excised roof of the cyst, which has been removed to facilitate tooth eruption. These show surface oral epithelium on the superior aspect. The underlying lamina propria shows a variable inflammatory cell infiltrate. The deep portion of the specimen, which represents the roof of the cyst, shows a thin layer of nonkeratinizing squamous epithelium (Fig. 15-10).
PRIMORDIAL CYST
The concept and meaning of the term primordial cyst often have been controversial and confusing. In the older classification of cysts widely used in the United States, the primordial cyst was considered to originate from cystic degeneration of the enamel organ epithelium before the development of dental hard tissue. Therefore, the primordial cyst occurs in place of a tooth.
In the mid 1950s, oral pathologists in Europe introduced the term odontogenic keratocyst to denote a cyst with specific histopathologic features and clinical behavior, which was believed to arise from the dental lamina (i.e., the dental primordium). Subsequently, this concept was widely accepted, and the terms odontogenic keratocyst and primordial cyst were used synonymously. The 1972 WHO classification used the designation primordial cyst as the preferred term for this lesion. The 1992 WHO classification, however, listed odontogenic keratocyst as the preferred designation.
Whether there is a primordial cyst that is not microscopically an odontogenic keratocyst is still unsettled. Many believe that all primordial cysts are odontogenic keratocysts, although some recognize the existence of a primordial cyst that does not have the histopathologic features of the odontogenic keratocyst. If such a lesion exists, then it must be exceedingly rare. Reference to this lesion is almost nonexistent in the current literature, and no reported series include a significant number of cases. In the authors’ experience, a cyst clinically considered to represent a primordial cyst, in the older meaning of the term, almost always is an odontogenic keratocyst after microscopic study (Fig. 15-11).
ODONTOGENIC KERATOCYST
The odontogenic keratocyst is a distinctive form of developmental odontogenic cyst that deserves special consideration because of its specific histopathologic features and clinical behavior. There is general agreement that the odontogenic keratocyst arises from cell rests of the dental lamina. This cyst shows a different growth mechanism and biologic behavior from the more common dentigerous cyst and radicular cyst. Most authors believe that dentigerous and radicular cysts continue to enlarge as a result of increased osmotic pressure within the lumen of the cyst. This mechanism does not appear to hold true for odontogenic keratocysts, and their growth may be related to unknown factors inherent in the epithelium itself or enzymatic activity in the fibrous wall. Several investigators have suggested that odontogenic keratocysts be regarded as benign cystic neoplasms rather than cysts, and in the latest WHO classification of odontogenic tumors, these lesions have been given the name “keratocystic odontogenic tumor.” The arguments to support this change in nomenclature largely rely on a few studies that have shown certain molecular genetic alterations that are also present in some neoplasms. Unfortunately, these studies have not examined other cystic lesions of the jaws; therefore, it is currently unknown whether these alterations are unique to the odontogenic keratocyst. Most oral and maxillofacial pathologists do not feel that sufficient evidence exists to justify renaming this widely recognized lesion, with the likely result of causing widespread confusion among the professional community.
Although there are wide variations in the reported frequency of odontogenic keratocysts compared with that of other types of odontogenic cysts, several studies that include large series of cysts indicate that odontogenic keratocysts make up 3% to 11% of all odontogenic cysts.
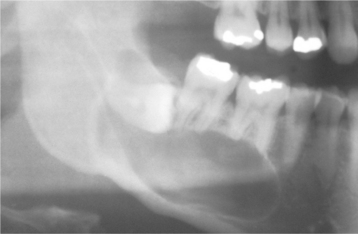
CLINICAL AND RADIOGRAPHIC FEATURES: Odontogenic keratocysts may be found in patients who range in age from infancy to old age, but about 60% of all cases are diagnosed in people between 10 and 40 years of age. There is a slight male predilection. The mandible is involved in 60% to 80% of cases, with a marked tendency to involve the posterior body and ascending ramus (Fig. 15-12).
Small odontogenic keratocysts are usually asymptomatic and discovered only during the course of a radiographic examination. Larger odontogenic keratocysts may be associated with pain, swelling, or drainage. Some extremely large cysts, however, may cause no symptoms.
Odontogenic keratocysts tend to grow in an anteroposterior direction within the medullary cavity of the bone without causing obvious bone expansion. This feature may be useful in differential clinical and radiographic diagnosis because dentigerous and radicular cysts of comparable size are usually associated with bony expansion. Multiple odontogenic keratocysts may be present, and such patients should be evaluated for other manifestations of the nevoid basal cell carcinoma (Gorlin) syndrome (see page 688).
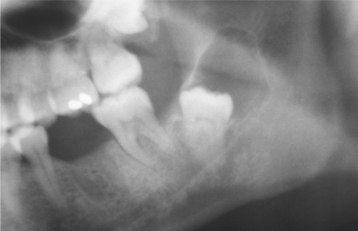
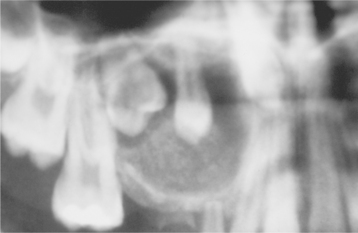
Odontogenic keratocysts demonstrate a well-defined radiolucent area with smooth and often corticated margins. Large lesions, particularly in the posterior body and ascending ramus of the mandible, may appear multilocular (Fig. 15-13). An unerupted tooth is involved in the lesion in 25% to 40% of cases; in such instances, the radiographic features suggest the diagnosis of dentigerous cyst (Figs. 15-14 and 15-15). In these cases, the cyst has presumably arisen from dental lamina rests near an unerupted tooth and has grown to envelop the unerupted tooth. Resorption of the roots of erupted teeth adjacent to odontogenic keratocysts is less common than that noted with dentigerous and radicular cysts.
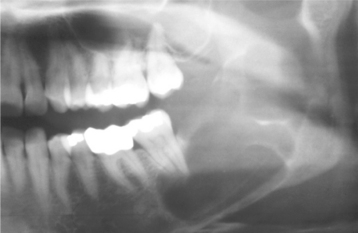
Fig. 15-13 Odontogenic keratocyst. Large, multilocular cyst involving most of the ascending ramus. (Courtesy of Dr. S.C. Roddy.)
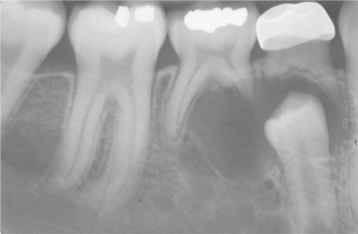
Fig. 15-14 Odontogenic keratocyst. This cyst involves the crown of an unerupted premolar. Radiographically, this lesion cannot be differentiated from a dentigerous cyst.
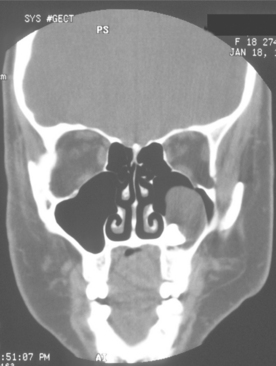
Fig. 15-15 Odontogenic keratocyst. Computed tomography (CT) scan showing a large cyst involving the crown of an unerupted maxillary third molar. The cyst largely fills the maxillary sinus. (Courtesy of Dr. E.B. Bass.)
The diagnosis of odontogenic keratocyst is based on the histopathologic features. The radiographic findings, although often highly suggestive, are not diagnostic. The radiographic findings in an odontogenic keratocyst may simulate those of a dentigerous cyst, a radicular cyst, a residual cyst, a lateral periodontal cyst (Fig. 15-16), or the so-called globulomaxillary cyst (which is no longer considered to be a true entity). Odontogenic keratocysts of the anterior midline maxillary region can mimic nasopalatine duct cysts. For unknown reasons, this particular subset of keratocyst usually occurs in older individuals with a mean age of nearly 70 years. Rare examples of peripheral odontogenic keratocysts within the gingival soft tissues have been reported.
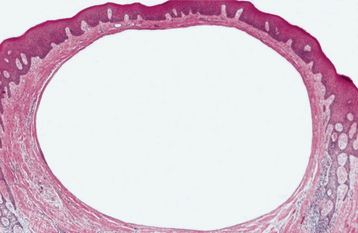
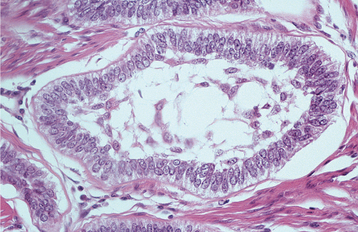
HISTOPATHOLOGIC FEATURES: The odontogenic keratocyst typically shows a thin, friable wall, which is often difficult to enucleate from the bone in one piece. The cystic lumen may contain a clear liquid that is similar to a transudate of serum, or it may be filled with a cheesy material that, on microscopic examination, consists of keratinaceous debris. Microscopically, the thin fibrous wall is essentially devoid of any inflammatory infiltrate. The epithelial lining is composed of a uniform layer of stratified squamous epithelium, usually six to eight cells in thickness. The epithelium and connective tissue interface is usually flat, and rete ridge formation is inconspicuous. Detachment of portions of the cyst-lining epithelium from the fibrous wall is commonly observed. The luminal surface shows flattened parakeratotic epithelial cells, which exhibit a wavy or corrugated appearance (Fig. 15-17). The basal epithelial layer is composed of a palisaded layer of cuboidal or columnar epithelial cells, which are often hyperchromatic. Small satellite cysts, cords, or islands of odontogenic epithelium may be seen within the fibrous wall. These structures have been present in 7% to 26% of cases in various reported series. In rare instances, cartilage has been observed in the wall of an odontogenic keratocyst.
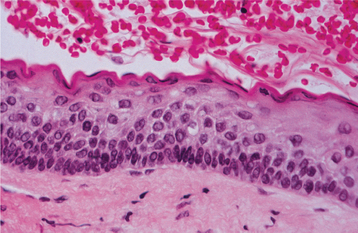
Fig. 15-17 Odontogenic keratocyst. The epithelial lining is 6 to 8 cells thick, with a hyperchromatic and palisaded basal cell layer. Note the corrugated parakeratotic surface.
In the presence of inflammatory changes, the typical features of the odontogenic keratocyst may be altered. The parakeratinized luminal surface may disappear, and the epithelium may proliferate to form rete ridges with the loss of the characteristic palisaded basal layer (Fig. 15-18). When these changes involve most of the cyst lining, the diagnosis of odontogenic keratocyst cannot be confirmed unless other sections show the typical features described earlier.
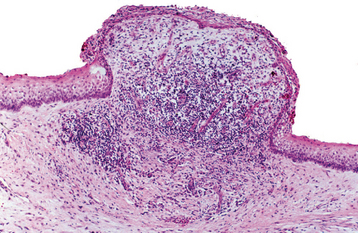
Fig. 15-18 Odontogenic keratocyst. The characteristic microscopic features have been lost in the central area of this portion of the cystic lining because of the heavy chronic inflammatory cell infiltrate.
Some investigators recognize a microscopic orthokeratotic variant and include this lesion as a subtype of the odontogenic keratocyst. However, these cysts do not demonstrate a hyperchromatic and palisaded basal cell layer, which is so characteristic of true odontogenic keratocysts. In addition, the clinical behavior of these orthokeratinized cysts differs markedly from that of the typical parakeratinized cysts described in this section. The authors believe that it is more logical to discuss these orthokeratinizing cysts separately (see following section).
TREATMENT AND PROGNOSIS: Although the presence of an odontogenic keratocyst may be suspected on clinical or radiographic grounds, histopathologic confirmation is required for the diagnosis. Consequently, most odontogenic keratocysts are treated similarly to other odontogenic cysts, that is, by enucleation and curettage. Complete removal of the cyst in one piece is often difficult because of the thin, friable nature of the cyst wall. In contrast to other odontogenic cysts, odontogenic keratocysts often tend to recur after treatment. Whether this is due to fragments of the original cyst that were not removed at the time of the operation or a “new” cyst that has developed from dental lamina rests in the general area of the original cyst cannot be determined with certainty.
The reported frequency of recurrence in various studies ranges from 5% to 62%. This wide variation may be related to the total number of cases studied, the length of follow-up periods, and the inclusion or exclusion of orthokeratinized cysts in the study group. Several reports that include large numbers of cases indicate a recurrence rate of approximately 30%. Recurrence is encountered more often in mandibular odontogenic keratocysts, particularly those in the posterior body and ascending ramus. Multiple recurrences are not unusual. Although many odontogenic keratocysts recur within 5 years of the original surgery, a significant number of recurrences may not be manifested until 10 or more years after the original surgical procedure. Long-term clinical and radiographic follow-up, therefore, is necessary.
Many surgeons recommend peripheral ostectomy of the bony cavity with a bone bur to reduce the frequency of recurrence. Others advocate chemical cauterization of the bony cavity with Carnoy’s solution after cyst removal. Intraluminal injection of Carnoy’s solution also has been used to free the cyst from the bony wall, thereby allowing easier removal with a lower recurrence rate. After cystotomy and incisional biopsy, some surgeons have treated large odontogenic keratocysts by insertion of a polyethylene drainage tube to allow decompression and subsequent re-duction in size of the cystic cavity (Fig. 15-19). Such decompression treatment results in thickening of the cyst lining, allowing easier removal with an apparently lower recurrence rate.
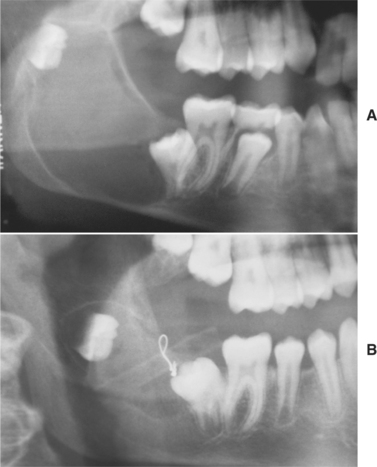
Fig. 15-19 Decompression of an odontogenic keratocyst. A, Large unilocular radiolucency associated with the right mandibular third molar. B, Six months after insertion of a polyethylene drainage tube to allow decompression, the cyst has shrunk and the third molar has migrated downward and forward. (Courtesy of Dr. Tom Szakal.)
Other than the tendency for recurrences, the overall prognosis for most odontogenic keratocysts is good. Occasionally, a locally aggressive odontogenic keratocyst cannot be controlled without local resection and bone grafting. In extremely rare instances, keratocysts have been seen to extend up into the skull base region. A few examples of carcinoma arising in an odontogenic keratocyst have been reported, but the propensity for an odontogenic keratocyst to undergo malignant alteration is no greater and is possibly less than that for other types of odontogenic cysts. Patients with odontogenic keratocysts should be evaluated for manifestations of the nevoid basal cell carcinoma syndrome (see page 688), particularly if the patient is in the first or second decade of life or if multiple keratocysts are identified.
ORTHOKERATINIZED ODONTOGENIC CYST
The designation orthokeratinized odontogenic cyst does not denote a specific clinical type of odontogenic cyst but refers only to an odontogenic cyst that microscopically has an orthokeratinized epithelial lining. Although such lesions were originally called the orthokeratinized variant of odontogenic keratocyst, it is generally accepted that they are clinicopathologically different from the more common parakeratinized odontogenic keratocyst and should be placed into a different category. Orthokeratinized odontogenic cysts represent 7% to 17% of all keratinizing jaw cysts.
CLINICAL AND RADIOGRAPHIC FEATURES: Orthokeratinized odontogenic cysts occur predominantly in young adults and show a 2:1 male-to-female ratio. The lesion occurs twice as frequently in the mandible than the maxilla, with a tendency to involve the posterior areas of the jaws. They have no clinical or radiographic features that differentiate them from other inflammatory or developmental odontogenic cysts. The lesion usually appears as a unilocular radiolucency, but occasional examples have been multilocular. About two thirds of orthokeratinized odontogenic cysts are encountered in a lesion that appears clinically and radiographically to represent a dentigerous cyst; they most often involve an unerupted mandibular third molar tooth (Figs. 15-20 and 15-21). The size can vary from less than 1 cm to large lesions greater than 7 cm in diameter.
HISTOPATHOLOGIC FEATURES: The cyst lining is composed of stratified squamous epithelium, which shows an orthokeratotic surface of varying thickness. Keratohyaline granules may be prominent in the superficial epithelial layer subjacent to the orthokeratin. The epithelial lining may be relatively thin, and a prominent palisaded basal layer, characteristic of the odontogenic keratocyst, is not present (Fig. 15-22).
TREATMENT AND PROGNOSIS: Enucleation with curettage is the usual treatment for orthokeratinized odontogenic cysts. Recurrence has rarely been noted, and the reported frequency is around 2%, which is in marked contrast with the 30% or higher recurrence rate associated with odontogenic keratocysts. It has been suggested that cysts with an orthokeratinized surface may be at slightly greater risk for malignant transformation, but evidence for this is scant. Orthokeratinized odontogenic cysts have not been associated with nevoid basal cell carcinoma syndrome.
NEVOID BASAL CELL CARCINOMA SYNDROME (GORLIN SYNDROME)
Nevoid basal cell carcinoma syndrome (Gorlin syndrome) is an autosomal dominant inherited condition that exhibits high penetrance and variable expressivity. The syndrome is caused by mutations in patched (PTCH), a tumor suppressor gene that has been mapped to chromosome 9q22.3-q31. Approximately 35% to 50% of affected patients represent new mutations. The chief components are multiple basal cell carcinomas of the skin, odontogenic keratocysts, intracranial calcification, and rib and vertebral anomalies. Many other anomalies have been reported in these patients and probably also represent manifestations of the syndrome. The prevalence of Gorlin syndrome is estimated to be about 1 in 60,000.
CLINICAL AND RADIOGRAPHIC FEATURES: There is great variability in the expressivity of nevoid basal cell carcinoma syndrome, and no single component is present in all patients. The most common and significant features are summarized in Box 15-2. The patient often has a characteristic facies, with frontal and temporoparietal bossing, which results in an increased cranial circumference (>60 cm in adults). The eyes may appear widely separated, and many patients have true mild ocular hypertelorism. Mild mandibular prognathism is also commonly present (Fig. 15-23).
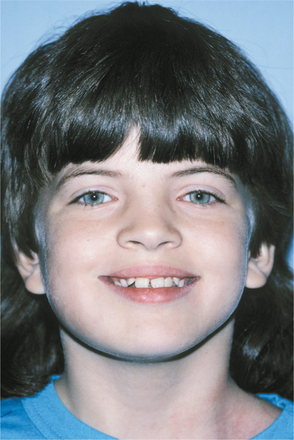
Fig. 15-23 Nevoid basal cell carcinoma syndrome. This 11-year-old girl shows hypertelorism and mandibular swelling. (Courtesy of Dr. Richard DeChamplain.)
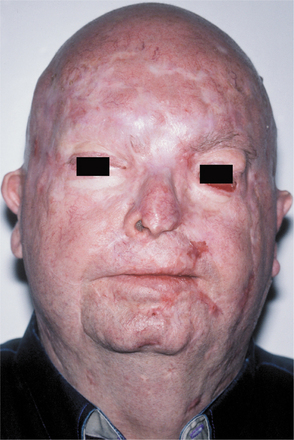
Basal cell carcinomas of the skin are a major component of the syndrome. Even though the microscopic appearance of the syndromic basal cell carcinomas is identical to that of nonsyndromic basal cell carcinoma, the syndromic lesions generally have a much less aggressive biologic behavior. The basal cell carcinomas usually begin to appear at puberty or in the second and third decades of life, although they can develop in young children. The tumors may vary from flesh-colored papules to ulcerating plaques. They often appear on skin that is not exposed to sunlight, but they are most commonly located in the midface area (Fig. 15-24). The number of skin tumors may vary from only a few to many hundreds. Blacks with the syndrome tend to develop basal cell carcinomas less frequently than whites (40% versus 90%), and they have fewer of these lesions, probably because of protective skin pigmentation.
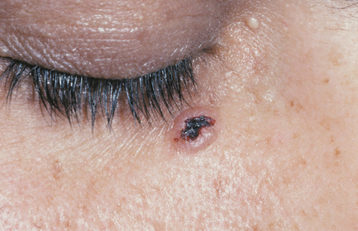
Fig. 15-24 Nevoid basal call carcinoma syndrome. An ulcerating basal cell carcinoma is present on the upper face.
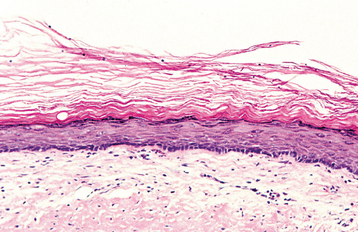
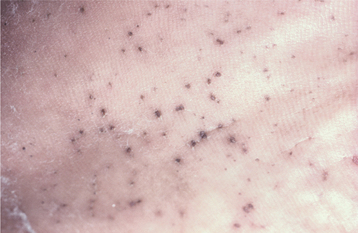
Palmar and plantar pits are present in about 65% to 80% of patients (Fig. 15-25). These punctate lesions represent a localized retardation of the maturation of basal epithelial cells, resulting in a focally depressed area as the result of a markedly thinned keratin layer. Basal cell carcinomas rarely may develop at the base of the pits.
Ovarian fibromas have been reported in 15% to 25% of women with this syndrome. A number of other tumors also have been reported to occur with lesser frequency. These include medulloblastoma within the first 2 years of life, meningioma, cardiac fibroma, and fetal rhabdomyoma.
Skeletal anomalies are present in 60% to 75% of patients with this syndrome. The most common anomaly is a bifid rib or splayed ribs (Fig. 15-26). This anomaly may involve several ribs and may be bilateral. Kyphoscoliosis has been observed in about 30% to 40% of patients, and a number of other anomalies, such as spina bifida occulta and shortened metacarpals, seem to occur with unusual frequency. A distinctive lamellar calcification of the falx cerebri, noted on an anteroposterior skull radiograph or computed tomography (CT) image, is a common finding and is present in most affected patients (Fig. 15-27).
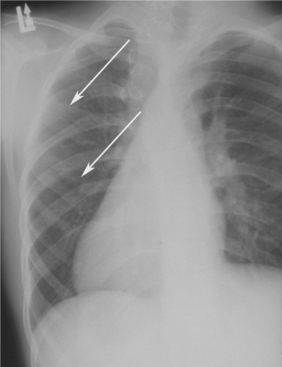
Fig. 15-26 Nevoid basal cell carcinoma syndrome. Chest film showing presence of bifid ribs (arrows).
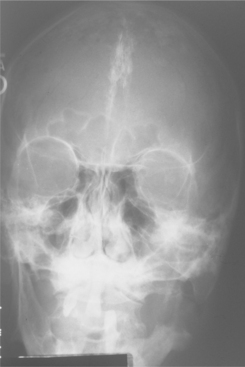
Fig. 15-27 Nevoid basal cell carcinoma syndrome. Anteroposterior skull film showing calcification of the falx cerebri. (Courtesy of Dr. Ramesh Narang.)
Jaw cysts are one of the most constant features of the syndrome and are present in at least 75% of the patients. The cysts are odontogenic keratocysts, although there are some differences between the cysts in patients with nevoid basal cell carcinoma syndrome and in those with isolated keratocysts. The cysts are frequently multiple; some patients have had as many as ten separate cysts. The patient’s age when the first keratocyst is removed is significantly younger in those affected by this syndrome than in those with isolated keratocysts. For most patients with this syndrome, their first keratocyst is removed before age 19. About one third of patients with nevoid basal cell carcinoma syndrome have only a solitary cyst at the time of the initial presentation, but in most cases additional cysts will develop over periods ranging from 1 to 20 years.
Radiographically, the cysts in patients with nevoid basal cell carcinoma syndrome do not differ significantly from isolated keratocysts. The cysts in patients with this syndrome are often associated with the crowns of unerupted teeth; on radiographs they may mimic dentigerous cysts (Fig. 15-28).
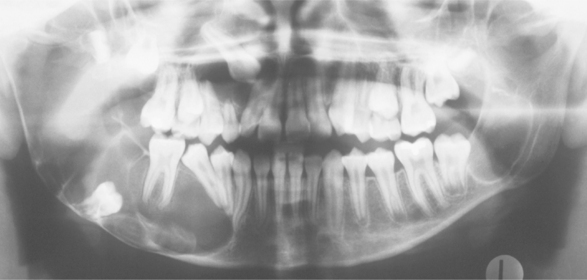
Fig. 15-28 Nevoid basal cell carcinoma syndrome. Large cysts are present in the right and left mandibular molar regions, together with a smaller cyst involving the right maxillary canine in the same patient shown in Fig. 15-23. (Courtesy of Dr. Richard DeChamplain.)
HISTOPATHOLOGIC FEATURES: The cysts in the nevoid basal cell carcinoma syndrome histopathologically are invariably odontogenic keratocysts. The keratocysts in patients with this syndrome tend to have more satellite cysts, solid islands of epithelial proliferation, and odontogenic epithelial rests within the fibrous capsule than do isolated keratocysts (Fig. 15-29). Foci of calcification also appear to be more common. These features, however, are not diagnostic for nevoid basal cell carcinoma syndrome because they may be seen in isolated keratocysts. Odontogenic keratocysts associated with this syndrome have been shown to demonstrate overexpression of p53 and cyclin D1 (bcl-1) oncoproteins when compared with nonsyndrome keratocysts.
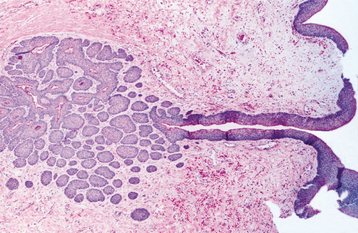
Fig. 15-29 Nevoid basal cell carcinoma syndrome. Odontogenic keratocyst showing numerous odontogenic epithelial rests in the cyst wall.
The basal cell tumors of the skin cannot be distinguished from ordinary basal cell carcinomas. They exhibit a wide spectrum of histopathologic findings, from superficial basal cell lesions to aggressive, noduloulcerative basal cell carcinomas.
TREATMENT AND PROGNOSIS: Most of the anomalies in nevoid basal cell carcinoma syndrome are minor and usually not life threatening. The prognosis generally depends on the behavior of the skin tumors. In a few cases, aggressive basal cell carcinomas have caused the death of the patient as a result of tumor invasion of the brain or other vital structures (Figs. 15-30 and 15-31). Because the development of the basal cell carcinomas seems to be triggered by ultraviolet (UV) light exposure, patients should take appropriate precautions to avoid sunlight. For the same reason, radiation therapy should be avoided if at all possible. The jaw cysts are treated by enucleation, but in many patients additional cysts will continue to develop. Varying degrees of jaw deformity may result from the operations for multiple cysts. Infection of the cysts in patients with this syndrome is also relatively common. Some investigators have suggested that affected children should have magnetic resonance imaging (MRI) studies every 6 months until 7 years of age to monitor for the development of medulloblastoma. Genetic counseling is appropriate for affected individuals.
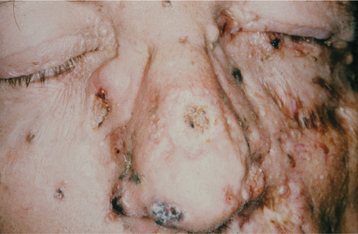
Fig. 15-30 Nevoid basal cell carcinoma syndrome. This 52-year-old man had more than 100 basal cell carcinomas removed from his face over a 30-year period. Several basal cell carcinomas are present in this photograph. The lesion at the inner canthus of the left eye was deeply invasive and was eventually fatal as a result of brain invasion.
GINGIVAL (ALVEOLAR) CYST OF THE NEWBORN
Gingival cysts of the newborn are small, superficial, keratin-filled cysts that are found on the alveolar mucosa of infants. These cysts arise from remnants of the dental lamina. They are common lesions, having been reported in up to half of all newborns. However, because they disappear spontaneously by rupture into the oral cavity, the lesions seldom are noticed or sampled for biopsy. Similar inclusion cysts (e.g., Epstein’s pearls and Bohn’s nodules) are also found in the midline of the palate or laterally on the hard and soft palate (see page 26).
CLINICAL FEATURES: Gingival cysts of the newborn appear as small, usually multiple whitish papules on the mucosa overlying the alveolar processes of neonates (Fig. 15-32). The individual cysts are usually no more than 2 to 3 mm in diameter. The maxillary alveolus is more commonly involved than the mandibular.
GINGIVAL CYST OF THE ADULT
The gingival cyst of the adult is an uncommon lesion. It is considered to represent the soft tissue counterpart of the lateral periodontal cyst (see next topic), being derived from rests of the dental lamina (rests of Serres). The diagnosis of gingival cyst of the adult should be restricted to lesions with the same histopathologic features as those of the lateral periodontal cyst. On rare occasions, a cyst may develop in the gingiva at the site of a gingival graft; however, such lesions probably represent epithelial inclusion cysts that are a result of the surgical procedure.
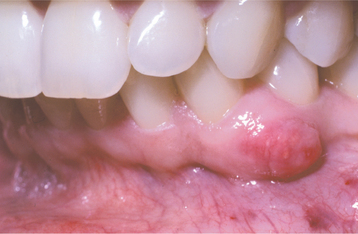
CLINICAL FEATURES: Like the lateral periodontal cyst, the gingival cyst of the adult shows a striking predilection to occur in the mandibular canine and premolar area (60% to 75% of cases). Gingival cysts of the adult are most commonly found in patients in the fifth and sixth decades of life. They are almost invariably located on the facial gingiva or alveolar mucosa. Maxillary gingival cysts are usually found in the incisor, canine, and premolar areas.
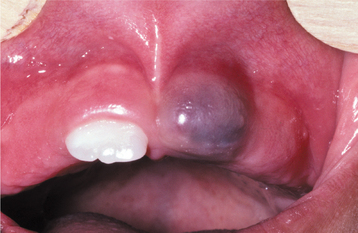
Clinically, the cysts appear as painless, domelike swellings, usually less than 0.5 cm in diameter, although rarely they may be somewhat larger (Fig. 15-33). They are often bluish or blue-gray. In some instances, the cyst may cause a superficial “cupping out” of the alveolar bone, which is usually not detected on a radiograph but is apparent when the cyst is excised. If more bone is missing, one could argue that the lesion may be a lateral periodontal cyst that has eroded the cortical bone rather than a gingival cyst that originated in the mucosa.
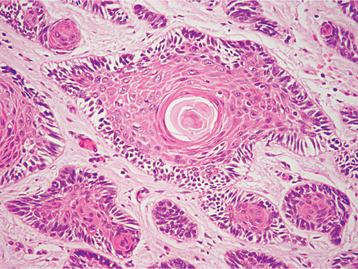
HISTOPATHOLOGIC FEATURES: The histopathologic features of the gingival cyst of the adult are similar to those of the lateral periodontal cyst, consisting of a thin, flattened epithelial lining with or without focal plaques that contain clear cells (Figs. 15-34 and 15-35). Small nests of these glycogen-rich clear cells, which represent rests of the dental lamina, also may be seen in the surrounding connective tissue. Sometimes the cystic lining is so thin that it is easily mistaken for the endothelial lining of a dilated blood vessel.
LATERAL PERIODONTAL CYST (BOTRYOID ODONTOGENIC CYST)
The lateral periodontal cyst is an uncommon type of developmental odontogenic cyst that typically occurs along the lateral root surface of a tooth. It is believed to arise from rests of the dental lamina, and it represents the intrabony counterpart of the gingival cyst of the adult. The lateral periodontal cyst accounts for less than 2% of all epithelium-lined jaw cysts.
In the past, the term lateral periodontal cyst was used to describe any cyst that developed along the lateral root surface, including lateral radicular cysts (see page 131) and odontogenic keratocysts (see page 683). However, the lateral periodontal cyst has distinctive clinical and microscopic features that distinguish it from other lesions that sometimes develop in the same location.
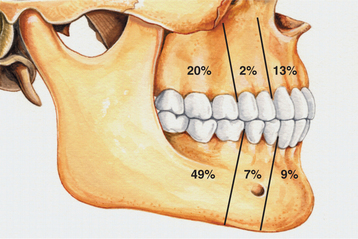
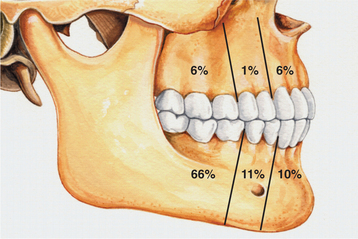
CLINICAL AND RADIOGRAPHIC FEATURES: The lateral periodontal cyst is most often an asymptomatic lesion that is detected only during a radiographic examination. It most frequently occurs in patients in the fifth through the seventh decades of life; rarely does it occur in someone younger than age 30. Around 75% to 80% of cases occur in the mandibular premolar-canine-lateral incisor area. Maxillary examples also usually involve this same tooth region (Fig. 15-36).
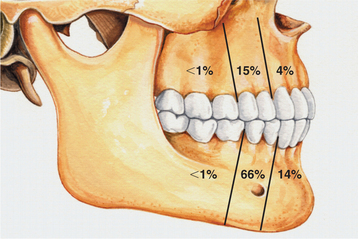
Fig. 15-36 Lateral periodontal cyst. Relative distribution of lateral periodontal cysts in the jaws.
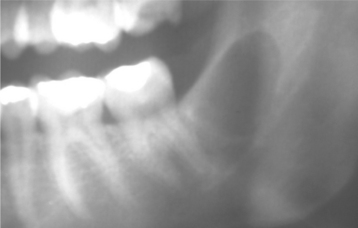
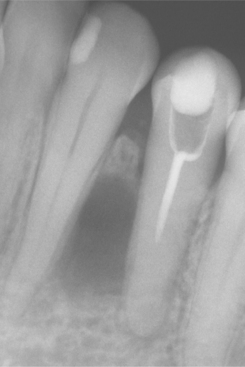
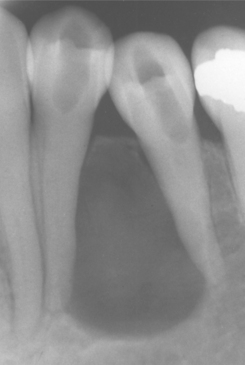
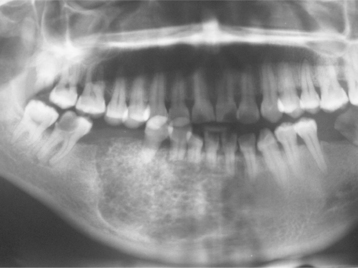
Radiographically, the cyst appears as a well-circumscribed radiolucent area located laterally to the root or roots of vital teeth. Most such cysts are less than 1.0 cm in greatest diameter (Figs. 15-37 and 15-38).
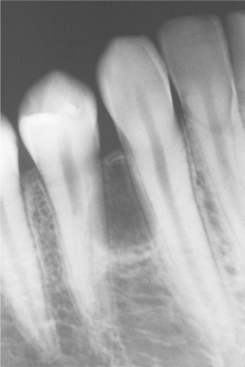
Fig. 15-37 Lateral periodontal cyst. Radiolucent lesion between the roots of a vital mandibular canine and first premolar.
Occasionally, the lesion may have a polycystic appearance; such examples have been termed botryoid odontogenic cysts. Grossly and microscopically, they show a grapelike cluster of small individual cysts (Fig. 15-39). These lesions are generally considered to represent a variant of the lateral periodontal cyst, possibly the result of cystic degeneration and subsequent fusion of adjacent foci of dental lamina rests. The botryoid variant often shows a multilocular radiographic appearance, but it also may appear unilocular.
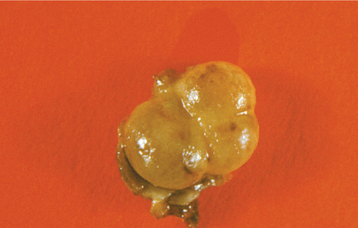
Fig. 15-39 Lateral periodontal cyst. Gross specimen of a botryoid variant. Microscopically, this grapelike cluster revealed three separate cavities.
The radiographic features of the lateral periodontal cyst are not diagnostic; an odontogenic keratocyst that develops between the roots of adjacent teeth may show identical radiographic findings. An inflammatory radicular cyst that occurs laterally to a root in relation to an accessory foramen or a cyst that arises from periodontal inflammation also may simulate a lateral periodontal cyst radiographically (see page 132). In one study of 46 cases of cystic lesions in the lateral periodontal region, only 13 met the histopathologic criteria for the lateral periodontal cyst; 8 were odontogenic keratocysts, 20 were inflammatory cysts, and 5 were of undetermined origin.
HISTOPATHOLOGIC FEATURES: The lateral periodontal cyst has a thin, generally noninflamed, fibrous wall, with an epithelial lining that is only one to three cells thick in most areas. This epithelium usually consists of flattened squamous cells, but sometimes the cells are cuboidal in shape. Foci of glycogen-rich clear cells may be interspersed among the lining epithelial cells. Some cysts show focal nodular thickenings of the lining epithelium, which are composed chiefly of clear cells (Fig. 15-40). Clear cell epithelial rests sometimes are seen within the fibrous wall. Rarely, botryoid odontogenic cysts exhibit focal areas that histopathologically are suggestive of the glandular odontogenic cyst (see page 697).
TREATMENT AND PROGNOSIS: Conservative enucleation of the lateral periodontal cyst is the treatment of choice. Usually, this can be accomplished without damage to the adjacent teeth. Recurrence is unusual, although it has been reported with the botryoid variant, presumably because of its polycystic nature. An exceedingly rare case of squamous cell carcinoma, which apparently originated in a lateral periodontal cyst, also has been reported.
CALCIFYING ODONTOGENIC CYST (GORLIN CYST; DENTINOGENIC GHOST CELL TUMOR; CALCIFYING CYSTIC ODONTOGENIC TUMOR; CALCIFYING GHOST CELL ODONTOGENIC CYST)
The calcifying odontogenic cyst is an uncommon lesion that demonstrates considerable histopathologic diversity and variable clinical behavior. Although it is widely considered to represent a cyst, some investigators prefer to classify it as a neoplasm. Some calcifying odontogenic cysts appear to represent nonneoplastic cysts; other members of this group, variously designated as dentinogenic ghost cell tumors or epithelial odontogenic ghost cell tumors, have no cystic features, may be infiltrative or even malignant, and are regarded as neoplasms.
In addition, the calcifying odontogenic cyst may be associated with other recognized odontogenic tumors, most commonly odontomas. However, adenomatoid odontogenic tumors and ameloblastomas have also been associated with calcifying odontogenic cysts. The WHO classification of odontogenic tumors groups the calcifying odontogenic cyst with all its variants as an odontogenic tumor rather than an odontogenic cyst. Given the innocuous clinical behavior of this lesion, the widely recognized historic categorization of the lesion as a cyst, and the cystic nature of the majority of these lesions, the change in terminology does not seem practical.
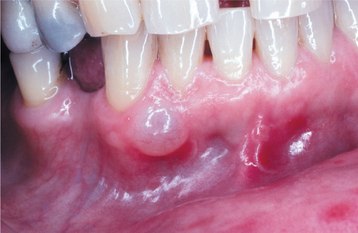
CLINICAL AND RADIOGRAPHIC FEATURES: The calcifying odontogenic cyst is predominantly an intraosseous lesion, although 13% to 30% of cases in reported series have appeared as peripheral (extraosseous) lesions. Both the intraosseous and extraosseous forms occur with about equal frequency in the maxilla and mandible. About 65% of cases are found in the incisor and canine areas (Fig. 15-41). Patients may range in age from infant to elder. The mean age is 33 years, and most cases are diagnosed in the second and third decades of life. Calcifying odontogenic cysts that are associated with odontomas tend to occur in younger patients, with a mean age of 17 years. The rare neoplastic variants of the calcifying odontogenic cyst appear to occur in older patients; because of the paucity of reported cases, however, this may not be significant.
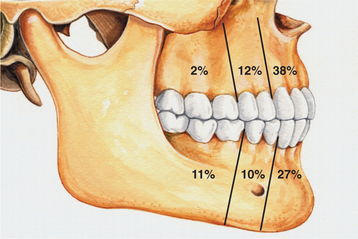
Fig. 15-41 Calcifying odontogenic cyst. Relative distribution of calcifying odontogenic cysts in the jaws.
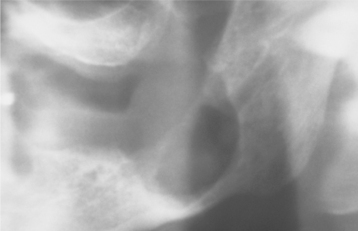
The central calcifying odontogenic cyst is usually a unilocular, well-defined radiolucency, although the lesion may occasionally appear multilocular. Radiopaque structures within the lesion, either irregular calcifications or toothlike densities, are present in about one third to one half of cases (Fig. 15-42). In approximately one third of cases, the radiolucent lesion is associated with an unerupted tooth, most often a canine. Most calcifying odontogenic cysts are between 2.0 and 4.0 cm in greatest diameter, but lesions as large as 12.0 cm have been noted. Root resorption or divergence of adjacent teeth is seen with some frequency (Fig. 15-43).
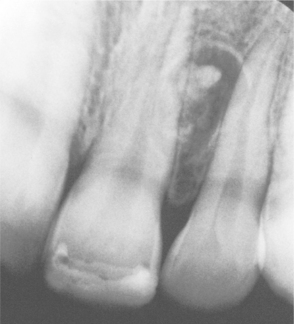
Fig. 15-42 Calcifying odontogenic cyst. Maxillary radiolucent lesion containing calcified structures.
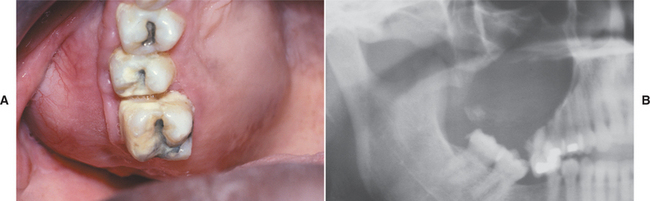
Fig. 15-43 Calcifying odontogenic cyst. A, Expansion of the posterior maxillary alveolus caused by a large calcifying odontogenic cyst. B, Panoramic radiograph of the same patient showing a large radiolucency in the posterior maxilla. A small calcified structure is seen in the lower portion of the cyst. (Courtesy of Dr. Tom Brock.)
Extraosseous calcifying odontogenic cysts are localized sessile or pedunculated gingival masses with no distinctive clinical features (Fig. 15-44). They can resemble common gingival fibromas, gingival cysts, or peripheral giant cell granulomas.
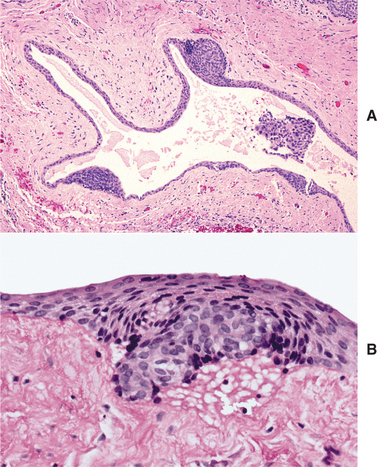
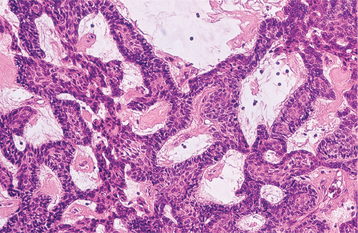
HISTOPATHOLOGIC FEATURES: The cystic (nonneoplastic) forms comprise 86% to 98% of all calcifying odontogenic cysts in various reported series. These may occur both intraosseously and extraosseously. Most commonly, a well-defined cystic lesion is found with a fibrous capsule and a lining of odontogenic epithelium of 4 to 10 cells in thickness. The basal cells of the epithelial lining may be cuboidal or columnar and are similar to ameloblasts. The overlying layer of loosely arranged epithelium may resemble the stellate reticulum of an ameloblastoma.
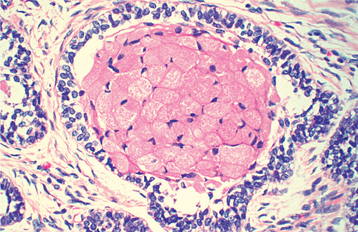
The most characteristic histopathologic feature of the calcifying odontogenic cyst is the presence of variable numbers of “ghost cells” within the epithelial component. These eosinophilic ghost cells are altered epithelial cells that are characterized by the loss of nuclei with preservation of the basic cell outline (Fig. 15-45).
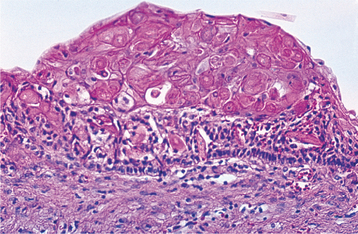
Fig. 15-45 Calcifying odontogenic cyst. The cyst lining shows ameloblastoma-like epithelial cells, with a columnar basal layer. Large eosinophilic ghost cells are present within the epithelial lining.
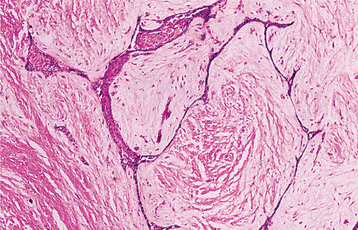
The nature of the ghost cell change is controversial. Some believe that this change represents coagulative necrosis or accumulation of enamel protein; others contend it is a form of normal or aberrant keratinization of odontogenic epithelium. Masses of ghost cells may fuse to form large sheets of amorphous, acellular material. Calcification within the ghost cells is common. This first appears as fine basophilic granules that may increase in size and number to form extensive masses of calcified material. Areas of an eosinophilic matrix material that are considered by some authors to represent dysplastic dentin (dentinoid) also may be present adjacent to the epithelial component. This is believed to be the result of an inductive effect by the odontogenic epithelium on the adjacent mesenchymal tissue (Fig. 15-46).
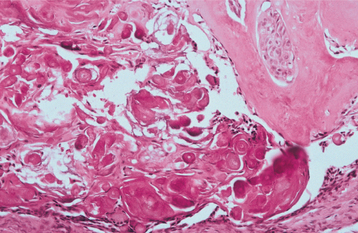
Fig. 15-46 Calcifying odontogenic cyst. Eosinophilic dentinoid material is present adjacent to a sheet of ghost cells.
Several variants of the cystic type of calcifying odontogenic cyst are seen. In some cases, the epithelial lining proliferates into the lumen so that the lumen is largely filled with masses of ghost cells and dystrophic calcifications. Multiple daughter cysts may be present within the fibrous wall, and a foreign body reaction to herniated ghost cells may be conspicuous.
In another variant, unifocal or multifocal epithelial proliferation of the cyst lining into the lumen may resemble ameloblastoma. These proliferations are intermixed with varying numbers of ghost cells. These epithelial proliferations superficially resemble, but do not meet, the strict histopathologic criteria for ameloblastoma.
About 20% of cystic calcifying odontogenic cysts are associated with odontomas. This variant is usually a unicystic lesion that shows the features of a calcifying odontogenic cyst together with those of a small complex or compound odontoma.
Neoplastic (solid) calcifying odontogenic cysts are uncommon, accounting for 2% to 16% of all calcifying odontogenic cysts in reported series. These may occur intraosseously or extraosseously.
The extraosseous forms of the solid variant appear to be more common. These show varying-sized islands of odontogenic epithelium in a fibrous stroma. The epithelial islands show peripheral palisaded columnar cells and central stellate reticulum, which resemble ameloblastoma. Nests of ghost cells, however, are present within the epithelium, and juxtaepithelial dentinoid is commonly present. These features differentiate this lesion from the peripheral ameloblastoma.
The rare intraosseous variant is a solid tumor that consists of ameloblastoma-like strands and islands of odontogenic epithelium in a mature fibrous connective tissue stroma. Variable numbers of ghost cells and juxtaepithelial dentinoid are present.
A small number of aggressive or malignant epithelial odontogenic ghost cell tumors (odontogenic ghost cell carcinoma) have been reported. These lesions have cellular pleomorphism and mitotic activity with invasion of the surrounding tissues.
TREATMENT AND PROGNOSIS: The prognosis for a patient with a calcifying odontogenic cyst is good; only a few recurrences after simple enucleation have been reported. The peripheral neoplastic calcifying odontogenic cyst appears to have the same prognosis as a peripheral ameloblastoma, with a minimal chance of recurrence after simple surgical excision.
When a calcifying odontogenic cyst is associated with some other recognized odontogenic tumor, such as an ameloblastoma, the treatment and prognosis are likely to be the same as for the associated tumor. Although few cases have been reported, odontogenic ghost cell carcinomas appear to have an unpredictable behavior. Recurrences are common, and a few patients have died from either uncontrolled local disease or metastases. An overall 5-year survival rate of 73% has been calculated for reported cases.
GLANDULAR ODONTOGENIC CYST (SIALO-ODONTOGENIC CYST)
The glandular odontogenic cyst is a rare type of developmental odontogenic cyst that can show aggressive behavior. Although it is generally accepted as being of odontogenic origin, it also shows glandular or salivary features that presumably are an indication of the pluripotentiality of odontogenic epithelium.
CLINICAL AND RADIOGRAPHIC FEATURES: The glandular odontogenic cyst occurs most commonly in middle-aged adults, with a mean age of 48 years at the time of diagnosis; rarely does it occur before the age of 20. Nearly 75% of reported cases have occurred in the mandible. The cyst has a strong predilection for the anterior region of the jaws, and many mandibular lesions will cross the midline.
The size of the cyst can vary from small lesions less than 1 cm in diameter to large destructive lesions that may involve most of the jaw. Small cysts may be asymptomatic; however, large cysts often produce clinical expansion, which sometimes can be associated with pain or paresthesia (Fig. 15-47).
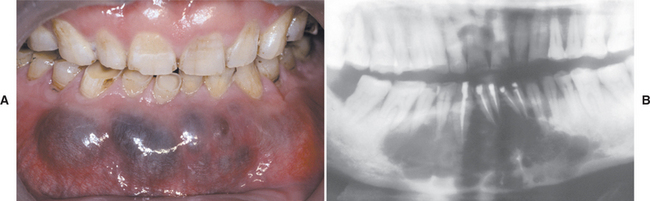
Fig. 15-47 Glandular odontogenic cyst. A, Expansile lesion of the anterior mandible. B, The panoramic radiograph shows a large multilocular radiolucency. (Courtesy of Dr. Cheng-Chung Lin.)
Radiographically, the lesion presents as either a unilocular or multilocular radiolucency. The margins of the radiolucency are usually well defined with a sclerotic rim.
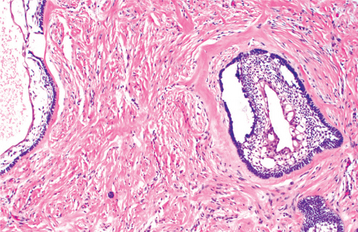
HISTOPATHOLOGIC FEATURES: The glandular odontogenic cyst is lined by squamous epithelium of varying thickness. The interface between the epithelium and the fibrous connective tissue wall is generally flat. The fibrous cyst wall is usually devoid of any inflammatory cell infiltrate. The superficial epithelial cells that line the cyst cavity tend to be cuboidal to columnar, resulting in an uneven hobnail and sometimes papillary surface (Fig. 15-48). Occasionally, cilia may be noted. Pools of mucicarminophilic material are often present within the epithelium. Cuboidal cells usually line these pools. Mucous cells may or may not be present within the epithelium. In focal areas, the epithelial lining cells may form spherical nodules, similar to those seen in lateral periodontal cysts.
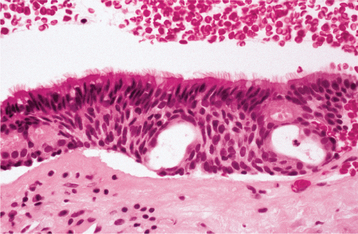
Fig. 15-48 Glandular odontogenic cyst. The cyst is lined by stratified squamous epithelium that exhibits surface columnar cells with cilia. Small microcysts and clusters of mucous cells are present.
There is some histopathologic overlap between the features of the glandular odontogenic cyst and those of some intraosseous, low-grade, predominantly cystic mucoepidermoid carcinomas (see page 490). In selected microscopic fields, the microscopic features may be identical. Examination of multiple sections, however, usually permits the differentiation of these lesions.
TREATMENT AND PROGNOSIS: Most cases of glandular odontogenic cyst have been treated by enucleation or curettage. However, this cyst shows a propensity for recurrence, which is observed in approximately 30% of all cases. Recurrence appears to be more common among the lesions that present in a multilocular fashion. Because of its potentially aggressive nature and tendency for recurrence, some authors have advocated en bloc resection, particularly for multilocular lesions.
BUCCAL BIFURCATION CYST
The buccal bifurcation cyst is an uncommon inflammatory odontogenic cyst that characteristically develops on the buccal aspect of the mandibular first permanent molar. The pathogenesis of this cyst is uncertain. Some of these lesions have been associated with teeth that demonstrate buccal enamel extensions into the bifurcation area (see page 93). Such extensions may predispose these teeth to buccal pocket formation, which could then enlarge to form a cyst in response to pericoronitis. It has been speculated that when the tooth erupts, an inflammatory response may occur in the surrounding follicular tissues that stimulates cyst formation.
The term paradental cyst sometimes has been used synonymously for the buccal bifurcation cyst. Such lesions typically occur distal or buccal of partially erupted mandibular third molars with a history of pericoronitis. The pathogenesis of the so-called paradental cyst also is uncertain. However, the distinction of paradental cysts from secondarily inflamed dentigerous cysts is difficult, if not impossible, in many instances (see page 679).
CLINICAL AND RADIOGRAPHIC FEATURES: The buccal bifurcation cyst typically occurs in children from 5 to 13 years of age. The patient has slight-to-moderate tenderness on the buccal aspect of the mandibular first molar, which may be in the process of erupting. The patient often notes associated clinical swelling and a foul-tasting discharge. Periodontal probing usually reveals pocket formation on the buccal aspect of the involved tooth. Around one third of patients have been reported to have bilateral involvement of the first molars.
Radiographs typically show a well-circumscribed unilocular radiolucency involving the buccal bifurcation and root area of the involved tooth (Fig. 15-49). The average size of the lucent defect is 1.2 cm, but the lesion may be as large as 2.5 cm in diameter. An occlusal radiograph is most helpful in demonstrating the buccal location of the lesion. The root apices of the molar are characteristically tipped toward the lingual mandibular cortex (Fig. 15-50). Many cases are associated with proliferative periostitis (see page 148) of the overlying buccal cortex, which is characterized by a single or multiple layers of reactive bone formation.
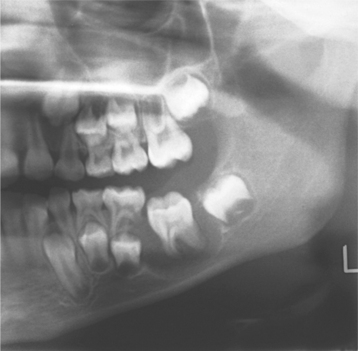
Fig. 15-49 Buccal bifurcation cyst. Well-circumscribed unilocular radiolucency superimposed on the roots of the mandibular first permanent molar. (Courtesy of Dr. Michael Pharoah.)
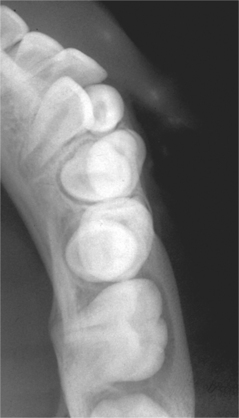
Fig. 15-50 Buccal bifurcation cyst. Occlusal radiograph of the lesion shown in Fig. 15-49. Note the lingual displacement of the roots of the first permanent molar. (Courtesy of Dr. Michael Pharoah.)
HISTOPATHOLOGIC FEATURES: The microscopic features are nonspecific and show a cyst that is lined by nonkeratinizing stratified squamous epithelium with areas of hyperplasia. A prominent chronic inflammatory cell infiltrate is present in the surrounding connective tissue wall.
TREATMENT AND PROGNOSIS: The buccal bifurcation cyst is usually treated by enucleation; extraction of the associated tooth is unnecessary. Within 1 year of surgery, there is usually complete healing with normalization of periodontal probing depths and radiographic evidence of bone fill. One report described three cases that resolved without surgery—either with no treatment at all or by daily irrigation of the buccal pocket with saline and hydrogen peroxide.
CARCINOMA ARISING IN ODONTOGENIC CYSTS
Carcinoma arising within bone is a rare lesion that is essentially limited to the jaws. Because the putative source of the epithelium giving rise to the carcinoma is odontogenic, these intraosseous jaw carcinomas are collectively known as odontogenic carcinomas. Odontogenic carcinomas may arise in an ameloblastoma, rarely from other odontogenic tumors, de novo (without evidence of a preexisting lesion), or from the epithelial lining of odontogenic cysts. Some intraosseous mucoepidermoid carcinomas (see page 490) also may arise from mucous cells lining a dentigerous cyst.
Most intraosseous carcinomas apparently arise in odontogenic cysts. Although infrequently documented in the literature, carcinomatous transformation of the lining of an odontogenic cyst may be more common than is generally appreciated. Several studies have shown that 1% to 2% of all oral cavity carcinomas seen in some oral and maxillofacial pathology services may originate from odontogenic cysts. The pathogenesis of carcinomas arising in odontogenic cysts is unknown. Occasionally, areas within the lining of odontogenic cysts histopathologically demonstrate varying degrees of epithelial dysplasia, and such changes likely give rise to the carcinoma.
CLINICAL AND RADIOGRAPHIC FEATURES: Although carcinomas arising in cysts may be seen in patients across a wide age range, they are encountered most often in older patients. The mean reported age is 57 to 61 years. This lesion is about twice as common in men as in women. Pain and swelling are the most common complaints. However, many patients have no symptoms, and the diagnosis of carcinoma is made only after microscopic examination of a presumed odontogenic cyst.
Radiographic findings may mimic those of any odontogenic cyst, although the margins of the radiolucent defect are usually irregular and ragged. Computed tomography of the lesion may demonstrate a destructive pattern that is not appreciated on viewing plain radiographs. A lesion considered to be a residual periapical cyst is apparently the most common type associated with carcinomatous transformation, although routine periapical cysts can also exhibit malignant change. In about 25% of reported cases, the carcinoma appeared to have arisen in a dentigerous cyst (Fig. 15-51). In one patient, the carcinoma appeared to originate in a lateral periodontal cyst.
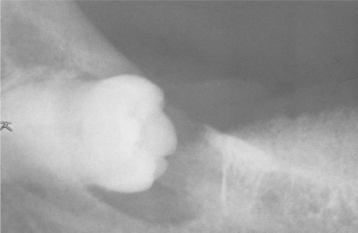
Fig. 15-51 Carcinoma arising in a dentigerous cyst. Radiolucent lesion surrounding the crown of an impacted third molar in a 56-year-old woman. This was clinically considered to be a dentigerous cyst. (Courtesy of Dr. Richard Ziegler.)
A few examples of carcinoma arising in an odontogenic keratocyst also have been documented (Fig. 15-52). However, some reported examples do not appear to have arisen in true parakeratinized odontogenic keratocysts, but rather in orthokeratinized odontogenic cysts.
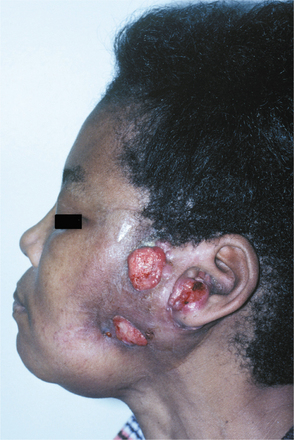
Fig. 15-52 Carcinoma arising in a cyst. There is a massive carcinoma of the mandible, with extension into the parotid gland, the face, and the base of the brain. Nineteen years previously, a large odontogenic keratocyst with areas of epithelial dysplasia had been removed from the ascending ramus. The patient had suffered multiple recurrences, with eventual change into invasive carcinoma.
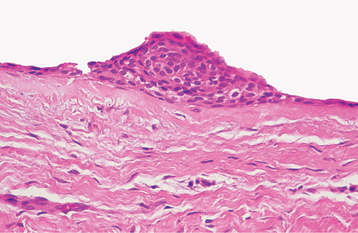
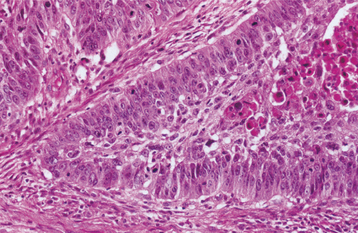
HISTOPATHOLOGIC FEATURES: Most carcinomas arising in cysts have histopathologically been well-differentiated squamous cell carcinomas. It is sometimes possible to identify a transition from a normal-appearing cyst lining to invasive squamous cell carcinoma (Figs. 15-53 and 15-54).
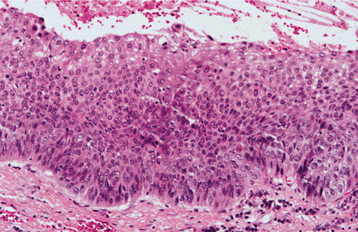
Fig. 15-53 Carcinoma arising in a cyst. High-power view of a dentigerous cyst from a 53-year-old man. The lining demonstrates full-thickness epithelial dysplasia.
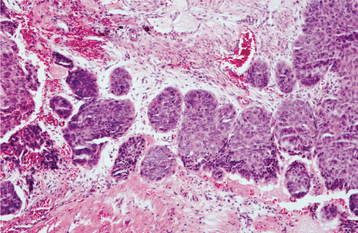
Fig. 15-54 Carcinoma arising in a cyst. Same case as Fig. 15-53 showing islands of invasive epithelial cells in the cyst wall.
TREATMENT AND PROGNOSIS: The treatment of patients with carcinomas arising in cysts has varied from local block excision to radical resection, with or without radiation or adjunctive chemotherapy. The prognosis is difficult to evaluate because most reports consist of isolated cases; often the follow-up is inadequate. Several larger studies indicate an approximate 50% 5-year survival rate after treatment. Metastases to regional lymph nodes have been demonstrated in a few cases.
Before a given lesion can be accepted as an example of primary intraosseous carcinoma, the possibility that the tumor represents metastatic spread from an intraoral or extraoral site must be ruled out by appropriate studies.
Odontogenic Tumors
Odontogenic tumors comprise a complex group of lesions of diverse histopathologic types and clinical behavior. Some of these lesions are true neoplasms and may rarely exhibit malignant behavior. Others may represent tumorlike malformations (hamartomas).
Odontogenic tumors, like normal odontogenesis, demonstrate varying inductive interactions between odontogenic epithelium and odontogenic ectomesenchyme. This ectomesenchyme was formerly referred to as mesenchyme because it was thought to be derived from the mesodermal layer of the embryo. It is now accepted that this tissue differentiates from the ectodermal layer in the cephalic portion of the embryo. Tumors of odontogenic epithelium are composed only of odontogenic epithelium without any participation of odontogenic ectomesenchyme.
Other odontogenic neoplasms, sometimes referred to as mixed odontogenic tumors, are composed of odontogenic epithelium and ectomesenchymal elements. Dental hard tissue may or may not be formed in these lesions.
A third group, tumors of odontogenic ectomesenchyme, is composed principally of ectomesenchymal elements. Although odontogenic epithelium may be included within these lesions, it does not appear to play any essential role in their pathogenesis.
Box 15-3 presents categories of odontogenic tumors modified from the 2005 WHO classification.
Tumors of Odontogenic Epithelium
Epithelial odontogenic tumors are composed of odontogenic epithelium without participation of odontogenic ectomesenchyme. Several distinctly different tumors are included in the group; ameloblastoma is the most important and common of them.
AMELOBLASTOMA
The ameloblastoma is the most common clinically significant odontogenic tumor. Its relative frequency equals the combined frequency of all other odontogenic tumors, excluding odontomas. Ameloblastomas are tumors of odontogenic epithelial origin. Theoretically, they may arise from rests of dental lamina, from a developing enamel organ, from the epithelial lining of an odontogenic cyst, or from the basal cells of the oral mucosa. Ameloblastomas are slow-growing, locally invasive tumors that run a benign course in most cases. They occur in three different clinicoradiographic situations, which deserve separate consideration because of differing therapeutic considerations and prognosis:
CONVENTIONAL SOLID OR MULTICYSTIC INTRAOSSEOUS AMELOBLASTOMA
CLINICAL AND RADIOGRAPHIC FEATURES: Conventional solid or multicystic intraosseous ameloblastoma is encountered in patients across a wide age range. It is rare in children younger than age 10 and relatively uncommon in the 10- to 19-year-old group. The tumor shows an approximately equal prevalence in the third to seventh decades of life. There is no significant sex predilection. Some studies indicate a greater frequency in blacks; others show no racial predilection. About 80% to 85% of conventional ameloblastomas occur in the mandible, most often in the molar-ascending ramus area. About 15% to 20% of ameloblastomas occur in the maxilla, usually in the posterior regions (Fig. 15-55). The tumor is often asymptomatic, and smaller lesions are detected only during a radiographic examination. A painless swelling or expansion of the jaw is the usual clinical presentation (Figs. 15-56 and 15-57). If untreated, then the lesion may grow slowly to massive or grotesque proportions (Fig. 15-58). Pain and paresthesia are uncommon, even with large tumors.
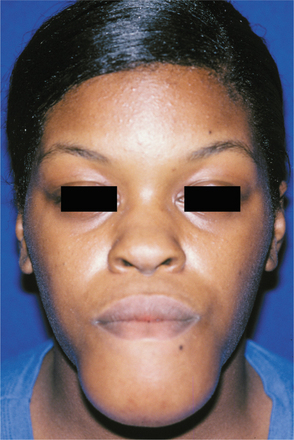
Fig. 15-56 Ameloblastoma. Large expansile mass of the anterior mandible. (Courtesy of Dr. Michael Tabor.)
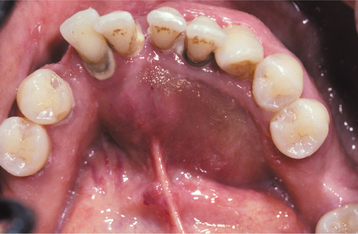
Fig. 15-57 Ameloblastoma. Prominent expansion of the lingual alveolus caused by a large ameloblastoma of the mandibular symphysis. The radiograph of the patient is shown in Fig. 15-61.
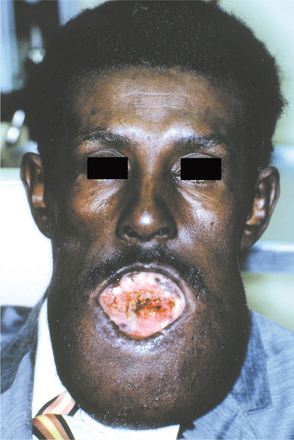
Fig. 15-58 Ameloblastoma. Massive tumor of the anterior mandible. (Courtesy of Dr. Ronald Baughman.)
The most typical radiographic feature is that of a multilocular radiolucent lesion. The lesion is often described as having a “soap bubble” appearance (when the radiolucent loculations are large) or as being “honeycombed” (when the loculations are small) (Figs. 15-59 to 15-61). Buccal and lingual cortical expansion is frequently present. Resorption of the roots of teeth adjacent to the tumor is common. In many cases an unerupted tooth, most often a mandibular third molar, is associated with the radiolucent defect. Solid ameloblastomas may radiographically appear as unilocular radiolucent defects, which may resemble almost any type of cystic lesion (Fig. 15-62). The margins of these radiolucent lesions, however, often show irregular scalloping. Although the radiographic features, particularly of the typical multilocular defect, may be highly suggestive of ameloblastoma, a variety of odontogenic and nonodontogenic lesions may show similar radiographic features (see Appendix).
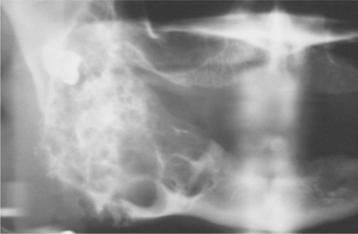
Fig. 15-59 Ameloblastoma. Large multilocular lesion involving the mandibular angle and ascending ramus. The large loculations show the “soap bubble” appearance. An unerupted third molar has been displaced high into the ramus.
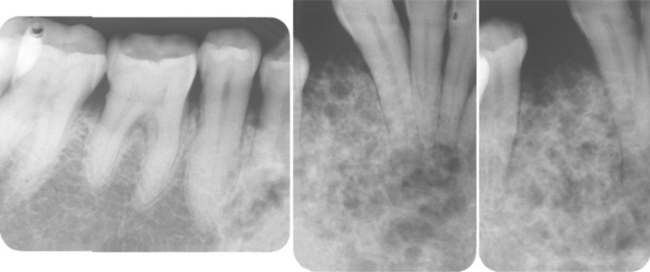
Fig. 15-60 Ameloblastoma. Periapical films showing the “honeycombed” appearance. (Courtesy of Dr. John Hann.)
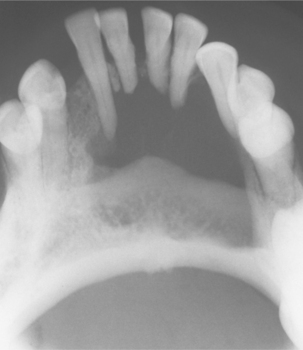
Fig. 15-61 Ameloblastoma. Destructive radiolucent lesion associated with root resorption of the anterior teeth. (Courtesy of Dr. Richard Brock.)
Fig. 15-62 Ameloblastoma. This small unilocular radiolucency lesion could easily be mistaken for a lateral periodontal cyst. (Courtesy of Dr. Tony Traynham.)
One form of ameloblastoma that does not have these characteristic features is the desmoplastic ameloblastoma, a variant that Eversole and colleagues documented initially in the literature in 1984. The desmoplastic ameloblastoma has a marked predilection to occur in the anterior regions of the jaws, particularly the maxilla. Radiographically, this type seldom suggests the diagnosis of ameloblastoma and usually resembles a fibro-osseous lesion because of its mixed radiolucent and radiopaque appearance (Fig. 15-63). This mixed radiographic appearance is due to osseous metaplasia within the dense fibrous septa that characterize the lesion, not because the tumor itself is producing a mineralized product.
HISTOPATHOLOGIC FEATURES: Conventional solid or multicystic intraosseous ameloblastomas show a remarkable tendency to undergo cystic change; grossly, most tumors have varying combinations of cystic and solid features. The cysts may be seen only at the microscopic level or may be present as multiple large cysts that include most of the tumor. Several microscopic subtypes of conventional ameloblastoma are recognized, but these microscopic patterns generally have little bearing on the behavior of the tumor. Large tumors often show a combination of microscopic patterns.
The follicular and plexiform patterns are the most common. Less common histopathologic patterns include the acanthomatous, granular cell, desmoplastic, and basal cell types.
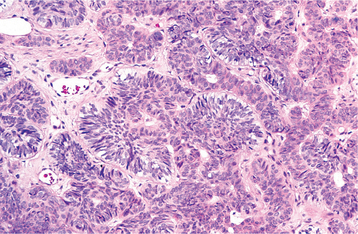
FOLLICULAR PATTERN: The follicular histopathologic pattern is the most common and recognizable. Islands of epithelium resemble enamel organ epithelium in a mature fibrous connective tissue stroma. The epithelial nests consist of a core of loosely arranged angular cells resembling the stellate reticulum of an enamel organ. A single layer of tall columnar ameloblast-like cells surrounds this central core. The nuclei of these cells are located at the opposite pole to the basement membrane (reversed polarity). In other areas, the peripheral cells may be more cuboidal and resemble basal cells. Cyst formation is common and may vary from microcysts, which form within the epithelial islands, to large macroscopic cysts, which may be several centimeters in diameter (Figs. 15-64 and 15-65).
PLEXIFORM PATTERN: The plexiform type of ameloblastoma consists of long, anastomosing cords or larger sheets of odontogenic epithelium. The cords or sheets of epithelium are bounded by columnar or cuboidal ameloblast-like cells surrounding more loosely arranged epithelial cells. The supporting stroma tends to be loosely arranged and vascular. Cyst formation is relatively uncommon in this variety. When it occurs, it is more often associated with stromal degeneration rather than cystic change within the epithelium (Fig. 15-66).
ACANTHOMATOUS PATTERN: When extensive squamous metaplasia, often associated with keratin formation, occurs in the central portions of the epithelial islands of a follicular ameloblastoma, the term acanthomatous ameloblastoma is sometimes applied. This change does not indicate a more aggressive course for the lesion; histopathologically, however, such a lesion may be confused with squamous cell carcinoma or squamous odontogenic tumor (Fig. 15-67).
GRANULAR CELL PATTERN: Ameloblastomas may sometimes show transformation of groups of lesional epithelial cells to granular cells. These cells have abundant cytoplasm filled with eosinophilic granules that resemble lysosomes ultrastructurally and histochemically. Although originally considered to represent an aging or degenerative change in long-standing lesions, this variant has been seen in young patients and in clinically aggressive tumors. When this granular cell change is extensive in an ameloblastoma, the designation of granular cell ameloblastoma is appropriate (Fig. 15-68).
DESMOPLASTIC PATTERN: This type of ameloblastoma contains small islands and cords of odontogenic epithelium in a densely collagenized stroma. Immunohistochemical studies have shown increased production of the cytokine known as transforming growth factor-b (TGF-b) in association with this lesion, suggesting that this may be responsible for the desmoplasia. Peripheral columnar ameloblast-like cells are inconspicuous about the epithelial islands (Fig. 15-69).
BASAL CELL PATTERN: The basal cell variant of ameloblastoma is the least common type. These lesions are composed of nests of uniform basaloid cells, and they histopathologically are very similar to basal cell carcinoma of the skin. No stellate reticulum is present in the central portions of the nests. The peripheral cells about the nests tend to be cuboidal rather than columnar (Fig. 15-70).
TREATMENT AND PROGNOSIS: Patients with conventional solid or multicystic intraosseous ameloblastomas have been treated by a variety of means. These range from simple enucleation and curettage to en bloc resection (Fig. 15-71). The optimal method of treatment has been the subject of controversy for many years. The conventional ameloblastoma tends to infiltrate between intact cancellous bone trabeculae at the periphery of the lesion before bone resorption becomes radiographically evident. Therefore, the actual margin of the tumor often extends beyond its apparent radiographic or clinical margin. Attempts to remove the tumor by curettage often leave small islands of tumor within the bone, which later manifest as recurrences. Recurrence rates of 50% to 90% have been reported in various studies after curettage. Recurrence often takes many years to become clinically manifest, and 5-year disease-free periods do not indicate a cure.
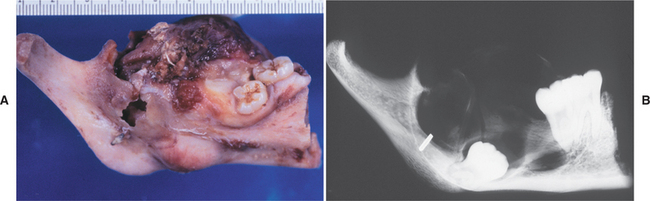
Fig. 15-71 Ameloblastoma. A, Gross photograph of a mandibular resection specimen. B, The radiograph of the specimen shows a large radiolucent defect associated with an inferiorly displaced third molar. (Courtesy of Dr. Mary Richardson.)
Marginal resection is the most widely used treatment, but recurrence rates of up to 15% have been reported after marginal or block resection. Some surgeons advocate a more conservative approach to treatment by planning surgery after careful evaluation of computed tomography (CT) scans of the tumor. Removal of the tumor, followed by peripheral ostectomy, often reduces the need for extensive reconstructive surgery. Some tumors may not be amenable to this approach because of their size or growth pattern.
Other surgeons advocate that the margin of the resection should be at least 1.0 to 1.5 cm past the radiographic limits of the tumor. Ameloblastomas of the posterior maxilla are particularly dangerous because of the difficulty of obtaining an adequate surgical margin around the tumor. Orbital invasion by maxillary ameloblastomas occasionally has been described. Although some studies suggest that the ameloblastoma may be radiosensitive, radiation therapy has seldom been used as a treatment modality because of the intraosseous location of the tumor and the potential for secondary radiation-induced malignancy developing in a relatively young patient population.
The conventional ameloblastoma is a persistent, infiltrative neoplasm that may kill the patient by progressive spread to involve vital structures. Most of these tumors, however, are not life-threatening lesions. Rarely, an ameloblastoma exhibits frank malignant behavior. These are discussed separately.
UNICYSTIC AMELOBLASTOMA
The unicystic ameloblastoma has for several decades been given separate consideration based on its clinical, radiographic, and pathologic features. Although its response to treatment in reports from the 1970s and 1980s suggested that this lesion might behave in a less aggressive fashion, recent reports have disputed this concept. Unicystic ameloblastomas account for 10% to 46% of all intraosseous ameloblastomas in various studies. Whether the unicystic ameloblastoma originates de novo as a neoplasm or whether it is the result of neoplastic transformation of nonneoplastic cyst epithelium has been long debated. Both mechanisms probably occur, but proof of which is involved in an individual patient is virtually impossible to obtain.
CLINICAL AND RADIOGRAPHIC FEATURES: Unicystic ameloblastomas are most often seen in younger patients, with about 50% of all such tumors diagnosed during the second decade of life. The average age in one large series was 23 years. More than 90% of unicystic ameloblastomas are found in the mandible, usually in the posterior regions. The lesion is often asymptomatic, although large lesions may cause a painless swelling of the jaws.
In many patients, this lesion typically appears as a circumscribed radiolucency that surrounds the crown of an unerupted mandibular third molar (Figs. 15-72 and 15-73), clinically resembling a dentigerous cyst. Other tumors simply appear as sharply defined radiolucent areas and are usually considered to be a primordial, radicular, or residual cyst, depending on the relationship of the lesion to teeth in the area. In some instances, the radiolucent area may have scalloped margins but is still a unicystic ameloblastoma. Whether a unicystic ameloblastoma can have a truly multilocular radiographic presentation is arguable.
Fig. 15-72 Unicystic ameloblastoma. A large radiolucency in a 7-year-old boy with displacement of the developing second molar to the inferior border of the mandible. This was believed to be a large dentigerous cyst. (Courtesy of Dr. Larry Chewning.)
Fig. 15-73 Unicystic ameloblastoma (intraluminal plexiform type). Coronal computed tomography (CT) image that shows a large cystic lesion with an intraluminal mass arising from the cyst wall (arrow).
The surgical findings may also suggest that the lesion in question is a cyst, and the diagnosis of ameloblastoma is made only after microscopic study of the specimen.
HISTOPATHOLOGIC FEATURES: Three histopathologic variants of unicystic ameloblastoma have been described. In the first type (luminal unicystic ameloblastoma), the tumor is confined to the luminal surface of the cyst. The lesion consists of a fibrous cyst wall with a lining that consists totally or partially of ameloblastic epithelium. This demonstrates a basal layer of columnar or cuboidal cells with hyperchromatic nuclei that show reverse polarity and basilar cytoplasmic vacuolization (Fig. 15-74). The overlying epithelial cells are loosely cohesive and resemble stellate reticulum. This finding does not seem to be related to inflammatory edema.
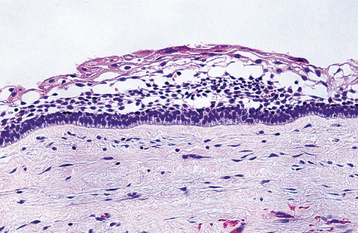
Fig. 15-74 Unicystic ameloblastoma (luminal type). The cyst is lined by ameloblastic epithelium showing a hyperchromatic, polarized basal layer. The overlying epithelial cells are loosely cohesive and resemble stellate reticulum.
In the second microscopic variant, one or more nodules of ameloblastoma project from the cystic lining into the lumen of the cyst. This type is called an intraluminal unicystic ameloblastoma. These nodules may be relatively small or largely fill the cystic lumen. In some cases, the nodule of tumor that projects into the lumen demonstrates an edematous, plexiform pattern that resembles the plexiform pattern seen in conventional ameloblastomas (Fig. 15-75). These lesions are sometimes referred to as plexiform unicystic ameloblastomas. The intraluminal cellular proliferation does not always meet the strict histopathologic criteria for ameloblastoma, and this may be secondary to inflammation that nearly always accompanies this pattern. Typical ameloblastoma, however, may be found in other, less inflamed parts of the specimen.
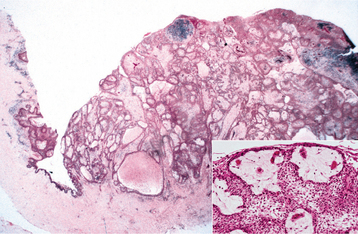
Fig. 15-75 Unicystic ameloblastoma (intraluminal plexiform type). Photomicrograph of the intraluminal mass arising from the cyst wall in the same patient shown in Fig. 15-72. The inset shows the intraluminal mass at higher magnification.
In the third variant, known as mural unicystic ameloblastoma, the fibrous wall of the cyst is infiltrated by typical follicular or plexiform ameloblastoma. The extent and depth of the ameloblastic infiltration may vary considerably. With any presumed unicystic ameloblastoma, multiple sections through many levels of the specimen are necessary to rule out the possibility of mural invasion of tumor cells (Fig. 15-76).
TREATMENT AND PROGNOSIS: The clinical and radiographic findings in most cases of unicystic ameloblastoma suggest that the lesion is an odontogenic cyst. These tumors are usually treated as cysts by enucleation. The diagnosis of ameloblastoma is made only after microscopic examination of the presumed cyst. If the ameloblastic elements are confined to the lumen of the cyst with or without intraluminal tumor extension, then the cyst enucleation has probably been adequate treatment. The patient, however, should be kept under long-term follow-up. If the specimen shows extension of the tumor into the fibrous cyst wall for any appreciable distance, then subsequent management of the patient is more controversial. Some surgeons believe that local resection of the area is indicated as a prophylactic measure; others prefer to keep the patient under close radiographic observation and delay further treatment until there is evidence of recurrence.
Recurrence rates of 10% to 20% were described after enucleation and curettage of unicystic ameloblastomas in many of the earlier series of cases. This range is considerably less than the 50% to 90% recurrence rates noted after curettage of conventional solid and multicystic intraosseous ameloblastomas. One recent series of unicystic ameloblastomas reported recurrence rates after conservative excision of 50% at one institution and 80% at another. A systematic review of the literature before 2005 determined that 30% of these lesions recurred after enucleation. These findings suggest that this lesion may not be as innocuous as previously thought. Alternatively, it is possible that some of those tumors that are designated as “unicystic” may, in fact, have a more characteristic invasive component that has not been detected histopathologically because it is essentially impossible to examine these lesions in every 360-degree plane of section.
PERIPHERAL (EXTRAOSSEOUS) AMELOBLASTOMA
The peripheral ameloblastoma is uncommon and accounts for about 1% to 10% of all ameloblastomas. This tumor probably arises from rests of dental lamina beneath the oral mucosa or from the basal epithelial cells of the surface epithelium. Histopathologically, these lesions have the same features as the intraosseous form of the tumor.
CLINICAL FEATURES: The peripheral ameloblastoma is usually a painless, nonulcerated sessile or pedunculated gingival or alveolar mucosal lesion. The clinical features are non-specific, and most lesions are clinically considered to represent a fibroma or pyogenic granuloma. Most examples are smaller than 1.5 cm, but larger lesions have been reported (Fig. 15-77). The tumor has been found in patients across a wide age range, but most are seen in middle-aged persons, with an average reported age of 52 years.
Peripheral ameloblastomas are most commonly found on the posterior gingival and alveolar mucosa, and they are somewhat more common in mandibular than in maxillary areas. In some cases, the superficial alveolar bone becomes slightly eroded, but significant bone involvement does not occur. A few examples of a microscopically identical lesion have been reported in the buccal mucosa at some distance from the alveolar or gingival soft tissues.
HISTOPATHOLOGIC FEATURES: Peripheral ameloblastomas have islands of ameloblastic epithelium that occupy the lamina propria underneath the surface epithelium (Fig. 15-78). The proliferating epithelium may show any of the features described for the intraosseous ameloblastoma; plexiform or follicular patterns are the most common. Connection of the tumor with the basal layer of the surface epithelium is seen in about 50% of cases. Whether this represents origin of the tumor from the basal layer of the epithelium or merging of the tumor with the surface epithelium has not been ascertained.
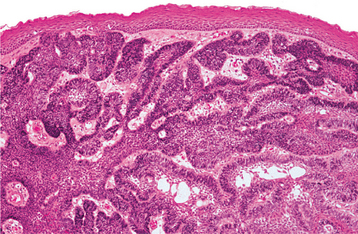
Fig. 15-78 Peripheral ameloblastoma. Interconnecting cords of ameloblastic epithelium filling the lamina propria.
Basal cell carcinomas of the oral mucosa have been reported, but most of these would be designated best as peripheral ameloblastomas. A peripheral odontogenic fibroma may be confused microscopically with a peripheral ameloblastoma, particularly if a prominent epithelial component is present in the former. The presence of dysplastic dentin or cementum-like elements in the peripheral odontogenic fibroma and the lack of peripheral columnar epithelial cells showing reverse polarity of their nuclei should serve to distinguish the two lesions.
TREATMENT AND PROGNOSIS: Unlike the intraosseous ameloblastoma, the peripheral ameloblastoma shows an innocuous clinical behavior. Patients respond well to local surgical excision. Although local recurrence has been noted in 15% to 20% of cases, further local excision almost always results in a cure. Several examples of malignant change in a peripheral ameloblastoma have been reported, but this is rare.
MALIGNANT AMELOBLASTOMA AND AMELOBLASTIC CARCINOMA
Rarely, an ameloblastoma exhibits frank malignant behavior with development of metastases. The frequency of malignant behavior in ameloblastomas is difficult to determine but probably occurs in far less than 1% of all ameloblastomas.
The terminology for these lesions is somewhat controversial. The term malignant ameloblastoma should be used for a tumor that shows the histopathologic features of ameloblastoma, both in the primary tumor and in the metastatic deposits. The term ameloblastic carcinoma should be reserved for an ameloblastoma that has cytologic features of malignancy in the primary tumor, in a recurrence, or in any metastatic deposit. These lesions may follow a markedly aggressive local course, but metastases do not necessarily occur.
CLINICAL AND RADIOGRAPHIC FEATURES: Malignant ameloblastomas have been observed in patients who range in age from 4 to 75 years (mean age, 30 years). For patients with documented metas-tases, the interval between the initial treatment of the ameloblastoma and first evidence of metastasis varies from 1 to 30 years. In nearly one third of cases, metastases do not become apparent until 10 years after treatment of the primary tumor. Ameloblastic carcinomas, in contrast, tend to develop later in life, with the mean age at diagnosis typically being in the sixth decade of life.
Metastases from ameloblastomas are most often found in the lungs. These have sometimes been regarded as aspiration or implant metastases. However, the peripheral location of some of these lung metas-tases suggests that they must have occurred by blood or lymphatic routes rather than aspiration.
Cervical lymph nodes are the second most common site for metastasis of an ameloblastoma. Spread to vertebrae, other bones, and viscera has also occasionally been confirmed.
The radiographic findings of malignant ameloblastomas may be essentially the same as those in typical nonmetastasizing ameloblastomas. Ameloblastic carcinomas are often more aggressive lesions, with ill-defined margins and cortical destruction (Fig. 15-79).
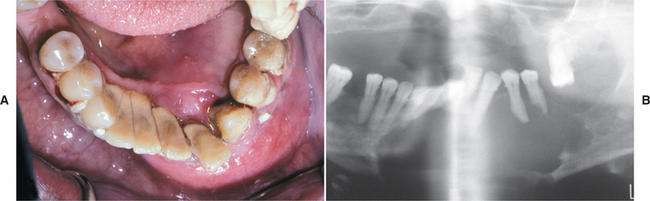
Fig. 15-79 Ameloblastic carcinoma. A, Rapidly growing tumor showing prominent labial expansion of the mandible in the incisor and premolar area. B, The panoramic radiograph shows irregular destruction of the mandible. (From Neville BW, Damm DD, White DK: Color atlas of clinical oral pathology, ed 2, Hamilton, 1999, BC Decker.)
HISTOPATHOLOGIC FEATURES: With malignant ameloblastomas, the primary jaw tumor and the metastatic deposits show no microscopic features that differ from those of ameloblastomas with a completely benign local course. With ameloblastic carcinomas, the metastatic deposits or primary tumor shows the microscopic pattern of ameloblastoma in addition to cytologic features of malignancy. These include an increased nuclear-to-cytoplasmic ratio, nuclear hyperchromatism, and the presence of mitoses (Fig. 15-80). Necrosis in tumor islands and areas of dystrophic calcification may also be present.
TREATMENT AND PROGNOSIS: The prognosis of patients with malignant ameloblastomas appears to be poor, but the paucity of documented cases with long-term follow-up does not permit accurate assumptions to be made. About 50% of the patients with documented metastases and long-term follow-up have died of their disease. Lesions designated as ameloblastic carcinoma have demonstrated a uniformly aggressive clinical course, with perforation of the cortical plates of the jaw and extension of the tumor into adjacent soft tissues.
CLEAR CELL ODONTOGENIC CARCINOMA (CLEAR CELL ODONTOGENIC TUMOR)
Clear cell odontogenic carcinoma is a rare jaw tumor that was first described in 1985. To date, approximately 50 examples have been documented. The tumor appears to be of odontogenic origin, but its histogenesis is uncertain. Histochemical and ultrastructural studies show that the clear cells, which are the prominent feature of this neoplasm, have similarities to glycogen-rich presecretory ameloblasts.
CLINICAL AND RADIOGRAPHIC FEATURES: The clear cell odontogenic carcinoma exhibits a variable clinical pattern. A wide age range (from 14 to 89 years of age) has been described, but most cases are diagnosed in patients older than age 50. More than 80% of the lesions develop in the mandible. Some patients complain of pain and bony swelling; others are relatively symptom free. Approximately 60% of patients will have evidence of soft tissue involvement by the tumor at the time of diagnosis because the lesion perforates bone.
Radiographically, the lesions appear as unilocular or multilocular radiolucencies. The margins of the radiolucency are often somewhat ill defined or irregular (Fig. 15-81).
HISTOPATHOLOGIC FEATURES: Three histopathologic patterns have been described for clear cell odontogenic carcinoma. The biphasic pattern consists of varying-sized nests of epithelial cells, with a clear or faintly eosinophilic cytoplasm admixed with more eosinophilic polygonal epithelial cells (Fig. 15-82). The second pattern is more monophasic, characterized by only clear cells that are arranged in nests and cords. Thin strands of hyalinized connective tissue often separate the clear cell nests. The third pattern has a resemblance to ameloblastoma in that the peripheral cells of the clear cell islands may infrequently demonstrate palisading (Fig. 15-83). Often the lesional cells do not exhibit a significant degree of nuclear or cytologic pleomorphism. Furthermore, mitoses are generally sparse and necrosis is not a prominent feature. The clear cells contain small amounts of glycogen, but mucin stains are negative. In some cases, islands more typical of ameloblastoma are interspersed among the other tumor elements.
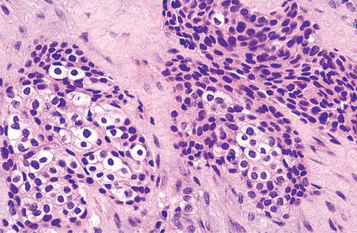
Fig. 15-82 Clear cell odontogenic carcinoma. Hyperchromatic epithelial nests including clusters of cells with abundant clear cytoplasm.
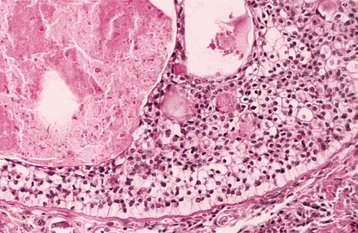
Fig. 15-83 Clear cell odontogenic carcinoma. Tumor island demonstrating cells with a clear cytoplasm. Note the peripheral columnar differentiation.
Clear cell odontogenic carcinoma may be difficult to distinguish from intraosseous mucoepidermoid carcinoma with a prominent clear cell component, although the negative mucin stains are consistent with the former. A clear cell variant of the calcifying epithelial odontogenic tumor may also present problems in the differential diagnosis, but amyloid stains should be negative in the case of clear cell odontogenic tumor. A metastatic clear cell neoplasm, such as a renal cell carcinoma, clear cell breast carcinoma, or clear cell melanoma, may also need to be ruled out before the diagnosis of clear cell odontogenic carcinoma can be established.
TREATMENT AND PROGNOSIS: Clear cell odontogenic carcinomas largely demonstrate an aggressive clinical course, with invasion of contiguous structures and a tendency to recur. Most patients require fairly radical surgery. Metastatic involvement of regional lymph nodes has been documented in 25% of these patients, and pulmonary metastases have been described as well.
ADENOMATOID ODONTOGENIC TUMOR
The adenomatoid odontogenic tumor represents 3% to 7% of all odontogenic tumors, and more than 750 examples have been reported in the literature. Although this lesion was formerly considered to be a variant of the ameloblastoma and was designated as “adenoameloblastoma,” its clinical features and biologic behavior indicate that it is a separate entity. Although there is evidence that the tumor cells are derived from enamel organ epithelium, investigators have also suggested that the lesion arises from remnants of dental lamina.
CLINICAL AND RADIOGRAPHIC FEATURES: Adenomatoid odontogenic tumors are largely limited to younger patients, and two thirds of all cases are diagnosed when patients are 10 to 19 years of age. This tumor is definitely uncommon in a patient older than age 30. It has a striking tendency to occur in the anterior portions of the jaws and is found twice as often in the maxilla as in the mandible (Fig. 15-84). Females are affected about twice as often as males.
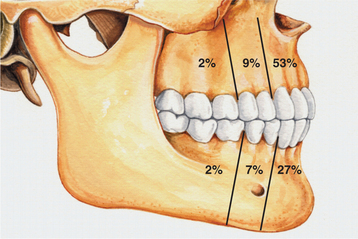
Fig. 15-84 Adenomatoid odontogenic tumor. Relative distribution of adenomatoid odontogenic tumor in the jaws.
Most adenomatoid odontogenic tumors are relatively small. They seldom exceed 3 cm in greatest diameter, although a few large lesions have been reported. Peripheral (extraosseous) forms of the tumor are also encountered but are rare. These usually appear as small, sessile masses on the facial gingiva of the maxilla. Clinically, these lesions cannot be differentiated from the common gingival fibrous lesions.
Adenomatoid odontogenic tumors are frequently asymptomatic and are discovered during the course of a routine radiographic examination or when films are made to determine why a tooth has not erupted. Larger lesions cause a painless expansion of the bone.
In about 75% of cases, the tumor appears as a circumscribed, unilocular radiolucency that involves the crown of an unerupted tooth, most often a canine. This follicular type of adenomatoid odontogenic tumor may be impossible to differentiate radiographically from the more common dentigerous cyst. The radiolucency associated with the follicular type of adenomatoid odontogenic tumor sometimes extends apically along the root past the cementoenamel junction. This feature may help to distinguish an adenomatoid odontogenic tumor from a dentigerous cyst (Fig. 15-85).
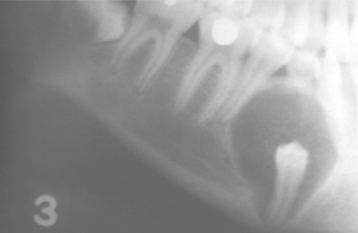
Fig. 15-85 Adenomatoid odontogenic tumor. Radiolucent lesion involving an unerupted mandibular first premolar. In contrast to the usual dentigerous cyst, the radiolucency extends almost to the apex of the tooth. (Courtesy of Dr. Tony Traynham.)
Less often the adenomatoid odontogenic tumor is a well-delineated unilocular radiolucency that is not related to an unerupted tooth, but rather is located between the roots of erupted teeth (extrafollicular type) (Fig. 15-86).
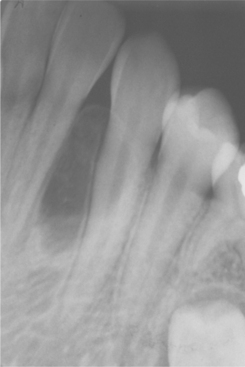
Fig. 15-86 Adenomatoid odontogenic tumor. A small radiolucency is present between the roots of the lateral incisor and canine. (Courtesy of Dr. Ramesh Narang.)
The lesion may appear completely radiolucent; often, however, it contains fine (snowflake) calcifications (Fig. 15-87). This feature may be helpful in differentiating the adenomatoid odontogenic tumor from a dentigerous cyst.
HISTOPATHOLOGIC FEATURES: The adenomatoid odontogenic tumor is a well-defined lesion that is usually surrounded by a thick, fibrous capsule. When the lesion is bisected, the central portion of the tumor may be essentially solid or may show varying degrees of cystic change (Fig. 15-88).
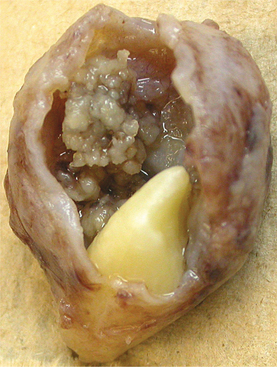
Fig. 15-88 Adenomatoid odontogenic tumor. A well-circumscribed cystlike mass can be seen enveloping the crown of a maxillary cuspid. Note the intraluminal vegetations, which represent nodular tumor growth.
Microscopically, the tumor is composed of spindle-shaped epithelial cells that form sheets, strands, or whorled masses of cells in a scant fibrous stroma. The epithelial cells may form rosettelike structures about a central space, which may be empty or contain small amounts of eosinophilic material. This material may stain for amyloid.
The tubular or ductlike structures, which are the characteristic feature of the adenomatoid odontogenic tumor, may be prominent, scanty, or even absent in a given lesion. These consist of a central space surrounded by a layer of columnar or cuboidal epithelial cells. The nuclei of these cells tend to be polarized away from the central space. The mechanism of formation of these tubular structures is not entirely clear but is likely the result of the secretory activity of the tumor cells, which appear to be preameloblasts. In any event, these structures are not true ducts, and no glandular elements are present in the tumor (Fig. 15-89).
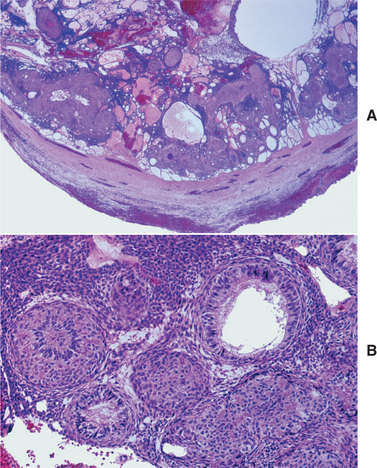
Fig. 15-89 Adenomatoid odontogenic tumor. A, Low-power view demonstrating a thick capsule surrounding the tumor. B, Higher magnification showing the ductlike epithelial structures. The nuclei of the columnar calls are polarized away from the central spaces.
Small foci of calcification may also be scattered throughout the tumor. These have been interpreted as abortive enamel formation. Some adenomatoid odontogenic tumors contain larger areas of matrix material or calcification. This material has been interpreted as dentinoid or cementum.
Some lesions also have another pattern, particularly at the periphery of the tumor adjacent to the capsule. This consists of narrow, often anastomosing cords of epithelium in an eosinophilic, loosely arranged matrix.
The histopathologic features of this lesion are distinctive and should not be confused with any other odontogenic tumor. Interestingly, some adenomatoid odontogenic tumors have been described with focal areas that resemble calcifying epithelial odontogenic tumor, odontoma, or calcifying odontogenic cyst. These lesions appear to behave as a routine adenomatoid odontogenic tumor, however. The chief problem relates to mistaking this tumor for an ameloblastoma by a pathologist who is not familiar with this lesion. This error can lead to unnecessary radical surgery.
CALCIFYING EPITHELIAL ODONTOGENIC TUMOR (PINDBORG TUMOR)
The calcifying epithelial odontogenic tumor, also widely known as the Pindborg tumor, is an uncommon lesion that accounts for less than 1% of all odontogenic tumors. Approximately 200 cases have been reported to date. Although the tumor is clearly of odontogenic origin, its histogenesis is uncertain. The tumor cells bear a close morphologic resemblance to the cells of the stratum intermedium of the enamel organ; however, some investigators have recently suggested that the tumor arises from dental lamina remnants based on its anatomic distribution in the jaws.
CLINICAL AND RADIOGRAPHIC FEATURES: Although the calcifying epithelial odontogenic tumor has been found in patients across a wide age range and in many parts of the jaw, it is most often encountered in patients between 30 and 50 years of age. There is no sex predilection. About two thirds of all reported cases have been found in the mandible, most often in the posterior areas (Fig. 15-90). A painless, slow-growing swelling is the most common presenting sign.
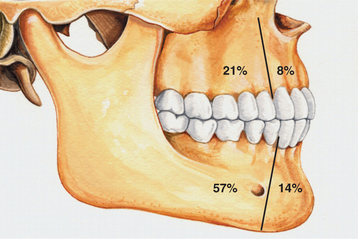
Fig. 15-90 Calcifying epithelial odontogenic tumor. Relative distribution of calcifying epithelial odontogenic tumor in the jaws.
Radiographically, the tumor exhibits either a unilocular or a multilocular radiolucent defect (Fig. 15-91), with the unilocular pattern encountered more commonly in the maxilla. The margins of the lytic defect are often scalloped and usually relatively well defined. However, approximately 20% of cases have an ill-defined periphery, and an additional 20% exhibit a corticated border. The lesion may be entirely radiolucent, but the defect usually contains calcified structures of varying size and density. The tumor is frequently associated with an impacted tooth, most often a mandibular molar. Calcifications are usually scattered within the tumor. Although some authors have suggested that these are often most prominent around the crown of the impacted tooth (Fig. 15-92), a recent review identified this feature in only 12% of published cases with adequate radiographic documentation. Similarly, the description of a “driven-snow” pattern of the calcifications appears to be much less common than previously believed.
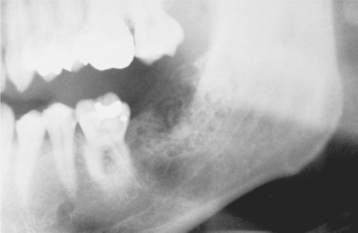
Fig. 15-91 Calcifying epithelial odontogenic tumor. Honeycombed multilocular radiolucency containing fine calcifications.
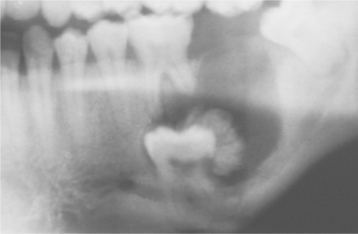
Fig. 15-92 Calcifying epithelial odontogenic tumor. Prominent calcification around the crown of an impacted second molar that is involved in the tumor. (Courtesy of Dr. Harold Peacock.)
A few cases of peripheral (extraosseous) calcifying epithelial odontogenic tumor have been reported. These appear as nonspecific, sessile gingival masses, most often on the anterior gingiva. Some of these have been associated with cupped-out erosion of the underlying bone.
HISTOPATHOLOGIC FEATURES: The calcifying epithelial odontogenic tumor has discrete islands, strands, or sheets of polyhedral epithelial cells in a fibrous stroma (Fig. 15-93). The cellular outlines of the epithelial cells are distinct, and intercellular bridges may be noted. The nuclei show considerable variation, and giant nuclei may be seen. Some tumors show considerable nuclear pleomorphism, but this feature is not considered to indicate malignancy. Large areas of amorphous, eosinophilic, hyalinized (amyloid-like) extracellular material are also often present. The tumor islands frequently enclose masses of this hyaline material; this results in a cribriform appearance. Calcifications, which are a distinctive feature of the tumor, develop within the amyloid-like material and form concentric rings (Liesegang ring calcifications) (Fig. 15-94). These tend to fuse and form large, complex masses.
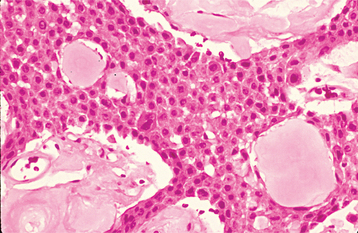
Fig. 15-93 Calcifying epithelial odontogenic tumor. Sheets of polyhedral tumor cells with prominent eosinophilic cytoplasm and intercellular bridging. Pools of amorphous eosinophilic amyloid are present.
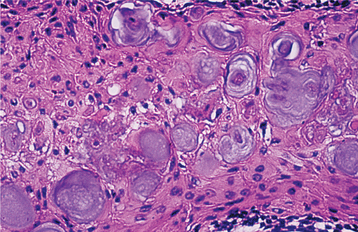
Fig. 15-94 Calcifying epithelial odontogenic tumor. Multiple concentric Liesegang ring calcifications.
Several microscopic variations may be encountered. Some tumors consist of large sheets of epithelial cells with minimal production of amyloid-like material and calcifications. Others show large diffuse masses of amyloid-like material that contain only small nests or islands of epithelium. A clear cell variant has been described, in which clear cells constitute a significant portion of the epithelial component, and this tumor also has been reported to have a cystic growth pattern.
The amyloid-like material in the Pindborg tumor has been extensively investigated by histochemical, immunohistochemical, and biochemical methods, as well as by electron microscopy. The material generally stains as amyloid (i.e., positive staining results with Congo red or thioflavine T). After Congo red staining, the amyloid will exhibit apple-green birefringence when viewed with polarized light (Fig. 15-95). A recent report has identified this material as a unique protein that is produced by this tumor, and both the protein structure and the DNA sequence of the responsible gene have been described.
TREATMENT AND PROGNOSIS: Although it was originally believed that the calcifying epithelial odontogenic tumor had about the same biologic behavior as the ameloblastoma, accumulating experience indicates that it tends to be less aggressive. Conservative local resection to include a narrow rim of surrounding bone appears to be the treatment of choice, although lesions in the posterior maxilla should probably be treated more aggressively. A recurrence rate of about 15% has been reported; tumors treated by curettage have the highest frequency of recurrence. The overall prognosis appears good, although rare examples of malignant or borderline malignant calcifying epithelial odontogenic tumor have been reported, with documented metastasis to regional lymph nodes and lung.
SQUAMOUS ODONTOGENIC TUMOR
Squamous odontogenic tumor is a rare benign odontogenic neoplasm that was first described in 1975 and is now recognized as a distinct entity. Fewer than 50 examples have been reported to date. Most of these have been located within bone, although a few peripheral examples have been described. Before 1975, this lesion was probably believed to represent an atypical acanthomatous ameloblastoma or even a squamous cell carcinoma. The squamous odontogenic tumor may arise from neoplastic transformation of dental lamina rests or perhaps the epithelial rests of Malassez. The tumor appears to originate within the periodontal ligament that is associated with the lateral root surface of an erupted tooth.
CLINICAL AND RADIOGRAPHIC FEATURES: Squamous odontogenic tumors have been found in patients whose ages ranged from 8 to 74 years (average age, 38). They are randomly distributed throughout the alveolar processes of the maxilla and mandible, with no site of predilection. A few patients have had multiple squamous odontogenic tumors that involved several quadrants of the mouth; one family with three affected siblings who each had multiple lesions has been reported. There is no apparent sex predilection. A painless or mildly painful gingival swelling, often associated with mobility of the associated teeth, is the most common complaint. About 25% of reported patients have had no symptoms, and their lesions were detected during a radiographic examination.
The radiographic findings are not specific or diagnostic and consist of a triangular radiolucent defect lateral to the root or roots of the teeth (Fig. 15-96). In some instances, this suggests vertical periodontal bone loss. The radiolucent area may be somewhat ill defined or may show a well-defined, sclerotic margin. Most examples are relatively small lesions that seldom exceed 1.5 cm in greatest diameter.
HISTOPATHOLOGIC FEATURES: The microscopic findings of squamous odontogenic tumor are distinctive and consist of varying-shaped islands of bland-appearing squamous epithelium in a mature fibrous connective tissue stroma. The peripheral cells of the epithelial islands do not show the characteristic polarization seen in ameloblastomas (Fig. 15-97). Microcystic vacuolization and individual cell keratinization within the epithelial islands are common features. Small microcysts are sometimes observed within the epithelial islands. Laminated calcified bodies and globular eosinophilic structures, which do not stain for amyloid, are present within the epithelium in some cases. The former probably represents dystrophic calcifications; the nature of the latter is unknown.
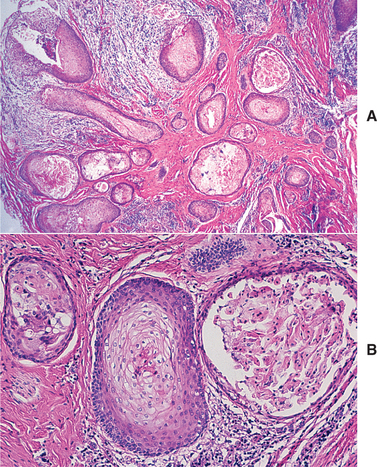
Fig. 15-97 Squamous odontogenic tumor. A, Low-power photomicrograph showing islands of bland-appearing squamous epithelium in a fibrous stroma. B, Higher-power photomicrograph showing bland appearance of the epithelium with microcyst formation.
Islands of epithelium that closely resemble those of the squamous odontogenic tumor have been observed within the fibrous walls of dentigerous and radicular cysts. These have been designated as squamous odontogenic tumorlike proliferations in odontogenic cysts. These islands do not appear to have any significance relative to the behavior of the cyst, and evaluation of the clinical, radiographic, and histopathologic features should permit differentiation from a squamous odontogenic tumor.
In published reports, some squamous odontogenic tumors have been misdiagnosed initially as ameloblastomas, resulting in unnecessary radical surgery.
TREATMENT AND PROGNOSIS: Conservative local excision or curettage appears to be effective for patients with squamous odontogenic tumors, and most reported cases have not recurred after local excision. A few instances of recurrence have been reported, but these have responded well to further local excision. Maxillary squamous odontogenic tumors may be somewhat more aggressive than mandibular lesions, with a greater tendency to invade adjacent structures. This may be because of the porous, spongy nature of the maxillary bone. The multicentric lesions have typically exhibited a less aggressive, almost hamartomatous behavior when compared with solitary lesions. A well-documented example of apparent malignant transformation of squamous odontogenic tumor has been reported.