Chapter 28 Movements of the Gastrointestinal Tract
1. Slow waves of electrical depolarization are a unique feature of gut smooth muscle.
2. When slow waves reach sensitized smooth muscle cells, action potentials and contraction result.
3. Coordinated motility enables the lips, tongue, mouth, and pharynx to grasp food and propel it down the gastrointestinal tract.
4. Motility of the esophagus propels food from the pharynx to the stomach.
5. The function of the stomach is to process food into a fluid consistency and release it into the intestine at a controlled rate.
6. The proximal stomach stores food awaiting further gastric processing in the distal stomach.
7. The distal stomach grinds and sifts food entering the small intestine.
8. Control of gastric motility differs in the proximal and distal stomach.
9. The rate of gastric emptying must match the small intestine’s rate of digestion and absorption.
10. Between meals, the stomach is cleared of indigestible material.
11. Vomiting is a complex reflex coordinated from the brainstem.
12. Motility of the small intestine has digestive and interdigestive phases.
13. The ileocecal sphincter prevents movement of colon contents back into the ileum.
14. Motility of the colon causes mixing, retropulsion, and propulsion of ingesta.
15. The colon is an important site of storage and absorption in all animals.
16. Despite large anatomical differences in the colons of herbivores compared to omnivores and carnivores, there are similarities in motility.
17. The anal sphincter has two layers with separate innervation.
18. The rectosphincteric reflex is important in defecation.
19. Major differences between avian and mammalian digestive systems include, in birds, both the lack of teeth and the separation of gastric functions into distinct anatomical regions.
The walls of the gastrointestinal (GI) tract, at all levels, are muscular and capable of movement. Movements of the GI muscles have direct actions on ingesta in the gut lumen. GI movements have several functions: (1) to propel ingesta from one location to the next; (2) to retain ingesta at a given site for digestion, absorption, or storage; (3) to break up food material physically and mix it with digestive secretions; and (4) to circulate ingesta so that all portions come into contact with absorptive surfaces.
The dynamics of fluid movement in the gut are not as well understood as in other organ systems, particularly the cardiovascular system. The heart and great vessels behave in a manner similar to that of most mechanical pumping systems: a central pump pushes fluid through a conduit of relatively fixed diameter. Because of this configuration, the cardiovascular system more or less conforms to physical laws that are well established and studied reasonably easily; sophisticated quantitative analyses of cardiovascular function can be made clinically. In contrast to the situation in the heart, the fluid pump and the conduit are the same organ in the gut. This makes study of the fluid dynamics of the gut extremely complex. At this time, the mathematically defined physical laws of fluid dynamics, as applied to the gut, are of little clinical usefulness. Therefore the physiology of GI motility is usually applied clinically on a qualitative, rather than a quantitative, basis.
Movement of the gut wall is referred to as motility, and motility may be of a propulsive, retentive, or mixing nature. The time it takes material to travel from one portion of the gut to another is referred to as the transit time. An increase in propulsive motility decreases the transit time, whereas an increase in retentive motility increases the transit time. Selectively increasing retentive motility and reducing propulsive motility are important aspects of diarrhea therapy.
Slow Waves of Electrical Depolarization Are a Unique Feature of Gut Smooth Muscle
The first level of control of GI motility lies in the intrinsic electrical properties of the smooth muscle mass. These electrical properties consist of spontaneously undulating waves of partial depolarization that sweep over the gut smooth muscle. The origin of this electrical activity is from specialized smooth muscle cells referred to as the interstitial cells of Cajal (ICC). The ICC form an interconnecting lattice of cells that surrounds the circular and longitudinal layers of muscle over the entire length of the gut. These cells are very similar in structure and function to the Purkinje cells of the heart. The ICC exhibit rhythmical and spontaneous oscillation in their transmembrane electrical potentials, as illustrated in Figure 28-1. They are connected to one another and to cells of the general smooth muscle mass by tight junctions or nexuses. These connections allow for the flow of ions from cell to cell. The resulting ionic movements lead to the propagation of waves of partial cell membrane depolarization across large numbers of cells. Within the ICC, fluctuations in intracellular calcium concentrations appear responsible for the spontaneous changes in membrane polarization. Figure 28-1 illustrates the concept of a fluctuating membrane potential in a single ICC. The property of spontaneous electrical rhythmicity, in combination with their electrical connection to the smooth muscle mass, imparts to the ICC their role as electrical “pacemakers” of the gut.
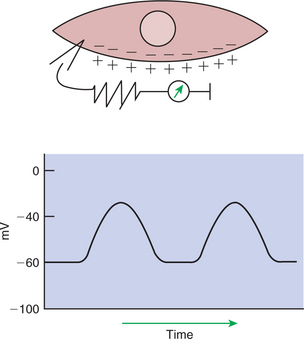
FIGURE 28-1 Spontaneous changes in membrane polarity of the interstitial cells of Cajal, specialized gastrointestinal (GI) smooth muscle cells that are responsible for spontaneous electrical rhythmicity in gut muscle. The upper illustration represents a single cell with a voltmeter measuring the transmembrane electrical potential. The graph illustrates spontaneous changes in electrical potential (in millivolts, mV) that would be measured across the cell membrane.
In GI smooth muscle cells the baseline membrane potential is usually −70 to −60 millivolts (mV). Under the influence of the ICC, the membrane potential fluctuates from this baseline level by as much as 20 to 30 mV. Thus, under resting conditions, the depolarization is only partial, and the membrane potential never reaches 0 mV. The smooth muscle cells are connected to the ICC and to each other by nexuses, allowing the changes in membrane potential to be spread, or propagated, over large areas of muscle. The ICC initiate these changes and thus determine their origin and direction of propagation. Under normal conditions in the small intestine, changes in membrane potential begin high in the duodenum and are propagated aborally (away from the mouth) along the length of the small intestine (Figure 28-2). These aborally moving waves of partial depolarization are called slow waves or the basic electrical rhythm of the gut. In the dog, slow waves occur about 20 times per minute in the small intestine. In the stomach and colon, slow waves occur less frequently, about five times per minute. However, the slow waves are present throughout the smooth muscle portions of the GI tract. The frequency of slow waves varies among the domestic species, but their presence does not vary.
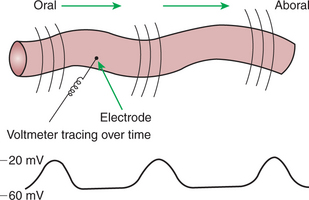
FIGURE 28-2 Partial membrane depolarizations of GI smooth muscle cells occur in a coordinated manner, creating waves of depolarization that sweep over large segments of muscle. Electrodes placed on or near the surface of the muscle record changes in potential as waves of depolarization pass toward or away from them. Coordinated changes in membrane potential among cells are necessary for these waves to be measured because random changes among cells would cancel each other out, and no changes would be recorded by electrodes placed extracellularly.
The slow waves are an intrinsic property of the GI smooth muscle and associated ICC. The presence of the slow waves depends only on the ICC, whereas the amplitude and to a lesser extent the frequency of the slow waves can be modulated by the enteric nervous system (ENS) and endocrine/paracrine system. The link between slow waves and muscle contractions, however, is under control of nervous, endocrine, and paracrine factors, as discussed next.
When Slow Waves Reach Sensitized Smooth Muscle Cells, Action Potentials and Contraction Result
Slow waves have an important relationship with muscle contractions, but they are not the direct stimuli for contractions. Slow waves are constantly passing over GI smooth muscle, whether it is actively contracting or not. GI smooth muscle cells, as with other muscle cells, contract in association with action, or spike, potentials. These potentials are characterized by complete depolarization of the membrane for a short time, in contrast to the slow waves, which are characterized by incomplete depolarization (see Chapter 4). Action potentials in the GI smooth muscle occur only in association with slow waves. Thus the presence of slow waves is necessary but not sufficient to cause muscle contractions. When slow waves pass over an area of smooth muscle without eliciting action potentials, no contractions occur. When slow waves pass over an area of smooth muscle and action potentials are superimposed on the slow waves, gut muscle contracts. Control and coordination of smooth muscle activity is achieved by influencing the likelihood that action potentials will be superimposed on slow waves. Such control is a function of the neurohumoral substances produced by the ENS and enteric endocrine/paracrine system.
Smooth muscle control and coordination are achieved by modulation of the baseline electrical potential in the smooth muscle cells. Neurohumoral regulatory molecules from ENS neurons or endocrine/paracrine cells are released in the vicinity of the smooth muscle cells, affecting membrane ion channels and influencing the baseline membrane potential (see Chapter 27). Excitatory molecules elevate the baseline (bring it closer to zero), and inhibitory molecules lower the baseline (make it more negative). The position of the baseline influences how close the overall potential will come to 0 mV at the crest of a slow wave. When the membrane potential of a smooth muscle becomes close to zero, action potentials occur and muscle contracts (Figure 28-3). Neurohumoral substances (neurocrines, paracrines, and hormones) that are excitatory elicit smooth muscle contraction by elevating the baseline, whereas inhibitory substances inhibit muscle contraction by lowering the baseline.
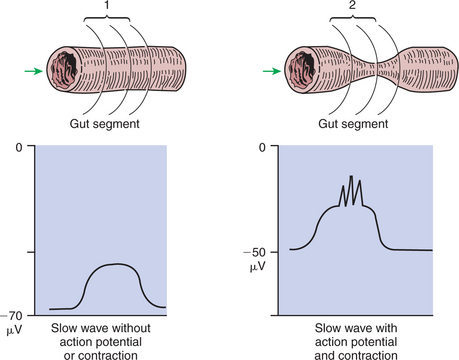
FIGURE 28-3 1, No muscle contraction occurs in the absence of action potentials. 2, Muscle contracts when the crest of the slow waves reaches a critical point of depolarization, allowing action potentials to occur. The probability of action potentials occurring during the passage of a slow wave over a segment of gut muscle is influenced by the degree of baseline depolarization. Norepinephrine lowers the baseline (increases its absolute value), whereas acetylcholine raises the baseline (decreases its absolute value). μV, Microvolts.
The integrated actions of the slow waves, ENS, and endocrine/paracrine system appear to function to synchronize the contractions of the GI muscle mass. In order for the muscle to perform efficiently, all or many of the muscle cells in one layer of a segment of gut must be synchronized to contract simultaneously. This can best be visualized by considering the circular muscle layer. The contents of the circle cannot be “squeezed” effectively unless all the muscles of the circumference contract simultaneously; it would have little effect on luminal pressure if one portion of the circle contracted while another portion relaxed. In any discrete area of gut, slow waves pass simultaneously over the entire circumference of the smooth muscle. If that area has been sensitized by an excitatory neurohumoral regulatory molecule, the entire circumference of circular muscle will contract in synchrony.
Muscle contractions can occur at a frequency no higher than the frequency of the slow waves. As an example of frequency modulation, consider the activity of muscle in the stomach of the dog. Slow waves in the canine stomach occur about five times per minute. The crest of each slow wave may or may not be accompanied by action potentials. Therefore, during a given minute, the muscle in a localized area may not contract at all or may contract up to five times. If the passing slow waves generate no action potentials, the muscle does not contract at all. In a given minute, if action potentials are associated with one slow wave, the muscle contracts once. Action potentials on two slow waves result in two contractions, and so on, up to a maximum of five contractions per minute, but no more than five, because there are no more slow waves.
The motility patterns of the gut vary in their complexity, as described in the following sections. In the stomach and colon, motility patterns are relatively complex compared with the small intestine. In all cases, motility patterns are programmed into the ENS and coordinated in conjunction with the slow waves.
Coordinated Motility Enables the Lips, Tongue, Mouth, and Pharynx to Grasp Food and Propel It down the Gastrointestinal Tract
Before digestion can begin, food must be directed into the GI tract. To ingest food, quadruped animals must first grasp it with the lips, teeth, or tongue. This involves highly coordinated activity of small, voluntary skeletal muscles. The muscles of the face, lips, and tongue appear to be among the most delicately controlled voluntary muscles of most domestic animals. The exact method of food prehension varies greatly among different species. For example, horses use their lips extensively, whereas cattle often use their tongues for grasping food. In all domestic animals, however, prehension is a highly coordinated process involving direct control by the central nervous system (CNS). Problems of prehension may develop because of abnormalities in the teeth, jaws, muscles of the tongue and face, cranial nerves, or CNS. The facial nerve, the glossopharyngeal nerve, and the motor branch of the trigeminal nerve control the muscles of prehension.
Mastication, or chewing, involves the actions of the jaws, tongue, and cheeks and is the first act of digestion. It serves not only to break food particles down to a size that will pass into the esophagus but also to moisten and lubricate food by thoroughly mixing it with saliva. Abnormalities of the teeth are a common cause of digestive disturbances in animals.
Deglutition, or swallowing, involves voluntary and involuntary stages and occurs after food has been well masticated. In the voluntary phase of swallowing, food is molded into a bolus by the tongue and then pushed back into the pharynx. When food enters the pharynx, sensory nerve endings detect its presence and initiate the involuntary portion of the swallow reflex.
The involuntary actions of the swallow reflex occur primarily within the pharynx and esophagus. The pharynx is the common opening of both the respiratory and the digestive tract. The major physiological function of the pharynx is to ensure that air, and only air, enters the respiratory tract and that food and water, and only food and water, enter the digestive tract. The involuntary portion of the swallow reflex is the action that directs food into the digestive system and away from the upper airway. This reflex involves the following series of highly coordinated actions (Figure 28-4). Breathing stops momentarily. The soft palate is elevated, closing the pharyngeal opening of the nasopharynx and preventing food from entering the internal openings of the nostrils. The tongue is pressed against the hard palate, closing off the oral opening of the pharynx. The hyoid bone and larynx are pulled forward; this action pulls the glottis under the epiglottis, blocking the laryngeal opening. Concurrently, the arytenoid cartilages constrict, further closing the opening of the larynx and preventing the movement of food into the respiratory system. When all openings to the pharynx are closed, a wave of muscular constriction passes over the walls of the pharynx, pushing the bolus of food toward the opening of the esophagus. As the food reaches the esophagus, the upper esophageal sphincter relaxes to accept the material.
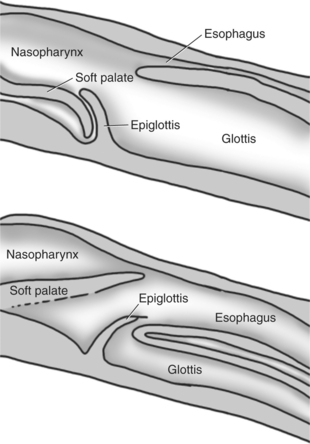
FIGURE 28-4 Midline cross-sectional schematics showing the position of the structures of the larynx and pharynx during breathing (top) and swallowing (bottom).
The complex reactions of deglutition are controlled by lower motor neurons located in various centers of the brainstem. Efferent nerve fibers from these centers travel in the facial, vagus, hypoglossal, and glossopharyngeal nerves, as well as the motor branch of the trigeminal nerve. Clinically, problems with prehension, mastication, and deglutition frequently are related to neurological lesions, either peripherally in the cranial nerves or centrally in the brainstem.
Motility of the Esophagus Propels Food from the Pharynx to the Stomach
The esophagus, as with other tubular portions of the gut, contains an outer longitudinal and inner circular layer of muscle. The esophagus is unique compared with other areas of the gut in that much of its muscular wall is composed of striated skeletal muscle fibers. In most domestic animals the entire length of esophageal musculature is striated. In horses, primates, and cats, however, a portion of the distal esophagus is smooth muscle. The striated muscle portions of esophagus are under control of somatic (not parasympathetic) motor neurons in the vagus nerve, whereas the smooth muscle portions are under direct control of the ENS and indirect control of the autonomic nervous system. A myenteric plexus exists throughout the entire length of the esophagus. In the area of striated muscle, the myenteric plexus probably serves a sensory function and acts to coordinate the movements of the striated muscle portion with the esophageal smooth muscle segments and stomach.
In terms of motor activity, the esophagus may be viewed as consisting of an upper sphincter, body, and lower sphincter. The upper esophageal sphincter is called the cricopharyngeal muscle. This muscle and the upper end of the esophagus are attached to the cricoid cartilage of the larynx. When deglutition is not taking place, the muscle compresses the end of the esophagus against the cartilage of the larynx, tightly closing the upper esophageal opening. During deglutition the cricopharyngeal muscle relaxes and the larynx is pulled forward. The ventral portion of the upper end of the esophagus is attached to the larynx and the dorsal portion to the cervical spine. Because of these attachments, the forward motion of the larynx in conjunction with the relatively fixed nature of the cervical spine tends to pull open the upper esophageal orifice passively (Figure 28-4).
The body of the esophagus serves as a relatively simple conduit, rapidly transferring food from the pharynx to the stomach. Food is propelled through the esophagus by propulsive movements known as peristalsis. Peristalsis consists of a moving ring of constriction in the wall of a tubular organ. In the esophagus, these rings start at the cranial end and progress toward the stomach. The rings reduce or obliterate the esophageal lumen, thus pushing the bolus of food ahead of them in much the same manner as a person would push material out of a soft rubber tube by stripping it with the fingers. In addition to the constriction of the circular muscles, there may be some contraction of longitudinal muscles just ahead of, or aboral to, the ring of circular muscle contraction. This longitudinal muscle activity increases the size of the esophageal lumen to accommodate the advancing food bolus (Figure 28-5). Peristalsis is a universal type of GI propulsive motility that exists at all levels of the gut.
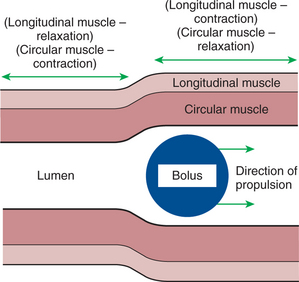
FIGURE 28-5 Peristalsis consists of a moving ring of luminal constriction preceded by an area of luminal distention. The area of constriction is created by contractions of the circular muscle, whereas the dilation is created by contractions of the longitudinal muscle. The net action is to propel a bolus of ingesta.
During deglutition the upper esophageal sphincter relaxes as the pharynx constricts; food is pushed into the upper portion of the esophageal body, and a wave of peristalsis propels the material toward the stomach. As the food bolus reaches the distal end of the esophagus, the lower sphincter relaxes, and the ingested matter enters the stomach. If the esophagus is not cleared of food material by the primary wave of peristalsis, secondary peristaltic waves are generated. One or more secondary waves are almost always adequate for pushing material into the stomach and clearing the esophagus. If food or foreign bodies become lodged in the esophagus, secondary waves of peristalsis may lead eventually to muscle spasms that constrict tightly around the lodged material. These spasms frequently interfere with therapeutic attempts to remove obstructing objects in the esophagus.
When deglutition is not taking place, the body of the esophagus is relaxed, but the upper and lower sphincters remain constantly constricted. The constriction of these sphincters is important because of the differences in external pressure applied to the esophagus at different points along its length. During the inspiratory phase of breathing, the portion of the esophagus within the thorax is subjected to less-than-atmospheric pressure. If the two esophageal sphincters were not tightly closed, inspiration would cause aspiration of air from the pharynx and reflux of ingesta from the stomach into the body of the esophagus, in the same manner as inspiration draws air into the lung. Stomach contents would be drawn into the esophagus because inspiratory pressures in the thorax are lower than intraabdominal pressure. It is particularly important that the lower esophageal sphincter remain closed during inspiration because the mucosa of the esophagus is not equipped to resist the caustic actions of gastric contents; thus movement of stomach contents into the esophagus would cause damage to the esophageal mucosa.
In many species the action of the lower esophageal sphincter is aided by the anatomical nature of the attachment of the esophagus and stomach. The esophagus enters the stomach obliquely, allowing distention of the stomach to block the esophageal opening in a valvelike manner. During deglutition the longitudinal muscle of the esophagus contracts, shortening the esophagus and opening the valve at the junction with the stomach. This anatomical arrangement, along with the lower esophageal sphincter, is particularly well developed in the horse, making reflux of stomach material into the esophagus extremely rare in this species. In many cases, when the intragastric pressure of the horse is pathologically raised, the stomach ruptures before vomiting or esophageal reflux takes place.
The Function of the Stomach Is to Process Food into a Fluid Consistency and Release It into the Intestine at a Controlled Rate
Among animals there is tremendous diversity in the anatomy and motility patterns of the stomach. The following discussion applies best to the animals with the simplest stomachs, such as the dog and cat, but is probably also a reasonable description of the activity of the somewhat more complex stomachs of the pig, horse, and rat. The complex motility patterns of the ruminant stomach are discussed in Chapter 31.
The function of the stomach is to serve food to the small intestine. There are two important aspects of this function: rate of delivery and consistency of material. The stomach serves both as a storage vat to control the rate of delivery of food to the small intestine and as a grinder and sieve that reduces the size of food particles and releases them only when they are broken down to a consistency compatible with small-intestinal digestion.
The stomach is divided into two physiological regions, each of which has a different impact on gastric function. The proximal region, at the esophageal end of the stomach, serves a storage function, retaining food as it awaits eventual entry into the small intestine. The distal region serves a grinding and sieving function, breaking solid pieces of food down into particles small enough for small-intestinal digestion.
The Proximal Stomach Stores Food Awaiting Further Gastric Processing in the Distal Stomach
The major muscular activity in the proximal region of the stomach is of a weak, continuous-contraction nature. These tonic contractions tend to shape the gastric wall to its contents and provide gentle propulsion of material into the distal stomach. The major muscular reflex of the proximal stomach is adaptive relaxation (Figure 28-6). This reflex is characterized by relaxation of the muscles as food enters the stomach. Because of this relaxation, the stomach can dilate to accept large quantities of food without an increase in intraluminal pressure. Thus the proximal stomach serves as a food storage area. Because of the rather passive muscular activity of the proximal stomach, little mixing occurs there. In fact, food boluses tend to become layered in the stomach in the order in which they were swallowed. As the stomach empties, tension on the wall of the proximal stomach increases slightly, pushing food distally in the stomach, where it can be processed for transport into the duodenum.
The Distal Stomach Grinds and Sifts Food Entering the Small Intestine
The muscular activity of the distal stomach and pylorus (sphincterlike junction between stomach and duodenum) is completely different from that of the proximal stomach. In the distal stomach, known as the antrum, there is intense slow-wave activity, and muscular contractions are frequently present. Strong waves of peristalsis begin at about the middle of the stomach and migrate, with the slow waves, toward the pylorus. As the waves of peristalsis near the pylorus, the pylorus constricts, blocking the gastric exit of all but the smallest particles (Figure 28-7). Particles leaving the stomach during the digestive phase of activity are less than 2 mm in diameter. Particles too large to pass the pylorus are crushed and ejected back into the antrum by the passing wave of peristalsis. Thus the peristaltic actions of the distal stomach walls serve not only to propel food but also, and perhaps more importantly, to grind and mix it.
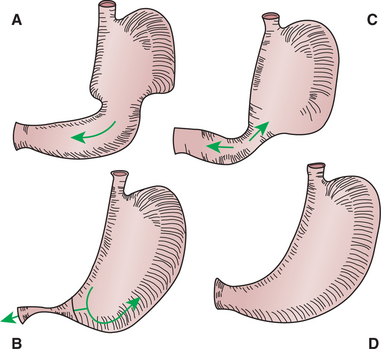
FIGURE 28-7 Grinding and churning activity of the distal stomach. A, Wave of peristalsis begins at the junction of the proximal and distal areas of the stomach and moves toward the pylorus. B, As the peristaltic wave approaches the pylorus, the pylorus constricts, causing some of the ingesta to be crushed within the peristaltic ring and propelled back toward the proximal stomach. C, As the peristaltic wave reaches the pylorus, some finely ground and liquefied material passes through into the duodenum, but the majority of material has been propelled back into the stomach. D, Between contractions, no gross movement of gastric contents occurs.
(From Johnson LR, editor: Gastrointestinal physiology, St Louis, 1985, Mosby.)
Control of Gastric Motility Differs in the Proximal and Distal Stomach
The motility of the stomach, as in other smooth muscle portions of the gut, is under neurohumoral control. Fibers of the vagus nerve synapse on nerve cell bodies of the extensive gastric myenteric plexus and exert a high degree of control over gastric motility. The effects of vagal stimulation on the proximal and distal regions of the stomach are opposite; in the proximal stomach, vagal activity suppresses muscular contractions and leads to adaptive relaxation, whereas in the distal stomach, vagal stimulation causes intense peristaltic activity. Vagal stimulation of distal antral motility is mediated by acetylcholine, but vagal inhibition of proximal stomach motility is not. The identity of the inhibitory mediator is not well established, but it may be vasoactive intestinal peptide.
Vagal action on the stomach is stimulated by events occurring within the CNS, as well as within the stomach and intestine. The anticipation of food consumption causes vagal stimulation of the stomach and thus primes the stomach to receive a meal. Reactions of the GI tract that originate in the CNS in response to anticipated food intake are often referred to as the cephalic phase of digestion. Reactions to the cephalic phase are then augmented as food enters the stomach. In response to food in the stomach, vagal activity increases as sensory receptors in the stomach create a positive feedback loop.
The exact role of hormones in regulation of gastric motility is not completely established. Gastrin, which is secreted from cells in the gastric antrum, appears to enhance gastric motility. Cholecystokinin (CCK), secretin, and gastric inhibitory peptide appear to suppress gastric motility, at least in the dog. The roles of the various GI hormones are difficult to determine from available information, because many of the experimental results reported have been in response to administration of GI hormones at amounts far greater than those normally occurring.
The Rate of Gastric Emptying Must Match the Small Intestine’s Rate of Digestion and Absorption
The rate at which food leaves the stomach must match the rate at which it can be digested and absorbed by the small intestine. Because some types of foods can be digested and absorbed more rapidly than others, the rate at which the stomach empties must be regulated by the contents of the small intestine. Thus there are reflexes that regulate gastric emptying and allow the stomach to serve as a storage site. The afferent receptors of these reflexes are in the duodenum and are activated by low pH, high osmolality, and the presence of fat. Separate sensory receptors apparently exist for each of these stimuli, but these receptors have not been identified anatomically.
Many reflexes occur within the GI system. Their names usually reflect the site of origin of the afferent stimulus and the site of the efferent response. Thus, reflex control of gastric emptying by the duodenum is referred to as the enterogastric reflex (“entero” referring to the intestine).
The arc of the enterogastric reflex probably involves both the CNS and the ENS, as well as the endocrine/paracrine system (Figure 28-8). The extrinsic reflex pathway appears to involve afferent fibers of the vagus nerve, which receive stimuli in the duodenum. These stimuli are integrated in the brainstem, and the response is mediated by vagal efferent fibers to the stomach. The enteric reflex arc involves receptors in the duodenum and nerve fiber connections in the ENS that directly affect gastric emptying.
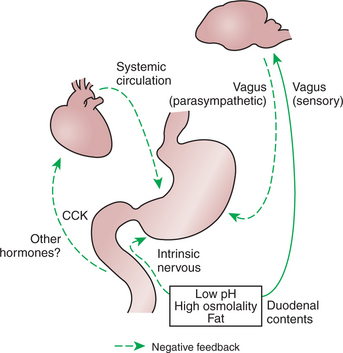
FIGURE 28-8 Inhibitory arcs of the enterogastric reflex. Low pH, high osmolality, and the presence of fat in the duodenum stimulate vagal, enteric neuronal, and hormonal reflexes that inhibit stomach emptying. After the duodenal pH and osmolality have moderated and some of the fat has been absorbed, the inhibitory influences on the stomach are removed. CCK, Cholecystokinin.
A contribution of the GI endocrine system to the enterogastric reflex has long been suspected, but the exact hormones responsible for the reflex are not known. CCK and secretin may be important. Both hormones are secreted by cells in the duodenum; CCK is secreted in response to fat and secretin in response to low pH; both appear to have suppression of gastric emptying as secondary effects. Gastric inhibitory peptide is a hormone produced in the duodenum in response to the presence of carbohydrate. In the dog, gastric inhibitory peptide may function as an inhibitor of gastric emptying, although stimulation of insulin secretion is probably its major action.
Enterogastric reflexes control gastric emptying by regulating stomach motility. The manner in which motility affects gastric emptying of solids is different from that for liquids. The rate at which solids are expelled from the stomach is regulated by the rate at which they are broken down into particles small enough to pass through the pylorus. This in turn is controlled by the motility of the antrum, or distal stomach; the greater the motility of the antrum, the faster material is broken down. Thus, antrum motility regulates the rate of release of solid material from the stomach. Liquid material leaves the stomach more quickly than solid matter, and the release of liquid may be less dependent on antral motility than on the motility of the proximal stomach.
Minimal mixing activity occurs in the proximal stomach. Therefore, liquids and solids tend to separate, the liquids moving to the outside and the solids to the center of the mass of food in the proximal stomach. Increased tension in the wall of the stomach body forces liquid into the antrum. Liquid may leave the antrum quickly, dependent on the activity of the pylorus. On the other hand, increased tension in the stomach body has little effect on the transport of solid material, because such material cannot leave the gastric body until sufficient space has been made available in the antrum. Thus, motility of the stomach body appears primarily responsible for the liquid-emptying rate, whereas motility of the antrum is most responsible for the solid-emptying rate. The effect of the pylorus itself on gastric emptying is not as great as might be expected; removal of the pylorus results in a slight increase in the liquid-emptying rate and little increase in the rate of emptying of solid material. It appears that the distal portion of the antrum can account for much of the sieving action usually attributed to the pylorus. The rate of emptying of an isotonic liquid from the stomach is exponential and dependent on the initial volume of the liquid meal. Under usual circumstances, a liquid meal in the canine stomach has a half-life of about 18 minutes and is essentially gone by 1 hour after ingestion. Solid material is emptied more slowly, and its rate depends on its fat content. Low-fat meat meals are usually gone from the stomach in 3 to 4 hours after ingestion.
Between Meals, the Stomach Is Cleared of Indigestible Material
Some types of ingested materials, such as bone and indigestible foreign objects, cannot be reduced to particles less than 2 mm in diameter. During the digestive phase of gastric motility, such material does not leave the stomach. To clear the stomach of indigestible debris, a particular type of motility occurs between meals. This motility pattern is called the interdigestive motility complex. In association with this complex, the pylorus relaxes as strong waves of peristalsis sweep over the antrum, forcing less digestible material into the duodenum. This type of motility appears to have a “housekeeping” function in clearing the stomach of indigestible material.
The peristaltic waves of the interdigestive motility complex occur at approximately 1-hour intervals during the periods when the stomach is relatively empty of digestible material. Eating disrupts the complex and causes the resumption of the digestive motility pattern. Herbivores, which eat almost constantly, have a slightly different pattern; the interdigestive motility complex occurs at approximately hourly intervals, even with digestible food present in the stomach.
Vomiting Is a Complex Reflex Coordinated from the Brainstem
Vomiting is a complex reflex activity, and its integration, or coordination, is centered in the brainstem. The act of vomiting involves many striated muscle groups and other structures outside the GI tract. Vomiting is associated with the following actions:
1. Relaxation of the muscles of the stomach and lower esophageal sphincter and closing of the pylorus.
2. Contraction of the abdominal musculature, creating an increase in intraabdominal pressure.
3. Expansion of the chest cavity while the glottis remains closed; this action lowers intrathoracic pressure and thus the pressure in the body of the esophagus.
4. Opening of the upper esophageal sphincter.
5. Antiperistaltic motility (peristaltic motility propelling ingesta toward the mouth) in the duodenum, which may precede the previous actions; thus vomiting may include ingesta of intestinal origin.
The efferent limb of this reflex arc involves motor fibers in many different peripheral nerves.
Afferent stimulation of the vomiting reflex comes from a large number of receptors. Of particular importance are mechanoreceptors in the pharynx and tension receptors and chemoreceptors in the gastric and duodenal mucosa. Stimulation of these receptors sends signals to the vomit center in the brainstem. Thus, noxious tactile or chemical stimulation of the GI mucosa can result in vomiting, clearing, or attempting to clear the offending stimulus from the GI tract. Direct irritation of GI structures, however, is not the only stimulus for vomiting. The vomit center receives afferent input from a variety of organs; thus vomiting is not always an indication of a primary GI problem.
An important structure outside the GI tract that supplies afferent input in the vomit center is the chemoreceptor trigger zone. This is an area of the brainstem that lies in contact with the third ventricle. The chemoreceptor trigger zone is sensitive to the presence of some drugs and toxins in the blood. When stimulated, this zone sends signals to the vomit center and induces vomiting. Some of the products of inflammation stimulate the chemoreceptor trigger zone. Thus, inflammatory disease, even outside the GI tract, can sometimes lead to vomiting.
The semicircular canals of the inner ear are other important structures that supply afferent input to the vomit center. Constant stimulation of the semicircular canals may induce vomiting, as occurs in motion sickness. Other sites in the body also may stimulate the vomit center; thus vomiting is a rather nonspecific sign of disease.
Motility of the Small Intestine Has Digestive and Interdigestive Phases
Motility of the small intestine occurs in two distinct phases: (1) during the digestive period after food intake and (2) during the interdigestive period when little food is present in the gut. In the digestive phase there are two primary motility patterns: propulsive and nonpropulsive. The nonpropulsive pattern is referred to as segmentation. Segmentation results from localized contractions of circular muscle. Portions of small intestine, usually 3 to 4 cm long, contract tightly, dividing the gut into segments of constricted and dilated lumen. Within a few seconds, the constricted portions relax and new areas constrict (Figure 28-9). This action tends to “milk” gut contents back and forth within the small intestine, mixing them with digestive juices and circulating them over the absorptive mucosal surfaces. This type of motility does not contribute much to the net aboral propulsion of ingesta. In fact, segmentation tends to slow down the aboral movement of material because of closure of the intestinal lumen in the constricted segments.
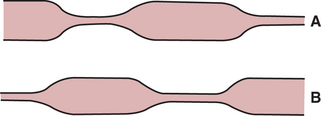
FIGURE 28-9 Segmentation in the small intestine. A, Areas of circular muscle constriction close the lumen and divide the gut into dilated segments containing ingesta. B, At periodic intervals the areas of constriction and dilation alternate, exerting a mixing and circulating action on the ingesta.
Propulsive activity during the digestive phase consists of peristaltic contractions that migrate down the gut in phase with the slow waves. Digestive-phase peristaltic contractions, in contrast to interdigestive-phase peristalsis, pass over short segments of intestine and then die out. Thus, ingesta are pushed down the gut for a short distance and then subjected to additional segmentation contractions and mixing activity.
The interdigestive phase of small-intestinal motility is characterized by waves of powerful peristaltic contractions that sweep over a large length of small intestine, sometimes traversing the entire organ. These waves are referred to as the migrating motility complex (MMC) or, alternatively, the migrating myoelectric complex. The MMC begins in the duodenum as groups of slow waves that stimulate intense action potential and muscular contraction activity. The complex migrates down the intestine at the rate of the slow waves. Some of the MMCs die out before reaching the ileum, but some travel the entire length of the small intestine.
The MMC probably has a “housekeeping” function and serves to push undigested material out of the small intestine. The MMC may also be important in controlling the bacterial population in the upper gut. Normally the duodenum harbors a relatively small population of bacteria, and the population increases distally into the ileum, which has a moderately large number of bacterial organisms. The colon is heavily colonized by numerous species of bacteria. It is important for digestive function that this relative distribution of bacteria be maintained within the gut. The MMC may help to impede the migration of bacteria from the ileum to the duodenum.
The Ileocecal Sphincter Prevents Movement of Colon Contents Back into the Ileum
The ileocecal sphincter is at the junction of the small and large bowel and prevents the retrograde movement of colon contents into the ileum. It consists of a well-developed ring of circular muscle that remains constricted at most times. In addition to the muscular sphincter, in many species there is a flap of mucosa that acts as a one-way valve, further blocking movement of colon contents into the ileum. During periods of peristaltic activity in the ileum, the sphincter relaxes, allowing movement of material into the colon. When colonic pressure increases, the ileocecal sphincter constricts more tightly.
Motility of the Colon Causes Mixing, Retropulsion, and Propulsion of Ingesta
The colon has multiple functions, including (1) absorption of water and electrolytes, (2) storage of feces, and (3) fermentation of organic matter that escapes digestion and absorption in the small intestine. The relative importance of these functions varies with the species, and tremendous differences in colon size and shape exist among animals. The major determinant of colon size is the importance of colonic fermentation to the energy needs of the animal. Some species, such as the horse and rabbit, make extensive use of fermentation products for nutritional needs and have large and complex colons. (The ruminant’s fermentation chamber is in the stomach.) Other species, such as the dog and cat, do not rely on fermentation products and have relatively simple colons. Figure 28-10 illustrates differences in colonic anatomy among four species with different needs for fermentative digestion.
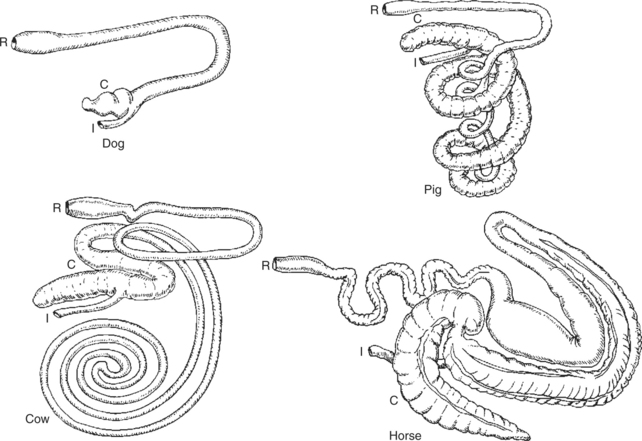
FIGURE 28-10 Variations of colon anatomy of four mammals. Animals with simple colons, such as the dog, are not dependent on colonic fermentation to supply energy needs. Horses, which have tremendous colonic development, rely on colonic fermentation for a large portion of their energy needs. In animals such as pigs and cattle, the importance of colonic fermentation to digestive needs is intermediate between that of the horse and dog, and this intermediate position is noted in their colon development. C, Cecum; I, ileum; R, rectum.
Considerable similarity appears to exist in colon motility patterns among animals, despite the anatomical diversity. Mixing activity is prominent in the colons of all species because mixing and circulating are important to both absorptive and fermentative functions. Mixing is achieved by segmentation contractions along with other types of motility. In many species, such as the horse and pig, colonic segmentation is pronounced and in some areas results in the formation of sacculations known as haustra, which are visible even after death.
A particular characteristic of colonic motility is retropulsion, or antiperistalsis. This type of peristaltic contraction migrates orally, the opposite of normal peristaltic movement. Such motility results from colonic slow-wave activity that is somewhat more complex than that of the small intestine. In the colon, as in the small intestine, the slow waves originate in the ICC. The colonic ENS, however, can influence the ICC in such a manner as to shift the site of origin of slow waves and the direction of their propagation. Under resting conditions in the colon, slow waves originate from “pacemakers” in one or more central sites. The pacemakers are not anatomical structures; rather, they are areas defined by activities of the ENS. Thus the pacemakers are not always the same areas; they can disappear and form in different locations in response to the need for different motility patterns. Antiperistaltic contractions occur in the segments in which slow waves migrate in an oral direction. Antiperistaltic contractions are retropulsive and impede the movement of ingesta, causing intense mixing activity and forcing material to accumulate in the proximal portions of the colon. Retropulsion appears to be particularly strong near the pacemakers, and the pacemakers therefore represent sites of high resistance to the flow of colonic ingesta.
Because of continued inflow of material from the ileum into the colon, some ingesta escape the retropulsive, antiperistaltic motility, move into areas of propulsive, peristaltic activity, and proceed along the colon. In addition, there are periods of intense propulsive activity that involve the entire colon. These are called mass movements and frequently involve the distal translocation of the entire colonic content.
The Colon Is an Important Site of Storage and Absorption in All Animals
The colon of the dog and cat is a relatively simple organ consisting of a short cecum, an ascending part, a transverse part, and a descending part. During the resting phase, there is a colonic pacemaker at about the junction of the transverse and descending colons (Figure 28-11). This gives rise to antiperistaltic activity in the proximal colon, with resultant accumulation of ingesta in the cecum and ascending colon areas. Moderate peristaltic activity usually occurs in the descending colon, whereas the distal colon and rectum are usually constricted and empty.
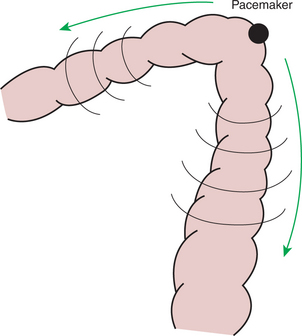
FIGURE 28-11 A pacemaker is present at the junction of the transverse and descending parts of the colon of the cat and probably in other mammals with similar colonic anatomy. Slow waves and peristaltic activity emanate in both directions from the pacemaker. Retrograde, or reverse, peristalsis in the proximal portions of the colon causes ingesta to be retained there, promoting the storage and absorptive functions of the colon.
Material entering the carnivore colon is of a fluid consistency. It is thoroughly mixed in the ascending and transverse colons, and much of the water and many of the electrolytes are absorbed. By the time it reaches the descending colon, it is semisolid and becoming feces.
Despite Large Anatomical Differences in the Colons of Herbivores Compared to Omnivores and Carnivores, There Are Similarities in Motility
There are important similarities in motility among various species, even those with extensive anatomical differences. The purpose of this discussion is to describe the similarities in motility between species with simple colons and those with complex colons. Chapter 31 provides a more extensive discussion of the highly developed colons of herbivores.
The equine hindgut, as an example of an herbivore hindgut, is complex and highly developed (see Figure 28-10). The cecum is large and separated into haustra. The equine cecum is unique among ceca of most species, even other herbivores, because a distinct, sphincterlike orifice joins it to the colon. The colon is divided into a large and a small portion, and the large colon is folded on itself so that three distinct flexures exist. The longitudinal muscles of the cecum and most areas of the colon are not evenly dispersed around the circumference of the gut. Instead, they form discrete bands, or teniae, that course along the longitudinal axis of the gut. The teniae divide the haustra longitudinally, giving the equine cecum and large colon a sacculated appearance.
Motility in the equine cecum consists of active segmentation and mixing, along with occasional mass movements that appear to transfer large amounts of ingesta to the colon. Motility in the colon consists of segmentation, antiperistalsis, and peristalsis. A colonic pacemaker appears to exist at the pelvic flexure and creates an area of high resistance to flow that results in prolonged retention of material in the ventral portions of the large colon. The pelvic flexure pacemaker in the equine colon is similar in function to the colonic pacemaker in the transverse colon of the dog and cat. Little is known about regulation of motility in the equine small colon. The characteristic ball-shaped form of equine feces probably represents intense segmentation-type motility in the small colon, where the feces are formed (see Chapter 31).
In ruminants and swine, the hindgut consists of a cecum of intermediate complexity, a spiral colon, and a straight colon. Compared with other species, less is known about the hindgut motility of animals with spiral colons. There seems to be an area of high flow resistance at the flexure, or central point, of the spiral colon. This site of flow resistance may represent a pacemaker that generates antiperistaltic motility in the centripetal portion of the colon.
The Anal Sphincter Has Two Layers with Separate Innervation
The anal opening is constricted by two sphincters: an internal sphincter of smooth muscle, which is a direct extension of the circular muscle layer of the rectum; and an external sphincter of striated muscle. The internal anal sphincter usually remains tonically contracted and is responsible for anal continence. The internal sphincter receives parasympathetic innervation from the sacral spinal segments through the pelvic nerve and sympathetic innervation from the lumbar spinal segments through the hypogastric nerve. In most species, sympathetic stimulation results in constriction of the sphincter, and parasympathetic stimulation results in relaxation.
The external sphincter maintains some degree of tonic contraction, but the consistent tone of the anus is primarily regulated by the internal sphincter. The external sphincter is innervated by general somatic efferent fibers that have cell bodies in the cranial sacral spinal segments and course in the pudendal nerve.
The Rectosphincteric Reflex Is Important in Defecation
The entry of feces into the rectum is accompanied by the reflex relaxation of the internal anal sphincter, followed by peristaltic contractions of the rectum. This is known as the rectosphincteric reflex and is an important part of the act of defecation (Figure 28-12). The reflex normally results in defecation, but in trained animals its effects can be blocked by voluntary constriction of the external anal sphincter. When defecation is voluntarily suppressed, the rectum soon relaxes to accommodate the fecal bolus, and the internal anal sphincter regains its tone. In humans, and presumably in dogs and cats, relaxation of the rectum and constriction of the internal sphincter is associated with fading of the urge to defecate, until another bolus of feces enters the rectum.
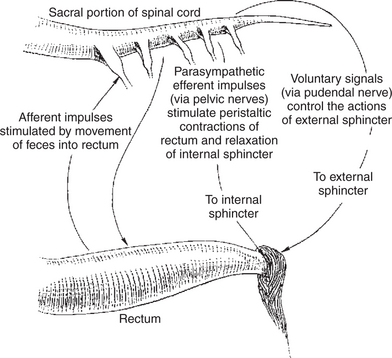
FIGURE 28-12 Arcs of the rectosphincteric reflex. The reflex is initiated by the movement of feces into the rectum and results in peristaltic movements of the rectal wall and relaxation of the internal anal sphincter. Fecal passage is the normal effect of the reflex, but voluntary constriction of the external anal sphincter can prevent the passage of feces and eventually override the reflex, apparently enabling trained animals to suppress the urge to defecate.
Uninhibited animals respond to the presence of feces in the rectum with a number of voluntary actions associated with defecation. In carnivores the diaphragm and abdominal muscles contract to increase intraabdominal pressure, and striated muscles of the anal canal relax as the animal assumes the defecation posture. These acts are important for complete evacuation of the rectum.
Major Differences Between Avian and Mammalian Digestive Systems Include, in Birds, Both the Lack of Teeth and the Separation of Gastric Functions into Distinct Anatomical Regions
Important anatomical differences exist between the digestive systems of birds and mammals. These differences affect motility functions more than other aspects of digestion, such as secretion, digestion, and absorption. Therefore the digestive tract of birds is covered in this chapter as a separate topic. In other chapters of this section, aspects of avian digestion are integrated into the general discussion.
Figure 28-13 illustrates the general anatomy of the avian digestive system. The pharynx of birds is simpler than that in mammals, with birds having no soft palate. There are no teeth, although in carnivorous species, the beak is modified for tearing food into pieces small enough to swallow. The esophagus has a large diameter so as to accommodate unmasticated food. An outpouching of the esophagus is known as the crop. The extent of development of the crop varies widely among avian species. The glandular portion of the stomach is the proventriculus, which is separated from the muscular stomach, known as the ventriculus or gizzard, by a short isthmus. The small intestine varies greatly in length among avian species but is generally rather short compared with that of mammals of similar size. The ceca are usually paired and vary tremendously in development among avian species. In some carnivorous species, such as the hawk, the ceca are rudimentary, whereas in some of the nonflying herbivores, such as the ostrich, cecal development is extensive (see Figure 28-13). The colon and rectum are very simple; the rectum ends in the cloaca, which is a common passageway for digestive, urinary, and reproductive discharges.
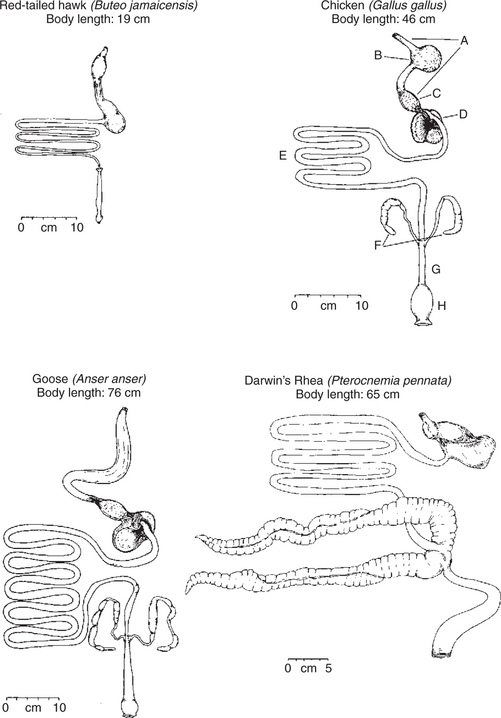
FIGURE 28-13 Comparative anatomy of the digestive tracts of four avian species. Note the variation in crop and cecal development. The carnivorous red-tailed hawk has a small crop and rudimentary ceca. The chicken has a well-developed crop, and the rhea has tremendous cecal development. A, Esophagus; B, crop; C, proventriculus; D, gizzard or ventriculus; E, small intestine; F, ceca; G, rectum; H, cloaca.
(From Stevens CD: Comparative physiology of the vertebrate digestive system, Cambridge, UK, 1988, Cambridge University Press.)
The crop performs a storage function. In some species the crop is little more than a pouch in the esophagus, whereas in others, such as the chicken, there is a distinct sphincterlike opening between the esophagus and crop. In general, ingesta do not begin to accumulate in the crop until the gizzard is full. The crop is richly populated with mucus-secreting cells, but no digestive glands are present. However, digestive glandular secretions originating from the salivary glands and proventriculus are present in the crop. In many species it appears that ingesta and secretions pass in a retrograde manner up the esophagus from the gizzard and proventriculus to the crop. The motility of the crop is under regulation of vagal impulses. Crop motility and rate of emptying are coordinated so as to release ingesta at a rate matching the emptying rate of the proventriculus and gizzard. In some avian species the crop also functions as a storage place for food being transported to the young. In this case, food is swallowed into the crop and later regurgitated as feed for offspring.
The proventriculus is a low-volume organ with a glandular epithelium resembling that of the stomach of mammals (see Chapter 29). Motility functions of the proventriculus are to propel ingesta and digestive secretions into the gizzard for mixing and grinding. The gizzard is a muscular organ that grinds and liquefies ingesta. In addition, particle-size discrimination occurs in the gizzard; small particles are passed into the duodenum, whereas large particles are retained for further comminution or ejected back into the proventriculus for further addition of digestive secretions. In carnivorous birds, concretions of bone, hair, feathers, and other indigestible material accumulate in the gizzard and are occasionally ejected orally in an action known as egestion. In grain-eating birds, small stones or gravel are swallowed and retained in the gizzard to aid in the comminution of ingesta. This inorganic material is referred to as grit, and its presence increases digestive efficiency, although it is not essential. The mucosa of the gizzard is covered by a tough coating known as koilin. This coating is composed of glandular secretions and desquamated cells. It protects the mucosa from the physical grinding actions of the gizzard.
The motility and function of the various stomach areas of birds are easily comparable with gastric motility and function in mammals. The crop and proventriculus function in much the same manner as the fundus and body of the mammalian stomach, with storage and secretory functions. The gizzard functions in much the same way as the antrum of the mammalian stomach, with grinding and particle-size discrimination functions. The major functional differences between birds and mammals include the physical separation of the stomach compartments in birds and the advanced grinding function of the gizzard.
The motility patterns of the avian small intestine appear to be generally similar to those in mammals. The motility of the hindgut also shares some characteristics of other animals. Reverse peristalsis is a dominant characteristic of the avian colon and rectum, moving ingesta into the ceca. Urinary excretions arriving at the cloaca become incorporated with ingesta and move in a retrograde manner into the ceca, thus facilitating reabsorption of the remaining water and electrolytes from urine. Cecal motility is characterized primarily by mixing and reverse peristalsis, with occasional mass movements resulting in the evacuation of the ceca. These mass movements in avian species are followed by defecation.
CLINICAL CORRELATIONS
Equine Rabies
History.
Owners report that their horse has not “been itself” for the past few days. Today the animal is extremely lethargic and stands with forelegs wide apart and head held low. The nostrils are soiled, and the owners report that water and feed come out of the nostrils when the animal attempts to eat or drink.
Clinical Examination.
From the history and presenting signs, you recognize that the horse may have paralysis of muscles of the pharynx and larynx. Because these lesions are typically associated with rabies in horses, you don a pair of plastic gloves and sleeves and proceed with your examination. To assess the function of the swallow reflex, you attempt to pass a stomach tube. You observe that the swallow reflex appears to be diminished, but that with some persistence the tube can be passed. This indicates that there is no physical obstruction in the pharynx or esophagus and that the problem is functional. These findings support, but do not confirm, a diagnosis of rabies.
Comment.
Rabies in herbivorous animals may take a number of forms. One of the most common signs in cattle and horses is paralysis of the pharynx and larynx as a result of viral lesions in the brainstem nuclei supplying the appropriate cranial nerves. If rabies is suspected, no one should come into direct contact with the excretions of the animal, especially saliva.
Treatment.
In this horse, treatment should consist of oral fluid and electrolyte therapy administered through an indwelling stomach tube. If there is no response to this conservative therapy, and if the animal’s condition appears to deteriorate, euthanasia is necessary, and the animal’s head should be submitted for evaluation for a positive diagnosis of rabies.
Biancani P, Harnett KM, Behar J. Esophageal motor function. Yamada T, ed. Textbook of gastroenterology, ed 4, vol. 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Clouse RE, Diamant NE. Motor function of the esophagus. Johnson LR, ed. Physiology of the gastrointestinal tract, ed 4, vol 1. Amsterdam: Elsevier Science & Technology Books, 2006.
Duke GE. Alimentary canal: anatomy, regulation of feeding and motility. In: Sturkie PD, ed. Avian physiology. New York: Springer-Verlag, 1986.
Hanani M, Freund HR. Interstitial cells of Cajal: their role in pacing and signal transmission in the digestive system. Acta Physiol Scand. 2000;170:177.
Hasler WL. Motility of the small intestine and colon. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Hasler WL. The physiology of gastric motility and gastric emptying. Yamada T, ed. Textbook of gastroenterology, ed 4, vol. 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Hasler WL. Small intestinal motility. Johnson LR, ed. Physiology of the gastrointestinal tract, ed 4, vol 1. Amsterdam: Elsevier Science & Technology Books, 2006.
Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol. 1999;61:19.
Kerlin P, Zinsmeister A, Phillips S. Relationship of motility to flow of contents in the human small intestine. Gastroenterology. 1982;82:701.
Makhlouf GM. Smooth muscle of the gut. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Makhlouf GM, Murthy KS. Cellular physiology of gastrointestinal smooth muscle. Johnson LR, ed. Physiology of the gastrointestinal tract, ed 4, vol 1. Amsterdam: Elsevier Science & Technology Books, 2006.
Merrit AM. Normal equine gastroduodenal secretion and motility. Equine Vet J Suppl. 1999;29:7.
Rasmussen OO, Christiansen J. Physiology and pathophysiology of anal function. Scand J Gastroenterol Suppl. 1996;216:169.
Sanders KM, Koh SD, Ward SM. Organization and electrophysiology of interstitial cells of Cajal and smooth muscle cells in the gastrointestinal tract. Johnson LR, ed. Physiology of the gastrointestinal tract, ed 4, vol 1. Amsterdam: Elsevier Science & Technology Books, 2006.
Sarna S. Function and regulation of colonic contractions in health and disease. Johnson LR, ed. Physiology of the gastrointestinal tract, ed 4, vol 1. Amsterdam: Elsevier Science & Technology Books, 2006.
Shaker R. Pharyngeal motor function. Johnson LR, ed. Physiology of the gastrointestinal tract, ed 4, vol 1. Amsterdam: Elsevier Science & Technology Books, 2006.
Stevens CE, Hume ID. Comparative physiology of the vertebrate digestive system, ed 2. Cambridge, UK: Cambridge University Press, 1996.
Weisbrodt NW. Gastric emptying. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Weisbrodt NW. Motility of the large intestine. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Weisbrodt NW. Motility of the small intestine. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Weisbrodt NW. Regulation: nerves and smooth muscle. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Weisbrodt NW. Swallowing. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
PRACTICE QUESTIONS
1. A unique feature of gastrointestinal (GI) smooth muscle cells is that:
2. The term slow waves as applied to the gut refers to:
3. An animal is presented to you with aspiration pneumonia (the result of food material entering the lower respiratory tract). Which of the following lesions would be a likely cause?
4. The term cephalic phase is used in reference to a number of activities occurring in the GI tract. In general, the term means:
5. Conditions in the duodenum, such as low pH or high fat concentration, can reflexively inhibit gastric emptying. Which reflex arc is involved in this inhibition?
6. Which of the following best describes the motility of the proximal region of the monogastric stomach?
7. Which of the following is characteristic of the interdigestive phase of small intestinal motility?
8. Which of the following aspects of colon physiology is common to many species, irrespective of interspecies anatomical differences in colon structure?