13 The Thorax of the Dog and Cat
CONFORMATION AND SURFACE ANATOMY
The shape of the thorax differs considerably among different breeds, as is well illustrated by the deep laterally compressed thorax of the Greyhound (Figure 13–1) and the broad, barrel-shaped one of the Pug (Figure 13–2). These differences are reflected in the form of the ribs, which are long and relatively straight in the Greyhound, and shorter and strongly curved in the contrasting type. In cats, corresponding but less pronounced variation distinguishes the Oriental breeds from the Persian.
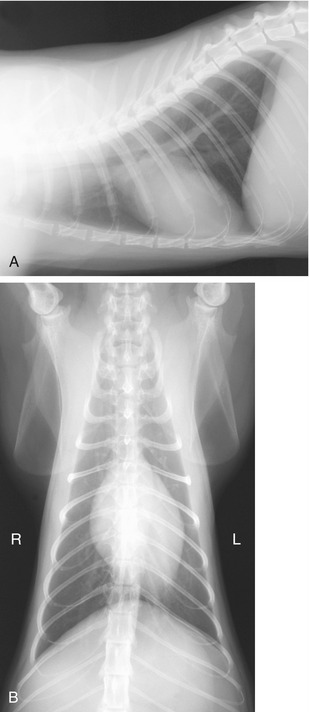
The small size of the cranial part of the bony thorax and thus of the thoracic inlet is masked by the enclosure of the upper parts of the forelimbs within the skin of the trunk (Figure 13–3) and by the height of the first few thoracic spinous processes (Figure 13–5). The dorsal contours of the neck and thorax generally meet without a noticeable elevation at the withers. The skin is loosely attached here, which makes this a suitable site for the subcutaneous infusion of large volumes of fluid when it is necessary to correct dehydration. The tips of the thoracic spinous processes are individually palpable, together with the spine and the cranial and caudal angles of the scapula to each side. In the standing dog these angles are placed opposite the spinous processes of the first thoracic vertebra and the bodies of the fourth and fifth thoracic vertebrae, respectively. The shoulder joint is located opposite the ventral end of the first rib, and the point of the shoulder is slightly behind the level of the manubrium sterni. The gently curved sternum rises between the forelimbs to the thoracic inlet, bringing the easily palpated manubrium a few centimeters cranial to the first pair of ribs. The olecranon projects on the thoracic wall immediately below the ventral end of the fifth intercostal space. However, breed and individual variations make it necessary to regard all these statements of projection with caution (Figure 13–5 and Figure 13–6).

Figure 13–3 Dorsal section of the canine trunk level with the base of the heart, dorsal view. 1, Cephalic vein; 2, proximal end of humerus; 3, triceps; 4, cranial, middle, caudal, and accessory lobes of the right lung; 5, liver; 6, stomach; 7, right atrium; 8, aortic arch; 9, cranial vena cava; 10, pulmonary valve; 11, left atrioventricular valve; 12, divided cranial and caudal lobes of the left lung; 13, caudal mediastinum; 14, diaphragm.

Figure 13–5 Left and right surface projections of the canine heart and lungs. Circled letters on the heart: puncta maxima of left atrioventricular valve (A), pulmonary valve (B), aortic valve (C), and right atrioventricular valve (D). 1, Apex of left lung (broken line) in cupula pleurae; 2, heart; 3, basal border of lung; 4, diaphragm.

Figure 13–6 Left and right surface projections of the feline heart and lung. 1, Apex of left lung; 2, heart; 3, basal border of lung; 4, diaphragm.
The epaxial muscles provide a thick covering to the thoracic vertebrae and the dorsal parts of the ribs. The caudal border of the scapula can be difficult to distinguish because of the triceps muscle occupying the angle between the scapula and humerus. Medial to the triceps and behind the limb, the lateral parts of the ribs are more thinly covered by the serratus ventralis, latissimus dorsi, scalenus, and obliquus abdominis externus muscles. The outlines of some of these generally flat muscles may be traced, and it is possible to feel the ribs through them (Figure 13–7). Although the ventral surface of the thorax is covered by pectoral muscles, the axilla is deep and permits palpation of the first five ribs and the axillary and accessory axillary lymph nodes when they are enlarged. The most extensive exposure of the chest is obtained when the limb is drawn forward.
The thorax of young dogs and cats yields considerably to external pressure, which accounts for the remarkable avoidance of major damage frequently observed after traffic accidents. The costochondral joints of certain rib pairs can be brought together by manual compression cranial to the heart. The freedom with which the forelimbs of the cat may be shifted against the trunk (exemplified by the position of the scapulae in the posture adopted by a cat stalking prey) deprives the projections of the skeletal features of much significance (Figure 13–6).
Pectus excavatum is an uncommon congenital anomaly in both dogs and cats. It is characterized by a concave inward deformation of the caudal sternum and costal cartilages, which may cause severe respiratory and circulatory abnormalities.
THE THORACIC WALL AND PLEURA
(See also pp. 41–43, 48–52, and 158–160.)
The dog generally has 13 rib pairs of which nine are sternal. Asymmetry of number and the presence of 12 or 14 pairs are both occasionally found. The first three to four ribs are almost vertical; behind this, the ribs slope increasingly caudoventrally (see Figure 2–1). The ribs are relatively narrow, resulting in wide intercostal spaces, which is an advantage in thoracic surgery. The costal cartilages at first continue the direction of the bony ribs but then bend forward, almost at right angles (see Figure 13–6), to form the rib “knees.” Those of the sternal ribs form synovial articulations with the sternum, which allow expansion of the thorax when the ribs are carried cranially in the “bucket-handle” movement. The cartilages of the four asternal ribs join to form the costal arch, which is easily palpated and may be followed to the vicinity of the xyphoid cartilage (Figure 13–8/5). The slender, cylindrical sternebrae are slightly thickened at their extremities where the costal cartilages attach. Only a thin layer of compact bone encloses the spongy interior, and this, combined with the superficial position, makes them ideal for bone marrow biopsy.
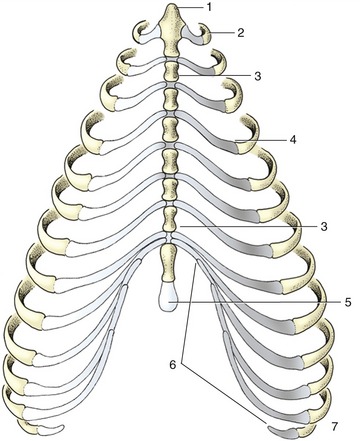
Figure 13–8 Canine sternum and costal cartilages, ventral view. 1, Manubrium; 2, first rib; 3, sternebra; 4, costochondral junction; 5, xiphoid cartilage; 6, costal arch; 7, floating rib.
The intercostal spaces have the usual construction. The principal intercostal vessels and nerves run caudomedially to the ribs, under the endothoracic fascia. Additional vessels from the internal thoracic trunks follow the cranial borders of the ribs in the ventral parts of the spaces (see Figure 13–4). These locations must be borne in mind when contemplating incision or puncture. When such procedures are contemplated, a useful guide to the topography is supplied by the boundary between the scalenus and external abdominal oblique muscles, which marks the fifth intercostal space. The space chosen for a lateral thoracotomy is not always that suggested by prior knowledge of the topography or by preliminary radiography; the ribs are so much more easily displaced cranially than caudally that a more favorable exposure of the “target” region may be gained by opening the space immediately caudal to the one that initially seemed most appropriate.
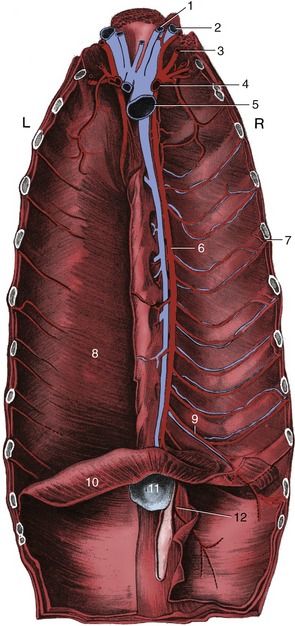
Figure 13–4 The vessels on the floor of the canine thorax; the transversus thoracis has been removed on the right. 1, Internal jugular vein; 2, external jugular vein; 3, vertebral artery; 4, right subclavian artery; 5, cranial vena cava; 6, internal thoracic artery; 7, intercostal artery; 8, transversus thoracis; 9, musculophrenic artery; 10, diaphragm; 11, xiphoid cartilage; 12, cranial epigastric artery.
The diaphragm arises by right and left crura from the first few lumbar vertebrae and attaches to the medial surfaces of the ribs close to the costal arches and to the sternum. Its strong curvature brings its most cranial point to the level of the sixth or seventh rib. The small, triangular tendinous center transmits the caudal vena cava a little to the right of the median plane. The openings for the esophagus and aorta lie in the fleshy lumbar part, and the former is opposite the upper palpable part of the tenth rib (Figure 13–9). In lateral radiographs the strongly convex ventral part of the diaphragm presents a simple border that is continued dorsally by the paired outlines of the cupulae (Figure 13–10, A/4); the more cranial outline of this double image is provided by the cupula on the “lower” side of a laterally recumbent animal, which is the side subjected to greater forward pressure from the abdominal viscera. A further guide to the correct identification of the twin elevations is provided by the gas bubble that is usually found in the gastric fundus, which is of course located on the left side. The doubling of the outline is less distinct in cats in which the lighter abdominal organs exert possibly less pressure.

Figure 13–9 Cranial view of the canine diaphragm. 1, Aorta; 2, esophagus; 3, caudal vena cava; 4, tendinous center; 5, sternal and costal parts of diaphragm; 6, attachment of plica venae cavae; 7, attachment of caudal mediastinum.
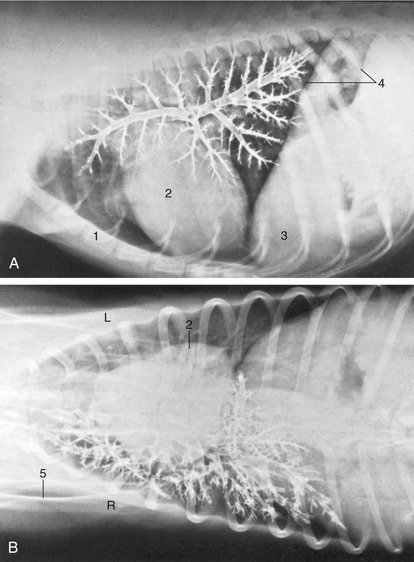
Figure 13–10 Lateral (A) and ventrodorsal (B) bronchograms of the right canine lung. 1, Sternum; 2, heart; 3, liver behind diaphragm; 4, paired shadows of the cranial extent of the diaphragm; 5, scapula.
A sudden increase in abdominal pressure, commonly produced by compression in traffic accidents, may tear the diaphragm and allow abdominal viscera to enter the thoracic cavity (diaphragmatic hernia).
At rest, ventilation principally depends on the diaphragm, but when respiratory demands increase, other muscles are called into play. Some or all of the external intercostal, sternocephalic, ventral serratus, and scalenus may be used to assist at inspiration, whereas the internal intercostal and abdominal muscles may assist at expiration.
The pleural cavities present the usual features, of which the most important clinically are the cupulae cranially, the caudal reflection of the costal pleura onto the diaphragm, and the presence and extents of the costomediastinal and costodiaphragmatic recesses. In the dog, the cupulae (see Figure 13–5) project only slightly in front of the first ribs, but this is sufficient to make it possible for air to be introduced into a pleural cavity by a penetrating wound that appears to be confined to the base of the neck, the result of which is the collapse of a lung.
The junction between costal and diaphragmatic pleura, the line of pleural reflection, defines the caudal extent of the pleural cavity. The line runs from the sternum along the eighth costal cartilage, crosses the middle of the ninth cartilage, and then proceeds in a curve that intersects the eleventh costochondral junction to reach the dorsal end of the last rib. The two recesses are of course never fully exploited by the lungs. Fluid may be collected through the ventral third of any of the fourth to seventh intercostal spaces of a dog standing or restrained in sternal recumbency. In cases of pneumothorax, air may be aspirated at the dorsal part of the seventh or eighth space of dogs similarly placed. The eighth space is optimal for this purpose in the cat.
The coupling of the lung to the thoracic wall, maintained by a thin layer of pleural fluid, is disrupted when air gains entry to the pleural cavity. This causes not only collapse of the lung but also expansion of the rib cage as the thoracic wall recoils outward. Although pneumothorax is generally produced by trauma of the thoracic wall, it may result from rupture of the lung or trachea or from perforation of the esophagus.
THE LUNGS
(See also pp. 160–165.)
The lungs of the dog obtain their distinctive appearance from the deep fissures that divide the lobes, sometimes so completely that they remain connected by little more than the branches of the bronchial tree and pulmonary vessels. In consequence, torsion of a lobe is a possible complication of thoracic trauma, perhaps most frequently seen after traffic accidents. In contrast, lobulation is not evident to the naked eye through the covering pleura. The right lung, always somewhat the larger, possesses cranial, middle, caudal, and accessory lobes (Figure 13–11, A–B); the left one has only a divided cranial lobe and a caudal lobe. In keeping with the difference in size, the cardiac impression on the medial surface of the left lung is shallower than that on the right. Despite the existence of a small notch between the two parts of the cranial lobe, the left lung, for all practical purposes, may be regarded as covering the lateral face of the pericardium. The notch on the right side, between the cranial and middle lobes, is larger, although it is restricted to the ventral part of the fourth intercostal space; it provides the site recommended for heart (right ventricular) puncture and for ultrasonic cardiac imaging.
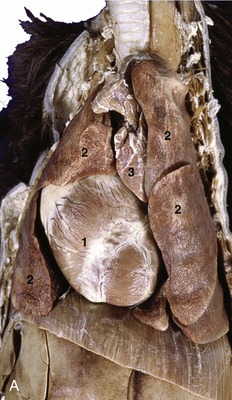
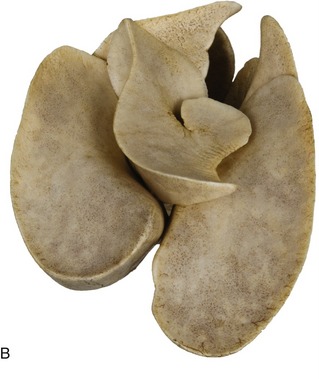
Figure 13–11 A, Thoracic viscera of the dog. 1, Heart; 2, pulmonary lobes; 3, thymus. In B (inflated specimen) the deep fissure between the lobes of the lung are clearly visible.
Pulmonary ligaments connect the hilar region of the left lung to the aorta and that of the right lung to the esophagus, which it follows to the hiatus in the diaphragm.
The fields for auscultation and percussion of the lungs are triangular: the cranial border is provided by the fifth rib (actually the caudal border of the triceps), the dorsal border is provided by the lateral margin of the back muscles from the fifth rib to the eleventh space, and the basal border is provided by the line joining the sixth costochondral junction, the middle of the eighth rib, and the dorsal end of the eleventh space. The forelimb may be drawn forward to increase the accessible area by the space of a couple of ribs.
In plain radiographs, the principal features of the lungs are made by the vessels and bronchi. The blood within the arteries and veins, which cannot be immediately differentiated, produces a pattern of light streaks radiating from the hilar region toward the periphery, branching and tapering as they go. The bronchi, being filled with air, provide dark streaks that contrast less definitely with the lung parenchyma. Their walls may be invisible or appear as narrow, whitish lines, especially in older animals in which the cartilage tends to have calcified. The relationships within the bronchial–vascular triads vary in different regions and in different radiographic views. The components are most clearly depicted when portrayed end-on; the dark circles of the bronchial lumina are then flanked by white circles representing the companion vessels. The subpleural connective tissue bordering the interlobar fissures may appear as fine lines when penetrated tangentially.
Both the bronchial tree and the pulmonary vasculature may be made more evident by the use of an appropriate contrast technique (contrast bronchography: Figure 13–10; angiocardiography: Figure 13–21). The larger divisions of the bronchial tree are then very clearly depicted, and if the normal pattern of branching is known, any deviation may reveal the existence of pathology. A more exact picture of the nature and extent of that pathology may obtained by the use of bronchoscopy, which also requires familiarity with the branching pattern. The principal bronchi produced at the bifurcation of the trachea are separated by a sharp ridge, the carina. The bronchi that initially branch from the principal bronchi supply the different lobes and are named accordingly. The divisions of the next order, the segmental bronchi, also arise according to a consistent pattern and are each associated with well-defined parts of the lobes. Subsequent divisions into smaller bronchi are less regular and less predictable. The parts of lung associated with the segmental bronchi (the bronchopulmonary segments) constitute the divisions of the lungs on which surgery is based. Various systems of nomenclature have been devised for the identification; one is based on topography, while a second employs a basically numerical code; their details may safely be left to those with a specialist interest.
The manner of branching is such that after each division the two daughter bronchi in combination provide a greater cross-sectional area, thus offering less resistance to the flow of air than their parent; resistance thus decreases progressively as air passes more deeply into the lung. This process is a continuation of that in the upper respiratory tract, where the nostrils, the nasal cavity, the pharynx, the larynx, and the trachea offer successively less obstruction than the preceding segment. According to one estimate, the resistance to the inspiratory airflow in dogs is 79% due to the nasal, 6% to the laryngeal, and 15% to the bronchopulmonary parts of the tract; the corresponding figures at expiration are given as 74%, 3%, and 23%, respectively. These findings offer a reminder that dogs of brachycephalic breeds in which nasopharyngeal resistance is pronounced may be severely compromised even when breathing normally.
The lungs of the cat, relatively shallow in comparison with those of the dog, show no significant differences in general or radiographic anatomy or in bronchopulmonary segmentation.
THE MEDIASTINUM
(See also pp. 158–160.)
The fibrous tissue associated with the thoracic organs and between the pleural sacs (fascia endothoracica) is so thin that the mediastinum is reduced in several places to a very delicate and transparent membrane (Figure 13–12, B) consisting only of apposed right and left pleural sheets. It ruptures easily, and although the two pleural sacs may be regarded as normally independent, most dogs in which pneumothorax has been induced unilaterally show bilateral pneumothorax in radiographs.


Figure 13–12 A, Mediastinum of a cat, right view. In the middle part the heart is the main component. The cranial and caudal mediastinum is thin and, in some places, fragile. B, Mediastinum, left view. A large opening in the caudal part, probably caused by dissection, indicates the fragility of the structure.
The cranial mediastinum is wide dorsally where it contains the trachea and esophagus lying side by side as they pass through the thoracic inlet; below these the cranial vena cava and brachiocephalic trunk, with their tributaries and branches, are embedded in generous quantities of fat. Ventrally the cranial mediastinum contains lymph nodes, the internal thoracic vessels, fat, and, in the young animal, the thymus. This part narrows with the regression of the thymus, providing more space for the apices of the lungs.
The dorsal part of the middle mediastinum is slightly narrower than the heart (Figure 13–13); it contains the termination of the trachea, the esophagus, the aortic arch, the structures comprising the roots of the lungs, and lymph nodes. Its right surface is flat, but the aorta (Figure 13–13/4) bulges laterally on the left, indenting the left lung. The middle part at this level contains the heart (within the pericardium), while the ventral part, between the pericardium and sternum, is folded, resembling the greater omentum, and empty but for the phrenicopericardiac ligament, which attaches the pericardium to the sternum and diaphragm more loosely than the tether provided by the corresponding sternopericardiac ligament of the larger species.


Figure 13–13 A, Transverse section of the canine trunk at the level of the sixth thoracic vertebra. B, Corresponding computed tomographic image at a slightly more caudal level. 1, Caudal angle of scapula; 2, sixth thoracic vertebra; 3, esophagus; 4, aorta; 5, tracheal bifurcation; 5′, large blood vessels accompanying principal bronchi are likely right and left pulmonary aa.; 6, right lung; 7, tracheobronchial lymph nodes and pulmonary a.; 8, right atrium; 9, origin of aorta; 10, right ventricle; 11, interventricular septum; 12, fifth rib; 13, sternum; 14, left auricle.
The triangular dorsal part of the caudal mediastinum contains the aorta and the right azygous vein and, more ventrally, the esophagus (Figure 13-12, A, through Figure 13-16). The delicate ventral part runs between the pericardium and the diaphragm, which it approaches along a line that is displaced so far to the left that it reaches the thoracic wall near the ninth costochondral junction. There is the usual recess between the mediastinum and the fold enclosing the caudal vena cava that is occupied by the accessory lobe of the right lung.
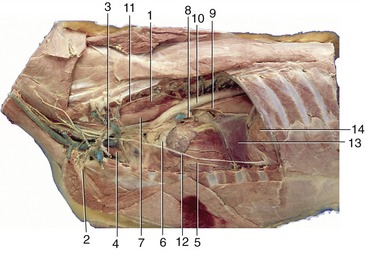
Figure 13–14 Left lateral view of the canine thoracic cavity; the lung and much of the pericardium have been removed. 1, Longus colli; 2, left subclavian artery; 3, internal thoracic vessels; 4, thymus; 5, vessels in paraconal interventricular groove; 6, pulmonary trunk; 7, esophagus; 8, pulmonary veins entering left atrium; 9, left principal bronchus and dorsal and ventral vagal trunks; 10, aorta; 11, sympathetic trunk; 12, phrenic nerve; 13, caudal mediastinum; 14, diaphragm.
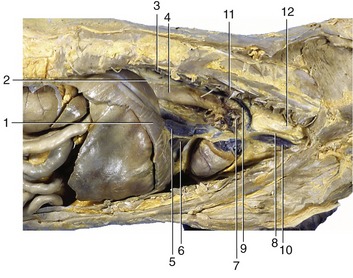
Figure 13–15 Right lateral view of the canine thoracic cavity; the lung and much of the pericardium have been removed. 1, Diaphragm; 2, infracardiac bursa; 3, sympathetic trunk; 4, esophagus; 5, caudal vena cava; 6, plica venae cavae; 7, root of lung and phrenic nerve; 8, right vagus; 9, right azygous vein; 10, cranial vena cava; 11, longus colli; 12, trachea.
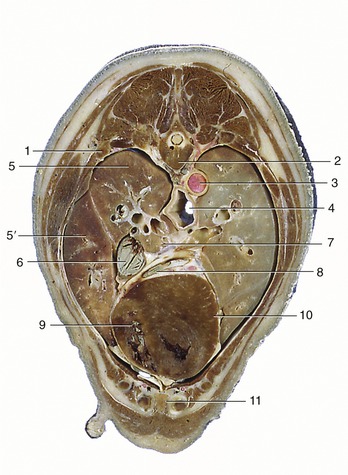
Figure 13–16 Transverse section of the canine trunk at the level of the seventh thoracic vertebra. 1, Sixth rib; 2, seventh thoracic vertebra; 3, aorta; 4, esophagus; 5, cranial lobe; 5′, middle lobe of right lung; 6, caudal vena cava; 7, pulmonary veins passing to left atrium; 8, great cardiac vein; 9, right ventricle; 10, left ventricle; 11, sternum.
A diverticulum of peritoneum, the infracardiac bursa, intrudes through the esophageal hiatus of the diaphragm to lie against the right face of the esophagus, extending from the diaphragm to the root of the lung. It is the occasional recipient of a herniated part of an abdominal organ, either as a congenital anomaly or as the result of trauma.
THE HEART
(See also pp. 228–234.)
The canine heart is ovoid. Its long axis forms an angle of about 45° with the sternum; the base thus faces craniodorsally, and the blunt apex lies near the junction of the sternum and the diaphragm, a little to the left of the midline (Figure13–17, A-B). The angle between the axis of the heart and the sternum and the space between the apex and the diaphragm vary both more considerably than many accounts suggest. The angle is greater and the shape of the heart more conical in deep-chested breeds. Because the position of the heart is biased, a thinner layer of lung tissue intervenes between the heart and the left thoracic wall, resulting in the heart sounds being more pronounced on the left side (see Figure 13–10, Figure 13–17, A-B, and Figure 13–21, A-B).
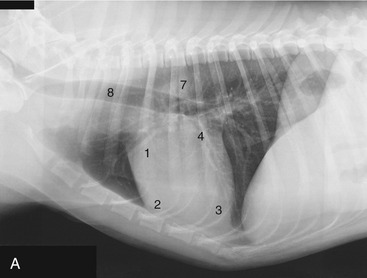
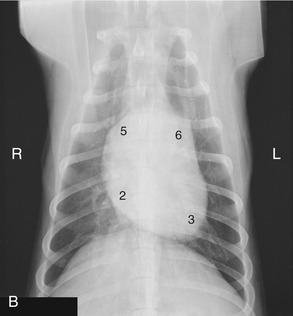
Figure 13–17 Lateral (A) and ventrodorsal (B) views of the position of the canine heart. 1, Right auricle; 2, right ventricle; 3, left ventricle; 4, left atrium; 5, right atrium; 6, pulmonary trunk; 7, aorta; 8, trachea.
The heart contributes about 0.7% of the body weight on average, but its weight, both absolute and relative, varies considerably. Dogs trained for hunting or racing have hearts two or three times heavier than those of fat and less athletic individuals of comparable size.
The left surface presents the auricles embracing the pulmonary trunk, and below the coronary groove the ventricles are divided by the paraconal interventricular groove (see Figure 13–14). The right surface presents the atria and the subsinuosal interventricular groove. Neither surface faces quite as its name suggests; the left surface is rotated a little more toward the sternum, and the right one is a little more toward the vertebrae. When one reads counterclockwise from the base, the periphery of the heart shadow in a left lateral radiograph presents the right auricle, the right ventricle, the left ventricle, and the left atrium (Figure 13–17/1-4); in a ventrodorsal radiograph the sequence is right atrium, right ventricle, left ventricle, and pulmonary trunk (Figure 13–17/2,3,5,6). The apex is formed only by the wall of the left ventricle.
It is clearly important to know the relationships of the parts of the heart to external landmarks. The heart extends from the third rib to the sixth intercostal space, and the latter limit roughly coincides with the most cranial extent of the diaphragm (Figure 13–17, A). The projection of the base intersects the middle of the fourth rib; the most dorsal part of the heart reaches approximately to the line connecting the acromion with the ventral end of the last rib. The apex lies just to the left of the second last sternebra. In the standing dog the apex beat is palpable on both sides, low in the fifth or sixth intercostal space. The main contractions are said to be strongest in the lower third of the fourth or fifth space and to be a little more pronounced on the left. The ductus arteriosus or its replacement, the ligamentum arteriosum (p. 255), is located where the pulmonary trunk is intersected by the left vagus, opposite the fourth rib (see Figure 13–14). These details are relevant to the diagnosis and surgical treatment of persistent ductus arteriosus, the most common congenital anomaly of the canine cardiovascular system. Among other signs, a persistent ductus produces a characteristic “machine” murmur. The condition can be treated by ligation and section of the duct. It may be reached by a left lateral thoracotomy with the use of the fourth intercostal space. The same approach provides access to the right ventricle, left auricle, pulmonary trunk, and descending aorta. (The fourth space on the right side may be used to gain access to the main part of the right ventricle, both atria, the ascending aorta, and both the caval and the azygous veins.)
The heart is more easily auscultated than in the larger species because it is less covered by the forelimbs and a stethoscope can be introduced deeply into the axilla. The puncta maxima for optimal perception of the valve sounds may be summarized: the left atrioventricular valve—low (at the costochondral junction) in the left fifth intercostal space; the pulmonary valve—low in the left third space; the aortic valve—high (just below the horizontal plane of the shoulder joint) in the left fourth space; the right atrioventricular valve—high (just a little lower than the location of the aorta at the left side) in the fourth space on the right side (Figure 13–5, A-D). These findings correspond surprisingly closely with those determined at postmortem examinations of dogs diagnosed in life as having valvular lesions, despite the distorting influence of tissues on the conduction of sound.
There are no significant structural peculiarities of the canine heart, although it may be noted that the right atrioventricular valve possesses only two major cusps in many (perhaps most) dogs. No clinical significance attaches to the variation.
In North America many dogs are infested with large heartworms (Dirofilaria immitis), which occupy the pulmonary trunk and, in severe cases, the right ventricle, atrium, and caudal vena cava.
The heart of the cat extends from the third (or fourth) to the sixth (or seventh) rib. Little is covered by the forelimb in the standing animal because the triceps reaches no farther than the fourth rib. The long axis of the heart forms a more acute angle with the sternum, which results in a greater area of sternal contact than in most dogs. The contractions are strongest near the ventral ends of the fourth to sixth ribs on the left and the fifth rib on the right (Figure 13–18). The corresponding puncta maxima are as follows: the left atrioventricular valve—in the fifth and sixth intercostal space, level with the shoulder joint; the pulmonary and aortic valves—low in the left second and third intercostal space; and the right atrioventricular valve—level with the shoulder joint in the fourth and fifth intercostal space. Puncture is difficult because the organ is so small; a needle inserted on either side of the right fifth costochondral junction should enter a ventricle.
THE ESOPHAGUS, TRACHEA, AND THYMUS
(See also pp. 119–121, 157–158, and 265–267.)
The esophagus enters the thoracic cavity to the left of the trachea but gradually assumes a median position above the trachea within the cranial mediastinum, where it is related to the left subclavian artery, which intervenes between it and the left lung (see Figure 13–14). It continues dorsal to the trachea and subsequently to the left principal bronchus, where it crosses the heart before passing between the aorta and the azygous vein. Inclusion between these vessels and perhaps also the slight rise over the tracheal bifurcation predispose this part to obstruction by foreign bodies. A potentially more serious interference with the passage of food may be provided by the anomaly in which the right aortic arch persists as part of a constricting ring composed of the aorta to the right, the ligamentum arteriosum dorsally, and the pulmonary trunk and right pulmonary artery to the left (see Figure 7–2, D). More caudally, the esophagus rests on the left atrium and then on the accessory lobe of the right lung before reaching the hiatus in the diaphragm below the tenth thoracic vertebra. A slight narrowing here provides another site for obstruction. The chief blood supply from the bronchesophageal artery is supplemented by direct branches from the aorta; the most caudal stretch is supplied by branches of the left gastric artery.
Cranial to the heart, a surgical approach to the esophagus is easier from the left; level with the heart, the approach from the right is favored because the azygous vein may be ligated with impunity, unlike the aorta to the left. The caudal section is equally approachable from either side.
The muscle is striated throughout the length of the esophagus in both dog and cat; only the caudal section is extensively covered by serosa. Glands are present in the submucosa only in the dog. The mucosa is thrown into ridges; these are predominately longitudinal throughout the length of the esophagus of the dog but acquire an oblique orientation in the caudal part of the esophagus of the cat. These differences are responsible for the radiographic appearance after a barium swallow: longitudinal streaks are replaced caudal to the heart in cats by a herring-bone pattern (Figure 13–19, B).
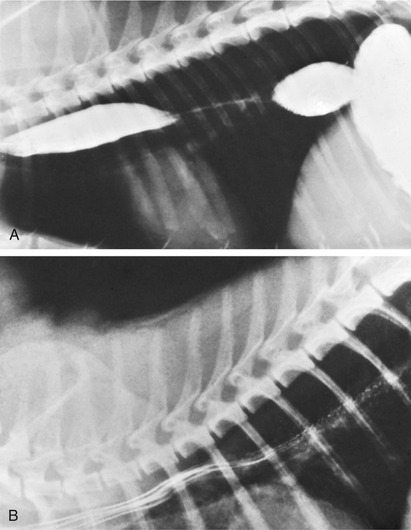
Figure 13–19 Contrast medium in the esophagus of the dog (A) and cat (B). Note the herring-bone pattern caused by the oblique folds in the caudal part of the feline esophagus.
The relationship of the trachea to the esophagus has been mentioned. The shift to a position ventral to the esophagus at the level of the aortic arch produces a caudally open angle that is a very prominent feature of lateral radiographs (Figure 13–17/8 and Figure 13–18). Changes in this angle may reveal abnormalities of various cranial mediastinal structures. The relations of the trachea in this region are with the brachiocephalic trunk, common carotid arteries, and cranial vena cava. The trachea bifurcates below the fifth or sixth thoracic vertebra where it lies above the base of the heart. It is continued by the divergent principal bronchi, of which the left one is at a slightly more dorsal level, despite having the esophagus resting on it.
There is some difficulty in deciding when the diameter of the trachea transgresses beyond its normal limits. Two measures have been proposed for the evaluation of its diameter in lateral radiographs. According to one, the tracheal diameter at the level of the third rib should be about three times the width of that rib; the alternative measure asserts that the height of the trachea should be about half that of the thoracic inlet. When the latter criterion is used, dogs with severe tracheal hypoplasia can exhibit a ratio that is only a small fraction of this. In this condition the deformed tracheal rings are small, thickened, and have ends that meet dorsally, displacing the tracheal muscle inward, toward the lumen. It may be part of a wider “brachycephalic syndrome.” Stretches of trachea reduced in size but otherwise normal have been recorded in dogs of certain large breeds. Collapse of the trachea along with abnormality of its cartilages, and sometimes also of those of the bronchi, occurs in dogs of miniature breeds.
In the dog, the thymus is confined to the thorax, where it occupies the ventral part of the cranial mediastinum, stretching from the thoracic inlet to the pericardium on which it is molded (see Figure 13–14 and Figure 13–11; see also Figure 13–20). A larger part extends onto the left surface of the pericardium than onto the right, which produces a characteristic shadow (sail sign) in dorsoventral radiographs of young dogs (those less than a year old). The thymus consists of right and left lobes, is distinctly lobulated, is pink when fresh, and attains its greatest development at about 6 to 8 weeks. Regression begins about the fourth month but is never complete. Thymic neoplasms may compress the cranial vena cava and esophagus at the thoracic inlet.
THE GREAT VESSELS AND NERVES WITHIN THE THORAX
(See also pp. 243, 253, 319, and 321.)
The aorta is slightly expanded where it arises from the base of the heart between the pulmonary trunk to the left and the right atrium to the right, which provides room for the aortic valve (Figure 13–21/4); it first passes craniodorsally before turning back to follow the vertebrae toward the diaphragm (see Figure 13–14). Its arch, which is a prominent feature on lateral radiographs (Figure 13–17/7), gives rise to the brachiocephalic trunk and, a short distance farther on at the level of the third intercostal space, to the left subclavian artery (Figure 13–14/2). The brachiocephalic trunk lies ventral to the esophagus and trachea and detaches the two common carotid arteries that accompany these organs through the thoracic inlet before it continues as the right subclavian artery; this gradually shifts to the right before winding round the first rib to enter the forelimb. It is reported that the loss of a subclavian artery will be compensated by the enlargement of collateral connections with the vertebral and other arteries.
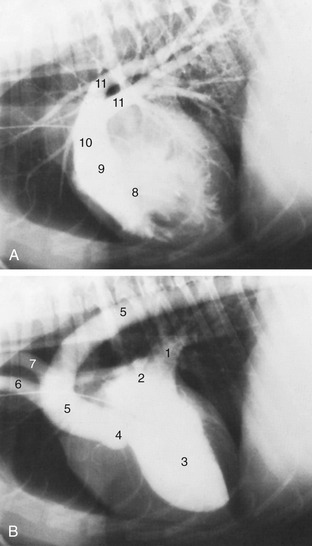
Figure 13–21 Contrast medium in the canine right (A) and left (B) ventricles marking the great vessels. The catheter is in the cranial vena cava. 1, Pulmonary veins; 2, left atrium; 3, left ventricle; 4, position of aortic valve; 5, aorta; 6, brachiocephalic trunk; 7, left subclavian artery; 8, right ventricle; 9, position of pulmonary valve; 10, pulmonary trunk; 11, pulmonary arteries.
The pulmonary trunk arises from the craniosinistral aspect of the base of the heart to the left of the aorta. It passes dorsocaudally before dividing into divergent left and right pulmonary arteries (Figure 13–21, A/10,11). Shortly before its division, it is connected to the aorta by the ligamentum arteriosum. The right pulmonary artery, slightly larger than the left, passes across the base of the heart between the venae cavae; each artery detaches a branch to the cranial lobe before entering the lung for further ramification.
The cranial vena cava passes ventral to the trachea, to the right of the brachiocephalic trunk and in contact with the esophagus on the left side (see Figure 13–15). It is the most ventral of the major structures that pass through the thoracic inlet and is formed cranial to the inlet by the union of the two brachiocephalic veins, each with tributaries corresponding to the branches of a subclavian artery (see Figure 7–36), and is augmented by the addition of an external jugular vein.
The caudal vena cava spans the gap between the right atrium and the diaphragm and provides a very conspicuous feature of lateral radiographs of the chest. The dog possesses a right azygous vein that receives the more cranial lumbar veins and, after entering the thorax, most intercostal veins; these provide potentially significant connections with the internal vertebral venous plexus (p. 252). The azygous vein ends by descending in front of the root of the right lung to join the cranial vena cava shortly before this opens into the right atrium opposite the third intercostal space.
There are no specific features of interest in the formation, course, or distribution of the phrenic, vagus, and sympathetic nerves.
LYMPHATIC STRUCTURES OF THE THORAX
(See also pp. 259–260.)
A single intercostal lymph node may be present under the pleura at the dorsal end of the fifth or sixth intercostal space. It drains the structures of the dorsal thoracic wall and sends its efferent vessels to the cranial mediastinal nodes (Figure 7–55/6).
The sternal lymph nodes are large—up to 2 cm in length—and lie embedded in fat beside the sternum at the level of the second rib. They receive lymph from the muscles of the ventral thoracic wall, the diaphragm, and the mediastinum and may collaborate with the axillary lymph nodes in draining the first three pairs of mammary glands. Their efferent vessels go to veins at the thoracic inlet (Figure 7–55/10).
The cranial mediastinal lymph nodes are variously related to the large blood vessels in front of the heart. They drain structures in the mediastinum (including the tracheobronchial nodes) and the deep muscles at the base of the neck. Their outflow also enters the veins at the thoracic inlet (Figure 7–55/8).
The tracheobronchial lymph nodes (Figure 13–13, A) are scattered about the termination of the trachea and the principal bronchi. They drain the lungs as well as mediastinal structures and part of the diaphragm. Their efferent vessels pass to the cranial mediastinal nodes.
The thin-walled thoracic duct begins between the crura of the diaphragm as the continuation of the cisterna chyli. It accompanies the aorta and azygous vein forward and, level with the heart, passes obliquely to the left, crossing the esophagus, to gain a position within the left side of the cranial mediastinum. It follows the esophagus to the thoracic inlet, where it opens into one or other of the larger veins; occasionally it ends more caudally, joining the azygous vein or even opening into one of the mediastinal lymph nodes. The duct, which has a diameter of 2 to 3 mm in a medium-sized dog, may be plexiform (Figure 7–57). Within the chest it receives additional lymph from various thoracic structures and nodes of the left side; a separate right lymphatic duct provides similar drainage for structures of the right side. One or both commonly receive the corresponding tracheal duct(s). In cats the thoracic duct courses from the left dorsal aspect of the aorta to terminate in the left jugular vein. In both species the thoracic duct may have multiple collaterals.