CHAPTER 7 Myocardial Diseases of the Dog
Heart muscle disease that leads to contractile dysfunction and cardiac chamber enlargement is an important cause of heart failure in dogs. Idiopathic or primary dilated cardiomyopathy (DCM) is most common and mainly affects the larger breeds. Secondary and infective myocardial diseases (see pp. 135 and 137) occur less often. Arrhythmogenic right ventricular cardiomyopathy (ARVC), also known as Boxer cardiomyopathy, is an important myocardial disease in Boxers. ARVC is uncommon in other breeds. Hypertrophic cardiomyopathy (HCM) is recognized infrequently in dogs (see p. 137).
DILATED CARDIOMYOPATHY
Etiology and Pathophysiology
DCM is an idiopathic disease characterized by poor myocardial contractility, with or without arrhythmias. A genetic basis is thought to exist for idiopathic DCM, especially in breeds that have a high prevalence or a familial occurrence of the disease. Large and giant breeds are most commonly affected, including Doberman Pinschers, Great Danes, Saint Bernards, Scottish Deerhounds, Irish Wolfhounds, Boxers, Newfoundlands, Afghan Hounds, and Dalmatians. Some smaller breeds such as Cocker Spaniels and Bulldogs are also affected. The disease in rarely seen in dogs that weigh less than 12 kg. In at least some Great Danes, DCM appears to be an X-linked recessive trait. An autosomal dominant inheritance pattern was found in Boxers with ventricular arrhythmias (discussed in more detail later in this chapter); however, a rapidly fatal familial DCM affecting very young Portuguese Water Dogs shows an autosomal recessive inheritance pattern. Doberman Pinschers appear to have the highest prevalence of DCM; although a genetic basis is suspected, the inheritance pattern is not clear.
Various biochemical defects, nutritional deficiencies, toxins, immunologic mechanisms, and infectious agents may be involved in the pathogenesis of DCM in different cases. Impaired intracellular energy homeostasis and decreased myocardial adenosine triphosphate (ATP) concentrations were found in myocardial biochemical studies of affected Doberman Pinschers. DCM as an entity probably represents the end-stage of different pathologic processes or metabolic defects involving myocardial cells or the intercellular matrix rather than a single disease. Idiopathic DCM has also been associated with prior viral infections in people. However, on the basis of polymerase chain reaction (PCR) analysis of myocardial samples from a small number of DCM-affected dogs, viral agents do not seem to be commonly associated with DCM in this species.
Decreased ventricular contractility (systolic dysfunction) is the major functional defect in dogs with DCM. Progressive cardiac chamber dilation (remodeling) develops as systolic pump function and cardiac output worsen and compensa tory mechanisms become activated. Poor cardiac output can cause weakness, syncope, and ultimately, cardiogenic shock. Increased diastolic stiffness also contributes to the development of higher end-diastolic pressures, venous congestion, and congestive heart failure (CHF). Cardiac enlargement and papillary muscle dysfunction often cause poor systolic apposition of mitral and tricuspid leaflets with valve insufficiency. Although severe degenerative atrioventricular (AV) valve disease is not typical in dogs with DCM, some have mild to moderate valvular disease, which exacerbates valve insufficiency.
As cardiac output decreases, sympathetic, hormonal, and renal compensatory mechanisms become activated. These mechanisms increase heart rate, peripheral vascular resistance, and volume retention (see Chapter 3). Chronic neurohormonal activation is thought to contribute to progressive myocardial damage, as well as to CHF. Coronary perfusion can be compromised by poor forward blood flow and increased ventricular diastolic pressure; myocardial ischemia further impairs myocardial function and predisposes to arrhythmia development. Signs of low-output heart failure and right- or left-sided CHF (see Chapter 3) are common in dogs with DCM.
Atrial fibrillation (AF) often develops in dogs with DCM. Atrial contraction contributes importantly to ventricular filling, especially at faster heart rates. The loss of the “atrial kick” associated with AF reduces cardiac output and can cause acute clinical decompensation. Persistent tachycardia associated with AF probably also accelerates disease progression. Ventricular tachyarrhythmias also occur frequently and can cause sudden death. In Doberman Pinschers serial Holter recordings have documented the appearance of ventricular premature contractions (VPCs) months to more than a year before early echocardiographic abnormalities were noted. Once left ventricular (LV) function begins to deteriorate, the frequency of tachyarrhythmias increases. Excitement-induced bradyarrhythmias have also been associated with low-output signs in Doberman Pinschers.
Dilation of all cardiac chambers is typical in dogs with DCM, although left atrial (LA) and LV enlargement usually predominate. The ventricular wall thickness may appear decreased compared with the lumen size. Flattened, atrophic papillary muscles and endocardial thickening are described. Concurrent degenerative changes of the AV valves are generally only mild to moderate, if present at all. Histopathologic findings include scattered areas of myocardial necrosis, degeneration, and fibrosis, especially in the left ventricle. Narrowed (attenuated) myocardial cells with a wavy appearance may be a common finding. Inflammatory cell infiltrates, myocardial hypertrophy, and fatty infiltration (mainly in Boxers and some Doberman Pinschers) are inconsistent features.
Clinical Findings
The prevalence of DCM increases with age, although most dogs presented with CHF are 4 to 10 years old. Males appear to be affected more often than females. However, in Boxers and Doberman Pinschers there may be no gender predilection once dogs with occult disease are included. Cardiomyopathy in Boxers is described in more detail later (see p. 134). Male Doberman Pinschers generally show signs at an earlier age than females.
DCM appears to develop slowly, with a prolonged preclinical (occult) stage that may evolve over several years before clinical signs become evident. Occult DCM often is recognized through the use of echocardiography. Some giant-breed dogs with mild-to-moderate LV dysfunction are relatively asymptomatic, even in the presence of AF.
Clinical signs of DCM may appear to develop rapidly, especially in sedentary dogs in which early signs may not be noticed. Sudden death before CHF signs develop is relatively common. Presenting complaints include any or all of the following: weakness, lethargy, tachypnea or dyspnea, exercise intolerance, cough (sometimes described as “gagging”), anorexia, abdominal distention (ascites), and syncope (see Fig. 1-2). Loss of muscle mass (cardiac cachexia), accentuated along the dorsal midline, may be severe.
Physical examination findings vary with the degree of cardiac decompensation. Dogs with occult disease may have a normal physical exam. Others have a soft murmur of mitral or tricuspid regurgitation or an arrhythmia. Dogs with advanced disease and poor cardiac output have increased sympathetic tone and peripheral vasoconstriction. A consequence is mucous membrane pallor and slowed capillary refill time. The femoral arterial pulse and precordial impulse are often weak and rapid. Uncontrolled AF and frequent VPCs cause an irregular and usually rapid heart rhythm, with frequent pulse deficits and variable pulse strength (see Fig. 4-1). Signs of left- and/or right-sided CHF include tachypnea, increased breath sounds, pulmonary crackles, jugular venous distention or pulsations, pleural effusion or ascites, and/or hepatosplenomegaly. Heart sounds may be muffled in association with pleural effusion or poor cardiac contractility. An audible third heart sound (S3 gallop) is a classic finding, although it may be obscured by an irregular heart rhythm. Systolic murmurs of mitral or tricuspid regurgitation that are soft to moderate in intensity are common.
RADIOGRAPHY
The stage of disease, chest conformation, and hydration status influence the radiographic findings. Generalized cardiomegaly is usually evident, although left heart enlargement may predominate (Fig. 7-1). In Doberman Pinschers the heart may appear minimally enlarged, except for the left atrium. In other dogs cardiomegaly may be severe and can mimic the globoid cardiac silhouette typical of large pericardial effusions. Distended pulmonary veins and pulmonary interstitial or alveolar opacities, especially in the hilar and dorsocaudal regions, accompany left heart failure with pulmonary edema. The distribution of pulmonary edema infiltrates may be asymmetric or widespread. Pleural effusion, caudal vena cava distention, hepatomegaly, and ascites usually accompany right-sided CHF.
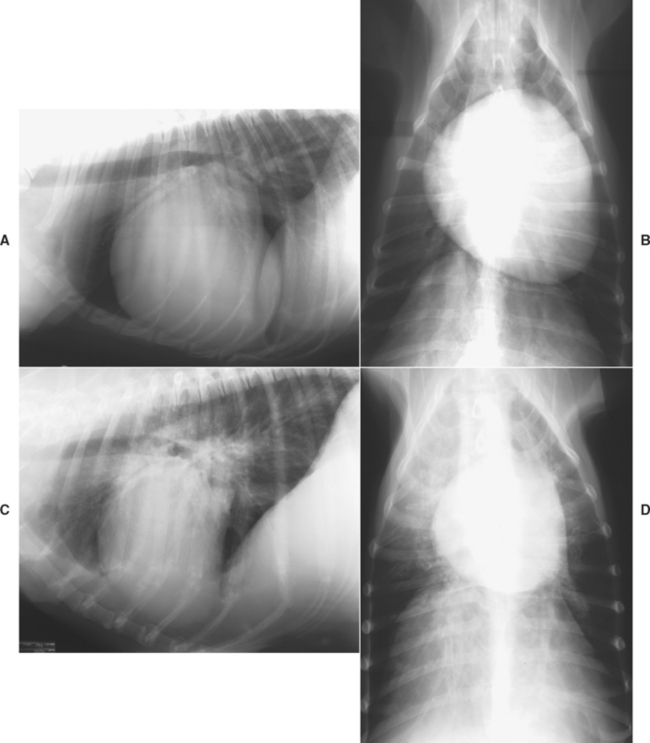
FIG 7-1 Radiographic examples of dilated cardiomyopathy in dogs. Lateral (A) and dorsoventral (B) views showing generalized cardiomegaly in a male Labrador Retriever. Note the cranial pulmonary vein is slightly larger than the accompanying artery in (A). Lateral (C) and dorsoventral (D) views of Doberman Pinscher depicting the prominent left atrial and relatively moderate ventricular enlargements commonly found in affected dogs of this breed. There is mild peribronchial pulmonary edema as well.
ELECTROCARDIOGRAPHY
The electrocardiogram (ECG) findings in dogs with DCM are also variable. Sinus rhythm is usually the underlying rhythm, although AF is often documented instead, especially in Great Danes and other giant breeds (see Fig. 2-11). Other atrial tachyarrhythmias, paroxysmal or sustained ventricular tachycardia, fusion complexes, and multiform VPCs are frequent findings. The QRS complexes may be tall (consistent with LV dilation), normal size, or small. Myocardial disease often causes a widened QRS complex with a slowed R-wave descent and slurred ST segment. A bundle-branch block pattern or other intraventricular conduction disturbance may be observed. The P waves in dogs with sinus rhythm are frequently widened and notched, suggesting LA enlargement.
Twenty-four–hour Holter monitoring is useful for documenting frequent ventricular ectopy. This has been used as a screening tool for cardiomyopathy in Doberman Pinschers and Boxers (see p. 135). The presence of >50 VPCs/day or any couplets or triplets is thought to predict future overt DCM in Doberman Pinschers. Nevertheless, some dogs with <50 VPCs/day on initial evaluation may develop DCM after several years. The frequency and complexity of ventricular tachyarrhythmias appear to be negatively correlated with fractional shortening; sustained ventricular tachycardia has been associated with increased risk of sudden death. Variability in the number of VPCs between repeated Holter recordings in the same dog can be high. If available, the technique of signal averaged electrocardiography can reveal the presence of ventricular late potentials, which may suggest an increased risk for sudden death in Doberman Pinschers with occult DCM.
ECHOCARDIOGRAPHY
Echocardiography is used to assess cardiac chamber dimensions and myocardial function and differentiate pericardial effusion or chronic valvular insufficiency from DCM. Dilated cardiac chambers and poor systolic ventricular wall and septal motion are characteristic findings in dogs with DCM. In severe cases only minimal wall motion is evident. All chambers are usually affected, but right atrial (RA) and right ventricular (RV) dimensions may appear normal, especially in Doberman Pinschers and Boxers. LV systolic (as well as diastolic) dimension is increased compared with normal ranges for the breed, and the ventricle appears more spherical. Fractional shortening and ejection fraction are decreased (Fig. 7-2). Other common features are a wide mitral valve E point–septal separation and reduced aortic root motion. LV free-wall and septal thicknesses are normal to decreased. The calculated end-systolic volume index (see p. 41) is generally over 80 ml/m2 in dogs with overt DCM (<30 ml/m2 is considered normal). Evidence for abnormal diastolic as well as systolic function can be found in dogs with advanced disease. Mild to moderate AV valve regurgitation is usually seen with Doppler echocardiography (Fig. 7-3).
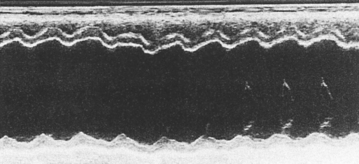
FIG 7-2 M-mode echocardiogram from a dog with dilated cardiomyopathy at the chordal (left side of figure) and mitral valve (right side of figure) levels. Note attenuated wall motion (fractional shortening = 18%) and the wide mitral valve E point–septal separation (28 mm).
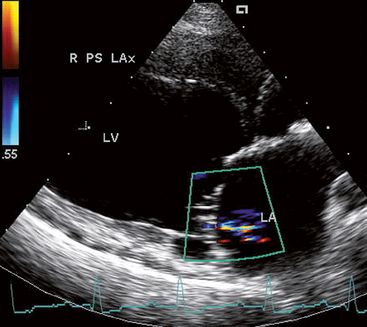
FIG 7-3 Mild mitral regurgitation is indicated by a relatively small area of disturbed flow in this systolic frame from a Standard Poodle with dilated cardiomyopathy. Note the LA and LV dilation. Right parasternal long axis view, optimized for the left ventricular inflow tract. LA, Left atrium; LV, left ventricle.
Echocardiography is also used to screen for occult disease. There may be no clear abnormalities early in the disease. Alternatively, apparently healthy Doberman Pinschers may have slightly reduced fractional shortening compared with what is considered normal for other breeds. The following echocardiographic criteria appear to indicate high risk for overt DCM within 2 to 3 years in asymptomatic Doberman Pinschers: LVIDd >46 mm (in dogs ≤42 kg) or >50 mm (in dogs >42 kg), LVIDs >38 mm, or VPCs during initial examination, FS < 25%, and/or mitral valve E point–septal separation >8 mm (LVID, left ventricular internal diameter; d, diastole; s, systole).
CLINICOPATHOLOGIC FINDINGS
Clinicopathologic findings are noncontributory in most cases. In others, prerenal azotemia resulting from poor renal perfusion or mildly increased liver enzyme activities resulting from passive hepatic congestion occur. Severe CHF may be associated with hypoproteinemia, hyponatremia, and hyperkalemia. Hypothyroidism with associated hypercholesterolemia occurs in some dogs with DCM. Others have a reduced serum thyroid hormone concentration without hypothyroidism (sick euthyroid); normal TSH and free T4 concentrations are common. Increased circulating neurohormones (e.g., norepiniphrine, aldosterone, endothelin, natriuretic peptides) occur mainly in DCM dogs with overt CHF. Natriuretic peptide elevations in dogs with occult DCM are also reported in some studies. Significant positive correlations have been identified between LV dimensions (in both diastole and systole) and atrial natriuretic peptide, as well as endothelin (O’Sullivan et al., 2007). Neurohormonal changes in occult DCM were not associated with time to CHF onset or sudden death in this study; however, in dogs with overt CHF, increases in NE and endothelin over a month were inversely associated with survival time. Serum cardiac troponin (cTnT or cTnI) concentrations are elevated in some dogs with DCM, as well as with other causes of myocyte injury.
OCCULT DILATED CARDIOMYOPATHY
Dogs with LV dilation or reduced FS are often treated with an angiotensin-converting enzyme inhibitor (ACEI), although it is unclear whether this prolongs the preclinical phase. Other therapy aimed at modulating early neurohormonal responses and ventricular remodeling processes have theoretical appeal, but their clinical usefulness is not clear. Further study of this using certain β-blockers (e.g., carvedilol, metoprolol), spironolactone, pimobendan, and other agents is ongoing.
The decision to use antiarrhythmic drug therapy in dogs with ventricular tachyarrhythmias is influenced by whether they result in clinical signs (e.g., episodic weakness, syncope) as well as the arrhythmia frequency and complexity seen on Holter recording. Various antiarrhythmic agents have been used, but the most effective regimen(s) and when to institute therapy are still not clear. It would seem that a regimen that increases ventricular fibrillation threshold and decreases arrhythmia frequency and severity is desired. Sotalol, amiodarone (both Class III agents), as well as the combination of mexiletine and atenolol or procainamide with atenolol, may be useful.
CLINICALLY EVIDENT DILATED CARDIOMYOPATHY
Therapy is aimed at improving the animal’s quality of life and prolonging survival to the extent possible by controlling CHF signs, optimizing cardiac output, and managing arrhythmias. Pimobendan (or digoxin), an ACEI, and furosemide are used for most dogs (Box 7-1). Severe heart failure may require additional therapy, including an intravenous (IV) inotropic agent. Antiarrhythmic drugs are used on the basis of individual need.
 BOX 7-1 Treatment Outline for Dogs with Dilated Cardiomyopathy
BOX 7-1 Treatment Outline for Dogs with Dilated Cardiomyopathy
ACEI, Angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AV, atrioventricular.
Mild to Moderate Signs of Congestive Heart Failure*
Severe, Acute Signs of Congestive Heart Failure*
AF and Inadequate Heart Rate Control with Digoxin**
Chronic Dilated Cardiomyopathy Management*
* See text and Chapter 3 for further details.
** See Chapter 4, p. 81.
Dogs with acute CHF are treated as outlined in Box 3-1, with parenteral furosemide, supplemental oxygen, 2% nitroglycerin ointment or sodium nitroprusside infusion, inotropic support, and cage rest, with or without aminophylline and morphine or butorphanol. Thoracocentesis is indicated if pleural effusion is suspected or identified.
Inotropic support can be in the form of oral pimobendan and/or digoxin if oral administration is not overly stressful and the delay in onset of effects is not critical. More acute and stronger inotropic support for dogs with very poor contractility, persistent hypotension, or fulminant CHF can be provided by IV infusion of dobutamine or dopamine for 2 (to 3) days. The phosphodiesterase inhibitors amrinone and milrinone may be helpful for short-term stabilization in some dogs and can be used concurrently with digoxin and a catecholamine. Long-term use of strong positive inotropic drugs is thought to have detrimental effects on the myocardium. During infusion of these drugs, the animal must be observed closely for worsening tachycardia or arrhythmias (especially VPCs).
If arrhythmias develop, the drug is discontinued or infused at up to half the original rate. In dogs with AF, catecholamine infusion is likely to increase the ventricular response rate because the drug enhances AV conduction. If dopamine or dobutamine is thought necessary in dogs with AF, rapid oral or cautious IV diltiazem can be used to slow AV conduction. Digoxin, either administered orally or by cautious IV loading doses, is an alternative.
Because clinical status may deteriorate rapidly, frequent patient evaluation is important. Respiratory rate and character, lung sounds, pulse quality, heart rate and rhythm, peripheral perfusion, rectal temperature, hydration status, body weight, renal function, mentation, pulse oxymetry, and blood pressure should be monitored. Ventricular contractility is abysmal in many dogs with severe DCM; because these patients have little cardiac reserve, diuretic and vasodilator therapy may lead to hypotension, and even cardiogenic shock.
Long-Term Therapy
Chronic inotropic therapy for dogs with DCM traditionally consisted of oral digoxin, but pimobendan now offers several advantages over digoxin (see p. 65). Pimobendan (Vetmedin, Boehringer Ingelheim) is a phosphodiesterase III inhibitor that increases contractility through a Ca++-sensitizing effect; the drug also has vasodilator and other beneficial effects. Digoxin, with its neurohormonal modulating and antiarrhythmic effects, may still be useful and can be given with pimobendan. Digoxin is indicated in dogs with AF to help slow the ventricular response rate. It can also suppress some other supraventricular tachyarrhythmias.
If digoxin is used, it is generally initiated with oral maintenance doses. Toxicity seems to develop at relatively low dosages in some dogs, especially Doberman Pinschers. A total maximum daily dose of 0.5 mg is generally used for large and giant-breed dogs, except for Doberman Pinschers, which are given a total maximum dose of 0.25 mg/day to 0.375 mg/day. Serum digoxin concentration should be measured 7 to 10 days after digoxin therapy is initiated or the dose is changed (see p. 66). Dogs with AF and a ventricular rate exceeding 200 beats/min can be cautiously given digoxin IV (see Box 3-1) or twice the oral maintenance dose on the first day to more rapidly achieve effective blood concentrations. However, the use of IV or rapid oral diltiazem is probably safer (see p. 81). If oral digoxin alone has not significantly reduced the heart rate after 36 to 48 hours, a β-blocker or diltiazem may be added (see Table 4-2). Because these agents can have negative inotropic effects, a low initial dose and gradual dosage titration to effect or a maximum recommended level is advised. Heart rate control in dogs with AF is important. A maximum ventricular rate of 140 to 150 beats/min in the hospital (i.e., stressful) setting is the recommended target; lower heart rates (e.g., ∼100 beats/min or less) are expected at home. Because accurate counting of heart rate by auscultation or chest palpation in dogs with AF is difficult, an ECG recording is recommended. Femoral pulses should not be used to assess heart rate in the presence of AF.
Furosemide is used at the lowest effective oral dose and at consistent time intervals for long-term therapy (see Table 3-3). Hypokalemia and alkalosis are uncommon sequelae, unless anorexia or vomiting occurs. Potassium supplements may be given if hypokalemia is documented. However, these should be used cautiously if an ACEI and/or spironolactone (see Table 3-3 and p. 62) are also being administered to prevent hyperkalemia, especially if renal disease is present.
Spironolactone is thought to be useful for chronic therapy because of its aldosterone-antagonist, as well as potential diuretic, effects. Increased aldosterone production develops as a component of neurohormonal activation in heart failure, but ACEIs do not fully suppress this. Aldosterone is known to promote cardiovascular fibrosis and abnormal remodeling and as such contributes to the progression of cardiac disease. Therefore spironolactone is advocated as adjunctive therapy in combination with an ACEI, furosemide, and pimobendan/digoxin for chronic DCM therapy.
An ACEI should be used in the chronic treatment of DCM. Angiotensin-converting enzyme inhibition can attenuate progressive ventricular dilation and secondary mitral regurgitation. ACEIs have a positive effect on survival in both people and dogs with myocardial failure. These drugs minimize clinical signs and increase exercise tolerance. Enalapril or benazepril are used most extensively, but other ACEIs have similar effects.
The pure arteriolar dilator hydralazine can also improve cardiac output and exercise tolerance, as well as help reduce congestion; however, it can precipitate hypotension and reflex tachycardia, and it tends to exacerbate neurohormonal activation. Hydralazine can be used in combination with a nitrate in dogs that do not tolerate an ACEI. Hydralazine or amlodipine (see Table 3-3) could also be useful as adjunct therapy for dogs with refractory CHF, although arterial blood pressure should be carefully monitored in such animals. Any vasodilator must be used cautiously in dogs with a low cardiac reserve because of the increased potential for hypotension. Therapy is initiated at a low dose; if this is well-tolerated, the next dose is increased to a low maintenance level. The patient should be evaluated for several hours after each incremental dose, ideally by blood pressure measurement. Signs of worsening tachycardia, weakened pulses, or lethargy also can indicate the presence of hypotension. The jugular venous PO2 can be used to estimate directional changes in cardiac output; a venous PO2 >30 mm Hg is desirable.
A number of other therapies may be useful in certain dogs with DCM, although additional studies are needed to define optimal recommendations. These include omega-3 fatty acids, l-carnitine (in dogs with low myocardial carnitine concentrations), taurine (in dogs with low plasma concentrations), long-term β-blocker therapy (e.g., carvedilol or metoprolol), and possibly others (see Chapter 3, p. 69). Several palliative surgical therapies for DCM have been described in dogs but are not widely used.
Monitoring
Many dogs can be maintained fairly well for a variable time with chronic oral therapy. Owner education regarding the purpose, dosage, and adverse effects of each drug used is also important. Monitoring the dog’s resting respiratory (and heart) rate at home helps assess how well the patient’s CHF is controlled. Periodic reevaluation is important, but the time frame depends on the animal’s status. Visits once or twice a week may be needed initially. Dogs with stable heart failure can be rechecked every 2 or 3 months. Serum electrolyte and creatinine (or BUN) concentrations, an ECG, pulmonary status, blood pressure, serum digoxin concentration, body weight, and other appropriate factors can be evaluated, and therapy adjusted as needed.
Prognosis
The prognosis for dogs with DCM is generally guarded to poor. Historically, most dogs do not survive longer than 3 months after the clinical manifestations of CHF, although approximately 25% to 40% of affected dogs live longer than 6 months if initial response to therapy is good. The probability of survival for 2 years is estimated at 7.5% to 28%. However, the advent of newer therapies may change this bleak picture. Pleural effusion and possibly ascites and pulmonary edema have been identified as independent indicators of poorer prognosis.
Sudden death may occur even in the occult stage, before heart failure is apparent. Sudden death occurs in about 20% to 40% of affected Doberman Pinschers. Although ventricular tachyarrhythmias are thought to precipitate cardiac arrest most commonly, bradyarrhythmias may be involved in some dogs.
Doberman Pinschers with occult DCM often experience deterioration within 6 to 12 months. Dobermans in overt CHF when initially presented generally do not live long, with a reported median survival of less than 7 weeks. The prognosis is worse if AF is present in dogs with CHF. Most symptomatic dogs are between 5 and 10 years old at the time of death.
In each case, however, it is reasonable to assess the animal’s response to initial treatment before pronouncing an unequivocally dismal prognosis. Early diagnosis may help prolong life; further cardiac evaluation is indicated for dogs with a history of reduced exercise tolerance, weakness, or syncope or in those in which an arrhythmia, murmur, or gallop sound is detected.
ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY
CARDIOMYOPATHY IN BOXERS
Myocardial disease in Boxers has similar features to those of people with ARVC. Histologic changes in the myocardium are more extensive than those in dogs of other breeds with cardiomyopathy and include atrophy of myofibers, fibrosis, and fatty infiltration. Focal areas of myocytolysis, necrosis, hemorrhage, and mononuclear cell infiltration are also common.
Although clinical features vary, the prevalence of ventricular arrhythmias and syncope is high in Boxers with myocardial disease. A genetic basis is believed to exist given that the disease is more prevalent in some bloodlines. Three disease categories have been described. The first consists of dogs with ventricular tachyarrhythmia but without clinical signs. The second consists of dogs that have syncope or weakness associated with paroxysmal or sustained ventricular tachycardia, despite normal heart size and LV function. The third group comprises Boxers with poor myocardial function and CHF, as well as ventricular tachyarrhythmias. Dogs with mild echocardiographic changes and those with syncope or weakness may later develop poor LV function and CHF. There appears to be geographical variation in the prevalence of these clinical presentations; for example, tachyarrhythmias with normal LV function are typical in affected U.S. Boxers, whereas LV dysfunction appears to be more common in parts of Europe.
Clinical Findings
Signs may appear at any age, but the mean age is reportedly 8.5 years (range <1-15 years). The most consistent clinical finding is a cardiac arrhythmia. When CHF occurs, left-sided signs are more common than ascites or other signs of right-sided heart failure. Many Boxers also develop a mitral insufficiency murmur.
The radiographic findings are variable; many Boxers have no visible abnormalities. Those with congestive signs generally show evidence of cardiomegaly and pulmonary edema. Echocardiographic findings also vary. Many Boxers have normal cardiac size and function; others show chamber dilation with reduced fractional shortening.
The characteristic ECG finding is ventricular ectopy. VPCs occur singly, in pairs, in short runs, or as sustained ventricular tachycardia. Most ectopic ventricular complexes appear upright in leads II and aVF. Some Boxers have multiform VPCs. There usually is an underlying sinus rhythm. AF is less common. Supraventricular tachycardia, conduction abnormalities, and evidence of chamber enlargement also are sometimes seen on ECG.
Twenty-four-hour Holter monitoring is often used as a screening tool for Boxer ARVC. It also is recommended to evaluate the efficacy of antiarrhythmic drug therapy. Frequent VPCs and/or complex ventricular arrhythmias are characteristic findings in affected dogs. However, an absolute number of VPCs/24-hour period that might separate normal from abnormal dogs is not (and may never be) clear. An arbitrary cut-off of >50 VPCs/24-hour period is often used to designate an abnormal frequency. However, there can be enormous variability in the number of VPCs between repeated Holter recordings in the same dog. Very frequent VPCs or episodes of ventricular tachycardia are thought to signal an increased risk for syncope and sudden death.
Treatment
Boxers with signs from tachyarrhythmias, but with normal heart size and LV function, are treated with antiarrhythmic drugs. Some asymptomatic dogs found to have ventricular tachycardia on Holter monitoring are also given an antiarrhythmic drug. The best regimen(s) and when to institute therapy are still not clear. Antiarrhythmic drug therapy that is apparently successful in reducing VPC number based on Holter recording may still not prevent sudden death. Sotalol, mexiletine with atenolol, amiodarone, or procainamide with atenolol have been advocated (see Chapter 4) because they might reduce the risk for sudden death from ventricular fibrillation, but further study is needed. Some dogs require treatment for persistent supraventricular tachyarrhythmias.
Therapy for CHF is similar to that described for dogs with idiopathic DCM. Myocardial carnitine deficiency has been documented in some Boxers with DCM and heart failure. Some of these dogs have responded to oral l-carnitine supplementation. Digoxin is used sparingly, if at all, when ventricular tachyarrhythmias are frequent.
Prognosis
The prognosis for affected Boxers is guarded. Survival is often <6 months in those with CHF. Asymptomatic dogs may have a more optimistic future, but the likelihood of developing serious arrhythmias is high. Sudden death is common, presumably from VPCs leading to ventricular fibrillation. The ventricular tachyarrhythmias may be refractory to drug therapy. Furthermore, even if most arrhythmias are suppressed, an increased survival is not assured.
ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY IN NONBOXER DOGS
A form of cardiomyopathy that mainly affects the right ventricle has been observed rarely in dogs. It appears similar to ARVC described in people and cats (see p. 154). Pathologic changes are characterized by widespread fibrous and fatty tissue replacement in the RV myocardium. In certain geographical areas, trypanosomiasis is a possible differential diagnosis. Clinical manifestations are largely related to right-sided CHF and severe ventricular tachyarrhythmias. Marked right heart dilation is typical. Sudden death is a common outcome in people with ARVC.
SECONDARY MYOCARDIAL DISEASE
Poor myocardial function may result from a variety of identifiable insults and nutritional deficiencies. Myocardial infections (see p. 137), inflammation, trauma (see p. 139), ischemia, neoplastic infiltrations, and metabolic abnormalities can impair normal contractile function. Hyperthermia, irradiation, electric shock, and other insults can also damage the myocardium. Some substances are known cardiac toxins.
MYOCARDIAL TOXINS
Doxorubicin
The antineoplastic drug doxorubicin induces both acute and chronic cardiotoxicity. Histamine, secondary catecholamine release, and free-radical production appear to be involved in the pathogenesis of myocardial damage, which leads to decreased cardiac output, arrhythmias, and degeneration of myocytes. Doxorubicin-induced cardiotoxicity is directly related to the peak serum concentration of the drug; administering the drug diluted (0.5 mg/ml) over 20 to 40 minutes minimizes the risk of developing cardiotoxicity. Progressive myocardial damage and fibrosis have developed in association with cumulative doses of >160 mg/m2 and sometimes as low as 100 mg/m2. In dogs that have normal pretreatment cardiac function, clinical cardiotoxicity is uncommon until the cumulative dose exceeds 240 mg/m2. It is difficult to predict whether and when clinical cardiotoxicity will occur. Increases in circulating cardiac troponin concentrations can be seen, but more work is needed to clarify the utility of this in monitoring dogs for doxorubicin-induced myocardial injury.
Cardiac conduction defects (infranodal AV block and bundle branch block) as well as ventricular and supraventricular tachyarrhythmias can develop in affected dogs. ECG changes do not necessarily precede clinical heart failure. Dogs with underlying cardiac abnormalities and those of breeds with a higher prevalence of idiopathic DCM are thought to be at greater risk for doxorubicin-induced cardiotoxicity. Recently, carvedilol has been shown to minimize or prevent the development of doxorubicin-induced cardiotoxicity in humans; we have had similar anecdotal experiences in dogs. Clinical features of this cardiomyopathy are similar to those of idiopathic DCM.
Other Toxins
Ethyl alcohol, especially if given intravenously for the treatment of ethylene glycol intoxication, can cause severe myocardial depression and death; slow administration of a diluted (20% or less) solution is advised. Other cardiac toxins include plant toxins (e.g., Taxus, foxglove, black locust, buttercups, lily-of-the-valley, gossypol), cocaine, anesthetic drugs, cobalt, catecholamines, and ionophores such as monensin.
METABOLIC AND NUTRITIONAL DEFICIENCY
l-carnitine
l-carnitine is an essential component of the mitochondrial membrane transport system for fatty acids, which are the heart’s most important energy source. It also transports potentially toxic metabolites out of the mitochondria in the form of carnitine esters. l-carnitine–linked defects in myocardial metabolism have been found in some dogs with DCM. Rather than simple l-carnitine deficiency, one or more underlying genetic or acquired metabolic defects are suspected. There may be an association between DCM and carnitine deficiency in some families of Boxers, Doberman Pinschers, Great Danes, Irish Wolfhounds, Newfoundlands, and Cocker Spaniels. l-carnitine is mainly present in foods of animal origin. DCM has developed in some dogs fed strict vegetarian diets.
Plasma carnitine concentration is not a sensitive indicator of myocardial carnitine deficiency. Most dogs with myocardial carnitine deficiency, diagnosed via endomyocardial biopsy, have had normal or high plasma carnitine concentrations. Furthermore, the response to oral carnitine supplementation is inconsistent. Subjective improvement may occur, but few dogs have echocardiographic evidence of improved function. Dogs that do respond show clinical improvement within the first month of supplementation; there may be some degree of improvement in echo parameters after 2 to 3 months. l-carnitine supplementation does not suppress preexisting arrhythmias or prevent sudden death. See p. 69 for supplementation guidelines.
Taurine
Although most dogs with DCM are not taurine deficient, low plasma taurine concentration is found in some. Low taurine, and sometimes carnitine, concentrations occur in Cocker Spaniels with DCM. Oral supplementation of these amino acids can improve LV size and function as well as reduce the need for heart failure medications in this breed. Low plasma taurine concentrations have also been found in some Golden Retrievers, Labrador Retrievers, Saint Bernards, Dalmatians, and other dogs with DCM. A normally adequate taurine content is found in the diets of some such cases, although others have been fed low-protein or vegetarian diets. The role of taurine supplementation is unclear. Although taurine-deficient dogs may show some echocardiographic improvement after supplementation, there is questionable effect on survival time. Nevertheless, measurement of plasma taurine or a trial of supplemental taurine for at least 4 months may be useful, especially in an atypical breed affected with DCM. (See p. 69 for supplementation guidelines.) Plasma taurine concentrations <25 (to 40) nmol/ml and blood taurine concentrations <200 (or 150) nmol/ml are generally considered deficient. Specific collection and submission guidelines should be obtained from the laboratory used.
Other Factors
Myocardial injury induced by free radicals may play a role in a number of diseases. Evidence for increased oxidative stress has been found in dogs with CHF and myocardial failure, but the clinical ramifications of this are unclear. Diseases such as hypothyroidism, pheochromocytoma, and diabetes mellitus have been associated with reduced myocardial function, but clinical heart failure is unusual in dogs secondary to these conditions alone. Excessive sympathetic stimulation stemming from brain or spinal cord injury results in myocardial hemorrhage, necrosis, and arrhythmias (brain-heart syndrome). Muscular dystrophy of the fasciohumoral type (reported in English Springer Spaniels) may result in atrial standstill and heart failure. Canine X-linked (Duchenne’s) muscular dystrophy in Golden Retrievers and other breeds also has been associated with myocardial fibrosis and mineralization. Rarely, nonneoplastic (e.g., glycogen storage disease) and neoplastic (metastatic and primary) infiltrates interfere with normal myocardial function. Immunologic mechanisms may also play an important role in the pathogenesis of myocardial dysfunction in some dogs with myocarditis.
ISCHEMIC MYOCARDIAL DISEASE
Acute myocardial infarction resulting from coronary embolization is uncommon. An underlying disease associated with increased risk for thromboembolism, such as bacterial endocarditis, neoplasia, severe renal disease, immune-mediated hemolytic anemia, acute pancreatitis, disseminated intravascular coagulopathy, and/or corticosteroid use, underlies most cases. Sporadic reports of myocardial infarction are associated with congenital ventricular outflow obstruction, patent ductus arteriosus, hypertrophic cardiomyopathy, and mitral insufficiency. Atherosclerosis of the major coronary arteries, which can accompany severe hypothyroidism in dogs, rarely leads to acute myocardial infarction. Clinical signs of acute major coronary artery obstruction are likely to include arrhythmias, pulmonary edema, marked ST segment change on ECG, and evidence of regional or global myocardial contractile dysfunction on echocardiogram. High circulating cardiac troponin concentrations and possibly creatine kinase activity occur after myocardial injury and necrosis.
Disease of small coronary vessels is recognized as well. Non-atherosclerotic narrowing of small coronary arteries could be more clinically important than previously assumed. Hyalinization of small coronary vessels and intramural myocardial infarctions have been described in dogs with chronic degenerative AV valve disease, but they can occur in older dogs without valve disease as well. Fibromuscular arteriosclerosis of small coronary vessels is also described. These changes in the walls of the small coronary arteries cause luminal narrowing and can impair resting coronary blood flow as well as vasodilatory responses. Small myocardial infarctions and secondary fibrosis lead to reduced myocardial function. Various arrhythmias can occur. Eventual CHF is a cause of death in many cases with intramural coronary arteriosclerosis. Sudden death is a less common sequela. Larger breeds of dog may be predisposed, although Cocker Spaniels and Cavalier King Charles Spaniels appear to be commonly affected smaller breeds.
TACHYCARDIA-INDUCED CARDIOMYOPATHY
The term tachycardia induced cardiomyopathy (TICM) refers to the progressive myocardial dysfunction, activation of neurohormonal compensatory mechanisms, and CHF that result from rapid, incessant tachycardias. The myocardial failure may be reversible if the heart rate can be normalized in time. TICM has been described in several dogs with AV nodal reciprocating tachycardias associated with accessory conduction pathways that bypass the AV node (e.g., WolffParkinson-White; see p. 27). Rapid artificial pacing (e.g., >200 beats/min) is a common model for inducing experimental myocardial failure that simulates DCM.
HYPERTROPHIC CARDIOMYOPATHY
In contrast to cats, hypertrophic cardiomyopathy (HCM) is quite uncommon in dogs. A genetic basis is suspected, although the cause is unknown. It is possible that several disease processes lead to similar ventricular changes. The pathophysiology is similar to that of HCM in cats (see Chapter 8). Abnormal, excessive myocardial hypertrophy increases ventricular stiffness and leads to diastolic dysfunction. The LV hypertrophy is usually symmetric, but regional variation in wall or septal thickness can occur. Compromised coronary perfusion is likely with severe ventricular hypertrophy. This leads to myocardial ischemia, which exacerbates arrhythmias, delays ventricular relaxation, and further impairs filling. High LV filling pressure predisposes to pulmonary venous congestion and edema. Besides diastolic dysfunction, systolic dynamic LV outflow obstruction occurs in some dogs. Malposition of the mitral apparatus may contribute to systolic anterior mitral valve motion and LV outflow obstruction as well as to mitral regurgitation. In some dogs asymmetric septal hypertrophy also contributes to outflow obstruction. LV outflow obstruction increases ventricular wall stress and myocardial oxygen requirement while also impairing coronary blood flow. Heart rate elevations magnify these abnormalities.
Clinical Features
HCM is most commonly diagnosed in young to middle-age large-breed dogs, although there is a wide age distribution. Various breeds are affected. There may be a higher prevalence of HCM in males. Clinical signs of CHF, episodic weakness, and/or syncope occur in some dogs. Sudden death is the only sign in some cases. Ventricular arrhythmias secondary to myocardial ischemia are presumed to cause the low-output signs and sudden death. A systolic murmur, related to either LV outflow obstruction or mitral insufficiency, may be heard on auscultation. The systolic ejection murmur of ventricular outflow obstruction becomes louder when ventricular contractility is increased (e.g., with exercise or excitement) or when afterload is reduced (e.g., from vasodilator use). An S4 gallop sound is heard in some affected dogs.
Diagnosis
Echocardiography is the best diagnostic tool for HCM. An abnormally thick left ventricle, with or without narrowing of the LV outflow tract area or asymmetrical septal hypertrophy, and LA enlargement are characteristic findings. Mitral regurgitation may be evident on Doppler studies. Systolic anterior motion of the mitral valve may result from dynamic outflow obstruction causes. Partial systolic aortic valve closure may be seen as well. Other causes of LV hypertrophy include congenital subaortic stenosis, hypertensive renal disease, thyrotoxicosis, and pheochromocytoma. Thoracic radiographs may indicate LA and LV enlargement, with or without pulmonary congestion or edema. Some cases appear radiographically normal. ECG findings may include ventricular tachyarrhythmias and conduction abnormalities, such as complete heart block, first-degree AV block, and fascicular blocks. Criteria for LV enlargement are variably present.
Treatment
The general goals of HCM treatment are to enhance myocardial relaxation and ventricular filling, control pulmonary edema, and suppress arrhythmias. A β-blocker (see p. 89) or Ca++-channel blocker (see p. 91) may lower heart rate, prolong ventricular filling time, reduce ventricular contractility, and minimize myocardial oxygen requirement. β-blockers can also reduce dynamic LV outflow obstruction and may suppress arrhythmias induced by heightened sympathetic activity, whereas Ca++-blockers may facilitate myocardial relaxation. Diltiazem has a lesser inotropic effect and would be less useful against dynamic outflow obstruction, especially in view of its vasodilating effect. Because β- and Ca++-channel blockers can worsen AV conduction abnormalities, they may be relatively contraindicated in certain animals. A diuretic and ACEI are indicated if congestive signs are present. Digoxin should not be used because it may increase myocardial oxygen requirements, worsen outflow obstruction, and predispose to the development of ventricular arrhythmias. Exercise restriction is advised in dogs with HCM.
MYOCARDITIS
A wide variety of agents can affect the myocardium, although disease manifestations in other organ systems may overshadow the cardiac involvement. The heart can be injured by direct invasion of the infective agent, by toxins it elaborates, or by the host’s immune response. Non-infective causes of myocarditis include cardiotoxic drugs and drug hypersensitivity reactions. Myocarditis can cause persistent cardiac arrhythmias and progressively impair myocardial function.
INFECTIVE MYOCARDITIS
Viral Myocarditis
Lymphocytic myocarditis has been associated with acute viral infections in experimental animals and in people. Cardiotropic viruses can play an important role in the pathogenesis of myocarditis and subsequent cardiomyopathy in several species, but this is not recognized commonly in dogs. The host animal’s immune responses to viral and nonviral antigens contribute to myocardial inflammation and damage.
A syndrome of parvoviral myocarditis was well-known in the late 1970s and early 1980s. It is characterized by a peracute necrotizing myocarditis and sudden death (with or without signs of acute respiratory distress) in apparently healthy puppies about 4 to 8 weeks old. Cardiac dilation with pale streaks in the myocardium, gross evidence of congestive failure, large basophilic or amorphophilic intranuclear inclusion bodies, myocyte degeneration, and focal mononuclear cell infiltrates are typical necropsy findings. This syndrome is uncommon now, probably as a result of maternal antibody production in response to virus exposure and vaccination. Parvovirus may cause a form of DCM in young dogs that survive neonatal infection; viral genetic material has been identified in some canine ventricular myocardial samples in the absence of classic intranuclear inclusion bodies.
Canine distemper virus may cause myocarditis in young puppies, but multisystemic signs usually predominate. Histologic changes in the myocardium are mild compared with those in the classic form of parvovirus myocarditis. Experimental herpesvirus infection of pups during gestation also causes necrotizing myocarditis with intranuclear inclusion bodies leading to fetal or perinatal death.
Bacterial Myocarditis
Bacteremia and bacterial endocarditis or pericarditis can cause focal or multifocal suppurative myocardial inflammation or abscess formation. Localized infections elsewhere in the body may be the source of the organisms. Clinical signs include malaise; weight loss; and, inconsistently, fever. Arrhythmias and cardiac conduction abnormalities are common, but murmurs are rare unless concurrent valvular endocarditis or another underlying cardiac defect is present. Serial bacterial (or fungal) blood cultures, serology, or PCR may allow identification of the organism. Bartonella vinsonii subspecies have been associated with cardiac arrhythmias, myocarditis, endocarditis, and sudden death.
Lyme Carditis
Lyme disease is more prevalent in certain geographic areas, especially the northeastern, western coastal, and north central United States, as well as in Japan and Europe, among other areas. The spirochete Borrelia burgdorferi (or related species) is transmitted to dogs by ticks (especially Ixodes genus) and possibly other biting insects. Third-degree (complete) and high-grade second-degree AV block have been identified in dogs with Lyme disease. Syncope, CHF, reduced myocardial contractility, and ventricular arrhythmias also are reported in affected dogs. Pathologic findings of Lyme myocarditis include infiltrates of plasma cells, macrophages, neutrophils, and lymphocytes, with areas of myocardial necrosis. These are similar to findings in human Lyme carditis. A presumptive diagnosis is made on the basis of the finding of positive (or increasing) serum titers or a positive SNAP test and concurrent signs of myocarditis, with or without other systemic signs. The findings from endomyocardial biopsy, if available, may be helpful in confirming the diagnosis. Treatment with an appropriate antibiotic should be instituted pending diagnostic test results. Cardiac drugs are used as needed. Resolution of AV conduction block may not occur in dogs despite appropriate antimicrobial therapy.
Protozoal Myocarditis
Trypanosoma cruzi, Toxoplasma gondii, Neosporum caninum, Babesia canis, and Hepatozoon canis are known to affect the myocardium. Trypanosomiasis (Chagas’ disease) has occurred mainly in young dogs in Texas, Louisiana, Oklahoma, Virginia, and other southern states in the United States. The possibility for human infection should be recognized; this is an important cause of human myocarditis and subsequent cardiomyopathy in Central and South America. The organism is transmitted by bloodsucking insects of the family Reduviidae and is enzootic in wild animals of the region. Amastigotes of T. cruzi cause myocarditis with a mononuclear cell infiltrate and disruption and necrosis of myocardial fibers. Acute, latent, and chronic phases of Chagas’ myocarditis have been described. Lethargy, depression, and other systemic signs, as well as various tachyarrhythmias, AV conduction defects, and sudden death, are seen in dogs with acute trypanosomiasis. Clinical signs are sometimes subtle. The disease is diagnosed in the acute stage by finding trypomastigotes in thick peripheral blood smears; the organism can be isolated in cell culture or by inoculation into mice. Animals that survive the acute phase enter a latent phase of variable duration. During this phase the parasitemia is resolved, and antibodies develop against the organism as well as cardiac antigens. Chronic Chagas’ disease is characterized by progressive right-sided or generalized cardiomegaly and various arrhythmias. Ventricular tachyarrhythmias are most common, but supraventricular tachyarrhythmias may occur. Right bundle branch block and AV conduction disturbances are also reported. Ventricular dilation and reduced myocardial function are usually evident echocardiographically. Clinical signs of biventricular failure are common. Antemortem diagnosis in chronic cases may be possible through serologic testing. Therapy in the acute stage is aimed at eliminating the organism and minimizing myocardial inflammation; several treatments have been tried with variable success. The therapy for chronic Chagas’ disease is aimed at supporting myocardial function, controlling congestive signs, and suppressing arrhythmias.
Toxoplasmosis and neosporiosis can cause clinical myocarditis in conjunction with generalized systemic infection, especially in the immunocompromised animal. The organism becomes encysted in the heart and various other body tissues after the initial infection. With rupture of these cysts, expelled bradyzoites induce hypersensitivity reactions and tissue necrosis. Other systemic signs often overshadow signs of myocarditis. Immunosuppressed dogs with chronic toxoplasmosis (or neosporiosis) may be prone to active disease, including clinically relevant myocarditis, pneumonia, chorioretinitis, and encephalitis. Antiprotozoal therapy may be successful.
Babesiosis can be associated with cardiac lesions in dogs, including myocardial hemorrhage, inflammation, and necrosis. Pericardial effusion and variable ECG changes are also noted in some cases. A correlation between plasma cardiac troponin I (cTnI) concentration and clinical severity, survival, and cardiac histopathologic findings was shown in dogs with babesiosis.
H. canis may involve the myocardium during part of its life cycle; this was found in dogs along the Texas coast. Infection occurs as a result of ingesting the organism’s definitive host, the brown dog tick (Rhipicephalus sanguineus). Clinical signs include stiffness, anorexia, fever, neutrophilia, and periosteal new bone reaction.
Other Causes
Rarely, fungi (Aspergillus, Cryptococcus, Coccidioides, Histoplasma, Paecilomyces), rickettsiae (Rickettsia rickettsii, Ehrlichia canis, Bartonella elizabethae), algaelike organisms (Prototheca sp.), and nematode larval migration (Toxocara sp.) cause myocarditis. Affected animals are usually immunosuppressed and have systemic signs of disease. Rocky Mountain spotted fever (R. rickettsii) occasionally causes fatal ventricular arrhythmias, along with necrotizing vasculitis, myocardial thrombosis, and ischemia. Angiostrongylus vasorum infection in association with immune-mediated thrombocytopenia has rarely caused myocarditis, thrombosing arteritis, and sudden death.
Clinical Findings and Diagnosis
Unexplained onset of arrhythmias or heart failure after a recent episode of infective disease or drug exposure is the classic clinical presentation of acute myocarditis. However, definitive diagnosis is difficult because clinical and clinicopathologic findings are usually nonspecific and inconsistent. A database including complete blood count, serum biochemical profile with creatine kinase activity, cardiac troponin concentration, thoracic and abdominal radiographs, and urinalysis are usually obtained. ECG changes could include an ST segment shift, T-wave or QRS voltage changes, AV conduction abnormalities, and various arrhythmias. Echocardiographic signs of poor regional or global wall motion, altered myocardial echogenicity, or pericardial effusion may be evident. In dogs with persistent fever, serial bacterial (or fungal) blood cultures may be useful. Serologic screening for known infective causes may or may not be helpful. Histologic criteria for a diagnosis of myocarditis include inflammatory infiltrates with myocyte degeneration and necrosis. Endomyocardial biopsy specimens are currently the only means of obtaining a definitive antemortem diagnosis, but if the lesions are focal, the findings may not be diagnostic.
Treatment
Unless a specific etiology can be identified and treated, therapy for suspected myocarditis is largely supportive. Strict rest, antiarrhythmic drugs (see Chapter 4), therapy to support myocardial function and manage CHF signs (see Chapter 3), and other support are used as needed. Corticosteroids are not proven to be clinically beneficial in dogs with myocarditis, and considering the possible infective cause, they are not recommended as nonspecific therapy. Exceptions would be confirmed immune-mediated disease, drug-related or eosinophilic myocarditis, or confirmed nonresolving myocarditis.
NON-INFECTIVE MYOCARDITIS
Myocardial inflammation can result from the effects of drugs, toxins, or immunologic responses. Although there is little clinical documentation for many of these in dogs, a large number of potential causes have been identified in people. Besides the well-known toxic effects of doxorubicin and catecholamines, other potential causes of non-infective myocarditis include heavy metals (e.g., arsenic, lead, mercury), antineoplastic drugs (cyclophosphamide, 5-fluorouracil, interleukin-2, alpha-interferon), other drugs (e.g., thyroid hormone, cocaine, amphetamines, lithium), and toxins (wasp or scorpion stings, snake venom, spider bites). Immune-mediated diseases and pheochromocytoma can cause myocarditis as well. Hypersensitivity reactions to many antiinfective agents and other drugs have also been identified as causes of myocarditis in people. Drug-related myocarditis is usually characterized by eosinophilic as well as lymphocytic infiltrates.
TRAUMATIC MYOCARDITIS
Nonpenetrating or blunt trauma to the chest and heart is more common than penetrating wounds. Cardiac arrhythmias are frequently observed after such trauma, especially in dogs. Cardiac damage can result from impact against the chest wall, compression, or acceleration-deceleration forces. Other possible mechanisms of myocardial injury and arrhythmogenesis include an autonomic imbalance, ischemia, reperfusion injury, and electrolyte and acid-base disturbances. Thoracic radiographs, serum biochemistries, circulating cardiac troponin concentrations, ECG, and echocardiography are recommended in the assessment of these cases. Echocardiography can define preexisting heart disease, global myocardial function, and unexpected cardiovascular findings, but it may not identify small areas of myocardial injury.
Arrhythmias usually appear within 24 to 48 hours after trauma, although they can be missed on intermittent ECG recordings. VPCs, ventricular tachycardia, and accelerated idioventricular rhythms (with rates of 60 to 100 beats/min or slightly faster) are more common than supraventricular tachyarrhythmias or bradyarrhythmias in these patients. Accelerated idioventricular rhythms usually are manifested only when the sinus rate slows or pauses; they are benign in most dogs with normal underlying heart function and disappear with time (generally within a week or so). Antiarrhythmic therapy for accelerated idioventricular rhythm in this setting is usually unnecessary. The patient as well as the ECG should be monitored closely. More serious arrhythmias (e.g., faster rate) or hemodynamic deterioration may require antiarrhythmic therapy (see Chapter 4).
Traumatic avulsion of AV valve papillary muscles, septal perforation, and rupture of the heart or pericardium have also been reported. Traumatic papillary muscle avulsion causes acute volume overload with acute onset of CHF. Signs of low-output failure and shock, as well as arrhythmias, can develop rapidly after cardiac trauma.
Noninfective Myocardial Disease
Backus RC, et al. Taurine deficiency in Newfoundlands fed commercially available complete and balanced diets. J Am Vet Med Assoc. 2003;223:1130.
Baumwart RD, et al. Clinical, echocardiographic, and electrocardiographic abnormalities in Boxers with cardiomyopathy and left ventricular systolic dysfunction: 48 cases (1985-2003). J Am Vet Med Assoc. 2005;226:1102.
Baumwart RD, Orvalho J, Meurs KM. Evaluation of serum cardiac troponin I concentration in boxers with arrhythmogenic right ventricular cardiomyopathy. Am J Vet Res. 2007;68:524.
Borgarelli M, et al. Prognostic indicators for dogs with dilated cardiomyopathy. J Vet Intern Med. 2006;20:104.
Calvert CA, et al. Results of ambulatory electrocardiography in overtly healthy Doberman Pinschers with echocardiographic abnormalities. J Am Vet Med Assoc. 2000;217:1328.
Calvert CA, Jacobs GJ, Pickus CW. Bradycardia-associated episodic weakness, syncope, and aborted sudden death in cardiomyopathic Doberman Pinschers. J Vet Intern Med. 1996;10:88.
Calvert CA, et al. Clinical and pathological findings in Doberman Pinschers with occult cardiomyopathy that died suddenly or developed congestive heart failure: 54 cases (1984–1991). J Am Vet Med Assoc. 1997;210:505.
Calvert CA, et al. Signalment, survival, and prognostic factors in Doberman Pinschers with end-stage cardiomyopathy. J Vet Intern Med. 1997;11:323.
Carroll MC, Cote E. Carnitine: a review. Compend Cont Educ. 2001;23:45.
Dambach DM, et al. Familial dilated cardiomyopathy of young Portuguese water dogs. J Vet Intern Med. 1999;13:65.
De Andrade JN, et al. Reduction of diameter of the left ventricle of dogs by plication of the left ventricular free wall. Am J Vet Res. 2001;62:297.
Dukes-McEwan J, et al. Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. J Vet Cardiol. 2003;5:7.
Driehuys S, Van Winkle TJ, Sammarco CD, et al. Myocardial infarction in dogs and cats: 37 cases (1985–1994). J Am Vet Med Assoc. 1998;213:1444.
Falk T, Jonsson L. Ischaemic heart disease in the dog: a review of 65 cases. J Small Anim Pract. 2000;41:97.
Fascetti AJ, et al. Taurine deficiency in dogs with dilated cardiomyopathy:12 cases (1997-2001). J Am Vet Med Assoc. 2003;223:1137.
Freeman LM, Brown DJ, Rush JE. Assessment of degree of oxidative stress and antioxidant concentration in dogs with idiopathic dilated cardiomyopathy. J Am Vet Med Assoc. 1999;215:644.
Freeman LM, et al. Relationship between circulating and dietary taurine concentration in dogs with dilated cardiomyopathy. Vet Therapeutics. 2001;2:370.
Kittleson MD, et al. Results of the multicenter spaniel trial (MUST): taurine- and carnitine-responsive dilated cardiomyopathy in American Cocker Spaniels with decreased plasma taurine concentration. J Vet Intern Med. 1997;11:204.
Mauldin GE, Fox PR, Patnaik AK. Doxorubicin-induced cardiotoxicosis: clinical features in 23 dogs. J Vet Intern Med. 1992;6:82.
Maxson TR, et al. Polymerase chain reaction analysis for viruses in paraffin-embedded myocardium from dogs with dilated cardiomyopathy or myocarditis. Am J Vet Res. 2001;62:130.
Meurs KM, et al. Familial ventricular arrhythmias in Boxers. J Vet Intern Med. 1999;13:437.
Meurs KM, et al. Comparison of the effects of four antiarrhythmic treatments for familial ventricular arrhythmias in Boxers. J Am Vet Med Assoc. 2002;221:22.
Meurs KM, Miller MW, Wright NA. Clinical features of dilated cardiomyopathy in Great Danes and results of a pedigree analysis: 17 cases (1990-2000). J Am Vet Med Assoc. 2001;218:729.
McEntee K, et al. Usefulness of dobutamine stress tests for detection of cardiac abnormalities in dogs with experimentally induced early left ventricular dysfunction. Am J Vet Res. 2001;62:448.
Minors SL, O’Grady MR. Resting and dobutamine stress echocardiographic factors associated with the development of occult dilated cardiomyopathy in healthy Doberman Pinscher dogs. J Vet Intern Med. 1998;12:369.
O’Sullivan ML, O’Grady MR, Minors SL. Plasma big endothelin-1, atrial natriuretic peptide, aldosterone, and norepinephrine concentrations in normal Doberman Pinschers and Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med. 2007;21:92-99.
O’Sullivan ML, O’Grady MR, Minors SL. Assessment of diastolic function by Doppler echocardiography in normal Doberman Pinschers and Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med. 2007;21:81.
Oyama MA, Sisson DD, Prosek R, et al. Carvedilol in dogs with dilated cardiomyopathy. J Vet Intern Med. 2007;21:1272-1279.
Sisson DD, Thomas WP, Keene BW. Primary myocardial diseases in the dog. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. ed 5. Philadelphia: WB Saunders; 2000:874-895.
Sleeper MM, Clifford CA, Laster LL. Cardiac troponin I in the normal dog and cat. J Vet Intern Med. 2001;15:501.
Sleeper MM, et al. Dilated cardiomyopathy in juvenile Portuguese water dogs. J Vet Intern Med. 2002;16:52.
Spier AW, Meurs KM. Evaluation of spontaneous variability in the frequency of ventricular arrhythmias in Boxers with arrhythmiogenic right ventricular cardiomyopathy. J Am Vet Med Assoc. 2004;24:538.
Tidholm A, Svensson H, Sylven C. Survival and prognostic factors in 189 dogs with dilated cardiomyopathy. J Am Anim Hosp Assoc. 1997;33:364.
Tidholm A, Haggstrom J, Jonsson L. Detection of attenuated wavy fibers in the myocardium of Newfoundlands without clinical or echocardiographic evidence of heart disease. Am J Vet Res. 2000;61:238.
Tidholm A, Haggstrom J, Hansson K. Effects of dilated cardiomyopathy on the renin-angiotensin-aldosterone system, atrial natriuretic peptide activity, and thyroid hormone concentrations in dogs. Am J Vet Res. 2001;62:961.
Vollmar AC. The prevalence of cardiomyopathy in the Irish Wolfhound: a clinical study of 500 dogs. J Am Anim Hosp. 2000;36:126.
Vollmar AC, et al. Dilated cardiomyopathy in juvenile Doberman Pinscher dogs. J Vet Cardiol. 2003;5:23.
Wright KN, et al. Radiofrequency catheter ablation of atrioventricular accessory pathways in 3 dogs with subsequent resolution of tachycardia-induced cardiomyopathy. J Vet Intern Med. 1999;13:361.
Barber JS, Trees AJ. Clinical aspects of 27 cases of neosporosis in dogs. Vet Rec. 1996;139:439.
Bradley KK, et al. Prevalence of American trypanosomiasis (Chagas disease) among dogs in Oklahoma. J Am Vet Med Assoc. 2000;217:1853.
Breitschwerdt EB, et al. Bartonella vinsonii subsp. Berkhoffii and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis, or myocarditis. J Clin Microbiol. 1999;37:3618.
Dvir E, et al. Electrocardiographic changes and cardiac pathology in canine babesiosis. J Vet Cardiol. 2004;6:15.
Fritz CL, Kjemtrup AM. Lyme borreliosis. J Am Vet Med Assoc. 2003;223:1261.
Gould SM, McInnes EL. Immune-mediated thrombocytopenia associated with Angiostrongylus vasorum infection in a dog. J Small Anim Pract. 1999;40:227.
Lobetti R, Dvir E, Pearson J. Cardiac troponins in canine babesiosis. J Vet Intern Med. 2002;16:63.
Meurs KM, et al. Chronic Trypanosoma cruzi infection in dogs: 11 cases (1987–1996). J Am Vet Med Assoc. 1998;213:497.
Pisani B, Taylor DO, Mason JW. Inflammatory myocardial diseases and cardiomyopathies. Am J Med. 1997;102:459.
Snyder PS, et al. Electrocardiographic findings in dogs with motor vehicle-related trauma. J Am Anim Hosp Assoc. 2001;37:55.