CHAPTER 8 Myocardial Diseases of the Cat
Myocardial disease in cats encompasses a diverse collection of idiopathic and secondary processes affecting the myocardium. The spectrum of anatomic and pathophysiologic features is wide. Disease characterized by myocardial hypertrophy is most common, although features of multiple pathophysiologic categories co-exist in some cats. Restrictive pathophysiology develops often. Classic dilated cardiomyopathy is now uncommon in cats; its features are similar to those of dilated cardiomyopathy in dogs (see Chapter 7). Myocardial disease in some cats does not fit neatly into the categories of hypertrophic, dilated, or restrictive cardiomyopathy and therefore is considered indeterminate or unclassified cardiomyopathy. Arterial thromboembolism is a major complication in cats with myocardial disease.
HYPERTROPHIC CARDIOMYOPATHY
Etiology
The cause of primary or idiopathic hypertrophic cardiomyopathy (HCM) in cats is unknown, but a heritable abnormality is likely in many cases. Disease prevalence appears to be high in several breeds, such as the Maine Coon, Persian, Ragdoll, and American Shorthair. There are also reports of HCM in litter mates and other closely related domestic shorthair cats. An autosomal dominant inheritance pattern has been found in some breeds. In human familial HCM, many different gene mutations are known to exist. Although several common human gene mutations have not yet similarly been found in feline HCM, others may be in the future. Reduced myomesin (a sarcomeric protein) occurs in some affected Maine Coon cats. The same researchers (Meurs et al. 2005) also found a mutation in cardiac myosin-binding protein C in this breed. Another mutation has been identified in Ragdoll cats; testing for these mutations is currently available (contact www.vetmed.wsu.edu/deptsVCGL/felineTests.aspx).
In addition to mutations of genes that encode for myocardial contractile or regulatory proteins, possible causes of the disease include an increased myocardial sensitivity to or excessive production of catecholamines; an abnormal hypertrophic response to myocardial ischemia, fibrosis, or trophic factors; a primary collagen abnormality; and abnormalities of the myocardial calcium-handling process. Myocardial hypertrophy with foci of mineralization occurs in cats with hypertrophic feline muscular dystrophy, an X-linked recessive dystrophin deficiency similar to Duchenne muscular dystrophy in people; however, congestive heart failure (CHF) is uncommon in these cats. Some cats with HCM have high serum growth hormone concentrations. It is not clear whether viral myocarditis has a role in the pathogenesis of feline cardiomyopathy. One study of myocardial samples from cats with HCM evaluated by polymerase chain reaction (PCR) showed evidence of panleukopenia virus DNA in approximately one third of the cats with myocarditis but in none of the healthy control cats (Meurs, 2000).
Pathophysiology
Thickening of the left ventricular (LV) wall and/or interventricular septum is characteristic, but the extent and distribution of hypertrophy in cats with HCM are variable. Many cats have symmetric hypertrophy, but some have asymmetric septal thickening, and a few have hypertrophy limited to the free wall or papillary muscles. The LV lumen usually appears small. Focal or diffuse areas of fibrosis occur within the endocardium, conduction system, or myocardium; narrowing of small intramural coronary arteries may also be noted. Areas of myocardial infarction as well as myocardial fiber disarray may be present.
Myocardial hypertrophy and the accompanying changes increase ventricular wall stiffness. Additionally, early active myocardial relaxation may be slow and incomplete, especially in the presence of myocardial ischemia. This further reduces ventricular distensibility and promotes diastolic dysfunction. This ventricular stiffness impairs LV filling and increases diastolic pressure. LV volume remains normal or decreased. Reduced ventricular volume results in a lower stroke volume, which may contribute to neurohormonal activation. Higher heart rates further interfere with LV filling, promote myocardial ischemia, and contribute to pulmonary venous congestion and edema by shortening the diastolic filling period. Contractility, or systolic function, is usually normal in affected cats. However, some cats experience progression to ventricular systolic failure and dilation.
Progressively higher LV filling pressures lead to increased left atrial (LA) and pulmonary venous pressures. Progressive LA dilation as well as pulmonary congestion and edema can result. The degree of LA enlargement varies from mild to massive. A thrombus is sometimes found within the LV or attached to a ventricular wall, although it is more commonly located in the LA. Arterial thromboembolism is a major complication of HCM as well as other forms of cardiomyopathy in cats (see Chapter 12). Mitral regurgitation develops in some affected cats. Changes in LV geometry, papillary muscle structure, or the systolic movement of the mitral valve (systolic anterior motion [SAM]) may prevent normal valve closure. Valve insufficiency exacerbates the increased LA size and pressure.
Systolic dynamic LV outflow obstruction occurs in some cats. This is also known as hypertrophic obstructive cardiomyopathy or functional subaortic stenosis. Excessive asymmetric hypertrophy of the basilar interventricular septum may be evident on echocardiograms or at necropsy. Systolic outflow obstruction increases LV pressure, wall stress, and myocardial oxygen demand and promotes myocardial ischemia. Mitral regurgitation is exacerbated by the tendency of hemodynamic forces to pull the anterior mitral leaflet toward the interventricular septum during ejection (SAM, see Figure 8-3). Increased LV outflow turbulence commonly causes an ejection murmur of variable intensity in these cats.
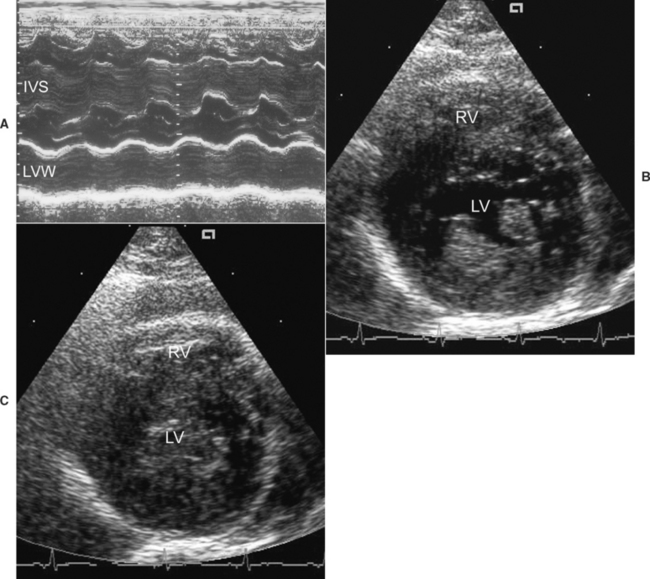
FIG 8-3 Echocardiographic examples of feline hypertrophic cardiomyopathy. M-mode image (A) at the left ventricular level from a 7-year-old male Domestic Shorthair cat. The left ventricular diastolic free-wall and septal thicknesses are about 8 mm. Two-dimensional right parasternal short-axis views during diastole (B) and systole (C) in male Maine Coon cat with hypertrophic obstructive cardiomyopathy. In (B) note the hypertrophied and bright papillary muscles. In (C) note the almost total systolic obliteration of the left ventricular chamber. IVS, interventricular septum; LV, left ventricle; LVW, left ventricular free wall; RV, right ventricle.
Several factors probably contribute to the development of myocardial ischemia in cats with HCM. These include narrowing of intramural coronary arteries, increased LV filling pressure, decreased coronary artery perfusion pressure, and insufficient myocardial capillary density for the degree of hypertrophy. Tachycardia contributes to ischemia by increasing myocardial O2 requirements while reducing diastolic coronary perfusion time. Ischemia impairs early, active ventricular relaxation, which further increases ventricular filling pressure, and over time leads to myocardial fibrosis. Ischemia can provoke arrhythmias and possibly thoracic pain.
Atrial fibrillation (AF) and other tachyarrhythmias fur-ther impair diastolic filling and exacerbate venous congestion; the loss of the atrial “kick” and the rapid heart rate associated with AF are especially detrimental. Ventricular tachycardia or other arrhythmias may lead to syncope or sudden death.
Pulmonary venous congestion and edema result from increasing LA pressure. Increased pulmonary venous and capillary pressures are thought to cause pulmonary vasoconstriction; increased pulmonary arterial pressure and secondary right-sided CHF signs may occur. Eventually, refractory biventricular failure with profuse pleural effusion develops in some cats with HCM. The effusion is usually a modified transudate, although it can be (or become) chylous.
Clinical Features
HCM may be most common in middle-age male cats, but clinical signs can occur at any age. Cats with milder disease may be asymptomatic for years. Symptomatic cats are most often presented for respiratory signs of variable severity or acute signs of thromboembolism (see p. 195). Respiratory signs include tachypnea; panting associated with activity; dyspnea; and, only rarely, coughing (which can be misinterpreted as vomiting). Disease onset may seem acute in sedentary cats, even though pathologic changes have developed gradually. Occasionally, lethargy or anorexia is the only evidence of disease. Some cats have syncope or sudden death in the absence of other signs. Stresses such as anesthesia, surgery, fluid administration, systemic illnesses (e.g., fever or anemia), or boarding can precipitate CHF in an otherwise compensated cat. Asymptomatic disease is discovered in some cats when a murmur or gallop sound is heard during routine auscultation.
Systolic murmurs compatible with mitral regurgitation or LV outflow tract obstruction are common. Some cats do not have an audible murmur, even those with marked ventricular hypertrophy. A diastolic gallop sound (usually S4) may be heard, especially if heart failure is evident or imminent. Cardiac arrhythmias are relatively common. Femoral pulses are usually strong, unless distal aortic thromboembolism has occurred. The precordial impulse often feels vigorous. Prominent lung sounds, pulmonary crackles, and sometimes cyanosis accompany severe pulmonary edema. Pulmonary crackles are not always heard with edema in cats. Pleural effusion usually attenuates ventral pulmonary sounds. The physical examination may be normal in subclinical cases.
RADIOGRAPHY
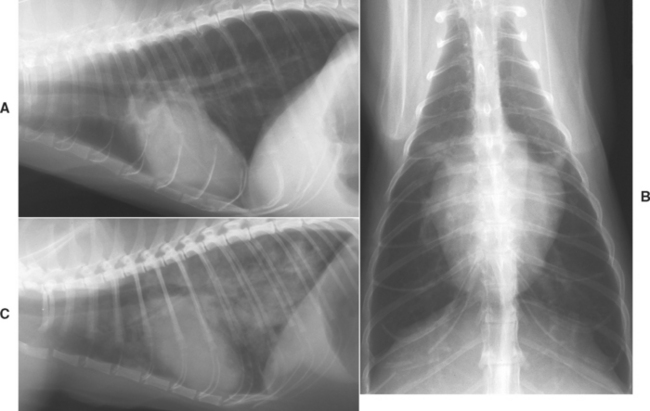
Radiographic features of HCM include a prominent left atrium and variable LV enlargement (Fig. 8-1). The classic valentine-shaped appearance of the heart on dorsoventral or ventrodorsal views is not always present, although usually the point of the left ventricular apex is maintained. The cardiac silhouette appears normal in most cats with mild HCM. Enlarged and tortuous pulmonary veins may be noted in cats with chronically high LA and pulmonary venous pressure. Left-sided CHF produces variable degrees of patchy interstitial or alveolar pulmonary edema infiltrates. The radiographic distribution of pulmonary edema is variable; a diffuse or focal distribution throughout the lung fields is common, in contrast to the characteristic perihilar distribution of cardiogenic pulmonary edema seen in dogs. Pleural effusion is common in cats with advanced or biventricular CHF.
ELECTROCARDIOGRAPHY
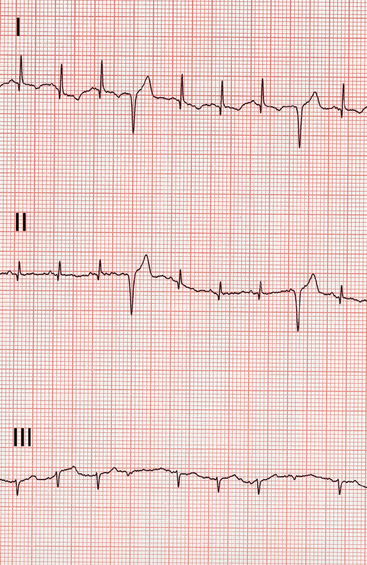
Many (up to 70%) cats with HCM have electrocardiogram (ECG) abnormalities. These include criteria for LA or LV enlargement, ventricular and/or (less often) supraventricular tachyarrhythmias, and a left anterior fascicular block pattern (Fig. 8-2). Atrioventricular (AV) conduction delay, complete AV block, or sinus bradycardia is occasionally found.
ECHOCARDIOGRAPHY
Echocardiography is the best means of diagnosis and differentiation of HCM from other disorders. The extent of hypertrophy and its distribution within the ventricular wall, septum, and papillary muscles is shown by two-dimensional and M-mode echo studies. Doppler techniques can demonstrate LV diastolic or systolic abnormalities.
Widespread myocardial thickening is common, and the hypertrophy is often asymmetrically distributed among various LV wall, septal, and papillary muscle locations. Focal areas of hypertrophy also occur. Use of two-dimensional-guided M-mode helps ensure proper beam position. Standard M-mode views and measurements are obtained, but thickened areas outside these standard positions should also be measured (Fig. 8-3). The diagnosis of early disease may be questionable in cats with mild or only focal thickening. Falsely increased thickness measurements (pseudohypertrophy) can occur with dehydration and sometimes tachycardia. Spurious diastolic thickness measurements also arise when the beam does not transect the wall/septum perpendicularly and when the measurement is not taken at the end of diastole, as can happen without simultaneous ECG record ing or when using two-dimensional imaging of insufficient frame rate. A (properly obtained) diastolic LV wall or septal thickness >5.5 mm is considered abnormal. Cats with severe HCM have diastolic LV wall or septal thicknesses of 8 mm or more, although the degree of hypertrophy is not necessarily correlated with the severity of clinical signs. Doppler-derived estimates of diastolic function, such as isovolumic relaxation time, and mitral inflow and pulmonary venous velocity patterns, as well as Doppler tissue imaging techniques are being employed more often to define disease characteristics.
Papillary muscle hypertrophy can be marked, and systolic LV cavity obliteration is observed in some cats. Increased echogenicity (brightness) of papillary muscles and subendocardial areas is thought to be a marker for chronic myocardial ischemia with resulting fibrosis. LV fractional shortening (FS) is generally normal to increased. However, some cats have mild to moderate LV dilation and reduced contractility (FS – 23%-29%; normal FS is 35%-65%). Right ventricular enlargement and pericardial or pleural effusion are occasionally detected.
Cats with dynamic LV outflow tract obstruction also often have SAM of the mitral valve (Fig. 8-4) or premature closure of the aortic valve leaflets on M-mode scans. Doppler modalities can demonstrate mitral regurgitation and LV outflow turbulence (Fig. 8-5), although optimal alignment with the maximal-velocity outflow jet is often difficult and it is easy to underestimate the systolic gradient.
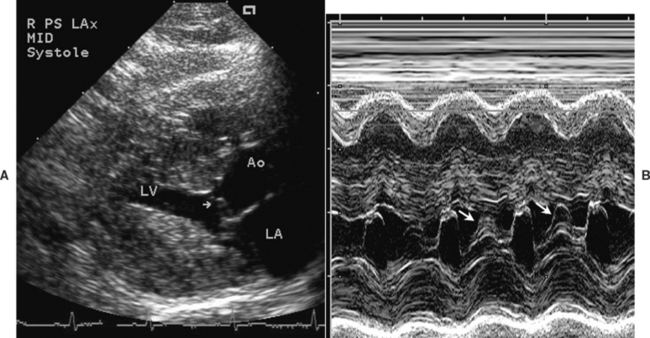
FIG 8-4 A, Two-dimensional echo image in midsystole from the cat in Fig. 8-3, B and C. Echoes from the anterior mitral leaflet appear within the LV outflow tract (arrow) because of abnormal systolic anterior (toward the septum) motion (SAM) of the valve. B, The M-mode echocardiogram at the mitral valve level also shows the mitral SAM (arrows). Ao, Aorta; LA, left atrium; LV, left ventricle.
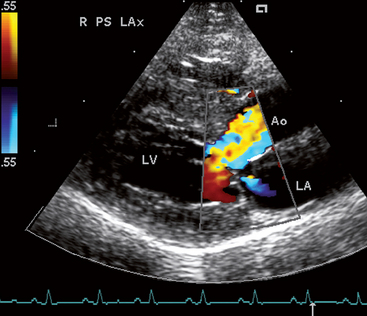
FIG 8-5 Color flow Doppler image taken in systole from a male Domestic Longhair cat with hypertrophic obstructive cardiomyopathy. Note the turbulent flow just above where the thickened interventricular septum protrudes into the left ventricular outflow tract and a small mitral insufficiency jet into the LA, common with SAM. Right parasternal long axis view. Ao, Aorta; LA, left atrium; LV, left ventricle.
LA enlargement may be mild to marked. Spontaneous contrast (swirling, smoky echos) is visible within the enlarged LA of some cats. This is thought to result from blood stasis with cellular aggregations and to be a harbinger of thromboembolism. A thrombus is occasionally visualized within the left atrium, usually in the auricle (Fig. 8-6).
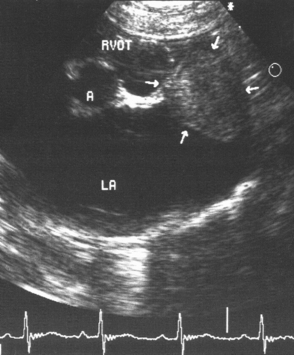
FIG 8-6 Echocardiogram obtained from the right parasternal short-axis position at the aortic-left atrial level in an old male Domestic Shorthair cat with restrictive cardiomyopathy. Note the massive left atrial enlargement and thrombus (arrows) within the auricle. A, Aorta; LA, left atrium; RVOT, right ventricular outflow tract.
Other causes of myocardial hypertrophy (see p. 149) should be excluded before a diagnosis of idiopathic HCM is made. Myocardial thickening can also result from infiltrative disease. Variation in myocardial echogenicity or wall irregularities may be noted in such cases. Excess moderator bands appear as bright, linear echos within the left ventricular cavity.
SUBCLINICAL HYPERTROPHIC CARDIOMYOPATHY
Whether (and how) asymptomatic cats should be treated is controversial. It is unclear if disease progression can be slowed or survival prolonged by medical therapy before the onset of clinical signs. According to anecdotal reports, some cats show increased activity or improved “attitude” after being treated with a β-blocker or diltiazem on the basis of echocardiographic findings or an arrhythmia. When moderate to severe LA enlargement is found, especially with spontaneous echocontrast, instituting antithrombotic prophylaxis is prudent (see Chapter 12).
Avoidance of stressful situations likely to cause persistent tachycardia and reevaluation on a semiannual or annual basis are usually advised. Secondary causes of myocardial hypertrophy, such as systemic arterial hypertension and hyperthyroidism, should be ruled out (or treated, if found).
CLINICALLY EVIDENT HYPERTROPHIC CARDIOMYOPATHY
Goals of therapy are to enhance ventricular filling, relieve congestion, control arrhythmias, minimize ischemia, and prevent thromboembolism (Box 8-1). Furosemide is used only at the dosage needed to help control congestive signs. Moderate to severe pleural effusion is treated by thoraco centesis, with the cat restrained gently in sternal position. Cats with severe CHF signs are given supplemental oxygen, parenteral furosemide, and sometimes other drugs to con-trol edema (discussed in more detail later). Once initial medications have been given, the cat should be allowed to rest. The respiratory rate is noted initially and then every 30 minutes or so without disturbing the cat. Catheter placement, blood sampling, radiographs, and other tests and therapies should be delayed until the cat’s condition is more stable.
 BOX 8-1 Treatment Outline for Cats with Hypertrophic Cardiomyopathy
BOX 8-1 Treatment Outline for Cats with Hypertrophic Cardiomyopathy
ACE, Angiotensin-converting enzyme; CHF, congestive heart failure; HR, heart rate; LMWH, low-molecular-weight heparin.
Severe, Acute Signs of CHF*
Mild To Moderate Signs of CHF*
Chronic HCM Management*
* See text and Chapters 3 and 4 for further details.
† See Chapter 4 for additional ventricular antiarrhythmic drug therapy.
‡ See Chapter 12 for further details.
Ventricular filling is improved by slowing the heart rate and enhancing relaxation. Stress and activity level should be minimized to the extent possible. Although the Ca++-channel blocker diltiazem, or a β-blocker (see Chapter 4 and Table 4-2) have historically formed the foundation of long-term oral therapy, an angiotensin-converting enzyme inhibitor (ACEI) may have greater benefit in cats with CHF. Optimal recommendations await further study. The decision to use one particular drug over another is influenced by echocardiographic or other findings in the individual cat or the response to medication. Diltiazem is often used when severe, symmetric LV hypertrophy is present. A β-blocker is currently preferred for cats with dynamic LV outflow obstruction, tachyarrhythmias, syncope, suspected myocardial infarction, or concurrent hyperthyroidism. An ACEI may reduce neurohormonal activation and abnormal cardiac remodeling. It is sometimes used alone or combined with diltiazem or a β-blocker. Long-term therapy generally also includes therapy to reduce the likelihood of arterial thromboembolism (see Chapter 12). Dietary sodium restriction is recommended if the cat will accept such a diet, but it is more important to forestall anorexia.
Certain drugs are generally discouraged in cats with HCM. These include digoxin and other positive inotropic agents because they increase the myocardial oxygen demand and can worsen dynamic LV outflow obstruction. Any drug that accelerates the heart rate is also potentially detrimental because tachycardia shortens ventricular filling time and predisposes to myocardial ischemia. Arterial vasodilators can cause hypotension and reflex tachycardia, and cats with HCM have little preload reserve. Hypotension can also exacerbate dynamic outflow obstruction. Although ACEIs have this potential, their vasodilating effects are usually mild.
Diuretic Therapy
Cats with severe pulmonary edema are usually given intramuscular (IM) furosemide initially (2 mg/kg q1-4h; see Box 3-1 and p. 58), until an IV catheter can be placed without excessive stress to the cat. The respiratory rate and effort are used to guide ongoing diuretic therapy. As respiratory distress resolves, furosemide can be continued at a reduced dose (∼1 mg/kg q8-12h). Once pulmonary edema is controlled, furosemide is given orally and the dose gradually titrated downward to the lowest effective level. A starting dose of 6.25 mg/cat q8-12h can be slowly reduced over days to weeks, depending on the cat’s response. Some cats do well with dosing a few times per week (or less), whereas others require it several times per day. Complications of excessive diuresis include azotemia, anorexia, electrolyte disturbances, and poor LV filling. If the cat is unable to rehydrate itself by oral water intake, cautious parenteral fluid administration may be needed (e.g., 15-20 ml/kg/day of 0.45% saline, 5% dextrose in water, or other low-sodium fluid).
Other Therapy for Acute Congestive Heart Failure
Nitroglycerin ointment may be used (q4-6h, see Box 3-1), although no studies of its efficacy in this situation have been done. The bronchodilating and mild diuretic effects of aminophylline (5 mg/kg q12h, IM, IV) may be helpful in cats with severe pulmonary edema, as long as the drug does not increase the heart rate.
Butorphanol may be used to reduce anxiety (see Box 3-1). Acepromazine may be used as an alternative and can promote peripheral redistribution of blood by its β-blocking effects. Hypothermia may be exacerbated by peripheral vasodilation. Morphine should not be used in cats. Airway suctioning and mechanical ventilation with positive end-expiratory pressure can be considered in extreme cases.
Angiotensin-converting enzyme inhibitors.
An ACEI appears to have beneficial effects, especially in cats with refractory heart failure. Renin-angiotensin system inhibition may mitigate angiotensin II–mediated ventricular hypertrophy. ACE inhibition might reduce LA size and ventricular/septal wall thickness, at least in some cats. Enalapril and benazepril are the agents used most often in cats, although others are available (see Chapter 3 and Table 3-3).
Ca++-channel blockers.
Ca++-channel blockers are thought to have beneficial effects in cats with HCM by modestly reducing heart rate and contractility (which reduces myocardial O2 demand). Diltiazem promotes coronary vasodilation and may have a positive effect on myocardial relaxation. Verapamil is not recommended because of its variable bioavailability and risk of toxicity in cats. Amlodipine has primarily vasodilatory effects and is not used for HCM because it can provoke reflex tachycardia and worsen a systolic outflow gradient.
Diltiazem is well-tolerated in many cases. Longer-acting diltiazem products are more convenient for chronic use, although the serum concentrations achieved can be variable. Dilacor (diltiazem) XR, dosed at half of an internal (60-mg) tablet from the 240-mg capsule size q24(-12)h, or Cardizem CD, compounded and dosed at 10 mg/kg q24h, are most often used.
β-adrenergic blockers.
β-blockers can reduce heart rate and dynamic LV outflow obstruction to a greater extent than diltiazem. They are also used to suppress tachyarrhythmias in cats. Sympathetic inhibition also leads to reduced myocardial O2 demand, which can be important in cats with myocardial ischemia or infarction. By inhibiting catecholamine-induced myocyte damage, β-blockers may reduce myocardial fibrosis. β-blockers can slow active myocardial relaxation, although the benefits of heart rate reduction may outweigh this.
Atenolol (see Chapter 4) is used most commonly. Propranolol or another nonselective β-blocker can also be used, but these should be avoided until pulmonary edema is largely resolved. Antagonism of airway β2-receptors leading to bronchoconstriction is a concern when using nonselective agents in CHF. Propranolol (a lipid soluble drug) causes lethargy and depressed appetite in some cats.
Occasionally, a β-blocker is added to diltiazem therapy (or vice versa) in cats with chronic refractory failure or to further reduce heart rate in cats with AF. However, care must be taken to prevent bradycardia or hypotension in animals receiving this combination.
CHRONIC REFRACTORY CONGESTIVE HEART FAILURE
Refractory pulmonary edema or pleural effusion is difficult to manage. Moderate to large pleural effusions should be treated by thoracocentesis. Various medical strategies may help slow the rate of abnormal fluid accumulation, including maximizing the dosage of (or adding) an ACEI; increasing the dosage of furosemide (up to 4 mg/kg q8h); increasing the dose of diltiazem or β-blocker for greater heart rate control; and adding spironolactone, with or without hydrochlorothiazide (see Table 3-3). Spironolactone can be compounded into a flavored suspension for more accurate dosing. Pimobendan or digoxin can also be used for treating refractory right-sided CHF signs in cats without LV outflow obstruction and those with progressive LV dilation and myocardial systolic failure in end-stage disease. Frequent monitoring for the development of azotemia or electrolyte disturbances is warranted.
Prognosis
Several factors influence the prognosis for cats with HCM, including the speed with which the disease progresses, the occurrence of thromboembolic events and/or arrhythmias, and the response to therapy. Asymptomatic cats with only mild to moderate LV hypertrophy and atrial enlargement often live well for several years. Cats with marked LA enlargement and more severe hypertrophy appear to be at greater risk for CHF, thromboembolism, and sudden death. LA size and age (i.e., older cats) appear to be negatively correlated with survival. Median survival time for cats with CHF is probably between 1 to 2 years. The prognosis is worse in cats with AF or refractory right-sided CHF. Thromboembolism and CHF confer a guarded prognosis (median survival of 2 to 6 months), although some cats do well if congestive signs can be controlled and infarction of vital organs has not occurred. Recurrence of thromboembolism is common.
SECONDARY HYPERTROPHIC MYOCARDIAL DISEASE
Myocardial hypertrophy is a compensatory response to certain identifiable stresses or diseases. Marked LV wall and septal thickening and clinical heart failure can occur in some of these cases, although they are generally not considered to be idiopathic HCM. Secondary causes should be ruled out whenever LV hypertrophy is identified.
Evaluation for hyperthyroidism is indicated in cats 6 years of age or older with myocardial hypertrophy. Hyperthyroidism alters cardiovascular function by its direct effects on the myocardium and through the interaction of heightened sympathetic nervous system activity and excess thyroid hormone on the heart and peripheral circulation. Cardiac effects of thyroid hormone include myocardial hypertrophy and increased heart rate and contractility. The metabolic acceleration that accompanies hyperthyroidism causes a hyperdynamic circulatory state characterized by increased cardiac output, oxygen demand, blood volume, and heart rate. Systemic hypertension can further stimulate myocardial hypertrophy. Manifestations of hyperthyroid heart disease often include a systolic murmur, hyperdynamic arterial pulses, a strong precordial impulse, sinus tachycardia, and various arrhythmias. Criteria for LV enlargement or hypertrophy are often found on ECG, thoracic radiographs, or echocardiogram. Signs of CHF develop in approximately 15% of hyperthyroid cats; most have normal to high FS, but a few have poor contractile function. Cardiac therapy, in addition to treatment of the hyperthyroidism, may be neces sary for these cats. A β-blocker can temporarily control many of the adverse cardiac effects of excess thyroid hormone, especially tachyarrhythmias. Diltiazem is an alternative therapy. Treatment for CHF is the same as that described for HCM. The rare hypodynamic (dilated) cardiac failure is treated in the same way as dilated cardiomyopathy. Cardiac therapy, including a β-blocker, is not a substitute for antithyroid treatment.
LV concentric hypertrophy is the expected response to increased ventricular systolic pressure (afterload). Systemic arterial hypertension (see Chapter 11) increases afterload because of high arterial pressure and resistance. Increased resistance to ventricular outflow also occurs with a fixed (e.g., congenital) subaortic stenosis or dynamic LV outflow tract obstruction (hypertrophic obstructive cardiomyopathy). Cardiac hypertrophy also develops in cats with hypersomatotropism (acromegaly) as a result of growth hormone’s trophic effects on the heart. CHF occurs in some of these cats. Increased myocardial thickness occasionally results from infiltrative myocardial disease, most notably from lymphoma.
RESTRICTIVE CARDIOMYOPATHY
Etiology and Pathophysiology
Restrictive cardiomyopathy (RCM) is associated with extensive endocardial, subendocardial, or myocardial fibrosis. The cause is not clear but probably is multifactorial. This condition may be a consequence of endomyocarditis or the end-stage of myocardial failure and infarction caused by HCM. Neoplastic (e.g., lymphoma) or other infiltrative or infectious diseases occasionally causes a secondary RCM.
There are a variety of histologic findings in cats with RCM, including marked perivascular and interstitial fibrosis, intramural coronary artery narrowing, and myocyte hypertrophy, as well as areas of degeneration and necrosis. Some cats have extensive LV endomyocardial fibrosis with chamber deformity, or fibrous tissue bridging between the septum and LV wall. The mitral apparatus and papillary muscles may be fused to surrounding tissue or distorted.
LA enlargement is prominent in cats with RCM, as a consequence of chronically high LV filling pressure from increased LV wall stiffness. The LV may be normal to reduced in size or mildly dilated. LV hypertrophy is variably present and may be regional. Intracardiac thrombi and systemic thromboembolism are common.
LV fibrosis impairs diastolic filling. Most affected cats have normal to only mildly reduced contractility, but this may progress with time as more functional myocardium is lost. Some cases develop regional LV dysfunction, possibly from myocardial infarction, which decreases overall systolic function. These cases are perhaps better considered unclassified rather than restrictive. If mitral regurgitation is present, it is usually mild. Arrhythmias, ventricular dilation, and myocardial ischemia or infarction also contribute to the development of diastolic dysfunction. Chronically elevated left heart filling pressures, combined with compensatory neurohormonal activation, leads to left-sided or biventricular CHF. The duration of subclinical disease progression in RCM is unknown.
Clinical Features
Middle-aged and older cats are most often diagnosed with RCM. Young cats are sometimes affected. Inactivity, poor appetite, vomiting, and weight loss of recent onset are common in the history. The clinical presentation varies but usually includes respiratory signs from pulmonary edema or pleural effusion. Clinical signs are often precipitated or acutely worsened by stress or concurrent disease that causes increased cardiovascular demand. Thromboembolic events are also common. Sometimes the condition is discovered by detecting abnormal heart sounds or arrhythmias on routine exam or radiographic evidence of cardiomegaly.
A systolic murmur of mitral or tricuspid regurgitation, a gallop sound, and arrhythmias are common physical examination findings. Pulmonary sounds can be abnormal in cats with pulmonary edema or pleural effusion. Femoral arterial pulses are normal or slightly weak. Jugular vein distention and pulsation are common in cats with right-sided CHF signs. Acute signs of distal aortic (or other) thromboembolism may be the reason for presentation.
Diagnosis
Diagnostic test results are frequently similar to those in cats with HCM. Radiographs indicate LA or biatrial enlargement (sometimes massive) and LV or generalized heart enlargement (Fig. 8-7). Mild to moderate pericardial effusion contributes to the cardiomegaly in some cats. Proximal pulmonary veins may appear dilated and tortuous. Other possible radiographic findings in cats with CHF signs include infiltrates of pulmonary edema, pleural effusion, and sometimes hepatomegaly.
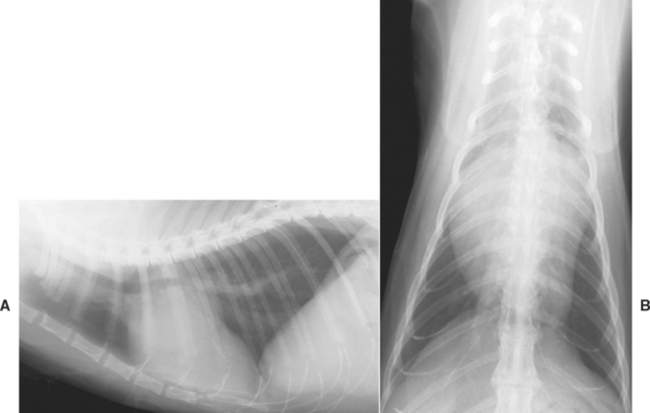
FIG 8-7 Lateral (A) and dorsoventral (B) radiographs from an older Domestic Shorthair cat with restrictive cardiomyopathy show marked left atrial enlargement and prominent proximal pulmonary veins.
Common ECG abnormalities include wide QRS complexes, tall R waves, evidence of intraventricular conduction disturbances, wide P waves, and atrial tachyarrhythmias or fibrillation. Echocardiography typically shows marked LA (and sometimes right atrial [RA]) enlargement. There is variable LV wall and interventricular septal thickening. Ventricular wall motion is often normal but may be somewhat depressed (FS usually >25%). Hyperechoic areas of fibrosis within the LV wall and/or endocardial areas may be evident. Extraneous intraluminal echos representing excess moderator bands are occasionally seen. Sometimes, extensive LV endocardial fibrosis, with scar tissue bridging between the free-wall and septum, constricts part of the ventricular chamber. Right ventricular (RV) dilation is often seen. Sometimes an intracardiac thrombus is found, usually in the left auricle or left atrium, but occasionally in the left ventricle (see Fig. 8-6). Mild mitral or tricuspid regurgitation and a restrictive mitral inflow pattern can be seen with Doppler studies. Some cats have marked regional wall dysfunction, especially of the left ventricular free wall, which depresses FS, along with mild left ventricular dilation. These may represent cases of myocardial infarction or unclassified cardiomyopathy rather than RCM.
The clinicopathologic findings are nonspecific. Pleural effusions are usually classified as modified transudate or chyle. Plasma taurine concentration is low in some affected cats and should be measured if decreased contractility is identified.
Treatment and Prognosis
Therapy for acute CHF is the same as for cats with HCM (see p. 148). Cats that require inotropic support can be given dobutamine by constant rate infusion (CRI). Management of thromboembolism is described on p. 197.
Long-term therapy for heart failure includes furosemide at the lowest effective dosage; the resting respiratory rate, activity level, and radiographic findings are used to monitor efficacy. An ACEI is also used, starting with a very low dose and increasing to the usual maintenance dose (see Table 3-3). Ideally, blood pressure should be monitored when initiating or adjusting this therapy. A β-blocker is usually used for tachyarrhythmias or if myocardial infarction is suspected. Alternatively, diltiazem can also be used, although its value in the face of significant fibrosis is controversial. Cats that need chronic inotropic support can be given digoxin or pimobendan (see Table 3-3). Taurine supplementation may be helpful. Prophylaxis against thromboembolism is recommended (see p. 199), and a low-sodium diet should be fed, if accepted. Creatinine or the blood urea nitrogen and electrolyte concentrations should be measured periodically. Furosemide and/or enalapril doses should be reduced if hypotension or azotemia occurs.
Cats with refractory heart failure and pleural effusion are difficult to manage. In addition to thoracocentesis as needed, the ACEI and furosemide dosages can be increased cautiously. Adding digoxin or pimobendan, if not already being used, may be helpful for cats with refractory failure. Other strategies include adding spironolactone (with or without hydrochlorothiazide) or nitroglycerin ointment to the regimen.
The prognosis is generally guarded to poor for cats with RCM and heart failure. Nevertheless, some cats survive more than a year after diagnosis. Thromboembolism and refractory pleural effusion commonly occur.
DILATED CARDIOMYOPATHY
Etiology
In the late 1980s taurine deficiency was identified as a major cause of dilated cardiomyopathy (DCM) in cats. This discovery prompted pet food manufacturers to increase the taurine content of commercial cat diets. Clinical DCM then became an uncommon disease in cats. Not all cats fed a taurine-deficient diet develop DCM. Other factors besides a simple deficiency of this essential amino acid are likely to be involved in the pathogenesis, including genetic factors and a possible link with potassium depletion. Relatively few cases of DCM are identified now; many of these cats are not taurine deficient. DCM in these cats may represent the end-stage of another myocardial metabolic abnormality, toxicity, or infection.
Doxorubicin causes characteristic myocardial histologic lesions in cats as it does in dogs. Cats appear less likely to develop clinical CHF from myocardial failure. Although in very rare cases cats have echocardiographic changes consistent with DCM after receiving cumulative doses of 170 to 240 mg/m2, clinically relevant doxorubicin-induced cardiomyopathy does not occur in the cat.
Pathophysiology
DCM in cats has a similar pathophysiology to that in dogs (see p. 129). Poor myocardial contractility is the characteristic feature (Fig. 8-8). Usually, all cardiac chambers become dilated. AV valve insufficiency occurs secondary to chamber enlargement and papillary muscle atrophy. As cardiac output decreases, compensatory neurohormonal mechanisms are activated, leading eventually to signs of CHF and low cardiac output. Besides pulmonary edema, pleural effusion and arrhythmias are common in cats with DCM.
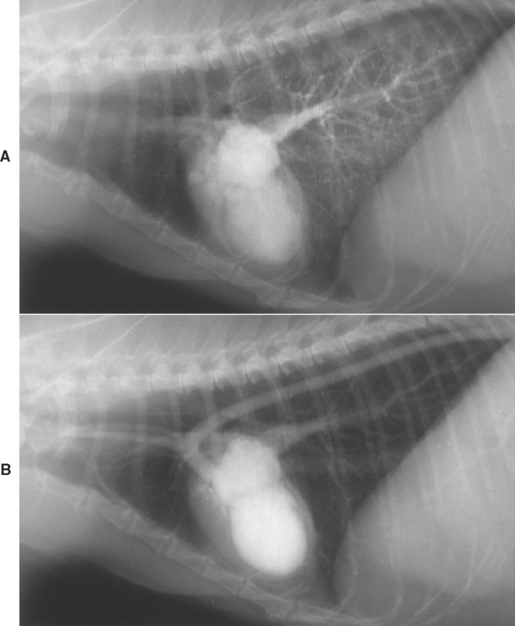
FIG 8-8 Nonselective angiogram from a 13-year-old female Siamese cat with dilated cardiomyopathy. A bolus of radiographic contrast material was injected into the jugular vein. A, Three seconds after injection, some contrast medium remains in the right ventricle and pulmonary vasculature. Dilated pulmonary veins are seen entering the left atrium. Note the dilated left atrium and ventricle. B, Thirteen seconds after the injection, the left heart and pulmonary veins are still opacified, illustrating the poor cardiac contractility and extremely slow circulation time. The thin left ventricular caudal wall and papillary muscles are better seen in this frame.
Clinical Features
DCM can occur at any age, although most affected cats are late-middle aged to geriatric. There is no breed or gender predilection. Clinical signs often include anorexia, lethargy, increased respiratory effort or dyspnea, dehydration, and hypothermia. Subtle evidence of poor ventricular function is usually found in conjunction with signs of respiratory compromise. Jugular venous distention, an attenuated precordial impulse, weak femoral pulses, a gallop sound (usually S3), and a left or right apical systolic murmur (of mitral or tricuspid regurgitation) are common. Bradycardia and arrhythmias are frequently heard, although many cats have a normal sinus rhythm. Increased lung sounds and pulmonary crackles can be auscultated in some cats, but pleural effusion may muffle ventral lung sounds. Some cats have signs of arterial thromboembolism (see p. 195).
Diagnosis
Generalized cardiomegaly with rounding of the cardiac apex is often seen on radiographs. Pleural effusion is common and may obscure the heart shadow and co-existing evidence of pulmonary edema or venous congestion. Hepatomegaly may be detected; ascites is occasionally found.
Typical ECG findings include a LV enlargement pattern, AV conduction disturbance, and tachyarrhythmias. Echocardiography is an important tool to differentiate DCM from other myocardial pathophysiology. Findings are analogous to those in dogs with DCM (see p. 131). Some cats have areas of focal hypertrophy with hypokinesis of only the LV wall or septum. These may represent indeterminant myocardial disease rather than typical DCM. An intracardiac thrombus is identified in some cats, more often within the left atrium.
Nonselective angiocardiography is a more risky alternative to echocardiography, as with other cardiomyopathies. Characteristic angiographic findings include generalized chamber enlargement, atrophied papillary muscles, small aortic diameter, and slow circulation time (see Fig. 8-8). Complications of angiography, especially in cats with poor myocardial function or CHF, include vomiting and aspiration, arrhythmias, and cardiac arrest. The pleural effusion in cats with DCM is usually a modified transudate, although it can be chylous. Prerenal azotemia, mildly increased liver enzyme activity, and a stress leukogram are common clinicopathologic findings. Cats with arterial thromboembolism often have high serum muscle enzyme activities and may have an abnormal hemostasis profile. Plasma or whole blood taurine concentration measurement is recommended. Specific instructions for sample collection and mailing should be obtained from the specific laboratory. Plasma taurine concentrations are influenced by the amount of taurine in the diet, the type of diet, and the time of sampling in relation to eating; however, a plasma taurine concentration of 20 to 30 nmol/ml or less in a cat with DCM is diagnostic for taurine deficiency. Non-anorexic cats with a plasma taurine concentration of <60 nmol/ml probably should receive taurine supplementation or a different diet. Whole blood samples produce more consistent results than plasma samples. Normal whole blood taurine concentrations exceed 200 nmol/ml; <140 nmol/ml is considered deficient.
Treatment and Prognosis
The goals of treatment are analogous to those for dogs with DCM. Pleural fluid is removed by thoracocentesis. In cats with acute CHF, furosemide is given to promote diuresis, as described for HCM. Overly aggressive diuresis is discouraged because it can markedly reduce cardiac output in these cases with poor systolic function. Supplemental O2 is recommended. The venodilator nitroglycerin may be helpful in cats with severe pulmonary edema. ACEI therapy is begun as soon as oral medication can be safely given. Other vasodilators (nitroprusside, hydralazine, or amlodipine) may help maximize cardiac output, although they increase the risk of hypotension (see Box 3-1). Blood pressure, hydration, renal function, electrolyte balance, and peripheral perfusion should be monitored closely. Hypothermia is common in cats with decompensated DCM; external warming is provided as needed.
Positive inotropic support is indicated. Dobutamine (or dopamine) is administered by CRI for critical cases (see p. 60 and Box 3-1). Possible adverse effects include seizures or tachycardia; if they occur, the infusion rate is decreased by 50% or discontinued. Oral inotropic therapy with pimobendan or digoxin (see p. 65 and Table 3-3) for maintenance therapy may be instituted. Digoxin tablets are usually used because the elixir is distasteful to many cats. Toxicity can easily occur, especially in cats receiving concurrent drug therapy; serum digoxin concentration should be monitored if this drug is used (see p. 66).
Sometimes the diuretic and vasodilator therapy used for acute CHF leads to hypotension and can predispose to cardiogenic shock in cats with DCM. Half-strength saline solution with 2.5% dextrose or other low-sodium fluids can be used intravenously with caution to help support blood pressure (e.g., 20 to 35 ml/kg/day in several divided doses or by CRI); potassium supplementation may be needed. Fluid can be administered subcutaneously if necessary, although its absorption from the extravascular space may be impaired in these cases.
Chronic therapy for DCM in cats that survive acute CHF includes oral furosemide (tapered to the lowest effective dosage), an ACEI, pimobendan or digoxin, antithrombotic prophylaxis (p. 199), and (if the patient is taurine deficient) supplemental taurine or a high-taurine diet. Taurine supplementation is instituted as soon as practical, at 250 to 500 mg orally q12h, when plasma taurine concentration is low or cannot be measured. Clinical improvement, if it occurs, is generally not apparent until after the first 1 to 2 weeks of taurine supplementation, so supportive cardiac care is vital.
Improved systolic function is seen echocardiographically within 6 weeks of starting taurine supplementation in most taurine-deficient cats. Drug therapy may become unnecessary in some cats after 6 to 12 weeks, but resolution of pleural effusion and pulmonary edema should be confirmed before weaning the cat from medications. If normal systolic fun ction, based on echocardiography, returns, the patient can be slowly weaned from supplemental taurine as long as a diet known to support adequate plasma taurine concentrations (e.g., most name-brand commercial foods) is consumed. Dry diets with 1000 to 1200 mg of taurine per kilogram of dry weight and canned diets with 2000 to 2500 mg of taurine per kilogram of dry weight are thought to maintain normal plasma taurine concentrations in adult cats. Reevaluation of the plasma taurine concentration 2 to 4 weeks after discontinuing the supplement is advised.
Taurine-deficient cats that survive a month after initial diagnosis often can be weaned from all or most medications and appear to have approximately a 50% chance for 1-year survival. The prognosis for cats that do not receive taurine supplements or do not respond to taurine is guarded to poor. Thromboembolism in cats with DCM is a grave sign.
OTHER MYOCARDIAL DISEASES
ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an idiopathic cardiomyopathy that is similar to the uncommon ARVC in people. Characteristic features include moderate to severe RV chamber dilation, with either focal or diffuse RV wall thinning. RV wall aneurysm is also common. Dilation of the right atrium and, less commonly, the left atrium may occur. Myocardial atrophy with fatty and/or fibrous replacement tissue, focal myocarditis, and evidence of apoptosis are typical histologic findings. These are most prominent in the RV wall. Fibrous tissue or fatty infiltration is sometimes found in the LV and atrial walls.
Signs of right-sided CHF are common, with labored respirations caused by pleural effusion, jugular venous distention, ascites or hepatosplenomegaly, and occasionally syncope. Lethargy and inappetence without overt heart failure are sometimes the presenting signs.
Thoracic radiographs indicate right heart and sometimes LA enlargement. Pleural effusion is common. Ascites, caudal vena caval distention, and evidence of pericardial effusion may also occur. The ECG can document various arrhythmias in affected cats, including ventricular premature complexes (VPCs), ventricular tachycardia, AF, and supraventricular tachyarrhythmias. A right bundle branch block pattern appears to be common; some cats have first-degree AV block. Echocardiography shows severe RA and RV enlargement. Other possible findings include abnormal muscular trabeculation, aneurysmal dilation, areas of dyskinesis, and paradoxical septal motion. Tricuspid regurgitation appears to be a consistent finding on Doppler exam.
The prognosis is guarded once signs of heart failure appear. Recommended therapy includes diuretics as necessary, an ACEI, and digoxin (or pimobendan). Additional antiarrhythmic therapy may be needed (see Chapter 4). In people with ARVC, various tachyarrhythmias are a prominent feature and sudden death is common.
CORTICOSTEROID-ASSOCIATED HEART FAILURE
Some cats develop CHF after receiving corticosteroid therapy. It is unclear whether this represents a previously unrecognized form of feline heart failure, unrelated to preexisting HCM, hypertension, or hyperthyroidism. An acute onset of lethargy, anorexia, tachypnea, and respiratory distress is described in affected cats. Most cats have normal auscultatory findings without tachycardia.
Moderate cardiomegaly, with diffuse pulmonary infiltrates and mild or moderate pleural effusion, appears to be typical on radiographic examination. Possible ECG findings include sinus bradycardia, intraventricular conduction abnormalities, atrial standstill, atrial fibrillation, and VPCs. On echocardiogram, most affected cats have some degree of LV wall or septal hypertrophy and LA enlargement. Some have AV valve insufficiency or abnormal systolic mitral motion.
CHF is treated in the same way as HCM; corticosteroids should be discontinued. Partial resolution of abnormal cardiac findings and successful weaning from cardiac medications are reported in some cats.
MYOCARDITIS
Inflammation of the myocardium and adjacent structures may occur in cats, as it does in other species (see also p. 137). In one study myocarditis was histologically identified in samples from more than half of cardiomyopathic cats but none from cats in the control group; viral DNA (panleukopenia) was found in about one third of the cats with myocarditis. However, the possible role of viral myocarditis in the pathogenesis of cardiomyopathy is not clear. Severe, widespread myocarditis may cause CHF or fatal arrhythmias. Cats with focal myocardial inflammation may be asymptomatic. Acute and chronic viral myocarditis have been suspected. A viral cause is rarely documented, although feline coronavirus has been identified as a cause of pericarditis-epicarditis.
Endomyocarditis has been documented mostly in young cats. Acute death, with or without preceding signs of pulmonary edema for 1 to 2 days, is the most common presentation. Histopathologic characteristics of acute endomyocarditis include focal or diffuse lymphocytic, plasmacytic, and histiocytic infiltrates with few neutrophils. Myocardial degeneration and lysis are seen adjacent to the infiltrates. Chronic endomyocarditis may have a minimal inflammatory response but much myocardial degeneration and fibrosis. RCM could represent the end stage of nonfatal endomyocarditis. Therapy involves managing CHF signs and arrhythmias, and other supportive care.
Bacterial myocarditis may develop in association with sepsis or as a result of bacterial endocarditis or pericarditis. Experimental Bartonella sp. infection can cause subclinical lymphoplasmacytic myocarditis, but it is unclear whether natural infection plays a role in the development of cardiomyopathy in cats. Toxoplasma gondii occasionally has been associated with myocarditis, usually in immunosuppressed cats as part of a generalized disease process. Traumatic myocarditis is recognized infrequently in cats.
Baty CJ, et al. Natural history of hypertrophic cardiomyopathy and aortic thromboembolism in a family of domestic shorthair cats. J Vet Intern Med. 2001;15:595.
Bright JM, Herrtage ME, Schneider JF. Pulsed Doppler assessment of left ventricular diastolic function in normal and cardiomyopathic cats. J Am Anim Hosp Assoc. 1999;35:285.
Ferasin L, et al. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994-2001). J Feline Med Surg. 2003;5:151.
Fox PR. Hypertrophic cardiopathy: clinical and pathologic correlates. J Vet Cardiol. 2003;5:39.
Fox PR. Endomyocardial fibrosis and restrictive cardiomyopathy: pathologic and clinical features. J Vet Cardiol. 2004;6:25-31.
Fox PR, et al. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: a new animal model similar to the human disease. Circulation. 2000;102:1863.
Gaschen L, et al. Cardiomyopathy in dystrophin-deficient hypertrophic feline muscular dystrophy. J Vet Intern Med. 1999;13:346.
Gavaghan BJ, et al. Quantification of left ventricular diastolic wall motion by Doppler tissue imaging in healthy cats and cats with cardiomyopathy. Am J Vet Res. 1999;60:1478.
Harvey AM, et al. Arrhythmogenic right ventricular cardiomyopathy in two cats. J Small Anim Pract. 2005;46:151.
Johnson LM, et al. Pharmacokinetic and pharmacodynamic properties of conventional and CD-formulated diltiazem in cats. J Vet Intern Med. 1996;10:316.
Kittleson MD, et al. Familial hypertrophic cardiomyopathy in a Maine Coon cat: an animal model of human disease. Circulation. 1999;99:3172.
Koffas H, et al. Pulsed tissue Doppler imaging in normal cats and cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2006;20:65.
Kraus MS, Calvert CA, Jacobs GJ. Hypertrophic cardiomyopathy in a litter of five mixed-breed cats. J Am Anim Hosp Assoc. 1999;35:293.
Liu SK, Keene BW, Fox PR. Myocarditis in the dog and cat. In: Bonagura JD, editor. Kirk’s current veterinary therapy XII. Philadelphia: WB Saunders; 1995:842-845.
MacDonald KA, et al. Tissue Doppler imaging and gradient echo cardiac magnetic resonance imaging in normal cats and cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2006;20:627.
MacLean HN, et al. N-terminal atrial natriuretic peptide immunoreactivity in plasma of cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2006;20:284.
Meurs KM, et al. Familial systolic anterior motion of the mitral valve and/or hypertrophic cardiomyopathy is apparently inherited as an autosomal dominant trait in a family of American shorthair cats. Abstr. J Vet Intern Med. 1997;11:138.
Meurs KM, et al. Molecular screening by polymerase chain reaction detects panleukopenia virus DNA in formalin-fixed hearts from cats with idiopathic cardiomyopathy and myocarditis. Cardiovasc Pathol. 2000;9:119.
Meurs KM, et al. Myomesin, a sarcomeric protein is reduced in Maine Coon cats with familial hypertrophic cardiomyopathy. J Vet Intern Med. 2001;15:281.
Meurs KM, et al. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet. 2005;14:3587.
Pion PD, Kittleson MD, Thomas WP. Response of cats with dilated cardiomyopathy to taurine supplementation. J Am Vet Med Assoc. 1992;201:275.
Rush JE, et al. The use of enalapril in the treatment of feline hypertrophic cardiomyopathy. J Am Anim Hosp Assoc. 1998;34:38.
Rush JE, et al. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc. 2002;220:202.
Sampedrano CC, et al. Systolic and diastolic myocardial dysfunction in cats with hypertrophic cardiomyopathy or systemic hypertension. J Vet Intern Med. 2006;20:1106.
Schober KE, Bonagura JD. Doppler echocardiographic assessment of E : Ea and E : Vp as indicators of left ventricular filling pressure in normal cats and cats with hypertrophic cardiomyopathy. Abstr. J Vet Intern Med. 2005;19:931.
Schober KE, Maerz I. Assessment of left atrial appendage flow velocity and its relation to spontaneous echocardiographic contrast in 89 cats with myocardial disease. J Vet Intern Med. 2006;20:120.
Smith SA, et al. Corticosteroid-associated congestive heart failure in 12 cats. Intern J Appl Res Vet Med. 2004;2:159.