Chapter 24 The prescription
Introduction
The access to medicines by the general public varies dependent on the laws of each country. In the UK, the Medicines Act 1968 classifies medicines into three categories, namely:
GSL medicines are available for sale to the public through many retail outlets. These medicines are for the treatment of minor ailments or conditions and have a history of being safe and effective for patients when they self-medicate with these products. P medicines are available only from pharmacies and are sold under the supervision of a pharmacist. Some P medicines are those that have recently been ‘deregulated’ from the POM classification. Other P medicines are restricted in their supply to the public due to the nature of the condition they are intended to treat or because they have a greater tendency to be misused or abused compared to GSL medicines. POM medicines are normally supplied to a patient after they have received a prescription from an authorized prescriber. In the past the authorized prescribers were doctors, dentists and veterinary surgeons but recently more professions have been authorized to write prescriptions, including nurses and pharmacists (see Chs 2, 14).
A prescription is a paper or electronic document detailing the medicine or medicines to be dispensed for an individually named patient and issued by an authorized prescriber. The medicine can be any of the above three legal categories. A prescription item is one named medicine on a prescription, e.g. aspirin tablets. A prescription may contain more than one prescription item, e.g. aqueous cream, pholcodine linctus and aspirin tablets (three prescription items), in which case the prescription may be referred to as a multiple item prescription. In addition to medicines, a prescription may contain other items or appliances required by the patient for their treatment, e.g. wound dressings, elastic hosiery, blood glucose monitoring equipment, needles and syringes, nutritionally complete feeds and gluten-free foods.
In the UK, a state-funded National Health Service (NHS) and a private system of health care run alongside each other. Prescriptions can be provided to patients by prescribers in both systems. In the NHS system, not all medicines or appliances available can be prescribed. Limiting the access to medicines and appliances has been used as a method to reduce the cost to the government of providing the NHS (see Ch. 6).
POM medicines can, under certain criteria, be supplied without a prescription to the public by way of an emergency supply or through a patient group direction (PGD). PGDs are used for the supply of POM and P medicines by designated healthcare professionals to individual patients, subject to any exclusion stated in the PGD. PGDs are written directions signed by a doctor or dentist and by a pharmacist relating to the supply and administration, or administration only, of certain POM and P medicines. The particulars to be included in a PGD are detailed in Box 24.1. Records of supply to individual patients are required as part of the PGD process (see Ch. 16). PGDs have been used in pharmacy for the supply of a number of different types of medication such as emergency hormonal contraception, nicotine replacement therapy and head lice treatments.
Although at present the majority of prescriptions are produced on paper, there will in future be a continued move towards electronic prescriptions in both the community and in hospitals. Electronic prescriptions have the same legal force as prescriptions signed in writing. The benefits of electronic prescriptions include patient convenience, easier ordering of repeat prescriptions and more complete information about prescribing. The production of electronic prescriptions might in the future mean an end to incomplete and illegible prescriptions. Although electronic prescriptions will become the norm in the NHS, private prescriptions will, for the foreseeable future, remain as paper documents. This chapter will concentrate on paper prescriptions in use at the time of writing. The information contained on both paper and electronic prescriptions and the method of dispensing and recording is essentially the same.
Information required on a prescription
When producing a prescription, the prescriber is giving information and instructions to the person who will supply the medicine to the patient. A prescription is in effect three types of document in one, in that it is a clinical document, a legal document and an invoice. Law may require some of the information on the prescription and some of the information is required to ensure the patient receives the correct medicine. The dispenser will also have to take payment for the medicine from the patient or send the prescription to the appropriate body for them to pay, hence it is also an invoice.
The information and instructions that are required as a minimum are detailed below.
Name and address of the prescriber
This identifies who the prescriber is and informs the pharmacist where to contact the prescriber should there be an issue related to the prescription. A telephone number on the prescription is helpful, but if the pharmacist suspects the prescription is a forgery this number should not be used as there have been cases where the telephone number has been changed so the pharmacist has contacted someone who has then pretended to be the prescriber.
Date of the prescription
This identifies when the prescription was written. The law usually defines the length of time from being written that a prescription remains valid. In the UK, all NHS prescriptions should be dispensed within 6 months of the date on the prescription except for certain types of controlled drugs where the requirement is they are dispensed within 28 days.
Name of the medicine (with strength and dosage form, if relevant)
Dose and dosage regimen
Directions should be as specific as possible. Ideally, the amount to be used and the number of times a day that it should be taken should be stated. Vague directions – in particular ‘Take as directed’ – are of little value to the patient and should be avoided. It such situations the pharmacist will have to ensure that the patient is clear about how to use or take their medication.
Directions for use
The directions for use will include the dose and dosage regimen but the prescriber may include additional information about the product. This can include how to use (e.g. spread thinly, dissolve in water), where to use (e.g. in the eye, in the ear, on the scalp), why they are using it (e.g. for pain, for sleeping). The prescriber may also indicate a maximum amount that should be taken, particularly if the medicine is dosed on a ‘when required’ basis.
Name and address of the patient
This identifies the patient who is to receive the medicine. The age of the patient would also be useful to enable the pharmacist to check the dose of the medicine, particularly if the person is very young or very old.
Prescriber’s signature
This is usually a legal requirement of prescriptions.
The above details are not totally inclusive, depending on the prescription type and the legal requirements of the country.
It is important that the person dispensing the product is aware of the information they require and is able to take the appropriate action to clarify and complete any missing or ambiguous information.
A number of specific terms, for example ‘dose’, ‘dosage regimen’, etc. are used in the above list and are often confused. These terms are explained below and Example 24.1 demonstrates the terms using an extract from a prescription.
Example 24.1
Examine the following details which have been abstracted from a prescription:
Using the above prescription as an example, the dosage form, strength, dose, dosage regimen, total daily dose, total amount, proprietary name, generic name and length of treatment are:
| Term | Example |
| Dosage form | Tablets |
| Strength | 200 mg |
| Dose | 400 mg (2 tablets of 200 mg) |
| Dosage regimen | 400 mg three times a day |
| Total daily dose | 1200 mg |
| Total amount | 84 tablets |
| Proprietary name | Brufen® |
| Generic name | Ibuprofen |
| Length of treatment | 14 days |
Dosage form
The term dosage form refers to the type of formulated product. Examples of different forms would be tablets, capsules, creams, ointments, ear/eye/nasal drops, aerosols, suppositories, vaginal pessaries and creams, mixtures, linctuses and patches and these are discussed in the following chapters. Each dosage form may also be presented in a number of specialist forms. For example, tablets are available as modified-release, enteric-coated, dispersible, buccal, soluble, and chewable. The dosage form should be stated on the prescription if there is more than one form available. For example, glyceryl trinitrate is available as tablets, modified-release tablets, a pump spray, an aerosol spray, an injection, ointment and patches. The prescriber will need to state the precise form to ensure that the required product is supplied.
Strength
Strength refers to the amount of drug in the dosage form or a unit of the dosage form (e.g. a capsule, a tablet, a patch). The strength of a dosage form can be expressed in a number of ways. For example, the strength of oral liquids is usually expressed as the amount of drug per usual dose volume (e.g. ampicillin suspension is available as 125 mg/5 mL and 500 mg/5 mL) or the strength may be expressed in units (e.g. nystatin suspension is available as 100 000 units/mL). External liquids, topical preparations and injections are usually expressed as an amount per millilitre or gram (e.g. naloxone hydrochloride injection 400 micrograms/mL, nystatin cream 100 000 units/g, terbutaline sulphate nebulizer solution 2.5 mg/mL) or as a percentage (e.g. chloramphenicol eye drops 0.5%, ketoconazole cream 2%, benzyl benzoate application 25%, lidocaine injection 0.5%). Single-dose unit forms, e.g. tablets or suppositories, are usually expressed as the amount of drug in one dose unit, e.g. diclofenac sodium suppositories are available in 12.5 mg, 25 mg, 50 mg and 100 mg strengths.
Dose
This is the amount of drug taken at any one time. This can be expressed as the weight of drug (e.g. 500 mg) or volume of drug solution (e.g. 5 mL, 2 drops) or as the number of dose unit forms (2 capsules, half a tablet, 1 sachet, 1 patch) or some other quantity (2 puffs, 1–2 inches of ointment).
Dosage regimen
Dosage regimen refers to the frequency of administration or the number of times the dose is to be taken in a period of time. Examples include: 5 mL twice a day; use the cream night and morning; 1 injection every 4 weeks; 3 tablets three times a week.
Total daily dose
The total daily dose can be calculated from the dose and the number of times per day that the dose is taken. Some maximum doses of drugs are expressed in terms of per day rather than each separate dose (e.g. the total daily dose for paracetamol by mouth in the British National Formulary (BNF) is 4 grams).
Total amount to be supplied
This refers to the total amount of the medicine to be supplied to the patient. This can be expressed as a number of units (e.g. 21 tablets, 12 suppositories), as a volume (e.g. 100 mL of mixture, 5 mL of eye drops) or as a weight (e.g. 30 g of cream) or as a single pack size or multiple thereof (e.g. 1 tube of ointment, 2 inhalers). It may mean that the person dispensing the prescription may have to calculate the total amount if the prescriber states a dosage regimen for the preparation and a number of days of treatment (e.g. one to be taken three times a day for 28 days).
Generic name
The generic name is also known as the approved name. All drugs are given an approved name which is usually related to their chemical structure and the medical classification of the drug. This name is adopted by the World Health Organization and is known as the recommended international non-proprietary name (rINN). In the UK these names are usually co-opted as a British approved name (BAN). European law (92/27/EEC) requires that only the rINN be used and as a result some BAN names have been modified to the rINN (e.g. frusemide has changed to furosemide). Prescribers in the UK are encouraged to use generic names for cost-saving reasons as generic products are usually less expensive than the equivalent proprietary product (see below) and hence the generic name is the most commonly used name on prescriptions. If the prescription is written by the generic name, in the UK, any equivalent product can be supplied even if it also has a proprietary name. The person who dispensed the prescription may lose money if they supply a more expensive proprietary product when the prescription is written generically.
Proprietary name
This can also be referred to as the brand name, manufacturer’s name or trade name. The company that first produces and markets a drug will give it a proprietary name. The company will apply for a trademark in respect of the proprietary name. The granting of a trademark means that no other company can use that name. Usually proprietary names are short, distinct and easy to remember and write. They may reflect the name of the company or the condition the medication is being used to treat or the type of medication. After expiry of the patent (in the UK, 20 years from first date of discovery or, under a certificate of supplementary protection, 15 years from the date of first marketing) the drug may be produced by other companies using the approved (or generic) name. Drugs may be prescribed by their proprietary name. If the prescriber, in the UK, states the proprietary name or the manufacturer of the product, this product or the product manufactured by the stated manufacturer must be supplied.
Length of treatment
The length of treatment may be stated on the prescription (e.g. use for 1 week) or it may be possible to calculate the length of time from the amount prescribed and the dosage regimen (e.g. 21 capsules to be taken ‘one three times a day’ will be sufficient for 7 days’ treatment).
Types of prescription forms
As stated previously, in the UK there are two providers of health care: the private sector and the NHS. Concomitantly there are two categories of prescriptions, namely private prescriptions and NHS prescriptions. Additionally, prescriptions may be provided in both the primary care sector (e.g. community) and the secondary care sector (e.g. hospitals). The format of prescriptions in these two sectors will be different.
Private prescription forms
Private prescriptions do not have a standard format and can, in fact, be a piece of paper containing all the required information. Normally some of the information, such as the name, the address and qualifications of the prescriber, are pre-printed on the paper. The symbol 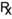 is often used on private prescription forms to indicate that the form is a prescription. Changes in the law relating to controlled drugs in the UK have led to the production of a standard form for private prescriptions for controlled drugs for human use. All veterinary prescriptions are private prescription.
is often used on private prescription forms to indicate that the form is a prescription. Changes in the law relating to controlled drugs in the UK have led to the production of a standard form for private prescriptions for controlled drugs for human use. All veterinary prescriptions are private prescription.
NHS prescription forms
NHS prescriptions should only be issued to NHS patients. There are a number of different types of NHS prescription forms available in the UK that may be dispensed at any community pharmacy. Each type of prescription form is given a different number and colour and might have a different format depending on where in the UK they originate from and the type of prescriber who generated the prescription.
Hospital prescription forms
There is no standard form for hospital prescribing for patients staying in hospital, and the actual format and design will depend on the individual hospital trust. However, the forms usually contain much of the same information. They usually have space for the prescription details, as well as space for confirmation of the administration of the medicine by nursing or other staff. For convenience, most hospital forms are divided into three separate areas, namely medicines to be administered on a regular basis, medicines to be administered once only and medicines to be administered on an ‘as required’ basis. Some forms may also have an area for listed medicines that can be administered by nursing staff at their discretion. Such medicines may be simple analgesics, sore throat lozenges, laxatives, etc. In addition all hospital prescription forms will require space for identification of the patient and will also have room for important details about the patient such as if they are allergic to any medicines.
Routine procedure for dispensing prescriptions
The dispensing of prescriptions requires a logical and very thorough approach in order to ensure the patient gets the right product in the right form, at the right dose with the right advice. It is imperative that the pharmacist conducts a thorough check of the prescription to ensure that it is complete and clinically acceptable. The product should then be assembled and labelled in a manner that ensures the product and all the information is accurate and that it is professional in its appearance. All stages involved in the dispensing process should be covered by a standard operating procedure.
The stages involved in dispensing a prescription are:
Receiving the prescription
It is important at this stage that the person receiving the prescription has checked the patient details so that:
The full name of the patient should be ascertained to ensure that the medicines reach the person for whom they are intended. The information should also include the sex of the patient, if it is not given elsewhere on the prescription. The sex of the patient may be necessary in the assessment of the appropriateness of the medicine for the patient (see Example 24.2).
Example 24.2
Examine the following details found on a prescription:
Hytrin is the proprietary name for terazosin, an alpha-adrenoceptor blocking drug which is used for the treatment of urinary retention in benign prostatic hyperplasia and for the treatment of mild to moderate hypertension. It is available as two starter packs with different numbers of different strength tablets in them. In the above prescription the patient is female and the drug is most likely to be prescribed for the treatment of hypertension. Thus an indication of the sex of the patient is useful to the pharmacist in this case, since it would indicate that the patient should receive the starter pack for hypertension. However, it would still be important to check with the prescriber that this is the case.
The full address of the patient is also checked and completed if required. It may be possible that two patients have the same name and so the address will identify the patient. If two patients have the same address and name, then it is helpful if the age or date of birth of the patient is stated. The age of the patient if they are under 12 years old is a legal requirement on prescriptions in the UK. Since the advent of computer-generated prescriptions and with electronic prescriptions, the date of birth of the patient is being supplied on most prescriptions. On private prescriptions, the age of very young children may be expressed as a fraction. If the age is expressed in days, weeks or months, then the denominator is 7, 52 and 12, respectively. For example, an age of 3 days may be abbreviated to 3/7, an age of 3 weeks may be abbreviated to 3/52 and an age of 3 months to 3/12.
As well as checking patient details, the person taking the prescription in should also be able to check if the product is available in the pharmacy or whether it needs to be ordered and, if so, how long it will take to arrive. This information is useful in advising the person collecting the prescription how long it will take before it is ready for collection.
The person taking in the prescription may also have to take payment for the prescription. They should understand how to calculate the cost of the prescription and may be required to understand the rules about who pays for their prescriptions and what checks may be required to ensure the patient is making a legitimate declaration of exemption.
Clinical and legal checking of the prescription
This is an essential role of the pharmacist to ensure that the prescription is legally complete and clinically correct for the patient. The pharmacist should check that all the information required to select and dispense the right product is available on the prescription. Any information which is missing or ambiguous will require the pharmacist to take some action before the prescription can go to the next stage in the dispensing process. If information is not present the pharmacist should ascertain this information and the prescription might have to be sent back to the prescriber for the prescription to be completed or altered. In the UK, if it is not possible to get the prescription back to the prescriber or the prescriber cannot be contacted, the pharmacist can add information relating to the dose, strength and quantity to be supplied using the endorsements ‘prescriber contacted’ and ‘prescriber not contacted’ as outlined in the BNF.
The pharmacist should also check that the prescription is appropriate for the patient and that it will be of benefit and not cause the patient harm. A number of court cases have identified that the pharmacist has a responsibility for what they have supplied under the directions of the prescriber. Therefore, in addition to ensuring the prescription is legal and complete, the pharmacist should be checking the clinical elements of the prescribing and contacting the prescriber to discuss any issues they have identified.
One suggested way to do this review is by using the mnemonic IDEAL CASE, as follows:
Interactions
Does the drug interact with any other items the patient is taking or with the patient’s condition? Many of the programmes which are used to produce labels and store a patient’s medication record in the pharmacy have the functionality that will identify possible interactions. The information is normally highlighted on the computer screen in the pharmacy with an indication of the possible importance of the interaction. The pharmacist needs to be able to interpret this information and, using any other information they have, decide what action would be appropriate to ensure that no harm comes to the patient.
Dose
Is the dose and dosage regimen appropriate for the patient and their condition? (See Example 24.3.) This is more significant when dealing with drugs that have a narrow therapeutic window (i.e. the dose difference between the therapeutic dose and the toxic dose is small) and when using medication in the very young or very old patient. Being able to calculate the appropriate dose for the patient is a key skill and will sometimes require knowledge about the weight of the patient. Doses should be checked against the maximum dosage information contained in the BNF in the UK. The BNF states maximum doses in a number of ways:
If the strength or dose and dosage regimen is missing or incorrect on the prescription, the pharmacist will have to calculate an appropriate strength, dose or dosage regimen. The pharmacist will then have to discuss this with the prescriber to ensure that this meets the patient’s requirements.
Example 24.3
Look at the following details which are written on a prescription:
On the above prescription there is no indication of the age of the patient. It cannot be assumed that the patient is an adult. If the patient is a child, then the dose may be inappropriate and the BNF for children would have to be consulted. Bendroflumethiazide is usually taken in the morning, not at night, but night workers may take the tablets at night. In this case it is essential to check with the patient and the prescriber for the correctness of the prescription. If the patient is taking the medication for hypertension then the normal dose would be 2.5 mg each morning and increasing the dose will have little enhanced effect, and this too may need to be discussed with the prescriber.
The dose and dosage regimen of the medication may be affected by other medication on the prescription, or the conditions the patient may be suffering from. This is particularly true if they have any degree of kidney failure, as these are the main routes of excretion of drugs from the body.
Evidence of benefit/harm
Is there any evidence the patient is benefiting from the treatment? Do they believe it is working and is there any evidence that their condition is improving? Could it be causing them any harm? Are they suffering from any adverse effects? Is another treatment on the prescription being used to treat these side-effects? (See Example 24.4.)
Example 24.4
Examine the following details which are written on a prescription:
On the above prescription you may find after talking to the patient that the ibuprofen is being prescribed to treat muscle pain. Muscle pain is a recognized adverse drug reaction (ADR) of a statin which the prescriber may have missed. You would then need to discuss with the prescriber the possibility that the symptoms may be due to an ADR to the statin. This would enable the prescriber to decide on an appropriate course of action to take which could mean undertaking further tests on the patient to establish if it is a true ADR.
Appropriate
Is the product the most appropriate medication available for the patient and their condition? This leads into the use of the mnemonic CASE, which helps to look at the specifics of the drug in terms of its cost, its acceptability to the patient and its safety and efficacy.
Legal
Check the prescription complies with any laws related to the supply of medicines. The legal requirements will be dependent on the legislation in place in the individual country at the time of writing the prescription. For example, the Medicines, Ethics and Practice guide covers the legislation relating to prescriptions generated in the UK adequately for most purposes. However, the legal requirements are likely to require the prescription, at the very least, to be signed and dated by the prescriber. Clearly it is the responsibility of the pharmacist to check that the signature is genuine and the date correct.
Cost-effective
Is there a more cost-effective product? Does this treatment offer the most cost-effective option? This might be worth considering if a cheaper medication has the same evidence of safety and efficacy (e.g. comparing the use of simvastatin and atorvastatin). Sometimes the prescriber prescribes a dose of two 10 mg tablets when it would be cheaper to provide one 20 mg tablet.
Acceptable to the patient
Ask the patient if they can take or use the medication? Ask if they are able to take their medication as they are directed to take it? Try and find out if they take their medication all the time and investigate how concordant they are. If the patient does not or cannot take the medicine, there is little reason for it to be dispensed in the first place. Some of the considerations might be, for example, can they swallow tablets or would a liquid or soluble product be more acceptable? Or, can they use their inhaler correctly? If the patient cannot use the inhaler after effective counselling then they should be considered for an alternative device which they might find easier to use. Can they get into child resistant containers? If not, could the medicine be supplied in a device that the patient can get into?
Safe
What do I have to do to ensure the product is safe for this patient? Which possible side-effects might they suffer? What should they do if they suffer from these side-effects? In some cases this will mean that they have to return to the prescriber, but should they stop their treatment or keep taking it until they can see the prescriber? Do I need to tell them about any cautions when taking the medication? (This might be telling them about driving or operating machinery or taking alcohol when taking their medicine.) Does the treatment need to be monitored? If so, when, how often and how should it be monitored? When should the treatment be reviewed and stopped? When would I be justified in looking for a safer alternative treatment? If the patient is currently on a treatment dose, when can that be reduced to a maintenance dose or even stopped?
Evidence based
Is the treatment the most effective treatment available? Is the treatment evidence based? Does the treatment concur with any available guidelines or protocols (see Chs 17 and 18)? The use of this mnemonic and considerations of the questions should help the pharmacist to clinically and legally review the prescription and thereby to ensure that the patient is being treated optimally. The pharmacist should then deal with any issue. Some issues will prevent the prescription from being dispensed, such as missing information, a potential overdose and a serious interaction. These will mean the pharmacist has to contact the prescriber before the prescription is sent to the next stage in the dispensing process. Some issues can be raised with the prescriber after the prescription has been dispensed, such as issues about the cost-effectiveness of the treatment. Ensuring the patient gets adequate counselling when the medicine is delivered to the patient might be enough to satisfy some of the issues, particularly in relation to information about possible side-effects and cautions in using the medication.
Assembly of the product and labelling
This stage involves producing a label for the product, selecting the product from where it is stored in the pharmacy and doing any assembly work and putting the label onto the product. Usually trained pharmacy technicians carry out this stage in the process and some pharmacies have introduced dispensing robots to select the products. The assembly work can range from picking a patient pack from a shelf to making the product from its ingredients. Accuracy in this stage of the assembly process is paramount in ensuring that the patient gets the product ordered on the prescription. Picking the wrong strength or form, or even the wrong product, are errors that can occur at this stage. The dispenser needs to take great care in selecting the products because many drugs have similar names, as outlined in Box 24.2 or they may be in containers with a similar appearance (see Ch. 9).
Box 24.2 A few products with similar names
| Aldactide | Aldactone |
| Betnesol | Betnelan |
| Co-amilofruse | Co-amilozide |
| Co-amoxiclav | |
| Cardene | Codeine |
| Daonil | Danol |
| Gliclazide | Glipizide |
| Nicardipine | Nifedipine |
| Promazine | Promethazine |
| Zocor | Zoton |
At this stage of the process the dispenser may be required to make records, either for legal purposes such as completion of a controlled drugs register, or as good practice, such as adding information to a patient medication record (PMR). The prescription may need to be priced if it is a private prescription or endorsed as required if it is an NHS prescription.
Accuracy checking the product against the prescription
This final accuracy check is present to ensure that there has not been an error in the dispensing process. It is an important stage in minimizing risk to the patient. The person involved in carrying out this check needs to work in a structured and methodical manner without interruption to have the best chance of working effectively. Until recently the pharmacist exclusively did the final check, but more and more accredited checking technicians are being trained to perform this task. This will free up the pharmacist to carry out other tasks which better match their skills, e.g. the delivery of medicines usage reviews (MURs) to patients (see Ch. 47).
Delivery of the product to the patient with the appropriate advice about the product
The final stage is to hand the product to the patient. This stage can be done by a pharmacist or can be delegated to another member of staff. It is important that whoever gives the medicine to the patient ensures the patient has all the advice they need. The pharmacist must be confident that the patient can take or use their medication correctly. The patient should be able to identify signs to tell if the medicine is having the desired effect or is causing a problem and they should know what to do about it. Once the prescription has been handed to the patient, the prescription form will be filed or sent to the appropriate place so the pharmacy receives payment.
Information sources
When dealing with a prescription it is important that the pharmacist knows where to look for up-to-date information relating to different aspects involved in checking the prescription. Drugs are continually being introduced to the market and the indications for some drugs change, as do doses, dosage regimens and formulations. In addition, some drugs are removed from the market for a variety of reasons. Prescribers will need independent, accessible and unambiguous reviews of effective treatments when writing prescriptions, while pharmacists will require similar information to clinically check the prescription and advise the patient. In addition the dispenser will also need information relating to the cost of the medication to the patient and any rules which they must apply to ensure appropriate remuneration and reimbursement, as well as information about the availability and where to order the products. Some of the most useful information sources in the UK are listed in Box 24.3 (see also Ch. 23 for more detailed information).
Box 24.3 Some of the reference sources most frequently used in dispensing
| British National Formulary (BNF) |
| Medicines, Ethics and Practice Guide |
| Stockley’s Drug Interactions |
| Drug and Therapeutics Bulletin |
| Drug Tariff |
| MeReC Bulletin |
| Effective Healthcare |
| Prescribers’ Journal |
| Pharmaceutical Codex |
| Current Problems in Pharmacovigilance |
| Martindale: the Extra Pharmacopoeia |
The BNF is published and updated every 6 months and lists the products available for dispensing in the UK. It is sent to all doctors, pharmacists and prescribing nurses in the NHS. The Nurse Prescribers’ Formulary and Dental Practitioners’ Formulary are included in the BNF and are also published as separate booklets. There is also a BNF for Children which has been produced to provide sound up-to-date information on the use of medicines for treating children. The BNF is the major source of easily available information on the characteristics of individual medicines, including proprietary and generic formulations, strengths, dose and dosage regimens, drug side-effects and drug interactions. The most recent copy should always be used. For more comprehensive information about medicines you should refer to Martindale.
The Royal Pharmaceutical Society of Great Britain (RPSGB) produces the Medicines, Ethics and Practice guide. It outlines the legal requirements for the sale and supply of medicines and poisons in the UK and contains the RPSGB Code of Ethics for Pharmacists and Pharmacy Technicians.
The Drug Tariff is the resource which details the rules for the NHS remuneration and reimbursement for pharmacy contractors in the UK and contains information on what is or is not allowed on NHS prescriptions.
If the pharmacist cannot find the information that they require in the reference sources they have available, they can contact the local medicines information department which will be listed in the BNF, or the manufacturer of the product where appropriate.