23 The Forelimb of the Horse
In the Western world horses are now mainly bred for use in sport and recreation, pursuits that often make heavy demands on their speed and endurance and expose their limbs to continual strain and repeated risk of injury. Even relatively minor incapacity may unfit a horse for this work, and the importance of soundness of limb is crisply stated by the old adage “no foot, no horse.” Since lameness accounts for much of the work of equine practitioners, it follows that they have need of a more detailed knowledge of the anatomy of the limbs than is necessary for those who deal with other species.
The limbs of the horse display extreme adaptations for fast running with a concomitant loss of versatility. Although both forelimbs and hindlimbs find their main indeed almost exclusive employment in supporting the body when at rest and in driving it forward when in motion, they do manifest significant division of labor. It is the forelimbs that carry the greater part (some 55% to 60%) of the body weight at rest; they also supply the principal shock absorbers that are necessary in the faster gaits and especially when landing from a jump. The hindlimbs are less committed to these tasks and furnish the main propulsive thrust. However, this distribution of duties is not invariable; in particular, the share of the load that is supported by each limb may be altered by varying the posture to shift the center of gravity. The most obvious maneuver is to raise the head, thus shortening the lever arm of the neck and displacing the center caudally; the reciprocal movement brings the center of gravity cranially. These alterations in the carriage of the head may be pronounced in a lame animal, which lifts the head when a painful forelimb is placed on the ground and lowers it when the sound limb bears weight. Since it is the latter movement that usually strikes an observer with more force, a horse with forelimb lameness is said to “nod on the sound foot.” When there is a painful condition of a hindlimb, the head is lowered as the affected limb assumes support.
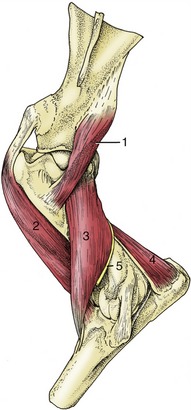
A forelimb with good conformation is straight when viewed from the front. A line dropped from the point of the shoulder bisects the limb and passes through the center of the hoof; the digit continues the cannon (metacarpus) in a straight line, neither “toeing-in” nor “toeing-out” (Figure 23–1). Much of the limb should also be straight when viewed from the side; a line dropped from the tuberosity of the scapular spine should bisect it to the fetlock and then pass just behind the hoof, whose slope should parallel that of the digit. Deviations from the normal conformation can result in abnormal movements, which in turn may cause interference between the feet, unequal and abnormal hoof wear, and development of lameness.
Figure 23–1 Desirable conformation and autonomous zones of cutaneous innervation of the forelimb. A, Cranial view; a vertical broken line dropped from the point of the shoulder bisects the limb. B, Right lateral view; a vertical line dropped from the tuberosity of the scapular spine bisects the limb down to the fetlock. The autonomous zones represent skin areas innervated solely by the nerves below.
1, Caudal cutaneous antebrachial nerve (ulnar); 2, medial cutaneous antebrachial nerve (musculocutaneous); 3, ulnar nerve; 4, median nerve; 5, chestnut; 6, ergot; 7, cephalic vein.
The more common deviations seen when viewing from the front are categorized as “base-wide,” in which the limbs slope laterally, and “base-narrow,” in which they slope medially. Deviations seen from the side include “standing under,” in which the limbs slope caudally, and “camped,” in which they slope cranially. Cranial, caudal, medial, and lateral deviations of the carpus are also recognized; the last two faults are “knock-knees” and “bowlegs.”
Retention of the full length of the shaft of the ulna is a congenital anomaly that is fairly common in Shetland ponies. It is associated with a valgus deformity*—sometimes very severe—of the limb.
The distinctive “leggy” appearance of the young foal must be familiar to every reader (Figure 23–2). The acquisition of the adult shape involves changes in the ratios of the lengths of the limbs (taken as a whole) to that of the trunk and in the ratios between the lengths of successive segments of the limbs—arm (thigh), forearm (leg), and metacarpus (metatarsus). According to one source, in the newborn Thoroughbred the ratio of the humerus (femur) to the metacarpus (metatarsus) is approximately 4 : 5 (4 : 5); in the adult the ratio is approximately 6 : 5 (6.5 : 5). These changes are achieved through a postnatal growth in length of the metacarpal (metatarsal) bones of about 20% and growth of the humerus and femur of about 100%.

Figure 23–2 This photograph of a 10-day-old foal with its dam illustrates the proportions of the limbs and trunk that account for the “leggy” appearance of the young foal.
1, Flaccid long and medial heads of triceps; 2, “poverty” line between biceps femoris and semitendinosus.
The cutaneous features known as chestnuts and ergots are described on page 362.
THE GIRDLE MUSCLES
The same muscles join the limb to the trunk as in other species, but there are certain differences in detail. The trapezius arises from the dorsal midline, extending almost from the poll to beyond the withers. Both cervical and thoracic parts insert on the spine of the scapula, and when they act in unison, they raise this bone against the trunk. The cervical part acting alone swings the scapula forward, which advances the limb, whereas the thoracic part acting alone swings it in the opposite direction. Both parts may be visibly outlined through the skin when contracted. The nerve supply is the accessory nerve.
The brachiocephalicus (Figure 23–3/4) arises from the mastoid region of the skull and inserts on a ridge of the humerus that extends distally from the deltoid tuberosity. It is intimately joined in the neck to the omotransversarius (Figure 23–3/6), which takes origin from the transverse processes of the more cranial cervical vertebrae and ends at the clavicular intersection that divides the brachiocephalicus into cervical (cleidomastoideus) and brachial (cleidobrachialis) parts. The dorsal edge of the omotransversarius is connected to the trapezius by the superficial fascia. The ventral edge of the brachiocephalicus is clearly delineated, at least in its cranial half, as it forms the upper margin of the jugular groove (see Figure 18–38, B).
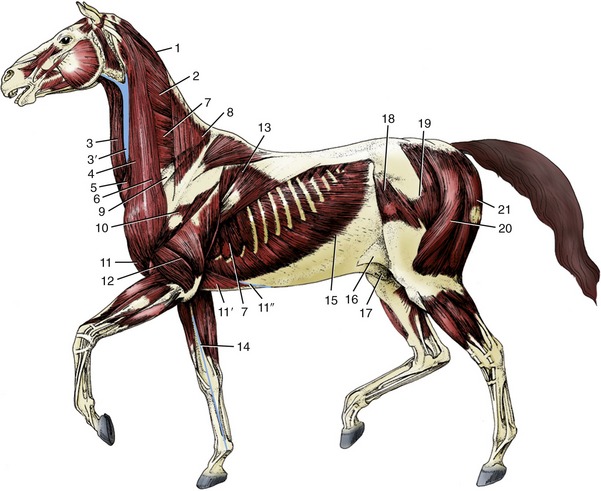
Figure 23–3 The superficial muscles and veins. The cutaneous muscles except for the cutaneous colli have been removed.
1, Rhomboideus; 2, splenius; 3, sternocephalicus; 3′, jugular vein; 4, brachiocephalicus; 5, cutaneous colli; 6, omotransversarius; 7, serratus ventralis; 8, trapezius; 9, subclavius; 10, deltoideus; 11, pectoralis descendens; 11′, pectoralis ascendens; 11″, superficial thoracic vein; 12, triceps; 13, latissimus dorsi; 14, cephalic vein; 15, external abdominal oblique; 16, stump of cutaneous trunci forming flank fold; 17, sheath; 18, tensor fasciae latae; 19, gluteus superficialis; 20, biceps femoris; 21, semitendinosus.
The muscle is broadest over the shoulder joint, where it covers the origin of the biceps and the insertions of the supraspinatus and infraspinatus. Bilateral action flexes the neck ventrally when that part is free to move. Unilateral action in the same circumstances bends the neck toward the active side; when the neck is fixed and it is the limb that is free, unilateral action advances the limb. The innervation is shared by the accessory, cervical, and axillary nerves.
The latissimus dorsi (Figure 23–3/13) arises from the supraspinous ligament and thoracolumbar fascia and converges to an insertion on the teres tuberosity of the humerus. The cranial strip covers the caudal angle of the scapula and holds it against the trunk. This muscle is commonly described as a retractor of the limb and thus is an antagonist of the brachiocephalicus; in fact, its most important role, especially in draft animals, may be to pull the trunk forward onto an advanced limb. It is supplied by the thoracodorsal nerve.
The superficial layer of girdle muscles is completed by the two superficial pectoral muscles. The cranial pectoralis descendens arises from the manubrium and divides its insertion between the humerus and fascia of the arm (Figure 23–4/4). It is well developed and clearly outlined in life; a median groove separates it from its contralateral fellow. The lateral groove that marks its boundary with the brachiocephalicus is occupied by the cephalic vein. It is primarily an adductor.
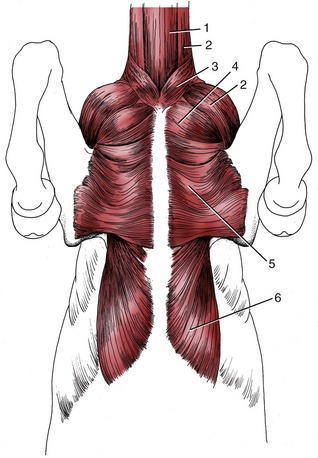
Figure 23–4 Muscles on the ventral surface of the thorax.
1, Sternocephalicus; 2, brachiocephalicus; 3, cutaneous colli; 4, pectoralis descendens; 5, pectoralis transversus; 6, pectoralis profundus.
The caudal pectoralis transversus (Figure 23–4/5) arises from the cranial sternebrae and inserts into the fascia over the medial aspect of the upper part of the forearm. The transverse course of its fibers makes it clear that it is essentially an adductor, which is a term that embraces the lateral shifting of the trunk toward a previously abducted limb. Both superficial pectoral muscles are supplied by pectoral branches of the brachial plexus.
Although the rhomboideus lies deep to the trapezius, it may, when contracted, form a visible surface feature. Its origin from the nuchal and supraspinous ligaments extends between the second cervical and seventh thoracic vertebrae. The entire muscle inserts on the deep face and dorsal edge of the scapular cartilage (Figure 23–5/4). Although it serves to raise the scapula, the course of the thoracic fascicles enables them to rotate the bone so that the ventral angle is carried caudally. The innervation is by dorsal branches of caudal cervical nerves.
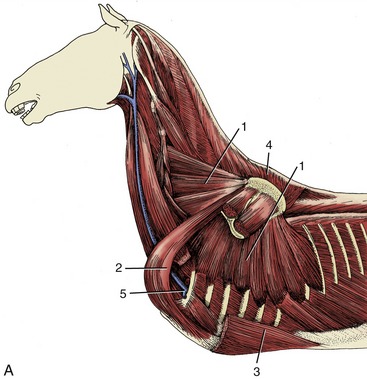

Figure 23–5 A, Deep muscles attaching the forelimb to the trunk.
1, Serratus ventralis; 2, subclavius; 3, pectoralis profundus; 4, rhomboideus; 5, axillary vessels turning around first rib into limb.
The serratus ventralis (Figure 23–5/1) is very strong, both actively because of its extent and bulk and passively because it is covered and intershot by stout connective tissue sheets. The origin spreads from the fourth cervical vertebra to the tenth rib. The insertion is confined to the scapular cartilage and to two triangular areas on the adjacent part of the medial surface of the scapula. The dominant function of the serratus is support of the trunk. However, the cervical and thoracic parts each have an additional (and antagonistic) function in rotating the scapula. The cervical part rotates the bone so that the ventral angle is carried caudally, thus retracting the limb; contraction of the thoracic part advances this angle and thus the limb. The serratus ventralis is supplied by the long thoracic nerve.
The pectoralis profundus has a widespread origin from the caudal part of the sternum and adjacent area of the abdominal floor (Figure 23–5/3). The fascicles converge, and the muscle thickens as it passes craniolaterally to a restricted insertion on the greater and lesser tubercles of the humerus. The relative heights of the origin and insertion suggest that the deep pectoral muscle may assist the serratus in supporting the weight of the trunk; the catastrophic results of rupture of the serratus suggest the limited effectiveness in this capacity (Figure 23–5, B). Its foremost uses are probably adduction, retraction of the limb when this is free to move, and advancement of the trunk onto an advanced and fixed limb. It is supplied by pectoral nerves.
The subclavius (Figure 23–5/2), to the front of the deep pectoral, takes origin from the cranial part of the sternum. It then bends dorsally to follow the cranial surface of the supraspinatus, over which it tapers to an extended insertion on the epimysium. Its presence along the leading edge of the scapula helps smooth the transition from the narrow neck to the greater breadth between the shoulders. The actions of the subclavius complement those of the deep pectoral (of which it was formerly regarded as a part). It too is supplied by pectoral nerves.
THE SHOULDER REGION AND UPPER ARM
The bases of the shoulder region and upper arm are the scapula and humerus, both wholly included within the skin of the trunk. The slope of the scapula, of interest to horsemen and horsewomen, varies considerably and is revealed by the orientation of its spine. A more sloping shoulder is preferred in saddle horses. The thickened middle portion (tuber spinae) of the spine is readily recognized on palpation and may even provide a visible landmark (Figure 23–6, A/3). The distal part of the spine subsides gradually and does not form an acromion. The bone is extended beyond its dorsal border by a large scapular cartilage that is incorporated within the withers. The margin of the cartilage and the cranial and caudal angles of the bone may be palpated in most subjects. The caudal angle is often quite prominent, even though it is covered by the latissimus (Figure 23–3/13).
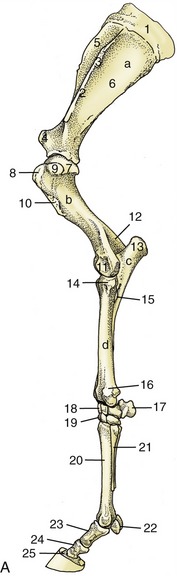
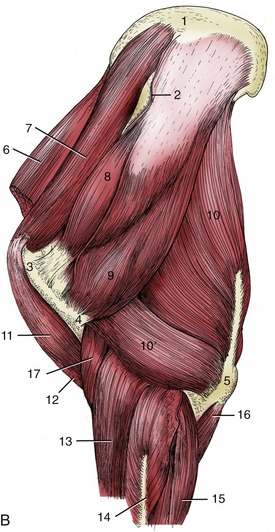
Figure 23–6 A, Skeleton of the left forelimb; lateral view.
a, Scapula; b, humerus; c, ulna; d, radius; 1, scapular cartilage; 2, scapular spine; 3, tuberosity of scapular spine; 4, supraglenoid tubercle; 5, 6, supraspinous and infraspinous fossae; 7, head of humerus; 8, 9, cranial and caudal parts of greater tubercle; 10, deltoid tuberosity; 11, condyle; 12, olecranon fossa; 13, olecranon; 14, tubercle for lateral collateral ligament; 15, interosseous space; 16, lateral styloid process; 17, accessory carpal; 18, 19, proximal and distal row of carpal bones; 20, large metacarpal (cannon) bone; 21, small metacarpal (splint) bone; 22, proximal sesamoid bones; 23, proximal phalanx; 24, middle phalanx; 25, distal phalanx.
B, Muscles associated with shoulder and elbow joints; lateral view.
1, Scapular cartilage; 2, scapular spine; 3, greater tubercle of humerus; 4, deltoid tuberosity of humerus; 5, olecranon; 6, subclavius; 7, supraspinatus; 8, infraspinatus; 9, deltoideus; 10, long head of triceps; 10′, lateral head of triceps; 11, biceps; 12, lacertus fibrosus; 13, extensor carpi radialis; 14, common digital extensor; 15, ulnaris lateralis; 16, ulnar head of deep digital flexor; 17, brachialis.
The humerus forms a right angle with the scapula and slopes less steeply than in the smaller species. Its surface relief is marked, and many features may be felt through the skin and musculature. The greater and lesser tubercles of the proximal extremity are both well developed and are more nearly equal than in most species. Each is divided into cranial and caudal parts. The cranial parts are separated by an intertubercular groove that is interrupted by an intermediate tubercle; there are thus five processes that together enclose the head on all but its caudal aspect. Although both parts of the greater tubercle are easily palpated, it is the cranial division that provides the surface feature known as the “point of the shoulder” (Figure 23–6, A/8). Distal to this, the deltoid tuberosity furnishes another easily found landmark (Figure 23–6/10).
The shoulder joint has the attributes of a spheroidal joint and is theoretically capable of considerable versatility of movement (Figure 23–7). In practice, it generally functions as a hinge joint whose excursions take place in a sagittal plane. The restriction on transverse movements, imposed by collateral ligaments at most hinge joints, is here provided by the tendons of the muscles that closely surround the shoulder, notably the infraspinatus (and, to a lesser degree, the supraspinatus) laterally and the subscapularis medially. The cavity is relatively capacious. It may be tapped by inserting a needle at the cranial margin of the palpable infraspinatus tendon about 2 cm proximal to the caudal part of the greater tubercle. The needle is directed ventromedially and must be introduced about 4 or 5 cm before its tip penetrates the capsule. The procedure requires some care because a cranial deflection may cause the needle to enter a quite separate synovial sac, the bursa that protects the biceps tendon within the intertubercular groove. This intertubercular bursa corresponds to the diverticulum of the joint capsule found in the dog and sheep.
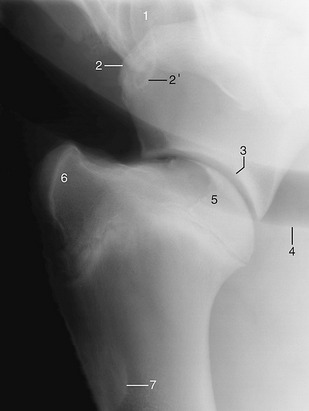
Figure 23–7 Lateral radiograph of a shoulder joint.
1, Sixth cervical vertebra; 2, supraglenoid tubercle of scapula; 2′, coracoid process; 3, glenoid cavity; 4, trachea; 5, head of humerus; 6, superimposed greater, lesser, and intertubercular tubercles; 7, deltoid tuberosity.
The muscles that act primarily on the shoulder may be considered as being arranged in lateral and medial groups, although they enclose the joint on all sides. The lateral group comprises the supraspinatus, infraspinatus, deltoideus, and teres minor (Figure 23–6, B).
The supraspinatus (Figure 23–6, B/8) arises from and occupies the supraspinous fossa of the scapula; it bulges beyond the bone cranially where its covering epimysium provides insertion to the subclavius. It splits before its insertion, forming two short tendons that straddle the origin of the biceps before attaching to the cranial parts of the tubercles of the humerus. The muscle is placed to extend the shoulder joint, but its most important function may be stabilization of the joint.
The infraspinatus (Figure 23–6, B/9) has a similar relationship to the infraspinous fossa. Its insertion crosses the lateral aspect of the shoulder joint before separating into deep and superficial tendons. The short deep tendon attaches to the edge of the caudal part of the greater tubercle. The superficial tendon crosses this projection to attach at a more distal level and is protected by a synovial bursa where it lies against the bone. Inflammation of the bursa may be painful and may cause the animal to stand with the affected limb abducted at the shoulder, which is a posture that relieves the pressure at the site. The infraspinatus is primarily a shoulder fixator whose tendon substitutes for a lateral collateral ligament. It has a secondary abductor action. Both supraspinatus and infraspinatus are supplied by the suprascapular nerve.
The deltoideus (Figure 23–6, B/9) arises from the caudal border and spine of the scapula; the latter origin is indirect and effected by way of an aponeurosis that covers the infraspinatus. The insertion is to the deltoid tuberosity. This muscle may be identified by first referring to that landmark and then following the belly proximally. It is partly recessed within a depression of the triceps, and the line between the muscles is sometimes visible in thin-skinned animals. The deltoideus is a shoulder flexor with a secondary role as abductor of the arm. Innervation is by the axillary nerve.
The unimportant teres minor is buried by the deltoideus over the caudolateral aspect of the shoulder joint.
The medial muscle group comprises the subscapularis, teres major, coracobrachialis, and capsularis, of which the last is of trivial significance. The subscapularis arises from and occupies the subscapular fossa (Figure 23–8/1). It inserts on the lesser tubercle and, though primarily employed to stabilize the joint, may also function as an adductor of the arm. It is supplied by the subscapular nerve.
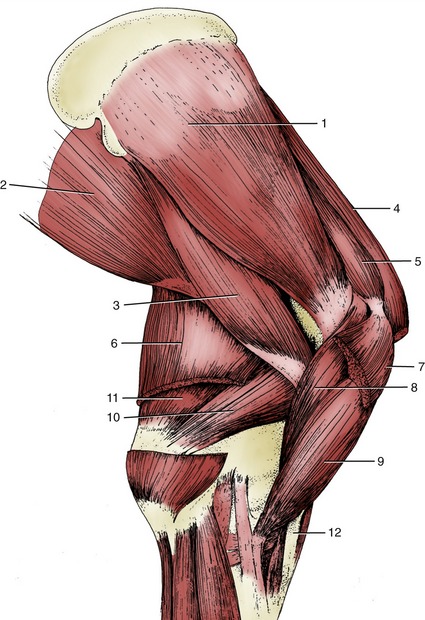
Figure 23–8 Muscles on the medial surface of the right shoulder and arm.
1, Subscapularis; 2, latissimus dorsi; 3, teres major; 4, subclavius; 5, supraspinatus; 6, tensor fasciae antebrachii; 7, deep pectoral; 8, coracobrachialis; 9, biceps; 10, medial head of triceps; 11, long head of triceps; 12, lacertus fibrosus.
The teres major (Figure 23–8/3) arises from the caudal angle of the scapula. It is contained between the subscapularis and the latissimus dorsi and inserts in common with the latter. It is chiefly a flexor of the shoulder but may also adduct the arm. It is supplied by the axillary nerve, as are all the true flexors of the shoulder.
The coracobrachialis (Figure 23–8/8) arises from the coracoid process on the medial aspect of the supraglenoid tubercle and inserts on the proximal part of the shaft of the humerus. It is an adductor of the arm but is of little consequence. It is supplied by the musculocutaneous nerve.
THE ELBOW JOINT AND THE MUSCLES OF THE ARM
The skeletal basis of the elbow joint is provided by the distal end of the humerus and proximal parts of the radius and ulna (Figure 23–6, A). Both epicondyles of the humerus may be palpated without much difficulty, but the medial one is especially prominent and projects to the inner aspect of the olecranon. The condyle may be identified more distally; it presents a deep fossa into which fits the anconeal process of the olecranon (Figure 23–9/4,6). A shallow radial fossa occupies the corresponding site on the cranial aspect.
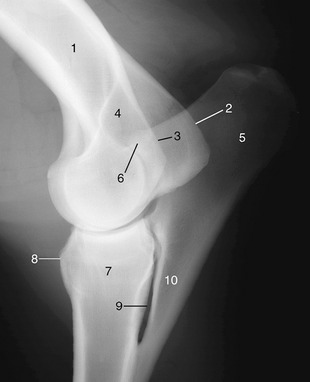
Figure 23–9 Lateral radiograph of an elbow joint.
1, Humerus; 2, medial epicondyle; 3, lateral epicondyle; 4, olecranon fossa; 5, olecranon; 6, anconeal process of olecranon; 7, radius; 8, radial tuberosity; 9, interosseous space; 10, ulna.
The powerful olecranon rises high above the joint to project on the lower part of the fifth rib (or following space) and is therefore a less direct guide to the position of the articulation. The shaft of the ulna is much reduced. It tapers distally to fusion and ultimate submergence within the shaft of the radius, but it leaves open an interosseous space in the proximal forearm. The proximal extremity of the radius is expanded. It carries an articular surface that engages with the cylindrical humeral condyle and, just distal to this, medial and lateral eminences that furnish attachment to the collateral ligaments. The radial tuberosity is present to the front (Figure 23–9/8). Both collateral ligaments may be palpated, although the medial one is covered by the relatively thick pectoralis transversus. A cranial division of this ligament represents a vestige of the pronator teres.
The shape of the articular surfaces and the presence of stout collateral ligaments restrict movement of the elbow joint to flexion and extension in a sagittal plane. The equine elbow is a good example of the “snap” joint, which abruptly moves from a stable to a more mobile position. This character depends on two features of its construction. The first is the unequal curvature of the humeral surface; the radius of curvature of the central part is longer than those of the parts in front and behind, which are in contact with the radius in the more flexed and more extended positions of the joint. The second is that the collateral ligaments insert eccentrically on the humerus and are taut only in the intermediate position (Figure 23–10).
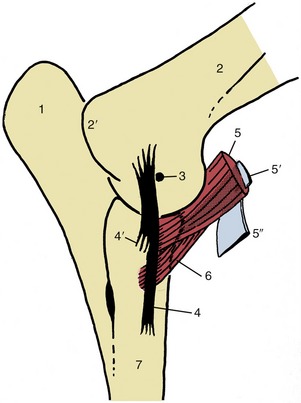
Figure 23–10 Medial view of left elbow joint to show the eccentrically placed collateral ligament and the insertions of biceps and brachialis. The internal tendon (5) of the biceps splits off the lacertus fibrosus (5″) from the surface of the muscle.
1, Olecranon; 2, humerus; 2′, medial epicondyle; 3, axis of rotation; 4, 4′, long superficial and short deep parts of medial collateral ligament; 5, biceps; 5′, internal tendon of biceps; 5″, lacertus fibrosus; 6, brachialis; 7, radius.
The joint is most conveniently punctured by passing a needle between the lateral epicondyle and the olecranon into a caudal pouching of the joint capsule within the olecranon fossa.
The muscles of the arm that operate the elbow joint are arranged in flexor and extensor groups.
THE FLEXOR MUSCLES
The flexor muscles comprise the biceps brachii and brachialis. Although largely under cover of the brachiocephalicus, the belly of the biceps is palpable as it lies against the cranial face of the humerus. The biceps takes origin from the supraglenoid tubercle of the scapula by means of a short, broad, and largely fibrocartilaginous tendon that is molded on the intertubercular groove. The (intertubercular) bursa that protects the tendon spreads from the groove onto the cranial aspect of the humerus; it may be a cause of shoulder lameness when inflamed. The bursa may be reached, certainly if overdistended, by inserting a needle between the muscle and the bone, slightly above the level of the deltoid tuberosity, and then directing it proximally (Figure 23–11/3).
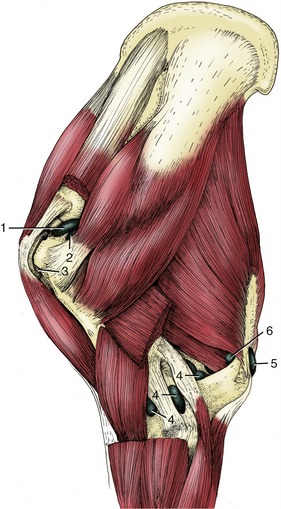
Figure 23–11 Synovial structures of the left shoulder and elbow regions; lateral view.
1, Shoulder joint capsule; 2, infraspinatus bursa; 3, intertubercular bursa (between biceps tendon and humerus); 4, elbow joint capsule; 5, subcutaneous olecranon bursa; 6, subtendinous olecranon bursa. (For identification of the muscles see Figure 23–6, B.)
The biceps inserts mainly on the radial tuberosity, but a branch of the attachment passes beneath the medial collateral ligament to the adjoining parts of the radius and ulna. A more important peculiarity is the existence within its belly of a fibrous strand (internal tendon; Figure 23–10/5′) that joins the tendons of origin and insertion; a part splits away to emerge on the surface and blend more distally with the epimysium of the extensor carpi radialis. The bridging band, known as the lacertus fibrosus, is easily found as a firm structure crossing the flexor aspect of the elbow (Figure 23–10/5″; Figure 23–8/12). It is taut in the standing animal but slackens as the joint is flexed. The internal tendon and the lacertus help maintain the carpal joint in extension when the biceps resists collapse of the shoulder under the weight of the trunk (Figure 23–38, A/2,6).
The biceps is a fixator and, potentially, an extensor of the shoulder; the construction and form of the tendon of origin suggest its particular fitness for the first task. Although it is regarded as the most important flexor of the elbow, the fibrous arrangements imply that its passive role may also be more significant at this joint. Recent work has furnished a more satisfactory explanation of the unusual structure and enigmatic role of the equine biceps brachii than has been available hitherto. Calculation has shown that the force necessary for the rapid protraction of the forelimb that is effected at the faster gaits is beyond the power of the available muscle as conventionally assessed. It is now suggested that the central tendon allied to the bipennate construction of the muscle enables it to store energy when stretched during the support phase of the stride and that this energy is later very rapidly released to accelerate the forward movement of the limb. Its nerve supply comes from the musculocutaneous nerve.
The brachialis is purely fleshy and crosses only one joint, the elbow. It arises from the caudoproximal part of the humerus, winds laterally within a spiral groove, and then crosses the flexor aspect of the elbow to insert on the craniomedial part of the proximal radius (Figure 23–12/3). Proximally, the muscle is covered by the triceps, but its distal part is superficial and may be palpated. The brachialis is purely an elbow flexor. It is supplied by the musculocutaneous nerve with, rather surprisingly, a contribution from the radial nerve.
THE EXTENSOR MUSCLES
The extensor muscles constitute a large mass that fills the triangle between the scapula and humerus. The group comprises the triceps, tensor fasciae antebrachii, and anconeus.
The triceps is by far the most important extensor of the elbow. It presents three heads (Figure 23–6, B/10,10′). The long head arises from the caudal border of the scapula by a short aponeurosis, and the lateral and medial heads arise from the shaft of the humerus. Together they insert on the olecranon where a small bursa is inserted between the tendon and the bone. The division between the long and lateral heads is sometimes visible in thin-skinned animals. A second, acquired (adventitious) bursa is commonly found subcutaneously, over the triceps insertion and expanded part of the olecranon tuber (“capped elbow”; Figure 23–11/5).
The triceps is extensor to the elbow. Since the long head spans the shoulder joint, it is theoretically available to flex this joint; however, it is probably little used for that purpose.
The tensor fasciae antebrachii (Figure 23–8/6) is a broad, thin sheet covering the medial aspect of the triceps. Its origin is from the caudal border of the scapula and the tendon of the latissimus, while its insertion is spread between the olecranon and forearm fascia. Since it crosses both shoulder and elbow joints, it must be considered as having a potential action at each; neither is likely to be of great importance.
The much smaller anconeus lies within the olecranon fossa, embedded within the deep face of the lateral head of the triceps and directly related to the capsule of the elbow joint. It may be supposed that its principal action is to tense the capsule, thus preventing it from being pinched between the humerus and ulna (Figure 23–12/4).
The radial nerve supplies all muscles of the extensor group.
THE FOREARM AND CARPUS
THE SKELETON AND CARPAL JOINT
The shaft of the radius is flattened from front to back and is covered by muscle on all but its subcutaneous medial border. The distal extremity broadens to meet the expanded carpus (commonly known as the “knee”). On each side it carries a styloid process and, proximal to this, an eminence for the attachment of a collateral ligament. The cranial aspect is grooved for the passage of the extensor tendons. These tendons, the adjacent molding of the bone, the styloid processes, and the eminences for ligamentous attachment are all very distinctly palpable.
The carpal skeleton is arranged in the usual two rows (see Figure 23–20, A). The proximal row comprises radial, intermediate, and ulnar carpal bones, concerned in weight-bearing, together with a laterally flattened, discoidal accessory bone that projects backward in a very conspicuous fashion. The accessory bone articulates with the lateral styloid process and the ulnar carpal but bears no weight. The distal row is also deep; in addition to three constant elements—second, third, and fourth carpal bones—there is often a pea-shaped first carpal. This bone is frequently isolated from the remainder of the skeleton, embedded in the palmar carpal ligament behind the second carpal; it may be mistaken for a bone fragment when shown in radiographs (Figure 23–13/6).

Figure 23–13 Dorsopalmar (A) and lateral (B) radiographs of the carpus.
1, Radius; 2, accessory carpal (faint); 3, radial carpal; 4, intermediate carpal; 5, ulnar carpal; 6, position of first carpal, when present; 7, 8, 9, second, third, and fourth carpals; 8′, 9′, superimposed third and fourth carpals; 10, 11, 12, second, third, and fourth metacarpals; 10′, 12′, superimposed second and fourth metacarpals; 13, metacarpal tuberosity.
The carpal joint is maintained in full extension in the standing posture but is capable of very considerable flexion. It presents three levels of articulation. Movement is most free at the radiocarpal (antebrachiocarpal) level, where as much as 90° or 100° of flexion is allowed. The midcarpal articulation is also mobile, allowing perhaps 45° of flexion, but no significant movement is possible at the carpometacarpal level (Figure 23–13, B).
The articular surfaces of the bones reflect these differences (Figure 23–14, A). The radial articular surface shows some demarcations corresponding to the three proximal carpal bones but overall presents a caudal hemicylindrical ridge and narrow cranial gutter. The upper surfaces of the proximal carpal bone row have the reciprocal conformation. Their lower surfaces are convex in front and concave behind. The surfaces at the distal joint are broadly flat. Figure 23–15, A illustrates these features and the two axes of rotation. The fronts of the bones are driven together in full extension of the joint and may splinter (“chip fractures”*) during the fast gaits.
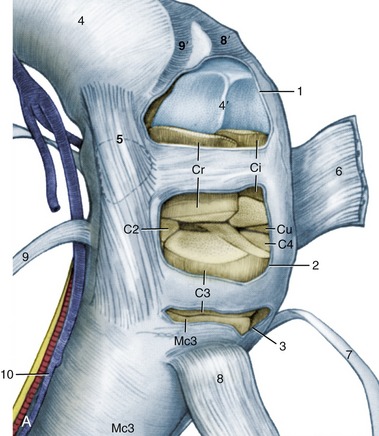
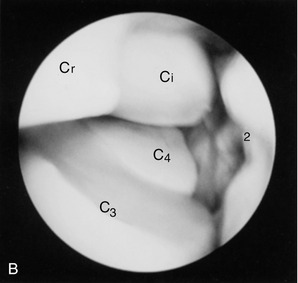

Figure 23–14 A, Flexed left carpus, dorsomedial view. The articular surfaces are stippled. B, Arthroscopic medial-to-lateral view of the left midcarpal joint. Cr, Ci, Cu, radial, intermediate, and ulnar carpal bones; C2, C3, C4, second, third, and fourth carpal bones; Mc3, third metacarpal (cannon) bone.
1, Radiocarpal joint capsule, fenestrated; 2, midcarpal joint capsule, fenestrated in A; 3, carpometacarpal joint capsule, fenestrated; 4, 4′, radius and its distal articular surface; 5, position of bursa between medial collateral ligament and extensor carpi obliquus (9); 6, extensor retinaculum, reflected; 7, common digital extensor; 8, 8′, extensor carpi radialis and its groove on radius; 9, 9′, extensor carpi obliquus and its groove on radius; 10, medial palmar nerve, artery, and vein.
C, Puncture of radiocarpal joint. D, Puncture of midcarpal joint.
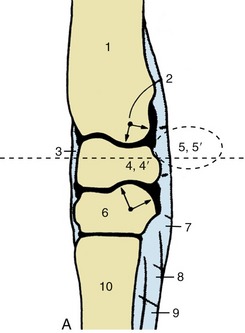
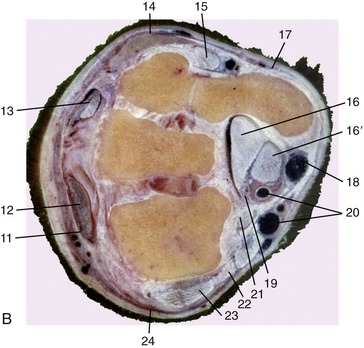
Figure 23–15 A, Axial section of the carpus. The broken transverse line indicates level of section in B. B, Transverse section of the right carpus, proximal surface, Both joints face to the left.
1, Radius; 2, axis of rotation; 3, fibrous joint capsule; 4, 4′, intermediate and radial carpal; 5, 5′, accessory and ulnar carpal; 6, third carpal; 7, palmar carpal ligament; 8, accessory (check) ligament of deep digital flexor; 9, interosseus; 10, large metacarpal; 11, extensor retinaculum; 12, extensor carpi radialis; 13, common digital extensor; 14, lateral digital extensor; 15, long tendon of ulnaris lateralis; 16, 16′, deep and superficial flexor tendons in carpal canal; 17, dorsal branch of ulnar nerve; 18, palmar branch of median artery and lateral palmar nerve; 19, median artery and medial palmar nerve; 20, radial artery and vein; 21, flexor carpi radialis; 22, flexor retinaculum; 23, medial collateral ligament; 24, extensor carpi obliquus.
The carpus is mainly supported by the cannon bone but also makes contact with the bases of the splint bones. Indeed, so large a part of the second carpal bone rests on the second metacarpal that it may tend to drive that bone away from its larger neighbor, which induces the painful acute inflammation mentioned later. Certainly the condition known as “splints” is more common at the medial intermetacarpal joint.
The three levels of articulation share a common fibrous capsule, but the synovial compartments are separate except for a narrow communication between the middle and distal levels (Figure 23–14). The fibrous capsule (Figure 23–15, A/3), which has extensive connections with all the bones involved in the joint, is of very unequal thickness. It is weakest dorsally, where it is rather loose in the extended position of the joint. It is much thicker over the palmar aspect (Figure 23–15/7), where it opposes overextension. This part, the palmar carpal ligament, fills the irregularities of the bones and smoothes the backward facing aspect of the carpal skeleton. Medial and lateral collateral ligaments extend between the lower end of the radius and the upper part of the metacarpus. They have intermediate attachments to the carpal bones and ensure that movement is confined to the sagittal plane. There are numerous additional ligaments. Some merely join adjacent bones in the same row or join distal bones to the metacarpus, and although they help stabilize the joint, they are not individually of interest. Others secure the accessory bone; one that runs obliquely from its distal edge to the metacarpus forms a conspicuous ridge. A larger transverse ligament (flexor retinaculum; Figure 23–15/22) extends from the palmar edge of the accessory bone to attach at the mediopalmar aspect of the joint. It completes the enclosure of a space, the carpal canal, through which pass the flexor tendons and other structures en route from the forearm to the distal part of the limb.
Distention of the radiocarpal joint capsule is not uncommon (Figure 23–16/1). The capsule pouches where support is weak, dorsally between the extensor tendons and proximally, above the accessory bone, just caudal to the lateral digital extensor tendon. It may be punctured here, but a more convenient approach is from the dorsal aspect. Flexion of the carpus opens up the joint space, facilitating the entry of a needle between the extensor tendons. A similar approach may be made to the middle compartment (Figure 23–14, C-D).
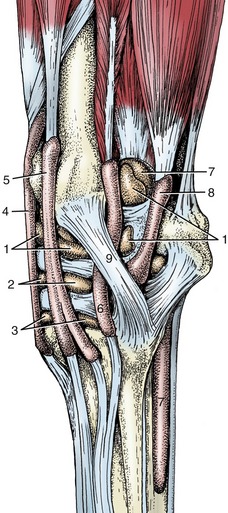
Figure 23–16 Synovial structures of the left carpus; lateral view.
1, Radiocarpal joint capsule; 2, midcarpal joint capsule; 3, carpometacarpal joint capsule; 4, tendon sheath of extensor carpi radialis; 5, tendon sheath of common digital extensor; 6, tendon sheath of lateral digital extensor; 7, tendon sheath of superficial and deep digital flexors (carpal sheath); 8, tendon sheath of ulnaris lateralis; 9, lateral collateral ligament.
THE MUSCLES OF THE FOREARM
The Extensor Group
With one exception—the extensor carpi obliquus—all carpal and digital extensors arise from the craniolateral aspect of the distal end of the humerus and occupy the craniolateral part of the forearm. Their insertion tendons begin a little above the carpus and are secured in their passage over the joint by condensed deep fascia known as the extensor retinaculum (Figure 23–15, B/11). Each is also individually protected by a synovial sheath, from just above to well below the carpus (Figure 23–16).
Except for the ulnaris lateralis, all are extensor to the carpus; the longer muscles also extend the joints of the digit. In addition, their origin provides them with some capacity to flex the elbow, although they are probably little used in this role. All are supplied by the radial nerve. They may each be identified on palpation, and several provide quite conspicuous visible features of the forearm of thin-skinned animals.
The extensor carpi radialis (Figure 23–17/5), the most medial member of the group, runs directly to the front of the subcutaneous border of the radius. Its epimysial covering is joined by the lacertus fibrosus that enables it passively to prevent flexion of the carpal joint when weight is on the limb.
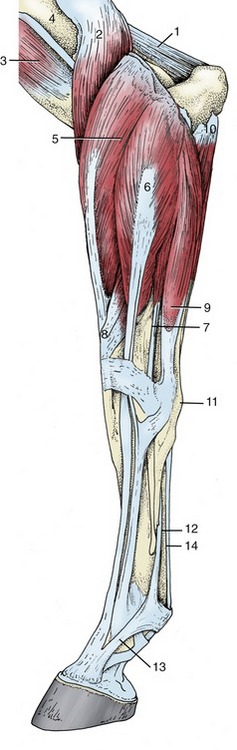
Figure 23–17 Distal muscles of the left forelimb; lateral view.
1, Anconeus; 2, brachialis; 3, biceps; 4, deltoid tuberosity of humerus; 5, extensor carpi radialis; 6, common digital extensor; 7, lateral digital extensor; 8, extensor carpi obliquus; 9, ulnaris lateralis; 10, ulnar head of deep digital flexor; 11, accessory carpal bone; 12, interosseus; 13, extensor branch of interosseus; 14, flexor tendons.
The common digital extensor (Figure 23–17/6) possesses a rather slight radial head in addition to the more substantial origin from the humerus. The radial head is never fully incorporated in the main mass and separates in the lower part of the forearm; its tendon joins that of the lateral digital extensor within the cannon. The main tendon continues down the dorsal aspect to the metacarpus and digit to insert on the extensor process of the distal phalanx. Just before this, it is joined by branches of the interosseus that wind around the sides of the digit from the palmar aspect (Figure 23–17/13).
The slighter lateral digital extensor (Figure 23–17/7) creates a prominent ridge on the lateral aspect of the forearm. It is joined by the contribution from the common extensor in the upper part of the cannon and then gently inclines toward the dorsal aspect of the limb to insert on the proximal end of the proximal phalanx.
The ulnaris lateralis (Figure 23–17/9) runs down the caudal aspect of the forearm. Its short tendon of insertion splits above the accessory carpal bone; a part at once inserts on this bone, while a longer branch descends over the lateral aspect of the bone, tunnels under the collateral ligament, and ends on the head of the lateral splint bone. The longer division requires the protection of a synovial sheath (Figure 23–16/8).
The extensor carpi obliquus is distinguished by arising from the shaft of the radius. It runs in a mediodistal direction to insert on the medial splint bone. Although largely covered by the other muscles, its tendon becomes superficial to that of the extensor carpi radialis (Figure 23–17/8).
The Flexor Group
The muscles of the flexor group also share several attributes. They arise from the caudomedial aspect of the humerus, occupy the caudal part of the forearm, obtain their innervation from the median and ulnar nerves, and are flexor to the carpal joint; those that proceed beyond this level are also flexor to the digital joints.
The flexor carpi radialis (Figure 23–18/8) follows the subcutaneous border of the radius and covers the important median vessels and nerve. The tendon of insertion tunnels through the flexor retinaculum where it obtains the necessary protection of a synovial sheath before attaching to the medial splint bone.
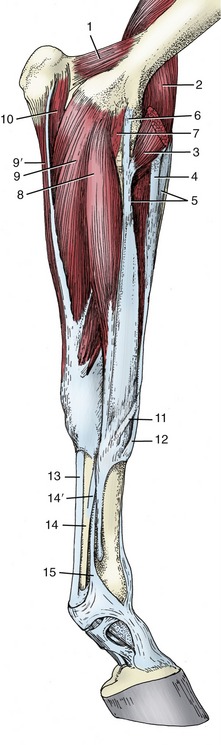
Figure 23–18 Distal muscles of the left forelimb; medial view.
1, Anconeus; 2, brachialis; 3, biceps; 4, lacertus fibrosus; 5, extensor carpi radialis; 6, long part of medial collateral ligament (pronator teres); 7, short part of medial collateral ligament; 8, flexor carpi radialis; 9, 9′, humeral and ulnar heads of flexor carpi ulnaris; 10, ulnar head of deep digital flexor; 11, tendon of extensor carpi obliquus; 12, tendon of extensor carpi radialis; 13, tendon of superficial digital flexor; 14, tendon of deep digital flexor; 14′, accessory (check) ligament; 15, interosseus.
The flexor carpi ulnaris (Figure 23–18/9) lies on the medial aspect of the forearm, partly under cover of the flexor carpi radialis. It arises by two heads—from the humerus and the ulna—and inserts on the proximal margin of the accessory carpal bone by means of a short tendon that has no need of synovial protection.
The superficial digital flexor occupies a central position within the flexor group, between the larger mass of the deep flexor and the flexor carpi ulnaris (Figure 23–19/9). A purely tendinous head, usually known as an accessory or check ligament (Figure 23–19/4), arises from the caudal surface of the radius to join the main tendon in the lower part of the forearm; it is a component of the passive stay-apparatus (see further on). The superficial and deep flexor tendons share a common synovial sheath, the carpal sheath, in their passage through the carpal canal.
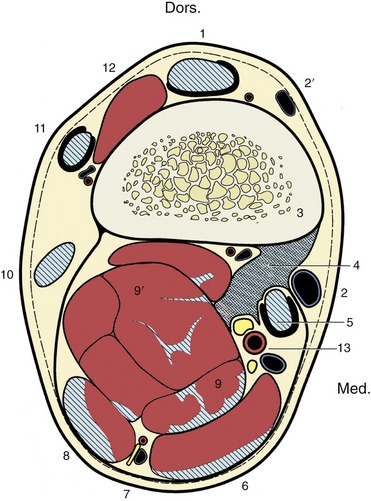
Figure 23–19 Transverse section of the right forearm 6 cm proximal to the proximal border of the accessory carpal, to demonstrate the topography of the accessory (check) ligament (4) of the superficial digital flexor; looking distally. The hatched, blue areas are tendons or tendinous tissue, and the dark pink areas are muscle tissue.
1, Extensor carpi radialis; 2, 2′, cephalic and accessory cephalic veins; 3, radius; 4, accessory (check) ligament of superficial digital flexor; 5, flexor carpi radialis; 6, flexor carpi ulnaris; 7, ulnar nerve and collateral ulnar vessels; 8, ulnaris lateralis; 9, 9′, superficial and deep digital flexors; 10, 11, 12, lateral, common, and oblique extensors; 13, median artery, medial and lateral palmar nerves.
The tendon is superficial to that of the deep tendon in the metacarpus, but at the fetlock it obtains the deeper position necessary for its insertion on neighboring parts of the proximal and middle phalanges (Figure 23–18/13).
The deep digital flexor is by far the largest of the flexors, although this is not apparent without dissection (Figure 23–19/9′). In addition to the humeral head, there are lesser heads of origin from the upper parts of the radius and ulna. The common tendon passes through the carpal canal and continues down the palmar aspect of the limb to find insertion on the palmar surface of the distal phalanx. In the metacarpus the tendon is joined by a stout tendinous band that arises from the thick fibrous joint capsule on the palmar aspect of the carpal joint (Figure 23–18/14,14′). This is almost invariably known as an accessory or check ligament; it provides an important element of the passive stay-apparatus that is of far greater significance than the analogous contribution to the superficial tendon.
THE DISTAL PART OF THE LIMB
The more distal structures of the limb not only have the greatest propensity to injury but also show many and important specific differences.
THE SKELETON AND JOINTS
The skeleton comprises the metacarpal bones and the proximal, middle, and distal phalanges. The metacarpophalangeal and the proximal and distal interphalangeal joints linking these bones are commonly referred to as the fetlock, pastern, and coffin joints. A pair of proximal sesamoid bones enlarges the concavity of the fetlock joint, and a single distal sesamoid bone enlarges that of the coffin joint.
The metacarpal skeleton comprises second, third, and fourth metacarpal bones. The third bone, the cannon bone, is much stronger than the other two and is the functional element. It carries a prominent tuberosity on its dorsal surface just distal to the joint. The bones to each side, generally known as the splint bones, are much reduced in size. Each has a small proximal base that continues into a tapering shaft. In young animals the splint and cannon bones are joined by fibrous tissue; this generally later ossifies, and the upper parts of the shafts are then fused together. The process is often accompanied by an acute inflammation (a condition known as “splints”), which leaves a palpable—and often visible—blemish on the dorsal surface.
The tapering second and fourth metacarpals end in slight but easily palpable buttons three quarters of the way down the cannon (see Figure 2–49, B). The lower parts of their shafts are free, and when a break occurs, it is a simple matter to remove the fragment below the fracture line.
The third metacarpal bone is exceptionally robust. It is oval in cross section (which distinguishes it from the longer but more rounded cannon bone of the hindlimb), and its thick compacta attests to its tremendous strength; it is in fact one of the strongest elements of the skeleton (see Figure 23–45/1).
The distal extremity presents an axially keeled condyle that articulates with the proximal phalanx and the paired sesamoid bones. When viewed from the side, the condyle encompasses some 220° of a circle, which is evidence of the great range of flexion and extension—the only movements allowed. The articular surface to each side of the keel is interrupted by a slight ridge that separates the more strongly curved palmar area from the larger dorsal one. Despite the obvious strength of the cannon bone, longitudinal fractures of the distal extremity are common racing injuries, more often involving the lateral than the medial side and the forelimb rather than the hindlimb. The degree of involvement of the joint surface is an important factor in prognosis.
The proximal sesamoid bones are three-sided pyramids whose bases face distally (Figure 23–20/10). The dorsal (articular) surface of each lies against the condyle, the palmar (flexor) surface tilts axially and faces the flexor tendons that ride over it, and the abaxial surface is hollowed for the reception of the thick branch of the interosseous (see further on). The palmar aspects of the bones are converted by thick fibrous tissue (palmar ligament) into a single bearing surface over which the flexor tendons change direction. Although close to the proximal phalanx, the sesamoid bones do not articulate with it.
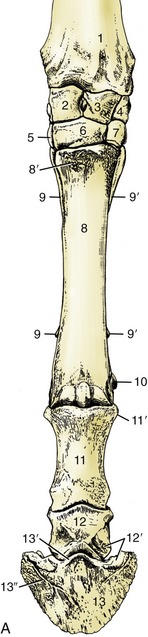
Figure 23–20 Skeleton of the distal part of the forelimb. A, Left limb, dorsal view. B, Palmar view.
1, Radius; 2, radial carpal; 3, intermediate carpal; 4, ulnar carpal; 5, 6, 7, second, third, and fourth carpals; 8, large metacarpal bone; 8′, metacarpal tuberosity; 9, 9′, medial and lateral splint bones; 10, proximal sesamoid bones; 11, proximal phalanx; 11′, proximal tubercle; 11″, attachment of distal digital annular and abaxial palmar ligaments; 11′″, attachment of axial palmar and oblique sesamoidean ligaments; 12, middle phalanx; 12′, attachments of collateral ligament of coffin joint; 12″, bearing surface for deep flexor tendon; 13, distal phalanx; 13′, extensor process; 13″, parietal groove; 14, navicular bone; 15, sole foramen and semilunar crest for attachment of deep flexor tendon; 16, palmar process and attachment of distal navicular ligament.
The proximal sesamoids fracture most often of all the bones in the forelimb, followed in frequency by the metacarpal and carpal bones. These fractures are known in racetrack practice as “the big three” for which, when serious, horses pay with their lives.
The strong proximal phalanx (PI for short) is compressed from front to back and is wider proximally than distally. Its proximal extremity is hollowed and deepened axially by a groove that allows it to conform to the condyle of the large metacarpal bone. Palpable tubercles to each side receive the collateral ligaments of the fetlock joint. The distal end is shaped as two condyles separated by a shallow axial groove and presents similar but smaller tubercles for the collateral ligaments of the pastern joint. The palmar surface of the bone is roughened for the attachment of several ligaments; a large triangular area and various smaller ones to each side stand out (see Figure 23–20/11,11′,11″,11′″).
The middle phalanx (PII) is generally similar to PI but, being only half as long, is proportionately very robust. Both extremities are of equal width. The proximal articular surface—hollowed with a slight axial ridge—is the reciprocal of the lower end of PI, whereas the distal one—two condyles separated by a groove—mimics that of PI. The distal articular surface extends onto the palmar aspect, where it articulates with the distal sesamoid bone. There are proximal collateral tubercles on PII for the collateral ligaments of the pastern joint; the corresponding distal sites from which the collateral ligaments of the coffin joint arise are excavated. The proximopalmar border presents a smooth area (Figure 23–20/12″) that is enlarged in the natural state by a complementary fibrocartilage that forms a bearing surface for the deep flexor tendon (see further on). The fibrocartilage enlarges the articular surface of the pastern joint and gives attachment to several ligaments.
The distal phalanx (PIII, coffin bone) generally conforms to the interior of the hoof in which it resides, “as in a coffin.” It is wedge shaped: sharp distally and to the sides and blunt proximally and toward the back. The dorsal (parietal) surface is convex from side to side and lies against the dermis that unites it to the inner surface of the hoof wall. It tapers caudally into medial and lateral palmar processes that are notched (or perforated) and grooved for the dorsal terminal branches of the digital arteries and accompanying nerves (Figure 23–20/13″). Depressions for the collateral ligaments of the coffin joint are present proximodorsal to the processes. The palmar (sole) surface is slightly concave to fit the domed sole of the hoof. Both parietal and sole surfaces are very porous to allow the passage of numerous small arteries from the interior of the bone into the overlying dermis. The articular surface faces proximally; it is very similar to the proximal articular surface of PII, consisting of two fossae separated by an axial ridge. Its dorsal border tapers to an extensor process, the highest point of the bone, where the common digital extensor tendon is attached. The palmar border is extended by a narrow articular zone for the distal sesamoid bone, which, in contrast to the proximal sesamoids, articulates with both major bones of the joint. Just distal to this, two prominent foramina lead to a U-shaped canal within the bone; this contains the anastomosis of the terminal palmar branches of the digital arteries. The deep flexor tendon ends on the semilunar crest just distal to the foramina (Figure 23–20/15).
The flat cartilages (of the hoof), which surmount and continue the palmar processes, lie mainly against the inner wall of the hoof, but their proximal borders are free, subcutaneous, and palpable to each side of the pastern joint (Figure 23–20, B/14).
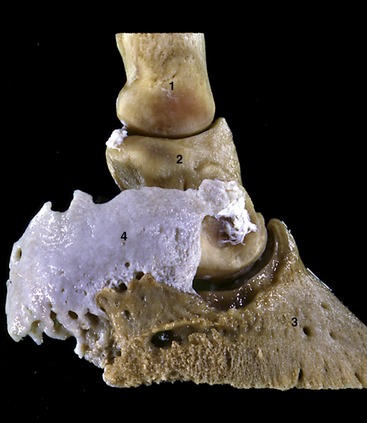
Figure 23–21 Hoof cartilage attached to palmar process of distal phalanx.
1, 2, 3, Proximal, middle, and distal phalanges; 4, hoof cartilage.
The distal sesamoid (navicular) bone (Figure 23–21/3) is boat shaped with straight proximal and convex distal borders. Its dorsal (articular) surface contacts the distal end of PII; a narrow distal facet touches PIII. The palmar (flexor) surface faces the wide tendon of the deep flexor, providing it with yet another bearing surface as it bends toward the semilunar crest on the undersurface of PIII. The navicular bone enlarges the distal articular surface of the coffin joint (see Figure 23–24/7′,7″).
The fetlock joint is formed between the large metacarpal bone, PI, and the proximal sesamoid bones (Figure 23–22). The large bones are connected by medial and lateral collateral ligaments, while additional smaller and triangular (collateral) ligaments anchor the sesamoid bones to the sides of the metacarpal condyle and the proximal tubercles of PI. A series of sesamoidean ligaments connects the bases of the sesamoid bones to the first phalanx and ensures that the sesamoids move against the metacarpal condyle in unison with PI. The deepest ligaments are short and pass to the proximopalmar border of PI; they are overlain by rather longer cruciate ligaments that end a little more distally, and these in turn are overlain by oblique ligaments that attach broadly to the central triangular area of the palmar surface of the same bone. Finally, an additional straight sesamoidean ligament, arising from the bases of the sesamoids, connects with the complementary fibrocartilage of PII (Figure 23–23/4). The cruciate, oblique, and straight ligaments are mentioned again in connection with the action of the interosseus.
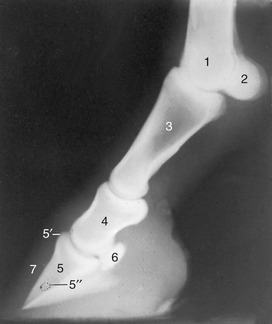
Figure 23–22 Lateral radiograph of fetlock joint and digit.
1, Large metacarpal bone; 2, proximal sesamoid bones; 3, proximal phalanx; 4, middle phalanx; 5, distal phalanx; 5′, extensor process; 5″, canal containing terminal arterial arch; 6, navicular bone; 7, wall of hoof.
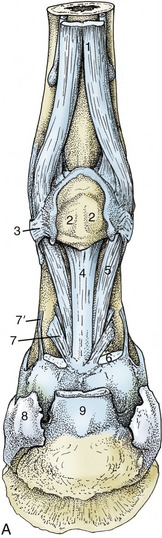
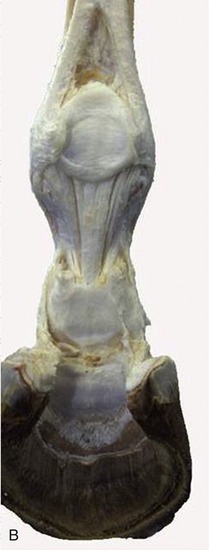
Figure 23–23 A, Structures supporting the fetlock joint.
1, Interosseus; 2, proximal sesamoid bones connected by thick palmar ligament; 3, collateral sesamoidean ligament; 4, straight sesamoidean ligament; 5, oblique sesamoidean ligament; 6, stump of superficial flexor; 7, 7′, axial and abaxial palmar ligaments of pastern joint; 8, hoof cartilage; 9, stump of deep flexor.
The sesamoid bones are connected to each other by a thick palmar ligament that extends the bearing surface for the flexor tendons proximally by about 2 cm (Figure 23–23/2). This extension supports the tendons when the sesamoids themselves slip below the condyle in maximal overextension of the fetlock joint (when the dorsal angle can be as small as 90°). When the joint is fully flexed, the sesamoid bones lose contact with the condyle and ride up on the back of the metacarpal bone, where bone-to-bone contact is prevented by the proximal extension of the palmar ligament.
The joint capsule is capacious, and to allow for the fetlock’s mobility, it extends large dorsal and palmar pouches proximally (see Figure 23–27/7). These lie against the shaft of the metacarpal bone and are easily punctured from the side; the end of the splint bone, the interosseus, and the sesamoid bone are convenient (almost visible) landmarks for entry into the palmar pouch. An other perhaps better place in the bowed limb is between the sesamoid bone and the metacarpus, directly through the collateral ligament of the flexed joint (see Figure 23–26, B-C/11). Distentions of the joint known as “wind puffs” or “galls” manifest themselves at this site. The interior of the dorsal pouch contains a so-called capsular fold (see Figure 23–27/7′). This arises from the shaft of the metacarpal bone and projects distally into the center of the pouch; its inflammation and enlargement can cause lameness. Short distal palmar pouches are palpable as small depressions in the angles between PI and the bases of the sesamoid bones.
The movement of the pastern joint is much more restricted. Paired (axial and abaxial) palmar ligaments connect the palmar aspect of PI with the complementary fibrocartilage of PII (Figure 23–23/7,7′); together with the straight sesamoidean ligament (Figure 23–23/4) they limit overextension. The capsule is similar to that of the fetlock joint, but the pouches are smaller and only the dorsal one is accessible for puncture, again from the side. The radiographic appearance of the pastern and coffin joints is shown in Figure 23–24, A).
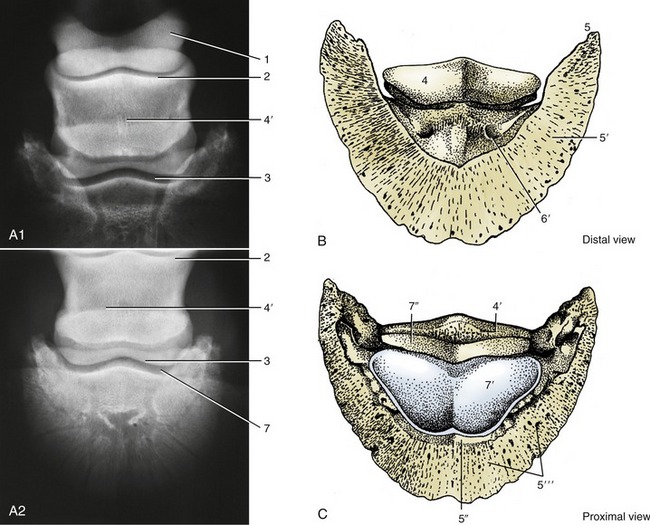
Figure 23–24 A1–A2, Dorsopalmar radiograph of hoof. B and C, Palmar and dorsal surfaces of distal phalanx (PI) and navicular bone.
1, Proximal phalanx; 2, proximal contour of middle phalanx; 3, distal contour of middle phalanx; 4, navicular bone (its flexor surface in B); 4′, proximal border of navicular bone; 5, palmar process of PIII; 5′, palmar (sole) surface of PI; 5″, extensor process and dorsal (parietal) surface of PI; 5″′, dorsal surface; 6, sole foramen; 7, coffin joint; 7′, articular surface of PI; 7″, articular surface of navicular bone.
The coffin joint allows flexion and extension to about the same degree as the pastern joint. The collateral ligaments are short and thick and are solidly anchored at both ends to depressions in the bones. The navicular bone, an integral part of the joint, is suspended from the distal extremity of PI by the collateral navicular ligaments (Figure 23–25/2). These cross the medial and lateral borders of PII and attach to the ends and proximal border of the navicular bone in a U-shaped fashion. A very short but wide distal navicular ligament (Figure 23–25/3) connects the distal border of the bone with PIII, attaching proximal to the prominent sole foramina. The capsule attaches to the articular margins of the three bones and resembles those of the other digital joints in having dorsal and palmar pouches. The pouches are small, and only the dorsal one is accessible for puncture (at the proximal border of the hoof); the procedure is not easy (see Figure 23–27, C-D).
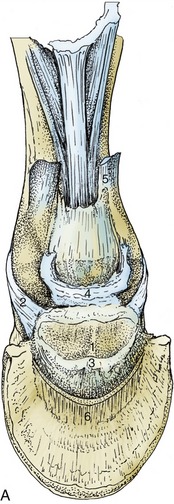

Figure 23–25 A, Ligaments of the navicular bone; palmar view.
1, Navicular bone; 2, collateral ligament of navicular bone; 3, distal navicular ligament; 4, connective tissue between coffin joint, digital sheath, and navicular bursa (see Figure 23–27/15); 5, stump of superficial digital flexor; 6, stump of deep digital flexor.
The incorporation of sesamoid bones in the fetlock and coffin joints divides the weight pressing onto the lower part of each joint over two bones, phalanx and sesamoid. The elasticity of the sesamoid ligaments and the flexor tendons behind them allows the joint to yield slightly during foot impact. This is but one of several mechanisms designed to dissipate the concussion generated by so heavy and swift an animal. The concussive effects may be accentuated by poor conformation: upright pasterns and small feet (in relation to body size) are a combination encountered frequently in animals afflicted with navicular disease, a relatively common cause of lameness. This condition is characterized by erosion at the margins of the navicular bone, where its ligaments attach, and by inflammation and degeneration of the navicular bursa (Figure 23–27/10) and the related part of the deep flexor tendon (Figure 23–27/13). However, the exact pathogenesis is still debated, and different authorities give quite contradictory explanations.
THE TENDONS, ANNULAR LIGAMENTS, AND INTEROSSEUS MUSCLE
The tendons of the common and lateral digital extensors enter the foot to the front of the metacarpal bone; those of the superficial and deep flexors enter behind it. A third very important element in the support of the fetlock, the tendinous interosseus muscle, is situated on the palmar aspect, between the bone and the flexor tendons. The structures on the palmar surface of the cannon are enclosed within a deep fascia that extends from one splint bone to the other. The fascia is thickest immediately below the carpus but gradually thins when followed distally, and toward the fetlock it offers little hindrance to the palpation of deeper structures.
The common extensor tendon is protected by a synovial bursa as it passes over the dorsal pouch of the fetlock joint. Broadening, it makes limited attachments at the proximal borders of PI and PII before receiving the extensor branches of the interosseus that wind around the digit. It ends on the extensor process of PIII (Figures 23–26/1 and 23–27/17).
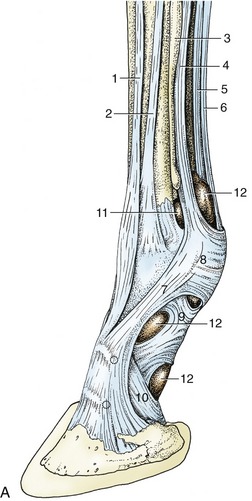
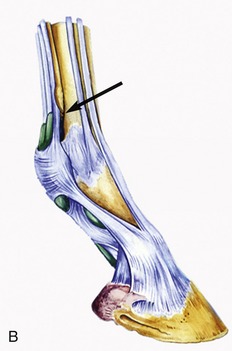

Figure 23–26 A, Tendons and annular ligaments of the left digit; dorsolateral view. The two dotted circles indicate the sites for injecting the pastern and coffin joints.
1, Common digital extensor; 2, lateral digital extensor; 3, lateral splint bone; 4, interosseus; 5, deep digital flexor; 6, superficial digital flexor; 7, extensor branch of interosseus; 8, palmar annular ligament; 9, proximal digital annular ligament; 10, distal digital annular ligament; 11, palmar pouch of fetlock joint; 12, digital sheath.
B, Schematic drawing of right digit showing digital sheath (green) and palmar pouch of fetlock joint (arrow). C, Puncture of fetlock joint.
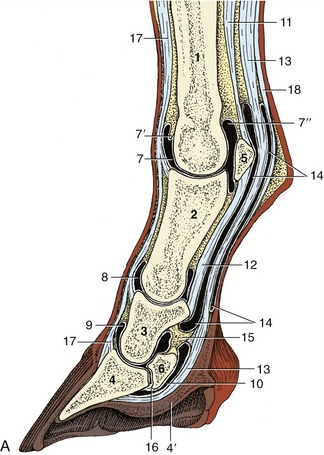
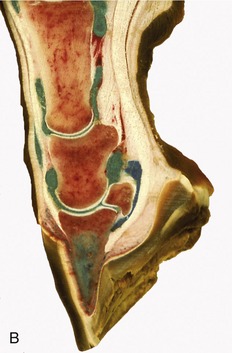


Figure 23–27 A, Axial section of digit, semischematic. B, Axial section of digit with latex-injected fetlock, pastern, and coffin joints. C, Corresponding magnetic resonance image. D, Puncture of coffin joint.
1, Large metacarpal bone; 2, proximal phalanx; 3, middle phalanx; 4, distal phalanx; 4′, digital cushion; 5, proximal sesamoid bone; 6, distal sesamoid (navicular) bone; 7, dorsal pouch of fetlock joint; 7′, capsular fold; 7″, palmar pouch of fetlock joint; 8, 9, dorsal pouch of pastern and coffin joints; 10, navicular bursa; 11, interosseus; 12, straight sesamoidean ligament; 13, deep flexor tendon; 14, digital sheath; 15, connective tissue bridge; 16, distal navicular ligament; 17, common digital extensor tendon; 18, superficial flexor tendon.
The lateral extensor tendon descends on the metacarpal bone lateral to the common tendon, crosses the fetlock joint, and ends on a roughening on the dorsal aspect of PI. Both extensor tendons, though easily palpated in the metacarpus, evade recognition beyond the fetlock joint, where they become broader and thinner. The extensor branches of the interosseus are more prominent below the skin.
The superficial digital flexor tendon becomes subcutaneous (except for the fascial investment of distally decreasing thickness) after emerging from the carpal canal and provides the caudal border of the cannon. It forms a sleeve around the deep flexor tendon at the level of the proximal sesamoid bones (Figure 23–28, B). The deep part of the sleeve splits opposite the middle of PI to allow the superficial flexor to attach to the distal tubercles of PI and the adjacent complementary fibrocartilage of PII. The palmar part of the sleeve ends at about the same level to allow the deep flexor tendon to gain the superficial position, where it is palpable for a few centimeters before it enters the hoof.
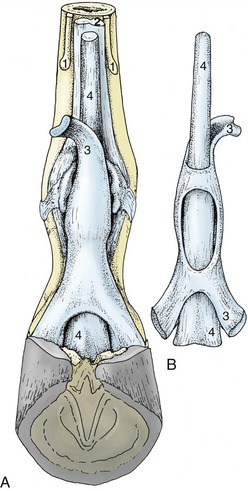
Figure 23–28 Relations and topography of the superficial and deep flexor tendons. A, Palmar view, in situ. B, Dorsal view, isolated.
1, Splint bones; 2, interosseus; 3, superficial digital flexor; 4, deep digital flexor.
Only the medial and lateral borders of the deep flexor tendon can be palpated above the fetlock. The tendon is most easily separated and distinguished from that of the superficial flexor muscle when the fetlock joint is flexed to relieve tension, but even in these circumstances, it is usually impossible to identify the very strong accessory (check) ligament that arises from the palmar carpal ligament to join the deep face of the tendon toward the middle of the cannon (Figure 23–18/14′). The tendon then passes the fetlock in the sleeve formed by the superficial tendon, and beyond the middle of PI it rides over the bearing surface provided by the complementary fibrocartilage of PII. It then widens before passing over the navicular bone to terminate on PIII.
The flexor tendons are held in place by three annular ligaments, which are local thickenings of the deep fascia. The first, the palmar annular ligament, arises from the abaxial borders of the proximal sesamoid bones; because it adheres to the superficial flexor tendon, the potential for movement between the tendon and the sesamoids is clearly restricted. The second, the proximal digital annular ligament, resembles an X when viewed from behind (Figure 23–29/6). The proximal margin of the X and the four corners, which attach near the proximal and distal tubercles of PI, are most easily distinguished because the body and the distal margin fuse with the superficial tendon. The third, the distal digital annular ligament, arises from the medial and lateral borders of PI together with the abaxial palmar ligaments of the pastern joint. It provides a sling that fuses with the palmar surface of the deep tendon, continuing to the insertion on PIII within the hoof; it separates the tendon from the digital cushion. Usually, only its free upper border can be demonstrated (Figure 23–29/7).
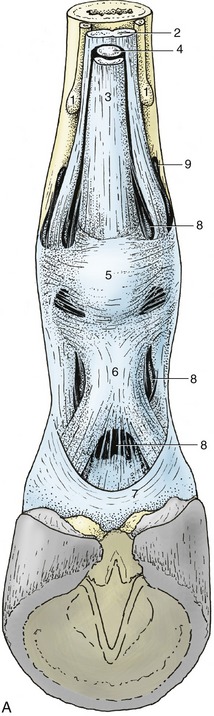
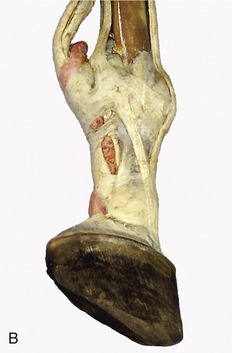
Figure 23–29 A, Annular ligaments of the digit.
1, Splint bones; 2, interosseus; 3, superficial digital flexor; 4, deep digital flexor; 5, palmar annular ligament; 6, proximal digital annular ligament; 7, distal digital annular ligament; 8, digital sheath; 9, palmar pouch of fetlock joint.
B, Digital sheath injected with pink, fetlock joint with red latex.
The navicular (podotrochlear) bursa protects the deep flexor tendon from excessive friction and pressure against the navicular bone (Figure 23–27/10). More proximally the tendon shares a complex synovial (digital) sheath (Figure 23–27/14) with the superficial flexor tendon. The sheath begins a few centimeters proximal to the fetlock joint and ends level with the middle of PII (Figure 23–27, B). It lubricates the passage of the tendons over the bearing surfaces and under the free parts of the annular ligaments and facilitates their movements against each other where they exchange position. It is a common site of inflammation and, when distended, bulges most noticeably above the proximal sesamoid bones. Although the sheath is in close proximity to the fetlock, pastern, and coffin joints and to the navicular bursa, these cavities do not communicate, except for a connection between the sheath and the coffin joint in the foal. Despite this, anesthetics injected into the coffin joint of adult horses reach the navicular bursa by diffusion.
The interosseus muscle is a strong, flat, predominantly tendinous band, better known as the suspensory ligament. Although it includes a small contingent of muscle fibers, there is little evidence that these fibers are gradually replaced by tendinous tissue as the animal becomes older and heavier. The interosseus arises from the palmar carpal ligament and adjacent part of the large metacarpal bone, descends between the splint bones, and divides a short distance above the fetlock. The two divisions are substantial—and easily palpable—and insert on the abaxial surface of the proximal sesamoid bones. Each detaches a weak (extensor) branch that winds around PI to join the common extensor tendon at the level of the pastern joint (Figures 23–23/1 and 23–26/7).
A functional continuation of the interosseus beyond the sesamoid bones is provided by the cruciate, oblique, and straight sesamoidean ligaments (Figure 23–23/4,5). These support the normally overextended fetlock joint; the inclusion of the sesamoids permits frictionless movement over the flexor aspect of the joint (Figure 23–27/5,11,12). Energy, stored within the apparatus (and in the flexor tendons) by stretching on hoof impact, is released at the end of the stride, which allows the joint to flex and impart forward impetus.
THE HOOF
The distal extremity of the limb is protected by the hoof, which is formed by epithelial keratinization over a greatly modified dermis*; this is continuous with the common dermis of the skin at the coronet (the term applied to the junction between skin and hoof). The hoof is conveniently divided into wall, periople, sole, and frog; the last is an integral part of the hoof capsule, although homologous with the digital pad of other species (see Figure 10–18).
The wall is the part of the hoof visible in the standing animal (see Figure 10–20). It is highest at its dorsal segment (toe) and decreases in height over the sides (quarters) until it is reflected on itself, forming the rounded heels at the back of the hoof. The inflected parts continue forward for a short distance as the bars that are visible beside the frog when the hoof is raised (Figure 23–30/1′″). The angle that the toe makes with the ground is about 50° in the forelimb and slightly more in the hindlimb; the quarters descend toward the ground more steeply, especially on the medial side. The wall is thickest at the toe and gradually thins toward the bars, which is an important point for farriers to bear in mind when rasping or driving nails.
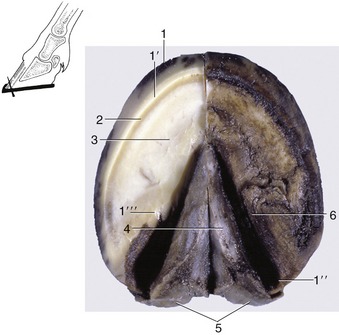
Figure 23–30 Ground surface of the hoof. The inset shows the direction of hoof nails started at the white line.
1, Wall; 1′, unpigmented part of wall; 1″, heel; 1′″, bar; 2, white line (union of wall and sole); 3, sole; 4, frog; 5, bulbs of the heels; 6, paracuneal groove.
The wall grows from the epithelium covering the coronary dermis (Figure 23–31/2) (which almost surrounds the digit at the coronet). It consists of horn tubules embedded in less structured intertubular horn and slides over the dermis covering the coffin bone and hoof cartilages to be worn away by contact with the ground. The greater part forms the generally pigmented stratum medium. The deeper, nonpigmented stratum internum comprises about 600 (homy) laminae that interdigitate with the sensitive laminae of the underlying laminar dermis (Figure 23–31/5). Trauma affecting the coronary dermis causes horn defects that descend with the wall, reaching the ground in about 8 months (a rate of growth of less than 1 cm per month).
Figure 23–31 The structure of the hoof wall and of the underlying laminar dermis.
1, Perioplic dermis; 2, coronary dermis; 3, horn tubules growing from epithelium over papillae (3′) of the coronary dermis (enlarged in left inset); 4, stratum medium of wall consisting of horn tubules embedded in less structured intertubular horn; 5, dermal laminae that interdigitate with the horny laminae of the hoof wall (see also insets to the right); 6, periople; 6′, stratum externum of wall (dried periople).
The periople contributes the stratum externum of the wall (Figure 23–31/6,6′). It consists of a band of soft, rubbery horn a few millimeters thick near the coronet but dries to a thin glossy layer distally. The band widens toward the palmar aspect where it covers the bulbs of the heels and blends with the base of the frog. The periople, which also consists of an admixture of tubular and intertubular horn, is produced over the narrow perioplic dermis (Figure 23–31/1) directly proximal to the coronary dermis.
The sole fills the space between the wall and frog and forms most of the undersurface of the hoof (see Figure 23–33). It is slightly concave so that only the distal edge of the wall and the frog make contact on firm ground. The parts between the bars and quarters, known as the angles of the sole, are the seat of “corns,” blood-soaked flecks resulting from trauma to the underlying dermis. Sole horn, though softer than that of the wall, again consists of an admixture of tubules and intertubular horn; it tends to become spongy and to flake in animals required to stand on soiled bedding.
The junction between the sole and the wall is known as the white line (zona alba; Figure 23–30 and Figure 23–32, A). It includes some of the nonpigmented stratum medium of the wall, the distal ends of the horny laminae (stratum internum), and, between these, pigmented horn produced over the terminal papillae of the laminar dermis (these project distally, level with the dermal papillae above the sole) (Figure 23–33/3). The startlingly white streak within the broad, so-called white line is provided by “cap horn” produced over the distal third of the dermal laminae. The internal rim of the white line is where farriers place nails when shoeing; the nails pass obliquely through the wall to emerge a few centimeters above the shoe, where they are cut and clinched (see Figure 23–30).





Figure 23–32 A, Section of the hoof. B, Transverse section of the part of the hoof at level indicated in A. C, Dermal lamellae that interdigitate with the horny lamellae of the hoofwall. D, Enlargement of the dermal lamellae. E, The small secunduring lamellae of the dermal lamellae are shown.
1, Stratum externum. 2, stratum medium. 3, Stratum internum; 3′, white line; 4, wall; 5, primary horny laminae; 6, primary dermal laminae; 7, interdigitating secondary laminae; 8, horn.
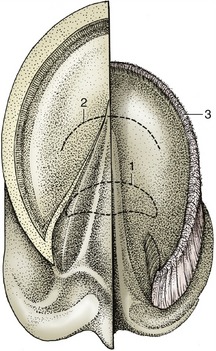
Figure 23–33 Ground surface of the hoof. Half of the hoof has been removed to expose the dermis.
1, Position of navicular bone; 2, position of the insertion of the deep flexor tendon; 3, terminal papillae.
The wedge-shaped frog (cuneus ungulae) projects into the sole from behind. Its wide base closes the gap between the heels, where it furnishes the palmar part of the hoof (Figure 23–30/4) and spreads upward to end in thickenings—the bulbs of the heels—that overhang the heels of the wall. Its external surface is marked by a central groove to which corresponds an internal spine (frog-stay) that juts proximally into the digital cushion (see further on). The frog is separated from the bars and the sole by deep (paracuneal) grooves (Figure 23–30/6) that accentuate its medial and lateral borders; the grooves are convenient for the application of hoof testers (large “pincers” used to detect soreness in deeper structures). The projection of these structures is shown in Figure 23–33. In horses stood on damp bedding, the grooves are often the site of “thrush,” a foul-smelling infection that may spread to deeper, sensitive tissues.
The frog horn is tubular and fairly soft and elastic, being kept pliable by the fatty secretion of glands in the underlying digital cushion. Though horses can be shod with the “frog off the ground” (as were city draft horses formerly), a sound hoof requires the frog pressure that is obtained through ground contact.
The dermis deep to the hoof capsule can be divided into five parts: perioplic, coronary, and laminar dermis and those of the sole and frog that are associated with the like-named segments of the hoof. Both the coronary and laminar dermis are associated with the wall.
The entire dermis (other than the laminar part) carries papillae that run parallel to each other and to the dorsal surface of the hoof, directed toward the ground. It is richly supplied with vessels and nerves, and an ill-directed farrier’s nail that penetrates the dermis (“quick”) therefore draws blood and causes pain. Because nerves are absent from the hoof capsule, the apposing dermal and epidermal tissues are often designated sensitive and insensitive, respectively.
The subcutis, generally thin, attaches the dermis to such deeper structures as the coffin bone, the hoof cartilages, and the tendons. It is greatly thickened in two places: beneath the coronary dermis (the coronary cushion) and beneath the frog dermis (the digital cushion). These cushions consist of a feltwork of collagenous and elastic fibers interspersed with small islands of fat and cartilage.
The narrow, raised perioplic dermis embraces the digit at the coronet. Studded with short papillae, it widens caudally where it covers the bulbs of the heels (Figure 23–34/1).
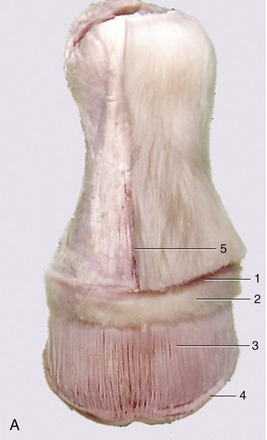



Figure 23–34 A, Dermis exposed by removal of the hoof. B, Hoof shoe removed from specimen A. C, Changes in the form of the hoof during locomotion. D, Shoe showing the heel part polished by movement of the hoof heel.
1, Perioplic dermis; 2, coronary dermis; 3, laminar dermis; 4, terminal papillae on the ends of the dermal laminae; 5, cut edge of skin.
The wider elevation of the coronary dermis (Figure 23–34/2) is separated from the perioplic dermis by a shallow groove. Its prominence is due to the rounded underlying coronary cushion. The coronary dermis also follows the coronet, but like the hoof wall, it folds on itself above the heels. It is widely known as the coronary band, although many clinicians interpret this term more widely to include the (external) coronet. The epithelium over most of its surface produces the bulk of the wall; that over the tucked-in distal margin produces most of the unstructured horn of the horny laminae.
The laminar dermis is composed of about 600 sensitive (dermal) laminae that interdigitate with the insensitive (horny) laminae on the deep surface of the wall (Figure 23–32/5-7). Both sets bear numerous secondary laminae that further secure the wall to the dermis, and ultimately to the coffin bone, while leaving it possible for the horn to slide over the bone.
Normally the epithelium covering the sensitive laminae proliferates just sufficiently to allow the wall to slide past. However, it has the capacity to produce additional amounts of (scar-) horn when a defect in the wall must be closed. This potential is utilized even more dramatically in chronic laminitis (founder), a disease in which the normal attachment is loosened and the coffin bone rotates away from the wall; the space in front of the bone becomes filled with irregular horn produced over a new set of sensitive laminae that form near the dorsal surface of the bone.
The dermis of the sole is firmly attached to the undersurface of the coffin bone.
The dermis of the frog lies between the frog and the digital cushion, which occupies the space below the deep flexor tendon and between the cartilages of the hoof (Figure 23–35/6).
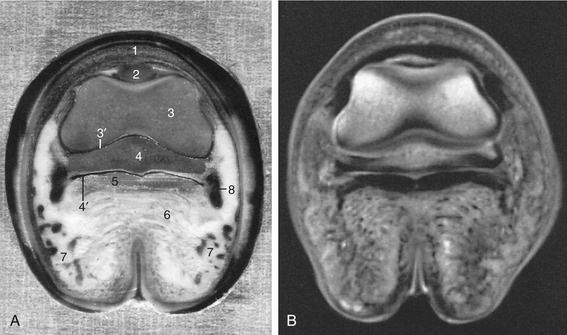
Figure 23–35 A, Transverse section of the digit at the level of the navicular bone, proximal surface. B, Magnetic resonance image taken at the same level.
1, Coronary dermis; 2, extensor process of distal phalanx (PIII); 3, distal end of middle phalanx (PII); 3′, coffin joint; 4, navicular bone; 4′, navicular bursa; 5, deep flexor tendon; 6, digital cushion; 7, cartilage of hoof and venous plexus; 8, position of digital vessels and nerve.
The blood supply of the dermis comes from three sets of vessels, all branches of the digital arteries that descend into the hoof to each side of the flexor tendons. Those that arise at the level of the coronet supply the perioplic and coronary dermis, and those that arise opposite the pastern joint supply branches to the digital cushion and the dermis of the caudal aspect of the hoof, including the frog; the vessels of the third set arise from the dorsal and palmar terminal branches (mentioned in connection with the sole foramina of PIII) and go to the laminar and sole dermis. Veins do not accompany the arteries but instead form extensive interconnected networks in the dermis* and underlying subcutis, particularly in the coronary band, in the laminar dermis, and under the palmar aspect of the hoof (the coronary, dorsal, and palmar plexuses, respectively). They combine to form medial and lateral digital veins that become satellite to the arteries at the level of the pastern joint.
The hoof is a flexible structure, yielding under pressure on impact with the ground and so dissipating much of the attending concussion. The load that presses on the coffin joint is split between PIII and the navicular bone. The force on PIII is transmitted by the interdigitating laminae to the wall of the hoof, whose distal border is thus a principal weight bearer, especially in horses shod with the frog off the ground. The force retracts the slanted toe while the heels are spread by the distortion of the wall. The force exerted on the navicular bone presses into the yielding “sling” provided by the deep flexor tendon, which in turn compresses the digital cushion and frog (see Figure 23–27). These redirect the force sideways: the cushion presses against the cartilages and the frog presses against the bars and sole, thus assisting the outward movement of the heels (Figure 23–34, C).
The to-and-fro movement of the heels is not obvious to the eye, but as any farrier can verify, it polishes the upper surface of the related parts of the shoe. It is to avoid interfering with this mechanism that farriers do not nail these parts of the shoe to the wall; if this precaution is neglected, the horse develops “contracted heels” and eventually goes lame (Figure 23–34, D).
The mechanism explains why the coffin bone is continued caudally by cartilage rather than by bone (Figure 23–21/4). Progressive calcification of the cartilage with subsequent replacement by bone is a common aging process known as “sidebone,” which is yet another cause of lameness.
The movements of the heels have a further benefit, aiding venous return. The dense plexuses on both sides of the cartilages (Figure 23–35/7) are compressed at each step and deliver blood into the valved digital veins. This has been shown experimentally by cannulating a digital vein under local anesthesia; blood is squirted at every step the horse takes. (In other species, contractions of striated muscles within the foot compress the veins and assist the venous return.)
Apart from minor differences in conformation, the forehoofs and hindhoofs are identical (Figure 23–36). In conformity with its larger weight-bearing role, the forehoof is somewhat wider and therefore more rounded in outline than the narrower, more pointed hindhoof (Figure 23–36, C-D). However, the distinction is less than the adjectives suggest, and the provenance—fore or hind—of a single specimen is not always obvious.
Figure 23–36 A, In former days horses at pasture were hobbled with a “pastern”; this is why the narrow part of the limb above the hoof is known today as the pastern. B, Palmar (plantar) view of the foot; the lateral (L) angle of the wall (with the ground) is more acute than the medial (M). C and D, The angle at the toe is more acute in the forelimb than in the hindlimb.
When the hoof capsule first forms early in fetal life, it consists of horn that is soft, unpigmented, and of uniform composition. Later, new hard and more structured horn is produced that pushes the soft horn distally, where it becomes a rather misshaped mass covering the entire ground surface of the hoof and (thinly) an adjoining strip of the hoof wall. When exposed to air at term, the soft mass soon dries and sloughs away. The soft mass over the hard horn of the fetal hoof is said to prevent injury to the fetal membranes and birth canal (Figure 23–37).
THE PASSIVE STAY-APPARATUS
It is well-known that horses can remain on their feet for much longer than other domestic animals. In fact, they are thought by many to sleep while standing. This is not quite true: they may rest or doze standing, but for a refreshing sleep they lie down, often only at night when unobserved. When horses stand quietly, most weight is carried by the tendons, ligaments, and deep fascia of the stay-apparatus, which do not tire; only a minimum of muscular energy is expended.
The bony column of the forelimb supports the cranial end of the trunk at the attachment of the serratus ventralis muscle to the medial surface of the scapula (Figure 23–38, A/1). A vertical line dropped from the center of this attachment passes caudal to the shoulder, through the elbow, through or slightly cranial to the carpal joint, and cranial to the fetlock and pastern joints. If unsupported, the column would collapse by flexion of the shoulder and elbow joints, by overextension (or possibly flexion [buckling forward]) of the carpal joint, and by overextension of the fetlock and pastern joints. (The coffin joint actually flexes when the fetlock sinks under weight and can be disregarded in this discussion.)
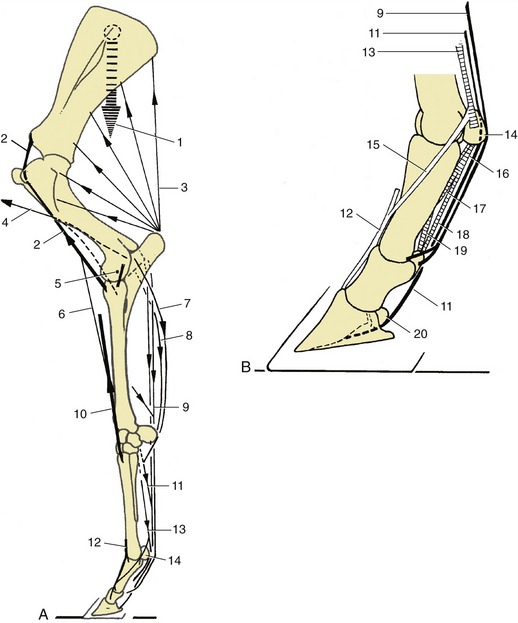
Figure 23–38 A, The stay-apparatus of the left forelimb; lateral view. B, Detail of digit; lateral view.
1, Weight of trunk; 2, internal biceps tendon; 3, triceps; 4, brachiocephalicus and brachial fascia to elbow joint; 5, axis of elbow rotation, next to eccentric collateral ligament; 6, lacertus fibrosus; 7, ulnaris lateralis; 8, flexor carpi ulnaris; 9, superficial digital flexor and accessory (check) ligament; 10, extensor carpi radialis; 11, deep digital flexor and accessory (check) ligament; 12, common digital extensor; 13, interosseus; 14, proximal sesamoid bones; 15, extensor branch of interosseus; 16, 17, 18, cruciate, oblique, and straight sesamoidean ligaments; 19, axial palmar ligament; 20, navicular bone.
The shoulder joint is prevented from flexion by the strong internal biceps tendon (Figure 23–38/2) that connects the supraglenoid tubercle of the scapula with the radius. The latter attachment can be regarded as fixed because it is very close to the axis of rotation of the elbow joint (Figure 23–38/5), which is stabilized by the weight on the limb. Tension in the wide biceps tendon puts great pressure on the intertubercular groove of the humerus. Indeed, some believe that the molding of the tendon to the intermediate tubercle actually causes the joint to lock. At its other end, the pull of the biceps is transmitted via the lacertus and extensor carpi radialis (Figure 23–38/6,10) to a second fixed point at the upper end of the large metacarpal bone. This pull augments the action of the extensors of the carpal joint and prevents that joint from buckling forward and collapsing the limb; any tendency toward overextension is prevented by close packing of the carpal bones in front and by the strong palmar carpal ligament behind (Figure 23–15, A-B/7).
The fetlock joint is prevented from overextension principally by the suspensory apparatus (comprising the interosseus, proximal sesamoid bones, and distal sesamoidean ligaments), which is tensed under load (Figure 23–38/13,14,16-18). The effect is reinforced by tension in the accessory (check) ligaments and distal parts of the superficial and deep flexor tendons (Figure 23–38/9,11).
Tension in the deep flexor tendon tends to flex the coffin joint, which causes the toe of the hoof to dig into the ground. The extensor branches of the interosseus (Figure 23–38/15), pulling on the extensor process of the bone at impact, counteract this and keep the hoof level.
Overextension of the pastern joint is opposed by the axial and abaxial palmar and straight sesamoidean ligaments (Figure 23–38/18,19), which span its palmar aspect. The taut deep flexor tendon gives additional support. (Buckling forward is prevented by the superficial flexor that attaches on the palmar aspect of the joint.)
With the shoulder joint fixed (by the biceps tendon), the weight of the trunk rests on the upper end of the nearly vertical radius. Therefore, unless the horse sways markedly forward, only small forces are required to prevent the elbow joint from flexing. These are mainly supplied by passive tension of the tendinous components of the carpal and digital flexors (the superficial digital flexor especially) and the eccentrically placed collateral ligaments (Figure 23–38/5,7-9). Recent information indicates that because of their muscle fiber composition—characteristic of postural muscles—the anconeus and the medial head of the triceps may also oppose flexion of the elbow joint. The large mass of the long and lateral heads of the triceps—the principal extensor of the elbow joint—remains flaccid even when the other forelimb is picked up to make the horse stand three-legged (see Figure 23–2/1, and the effects of radial paralysis on page 621).
THE BLOOD VESSELS AND LYMPHATIC STRUCTURES OF THE FORELIMB
The axillary artery, the main supply of the limb, enters the axillary space after crossing the cranial border of the first rib, where it may be punctured (p. 539). It descends on the medial aspect of the arm in company with the median and ulnar nerves and shortly becomes known as the brachial artery. The trunk releases several branches to the muscles of the shoulder and arm, the most prominent being the subscapular artery, which follows the caudal border of the scapula, and the deep brachial, which disappears between the heads of the triceps (see Figure 23–8). Just proximal to the elbow joint, lesser cranial and caudal branches (transverse cubital and collateral ulnar arteries, respectively) are detached for the muscles in the forearm; their further courses and connections are shown in Figure 23–39/11,12. The brachial artery crosses the elbow cranial to the medial collateral ligament, where it can be palpated and the pulse evaluated, through the pectoralis transversus (Figure 23–40/5). Together with the median nerve it dips under the flexor carpi radialis caudal to the radius and soon gives off the common interosseous artery, which passes through the interosseous space to reach the craniolateral muscles of the forearm.

Figure 23–39 The major arteries of the right forelimb. A, Medial view. B, Palmar view.
1, Axillary a.; 2, suprascapular a.; 3, subscapular a.; 5, thoracodorsal a.; 6, 7, caudal and cranial circumflex humeral aa.; 8, brachial a.; 9, deep brachial a.; 10, collateral radial a.; 11, collateral ulnar a.; 12, transverse cubital a.; 13, common interosseous a.; 14, median a.; 15, radial a.; 16, 16′, medial and lateral palmar aa.; 17, 17′, medial and lateral palmar metacarpal aa.; 18, 18′, medial and lateral digital aa.
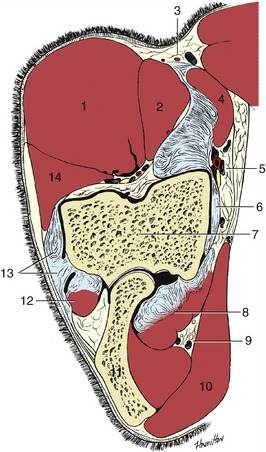
Figure 23–40 Transverse section of the left elbow.
1, Extensor carpi radialis; 2, brachialis; 3, medial cutaneous antebrachial nerve and cephalic vein lying on lacertus fibrosus; 4, biceps; 5, brachial vessels and median nerve; 6, medial collateral ligament; 7, humerus; 8, flexors arising from medial epicondyle of humerus; 9, ulnar nerve and collateral ulnar vessels; 10, tensor fasciae antebrachii; 11, olecranon; 12, ulnaris lateralis; 13, lateral collateral ligament; 14, common digital extensor.
The main trunk, now redesignated the median artery (Figure 23–41/12), gradually works its way to the caudal surface of the forearm before dividing into three above the carpus. The lesser branch (palmar branches of the median and radial artery) contribute the small palmar metacarpal arteries that accompany the interosseus muscle, while the main trunk passes through the carpal canal with the digital flexor tendons (Figure 23–15, B/19). It continues with these in the cannon where it becomes the medial palmar artery, the main artery to the digit and hoof. This inclines axially before splitting into the medial and lateral digital arteries above the fetlock. The digital arteries pass over the abaxial surfaces of the sesamoid bones (where they are palpable) and continue into the digit on each side of the flexor tendons; the lateral artery is reinforced by the small metacarpal arteries that join above the sesamoid bone (Figure 23–39/18′). The branches of the digital arteries distal to the fetlock are symmetrical. Dorsal and palmar branches are given off opposite PI, and these supply adjacent structures while forming a circle about the bone. A branch to the digital cushion is detached level with the pastern joint before the digital artery disappears by passing deep to the hoof cartilage. Dorsal and palmar branches detached opposite the middle of PII comport themselves similarly to the branches about PI but also take part in the supply of the dermis of the hoof. The dorsal and palmar terminal branches (to PIII) have been described (pp. 602 and 612); the palmar branches anastomose to form a terminal arch within the bone.
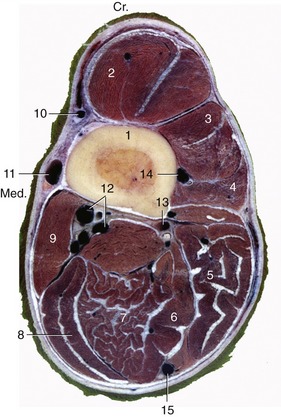
Figure 23–41 Transverse section of the right forearm at the level shown in Figure 23–42.
1, Radius; 2, extensor carpi radialis; 3, common digital extensor; 4, lateral digital extensor; 5, ulnaris lateralis; 6, deep digital flexor; 7, superficial digital flexor; 8, flexor carpi ulnaris; 9, flexor carpi radialis; 10, accessory cephalic vein and medial cutaneous antebrachial nerve (from musculocutaneous); 11, cephalic vein; 12, median artery, veins, and nerve; 13, muscular branches of median vessels; 14, cranial interosseous vessels; 15, ulnar nerve and collateral ulnar vessels.
Most veins of the forelimb are satellite, although they are often duplicated or further replicated where they accompany the larger arteries (Figure 23–42/1). Some superficial veins seek independent courses, and those coming from the hoof have already been mentioned. The superficial veins include the cephalic and accessory cephalic veins, which are prominent and palpable in the forearm (Figure 23–42/10,10′). The cephalic vein is joined to the brachial vein via the median cubital at the elbow and continues to ascend in the groove between brachiocephalicus and pectoralis descendens, where it is at risk in “staking” injuries. It joins the external jugular vein at the base of the neck.
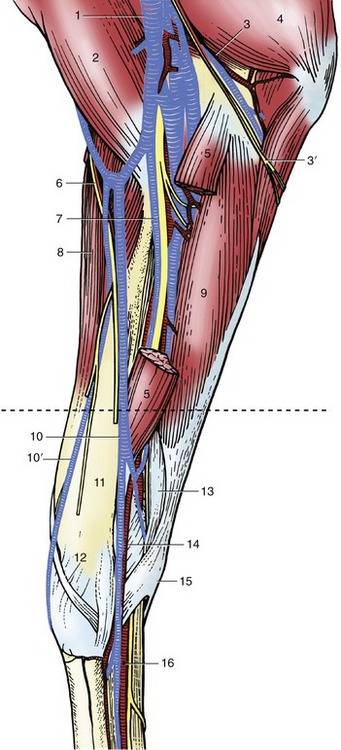
Figure 23–42 Dissection of the medial surface of the right forearm. (The broken transverse line indicates level of section in Figure 23–41.)
1, Multiple brachial veins; 2, biceps; 3, ulnar nerve and collateral ulnar vessels; 3′, caudal cutaneous antebrachial nerve; 4, triceps; 5, flexor carpi radialis, resected; 6, medial cutaneous antebrachial nerve; 7, median nerve and vessels; 8, extensor carpi radialis; 9, flexor carpi ulnaris; 10, 10′, cephalic and accessory cephalic veins; 11, radius; 12, extensor carpi obliquus; 13, superficial digital flexor; 14, radial artery and vein; 15, accessory carpal bone; 16, medial palmar nerve and vessels.
Two clusters of lymph nodes drain the free part of the limb. The cubital nodes lie on the medial aspect of the humerus just proximal to the elbow joint. They drain more distal parts of the limb and channel their outflow to the axillary nodes. These lie medial to the shoulder joint in the angle between the axillary and subscapular arteries and drain the arm and shoulder, together with a part of the thoracic wall caudal to the limb. Their efferent vessels go to the caudal deep cervical nodes, and thence the lymph flows directly or indirectly to the veins at the thoracic inlet. The superficial cervical nodes are arranged in a long chain that crosses the deep surface of the omotransversarius and brachiocephalicus (see Figure 18–38/8). The group consists of many small nodes, and because these are embedded in fat and do not form a firm compact mass, the group is not always easily located. Palpation should be directed to drawing the nodes forward, away from the subclavius against which they lie. The superficial cervical nodes mainly drain skin over the upper part of the limb but also receive some lymph from deeper structures.
THE NERVES OF THE FORELIMB
THE BRANCHES OF THE BRACHIAL PLEXUS
With few exceptions, the structures of the forelimb are innervated from the brachial plexus formed by contributions from the last three cervical and first two thoracic nerves (C6–T2). The plexus reaches the axilla as a broad band that emerges between the parts of the scalenus, but this soon divides into the usual dozen or so trunks. The major trunks, of clinical interest because of their vulnerability to injury or availability for nerve-blocking techniques, are described even though there are few specific features of significance above the carpus.
The suprascapular nerve (C6–7) leaves the axilla by sinking between the subscapular and supraspinatus muscles. It then winds around the neck of the scapula before expending itself in the supraspinatus and infraspinatus (Figure 23–43/2). A direct relationship to bone always carries a risk of injury, and the suprascapular nerve may be damaged where it lies against the scapula; apparently this is usually the result of pulling on the nerve as the animal stumbles with the limb stretched back. Injury is therefore most frequent in horses worked over uneven ground. Even serious damage to the nerve may have little immediate effect, although an observer stationed in front of an affected horse may notice a lateral deviation of the shoulder joint at each stride. After a time, muscular atrophy markedly alters the conformation of the shoulder region, causing the scapular spine to project above the wasted muscles. Suprascapular paralysis is commonly known as sweeny or shoulder slip.
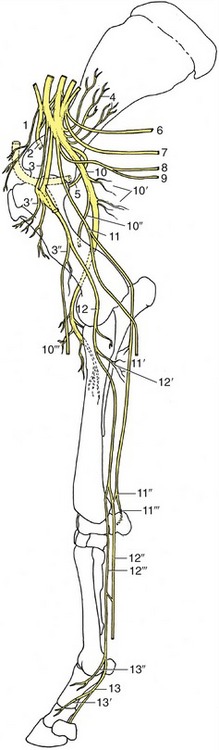
Figure 23–43 Distribution of the nerves in the right forelimb; medial view. The axillary artery at the shoulder joint is stippled.
1, Cranial pectoral nn.; 2, suprascapular n.; 3, musculocutaneous n.; 3′, proximal branches; 3″, distal branches with medial cutaneous antebrachial n.; 4, subscapular n.; 5, axillary n.; 6, long thoracic n.; 7, thoracodorsal n.; 8, lateral thoracic n.; 9, caudal pectoral nn.; 10, radial n.; 10′, proximal muscular branches (triceps); 10″, lateral cutaneous antebrachial n.; 10′″, distal muscular branches; 11, ulnar n.; 11′, caudal cutaneous antebrachial n.; 11″, palmar branch; 11′″, dorsal branch; 12, median n.; 12′, muscular branches; 12″, lateral palmar n.; 12′″, medial palmar n.; 13, medial palmar digital n.; 13′, 13″, dorsal branches.

Figure 23–44 Distribution of the medial palmar nerve.
1, Medial palmar n.; 2, communicating branch; 3, medial digital palmar n.; 3′, dorsal branch; 4, medial palmar artery and vein; 5, medial digital artery and vein.
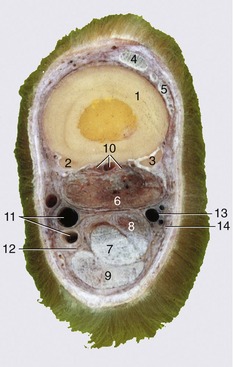
Figure 23–45 Transverse section of the middle of the right metacarpus.
1, 2, 3, Large and small metacarpal bones; 4, common digital extensor; 5, lateral digital extensor; 6, interosseus; 7, deep digital flexor; 8, accessory (check) ligament; 9, superficial digital flexor; 10, palmar metacarpal vessels and nerves; 11, medial palmar artery and vein; 12, medial palmar nerve; 13, lateral palmar artery; 14, lateral palmar nerve.
The musculocutaneous nerve (C7–8) (Figure 23–43/3,3′,3″) first runs craniolateral to the axillary artery before turning below the vessel to unite with the median nerve. A branch to the coracobrachialis and biceps is detached before the union. The part incorporated in the median trunk separates in the distal arm and supplies the brachialis and a medial cutaneous antebrachial nerve that crosses the lacertus fibrosus, where it is easily palpated, before being distributed to the skin over the cranial and medial aspects of the forearm and carpus. Damage to the musculocutaneous nerve cannot be common; however, should such occur, it is unlikely that loss of activity by the principal elbow flexors would greatly affect the gait.
The axillary nerve (C7–8) (Figure 23–43/5) has the usual course and distribution—to the principal flexors of the shoulder and the skin over the lateral aspect of the arm and forearm. There appear to be no records of traumatic damage to this nerve in the horse; it is known that in other species section does not impair the gait because other muscles are potentially able to flex the shoulder.
The radial nerve (C8–T1) is one of the larger branches of the plexus (Figure 23–43/10). It follows the caudal border of the brachial artery in the upper arm and later sinks between the medial and long heads of the triceps, rounding the caudal surface of the humerus to gain the lateral aspect of the limb. The nerve detaches branches to the triceps group in the proximal part of the arm; more distally, where it is covered by the lateral head of the triceps, it detaches other branches to the extensor muscles of the carpus and digit. A purely sensory continuation (lateral cutaneous antebrachial nerve) supplies skin over the lateral aspect of the forearm; contrary to the pattern in other species, this branch fades at the carpal level.
The radial nerve is the sole supply to the extensor muscles of all joints distal to the shoulder; the effects of damage are therefore proportionately severe. When injury is proximal to the origin of the tricipital branches, the animal is unable to support weight on the affected limb. It stands with the joints uncharacteristically flexed; the angle between scapula and humerus is enlarged, and the elbow is dropped in relation to the trunk. The hoof is rested on its dorsal aspect. High radial paralysis may arise from injury to or disease of the humerus or from damage to the brachial plexus itself. If other components of the plexus are affected, the signs may be complicated by simultaneous paralysis of the flexor muscles of the distal joints.
The results of injury distal to the origin of the tricipital branches are naturally less severe. Normal stances of the shoulder and elbow are maintained (Figure 23–46). The animal may rest the dorsal surface of the hoof on the ground but supports weight on the limb if the hoof is first restored to the normal position. Many horses learn to compensate for this disability by setting the hoof down before the impetus—gained when the limb is swung forward during the stride—is lost; the gait may appear almost normal when the terrain is flat, but unevenness quickly brings an affected animal into difficulties. Low radial paralysis may be simulated by the ischemia that sometimes results from prolonged lateral recumbency.
The median nerve (C8–T2) is the largest branch of the brachial plexus (Figure 23–46/12). It follows the cranial border of the brachial artery for most of its course through the arm but shifts to the caudal margin on approaching the elbow. It is covered by the pectoralis transversus as it crosses this joint, but even so, the nerve and artery together form a palpable cord (Figure 23–42/7). The two structures continue together as they descend the forearm, buried within the flexor mass of muscle; they divide at the same level, a little above the radiocarpal joint. The end branches, known as the medial and lateral palmar nerves, are described in the next section. The muscular branches to the flexor muscles of the carpus and digit are detached in the very proximal part of the forearm; beyond these detachments the nerve is purely sensory.
The ulnar nerve (T1–2) follows the caudal border of the brachial artery in the proximal part of the arm (Figure 23–43/11). It then diverges caudally, detaches the caudal cutaneous antebrachial nerve (for the caudal aspect of the forearm), and passes over the medial epicondyle of the humerus before entering the forearm. As it does so, it releases branches to the flexor muscles. The much depleted and now purely sensory nerve follows the ulnar head of the deep flexor at the caudal margin of the limb, under cover of deep fascia (Figure 23–41/15). A few centimeters above the carpus it divides into dorsal and palmar branches. The dorsal branch comes to the surface a short distance proximal to the accessory carpal bone and can be palpated against the ulnaris lateralis tendon attaching here; it passes over the lateral aspect of the carpus to expend itself in the skin over the lateral surface of the metacarpus. The palmar branch passes the carpus within the flexor retinaculum, where it exchanges fibers with the lateral palmar nerve, one of the terminal branches of the median.
The overlap of the median and ulnar nerves in their motor distribution makes it unlikely that damage restricted to either one would much affect the gait.
INNERVATION OF THE FOREFOOT
Four nerves attend to the innervation of most of the structures distal to the carpus: the medial and lateral palmar nerves from the median nerve, and the palmar and dorsal branches of the ulnar nerve. All but the dorsal branch of the ulnar lie palmar to the large metacarpal bone. The medial palmar nerve lies in the groove between the interosseus and the flexor tendons. In midcannon it detaches a communicating branch that crosses obliquely over the superficial flexor tendon (where it is palpable) to join the lateral palmar nerve. A little above the fetlock the medial palmar becomes the medial digital nerve, which immediately gives rise to one or two dorsal branches that ramify over the dorsomedial aspect of the digit and coronet. The main trunk of the digital nerve continues with the like-named artery over the outer aspect of the proximal sesamoid bone, passes under the ligament of the ergot (Figure 23–44), and then disappears into the hoof. The neurovascular bundle may be palpated against the sesamoid bone. Small branches supply the structures caudal to the phalanges. The nerve ends by supplying the laminar and sole dermis.
The lateral palmar nerve, it will be recalled, exchanged fibers with the palmar branch of the ulnar nerve at the carpus. It emerges from the short (1- to 2-cm) union and takes a course and has a distribution similar to that of the medial palmar nerve, including the ramifications in the digit. The first branch of this composite nerve arises at the carpus and soon splits into thin medial and lateral palmar metacarpal nerves that descend, deeply embedded, along the axial surface of the splint bones. These nerves supply the interosseus and the palmar pouch of the fetlock joint before becoming subcutaneous at the distal ends of the splint bones. They now supply the dorsal pouch of the joint before mingling with the dorsal branches of the digital nerves; they do not reach the coronet.
All of these nerves can be blocked at various levels—mainly for the diagnosis of lameness. The rationale of the procedure is that a lame horse temporarily becomes sound when the area that contains the undetected lesion is desensitized. A sequence of injections, in which increasingly larger territories are desensitized, is therefore required. Four sites are commonly used.
The autonomous zones of skin innervation are shown in Figure 23–1. A skin prick in the center of a zone tests for the integrity of the particular nerve.