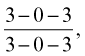18 The Head and Ventral Neck of the Horse
CONFORMATION AND EXTERNAL FEATURES
The general character of the head is determined by the age, the sex, and the breed. In young foals the cranial vault is domed to match the contours of the brain and projects above a face that is both short and shallow (Figure 18–1). The adult conformation develops as the face lengthens and deepens to accommodate the full complement of teeth and expanding paranasal sinuses; enlargement of the frontal sinus smoothes the dorsal profile at the junction of the face and cranium. Sex and breed differences are not wholly separable from those due to age, as the face is disproportionately increased in larger animals; a longer face is therefore characteristic of the adult compared with the juvenile, the stallion with the mare, and the heavy draft horse with the pony. The other very obvious breed difference concerns the dorsal profile; a relatively straight profile is generally preferred but some convexity (“ram’s head”) is characteristic of certain heavy breeds, whereas concavity (“dishing”) is the rule in Arabs and common in horses with admixture of Arab blood (see Figure 18–1). The ventral margin of the lower jaw of young horses may be disfigured by one or more rounded swellings, each corresponding in position to the root of an unerupted permanent cheek tooth. The temporary irregularities, though unsightly, are part of a normal process (p. 514).

Figure 18–1 Variations in the profile of the equine head. A, The common straight profile. B, The dished Arabian profile. C, The domed contour of the foal.
The skin is thinner and more firmly bound down than over most other parts of the body and is especially tight where it lies directly on bone. The coat is generally short, but a forelock continuing the mane may be prominent; a “mustache” is a feature of some animals, especially the larger breeds. Tactile hairs are numerous and widely scattered on the lips and chin and about the margins of the nostrils.
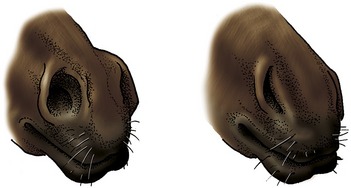
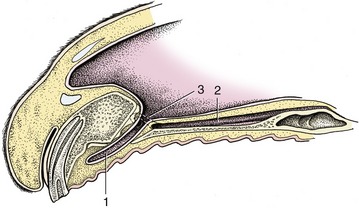
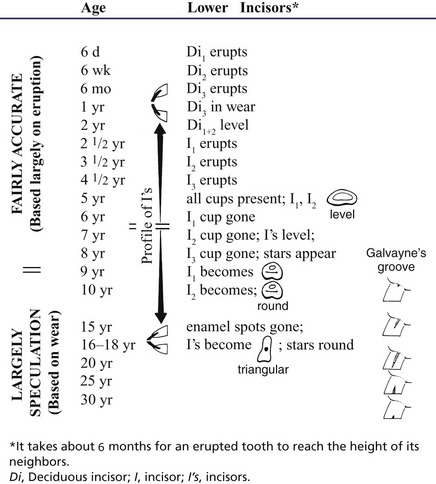
The nostrils are large and widely spaced, especially in the Thoroughbred (Figure 18–2). Their peculiar form is imposed by the supporting alar cartilages (Figure 18–3, B/1′,2′). The upper part of the opening leads to a blind nasal diverticulum (Figure 18–3/1″) that occupies the nasoincisive notch (Figure 18–3/6) and is without counterpart in other domestic species.* The lower part leads directly to the nasal cavity. It is therefore essential when a stomach tube is passed to ensure that it is guided into this lower part. The margins of the nostril are very flexible and allow the opening to be dilated, both actively when breathing is strenuous and passively on manipulation. The dilated nostril is rounded, and the change in form is achieved by apposition of the walls of the diverticulum. The pliancy of the tissues facilitates examination of the nasal vestibule and exposure of the opening of the nasolacrimal duct, which is found on the floor, about 5 cm internal to the entrance and near the mucocutaneous junction. Occasionally the duct has more than one opening.
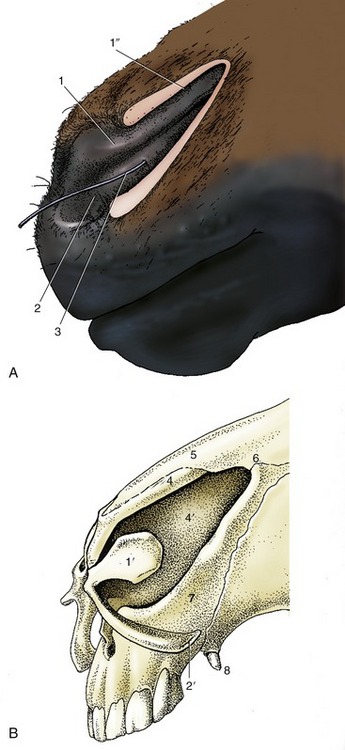
Figure 18–3 A, Left nostril opened laterally to expose nasal diverticulum. B, Nasal cartilages. 1, Alar fold, supported by the lamina (1)′ of the alar cartilage; dorsal to the alar fold is the nasal diverticulum (1″); 2, floor of nostril supported by the cornu of the alar cartilage (2′)—the floor leads into the nasal cavity; 3, probe in nasolacrimal duct; 4, dorsal lateral nasal cartilage; 4′, nasal septum; 5, nasal bone; 6, nasoincisive notch; 7, incisive bone; 8, canine tooth.
The entrance to the mouth is small, and the commissure is a little in front of the first cheek teeth (P2). The skin of the lips and adjacent part of the muzzle is sparsely covered by short, fine hairs that impart a velvety texture. The lips are both mobile and sensitive and are used in the selection and prehension of food. The sensitivity of the upper lip is exploited when a twitch is applied to control a horse during procedures (e.g., injections) elsewhere on the body. The application of acupressure causes the animal to become somewhat sedated while its heart rate is lowered and endorphins are released. It is suggested that the endorphins activate a pain-decreasing mechanism. The lower lip surmounts the chin swelling, which is based on a pad of fatty fibrous tissue.
The eyes are prominent and placed to each side of the head, indicating that the horse, like other herbivores, enjoys a panoramic field of vision. Indeed, horses may view almost all around by making only slight movements of the head. This ability to survey widely—perhaps through 330°—is obtained at the expense of the binocular field, which is limited to some 65°. The field of overlap is further reduced by the length and shape of the muzzle, which creates a blind area directly to the front (see Figure 9–1).
The upper and lower eyelids and adjacent skin carry a few scattered tactile hairs. The palpebral skin is thin and, being loosely attached, is thrown into folds when the eye is open. The lid margins carry numerous lashes, longer and more prominent on the upper than on the lower lid (Figure 18–4). The tarsal glands, which open at the junction of the skin with the conjunctiva, number about 50 in the upper lid, rather fewer below, and are clearly visible in palisade formation when the lids are everted. The palpebral conjunctiva is well vascularized, the bulbar part less generously; the bulbar conjunctiva is strongly pigmented toward the corneoscleral region. The third eyelid (Figure 18–5/1) in the medial angle can be exposed in the usual way by pressing on the eyeball through the upper eyelid; a small accessory lacrimal gland is associated with it. The lacrimal caruncle is prominent. The features of the eyeball are considered later (p. 527).
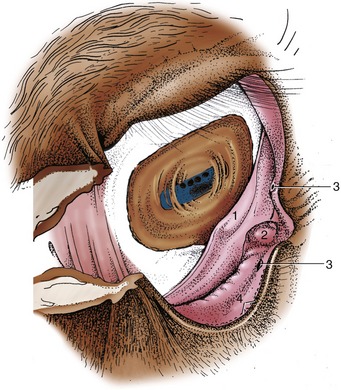
Figure 18–5 The right conjunctival sac. 1, Third eyelid; 2, lacrimal caruncle; 3, lacrimal puncta; 4, openings of the tarsal glands.
A depression caudal to the eye (behind the palpable postorbital bar of bone) is prominent in the animal at rest. It disappears and reappears during feeding in rhythm with the movements of the jaws; the effects are due to the displacement of a pad of fat interposed between the temporalis and the periorbita. The fat is depleted in horses in poor condition when exaggeration of the hollow contributes significantly to the haggard appearance.
Deposition of fat above the upper eyelid may produce a conspicuous swelling seen in animals suffering from Cushing disease.
Little need be said concerning the external ears, which are prominent and capable of being swiveled when attempts are made to locate the origin of a sound. Their carriage is also very expressive of emotion.
SUPERFICIAL STRUCTURES
THE MUSCLES OF FACIAL EXPRESSION
Many clinically important features are revealed as soon as the skin is removed. Large areas of the skull are not covered by any considerable thickness of soft tissue and are therefore vulnerable to injury. These areas include the dorsal aspect of the nose, the forehead, and part of the temple, in addition to much of the mandible. Prominent landmarks include the facial crest, which runs parallel to the dorsum of the nose; it begins above the rostral margin of the fourth cheek tooth, continues into the zygomatic arch, which forms the lower margin of the orbit, and extends to the temporomandibular joint (Figure 18–6/4). The joint itself is easily located by the salience of the lateral aspect of the condyle, directly before the palpable caudal margin of the mandible. The identification becomes more certain if the animal can be induced to perform chewing movements. The ventral margin of the mandible is also prominent, particularly the half that lies rostral to the masseter muscle. A shallow notch in the bone directly in front of the muscle conveys the facial vessels and parotid duct from the intermandibular space to the face.
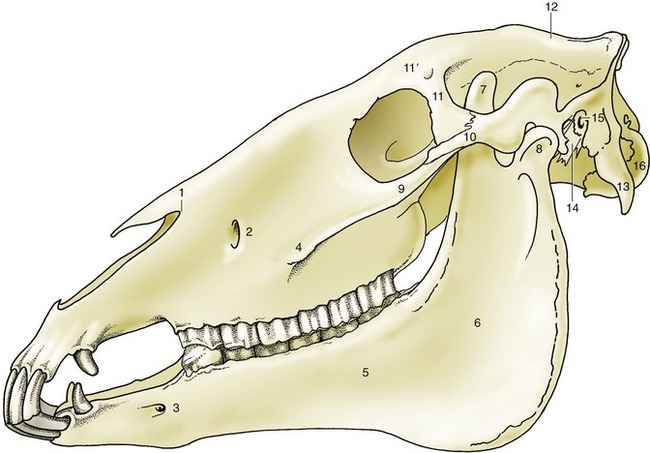
Figure 18–6 Lateral view of the skull. 1, Nasoincisive notch; 2, infraorbital foramen; 3, mental foramen; 4, facial crest; 5, body of mandible; 6, ramus of mandible; 7, coronoid process; 8, condylar process; 9, temporal process of zygomatic bone; 10, zygomatic process of temporal bone; 11, zygomatic process of frontal bone; 11′, supraorbital foramen; 12, external sagittal crest; 13, paracondylar process; 14, styloid process; 15, external acoustic meatus; 16, occipital condyle.
The incomplete sheet of cutaneous muscle over the lateral aspect of the head is best developed where it merges with the orbicularis oris around the opening of the mouth.
A few individual mimetic muscles deserve notice. The levator labii superioris arises over the maxilla and runs dorsorostrally to form a common tendon with its fellow of the other side (Figure 18–7/7); the tendon, which is enclosed within a synovial sheath, descends between the nostrils to splay out within the upper lip. This muscle is responsible for the lip curl (Flehmen) seen in certain circumstances, including sexual excitement. The levator belly is easily palpated, and because it covers the infraorbital foramen, it must be pushed dorsally to locate the emergent infraorbital nerve. This foramen lies along the line joining the nasoincisive notch to the rostral end of the facial crest.
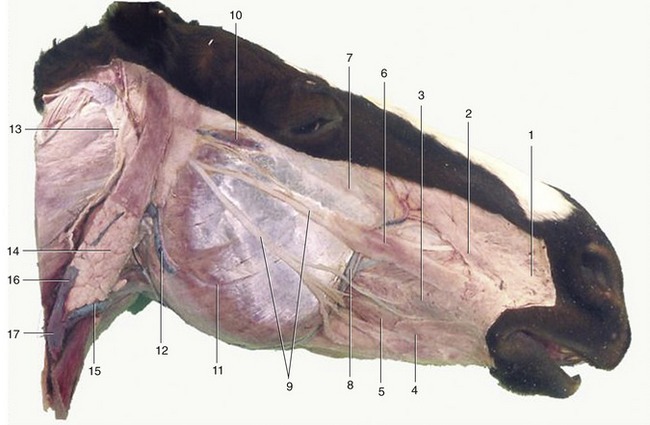
Figure 18–7 Superficial dissection of the head. 1, Caninus; 2, levator nasolabialis; 3, buccinator; 4, stump of cutaneous muscle joining orbicularis oris; 5, depressor labii inferioris; 6, zygomaticus; 7, levator labii superioris; 8, facial artery and vein; 9, buccal branches of facial nerve; 10, transverse facial artery and vein and transverse facial branch of auriculotemporal nerve; 11, masseter; 12, masseteric artery and vein; 13, great auricular nerve (C2); 14, parotid gland; 15, linguofacial vein; 16, maxillary vein; 17, external jugular vein.
The depressor labii inferioris (Figure 18–7/5) arises with the buccinator from the alveolar margin and adjacent part of the mandible under cover of the masseter. It can be identified as a rounded cord running rostrally over the body of the bone. The tendon covers the mental foramen, located about 2 to 3 cm caudal to the angle of the mouth, and this is readily palpable when the muscle is slid aside. The buccinator (Figure 18–7/3) has a well-marked herring-bone structure and is partly covered by the masseter. It is important in returning food to the central cavity of the mouth, preventing its accumulation in the oral vestibule.
SUPERFICIAL VESSELS
The facial artery and vein enter the face in company with the parotid duct (Figure 18–7/8). The artery is easily found where it is in contact with the bone and is convenient for taking the pulse (see Figure 18–40/7). The artery can best be palpated just before it crosses the lower border of the mandible (on the medial side of the mandible). The artery then ascends along the rostral margin of the masseter before terminating in divergent branches; although the pattern of collateral and terminal branches varies, it is usually possible to identify inferior and superior labial, lateral and dorsal nasal, and angularis oculi arteries.
The arrangement of the veins is similar, and their pattern may be visible in life in thin-skinned horses. Certain of the tributaries turn caudally, deep to the masseter, to anastomose with other veins of the head. The most dorsal connection, the transverse facial vein (see Figure 18–9/4), joins the superficial temporal vein. The rostral part lies deep to the masseter, but it then penetrates the muscle; in the caudal part of its course it lies superficially and follows the ventral edge of the zygomatic arch. This caudal stretch is accompanied by an artery (an alternative site for examination of the pulse) and a nerve. Another site for pulse taking is the subcutaneous segment of the masseteric artery (see Figure 18–7/12).
The second connection, the deep facial vein (see Figure 18–9/5), burrows below the masseter and perforates the periorbita before passing through the orbital fissure to join the cavernous venous sinus within the cranial cavity. Two features of this vein are believed to possess functional significance. The discharge into the cavernous sinus contains relatively cool blood drained from the hard palate and nasal cavity; because the sinus envelops the internal carotid artery, this cools the arterial blood passing to the brain, where the temperature is monitored as part of the heat control mechanism. Secondly, an expansion of the vein deep to the masseter may form the basis of a pumping mechanism. It is liable to compression by the masseter, and it is asserted that this helps prevent stagnation of the venous return from the lowered head of the grazing animal.
There is a similar expansion on the third connection, the buccal vein (see Figure 18–9/6), which also runs deep to the masseter to join the superficial temporal tributary of the maxillary vein.
There are two superficial groups of lymph nodes. The parotid group under cover of the rostral part of the parotid gland is not usually palpable unless enlarged. The second group comprises numerous mandibular nodes arranged in a spindle within the intermandibular space. Together with their contralateral fellows, these nodes form a forward-pointing V that is always very distinctly palpable (see Figure 18–39/2). The course of the lymph flow is dealt with later (p. 531).
SUPERFICIAL NERVES
Only a few features of the superficial nerves require notice. The facial nerve detaches its auriculopalpebral branch before it enters the face (see Figure 18–36/24). This branch then takes an independent course across the zygomatic arch (where it is palpable), which leads it between the eye and the ear. The branch may be blocked by injection between the caudal end of the arch and the base of the ear. The procedure facilitates examination of the eye because it eliminates blinking and closure of the lids (p. 345).
The facial trunk divides into dorsal and ventral buccal branches before or, more commonly, shortly after emerging from under the protection of the parotid gland (Figure 18–7/9). These branches and the smaller divisions into which they soon assort run forward over the masseter, where they are palpable and sometimes even visible through the skin. Blows over the masseter or pressure in prolonged recumbency may damage some or all of the divisions. The asymmetry of the face that results when the muscles of the lips, cheek, and nose are paralyzed is usually more striking than in other species. Because the auriculopalpebral branch is precociously detached, such trauma generally spares the muscles of the eyelids and external ear; their involvement points to injury at a more proximal level, which suggests a more sinister causation (Figure 18–8).
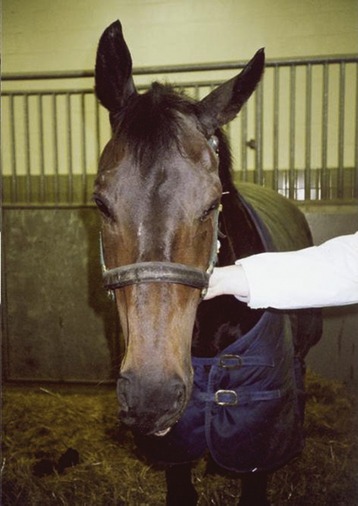
Figure 18–8 Injury to the facial nerve. Note pronounced drooping of ear, moderate drooping of upper eyelid of affected side, and distortion of the nose, which is drawn toward the sound side.
The sensory innervation of the face is the duty of the trigeminal nerve. It is an easy matter to locate some of the principal branches concerned—the supraorbital, infraorbital, and mental nerves—where they emerge from the corresponding foramina (Figure 18–9/1,3). The supraorbital nerve leaves the supraorbital foramen within an easily located dimple in the root of the zygomatic process of the frontal bone. The nerve supplies the upper eyelid and adjacent part of the forehead skin. Directions for location of the infraorbital and mental nerves have already been given (pp. 504 and 505). Anesthetic deposited about the infraorbital nerve at its emergence will desensitize the skin of the upper lip, nostril, and much of the nose extending well caudal to the foramen. Blockage of the mental nerve desensitizes the skin of the lower lip and chin region. During blockage of either of these nerves, it is possible to insert the tip of the needle through the foramen into the bony canal within the jaw. If this is done, injection of anesthetic will also deaden the more rostral teeth (from P2 forward).
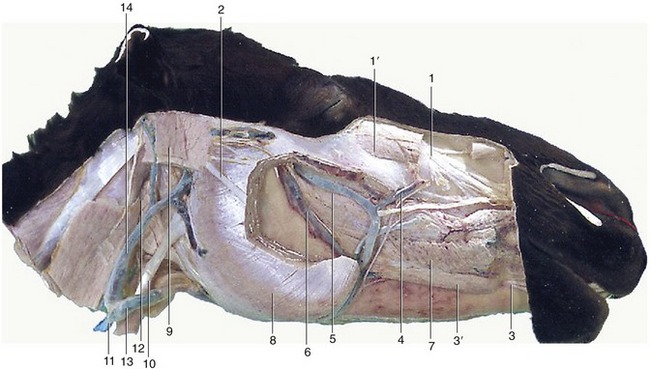
Figure 18–9 Deeper dissection of the head. Parts of the superficial muscles, masseter, and parotid gland have been removed. 1, Infraorbital nerve; 1′, levator labii superioris; 2, dorsal buccal branch of facial nerve; 3, mental nerve; 3′, depressor labii inferioris; 4, facial vein; 5, deep facial vein; 6, buccal vein; 7, buccinator; 8, masseter; 9, occipitomandibularis; 10, sternocephalicus; 11, external jugular vein; 12, mandibular gland; 13, linguofacial vein; 14, maxillary vein.
THE NASAL CAVITY AND PARANASAL SINUSES
THE NASAL CAVITY
Some features of the external nose have been described (p. 501). The ventral part of the nostril leads through a constricted vestibule into a nasal cavity considerably less roomy than might be supposed from the exterior. The factors that determine this are common to all species, but their importance is exaggerated in the horse by the reserve portions of the cheek teeth and the extensive development of the paranasal sinus system (see Figure 3–14).
The dorsal and ventral conchae form delicate scrolls that coil in opposite directions from their lateral attachments (Figure 18–10). The space enclosed within each is divided into two compartments by an internal septum. The caudal part of the dorsal concha is occupied by a rostral extension of the frontal sinus with which it enjoys free communication. The caudal space within the ventral concha communicates with the rostral maxillary sinus. The space within the rostral part of each major concha is in direct communication with the nasal cavity. Numerous small ethmoidal conchae projecting into the fundus serve to enlarge the olfactory area (Figure 18–11/3).
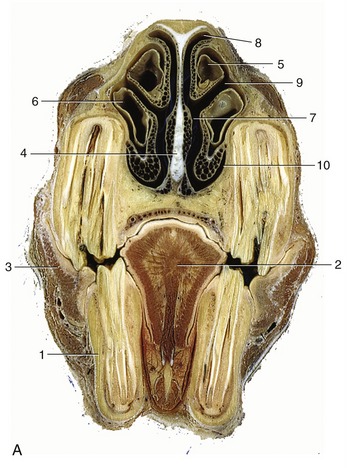
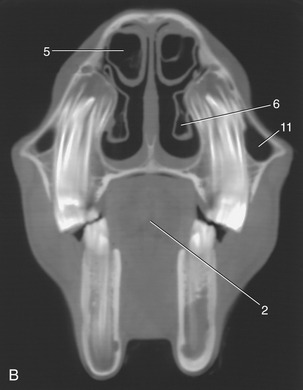
Figure 18–10 A, Transverse section of the head at the level of the rostral maxillary sinus. B, Computed tomographic scan (bone window) at about the same level. 1, P4; 2, tongue; 3, buccinator; 4, nasal septum; 5, dorsal nasal concha; 6, ventral nasal concha; 7, common nasal meatus; 8, dorsal nasal meatus; 9, middle nasal meatus; 10, ventral nasal meatus; 11, rostral maxillary sinus.

Figure 18–11 Median section of the head; most of the nasal septum has been removed. 1, Dorsal nasal concha; 2, ventral nasal concha; 3, ethmoidal conchae; 4, right choana; 5, hard palate with prominent ridges (rugae); 6, soft palate; 7, nasopharynx; 8, pharyngeal opening of auditory tube; 9, geniohyoideus; 10, genioglossus; 11, epiglottis; 12, medial wall of guttural pouch; 13, pharyngeal muscles; 14, cerebellomedullary cistern; 15, basihyoid.
The major conchae divide the cavity into the usual pattern of meatuses (see Figure 18–10). It may be presumed (for direct evidence is lacking) that air moves from the dorsal meatus to the olfactory mucosa and from the middle meatus to the sinuses, while the ventral and common meatuses supply the principal respiratory passage. The conjunction of the last two provides the widest and most convenient route for the introduction of a stomach tube, endoscope, or other instrument. The fragility of the ventral concha and the vascularity of the covering mucosa require that the procedure be performed with care.
Because breathing through the mouth is impossible, augmentation of the air intake in conditions of stress depends on reduction of the obstruction offered by the nose itself. The nostrils may be greatly widened by obliteration of the nasal diverticulum (see Figure 18–2), while contraction of the mucosal venous plexuses thins (and blanches) the membrane. Conversely, congestion of the mucosal vessels seriously impedes air flow. In infections, thickening of the mucosa around the slitlike entrance to the sinus system may obstruct its drainage, damming back a catarrhal exudate.
The vomeronasal organ does not communicate with the mouth in the horse but maintains the usual connection with the nasal cavity (Figure 18–12/2).
THE PARANASAL SINUSES
The extensive sinus system possesses considerable clinical interest as it is susceptible to infection that may spread from the nose or from an alveolar abscess. It also provides a means of access to the unerupted portions of the caudal cheek teeth (Figure 18–13).
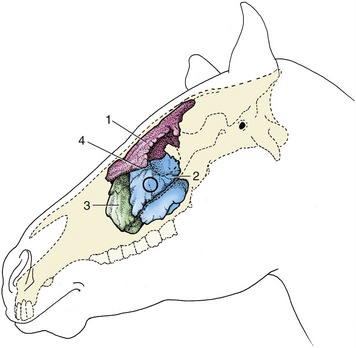
Figure 18–13 Topography of the conchofrontal and maxillary sinuses, which are filled with casting material. The circle indicates where the caudal maxillary sinus can be trephined. 1, Conchofrontal sinus; 2, caudal maxillary sinus; 3, rostral maxillary sinus; 4, position of frontomaxillary opening between 1 and 2.
On each side there are frontal, caudal maxillary, and rostral maxillary sinuses of importance and sphenopalatine and ethmoidal spaces of less account. The layout is complicated and, in one important respect, unique (among domestic species); the frontal sinus communicates with the nasal cavity indirectly via the caudal maxillary sinus.
The frontal sinus occupies the dorsal part of the skull medial to the orbit. It overlaps both cranial and nasal cavities, and because it also occupies the closed part of the dorsal concha, it is more correctly known as the conchofrontal sinus. Its extent is shown in Figure 18–14/1,1′. From this it will be seen that the interior of the frontal part is incompletely divided by several bony lamellae. The floor of this part is molded over the ethmoidal labyrinth, and rostrolateral to these areas of unevenness, it displays the large oval communication (frontomaxillary opening) with the caudal maxillary sinus. The opening normally allows easy natural drainage. A window may be opened, usually by trephination, in the roof of the frontal sinus to allow for irrigation or for removal of a molar by repulsion, when a punch introduced through the frontomaxillary opening is brought to bear on the appropriate alveolus. Such a window also allows introduction of a fiberoptic endoscope to inspect the interior of this large sinus.
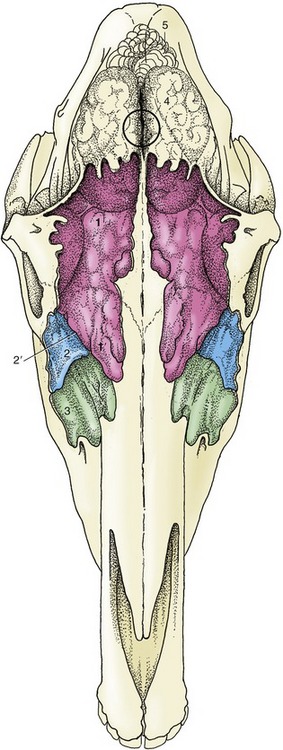
Figure 18–14 Projection of the brain and frontal and maxillary sinuses on the dorsal surface of the skull. The sinuses are filled with casting material. The frontal sinus extends caudally over the rostral part of the brain and rostrally beyond the level of the orbit. The circle indicates the center of the brain and the location where a horse may be shot. 1, 1′, Conchofrontal sinus; 1, frontal part; 1′, dorsal conchal part; 2, caudal maxillary sinus; 2′, position of frontomaxillary opening; 3, rostral maxillary sinus; 4, cerebrum; 5, cerebellum.
The two maxillary sinuses together occupy a large part of the upper jaw, where they have a critically important relationship to the embedded portions of the caudal cheek teeth. They share a slitlike communication (nasomaxillary opening) with the middle meatus of the nasal cavity but are otherwise completely divided by an oblique septum. This is variable in position but most commonly located about 5 cm caudal to the rostral end of the facial crest. The ventral part of each sinus is also divided into medial and lateral spaces by an upright longitudinal plate supporting the infraorbital canal and fused in young animals to the alveoli containing the roots and unerupted portions of the cheek teeth. The medial part of the caudal sinus continues into the irregular sphenopalatine sinus. The corresponding part of the rostral sinus extends into the ventral concha.
It is impossible to define the exact extent and projections of the maxillary sinuses, which enlarge considerably after birth as the teeth are extruded (Figure 18–15). Their relationship to the teeth is also affected by the forward migration of the teeth as they develop and come into wear. As Figure 18–15 shows, the relationship is confined to the last premolar and first molar tooth in the newborn foal; it later extends to involve the last four teeth but finally retains contact only with the three molars. There is much variation, and attention to the varying inclination of the embedded parts of different teeth is required.
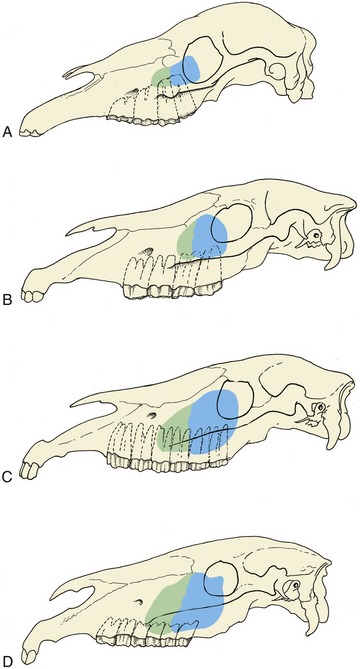
Figure 18–15 Projection of the maxillary sinuses at various ages. In older horses the cheek teeth are more rostrally placed. A, 1 month. B, 1 year. C, 4 to 6 years. D, Older than 12 years.
The surface projection of the maxillary sinuses is considerably larger than the safe surgical field. The latter is determined by several factors, not least the routes followed by the very vulnerable nasolacrimal duct and infraorbital nerve. The potential operating area is defined by the following boundaries: (1) the vertical line tangential to the rostral limit of the orbit; (2) the facial crest; (3) the oblique line joining the rostral limit of the crest to the infraorbital foramen; and (4) the line parallel to the facial crest that intersects the infraorbital foramen. Entry to the sinus may be required either to effect drainage (because the natural route, the nasomaxillary opening, is placed high in the wall) or to give access to certain teeth.
THE MOUTH
The small size of the entrance makes it impossible to open the mouth wide; this limitation, coupled with the great depth of the cavity, severely hampers clinical inspection.
The vestibule communicates with the mouth cavity proper only between the incisor and cheek teeth (where the diastema may be interrupted by the canine) and by small gaps behind the last molars. The hard palate is therefore largely bounded by the alveolar processes and teeth. It is almost uniformly broad and is marked by two more or less symmetrical series of ridges (Figure 18–11/5). The incisive papilla is found directly behind the central incisors; grooves that flank the elevation end blindly and do not communicate with the nasal cavity and vomeronasal organs (see Figure 18–12). The mucosa of the hard palate is thick, particularly in its most rostral part, and incorporates a very generous venous plexus, which may become engorged (lampas) at the time of tooth replacement when it may project above the occlusal surfaces of the neighboring teeth. The appearance is striking and laypeople are sometimes alarmed by this purely physiological phenomenon.
The soft palate continues the hard palate beyond the level of the second molar tooth. It is remarkably long and hangs down before the epiglottis; its free margin is closely applied to the tongue. The palatopharyngeal arches extend caudally from the palate, completing a sphincter about the structures that bound the entrance to the larynx, which thus projects some way into the nasopharynx. The application of the palate to the tongue is so firm that an air-tight seal is created that closes the oropharynx, which then provides a barrier between the mouth and the pharynx. This ensures that breathing is through the nose, precluding use of the oral route and incidentally resulting in ingesta passing into the nasal passages on the rare occasions when horses vomit. These relationships of the palate are normally maintained except during deglutition. The obstructions of the upper respiratory tract commonly recognized in horses worked at a fast pace are often due to anomalous position and relations of the soft palate.
Understanding of these matters has been greatly improved by video endoscopy of the nasopharynx and larynx of affected horses undertaken while they were strenuously exercised on a treadmill. Two abnormal conditions of the soft palate are now recognized; both apparently occur after admission of air into the oropharynx breaks the seal that normally maintains the parts in close apposition. In the less severe form, there is abnormal movement of the caudal part of the palate, aptly described as “billowing.” In the more severe form, of which billowing is probably a precursor, the soft palate is displaced dorsally, losing contact with the ventral side of the epiglottis and narrowing the nasopharyngeal airway. At endoscopy the epiglottis is no longer visible. Both forms may be accompanied abnormal respiration sounds. The impairment of respiratory efficiency inevitably leads to diminished physical performance. It is uncertain how the seal comes to be broken, but among the factors blamed are the following: the extreme negative pressure developed in the rostral nasopharynx at one stage of the respiratory cycle; dysfunction of the palate musculature weakening the contact between tongue and palate; overactivity of those ventral cervical muscles that attach to the larynx and hyoid drawing the larynx caudally, freeing the palate from entrapment by the epiglottis; and abnormal activity of the hyoepiglottic muscle, tilting the epiglottis caudally with the same effect. There is some evidence that obstructions are commoner at the palatopharyngeal level in younger animals and at the laryngeal level in older animals and that frequently both occur together.
The mucosa on the oral surface of the soft palate is marked by numerous pits where the palatine glands open. It also exhibits a rostral median tonsillar swelling (see Figure 3–25).
The tongue is long, conforming to the shape of the cavity, and is spatulate at its apex, which is incompletely restrained by a narrow frenulum. Its upper surface is thickly strewn with delicate filiform papillae that confer a velvetlike texture; the larger papillae with gustatory function are less widely spread (Figure 18–16/9,10,11). A scattering of lymphoid tissue over the root constitutes a diffuse lingual tonsil. Each of two low mucosal folds beneath the apex of the tongue carries a fleshy sublingual caruncle where the mandibular duct opens.
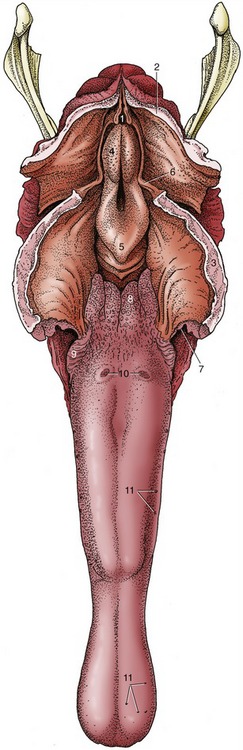
Figure 18–16 The tongue and pharynx; the latter has been opened dorsally to expose the entrance to the larynx. 1, Entrance into esophagus; 2, dorsal wall of nasopharynx (split in median plane); 3, soft palate (split in median plane); 4, corniculate process of arytenoid cartilage; 5, epiglottis; 6, free border of soft palate, continued caudally by palatopharyngeal arch; 7, palatoglossal arch; 8, lingual tonsil; 9, foliate papillae; 10, vallate papillae; 11, examples of fungiform papillae.
THE DENTITION AND MASTICATORY APPARATUS
THE DENTITION
The dentition of the horse is admirably suited to a diet of grass, a surprisingly abrasive material. The masticatory area is increased by the enlargement of the premolars and their assimilation to the molars with which they present a continuous grinding surface. Both cheek teeth and incisors have high crowns, which ensure a long working life, despite the considerable attrition that takes place at the occlusal surfaces. Delayed formation of the roots also allows the cheek teeth to grow for some years after they come into wear. Attrition wastes the cheek tooth by 2 to 3 mm each year; to allow for this the greater part of the crown is initially embedded within the jaw and only gradually extruded to compensate for this loss. The enamel casing of the incisor and cheek teeth is also folded, although in different ways in the incisor, upper cheek, and lower cheek teeth series. The folding increases the area of the durable enamel presented at the working surface, where it stands proud of the neighboring dentine; the alternation of harder and softer tissues provides efficient grinding instruments (see Figure 18–19).
The formula of the temporary dentition is
and that of the permanent dentition is
The incisor teeth are ranked together to form a continuous arch in each jaw and are so implanted that their roots converge (Figure 18–17). Each is curved lengthwise, presenting a labial convexity. When in occlusion, the upper and lower incisors of the young animal form a continuous arch when viewed in profile. Later, as they wear, the upper and lower teeth meet at an increasingly pronounced angle. The occlusal surface recently brought into use is a broad transverse oval (Figure 18–18, B) and presents an outer enamel casing and an inner enamel ring lining the infolding known as the infundibulum; this is partially filled with cement, leaving a small cavity, the cup (Figure 18–18/1). Because the enamel lining is more resistant, it projects above the surrounding dentine. Changes in the appearance of the occlusal surface provide the information principally used in aging older horses. The points to note are the depth of the infundibulum and its overlap with the dental cavity. Although it may appear that wear would eventually expose the pulp, this is prevented by the timely formation of secondary dentine, distinguishable from primary dentine by its darker color; this secondary dentine provides the feature known as the dental star (Figure 18–18/3).

Figure 18–17 Root convergence of permanent lower incisors; radiograph of a bone specimen from a 5-year-old (estimated) horse. Note the funnel-shaped infundibulum visible in each of the first and second incisors. I1, I2, and I3, Lower first, second, and third incisors; C, lower canine tooth, present only in the male; 1, mounting wire of specimen.
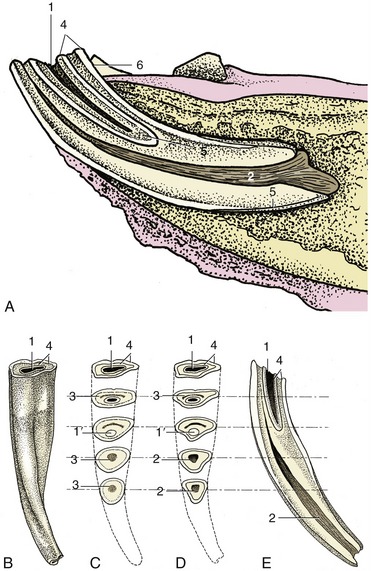
Figure 18–18 Structure of a lower incisor. A, In situ, sectioned longitudinally; the clinical crown is short in relation to the embedded part of the tooth. B, Caudal view; the junction between the clinical crown and the rest of the tooth is not marked. C, As a result of wear the occlusal surface changes; the cup gets smaller and disappears, leaving, for a time, the enamel spot; the dental star appears and changes from a line to a large round spot. D, These are sawn sections of a young tooth for comparison. E, Longitudinal section of incisor, showing the relationship between the infundibulum and dental cavity; the latter is rostral. 1, Cup, black cavity in center of infundibulum; 1′, enamel spot, proximal end of infundibulum; 2, dental cavity; 3, dental star, changing in shape from a linear to a rounded form; 4, outer and inner enamel rings; 5, cement; 6, lingual surface.
Although canine teeth generally form in both sexes, they are rudimentary and commonly fail to erupt in mares. In male animals they are low, laterally compressed cones placed within the diastemas rather closer to the corner incisors than to the cheek teeth. The embedded portions are disproportionately large in relation to the exposed crowns.
The first premolar (“wolf” tooth) often fails to develop, and when present, it is vestigial and almost invariably confined to the upper jaw. Although it is without functional significance, it does have a potential nuisance value because it may shift under the pressure of the bit and so irritate the gum. It is easily extracted.
The remaining premolars (P2–P4) form a continuous row with the molars. The first and last of the six cheek teeth are somewhat triangular in section, the others rectangular; nonetheless, each is so like its neighbors that only an expert may distinguish isolated teeth (see Figure 18–21). There are, however, important differences between the upper and lower sets; the upper teeth are much wider and exhibit a more complicated enamel folding, which creates two infundibula that fill with cement before eruption. The enamel of the lower teeth is also much folded but forms no infundibula (Figure 18–19, B). Most teeth occlude with two members of the opposing set along a relatively narrow area of contact that follows the lingual edge of the upper teeth and buccal edge of the lower teeth. The occlusal plane slopes ventrobuccally (see Figure 18–10). Irregular or incomplete chewing movements may cause the buccal edge of the upper cheek teeth and the lingual edge of the lower cheek teeth to escape wear (sharp teeth); the resulting protrusions must be filed down (floated) to prevent injury to cheeks and tongue.
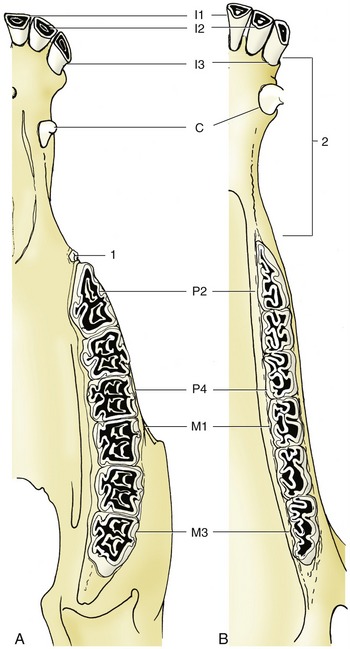
Figure 18–19 The permanent teeth of the upper (A) and lower (B) jaws. 1, “Wolf” tooth (P1); 2, diastema.
The structure of the cheek teeth is shown in Figure 18–20. The upper teeth are anchored by three or four roots and are so implanted that the reserve portions slope caudally at varying angles (Figure 18–21). The relationship to the maxillary sinuses and other features of the skull is very helpfully revealed in radiographs. Only a thin plate of alveolar bone separates the molars from the sinus; in consequence, infection may easily spread to the sinus from tooth or alveolar abscesses. The relationship changes with age, partly because gradual extrusion lowers the alveolar floor, enlarging the sinus, and partly because the teeth migrate rostrally (see Figure 18–15).
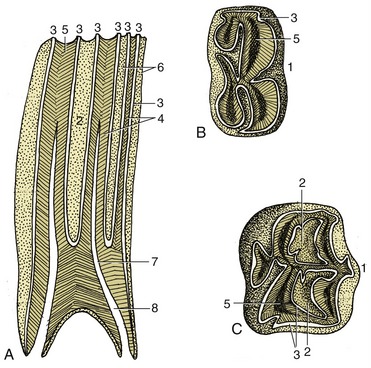
Figure 18–20 Structure of the cheek teeth shown in sagittal section (A) and by views of the occlusal surface of lower (B) and upper (C) molars. 1, Buccal (labial) surface; 2, infundibulum; 3, enamel; 4, dentine; 5, secondary dentine; 6, cement; 7, dental cavity; 8, root canal.
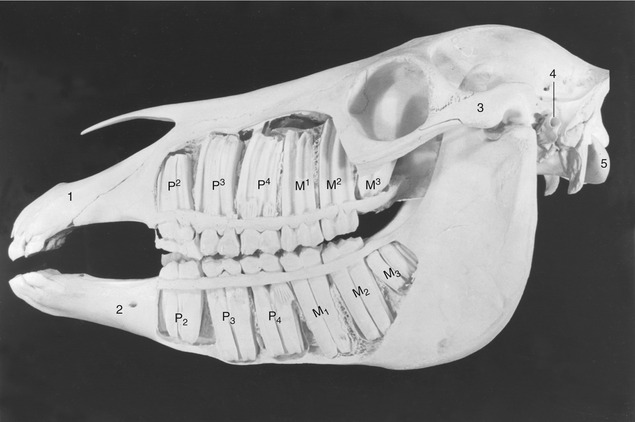
Figure 18–21 Exposed cheek teeth of a horse 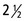 years old (estimated). Upper jaw: The deciduous premolars are still present, p2 in the form of a cap; M3 has not yet erupted. Lower jaw: The deciduous premolars 3 and 4 are still present in the form of caps; M3 has not yet erupted. 1, Incisive bone; 2, mental foramen; 3, zygomatic arch; 4, external acoustic meatus; 5, occipital condyle.
years old (estimated). Upper jaw: The deciduous premolars are still present, p2 in the form of a cap; M3 has not yet erupted. Lower jaw: The deciduous premolars 3 and 4 are still present in the form of caps; M3 has not yet erupted. 1, Incisive bone; 2, mental foramen; 3, zygomatic arch; 4, external acoustic meatus; 5, occipital condyle.
The transitory swellings occasionally seen on the ventral margin of the mandible of 2- to 4-year-old horses are produced by modeling of the mandible to accommodate the formation of the roots of permanent teeth, which are prevented from rising within the jaw by remnants (caps) of deciduous predecessors blocking the way (Figure 18–22). When the remnants are shed, their successors can move into place. Further modeling of the mandibular border erases the swellings.
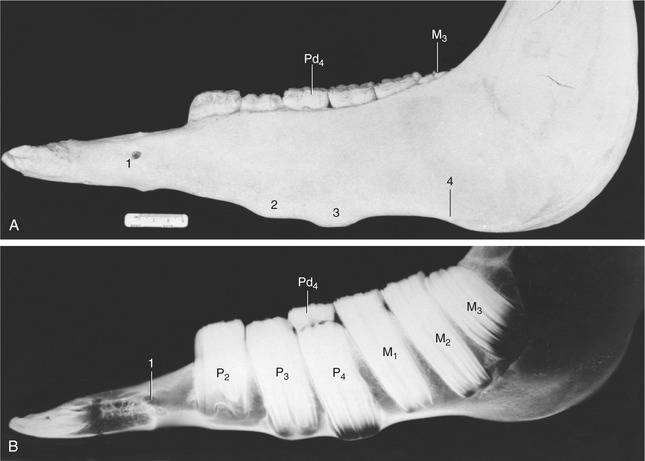
Figure 18–22 Photograph (A) and radiograph (B) of the left half-mandible of a horse 3 years old (estimated). Note the transitory tubercles on the ventral border and the wedged-in cap (Pd4) that retards the advance of P3 and P4. 1, Mental foramen; 2, 3, tubercles over the proximal ends of P3 and P4, respectively; 4, notch for facial artery and vein.
Simple extraction of cheek teeth is more or less impossible. Their length, curvature, and close fit would hamper any effort to draw one out past its neighbor(s), even were the attempt permitted by the small size of the opening between the lips and the depth of the oral cavity (Figure 18–23). Instead, they must be removed by expulsion, that is, by means of a punch brought to bear over the root in an operation of some severity and difficulty involving the opening of a window through bone. Accurate determination of the position of the root of the tooth involved is essential, and for this it is necessary to be mindful of how the dispositions of the teeth change with age. The approach to a caudal member of the upper cheek teeth series is made via the caudal maxillary sinus or the frontal and caudal maxillary sinuses when M3 is involved.
Figure 18–24 Characteristic appearance of lower incisors of Standardbred horses of accurately known ages. A, 1.5 years. B, 2.5 years. C, 3 years. D, 4 years. E, 5 years. F, 6 years. G, 7 years. H, 8 years. I, 9 years. 1, Deciduous teeth; 2, newly erupted I1; 3, dental cup; 4, dental star; 5, enamel spot (proximal end of infundibulum).
The deciduous teeth generally resemble the permanent teeth but are much smaller and significantly shorter in relation to their breadth. The deciduous incisors are constricted at the neck and are much whiter than their replacements because the porcelain-like enamel is unobscured by the cement encrustation that gives permanent teeth a slightly yellow and porous appearance. Some longitudinal striation is apparent on the temporary incisor crown.
THE ESTIMATION OF AGE FROM THE TEETH
Examination of the teeth provides the traditional and sole convenient means of estimating age. Because there is copious specialist literature, the subject is treated very briefly here (Table 18–1). The eruption dates and changes in appearance of the occlusal surfaces, specifically those of the lower incisors, are the main criteria. Neither is wholly dependable but the first is more reliable, although limited in application to younger animals; the second may be used throughout the life span but becomes increasingly inaccurate.
Table 18–1 A Rough Guide for the Aging of the Horse by Its Teeth
The initially oval occlusal surface of the incisors becomes rounded and finally forms a triangle elongated in the labiolingual direction. The enamel casing is intact when the tooth erupts, and the occlusal surface then presents a central depression (cup) that is soon stained by food debris. Wear first abrades the labial edge but quickly extends all around, isolating the infundibular from the external enamel; the tooth is then said to be level. Further wear reduces the depth of the cup, although its thick base (the enamel “spot”) resists attrition for a considerable time. The dental star appears on the labial aspect of the cup meanwhile and persists after the cup and the enamel spot have been entirely lost.
Less reliable criteria are a “hook” on I3 (see Table 18–1) and Galvayne’s groove on the labial surface of the same tooth. The hook is present when the horse is about 7 years old; unfortunately it may recur at 11 years. The appearance, progression, and disappearance of Galvayne’s groove are also depicted in Table 18–1. Although unreliable by themselves, both features may enhance accuracy when combined with the appearance of the occlusal surfaces and the profile of the incisors (Figure 18–24 and Figure 18–25).
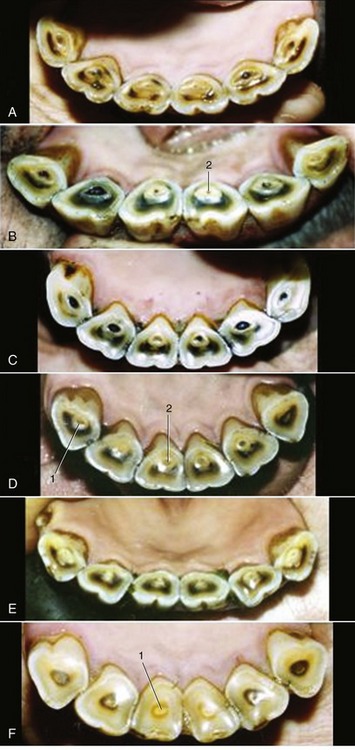
Figure 18–25 Characteristic appearance of lower incisors of Standardbred horses of accurately known ages. A, 11 years. B, 12 years. C, 14 years. D, 16 years. E, 17 years. F, 20 years. Note particularity of the changes in form of the occlusal surface: from round to triangular. 1, Dental star; 2, enamel spot.
It has to be emphasized that the variation in these (and in other undescribed) features is extremely large, and in a horse more than 8 years old the assessment may be at fault by several years.
THE MUSCLES OF MASTICATION AND THE TEMPOROMANDIBULAR JOINT
The muscles of mastication are well developed. The masseter takes origin along the whole length of the facial crest and zygomatic arch and inserts on the mandible between the vascular notch and condyle (Figure 18–7/11). It is a multipennate muscle constructed so that the fibers of the superficial strata run caudoventrally, while those more deeply placed are nearly vertical. Its cranial margin produces a very prominent surface contour that serves as a guide to the location of the facial vessels and parotid duct. Its caudodorsal part is overlain by the parotid gland but to a variable depth and extent, which affect the accessibility to palpation of the parotid lymph nodes. Laterally, the masseter is traversed by buccal branches of the facial nerve.
The temporalis almost fills the temporal fossa, where it is easily palpated despite the partial covering of thin muscles concerned with the movement of the external ear (Figure 18–23/1). It arises from the wall of the fossa and from the sagittal crest that forms its median margin, and it envelops the coronoid process of the mandible. On contraction it raises the mandible.
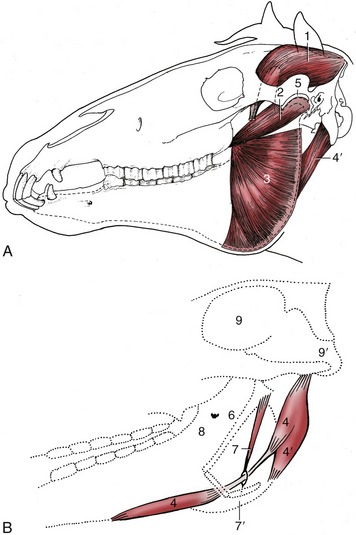
Figure 18–23 A, The deep masticatory muscles of the left side have been exposed by removal of the left mandibular ramus (stippled). B, Medial view of the right digastricus and some related structures. 1, Temporalis; 2, pterygoideus lateralis; 3, lateral surface of pterygoideus medialis; 4, digastricus; 4′, occipitomandibularis; 5, left temporomandibular joint; 6, stylohyoid; 7, stylohyoideus; 7′, insertion of 7 on thyrohyoid; 8, medial surface of right mandible and mandibular foramen; 9, cranial cavity; 9′, foramen magnum.
The pterygoideus medialis and lateralis, deep to the mandible, broadly correspond to the masseter in position, orientation, and attachments (Figure 18–23/2,3). The medial muscle, always the larger, extends from the pterygoid process to the mandibular margin. The lateral muscle runs more horizontally to insert close to the condyle. The masseter and contralateral pterygoid muscles act together to produce the horizontal shifts that supply the principal grinding movement.
The digastricus and occipitomandibularis (strictly a part of the digastricus; Figure 18–23/4,4′) are responsible for active opening of the mouth. Despite its much greater bulk, the latter may be regarded as a detachment from the caudal belly of the digastricus. It extends between the paracondylar process of the occipital bone and the caudal border of the mandible. The much more slender digastricus has a similar origin. It presents an intermediate tendon that passes through a split in the insertion of the stylohyoideus. The rostral belly attaches to the ventromedial part of the molar region of the mandible. When the mouth is closed, contraction of the digastricus raises the hyoid apparatus (by virtue of its association with the stylohyoideus) and thus the root of the tongue (Figure 18–23, B).
A thick intraarticular disk is interposed between the expanded and rather flat facets of the mandibular condyle and articular tubercle of the temporal bone (Figure 18–23, A/5). Hinge movements occur at the lower level, which is supported by a tight capsule; the lateral and slight protrusive movements occur at the upper level where the joint cavity is more capacious. The whole joint is supported by a fibrous lateral ligament and an elastic caudal one.
THE SALIVARY GLANDS
The parotid is clearly lobulated and has a firm texture and a yellow-gray or yellow-pink color. It is the largest salivary gland and extends ventrally from the base of the ear and wing of the atlas into the angle formed by the convergence of the maxillary and linguofacial veins and may possibly extend beyond because the maxillary vein frequently tunnels through the gland substance (Figure 18–7/14). The cranial margin is largely contained by the caudal border of the mandible, but a thin flange extends some distance over the masseter directly ventral to the jaw joint, where it covers the parotid lymph nodes. The lateral surface is overlain by a well-developed fascia that gives attachment to the parotidoauricularis muscle. The deep surface is related to the guttural pouch, the stylohyoid, the muscles that run to the corner of the jaw and open the mouth, and the combined insertion tendon of the brachiocephalicus and sternocephalicus, which separates it from the more deeply placed mandibular gland (see Figure 18–9).
The serous secretion of the parotid is drained by several sizable ducts that come together at the rostroventral angle of the gland to form a single channel. This crosses the tendon of the sternocephalicus before turning forward to run medial to the ventral border of the mandible. Accompanied by the facial vessels, it turns onto the face, where it ascends along the rostral margin of the masseter. It first lies caudal to the artery and vein but later shifts rostral to them. It ends by opening into the vestibule opposite the third upper cheek tooth. The duct is relatively exposed in the last part of its course and may be damaged in superficial wounds. Leakage is most profuse when feeding stimulates the flow of saliva.
The much smaller and crescentic mandibular gland extends from the basihyoid to the atlantal fossa and is thus partly under cover of the mandible (Figure 18–9/12 and Figure 18–30/5). The superficial relations include the parotid gland and the medial pterygoid, sternocephalic, digastric, and occipitomandibular muscles. Its deep location puts it out of reach on palpation. The mandibular duct is formed along the concave rostral margin of the gland by the confluence of several ductules. It runs rostrally, covered by the mylohyoideus, and follows the medial aspect of the sublingual gland until it opens on the floor of the mouth at the small sublingual caruncle. The secretion is mixed.
The sublingual gland lies directly below the oral mucosa, between the body of the tongue and the medial surface of the mandible, extending as a thin strip from the symphysis to the level of the fifth cheek tooth (Figure 18–30/1). It drains through numerous small ductules that open below the tongue.
Two rows of buccal glands are scattered along the dorsal and ventral margins of the buccinator. The glands of the dorsal series are more considerable and clump together caudally. Small salivary glands are found in the lips, soft palate, and tongue.
THE PHARYNX AND GUTTURAL POUCH
THE PHARYNX
The pharynx lies wholly beneath the skull to which the rostral third of its roof is directly applied. The remaining part of the roof and the lateral walls are enveloped by the guttural pouches (see further on). The lumen is clearly divided into upper and lower compartments by the soft palate and the palatopharyngeal arches, which extend over the lateral walls to meet directly above the entrance to the esophagus (see Figure 18–11). The most prominent features of the nasopharynx are the flaps guarding the entrances to the auditory tubes. Each is about 3 cm long and is pressed against the pharyngeal wall, presenting an oblique and rather sinuous ventral free edge (Figure 18–26, A). It is stiffened by a flange of cartilage, the expansion of the medial cartilage that supports the auditory tube. The slitlike opening lateral to the flap is normally held closed but becomes patent when the animal swallows. This provides an opportunity for equalizing the pressure on the two sides of the tympanic membrane. The maneuver, which can be observed endoscopically, involves the flap swinging medially while the soft palate rises and momentarily narrows the lumen of the nasopharynx (Figure 18–27). The flap can also be elevated passively, and it is a relatively simple matter to introduce an endoscope to examine, or a catheter to drain or irrigate, the guttural pouch. The entrance to the tube lies in the transverse plane of the lateral angle of the eye, which is a useful external guide to its position. An indication of the progress of the instrument through the ventral meatus and nasopharynx is provided by the resistance encountered; the firm support offered to its tip by the vertical lamina of the pterygoid bone is lost only a short distance rostral to the opening. Advancement of the instrument to this level generally provokes a swallowing movement when deflection of the cartilage flap facilitates entry to the pouch. When the procedure is performed blind, the absence of resistance to deeper penetration indicates that the pharyngotubal opening has been successfully passed.
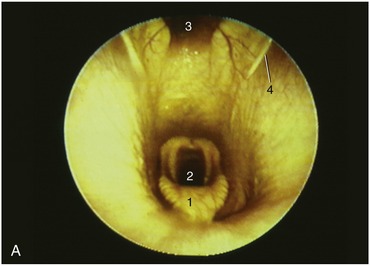
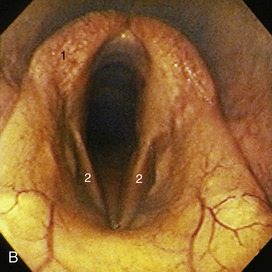
Figure 18–26 A, Endoscopic view of equine nasopharynx. 1, Epiglottis; 2, laryngeal entrance; 3, pharyngeal recess; 4, entrance to auditory tube. B, Endoscopic view of larynx. 1, Arytenoid cartilage; 2, left and right vocal folds.
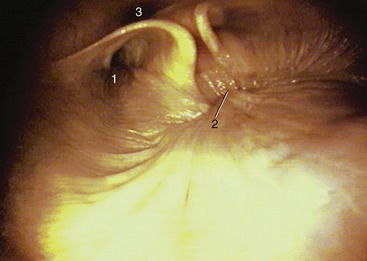
Figure 18–27 Endoscopic view of the caudal part of equine nasopharynx (foal). 1, Entrance to auditory tube; 2, closure of the intrapharyngeal ostium between the nasopharynges and laryngopharynges (during swallowing); 3, cartilage flange supporting the auditory tube.
The lower compartment of the pharynx is divided between the oropharynx and the laryngopharynx (Figure 18–28/4,5). The narrow oropharynx extends between the attachment of the palatoglossal arches to the tongue and the epiglottis; its lateral walls and floor contain much diffuse tonsillar tissue, including the long palatine tonsil (see Figure 3–25). The laryngopharynx is largely occupied by the projection of the larynx, and its floor is reduced to the narrow flanking piriform recesses. The laryngopharynx narrows abruptly to the origin of the esophagus.
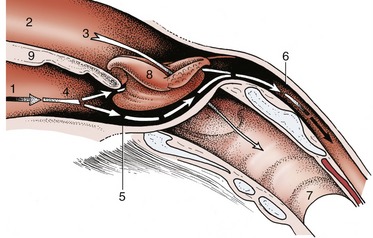
Figure 18–28 The communications of the pharynx, rostrally with the oral and nasal cavities, caudally with the esophagus; schematic. The broken arrows mark the digestive pathway; the unbroken arrow marks the respiratory pathway. 1, Oral cavity; 2, nasal cavity; 3, nasopharynx; 4, oropharynx; 5, laryngopharynx; 6, esophagus; 7, trachea; 8, epiglottis, laryngeal entrance; 9, soft palate.
The structure and musculature follow the common pattern (Figure 18–29). Difficulties in swallowing sometimes arise from malfunction of palatine and pharyngeal muscles. The cause frequently lies in involvement of the relevant glossopharyngeal and vagus nerves in infections of the guttural pouch; because the nerves run together, they are equally susceptible (Figure 18–30/7,14).
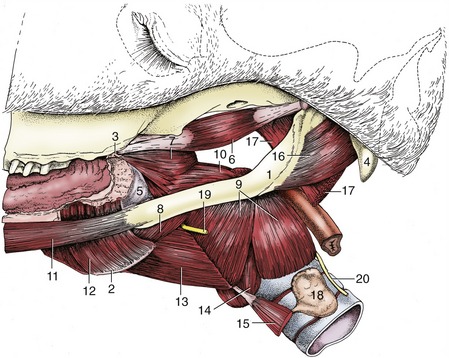
Figure 18–29 Muscles of the pharynx, soft palate, and hyoid apparatus. 1, Stylohyoid; 2, thyrohyoid; 3, hamulus of pterygoid bone; 4, paracondylar process; 5, buccopharyngeal fascia; 6, tensor veli palatini; 7, rostral pharyngeal constrictor; 8, middle pharyngeal constrictor; 9, caudal pharyngeal constrictor (thyropharyngeus and cricopharyngeus); 10, stylopharyngeus caudalis; 11, styloglossus; 12, hyoglossus; 13, thyrohyoideus; 14, cricothyroideus; 15, sternothyroideus; 16, occipitohyoideus; 17, longus capitis (stump); 18, thyroid gland; 19, cranial laryngeal nerve; 20, caudal (recurrent) laryngeal nerve.
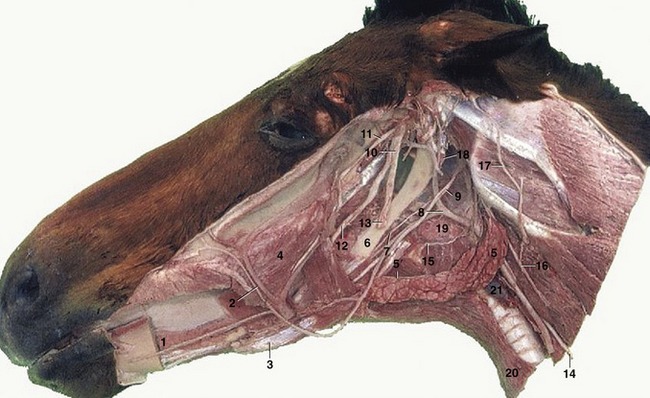
Figure 18–30 Deep dissection of the head. The mandible and masticatory muscles have been removed. 1, Sublingual gland; 2, facial artery and vein; 3, rostral belly of digastricus; 4, buccinator; 5, mandibular gland; 5′, mandibular duct; 6, stylohyoid; 7, glossopharyngeal nerve; 8, linguofacial artery; 9, hypoglossal nerve; 10, mandibular nerve; 11, masseteric nerve; 12, lingual nerve; 13, inferior alveolar nerve, cut where it enters the mandibular foramen; 14, vagus and sympathetic trunk; 15, cranial laryngeal nerve; 16, dorsal branch of spinal accessory nerve; 17, great auricular nerve; 18, guttural pouch; 19, medial retropharyngeal lymph nodes; 20, sternohyoideus; 21, thyroid gland.
THE GUTTURAL POUCH
A diverticulum of the auditory tube, the guttural pouch, is found in the horse and other Perissodactyla* (Figure 18–31/9). It is formed by the escape of the mucosal lining of the tube through a ventral slit between medial and lateral supporting cartilages and attains a capacity of some 300 to 500 mL. It lies between the base of the skull and atlas dorsally and the pharynx and commencement of the esophagus ventrally; it is covered laterally by the pterygoid muscles and parotid and mandibular glands. Medially, the dorsal parts of the right and left sacs are separated by the ventral straight muscles of the head, but below this they meet, forming a thin median septum. The floor lies mainly on the pharynx but also covers and is molded to the stylohyoid, which raises a ridge that incompletely divides medial and lateral compartments (Figure 18–32).
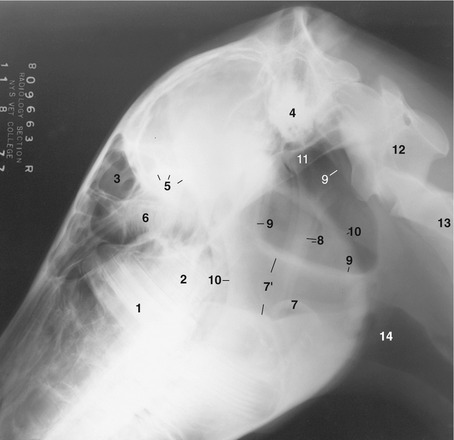
Figure 18–31 Lateral radiographic view of the head to show the position of the guttural pouches (9) in a horse 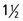 years old (estimated). 1, M1; 2, unerupted M2; 3, frontal sinus; 4, petrous temporal bone; 5, caudal border of orbit; 6, ethmoid labyrinth; 7, epiglottis; 7′, nasopharynx; 8, stylohyoid bones; 9, borders of guttural pouches; 10, rostral and caudal borders of mandible; 11, base of skull; 12, atlas; 13, axis; 14, larynx.
years old (estimated). 1, M1; 2, unerupted M2; 3, frontal sinus; 4, petrous temporal bone; 5, caudal border of orbit; 6, ethmoid labyrinth; 7, epiglottis; 7′, nasopharynx; 8, stylohyoid bones; 9, borders of guttural pouches; 10, rostral and caudal borders of mandible; 11, base of skull; 12, atlas; 13, axis; 14, larynx.
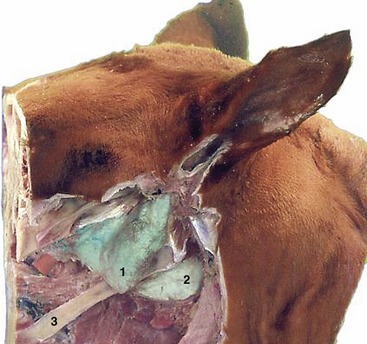
Figure 18–32 Position of the guttural pouch in relation to the skull and stylohyoid. 1, Lateral compartment of guttural pouch; 2, medial compartment of guttural pouch; 3, stylohyoid.
More detailed relations include several cranial nerves and arteries that lie directly against the pouch as they pass to and from foramina in the caudal part of the skull. The glossopharyngeal, vagus, accessory, and hypoglossal nerves; the continuation of the sympathetic trunk beyond the cranial cervical ganglion; and the internal carotid artery are closely related for a stretch and together raise a mucosal fold that indents the medial compartment from behind; this is a conspicuous feature when the interior of the pouch is viewed endoscopically (Figure 18–33/4). The facial nerve has a more limited contact with the dorsal part of the pouch. The large external carotid artery passes ventral to the medial compartment before crossing the lateral and then rostral walls of the lateral compartment (Figure 18–33/6) in its approach (as the maxillary artery) to the alar canal. The pouch also directly covers the temporohyoid joint.
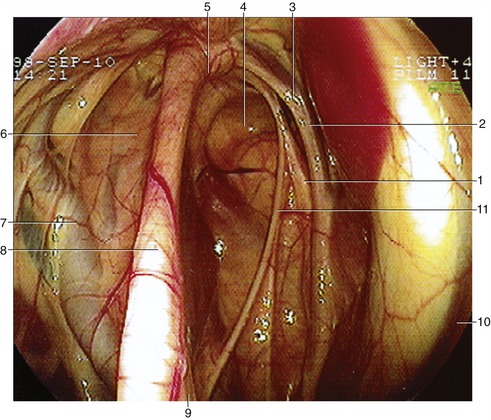
Figure 18–33 Endoscopic view of the interior of the guttural pouch. 1, Hypoglossal; 2, vagus nerve; 3, internal carotid artery; 4, medial compartment; 5, articulation of the stylohyoid and petrous temporal bone; 6, lateral compartment; 7, external carotid artery; 8, stylohyoid bone; 9, stylopharyngeus muscle; 10, longus capitus muscle; 11, glossopharyngeal nerve.
The mucous secretion of the lining normally drains into the pharynx through the pharyngotubal opening (Figure 18–11/8) placed at the rostral end of the pouch, the most dependent part when the head is lowered. The connection opens when the horse swallows, and grazing normally promotes drainage. When the exit is blocked or the secretion accumulates for any reason, the pouch distends, producing a palpable, often visible swelling behind the jaw (Figure 18–34). The exudate may be contaminated by microorganisms that invade along the tube or spread from infection of the neighboring retropharyngeal lymph nodes; Streptococcus equi equi is commonly involved. Mycotic infections of the guttural pouch also occur. Apart from such obvious signs as painful swelling of the parotid region, abnormal carriage of the head and neck, and nasal discharge, affected animals may exhibit a variety of specific abnormalities that result from involvement of structures directly related to the pouch. Fusion of the stylohyoid with the adjacent portion of the petrous temporal bone, eliminating the intervening joint, may expose the complex to abnormal stress, for example, during movements of the tongue, and fractures of these bones have been reported. More frequent possible sequelae include inflammation of the middle ear (by extension of infection along the auditory tube); epistaxis (nasal bleeding) from erosion of the internal carotid artery; difficulty in swallowing following involvement of the glossopharyngeal and vagus nerves (or their pharyngeal branches); laryngeal hemiplegia (roaring) following vagus involvement; and various signs, collectively known as Horner syndrome, that may result from involvement of the sympathetic nerve—nasal congestion, drooping of the upper eyelid, pupillary constriction, sweating, and increased skin temperature over the affected side of the head and neck. Signs of involvement of the facial nerve are relatively rare, and none suggestive of damage to the hypoglossal nerve have been recorded. The external carotid artery also seems to enjoy a relative immunity.
The pouch can be inspected or drained via the pharyngotubal opening or approached by open surgery. A favored route of access is provided by Viborg’s triangle, bounded by the caudal border of the mandible (more deeply the occipitomandibularis), the tendon of the sternocephalicus, and the linguofacial vein. The distance between the triangle and the pouch is greatly reduced when the pouch is enlarged. An alternative, more dorsal approach, involving reflection of the parotid gland, is also employed.
Hemorrhage from the internal carotid artery is frequently fatal unless treated in good time by closure of the vessel to each side of the leak. A proximal ligature is easily applied, but direct access to a site distal to the lesion may be impossible. Recourse may then be had to a balloon-tipped catheter, which is introduced beyond the proximal ligature and advanced into the siphonlike formation that the artery displays immediately before entering the cranial cavity. The catheter is left in place until it is judged that thrombosis will have sealed the damaged segment of the artery.
In foals, malfunction of the pharyngotubal opening may result in the pouch becoming distended with air to the extent that a swelling is visible externally (Figure 18–34). It appears that in some horses there may be a redundancy of the mucosal fold (plica salpingopharyngea) that is normally present at the entry of the tube. In these individuals the excess mucosa creates a one-way valve that allows air to be drawn into the pouch but not expelled from it. Unilateral tympany may be relieved by forcing an opening in the median septum so that both pouches communicate with the pharynx through a single opening. When swelling is bilateral, an alternative surgical method has to be used.
Although the clinical importance of the guttural pouch has long been appreciated, its functional significance has resisted convincing explanation until very recently. Inevitably, the absence of hard fact has prompted speculative interpretations of different degrees of plausibility. These speculations can be disregarded after recent experimental investigations that identify the pouch as a mechanism for cooling the cerebral blood supply, a mechanism that is peculiar to the horse (at least among domestic species) and additional to other devices found in mammals generally (pp. 312, 505). These studies emphasize the relevance of the extensive contact between the extracranial part of the internal carotid artery and the exceedingly thin pouch wall. That this intimacy provides the potential for cooling the major (internal carotid) contribution to the cerebral blood supply was revealed in these experiments, in which thermocouples were implanted at various points along the course of the vessel. No local differences in blood temperature were registered in the resting animal, but a significant drop in temperature (of about 2°C) at the distal end of the artery was demonstrated in horses engaged in 15 minutes of strenuous exercise. Physical activity of course raises body temperature, ultimately to a level that could endanger brain function unless effective countermeasures exist. The transfer of heat from internal carotid blood to adjacent air is facilitated by the more vigorous ventilation of the pouch that accompanies exertion.
THE LARYNX
The larynx is suspended by the hyoid apparatus and is partly contained within the intermandibular space (see Figure 4–8). Although few distinguishing features of the cartilages are important, attention must be drawn to the deep notch in the ventral part of the thyroid cartilage because this provides very convenient access to the interior after incision of the cricothyroid ligament. A prominence rostral to the notch and the ventral part of the cricoid arch provide the necessary landmarks (see Figure 4–13/7). A prudent operator will also identify the basihyoid to confirm the site before making the initial skin incision. The normally retrovelar position of the leaf-shaped epiglottis has been pointed out (Figure 18–11/11).
The mucosa forms outpouchings (ventricles) that pass laterally between the vocal and vestibular folds but remain within the protection of the thyroid laminae. The ventricular entrance is sufficiently large to admit the burr that is used to evert the sac in one of the “roaring” operations (Figure 18–35/1).
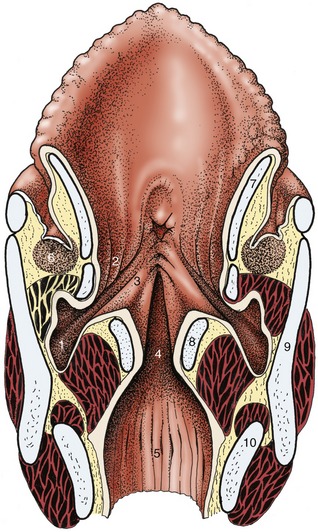
Figure 18–35 Dorsal section of the larynx. 1, Laryngeal ventricle; 2, vestibular fold with ventricularis; 3, vocal fold with vocalis; 4, glottic cleft; 5, infraglottic cavity; 6, caudal end of palatine tonsil; 7, epiglottic cartilage; 8, arytenoid cartilage; 9, thyroid cartilage; 10, cricoid cartilage.
Dilation of the glottis is a normal accompaniment of inspiration, and any interference with the process is harmful to respiratory efficiency. The condition known as roaring, from the strident sound emitted at inspiration, is a common manifestation of this in high-performance horses. In its severe form it is characterized by unilateral adduction of the arytenoid cartilage and vocal cord; in less severe forms it is identified by limited abduction of these structures. The abnormal sound is produced by passive vibration of a lax vocal cord in the airstream. The cause lies in dysfunction, proceeding to atrophy, of part of the intrinsic laryngeal musculature. For reasons that are unclear, the pathology is almost always limited to the left side and is initially manifested in the cricoarytenoideus dorsalis, the abductor muscle of the cartilage, before possibly proceeding to affect other muscles with adductor actions.
The term roaring is applied to a strident sound produced at inspiration in affected animals. The sound is caused by the flow of air passively vibrating a lax, adducted vocal fold (Figure 18–26, A-B). The laxity results from paralysis of certain laryngeal muscles. A polyneuropathy is considered to be the underlying cause. The paralysis is almost always limited to the left side, and though it initially affects the cricoarytenoideus dorsalis—abductor of the arytenoid cartilage and vocal fold (see Figure 4–15/5)—other muscles may later become involved.
The asymmetry in incidence directs attention to differences in the courses and relations of the right and left recurrent laryngeal nerves. The left nerve loops around the aortic arch and has a closer relationship to tracheobronchial and other lymph nodes within the chest. Because the condition often follows a respiratory infection, the relationship to the nodes is perhaps the more relevant; it is not a complete explanation, however, as laryngeal muscular atrophy has been recognized in unborn foals. Wastage of the cricoarytenoideus dorsalis alters the contours of the larynx in a manner that can be appreciated on external palpation; it hollows the space above the arytenoid cartilage, which makes the muscular process of that cartilage more prominent. One of the operations for the relief of roaring is the reinforcement of the wasted dorsal cricoarytenoideus muscle by a suture tightened to fix the arytenoid cartilage in permanent abduction. An older alternative was the eversion and excision of the lateral laryngeal ventricle in the expectation that the resulting scar tissue would bind this cartilage to the thyroid cartilage. Both operations result in tightening the vocal fold and widening the glottic cleft. Neither operation effects a cure of the condition, which has human and canine parallels. Other defects, such as partial collapse of an arytenoepiglottic fold or prolapse of the cricotracheal membrane, may also cause obstruction. It is noteworthy that multiple defects, possibly involving nasal, pharyngeal, and laryngeal levels, are quite common. Recently a syndrome of deformaties that may afflict the derivates of the fourth branchial arch has been described. This 4-BAD syndrome may also result in multiple anomalies of the pharynx, larynx, and upper esophagus.
THE EYE
Some account has been given of the external features (p. 501). The adnexa call for little comment. The lacrimal gland is relatively large and placed over the dorsolateral aspect of the bulbus, where it is protected by the adjacent part of the orbital rim (Figure 18–36/1). A small accessory lacrimal gland is associated with the deep part of the cartilage of the third eyelid.
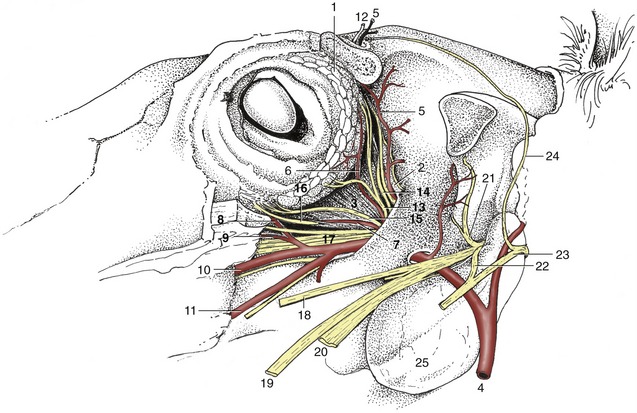
Figure 18–36 Dissection of the orbit; the zygomatic arch and periorbita have been removed. 1, Lacrimal gland; 2, periorbita; 3, lateral rectus; 4, maxillary a.; 5, supraorbital a.; 6, lacrimal a.; 7, muscular branch of external ophthalmic a.; 8, malar a.; 9, infraorbital a.; 10, major palatine a.; 11, buccal a.; 12, supraorbital n.; 13, lacrimal n.; 14, trochlear n.; 15, zygomatic n.; 16, oculomotor n.; 17, rostral branches of maxillary n.; 18, buccal n.; 19, lingual n.; 20, inferior alveolar n.; 21, masticatory n.; 22, auriculotemporal n.; 23, facial n.; 24, auriculopalpebral n.; 25, guttural pouch.
The nasolacrimal duct, already mentioned in relation to surgical access to the maxillary sinus, provides a conspicuous feature where it opens on the floor of the nostril (see Figure 18–3). The extraocular muscles show little that is distinctive; as is common in ungulates, the retractor bulbi is relatively large (see Figure 9–19/7).
The eyeball shows significant departure from the spheroidal form—it is compressed from front to back and is higher than it is wide—which is relevant to the concept of the ramp retina (see further on). It is constructed of the usual layers. The sclera is relatively thin toward the equator, where it obtains a bluish tint from the pigmentation of the underlying choroid. The cornea is relatively small and ovoid; its pointed end is lateral.
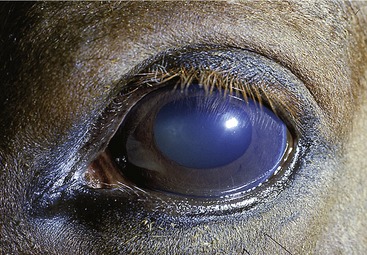
The choroid exhibits a triangular green or bluish-green tapetum dorsal to the optic disc (Figure 18–37). The ciliary muscle is poorly developed; a second point is adduced in support of the theory of the ramp retina as the means of accommodation. The iris is generally dark brown; in the absence of pigmentation (a rather uncommon anomaly) it is a rather unattractive bluish color (“walleye”) (see Figure 9–10, B). Both the iris and the pupillary opening within it are oval (with the long axes horizontal), but the pupil becomes rounder when contracted. The pupil of the newborn is almost round. Both margins of the pupil, but particularly the upper one, carry irregular granular excrescences interpreted as “shades” that limit the entry of light (see Figure 9–9/3).
The optic disc, very prominent on ophthalmoscopic examination of the fundus, is placed ventral to the tapetum and ventrolateral to the posterior pole of the bulb (see Figure 18–37). The macula is said to comprise both round and elongated parts; it is asserted that the former is concerned with binocular vision, the latter with monocular vision. The central artery of the retina is poorly developed, and the few straight branches that radiate from the margins of the disc soon fade. Much the larger part of the retina is nourished by the vessels of the middle tunic. There is nothing noteworthy in the refractive media.
It is believed that the poor development of the ciliary muscle compels the horse to rely on the distorted form of the bulb for accommodation. The upper part of the retina, which is at a greater distance from the lens, serves for near vision; the lower part, closer to the lens, serves for distance vision. The animal therefore adjusts the carriage of the head—and thereby the location of the image on the retina—as a means of focusing. The technique is sometimes well-illustrated by a horse approaching and jumping an obstacle.
THE VENTRAL PART OF THE NECK
The ventral part of the neck contains the visceral space occupied by the esophagus, trachea, and other structures passing between the head and the thorax. This space is bounded dorsally by the muscles below the vertebrae and laterally and ventrally by flatter muscles united by stout fasciae. The foremost lateroventral muscles are the brachiocephalicus and sternocephalicus, which bound the groove occupied by the (external) jugular vein (Figure 18–38/12). The caudal part of this groove is covered by the cutaneous muscle of the neck, which radiates from a manubrial origin; the muscle thins as it passes from its origin, which increases the prominence of the cranial part of the vein, the obvious target when the vein is raised for puncture (Figure 18–39/9,11). The brachiocephalicus is described on page 587.
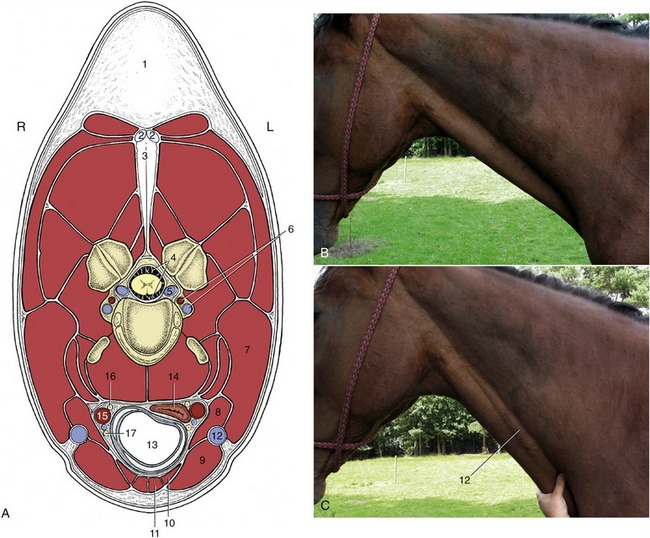
Figure 18–38 A, Transection of the neck at the level of the fourth cervical vertebra. B, The external jugular vein is not visible, but it is raised (c) when occluded in the jugular groove. 1, Crest; 2, 3, funicular and laminar parts of nuchal ligament; 4, subarachnoid space; 5, internal vertebral venous plexus; 6, vertebral artery and vein; 7, brachiocephalicus; 8, omohyoideus; 9, sternocephalicus; 10, sternothyroideus; 11, sternohyoideus; 12, external jugular vein; 13, trachea; 14, esophagus; 15, common carotid artery; 16, vagosympathetic trunk; 17, recurrent laryngeal nerve.
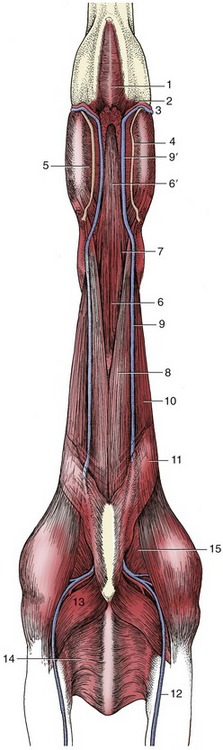
Figure 18–39 Ventral view of the neck and intermandibular space. 1, Mylohyoideus; 2, mandibular lymph nodes; 3, facial artery and vein; 4, parotid duct; 5, medial pterygoid; 6, sternohyoideus and sternothyroideus; 6′, combined sternohyoideus and omohyoideus; 7, omohyoideus; 8, sternocephalicus; 9, external jugular vein; 9′, linguofacial vein; 10, brachiocephalicus; 11, cutaneous colli; 12, cephalic vein; 13, pectoralis descendens; 14, pectoralis transversus; 15, subclavius.
The right and left sternocephalicus muscles arise from the manubrium side-by-side but diverge toward their mandibular insertions (Figure 18–39/8). This leaves a median space through which the trachea may be palpated, although it is still covered by the thin sternothyroideus and sternohyoideus (Figure 18–39/6). These are combined at their origin from the sternum but branch into slips that diverge to attach to the thyroid cartilage and the basihyoid. The omohyoideus (Figure 18–39/7), which extends between the medial aspect of the shoulder and the basihyoid, forms the floor of the jugular groove. It is said, unconvincingly, to protect the more deeply placed common carotid artery in unskillful venipuncture (Figure 18–38/15). The muscles ventral to the trachea constitute the “strap muscles” that are resected in Forsell’s operation for cribbing, which is a condition of stabled horses in which a horse hangs onto the crib with its teeth and dilates the pharynx to swallow air.
The trachea occupies a median position in the visceral space. Its size bears no constant relation to that of the body, which is an important point when selecting an endotracheal tube because the generous size of the glottis is not a limiting factor. The tracheal lumen is slightly flattened dorsoventrally and is of course maintained patent by the tracheal rings. It is therefore customary to completely transect as few cartilages as possible to avoid collapse of the wall in tracheotomy operations, such as those performed to allow the air intake to bypass an obstructed larynx.
The esophagus begins dorsal to the trachea but slips to the left side by the middle of the neck (Figure 18–38/14). It then slowly creeps back toward a median position, though it is often ventral to the trachea just before it enters the chest. It takes a more direct course when the neck is extended. The esophagus is too soft to identify easily on palpation, but its position is revealed when the animal swallows.
The common carotid artery lies ventral to the trachea at the base of the neck but gradually ascends to a more dorsal position (Figure 18–38/15); it divides above the pharynx into occipital, internal carotid, and external carotid arteries. The internal carotid supplies the brain, and the occipital supplies the region of the poll. The clinically relevant branches of the external carotid have been noted in previous contexts; the overall pattern of distribution is shown in Figure 18–40. Pulsations of the common carotid may sometimes be felt in the middle of the neck when the artery is pressed against the subvertebral muscles. Nowadays, puncture at this site may be employed for the provision of a sample of arterial blood. The artery is enclosed in a thick fascial sheath shared with the vagosympathetic trunk, which follows its dorsal border. The recurrent laryngeal nerve lies ventral to it in the tracheal fascia (Figure 18–38/16,17).
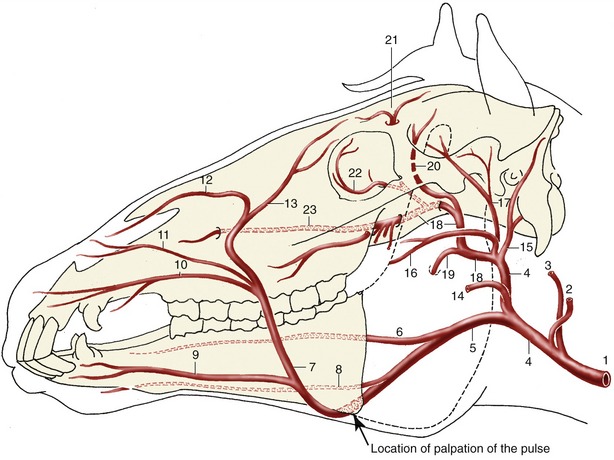
Figure 18–40 Principal arteries of the head, schematic. 1, Common carotid a.; 2, occipital a.; 3, internal carotid a.; 4, external carotid a.; 5, linguofacial a.; 6, lingual a.; 7, facial a.; 8, sublingual a.; 9, inferior labial a.; 10, superior labial a.; 11, lateral nasal a.; 12, dorsal nasal a.; 13, angularis oculi a.; 14, masseteric a.; 15, caudal auricular a.; 16, transverse facial a., displaced ventrally for clarity; 17, superficial temporal a.; 18, maxillary a.; 19, inferior alveolar a.; 20, caudal deep temporal a.; 21, supraorbital a.; 22, malar a.; 23, infraorbital a.
The deep cervical lymph nodes are scattered in packets—cranial, middle, and caudal—along the course of the tracheal lymph duct. The caudal group receives the outflow from the superficial cervical nodes (Figure 18–41).
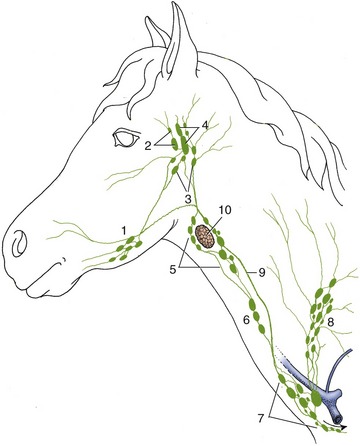
Figure 18–41 Lymphatic structures of the head and neck, schematic. 1, Mandibular lymph nodes; 2, parotid lymph nodes; 3, medial retropharyngeal lymph nodes; 4, lateral retropharyngeal lymph nodes; 5, 6, 7, cranial, middle, and caudal deep cervical lymph nodes; 8, superficial cervical lymph nodes; 9, tracheal duct; 10, thyroid gland.
The external jugular vein is supplemented by the vertebral vein and the plexus within the vertebral canal in the drainage of the head. It is formed at the caudoventral angle of the parotid gland by the confluence of maxillary and linguofacial veins. It stands out very prominently and very conveniently for injection and sampling when raised by pressure over the jugular groove.
The lobes of the thyroid gland can be recognized on palpation as soft ovoid structures placed dorsolateral to the first part of the trachea (Figure 18–30/21). They are joined ventrally by a narrow isthmus.
Although rarely as well-developed as in the calf, a cervical part of the thymus may extend beside the trachea in the caudal part of the neck of the foal. It is often separated from the thoracic part and may be broken into several masses.
THE LYMPHATIC STRUCTURES OF THE HEAD AND NECK
The parotid, mandibular, and deep cervical lymph nodes have been encountered (pp. 506 and 530). The superficial cervical nodes are described on page 619.
The retropharyngeal nodes are arranged in clumps on the pharyngeal wall (Figure 18–30/19). The lateral group is also related to the guttural pouch, lying caudal to it within the atlantal fossa. Infection of these nodes, frequently leading to abscess formation (strangles), may be followed by contamination of the guttural pouch with the potential sequelae already mentioned (p. 524). The pattern of drainage is such that the medial retropharyngeal nodes serve as the collecting center for all lymph emanating from the upper part of the head (Figure 18–41).