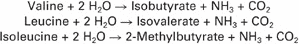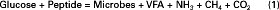Chapter 31 Digestion: the Fermentative Processes
1. Fermentation is the metabolic action of bacteria.
2. The sites of fermentative digestion must be conducive to microbial growth.
Microbial Ecosystem of Fermentative Digestion
1. The microbes responsible for fermentative digestion include bacteria, fungi, and protozoa.
2. Cooperation and interplay among the many species of microbes give rise to a complex ecosystem in the forestomach and hindgut.
Substrates and Products of Fermentative Digestion
1. Plant cell walls are important substrates for fermentative digestion and significant nutrient sources for many species.
2. Nutrients other than cell walls are also subject to fermentative digestion.
3. Anaerobic conditions in the rumen result in metabolic activities leading to the production of volatile fatty acids.
4. Volatile fatty acids are important energy substrates for the host animal.
5. Fermentative digestion of protein results in the deamination of a large portion of amino acids.
6. When protein and energy availability in the forestomach are well matched, rapid microbial growth and efficient protein utilization result.
7. Microbial protein can be synthesized in the rumen from nonprotein nitrogen sources.
Reticulorumen Motility and Maintenance of the Rumen Environment
1. The physiological functions of the reticulorumen maintain an environment favorable to fermentation patterns that are beneficial to the host.
2. Rumen fermentation is maintained by selectively retaining actively fermenting material while allowing unfermentable residue to pass on to the abomasum.
3. Gravity and reticulorumen motility combine to create the selective flow of particulate matter out of the rumen.
4. Functional specific gravity determines the rate at which particulate matter (solids) moves through the zones of the reticulorumen.
5. Digestibility and physical characteristics of feed have important influences on both the rate of particle passage from the rumen and the rate of feed intake.
6. Rumination, or cud chewing, has an important effect on the reduction of particle size and the movement of solid material through the rumen.
7. Water moves through the rumen at a much faster rate than particulate matter.
8. Rumen dilution rate has important influences on fermentation and microbial cell yield.
Control of Reticulorumen Motility
1. Reticulorumen motility is controlled by the central nervous system and affected by intraluminal conditions.
Volatile Fatty-Acid Absorption
1. Volatile fatty acids, representing 60% to 80% of the energy needs of the animal, are absorbed directly from the forestomach epithelium.
Rumen Development and Esophageal Groove Function
1. Significant changes in forestomach size and function occur with dietary changes in early life.
2. The esophageal groove diverts the flow of ingested milk past the forestomach and into the abomasum.
Function of the Equine Large Hindgut
1. The equine hindgut has a great capacity for fermentation.
2. The types of substrate and fermentation patterns are essentially identical for forestomach and hindgut fermentation.
3. The motility functions of the cecum and colon retain material for fermentation and separate particles by size.
4. The rate of fermentation and volatile fatty-acid production in the equine colon is similar to that in the rumen.
5. Hindgut anatomy and function vary greatly among the many species of veterinary interest.
Fermentation Is the Metabolic Action of Bacteria
In fermentative digestion, molecular substrates are broken down by the action of bacteria and other microorganisms. Enzymatic hydrolysis of large molecules is an essential part of fermentative digestion, just as it is for glandular digestion. The major difference between the two processes is that the enzymes of fermentative digestion are microbial in origin, rather than coming from the host animal. Other major differences between fermentative and glandular digestion involve the rate of reactions and the extent of alteration of the substrate molecules. In general, fermentative digestion is much slower than glandular digestion, and the substrates are altered to a much greater degree.
The Sites of Fermentative Digestion Must Be Conducive to Microbial Growth
Fermentative digestion occurs in specialized compartments that are positioned either before or after the stomach and small intestine. Fermentative compartments positioned before the stomach are called forestomachs and are most highly developed in the ruminants and camelids. The size and development of the forestomach fermentation compartments vary greatly among species; many species have distinct forestomachs that are less developed than those of ruminants. Some species, including the horse and rat, have no anatomically distinct forestomach; however, some fermentative digestion may occur in a nonglandular portion of the proximal stomach.
Fermentation compartments positioned distal to the small intestine are the cecum and colon, often collectively called the hindgut. As with the forestomach, great anatomical differences exist in the hindgut of various species. This variation can be so extensive that the cecum and colon may appear to be functionally different organs in different species; however, when the variations are evaluated critically, important similarities can be seen in hindgut function among species.
The forestomach and hindgut can support fermentative digestion because their pH, moisture, ionic strength, and oxidation-reduction conditions are maintained in a range compatible for the growth of suitable microbes. In addition, the flow of ingesta through these areas is comparatively slow, allowing microbes time to maintain their population size. The importance of these factors can be illustrated through comparison of the forestomach and colon to the stomach and small intestine. In the stomach, bacterial numbers are kept low by the acid pH, whereas in the small intestine, bacterial numbers are kept in check by the constant flushing action of ingesta and secretions. In contrast, the pH in the forestomach and large colon is close to neutral, and the flow rate is comparatively slow.
In general, the fermentative patterns of the hindgut appear to be similar to those of the forestomach, although forestomach fermentation, especially that of the rumen, appears to be the better studied of the two. The following discussion focuses on rumen digestion but includes comments on hindgut digestion. Digestion in the equine cecum and colon is discussed at the end of the chapter.
MICROBIAL ECOSYSTEM OF FERMENTATIVE DIGESTION
The Microbes Responsible for Fermentative Digestion Include Bacteria, Fungi, and Protozoa
The bacterial population associated with fermentative digestion is vast, with at least 28 functionally important species occurring in the rumen. Box 31-1 lists some of the major species found in the rumen and their preferred substrates. Total bacterial numbers in the forestomach or hindgut normally range from 1010 to 1011 cells per gram of ingesta. Most of these bacteria are strict anaerobes that cannot survive in the presence of oxygen, although facultative organisms are also present. In the rumen, fungi are present, and research suggests that fungi may play an important role in the digestion of plant cell walls.
Box 31-1 Grouping of Rumen Bacterial Species According to Type of Substrates Fermented
From Church DC, editor: The ruminant animal. digestive physiology and nutrition, Englewood Cliffs, NJ, 1988, Prentice-Hall.
There is also a large population of protozoa in the rumen as well as in the cecum and colon. Protozoal numbers average about 105 to 106 cells per gram of rumen contents. Although this number is considerably smaller than the number of bacteria, the relatively larger size of the individual protozoa compared with bacteria results in a total rumen protozoal cell mass approximately equal to the bacterial cell mass, under most dietary conditions. Most of the rumen protozoa are ciliated and belong to the genus Isotricha or Entodinium, although flagellate species are also present, especially in young ruminants. As with the other organisms of the rumen, the protozoa are anaerobic.
The digestive abilities, or capacities, of protozoa and bacteria are similar; thus either type of organism can perform most of the fermentative functions of the rumen. Protozoa ingest large numbers of bacteria and hold rumen bacterial numbers in check. However, none of the actions of protozoa appears essential to rumen function because ruminants can survive well without protozoa. Thus the role of protozoa in the total ecological picture of the rumen is uncertain. One potentially important function of protozoa involves their ability to slow down the digestion of rapidly fermentable substrates, such as starch and some proteins. Protozoa are capable of ingesting particles of starch and protein and storing them in their bodies, protected from bacterial action. The starch and protein remain engulfed until digested by the protozoa, or until the protozoa die or are swept from the rumen into the lower digestive tract. Thus, protozoa may have the effect of delaying or prolonging the digestion of these substrates. Especially in the case of starch, this proto-zoal effect may be beneficial to the host through modulation or delay of the digestion of rapidly fermentable substrate.
Cooperation and Interplay Among the Many Species of Microbes Give Rise to a Complex Ecosystem in the Forestomach and Hindgut
The digestive process in the rumen or colon involves the interplay among the many species of bacteria and other microbes. The ecosystem of fermentative digestion is extremely complex, with the waste products of one microbial species serving as substrate for another. For example, Ruminococcus albus and Bacteroides ruminicola appear to exist synergistically. R. albus digests cellulose (is cellulolytic) but cannot digest protein. B. ruminicola, on the other hand, can digest protein but cannot digest cellulose. When the microbes are grown together, cellulose digestion by R. albus provides hexoses for the energy needs of B. ruminicola, and protein digestion by B. ruminicola provides ammonia and branch-chain fatty acids for the growth needs of R. albus.
In addition to substrate needs, growth factor needs are also supplied synergistically within the rumen ecosystem. For example, B vitamins are necessary for the growth of several rumen microbes, but these nutrients are generally not necessary in ruminant diets. The synergistic effect of B vitamins results from cross-feeding between species of those microbes that produce various B vitamins and those microbes that require them.
Despite tremendous ecological complexity, however, the entire pattern of fermentation may be viewed as a holistic process, without consideration of the roles and interactions of individual microbial species. Fermentative digestion is examined here from this viewpoint, with the actions of the entire rumen biomass considered as an overall digestive process, irrespective of the specific needs and actions of individual microbial species.
SUBSTRATES AND PRODUCTS OF FERMENTATIVE DIGESTION
Plant Cell Walls Are Important Substrates for Fermentative Digestion and Significant Nutrient Sources for Many Species
Forages, or the foliage of plants, are both the major feedstuff of large herbivores and an important substrate for fermentative digestion. Some appreciation of the physical and chemical nature of plants is important to an understanding of the fermentative digestion of forages. This understanding may be aided by a brief comparison of plant and animal tissue structure.
At the cellular level, a major difference between plants and animals is the existence of a cell wall in plants. The cell wall is a complex of various carbohydrate molecules. The structural parts of plants, the leaves and stems, contain a large portion of cell-wall material. This material gives the plants their rigid framework and protects them from weather and other elements during growth. The cell-wall structure of plants can be roughly compared to the connective tissue structure of animals. Long, fiberlike molecules of cellulose have a strength-giving role similar to that of collagen, whereas hemicellulose, pectin, and lignin cement the cellulose together, much as hyaluronic acid and chondroitin sulfate do in animal connective tissue. With the exception of lignin, all these cell-wall molecules are carbohydrate.
Cellulose is composed of nonbranching chains of glucose monomers joined by β[1–4] glycosidic linkages, in contrast to the α[1–4] linkages in starch. Pectin and hemicellulose are chemically more heterogeneous than cellulose, being composed of various proportions of several sugars and sugar acids. None of the cell-wall materials is subject to hydrolytic digestion by mammalian glandular digestive enzymes. However, cellulose, hemicellulose, and pectin are subject to the hydrolytic action of a complex of microbial enzymes known as cellulase. This enzyme system releases monosaccharides and oligosaccharides from the complex carbohydrates of cell walls, but the released saccharides are not directly available for absorption by the animal. Rather, they are further metabolized by the microbes, as discussed later.
Lignin, a heterogeneous group of phenolic chemicals, is resistant to the action of either mammalian or microbial enzymes, and only a small portion of lignin is digested by either process. Lignin is important not only because it is indigestible itself, but also because it tends to encase the cell-wall carbohydrates, reducing their digestibility by protecting them from the action of bacterial cellulase. The lignin concentration of plants increases with age and ambient temperature; thus young, cool-season plants are more digestible than mature plants grown in hot weather.
Nutrients Other Than Cell Walls Are Also Subject to Fermentative Digestion
The fermentative digestion of plant cell-wall material and its importance to herbivore digestion are well known. In addition, however, essentially all protein and carbohydrate nutrients that can provide substrate for energy and growth in mammals can also support the similar needs of microbes. Therefore, almost all dietary protein and carbohydrate are potentially subject to fermentative digestion. This fact is especially important in ruminants, in which food is exposed to fermentative digestion in the forestomach before its arrival at sites of glandular digestion. This temporal arrangement leads to the fermentative digestion of many nutrients that would otherwise have been available to the animal through glandular digestion. Thus, forestomach fermentative digestion, which provides for the efficient use of plant cell walls, can potentially lead to the inefficient use of other nutrients because of microbial alteration.
Anaerobic Conditions in the Rumen Result in Metabolic Activities Leading to the Production of Volatile Fatty Acids
When carbohydrate material enters the rumen or colon, it is attacked by hydrolytic microbial enzymes. In the case of insoluble carbohydrates, attack requires the physical attachment of bacteria to the surface of the plant particle, with the enzymes themselves part of the surface coating of the bacteria. Enzymatic action liberates glucose, other monosaccharides, and short-chain polysaccharides into the fluid phase, outside the microbial cell bodies. Although free in solution, these products of microbial enzyme action do not become immediately available to the host animal; rather, they are quickly subjected to further metabolism by the microbial mass. Glucose and other sugars are absorbed into the cell bodies of the microbes.
Once within the microbial cells, glucose enters the glycolytic, or Embden-Meyerhof, pathway. This is the same glycolytic pathway that exists in mammalian cells, and as in mammalian tissues, catabolism of glucose through this pathway yields two molecules of pyruvate for each molecule of glucose metabolized. In the process, two molecules of oxidized nicotinamide adenine dinucleotide (NAD) are reduced to NAD hydrogen (NADH), and two molecules of adenosine triphosphate (ATP) are formed from adenosine diphosphate (ADP). The potential energy represented by the ATP formed in this reaction is not directly available to the host animal but is the major source of energy for maintenance and growth of microbes.
If fermentative digestion were to occur under aerobic conditions, which it does not, the pyruvate produced by the glycolytic process would enter the citric acid (Krebs) cycle and would be metabolized to carbon dioxide and water, as occurs under the aerobic conditions in mammalian cells. Furthermore, in an aerobic system, the NADH produced would be oxidized in the cytochrome oxidase system with additional production of ATP and the regeneration of NAD. However, fermentative digestion is not an aerobic system; on the contrary, it proceeds in a reductive, highly anaerobic environment. Therefore a different mechanism must be provided for the oxidation of NADH and other reduced cofactors. If such a mechanism were not available, all the oxidized cofactors present would soon be reduced, and metabolism would come to a halt. Because no atmospheric oxygen is available, some other compound must serve as an “electron sink” for the oxidation of enzyme cofactors.
In fermentative digestion, pyruvate can act as an electron sink, being further reduced to provide for regeneration of NAD and the general removal of excess electrons, with an additional yield of ATP. Also, carbon dioxide can be reduced to methane, accepting electrons for the regeneration of NAD. Figure 31-1 illustrates the metabolic pathways of these reactions. These pathways lead to the major end products of the fermentative digestion of carbohydrate, the volatile fatty acids (VFAs). The primary VFAs are acetic acid, propionic acid, and butyric acid; the VFAs are often referred to as their dissociated ions: acetate, propionate, and butyrate, respectively. Other quantitatively minor but metabolically important VFAs are valeric acid, isovaleric acid, isobutyric acid, and 2-methylbutyric acid. Figure 31-2 shows the chemical structures of the VFAs.
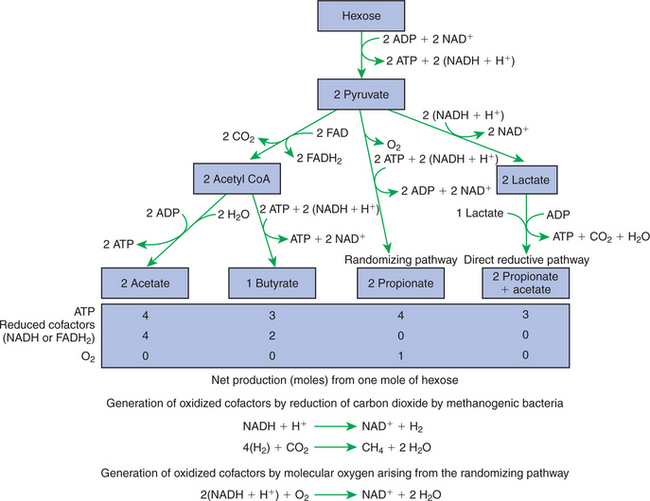
FIGURE 31-1 Pathways of volatile fatty-acid (VFA) production by the rumen or colonic biomass. The production of methane is necessary for the production of oxidized cofactors in the pathways leading to acetate and butyrate production, but not in the pathways leading to propionate production. The production of oxygen by the randomizing pathway results in the net production of oxidized cofactors. ADP, Adenosine diphosphate; ATP, adenosine triphosphate; NAD, nicotinamide adenine dinucleotide; FAD, flavin adenine dinucleotide; H, hydrogen; CoA, coenzyme A; CO2, carbon dioxide.
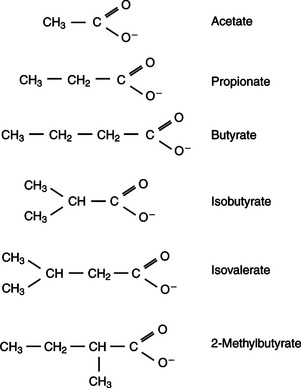
FIGURE 31-2 Chemical structures of the major volatile fatty acids (VFAs) produced by fermentative digestion.
Production of propionic acid from pyruvate results in the efficient regeneration of NAD with no net production of NADH. In fact, production of available oxygen by the randomizing branch of the propionic acid pathway leads to oxidation of excess NADH originating from the acetic or butyric acid pathways (see Figure 31-1). The production of acetic acid leads to the efficient generation of ATP but, in contrast to the production of propionic acid, does not result in the regeneration of NAD from NADH. In the acetic acid pathway, excess NADH is produced. In this case, NAD is regenerated by the formation of free hydrogen, which is subsequently used to reduce carbon dioxide to methane and water (see Figure 31-1, lower portion).
Thus a direct relationship exists between acetic acid production and methane production; as the amount of pyruvate entering the acetic acid pathway increases, there must be a concomitant rise in methane production. Likewise, a reciprocal relationship exists between methane production and propionic acid production; as pyruvate is diverted to propionic acid production, there is less need for methane synthesis. These relationships are shown in the stoichiometric equations of Box 31-2. These reactions do not, however, fully describe the flow of hydrogen, or reducing substances, in rumen or colonic metabolism. The chemical reactions of fermentation are extremely complex and interdependent, and NADH can donate its electrons to reactions other than those described in Box 31-2, such as the synthesis of microbial protein and the saturation of unsaturated fatty acids.
Box 31-2 Theoretical Stoichiometric Carbon-Hydrogen Balance Equations Describing Conversion of Glucose in Rumen
Case 1
Case 2
From Van Soest PJ: Nutritional ecology of the ruminant, Ithaca, NY, 1982, Cornell University Press.
* Note that in Case 1, the acetate/propionate ratio is 1:1 and the methane/glucose ratio 1:3, whereas in Case 2, the acetate/propionate ratio is 3:1 and the methane/glucose ratio 3:5.
In the rumen, methane production is facilitated by methanogenic bacteria, such as Methanobacterium ruminantium. This fragile bacterium is sensitive to changing conditions in the rumen. When conditions are unfavorable for the survival of M. ruminantium, methane production is reduced, shifting the metabolic pathways toward propionic acid production. Some conditions that suppress methanogenic species are high levels of feed intake, use of finely ground or pelleted feeds, and high-grain or high-starch diets. Under these circumstances the rate of methane production is reduced, resulting in a lower rate of acetic acid production with a concomitant increase in the propionic acid production rate.
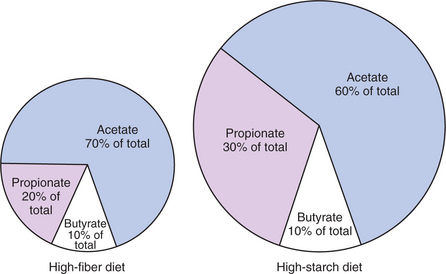
The proportional rates at which acetic acid, propionic acid, and butyric acid are produced are reflected in their relative concentrations in the rumen fluid. The relative concentrations of the VFAs have important nutritional and metabolic con-sequences, and although seldom measured for medical purposes, VFA concentrations are frequently reported in research literature. Typically, the ruminal acetic/propionic/butyric acid concentration ratio in ruminants ranges from 70:20:10 for animals eating high-forage diets to 60:30:10 for animals eating high-grain diets. One must remember that these values represent relative proportions and not absolute amounts. The total amount of VFA produced with a high-starch diet is usually much higher than that produced with a high-fiber diet, such that total acetic acid production may be higher with a high-starch diet than with a high-fiber diet, even though the acetic acid production relative to the other VFAs may be reduced. Figure 31-3 illustrates this principle.
Volatile Fatty Acids Are Important Energy Substrates for the Host Animal
One can appreciate the elegance and beauty of the symbiotic relationship represented by fermentative digestion by considering the metabolism of VFAs. These molecules are the end products, indeed, the waste products, of anaerobic microbial metabolism, just as carbon dioxide is the waste product of aerobic metabolism. If the VFAs were allowed to accumulate, they would suppress or alter the fermentative process by lowering the pH of the gut or forestomach. However, the host animal maintains conditions for fermentation both by buffering pH changes and by removing VFAs from the gut by absorption. The benefit derived by the host is from the chemical energy that is contained in the VFAs. These bacterial “waste products” represent spent compounds within the framework of the anaerobic fermentation system, but they still contain considerable energy that can be derived from aerobic metabolism. In ruminants and other large herbivores, the VFAs are the major energy fuels, to a large extent serving the role played by glucose in omnivorous monogastric animals. The metabolic fates of the VFAs are discussed further in Chapter 32.
Fermentative Digestion of Protein Results in the Deamination of a Large Portion of Amino Acids
To this point, the discussion of fermentative digestion has centered primarily on carbohydrates, but as previously mentioned, other energy-yielding substrates are subject to microbial attack as well. Proteins are particularly vulnerable because they are composed of carbon compounds that can be further reduced to provide energy for anaerobic microbes. As proteins enter fermentative areas of the gut, they are attacked by extracellular microbial proteases. The majority of these enzymes are “trypsin-like” endopeptidases that form short-chain peptides as end products. These peptides are formed extracellularly and are absorbed into the microbial cell bodies, much as glucose is formed from carbohydrate and then absorbed. Within the microbial cells, the peptides can be used to form microbial protein or can be further degraded for the production of energy through the VFA pathways (Figure 31-4).
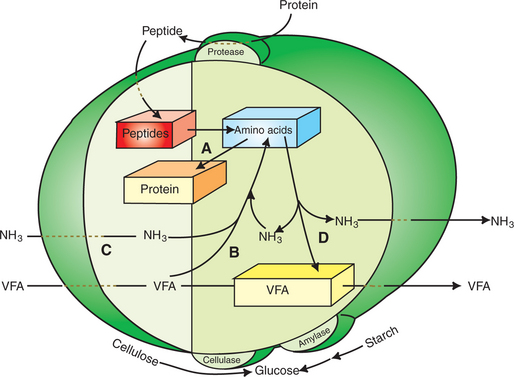
FIGURE 31-4 Protein metabolism by rumen microbes. Protease enzymes on the microbe surfaces generate peptides that are then taken up by many types of organisms. Absorbed peptides contribute to an intracellular pool of amino acids from which microbial proteins are synthesized (A). Another source of amino acids is from intracellular synthesis (B), using ammonia (NH3) and volatile fatty acid (VFA). Many microbes appear capable of deriving their amino acids from either extracellular peptides or intracellular synthesis; however, several types of bacteria seem incapable of using peptides for an amino acid source and are thus dependent on an extracellular source of ammonia (C) for amino acid synthesis. Amino acids not used for protein synthesis can be metabolized to VFA and ammonia (D).
To enter the VFA pathways, the individual amino acids are first deaminated to yield ammonia (NH3) and a carbon skeleton. The carbon structures of many of the amino acids can fit directly into various steps of the pathways leading to the production of the three major VFAs. The three branch-chain amino acids (BCAAs) are exceptions, however, and lead to the production of branch-chain VFAs by the following reactions:
These branch-chain VFAs are important growth factors for several species of bacteria, as described later.
Although many species of rumen microbes appear capable of using preformed amino acids for the synthesis of protein, several species cannot do so. These species must synthesize amino acids from ammonia and the various carbon metabolites of the VFA pathways. For synthesis of the BCAAs, the branch-chain VFAs are required. Among the microbial species that require ammonia and branch-chain fatty acids are some of the important cellulose-digesting bacteria.
When Protein and Energy Availability in the Forestomach Are Well Matched, Rapid Microbial Growth and Efficient Protein Utilization Result
Because a large part of preformed dietary protein is fermented in the rumen, ruminant animals depend, to a large extent, on microbial protein to meet their own protein needs. Microbial protein reaches the abomasum and small intestine when microbes are washed out of the rumen and into the lower tract. Digestive efficiency is optimized in ruminants when the growth rate of the microbial mass is maximal, resulting in maximal delivery of microbial protein to the host animal. These conditions are best met by rapidly growing populations of microbes. The microbial growth rate depends on the supply of nutrients and the rate at which microbes are washed from the rumen. Here we consider the effect of nutrient supply on microbial growth rate; factors affecting the rate of microbial removal are discussed later.
The overall reaction in the rumen may be greatly simplified, for the purposes of this discussion, to Equation 1:
Glucose and peptide represent ruminally available carbohydrate and protein, respectively. In this context, available means available to the microbes for fermentation. Carbohydrate or protein that is not susceptible, or accessible, to microbial attack is classified as unavailable and is not included in Equation 1. Glucose was chosen to represent carbohydrate, and peptide to represent protein, because all carbohydrates must be broken down to simple sugars, and proteins to peptides, before becoming available to bacteria. The term peptide in this equation could be replaced by other forms of nitrogen, but for now, the discussion is confined to peptide as a nitrogen source. Peptide is the only nitrogen-containing substrate on the left in the equation, but there are two nitrogen-containing products on the right: microbes (as protein) and NH3 (ammonia). Both substrates, glucose and peptide, contain carbon, oxygen, and hydrogen and thus can contribute to the formation of microbial carbon, VFA, CH4, and CO2.
Equation 1 always balances, but the distribution of products varies according to the relative concentrations of substrates, as illustrated in Figure 31-5. For microbial cells to be produced, both energy and nitrogen are required. Energy can come from either glucose or peptide, but nitrogen must come from peptide. When glucose and peptide availability are appropriately matched (Figure 31-5, a), energy for cellular growth comes primarily from glucose, with peptides directed toward microbial protein synthesis. Under these conditions, the products of Equation 1 favor microbial cells with little ammonia production. Glucose fermentation with accompanying VFA production must be high to meet the large energy demands necessary to support the rapid growth of the microbial mass. Ammonia production is low because most peptide nitrogen is being incorporated into microbial protein.
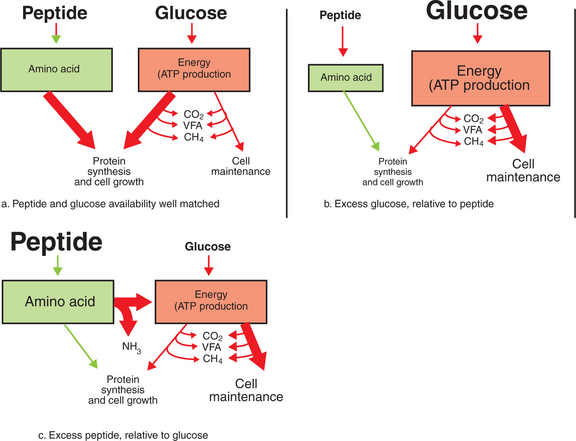
FIGURE 31-5 The efficiency with which dietary energy is used for protein synthesis in the rumen depends on the balance between energy and nitrogen sources. The proportion of energy used for protein synthesis and cell maintenance (as indicated by the size of the arrows) changes in relation to the balance of peptide (nitrogen) and glucose supplies. ATP, Adenosine triphosphate; VFA, volatile fatty acid.
When the availability of glucose is high relative to peptide (Figure 31-5, b), there is ample energy but insufficient nitrogen to support adequate protein synthesis, and thus microbial replication is not maximal. In this case, microbial energy utilization becomes inefficient as energy is used for the maintenance of nondividing cells, rather than for the energy-requiring synthetic processes of growing cells. The maintenance energy needs of the microbes still drive some fermentation of glucose with moderate VFA production, but production of both microbial cells and ammonia is limited because of lack of nitrogen.
When peptide availability exceeds glucose availability (Figure 31-5, c), there is ample nitrogen to support growth, but growth is limited because of insufficient energy supplies. These conditions force the microbes to use peptides to meet energy needs instead of for protein synthesis. Microbial growth rate is low, and VFA production is moderate, because fermentation is driven only by the maintenance energy needs of the microbes. Much of the VFA production comes from the carbon portions of the peptides, whereas the amine groups are shunted to ammonia production; thus the products of Equation 1 favor ammonia.
The relationship between available glucose (carbohydrate) and peptide (or nitrogen) has a tremendous effect on the production of microbial cells and thus a profound impact on the nutrition of the host. This relationship, as illustrated in Figure 31-5, is quantified by expressing microbial growth in terms of grams of microbial dry matter produced per mole of energy-producing substrate used. This value, referred to as microbial yield, is usually designated by a capital Y subscripted with the abbreviation of the energy substrate to which it is referenced. A convenient but somewhat theoretical substrate with which to reference microbial cell yield is ATP. Microbial yield is then written as YATP = x, where x is the number of grams of microbial dry matter produced per mole of ATP used. The value of YATP varies between about 10 and 20 g of microbes per mole of ATP. Nitrogen availability, from either peptide or nonprotein sources, has an important effect on the YATP value. When microbial growth is limited by low nitrogen availability, a large portion of available ATP is used for maintenance rather than cell growth; thus the number of cells produced per ATP is small, and the YATP value is low.
Microbial Protein Can Be Synthesized in the Rumen from Nonprotein Nitrogen Sources
If sufficient carbohydrate is available, most rumen microbes, even those capable of utilizing preformed peptides, can synthesize protein from ammonia (see Figure 31-4). Thus, protein can be produced in the rumen from such nonprotein sources as ammonia, nitrates, and urea. From a nutritional and economic point of view, this capability has been exploited by the inclusion of inexpensive nonprotein nitrogen sources in place of expensive protein in ruminant diets, allowing the microbes to synthesize protein for the amino acid needs of the host. This process also can be exploited physiologically by the recycling of endogenous urea.
Urea, the nitrogenous waste product of protein catabolism, is formed in the liver. In ruminant animals, hepatic urea production is from two sources: (1) nitrogen arising from the deamination of endogenous amino acids and (2) nitrogen absorbed as ammonia from the rumen (Figure 31-6). Ammonia absorption from the rumen is proportional to the ruminal ammonia production rate, which is subject to the influences of ruminal carbohydrate and protein availability, as discussed earlier. Ammonia, which is toxic at moderate concentrations, is absorbed from the rumen and delivered to the liver through the hepatic-portal blood vascular system. The liver extracts ammonia from the portal blood efficiently; thus little of the potentially toxic ammonia reaches the systemic circulation.
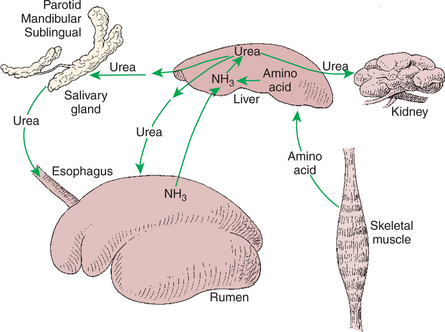
FIGURE 31-6 Interorgan nitrogen cycling in ruminants. The diagram shows the effects of rumen ammonia (NH3) concentration on the formation and utilization of urea. When rumen ammonia concentrations are high, the net movement of nonprotein nitrogen is toward the liver, resulting in high urea production rates and poor nitrogen conservation. When rumen ammonia concentrations are low, the net movement of nonprotein nitrogen is from liver to rumen, resulting in protein production from endogenous urea.
In monogastric animals, urea is excreted from the body almost exclusively by the kidneys. In ruminants, however, urea also may be excreted into the rumen (see Figure 31-6). Such excretion can occur by direct absorption of urea into the rumen from the blood or by excretion of urea into saliva. In either case, the urea reaches the rumen, where it is quickly transformed to ammonia, and enters the general pool of rumen nitrogen from which microbial proteins are synthesized.
The direction of nonprotein nitrogen flow, either into the rumen as urea or out of the rumen as ammonia, depends on rumen ammonia concentrations. During times of high nitrogen availability in the rumen, relative to carbohydrate availability, this system results in high blood urea concentrations and the extensive loss of precious nitrogen through urinary excretion, making ruminants nutritionally inefficient under these dietary conditions. However, during times of high carbohydrate availability relative to nitrogen availability, the major flow of urea nitrogen is from the blood into the rumen. Under these circumstances, in which ruminal ammonia concentrations are low, most of the blood urea is from endogenous protein catabolism. A portion of this urea, which in monogastric animals would be unavailable for protein synthesis, is excreted into the rumen, where it can be resynthesized into protein that will contribute eventually to the amino acid needs of the host. Thus, under conditions of low dietary protein, ruminants are efficient conservers of nitrogen.
RETICULORUMEN MOTILITY AND MAINTENANCE OF THE RUMEN ENVIRONMENT
The Physiological Functions of the Reticulorumen Maintain an Environment Favorable to Fermentation Patterns That Are Beneficial to the Host
The host animal has no direct control over the metabolism of the microbes in its gut. However, important physiological factors influence the gastrointestinal (GI) fermentation process. For the host to ensure that the proper type of fermentation patterns occur, it must maintain within the rumen (or colon) conditions that promote the growth and favorable metabolic patterns of the most beneficial bacteria and other microbes. The following requirements must be met by the host for proper fermentation to occur:
1. Substrate for fermentation must be supplied.
2. Temperature must be maintained at or near 37° C.
3. Ionic strength (osmolality) of the rumen fluid must be kept within an optimal range (near 300 mOsm).
4. A negative oxidation-reduction potential must be maintained (–250 to −450 mV).
5. Indigestible waste (solid material) must be removed.
6. The rate of removal of microbes must be compatible with the regeneration times of the most favorable microbes.
7. Acid products of anaerobic fermentation (VFAs) must be buffered or removed.
The first of these requisites, delivery of substrate, requires only eating; others (e.g., temperature, ionic strength) are met by the same homeostatic mechanisms that maintain these physiological conditions within the host body in general. Maintenance of an appropriate oxidation-reduction potential requires only that oxygen be kept away from the fermentation site. The remaining requisites for fermentation, however, have necessitated the development of special physiological functions associated with the forestomach (or hindgut). These specialized functions include the motility patterns characteristic of the reticulorumen, the direct absorption of VFA, and the production of copious amounts of saliva.
Rumen Fermentation Is Maintained by Selectively Retaining Actively Fermenting Material While Allowing Unfermentable Residue to Pass on to the Abomasum
The walls of the reticulorumen are muscular, possess an extensive intrinsic nervous system, and are capable of highly complex and coordinated motility patterns. The selective ruminal retention of fermenting material and the release of unfermentable residue are accomplished by these motility patterns. An understanding of reticulorumen anatomy is necessary to appreciate the effects of reticulorumen motility patterns. Figure 31-7 illustrates the division of the reticulorumen into compartments, or sacs. These divisions are created by muscular pillars that project into the lumen of the organ. The reticular fold and rumen pillars, in addition to the walls themselves, are motile. During reticulorumen contractions the pillars alternately elevate and relax, either accentuating or reducing the divisions within the lumen of the reticulorumen. Students accustomed to studying the reticulorumens of embalmed specimens may find it difficult to visualize the extent of rumen movement. At times during contractions, the excursions of the walls and pillars are so great that the total shape of the reticulorumen is distorted; sacs and compartments are almost obliterated, and pillars elevate to the extent that compartmental divisions become nearly complete. When the magnitude of these contractions is recognized, it is not difficult to appreciate the tremendous effect that reticulorumen motility has on the flow of rumen ingesta.
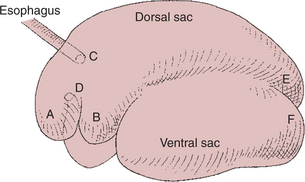
FIGURE 31-7 Rumen anatomy. A, Reticulum; B, cranial sac; C, cardia; D, reticulo-omasal orifice; E, caudal-dorsal blind sac; F, caudal-ventral blind sac.
Two patterns of reticulorumen motility are generally described: primary (or mixing) contractions and secondary (or eructation) contractions. Primary contractions start with a double, or biphasic, contraction of the reticulum. In the first phase of this reticular contraction, the organ is reduced to about half its relaxed size, whereas the second contraction is strong, nearly obliterating the lumen of the reticulum. The next action of the primary contraction pattern is a caudal-moving peristaltic contraction of the dorsal sac. On completion of the dorsal sac contraction, a similar caudal-moving contraction of the ventral sac occurs, followed by a cranial-moving contraction of the dorsal sac. The primary contraction pattern is completed by a cranial-moving contraction of the ventral sac (Figure 31-8). The primary contraction pattern serves to mix ingesta and to aid in the separation of large and small particles. Secondary contractions, when they occur, follow immediately after the primary contractions.
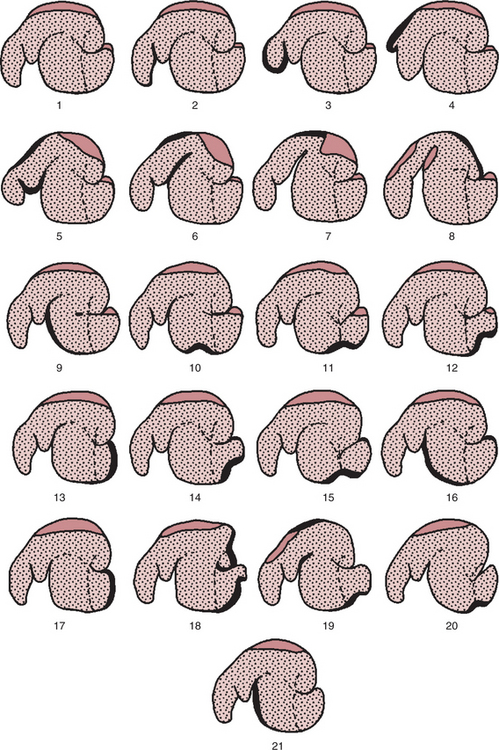
FIGURE 31-8 Contraction sequence in the reticulorumen. These drawings were derived by taking tracings directly from radiographs. The open regions represent the rumen gas cap (zone), whereas the stippled region represents ingesta. The heavy lines indicate portions of the wall that are actively contracting. Drawings 1 through 16 represent the sequence of events in a primary contraction in a normally fed sheep. Drawings 17 through 21 represent the sequence of events in a secondary or eructation contraction. 1, Resting stage. 2, Initiation of sequence with elevation of reticuloruminal fold. 3, End of first phase of reticular contraction. 4, End of second phase of reticular contraction; note dilation of cranial sac. 5 to 7, Contraction of cranial sac followed by contraction of cranial pillar and dorsal sac. 8, Contraction of caudal-dorsal blind sac and caudal pillar, causing cranial displacement of gas cap toward reticulum, under cranial pillar, and into caudal-ventral blind sac. 9, Contraction of longitudinal pillar and cranial ventral rumen; in anorectic sheep, the sequence frequently ceases at this point, and the occurrence of the remaining steps in the sequence varies according to the degree of filling of the reticulorumen. 10 to 12, Wave of contraction migrating caudally onto the caudal-ventral blind sac, associated with a ventral displacement of the caudal pillar. 13, Contraction of the pole of the caudal-ventral blind sac displacing gas cap around the caudal pillar. 14 to 16, Cranial migration of contraction if no secondary contraction sequence occurs. 17, When a secondary contraction follows a primary, the terminal contraction of the caudal-ventral blind sac may be maintained over a prolonged period or may be repeated simultaneously with a second contraction of the caudal pillar. 18, Contraction of caudal pillar and dorsal blind sac start to push gas cap cranially; contraction starts to move cranially across caudal-ventral blind sac. 19, Contraction has moved rapidly across dorsal rumen, and cranial pillar has moved for the second time; eructation, if it occurs, occurs at this point. 20 and 21, Contraction migrates cranially onto ventral rumen, causing contraction of ventral coronary pillars and second ventral displacement of the caudal pillar; cycle terminates with a contraction of the cranial ventral rumen.
(From Ruckebusch Y, Thivend P: Digestive physiology and metabolism in ruminants, Westport, Conn, 1980, AVI Publishing.)
Secondary contractions consist of a cranial-moving wave that starts in the caudal-dorsal blind sac and continues over the dorsal sac (Figure 31-8). The function of the secondary contraction is to force gas toward the cranial portion of the rumen. As the secondary contraction moves gas toward the cardia, the cranial sac relaxes and the cranial pillar elevates, allowing liquid ingesta to move away from the cardia so that gas can enter the esophagus and be eructated. Secondary contractions are important because large amounts of gas, primarily CO2 and CH4, are formed during fermentation and must be removed rapidly to prevent distention of the rumen.
In general, one to three reticulorumen contractions occur per minute. Contractions occur most frequently during eating and disappear entirely during deep sleep. The rate and strength of contractions depend on the character of the diet: coarse, fibrous feeds stimulate the most frequent and strongest contractions. Secondary contractions usually occur in association with half the primary contractions, although this relationship is variable and depends on the rate of gas formation. Reticulorumen contractions have an important influence on the flow of fluid and particulate matter through the rumen.
Gravity and Reticulorumen Motility Combine to Create the Selective Flow of Particulate Matter out of the Rumen
Rumen ingesta are stratified and segregated by the effects of gravity and reticulorumen motility. Cattle receiving forage diets have distinct zones, or phases, of rumen ingesta. In the dorsal rumen a gas cap, or gas zone, is created by the fermentation gases. Below this is a solid zone composed of intertwined particles of fermenting forage. The solid zone is sometimes referred to as the rumen mat because of the braided or woven nature of its particles. The solid zone is kept afloat by buoyancy created by air trapped in the feed particles, as well as by small bubbles of fermentation gases that form around bacteria that adhere to the plant material as fermentation takes place. At the bottom of the rumen there is a liquid zone with a waterlike consistency. The area between the solid and liquid zones is the slurry zone. The slurry zone has indistinct boundaries and forms a continuum of consistency from the liquid to the solid zones. These four major zones are created primarily by the effect of gravity; two additional functional zones are created by the motility patterns. These are the ejection zone and the zone of potential escape, which constitute the dorsal and ventral areas, respectively, of the reticulum and cranial sac (Figure 31-9).
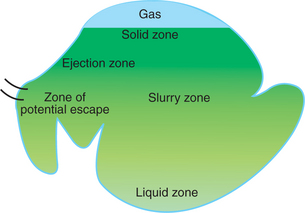
FIGURE 31-9 The rumen is stratified into indistinct zones, varying in consistency of ingesta. The dorsal solid zone contains relatively undigested forage material and continues imperceptibly into the slurry and liquid zones. The ejection zone, near the cardia, is the area that receives newly swallowed feed; contractions of the reticulum eject feed material from this area into the solid zone. As material becomes digested, it sinks into the liquid zone, eventually returning from the liquid zone to the cranial sac and reticulum. Once in the reticulum and cranial sac, material is in a zone of potential escape, from which it may enter the reticulo-omasal orifice.
Functional Specific Gravity Determines the Rate at Which Particulate Matter (Solids) Moves Through the Zones of the Reticulorumen
As forage is consumed by ruminants, the particles are only partially comminuted by the initial mastication and thus arrive at the reticulum as a tangled, masticated bolus of fairly long forage pieces. The bolus has a functional specific gravity of less than 1 because of air that is trapped both within and between the feed particles. (The term functional is applied to specific gravity in this context to indicate that the effects of trapped air are taken into consideration.) Because of the low specific gravity, the bolus floats in the ejection zone until a reticulum contraction occurs, when the pressure exerted by the reticular contraction washes, or ejects, the bolus from the reticulum into the solid zone of the dorsal sac.
In the dorsal sac, bacteria adhere to the forage particles, and fermentation begins. As fermentation proceeds, small bubbles of fermentation gases form and help keep the functional specific gravity of the particles low. Motility in the dorsal sac mixes ingesta in the solid zone in a counterclockwise (when viewed from the left side of the animal) movement (Figure 31-10). As the ingesta are mixed, the particles begin to break up because of fermentative destruction of structural carbohydrates in the plants. As fermentation proceeds, particle size is reduced, entrapped air escapes, and there is a lower rate of fermentation gas formation. Because of these events, the functional specific gravity of the feed particles increases.
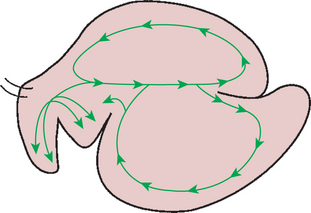
FIGURE 31-10 Patterns of movement of rumen ingesta. Rumen motility results in more or less circular patterns of ingesta movement.
(From Ruckebusch Y, Thivend P: Digestive physiology and metabolism in ruminants, Westport, Conn, 1980, AVI Publishing.)
As functional specific gravity rises, particles tend to sink and separate into a slurry zone between the solid and liquid zones of the rumen, where further fermentation and size reduction occur. In the ventral sac the motility pattern creates a clockwise movement of ingesta (Figure 31-10). As the flowing mass of ingesta moves against the cranial pillar of the rumen, material that still has a relatively low functional specific gravity tends to remain in suspension in the slurry zone and is retained in the circulating mass within the ventral sac. Material that has become relatively dense tends to fall over the cranial pillar and into the cranial sac, thus into the zone of potential escape. During contractions of the cranial sac, dense material can move into the reticulum, from which it can exit the rumen through the reticulo-omasal orifice.
You can appreciate the effectiveness of the particle separation system in the rumen by considering particle sizes at different points in the digestive process. Long forage material is reduced by initial mastication to particles of 1 to 2 cm or shorter. Most of the material in the dorsal rumen is of similar particle size. Particle size diminishes in the more ventral portions of the rumen. Most particles that move through the reticulo-omasal orifice are 2 to 3 mm long. The selection of small particles for passage into the omasum occurs even though the reticulo-omasal orifice, when dilated for food passage, is probably about 2 cm in diameter, indicating that size discrimination is not based on sieving action at the orifice.
Digestibility and Physical Characteristics of Feed Have Important Influences on Both the Rate of Particle Passage from the Rumen and the Rate of Feed Intake
As indicated earlier, feed does not leave the rumen until it is broken down into small particles. Microbial action and remastication (as discussed later) are primarily responsible for particle size reduction in the rumen, and the rate of breakdown of fiber is chiefly a function of its digestibility. Poorly digestible fiber takes longer to be broken down sufficiently to enter the zone of potential escape compared with fiber of greater digestibility. This means that poorly digestible fiber remains in the rumen longer than fiber of greater digestibility. Because there are fixed limits to the volume of the rumen, the rate of feed intake cannot exceed the rate of ingesta outflow; therefore, intake of poorly digestible feeds is always less than intake of highly digestible feeds.
Feed preparation can influence this relationship. Chopping or grinding of poorly digestible forages increases their rate of passage from the rumen because less particle size reduction is necessary before pieces can pass into the omasum. Chopping or grinding also usually increases the amount of material that an animal can eat because the rumen throughput is increased. Often, however, digestibility is decreased by chopping or grinding of forages, because the duration of exposure to microbial action is reduced as a result of rapid passage of feed through the rumen. Thus, physical form (length) and digestibility each have an effect on rate of passage from the rumen and also on feed intake. In general, forage material of relatively high digestibility has a rumen half-life of approximately 30 hours, whereas poorly digestible material has a half-life of up to 50 hours.
Rumination, or Cud Chewing, Has an Important Effect on the Reduction of Particle Size and the Movement of Solid Material Through the Rumen
Rumination is the act of remasticating rumen ingesta. The initial act of rumination is regurgitation, which occurs just before the initiation of a primary rumen contraction. When regurgitation occurs, there is an extra contraction of the reticulum, which takes place just before the regular biphasic reticular contraction that initiates the primary cycle. Simultaneous with the extra reticular contraction, the cardia relaxes, and there is an inspiratory excursion of the ribs with the glottis closed. The latter action creates a negative pressure within the thorax, favoring the movement of food into the esophagus. When food enters the esophagus, a reverse peristaltic wave propels the material cranially into the mouth. As soon as the food bolus reaches the mouth, excess water is expressed by action of the tongue, the water is swallowed, and remastication of the material begins. The duration of remastication depends on the character of the diet, with coarse material requiring more time for remastication than finely ground or highly digestible feeds.
Regurgitated material comes from the dorsal portion of the reticulum, where particle size and functional specific gravity are characteristic of the slurry zone. Thus the ingesta selected for remastication are not the coarsest material in the rumen, but rather the material that has already been through the digestive actions of the solid zone. This appears to be an efficient system in which some of the structural material of the plant is softened by soaking and removed or weakened by microbial action in the solid zone. The partially fermented material then reaches the slurry zone and is subjected to remastication, causing further comminution and exposing additional fermentable substrate that may not have been directly exposed to previous microbial action.
Rumination may also aid the particle separation process: as the regurgitated bolus reaches the mouth, it is squeezed by the tongue and cheeks before mastication begins. Water and small particles are expressed from the bolus by this squeezing action and are swallowed before mastication of the remaining bolus. This action tends to separate small particles from large particles. The small particles, when reswallowed, sink into the zone of potential escape, whereas the larger particles, when swallowed after remastication, are ejected back into the slurry zone.
Rumination occurs when the animal is not actively eating, usually during times of rest, but not during deep sleep. The time spent ruminating depends on the type of diet and ranges from almost none for high-grain diets to a maximum of about 10 hours per day for high-forage diets. The feed intake level also influences the amount of rumination time, with high intakes stimulating greater rumination.
Water Moves Through the Rumen at a Much Faster Rate Than Particulate Matter
The flow of water has important effects on rumen dynamics. In order for small particles and soluble material to exit the rumen, liquid from the liquid zone of the ventral sac, cranial sac, and reticulum must constantly be moving through the reticulo-omasal orifice. This means that water must be constantly flowing through the mass of solid material. In effect, the reticulorumen functions as a giant strainer or sieve, holding the fermenting mass of particulate matter while water flows through it and washes small particles and soluble material away. Therefore the transit rate of water must be considerably greater than the transit rate of particulate matter through the rumen. The relative differences in the rates of movement of solid-phase and liquid-phase material through the rumen can be appreciated from their respective rumen half-lives: 30 to 50 hours for particulate matter and about 15 to 20 hours for liquid.
The rate of liquid flow through the rumen is often measured as the dilution rate, which is expressed as the percentage of total liquid that leaves the rumen in an hour. The term dilution rate comes from the way liquid turnover is measured; some soluble marker substance is mixed into the rumen, and its concentration is measured as soon as it is thoroughly dispersed into the liquid phase. Samples are then taken over time, and the rate at which the marker substance becomes diluted is measured. The rate of dilution depends on the rate at which water that contains marker leaves the rumen and is replaced with new, unmarked water; thus the dilution rate is an indirect measure of the rate of water flow through the rumen. Normal dilution rate values vary with diet and feed intake and are usually in the range of 5% to 30% per hour. One other point should be appreciated from the concept of dilution rate: water leaves the rumen only as it is replaced from some other source.
Almost all water that enters the rumen does so through the esophagus, from salivary flow, drinking, or succulent feeds. Thus the dilution rate depends on rates of salivation and drinking. The salivation rate is influenced by the chewing time and feed type; feeds such as long-stemmed dry roughages, which require relatively high rates of mastication, stimulate high rates of both salivary flow and dilution. Salivation occurs during rumination as well as during initial mastication; therefore those feeds that stimulate high rumination rates, such as forages, also stimulate high dilution rates. Conversely, feeds that do not stimulate extensive rumination (e.g., concentrates) result in relatively low dilution rates. The rate of drinking is influenced by (1) the rate of feed intake and (2) the salt, or electrolyte, content of the diet. Thus, high rates of intake or diets with high electrolyte contents stimulate high dilution rates.
Little water enters the rumen by way of the mucosa. The mucosa of the forestomachs is stratified squamous epithelium and is aglandular; thus there is no direct fluid secretion. Some water can enter the rumen through osmosis, but under normal conditions, the amount appears to be minimal. Normal rumen osmolality is about 280 mOsm/kg, slightly less than the 300-mOsm/kg osmolality of blood and extracellular fluid. Thus the usual osmotic flow of water is out of the rumen. After consumption of relatively digestible feeds, rumen osmolality increases briefly because of VFA production; however, it appears that osmolalities in excess of 340 mOsm/kg are necessary for water to flow osmotically into the rumen. Under normal conditions, osmolalities this high are not sustained for long, and thus there is usually little osmotic flow of water into the rumen.
Rumen Dilution Rate Has Important Influences on Fermentation and Microbial Cell Yield
Small particles, including microbes, leave the rumen with the liquid phase. Therefore, high dilution rates result in rapid removal of microbes and reductions in microbial cell concentrations. Because high microbe concentrations suppress microbial cell division, the growth of microbes is stimulated by high dilution rates. High growth rates are nutritionally desirable because a larger portion of the energy available to the microbes is used for growth instead of for maintenance, as occurs in older, relatively stable microbial populations. Thus, high dilution rates usually increase YATP values, provided that adequate protein is available to support cell growth.
In addition to its effect on YATP, the dilution rate may affect the microbial makeup of the rumen biomass and also may have some influence on the fermentation pattern. The rate of microbial washout increases with the dilution rate. At high dilution rates, microbial species with slow growth rates diminish in population size because their replication rate is not great enough to match the rate at which they are removed. Thus, selection pressure favors species with faster growth rates during times of high rumen dilution rates. Exceptions to this pattern occur because some microbes are able to attach themselves to the particulate matter in the solid and slurry zones. Such microbes then exit the rumen according to the kinetics of particle size reduction rather than dilution rate. In general, the changes occurring in the rumen microbial population with high dilution rates appear to favor acetic acid production and to increase the acetic/propionic acid ratio.
CONTROL OF RETICULORUMEN MOTILITY
Reticulorumen Motility Is Controlled by the Central Nervous System and Affected by Intraluminal Conditions
In the dorsal vagal nucleus of the brainstem, there is a motility control center for the regulation of reticuloruminal motility. This center sends action potentials along afferent fibers to the forestomach by way of the vagus nerve. There is an extensive enteric nervous system within the reticulorumen, but vagal innervation is necessary for coordination of normal motility patterns. When the vagal nerves are destroyed, motility of the rumen musculature ceases initially but returns within several days; however, the motility that develops after vagotomy is erratic, uncoordinated, and incapable of supporting the normal flow of ingesta through the reticulorumen. Vagotomized ruminants do not survive.
The dorsal vagal nucleus receives afferent stimuli that affect the control of forestomach motility. Important afferent signals come from the lumen of the reticulorumen and monitor distention, ingesta consistency, pH, VFA concentration, and ionic strength. Rumen volume, or distention, appears to be monitored by stretch receptors in the walls and especially in the pillars. Moderate distention increases rumen motility and rumination. Increased motility and rumination have the effect of raising the rate at which particles are broken down, leading to a higher passage rate. Thus, rumen throughput is enhanced when increased intake expands rumen volume. Severe distention, as occurs pathologically in bloat, causes cessation of rumen motility.
The consistency of ingesta also has an important influence on rumen motility. Consistency is determined largely by diet type. When the diet consists of succulent plants, grain, or finely chopped forage, there is little material in the solid zone, or rumen mat, and the slurry zone is fluid. This type of fluid ingesta offers little resistance to the movement of the rumen pillars; thus the rumen musculature has to apply relatively little force to mix and circulate the rumen contents. Tension receptors in the reticuloruminal muscle appear to monitor the force necessary to move the pillars through the ingesta. Highly fluid rumen ingesta are associated with low muscle tension and have a negative influence on reticulorumen motility. At the other dietary extreme, when animals are eating dry, long-stem hay, the rumen contents are solid and create a large and highly interwoven rumen mat. Resistance to movement of the pillars through the solid mass of ingesta is high and leads to stimulation of tension receptors, resulting in a positive feedback on motility. The motility rate is directly related to the rate of particle breakdown; this arrangement appears to be a self-regulatory mechanism that increases the rate of particle comminution when animals consume diets with large particle size.
Chemoreceptors in the walls of the rumen and reticulum monitor pH, VFA concentration, and ionic strength (or osmolality). The pH of the reticulorumen is normally slightly acid, reflecting the acidity of the VFAs, but extreme acid conditions are undesirable. Increasing VFA concentrations or decreasing pH results in a suppression of rumen motility. The normal rumen pH is in the range of 5.5 to 6.8, depending on the type of diet. When the rumen pH falls much below 5.0, motility is severely depressed. This response appears to be protective because fermentation tends to be enhanced by motility-induced mixing; thus suppression of motility slows down fermentation, allowing VFA absorption to catch up with VFA production.
Osmolality may influence rumen motility as well, although motility appears less sensitive to osmotic changes than it does to pH changes. Normal osmolality in the rumen is about 280 mOsm, but the osmolality increases during active fermentation. Osmotically active solutes in the rumen include organic acids as well as salivary and dietary electrolytes. As organic acid formation increases during fermentation, osmolality also increases, tending to reduce motility. The rumen epithelium creates a relatively impermeable barrier to water, so wide swings in rumen osmolality can occur without large shifts in water between the rumen and the vascular compartment. At abnormally high osmolalities, however, water can be drawn into the rumen.
OMASAL FUNCTION
Passage of Material from the Reticulum to the Omasum Occurs During Reticular Contraction
The omasum is composed of a body and a canal. The body is filled with multiple muscular folds, or leaves, that project from the greater curvature into the lumen. The canal, which is located on the lesser curvature, connects the reticulum to the abomasum. Ingesta move into the omasum during reticular contractions. The omasal orifice usually remains open, but dilates especially during the second phase of the reticular contraction, during which ingesta flow rapidly into the omasal canal. After the reticular contraction, the omasal orifice closes briefly as the canal contracts, forcing newly arrived ingesta up into the leaves. Intermittently, the body and leaves of the omasum contract, forcing the material from the body of the organ into the canal and on into the abomasum.
Proper functioning of the omasum and reticulum appears to be particularly important to the passage of ingesta out of the rumen. Occasionally, traumatic injury resulting from ingested foreign bodies causes severe adhesions of the reticulum and omasum to the body wall. In addition, damage to vagal fibers entering the organs may occur. In such cases, motility of the rumen proper may continue normally, but the ability to move food out of the forestomachs and into the abomasum is severely impaired. The rumen becomes greatly distended with finely comminuted feed, and the entire rumen becomes a slurry zone. Despite the distended rumen, little movement of ingesta occurs into the abomasum, and the animals eventually suffer severe inanition. This condition is variably known as omasal transport failure and vagal indigestion; usually, little can be done to correct it.
The structure of the omasum, with its many leaves and large mucosal surface area, suggests that it has an absorptive function, but the exact nature of this function is still incompletely understood. One important possibility is that it exists to remove residual VFAs and bicarbonate from ingesta before material is transported to the abomasum. VFAs appear to cause unfavorable reactions in the abomasum, so it is important that the major portion of them be removed before abomasal entry. Also, it appears desirable to absorb, before abomasal entry, any bicarbonate remaining in the ingesta. Bicarbonate remaining in ingesta and entering the abomasum would only neutralize abomasal hydrochloric acid, making the abomasal glands work harder to maintain appropriate abomasal pH.
VOLATILE FATTY ACID ABSORPTION
Volatile Fatty Acids, Representing 60% to 80% of the Energy Needs of the Animal, Are Absorbed Directly from the Forestomach Epithelium
VFAs are bacterial waste products and, if allowed to accumulate, will suppress fermentation. Furthermore, the VFAs are extremely important energy substrates for the host, supplying 60% to 80% of the dietary energy to ruminants with most types of diets. Therefore the presence of an efficient and high-capacity mechanism for VFA absorption is important to both digestion and host metabolism. The forestomach epithelium supplies such a system, absorbing almost all the VFAs, with only small amounts escaping to the lower digestive tract. In addition, the absorptive process helps maintain rumen pH by removing acid from the forestomach ingesta and contributing bicarbonate in the process.
The epithelium responsible for this tremendous absorption is structurally much different from other absorptive epithelia of the GI system. However, the nature of the rumen epithelium may give it functional characteristics similar to those of the absorptive epithelium of the small intestine and colon. The forestomach surface is of the stratified squamous type and, as with the stratified squamous epithelium of the skin and other surfaces, consists of several layers of cells of varying maturity. The deepest layer is the stratum basale, from which cells divide and migrate into the stratum spinosum. Cells of the stratum spinosum begin the process of keratinization and continue into the stratum granulosum, which is covered by the outermost and most keratinized layer, the stratum corneum. Although the forestomach epithelium seems completely different from the columnar epithelium of the small intestine, an interesting similarity between forestomach and intestinal epithelia is noted when the cellular attachments and intercellular spaces of the forestomach are examined (Figure 31-11).
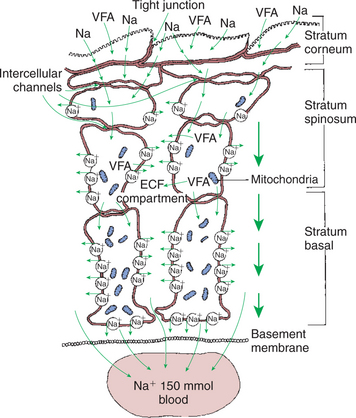
FIGURE 31-11 The stratified squamous epithelium of the rumen, although anatomically much different, shares functional similarities with the columnar epithelium of the intestine. Note the tight junctions of the cells of the stratum corneum and the lateral space–like compartment between adjacent cells of the stratum spinosum and stratum basale. Although the cells of the stratum spinosum are metabolically inactive, the intercellular channels allow the metabolic actions of the stratum basale to be reflected in the more superficial layers. VFA, Volatile fatty acid; Na, sodium; ECF, extracellular fluid.
(Modified from Steven DH, Marshall AB: Organization of the rumen epithelium. In Phillipson AT, editor: Physiology of digestion and metabolism in the ruminant, Newcastle upon Tyne, UK, 1970, Oriel Press.)
The cells of the stratum granulosum are tightly joined by junctions that may functionally resemble the tight junctions of the enterocytes (see Chapter 30). Deeper in the epithelium, the cells of the stratum spinosum and stratum basale are separated by intercellular spaces that increase in size as the basement membrane is approached. These intercellular spaces are reminiscent of the lateral spaces of columnar absorptive epithelia. If these observations are combined with the existence of the intercellular bridges that characterize the forestomach epithelium, an interesting analogy to columnar absorptive epithelia can be constructed. VFAs, electrolytes, and water apparently are initially absorbed through the stratum corneum and passed cell to cell by way of intercellular bridges to the cells of the stratum spinosum and stratum basale, from which the absorbed substances are passed into the intercellular spaces before entering the capillaries.
This arrangement of the forestomach epithelium is very similar to the three-compartment characteristics of the columnar absorptive epithelia, with solutes passing from lumen to cell to lateral spaces. Although the keratinized cells of the stratum corneum do not appear to retain adequate metabolic machinery (e.g., mitochondria) to maintain appropriate gradients for diffusion, the cells of the stratum spinosum and stratum basale are metabolically active. Because of the intercellular bridges, absorbed solute can be transferred directly from the outer keratinized cells to the deeper, more metabolically active cells. Thus the metabolic activity deep in the epithelium appears to maintain conditions for absorption at the epithelial surface.
The molecular mechanism of VFA absorption is incompletely understood but seems to involve local alterations in pH near the absorptive surface. Differences in pH can have an important influence on VFA absorption because of shifts in the dissociation state of the VFA molecules. The pKa of the VFA is approximately 4.8, well below the normal pH of the rumen; thus most of the VFAs exist in the rumen in the dissociated, or ionic, form. However, sodium-hydrogen ion exchange by the epithelial cells may decrease the local pH at the absorptive surface. Such a drop in pH would lead to a shift in the VFA from the ionic to the free-acid state. Cell membranes are permeable to VFA free acids, and absorption proceeds because of the concentration gradient between the lumen and cells. The high CO2 tension in the rumen, caused by the production of fermentation gases, may also enhance the conversion of VFA to the free-acid state. As shown in Figure 31-12, when one VFA molecule is absorbed, one molecule of bicarbonate (HCO3−) is generated in the lumen; thus VFA absorption helps buffer rumen pH both by generating base and by removing acid.
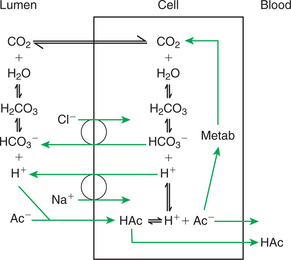
FIGURE 31-12 VFA absorption is promoted by the conversion of VFA anions (Ac−) to free acids (HAc) in the microenvironment near the epithelial surface. This diagram illustrates two proposed means, one intracellular and one extracellular, by which hydrogen ions could be locally generated to effect the formation of VFA free acids; both mechanisms could exist simultaneously.
(From Stevens CE, Argenzio RA, Roberts MC: Comparative physiology of mammalian colon and suggestions for animal models of human disorders, Clin Gastroenterol 15:763, 1986.)
All the VFAs appear to be absorbed by the same mechanism, but they are handled differently within the epithelial cells. Some acetate seems to be completely oxidized within the cells, with the remainder absorbed unchanged. Most propionate is absorbed, but a small portion is converted to lactate by the epithelial cells. Butyrate is modified extensively, and essentially all molecules are changed to β-hydroxybutyrate before absorption. β-Hydroxybutyrate is an important metabolite known as a ketone body. Ketone bodies are metabolites that frequently have special medical significance (see Chapter 32). In ruminants the rumen itself is a significant source of ketone bodies. In monogastric animals, however, ketone bodies arise exclusively from the partial oxidation of long-chain fatty acids.
The rumen epithelium is arranged in papillae, fingerlike projections that increase the absorptive surface area. Although they serve the same area-expanding function as the villi of the small intestine, papillae are much larger and easily visible to the unaided eye. The size and shape of the papillae are quite dynamic and responsive to changes in diet. Papillary growth is stimulated by VFAs, especially butyrate and propionate. Diets with high digestibility result in high rumen VFA concentrations, which stimulate the growth of long papillae. In contrast, animals receiving little feed or diets of low digestibility have short rumen papillae. It is important to adapt ruminants gradually when changing them from diets of low digestibility to high digestibility; this may allow time for sufficient adjustment of papillary size so that VFA absorption will match VFA production.
RUMEN DEVELOPMENT AND ESOPHAGEAL GROOVE FUNCTION
Significant Changes in Forestomach Size and Function Occur with Dietary Changes in Early Life
At birth the forestomach is about equal in size to the abomasum in both lambs and calves, a stark contrast to the normal adult proportions, in which the forestomach accounts for more than 90% of the total stomach volume. Enlargement of the forestomach occurs rapidly after birth, but the rate depends on diet type. When young ruminants are given access to solid feeds soon after birth, the forestomach development rate is maximal.
In cattle the period of forestomach development is arbitrarily divided into the nonruminant period, from birth to 3 weeks, and the transitional period, from 3 to 8 weeks. Approximate adult distribution of stomach proportions is achieved usually by 8 weeks, if calves have access to solid feeds. Calves can be seen eating grain and forage as early as 2 weeks of age and frequently ruminate by 3 weeks, indicating considerable forestomach development by this time. Withholding solid feed dramatically reduces the rate of rumen development. In calves that are given diets of only milk or milk substitute (“replacer”), forestomach development remains rudimentary for 14 to 15 weeks or longer.
Development of forestomach epithelium parallels the general development of the organ. At birth the epithelium is thin, with small or nonexistent papillae. Exposure of the epithelium to VFAs appears to stimulate papillary development and general organ development as well. Highly digestible feeds, such as concentrates, result in the greatest VFA production and fastest epithelial development. Some dietary forage may aid in muscular development of the fore-stomachs, but calves and lambs in the transitional period should receive most of their solid feed as grain because their energy needs are high compared with their ability to ferment forages.
The forestomach is sterile at birth but is quickly colonized by environmental bacteria, mostly facultative organisms. As bacterial fermentation proceeds in the anaerobic confines of the forestomach, the electromotive force decreases; the typical reductive environment of the rumen is created by bacterial action. This environment creates conditions necessary for the growth and establishment of the strict anaerobes. The development of forestomach bacterial flora occurs independently of any special inoculation process, and indeed, it is impossible to prevent it from occurring except by raising calves under gnotobiotic conditions. Protozoal inoculation, in contrast to bacterial inoculation, seems to require some exposure to other cattle; calves raised in complete isolation do not develop protozoal fauna. It appears that aerosol spread of protozoa can occur because no direct physical contact among cattle is necessary to establish a protozoal fauna.
The Esophageal Groove Diverts the Flow of Ingested Milk Past the Forestomach and into the Abomasum
For proper rumen development in the suckling animal, it is important for milk to be diverted away from the developing rumen. This is accomplished by the actions of the reticular groove (also called the esophageal groove). This structure is a gutterlike invagination traversing the wall of the reticulum from the cardia to the reticulo-omasal orifice. When stimulated, muscles of the groove contract, causing it to shorten and twist. The twisting action causes the lips of the groove to close together, forming a nearly complete tube from the cardia to the omasal canal. When the groove is contracted, milk entering the cardia is directed into the omasum, with 10% or less entering the rumen. Milk quickly traverses the omasum and enters the abomasum.
Reticular groove closure is a reflex action, with efferent impulses arriving from the brainstem through the vagus nerve. Afferent stimuli arise centrally and from the pharynx. Anticipation of suckling invokes central stimulation of reticular groove closure, which may be considered a cephalic phase. Fluid, especially sodium-containing fluid in the pharynx, stimulates afferent fibers that reinforce the cephalic phase of groove closure. The posture of the calf or lamb when suckling does not appear to have much influence on reticular groove function, but rapid drinking from an open pail, in contrast to suckling from a nipple, frequently results in inefficient groove function and spillage of milk into the rumen. Milk in the rumen results in the formation of improper fermentation patterns.
The reticular groove has its primary function in suckling animals, and the activity of the groove reflex appears to diminish after weaning and with advancing age. However, the groove reflex is stimulated by antidiuretic hormone (ADH; see Chapter 43), indicating that it may have some physiological function in adult life. ADH is secreted by the posterior pituitary in response to dehydration or increases in plasma osmolality. ADH is associated with thirst, and because it stimulates the reticular groove, a large portion of the water drunk by water-deprived animals may bypass the rumen. This may be a functional mechanism to ensure that water arrives quickly at the site of most rapid absorption, the small intestine.
FUNCTION OF THE EQUINE LARGE HINDGUT
The Equine Hindgut Has a Great Capacity for Fermentation
A general function of the cecum and colon, as mentioned in Chapter 30, is to recover fluid and electrolytes from ingesta leaving the ileum. In many herbivorous species, this function has been expanded to include fermentative digestion. Absorptive and fermentation functions complement each other in the colons of nonruminant herbivores. This arrangement leads to an elegantly interactive system of fermentation and absorption; however, it also results in an interdependence between the two processes, meaning that disturbances in fermentation can result in important abnormalities in absorption, and vice versa.
The Types of Substrate and Fermentation Patterns Are Essentially Identical for Forestomach and Hindgut Fermentation
Structural and nonstructural carbohydrates as well as proteins form the major substrates for hindgut fermentation. However, the passage of material through the stomach and small intestine before its arrival at the cecum and colon may have some important effects on fermentative digestion. First, hindgut fermentation may be aided by prior gastric action. The effects of soaking and acid exposure on plant particles in the stomach may increase their susceptibility to microbial attack and thus raise their rate of digestion in the hindgut. Second, some of the readily available carbohydrate, particularly sugars and starches, may be digested and absorbed before the other material arrives in the cecum. Most evidence indicates, however, that glandular digestion of carbohydrate in the horse is not extremely efficient and that substantial amounts of starch and sugars reach the cecum. Further, cell-wall carbohydrate appears to interfere with the digestion or absorption of nonstructural carbohydrate, so that diets high in cell-wall content result in relatively little starch digestion and absorption in the equine small intestine. Even with a high-grain diet, up to 29% of dietary starch may reach the cecum and colon.
Protein as well as carbohydrate is absorbed in the small intestine, potentially leading to a deficiency of nitrogen for colonic microbes. However, there is extensive urea recycling into the colon and cecum, similar to that occurring in the rumen (see Figure 31-6). Thus, urea plus protein escaping small-intestinal digestion supplies the nitrogen needs of the microbes. In contrast to ruminants, horses do not have an efficient means of recovering the microbial protein synthesized in the hindgut, and most of it passes out in the feces. Some experiments have shown a small amount of amino acid absorption from the equine cecum or colon, but the amount does not compare with microbial protein availability in the ruminant.
The Motility Functions of the Cecum and Colon Retain Material for Fermentation and Separate Particles by Size
The functions of the equine hindgut in maintaining fermentation are similar to those of the rumen: favorable conditions must be maintained to support optimal fermentation. As in the rumen, these conditions are (1) substrate supply, (2) control of pH and osmolality, (3) anaerobiosis, (4) retention of fermenting material, and (5) continual removal of waste products and the residue of spent fermentation substrate. Separation of fermenting material from residue appears to be accomplished by selective retention of particles according to size, just as in the rumen; however, the means by which the cecum and colon accomplish size separation and discriminate passage are quite different from those of the forestomach. Anatomical characteristics and motility patterns in the cecum and colon are responsible for selective retention of long particles, allowing sufficient exposure for microbial digestion to occur. In general, the fermentative digestive process in the horse is not as efficient as that in the ruminant, and digestible energy values for forages are usually lower for horses than for cattle.
Before the motility of the equine cecum and colon is discussed, a brief review of the anatomy of the equine hindgut is important. Figure 31-13 shows the equine digestive system, separated from its mesenteric attachments and laid out in linear fashion. The hindgut commences with the cecum, which is separated from the large colon by a well-defined orifice. The large colon is folded on itself three times, forming four major anatomical divisions: the right ventral and left ventral and the left dorsal and right dorsal colon segments. Ingesta enter the right ventral colon and course to the left ventral colon, from which the material enters the left dorsal portion through the pelvic flexure. From the left dorsal colon, material moves to the right dorsal colon before entering the small colon. (Anatomy textbooks describe the arrangement of the large colon in the abdomen.) For the purposes of physiological study, the reader should note in Figure 31-13 the tremendous size and volume of the cecum and colon compared with the small intestine. The differences in diameter that occur throughout the colon should also be noted, particularly the reductions in diameter that occur at the pelvic flexure and at the junction of the large and small colons. The saclike evaginations that occur in the wall of the cecum and most segments of the colon are called haustra. Functionally, the equine hindgut can be divided into four sections: cecum, ventral colon, dorsal colon, and small colon.
FIGURE 31-13 Equine gut. Note the tremendous development of the colon compared with the small intestine. Note also the relative areas of constriction at the junctions of the ventral and dorsal colons (A) and the large and small colons (B).
(From Stevens CE: Comparative physiology of the digestive system. In Swenson MJ, editor: Dukes’ physiology of domestic animals, ed 9, Ithaca, NY, 1977, Cornell University Press.)
Ingesta reach the cecum after a relatively short time in the stomach and small intestine. A large portion of soluble ingesta usually reaches the cecum by 2 hours after ingestion, whereas solids take somewhat longer, depending on particle size and consistency. The material in the cecum and throughout the large colon has a high water content and a slurrylike consistency.
The majority of cecal motility is of a mixing nature, with frequent low-amplitude contractions that transport ingesta from haustrum to haustrum and back in a mixing pattern. The mixing action of the cecum maintains the cecal contents in a homogeneous state. About once every 3 to 4 minutes, there is a strong contraction of cecal muscles in a mass-movement type of action (see Chapter 28 for a description of mass movement) in which the body and apex of the organ shorten and constrict, lifting ingesta into the base. Constriction of the base forces material through the cecocolic orifice and into the right ventral colon. The motility pattern functionally separates the cecum from the ventral colon, with no apparent mixing of contents between the two hindgut segments. Thus, there is no retrograde flow of material from the colon to the cecum, so the composition of ingesta in these two organs usually differs somewhat.
Three types of motility patterns exist in the right and left ventral colon: haustral segmentation, propulsive peristalsis, and retropulsive peristalsis. Segmentation serves a mixing function that aids in promoting fermentation and bringing VFAs in contact with the mucosa for absorption. Mixing occurs throughout the ventral colon, and the right and left segments may be regarded as one functional unit with homogeneous ingesta. Propulsive activity, or aboral peristalsis, in the ventral colon originates near the cecum and appears to occur as a continuation of the cecal mass movements. Peristaltic activity in the proximal ventral colon propels ingesta distally into the left ventral colon. In the left ventral colon, retropulsive or antiperistaltic movements resist the flow of ingesta and result in the retention of material in the ventral colon, allowing time for microbial digestion and preventing the washout of microbes. In addition, the retropulsive actions of the left ventral colon aid in creating differential flow rates of liquid and particulate matter through the colon. The antiperistaltic motility appears to originate from a pacemaker in the pelvic flexure, the area of restricted diameter where the left ventral and left dorsal colons meet.
The motility of the ventral colon can be roughly compared with that of the stomach, with the pelvic flexure and distal left ventral colon acting as the pylorus and antrum, respectively. The pumping action of cecal mass movements, combined with the propulsive action of the proximal ventral colon, continually moves ingesta toward the pelvic flexure. In the distal ventral colon, however, antiperistaltic activity and the narrow diameter of the pelvic flexure impede the movement of material, causing it to be retained in the ventral colon. The squeezing action of the pelvic flexure mimics the action of the pylorus in selectively retaining relatively large particulate matter while allowing liquid and small particles to pass. As particle size is reduced by fermentative action and the mixing activity of the colon, particles eventually become small enough to flow with the fluid phase and leave the colon. The action of the pelvic flexure is not as efficient as that of the pylorus, and some large particles do escape the ventral colon. In addition, there are periods during which propulsive movements occur in the left ventral colon and pelvic flexure. These factors allow the movement of particulate matter into the left dorsal colon.
The actions of the dorsal colon appear to mimic those of the ventral colon. Impedance to ingesta flow is created by the size restriction at the junction of the right dorsal colon and small colon. In addition, retropulsive motility may originate in the area of the distal right dorsal colon, near the junction with the small colon. These actions tend to impede the movement of ingesta through the dorsal colon, subjecting the material to another round of fermentative digestion, as occurred in the ventral colon. The delay in the flow of ingesta created by the combined actions of the ventral and dorsal colons results in significant retention of material, with most particulate matter taking from 24 to 96 hours to pass the large colon. The efficiency of the large colon in retaining and separating ingesta of different particle sizes can be understood from Figure 31-14.
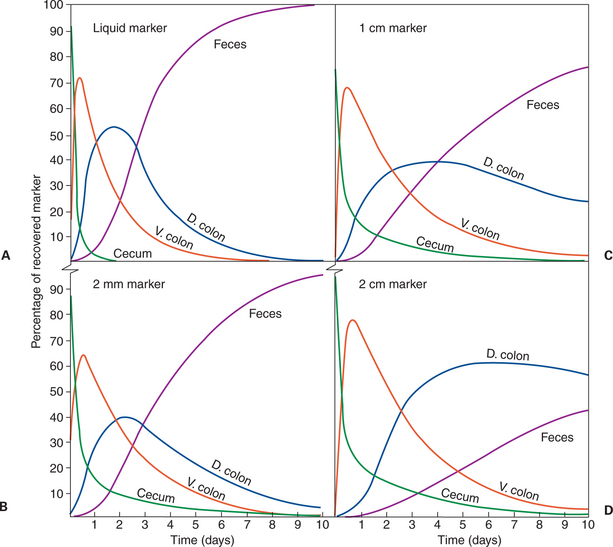
FIGURE 31-14 Retention of liquid and particles of various sizes in the compartments of the equine large intestine. Marker liquid (A) and marked particles of various sizes (B, 2 mm; C, 1 cm; D, 2 cm) were placed in the cecum of ponies, and the distribution of marked materials among colon segments was measured at 2-hour intervals. The lines of the graph were mathematically fitted to the data. Each line indicates the percentage of marker in a given segment at any time. Note in graph A that at 7 days after infusion, almost all the liquid marker had been recovered in the feces, with little or none remaining in the intestinal segments. Increasing particle size has a relatively small effect on the movement of particles out of the cecum. In contrast, as particle size increases, there is significant retention of material in the colon and slow passage to the feces. D, Dorsal; V, ventral.
(From Argenzio RA, Lowe JE, Pickard DW, et al: Digesta passage and water exchange in the equine large intestine, Am J Physiol 226:1035, 1974.)
Understanding the motility of the equine colon is important because problems of colon impaction in horses are common. Impactions usually occur near or within the pelvic flexure, probably because the pelvic flexure is a site of flow restriction and differential flow of solid and liquid material. One can easily appreciate how the normal motility pattern could allow solid material to accumulate in this area and cause obstructions.
Although general understanding of small colon motility is limited, it appears to consist primarily of segmentation and propulsion. The characteristic fecal balls of horses are formed by segmentation within the small colon.
The Rate of Fermentation and Volatile Fatty-Acid Production in the Equine Colon Is Similar to That in the Rumen
In the equine colon, efficient means of buffering and VFA absorption must be present. Salivary buffering, as occurs in ruminants, cannot aid in buffering the colon. In the horse, large quantities of fluid, rich in bicarbonate and phosphate buffers, are secreted by the ileum and transferred to the cecum, thus mimicking the actions of the salivary glands in ruminants. Also, because of the glandular nature of the colonic mucosa, bicarbonate and other electrolytes are added more directly to the lumen fluid in the cecum and colon than in the rumen.
Large fluxes of water traverse the cecal and colonic mucosa during the course of digestion. When horses are meal-fed, feed starts to enter the cecum about 2 hours after eating, and VFA production rapidly commences. As ingesta are transported from the cecum, VFA production continues in the large colon. During the period of active VFA production, large quantities of water enter the hindgut from the blood through the mucosa. Although this water flux may be a response to increased osmolality created by the generation of osmotically active VFA molecules, it is more likely a response to direct fluid secretion from the crypts of the colonic epithelium (see Chapter 29). Secretion of fluid containing sodium, bicarbonate, and chloride from the colonic mucosa appears to occur in response to high concentrations of VFA in the lumen. This secretory response, in combination with the ileal secretions, is responsible for buffering of the lumen contents. Figure 31-15 illustrates the magnitude of water fluxes that occur during hindgut digestion in the pony. Note that considerable inward and outward movement of water occurs across the mucosa in each of the major fermentation compartments, ventral and dorsal colons, and cecum. Inward (into the lumen) water movement results from mucosal secretion, whereas outward water movement occurs in association with absorption of VFA.
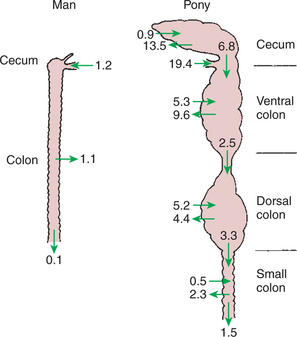
FIGURE 31-15 Net movement of water through the large intestine of a 70-kg man and a 160-kg pony. Values are in liters per day. Note the relatively large amount of fluid delivered to the pony’s colon from the ileum (19.4 L/day) compared with the human colon. Also note the inward and outward movement of fluid in the various compartments of the pony’s large intestine.
(From Argenzio RA, Lowe JE, Pickard DW, Stevens CE: Digesta passage and water exchange in the equine large intestine, Am J Physiol 226:1035, 1974.)
The molecular mechanisms of VFA absorption in the equine colon appear to be identical to those in the rumen (see Figure 31-12). Note in Figure 31-12 that sodium absorption accompanies VFA absorption and that bicarbonate is generated in the lumen. The absorption of VFA and sodium leads to osmotic absorption of water, probably through the transcellular pathway. (See Chapter 30 for a review of the dynamics of water and electrolyte absorption in the gut.)
The function of the small colon is to recover water, electrolytes, and VFAs that were not absorbed in the large colon. VFA production appears minimal in the small colon, but considerable absorption of water, sodium, and phosphate occurs there.
The large water and electrolyte fluxes in the colon make horses vulnerable to colonic diseases, resulting in fluid and electrolyte losses that are more characteristic of small-intestinal disease in many other animals.
Hindgut Anatomy and Function Vary Greatly Among the Many Species of Veterinary Interest
All the variations in hindgut anatomy and function among the species are beyond the scope of this discussion, but you should remember that rabbits, rats, guinea pigs, swine, and some large birds, in addition to Equidae, depend on hindgut fermentation for a significant portion of their energy needs. Also, ruminants have a reasonably extensive hindgut, and fermentative digestion occurs there, even after material has been through the rumen.
Scientific understanding of colonic function in general is not as advanced as that of small-intestinal function. This dichotomy probably exists because in humans, diseases of the small intestine are more frequent and severe than diseases of the colon. However, interest in colonic physiology and pathophysiology has increased among basic scientists and physicians. Veterinary physiologists have long been interested in colonic function and have become leaders in the exploration of this area.
CLINICAL CORRELATIONS
Grain Engorgement Toxemia
History.
In mid-January a cattle feeder has asked you to examine a feedlot full of 400-kg steers. The steers have been on free-choice grain from a self-feeder for several weeks. Three days ago a blizzard prevented the caretaker from delivering feed to the feeders, and they were empty for 36 hours. Yesterday the feeders were filled, and all the steers ate ravenously. Today, two of the 40 steers are dead, and many appear lethargic and uncoordinated and have diarrhea.
Clinical and Laboratory Examination.
Two steers are isolated for physical examination; they are lethargic and must be coaxed to move. Their heart rates are all above 100 beats/min (normal, <80 beats/min), and their body temperatures are less than 101.0°F (normal, 101.5°–103.0°F). Their rumens appear distended, and there is no evidence of rumen motility. The eyes are sunken in the orbits, and the oral mucosa is dry and sticky, indicating clinical dehydration. Necropsy examination of a dead steer reveals a greatly distended rumen filled with grain and fluid. A pH-paper test indicates that the rumen fluid pH of the dead animal is below 4.5 but above 3 (normal, 5.5–7.0).
Comment.
Ruminants may be fed large amounts of grain if they are accustomed to it and receive it on a regular and frequent basis. In this case, even though the steers had been accustomed to a high-grain diet, the lack of grain for more than a day, followed by a large grain intake, set up conditions for grain engorgement toxemia.
In grain engorgement there is an abundant supply of starch, leading to the rapid growth and proliferation of rumen streptococci. These bacteria produce VFA rapidly, causing the rumen pH to diminish. As the rumen pH becomes lower, conditions become unfavorable for the growth and survival of cellulose-fermenting organisms and favorable for the growth of lactic acid–producing bacteria. This leads to the accumulation of lactic acid, a stronger acid than the VFAs. Thus, rumen pH becomes even lower, killing many of the normal microflora. Some of the lactic acid is absorbed, leading to a reduced blood pH and a life-threatening situation. Moreover, the large ruminal concentration of lactic acid and VFA results in a high osmotic pressure, drawing water out of the vascular fluid compartment and into the rumen. This leads to systemic hypovolemia, which may proceed to hypovolemic shock.
Treatment.
This is a life-threatening situation, and the farmer will likely lose additional steers. Treatment is aimed at expanding the intravascular fluid volume, correcting the systemic acidosis, and reestablishing a normal rumen environment. Severely affected steers should be evaluated to determine whether their prognosis is good enough to warrant the expense of therapy; if not, euthanasia should be employed. Initial treatment should consist of rapid intravenous administration of large quantities of alkalizing fluid. After the correction of fluid and acid-base disturbances, ideally the rumen should be emptied, either by intubation with a large-bore stomach tube or by rumenotomy. In some cases, oral administration of an antifermentation agent, such as oil of turpentine, mineral oil, or an antibiotic, along with an alkalizing agent, is an acceptable alternative to emptying the rumen. After the rumen environment is brought back to normal, it may be helpful to re-inoculate the rumen with material taken from the rumen of a normal animal.
Impaction Colic
History.
You are presented with a 20-year-old gelding that has been showing signs of abdominal discomfort (colic) for 16 hours. When left alone in the stall, the horse lies down, frequently preferring to lie on his back. There is little fresh manure in the stall. When taken out of the stall, the gelding leads normally, but then lies down and rolls on release from the lead rope.
Clinical and Laboratory Examination.
The heart rate is slightly elevated, at 60 beats/min; respiratory rate and temperature are normal. The hydration state, as well as the color and perfusion of the mucous membranes, is normal. Simple laboratory evaluation reveals the packed cell volume to be 41% (normal, 35%-45%) and the plasma total solids to be 7.8 g/dL (normal, 6.5-8.0 g/dL). Borborygmi (intestinal sounds) are softer and less frequent than normal, especially on the left side. Examination by rectal palpation reveals the pelvic flexure to be firm with a doughlike consistency; normally, the contents of the pelvic flexure have a fluid consistency. When you examine the teeth, you find that the molar surfaces are irregular, and one of the molars has a crack extending from the table surface to below the gum line.
Comment.
The pelvic flexure is a site of flow restriction and particle size separation. As water moves through the pelvic flexure, large forage particles accumulate and are retained for further fermentation and mixing in the ventral colon. A horse with poor teeth may not chew its forage adequately; as a result, many large particles may be swallowed. These particles tend to accumulate in the pelvic flexure and may cause an impaction and obstruction, as occurred in this case.
Treatment.
Treatment involves the oral administration of softening agents, such as mineral oil. Drugs such as dioctyl sodium sulfosuccinate, which stimulate water secretion from the intestinal mucosa, are also beneficial. Prevention in this case involves correction of the dental problems so that forage is more thoroughly chewed. Feeding pelleted feeds may also be beneficial.
Argenzio RA. Functions of the equine large intestine and their interrelationship in disease. Cornell Vet. 1975;65:303.
Church DC. The ruminant animal: digestive physiology and nutrition. Prospect Heights, Ill: Waveland Press, 1993.
Forbes JM, ed. Quantitative aspects of ruminant digestion and metabolism. Wallingford, UK: CAB International, 1993.
Hungate RE. The rumen and its microbes. New York: Academic Press, 1966.
McDonald W, Warner ACI. Digestion and metabolism in the ruminant. Armidale, Australia: University of New England Publishing, 1975.
Phillipson AT, ed. Physiology of digestion and metabolism in the ruminant. Newcastle upon Tyne, UK: Oriel Press, 1970.
Russell JB. Rumen microbiology and its role in ruminant nutrition. Ithaca, NY: Department of Microbiology, Cornell University, 2002.
Ryckebusch Y, Thivend P. Digestive physiology and metabolism in ruminants. Westport, Conn: AVI Publishing, 1980.
Sejrsen K, Hvelplund T, Nielsen MO. Ruminant physiology: digestion, metabolism and impact of nutrition on gene expression, immunology and stress. Wageningen, The Netherlands: Wageningen Academic Publishers, 2006.
Stevens CE, Hume ID. Comparative physiology of the vertebrate digestive system, ed 2. Cambridge, UK: Cambridge University Press, 1995.
Van Soest PJ. Nutritional ecology of the ruminant, ed 2. Ithaca, NY: Comstock Publishing, 1994.
Von Engelhardt W, Leonhard-Marek S, Breves G, Giesecke D. Ruminant physiology: digestion, metabolism, growth and reproduction. Albany, NY: Delmar Publishers, 1995.
PRACTICE QUESTIONS
1. In which of the following respects is fermentative digestion different from glandular digestion?
2. In a comparison of hindgut fermentation and forestomach fermentation, which of the following statements is true?
3. The three VFAs—acetate, propionate, and butyrate—are:
4. Matching protein and energy availability in the rumen is an important nutritional goal in feeding ruminants. Which of the following completions of this statement concerning protein and energy availability in the rumen is false? Diets well matched in available protein and energy result in:
5. Which of the following statements is true of both methane and propionate?