2 The Locomotor Apparatus
This chapter is concerned with the descriptive anatomy of the bones, joints, and muscles, which is the study of systematic osteology, arthrology, and myology, respectively.* The accounts of these three classes of organs are grouped according to the major divisions of the body—the trunk, the head, the forelimb, and the hindlimb—as this breaks them into more manageable, and possibly more palatable, fragments. The system has the further advantage of better suiting the needs of any reader who is concurrently engaged in dissection. The descriptions are based on the structures of the dog, and only the most salient comparative features are noted. They omit much that is commonly included in books of systematic anatomy, but many additional details, particularly those that have an applied value, are found in the regional chapters. The introduction to each section mentions those features of development that are likely to be immediately helpful in understanding adult anatomy. These digressions are intended to recapitulate, not to supplant, the descriptions in the standard embryology texts.
THE TRUNK
BASIC PLAN AND DEVELOPMENT
The trunk is the large part of the carcass that remains after the removal of the head and neck, the tail, and the forelimbs and hindlimbs; in common speech, it is the body of the animal (Figure 2–1). It consists of three segments—thorax, abdomen, and pelvis—which are not clearly divided externally. Each is bounded by the body wall, and each contains a cavity, or a potential cavity, since, in life, the space is more or less obliterated by the close apposition of the walls and contents. The thoracic cavity lies cranial to the diaphragm, a domed sheet of muscle and tendon with a peripheral attachment to the body wall and a free center that bulges cranially. The abdominal cavity lies caudal to the diaphragm and corresponds to the belly. It communicates freely with the pelvic cavity within the enclosure of the bony pelvis (Figure 2–2).

Figure 2–1 The skeleton of the dog. 1, Wing of atlas, first cervical vertebra (C1); 2, spine of axis (C2); 3, ligamentum nuchae; 4, scapula; 5, last cervical vertebra (C7); 6, cranial end (manubrium) of sternum; 7, humerus; 8, ulna; 8′, olecranon (point of elbow); 9, radius; 10, carpal bones; 11, metacarpal bones; 12, proximal, middle, and distal phalanges; 13, sacrum; 14, hip bone (os coxae); 15, femur; 16, patella; 17, fibula; 18, tibia; 19, tarsal bones; 19′, calcanean tuber (point of hock); 20, metatarsal bones; T1, L1, and Cd1, first thoracic, lumbar, and caudal (tail) vertebrae.
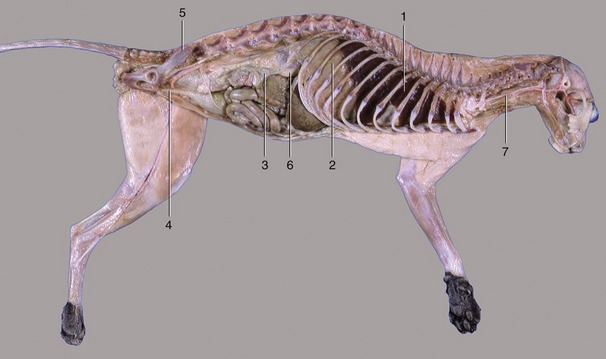
Figure 2–2 The thoracic, abdominal, and pelvic cavities of a cat; viewed from the left. 1, Thoracic cavity (with lung); 2, diaphragm; 3, abdominal cavity; 4, pelvic cavity; 5, sacrum; 6, right kidney; 7, esophagus.
The dorsal part of the body wall that roofs the thoracic, abdominal, and pelvic cavities is known as the back. It is formed by the vertebral column and associated muscles, which are structures that also extend through the neck and tail. It is therefore convenient, if not entirely appropriate, to consider the vertebrae and associated structures of the neck and tail in this section. The structures of the ventral part of the neck are included with the head.
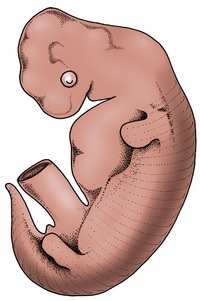
The neck, back, and tail exhibit a serial repetition of like elements, most notably the vertebrae. This apparent segmentation is, as reference to a young embryo shows (Figure 2–3), a legacy of the somites, the blocks into which the paraxial mesoderm is segregated to each side of the neural tube and notochord. The appearance in the adult is somewhat misleading; the vertebrae are, in fact, each formed by contributions from two somites of each side and are therefore more accurately described as intersegmental. Together with the ribs and sternum, they are produced from the medial portions of the somites known as sclerotomes. The muscles of the vertebral column are derived from the lateral portions of the somites, the myotomes. Many adult muscles are polysegmental and combine contributions from several or even many myotomes, but certain groups of deeper units retain the unisegmental pattern. Because the vertebrae are intersegmental, even the shortest muscles bridge, and thus can move, the joint between two successive bones.
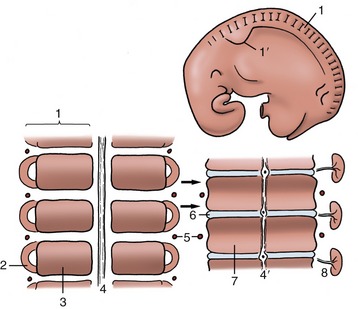
Figure 2–3 Segmentation of the paraxial mesoderm shown in a 10-mm bovine embryo (above) together with two stages in the development of the vertebrae and related vessels and nerves. The arrows show the formation of each vertebra from two pairs of adjacent somites. 1, Somite; 1′, forelimb bud; 2, myotome; 3, sclerotome; 4, notochord; 4′, notochord giving rise to the nucleus pulposus in the center of the intervertebral disk (6); 5, intersegmental artery; 6, intervertebral disk; 7, body of vertebra; 8, myotome with segmental nerve.
Early on, each myotome attracts a single nerve (Figure 2–3/8) that grows out from the adjacent neural tube; from this, it follows that the motor innervation of the muscles is also segmental and that polysegmental muscles will have a multiple innervation. A similar pattern is apparent in the sensory innervation of the skin. It was formerly believed that the connective tissue component of the skin, the dermis, derived exclusively from third portions of the somites, the dermatomes. Cells from these were supposed to migrate to underlie specific regions of the surface ectoderm. This ordered pattern of migration is now in question, and it is thought that the dermis may be, in part, produced through mesenchyme differentiating in situ. Be that as it may, a segmental innervation of skin (Figure 2–4) exists in the adult that is very regular in some places and less so in others. The bands of skin that are the provinces of particular pairs of spinal nerves are also known as dermatomes. Many overlap their neighbors. The associations between these bands and particular sensory nerves develop quite separately from those between the motor nerves and the muscles. The sensory component of the spinal nerve develops from a group of ganglion cells of neural crest origin; central branches of these cells form the dorsal root, which grows into the segment of the neural tube already defined by the outgrowth of the motor root. Together, the dorsal and ventral roots constitute the mixed spinal nerve.
In contrast to the segmental pattern of the nerves, the arteries to the body wall are branches of the aorta that initially pass intersegmentally between the somites (Figure 2–3/5). Despite this, the arteries and nerves later associate in a way that fails to reflect the different patterns of their origins.
The lateral and ventral parts of the body wall are initially unsegmented (see Figure 2–3). The tissues of these parts develop in the somatopleure, which is formed by the association of the ectoderm and the outer of the two sheets into which the lateral plate mesoderm is split. The inner sheet of the lateral mesoderm is, of course, combined with the endoderm to constitute the splanchnopleure or gut wall. The separation of these sheets is achieved by the coalescence of initially scattered spaces to form a continuous cavity (Figure 2–5/9). The cavity, known as the celom, is afterward divided to yield the pericardial and pleural spaces of the thorax and the peritoneal space of the abdomen and pelvis. The somatopleure is later invaded by cells that migrate ventrally from local somites. Cells that migrate from the sclerotomes of thoracic somites differentiate to form the ribs and sternum. Cells that migrate from the myotomes of both thoracic and abdominal somites differentiate to form the muscles of the thoracic and abdominal walls. The presence of the ribs ensures that the thoracic wall retains a segmental pattern, which is almost completely lost by the abdominal wall.

Figure 2–5 Transections of an early discoidal embryo (above) and of an older ventrally closed one to show the splitting of the lateral mesoderm and the development of the celom. 1, Ectoderm; 2, lateral plate of mesoderm; 3, endoderm; 4, notochord; 5, neural tube; 5′, neural crest cells; 6, somite; 7, somatopleure; 8, splanchnopleure; 9, celom; 10, primitive gut.
The embryo is still open ventrally while these events are proceeding. The ventral aspect of the body wall closes only in the final stage of the folding (reversal) process (p. 100) that converts the embryonic disk into a more or less cylindrical body. Ventral midline structures including the sternum and the linea alba—the median connective tissue strip of the abdominal floor—are therefore initially represented bilaterally. The umbilical scar, our “belly button,” betrays the site of final closure of the body wall.
The clinician’s chief interest in the umbilical scar relates to the prevalence of umbilical hernia, a congenital (possibly inherited) defect that frequently occurs in domestic species. Some delay in the closure of the ventral abdominal wall is always necessary to allow for the temporary physiological herniation (p. 145) of a part of the gut into the extraembryonic celom (within the umbilical cord). Normally the herniated loops of intestine are soon drawn back into the abdomen, and narrowing and, eventually, closure of the peritoneal ring at the junction of the intraembryonic and extraembryonic parts of the celom then follow. This, in turn, allows the closure of the defect in the mesodermal tissues, creating the umbilical scar. These processes may be faulty. The intestine may fail to complete its return to the abdomen or, once returned, may make a second escape into the umbilical cord through a persistent peritoneal ring and thus be exposed when the cord is ruptured at birth. More commonly, the peritoneal ring closes, but the overlying tissues remain defective and herniation occurs into a protuberant sac formed by stretching of the peritoneum and covering fasciae and skin. Fortunately, umbilical hernia is usually amenable to simple surgical correction.
THE SKELETON AND JOINTS OF THE TRUNK
The Vertebral Column
The vertebral column (or spine) extends from the skull to the tip of the tail. It consists of a large number of separate bones, the vertebrae, firmly but not rigidly joined together. It serves to stiffen the body axis and thus contributes to the maintenance of posture; by alternate flexion and extension, and sometimes by torsion, it plays a part in progression and other activities. The vertebral column encloses and protects the spinal cord and accessory structures within a central canal; in a more general way, it shields the structures of the neck, thorax, abdomen, and pelvis (see Figure 2–1).
Most vertebrae conform to a common pattern on which are superimposed features that distinguish the several regions: cervical (neck), thoracic (back, in the narrow sense), lumbar (loins), sacral (croup), and caudal (tail). The numbers of vertebrae that compose these regions vary among species and also, although to a much smaller extent, individually. They can be represented by a formula: that for the dog is C7, T13, L7, S3, Cd20–23.
A typical vertebra (Figure 2–6) consists of a massive body surmounted by an arch that completes the enclosure of a vertebral foramen; it is the summation of these foramina that constitutes the vertebral canal. The body, broadly cylindrical, is somewhat flattened on its dorsal surface, which faces into the vertebral canal; it may carry a median crest ventrally. Its extremities are usually curved: the cranial one is convex, the caudal one concave. The arch consists of two upright pedicles, and from each of these a lamina projects medially to meet its fellow and thus complete the ring about the spinal cord. The bases of the pedicles are notched, and when successive bones articulate, these notches combine to outline intervertebral foramina, openings through which pass both the spinal nerves and the vessels that supply the structures within the vertebral canal. Sometimes an additional lateral vertebral foramen perforates the pedicle next to the intervertebral foramen.

Figure 2–6 Lumbar vertebra of the dog, left lateral view. 1, Spinous process; 2, cranial articular process; 3, transverse process; 4, body; 5, caudal vertebral notch; 6, arch; 7, caudal articular process.
Each vertebra also carries a number of processes. The dorsal or spinous process springs from the union of the laminae and is generally prominent, although its form, its length, and its inclination vary with the region and with the species. Transverse processes project to each side at the junction of the body and the arch; these processes arise at the level of the intervertebral foramina and divide the muscles of the trunk into dorsal and ventral divisions. Synovial joints connect restricted parts of the arches. Sometimes the articular facets hardly rise above the level of their surroundings, but elsewhere, and especially in the caudal thoracic and lumbar region, the facets are carried on articular processes that project cranially and caudally from the dorsal portions of the arches (Figure 2–6/2,7).
In domestic as in almost all mammals there are seven cervical vertebrae. The first two, the atlas and the axis, are much modified to allow free movement of the head and require individual description. The remaining five are more typical.
The atlas is the most unusual of all the vertebrae because it appears to possess no body but to consist of two lateral masses joined by dorsal and ventral arches (Figure 2–7, A). This form results from the fusion (in early embryonic life) of a component of the atlantal body with the corresponding part of the following bone, the axis. This addition provides the axis with a cranial projection (dens; Figure 2–7, B/5), which fits into the vertebral foramen of the atlas and serves as a pivot around which the atlas (and the head) may be rotated. A plate of bone, the wing of the atlas (ala atlantis, transverse process), projects laterally from each mass, constituting a landmark that is often visible and always palpable in the living animal. The cranial aspect of the ventral arch and the adjacent areas on the wings carry two deep excavations that receive the occipital condyles of the skull. These facets approach ventrally, and in some species they merge. The caudal aspect of the ventral arch is hollowed transversely to provide an articular surface that engages with the cranial extremity of the axis. An extension (fovea dentis; Figure 2–7, A/2) of this facet onto the dorsal surface of the ventral arch accommodates the dens. The dorsal arch is perforated by openings that correspond with the transverse and intervertebral foramina of more typical cervical vertebrae; in some species a third (alar) foramen perforates the wing.
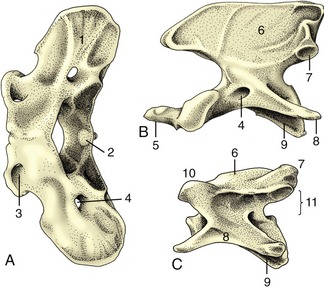
Figure 2–7 Cervical vertebrae of the dog; cranial is to the left. A, Atlas, dorsal view. B, Axis, lateral view. C, Fifth vertebra, lateral view. 1, Wing of atlas; 2, fovea dentis; 3, lateral vertebral foramen; 4, transverse foramen; 5, dens; 6, spinous process; 7, caudal articular process; 8, transverse process; 9, body; 10, cranial articular process; 11, position of vertebral foramen.
The axis is the longest vertebra. Its cranial extremity carries the dens, which is rodlike in carnivores and more spoutlike in some other species. The cranial extremity of the body and the ventral surface of the dens concur in forming a single wide articular surface for the atlas. Dorsally the dens is roughened for the attachment of ligaments that hold it in place. The arch carries a very high (and in the dog, long) spinous process that bears articular facets at its caudal extremity; these meet corresponding facets on the third cervical vertebra. The transverse processes are large; each is perforated toward its root by a transverse foramen that transmits the vertebral artery, vein, and nerve.
The remaining cervical vertebrae become progressively shorter as the series is followed toward its junction with the thorax. The extremities of the body are more strongly curved than in other regions and slope obliquely. The ventral surface carries a stout crest. The arch is strong and wide, but the spinous process is poorly developed except on the last (considerable variation, however, exists among species). The large transverse process (Figure 2–7/8) branches into dorsal and ventral tubercles, the latter commonly developing a caudal platelike extension (Figure 2–8/5). On the third to sixth bones the process is perforated by a transverse foramen through which the vertebral vessels and nerve pass. The articular facets are large and flat but do not rise above the surrounding level. The seventh cervical vertebra, transitional to those of the thoracic region, is distinguished by its taller spinous process, unperforated transverse process, and the presence of facets on the caudal extremity of its body for articulation with the first pair of ribs.
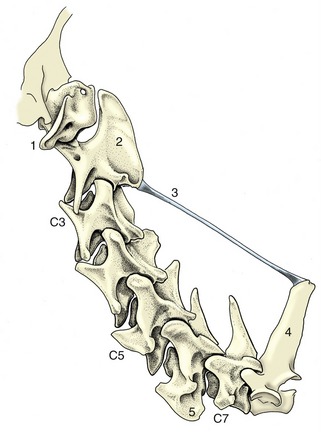
Figure 2–8 Nuchal ligament of the dog. 1, Wing of atlas; 2, spinous process of axis; 3, nuchal ligament; 4, spinous process of first thoracic vertebra; 5, platelike extension of transverse process.
The thoracic vertebrae (Figure 2–9) articulate with the ribs and correspond with these in number. Minor variations in number are not uncommon; they are often compensated by a reciprocal change in the lumbar region that leaves the thoracolumbar total unaffected. All thoracic vertebrae share common features, but serial changes also occur that gradually (and on some points abruptly) distinguish the more cranial from the more caudal bones. Common thoracic features are short bodies with flattened extremities; costal facets, on both extremities for the rib heads and on the transverse processes for the rib tubercles; short, stubby transverse processes; closely fitting arches; very prominent spinous processes; and low articular processes.
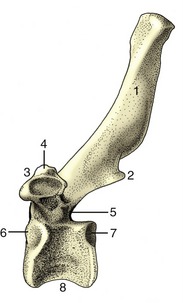
Figure 2–9 Thoracic vertebra of the dog; left lateral view. 1, Spinous process; 2, caudal articular process; 3, transverse process with costal fovea; 4, mamillary process; 5, caudal vertebral notch; 6, 7, costal foveae; 8, body.
Conspicuous serial features are a rapid increase in the height of the spinous processes, which reach a maximum a few vertebrae behind the cervicothoracic junction and gradually decline thereafter; progressive simplification of the costal facets (those on the transverse processes approach and finally merge with those on the cranial extremity); reduction (and eventual disappearance) of the caudal costal facets; and appearance of an additional (mamillary) process as a projection from the transverse process and its gradual migration to join the cranial articular process. More abrupt changes toward the end of the thoracic series include sudden alteration from a caudodorsal to a craniodorsal orientation of the spinous processes and a change in the character of the articular facets from the cervical to the lumbar pattern (Figure 2–10). In some species, including the dog, the last members of the thoracic series possess yet other (accessory) processes that spring from the caudal part of the arch to overlap the following bone.
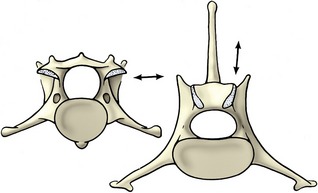
Figure 2–10 Contrast the orientation (arrows) of the articular surfaces of a cervical (left) and a lumbar (right) vertebra of the dog, caudal view.
The lumbar vertebrae (Figure 2–11) differ from the thoracic vertebrae in the greater length and more uniform shape of their bodies. Other regional features are absence of costal facets; a shorter height and generally forward slope of the spinous processes; long, flattened transverse processes that project laterally, sometimes (as in the dog) with a cranioventral inclination; interlocking articular processes; and prominent mamillary, and sometimes also accessory, processes.
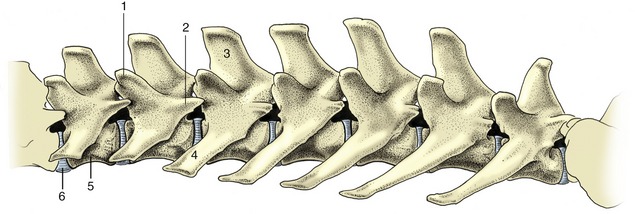
Figure 2–11 Lumbar vertebrae of the dog, left lateral view. 1, Mamillary process; 2, accessory process; 3, spinous process; 4, transverse process; 5, body; 6, intervertebral disk.
Caudal to the loins the vertebral column is continued by the sacrum, a single bone formed by the fusion of several vertebrae. The sacrum forms a firm articulation with the pelvic girdle through which the thrust of the hindlimbs is transmitted to the trunk. Usually only one or two of the constituent vertebrae directly participate in the articulation. The more caudal bones project behind this to furnish the greater part of the roof of the pelvic cavity. In some animals (especially pigs) one or more tail vertebrae may be incorporated into the sacrum in later life. In the dog the three sacral vertebrae form a short quadrilateral block (Figure 2–12).
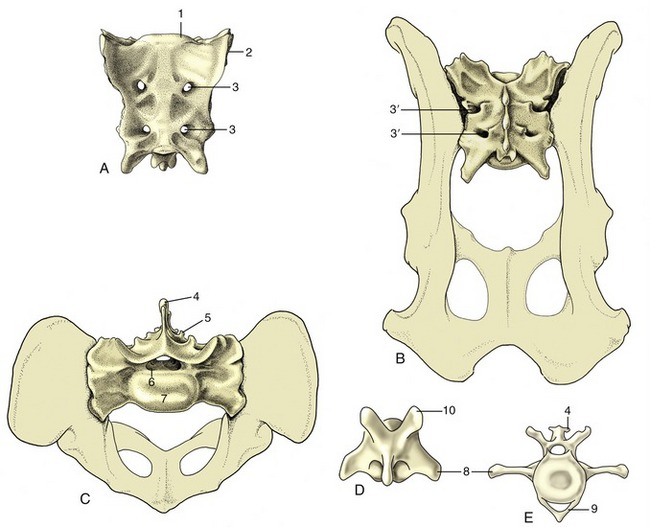
Figure 2–12 Canine sacrum and caudal vertebrae. A, Sacrum, ventral view. B, Sacrum, dorsal view. C, Sacrum, cranial view. D, Caudal vertebra, dorsal view. E, Caudal vertebra, cranial view. 1, Promontory; 2, auricular articular surface; 3, ventral (3′ dorsal) sacral foramina for ventral (3′ dorsal) branches of sacral nerves; 4, spinous process; 5, rudimentary articular process; 6, vertebral canal; 7, body; 8, transverse process; 9, hemal arch, also called chevron; 10, cranial articular process.
The sacrum commonly narrows from its cranial to its caudal extremity and is curved along its length to present a smooth, slightly concave face toward the pelvic cavity. In most species the dorsal surface is marked by the appropriate number of spinous processes, although these may be much reduced or even absent (e.g., pig). When present, they may preserve their independence (e.g., dog or horse) or fuse to form a continuous crest (e.g., ruminants). Lateral to this, a lower irregular crest usually marks the site of the redundant articular processes. The margin of the bone is formed by the fused transverse processes and carries toward its cranial extremity the articular surface for the ilium; this is often “ear-shaped,” hence the name auricular surface (Figure 2–12/2).
The degree of fusion of the sacral vertebrae varies among species; it is least complete in the pig. Even when fusion is total, the composition of the sacrum is betrayed by the number of foramina that mark both surfaces; the dorsal and the ventral branches of the sacral nerves issue separately through these. The junction of the ventral surface with the cranial extremity forms a lip known as the promontory (Figure 2–12/1); though often inconspicuous, it is a reference point in obstetrics.
The number of caudal vertebrae varies greatly, even within a single species. These vertebrae show a progressive simplification in form, and although the first few resemble miniature lumbar vertebrae, the middle and later members of the series are reduced to simple rods. In addition to the usual features, the more cranial vertebrae of some species provide protection to the main artery of the tail in the form of ventral (hemal) arches, separate small chevron (V-shaped) bones connected to the undersurfaces of the bodies, or paired ventral (hemal) processes (Figure 2–12, E).
The contours of the vertebral column vary with the posture, the species, and the breed. In general, the vertebrae from the caudal thoracic region to the tail head follow a more or less horizontal line. The more cranial thoracic vertebrae slope downward to reach the lowest point at the entrance to the chest, where an abrupt change in direction puts the spine on a course that ascends toward the head. The ventral inclination of the cranial thoracic vertebrae is masked in the live animal by the height of the spinous processes; indeed, in some species, the horse most notably, the spines are so long that the contour of this part of the back is raised to constitute the withers. Except toward the poll, the cervical vertebrae run at some distance from the dorsal skin. This is not apparent in the live subject, and in larger animals it may not be easy to determine, even on palpation. The greater part of the tail hangs down in large animals, but its posture is more variable in dogs and cats, being an expression of emotion in both species and influenced by breed in the former.
The Joints of the Vertebral Column
The vertebrae form two sets of joints: one cartilaginous, involving the direct connection of the vertebral bodies, the other synovial, existing between facets carried on the vertebral arches. In addition, certain long ligaments extend over many vertebrae. This pattern is modified in two regions; cranially, allowance is made for the free movement of the head, and in the pelvic region, sacral fusion occurs.
The two joints of the atlas are described first. The atlantooccipital joint (Figure 2–13) is formed between the condyles of the skull and the corresponding concavities of the atlas. Although the separate right and left articular surfaces converge ventrally, they do not always merge; despite this, a single synovial cavity generally exists. The synovial membrane attaches around the occipital and atlantal facets. It is strengthened externally by dorsal and ventral atlantooccipital membranes, which pass from the arches of the atlas to corresponding parts of the margin of the foramen magnum (see Figure 2–32/12), and by lesser lateral ligaments, which pass between the atlas and adjacent regions of the skull. Despite its odd character, the joint functions as a ginglymus: movement is virtually restricted to flexion and extension in the sagittal plane (the nodding movement that in ourselves conveys agreement).
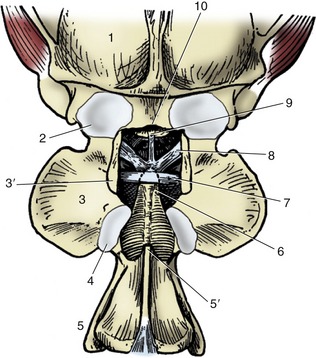
Figure 2–13 Canine atlantooccipital joint, dorsal view; the dorsal arch of the atlas has been removed. 1, Skull; 2, atlantooccipital joint capsule; 3, wing of atlas; 3′, dorsal arch of atlas, resected; 4, atlantoaxial joint capsule; 5, axis; 5′, spine of axis, its overhanging cranial portion having been removed; 6, dens; 7, transverse ligament of atlas; 8, alar ligaments; 9, apical ligament of dens; 10, dorsal margin of foramen magnum.
The atlantoaxial joint is even more peculiar. The extensive articular surfaces of the ventral arch of the atlas and of the body and dens of the axis face into a single synovial cavity. The surfaces are so formed that only limited areas are in contact in any position of the head. This limitation of contact, together with the roomy capsule, allows some versatility of movement, although free excursion is confined to rotation about a longitudinal axis (the head-shaking movement that implies negation). The dorsal atlantoaxial ligament that joins adjacent parts of these vertebrae imposes little restraint. The dens of the axis, which occupies a potentially dangerous position in relation to the spinal cord, is secured by one or more ligaments that strap it to the adjacent part of the upper surface of the ventral atlantal arch and sometimes also to the occipital bone (as in the dog). It is rupture of these ligaments—or fracture of the dens itself—that allows the axis to strike against the cord and procure death in judicial hanging, according to traditional accounts (other forms of cervical fracture or dislocation may be at least as common).
A single description serves for the articulations of most other vertebrae. The intervertebral articulations combine symphyses between the bodies and synovial joints between the articular processes. The bodies of adjacent bones are connected through thick but flexible pads, the intervertebral disks, which make an appreciable contribution to the articulated column. They account for about 10% of its length in ungulates, about 16% in dogs, and about 25% in ourselves, which are proportions that are clearly correlated with different degrees of suppleness of the trunk. The disks are among the organs that most consistently show degenerative changes with advancing age; disk lesions are a common source of back trouble, long recognized in ourselves and in dogs, now also diagnosed in other domestic and even wild animals. Therefore, their structure has considerable importance, and it may be wise to stress that the details of anatomy and the nature of the troubles that may occur are not the same in ourselves as in quadrupeds.
Each disk consists of two parts, a nucleus pulposus and an anulus fibrosus (Figure 2–14). The nucleus occupies a slightly eccentric position. In the young animal, it consists of an unusual semifluid tissue derived from the embryonic notochord and retains some resemblance to this in structure. It is contained under pressure and escapes if afforded opportunity. The anulus fibrosus consists of encircling bundles of fibrous tissue that pass obliquely from one vertebra to the other, in most species merging with cartilage plates that cap the bones. The orientation of the fibers changes between successive lamellae, of which about a score exist. The distinction between anulus and nucleus is not always very clear, particularly in the larger species. Retention of the nucleus within the fibrous ring absorbs shock and spreads the compressive forces to which the column is subjected over a wider part of the vertebrae.
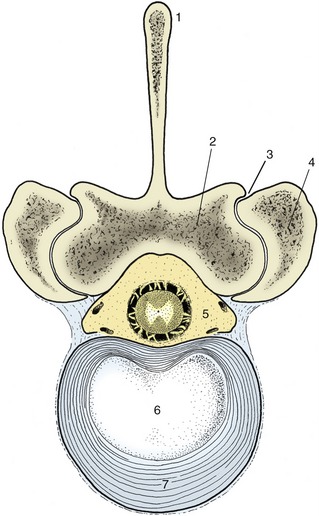
Figure 2–14 Bovine lumbar intervertebral disk. 1, Spinous process; 2, lamina; 3, synovial intervertebral joint; 4, articular process of adjacent vertebra; 5, vertebral canal with contents (spinal cord and meninges surrounded by epidural fat); 6, nucleus pulposus; 7, anulus fibrosus.
Insidious changes involving both nucleus and anulus commence relatively early in life. Fragmentation of the ring may allow the nucleus to escape, usually in the direction of the vertebral canal, where, directly or indirectly, it may press on the cord. Calcification of the nucleus diminishes the normal resilience and flexibility of the spine. Degenerative changes may affect any disk, but the effects are naturally likely to be most severe when they involve the disks at the most mobile regions; those of the neck and, in large animals, that at the lumbosacral junction are especially susceptible. Most thoracic disks are crossed dorsally by the intercapital ligaments that unite the heads of the right and left ribs (p. 43), and these are alleged to mitigate the effects of disk rupture at these levels.
The joints between the facets on the vertebral arches are conventional synovial joints. The nature and degree of mobility vary with the region and, to some extent, also with the species. In the cervical and cranial thoracic regions the joint surfaces are arranged tangential to the circumference of a circle centered in the vertebral body (see Figure 2–10); in these regions, rotation is possible in addition to the usual flexion and extension. In the caudal thoracic and lumbar regions the surfaces have a radial alignment, and movement is more or less restricted to the median plane. Movement is most free in the neck, where the articular surfaces are largest and the capsules most loose. The elastic interarcuate ligaments that fill the dorsal spaces between the arches of successive vertebrae may be regarded as accessory to these joints; their extent is inversely related to the width of the arches. In certain regions, interspinous and intertransverse ligaments also exist, but these are of less importance.
Three long ligaments extend along substantial portions of the column. A dorsal longitudinal ligament (Figure 2–15/7) runs along the floor of the vertebral canal from the axis to the sacrum. Narrow over the middle of each vertebral body, it widens where it crosses each intervertebral disk. A ventral longitudinal ligament follows the ventral aspect of the vertebrae from the midthoracic region to the sacrum; more cranially, its role is filled by the longus colli muscles. It also widens over and fuses with the intervertebral disks.
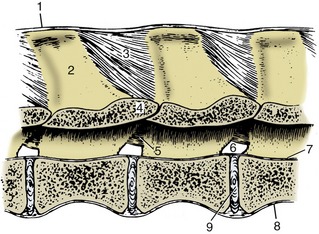
Figure 2–15 Ligaments of the vertebral column. Paramedian section of lumbar vertebrae of a dog; viewed from the left. 1, Supraspinous ligament; 2, spinous process; 3, interspinous ligament; 4, arch of vertebra; 5, interarcuate ligament; 6, intervertebral foramen; 7, dorsal longitudinal ligament; 8, ventral longitudinal ligament; 9, intervertebral disk.
A third (supraspinous) common ligament runs over (or to each side of) the summits of the spinous processes of the thoracic and lumbar vertebrae. It merges with the tendons of the epaxial muscles so completely that some dispute its independent existence. Except in the pig and cat, a cranial continuation of this ligament leaves the highest spines of the withers and runs by the shortest route to attach to the nuchal surface of the skull or, as in the dog, the spinous process of the axis (see Figure 2–8). This nuchal ligament runs close to the upper contour of the neck, and for most of its length, it is well separated from the more ventral course followed by the cervical vertebrae. Unlike the other long ligaments, it is elastic and thus able to accept much of the burden of the head when this is held high without interfering with the animal’s ability to lower the head to feed or drink from the ground. There is an obvious correlation between the strength of this ligament and the weight of the head and the length of the lever arm of the neck; the nuchal ligament is therefore much more powerfully developed in the larger species (see Figure 19–3), in which it is also more complicated in structure.
The Ribs and Sternum
The thoracic skeleton is completed by the ribs and sternum. The ribs (costae) are arranged in pairs and generally articulate with two successive vertebrae: the caudal one is that with the same numerical designation as the rib. Each rib consists of a bony dorsal part, the rib proper, and a cartilaginous ventral part, the costal cartilage (Figure 2–16, A). The two parts meet at a costochondral junction. The dorsal part of the rib articulates with the vertebral column, while the cartilage articulates with the sternum either directly, as do the first eight or so sternal or “true” ribs, or indirectly through connection of the cartilage with that in front, as do the asternal or “false” ribs. In this way, the cartilages of the asternal ribs combine to form the costal arch (Figure 2–17, A/6), the cranial boundary of the flank. The cartilage of the last rib may fail to make contact with its neighbor, and this rib is then said to be “floating.”

Figure 2–16 A, Left rib of a dog, caudal view. B, Left rib of a dog articulating with two vertebrae, lateral view. 1, Tubercle; 2, head; 3, neck; 4, angle; 5, body; 6, costochondral junction; 7, costal cartilage; 8, intervertebral disk; 9, vertebra of same number as rib.
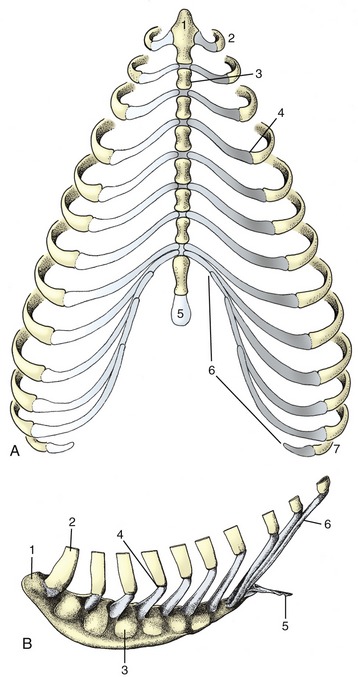
Figure 2–17 A, Canine and B, equine sternum and costal cartilages, ventral and left lateral views. 1, Manubrium; 2, first rib; 3, sternebra; 4, costochondral junction; 5, xiphoid cartilage; 6, costal arch; 7, floating rib.
The dorsal extremity of the rib terminates in a rounded head that carries two facets, one for articulation with the body of each of the two vertebrae with which it is connected. These facets are separated by a rougher area (crest) that makes contact with the intervertebral disk and on most ribs also gives origin to the intercapital ligament. The head is joined to the body of the rib by a short constricted neck whose lower part carries a lateral tubercle. The tubercle bears a third articular facet, which meets that on the transverse process of the more caudal of the associated vertebrae (Figure 2–16, B).
The body of the rib begins beyond the tubercle. It is long, curved in its length, and usually laterally flattened, particularly in the larger species and toward the lower extremity. It is most strongly bent at a region known as the angle (Figure 2–16/4), where the lateral surface is roughened for the attachment of the iliocostalis. The cranial and caudal margins of the body are often sharply defined and give attachment to the intercostal muscles that fill the space between successive ribs. The caudal margin may also be grooved to give protection to the neurovascular bundle of the intercostal space.
The costal cartilage is flexible in the young animal, especially if it is long and thin, as in the dog. It becomes more rigid as calcification develops and increases with age. The cartilage either meets the bony rib at an angle (knee, genu) or is itself flexed cranioventrally some way beyond the costochondral junction.
Serial changes are obvious. The first rib is always relatively strong, short, and straight. Its cartilage is also stumpy and articulates with the sternum at a tight joint that fixes the rib; this allows it to act as a firm base toward which the other ribs may be drawn on inspiration. The succeeding ribs increase in length, in curvature, and in caudoventral inclination, most markedly over the caudal part of the thoracic wall, although the very last two or three may again be somewhat shorter. The three articular facets of the upper end approach and eventually merge on the ribs toward the end of the series. The cartilages of the sternal ribs are short and about as thick as the bony ribs; those of the asternal ribs are mostly slender and taper toward their ventral extremities.
The sternum is composed of three parts. The most cranial part, known as the manubrium (Figure 2–17/1), generally projects in front of the first ribs and may be palpated at the root of the neck. It is rodlike in the dog and cat but is laterally compressed in the larger animals. The body of the bone is composed of several segments (sternebrae), in youth joined by cartilage that is later replaced by bone. It is cylindrical in the dog, wide and flat in ruminants, and carries a ventral keel in the horse (Figure 2–17, B). Its dorsolateral margin bears a series of depressions in which the extremities of the costal cartilages are lodged. The more cranial of these depressions alternate with the sternebrae, and each receives a single cartilage; the more caudal depressions are crowded more closely together and may receive more than one cartilage. The caudal part of the sternum consists of flat (xiphoid) cartilage (Figure 2–17/5) that projects between the lower parts of the costal arches. It supports the most cranial part of the abdominal floor and gives attachment to the linea alba.
The Joints of the Thoracic Wall
Most ribs make two separate articulations with the vertebral column. The head participates in a ball-and-socket costovertebral joint of unusually restricted mobility. The joint cavity is divided into two compartments by the intercapital ligament (Figure 2–18/2), which arises from the interarticular crest. This ligament passes through the intervertebral foramen, crosses the floor of the vertebral canal, and ends by inserting on the corresponding region of the rib of the other side. In its passage, it detaches slips that anchor to the intervertebral disk and the adjacent parts of the vertebrae. It passes below the dorsal longitudinal ligament (Figure 2–18/6) and offers some protection against nuclear material from a ruptured disk protruding into the vertebral canal. An intercapital ligament is not found at the first costovertebral joint or at the last few. Additional short and tight ligaments support the joint dorsally and ventrally.

Figure 2–18 Costovertebral articulations; transverse section of the vertebral column of the dog (about T8). 1, Lamina of vertebra; 2, intercapital ligament; 3, tubercle of rib; 4, head of rib; 5, intervertebral disk; 6, dorsal longitudinal ligament; 7, costovertebral joint; 8, costotransverse joint covered by costotransverse ligament.
The costotransverse joint in which the tubercle participates is of the sliding variety. It is supported by a ligament that passes between the neck of the rib and the transverse process of the vertebra (Figure 2–18/8).
The costosternal joints are synovial joints of the pivot variety. The interchondral joints of the asternal ribs are syndesmoses of a rather elastic nature. The intersternal joints are mostly impermanent synchondroses, although in some species the manubrium articulates with the body at a synovial joint.
The movements possible at these joints are discussed with the actions of the muscles of the thoracic wall.
The Pelvic Girdle
Although the pelvic girdle is formally a part of the hindlimb skeleton, it seems more sensible to treat it here since it is fully integrated into the construction of the trunk. The girdle consists of symmetrical halves, the hip bones (ossa coxarum), which meet at the pelvic symphysis ventrally and form firm, though not rigid, articulations with the sacrum dorsally. When augmented by the sacrum and first few tail vertebrae, it forms a ring known as the bony pelvis around the pelvic cavity. The close association with the pelvic organs exposes the girdle to visceral influences of which those related to giving birth are most important; the form of the bony pelvis therefore reflects a compromise between these and the requirements of locomotion and posture.
Each hip bone is composed of three bones that develop from separate ossifications within a single cartilage plate. In the young animal, strips of cartilage demarcate the boundaries to allow for growth, but they disappear once growth is complete. It is therefore artificial to describe the three components—ilium, pubis, and ischium—as separate units; the practice can be justified only by its convenience in facilitating description. The ilium (Figure 2–19/1) is the craniodorsal part that extends obliquely forward from the hip joint to articulate with the sacrum. The pubis (Figure 2–19/6) extends medially from the joint to form the cranial part of the pelvic floor. The ischium (Figure 2–19/8) is more caudal and forms the larger part of the floor, although it also sends a branch to the joint. Both pubis and ischium participate in the symphysial joint in domestic species, although only the pubis does so in the human pelvis.
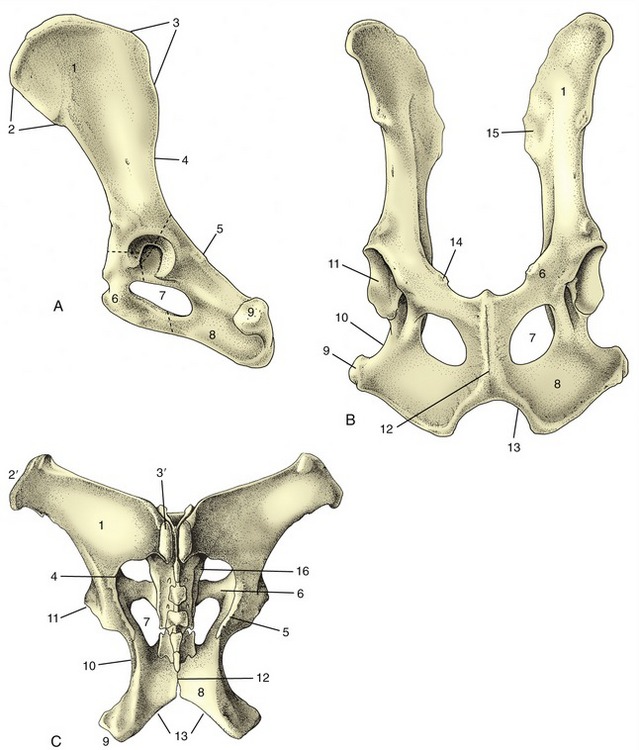
Figure 2–19 Canine hip bones in left lateral (A) and ventral (B) views. Dorsal (C) view of equine pelvis. The broken lines give the approximate extents of ilium, pubis, and ischium. 1, Wing of ilium; 2, ventral iliac spines; 2′, coxal tuber; 3, dorsal iliac spines; 3′, sacral tuber; 4, greater sciatic notch; 5, ischial spine; 6, pubis; 7, obturator foramen; 8, ischium; 9, ischial tuber; 10, lesser sciatic notch; 11, acetabulum; 12, pelvic symphysis; 13, ischial arch; 14, iliopubic eminence; 15, auricular articular surface; 16, sacrum.
The ilium consists of a cranial expansion or wing and a caudal shaft or body. The wing varies much among species; it is oblong with a more or less sagittal orientation in the dog and cat and is triangular and almost vertical in the horse and ruminants (see Figure 2–19). Its margin forms saliences, generally thickened, at certain points. Dorsally (dorsomedially in the larger species), it forms a sacral tuber; this is reduced to two low (cranial and caudal dorsal iliac) spines in the dog and cat (Figure 2–19/3) but is prominent in the large animals, in which it is close to the spinous processes of the vertebrae (Figure 2–19/3′). Ventrally (ventrolaterally in the larger species), the ilium forms a coxal tuber (Figure 2–19/2′,2); this is also reduced to low (cranial and caudal ventral iliac) spines in the carnivores but is prominent in large species, forming the point of the hip at the dorsocaudal corner of the flank (Figure 2–20, B/8). Including these projections, the margin of the wing is known as the iliac crest; thickened and convex in carnivores, it is thin and concave in large animals. Some of these features form important landmarks in the living animal.
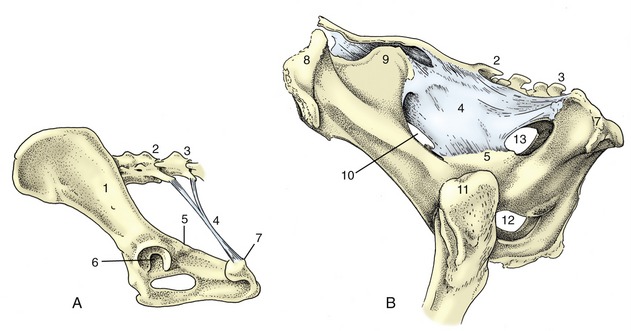
Figure 2–20 Canine sacrotuberous ligament (A) and bovine sacrosciatic ligament (B), left lateral views. 1, Ilium; 2, sacrum; 3, caudal vertebra(e); 4, sacrotuberous ligament (in A), sacrosciatic ligament (in B); 5, ischial spine; 6, acetabulum; 7, ischial tuber; 8, coxal tuber; 9, sacral tuber; 10, greater sciatic foramen; 11, greater trochanter; 12, obturator foramen; 13, lesser sciatic foramen.
The lateral (dorsolateral) surface is excavated and largely given over to the origin of the gluteus medius, whose attachment may raise one or more quite prominent ridges. The medial (ventromedial) surface faces toward the body cavity. The ventral part gives origin to the iliacus, while more dorsally it bears the roughened auricular articular surface (see Figure 2–19, B/15) for the sacrum. The dorsal border of the wing is cut away at its junction with the shaft, forming the greater sciatic notch (incisura; see Figure 2–19/4), over which the sciatic nerve runs in passage to the hindlimb.
The shaft of the ilium is robust and columnar. Its caudal extremity contributes to the acetabulum, the deep cavity that receives the head of the femur. Its ventral border is marked by the low arcuate line that serves as part of the arbitrary boundary (“terminal line”) between the abdominal and pelvic cavities. Except in the dog, the line carries the psoas tubercle midway along its length; the psoas minor attaches here.
The pubis (Figure 2–19/6), essentially L-shaped, consists of cranial (acetabular) and caudal (symphysial) branches. The lateral end of the cranial branch contributes to the acetabulum and is known as the body. Its cranial edge, known as the pecten of the pubis, bears the iliopubic eminence and gives attachment to the abdominal muscles. Between them, the two branches account for about half the circumference of the obturator foramen (Figure 2–19/7), the large opening in the pelvic floor through which the obturator nerve emerges. The foramen is closed by muscle and membrane in the fresh state.
The ischium (Figure 2–19/8) consists of a horizontal plate extended cranially by symphysial and acetabular branches, one to each side of the obturator foramen. The extremity of the acetabular branch that contributes to the articular cup is known as the body. The body and the cranial part of this branch are surmounted by a crest, the ischial spine (Figure 2–19/5), which also extends onto the caudal part of the ilium. Marked by the origin of the gluteus profundus, it is relatively low in the dog and particularly high in ruminants. The caudolateral corner of the plate forms the ischial tuber (Figure 2–19/9); the border between this and the spine is indented by the lesser sciatic notch (Figure 2–19/10). The ischial tuber is a horizontal thickening in the dog, and a conspicuously triangular swelling in cattle. In most species it is subcutaneous, and it may be a visible landmark. The remaining part of the caudal border forms with its fellow the ischial arch, a notch that is broad and, except in the horse, shallow.
The acetabulum is a deep articular cup to which all three bones contribute; an additional small acetabular bone may be found in young animals. The acetabulum is contained by a prominent rim that is interrupted by a notch caudoventrally. It carries a lunate articular surface internally, but the depth of the cup is nonarticular and rough.
Species differences in the general form of the pelvic girdle are very pronounced. The ilium is most vertical in the larger and heavier species, which is a conformation that brings the sacroiliac joint, and therefore the weight of the trunk, more nearly above the hip joint (Figure 2–20, B). In smaller species, in which this consideration is of less importance, the ilium is very oblique (see Figure 2–1). This displaces the pelvic floor caudally relative to the vertebral column and increases the effectiveness of the abdominal muscles that flex the column in bounding gaits. Caudal displacement of the ischial tuber also increases the leverage that may be exerted by the hamstring muscles, the powerful extensors of the hip that arise here.
The dimensions of the girdle are most important in species that carry a single large offspring. They are of little significance in polytocous species (those that normally carry a litter), in which the full-term fetuses are relatively small. These aspects of pelvic conformation are discussed in later chapters.
The Joints and Ligaments of the Pelvic Girdle
The pelvic symphysis is a secondary cartilaginous joint that ossifies with advancing age. The process of ossification is irregular; it commences at different ages and advances at different rates, even in a single species. It is usually more precocious in onset and more advanced at any stage in the pubic than in the ischial part. It is sometimes asserted that in certain domestic species changes can be detected in the tissues of the symphysis (and sacroiliac joint) in advance of parturition. If this is so, and it is not universally accepted, these changes are minor in comparison with those that occur in guinea pigs and many other small animals at this time; in these, complete dissolution of the symphysis, which allows the two halves of the girdle to move apart to enlarge the birth passage, may occur.
The sacroiliac joints are curious in combining a synovial joint with an adjacent region of extensive fibrous union. The arrangement appears designed to combine firmness of attachment with some shock-absorbent capacity, for these joints are required to transmit the weight of the trunk to the hindlimbs when standing and the thrust of the limbs to the trunk in progression. The sacrum is wedged between the two halves of the pelvic girdle; each sacral wing carries an articular surface that is broadly flat (but irregular in detail) to match the corresponding iliac surface. The joint capsule is tight and is surrounded and supported by short fascicles of connective tissue that join adjacent parts of the two bones. It is a matter of preference whether certain longer sacroiliac ligaments, at a greater distance from the synovial articulation, are to be regarded as components of that joint or as independent structures. They may include long and short dorsal ligaments passing between the wing of the ilium and the spinous processes and other features of the sacrum. A ventral ligament offers more immediate support to the joint.
The sacrotuberous ligament (Figure 2–20/4) is of considerably greater interest. In the dog, it is a stout rounded cord extending between the caudolateral angle of the sacrum and the lateral part of the ischial tuber; no such ligament is present in the cat. In ungulates, it is better named the sacrosciatic ligament because it is expanded to a broad sheet that largely fills the space between the lateral border of the sacrum and the dorsal border of the ilium and ischium, which leaves open two foramina adjacent to the greater and lesser sciatic notches. The caudal edge is palpable in dogs and cattle (see p. 490 and p. 698).
THE MUSCLES OF THE TRUNK
The Cutaneous Muscle of the Trunk
The cutaneous muscle of the trunk (Figure 2–21) varies in relative thickness and extent but generally covers the lateral aspect of the thorax and abdomen with fascicles of a predominately horizontal course. It is contained within the superficial fascia and has as its main function tension and twitching of the skin. In some animals detachments are associated with the prepuce, and in horses and cattle a separate lamella covers the shoulder and arm regions. The innervation comes from the brachial plexus.
The Muscles of the Vertebral Column
These can be separated into two divisions according to their position and innervation. The epaxial division (Figure 2–22, B/12) is placed dorsal to the line of the transverse processes of the vertebrae and receives its nerve supply from dorsal branches of the spinal nerves. The hypaxial division (Figure 2–22/14) lies ventral to the transverse processes and is supplied by the ventral branches of these nerves; it includes the muscles of the thoracic and abdominal walls in addition to those placed closely on the vertebrae. The thoracic and abdominal muscles are considered in later sections.
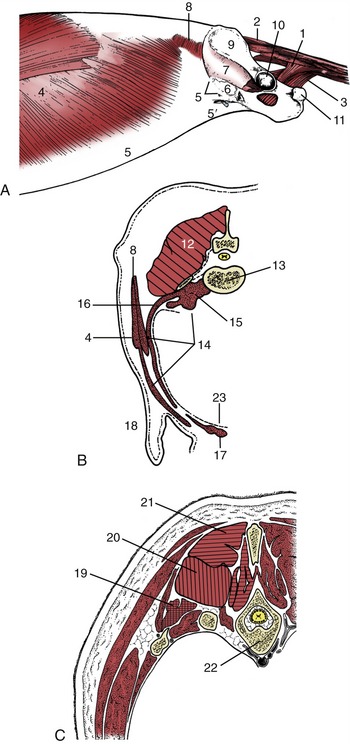
Figure 2–22 A, Trunk muscles of the dog, lateral view; the limbs have been removed. B, Epaxial (hatched) and hypaxial (stippled) muscles shown in a transverse section of the lumbar region. C, The three systems of epaxial muscles at the level of the thorax. 1, Coccygeus; 2, dorsal sacrocaudal; 3, levator ani; 4, external abdominal oblique; 5, its aponeurosis, pelvic tendon, and inguinal ligament; 5′, abdominal tendon; 6, vascular lacuna; 7, iliopsoas; 8, internal abdominal oblique; 9, wing of ilium; 10, acetabulum; 11, ischial tuber; 12, epaxial muscles; 13, lumbar vertebra—its transverse process appears as detached section; 14, hypaxial muscles; 15, psoas muscles; 16, transverses abdominis; 17, rectus abdominis; 18, flank fold; 19, iliocostalis system (crosshatched); 20, longissimus system (vertically hatched); 21, transversospinalis system (horizontally hatched); 22, thoracic vertebra and ribs; 23, peritoneum.
The Epaxial Muscles
These are numerous and complicated but fortunately do not require detailed description as they are rarely of clinical importance, except in the dog (p. 415). The major muscles are arranged in three parallel columns (Figure 2–22, C/19-21), which show some tendency to fuse over the loins and to split into additional units in the neck. They are extensors of the vertebral column, locally or more generally according to their extent, and are relatively more powerful in animals that make use of a bounding gait when traveling at speed (e.g., the dog).
The lateral column, the iliocostalis, arises from the ilium and transverse processes of the lumbar vertebrae and inserts on the more cranial lumbar vertebrae and ribs with, in most species, a weaker continuation into the neck. It is composed of many fascicles that overlap; for the most part they span about four vertebrae. Its lateral position also makes it effective in bending the trunk to the side (Figure 2–23, B/17).
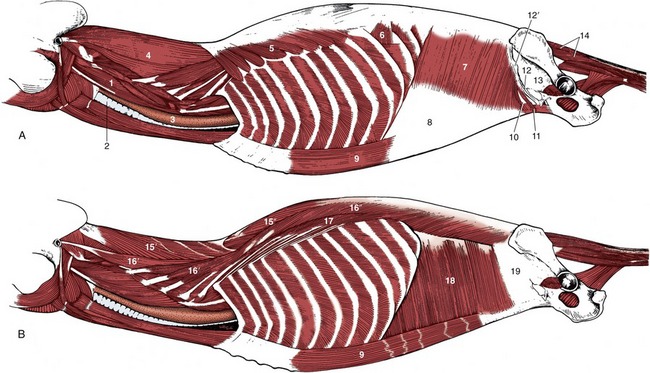
Figure 2–23 A and B, Trunk muscles of the dog, deeper layers. 1, Longus capitis; 2, trachea; 3, esophagus; 4, splenius; 5, 6, serratus dorsalis cranialis and caudalis; 7, internal abdominal oblique; 8, its aponeurosis; 9, rectus abdominis; 10, caudal free border of internal abdominal oblique; 11, cremaster; 12, inguinal ligament; 12′, external abdominal oblique aponeurosis, cut and reflected; 13, fascia iliopsoas; 14, dorsal sacrocaudal muscles; 15, transversospinalis system; 15′, semispinalis capitis; 15″, spinalis et semispinalis; 16, longissimus system; 16′, longissimus capitis and cervicis; 16″, longissimus thoracis; 17, iliocostalis; 18, transversus abdominis; 19, transverse fascia.
The middle column, the longissimus (Figure 2–23/16), is strongest and can be followed into the neck, even to the head. Some of its more cranial parts are independent to a greater or lesser degree. The caudal attachments, which are the conventional origin, are from the ilium, the sacrum, and the mamillary processes, whereas the insertions are to the transverse processes and ribs. The fascicles thus pursue a cranial, lateral, and ventral course, and each bridges several vertebrae; the longest fascicles span the especially mobile thoracolumbar junction. Different parts may be designated longissimus lumborum, longissimus dorsi, longissimus cervicis, longissimus atlantis, and longissimus capitis, but usually the generic term is sufficient. The muscle tends to fuse with its medial and lateral neighbors in the lumbar region.
In addition to the more or less direct continuation, the cervical part of the longissimus is closely associated with the more superficial splenius (Figure 2–23, A/4). This passes from the highest spines of the withers and thoracolumbar fascia to the occipitomastoid region of the skull. It is covered by certain muscles of the thoracic girdle, especially the trapezius and rhomboideus.
The longissimus complex also includes certain small muscles passing between adjacent transverse processes as well as the dorsal (sacrocaudal) muscles of the tail (Figure 2–23/14); the latter are fleshy at their origin and are continued by tendons that run the length of the tail.
The medial column, the transversospinalis system (Figure 2–24/2), is the most complex, although the number of discrete units into which it may be divided varies among species. It lies on and between the medial parts of the vertebral arches and the spinous processes. Some fascicles run sagittally; others pursue a cranial, medial, and dorsal course from their caudal origin. The sagittal bundles include small units, often converted into ligaments, passing between adjacent spinous processes as well as larger units that span several vertebrae. The oblique bundles run from mamillary to spinous processes and may be distinguished by name according to whether they span one, two, three, or more joints. The longest fascicles are again concentrated at the middle, most mobile region of the back.

Figure 2–24 A and B, Trunk muscles of the dog, deepest layers. 1, Longus capitis; 2, transversospinalis system; 2′, multifidus; 2″, spinalis cervicis; 2′″, spinalis et semispinalis; 3, quadratus lumborum; 4, rectus abdominis; 5, transversus abdominis; 5′, its aponeurosis; 6, external intercostal muscles; 7, internal intercostal muscles; 8, rectus capitis ventralis; 9, longus colli; 10, psoas minor; 11, iliopsoas (psoas major and iliacus).
A number of specialized units bridge the joints between the axis, the atlas, and the skull and are responsible for the special movements in this region. Those of the dog are briefly described later (p. 415).
The Hypaxial Muscles
These are flexor muscles of the neck or tail. The longus colli (Figure 2–24/9) runs from the cranial thoracic region to the atlas, covering the ventral surfaces of the vertebral bodies. It has a complex organization, and most of its constituent bundles are relatively short and cross only a few joints; their orientation varies. It is complemented by the rectus capitis ventralis (Figure 2–24/1), which extends from the atlas to the ventral aspect of the skull, and the longus capitis (Figure 2–24/1), which lies lateral to the longus colli and extends from the transverse processes of the midcervical vertebrae to the skull. The scalenus group occupies a similar position in relation to the caudal cervical vertebrae. It passes to the first one or few ribs, which it helps stabilize during inspiration. In some species the scalenus is readily divisible into dorsal, middle, and ventral parts.
The ventral muscles of the tail are close counterparts of the dorsal muscles.
The Muscles of the Thoracic Wall
The muscles of the thoracic wall are primarily concerned with respiration. Most are inspiratory and enlarge the thoracic cavity, causing air to flow into the lungs. Some are expiratory and diminish the cavity, expelling air. They comprise muscles that fill the spaces between the ribs, certain small units placed lateral to the ribs, and, by far the most important, the diaphragm.
The intercostal muscles are theoretically arranged in three layers that correspond to the layers of the abdominal wall. The external intercostal muscles are outermost (Figure 2–24/6). Each of these muscles is confined to a single intercostal space in which its fibers run caudoventrally from an origin on one rib to a termination on the following rib. They fill the spaces from the upper ends to the costochondral junctions and sometimes beyond these but fail to reach the sternum. The parts between the cartilages are sometimes separately named. The internal intercostal muscles (Figure 2–24/7) are placed more deeply within the intercostal spaces and run cranioventrally, approximately perpendicular to the course of the external muscles. They do not occupy the most dorsal parts of the spaces, but, as if in compensation, they do reach the margin of the sternum. The third (subcostal) layer is so weak and so inconsistently developed that it may be ignored. The transversus thoracis is a triangular sheet that arises from and covers the dorsal surface of the sternum. The apex points cranially, and the muscle splits into slips that run caudolaterally to insert on the sternal ribs close to the costochondral junctions. It is morphologically the equivalent of the ventral part of the transversus abdominis.
Two muscles lie on the lateral surface of the thoracic wall. The rectus thoracis is a small quadrilateral sheet placed over the lower ends of the first four ribs in apparent continuation of the rectus abdominis. The serratus dorsalis (Figure 2–23, A/5,6) lies over the dorsal parts of the ribs. It takes origin from the fascia of the back and inserts on the ribs by a series of slips. The slips of the cranial part of the muscle slope caudoventrally, and those of the caudal part slope cranioventrally, which points to antagonistic functions. The two parts are sometimes quite widely separated. The scalenus, mentioned in the preceding section, has an attachment to the first rib; in some species it also passes quite extensively over the rib cage.
The diaphragm separates the thoracic and abdominal cavities. It is dome-shaped, being convex in all directions on its cranial surface, and bulges cranially under cover of the ribs to enlarge the abdomen at the expense of the thoracic cavity (Figures 2–2 and 2–25, A). It consists of a heart-shaped (trefoil-shaped in the dog) central tendon (Figure 2–25/7) and a muscular periphery that is divisible into portions that arise from the lumbar vertebrae, the caudal ribs, and the sternum.

Figure 2–25 A, Cranial view of the canine diaphragm. B, Lateral view of the canine thorax showing ribs and cranial extent of diaphragm in inspiration (broken lines) and expiration (solid lines). 1, Left crus; 2, right crus; 3, aorta; 4, esophagus; 5, attachment of caudal mediastinum to diaphragm; 6, sternal and costal parts of diaphragm; 7, tendinous center; 8, attachment of plica venae cavae; 9, caudal vena cava.
The central tendon is the most cranial part and forms the vertex. In the neutral position between full inspiration and full expiration, it reaches the level of the lower part of the sixth rib (or following space) and is thus only a little behind the plane of the olecranon in an animal standing square. Knowledge of this fact and of the line of the costal attachment is indispensable in appreciating the extent of the thoracic cavity (Figure 2–25, B).
The powerful lumbar portion of the peripheral muscle consists of left and right crura (Figure 2–25/1,2) that arise from the ventral aspect of the first three or four lumbar vertebrae by means of stout tendons. The right crus is considerably the larger, and it divides into three branches that radiate ventrally to join the central tendon. The left crus is undivided.
The much thinner costal part arises by serial digitations from the inner surfaces of the ribs and costal cartilages. The most caudal slip, which is also the most dorsal, arises close to the dorsal end of the last rib; those in front arise at successively more ventral levels, and the last costal digitation follows the cartilage of the eighth rib to the sternum. A final sternal slip arises from the dorsal surface of the sternum and runs dorsally to meet the tendon, which is thus bordered by muscle on all sides.
The diaphragm has three openings. The most dorsal, the aortic hiatus (Figure 2–25/3), is between the lumbar vertebrae and the crural tendons. It transmits the aorta, the azygous vein, and the thoracic duct. The esophageal hiatus (Figure 2–25/4) lies more ventrally, between the two medial divisions of the right crus. It transmits the esophagus, the dorsal and ventral vagal trunks that accompany the esophagus, and the vessels that supply it. The third opening, the caval foramen (Figure 2–25/9), lies within the central tendon, somewhat dorsal to the vertex and to the right of the median plane. It conveys the caudal vena cava and is of a rather different nature from the other openings because the adventitia of the vessel fuses with the tendon to leave no surrounding space. The margins of the other openings can slide over the structures passing through.
The diaphragm is supplied by the phrenic nerves formed from contributions by ventral branches of caudal cervical nerves (usually C5–C7). Despite the apparently involuntary nature of breathing, these are ordinary somatic nerves of mixed composition. The other muscles of the chest wall are supplied by intercostal nerves (ventral branches of thoracic spinal nerves).
Functional Considerations
The form and construction of the thorax represent a compromise between the requirements of posture and locomotion and the more specialized needs of respiration. In most domestic mammals the advantages of a barrel-shaped thorax for respiration are largely sacrificed to the easier movement allowed to the scapulae by flattening the cranial part of the rib cage. The potential for movement of the cranial ribs is also reduced in favor of the more rigid construction that provides a stable origin for the muscles that pass between the trunk and the forelimbs.
Respiratory activity is therefore most evident in changes in the form of the caudal part of the rib cage and abdomen. All species exhibit both costal and abdominal (i.e., diaphragmatic) modes of breathing, but their relative importance varies with the species, with the prevailing circumstances, and with the individual, as breathing pattern is as distinctive as stance or gait. It is commonly stated that, in ourselves, about 70% of the air flow is attributable to movements of the diaphragm; the proportion is unlikely to be very different in the domestic species, although such matters have received little attention. It is certainly safe to conclude that normal respiration is always accompanied by contraction of the diaphragm, while involvement of the intercostal and other accessory respiratory muscles is less certain.
The diaphragm contracts against the resistance of the abdominal viscera; for practical purposes these can be regarded as incompressible, and they must be displaced caudally into space provided by relaxation of the abdominal floor and flanks. In the course of this movement the central part of the dome of the diaphragm shifts backward, perhaps half a vertebral length in quiet breathing, while additional thoracic enlargement is obtained through flattening its peripheral parts. Contraction of the sternocostal parts of the diaphragm, which attach to the last ribs, tends to pull these ribs inward in opposition to the outward and forward pull exerted on them by the intercostal muscles. It is a common observation (easily confirmed by watching a sleeping dog) that the last rib may actually be tucked inward during inspiration while its more cranial fellows move outward to broaden the thorax.
The actual movements undertaken by the ribs and the forces that produce them are controversial. The caudal inclination of the lower part of the rib (before it is turned forward by the cartilage) results in the rib performing a movement that is compared to raising a bucket handle. Just how the articular surfaces engage during this movement and where the axes of rotation may be found are matters in dispute; it is clear, however, that the overall effect is to widen while shortening the rib cage. In humans and some quadrupeds (including the dog), a concurrent ventral displacement of the sternum occurs.
A considerable number of the muscles attaching to the ribs and sternum appear from their geometry to be capable of producing the necessary movements. Electromyographic studies, admittedly performed mainly in humans, have shown that little of this potential is actually employed in quiet breathing. During inspiration the superficial layer of intercostal musculature is most consistently engaged, that is, the external intercostals and the interchondral parts of the internal intercostals. The scalenus (and possibly also muscles that pass forward from the manubrium) may assist in fixing the thoracic inlet. Expiration is mainly passive, and the elastic recoil of the lungs is the major force. The muscles of the abdominal wall may contract to reinforce the passive tension in the tendinous parts that raises the viscera and that indirectly helps to restore the diaphragm to its former position. Sometimes the deeper layer of intercostal muscle—the interosseous parts of the internal intercostals and the transversus thoracis—is also engaged.
Contrary to common belief, the diaphragm is not indispensable. Evidence obtained from experimental and clinical subjects (dogs and ruminants) in which both phrenic nerves have been sectioned or paralyzed indicates little obvious loss of respiratory efficiency even under moderate stress. This of course does not deny the diaphragm the major role in normal animals; it confirms that there is an ample reserve of inspiratory muscle.
The Muscles of the Abdominal Wall
The muscles of the abdominal wall are conveniently divided into ventrolateral and dorsal (sublumbar) groups (Figure 2–22, B). The first comprises the muscles of the flanks and abdominal floor; these muscles possess a particular importance because they are encountered and incised in almost all surgical approaches to abdominal organs. Most muscles of the second group properly belong to the girdle division of hindlimb musculature. They are included here because they constitute part of the body wall, namely, the roof of the abdomen to each side of the vertebral column.
The Ventrolateral Group
The intrinsic musculature of the flank comprises three broad fleshy sheets superimposed on each other with contrasting orientation of their fibers. Each is continued ventrally by an aponeurotic tendon that proceeds to a principal insertion within a fibrous cord, the linea alba, which runs in the ventral midline from the xiphoid cartilage to the cranial end of the pelvic symphysis (via the prepubic tendon). In so doing, the tendons ensheathe the fourth muscle, the rectus abdominis, which pursues a sagittal course within the abdominal floor directly to the side of the linea alba. The following account is of the basic arrangement. The details vary among species and may have surgical importance, especially in the small species (Figure 2–26; see also pp. 435–436).
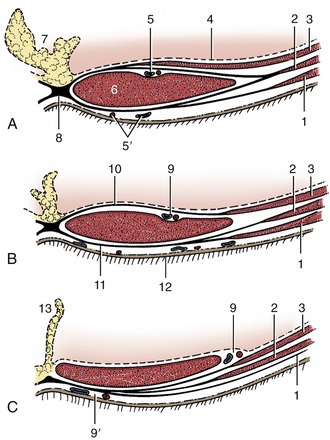
Figure 2–26 Rectus sheath of the dog in transverse sections taken cranially (A) and caudal (B) to the umbilicus and near the pubis (C). 1, External abdominal oblique; 2, internal abdominal oblique; 3, transversus abdominis; 4, peritoneum; 5, cranial epigastric vessels; 5′, cranial superficial epigastric vessels; 6, rectus abdominis; 7, fat-filled falciform ligament; 8, linea alba; 9, caudal epigastric vessels; 9′, caudal superficial epigastric vessels; 10, internal lamina of rectus sheath; 11, external lamina of rectus sheath; 12, skin; 13, median ligament of the bladder.
The outermost external abdominal oblique muscle (Figure 2–22/4) arises from the lateral surfaces of the ribs and from the lumbar fascia. The majority of its fibers run caudoventrally; however, some radiation is present and allows the most dorsal bundles to follow a more horizontal course. The aponeurosis (Figure 2–22/5) that succeeds the fleshy part divides into two parts (tendons) before its insertion. The larger abdominal tendon terminates on the linea alba after passing ventral to the rectus muscle; the smaller pelvic tendon proceeds to attach on the fascia over the iliopsoas and on the pubic brim lateral to the insertion of the rectus (Figure 2–27/3′,4).
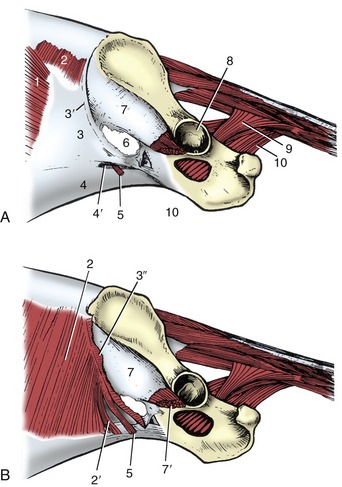
Figure 2–27 Inguinal canal and pelvic diaphragm of the dog, left lateral view. The external abdominal oblique muscle, present in A, has been removed in B. 1, External abdominal oblique; 2, internal abdominal oblique; 2′, free caudal edge of internal oblique, forming border of deep inguinal ring; 3, pelvic tendon of external oblique aponeurosis; 3′, caudal border of 3 (inguinal ligament) ending on 7; 3″, stump of external oblique aponeurosis reflected caudally (B); 4, abdominal tendon of external oblique aponeurosis; 4′, superficial inguinal ring; 5, cremaster derived from internal oblique; 6, vascular lacuna; 7, iliac fascia covering iliopsoas; 7′, iliopsoas; 8, acetabulum; 9, coccygeus; 10, levator ani.
The second muscle, the internal abdominal oblique (Figure 2–23/7), arises mainly from the coxal tuber (or the equivalent region of the ilium) but to lesser extents from the insertion of the pelvic tendon of the external oblique, the thoracolumbar fascia, and the tips of the lumbar transverse processes. This muscle fans out more obviously: its most caudal fascicles pass ventrocaudally, and although the next group runs more or less transversely in the plane of the coxal tuber, most pass ventrocranially. Some cranial fascicles insert directly on the last rib, but the bulk are continued by an aponeurosis (Figure 2–23/8) that passes ventral to the rectus to reach the linea alba. Toward the midline some interchange of fibers between the aponeuroses of the two oblique muscles usually occurs. The origin from the pelvic tendon allows the muscle a free caudal edge (Figure 2–23/10) that is mentioned again shortly in connection with the inguinal canal. A caudal slip (cremaster; Figure 2–23/11) detached from the internal oblique passes onto the spermatic cord (p. 191).
The deepest muscle of the flank, the transversus abdominis (Figure 2–24/5), arises from the inner surfaces of the last ribs and the transverse processes of the lumbar vertebrae. Its fibers run more or less transversely and are succeeded by an aponeurosis (Figure 2–24/5′) that passes dorsal to the rectus abdominis before terminating on the linea alba. This muscle does not extend caudal to the coxal tuber. The cauda part of the tendon passes ventral to the rectus so that the most caudal part of that muscle is left uncovered dorsally.
The fourth muscle, the rectus abdominis (Figure 2–23/9), forms a broad band to the side of the linea alba in the abdominal floor. It arises from the ventral surfaces of the rib cartilages and sternum and inserts on the pubic brim by means of a prepubic tendon. The fleshy part, which is widest about the middle of the abdomen, is divided into a series of segments by irregular transverse septa (tendinous intersections) that recall, even if they do not exactly reproduce, its polysegmental origin. The prepubic tendon serves as a common insertion for the abdominal muscles and the linea alba and may incorporate part of the tendons of origin of adductor (pectineus and gracilis) muscles of the thigh.
The rectus sheath (vagina musculi recti abdominis), the arrangement of the aponeurotic tendons of the flank muscles about the rectus abdominis, varies in detail among species. In the basic arrangement, the tendons of the two oblique muscles form a layer on the external (ventral) surface of the rectus, while that of the transversus lies against the internal surface; both layers merge with the linea alba to complete the enclosure (see Figure 2–26 and p. 436 for a fuller description of the rectus sheath in the dog).
The abdominal wall is perforated in the region of the groin by a passage known as the inguinal canal (Figures 2–27 and 21–5). Before or shortly after birth this transmits the testis in its descent toward the scrotum; in the adult male it contains the spermatic cord, consisting of the duct from the testis, and associated structures within an outpouching of the peritoneum. In both sexes, it also transmits the external pudendal artery and (usually) vein, efferent vessels from the superficial inguinal lymph nodes, and the genitofemoral nerve, which are all structures associated with the groin.
The term canal is misleading because it suggests a roomier passage than actually exists. The canal is a potential flat space between the fleshy part of the internal oblique on the one side and the pelvic tendon of the external oblique aponeurosis on the other (Figure 2–27/2,3). The walls are apposed and joined by areolar tissue except where the transmitted structures hold them apart. The slitlike abdominal entrance to the canal (the deep inguinal ring) lies along the free caudal edge of the internal oblique muscle (Figure 2–27/2′). The exit from the canal (the superficial inguinal ring; Figure 2–27/4′) is contained between the two divisions of the external oblique tendon. (The edges of the superficial inguinal ring are known as medial and lateral crura.) Species differences are mentioned in later chapters and may be of great importance since some explain why the escape of organs into and through the canal (inguinal hernia) occurs more readily in certain animals. Other differences are of immediate relevance to surgery in this area, most obviously in connection with castration, whether of the normal male or of one in which the testis has failed to descend and remains hidden within the abdomen or within the canal itself (a condition known as cryptorchidism).
Functional Considerations
Observation and palpation suggest that animals standing quietly make little active use of the abdominal muscles in support of the viscera; the support is obtained from passive tension. Some electromyographic studies have revealed slight though continuous activity in the internal oblique and sporadic bursts in other muscles of the flank. A similar observation in ourselves has provoked the suggestion that the internal oblique muscle guards the entrance to the inguinal canal. Greater activity of the abdominal muscles may occur toward the end of quiet expiration and is more pronounced when breathing is labored, as the muscles then contract to assist the forward recovery of the diaphragm.
When the abdominal muscles are contracted against a fixed diaphragm, the animal is said to “strain.” The resulting increase in intraabdominal pressure reinforces the efforts of visceral muscle to expel urine, feces, or a fetus. The use made of straining varies with the species and conditions. Those animals that adopt a squatting posture for micturition (e.g., goat) or defecation (e.g., dog) obviously use the abdominal muscles to assist expulsion; other species adopt no special posture for these functions and presumably do not require this assistance.
The rigidity of the abdominal wall produced by contraction of these muscles may be used to protect the viscera. This defense is used by a nervous dog when efforts, particularly if unskillful, are made to palpate its abdomen; gentle massage may be necessary to allay the fear before the muscles relax. Abdominal visceral pain may spontaneously provoke local or general contraction with ensuing rigidity, presumably to prevent the organs from sliding against each other.
These muscles are also used in the adjustment of posture and in progression. Acting unilaterally, the muscles of the flank bend the trunk to that side. Acting bilaterally, they may assist in arching the back, which is a movement of great importance in bounding gaits.
The ventrolateral abdominal muscles are supplied by caudal intercostal nerves and the ventral branches of the lumbar nerves, particularly those more cranial in the series.
The Sublumbar Muscles
The psoas minor (Figure 2–24/10) arises from the bodies of the thoracolumbar vertebrae and inserts on the psoas minor tubercle on the ilium. Much tendon is intermingled in the flesh, which supports the contention that the muscle is probably mainly employed to stabilize the vertebral column. It may also rotate the pelvis at the sacroiliac joint.
The psoas major and iliacus muscles may be regarded as vertebral and pelvic heads of a single muscle (iliopsoas; Figure 2–24/11) that terminates on the lesser trochanter of the femur. The psoas major arises from the bodies and ventral surfaces of the transverse processes of the lumbar vertebrae lateral to the psoas minor. The iliacus arises from the ventral aspect of the wing and shaft of the ilium. The tendons of the two heads combine shortly before insertion. The iliopsoas is a flexor of the hip and an outward rotator of the thigh. The psoas head probably also contributes to the stability of the vertebral column.
The quadratus lumborum (Figure 2–24/3) arises from the last ribs and from the transverse processes of the lumbar vertebrae and inserts on the wing of the sacrum (sometimes also on the ilium). It stabilizes the lumbar portion of the vertebral column.
These muscles are principally innervated by direct twigs from the ventral branches of the last few thoracic and the lumbar nerves. Other twigs detach from named branches of the lumbosacral plexus, principally the femoral nerve.
The Muscles of the Pelvic Outlet
The pelvic outlet is closed about the terminal parts of the digestive and urogenital tracts by a portion of the body wall known as the perineum. The projection of the perineum on the skin outlines the perineal region, which has as its principal features the anus and the vulva (in the female, to which we principally refer here). Because the ventral part of the vulva falls below the level of the pelvic floor, it is usual to enlarge the concept of the perineal region to embrace the whole vulva. Very often the dorsocaudal part of the udder (in animals such as the cow) is also included. Several muscles and fasciae interlace in a node between the anus and the vulva and vestibule, and this formation is properly known as the perineal body or center; however, in clinical, especially obstetrical, literature the perineal body is frequently known simply, though incorrectly, as “the perineum.” The three concepts—perineum, perineal region, and perineal body—should be kept distinct. Another potential source of confusion exists. In human anatomy, the structures that occupy the pelvic outlet are said to form a “floor” to the pelvic cavity. In quadrupeds, the “floor” is provided by the pelvic girdle. The difference in posture not only affects the appropriate use of vernacular terms but, more important, also modifies the function of homologous structures. The principal component of the dorsal part of the perineum is the pelvic diaphragm, an arrangement of striated muscles contained between fasciae, which closes about the anorectal junction. A similar but less conspicuous arrangement in the ventral part of the perineum, the urogenital diaphragm, closes about the vestibule.
The pelvic diaphragm attaches laterally to the pelvic wall and spreads caudomedially to close about the anal canal. The term diaphragm aptly describes the human arrangement, which forms a basin in which the pelvic organs rest. It is less appropriate in domestic species, in which the “halves” of the diaphragm have more sagittal courses and converge more gently on the anus, which is the result of the relatively greater length of the pelvic girdle.
The more lateral of the two muscles of the diaphragm, the coccygeus (Figure 2–27/9), is essentially a muscle of the tail. Rhomboidal in outline, it arises from the ischial spine, crosses the sacrotuberous ligament medially, and inserts on and about the transverse processes of the first few tail vertebrae.
The medial muscle, the levator ani, is thinner and more extensive and runs more obliquely in a dorsocaudal direction; it is only partly covered by the coccygeus. The two muscles arise close together or by a common tendon in ungulates. In the dog, the levator has a more widely spread origin that continues from the iliac shaft over the cranial ramus of the pubis to follow the pelvic symphysis (Figure 2–27/10). The insertion is divided between the fascia and vertebrae of the tail (extending distal to the insertion of the coccygeus) and the fascia about the anus and external anal sphincter. The tail attachment predominates in carnivores, the anal one in ungulates, in which considerable exchange of fascicles with the anal sphincter and constrictor vestibuli muscles occurs.
The coccygeus flexes the tail laterally or, when acting in concert with its fellow, draws the tail ventrally to cover the perineum, an attitude familiar in the nervous dog. The action of the levator is best known from an electromyographic study in the goat, and it is possible that important species’ differences exist. In the goat it is active whenever the intraabdominal pressure is raised, presumably to oppose the tendency to displace pelvic organs caudally. Although also involved in other visceral functions, it has a very definite relationship to defecation; it is active before the event (when it may fix the position of the anus against the contraction of the smooth muscle of the colon), becomes inactive during the event, and regains activity following the event (when it may restore the parts to their resting positions). The jerky movements of the dog’s tail after defecation are probably evidence of levator activity in this species. Both muscles are supplied by ventral branches of the sacral nerves.
The smaller urogenital diaphragm (membrana perinei) contains more slender muscles, which are more appropriately described later with the reproductive organs. The fascia of the urogenital diaphragm attaches to the ischial arch and curves cranially, dorsally, and medially to blend with the ventral edge of the pelvic diaphragm and embrace the vestibule. It helps anchor the reproductive tract against a forward drag when the pregnant uterus sinks within the abdomen and against a backward displacement during parturition.
It may now be evident that to each side there is a space that is enclosed by the pelvic girdle but excluded from the pelvic cavity by the pelvic diaphragm. This space is pyramidal and has a cranial apex, a lateral wall furnished by the ischial tuber and sacrotuberous ligament, a medial wall furnished by the pelvic diaphragm, a ventral wall furnished by the pelvic floor, and a base directed toward the skin. It is appropriately known as the ischiorectal fossa and is normally occupied by fat (see Figure 29–10/12). When this fat is depleted, a pronounced sinking of the skin to the side of the anus is apparent (except in the horse and pig, in which the vertebral head of the semimembranosus covers the region).
THE HEAD AND VENTRAL PART OF THE NECK
BASIC PLAN AND DEVELOPMENT
Even a cursory examination of the head, intact or in sagittal section, shows that it consists of two principal parts. One, the neural part, comprises the brain together with the encasing structures; the other, the facial part, is much larger in most adult mammals and is formed by the jaws and the initial parts of the respiratory and digestive systems. The distinction between neural and facial parts is already plain in embryos at the somite stage (Figure 2–28).

Figure 2–28 Pig embryo (1.5 cm) to show dominance of the neural over the facial part of the head at this stage.
At this stage of development the dorsal structures predominate, and the size and form of the head are largely determined by the brain.
The neural part (cranium) of the skull has its primordium in a series of cartilages that form ventral to the brain and are supplemented by cartilaginous capsules enclosing the primitive olfactory organs, eyeballs, and labyrinths of the ears. Later, “dermal bones” appear by ossification within the membrane that covers the brain to the sides and above; ultimately, all of these elements fuse with each other and with the bones of the face.
The ventral part of the head—the future face—is much smaller and at this stage blends smoothly with the neck, largely occupied by the heart. It exhibits a quite different pattern of segmentation imposed by the pharyngeal arches, serial thickenings of the unsplit mesoderm lateral and ventral to the rostral part of the foregut that becomes the pharynx.
The formation, significance, and detailed fate of these arches is not described here; at present it is sufficient to recall that a cartilaginous skeleton with associated musculature innervated by a specific cranial nerve develops within the core of each arch. Each arch is also supplied by an arterial loop connecting the ventral to the dorsal aorta. The structures formed within the various pharyngeal arches are listed in Table 2–1; from this it can be seen that the cartilaginous parts ultimately make only a small contribution to the skeleton of the face. The definitive facial skeleton is mainly provided by dermal bones formed in the connective tissue of the jaws, although certain elements for a time obtain support from cartilaginous precursors such as the cartilage of the first arch and the nasal capsule.
In most mammals the facial part enlarges disproportionately and comes to lie as much before as below the brain. Despite many qualitative and quantitative differences the basic arrangement is the same in all species. The relationships and topography of the major organs and cavities of the head should be studied before passing on to more detailed matters. Figures 4–2 and 4–3 provide the necessary information.
THE SKULL
The complete skeleton of the head comprises the skull,* the mandible or lower jawbone, the hyoid apparatus, the ossicles of the middle ear, and the cartilages of the external ear, nose, and larynx.
The skull (in the narrower sense) is a mosaic of many bones, mostly paired but some median and unpaired, that fit closely together to form a single rigid construction. The separate elements, which are named individually, develop from independent centers of ossification and have, for the most part, well established homologies. In the young animal they are separated from each other by narrow strips of fibrous tissue—cartilage in a few situations—and this pattern of joints or sutures provides for growth. Once growth has ceased, sutures are no longer necessary and ossification extends into the connective tissue, finally welding the bones together. This process is drawn out, and it may never be completed; the outlines of most bones are therefore discernible, even in skulls of old animals. Acquaintance with the names, positions, and approximate extents of the individual bones (Figure 2–29) is essential as it provides a useful system of reference to regions of the head, but a detailed knowledge of the disarticulated units has little practical value; most readers are better served by an appreciation of the skull as a whole.

Figure 2–29 Lateral (A), dorsal (B), and ventral (C) views of the canine skull to show the extents of the cranial bones. 1, Nasal bone; 2, incisive bone; 3, maxilla; 4, lacrimal bone; 5, orbit; 6, frontal bone; 7, parietal bone; 8, occipital bone; 9, temporal bone; 10, zygomatic bone; 11, palatine bone; 12, presphenoid; 12′, wing of presphenoid; 13, pterygoid bone; 14, basisphenoid; 14′, pterygoid process of basisphenoid; 15, vomer.
Conventional descriptions are based on the views obtained from various directions with the skull resting on a flat surface, even though this may not be its habitual orientation in life. In most views the two distinct portions of the skull are immediately apparent: the caudal part encasing the brain and the rostral part supporting the face. The orbits, the fossae containing the eyeballs, are part of the face but lie at the boundary. In most domestic animals the facial part of the skull is larger than the neural part and is situated mainly in front of this. However, the ratio varies among species and also with breed, age, and individual conformation. The many particular differences make it impossible to provide even a general description of the skull that is valid for all species.
The Skull of the Dog
This initial account is of the skull of an adult dog of average (mesaticephalic) conformation, neither short-headed (brachycephalic) like a Pekingese nor long-headed (dolichocephalic) like a Borzoi. Some salient breed differences are mentioned later (p. 374).
In the dorsal view (Figure 2–30), the ovoid cranium meets the bones of the face where the zygomatic processes (Figure 2–30/4′) of the frontal bones project laterally to form the dorsocaudal parts of the orbital walls. The caudal extremity of the cranium is marked by the external occipital protuberance in the midline; its demarcation from the caudal (nuchal) surface is completed by the nuchal crests that extend laterally to each side. The median sagittal crest that extends forward from the occipital protuberance is most prominent in robust, well-muscled animals. All these features are easily palpated in life. The dorsal and lateral surfaces of each half of the cranium blend in a continuous and slightly roughened surface from which the temporalis muscle arises. Rostral to the zygomatic processes of the frontal bones the dorsal surface of the skull dips, sometimes quite markedly, before continuing as the straight and narrow dorsum of the nose. This ends at the wide nasal aperture beyond which the bony skull is prolonged by pliant nasal cartilages.
Figure 2–30 Dorsal view of canine skull. 1, Nasal aperture; 2, infraorbital foramen; 2′, maxillary foramen; 3, fossa for lacrimal sac; 4, orbit; 4′, zygomatic process of frontal bone; 5, zygomatic arch; 6, external sagittal crest; 7, nuchal crest; 8, external occipital protuberance, 9, cranium.
The orbit is the most prominent feature of the lateral view (Figure 2–31). Behind the orbit, the dorsolateral part of the braincase forms the wall of the temporal fossa (Figure 2–31/16). The ventrolateral part is more complicated and presents the zygomatic arch and ear regions. The zygomatic arch (Figure 2–31/15) springs free from the braincase and, bowing laterally, passes below the orbit to rejoin the facial part of the skull. It is formed by two bones, the squamous temporal and zygomatic, which meet at an overlapping suture. The ventral surface of the caudal part of this arch carries the articular surface for the mandible, shaped as a transverse gutter in this species; the articular area continues caudal to this onto the rostral surface of a ventral projection, the retroarticular process (Figure 2–31/6). The large, smooth dome of the tympanic bulla (Figure 2–31/9) (enclosing part of the cavity of the middle ear) and the rough mastoid process lie behind the retroarticular process. Three openings are present in this region of the skull: the retroarticular foramen emits a major vein draining the cranial cavity, the stylomastoid foramen gives passage to the facial nerve, and the external acoustic meatus is, in the fresh state, closed by a membrane (eardrum) that separates the canal of the external ear from the cavity of the middle ear. The paracondylar process (Figure 2–31/11) is conspicuous at the caudal limit of the skull.
Figure 2–31 Lateral view of canine skull. 1, Orbital ligament (inset); 2, infraorbital foramen; 3, orbit; 4, pterygopalatine fossa; 5, optic canal, orbital fissure, and rostral alar foramen; 6, retroarticular process; 7, retroarticular foramen; 8, external acoustic meatus; 9, tympanic bulla; 10, stylomastoid foramen; 11, paracondylar process; 12, occipital condyle; 13, nuchal surface; 14, mastoid process; 15, zygomatic arch; 16, temporal fossa; 17, nuchal crest.
The orbit is funnel shaped, and in the macerated state its walls are very incomplete. In life the orbital rim is completed by a ligament (Figure 2–31/1) that connects the zygomatic process of the frontal bone to the zygomatic arch. Ventrally the orbital cavity is continuous with the pterygopalatine fossa (Figure 2–31/4), but in the fresh state these regions are separated by the periorbita, a dense fascial sheet that completes the definition of the orbit. Two groups of foramina are visible in this region. The caudal group (Figure 2–31/5) comprises the optic canal, orbital fissure, and rostral alar foramen. The optic opening, placed at the apex of the conical orbital cavity, is the portal of entry of the optic nerve. The more ventral orbital fissure transmits the nerves (ophthalmic, oculomotor, trochlear, and abducent) that supply ancillary structures of the eye and the external ophthalmic vein. Most ventrally the rostral alar foramen provides a common opening for the maxillary nerve, passing from the cranial cavity, and the maxillary artery, which transverses a canal (alar canal) in the sphenoid bone.
The rostral group of foramina comprises the maxillary, sphenopalatine, and caudal palatine foramina. The maxillary foramen (Figure 2–30/2′) leads to the infraorbital canal, the sphenopalatine foramen to the nasal cavity, and the caudal palatine to the palatine canal, which emerges on the hard palate; each opening conveys like-named branches of the maxillary artery and nerve. More dorsally the rostral orbital wall contains the lacrimal fossa for the lacrimal sac (Figure 2–30/3). An opening in the depth of the fossa leads to a passage that conveys the nasolacrimal (tear) duct to the nose.
The infraorbital foramen (Figure 2–30/2) is the most prominent feature of the lateral aspect of the face and is easily palpable in the live animal; it is the site of emergence of the infraorbital nerve, which continues from the maxillary nerve through the infraorbital canal. Toward the alveolar margin the facial skeleton is molded over the roots of the teeth, most especially over the large root of the canine tooth.
In the ventral view (Figure 2–32), three regions of the skull are distinct: the base of the cranium, the choanal region where the nasal cavities open into the pharynx, and the hard palate. The first shows at its caudal limit the ovoid, obliquely oriented occipital condyles that flank the foramen magnum (Figure 2–32/12) through which the spinal cord connects with the brain. Rostral to this the median area is generally flat, although midway along its length, tubercles are present for the attachment of muscles that flex the head on the neck. The tympanic bulla and paracondylar process occupy much space to each side. The medial aspect of the bulla (Figure 2–32/7) meets the occipital bone, and this fusion separates two openings that are confluent in some other species (e.g., horse; see Figure 2–37), namely, the more caudal jugular foramen and the more rostral foramen lacerum (Figure 2–32/8,6). The glossopharyngeal, vagus, and accessory nerves emerge through the jugular foramen together with a large vein draining the interior of the cranium. Between the jugular foramen and the condyle is the hypoglossal canal, which transmits the hypoglossal nerve.

Figure 2–32 Ventral view of canine skull. 1, Palatine fissure; 2, hard palate; 3, choanal region; 4, oval foramen; 5, base of cranium; 6, foramen lacerum; 7, tympanic bulla; 8, jugular foramen; 9, paracondylar process; 10, hypoglossal canal; 11, occipital condyle; 12, foramen magnum.
Lateral to the foramen lacerum, small fissures exist for the exit of the chorda tympani (a branch of the facial nerve) and for the communication of the cartilaginous auditory tube with the cavity of the middle ear. Rostral to these is the prominent oval foramen (Figure 2–32/4), through which the mandibular nerve emerges.
The openings (choanae) that lead from the nasal cavities to the nasopharynx are the main features of the middle part of the ventral aspect. The choanal region is bounded dorsally by the floor of the cranium and laterally by the thin plates of bone whose outer surfaces were earlier noted as forming the medial walls of the pterygopalatine fossae. The soft palate, which arises from the free margin of the hard palate, in life provides the floor of the space—essentially the first part of the nasopharynx—enclosed by these formations. The palate, which lies rostral to this, is broad behind and narrower in front. It is margined by the alveoli or sockets in which the upper teeth are implanted. Toward its rostral extremity, it is perforated by the large bilateral palatine fissures. Several smaller foramina toward the caudal extremity of the palate are rostral openings of the palatine canal.
The nuchal surface (Figure 2–31/13), broadly triangular, is limited dorsally by the external occipital protuberance and the nuchal crests. Its lower part presents the foramen magnum, the occipital condyles, and the paracondylar processes. The remainder of the surface is roughened for the attachment of dorsal muscles of the neck.
The apex of the skull is formed by the nasal aperture situated dorsal to the rostral extremities of the jaws that carry the incisor teeth.
The cavities of the skull are described with the respiratory system (Chapter 4), central nervous system (Chapter 8), and ear (Chapter 9).
The lower jaw or mandible comprises two parts (Figure 2–33). In the dog these are firmly but not rigidly united by the connective tissues of the mandibular symphysis. Each half is divided between a body, or horizontal part, and a ramus, or vertical part. The body carries the alveoli of the lower teeth and is laterally compressed. Except at its rostral extremity, it diverges from its fellow to bound an intermandibular space. Toward its rostral extremity the lateral surface presents several mental foramina, one generally much larger than the rest; through these emerge the mental branches of the inferior alveolar nerve and vessels. The ramus (Figure 2–33/2) is wider but less robust. Its dorsal extremity ends in the high recurved coronoid process, which projects into the temporal fossa and gives attachment to the temporalis muscle, and the lower and more caudal condylar process (Figure 2–33/3), which carries an articular head shaped like a portion of a truncated cone. The lower part of the caudal margin of the ramus carries the projecting angular process that enlarges the areas of attachment of the masseter and medial pterygoid muscles. The lateral surface is scooped out to provide a roughened depression where the masseter inserts. The medial surface gives insertion to the pterygoid muscles and also presents the large mandibular foramen (Figure 2–33/7), where the inferior alveolar vessels and nerve enter the bone.

Figure 2–33 Lateral (A) and medial (B) views of the left half of the canine mandible. 1, Coronoid process; 2, vertical part (ramus); 3, condylar process; 4, angular process; 5, horizontal part (body); 6, mental foramina; 7, mandibular foramen; 8, symphysial surface.
The hyoid apparatus consists of a series of bony rods, jointed together and forming a means of suspending the tongue and larynx from the skull. The names given to the several parts are shown in Figure 2–34, which illustrates their arrangement and the attachment of the apparatus as a whole to the temporal region of the skull. The transversely placed basihyoid may be palpated within the intermandibular space; other parts are palpable—indeed their positions are visible—when the walls of the pharynx are inspected through the mouth.
Some Comparative Features of the Skull
When equipped with the mandible the skull of the cat (Figure 2–35) appears globular. Several features combine to create this conformation: the rounded cranial capsule, surmounted by a short, often weak sagittal crest, and corresponding closely to the contours of the brain; the very salient convex zygomatic arches; and the relative shortness of the face, which may account for as little as 20% of the total length. Breed differences are more pronounced than sometimes supposed. The skulls of Siamese and similar cats have much longer faces, which often blend smoothly with the cranium without any break (stop) in the dorsal contour. In contrasting types, for example, the Persian, the face is short and shallow and the stop is prominent.
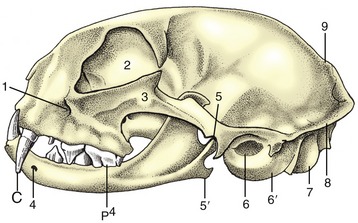
Figure 2–35 Feline skull with mandible. 1, Infraorbital foramen; 2, orbit; 3, zygomatic arch; 4, mental foramen; 5, temporomandibular joint; 5′, angular process of mandible; 6, external acoustic meatus; 6′, tympanic bulla; 7, occipital condyle; 8, nuchal crest; 9, sagittal crest; C, canine tooth; P4, upper fourth premolar.
The orbital region is distinctive. The orbits are large, face more directly forward than in the dog, and have more complete bony margins. The frontal process of the zygomatic bone and the zygomatic process of the frontal bone leave only a small gap in the ovoid margin to be closed by the orbital ligament. The zygomatic arch is surprisingly strong where it contributes to the orbital rim. The infraorbital foramen is placed close to the rostroventral part of the orbit, where it may be palpated.
On the ventral aspect, the hard palate is short, wide, and carries alveoli for only four cheek teeth. That for the largest (P4) of these teeth is located dangerously close to the orbit, which may become involved in a spreading alveolar abscess. Caudally, the deep gutter of the temporomandibular articulation is bounded by a prominent retroarticular process. The very large tympanic bulla is so salient that it may be palpated between the caudal part of the zygomatic arch and the wing of the atlas.
As in the dog, the halves of the mandible do not fuse, even in old age, and a small degree of movement is allowed at the mandibular symphysis. Each half carries sockets for only three cheek teeth.
The equine skull (Figure 2–36) is characterized by a relatively long face, a feature that develops further with increasing size; it is therefore more pronounced in mature than in juvenile animals and in large than in small breeds. The cranium is relatively narrow and generally not unlike that of the dog. The external sagittal crest is weaker. The forehead is wide between the origins of the zygomatic processes of the frontal bones, which bend ventrally to join the zygomatic arches.

Figure 2–36 A, Equine skull, and B, equine mandible. 1, Incisive bone; 2, nasoincisive notch; 3, nasal bone; 4, infraorbital foramen; 4′, cheek teeth; 5, facial crest; 6, hamulus of pterygoid bone; 7, zygomatic arch; 8, retroarticular process; 9, external acoustic meatus; 10, paracondylar process; 11, occipital condyle; 12, horizontal part (body) of mandible; 12′, mental foramen; 12″, vascular notch; 13, vertical part (ramus) of mandible; 13′, coronoid process; 13″, mandibular foramen; I, incisors; C, canine tooth (present only in the male).
The zygomatic arch (Figure 2–36/7) is conspicuously strong, even without taking into account the extra support it obtains from the zygomatic process connecting it with the frontal bone. It is not bowed laterally to any extent and carries a rather complicated articular surface on its caudoventral aspect; this comprises a rostral tuber, an intermediate fossa, and a salient retroarticular process (Figure 2–36/8). The orbit faces almost laterally and has a complete bony rim. A large maxillary tuberosity appears to continue the alveolar process directly. The zygomatic arch is continued rostrally, beyond the orbit, as a prominent ridge on the lateral surface of the face. This ridge, the facial crest (Figure 2–36/5), runs parallel to the dorsal contour of the nose and ends above a septum between the alveoli of the third and fourth cheek teeth in the adult.
A deep (nasoincisive) notch separates the pointed nasal bone from the incisive bone (Figure 2–36/1,2,3). This notch and the rostral end of the facial crest are both very easily identified landmarks; they are used as guides to the position of the infraorbital foramen, which lies a little caudal to the middle of the connecting line (Figure 2–36/4).
The features visible on the ventral view lie more or less on one level. The caudal part of this surface is distinguished by the large and very salient paracondylar processes (Figure 2–36/10) and the jagged outlines of the large openings to each side of the occipital bone. Each opening results from the failure of the temporal bone to reach the lateral margin of the occipital bone, which permits the confluence of several foramina that are distinct in the dog. The caudal part is the equivalent of the jugular foramen; the cranial part (foramen lacerum) combines the oval and carotid foramina (Figure 2–37/7,6). In life the greater part of the large opening is occluded by membrane that leaves barely sufficient passage for the various nerves and vessels. The tympanic bulla is not prominent, but styloid (for the hyoid apparatus) and muscular processes of the temporal bone are well developed.

Figure 2–37 Left caudolateral parts of the base of the equine (A) and canine (B) cranium, showing portions of the occipital (O), sphenoid (S), and temporal (T) bones; ventral view (schematic). 1, Foramen magnum; 2, occipital condyle; 3, hypoglossal canal; 4, jugular foramen; 5, foramen lacerum; 5′, petrooccipital suture; 6, carotid canal; 6′, carotid notches; 7, oval foramen; 7′, oval notch.
The choanae lie almost in the plane of the hard palate. The vertical plate of bone that separates the choanal from the pterygopalatine region carries a prominent hamular process (Figure 2–36/6). The palate is flat and unremarkable. The greater part of its margin is occupied by the alveoli of the incisor and cheek teeth.
A well-marked external occipital protuberance is present on the nuchal surface, midway between the nuchal crest and the dorsal margin of the foramen magnum.
The mandible is massive, and its right and left halves diverge at a relatively small angle (Figure 2–36, B). The symphysis becomes obliterated quite early, usually about 2 years after birth. The lower margin carries a prominent vascular notch where the facial vessels wind onto the face (Figure 2–36/12″). The ramus is high, the coronoid process projects far into the temporal fossa, and the articular process carries the ovoid articular surface well above the occlusal plane of the cheek teeth.
The parts of the hyoid apparatus (see Figure 4–8) are of different proportions to their counterparts in the dog and are laterally compressed. A substantial lingual process projects from the basihyoid into the root of the tongue.
The bovine skull (Figure 2–38) is relatively short and wide: its general form is pyramidal. Cornual (horn) processes project from the frontal bones of horned breeds where the dorsal, lateral, and nuchal surfaces meet; their size and direction vary greatly with breed, age, and sex. The very wide and flat frontal region is bounded by a prominent temporal line that overhangs the deep temporal fossa and confines this to the lateral aspect of the skull. The forehead continues smoothly into the dorsal contour of the nose.

Figure 2–38 Bovine skull with mandible. 1, Incisive bone; 2, mental foramen; 3, infraorbital foramen; 4, facial tuberosity; 5, nasal bone; 6, orbit; 7, frontal bone; 7′, horn surrounding cornual process of frontal bone; 7″, temporal line; 8, temporal fossa; 9, zygomatic arch; 10, external acoustic meatus; 10′, tympanic bulla; 11, paracondylar process; 12, occipital condyle; I, incisors; C, canine tooth, incorporated in the row of incisors.
The principal features of the lateral aspect are the confinement of the temporal fossa and the elevation of the orbital rim above its surroundings. The rim is complete and is formed by the meeting of processes from the zygomatic and frontal bones in its caudal part. There is no facial crest, only a discrete facial tuberosity from which the rostral part of the masseter arises. The infraorbital foramen is directly above the first cheek tooth, rather low toward the palate.
The ventral surface is very uneven, and the cranial base is located in a considerably more dorsal plane than the palate. The temporal and occipital bones are separated by a narrow fissure, which is an arrangement intermediate between the suture of the dog and the wide opening of the horse and pig. The tympanic bulla is prominent and laterally compressed. The choanae are separated by the caudal prolongation of the ventral part of the nasal septum and are enclosed laterally by very extensive plates of bone. The palate, long and narrow, is bounded by high alveolar processes. Of course, no alveoli are present for incisor or canine teeth, which are lacking in the upper jaws of ruminants.
The mandibular symphysis ossifies late, if at all, in ruminants. In general, the mandible is weaker than that of the horse, which is a feature very apparent in the body of the bone with its gently convex ventral border. The coronoid process is high and caudally inflected. The articular surface is concave and widened laterally.
The few remarks necessary regarding the skulls of the small ruminants and pig are found on pages 646 and 752, respectively.
The Joints of the Head
The articulations between the skull and mandible (temporomandibular joints) and that between the halves of the mandible (mandibular symphysis) are appropriately considered in the following chapter (p. 112) because the teeth, the muscles of mastication, and the joints form a single functional complex.
THE MUSCLES OF THE HEAD AND VENTRAL PART OF THE NECK
The principal groups into which the muscles of the head may be divided are given in Table 2–2, which draws attention to the correspondence between embryological origin, innervation, and function. The functional associations are so well defined and specific that it is both more convenient and more profitable to refer treatment of most groups to other chapters, where they are considered together with related organs.
Table 2–2 Source and Innervation of the Principal Muscle Groups of the Head
| Muscle Group | Source | Innervation |
|---|---|---|
| Masticatory musculature | First pharyngeal arch | Mandibular division of trigeminal nerve (V3) |
| Mimetic musculature | Second pharyngeal arch | Facial nerve (VII) |
| Pharyngeal and palatine musculature | Third and fourth pharyngeal arches | Glossopharyngeal (IX) and vagus (X) nerves |
| Laryngeal musculature | Sixth pharyngeal arch | Vagus nerve (X) |
| External ocular musculature | Hypothetical preotic somites | Oculomotor (III), trochlear (IV), and abducent (VI) nerves |
| Lingual musculature | Hypothetical postotic somites | Hypoglossal nerve (XII) |
The first four groups take origin in the unsplit mesoderm, which covers the lateral and ventral walls of the pharynx and condenses to form the cores of the pharyngeal arches.
In lower vertebrates the muscles equivalent to the last two groups in Table 2–2 are known to develop from somites that appear to each side of the hindbrain, some rostral to the otocyst, the primordium of the inner ear, and the others caudal to it. A similar origin may be assumed in mammals, although the evidence for the formation of these somites is unconvincing at the least. They are of course somatic muscles with the appropriate type of innervation.
The Trigeminal Musculature
The muscles of mastication constitute the greater part of the musculature supplied by the mandibular division of the trigeminal nerve, the motor nerve to the first pharyngeal arch. They are described in the chapter on the digestive system (p. 113). The same chapter deals with the digastricus—a composite muscle to which the mandibular field makes a contribution; the mylohyoideus (p. 105), which slings the tongue between the lower jaws; and one (tensor veli palatini) of the muscles of the soft palate (p. 119). The tensor tympani is considered with the middle ear (p. 346).
The Facial Musculature
The musculature supplied by the facial nerve, the nerve of the second pharyngeal arch, is resolvable into two divisions. The superficial division comprises the cutaneous muscle of the head and neck in addition to many small units that control the posture of the lips, cheeks, nostrils, eyelids, and external ears. The deep division is rather scattered but includes some muscles associated with the hyoid apparatus, a contribution to the digastricus (p. 114), and the stapedius (p. 348) of the middle ear.
The Superficial Division
The muscles of this division are conjectured to have their source in an ancestral deep sphincter muscle of the neck, which may be envisaged as arranged in three incomplete overlapping layers. The outermost layer, consisting of transversely disposed fascicles, is reduced to insignificance or is entirely lacking in domestic mammals. A remnant (sphincter colli) survives in the dog. A more substantial portion of the middle layer commonly persists in the form of a sheet of longitudinally disposed fibers that covers the ventral part of the face and extends onto the neck, even reaching the nape in the dog. It is known as the platysma. Detached slips are believed to provide the small muscles that attach to the caudal aspect of the external ear.
The third and deepest layer is again transverse. Although little of it remains in sheet form, it is believed to be the origin of the many discrete muscles of the mammalian face. These are extremely variable among species, but fortunately, few units, and even fewer differences, require detailed notice. Because of their effect on the appearance of the face, they are collectively known as the muscles of facial expression or mimetic musculature.
The principal muscles of the lips and cheeks are the buccinator, orbicularis oris, caninus, levator nasolabialis, levator labii superioris, and depressor labii inferioris (Figures 2–39 and 11–6). The buccinator (Figure 2–39/4) passes between the margins of the upper and lower jaws and is partly covered by the masseter. It forms the basis of the cheek and acts in opposition to the tongue, preventing food from collecting in the vestibule by returning it to the central cavity of the mouth. The buccal salivary glands are scattered among its fascicles, and discharge of their secretion into the mouth may be assisted by contraction of the muscle. The orbicularis oris (Figure 2–39/1) surrounds the mouth opening, where it is closely attached to the skin and mucosa of the lips. It closes the opening of the mouth by pursing the lips and is important in sucking. The caninus (Figure 2–39/2) arises ventral to the infraorbital foramen and radiates into the wing of the nostril and the upper lip. It dilates the nostril and elevates the corner of the mouth in the snarling gesture, especially in the dog. The levator nasolabialis (Figure 2–39/5) arises over the dorsum of the nose and inserts partly on the wing of the nostril and partly into the lateral part of the upper lip. It is able to dilate the nostril and to elevate and retract the upper lip. The medial part of the upper lip is elevated by the separate levator labii superioris (Figure 2–39/6). This muscle arises on the lateral aspect of the face and runs dorsorostrally to form with its fellow a common tendon that descends into the lip between the nostrils. A special depressor labii inferioris is present in the lower lip of certain species (excluding the dog and cat). It appears to be a detachment from the buccinator muscle. Other muscles associated with the lips and nostrils do not merit specific mention, although some are identified in various illustrations.

Figure 2–39 Superficial muscles of the equine head. The cutaneous muscle has been removed. 1, Orbicularis oris; 2, caninus; 3, depressor labii inferioris; 4, buccinator; 5, levator nasolabialis; 6, levator labii superioris; 7, orbicularis oculi; 7′, levator anguli oculi medialis; 8, temporalis; 9, occipitomandibular part of digastricus; 10, masseter.
The muscles of the eyelids include one, the levator palpebrae superioris, that is clearly foreign to the facial group because it arises within the orbit and is supplied by the oculomotor nerve. It is described on page 342. The muscles of the lids that are supplied by the facial nerve include a sphincter—the orbicularis oculi (Figure 2–39/7)—that surrounds the palpebral fissure, the opening between the lids. It is anchored at the medial and lateral commissures and therefore narrows the opening to a horizontal slit when it contracts. Other muscles are present to raise the upper (levator anguli oculi) lid and to depress the lower (malaris) lid, enlarging the eye opening.
The muscles of the external ear are especially numerous but of little account individually. A caudal group has already been mentioned. Others converge on the auricle—the skin-covered cartilaginous ear “trumpet”—from medial, rostral, and lateral directions; they lie between the skin and the temporalis muscle and skull and form a thin, incomplete sheet that includes a (scutiform) cartilage plate. The scattered origins and precisely located insertions provide for displacement and rotation of the ear in all directions. One, the parotidoauricularis, is of somewhat greater importance because it is encountered in the operation for drainage of infections of the external ear of the dog (p. 399). As its name suggests, it arises from the fascia over the parotid gland and approaches the auricle from the ventrolateral direction.
Besides the individual functions mentioned or implied in the preceding paragraphs, these muscles have a collective function in communication, mainly within the species but also between species. Human observers can intuitively, or as the result of experience, interpret many facial gestures of animals: one need only recall the hangdog expression of submission, the evident threat conveyed by snarling or laying back the ears, or the quizzical look a dog may adopt. The analysis of the more subtle expressions in terms of specific muscle activity is not yet possible for domestic species.
Paralysis of these muscles is not uncommon after damage to the facial nerve. Since different groups are supplied by branches of the nerve that arise at different levels, the particular pattern of distortions can be a valuable pointer to the location of the nerve lesion (p. 318).
The Deep Division
The muscles attaching to the hyoid apparatus are a rather heterogeneous assemblage. Certain small units are supplied by the facial nerve and elevate the hyoid, in consequence drawing the tongue backward. Although it cannot be denied that these activities have significance in swallowing, the muscles do not appear to merit description. The digastricus, in part derived from the facial musculature, is described on page 114; the stapedius of the middle ear is described on page 348.
The Muscles of the Ventral Part of the Neck
The neck connects the head with the trunk and is usually distinguished by its relatively slender construction, although this is hardly true of the pig. It has a generally cylindrical form in the dog and cat but is quite obviously compressed from side to side in the larger animals, in which it deepens considerably toward its junction with the thorax (Figure 2–40). The core structures of the neck—the cervical vertebrae and the muscles closely applied to them—were described with the trunk (p. 47). Certain superficial muscles are considered under the heading of girdle muscles of the forelimb (p. 82). The present section is therefore concerned only with the ventral part of the neck, a region of considerable clinical importance on account of the numerous visceral, vascular, and nervous structures that traverse it en route between the head and thorax.
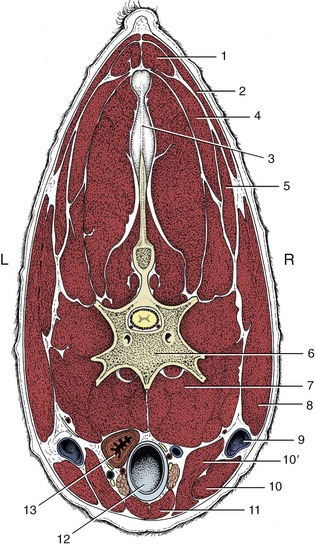
Figure 2–40 Transverse section of the bovine neck. 1, Rhomboideus; 2, trapezius; 3, nuchal ligament; 4, splenius; 5, omotransversarius; 6, vertebra; 7, longus colli; 8, brachiocephalicus; 9, external jugular vein in jugular groove; 10, 10′, sternocephalicus, mandibular, and mastoid parts; 11, combined sternohyoideus and sternothyroideus; 12, trachea; 13, esophagus (ventral to it, nerves, blood vessels, and thymus).
These structures, with the important exception of the external jugular veins (Figure 2–40/9), occupy a central visceral space. The roof of this space is provided by the muscles immediately ventral to the vertebrae, namely the longus colli, longus capitis, rectus capitis ventralis, and scalenus (p. 48). The side and ventral walls blend together and are provided by thinner muscles disposed with a sagittal course and joined by stout fasciae.
The cervical part of the cutaneous muscle (m. cutaneous colli) is unimportant in the dog and cat. It is much better developed in the ungulates, in which it radiates from a stout origin on the manubrium of the sternum; it thins as it passes cranially and laterally and eventually fades away. In the horse, the cutaneous muscle provides a relatively thick cover to the caudal third or so of the jugular groove.
The straplike sternocephalicus (Figure 2–41/2) is the most ventral of the other muscles. It also arises from the manubrium and is first pressed against its fellow. As it ascends the neck, however, it diverges laterally toward its insertion, which varies among species but includes one or the other (or both) of the angle of the mandible and the mastoid process of the skull. The divergence of the right and left muscles exposes the upper part of the trachea to palpation through the skin, although a very thin layer of deeper muscle still intervenes. The sternocephalicus is supplied by the ventral branch of the accessory nerve. Unilateral contraction draws the head and neck to that side. Bilateral contraction flexes the head and neck ventrally. In species with a mandibular insertion the sternocephalicus may assist in opening the mouth.
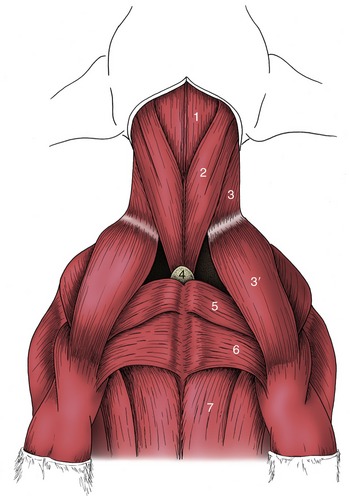
Figure 2–41 Ventral muscles of the canine neck and thorax. 1, Combined sternohyoideus and sternothyroideus; 2, sternocephalicus; 3, 3′, brachiocephalicus: cleidocervicalis, cleidobrachialis; 4, manubrium of sternum; 5, pectoralis descendens; 6, pectoralis transversus; 7, pectoralis profundus.
The sternocephalicus forms the ventral border of the jugular groove. The dorsal border of the groove is furnished by the brachiocephalicus, described more fully elsewhere (p. 83). The groove is often visible in life, particularly toward the upper part of the neck. It accommodates the external jugular vein (Figure 2–42).

Figure 2–42 Plastination specimen of the ventral part of the neck of a dog. Notice the external jugular vein (1) in the groove formed by the brachiocephalic muscle (2) dorsally, and the sternocephalic muscle (3) ventrally.
The deeper muscles constitute an infrahyoid group closely integrated in arrangement and function. They provide an incomplete cover to the lateral and ventral aspects of the trachea and insert, directly or indirectly, on the hyoid apparatus, which they stabilize and retract toward the thorax during swallowing. The obvious members of the group are the sternothyroideus, sternohyoideus, and omohyoideus; the thyrohyoideus on the lateral aspect of the larynx may be regarded as a detached member. The nerve supply is mainly, although possibly not entirely, from the first and second cervical nerves.
The sternothyroideus and sternohyoideus are very thin ribbonlike muscles that take a common origin from the manubrium of the sternum. The caudal parts of the right and left muscles are not always distinctly divided, and in the middle of the neck they may share a common intermediate tendon from which three or four slips diverge cranially. The sternothyroideus inclines laterally to terminate on the lateral aspect of the thyroid cartilage. The sternohyoideus, not always separable from its fellow, passes beside the midline to insert on the basihyoid.
The omohyoideus, lacking in carnivores, is also thin and straplike. Its absence is compensated by the relative enlargement of the other muscles. In the horse it arises from the subscapular fascia, and in the ruminants from the deep fascia of the neck; thereafter it edges medially to join the lateral margin of the sternohyoideus beside which it inserts. In the horse it provides a floor to the caudal part of the jugular groove, separating the vein from the structures within the visceral space.
THE LIMBS
BASIC PLAN AND DEVELOPMENT
Although the forelimbs and hindlimbs are not homologous, they have a similar organization and segmentation with a remarkably close correspondence of analogous parts. Each first appears as a bud that grows out from the ventrolateral surface of the body of the young embryo at a level corresponding to the origin of the nerves by which it will later be supplied. The bud of the forelimb appears before that of the hindlimb, and its development maintains this advantage for some time—indeed, until after birth in puppies and other animals born in a rather immature state. These animals initially confine their locomotor activities to dragging themselves, using the forelimbs only, toward their dam’s teats.
When first formed, a limb bud consists of a mass of mesenchyme, the loose embryonic connective tissue, within an ectodermal covering. The ectoderm becomes the epidermis, including its derivatives; the mesenchyme differentiates to form skeletal tissues, muscles and tendons, fasciae, and blood vessels. Thus it is only the limb nerves that invade from outside; all other structures develop in situ. The limb bud lengthens, and its free distal part expands to form a flattened hand (foot) plate while the more proximal part acquires a more columnar form. Thickenings corresponding to the digital rays soon appear in the plate and are accentuated when the intervening tissues are reduced. The details of this development naturally vary with the species, for it is only some that retain the primitive pentadactyl (five-digit) pattern and only a few that show a complete separation of digits. It is interesting to note that five digits appear in most species; when evolution has reduced the complement to fewer, the adult condition is usually attained by fetal regression of some digits. Creases formed in the proximal part of the bud soon allow recognition of segments corresponding to the arm and forearm (or thigh and leg) regions of the adult.
The first indication of the future limb skeleton is provided by an axial condensation of the mesoderm to produce a denser core. In the early stages of development (but not always later) a definite proximodistal gradient of differentiation occurs. This establishes and then maintains the girdle elements in advance of those of the arm or thigh, and the latter in advance of more distal parts.
In the next stage of development the mesoderm is locally transformed to create a series of cartilaginous models in the pattern of the adult bones. These precursors soon come to resemble the final forms in broad outline; they remain ensheathed by thin coverings of the unmodified mesoderm, now appropriately known as perichondrium. Dense mesoderm also remains between the cartilages where the joints will develop.
The cartilage models grow mainly by interstitial growth, in which each part expands more or less uniformly to maintain the general form. The next stage involves the replacement of the cartilage by bone tissue—not its transformation into bone, a distinction that deserves to be emphasized. The process does not occur identically or synchronously in different bones, and the remarks that follow concern that hypothetical concept, the “typical long bone.”
The initial ossification involves two processes. In one, the perichondrium around the middle of the shaft lays bone down on the cartilage. This process of bone formation is known as intramembranous ossification because it occurs within the connective tissue membrane. Its details must be sought in textbooks of histology. A tubular bony sheath, the periosteal collar, is thus formed about the center of the shaft; it is gradually extended toward each extremity (Figure 2–43). In the other process, the cartilage of the center of the shaft shows aging or degenerative changes; its cells hypertrophy, come to occupy enlarged lacunae (spaces) in the matrix, and then die, while the matrix becomes impregnated with calcium salts. This central patch of dead cartilage is now invaded by a connective tissue sprout that pushes in from the periosteum (as the perichondrium is now more appropriately known in the region of the collar). The progress of this sprout, which is rather cellular and well vascularized, is facilitated by the spongy texture given to the dead cartilage by the enlarged lacunae. Some of the cells that are carried inward have the capacity to engulf and remove calcified matrix, others have the capacity to lay bone down on the surviving framework, while a third group are precursors of marrow cells. The processes of construction and destruction continue in parallel and transform the whole middle portion of the shaft into a parcel of bone known as the primary or diaphysial center of ossification.

Figure 2–43 Development of a long bone, schematic. 1, Cartilage model with perichondral membrane (arrow); 2, intramembranous ossification of diaphysis; 3, 4, endochondral (primary) ossification of diaphysis, replaces cartilage; 5, beginning of medullary cavity (arrow); 6, epiphysial ossification centers appear; 7, endochondral (secondary) ossification of epiphyses; 8, narrow epiphysial cartilages (arrows) separate the diaphysis from epiphyses: these and the articular cartilages are all that remain of the cartilage model (1); note circumferential growth of diaphysis by removal (−) and addition (+) of compact bone; 9, mature bone consisting of articular cartilage, spongy bone, and compact bone; the epiphysial cartilages have disappeared.
Later (much later in some species and mainly after birth in ourselves), similar sprouts from the perichondrium invade the centers of the two extremities; they establish secondary or epiphysial centers of ossification. The secondary centers are not preceded by the formation of any equivalent to the periosteal collar of the shaft. The general stage of development of the long bone at this time is shown in Figure 2–43/8. This reveals that the original cartilage now survives only as two plates, the epiphysial or growth cartilages, that intervene between the primary and secondary centers. These have a special significance since they are responsible for the growth in length of the bone. They are clearly polarized; cell division and matrix expansion are confined to the epiphysial aspect while degeneration, calcification, and replacement occur at the central or diaphysial side (Figure 2–44). The replacement adds continuously to the length of the diaphysis while the growth of the cartilage continues to shift the epiphyses away from this. The two processes are balanced until finally growth fails to keep pace with replacement. The plate thins and ultimately is quite destroyed. The epiphysis and diaphysis have now fused as one, and further longitudinal growth is impossible. Neither the rates of growth nor the times of final disappearance are necessarily the same in the two growth cartilages of a long bone. Meanwhile, however, the bone has also been increasing in its girth, which is the result of further lamellae being laid in succession on the existing bone within the periosteal sheath. Some of the larger projections on long bones develop from independent centers of ossification and remain separated from the shaft by cartilage growth plates while growth continues. The projections distinguished in this way are known as apophyses.

Figure 2–44 Equine (pony) fetus 80 days. The developing skeleton has been colored with Alcian blue and Alizarin red. The calcified parts are red (Alizarin) and the epiphyses have not begun to calcify. These cartilaginous parts are blue.
Little reflection is necessary before deciding that bone growth must be more complicated than this. The form established by the original model would not be maintained by continuous accretion. A simultaneous process of destruction must exist, especially to maintain the shape of the metaphyses (the regions of the shaft adjoining the growth cartilages), to keep surface features in the same relationship to each other, and to establish and then enlarge the medullary cavity. Although we have no space to elaborate on this statement, one point can be made: bone grows by apposition, the deposition of new material on that previously existing. In this it differs from the periosteum, which grows interstitially as though uniformly stretched. The periosteal sheath therefore shifts relative to the underlying bone, and the consequent drag on the nutrient vessels explains the generally oblique orientation of the adult nutrient foramina. By the time of birth, skeletal development has reached very different stages in different mammalian species. In the precocious ungulates, immediately active after birth, almost all epiphyses are well established at term. This contrasts sharply with the much less mature condition of the canine and, most especially, human neonates, in which many of the secondary ossification centers have yet to appear. The individual rate of skeletal development is affected by many factors—inherited, nutritional, and hormonal, the last covering a complex situation in which hormones of hypophysial, thyroid, adrenal, and gonadal origin are involved. It is hardly surprising that abnormality of skeletal development is common.
The important features of the development of joints can be discussed more briefly. The joint tissues derive from the mesoderm left between the cartilaginous primordia of the bones. Spaces that develop in this tissue coalesce to form a single synovial cavity bounded by articular cartilage and synovial membrane. The former is probably produced by delayed chondrification of the mesoderm bordering the cartilaginous models; structural differences suggest that it is not the outer shell of this model left over after completion of epiphysial ossification. The synovial membrane is a more direct transformation of the mesoderm bordering the space. The fibrous part of the capsule and periarticular ligaments develop from more peripheral mesoderm.
It is now generally agreed that the limb muscles develop within the buds. The attractive notion that portions of myotomes migrate into these buds, pulling along the appropriate nerves, has been abandoned. Certain mesenchymal cells outside the denser axial core differentiate into precursor muscle cells (myoblasts); these then increase in number through mitosis while recruitment from the mesenchyme continues. These myoblasts then form myocytes or muscle cells by a maturation in which the nuclei increase in number and migrate to the periphery of the cells. The final number of muscle cells seems, in most species, to be established before birth, perhaps well before birth. The later growth of muscles therefore depends on an increase in the size of existing elements.
The limb nerves grow in from the ventral rami of certain spinal nerves: generally C6–T2 for the forelimb and L4–S2 for the hindlimb. The segmental pattern becomes disturbed by the development of the limb plexuses in which fibers from the several ventral rami reassort before combining as the named peripheral trunks. As a consequence, all but a few very small muscles are supplied by fibers that lead from neurons in more than one spinal segment. The sensory fibers to the skin arrange themselves so that specific regions are more or less the territory of particular spinal segments. The basis for this has become more difficult to understand now that it is believed that the dermis of the limb skin develops from cells of local origin, not from cells that migrated from particular somites.
Table 2–3 lists in parallel columns the bones of the forelimb skeleton and the parts to which they give support; for comparison, columns for the corresponding bones and parts of the hindlimb (which, it will be recalled, are analogous and not homologous) are also included. A central column gives additional terms, more common in zoological than in veterinary literature, that are common to both limbs; most are not used in this text but may be encountered elsewhere.
Some entries in the first and last columns may include three terms. Those printed in plain type are the technical words used when referring to domestic animals, the terms commonly employed by veterinarians; those italicized are the corresponding words used in human anatomy; and those in brackets are the more elevated Latin terms. Probably the most surprising feature of the table is the apparent absence of vernacular terms for certain regions of animals. The situation is in fact rather better, or rather worse according to one’s point of view, than it appears. Many additional vernacular terms are restricted by custom to certain species; for example, the metacarpus of the horse is known as the cannon, but that of the dog is not. A particular difficulty is presented by the lack of handy equivalents to “paw” in description of farm animals: manus and pes are unacceptably pedantic (hence enclosed in brackets), and forefoot and hindfoot are usually (if not entirely logically) preferred; however, to the horse owner the foot generally means only the hoof and its contents. It is impossible to avoid all inconsistency.
In this book we employ the more elevated terms where it seems that vernacular equivalents might be ambiguous, risking the charge of pedantry. It is of course more sensible to use the everyday terms in conversation with laypeople.
THE SKELETON OF THE FORELIMB
Pectoral Girdle
The scapula, or shoulder-blade (Figure 2–45), is a flat bone that lies over the laterally compressed, craniodorsal part of the thorax, where it is held in place by an arrangement (synsarcosis) of muscles without forming a conventional articulation with the trunk. It is the basis of the shoulder region, a term that embraces much more than the immediate neighborhood of the shoulder joint. In ungulates, the scapula is extended dorsally by an unossified portion, the scapular cartilage (Figure 2–45, E/13), which enlarges the area for muscular attachment. The cartilage becomes increasingly calcified and thus more rigid with age.

Figure 2–45 Left scapula of the dog; lateral (A), ventral (B), and medial (C) views. Distal end (D) of left feline scapula. Left equine scapula (E). 1, Cranial angle; 2, spine; 2′, tuber of spine; 3, supraspinous fossa; 4, infraspinous fossa; 5, neck; 6, supraglenoid tubercle; 7, acromion; 7′,7″, hamate and suprahamate processes of acromion; 8, infraglenoid tubercle; 9, caudal angle; 10, facies serrata; 11, coracoid process; 12, glenoid cavity; 13, scapular cartilage.
The bone is roughly triangular, though less so in the dog and cat than in the other domestic species. Its lateral surface is unequally divided by a prominent spine into supraspinous and infraspinous fossae, each occupied by the like-named muscle. The spine extends from the dorsal border almost to the articular angle and may bear a thickening for the insertion of the thoracic part of the trapezius; it is generally palpable through the skin. In all but the horse and pig, it ends in a prominent process (acromion), laterally flattened to form a hamate process in the carnivores (Figure 2–45/7′) and furnished with an additional projection (suprahamate process; Figure 2–45/7″) in the cat. The medial surface of the bone is largely given over to the origin of the subscapularis, which occupies a shallow fossa; a more dorsal roughened area, where the serratus ventralis attaches, extends onto the cartilage in the larger species.
The caudal border is thickened and almost straight. The thinner and sinuous cranial border is notched toward its distal end for the passage of the suprascapular nerve. The dorsal border is also generally straight and extends between cranial and caudal angles; the latter is thickened and more easily identified on palpation. The ventral or articular angle is joined to the body of the bone by a slightly constricted neck. Its caudal part carries a shallow glenoid cavity (Figure 2–45/12) for articulation with the head of the humerus. The cavity, which is somewhat extended in the sagittal direction, faces more or less ventrally. A large muscular process, the supraglenoid tubercle, projects in front of the cavity; it gives origin to the biceps brachii.
The clavicle is reduced to a fibrous intersection in the brachiocephalicus. A nubbin of bone in the dog and a slender rodlet in the cat are embedded in the intersection; their sole importance lies in the risk of misinterpretation when they are seen on radiographs.
Skeleton of the Free Appendage
The humerus (Figure 2–46) forms the skeleton of the arm. It is a long bone that lies obliquely against the ventral part of the thorax, more horizontally in the large species than in the small. It is also relatively shorter and more robust in horses and cattle than in the small ruminants and carnivores. The proximal extremity carries a large articular head (Figure 2–46/2), facing toward the glenoid cavity of the scapula and thus offset to the shaft to which it is joined by a neck. The head is shaped like a segment of a sphere and is considerably larger than the fossa with which it articulates. Two processes, the greater (lateral) and lesser (medial) tubercles, are placed in front and to the side of the articular area. They are separated by the intertubercular groove (Figure 2–46/13) through which the biceps tendon runs. The processes are sometimes more or less equal, as in the horse. More often the lateral one, which forms the basis of the surface feature known as the point of the shoulder, is larger; it is so in the dog. In the horse and in cattle, both tubercles are divided into cranial and caudal parts (Figure 2–46/1′,1″,3′); the intertubercular groove is also molded by an intermediate tubercle in the horse (Figure 2–46/13′). The medial and lateral tubercles give attachment to the muscles that brace and support the shoulder joint, substituting for collateral ligaments.
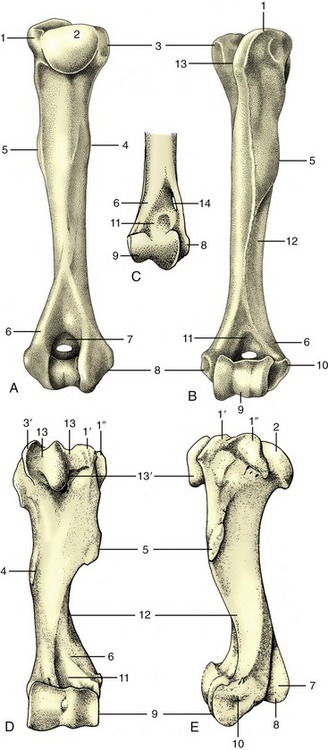
Figure 2–46 Left humerus of the dog; caudal (A) and cranial (B) views. C, Distal end of right feline humerus; cranial view. Cranial (D) and lateral (E) views of left equine humerus. 1, Greater tubercle; 1′,1″, cranial and caudal parts of greater tubercle; 2, head; 3, lesser tubercle; 3′, cranial part of lesser tubercle; 4, teres (major) tuberosity; 5, deltoid tuberosity; 6, lateral supracondylar crest; 7, olecranon fossa (with supratrochlear foramen in dog); 8, medial epicondyle; 9, condyle; 10, lateral epicondyle; 11, radial fossa; 12, groove for brachialis; 13, intertubercular groove; 13′, intermediate tubercle; 14, supracondylar foramen.
A twisted appearance is imparted to the shaft by a groove (Figure 2–46/12) that spirals over the lateral aspect and carries the brachialis and the radial nerve. Laterally, toward its upper end, the shaft carries the large, easily palpated deltoid tuberosity (Figure 2–46/5), which is joined to the greater tubercle by a prominent ridge. A less prominent, gradually subsiding ridge, the crest of the humerus, continues distally beyond the deltoid tuberosity. The medial aspect of the shaft is marked by the much less salient roughening, the teres (major) tuberosity.
The distal extremity bears an articular condyle (Figure 2–46/9) that is also set at an angle to the axis of the shaft. In large animals it engages with the radius and has the form of a trochlea. In the dog and cat it is divided into a medial area (trochlea) for the ulna and a lateral area (capitulum) for the radius. In all species the caudal part of the groove of the trochlea is continued proximally into a deep (olecranon) fossa (Figure 2–46/7) that receives the anconeal process of the ulna. Two saliences proximal to the articular surface are known as epicondyles. The medial one (Figure 2–46/8) is prominent and forms a right-angled, caudally directed projection that gives origin to the flexor muscles of the carpus and digit. The cranial aspect of the lateral epicondyle (Figure 2–46/10) gives origin to the extensor muscles of the carpus and digit. To the side, each epicondyle gives origin to the corresponding collateral ligament of the elbow joint. In the dog the floor of the olecranon fossa is perforated by a supratrochlear foramen that opens to a much shallower radial fossa on the cranial aspect of the shaft (Figure 2–46/7,11). In the cat alone, the mediodistal part of the humerus is pierced by a supracondylar foramen (Figure 2–46/14) that gives passage to the median nerve and brachial artery.
The skeleton of the forearm is provided by two bones, the radius and the ulna (Figure 2–47). In the standing position they are arranged with the ulna caudal to the radius in the upper part of the forearm but lateral in the lower part. In the primitive condition these bones articulate only at their extremities, leaving an interosseous space between their shafts; rotational movements of the human forearm bones result in turning the hand so that the palm is brought to face forward (supination) or backward (pronation). In most domestic animals the capacity for these movements is reduced or lost, and the two bones are firmly held together by ligaments or by fusion in the prone position. When supination is possible, it consists of rotation of the upper extremity of the radius within the embrace of the ulna while the distal extremity is carried in an arc around the ulna.
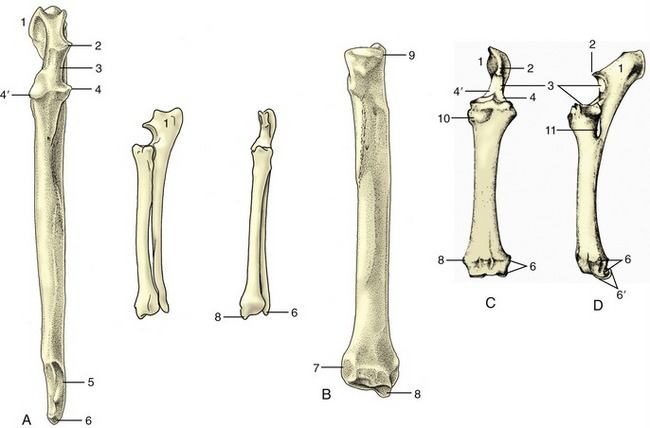
Figure 2–47 Left ulna (A) and left radius (B) of the dog. In sequence from the left: cranial view of the ulna, craniolateral and cranial views of the radius and ulna, and caudal view of the radius alone. Cranial (C) and lateral (D) views of fused left radius and ulna of the horse. 1, Olecranon; 2, anconeal process; 3, trochlear notch; 4, 4′, lateral and medial coronoid processes; 5, distal articular facet for radius; 6, lateral styloid process (with facet for the ulnar carpal bone in the dog); 6′, distal end of ulna incorporated within radius; 7, articular facet for ulna; 8, medial styloid process; 9, circumferential facet; 10, radial tuberosity; 11, interosseous space.
Clearly, no movement is possible when the bones are fused, which is the condition prevailing in ungulates and reaching its extreme in the horse, in which only the upper end of the ulna remains distinct (Figure 2–47, D/1). About 45° of supination is allowed to the dog, and somewhat more to the cat. (Rotation at the carpus contributes a substantial extra component to the movement subjectively interpreted as supination.)
The radius is a rather simple rodlike bone, usually much stronger than the ulna in ungulates, but less dominant in carnivores, in particular the cat. The proximal extremity is transversely widened, though tending to a more circular plan in carnivores, in which some supinatory capacity remains. It articulates with the distal articular surface of the humerus and is shaped to match this. A circumferential facet (Figure 2–47, B/9) on the caudal part of the extremity articulates with the ulna and is present even when no supination is allowed. The shaft is craniocaudally compressed and slightly bowed in its length. The distal part of the cranial surface is grooved for the passage of the extensor tendons (Figure 2–47, C), whereas the caudal surface is roughened for muscular attachment. The medial border is subcutaneous and therefore palpable.
The distal extremity of the radius is somewhat expanded. It carries an articular surface that is concave in its cranial part and convex in its caudal part in ungulates; it has a slightly concave ovoid form in carnivores, in which some abduction, adduction, and rotation are allowed to the antebrachiocarpal joint in addition to the major movements of flexion and extension. Medial to the articulation, the radius is prolonged to form a styloid process (Figure 2–47, B/8). The corresponding lateral projection is furnished by the ulna and, in the horse, by the portion of the radius representing the incorporated ulna.
The ulna has an unusual appearance, as its shaft is greatly reduced and its proximal extremity is prolonged beyond the articular surface to form the high olecranon, the point of the elbow. This process, which constitutes a very prominent landmark, gives attachment to the triceps. Distal to this, the cranial margin carries the beaklike anconeal process (Figure 2–47/2), which fits into the olecranon fossa of the humerus, above an articular notch that engages with the humeral trochlea; yet farther from the extremity, there is a facet for the circumferential articular area of the radius. In the dog the shaft, though slender, runs the full length of the radius from which it is separated by an interosseous space that is bridged by membrane in life. The distal extremity carries a small articular facet for the radius and beyond this is continued as the lateral styloid process (Figure 2–47/6), which makes contact with the ulnar carpal bone.
Reduction of the ulna is greatest in the horse, in which the shaft tapers to end at midforearm level (Figure 2–47, D). The distal part became incorporated within the radius in fetal life (Figure 2–47/6′). The ruminants and pig show intermediate conditions. Of course the fusion of the ulna with the radius prohibits the movements of supination and pronation in the domestic mammals other than the dog and cat.
The short carpal bones articulate in complex fashion. The plan of the primitive carpal skeleton is uncertain, but in domestic species the bones are clearly arranged in two rows (Figure 2–48). The proximal row comprises (in mediolateral sequence) radial, intermediate, ulnar, and accessory bones; the last appears as an appendage projecting behind the carpus and is a prominent landmark in the live animal. The radial and intermediate carpals fuse in the dog and cat. The elements of the distal row are numbered from one to five (again in mediolateral sequence), although the fifth never appears as a separate bone but is either suppressed or fused with the fourth. The first is also often lacking while the second and third fuse in ruminants. The diagrams illustrate the carpal formulae in different species. Apart from the accessory carpal bone, which is probably a sesamoid by origin, a small sesamoid bone is embedded in the medial tissues of the joint of the dog. Intrinsically unimportant, it can confuse radiographic interpretation by wrongly suggesting a “chip” fracture.

Figure 2–48 The bones of the carpal skeleton in the carnivores (Car), horse (eq), cattle (bo), and pig (su), schematic. Roman numerals identify the metacarpal bones; Arabic numerals, the distal carpal bones. R, Radius; U, ulna; a, accessory carpal bone; i, intermediate carpal bone; r, radial carpal bone; u, ulnar carpal bone.
Viewed as a whole, the carpus is convex from side to side on its cranial aspect and flat and very irregular caudally, although in life these irregularities are smoothed by thick ligaments. Most movement occurs at the antebrachiocarpal level, some at the intercarpal level, and virtually none at the carpometacarpal level or between neighboring bones in a row. The combined proximal articular surface is the reciprocal of that of the radius (see earlier) and in carnivores has a convex ovoid form.
The primitive pattern for the skeleton of the mammalian manus exhibits five more or less equal rays, each consisting of a metacarpal bone and proximal, middle, and distal phalanges in line (Figure 2–49, A). This pattern has been modified in all domestic species, each of which (not excepting the pig) is to some degree specialized for fast running. Cursorial specialization involves raising the manus (and pes) from the primitive “flatfooted” (plantigrade) posture demonstrated by bears (Figure 2–50). An intermediate stage, the digitigrade posture, has been attained by dogs, which support themselves by the digits only; it culminates in the unguligrade posture attained by ruminants, pigs, and horses, in which only the tips of the digits, protected by hooves (ungulae), give support. The process results in the abaxial digits first losing permanent contact with the ground; a compensating development of the remaining digits enables them to carry an increased proportion of the weight. The process has not progressed very far in the dog and cat, in which only the most medial (first) digit has lost contact and is retained as a nonfunctional dewclaw (Figure 2–51). The four functional digits are broadly equal, with the axis of the manus passing between the third and fourth digits (a paraxonic position). Pigs have entirely lost the first digit; the second and fifth digits are very much reduced, although each retains a complete skeleton. In ruminants the process has gone further, and although elements of four digits are present, those of the abaxial pair are vestigial; the metacarpal bones of the functional third and fourth digits are fused in a single bone that retains evidence of its composite origin (Figure 2–49, C).
Figure 2–49 Right manus (human hand; A) of horse (B) and ruminant (C), palmar views. The Roman numerals number the rays. 1, Radius; 2, ulna; 3, metacarpal; 4, 5, 6, proximal, middle, and distal phalanges; 7, carpal bones; 8, rudimentary metacarpal V; 9, accessory carpal bone; 10, rudimentary metacarpals II and IV (medial and lateral splint bones); 11, axis in line with ray III (mesaxonic), in C paraxonic.
Figure 2–50 Hindlimbs of bear, dog, and horse (from left to right) illustrating plantigrade, digitigrade, and unguligrade postures.
Figure 2–51 Skeleton of the right manus of the dog, lateral (A) and dorsal (B) views. The Roman numerals identify the metacarpal bones. 1, Radius; 2, ulna; 3, accessory carpal; 4, ulnar carpal; 5, radial carpal (intermedioradial in the dog); 5′, intermediate carpal; 6, 7, first and fourth of the distal row of carpal bones; 8, sesamoid bone; 9, proximal sesamoid bones; 9′, ridged articular surface of equine metacarpus III, articulates with proximal sesamoid bones (not shown); 10, dorsal sesamoid bone; 11, 12, 13, proximal, middle, and distal phalanges; 13′, claw; 14, axis of manus.
In the horse (Figure 2–49, B), only the third ray survives in functional form and its axis coincides with that of the limb; the manus is said to be mesaxonic. Remnants of the second and fourth metacarpal bones survive as the splint bones that flank the third metacarpal or cannon bone; they end in nodules, but the assumption that these incorporate greatly reduced elements of all three phalanges of the lost digits is unfounded.
The differences in the metacarpal and digital skeleton are very striking as a consequence of these changes, and the short description that follows is amplified in later chapters by details of a species-specific nature.
As the number of metacarpal bones diminishes, the relative stoutness of the surviving members of the series increases. The single (third) metacarpal bone of the horse therefore has a particularly strong shaft, whereas the individual metacarpal bones of the dog are relatively much weaker. The dog’s bones are also shaped by their mutual contacts; the third and fourth bones are square in section, and the flanking second and fifth bones are triangular. Taken as a whole, the metacarpal skeleton of all species is somewhat compressed in the dorsopalmar direction. Each bone has a proximal extremity (base), a shaft, and a distal extremity (caput). The base has a flattish articular surface for the distal row of carpal bones and may, according to its position in the metacarpal series, have medial and lateral facets where it makes contact with neighbors. The distal extremity articulates with the proximal phalanx by a hemicylindrical surface with a central ridge. Various roughenings for ligamentous attachment are present at both extremities.
The proximal phalanx is a short cylindrical bone with a proximal extremity adapted to the caput of the metacarpal bone and a distal articulation in the form of a shallow trochlea. Again, the bone may be shaped by its position in the digital series.
The middle phalanx is shorter than, but basically very similar to, the first phalanx. The distal phalanx corresponds to the form of the hoof or claw in which it is wholly (hoof) or partly (claw) contained. The digital skeleton is completed by paired proximal sesamoid bones at the palmar aspect of the metacarpophalangeal joint and by a distal sesamoid bone (cartilage in the dog) at the palmar aspect of the distal interphalangeal joint. In the dog small sesamoids also exist within the extensor tendons over the dorsal aspect of the metacarpophalangeal joints.
THE JOINTS OF THE FORELIMB
The shoulder joint (Figure 2–52, A) links the scapula and humerus, and although it has attributes of the spheroidal variety, sagittal excursions predominate in practice. The glenoid cavity of the scapula is considerably smaller than the head of the humerus. In large animals, both surfaces may be indented peripherally by naked areas (synovial fossae) simulating, to the inexperienced eye, lesions of the cartilage. The joint capsule is roomy and is fused here and there with the tendons of the surrounding muscles, particularly the subscapularis. In all but the horse and ox it sends a prolongation or diverticulum around the tendon of origin of the biceps brachii, where this lies within the intertubercular groove. The diverticulum protects the tendon in the manner of a synovial sheath; it is replaced by a discrete intertubercular bursa in the two large species. Although the fibrous layer of the capsule is locally strengthened, it is usual to say that the joint is without pericapsular ligaments. Tendons of immediately adjacent muscles, notably the subscapularis medially and infraspinatus laterally, take the place of ligaments in bracing the joint.
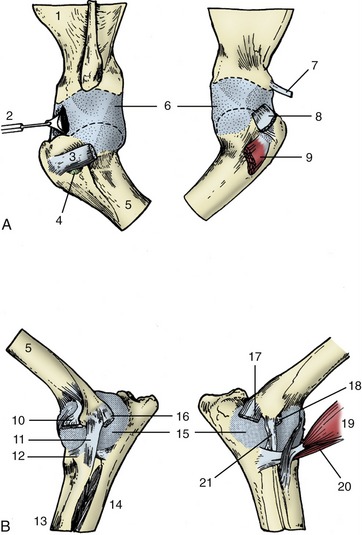
Figure 2–52 Left shoulder (A) and elbow (B) joints of the dog. The drawings on the left are lateral views, those on the right medial. 1, Scapula; 2, joint capsule opened to expose biceps tendon; 3, tendon of infraspinatus; 4, infraspinatus bursa; 5, humerus; 6, joint capsule, stretched by pulling bones apart; 7, tendon of coracobrachialis; 8, tendon of subscapularis, reflected ventrally; 9, biceps tendon emerging from intertubercular groove; 10, stump of extensor carpi radialis and common digital extensor; 11, lateral collateral ligament; 12, annular ligament of radius; 13, radius; 14, ulna; 15, joint capsule; 16, stump of ulnaris lateralis; 17, common stump of carpal and digital flexors; 18, stump of pronator teres; 19, biceps; 20, brachialis; 21, medial collateral ligament.
Movement is most free in the sagittal direction, but significant amounts of rotation, abduction, and adduction, and therefore also of circumduction, are possible, particularly in the dog and cat; in these animals a component of the movement interpreted as supination probably occurs at shoulder level.
The elbow joint (Figure 2–52, B) combines within a single capsule the hinge joint between the humerus and the radius and ulna and, at least in carnivores, the pivot joint between the proximal extremities of the latter pair of bones. The humeral surface is broadly trochlear, and the lower surface, variously furnished by the radius and ulna, is its reciprocal. Ridging of the surfaces, most pronounced in the larger animals, impedes other than hinge movements. A proximal radioulnar articulation between a circumferential facet on the radius and a corresponding but smaller area on the ulna is present even when more distal fusion precludes the possibility of movement. The joint capsule is surprisingly roomy and, when distended, bulges to each side of the ulna within the olecranon fossa. The strongest ligaments are medial and lateral collateral ligaments, which is a predictable arrangement in what is basically a hinge joint.
The lateral of these ligaments is short and thick (Figure 2–52/11), and the medial one is longer, more slender, and divisible into two parts (Figure 2–52/21)—radial and ulnar in the dog and cat and superficial and deep in the larger animals. An additional oblique ligament is placed over the flexor aspect of the joint of the dog and cat. In these species there is also an annular ligament (Figure 2–52/12) extending between the collateral ligaments and completing the enclosure of the head of the radius within an osseoligamentous ring.
In the large species, most notably the horse, the curvature of the humeral surface is not uniform. This feature, combined with the eccentric proximal attachment of the collateral ligaments (see Figure 23–10), makes the joint more stable in the normal standing position (which approaches but does not reach maximal extension); some effort is required to “unlock” the joint before it can be flexed.
The shafts of the radius and ulna are joined by an interosseous membrane that ossifies early in life in ungulates. In the dog and cat the membrane is sufficiently long to allow the limited rotation possible in these species.
The carpal joint includes antebrachiocarpal, midcarpal, and carpometacarpal levels of articulation and also a distal radioulnar joint. The antebrachiocarpal and the radioulnar joints share a common joint cavity. The midcarpal and carpometacarpal joint cavities are interconnected. In hoofed species the proximal joint may be regarded as being of the hinge variety (although the form of the surfaces introduces a certain obliquity of movement in ruminants), but in dogs and cats it is more versatile and can be regarded as an ellipsoidal joint, although a poor example of the type. The hinge movement is quite free at the antebrachiocarpal level (horse: ca. 90°). Considerable movement is also possible at the midcarpal level (ca. 45°), but virtually no movement is allowed at the carpometacarpal level. Medial and lateral collateral ligaments are well developed in ungulates but are necessarily much weaker in the dog and cat to allow for some adduction and abduction. On the dorsal aspect, a number of short ligaments join neighboring bones in the same row and those of the row distal to the metacarpus. More robust ligaments are found on the palmar aspect, where a deep ligament (Figure 2–53/6) covers the entire palmar surface of the skeleton, burying the unevenness of the bones. A second, superficial, transverse ligament (flexor retinaculum) passes obliquely from the free extremity of the accessory carpal bone to the medial aspect of the carpus (Figure 2–53/7), completing the enclosure of a passage behind the carpus. This, the carpal canal, conveys the flexor tendons and other structures continuing into the foot from the forearm. Additional small ligaments (Figure 2–53/5) join the accessory bone to the adjacent carpal and metacarpal bones. These palmar ligaments do not interfere with flexion but assist in preventing overextension.
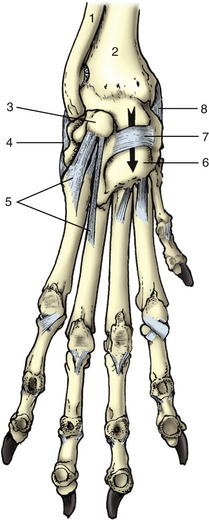
Figure 2–53 Left carpal joint of the dog, palmar view. 1, Ulna; 2, radius; 3, accessory carpal; 4, lateral collateral ligament; 5, distal ligaments of accessory carpal; 6, palmar carpal ligament; 7, flexor retinaculum; 8, medial collateral ligament; the arrow is in the carpal canal.
Description of the more distal joints is best deferred because of the marked interspecific variation. These joints are only important in the large species.
THE MUSCLES OF THE FORELIMB
The muscles of the forelimb comprise the girdle musculature, passing between the trunk and the limb, and the intrinsic musculature.
Girdle Muscles
The girdle muscles join the forelimb to the trunk, forming a connection known as a synsarcosis that substitutes for a conventional joint. When the animal is standing, some of the muscles of the synsarcosis (the serratus ventralis and pectoralis profundus) sling the body between the forelimbs to which they transmit the weight of the head, neck, and cranial part of the trunk (Figure 2–54). These and other girdle muscles can also stabilize the scapula against external forces, preventing its displacement or rotation. A good example of this role is supplied by a cat pouncing on a mouse or plaything with forelimbs rigidly braced against the trunk. During progression the same muscles resolve into antagonistic groups that control the swing of the limb; one group advances (protracts) the limb, the other retracts it. For these actions to be understood, it is necessary to appreciate that the scapula may be moved against the chest wall in two different ways. In one, the bone is rotated about a transverse axis located toward its upper end. The position of this axis, which is of course imaginary, is fixed by the balance of opposing muscles, chiefly the rhomboideus and serratus ventralis, which both attach on the dorsal part of the scapula. In the other movement, the whole bone is shifted on the thoracic wall. It is slid downward and forward as the limb is advanced and upward and backward in recovery during retraction. This movement of the scapula, which adds usefully to the length of the stride, is permitted by the looseness of the connective tissue that intervenes between the limb and the trunk where there exists a potential space, the axilla, corresponding to the human armpit. The axilla also gives passage to the nerves and vessels entering the limb from the trunk, and it contains the axillary lymph nodes.
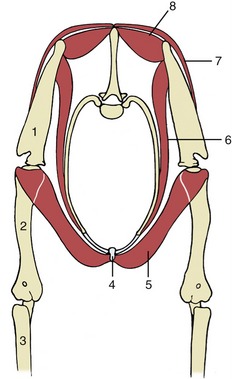
Figure 2–54 Muscular suspension of the thorax between the forelimbs (dog). 1, Scapula; 2, humerus; 3, radius and ulna; 4, sternum; 5, pectoralis profundus (ascendens); 6, serratus ventralis; 7, trapezius; 8, rhomboideus.
For the purpose of description, the girdle muscles can be considered in two layers.
The Superficial Layer
This consists of a cranial group supplied mostly by the accessory nerve, the latissimus dorsi more caudally, and the two superficial pectoral muscles ventrally. The cranial group comprises the trapezius, omotransversarius, and brachiocephalicus.
The trapezius (Figure 2–55/5,5′) is thin. It takes origin from the middorsal raphe and supraspinous ligament, extending from about the level of the second cervical to that of the ninth thoracic vertebra, and converges to insert on the spine of the scapula. It consists of two fleshy parts, cervical and thoracic, usually separated by an intermediate aponeurosis. The fibers of the cervical part run caudoventrally to attach along the greater part of the length of the scapular spine; those of the thoracic part run cranioventrally to a more confined insertion on the tuberous thickening of the spine. The trapezius may raise the scapula against the trunk and swing the ventral angle of the bone cranially, thus advancing the limb.
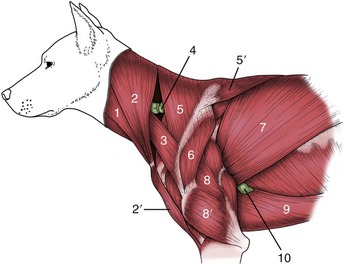
Figure 2–55 Superficial muscles of the shoulder and arm. 1, Sternocephalicus; 2, 2′, brachiocephalicus: cleidocervicalis and cleidobrachialis; 3, omotransversarius; 4, superficial cervical lymph node; 5, 5′, cervical and thoracic parts of trapezius; 6, deltoideus; 7, latissimus dorsi; 8, 8′, long and lateral heads of triceps; 9, pectoralis profundus (ascendens); 10, accessory axillary lymph node.
The omotransversarius (Figure 2–55/3) is a narrow muscle that extends between the transverse processes of the atlas (and possibly also the succeeding vertebrae) and the acromion and adjacent part of the scapula. It assists in advancement of the limb.
The brachiocephalicus (Figure 2–55/2,2′) is more complex, being formed by the union of two elements that are separated by the clavicle in less specialized mammals. In these the caudal part (cleidobrachialis) passes between the clavicle and the humerus and is a component of the deltoideus muscle. The cranial part passes cranially from the clavicle to several attachments in the head and neck. These attachments vary among species and hence a rather bewildering array of names for particular units exists: cleidooccipitalis, cleidomastoideus, and so forth. In domestic species the two parts join in tandem, and the clavicle is generally reduced to a fibrous intersection in the combined muscle at the level of the shoulder joint, although vestigial ossifications are present in the dog and cat. Brachiocephalicus is a most appropriate name for the whole complex since it does not specify precise attachments. The brachiocephalicus advances the limb, possibly also extending the shoulder joint, when the cranial attachment is fixed and the limb is free to move; in contrast, when the limb is fixed and the head is free, it draws the head and neck ventrally when acting bilaterally and toward the side when acting unilaterally.
The muscles supplied by the accessory nerve split from a single primordium in the embryo. However, the caudal part of the brachiocephalicus of deltoid origin retains the appropriate innervation by the axillary nerve.
The latissimus dorsi (Figure 2–55/7) has a very broad origin from the thoracolumbar fascia and converges to an insertion on the teres tuberosity of the humerus. The most cranial fibers, which are also the most vertical, cover the caudal angle of the scapula and strap it against the chest. The muscle retracts the free limb and may also flex the shoulder joint. On the other hand, when the limb is advanced and the foot firmly planted on the ground, the latissimus may draw the trunk forward. It may be regarded as antagonist to the brachiocephalicus. It is supplied by a local branch (thoracodorsal nerve) of the brachial plexus.
Two superficial pectoral muscles (Figure 2–41/5,6) arise, one behind the other, from the cranial part of the sternum. The cranial muscle (pectoralis descendens) terminates on the crest of the humerus, distal to the deltoid tuberosity. The caudal muscle (pectoralis transversus) descends over the medial aspect of the arm and in the larger species continues distally over the elbow joint, covering the median artery and nerve, to insert into the medial fascia of the forearm. Both muscles adduct the limb, which is an action that may be understood to embrace the sideways shift of the trunk toward a previously abducted limb. It seems probable that they may also assist protraction or retraction, depending on the initial position of the limb relative to the trunk. They are supplied by local branches (cranial pectoral nerves) from the brachial plexus.
The Deep Layer
This comprises the rhomboideus dorsally, the serratus ventralis medially, and the pectoralis profundus ventrally.
The rhomboideus (Figure 2–54/8) takes origin from median connective tissue structures extending from the poll to the withers and lies deep to the trapezius. It always presents cervical and thoracic parts and in carnivores has an additional, capital, part. All attach to the dorsal border and adjacent area on the medial surface of the scapula. Although the fiber courses differ in their relation to the axis of rotation of the scapula, most seem able to draw the dorsal part of the bone cranially, thereby retracting the limb. The muscle may also raise the limb and hold it firmly against the trunk. It is supplied from the brachial plexus in the dog, but in some species it is also supplied by dorsal branches of local spinal nerves, which is unusual for a limb muscle.
The serratus ventralis (Figure 2–54/6) is a large fan-shaped muscle that takes an extensive origin by separate digitations from the fourth cervical vertebra to the tenth rib. The fibers run dorsally to terminate on a well-defined area on the medial aspect of the scapula and scapular cartilage. The direction of the fibers indicates that this muscle must play a large part in supporting the weight of the trunk, and in the larger species it is better adapted to this function by the presence of a strong fascial covering and intersections. The cervical portion of the muscle, which inserts craniodorsal to the axis of scapular rotation, can retract the limb; the caudal portion, which inserts caudodorsal to the axis, can advance the limb. When acting unilaterally, the cervical fibers may also draw the neck to that side; when acting bilaterally, they raise the neck. The thoracic part is a potential inspiratory muscle, although it is not normally used in that capacity. The innervation is mainly by a branch (long thoracic nerve) of the brachial plexus.
The pectoralis profundus (Figure 2–55/9) may be considered as having cranial and caudal parts. The cranial part, well formed only in the horse and pig, probably corresponds to the subclavius of other mammals and is now so named officially. Both parts (or muscles) arise from the ventral aspect of the length of the sternum and adjacent cartilages, and the most caudal fibers extend beyond this onto the abdominal floor. In the horse and pig the subclavius passes dorsally along the leading edge of the scapula, attaching to the supraspinatus (see Figure 23–5, A/2). The larger caudal part, also known as the pectoralis ascendens, inserts on the lesser tubercle of the humerus. Both play a role, secondary to that of the serratus ventralis, in slinging the trunk between the forelimbs. They may also act as retractors of the forelimb when this is free. When the limb is advanced and fixed, they draw the trunk forward, toward the limb. The nerves are local branches (caudal pectoral nerves) of the brachial plexus.
Intrinsic Muscles of the Forelimb
The intrinsic muscles are conveniently grouped by their common location, actions, and innervations.
Muscles Acting Primarily on the Shoulder Joint
The muscles acting on the shoulder joint are arranged in lateral, medial, and caudal groups.
The lateral group comprises the supraspinatus and infraspinatus, which arise from and fill the corresponding fossae of the scapula. The supraspinatus (Figure 2–56/3) terminates on the summits of both tubercles of the humerus. The infraspinatus inserts by a tendon that splits into a shorter deep part, which attaches to the summit, and a longer superficial part, which attaches to the lateral face of the (caudal part of the) greater tubercle; a bursa between the bone and the longer tendon may be the seat of a painful inflammation. Both muscles brace the joint laterally. The supraspinatus tendon passes cranial to the axis of rotation, and it may therefore also extend the shoulder. It is sometimes asserted that the infraspinatus tendon passes cranial or caudal to the axis of rotation depending on the actual position of the joint and may then further extend the already extended joint or further flex the already flexed joint; clearly, it is unlikely to be very effective in either role. Both muscles are supplied by the suprascapular nerve from the brachial plexus.
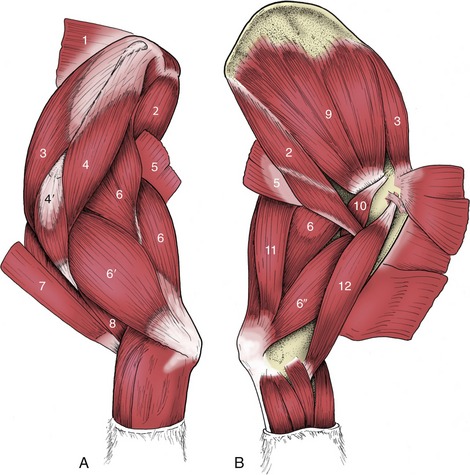
Figure 2–56 Intrinsic muscles of the left shoulder and arm of the dog, lateral (A) and medial (B) views. 1, Rhomboideus; 2, teres major; 3, supraspinatus; 4, 4′, scapular and acromial parts of deltoideus; 5, latissimus dorsi; 6, 6′,6″, long, lateral, and medial heads of triceps; 7, brachiocephalicus; 8, brachialis; 9, subscapularis; 10, coracobrachialis; 11, tensor fasciae antebrachii; 12, biceps.
The medial group comprises the subscapularis and coracobrachialis. The subscapularis (Figure 2–56/9) arises over much of the deep surface of the scapula and inserts on the medial tubercle of the humerus, distal to the axis of the shoulder joint. It braces the medial aspect of the joint. It is also a potential adductor of the arm and, like the infraspinatus, has an equivocal relationship to flexion and extension of the shoulder. It is supplied by the subscapular nerve from the brachial plexus. The coracobrachialis (Figure 2–56/10) extends between the medial aspect of the supraglenoid tubercle and the proximal part of the shaft of the humerus. Too small to be of real significance, it is a fixator of the shoulder with the same equivocal relationship to shoulder flexion and extension. It is supplied by the proximal branch of the musculocutaneous nerve from the brachial plexus.
The caudal or flexor group comprises the deltoideus, teres major, and teres minor. The deltoideus has one head of origin in the horse and two in species possessing an acromion (Figure 2–56/4,4′). The constant head arises from the caudal border and spine of the scapula; the inconstant second head arises from the acromion. Both insert on the deltoid tuberosity of the humerus. The teres major (Figure 2–56/2) arises from the dorsal part of the caudal margin of the scapula and terminates on the teres tuberosity, midway down the humerus. The relatively insignificant teres minor lies over the caudolateral aspect of the joint between the deltoideus and infraspinatus. These three muscles are clearly primarily flexor; the deltoideus may also be an abductor and an outward rotator of the arm. The group is supplied by the axillary nerve from the brachial plexus.
In contrast to the well-defined group of flexors, it seems that no muscles are clearly established as primarily extensors of the shoulder. The potential candidates, brachiocephalicus, biceps brachii, supraspinatus, and pectoralis ascendens, have other, apparently more important roles.
Muscles Acting Primarily on the Elbow Joint
There are extensor and flexor groups. The extensor group, which largely fills the angle between the scapula and humerus, consists of the triceps brachii, tensor fasciae antebrachii, and anconeus. The large and powerful triceps brachii (Figure 2–56/6,6′,6″) possesses three heads of origin (four in the dog). The long head, which arises from the caudal margin of the scapula, is potentially also a flexor of the shoulder. The lateral, medial, and (in the dog) accessory head(s) arise from the shaft of the humerus and have an action restricted to the elbow. The several heads combine to make a stout tendon that inserts on the summit of the olecranon, where it is protected on its deep aspect—against the bone—by the tricipital bursa. A second, subcutaneous bursa often lies between the tendon and the skin.
The tensor fasciae antebrachii (Figure 2–56/11) is a thin sheet, partly muscular, partly aponeurotic, that lies over the medial aspect of the long head of the triceps, extending from the scapula to the olecranon. The anconeus is much smaller and arises from the distal part of the humerus to insert on the lateral part of the olecranon; it is directly related to the elbow joint capsule and may have the additional function of tensing this so that it is not pinched between the humerus and ulna. All parts of the extensor group are supplied by the radial nerve from the brachial plexus.
The flexor group comprises the biceps brachii and brachialis. The biarticular biceps brachii (Figure 2–56/12) arises from the supraglenoid tubercle of the scapula and runs through the intertubercular groove of the humerus before continuing distally to insert on the medial tuberosity of the proximal extremity of the radius and on the adjacent part of the ulna. It is thus also a potential extensor of the shoulder. The brachialis (Figure 2–56/8) arises from the proximocaudal part of the humerus and winds laterally in the spiral groove of this bone before inserting next to the biceps. Both are supplied by the musculocutaneous nerve.
Pronator and Supinator Muscles of the Forearm
Generalized mammals possess muscles that have supination or pronation as a prime function, but these muscles tend to become vestigial or to disappear when the capacity for the movements is reduced or lost. Among domestic species significant movement is possible only in the dog and cat in which there are two supinator muscles and two pronators. The brachioradialis or long supinator is a thin fleshy ribbon that extends from the lateral epicondyle of the humerus to the distal medial part of the forearm within the superficial fascia. It is quite prominent in the cat but is slight, often lost, in the dog. The short supinator muscle is more consistently developed. It is a small fusiform muscle, placed deep to the extensor muscles and passing obliquely over the flexor aspect of the elbow from the lateral humeral epicondyle to the upper quarter of the medial border of the radius. The supinator muscles are supplied by the radial nerve.
The pronator teres (Figure 2–57/12) arises from the medial epicondyle of the humerus and converges on the insertion of the supinator on the radius. It is functional only in the dog and cat. The pronator quadratus is found only in carnivores. It passes from the shaft of the ulna to that of the radius, bridging the medial aspect of the interosseous space of the forearm. The pronator muscles are supplied by the median nerve.

Figure 2–57 Muscles of the left forearm of the dog, lateral (A) and medial (B) views. 1, Extensor carpi radialis; 2, common digital extensor; 3, lateral digital extensor; 4, ulnaris lateralis; 5, flexor carpi ulnaris; 6, extensor carpi obliquus; 7, extensor retinaculum; 8, carpal pad; 9, biceps; 10, superficial digital flexor; 11, flexor carpi radialis; 12, pronator teres; 13, radius; 14, deep digital flexor; 15, flexor retinaculum.
The rotation from the neutral position that may be produced by these muscles is most free when the elbow is flexed. The movements are limited to about 40° of pronation and about 45° of supination in the dog, although the cat has a somewhat larger range.
Muscles Acting Primarily on the Carpal and Digital Joints
These are simply classified as flexor or extensor, although the action of one muscle is equivocal.
The Extensor Muscles of the Carpus and Digits
These include digital extensor muscles in addition to those whose action is confined to the carpus. They have the following features in common: an extensor action at the carpus, a craniolateral position in the forearm, a radial nerve supply, and, with one exception, an origin from the cranial aspect of the lateral epicondyle of the humerus. The extensor carpi radialis (Figure 2–57/1), the most medial member of the group, is situated directly cranial to the subcutaneous border of the radius. It inserts on the proximal extremity of the third (sometimes also second) metacarpal bone. The ulnaris lateralis (Figure 2–57/4) [extensor carpi ulnaris] is the most lateral member and runs parallel to the ulnar flexor of the carpus on the outer aspect of the limb to insert on the accessory carpal and the upper end of the most lateral metacarpal bone. It may extend an already extended carpus but further flexes the joint that is in a flexed position. It may also deviate the paw laterally. Despite its equivocal character the ulnaris lateralis retains the extensor nerve supply. The extensor carpi obliquus (Figure 2–57/6) (also known as the abductor pollicis longus) is distinguished by its origin from the cranial surface of the radius and by the oblique mediodistal course pursued by its tendon, which attaches to the most medial metacarpal bone present. It functions as an extensor of the carpus with a potential, in the dog and the cat, for medial deviation of the paw.
The long digital extensor muscles vary in arrangement because, although all species possess a common and a lateral muscle, the common one may be subdivided. The common digital extensor (Figure 2–57/2) inserts on the extensor process of the distal phalanx of each functional digit: the tendon is therefore unbranched in the horse; divides into two in the ruminants; divides into four in the pig and dog; and into five in the cat. A subdivision of the common extensor, which is present in all species but the horse and cat, inserts on the most medial of the functional digits; it sends an oblique branch to the dewclaw in the dog. It is sometimes usefully termed medial digital extensor, but this term is not official. The lateral digital extensor (Figure 2–57/3) runs along the lateral edge of the common extensor; the undivided tendon inserts on the dorsal surface of the proximal phalanx in the horse. The muscle also has one insertion tendon in the ruminants, two in the pig, three in the dog, and four in the cat; in these species the insertion is in common with the branch of the common extensor to the distal phalanx of the most lateral one, two, three, or four functional digits. In the smaller species, separation of the digital divisions begins more proximally and is more complete.
The Flexor Muscles of the Carpus and Digits
The carpal flexor group includes digital flexor muscles in addition to muscles that act only at the carpus. They have certain common features: a flexor action at the carpus; a caudal position in the forearm; an origin, in part at least, from the caudal aspect of the medial epicondyle of the humerus; and an innervation from the median or ulnar nerve, or from both these nerves. Some have additional, even principal, origins in the forearm and also act on the digital joints. The flexor carpi radialis (Figure 2–57/11) is most medial and runs directly caudal to the subcutaneous border of the radius. It ends on the upper end of the second (sometimes third) metacarpal bone. The flexor carpi ulnaris (Figure 2–57/5) is lateral and ends on the accessory carpal bone. Both muscles are solely carpal flexors.
The superficial digital flexor (Figure 2–57/10) lies in the caudomedial part of the forearm and is not enclosed in a synovial sheath where it passes the carpus; later it divides into a branch for each functional digit that inserts in the region of the proximal interphalangeal joint. To reach these positions the branches of the tendon must first change position with those of the deep flexor that continue to more distal terminations. In principle (although the details vary), each branch of the superficial flexor tendon splits into two slips that diverge to the sides of the deep tendon, which then passes through the resulting arch. The deep digital flexor (Figure 2–57/14) lies more deeply in the forearm and passes the carpus through the carpal canal before dividing into one to four digital branches; each perforates the corresponding branch of the superficial flexor tendon and then continues to its insertion on the palmar aspect of a distal phalanx.
Short Digital Muscles
Interosseous muscles support the metacarpophalangeal joints. They show marked species differences in number, structure (they are largely tendinous in the large species), and function. They arise from the palmar aspect of the proximal ends of the metacarpal bones and find initial insertion on the sesamoid bones at the metacarpophalangeal joints; from here they are continued by distal sesamoidean ligaments that attach to the phalanges and by extensor branches that wind around to the dorsal aspect of the digit to join the extensor tendons. They are considered in detail later for the species in which they are important.
In the carnivores and pig a number of small digital muscles assist in the extension, flexion, abduction, or adduction of the abaxial digits—one, two, and five in the dog and the cat and two and five in the pig. It is unnecessary to describe them.
THE SKELETON OF THE HINDLIMB
Pelvic Girdle
The pelvic girdle has been described with the trunk (p. 43) for the reason previously given.
Skeleton of the Free Appendage
The femur (os femoris; Figure 2–58), the skeleton of the thigh, is the strongest of the long bones. The proximal end curves medially so that the proximal articular surface, the head, is offset to the long axis of the shaft. The femoral head is hemispherical and is joined to the shaft by a neck, which is best defined in the smaller species. The articular surface is interrupted by a nonarticular area (fovea) to which the intracapsular ligament(s) attach(es); the fovea is round and central in the dog, and wedge-shaped and extended to the medial periphery in the horse. A large process, the greater trochanter (Figure 2–58/3), is placed lateral to the head; it rises level with the head in small animals but projects high above it in larger species (Figure 2-58/3′,3″); it gives attachment to the bulk of the gluteal muscles, providing these extensors of the hip with a long lever arm. A plate of bone between the trochanter and the femoral neck helps bound the trochanteric fossa (Figure 2–58/5), an excavation that is open caudally, and the site of insertion of the small rotator muscles of the hip.
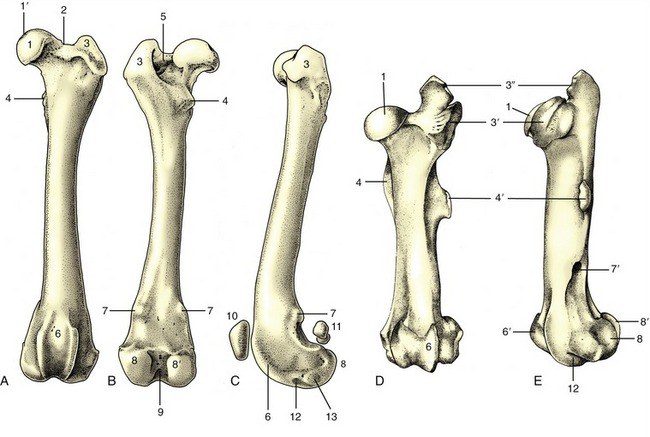
Figure 2–58 Left femur of the dog, cranial (A), caudal (B), and lateral (C) views. Cranial (D) and lateral (E) views of left equine femur. 1, Head; 1′, fovea; 2, neck; 3, greater trochanter; 3′,3″, cranial and caudal parts of greater trochanter; 4, lesser trochanter; 4′, third trochanter; 5, trochanteric fossa; 6, trochlea; 6′, enlarged proximal end of medial trochlear ridge; 7, supracondylar tuberosities; 7′, supracondylar fossa; 8, 8′, lateral and medial condyles; 9, intercondylar fossa; 10, patella; 11, sesamoid bones (in gastrocnemius); 12, extensor fossa; 13, fossa for popliteus.
The caudal aspect of the shaft is flattened, but the other aspects combine in a continuous smooth surface. The borders between the flat and rounded areas are emphasized by rough lines indicating muscular attachment. Two processes mark the proximal half of the shaft. A low and rough lesser trochanter (Figure 2–58/4) projects from the medial border and gives insertion to the iliopsoas muscle. An inconspicuous ridge at the base of the greater trochanter is known as the third trochanter (trochanter tertius; Figure 2–58/4′). It is salient only in the horse and gives attachment to the gluteus superficialis. In the large animals the caudodistal part of the shaft exhibits a deep supracondylar fossa that increases the area of origin of the superficial digital flexor (Figure 2–58/7′). The same function is fulfilled by tuberosities in the dog.
The distal extremity articulates with the tibia and the patella. The articulation with the tibia is accomplished by two condyles directed caudodistally and separated by a deep intercondylar fossa. The abaxial surfaces of the condyles are roughened and give attachment to the collateral ligaments of the stifle. The lateral condyle also carries two depressions close to the articular margin: the cranial one, the extensor fossa (Figure 2–58/12), gives origin to the long digital extensor and peroneus tertius muscles; the caudal one (Figure 2–58/13) gives origin to the popliteus. In the dog and cat the caudal aspect of each condyle is surmounted by a small flat facet for articulation with one of the small sesamoid bones (Figure 2–58/11; formerly fabellae) in the origin of the gastrocnemius (see Figure 17–3). A cranial trochlea (Figure 2–58/6) articulates with the patella and extends proximally on the cranial surface. The bounding ridges are low and more or less equal in size in the dog and relatively larger and disparate in the horse and in cattle, in which the stouter medial ridge ends in a proximal enlargement (Figure 2–58/6′).
The patella, the kneecap, is a sesamoid developed within the insertion of the quadriceps femoris, the main extensor of the stifle. It is ovoid in the dog but prismatic in the horse and in cattle. It is extended medially and laterally by parapatellar cartilages in the fresh state.
The skeleton of the leg consists of the tibia and fibula (Figure 2–59), which, unlike the analogous elements of the forelimb, run side by side without any tendency to cross. The medial bone, the tibia, is always by far the larger of the two. The fibula is excluded from articulation with the femur and has only restricted contact with the hock skeleton.

Figure 2–59 Left tibia and fibula of the dog, lateral (A), cranial (B), and caudal (C) views. Cranial (D) and lateral (E) views of left equine tibia and fibula. 1, Tibial tuberosity; 2, 2′, lateral and medial condyles; 3, extensor groove; 4, intercondylar eminence; 5, fibula; 6, 6′, medial and lateral malleoli; 6″, lateral malleolus in the horse (representing distal end of fibula); 7, cochlea.
The expanded proximal extremity of the tibia presents two condyles divided by a caudal popliteal notch that accommodates the like-named muscle. Each condyle has a gently undulating articular surface facing the corresponding condyle of the femur; a narrow intermediate nonarticular area carries a central eminence (Figure 2–59/4) onto which the articular surfaces slope. A depression of the eminence and less defined areas cranial and caudal to it indicate ligamentous attachments. The very robust tibial tuberosity (Figure 2–59/1) projecting from the cranial aspect of this extremity is a prominent landmark in life; it is continued by a gradually subsiding crest. A groove (Figure 2–59/3) lodging the tendons of certain muscles of the leg (crus) separates the tuberosity from the cranial aspect of the lateral condyle. Caudal to this, the edge of the condyle carries a small facet for articulation with the fibula, although in some species the joint space is obliterated by fusion.
The proximal part of the tibial shaft is three-sided, but more distally the bone is craniocaudally compressed; the change is brought about by the smooth surface that faces craniolaterally in its proximal part but then twists to face directly forward. The entire medial surface (border distally) is subcutaneous and flat. The caudal surface is ridged for muscular attachment.
The distal extremity carries an articular area known as the cochlea (Figure 2–59/7), which is shaped to receive the trochlea of the talus. The central ridge and the flanking grooves of the cochlea have a craniolateral deflection, although the angle varies among species. A bony salience, the medial malleolus (Figure 2–59/6), is present to the medial side of the cochlea. A similar lateral swelling is found only in the horse and represents the assimilated distal part of the fibula (Figure 2–59/6″). In other species the corresponding feature (lateral malleolus) is provided by the fibula.
In carnivores and the pig the fibula is reduced in robustness but not in length. It is separated from the tibia by an interosseous space that runs the whole length of the leg in the pig but is limited to the proximal half in the dog. The shaft of the fibula regresses in ruminants: the proximal extremity persists as a tear-shaped process fused to the lateral condyle of the tibia; the distal extremity is isolated as a small compact malleolar bone that forms an interlocking joint with the tibia, completing the articular surface for the talus. The flattened proximal head of the fibula of the horse is closely applied to the tibia, and the slender shaft that leads from it converges on the tibia but fades toward the middle of the leg.
The tarsal bones are arranged in three tiers. The proximal tier consists of two relatively large bones: the talus medially and the calcaneus laterally. The middle tier comprises only a single central tarsal bone, but the distal tier comprises up to four bones, which are numbered in mediolateral sequence. The lateral fourth tarsal bone is constantly present and, being much deeper than the others, intrudes into the middle tier (Figure 2–60).
Figure 2–60 The bones of the tarsal skeleton in the carnivores (Car), horse (eq), cattle (bo), and pig (su), schematic. Roman numerals identify the metatarsal bones, Arabic numerals the distal tarsal bones. Tib., Tibia; F, fibula; T, talus; C, calcaneus; c, central tarsal bone.
The talus (Figure 2–61) has a proximal trochlear surface shaped to fit the tibia. The distal surface, which articulates with the central bone, is flattened in the horse and more rounded in other species. The calcaneus lies mainly lateral to the talus but extends a shelflike process that overlaps the talus on its plantar surface; the process (sustentaculum tali; Figure 2–61/3′) supports the deep digital flexor tendon. The larger part of the bone projects proximally behind the tibia as a free lever arm to which the common calcanean tendon attaches. It ends in a thickening that is the basis of the point of the hock (Figure 2–61/3″) and corresponds to the human heel. The distal extremity of the calcaneus rests on the fourth tarsal bone (Figure 2–61/6). The central tarsal bone is interposed between the talus proximally and the first, second, and third tarsal bones distally; its proximal surface conforms to the talus and is concave in most animals but flat in the horse. Its distal articular surface is flattened. The central and fourth tarsal bones fuse in ruminants.

Figure 2–61 Skeleton of right pes of the dog, lateral (A) and dorsal (B) views. Dorsal (C) view of left equine tarsus. Roman numerals identify the metatarsal bones. 1, Tibia; 2, fibula; 2′, lateral malleolus; 3, calcaneus; 3′, sustentaculum tali; 3″, calcanean tuber (point of hock); 4, talus; 5, central tarsal; 6, fourth tarsal; 7, first, second, and third tarsal bones in distal row; 7′, third tarsal in the horse; 8, proximal sesamoid bones; 9, dorsal sesamoid bones; 10, 11, 12, proximal, middle, and distal phalanges; 12′, claw.
The distal tarsal bones are not always separate: the first and second are fused in the horse, and the second and third are fused in ruminants. Individually irregular, these bones together form a more or less flattened disk interposed between the central tarsal and the metatarsal bones. The cuboidal fourth tarsal bone is interposed between the calcaneus and the lateral metatarsal bones; in some species it also gives support to the talus.
The remaining bones of the hindlimb closely resemble those of the forelimb. The metatarsal bones are longer (by about 20%) than the metacarpals and are more rounded in cross section. The first metatarsal is rudimentary in the dog, in which only a few breeds consistently possess a dewclaw in the hindlimb.
THE JOINTS OF THE HINDLIMB
The hip joint (Figure 2–62) is a spheroidal joint formed between the lunate surface of the acetabulum and the head of the femur. The acetabular surface is enlarged by an articular labrum (Figure 2–62/2′) continuous with the transverse acetabular ligament (Figure 2–62/2″) that bridges the notch interrupting the medial wall of the socket. The walls of the articular cavity are completed by a synovial membrane supported externally by a fibrous covering. Although the fibrous capsule is not uniformly strong, there are no thickenings so definite that they need be recognized as specific ligaments. However, the head of the femur is joined to the depth of the acetabulum by the intracapsular ligament of the femoral head, which is covered by a reflection of the synovial membrane. In some species this ligament is known to convey blood vessels, but the importance of these to the nutrition of the head remains uncertain. In the horse a second (accessory) ligament inserts on the nonarticular area of the head (p. 624).

Figure 2–62 Schematic transverse section through the left hip joint of a dog. The femur has been drawn in relief. 1, Gluteus medius; 2, acetabulum, connected to the femoral head by the ligament of the head of the femur; 2′, fibrous rim (labrum) of acetabulum; 2″, transverse acetabular ligament; 3, femur; 4, biceps; 5, rectum; 6, vagina; 7, urethra; 8, obturator foramen; 9, pelvic floor.
Although a spheroidal joint, the hip does not enjoy the full range of movement expected of this class of joint. In the large animals movement is largely restricted to flexion and extension; the capacity for rotation, abduction, and, especially, adduction is limited. In conformity with the dominance of sagittal movement, the articular area tends to extend onto the neck in ruminants. The restriction on movement owes much to the intraarticular ligament(s) but something to the massive medial muscles of the thigh. The joint has a more versatile employment in the dog.
The stifle joint (Figure 2–63), corresponding to the human knee, comprises femorotibial, femoropatellar, and proximal tibiofibular joints; in the dog it also includes the joints between the femur and paired sesamoids in the origins of the gastrocnemius and that between the tibia and the sesamoid in the popliteus tendon. In the dog, all these articulations share a common synovial cavity; in the large species the femoropatellar and the medial and lateral femorotibial compartments have more restricted communication with each other.

Figure 2–63 Left stifle joint of the dog, cranial view (A-C). The extent of the joint capsule is shown in B. The patella has been removed in C. D shows the crossing of the cruciate ligaments in a medial view. E is a caudal view. 1, Femur; 2, sesamoids in gastrocnemius; 3, patella; 4, extensor groove; 5, tibial tuberosity; 6, fibula; 7, tibia; 8, patellar ligament; 9, tendon of long digital extensor passing through extensor groove; 10, medial meniscus; 11, medial collateral ligament; 12, lateral femoropatellar ligament; 13, lateral collateral ligament; 14, trochlea; 15, caudal cruciate ligament; 16, cranial cruciate ligament; 17, lateral meniscus; 18, stump of 9; 19, popliteus tendon; 20, meniscofemoral ligament.
The femorotibial joint is unusual in having two fibrocartilaginous menisci (Figure 2–63/10,17) interposed between the femoral and tibial condyles. The menisci, which compensate for the incongruence of the articular surfaces, are each semilunar in plan and wedge-shaped in section and have concave proximal and flattened distal surfaces. Each is secured by ligaments that extend between its cranial and caudal extremities and the central nonarticular area of the proximal extremity of the tibia; the lateral meniscus is also attached caudally to the intercondylar fossa of the femur.
Four ligaments join the femur to the bones of the leg. A medial collateral ligament passes between the femoral epicondyle and the proximal part of the tibia, toward the caudal part of the joint. The corresponding lateral ligament has a similar disposition but attaches to the fibular head. The cruciate ligaments are centrally placed. The cranial (lateral) cruciate ligament (Figure 2–63/16) arises from the lateral condyle of the femur within the intercondylar fossa and runs craniodistally to attach on the tibia. The caudal (medial) cruciate ligament (Figure 2–63/15) runs at right angles to the cranial one and attaches far back on the tibia near the popliteal notch.
The femoropatellar joint is formed between the femoral trochlea and the patella and is extended by its parapatellar cartilages, of which the medial one is especially well developed in the large animals. Relatively weak collateral femoropatellar ligaments (Figure 2–63/12) run between the cartilages and the femur. Distally the patella is joined to the tibial tuberosity by a single patellar ligament, except in the horse and ox, in which three ligamentous thickenings are present—medial, intermediate, and lateral—connected by a fibrous sheet (see Figure 24–4). The middle (or single) patellar ligament represents the insertion tendon of the quadriceps femoris; the others, when present, represent the continuation of other muscles inserting about the joint.
The synovial membrane attaches around the peripheries of the articular surfaces and the menisci. It covers the cruciate ligaments and here forms a partition, complete only in the horse, between the medial and lateral femorotibial joints. The femoropatellar portion of the cavity extends proximally between the femur and the quadriceps. In the horse it generally communicates only with the medial femorotibial compartment, but in other species it has free communication with both. Diverticula of the capsule embrace the lesser joints with the fibula and the sesamoid bones and extend along the tendons of origin of the long digital extensor and popliteus muscles.
Despite its complexity, the stifle functions as a hinge joint whose free movement is restricted to flexion and extension. The femoral condyles roll on the menisci, and these in turn slide over the tibial plateau—cranially on extension, caudally on flexion. The travel between the femur and menisci is about three times that between the menisci and the tibia. The spiral configuration of the femoral condyles, when viewed from the side, tightens the ligaments and slows the movement when the joint moves toward the extended position. The stability of the articulation depends much on the cruciate ligaments. Rupture of one of these, which is not an uncommon misfortune, allows the tibia unusual mobility; it may slip forward when the cranial ligament is torn and backward when the caudal ligament is torn. Rotation imposed on the joint, particularly when the joint is extended, places great strain on the menisci and their attachments.
The tarsal joint of quadrupeds is usually known as the hock. It possesses four levels of articulation, but in most species almost all movement occurs at the crurotarsal level. This is a hinge joint but not a typical one as the obliquity of the interlocking ridges and grooves of the tibia and talus imposes a lateral deviation of the foot when it is carried forward on flexion. In ruminants and carnivores, limited flexion is also possible at the curved surfaces of the talocentral joint.
The ligaments are numerous. Those most important are the medial and lateral collateral ligaments, which extend, with intermediate attachments, from the tibia (and fibula) to the proximal extremity of the metatarsus. Each comprises a long superficial part of full extent and a shorter deeper part restricted to the proximal level of articulation. Another long ligament is found caudally, extending from the plantar surface of the calcaneus over the fourth tarsal bone to the metatarsus. The remaining smaller ligaments firmly hold the tarsal bones together.
There are several compartments to the joint. That between the tibia and talus is most capacious and may possess a number of local pouches, as the less supported parts of joint capsules are known. The other synovial sacs are much tighter and often communicate. The details are most important in the horse (p. 631).
The remaining joints of the hindlimb are considered in the regional chapters, insofar as they require to be differentiated from the corresponding forelimb joints.
THE MUSCLES OF THE HINDLIMB
The girdle musculature has been described (p. 55).
The Intrinsic Muscles of the Hindlimb
Muscles Acting Primarily on the Hip Joint
The muscles acting at the hip are arranged in gluteal, medial, deep, and caudal (hamstring) groups; it is a classification based primarily on topography.
The gluteal group comprises superficial, middle, and deep gluteal muscles and the tensor fasciae latae. The gluteus superficialis varies greatly. In the dog it is a relatively narrow muscle that covers the caudal part of the gluteus medius, extending from the gluteal and caudal fascia to the third trochanter of the femur (Figure 2–64/4). In ungulates a part becomes incorporated within the biceps femoris, and sometimes also the semitendinosus, supplying these with vertebral heads of origin. It is an extensor of the hip and therefore a retractor of the limb. It is supplied by the caudal gluteal nerve.

Figure 2–64 Muscles of the canine hindquarter and thigh, lateral (A) and medial (B) views. 1, Sartorius; 2, tensor fasciae latae; 3, gluteus medius; 4, gluteus superficialis; 5, biceps; 6, semimembranosus; 7, semitendinosus; 8, pelvic symphysis; 9, internal obturator; 10, levator ani; 11, rectus abdominis; 12, quadriceps; 13, pectineus; 14, adductor; 15, gracilis.
The gluteus medius (Figure 2–64/3) is by far the largest of the group. It arises from the outer surface of the ilium and the gluteal fascia and inserts on the greater trochanter. It is an exceptionally powerful extensor of the hip with some abduction potential. A deeper subdivision is known as gluteus accessorius. Neither it nor the small, more caudal piriformis need be considered separately; their actions are similar to those of the main mass. The muscle is principally supplied by the cranial gluteal nerve.
The much smaller gluteus profundus is completely covered by the gluteus medius. It arises from the ischial spine and adjacent region of the os coxae and inserts on the cranial part of the greater trochanter. It may also extend the hip, but because most fibers run more or less transversely, it is more advantageously placed to abduct the limb. It is also supplied by the cranial gluteal nerve.
The tensor fasciae latae (Figure 2–64/2) is the most cranial muscle of the group. It arises from the coxal tuber (or equivalent) and from the adjacent part of the ilium and extends down the cranial border of the thigh before inserting into the heavy lateral femoral fascia, which serves as its insertion tendon and provides it with attachment to the patella and other structures of the stifle region. Supplied by the cranial gluteal nerve, it is primarily a flexor of the hip. In the horse its most caudal part extends toward and fuses with a cranial slip of the gluteus superficialis.
The medial group is principally employed to adduct the hindlimb, adduction is, of course, a term that also embraces the prevention of unwanted abduction. Most muscles of this group are supplied by the obturator nerve and these—gracilis, pectineus, adductor, and external obturator—are sometimes specifically termed the adductors. The sartorius has a rather different origin and relationship.
The gracilis, a broad but thin muscle, takes an aponeurotic origin from the symphysial region of the pelvis (Figure 2–64/15). Its insertion, also aponeurotic, merges with the crural fascia through which it finds attachment to the tibial crest and other medial structures of the stifle region.
The pectineus is a small fusiform muscle, which in the dog forms a prominent surface feature of the proximal part of the thigh (Figure 2–64/13). It arises from the cranial branch of the pubis and from the prepubic tendon and inserts on the proximal part of the medial “rough line” of the femur. In the larger species, but not in the dog, a considerable part of the tendon of origin decussates with its fellow within the prepubic tendon.
The adductor is often divided into several individually named parts, but these distinctions are unnecessary. The muscle arises over an extensive area of the ventral aspect of the pelvic floor and inserts along the distal two thirds of the medial “rough line” of the femur and to the fascia and ligaments of the medial aspect of the stifle (Figure 2–64/14).
The obturator externus is conveniently included here, although it has obvious affinities with the following deep group. It arises from the ventral surface of the pelvic floor, over and around the obturator foramen, and inserts within the ventral part of the trochanteric fossa. In addition to being an adductor, it is potentially an outward rotator of the thigh.
The sartorius is set apart from the other medial muscles by its innervation from the saphenous branch of the femoral nerve. It is superficial and follows the craniomedial aspect of the thigh; in the dog it consists of two parallel bellies, one of which forms the cranial contour of the thigh (Figure 2–64/1). Except in the horse (in which it arises from the iliac fascia on the abdominal roof), it arises from the iliac crest and its insertion is to the medial structures of the stifle region. Flexion of the hip is probably its main action, but it has some capacity for adduction of the thigh and extension of the stifle. The superficial space between the caudal margin of the sartorius and the pectineus is often designated the femoral canal.
The deep muscles of the hip form a rather heterogeneous community of small and essentially trivial muscles: the obturator internus, gemelli, quadratus femoris, and articularis coxae. Most are supplied by the sciatic nerve.
The obturator internus (Figure 2–64/9) is a thin muscle that arises from the dorsal surface of the hip bone in the vicinity of the obturator foramen; in carnivores and in the horse its tendon leaves the pelvis by passing over the ischium, caudal to the acetabulum, to end in the trochanteric fossa. In other species the tendon passes through the obturator foramen; in this arrangement, the muscle may have its origin as a detachment from the external obturator. The muscle is an external rotator of the thigh.
The gemelli are two small “twin” bundles that pass from the ischial spine to the trochanteric fossa. They are also external rotators.
The quadratus femoris passes from the ventral aspect of the ischium to end on the femoral shaft close to the trochanteric fossa. It is described as an extensor but can be of no significance in this role.
The articularis coxae lies on the capsule over the cranial aspect of the hip and protects this from being nipped between the femoral and acetabular surfaces.
The muscles of the caudal (hamstring) group—biceps femoris, semitendinosus, and semimembranosus—flesh the caudal part of the thigh. They extend from the ischial tuber and adjacent part of the sacrotuberous ligament to a broad insertion both proximal and distal to the joint space of the stifle; certain components continue within the common calcanean tendon to the calcaneus. In ungulates, one (or more) muscle is also extended proximally through the acquisition of an origin (vertebral head) from the sacrocaudal vertebrae. The vertebral heads are best developed in the horse and account for the full, rounded contour of the rump of this animal, which contrasts with the more angular appearance in the ox or dog. At least part of the vertebral extension is due to assimilation of a superficial gluteal component. The term gluteobiceps may be encountered for the combination.
The biceps femoris is most lateral (Figure 2–64/5). In the horse and in ruminants, but not in the dog, it has both vertebral and pelvic heads. In the lower part of the thigh the united muscle divides into insertions that attach, by way of the femoral and crural fascia, to the patella and ligaments of the stifle joint both proximal and distal to the joint space; an additional insertion to the point of the hock is achieved through a contribution (tarsal tendon) to the common calcanean tendon.
The semitendinosus (Figure 2–64/7) forms the caudal contour of the thigh. It has a vertebral head only in the horse and pig. The insertion is to the medial aspect of the proximal extremity of the tibia and to the calcaneus. The insertions of the biceps and semitendinosus, one to each side of the depression (popliteal fossa) behind the stifle, can be palpated in life—they are the “strings of the ham” that give the group its name.
The semimembranosus (Figure 2–64/6) is most medial and has a vertebral head only in the horse. The insertion is divided between a cranial part attaching to the medial femoral condyle and a caudal part attaching to the medial tibial condyle.
In the dog a ribbon-like abductor cruris caudalis lies on the deep face of the biceps and is probably derived from it. It has no great functional significance.
The vertebral heads of these muscles are generally supplied by the caudal gluteal nerve, and the pelvic heads are generally supplied by the sciatic nerve (or its tibial division).
Certain functions of these muscles are difficult to analyze, but their main role is undoubtedly the forceful extension of the hip joint that thrusts the trunk forward. In addition, the biceps has an abductor potential, and the semimembranosus an adductor potential, at the hip.
When consideration is given to muscle action on the stifle, it is probably more useful to divide the muscles into a cranial division inserting proximal to the joint axis and a caudal division inserting distal to this axis rather than to consider the named units. The cranial division extends the stifle when the foot is planted on the ground. The caudal division has the same action when the foot is fixed but flexes the joint when the foot is free to move. The parts of the biceps and semitendinosus that insert on the calcaneus can obviously extend the hock. It is clear that not all these effects can be accomplished simultaneously; apart from the potential antagonism of the cranial and caudal divisions at the stifle, it is unlikely that an animal would wish to flex the stifle while extending the hock. Indeed, in the horse in particular, this combination of actions is precluded by the reciprocal mechanism (p. 637). Different parts of these muscles must therefore be used at different times and in different combinations.
Muscles Acting Primarily on the Stifle Joint
There are extensor and flexor groups. The quadriceps femoris, the principal extensor of the stifle, forms the mass of muscle cranial to the femur (see Figure 17–2/9). It consists of four parts, separate at their origins but joined distally. One, the rectus femoris, arises from the shaft of the ilium immediately cranial to the acetabulum. The others, vastus medialis, intermedius, and lateralis, arise from the medial, cranial, and lateral aspects of the femoral shaft. The common insertion appears to be on the patella but is actually on the tibial tuberosity because the muscle is continued distal to the patella by the patellar ligament(s). The rectus femoris has the potential secondary action of flexion of the hip, although it is ill-placed for this purpose. The quadriceps is supplied by the femoral nerve.
The small popliteus muscle lies directly over the caudal aspect of the joint. It takes a tendinous and confined origin from the lateral condyle of the femur and fans out to a broad fleshy insertion on the proximal third of the caudal surface of the tibia (Figure 2–65/15). Its tendon of origin contains a sesamoid in the dog and cat. In addition to being a flexor of the stifle, the popliteus rotates the distal part of the limb. It is supplied by the tibial nerve.

Figure 2–65 Muscles of the left canine leg, lateral (A) and medial (B) views. 1, Biceps; 2, semitendinosus; 3, peroneal nerve; 4, gastrocnemius; 5, tibialis cranialis; 6, peroneus longus; 7, lateral deep digital flexor, 7′, tendon of the smaller medial deep digital flexor; 8, superficial digital flexor; 9, long digital extensor; 10, peroneus brevis; 11, extensor brevis; 12, tendon of lateral digital extensor; 13, interossei; 14, tibia; 15, popliteus.
Muscles Acting Primarily on the Tarsal and Digital Joints
These comprise extensors and flexors of the hock and extensors and flexors of the digits. They are grouped in two masses: one craniolateral to the tibia and the other caudal to the tibia.
Craniolateral Muscles of the Leg
The craniolateral group comprises muscles with an action confined to flexion of the hock and others that have this action but continue to extend the digits. This arrangement contrasts with that of the digital extensor muscles of the forelimb, which extend both carpal and distal joints. In addition to their position and action, the craniolateral crural muscles have their innervation in common—through the peroneal* nerve (Figure 2–65/3).
A full set of the muscles that are pure flexors of the hock is not found in any domestic species; it would comprise the tibialis cranialis, peroneus tertius, peroneus longus, and peroneus brevis. The dog and cat lack the peroneus tertius, and ungulates lack the peroneus brevis; the horse also lacks the peroneus longus and has its peroneus tertius reduced to a tendinous cord.
The tibialis cranialis, always substantial, lies immediately cranial to the subcutaneous medial surface of the tibia (Figure 2–65/5). It takes origin from the lateral condyle of the tibia and inserts on the mediodistal tarsal and adjacent metatarsal skeleton. It is a flexor of the hock with a secondary supinator role. The peroneus tertius is most important in the horse, in which it constitutes an essential component of the so-called reciprocal mechanism.
The weak peroneus longus arises from and around the distal part of the lateral collateral ligament of the stifle joint (Figure 2–65/6). It crosses the lateral aspect of the tarsus before turning medially, over the plantar aspect, to end on the proximal parts of the medial metatarsal bone. It is primarily a pronator of the foot but may also flex the hock. The peroneus brevis is of no practical importance.
The number and the arrangement of the extensor muscles of the digits are naturally correlated with the digital pattern. A long digital extensor muscle (Figure 2–65/9) arises from the distal extremity of the femur and follows the lateral border of the tibialis cranialis. Its tendon crosses the dorsal surface of the hock, where it is held down by retinacula; later it splits into branches, one for each functional digit. Each branch inserts on the extensor process of a distal phalanx. In the dog, the tendons develop small sesamoid bones similar to those of the forelimb.
A lateral digital extensor (Figure 2–65/12) arises from the head of the fibula, crosses the lateral aspect of the hock, and enters the most lateral functional digit, where it terminates either on the proximal phalanx (dog) or by joining the long extensor tendon (horse). In certain species, including the dog, a small discrete extensor hallucis longus is associated with the medial digit; it arises on the cranial border of the fibula and inserts on the proximal part of the digit.
Caudal Muscles of the Leg
The caudal group comprises the twin-bellied gastrocnemius, the soleus, and the superficial and deep digital flexors. All are supplied by the tibial nerve.
The gastrocnemius and the soleus, the latter insignificant except in the cat and absent in the dog, are sometimes collectively known as the triceps surae. The two heads of the gastrocnemius (Figure 2–65/4) spring from the caudal aspect of the femur proximal to the condyles; two sesamoid bones are included in the origins in carnivores. The heads combine in the upper part of the crus and give rise to a single stout tendon that inserts on the point of the hock. It is the principal component of the common calcanean (Achilles) tendon. Despite its inclusion among the extensors of the hock, the role of the gastrocnemius is enigmatic because its proximal attachment suggests that it is a potential flexor of the stifle; stifle and hock, however, normally move in unison. The apparent contradiction in these actions is not easily explained. It has been suggested that the prime function of the muscle is not to move either joint but to oppose bending of the tibia, ensuring that the strain is always directed along its long axis.
The superficial digital flexor (Figure 2–65/8) arises from a supracondylar fossa or tubercle on the caudal aspect of the femur, close to the origin of the gastrocnemius. It first runs deeply, between the two parts of the latter muscle; its tendon later winds around the medial border of the gastrocnemius tendon to gain the more superficial position. It forms a broad cap over the point of the hock, where part finds attachment through medial and lateral slips, before continuing over the plantar aspect of the calcaneus to enter the foot; it is then disposed like the corresponding tendon of the forelimb. The muscle is heavily infiltrated by connective tissue, especially in the horse, in which it becomes almost entirely tendinous and forms the caudal component of the reciprocal mechanism.
There are three deep digital flexor muscles whose independence varies among species. The three—lateral and medial flexors and the tibialis caudalis—lie close together on the caudal surface of the tibia (and fibula), from which they take origin (Figure 2–65/7). In the ungulates, the tendons of the lateral muscle and the tibialis caudalis unite above the tarsus and then run over the plantar aspect of the joint, medial to the calcaneus; this common tendon is then joined in the upper part of the metatarsus by that of the medial muscle, which descends over the medial malleolus. The combined deep flexor tendon ends as the corresponding tendon of the forelimb. In carnivores, only the lateral (Figure 2–65/7) and medial (Figure 2–65/7′) muscles unite. The rather small tibialis caudalis remains aloof and inserts separately on the hock; this truncated course transforms it into an extensor of the hock and supinator of the foot.
The most important short digital muscles are the interossei (Figure 2–65/13), which resemble those of the forelimb. A number of other small muscles that occur, especially in the dog, are of trivial significance.