Chapter 9 Pharyngeal Apparatus, Face, and Neck
The pharyngeal apparatus consists of pharyngeal arches, pouches, grooves, and membranes (Fig. 9-1). These early embryonic structures contribute to the formation of the face and neck.
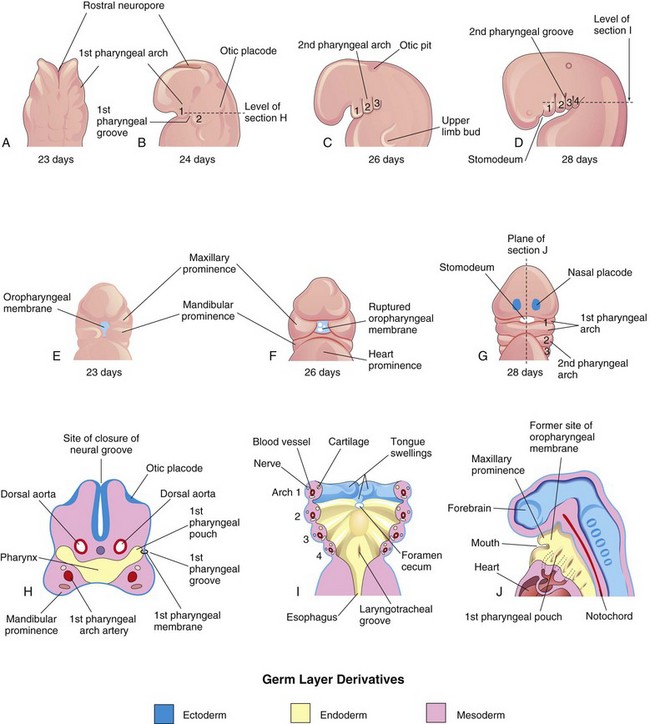
FIGURE 9–1 Illustrations of the human pharyngeal apparatus. A, Dorsal view of the upper part of a 23-day embryo. B to D, Lateral views showing later development of the pharyngeal arches. E to G, Ventral or facial views illustrating the relationship of the first pharyngeal arch to the stomodeum. H, Horizontal section through the cranial region of an embryo. I, Similar section illustrating the pharyngeal arch components and the floor of the primordial pharynx. J, Sagittal section of the cranial region of an embryo, illustrating the openings of the pharyngeal pouches in the lateral wall of the primordial pharynx.
 Pharyngeal Arches
Pharyngeal Arches
The pharyngeal arches begin to develop early in the fourth week as neural crest cells migrate into the future head and neck regions (see Fig. 5-5, Chapter 5). Sonic hedgehog signaling plays an important role in the formation of the first pharyngeal arches. The first pair of pharyngeal arches, the primordium of the jaws, appears as surface elevations lateral to the developing pharynx (Fig. 9-1A and B). Soon other arches appear as obliquely disposed, rounded ridges on each side of the future head and neck regions (Fig. 9-1C and D). By the end of the fourth week, four pairs of pharyngeal arches are visible externally (Fig. 9-1D). The fifth and sixth arches are rudimentary and are not visible on the surface of the embryo. The pharyngeal arches are separated from each other by the pharyngeal grooves (clefts). Like the pharyngeal arches, the grooves are numbered in a craniocaudal sequence.
The first pharyngeal arch separates into two prominences, the maxillary and mandibular prominences (Figs. 9-1E and 9-2):
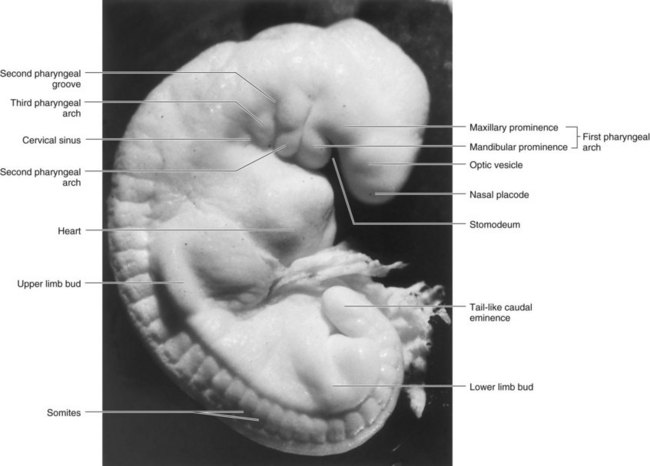
FIGURE 9–2 Photograph of a stage 13, 4-1/2-week human embryo.
(Courtesy of the late Professor Emeritus Dr. K.V. Hinrichsen, Medizinische Fakultät, Institut für Anatomie, Ruhr-Universität Bochum, Bochum, Germany.)
The second pharyngeal arch (hyoid arch) contributes, along with parts of the third and fourth arches, to the formation of the hyoid bone.
The pharyngeal arches support the lateral walls of the primordial pharynx, which is derived from the cranial part of the foregut. The stomodeum (primordial mouth) initially appears as a slight depression of the surface ectoderm (Fig. 9-1D and E). It is separated from the cavity of the primordial pharynx by a bilaminar membrane—the oropharyngeal membrane, which is composed of ectoderm externally and endoderm internally. The oropharyngeal membrane ruptures at approximately 26 days, bringing the pharynx and foregut into communication with the amniotic cavity (Fig. 9-1F and G). The ectodermal lining of the first arch gives rise to the oral epithelium.
Pharyngeal Arch Components
Each pharyngeal arch consists of a core of mesenchyme (embryonic connective tissue) and is covered externally by ectoderm and internally by endoderm (Fig. 9-1H and I). Originally, this mesenchyme is derived during the third week from mesoderm. During the fourth week, most of the mesenchyme is derived from neural crest cells that migrate into the pharyngeal arches. It is the migration of neural crest cells into the arches and their differentiation into mesenchyme that produces the maxillary and mandibular prominences (Fig. 9-2), in addition to all connective tissue including the dermis and smooth muscle.
Coincident with immigration of neural crest cells, myogenic mesoderm from paraxial regions moves into each pharyngeal arch, forming a central core of muscle primordium. Endothelial cells in the arches are derived from both the lateral mesoderm and the invasive angioblasts (cells taking part in blood vessel formation) that move into the arches. The pharyngeal endoderm plays an essential role in regulating the development of the pharyngeal arches.
A typical pharyngeal arch contains:
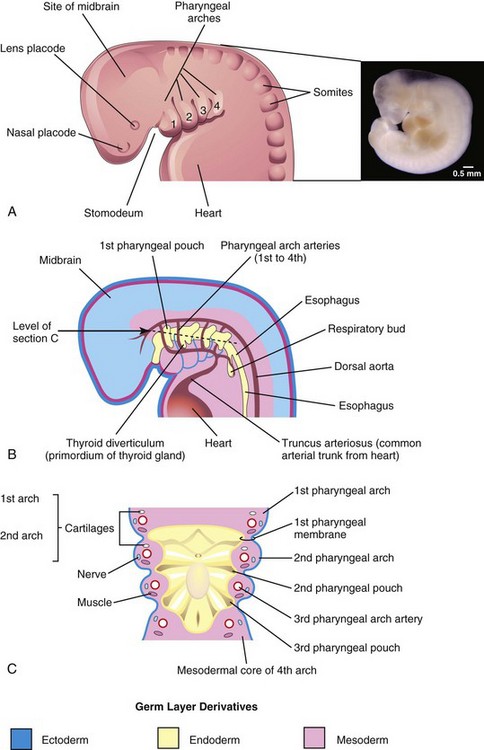
FIGURE 9–3 A, Drawing of the head, neck, and thoracic regions of a human embryo (approximately 28 days), illustrating the pharyngeal apparatus. Inset, Photograph of a human embryo approximately the same age as A. B, Schematic drawing showing the pharyngeal pouches and pharyngeal arch arteries. C, Horizontal section through the embryo showing the floor of the primordial pharynx and illustrating the germ layer of origin of the pharyngeal arch components.
( Inset, Courtesy of Dr. Bradley R. Smith, University of Michigan, Ann Arbor, MI.)
The nerves that grow into the arches are derived from neuroectoderm of the primordial brain.
Fate of Pharyngeal Arches
The pharyngeal arches contribute extensively to the formation of the face, nasal cavities, mouth, larynx, pharynx, and neck (Figs. 9-3 and 9-4). During the fifth week, the second pharyngeal arch enlarges and overgrows the third and fourth arches, forming an ectodermal depression—the cervical sinus (Figs. 9-2 and 9-4A to D). By the end of the seventh week, the second to fourth pharyngeal grooves and the cervical sinus have disappeared, giving the neck a smooth contour.
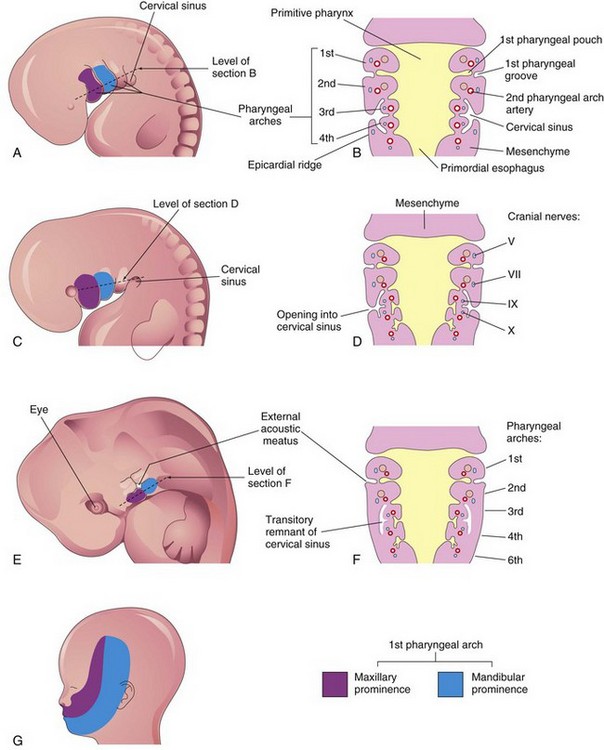
FIGURE 9–4 A, Lateral view of the head, neck, and thoracic regions of an embryo (approximately 32 days), showing the pharyngeal arches and cervical sinus. B, Diagrammatic section through the embryo at the level shown in A, illustrating the growth of the second arch over the third and fourth arches. C, An embryo of approximately 33 days. D, Section of the embryo at the level shown in C, illustrating the early closure of the cervical sinus. E, An embryo of approximately 41 days. F, Section of the embryo at the level indicated in E, showing the transitory cystic remnant of the cervical sinus. G, Drawing of a 20-week fetus illustrating the area of the face derived from the first pair of pharyngeal arches.
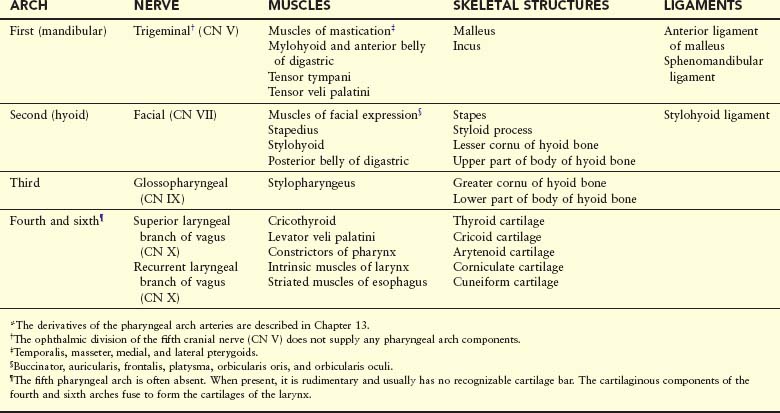
Derivatives of Pharyngeal Arch Cartilages
The dorsal end of the first pharyngeal arch cartilage (Meckel cartilage) is closely related to the developing ear. Early in development, small nodules break away from the proximal part of this cartilage and form two of the middle ear bones, the malleus and incus (Fig. 9-5, Table 9-1). The middle part of the cartilage regresses, but its perichondrium forms the anterior ligament of malleus and the sphenomandibular ligament.

FIGURE 9–5 A, Schematic lateral view of the head, neck, and thoracic regions of a 4-week embryo, illustrating the location of the cartilages in the pharyngeal arches. B, Similar view of a 24-week fetus illustrating the derivatives of the pharyngeal arch cartilages. The mandible is formed by intramembranous ossification of mesenchymal tissue surrounding the first arch cartilage. This cartilage acts as a template for development of the mandible, but does not contribute directly to its formation. Occasionally ossification of the second pharyngeal arch cartilage may extend from the styloid process along the stylohyoid ligament. When this occurs, it may cause pain in the region of the palatine tonsil.
Ventral parts of the first arch cartilages form the horseshoe-shaped primordium of the mandible and, by keeping pace with its growth, guide its early morphogenesis. Each half of the mandible forms lateral to and in close association with its cartilage. The cartilage disappears as the mandible develops around it by intramembranous ossification (Fig. 9-5B). Multiple signaling involving expression of homeobox genes (BMPs, Prxl and Prx2) and fibroblast growth factors (FGFs) regulate the morphogenesis of the mandible.
An independent cartilage anlage (primordium), near the dorsal end of the second pharyngeal arch cartilage (Reichert cartilage), is closely related to the developing ear. It ossifies to form the stapes of the middle ear and the styloid process of the temporal bone (Fig. 9-5B). The part of cartilage between the styloid process and hyoid bone regresses; its perichondrium (connective tissue membrane) forms the stylohyoid ligament. The ventral end of the second arch cartilage ossifies to form the lesser cornu (Latin, horn) and the superior part of the body of the hyoid bone (Fig. 9-5B).
The third pharyngeal arch cartilage, located in the ventral part of the arch, ossifies to form the greater cornu and the inferior part of the body of the hyoid bone.
The fourth and sixth pharyngeal arch cartilages fuse to form the laryngeal cartilages (Fig. 9-5B, Table 9-1), except for the epiglottis. The cartilage of the epiglottis develops from mesenchyme in the hypopharyngeal eminence (see Fig. 9-24A), a prominence in the floor of the embryonic pharynx that is derived from the third and fourth pharyngeal arches.
The fifth pharyngeal arch is rudimentary (if present) and has no derivatives.
Derivatives of Pharyngeal Arch Muscles
The muscular components of the arches derived from unsegmented paraxial mesoderm and the prechordal plate form various muscles in the head and neck. The musculature of the first pharyngeal arch forms the muscles of mastication and other muscles (Fig. 9-6, Table 9-1). The musculature of the second pharyngeal arch forms the stapedius, stylohyoid, posterior belly of digastric, auricular, and muscles of facial expression. The musculature of the third pharyngeal arch forms the stylopharyngeus. The musculature of the fourth pharyngeal arch forms the cricothyroid, levator veli palatini, and constrictors of the pharynx. The musculature of the sixth pharyngeal arch forms the intrinsic muscles of the larynx.
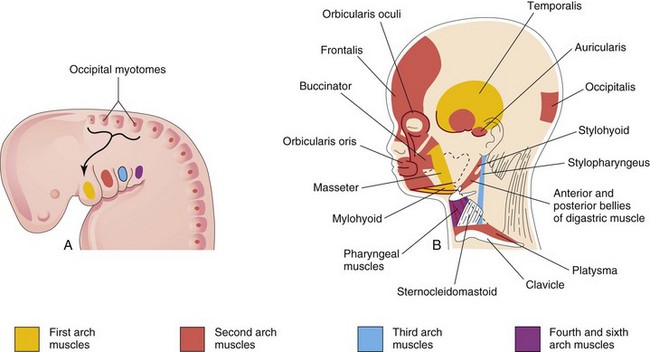
FIGURE 9–6 A, Lateral view of the head, neck, and thoracic regions of a 4-week embryo showing the muscles derived from the pharyngeal arches. The arrow shows the pathway taken by myoblasts from the occipital myotomes to form the tongue musculature. B, Sketch of the head and neck regions of a 20-week fetus, dissected to show the muscles derived from the pharyngeal arches. Parts of the platysma and sternocleidomastoid muscles have been removed to show the deeper muscles. The myoblasts from the second pharyngeal arch migrate from the neck to the head, where they give rise to the muscles of facial expression. These muscles are supplied by the facial nerve (cranial nerve VII), the nerve of the second pharyngeal arch.
Derivatives of Pharyngeal Arch Nerves
Each arch is supplied by its own cranial nerve (CN). The special visceral efferent (branchial) components of the CNs supply muscles derived from the pharyngeal arches (Fig. 9-7, Table 9-1). Because mesenchyme from the pharyngeal arches contributes to the dermis and mucous membranes of the head and neck, these areas are supplied with special visceral afferent nerves.
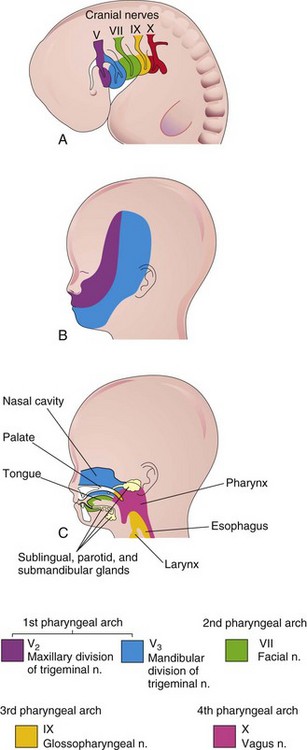
FIGURE 9–7 A, Lateral view of the head, neck, and thoracic regions of a 4-week embryo showing the cranial nerves supplying the pharyngeal arches. B, Sketch of the head and neck regions of a 20-week fetus showing the superficial distribution of the two caudal branches of the first pharyngeal arch nerve (cranial nerve V). C, Sagittal section of the fetal head and neck showing the deep distribution of sensory fibers of the nerves to the teeth and mucosa of the tongue, pharynx, nasal cavity, palate, and larynx.
The facial skin is supplied by the CN V—the trigeminal nerve. However, only its caudal two branches (maxillary and mandibular) supply derivatives of the first pharyngeal arch (Fig. 9-7B). CN V is the principal sensory nerve of the head and neck and is the motor nerve for the muscles of mastication (see Table 9-1). Its sensory branches innervate the face, teeth, and mucous membranes of the nasal cavities, palate, mouth, and tongue (Fig. 9-7C).
CN VII—the facial nerve, CN IX—the glossopharyngeal nerve, and CN X—the vagus nerve—supply the second, third, and caudal (fourth to sixth) arches, respectively. The fourth arch is supplied by the superior laryngeal branch of the vagus (CN X) and by its recurrent laryngeal branch. The nerves of the second to sixth pharyngeal arches have little cutaneous distribution (Fig. 9-7C); however, they innervate the mucous membranes of the tongue, pharynx, and larynx.
 Pharyngeal Pouches
Pharyngeal Pouches
The primordial pharynx, derived from the foregut, widens cranially where it joins the stomodeum (Figs. 9-3A and B and 9-4B), and narrows as it joins the esophagus. The endoderm of the pharynx lines the internal aspects of the pharyngeal arches and passes into the pharyngeal pouches (Figs. 9-1H to J and 9-3B and C). The pouches develop in a craniocaudal sequence between the arches. The first pair of pouches, for example, lies between the first and second pharyngeal arches. There are four well-defined pairs of pharyngeal pouches; the fifth pair is rudimentary or absent. The endoderm of the pouches contacts the ectoderm of the pharyngeal grooves and together they form the double-layered pharyngeal membranes that separate the pharyngeal pouches from the pharyngeal grooves (Figs. 9-1H and 9-3C).
 Derivatives of Pharyngeal Pouches
Derivatives of Pharyngeal Pouches
The endodermal epithelial lining of the pharyngeal pouches gives rise to important organs in the head and neck.
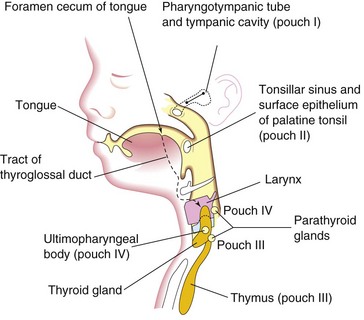
First Pharyngeal Pouch
The first pharyngeal pouch expands into an elongate tubotympanic recess (Fig. 9-8B). The expanded distal part of this recess contacts the first pharyngeal groove, where it later contributes to the formation of the tympanic membrane (eardrum). The cavity of the tubotympanic recess becomes the tympanic cavity and mastoid antrum. The connection of the tubotympanic recess with the pharynx gradually elongates to form the pharyngotympanic tube (auditory tube).
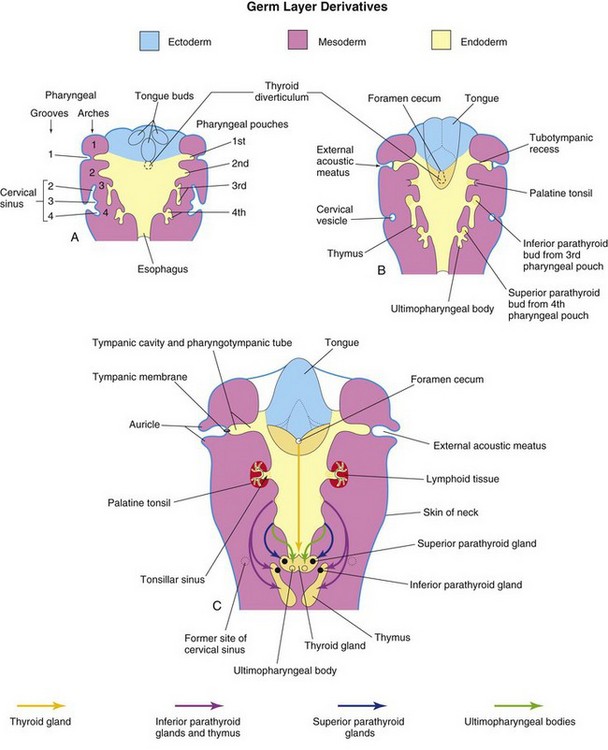
FIGURE 9–8 Schematic horizontal sections at the level shown in Figure 9-4A, illustrating the adult derivatives of the pharyngeal pouches. A, At 5 weeks. Note that the second pharyngeal arch grows over the third and fourth arches, burying the second to fourth pharyngeal grooves in the cervical sinus. B, At 6 weeks. C, At 7 weeks. Note the migration of the developing thymus, parathyroid, and thyroid glands into the neck.
Second Pharyngeal Pouch
Although the second pharyngeal pouch is largely obliterated as the palatine tonsil develops, part of the cavity of this pouch remains as the tonsillar sinus (fossa), the depression between the palatoglossal and palatopharyngeal arches (Figs. 9-8C and 9-9). The endoderm of the second pouch proliferates and grows into the underlying mesenchyme. The central parts of these buds break down, forming tonsillar crypts (pit-like depressions). The pouch endoderm forms the surface epithelium and the lining of the tonsillar crypts. At approximately 20 weeks, the mesenchyme around the crypts differentiates into lymphoid tissue, which soon organizes into the lymphatic nodules of the palatine tonsil.
Third Pharyngeal Pouch
The third pharyngeal pouch expands and develops a solid, dorsal bulbar part and a hollow, elongate ventral part (Fig. 9-8B). Its connection with the pharynx is reduced to a narrow duct that soon degenerates. By the sixth week, the epithelium of each dorsal bulbar part of the pouch begins to differentiate into an inferior parathyroid gland. The epithelium of the elongate ventral parts of the pouch proliferates, obliterating their cavities. These come together in the median plane to form the thymus. The bilobed structure of this lymphatic organ remains throughout life, discretely encapsulated; each lobe has its own blood supply, lymphatic drainage, and nerve supply. The developing thymus and inferior parathyroid glands lose their connections with the pharynx when the brain and associated structures expand rostrally while the pharynx and cardiac structures generally expand caudally. The derivatives of pharyngeal pouches two to four become displaced caudally. Later, the parathyroid glands separate from the thymus and lie on the dorsal surface of the thyroid gland (Figs. 9-8C and 9-9). The FGF signaling pathways, acting through FRS2-α, are involved in the development of the thymus and parathyroid glands.
Histogenesis of Thymus
This primary lymphoid organ develops from epithelial cells derived from endoderm of the third pair of pharyngeal pouches and from mesenchyme into which epithelial tubes grow. The tubes soon become solid cords that proliferate and give rise to side branches. Each side branch becomes the core of a lobule of the thymus. Some cells of the epithelial cords become arranged around a central point, forming small groups of cells—the thymic corpuscles (Hassall corpuscles). Other cells of the epithelial cords spread apart but retain connections with each other to form an epithelial reticulum. The mesenchyme between the epithelial cords forms thin incomplete septa between the lobules. Lymphocytes soon appear and fill the interstices between the epithelial cells. The lymphocytes are derived from hematopoietic stem cells. The thymic primordium is surrounded by a thin layer of mesenchyme that is essential for its development. Neural crest cells also contribute to thymic organogenesis.
Growth and development of the thymus are not complete at birth. It is a relatively large organ during the perinatal period and may extend through the superior thoracic aperture at the root of the neck. As puberty is reached, the thymus begins to diminish in relative size (i.e., undergoes involution). By adulthood, it is often scarcely recognizable because of fat infiltrating the cortex of the gland; however, it is still functional and important for the maintenance of health. In addition to secreting thymic hormones, the thymus primes thymocytes (T-cell precursors) before releasing them to the periphery.
Fourth Pharyngeal Pouch
The fourth pharyngeal pouch expands into dorsal bulbar and elongate ventral parts (Figs. 9-8 and 9-9). Its connection with the pharynx is reduced to a narrow duct that soon degenerates. By the sixth week, each dorsal part develops into a superior parathyroid gland, which lies on the dorsal surface of the thyroid gland. Because the parathyroid glands derived from the third pouches accompany the thymus, they are in a more inferior position than the parathyroid glands derived from the fourth pouches (Fig. 9-9).
Histogenesis of Parathyroid and Thyroid Glands
The epithelium of the dorsal parts of the third and fourth pouches proliferates during the fifth week and forms small nodules on the dorsal aspect of each pouch. Vascular mesenchyme soon grows into these nodules, forming a capillary network. The chief or principal cells differentiate during the embryonic period and are believed to become functionally active in regulating fetal calcium metabolism. The oxyphil cells differentiate 5 to 7 years after birth.
The elongated ventral part of each fourth pouch develops into an ultimopharyngeal body, which fuses with the thyroid gland. Its cells disseminate within the thyroid, giving rise to the parafollicular cells. These cells are also called C cells to indicate that they produce calcitonin, a hormone involved in the regulation of calcium levels. C cells differentiate from neural crest cells that migrate from the pharyngeal arches into the fourth pair of pharyngeal pouches. Recent findings suggest that the basic helix-loop-helix (bHLH) transcription factor Mash1 regulates the differentiation of C cells.
 Pharyngeal Grooves
Pharyngeal Grooves
The head and neck regions of the human embryo exhibit four pharyngeal grooves (clefts) on each side during the fourth and fifth weeks (Figs. 9-1B to D and 9-2). These grooves separate the pharyngeal arches externally. Only one pair of grooves contributes to postnatal structures; the first pair persists as the external acoustic meatus or ear canals (Fig. 9-8C). The other grooves lie in a slit-like depression—the cervical sinus—and are normally obliterated along with the sinus as the neck develops (Fig. 9-4B, D, and F).
 Pharyngeal Membranes
Pharyngeal Membranes
The pharyngeal membranes appear in the floors of the pharyngeal grooves (Figs. 9-1H and 9-3C). These membranes form where the epithelia of the grooves and pouches approach each other. The endoderm of the pouches and the ectoderm of the grooves are soon infiltrated and separated by mesenchyme. Only one pair of membranes contributes to the formation of adult structures; the first pharyngeal membrane, along with the intervening layer of mesenchyme, becomes the tympanic membrane (Fig. 9-8C).
Cervical (Branchial) Sinuses
External cervical (branchial) sinuses are uncommon and almost all result from failure of the second pharyngeal groove and the cervical sinus to obliterate (Figs. 9-10D and 9-11A and B). The sinus typically opens along the anterior border of the sternocleidomastoid muscle in the inferior third of the neck. Anomalies of the other pharyngeal grooves occur in approximately 5% of neonates. External branchial sinuses are commonly detected during infancy because of the discharge of mucous material from them (Fig. 9-11A). These external cervical sinuses are bilateral in approximately 10% of affected neonates and are commonly associated with auricular sinuses.
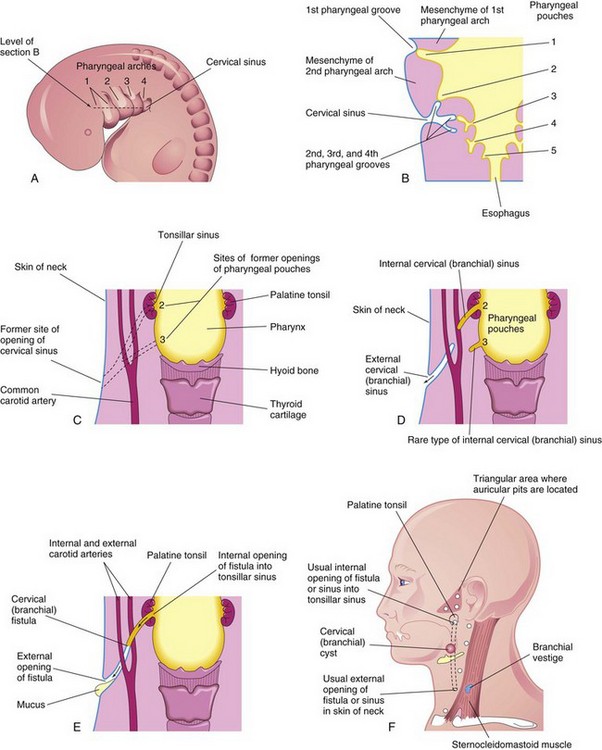
FIGURE 9–10 A, Lateral view of the head, neck, and thoracic regions of a 5-week embryo showing the cervical sinus that is normally present at this stage. B, Horizontal section of the embryo, at the level shown in A, illustrating the relationship of the cervical sinus to the pharyngeal arches and pouches. C, Diagrammatic sketch of the adult pharyngeal and neck regions indicating the former sites of openings of the cervical sinus and pharyngeal pouches. The broken lines indicate possible courses of cervical fistulas. D, Similar sketch showing the embryologic basis of various types of cervical sinus. E, Drawing of a cervical fistula resulting from persistence of parts of the second pharyngeal groove and second pharyngeal pouch. F, Sketch showing possible sites of cervical cysts and the openings of cervical sinuses and fistulas. A branchial vestige is also illustrated (also Fig. 9-14).
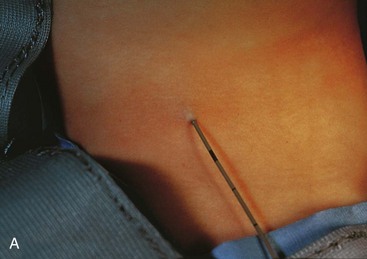
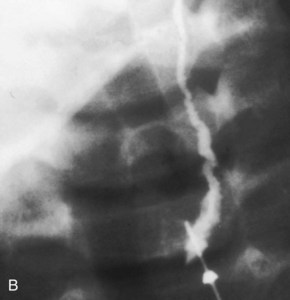
FIGURE 9–11 A, A child’s neck showing a catheter inserted into the external opening of a cervical (branchial) sinus. The catheter allows definition of the length of the tract, which facilitates surgical excision. B, A fistulogram of a complete cervical (branchial) fistula. The radiograph was taken after injection of a contrast medium showing the course of the fistula through the neck.
(Courtesy of Dr. Pierre Soucy, Division of Paediatric Surgery, Children’s Hospital of Eastern Ontario, Ottawa, Ontario, Canada.)
Internal cervical (branchial) sinuses open into the tonsillar sinus or near the palatopharyngeal arch (Fig. 9-10D and F ). These sinuses are rare. Almost all these sinuses result from persistence of the proximal part of the second pharyngeal pouch. Normally this pouch disappears as the palatine tonsil develops; its normal remnant is the tonsillar sinus.
Cervical (Branchial) Fistula
An abnormal canal that opens internally into the tonsillar sinus and externally in the side of the neck is a cervical fistula. This canal results from persistence of parts of the second pharyngeal groove and second pharyngeal pouch (see Figs. 9-10E and F and 9-11B). The fistula ascends from its opening in the neck through the subcutaneous tissue and platysma muscle to reach the carotid sheath. The fistula then passes between the internal and external carotid arteries and opens into the tonsillar sinus.
Piriform Sinus Fistula
A piriform sinus fistula is thought to result from the persistence of remnants of the ultimopharyngeal body along its path to the thyroid gland (Fig. 9-8C ).
Cervical (Branchial) Cysts
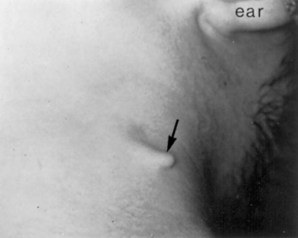
Remnants of parts of the cervical sinus and/or the second pharyngeal groove may persist and form a spherical or elongate cyst (Fig. 9-10F ). Although they may be associated with cervical sinuses and drain through them, cervical cysts often lie free in the neck just inferior to the angle of the mandible. However, they can develop anywhere along the anterior border of the sternocleidomastoid muscle. Cervical cysts do not usually become apparent until late childhood or early adulthood, when they produce a slowly enlarging, painless swelling in the neck (Fig. 9-12). The cysts enlarge because of the accumulation of fluid and cellular debris derived from desquamation of their epithelial linings (Fig. 9-13). Cervical cysts have also been observed in the parathyroid glands.
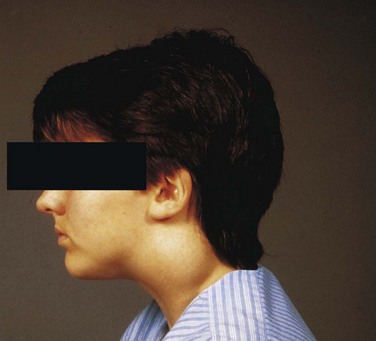
FIGURE 9–12 A swelling in a boy’s neck produced by a cervical cyst. These large cysts often lie free in the neck just inferior to the angle of the mandible, but they may develop anywhere along the anterior border of the sternocleidomastoid muscle as in this case.
(Courtesy of Dr. Pierre Soucy, Division of Paediatric Surgery, Children’s Hospital of Eastern Ontario. Ottawa, Ontario, Canada.)
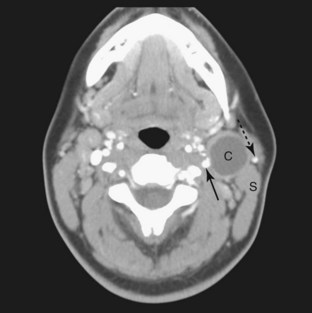
FIGURE 9–13 This is a computed tomography (CT) image of the neck region of a 24-year-old woman who presented with a 2-month history of a “lump” in the neck. The low-density cervical cyst, (C) is anterior to the sternocleidomastoid muscle (S). Note the external carotid artery (arrow) and the external jugular vein (dotted arrow).
(Courtesy of Dr. Gerald S. Smyser, Altru Health System, Grand Forks, ND.)
Branchial Vestiges
Normally the pharyngeal cartilages disappear, except for parts that form ligaments or bones; however, in unusual cases, cartilaginous or bony remnants of pharyngeal arch cartilages appear under the skin in the side of the neck (Fig. 9-14). These are usually found anterior to the inferior third of the sternocleidomastoid muscle (Fig. 9-10F ).
First Pharyngeal Arch Syndrome
Abnormal development of the components of the first pharyngeal arch results in various congenital anomalies of the eyes, ears, mandible, and palate that together constitute the first pharyngeal arch syndrome (Fig. 9-15). This syndrome is believed to result from insufficient migration of neural crest cells into the first arch during the fourth week. There are two main manifestations of the first pharnygeal arch syndrome:
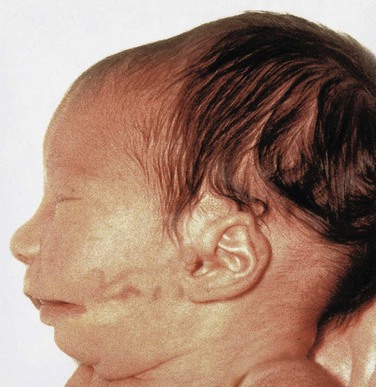
FIGURE 9–15 An infant with first arch syndrome, a pattern of anomalies resulting from insufficient migration of neural crest cells into the first pharyngeal arch. Note the deformed auricle, the preauricular appendage, the defect in the cheek between the auricle and the mouth, hypoplasia of the mandible, and macrostomia (large mouth).
DiGeorge Syndrome
Infants with DiGeorge syndrome are born without a thymus and parathyroid glands and have defects in their cardiac outflow tracts. In some cases, ectopic glandular tissue has been found (Fig. 9-16). Clinically, the disease is characterized by congenital hypoparathyroidism, increased susceptibility to infections (from immune deficiency, specifically defective T-cell function), anomalies of the mouth (shortened philtrum of upper lip [fish-mouth deformity]), low-set notched ears, nasal clefts, thyroid hypoplasia, and cardiac abnormalities (defects of the arch of the aorta and heart).
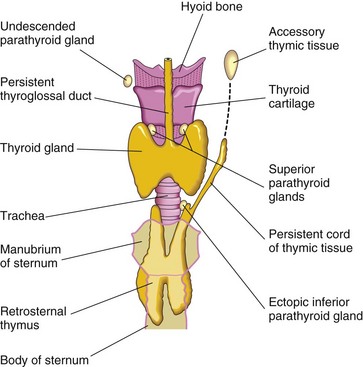
FIGURE 9–16 Anterior view of the thyroid gland, thymus, and parathyroid glands, illustrating various congenital anomalies that may occur.
DiGeorge syndrome occurs because the third and fourth pharyngeal pouches fail to differentiate into the thymus and parathyroid glands. This is the result of a breakdown in signaling between pharyngeal endoderm and adjacent neural crest cells. The facial abnormalities result primarily from abnormal development of the first arch components because neural crest cells are disrupted, and the cardiac anomalies arise in those sites normally occupied by neural crest cells. In most cases of DiGeorge syndrome, there is a microdeletion in the q11.2 region of chromosome 22, HIRA and UFDIL gene mutation, and neural crest cell defects.
Accessory Thymic Tissue
An isolated mass of thymic tissue may persist in the neck, often close to an inferior parathyroid gland (Fig. 9-16). This tissue breaks free from the developing thymus as it shifts caudally in the neck.
Ectopic Parathyroid Glands
The location of the parathyroid glands is highly variable. They may be found anywhere near or within the thyroid gland or thymus. The superior glands are more constant in position than the inferior ones. Occasionally, an inferior parathyroid gland remains near the bifurcation of the common carotid artery. In other cases, it may be in the thorax.
Abnormal Number of Parathyroid Glands
Uncommonly there are more than four parathyroid glands. Supernumerary parathyroid glands probably result from division of the primordia of the original glands. Absence of a parathyroid gland results from failure of one of the primordia to differentiate or from atrophy of a gland early in development.
 Development of Thyroid Gland
Development of Thyroid Gland
The thyroid gland is the first endocrine gland to develop in the embryo. It begins to form, under the influence of FGF signaling pathways, approximately 24 days after fertilization from a median endodermal thickening in the floor of the primordial pharynx. This thickening soon forms a small outpouching—the thyroid primordium (Fig. 9-17A). As the embryo and tongue grow, the developing thyroid gland descends in the neck, passing ventral to the developing hyoid bone and laryngeal cartilages. For a short time, the thyroid gland is connected to the tongue by a narrow tube, the thyroglossal duct (see Fig. 9-17B and C). At first the thyroid primordium is hollow, but it soon becomes a solid mass of cells and divides into right and left lobes that are connected by the isthmus of the thyroid gland (Fig. 9-18), which lies anterior to the developing second and third tracheal rings.
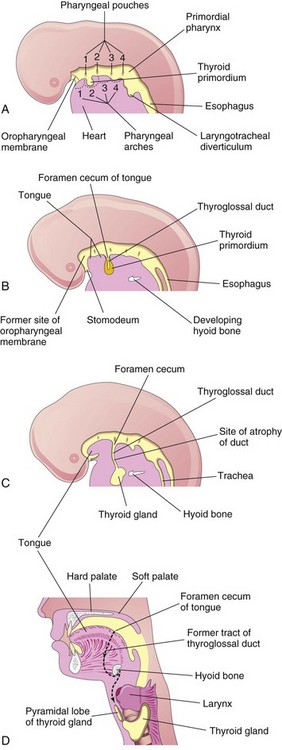
FIGURE 9–17 Development of the thyroid gland. A to C, Schematic sagittal sections of the head and neck regions of embryos at 4, 5, and 6 weeks illustrating successive stages in the development of the thyroid gland. D, Similar section of an adult head and neck showing the path taken by the thyroid gland during its embryonic descent (indicated by the former tract of the thyroglossal duct).
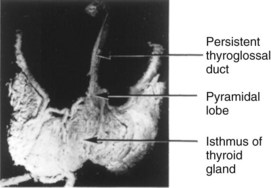
FIGURE 9–18 The anterior surface of a dissected adult thyroid gland showing persistence of the thyroglossal duct. Observe the pyramidal lobe ascending from the superior border of the isthmus. It represents a persistent portion of the inferior end of the thyroglossal duct that has formed thyroid tissue.
By 7 weeks, the thyroid gland has assumed its definitive shape and is usually located in its final site in the neck (Fig. 9-1D). By this time, the thyroglossal duct has normally degenerated and disappeared. The proximal opening of the thyroglossal duct persists as a small pit in the dorsum (posterosuperior surface) of the tongue—the foramen cecum. A pyramidal lobe of the thyroid gland extends superiorly from the isthmus in approximately 50% of people.
Histogenesis of Thyroid Gland
The thyroid primordium consists of a solid mass of endodermal cells. This cellular aggregation later breaks up into a network of epithelial cords as it is invaded by the surrounding vascular mesenchyme. By the 10th week, the cords have divided into small cellular groups. A lumen soon forms in each cell cluster and the cells become arranged in a single layer around thyroid follicles. During the 11th week, colloid begins to appear in these follicles; thereafter, iodine concentration and the synthesis of thyroid hormones can be demonstrated. By 20 weeks, the levels of fetal thyroid-stimulating hormone and thyroxine begin to increase, reaching adult levels by 35 weeks.
Congenital Hypothyroidism
Congenital hypothyroidism is the most common metabolic disorder in neonates. It is a heterogeneous disorder for which several candidate genes, including TSH receptor and thyroid transcription factors (TTF-1, TF-2, and Pax8) have been identified. Congenital hypothyroidism may result in neurodevelopmental disorders and infertility if untreated. An increased incidence of renal and urinary tract anomalies in infants has been reported in association with congenital hypothyroidism.
Thyroglossal Duct Cysts and Sinuses
Cysts may form anywhere along the course of the thyroglossal duct (Fig. 9-19). Usually, the thyroglossal duct atrophies and disappears, but a remnant of it may persist and form a cyst in the tongue or in the anterior part of the neck, usually just inferior to the hyoid bone (Fig. 9-20). Most thyroglossal duct cysts are observed by the age of 5 years. Unless the lesions become infected, most of them are asymptomatic. The swelling produced by a thyroglossal duct cyst usually develops as a painless, progressively enlarging, movable mass (Fig. 9-21). The cyst may contain some thyroid tissue. If infection of a cyst occurs, a perforation of the skin may develop, forming a thyroglossal duct sinus that usually opens in the median plane of the neck, anterior to the laryngeal cartilages (Fig. 9-19A).
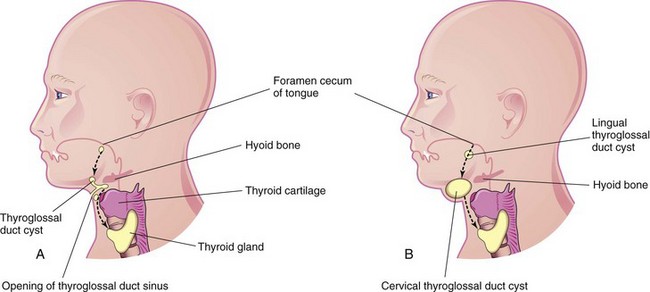
FIGURE 9–19 A, Sketch of the head and neck showing the possible locations of thyroglossal duct cysts. A thyroglossal duct sinus is also illustrated. The broken line indicates the course taken by the thyroglossal duct during descent of the developing thyroid gland from the foramen cecum to its final position in the anterior part of the neck. B, Similar sketch illustrating lingual and cervical thyroglossal duct cysts. Most thyroglossal duct cysts are located just inferior to the hyoid bone.
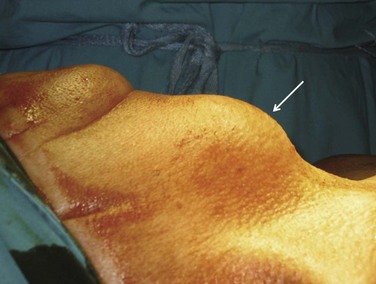
FIGURE 9–20 Large thyroglossal duct cyst (arrow) in a male patient.
(Courtesy of Dr. Srinivasa Ramachandra.)

FIGURE 9–21 Computed tomography images. A, Level of the thyrohyoid membrane and base of the epiglottis. B, Level of thyroid cartilage, which is calcified. The thyroglossal duct cyst extends cranially to the margin of the hyoid bone.
(Courtesy of Dr. Gerald S. Smyser, Altru Health System, Grand Forks, ND.)
Ectopic Thyroid Gland
An ectopic thyroid gland is an infrequent congenital anomaly and is usually located along the course of the thyroglossal duct (Fig. 9-17D). Lingual thyroid tissue is the most common of ectopic thyroid tissues; intralingual thyroid masses are found in as many as 10% of autopsies, although they are clinically relevant in only one in 4000 persons with thyroid disease. Incomplete movement of the thyroid gland results in the ectopic thyroid gland appearing high in the neck (sublingual), at or just inferior to the hyoid bone (Figs. 9-22 and 9-23). Usually, an ectopic sublingual thyroid gland is the only thyroid tissue present. It is clinically important to differentiate an ectopic thyroid gland from a thyroglossal duct cyst or accessory thyroid tissue to prevent inadvertent surgical removal of the thyroid gland. Failure to do so may leave the person permanently dependent on thyroid medication.
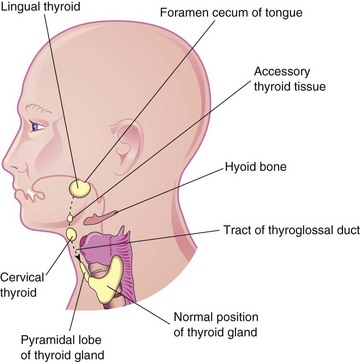
FIGURE 9–22 Sketch of the head and neck showing the usual sites of ectopic thyroid tissue. The broken line indicates the path followed by the thyroid gland during its descent and the former tract of the thyroglossal duct.
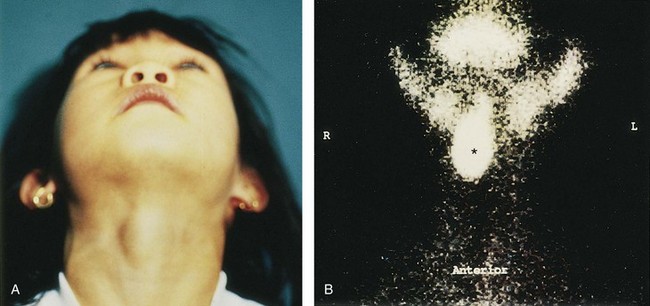
FIGURE 9–23 A, A sublingual thyroid mass in a 5-year-old girl. B, Technetium-99m pertechnetate scan (scintigraphy) showing a sublingual thyroid gland (*) without evidence of functioning thyroid tissue in the anterior part of the neck.
(From Leung AKC, Wong AL, Robson WLLM: Ectopic thyroid gland simulating a thyroglossal duct cyst. Can J Surg 38:87, 1995.)
 Development of Tongue
Development of Tongue
Near the end of the fourth week, a median triangular elevation appears in the floor of the primordial pharynx, just rostral to the foramen cecum (Fig. 9-24A). This median lingual swelling (tongue bud) is the first indication of tongue development. Soon, two oval lateral lingual swellings (distal tongue buds) develop on each side of the median tongue bud. The three lingual swellings result from the proliferation of mesenchyme in ventromedial parts of the first pair of pharyngeal arches. The lateral lingual swellings rapidly increase in size, merge with each other, and overgrow the median lingual swelling. The merged lateral lingual swellings form the anterior two thirds (oral part) of the tongue (Fig. 9-24C). The fusion site of the lateral lingual swellings is indicated by the midline groove of the tongue and internally by the fibrous lingual septum. The median lingual swelling does not form a recognizable part of the adult tongue.
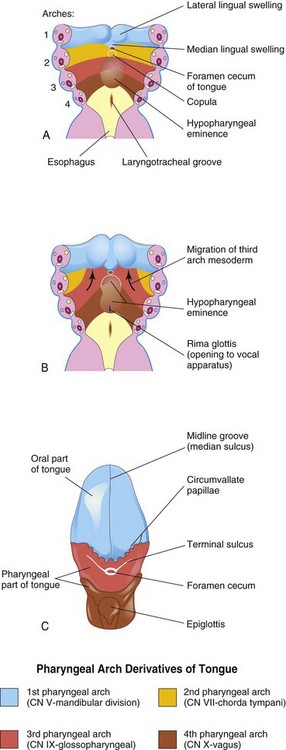
FIGURE 9–24 A and B, Schematic horizontal sections through the pharynx at the level shown in Figure 9-4A showing successive stages in the development of the tongue during the fourth and fifth weeks. C, Drawing of the adult tongue showing the pharyngeal arch derivation of the nerve supply of its mucosa.
Formation of the posterior third (pharyngeal part) of the tongue is indicated in the fetus by two elevations that develop caudal to the foramen cecum (Fig. 9-24A):
As the tongue develops, the copula is gradually overgrown by the hypopharyngeal eminence and disappears (see Fig. 9-24B and C). As a result, the posterior third of the tongue develops from the rostral part of the hypopharyngeal eminence.
The line of fusion of the anterior and posterior parts of the tongue is roughly indicated by a V-shaped groove—the terminal sulcus (see Fig. 9-24C). Pharyngeal arch mesenchyme forms the connective tissue and vasculature of the tongue. Most of the tongue muscles are derived from myoblasts that migrate from the occipital myotomes (Fig. 9-6A). The hypoglossal nerve (CN XII) accompanies the myoblasts during their migration and innervates the tongue muscles as they develop. Both anterior and posterior parts of the tongue are located within the oral cavity at birth; the posterior third of the tongue descends into the oropharynx by 4 years of age.
Lingual Papillae and Taste Buds
Lingual papillae appear toward the end of the eighth week. The vallate and foliate papillae appear first, close to terminal branches of the glossopharyngeal nerve (CN IX). The fungiform papillae appear later near terminations of the chorda tympani branch of the facial nerve (CN VII). The long and numerous lingual papillae are called filiform papillae because of their thread-like shape. They develop during the early fetal period (10–11 weeks). They contain afferent nerve endings that are sensitive to touch.
Taste buds develop during weeks 11 to 13 by inductive interaction between the epithelial cells of the tongue and invading gustatory nerve cells from the chorda tympani, glossopharyngeal, and vagus nerves. Most taste buds form on the dorsal surface of the tongue, and some develop on the palatoglossal arches, palate, posterior surface of the epiglottis, and the posterior wall of the oropharynx. Fetal facial responses can be induced by bitter-tasting substances at 26 to 28 weeks, indicating that reflex pathways between taste buds and facial muscles are established by this stage.
Nerve Supply of Tongue
The development of the tongue explains its nerve supply. The sensory supply to the mucosa of almost the entire anterior two thirds of the tongue is from the lingual branch of the mandibular division of the trigeminal nerve (CN V), the nerve of the first pharyngeal arch. This arch forms the median and lateral lingual swellings (Fig. 9-24). Although the facial nerve is the nerve of the second pharyngeal arch, its chorda tympani branch supplies the taste buds in the anterior two thirds of the tongue, except for the vallate papillae. Because the second arch component, the copula, is overgrown by the third arch, the facial nerve (CN VII) does not supply any of the tongue mucosa, except for the taste buds in the anterior part of the tongue. The vallate papillae in the anterior part of the tongue are innervated by the glossopharyngeal nerve (CN IX) of the third pharyngeal arch (see Fig. 9-24C). The reason usually given for this is that the mucosa of the posterior third of the tongue is pulled slightly anteriorly as the tongue develops.
The posterior third of the tongue is innervated mainly by the glossopharyngeal nerve of the third pharyngeal arch. The superior laryngeal branch of the vagus nerve (CN X) of the fourth arch supplies a small area of the tongue anterior to the epiglottis (Fig. 9-24C). All muscles of the tongue are supplied by the hypoglossal nerve (CN XII), except for the palatoglossus, which is supplied from the pharyngeal plexus by fibers arising from the vagus nerve (CN X).
Congenital Anomalies of Tongue
Abnormalities of the tongue are uncommon, except for fissuring of the tongue and hypertrophy of the lingual papillae, which are characteristics of infants with Down syndrome (see Chapter 20).
Congenital Lingual Cysts and Fistulas
Cysts in the tongue may be derived from remnants of the thyroglossal duct (Fig. 9-19). They may enlarge and produce symptoms of pharyngeal discomfort and/or dysphagia (difficulty in swallowing). Fistulas are also derived from persistence of lingual parts of the thyroglossal duct; they open through the foramen cecum into the oral cavity.
Ankyloglossia
The lingual frenulum normally connects the inferior surface of the tongue to the floor of the mouth. Sometimes the frenulum is short and extends to the tip of the tongue (Fig. 9-25). This interferes with its free protrusion and may make breast-feeding difficult. Ankyloglossia (tongue-tie) occurs in approximately 1 in 300 North American infants, but is usually of no permanent functional significance. A short frenulum usually stretches with time, making surgical correction of the anomaly unnecessary.
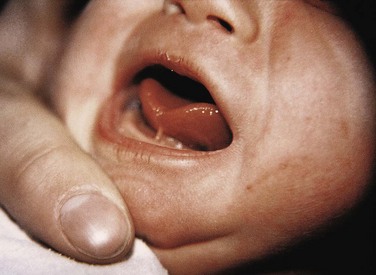
FIGURE 9–25 An infant with ankyloglossia (tongue-tie). Note the short frenulum, which extends to the tip of the tongue. Ankyloglossia interferes with protrusion of the tongue and may make breast-feeding difficult.
(Courtesy of Dr. Evelyn Jain, Lakeview Breastfeeding Clinic, Calgary, Alberta, Canada.)
Macroglossia
An excessively large tongue is not common. It results from generalized hypertrophy of the developing tongue, usually resulting from lymphangioma (a lymph tumor) or muscular hypertrophy. Macroglossia is often seen in individuals with Down syndrome.
Development of Salivary Glands
During the sixth and seventh weeks, the salivary glands, under the influence of the Notch signaling pathway, begin as solid epithelial buds from the primordial oral cavity (Fig. 9-7C). The club-shaped ends of these epithelial buds grow into the underlying mesenchyme. The connective tissue in the glands is derived from neural crest cells. All parenchymal (secretory) tissue arises by proliferation of the oral epithelium.
The parotid glands are the first to appear (early in the sixth week). They develop from buds that arise from the oral ectodermal lining near the angles of the stomodeum. Elongation of the jaws causes lengthening of the parotid duct, with the gland remaining close to its site of origin. Later the elongated buds canalize—develop lumina (canals)—and become ducts by approximately 10 weeks. The rounded ends of the cords differentiate into acini (grape-shaped structures). Secretions commence at 18 weeks. The capsule and connective tissue develop from the surrounding mesenchyme.
The submandibular glands appear late in the sixth week. They develop from endodermal buds in the floor of the stomodeum. Solid cellular processes grow posteriorly, lateral to the developing tongue. Later they branch and differentiate. Acini begin to form at 12 weeks and secretory activity begins at 16 weeks. Growth of the submandibular glands continues after birth with the formation of mucous acini. Lateral to the tongue, a linear groove forms that soon closes over to form the submandibular duct.
The sublingual glands appear in the eighth week, approximately 2 weeks later than the other salivary glands (Fig. 9-7C). They develop from multiple endodermal epithelial buds in the paralingual sulcus. These buds branch and canalize to form 10 to 12 ducts that open independently into the floor of the mouth.
 Development of Face
Development of Face
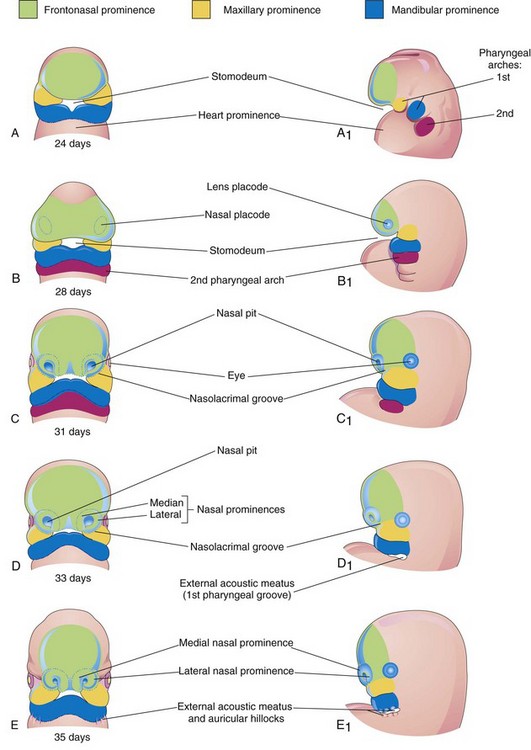
The facial primordia appear early in the fourth week around the stomodeum—the primordium of the mouth (Fig. 9-26A and B). Facial development depends on the inductive influence of the prosencephalic and rhombencephalic organizing centers. The prosencephalic organizing center includes prechordal mesoderm located in the midline rostral to the notochord and overlying the presumptive prosencephalic neural plate (see Chapter 17). The midbrain-hindbrain (rhombencephalon) boundary is a signaling center that directs the spatial organization of the caudal midbrain and the rostral hindbrain structures.
The five facial primordia that appear as prominences around the stomodeum (Fig. 9-26A) follow:
The maxillary and mandibular prominences are derivatives of the first pair of pharyngeal arches. The prominences are produced mainly by the expansion of neural crest populations that originate from the mesencephalic and rostral rhombencephalic neural folds during the fourth week. These cells are the major source of connective tissue components, including cartilage, bone, and ligaments in the facial and oral regions. The frontonasal prominence (FNP) surrounds the ventrolateral part of the forebrain, which gives rise to the optic vesicles that form the eyes (Fig. 9-26C). The frontal part of the FNP forms the forehead; the nasal part forms the rostral boundary of the stomodeum and nose. The maxillary prominences form the lateral boundaries of the stomodeum and the mandibular prominences constitute the caudal boundary of the stomodeum (Fig. 9-27).
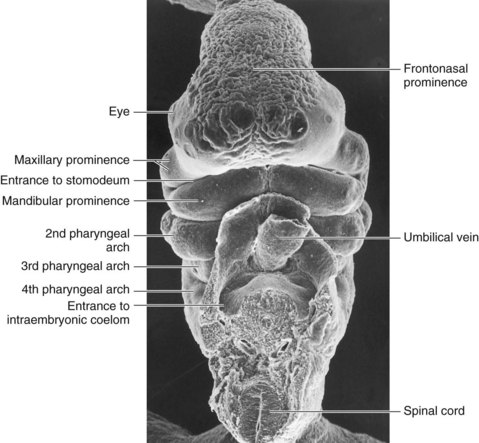
FIGURE 9–27 Scanning electron micrograph of a ventral view of a Carnegie stage 14 embryo (30–32 days).
(Courtesy of the late Professor Emeritus Dr. K.V. Hinrichsen, Medizinische Fakultät, Institut für Anatomie, Ruhr-Universität Bochum, Bochum, Germany.)
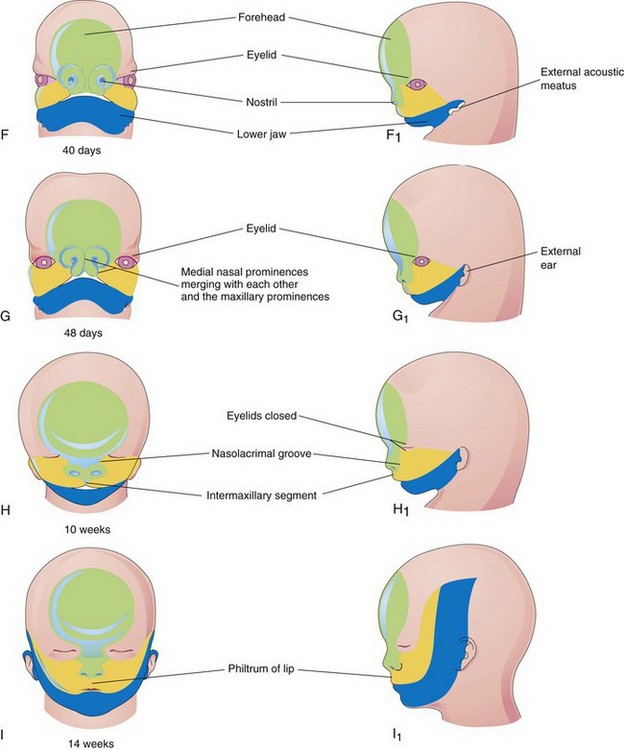
The facial prominences are active centers of growth in the underlying mesenchyme. This embryonic connective tissue is continuous from one prominence to the other. Facial development occurs mainly between the fourth and eighth weeks (Fig. 9-26A to G). By the end of the embryonic period, the face has an unquestionably human appearance. Facial proportions develop during the fetal period (Fig. 9-26H and I). The lower jaw and lower lip are the first parts of the face to form. They result from merging of the medial ends of the mandibular prominences in the median plane.
By the end of the fourth week, bilateral oval thickenings of the surface ectoderm—nasal placodes—the primordia of the nasal epithelium, have developed on the inferolateral parts of the FNP (Figs. 9-28 and 9-29A and B). Initially these placodes are convex, but later they are stretched to produce a flat depression in each placode. Mesenchyme in the margins of the placodes proliferates, producing horseshoe-shaped elevations—the medial and lateral nasal prominences. As a result, the nasal placodes lie in depressions—nasal pits (Fig. 9-29C and D). These pits are the primordia of the anterior nares (nostrils) and nasal cavities (Fig. 9-29E) while lateral nasal prominences form the alae (sides) of the nose.
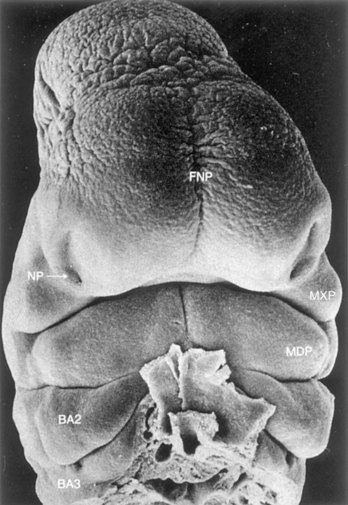
FIGURE 9–28 Scanning electron micrograph of a ventral view of a human embryo of approximately 33 days (Carnegie stage 15, crown–rump length 8 mm). Observe the prominent frontonasal process (FNP) surrounding the telencephalon (forebrain). Also observe the nasal pits (NP) located in the ventrolateral regions of the frontonasal prominence. Medial and lateral nasal prominences surround these pits. The maxillary prominences (MXP) form the lateral boundaries of the stomodeum. The fusing mandibular prominences (MDP) are located just caudal to the stomodeum. The second pharyngeal arch (BA2) is clearly visible and shows overhanging margins (opercula). The third pharyngeal arch (BA3) is also clearly visible.
(From Hinrichsen K: The early development of morphology and patterns of the face in the human embryo. Adv Anat Embryol Cell Biol 98:1, 1985.)
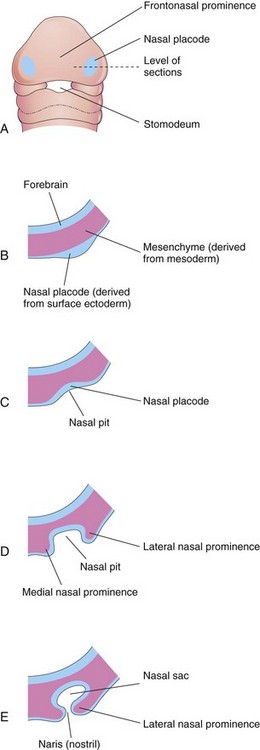
FIGURE 9–29 Progressive stages in the development of a human nasal sac (primordial nasal cavity). A, Ventral view of embryo of approximately 28 days. B to E, Transverse sections through the left side of the developing nasal sac.
Proliferation of mesenchyme in the maxillary prominences causes them to enlarge and grow medially toward each other and the nasal prominences (Figs. 9-26D to G, 9-27, and 9-28). This proliferation-driven expansion results in movement of the medial nasal prominences toward the median plane and each other. Each lateral nasal prominence is separated from the maxillary prominence by a cleft called the nasolacrimal groove (Figs. 9-26C and D).
By the end of the fifth week, the primordia of the auricles (external part of the ears) have begun to develop (Figs. 9-26E and 9-30). Six auricular hillocks (three mesenchymal swellings on each side) form around the first pharyngeal groove, the primordia of the auricle, and the external acoustic meatus, respectively. Initially the external ears are located in the neck region (Fig. 9-31); however, as the mandible develops, they are located on the side of the head at the level of the eyes (Fig. 9-26H).
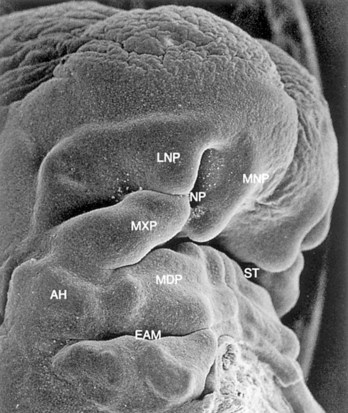
FIGURE 9–30 Scanning electron micrograph of the craniofacial region of a human embryo of approximately 41 days (Carnegie stage 16, crown–rump length 10.8 mm) viewed obliquely. The maxillary prominence (MXP) appears puffed up laterally and wedged between the lateral (LNP) and medial (MNP) nasal prominences surrounding the nasal pit (NP). The auricular hillocks (AH) can be seen on both sides of the pharyngeal groove between the first and second arches, which will form the external acoustic meatus (EAM). MDP, Mandibular prominence; ST, stomodeum.
(From Hinrichsen K: The early development of morphology and patterns of the face in the human embryo. Adv Anat Embryol Cell Biol 98:1, 1985.)
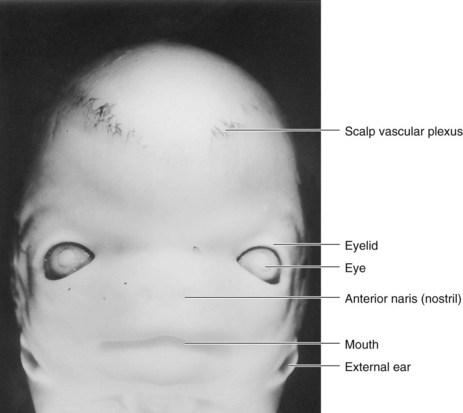
FIGURE 9–31 Ventral view of the face of an embryo at Carnegie stage 22, approximately 54 days. Observe that the eyes are widely separated and the ears are low-set at this stage.
(From Nishimura H, Semba R, Tanimura T, Tanaka O: Prenatal Development of the Human with Special Reference to Craniofacial Structures: An Atlas. Bethesda, MD, U.S. Department of Health, Education, and Welfare, National Institutes of Health, 1977.)
By the end of the sixth week, each maxillary prominence has begun to merge with the lateral nasal prominence along the line of the nasolacrimal groove (Figs. 9-32 and 9-33). This establishes continuity between the side of the nose, formed by the lateral nasal prominence, and the cheek region formed by the maxillary prominence.
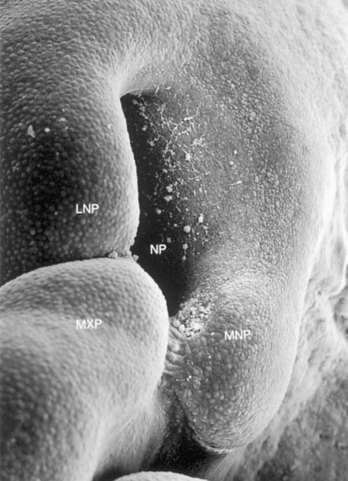
FIGURE 9–32 Scanning electron micrograph of the right nasal region of a human embryo of approximately 41 days (Carnegie stage 17, crown–rump length 10.8 mm) showing the maxillary prominence (MXP) fusing with the medial nasal prominence (MNP). Observe the large nasal pit (NP). Epithelial bridges can be seen between these prominences. Observe the furrow representing the nasolacrimal groove between the MXP and the lateral nasal prominence (LNP).
(From Hinrichsen K: The early development of morphology and patterns of the face in the human embryo. Adv Anat Embryol Cell Biol 98:1, 1985.)
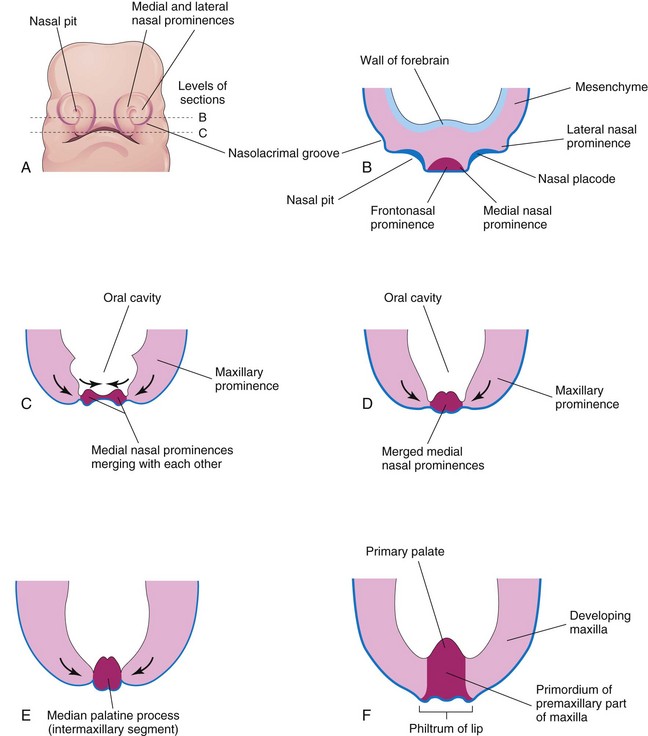
FIGURE 9–33 Early development of the maxilla, palate, and upper lip. A, Facial view of a 5-week embryo. B and C, Sketches of horizontal sections at the levels shown in A. The arrows in C indicate subsequent growth of the maxillary and medial nasal prominences toward the median plane and merging of the prominences with each other. D to F, Similar sections of older embryos illustrating merging of the medial nasal prominences with each other and the maxillary prominences to form the upper lip. Recent studies suggest that the upper lip is formed entirely from the maxillary prominences.
The nasolacrimal duct develops from a rod-like thickening of ectoderm in the floor of the nasolacrimal groove. This thickening gives rise to a solid epithelial cord that separates from the ectoderm and sinks into the mesenchyme. Later, as a result of apoptosis (programmed cell death), this epithelial cord canalizes to form a duct. The superior end of this duct expands to form the lacrimal sac (see Chapter 18). By the late fetal period, the nasolacrimal duct drains into the inferior meatus in the lateral wall of the nasal cavity. The duct becomes completely patent after birth.
Between the seventh and 10th weeks, the medial nasal prominences merge with each other and with the maxillary and lateral nasal prominences (Fig. 9-26G and H). Merging of these prominences requires disintegration of their contacting surface epithelia. This results in intermingling of the underlying mesenchymal cells. Merging of the medial nasal and maxillary prominences results in continuity of the upper jaw and lip and separation of the nasal pits from the stomodeum.
As the medial nasal prominences merge, they form an intermaxillary segment (Figs. 9-26H and 9-33E and F). This segment gives rise to the:
Clinical and embryologic studies indicate that the upper lip is formed entirely from the maxillary prominences. The lower parts of the medial nasal prominences appear to have become deeply positioned and covered by medial extensions of the maxillary prominences to form the philtrum.
In addition to connective tissue and muscular derivatives, various bones are derived from mesenchyme in the facial prominences. Until the end of the sixth week, the primordial jaws are composed of masses of mesenchymal tissue. The lips and gingivae begin to develop when a linear thickening of the ectoderm, the labiogingival lamina, grows into the underlying mesenchyme (see Fig. 9-37B). Gradually, most of the lamina degenerates, leaving a labiogingival groove between the lips and the gingivae (see Fig. 9-37H). A small area of the labiogingival lamina persists in the median plane to form the frenulum of the upper lip, which attaches the lip to the gingiva.
Further development of the face occurs slowly during the fetal period and results mainly from changes in the proportion and relative positions of the facial components. During the early fetal period, the nose is flat and the mandible is underdeveloped (Fig. 9-26H); they obtain their characteristic form as facial development is completed (Fig. 9-26I). As the brain enlarges, the cranial cavity expands bilaterally. This causes the orbits, which were oriented laterally, to assume their forward-facing orientation. The opening of the external acoustic meatus (auditory canal) appears to elevate, but in reality remains stationary. Rather, it is the elongation of the lower jaw that creates this impression.
Summary of Facial Development
Atresia of Nasolacrimal Duct
Part of the nasolacrimal duct occasionally fails to canalize, resulting in congenital atresia of the nasolacrimal duct. Obstruction of this duct with clinical symptoms occurs in approximately 6% of neonates.
Congenital Auricular Sinuses and Cysts
Small auricular sinuses and cysts are usually located in a triangular area of skin anterior to the auricle of the external ear (Fig. 9-10F ); however, they may occur in other sites around the auricle or in the lobule (earlobe). Although some sinuses and cysts are remnants of the first pharyngeal groove, others represent ectodermal folds sequestered during formation of the auricle from six auricular hillocks (nodular masses of mesenchyme from the first and second pharyngeal arches that coalesce to form the auricle). These sinuses and cysts are classified as minor anomalies that are of no serious medical consequence.
 Development of Nasal Cavities
Development of Nasal Cavities
As the face develops, the nasal placodes become depressed, forming nasal pits (Figs. 9-28, 9-29, and 9-32). Proliferation of the surrounding mesenchyme forms the medial and lateral nasal prominences, which results in deepening of the nasal pits and formation of primordial nasal sacs. Each nasal sac grows dorsally, ventral to the developing forebrain (Fig. 9-34A). At first, the sacs are separated from the oral cavity by the oronasal membrane. This membrane ruptures by the end of the sixth week, bringing the nasal and oral cavities into communication (Fig. 9-34C). Temporary epithelial plugs are formed in the nasal cavities from proliferation of the cells lining them. Between 13 to 15 weeks, the nasal plugs disappear.
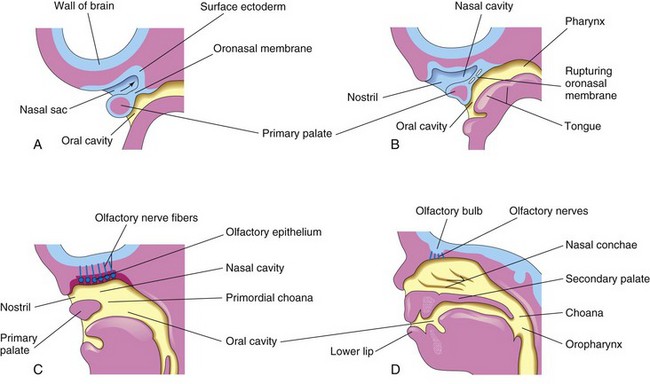
FIGURE 9–34 Sagittal sections of the head showing development of the nasal cavities. The nasal septum has been removed. A, 5 weeks. B, 6 weeks, showing breakdown of the oronasal membrane. C, 7 weeks, showing the nasal cavity communicating with the oral cavity and development of the olfactory epithelium. D, 12 weeks, showing the palate and the lateral wall of the nasal cavity.
The regions of continuity between the nasal and oral cavities are the primordial choanae (the right or left openings from the nasal cavity into the nasal pharynx). After the secondary palate develops, the choanae are located at the junction of the nasal cavity and pharynx (Figs. 9-34D and Fig. 9-37). While these changes are occurring, the superior, middle, and inferior nasal conchae develop as elevations of the lateral walls of the nasal cavities (Fig. 9-34D). Concurrently, the ectodermal epithelium in the roof of each nasal cavity becomes specialized to form the olfactory epithelium. Some epithelial cells differentiate into olfactory receptor cells (neurons). The axons of these cells constitute the olfactory nerves, which grow into the olfactory bulbs of the brain (Fig. 9-34C and D).
Most of the upper lip, maxilla, and secondary palate form from the maxillary prominences (Fig. 9-26H). These prominences merge laterally with the mandibular prominences. The primordial lips and cheeks are invaded by mesenchyme from the second pair of pharyngeal arches, which differentiates into the facial muscles (Fig. 9-6, Table 9-1). These muscles of facial expression are supplied by the facial nerve (CN VII), the nerve of the second pharyngeal arch. The mesenchyme in the first pair of arches differentiates into the muscles of mastication (chewing) and a few others, all of which are innervated by the trigeminal nerves (CN V), which supply the first pair of pharyngeal arches.
Paranasal Sinuses
Some paranasal sinuses, such as the maxillary sinuses, begin to develop during late fetal life; the remainder of them develop after birth. They form from diverticula (outgrowths) of the walls of the nasal cavities and become pneumatic (air-filled) extensions of the nasal cavities in the adjacent bones, such as the maxillary sinuses in the maxillae and the frontal sinuses in the frontal bones. The original openings of the diverticula persist as the orifices of the adult sinuses.
The first appearance of the vomeronasal primordia is in the form of bilateral epithelial thickenings on the nasal septum. Further invagination of the primordia and their breaking away from the nasal septal epithelium gives rise to a tubular vomeronasal organ (VNO) between days 37 and 43. This chemosensory structure, which ends blindly posteriorly, reaches its greatest development between 12 and 14 weeks. Later, a gradual replacement of the receptor population with patchy ciliated cells occurs. The VNO is consistently present in the form of a bilateral duct-like structure on the nasal septum, superior to the paraseptal cartilage (Fig. 9-35). The tubular human VNO with its minute anterior opening are true homologs of the VNO in other mammals, reptiles, and amphibians.
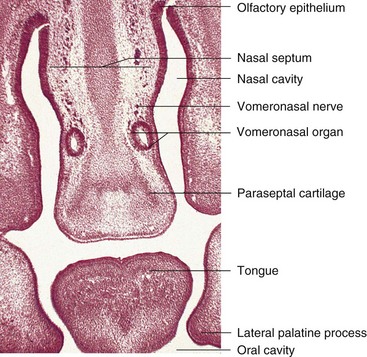
FIGURE 9–35 Photomicrograph of a frontal section through the developing oral cavity and nasal regions of a 22-mm human embryo of approximately 54 days. Observe the bilateral, tubular vomeronasal organ.
(Courtesy of Dr. Kunwar Bhatnagar, Department of Anatomical Sciences and Neurobiology, School of Medicine, University of Louisville, Louisville, KY.)
Postnatal Development of Paranasal Sinuses
Most of the paranasal sinuses are rudimentary or absent in neonates. The maxillary sinuses are small at birth. These sinuses grow slowly until puberty and are not fully developed until all the permanent teeth have erupted in early adulthood.
No frontal or sphenoidal sinuses are present at birth. The ethmoidal cells are small before the age of 2 years and do not begin to grow rapidly until 6 to 8 years of age. At approximately 2 years of age, the two most anterior ethmoidal cells grow into the frontal bone, forming a frontal sinus on each side. Usually the frontal sinuses are visible in radiographs by the seventh year.
The two most posterior ethmoidal cells grow into the sphenoid bone at approximately 2 years of age, forming two sphenoidal sinuses. Growth of the paranasal sinuses is important in altering the size and shape of the face during infancy and childhood and in adding resonance to the voice during adolescence.
 Development of Palate
Development of Palate
Palatogenesis begins in the sixth week; however, development of the palate is not completed until the 12th week. The critical period of palate development is from the end of the sixth week until the beginning of the ninth week. The palate develops in two stages: development of a primary palate and development of a secondary palate.
Primary Palate
Early in the sixth week, the primary palate—median process—begins to develop (Figs. 9-33F and 9-34). Initially this segment, formed by merging of the medial nasal prominences, is a wedge-shaped mass of mesenchyme between the internal surfaces of the maxillary prominences of the developing maxillae. The primary palate forms the anterior/midline aspect of the maxilla, the premaxillary part of the maxilla (Fig. 9-36). It represents only a small part of the adult hard palate (i.e., anterior to the incisive fossa).
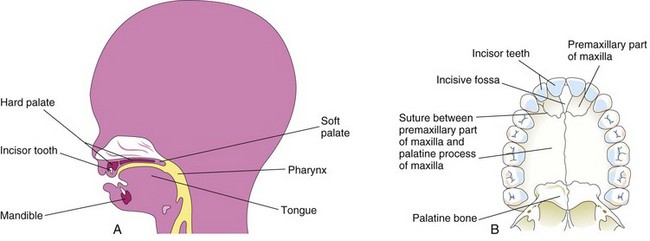
FIGURE 9–36 A, Sagittal section of the head of a 20-week fetus illustrating the location of the palate. B, The bony palate and alveolar arch of a young adult. The suture between the premaxillary part of the maxilla and the fused palatal processes of the maxillae is usually visible in crania (skulls) of young persons. It is not visible in the hard palates of most dried crania because they are usually from old adults.
Secondary Palate
The secondary palate (definitive palate) is the primordium of the hard and soft parts of the palate (Fig. 9-36). It begins to develop early in the sixth week from two mesenchymal projections that extend from the internal aspects of the maxillary prominences. Initially these structures—the lateral palatine processes (palatal shelves)—project inferomedially on each side of the tongue (Figs. 9-37B and 9-38A and B). As the jaws elongate, they pull the tongue away from its root, and, as a result, it is brought lower in the mouth.
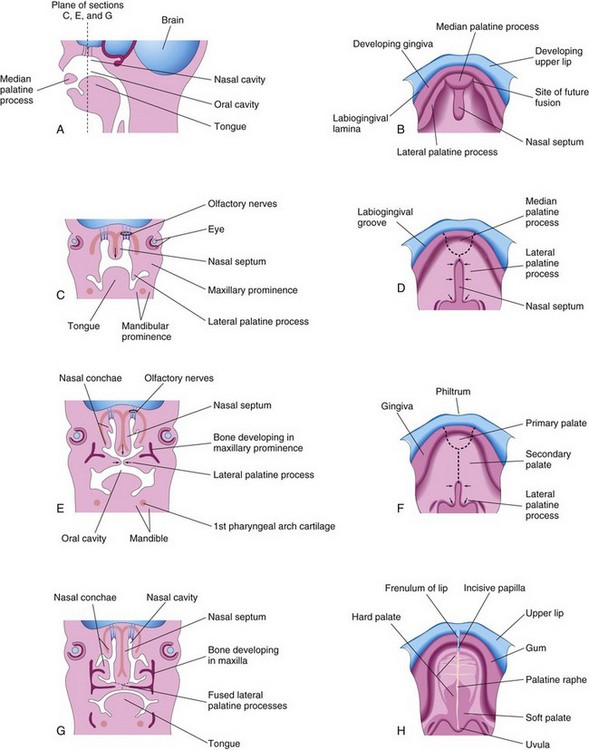
FIGURE 9–37 A, Sagittal section of the embryonic head at the end of the sixth week showing the median palatal process. B, D, F, and H, Roof of the mouth from the sixth to 12th weeks illustrating the development of the palate. The broken lines in D and F indicate sites of fusion of the palatine processes. The arrows indicate medial and posterior growth of the lateral palatine processes. C, E, and G, Frontal sections of the head illustrating fusion of the lateral palatine processes with each other and the nasal septum and separation of the nasal and oral cavities.
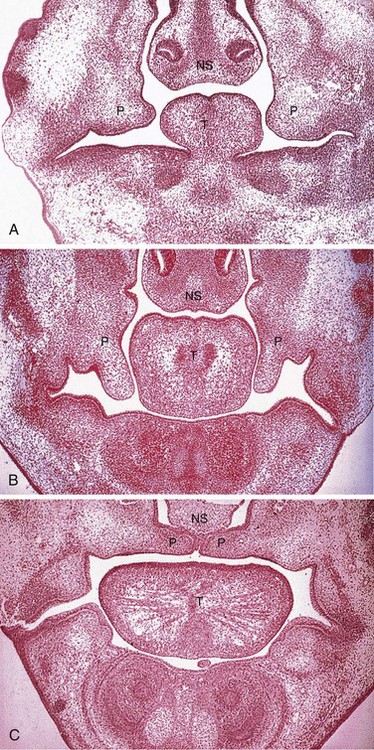
FIGURE 9–38 Frontal sections of human embryonic heads showing development of the lateral palatal processes (P), nasal septum (NS), and tongue (T) during the eighth week. A, Embryo with a crown–rump length (CRL) of 24 mm. This section shows early development of the palatine processes. B, Embryo with a CRL of 27 mm. This section shows the palate just before palatine process elevation. C, Embryo with a CRL of 29 mm (near the end of the eighth week). The palatine processes are elevated and fused.
(From Sandham A: Embryonic facial vertical dimension and its relationship to palatal shelf elevation. Early Hum Dev 12:241, 1985.)
During the seventh and eighth weeks, the lateral palatine processes assume a horizontal position above the tongue (Figs. 9-37E to H and 9-38C). This change in orientation occurs by a flowing process facilitated in part by the release of hyaluronic acid by the mesenchyme of the palatine processes.
Bone gradually develops in the primary palate, forming the premaxillary part of the maxilla, which lodges the incisor teeth (Fig. 9-36B). Concurrently, bone extends from the maxillae and palatine bones into the lateral palatine processes to form the hard palate (Fig. 9-37E and G). The posterior parts of these processes do not become ossified. They extend posteriorly beyond the nasal septum and fuse to form the soft palate, including its soft conical projection—the uvula (Fig. 9-37D, F, and H). The median palatine raphe indicates the line of fusion of the palatine processes.
A small nasopalatine canal persists in the median plane of the palate between the anterior part of the maxilla and the palatine processes of the maxillae. This canal is represented in the adult hard palate by the incisive fossa (Fig. 9-36B), which is the common opening for the small right and left incisive canals. An irregular suture runs on each side from the incisive fossa to the alveolar process of the maxilla, between the lateral incisor and canine teeth on each side (Fig. 9-36B). It is visible in the anterior region of the palates of young persons. This suture indicates where the embryonic primary and secondary palates fused.
The nasal septum develops as a downgrowth from internal parts of the merged medial nasal prominences (Figs. 9-37 and 9-38). The fusion between the nasal septum and the palatine processes begins anteriorly during the ninth week and is completed posteriorly by the 12th week, superior to the primordium of the hard palate.
Cleft Lip and Cleft Palate
Clefts of the lip and palate are common craniofacial anomalies. The defects are usually classified according to developmental criteria, with the incisive fossa as a reference landmark. These clefts are especially conspicuous because they result in an abnormal facial appearance and defective speech. There are two major groups of cleft lip and cleft palate (Figs. 9-39 to 9-41):
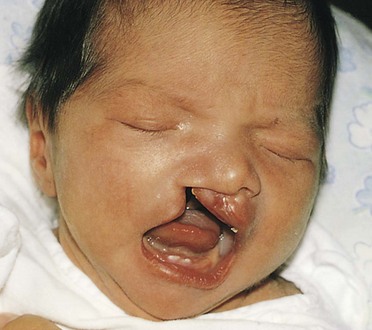
FIGURE 9–39 Infant with unilateral cleft lip and cleft palate. Clefts of the lip, with or without cleft palate, occur approximately once in 1000 births; most affected infants are males.
(Courtesy of A.E. Chudley, Section of Genetics and Metabolism, Department of Pediatrics and Child Health, Children’s Hospital, Winnipeg, Manitoba, Canada.)
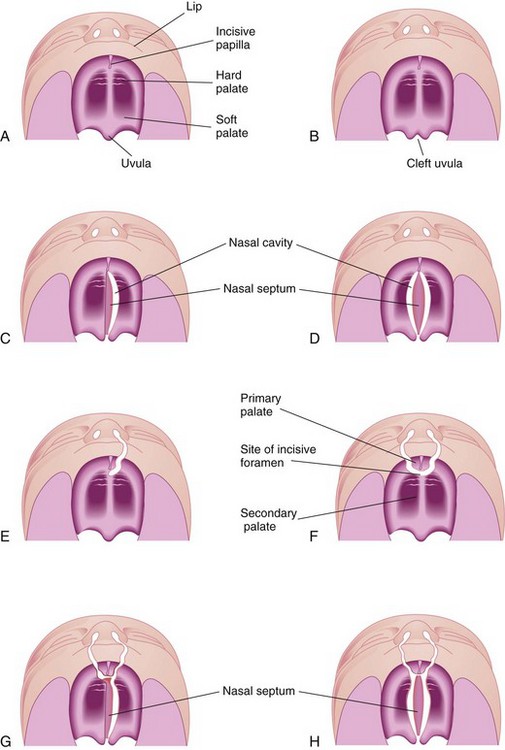
FIGURE 9–40 Various types of cleft lip and palate. A, Normal lip and palate. B, Cleft uvula. C, Unilateral cleft of the secondary (posterior) palate. D, Bilateral cleft of the posterior part of the palate. E, Complete unilateral cleft of the lip and alveolar process of the maxilla with a unilateral cleft of the primary (anterior) palate. F, Complete bilateral cleft of the lip and alveolar processes of the maxillae with bilateral cleft of the anterior part of the palate. G, Complete bilateral cleft of the lip and alveolar processes of the maxillae with bilateral cleft of the anterior part of the palate and unilateral cleft of the posterior part of the palate. H, Complete bilateral cleft of the lip and alveolar processes of the maxillae with complete bilateral cleft of the anterior and posterior palate.
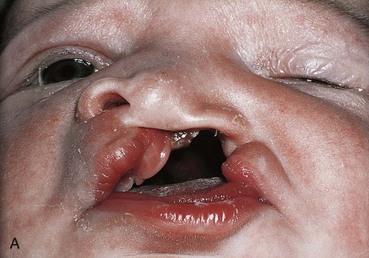
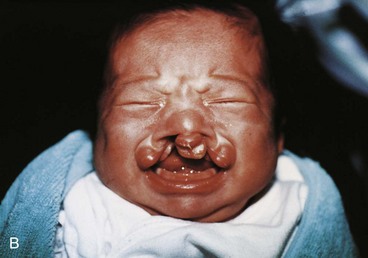
FIGURE 9–41 Congenital anomalies of the lip and palate. A, Infant with a left unilateral cleft lip and cleft palate. B, Infant with bilateral cleft lip and cleft palate.
(Courtesy of Dr. Barry H. Grayson and Dr. Bruno L. Vendittelli, New York University Medical Center, Institute of Reconstructive Plastic Surgery, New York, NY.)
A cleft lip, with or without a cleft palate, occurs approximately once in 1000 births; however, the frequency varies widely among ethnic groups; 60% to 80% of affected infants are males. The clefts vary from incomplete cleft lip to ones that extend into nose and through the alveolar part of the maxilla (Figs. 9-39 and 9-41A and B). Cleft lip may be unilateral or bilateral.
A unilateral cleft lip (Figs. 9-39, 9-40E and F, and 9-41A) results from failure of the maxillary prominence on the affected side to unite with the merged medial nasal prominences. This is the consequence of failure of the mesenchymal masses to merge and the mesenchyme to proliferate and smooth out the overlying epithelium. This results in a persistent labial groove (Fig. 9-42D). In addition, the epithelium in the labial groove becomes stretched and the tissues in the floor of the groove break down. As a result, the lip is divided into medial and lateral parts (see Fig. 9-42G and H ). Sometimes a bridge of tissue, called a Simonart band, joins the parts of the incomplete unilateral cleft lip.
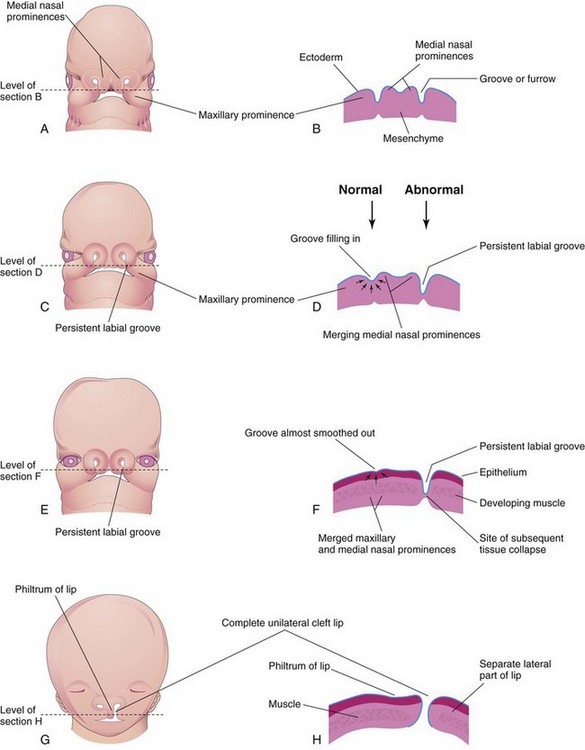
FIGURE 9–42 Drawings illustrating the embryologic basis of complete unilateral cleft lip. A, A 5-week embryo. B, Horizontal section through the head illustrating the grooves between the maxillary prominences and the merging medial nasal prominences. C, A 6-week embryo showing a persistent labial groove on the left side. D, Horizontal section through the head showing the groove gradually filling in on the right side after proliferation of mesenchyme (arrows). E, A 7-week embryo. F, Horizontal section through the head showing that the epithelium on the right has almost been pushed out of the groove between the maxillary and medial nasal prominences. G, A 10-week fetus with a complete unilateral cleft lip. H, Horizontal section through the head after stretching of the epithelium and breakdown of the tissues in the floor of the persistent labial groove on the left side, forming a complete unilateral cleft lip.
A bilateral cleft lip (Figs. 9-41B and 9-43C and D) results from failure of the mesenchymal masses in both maxillary prominences to meet and unite with the merged medial nasal prominences. The epithelium in both labial grooves becomes stretched and breaks down (Fig. 9-42H ). In bilateral cases, the defects may be dissimilar, with varying degrees of defect on each side. When there is a complete bilateral cleft of the lip and alveolar part of the maxilla, the median palatal process hangs free and projects anteriorly (Fig. 9-41B). These defects are especially deforming because of the loss of continuity of the orbicularis oris muscle (Fig. 9-6B), which closes the mouth and purses the lips.
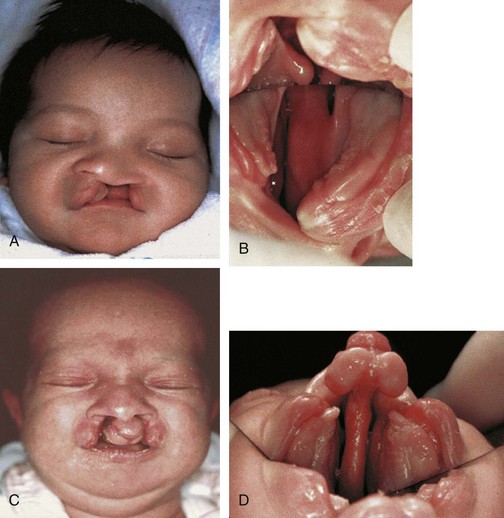
FIGURE 9–43 Congenital anomalies of the lip and palate. A, Newborn male infant with unilateral complete cleft lip and cleft palate. B, Intraoral photograph (taken with mirror) showing left unilateral complete cleft of the primary and secondary parts of palate. C, Newborn female infant with bilateral complete cleft lip and cleft palate. D, Intraoral photograph showing bilateral complete cleft palate. Note maxillary protrusion and natal tooth at gingival apex in each lesser segment.
(Courtesy of Dr. John B. Mulliken, Children’s Hospital Boston, Harvard Medical School, Boston, MA.)
A median cleft lip is a rare defect that results from a mesenchymal deficiency. This defect causes partial or complete failure of the medial nasal prominences to merge and form the median palatal process. A median cleft lip is a characteristic feature of the Mohr syndrome, which is transmitted as an autosomal recessive trait.
A median cleft of the lower lip is also very rare and results from failure of the mesenchymal masses in the mandibular prominences to merge completely and smooth out the embryonic cleft between them (Fig. 9-26A).
A cleft palate, with or without a cleft lip, occurs approximately once in 2500 births and is more common in females than in males. The cleft may involve only the uvula; a cleft uvula has a fishtail appearance (Fig. 9-40B), or the cleft may extend through the soft and hard regions of the palate (Figs. 9-40C and D and 9-43). In severe cases associated with a cleft lip, the cleft in the palate extends through the alveolar part of the maxilla and lips on both sides (Figs. 9-40G and H and 9-41B).
A complete cleft palate indicates the maximum degree of clefting of any particular type; for example, a complete cleft of the posterior palate is a defect in which the cleft extends through the soft palate and anteriorly to the incisive fossa. The landmark for distinguishing anterior from posterior cleft anomalies is the incisive fossa.
Unilateral and bilateral clefts of the palate are classified into three groups:
Most clefts of the lip and palate result from multiple factors (multifactorial inheritance; see Chapter 20), including genetic and nongenetic, each causing a minor developmental disturbance. Several studies show that the interferon regulatory factor-6 (IRF-6) gene is involved in the formation of isolated clefts.
Some clefts of the lip and/or palate appear as part of syndromes determined by single mutant genes. Other clefts are parts of chromosomal syndromes, especially trisomy 13 (see Chapter 20). A few cases of cleft lip and/or palate appear to have been caused by teratogenic agents (e.g., anticonvulsant drugs). Studies of twins indicate that genetic factors are of more importance in a cleft lip, with or without a cleft palate, than in a cleft palate alone.
A sibling of a child with a cleft palate has an elevated risk of having a cleft palate, but no increased risk of having a cleft lip. A cleft of the lip and alveolar process of the maxilla that continues through the palate is usually transmitted through a male sex-linked gene. When neither parent is affected, the recurrence risk in subsequent siblings (brother or sister) is approximately 4%.
Other Facial Defects
Congenital microstomia (small mouth) results from excessive merging of the mesenchymal masses in the maxillary and mandibular prominences of the first pharyngeal arch. In severe cases, the defect may be associated with underdevelopment (hypoplasia) of the mandible. A single nostril results when only one nasal placode forms. A bifid nose results when the medial nasal prominences do not merge completely; the nostrils are widely separated, and the nasal bridge is bifid. In mild forms of bifid nose, there is a groove in the tip of the nose.
By the beginning of the second trimester (Fig. 9-26), features of the fetal face can be identified sonographically. Using this imaging technique (Fig. 9-44), facial defects such as a cleft lip are readily recognizable.
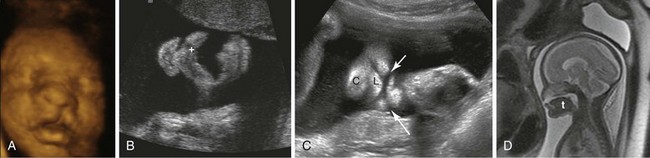
FIGURE 9–44 A, Three-dimensional ultrasound surface rendering of a fetus with unilateral cleft lip. B, Coronal sonogram of a fetal mouth with a cleft lip extending into the left nostril (+). Coronal plane. C, Coronal sonogram of a fetus showing a bilateral cleft lip (arrows), lower lip (L), and chin (C). D, Sagittal magnetic resonance image of a fetus showing the absence of the middle part of the hard palate. Note the fluid above the tongue (t) without the intervening palate.
(A and B, Courtesy of Dr. G.J. Reid, Department of Obstetrics, Gynecology and Reproductive Sciences, University of Manitoba, Women’s Hospital, Winnipeg, Manitoba, Canada; C and D, Courtesy of Deborah Levine, M.D., Director of Obstetric and Gynecologic Ultrasound, Beth Israel Deaconess Medical Center, Boston, MA.)
Facial Clefts
Various types of facial clefts occur, but all are rare. Severe clefts are usually associated with gross defects of the head. Oblique facial clefts are often bilateral and extend from the upper lip to the medial margin of the orbit. When this occurs, the nasolacrimal ducts are open grooves (persistent nasolacrimal grooves) (Fig. 9-45). Oblique facial clefts associated with a cleft lip result from failure of the mesenchymal masses in the maxillary prominences to merge with the lateral and medial nasal prominences. Lateral or transverse facial clefts run from the mouth toward the ear. Bilateral clefts result in a very large mouth, a condition called macrostomia. In severe cases, the clefts in the cheeks extend almost to the ears.
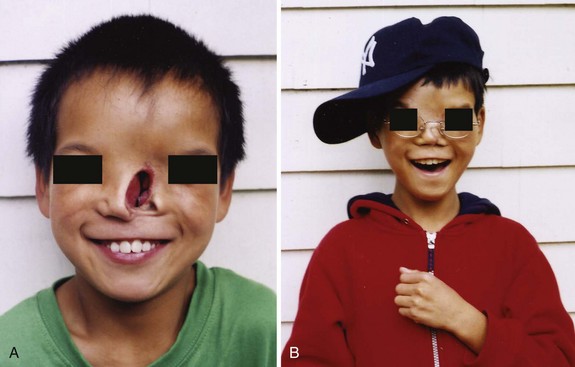
FIGURE 9–45 Photographs of a child with an oblique facial cleft. Note the persistent nasolacrimal cleft. A, Before surgical correction. B, After surgical correction.
(With permission from Columbia University Medical Center. Courtesy of Dr. J.A. Ascherman, Department of Surgery, Division of Plastic Surgery, Columbia University Medical Center, New York, NY. © Pat Farrell.)
Summary of Pharyngeal Apparatus, Face, and Neck
Clinically Oriented Problems
Case 9–1
The mother of a 2-year-old boy consulted her pediatrician about an intermittent discharge of mucoid material from a small opening in the side of the boy’s neck. There was also extensive redness and swelling in the inferior third of the neck, just anterior to the sternocleidomastoid muscle.
Case 9–2
During a subtotal thyroidectomy, a surgeon could locate only one inferior parathyroid gland.
Case 9–3
A young woman consulted her physician about a swelling in the anterior part of her neck, just inferior to the hyoid bone.
Case 9–4
A male infant was born with a unilateral cleft lip extending into his nose and through the alveolar process of his maxilla.
Discussion of these problems appears at the back of the book.
References and Suggested Reading
Abbott BD. The etiology of cleft palate: a 50-year search for mechanistic and molecular understanding. Birth Defects Res (Part B). 2010;89:266.
Arnold JS, Werling U, Braunstein EM, et al. Inactivation of Tbx1 in the pharyngeal endoderm results in 22q11DS malformations. Development. 2006;133:977.
Berkovitz BKB, Holland GR, Moxham B. Oral Anatomy, Histology, and Embryology, ed 4. Edinburgh and London: Elsevier/Mosby; 2009.
Bothe I, Tenin G, Oseni A, et al. Dynamic control of head mesoderm patterning. Development. 2011;138:2807.
Francis-West PH, Robson L, Evans DJR. Craniofacial development: The tissue and molecular interactions that control development of the head. Adv Anat Embryol Cell Biol. 2003;169:1-138.
Gartner LP, Hiatt JL. Color Textbook of Histology, ed 3. Philadelphia: WB Saunders; 2007.
Gitton Y, Heude E, Vieux-Rochas M, et al. Evolving maps in craniofacial development. Sem Cell Develop Biol. 2010;21:301.
Gorlin RJ, Cohen MMJr, Levin LS. Syndromes of the Head and Neck, ed 3. New York: Oxford University Press; 1990.
Greene RM, Pisano MM. Palate morphogenesis: current understanding and future directions. Birth Defects Res C. 2010;90:133.
Gross E, Sichel J-Y. Congenital neck lesions. Surg Clin North Am. 2006;86:383.
Hinrichsen K. The early development of morphology and patterns of the face in the human embryo. Adv Anat Embryol Cell Biol. 1985;98:1-79.
Hong P, Lago D, Seargeant J, et al. Defining ankyloglossis: a case series of anterior and posterior tongue ties. Int J Pediatr Otorhinolaryngol. 2010;74:1003.
Jirásel JE. An Atlas of Human Prenatal Developmental Mechanics. Anatomy and Staging. London and New York: Taylor & Francis; 2004.
Jones KL. Smith’s Recognizable Patterns of Human Malformation, ed 6. Philadelphia: Elsevier/WB Saunders; 2006.
Lale SM, Lele MS, Anderson VM. The thymus in infancy and childhood. Chest Surg Clin N Am. 2001;11:233.
Minoux M, Rijii FM. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development. 2010;137:2605.
Moore KL, Dalley AD, Agur AMR. Clinically Oriented Anatomy, ed 6. Baltimore: Williams & Wilkins; 2010.
Mueller DT, Callanan VP. Congenital malformations of the oral cavity. Otolaryngol Clin North Am. 2007;40:141.
Nishimura Y. Embryological study of nasal cavity development in human embryos with reference to congenital nostril atresia. Acta Anat. 1993;147:140.
Noden DM. Cell movements and control of patterned tissue assembly during craniofacial development. J Craniofac Genet Dev Biol. 1991;11:192.
Noden DM. Vertebrate craniofacial development: novel approaches and new dilemmas. Curr Opin Genet Dev. 1992;2:576.
Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. J Anat. 2005;207:575.
Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194-1218.
Ozolek JA. Selective pathologies of the head and neck in children—a developmental perspective. Adv Anat Pathol. 2009;16:332.
Passos-Bueno MR, Ornelas CC, Fanganiello RD. Syndromes of the first and second pharyngeal arches: a review. Am J Med Genet (Part A). 2009;149A:1853.
Pilu G, Segata M, Perola A. Fetal craniofacial and neck anomalies. In Callen PW, editor: Ultrasonography in Obstetrics and Gynecology, ed 5, Philadelphia: WB Saunders, 2008.
Rice DPC. Craniofacial anomalies: from development to molecular pathogenesis. Curr Mol Med. 2009;5:699.
Rodriguez-Vázquez JF. Development of the stapes and associated structures in human embryos. J Anat. 2005;207:165.
Smith TD, Bhatnagar KP. The human vomeronasal organ. Part II: Prenatal development. J Anat. 2000;197:421.
Sperber GH, Sperber SM, Guttmann GD. Craniofacial Embryogenetics and Development, ed 2. Beijing: People’s Medical Publishing House/PMPH-Global; 2010.
Tovar JA. The neural crest in pediatric surgery. J Pediatr Surg. 2007;42:915.
Wyszynski DF, Sarkozi A, Czeizel AE. Oral clefts with associated anomalies: Methodological issues. Cleft Palate Craniofac J. 2006;43:1.
Yatzey KE. Di George syndrome, Tbx1, and retinoic acid signaling come full circle. Circ Res. 2010;106:630.