Chapter 30 Digestion and Absorption: the Nonfermentative Processes
1. Digestion and absorption are separate, but related, processes.
2. The small-intestinal mucosa has a large surface area and epithelial cells with “leaky” junctions between them.
3. The intestinal surface microenvironment consists of glycocalyx, mucus, and an unstirred water layer.
1. Breaking down food particle size by physical action is an important part of the digestive process.
2. Chemical digestion results in the reduction of complex nutrients into simpler molecules.
3. Luminal-phase carbohydrate digestion results in the production of short-chain polysaccharides.
4. Luminal-phase digestion of carbohydrates applies only to starches, because sugars are digested in the membranous phase.
5. Proteins are digested by a variety of luminal-phase enzymes.
6. Membranous-phase digestive enzymes are a structural part of the intestinal surface membrane.
7. Membranous-phase digestion occurs within the microenvironment of the unstirred water layer, intestinal mucus, and glycocalyx.
8. A specific membranous-phase enzyme exists for the digestion of each type of polysaccharide.
9. Complete digestion of peptides to free amino acids takes place both on the enterocyte surface and within the cells.
1. Specialized nutrient transport systems exist in the apical and basolateral membranes.
2. Secondary and tertiary active transport mechanisms utilize the transcellular sodium ion electrochemical gradient as their source of energy.
3. Passive transport occurs either through ion channels in cell membranes or directly through the tight junctions.
4. The products of membranous-phase digestion are absorbed by sodium co-transport.
Absorption of Water and Electrolytes
1. There are at least three distinct mechanisms of sodium absorption.
2. There are three major mechanisms of chloride absorption.
3. Bicarbonate ion is secreted by several digestive glands and must be recovered from the gut if body acid-base balance is to be maintained.
4. Potassium is absorbed primarily by passive diffusion through the paracellular route.
5. The major mechanisms of electrolyte absorption are selectively distributed along the gut.
6. All intestinal water absorption is passive, occurring because of the absorption of osmotically active solutes.
Intestinal Secretion of Water and Electrolytes
1. Passive increases in luminal osmotic pressure occur during hydrolytic digestion and result in water secretion.
2. Active secretion of electrolytes from the crypt epithelium leads to intestinal water secretion.
1. Water and solute movement between the lateral spaces and villous capillaries is subject to the same forces that govern water and solute movement between the extracellular and vascular fluids in other tissues.
2. Absorbed nutrients enter the capillaries by diffusion from the lateral spaces.
3. A countercurrent, osmotic-multiplier system may increase the osmolality of blood at the tips of the villi, further promoting absorption of water into the blood.
4. Disturbances in the venous drainage from the intestine can greatly affect the mechanisms of capillary absorption in the villi.
Digestion and Absorption of Fats
1. Detergent action as well as enzymatic action is necessary for the digestion and absorption of lipids.
2. Lipids are absorbed through the apical membrane by carrier proteins and simple diffusion.
3. Bile acids are reabsorbed from the ileum by a sodium co-transport system.
4. Absorbed lipids are packaged into chylomicrons before leaving the enterocytes.
Growth and Development of the Intestinal Epithelium
1. The length of intestinal villi is determined by the relative rates of cell loss at the tips and cell replenishment at the base.
Digestion and Absorption Are Separate, but Related, Processes
Digestion is the process of breaking down complex nutrients into simple molecules. In contrast, absorption is the process of transporting those simple molecules across the intestinal epithelium (Figure 30-1). The two processes are the result of different biochemical events occurring within the gut. Both processes are necessary for the assimilation of nutrients into the body; absorption cannot occur if food is not digested, and the process of digestion is fruitless if the digested nutrients cannot be absorbed.
FIGURE 30-1 Digestion is the process of reducing macromolecules to their constituent monomers. Absorption is the transport of the resultant monomers across the intestinal epithelium into the bloodstream
Disturbances of nutrient assimilation are common in veterinary medicine and may be caused by a variety of diseases, with some affecting digestion and others affecting absorption. The overt signs of failure of nutrient assimilation are often similar, but the biochemical lesions and specific therapies associated with maldigestive disease can be quite different from those associated with malabsorptive disease. Therefore, diagnosing the cause of failure of nutrient assimilation is a frequent challenge faced by veterinary clinicians, a challenge that requires a thorough understanding of the physiology of nutrient digestion and absorption. This chapter first reviews the structural characteristics of the small-intestinal epithelium that are of particular importance to the digestive and absorptive processes.
The Small-Intestinal Mucosa Has a Large Surface Area and Epithelial Cells with “Leaky” Junctions Between Them
Contact between the small-intestinal mucosa and the luminal contents is facilitated by an extensive intestinal surface area. Three levels of surface convolutions serve to expand the surface area of the small intestine (see Figure 27-2). First, large folds of mucosa known as plicae circulares add to the intestinal surface area of some animals, but these are not present in all species. Second, the mucosal surface is covered with fingerlike epithelial projections known as villi. These structures are present in all species and increase the intestinal surface area by 10-fold to 14-fold compared with a flat surface of equal size. Third, the villi themselves are covered with a brushlike surface membrane known as the brush border. The brush border is composed of submicroscopic microvilli that further enlarge the surface area (Figure 30-2). At the base of the villi are glandlike structures known as crypts of Lieberkühn (Figure 30-3). The villi and crypts are covered with a continuous layer of cellular epithelium.
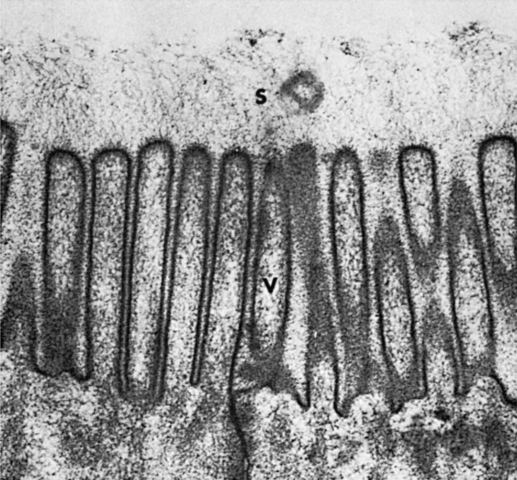
FIGURE 30-2 Electron micrograph of the microvilli of the intestinal brush border. The brush border is composed of the apical membranes of enterocytes. Note the indistinct array of molecular material (S) that radiates away from the microvilli (V); membranous-phase digestion occurs within this array of molecular material, which includes the membrane-bound digestive enzymes.
(From Johnson LR, Christensen J, Jacobsen ED, et al, editors: Physiology of the gastrointestinal tract, ed 2, New York, 1987, Raven Press.)
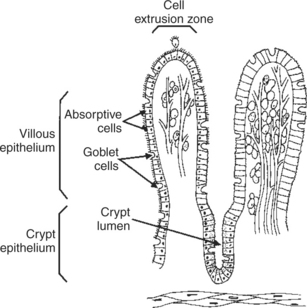
FIGURE 30-3 The one-cell-thick layer of intestinal epithelium is continuous over the villi and the crypts of Lieberkühn.
The epithelial cells covering the villi and crypts are called enterocytes. Each enterocyte has two distinct types of cell membranes (Figure 30-4). The cell surface facing the lumen is called the apex and is covered by the apical membrane. The apical membrane contains the microvilli. Under the light microscope, the microvilli give the cell surface its brushlike appearance (brush border, synonymous with apical membrane). Covering the apical membrane, and encasing the microvilli, is a jellylike layer of glycoprotein known as the glycocalyx. Important digestive enzymes and other proteins are attached to the microvilli and project into the glycocalyx. Under the intense magnification of the electron microscope, these enzyme molecules, as well as other proteins, give the glycocalyx a fuzzy appearance (see Figure 30-2). The apical membrane is a complex and unusual cellular membrane with high protein content.
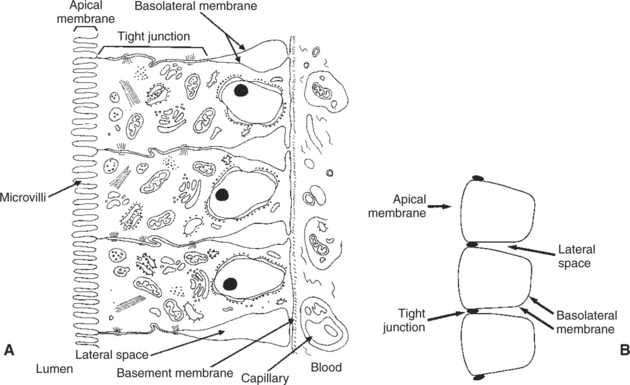
FIGURE 30-4 Understanding the anatomical relationships of the enterocytes, tight junctions, apical membrane, basolateral membrane, and lateral spaces is critical to an understanding of the physiology of intestinal absorption. A, Anatomic illustration of the intestinal epithelium. B, Stylized sketch of the epithelium, including a capillary containing formed elements of blood. It is important to understand the relationship between part A and part B of this diagram
The remaining portion of the enterocyte plasma membrane, that part not facing the gut lumen, is called the basolateral membrane, referring to the base and sides of the cell. This membrane is not especially unusual and indeed has many similarities to cell membranes of other tissues. Although the basolateral membrane is not in direct contact with ingesta in the gut lumen, it serves an important role in intestinal absorption; nutrients that are absorbed into the enterocytes through the apical membrane must exit the cell through the basolateral membrane before gaining access to the bloodstream.
The attachments between adjacent enterocytes are called tight junctions. These connections serve a special function in the process of digestion and absorption. The tight junctions form a narrow band of attachment between adjacent enterocytes. The band is near the apical end of the cells and divides the apical membrane from the basolateral membrane. The junctions may be called “tight,” but from a molecular standpoint, they are rather loose. This is especially true in the duodenum and jejunum, where the tight junctions are loose enough to allow the free passage of water and small electrolytes. Recent evidence indicates that the relative impermeability, or “tightness,” of the tight junctions is not constant and can be altered by neurohumoral regulatory substances in the gut. These selective changes in permeability can affect the rates of water and ion movement across the gastrointestinal (GI) epithelium, depending on the physiological needs for secretion or absorption. However, the tight junctions are never permeable enough to permit the passage of organic molecules.
The narrow band of tight junctions leaves the majority of the basolateral membrane unattached to its neighboring membrane on the adjacent enterocyte. This arrangement creates a potential space between enterocytes. This area between the lateral surfaces of the enterocytes is called the lateral space. The lateral spaces are normally distended and filled with extracellular fluid (ECF). At the end of the lateral spaces nearest the apical membrane, the ECF is separated from the fluid of the intestinal lumen only by the tight junctions. At the opposite end of the lateral spaces, the ECF is separated from the blood only by the basement membrane of the intestinal capillaries. Both the tight junctions and the capillary endothelium are permeable barriers that allow the free passage of water and small molecules. Thus, there is relatively free flow of water and most electrolytes among the fluid in the lumen of the intestine, the ECF in the lateral spaces, and the blood.
The Intestinal Surface Microenvironment Consists of Glycocalyx, Mucus, and an Unstirred Water Layer
Liberally interspersed among the enterocytes are goblet cells, which secrete a rich layer of mucus that covers the mucosa. At the brush border surface, the mucus blends into the glycocalyx, with the two layers forming a viscous coating that tends to trap molecules near the apical membrane. In addition to the mucus and glycocalyx layers, there is an area near the intestinal surface known as the unstirred water layer. With respect to the unstirred water layer, the intestine can be likened to a large stream or river; that is, the water in the center flows relatively rapidly, whereas the water near the edge or bank is quiet and flows slowly. Because of the same fluid-friction phenomenon that causes the water on the banks of rivers to be less turbulent and to flow at a slower rate than water in center stream, the water very near the intestinal surface is quiet and flows at a much slower rate than water in the central part of the lumen. The unstirred water layer, mucus, and glycocalyx form an important diffusion barrier through which nutrients must pass before entering the enterocytes.
DIGESTION
Breaking Down Food Particle Size by Physical Action Is an Important Part of the Digestive Process
The overall process of digestion is the physical and chemical breaking down of food particles and molecules into subunits suitable for absorption. Physical reduction of food particle size is important not only because it allows food to flow through the relatively narrow digestive tube, but also because it enlarges the surface area of the food particles, thus increasing the area exposed to the actions of digestive enzymes. Physical reduction of food particle size begins with mastication (chewing) but is completed by the grinding action of the distal stomach. In the distal stomach the physical action of grinding is aided by the chemical actions of pepsin and hydrochloric acid. The chemical actions of these stomach secretions break down connective tissue and thus aid in breaking apart food particles, especially foods of animal origin. The reduction of food particle size by physical means is essentially complete when food leaves the stomach, as described in Chapter 28 in the discussion of motility of the distal stomach.
Chemical Digestion Results in the Reduction of Complex Nutrients into Simpler Molecules
Chemical digestion of each major nutrient is accomplished by the process of hydrolysis, the splitting of a chemical bond by the insertion of a water molecule. Glycosidic linkages in carbohydrates, peptide bonds in proteins, ester bonds in fats, and phosphodiester bonds in nucleic acids are all cleaved by hydrolysis during digestion. Figure 30-5 illustrates hydrolytic splitting of these various chemical bonds.
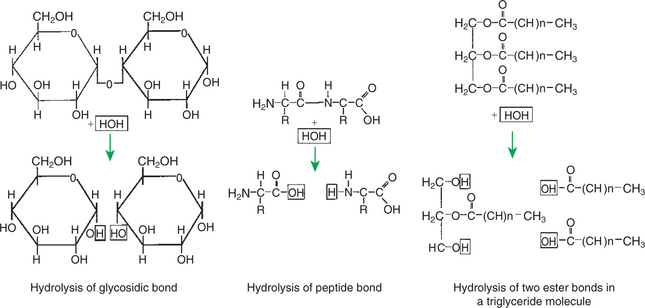
FIGURE 30-5 Major polymeric molecules forming food nutrients can be split into their constituent monomers by the insertion of a water molecule. This process, referred to as hydrolysis, is the major action of the digestive enzymes.
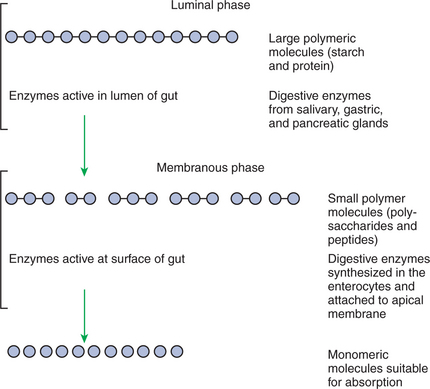
Hydrolysis in the digestive tract is catalyzed by the action of enzymes. There are two general classes of digestive enzymes: those that act within the lumen of the gut, and those that act at the membrane surface of the epithelium. Enzymes acting within the lumen originate from the major GI glands, including the salivary gland, gastric glands, and especially the pancreas. The secretions of these glands become thoroughly mixed with ingesta and exert their actions throughout the lumen of their associated gut segments; thus the actions they catalyze are referred to as the luminal phase of digestion. In general, luminal-phase digestion results in the incomplete hydrolysis of nutrients, resulting in the formation of short-chain polymers from the original macromolecules (Figure 30-6).
The hydrolytic process is completed by enzymes that are chemically bound to the surface epithelium of the small intestine. These enzymes break the short-chain polymers resulting from luminal-phase digestion into monomers that can be absorbed across the epithelium. This final phase, which occurs at the epithelial membrane surface, is referred to as the membranous phase of digestion. Membranous-phase digestion is followed closely by absorption.
Luminal-Phase Carbohydrate Digestion Results in the Production of Short-Chain Polysaccharides
Carbohydrates are nutrients containing carbon, hydrogen, and oxygen atoms arranged as long chains of repeating simple-sugar molecules. Dietary carbohydrates originate primarily from plants. There are three general types of plant carbohydrates: fibers, sugars, and starches. Fibers, the structural parts of plants, form an important energy source for herbivorous animals; however, plant fibers are not subject to hydrolytic digestion by mammalian enzymes and therefore cannot be digested directly by animals (see Chapter 31).
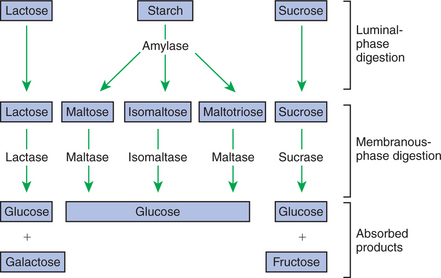
Sugars are energy-transport molecules in plants. Sugars, or saccharides, may be simple (made up of a single molecular unit, monosaccharides) or complex (made up of two or more repeating saccharide subunits, polysaccharides). Glucose, galactose, and fructose are the most important simple sugars in animal diets. These monosaccharides are present, preformed in small quantities, in normal diets; however, most monosaccharides absorbed from the gut arise from the enzymatic hydrolysis of more complex carbohydrates. Complex sugars are referred to as disaccharides, trisaccharides, and oligosaccharides, depending on the number of repeating simple-sugar subunits. Oligosaccharides contain several monomer units, usually between 3 and 10. Important complex sugars in animal diets are lactose, or milk sugar, and sucrose, or table sugar. Lactose is a disaccharide composed of glucose and galactose, whereas sucrose is a disaccharide composed of glucose and fructose. Other important complex sugars are maltose, isomaltose, and maltotriose; these three sugars are composed of two or three repeating glucose units (Figure 30-7). They are seldom present preformed in the diet but rather are formed in the gut as intermediate products of starch digestion.
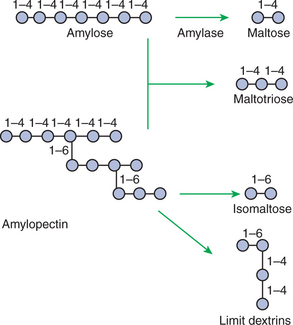
FIGURE 30-7 Two major forms of dietary starch are amylose and amylopectin. Amylose is composed of repeating glucose units joined by α[1–4] linkages. Amylopectin is a similar molecule, except that it has branch points formed by α[1–6] linkages. Because of the different linkage points, various polysaccharides result from luminal-phase digestion, as illustrated
Starch is an energy-storage carbohydrate of plants that forms the major energy-yielding nutrient in the diets of many omnivorous animals, such as pigs, rats, and primates. There are two chemical forms of starch, amylose and amylopectin. Both are long-chain glucose polymers, but amylose is a straight-chain molecule containing glucose monomers linked by α[1–4] glycosidic linkages. Amylopectin also contains glucose chains joined by α[1–4] glycosidic linkages, but the amylopectin chains are branched, having an α[1–6] linkage at each branch point (Figure 30-7). Although the chemical structure of starch is limited to these two molecular types, the physical structure and encapsulation of starches vary among plant sources. This variation results in the unique characteristics of starches from different sources, such as wheat, corn, and barley.
Luminal-Phase Digestion of Carbohydrates Applies Only to Starches, Because Sugars Are Digested in the Membranous Phase
The enzyme involved in luminal starch digestion is α-amylase, which is actually a mixture of several similar molecules. This enzyme arises from the pancreas in all species, as well as from the salivary glands of some species (see Chapter 29). The α[1–4] linkages of either amylose or amylopectin are attacked by α-amylase. Characteristic of luminal-phase digestion, α-amylase does not break off, or cleave, single glucose units from the ends of the chain. Rather, the starch chains are broken in their midsections, resulting in the production of polysaccharides of intermediate chain length, known as dextrins. These chains continue to be attacked until disaccharide (maltose) and trisaccharide (maltotriose) units are formed.
This digestive process proceeds for amylopectin in the same way as it does for amylose, except that the α[1–6] linkages at the chain branch points of amylopectin are not hydrolyzed. Because these branch points are not hydrolyzed, branch-chain oligosaccharides, known as limit dextrins, as well as an α[1–6]-linked disaccharide, known as isomaltose, are formed (see Figure 30-7). The end result of luminal-phase carbohydrate digestion is the creation of many disaccharides, trisaccharides, and oligosaccharides from large starch molecules. These complex sugars are not hydrolyzed further in the luminal phase.
Proteins Are Digested by a Variety of Luminal-Phase Enzymes
Proteins are a source of amino acids, which are essential components of all animal diets. Dietary proteins come from both plant and animal sources. The general pattern of protein digestion is similar to that of carbohydrate digestion, in that large molecular proteins are broken down into small peptide chains by luminal digestion. Subsequent digestion of the peptide chains to individual amino acids occurs to a large extent by membranous-phase digestion, although unlike in carbohydrate digestion, a portion of free monomers, that is, amino acids, is released in the luminal phase.
A major difference between protein digestion and carbohydrate digestion is in the number of different enzyme types involved. The relatively larger number of enzymes involved in protein digestion is expected, considering that starch molecules are made up of only one type of monomer, glucose. Therefore, only bonds between glucose molecules need to be broken. On the other hand, proteins are made up of infinite combinations of up to 20 individual types of amino acids; the various proteolytic enzymes are necessary for digestion because they differ in their efficiency in cleaving peptide bonds between specific types of amino acids.
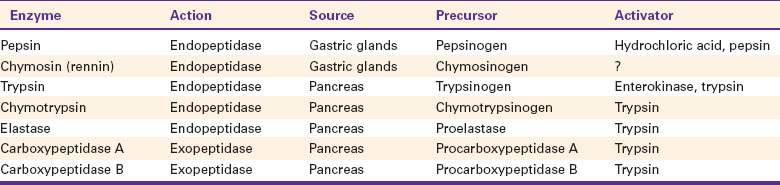
The major luminal-phase proteolytic enzymes are listed in Table 30-1. Most proteolytic enzymes are endopeptidases, meaning that they break proteins at internal points along the amino acid chains, resulting in the production of short-chain peptides from complex proteins. Endopeptidases produce essentially no free amino acids. Two exopeptidases, which release individual amino acids from ends of peptide chains, are secreted also from the pancreas and are active in luminal-phase digestion.
The proteolytic enzymes are secreted from the stomach glands or pancreas in the form of inactive zymogens (see Chapter 29), which are activated in the stomach or intestinal lumen, respectively. These enzymes must be secreted in an inactive form; otherwise, the active enzymes would digest the cells in which they are synthesized. Activation of the zymogens occurs in the gut lumen. The stomach enzymes pepsinogen and chymosinogen are activated by hydrochloric acid (HCl) in the stomach. Pepsinogen is also activated by pepsin in an autocatalytic feedback loop. Trypsinogen from the pancreas is activated by enterokinase, an enzyme elaborated by duodenal mucosal cells. The active enzyme, trypsin, then serves as an autocatalytic agent to activate additional trypsinogen as well as the other pancreatic protein-digesting enzymes. Figure 30-8 illustrates the cascade of intraluminal zymogen activation.
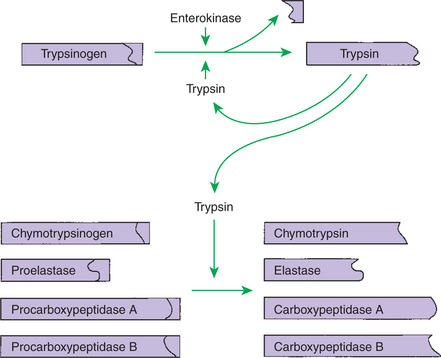
FIGURE 30-8 Activation of pancreatic zymogens. Note that trypsinogen is activated by trypsin as well as by the duodenal enzyme enterokinase. The autocatalytic action of trypsin on trypsinogen forms a positive feedback loop that ensures the rapid and complete activation of trypsinogen in the gut. Trypsin then activates the other zymogens.
Luminal-phase protein digestion begins in the stomach. Gastric digestion of protein is facilitated not only by the stomach enzymes but also by HCl, which has hydrolytic properties of its own. The acid environment of the stomach is suited to the action of pepsin, which has its optimal activity at pH 1 to 3. Gastric hydrolysis of protein is probably important to the physical as well as the chemical digestion of protein, because most connective tissue of animal origin is protein; digestion of connective tissue aids in breaking food down into particles small enough to pass the pylorus. Although stomach action is important in initiating protein digestion, it is not essential; animals without stomachs can digest proteins, provided they have a functional pancreas and are fed small, frequent meals of soft, moist food. Luminal-phase digestion of proteins is completed in the small intestine by the action of pancreatic enzymes.
Membranous-Phase Digestive Enzymes Are a Structural Part of the Intestinal Surface Membrane
Membranous-phase digestion, as with its luminal counterpart, occurs because of the hydrolytic action of enzymes. The difference between the two phases is that membranous-phase enzymes are chemically bound to the surface membrane of the intestine; thus the enzyme substrates must be in contact with the epithelium before hydrolysis can occur. These membrane-bound digestive enzymes are synthesized within the enterocytes and are subsequently transported to the luminal surface of the apical membrane. They remain attached to the surface by a short anchor segment while the large, catalytic portion of the enzyme molecule projects away from the surface, toward the gut lumen.
Membranous-Phase Digestion Occurs Within the Microenvironment of the Unstirred Water Layer, Intestinal Mucus, and Glycocalyx
As previously described, the unstirred water layer, mucus, and glycocalyx form a diffuse zone separating the mucosal surface from the lumen of the intestine. The membranous-phase digestive enzymes project from the apical membrane into this surface layer. The quiet surface layer forms a microenvironment in which membranous-phase digestion occurs. Peptides and polysaccharides in the intestinal lumen must diffuse into the surface layer before membranous-phase digestion can take place. Furthermore, most of the products of membranous-phase digestion never diffuse away from the surface environment back into the lumen of the intestine; instead, they are absorbed, soon after formation, into the underlying epithelial cells. This arrangement is efficient because it ensures that the final products of carbohydrate and protein digestion are formed near their site of absorption, avoiding the need for long diffusion distances (Figure 30-9).
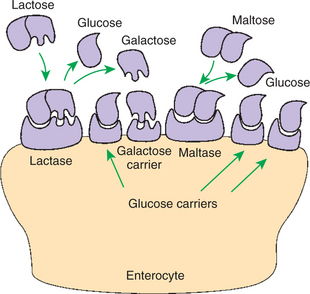
FIGURE 30-9 Relationship of membranous-phase digestion to absorption. The enzymes responsible for digestion and the carrier molecules responsible for absorption are both part of the apical membrane. The products of digestion are thus formed in the immediate vicinity of the carrier proteins, avoiding long diffusion distances. Specific enzymes and carrier molecules are present for the various substrates, as illustrated
A Specific Membranous-Phase Enzyme Exists for the Digestion of Each Type of Polysaccharide
The membranous-phase enzymes of carbohydrate digestion have as their substrates dietary complex carbohydrates, such as sucrose and lactose, as well as the polysaccharide products of luminal-phase starch digestion, including maltose and isomaltose. The specific membranous-phase enzymes are named according to their substrates and include maltase, isomaltase, sucrase, and lactase. The sole product of maltose and isomaltose digestion is glucose, whereas in addition to glucose, fructose and galactose are produced from the digestion of sucrose and lactose, respectively. All polysaccharides are digested to monosaccharides before absorption (Figure 30-10).
Complete Digestion of Peptides to Free Amino Acids Takes Place Both on the Enterocyte Surface and Within the Cells
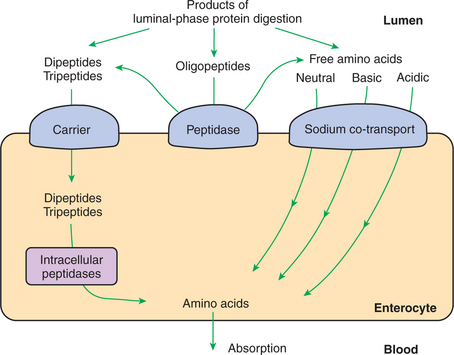
Membranous-phase digestion of peptides is, in some respects, similar to that of carbohydrates; peptide-digesting enzymes, or peptidases, are present on the enterocyte surface membrane and extend into the glycocalyx. These enzymes hydrolyze the peptide products of luminal-phase protein digestion, yielding free amino acids. Some of the longer-chain peptides are incompletely digested, yielding dipeptides and tripeptides. A large portion of dietary amino acids is absorbed directly in the form of dipeptides and tripeptides. This mode of absorption contrasts with that of carbohydrates, in which only monomeric, simple sugars may pass the apical membrane. Dipeptides and tripeptides that are absorbed intact are subsequently hydrolyzed by the action of intracellular peptidases, which results in the formation of free amino acids that are then available for passage into the blood. Thus the final digestion of peptides to free amino acids may occur at either of two sites: on the surface membrane of the enterocyte or within the cell. In either case, the final product of protein digestion is free amino acid (Figure 30-11).
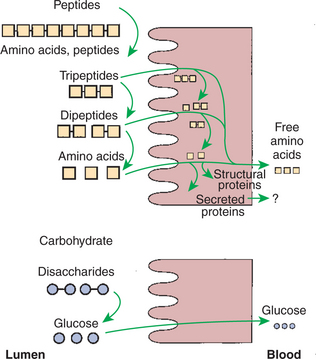
FIGURE 30-11 Membranous-phase digestion of peptides and carbohydrates. Note that tripeptides and dipeptides may be hydrolyzed to their constituent amino acids either on the apical membrane or within the enterocyte. In carbohydrate digestion, however, all disaccharide hydrolysis occurs at the apical membrane. Regardless of the site at which the final hydrolysis of peptides occurs, the product absorbed into the blood is free amino acid (see Figure 30-16).
INTESTINAL ABSORPTION
Absorption refers to the movement of the products of digestion across the intestinal mucosa and into the vascular system for distribution. To better understand the physiologically eloquent and clinically important processes of intestinal absorption, the reader might need to review the processes of diffusion across membranes, the difference in composition of intracellular and extracellular fluid (Chapter 1), the electrical polarity across cell membranes, the function of the sodium-potassium (Na+,K+) adenosine triphosphatase (ATPase) pump, and the function of selective ion channels (Chapters 1 and 4).
In considering intestinal absorption, keep in mind that molecules move across membrane barriers in response to chemical and electrical gradients. When molecules can freely penetrate a membrane, their movement across it is completely determined by the laws of diffusion and differences in chemical and electrical gradients: molecules flow to areas of lower concentration and charged particles move to areas of opposite charge. However, charged ions and most organic nutrient molecules do not freely penetrate the GI epithelium. Therefore, they do not move in accordance with the laws of diffusion unless there is some mechanism to facilitate their transport across membranes.
Specialized Nutrient Transport Systems Exist in the Apical and Basolateral Membranes
Specialized transport mechanisms exist for the movement of molecules across membranes in the intestinal epithelium. These mechanisms are interactions of events involving specific proteins that lie embedded in the matrix of the cell membranes of the epithelial cells. These proteins provide the transport pathway for the passage of ions and organic molecules across the plasma membranes of cells. As discussed here, there are many transport pathways. In general, the different pathways are polarized within the enterocytes, meaning that specific transport pathways exist on either the apical or the basolateral membrane, but not both. The transport pathway proteins chemically interact with specific organic nutrients and inorganic ions to effect their transport across the membrane. The transport mechanisms can be classified as active transport, secondary active transport, tertiary active transport, and passive transport.
Active transport involves the direct consumption of metabolic energy. During active transport, energy stored as ATP is expended to move ions or molecules across membranes against an electrical or chemical gradient. In the large and small intestine, the active transport pathway of greatest importance is the Na+,K+-ATPase pump. This protein pathway lies on the basolateral membrane and uses energy from the hydrolysis of one molecule of ATP to drive three ions of sodium out of the cell, in exchange for the entry of two potassium ions into the cell. This important transport pathway exists in a wide variety of cells, in addition to enterocytes. The Na+,K+-ATPase pump is the mechanism by which (1) the interior of cells is kept electrically negative with respect to the extracellular fluid and (2) the concentration of sodium is kept very low in the intracellular fluid (see Chapter 1).
Secondary and Tertiary Active Transport Mechanisms Utilize the Transcellular Sodium Ion Electrochemical Gradient as Their Source of Energy
Just as a large stone resting atop a hill represents potential energy, so does the electrochemical gradient of sodium ions (Na+) across the enterocyte membrane. Gravity imparts potential energy to the stone, whereas diffusion forces impart potential energy to Na+ outside cells. Transport mechanisms that harness the potential energy of the sodium gradient are referred to as secondary active transport. Various transport pathway proteins exist for secondary active transport.
One type is referred to as a co-transport protein or symport. The characteristic of a co-transport protein is that it has binding sites for one or more Na+ ions as well as an additional binding site for some other specific molecule. For example, the glucose co-transport protein has one binding site for glucose and two for Na+. Co-transport proteins exist in the apical membrane of enterocytes. When the binding sites are unoccupied, they face the intestinal lumen. When all binding sites are occupied, a change in molecular configuration results in the translocation of binding sites, with their ligand molecules, to the interior of the cell. When this happens, the Na+ ions along with the co-transported molecule are released into the intracellular fluid. Thus, there is transport of sodium and another molecule, such as glucose, across the apical membrane. When the ligand molecules are released, the protein assumes its original configuration so that the binding sites are again on the extracellular surface of the apical membrane, ready to transport additional molecules (Figure 30-12).
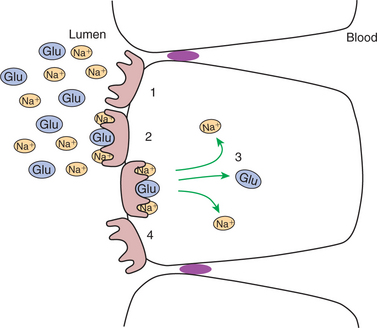
FIGURE 30-12 Co-transport is made possible by the allosteric transformation of transport proteins that lie in the apical membrane. The co-transport protein has two binding sites for sodium ions (Na+) and one for glucose (Glu). When all three binding sites are occupied, the protein changes configuration in such a way as to transport the three ligands into the cell. The favorable gradient for sodium movement is maintained by the continuous action of the Na+,K+-ATPase pump (see FIGURE 30-13)
This process proceeds only as long as there is an electrochemical gradient for the Na+. When this gradient is large, as is normally the case, it can provide the energy to “pull” the co-transported molecule, such as glucose, from an area of lower concentration to one of higher concentration, as illustrated in Figure 30-13 and Figure 30-14. Although the movement of a molecule against its concentration gradient represents expenditure of energy, there is no direct expenditure of metabolic energy by the sodium co-transport process. The energy expenditure is indirect and results from the direct expenditure of energy by the Na+,K+-ATPase pump in creating and maintaining the sodium electrochemical gradient. This is the definition of secondary active transport, with glucose transport being secondary to the active transport of sodium. Many organic nutrients, including glucose, amino acids, several vitamins, and bile acids, are absorbed by sodium co-transport processes.
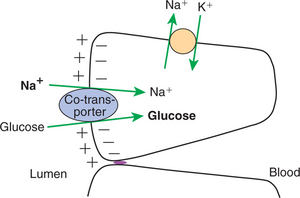
FIGURE 30-13 During co-transport, glucose is transported against an unfavorable concentration gradient. This diagram illustrates that the large sodium concentration difference across the apical membrane provides energy to transport glucose against its concentration gradient. The sodium concentration gradient, created by the action of the Na+,K+ ATPase pump, provides energy to drive this reaction.
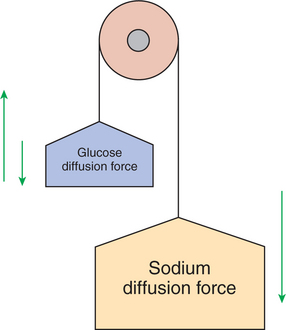
FIGURE 30-14 Secondary transport is an important concept. The tremendous sodium concentration difference between intracellular and extracellular fluid could be likened to the force of gravity; a pervasive force that affects many relationships in our environment. Movement of most ions, glucose, and many other organic molecules across the intestinal epithelium is driven by the strength of the sodium concentration difference.
In addition to sodium co-transport, there are other types of secondary active transport pathways. These pathway proteins are known as exchangers or antiports. Exchangers are usually involved with ion transport and are similar to co-transport proteins in that they have binding sites for selected ions. The difference between exchangers and co-transport proteins is that for exchangers, the binding sites for the two different ligands are on opposite sides of the plasma membrane. For example, an important exchanger is the sodium/ hydrogen (Na+/H+) exchanger in the apical membrane. The protein has a binding site for Na+ and another for H+. When the sites are unoccupied, the Na+ site faces the intestinal lumen, and the H+ site faces the interior of the enterocyte. When both sites are occupied, the protein flips, transporting H+ out of and Na+ into the cell, thus explaining the name exchanger, with H+ exchanged for Na+. As with co-transport, the force driving the exchange is the Na+ electrochemical gradient across the cell membrane.
Another form of active transport, tertiary active transport, occurs via transport pathway proteins and is driven by electrochemical gradients that are established by secondary active transport. The best example of tertiary active transport is the chloride/bicarbonate (Cl−/HCO3−) exchanger. This mechanism occurs in response to gradients established by the Na+/H+ exchanger, a secondary active transport mechanism. The Cl−/HCO3− exchanger is discussed in more detail later, in the section on absorption. In essence, the term tertiary is used because the Na+,K+-ATPase system (primary) establishes the gradient that drives the Na+/H+ exchanger (secondary), which then establishes the gradient that drives the Cl−/HCO3− exchanger (tertiary).
Passive Transport Occurs Either Through Ion Channels in Cell Membranes or Directly Through the Tight Junctions
Ion channels, which are protein constituents of cell plasma membranes, are the transport pathways of passive diffusion into cells. Ions move through the channels in a completely passive manner, responding only to electrochemical gradients. No metabolic energy is directly required to effect ion movement. The only regulatory influence the cell can exert over this form of transport is in opening or closing of the channels (see Chapter 1).
A second form of passive molecular movement through the intestinal epithelium is through the tight junctions. As previously mentioned, the “tight” junctions are not so tight, especially in the duodenum and upper jejunum. In these areas the tight junctions are freely permeable to water and small, inorganic ions. Thus, water and ions move across the tight junctions in response to osmotic pressure and electrochemical gradients. Movement of materials through the tight junctions is called paracellular (around the cells) absorption, in contrast to absorption through the apical membrane, which is called transcellular (through the cells) absorption. Transcellular absorption and paracellular absorption work in a complementary manner to produce an efficient absorptive process (Figure 30-15).
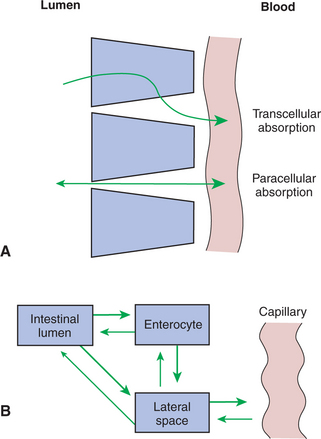
FIGURE 30-15 Transcellular and paracellular absorption. A, Substances move from the intestinal lumen to the capillary by either transcellular (through the enterocyte) or paracellular (through the tight junction) absorption. B, Intestinal lumen, enterocytes, and lateral spaces form three separate pools that may contain nutrients in different concentrations. Note that nutrients move into the capillaries from the lateral spaces and that reverse transport (from the capillary to the intestinal lumen) is possible for some substances
The Products of Membranous-Phase Digestion Are Absorbed by Sodium Co-Transport
Sodium co-transport proteins for glucose and galactose are located in the apical membrane, in proximity to the membranous-phase digestive enzymes. Because these saccharide monomers are produced by the action of membranous-phase enzymes on polysaccharides, they move very short distances to binding sites on co-transport proteins. When both the glucose-binding (or galactose-binding) sites and the sodium-binding sites on these proteins are occupied, absorption occurs as previously described in the description of transport proteins.
In the initial phases of digestion of a starch-containing meal, the glucose concentration at the apical membrane is very high because there is ample substrate. Sodium is also readily available as a result of its presence in the various GI secretions. At this time, movement of both sodium and glucose into the enterocytes is down a concentration gradient. As digestion and absorption proceed, the glucose concentration at the apical membrane diminishes. Thus, toward the end of the digestive and absorptive process, the concentration of glucose at the luminal surface of the enterocyte apical membrane becomes small. At this point the concentration of glucose within the enterocyte can be higher than in the intestinal lumen, thus creating an unfavorable concentration gradient for glucose absorption. However, the transcellular sodium concentration gradient is maintained, driving the continued absorption of glucose (see Figure 30-14). The process of glucose absorption by this mechanism is very efficient, and little free glucose escapes the absorptive process.
To complete the process of carbohydrate absorption, the glucose must move through the basolateral membrane, into the lateral spaces, and then into the capillaries. Movement of glucose through the basolateral membrane occurs by facilitated diffusion, in which there is a transport pathway protein, but the direction of transport is driven only by the concentration gradient for glucose. As the intracellular glucose concentration in the enterocytes increases because of the action of sodium-glucose co-transport from the gut lumen, glucose diffuses from the cells into the lateral spaces. From the lateral spaces, it diffuses through the capillary basement membrane into the blood.
Absorption of the products of membranous-phase protein digestion occurs in a manner similar to that of carbohydrates. Sodium co-transport systems exist for free amino acids and might also exist for dipeptides and tripeptides. At least three co-transport proteins are necessary for absorption of free amino acids. The mechanism of transport for dipeptides and tripeptides might also involve sodium co-transport, but this issue is not established with certainty (Figure 30-16).
ABSORPTION OF WATER AND ELECTROLYTES
Conservation of the body’s supply of water and electrolytes, primarily sodium, potassium, chloride, and bicarbonate, is a high priority for sustaining life. The gut plays a major role in this conservation, not only because it is the portal of entry for replenishment of the nutrients, but also because water and electrolytes in GI secretions must be efficiently reclaimed to maintain body composition. The most immediate clinical ramifications of GI disease usually involve the loss of water and electrolytes. This section discusses the absorption of the major ions and electrolytes sequentially.
There Are at Least Three Distinct Mechanisms of Sodium Absorption
The first pathway of sodium absorption is through sodium co-transport proteins, as previously discussed. This secondary active transport pathway is not only the mechanism for glucose and amino acid absorption (Figure 30-17, A), but also a major means of sodium absorption.
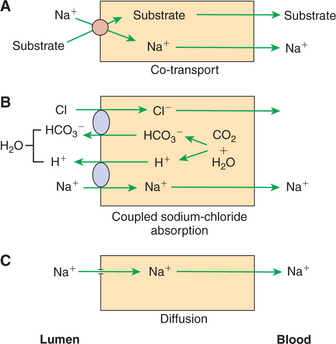
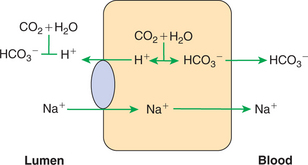
FIGURE 30-17 Three mechanisms of sodium (Na+) absorption. A, Sodium co-transport with organic molecules is a major means of sodium uptake during active digestion and absorption. B, Chloride-coupled sodium absorption is also an important means of sodium absorption and requires the action of carbonic anhydrase and the existence on the apical membrane of bicarbonate-chloride (HCO3−/Cl−) and sodium-hydrogen (Na+/H+) exchange mechanisms. C, Simple diffusion of sodium across the apical membrane may occur because of the large, favorable concentration gradient, but it is a relatively minor means of sodium absorption. H2O, Water; CO2, carbon dioxide.
The second sodium absorption mechanism is through the Na+/H+ exchanger (Figure 30-17, B), mentioned previously as an example of an ion exchanger, or antiport. Through this mechanism, intracellular H+ is exchanged for luminal Na+ across the apical membrane. The H+ for this exchange is formed by the action of carbonic anhydrase, which generates HCO3− as well as H+. As H+ is exchanged for Na+, HCO3− concentrations build up in the cell. The resulting transcellular HCO3− gradient drives the action of the Cl−/HCO3− exchanger, which results in the exchange of intracellular HCO3− for luminal Cl−. Because of the close connection between Na+ and Cl− absorption by these pathways, this transport mechanism is often called coupled sodium chloride transport, as illustrated in Figure 30-17, B. One must appreciate, however, that it is only the intracellular balance of H+ and HCO3− that couples the two exchange pathways. There are instances in which the intracellular pH is such that Na+/H+ exchange occurs without Cl−/HCO3− exchange, and vice versa.
Coupled sodium chloride absorption is usually most active in the ileum and colon, where the sodium concentration in the gut is usually relatively low compared with that in the duodenum and jejunum. As usual, sodium entering the enterocytes is transported across the basolateral membrane to the lateral spaces by the action of the Na+,K+-ATPase pump. Chloride, however, remains in the enterocyte until its concentration is high enough to promote the diffusion of chloride through special channels, or gates, in the basolateral membranes. The rate of absorption of sodium and chloride by the coupled mechanism appears to depend on the permeability of the chloride channels; when the permeability is high, chloride passes rapidly out of the enterocyte, allowing continued chloride absorption. Conversely, when chloride channels are relatively closed, the intracellular chloride concentration rises, diminishing chloride absorption by the creation of an unfavorable concentration gradient across the apical membrane.
The third mechanism of sodium absorption is by simple diffusion through ion channels in the apical membrane (Figure 30-17, C). The large electrochemical gradient that can exist for sodium across the enterocyte apical membrane allows direct, uncoupled movement of sodium across the membrane when the ion channels are open. Although some sodium absorption probably occurs by this mechanism, its overall importance in body sodium homeostasis is probably not great.
There Are Three Major Mechanisms of Chloride Absorption
One mechanism of chloride absorption is coupled sodium chloride absorption, as discussed previously in relation to sodium (Figure 30-18, A). Another mechanism is paracellular chloride absorption, which occurs in association with sodium co-transport of glucose and amino acids (Figure 30-18, B). Paracellular chloride transport occurs because of an electrical gradient. Sodium co-transport leads to the net movement of positive electrical charges (Na+) across the apical membrane, because neither glucose nor most amino acids are charged molecules. As the sodium cations are transferred to the lateral spaces, the spaces develop a positive polarity with respect to the gut lumen. Chloride from the gut lumen passes directly into the lateral spaces through the tight junctions because these junctions are readily permeable to small anions. This provides a major mechanism for the absorption of Cl− while maintaining electrical neutrality, although a small electrical potential is maintained across the gut surface, the lumen being negative with respect to the lateral spaces.
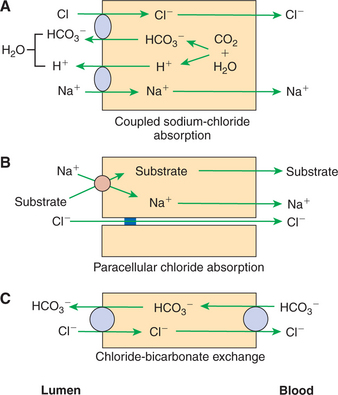
FIGURE 30-18 Three mechanisms of chloride (Cl−) absorption. A, Chloride-coupled sodium absorption is directly related to sodium (Na+) uptake. B, Paracellular chloride absorption is indirectly related to sodium absorption that occurs during co-transport. C, Chloride-bicarbonate (Cl−/HCO3−) exchange occurs especially in areas where bicarbonate secretion into the intestinal lumen is important.
The last mechanism of chloride absorption is by direct exchange for bicarbonate (Figure 30-18, C) without coupled sodium absorption. With this mechanism, there is a net movement of bicarbonate into the gut lumen, resulting in an increase in luminal pH. This can be particularly important in the colon of large herbivores where large concentrations of fermentation acids are created and require buffering.
Bicarbonate Ion Is Secreted by Several Digestive Glands and Must Be Recovered from the Gut if Body Acid-Base Balance Is to Be Maintained
Much bicarbonate is in essence “absorbed” by the neutralization of HCl from the stomach. Sodium bicarbonate entering the intestine reacts with HCl to form water, carbon dioxide, and sodium chloride, effectively resulting in the absorption of bicarbonate (HCO3−) and hydrogen (H+) ions. (See Chapter 28 for an explanation of the counterbalancing effects of gastric acid secretion and pancreatic bicarbonate secretion.) However, considerable bicarbonate remains in the intestine after the neutralization of stomach acid. This remaining bicarbonate is reabsorbed, primarily in the ileum and colon via an ion-exchange mechanism.
Bicarbonate anions in the gut are electrically balanced, primarily with sodium cations, and reabsorbed essentially as sodium bicarbonate. In the absorptive process, H+ and HCO3− ions are first generated within the enterocytes from water and carbon dioxide. H+ is then exchanged for Na+ across the apical membrane. Within the cell, Na+ is electrically balanced by the remaining HCO3−, whereas the HCO3− remaining in the gut lumen is neutralized by the secreted H+ (Figure 30-19). The result is that sodium is transferred through the membrane. However, luminal bicarbonate is converted to water and carbon dioxide in the gut lumen, whereas bicarbonate anion is regenerated intracellularly. The net effect is the absorption of sodium bicarbonate. This mechanism is essentially one half of chloride-coupled sodium absorption, except that the bicarbonate ion remains within the enterocyte rather than being exchanged for chloride.
Potassium Is Absorbed Primarily by Passive Diffusion Through the Paracellular Route
Potassium (K+), although a highly important ion in the body, is present in abundance in most animal diets. This is in contrast to sodium (Na+), which is present in nutritionally inadequate amounts in most natural animal feeds. Therefore, frequently the K+ concentration in the material entering the intestinal lumen is relatively high compared with Na+ concentration. In addition, dietary potassium is concentrated in the gut lumen because of the absorption of other nutrients, electrolytes, and water, unaccompanied by active potassium absorption. Thus, K+ concentration within the gut lumen increases as digestion and absorption of other osmotically active molecules proceeds.
As K+ reaches relatively high concentrations in the intestinal lumen, a concentration gradient favorable for the diffusion of potassium across the intestinal epithelium is created. Furthermore, the concentration gradient is enhanced by the normally low K+ concentration in the lateral spaces. The primary mechanism of potassium absorption is paracellular passive diffusion, which occurs in response to this concentration gradient (Figure 30-20). A clinical ramification of this absorptive mechanism is that potassium absorption is directly coupled to water absorption. That is, the movement of water out of the intestinal lumen results in an increase in the luminal K+ concentration, which in turn drives K+ absorption. In diarrhea conditions, in which net absorption of water is impaired, K+ absorption is impaired as well, because potassium in the gut lumen is diluted so that a concentration gradient favorable for passive diffusion of K+ never develops. In addition to passive diffusion, it appears that an H+,K+-ATPase pump exists in the distal colon. This transport pathway may be important for recovering the last remaining potassium from the colonic ingesta of animals with diets low in potassium.
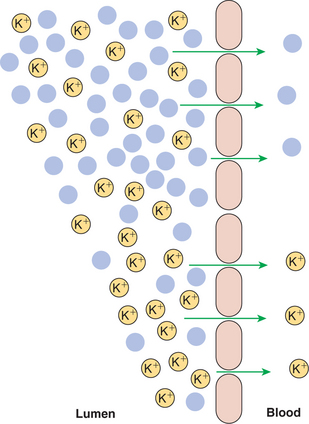
FIGURE 30-20 Potassium (K+) is absorbed by simple diffusion through the paracellular route. Water absorption in the upper intestine increases K+ concentration in the lower intestine, creating a favorable diffusion gradient for potassium. Note that the removal of water (solid blue circles) in the upper part results in a relative increase in the number of K+ ions in the lower part.
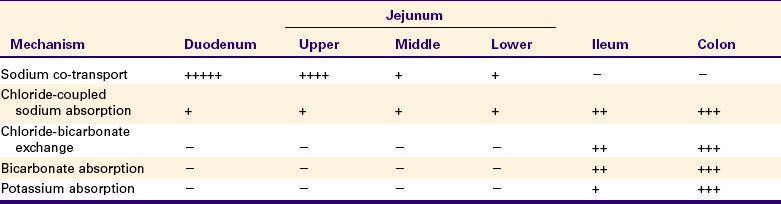
The Major Mechanisms of Electrolyte Absorption Are Selectively Distributed Along the Gut
The activity of the assorted electrolyte absorption mechanisms discussed earlier varies along the length of the gut. The distribution of activity is listed in Table 30-2.
All Intestinal Water Absorption Is Passive, Occurring Because of the Absorption of Osmotically Active Solutes
Water moves through the intestinal mucosa by either the paracellular or the transcellular route, but always by osmosis. The general discussion of osmosis in Chapter 1 should be reviewed by those who do not have a clear understanding of the process. The intestinal mucosa is freely permeable to water, allowing it to move in whatever direction is dictated by changes in osmotic pressure. As electrolytes and other soluble nutrients are actively absorbed, water is drawn along passively from lumen to intestinal capillaries. Water may move also into the intestinal lumen at times when the intraluminal osmotic pressure is high, as discussed later.
INTESTINAL SECRETION OF WATER AND ELECTROLYTES
In addition to the water and electrolytes that are secreted into the intestine by the pancreas, liver, and other glandular organs, a considerable portion of GI water and electrolyte secretion occurs directly from the intestinal surface. All water secretion is osmotic, but the osmotic gradient driving water secretion may occur in response to either passive or active processes.
Passive Increases in Luminal Osmotic Pressure Occur During Hydrolytic Digestion and Result in Water Secretion
Food entering the intestine may be hyperosmotic because of its composition, such as salty foods and foods with high sugar content. Alternatively, food may become hyperosmotic after digestion. Digestion of foods creates many osmotically active molecules from one giant precursor molecule; thus the osmotic activity of ingesta is increased initially by digestion. When starchy meals, for example, first enter the duodenum, intraluminal digestion creates thousands of osmotically active disaccharide and trisaccharide molecules from single starch molecules. These osmotically active saccharide molecules draw water from the lateral spaces into the intestinal lumen. Water in the lateral spaces is quickly replaced by water from the intestinal capillaries, so water is essentially drawn into the intestine from the vascular system. As digestion proceeds, the saccharide molecules are absorbed, thus reducing the number of particles and lowering the osmotic pressure of the intestinal lumen. As solute molecules are absorbed, water follows them osmotically back through the epithelium and into the blood vascular system. The cardinal rule of water movement in the intestine is that water moves in whatever direction necessary to keep ingesta iso-osmotic, entering the gut when ingesta is hyperosmotic and leaving the gut when ingesta is hypo-osmotic. This fact has important clinical implications in the pathophysiology of diarrhea, as discussed later.
Active Secretion of Electrolytes from the Crypt Epithelium Leads to Intestinal Water Secretion
In contrast to the absorptive function of the villous cells, the crypt cells have a secretory function. This secretory function appears to use a chloride transport mechanism. The mechanism seems to be similar to coupled sodium chloride transport, as occurs in the villous enterocytes, except that the direction of transport is reversed. In the crypt cells the coupled sodium chloride transport mechanism is on the basolateral membrane, in contrast to its position on the apical membrane of the villous cells. The effect of this arrangement is to pump Na+ and Cl− into the crypt enterocytes from the lateral spaces. As these ions are transported into the enterocytes, Na+ is quickly pumped out by the Na+,K+-ATPase pump. In contrast, Cl− is trapped within the cells, reaching relatively high intracellular concentrations. Under appropriate stimuli, Cl− channels in the apical membranes of the crypt cells are opened, and the pent-up chloride from within the cells flows down its concentration gradient into the lumen of the crypt. (Ion channels and their regulation in cellular membranes are discussed in Chapter 1.) Movement of Cl− into the lumen of the crypts creates an electrical attraction for Na+, which move into the luminal fluid from the lateral spaces through the paracellular route. Water follows Na+ and Cl− osmotically; thus chloride, sodium, and water are secreted from the crypt epithelium (Figure 30-21).
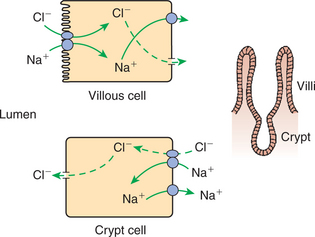
FIGURE 30-21 Water and electrolyte secretion in the crypts is affected by the secretion of chloride (Cl−) from the apical membrane of crypt enterocytes. Sodium (Na+) moves into the lumen by the paracellular route and electrically balances Cl− secretion. Water follows osmotically, the net effect being secretion of a sodium chloride (NaCl) solution into the crypt lumen. In the crypts the coupled NaCl absorption mechanism appears to exist on the basolateral membrane, with Cl− gates present on the apical membrane. The opening of Cl− gates on the crypt cell apical membranes initiates crypt secretion. The membrane position of the coupled NaCl transport process reverses itself, moving from the basolateral membrane to the apical membrane as the cells mature and move up the villi.
You may find the concept of ion transport processes jumping from one side of the cell to another intuitively “unappealing,” especially when you consider that the cells of the intestinal crypts will eventually mature and migrate up the villi to assume absorptive, as opposed to secretive roles. Consider, however, that ion transport mechanisms are simply proteins inserted into the cell membranes. As with other cellular proteins, they are synthesized within the cell under the direction of genetic code. The state of maturity and cellular differentiation dictates the membrane position into which the newly synthesized proteins are directed. The differential distribution of membrane proteins to one side of a cell or another is called polarization. Enterocytes are said to be “polarized” with respect to membrane function.
The triggering mechanism that activates water secretion from the crypts is the opening of the chloride gates in the crypt enterocyte apical membrane. Much study has been devoted to determining the factors controlling the opening of the chloride gates in crypt cells. One important factor in regulating chloride gates appears to be the activity of the adenylate cyclase enzyme and the intracellular concentration of cyclic adenosine 3′,5′-monophosphate (cyclic AMP, or cAMP). (The role of adenyl cyclase and cAMP in cellular regulation is discussed in Chapter 1.) As cAMP concentrations rise, chloride gates open, and secretion of water and electrolytes is stimulated. Vasoactive intestinal peptide originating from effector neurons of the mucosal plexus is probably an important normal regulator of cAMP and chloride gates in crypt apical membranes. Of perhaps greater medical significance than the normal regulation of this process is the existence of pathological, or abnormal, activators of crypt cell adenyl cyclase (see later section on the pathophysiology of diarrhea).
The physiological function of water and electrolyte secretion by the crypts is to maintain an appropriate hydration and ionic environment for digestion and absorption. Ingesta must be kept sufficiently moist to allow for the mixing of nutrients with digestive enzymes and for the circulation of digested nutrients in contact with absorptive surfaces. In addition, a constant supply of sodium must be available to promote the sodium co-transport necessary for the absorption of several nutrients. The regulated process of water and electrolyte secretion from the crypts ensures the continual availability of water and sodium in the gut lumen.
GASTROINTESTINAL BLOOD FLOW
Water and Solute Movement Between the Lateral Spaces and Villous Capillaries Is Subject to the Same Forces That Govern Water and Solute Movement Between the Extracellular and Vascular Fluids in Other Tissues
Water and all other nutrients, whether they are absorbed through the transcellular or paracellular routes, enter the extracellular fluid of the lateral spaces before entering the vascular system. Therefore the movement of extracellular fluid components into capillaries is of particular importance to intestinal absorption. The physical laws determining the distribution of water between the intravascular and extravascular fluid are the same in the villi as in other tissues. These Starling laws (which can be reviewed in Chapters 1 and 23) simply state that the movement of water is determined by the algebraic sum of osmotic and hydrostatic (created by water pressure) forces.
Absorbed Nutrients Enter the Capillaries by Diffusion from the Lateral Spaces
The collective action of the various intestinal absorptive mechanisms concentrates solutes (nutrients) in the lateral spaces. When the concentrations of individual solutes in the lateral spaces exceed their concentrations in blood, a gradient favoring the diffusion of nutrients from lateral spaces into the capillaries is established. The movement of solutes by diffusion into the capillaries creates an osmotic force that draws water into the capillaries (water follows solute). In addition, the oncotic force (the osmotic force exerted by plasma proteins; see Chapters 1 and 23) also tends to draw water into the capillary lumen. Moreover, hydrostatic pressure in the lateral spaces may force water directly into the capillaries. Lateral-space hydrostatic pressure can be created by the osmotic effect of absorbed solutes. As these solutes attract water from the intestinal lumen, the lateral spaces become distended, developing a small amount of hydrostatic pressure. There are two exits for the relief of this pressure: the tight junctions and the capillary endothelium, with the endothelium presenting the route of least resistance to water flow. Thus, water under slight pressure within the lateral spaces tends to flow into the capillaries rather than into the intestinal lumen (Figure 30-22).
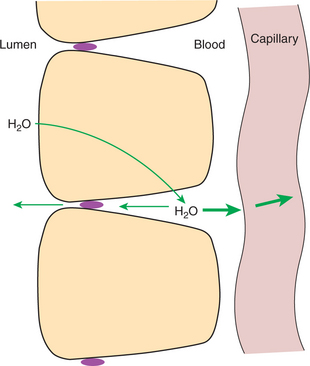
FIGURE 30-22 Water (H2O) enters the lateral spaces because of osmotic effects created by absorbed solutes, thus creating a hydrostatic pressure head in the lateral spaces. Under pressure, the lateral-space solution can exit through the tight junctions or through the basement membrane of the capillaries. Under normal conditions the route of least resistance is into the capillaries, resulting in little movement of water from the lateral space into the intestinal lumen
A Countercurrent, Osmotic-Multiplier System May Increase the Osmolality of Blood at the Tips of the Villi, Further Promoting Absorption of Water into the Blood
The villous vascular system consists of an arteriole rising up the central portion of the villi and dividing at the tip into many capillaries, which course down the outer portion of the villous stroma between the mucosa and artery. This arrangement provides for direct countercurrent flow of blood; that is, blood coming down the venules passes close to blood flowing in the opposite direction up the arteriole. Because blood in the venules contains absorbed nutrients, its osmolarity could be expected to be slightly higher than that of blood entering the villi in the arteriole. This slight difference in osmolarity can be multiplied and perpetuated by the countercurrent flow characteristics of the arterial and venous blood supplies. These conditions create a potential for the creation of an osmotic gradient along the villi; some researchers calculate osmolalities near the tips of the villi to be as high as 600 mOsm, approximately twice that of blood entering the base of the villi. (The characteristics of a countercurrent osmotic multiplier are further explained in Chapter 43 in reference to the renal loop of Henle.) The existence of the villous countercurrent osmotic multiplier is still somewhat controversial, and its presence may depend on the species in question. The effect of this osmotic-multiplier system would be to accentuate all the osmotic forces that result in the movement of water from lumen to lateral spaces and from lateral spaces to capillaries.
Disturbances in the Venous Drainage from the Intestine Can Greatly Affect the Mechanisms of Capillary Absorption in the Villi
With the exception of blood from the terminal colon and rectum, all venous blood from the GI tract is collected into the hepatic portal vein and passes through the liver before entering the vena cava and returning to the heart (Figure 30-23). Because of this system, the nutrient-rich blood leaving the intestine may be modified by the liver. The liver can thus regulate the nutrient concentration of blood reaching general body tissues, keeping it relatively constant. This particular vascular arrangement of the GI system results in the passage of blood through two capillary beds, one in the gut wall and one in the liver, before its return to the heart. In most tissues, arterial hydrostatic pressure forces blood through the capillary beds. In the liver, however, this is not the case, because most of the arterial hydrostatic pressure has been dissipated during flow of blood through the intestinal capillaries. The following two circumstances tend to overcome this problem and allow hepatic blood flow to occur:
1. The capillaries (referred to as sinusoids) of the liver are comparatively large and thus offer little resistance to flow; therefore they can function in a low-pressure system.
2. The venous outflow of the liver goes directly into the thoracic vena cava.
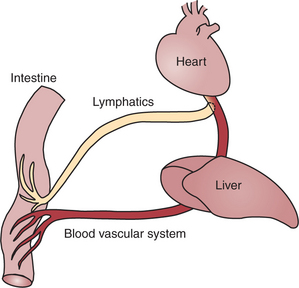
FIGURE 30-23 All blood exiting the gut flows through the liver before returning to the heart. Lymphatic drainage from the gut bypasses the liver, entering the bloodstream through the thoracic duct.
The bellows-like action of the thorax transmits a negative pressure to the thoracic vena cava, which tends to aspirate blood from the hepatic veins and abdominal vena cava. Under normal circumstances, these conditions allow blood to flow readily from the intestine through the liver. However, small changes in circulatory function can have a large impact on GI blood flow. If the pumping capacity of the heart becomes reduced, it cannot remove returning venous blood quickly. Accumulation of blood and an increase in pressure in the thoracic vena cava result. This increase in pressure interferes with the flow of blood out of the liver, which in turn reduces blood flow out of the intestine. This sequence of events makes the GI system particularly susceptible to right-sided heart failure, in which the heart’s pumping action is compromised.
Diffuse liver disease, in addition to right-sided heart failure, can also interfere with GI blood flow. In this condition the resistance to blood flow through the liver is increased because of pressure on the sinusoids. Small rises in hepatic flow resistance can have large effects on intestinal blood flow because the pressure gradient across the hepatic portal vein is normally small. When the flow of blood out of the intestine is impaired, hydrostatic pressure in the capillaries of the villi is increased; the higher pressure tends to offset the osmotic and hydrostatic forces promoting water absorption, and thus water absorption is impaired.
DIGESTION AND ABSORPTION OF FATS
Detergent Action as Well as Enzymatic Action Is Necessary for the Digestion and Absorption of Lipids
Lipids, or fats, present a special digestive problem to the animal because they do not dissolve in water, the major medium in which most body processes, including digestion, occur. Detergent action is necessary to emulsify or dissolve lipids so that they may be subjected to the actions of water-soluble hydrolytic enzymes in the gut. The problem of solubility makes the mechanics of digestion and absorption of lipids somewhat different from that of proteins and carbohydrates. For that reason, lipid assimilation is discussed here in a separate section.
Lipids make up a large portion of the diets of carnivores and omnivores, whereas they usually form a minor portion of the natural diets of adult herbivores. Nonetheless, it appears that herbivorous species have the capacity to digest and absorb lipids in quantities considerably higher than found in their natural diets, and frequently, supplemental lipids are added to the diets of performance horses and high-producing dairy cows. The neonates of all mammalian species have a high capacity for lipid digestion and absorption because milk has a high fat content.
The primary dietary lipid is triglyceride, which may originate from either plant or animal sources. Other important dietary lipids are cholesterol and cholesteryl ester from animal sources, waxes from plant sources, and phospholipids from both plant and animal sources. Figure 30-24 illustrates the structures of these dietary lipids. In addition, the lipid-soluble vitamins A, D, E, and K are absorbed along with the other dietary lipids.
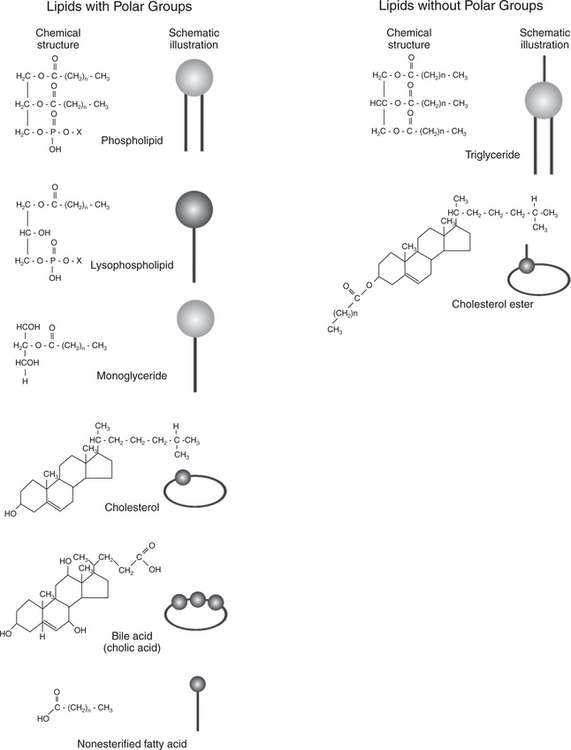
FIGURE 30-24 Chemical structures and their schematic representations for lipid molecules involved in fat digestion and absorption. n, Number of carbon atoms in fatty-acid chains; X, phospholipid head group, most often choline
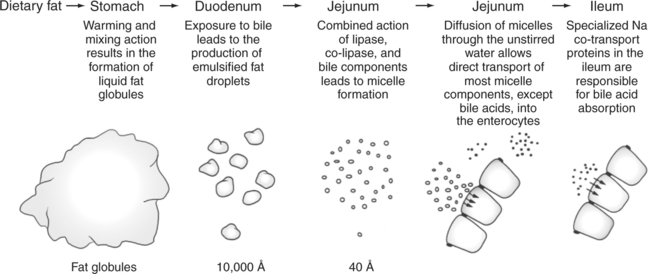
Lipid assimilation can be divided into four phases: (1) emulsification, (2) hydrolysis, (3) micelle formation, and (4) absorption. Emulsification is the process of reducing lipid droplets to a size that forms stable suspensions in water or water-based solutions. In the gut the emulsification phase begins in the stomach as the lipids are warmed to body temperature and subjected to the intense mixing, agitating, and sieving actions of the distal stomach. This distal-stomach activity tends to break lipid globules up into droplets that pass into the small intestine. In the small intestine, emulsification is completed by the detergent action of bile acids and phospholipids. (See Chapter 29 for a discussion of bile formation and secretion.) These bile products reduce the surface tension of the lipids and allow the droplets to become even further divided and reduced in size (Figure 30-25).
While in the bile-coated, or emulsified-droplet, stage, the lipids are subject to the actions of hydrolytic enzymes. Hydrolysis of triglyceride, the major dietary lipid component, occurs because of the combined action of the pancreatic enzymes lipase and co-lipase. Lipase is an enzyme secreted, in its active form, from the pancreas. However, lipase cannot directly attack the emulsified lipid droplets in the gut because it cannot penetrate the coat of bile products surrounding the droplets. The function of co-lipase, a relatively short peptide, is to “clear a path” through the bile products, giving lipase access to the underlying triglycerides. Lipase cleaves the fatty acids off each end of the triglyceride molecule but does not attack the central fatty acid, resulting in the formation of two free, or nonesterified, fatty acids and a monoglyceride from each molecule of triglyceride hydrolyzed (Figure 30-26).
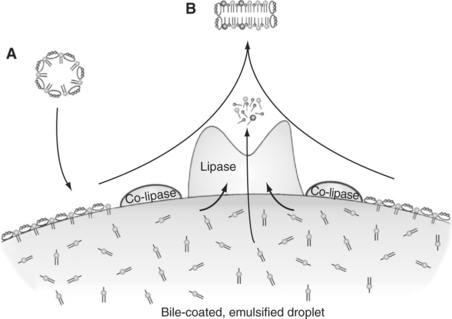
FIGURE 30-26 Portion of the surface of a bile-coated, emulsified fat droplet. Bile components reach the surface of the droplet through micelles (A) coming from the gallbladder. Co-lipase clears bile constituents from an area of the surface of the droplet, allowing the attachment of lipase. Lipase catalyzes the formation of fatty acids and monoglycerides from triglycerides. The surface components and products of lipase action combine to form micelles (B) containing fatty acids and monoglycerides as well as bile constituents.
Other lipid-digesting pancreatic enzymes are cholesterol esterase and phospholipase. The products of these enzymes are nonesterified fatty acids, cholesterol, and lysophospholipids.
The products of hydrolytic lipid digestion (fatty acids, monoglycerides, etc.) combine with bile acids and phospholipids to form micelles, small water-soluble aggregations of bile acids and lipids. Micelles are considerably smaller than the emulsified fat droplets from which they are derived (see Figure 30-25). The soluble micelles allow the lipids to diffuse through the gut lumen into the unstirred water layer and into close contact with the absorptive surface of the apical membrane (see Figures 30-26 and 30-27).
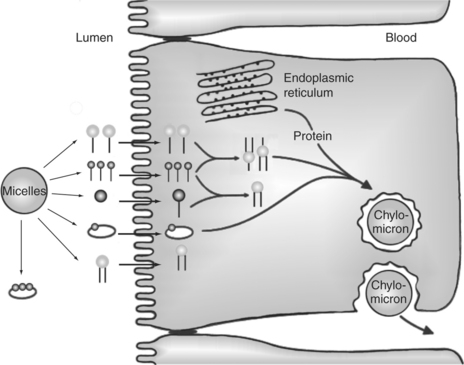
FIGURE 30-27 Lipid absorption from micelles with subsequent formation of chylomicrons. As micelles come close to the apical membrane, lipid constituents, except bile acids, are transported through the membrane into the cell. Once in the enterocyte, triglycerides are re-formed from fatty acids and monoglycerides. Triglycerides are then packaged into the core of chylomicrons for transport out of the cell. The chylomicron surface is coated with phospholipids, cholesterol, and proteins.
Lipids Are Absorbed Through the Apical Membrane by Carrier Proteins and Simple Diffusion
The process of lipid absorption into the enterocytes is incompletely understood. As the micelles come close to the surface of the enterocytes, the various lipid components diffuse the short distance through the glycocalyx to the apical membrane by means of special fatty acid–binding proteins (not shown in Figure 30-27). Fatty acids in the micelles appear to be taken up and transported across the apical membrane by special fatty acid–binding proteins in the apical membrane. Other micellar components appear to simply diffuse into the apical membrane; they include such lipids as monoglycerides, cholesterol, and vitamin A. The apical membrane, as with other cellular membranes, is composed primarily of phospholipids (see Chapter 1). The highly hydrophobic products of lipid digestion are soluble in the phospholipid matrix of the membrane and thus may diffuse freely through the apical membrane and into the cell. Figure 30-27 illustrates lipid absorption from micelles.
Bile Acids Are Reabsorbed from the Ileum by a Sodium Co-Transport System
All components of the micelle diffuse into the enterocytes except the bile acids. Bile acids remain in the lumen of the gut, being separated from the other micellar elements as absorption proceeds. By the time bile acids reach the ileum, they are in a relatively free state, devoid of other lipids. Localized in the ileum is a specific bile acid transport system. This system operates by sodium co-transport and results in the nearly complete reabsorption of bile acids. After absorption, bile acids are transported directly back to the liver by the portal vasculature. The liver efficiently extracts bile acids from the portal blood, so normally the concentration of bile acids in the nonportal blood (systemic circulation) is small. The bile acids extracted by the liver are recycled into the bile. This recycling process occurs repeatedly, so the entire mass of bile acids within the body is circulated through the intestine several times per day.
Absorbed Lipids Are Packaged into Chylomicrons Before Leaving the Enterocytes
After passing the apical membrane, the absorbed lipids are quickly picked up by carrier molecules and transported within the cell to the endoplasmic reticulum. Once on the smooth endoplasmic reticulum, the major lipids are re-esterified to form triglyceride and phospholipids. The re-esterified lipids are then packaged with cholesterol, minor dietary lipids, and proteins from the rough endoplasmic reticulum into structures known as chylomicrons. Chylomicrons are spherical structures with a core of triglyceride and cholesterol ester and a surface of phospholipid and cholesterol. The phospholipid and cholesterol are arranged with their hydrophobic (water-repelling) ends facing the core lipids and their hydrophilic (water-attracting) ends facing the surface of the chylomicron particle (Figure 30-28). This arrangement of surface lipid makes the chylomicron water soluble. A small number of special protein molecules are also present on the chylomicron surface. These proteins help to stabilize the surface and to direct the metabolism of the particle.
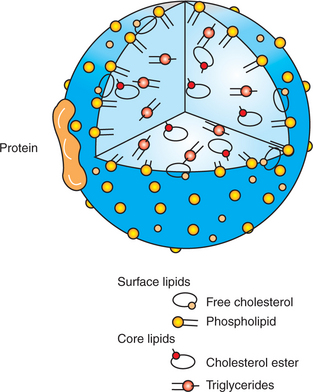
FIGURE 30-28 Chylomicron structure. Special proteins and lipids with polar groups form the surface coat, whereas nonpolar lipids form the core of the particle
After their formation, chylomicrons are expelled from the basolateral membrane into the lateral spaces. Unlike most other nutrients entering the lateral spaces, chylomicrons are too large to pass through the basement membrane of the intestinal capillaries. Thus, chylomicrons cannot be absorbed through the intestinal blood system. Rather, they travel through the intestinal lymphatics, which eventually form a major abdominal lymph duct that passes through the diaphragm and into the thoracic duct. The major lymph-collecting vessel of the body, the thoracic duct, empties into the vena cava. Through this means, chylomicrons eventually reach the blood vascular system. During absorption of a fatty meal, the character of intestinal lymph changes from water-clear to milky white because of the presence of chylomicrons. After a fatty meal, this milky white color can even be seen in blood plasma. In normal animals this white color in blood plasma, known as lipemia, is transient, disappearing within 1 to 2 hours after digestion of the meal. The metabolic fate of the chylomicrons is discussed in Chapter 32.
GROWTH AND DEVELOPMENT OF THE INTESTINAL EPITHELIUM
The Length of Intestinal Villi Is Determined by the Relative Rates of Cell Loss at the Tips and Cell Replenishment at the Base
Division and replication of enterocytes occur in the crypts only. Crypt enterocytes are highly mitotic and regenerate rapidly. In fact, the intestinal crypt cells are among the most rapidly regenerating cells of the body, representing the single greatest need for protein synthesis in nongrowing animals. As crypt cells multiply, they migrate upward onto the villi, pushing other villous cells ahead of them, so there is a continuous progression of cells migrating up the villi. As the cells migrate they mature, changing from relatively undifferentiated cells in the crypts to highly specialized absorptive cells on the villi. As the cells reach the tips of the villi, they are lost because of age and exposure to gut contents. The length of villi is determined by the rate at which cells are lost at the tips and the rate at which they are replaced by cells from the crypts. An increase in cell loss at the villi tips, relative to crypt cell replication, results in shortening of the villi. Conversely, fast replication of crypt cells, relative to cell loss, leads to lengthening of the villi. The time taken for an enterocyte to migrate from its site of origin in the crypt to the tip of a villus varies with species and physiological state; on average, however, the turnover time of enterocytes is 4 to 7 days.
The rate of cell replication in the crypts appears to be stimulated by several GI hormones. When appetite and feed intake increase, there is an overall increase in the secretion of GI hormones. This in turn leads to a rise in crypt cell proliferation. An increase in the rate of crypt cell replication adds cells to the villus at a rate faster than they are lost at the tips, resulting in longer villi. Appetite and feed intake may increase because of conditions of greater energy need, such as lactation, exercise, and cold environmental temperatures. The greater villi length provides greater digestive and absorptive capacity to match the need created by higher feed intake. Thus the functional capacity of the intestine is adjusted to match the nutrient needs of the animal.
DIGESTION IN THE NEONATE
During the First Few Hours of Life, Proteins Are Not Digested but Are Absorbed Intact
In general, a major function of digestion is to break down proteins by hydrolysis. Under most circumstances, this process is a benefit to the animal not only from a nutritional and digestive standpoint, but also from a toxicologic and allergic standpoint; potentially toxic or allergenic proteins are broken down before they are absorbed into the body. In the special case of some neonates, however, there is a need to absorb proteins intact. In most livestock species, including horses, cattle, sheep, and swine, essentially no antibodies are passed through the placenta from the dam to fetus. Thus, young livestock are born without the immunological protection of their mother’s antibodies. In these species, antibodies from the mother must be acquired through ingestion of colostrum, the special mammary secretion present at birth. In these animals the digestive tract at birth is altered from the adult state, so the antibody proteins are absorbed intact rather than after digestion. There are three primary alterations, as follows:
1. Acid secretion from the stomach is delayed for several days after birth.
2. A similar delay appears in the development of pancreatic function, and thus acid and trypsin digestion of proteins is avoided.
3. A specialized intestinal epithelium present at birth only is capable of engulfing soluble proteins in the intestinal lumen and discharging them into the lateral spaces.
The fetal intestinal epithelium has the same villous structure as the mature epithelium, but the villi are covered with special enterocytes capable of protein absorption. Immediately after birth, this special epithelium starts to disappear, and it is essentially gone after 24 hours. The loss of the protein-absorptive function in the neonate is referred to as gut closure.
The Major Intestinal Disaccharidase Switches from Lactase to Maltase with Maturity
Lactose from milk is the major carbohydrate in the diets of neonatal and young mammals; thus all mammals are born with high intestinal lactase activity. In contrast, maltase activity, necessary for digesting the products of luminal starch digestion, is weak or absent for several weeks after birth. As animals progress toward weaning, lactase activity wanes and maltase activity increases, allowing the animals to shift from lactose to starch as a carbohydrate source. In many species of adult animals, lactase activity is practically nonexistent.
PATHOPHYSIOLOGY OF DIARRHEA
Diarrhea refers to an increase in frequency of defecation or fecal volume. Volume often increases in diarrhea primarily because of increased water content. The amount of water passed in feces is the algebraic sum of GI water input and water absorption. As discussed earlier, water in the gut results from (1) ingested water, (2) water secreted by glands of the GI system, and (3) water secreted or lost directly through the mucosal epithelium. Under most circumstances the amount of water secreted into the gut greatly exceeds the amount ingested. Normally, the amount absorbed is just slightly less than the sum of the amounts secreted and ingested, leaving a small remainder for passage in the feces (Figure 30-29, A).
FIGURE 30-29 Pathophysiology of diarrhea. The bars represent the relative amounts of water entering or leaving the gut. Fecal volume is the sum of the water ingested and the water secreted minus the water absorbed. Therefore, fecal volume depends not on the amount of water entering the gut, but rather on the balance between water influx and efflux.
Diarrhea Occurs When There Is a Mismatch Between Secretion and Absorption
The amount of water in the feces is the result of the balance between secretion and absorption. Malabsorptive diarrhea occurs when absorption is inadequate to recover a sufficient portion of secreted water, as illustrated in Figure 30-29, C. Malabsorptive diarrhea usually occurs because of the loss of GI epithelium. In most instances, such losses occur because of viral, bacterial, or protozoal infections. Viral infections often cause particularly severe destruction of villous epithelium. These infections result in the loss of enterocytes from the villi. As noted previously, villous length is determined by the relative rates of cell loss and cell replacement (Figure 30-30).
FIGURE 30-30 Shortening of villi caused by increased cell loss. Many infectious diseases result in an increased rate of cell sloughing from the villi. As cells are lost, the villus shrinks to fill in the gap in the epithelial coat. If villous height is to be maintained in the presence of rapid loss of enterocytes, the rate of recruitment of new cells, generated in the crypts, must be increased. Therefore, when the rate of cell loss exceeds the capacity for cell replacement, shortened villi with reduced absorptive surfaces and relatively immature enterocytes appear.
Intestinal infections result in decreased villous length because the rate of cell loss is higher than the rate of cell replacement. Short villi cause impaired absorption for two reasons: (1) there is an absolute loss of absorptive intestinal surface area and (2) the cells that are lost are the mature cells from the upper regions of the villi. It is these mature cells that possess the enzymes of membranous-phase digestion and the transport proteins for sodium co-transport; loss of these cells results in impairment of digestion and absorption of nutrients. Because nutrient absorption is necessary for the osmotic absorption of water, water absorption is diminished when nutrient absorption is impaired.
Secretory diarrhea occurs when the rate of intestinal secretion increases and overwhelms the absorptive capacity. Most cases of hypersecretory diarrhea result from inappropriate secretion from the small intestinal crypts. This occurs when the normal secretory mechanism of the crypt epithelium (as discussed earlier) is abnormally stimulated. Toxins known as enterotoxins are produced by some types of pathogenic bacteria. These toxins bind to enterocytes and stimulate adenyl cyclase activity and the production of cAMP within the cells, resulting in opening of the chloride gates and the secretion of water and electrolytes from crypt epithelium. If the stimulation is mild, the gut may respond with an increase in absorption, and diarrhea does not result. However, when the secretion exceeds the capacity of the gut to increase absorption, as illustrated in Figure 30-29, B, diarrhea results. Hypersecretory diarrhea has devastating effects on the water, electrolyte, and acid-base status of animals, especially neonates. Hypersecretory diarrhea caused by enterotoxin-producing Escherichia coli is an extremely common disease of neonatal calves and pigs. This disease causes large economic losses in the cattle and swine industries as a result of treatment costs and death.
CLINICAL CORRELATIONS
Diarrhea with Dehydration and Acidosis in a Calf
History.
You are asked to examine a 2-day-old calf. The owner reports that the calf appeared normal the night before, but this morning she is recumbent and will not rise. In addition, she shows no interest in suckling a bottle.
Clinical and Laboratory Examination.
The calf’s body temperature is subnormal. The mouth is dry, and the eyes are sunken into the orbits. The ears, tail, and distal legs are cool to the touch. The tail and perineum of the calf are wet. As you remove your thermometer, the calf passes a stream of liquid feces. The feces are almost clear and slightly yellow, with the consistency of water. Simple laboratory tests indicate that the packed cell volume is 50% (normal, 30%-35%) and the serum total-solids concentration 7.5% (normal, 5.5%-6.5%).
Comment.
The calf has diarrhea, and the physical examination and laboratory findings indicate a state of advanced dehydration. Loss of body fluid volume is so severe that the calf appears to be in or near a state of hypovolemic shock. Although you cannot be certain from your examination of the calf at the farm, the severity of dehydration, the rapidity of onset, and the age of the calf all suggest a hypersecretory diarrhea caused by enterotoxigenic E. coli bacteria. Animals with such clinical signs are usually severely acidotic, although blood pH is seldom measured in field cases. Diarrhea, acidosis, and dehydration occur because toxins produced by the bacteria stimulate opening of chloride gates in the apical membranes of crypt cells, stimulating copious secretion of water and electrolytes, including bicarbonate. The sodium co-transport system on the villi is unaffected by the bacterial toxin, but the simultaneous presence of glucose and sodium in the lumen is necessary to promote co-transport, which can offset some of the fluid and electrolyte losses caused by hypersecretion from the intestinal crypts.
Treatment.
Vascular volume expansion and correction of acidosis are primary concerns in such cases. This calf should receive 2L of alkalizing fluids by rapid intravenous (IV) administration, with an additional 2 L or more over the next 24 hours. Frequently, the response of calves to such treatment is remarkable, and calves that appear almost dead can often be saved by vigorous fluid therapy. After the initial replacement of lost fluids by IV therapy, further dehydration caused by ongoing fluid losses can be prevented by oral administration of fluids containing glucose and sodium.
Juvenile Pancreatic Atrophy in a Dog
History.
You are presented with a thin, 3-year-old German shepherd. The owners report that the dog appeared normal until 6 months ago. At that time they noticed that he started losing weight and began to eat his own feces. Recently the weight loss has become more severe, even though he has had a good appetite and seems normal otherwise. Lately, the owners have noted that the dog seems to pass a large amount of feces, which is soft and gray with a claylike consistency.
Clinical and Laboratory Examination.
Physical examination reveals an extremely thin dog with a dull, uneven hair coat. Other physical findings are unremarkable, and the dog seems bright and friendly. You hospitalize the animal for further testing and note that he readily eats two cans of commercial dog food per day. Laboratory analysis of feces collected over a 24-hour period reveals that the dog is passing 25 g of fat in the feces per day (normal, 5 g, assuming normal diet).
Comment.
This degree of fat malabsorption is characteristic of pancreatic exocrine insufficiency. Because there is insufficient pancreatic lipase, fats cannot be hydrolyzed to fatty acids for absorption and thus pass unabsorbed through the gut. Other laboratory tests for assessing pancreatic exocrine function include (1) examination of blood for the presence of orally administered markers that require pancreatic enzymes for digestion and absorption and (2) direct examination of feces for the presence of pancreatic enzymes or undigested nutrients. Currently, the most definitive laboratory test for the diagnosis of pancreatic exocrine insufficiency is serum trypsin-like immunoreactivity. Under normal conditions, a very small proportion of the trypsinogen synthesized by the pancreatic acinar cells escapes into the peripheral blood stream, and a similar small portion of trypsin is absorbed from the intestine. Although these concentrations are too small to have an effect on the body, they can be measured in blood. The assay is based on an antibody reaction and the quantitative result is referred to as trypsin-like immunoreactivity. A low value for trypsin-like immunoreactivity is indicative of pancreatic exocrine insufficiency.
Treatment.
Feeding highly digestible diets mixed with commercially prepared pancreatic enzymes is usually successful in promoting adequate nutrient absorption in animals with juvenile pancreatic atrophy. Digestion may not be completely normal but is sufficient for the dog to attain a normal body weight. Treatment must be continued for life. You may question how orally administered pancreatic enzymes can survive intact through the proteolytic environment of the stomach. Undoubtedly, portions are destroyed, but sufficient enzyme appears to make it through the stomach to be effective.
Davidson NO. Intestinal lipid absorption. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Ganapathy V, Ganapathy ME, Leibach FH. Protein digestion and assimilation. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Hall EJ. Clinical laboratory evaluation of small intestinal function. Vet Clin North Am Small Anim Pract. 1999;29:441.
Johnson LR. Digestion and absorption. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Johnson LR. Fluid and electrolyte absorption. Johnson LR, ed. Gastrointestinal physiology, ed 6, St Louis: Mosby, 2001.
Johnson LR, Alpers DH, Christensen J, et al. Physiology of the gastrointestinal tract, ed 3, New York: Raven Press, 1994.
Lundgren O. Enteric nerves and diarrhea. Pharmacol Toxicol. 2002;90:109.
Montrose MH, Keely SJ, Barrett KE. Electrolyte secretion and absorption: small intestine and colon. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
Nagy B, Fekete PZ. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet Res. 1999;30:259.
Naylor JM. Oral electrolyte therapy. Vet Clin North Am Food Anim Pract. 1999;15:487.
Rao MC. Oral rehydration therapy: new explanations for an old remedy. Annu Rev Physiol. 2004;66:385.
Stevens CE, Hume ID. Comparative physiology of the vertebrate digestive system. Argenzio RA: Comparative physiology of the gastrointestinal system. In: Anderson NV, ed. Veterinary gastroenterology. Philadelphia: Lea & Febiger, 1980.
Traber PG. Carbohydrate assimilation. Yamada T, ed. Textbook of gastroenterology, ed 4, vol 1. Philadelphia: Lippincott Williams & Wilkins, 2003.
PRACTICE QUESTIONS
1. Finding triglycerides and starch in the feces of a thin dog with a normal feed intake would suggest:
2. Which statement about the tight junctions is false?
3. Which of the following molecules is consumed during the process of hydrolytic digestion?
4. A drug that blocks the activity of the Na+,K+-ATPase pump could be expected to have what effect on sodium-glucose co-transport?
5. During sodium absorption by glucose co-transport:
6. Before entering the intestinal capillaries, all nutrients pass through the: