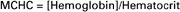Chapter 18 Overview of Cardiovascular Function
1. Because normal cardiovascular function is essential for life and health, a practical understanding of cardiovascular function and dysfunction is vital to the veterinary clinician.
2. Cardiovascular dysfunctions sometimes reflect primary cardiovascular disturbances or diseases, but more often they are secondary consequences of noncardiovascular disturbances or diseases.
3. Substances transported by the cardiovascular system include nutrients, waste products, hormones, electrolytes, and water.
4. Two modes of transport are used in the cardiovascular system: bulk flow and diffusion.
5. Because diffusion is very slow, every metabolically active cell in the body must be close to a capillary carrying blood by bulk flow.
6. The pulmonary and systemic circulations are arranged in series, but the various organs within the systemic circulation are arranged in parallel.
7. Cardiac output is the volume of blood pumped each minute by one ventricle.
8. The perfusion pressure for the systemic circulation is much greater than the perfusion pressure for the pulmonary circulation.
9. Each type of blood vessel has physical properties suited to its particular function.
10. Blood is a suspension of cells in liquid (plasma).
11. The cellular component of blood includes red blood cells, white blood cells, and platelets.
12. Most of the oxygen in blood is carried in chemical combination with the protein hemoglobin within red blood cells.
Because Normal Cardiovascular Function Is Essential for Life and Health, a Practical Understanding of Cardiovascular Function and Dysfunction Is Vital to the Veterinary Clinician
Cardiovascular physiology is the study of the function of the heart, the blood vessels, and the blood. The primary function of the cardiovascular system can be summarized in one word: transport. The bloodstream transports numerous substances that are essential for life and health, including the oxygen and nutrients required by every cell in the body. Blood also carries carbon dioxide and other metabolic waste products away from metabolically active cells and delivers them to the lungs, kidneys, or liver, where they are excreted.
To appreciate the importance of cardiovascular transport, the reader need only consider what happens if the heart stops contracting and circulation ceases: unconsciousness results within about 30 seconds, and irreversible damage to the brain and other sensitive body tissues occurs within a few minutes. However, circulation does not have to stop completely for significant dysfunction to occur. For example, the loss of as little as 10% of the normal blood volume can impair exercise performance. In each tissue of the body, normal function depends on the delivery of blood flow. The higher the rate of metabolism in a tissue, the greater is the requirement for blood flow. The condition of inadequate blood flow to any tissue is called ischemia. Even transient ischemia leads to dysfunction. Persistent ischemia leads to permanent tissue damage (infarction) or cell death (necrosis).
Impairment in the transport functions of the cardiovascular system is encountered frequently in veterinary medicine. Because any cardiovascular impairment inevitably leads to significant dysfunction and loss of health, a practical understanding of cardiovascular function and dysfunction is vital to the veterinary clinician.
Many veterinary students have difficulty understanding cardiovascular physiology. They tend to agree with William Harvey, the father of cardiovascular physiology, whose initial impression was that the motions of the heart and the blood were so complicated that they could be comprehended only by God! Harvey persisted, however, in a careful, deliberate study of cardiovascular function and in 1628 set forth the first proof that the heart propels blood through the blood vessels in a circulatory pattern. Before Harvey’s time, it was thought that blood flowed out of the heart into the blood vessels and then returned to the heart by backward flow in the same vessels. In other words, blood was thought to flow in a tidal manner, in much the same way that air flows through a single set of airways: first into the lungs and then back out.
We now take for granted that the cardiovascular system is a circulatory system, not a tidal system. However, the circularity of the cardiovascular system is precisely what makes it difficult to understand. It has no clear beginning or ending, and disturbances in one part of the cardiovascular system end up affecting all other parts as well. In recognition of this complexity, Chapters 18 to 26 are written with the goal of identifying the most basic and important concepts of normal cardiovascular function and explaining them in a way that best prepares the reader to understand, diagnose, and treat cardiovascular dysfunction (cardiovascular disease).
Cardiovascular Dysfunctions Sometimes Reflect Primary Cardiovascular Disturbances or Diseases, but More Often They Are Secondary Consequences of Noncardiovascular Disturbances or Diseases
Some of the cardiovascular dysfunctions encountered in veterinary medicine are primary, in that the fundamental disturbance or disease process affects the cardiovascular system directly. One example of primary cardiovascular dysfunction is hemorrhage (loss of blood from blood vessels). Another is myocarditis (literally, “muscle-heart-inflammation”). Myocarditis is caused when a viral or bacterial infection inflames the heart muscle, impairing the ability of the heart to pump blood.
Cardiovascular dysfunction and disease can be either congenital (present at birth) or acquired (developing after birth). Myocarditis is an example of an acquired cardiovascular disease, and there are many others. Congenital cardiovascular diseases frequently involve defective heart valves, which either cannot open fully or cannot close completely. Congenital cardiac defects are common in certain breeds of dogs and horses. Although a heart that has a congenital defect or an acquired disease may be able to pump an adequate amount of blood when the animal is at rest, it usually cannot deliver the increased blood flow required by the body during exercise. When a dysfunction in the heart impairs its ability to pump the amount of blood flow normally needed by the body, the condition is called heart failure (“pump failure”). The patient with heart failure classically exhibits exercise intolerance.
Parasites are another common cause of acquired cardiovascular dysfunction. In dogs, for example, adult heartworms (Dirofilaria immitis) lodge in the right ventricle and pulmonary artery, where they impede the flow of blood. These worms also release substances into the circulation that disrupt the body’s ability to control blood pressure and blood flow. In horses, bloodworms (Strongylus vulgaris) lodge in the mesenteric arteries and decrease the blood flow to the intestine. The resulting intestinal ischemia depresses digestive functions (motility, secretion, and absorption), and the horse exhibits signs of gastrointestinal distress (colic).
In many other disease states, cardiovascular complications develop even though the cardiovascular system is not the primary target of the disease. These secondary cardiovascular dysfunctions often become the most serious and life-threatening aspects of the disease. For example, severe burns or persistent vomiting or diarrhea leads to substantial losses of water and electrolytes (e.g., Na+, Cl−, K+, Ca2+) from the bloodstream. Even if the blood volume is not depleted to dangerously low levels in these conditions, the alteration in electrolyte concentrations can lead to abnormal heart rhythms (cardiac arrhythmias) and ineffective pumping of blood by the heart (heart failure). The electrolyte abnormalities in such a patient can be made even worse if incorrect fluid therapy is given. Incorrect fluid therapy can also lead to an accumulation of excess fluid in the tissues of the body; this “waterlogging” of tissues is called edema. If the excess fluid gathers in the lung tissue, the condition is called pulmonary edema. Pulmonary edema is life threatening because it slows the flow of oxygen from the pulmonary air sacs (alveoli) into the bloodstream.
Pulmonary edema is a secondary complication in many disease states. A further example is shock-lung syndrome, which results when toxic substances in the body trigger an increase in the permeability of the lung blood vessels. These “leaky” vessels allow water, electrolytes, plasma proteins, and white blood cells to leave the bloodstream and accumulate in the lung tissue and airways. The resulting pulmonary edema can lead to death.
Whereas the effects of shock-lung syndrome are most serious in the pulmonary circulation, other types of shock depress the cardiovascular system in general. Hemorrhagic shock is a generalized cardiovascular failure caused by severe blood loss. Cardiogenic shock is a cardiovascular collapse caused by heart failure. Septic shock is caused by bacterial infections in the bloodstream (bacteremia). Endotoxic shock occurs when endotoxins (fragments of bacterial cell walls) enter the bloodstream; this often occurs when the epithelial cells lining the intestines (intestinal mucosa) become damaged. Epithelial damage can result from bacterial infections in the intestines or from ischemia in the intestinal walls (as with bloodworms in horses). When the intestinal epithelium breaks down, endotoxins from the intestine enter the bloodstream. These endotoxins then cause the body to produce substances that depress the pumping ability of the heart. The resulting heart failure leads to low blood flow and ischemia in all the vital body organs. Kidney (or renal) failure, respiratory failure, central nervous system (CNS) depression, and death follow.
Anesthetic overdose is another common clinical problem in which the most serious and life-threatening symptoms are the secondary cardiovascular complications. Most anesthetics depress the CNS, and the resulting abnormal neural signals to the heart and the blood vessels can depress cardiac output and lower blood pressure. Some anesthetics, particularly the barbiturates, also depress the pumping ability of the heart directly.
There are many other examples of primary and secondary cardiovascular dysfunction, but those just mentioned illustrate the importance and variety of cardiovascular dysfunctions encountered in veterinary medicine. The distinction between primary and secondary cardiovascular dysfunction is sometimes unclear, but this difficulty simply emphasizes how intimately the cardiovascular system is interconnected with all the other body systems and how dependent all the other systems are on the normal functioning of the cardiovascular system.
The remainder of this chapter reviews the general features of the cardiovascular system. Chapters 19 to 25 discuss the various elements of the cardiovascular system in detail. Chapter 26 summarizes cardiovascular function and dysfunction by describing the overall effects of heart failure, hemorrhage, and exercise.
Substances Transported by the Cardiovascular System Include Nutrients, Waste Products, Hormones, Electrolytes, and Water
The blood transports the metabolic substrates needed by every cell of the body, including oxygen, glucose, amino acids, fatty acids, and various lipids. The blood also carries away from each cell in the body various metabolic waste products, including carbon dioxide, lactic acid, the nitrogenous wastes of protein metabolism, and heat. (Although the heat produced by metabolic processes within cells is not a material waste product, its transport by the cardiovascular system to the body surface is essential, because tissues deep within the body would otherwise become overheated and dysfunctional.)
Blood also transports vital chemical messengers: the hormones. Hormones are synthesized and released by cells in one organ and are carried by the bloodstream to cells in other organs, where they alter organ function. For example, insulin, which is produced by cells of the pancreas, is carried by the blood to cells throughout the body, where it promotes the cellular uptake of glucose. Inadequate insulin production (as in type 1 diabetes) results in inadequate entry of glucose into cells, whereas glucose concentrations in the blood rise to very high levels. The low intracellular glucose concentration is particularly disruptive to neural function, and the consequences can be serious (diabetic coma) or lethal. Another hormone, adrenaline (a mixture of epinephrine and norepinephrine), is released into the bloodstream by cells in the adrenal medulla during periods of stress. The epinephrine and norepinephrine circulate to various body organs, where they have effects that prepare a threatened animal for “fight or flight.” These effects include an increase in heart rate and cardiac contractility, dilation of skeletal muscle blood vessels, an increase in blood pressure, increased glycogenolysis, dilation of the pupils and airways, and piloerection (hair standing on end).
Finally, the blood transports water and essential electrolytes, including Na+, Cl−, K+, Ca2+, H+, and HCO3−. The kidneys are the organs primarily responsible for maintaining normal water and electrolyte composition in the body. The kidneys accomplish this by altering the electrolyte concentrations in blood as it flows through the kidneys. The altered blood then circulates to all other organs in the body, where it normalizes the water and electrolyte content in the extracellular fluids of each tissue.
Two Modes of Transport Are Used in the Cardiovascular System: Bulk Flow and Diffusion
Blood moves through blood vessels by bulk flow. The most important feature of bulk flow is that it is rapid over long distances. Blood that is pumped out of the heart travels quickly through the aorta and its various branches, and it reaches distant parts of the body, including the head and limbs, within 10 seconds. Transport requires energy, and the source of energy for bulk flow is a hydrostatic pressure difference; unless the pressure at one end of a blood vessel is higher than the pressure at the other end, flow will not occur. The difference in pressure between two points in a blood vessel is called the perfusion pressure difference or, more often, simply perfusion pressure. Perfusion literally means “flow through,” and the perfusion pressure is the pressure difference that causes blood to flow through blood vessels. The muscular pumping action of the heart creates the perfusion pressures that constitute the driving force for bulk flow of blood through the circulation.
It is important to distinguish between perfusion pressure difference and transmural pressure difference (usually shortened to transmural pressure). Transmural means “across the wall,” and transmural pressure is the difference between the blood pressure inside a blood vessel and the fluid pressure in the tissue immediately outside the vessel (transmural pressure equals inside pressure minus outside pressure). Transmural pressure is the pressure difference that would cause blood to flow out of a vessel if a hole were poked in the vessel wall. Transmural pressure is also called distending pressure, because it corresponds to the net outward “push” on the wall of a blood vessel. Figure 18-1 emphasizes the distinction between perfusion pressure and transmural pressure.
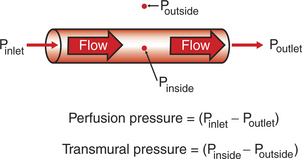
FIGURE 18-1 Fluid pressures associated with a blood vessel. Pinlet, Poutlet, and Pinside refer to blood pressure within the vessel. Poutside refers to the pressure in the tissue fluid (interstitial fluid) immediately outside the blood vessel. Perfusion pressure is the pressure difference along the length of a blood vessel. Transmural pressure (distending pressure) is the pressure difference across the wall of the vessel, indicated here at the midpoint of the vessel. Perfusion pressure is the driving force for blood flow through the vessel, whereas transmural pressure is the driving force that would cause blood to spill out of the vessel if there were a hole in it.
Diffusion is the second mode of transport in the cardiovascular system. Diffusion is the primary mechanism by which dissolved substances move across the walls of blood vessels, from the bloodstream into the interstitial fluid, or vice versa. Interstitial fluid is the extracellular fluid outside capillaries. It is the fluid that bathes each cell of a tissue. Most of the movement of substances between the blood and the interstitial fluid takes place across the walls of the capillaries, the smallest blood vessels. For a substance (e.g., oxygen) to move from the bloodstream to a tissue cell, it first diffuses across the wall of a capillary and into the tissue interstitial fluid, then diffuses from the interstitial fluid into the tissue cell.
The source of energy for diffusion is a concentration difference. A substance diffuses from the bloodstream, across the wall of a capillary, and into the interstitial fluid only if the concentration of the substance is higher in the blood than in the interstitial fluid (and if the capillary wall is permeable to the substance). If the concentration of a substance is higher in the interstitial fluid than in the blood, the substance will diffuse from the interstitial fluid into the capillary blood. It is important to distinguish diffusion, in which a substance moves passively from an area of high concentration toward an area of low concentration, from active transport, in which substances are forced to move in a direction opposite to their concentration gradient. In general, substances are not transported actively across the walls of capillaries. The movement of substances between the bloodstream and the interstitial fluid occurs by passive diffusion.
Because Diffusion Is Very Slow, Every Metabolically Active Cell in the Body Must Be Close to a Capillary Carrying Blood by Bulk Flow
To understand more fully how the two types of transport (bulk flow and diffusion) are used in the cardiovascular system, consider the transport of oxygen from the outside air to a neuron in the brain. With each inspiration, fresh air containing oxygen (O2) moves (by bulk flow) through the trachea, bronchi, and bronchioles and into the alveolar air sacs (Figure 18-2, A). The thin walls separating alveoli contain a meshwork of capillaries (Figure 18-2, B). Blood flowing through these alveolar capillaries passes extremely close (within 1 μm) to the air in the alveoli (Figure 18-2, C). This blood has just returned from the body tissues, where it gave up some of its oxygen. Therefore the concentration of oxygen in alveolar capillary blood is lower than the concentration of oxygen in alveolar air. This concentration difference causes some oxygen to diffuse from the alveolar air into the capillary blood.
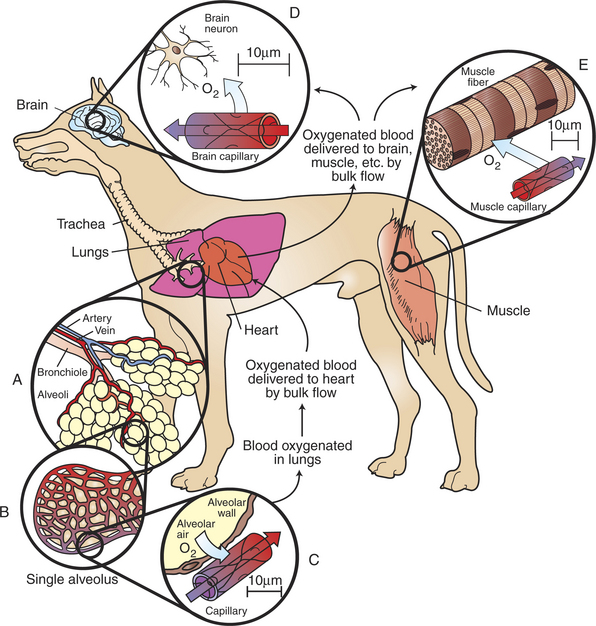
FIGURE 18-2 Oxygen (O2) is transported from the atmosphere to cells throughout the body by a combination of bulk flow and diffusion. First, O2 moves by bulk flow through the airways, from the atmosphere to the alveolar air sacs of the lungs (inset A). From the alveolar air, O2 next diffuses into the blood that is flowing through pulmonary capillaries (insets B and C). Bulk flow of blood carries this O2 to the heart; from there it is delivered by bulk flow into the capillaries of all the body organs (except the lungs). In the brain (inset D), skeletal muscle (inset E), and other tissues, O2 moves by diffusion from the capillary blood into the interstitial fluid and then into the tissue cells, where it is utilized to support oxidative metabolism. Bulk flow is rapid; it can transport O2 to all parts of the body within a few seconds. Diffusion is slow; it can transport O2 efficiently only over distances less than 100 μm (note distance scales in insets C, D, and E). Oxygenated blood has a bright-red color; deoxygenated blood is darker and bluish red.
A large dog has about 300 million alveoli, with a total surface area of about 130 m2 (equal to half the surface area of a tennis court). This huge surface area is laced with pulmonary capillaries. Thus, even though only a tiny amount of oxygen diffuses into each pulmonary capillary, the aggregate uptake of oxygen into the pulmonary bloodstream is substantial (typically, 125 mL O2/minute in a large, resting dog, increasing by a factor of 10 or more during strenuous exercise). In summary, both the large surface area and the proximity of alveolar air to capillary blood promote efficient diffusion of oxygen; it takes less than 1 second for the blood in a pulmonary capillary to become oxygenated.
As it leaves the lungs, each 100 mL of oxygenated blood normally carries 20 mL of oxygen. About 1.5% of this oxygen is carried in solution; the other 98.5% is bound to the protein hemoglobin within the erythrocytes (red blood cells). The oxygenated blood moves by bulk flow from the lungs to the heart. The heart pumps this oxygenated blood out into a system of branching arteries, and it is thereby delivered to all parts of the body, including the brain and skeletal muscles (Figure 18-2). Capillaries in the brain bring the oxygenated blood close to each brain neuron (Figure 18-2, D). Metabolic processes within the neurons consume oxygen, so the oxygen concentration inside neurons is low. The gradient of oxygen concentration between the capillary blood (high) and the neurons (low) provides the driving force for oxygen to diffuse first from the blood into the interstitial fluid and then into the neurons.
Each brain neuron must be within about 100 μm of a capillary carrying blood by bulk flow if diffusion is to deliver oxygen rapidly enough to sustain normal metabolism in the neuron. Diffusional exchange over distances up to 100 μm typically takes only 1 to 5 seconds. If the distance involved were a few millimeters, diffusion would take minutes to occur. Diffusion of oxygen a few centimeters through body fluid would take hours. Therefore, normal life processes require that every metabolically active cell of the body be within about 100 μm of a capillary carrying blood by bulk flow. If this bulk flow is interrupted for any reason, perhaps because of a thrombus (blood clot) in the artery that delivers blood to a particular region of a tissue, that region of tissue becomes ischemic. Again, as stated earlier, ischemia leads to dysfunction, and persistent, severe ischemia leads to infarction and eventually to necrosis. Cerebral infarction causes the condition commonly known as stroke.
Figure 18-2, E, shows a capillary carrying bulk flow of blood past a skeletal muscle cell (muscle fiber). Oxygen moves by diffusion from the capillary blood into the muscle interstitial fluid and then into the muscle cell, where it is consumed in the metabolic reactions that provide energy for muscle contraction. The oxygen consumption of a skeletal muscle depends on the severity of its exercise; at a maximum, oxygen consumption may reach levels 40 times greater than the resting level. Because of its tremendous metabolic capacity, muscle tissue has an especially high density of capillaries. In fact, several capillaries are typically arrayed around each skeletal muscle fiber. This arrangement provides more surface area for diffusional exchange than would be possible with a single capillary and also brings the bulk flow of blood extremely close to all parts of each skeletal muscle cell.
Heart muscle, as with skeletal muscle, consumes a large amount of oxygen. Oxygenated blood is carried from the aorta to the heart muscle by a network of branching coronary arteries. This blood moves by bulk flow through capillaries that run close to each cardiac muscle cell. If a thrombus interrupts the bulk flow of blood in a coronary artery, the heart muscle cells supplied by that artery become ischemic. Ischemia develops even if the cardiac muscle deprived of blood flow lies within a few millimeters of the left ventricular chamber, which is filled with oxygen-rich blood. Oxygen simply cannot diffuse rapidly enough from the ventricular chamber to the ischemic cells to sustain their metabolism. Ischemic cardiac muscle loses its ability to contract forcefully, and cardiac arrhythmias may develop. Severe myocardial ischemia causes a myocardial infarction, or heart attack.
Coronary artery disease and cerebrovascular disease are encountered more often in human medicine than in veterinary medicine. In contrast, cardiac disease (dysfunction of the heart muscle or valves, as distinguished from disease of the coronary arteries) is encountered more often in veterinary medicine than in human medicine. Therefore, Chapters 19 to 26 place more emphasis on cardiac physiology than on vascular physiology.
The Pulmonary and Systemic Circulations Are Arranged in Series, but the Various Organs Within the Systemic Circulation Are Arranged in Parallel
As shown in Figure 18-3, blood is pumped from the left ventricle into the aorta. The aorta divides and subdivides to form many arteries, which deliver fresh, oxygenated blood to each organ of the body, except the lungs. The pattern of arterial branching that delivers blood of the same composition to each organ is called parallel. After blood passes through the capillaries within individual organs, it enters veins. Small veins combine to form progressively larger veins, until the entire blood flow is delivered to the right atrium by way of the venae cavae. The blood vessels between the aorta and the venae cavae (including the blood vessels in all organs of the body except the lungs) are collectively called the systemic circulation. From the right atrium, blood passes into the right ventricle, which pumps it into the pulmonary artery. The pulmonary artery branches into progressively smaller arteries, which deliver blood to each lung capillary. Blood from lung capillaries is collected in pulmonary veins and brought to the left atrium. Blood then passes into the left ventricle, completing the circuit. The blood vessels of the lungs, including the pulmonary arteries and veins, constitute the pulmonary circulation. The pulmonary circulation and the heart are collectively termed the central circulation. The pulmonary circulation and the systemic circulation are arranged in series; that is, blood must pass through the pulmonary vessels between each passage through the systemic circuit.
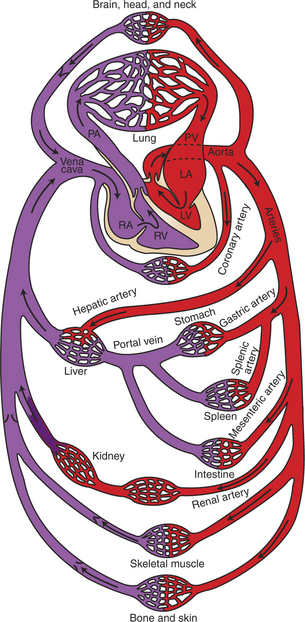
FIGURE 18-3 General layout of the cardiovascular system, showing that the systemic and pulmonary circulations are arranged in series and that the organs within the systemic circulation are arranged in parallel. LA, Left atrium; LV, left ventricle; PA, pulmonary artery; PV, pulmonary vein; RA, right atrium; RV, right ventricle. Oxygenated blood has a bright-red color; deoxygenated blood is darker and bluish red.
(Modified from Milnor WR: Cardiovascular physiology, New York, 1990, Oxford University Press.)
In one pass through the systemic circulation, blood generally encounters only one capillary bed before being collected in veins and returned to the heart, although a few exceptions to this rule exist. One exception occurs in the splanchnic circulation (blood supply of the digestive organs). As shown in Figure 18-3, blood that leaves the gastric, splenic, or mesenteric capillaries enters the portal vein. The portal vein carries splanchnic venous blood to the liver, where the blood passes through another set of capillaries before it returns to the heart. This arrangement of two systemic capillary beds in series is called a portal system. The splanchnic portal system allows nutrients that have been absorbed from the gastrointestinal tract to be delivered directly to the liver. There the nutrients are transformed for storage or allowed to pass into the general circulation. The liver also receives some blood directly from the aorta through the hepatic artery.
The kidneys also contain a portal system. As shown in Figure 18-3, blood enters a kidney by way of a renal artery and passes through two sets of capillaries (called glomerular and tubular) before returning to the venous side of the systemic circulation. Large amounts of water, electrolytes, and other solutes are filtered out of the blood as it passes through the glomerular capillaries. Most of this filtered material is subsequently reabsorbed into the bloodstream as it flows through the peritubular capillaries. The remainder becomes urine. The kidneys use the renal portal system to adjust the amounts of water, electrolytes, and other critical solutes in the blood.
A third portal system is found in the brain and is important in the control of hormone secretion by the pituitary gland. After traversing capillaries in the hypothalamus, blood enters portal vessels that carry it to the anterior pituitary gland (adenohypophysis) and to another set of capillaries (see Figures 33-16 and 33-17). As blood traverses the hypothalamic capillaries, it picks up substances that control the release of pituitary hormones. When this blood reaches capillaries in the anterior pituitary gland, these substances diffuse out of the bloodstream and into the pituitary interstitial fluid, where they act on pituitary cells to increase or decrease their secretion of specific hormones. This system is called the hypothalamic-hypophyseal portal system.
To summarize, except for a few specialized portal systems, blood encounters only one capillary bed in a single pass through the systemic circulation.
Cardiac Output Is the Volume of Blood Pumped Each Minute by One Ventricle
In a resting dog, it takes about 1 minute for blood to traverse the entire circulation (from the left ventricle back to the left ventricle). Because the pulmonary and systemic circulations are in series, the volume of blood pumped by the right side of the heart each minute must equal the volume of blood pumped by the left side of the heart each minute. The volume of blood pumped per minute by either the left ventricle or the right ventricle is called cardiac output. Among the mammalian species typically encountered in veterinary medicine, cardiac output at rest is approximately 3 liters per minute per square meter (L/min/m2) of body surface area. A large dog (e.g., German shepherd) typically has a body surface area a little less than 1 m2 and a cardiac output at rest of about 2.5 L/min.
In an animal at rest, blood entering the aorta is divided so that approximately 20% of it flows through the splanchnic circulation and 20% to the kidneys. Another 20% goes to the skeletal muscles. The brain receives about 15% of the cardiac output, and the coronary arteries carry about 3% of the cardiac output. The remainder goes to skin and bone.
The Perfusion Pressure for the Systemic Circulation Is Much Greater Than the Perfusion Pressure for the Pulmonary Circulation
When the left ventricle contracts and ejects blood into the aorta, the aorta becomes distended with blood, and aortic pressure rises to a peak value called systolic pressure (typically 120 mm Hg). Between ejections, blood continues to flow out of the aorta into the downstream arteries. This outflow of blood from the aorta causes aortic pressure to decrease. The minimal value of aortic pressure, just before the next cardiac ejection, is called diastolic pressure (typically 80 mm Hg). The mean aortic pressure (average value of the pulsatile pressure in the aorta) is about 98 mm Hg. The mean aortic pressure represents a potential energy for driving blood through the systemic circulation. As blood flows through the systemic blood vessels, this pressure energy is dissipated through friction. The potential energy (blood pressure) remaining by the time the blood reaches the venae cavae is only 3 mm Hg. Therefore the perfusion pressure for the systemic circuit is typically 98 − 3 mm Hg, or 95 mm Hg.
The pressures in the pulmonary artery are typically 20 mm Hg (systolic) and 8 mm Hg (diastolic); the typical mean value is 13 mm Hg. Pulmonary venous pressure is typically 5 mm Hg. Under these conditions the perfusion pressure for blood flow through the lungs is 8 mm Hg (i.e., 13 − 5 mm Hg).
The same volume of blood (the cardiac output) flows each minute through the systemic circulation and through the lungs; however, the perfusion pressure for the systemic circuit is much greater than the perfusion pressure for the lungs. The reason for this difference in perfusion pressure is that the systemic vessels offer more friction against blood flow (have a higher resistance) than do the pulmonary vessels. Therefore the systemic circulation is referred to as the high-pressure, high-resistance side of the circulation. The pulmonary circuit is called the low-pressure, low-resistance side.
By convention, blood pressures are always measured with reference to atmospheric pressure. Thus an aortic pressure of 98 mm Hg means that the blood pressure in the aorta is 98 mm Hg higher than the atmospheric pressure outside the body. Also, by convention, blood pressure is measured at heart level. This is why, in human medicine, blood pressure cuffs are typically applied over the brachial artery (in the upper arm); the brachial artery is at the same level as the heart. If blood pressure is measured in an artery or vein at a level different from heart level, an arithmetic correction should be made so that the pressure is reported as if it had been measured at heart level. This correction is necessary because gravity pulls downward on blood and therefore affects the actual pressure of blood within vessels. Gravity increases the actual pressure in vessels lying below heart level and decreases the actual pressure in vessels above heart level. The gravitational effect is significant in an animal the size of a dog and substantial in an animal the size of a horse. The correction factor for the effect of gravity is 1 mm Hg for each 1.36 cm above or below heart level.
Each Type of Blood Vessel Has Physical Properties Suited to Its Particular Function
In a resting animal, at any one moment, about 25% of the blood volume is in the central circulation and about 75% is in the systemic circulation (Table 18-1). Most of the blood in the systemic circulation is found in the veins. Only 20% of the systemic blood is found in the arteries, arterioles, and capillaries. Therefore, systemic veins are known as the blood reservoirs of the circulation. Arteries function as high-pressure conduits for rapid distribution of blood to the various organs. Arterioles are the “gates” of the systemic circulation; they constrict or dilate to control the blood flow to each capillary bed. Although only a small fraction of the systemic blood is found in capillaries at any one time, it is within these exchange vessels that the important diffusional exchange takes place between the blood stream and the interstitial fluid.
Table 18-1 Distribution of Blood Volume in the Cardiovascular System of a Normal Dog
| Distribution | Percent | |
|---|---|---|
| Between central and systemic circulations | ||
| Central circulation | 25 | |
| Systemic vessels | 75 | |
| TOTAL | 100 | |
| Within the systemic circulation | ||
| Arteries and arterioles | 15 | |
| Capillaries | 5 | |
| Venules and veins | 80 | |
| TOTAL | 100 | |
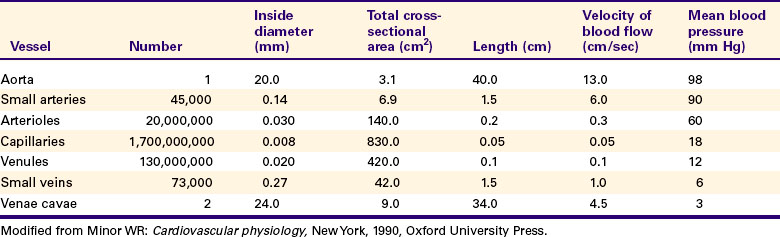
Table 18-2 compares the various types of vessels in the systemic circulation of a dog. As the aorta branches into progressively smaller vessels, the diameters of the vessels become smaller, but the number of vessels increases. One aorta supplies blood to 45,000 terminal arteries, each of which gives rise to more than 400 arterioles. Each arteriole typically branches into about 80 capillaries. The capillaries are so small in diameter that red blood cells must pass through in single file. However, because of the sheer number of capillaries, the total cross-sectional area of the capillaries is much greater than the cross-sectional area of the preceding arteries and arterioles. Because capillary blood flow is spread out over such a large cross-sectional area, the flow velocity is low. Blood moves rapidly (about 13 cm/sec) through the aorta and large arteries. At this speed, blood is delivered from the heart to all parts of the body in less than 10 seconds. The velocity of blood flow decreases as the blood leaves arteries and enters arterioles and capillaries in each tissue. The velocity of blood flow in capillaries is so slow that blood typically takes about 1 second to travel the 0.5-mm length of a capillary. During this time, diffusional exchange takes place between the capillary blood and the interstitial fluid. Blood from the capillaries is collected by venules and veins and is carried quite rapidly back to the heart.
Figure 18-4 depicts the branching pattern in the systemic circulation and graphs the velocity of blood flow within the different types of vessels. This figure emphasizes the rapidity of bulk flow through large vessels and the relatively slow flow through the capillaries. Note that the velocity of blood flow, not the total flow per minute, is lower in the capillaries. The same volume of blood necessarily flows each minute through an artery, the capillaries that it feeds, and the veins draining the capillaries.
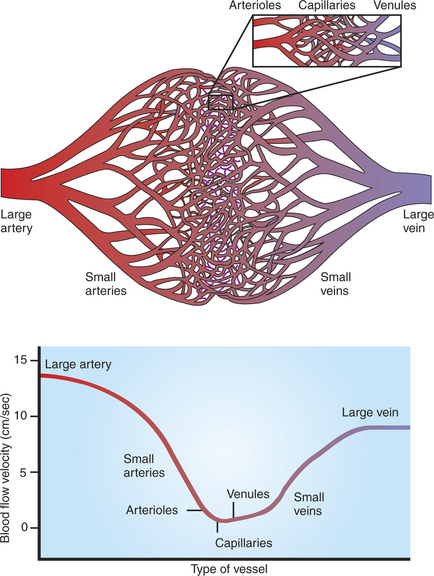
FIGURE 18-4 As the systemic arteries branch to form small arteries, arterioles, and capillaries (top), the total cross-sectional area of the vessels increases, so the forward velocity of blood flow decreases (bottom). As blood from the capillaries is collected into venules and veins, the total cross-sectional area is reduced, so the velocity of blood flow increases again. Therefore, blood moves quickly from the heart to the microvessels, where it stays for a few seconds before moving rapidly back to the heart.
In addition to having a large cross-sectional area (and therefore slow velocity of blood flow), capillaries have a large surface area. The total surface area of the walls of all the capillaries in the systemic circulation of a large dog is about 20 m2, which is nearly 30 times greater than the dog’s body surface area. The large surface area of capillaries helps promote efficient diffusional exchange between the capillary blood and the interstitial fluid.
Blood Is a Suspension of Cells in Liquid (Plasma)
As shown in Figure 18-5, blood can be separated into its cellular and liquid components by centrifugation. The liquid phase of blood is lighter in weight than the cells and therefore ends up on the top of the centrifuge tube. This acellular or extracellular liquid in blood is called plasma. Water constitutes 93% of the plasma volume. About 5% to 7% of the plasma volume is made up of protein molecules. The presence of proteins gives plasma its typical pale-yellow color. The plasma proteins are synthesized in the liver and added to the bloodstream as it passes through the liver capillaries. Globulin, albumin, and fibrinogen are the primary plasma proteins. Globulin and albumin are important in the immune responses of the body. Fibrinogen is important in the process of blood clotting. If blood is removed from the body and allowed to stand for a few moments, the soluble fibrinogen molecules polymerize to form an insoluble matrix of fibrin. This causes the blood to congeal, or coagulate. Coagulation can be prevented by adding an anticoagulant to the blood; the most common anticoagulants are heparin and citrate. An anticoagulant must be added in preparation for separating blood into its cellular and plasma fractions by centrifugation.
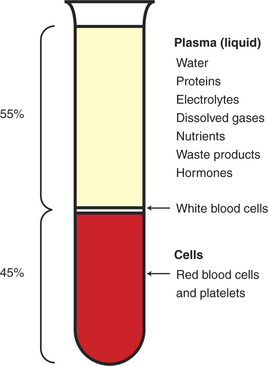
FIGURE 18-5 Anticoagulated blood can be separated into a liquid component (plasma) and a cellular component (cells) by centrifugation. Plasma is a solution of many important substances in water. The presence of proteins gives plasma its typical pale-yellow color. The cells are heavier than the plasma, and they settle to the bottom. Most of the cells are red blood cells. The white blood cells are slightly lighter in weight than the red blood cells, and they form a thin buffy coat on the top of the red cell layer. The fraction of cells in blood is called the hematocrit. In this example the hematocrit is 45%.
Many important substances, in addition to plasma proteins, are dissolved in plasma. Plasma contains several ions (electrolytes) in solution. The dominant cation is sodium (Na+). The predominant anions are chloride (Cl−) and bicarbonate (HCO3−). Other ions are present in lesser amounts, as indicated in Table 18-3. The concentration of each plasma electrolyte must be kept within narrow limits for body function to be normal, and numerous control systems accomplish this regulation. In general, the plasma electrolytes can diffuse readily across capillary walls; therefore, interstitial fluid and plasma typically have similar electrolyte concentrations.
Table 18-3 Some Constituents of Canine Plasma (in Addition to Water, the Main Constituent)
| Component | Normal range | Units |
|---|---|---|
| Plasma proteins (carried in colloidal suspension) | ||
| Globulin (total) | 2.7–4.4 | g/dL |
| Albumin | 2.3–3.1 | g/dL |
| Fibrinogen | 0.15–0.30 | g/dL |
| Electrolytes (dissolved) | ||
| Na+ | 140–150 | mmol/L |
| K+ | 3.9–5.1 | mmol/L |
| Ca2+ (ionized) | 1.2–1.5 | mmol/L |
| Mg2+ (ionized) | 0.5–0.9 | mmol/L |
| Cl− | 110–124 | mmol/L |
| HCO3− | 17–24 | mmol/L |
| HPO42− and H2PO4− | 1–1.4 | mmol/L |
| H+ | 38–49 | nmol/L* |
| (H+ expressed as pH)† | (7.31–7.42) | |
| Dissolved gases (values for arterial plasma) | ||
| O2 | 0.26–0.30 | mL/dL |
| CO2 | 2–2.5 | mL/dL |
| Examples of nutrients, waste products, hormones | ||
| Cholesterol | 140–280 | mg/dL |
| Glucose | 76–120 | mg/dL |
| Triglycerides | 40–170 | mg/dL |
| Urea nitrogen | 8–28 | mg/dL |
| Creatinine | 0.5–1.7 | mg/dL |
| Bile acids (fasting) | 0–8 | μmol/L |
| Thyroxine (T4) | 1.5–4 | nmol/L* |
* Note that [H+] and [Thyroxine] are in nanomolar units; 103 nmol = 1 μmol, and 103 μmol = 1 mmol.
† pH = −log [H+], where [H+] is expressed in molar units; pH is dimensionless.
Modified from Latimer KS, Mahaffey EA, Prasse KW: Duncan & Prasse’s veterinary laboratory medicine: clinical pathology, ed 4, Ames, 2003, Iowa State University Press.
Plasma contains small amounts of gases (O2, CO2, and N2) in solution. In the lungs, O2 enters the blood as dissolved O2, but most of this O2 quickly combines with hemoglobin (in the red blood cells). As a consequence, about 99% of the total O2 in blood is carried as oxyhemoglobin and only about 1% as dissolved O2. Likewise, only a small portion of the carbon dioxide (CO2) in blood is carried in the dissolved form. Most of the CO2 becomes hydrated to form HCO3− or combines with hemoglobin or plasma proteins to form carbamino compounds.
Nutrient substances dissolved in plasma include glucose, amino acids, lipids, and some vitamins. Dissolved metabolic waste products (in addition to CO2) include urea, creatinine, uric acid, and bilirubin. Plasma also contains many hormones (e.g., insulin, epinephrine, thyroxine), which are present in exceedingly tiny, but critically important amounts. Table 18-3 lists some of the normal constituents of plasma.
The Cellular Component of Blood Includes Red Blood Cells, White Blood Cells, and Platelets
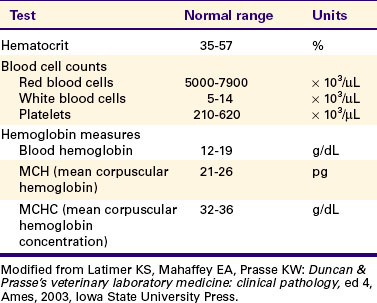
Cells normally constitute 30% to 60% of the blood volume (depending on the species). The fraction of cells in blood is called the hematocrit (see Figure 18-5). The hematocrit is determined by adding an anticoagulant to some blood and then centrifuging it in a tube. The cells are somewhat heavier than plasma and settle to the bottom of the tube during centrifugation. Because centrifugation results in a packing of the blood cells in the bottom of the tube, the hematocrit is sometimes called the packed cell volume. Most of the cell component looks red because most of the blood cells are erythrocytes (red blood cells, RBCs). Erythrocytes acquire their red color from hemoglobin.
The leukocytes (white blood cells, WBCs) are slightly lighter in weight than the RBCs; in a centrifuge tube the WBCs gather in a white “buffy coat” on top of the RBCs. The buffy coat is normally very thin because there are about 1000 times more RBCs than WBCs. Leukocytes are critical in immune and allergic responses of the body. The subtypes of leukocytes include neutrophils, lymphocytes, monocytes, eosinophils, and basophils. A laboratory analysis of the total number and relative distribution of the various WBC subtypes (differential white blood cell count) provides important clues in the diagnosis of disease. Both erythrocytes and leukocytes are made in the bone marrow. They develop, by mitosis and differentiation, from a common line of progenitor cells, the pluripotent (uncommitted) stem cells.
The cellular component in a centrifuge tube also contains platelets, or thrombocytes, which are fragments of the membranes of their precursor cells, the megakaryocytes. The megakaryocytes reside in the bone marrow and shed platelets into the bloodstream. Platelets participate in hemostasis (the control of bleeding through coagulation and clotting). In this process a clumping together of platelets (platelet aggregation) begins to create a physical barrier across openings in blood vessels. Substances released from the platelets, along with fibrinogen and several clotting factors in the plasma, lead to the coagulation of blood and the formation of a stable, fibrin-based blood clot.
Coagulation and clotting involve complex, interconnected sequences of chemical reactions (the coagulation cascade). A key step in the coagulation cascade is the formation in the plasma of thrombin, an enzyme that catalyzes the transformation of fibrinogen to fibrin. Several laboratory tests are used to assess the status of an animal’s coagulation system. The two most common tests involve determination of the prothrombin time (PT) and the partial thromboplastin time (PTT).
If blood is allowed to coagulate and then is centrifuged, the fibrin and other plasma clotting factors settle to the bottom along with the RBCs, WBCs, and platelets. The liquid portion remaining above (essentially plasma without fibrinogen and other clotting factors) is called serum. Most of the common clinical blood chemistry analyses are performed on serum. Examples include the determination of concentrations of electrolytes and cholesterol.
If blood is treated with an anticoagulant and then allowed simply to sit in a tube (without centrifugation), the erythrocytes slowly begin to settle. For reasons that are not completely understood, the rate of their settling tends to be increased to above normal in certain disease states and decreased to below normal in others. Therefore the erythrocyte sedimentation rate (ESR) is a clinically useful diagnostic measurement. The normal ESR varies substantially between species; for example, it is much more rapid in equine blood than in canine blood.
Blood cell counts are performed by manual or automated scanning of a very small volume (e.g., 1 μL) of anticoagulated whole blood. Table 18-4 presents a summary of normal hematologic values for the dog.
Most of the Oxygen in Blood Is Carried in Chemical Combination with the Protein Hemoglobin Within Red Blood Cells
Of the 20 mL of O2 normally carried in each 100 mL of oxygenated blood, only 1.5% (0.3 mL) is carried in dissolved form. The remaining 98.5% is carried in chemical combination with hemoglobin (in RBCs). Oxygenated hemoglobin (oxyhemoglobin, HbO2) is bright red. When O2 is released, HbO2 becomes reduced hemoglobin (Hb), which is dark bluish red. The adequacy of oxygenation of an animal’s blood can be judged somewhat by looking at the color of its nonpigmented epithelial membranes (e.g., gums, nostrils, or inside surfaces of eyelids). Well-oxygenated tissues appear pink. Poorly oxygenated tissues appear bluish (cyanotic) because of the prevalence of reduced Hb.
The ability of blood to carry oxygen is determined by the amount of hemoglobin in the blood and by the chemical characteristics of that Hb. For example, each deciliter (dL) of normal dog blood contains about 15 g of Hb. Each gram of Hb can combine with 1.34 mL of O2, when fully saturated. Thus, each deciliter of fully oxygenated, normal blood carries 20 mL of O2. Several disease states result in the synthesis of chemically abnormal Hb, with a diminished capacity to bind O2. In addition to these hemoglobinopathies, several common toxins, including carbon monoxide (CO) and nitrates, cause life-threatening alterations in the ability of Hb to bind O2.
Because hemoglobin is localized inside RBCs, it is possible to derive several clinically useful relationships among the blood Hb content, RBC count, Hb content of each RBC, and hematocrit. For example, if a dog has 15 g of Hb/dL of blood and RBC count of 6 million cells per microliter (μL) blood, it follows that each RBC (on average) contains 25 picograms (pg) of Hb:
The value calculated in this way is called the mean corpuscular hemoglobin (MHC).
An easier calculation, which serves the same purpose, is to determine how much hemoglobin is contained in each deciliter of packed RBCs. For example, if a dog has 15 g of Hb in each deciliter of blood and has a hematocrit of 50%, the Hb concentration in the RBC portion of the blood must be 30 g of Hb/dL of packed RBCs:
The value calculated in this way is called the mean corpuscular hemoglobin concentration (MCHC). For simplicity, the calculation is often summarized as follows:
The brackets denote concentration.
An abnormally low value of MCH or MCHC is clinically important because it points to a deficit in hemoglobin synthesis (i.e., not enough Hb being made to load up each RBC). In contrast, an abnormally low value for Hb by itself is less helpful; hemoglobin concentration in the blood could fall below normal for several reasons, including a deficit in Hb synthesis, a deficit in RBC synthesis, or a “watering down” of the blood either by addition of excess plasma fluid or by loss of RBCs.
Deviations from a normal hematocrit (Hct) can have important consequences in terms of the ability of blood to carry oxygen. Hematocrit also affects the viscosity of blood, as shown in Figure 18-6. Viscosity is a measure of resistance to flow. For example, honey is more viscous (more resistant to flow) than water. Plasma, by itself, is about 1.5 times more viscous than water because of the presence of plasma protein molecules (albumin, globulin, fibrinogen). The presence of cells in blood has an even greater effect on viscosity. Blood with a Hct of 40% has twice the viscosity of plasma. For Hct exceeding 50%, viscosity increases rapidly. An abnormally high hematocrit is called polycythemia, which literally means “many cells in the blood.” The blood of a patient with polycythemia can carry more than the normal 20 mL of O2/dL of blood (provided that the MCHC is normal), and this may be viewed as beneficial. However, the increased viscosity makes it difficult for the heart to pump the blood. Therefore, polycythemia creates a heavy workload for the heart and can lead to heart failure, particularly if the cardiac muscle is not healthy.
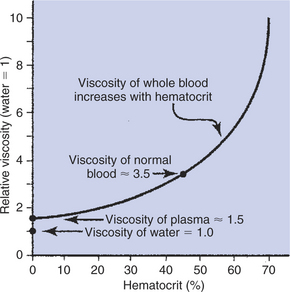
FIGURE 18-6 Plasma is more viscous than water because of the presence of plasma proteins. Blood is more viscous than plasma because of the presence of blood cells. Blood viscosity increases sharply when the fraction of cells (hematocrit) increases above 50%.
The opposite problem, in which the hematocrit is too low, is called anemia. Anemia literally means “no blood,” but the word is used to refer to any condition in which there are abnormally few RBCs in blood. Each deciliter of blood of an anemic patient carries less than the normal 20 mL of O2. Therefore, cardiac output must be increased above normal to deliver the normal amount of O2 to the tissues each minute. This need to increase cardiac output also imposes an increased workload on the heart and can lead to the failure of a diseased heart. Thus, Hct in the range of 40% to 50% provides the blood with enough Hb to carry an adequate amount of O2 without putting an undue workload on the heart. For additional information about the transport of O2 and CO2 in blood, see Chapter 48.
Figure 18-7 provides an idea of the relative sizes and shapes of the major constituents of blood. The plasma proteins are much, much larger than the ions and nutrient molecules that are dissolved in plasma. RBCs and WBCs are many, many times larger than the plasma proteins. In fact, as mentioned earlier, blood cells are so large that they can barely squeeze through a typical capillary.
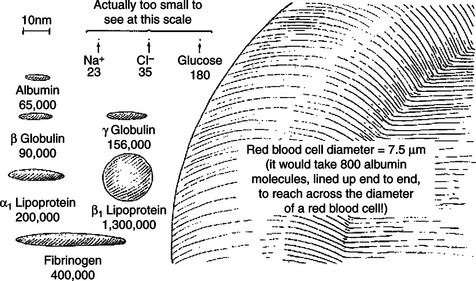
FIGURE 18-7 Relative size and shape of the major constituents of blood. The figure emphasizes that the plasma protein molecules are huge compared with the other plasma solutes, such as glucose, Na+, and Cl−. Furthermore, the blood cells (red and white) are huge compared with plasma protein molecules. Numbers under constituents are their molecular weights (in daltons). The scale (upper left) indicates a length of 10 nm. In comparison, the diameter of the red blood cell is 7.5 μm (7500 nm).
CLINICAL CORRELATIONS
Lethargic Kid Goat
History.
A 6-month-old female kid goat is presented for lethargy and difficulty breathing. Two months ago, in April, the owners bought this goat and another at a sale as pets for their children. The goats have been provided with a small amount of goat feed daily, along with access to a pasture. The owners noticed that both goats were initially very playful, but both have seemed progressively lethargic during the last month. Also, they seem to have more difficulty breathing, even at rest. No vaccinations, deworming, or other treatments have been given.
Clinical Examination.
The goat is somewhat thin and is reluctant to stand. There is a swelling (likely edema fluid) under the jaw. The goat’s temperature is slightly elevated. The pulse and respiratory rates are moderately increased. The mucous membranes are very pale, which makes the capillary refill time difficult to assess. Respiratory sounds are increased (suggesting possible pulmonary edema). There are no other abnormal findings on physical examination.
Comment.
The very pale mucous membranes suggest marked anemia. Indeed, centrifugation of a blood sample reveals that the goat’s packed cell volume (Hct) is only 12%. Plasma protein concentration is also below normal, at 4.5 g/dL. Given the lack of deworming, you suspect parasitic infection associated with Hemonchus contortus, Ostertagia, or Trichostrongylus. A fecal analysis is positive for Hemonchus and Ostertagia.
Parasitism is a common problem in sheep and goats. The parasites mentioned damage the abomasum, which results in blood loss. The consequent anemia would explain the goat’s lethargy, because anemia limits O2 delivery to the organs, especially during exercise. The elevated respiratory rate and heart rate reflect the animal’s attempts to compensate for low O2 delivery to the tissues by increasing air flow into the lungs and blood flow through the circulation. Plasma protein is lost along with RBCs. This hypoproteinemia could account for the edema, because the proteins in plasma exert an important osmotic effect to oppose the tendency for plasma water to leak out of capillaries and into the tissue (interstitial) fluid (see Chapter 23).
Treatment.
Ideally, a transfusion of whole blood would be given to help restore both RBCs and plasma proteins; the kid would then be dewormed. However, even if appropriate whole blood were available, transfusion in such an animal is risky. This goat’s ability to deal with stress has been severely compromised, and even the physical restraint needed to administer a transfusion might trigger physical collapse or even death. On the other hand, without the transfusion, the animal has little chance of recovery if only treated for the parasites.
Colic and Endotoxic Shock in Horse Secondary to Strongylus Parasitism
History.
A 1-year-old Standardbred filly is brought to your clinic by its new owner because the horse has been restless, rolling, kicking at its belly, and pawing the ground. The owner reports that the horse has had a poor appetite for several days and now refuses both hay and grain. The owner says he has dewormed the filly recently, but her previous worming history is unknown.
Clinical Examination.
The horse is underweight and has a dull hair coat. It is obvious that she is in pain. Physical examination reveals an abnormally high temperature (103.5° F), rapid labored breathing (40 breaths/min), and an elevated heart rate (80 beats/min). All limbs feel cool to the touch. The mucous membranes are abnormally dark, and the capillary refill time is prolonged (both these observations indicate sluggish circulation). Gastrointestinal auscultation of all four quadrants yields abnormal findings; no abdominal sounds are heard on either the left or the right side, dorsally or ventrally. A rectal examination reveals several distended loops of bowel.
You perform abdominocentesis and withdraw some peritoneal fluid. Normally, peritoneal fluid is clear and straw colored; the fluid from this horse is darker yellow than normal and has a turbid appearance. Measurements with a refractometer indicate that the peritoneal fluid contains five times more protein than normal. Microscopic examination of the fluid reveals the presence of four times the normal number of WBCs, specifically neutrophils, and the cells contain bacteria.
Outcome.
You tell the owner that the filly appears to have a badly damaged bowel and that the prognosis is poor. You inform him that surgical treatment is possible, but expensive postoperative complications are likely because infection appears to have spread into the peritoneum. After considering the options, the owner decides against surgery. You institute supportive therapy with intravenous (IV) fluids and analgesics. Depending on the extent of compromise to the bowel, horses can respond to medical management. However, based on the signs that this filly is showing, the prognosis is grave.
The horse’s condition deteriorates over the next 12 hours. The heart rate increases progressively to 100 beats/min. The mucous membranes show evidence of declining blood flow (darker color and longer capillary refill time). The horse begins to wheeze and becomes lethargic. Bowel sounds continue to be absent. Despite the delivery of IV fluids, there is no output of urine. With the owner’s consent, you euthanize the horse.
Necropsy examination indicates that this horse had thrombi (vascular obstructions) in several major branches of her mesenteric arteries, probably secondary to a severe infestation of bloodworms (Strongylus vulgaris). Several areas of the intestine were necrotic. Gram-negative bacteria were cultured from both the peritoneal fluid and the blood. The lungs were edematous, and excessive fluid was found in the airways and intrapleural space.
Comment.
In horses, S. vulgaris lodges in mesenteric arteries and decreases the blood flow to the intestine. Deworming a severely infested horse can precipitate acute intestinal ischemia, because the worms break away from the walls of major mesenteric arteries and drift into smaller arteries, which they occlude. Also, the dying worms release substances that trigger the formation of blood clots in the arteries. Digestive processes become disrupted and may cease entirely. Intestinal ischemia and gaseous distention of the bowel cause severe pain. With persistent ischemia, segments of the bowel become permanently damaged (infarcted). Ischemic damage to the intestinal epithelium allows intestinal bacteria and bacterial products (endotoxins) to enter the peritoneum and the blood. WBCs move from the bloodstream into the peritoneal fluid, where they combat the bacteria by engulfing them (phagocytosis). However, the infection overwhelms the immune system. Bacteria and endotoxins (from gram-negative bacteria) cause the body to produce substances that depress the heart and disrupt the capillary endothelium, especially in the lungs. The resultant combination of heart failure and pulmonary edema leads to respiratory failure and subsequent renal failure. The progression of dysfunction becomes irreversible.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Hill R, Wyse GA, Anderson M. Animal physiology, Sunderland. Mass: Sinauer Associates, 2004.
Jain NC. Essentials of veterinary hematology. Philadelphia: Lea & Febiger, 1993.
Latimer KS, Mahaffey EA, Prasse KW. Duncan & Prasse’s veterinary laboratory medicine: clinical pathology, ed 4. Ames: Iowa State University Press, 2003.
Levy MN, Pappano A. Cardiovascular system. In: Levy MN, Stanton BA, Koeppen BM. Berne & Levy principles of physiology. Philadelphia: Mosby, 2006.
Milnor WR. Cardiovascular physiology. New York: Oxford University Press, 1990.
Mohrman DE, Heller LJ. Cardiovascular physiology, ed 6. New York: Lange Medical Books/McGraw-Hill, 2006.
Patteson MW. Equine cardiology (Library of Veterinary Practice). Oxford, UK: Blackwell Scientific, 1996.
Physick-Sheard PW. Parasitic arteritis. Colahan PT, Merritt AM, Moore JN, et al, eds. Equine medicine and surgery, ed 5, vol 1. St Louis: Mosby–Year Book, 1999.
Reagan WJ, Sanders TG, Denicola DB. Veterinary hematology: atlas of common domestic species. Ames: Iowa State University Press, 1998.
Reece WO. Dukes’ physiology of domestic animals, ed 12. Ithaca, NY: Comstock Cornell University Press, 2004.
Schmidt-Nielsen K. Animal physiology: adaptation and environment. Cambridge, UK: Cambridge University Press, 1997.
Thrall MA. Veterinary hematology and clinical chemistry: text and case presentations (set). Philadelphia: Lippincott Williams & Wilkins, 2004.
PRACTICE QUESTIONS
1. According to Table 18-2, how long does it take for blood to travel the length of a canine capillary?
2. The amount of blood pumped by the left ventricle in 1 minute would equal:
3. A transfusion of normal plasma into a normal dog would:
4. Which of the following sequences of capillary beds might a red blood cell encounter in a normal circulation?
5. The walls of most capillaries have pores or clefts in them, which are approximately 4 nm in diameter (4 × 10−9 m). According to Figure 18-7:
6. Suppose that the following conditions exist in a particular blood vessel: blood pressure (BP) inside vessel at inlet = 60 mm Hg, BP inside vessel at midpoint = 45 mm Hg, BP inside vessel at outlet = 30 mm Hg, BP outside vessel at midpoint = 5 mm Hg. Under these conditions:
7. Compared with the systemic circulation, the pulmonary circulation: