Chapter 32 Postabsorptive Nutrient Utilization
1. The tricarboxylic acid (or Krebs) cycle is the major energy-yielding pathway of fuel utilization in the body.
1. The major metabolic fuels consist of glucose, amino acids, fatty acids, and ketone bodies; various storage and transport forms exist for these compounds.
2. Glucose is the central fuel in the energy metabolism of most animals.
3. Amino acids are important fuels in addition to being the building blocks of protein.
4. Fatty acids are the major form of energy storage in the animal body.
5. Ketone bodies are fat-derived, water-soluble metabolites that serve as glucose substitutes.
Nutrient Utilization During the Absorptive Phase
1. During the absorptive phase, the liver takes up glucose and converts it into glycogen and triglyceride.
2. The conversion of glucose to fatty acids is an irreversible process.
3. Transport of fatty acids out of the liver is through chylomicron-like particles known as very-low-density lipoproteins.
4. Amino acids can be classified into groups on the basis of metabolic characteristics.
5. Amino acids are extensively modified during absorption.
6. Many amino acids are removed by the liver on “first pass,” never reaching the systemic circulation.
7. Some amino acids taken up by the liver are used for protein synthesis.
8. Most amino acids taken up by the liver are converted to carbohydrates.
9. Not all amino acids are subject to hepatic destruction.
10. Metabolism at the tissue level is coordinated with hepatic metabolism and results in the deposition of fuel into storage tissues during the absorptive period.
11. Insulin promotes the synthesis of protein and the deposition of glycogen in muscle.
12. Insulin-stimulated uptake of amino acids by muscle results in a net increase in muscle protein synthesis.
13. During the absorptive phase, triglyceride accumulation in adipose tissue occurs by two mechanisms: uptake from very-low-density lipoproteins and direct lipid synthesis from glucose.
Nutrient Utilization During the Postabsorptive Phase
1. Hepatic metabolism switches from glucose utilization to glucose production during the postabsorptive phase.
2. Fuel mobilization in peripheral tissues occurs when the blood insulin concentration declines.
3. Muscle reacts to a metabolic demand for glucose by mobilizing amino acids to support hepatic gluconeogenesis.
4. Muscle release of amino acids is related to reduced glucose and amino acid uptake.
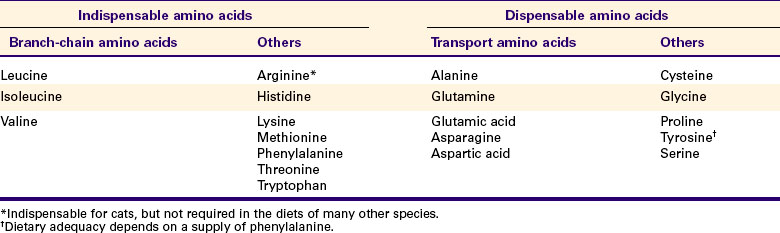
5. The complex pattern of muscle amino acid catabolism and release is necessary to accommodate the liver’s limited capacity for uptake of branch-chain amino acids and to facilitate the removal of amino nitrogen from the muscle.
6. The reaction of adipose tissue during the postabsorptive phase is to mobilize fatty acids.
Nutrient Utilization During Prolonged Energy Malnutrition or Complete Food Deprivation
1. During prolonged periods of fasting or undernutrition, glucose and amino acids are conserved by extensive utilization of fats and ketone bodies for energy production.
2. A large portion of the fatty acids released from adipose tissue is taken up directly by the liver.
3. Hepatic ketone body formation is promoted by low glucose availability, a high glucagon/insulin ratio, and a ready supply of fatty acids.
4. Glucagon plays an important role in the excessive production of ketone bodies in diabetes mellitus.
5. Fatty acids cannot be used for glucose synthesis.
6. Ketone bodies are formed in the mitochondria from acetyl coenzyme A.
7. Hepatic very-low-density lipoproteins may be synthesized from adipose-derived fatty acid as well as from newly synthesized fatty acid.
8. Hormonal conditions direct the distribution of very-low-density lipoprotein fatty acids in the body.
9. Changes in growth hormone concentrations may aid in shifting peripheral fuel utilization from glucose and amino acids to ketone bodies and fatty acids.
The rate of absorption of nutrients from the gut is not constant, but rather fluctuates greatly with food intake. Meals are digested at a rate dependent on their chemical composition, regardless of the animal’s nutrient needs. The nature of digestion dictates that nutrient absorption from the gut is rapid during digestion and then ceases during interdigestive periods. In other words, the gut is not a storehouse for nutrients, and digestion is not modulated by the animal’s nutritional demands. Nutrient needs are not well matched to the wide fluctuations that occur in nutrient absorption from the gut. In fact, a vital need exists for a constant, steady supply of fuel-providing nutrients to maintain the basal metabolic functions of the body. In addition, the periods when the animal’s metabolic needs are greatly elevated often do not coincide with times of rapid absorption of nutrients from the gut. Therefore, animals must have a sophisticated system for maintaining the supply of nutrients, particularly energy-supplying nutrients, and buffering both the short-term and the long-term “feast or famine” effects associated with the absorptive and postabsorptive periods of digestion.
Homeostatic Mechanisms Balance the Supply and Demand of Almost All Nutrients
This chapter focuses on supply regulation of the major energy-supplying nutrients; however, other nutrients, including vitamins and minerals, also are subject to homeostatic regulatory mechanisms. Although many of these mechanisms directly involve the digestive system, a complete discussion is beyond the scope of this book. The homeostatic mechanisms regulating the supply of minerals and vitamins are described in select references of the Bibliography.
Energy-supplying nutrients are referred to as metabolic fuels, and the physiological mechanisms for maintaining the supply of fuels and matching it to demand constitute fuel homeostasis. Fuel homeostasis is maintained by several mechanisms: the insulin-glucagon axis, the hypothalamic-pituitary axis, and the central nervous system (CNS). This chapter discusses some of the ways in which fuel is stored during the absorptive period of digestion and subsequently mobilized when needed to supply energy requirements. You may want to review Chapter 1 and the section on insulin and glucagon in Chapter 34 before reading this chapter.
THE FURNACE
The Tricarboxylic Acid (or Krebs) Cycle Is the Major Energy-Yielding Pathway of Fuel Utilization in the Body
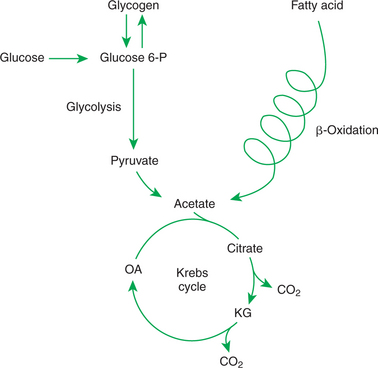
The Krebs cycle and the major pathways leading into it are briefly outlined in Figure 32-1. You probably have studied the Krebs cycle, glycolysis, and β-oxidation of fats in a basic course of biochemistry. Often, however, you become so intent on memorizing enzyme names and chemical changes in metabolites that you lose sight of the physiological significance of the pathways to whole-body metabolism. For the purposes of this discussion, it is important only to follow the flow of the major carbon-containing nutrients into and out of the various pathways. The only specific metabolic steps emphasized are those points at which the flow of fuels is directed or regulated. Note that the Krebs cycle and associated pathways of intermediary metabolism are sites not only of fuel utilization and energy production, but also of transformation from one fuel type to another. These transformations are important in the overall scheme of fuel homeostasis.
THE FUELS
The Major Metabolic Fuels Consist of Glucose, Amino Acids, Fatty Acids, and Ketone Bodies; Various Storage and Transport Forms Exist for These Compounds
Glucose, the digestion product of carbohydrate, is the basic metabolic fuel during periods of adequate nutrition in omnivorous monogastric animals, such as dogs and rats. Although there are other important fuels in the body, glucose has special significance because under most conditions it is the only fuel that is consumed by the CNS. Therefore, maintaining a steady supply of glucose for brain metabolism is of paramount importance to the body. It is not surprising that an elegant system of homeostasis exists to regulate the availability of glucose to the brain and other tissues. This system of maintaining glucose availability is a major focus of this chapter.
Glucose Is the Central Fuel in the Energy Metabolism of Most Animals
Glucose can be stored in the body as glycogen, a highly branched starch found in liver and skeletal muscle. Glycogen is the only direct storage form of glucose in the body, although glucose can be synthesized from other compounds. Directing glucose to and from glycogen depots is a major function of fuel homeostasis. Glucose is released from glycogen through the process of glycogenolysis.
The major means by which glucose is used as fuel is through the Embden-Meyerhof pathway, or glycolysis, the series of biochemical steps that initiate the oxidation of glucose. Glycolysis leads directly into the Krebs cycle, the site of complete fuel oxidation and the major energy-yielding metabolic pathway of the body. For the study of fuel homeostasis, you should appreciate that the process of glycolysis is reversible overall, meaning that glucose can be produced from the compounds that constitute the end products of glycolysis. Because of the close link between glycolysis and the Krebs cycle, any of the Krebs cycle intermediates can potentially move “backward” into the glycolytic pathway to produce glucose. The synthesis of glucose from end products of glycolysis and intermediates of the Krebs cycle is referred to as gluconeogenesis, a critical part of fuel homeostasis. Although Krebs cycle activity occurs in virtually all tissues except red blood cells (RBCs), the process of gluconeogenesis occurs only in liver and, to a limited extent, kidney.
Another pathway for glucose oxidation is the pentose-phosphate pathway. This is a quantitatively minor pathway that does not have great impact on fuel homeostasis. However, it is an important metabolic pathway in erythrocytes (RBCs), which have an absolute need for glucose, although these cells’ overall need for energy is small compared with the rest of the body.
Amino Acids Are Important Fuels in Addition to Being the Building Blocks of Protein
Amino acids are important fuels. Whereas these monomers are the building blocks of proteins, amino acids are also carbon-containing compounds that can provide energy to the body. In addition, they are important substrates for gluconeogenesis, indicating that most amino acids can be converted to glucose when the available glucose supply is limited. Although it is sometimes said that there is no storage site of amino acids in the body, the protein of skeletal muscle could be considered to have an amino acid storage function in addition to its locomotor functions.
Fatty Acids Are the Major Form of Energy Storage in the Animal Body
Fatty acids are stored in adipose tissue in the form of triglycerides (also called triacylglycerols), which consist of three fatty-acid molecules linked to a glycerol molecule by ester bonds (see Figure 30-24). Triglycerides are an ideal form of energy storage for animals. They are highly reduced molecules (there is little oxygen compared with the amount of carbon and hydrogen), which means they are a concentrated energy source, having more than twice the caloric value per gram than carbohydrates or amino acids. In addition, adipose tissue contains little water compared with protein or glycogen, the storage forms of the other two potential fuels. Thus, adipose tissue is undiluted by bulky water, allowing it to be a concentrated form of energy storage that permits animals to carry with them a maximal amount of energy at a minimal amount of weight. Fats, however, have a metabolic disadvantage; they are not water soluble. Therefore, special transport systems are needed to enable fats to be distributed among the tissues through the blood and lymph systems. In addition, fatty acids cannot be converted to glucose, so they cannot, under usual circumstances, contribute to the energy supply of the CNS. However, fatty acids can be converted to ketone bodies.
Ketone Bodies Are Fat-Derived, Water-Soluble Metabolites That Serve as Glucose Substitutes
Although glucose cannot be formed from fat, the fat-derived ketone bodies do have some glucoselike attributes. For example, ketone bodies can pass the blood-brain barrier, and during prolonged periods of dietary energy deprivation, they can provide a large portion of the energy supply to the CNS, at least in some species. It does appear, however, that ketone bodies cannot totally replace glucose in this function, and that a small amount of glucose is always needed by the CNS.
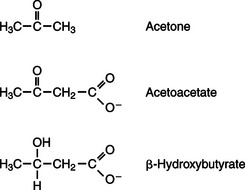
In monogastric species, ketone bodies are formed exclusively in the liver and are used by a wide variety of tissues. Some tissues, including cardiac muscle, use ketone bodies instead of glucose. In ruminants the ketone body β-hydroxybutyrate is formed from butyrate in the rumen epithelium. Thus, in ruminants, ketone bodies are not only products of fatty-acid metabolism, but also products of normal digestion. Elevated serum concentrations of ketone bodies are characteristic of several diseases associated with abnormalities of fuel homeostasis. This fact might lead one to conclude that ketone bodies are abnormal, or even toxic, metabolites. In fact, when present in physiological concentrations, ketone bodies are important fuels that occupy an integral part of the scheme of fuel homeostasis. Figure 32-2 illustrates the chemical structure of the three major ketone bodies.
NUTRIENT UTILIZATION DURING THE ABSORPTIVE PHASE
As absorption takes place, metabolic events in the liver and peripheral organs are coordinated to direct nutrients into storage molecules and storage sites. Figure 32-3 illustrates the general scheme of metabolism during the absorptive phase.
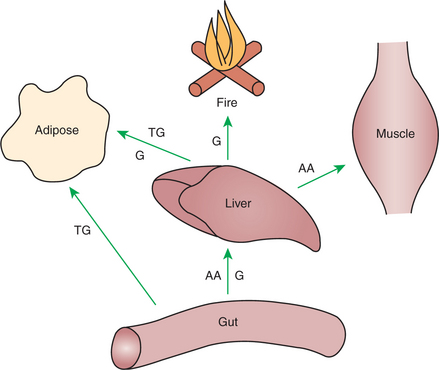
FIGURE 32-3 Metabolism during the absorptive period is characterized by the movement of potential fuels into depot sites and the utilization of glucose (G) as a fuel. AA, Amino acid; TG, triglyceride.
During the Absorptive Phase, the Liver Takes up Glucose and Converts It into Glycogen and Triglyceride
When a meal is ingested, insulin secretion begins even before maximal absorption of glucose is achieved. This secretion is stimulated by the action of gastric inhibitory peptide (see Chapter 27) and perhaps other enteric hormones. Early insulin secretion ensures that the liver and other tissues will be “primed” and ready for the arrival of glucose from the gut. A large portion of the glucose absorbed postprandially is taken up by the liver as portal blood traverses the hepatic sinusoids. Under the influence of insulin, glucose in the liver is directed into glycogen synthesis. The net effect is that glucose from the digestion and absorption of carbohydrate is stored in the liver during absorptive periods. This process moderates the flow of glucose from the gut to the general circulation and keeps blood glucose concentrations from becoming excessively high during the digestion of a carbohydrate meal. Insulin exerts its stimulatory effect on hepatic glycogen synthesis by stimulating intracellular metabolic pathways that lead to the formation of glycogen. These effects are discussed further in reference to the counterbalancing effects of glucagon.
The amount of glycogen that can be stored in the liver is limited and, under normal conditions, probably never exceeds 10% of the total weight of the liver. In humans, this represents about 100 g of glycogen, and a proportionately similar limit likely exists for the storage of glycogen in the livers of other species. This amount of glycogen does not account for all the glucose taken up by the liver during the digestion and absorption of a large carbohydrate meal; therefore, some additional mechanism must exist for the disposal of excess glucose. If there were no such alternatives for glucose disposal, blood glucose concentrations could rise out of control after glycogen stores had reached their maximum. Fatty-acid synthesis offers an alternative mechanism for glucose removal.
The Conversion of Glucose to Fatty Acids Is an Irreversible Process
The synthesis of fatty acids from glucose begins with glycolysis. This pathway leads to the production of two pyruvate molecules for each molecule of glucose consumed. Pyruvate can then enter the mitochondria to be activated to acetyl coenzyme A (acetyl CoA) for entry into the Krebs cycle. However, the Krebs cycle is for energy generation, and during the absorptive period, there is more than enough acetyl CoA and Krebs cycle activity to provide for energy needs; therefore the excess acetyl CoA must be shunted away from the Krebs cycle. The excess acetyl CoA combines with oxaloacetate to form citrate in what is essentially the first reaction of the Krebs cycle. Instead of continuing through the Krebs cycle reactions, however, during the absorptive period much of the citrate is transported out of the mitochondria into the cytosol. Once in the cytosol, each citrate molecule contributes two carbons toward the synthesis of fatty acids. The remaining portion of the citrate molecule cycles back into the mitochondria for further use. Citrate serves as a carrier molecule to transport two-carbon units out of the mitochondria because acetyl CoA cannot pass the mitochondrial membrane directly (Figure 32-4).
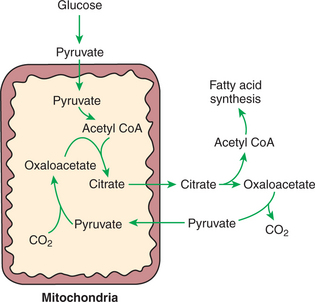
FIGURE 32-4 Hepatic synthesis of fatty acid from carbohydrate requires the passage of carbohydrate carbons through the mitochondria. Citrate forms a shuttle to transport the carbons of acetyl coenzyme A (acetyl CoA) out of the mitochondria because acetyl CoA cannot pass directly through the mitochondrial membrane. The formation of citrate from oxaloacetate and acetyl CoA is the first reaction of the Krebs cycle; thus fatty-acid formation is an alternative to Krebs cycle oxidation when there is more than enough acetyl CoA to provide cellular energy through Krebs cycle activity.
Several important steps in this conversion of glucose to fatty acids are promoted by insulin and are discussed in detail later. It is important to recognize that the conversion of glucose to fatty acids is irreversible; thus carbohydrate can form fat, but fat cannot form carbohydrate. The discussion here concerns hepatic metabolism, and the liver is an important site of fatty-acid synthesis in several species. Direct synthesis of fatty acids also occurs in adipose tissue. The relative importance of liver and adipose tissue as sites of fatty-acid synthesis varies with species, as discussed later.
Transport of Fatty Acids out of the Liver Is Through Chylomicron-Like Particles Known as Very-Low-Density Lipoproteins
Once formed in the liver, fatty acids must be transported either to adipose tissue for storage or to other tissues (e.g., muscle) for direct utilization for energy production. Because fatty acids are insoluble in blood, some special transport mechanism for their distribution is necessary. This mechanism is through the hepatic formation of triglyceride-rich serum lipoproteins, also known as very-low-density lipoproteins (VLDLs); these triglyceride-rich lipoproteins are much less dense than other lipoproteins in blood serum. In the synthesis of VLDLs, fatty acids are first esterified to form triglycerides, and the triglycerides are wrapped in a coat of phospholipid, cholesterol, and specific proteins (Figure 32-5). This is essentially the same mechanism by which fatty acids are transported out of the enterocytes after absorption from the gut. In the latter case the lipoproteins are called chylomicrons. The VLDLs of the liver are smaller than chylomicrons but have a similar structure and function. The mechanisms by which VLDLs and chylomicrons deliver fatty acids to peripheral tissues are further discussed in relation to peripheral tissues.
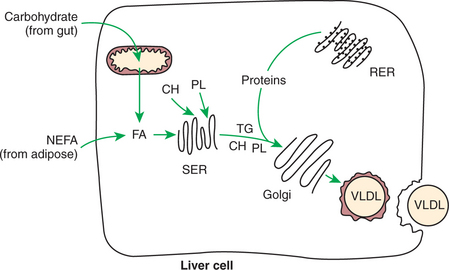
FIGURE 32-5 Formation of very-low-density lipoprotein (VLDL). Fatty acids (FA) for triglyceride (TG) formation may come from synthesis by carbohydrate or amino acids or from adipose tissue FA arriving at the liver in the form of nonesterified fatty acids (NEFA). Note the similarity to chylomicron formation (see Figure 30-27). CH, Cholesterol; PL, phospholipid; SER, smooth endoplasmic reticulum; RER, rough endoplasmic reticulum.
Amino Acids Can Be Classified into Groups on the Basis of Metabolic Characteristics
The discussion of amino acid absorption and metabolism becomes complicated because not all amino acids are subject to the same reactions. For this discussion the amino acids are divided into two groups, each containing two subgroups (Table 32-1). The major groups are the “nutritionally dispensable” amino acids and the “nutritionally indispensable” amino acids. Within the dispensable amino acid group, glutamate, aspartate, and alanine are separated out as transport amino acids; within the indispensable amino acid group, leucine, isoleucine, and valine form a special subgroup known as the branch-chain amino acids (BCAAs). The transport amino acids are utilized in several reactions in which amino groups are transferred from molecule to molecule or organ to organ.
Amino Acids Are Extensively Modified During Absorption
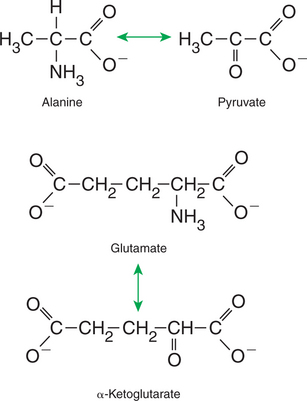
The profile of amino acids in the portal vein is considerably different from that of the diet, indicating that amino acid destruction and transformation occur during the absorptive process. Essentially all the glutamate and much of the aspartate in the diet are removed by the intestinal epithelial cells during absorption, so the portal blood is almost devoid of glutamate and contains little aspartate. Much of the nitrogen from glutamate and aspartate is transferred to pyruvate to form the amino acid alanine, which is present in high concentrations in portal blood. The metabolism of the transport amino acids in the intestinal epithelium is a good example of both the way in which amino groups can be gained and lost and how the metabolism of amino acids interfaces with the metabolism of carbohydrate. Glutamate and aspartate are similar to two Krebs cycle intermediates, α-ketoglutarate and oxaloacetate, differing only by the presence of an amino group or a keto-oxygen. Carbohydrates and amino acids having this relationship are said to be analogues; thus, α-ketoglutarate is the keto-analogue of glutamate, and pyruvate is the keto-analogue of alanine (Figure 32-6). All amino acids can form keto-analogues, and all keto-analogues can be readily converted back to their parent amino acids.
Many Amino Acids Are Removed by the Liver on “First Pass,” Never Reaching the Systemic Circulation
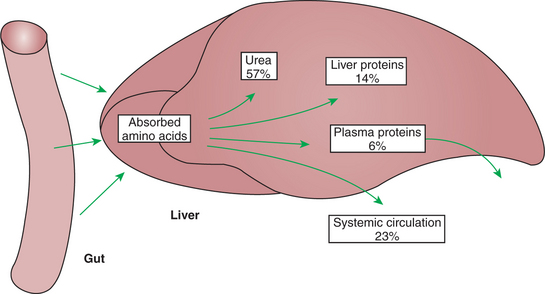
The hepatic-portal circulation is arranged in such a way that all nutrients leaving the gut via the blood pass through the liver before entering the systemic circulation (see Figure 30-23). This arrangement places the liver in a “sentinel” position, from which it can modify the nutrient composition of portal blood before the blood is distributed to other tissues. The function of the liver in modifying portal blood composition is well illustrated in the case of amino acid absorption. Many of the amino acids absorbed into portal blood are removed as the blood passes the liver, so they never reach the general circulation. Figure 32-7 illustrates that in the dog, only about 23% of the amino acids reaching the liver during the absorptive period pass into the general circulation; the liver thus helps keep blood amino acid concentrations stable during periods of amino acid absorption. The blood amino acid concentration, as with the blood glucose concentration, is usually kept relatively constant.
Some Amino Acids Taken up by the Liver Are Used for Protein Synthesis
The liver is an important site of protein synthesis, and thus its priority position for amino acid uptake seems reasonable. Figure 32-7 shows that approximately 20% of the portal blood amino acid supply is used for protein synthesis in the liver, although this proportion varies with dietary protein intake. Almost all the serum proteins are synthesized in the liver, including such critical proteins as albumin and the blood-clotting factors. Although the liver-derived serum proteins serve many important functions, one function they do not serve is that of amino acid transport. The direct amino acid supply for protein synthesis in nonhepatic tissue comes from free amino acids in the blood, not from preformed serum proteins.
Most Amino Acids Taken up by the Liver Are Converted to Carbohydrates
Most amino acids entering the liver undergo deamination, which means the amino groups are removed and the molecules converted to their keto-analogues. The keto-analogues enter the pathways of carbohydrate metabolism, from which they may be completely metabolized for energy, converted to glucose or glycogen, or shunted to fatty-acid synthesis. All these reactions proceed in the same manner as previously described for carbohydrate metabolism. Figure 32-8 illustrates the sites at which the various amino acids enter the carbohydrate pathways.
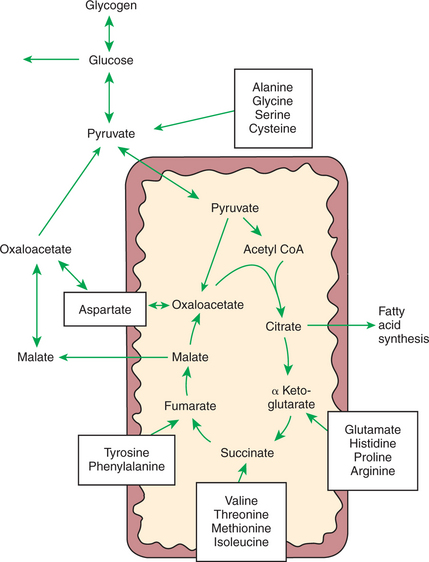
FIGURE 32-8 Sites of entry of various amino acids into the scheme of carbohydrate metabolism. This figure illustrates the means by which glucose can be synthesized from amino acids in the process of gluconeogenesis. In the case of the dispensable amino acids, the reactions are reversible, allowing amino acid production from carbohydrate.
Deamination of amino acids for the production of carbohydrate or energy may seem like a waste of expensive dietary protein; in some species, however, the deamination of amino acids is important for homeostasis of glucose and other fuels. For example, the natural diets of the true carnivores (e.g., cats, mink) contain a large portion of protein and little carbohydrate, but their glucose needs are no less than those of other animals, so it is extremely important that they synthesize glucose from amino acids. Ruminants are in a similar situation because most of the carbohydrates they consume undergo fermentative digestion and are absorbed as volatile fatty acids rather than glucose. As with carnivores, ruminants depend on amino acids for some of their glucose needs, although a large portion of ruminant glucose requirements may be met through conversion of propionate.
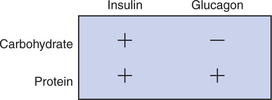
To allow carbohydrate production and the deamination of excess amino acids, the endocrine reactions to high-protein meals are somewhat different from those to meals containing substantial amounts of carbohydrate. During the digestion of high-protein meals, insulin and glucagon secretion does not occur in its usual reciprocal pattern. Insulin secretion is stimulated by amino acids as well as by glucose. Glucagon secretion, which is inhibited by glucose, is stimulated by amino acids as long as glucose concentrations are moderately low. This relationship means that during the digestion of a high-protein, low-carbohydrate meal, there is simultaneous secretion of insulin and glucagon. One of the effects of insulin is the greater cellular uptake of amino acids as well as glucose. Thus the effect of insulin in this situation is to increase transport of amino acids into tissues.
If insulin secretion were the only action stimulated by amino acid absorption, however, the animal would risk insulin-stimulated hypoglycemia when it consumed a high-protein, low-carbohydrate diet. An important action of glucagon is to stimulate gluconeogenesis through the deamination of amino acids in the liver. This process ensures that adequate glucose will be available to counterbalance the effects of amino acid– stimulated insulin secretion. Figure 32-9 illustrates the relationship between insulin secretion and glucagon secretion during the absorption of diets with different carbohydrate and protein concentrations.
Not All Amino Acids Are Subject to Hepatic Destruction
During the absorptive period, amino acids for peripheral (nonhepatic) protein synthesis must come from that portion of amino acids that escape hepatic destruction. As seen from Figure 32-7, this portion amounts to only about 23% of the amino acids absorbed from the gut. Although this may seem like a meager portion of amino acids to be allocated for protein synthesis by all body tissues except liver, two considerations make it more appropriate. First, amino acids are selectively taken up by the liver, so the distribution of individual amino acids in blood leaving the liver is not the same as that in blood reaching the liver. The indispensable amino acids, especially the BCAAs, are not avidly extracted by the liver, whereas some of the dispensable amino acids (e.g., alanine) are extensively taken up by hepatic tissue. The dispensable amino acids can be synthesized by protein-producing tissues; thus the relatively low concentration of serum amino acids resulting from hepatic amino acid removal is not rate limiting for tissue protein synthesis. Second, the proportion of amino acids taken up by the liver, as well as the fate of the amino acids that are taken up, is not constant and can be adjusted according to the body’s protein needs. Low-protein diets lead to reductions in hepatic amino acid uptake, protein synthesis, and amino acid destruction by the liver.
Metabolism at the Tissue Level Is Coordinated with Hepatic Metabolism and Results in the Deposition of Fuel into Storage Tissues During the Absorptive Period
The overall effects of hepatic metabolism during the absorption of a meal are the removal of glucose and amino acids and the synthesis of protein and fat. Complementary changes occur in peripheral tissues, so additional glucose and amino acids are removed by skeletal muscle and adipose tissue. In addition, fatty acids secreted by the liver as VLDL triglycerides are deposited in adipose tissue, as are the triglycerides of chylomicrons.
Insulin Promotes the Synthesis of Protein and the Deposition of Glycogen in Muscle
The absorptive period is dominated by the effects of insulin. In skeletal muscle, the largest tissue mass of the body, insulin promotes the uptake of glucose and amino acids and thus tends to moderate the increase in blood concentration of these nutrients during absorption of a meal. The uptake of glucose by muscle is associated with glycogen synthesis, just as in the liver. Muscle glycogen, in contrast to liver glycogen, cannot be made directly available to augment blood glucose concentrations during periods of low glucose availability. Muscle glycogen is primarily for metabolism in the muscle.
Insulin-Stimulated Uptake of Amino Acids by Muscle Results in a Net Increase in Muscle Protein Synthesis
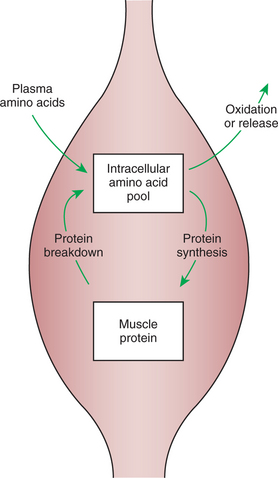
The term net increase is used in reference to muscle protein synthesis because muscle protein is in a state of dynamic equilibrium, that is, a constant state of flux. Protein molecules are continuously being broken down and their amino acids added to an intracellular amino acid pool. Simultaneously, new proteins are constantly being made, deriving their amino acids from the same pool (Figure 32-10). The size of the amino acid pool depends on the relative rates of entry and exit of amino acids. Amino acids enter the pool from the blood during the absorptive phase and at all times from the breakdown of body protein. Exit of amino acids from the pool results from protein synthesis and oxidative catabolism. In the absorptive phase of digestion, the amino acid pool is large because amino acids are being taken up from the blood. In addition, few of the amino acids leaving the pool are directed toward oxidative catabolism because sufficient glucose is available for oxidation and energy generation. Therefore the amino acid pool is large, and a high proportion of amino acids is directed to protein synthesis. When the rate of protein synthesis exceeds the rate of protein breakdown, there is a net increase in the amount of muscle protein. Thus, during the absorptive phase, amino acids are stored in muscle protein, protein that has a functional role not only for locomotion and posture, but also for amino acid storage.
During the Absorptive Phase, Triglyceride Accumulation in Adipose Tissue Occurs by Two Mechanisms: Uptake from Very-Low-Density Lipoproteins and Direct Lipid Synthesis from Glucose
Triglyceride fatty acids are transferred from chylomicrons and VLDLs to adipose tissue by the action of lipoprotein lipase (LPL). This enzyme resides on endothelial surfaces of capillaries and, when activated, binds to chylomicrons and VLDLs, catalyzing the hydrolysis of fatty acids from their core triglycerides and allowing the transfer of those fatty acids to the surrounding tissues. The sensitivity of LPL to specific hormones varies in different tissues. Adipose tissue LPL is stimulated by insulin; thus, during the absorptive phase, fatty acids from chylomicrons and VLDLs are selectively transferred to adipose tissue. Therefore, under the influence of insulin, excess carbohydrate and amino acids are converted to fatty acids in the liver, and those fatty acids are subsequently transported, via VLDLs, to the adipose tissue. Similarly, chylomicron triglyceride arising from intestinal fatty-acid absorption is also selectively transported to adipose tissue, under the influence of insulin.
Adipose tissue fatty acids may also arise from direct synthesis in addition to uptake from chylomicrons and VLDLs. Adipose tissue cells are metabolically active, and under the influence of insulin, they take up glucose. Within the adipocytes, glucose can be converted to fatty acids by the same metabolic mechanisms by which fatty acids were synthesized in the liver. In addition, acetate from fermentative digestion also can serve as a substrate for fatty-acid synthesis in adipose tissue (see later discussion of the special fuel considerations of ruminants). Thus there are two major sites of fatty-acid synthesis in the body: liver and adipose tissue. The relative importance of these sites varies with species.
NUTRIENT UTILIZATION DURING THE POSTABSORPTIVE PHASE
The postabsorptive phase is the relatively brief period (usually a few hours) between meals in well-fed animals. It is characterized by short-term changes that mobilize nutrients from storage pools to maintain fuel availability for metabolically active tissue. Figure 32-11 illustrates the general scheme of postabsorptive metabolism.
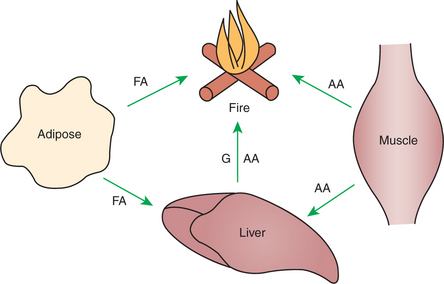
FIGURE 32-11 Postabsorptive metabolism is characterized by movement of fuels out of depot sites for immediate use. Glucose (G) arising from glycogenolysis or gluconeogenesis is a major fuel, although some fatty acid (FA) is consumed as well. Amino acid (AA) forms the substrate for gluconeogenesis.
Hepatic Metabolism Switches from Glucose Utilization to Glucose Production During the Postabsorptive Phase
As the absorption of a meal is completed, the rate of glucose absorption from the gut wanes, and the blood glucose concentration diminishes, removing the stimulus for insulin production. As blood glucose concentrations decline, glucagon secretion is stimulated. The primary target organ of glucagon is the liver, in which glucagon creates marked metabolic changes. Through stimulation of specific cell surface receptors on hepatocytes, glucagon activates adenyl cyclase, leading to the phosphorylation of numerous cellular enzymes (see Chapter 1). Some enzymes are activated by phosphorylation, whereas others are inactivated, and unless the overall scheme of substrate flow is considered, the whole phosphorylation-dephosphorylation system appears to be quite random and to make little sense. Considering the actions of the individual enzymes in light of their effect on the flow of energy substrate through the liver, however, reveals that the system is an elegant and incredibly well-orchestrated mechanism for the maintenance of fuel homeostasis.
The enzymes that stimulate mobilization and utilization of fuels are activated by phosphorylation, whereas those stimulating storage of fuels are inactivated by phosphorylation. It must be understood that many enzymes of intermediary metabolism serve a passive role, catalyzing reactions that can go in either direction, depending on substrate concentrations. A relatively small number of regulatory enzymes usually stand at the head of metabolic pathways and determine the substrate concentrations to which the other, unregulated enzymes are exposed. Through its effect on several key regulatory enzymes, glucagon (a stimulator of phosphorylation) places the liver in a fuel-mobilization state. In contrast, insulin (an inhibitor of phosphorylation) promotes a hepatic metabolic pattern that favors fuel storage, as previously discussed.
The opposing actions of insulin and glucagon on hepatic metabolism are evident from their actions on two key regulatory enzyme pairs: glycogen synthase and glycogen phosphatase, and phosphofructokinase and fructose-1,6-bisphosphatase. The first of these pairs regulates glycogen synthesis and breakdown, whereas the second regulates glycolysis and gluconeogenesis, respectively. Figure 32-12 illustrates the actions of these enzymes and their regulatory effects. Glycogen synthase and phosphofructokinase are inhibited by phosphorylation and thus are stimulated by insulin. Glycogen phosphatase and fructose-1,6-bisphosphatase are stimulated by phosphorylation and thus stimulated by glucagon. The actions of insulin and glucagon on these antagonistic enzyme pairs emphasize the importance of the insulin/glucagon ratio to which the liver is exposed. Neither hormone elicits an “all-or-none” reaction, but rather alters the balance of opposing reactions by influencing the relative activity of antagonistic enzymes. Thus the fuel-mobilizing or fuel-storing activity of the liver depends on which hormone is most dominant. For this reason, the insulin/glucagon ratio appears to be more important to liver metabolism than the absolute concentration of either hormone.
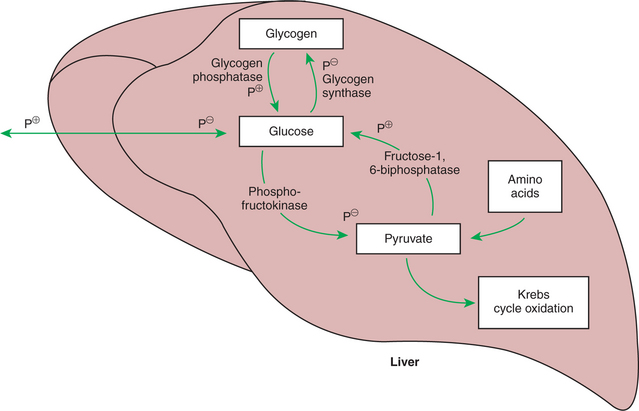
FIGURE 32-12 Effects of phosphorylation on four key enzymes of glucose production and utilization. All four enzymes are phosphorylated under the influence of cyclic adenosine monophosphate (cAMP). Note, however, that the enzymes that favor glucose formation are stimulated by phosphorylation (P+), whereas those that favor glucose utilization and storage are inhibited by phosphorylation (P−).
Under the influence of glucagon, glycogen phosphatase is activated by phosphorylation, promoting glycogenolysis and the elevation of intracellular glucose concentrations. As glucose accumulates, it is prevented from cycling back into glycogen because the major enzyme catalyzing that reaction, glycogen synthase, is blocked by phosphorylation. In addition, the flow of glucose into glycolysis is also blocked by phosphorylation inhibition of phosphofructokinase (Figure 32-12). Thus the normal pathways for glucose utilization within the hepatocyte are all inhibited by glucagon, allowing glucose from glycogen breakdown to accumulate in the cells. Eventually, intracellular glucose escapes into the extracellular fluid and on into the blood. In this manner, hepatic glycogen is mobilized to elevate and maintain blood glucose concentrations when they begin to decline.
The liver stores of glycogen are relatively limited and cannot maintain blood glucose concentrations for a long period. Estimates in humans are that hepatic glycogen will serve blood glucose needs for 6 to 12 hours under conditions of light exertion and for only about 20 minutes under conditions of heavy exertion. Values for animals are probably similar. Therefore, in addition to glycogen mobilization, some other means must exist for maintaining the body’s glucose supply during periods of exertion and prolonged periods between meals. Under these conditions of greater demand, glucose is provided by gluconeogenesis. Gluconeogenesis is promoted by the phosphorylation-stimulated enzyme fructose-1,6-bisphosphatase. This enzyme essentially puts the glycolytic pathway into reverse, leading to glucose production from the same molecules that are intermediates in its oxidative destruction. Important substrates are pyruvate and all the intermediates of the Krebs cycle.
At this point, it is important to remember that most of the Krebs cycle intermediates or pyruvate can be supplied by the deamination of amino acids. The entry point of the various amino acids into the scheme of carbohydrate metabolism is illustrated in Figure 32-8. Pyruvate and all the Krebs cycle intermediates can flow backward through the oxidative pathway (not all the reactions of gluconeogenesis are the exact reverse of the corresponding reactions in glycolysis, but the net result of gluconeogenesis is the reverse of glycolysis), resulting in the production of glucose. Thus, amino acids provide a large store of precursors for glucose formation. The end result of glucagon stimulation is to promote the production of glucose through glycogenolysis and gluconeogenesis, turning the liver into a glucose-synthesizing organ.
Fuel Mobilization in Peripheral Tissues Occurs when the Blood Insulin Concentration Declines
The pattern of metabolism in the peripheral tissues changes in the postabsorptive phase to support the liver’s capacity to maintain fuel supplies.
Muscle Reacts to a Metabolic Demand for Glucose by Mobilizing Amino Acids to Support Hepatic Gluconeogenesis
Mobilization of amino acid from muscle appears to be stimulated largely by a relative lack of insulin; thus mobilization occurs when blood glucose concentrations are low. Amino acids mobilized from skeletal muscle come from the intracellular amino acid pool cited earlier (see Figure 32-10). However, the mobilizing reactions are complex, and the distribution of amino acids leaving the muscle does not reflect the distribution of amino acids in the intracellular pool, as explained later.
Muscle Release of Amino Acids Is Related to Reduced Glucose and Amino Acid Uptake
The postabsorptive decline in the serum insulin concentration has a twofold effect on muscle: the entry of amino acids from the serum into the intracellular amino acid pool is diminished, and the entry of glucose into muscle cells for energy production declines. Reduced amino acid entry results in conditions favoring net protein degradation to maintain the cellular amino acid pool size. Reduced glucose entry results in increased utilization of amino acids from the pool for energy production.
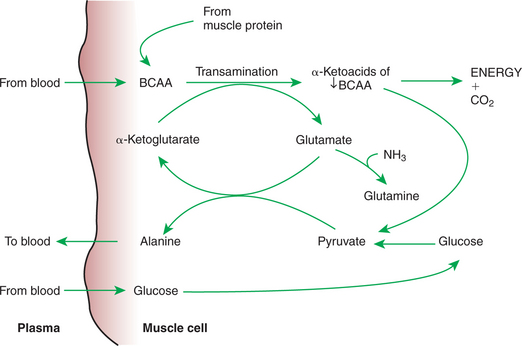
The pattern of utilization of amino acids for energy by muscle may at first seem unnecessarily complex, involving selective use and extensive transformation of amino acids. BCAAs serve as primary sources of energy in muscle cells during the postabsorptive phase because these amino acids account for approximately one third of all muscle amino acid. Catabolism of BCAAs begins with deamination and the formation of the α-keto-acid of the BCAA. The α-keto-acids then enter the Krebs cycle for energy production. Deamination of the BCAA requires that some acceptor be available to receive the amino group, and this acceptor is ultimately pyruvate, resulting in the formation of alanine. The source of pyruvate can be muscle glycogen, blood glucose, or the metabolic products of BCAA α-keto-acids. When metabolism of BCAA α-keto-acids serves as the supply of pyruvate for alanine synthesis, the net reaction is conversion of BCAA to alanine (Figure 32-13). Thus the overall metabolic activity in muscle during the postabsorptive phase is the destruction of BCAAs and the formation of alanine. The alanine formed is released from the muscle cells into the blood, from which it may be taken up by the liver for gluconeogenesis.
The Complex Pattern of Muscle Amino Acid Catabolism and Release Is Necessary to Accommodate the Liver’s Limited Capacity for Uptake of Branch-Chain Amino Acids and to Facilitate the Removal of Amino Nitrogen from the Muscle
It might appear that a simpler system of amino acid transfer to the liver would suffice. Why are amino acids not just released from muscle cell amino acid pools into the blood and transported to the liver for glucose synthesis? The answer lies in the limited uptake capacity of the liver for BCAAs and the need to transport amino nitrogen out of the muscle. BCAAs, the predominant amino acids of skeletal muscle, are not taken up readily by the liver; thus, if BCAAs were not transformed to alanine, amino acid transfer to the liver would be limited.
In addition, alanine is a convenient means by which nitrogen from the deamination of muscle amino acid can be transported to the liver. This is important because free amino groups liberated by the catabolism of amino acids in muscle, if not removed, could lead to the formation of toxic levels of ammonia. Ammonia is detoxified in the body by the formation of urea, but urea formation occurs only in the liver. Thus, alanine forms a gluconeogenic precursor that also transports nitrogen to the liver for urea synthesis. Figure 32-13 and Figure 32-14 illustrate the role of alanine in the transport of amino acid nitrogen and carbon to the liver for synthesis of urea and glucose, respectively.
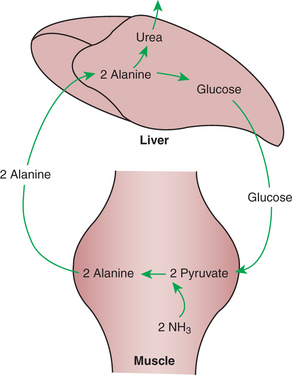
FIGURE 32-14 Alanine arising from BCAA catabolism in muscle is converted to glucose and urea in the liver. The glucose produced can potentially return to the muscle for alanine production. Thus the cycle of alanine to glucose forms a shuttle to transport nitrogen from the muscle to the liver for urea synthesis. NH3′ Ammonia.
The regulation of muscle protein mobilization is influenced to a large extent by the lack of insulin. However, the adrenocortical hormone cortisol has an important effect of stimulating protein breakdown and amino acid mobilization. Through the mobilization of muscle protein and stimulation of hepatic gluconeogenesis, cortisol exerts one of its major effects, raising blood glucose concentration. Under normal conditions, glucagon, the other major gluconeogenic hormone, exerts its effects on the liver and does not appear to have a direct effect on muscle.
The Reaction of Adipose Tissue During the Postabsorptive Phase Is to Mobilize Fatty Acids
Fatty acids are released from adipose tissue because of the action of the phosphorylation-stimulated enzyme hormone-sensitive lipase (HSL). This enzyme is stimulated by the relative lack of insulin in the postabsorptive period; insulin suppresses the action of HSL by promoting its dephosphorylation. Glucagon may have some adipose tissue activity in promoting triglyceride breakdown by stimulating the phosphorylation and activation of HSL. More likely, however, glucagon’s effects are restricted to the liver, and the normal stimulation of HSL comes from epinephrine or norepinephrine; norepinephrine originates from sympathetic nerves in the adipose tissue. The exact means by which sympathetic nerve activity in adipose tissue is coordinated with body fuel availability is not well established, but the catecholamine hormones and neuroregulators appear to be the primary positive stimulus for breakdown of adipose triglyceride. However, the negative stimulus provided by the absence of insulin may be the most important regulator of adipose fat mobilization.
Stimulation of HSL in the postabsorptive state leads to the release of fatty acids from adipose tissue into the blood. Fatty acids in blood are reversibly bound to albumin because they are not otherwise soluble in water. Albumin-bound fatty acids in blood are usually referred to as nonesterified fatty acids (NEFAs) to distinguish them from triglyceride fatty acids in chylomicrons and lipoproteins. NEFAs in blood may be used directly for energy by many tissues. However, many NEFAs are taken up by the liver and used for either ketone body production or VLDL synthesis, as discussed in the next section.
NUTRIENT UTILIZATION DURING PROLONGED ENERGY MALNUTRITION OR COMPLETE FOOD DEPRIVATION
During Prolonged Periods of Fasting or Undernutrition, Glucose and Amino Acids Are Conserved by Extensive Utilization of Fats and Ketone Bodies for Energy Production
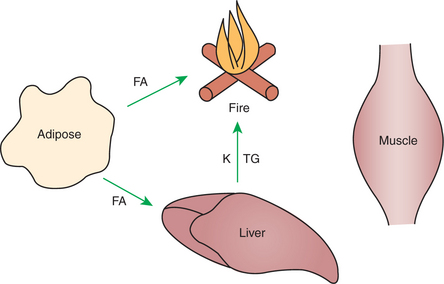
From the previous discussion of postabsorptive metabolism, you can appreciate that amino acids form an important depot for glucose precursors and energy-producing substrate. During prolonged fasting or undernutrition, however, it would not be advantageous for animals to rely heavily on their skeletal muscle for energy and glucose production; doing so would soon lead to severe weakness as the skeletal muscle protein was consumed. Thus, protective mechanisms have developed by which skeletal muscle is preserved during periods of insufficient energy intake. In utilization of stored fuels, shifts away from glucose and toward adipose fat stores are necessary for protein sparing. Figure 32-15 illustrates the general scheme of metabolism during prolonged catabolic periods.
A Large Portion of the Fatty Acids Released from Adipose Tissue Is Taken up Directly by the Liver
During prolonged periods of undernutrition, low glucose availability leads to rapid mobilization of adipose fatty acids in the form of NEFAs. Although NEFAs are metabolized by various tissues, many are extracted from the blood by the liver, which receives much of the total blood flow and has an efficient hepatic NEFA extraction mechanism. Once the NEFAs are in the hepatocytes, they may follow any of three potential metabolic paths. The first is complete oxidation for energy production; however, the hepatic requirements for energy are such that only a small amount of the total fatty-acid supply during adipose mobilization needs to be used for complete oxidation. The second pathway is esterification leading to triglyceride formation, and the third is production of ketone bodies. Triglyceride synthesis is discussed later; the focus here is ketone body production.
Hepatic Ketone Body Formation Is Promoted by Low Glucose Availability, a High Glucagon/Insulin Ratio, and a Ready Supply of Fatty Acids
Ketone body formation occurs within the hepatic mitochondria, and the rate of ketone body synthesis is controlled by the regulated transport of fatty acids across the mitochondrial membrane (Figure 32-16). Fatty acids enter mitochondria in combination with a molecule known as carnitine, and transport depends on an enzyme known as carnitine palmitoyltransferase I (CPT-I). The activity of this enzyme, along with the availability of fatty acid, is the primary determinant of the rate of ketone body formation. CPT-I activity is regulated in an interesting manner, being inhibited by an intermediate of the fatty-acid synthesis pathway, malonyl CoA. Malonyl CoA concentrations are high when the liver is responding to insulin and glucose is being used for fatty-acid synthesis. When glucagon concentrations are high relative to insulin, little fatty acid is synthesized in the liver. Thus, malonyl CoA concentrations are low, and CPT-I is fully active when the insulin/glucagon ratio is low. Ketene body synthesis is stimulated under these hormonal conditions.
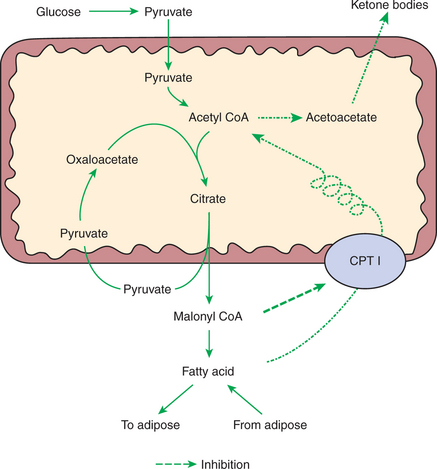
FIGURE 32-16 The liver is a site of both destruction and synthesis of fatty acids. To keep both processes from occurring simultaneously, fatty-acid destruction is inhibited during periods of fatty-acid synthesis. Solid lines, Pathway of fatty-acid synthesis; irregular broken line, fatty-acid destruction. Oxidative destruction is suppressed by the action of malonyl CoA, an intermediate in the synthesis of fatty acid. Malonyl CoA blocks the transport of fatty acids into the mitochondria at the translocation enzyme carnitine palmitoyltransferase I (CPT I).
Under conditions when CPT-I is active, most available fatty acid is transported into the mitochondria for ketone body synthesis. This well-orchestrated but somewhat complex regulatory system is important because the liver can both produce and consume fatty acids. If there were not a way of “turning off” fatty-acid destruction during periods of synthesis, a futile cycle of synthesis and destruction would occur. The inhibition of CPT-I by malonyl CoA provides a system that blocks the metabolic destruction of newly synthesized fatty acid while still providing a mechanism for the utilization of fatty acids derived from adipose tissue. The overall pattern of metabolism results in a reciprocal relationship between glucose availability and ketone body production. Although ketone bodies are produced in the liver, they cannot be used there for energy production. Therefore, all ketone bodies are transported to peripheral tissues for utilization. When the concentration of ketone bodies in the blood becomes abnormally high, some are excreted in urine.
Glucagon Plays an Important Role in the Excessive Production of Ketone Bodies in Diabetes Mellitus
If untreated, diabetes mellitus in animals, especially dogs, leads to high concentrations of ketone bodies in the blood. Diabetes mellitus occurs because of a lack of insulin, but the hepatic production of ketone bodies results from the unrestrained action of glucagon. Even though serum concentrations of glucose are high in diabetes mellitus, the inability of the pancreas to secrete insulin leads to a low insulin/ glucagon ratio; thus the liver is functioning solely under the direction of glucagon. Glucagon inhibits fatty-acid production from glucose, so malonyl CoA concentrations are low and CPT-I activity is high. Because of the lack of insulin to suppress adipose HSL, blood NEFA concentrations are high. The combination of high NEFA availability and unrestrained CPT-I activity results in rapid transport of fatty acids into the mitochondria with extensive ketone body production, even though blood glucose concentrations are high.
Fatty Acids Cannot Be Used for Glucose Synthesis
It is important to understand that the metabolism of fat within the mitochondria cannot contribute directly to gluconeogenesis. Once across the mitochondrial membrane, fatty acids undergo β-oxidation, which leads to the successive removal of two-carbon acetyl CoA units from the carbon chains of the fatty acids. The resulting acetyl CoA can enter the Krebs cycle through condensation with oxaloacetate. Because any of the Krebs cycle intermediates can lead to glucose production, it may first appear that acetyl CoA from fatty-acid β-oxidation could lead to the production of glucose. However, this is not the case; there is no net production of oxaloacetate associated with the consumption of acetyl CoA by the Krebs cycle (Figure 32-17). Existing oxaloacetate combines with acetyl CoA to form citrate in the initial step of the cycle. At the end of the cycle, the original oxaloacetate is re-formed as the two carbons from the acetyl CoA are converted to carbon dioxide. No new oxaloacetate can be produced by this process.
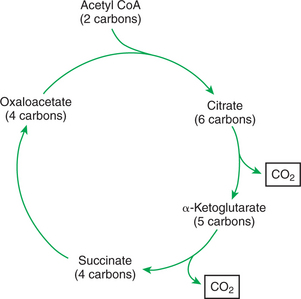
FIGURE 32-17 Oxidation of acetyl CoA (from acetate) by the Krebs cycle. The two carbons of acetyl CoA result in the formation of carbon dioxide; there is no net synthesis of oxaloacetate. Because oxaloacetate forms the precursor for glucose synthesis, acetyl CoA (and thus acetate) cannot lead to glucose formation.
Ketone Bodies Are Formed in the Mitochondria from Acetyl Coenzyme A
Not all mitochondrial acetyl CoA must enter the Krebs cycle. In fact, when fatty acids are rapidly entering the mitochondria, there is much more acetyl CoA available than necessary for Krebs cycle activity. It is this excess acetyl CoA, originating from fatty acids, from which the ketone bodies are synthesized (see Figure 32-16). Ketone bodies are able to leave the mitochondria freely.
Ketone bodies affect fuel homeostasis in peripheral tissues, where they may serve as a substitute for glucose. In this way, they conserve available glucose and reduce the need for gluconeogenesis.
Hepatic Very-Low-Density Lipoproteins May Be Synthesized from Adipose-Derived Fatty Acid as Well as from Newly Synthesized Fatty Acid
The section on absorptive-phase metabolism discusses the hepatic production of VLDL. During the absorptive phase, triglyceride for VLDL synthesis comes from fatty acids synthesized from glucose. During catabolic periods, VLDLs may continue to be produced, but fatty acids derived from serum NEFAs are used for VLDL synthesis (see Figure 32-5). This may initially appear to be an unnecessary and inefficient metabolic step. Why should fatty acids from adipose tissue be transported to the liver for VLDL formation when they can be directly metabolized for energy by the tissues? The need for VLDL synthesis occurs because of the need for a better transport system. The capacity of the serum to transport NEFA is limited because NEFA must circulate bound to albumin. The NEFA-binding capacity of albumin is finite and may become almost saturated during periods of rapid adipose mobilization. VLDLs provide a transport system for fatty acids that is independent of serum albumin.
Hormonal Conditions Direct the Distribution of Very-Low-Density Lipoprotein Fatty Acids in the Body
During the absorptive phase, VLDLs are directed to adipose tissue by the action of adipose tissue LPL, an insulin-stimulated enzyme. LPL also exists in muscle tissue, but does not depend on insulin stimulation for activity. Thus, during periods of low glucose availability, adipose tissue LPL is inhibited because of a lack of insulin, but muscle tissue LPL is fully active. This situation leads to the selective direction of VLDL fatty acids to muscle tissue during times of adipose mobilization.
Changes in Growth Hormone Concentrations May Aid in Shifting Peripheral Fuel Utilization from Glucose and Amino Acids to Ketone Bodies and Fatty Acids
The fat mobilization–induced changes in hepatic metabolism are effective in conserving protein only because of changes that occur in glucose and amino acid utilization in peripheral tissues. As ketone bodies, NEFAs, and VLDL triglycerides become the major energy supplies, there is less tissue demand for glucose or amino acids as energy substrates. Endocrine alterations, in addition to low insulin concentrations, may aid in promoting this switch in peripheral fuel utilization. In several species, growth hormone concentrations rise during a prolonged period of energy deprivation. Growth hormone is antagonistic to insulin, thus promoting an increase in the serum glucose concentration even in the presence of normal or near-normal serum insulin levels. In addition, growth hormone may have some direct effect on conserving protein and mobilizing lipid.
SPECIAL FUEL CONSIDERATIONS OF RUMINANTS
Ruminants Exist in a Perpetual State of Gluconeogenesis Because of Their Unique Digestive Process
Most carbohydrate digestion in ruminants occurs in the forestomach through fermentative digestion. The result is that almost no digestible carbohydrate enters the intestine for glandular digestion and absorption as glucose. Therefore, ruminants exist in a constant state of potential glucose deficiency. To cope with this situation, ruminants have developed efficient systems of both production and conservation of glucose.
Essentially, all the glucose available to ruminants with typical diets originates from gluconeogenesis. Quantitatively, the most important glucose precursor is the volatile fatty acid (VFA) propionate. Propionate contributes to glucose synthesis after entering the Krebs cycle at the level of succinate (Figure 32-18). Note that succinate is a four-carbon Krebs cycle intermediate that can lead to net formation of oxaloacetate, the entry metabolite for gluconeogenesis. The other VFAs, acetate and butyrate, also enter the Krebs cycle, although they enter as acetyl CoA. As previously discussed, acetyl CoA cannot lead to the net production of oxaloacetate or glucose. Therefore, of the ruminant’s major energy sources—acetate, propionate, and butyrate—only propionate can support glucose production. Almost all propionate absorbed from the rumen is extracted from the portal blood by the liver, never entering the systemic circulation.
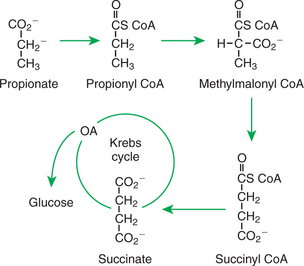
FIGURE 32-18 Gluconeogenesis from propionate involves its initial conversion to succinate. Succinate is a four-carbon Krebs cycle intermediate that can lead to net glucose synthesis.
In addition to constant gluconeogenesis, ruminants also support their glucose needs by efficiently conserving glucose. Fatty acids are synthesized in the liver of some animals (e.g., primates, rats, dogs) but only in the adipose tissue of ruminants. Furthermore, glucose is essentially not used for fatty-acid synthesis. Rather, fatty acids are synthesized from acetate, which is the most abundant energy source in ruminants. The only glucose used by adipose tissue is for the synthesis of the glycerol backbone for triglycerides. In lactating animals, fatty acids produced in the udder for milk fat are synthesized from either acetate or ketone bodies, never from glucose.
Some important metabolic diseases of ruminants occur during periods when their system of glucose homeostasis is stressed. Dairy cows are especially vulnerable in early lactation because the synthesis of lactose (milk sugar) requires glucose. In high-producing cows, nearly all the glucose they produce goes to lactose synthesis, whereas the remaining tissues function on alternative fuels. Sheep experience a similar stress on glucose synthesis in late gestation. The energy needs of the fetus and placenta can be met only by glucose (or glucose-derived lactate) and amino acids. Compared with many other animals, sheep have a high ratio of fetal mass to body size; thus their fuel homeostatic mechanisms are particularly stressed by pregnancy. Failure of the glucose homeostatic mechanism frequently occurs under these circumstances, resulting in conditions known as lactational ketosis in dairy cows and pregnancy toxemia in ewes.
CLINICAL CORRELATIONS
Hepatic Lipidosis in a Cat
History.
You are asked to examine a 3-year-old intact female cat. She apparently had been normal, even fat and happy, until 2 weeks ago, when she disappeared from her owner’s apartment for 4 days. When she returned, the cat seemed depressed and would not eat. Over the next few days she became progressively more listless, almost somnolent.
Clinical and Laboratory Examination.
The cat has a normal pulse, temperature, and respiratory rate, but she is depressed and responds little to handling. The ocular sclerae (whites of the eyes) appear icteric, or jaundiced. The latter physical sign leads you to suspect liver disease, so you submit blood samples for biochemical analysis. Analysis of blood taken from the jugular vein reveals a higher-than-normal concentration of bile acids and bilirubin, confirming a diagnosis of liver disease. A needle-aspiration biopsy of the liver reveals hepatocytes that are distended with large droplets of nonstaining material, probably fat.
Comment.
The presence of significant concentrations of bile acids in blood, other than in the hepatic-portal circulation, is evidence of reduced liver function. Recall that bile acids are absorbed from the ileum into the portal vein, in which they return to the liver. The normal liver extracts bile acids from portal blood efficiently, allowing only small amounts to escape into the systemic circulation; thus, elevated concentrations of bile acids in jugular blood indicate liver disease.
Hepatic lipidosis, or “fatty liver,” is a common disease of cats, initiated by a period of stress combined with either an unwillingness to eat or a lack of available food. In either situation the cat begins to mobilize large quantities of fat to support metabolic energy needs. Normally, much of the mobilized NEFA would be expected to be taken up by the liver and converted to VLDL for export to energy-using tissues. In cats that experience fatty liver, the hepatic influx of NEFA appears to overwhelm the liver’s capacity to synthesize and secrete VLDL, so fat accumulates in the liver. When the fat accumulation becomes severe, hepatic function is compromised, and the cat becomes systemically ill. Appetite becomes severely depressed, leading to a downward spiral of events in which the hepatic lipidosis becomes more and more severe.
Treatment.
Treatment consists of reversing the state of negative energy balance by force-feeding. Various methods of force-feeding exist; the most practical approach is placement of an indwelling gastric tube. The tube is often passed through the nostrils but may be placed by a number of different techniques, including direct intubation through the wall of the abdomen. The latter technique is facilitated by the use of a fiberoptic gastroscope. Once the cat is in positive energy balance, adipose mobilization ceases, and the liver eventually clears of fat. Tube feeding may need to continue for several days before the cat begins to eat on its own. Tube feeding has greatly improved the prognosis for hepatic lipidosis, although it is still a life-threatening condition.
Bauman DE, Currie WB. Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorrhesis. J Dairy Sci. 1980;63:1514.
Bender DA. Introduction to nutrition and metabolism, ed 2. Bristol, Pa: Taylor & Francis, 1997.
Brody T. Nutritional biochemistry, ed 2. San Diego: Academic Press, 1999.
Cahill GF. Starvation in man. Clin Endocrinol Metab. 1976;5:397.
DeGroot LJ, ed. Endocrinology. Philadelphia: Saunders, 1989.
Gillham B, Papachristodoulou DK, Thomas JH. Will’s bio-chemical basis of medicine, ed 3. Boston: Butterworth-Heinemann, 1996.
Herdt TH. Fuel homeostasis in the ruminant. Vet Clin North Am Food Anim Pract. 1988;4:213.
Kaneko JJ, Harvey JW, Bruss ML. Clinical biochemistry of domestic animals, ed 5. San Diego: Academic Press, 1997.
Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999;19:379.
Storey KB, ed. Functional metabolism: regulation and adaptation. Ottawa: John Wiley & Sons, 2004.
PRACTICE QUESTIONS
1. All the following are metabolites that can be oxidized for fuel in the animal body. Which one is not important in the transport of energy between organs and organ systems?
2. Which of the following reactions is not characteristic of the absorptive phase of digestion?
3. Which of the following reactions in the liver could be expected to occur during both the digestive phase and a prolonged fast?
4. Which of the following statements is true of both ketone bodies and nonesterified fatty acids?
5. Which of the following amino acids is not extensively catabolized by the liver?