7 The Cardiovascular System
The blood vascular and lymphatic systems are combined under a single heading, angiologia, in the official terminology. Angiology strictly means the study of vessels, but its scope is conveniently enlarged to include the heart, spleen, and various lymphatic organs in addition to the arteries, veins, and other vessels.
A circulatory system is essential to any organism that exceeds that relatively trivial size in which diffusion can deliver the metabolic fuel and other substances required by the tissues and convey away their products, whether waste for excretion or materials that are utilized elsewhere. Obviously, the critical mass must vary with the level of metabolic activity. It is soon reached in the rapidly growing mammalian embryo, in which the circulatory system, although not the first to be laid down, is the first body system to reach a “working state.”
The circulatory organs and the blood cells have a common origin in clusters of mesenchymal cells that first appear in the wall of the yolk sac. The outermost cells of these “blood islets” flatten and become arranged as an endothelium that lines spaces in which the remaining cells, hemocytoblasts or stem blood cells, float within a fluid plasma. The islets first formed are soon supplemented by others that appear in the mesoderm of the chorioallantois and within the body of the embryo; as the various patches spread and link up they form a diffuse system of connecting vessels that is then extended further by branching from existing channels. The principal vessels thus form independently of each other and in relation to the appearance and growth of the regions and organs of the embryo.
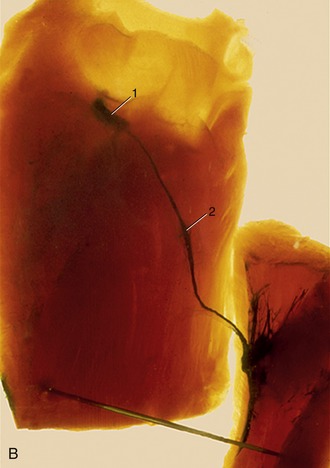
Because no proper circulation through this system can occur until a means of pumping blood is created, the heart necessarily makes a very early appearance. It is formed by differentiation of channels within a part of the mesoderm appropriately known as the cardiogenic area. This area lies in front of the oral membrane of the discoidal embryo, and the heart rudiments are related from the outset to the most rostral of the tissue spaces that later coalesce to form the celomic cavity, which divides the somatopleure from the splanchnopleure. The cardiogenic area, including both heart and pericardial rudiments, becomes folded ventrally and carried caudally in the process that converts the embryonic disk into a cylindrical body (p. 100). At this stage the heart consists of paired endothelial (endocardial) tubes placed ventral to the foregut, but these shortly fuse to form a single median organ that gradually shifts caudally to the level of the thoracic somites (Figure 7–1/5,7).
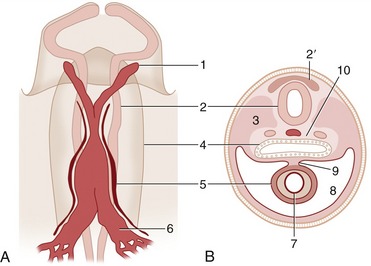
Figure 7–1 A, Ventral view of the cranial part of a 15-day-old pig embryo after fusion of the endocardial tube. B, Transverse section of a seven- to eight-somite embryo taken at the level of 5. 1, First aortic arch; 2, neural tube; 2′, neural crest; 3, somite; 4, foregut; 5, epimyocardial wall of the fused endocardial tubes; 6, vitelline vein; 7, endocardial tube; 8, pericardial cavity; 9, dorsal mesocardium; 10, notochord and dorsal aortae.
From the beginning the heart is connected at one extremity with the vessels that become the aorta and at the other with those that form three sets of veins: the vitelline (omphalomesenteric) veins that drain the yolk sac, the umbilical veins that drain the chorioallantoic placenta, and the cardinal veins that drain the body. The ventral aorta, continuous with the heart, is soon joined to an independently formed dorsal aorta by a system of aortic loops contained within the pharyngeal (branchial) arches lateral to the pharynx (Figure 7–2). It is possible to trace the origin of certain arteries of adult anatomy from the six pairs of aortic arches that develop (although not all persist), but the reader must refer to textbooks of embryology for details of this process and for a description of the even more complicated evolution of the veins. The reader is reminded that a hallmark of the developing circulatory system is its ability to respond to changing functional requirements by refashioning the pattern of vessels, always retaining obsolescent parts until their replacements have become operative.
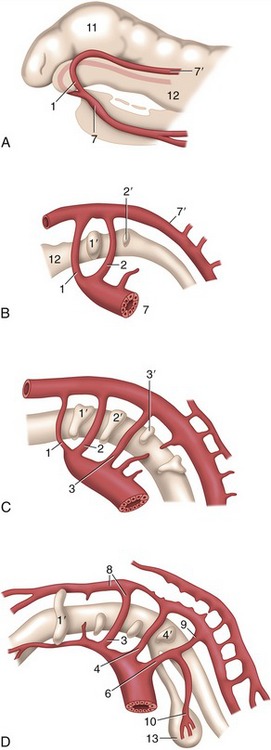
Figure 7–2 Left lateral view of the aortic arches and their transformation. A, Dorsal and ventral aortae are connected by the first aortic arches. B, First and second aortic arches are present. C, The first arch begins to disappear, the third is complete, and the fourth and sixth develop. D, The third arch and the cranial part of the dorsal aorta are now transformed into the internal carotid artery, while the sixth gives rise to the pulmonary trunk and ductus arteriosus. 1–4, 6, Aortic arches; 1′–4′, pharyngeal pouches; 7, 7′, ventral and dorsal aortae; 8, internal carotid artery; 9, ductus arteriosus; 10, left pulmonary artery; 11, brain vesicle; 12, foregut; 13, lung bud.
Descriptions of the development of the heart itself (p. 234) and of the particularly dramatic changes that occur in the circulation at birth (p. 256) are found later in this chapter.
THE HEART
The heart (cor) is the central organ that pumps blood continuously through the blood vessels by rhythmic contraction. In the adult it consists of four chambers: right atrium, left atrium, right ventricle, and left ventricle (Figure 7–3). The two atria are separated by an internal septum as are the two ventricles, but the atrium and ventricle of each side communicate through a large opening. The heart consists of two pumps that are combined within a single organ. The right pump receives deoxygenated (venous) blood from the body and ejects it into the pulmonary trunk, which carries it to the lungs for reoxygenation. the left pump receives the oxygenated blood from the lungs via the pulmonary veins and ejects it into the aorta, which distributes it to the body (Figure 7–4).
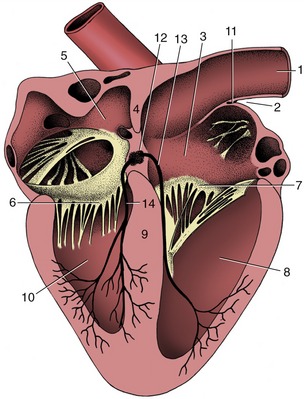
Figure 7–3 Section of the heart exposing the four chambers. 1, Cranial vena cava; 2, terminal sulcus; 3, right atrium; 4, interatrial septum; 5, left atrium; 6, left atrioventricular valve; 7, right atrioventricular valve; 8, right ventricle; 9, interventricular septum; 10, left ventricle; 11, sinuatrial node; 12, atrioventricular node; 13, 14, right and left limbs of atrioventricular bundle.

Figure 7–4 Schematic drawing of the systemic and pulmonary circulation. 1, Left ventricle; 2, aorta; 3, capillary bed of head, neck, and forelimb; 4, abdominal aorta; 5, liver; 6, capillary bed of intestines; 7, portal vein; 8, capillary bed of kidneys; 9, capillary bed of caudal part of the body; 10, caudal vena cava; 11, cranial vena cava; 12, right ventricle; 13, pulmonary trunk; 14, capillary bed of lungs; 15, pulmonary vein; 16, hepatic veins.
The size of the heart varies considerably among species and also among individuals; as a rule it is relatively larger in smaller species and in smaller individuals, but it may become markedly hypertrophied by hard training. As a rough guide it may be said to provide about 0.75% of the body weight but less than that in lethargic animals and considerably more in those renowned athletes—the Thoroughbred horse and racing Greyhound.
The construction, the form, and the general position of the heart are similar in all mammals, and as most differences in the first two have only theoretical implications, they receive little attention. Differences in topography do have practical importance because they modify the methods used for clinical examination and the interpretation of the evidence that this examination provides; these points are mentioned in later chapters.
THE PERICARDIUM AND THE TOPOGRAPHY OF THE HEART
The heart is almost completely invested by the pericardium, which fits snugly about it (Figure 7–5). The pericardium is essentially a closed serous sac that is so deeply invaginated by the heart that its lumen is reduced to a mere capillary cleft (Figure 7–5/4). The space contains serous fluid, normally just sufficient in amount to allow easy movement of the heart wall against its covering. The visceral and parietal layers of the pericardium continue into each other at a complicated reflection that runs over the atria and the roots of the great vessels. The visceral layer is so closely adherent to the heart wall that it may be described as a component of this, the epicardium. The parietal layer obtains a thick external fibrous covering (Figure 7–5/6) that blends with the adventitia of the great vessels dorsally and continues into a ligament at the ventral apex of the sac. This usually attaches to the sternum (sternopericardial ligament; Figure 7–5/8) but attaches to the diaphragm (phrenicopericardial ligament) in species in which the heart axis is more oblique. These attachments place a severe restraint on the mobility of the heart, although slight movement does occur with each respiratory excursion.
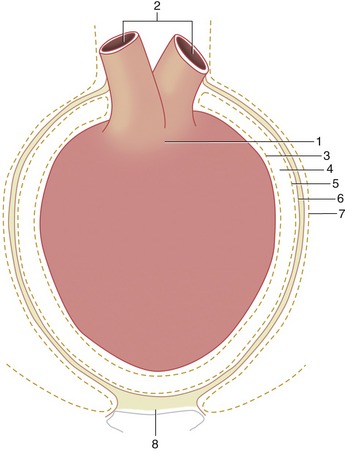
Figure 7–5 Schematic illustration of the pericardium. 1, Heart; 2, great vessels; 3, visceral pericardium (epicardium); 4, pericardial cavity (exaggerated in size); 5, parietal pericardium; 6, connective tissue layer of the parietal pericardium; 7, mediastinal pleura; 8, sternopericardial ligament.
Although the pericardium distorts to accommodate the changing form of the heart during the cardiac cycle, its fibrous component prevents any significant distention in the short term. It may stretch over longer periods should the heart become enlarged by exercise or disease or should effusion or pus collect within the pericardial cavity.
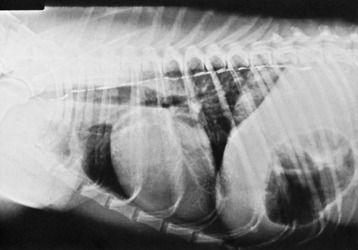
The heart (within the pericardium)* is included within the mediastinum, the partition that separates the right and left pleural cavities (see Figure 4–20, A). It is conical and is placed asymmetrically within the thorax, and the larger part (about 60%) lies to the left of the median plane (see Figures 13–13, B, and 20–8). The base is dorsal and reaches approximately to the horizontal (dorsal) plane that bisects the first rib; in some species (e.g., the dog) it is tilted in varying degree to face craniodorsally. The apex is placed close to the sternum, opposite the sixth costal cartilage. The long axis that joins the center of the base to the apex thus slopes caudoventrally, with some deviation to the left imposed by the skewed orientation (Figure 7–6). The projection of the heart on the chest wall extends between the third and sixth ribs (or thereabouts); thus, much of the heart is under cover of the forelimbs, which is a considerable handicap to clinical examination, especially in larger species (see Figures 20–1, 20–2, and 27–2).
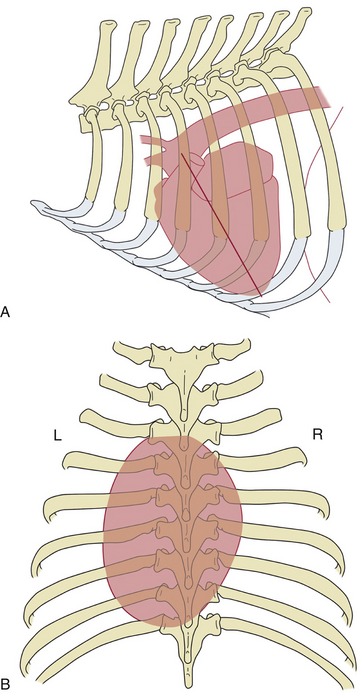
Figure 7–6 Schematic drawings to show the position of the canine heart, based on radiographs. A, Left lateral view; the caudoventrally sloping long axis (straight line) of the heart is indicated. B, Dorsoventral view showing the asymmetrical position of the heart.
Although generally conical, the heart displays some lateral compression to conform to the similar compression of the thorax of most quadrupeds. This better defines right and left surfaces that face toward the corresponding lungs, which are shaped to fit. The cardiac notch in the ventral border of each lung allows the heart a restricted contact with the chest wall, which is normally greater on the left side because of the asymmetrical position (see Figure 13–5). Each lateral surface is also crossed by the corresponding phrenic nerve. The cranial aspect is extensively related to the thymus (in the young animal), but the caudal surface faces toward the diaphragm and may be indirectly related through this to cranial abdominal organs (see Figure 28–14), which is a point of importance in certain species (p. 687).
GENERAL ANATOMY OF THE HEART
The base of the heart is formed by the thin-walled atria, which are clearly separated from the ventricles by an encircling coronary groove that contains the main trunks of the coronary vessels within a concealment of fat. The right and left atria combine in a continuous U-shaped formation that embraces the origin of the aorta; the formation is interrupted craniosinistrally where each atrium ends in a free blind appendage, the auricle (Figure 7–7, A/1), which overlaps the origin of the pulmonary trunk. The margins of the atria are often crenated.
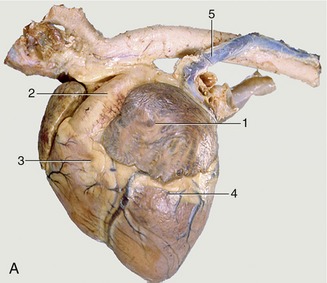
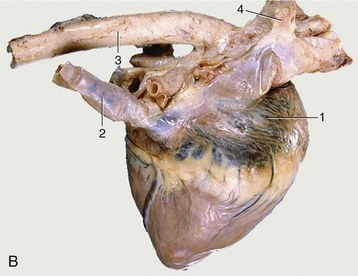
Figure 7–7 Left (A) and right (B) views of the heart. A, 1, Left auricle; 2, pulmonary trunk; 3, right ventricle; 4, left ventricle; 5, left azygous vein. B, 1, Right atrium; 2, caudal vena cava; 3, aorta; 4, right azygous vein (opening into the cranial vena cava).
The ventricles provide a much larger part of the heart that is also much firmer because of the greater thickness of the walls. Although the ventricles merge externally, their separate extents are defined by shallow grooves that descend toward the apex. The paraconal (left) groove runs close to the cranial aspect of the heart (Figure 7–7, A); the subsinuosal (right) groove runs close to the caudal aspect (Figure 7–7, B). Both convey substantial vessels that follow the edges of the interventricular septum; together they reveal the asymmetrical disposition of the ventricles. The right chamber lies as much cranially as to the right of the left one (see Figure 7–10). Additional branches of the coronary vessels extend some distance over the ventricular surface in a less constant pattern, but these apart, the external surface is smooth and featureless. Although it is not apparent externally, a fibrous skeleton separates the atrial from the ventricular muscle mass.
The Right Atrium
This chamber lies mainly on the right, although the auricular cul-de-sac extends to the cranial face of the pulmonary trunk to appear on the left side. The greater part forms a chamber (sinus venarum) into which the principal systemic veins discharge (Figure 7–8/1). The caudal vena cava enters the caudodorsal part of this chamber, above the opening of the much smaller vein (coronary sinus) that drains the heart itself. The cranial vena cava opens craniodorsally at the terminal crest (Figure 7–8/7). An azygous vein enters variously. When a right azygous is present (as in the horse, dog, and ruminants), it enters dorsally, either by joining the cranial vena cava (Figure 7–8/6) or discharging between the caval openings; when a left azygous is present (as in ruminants and the pig), it joins the coronary sinus close to its termination after winding around the caudal aspect of the base from the left side (Figure 7–9, A/12).
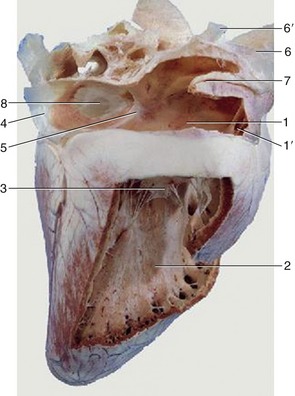
Figure 7–8 Overview of the interior of the right atrium and right ventricle of the equine heart. 1, Right atrium; 1′, right auricle; 2, right ventricle; 3, right atrioventricular valve; 4, caudal vena cava; 5, intervenous tubercle; 6, cranial vena cava; 6′, right azygous vein; 7, terminal crest; 8, fossa ovalis.
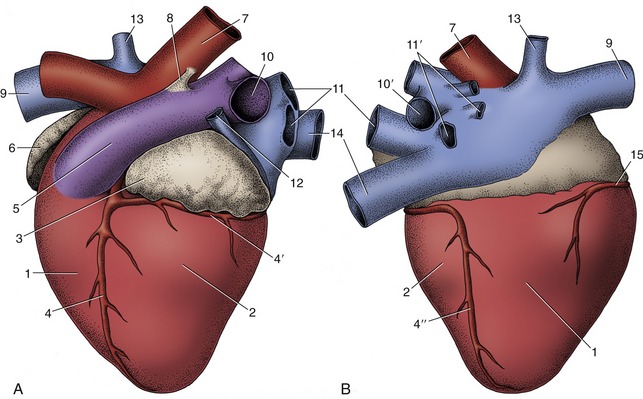
Figure 7–9 Left (A) and right (B) views of the bovine heart. 1, Right ventricle; 2, left ventricle; 3, left auricle; 4, paraconal interventricular branch of left coronary artery; 4′, circumflex branch of left coronary artery; 4″, subsinuosal interventricular branch of left coronary artery; 5, pulmonary trunk; 6, right auricle; 7, aorta; 8, ligamentum arteriosum; 9, cranial vena cava; 10, 10′, left and right pulmonary arteries; 11, 11′, left and right pulmonary veins; 12, left azygous vein; 13, right azygous vein; 14, caudal vena cava; 15, right coronary artery.
The interior of the atrium is smooth between the vein entrances, which are unobstructed by valves. Its roof dips between the caval openings, being indented by the passage of pulmonary veins returning across the right atrium to enter the left atrium. The ridge (intervenous tubercle; Figure 7–8/5) produced by the indentation prevents confrontation between the caval streams by deflecting both ventrally, toward the atrioventricular ostium (Figure 7–8/3) that occupies much of the floor. A depressed membranous area (fossa ovalis; Figure 7–8/8) of the septal wall is present caudal to the tubercle; it corresponds to the foramen ovale of fetal life. In sharp contrast, the interior of the auricle (Figure 7–8/1′) is made irregular by a series of ridges (musculi pectinati) that branch from the terminal crest that marks the boundary between the auricle and the main compartment.
The Left Atrium
This has a generally similar form. It receives the pulmonary veins, which enter, separately or in groups, at two or three sites: craniosinistral, craniodextral, and in some species, caudal (Figure 7–9/11,11′). The septal wall may present a scar marking the position of the valve of the fetal foramen ovale. The auricle resembles that of the right side.
The Right Ventricle
This chamber, crescentic in transverse section, is wrapped around the right and cranial aspects of the left ventricle (Figure 7–10). It is incompletely divided by a stout muscular beam (supraventricular crest) that projects from the roof cranial to the atrioventricular ostium. The main part of the chamber lies below this large elongated opening while the extension to the left, the conus arteriosus (Figure 7–12), leads directly to the much smaller circular exit into the pulmonary trunk.
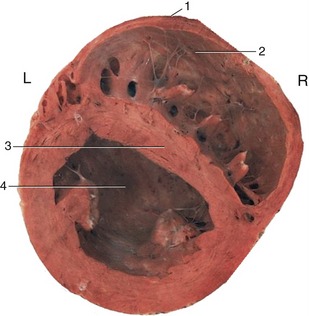
Figure 7–10 Transverse section through the ventricles. Note the different thicknesses of the walls of the right and left ventricles. 1, Most cranial point; 2, right ventricle; 3, interventricular septum; 4, left ventricle.
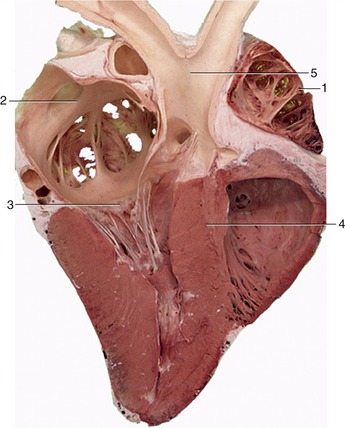
Figure 7–11 Section of the heart (cow). 1, Right auricle; 2, left atrium; 3, left atrioventricular valve; 4, interventricular septum; 5, aorta.
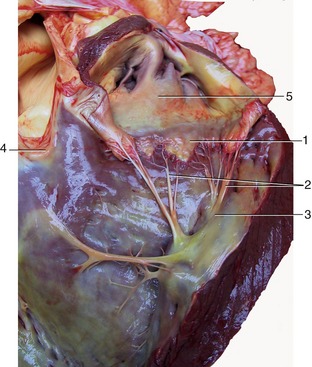
Figure 7–12 Cranioventral view of the interior of the right ventricle. 1, Cusp of right atrioventricular valve; 2, chordae tendineae; 3, papillary muscles; 4, pulmonary valve; 5, right auricle.
The right atrioventricular (tricuspid) valve is composed of three flaps or cusps that attach to a fibrous ring that encircles the opening. The cusps are fused at their attachment but part toward the center of the opening, where their free margins are thick and irregular, especially in later life. Each cusp is joined by fibrous strands (chordae tendineae) that descend into the ventricular cavity to insert on projections from the walls (papillary muscles). Generally, three of these muscles are present, and the chordae tendineae are so arranged that they connect each cusp to two muscles and each muscle to two cusps (Figure 7–12/2,3). The arrangement prevents eversion of the cusps into the atrium during ventricular contraction (systole). The lumen of the ventricle is crossed by a thin band of muscle (trabecula septomarginalis) that passes from the septal to the outer wall (see Figure 7–16, B/2). It provides a shortcut for a bundle of the conducting tissue, thus ensuring a more nearly simultaneous contraction of all parts of the ventricle (see Figure 7–3). A further modification of the muscle is provided by the many irregular ridges (trabeculae carneae) that give the lower part of the wall a spongy appearance. These are confined to the “inflow” part of the cavity and are thought to reduce blood turbulence.
The opening into the pulmonary trunk lies at a more dorsal level than the atrioventricular ostium and is craniosinistral to the origin of the aorta. It is closed during ventricular relaxation (diastole) by the backflow of blood forcing together the three cusps that arise around its margin and constitute the pulmonary valve (Figure 7–13/4). The cusps are semilunar and deeply hollowed on the arterial side, fitting together tightly when the valve is closed; thickenings of the contact areas, sometimes pronounced in older animals, improve the seal.
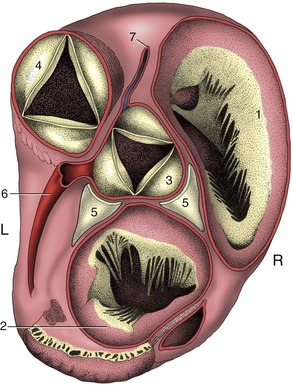
Figure 7–13 Dorsal view of the base of the bovine heart after removal of the atria. The ossa cordis on both sides of the aortic valve have been exposed. 1, Right atrioventricular valve; 2, left atrioventricular valve; 3, aortic valve; 4, pulmonary valve; 5, ossa cordis; 6, left coronary artery; 7, right coronary artery.
The Left Ventricle
This chamber is circular in section (see Figure 7–10) and forms the apex of the heart as a whole. Except toward the apex, its wall is much thicker than that of the right ventricle in conformity with the greater work it must perform; however, the impression that the chamber is also much smaller is illusory. The left atrioventricular (bicuspid or mitral) valve that closes the atrioventricular ostium generally has only two major cusps but is otherwise comparable to that of the right side. It lies largely to the left of the median plane (Figure 7–11/3 and 7–13/2). The exit to the aorta takes a more central position within the heart.
The aortic valve, generally resembling the pulmonary valve, shows a different orientation of its cusps (Figure 7–13/3). The nodular thickenings in the free margins of the aortic cusps are conspicuous.
THE STRUCTURE OF THE HEART
The thick middle layer of the wall (myocardium) is composed of cardiac muscle, which is a variety of striated muscle peculiar to this organ. It is covered externally by the visceral pericardium (epicardium) and internally by the endocardium, a thin smooth-surfaced layer continuous with the lining of the blood vessels.
The atrial and ventricular parts of the muscle are separated by a fibrous skeleton that is mainly formed by the conjunction of the rings that encircle the four heart orifices. The skeleton contains islands of fibrocartilage in which nodules of bone (ossa cordis) may develop (Figure 7–13/5). Although these bones appear precociously in the hearts of cattle, they are not confined to this species as is sometimes suggested. The fibrous skeleton is perforated in one place (near the entrance of the coronary sinus) to allow passage to the atrioventricular bundle of specialized tissue that conducts the impulse to contract and constitutes the only direct connection between the atrial and ventricular muscles. Delicate extensions of the fibrous tissue also provide the cores of the cusps of the various valves.
The atrial muscle is thin—indeed, the auricular wall may be translucent between the pectinate ridges. It is arranged in superficial and deep bundles; some of the former are common to both atria, but the remainder, and all of the deep bundles, are confined to one. It has been postulated that the fascicles that surround the various venous inlets, both systemic and pulmonary, act as throttles to oppose reflux of blood into the veins during atrial systole.
The much thicker ventricular muscle is also arranged in superficial and deep bundles. Some superficial bundles coil around both chambers, utilizing the septum to complete a figure-of-eight course. Others, like the deeper bundles, encircle only the one chamber. The arrangement of the muscle is actually very complicated, and analyses of the contraction mechanism still leave much obscure.
The inherent rhythm of the heart is controlled by a pacemaker, a small, richly innervated sinuatrial node of modified cardiac fibers (nodal myofibers) that provide the conducting tissue (Figure 7–15, A). This node, which is not apparent to the naked eye, lies below the epicardium of the right atrial wall ventral to the cranial caval opening (Figure 7–3/11). With each heart cycle a wave of excitation, which arises in the sinuatrial node and spreads throughout the atrial muscle, reaches the atrioventricular node (Figure 7-14 and Figure 7-15, B-C). In ungulates, specialized conductive tissue is present subendocardially in the atrium, mainly on the pectinate muscle. From the atrioventricular node an excitatory stimulus passes rapidly throughout the whole ventricular myocardium via the atrioventricular bundle, largely composed of Purkinje fibers, modified cardiac muscle fibers that conduct impulses much more rapidly than those of the common sort (see Figure 7–14). The atrioventricular node consists of modified nodal and Purkinje fibers and is found within the interatrial septum, cranial to the opening of the coronary sinus; it is richly innervated. This node gives origin to the atrioventricular bundle, which penetrates the fibrous skeleton before dividing into right and left limbs (crura) that straddle the interventricular septum (Figure 7–16, A-B). Each limb continues ventrally close to the endocardium and branches to reach all parts of the heart muscle; part of the right bundle travels to the outer wall by way of the septomarginal band. The main conducting structures are not difficult to display by dissection of the beef heart.

Figure 7–14 Schematic drawing of the conducting system of the heart. The broken lines suggest the passage of the excitation wave through the atrial wall. 1, Sinuatrial node; 2, atrioventricular node; 3, atrioventricular bundle; 4, left limb; 5, right limb; 6, branch of right limb traversing the septomarginal band.
CARDIAC VESSELS AND NERVES
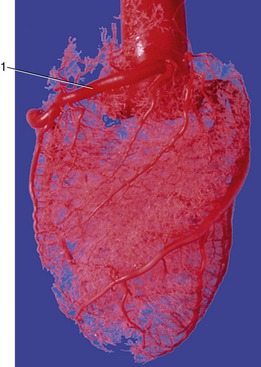
The heart is lavishly supplied with blood, receiving about 15% of the output of the left ventricle. The supply is led through the coronary arteries that spring from two of the three sinuses above the semilunar cusps at the beginning of the aorta (Figure 7–17).
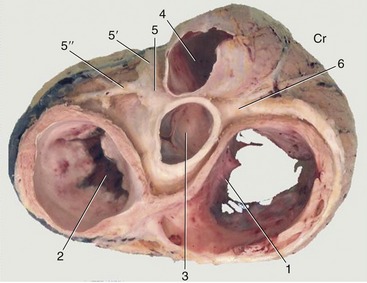
Figure 7–17 Dorsal view of the base of the heart after removal of the atria. The coronary arteries are exposed. 1, Right atrioventricular valve; 2, left atrioventricular valve; 3, aortic valve; 4, pulmonary valve; 5, left coronary artery; 5′, paraconal interventricular branch; 5″, circumflex branch; 6, right coronary artery. Cr, cranial.
The left coronary artery is usually the larger. It arises above the caudosinistral cusp and reaches the coronary groove by passing between the left auricle and the pulmonary trunk; it divides almost at once. The left (paraconal) interventricular branch follows the like-named groove toward the apex of the heart (Figure 7–18/2′). The trunk continues as a circumflex branch (Figure 7–18/2″) that follows the coronary groove toward the caudal aspect of the heart, where it may terminate close to the origin of the right (subsinuosal) interventricular groove (horse and pig) or continue into this (carnivores and ruminants) (Figure 7–19, A-B, and Figure 7–20).
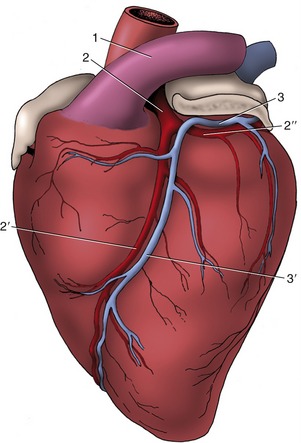
Figure 7–18 Branching of the left coronary artery of the heart, viewed from the left. The left auricle has been shortened. 1, Pulmonary trunk; 2, left coronary artery; 2′, paraconal interventricular branch; 2″, circumflex branch; 3, great cardiac vein (continued by the coronary sinus on the right side of the heart); 3′, paraconal interventricular tributary of 3.
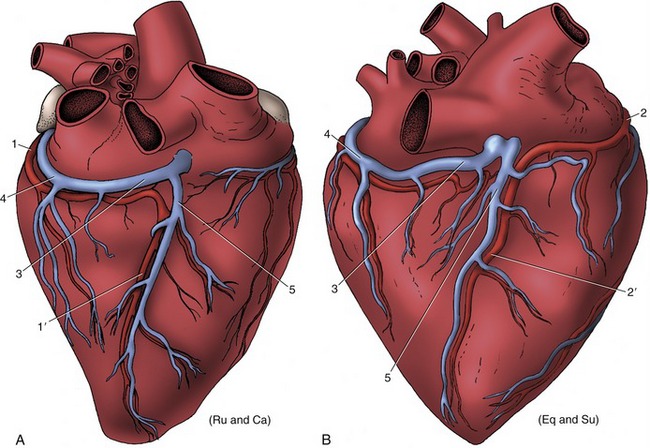
Figure 7–19 Patterns of coronary circulation of the heart viewed from the right. A, Situation in ruminants and carnivores; the right (subsinuosal) interventricular branch (1′) is a continuation of the left coronary artery. B, Situation in the horse and pig; the right (subsinuosal) interventricular branch (2′) is a continuation of the right coronary artery. Ru (ruminants), Ca (cat), su (pig), eq (horse). 1, Circumflex branch of left coronary artery; 1′, right (subsinuosal) interventricular branch; 2, right coronary artery; 2′, right (subsinuosal) interventricular branch; 3, coronary sinus; 4, great cardiac vein; 5, middle cardiac vein.
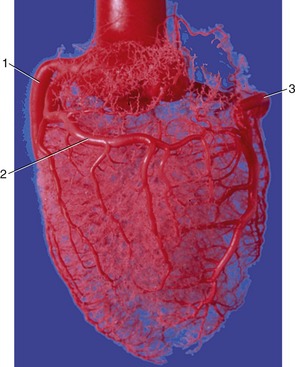
Figure 7–20 Corrosion cast of aorta and coronary circulation (pig). 1, Left coronary artery; 2, ramus circumflexus; 3, right coronary artery.
The right coronary artery arises above the cranial cusp (Figure 7–17/6) and reaches the coronary groove after passage between the right auricle and pulmonary trunk. It pursues a circumflex course that either fades toward the origin of the subsinuosal groove or turns into it in those species in which the left artery has the restricted distribution. Both coronary arteries send other branches, of varying size and constancy of position, to neighboring parts of the atrial and ventricular walls. Very small twigs extend some distance into the cores of the valve cusps (Figure 7–21).
Anastomoses are not formed between the main branches of the coronary arteries but are numerous between the lesser branches. Even so, sudden closure of one of these small vessels cannot usually be compensated; it leads to local infarction of the cardiac muscle.
Blood is principally returned to the heart through the great cardiac vein that opens separately into the right atrium via the coronary sinus (Figure 7–19/3,4). Rather surprisingly, many very small (thebesian) veins open directly into all four heart chambers.
The innervation of the heart is complicated topographically, but happily the details mainly concern physiologists. A sympathetic contribution is routed through the caudal cervical and first few thoracic ganglia of the sympathetic trunk. The postganglionic fibers form cardiac plexuses within the cranial mediastinum before extending to the heart wall (Figure 7–22). Parasympathetic fibers branch from the vagus nerves, either directly or after short passage within the recurrent laryngeal nerves. They end on nerve cells in the heart wall, especially within and about the sinuatrial and atrioventricular nodes. Many of the postganglionic fibers pass to the nodes, but others reach the periphery of the heart by following the atrioventricular bundle and its branches.
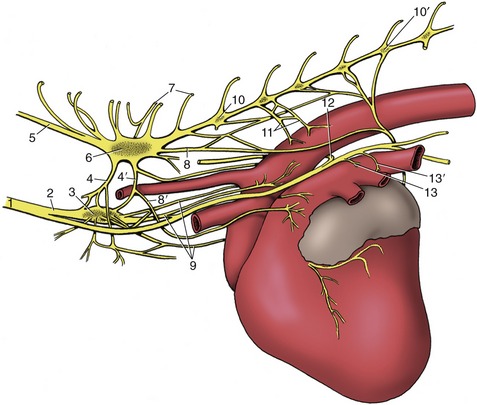
Figure 7–22 Cardiac nerves and related ganglia of the dog; left lateral view. 1, Vagosympathetic trunk; 2, sympathetic trunk; 3, middle cervical ganglion; 4, 4′, cranial and caudal limbs of ansa subclavia; 5, vertebral node; 6, cervicothoracic ganglion; 7, communicating branches; 8, 8′, caudodorsal and caudoventral cervicothoracic cardiac nodes; 9, vertebral cardiac nodes; 10, 10′, third and seventh thoracic ganglia; 11, thoracic cardiac nodes; 12, left recurrent laryngeal node; 13, 13′, cranial and caudal vagal cardiac nodes.
FUNCTIONAL ANATOMY
An indication of the exacting task required of the heart is provided by the following figures, culled from various sources: 60% of the total volume of blood within the human body passes through the heart each minute, and the corresponding figures for dogs and horses are 80% and 100%, respectively.
Coordinated contraction is essential for efficient pumping; asynchronous contraction of muscle fascicles (fibrillation) is ineffectual and is rapidly fatal when it involves the ventricular muscle. The sinuatrial node is the pacemaker from which the wave of excitation normally spreads to all parts of the muscle; it has the highest rate of spontaneous activity when relieved from external stimuli, but in normal circumstances its discharge is determined by the fine balance of accelerating sympathetic and retarding vagal inputs. The wave of excitation that spreads from the sinuatrial node through the atrial muscle soon reaches the atrioventricular node (Figure 7–14/2 and Figure 7–16, A-B). This does not respond at once, and the short delay permits completion of atrial contraction. The impulse then spreads to the ventricular muscle through the atrioventricular conducting tissue. Although ventricular contraction is almost synchronous, the subendocardial layer, which includes the papillary muscles, gains a slight lead.
The flow of blood is related to these activities. Blood enters the atria for as long as the pressure within the veins exceeds that within the heart. Several factors of uncertain and varying magnitude contribute to the venous pressure. The force exerted upstream (vis a tergo) is the summation of the following: the residual pressure imparted to the blood by ventricular contraction; the forces exerted by muscles, visceral activity, and arterial pulsation; and the contraction of the diaphragm (the so-called abdominal pump) expelling blood from the caudal vena cava and its large tributaries within the abdomen. The downstream force (vis a fronte) oscillates between a negative aspirating effect (provided by thoracic expansion and atrial relaxation) and a positive pressure developed on atrial systole. A lateral pressure may be exerted by contraction of the muscular coat of the great veins. Gravity also plays a part, sometimes assisting and sometimes impeding flow according to posture. Much blood flows directly into the ventricles through open atrioventricular ostia, and only a “topping-up” effect is exerted by the atrial contraction, which coincides with the last stage of ventricular relaxation. When the atria do contract, some blood may reflux into veins (despite the conjectured throttle mechanism already mentioned); a jugular pulse may be visible evidence of this, most seen in cattle.
The pulmonary and aortic (arterial) valves are closed during ventricular relaxation when the arterial pressure exceeds that within these chambers. Ventricular contraction closes the atrioventricular valves; eversion of the cusps into the atria is prevented by the timely contraction of the papillary muscles. As the contraction develops, blood forces the arterial valves open and the conducting arteries are expanded by this sudden input. The two ventricles do not contract identically. The right ventricular lumen is squeezed in a bellows action in which the outer wall is drawn toward the septum (Figure 7–23). The more cylindrical left ventricle contracts radially and in length; radial contraction is believed to have the greater effect.
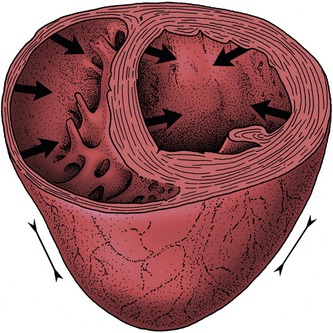
Figure 7–23 Schematic drawing of the mode of contraction of the left and right ventricles. The wall of the left ventricle contracts radially, while the right ventricular lumen is squeezed in a bellows action.
Closure of the heart valves produces distinctive sounds that are audible on auscultation. Their character provides valuable information on the condition of the valves. Because of the vagaries of sound conduction through tissues of different densities, the projections of the heart valves on the chest wall are not necessarily the spots (puncta maxima) where the sounds are most clearly heard. As a rough guide, if species and breed variations and other factors are not considered, it may be said that the pulmonary, aortic, and left atrioventricular valves are best auscultated over the third, fourth, and fifth ribs of the left side, and the right atrioventricular valve is best auscultated over the fourth rib on the right. The arterial valves are somewhat dorsal to the atrioventricular valves, although the slope of the heart is clearly relevant to this detail. Percussion is also used as a means of evaluating the size of the heart. The quality of cardiac dullness contrasts with the high pitch obtained when percussion is performed over the lungs. The boundary of the cardiac area is not sharply defined because the lung tissue covering the heart grades in thickness about the cardiac notch.
THE DEVELOPMENT OF THE HEART
The primitive heart, the single median structure formed by the fusion of paired rudiments, is carried ventral to the foregut by the reversal process reshaping the head end of the embryo (p. 100). Though initially consisting of a simple endothelial tube, the heart soon acquires an investment of mesoderm that forms the myocardial and epicardial components of its wall. The cranial part of the tube, which will later form the truncus arteriosus and ventricles, is at this stage contained within the pericardial cavity and suspended by a fold (dorsal mesocardium) extending between the myoepicardium and the pericardial wall (Figure 7–1, B/9). The caudal part, which forms the atria and sinus venosus, first lies caudal to the pericardial cavity embedded within the septum transversum. The enclosed (truncoventricular) part of the heart grows more rapidly than the pericardial space and is forced into a flexure whose apex is directed ventrocaudally and somewhat to the right. The atrial expansions of the initially paired endothelial tubes have now fused in a single common atrium continuous with the sinus venosus; this presents an unpaired transverse part that receives the paired horns created by the entry of the veins (Figure 7–24).
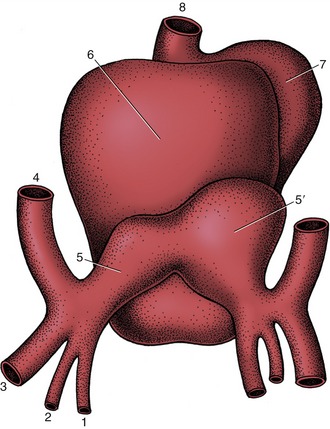
Figure 7–24 Dorsal view of the developing heart. 1, Vitelline vein; 2, umbilical vein; 3, caudal cardinal vein; 4, cranial cardinal vein; 5, 5′, left and right horns of sinus venosus; 6, atrium, 7, ventricle; 8, truncus arteriosus.
Four heart chambers are apparent at this stage: sinus venosus, atrium, ventricle, and truncus arteriosus, in caudocranial sequence. The last three are separated by regions of constriction; that between the atrium and ventricle is known as the atrioventricular canal, whereas the transition from ventricle to truncus forms the arterial conus (bulb of the heart). The truncus continues rostrally into the aortic arches, which now appear in the mesoderm to each side of the pharynx (see Figure 7–2, B). The sinus venosus receives the cardinal, vitelline, and umbilical systems of veins that extend from the body of the embryo, the yolk sac, and the chorioallantois, respectively (see Figure 7–24). The bifid character of the sinus venosus persists for a time, but its wide communication with the atrium gradually shifts toward the right as the amount of blood entering the left horn is diminished after the obliteration of the left umbilical and left vitelline veins. When the sinus is eventually incorporated within the atrium, it is the undivided part and the right horn that contribute the sinus venarum, the smooth-walled portion of the adult right atrium; the left horn is reduced to the coronary sinus. By this stage the sinus venosus and common atrium have also become included within the pericardial cavity, where they lie dorsal to the ventricle.
Division of the common atrium into right and left chambers is first achieved by the appearance and subsequent growth of a crescentic ridge (Figure 7–25/2). This projects ventrally into the lumen, and at its ends it grows toward thickenings of the wall of the atrioventricular canal known as the endocardial cushions (Figure 7–25/6). The ridge is known as the septum primum; the opening between its free margin and the cushions is known as the ostium primum (Figure 7–25/4). The ostium primum is gradually occluded by the further enlargement of the cushions, but before closure is complete, a number of perforations appear within the septum and coalesce to form a fresh communication, the ostium secundum (Figure 7–25/5), between the two atria. The definitive division of the atria is achieved by a second crest (Figure 7–25/3) that now appears to the right of the primary partition. The concave free ventral margin of this second crest overlaps the ostium secundum; the passage between the atria is reduced to a narrow space between the second septum and the remains of the first (Figure 7–25, C). The passage is known as the foramen ovale; the covering provided by the remnant of the septum primum forms the valve of the foramen ovale. The final closure of the opening is accomplished after birth by the apposition and subsequent fusion of the valve to the septum secundum (p. 256).
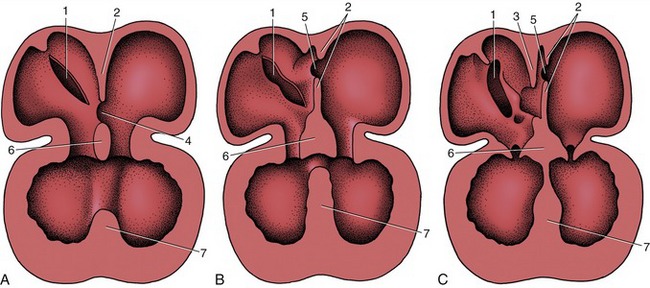
Figure 7–25 The partitioning of the atrium and ventricle, schematic. A, The primary atrial septum has formed, and development of the interventricular septum has begun. B, The primary atrial septum has fused with the endocardial cushions, and a secondary foramen (5) has been formed. C, The secondary atrial septum has formed, and a passage (foramen ovale) between primary and secondary septa connects the right and left atria. Note the fusion of the interventricular septum with the endocardial cushions. 1, Sinuatrial opening; 2, primary atrial septum; 3, secondary atrial septum; 4, ostium primum; 5, ostium secundum; 6, fused endocardial cushions; 7, interventricular septum.
Further growth and eventual mergence of the endocardial cushions divide the canal into the two openings that become the right and left atrioventricular ostia (Figures 7–26 and 7–27, B).

Figure 7–26 Partitioning of the atrioventricular canal by the endocardial cushions. The single atrioventricular canal is gradually divided into right and left atrioventricular openings.
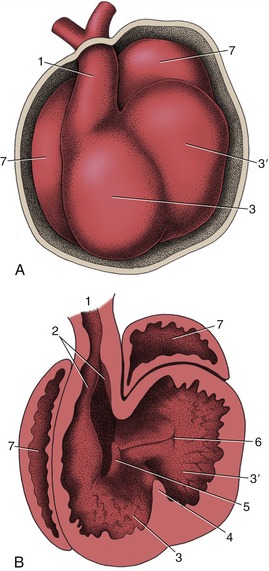
Figure 7–27 The partitioning of the truncus arteriosus. A, Ventral view of the developing heart. B, The ventral part of the heart has been removed to expose the developing ridges (2) in the truncus arteriosus. 1, Truncus arteriosus; 2, ridges in truncus; 3, right ventricle; 3′, left ventricle; 4, interventricular septum; 5, right atrioventricular canal; 6, left atrioventricular canal; 7, atrium.
The septation of the truncus arteriosus and bulbus is achieved by the appearance, growth, and fusion of two endocardial ridges that run along the length of the truncus. The left one is known as the septal ridge, the right one as the parietal or dorsal ridge. Fusion of the ridges commences at the distal extremity of the truncus and gradually extends proximally, producing a partition that ends in a free edge arched over the common ventricle (Figure 7–27, B/2,3). The lower end of the parietal ridge expands within the ventricle and contributes to the closure of the atrioventricular ostium. The septal ridge fuses with the most cranial part of the interventricular septum that has been developing in the meantime.
This interventricular septum first appears as a falciform crest formed by local thickening of the myocardium at the apex of the ventricle; as it extends it divides the common cavity into right and left chambers (Figure 7–25/7). Although the external conformation of the heart at this stage already approximates its final form, the truncus arteriosus (although now divided internally) appears to arise solely from the right ventricle (Figure 7–27, A). The two ventricles still communicate with each other over the free edge of the interventricular septum but are in separate communication with the atria through the paired slitlike openings created by the subdivision of the atrioventricular canal. The right atrioventricular opening is substantially bounded by the right part of the caudal endocardial cushion, less extensively by the cranial cushion, and partly, as already mentioned, by the parietal ridge of the truncus. These three contributions each form a separate cusp of the valve, and the truncus ridge contributes the parietal cusp.
The left atrioventricular valve has a similar origin, mainly from the cranial and caudal endocardial cushions but with a small additional (lateral) cushion forming the parietal cusp. The division of the ventricles is largely completed by fusion of the interventricular septum with the caudal cushion; it is finally achieved by fusion of the lower edge of the truncus septum with the right part of the caudal cushion and with the interventricular septum. Because the same process completes the aortic part of the truncus, the output of the heart is now divided into two streams: one from the left ventricle into the aorta and one from the right ventricle into the pulmonary trunk.
Requiring the meeting and fusion of various elements in precisely the right place at precisely the right time, the process is so complicated that it is clearly open to mishap; it is therefore unsurprising that heart malformations are among the most common congenital abnormalities. Various surveys suggest that their incidence approaches 1% of all human births; although reliable figures are not available, heart malformations are also frequent in domestic animals. The more common malformations are defects of the cardiac septa, atresia or stenosis of the pulmonary or aortic trunks, or some combination of these anomalies (e.g., the tetralogy of Fallot: pulmonary stenosis, enlarged overriding aorta, ventricular septal defect, and hypertrophy of the right ventricle). Failure of closure of the oval foramen is generally without functional significance, but most other malformations are incompatible with normal life after birth. Surgical correction is neither practicable nor advisable in those affected animals that do not die spontaneously.
THE BLOOD VESSELS
The arteries, capillaries, and veins form a continuous system lined by an unbroken low-friction endothelium. The other layers of their walls vary greatly in construction, thickness, and even presence, in evident or presumed adaptation to different functional requirements.
THE ARTERIES
The arterial wall is composed of three concentric tunics (Figure 7–28). The endothelium of the inner one (tunica interna) is supported by a thin layer of specialized connective tissue that is bounded externally by a well-developed, fenestrated elastic sheet, the inner elastic membrane (Figure 7–28/2). The subendothelial connective tissue is frequently affected by arteriosclerotic changes (hardening of the arteries), particularly, though not exclusively, in human subjects. The middle tunic (tunica media) is the thickest and most variable layer. It is composed of an elaborately organized admixture of elastic tissue and smooth muscle in varying proportions (Figure 7–28/3). The outer tunic (tunica adventitia) is predominantly fibrous and grades into the fibroareolar tissue within which many arteries are embedded (Figure 7–28/4). Its importance in limiting expansion of the artery, which safeguards against spontaneous rupture, is not always sufficiently recognized.
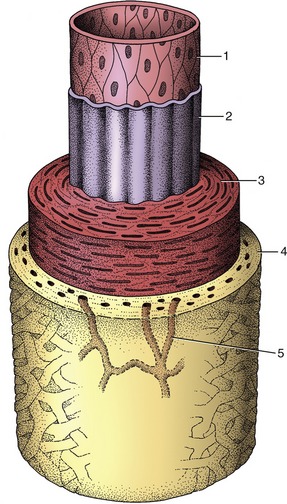
Figure 7–28 The components of the arterial wall. 1, 2, Tunica interna (1, endothelium; 2, inner elastic membrane); 3, tunica media; 4, tunica adventitia; 5, vasa vasorum.
Differences in the structure of the media allow the convenient recognition of three major classes of arteries, although it should not be assumed that these are sharply distinguished. A few very large arteries—those that are required to expand considerably when they receive the systolic output of the ventricles—have a media predominantly composed of concentric, fenestrated elastic membranes with relatively little muscle interspersed. The elastic tissue stretches to absorb and store the energy contained in the moving bloodstream; later, on recoil, it releases this energy to forward the flow of blood toward the periphery. These elastic or conducting arteries comprise the first part of the aorta, certain of its major branches, and the pulmonary trunk.
Most named arteries and others of smaller size have a media that consists largely of smooth muscle arranged in many closely spiraled layers. The caliber of these muscular or distributing arteries is closely controlled by an autonomic innervation.
The smallest arteries, known as arterioles, principally regulate the resistance to the flow of blood and hence the peripheral blood pressure. The muscle is reduced to a few layers that are progressively shed. Although arterioles may be little wider than the capillaries into which they open, they are distinguished from these by the retention of some muscle in their walls. The sphincters about the openings to the capillaries are the means of determining the fraction of the capillary bed that is open to perfusion at any time (Figure 7–29).
THE CAPILLARIES AND SINUSOIDS
The capillaries are reduced to narrow endothelial tubes supported by a very delicate connective tissue investment. They are the exchange vessels from which fluid passes from the blood into the tissue interstitium at the arterial end of the loop and into which some fluid is resorbed toward the venous end (see Figure 7–29). They permeate almost every tissue, although the density of the network varies considerably. The endothelium is described as complete, but minute pores are present in the (fenestrated) capillaries that are typical of some situations (e.g., in intestinal villi and in renal glomeruli).
Sinusoids constitute a special type of capillary found in certain organs, including the liver, spleen, and bone marrow. They are wider, less regular, and more commonly fenestrated than the ordinary capillary, and their endothelial cells are able to extract colloidal substances from the blood.
THE VEINS
Although thinner walled, the larger veins have a construction similar to that of arteries. The smallest ones, the venules, do not possess muscle and may pass through several successive confluences before acquiring this component of the wall. The tunica interna is always thin and lacks an elastic membrane; its chief distinction is its involvement in the formation of the valves whose form, disposition, and function have already been noted (p. 27). The media is relatively weak, is mainly muscular, and has little admixture of elastic elements. Elastic fibers are more plentiful in the adventitia.
The structure of veins is much less uniform than that of arteries, but although many specializations have been described, it has not yet been possible to assign specific adaptive significance to all. However, longitudinal bundles of smooth muscle within the adventitia of some veins can be correlated with a capacity to alter in length with changes in circumstance. Clear indications exist that the muscular layer can increase in thickness in response to elevated venous pressure (e.g., the digital veins of horses).
ARTERIOVENOUS ANASTOMOSES
Direct connections between small arteries and veins exist in many parts of the body where they are used to short-circuit the capillary bed (Figure 7–30). One purpose is to shunt blood away from tissues of intermittent activity when they are resting; good examples are supplied by the thyroid gland and the gastric mucosa. Arteriovenous anastomoses are also concerned with temperature regulation. To this end, they are plentiful in the exposed appendages of the body: the digits, external ears, and nose. Paradoxically, they appear to be used in two ways. They open in a cold environment to prevent local overchilling of the appendages; they also open when the animal is overheated, thus promoting heat loss by increasing the throughput of blood close to the body’s surface. A special example of the last use is provided by the panting dog; the circulation of blood through the many arteriovenous anastomoses within the tongue promotes the evaporation of saliva from the surface, which compensates to some degree for the restricted distribution of sweat glands in canine skin.
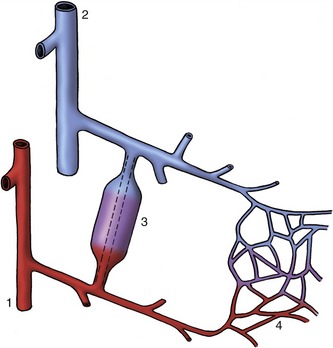
Figure 7–30 A precapillary arteriovenous anastomosis. 1, Artery; 2, vein; 3, arteriovenous anastomosis; 4, capillary plexus.
The use of radioactive-labeled microspheres has made it possible to estimate the amount of blood that can circumvent the capillary bed. In the pig, up to 30% of the total cardiac output sometimes passes through arteriovenous anastomoses.
The structure of these interconnecting channels is not uniform. Some are distinguished by having very muscular walls, others by the muscle cells taking on a peculiar epithelioid character; these epithelioid cells are believed to swell on response to specific chemical stimuli, thereby closing the channel.
ERECTILE TISSUE
Erectile or cavernous tissue is a vascular specialization in which many close-packed, endothelium-lined spaces are set in continuity with the bloodstream. The spaces are usually closed, but as they are directly fed by arterioles they rapidly engorge under appropriate nervous stimulation. Erectile tissue is best known in connection with the genital system; it provides a large part of the structure of the penis (p. 193) and of the smaller but comparable female equivalent. In modified form it is also found in the teat wall, the nasal mucosa, the vomeronasal organ, and a few other sites. A simultaneous response of the genital and nasal erectile tissue is common and has provoked curious speculation; the association is less surprising than it may seem at first because the perception of odors plays a significant part in the sexual behavior of many animals.
“Blood-cushions” formed by a concentration of veins, although not strictly comparable, may be mentioned here. Several of these arrangements are associated with the gastrointestinal tract. One of veterinary interest is provided by the ileal papilla of the horse (p. 556), which has a considerable capacity for engorgement. Another, less relevant example is supplied by the human anal mucosa; pads formed by the underlying veins are believed to contribute to closure of the orifice, and it has been claimed that the postnatal elaboration of these veins is correlated with the development of continence by the infant.
VASCULARIZATION AND INNERVATION OF THE VESSEL WALL
Like other tissues, blood vessel walls require nutrition. Diffusion from the lumen is sufficient to supply the needs of smaller vessels but requires supplementation by an intramural circulation in those of larger size. The supplying arteries (vasa vasorum) most often arise at some distance from the stretch of wall they feed, frequently coming from collateral branches. They penetrate the adventitia from outside and ramify within this layer and the adjoining part of the media (Figure 7–31/1). They do not penetrate beyond the middle of the media in arteries, probably because capillaries in the inner part of the wall would be closed by the radial pressure generated by the bloodstream within the lumen. The tunica intima is never vascularized unless diseased.
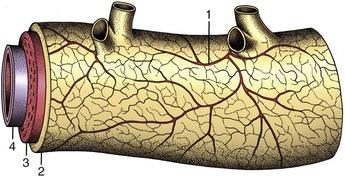
Figure 7–31 Vasa vasorum in the wall of a large artery. 1, Vasa vasorum; 2, tunica adventitia; 3, tunica media; 4, tunica interna.
Arteries and veins receive both a motor and a sensory innervation. The vasomotor nerves to the arteries are particularly important because they control the diameters of the lumina and hence the peripheral resistance. Most are vasoconstrictor fibers of sympathetic origin. Some pass directly to the great arteries from sympathetic plexuses within the mediastinum, but most first travel within local nerve trunks from which they later emerge to enmesh the peripheral arteries. The afferent supply is concerned in local and general vascular reflexes; some fibers mediate the sensation of pain perceived from arterial lesions.
In addition, certain specific sites are much more richly supplied with nerves whose endings respond to pressure or chemical stimuli. These baroreceptor and chemoreceptor concentrations, of great importance in the regulation of the circulation, are confined to arteries originating in the pharyngeal (branchial) arches: the internal carotid arteries, the aortic arch, the right subclavian artery, and the pulmonary trunk. The best-known examples of each type, the carotid sinus and carotid body (glomus caroticum), are found in close association at the origin of the internal carotid artery (Figure 7–32).
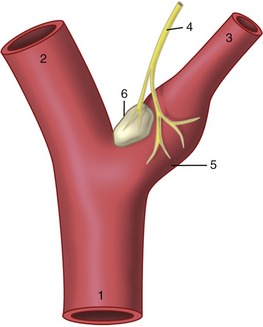
Figure 7–32 Baroreceptors and chemoreceptors at the origin of the internal carotid artery. 1, Common carotid artery; 2, external carotid artery; 3, internal carotid artery; 4, carotid sinus branch of the glossopharyngeal nerve; 5, carotid sinus (baroreceptor); 6, carotid body (chemoreceptor).
The carotid sinus may be recognized in the cadaver as a slightly expanded and especially distensible stretch at the origin of the internal carotid. Its receptors are stimulated by pressure changes that alter the mechanical tension in its wall. The carotid body is a neighboring nodule (sometimes palpable) that is composed of a richly vascularized mass of epithelioid cells. The chemoreceptors respond to changes in oxygen and carbon dioxide tension and hydrogen ion concentration in the perfusing blood. The afferent fibers from both receptor types travel in the carotid sinus branch (known to physiologists as the nerve of Hering) of the glossopharyngeal nerve to project on centers within the brainstem.
The less familiar receptor areas in the other arteries named are similar but less important. Specific differences exist, and in some animals they appear to decline in importance with the attainment of maturity.
PATTERNS OF ARTERIAL DISTRIBUTION
We have already mentioned certain more obvious features of arterial distribution: the increase in total cross-sectional area at each branching, the variation in the angle of branching, the preference for protected courses within the limbs, and the generosity of interarterial anastomoses (p. 27). Amplification of the description of certain features is required.
Collateral Circulation
Few arteries of any size proceed to their terminations in capillary beds without first detaching side or collateral branches. Most collateral branches, whether large or small, connect with their neighbors, although the profusion of anastomoses may not be apparent on dissection because so many are concealed within muscles and other organs (Figure 7–33). The anastomoses enlarge when the bloodstream is diverted from its normal route by occlusion of a principal trunk; initially the widening is due to relaxation and stretching of the wall but later is due to reconstruction of the anastomotic links. Thus, provided that sufficient blood can pass in the meantime, tissues deprived of their usual sources of supply generally survive, though possible temporary loss of function of the ischemic parts may occur. Experiments have shown that in healthy dogs even the aorta can be ligated (caudal to the origin of the renal arteries) leaving a fair, perhaps 50%, expectation of survival. This does not mean that any artery can be ligated with impunity. The ability to develop an adequate collateral circulation is increased when the obstruction develops slowly; it is lessened by sudden onset, aging, or frankly pathological changes in the vessel wall.

Figure 7–33 This illustration of the arterial pattern of the equine limb shows the generosity of interarterial anastomoses.
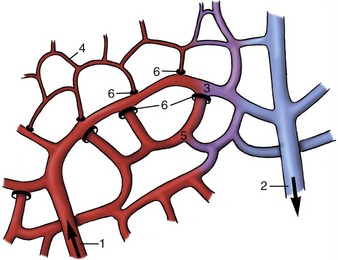
Some arteries have a patency that is essential: interruption to flow produces an infarct, the death of a block of tissue (typically shaped like a cone about the vascular axis). These arteries, known as end-arteries, are paradoxically more numerous among smaller arteries than their parent trunks, which generally have more extensive collateral connections. By strict definition, the end-artery is a rarity, but “functional” end-arteries, in which the collateral connections are of insufficient caliber, are more common (Figure 7–34). It is impossible to assess the adequacy of collateral circulation from purely morphological evidence; for example, although intramuscular arteries appear to anastomose freely, occlusion of one frequently leads to local necrosis. Other good examples of arteries in which anastomoses are poor are the central artery of the retina and many small vessels within the brain; the consequence of their obstruction may be immediate and catastrophic: destruction of the retina or the death of a nucleus or tract along with permanent sensory or motor disability. This may be contrasted with the freedom of anastomoses between the major arteries that conjoin to form the arterial circle on the ventral surface of the brain. Anastomoses between finer branches of the coronary arteries are also poor and usually incapable of maintaining an adequate collateral circulation; even so, not all coronary embolisms are fatal. Much may depend on the size and specific site of the infarct and on immediate medical care.
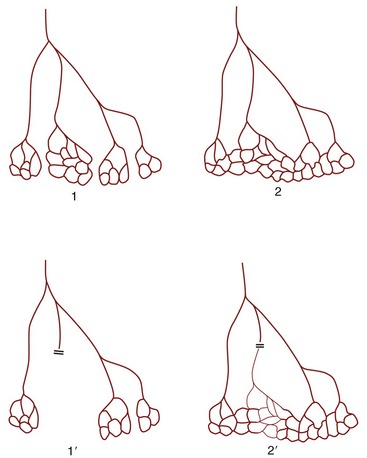
Figure 7–34 True (1) and functional (2) end-arteries. Closure of an end-artery leads to necrosis of the tissue it supplies (1′). In the case of a functional end-artery (2), a potential but inadequate alternative route exists (2′).
Anastomoses between small arteries within the limbs are especially numerous in the regions of the joints and sometimes form visible networks or retia; a prominent example exists over the dorsal aspect of the carpus of the horse (rete carpi dorsale).
The retia just described are not to be confused with the so-called retia mirabilia of more restricted occurrence. Retia mirabilia are found where a main trunk splits more or less at once into a leash of parallel vessels. In one variety the parallel trunks later reunite; this is a “bipolar” arrangement found on the arteries to the brain (in certain species) (Figure 7–35/7) and, on a diminished scale, in the renal glomeruli (see Figure 5–28). Other examples are “unipolar,” that is, the branches remain separate. Examples are found within the limbs of slow-moving arboreal creatures (sloths, lemurs) and in the thoracic cavity of whales and other diving mammals. No convincing explanations exist of the adaptive value of most of these; the renal glomeruli, however, are the obvious exception (p. 181).
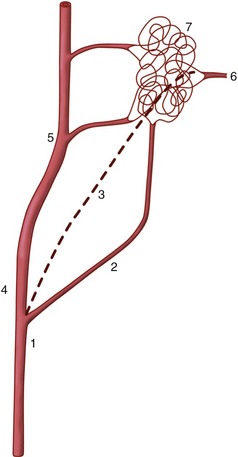
Figure 7–35 A rete mirabile interposed on the blood supply to the bovine brain. 1, Common carotid artery; 2, occipital artery; 3, internal carotid artery (regresses after birth); 4, external carotid artery; 5, maxillary artery; 6, branch from rete to arterial circle of the brain; 7, rostral epidural rete mirabile.
SYSTEMATIC ANGIOLOGY
It is not our intention to provide in one place a comprehensive description of all the blood vessels. Few things would be more tedious, and there seems to be a pragmatic advantage in fragmenting the account: dealing with the vascularization of particular organs and regions in other chapters makes it is easier to emphasize those features that have a special functional importance or clinical interest. Even so, it is advisable to have an outline of the arterial and venous trees. Because species differences are numerous and would, if given attention, require many qualifications of the description, the dog is used as model; only a few most salient comparative features are noted.
THE PULMONARY CIRCULATION
The Pulmonary Arteries
The pulmonary trunk arises from the pulmonary orifice of the right ventricle on the craniosinistral aspect of the heart. It is slightly expanded at its origin where it presents a small sinus above each cusp of the pulmonary valve. The trunk (Figure 7–9, A/5) passes between the two auricles then bends caudally over the base of the heart, where it is joined on its right face by the ligamentum arteriosum, the fibrosed remnant of the ductus arteriosus (p. 256). After penetrating the pericardium, it divides into right and left pulmonary arteries, each directed to the hilus of the corresponding lung in company with the principal bronchus and pulmonary veins (Figure 7–9/10,10′). The course of the right artery carries it ventral to the trachea.
The pulmonary arteries make their initial branching before entering the lung (Figure 4–23); their further ramifications have already been briefly noted (p. 164).
The Pulmonary Veins
The pulmonary veins open variously into the roof of the left atrium. They form two clusters in the dog: one for the veins draining each lung. In some other species the veins draining the caudal lobes of both lungs form a separate third cluster. Valves are absent from these veins.
THE SYSTEMIC CIRCULATION
The Systemic Arteries
The Aortic Arch
The origin of the aorta is similar to that of the pulmonary trunk but is from the left ventricle. The initial portion, the aortic bulb, is concealed between the atria and forms sinuses above the three cusps of the aortic valve; the right coronary artery arises from the cranial sinus, the left artery from the caudosinistral sinus (Figure 7–17/5,6). Beyond this, the aorta arches cranially, dorsally, and caudally, penetrating the pericardium to ascend within the mediastinum to reach the sinistroventral aspect of the vertebral column about the level of the seventh thoracic vertebra (Figure 7–36). In addition to the coronary arteries (p. 231) the first part of the aorta gives origin to the paired subclavian and paired common carotid arteries. These vessels amalgamate at their origins to form a short, cranially directed brachiocephalic trunk in the larger species (Figure 7–37); in the dog and pig the left subclavian artery remains distinct and takes a separate, more distal origin (Figure 7–36/4). The common carotid arteries supply structures of the head (p. 246).

Figure 7–36 Branching of the aortic arch in the dog. (In this series of figures, not all arteries depicted are named.) 1, Pulmonary trunk; 2, aorta; 3, intercostal aa.; 4, left subclavian a.; 4′, right subclavian a.; 5, brachiocephalic trunk; 6, vertebral a.; 7, costocervical trunk; 8, left and right common carotid aa.; 9, superficial cervical a.; 10, axillary a.; 11, internal thoracic a.
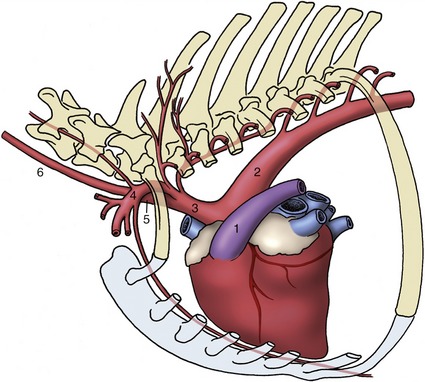
Figure 7–37 Branching of the aortic arch in the horse. The arteries to the head and neck and to the forelimbs originate from a short brachiocephalic trunk (3). 1, Pulmonary trunk; 2, aortic arch; 3, brachiocephalic trunk; 4, left subclavian a.; 5, bicarotid trunk; 6, left common carotid a.
The subclavian artery (Figure 7–36/4) supplies blood to the forelimb and to structures of the neck and cervicothoracic junction. It winds around the cranial border of the first rib to enter the limb through the axilla; it changes its name to axillary at this point. The subclavian detaches four branches in its intrathoracic course. The first, the vertebral artery (Figure 7–36/6), runs craniodorsally, dives between the scalenus and longus colli muscles, and then passes through the successive transverse foramina of the sixth to first cervical vertebrae. After receiving the termination of the occipital artery, it enters the vertebral canal within the atlas and there divides into a basilar artery to the brain and the ventral artery of the spinal cord (p. 312). Twigs are detached en route to the vertebral column, covering muscles, and contents of the vertebral canal.
The larger second branch, the costocervical trunk (Figure 7–36/7), provides the first few dorsal intercostal arteries and the deep cervical artery, which ascends the neck within the dorsal cervical musculature that it supplies.
The internal thoracic artery (Figure 7–36/11), the third branch, curves ventrally within the mediastinum to pass between the transversus thoracis and the sternum. It follows the sternum and tunnels below the diaphragm to continue as the cranial epigastric artery of the abdominal floor. Collateral branches include twigs to the pleura, thymus, and pericardium; perforating branches to the pectoral muscles and thoracic mammary glands; and ventral intercostal arteries. The more caudal ventral intercostal branches arise from a common trunk, the musculophrenic artery, which follows the lateral attachment of the diaphragm. The cranial epigastric artery divides into superficial and deep branches; the latter follows the deep face of the rectus abdominis to an anastomosis with the caudal epigastric artery within the substance of this muscle. The superficial branch passes to the superficial fascia, where it assists in the supply of the abdominal mammary glands.
The superficial cervical artery (Figure 7–36/9), the fourth branch, arises from the subclavian opposite the origin of the internal thoracic. It supplies muscles of the ventral part of the neck, the cranial part of the shoulder, and the upper arm.
The Axillary Artery
The axillary artery (Figure 7–38/1), the magistral trunk of the forelimb, crosses the axilla to continue distally over the medial surface of the arm, caudal to the humerus. It changes its name again when level with the teres major tuberosity, where it becomes the brachial artery (Figure 7–38/6). The axillary gives external and lateral thoracic arteries to the chest wall and one important collateral branch to the limb, the subscapular artery (Figure 7–38/3). This runs dorsally along the caudal border of the scapula between the subscapularis and teres major. It supplies branches to the muscles of the shoulder.
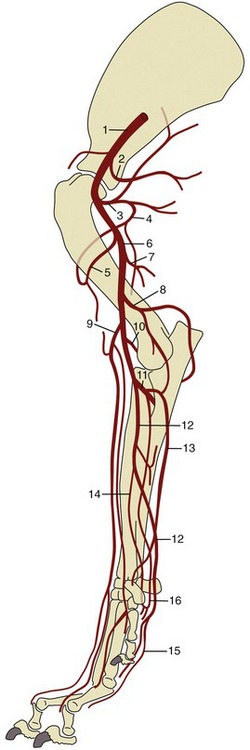
Figure 7–38 Arteries of the canine forelimb. 1, Axillary a.; 2, lateral thoracic a.; 3, subscapular a.; 4, caudal circumflex humeral a.; 5, cranial circumflex humeral a.; 6, brachial a.; 7, deep brachial a.; 8, collateral ulnar a.; 9, superficial brachial a.; 10, transverse cubital a.; 11, common interosseous a.; 12, median a.; 13, ulnar a.; 14, radial a.; 15, superficial palmar arch; 16, deep palmar arch.
The brachial artery (Figure 7–38/6) passes obliquely over the medial surface of the humerus to reach the craniomedial aspect of the elbow; it continues into the forearm where it shortly changes its name yet again, becoming the median artery. Its collateral branches include several to the muscles of the arm, principally the deep brachial (Figure 7–38/7) to the tricipital mass; toward the elbow it detaches collateral ulnar and superficial brachial arteries (Figure 7–38/8,9) that pass to the caudal and cranial aspects of the forearm, respectively. Branches of the superficial brachial run subcutaneously beside the cephalic vein and superficial branch of the radial nerve to reach the dorsum of the paw. The transverse cubital artery (Figure 7–38/10) is detached just proximal to the elbow joint. A substantial branch, the common interosseous artery, originates from the main artery distal to the elbow.
The common interosseous artery (Figure 7–38/11) detaches the ulnar artery (Figure 7–38/13) for the digital and carpal flexors and the caudal interosseous artery, which runs between the radius and ulna to reach the palmar arches of the proximal metacarpus. A cranial interosseous penetrates the interosseous space to supply the dorsal muscles of the forearm.
The median artery (Figure 7–38/12) runs down the caudomedial aspect of the forearm in company with the median nerve and under protection of the flexor carpi radialis. It passes through the carpal canal to end by concurring with branches of the common interosseous in forming palmar arterial arches (Figure 7–38/15,16) from which the palmar aspect of the forepaw is supplied.
The paw receives its principal blood supply on its palmar aspect where (deep) palmar metacarpal and (more superficial) palmar common digital arteries run at the boundaries of the metacarpal bones before dividing at their distal ends into proper palmar digital arteries that follow the axial borders of the digits. The corresponding but narrower dorsal common and proper digital arteries follow a similar pattern.
(The small arteries of the forepaw arise from anastomoses not listed.)
The Common Carotid Artery
The common carotid arteries arise separately in the dog (Figure 7–36/8) and by a short common (bicarotid) trunk in ungulates (Figure 7–37/5). Each crosses the ventrolateral face of the trachea (or esophagus on the left) in its ascent of the neck where it is accompanied by the vagosympathetic trunk. The artery ends by dividing above the larynx into external and internal carotid arteries. The only significant collateral branches of the common carotid are detached close to its termination; they are the caudal and cranial thyroid arteries, of which the latter is the origin of the laryngeal and pharyngeal branches.
The external carotid artery is the larger of the terminal branches and appears as the direct continuation of the parent trunk (Figure 7–39/1,2). In the dog it shortly detaches the occipital artery, which branches from the internal carotid in some other species. The external carotid is continued as the maxillary artery (Figure 7–39/11); this distinction is rather arbitrarily determined by the origin of the superficial temporal artery.
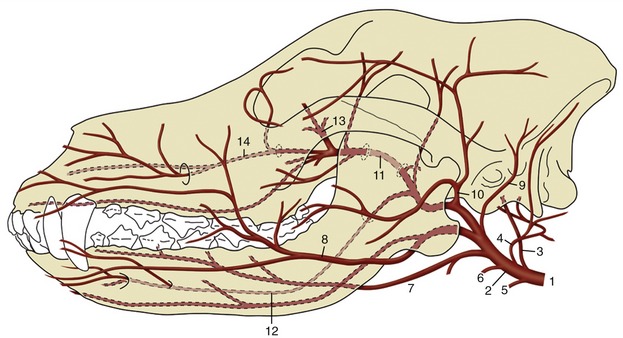
Figure 7–39 Arteries of the canine head. 1, Common carotid a.; 2, external carotid a.; 3, internal carotid a.; 4, occipital a.; 5, cranial laryngeal a.; 6, ascending pharyngeal a.; 7, lingual a.; 8, facial a.; 9, caudal auricular a.; 10, superficial temporal a.; 11, maxillary a.; 12, inferior alveolar a.; 13, external ophthalmic a.; 14, infraorbital a.
The external carotid in this narrow sense forms a short dorsally convex arch resting on the pharynx and covered by the mandibular gland and digastricus. Its branches are the occipital, cranial laryngeal, ascending pharyngeal, lingual, facial, caudal auricular, parotid, and superficial temporal arteries.
The occipital artery (Figure 7–39/4) runs to the condyloid fossa where it divides into several branches that supply, among other structures, the middle and internal ear and the caudal meninges. The largest branch, effectively the continuation of the stem, passes to the atlantal fossa to an anastomosis with the vertebral; it thus takes part in the supply to the brain (p. 312).
The cranial laryngeal and ascending pharyngeal arteries (Figure 7–39/5,6) are the principal supplies to these organs (i.e., the larynx and pharynx). The large lingual artery (Figure 7–39/7) pursues a rostroventral course over the pharynx to enter the tongue between the genioglossus and hyoglossus muscles. It principally supplies the tongue, but collateral branches detached en route include one to the palatine tonsil that is of potential importance to the surgeon (p. 393).
The facial artery (Figure 7–39/8) arises near the angle of the jaw and runs within the intermandibular space before winding around the ventral border of the mandible where it is conveniently located for pulse taking in larger species; it then divides into various branches for the lips, lateral nose, and angle of the mouth. The relatively large caudal auricular artery (Figure 7–39/9) generously supplies the external ear and associated muscles. The parotid artery supplies the parotid gland.
The superficial temporal artery (Figure 7–39/10) winds onto the face and runs forward to supply the masseter. In the dog it branches to the upper and lower eyelids and dorsum of the nose. The position and firm support of one of the branches (transverse facial artery) suit it to pulse taking in larger species.
The maxillary artery (Figure 7–39/11) heads in the direction of the alar canal through which it passes to enter the pterygopalatine fossa. Before reaching the canal, its main branch is the inferior alveolar (Figure 7–39/12), which enters the mandible to supply the alveoli and teeth and, through mental branches that emerge from the bone, the lower lip and the chin region. Other maxillary branches pass to the tympanic cavity, muscles of mastication, and cranial meninges (the last passing through the oval foramen). No branches are detached from the stretch of artery within the canal, but a sheaf of diverging vessels comes off directly as it reaches the pterygopalatine fossa. The most important is the external ophthalmic artery (Figure 7–39/13) going to the contents of the orbit (p. 344). Others include the ethmoidal artery to the nasal cavity, the major and minor palatine arteries to the hard and soft palates, respectively, and the continuation (infraorbital artery) of the main trunk into the superior alveolar canal (Figure 7–39/14).
The internal carotid artery (Figure 7–39/3) enters the cranial cavity through the jugular foramen and carotid canal, taking a rather indirect course in the dog (p. 311). It divides within the cavity into divergent caudal and rostral branches that concur with their contralateral counterparts and with the basilar artery in forming the arterial circle from which the brain is supplied (p. 311).
The Thoracic Aorta
The thoracic aorta runs caudally below the roof of the thorax to enter the abdomen by the aortic hiatus of the diaphragm. It continues as the abdominal aorta in company with the azygous vein and thoracic duct. The branches of the thoracic aorta are dorsal intercostal arteries (excepting those to the first few spaces), which arise variously and often by common trunks for the right and left vessels, and a bronchoesophageal artery, which is rather erratic in its origin.
Despite their names, which suggest rather restricted distribution within the intercostal spaces, the dorsal intercostal arteries detach substantial branches to the vertebral column and associated structures. They end by anastomosing with ventral intercostal arteries from the internal thoracic artery and its musculophrenic branch, thereby completing arterial loops within the spaces. The corresponding artery behind the last rib is known as the dorsal costoabdominal. The bronchoesophageal artery descends to the root of the lungs where it gives rise to bronchial branches for the tissues of the lungs and esophageal branches for much of the thoracic esophagus.
The Abdominal Aorta
The abdominal aorta follows the roof of the abdomen, related to the caudal vena cava on its right and the psoas muscles on its left. Shortly after releasing the paired external iliac arteries, the abdominal aorta terminates in the dog below the last lumbar vertebra by branching off the internal iliac arteries and continues as the much smaller median sacral artery that extends into the tail (Figure 7–40/2,3,4). Along its course the abdominal aorta detaches both visceral and parietal branches.
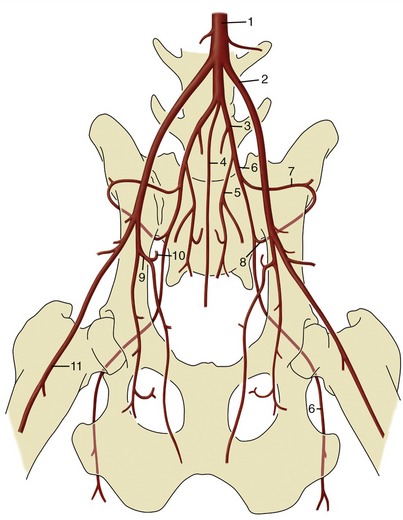
Figure 7–40 Termination of the canine abdominal aorta (ventral view). 1, Aorta; 2, external iliac a.; 3, internal iliac a.; 4, median sacral a.; 5, internal pudendal a.; 6, caudal gluteal a.; 7, iliolumbar a.; 8, cranial gluteal a.; 9, deep femoral a.; 10, pudendoepigastric trunk; 11, femoral a.
The visceral arteries have been considered with the organs they supply. They comprise the unpaired celiac (p. 126), cranial mesenteric (p. 134), and caudal mesenteric (p. 134) arteries and the paired renal (p. 180) and testicular (p. 189 or ovarian [p. 203]) arteries. The unpaired vessels represent the arteries of the caudal foregut, midgut, and hindgut of the embryo (see Figure 3–65).
The collateral parietal branches begin with the caudal phrenic and cranial abdominal arteries, which share a common phrenicoabdominal origin in the dog. They also include the paired lumbar arteries to the tissues and structures of the back, the deep circumflex iliac to the flank, the external iliac artery to the hindlimb, and the internal iliac artery, which serves both pelvic viscera and pelvic walls.
It is worth drawing attention at this point to the existence of several pathways, established by anastomosis, that mitigate the effects of constriction or blockage of the aorta (e.g., by thrombosis, especially common in the cat). The collateral pathways include those formed along the spinal cord by anastomoses between successive lumbar arteries, those along the gut formed by connections between the principal visceral arteries, and those within the abdominal floor formed by the cranial and caudal epigastric arteries.
The External Iliac Artery
This is the principal artery of the hindlimb. It arises close to the termination of the aorta and runs obliquely over the abdominal roof to leave the abdomen by the vascular lacuna above the caudodorsal corner of the flank (Figure 7–41/3). It detaches one branch within the abdomen, the deep femoral artery (Figure 7–41/12), which is the common origin of the pudendoepigastric trunk and an important branch to the adductor muscles of the thigh. The short pudendoepigastric trunk (Figure 7–41/13) ends by giving rise to the caudal epigastric and external pudendal arteries. The former divides in similar fashion to the cranial epigastric; the latter passes through the inguinal canal to supply structures in the groin, including the prepuce in the male and the caudal mammary glands (via the caudal superficial epigastric artery) in the bitch (see Figure 14–2).
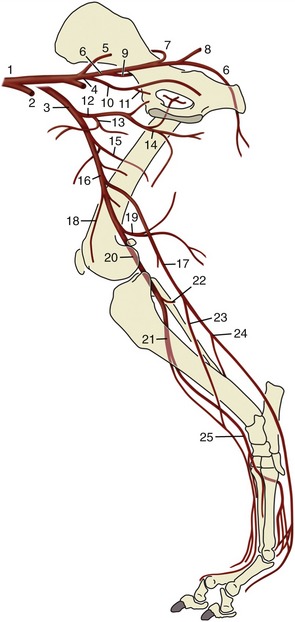
Figure 7–41 Arteries of the canine hindlimb. 1, Abdominal aorta; 2, left external iliac a.; 3, right external iliac a.; 4, left and right internal iliac aa.; 5, median sacral a.; 6, caudal gluteal a.; 7, cranial gluteal a.; 8, lateral caudal a.; 9, iliolumbar a.; 10, internal pudendal a.; 11, vaginal (prostatic) a.; 12, deep femoral a.; 13, pudendoepigastric trunk; 14, medial circumflex femoral a.; 15, lateral circumflex femoral a.; 16, femoral a.; 17, saphenous a.; 18, descending genicular a.; 19, distal caudal femoral a.; 20, popliteal a.; 21, cranial tibial a.; 22, caudal tibial a.; 23, cranial branch of the saphenous a.; 24, caudal branch of the saphenous a.; 25, dorsal pedal a.
The external iliac continues as the femoral artery (Figure 7–41/16) on leaving the abdomen. Its first part has a superficial position in the femoral triangle—between the sartorius and pectineus, where it raises a visible ridge and is ideally located for pulse taking. It then burrows more deeply among the muscles to cross the medial surface of the femur to gain the caudal aspect of the thigh; it continues directly over the capsule of the stifle joint as the popliteal artery. The femoral artery has many branches, named and unnamed, to the muscles of the thigh but most do not require individual notice. One branch that does merit attention is the saphenous artery (Figure 7–41/17), which is detached in midthigh. This is a more important vessel in carnivores than in the larger species; it descends over the medial aspect of the limb before dividing into cranial and caudal branches. The cranial branch (Figure 7–41/23) supplies the dorsal crural muscles before crossing the dorsal aspect of the hock to continue as the dorsal common digital arteries. The caudal branch (Figure 7–41/24) takes a deep course between the muscles of the caudal aspect of the leg (crus), which it supplies, crosses the caudal face of the hock, and terminates as the plantar common digital arteries, which are comparable to the corresponding forelimb arteries.
The popliteal (Figure 7–41/20) divides into cranial and caudal tibial arteries. The cranial tibial artery (Figure 7–41/21) passes through the interosseous space between the tibia and fibula to run distally with the deep peroneal nerve. It crosses the dorsal aspect of the hock (as the dorsal pedal artery; Figure 7–41/25) and gives rise to the dorsal metatarsal arteries among other branches. One of these metatarsal arteries reinforces the caudal branch of the saphenous on the plantar aspect of the limb after passing between the second and third metatarsal bones. The caudal tibial artery (Figure 7–41/22) is of little account in carnivores. The following list includes various muscular branches not mentioned in the text.
The Internal Iliac Artery
This is the supply of the pelvic viscera and walls, including the overlying muscles of the gluteal region and those of the proximocaudal part of the thigh. The internal iliac artery continues caudoventrally from its origin, and in the dog it has a single branch, the umbilical artery (Figure 7–42/5), a rather unimportant vestige of the placental supply of the fetus (p. 255). The proximal part of the umbilical artery carries a little blood to the cranial part of the bladder; the distal part is transformed into the round ligament of the bladder within the lateral vesical fold.
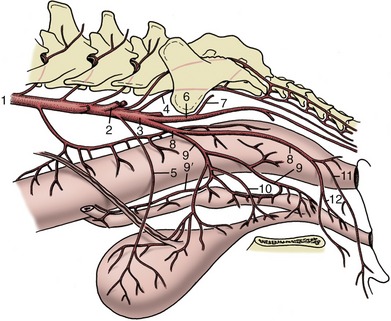
Figure 7–42 Arteries of the female pelvis, left lateral view (bitch). 1, Abdominal aorta; 2, external iliac a.; 3, internal iliac a.; 4, median sacral a.; 5, umbilical a.; 6, caudal gluteal a.; 7, cranial gluteal a.; 8, internal pudendal a.; 9, vaginal a.; 9′, uterine a.; 10, urethral a. (frequently a branch of the vaginal a.); 11, ventral perineal a.; 12, a. of the clitoris.
The internal iliac artery terminates by dividing into the caudal gluteal and internal pudendal arteries. The parietal branch, the caudal gluteal artery (Figure 7–42/6), turns out of the pelvis with the sciatic nerve. This trunk, with its iliolumbar and cranial gluteal (Figure 7–42/7) branches, supplies the muscles about the lumbosacral junction and those of the gluteal and proximocaudal femoral regions; the structures of the last-named region include the proximal parts of the hamstring muscles in which the caudal gluteal terminates.
The second terminal branch is the internal pudendal artery (Figure 7–42/8) to the pelvic viscera (see also pp. 564 and 698). Its branches are differently named and disposed in the two sexes. The first branch is the prostatic artery in the male dog and the vaginal artery (Figure 7–42/9) in the female. The prostatic artery supplies the middle rectal artery to the penultimate part of the rectum and various branches to the caudal parts of the ureter and bladder, the prostate, and the first part of the urethra. The vaginal artery also supplies the rectum and urinary organs in addition to the uterus and vagina. Its cranial branch, the uterine artery, forms the caudal part of the arterial arcade within the broad ligament (p. 203).
The next artery, the urethral artery (Figure 7–42/10), is the same in both sexes. It supplies the caudal part of the pelvic urethra. The terminal branches of the internal pudendal are the ventral perineal artery and the artery of the penis or clitoris. The ventral perineal artery (Figure 7–42/11) supplies a caudal rectal artery to the last part of the rectum and branches to the scrotum (or labia of the vulva). The artery of the penis runs the length of the upper border of this organ to the region of the bulbus glandis; it becomes known as the dorsal artery of the penis after detachment of a branch to the penis bulb, which also supplies the corpus spongiosum and pars longa glandis, and a deep branch to the corpus cavernosum (p. 469 and Figure 15–20). The artery of the clitoris (Figure 7–42/12) is similar but on a less substantial scale.
The Systemic Veins
The systemic veins return blood to the heart through the cranial vena cava, caudal vena cava, and coronary sinus. The coronary sinus returns the bulk of the blood from the heart wall (p. 233); in ruminants and pigs it is joined by the left azygous vein. In the horse and the dog the equivalent (azygous) territory is drained by the right azygous.
The Cranial Vena Cava
The cranial vena cava is formed close to the entrance to the chest by the union of the external jugular and subclavian veins, which drain the head and neck and the forelimb, respectively. In the dog the subclavian and jugular veins of each side join in a common trunk, which then combines with its fellow; another arrangement is the union of the two jugulars in a single bijugular trunk, which is then joined by the subclavian veins. The cranial vena cava runs through the cranial mediastinum, ventral and to the right of the trachea, and is related to the brachiocephalic trunk (dorsally at its origin, later at its left face). It is joined by various tributaries broadly corresponding to branches of the subclavian artery and by the larger right azygous vein toward its termination (Figure 7–43/3)—unless this makes separate entry to the right atrium as in the horse.
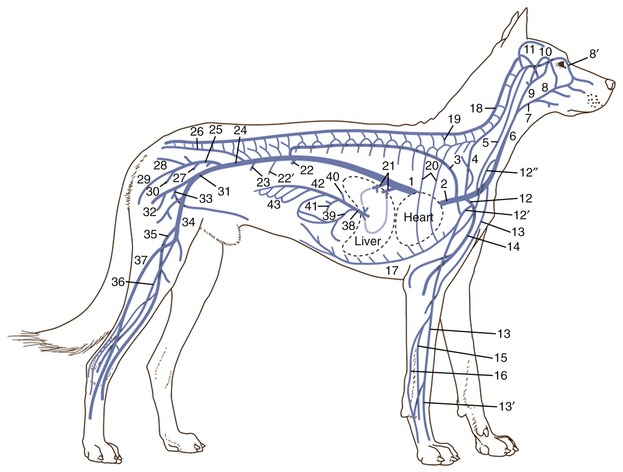
Figure 7–43 Schematic representation of the venous system (dog). 1, Caudal vena cava; 2, cranial vena cava; 3, azygous v.; 4, vertebral v.; 5, internal jugular v.; 6, external jugular v.; 7, linguofacial v.; 8, facial v.; 8′, angularis oculi v.; 9, maxillary v.; 10, superficial temporal v.; 11, dorsal sagittal sinus; 12, subclavian v.; 12′, axillobrachial v.; 12″, omobrachial v.; 13, cephalic v.; 13′, accessory cephalic v.; 14, brachial v.; 15, radial v.; 16, ulnar v.; 17, internal thoracic v.; 18, vertebral venous plexus; 19, intervertebral v.; 20, intercostal vv.; 21, hepatic vv.; 22, renal v.; 22′, testicular or ovarian v.; 23, deep circumflex iliac v.; 24, common iliac v.; 25, right internal iliac v.; 26, median sacral v.; 27, prostatic or vaginal v.; 28, lateral caudal v.; 29, caudal gluteal v.; 30, internal pudendal v; 31, right external iliac v.; 32, deep femoral v.; 33, pudendoepigastric trunk; 34, femoral v.; 35, medial saphenous v.; 36, cranial tibial v.; 37, lateral saphenous v.; 38, portal v.; 39, gastroduodenal v.; 40, splenic v.; 41, caudal mesenteric v.; 42, cranial mesenteric v.; 43, jejunal vv.
The azygous vein (Figure 7–43/3) is formed by the union of the first lumbar veins and passes through the aortic hiatus into the chest where it is reinforced by intercostal veins from the caudal and middle intercostal spaces. Right and left veins are present in the embryo, but the pattern is later commonly simplified: the main trunk is the right azygous vein in horses and dogs and the left one in ruminants and pigs—unless, as is usual in ruminants, both remain of some size. The right azygous vein arches ventrally, passing in front of the root of the right lung to reach the terminal part of the cranial vena cava or the adjacent part of the right atrium (horse). The left vein arches in front of the root of the left lung and must then run caudally, over the left atrium, to reach its confluence with the coronary sinus (Figure 7–9, A/12). The cranial intercostal veins that do not drain into this system join various tributaries of the subclavian or go directly to the cranial vena cava. The special importance of the azygous system in draining the plexus within the vertebral canal is considered elsewhere (p. 314).
The subclavian vein generally corresponds to the subclavian artery, and most tributaries in the upper part of the limb are satellite to arterial branches. The pattern is different in the distal part of the limb where important unaccompanied superficial veins are present. Although these are connected with the deeper veins at various levels, they also continue into the cephalic vein (Figure 7–43/13), which runs between the pectoral and brachiocephalic muscles in the arm to join the external jugular vein in the lower part of the neck.
Two pairs of jugular veins exist within the neck. The deep internal jugular (Figure 7–43/5) runs with the common carotid artery within the visceral space of the neck; however, except in the dog and cat, it is very much reduced in size or even absent in postnatal animals. Even in the dog and cat it is of minor importance. The external jugular vein (Figure 7–43/6) is formed near the angle of the jaw by the union of linguofacial and maxillary veins. Its course through the neck occupies a (jugular) groove between the brachiocephalicus dorsally and the sternocephalicus ventrally in the larger species; in the dog it lies on the sternocephalicus. It is easily raised for intravenous injection and blood sampling, and in the larger species it is the first choice for these procedures. The territories of its linguofacial and maxillary tributaries show considerable overlap and some species variation; the former vein is in general the principal drainage of the more superficial and more rostral structures of the head, the latter of those deeper and more caudal, including the contents of the cranial cavity (see Figure 11–44).
The Caudal Vena Cava
The caudal vena cava is formed on the roof of the abdomen, near the pelvic inlet, by the union of right and left common iliac veins, each formed in its turn by the union of an internal iliac vein, which drains the pelvic walls and much of the contents of the pelvic cavity, and an external iliac vein, which drains the hindlimb (Figure 7–43/25,31). The external iliac vein and the bulk of its tributaries are satellite to arteries. The independent medial and lateral saphenous veins of the leg (Figure 7–43/35,37) drain the superficial veins of the foot.
In its intraabdominal course the caudal vena cava is joined by additional tributaries draining the abdominal roof, including large renal veins, before it dips ventrally to tunnel through the liver and subsequently the diaphragm at the caval foramen. It enters the thoracic cavity at a relatively ventral level and pursues a course within the free edge of the plica venae cavae between the caudal and accessory lobes of the right lung (see Figure 4–20, B/9). It joins the right atrium dorsal to the inlet of the coronary sinus.
In its intrahepatic course the caudal vena cava receives the hepatic veins, which drain the liver (Figure 7–43/21).
The portal vein drains the spleen, the intraabdominal digestive organs, the caudal part of the thoracic esophagus, and the bulk of the rectum (Figure 7–43/38 and Figure 7–44). It is formed variously from three main tributaries (see Figure 3–50/2,4,5). The splenic tributary corresponds to the celiac artery (excluding its hepatic branches) and therefore drains the last part of the esophagus, the stomach, parts of the duodenum and pancreas, and the spleen. The cranial and caudal mesenteric veins drain the territories of the like-named arteries and usually join in a common trunk before combining with the splenic.
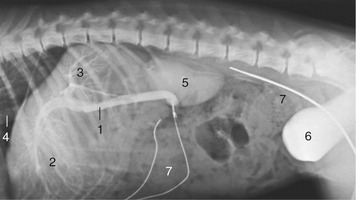
Figure 7–44 Cannulation of the portal vein of a dog. 1, Portal vein; 2, branches to the quadrate, left medial and lateral liver lobes; 3, branches to the remaining liver lobes; 4, caudal vena cava; 5, kidney; 6, bladder, filled with opaque medium; 7, catheters.
The last part of the rectum and the anal region differ from the remainder of the gut in draining toward the internal iliac vein. The veins of this part form one of the portosystemic connections that provide alternative (although not very capacious) outlets from the portal drainage territory that are used when the intrahepatic circulation is impaired, as, for example, by cirrhosis (hepatic fibrosis).
THE CIRCULATION IN THE FETUS AND THE CHANGES AFTER BIRTH
During fetal life the placenta combines the roles that are later performed by the lungs, the digestive tract, and the kidneys. The blood is therefore replenished with oxygen, provided with nutrients, and cleansed of waste in its circulation through the placenta. It is returned to the fetus by two large umbilical veins that wind within the umbilical cord and join as one where they enter the body at the navel (Figure 7–45/11). The single intraabdominal umbilical vein runs forward to penetrate the liver at the umbilical fissure before it divides. It detaches collateral branches that vascularize the left portions (umbilical moiety) of the liver while a further branch bends toward the right to make a wide connection with the portal vein (Figure 7–45/12), which vascularizes the right portions (portal moiety). A direct continuation of the umbilical trunk, the ductus venosus (Figure 7–45/9), tunnels through the substance of the liver, bypassing the hepatic circulation, to join the caudal vena cava. The ductus venosus, present in all young embryos, soon becomes vestigial in those of the horse and pig. It persists in other species but varies in caliber and importance and tends to become reduced toward term. The division of the liver into umbilical and portal moieties has obvious functional and possibly also clinical importance. The portal moiety is less generously supplied with oxygen, and this stimulates more active hemopoiesis; the umbilical moiety is more likely to suffer from infections acquired in utero.
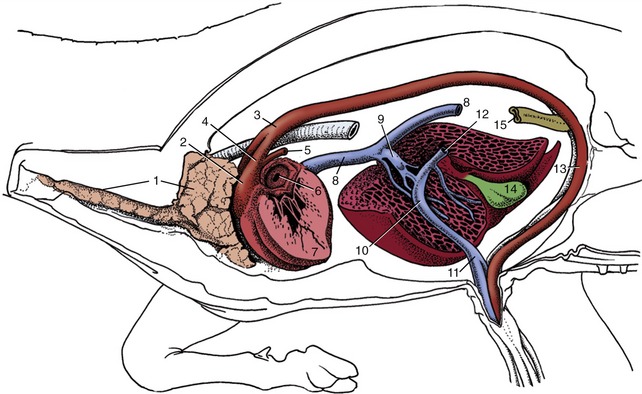
Figure 7–45 Semischematic drawing of fetal circulation (calf). 1, Thymus; 2, pulmonary trunk; 3, aortic arch; 4, ductus arteriosus; 5, pulmonary artery; 6, foramen ovale; 7, wall of left ventricle; 8, caudal vena cava; 9, ductus venosus; 10, junction of umbilical and portal branches within the liver; 11, umbilical vein; 12, stump of portal vein; 13, left umbilical artery; 14, gallbladder; 15, descending colon.
The caudal vena cava (Figure 7–45/8) receives the umbilical blood after its passage through the liver and adds it to the deoxygenated blood returned from the hindpart of the body. The oxygen content of the caudal caval stream is therefore already reduced below that of the placental return before it reaches the heart, where the stream impinges on the cranial margin of the foramen ovale (Figure 7–46/2,4). This divides it into two: one part continues into the right atrium (Figure 7–46/3), the other passes through the foramen ovale into the left atrium (Figure 7–46/8). The relative sizes of the two streams change as gestation advances: a continuing shift of the margin of the foramen to the left increases the flow into the right atrium. The right stream mixes with the return from other systemic veins (Figure 7–46/1), and the oxygen content of the blood passed to the right ventricle is thus further diminished. This blood is ejected into the pulmonary trunk (Figure 7–46/6), which in the fetus communicates with the aorta through a wide channel, the ductus arteriosus (Figure 7–46/7′). The ductus enters the aorta beyond the origin of the brachiocephalic trunk and is as wide as the pulmonary trunk (it is in fact its direct continuation—the right and left pulmonary arteries [Figure 7–46/7] are the side branches). The ductus arteriosus receives most of the output of the right ventricle because the vascular bed of the unexpanded lungs offers considerable resistance to blood flow.
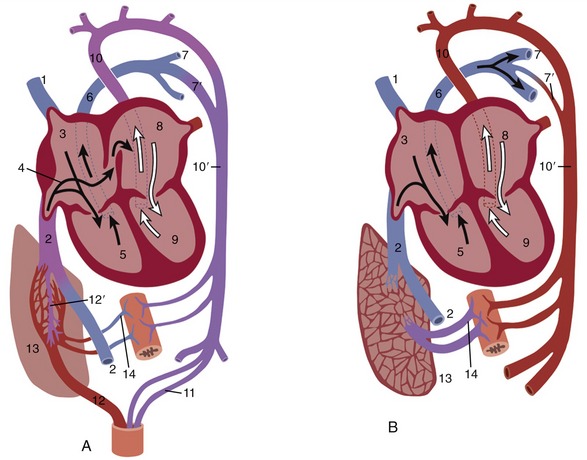
Figure 7–46 Diagrams of the fetal (A) and postnatal (B) circulatory systems. 1, Cranial vena cava; 2, caudal vena cava; 3, right atrium; 4, arrow entering oval foramen; 5, right ventricle; 6, pulmonary trunk; 7, pulmonary artery; 7′, ductus arteriosus (in B, vestige); 8, left atrium; 9, left ventricle; 10, aortic arch; 10′, descending aorta; 11, umbilical artery; 12, umbilical vein; 12′, ductus venosus; 13, liver; 14, portal vein.
The small flow that is returned to the left atrium from the lungs mixes there with the greater volume of blood that passed through the foramen ovale. The blood that enters the aorta (Figure 7–46/10) is therefore relatively well oxygenated; part of this stream enters the coronary and carotid arteries. The head and brain are therefore favored by receiving a richer supply of oxygen than is given to organs supplied from those branches of the aorta that arise distal to the entry of the ductus arteriosus; these later branches receive the mixed output of both ventricles. The placenta receives the greater share of the flow through the descending aorta (Figure 7–46/10′) by way of the umbilical arteries (Figure 7–46/11); these branch from the internal iliac arteries and leave the fetus at the umbilicus, together with the allantoic duct (Figure 7–45/13). The fetal bloodstream is brought into close apposition with the maternal bloodstream within the placenta, although the intervening tissue barrier varies in thickness and permeability among species (p. 209).
The changes in the circulation that follow birth are not completed as promptly as many believe, and some hours, or even days, may be necessary before a stable circulation of adult pattern is established. The permanent closure of the redundant fetal channels requires a much longer time. The arrest of the placental circulation may precede or follow the initiation of pulmonary ventilation according to the circumstances of parturition. The umbilical vessels are either bitten across by the mother (e.g., puppy) or are ruptured, being unable to support the weight of the offspring (e.g., calf); in species in which the latter fate is usual, they divide at predetermined levels. In both circumstances little hemorrhaging occurs because the rough treatment stimulates contraction of the muscle in the vessel wall. The arterial stumps are slowly transformed into the round ligaments of the bladder. The stump of the umbilical vein outside the abdomen shrivels, and the intraabdominal part is in time transformed into the round ligament of the liver (p. 436). The raw umbilical surfaces provide potential entry to infection (“navel ill”), and the allantoic duct and thrombosed vein are convenient routes for its spread.
The ductus venosus closes within a short time, but how this is achieved and whether closure is to be measured within hours or days are controversial points. Its elimination from the circulation allows the portal vein to perfuse all parts of the liver.
The loss of the umbilical return reduces both the volume and the pressure of the caudal caval stream. This, combined with the concurrent increase in left atrial pressure, halts the shunt through the foramen ovale. Contraction of the muscular wall of the ductus arteriosus is stimulated by the raised oxygen tension of the perfusing blood; it is not effected at once, and for some hours or days blood may shunt in either direction according to the relative pressures in the aorta and pulmonary artery. Expansion of the lungs reduces the resistance of their vascular bed, and the drop in pulmonary arterial pressure results in the flow through the ductus normally being from the aorta. The passage of blood through the constricted tube causes vibration of its wall, which may be detected on auscultation as a continuous murmur during the first day or two of postnatal life in calves and foals. Permanent structural changes eventually obliterate the lumen, converting the duct into a fibrous structure (ligamentum arteriosum); however, for some time after birth the ductus dilates in circumstances that produce hypoxia, and it is often found widely open in the neonatal postmortem specimen.
The increased venous return from the lungs raises the pressure within the left atrium, and this forces the valve of the foramen ovale against the atrial septum, which closes the foramen (Figures 7–25 and 7–46). The valve is a simple flap in carnivores but more elaborate and tubular in ungulates, in which muscle causes it to crumple, improving closure. Although fibrosis eventually seals the valve in place, this takes some time, and it is not uncommon for the opening to be patent to a probe for months or even years; such patency is rarely of significance.
Hypertrophy of the left ventricular wall occurs as a response to the increased workload that is now placed on that chamber. Although little exact information is available on this point for most species, significant relative thickening of the left ventricular wall is already apparent by the end of the first postnatal week in puppies.
THE ORGANIZATION OF THE LYMPHATIC SYSTEM
The lymphatic system is responsible for the immunological defense of the body. It protects the body from exogenous (foreign) and abnormal endogenous macromolecules and from viruses, bacteria, and other invasive microorganisms. It includes all the lymphatic organs: thymus, tonsils, spleen, lymph nodes and hemal nodes, and the diffuse lymphatic tissue and lymphatic nodules present in many mucous membranes. The circulating lymphocytes, as well as the lymphocytes and plasma cells that are widely disseminated throughout the organism, also participate in this protective system.
Two types of functionally distinct lymphocytes are recognized: T lymphocytes and B lymphocytes. Both result from antigen-independent proliferation and differentiation of stem cells in the primary lymphatic organs: T cells come from the thymus, and B cells come from the bursa of Fabricius in birds and the bone marrow in mammals. From the primary organs, both types of lymphocytes seed the secondary lymphatic organs, and within these, B and T lymphocytes undergo antigen-dependent proliferation and differentiation into effector cells that either attend to the disposal of particular antigens or provide the memory cells that become temporarily inactive. There is, in addition, a reserve population of undifferentiated lymphocytes.
The brief introduction to the system presented in Chapter 1 emphasizes the role of the lymphatic capillaries and larger vessels in returning an important fraction of the tissue fluid to the circulating blood. This role justifies the inclusion of these vessels, and of the nodes through which the lymph is passed, within the broad concept of a circulatory system (see Figure 1–34). The framework that supports the lymphatic nodules (germinal centers) contains phagocytic cells that remove particulate matter, including microorganisms on occasion, from the percolating lymph; this element must be included within the widely diffused macrophage or reticuloendothelial system that also includes the tissue macrophages and the endothelium of the hepatic, splenic, and bone marrow sinusoids. The vital uniting theme is defense, both humoral and cellular, against foreign invasion of the body. Because some of these functions do not intrude on the scope of gross anatomy, the present account concentrates on the lymphatic vessels and nodes as drainage and filtration mechanisms.
Before considering the topographical layout of the lymphatic system, mention must be made of the so-called lymphoepithelial structures comprising aggregations of unencapsulated lymph nodules within various mucosae. These are conveniently genetically termed tonsils, although the name is most often used specifically for those in the pharyngeal region where they guard against the passage of infection to deeper parts of the respiratory and digestive systems (Figure 7–47/2). Pharyngeal and palatine tonsils are mentioned on pages 116 and 117. Other tonsils are found in the mucosae of the larynx, intestine, prepuce, and vagina and other parts of the female tract. The common features that distinguish tonsils from lymph nodes are the absence of a capsule, the close relationship to a moist epithelial surface, and the position at the origin of a lymphatic drainage pathway.
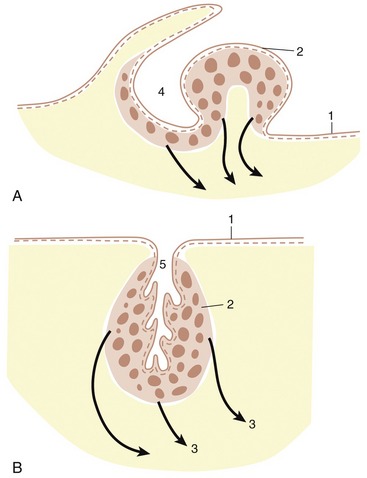
Figure 7–47 Schematic drawing of the palatine tonsils of the dog (A) and cattle (B). The tonsils of the dog develop around a fossa but protrude into the oropharynx. Those of cattle surround the tonsillar sinus within the oropharyngeal wall. 1, Epithelium; 2, palatine tonsil; 3, efferent vessels (arrows); 4, tonsillar fossa; 5, tonsillar sinus.
In addition to the ordinary lymph node, a second variety of similar structure exists but is positioned athwart the bloodstream. These hemal nodes (Figure 7–48) are not found in all species and are most familiar in sheep, in which their dark color (due to the contained blood) contrasts them with the white fat in which they are commonly embedded. They are mainly found below the roof of the abdomen and thorax. A so-called third variety, the hemolymph node, is probably only a lymph node that contains red blood cells in its sinuses as a result of hemorrhage in its tributary field.
It is uncertain whether lymph vessels develop independently and later make secondary entry to veins, bud from existing veins, or arise by a combination of these methods. Both methods account for the existence of the lymphaticovenous connections between the major lymphatic trunks and the great veins at the entrance to the chest. In some (nondomestic) mammals additional connections are described, often with renal veins. Such additional openings into the venous system can develop in later life when the normal flow is obstructed.
Lymph nodes initially form as mesenchymal condensations placed along the lymphatic capillary plexus. They are later populated by lymphocytes that emigrate from the central lymphoid organ, the thymus. All lymphoid structures are especially well developed in juveniles.
As already mentioned (p. 28) there are important species differences in the disposition of the components of the lymph nodes. In most animals, the lymph nodules are located in the peripheral cortex close to where the afferent lymph vessels penetrate the capsule (Figure 7-49 and Figure 7-50). The central medulla consists of loose lymphoreticular tissue where the efferent vessels take origin to leave the node in the indented hilar region. In contrast, in porcine nodes, the “cortical’’ tissue is central where most nodules lie alongside the trabecular sinuses. The afferent vessels penetrate the capsule at one or more sites and follow the trabeculae to reach the centrally located nodules. The periphery of the node is largely occupied by loose lymphoreticular tissue (Figure 7–51), and it is from here that the efferent lymph vessels emerge.
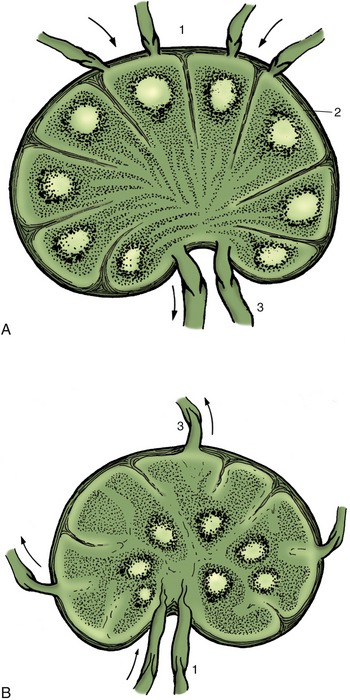
Figure 7–49 Structure of a lymph node (A) in which the germinal centers (lymph nodules) occupy the cortical region. In the pig (B) the germinal centers lie centrally. The arrows indicate the direction of lymph flow. 1, Afferent lymphatics; 2, subcapsular sinus; 3, efferent lymphatics.
Figure 7–50 Lymph node (dog) (28×). 1, Cortex with lymph nodules; 2, medulla; 3, afferent lymph vessels.
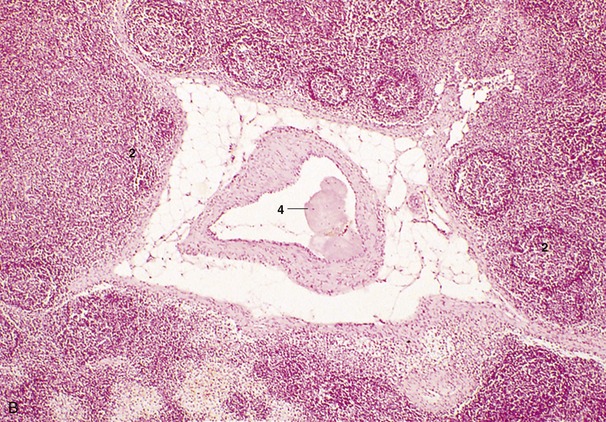
Figure 7–51 A and B, Lymph node (pig) (28×). 1, Loose lymphoreticular tissue; 2, lymph nodules in centrally located “cortex”; 3, efferent lymph vessels; 4, centrally located afferent lymph vessel, with valve.
THE TOPOGRAPHY OF LYMPHATIC DRAINAGE
The applied importance of the lymphatic drainage has been stressed, and accounts of its organization in different species are presented later. Since these accounts are necessarily fragmented by the regional character of the later chapters, it may be useful to give a short general account in which species variations and clinical significance are subordinated to the presentation of a view of the system as a whole. We begin with Figs. 7-52 and 7-53, which show the palpable lymph nodes of the dog and the cat.
Figure 7–52 Palpable lymph nodes of the dog. 1, Parotid; 2, mandibular; 3, lateral retropharyngeal (inconstant); 4, superficial cervical; 5, axillary; 6, accessory axillary (inconstant); 7, superficial inguinal; 8, popliteal; 9, femoral (inconstant).
Figure 7–53 Palpable lymph nodes of the cat. 1, Mandibular; 2, lateral retropharyngeal; 3, dorsal superficial cervical; 4, axillary; 5, accessory axillary; 6, superficial inguinal; 7, caudal epigastric; 8, popliteal.
The Lymph Nodes of the Head
Three lymphocenters are present in the head. The parotid center consists of one or more nodes placed on the masseter close to the temporomandibular joint and commonly covered by the parotid gland (Figure 7–54/2). These nodes receive lymph from dorsal structures of the head, including skin, the dorsal bones of the skull, the contents of the orbit, and the masticatory muscles (in part).
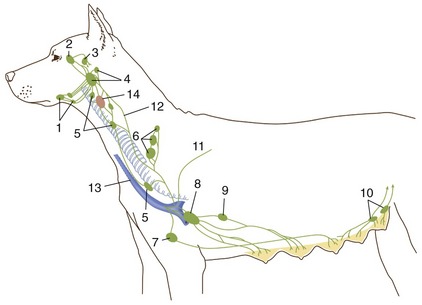
Figure 7–54 Lymph drainage of the head, neck, and mammary glands of the dog. 1, Mandibular nodes; 2, parotid node; 3, lateral retropharyngeal node; 4, medial retropharyngeal nodes; 5, cranial and caudal deep cervical nodes; 6, superficial cervical nodes; 7, sternal node; 8, axillary node; 9, accessory axillary node; 10, superficial inguinal nodes; 11, thoracic duct; 12, tracheal duct; 13, external jugular vein; 14, thyroid gland.
The mandibular center (Figure 7–54/1) comprises a group of nodes placed within the intermandibular space or more caudally by the angle of the jaw. They drain structures of the muzzle, the salivary glands, the intermandibular space (including the tongue), and a further part of the masticatory muscles.
The retropharyngeal center comprises two groups of nodes, medial and lateral; the former (Figure 7–54/4) lie against the roof of the pharynx, and the latter (Figure 7–54/3) are contained within the atlantal fossa. Together, they drain deeper structures of the head and adjacent parts of the neck, including the pharynx and larynx; one or the other also receives lymph that has already passed through the more peripheral centers. In most species the medial group serves as the collecting center for the head, receiving the output from the lateral retropharyngeal, parotid, and mandibular nodes; in cattle this role is taken by the lateral group (see Figure 25–26).
The Lymph Nodes of the Neck
The superficial cervical center (Figure 7–54/6) lies in front of the shoulder, under cover of the lateral superficial muscles of the neck; it consists of one or more nodes that drain a very wide but predominantly superficial territory. This extends from the nape to the middle of the trunk and includes the proximal part of the forelimb. The outflow is usually to the lymphatics at the thoracic inlet (Figure 7–54/12).
The deep cervical center (Figure 7–54/5) comprises a chain of nodes, usually described as packeted in cranial, middle, and caudal groups but often irregular in disposition. The nodes are placed along the trachea within the visceral space of the neck and mainly drain deeper and more ventral structures; much of this lymph percolates through successive nodes of the chain before entering one of the major lymphatic channels at the entrance to the chest.
The Tracheal Duct
In most species the tracheal duct (Figure 7–54/12) is a large paired vessel that follows the course of the trachea within the neck. Except in the horse, it takes origin in the retropharyngeal nodes that serve as the collecting center of the head; it may be augmented by tributaries from deep cervical nodes before it joins the thoracic (on the left side) or right lymphatic duct. Alternatively, one or both tracheal ducts may enter the corresponding jugular or other vein at the venous confluence at the entrance to the thorax (see Figure 1–34). In the horse the flow may be interrupted by serial passage through deep cervical nodes (see Figure 18–41/7).
The Lymph Nodes of the Forelimb
One axillary center exists. The principal nodes are contained within the axilla where they lie on the medial muscles of the shoulder; additional nodes may be found in relation to the first rib or more caudally on the chest wall. In the horse alone, a more distal group of cubital nodes is placed over the medial aspect of the elbow. The center drains the deeper structures of the entire limb and the more superficial structures of the distal segments. The efferent vessels pass directly, or after serial passage through several nodes, to one of the major lymphatic or venous channels at the entrance to the chest.
The Lymph Nodes of the Thorax
Four lymphocenters attend to the drainage of the thoracic walls and contents. The nodes within certain groups are rather diffusely spread, and it is not always easy to decide their correct designation.
The dorsal thoracic center comprises two groups of small, inconstant nodes. The intercostal set (Figure 7–55/6) is found within the upper parts of a few intercostal spaces; the thoracic aortic set is dispersed along the course of the vessel. The center drains the back and deeper tissues of the thoracic wall and sends its outflow, possibly after serial passage through several nodes, to the thoracic duct or the mediastinal nodes (Figure 7–55/8).
The ventral thoracic center comprises cranial sternal nodes (Figure 7–55/10) by the manubrium of the sternum and, only in ruminants, caudal sternal nodes placed against both surfaces of the transversus thoracis muscle. The center drains the deeper structures of the ventral part of the thoracic wall and sends its efferent flow either to mediastinal nodes or to one of the larger collecting vessels.
The mediastinal center is divided into a group of nodes within the cranial mediastinum (Figure 7–55/8), a middle group about the base of the heart, and a caudal group (absent in carnivores) near the esophagus as it approaches the diaphragm (see Figure 27–8/5,6). The various nodes drain structures of the thoracic wall, mainly after first passage of the lymph through other primary nodes, and thoracic viscera; they provide a secondary station for lymph from the lungs that has already passed through tracheobronchial nodes. The outflow goes to the large collecting vessels at the entrance to the chest, in part after serial passage through several nodes.
The bronchial center consists of groups of tracheobronchial nodes placed about the tracheal bifurcation and, in many animals, small pulmonary nodes embedded within the substance of the lung (Figures 7–55/5 and 7–56). The former groups are individually named (left, middle, right, and [in ruminants and pigs] cranial tracheobronchial nodes) according to their relationships to the major bronchi. They collect lymph from the lungs and send it in inconstant fashion to middle and caudal mediastinal nodes and sometimes directly to the thoracic duct.
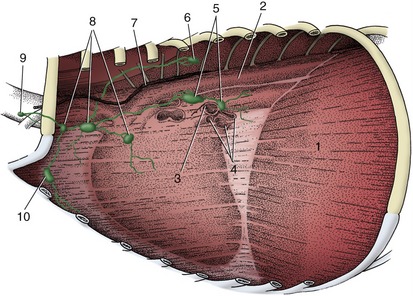
Figure 7–55 Thoracic lymph nodes in the dog. Left lung removed; the outline of the heart is visible within the mediastinum. 1, Diaphragm; 2, thoracic aorta; 3, left bronchus; 4, pulmonary vessels; 5, tracheobronchial nodes; 6, intercostal node; 7, thoracic duct; 8, cranial mediastinal nodes; 9, caudal deep cervical node; 10, sternal node.
The Thoracic Duct
The thoracic duct is the major lymph-collecting channel. It arises from the cisterna chyli, which receives lymph from the abdomen, pelvis, and hindlimbs (see Figure 1–34/5,7). The cisterna has a very irregular, even plexiform, shape, and although it is mainly contained between the aorta and the vertebrae at the thoracolumbar junction, it may also extend ventrally around the vena cava and the origin of the celiac artery. The thoracic duct passes through the aortic hiatus into the mediastinum. Its further course takes it cranially and ventrally, over the left face of the trachea, to a termination within one or other vein of the confluence that forms the cranial vena cava; it most often enters the left jugular vein or the vena cava itself (Figure 7–57). The duct receives additional lymph from the structures and nodes of the left side of the chest. A separate right lymphatic duct provides similar drainage for cranial thoracic structures of the right side and proceeds to a similar termination. One or both commonly receive the corresponding tracheal duct(s).
The Lymph Nodes of the Abdominal Viscera and Loins
The roof of the abdomen is drained by a lumbar center comprising various nodes spread along the abdominal aorta and possibly also within the spaces between the lumbar transverse processes (Figure 7–58). Usually those (renal) nodes (Figure 7–58/7) that are associated with the kidneys are larger than others in the series. In addition to draining the structures of the loins, kidneys, and adrenal glands, these nodes may receive some lymph from reproductive organs. The flow is to the cisterna chyli (Figure 7–58/5) directly or after serial passage.
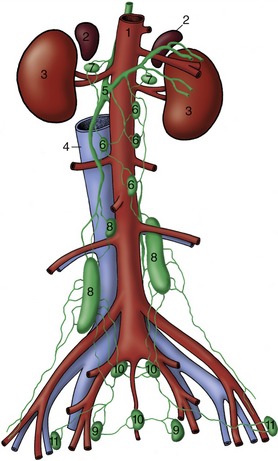
Figure 7–58 Lymph drainage of the canine lumbosacral area; ventral view. 1, Aorta; 2, adrenals; 3, kidneys; 4, caudal vena cava; 5, cisterna chyli; 6, lumbar aortic nodes; 7, renal nodes; 8, medial iliac nodes; 9, hypogastric nodes; 10, sacral nodes; 11, deep inguinal (iliofemoral) nodes.
Three centers associated with the drainage of the abdominal viscera have territories broadly corresponding to those of the celiac, cranial mesenteric, and caudal mesenteric arteries. They show very considerable interspecific distinctions, and bare mention of the nodes assigned to each center must be sufficient in this general account (Figure 7–59). The celiac center comprises splenic, gastric (subdivided in ruminants), hepatic, and pancreaticoduodenal nodes (Figure 7–59/1,2,3,4). The cranial mesenteric center comprises cranial mesenteric nodes toward the root of the mesentery and more peripheral jejunal, cecal, and colic nodes (Figure 7–59/5,6,7). The caudal mesenteric center comprises caudal mesenteric nodes associated with the descending colon (Figure 7–59/8). The three centers give rise to various visceral trunks that converge on the cisterna chyli.
Figure 7–59 Lymph drainage from the organs in the canine abdominal and pelvic cavities (schematized). 1, 1′, Right and left hepatic nodes; 2, gastric node; 3, splenic nodes; 4, pancreaticoduodenal nodes; 5, jejunal nodes; 6, right colic node; 7, middle colic node; 8, caudal mesenteric nodes; 9, lumbar aortic nodes; 9′, renal nodes; 10, efferents from the iliosacral region; 11, continuation of cisterna chyli as thoracic duct.
The Lymph Nodes of the Hindlimb, Pelvis, and Abdominal Wall
Although an inconveniently large territory to consider together, this cannot be subdivided because the responsibilities of certain nodes do not coincide with the usual division of the body. The description is most suitably begun with the most peripheral popliteal center, which consists of a node (or nodes) placed within the popliteal fossa caudal to the stifle (Figures 7–52/8 and 7–60/5). The nodes drain the distal part of the limb and direct their efferent flow to the medial iliac center (except in the horse, in which it passes to deep inguinal nodes).
Figure 7–60 Lymphangiogram of the canine lumbar area, pelvis, and thigh. 1, Lumbar aortic lymph node; 1′, lumbar trunks; 2, medial iliac nodes; 3, hypogastric node; 4, thigh muscles; 5, popliteal nodes; L6, sixth lumbar vertebra.
The ischial center has one element: the ischial node placed on the lateral aspect of the sacrosciatic ligament (of ungulates [see Figure 31–10/6]–no comparable node exists in carnivores). It collects from the muscles and skin of the rump and proximal thigh and sends its outflow to various nodes of the iliosacral center.
The deep inguinal (iliofemoral) center comprises nodes placed along the course of the external iliac artery or its femoral continuation (Figure 7–58/11). They primarily drain part of the thigh but also accept lymph from the popliteal nodes for onward passage to the iliosacral center.
The superficial inguinal center is more peripheral. It includes the superficial inguinal nodes of the groin, the subiliac nodes of the flank fold (except in the dog), the coxal node, and those of the paralumbar fossa of cattle (Figures 7–54/10 and 31–9/2,10). The superficial inguinal nodes are also named scrotal or mammary because they drain the external male reproductive organs or the udder (in dogs, caudal mammary glands) in addition to the groin region. The subiliac node drains skin and deeper structures extending from the midflank to the thigh. The efferent lymph passes to the iliosacral center, directly or after passage through the deep inguinal nodes.
The iliosacral center is a very large, widely spread collection of nodes placed against the roof of the caudal part of the abdomen and within the pelvic cavity (see Figure 7–58). The main components are the medial iliac nodes (Figure 7–58/8), near the origin of the external and internal iliac arteries and, though not in the dog, the lateral iliac about the branching of the deep circumflex iliac vessels. Other nodes are found within the pelvic cavity, both on the walls (sacral nodes) and about the viscera (hypogastric and anorectal nodes). These various small nodes are the primary filtration centers for adjacent structures and secondary stages in the drainage of the hindlimb and reproductive and other pelvic organs; the flow is funneled toward the medial iliac nodes that give origin to the lumbar trunks.
The Lumbar Trunks
These are mainly formed by efferent vessels from the medial iliac nodes. They form a plexus on the roof of the abdomen where they are augmented by part of the lumbar outflow before they expand as the cisterna chyli (Figure 7–58/5 and Figure 7–60/1′). This also receives visceral trunks from the digestive organs.
THE SPLEEN
The spleen* is contained within the left cranial part of the abdomen where it is joined to the greater curvature of the stomach by inclusion within the greater omentum. This helps fix its position, which cannot be defined with great precision as it is dependent on the degree of filling of the stomach and on its own blood content. The basic form is very dissimilar in the various domestic species, being dumbbell-shaped in the dog and cat, straplike in the pig, a broader oblong shape in cattle, and falciform in the horse (Figure 7–61). Its capsule extends trabeculae into the interior. In some species (carnivores) the capsule and trabeculae are very muscular, in others (ruminants) much less so; these differences determine the extent of the physiological variation in size that may occur. When relaxed, the spleen of the dog and cat increases severalfold from its contracted state; it is therefore particularly effective as a reservoir from which the cell content of the circulation may be recruited in times of stress.
Figure 7–61 Visceral surface of the spleens of horse (A), cattle (B), and dog (C) to show the distribution of the splenic arteries. Branches to other structures are shown in blue.
The soft tissue contained within the supporting framework is divided between red and white pulp; the former consists of spaces in series with the blood vessels and is occupied by a concentration of the cellular elements of the blood. The white pulp, which is divided into foci that are usually just visible to the naked eye, is formed of lymph nodules within a supporting reticuloendothelial framework. This tissue has the usual lymphogenic and phagocytic properties.
The functions of the spleen are blood storage, the removal of particulate matter from the circulation, the destruction of worn-out erythrocytes, and the production of lymphocytes. The first role is familiar to all who have experienced a “stitch,’’ the pain that sometimes accompanies physical stress and is associated with contraction of the splenic capsule.
The spleen is supplied by the splenic artery, a branch of the celiac artery that is generously sized in relation to the organ (see Figure 3–39/4). The venous drainage through the splenic vein leads to the portal vein (see Figure 3–50/1,2). Important specific features in the arrangement of these vessels exist. The artery and vein may pass undivided through a confined hilus (ruminants; Figure 7–61, B); run the length of the organ, detaching branches at intervals (horse, pig; Figure 7–61, A); or divide as they approach the spleen into branches that vascularize splenic compartments that are normally independent, although they do communicate (dog, cat; Figure 7–61, C). The lymph vessels found in the capsule and trabeculae do not extend into the pulp. The sympathetic and parasympathetic nerves approach with the artery.
The spleen develops from a mesodermal condensation within the dorsal mesogastrium (which becomes the greater omentum) (see Figure 3–65/6). The part of the sheet intervening between the stomach and the spleen may be specifically distinguished as the gastrosplenic ligament.
THE THYMUS
The thymus is an organ whose importance is greatest in the young animal. It begins to regress about the time of puberty and may eventually almost disappear. Even when a more sizable vestige persists, this will be found to consist largely of fat and fibrous elements and the thymic tissue is suppressed.
The thymus has a paired origin from the third pharyngeal pouch (see Figure 6–5/6), although some uncertainty exists about the precise contribution made by the endoderm and subjacent mesoderm; an ectodermal contribution is even conjectured in some species. The buds grow down the neck beside the trachea and invade the mediastinum, in which they extend to the pericardium. The cervical part regresses prematurely in many species (including the dog), and the thymus then appears as a single, median organ whose bilateral nature is anything but obvious. At its apogee it is a lobulated structure (with some resemblance to a salivary gland) that fills the ventral part of the cranial mediastinum, fitting about the other contents of this space.
The thymus is divisible in microscopic preparations into a cortex and medulla. The cortex produces the immunocompetent T lymphocytes, which enter the bloodstream for distribution to the peripheral lymphoid organs (nodes and scattered lymph nodules) where they settle and multiply. The medulla is formed of epithelioid cells of more speculative significance (Figure 7–62). Because of its relevance to the postnatal development and maintenance of immunological competence, the thymus is of vital importance.