CHAPTER 34 Disorders of the Peritoneum
INFLAMMATORY DISEASES
SEPTIC PERITONITIS
Etiology
Spontaneous septic peritonitis is usually caused by alimentary tract perforation or devitalization caused by neoplasia, ulceration, intussusception, foreign objects, or dehiscence of suture lines. Septic peritonitis can also develop after abdominal gunshot wounds, surgery, or hematogenous spread from elsewhere. Cats seemingly can develop spontaneous septic peritonitis.
Clinical Features
If septic peritonitis occurs secondary to suture line dehiscence, it classically manifests 3 to 6 days postoperatively. Dogs with two or more of the following have been reported to be at increased risk for dehiscence: serum albumin <2.5 g/dl, intestinal foreign body, and preoperative peritonitis. Dogs with septic peritonitis are usually depressed, febrile, and vomiting and may have abdominal pain (if they are not too depressed to respond). Abdominal effusion is usually mild to modest in amount. Signs usually progress rapidly until septic shock (i.e., systemic inflammatory response syndrome [SIRS]) occurs. However, some animals with septic peritonitis may have mild vomiting, slight fever, and copious volumes of abdominal fluid and feel relatively well for days or longer. In particular, cats with septic peritonitis may not show signs of abdominal pain and may be bradycardic.
Diagnosis
Most animals with septic peritonitis have small amounts of abdominal fluid that cannot be detected by physical examination but that decrease serosal detail on plain abdominal radiographs (much like what is seen in animals with a lack of body fat). Ultrasonography is a sensitive means for detecting such small fluid volumes. Free peritoneal gas not related to recent abdominal surgery strongly suggests alimentary tract leakage (Fig. 34-1) or infection with gas-forming bacteria. Ultrasonography may detect masses (e.g., tumors) responsible for such leakage. Neutrophilia is common but nonspecific in dogs and cats with septic peritonitis.
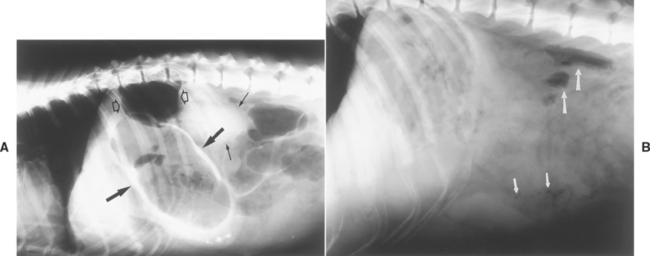
FIG 34-1 A, Plain lateral abdominal radiograph of a dog. Visceral margins of kidney (small solid arrows) and stomach (large solid arrows) are outlined by negative contrast (i.e., air). In addition, there are pockets of free air in the abdomen (open arrows). This dog had a gastric ulcer that spontaneously perforated. B, Plain lateral radiograph of a dog with a splenic abscess. There are air bubbles in the region of the spleen (short arrows) and free gas in the dorsal peritoneal cavity (long arrows).
Abdominocentesis is indicated if free abdominal fluid is detected or if septic peritonitis is suspected. Retrieved fluid is examined cytologically and cultured. Ultrasound guidance should allow the clinician to sample effusions, even when only minimal amounts are present.
Bacteria (especially if phagocytized by white blood cells) or fecal contents in abdominal fluid are diagnostic for septic peritonitis (Fig. 34-2). However, fecal contents and bacteria are often not seen despite severe infection. Prior antibiotic use may greatly suppress bacterial numbers and the percentage of neutrophils demonstrating degenerative changes. Furthermore, mild degenerative changes are common after recent abdominal surgery. More important, it is almost impossible to quickly distinguish septic peritonitis from sterile pancreatitis in some dogs without exploratory laparotomy. Both can cause SIRS, and ultrasound is not as sensitive in detecting pancreatitis as desired. Effusion lactate levels are not accurate in distinguishing septic from nonseptic effusions. Degenerative neutrophils are suggestive of septic peritonitis, but severe sterile pancreatitis can produce degenerative changes identical to that seen with infection. Unfor tunately, when septic peritonitis is strongly suspected, the clinician typically cannot wait for results of abdominal fluid culture. At this time, the ability of canine pancreatic lipase-immunoreactivity determinations to discriminate between the two is uncertain, especially since dogs with septic peritonitis may have secondary pancreatitis if the intestinal perforation is close to the pancreas. Therefore the clinician should always warn the client that the patient may or may not need the procedure but that there is no quick, reliable way to distinguish before surgery.
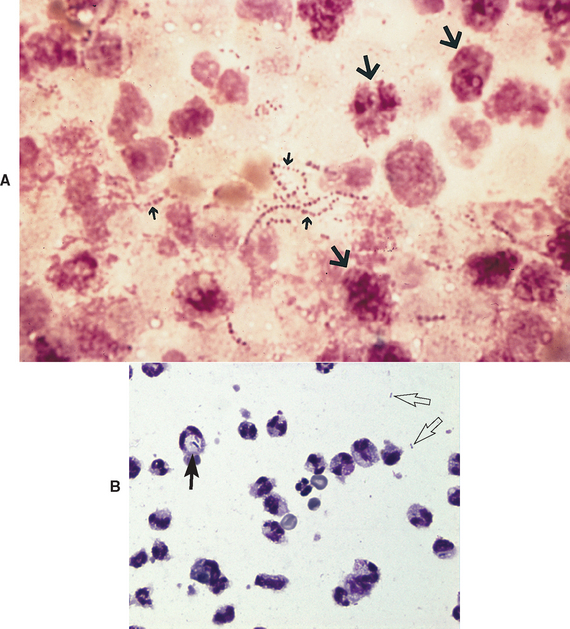
FIG 34-2 A, Photomicrograph of peritoneal exudate from a dog with septic peritonitis. Note bacteria (small arrows) and neutrophils that have degenerated so much that it is difficult to identify them as neutrophils (large arrows). (Wright’s stain; magnification ×1000.) B, Photomicrograph of septic peritoneal fluid. There is one intracellular bacterium (large arrow) and two things (small, clear arrows) that may or may not be bacteria. The neutrophils are not nearly as degenerated as in A.
(Courtesy Dr. Claudia Barton, Texas A & M University.)
Treatment
Animals with spontaneous septic peritonitis usually have an alimentary tract leak and should be surgically explored as soon as they are stable. A preanesthetic complete blood count (CBC), serum biochemistry profile, and urinalysis are desirable; however, surgery usually should not be delayed even if the laboratory results are. During surgery a careful search should be made for intestinal or gastric defects. Biopsy of tissue surrounding a perforation should be performed to search for underlying neoplasia or inflammatory bowel disease (IBD). After the defect is corrected, the abdomen should be repeatedly lavaged with large volumes of warm crystalloid solutions to dilute and remove debris and bacteria. The abdomen cannot be adequately lavaged via a drain tube or even a peritoneal dialysis catheter except in the mildest cases. Adhesions re-form quickly; they should not be broken down unless it is necessary to examine the intestines. Intestines should be resected only if they are truly devitalized. Intestines are sometimes unnecessarily removed because of adhesions, resulting in short bowel syndrome (see p. 466), which has substantial morbidity.
Substantial abdominal contamination may require protracted drainage. Penrose drains are typically inadequate for this purpose. Open abdominal drainage may be done, but it is very time and labor intensive. A nonabsorbable suture is used to close the abdomen except for a 6- to 8-cm opening at its most dependent aspect. This open incision is covered with sterile absorbent dressings (e.g., a sterile sanitary napkin held in place by sterile cast padding and sterile gauze) that are changed as needed, usually two to four times per day initially. Eventually, only one change per day will be needed. When the dressing is changed, a sterile, gloved hand should explore the opening to ensure that omentum and intestines have not blocked the site. This dressing change regimen is continued until abdominal drainage decreases and most or all of the peritoneal contamination is gone. Then a second surgery is performed to close the abdomen. The opening sometimes closes spontaneously. The abdomen should be recultured at the time of the second surgery. Alternatively, closed suction drains have been used postoperatively with success, and some clinicians advocate closure of such abdomens without drainage.
Systemic antimicrobial therapy should consist of broad-spectrum, parenteral antibiotics. A combination of a β-lactam drug (e.g., ticarcillin plus clavulinic acid) and metronidazole plus an aminoglycoside (e.g., amikacin) is usually an excellent choice (see the discussion of antibacterial drugs used in gastrointestinal disorders, p. 409). Enrofloxacin may be substituted for the aminoglycoside, but it must be given over 30 to 40 minutes in a diluted form. Aminoglycosides and quinolones are dose-dependent drugs; administration of the entire daily dose in one injection is safer and probably as or more effective than administering smaller doses two to three times daily. Cefoxitin (30 mg/kg q6-8h) and meropenem (24 mg/kg once daily) are other β-lactam drugs that may be used. Fluid and electrolyte support helps prevent aminoglycoside-induced nephrotoxicity. Hypoalbuminemia can occur, especially if open abdominal drainage is used. If disseminated intravascular coagulation (DIC) is present, administration of fresh frozen plasma to replenish antithrombin III (AT III) and other clotting factors is optimal; plasma is given until the AT III concentration and the prothrombine time (PT) and partial thromboplastin time (PTT) are normal or clearly much improved. Heparin may also be administered; low molecular weight heparin is believed to be more effective than unfractionated heparin.
SCLEROSING, ENCAPSULATING PERITONITIS
Etiology
Reported causes include bacterial infection, steatitis, and fiberglass ingestion. This form of peritonitis is rare.
Clinical features
Sclerosing, encapsulating peritonitis is a chronic condition in which abdominal organs are covered and encased in heavy layers of connective tissue. Typical clinical signs usually include vomiting, abdominal pain, and ascites. During exploratory surgery the lesions may mimic those of a mesothelioma. Analysis of abdominal fluid usually reveals red blood cells, mixed inflammatory cells, and macrophages. Diagnosis is confirmed by surgical biopsy of the thick covering of the abdominal organs.
HEMOABDOMEN
Most red effusions are blood-tinged transudates, not hemoabdomen. Hemoabdomen is usually indicated by a fluid with a hematocrit greater than or equal to 10% to 15%. Blood in the abdominal cavity can be iatrogenic (i.e., caused by abdominocentesis), traumatic (e.g., automobile-associated trauma), or toxic (e.g., ingestion of vitamin K antagonist) in origin, or can represent spontaneous disease. Clots or platelets in the sample mean that the bleeding is iatrogenic or is currently occurring near the site of the abdominocentesis. Spontaneous hemoabdomen is usually the result of a bleeding neoplasm (e.g., hemangiosarcoma, hepatocellular carcinoma). History, physical examination, coagulation studies, and/or abdominal ultrasonography usually establish the diagnosis. It should be noted that thrombocytopenia may cause or be caused by vigorous abdominal bleeding. Also, even when a coagulopathy is secondary to the original cause of the hemoabdomen (e.g., tumor), it may become severe enough to cause bleeding by itself.
ABDOMINAL HEMANGIOSARCOMA
Etiology
Abdominal hemangiosarcoma often originates in the spleen (see Chapter 82). It can spread throughout the abdomen by implantation, causing widespread peritoneal seepage of blood, or it can metastasize to distant sites (e.g., liver, lungs).
Clinical features
Abdominal hemangiosarcoma is principally found in older dogs, especially German Shepherd Dogs and Golden Retrievers. Anemia, abdominal effusion, and periodic weakness or collapse from poor peripheral perfusion are common presenting complaints. Some animals have bicavity hemorrhagic effusion.
Diagnosis
Ultrasonography is the most sensitive test for splenic and hepatic masses, especially when there is copious abdominal effusion. Radiographs may reveal a mass if there is minimal free peritoneal fluid. Abdominocentesis typically reveals hemoabdomen but not neoplastic cells. Definitive diagnosis requires biopsy (via laparotomy) because splenic hematoma, hemangioma, and widespread accessory splenic tissue masquerade as hemangiosarcoma but have a much better prognosis. Two or more large tissue samples should always be submitted, and the clinician should be prepared to request recuts; hemangiosarcoma may be difficult to find histologically. Fine-needle biopsy (especially fine-needle core biopsy) is sometimes diagnostic. However, there is the risk of inducing life-threatening hemorrhage, and the patient must be watched closely after the procedure for evidence of hypovolemia.
Treatment
Solitary masses should be excised. Chemotherapy may be palliative for some animals with multiple masses; chemotherapy is also indicated as an adjuvant postoperative treatment modality (see Chapter 82).
MISCELLANEOUS PERITONEAL DISORDERS
ABDOMINAL CARCINOMATOSIS
Etiology
Abdominal carcinomatosis involves widespread, miliary peritoneal carcinomas that may have originated from various sites; intestinal and pancreatic adenocarcinomas are common neoplasms that may result in carcinomatosis.
Clinical Features
Weight loss may be the predominant complaint, although some animals are presented because of obvious abdominal effusion.
Diagnosis
Physical examination and radiography rarely help to establish the diagnosis. Ultrasonography may reveal masses or infiltrates if they are large enough; however, small, miliary lesions can be missed by ultrasound. Fluid analysis reveals a nonseptic exudate or a modified transudate; epithelial neo plastic cells are occasionally found (see Chapter 36). Laparoscopy or abdominal exploratory surgery with histologic examination of biopsy specimens is usually needed for diagnosis.
Treatment
Intracavitary chemotherapy has been palliative for some animals, although generally there is no effective treatment for this disorder. Cisplatin (50 to 70 mg/m2 every 3 weeks) and 5-fluorouracil (150 mg/m2 every 2 to 3 weeks) are frequently effective in decreasing fluid accumulation in dogs with carcinomatosis but should not be used in cats; carboplatin (150 to 200 mg/m2 every 3 weeks) may be effective in cats.
MESOTHELIOMA
Clinical Features
Mesothelioma often causes bicavity effusion. The tumor may appear as fragile clots adhering to the peritoneal surface of various organs.
Diagnosis
Imaging reveals only fluid accumulations. Fluid cytology rarely is beneficial because reactive mesothelial cells are notorious for mimicing malignancy, and pathologists generally acknowledge the inability to cytologically distinguish neoplastic cells from nonneoplastic cells in abdominal fluid. Laparoscopy or laparotomy are typically needed to make a definitive diagnosis.
FELINE INFECTIOUS PERITONITIS
Feline infectious peritonitis (FIP) is a viral disease of cats, which is discussed in detail in Chapter 97. Only the abdominal effusion of FIP is discussed here. Although a major cause of feline abdominal effusion, FIP is not the only cause. Furthermore, not all cats with FIP have effusions. FIP effusions are classically pyogranulomatous (i.e., macrophages and nondegenerate neutrophils) with a relatively low nucleated cell count (i.e., ≤10,000/μl). However, some cats with FIP have effusions that primarily contain neutrophils. A nonseptic exudate in a nonazotemic cat suggests FIP until proven otherwise.
Boysen SR, et al. Evaluation of a focused assessment with sonography for trama protocol to detect free abdominal fluid in dogs involved in motor vehicle accidents. J Am Vet Med Assoc. 2004;225:1198.
Brockman DJ, et al. A practical approach to hemoperitoneum in the dog and cat. Vet Clin N Am. 2000;30:657.
Costello MF, et al. Underlying cause, pathophysiologic abnormalities, and response to treatment in cats with septic peritonitis: 51 cases (1990-2001). J Am Vet Med Assoc. 2004;225:897.
Hinton LE, et al. Spontaneous gastroduodenal perforation in 16 dogs and seven cats (1982–1999). J Am Anim Hosp Assoc. 2002;38:176.
Lanz OI, et al. Surgical treatment of septic peritonitis without abdominal drainage in 28 dogs. J Am Anim Hosp Assoc. 2001;37:87.
Levin GM, et al. Lactate as a diagnostic test for septic peritoneal effusions in dogs and cats. J Am Anim Hosp Assoc. 2004;40:364.
Merlo M, et al. Radiographic and ultrasonographic features of retained surgical sponge in eight dogs. Vet Radiol Ultrasound. 2000;41:279.
Mueller MG, et al. Use of closed-suction drains to treat generalized peritonitis in dogs and cats: 40 cases (1997–1999). J Am Vet Med Assoc. 2001;219:789.
Pintar J, et al. Acute nontraumatic hemoabdomen in the dog: a retrospective analysis of 39 cases (1987-2001). J Am Anim Hosp Assoc. 2003;39:518.
Ralphs SC, et al. Risk factors for leakage following intestinal anastomosis in dogs and cats: 115 cases (1991-2000). J Am Vet Med Assoc. 2003;223:73-77.
Saunders WB, et al. Penumperitoneum in dogs and cats: 39 cases (1983-2002). J Am Vet Med Assoc. 2003;223:462.
Shales CJ, et al. Complications following full-thickness small intestinal biopsy in 66 dogs: a retrospective study. J Small Anim Pract. 2005;46:317.
Sharpe A, et al. Intestinal haemangiosarcoma in the cat: clinical and pathological features of four cases. J Small Anim Pract. 2000;41:411.
Smelstoys JA, et al. Outcome of and prognostic indicators for dogs and cats with pneumoperitoneum and no history of penetrating trauma: 54 cases (1988-2002). J Am Vet Med Assoc. 2004;225:251.
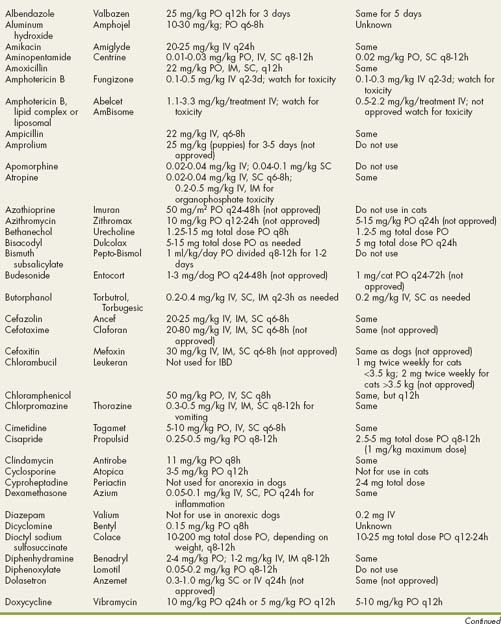
 Drugs Used in Gastrointestinal Disorders
Drugs Used in Gastrointestinal Disorders