Chapter 29 Secretions of the Gastrointestinal Tract
1. Saliva moistens, lubricates, and partially digests food.
2. Salivary secretions originate in the gland acini and are modified in the collecting ducts.
3. Salivary glands are regulated by the parasympathetic nervous system.
4. Ruminant saliva is a bicarbonate-phosphate buffer secreted in large quantities.
1. Depending on the species, there may be two general types of gastric mucosa: glandular and nonglandular.
2. The gastric mucosa contains many different cell types.
3. The gastric glands secrete hydrochloric acid.
4. Pepsin is secreted by gastric chief cells in an inactive form and is subsequently activated in the gut lumen.
5. The parietal cells are stimulated to secrete by the action of acetylcholine, gastrin, and histamine.
1. Pancreatic exocrine secretions are indispensable for the digestion of the complex nutrients: proteins, starches, and triglycerides.
2. Acinar cells secrete enzymes, whereas centroacinar cells and duct cells secrete a sodium bicarbonate solution.
3. Pancreatic cells have cell surface receptors stimulated by acetylcholine, cholecystokinin, and secretin.
1. The liver is an acinar gland with small acinar lumina known as canaliculi.
2. Bile contains phospholipids and cholesterol maintained in aqueous solution by the detergent action of bile acids.
3. The gallbladder stores and concentrates bile during the periods between feeding.
4. Bile secretion is initiated by the presence of food in the duodenum and stimulated by the return of bile acids to the liver.
Digestion and absorption can take place only in the aqueous milieu of digestive secretions. Synthesis and secretion of these fluids represent a well-controlled process regulated by endocrine, paracrine, and neural events. The total volume of digestive secretions is large, with the daily amount substantially larger than the volume of fluid ingested over a similar period. In addition, most of the digestive secretions have a relatively large concentration of electrolytes. This large outpouring of fluid and electrolyte into the gut makes reabsorption of these secretions imperative if fluid and electrolyte homeostasis of the body is to be maintained. Indeed, one of the major life-threatening ramifications of digestive diseases is the loss of water and electrolytes from the body caused by inadequate reabsorption of digestive secretions.
THE SALIVARY GLANDS
Saliva Moistens, Lubricates, and Partially Digests Food
As food is chewed, it is mixed with salivary secretions that allow it to be molded into well-lubricated boluses that facilitate swallowing. In addition, saliva may have antibacterial, digestive, and evaporative cooling functions, depending on the species.
The antibacterial activity of saliva results from antibodies and antimicrobial enzymes known as lysozymes. Initially, you may think that the antibacterial properties of saliva are inefficient because the mouth normally contains a large, thriving population of bacteria. However, saliva aids in keeping this population in check, and animals with impaired salivary function are prone to infectious diseases of the oral cavity.
In omnivorous animals, such as rats and pigs, saliva contains a starch-digesting enzyme known as salivary amylase. This enzyme is usually absent from the saliva of carnivorous animals, such as cats. The saliva of some species also contains a fat-digesting enzyme known as lingual lipase. This enzyme is frequently present in young animals, such as calves, while they are on a milk diet; the enzyme disappears as they mature.
Salivary enzymes probably have their major digestive effect in the proximal stomach, because food is not retained in the mouth long enough to permit extensive digestion. The lack of mixing activity in the proximal stomach may be essential for the starch-digesting function of saliva. This is because the amylase enzyme is functional at neutral to slightly basic pH, which characterizes saliva. The low pH of the distal stomach probably inactivates the enzyme; therefore it may be important that food entering the stomach initially not be mixed with gastric secretions, so as to allow the salivary enzymes some time to work before being inactivated by gastric acid. Some birds have salivary amylase that is active in the environment of the crop.
The evaporative cooling function of saliva is covered in Chapter 53.
Salivary Secretions Originate in the Gland Acini and Are Modified in the Collecting Ducts
The salivary gland is a typical acinar gland composed of an arborizing system of collecting ducts that end in cellular evaginations known as acini (Figure 29-1). The cellular epithelium of the acini is functionally distinct from that of the collecting ducts. Saliva is initially secreted into the lumen of the acini. The glandular cells lining the acini secrete water, electrolytes, enzymes, and mucus. As the newly formed saliva progresses through the collecting ducts, its composition is modified. The duct epithelium reabsorbs electrolytes, especially sodium and chloride, in a manner similar to that in the proximal tubules of the kidneys. The final product, saliva, is hypotonic and has a sodium concentration substantially less than that of extracellular fluid. The extent to which the acinar secretion is modified in the collecting ducts depends on the rate of saliva production. At high rates of salivary flow, there is little modification, which results in higher tonicity and electrolyte concentration, in comparison to low rates of flow.
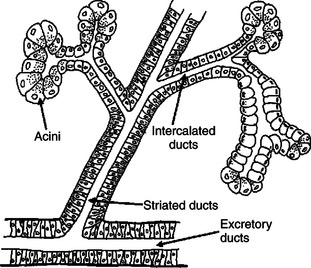
FIGURE 29-1 Schematic illustration of the salivary gland. Saliva is initially secreted by the acinar cells and is then modified as it passes through the intercalated, or collecting, ducts. Modification of acinar secretions by duct epithelia is a physiological phenomenon common among several types of glands, including the pancreas.
Most mammals have at least three pairs of salivary glands: the parotid glands, which lie just under the ear and behind the vertical ramus of the mandible; the mandibular glands, which are in the intramandibular space; and the lingual glands, which lie in the base of the tongue. Each of these glands drains into a main duct that has a single opening into the mouth. In addition to these major glands, there are minor glands in the tongue and buccal mucosa. These small, indistinct glands often have numerous secretory ducts emptying into the mouth. The concentration of mucus is different in the secretions of the various salivary glands. The parotid gland secretes watery, or serous, saliva, whereas many of the minor glands secrete highly mucous saliva. Other glands secrete a mixed type of saliva containing both mucous and serous material. Avian salivary glands secrete a copious amount of mucus to lubricate unmasticated food for swallowing.
Salivary Glands Are Regulated by the Parasympathetic Nervous System
Autonomic, parasympathetic nerve fibers of the facial and glossopharyngeal nerves end on the secretory cells of the salivary gland acini and stimulate the cells through cholinergic receptors. All phases of salivary activity are stimulated by this mechanism, including electrolyte, water, and enzyme secretion. The anticipation of eating can initiate a parasympathetic response that results in salivary secretion. In Pavlov’s famous experiment, parasympathetic stimulation of the salivary gland was evoked in dogs by the sound of a ringing bell. The dogs had been trained to anticipate eating after hearing the bell. This well-known experiment was one of the first demonstrations that the central nervous system (CNS) could regulate digestive functions. Chewing and stimulation of taste buds, in addition to the anticipation of eating, are afferent stimuli for salivation.
Salivary secretory cells also contain β-adrenergic receptors that are activated by sympathetic nerve stimulation or circulating catecholamines. This form of stimulation probably has little association with normal digestive activity but is related to the salivation and drooling seen in carnivores preparing to attack. Among digestive glands, the salivary glands are unique because there is no endocrine regulatory component.
Ruminant Saliva Is a Bicarbonate-Phosphate Buffer Secreted in Large Quantities
The normal composition of ruminant parotid saliva is quite different from the saliva of monogastric animals. Bovine and canine saliva are compared in Figure 29-2. Ruminant saliva is isotonic and, compared with blood serum, has high concentrations of bicarbonate and phosphate and a high pH. This well-buffered solution is necessary for neutralizing acids formed by fermentation in the rumen, and ruminants secrete it in enormous quantities. An adult cow may secrete 100 to 200 L of saliva per day. This volume is approximately equivalent to the extracellular fluid volume of most adult cattle. It is obvious that much of the water and electrolytes secreted in saliva must be reabsorbed rapidly and recirculated through the total body water, or the cow would die of dehydration. In abnormal circumstances, such as blockage of the esophagus, in which the flow of saliva is diverted from the gastrointestinal (GI) tract, cattle quickly become dehydrated and acidotic.
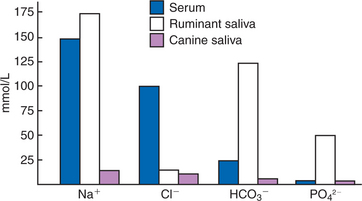
FIGURE 29-2 Electrolyte composition of blood serum and of canine and ruminant saliva. Note that the electrolyte concentration of canine saliva is much lower than that of serum, in contrast to the concentration in ruminant saliva. Also note the high concentrations of bicarbonate (HCO3−) and phosphate (PO42−) in ruminant saliva; these ions give ruminant saliva its alkalizing quality.
In general, the salivary glands of domestic animals are seldom involved in disease processes and infrequently require veterinary attention.
GASTRIC SECRETION
Depending on the Species, There May Be Two General Types of Gastric Mucosa: Glandular and Nonglandular
Most domestic, monogastric animals have only glandular mucosa in the stomach, but horses and rats have an area in the proximal portion of their stomachs that is covered by nonglandular, stratified squamous epithelium. This area is visibly different from the glandular area, to which it adjoins with a sharp line of demarcation. The function of the nonglandular area of gastric mucosa is unclear. The nonglandular area may serve as a place where a small amount of fermentative (rumenlike) digestion could occur. Because there is little mixing activity in the proximal stomach, food in the nonglandular area would be protected from the secretions of the gastric glands. These acid secretions kill bacteria, and thus their presence would prevent fermentation. Fermentative digestion is discussed in detail in Chapter 31.
The glandular area of the stomach is divided into three regions: cardiac mucosa, parietal mucosa, and pyloric mucosa. These areas contain glands of similar structure but with different types of secretions, as described later. In most species the cardiac mucosa forms a narrow band around the gastric opening of the esophagus. In the pig, however, the cardiac mucosa covers a substantial portion of the proximal stomach.
The Gastric Mucosa Contains Many Different Cell Types
The glandular mucosa of the stomach has frequent invaginations, or pores, known as gastric pits. The size of the pits is such that the pores leading into them can be seen with a hand-held magnifying glass. At the base of each pit is a narrowing, or isthmus, that continues into the opening of one or more gastric glands (Figure 29-3).
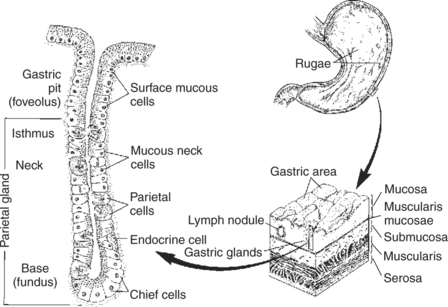
FIGURE 29-3 Anatomical illustration of glands of the stomach body. Other portions of the glandular stomach mucosa have similar structures but may differ somewhat in the cell types present in the glands. The gland openings are large enough to be seen with a hand-held magnifying glass.
The major surface areas of the stomach, as well as the lining of the pits, are covered with surface mucous cells. These cells produce thick, tenacious mucus that is a special characteristic of the stomach lining. The mucous cells and their associated secretion are important for protecting the stomach epithelium from the acid conditions and grinding activity present in the lumen. When the mucous cells are injured, stomach ulcers result.
Each region of the mucosa contains glands with characteristic cell types. Within the parietal area, the glands contain parietal cells. These cells are clustered in the neck, or proximal area, of the gland. Their function is to secrete hydrochloric acid (HCl). Distributed among the parietal cells in the neck of the gland is another type of cell, the mucous neck cells. These mucous cells secrete thin mucus, less viscous than that of the surface mucous cells. The mucous neck cells, in addition to their secretory function, appear to be the progenitor cells for the gastric mucosa. They are the only cells of the stomach lining capable of division. As they divide, they migrate either down into the glands or up into the pits and onto the surface epithelium. As they migrate, the mucous neck cells differentiate into any of the several types of mature cells of the gastric surface and glands. In the base of the gastric glands is yet a third type of cell, the chief cells. These cells secrete pepsinogen, the precursor to the digestive enzyme pepsin.
The glands of the cardiac and pyloric mucosal regions resemble those of the parietal area in structure but contain different cell types. The cardiac glands secrete only mucus. Their mucus is alkaline and probably serves to protect the adjacent esophageal mucosa from the acid secretions of the stomach. The pyloric glands have no parietal cells but contain the gastrin-producing G cells. According to most reports, pyloric glands do secrete pepsinogen.
The Gastric Glands Secrete Hydrochloric Acid
When the gastric glands are stimulated maximally, the HCl solution secreted into the lumen is isotonic and has a pH of less than 1. Both the hydrogen (H+) and the chloride (Cl−) ions are secreted by the parietal cells but apparently by different cellular mechanisms. H+ is secreted through an H+,K+-ATPase (adenosine triphosphatase) enzyme located on the luminal surface of the cell. This enzyme, sometimes referred to as a “proton pump,” exchanges H+ for potassium ions (K+), pumping one K+ into the cell for each H+ secreted into the lumen. In the exchange process, one molecule of adenosine triphosphate (ATP) is hydrolyzed to adenosine diphosphate (ADP), representing an expenditure of energy. The K+ cations that accumulate within the cells are released back into the lumen in combination with Cl− anions. This allows the recycling of K+ ions as they are pumped back into the cells in exchange for H+, resulting in the net secretion of H+ and Cl−, with little net movement of K+.
Hydrogen ions for secretion come from the dissociation of intracellular carbonic acid (H2CO3), leaving a bicarbonate ion (HCO3−) in the cell for each H+ secreted into the lumen (Figure 29-4). Carbonic acid originates from water and carbon dioxide through the action of carbonic anhydrase, an enzyme found in high concentration in the gastric mucosa.
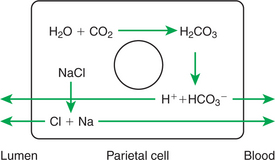
FIGURE 29-4 Electrolyte movements during gastric acid secretion. The production of hydrogen and bicarbonate ions from water and carbon dioxide is stimulated by the action of the enzyme carbonic anhydrase, the activity of which is high in the gastric mucosa.
As hydrogen cations are secreted, bicarbonate anions accumulate in the cell. To counterbalance this accumulation, bicarbonate anions are exchanged for chloride anions at the cell’s nonluminal surface. In this manner, additional chloride is made available to the cell for secretion into the glandular lumen, and bicarbonate is secreted into the blood. During periods of intense secretion by the gastric glands, large amounts of bicarbonate are released into the blood. This transient and mild alkalization of the blood during digestion is known as the “alkaline tide.” Normally, the alkaline tide is reversed when bicarbonate in the blood is consumed indirectly during the neutralization of gastric secretions as they enter the intestine (see the section on pancreatic secretions later in this chapter). Thus, on a total-body basis, gastric acid production results in only small and transient changes in blood pH. In disease states, however, in which the secretions of the stomach are prevented from entering the intestine or are lost from the body because of vomiting, the pH of the blood can rise to dangerously high values.
Pepsin Is Secreted by Gastric Chief Cells in an Inactive Form and Is Subsequently Activated in the Gut Lumen
Pepsin is usually referred to as a single compound, but it is actually a family of protein-digesting enzymes that are secreted from the gastric glands. They are formed in the chief cells as inactive proenzymes called pepsinogens. Pepsinogens are stored in the chief cells as granules until secreted into the lumen of the gastric glands. After secretion, pepsinogens are exposed to the acid contents of the stomach, resulting in cleavage of a small portion of the protein molecule, which leads to activation of the enzymes.
Digestive enzymes that are synthesized and stored as inactive proenzymes and activated in the lumen of the gut are known by the general name of zymogens. The general pattern of zymogen formation and activation is necessary because the active enzymes could digest and destroy the cells that synthesize them.
The Parietal Cells Are Stimulated to Secrete by the Action of Acetylcholine, Gastrin, and Histamine
Gastric acid secretion is stimulated by the anticipation of eating and the presence of undigested food in the stomach. When an animal anticipates eating, parasympathetic vagal impulses stimulate cells of the enteric nervous system (ENS), which in turn release acetylcholine (ACh) into the vicinity of G cells and parietal cells. These secretory cells have ACh receptors on their surfaces and respond by secreting gastrin and HCl, respectively. Gastrin circulates in the bloodstream and eventually finds its way to the parietal cells, which have gastrin receptors, in addition to ACh receptors, on their surfaces. The combined actions of gastrin and ACh on the parietal cells result in high rates of HCl flow. The response of the stomach to anticipatory stimuli originating in the brain is referred to as the cephalic phase of gastric secretion.
Food entering the stomach initiates the second phase, or “gastric phase,” of gastric secretion. Distention of the stomach by food stimulates stretch receptors, providing afferent stimulation of the ENS; the system responds by direct nervous (ACh) stimulation of the G and parietal cells. In addition, food acts as a buffer, raising the stomach pH. This removes the inhibiting effect of acid on G-cell secretion, further stimulating the production of gastrin, which leads to even greater enhancement of acid production by the parietal cells.
Histamine plays a role as an amplifying substance in gastric acid secretion. Parietal cells have surface receptors for gastrin, ACh, and histamine. They are stimulated maximally when all three receptors are occupied. Histamine is secreted by mast cells and enterochromaffin-like cells in the parietal mucosa. The histamine-secreting cells are stimulated to secrete by gastrin and ACh. Thus the effects of gastrin and ACh on gastric acid secretion are amplified through their stimulation of histamine secretion.
As gastric secretion and digestion proceed, the pH of the stomach decreases. When the stomach pH falls to about 2, gastrin secretion is suppressed, and at a pH of 1, gastrin secretion is completely abolished. Thus the gastrin stimulus to the parietal cells is removed, and acid secretion is reduced (see Figure 27-9).
The intestinal environment also influences the secretion of gastric acid. As acid contents from the stomach flow into the duodenum and the duodenal pH is reduced, gastric acid production is suppressed. The exact mechanism by which duodenal acidification exerts negative feedback on the parietal cells is unclear. The hormone secretin, produced in the duodenum, may be involved, as well as neuronal reflexes acting through the ENS.
The secretion of pepsinogen appears to be under the same regulatory influences as the secretion of HCl. However, regulation of pepsin secretion has been researched much less than regulation of HCl secretion.
THE PANCREAS
Pancreatic Exocrine Secretions Are Indispensable for the Digestion of the Complex Nutrients: Proteins, Starches, and Triglycerides
The pancreas is composed of two functionally separate types of glandular tissue. A small but important portion of the pancreatic tissue is arranged into discrete islets within the parenchyma of the gland. These cells are collectively called the endocrine pancreas because they secrete hormones into the bloodstream; the endocrine pancreas is discussed in Chapter 34. The great majority of the pancreatic tissue is involved with the elaboration of digestive secretions. This portion is known as the exocrine pancreas because its secretions are delivered into the intestinal lumen; the exocrine pancreas is the subject of this section.
Acinar Cells Secrete Enzymes, Whereas Centroacinar Cells and Duct Cells Secrete a Sodium Bicarbonate Solution
The exocrine pancreas is a typical acinar gland in which the end-pieces, or acini, are connected by an arborizing system of ducts; thus the gland conceptually resembles a bunch of grapes. Its general structure resembles the salivary gland, as illustrated in Figure 29-1. The cells of the acini contain a generous portion of rough endoplasmic reticulum, on which large amounts of secretory proteins, the digestive enzymes, are synthesized. Each pancreatic acinar cell can produce all the more than 10 different enzymes secreted by the pancreas. Chapter 30 discusses the functions of the major digestive enzymes of the pancreas (see Table 30-1). Protein-digesting enzymes, which are potentially harmful to the pancreatic cells, are synthesized as zymogens in the same manner as pepsinogen synthesis in the gastric glands. After synthesis, the enzymes and proenzymes are stored in vesicles, or zymogen granules, near the cellular apex. When the cells are stimulated, the zymogen granules fuse with the plasma membrane and release their contents into the lumen of the gland and eventually into the duodenal lumen, where they are converted to the activated form of the enzyme.
Specialized cells near the junction of the acini and ducts are called centroacinar cells. The function of these cells, and to a lesser degree the duct epithelial cells, is to modify the electrolyte composition of the fluid secreted by the acinar cells. The electrolyte makeup of the acinar secretion initially resembles extracellular fluid, having a relatively high concentration of sodium and chloride. The centroacinar cells have on their luminal surface a chloride-bicarbonate exchange protein that transports bicarbonate out of the cell in exchange for chloride, thus greatly enriching the bicarbonate concentration of pancreatic fluid. This exchange protein does not require an energy input, and its action is driven by a high intracellular concentration of bicarbonate. This system is facilitated by electrolyte transport proteins on the basolateral surface of the cell (see Chapter 30). These transport proteins consist of Na+,K+-ATPase, an Na+-HCO3− co-transporter, an H+-Na+ exchanger, and an H+-ATPase. The Na+-HCO3− co-transporter, in combination with carbonic anhydrase, generates bicarbonate within the cell, thus driving the chloride-bicarbonate exchange at the luminal membrane. The H+ remaining from the dissociation of carbonic acid is removed from the cell at the basolateral membrane by Na+-H+ exchange and by the H+-ATPase pump. The net result is that pancreatic fluid is a bicarbonate-rich, alkaline fluid that neutralizes the acid ingesta arriving in the duodenum from the stomach. In addition, the H+ ions transported into the basal interstitial fluid of the pancreas are absorbed into the blood, balancing the “alkaline tide” that was created by gastric acid secretion.
In broad generality, the ion transport activities of the pancreatic duct cells are similar, but directionally opposite, to those of the parietal cells, as illustrated in Figure 29-4. The overall effect of the two secretory cell types is to mix hydrochloric acid with ingesta in the stomach and to neutralize the acid with sodium bicarbonate in the duodenum.
The ducts of the pancreatic lobules coalesce in an arborizing pattern to form either one or two main pancreatic ducts, depending on the species. The pancreatic duct or ducts may empty directly into the duodenum or, as in sheep, into the common bile duct. In the latter case, the pancreatic secretions enter the intestinal lumen along with bile.
Pancreatic Cells Have Cell Surface Receptors Stimulated by Acetylcholine, Cholecystokinin, and Secretin
When binding sites on the surfaces of pancreatic acinar, centroacinar, or duct cells are occupied, the cells are stimulated to secrete. Each type of cell appears to have receptors for ACh as well as for cholecystokinin (CCK) and secretin. ACh, released from nerve endings near the cells, stimulates secretion, as do CCK and secretin arriving in the blood. CCK is the primary hormonal stimulus for acinar cells, whereas secretin is the primary hormonal stimulus for centroacinar and duct cells. It appears, however, that maximal stimulation of the cells occurs when all receptors are occupied. Thus, acinar cells secrete most actively in the presence of all three ligands: ACh, CCK, and secretin. In this manner, secretin is said to potentiate, or increase, the action of CCK on acinar cells, and CCK potentiates the action of secretin on centroacinar and duct cells.
Nerve fibers ending in the vicinity of pancreatic acinar glands originate from cell bodies in the ENS, traveling outside the gut wall and into the pancreas. These neurons are stimulated to release ACh by impulses arriving from other neurons of the ENS, or by parasympathetic fibers arriving through the vagus nerve. Vagal stimulation of pancreatic secretion may arise as the result of several stimuli. The sight and smell of food induce centrally integrated vagal responses, leading to pancreatic secretion. This is known as the cephalic phase of pancreatic secretion, which is analogous in concept to the cephalic phase of salivary and gastric secretion. Distention of the stomach causes a vagovagal reflex stimulating pancreatic secretion, called the gastric phase of pancreatic secretion. The effects of the cephalic and gastric phases of pancreatic secretion are to “ready” the intestine for the imminent arrival of food by prior stimulation of pancreatic secretions.
The third phase, or intestinal phase, of pancreatic secretion is the most intense and involves endocrine as well as neuronal stimuli. This phase commences as food material from the stomach enters the duodenum. This leads to distention of the duodenum, which appears to produce enteric nerve impulses, resulting in ACh stimulation of pancreatic secretory cells. This stimulation reinforces and enhances the vagally mediated neuronal stimulation of the cephalic and gastric phases. The endocrine portion of the intestinal phase of pancreatic secretion occurs in response to the chemical stimulation that results from the presence of gastric contents in the duodenum. Peptides in the duodenal lumen, arising from the digestion of food protein, stimulate CCK production from endocrine cells in the duodenum. Fats in gastric ingesta also stimulate CCK secretion, whereas the low pH of material entering the duodenum from the stomach stimulates the secretion of secretin.
This stimulatory pattern is logical and results in a coordinated pattern of digestion. Proteins (peptides) and fats stimulate, through CCK, the secretion of protein-digesting and fat-digesting enzymes. These enzymes function best in an alkaline environment, and so the acid secretions of the stomach must be neutralized in order for these enzymes to be effective. Acid conditions in the duodenum stimulate pancreatic bicarbonate secretion through secretin, leading to alkalization of the ingesta. As food is digested and absorbed and acid is neutralized, the stimuli for pancreatic secretion are removed, and the amount of secretion diminishes to low, basal rates.
BILE SECRETION
One function of the liver is being a secretory gland of the digestive system. Its secretion, bile, has an important role in fat digestion.
The Liver Is an Acinar Gland with Small Acinar Lumina Known as Canaliculi
The liver is composed of “plates,” or one-cell-thick layers of hepatocytes that are bathed on either side by blood from the hepatic sinusoids. Between each row of cells is a small space created by cavitations in the plasma membranes of two apposing cells. The portions of the plasma membranes lining the spaces are isolated from the remainder of the plasma membrane by tight junctions, which seal off the spaces from the surrounding extracellular environment. Within the plates of cells, these spaces join to form channels, or canaliculi, that connect to the bile ductules. Bile is secreted from the hepatocytes into the canaliculi, from which it flows into the bile duct system. From a functional standpoint, the canaliculi may be perceived as acini lined by hepatocytes and emptying into the biliary duct system, as illustrated in Figure 29-5. The bile duct epithelium is metabolically active and capable of altering the composition of canalicular bile by adding additional water and electrolytes, especially bicarbonate. In this function, the bile duct epithelial cells function in a manner similar or identical to the centroacinar and duct cells of the pancreas. In fact, they even respond to secretin by increasing their bicarbonate secretion.
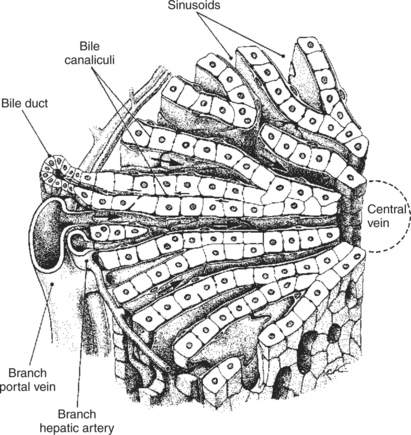
FIGURE 29-5 Hepatic microanatomy is complex and can be visualized in several ways. Note the relationship of the bile canaliculi to the bile ducts; the biliary system may be viewed as an acinar gland with the bile canaliculi forming a long, narrow acinus.
(Modified from Ham AW: Textbook of histology, ed 5, Philadelphia, 1965, Lippincott.)
Bile Contains Phospholipids and Cholesterol Maintained in Aqueous Solution by the Detergent Action of Bile Acids
Hepatocytes form bile acids from cholesterol. The chemical changes necessary to convert cholesterol to cholic acid, a representative bile acid, are shown in Figure 29-6. Cholesterol is almost totally insoluble in water, but the chemical changes involved in the conversion of cholesterol to bile acids result in a molecule with a water-soluble (hydrophilic, or “water-loving”) side and a lipid-soluble (hydrophobic, or “water-hating”) side. This combination hydrophobic-hydrophilic attribute is the characteristic property of a detergent. Because of this dual solubility, detergents can render lipids soluble in water. The function of the bile acids is to emulsify dietary lipids and to solubilize the products of fat digestion.
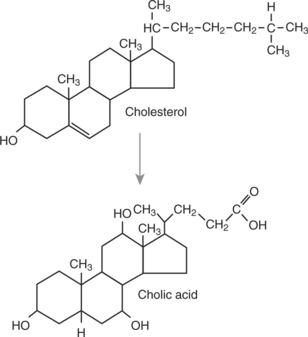
FIGURE 29-6 Conversion of cholesterol to cholic acid, a representative bile acid. Note the presence of two additional hydroxyl groups on the ring structure of cholic acid compared with cholesterol. These hydroxyl groups enhance the water solubility and detergent action of the bile acid molecule. Other bile acids differ from cholic acid in the number and position of hydroxyl groups.
Bile acids are produced in the smooth endoplasmic reticulum of the hepatocytes. As they are secreted from the cells into the lumen of the canaliculi, bile acids “dissolve away” some of the cell membrane components: phospholipids and cholesterol. These constituents—phospholipids, cholesterol, and bile acids—are the major functional components of bile and are important for the digestion and absorption of fats. The mechanism by which bile aids in fat digestion is discussed in Chapter 30.
Bile acids are secreted into the canaliculi as their sodium salts. The presence of bile acid salts and sodium in the canaliculi draws water, by osmosis, from the cells into bile. The electrolyte composition of canalicular bile usually resembles that of plasma but may be somewhat lower in chloride. As bile flows through the bile ducts, water and electrolytes are added. Bicarbonate may be secreted by the duct cells, so the bicarbonate concentration of bile is often higher than that in blood serum.
In addition to bile acids, phospholipids, and cholesterol, bile contains other lipid-soluble organic substances. Of these, the bile pigments are present in the highest concentration. Bile pigments are breakdown products of heme porphyrin, a portion of the hemoglobin molecule. The principal bile pigment is bilirubin, which is produced during the normal process of red blood cell turnover. Bilirubin gives bile its characteristic green color. In the lumen of the gut, bilirubin is converted by bacterial action to other compounds. These secondary compounds are responsible for the characteristic brown color of the feces of nonherbivorous animals. Bile pigments serve no useful digestive function: the body simply uses bile, and ultimately feces, as a route for the excretion of these waste products.
The liver serves as an excretory organ for many lipid-soluble substances in addition to bilirubin. The detergent action of the bile acids makes the liver an ideal excretory organ, in comparison with the kidney, for these types of compounds. Substances metabolized and secreted by the liver include many important drugs and toxins. This is important clinically because the actions of these agents can be potentiated by impaired liver function.
The Gallbladder Stores and Concentrates Bile During the Periods Between Feeding
When there is little or no food in the intestinal lumen, the sphincter of Oddi, at the union of the common bile duct and duodenum, is closed. With this sphincter closed, bile cannot enter the intestine and is diverted into the gallbladder. The gallbladder epithelium absorbs sodium, chloride, and bicarbonate from bile; water is absorbed passively. Thus, in the gallbladder the organic constituents of bile are concentrated, and the volume of bile is reduced. In species that have no gallbladder, such as horses and rats, the sphincter of Oddi is apparently nonfunctional, and bile is secreted into the intestine during all phases of the digestive cycle.
Bile Secretion Is Initiated by the Presence of Food in the Duodenum and Stimulated by the Return of Bile Acids to the Liver
When food, especially fat-containing food, reaches the duodenum, the GI endocrine cells are stimulated to secrete CCK, which in turn causes relaxation of the sphincter of Oddi and contraction of the gallbladder. These actions force stored bile into the intestine. Bile acids aid in the digestion and absorption of fats in the jejunum (see Chapter 30) but are not absorbed themselves until they reach the ileum. After absorption in the ileum, the bile acids travel via the hepatic portal vein to the liver. In the liver, bile acids are almost completely absorbed from the portal blood. As a result, almost no bile acids reach the posterior vena cava, and they are consequently found only in low concentrations in the systemic circulation. The flow of bile acids from liver to intestine to portal blood to liver and back to intestine is known as enterohepatic circulation (Figure 29-7).
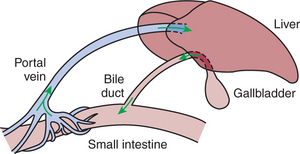
FIGURE 29-7 Bile acids and other molecules circulate in an enterohepatic cycle. Phases of the cycle include the portal vein, biliary system, and intestinal lumen.
Bile acids arriving at the liver, by way of the portal circulation, stimulate further bile synthesis. Thus a positive feedback system is initiated when the gallbladder contracts: the absorption of gallbladder bile from the intestine stimulates additional bile synthesis by the hepatocytes. Rapid bile synthesis and secretion continue as long as the sphincter of Oddi is open and the gallbladder is contracted. When fats have been digested and absorbed, the stimulus for CCK secretion is removed, resulting in closure of the sphincter of Oddi and diversion of bile to the gallbladder. Because bile acids are no longer reaching the intestine, they are no longer being absorbed, and thus the stimulus for bile secretion is reduced, and bile flow slows down.
In addition to the effect of CCK on bile secretion, secretin influences secretion from the bile duct epithelium. Secretin stimulates water and bicarbonate secretion from the bile ducts in a manner similar to that of its effects on the duct cells of the pancreas. Thus, bile may participate in the neutralization of stomach acids.
CLINICAL CORRELATIONS
Horse in Pain with Weight Loss
History.
A 4-year-old Thoroughbred mare presents for weight loss, inappetence, grinding of teeth, and low-grade colic. She is off the track now but was winning until 1 month ago, and thus the trainer is concerned.
Clinical Examination.
The mare seems to be quieter than expected. Her temperature, pulse, and respiration are normal. She appears thin for a horse from the track, and the trainer thinks the mare has lost 100 to 150 pounds in the last month. Her hair coat is poor. Your examination reveals no other abnormal findings.
With this history, gastric ulcers are a likely differential diagnosis. You discuss this with the trainer and decide to perform gastroscopy before doing any other diagnostic tests.
Comment.
On endoscopy the mare has several ulcers along the junction of the squamous and glandular sections of the gastric epithelium. In addition, she has two large and a few small ulcers on the nonglandular squamous compartment of the stomach. The squamous epithelium of the equine stomach has no mucus-secreting glands, in contrast to the glandular mucosa. The thick alkaline mucus that coats the glandular surface is an important component of the stomach epithelium’s natural defense against acid damage. The lack of surface mucus makes the squamous portion of the equine stomach particularly prone to ulcer development. Ulcers are likely the cause of this horse’s weight loss, colic, and poor performance. She will be treated, and her management will be modified to encourage healing of the ulcers.
Horses continuously secrete hydrochloric acid (HCl) in the stomach, in contrast to many other species that can modulate acid secretion based on food intake. Horses are thus suited to be constant grazers with access to food 24 hr/day. This mare is kept in the stall 24 hr/day unless she is being worked, and she is given a high-grain low-hay diet. Thus, she eats two high-grain meals per day, and she only has a small amount of grass hay, which she typically eats quickly. When horses do not eat, the pH of the stomach decreases rapidly. Furthermore, being inside all the time adds to her stress, making this mare more prone to gastric ulcers. Histamine and gastrin also stimulate HCl secretion, whereas somatostatin inhibits it. Therefore, treatment is multifactorial, aimed at changing the management to increase the pH of the stomach as well as administering drugs to help decrease acid secretion.
The parietal cells secrete HCl through H+,K+-ATPase (proton pump). Omeprazole, a once-daily medication, inhibits the proton pump. Other anti-ulcer medications include histamine type 2 receptor antagonists, such as cimetidine and ranitidine, which block histamine attachment to stimulatory receptors on the surface of parietal cells, decreasing HCl release. Another way to protect the stomach from acid damage is to coat the gastric lining with medications such as sucralfate, which forms a protective barrier between the mucosa and luminal contents.
Treatment.
A common treatment choice in this case currently is omeprazole, which specifically decreases HCl secretion. Treatment depends on the severity of the ulcers and the inciting cause. In many cases, treatment may be recommended for up to 28 days. Additional management changes to enhance healing would include increasing the time per day the mare spends eating. Pasture would be ideal, particularly alfalfa forage, which has inherent buffering capacity. Gradually decreasing the amount of grain fed daily and increasing the amount of forage would also be beneficial. Although these management changes are ideal, they can be difficult to maintain because of the typical management of these horses.
Del Valle J, Todisco A. Gastric secretion. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Johnson LR. Gastric secretion. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Johnson LR. Pancreatic secretion. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Johnson LR. Salivary secretion. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Johnson LR, Alpers DH, Christensen J, et al. Physiology of the gastrointestinal tract, ed 3, New York: Raven Press, 1994.
Owyang C, Williams JA. Pancreatic secretion. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Parsons ME. Control of gastric secretion. Proc Nutr Soc. 1996;55:251.
Prinz C, Zanner R, Gerhard M, et al. The mechanism of histamine secretion from gastric enterochromaffin-like cells. Am J Physiol. 1999;277:C845.
Stevens CE, Hume ID. Comparative physiology of the vertebrate digestive system, ed 2. Cambridge, UK: Cambridge University Press, 1995.
Weinman SA, Kemmer N. Bile excretion and cholestasis. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Weisbrodt NW. Bile production, secretion, and storage. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
PRACTICE QUESTIONS
1. In monogastric animals, saliva produced during periods of rapid secretion has a higher electrolyte concentration than saliva produced during periods of slow salivary secretion. From your understanding of salivary gland physiology, which appears to be the most likely explanation?
2. Some nutritionists are experimenting with a drug that increases salivary secretion in cattle. What effect do you think this would have on rumen pH?
3. Inhibition of the enzyme carbonic anhydrase is likely to have what effect on gastric pH?
4. Which of the following is not a potential stimulus for gastric acid secretion?
5. Which of the following is not a natural ligand for receptors in the pancreas?