CHAPTER 38 Hepatobiliary Diseases in the Dog
GENERAL CONSIDERATIONS
There are marked differences between dogs and cats in the causes, types, and presentations of liver disease (see Table 37-2). In dogs chronic liver disease is more common than acute disease, and notably, chronic parenchymal disease (chronic hepatitis) is much more common in dogs than cats; it almost invariably leads to progressive fibrosis and cirrhosis. This contrasts with cats, in which primary biliary disease is more common and fibrosis and cirrhosis extremely uncommon. The clinical signs of liver disease in dogs therefore tend to be even more nonspecific than in cats—jaundice is less common in association with parenchymal disease, and, because of the enormous reserve capacity of the liver, signs may not be seen until 75% of the liver mass is lost. The cause of chronic hepatitis in dogs is usually unknown, with a few notable exceptions, and treatment focuses on attempting to slow progression of disease and treating the clinical signs. Dogs with chronic hepatitis often develop portal hypertension, and treatment of the associated complications are central to treatment of disease in dogs (see also Chapter 39), whereas portal hypertension is very uncommon in cats. Congenital portosystemic shunts (PSSs) are more commonly recognized in dogs than in cats; in addition, vacuolar and secondary hepatopathies are very common in dogs and can be confused with primary liver disease on presentation. The most common primary and secondary liver diseases in dogs are outlined in Table 38-1.
 TABLE 38-1 Liver Diseases in Dogs
TABLE 38-1 Liver Diseases in Dogs
| PRIMARY | SECONDARY |
|---|---|
| Chronic hepatitis | Steroid-induced hepatopathy |
| Copper storage disease | Hepatic steatosis (lipidosis) (secondary to diabetes mellitus or hypothyroidism) |
| Congenital portosystemic shunt | Congestion: heart failure or heartworm disease |
| Drug/toxin-induced hepatopathy | “Idiopathic” vacuolar hepatopathy in Scottish Terriers and others |
| Reactive hepatitis (secondary to pancreatitis, inflammatory bowel disease, etc.) | |
| Metastatic neoplasia | |
| UNCOMMON OR RARE | |
| Biliary tract disease, all types | Hepatocutaneous syndrome |
| Hepatic infections (see text) | |
| Portal vein hypoplasia/microvascular dysplasia | |
| Hepatic arteriovenous fistula | |
| Acute fulminant hepatitis (all causes) | |
| Hepatic abscess | |
| Primary neoplasia | |
CHRONIC HEPATITIS
Chronic hepatitis is predominantly a histological definition. It is defined by the World Small Animal Veterinary Association (WSAVA) Liver Standardization group as being characterized by hepatocellular apoptosis or necrosis, a variable mononuclear or mixed inflammatory cell infiltrate, regeneration, and fibrosis (Van Den Ingh et al., 2006; Fig. 38-1). The histological definition says nothing about temporal chronicity, and some authors have suggested that increases in liver enzyme activities for more than 4 months associated with inflammatory liver disease might constitute a definite diagnosis of “chronic” hepatitis in dogs.
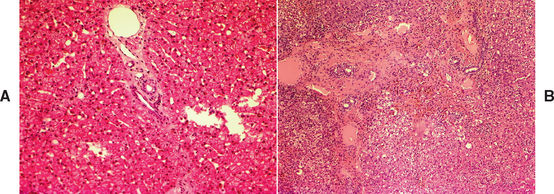
FIG 38-1 A, Histopathology of normal liver from a middle-aged Yorkshire terrier. Note normal portal triad with hepatic portal vein, artery, and bile duct and hepatocytes arranged in neat cords with sinusoids between (white holes in bottom right are a sectioning artefact) Hematoxylin and eosin ×200. B, Histopathology of liver in a 3-year-old female English Springer Spaniel with severe chronic hepatitis. There is marked distortion of the normal lobular structure (compare to A) with inflammation and fibrosis and hepatocyte vacuolation and necrosis. There is also some ductular hyperplasia and disruption of the limiting plate. Hematoxylin and eosin ×100.
(Courtesy the Pathology Department, Department of Veterinary Medicine, University of Cambridge.)
Chronic hepatitis is common in dogs and shows some noticeable breed predilections, suggesting a genetic basis to the disease. Box 38-1 lists dog breeds reported to have a high prevalence of chronic hepatitis, and Box 38-2 lists possible reasons for genetic increases in susceptibility, all of which have been demonstrated in humans with chronic hepatitis and some of which have been recognized in other diseases in dogs. Young to middle-aged dogs are most commonly affected, and the sex ratio varies among breeds. It should also be noted that there are some notable geographical differences in breed-related liver diseases, which likely reflect differences in breeding in different countries: Diseases common in the United States may be unusual in the United Kingdom and vice versa. It is also important to remember that chronic hepatitis can affect mixed breed as well as purebred dogs and that recognition of one cause in a breed does not necessarily mean that chronic hepatitis in all dogs of that breed are due to the same cause. For example, in many Doberman Pinschers and West Highland White Terriers chronic hepatitis is due to copper accumulation, but in others it is not. In many cases of canine chronic hepatitis, the cause is unknown. This contrasts with the situation in human medicine, wherein most cases of chronic hepatitis are viral and some have defined and often effective treatments that can reverse the disease process. In dogs chronic viral causes have not been convincingly demonstrated, but the histology in some cases is suggestive of this and the search for infectious agents con tinues. Most cases therefore remain a nonspecific diagnosis of “chronic hepatitis,” and the treatment remains nonspecific and symptomatic. However, in a few notable exceptions, such as copper storage disease and toxic hepatitis, the cause may be known and may be treated specifically. These are outlined in separate sections of this chapter.
 BOX 38-1 Dog Breeds with a Reported Increased Risk of Chronic Hepatitis*
BOX 38-1 Dog Breeds with a Reported Increased Risk of Chronic Hepatitis*
* No reported sex ratio unless stated.
IDIOPATHIC CHRONIC HEPATITIS
Etiology and Pathogenesis
Idiopathic chronic hepatitis likely represents an unidentified viral, bacterial, or other infection; an unidentified previous toxic event; or, in some cases, autoimmune disease. However, because autoimmune chronic hepatitis has not yet been convincingly demonstrated in dogs, immunosuppressive drugs should not be used or used only very cautiously.
The pathogenesis of chronic hepatitis relates to loss of hepatic mass resulting in loss of function and, late in the disease process, development of portal hypertension. In many cases hepatocyte swelling, fibrosis, and portal hypertension also contribute to cholestasis and jaundice. Ongoing inflammation may also result in bouts of pyrexia and hepatic pain with associated gastrointestinal (GI) and other signs, and many dogs with chronic hepatitis develop negative nitrogen balance and protein-calorie malnutrition. Loss of hepatic function accounts for coagulopathies and adverse drug reactions in affected dogs.
Portal hypertension is an important consequence of chronic hepatitis and fibrosis, and its effects contribute to the clinical signs and death of many affected animals (see also Chapter 39). It causes a typical triad of clinical signs of ascites, GI ulceration, and hepatic encephalopathy (HE). In a healthy dog the pressure in the portal vein is lower than the pressure in the caudal vena cava. However, in association with obstruction and disruption of sinusoids by fibrosis and hepatocyte swelling, portal pressure rises until it exceeds that in the caudal vena cava (portal hypertension). This results in splanchic congestion with splenic congestion, gut wall edema, and eventually ascites. The mechanisms of ascites formation in dogs with liver disease are complex but involve activation of the renin-angiotensin-aldosterone system (RAAS) with sodium retention in the kidneys and increased circulating fluid volume.
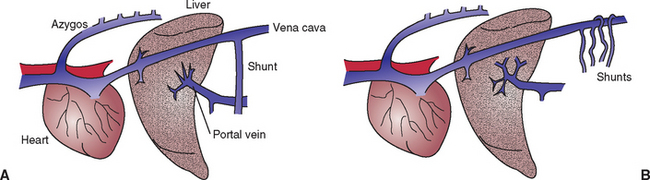
If the rise in portal pressure is sustained, multiple acquired PSSs will develop by the opening up of previously nonfunctional vessels; this allows for some of the portal blood to bypass the liver and enter the portal vein directly (Fig. 38-2). These acquired PSSs differ from congenital PSSs in that they are multiple and exist in the presence of increased portal pressure, whereas in patients with congenital PSSs the portal pressure is low. Acquired PSSs lead to HE by a similar mechanism to congenital PSS (see Chapter 39). However, the HE must be medically managed because ligation of acquired PSSs is contraindicated. This is because acquired PSSs are important escape valves to allow dissipation of some of the portal hypertension; therefore any attempt to ligate them will result in fatal splanchic congestion. Acquired PSSs in humans are also recognized to reduce the risk of serious GI ulceration associated with portal hypertension; because of this, they are sometimes created surgically in humans with cirrhosis to reduce the risk of serious bleeds. The same is likely to be true in dogs: GI ulceration is one of the most common causes of death in dogs with chronic hepatitis; acquired PSSs will help reduce this risk.
Clinical Features
Dogs of any age or breed can be affected with idiopathic chronic hepatitis, but there is an increased suspicion in middle-aged dogs of the breeds outlined in Box 38-1. The functional and structural reserve capacity of the liver implies that dogs with chronic hepatitis usually have no clinical signs until late in the disease process, when more than 75% of liver function has gone. By this stage, there is already extensive destruction of liver mass and treatment will be less effective than it would have been earlier in the disease (Fig. 38-3). It is therefore beneficial to diagnose the disease earlier, and dogs with persistently high liver enzyme activities (particularly hepatocellular enzymes such as ALT) should not be ignored. If liver enzyme activities are high for several months and other causes have been ruled out (see the section on secondary hepatopathy), then a liver biopsy should be obtained. This is even more important in breeds at high risk and in those predisposed to treatable diseases, such as copper storage disease.
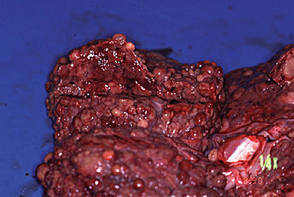
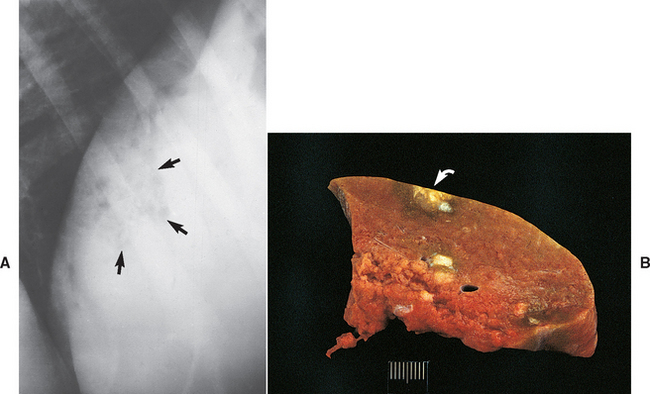
FIG 38-3 Liver from a 6-year-old Bearded Collie that had shown clinical signs for only 1 month before dying from end-stage liver disease. The diagnosis was chronic hepatitis with macronodular cirrhosis and very little normal liver tissue remaining.
Once dogs have lost a significant amount of liver mass, they will display clinical signs, but these are typically low-grade, waxing and waning, and nonspecific, making differential diagnosis from other diseases a challenge. Vomiting and diarrhea, anorexia, and polydipsia/polyuria are common. Jaundice and ascites occur in some dogs at presentation and develop later in others, but not in all cases. Ascites at presentation has been identified as a poor prognostic indicator in humans and dogs because it may represent more advanced disease with secondary portal hypertension. HE is uncommon and usually seen only in end-stage disease. The presence of HE strongly suggests the development of acquired PSS. Dogs with chronic hepatitis usually have some degree of protein-calorie malnutrition as a result of chronic hepatic functional impairment and concurrent GI signs. They are often overtly thin. They may be depressed, but they are also often surprisingly alert considering the severity of their disease.
Diagnosis
Ultimately, a definitive diagnosis requires a liver biopsy, but suspicion of disease is gained from the clinical signs and clinicopathologic features. Clinical signs, clinicopathologic findings, and imaging may be supportive of chronic hepatitis but are not specific. A serum biochemical profile may show a combination of high activities of hepatocellular (alanine transaminase [ALT] and aspartate aminotransferase [AST]) and cholestatic (alkaline phosphatase [ALP] and γ-glutamyltransferase [GGT]) enzymes, and evidence of decreased parenchymal liver function (low urea, low albumin, and sometimes high bilirubin and bile acid concentrations). Persistent increases in ALT are the most consistent finding in dogs with chronic hepatitis, but they can also be found in other primary and secondary hepatopathies. A high ALP activity is much less specific in dogs, particularly because there is a steroid-induced isoenzyme. Hepatocellular enzymes can become normal in end-stage disease because of a lack of liver mass, but by that stage function tests (e.g., ammonia and bile acid concentrations) will be abnormal, and the dog may even be jaundiced.
Radiographic findings are nonspecific. Dogs with chronic hepatitis often have a small liver (contrasting with cats, in which hepatomegaly is more common), but there is an overlap with normal, and the assessment of liver size is further confused by the variation in gastric axis in deep-chested dogs. If ascites is present, radiographs are not helpful because the fluid obscures all abdominal detail. Ultrasonography is much more useful in assessing hepatic architecture (see Chapter 36). Dogs with chronic hepatitis often have a small, diffusely hyperechoic liver on ultrasonography, although the liver may look ultrasonographically normal in some cases. In other cases it may appear nodular because of macronodular cirrhosis and/or concurrent benign nodular hyperplasia. It is impossible to definitively differentiate benign from malignant nodules on ultrasonographic appear ance alone; cytology or biopsy is essential to obtain a definitive diagnosis.
End-stage chronic hepatitis with cirrhosis may appear very similar to noncirrhotic portal hypertension from a diagnostic standpoint, and yet the treatment of the latter is very different and the long-term prognosis much more favorable than with cirrhosis. Therefore a liver biopsy is necessary for a definitive diagnosis and appropriate treatment. It is important to perform a hemostasis profile (one stage prothrombin time; activated partial thromboplastin time, and platelet count) before obtaining a biopsy and to address any coagulopathies or thrombocytopenia before the procedure. Fine needle aspirate (FNA) cytology is of limited value in the diagnosis of chronic hepatitis; the most representative biopsies are wedge biopsies obtained during laparotomy or laparoscopy, although ultrasonographically guided Tru-Cut needle biopsies can be of some benefit.
Treatment
The aims of treatment of dogs with chronic hepatitis are to treat any identified underlying cause (see subsequent sections), slow progression of the disease if possible, and support liver function and the animal’s nutritional and metabolic needs.
Diet
Dietary management is always an important part of treatment in patients with liver disease because the liver is the “first stop” for nutrients on their way from the gut to the systemic circulation and it is intimately involved in the metabolism of nutrients. This metabolism is compromised in patients with liver disease; in addition, dogs with chronic hepatitis typically have protein-calorie malnutrition, so excessive restriction of nutrients can be harmful. The nutritional requirements in dogs with liver disease are outlined in Table 38-2. The most important consideration is dietary protein concentration. It is now recognized in both people and dogs with liver disease that, in order to avoid negative nitrogen balance, dietary protein should not be restricted. However, it is important to feed a high-quality, highly digestible protein to reduce hepatic work and to decrease the amount of undigested protein that reaches the colon, where it is converted to ammonia. Most ammonia reaching the systemic circulation in the portal blood of animals with congenital and acquired PSSs originates not from dietary protein but from enterocyte catabolism of glutamine as their main source of energy. This cannot be avoided without starving the enterocytes, so other means of control of HE are recommended in addition to dietary restriction. Clinical diets available for dogs with liver disease (Hills LD and Royal Canin-Waltham Hepatic support) are ideally formulated, except that they have lower protein than is ideal for a dog with chronic hepatitis. Therefore these diets should be fed as a baseline little and often, with the addition of high-quality protein to the food. Dairy and vegetable protein produce the best results in humans and dogs with liver disease; cottage cheese is a good choice to add to the diet. The amount to add to the food is difficult to estimate. It is advisable to start with 1 or 2 tablespoons of cottage cheese per meal, monitor clinical signs and blood protein levels, and adjust accordingly.
 TABLE 38-2 Dietary Considerations for Dogs with Liver Disease
TABLE 38-2 Dietary Considerations for Dogs with Liver Disease
| The diet should be fed little and often (4-6 times a day) and needs to be palatable. A good and sufficient diet is essential for hepatic regeneration and optimal hepatic function. |
Drugs
Drug support in dogs with idiopathic chronic hepatitis is nonspecific and attempts to slow progression of disease and control clinical signs. Specific drug treatments are reserved for patients with an identified underlying cause. Without a biopsy, nonspecific treatment should consist of choleretics, antioxidants, and diet. The use of glucocorticoids must be reserved for biopsy-confirmed cases only.
Glucocorticoids.
Glucocorticoids are commonly used in dogs with idiopathic chronic hepatitis, but they should never be used without a biopsy. Biopsies are necessary not only to confirm the presumptive diagnosis but also to rule out any contraindications. There is currently no evidence that idiopathic chronic hepatitis is an autoimmune disease, so glucocorticoids are used in this context for their antiinflammatory and antifibrotic role rather than as immunosuppressives. Fibrous tissue is laid down in the liver by transformed Ito (stellate) cells, and in dogs these are usually stimulated indirectly by cytokines produced by inflammatory cells to transform to collagen-producing cells. The chain of events in idiopathic chronic hepatitis is therefore usually as outlined in Fig. 38-4. Glucocorticoids have an important role to play early in the disease process: Their antiinflammatory effect reduces cytokine formation and Ito cell stimulation, thus reducing fibrous tissue deposition. They are therefore indicated early in the disease process, when there is inflammation and minimal fibrosis, and once infectious etiologies have been ruled out. In these situations they may slow the progression of the disease (although that has not been proved). The logical dose to use is antiinflammatory (equivalent to 0.5 mg/kg of prednisone and gradually reducing over several weeks by halving the dose and reducing to every-other-day treatment), although immunosuppressive doses also have been used; there is currently insufficient evidence in dogs to advise which is correct.
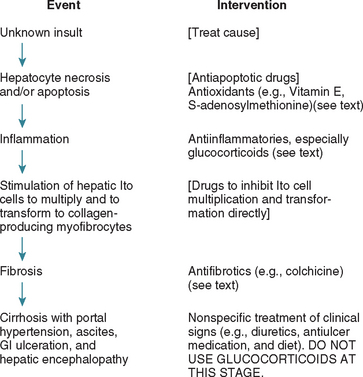
FIG 38-4 Chain of events in typical idopathic hepatitis in dogs and points for therapeutic intervention (those in brackets are potential treatments not yet available for clinical use in dogs).
Glucocorticoids are contraindicated later in the disease, when there is portal hypertension and end-stage fibrosis, or in conditions with noninflammatory fibrosis (e.g., noncirrhotic portal hypertension), in which there is no reason for their use. In these circumstances they are also likely to shorten the life expectancy by increasing the risk of serious GI ulceration (see Fig. 39-1). Hence glucocorticoids should never be used without a histopathologic diagnosis and staging of disease.
Other antiinflammatory or immunosuppressive drugs.
Some of the other drugs used in dogs with liver disease also have antiinflammatory activity, particularly zinc, S-adenosylmethionine, and ursodiol (discussed in more detail later). Azathioprine has occasionally been used in dogs with chronic hepatitis, but there is no evidence that it is beneficial; until immune-mediated causes of chronic hepatitis have been proved, it would be wise to avoid the use of this or other potent immunosuppressive medications.
Choleretics.
Ursodiol is widely and commonly used in dogs with chronic hepatitis. It is a synthetic hydrophilic bile acid that is choleretic and also modulates the bile acid pool in biliary stasis, making the bile less toxic to hepatocytes. It also has antiinflammatory and antioxidant properties, and recent studies suggest that it is synergistic with S-adenosylmethionine and vitamin E. The only absolute contraindication is complete biliary obstruction, which is very rare in dogs and would usually result in obvious acholic feces. It is logical to use it in any dog with chronic hepatitis, particularly in those associated with biliary stasis, and it can safely be used without a biopsy. However, as with other drugs used in canine liver disease, there is very limited (although encouraging) evidence as to its efficacy. It may be more helpful in some diseases than others, but this is not known yet in dogs. The recommended dose is 10 to 15 mg/kg q12h (or split into two doses given q12h).
Antioxidants.
A variety of antioxidants are used in dogs with chronic hepatitis. The most well-documented are vitamin E and S-adenosylmethionine. Vitamin E appears to be beneficial at a dose rate of 400 IU/day for a 30-kg dog given as a water-soluble preparation once a day. Doses for smaller dogs are scaled appropriately. S-Adenosylmethionine is a glutathione precursor and is of particular benefit in dogs with toxic hepatopathy (discussed in more detail later) and those with biliary stasis because bile is a potent oxidant. It is synergistic with Vitamin E and ursodiol, and an argument could be made for it being beneficial in any dog with chronic hepatitis. The recommended dose is 20 mg/kg PO q24h. There are some studies documenting its use in dogs, but more are needed to define in which diseases it is most useful. S-Adenosylmethionine is a very unstable molecule (because it is a methyl donor) and must therefore be carefully packaged and given on an empty stomach. The pharmokinetics and GI availability in dogs are known for the pure preparation (Denosyl SD4; Nutramax Laboratories), but it is increasingly being marketed as a polypharmacy nutraceutical in preparations with other nutraceuticals and vitamins mixed together. Pharmacokinetic and absorption data should be sought from the manufacturers of these products to ensure that the S-adenosylmethionine is absorbed in effective amounts.
Another antioxidant commonly used in dogs with chronic hepatitis is milk thistle (Silybum marianum). The active ingredients are flavonoids, commonly referred to as silymarin, and the most effective of these is believed to be silybin. There are very few studies of the use of flavonoids in dogs, and the only clinical studies are on acute toxic hepatitis. Silybin undoubtedly has the potential to be a helpful adjunct to therapy in some cases, but much more information on absorption, availability, and ideal dosage is necessary. Silybin is included in many nutraceuticals marketed for dogs with liver disease. One recent study (Filburn et al., 2007) showed that it had very poor absorption alone but was much more bioavailable when complexed with phosphatidylcholine.
Therefore, although antioxidant nutraceuticals have great potential benefits in the treatment of chronic liver disease in dogs and can be safely used without a biopsy, the clinician must be aware of the emerging nature of the information about their bioavailability and efficacy and choose products carefully with this in mind.
Antifibrotics.
In inflammatory liver disease and early fibrosis, glucocorticoids have a potent indirect antifibrotic activity by reducing inflammation, as outlined in the preceding sections. Later in the disease process, when there is extensive fibrosis, the direct antifibrotic agent colchicine can be used; there is limited but encouraging anecdotal evidence supporting its effectiveness in dogs. It is an alkaloid derivative that binds tubulin and has the potential to reverse fibrosis. The recommended dose in dogs is 0.03 mg/kg/day PO. Adverse effects are uncommon in dogs but include bone marrow suppression and anorexia/diarrhea; it is the latter that often limits its use in clinical cases.
Antibiotics.
There is a primary indication for the use of antibiotics in dogs with ascending biliary tract infections or suspected bacterial infection as a cause of the chronic hepatitis. The latter is rarely proved, but if it is possible that atypical leptospiral infection may be present (e.g., if chronic hepatitis is seen in a dog with access to sources of infection such as rivers or ditches), a course of appropriate antibiotics would be wise to rule this out. The recommended therapy for leptospiral infections is to start with intravenous (IV) amoxicillin at a dose of 22 mg/kg q12h to terminate replication and reduce potentially fatal liver and kidney complications. If leptospiral infection is subsequently confirmed (on rising titres on serology, dark field microscopy, or PCR of the urine for organisms), this should be followed by doxycycline therapy (5 mg/kg PO q12h for 3 weeks) once liver function is normal to eliminate the chronic renal carrier state. Bartonella spp. have occasionally been associated with chronic liver disease in dogs. The optimal treatment for Bartonella spp. in dogs has not been established. Macrolides (e.g., erythromycin) or alternatively fluoroquinolones or doxycycline have been shown to have some efficacy against some Bartonella spp. in dogs. It has been suggested that 4 to 6 weeks of treatment might be necessary to eliminate infection.
Antibiotics are also used as part of supportive treatment in dogs with HE caused by acquired PSS in end-stage chronic hepatitis, in a similar way to dogs with congenital PSS to reduce toxin absorption from the gut and the risk of systemic infections (see Chapter 39). Ampicillin is often used long term in these cases at a dose of 10 to 20 mg/kg, PO or IV q8-12h.
As with other drugs, the clinician should avoid any antibiotics that increase hepatic work or the risk of hepatotoxicity. Thus tetracyclines, potentiated sulphonamides, nitrofurantoin, and erythromycin should be avoided unless necessary (e.g., with confirmed leptospirosis or bartonellosis) because they are potentially hepatotoxic.
COPPER STORAGE DISEASE
Pathogenesis and Etiology
Copper storage disease has been recognized as a cause of acute and chronic hepatitis in several breeds, the best researched of which is the Bedlington Terrier (see Box 38-1). Other breeds in which copper storage disease has been reported are Dalmatians (in the United States and Canada), Labrador Retrievers (in the United States and Holland), and some Doberman Pinschers (in Holland), although individual members of all these breeds have also been reported with chronic hepatitis without copper accumulation. In addition, copper storage disease has been suspected but not extensively investigated in West Highland White Terriers and Skye Terriers. It is also possible for seemingly normal dogs without a recognized copper storage disease to develop copper-associated chronic hepatitis if fed a diet very high in copper, such as dry calf feed (Van den Ingh et al., 2007).
Copper is excreted in the bile and can build up as a secondary phenomenon in any type of chronic hepatitis associated with cholestasis. In these cases the accumulation is usually mild, often in zone 1 (peribiliary), and the amount of copper does not correlate with the severity of the disease. It is unclear whether copper chelation is helpful in dogs with secondary copper build-up, but probably it is not. The peribiliary distribution and lack of correlation between amount of copper build up and clinical signs helps to distinguish these cases from “true” copper storage disease, in which the copper accumulation is the cause rather than an epiphenomenon of the disease and accumulation is usually marked, progressive, correlated with disease severity, and in Zone 3 (perivenous; see Fig. 35-4 for an explanation of hepatic zonation).
True copper storage disease likely represents a genetic defect in copper transport and/or storage, but the only breed in which this has been defined is the Bedlington Terrier. In this breed it is inherited as an autosomal recessive trait, and up to 60% of Bedlington Terriers in some countries have been affected in the past, although the prevalence is now decreasing as a result of selective breeding. The disease is confined to the liver, and there appears to be a specific defect in hepatic biliary copper excretion (probably in transport from the hepatocyte lysosomes to the biliary tract). Recent work has identified at least one genetic defect associated with the disease: a deletion in the MURR1 gene (now COMMD1; Van de Sluis et al., 2002), which codes for a protein of unknown function. However, Bedlington Terriers with copper storage disease but without a COMMD1 deletion are now being reported in the United States, United Kingdom, and Australia (Coronado et al., 2003; Heywood, 2006; Hyun et al., 2004), suggesting that there is at least one other mutation involved in the breed that has yet to be identified.
Clinical Features
Affected Bedlington Terriers can present with either acute or chronic clinical signs, depending on individual factors, such as the amount of copper in the diet, and also likely other factors, including concurrent stress and disease. If there is rapid and marked build-up, dogs may present with acute fulminant hepatic necrosis and no previous clinical signs. This is usually seen in young to middle-aged dogs and is often accompanied by acute hemolytic anemia caused by the rapid release of copper into the circulation. The prognosis is poor, and most animals die within a few days. Fortunately, this is uncommon; most dogs have a more chronic, protracted course with several years of copper build-up and persistently high ALT activity, culminating in the development of chronic hepatitis with piecemeal necrosis, inflammation, and bridging fibrosis. Clinical signs are therefore recognized in these individuals only late in the disease process and are usually those of canine chronic hepatitis. These dogs usually present at about 4 years old but may be younger (Fig. 38-5). Eventually, if not treated, affected dogs will develop cirrhosis.

FIG 38-5 Beddlington Terrier with copper storage disease.
(From Hall EJ, Simpson JW, Williams DA, editors: BSAVA manual of canine and feline gastroenterology, ed 2, Gloucestershire, United Kingdom, 2005, British Small Animal Veterinary Association.)
The clinical signs and progression in other breeds with copper storage disease are similar to those in Bedlington Terriers. The disease in Dalmatians is associated with acute onset, rapid progression, and very high levels of hepatic copper in the absence of significant clinical, clinicopathological, or histological evidence of cholestasis. Affected dogs usually present as young adults with acute onset of GI signs and polydipsia/polyuria, by which time severe liver disease is already present. Labrador Retrievers with copper storage disease have an average age at presentation of 7 to 9 years (range, 2.5 to 14 years). The clinical signs are relatively mild and included anorexia, vomiting, and lethargy. Doberman Pinschers appear to have a long phase of subclinical disease culminating, in untreated cases, in an acute-on-chronic disease and rapidly progressive deterioration. However, it is unclear how many of the clinically affected Doberman Pinschers described in the literature had copper storage disease and how many had idiopathic chronic hepatitis, so the true presenting signs of copper storage disease in this breed are unclear. Most published studies on true copper storage disease in Doberman Pinschers describe diagnosis and treatment of subclinical disease.
Diagnosis
The magnitude of increase in liver enzyme activities and the diagnostic imaging findings in dogs with chronic copper storage disease are very similar to those of dogs with idiopathic chronic hepatitis. Therefore a definitive diagnosis requires a liver biopsy and estimation of the copper concentration in the liver. This can be done qualitatively on formalin fixed sections using rhodanine staining to detect copper; correlations between quantitative and qualitative estimation of copper accumulation have been published (Shih et al., 2007). The finding of large accumulations of copper in hepatocytes on cytology with rubeanic acid is also very suggestive of copper storage disease (Fig. 38-6; Teske et al., 1992). Quantitative measurement of copper content can also be performed, but this requires a large biopsy specimen carefully taken and stored in copper-free tubes. In addition to estimating copper content, the liver biopsy will give an indication of the chronicity and extent of liver damage, which will affect treatment decisions in a very similar way to chronic hepatitis. Bedlington Terriers can be tested for the COMMD1 deletion either before breeding or when newly acquired to assess their risk for this disease, but an absence of the COMMD1 deletion does not guarantee that the dog will not be affected. The genetic test is currently offered via mouth swabs at the Animal Health Trust in Newmarket, U.K. (details at www.aht.org.uk/sci_diag_disc_genetic_main.htm) and by Vet Gen in the United States (www.vetgen.com). To rule out copper storage disease through a liver biopsy in a breeding animal, clinicians should obtain a biopsy when the dog is about 12 months old, by which time there will be sufficient copper build-up to diagnose the disease. In much older animals, cirrhosis with nodular regeneration can develop, and the nodules will have a lower copper content than the rest of the liver, confusing diagnosis if a regenerative nodule is inadvertently biopsied.
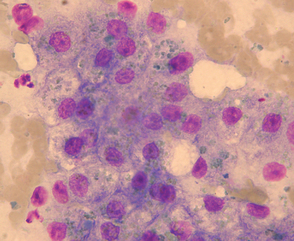
FIG 38-6 Cytology of hepatocytes from Bedlington terrier with copper storage disease demonstrating copper granules (rubeanic acid stain).
(Courtesy Elizabeth Villiers; from Hall EJ, Simpson JW, Williams DA, editors: BSAVA manual of canine and feline gastroenterology, ed 2, Gloucestershire, United Kingdom, 2005, British Small Animal Veterinary Association.)
Treatment
The ideal treatment in a dog known to be affected is prevention. Bedlington Terriers with the COMMD1 mutation should be fed a low-copper, high-zinc diet. The proprietary liver diets formulated for dogs (Royal-Canin Hepatic support or Hills canine LD) have low copper and high zinc concentrations but are also moderately protein restricted, so it would be wise to supplement with a low-copper protein source (e.g., cottage cheese) in growing dogs. It is also important to avoid giving the dog tap water from copper pipes in soft water areas; bottled water should be used instead. Box 38-3 gives a list of common high-copper foods that should be avoided and high-zinc foods that could be supplemented.
Dogs that present with an acute crisis should be treated with intensive support in exactly the same way as dogs with acute hepatitis (Box 38-4). Blood transfusion may be necessary if hemolysis is severe. Copper chelation is unlikely to be beneficial acutely, but chelation with 2,2,2-tetramine (trientine) could be considered (or 2,3,2-tetramine if obtainable) because this can chelate rapidly. Trientine is available as a drug licensed for humans (Syprine,® Merck Sharp and Dohme). The recommended dose in dogs is 10 to 15 mg/kg PO q12h 30 minutes before a meal. 2,3,2-Tetramine is difficult to obtain. Penicillamine is not helpful in an acute crisis because chelation takes weeks to months. However, it should be noted that there is much less information available about the pharmacokinetics, drug interactions, and toxicity of trientine in dogs than there is for D-penicillamine. Reported adverse effects include nausea, gastritis, abdominal pain, melena, and weakness. On recovery, the animal should continue on long-term treatment, as outlined in the following sections.
 BOX 38-4 Outline of Treatment Recommendations for Acute Fulminant Hepatitis
BOX 38-4 Outline of Treatment Recommendations for Acute Fulminant Hepatitis
Treatment of dogs that already have high hepatic copper concentrations documented by biopsy but are not in an acute crisis consists of active copper chelation, zinc supplementation, and use of a low-copper diet and additional supportive therapy. The chronic hepatitis secondary to copper storage disease should be treated the same way as in dogs with idiopathic chronic hepatitis, using antioxidants, ursodiol, and other supportive medication (see the section on chronic idiopathic hepatitis). There is a particular role for antioxidants such as vitamin E and S-adenosylmethionine in metal-induced liver injury. Chelation can be achieved using either D-penicillamine or trientine. D-penicillamine takes months to have a significant effect on the copper content of the liver but is easily available and its pharmacokinetics and toxicity in dogs are well documented. The recommended dose is 10 to 15 mg/kg PO q12h 30 minutes before meals. It also has weak antifibrotic and antiinflammatory properties. Starting at the lower end of the dose range and increasing the dose after 1 week (or dividing the dose and giving it more frequently) can reduce the common adverse effects of vomiting and anorexia. It has also been reported to cause nephrotic syndrome, leukopenia, and thrombocytopenia in dogs, so a complete blood count and urine samples should be monitored regularly during therapy. A decrease in liver copper content of about 900 μg/g dry weight per year can be anticipated in dogs treated with D-penicillamine. Trientine (2,2,2 tetramine) is another efficacious copper chelator that may be used and can remove copper from the liver more rapidly than D-penicillamine. Details of dose and potential adverse effects are given in a preceding section.
Copper chelation treatment is continued until normal liver copper concentration is reached; this is best determined by liver biopsy and copper quantification or cytologic estimate. Treatment should then be stopped to prevent copper deficiency, which can occur after prolonged, overzealous copper chelation and can result in severe effects of copper deficiency with weight loss and hematemesis. The regimen can then be changed to a preventive protocol consisting of a copper-restricted diet and zinc administration.
INFECTIOUS CAUSES OF CANINE CHRONIC HEPATITIS
Primary chronic hepatitis caused by infectious agents is uncommon in dogs, although there may be a yet unidenti fied infectious cause in some dogs with what appears to be idiopathic chronic hepatitis. Clinicians should keep this possibility in mind before prescribing immunosuppressive medication.
To date, there has been no convincing demonstration of a viral cause of canine chronic hepatis, although it has been suspected in several cases. The most common viral cause of chronic hepatitis in people is hepatitis B virus, a hepadnavirus. Similar hepadnaviruses associated with hepatitis have been identified in woodchucks, ground squirrels, tree squirrels, and ducks, but attempts to identify hepadnaviruses by PCR in the liver of dogs with chronic hepatitis or hepatocellular carcinoma have failed. Two other viruses have been suggested as a possible cause of canine chronic hepatitis: canine adenovirus type 1 (CAV1) and canine acidophil cell hepatitis virus. CAV1 causes acute fulminant hepatitis in immunologically naive dogs, but it can also cause chronic hepatitis experimentally in partially immune dogs. However, its importance in naturally occurring chronic hepatitis is unclear, and studies are conflicting. An alternative viral cause of canine acute, persistent, and chronic hepatitis was proposed in Glasgow by Jarrett et al. in 1985 and named canine acidophil cell hepatitis virus pending isolation and identification. The virus appeared to be transmissible by subcutaneous injection of liver homogenate and serum and was apparently capable of producing a chronic hepatitis marked by fibrosis and hepatocyte necrosis, but sparse inflammatory changes (Jarrett et al., 1985, 1987). It was proposed at the time that this was the most important cause of hepatitis in Glasgow. However, there have been no further published studies by either these or other workers regarding the identity or significance of this virus, so its identity and role remain unknown.
Bacterial infections have been sporadically reported as a cause of canine chronic hepatitis, but their importance is unclear. Bile-tolerant Helicobacter spp. can cause hepatitis centered on the bile ducts in rodents; there is one report of necrotizing hepatitis associated with Helicobacter canis infection in a pup (Fox et al., 1996). However, no further work has been reported in dogs, and a clear association between Helicobacter infection and liver disease has yet to be demonstrated.
Infections with apparently atypical leptospires may be a clinically relevant and underestimated cause of chronic hepatitis in dogs. Most dogs in the United States are vaccinated regularly against Leptospira interrogans serovars canicola and icterohaemorrhagiae, so it is assumed that leptospiral infection is now a rare disease. However, recent studies have shown an emergence of diseases associated with other serovars; in addition, there is little immunologic cross-reaction with the vaccine serovars. Infection with “atypical” leptospires, particularly L. grippotyphosa, can cause a chronic hepatitis with ascites, particularly in young dogs; azotemia is uncommon in these dogs. Histologically, the liver of dogs with confirmed atypical leptospire infection has portal and intralobular inflammation (i.e., mainly lymphocyticplasmacytic with some neutrophils and macrophages). There may also be periportal and portoportal fibrosis that may disrupt the hepatic architecture. The organisms are sparse and difficult to find with conventional staining techniques, so it is very possible that some cases of leptospiral hepatitis are misdiagnosed as immune-mediated disease on the basis of the histological appearance. There is also often a poor serological response in affected dogs, further complicating diagnosis.
Adamus et al. (1997) noted the similarity in age bias (6 to 9 months) and histological appearance between leptospiral hepatitis and lobular dissecting hepatitis, and it has been suggested that undiagnosed infections may be a cause of lobular dissecting hepatitis in some young dogs (discussed in more detail later). There have also been recent sporadic reports of Bartonella henselae and Bartonella clarridgeiae in dogs with chronic liver disease, but again their significance as a cause of the disease is unclear. Peliosis hepatis, rather than chronic hepatitis, is the more classical histological appearance associated with Bartonella spp. infection in humans and has been reported in one dog (Kitchell et al., 2000). Serology or PCR for Bartonella spp. is available.
A recent study (Boomkens et al., 2005) evaluated 98 liver samples from dogs with chronic hepatitis using nested PCR for Hepadnaviridae, Helicobacter spp., Leptospira spp., Borrelia spp., hepatitis A virus, hepatitis C virus, hepatitis E virus, canine adenovirus, and canine parvovirus and failed to find evidence of infection in any of the dogs. More work is needed before potential infectious causes of chronic hepatitis in dogs can be completely ruled out.
LOBULAR DISSECTING HEPATITIS
Lobular dissecting hepatitis is an idiopathic inflammatory disorder recognized predominantly in young dogs; it has a typical histological appearance of fibrotic dissection of lobular parenchyma into individual and small groups of hepatocytes. It has been reported in several breeds, including families of Standard Poodles and Finnish Spitzes. It has been proposed that lobular dissecting hepatitis does not represent a distinctive disease but rather a response of the juvenile liver to a variety of insults. Infectious etiologies have been suggested, although not proved, and the age of onset and histological appearance bear a striking resemblance to atypical leptospiral infection in dogs. Treatment recommendations are similar to those for canine chronic hepatitis (see preceding section).
TOXIC CAUSES OF CHRONIC HEPATITIS
Toxins and drug reactions more commonly cause acute, necrotizing hepatitis than chronic disease. Phenobarbital or primidone can cause either acute or chronic hepatotoxicity (see later discussion). Lomustine (CCNU) can also cause delayed, cumulative dose-related, chronic hepatotoxicity that is irreversible and can be fatal. Another occasional reported cause of chronic liver damage is phenylbutazone. Most other reported hepatotoxic drugs and toxins cause an acute hepatitis (see section on acute hepatitis and Box 38-5). Certain mycotoxins, including aflatoxins, can cause acute or chronic liver disease in dogs depending on the dose ingested and period of exposure. Dogs scavenge and eat contaminated food more often than humans do, so it is possible that a number of cases of canine chronic hepatitis are due to acute or chronic ingestion of unidentified toxins. Because a wide variety of drugs have been reported as causing hepatic adverse reactions in humans and dogs, a drug reaction should be considered in any dog with chronic hepatitis that is also on long-term therapy of any sort, although care should be taken not to overdiagnose drug reactions; chronic hepatitis should be considered as possibly drug related only when there is a clear temporal relationship with drug intake and likely alternative causes have been excluded.
 BOX 38-5 Potential Causes of Acute Fulminant Hepatitis in Dogs
BOX 38-5 Potential Causes of Acute Fulminant Hepatitis in Dogs
Metabolic
ACUTE HEPATITIS
Etiology and Pathogenesis
Acute hepatitis is much less common than chronic hepatitis in dogs but, when severe, carries a much poorer prognosis. Treatment focuses on providing supportive measures and allowing the liver to recover. Dogs with acute hepatitis are at high risk of disseminated intravascular coagulation (DIC). Severe loss of liver function is also fatal because it cannot be replaced artificially while awaiting recovery; there is no such thing as liver dialysis. However, because of the remarkable regenerative capacity of the liver, animals that recover from the acute phase of the disease can recover completely, with no permanent hepatic injury, as long as they are fed and supported properly.
Most causes of acute fulminating hepatitis in dogs are infectious or toxic (see Box 38-5). In unvaccinated dogs CAV-1 and leptospira are important differential diagnoses. Dogs with copper storage disease can present acutely and often will have hemolysis associated with high serum copper concentration, in addition to acute hepatic necrosis. Xylitol, an artificial sweetener, has recently been reported to cause acute hepatic necrosis in dogs (Dunayer et al., 2006) with a high mortality. Aflatoxin in contaminated feed-stuffs also recently caused acute and subacute hepatitis with a high mortality in dogs (Newman et al., 2007). The most common drugs implicated in causing acute hepatic necrosis in dogs are listed in Box 38-5, but potentially any drug could cause idiosyncratic hepatic necrosis in an individual dog. Recently, a case of destructive cholangitis (“disappearing bile duct syndrome”) was reported in a dog as a suspected drug reaction to either one or a combination of amoxicillinclavulanate, amitraz and milbemycin oxime (Gabriel et al., 2006), and we have seen this in a clinical case likely caused by an indiosyncratic reaction to amoxicillin-clavulanate.
Clinical Features
The clinical features of acute fulminating hepatitis, independent of the cause, relate to the acute loss of hepatic function together with the effects of generalized cell necrosis and release of inflammatory cytokines and tissue factors. Dogs usually present with acute onset of one or more of the following: anorexia; vomiting; polydipsia; dehydration; hepatic encephalopathy with depression progressing to seizures and/or coma; jaundice; fever; cranial abdominal pain; coagulopathy with petechiae and possible hematemesis and melena; and, in some cases, ascites and splenomegaly resulting from acute portal hypertension. Renal failure is a severe complication in some cases with both prerenal and intrinsic renal components. In humans with acute hepatic failure, hypotension, cardiac arrhythmias, cerebral and pulmonary edema, and pancreatic inflammation also have been reported; these may occur in some dogs, although they have not been specifically reported.
Diagnosis
Diagnosis is usually made on the basis of history, clinical signs, and clinicopathologic findings. Liver histopathology should be confirmatory, but results are often not obtained until recovery (or postmortem) because of the severe acute nature of the disease. A history of recent drug or toxin exposure is important in implicating these as a cause; vaccination status is an important consideration for infectious causes.
On clinical pathology dogs with acute hepatitis often have early, marked increases in hepatocellular enzyme ALT and AST activities (tenfold to >100-fold). Jaundice and increases in markers of cholestasis may also occur; the rare cases of destructive cholangitis are characterized by early, severe jaundice and marked increases in ALP activity and hyperbilirubinemia. Hypoglycemia and hypokalemia are common in dogs with acute hepatitis, and azotemia is seen in some cases, as a result of both prerenal and renal causes. Hemostatic abnormalities, with both prolonged clotting times and thrombocytopenia, are frequently present and can be a sign of developing DIC (see Chapter 87). Diagnostic imaging is not usually very helpful in dogs with acute hepatitis. There may be hepatomegaly and a diffuse change in hepatic echogenicity; in some cases there may be splenic congestion and/or ascites, but these changes are not specific and do not help define the cause or extent of the damage. In some patients the ultrasonographic exam is unremarkable.
Treatment and Prognosis
Treatment of acute fulminant hepatitis in dogs is largely supportive and is outlined in Box 38-4. Every attempt should be made to identify and treat the primary cause at the same time that supportive therapy is instituted. Corticosteroid treatment is not indicated in these cases and may in fact worsen the prognosis by increasing the risk of GI ulceration and thrombosis. The owner should be warned of the poor prognosis for recovery in spite of intensive support, and in severe cases, early referral to an intensive care unit should be considered. However, dogs that recover from the acute phase have a good chance of complete recovery. Some research in humans and animals has suggested that chronic liver lesions are less likely to develop if a single-protein milk or soybean-based diet is fed in the recovery phase.
BILIARY TRACT DISORDERS
Biliary tract disorders are less common in dogs than in cats, but both primary biliary tract disorders and extrahepatic bile duct obstruction are recognized in dogs. In addition, destructive cholangitis caused by drug reactions leading to severe cholestasis and icterus has been recognized occasionally in dogs (but not cats). Dogs occasionally develop congenital hepatic and renal cysts, similar to Caroli’s disease in humans.
CHOLANGITIS AND CHOLECYSTITIS
As discussed in the preceding section, primary biliary tract disease is less common in dogs than in cats. The clinical signs and diagnostic evaluation are very similar to those in cats with neutrophilic cholangitis (see Chapter 37). Dogs can be of any age or breed, and the typical presentation is acute onset of anorexia, jaundice, and vomiting, with or without pyrexia. In some cases there may have been a previous history of acute enteritis or pancreatitis, suggesting a potential cause for ascending biliary infection from the gut. Mechanical obstruction and gallbladder mucocele (discussed in more detail later) should be ruled out first, usually by ultrasonography, and then liver and bile and/or gallbladder mucosa specimens should be obtained for histopathology and microbial culture and sensitivity testing, preferably before antibiotic treatment is initiated.
Liver biopsies and bile samples can be obtained by direct visualisation during surgery or laparoscopy or via ultrasonographic guidance. The latter method carries a greater risk of bile leakage; to minimize this, a 22-gauge needle attached to a 12-ml syringe is used for cholecystocentesis (bile retrieval), and an attempt is made to evacuate the gallbladder. The procedure is best performed under general anesthesia rather than heavy sedation to minimize the chance of patient motion during aspiration. The risk of iatrogenic bile or septic peritonitis is greatest with patients with a severely diseased gallbladder wall (determined ultrasonographically); surgical treatment is necessary if bile peritonitis occurs. Enteric organisms similar to those found in cats are most commonly found, and the most common isolate in several studies is Escherichia coli. Other organisms reported are all of gut origin and include Enterococcus sp., Klebsiella sp., Clostridium sp. (which may be a gas-forming species causing emphysematous changes in the gallbladder wall visible radiographically), fecal Streptococcus sp., Corynbacterium spp., and Bacteroides sp. Antibiotic resistance is relatively common among isolates and can also develop during therapy, underscoring the importance of obtaining bile samples for culture and sensitivity whenever possible. Choleliths can be found in association with cholecystitis or cholangitis; the cause-and-effect relationship is not always clear.
GALLBLADDER MUCOCELE
Gallbladder mucocele has recently been reported as a common cause of clinical signs of biliary tract disease in dogs (Figure 38-7). The cause is unclear, but it is most common in middle-aged to older dogs; there appears to be a breed predisposition in Shetland Sheepdogs in the United States (Aguirre et al., 2007). Other suggested breed associations are Cocker Spaniels and Miniature Schauzers. It has been proposed that sterile or septic inflammation of the gallbladder wall and/or disordered gallbladder motility predispose to mucocele formation. In the Shetland Sheepdogs there appeared to be an association between gallbladder mucocele and dyslipidemias, usually caused by other concurrent diseases such as pancreatitis, hyperadrenocorticism, hypothyroidism, and diabetes mellitus.
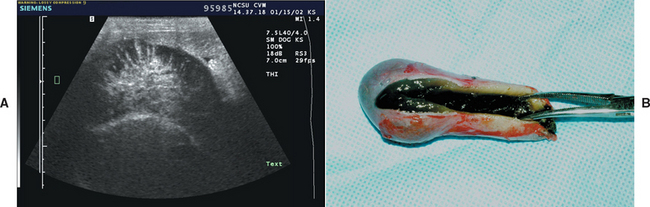
FIG 38-7 A, Ultrasonographic transverse image of the gallbladder of a dog with a mucocele; note the stellate pattern to the bile. The mucinous material does not move with change in patient position. B, Appearance of the gallbladder and contents after surgical removal.
(Courtesy Dr. Kathy A. Spaulding, North Carolina State University, College of Veterinary Medicine.)
Clinical signs vary. In some dogs mucocele is clinically silent and is an incidental finding on abdominal ultrasonography (Fig. 38-7). In others nonspecific clinical signs are seen similar to those of other biliary tract diseases with anorexia, lethargy, vomiting, and icterus. Some dogs present acutely because of gallbladder rupture and bile peritonitis.
Treatment is usually surgical for clinically affected dogs with cholecystectomy with or without biliary diversion. There is a high perioperative mortality, particularly for dogs that have biliary diversion surgery. However, those that survive the perioperative period have a good long-term prognosis. Medical management of subclinical mucoceles has been reported in Shetland Sheepdogs (Aguirre et al., 2007). This consisted of a low-fat diet (such as Hills ID or Royal-Canin Waltham intestinal low fat or Eukanuba intestinal diets) with a choleretic (ursodeoxycholic acid 10-15 mg/kg total dose daily, preferably split twice daily) and an anti-oxidant (S-adenosylmethionine 20 mg/kg PO q24h). In one dog this resulted in resolution of the mucocele, in two dogs the mucocele remained static, one dog died as a result of gallbladder rupture, and one dog died as a result of pulmonary thromboembolism, both within 2 weeks of diagnosis; two dogs were lost to follow-up. It would seem sensible also to address the underlying cause of the dyslipidemia in all cases, whether surgically or medically managed.
EXTRAHEPATIC BILE DUCT OBSTRUCTION
The causes of extrahepatic bile duct obstruction (EBDO) in dogs are very similar to those in cats (see Box 37-4) with the exception of liver flukes, which are uncommon in dogs. The most common cause of EBDO in dogs is extraluminal obstruction from acute-on-chronic pancreatitis (see Chapter 40), but intestinal foreign bodies, neoplasia, bile duct involvement in a diaphragmatic hernia, and other processes can also cause EBDO (Fig. 38-8). Bile duct injuries that heal and result in stricture formation several weeks later are also seen in dogs; the common bile duct (CBD) may be compressed when carried with the liver into the thorax in dogs with diaphragmatic hernia. Extraluminal compressive lesions, such as pancreatic, biliary, or duodenal neoplasms, are less common causes, and cholelithiasis as a cause of EBDO is rare. To be considered EBDO, a pathologic process must exist at the level of the CBD that impedes bile flow into the duodenum. Only if bile flow has been completely interrupted for several weeks do acholic feces, vitamin K–responsive coagulopathy, and repeated absence of urobilinogen in properly processed urine specimens occur. If obstruction is incom plete, these features are not present and the constellation of signs and clinicopathologic test results resembles those of other, nonobstructive biliary tract disorders.
BILE PERITONITIS
Bile peritonitis results most often from abdominal trauma damaging the common bile duct (e.g., penetrating injury, horse kick, automobile accident) or pathologic rupture of a severely diseased gallbladder, which sometimes occurs after diagnostic ultrasonography-guided aspiration. Early signs of bile peritonitis are nonspecific, but with progression, jaundice, fever, and abdominal effusion are seen. When bile, which is normally sterile, comes in contact with the peritoneal surface, resultant cell necrosis and changes in permeability predispose to infection with bacteria that move across the intestinal wall. Hypovolemia and sepsis may occur in animals with undetected bile peritonitis.
Clinical Features
Presenting clinical signs and clinicopathologic and physical examination findings of all these disorders may not differ greatly unless the underlying condition has caused EBDO or bile peritonitis. Regardless of the underlying disorder, typical clinical signs are jaundice, acute or chronic vomiting, anorexia, depression, weight loss, and occasionally vague cranial abdominal pain. Because of the protected location of the gallbladder in the abdomen, it is rarely possible to be able to palpate it in a dog with EBDO, unless the gallbladder is greatly enlarged.
Diagnosis
The pattern of clinicopathologic findings typical of biliary tract disorders is that of hyperbilirubinemia, high serum AP and GGT activities, high fasting and postprandial serum bile acid (SBA) concentrations, and less severe changes in serum ALT activity. SBA concentrations increase early in dogs with biliary stasis; in these circumstances the degree of SBA elevation gives no indication of liver function. Generally, more severe cholestatic lesions are associated with more severe clinicopathologic changes. Fractionating the total bilirubin concentration into direct- and indirect-reacting components (i.e., the van den Bergh reaction) does not distinguish intrahepatic from extrahepatic cholestasis or obstructive from nonobstructive cholestasis. Radiographically, there may be evidence of hepatomegaly and a mass effect in the area of the gallbladder on survey abdominal films. Gas shadows associated with the gallbladder and other biliary tract structures could be ascribed to ascending infection with gas-forming organisms. Findings consistent with acute-on-chronic pancreatitis as an underlying cause of EBDO are loss of serosal detail in the area of the pancreas as an indication of localized peritonitis, trapped pockets of gas in the duodenum, and duodenal displacement. However, in many cases of chronic pancreatitis imaging findings may be less severe or normal in spite of extensive fibrosis around the bile duct. Choleliths form in dogs in a manner similar to the way they form in cats, usually as a sequela to cholestasis and infection, but they may also be found in asymptomatic dogs. These concretions are radiolucent unless they contain calcium, which occurs about 50% of the time. Inflammatory abdominal effusion is expected in dogs with bile peritonitis but not in those with most causes of EBDO (except for effusions associated with pancreatitis or pancreatic cancer).
The ability to differentiate medical from surgical causes of jaundice has been refined with the development of ultrasonography, although this imaging modality is certainly not foolproof. Dilated and tortuous hepatic bile ducts and CBD, as well as gallbladder distention, are convincing ultrasonographic evidence of EBDO at the CBD or sphincter of Oddi. When dilated biliary structures are seen, it might be difficult to distinguish EBDO that requires surgical intervention from resolving, transient EBDO associated with severe acute-on-chronic pancreatitis or from nonobstructive biliary disease (e.g., bacterial cholecystitis/cholangitis) unless a source of obstruction is specifically identified (e.g., pancreatic mass, cholelith in the CBD). Prolonged fasting causes gallbladder enlargement because of delayed evacuation and should not be overinterpreted. In addition, cystic hyperplasia and epithelial polyp formation are common lesions in older dogs, not to be confused with choleliths in the gallbladder. A stellate appearance to the contents of the gallbladder is characteristic of gallbladder mucocele. Monitoring the serum bilirubin concentration to determine when to intervene surgically is not worthwhile because it begins to decline over days to weeks, without relief of obstruction, in both cats and dogs with experimentally induced EBDO. Conversely, in some dogs a significant proportion of bilirubin becomes irreversibly bound to albumin in the circulation (“biliprotein”), resulting in delayed clearance and continued elevation of serum bilirubin concentration for up to 2 weeks after the initial insult has resolved.
Treatment and Prognosis
If the distinction between medical and surgical causes of jaundice is not clear, it is safer to proceed surgically to avoid excessive delays in diagnosis. Surgery is required in dogs with persistent EBDO, bile peritonitis, and gallbladder mucocele. As with any other form of liver disease, it is important to stabilize the patient with fluids and electrolytes and perform a hemostasis profile and platelet count before surgery. Prolonged coagulation times may respond to vitamin K injections (1 mg/kg SQ q24h for 24 to 48 hours before and after surgery), but if not, a plasma transfusion is advisable before surgery to replace clotting factors. If surgery for bile peritonitis is to be delayed, peritoneal drainage should be established to remove noxious, bile-containing abdominal fluid and for lavage. Should a site of obstruction or biliary injury not be identified, at least tissue (i.e., liver, gallbladder mucosa) and bile specimens can be obtained for histopathologic and cytologic evaluation and bacterial culture and sensitivity testing. Any abdominal fluid should be analyzed cytologically and cultured for aerobic and anaerobic bacteria. A liver biopsy specimen should also be obtained in all cases. Typical hepatic histopathologic findings in dogs with early EBDO are canalicular bile plugs and bile ductular proliferation, with degrees of periportal inflammation and fibrosis in chronic cases. Confounding biliary infection can incite a stronger inflammatory reaction in the periportal region. However, it is impossible to diagnose a primary biliary tract infection from a liver biopsy alone. Aerobic and anaerobic culture and cytological examination of bile are required to diagnosis infectious cholangitis.
Surgical goals are to relieve biliary obstruction or leakage and restore bile flow. Reconstructive procedures to divert bile flow can be performed if the cause of EBDO cannot be corrected. However, because these carry a poor long-term prognosis, less invasive procedures such as stenting are preferred whenever possible (Amsellem et al., 2006).
Antibiotic therapy is started immediately after bile samples are obtained; ampicillin or amoxicillin (22 mg/kg IV, SQ, or PO q8h), first-generation cephalosporins (22 mg/kg IV or PO q8h), or metronidazole (7.5 to 10 mg/ kg PO q12h; use lower dose when severe hepatobiliary dysfunction is present) are good empiric choices as single agents initially in animals without a long history of antibiotic administration.
In cases without complete biliary obstruction (e.g., ascending cholangitis) or with transient obstruction (e.g., some cases of acute-on-chronic pancreatitis), medical management alone is indicated. The choleretic ursodiol is indicated as additional treatment in these cases, provided that complete EBDO has been ruled out. The recommended dose is 10 to 15 mg/kg total daily, preferably split into two doses. In addition, all cases (both medical and surgical) should receive antioxidant therapy, preferably with vitamin E (400 IU for a 30-kg dog, scaled appropriately to the size of the dog; tablets usually come as 100 IU, 200 IU, or 400 IU) and S-adenosylmethionine (20 mg/kg PO q24h) because it has been demonstrated that bile reflux in the liver is a potent oxidant toxin. Dogs should be fed a high quality diet which is not protein-restricted: in most cases, a diet designed for critical care feeding is more appropriate than a manufactured liver support diet, because the dog is suffering an inflammatory and/or septic process whereas hepatocyte function is usually good.
The prognosis for dogs with EBDO or bile peritonitis depends on the underlying cause. If the cause can be addressed without surgical reconstruction, the prognosis is fair to good. If extensive biliary reconstruction is needed, the prognosis is guarded.
CONGENITAL VASCULAR DISORDERS
Congenital disorders of hepatic vasculature, both intrahepatic and extrahepatic, are more common in dogs than in cats. There are some breed-related tendencies, suggesting a genetic basis to some disorders, but it is also assumed that most of them result from some type of (as yet undefined) insult in utero. It is known that experimental reduction in flow in the umbilical vein in sheep and other species can result in the development of PSSs and asymmetry of hepatic lobular and vascular supplies; this is likely also true in dogs. This would explain why it is relatively common to see dogs with more than one co-existent congenital vascular disorder in the liver (e.g. a congenital PSS combined with intrahepatic portal vein hypoplasia or microvascular dysplasia [MVD]) and would also explain why dogs with congenital PSSs have a higher prevalence of other congenital defects, such as cryptorchidism and cardiac disorders.
For ease of categorization and because they have different clinical presentations, congenital vascular disorders have been divided into disorders associated with low portal pressure and those with high portal pressure. However, it is important to remember than when two or more congenital hepatic defects occur concurrently, the differentiation will be less obvious.
CONGENITAL VASCULAR DISORDERS ASOCIATED WITH LOW PORTAL PRESSURE: CONGENITAL PORTOSYSTEMIC SHUNT
Etiology and Pathogenesis
Congenital PSSs are the most common congenital portovascular disorder in dogs. The etiology and pathogenesis are very similar to those in cats; the reader is referred to Chapter 37 for more details. Many different types of congenital portovascular anomalies have been reported in dogs; sometimes they co-exist with intrahepatic or extrahepatic portal vein hypoplasia or intrahepatic MVD (discussed in more detail later). However, a distinguishing feature of isolated congenital PSS is that it results in low portal pressure, because some blood is shunted away from the high resistance sinusoidal circulation by the shunting vessel. Dogs with isolated congenital PSS therefore do not present with ascites unless they are severely hypoalbuminemic. This allows differentiation from the congenital vascular disorders associated with increased portal pressure, and therefore acquired PSS, outlined below, in which portal hypertension and associated ascites are common at presentation.
Canine congenital PSS can be extrahepatic or intrahepatic. Extrahepatic PSSs are anomalous vessels connecting the portal vein or one of its contributors (left gastric, splenic, cranial or caudal mesenteric, or gastroduodenal veins) to the caudal vena cava or azygos vein. They are most commonly recognized in small-breed dogs and have a high prevalence in Cairn Terriers, Yorkshire Terriers, West Highland White Terriers, Maltese, Havanese, other terriers, and Miniature Schnauzers (Fig. 38-9). Intrahepatic PSSs may be left-sided, in which case they are thought to represent persistence of the fetal ductus venosus, or they can be right-sided or central, in which case they likely have a different embryological origin. Intrahepatic PSS is more commonly seen in large-breed dogs, but Collies also tend to have extrahepatic PSSs, despite being large dogs. Increased breed prevalence suggests a genetic basis to the disease, but this has only been investigated in Irish Wolfhounds, in which an inherited basis of patent ductus venosus has been demonstrated, and in Cairn Terriers with extrahepatic PSS, in which an autosomal polygenic inheritance or monogenic with variable expression is suspected (Van Straten et al., 2005). Affected Irish Wolfhounds tend to have smaller litters and can also produce more than one puppy with a PSS in a litter.
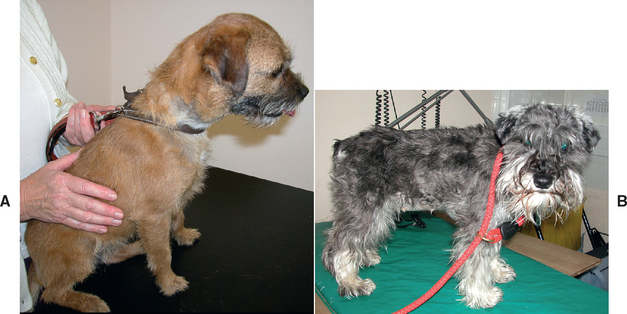
FIG 38-9 Typical small-breed dogs with congenital extrahepatic portosytemic shunts. A, An 8-month-old female Border Terrier. B, A 9-month-old female Miniature Schnauzer.
One study reported that dogs from breeds that were not usually recognized as having a high risk of PSS were more likely to present with unusual anatomical forms of PSS that were less often amenable to surgical management (Hunt, 2004).
Clinical Features
Clinical signs are very similar to those in cats; neurological, gastrointestinal, and urinary tract signs predominate (see Chapter 37 for more details). About 75% of dogs present before 1 year of age, but some present at an older age, with some as old as 10 years of age before signs are recognized. There is a spectrum of severity of neurological signs ranging from severely affected young puppies that persistently circle, become centrally blind, and can even have seizures or become comatose to very mildly affected individuals. It is likely that this variation reflects differences in shunt fraction and also dietary and other environmental differences among dogs. Polydipsia and polyuria with hyposthenuric urine are relatively common; this is largely due to high cortisol concentration in affected dogs (see Chapter 35) and also increases in antidiuretic hormone and reduced renal medullar concentrating gradient (see Chapter 35). Urate uroliths are also common and can be renal. Anecdotally, urate renal calculi seem to be more common in terriers, and dogs presenting with calculi often do not have prominent neurological signs. On physical examination animals are often (but not always) smaller than their littermates and may have non-localizing neurological signs and (in some cases) palpable renomegaly. The latter is due to circulatory changes and is not a reflection of renal disease; it is of no clinical significance and regresses after shunt ligation. Other congenital defects may be apparent, particularly cryptorchidism.
Diagnosis
Diagnosis of congenital PSS in dogs is the same as in cats (see Chapter 37) and relies on visualizing the shunting vessel ultrasonographically, with portovenography (Fig. 38-10), or grossly at surgery. Scintigraphy can demonstrate shunting but is not helpful to differentiate congenital from acquired PSS, so some other imaging method is necessary for treatment decisions. See Chapter 36 for more information on imaging PSS.
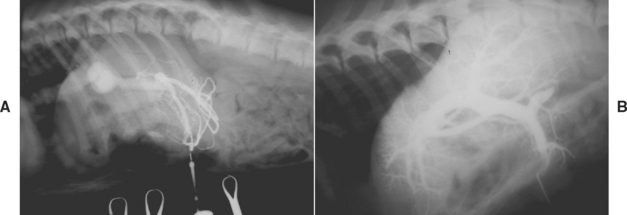
FIG 38-10 A, Portovenogram in a 1-year-old Golden Retriever with an intrahepatic portosystemic shunt. This was a central divisional shunt and had a venous sinus–like structure, as demonstrated well in this radiograph. B, Normal portovenogram in a dog for comparison.
(Courtesy the Diagnostic Imaging Department, the Queen’s Veterinary School Hospital, University of Cambridge.)
It is important, if possible, to try to estimate how well-developed the remaining hepatic portal vasculature is by repeating the portovenography after ligation and/or by evaluating the histological findings on liver biopsies taken at the time of ligation. This is a work in progress, but there is a strong suspicion that the prognosis postligation may depend on the potential for the intrahepatic vasculature to open up after surgery and that dogs that do poorly postoperatively may have concurrent portal vein hypoplasia and/or MVD (discussed in more detail later).
Nonspecific clinicopathologic findings in more than 50% of affected dogs, regardless of the type of vascular anomaly, are microcytosis, hypoalbuminemia, mild increases in serum AP and ALT activities, hypocholesterolemia, and low BUN concentration. Fasting bile acid concentrations may be normal or high, but postprandial bile acid concentrations are high in all cases. However, this does not distinguish congenital PSS from acquired PPS or early cholestasis, which also causes increases in bile acid concentrations. Postpran dial ammonia concentration can also be measured and will be high, whereas fasting ammonia concentration may be high or normal (see Box 36-1 for details of how to perform an ammonia challenge test). Ammonia tolerance or challenge tests are potentially dangerous because they can precipitate an encephalopathic crisis. Other tests have been evaluated for their sensitivity and specificity in the diagnosis of PSS. Protein C, a liver-derived anticoagulant factor, is also decreased in dogs with PSS and increases after ligation; this can help differentiate PSS from MVD.
Puppies of high-risk breeds could be screened for congenital PSS by measuring bile acid or ammonia concentrations before they are placed into homes, but there are potential false positives with both of these tests and no puppy should be euthanized or labeled as having a definite congenital PSS on the basis of high bile acid and/or ammonia concentrations without further evidence. Normal Irish Wolfhounds can have a transiently high blood ammonia concentration between the ages of 6 to 8 weeks; this normalizes at 3 to 4 months of age. Zandlivet et al. (2007) have demonstrated that this is due to a clinically insignificant urea cycle defect. Postprandial bile acid concentrations can be falsely elevated in Maltese puppies without PSS for unknown reasons, again confusing any efforts at screening tests in this breed (Tisdall et al., 1995).
On diagnostic imaging the liver is frequently (but not always) small. Ultrasonography now has a high sensitivity and specificity for the diagnosis of both intrahepatic and extrahepatic PSS; furthermore, their anatomy can usually also be described ultrasonographically.
Treatment and Prognosis
Surgical occlusion of the anomalous vessel to restore normal portal circulation has long been recommended as the treatment of choice. In many cases this will restore normal or near normal liver function. However, owners need to be aware of the small but definite risk of postoperative mortality as a result of portal hypertension and/or refractory seizures and of the potential that the PSS may be only partially and not totally ligated. In fact, it is more common to be able to partially ligate the PSS at the first surgery because the portal vasculature cannot initially accommodate all the shunting blood. In some cases it is possible to repeat the surgery at a later date to ligate the PSS further, but this is often not necessary to control clinical signs. A few dogs with partially ligated shunts develop portal hypertension and multiple acquired PSS with a recurrence of their clinical signs. There are several different surgical procedures described for ligation of PSS, but they are outside the scope of this book. In addition to surgical ligation, PSS may be attenuated with ameroid constrictors (Fig. 38-11) or embolized with coils. Laparoscopic ligation of PSS has been reported in two dogs (Miller et al., 2006). Ligation of a PSS requires an experienced surgeon.
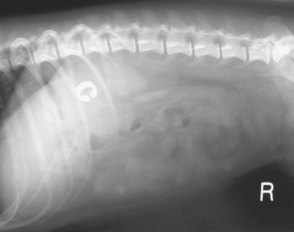
FIG 38-11 Lateral abdominal radiograph of a 3-year-old Miniature Schnauzer that had an extrahepatic portosystemic shunt ligated with an ameroid constrictor 2 years previously. Note the ameroid is visible as a radiodense ring in the craniodorsal abdomen.
(Courtesy the Diagnostic Imaging Department, the Queen’s Veterinary School Hospital, University of Cambridge.)
Medical management is required to stabilize the patient before surgery and also for about 8 weeks after surgery while the hepatic vasculature and mass recover. This involves careful dietary management combined, in many cases, with antibiotics and soluble dietary fiber. The details are outlined in Chapter 39. In some cases medical management may continue successfully over the course of the patient’s life as an alternative to surgery (Watson et al., 1998). Usually, this is because the client cannot afford referral or is unhappy about the risks associated with surgery or because the patient has multiple or intrahepatic shunts. Mildly affected and older animals are good candidates for medical management; generally, these are the individuals with smaller shunting fractions. Dogs (particularly terriers) that present at an older age with urate stones but no neurological signs, are also good candidates for medical management alone. In addition, dogs with concurrent portal vein hypoplasia and/or MVD tend to have a higher surgical risk and are best managed medically. Medical management does not reverse the underlying disorder but can result in good long-term results. Once the dog has reached adulthood, there is no evidence that the liver progressively atrophies throughout life. Ultimately, more studies are needed to identify the factors that are most important in determining prognosis after medical and/or surgical management and to help identify preoperatively the small number of animals that will have a poor outcome after surgery.
CONGENITAL VASCULAR DISORDERS ASSOCIATED WITH HIGH PORTAL PRESSURE
There are a number of less common congenital vascular disorders of the liver in dogs that present with normal or high portal pressure, rather than the low portal pressure seen in association with congenital PSS. Because of the portal hypertension, the affected dog may present with the constellation of typical clinical signs (see Chapter 39), including ascites, and the potential for GI ulceration in addition to multiple acquired PSS and HE. With the exception of arteriovenous fistulae, none of these conditions can be treated surgically; however, some of them have a good long-term prognosis with medical management.
Etiology and Pathogenesis
There are several reports of vascular disorders in young dogs associated with portal hypertension, usually ascites, and characteristic histopathological changes in the liver of a reduction in smaller portal vein branches, increased numbers of arterioles, and a variable amount of mild fibrosis. There are some reports of overt hypoplasia of the extrahepatic portal vein, but most reports of noncirrhotic portal hypertension and MVD appear to describe portal vein hypoplasia confined to the intrahepatic vasculature. These diseases may all be different abnormalities or they may represent different spectra of the same abnormalities, but their clinical presentation, treatment, and prognosis are similar. A lack of intrahepatic or extrahepatic portal vein branches results in portal hypertension, with the same potential consequences as those of chronic hepatitis (see preceding section), including ascites, gut wall edema, and often GI ulceration and acquired PSS. Dogs with MVD often do not present with notable portal hypertension, but it is grouped with these diseases by the WSAVA liver standardization group (Cullen et al., 2006). Dogs reported with MVD typically have shunting at the level of the hepatic lobule but do not have clinical signs of overt portal hypertension.
Any breed can be affected, but MVD particularly affects small-breed dogs, with Yorkshire Terriers and Cairn Terriers showing a particularly high incidence.
Clinical Signs
Dogs with all these conditions typically present at a young age with a combination of signs of portal hypertension and PSS, the severity of which depends on that of their lesions. Because of the acquired PSS seen in these patients, some of the clinical signs and clinicopathologic findings overlap with those of congenital PSS, particularly because all these disorders typically present in young dogs. Therefore presence of other signs of portal hypertension (e.g., ascites) is an important clinical clue that one of these disorders with acquired PSS may be present, rather than a congenital PSS.
Dogs with portal vein hypoplasia or idiopathic noncirrhotic portal hypertension typically present between 1 and 4 years of age and are often purebreds of either gender; large breeds predominate. Early reports of “congenital” or juvenile hepatic fibrosis in German Shepherd Dogs may also have represented a form of noncirrhotic portal hypertension. Presenting signs are typically those of portal hypertension, with abdominal distention associated with effusion; GI signs; polydipsia; weight loss; and, less consistently, signs of HE. Dogs are often surprisingly alert (Fig. 38-12).
FIG 38-12 A female German Shepherd Dog with noncirrhotic portal hypertension. A, At 14 months of age, with ascites and in poor body condition but remarkably alert B, 5 years later on medical management only—very stable and in good body condition with no detectable ascites. The dog lived for 8 years with a good quality of life before developing a gastroduodenal ulcer (see Chapter 39). C, Drugs that the dog received long term, in addition to dietary management.
(B and C reproduced by permission from UK Vet, 9(7):41, 2004.)
Dogs with MVD present with similar clinicopathological findings but usually without overt evidence of portal hypertension or ascites. MVD tends to affect terriers and thus overlaps with breeds at high risk for congenital PSS. In addition, some dogs may have both congenital PSS and MVD or portal vein hypoplasia, further confusing the diagnosis. Cairn Terriers and Yorkshire Terriers in particular have been reported with MVD. In one breed (the Cairn Terrier), the site of anatomic abnormality has been identified as the terminal portal veins. In this breed it is believed to be an autosomal, inherited trait, but the specific mode of inheritance has not been established. Typical signs include vomiting, diarrhea, and signs of HE, although the clinical signs, particularly the HE, are notably milder in dogs with MVD than in those with congenital PSS unless both disorders occur concurrently. Dogs with only MVD are somewhat older, and many have mild to no signs of illness. In the case of young purebred dogs that have been screened for congenital PSS before sale or that are ill for nonhepatic reasons, high SBA concentration may be the only finding.
Diagnosis
Diagnosis of MVD/intrahepatic portal vein hypoplasia and noncirrhotic portal hypertension relies ultimately on liver biopsy findings of intrahepatic portal vein hypoplasia in the absence of a grossly demonstrable shunting vessel. The liver biopsy findings alone can be indistinguishable from the changes that occur secondary to congenital PSS, and therefore the clinical findings of concurrent portal hypertension and ruling out a shunting vessel are important parts of the final diagnosis. Clinicopathologic findings are very similar to those in dogs with congenital PSS and include microcytosis, evidence of hepatic dysfunction (e.g., hypoalbuminemia), and low urine specific gravity. Microhepatia and hypoechogenic abdominal fluid are the notable abdominal ultrasonographic findings in dogs with noncirrhotic portal hypertension; it may be possible to visualize multiple acquired PSSs ultrasonographically. Dogs with MVD alone tend not to have ascites and have less marked increases in SBA concentrations than dogs with true congenital PSS.
The most important aspects of identifying a dog with MVD/portal vein hypoplasia/noncirrhotic portal hypertension are ruling out a surgically correctable PSS, identifying portal hypertension (which requires treatment, see Chapter 39), and obtaining a liver biopsy for confirmation or exclusion of other hepatopathies. Portal vein hypoplasia and noncirrhotic portal hypertension are very similarly clinically, on clinical pathology, and on diagnostic imaging to end-stage chronic hepatitis with cirrhosis, and the only way to differentiate the two is on liver histology. In general, portal vein hypoplasia/noncirrhotic portal hypertension carries a much better long-term prognosis than cirrhosis, so the differentiation is important prognostically.
Treatment and Prognosis
The prognosis for all these conditions appears to be relatively good, provided the clinical signs can be controlled. They are nonprogressive, and there is no surgical treatment for any of them; symptomatic therapy of HE, ascites, and GI ulceration (if present) is usually successful (see Chapter 39). It should be noted that glucocorticoid therapy is absolutely contraindicated in these dogs and is likely to worsen the outcome because of the associated portal hypertension and high risk of GI ulceration. This underlines the importance of liver biopsy in these dogs, allowing differentiation from chronic hepatitis.
One study of dogs with noncirrhotic portal hypertension concluded that affected dogs might live as long as 9 years after diagnosis with appropriate symptomatic therapy. A few dogs were euthanized because of problems related to persistent portal hypertension (e.g., duodenal ulceration). Dogs with MVD tend to have milder clinical signs than dogs with congenital PSS and can be managed medically with success over the long term. Affected dogs seem to live comfortably in good to excellent condition for at least 5 years (Christiansen et al., 2000).
Arterioportal Fistula
Intrahepatic arterioportal fistula, causing marked volume overload of the portal circulation resulting in portal hypertension, acquired PSSs, and ascites, is seen occasionally. Abdominal ultrasonography with Doppler can frequently detect the tortuous tubular structures representing the connection between an artery and overperfused portal vein or veins; sometimes the turbulent blood flow through the fistula can be auscultated through the body wall. If only one lobe of the liver is affected, the lobe containing the arterioportal fistula can be removed surgically; assuming that there is adequate intrahepatic portal vasculature, acquired PSSs regress once portal overcirculation subsides. More often, multiple liver lobes are involved, making surgical treatment impossible.
FOCAL HEPATIC LESIONS
ABSCESSES
Etiology
Hepatic abscesses are usually the result of septic embolization from an intraabdominal bacterial infection. In puppies they are a frequently a consequence of omphalophlebitis, whereas in adult dogs they arise most often subsequent to inflammatory conditions of the pancreas or hepatobiliary system. Adult dogs with certain endocrine diseases, such as diabetes mellitus or hyperadrenocorticism, are also at risk. Occasionally, infection arising from a location other than the abdominal cavity, such as the endocardium, lung, or blood, may disseminate to the liver, causing abscessation.
In a review (Farrar et al., 1996) of 14 dogs with hepatic abscesses, aerobic bacteria were isolated in 9 of 10 cases in which material from the hepatic lesions was submitted for culture. Although the most common isolates were gram-negative organisms, Staphylococcus spp. were identified in two dogs. Clostridium sp. was the only isolate cultured anaerobically from abscess fluid in 4 of 7 dogs.
Clinical Features
The typical signalment and physical examination findings in dogs with hepatic abscesses depend on the underlying cause. Dogs over 8 years old are most often affected because the predisposing causes of liver abscesses are seen more commonly in older dogs. Regardless of the initiating event, anorexia, lethargy, and vomiting are consistent presenting complaints. Expected physical examination findings include fever, dehydration, and abdominal pain. Hepatomegaly may be detected in dogs with diabetes mellitus or hyperadrenocorticism and in some dogs with primary hepatobiliary disease.
Diagnosis
Neutrophilic leukocytosis with a left shift, with or without toxic changes, and high serum ALP and ALT activities are dependable but nonspecific clinicopathologic abnormalities. Survey abdominal radiographs may reveal evidence of irregular hepatomegaly, a mass, or gas opacities within the area of the hepatic parenchyma (Fig. 38-13), but ultrasonography is the imaging modality of choice. One or more hypoechoic or anechoic hepatic masses and perhaps a hyperechoic rim surrounding the mass or masses are characteristic findings. If there are multiple masses that would preclude surgical removal or if the owner declines surgery, FNA cytologic analysis of the contents of a representative lesion will distinguish an abscess from nodular hyperplasia, neoplasm (e.g., hemangiosarcoma), or granuloma. Ideally, material should be obtained for cytologic analysis and aerobic and anaerobic bacterial culture from a representative lesion deep in the liver parenchyma to prevent abscess rupture and abdominal contamination. Abscess material should also be obtained by this approach during surgery so that antibiotic treatment can be initiated postoperatively. Ultrasound-guided drainage of the abscess can also be used as treatment in combination with appropriate antibiotics (discussed in more detail later). Results of the preliminary clinicopathologic and radiographic evaluation should be scrutinized for evidence of previously mentioned associated or predisposing illnesses.
Treatment and Prognosis
Treatment for liver abscesses consists of surgical removal of infected tissue, administration of appropriate antibiotics, supportive care, and resolution of underlying predisposing conditions. Infected liver tissue should be removed, if possible, and submitted for histopathologic examination and bacterial culture if this was not done preoperatively. Fluid, electrolyte, and acid-base abnormalities are addressed. Administration of a combination of antibiotics with a gram-negative and anaerobic spectrum is initiated until culture and sensitivity test results are available. Because staphylococci and clostridia are the most common isolates, amoxicillin (10 to 20 mg/kg IV q8h) or enrofloxacin (2.5 mg/kg IV or PO q12h) combined with metronidazole (10 mg/kg PO q8-12h or 7.5 mg/kg PO q8-12h for dogs with hepatic dysfunction) or clindamycin (10 mg/kg IV or PO q12h) is a good empiric choice. Surgery is not indicated in animals with multiple abscesses; ultrasound-guided centesis and abscess evacuation may be a reasonable adjunct to treatment. Antibiotic treatment is continued on a long-term basis, usually for 6 to 8 weeks or until clinicopathologic and ultrasonographic indicators of abscessation are resolved. From the limited information available about this rare condition, it seems that with aggressive medical and surgical management the prognosis for dogs with liver abscesses may not be as poor as once thought.
NODULAR HYPERPLASIA
Hepatic nodular hyperplasia is a benign condition of older dogs that does not cause clinical illness; clinicians should be aware of it, however, because hyperplastic nodules may be misinterpreted as a more serious condition, such as primary or metastatic malignancy or regenerative nodules associated with cirrhosis. The prevalence increases with age, and as many as 70% to 100% of dogs older than 14 years of age have some microscopic or macroscopic hyperplasia. Affected dogs have high serum ALP activity (usually 2.5-fold elevation but may be as high as fourteenfold), which prompts investigation for hyperadrenocorticism. There is no evidence of hepatic dysfunction on serum biochemical analysis. Many dogs have multiple macroscopic nodules found ultrasonographically or at surgery, ranging in size from 2 to 5 cm in diameter; some dogs have a single nodule. Micronodular change occurs much less frequently and would be identified in liver biopsy specimens. The lesion consists of increased numbers of normal to vacuolated hepatocytes with more mitotic figures and fewer binucleate cells than expected in normal liver; components of normal lobular architecture (e.g., portal tracts, central vein) remain. Adjacent parenchyma is compressed by growth of the nodules; fibrosis, necrosis, inflammation, and bile ductule hyperplasia are absent. Because the prognosis for each of these nodular conditions is different and the margin of the lesion with adjacent hepatic tissue is important to establish a diagnosis, a wedge biopsy is recommended. Needle specimens are likely to be too small to confidently differentiate nodular hyperplasia from primary hepatocellular carcinoma or adenoma. The cause of this lesion is unknown; on the basis of experimental development of nodular hyperplasia in rodent species, some have speculated a dietary role (low protein).
NEOPLASIA
Etiology
Primary hepatic neoplasms are rare in dogs, accounting for fewer than 1.5% of all canine tumors. Unlike in cats, malignant tumors are more common than benign tumors, and metastatic tumors are 2.5 times more common than primary tumors in dogs. Metastases particularly arise from primary neoplasms in the spleen, pancreas, and GI tract (Fig. 38-14); the liver can also be involved in systemic malignancies such as lymphoma, malignant histiocytosis, and mastocytosis.
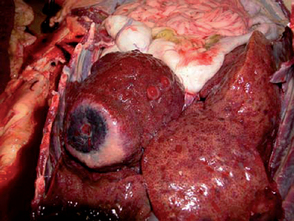
FIG 38-14 Gross appearance of liver post-mortem from a 2-year-old male Husky with a metastic carcinoma.
Although certain chemicals can induce hepatic neoplasms experimentally and infectious hepatitis is also a predisposing cause in other species, the cause of naturally occurring canine hepatic neoplasms is unknown. The types of primary hepatic tumors seen in dogs and their relative importance and metastatic potential are outlined in Table 38-3.
 TABLE 38-3 Primary Liver Tumors in Dogs
TABLE 38-3 Primary Liver Tumors in Dogs
| Note that malignant tumors are more common than benign tumors and that metastases to the liver are more common than primary liver tumors in dogs. |
| TYPE OF TUMOR | COMMENTS |
|---|---|
| Hepatocellular tumors: | HCC most common primary liver tumor in dogs (50%). Most are massive; some are nodular or diffuse. Miniature Schnauzers and male dogs may be at increased risk. MR 0% to 37% for massive and 93% to 100% for nodular and diffuse forms. Adenoma uncommon and usually incidental. |
| Hepatocellular carcinoma (HCC) | |
| Hepatocellular adenoma/hepatoma | |
| (Hepatoblastoma—very rare) | |
| Bile duct carcinomas second most common primary tumor in dogs (22% to 41% of malignant canine liver tumors). Labrador Retrievers and females may be at increased risk. Usually aggressive. MR up to 88%. Adenomas uncommon and gallbladder tumors very rare. | |
| Very rare, but always diffuse or nodular, and very aggressive. | |
| Uncommon. Most locally aggressive, diffuse or nodular and high MR. |
MR, Metastatic rate.
Clinical Features
Clinical signs and physical examination findings in dogs with primary or secondary liver tumors are nonspecific, except for diffuse or nodular hepatomegaly. Even this can be confused with other conditions, such as macronodular cirrhosis or benign nodular hyperplasia, which are also common in older dogs. Therefore no dog should be euthanized on the basis of a presumptive diagnosis of a liver mass on clinical examination or diagnostic imaging without supportive histology. The left liver lobes are often affected by hepatocellular carcinoma which can occur in three different patterns: massive (single, large nodule; most common), nodular (multiple smaller nodules), and diffuse (indistinct nodules throughout). The behavior of each type of tumor tends also to be different, as outlined in Table 38-3.
Clinicopathologic abnormalities are likewise not specific for neoplasia and blood tests may be normal, even in dogs with extensive involvement. Dogs with lymphoma infiltrating the liver usually have marked increases in ALT and ALP activities but are rarely jaundiced; moreover, they may have normal liver echotexture. Hypoglycemia has been described in association with hepatocellular carcinoma in dogs and can be due to paraneoplastic production of insulin-like growth factor. Massive forms of hepatocellular carcinoma have a low metastatic rate. Metastases from other diffuse and nodular forms of hepatocellular carcinoma or biliary carcinoma usually occur early; the most common sites are regional lymph nodes, lung, and peritoneal surfaces. Hepatocellular adenoma (hepatoma) is a benign tumor that most often occurs as a single mass that is typically smaller than the massive form of hepatocellular carcinoma but can be multifocal. Histologic features of hepatocellular adenoma are very similar to those of nodular hyperplasia (or indeed normal liver) except for the presence of a fine rim of reticulin surrounding the adenoma and lack of apparent normal architecture (i.e., few portal tracts, no central veins).
Treatment and Prognosis
When a single large hepatic mass is identified, it can be very difficult to distinguish well-differentiated hepatocellular carcinoma from nodular hyperplasia and hepatocellular adenoma. Surgical resection is the treatment of choice for primary hepatic neoplasms and for massive hepatocellular carcinoma. In the latter, it usually carries a good prognosis because they have a lower metastatic rate than the more diffuse and nodular forms of the tumor and local recurrence rate after liver lobectomy is reportedly less than 13%. Long-term (2- to 3-year) survival rates after surgical resection are common in dogs with massive hepatocellular carcinoma Surgical excision is therefore the treatment of choice for single tumors involving one liver lobe because this allows both diagnosis and, in many cases, cure. The prognosis for diffuse and nodular hepatocellular carcinoma and other forms of primary malignant liver tumors is poor because there is no effective therapy. Radiation therapy is not effective because the liver cannot tolerate cumulative doses of radiation. Hepatic tumors also respond poorly to chemotherapy, likely partly because of development of rapid drug resistance by neoplastic hepatocytes. The response of secondary (metastatic) liver tumors depends on the type and location of the primary; responses in dogs with lymphoma are very good to excellent, and in dogs with hemangisoarcoma they are good. Metastatic carcinomas or carcinoids of the liver rarely respond to chemotherapy.
HEPATOCUTANEOUS SYNDROME/SUPERFICIAL NECROLYTIC DERMATITIS
Etiology and Pathogenesis
Hepatocutaneous syndrome (also known as superficial necrolytic dermatitis, metabolic epidermal necrosis, and necrolytic migratory erythema) is a skin condition reported in association with certain liver diseases that usually carries a poor prognosis. The pathophysiology and underlying causes in dogs remain unclear, and it is likely multifactorial. It occurs in association with certain classical findings on hepatic ultrasonography and histopathology, and often no underlying cause is found. However, because it is likely that many cases represent a hepatic reaction to an underlying endocrine tumor or disorder, superficial necrolytic dermatitis represents an intermediate disorder between primary liver disease and secondary hepatopathies.
The underlying pathogenesis in the skin appears to be due to abnormally low circulating amino acid concentrations and thus malnutrition of the skin, particularly in areas of poor blood supply, such as the extremities. Zinc deficiency may also be involved because the histological appearance of the skin is very similar to that in dogs with zinc-responsive dermatosis; fatty acid deficiencies have also been implicated. In humans the disorder is usually associated with a glucagon-secreting tumor of the pancreas. However, glucagonomas are rarely reported in affected dogs, and circulating glucagon concentrations are usually normal (although they may be occasionally high). Plasma amino acid concentrations have been reported to be very low in all affected dogs in which they have been measured, both in dogs with pancreatic tumors and dogs without. It has been proposed that canine superficial necrolytic dermatitis represents a metabolic hepatopathy with increased hepatic catabolism of amino acids that decreases their peripheral availability.
Recently, 11 dogs with superficial necrolytic dermatitis secondary to chronic phenobarbital administration for epilepsy were reported (March et al., 2004). The median age of the affected dogs was 10 years, and the median duration of phenobarbital therapy was 6 years. No other underlying cause could be found. Plasma amino acid concentrations were markedly decreased in the only dog in which they were measured.
Whatever the underlying pathogenesis, dogs with superficial necrolytic dermatitis are at high risk of becoming diabetic, which is reported in 25% to 40% of cases. This is easy to explain if blood glucagon concentrations are high, because glucagon is a diabetogenic hormone, but is difficult to explain on the basis of simple amino acid alterations.
Clinical Findings
Idiopathic superficial necrolytic dermatitis is reported most often in older dogs of small breeds; in one study 75% of the affected dogs were male (Outerbridge et al., 2002). Most dogs present because of their skin disease rather than their primary liver disease. Typically, there is erythema; crusting; and hyperkeratosis affecting the footpads, the nose, and periorbital, perianal, and genital areas and also often pressure points on the limbs. The paw lesions can be extremely painful because of associated fissures and may result in lameness and secondary infection. Signs of liver disease may also be present (although usually not), and diabetes mellitus often develops later in the disease process, especially if the animal is given diabetogenic drugs such as glucocorticoids in an attempt to control the skin disease.
Diagnosis
Definitive diagnosis is based on skin biopsy findings that are very characteristic and unique: The only syndrome with a similar appearance on skin histopathology is zinc-responsive dermatosis. There is a marked parakeratotic hyperkeratosis with intercellular and intracellular edema and hyperplastic basal cells, producing a characteristic “red, white, and blue” appearance on hematoxylin and eosin staining.
The associated hepatic findings are more nonspecific, except for the ultrasonographic findings. There are usually increases in liver enzyme activities, and there may be hypoalbuminemia in some cases. In dogs that are diabetic there is hyperglycemia and glycosuria. The classical ultrasonographic appearance is a “Swiss-cheese” liver consisting of multiple hypoechoic regions with hyperechoic borders (Fig. 38-15). Hepatic histology in all cases is remarkably similar, showing what has been described as a distinctive form of macronodular cirrhosis. The liver is divided into regenerative hyperplastic nodules with fibrous septa and bordered by characteristic ballooned, vacuolated hepatocytes but with minimal or no inflammation or necrosis.
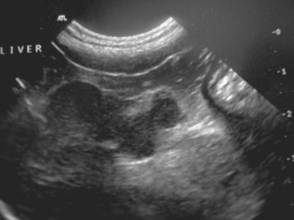
FIG 38-15 Ultrasonographic appearance of the liver of a 6-year-old Border Terrier with hepatocutaneous syndrome secondary to chronic phenobarbital medication for idiopathic epilepsy. Note the typical hypoechoic “holes” in the liver parenchyma on the left.
(Courtesy Diagnostic Imaging Department, Queen’s Veterinary School Hospital, University of Cambridge.)
Treatment and Prognosis
The prognosis is very poor unless the underlying cause can be identified and treated; most dogs live for less than 6 months. There have been reports of resolution of disease if a pancreatic tumor is identified and removed. Dogs with phenobarbital-associated hepatocutaneous syndrome may improve when the drug is withdrawn, although this has not yet been demonstrated. An alternative nonhepatotoxic therapy for their epilepsy will need to be instituted; potassium bromide might be an alternative choice, but it takes weeks to reach steady-state. Gabapentin might also be used, although this is only effective in some dogs. For additional information, please see Chapter 67.
When an underlying cause cannot be identified and treated, therapy is symptomatic and supportive. The most important aspect is amino acid/protein supplementation; in a few cases this may lead to long-term survival. There are single case reports of humans with resolution of the disease after amino acid infusions and/or regular dietary supplementation of egg protein; feeding egg yolks has also been reported as resulting in a clinical improvement in some dogs. It is unclear whether eggs are beneficial simply because they are a high-quality amino acid supplement or whether there are other beneficial micronutrients in the eggs. Dogs with hepatocutaneous syndrome should not be fed proprietary diets for liver disease because these are protein restricted. Other support included antibiotics for secondary skin infections (such as cefalexin 20 mg/kg q12h) and antioxidants (see the section on the treatment of chronic hepatitis). In addition, zinc and fatty acid supplementation may be helpful in some cases. Glucocorticoids should be avoided because they will precipitate diabetes mellitus. We have treated two dogs with hepatocutaneous syndrome that survived for several years on a high-quality digestible diet (marketed for GI disease) supplemented with extra egg and vitamin E and S-adenosylmethionine supplementation with antibiotics; however, one dog did become diabetic a month after diagnosis.
SECONDARY HEPATOPATHIES
Secondary (reactive and vacuolar) hepatopathies are very common in dogs. In fact, in pathology studies it is clear that they are more common than primary hepatic disease. Many of these hepatopathies result in elevations in liver enzymes, but the liver changes are usually not clinically significant and usually do not result in compromised liver function. However, they are often confused with primary liver disease, and it is important to rule out secondary hepatopathies as much as possible in the workup of dogs with elevated liver enzymes to allow identification and treatment of the underlying primary disease (e.g., endocrine disease or inflammatory disease elsewhere in the splanchic bed). It is also important to be aware that raised liver enzymes in an old dog have many other causes apart from primary liver disease and to resist the tendency to immediately put such dogs on a protein-restricted diet and other medication for liver disease before working up the case properly. Many dogs with secondary hepatopathies will not have hepatic histopathology performed because the primary cause will be identified with other tests. However, it is convenient from a classification point of view to split secondary hepatopathies into three groups on the basis of their appearance histopathologically: secondary hepatopathies associated with hepatocyte swelling and/or vacuolation, hepatic congestion/edema, and reactive hepatitis.
HEPATOCYTE VACUOLATION
Secondary hepatopathies associated with hepatocyte vacuolation are divided into steroid-induced hepatopathy and hepatocellular steatosis (lipidosis/fatty change). Steroid-induced hepatopathy is characterized by hepatocellular glycogen accumulation, which is distinctive from steatosis, in which fat (rather than glycogen) accumulates in hepatocytes. The difference can be demonstrated with special stains (Periodic acid Schiff for glycogen and Oil red O or Sudan black for fat), but there are some differences also on routine hematoxylin and eosin staining that help with differentiation: Glycogen vacuoles tend not to displace the nucleus from the center of the cell and often contain strands of eosinophilic material, whereas classic steatosis is associated with clear, empty vacuoles (because the fat is lost in processing) and the nucleus is often displaced to the edge of the cell (Fig. 38-16).
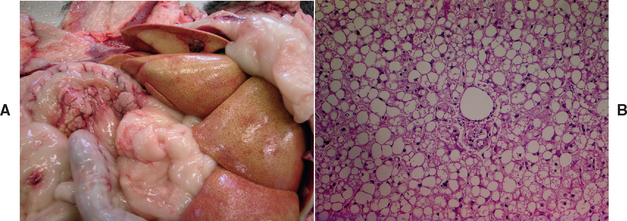
FIG 38-16 Gross (A) and histological (B) appearance of the liver postmortem in a middle-aged Miniature Poodle with poorly controlled diabetes mellitus. Note the pale, yellowish appearance of the liver associated with generalized hepatic steatosis. Histologically, the hepatocytes are markedly swollen with fat that displaces the nuclei to the edge of the cells. Portal triad in the center (Hematoxylin and eosin x 200).
(Courtesy Pathology Department, Department of Veterinary Medicine, University of Cambridge.)
Both types of vacuolar hepatopathy are reversible when the underlying cause is taken away. The most common causes are endocrine diseases (see Table 38-1). Steroid-induced hepatopathy is seen in hyperadrenocorticism and dogs being given exogenous corticosteroids. It has also been associated with other hormone therapies and administration of some other drugs, such as D-penicillamine. There have been reports of idiopathic vacuolar hepatopathy in Scottish terriers causing marked elevations in ALP, but the underlying cause is unknown. The vacuolation seen as part of the hepatocutaneous syndrome looks very similar to glycogen vacuolation. Steatosis is classically associated with diabetes mellitus in dogs, in which it starts centrilobularly and then spreads. It has also been reported in juvenile hypoglycemia of small-breed dogs. However, although hepatic steatosis can sometimes appear very marked in dogs, it does not appear to become a clinically significant disease in its own right, unlike in cats, in which primary or secondary hepatic lipidosis are important clinical syndromes (Chapter 37).
HEPATIC CONGESTION/EDEMA
Hepatic congestion is a common finding with right-sided congestive heart failure and other causes of posthepatic venous congestion, such as heartworm disease. This results again in elevation in liver enzymes. It is usually reversible, but in a few very chronic cases of congestion associated with heart disease, it can result in fibrosis and permanent compromise (“hepatic cirrhosis”).
NONSPECIFIC REACTIVE HEPATITIS
Nonspecific reactive hepatitis is a nonspecific hepatic response to a number of extrahepatic processes, particularly inflammatory processes in the splanchic bed such as pancreatitis and inflammatory bowel disease. There is a mild inflammatory infiltrate in the sinusoids and portal areas and/or parenchyma but no associated hepatocyte necrosis or fibrosis and therefore no evidence of primary (significant) hepatitis. This could be viewed as the hepatic equivalent of a “reactive lymph node” and should stimulate a search for an underlying cause.
Diagnosis
The diagnosis of all types of secondary hepatopathy relies on diagnosing the underlying cause. The clinical signs will be those of the primary cause and not related to the liver. However, sometimes there will be an overlap in clinical signs—notably with hyperadrenocorticism or diabetes mellitus in which the polydipsia, poluria, and abdominal enlargement together with raised liver enzymes might increase the suspicion of primary liver disease. Recognizing that there is a secondary hepatopathy involves initial pattern recognition of the enzyme elevation and clinical signs (e.g., in a dog with polydipsia/polyuria, a pot-belly, and dermatological signs, a pattern of a very marked elevation in ALP and less marked elevation in ALT should raise the suspicion of hyperadrenocorticism). This is followed by appropriate diagnostic tests for the underlying condition. Liver biopsies are usually not indicated or taken. However, there will inevitably be cases with mild or nonclassical changes of the primary condition in which liver biopsies will be taken on suspicion of primary hepatopathy. Finding nonspecific secondary changes in the liver should then stimulate a repeat search for an underlying cause.
Adamus C, et al. Chronic hepatitis associated with leptospiral infection in vaccinated beagles. J Comp Path. 1997;117:311.
Aguirre AL, et al. Gallbladder disease in Shetland Sheepdogs: 38 cases (1995-2005). J Am Vet Med Assoc. 2007;231:79.
Allen L, et al. Clinicopathological features of dogs with hepatic microvascular dysplasia with and without portosystemic shunts: 42 cases (1991–1996). J Am Vet Med Assoc. 1999;15:218.
Amsellem PM, et al. Long-term survival and risk factors associated with biliary surgery in dogs: 34 cases (1994-2004). J Am Vet Med Assoc. 2006;229:1451.
Boomkens SY, et al. PCR screening for candidate etiological agents of canine hepatitis. Vet Microbiol. 2005;108:49.
Bunch SE. Hepatotoxicity associated with pharmacologic agents in dogs and cats. Vet Clin N Am Small Anim Pract. 1993;23:659.
Bunch SE, et al. Idiopathic noncirrhotic portal hypertension in dogs: 33 cases (1982–1988). J Am Vet Med Assoc. 2001;218:392.
Christiansen JS, et al. Hepatic microvascular dysplasia in dogs: a retrospective study of 24 cases (1987–1995). J Am Anim Hosp Assoc. 2000;36:385.
Coronado VA, et al. New haplotypes in the Bedlington terrier indicate complexity in copper toxicosis. Mammalian Genome. 2003;14:483.
Cullen JM, et al. Morphological classification of circulatory disorders of the canine and feline liver. In: Rothuizen J, et al, editors. WSAVA standards for clinical and histological diagnosis of canine and feline liver disease. Oxford, England: Saunders Ltd, 2006.
Dunayer EK, et al. Acute hepatic failure and coagulopathy associated with xylitol ingestion in eight dogs. J Am Vet Med Assoc. 2006;229:1113.
Farrar ET, et al. Hepatic abscesses in dogs: 14 cases (1982–1994). J Am Vet Med Assoc. 1996;208:243.
Filburn CR, et al. Bioavailability of a silybin-phosphatidylcholine complex in dogs. J Vet Pharmacol Ther. 2007;30:132.
Fox JA, et al. Helicobacter canis isolated from a dog liver with multifocal nectrotizing hepatitis. J Clin Microbiol. 1996;34:2479.
Gabriel A, et al. Suspected drug-induced destructive cholangitis in a young dog. J Small Anim Pract. 2006;47:344.
Gillespie TN, et al. Detection of Bartonella henselae and Bartonella clarridgeiae DNA in hepatic specimens from two dogs with hepatic disease. J Am Vet Med Assoc. 2003;222:47.
Görlinger S, et al. Congenital dilatation of the bile ducts (Caroli’s disease) in young dogs. J Vet Intern Med. 2003;17:28.
Haywood S. Copper toxicosis in Bedlington terriers. Vet Rec. 2006;159:687.
Hartmann K, Greene CE. Diseases caused by systemic bacterial infections. In Ettinger SJ, Feldman EC, editors: Textbook of veterinary internal medicine, ed 6, St Louis: Saunders Elsevier, 2005.
Hoffmann G, et al. Copper-associated chronic hepatitis in Labrador Retrievers. J Vet Intern Med. 2006;20:856.
Hyun C, et al. Evaluation of haplotypes associated with copper toxicosis in Bedlington terriers in Australia. Am J Vet Res. 2004;65:1573.
Hunt GB. Effect of breed on anatomy of portosystemic shunts resulting from congenital diseases in dogs and cats: a review of 242 cases. Aust Vet J. 2004;82:746.
Jarrett WFH, et al. A new transmissible agent causing acute hepatitis, chronic hepatitis and cirrhosis in dogs. Vet Rec. 1985;116:629.
Jarrett WFH, et al. Persistent hepatitis and chronic fibrosis induced by canine acidophil cell hepatitis virus. Vet Rec. 1987;120:234.
Kitchell BE, et al. Peliosis hepatis in a dog infected with Bartonella henselae. J Am Vet Med Assoc. 2000;216:519.
Kristal O, et al. Hepatotoxicity associated with CCNU (lomustine) chemotherapy in dogs. J Vet Intern Med. 2004;18:75.
Lee KC, et al. Association of portovenographic findings with outcome in dogs receiving surgical treatment for single congenital portosystemic shunts: 45 cases (2000–2004). J Am Vet Med Assoc. 2006;229:1122.
Liptak JM. Hepatobiliary tumours. In Withrow SJ, Vail DM, editors: Withrow and MacEwan’s small animal clinical oncology, ed 4, St Louis: Saunders Elsevier, 2007.
Mandigers PJ, et al. Association between liver copper concentration and subclinical hepatitis in Doberman Pinschers. J Vet Intern Med. 2004;18:647.
Mandigers PJ, et al. Improvement in liver pathology after 4 months of D-penicillamine in 5 doberman pinschers with subclinical hepatitis. J Vet Intern Med. 2005;19:40.
March PA, et al. Superficial necrolytic dermatitis in 11 dogs with a history of phenobarbital administration (1995-2002). J Vet Intern Med. 2004;18:65.
Miller JM, et al. Laparoscopic portosystemic shunt attenuation in two dogs. J Am Anim Hosp Assoc. 2006;42:160.
Newman SJ, et al. Aflatoxicosis in nine dogs after exposure to contaminated commercial dog food. J Vet Diagn Invest. 2007;19:168.
O’Neill EJ, et al. Bacterial cholangitis/cholangiohepatitis with or without concurrent cholecystitis in four dogs. J Small Anim Pract. 2006;47:325.
Outerbridge CA, et al. Plasma amino acid concentrations in 36 dogs with histologically confirmed superficial necrolytic dermatitis. Vet Dermatol. 2002;13:177.
Pike FS, et al. Gallbladder mucocele in dogs: 30 cases (2000–2002). J Am Vet Med Assoc. 2004;224:1615.
Schermerhorn T, et al. Characterization of hepatoportal microvascular dysplasia in a kindred of cairn terriers. J Vet Intern Med. 1996;10:219.
Schwarz LA, et al. Hepatic abscesses in 13 dogs: a review of the ultrasonographic findings, clinical data and therapeutic options. Vet Radiol Ultrasound. 1998;39:357.
Seguin MA, et al. Iatrogenic copper deficiency associated with long-term copper chelation for treatment of copper storage disease in a Bedlington Terrier. J Am Vet Med Assoc. 2001;15:218.
Sepesy LM, et al. Vacuolar hepatopathy in dogs: 336 cases (1993-2005). J Am Vet Med Assoc. 2006;229:246.
Shawcross D, et al. Dispelling myths in the treatment of hepatic encephalopathy. Lancet. 2005;365:431.
Shih JL, et al. Chronic hepatitis in Labrador Retrievers: clinical presentation and prognostic factors. J Vet Intern Med. 2007;21:33.
Szatmari V, Rothuizen J. Ultrasonographic identification and characterization of congenital portosystemic shunts and portal hypertensive disorders in dogs and cats. In: Rothuizen J, et al, editors. WSAVA standards for clinical and histological diagnosis of canine and feline liver disease. Oxford, England: Saunders, 2006.
Teske E, et al. Cytological detection of copper for the diagnosis of inherited copper toxicosis in Bedlington terriers. Vet Rec. 1992;131:30.
Tisdall PL, et al. Post-prandial serum bile acid concentrations and ammonia tolerance in Maltese dogs with and without hepatic vascular anomalies. Aust Vet J. 1995;72:121.
Tobias KM, et al. Association of breed with the diagnosis of congenital portosystemic shunts in dogs: 2,400 cases (1980-2002). J Am Vet Med Assoc. 2003;223:1636.
Toulza O, et al. Evaluation of plasma protein C activity for detection of hepatobiliary disease and portosystemic shunting in dogs. J Am Vet Med Assoc. 2006;229:1761.
Van den Ingh TSGAM, et al. Morphological classification of parenchymal disorders of the canine and feline liver. In: Rothuizen J, et al, editors. WSAVA standards for clinical and histological diagnosis of canine and feline liver disease. Oxford, England: Saunders, 2006.
Van den Ingh TSGAM, et al. Possible nutritionally induced copper-associated chronic hepatitis in two dogs. Vet Rec. 2007;161:728.
Van de Sluis B, et al. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Molecr Genets. 2002;11:165.
van Straten G, et al. Inherited congenital extrahepatic portosystemic shunts in Cairn terriers. J Vet Intern Med. 2005;19:321.
Watson PJ. Decision making in the management of portosystemic shunts. In Practice. 1997;19:106.
Watson PJ. Canine chronic liver disease: a review of current understanding of the aetiology, progression and treatment of chronic liver disease in the dog. The Vet Journal. 2004;167:228.
Watson PJ, et al. Medical management of congenital portosystemic shunts in 27 dogs—a retrospective study. J Small Anim Pract. 1998;39:62.
Webb CB, et al. Copper-associated liver disease in Dalmatians: a review of 10 dogs (1998-2001). J Vet Intern Med. 2002;16:665.
Zandvliet MM, et al. Transient hyperammonemia due to urea cycle enzyme deficiency in Irish wolfhounds. J Vet Intern Med. 2007;21:215.