4 The Respiratory Apparatus
The essential organs of respiration are the lungs, in which gaseous exchange takes place between the inspired air and the bloodstream. The ancillary organs comprise the passages through which air is led to and from the lungs. The nose is included, although it may alternatively be considered among the organs of special sense as it evolved as the organ of olfaction. The pharynx, in which the air and food streams cross, is more conveniently considered among the digestive organs, although its upper part (nasopharynx) is purely an airway. A short account of the development follows the description of the adult anatomy.
THE NOSE
The nose* (nasus) in the broad sense comprises the external nose, the paired nasal cavities, and the paranasal sinuses. A case may be argued for also including the nasopharynx.
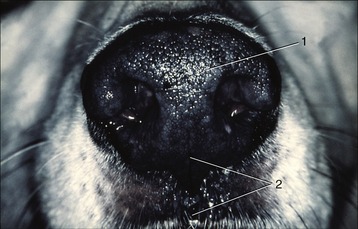
An external nose such as forms so conspicuous a feature of the human face is hardly to be recognized in the domestic species, in which it is merged within the general contours of the muzzle (Figure 4–1). Its extent is more easily determined on palpation as it more or less corresponds with the part of the muzzle skeleton that is cartilaginous and therefore flexible. It is divided internally into two cavities, the nasal vestibules, each of which is entered through a nostril and leads through a region of constriction to the much larger nasal cavity placed beyond. The form and size of the nostrils, their orientation, and the nature of the surrounding integument all show considerable species differences. The integument around the nostrils is naked and sharply demarcated from the unmodified skin in all domestic species other than the horse. According to its extent, the modified region is variously known as the nasal (carnivores, small ruminants), nasolabial (cattle), or rostral (pigs) plate. The nasal plate may be divided by a median groove or philtrum (Figure 4–1/2). The plate is kept moist in cattle, pigs, and dogs; in the first two species the moisture is derived from closely packed underlying glands, whereas in the dog it is an overflow of the secretion of glands of the nasal mucosa, principally the lateral nasal glands.
The cartilages that support the external nose are variable in form, relative size, and even number. The rostral end of the nasal septum forms the median partition between the right and left vestibules and includes a small bone (os rostrale) in the pig. The free edge of the septum gives attachment to other cartilages that support the dorsal and lateral margins of the nostril and determine the form of the opening. One, the alar cartilage, is especially large in the horse and accounts for the curious comma form of the nostril, which is divided in this species into a ventral part, the so-called true nostril leading to the nasal cavity, and a dorsal part, the false nostril leading to a skin-lined diverticulum occupying the nasoincisive notch (see Figure 18–3). The nostril is round in the pig, but in most other species it is prolonged laterally by a slitlike extension. The form of the nostril may be altered, principally by the lateral “wing” (ala) actively being raised by certain facial muscles or passively when the air flow is increased in strenuous breathing or sniffing. These changes can be very pronounced in the horse, leading to compression and almost complete obliteration of the diverticulum.
The integument is carried some distance into the vestibule, where it meets the nasal mucosa at a sharply defined line near which several ducts may open. In the horse these include the nasolacrimal (tear) duct, whose opening is very evident on inspection of the vestibular floor of the live animal; the opening is less easily found in other species, either because the tissues are less pliant (cattle) or because it is placed more deeply (dog). The much smaller openings of the long ducts of the serous lateral nasal glands also discharge in this area. This arrangement aids humidification of the incoming air because the acceleration of flow at the constriction favors vaporization of tears and other watery discharge.
The two nasal cavities occupy a large part of the face: they extend caudally to the transverse bony septum at the rostral end of the cranial cavity (Figure 4–2). Their size may be gauged from the conformation of the head, but the first impression is apt to be grossly misleading. Several features greatly reduce the extent of the cavities below expectation. Firstly, certain bones bounding the cavity are thickened by air spaces (paranasal sinuses) that communicate with the cavity but do not form part of it. Secondly, the embedded portions of the upper cheek teeth occupy a surprising amount of space, especially in the horse. The potential space is also much reduced by certain very delicate mucosa-covered turbinate bones (conchae) that project into the interior from the dorsal and lateral walls. Finally, the walls are covered by a mucosa locally thickened by vascular plexuses (Figures 4–3, 4–4, and 4–5).
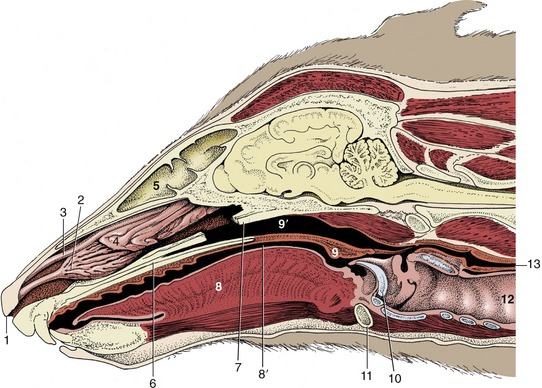
Figure 4–2 Paramedian section of the canine head; the nasal septum has been removed. 1, Right nostril; 2, ventral nasal concha; 3, dorsal nasal concha; 4, ethmoidal conchae; 5, frontal sinus; 6, hard palate; 7, vomer, resected; 8, tongue; 8′, oropharynx; 9, soft palate; 9′, nasopharynx; 10, epiglottis; 11, basihyoid; 12, trachea; 13, esophagus.
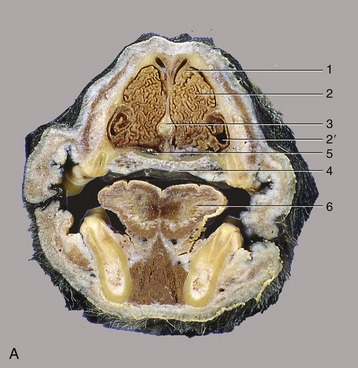
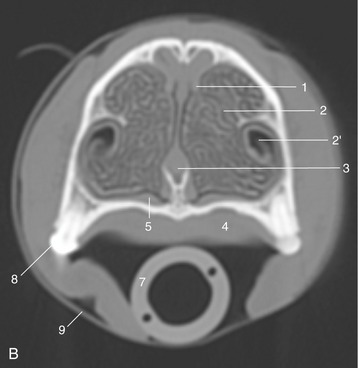
Figure 4–3 A, Transverse section of the canine head at the level of P2. B, CT image taken at the same level but without tongue and structures of the lower jaw. 1, Dorsal concha; 2, ventral concha; 2′, recess of ventral concha; 3, nasal septum; 4, hard palate; 5, venous plexus in nasal mucosa; 6, tongue; 7, endotracheal tube; 8, P2; 9, tape to keep endotracheal tube against hard palate during CT procedure.
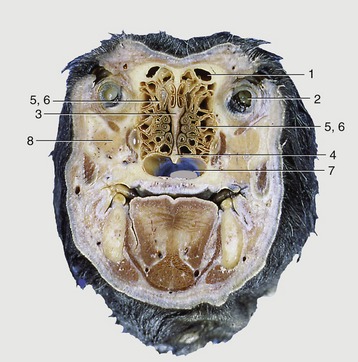
Figure 4–4 Transverse section of the canine head at the level of the eyeball. 1, Frontal sinus; 2, eyeball; 3, ethmoid bone; 4, vomer; 5, 6, ethmoidal conchae; 7, choana; 8, zygomatic gland.
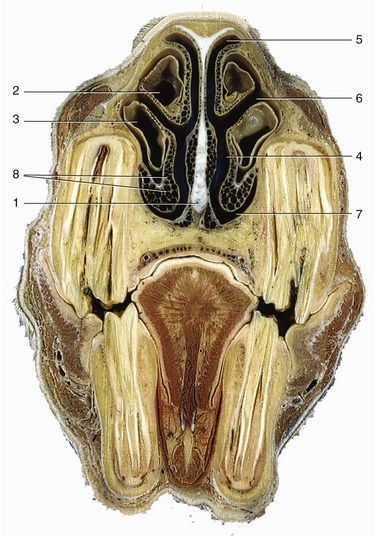
Figure 4–5 Transverse section of the equine head at the level of P4. 1, Nasal septum; 2, dorsal concha; 3, ventral concha; 4, common meatus; 5, dorsal meatus; 6, middle meatus; 7, ventral meatus; 8, venous plexus in nasal mucosa.
The right and left cavities are divided by the nasal septum, which is largely cartilaginous but ossified in its most caudal part (the perpendicular plate of the ethmoid bone). The septum meets the upper surface of the hard palate, which separates the nasal and mouth cavities, but the details vary greatly between species (see Figure 4–5). In the horse the septum meets the whole length of the hard palate so that each nasal cavity communicates with the pharynx through a separate opening (choana) (see Figure 18–11). In other species (e.g., ox, dog) the caudal part of the septum fails to meet the palate and a single opening is shared by the two sides (Figures 4–4/7 and 25–9).
The conchae, which intrude on the cavity, have a complicated and variable pattern. Classified by topography (and not by morphology), they comprise a caudal system (of ethmoidal conchae) constituting the lateral mass or labyrinth of the ethmoid bone and a rostral (nasal) system in which large dorsal and ventral (and a much smaller middle) conchae predominate (Figures 4–2 and 25–9). The numerous ethmoidal conchae are separated by narrow clefts (ethmoidal meatuses), and their pattern is most complicated in species that place much reliance on the sense of smell (Figure 4–4/5,6). The dorsal and ventral nasal conchae impose the meatal pattern of the middle and more rostral parts of the cavity. They are formed of fragile laminae coiled on themselves in a manner that varies with the species and the location. Rostrally, the lamina does not recurve to meet itself and thus bounds a recess of the nasal cavity; more caudally the coil meets itself or the lateral nasal wall to enclose a space that is part of the paranasal sinus system. The conchae reduce the cavity to a series of clefts or meatuses in an arrangement that may be likened to the letter E in transverse section (see Figure 4–5); in other words, the major conchae define dorsal, middle, and ventral meatuses branching from a common meatus against the septum. The dorsal meatus leads directly to the fundus of the nasal cavity and presents air to the olfactory mucosa. The middle meatus usually gives access to the sinus system. The ventral and common meatuses provide the principal airway leading to the pharynx. The relatively wide space at their junction is the route chosen for passage of an instrument such as a stomach tube.
The nasal mucosa blends with the underlying periosteum and varies in thickness. In some parts it is thin, but elsewhere, and especially ventrally, it is much thickened by the inclusion of cavernous blood spaces that make it a semierectile tissue (Figure 4–5/8). The thickness of the mucosa varies with the degree of vascular congestion; when the vessels are most congested, they greatly impede the air flow, causing the stuffiness associated with a head cold.
Apart from olfaction, the nasal cavity has the important function of modifying the incoming air before it is presented to the lower respiratory passages. The air is warmed by passing over the very vascular mucosa, humidified by the vaporization of the tears and serous nasal secretion, and cleansed by contact with the secretion of numerous scattered mucous glands. These glands spread a carpet of mucus over the nasal mucosa that entraps particles and droplets that come into contact with it. The carpet is moved toward the pharynx by the ciliary action of the lining epithelium and is then swallowed. It is said that in the human species as much as half a liter of mucus is swallowed unconsciously each day.
The paranasal sinuses are diverticula of the nasal cavity that excavate the skull bones (Figure 4–6), largely after birth. The separation of the inner and outer tables of the bones alters the conformation of the head and is especially striking in pigs and cattle (Figures 4–7 and 25–11), in which certain sinuses eventually extend dorsal and even caudal to the cranial cavity. The sinuses retain their connections with the nasal cavity, but because the openings are generally narrow, a relatively slow exchange of air occurs. The narrowness and locations of the openings make them prone to blockage when the mucosa is thickened by inflammation or congestion. Not all the sinuses are of equal clinical importance; the surface projections of those commonly involved in disease are considered in the topographical chapters.
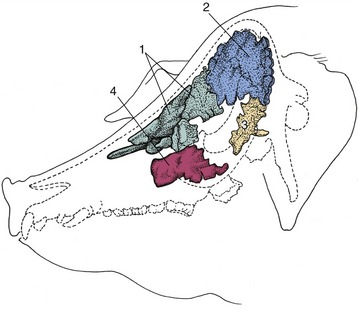
Figure 4–7 Paranasal sinuses in the pig. 1, Rostral frontal sinus; 2, caudal frontal sinus; 3, sphenoidal sinus; 4, maxillary sinus.
All species have frontal and maxillary systems, neither communicating with its contralateral counterpart. The frontal system consists of one or more spaces within the bones at the border between the nasal and cranial cavities. In most species the various frontal compartments open separately into the ethmoidal meatuses in the nasal fundus, but in the horse the frontal sinus communicates with the nasal cavity indirectly via the caudal maxillary sinus.
The maxillary sinus system occupies the caudolateral part of the upper jaw, above the caudal cheek teeth; in some species it sends extensions, variously described as separate sinuses or as diverticula, into the hard palate, the sphenoid bones, the medial aspect of the orbit, and the ventral concha. In the horse the maxillary sinus is divided into caudal and rostral parts, both connected to the middle nasal meatus. In the dog the cavity communicates freely with that of the nose and is known as the maxillary recess.
The function of the sinuses is obscure: they offer some thermal and mechanical protection to the orbit and nasal and cranial cavities, enlarge the skull areas available for muscular attachment without unduly increasing weight, and affect the resonance of the voice.
THE LARYNX
The larynx forms the connection between the pharynx and the tracheobronchial tree. It lies below the pharynx and behind the mouth, suspended from the cranial base by the hyoid apparatus; in most species it is partly contained between the rami of the mandible and partly extended into the neck, where its cartilaginous skeleton is easily recognized on palpation of the living animal (Figure 4–8). Because of its connection with the tongue and hyoid apparatus, the larynx shifts its position when the animal swallows.
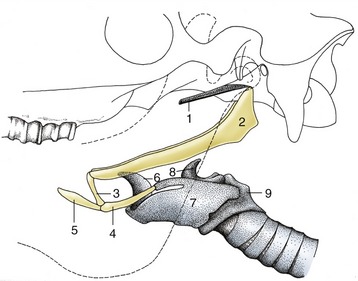
Figure 4–8 Hyoid apparatus suspending the larynx from the base of the skull (horse). The broken line indicates the mandible. 1, Cartilage of auditory tube; 2, stylohyoid; 3, keratohyoid; 4, thyrohyoid; 5, lingual process of basihyoid; 6, epiglottic cartilage; 7, thyroid cartilage; 8, arytenoid cartilage; 9, cricoid cartilage.
THE CARTILAGES
The forms of the laryngeal cartilages, and even the number of the minor elements, vary from species to species, but few differences are of great practical significance. The major, consistently present cartilages comprise the median epiglottic, thyroid, and cricoid cartilages and the paired arytenoid cartilages (Figures 4–9 and 4–10).
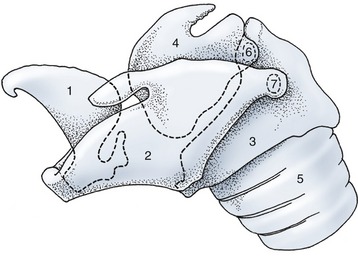
Figure 4–9 Lateral view of the equine laryngeal skeleton. The outlines of those parts of the cartilages that are covered by others are indicated by broken lines. 1, Epiglottic cartilage; 2, thyroid cartilage; 3, cricoid cartilage; 4, arytenoid cartilage; 5, trachea; 6, cricoarytenoid joint; 7, cricothyroid joint.
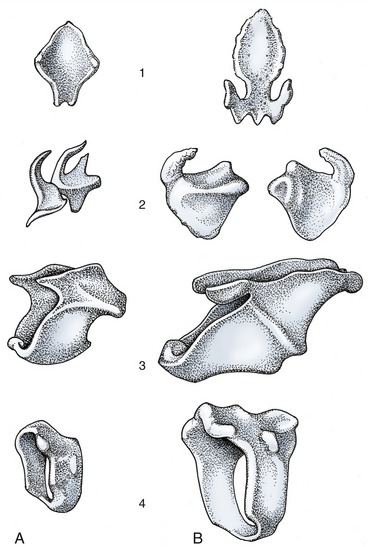
Figure 4–10 Laryngeal cartilages of the dog (A) and the horse (B). 1, Epiglottic cartilage; 2, arytenoid cartilage; 3, thyroid cartilage; 4, cricoid cartilage.
The epiglottic cartilage is most rostral. It consists of a small stalk and a large leaflike blade. The stalk is embedded between the root of the tongue, the basihyoid, and the body of the thyroid cartilage and is attached to all of these structures. At rest, the blade inclines dorsorostrally behind the soft palate (the retrovelar position), but it may be tilted backward to partially cover the entrance to the larynx when the animal swallows. It is composed of elastic cartilage and is flexible.
The thyroid cartilage is the largest of the series. It consists of two lateral plates that meet ventrally, where they fuse to a varying degree, forming a major part of the laryngeal floor (Figure 4–10/3). The body formed by this ventral fusion is least extensive in the horse, in which a large, forward-pointing notch provides a convenient route of entry for laryngeal surgery. The most rostral part of the body is generally thickened and corresponds to the “Adam’s apple,” which is more salient in the human than in domestic species. The rostral and caudal extremities of the dorsal edge of each lamina articulate with the thyrohyoid and arch of the cricoid cartilage, respectively. The thyroid cartilage is hyaline and susceptible to the age changes that affect this tissue; islands of calcification and even ossification make it more brittle with advancing age.
The cricoid cartilage is fashioned like a signet ring and consists of an expanded dorsal “seal” (lamina) and a narrower ventral arch (Figure 4–10/4). The dorsal part carries a median crest and, on its rostral rim, two facets for the arytenoid cartilages. The arch carries a facet on each side for articulation with the thyroid cartilage. The cricoid cartilage is also hyaline and subject to the aging process.
The arytenoid cartilages have a very irregular form best described as pyramidal (Figure 4–10/2). However, the details are of little importance, and for most purposes it is sufficient to recognize only a few features. A caudal facet articulates with the rostral margin of the cricoid lamina, and from this radiate (1) a vocal process that projects ventrally into the laryngeal lumen, and to which the vocal fold attaches; (2) a muscular process that extends laterally; and (3) a corniculate process that extends dorsomedially, forming the caudal margin of the laryngeal entrance with its fellow of the other side. The arytenoid cartilage is mainly hyaline, but the corniculate process is elastic.
Among the smaller and less prominent cartilages are the elastic cuneiform processes that support mucosal folds passing from the epiglottis to the arytenoids. These processes do not occur in all species, and when present, they may be free or fused with the epiglottis or with the arytenoid cartilages. A discrete nodule of hyaline cartilage, the interarytenoid cartilage, may be found between the arytenoid cartilages dorsally.
THE ARTICULATIONS, LIGAMENTS, AND MEMBRANES
In most mammals a synovial articulation is present between the thyrohyoid and the dorsorostral angle of the thyroid cartilage. Rotation occurs about a transverse axis common to the right and left joints. The joints between the dorsocaudal angles of the thyroid cartilage and the lateral facets of the cricoid cartilage also allow rotation about a common transverse axis. The third pair of synovial joints is formed between the arytenoid and cricoid cartilages (Figures 4–9 and 4–11). They are more complex and allow rotation about both sagittal and transverse axes as well as sliding movements that bring the two arytenoid cartilages closer together or carry them farther apart. Movement at the cricoarytenoid joints is the most important factor in regulating the size of the glottic opening, the narrow stretch of the lumen of the larynx. All of these joints possess the usual attributes of synovial joints.
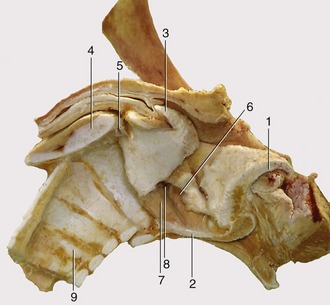
Figure 4–11 Median section of the equine larynx after removal of the mucosa. 1, Epiglottic cartilage; 2, sectioned body of thyroid cartilage; 3, corniculate process of arytenoid cartilage; 4, sectioned lamina of cricoid cartilage; 5, cricoarytenoid joint; 6, ventricularis; 7, vocalis; 8, laryngeal ventricle; 9, tracheal rings.
The cartilages are additionally joined by various membranes and ligaments that balance the laryngeal musculature and determine the resting posture of the larynx when the latter is inactive.
Elastic membranes join the epiglottis to the thyroid and arytenoid cartilages, the thyroid to the cricoid cartilage, and the cricoid to the first tracheal ring. Other less elastic ligaments form the basis of the vocal folds (and the vestibular folds when these are present) that pass between the arytenoid cartilages and the laryngeal floor.
THE MUSCULATURE
In addition to the extrinsic laryngeal muscles that pass between this organ and the pharynx, tongue, hyoid bone, and sternum, a suite of small, paired, intrinsic muscles connects the laryngeal cartilages and influences their mutual relations (Figure 4–12).

Figure 4–12 Intrinsic muscles of the equine larynx. 1, Cricothyroideus; 2, cricoarytenoideus dorsalis; 3, cricoarytenoideus lateralis; 4, vocalis; 5, ventricularis (4,5: thyroarytenoideus); 6, arytenoideus transversus; 7, laryngeal ventricle.
One of these muscles, the cricothyroideus (Figure 4–12/1), is somewhat set apart from the rest through its superficial position and its innervation by the cranial laryngeal nerve, a branch of the vagus. It runs between the lateral surfaces of the thyroid lamina and cricoid arch ventral to the cricothyroid joint; on contraction it approximates these attachments and thus carries the dorsal part of the cricoid (and the attached arytenoid cartilages) caudally, which tenses the vocal folds.
The other muscles lie more deeply, attach to the arytenoid cartilage, and are innervated by the caudal (recurrent) laryngeal branch of the vagus nerve. The cricoarytenoideus dorsalis (Figure 4–12/2) arises from the dorsal surface of the cricoid lamina, and its fibers converge rostrolaterally to insert on the muscular process of the arytenoid cartilage. On contraction it abducts the vocal process and thereby the vocal fold and so widens the glottis. The cricoarytenoideus lateralis (Figure 4–12/3) takes origin from the rostroventral part of the cricoid arch and passes dorsally to an insertion on the muscular process. It is therefore an adductor of the vocal processes and thus narrows the glottis. The thyroarytenoideus arises from the cranial part of the laryngeal floor (chiefly the thyroid cartilage) and runs dorsocaudally to insert on the muscular process and adjacent part of the arytenoid cartilage. In certain species (horse and dog included) it is divided into two units, a rostral ventricularis (Figure 4–12/5) and a caudal vocalis (Figure 4–12/4), which occupy the vestibular and vocal folds. This muscle adjusts the tension of the fold(s) and forms part of the sphincter arrangement. The arytenoideus transversus (Figure 4–12/6) runs from the muscular process of the arytenoid cartilage to a median raphe (sometimes containing the interarytenoid nodule); some fibers may cross the midline to reach the arytenoid cartilage of the other side. It approximates the arytenoid cartilages and completes the sphincter.
THE CAVITY OF THE LARYNX
The cavity of the larynx may be divided into three sections arranged in series (Figures 4–13 and 18–35). The vestibule extends from the laryngeal entrance to the rostral margin of the arytenoid cartilages and vocal folds. The glottic cleft is bounded by the arytenoid cartilages dorsally and the vocal folds ventrolaterally and can be varied in size. The third, infraglottic, cavity is of fixed dimensions and leads smoothly to the lumen of the trachea (Figure 4–14).
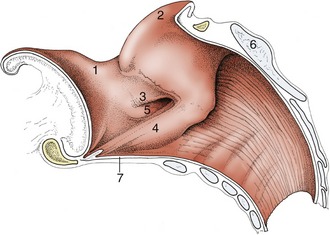
Figure 4–13 Median section of the equine larynx. 1, Epiglottis; 2, corniculate process of arytenoid cartilage; 3, vestibular fold; 4, vocal fold; 5, laryngeal ventricle; 6, lamina of cricoid cartilage; 7, cricothyroid ligament.
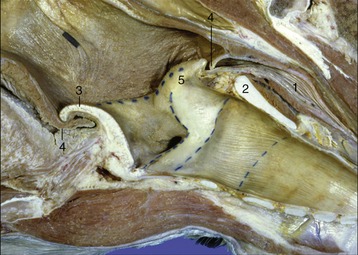
Figure 4–14 Sagittal section of junction of pharynx with larynx (horse). 1, Esophagus; 2, cricoid lamina; 3, epiglottis; 4, palatopharyngeal arch; 5, corniculate process of arytenoid cartilage.
The structures bounding the entrance to the larynx (aditus laryngis) project into the lumen of the pharynx; they may protrude through the intrapharyngeal ostium into the nasopharynx, where they may be grasped by the free margin of the soft palate and its continuation by the palatopharyngeal arches. The rostral part of the wall of the entrance is provided by the epiglottis, the lateral parts by the (aryepiglottic) folds extending between the epiglottis and the arytenoid cartilages, and the caudal part by the corniculate processes of the arytenoid cartilages. The interior of the vestibule may present a number of important features, but none of these is found in every species. In some animals a vestibular fold runs roughly parallel to the vocal fold but at a more rostral level (Figure 4–13/3). This fold pairs with an outpouching of the mucosa to form a ventricle or diverticulum that is entered between the vestibular and vocal folds (Figure 18–35). These features are especially prominent in the horse and receive more attention later. The mucous membrane bounding the vestibule is tightly adherent to the epiglottic and arytenoid cartilages but is looser elsewhere where it rests on fat.
The glottic cleft (rima glottidis) is narrower than the vestibule: the dorsal part is bounded by the vocal processes and adjacent parts of the arytenoid cartilages, and the ventral part is bounded by the vocal folds (the folds and the arytenoid cartilages constitute the glottis). The cleft, laterally compressed and diamond-shaped, varies in dimensions and disappears when the glottis is closed. The vocal folds run caudodorsally from the rostral part of the laryngeal floor to their attachments on the arytenoid cartilages. Each fold contains a ligament in its free margin and, lateral to this, the vocalis muscle, which is surrounded on most sides by fat. The vestibular folds, when present, have a similar construction but form no part of the glottis in the strict sense. The mucosa is tightly adherent to the arytenoid cartilages and along the free margin of the folds; it is much looser elsewhere.
The infraglottic cavity has few features of interest: its form reflects that of the cricoid cartilage. It may be slightly reduced in size where it continues into the trachea. The mucosa is relatively firmly attached.
The laryngeal mucous membrane contains numerous mucous glands (especially massed within the ventricles when these are present) and also lymphoid aggregations (especially in the infraglottic region). It is surfaced by an epithelium whose character varies from region to region according to its use. This epithelium is stratified squamous about the entrance, where it risks abrasion from the passage of food, and also on the free edges of the folds, which at times are abruptly brought together; elsewhere it is pseudostratified and ciliated like the epithelium lining most respiratory passages. The sensory innervation is from the cranial and caudal (recurrent) laryngeal nerves; the boundary between the territories coincides with the glottis.
THE MECHANISM OF THE LARYNX
The larynx originally developed as a device to protect the lower respiratory passages against inundation. Protection remains its primary role, although phonation—the production of voice—is the function that most often comes to mind.
Protection of the lower passages against the entrance of food and drink is achieved in two ways. On swallowing, the larynx is drawn forward, and the epiglottis, tilted somewhat backward by coming against the root of the tongue, forms a partial cover to the laryngeal entrance. The resemblance between the outlines of the epiglottis and the aditus suggests a much closer fit than actually occurs. Solid foods are swiftly carried over the laryngeal entrance by the pharyngeal muscles, whereas fluids are deflected by the epiglottis through the piriform recesses of the pharyngeal floor. It is known that removal of the larger part of the human epiglottis does not interfere with normal swallowing. A second, active protection is provided at a deeper level by the glottis, which is closed by the adduction of the vocal folds. Inhibition of inspiration at this time further reduces the risk of food being drawn into the larynx. In fact, food comparatively rarely “goes down the wrong way,” but, when it does, contact with the vestibular mucosa initiates reflex coughing.
On inspiration, abduction of the vocal folds may widen the rima glottidis, but the effect is pronounced only when breathing is unusually vigorous. Abduction is the task of the dorsal cricoarytenoideus, and subsequent adduction is the task of the lateral cricoarytenoideus muscle (Figure 4–15/5,6 and arrows). It should be noticed that these antagonistic muscles are supplied by the same nerve, which is contrary to the common arrangement.
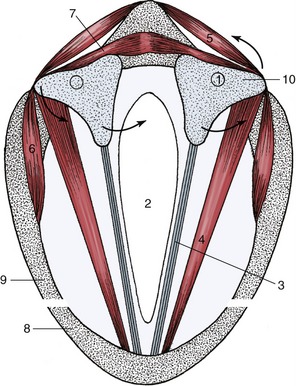
Figure 4–15 Schematic transverse section of the larynx. Arrows on the left: action of cricoarytenoideus lateralis (6) on arytenoid cartilage; arrows on the right: action of cricoarytenoideus dorsalis (5) on arytenoid cartilage (10). 1, Location of the cricoarytenoid joint; 2, glottic cleft; 3, vocal ligament in vocal fold; 4, thyroarytenoideus; 5, cricoarytenoideus dorsalis; 6, cricoarytenoideus lateralis; 7, arytenoideus transverse; 8, thyroid cartilage; 9, cricoid cartilage; 10, arytenoid cartilage.
Closure of the glottis also occurs in a number of other functional contexts in which free passage of air to or from the lungs must be prevented. A build-up of expiratory forces against a closed glottis allows for a forceful expulsion when the air is eventually released; this is the mechanism used when coughing to clear the lower passages of mucus accumulations or foreign matter. Sustained closure with elevation of the intrathoracic pressure is also used in activities involving straining: defecation, micturition, and parturition. The blockage of the escape route for air helps maintain the intrathoracic pressure and by so stabilizing the diaphragm aids the action of the muscles of the abdominal wall.
The skeleton of the thorax can also be more effectively fixed to provide a firm base for muscles attaching to the ribs when the glottis is closed. This combination of activities is well illustrated in ourselves when we attempt to lift a heavy weight or to draw the trunk toward a handhold above the head.
The production of voice is a further important function of the larynx. The sounds of human speech are more complex than those produced by other species, although no greater complexity of laryngeal structure is present. Indeed the complex laryngeal mechanism is not indispensable to this task; after the surgical removal of the larynx, an operation sometimes required by malignant disease, the human patient can learn to use the expulsion of air from the esophagus to produce voice, although it may be sadly unnatural. Even in normal circumstances the voice does not issue from the larynx in its final form but is much modified and “colored” by the resonance chambers provided by other cavities of the head. Some controversy exists over the manner in which the basic sound is produced in the larynx. The airstream is made to vibrate as it passes through the glottis. The pitch is controlled by the thickness, the length, and the tension of the vocal folds and is thus to some extent variable and to some extent determined by permanent (or semipermanent, since a boy’s voice breaks with growth) and individual features of laryngeal anatomy. The tension of the folds, or of part of them, is varied by the cricothyroideus muscle acting as the coarse adjustment and the vocalis muscle as the fine adjustment. Most believe the folds are made to vibrate passively by the flow of air passing between them. An alternative theory suggests that the muscles contract and relax at the appropriate rate; however, as some tones of the human voice exceed 200 cycles per second and tonic contraction of the vocalis muscle occurs with stimuli repeated 67 times per second, this theory is untenable.
Electromyographic studies show that purring in cats is produced by fast twitching of the laryngeal muscles and the diaphragm. The laryngeal muscles rapidly narrow and widen the glottis, which causes the respiratory air to vibrate and make the sound.
THE TRACHEA
The trachea and bronchi form a continuous system of tubes conducting air between the larynx and the smaller passages (bronchioli) in the lungs. They have a very similar construction and together are sometimes termed the tracheobronchial tree.
The trachea leads from the larynx through the visceral space of the neck, enters the mediastinum at the thoracic inlet, and continues to its terminal bifurcation above the heart. The two chief bronchi diverge from the line of the trachea to enter the corresponding lungs at their roots. In ruminants and pigs a separate tracheal bronchus arises proximal to the tracheal bifurcation and separately aerates the cranial lobe of the right lung. The cervical part of the trachea maintains a more or less median position, although its relationship to the esophagus alters at different levels and in different postures of the head and neck (see Figures 3–29 and 4–16/1). Other relations in the neck include the ventral strap muscles of the neck and the carotid sheath and its contents; the common carotid artery commences ventrolaterally but gradually climbs to a dorsolateral position where the trachea originates from the larynx.
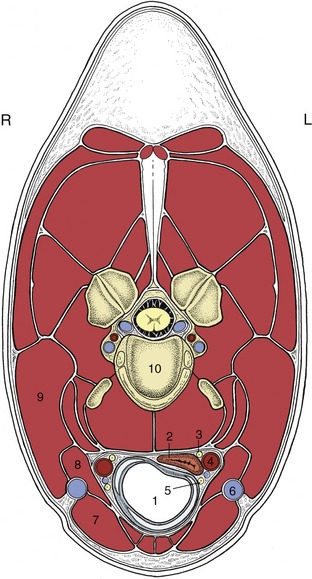
Figure 4–16 Transverse section of the neck (horse) at the level of the fourth cervical vertebra. 1, Trachea; 2, esophagus; 3, vagosympathetic trunk; 4, common carotid artery; 5, caudal (recurrent) laryngeal nerve; 6, external jugular vein; 7, sternocephalicus; 8, omohyoideus; 9, brachiocephalicus; 10, body of the fourth cervical vertebra.
The thoracic part of the trachea is deflected slightly to the right where it crosses the aortic arch. It is related ventrally to the cranial vena cava, to the arteries arising from the aortic arch, and to various tributaries and branches of these vessels; it is related dorsally to the esophagus and related variously to mediastinal lymph nodes. In young subjects it is related to the thymus. The bifurcation lies in the region of the fourth to sixth intercostal spaces but varies with the species and with the respiratory phase.
The chief bronchi very quickly enter the lungs (Figure 4–17), in which they ramify according to a pattern described later (p. 162).
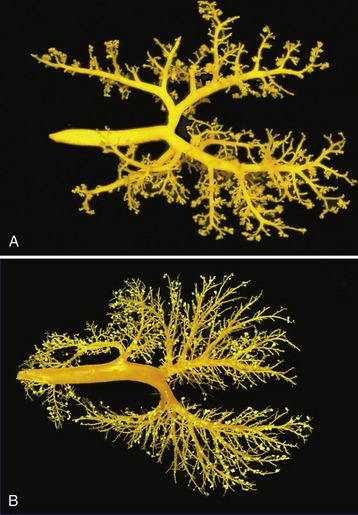
Figure 4–17 Dorsal views of corrosion casts of the bronchial tree and lungs of the cat (A) and calf (B).
The wall of the trachea is composed of an inner mucosa, a fibrocartilaginous middle layer, and an adventitia (in the neck) or serosa (in the thorax) (Figure 4–18). The mucosa, which continues that lining the infraglottic part of the larynx, may show slight longitudinal folding when the lumen is narrowed. It contains both unicellular and multicellular mucous glands that produce a protective covering of mucus that is continuously moved toward the larynx by the ciliary action of the epithelium. This mucus eventually reaches the pharynx and is swallowed without being noticed. Excessive mucus accumulations may irritate the mucosa, stimulating coughing to clear the airway. The fibrocartilaginous coat is composed of numerous strips of cartilage that are bent to form “rings” that are incomplete dorsally where the ends may fail to meet or may overlap. The edges of the strips are connected to each other by sheets of rather elastic connective tissue continuous with the perichondrium. The ends are joined by the smooth tracheal muscle (Figure 4–18/4), which bridges the gap within the “ring” in most species but is placed externally in the dog and the cat.
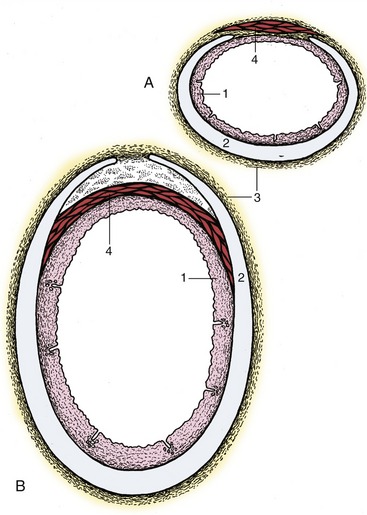
Figure 4–18 Transverse sections of the canine (A) and bovine (B) trachea. 1, Mucous membrane; 2, tracheal cartilage; 3, adventitia; 4, tracheal muscle (external in dogs, internal in cattle).
The construction of the trachea prevents it from collapsing and allows it to make the necessary adjustment in length when the neck is extended and also when the diaphragm contracts. It is attached to the diaphragm indirectly by the pulmonary ligaments and mediastinal connective tissue and also, more effectively, by the negative intrapleural pressure that couples the lungs to the chest wall, including the diaphragm. Variations in diameter are regulated by the tracheal muscle. In addition to these functional changes, there are permanent species and regional variations in the cross-sectional form and area of the trachea.
The structure of the larger bronchi is identical to that of the trachea if allowance is made for the mergence of their outer surfaces with the peribronchial connective tissue (and through this with the stroma of the lung). On the smaller bronchi the cartilage rings are gradually replaced by irregular plaques, and it is the shedding of the last of these that defines the bronchobronchiolar transition.
Variations in the diameter of the bronchi and bronchioli are relatively greater and more significant than those of the trachea.
Before proceeding, one may need to reread the section on the shape and function of the thoracic cavity (p. 52).
THE PLEURA
Each lung is invested by a serous membrane, the pleura, which also lines the corresponding “half” of the thoracic cavity. Thus, two pleural membranes exist, each arranged as a closed invaginated sac. The space between the right and left sacs forms the mediastinum, a more or less median partition in the thorax within which the heart and other thoracic organs are situated (Figure 4–19/7).
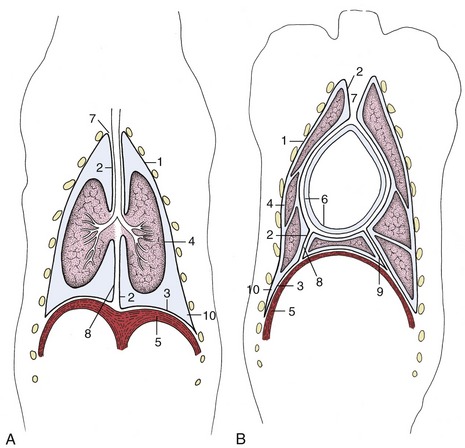
Figure 4–19 Schematic dorsal sections of the pleural cavities (dog); at the level of the tracheal bifurcation (A) and at the level of the heart (B). 1–3, Parietal pleura, later subdivided; 1, costal pleura; 2, mediastinal pleura; 3, diaphragmatic pleura; 4, visceral pleura; 5, diaphragm; 6, parietal and visceral pericardium; 7, cranial mediastinum; 8, caudal mediastinum; 9, plica venae cavae; 10, costodiaphragmatic recess.
The part of the pleura that clothes the lung directly is known as the visceral or pulmonary pleura (Figure 4–19/4). It is reflected around, and also behind, the root of the lung to become continuous with the mediastinal pleura which, in turn, is continuous with the costal and diaphragmatic pleura; these last three parts are together termed the parietal pleura.
In the healthy animal the pleural cavity is a potential rather than an actual space, and it contains only a small amount (a few milliliters) of serous fluid, which is thinly spread over the pleural surface and facilitates the smooth movement of the lung against the chest wall and of one lung lobe against another. The pressure within the pleural cavity, which is about –5 cm H2O in the neutral resting position of the chest, represents the difference between the forces that tend to recoil the lung and those that tend to expand the chest. The pressure is not uniform throughout the pleural cavity, and in addition to the expected dorsoventral gradient, local and partly unexplained differences exist; these variations in intrapleural pressure account for regional differences in the expansion and aeration of the lungs. The prevailing negative pressure explains why a surgical or traumatic opening in the chest wall causes an inrush of air into the pleural cavity, collapsing the lung and producing the condition known as pneumothorax.
The pleural sac is always more extensive than the lung, and in certain regions, facing surfaces of parietal pleura are directly applied to each other. The most important example of such an arrangement is found caudal to the basal border of the lung, where the peripheral part of the diaphragmatic pleura rests against the costal pleura lining the chest wall (the costodiaphragmatic recess; Figure 4–19/10). Although the extent of the recess varies with the phase of respiration, it remains considerable even in full inspiration, and the potential of this portion of the pleural sac is therefore never realized (see Figure 4–22/6). A similar but smaller costomediastinal recess is present ventral to the lung (Figure 4–20/12).
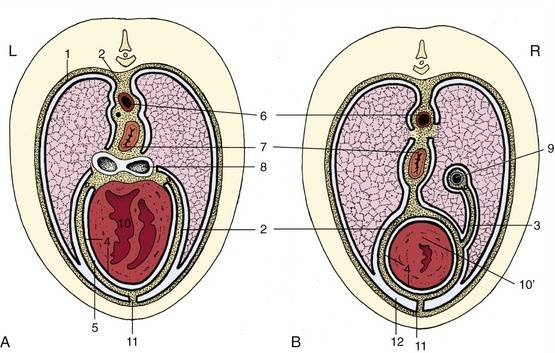
Figure 4–20 Schematic transverse section of the thorax at the level of the heart (A) and at the transition of heart to caudal mediastinum (B). 1, Costal pleura; 2, mediastinal pleura; 3, plica venae cavae; 4, parietal and visceral pericardium; 5, pericardial space; 6, aorta; 7, esophagus; 8, tracheal bifurcation; 9, caudal vena cava; 10, heart; 10′, apex of heart; 11, sternopericardial ligament; 12, costomediastinal recess.
Cranially, the costal and mediastinal portions of the pleura come together to form a dome, the cupula pleurae, which may extend in front of the first rib, where it is obviously vulnerable to injury (Figure 4–21/8′). The mediastinum is not symmetrical but is deflected to the left at certain levels. The important deflection of the caudal mediastinum is produced by the greater size of the base of the right lung.
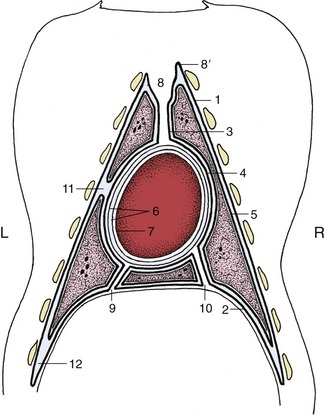
Figure 4–21 The distribution of the pleura and pericardium, schematic. The heavy lines indicate the pleura. 1–4, Parietal pleura, later subdivided; 1, costal pleura; 2, diaphragmatic pleura; 3, mediastinal pleura; 4, pericardial pleura; 5, visceral (pulmonary) pleura; 6, parietal pericardium; its outer fibrous layer tightly adheres to its inner serous layer; 7, visceral pericardium, adherent to heart (epicardium); 8, cranial mediastinum; 8′, cupula pleurae; 9, caudal mediastinum; 10, plica venae cavae; 11, left cardiac notch; 12, costodiaphragmatic recess.
A special fold (plica venae cavae) of the pleura of the right sac extends between the diaphragm and pericardium and carries the caudal vena cava in its free dorsal border (Figure 4–20/3,9). This triangular partition helps define a recess into which the accessory lobe of the right lung fits (Figure 4–21).
Considerable practical significance attaches to the strength of the mediastinum, which varies much between species. In some, for example, the ruminants, the mediastinum is thick and able to withstand a considerable pressure difference between the two pleural cavities; consequently, collapse of one lung may be tolerated. In others, for example, the dog, cat, and horse, it is very delicate and ruptures readily. Indeed the horse is among those species in which the mediastinum of the dead specimen always presents numerous small openings that place the right and left pleural cavities in communication.
THE LUNGS
The right and left lungs (pulmones,* pl.) are each invaginated into the corresponding pleural sac and are free, except at the roots where they are attached to the mediastinum. They have no fixed size or shape since they comply with respiratory changes in the dimensions of the thorax. The lungs are normally kept expanded by the air pressure within the respiratory tree, and being elastic, they recoil and collapse as soon as air is admitted into the pleural cavities by trauma, surgery, or dissection. They have a soft, spongy texture, and the residual air they contain, even when collapsed, causes them to crepitate when squeezed and to float when placed in water. In contrast, the unexpanded lungs of the fetus or stillborn animal feel solid; they sink when immersed, and this provides the pathologist with an easy means of determining that the animal from which they came had not breathed. The color of healthy lungs varies in intensity with the blood content and therefore with the manner of death; it is a fresh pink in many slaughterhouse specimens but a much deeper red in lungs obtained from animals that were not bled. The frequently patchy coloration is produced by uneven distribution of blood, which is often the result of gravitation after death. The lungs of animals that spent their lives in heavily polluted atmospheres acquire a grayish tinge from deposition of soot or other inhaled particles.
Anatomical descriptions are generally based on specimens hardened in situ before the thorax was opened; at death such lungs retain their size, which is intermediate between those adopted in full inspiration and full expiration (Figure 4–22). The two lungs are grossly alike and mirror each other in shape, although the right one is always larger; this asymmetry, partly due to the skewed position of the heart, is most obvious in the lungs of cattle. Each has some resemblance to the half of a cone, which makes it possible to recognize the following features: an apex presented toward the thoracic inlet; a wide, concave base related to the face of the diaphragm; a convex costal surface fitted against the lateral chest wall; an irregular medial surface modeled on the contents of the mediastinum; a thick dorsal border occupying the gutter between the vertebrae and ribs; and a thin border that comprises a ventral part bordering the costomediastinal recess and a basal (caudoventral) part bordering the costodiaphragmatic recess (Figures 4–20 and 4–22). The ventral part is indented over the heart (cardiac notch; incisura cardiaca).
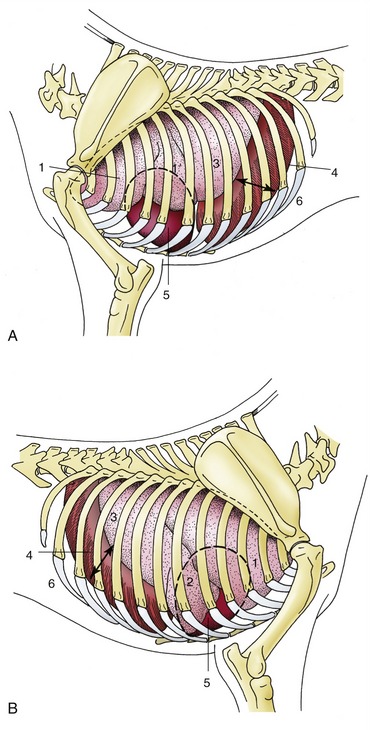
Figure 4–22 Semischematic drawings of the thoracic organs of the dog on the left (A) and right (B) sides. The outline of the heart is indicated by a broken line. 1, Cranial lobe; 1′, caudal part of left cranial lobe; 2, middle lobe; 3, caudal lobe; 4, diaphragm; 5, heart; 6, costodiaphragmatic recess (arrow).
Certain features of the mediastinal surface and base require further attention. The many indentations carried by the mediastinal surface include the large and deep cardiac impression, which is created by the heart and naturally larger on the left lung because the heart itself is biased to this side. The impression extends to the ventral border, which is deeply notched at this level in most species and which, in turn, allows the heart (or more accurately, the pericardium) direct contact with the thoracic wall (see Figure 4–22). The root of the lung, situated dorsal to the cardiac impression, is formed by the bunching together of the chief bronchus and the pulmonary artery, veins, lymphatics, and nerves within a covering of pleura provided by the reflection of the mediastinal pleura onto the lung. The reflection extends caudal to the root in a tapering fashion that leaves bare an area of lung that is directly joined by mediastinal connective tissue to the corresponding part of its partner. In some species, including the dog and the cat, the empty part of the reflection, which is known as the pulmonary ligament, extends onto the base of the lung, which thus finds additional attachment to the diaphragm. In ruminants and pigs the bronchus that arises from the trachea before its bifurcation together with the associated vessels creates a smaller second root of the right lung (Figure 4–23 and Figure 4–17, B).

Figure 4–23 Ventral (A) and dorsal (B) surface of the lung of a pig. C, dorsal and D, ventral surface of the lung of a dog. Notice the deep indentations between the lobes of the canine lung. 1, Trachea; 2, cranial lobe; 3, middle lobe; 4, caudal lobe; 5, accessory lobe; 6, opening for caudal vena cava.
The base of the right lung reveals the small accessory lobe, which is separated from the medial surface of the caudal lobe by a fissure that widens at its dorsal limit to accommodate the caudal vena cava in its passage between the caval foramen of the diaphragm and the right atrium. The accessory lobe sits, as it were, astride the vein.
In most species one or more fissures extend into the substance toward the root, dividing each lung into parts that are commonly equated with lobes. The lobes are properly defined by the ramification of the bronchial tree, and scope for confusion exists because many older texts employed the external demarcations for this purpose. According to the current practice, the left lung consists of cranial and caudal lobes and the right one of cranial, middle, caudal, and accessory lobes; however, the cranial lobe is commonly subdivided by an external fissure, whereas the right lung of the horse lacks a middle lobe. The fissures are much deeper in the lungs of the dog and the cat than in those of other species, but it is difficult to find convincing functional significance in such differences. The deeper fissures may allow the parts to slip over each other more easily and facilitate the adaptation of the lungs to the pronounced changes in thoracic form that occur in animals that employ a bounding gallop.
The bulk of the lung substance is provided by the bronchi, pulmonary vessels, and peribronchial and perivascular connective tissue. The right and left chief bronchi arise at the tracheal bifurcation above the heart, and after entering the lung at its root, each detaches a bronchus to the cranial lobe before continuing caudally (see Figures 4–17 and 4–24). The two generations of subdivisions that follow next have a fairly consistent pattern of origin, but subsequent ramifications are less predictable. The number of bronchial generations before the smaller bronchi are succeeded by bronchioles varies among species and also among parts of the one lung. In mice and other small animals only four or five generations of bronchi are present, whereas more than a dozen may be necessary in large animals. The consistency in the pattern of the first branchings allows the recognition of the so-called bronchopulmonary segments, specific portions of the lung supplied by identifiable bronchi and partly defined by connective tissue septa that extend from the peribronchial and perivascular tissue (and are responsible for the surface marbling where they impinge on the visceral pleura). Although bronchopulmonary segmentation has been studied in domestic species, it has yet to find important application; it is not yet common veterinary practice to resect portions of diseased lungs. It is the elasticity of the connective tissue stroma that allows the lungs to expand on inspiration and collapse on subsequent expiration. Loss of this elasticity, which occurs naturally with aging (but also in certain pathological conditions), reduces respiratory efficiency.
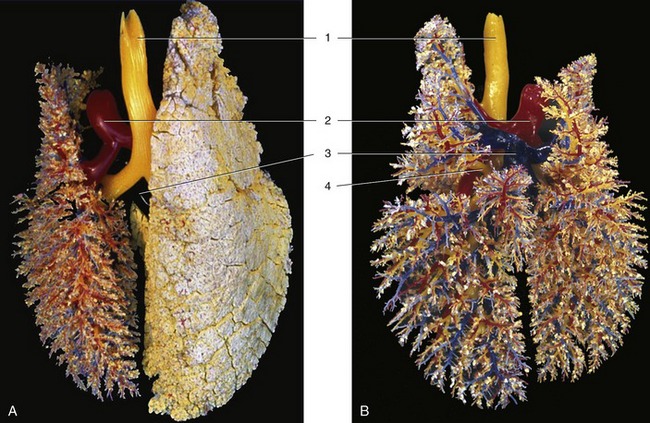
Figure 4–24 A, B, Dorsal view of the bronchial tree (yellow) and accompanying blood vessels of the pig (corrosion cast). 1, Trachea; 2, pulmonary trunk; 3, pulmonary veins; 4, tracheal bronchus.
The structure of the major bronchi resembles that of the trachea, but with each successive division the supporting cartilages become smaller and more irregular, while the muscle expands to enclose the lumen on all sides. The lumen is lined by a pseudostratified epithelium comprising tall ciliated columnar cells interspersed with goblet and serous-secreting cells and with stem cells that proliferate to repair depletions of the other types. Larger glands are included within the submucosa of the major bronchi. The transition from bronchus to bronchiole is defined by the disappearance of the last cartilage plate and by the submucosal glands. Bronchioles are narrow—less than 1 mm in diameter—and also pass through several generations. The last of these is characterized by the loss of goblet cells and their replacement by the exocrinocytes (Clara cells) thought to secrete a component of lung surfactant. The terminal bronchioles present scattered alveolar outpouchings of their walls (and are continued by alveolar ducts), thence alveolar sacs, and ultimately by the saclike alveoli, the spaces where gaseous exchange takes place through a flattened epithelium closely related to the pulmonary capillaries. Patency of the finer passages, which are unsupported by cartilage, is ensured by elastic fibers that anchor them to the pulmonary stroma. At the first breath, the alveoli fill with air and dilate, although for a time they remain significantly smaller than those of the adult (Figure 4–25).
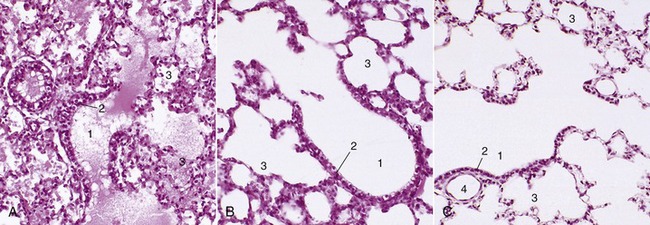
Figure 4–25 A, Lung of pig fetus (140×); note presence of fluid in bronchioles and alveoli. B, Lung of 1-day old piglet (140×). C, Lung of an adult pig (140×). 1, Terminal bronchioles; 2, bronchiolar exocrinocyte (Clara) cells; 3, alveolar sac; 4, bronchiole.
The identification of the lungs of individual species is most conveniently based on the degrees of lobation and lobulation. The lungs of horses show almost no lobation and very inconspicuous lobulation externally (Figure 4–26), those of ruminants (Figure 4–27) and pigs are conspicuously lobated and lobulated (though not uniformly in sheep and goats), and those of carnivores are very deeply fissured into lobes but show little external evidence of lobulation (see Figure 4–23).
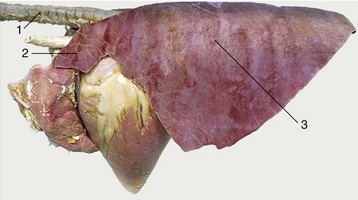
Figure 4–26 Left lateral view of the equine lungs. Note the poor lobation and lobulation. 1, Trachea; 2, cranial lobe; 3, caudal lobe.
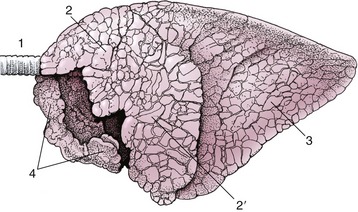
Figure 4–27 Left lateral view of the bovine lungs. Note the definite lobation and lobulation. 1, Trachea; 2, 2′, cranial and caudal parts of left cranial lobe; 3, caudal lobe; 4, right cranial lobe.
The pulmonary arteries generally follow the bronchi (see Figure 4–24), while the pulmonary veins sometimes run separately, alternating in position with the bronchoarterial associations. The pattern varies not only with the species but also with location in the one lung. These differences may find clinical significance if lung surgery becomes more common. Then it will be important to know the vascular arrangements and to be aware that both interarterial and intervenous anastomoses are to be found crossing the connective tissue partitions. A set of bronchial arteries arises from the aorta to supply the bronchi and associated connective tissue wholly independently of the pulmonary arteries (Figure 4–28). A corresponding set of bronchial veins may return this blood to the right atrium via the azygous vein, but often the bronchial flow is entirely returned to the left atrium. Arteriovenous anastomoses appear to be absent, and this makes the lung an effective filter for preventing the further spread of emboli and tumor cells. This accounts for the frequent occurrence of abscesses and tumor metastases in lung tissue, secondary to disease of other organs.
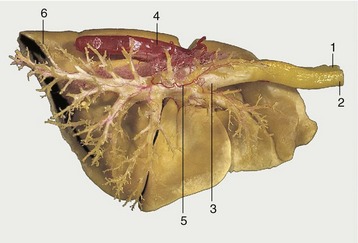
Figure 4–28 Corrosion specimen of the lungs and part of the aorta of a dog. On the right side the resin in the bronchioli and smaller bronchi has been removed to expose the main tracheobronchial tree. 1, Esophagus; 2, trachea; 3, tracheal bifurcation; 4, aorta; 5, bronchial artery; 6, caudal lobe of left lung.
Lymph drains to the tracheobronchial and mediastinal lymph nodes, directly or after initial passage through small pulmonary nodes set on the bronchial tree within the lung substance; the details are complicated, vary among species, and receive later notice when of pathological relevance.
The nerves to the lungs are delivered through a pulmonary plexus within the mediastinum to which both sympathetic and parasympathetic (vagal) fibers contribute. The efferent fibers pass to the bronchial glands and musculature and to the blood vessels. Afferent fibers come from the bronchial mucosa (cough reflex), from vessels, and from stretch receptors. Vagal section has been found to relieve pain in inoperable bronchial carcinoma of human patients.
The features of the lungs of greatest clinical significance are their projection on the surface of the body and their radiographic appearance. The projections vary among species and are described later; meanwhile, it may be stressed that they obviously vary with the phase of respiration. Moreover, the areas over which auscultation and percussion can usefully be employed are more limited than might initially be supposed; this is partly because intervention of the upper part of the forelimb denies access to part of the lung field and partly because the lower border of the lung is too thin to provide much useful information.
Because radiography of the lungs is done mainly in small animals (dogs and cats), the relevant observations on their appearance on radiographs and figures will be found in Chapter 13.
THE DEVELOPMENT OF THE RESPIRATORY APPARATUS
The development of the nose was considered in the previous chapter in relation to the development of the mouth and face (p. 141). The larynx, trachea, and lungs find a common origin in a ventral outgrowth from the foregut, directly caudal to the second of the two swellings that form the tongue (Figure 4–29). The primordium extends caudally as a (tracheobronchial) groove in the pharyngoesophageal floor; the groove is later converted into a tube by infolding and fusion of its lips. Fusion commences caudally and extends forward until the esophagus and pharynx are divided from the respiratory tract, except for a small cranial opening that persists as the entrance to the larynx. The fact that the initial development has the form of a groove rather than a tube is important because it explains the wide variety of communications between the esophagus and trachea that may occur as congenital anomalies when the process of division has been locally unsuccessful.

Figure 4–29 Five stages in the development of the trachea and lungs (ventral view). A, Caudal growth of the tracheobronchial tube. B, Its division into two lung buds. C, Further division into three bronchi on the right and two on the left. D, E, Further development of the bronchial tree.
The further differentiation of the larynx includes the appearance of the separate cartilages and muscles by condensation and differentiation of the mesoderm of the neighboring pharyngeal arches. The epiglottis has a somewhat different origin, developing as a caudal division of the second of the two median swellings that give rise to the tongue.
After separation from the esophagus, the caudal end of the respiratory tract grows down the neck and comes to lie in the median mesoderm that intervenes between the two forward-pointing extensions of the celom that become the pleural cavities. The apex of the tract splits into two lung buds (Figure 4–29, B), whose further splitting first reproduces the pattern of the bronchial tree and then creates the smaller respiratory passages that succeed the bronchi. In babies about 18 divisions succeed the stem bronchi by the time of birth; however, the process is not yet complete, and further divisions are added during infancy. The branches of the lung buds become invested by the splanchnic mesoderm into which they thrust, and it is this mesoderm that forms the tissues of the respiratory organs other than the lining epithelium (which is, of course, supplied by the foregut endoderm). The histological development of the lungs encompasses three phases named after the dominant microscopic characters: the first (glandular) phase establishes the bronchial pattern, the second (canalicular) phase establishes the respiratory portion of the lung, and the third and final (alveolar) phase is concerned with the development of the alveoli.
The production of surfactant, a substance secreted by certain alveolar cells that reduces the surface tension to allow alveolar expansion when breathing commences, is of rather late occurrence. The respiratory distress syndrome of the newborn is associated with immaturity of this feature of development.