Chapter 6 Ninth Week to Birth
The Fetal Period
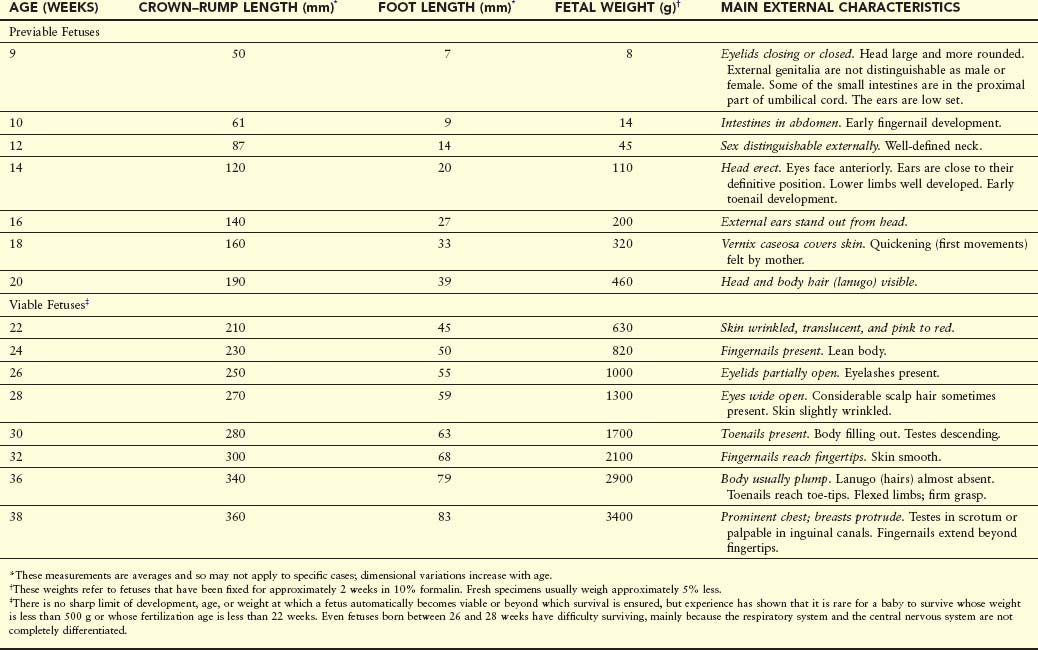
The transformation of an embryo to a fetus is gradual, but the name change is meaningful because it signifies that the embryo has developed a recognizable human appearance and that the primordia of all major systems have formed. Development during the fetal period is primarily concerned with rapid body growth and differentiation of tissues, organs, and systems. A notable change occurring during the fetal period is the relative slowdown in the growth of the head compared with the rest of the body. The rate of body growth during the fetal period is very rapid (Table 6-1), and fetal weight gain is phenomenal during the terminal weeks. Periods of normal continuous growth alternate with prolonged intervals of absent growth.
Viability of Fetuses
Viability is defined as the ability of fetuses to survive in the extrauterine environment (i.e., after birth). Most fetuses weighing less than 500 g at birth do not usually survive. Many full-term, low-birth-weight babies result from intrauterine growth restriction (IUGR). Consequently, if given expert postnatal care, some fetuses weighing less than 500 g may survive; they are referred to as extremely low birth weight or immature infants.
Most fetuses weighing between 750 and 1500 g survive, but complications may occur; they are referred to as preterm infants. Each year, approximately 500,000 preterm infants are born in the United States. Many of these preterm infants suffer from severe medical complications or early mortality. The use of antenatal steroids and postnatal administration of endotracheal surfactant has greatly improved acute and long-term morbidity. Prematurity is one of the most common causes of morbidity and perinatal death.
Estimation of Fetal Age
Ultrasound measurements of crown-rump length (CRL) of fetuses are taken to determine the size and probable age of the fetus and to provide a prediction of the expected date of delivery. Fetal head measurements and femur length are also used to evaluate age. Gestational age is commonly used clinically, and it may be confusing because the term seems to imply the actual age of the fetus from fertilization of the oocyte. In fact, this term is most often meant to be synonymous with last normal menstrual period (LNMP) age. It is important that the person ordering the ultrasound examination and the ultrasonographer use the same terminology.
The intrauterine period may be divided into days, weeks, or months (Table 6-2), but confusion arises if it is not stated whether the age is calculated from the onset of the LNMP or the estimated day of fertilization of the oocyte. Uncertainty about age arises when months are used, particularly when it is not stated whether calendar months (28–31 days) or lunar months (28 days) are meant. Unless otherwise stated, fetal age in this book is calculated from the estimated time of fertilization.
Trimesters of Pregnancy
Clinically, the gestational period is divided into three trimesters, each lasting 3 months. At the end of the first trimester, one third of the length of the pregnancy, all major systems are developed (Fig. 6-1B). In the second trimester, the fetus grows sufficiently in size so that good anatomic detail can be visualized during ultrasonography. During this period, most major birth defects can be detected using high-resolution real-time ultrasonography. By the beginning of the third trimester, the fetus may survive if born prematurely. The fetus reaches a major developmental landmark at 35 weeks of gestation and weighs approximately 2500 g, which is used to define the level of fetal maturity. At this stage, the fetus usually survives if born prematurely.
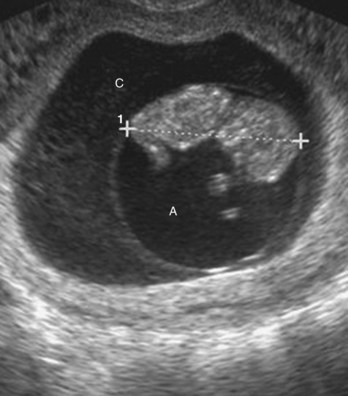
FIGURE 6–1 Ultrasound image of 9-week fetus (11 weeks gestational age). Note the amnion, amniotic cavity (A), and chorionic cavity (C). CRL 4.2 cm (calipers).
(Courtesy of Dr. E.A. Lyons, professor of radiology and obstetrics and gynecology and anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
Measurements and Characteristics of Fetuses
Various measurements and external characteristics are useful for estimating fetal age (see Table 6-1). CRL is the method of choice for estimating fetal age until the end of the first trimester because there is very little variability in fetal size during this period. In the second and third trimesters, several structures can be identified and measured ultrasonographically, but the most common measurements are biparietal diameter (diameter of the head between the two parietal eminences), head circumference, abdominal circumference, femur length, and foot length. Weight is often a useful criterion for estimating age, but there may be a discrepancy between the age and the weight, particularly when the mother had metabolic disturbances such as diabetes mellitus during pregnancy. In these cases, weight often exceeds values considered normal for the corresponding CRL.
Fetal dimensions obtained from ultrasound measurements closely approximate CRL measurements obtained from spontaneously aborted fetuses. Determination of the size of a fetus, especially of its head, is helpful to the obstetrician for management of patients.
Highlights of Fetal Period
There is no formal staging system for the fetal period; however, it is helpful to describe the changes that occur in periods of 4 to 5 weeks.
Nine to Twelve Weeks
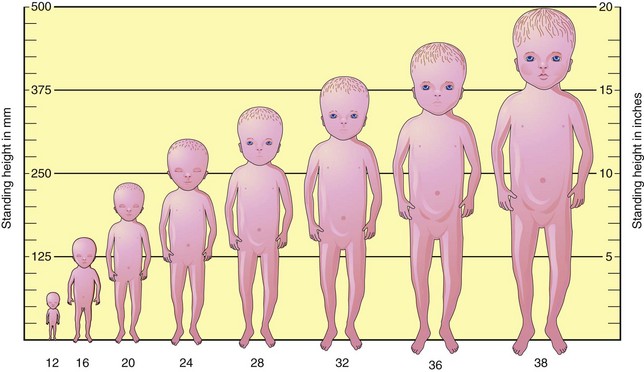
At the beginning of the ninth week, the head constitutes approximately half the CRL of the fetus (Figs. 6-1 and 6-2A). Subsequently, growth in body length accelerates rapidly so that by the end of 12 weeks, the CRL has more than doubled (Fig. 6-1B; Table 6-1). Although growth of the head slows down considerably by this time, it is still disproportionately large compared with the rest of the body.
At 9 weeks, the face is broad, the eyes are widely separated, the ears are low set, and the eyelids are fused (Fig. 6-2B). By the end of 12 weeks, primary ossification centers appear in the skeleton, especially in the cranium (skull) and long bones. Early in the ninth week, the legs are short and the thighs are relatively small. By the end of 12 weeks, the upper limbs have almost reached their final relative lengths, but the lower limbs are still not so well developed and are slightly shorter than their final relative lengths.
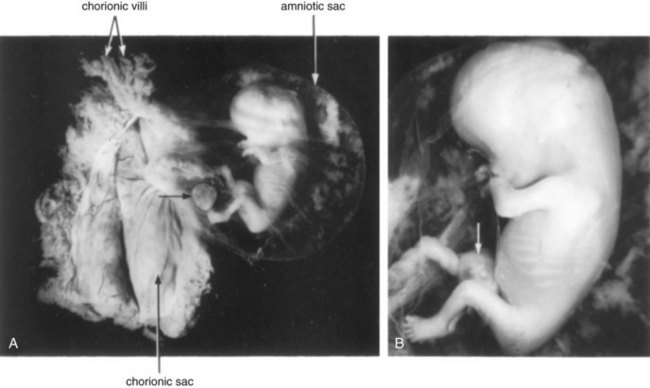
FIGURE 6–2 A 9-week fetus in the amniotic sac exposed by removal from the chorionic sac. A, Actual size. The remnant of the umbilical vesicle is indicated by an arrow. B, Enlarged photograph of the fetus (x2). Note the following features: large head, fused eyelids, cartilaginous ribs, and intestines in umbilical cord (arrow).
(Courtesy of Professor Jean Hay [retired], Department of Human Anatomy and Cell Science, University of Manitoba, Winnipeg, Manitoba, Canada.)
The external genitalia of males and females appear similar until the end of the ninth week. Their mature fetal form is not established until the 12th week. Intestinal coils are clearly visible in the proximal end of the umbilical cord until the middle of the tenth week (see Fig. 6-2B). By the 11th week, the intestines have returned to the abdomen (Fig. 6-3).
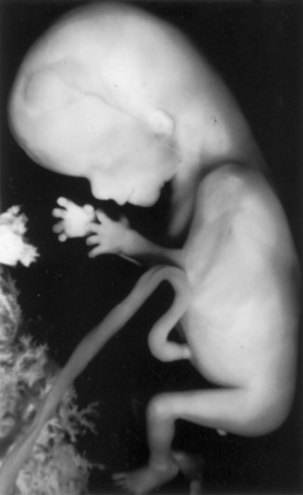
FIGURE 6–3 An 11-week fetus (x1.5). Note its relatively large head and that the intestines are no longer in the umbilical cord.
(Courtesy of Professor Jean Hay [retired], Department of Human Anatomy and Cell Science, University of Manitoba, Winnipeg, Manitoba, Canada.)
At 9 weeks, the liver is the major site of erythropoiesis (formation of red blood cells). By the end of 12 weeks, this activity has decreased in the liver and has begun in the spleen. Urine formation begins between the 9th and 12th weeks, and urine is discharged through the urethra into the amniotic fluid. The fetus reabsorbs some amniotic fluid after swallowing it. Fetal waste products are transferred to the maternal circulation by passing across the placental membrane (see Chapter 7).
Thirteen to Sixteen Weeks
Growth is rapid during this period (Figs. 6-4 and 6-5; see Table 6-1). By 16 weeks, the head is relatively small compared with that of the 12-week fetus and the lower limbs have lengthened. Limb movements, which first occur at the end of the embryonic period, become coordinated by the 14th week but are too slight to be felt by the mother. Limb movements are visible during ultrasound examinations.
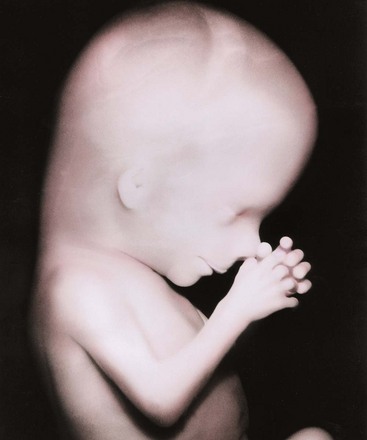
FIGURE 6–5 Enlarged photograph of the head and superior part of the trunk of a 13-week fetus.
(Courtesy of Professor Jean Hay [retired], Department of Human Anatomy and Cell Science, University of Manitoba, Winnipeg, Manitoba, Canada.)
Ossification of the fetal skeleton is active during this period, and the bones are clearly visible on ultrasound images by the beginning of the 16th week. Slow eye movements occur at 14 weeks. Scalp hair patterning is also determined during this period. By 16 weeks, the ovaries are differentiated and contain primordial ovarian follicles that contain oogonia (see Chapter 12). The genitalia of the fetuses can be recognized by 12 to 14 weeks. By 16 weeks, the eyes face anteriorly rather than anterolaterally. In addition, the external ears are close to their definitive position on the sides of the head.
Seventeen to Twenty Weeks
Growth slows down during this period, but the fetus still increases its CRL by approximately 50 mm (Figs. 6-4 and 6-6; see Table 6-1). Fetal movements—quickening—are commonly felt by the mother. The skin is now covered with a greasy, cheese-like material—vernix caseosa. It consists of a mixture of dead epidermal cells and a fatty substance (secretion) from the fetal sebaceous glands. The vernix caseosa protects the delicate fetal skin from abrasions, chapping, and hardening that result from exposure to the amniotic fluid.
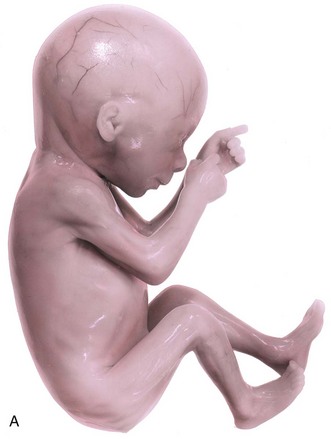

FIGURE 6–6 A, A 17-week fetus. Because there is little subcutaneous tissue and the skin is thin, the blood vessels of the scalp are visible. Fetuses at this age are unable to survive if born prematurely, mainly because their respiratory systems are immature. B, A frontal view of a 17-week fetus. Note that the eyes are closed at this stage.
(A, From Moore KL, Persaud TVN, Shiota K: Color Atlas of Clinical Embryology, 2nd ed. Philadelphia, WB Saunders, 2000; B, Courtesy of Dr. Robert Jordan, St. George’s University Medical School, Grenada.)
Eyebrows and head hair are visible at 20 weeks. The fetuses are usually completely covered with fine downy hair—lanugo—that helps to hold the vernix caseosa on the skin. Brown fat forms during this period and is the site of heat production, particularly in the newborn infant. This specialized adipose tissue, found chiefly at the root of the neck, posterior to the sternum, and in the perirenal area, produces heat by oxidizing fatty acids.
By 18 weeks, the fetal uterus is formed and canalization of the vagina has begun and many primordial ovarian follicles containing oogonia are visible. By 20 weeks, the testes have begun to descend, but they are still located on the posterior abdominal wall, as are the ovaries in female fetuses.
Twenty-One to Twenty-Five Weeks
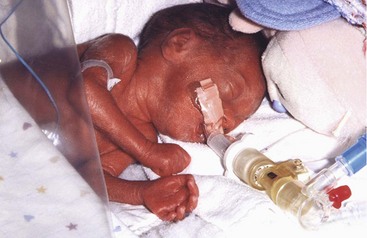
There is a substantial weight gain during this period and the fetus is better proportioned (Fig. 6-7). The skin is usually wrinkled and more translucent, particularly during the early part of this period. The skin is pink to red because blood is visible in the capillaries. At 21 weeks, rapid eye movements begin and blink-startle responses have been reported at 22 to 23 weeks. The secretory epithelial cells (type II pneumocytes) in the interalveolar walls of the lung have begun to secrete surfactant, a surface-active lipid that maintains the patency of the developing alveoli of the lungs (see Chapter 10). Fingernails are present by 24 weeks. Although a 22- to 25-week fetus born prematurely may survive if given intensive care (Fig. 6-7), it may die because its respiratory system is still immature. The risk for neurodevelopmental disability is high in infants born before 26 weeks of gestation.
Twenty-Six to Twenty-Nine Weeks
During this period, fetuses usually survive if born prematurely and given intensive care (Fig. 6-8). The lungs and pulmonary vasculature have developed sufficiently to provide adequate gas exchange. In addition, the central nervous system has matured to the stage where it can direct rhythmic breathing movements and control body temperature. The highest neonatal mortality occurs in infants classified as low (≤2500 g) and very low (≤1500 g) birth weight.
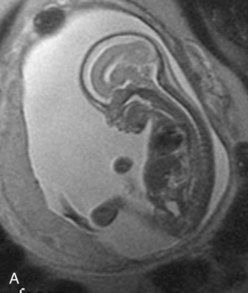
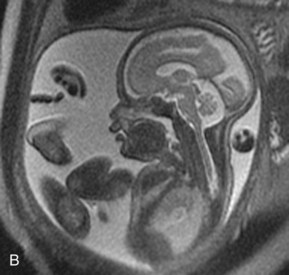
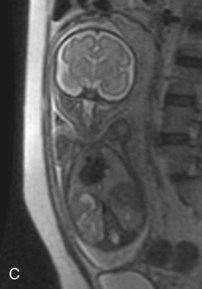
FIGURE 6–8 Magnetic resonance images (MRIs) of normal fetuses. A, At 18 weeks (20-week gestational age). B, At 26 weeks. C, At 28 weeks.
(Courtesy of Dr. Deborah Levine, Director of Obstetric and Gynecologic Ultrasound, Beth Israel Deaconess Medical Center, Boston, MA.)
The eyelids are open at 26 weeks, and lanugo and head hair are well developed. Toenails become visible and considerable subcutaneous fat is now present under the skin, smoothing out many of the wrinkles. During this period, the quantity of white fat increases to approximately 3.5% of body weight. The fetal spleen has been an important site of erythropoiesis (formation of red blood cells). This ends by 28 weeks, by which time bone marrow has become the major site of this process.
Thirty to Thirty-Four Weeks
The pupillary reflex (change in diameter of pupil in response to stimulus caused by light) can be elicited at 30 weeks. Usually by the end of this period, the skin is pink and smooth and the upper and lower limbs have a chubby appearance. At this age, the quantity of white fat is approximately 8% of body weight. Fetuses 32 weeks and older usually survive if born prematurely.
Thirty-Five to Thirty-Eight Weeks
Fetuses born at 35 weeks have a firm grasp and exhibit a spontaneous orientation to light. As term approaches, the nervous system is sufficiently mature to carry out some integrative functions. Most fetuses during this “finishing period” are plump (Fig. 6-9). By 36 weeks, the circumferences of the head and abdomen are approximately equal. After this, the circumference of the abdomen may be greater than that of the head. The fetal foot length is usually slightly larger than femoral length at 37 weeks, and is an alternative parameter for confirmation of fetal age (Fig. 6-10). There is a slowing of growth as the time of birth approaches (Fig. 6-11).
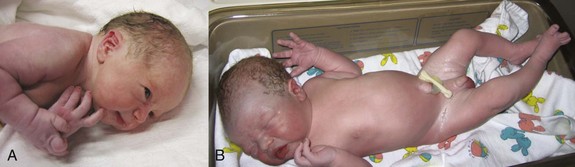
FIGURE 6–9 Healthy newborns. A, At 34 weeks (36-week gestational age). B, At 38 weeks (40-week gestational age).
(A, Courtesy of Michael and Michele Rice; B, Courtesy of Dr. Jon and Mrs. Margaret Jackson.)
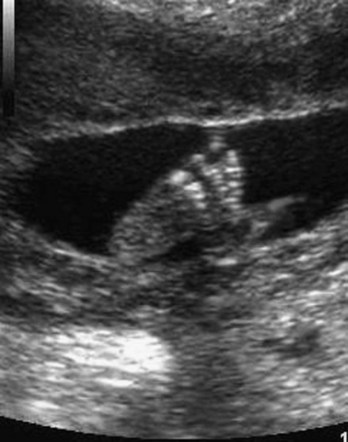
FIGURE 6–10 Ultrasound scan of the foot of a fetus at 19 weeks.
(Courtesy of Dr. E.A. Lyons, Professor of Radiology, Obstetrics and Gynecology, and Anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
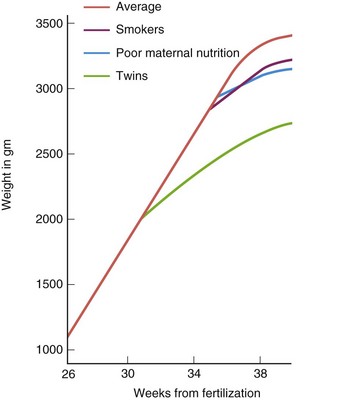
FIGURE 6–11 Graph showing the rate of fetal growth during the last trimester (3 months). Average refers to babies born in the United States. After 36 weeks, the growth rate deviates from the straight line. The decline, particularly after full term (38 weeks), probably reflects inadequate fetal nutrition caused by placental changes.
(Adapted from Gruenwald P: Growth of the human fetus. I. Normal growth and its variation. Am J Obstet Gynecol 94:1112, 1966.)
By full term, most fetuses usually reach a CRL of 360 mm and weigh approximately 3400 g. The amount of white fat is approximately 16% of body weight. A fetus adds approximately 14 g of fat per day during these last weeks. The thorax (chest) is prominent and the breasts often protrude slightly in both sexes. The testes are usually in the scrotum in full-term male infants; premature male infants commonly have undescended testes. Although the head is smaller at full term in relation to the rest of the body than it was earlier in fetal life, it still is one of the largest regions of the fetus. In general, male fetuses are longer and weigh more at birth than females.
Low Birth Weight
Not all low-birth-weight babies are premature. Approximately one third of those with a birth weight of 2500 g or less are actually small for gestational age. These “small for dates” infants may be underweight because of placental insufficiency (see Chapter 7). The placentas are often small or poorly attached and/or have undergone degenerative changes that progressively reduce the oxygen supply and nourishment to the fetus.
It is important to distinguish between full-term infants who have a low birth weight because of intrauterine growth restriction (IUGR), and preterm infants who are underweight because of a shortened gestation (i.e., premature by date). IUGR may be caused by placental insufficiency, preeclampsia (hypertension), multiple gestations (e.g., triplets), infectious diseases, cardiovascular anomalies, inadequate maternal nutrition, and maternal and fetal hormones. Teratogens (drugs, chemicals, and viruses) and genetic factors are also known to cause IUGR (see Chapter 20). Infants with IUGR show a characteristic lack of subcutaneous fat and their skin is wrinkled, suggesting that white fat has actually been lost.
Expected Date of Delivery
The expected date of delivery of a fetus is 266 days or 38 weeks after fertilization, that is, 280 days or 40 weeks after LNMP (see Table 6-2). Approximately 12% of babies are born 1 to 2 weeks after the expected time of birth.
Postmaturity Syndrome
Prolongation of pregnancy for 3 or more weeks beyond the expected date of delivery occurs in 5% to 6% of women. Some infants in such pregnancies develop postmaturity syndrome and have an increased risk of mortality. Because of this, labor is often induced (see Chapter 7). These fetuses have dry, parchment-like skin; are often overweight; and have no lanugo, decreased or absent vernix caseosa, long nails, and increased alertness.
Factors Influencing Fetal Growth
The fetus requires substrates (nutrients) for growth and production of energy. Gases and nutrients pass freely to the fetus from the mother through the placental membrane (see Chapter 7). Glucose is a primary source of energy for fetal metabolism and growth; amino acids are also required. These substances pass from the mother’s blood to the fetus through the placental membrane. Insulin required for the metabolism of glucose is secreted by the fetal pancreas; no significant quantities of maternal insulin reach the fetus because the placental membrane is relatively impermeable to this hormone. Insulin, insulin-like growth factors, human growth hormone, and some small polypeptides (such as somatomedin C) are believed to stimulate fetal growth.
Many factors may affect prenatal growth: maternal, fetal, and environmental. Some factors operating throughout pregnancy, such as maternal vascular disease, intrauterine infection, cigarette smoking, and consumption of alcohol, tend to produce intrauterine growth restriction (IUGR) infants or small for gestational age (SGA) infants, whereas factors operating during the last trimester, such as maternal malnutrition, usually produce underweight infants with normal length and head size. The terms IUGR and SGA are related, but they are not synonymous.
IUGR refers to a process that causes a reduction in the expected pattern of fetal growth as well as fetal growth potential. Constitutionally small for gestational age infants have a birth weight that is lower than a predetermined cutoff value for a particular gestational age (<2 standard deviations below the mean or less than the third percentile). Severe maternal malnutrition resulting from a poor-quality diet is known to cause restricted fetal growth (see Fig. 6-11).
Cigarette Smoking
Smoking is a well-established cause of IUGR. The growth rate for fetuses of mothers who smoke cigarettes is less than normal during the last 6 to 8 weeks of pregnancy (Fig. 6-11). On average, the birth weight of infants whose mothers smoke heavily during pregnancy is 200 g less than normal, and perinatal morbidity (medical complications) is increased when adequate medical care is unavailable. The effect of maternal smoking is greater on fetuses whose mothers also receive inadequate nutrition.
Multiple Pregnancy
Individuals of multiple births usually weigh considerably less than infants resulting from a single pregnancy (Fig. 6-11). It is evident that the total metabolic requirements of two or more fetuses exceed the nutritional supply available from the placenta during the third trimester.
Alcohol and Illicit Drugs
Infants born to alcoholic mothers often exhibit IUGR as part of the fetal alcohol syndrome (see Chapter 20). Similarly, the use of marijuana and other illicit drugs (e.g., cocaine) can cause IUGR and other obstetric complications.
Impaired Uteroplacental and Fetoplacental Blood Flow
Maternal placental circulation may be reduced by conditions that decrease uterine blood flow (e.g., small chorionic vessels, severe maternal hypotension, and renal disease). Chronic reduction of uterine blood flow can cause fetal starvation resulting in IUGR. Placental dysfunction or defects (e.g., infarction; see Chapter 7) can also cause IUGR. The net effect of these placental abnormalities is a reduction of the total area for exchange of nutrients between the fetal and maternal blood streams. It is very difficult to separate the effect of these placental changes from the effect of reduced maternal blood flow to the placenta. In some instances of chronic maternal disease, the maternal vascular changes in the uterus are primary and the placental defects are secondary.
Genetic Factors and Growth Retardation
It is well established that genetic factors can cause IUGR. Repeated cases of this condition in one family indicate that recessive genes may be the cause of the abnormal growth. Structural and numerical chromosomal aberrations have also been shown to be associated with cases of retarded fetal growth. IUGR is pronounced in infants with Down syndrome and is very characteristic of fetuses with trisomy 18 syndrome (see Chapter 20).
Procedures for Assessing Fetal Status
By accepting the shelter of the uterus, the fetus also takes the risk of maternal disease or malnutrition and of biochemical, immunological and hormonal adjustment.
– George W. Corner, renowned American embryologist, 1888–1981
Perinatology is the branch of medicine that is concerned with the well-being of the fetus and newborn infant, generally covering the period from approximately 26 weeks after fertilization to 4 weeks after birth. This subspecialty of medicine combines aspects of obstetrics and pediatrics.
Ultrasonography
Ultrasonography is the primary imaging modality in the evaluation of the fetus because of its wide availability, low cost, and lack of known adverse effects. The chorionic sac and its contents may be visualized by ultrasonography during the embryonic and fetal periods. Placental and fetal size, multiple births, abnormalities of placental shape, and abnormal presentations can also be determined. Ultrasound scans give accurate measurements of the biparietal diameter of the fetal cranium (skull), from which close estimates of fetal age and length can be made. Figures 6-10 and 6-12 illustrate how details of the fetus can be observed in ultrasound scans. Ultrasound examinations are also helpful for diagnosing abnormal pregnancies at a very early stage. Rapid advances in ultrasonography have made this technique a major tool for prenatal diagnosis of fetal abnormalities. Biopsy of fetal tissues, such as skin, liver, kidney, and muscle, can be performed with ultrasound guidance.
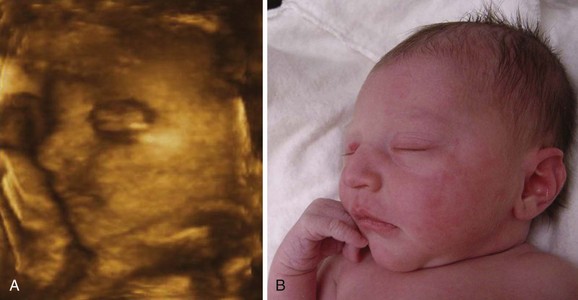
FIGURE 6–12 A, Three-dimensional ultrasound (sonogram) of a 28-week fetus showing the face. The surface features are clearly recognizable. B, Photograph of the newborn infant (from A) 3 hours after birth.
(Courtesy of Dr. E.A. Lyons, Professor of Radiology, Obstetrics and Gynecology, and Anatomy, Health Sciences Centre and University of Manitoba, Winnipeg, Manitoba, Canada.)
Diagnostic Amniocentesis
This is a common invasive prenatal diagnostic procedure, usually performed between 15 and 18 weeks gestation. Amniotic fluid is sampled by inserting a 22-gauge needle through the mother’s anterior abdominal and uterine walls into the amniotic cavity by piercing the chorion and amnion (Fig. 6-13A). Because there is relatively little amniotic fluid before the 14th week, amniocentesis is difficult to perform before this time. The amniotic fluid volume is approximately 200 ml, and 15 to 20 ml can be safely withdrawn. Amniocentesis is relatively devoid of risk, especially when the procedure is performed by an experienced physician using real-time ultrasonography guidance for outlining the position of the fetus and placenta.
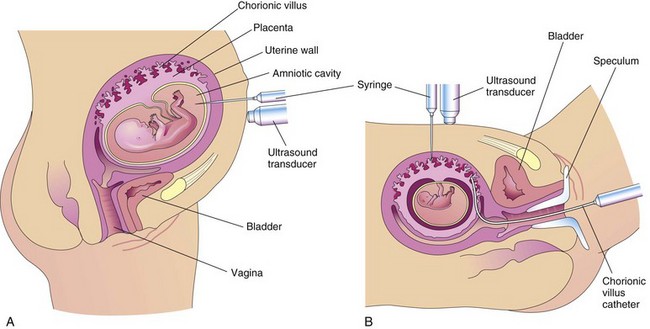
FIGURE 6–13 A, Illustration of amniocentesis. A needle is inserted through the lower abdominal and uterine walls into the amniotic cavity. A syringe is attached and amniotic fluid is withdrawn for diagnostic purposes. B, Drawing illustrating chorionic villus sampling. Two sampling approaches are illustrated: through the maternal anterior abdominal wall with a needle and through the vagina and cervical canal using a malleable catheter. A speculum is an instrument for exposing the vagina.
Alpha-Fetoprotein Assay
Alpha-fetoprotein (AFP) is a glycoprotein that is synthesized in the fetal liver, umbilical vesicle, and gut. AFP is found in high concentration in fetal serum, peaking 14 weeks after the LNMP. Small amounts of AFP normally enter the amniotic fluid.
Alpha-Fetoprotein and Fetal Anomalies
The concentration of AFP is high in the amniotic fluid surrounding fetuses with severe anomalies of the central nervous system and ventral abdominal wall. Amniotic fluid AFP concentration is measured by immunoassay, and, when used with ultrasonographic scanning, approximately 99% of fetuses with these severe defects can be diagnosed prenatally. When a fetus has an open neural tube defect, the concentration of AFP is also likely to be higher than normal in the maternal serum. Maternal serum AFP concentration is lower than normal when the fetus has Down syndrome (trisomy 21), trisomy 18, or other chromosome defects.
Spectrophotometric Studies
Examination of amniotic fluid by this method may be used for assessing the degree of erythroblastosis fetalis, also called hemolytic disease of newborn. This disease results from destruction of fetal red blood cells by maternal antibodies (see Chapter 7).
Chorionic Villus Sampling
Biopsies of trophoblastic tissue (5–20 mg) may be obtained by inserting a needle, guided by ultrasonography, through the mother’s abdominal and uterine walls (transabdominal) into the uterine cavity (see Fig. 6-13B). Chorionic villus sampling (CVS) can also be performed transcervically by passing a polyethylene catheter through the cervix and guided by real-time ultrasonography. For assessing the condition of a fetus at risk, the fetal karyotype (chromosome characteristics) can be obtained and a diagnosis made weeks earlier when CVS is performed, compared with amniocentesis. The risk of miscarriage with CVS is approximately 1%, more than with amniocentesis.
Diagnostic Value of Chorionic Villus Sampling
Biopsies of chorionic villi are used for detecting chromosomal abnormalities, inborn errors of metabolism, and X-linked disorders. Chorionic villus sampling (CVS) can be performed between 10 and 12 weeks of gestation. The rate of fetal loss is approximately 1%, slightly more than the risk from amniocentesis. Reports regarding an increased risk of limb defects after CVS are conflicting. The advantage of CVS over amniocentesis is that it can be carried out sooner and the results of chromosomal analysis are available several weeks earlier.
Sex Chromatin Patterns
Fetal sex can be determined by noting the presence or absence of sex chromatin in the nuclei of cells recovered from amniotic fluid. These tests were developed after it was discovered that sex chromatin was visible in nuclei of normal female cells but not in normal male cells (Fig. 6-14A and B). Females with three X chromosomes (46, XXX) have two masses of sex chromatin (Fig. 6-14C). By use of a special staining technique, the Y chromosome can also be identified in cells recovered from the amniotic fluid surrounding male fetuses (Fig. 6-14D). Knowledge of fetal sex can be useful in diagnosing the presence of severe sex-linked hereditary diseases, such as hemophilia and muscular dystrophy.

FIGURE 6–14 Oral epithelial nuclei stained with cresyl echt violet (A, B, and C) and quinacrine mustard (D) (x2000). A, From normal male. No sex chromatin is visible (chromatin negative). B, From normal female. The arrow indicates a typical mass of sex chromatin (chromatin positive). C, From female with 47, XXX trisomy. The arrows indicate two masses of sex chromatin. D, From normal male. The arrow indicates a mass of Y chromatin as an intensely fluorescent body.
(A and B, From Moore KL, Barr ML: Smears from the oral mucosa in the detection of chromosomal sex. Lancet 2:57, 1955.)
Cell Cultures and Chromosomal Analysis
The prevalence of chromosomal disorders is approximately one in 120 live-born infants. Fetal sex and chromosomal aberrations can be determined by studying the sex chromosomes in cultured fetal cells obtained during amniocentesis. These cultures are commonly done when an autosomal abnormality, such as occurs in Down syndrome, is suspected. Moreover, microdeletions and microduplications, as well as subtelomeric rearrangements, can now be detected with fluorescence in situ hybridization technology. Inborn errors of metabolism in fetuses can also be detected by studying cell cultures. Enzyme deficiencies can be determined by incubating cells recovered from amniotic fluid and then detecting the specific enzyme deficiency in the cells (see Chapter 20).
Fetal Transfusion
Fetuses with hemolytic disease of the newborn (HDN) can be treated by intrauterine blood transfusions. The blood is injected through a needle inserted into the fetal peritoneal cavity. With recent advances in percutaneous umbilical cord blood sampling (PUBS), blood and packed red blood cells are transfused directly into the umbilical vein for the treatment of fetal anemia due to isoimmunization. The need for fetal blood transfusions is reduced nowadays owing to the treatment of Rh-negative mothers of Rh-positive fetuses with anti-Rh immunoglobulin, which in many cases prevents development of this disease of the Rh system. Fetal transfusion of platelets directly into the umbilical cord vein is carried out for the treatment of alloimmune thrombocytopenia. Also, fetal infusion of drugs in a similar manner for the treatment of a few medical conditions in the fetus has been reported.
Fetoscopy
Using fiberoptic instruments, parts of the fetal body may be directly observed. It is possible to visualize the entire fetus looking for birth defects, such as cleft lip and limb defects. The fetoscope is usually introduced through the abdominal and uterine walls into the amniotic cavity, similarly to the way in which the needle is inserted during amniocentesis. Fetoscopy is usually carried out at 17 to 20 weeks of gestation, but with new approaches such as transabdominal thin-gauge embryo fetoscopy, it is possible to detect certain defects in the embryo or fetus during the first trimester. Because of the risk to the fetus compared with other prenatal diagnostic procedures, fetoscopy now has few indications for routine prenatal diagnosis or treatment of the fetus. Fetoscopy combined with laser coagulation has been used to treat fetal conditions such as twin–twin transfusion syndrome (TTTS). Fetoscopy has also been used for the release of amniotic bands (see Fig. 7-21).
Percutaneous Umbilical Cord Blood Sampling
Fetal blood samples may be obtained directly from the umbilical vein by percutaneous umbilical cord blood sampling (PUBS) or cordocentesis for the diagnosis of many fetal abnormal conditions, including aneuploidy, fetal growth restriction, fetal infection, and fetal anemia. PUBS is usually performed after 18 weeks of gestation under continuous direct ultrasound guidance, which is used to locate the umbilical cord and its vessels. Moreover, the procedure permits treating the fetus directly, including the transfusion of packed red blood cells for the management of fetal anemia resulting from isoimmunization.
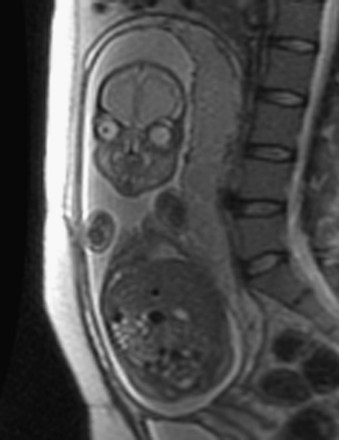
Magnetic Resonance Imaging
When planning fetal treatment, magnetic resonance imaging (MRI) may be used to provide more information about an abnormality that has been detected in ultrasonic images. Important advantages of magnetic resonance imaging are that it does not use ionizing radiation and that it has high soft-tissue contrast and resolution (Fig. 6-15).
Fetal Monitoring
Continuous fetal heart rate monitoring in high-risk pregnancies is routine and provides information about the oxygenation of the fetus. There are various causes of prenatal fetal distress, such as maternal diseases that reduce oxygen transport to the fetus (e.g., cyanotic heart disease). Fetal distress (e.g., indicated by an abnormal heart rate or rhythm) suggests that the fetus is in jeopardy. A noninvasive method of monitoring uses transducers placed on the mother’s abdomen.
Summary of Fetal Period
Clinically Oriented Problems
Case 6–1
A woman in the 20th week of a high-risk pregnancy was scheduled for a repeat cesarean section. Her physician wanted to establish an expected date of delivery.
Case 6–2
A 44-year-old pregnant woman was worried that she might be carrying a fetus with major birth defects.
Case 6–3
A 19-year-old woman in the second trimester of pregnancy asked a physician whether her fetus was vulnerable to over-the-counter drugs and street drugs. She also wondered about the effect of her heavy drinking and cigarette smoking on her fetus.
Discussion of these problems appears at the back of the book.
References and Suggested Reading
Anderson MS, Hay WW. Intrauterine growth restriction and the small-for-gestational-age infant. In MacDonald MG, Seshia MMK, Mullett MD, editors: Avery’s Neonatology: Pathophysiology & Management of the Newborn, ed 6, Philadelphia: Lippincott Williams & Wilkins, 2005.
Arroyo JA, Winn VD. Vasculogenesis and angiogenesis in the IUGR placenta. Semin Perinatol. 2008;32:172.
Chiu RW, Lo YM. Non-invasive prenatal diagnosis by fetal nucleic acid anlysis in maternal plasma: the coming of age. Semin Fetal Neonatal Med. 2011;16:88.
Chung R, Kasprian G, Brugger PC, et al. The current state and future of fetal imaging. Clin Perinatol. 2009;36:685.
Claris O, Beltrand J, Levy-Marchal C. Consequences of intrauterine growth and early neonatal catch-up growth. Semin Perinatol. 2010;34:207.
Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics, ed 23. New York: McGraw-Hill; 2009.
Deprest JA, Devlieger R, Srisupundit K, et al. Fetal surgery is a clinical reality. Semin Fetal Neonatal Med. 2010;15:58.
Drugan A, Isada NB, Evans MI. Prenatal diagnosis in the molecular age—indications, procedures, and laboratory techniques. In MacDonald MG, Seshia MMK, Mullett MD, editors: Avery’s Neonatology: Pathophysiology & Management of the Newborn, ed 6, Philadelphia: Lippincott Williams & Wilkins, 2005.
Durkin EF, Shaaban A. Commonly encountered surgical problems in the fetus and neonate. Pediatr Clin N Am. 2009;56:647.
Evans MI, Johnson MP, Flake AW, et al. Fetal therapy. In MacDonald MG, Seshia MMK, Mullett MD, editors: Avery’s Neonatology: Pathophysiology & Management of the Newborn, ed 6, Philadelphia: Lippincott Williams & Wilkins, 2005.
Filly RA, Feldstein VA. Ultrasound evaluation of normal fetal anatomy. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Hinrichsen KV, editor. Humanembryologie. Berlin: Springer-Verlag, 1990.
Jirásel JE. An Atlas of Human Prenatal Developmental Mechanics: Anatomy and Staging. London and New York: Taylor & Francis; 2004.
Lyons EA, Levi CS. Ultrasound of the normal first trimester of pregnancy. Syllabus: Special Course Ultrasound. Radiological Society of North America; 1991.
O’Rahilly R, Müller F. Development Stages in Human Embryos. Publication 637. Washington, DC: Carnegie Institution of Washington; 1987.
Pergament E. First-trimester genetic counseling: perspectives and considerations. Clin Lab Med. 2010;30:557.
Persaud TVN, Hay JC. Normal embryonic and fetal development. In: Reece EA, et al, editors. Clinical Obstetrics: The Fetus and Mother. ed 3. Malden, MA: Blackwell Publishing; 2006:19-32.
Pooh RK, Shiota K, Kurjak A. Imaging of the human embryo with magnetic resonance imaging microscopy and high-resolution transvaginal 3-dimensional sonography: human embryology in the 21st century. Am J Obstet Gynecol. 2011;204:77. e1-16
Reed MD, Blumer JL. Pharmacologic treatment of the fetus. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s Neonatal-Perinatal Medicine. Diseases of the Fetus and Infant, ed 8, Philadelphia: Mosby, 2006.
Steding G. The Anatomy of the Human Embryo. A Scanning Electron-Microscopic Atlas. Basel: Karger; 2009.
Streeter GL. Weight, sitting height, head size, foot length and menstrual age of the human embryo. Contrib Embryol Carnegie Inst. 1920;11:143.
Whitworth M, Bricker L, Neilson JP, et al: Ultrasound for fetal assessment in early pregnancy, Cochrane Database Syst Rev 4: CD007058, 2010.
Zhang J, Merialdi M, Platt LD, et al. Defining normal and abnormal fetal growth: promises and challenges. Am Obstet Gynecol. 2010;202:522.