Chapter 27 Regulation of Gastrointestinal Function
1. An independent, intrinsic enteric nervous system lies within the wall of the gut.
2. The enteric nervous system contains receptors, sensory neurons, interneurons, and motor neurons.
3. The gut receives extrinsic innervation from the autonomic nervous system.
4. The gut contains afferent neurons that relay information to the central nervous system.
5. The gastrointestinal system has an intrinsic endocrine system.
6. The immune system participates in regulation of gastrointestinal activity.
7. The regulation of gastrointestinal function is integrated by the interaction of many regulatory molecules on multiple cell types in the gut.
The gastrointestinal (GI) system is regulated in an integrated manner from two control systems. One level of control is applied by the central nervous and endocrine systems and is exerted in a manner similar to that for other organ systems. The second level of control is unique to the GI system and is exerted by intrinsic nervous and endocrine components located within GI organs. This intrinsic level of control allows the gut to regulate its functions autonomously based on local conditions, such as the amount and type of food contained in the lumen. Coordination of GI function with the rest of the body is achieved by integration of intrinsic (within the gut) and extrinsic (outside of the gut) influences (Figure 27-1).
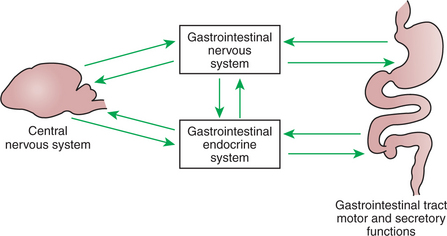
FIGURE 27-1 Gut function is under direct regulation of the enteric nervous system (ENS) and the gastrointestinal (GI) endocrine system. Most central nervous system influence on the gut is mediated through indirect effects on the ENS and GI endocrine systems.
An Independent, Intrinsic Enteric Nervous System Lies Within the Wall of the Gut
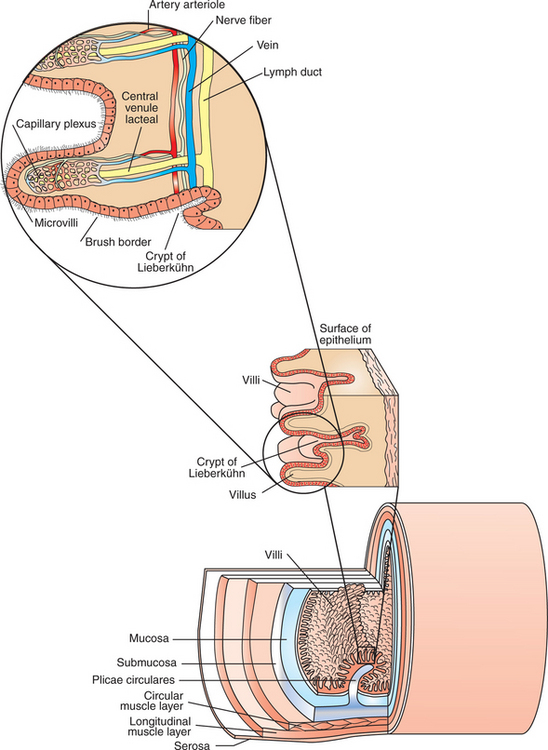
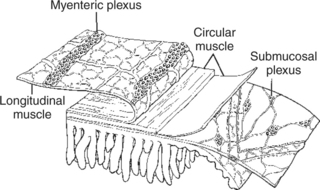
The enteric nervous system (ENS) is extensive and highly sophisticated, containing about as many neurons as the spinal cord. The ENS consists of cell bodies and their associated neurons, all of which lie within the gut wall. Anatomical characteristics of the GI wall are illustrated in Figure 27-2, which depicts the small intestine as an example. Within the gut wall, cell bodies of the ENS are arranged into two systems of ganglia: the myenteric (Auerbach) plexus and the submucosal (Meissner) plexus. (See Chapter 13 for a more general discussion of ganglia.) The myenteric plexus consists of ganglia located between the circular and longitudinal muscle layers. The submucosal plexus has its ganglia in the submucosal layer. Axons from the cell bodies project in rich networks near the ganglia. A thick net of neurons runs in the plane between the circular and longitudinal muscle layers, connecting the ganglia of the myenteric plexus. Individual neurons leave the neuronal network to innervate structures within the gut wall and to intercommunicate between the myenteric and submucosal plexuses (Figure 27-3). Interneuronal connections within the myenteric plexus are extensive and traverse long segments of gut, whereas interneuronal connections are limited within the submucosal plexus. The intricacy of the ENS has earned it the name “little brain” within the gut.
The Enteric Nervous System Contains Receptors, Sensory Neurons, Interneurons, and Motor Neurons
The plexuses of the ENS contain sensory (afferent) neurons, interneurons, and motor (efferent) neurons. Sensory input comes from mechanoreceptors within the muscular layers and chemoreceptors within the mucosa. Mechanoreceptors monitor distention of the gut wall, whereas chemoreceptors in the mucosa monitor chemical conditions in the gut lumen (Figure 27-4).
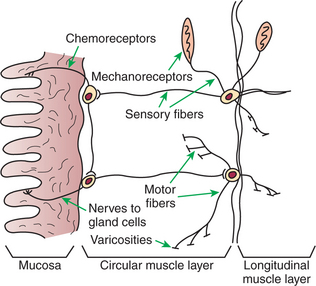
FIGURE 27-4 Arrangement of nerve fibers and receptors within the enteric nervous system. Varicosities release regulatory substances known as neurocrines in the vicinity of muscle fibers.
Enteric motor nerves supply vascular muscle, gut muscle, and glands within the gut wall. Motor innervation of gut structures is less intimate than that found, for example, in skeletal muscle; no direct synaptic type of junction exists between enteric nerve endings and the structures they innervate. Rather, axons end in arborizations that contain many vesicular structures called varicosities (see Figure 27-4). The varicosities contain regulatory substances collectively known as neurocrines that are secreted in response to action potentials and that affect the activities of nearby muscle or glandular cells. Efferent neurons of the ENS may be stimulatory or inhibitory, and the nature of their action is largely determined by the type of neurocrine substance they secrete. Many of the neurocrine substances secreted by the ENS are peptides, and many are identical in chemical structure to regulatory molecules secreted by the endocrine and paracrine cells of the gut. These cells and their secretions are discussed in the following section.
Many of the stimulatory, or excitatory, neurons are cholinergic, having acetylcholine as their neurocrine transmitter substance. A peptide known as substance P is another common excitatory neurocrine, and other minor excitatory neurocrines may exist. Inhibitory enteric neurons contain different neurocrine transmitters, most of which are peptides. These inhibitory neurocrines vary widely and include such peptides as somatostatin and pituitary adenylate cyclase– activating peptide (PACAP), as well as nonpeptide neuro-crines such as nitric oxide (NO) and adenosine triphosphate (ATP). Some neurocrines, such as vasoactive intestinal peptide (VIP), are inhibitory to gut muscle but stimulatory to the secretion of mucosal glands. The substances mentioned here are major neurocrines in the GI tract; the complete list of neurocrines and other regulatory substances in the gut is long and still expanding. Many of their names, such as calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase– activating peptide (PACAP), are obscure, nondescriptive, and probably relate to historical events in their discovery rather than to their function in GI physiology. Box 27-1 illustrates the multiplicity and complexity of neuocrines and other regulatory molecules in the gut. The physiological basis for this complex and seemingly redundant system of regulatory molecules is discussed later, after the GI endocrine and immune systems.
Box 27-1 Neurohumoral Regulatory Molecules of the Gut*
Peptide
* Most of these substances can act as neurocrines, endocrines (hormones), or paracrines, depending on their site of synthesis and mechanism of distribution. Those substances known to function as hormones are italicized. Their actions on smooth muscle activity are indicated as inhibitory (I) or excitatory (E).
The Gut Receives Extrinsic Innervation from the Autonomic Nervous System
The parasympathetic and sympathetic nervous systems form the link between the central nervous system (CNS) and the ENS. Most of the GI tract receives parasympathetic innervation by way of the vagus nerve, except the terminal portions of the colon, which receive parasympathetic innervation from the sacral cord through the pelvic nerve (Figure 27-5). By classic description, the parasympathetic nervous system is composed of preganglionic and postganglionic fibers (see Chapter 13). However, this division of parasympathetic fiber types is not clearly defined for the gut, because extrinsic preganglionic fibers of the parasympathetic system become integrated with fibers of the ENS. Parasympathetic, preganglionic fibers reach the gut and synapse on cell bodies of the ENS, and thus the enteric ganglia of the gut serve as peripheral autonomic ganglia of the parasympathetic system. However, the ENS is much more than postganglionic parasympathetic neurons. Unlike typical postganglionic parasympathetic neurons, the ENS neurons receive input from sources other than preganglionic parasympathetic fibers. These include afferent neurons and interneurons of the ENS, as well as humoral (chemical) influences from other cells in the gut (see next section). Thus the classic description of the organization of the parasympathetic nervous system does not strictly apply to the gut (Figure 27-6).
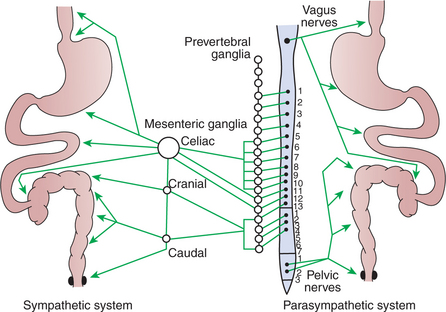
FIGURE 27-5 Distribution of autonomic nerve fibers to the gut. The spinal cord is represented in the center, with the sympathetic system extended to the left and the parasympathetic system to the right.
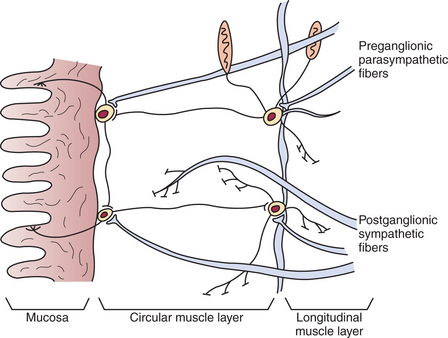
FIGURE 27-6 Interface between the autonomic and enteric nervous systems. Note that parasympathetic fibers reaching neurons of the enteric system are preganglionic, whereas sympathetic fibers are postganglionic.
In contrast to the extrinsic parasympathetic fibers, extrinsic sympathetic fibers that enter the gut are primarily postganglionic. Postganglionic sympathetic fibers arise from cells in the prevertebral ganglia (see Chapter 13) and follow the splanchnic nerves and vascular arteries into the gut wall (see Figure 27-5). Some sympathetic fibers synapse on neurons of the ENS, whereas others exert a direct effect on GI muscles and glands.
Neurons of the sympathetic and parasympathetic nervous system influence GI function through the release of neurocrines. In general, parasympathetic neurocrines are stimulatory (i.e., they increase gut blood flow, motility, and glandular secretions), whereas those of the sympathetic system are inhibitory.
The Gut Contains Afferent Neurons That Relay Information to the Central Nervous System
The gut contains afferent neurons that course to CNS centers through nerves associated with the autonomic nervous system. Vagal afferent nerves of the GI tract are associated with both mechanoreceptors and chemoreceptors and thus provide input to the CNS about changes in gut muscle tension and chemical conditions in the gut lumen. These signals allow the CNS to function in a coordinated manner with the ENS in the regu-lation of gut function. Chapters 28 to 32 discuss numerous examples of parasympathetic modulation and reinforcement of GI activities. These parasympathetic inputs depend on sensory signals received at the level of the brainstem. These signals and actions are part of the normal or physiological functions of the gut, and most of the signals do not reach the conscious awareness of the animal.
There are also afferent neurons from the gut that connect with the CNS through the splanchnic nerves, which also contain sympathetic efferent fibers. These afferent neurons convey signals arising from mechanoreceptors and chemoreceptors in the gut. In contrast to the vagal afferent nerves, however, the splanchnic afferents usually signal the presence of pathological conditions, such as overdistention of the gut wall, inflammation, or the presence of noxious chemicals or substances in the gut lumen. These nerves are distributed into the serosal surfaces and mesentery of the gut, in addition to the muscular and mucosal layers. These splanchnic afferent nerves convey to the CNS the conscious perception of pain caused by abnormal pressure within the gut lumen, inflammation, the presence of noxious stimuli in the gut lumen, or the stretching of mesenteric attachments. Such nerve signals result in the intense pain response characteristic of such conditions as equine colic and gastric distention and volvulus in dogs. Painful stimuli from the gut usually evoke a sympathetic motor response, inhibiting gut motility and many glandular secretions.
The Gastrointestinal System Has an Intrinsic Endocrine System
The GI system has an extensive number and variety of endocrine and paracrine cells, which are distributed diffusely throughout the gut epithelium. Endocrine cells are those that produce true hormones, which by definition are molecules that travel through the blood from their site of synthesis to their site of action. Paracrine substances, on the other hand, are molecules that are secreted by one cell and exert their effect locally, traveling by diffusion to nearby target cells. Typically, both the GI endocrine cells and the GI paracrine cells are columnar with a broad base and a narrow apex (Figure 27-7). They are positioned individually among the other mucosal cells in both the secretory and the absorptive areas of the mucosa. The narrow apex of these cells is exposed to the lumen of the gut, allowing them to “sample” or “taste” the luminal contents. This anatomical arrangement provides a mechanism for the cells to sense changes in gut luminal contents and to respond by releasing hormones and other regulatory substances. The base of these cells contains secretory granules, which are storage forms of hormones and paracrine substances. Endocrine cells secrete near blood vessels, whereas paracrine cells secrete into the interstitial fluid. Some paracrine cells have long basal appendages that direct their secretions to the vicinity of target cells.
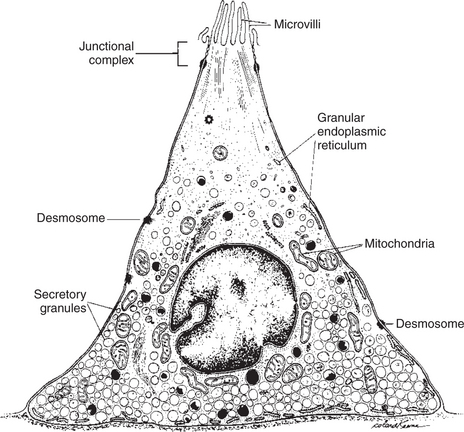
FIGURE 27-7 Gastrointestinal endocrine cell. All the GI endocrine cells have a similar structure, but each cell produces only one type of hormone. Note the narrow apex that is exposed to the intestinal luminal contents and the broad base for storage of secretory granules.
(From Johnson LR, Christensen J, Jacobsen ED, et al, editors: Physiology of the gastrointestinal tract, vol 1, ed 2, New York, 1987, Raven Press.)
It is important for students to appreciate that the secretory molecules of these cells are regulatory, not digestive. The products of these cells are secreted into either the blood or the interstitial fluid, not into the lumen of the gut. The many types of endocrine and paracrine cells in the gut epithelium are morphologically similar. However, despite their similar appearance, many distinct populations of cells produce a wide variety of hormones and other regulatory substances. Many of these substances are peptides in structure and can be referred to collectively as regulatory peptides, an inclusive term that may include molecules of neurocrine, endocrine, or paracrine origin (see previous definition of neurocrine). There are at least 28 peptides that have, or that are suspected to have, gut regulatory functions. Not all regulatory molecules in the gut are peptides, however, and several important nonpeptide paracrine and neurocrine substances exist. See Box 27-1 for a listing of peptide and nonpeptide neurocrine, paracrine, and endocrine molecules of importance in the regulation of gut function. For convenience and clarity, all these substances are frequently referred to collectively as neurohumoral regulatory molecules.
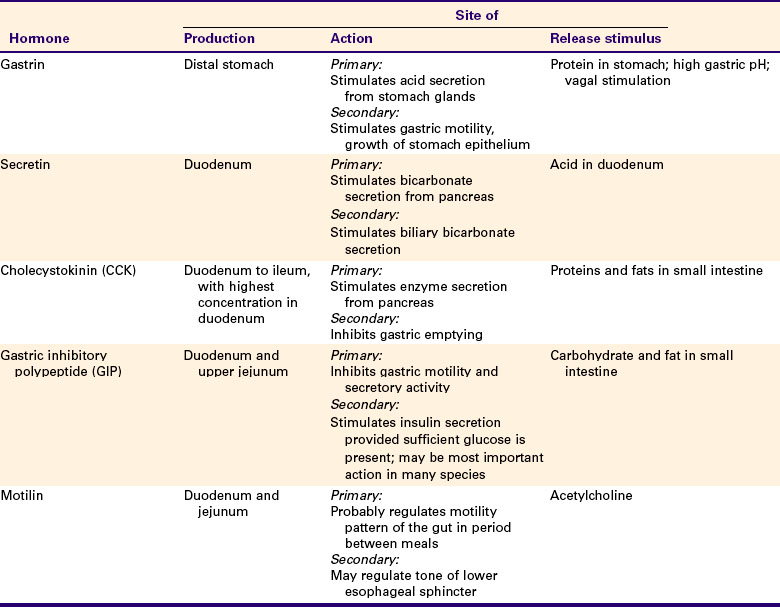
Each type of endocrine or paracrine cell has a characteristic distribution within the GI tract. For example, gastrin-producing cells, called G cells, are found primarily in the distal portion of the stomach, and few are found elsewhere in the gut. Cholecystokinin-producing cells are found in the small intestine, especially in the proximal region. Thus, although endocrine cells are distributed throughout the GI tract, the production of individual regulatory molecules may be confined to specific areas. This is not always the case, however, as with somatostatin- and serotonin-producing cells, which are distributed along the entire length of the gut (Figure 27-8). Table 27-1 lists the sites of production and actions of the major GI hormones.
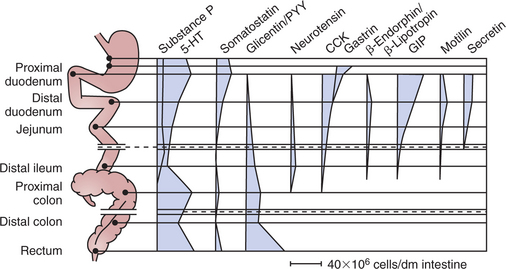
FIGURE 27-8 Distribution of gastrointestinal endocrine and paracrine cells throughout the gut, based on the hormone or paracrine substance they secrete.
(Modified from Brown DR, editor: Gastrointestinal regulatory peptides. In Handbook of experimental pharmacology, vol 106, Berlin, 1993, Springer-Verlag.)
A subset of GI paracrine cells is known as enterochromaffin cells because of their particular histological staining characteristics. These cells are found extensively throughout the gut, although their density varies from region to region (see Figure 27-8). These mucosal cells are similar in structure to the endocrine and paracrine cells previously discussed, being exposed to the luminal contents of the gut through their apical membranes. These cells secrete a regulatory molecule known as serotonin or 5-hydroxytryptamine (5-HT). This regulatory substance is particularly important in signaling neurons that have excitatory influence on gut muscle, as well as in generating sensory signals from the gut mucosa.
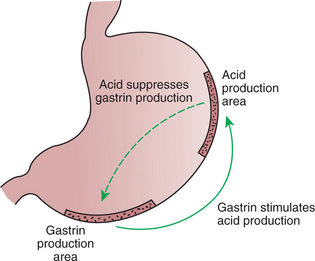
The GI regulatory peptides influence various gut functions and in many cases form part of regulatory feedback loops. An example is the feedback loop that involves gastrin and gastric acid. After a meal, gastrin-producing cells, which are located in the antrum of the stomach, secrete gastrin. Gastrin then acts as a hormone traveling through the blood to stimulate gastric acid production from specialized cells in the body of the stomach. The acid is secreted into the lumen of the stomach, lowering the pH of stomach contents. The feedback loop is completed as acidified stomach contents come into contact with the apical portion of the G cells. The reduced pH of the stomach contents signals the cells to stop gastrin secretion, removing a major stimulus for the production of gastric acid and thus stabilizing stomach pH. The actions of the G cells therefore closely regulate the pH of the stomach (Figure 27-9). Throughout this discussion of the GI system, you will see that this general pattern of endocrine or paracrine stimulus and feedback inhibition is inherent in the function of most GI regulatory peptides and other molecules. Other specific examples of GI neurohumoral regulatory effects are discussed in subsequent chapters, in the context of the actions they regulate.
The Immune System Participates in Regulation of Gastrointestinal Activity
The ENS and GI endocrine/paracrine systems monitor the physical (wall distention and pressure) and chemical environment within the gut. Another important aspect of the gut environment, however, is the antigenic milieu. It is the function of the GI immune system to monitor the antigenic environment of the gut. The mucosal surface of the gut is exposed to a vast number of microorganisms and other antigens, and the means necessary to control their numbers and their access to the body is provided by the many immune cells in the gut mucosa. In fact, the majority of the immune system cells in the body reside in the gut mucosa. These cells span the entire range of immune cell types. On one hand, the immune cells of the gut respond to antigenic stimulation similar to immune cells in other body sites: antigenic memory is created; neutralizing and opsonizing antibodies are synthesized; and killer cells are recruited (see Chapters 54 and 55). On the other hand, inflammatory mediators such as prostaglandins, histamine, and cytokines, the products of immune cells, can interact directly with the ENS and GI endocrine/paracrine cells to modulate gut activities. For example, if a microbe begins to invade an area of the gut, sensitized immune cells will secrete prostaglandins, cytokines, and other immune mediator substances. These immune mediators can interact directly with cells of the ENS and the GI endocrine/paracrine system, eliciting a response of increased fluid secretion and motility in the stimulated area of gut. The result is that the pathogenic microbe is washed downstream in the gut and eventually passed in the feces, thus protecting the gut and ridding the animal of the pathogen.
The Regulation of Gastrointestinal Function Is Integrated by the Interaction of Many Regulatory Molecules on Multiple Cell Types in the Gut
You should now appreciate that the physical, chemical, and antigenic environments of the gut are monitored by multiple systems, and that responses to various stimuli are mediated by a bewildering and seemingly redundant array of neurohumoral regulatory molecules. From a clinical standpoint, it is most important to realize that all the regulatory molecules of the gut form a highly integrated scheme for the overall control of gut function (Figure 27-10). This integration is achieved in a milieu of stimulatory and inhibitory molecules that can arise from nervous or glandular (endocrine/paracrine) elements of the gut. All these regulatory molecules act by occupying ligand sites on target cells. These target cells may be glands, muscles, and vascular muscle cells of the GI tract, but they may also be other regulatory cells, such as neurons and endocrine/paracrine cells (Figure 27-11). Thus, nerve cells can influence endocrine/paracrine cells, and vice versa. This provides a system of positive and negative feedback controls that can orchestrate a fine degree of regulation over gut function. The overall effect is that the balance of inhibitory versus excitatory neurohumoral regulatory molecules determines the activity of muscles, glands, and blood vessels in a given area of the gut.
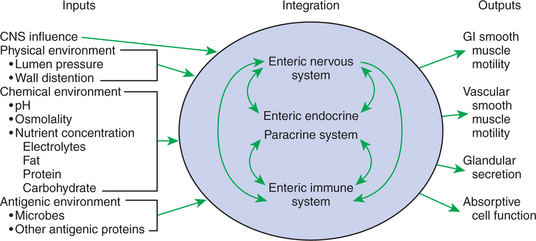
FIGURE 27-10 Stimulus-response coupling of gut reflexes and reactions at their most basic level. Despite its apparent complexity, the gut receives a limited array of afferent information and can respond with a similarly limited number of reactions. Afferent information supplied to the gut includes signals from the central nervous system, mechanical stimulation generated by wall distention or luminal pressure, chemical signals generated by luminal contents, and antigenic stimulation provided by microbial products or ingested antigens. This information is received by three integrating systems: the enteric nervous system (ENS), the endocrine/paracrine system, and the immune system of the gut. Among these systems there is considerable cross-communication that aids in integrating information and refining the efferent signals to the response elements. The responses of the gut are equally limited. Smooth muscle of the gastrointestinal wall can contract or relax; vascular smooth muscle can adjust blood supply; and absorptive and secretory cells can become more or less active. Although the gut is indeed complex, all functions may be reduced to these elements.
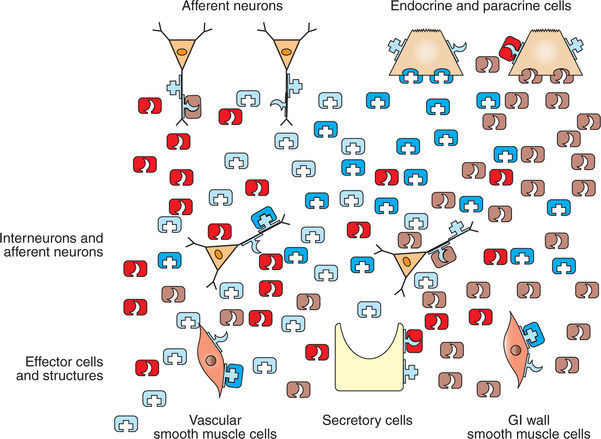
FIGURE 27-11 Role of neurohumoral regulatory substances in stimulus-response coupling in the gut. No attempt is made to distinguish individual regulatory substances, other than to indicate that some are stimulatory and others inhibitory. Of particular importance is the existence of ligand receptors on the surfaces of the nerve and endocrine/paracrine cells that are similar to those on the response cells. This indicates considerable feedback among the regulatory cell types of the gut. For example, a regulatory substance secreted by a neuron could influence the sensitivity of a paracrine cell, either blunting or augmenting its response to stimulation. This type of cellular feedback leads to a fine level of integration and control. The response reactions of the gut are the result of the overall balance of stimulatory and inhibitory neurohumoral regulatory substances.
From a clinical standpoint, it is important to understand that specific neurohumoral regulatory molecules provide opportunities for pharmacological intervention. Several highly effective drugs that either mimic or block the actions of GI neurohumoral regulatory molecules are now available for the treatment of GI diseases in animals.
Beyak MJ, Bulmer DCE, Jiang W, et al. Extrinsic sensory afferent nerves innervating the gastrointestinal tract. Johnson LR, ed. Physiology of the gastrointestinal tract, ed 4, vol 1. Amsterdam: Elsevier Science & Technology Books, 2006.
Buchan AM. Nutrient tasting and signaling mechanisms in the gut. III. Endocrine cell recognition of luminal nutrients. Am J Physiol. 1999;277:G1103.
Furness JB, Clerc N, Vogalis F, Stebbing MJ. The enteric nervous system and its extrinsic connections. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Furness JB, Stebbing MJ, Clerc N. Sensory neurons of the gastrointestinal tract. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surg. 2003;186:253.
Gershon MD. The second brain: a groundbreaking new understanding of nervous disorders of the stomach and intestine. New York: HarperCollins, 2003.
Johnson LR. Regulation: peptides of the gastrointestinal tract. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117.
Miller LJ. Gastrointestinal hormones and receptors. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Stevens CE, Hume ID. Comparative physiology of the vertebrate digestive system, ed 2. Cambridge, UK: Cambridge University Press, 1996.
Weisbrodt NW. Regulation: nerves and smooth muscle. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Wood JD. Integrative functions of the enteric nervous system. Johnson LR, ed. Physiology of the gastrointestinal tract, ed 4, vol 1. Amsterdam: Elsevier Science & Technology Books, 2006.
PRACTICE QUESTIONS
1. Which statement is the most accurate anatomical description of the enteric nervous system (ENS) of the gut?
2. Which statement is true with regard to the parasympathetic fibers that innervate the cells of the ENS?
3. Which statement is true with regard to the GI endocrine cells?
4. Which of the following statements concerning the neurohumoral regulatory molecules of the gut is true?
5. Which of the following conditions in the gut would NOT provide direct sensory input to the ENS?
6. Which of the following neurocrine transmitter molecules is most consistently excitatory relative to gut functions?
7. All neurocrine transmitter molecules are peptides.
8. The conscious sensation of pain due to excessive distention of a segment of the gut arises from afferent impulses traveling to the brain through:
9. Which anatomical description best applies to the organization of the GI endocrine cells?
10. The influence of the GI mucosal immune system on gut functions is mediated by: