CHAPTER 40 The Exocrine Pancreas
GENERAL CONSIDERATIONS
The pancreas is located in the cranial abdomen, with the left limb positioned between the transverse colon and the greater curvature of the stomach and the right limb running alongside the proximal duodenum. Any or all of these neighboring structures can be affected when there is pancreatic inflammation. The exocrine acini make up about 90% of pancreatic tissue, and the endocrine islets interspersed among the acini make up the other 10% (Fig. 40-1). The close anatomical association between the acini and islets allows subtle signaling between them to coordinate digestion and metabolism, but it also means that there is a complex cause-and-effect relationship between diabetes mellitus and pancreatitis. The major function of the exocrine pancreas is to secrete digestive enzymes, bicarbonate, and intrinsic factor (IF) into the proximal duodenum. Pancreatic enzymes are responsible for the initial digestion of larger food molecules and require an alkaline pH to function (hence the concurrent bicarbonate secretion by pancreatic duct cells). The pancreas secretes several proteases, phospholipases, ribonucleases, and deoxyribonucleases as inactive precursors (zymogens) and also α-amylase and lipase as intact molecules. The pancreas is the only significant source of lipase, and hence steatorrhea (fatty feces) is a prominent sign of exocrine pancreatic insufficiency (EPI). Trypsin is central to the pathogenesis of pancreatitis, as outlined in the subsequent sections, and inappropriate early activation of the zymogen trypsinogen to trypsin within the pancreatic acini is the final common pathway triggering pancreatic inflammation. In the normal animal pancreatic secretion is triggered by the thought of food and stomach filling and most potently by the presence of fat and protein in the small intestinal lumen. The vagus nerve, the local enteric nervous system, and the hormones secretin and cholecystokin from the small intestine all stimulate pancreatic secretion. Trypsinogen is activated within the small intestine by the brush border enzyme enterokinase, which cleaves a peptide (the “trypsin-activation peptide” [TAP]) from trypsinogen. Activated trypsin then activates the other zymogens within the intestinal lumen. IF, which is necessary for vitamin B12 absorption in the ileum, is secreted only by the pancreas in the cat. In the dog the pancreas is the main source of IF, but a small amount is also secreted by the gastric mucosa.
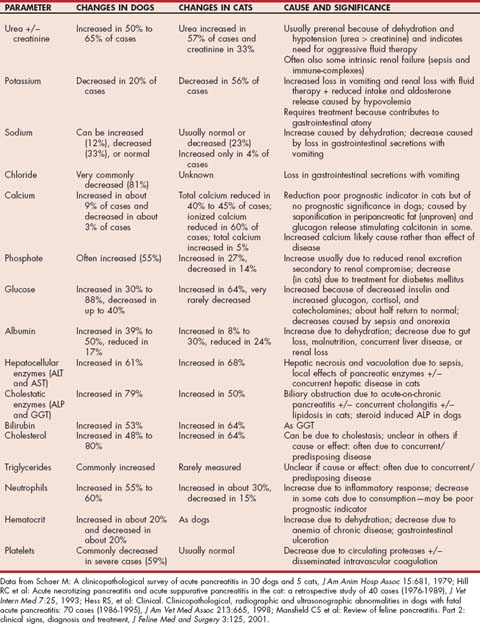
FIG 40-1 Histopathology of a section of normal canine pancreas showing two paler staining islets of Langerhans and exocrine acini surrounding them. Note that the islets make up only 10% to 20% of the volume of the pancreas.
Diseases of the exocrine pancreas are relatively common but often misdiagnosed in both dogs and cats because of nonspecific clinical signs and a lack of sensitive and specific clincopathological tests. Pancreatitis is the most common disease of the exocrine pancreas in both cats and dogs; EPI, although less common, is also recognized frequently. Uncommon diseases of the pancreas include pancreatic abscess, pseudocyst, and neoplasia.
Recent advances in the understanding of the pathophysiology, prevalence, and potential causes of pancreatitis in dogs and cats may give clues to treatment in the future, although treatment of acute pancreatitis remains largely nonspecific and supportive in all species.
Important differences in the anatomy of the pancreas and associated areas between the dog and cat are outlined in Table 40-1.
 TABLE 40-1 Differences in Pancreatic Structure, Function, and Diseases Between Dogs and Cats
TABLE 40-1 Differences in Pancreatic Structure, Function, and Diseases Between Dogs and Cats
| FEATURE | DOGS | CATS |
|---|---|---|
| Usually two pancreatic ducts: | ||
| Pancreatic function | Intrinsic factor secreted largely by pancreas but also some in stomach; vitamin B12 deficiency common in exocrine insufficiency but sometimes normal | Intrinsic factor secreted entirely by pancreas so Vitamin B12 deficiency very common in exocrine insufficiency; vitamin K deficiency also common because of concurrent liver and intestinal disease further reducing absorption |
| Pancreatitis: disease associations | ||
| Exocrine pancreas: other pathology | ||
| Pancreatitis: spectrum of disease | ||
| Pancreatitis: diagnosis | ||
| Causes of exocrine pancreatic insufficiency | Most cases end-stage chronic pancreatitis Pancreatic acinar atrophy not reported |
PANCREATITIS
Pancreatitis may be acute or chronic. As with acute and chronic hepatitis, the difference is histological and not necessarily clinical (Table 40-2 and Fig. 40-2), and there is some clinical overlap between the two. Chronic disease may present initially as an acute-on-chronic episode; in postmortem studies of fatal acute pancreatitis in dogs and cats, up to half of the cases were actually acute-on-chronic disease. Differentiation of truly acute disease from an acute flare-up of chronic disease is not important for initial management, which is the same in all cases, but is important to allow recognition of the potential long-term sequelae of chronic disease, as outlined in the following sections. The causes of acute and chronic pancreatitis may be different, but there may also be some overlap between them.
 TABLE 40-2 Differences Between Acute and Chronic Pancreatitis in Dogs and Cats
TABLE 40-2 Differences Between Acute and Chronic Pancreatitis in Dogs and Cats
| ACUTE PANCREATITIS | CHRONIC PANCREATITIS | |
|---|---|---|
| Histopathology | ||
| Clinical appearance | Spectrum from severe and fatal (usually necrotizing) to mild and subclinical (less common) | Spectrum from mild, low-grade intermittent gastrointestinal signs (most common) to an acute-on-chronic episode indistinguishable from classical acute pancreatitis |
| Diagnostic challenge | Higher sensitivity of enzyme tests and ultrasonography than in chronic disease | Lower sensitivity of enzyme tests and ultrasonography than in acute disease: diagnosis much more challenging |
| Mortality and long-term sequelae | High immediate mortality but no long-term sequelae |
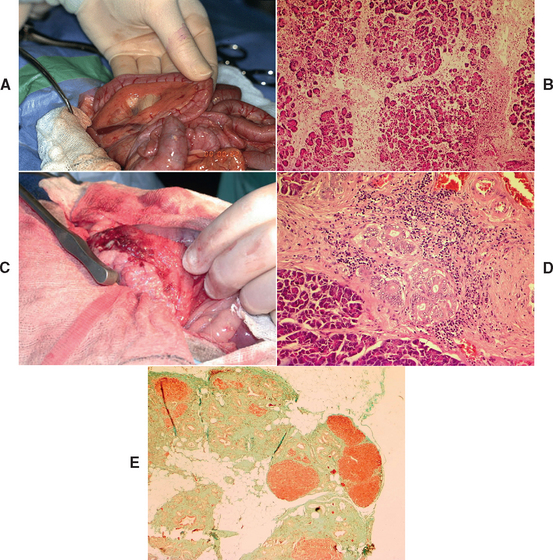
FIG 40-2 A, Gross appearance of acute pancreatitis in a cat at laparotomy demonstrating generalized hyperemia. It is also possible for acute pancreatitis to appear normal grossly. B, Histopathological appearance of acute pancreatitis in a young adult female West Highland White Terrier. Note prominent edema and inflammation disrupting the acini. This case was fatal, but it would have been potentially completely reversible if the dog had survived the acute phase. Hematoxylin and eosin ×100. C, Gross appearance of chronic pancreatitis in a middle-aged Jack Russell Terrier. Note nodular appearance of pancreas and extensive adhesions to the duodenum obscuring the mesentery. It is also possible for chronic pancreatitis to appear normal grossly. D, Histological appearance of chronic pancreatitis from a 10-year-old male Cavalier King Charles Spaniel. Note fibrosis, mononuclear inflammatory cells, and ductular hyperplasia. Hematoxylin and eosin ×200. E, Histological appearance of end-stage chronic pancreatitis in an 11-year-old neutered female Cavalier King Charles Spaniel with diabetes mellitus and exocrine pancreatic insufficiency. Note extensive fibrosis (green) and small islands of remaining acini (red). Massons Trichrome ×40.
(A and C, From Villiers E, Blackwood L, editors: BSAVA manual of canine and feline clinical pathology, ed 2, Gloucestershire, United Kingdom, 2005, British Small Animal Veterinary Association.)
ACUTE PANCREATITIS
Etiology and Pathogenesis
Understanding of the pathophysiology of acute pancreatitis in humans has increased in recent years with the discovery of hereditary mutations of trypsin, which predispose to pancreatitis; the pathophysiology of this disease is believed to be similar in dogs and cats. The final common pathway in all cases is the inappropriate early activation of trypsinogen within the pancreas as a result of increased autoactivation and/or reduced autolysis. Trypsin is the major protease secreted by the pancreas, and inappropriate early activation within the acinar cells would obviously cause autodigestion and severe inflammation. Protective mechanisms therefore exist to prevent early activation: Trypsin is stored within zymogen granules in the pancreatic acini as the inactive precursor trypsinogen; up to 10% of trypsinogen gradually autoactivates within the granules but is inactivated by the action of other trypsin molecules and by the co-segregating protective molecule pancreatic secretory trypsin-inhibitor (PSTI; also known as serine protease inhibitor Kazal type 1, or SPINK1). Genetic mutations of trypsinogen, which make it resistant to hydrolysis, and/or of PSTI predispose to pancreatitis in people and are also likely to occur in some dogs (Table 40-3). If too much trypsin autoactivates within the pancreas, the protective mechanisms are overwhelmed and a chain reaction occurs, whereby activated trypsin activates more trypsin and the other enzymes within the pancreas, with the resulting pancreatic autodigestion, inflammation, and peripancreatic fat necrosis that leads to focal or more generalized sterile peritonitis. There is an associated systemic inflammatory response (SIR) in even the mildest cases of pancreatitis. Many other organs may be involved, and in the most severe cases, there is multiorgan failure and diffuse intravascular coagulation (DIC). The circulating protease inhibitors α1-antitrypsin (= α1-protease inhibitor) and α-macroglobulin play a role in removing trypsin and other proteases from the circulation. Saturation of these protease inhibitors by excessive amounts of circulating proteases contributes to the systemic inflammation, but generalized neutrophil activation and cytokine release is probably the primary cause of SIR.
 TABLE 40-3 Causes of Acute Pancreatitis in Dogs and Cats
TABLE 40-3 Causes of Acute Pancreatitis in Dogs and Cats
| RISK FACTOR | CAUSE |
|---|---|
| Idiopathic 90% | Unknown (some may be hereditary) |
| Duct obstruction ± hypersecretion ± bile reflux into pancreatic duct | Experimental; neoplasia; surgery ± cholangitis + role in chronic pancreatitis |
| Hypertriglyceridemia | Inherent abnormal lipid metabolism (breed related, e.g., Min. Schnauzers) |
| Endocrine: diabetes mellitus, hyperadrenocortism, hypothyroidism | |
| Breed/sex? | Increased risk terriers ± spayed females—may reflect risk of hypertriglyceridemia (also Min. Schnauzers; see above) |
| Diet | Dietary indiscretion/high-fat diet |
| Malnutrition; Obesity? | |
| Trauma | Road traffic accident, surgery, “high rise syndrome” |
| Ischemia/reperfusion | Surgery (not just pancreas), gastric dilatation and volvulus; shock, severe immune-mediated hemolytic anemia (common association if anemia severe) |
| Hypercalcemia | Experimental; hypercalcemia of malignancy (uncommon association clinically); primary hyperparathyroidism |
| Drugs/toxins | Organophosphates; azathioprine; asparaginase; thiazides; furosemide; estrogens; sulpha drugs; tetracycline; procainamide, potassium bromide. |
| Infections | Toxoplasma, others (uncommon) |
From Villiers E, Blackwood L, editors: BSAVA manual of canine and feline clinical pathology, ed 2, Gloucestershire, United Kingdom, 2005, British Small Animal Veterinary Association.
The previous paragraph describes the final common pathway of acute pancreatitis in dogs and cats, but the underlying cause of the disease is often unknown (see Table 40-3). There appears to be a strong breed relationship in dogs with pancreatitis, so hereditary causes are likely to be a factor in this species. Many of the previously reported supposed causes in dogs are likely triggers for disease in genetically susceptible individuals.
Clinical Features
Acute pancreatitis typically affects middle-aged dogs and cats, although very young and very old individuals may also be affected. Terrier breeds, Miniature Schauzers, and domestic short-haired cats appear to be at increased risk for acute pancreatitis, although any breed or cross-breed can be affected. Some dog breeds appear to be underrepresented in clinical studies, particularly large and giant breeds, although Labrador Retrievers are sometimes affected and also sometimes Husky-types (particularly in Australia). Breed relationships suggest an underlying genetic tendency, mirroring the situation in humans. It is likely that the disease is multifactorial with a genetic tendency and superimposed triggering factors. For example, eating a high-fat meal may be a trigger for a susceptible terrier. Some studies suggest a slight increase in risk in female dogs, whereas others show no sex predisposition. Obesity has been suggested as a predisposing factor in dogs, but it is unclear whether this is a cause or whether it is co-segregating with disease (i.e., breeds at high risk for acute pancreatitis may coincidentally also be breeds with a high risk for obesity). In cats there is a recognized association with concurrent cholangitis, inflammatory bowel disease, or renal disease in some cases. Cats with acute pancreatitis are also at high risk for hepatic lipidosis.
The history in dogs often includes a trigger such as a high-fat meal or engorging (see Table 40-3). Recent drug therapy may also be a trigger, particularly potassium bromide, azathioprine or asparaginase in dogs. Concurrent endocrine diseases such as hypothyroidism, hyperadrenocorticism, or diabetes mellitus (DM) increase the risk of severe fatal pancreatitis in dogs; therefore it is important to identify these in the history. In cats the history may include features of concurrent cholangiohepatitis, inflammatory bowel disease, or hepatic lipidosis (or any combination thereof).
The clinical signs in dogs vary with the severity of the disease from mild abdominal pain and anorexia at one end of the spectrum to an “acute abdomen” and potential multiorgan failure and DIC at the severe end of the spectrum. Dogs with severe acute disease usually present with acute vomiting, anorexia, marked abdominal pain, and varying degrees of dehydration, collapse, and shock. The vomiting is initially typical of delayed gastric emptying resulting from peritonitis, with emesis of undigested food a long time after feeding progressing to vomiting only bile. The main differential diagnoses in these cases are other causes of acute abdomen, particularly intestinal foreign body or obstruction; the vomiting may be so severe that the dog may undergo an unnecessary laparotomy for a suspected obstruction if a careful workup was not performed first. Some patients may show the classic “praying stance,” with the forelegs on the floor and the hindlegs standing (Fig. 40-3), but this is not pathognomonic for pancreatitis and can be seen in association with any pain in the cranial abdomen, including hepatic, gastric, or duodenal pain. By contrast, cats with severe, fatal, necrotizing pancreatitis usually have surprisingly mild clinical signs, such as anorexia and lethargy; vomiting and abdominal pain occur in fewer than half the cases.

FIG 40-3 Dog exhibiting evidence of cranial abdominal pain by assuming the “position of relief.”
(Courtesy Dr. William E. Hornbuckle, Cornell University, College of Veterinary Medicine.)
At the milder end of the spectrum, dogs and cats may present with mild gastrointestinal signs—typically anorexia and sometimes some mild vomiting, followed by the passage of some colitic-like feces accompanied by some fresh blood resulting from local peritonitis in the area of the transverse colon. Inflammatory bowel disease, low-grade infectious enteritis, and chronic hepatitis would be major differential diagnoses for this presentation in dogs as well as cats. Animals that are still eating may show prominent postprandial discomfort.
Both cats and dogs with acute pancreatitis can present with jaundice, either at initial examination or often developing a few days later, when the initial acute signs are resolving. In fact, most, if not all, animals with jaundice have acute-on-chronic disease (see the section on chronic pancreatitis).
Careful clinical examination should focus on identification of the degree of dehydration and shock, careful assess ment for any concurrent diseases (particularly endocrine disease), and careful abdominal palpation. In severe cases petechiae or ecchymoses suggestive of DIC may be identified, and there may be respiratory distress associated with acute respiratory distress syndrome. Careful clinical and clinicopathological assessment of the degree of shock and concurrent organ damage is important for prognosis and treatment decisions, as outlined in the following sections. Abdominal palpation should identify pancreatic pain and rule out, if possible, any palpable foreign bodies or intussusceptions, although abdominal imaging will be required to rule these out with confidence. In severe cases generalized peritonitis will result in generalized unmistakable abdominal pain, whereas in milder cases careful palpation of the cranial abdomen is required to identify a focus of abdominal pain, as indicated in Fig. 40-4. Occasionally, a cranial abdominal mass may be palpated, particularly in cats, representing a focus of fat necrosis.
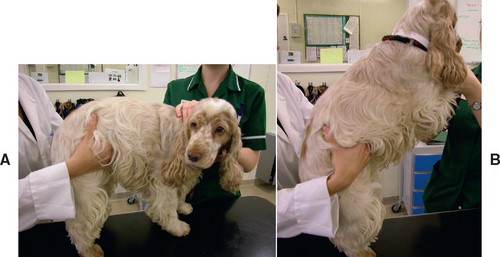
FIG 40-4 Carefully palpating a Cocker Spaniel for cranial abdominal pain. A, The clinician should palpate craniodorsally under the rib cage for evidence of focal pancreatic pain (as shown in this dog by turning of the head). B, With deep-chested dogs it helps to ask an assistant to elevate the head of the dog to displace the pancreas caudally (effectively achieving the opposite of the dog in Fig. 40-3).
Routine Clinical Pathology
Routine laboratory analysis (i.e., complete blood count [CBC], serum biochemical profile, and urinalysis) typically does not help in arriving at a specific diagnosis, but it is very important to perform these in all but the mildest cases because they give important prognostic information and aid in effective treatment, as outlined in the following sections. Typical clinicopathologic abnormalities in dogs and cats with acute pancreatitis are shown in Table 40-4.
More Specific Pancreatic Enzyme Assays
More specific laboratory tests for the pancreas are the catalytic assays amylase and lipase and the immunoassays trypsinlike immunoreactivity (TLI) and pancreatic lipase immunoreactivity (PLI). Catalytic assays rely on the ability of the molecule to catalyze a reaction in vivo and thus rely on presence of active enzyme; however, they are not species specific. In cats amylase and lipase are of very questionable diagnostic value. Immunoassays, however, use an antibody against a part of the enzyme molecule distant from the active site and thus will also measure inactive precursors (e.g., trypsinogen) and tend to be organ and species specific. The advantages and disadvantages of the different assays are outlined in Table 40-5. Overall, PLI has the highest sensitivity and likely the highest specificity in both species and is the only reliable test for pancreatitis currently available in cats. A SNAP® test for canine PLI has recently been released by IDEXX (see details at www.idexx.com/animalhealth/testkits/snapcpl/index.jsp), which should aid in rapid diagnosis.
 TABLE 40-5 The Use of Specific Catalytic Enzyme Tests and Immunoassays in the Diagnosis of Acute and Chronic Pancreatitis in Dogs and Cats
TABLE 40-5 The Use of Specific Catalytic Enzyme Tests and Immunoassays in the Diagnosis of Acute and Chronic Pancreatitis in Dogs and Cats
| ASSAY | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Either may be normal in severe ± chronic pancreatitis due to enzyme depletion ± loss of tissue; degree of elevation of no prognostic value, except where stated; both renally excreted and elevated 2 or 3 times in azotemia | ||
| Amylase | Low sensitivity and specificity because of high background level from other sources, including small intestine | |
| Lipase | Widely available on practice analyzers; more sensitive than amylase; degree of elevation may have prognostic significance | Extrapancreatic sources so high background level. Steroids elevate up to 5×. |
| Immunoassays | ||
| Canine TLI | Elevations high specificity for pancreatitis | |
| Feline TLI | One of only two assays available for cats | Lower sensitivity and specificity than canine TLI—better used for diagnosis of EPI; renally excreted so elevated in azotemia |
| Canine PLI | Increased in renal disease but may not be significantly so? (Unclear yet if affected by steroids) | |
| Feline PLI | Very new test but appears most sensitive and specific test available for feline pancreatitis | Very little published data available on its use |
TLI, Trypsinlike immunoreactivity; PLI, pancreatic lipase immunoreactivity
Diagnostic Imaging
The most sensitive way to image the canine and feline pancreas noninvasively is by ultrasonography. Abdominal radiographs in patients with pancreatitis usually show mild or no changes, even in those with severe disease (Fig. 40-5). However, in patients with acute disease, abdominal radiography plays an important role in ruling out acute intestinal obstruction, which would result in obvious changes, primarily dilated, gas-filled, stacking loops of intestine. Classical radiographic changes in dogs and cats with acute pancreatitis include focal decrease in contrast in the cranial abdomen associated with local peritonitis; a dilated, fixed (C-shaped), and laterally displaced proximal duodenum on ventrodorsal views; and caudal displacement of the transverse colon. Occasionally, a “mass” effect may be seen in the region of the pancreas, usually the result of fat necrosis. Pancreatic tumors by contrast are usually small, but it is impossible to differentiate fat necrosis from neoplasia using imaging alone. Abdominal radiographs appear normal in many dogs and cats with acute or chronic pancreatitis. Barium studies should be avoided, if possible, because they do not contribute to diagnosis and the associated gut filling provides further stimulus for pancreatic enzyme release.
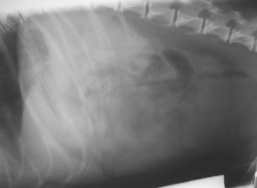
FIG 40-5 Lateral abdominal radiograph from a 7-year-old Jack Russell Terrier with acute pancreatitis. There are minimal changes apparent apart from a mild loss of abdominal contrast, in spite of the severity of the disease. This does, however, help to rule out acute obstruction because the intestinal loops are not dilated and gas filled.
(Courtesy the Diagnostic Imaging Department, Queen’s Veterinary School Hospital, University of Cambridge.)
The most sensitive imaging modalities in humans with pancreatitis are magnetic resonance imaging (MRI), computed tomography (CT), or transendoscopic ultrasonography. CT has so far proved disappointing in both cats and dogs. Pancreatic MRI has not been reported in small animal species, and transendoscopic ultrasonography is not widely available, although it would be expected to be useful insofar as the pancreas can be imaged very closely from the adjacent stomach or duodenum. Because all these techniques require general anesthesia, they may never become widely used in small animal patients with severe acute pancreatitis. Transcutaneous ultrasonography has a high specificity for pancreatic disease (i.e., if a lesion is found, it is real) but a variable sensitivity depending on the skill of the operator and the severity of the disease. Ultrasonography has a higher sensitivity for classical acute pancreatitis in both dogs and cats because associated edema and peripancreatic fat necrosis result in visible interfaces. The sensitivity is much lower for chronic pancreatitis in both cats and dogs (Fig. 40-6).
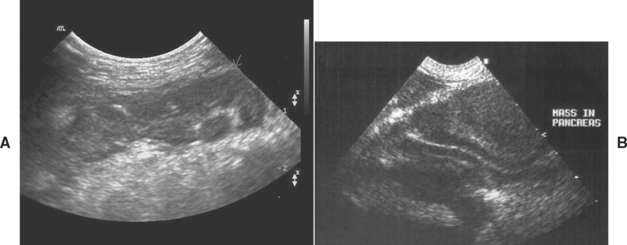
FIG 40-6 A, Typical ultrasonographic appearance of acute pancreatitis in a Miniature Schnauzer with a diffusely hypoechoic pancreas (white arrows) with surrounding hyperechoic mesentery. B, Typical ultrasonographic appearance of chronic pancreatitis in an English Cocker Spaniel. There is a masslike effect displacing the duodenum. Many dogs and cats with chronic pancreatitis have an unremarkable abdominal ultrasound.
(Courtesy the Diagnostic Imaging Department, Queen’s Veterinary School Hospital, University of Cambridge.)
Fluid Analysis
Some dogs and cats with pancreatitis have abdominal effusion. Fluid analysis usually reveals serosanguineous sterile exudates, although transudates and chylous effusions have also been reported in cats. Amylase and lipase activities in the fluid may be higher than in the serum, and elevated lipase in the effusion can be diagnostically helpful (Guija de Arespacochaga et al., 2006). Pleural effusions also occur in a small number of dogs with acute pancreatitis as a result of generalized vasculitis.
The search continues for the ideal diagnostic test for pancreatitis. Trypsin-activation peptide (TAP) is well conserved between species, so human ELISAs can be used for dogs and cats. However, elevations in either plasma or urine TAP are no more sensitive or specific than currently available blood tests. In dogs the best prognostic indicator is the modified organ score, as shown in Tables 40-6 and 40-7. This system has been extrapolated from humans, but its use as a prognostic and treatment indicator in cats has not been critically evaluated. Of the individual diagnostic tests, the following were found to be negative prognostic indicators in dogs: high urinary TAP : creatinine ratio, marked increases in serum lipase activity, marked increases in serum creatinine and phosphate concentrations, and low urine specific gravity. In cats, the following negative prognostic indicators were found: low ionized calcium and leukopenia. Urinary or plasma TAP do not appear to be prognostically useful in cats, and neither does the degree of elevation of TLI in either species. The prognostic significance of degree of elevation of cPLI activity is currently unknown.
 TABLE 40-6 Modified Organ Scoring System for Treatment and Prognostic Decisions in Acute Pancreatitis
TABLE 40-6 Modified Organ Scoring System for Treatment and Prognostic Decisions in Acute Pancreatitis
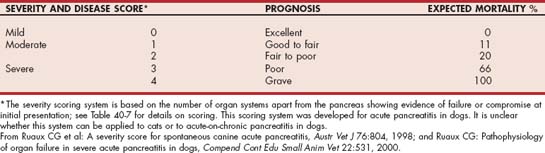
 TABLE 40-7 Criteria to Assess Organ System Compromise for Severity Scoring System in Canine Acute Pancreatitis
TABLE 40-7 Criteria to Assess Organ System Compromise for Severity Scoring System in Canine Acute Pancreatitis
| ORGAN SYSTEM | CRITERIA FOR COMPROMISE | LAB REFERENCE RANGE |
|---|---|---|
| Hepatic | One or more of alkaline phosphatase, aspartate aminotransferase, or alanine aminotransferase >3× upper reference range | |
| Renal | Blood urea >84 mg/dl | Blood urea 15-57 mg/dl |
| Creatinine >3.0 mg/dl | Creatinine 0.6-1.8 mg/dl | |
| Leukocytic | >10% band neutrophils or total white cell count >24 × 103/μl | Band neutrophils 0.0-0.2 × 103/μl |
| Total white cell count 4.5-17 × 103/μl | ||
| Endocrine pancreas* | Blood glucose >234 mg/dl and/or β-OH butyrate >1 mmol/l | Blood glucose 59-123 mg/dl |
| β-OH butyrate 0.0-0.6 mmol/l | ||
| Acid/base buffering* | Bicarbonate <13 or >26 mmol/l and/or anion gap <15 or >38 mmol/l | Bicarbonate 15-24 mmol/l |
| Anion gap 17-35 mmol/l |
* If increased glucose, butyrate, and acidosis co-exist, count as one system.
From Ruaux CG et al: A severity score for spontaneous canine acute pancreatitis, Austr Vet J 76:804, 1998.
Histopathology
Definitive diagnosis of acute pancreatitis can be achieved only via histopathology of a pancreatic biopsy, but this is invasive and not indicated in most cases. However, if the animal has a laparotomy during its investigation, the clinician should always remember to visually inspect the pancreas and also, preferably, to obtain a small biopsy. The pancreas usually appears grossly inflamed and may have a masslike appearance. The latter is usually due to fat necrosis and/or fibrosis and not neoplasia; therefore no animal should be euthanized on the basis of a tumorlike appearance in the pancreas without supportive cytology or pathology because most large masses in the pancreas are not tumors. As in the small intestine, it is possible for the pancreas to appear grossly normal despite having clinically relevant disease, particularly in cats and in dogs and cats with low-grade chronic disease. Pancreatic biopsy is safe and does not carry a high risk of postoperative pancreatitis, provided that the pancreas is handled gently and the blood supply is not disrupted. It is best to take a small biopsy from the tip of a lobe and not to ligate any vessels, particularly on the right limb, which shares a blood supply with the proximal duodenum.
However, in most cases a biopsy will not be performed and diagnosis is based on a combination of clinical suspicion, specific enzyme tests, and diagnostic imaging. No one noninvasive test is 100% sensitive and specific for pancreatitis in dogs and cats; in a few cases of even severe disease, all the tests may be negative.
Treatment and Prognosis
The treatment and prognosis of dogs and cats with acute pancreatitis depends on the severity of the condition at presentation. Severe acute pancreatitis is a very serious disease, has a very high mortality, and requires intensive management, whereas more moderate disease can be managed with intravenous fluids and analgesia, and patients with mild disease can sometimes be managed on an outpatient basis.
The easiest and most practical way to scale treatment and prognosis in dogs is to use the organ-scoring system modified from human medicine by Ruaux and Atwell (1998) and Ruaux (2000; see Tables 40-6 and 40-7). Cats, even those with severe disease, are more difficult to assess because of their mild clinical signs and because the utility of the organ-scoring system has not yet been assessed in this species. It therefore seems prudent to assume that all cats have severe disease unless proved otherwise and treat them intensively, with the intent of preventing hepatic lipidosis and other fatal complications.
The inciting cause of the pancreatitis should be treated or removed in the few cases where it is known (e.g., hypercalcemia or drug-induced), and every effort should be made during treatment to avoid further potential triggers, as outlined in Table 40-3. Most cases of pancreatitis are, however, idiopathic, and treatment is largely symptomatic. The one exception is chronic pancreatitis in English Cocker Spaniels, which may be an immune-mediated disease in which steroids and other immunosuppressive drugs may be indicated as a specific treatment (see the section on chronic pancreatitis for more details). Occasionally, Cocker Spaniels with chronic pancreatitis present with acute clinical signs, and judicious corticosteroid therapy might be considered in these individuals. However, there is no evidence that corticosteroid therapy helps in other breeds of dogs, including terriers, and in these the use of such drugs might actually worsen prognosis by increasing the risk of gastric ulceration and reducing the activity of the reticuloendothelial system in the removal of circulating α2-macroglobulin-protease complexes. In some instances, a dog or cat may need corticosteroid therapy for a concurrent condition, such as immune-mediated hemolytic anemia or inflammatory bowel disease, in which case the benefits of corticosteroids may outweigh their potential deleterious effects.
Severe, necrotizing pancreatitis (scores 3 and 4; Tables 40-6 and 40-7) carries a poor to very poor prognosis in both cats and dogs. These patients have severe fluid and electrolyte abnormalities associated with systemic inflammatory disease, renal compromise, and a high risk of DIC. Intensive management is required, including plasma transfusions in many cases and enteral tube feeding or total parenteral nutrition in some (see next section). These patients will likely benefit from referral to a specialist. If referral is not an option, intensive therapy can be attempted in the practice, but the owner must be warned of the very poor prognosis and expense of treatment.
At the other end of the spectrum, patients with very mild pancreatitis (score 0) may simply need hospitalization for 12 to 24 hours of intravenous fluid therapy if they are vomiting and dehydrated; if they are alert and well-hydrated, they may be managed at home with 24 to 48 hours of pancreatic rest (fluids only by mouth) and analgesia followed by long-term feeding of an appropriate diet.
It is important to give consideration to the following aspects of treatment in all patients: intravenous fluid and electrolyte replacement; analgesia; nutrition; and other supportive therapy, as indicated, such as antiemetics and antibiotics.
Intravenous Fluids and Electrolytes
Intravenous fluid therapy is very important in all but the mildest cases of pancreatitis to reverse dehydration, address electrolyte imbalances associated with vomiting and fluid pooling in the hypomotile gastrointestinal tract, and maintain adequate pancreatic circulation. It is vital to prevent pancreatic ischemia associated with reduced perfusion because it contributes to necrosis. Replacement fluids (e.g., lactated Ringer’s or Plasmalyte) are usually used at rates and volumes that depend on the degree of dehydration and shock—twice maintenance (100 to 120 ml/kg/day) rates are adequate for mild to moderately affected animals (grades 0 and 1), but more severely affected animals may need initial shock rates (90 ml/kg/hour for 30 to 60 minutes) followed by synthetic colloids. It is important to measure urine output concurrently. Rapid crystalloid infusion in severely affected animals that have a pathological increase in vascular permeability carries an increased risk of pulmonary edema, so patients should be closely monitored; central venous pressure ideally should be measured in the most severely affected dogs.
Serum electrolyte concentrations should be carefully monitored. Potential electrolyte abnormalities are outlined in Table 40-4, but the most clinically important abnormality in most cases is hypokalemia caused by vomiting and reduced food intake. Hypokalemia can significantly impair recovery and contribute to mortality because it causes not only skeletal muscle weakness but also gastrointestinal atony, which will contribute to the clinical signs of the disease and delay successful feeding. Aggressive fluid therapy further increases renal potassium loss, particularly in cats, so it is important to measure serum potassium concentrations frequently (at least daily while the patient is vomiting) and add supplemental potassium chloride to the fluids as necessary. A scaled approach is best, based on the degree of hypokalemia. Lactated Ringer’s or Plasmalyte contains only 4 mEq/l potassium, and most cases require supplementing at least to replacement rates (20 mEq/l). Even if serum potassium concentration cannot be measured, a vomiting anorexic dog with no evidence of renal failure should receive replacement rates of potassium in the fluids. More severely hypokalemic dogs should be supplemented more, as long as serum concentrations can be regularly measured and infusion rates carefully controlled. A dog or cat with a serum potassium concentration of 2.0 mEq/l or less should receive between 40 and 60 mEq/l in the fluids at a controlled infusion rate. As a general rule, the infusion rate of potassium should still not be increased above 0.5 mEq/kg/hour.
A plasma transfusion is indicated in dogs and cats with severe pancreatitis (organ score 2 to 4) to replace α1antitrypsin and α2-macroglobulin. It also supplies clotting factors and may be combined with heparin therapy in animals at high risk of DIC, although the efficacy of heparin therapy in DIC in humans and animals has recently been questioned and there are no controlled trials that either support or refute its use in pancreatitis in dogs and cats at present.
Analgesia
Pancreatitis is usually a very painful condition in humans and animals. Hospitalized patients should therefore be monitored carefully for pain, and analgesia should be administered as necessary. In practice, analgesia is indicated in almost all patients with pancreatitis and should be given routinely to cats with pancreatitis because pain is difficult to assess in this species. Morphine agonists or partial agonists are often used, particularly buprenorphine. Morphine, meperidine, and fentanyl (intravenous or patches) can also be used (Table 40-8). Concerns that the effects of opiates on the sphincter of Oddi might exacerbate disease have often been cited with regard to dogs as well as humans, but more recent studies have suggested minimal clinically relevant effects, except when high and repeated doses of morphine are used; these drugs are regularly used now in humans with pancreatitis with no obvious problems. Fentanyl patches take time to achieve effect (on average, 24 hours in dogs and 7 hours in cats), so concurrent use of an opiate for the first few hours after application is recommended. Nonsteroidal antiinflammatory drugs (NSAIDs) should be avoided if possible because of the increased risk of gastroduodenal ulceration in patients with pancreatitis and also the potential of some NSAIDs to precipitate renal failure in animals with hypotension and/or shock. In people acute pancreatitis has been associated with the use of NSAIDs. Cyclo-oxygenase-2 inhibitors have a lower risk ratio than the conventional NSAIDs in this respect. Alternative analgesics that could be considered in severe cases include a low-dose intravenous ketamine infusion, which has the advantage of minimal effect on gastrointestinal motility (Bares et al., 1995) or intravenous lidocaine. Details of analgesia are given in Table 40-8.
Providing analgesia that can be dispensed for the client to take home in patients with milder or resolving disease can be a challenge. The pain should not be underestimated in these patients. However, it is difficult to find effective and safe analgesia that can be dispensed for use at home. Administration of opioids during visits to the clinic is wise, and one of the less ulcerogenic NSAIDs could be used cautiously at home. Cats can be effectively dosed with buprenorphine orally (Robertson et al., 2003), allowing simple home medication, but the oral route is not effective in dogs. Anecdotally, Tramadol has been found to be helpful in dogs. Feeding a low-fat diet helps reduce postprandial pain in humans and anecdotally helps some dogs significantly. However, administering pancreatic enzymes in the food does not seem to reduce pain in dogs, and there is little evidence in support of their use for pain relief in either dogs or cats.
Nutrition
It is very important to consider appropriate nutritional management of the patient with pancreatitis. Complete pancreatic rest by starvation, avoiding anything by mouth (including water or barium), has traditionally been advised for patients with acute pancreatitis. Initially, it was believed that early enteral nutrition was contraindicated because it was likely to result in cholecystokinin and secretin release, with consequent release of pancreatic enzymes and worsening of pancreatitis and associated pain. Total parenteral nutrition (TPN) seemed a more logical route early in the disease process, with jejunal tube feeding later in the disease aiming to bypass the areas of pancreatic enzyme stimulation. However, recent studies have suggested that early enteral nutrition is preferable to TPN, and current best practice in human medicine is outlined in Box 40-1 along with relevance to veterinary patients. It is no longer appropriate or acceptable to starve the patient for days and days while awaiting resolution of disease. Increasing evidence is accumulating in human medicine of the importance of early enteral nutrition in patients with pancreatitis, and emerging work in humans suggests that immunomodulating nutrients may also be of benefit. There are no studies evaluating the efficacy of early or late enteral or parental nutrition in naturally occurring pancreatitis in dogs or cats. Therefore the advice currently given is based on anecdotal evidence, extrapolation from humans, and on experimental studies in dogs only.
 BOX 40-1 Best Practice for Feeding Patients with Acute Pancreatitis
BOX 40-1 Best Practice for Feeding Patients with Acute Pancreatitis
Recent studies and metaanalyses of studies of nutrition in human acute pancreatitis have led to changes in advice for best-practice feeding in these cases (Meier and Beglinger, 2006). Note that early enteral nutrition is particularly indicated in severe disease, which is perhaps unexpected and counter to our current practice in dogs.
However, early feeding of an appropriate diet is now indicated in dogs. In addition, starvation is contraindicated in cats because of the high risk of hepatic lipidosis. The current advice is therefore to institute some form of enteral feeding, whenever possible, within 48 hours in both dogs and cats. The more severe the disease, the more important it is to feed early. In severe cases this is best achieved with jejunostomy tube feeding by continuous infusion of an elemental diet, although frequent small-volume feeds of a low-fat food via a gastrostomy tube is also well tolerated in most dogs and cats with moderate pancreatitis. A good initial choice is baby rice mixed with water followed by a low-fat proprietary veterinary diet (such as Eukanuba Intestinal Formula; Hill’s i/d; Royal-Canin-Waltham Digestive low fat or Purina EN-formula) (Fig. 40-7). Concurrent antiemetics are also essential to allow effective feeding in many cases (see next section). In patients in which enteral nutrition is not possible or when only a small percentage of the daily caloric requirements can be given enterally, some form of supplemental parenteral nutrition should be considered. This is most practically administered as peripheral parenteral nutrition (see Chandler et al., 2000).
Antiemetics
Antiemetics are often necessary to manage acute vomiting in dogs and cats with pancreatitis. Metoclopramide has been used successfully in dogs with pancreatitis (0.5 to 1 mg/kg, administered intramuscularly, subcutaneously, or orally three times a day, or 1 to 2 mg/kg, administered intravenously over 24 hours as a slow infusion), but its effect on stimulating gastric motility may increase pain and pancreatic enzyme release in some animals. A phenothiazine antiemetic such as chlorpromazine may be more effective in some patients, but phenothiazines have sedative and hypotensive effects, which may be particularly marked if they are used together with opioid analgesia, so care should be taken in these cases. 5-HT3 receptor antagonists such as ondansetron are useful in other forms of vomiting in dogs (such as chemotherapy-induced emesis) but are best avoided in pancreatitis because they have occasionally been reported to trigger pancreatitis in humans. The newly available NK1 receptor antagonist maropitant, licensed for use in dogs, has both central and peripheral antiemetic effects and is showing promise as an antiemetic in dogs with pancreatitis, although it is not licensed for use in cats. (Maropitant is available as Cerenia (Pfizer) in either an injectable solution (10 mg/ml) or tablets (16 mg, 24 mg, and 60 mg). The dose of injection is 1 mg/kg (i.e., 1 ml per 10 kg body weight once a day for up to 5 days). The dose of the tablets is 2 mg/kg once a day for up to 5 days.
Gastroprotectants
Patients with acute pancreatitis have an increased risk of gastroduodenal ulceration caused by local peritonitis; they should be monitored carefully for evidence of this (melena, hematemesis) and treated as necessary with sucralfate and acid secretory inhibitors (H2 blockers such as cimetidine, famotidine, ranitidine, or nizatidine or the proton pump inhibitor omeprazole). Cimetidine should be avoided in animals with concurrent liver disease because of its effect on the cytochrome P450 system. Ranitidine can be used instead in these animals, but its additional gastric prokinetic effect can cause vomiting in some individuals; it should be discontinued if this occurs. Because famotidine does not have these prokinetic effects, it may be preferable.
Antibiotics
Infectious complications are reportedly rare in dogs and cats with pancreatitis, but when they occur, they can be serious; antibiotic therapy has been shown to improve survival in such cases in humans. It is therefore advisable to use broad-spectrum antibiotics in dogs and cats with acute pancreatitis because it is not always possible to assess the occurrence or risk of septic complications. Fluroquinolones or potentiated sulphonamides have been used in humans because they penetrate the pancreas well and are effective against most human bacterial isolates from this region. However, because potentiated sulphonamides are potentially hepatotoxic, they are best avoided if there is concurrent hepatic involvement; fluroquinolones are effective against only aerobes, so combination with another antibiotic with action against anaerobes, such as metronidazole or amoxicillin, may be necessary. Metronidazole has the added benefit of being beneficial if there is concurrent inflammatory bowel disease or small intestinal bacterial overgrowth secondary to intestinal ileus.
Treatment of Biliary Tract Obstruction Associated with Pancreatitis
Most cases of extrahepatic biliary obstruction secondary to acute-on-chronic pancreatitis resolve with conservative management, and surgical or needle decompression of the gallbladder and stenting of the bile duct are usually unnecessary in dogs and cats. In humans it has now been demonstrated that there is no advantage to surgical intervention in most patients and no difference in the severity and chronicity of secondary liver disease between those treated medically and those treated surgically, provided the jaundice resolves within a month (Addallah et al 2007). No such study has been done in small animals, so treatment advice has to be empirical: If the feces remain colored (not white or acholic, which implies complete biliary obstruction) and the jaundice gradually resolves over a week to 10 days, then surgical intervention is not indicated and conservative management with antioxidants and ursodeoxycholic acid are advised (see Chapters 37 and 38).
CHRONIC PANCREATITIS
Etiology and Pathogenesis
Chronic pancreatitis is defined as “a continuing inflammatory disease characterized by the destruction of pancreatic parenchyma leading to progressive or permanent impairment of exocrine or endocrine function or both.” The gold standard for diagnosis is histology (see Fig. 40-2), but this is rarely indicated or performed in dogs or cats. Noninvasive diagnosis is difficult with the currently available diagnostic imaging, and blood tests have a lower sensitivity than for acute disease.
Chronic pancreatitis has been considered a rare and not particularly important disease in dogs, whereas it is recognized as the most common form of pancreatitis in cats. However, the early literature published on canine pancreatic disease in the 1960s and 1970s recognized it as a common disease of clinical significance. It was noted that a high proportion of cases of EPI in dogs were caused by chronic pancreatitis and also that it might be responsible for up to 30% or more of cases of diabetes mellitus (DM). More recent pathological and clinical studies in both dogs (Newman et al., 2004; Watson et al., 2007) and cats (DeCock et al., 2007) have reconfirmed it as a common and clinically relevant disease in both dogs and cats. It is likely to cause intermittent and/or ongoing recurrent gastrointestinal signs and epigastric pain in a high number of dogs and cats, but it is frequently underrecognized because of the difficulty of obtaining a noninvasive diagnosis. In dogs the postmortem prevalence of chronic pancreatitis is up to 34%, particularly in susceptible breeds, and even in studies of fatal acute pancreatitis, acute-on-chronic disease accounts for 40% of cases. In cats an even higher postmortem prevalence of chronic pancreatitis of 60% has been reported. It must be noted that postmortem studies tend to overestimate the prevalence of chronic diseases, which leave permanent architectural changes in the organ, whereas the prevalence of acute, totally reversible diseases will be underestimated, unless the animal dies during the episode. Nevertheless, it is clear that there are many more cases of chronic pancreatitis in veterinary practice than currently recognized and that a number of these are clinically relevant.
Idiopathic Chronic Pancreatitis
As in acute pancreatitis, the cause of chronic pancreatitis in dogs is usually unknown (see Table 40-3). Any age or breed of dog can be affected, but the most typical signalment is a middle-aged to old dog, particularly a Cavalier King Charles Spaniel, Cocker Spaniel, Collie, or Boxer in the U.K. (Watson et al., 2007; Fig. 40-8). The breed prevalence in the U.S. has not been investigated, but an independent large study of EPI in the U.K. found an increased prevalence in older Cavalier King Charles Spaniels, supporting this breed association. Other parts of the world have also reported a high incidence in arctic-type breeds such as Huskies. There is likely to be some overlap with acute disease, although some cases will have a separate etiology. Some cases may represent chronic relapsing cases of acute disease, but many cases are truly chronic from the outset, with an initial mononuclear infiltrate. Genetic causes are likely to be important in dogs, which explains the increased risk in certain breeds.
No particular breed prevalence has been reported for cats with chronic pancreatitis, and domestic shorthairs are most commonly affected.
Autoimmune Chronic Pancreatitis
The particular form of chronic pancreatitis recognized in English Cocker Spaniels in the U.K. is thought to be an autoimmune disorder (Watson et al., 2006b; see Fig. 40-8). As in human autoimmune pancreatitis, it typically affects middle-aged to older dogs, with a higher prevalence in males, and at least 50% of affected dogs subsequently develop DM, EPI, or both. Dogs also often have other concurrent autoimmune disease, particularly keratoconjunctivitis sicca. There is often a mass-like lesion on ultrasound (see Fig. 40-6, B), and biopsies show a typical perilobular diffuse fibrotic and lymphocytic disease centered on perilobular ducts and vessels, with loss of large ducts and hyperplasia of smaller ducts. Immunohistochemistry shows a preponderance of duct and vein-centered CD3+ lymphocytes (i.e., T-cells). The human disease is believed to be a duct-centered immune reaction and responds to steroid therapy, including a reduction in insulin requirement in some diabetics. This is clearly differentiated from the proposed autoimmunity in young German Shepherd Dogs with pancreatic acinar atrophy, which is acinar-centered and does not result in DM (discussed in more detail later). There are not yet any controlled trials evaluating the use of immunosuppressive drugs in English Cocker Spaniels with chronic pancreatitis, but there is now enough circumstantial evidence to justify their use in this particular breed. However, the clinician should note that this is very breed specific; terriers in the U.K., for example, have a very different histopathological and clinical picture of disease that does not appear to be autoimmune, and the use of steroids in terriers with chronic pancreatitis is not recommended.
Clinical Features
Dogs with chronic pancreatitis, regardless of the cause, most commonly present with mild intermittent gastrointestinal signs. Typically, they have bouts of anorexia, occasional vomiting, mild hematochezia, and obvious postprandial pain, which often goes on for months to years before a veterinarian is consulted. The trigger for finally seeking veterinary attention is often an acute-on-chronic bout or the development of DM or EPI. The main differential diagnoses in the low-grade cases are inflammatory bowel disease and primary gastrointestinal motility disorders. Dogs may become more playful and less picky with their food when they are switched to a low-fat diet, which suggests that they previously had postprandial pain. Chronic epigastric pain is a hallmark of the human disease and is sometimes severe enough to lead to opiate addiction or surgery, so it should not be overlooked or underestimated in small animal patients. In more severe, acute-on-chronic cases, the dogs are clinically indistinguishable from those with classical acute pancreatitis (see preceding section), with severe vomiting, dehydration, shock, and potential multiorgan failure. The first clinically severe bout tends to come at the end of a long (often years) subclinical phase of quietly progressive and extensive pancreatic destruction in dogs. It is very important for clinicians to be aware of this because these dogs are at much higher risk for developing exocrine and/or endocrine dysfunction than those with truly acute pancreatitis; in addition, they usually already have protein-calorie malnutrition at presentation, which makes their management even more challenging. It is also relatively common for dogs with chronic pancreatitis to first present with signs of DM and a concurrent acute-on-chronic bout of pancreatitis resulting in a ketoacidotic crisis. In some dogs there are no obvious clinical signs until the development of EPI, DM, or both. The development of EPI in a middle-aged to older dog of a breed not typical for pancreatic acinar atrophy has to increase the index of suspicion for underlying chronic pancreatitis. The development of EPI or DM in a dog or cat with chronic pancreatitis requires the loss of approximately 90% of exocrine or endocrine tissue function, respectively, which implies considerable tissue destruction and end-stage disease.
In cats the clinical signs of chronic pancreatitis are usually very mild and nonspecific. This is not surprising considering that cats display mild clinical signs, even in association with acute necrotizing pancreatitis. One study showed that the clinical signs of histologically confirmed chronic nonsuppurative pancreatitis in cats were indistinguishable from those of acute necrotizing pancreatitis (Ferreri et al., 2003). However, chronic pancreatitis in this species is significantly more often associated with concurrent disease than acute pancreatitis, particularly inflammatory bowel disease, cholangiohepatitis, hepatic lipidosis, and/or renal disease. The clinical signs of these concurrent diseases may predominate, further confusing diagnosis. Nevertheless, some cats will eventually develop end-stage disease with resultant EPI and/or DM.
Chronic pancreatitis is the most common cause of extrahepatic biliary obstruction in dogs (see Chapter 38), and dogs and cats with acute-on-chronic pancreatitis frequently develop jaundice.
NonInvasive Diagnosis
In the absence of a biopsy, which is the gold standard, the clinician must rely on a combination of clinical history, ultrasonography, and clinical pathology. The findings on diagnostic imaging and clinical pathology are similar to those outlined in the section on acute pancreatitis and Tables 40-4 and 40-5. However, changes tend to be less marked in dogs and cats with chronic pancreatitis, and the diagnostic sensitivity of all tests is lower. Ultrasonography has a lower sensitivity in dogs and cats with chronic disease because there is less edema than in those with acute disease. A variety of ultrasonographic changes may be seen in patients with chronic pancreatitis, including a normal pancreas, a mass lesion, a mixed hyperechoic and hypoechoic appearance to the pancreas, and sometimes an appearance resembling that of classical acute pancreatitis with a hypoechoic pancreas and a bright surrounding mesentery (Watson et al 2006b; see Fig. 40-6). In addition, in patients with chronic disease adhesions to the gut may be apparent, and the anatomy of the pancreatic and duodenal relationship may be changed by these adhesions. Some patients (particularly Cocker Spaniels) have large mass-like lesions associated with fibrosis and inflammation; some cases have tortuous and dilated, irregular ducts; and many cases have completely normal pancreatic ultrasonographic findings in spite of severe disease.
Likewise, clinical pathology can be helpful, but the results may also be normal. Increases in pancreatic enzyme acitivities are most likely to be seen during an acute-on-chronic bout than during a quiescent phase of disease (very similar to the waxing-and-waning increases in liver enzyme activities in patients with ongoing chronic hepatitis). Again, similar to the situation in hepatic cirrhosis, in end-stage chronic pancreatitis there may not be enough pancreatic tissue left to produce increases in enzyme activities, even in acute flare-ups. On the other hand, occasionally serum TLI can temporarily increase into or above the normal range in dogs with EPI as a result of end-stage chronic pancreatitis, confusing the diagnosis of EPI in these dogs. cPLI appears to have the highest sensitivity for the diagnosis of canine chronic pancreatitis, but even this has a lower sensitivity than in acute disease. The diagnostic sensitivity of feline PLI for chronic pancreatitis in cats is unknown.
It is important to measure serum B12 concentrations in dogs and cats with chronic pancreatitis. The gradual development of EPI, combined often with concurrent ileal disease particularly in cats, predisposes to cobalamin deficiency, as outlined in the section on EPI. If serum B12 concentration is low, cobalamin should be supplemented parenterally (0.02 mg/kg, administered intramuscularly or subcutaneously every 2 weeks in dogs and cats until serum concentration is normalized).
Biopsy
The diagnosis of chronic pancreatitis can be very difficult in dogs and cats, and difficulties in diagnosis likely result in under-recognition of the disease. Establishing a definitive diagnosis relies on obtaining a pancreatic biopsy. However, this will not be indicated in most cases until there are effective treatments because a biopsy is a relatively invasive procedure, the results of which do not alter treatment or outcome. However, with the potential for some more specific therapies, routine biopsy may be indicated in the future. In humans the preferred method is needle-biopsy via transendoscopic ultrasonographic guidance. Transendoscopic ultrasonography is very expensive and of limited availability in veterinary medicine, so in dogs and cats surgical or laparo scopic biopsies remain the most applicable. Cytology of ultrasound-guided transcutaneous fine needle aspirates of the pancreas may help differentiate neoplasia or dysplasia from inflammation, but veterinary experience in this area is very limited. If the clinician is performing a laparotomy to obtain other biopsies, it makes perfect sense to obtain a pancreatic biopsy at that time as well. Pancreatitis is not a risk, provided the pancreas is handled gently and the blood supply is not disrupted. However, the biopsy should be small and from the tip of a lobe and may therefore miss the area of disease, which is usually patchy, particularly early on, and can also be centered on large ducts. Unfortunately therefore, even biopsy has its limitations.
Treatment and Prognosis
Dogs and cats with chronic, intermittent pancreatitis may have intermittent bouts of mild gastrointestinal signs and anorexia, and the owner’s primary concern is often that the pet has missed a meal. These animals can be managed at home, as long as anorexia is not long lasting, and the owner should be reassured that a short period of self-induced starvation is actually beneficial because it provides pancreatic rest.
As in patients with acute pancreatitis, the current preference is for symptomatic treatment. Dogs and cats with acute flare-ups require the same intensive treatment as dogs and cats with classical acute pancreatitis and have the same risk of mortality (see preceding section). The difference from isolated acute pancreatitis is that if the animal recovers from the acute bout, it is likely to remain with considerable exocrine and/or endocrine functional impairment. In the milder cases symptomatic treatment can make a real difference in the animal’s quality of life. Changing to a low-fat diet (such as Hill’s ID, Royal-Canin-Waltham Digestive low fat, or Eukanuba Intestinal) apparently reduces postprandial pain and acute flare-ups in many cases. Owners often underestimate the effects of fatty treats, which can precipitate recurrences in susceptible individuals. Some animals need analgesia, either intermittently or continuously (see section on acute pancreatitis and Table 40-8). According to anecdotal reports, short courses of metronidazole (10 mg/kg, PO q12h) seem to help some patients after acute bouts—presumably because they develop secondary bacterial overgrowth as a result of a “stagnant loop” phenomenon in the adjacent duodenum. Serum B12 concentration should be measured regularly, and cobalamin should be supplemented parenterally as necessary (0.02 mg/kg, administered intramuscularly 2 to 4 weeks until serum concentration normalizes).
Treatment of extrahepatic biliary tract obstruction associated with acute-on-chronic disease should be as outlined in the acute pancreatitis section.
In patients with end-stage disease, exocrine and/or endocrine deficiency may develop. Dogs and cats with EPI and/or DM are managed with enzymes (discussed in more detail later) and insulin as necessary in the usual way (see Chapter 52), and most do surprisingly well long term.
EXOCRINE PANCREATIC INSUFFICIENCY
EPI is a functional diagnosis that results from a lack of pancreatic enzymes. As such, unlike pancreatitis, it is diagnosed on the basis of clinical signs and pancreatic function tests and not primarily the results of pancreatic histopathology, although finding a marked reduction in pancreatic acinar mass on histology is supportive of a diagnosis of EPI. The pancreas is the only significant source of lipase, so fat maldigestion with fatty feces (steatorrhea) and weight loss are the predominant signs of EPI.
Pathogenesis
Pancreatic acinar atrophy (PAA) is believed to be the predominant cause of EPI in dogs, but recent work has shown that end-stage chronic pancreatitis is also important in this species (Fig. 40-9; Watson and Herrtage, 2006a; Batchelor et al., 2007a). PAA has not been recognized in cats; end-stage pancreatitis is the most common cause of feline EPI (Fig. 40-10). The development of clinical EPI requires approximately a 90% reduction in lipase production and thus extensive loss of pancreatic acini. It is therefore extremely unlikely to occur after a severe bout of pancreatitis; it tends to result from chronic, ongoing disease. However, the chronic disease may be largely subclinical or only present as occasional clinical acute-on-chronic episodes, so the degree of underlying pancreatic damage may be underestimated.
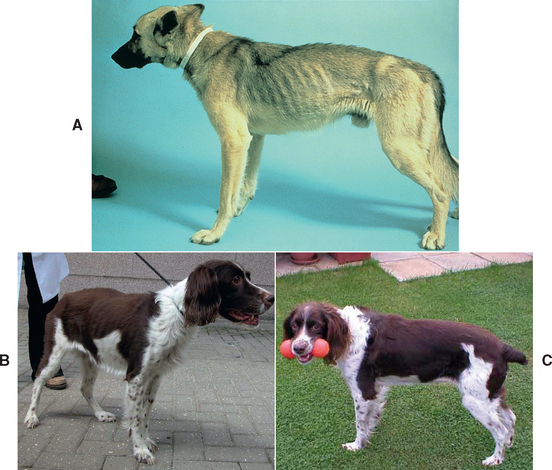
FIG 40-9 A, Physical appearance of a 2-year-old male German Shepherd Dog with exocrine pancreatic insufficiency (EPI). B, An 11-year-old neutered female English Springer Spaniel with EPI caused by end-stage chronic pancreatitis. This dog also had diabetes mellitus (DM) but was still losing weight in spite of good control of the DM. EPI had not initially been suspected, but once it was diagnosed and treated with enzyme supplements, the dog returned to normal weight and coat condition within 6 months (C).
(A, Courtesy Dr. William E. Hornbuckle, Cornell University, College of Veterinary Medicine. B, From Journal of Small Animal Practice vol. 44, 2003.)
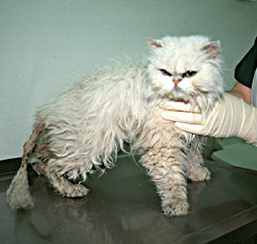
FIG 40-10 A middle-aged Persian cat with end-stage chronic pancreatitis and exocrine pancreatic insufficiency. Note matting of coat with feces and poor body condition.
PAA is particularly recognized in young German Shepherd Dogs (see Fig. 40-9, A), in which an autosomal mode of inheritance has been demonstrated, and has also been described in Rough Collies, suspected in English Setters, and sporadically reported in other breeds. A recent large study of EPI in the U.K. reported that young Chow Chows were overrepresented (Batchelor et al., 2007a). The pathogenesis was unknown, but the juvenile onset suggested PAA or perhaps a congenital defect in this breed.
Histological studies in German Shepherd Dogs suggest that PAA is an autoimmune disease directed against the acini (Wiberg et al., 2000). Therefore the islets are spared, and dogs with PAA are not typically diabetic. However, affected dogs do not respond to immunosuppressive therapy. Most dogs develop the disease in young adulthood, but a proportion of German Shepherd Dogs remain subclinical for a prolonged period of time and present only late in life.
In contrast, many dogs with end-stage chronic pancreatitis also develop DM either before or after EPI as a result of concurrent islet cell destruction (Watson, 2003; Watson et al., 2006a). The situation is similar in cats with end-stage chronic pancreatitis. There is no breed relationship in cats, but dogs with EPI as a result of end-stage chronic pancreatitis tend to be middle-aged to older medium- or small-breed dogs, particularly Cavalier King Charles Spaniels, English Cocker Spaniels, and Collies (see Fig. 40-8). Interestingly, although Boxers in the U.K. were reported to have an increased prevalence of chronic pancreatitis in one study, they have also been reported to be significantly under represented among dogs with EPI, which suggests that their chronic pancreatitis does not progress to end-stage disease. Other underrepresented breeds in a large study of EPI were Golden Retrievers, Labrador Retrievers, Rottweilers and Weimaraners (Batchelor et al., 2007a). Finding compatible clinical signs in these breeds should first trigger a search for other possible causes, such as chronic infections or inflammatory bowel disease.
Other causes of EPI in dogs and cats are pancreatic tumors, hyperacidity of the duodenum inactivating lipase, and isolated enzyme (particularly lipase) deficiency. These are all rare causes. Patients with pancreatic tumors usually present for other reasons, but tumors can result in EPI owing to a combination of compression of pancreatic ducts by the mass, destruction of acinar tissue, and associated pancreatitis.
Up to 70% of dogs with EPI have concurrent small intestinal bacterial overgrowth (SIBO). This will contribute to clinical signs and should be considered when treating an affected dog. In SIBO bacteria deconjugate bile salts, thus decreasing fat emulsification and therefore fat digestion. Bacteria also break down the undigested fat to hydroxy fatty acids. These and deconjugated bile salts irritate the colonic mucosa and may cause large intestinal diarrhea by stimulating secretion. Dogs with EPI therefore tend to present with signs of both small and large bowel diarrhea.
A high proportion of dogs (particularly those presenting with low body condition scores) also have reduced duodenal enzyme activity, which may be partly due to the SIBO but also to the effects of malnutrition on the gut and possibly to the loss of the trophic influence of pancreatic secretions. Cobalamin deficiency is common in both dogs and cats with EPI and seems to be a negative prognostic indicator in dogs if untreated (Batchelor et al 2007b). Vitamin B12 is absorbed from the distal ileum using a carrier-mediated process that requires the vitamin to be bound to intrinsic factor (IF). The latter is produced entirely by the pancreas in cats and mainly by the pancreas in dogs, although the canine stomach can also produce a small amount. Therefore most cats with EPI are expected to be B12-deficient, whereas most but not all of dogs with EPI have hypocobalaminemia. In one large study of dogs with EPI, 82% of dogs had low serum cobalamin concentration (Batchelor et al 2007b). In cats with end-stage pancreatitis, the hypocobalaminemia is compounded by the high prevalence of concurrent inflammatory bowel disease, which often decreases ileal absorption of vitamin B12. Cobalamin deficiency causes villous atrophy and reduced gastrointestinal function, weight loss, and diarrhea in cats; therefore it is important not only to document hypocobalaminemia but also to treat it with parenteral B12 injections (0.02 mg/kg, administered intramuscularly 2 to 4 weeks until serum concentration normalizes).
Clinical Features
Most dogs and cats with EPI present because of chronic diarrhea and emaciation in tandem with a ravenous appetite (see Fig. 40-9). The diarrhea tends to be fatty (steatorrhea) because of prominent fat maldigestion but is variable from day to day and among individuals. Sometimes diarrhea is not a prominent feature because digestion is interrupted so early in the process that the osmotic effect of molecules is relatively small. Affected dogs and cats also often have chronic seborrheic skin disease resulting from deficiency of essential fatty acids and cachexia, and some patients present to a dermatology clinic for this reason. If EPI is due to chronic pancreatitis, the diagnosis may be complicated by concurrent ongoing pancreatitis that may cause intermittent anorexia and vomiting. Animals with end-stage chronic pancreatitis may also develop DM either before or months to years after the development of EPI.
Concurrent diseases are common in dogs with EPI, either related or unrelated to the pancreatic deficiency. In one study in dogs concurrent gastrointestinal, skeletal, and skin conditions were common (Batchelor et al 2007b). Cats with pancreatitis often have concurrent cholangitis and/or inflammatory bowel disease, and it is often difficult to differentiate the clinical signs of the three conditions because they are so similar.
ROUTINE CLINICAL PATHOLOGY
CBCs and serum biochemistry profiles are often normal in dogs and cats with EPI. In very cachectic animals there may be subtle nonspecific changes consistent with malnutrition, negative nitrogen balance, and breakdown of body muscle such as low albumin and globulin concentrations, mildly increased liver enzyme activities, low cholesterol and triglyceride concentrations, and lymphopenia.
Finding marked hypoproteinemia or more severe changes on the CBC and biochemistry profiles in an animal with EPI should trigger a search for another concurrent disease. Cats and dogs with end-stage pancreatitis may present with more severe secondary clinicopathologic changes, as outlined in the pancreatitis section. A high percentage of these patients with end-stage pancreatitis (up to 50%) also have concurrent DM, so they have clinicopathological changes typical of DM (see Chapter 52).
PANCREATIC ENZYMES
The diagnosis of EPI in dogs and cats relies on demonstrating reduced pancreatic enzyme output. The most sensitive and specific way of doing this is by measuring reduced circulating enzyme activity. Blood tests that indirectly measure gut enzyme activity, such as the BT-PABA test, are now rarely used because they have been replaced by the specific immunoassays for serum activities of pancreatic enzymes. Readers who would like more information on the BT-PABA test are referred to Batt et al. (1981). The plasma turbidity test, used historically after feeding a high-fat meal, with and without pancreatic enzymes, had a very low sensitivity and specificity for EPI and has been completely superseded by the enzymatic test.
Measurement of reduced TLI in the blood has a high sensitivity and specificity for the diagnosis of EPI in dogs and cats and is currently the single test of choice for the diagnosis of EPI in small animals. It is important to measure it on a fasting sample because the release of pancreatic enzymes associated with feeding can raise the levels in the serum. It is not necessary to stop exogenous pancreatic enzyme supplementation before measuring TLI because exogenous enzymes should not be absorbed from the gut into the circulation; even if they are, the test is an immunoassay that does not cross-react with the tryspin/trypsinogen of other species in the supplement. However, there are some problems in interpreting the results, as listed in Box 40-2.
 BOX 40-2 Interpretation of TLI Results in the Diagnosis of Canine Exocrine Pancreatic Insufficiency
BOX 40-2 Interpretation of TLI Results in the Diagnosis of Canine Exocrine Pancreatic Insufficiency
Note: A TLI stimulation test could be used in animals with subclinical EPI (low TLI but no clinical signs) or animals with a TLI persistently in the grey area. Pancreatic enzyme output is stimulated either with intravenous cholecystokinin and secretin or with a test meal, and TLI concentrations are measured before and after stimulation (Wiberg et al., 1999). Animals with true clinical EPI show no stimulation, whereas animals with subclinical EPI still have enough enzyme activity to increase their TLI after stimulation. The value of a stimulation test in clinical cases is limited because the decision to treat is based on the clinical signs. It is of more value in monitoring progression of disease for clinical research.EPI, Exocrine pancreatic insufficiency; PAA, pancreatic acinar atrophy.
Unlike in humans, amylase and lipase activities are not consistently low in dogs and cats with EPI because of the high background levels of enzymes from other organs. A low cPLI also has a good sensitivity and specificity for the diagnosis of EPI in dogs (Steiner et al., 2001). However, this test is not superior to TLI. PLI is also likely to be low in cats with EPI.
Fecal tests for EPI are rarely used because of low sensitivity and specificity compared with serum tests. Measuring fecal trypsin activity has a very low sensitivity and specificity for EPI, as do assessment of fecal proteolytic activity or microscopic examination of feces for undigested fat, starch, and muscle fibers. All these tests have been superseded by measurement of serum TLI and cPLI. Measurement of fecal elastase may have some utility in dogs with EPI as a result of chronic pancreatitis or duct blockage, in which TLI results may be misleading. Elastase appears to have higher sensitivity and specificity than the other fecal tests for the diagnosis of EPI in dogs. Elastase is a pancreatic enzyme, and a species-specific ELISA for canine elastase has been developed and is available for commercial use in dogs (Spillman et al., 2000 and 2001). As with canine TLI, because there is no cross-reaction with elastase from other species, dogs can remain on enzyme supplementation while the test is performed. There is marked variation in elastase levels in normal canine feces compared with humans. The sensitivity and specificity of the test are improved by taking three separate fecal samples on three days or using a cut-off value for diagnosis of EPI, which is below this variation in most dogs.
OTHER DIAGNOSTIC TESTS
It is also advisable to measure serum cobalamin concentration in animals with EPI because cobalamin concentration is often reduced because of a deficiency of pancreatic intrinsic factor, as previously explained. If serum B12 concentration is low, it should be supplemented parenterally. (0.02 mg/kg, administered intramuscularly every 2 to 4 weeks until serum concentration normalizes).
Serum folate concentrations are high in about a third of dogs with EPI. This may indicate SIBO, although the sensitivity and specificity of high serum folate concentration for the diagnosis of SIBO is poor. The definition and diagnosis of SIBO is problematic, and it is better to assume that a newly diagnosed dog with EPI has SIBO and treat it appropriately than to rely on the results of diagnostic tests. The importance of SIBO in cats with EPI is unknown. Occasionally in dogs and cats with EPI, serum folate concentration may be low; this can suggest either dietary deficiency or concurrent inflammatory or infiltrative disease in the jejunum. Unlike cobalamin, there is no clear evidence that folate should be supplemented in dogs when it is low.
DRUGS
All dogs and cats with clinical EPI require enzyme supplementation for life. In most cases this is provided as a powder or in the form of a capsule, which is opened and then sprinkled on the food. Fresh raw pancreas (which can be frozen in aliquots) may be used as an alternative and can be very effective, but there is also the potential for acquiring gastrointestinal infections (such as Salmonella and Campylobacter). The dose of enzymes is initially as recommended by the manufacturer and then titrated to the individual. A large proportion of enzyme activity is lost in the acid pH of the stomach (up to 83% of lipase activity and 65% of trypsin activity). To overcome this, either the dose of enzymes given is increased or an H2 blocker is administered concurrently to increase gastric pH. Preincubating enzymes with the food is not indicated because they require the alkaline environment of the small intestine to work properly. In the long term it is often possible to decrease the dose of enzymes given (but not stop altogether). This may be due to resolution of the secondary bacterial overgrowth and the effects of chronic malnutrition and cobalamin deficiency on enterocytes and brush border enzymes. Reports suggest that enzyme replacement may be reduced over the long term by between 6% and 58% but not stopped altogether.
Dogs and cats with EPI and concurrent SIBO require courses of appropriate antibiotics (oxytetracycline, tylosin, or metronidazole). It is advisable to administer prophylactic medication for presumed SIBO in all newly diagnosed cases for 3 to 4 weeks in view of the high prevalence of concurrent bacterial overgrowth and the difficulty in diagnosing it, although it remains unclear whether initial antibiotic therapy improves prognosis.
Dogs and cats with documented hypocobalaminemia will require parenteral vitamin B12 injections (0.02 mg/kg, administered intrumuscularly every 2 to 4 weeks until serum concentration normalizes). It is relatively common for German Shepherd Dogs with PAA to have concurrent inflammatory bowel disease, and this must also be addressed. Animals with EPI as a result of chronic pancreatitis may require insulin therapy for concurrent DM and other therapies for acute flare-ups, including analgesics, as outlined in the section on pancreatitis.
DIET
Disruption of fat digestion is the most important feature of EPI. A low-fat food has therefore been traditionally recommended, but it may not contain enough calories to feed a large-breed dog (e.g., a German Shepherd Dog) effectively. Fat usually contributes a significant proportion of daily energy intake because it is more energy-dense than carbohydrates. In large-breed dogs with EPI and cachexia, weight gain may be very difficult to achieve with a low-fat diet. There is no convincing evidence in the literature that long-term feeding of a low-fat diet improves outcome in dogs with PAA, although there is some evidence that it may result in faster resolution of clinical signs. However, high-fat diets (such as proprietary renal diets) should obviously be avoided. We therefore recommend that dogs with PAA be fed a normal to moderately fat-restricted, highly digestible diet with reasonable calorie density. The diet should also be low in fiber because fiber impairs the activity of pancreatic enzymes and soluble fiber may actually absorb pancreatic enzymes. Fiber may also reduce small intestinal absorption and activity of brush border enzymes. The proprietary veterinary diets marketed for gastrointestinal disease in dogs (e.g., Hill’s ID, Royal-Canin Digestive low fat HE, and Eukanuba Intestinal or Dermatosis FP) fulfill these requirements and are recommended, at least for initial stabilization. In the long term, after the gut wall recovers, these dogs can be maintained on a normal fat level in most cases and can often return to their normal diet. In some individuals with PAA extra calories can be added to the diet between meals in the form of medium chain triglycerides, such as coconut oil. They should not be used in cats and should not be given in overly high doses in dogs because of the risk of osmotic diarrhea. The recommended daily amount is 1/4 to 4 teaspoons in dogs in divided doses. Medium chain triglycerides also cannot carry fat-soluble vitamins, will cause vomiting in some individuals, and are contraindicated in dogs with liver disease because they may worsen encephalopathy.
In dogs with EPI as a result of CP, dietary advice is slightly different. Many of these dogs benefit from long-term feeding of a low-fat diet, which seems to reduce postprandial pain and acute flare-ups of disease (Hill’s ID; Royal-Canin Digestive low fat, or Eukanuba Intestinal). Therefore proprietary low-fat diets would be preferred in these patients. The use of medium chain triglycerides is not recommended in dogs with chronic pancreatitis, but fortunately these are often small-breed dogs with less cachexia than the German Shepherd Dogs with PAA.
It is best to feed two or more meals a day, each with enzymes added, and the dog should not be allowed to scavenge. This is often difficult because they are polyphagic, but scavenging, especially of fatty foods, causes recurrence of diarrhea and sets back recovery.
Cats with EPI are often best managed on a hypoallergenic intestinal type diet (e.g., Hill’s DD, Eukanuba dermatosis LB, or Royal-Canin limited ingredient diets) because there is a high incidence of concurrent inflammatory bowel disease. If they are also diabetic, it is unclear whether they should be fed an intestinal diet or a proprietary feline diabetes diet (e.g., Hill’s MD, Royal-Canin diabetic diet, or Purina DM).
Prognosis
The prognosis for dogs with EPI is good because the disease can be treated. However, a surprising number of dogs (19% in one recent study) are euthanized within the first year of treatment because of poor response to therapy (Batchelor et al., 2007b). The same study showed that the median survival time of dogs that responded to treatment was very good, at 1919 days. This underlines the importance of scheduling regular follow-up appointments, particularly in the initial stages of therapy, to evaluate progress and change management as necessary. Prognosis for dogs and cats with EPI as a result of end-stage chronic pancreatitis is surprisingly good in most cases, even if it is complicated by concurrent DM, with survival times of several years in most cases.
EXOCRINE PANCREATIC NEOPLASIA
Neoplasms of the exocrine pancreas are uncommon in both cats and dogs. Pancreatic adenocarcinomas have a very aggressive biological behavior and have usually disseminated widely by the time of diagnosis. They are often subclinical until they have disseminated, but they can result in single or repeat bouts of pancreatitis and/or the development of EPI. Some pancreatic tumors have been associated with paraneoplastic syndromes such as sterile panniculitis in dogs, alopecia with shiny skin in cats, and hypercalcemia. Chronic pancreatitis is a risk factor for the development of pancreatic adenocarcinomas in humans, and this may also be true in dogs because the published reports of these tumors in dogs show a predominance of Cocker and Cavalier King Charles spaniels.
Pancreatic adenomas are rare in small animals but have been reported in cats. Nodular hyperplasia of the exocrine pancreas is also common in older dogs and cats. This usually presents as multiple small masses, whereas pancreatic tumors are usually single, but histopathology or cytology is necessary to definitively differentiate hyperplasia from neoplasia. Both dogs and cats with acute and chronic pancreatitis sometimes present with a large pancreatic “mass” as a result of fat necrosis and/or associated fibrosis, and it is important not to confuse these with neoplasia. Again, histopathology is required to differentiate these conditions. Ultrasound-guided fine needle aspiration cytology has been suggested as a useful means of differentiating inflammatory and neoplastic lesions of the pancreas (Bjorneby and Kari, 2002). Clinical use in dogs and cats is limited, but it has been reported to be helpful in diagnosis in some studies (Bennet et al., 2001).
Pancreatic tumors are not associated with any specific clinicopathological changes and may cause no changes in enzymes at all. Alternatively, they can result in recurrent bouts of pancreatitis with typical associated blood changes, and EPI can develop. In some cases biliary tract obstruction may occur with associated jaundice and marked elevations in liver enzyme activities. Occasionally, pancreatic tumors have been reported associated with marked hyperlipasemia.
The prognosis in dogs and cats with pancreatic adenocarcinoma is very poor. The tumors are extremely aggressive, poorly sensitive to chemotherapy or radiotherapy, and have usually disseminated widely by the time of diagnosis.
Neuroendocrine tumors such as insulinomas and gastrinomas appear to be more common than pancreatic adenocarcinomas in dogs and tend to be seen in different breeds of dog, predominantly large breeds (Watson et al., 2007). These are tumors of the endocrine pancreas that produce clinical signs related to secretion of hormones and are therefore outside the scope of this chapter.
PANCREATIC ABSCESSES, CYSTS, AND PSEUDOCYSTS
Pancreatic abscesses, cysts, and pseudocysts are uncommonly reported in dogs and cats and are usually a complication or sequela of pancreatitis. Pancreatic cysts may be congenital (e.g., as part of the polycystic renal disease in Persian cats) or secondary to cystic neoplasia, but the most common are pseudocysts secondary to pancreatitis. A pancreatic pseudocyst is a collection of fluid containing pancreatic enzymes and debris in a nonepithelialized sac. Pseudocysts have been recognized in association with pancreatitis in both cats and dogs, although they appear to be rare, and microscopic acinar cysts were found frequently in feline chronic pancreatitis. Pseudocysts are not associated with any distinct clinicopathological findings other than those associated with the underlying pancreatitis. Analysis of fluid obtained from a pseudocyst by fine needle aspiration generally shows a modified transudate. The activities of amylase and lipase can be measured in the pseudocyst fluid. In humans the enzymes are higher in pseudocysts associated with pancreatitis than in those associated with cystic carcinomas, but the value of this measurement in small animals is unknown. Cytology can differentiate a pseudocyst from a true abscess because a pseudocyst contains amorphous debris; some neutrophils and macrophages; and, rarely, small numbers of reactive fibroblasts, whereas an abscess contains many degenerative neutrophils and variable numbers of pancreatic acinar cells, which may appear very atypical as a result of inflammation.
A true pancreatic abscess is a collection of septic exudate that results from secondary infection of necrotic pancreatic tissue or a pancreatic pseudocyst. They are associated with a poor prognosis but fortunately are rare in dogs and cats.
Treatment of pancreatic pseudocysts can be surgical or medical. Medical treatment by ultrasound-guided cyst aspiration has had a reasonable success rate. Pancreatic abscesses should be treated surgically with omentalization or open peritoneal drainage. Both carry a high mortality rate, but a recent study suggested that omentalization may be preferable (Johnson et al., 2006).
Addallah AA, et al. Biliary tract obstruction in chronic pancreatitis. HPB. 2007;9:421.
Barres FJ, et al. Effects of intravenous ketamine on gastrointestinal motility in the dog. Int Med. 1995;7:584.
Batchelor DJ, et al. Breed associations for canine exocrine pancreatic insufficiency. J Vet Intern Med. 2007;21:207.
Batchelor DJ, et al. Prognostic factors in canine exocrine pancreatic insufficiency: prolonged survival is likely if clinical remission is achieved. J Vet Intern Med. 2007;21:54.
Batt RM, et al. Specificity of the BT-PABA test for the diagnosis of exocrine pancreatic insufficiency in the dog. Vet Rec. 1981;108:303.
Bennett PF, et al. Ultrasonographic and cytopathological diagnosis of exocrine pancreatic carcinoma in the dog and cat. JAAHA. 2001;37:466.
Bjorneby JM, et al. Cytology of the pancreas. Vet Clin N Am. 2002;32:1293.
Chandler ML, et al. A pilot study of protein sparing in healthy dogs using peripheral parenteral nutrition. Res Vet Sci. 2000;69:47.
De Cock HE, et al. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol. 2007;44:39.
Etemad B, et al. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682.
Hall EJ, et al. A survey of the diagnosis and treatment of canine exocrine pancreatic insufficiency. J Small Anim Prac. 1991;32:613.
Ferreri JA, et al. Clinical differentiation of acute necrotizing from chronic non-suppurative pancreatitis in cats: 63 cases (1996-2001). J Am Vet Med Assoc. 2003;223:469.
Gerhardt A, et al. Comparison of the sensitivity of different diagnostic tests for pancreatitis in cats. J Vet Intern Med. 2001;15:329.
Guija de Arespacochaga A, et al. Comparison of lipase activity in peritoneal fluid of dogs with different pathologies—a complementary diagnostic tool in acute pancreatitis? J Vet Med. 2006;53:119.
Hall EJ, et al. A survey of the diagnosis and treatment of canine exocrine pancreatic insufficiency. J Small Anim Prac. 1991;32:613.
Hess RS, et al. Clinical, clinicopathological, radiographic and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986–1995). J Am Vet Med Assoc. 1998;213:665.
Hess RS, et al. Evaluation of risk factors for fatal acute pancreatitis in dogs. J Am Vet Med Assoc. 1999;214:46.
Hill RC, et al. Acute necrotizing pancreatitis and acute suppurative pancreatitis in the cat: a retrospective study of 40 cases (1976–1989). J Vet Intern Med. 1993;7:25.
Jennings M, et al. Successful treatment of feline pancreatitis using an endoscopically placed gastrojejunostomy tube. J Am Anim Hosp Assoc. 2001;37:145.
Johnson MD, et al. Treatment for pancreatic abscesses via omentalization with abdominal closure versus open peritoneal drainage in dogs: 15 cases (1994-2004). J Am Vet Med Assoc. 2006;228:397.
Kimmel SE, et al. Incidence and prognostic value of low plasma ionised calcium concentration in cats with acute pancreatitis: 46 cases (1996–1998). J Am Vet Med Assoc. 2001;219:1105.
Mansfield CS, et al. Trypsinogen activation peptide in the diagnosis of canine pancreatitis. J Vet Intern Med. 2000;14:346.
Mansfield CS, et al. Review of feline pancreatitis. Part 2: clinical signs, diagnosis and treatment. J Feline Med Surg. 2001;3:125.
Meier RF, et al. Nutrition in pancreatic diseases. Best Pract Res Clin Gastroenterol. 2006;20:507.
Mohr AJ, et al. Effect of early enteral nutrition on intestinal permeability, intestinal protein loss, and outcome in dogs with severe parvoviral enteritis. J Vet Intern Med. 2003;17:791.
Newman S, et al. Localization of pancreatic inflammation and necrosis in dogs. J Vet Intern Med. 2004;18:488.
Pearce CB, et al. A double-blind, randomised, controlled trial to study the effects of an enteral feed supplemented with glutamine, arginine, and omega-3 fatty acid in predicted acute severe pancreatitis. J Pancreas. 2006;7:361.
Ruaux CG. Pathophysiology of organ failure in severe acute pancreatitis in dogs. Compend Cont Educ Small Animl Vet. 2000;22:531.
Ruaux CG, et al. A severity score for spontaneous canine acute pancreatitis. Aus Vet J. 1998;76:804.
Robertson SA, et al. Systemic uptake of buprenorphine by cats after oral mucosal administration. Vet Rec. 2003;152:675.
Schaer M. A clinicopathological survey of acute pancreatitis in 30 dogs and 5 cats. J Am Anim Hosp Assoc. 1979;15:681.
Spillmann T, et al. Canine pancreatic elastase in dogs with clinical exocrine pancreatic insufficiency, normal dogs and dogs with chronic enteropathies. Eur J Comp Gastroenteroly. 2000;5:1.
Spillmann T, et al. An immunoassay for canine pancreatic elastase 1 as an indicator of exocrine pancreatic insufficiency in dogs. J Vet Diagnost Invest. 2001;13:468.
Steiner JM, et al. Serum canine lipase immunoreactivity in dogs with exocrine pancreatic insufficiency. J Vet Intern Med. 2001;15:274.
Swift NC, et al. Evaluation of serum feline tryspin-like immunoreactivity for diagnosis of pancreatitis in cats. J Am Vet Med Assoc. 2000;217:37.
Watson PJ. Exocrine pancreatic insufficiency as an end stage of pancreatitis in four dogs. J Small Anim Pract. 2003;44:306.
Watson PJ, et al. A prospective observational study of 30 cases of canine chronic pancreatitis. Proceedings of the 16th ECVIM-CA Congress Amsterdam, and published in the. Journal of Veterinary Internal Medicine. 2006;20:1519.
Watson PJ, et al. Prevalence and breed distribution of chronic pancreatitis at post-mortem examination in first opinion dogs. J Small Anim Prac. 2007;48:609.
Watson PJ, et al. Chronic pancreatitis in cocker spaniels shows features of human autoimmune pancreatitis. Proceedings of the 16th ECVIM-CA Congress Amsterdam, and published in the. Journal of Veterinary Internal Medicine. 2006;20:1518.
Weiss DJ, et al. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis and nephritis in cats. J Am Vet Med Assoc. 1996;206:1114.
Westermarck E, et al. Exocrine pancreatic insufficiency in dogs. Vet Clin Nh Am Small Anim Pract. 2003;33:1165.
Wiberg ME. Pancreatic acinar atrophy in German shepherd dogs and rough-coated collies. Etiopathogenesis, diagnosis and treatment. A review. Vet Q. 2004;26:61.
Wiberg ME, et al. Cellular and humoral immune responses in atrophic lymphocytic pancreatitis in German shepherd dogs and rough-coated collies. Vet Immunol Immunopathol. 2000;76:103.
Wiberg ME, et al. Serum trypsinlike immunoractivity measurement for the diagnosis of subclinical exocrine pancreatic insufficiency. J Vet Intern Med. 1999;13:426.
Williams DA, Batt RM. Sensitivity and specificity of radioimmunoassay of serum trypsin-like immunoreactivity for the diagnosis of canine exocrine pancreatic insufficiency. J Am Vet Med Assoc. 1988;192:195.
 Drugs Used in Hepatobiliary and Pancreatic Disorders
Drugs Used in Hepatobiliary and Pancreatic Disorders
| DRUG NAME (TRADE NAME) | DOSAGE | INDICATIONS AND COMMENTS |
|---|---|---|
| ANALGESICS | See Table 40-8 | |
| ANTIBACTERIALS | ||
| Amoxicillin and ampicillin | 10-20 mg/kg PO, SC, or IV q8-12h dogs and cats | |
| Cephalexin | 10-20 mg/kg PO, SC, or IV q8-12h dogs and cats | |
| Enrofloxacin (Baytril) | 5 mg/kg SC or PO q24h dogs and cats | |
| Marbofloxacin (Zeniquin) | 2 mg/kg SC, PO, or IV q24h dogs and cats | As enrofloxacin |
| Metronidazole | Bactericidal particularly effective against anaerobes; often used in combination with ampicillin for biliary tract infections or to control gut bacteria in hepatic encephalopathy | |
| Neomycin | 20 mg/kg q6-8h PO or as a retention enema dogs and cats | Particularly used in acute hepatic encephalopathy; systemic absorption and oto- and nephrotoxicity can occur if there is concurrent GI ulceration, particularly in cats. |
| Potentiated sulphonamides, (e.g., trimethoprim-sulpha) | 15 mg/kg of combined ingredients (trimethoprim + sulphonamide) PO q12h | Bactericidal, broad-spectrum and probably drug of choice with infectious complications of pancreatitis; should not be used in liver disease if possible because hepatotoxic in susceptible individuals; should not be used in Doberman Pinschers because of reduced hepatic clearance; immune-mediated diseases occasional adverse effects |
| ANTIEMETICS | ||
| Chlorpromazine | 0.2-0.4 mg/kg SC q8h dogs and cats | Indicated in vomiting associated with pancreatitis and some cases of hepatitis, but only if other anti-emetics tried and ineffective because it is a phenothiazine sedative; effective antiemetic but also sedative, so ensure adequate hydration and avoid or use very low dose in encephalopathy and cardiovascular compromise |
| Metoclopramide | 0.2-0.5 mg/kg PO or SC q8h or 1 mg/kg q24h as a constant rate infusion | Indicated in vomiting associated with liver disease and some cases of pancreatitis; however, peripheral prokinetic effect may increase pain in pancreatitis; neurological adverse effects occasionally seen; avoid in encephalopathy |
| Maropitant (Cerenia) | Dogs only: 1 mg/kg SC q24h for up to 5 days or 2 mg/kg orally q24h for up to 5 days | Centrally acting antiemetic in new class (NK1 receptor antagonist); antiemetic of choice in canine pancreatitis as no obvious prokinetic effect; used with care in liver disease because metabolized in the liver, so do not use if significant liver dysfunction; not licensed for cats |
| Ondansetron (Zofran) | Cats and dogs: 0.5 mg/kg IV loading dose followed by 0.5 mg/kg/hour infusion q6h or 0.5-1 mg/kg PO q12-24h | |
| ANTIENCEPHALOPATHIC | ||
| Lactulose | Hepatic encephalopathy with acquired or congenital portosystemic shunts; overdose produces diarrhea; titrate to effect (= 2-3 soft bowel movements a day) | |
| Antibiotics (e.g., ampicillin, metronidazole, neomycin) | See antibacterial section | |
| Propofol | Constant rate infusion; rate calculated by giving an initial bolus to effect (usually about 1 mg/kg) and timing duration of action; usually about 0.1-0.2 mg/kg/min | Drug of choice for seizures because of liver disease/hepatic encephalopathy; should not be used in pancreatitis because it is a lipid vehicle |
| Phenobarbital | 5-10 mg/kg PO q24h preoperatively followed by 3-5 mg/kg q12h postoperatively for 3 weeks | Can be used prophylactically before and immediately after surgery to reduce risk of postoperative seizures after ligation of PSS, but evidence of effectiveness is anecdotal |
| ANTIINFLAMMATORY and ANTIFIBROTIC | ||
| Prednisolone (prednisone) | Antiinflammatory dose: 0.5 mg/kg q24h; immunosuppressive dose: 1-2 mg/kg q24h. Taper at 0.5 mg/kg q24h or eod | |
| Colchicine | Dogs only 0.03 mg/kg/day PO | Antifibrotic of choice in moderate hepatic fibrosis in dogs, but efficacy unclear; monitor blood samples for bone marrow suppression; GI side effects common and most likely reason to stop therapy |
| ANTIOXIDANTS | ||
| Dogs: 20 mg/kg PO q24h or higher; cats: 20 mg/kg or 200-400 mg total daily | Indicated in any liver disease but particularly hepatic lipidosis in cats and toxic hepatitis and diseases causing biliary stasis in dogs and cats; tablets must be given whole on an empty stomach for effective absorption | |
| Sylmarin (silymarin, silibin) | 50-200 mg/dog PO q24h | Antioxidant derived from milk thistle; likely effective and safe, but very limited studies to base dose advice on in dogs; studies were in toxic hepatitis |
| Vitamin E (tocopherol) | 400 IU per day for medium-sized dogs (titrate accordingly for other sizes) or 5-25 IU/kg PO daily dogs and cats | Indications as SAMe but including any chronic hepatitis in dogs |
| Zinc (see copper chelating) and ursodeoxycholic acid (see choleretic) also have antioxidant activities | ||
| ANTIDOTES | ||
| N-acetylcysteine | Cats and dogs: 140 mg/kg IV or PO as a loading dose and then continued at 70 mg/kg q6h for a total of 7 treatments or for up to 5 days | Antidote for acetaminophen toxicity that binds the toxic metabolite and increases the glucuronidation process; can cause nausea and vomiting when given orally; foul taste makes oral dosing difficult without nasogastric tube |
| Cimetidine | Dogs: 5-10 mg/kg IV, IM, or PO q6-8h; Cats 2.5-5 mg/kg IV, IM, or PO q8-12h | Slows oxidative hepatic drug metabolism by binding to microsomal cytochrome P450; therefore useful additional antidote in acetaminophen toxicity in dogs and cats |
| Also antioxidants such as S-adenosylmethionine and vitamins E and C supportive for oxidant toxins such as acetaminophen | See sections on antioxidants and vitamins | |
| ANTIULCER TREATMENT | ||
| 2 mg/kg PO or slowly IV q12h dogs and cats | Acid secretory inhibitor of choice in liver disease; may not be necessary if gastric pH is high; Cimetidine should be avoided because of action on P450 enzymes, except as an antidote (see above) | |
| Sucralfate (Carafate) | Gastric ulceration associated with liver or pancreatic disease | |
| COPPER CHELATING | ||
| Penicillamine | Dogs only: 10-15 mg/kg PO q12h | Copper chelator for copper storage disease; takes months to de-copper liver; give on an empty stomach; vomiting common; Immune-mediated, renal, and skin disease possible |
| Dogs only: 10-15 mg/kg PO q12h | Copper chelator for copper storage disease in dogs; more rapid effect than penicillamine so may be more useful in acute disease; 2,3,2-tetramine produces greater copper loss but is not available as a drug; isolated case reports of their use in dogs but no extensive trials; toxicity data unclear except that prolonged use may lead to clinical signs resulting from low copper levels | |
| Zinc acetate or sulphate | 1-20 mg/kg/day of elemental zinc dogs; 7 mg/cat/day of elemental zinc cats | Indicated in copper storage disease to reduce copper absorption; also antioxidant, antifibrotic, and increases ammonia detoxification, so may be helpful in any chronic hepatitis or hepatic encephalopathy; monitor blood levels every 1-2 weeks and keep below 200-300 μg/dl to avoid toxicity (iron deficiency and hemolysis); main side effect is vomiting—give 1 hour before food to minimize this |
| CHOLERETIC | ||
| Ursodeoxycholic acid (Ursodiol) | 4-15 mg/kg per day split into two doses 12 hours apart (dogs); 15 mg/kg PO once a day (cats) | Choleretic + also moderates bile acid pool to be less toxic + antiinflammatory and antioxidant; indicated in conditions associated with biliary stasis but without complete bile duct obstruction; contraindicated with obstruction in case of gallbladder rupture |
| DIURETIC | ||
| Furosemide | 2 mg/kg PO q8-12h dogs and cats | Use as additional diuretic where necessary in ascites of liver disease; always use concurrent spironolactone to avoid compensatory increase aldosterone action with further water retention and hypokalaemia |
| Spironolactone | 2-4 mg/kg day in divided doses dogs and cats | Diuretic of choice in ascites of liver disease (see text Chapter 39); Gradual onset of action over 2-3 days; may be combined with furosemide for more marked diuresis |
| TREATMENT OF COAGULOPATHIES | ||
| Fresh frozen plasma | Dogs and cats: starting dose of 10 ml/kg; the dose of plasma is titrated based on the results of the OSPT and APTT | Replenish depleted clotting factors in severe acute or chronic liver disease, particularly if prolonged OSPT and/or APTT and no response to vitamin K treatment alone |
| Vitamin K1(Phytomenadione) (Konakion) | 0.5-2 mg/kg SC or IM 12 hours before biopsy and then q12h for 3 days | |
| VITAMINS | ||
| Vitamin B12 (Cyanocobalamin) | Dogs and cats: 0.02 mg/kg IM or SC every 2-4 weeks until serum concentration normalizes (oral dosing ineffective in EPI because of ineffective absorption) | Treatment of vitamin B12 deficiency, particularly associated with EPI and lack of pancreatic intrinsic factor |
| Vitamin K1(Phytomenadione) | See treatment of coagulopathy section | |
| Vitamin E | See antioxidant section | |
| Vitamin C (ascorbic acid) | Cats and dogs oxidant toxins: 30-40 mg/kg SC q6h for 7 treatments | |
PO, By mouth; SC, subcutaneous; IV, intravenous; GI, gastrointestinal; PSS, portosystemic shunt; IM, intramuscular; EPI, exocrine pancreatic insufficiency.