CHAPTER 52 Disorders of the Endocrine Pancreas
HYPERGLYCEMIA
Etiology
Hyperglycemia is present if the blood glucose concentration is greater than 130 mg/dl, although clinical signs of hyperglycemia do not develop until the renal tubular threshold for the resorption of glucose is exceeded. In dogs this typically occurs whenever the blood glucose concentration exceeds 180 to 220 mg/dl. The threshold for glucose resorption appears to be more variable in cats, ranging from 200 to 280 mg/dl. Glycosuria causes an osmotic diuresis, which in turn causes polyuria and polydipsia, the hallmark clinical signs of severe hyperglycemia (greater than 180 mg/dl in dogs and greater than 200 to 280 mg/dl in cats). The most common cause of hyperglycemia and glycosuria is diabetes mellitus. Severe hyperglycemia without glycosuria also occurs commonly in cats with stress-induced hyperglycemia, presumably resulting from the secretion of catecholamines and possibly lactate. Transient glycosuria (typically less than 1% on urine glucose test strips) may occur in some cats with severe or prolonged stress-induced hyperglycemia.
Clinical Features
Hyperglycemia of between 130 and 180 mg/dl (possibly as high as 280 mg/dl in cats) is clinically silent and is often an unsuspected finding encountered during blood testing for another reason. If a dog or cat with mild hyperglycemia (less than 180 mg/dl) and no glycosuria is seen because of poly uria and polydipsia, a disorder other than overt diabetes mellitus should be suspected. Mild hyperglycemia can occur in some dogs and cats up to 2 hours after consumption of diets containing increased quantities of monosaccharides and disaccharides, corn syrup, or propylene glycol; during intravenous (IV) administration of total parenteral nutrition fluids; in stressed, agitated, or excitable cats and dogs; in animals in the early stages of diabetes mellitus; and in animals with disorders and drugs causing insulin resistance (Box 52-1). A diagnostic evaluation for disorders causing insulin resistance is indicated if mild hyperglycemia is found to persist in a fasted, unstressed dog or cat, especially if the blood glucose concentration is increasing over time (see p. 783).
 BOX 52-1 Causes of Hyperglycemia in Dogs and Cats
BOX 52-1 Causes of Hyperglycemia in Dogs and Cats
* Common cause.
HYPOGLYCEMIA
Etiology
Hypoglycemia is present if the blood glucose concentration is less than 60 mg/dl. It typically results from the excessive use of glucose by normal cells (e.g., during periods of hyperinsulinism) or neoplastic cells, impaired hepatic gluconeogenesis and glycogenolysis (e.g., portal shunt, hepatic cirrhosis), a deficiency in diabetogenic hormones (e.g., hypocortisolism), an inadequate dietary intake of glucose and other substrates required for hepatic gluconeogenesis (e.g., anorexia in the neonate or toy breeds), or a combination of these mechanisms (e.g., sepsis; Box 52-2). Iatrogenic hypoglycemia is a common problem resulting from overzealous insulin administration in diabetic dogs and cats.
 BOX 52-2 Causes of Hypoglycemia in Dogs and Cats
BOX 52-2 Causes of Hypoglycemia in Dogs and Cats
* Common cause.
Prolonged storage of blood before separation of serum or plasma causes the glucose concentration to decrease at a rate of approximately 7 mg/dl/h. Glycolysis by red and white blood cells becomes even more apparent in dogs and cats with erythrocytosis, leukocytosis, or sepsis. Therefore whole blood obtained for the measurement of the glucose concentration should be separated soon after collection (within 30 minutes), and the serum or plasma should be refrigerated or frozen until the assay is performed to minimize artifactual lowering of the blood glucose concentration. Glucose determinations from separated and refrigerated plasma or serum are reliable for as long as 48 hours after the separation and refrigeration of the specimen. Alternatively, plasma can be collected in sodium fluoride tubes. Unfortunately, hemolysis is common in blood collected in sodium fluoride-treated tubes, which can result in slight decrements in glucose values owing to methodologic problems in laboratory determinations. Blood glucose values determined by many portable home blood glucose–monitoring devices are typically lower than actual glucose values determined by bench-top methodologies, and this may result in an incorrect diagnosis of hypoglycemia. Finally, a laboratory error may also result in an incorrect value. It is wise to confirm hypoglycemia by determining the blood glucose concentration from a second blood sample and using bench-top methodology before embarking on a search for the cause of hypoglycemia.
Clinical Features
Clinical signs of hypoglycemia usually develop when the blood glucose concentration is less than 45 mg/dl, although this can be quite variable. The development of clinical signs depends on the severity and duration (acute versus chronic) of hypoglycemia and the rate of decline in the blood glucose concentration. Clinical signs are a result of neuroglycopenia and hypoglycemia-induced stimulation of the sympathoadrenal nervous system. Neuroglycopenic signs include seizures; weakness; collapse; ataxia; and, less commonly, lethargy, blindness, bizarre behavior, and coma. Signs of increased secretion of catecholamines include restlessness, nervousness, hunger, and muscle fasciculations.
Depending on the cause, the signs of hypoglycemia may be persistent or intermittent. The hallmark clinical sign of hypoglycemia (i.e., seizures) tends to be intermittent, regardless of the cause. Dogs and cats usually recover from hypoglycemic seizures within 30 seconds to 5 minutes as a result of activation of counterregulatory mechanisms (e.g., secretion of glucagon and catecholamines) that block the effects of insulin, stimulate hepatic glucose secretion, and promote an increase in the blood glucose concentration.
Diagnostic Approach
Hypoglycemia should always be confirmed before beginning diagnostic studies to identify the cause. Careful evaluation of the animal’s history, physical examination findings, and results of routine blood tests (i.e., complete blood count [CBC], serum biochemistry panel, urinalysis) usually provides clues to the underlying cause. Hypoglycemia in the puppy or kitten is usually caused by idiopathic hypoglycemia, starvation, liver insufficiency (i.e., portal shunt), or sepsis. In young adult dogs or cats hypoglycemia is usually caused by liver insufficiency, hypoadrenocorticism, or sepsis. In older dogs or cats liver insufficiency, β-cell neoplasia, extrapancreatic neoplasia, hypoadrenocorticism, and sepsis are the most common causes.
Hypoglycemia tends to be mild (greater than 45 mg/dl) and is often an incidental finding in dogs and cats with hypoadrenocorticism or liver insufficiency. Additional clinical pathologic alterations are usually present (e.g., hyponatremia and hyperkalemia in animals with Addison’s disease or increased alanine aminotransferase [ALT] activity, hypocholesterolemia, hypoalbuminemia, and a low blood urea nitrogen [BUN] concentration in animals with liver insufficiency). An adrenocorticotropic hormone (ACTH) stimulation test or liver function test (i.e., preprandial and postprandial bile acids) may be required to confirm the diagnosis. Severe hypoglycemia (less than 40 mg/dl) may develop in neonates and juvenile kittens and puppies (especially toy breeds) and in animals with sepsis, β-cell neoplasia, and extrapancreatic neoplasia, most notably hepatic adenocarcinoma and leiomyosarcoma. Sepsis is readily identified on the basis of physical examination findings and abnormal CBC findings, such as a neutrophilic leukocytosis (typically greater than 30,000/μl), a shift toward immaturity, and signs of toxicity. Extrapancreatic neoplasia can usually be identified on the basis of the physical examination, abdominal or thoracic radiography, and abdominal ultrasonography findings. Dogs with β-cell neoplasia typically have normal physical examination findings and no abnormalities other than hypoglycemia identified on routine blood and urine tests. Measurement of baseline serum insulin concentration when the blood glucose is less than 60 mg/dl (preferably less than 50 mg/dl) is necessary to confirm the diagnosis of a β-cell tumor.
Treatment
Whenever possible, therapy should always be directed at eliminating the underlying cause of the hypoglycemia. If the disorder cannot be eliminated and the clinical signs of hypoglycemia persist, long-term symptomatic therapy designed to increase the blood glucose concentration may be necessary to minimize clinical signs (see Box 52-12). Such therapy is usually required for animals with metastatic β-cell or extrapancreatic neoplasia.
 BOX 52-12 Long-term Medical Therapy for Dogs with β-Cell Neoplasia
BOX 52-12 Long-term Medical Therapy for Dogs with β-Cell Neoplasia
Symptomatic therapy for animals with severe hypoglycemia of acute onset relies on the administration of glucose (Box 52-3). If the dog or cat is having a hypoglycemic seizure at home, the client should rub a sugar mixture on the pet’s buccal mucosa. Most animals respond within 1 to 2 minutes. Clients should be instructed never to place fingers in, or pour the sugar solution down, the pet’s mouth. Once the dog or cat is sternal and cognizant of its surroundings, it should be fed a small meal and brought to the veterinarian.
 BOX 52-3 Medical Therapy for Acute Hypoglycemic Seizures
BOX 52-3 Medical Therapy for Acute Hypoglycemic Seizures
IV, Intravenous.
Intractable Seizures in Hospital
If collapse, seizures, or coma develops in the hospital, a blood sample should be obtained to measure the glucose concentration and other variables before reversing the signs with the IV administration of 50% dextrose. Dextrose should be administered in small amounts slowly rather than in large boluses rapidly. This is especially important in dogs with suspected β-cell neoplasia in which aggressive glucose administration can result in severe hypoglycemia after excessive insulin secretion by the tumor in response to the glucose. Commonly, 2 to 15 ml of 50% dextrose is required to alleviate the signs. Dogs and cats with hypoglycemia usually respond to glucose administration within 2 minutes. Recurrence of hypoglycemia is dependent on the ability to correct the underlying etiology.
Occasionally, a dog or cat with severe central nervous system signs (e.g., blindness, coma) does not respond to initial glucose therapy. Irreversible cerebral lesions may result from prolonged severe hypoglycemia and the resultant cerebral hypoxia. The prognosis in these animals is guarded to poor. Therapy is directed at providing a continuous supply of glucose by administering a 2.5% to 5% solution intravenously or increasing hepatic gluconeogenesis with a constant rate infusion of glucagons (see p. 805). Seizure activity is controlled with diazepam or a stronger anticonvulsant medication. Glucocorticoids and mannitol may be necessary to combat cerebral edema.
DIABETES MELLITUS IN DOGS
Etiology
Virtually all dogs with diabetes have insulin-dependent diabetes mellitus (IDDM) at the time of diagnosis. IDDM is characterized by hypoinsulinemia, essentially no increase in the endogenous serum insulin concentration after the administration of an insulin secretagogue (e.g., glucose or glucagon) at any time after the diagnosis of the disease, failure to establish glycemic control in response to diet or treatment with oral hypoglycemic drugs (or both), and an absolute need for exogenous insulin to maintain glycemic control. The cause of diabetes mellitus has been poorly characterized in dogs but is undoubtedly multifactorial. A genetic predisposition, infection, insulin-antagonistic diseases and drugs, obesity, immune-mediated insulitis, and pancreatitis have been identified as inciting factors. The end result is a loss of β-cell function, hypoinsulinemia, impaired transport of circulating glucose into most cells, and accelerated hepatic gluconeogenesis and glycogenolysis. The subsequent development of hyperglycemia and glycosuria causes polyuria, polydipsia, polyphagia, and weight loss. Ketoacidosis develops as the production of ketone bodies increases to compensate for the underutilization of blood glucose (see p. 794). Loss of β-cell function is irreversible in dogs with IDDM, and lifelong insulin therapy is mandatory to maintain glycemic control of the diabetic state.
Unlike cats, dogs very rarely have a transient or reversible form of diabetes mellitus. The most common scenario for transient diabetes mellitus in dogs is correction of insulin antagonism after ovariohysterectomy in a bitch in diestrus. Progesterone stimulates secretion of growth hormone in the bitch. Ovariohysterectomy removes the source of progesterone, plasma growth hormone concentration declines, and insulin antagonism resolves. If an adequate population of functional β cells are still present in the pancreas, hyperglycemia may resolve without the need for insulin treatment. These dogs have a significant reduction in β-cell numbers (i.e., subclinical diabetes) compared with healthy dogs, before the development of hyperglycemia during diestrus, and are prone to redevelopment of hyperglycemia and diabetes mellitus if insulin antagonism recurs for any reason after ovariohysterectomy. Although uncommon, a similar situation can occur in dogs with subclinical diabetes treated with insulin-antagonistic drugs (e.g., glucocorticoids) or in the very early stages of an insulin-antagonistic disorder (e.g., hyperadrenocorticism). Failure to quickly correct the insulin antagonism will result in IDDM and the lifelong requirement for insulin treatment to control the hyperglycemia.
A honeymoon period occurs in some dogs with newly diagnosed IDDM. It is characterized by excellent glycemic control in response to small doses of insulin (less than 0.2 U/kg/injection), presumably because of the presence of residual β-cell function. However, glycemic control becomes more difficult and insulin doses usually increase within 3 to 6 months of starting treatment as residual functioning β cells are destroyed and endogenous insulin secretion declines. It is very uncommon for non–insulin-dependent diabetes mellitus (NIDDM) to be recognized clinically in dogs, despite the documentation of obesity-induced carbohydrate intolerance in dogs and the identification of residual β-cell function in some diabetic dogs.
SIGNALMENT
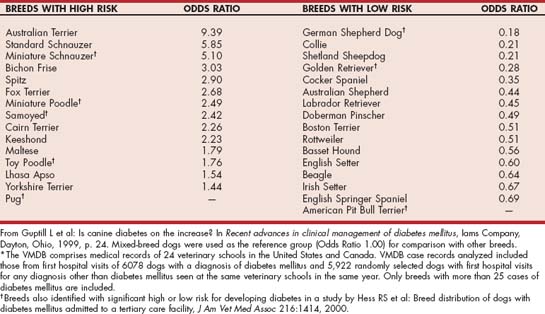
Most dogs are 4 to 14 years old at the time diabetes mellitus is diagnosed, with a peak prevalence at 7 to 9 years of age. Juvenile-onset diabetes occurs in dogs younger than 1 year of age and is uncommon. Female dogs are affected about twice as frequently as male dogs. Genetic predispositions to the development of diabetes are suspected in some breeds on the basis of familial associations and pedigree analysis (Table 52-1).
HISTORY
The history in virtually all diabetic dogs includes polydipsia, polyuria, polyphagia, and weight loss. Polyuria and polydipsia do not develop until hyperglycemia results in glycosuria. Occasionally, a client brings in a dog because of sudden blindness caused by cataract formation (Fig. 52-1). The typical clinical signs of diabetes were either unnoticed or considered irrelevant by the client. If the clinical signs associated with uncomplicated diabetes are not observed by the client and impaired vision caused by cataracts does not develop, a diabetic dog is at risk for the development of systemic signs of illness as progressive ketonemia and metabolic acidosis develop. The time sequence from the onset of initial clinical signs to the development of diabetic ketoacidosis (DKA) is unpredictable, ranging from days to weeks.
PHYSICAL EXAMINATION
Physical examination findings depend on the presence and severity of DKA, on the duration of diabetes before its diagnosis, and on the nature of any other concurrent disorder. The nonketotic diabetic dog has no classic physical examination findings. Many diabetic dogs are obese but are otherwise in good physical condition. Dogs with prolonged untreated diabetes may have lost weight but are rarely emaciated unless concurrent disease (e.g., pancreatic exocrine insufficiency) is present. The haircoat may be sparse; the hairs may be dry, brittle and lusterless; and scales from hyperkeratosis may be present. Diabetes-induced hepatic lipidosis may cause hepatomegaly. Lenticular changes consistent with cataract formation are common. Additional abnormalities may be identified if DKA is present (see p. 796).
Diagnosis
The diagnosis of diabetes mellitus is based on three findings: appropriate clinical signs, persistent fasting hyperglycemia, and glycosuria. Measurement of the blood glucose concentration using a portable blood glucose–monitoring device and testing for the presence of glycosuria using urine reagent test strips (e.g., KetoDiastix; Ames Division, Miles Laboratories) provides rapid confirmation of diabetes mellitus. Concurrent documentation of ketonuria establishes a diagnosis of diabetic ketosis (DK), and documentation of metabolic acidosis establishes a diagnosis of DKA.
It is important to document both persistent hyperglycemia and glycosuria to establish a diagnosis of diabetes mellitus because hyperglycemia differentiates diabetes mellitus from primary renal glycosuria and glycosuria differentiates diabetes mellitus from other causes of hyperglycemia (see Box 52-1), most notably epinephrine-induced stress hyperglycemia that may develop around the time of blood sampling. Stress-induced hyperglycemia is a common problem in cats and occasionally occurs in dogs, especially those that are very excited, hyperactive, or aggressive. The reader is referred to p. 792 for more information on stress-induced hyperglycemia.
A thorough evaluation of the dog’s overall health is recommended once the diagnosis of diabetes mellitus has been established to identify any disease that may be causing or contributing to the carbohydrate intolerance (e.g., hyperadrenocorticism), that may result from the carbohydrate intolerance (e.g., bacterial cystitis), or that may mandate a modification of therapy (e.g., pancreatitis). The minimum laboratory evaluation should include a CBC, serum biochemistry panel, measurement of serum pancreatic lipase immunoreactivity, and urinalysis with bacterial culture. Serum progesterone concentration should be determined if diabetes mellitus is diagnosed in an intact bitch, regardless of her cycling history. If available, abdominal ultrasound is indicated to assess for pancreatitis, adrenomegaly, pyometritis in an intact bitch, and abnormalities affecting the liver and urinary tract (e.g., changes consistent with pyelonephritis or cystitis). Measurement of baseline serum insulin concentration or an insulin response test is not routinely done. Additional tests may be warranted after obtaining the history, performing the physical examination, or identifying ketoacidosis. Potential clinical pathologic abnormalities are listed in Box 52-4.
 BOX 52-4 Clinicopathologic Abnormalities Commonly Found in Dogs and Cats with Uncomplicated Diabetes Mellitus
BOX 52-4 Clinicopathologic Abnormalities Commonly Found in Dogs and Cats with Uncomplicated Diabetes Mellitus
IDDM, Insulin-dependent diabetes mellitus; NIDDM, non–insulin-dependent diabetes mellitus.
Treatment
The primary goal of therapy is elimination of client-observed clinical signs of diabetes. Persistence of clinical signs and development of chronic complications (Box 52-5) are directly correlated with the severity and duration of hyperglycemia. In the diabetic dog establishing control of hyperglycemia can be accomplished with insulin, diet, exercise, prevention or control of concurrent insulin antagonistic diseases, and discontinuation of medications that cause insulin resistance. The veterinarian must also guard against development of hypoglycemia, a serious and potentially fatal complication of therapy. Hypoglycemia is most apt to occur as the result of overzealous insulin therapy. The veterinarian must balance the benefits of tight glucose control obtainable with aggressive insulin therapy against the risk of hypoglycemia.
OVERVIEW OF INSULIN PREPARATIONS
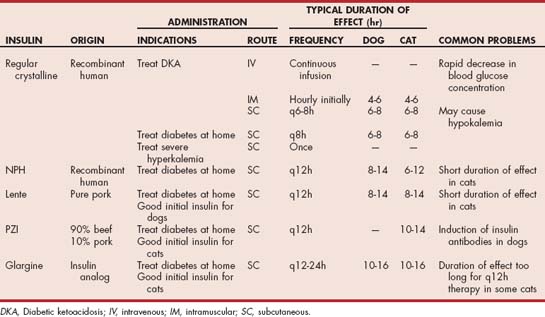
Types of insulin typically used for the home treatment of diabetes in dogs and cats include intermediate-acting insulin (NPH, lente) and long-acting basal insulin (PZI, insulin glargine; (Table 52-2). NPH (Humulin N®, Eli Lilly) is a recombinant human insulin, lente (Vetsulin®, Intervet) is a purified pork-source insulin, and PZI (PZI Vet®, IDEXX) is a beef/pork-source insulin with approximately 90% being beef-source insulin. Insulin glargine (Lantus®, Aventis Pharmaceuticals) is a long-acting insulin analog in which the amino acid sequence has been altered, compared with human insulin, making glargine more soluble at a slightly acidic pH and less soluble at a physiological pH than human insulin. The solution in the bottle of glargine is acidic, which keeps glargine soluble and suspended in the solution (i.e., the solution is clear, and the bottle does not need to be rolled before the insulin is drawn into the syringe). Because of this dependency on pH, glargine cannot be diluted or mixed with anything that may change the pH of the solution. Glargine forms microprecipitates in the subcutaneous tissue at the site of injection, from which small amounts of insulin glargine are slowly released and absorbed into the circulation. In humans the slow, sustained release of insulin glargine from these microprecipitates results in a relatively constant concentration/time profile over a 24-hour period with no pronounced peak in serum insulin. Insulin glargine is currently recommended as a basal insulin (i.e., sustained long-acting insulin used to inhibit hepatic glucose production) administered once a day at bedtime and used in conjunction with either prandial insulin analogs or oral hypoglycemic drugs in human diabetics.
STORAGE AND DILUTION OF INSULIN
Freezing, heating, and shaking the insulin bottle inactivate insulin in the bottle. Although keeping the substance at “room temperature”: does not inactivate insulin, I instruct clients to store insulin in the door of the refrigerator to maintain a consistent environment and prolong the life of the insulin preparation. Some veterinarians advocate replacing insulin with a new bottle every month to prevent problems caused by loss of activity or sterility. I have not appreciated a clinically significant loss of insulin action with time when insulin preparations, including glargine, are maintained in a constant environment (i.e., refrigerator) and handled appropriately. I do not routinely recommend purchasing a new bottle of insulin every month, especially if the diabetic dog or cat is doing well. However, development of cloudiness or discoloration suggest contamination, change in pH of the solution (glargine), and/or loss of insulin activity. The vial of insulin should be discarded and replaced with a new bottle of insulin. Similarly, loss of insulin activity in the bottle should always be considered whenever clinical signs recur, regardless of the quantity of insulin remaining in the bottle.
Dilution of insulin is a common practice, especially in very small dogs and cats. Although studies evaluating the shelf-life of diluted insulin have not been published, I recommend replacing diluted insulin preparations every 4 to 8 weeks. Even when these guidelines are observed, insufficient amounts of insulin are administered when diluted insulin is used in some dogs and cats, despite appropriate dilution and insulin administration techniques—inadequacies that are corrected when full-strength insulin is used. It is important to remember that insulin glargine is pH dependent and cannot be diluted.
INITIAL INSULIN RECOMMENDATIONS FOR DIABETIC DOGS
Lente and NPH are the initial insulins of choice for treating diabetes in dogs (see Table 52-2). Recombinant human-source or pork-source insulin should be used to prevent insulin antibodies (see p. 782). My starting dosage for both types of insulin is approximately 0.25 U/kg of body weight. Because the overwhelming majority of diabetic dogs require lente or NPH insulin twice a day, the preference is to start with twice-daily insulin therapy. Establishing control of glycemia is easier and problems with hypoglycemia and the Somogyi response (see p. 780) are less likely when twice-daily insulin therapy is initiated while the insulin dose is low (i.e., at the time insulin treatment is initiated). The initial dosage recommendation (1 U/kg) on the package insert for Vetsulin® is too high. In a recent study by Monroe et al. (2005) evaluating the efficacy of Vetsulin® using the dosage recommendations on the package insert, approximately 40% of the dogs developed clinical signs of hypoglycemia at home and a blood glucose concentration of less than 60 mg/dl was identified in 36% of the dogs during generation of a blood glucose curve in the hospital.
I currently use insulin glargine in poorly controlled diabetic dogs in which NPH and lente insulin are ineffective because of problems with short duration of insulin effect. I rarely use beef/pork-source PZI insulin in dogs because of the potential for development of insulin antibodies directed against the beef insulin in the preparation that may create problems with diabetic control (see p. 782).
DIET
Correction of obesity and increasing the fiber content of the diet are the two most beneficial steps that can be taken to improve control of glycemia in diabetic dogs. Obesity causes insulin resistance in dogs and is an important factor accounting for variations in response to insulin therapy in diabetic dogs. Weight loss improves insulin resistance in obese diabetic dogs. Weight loss usually requires a combination of the following: restricting caloric intake, feeding low calorie-dense diets, and increasing caloric expenditure through exercise.
Diets containing increased fiber content are beneficial for treating obesity and improving control of glycemia in diabetics dogs. The ability of the fiber to form a viscous gel appears to be of greatest importance in slowing intestinal glucose absorption. More viscous soluble fibers (e.g., gums, pectin) slow glucose absorption to a greater degree than less viscous insoluble fibers (e.g., cellulose, peanut hulls) and, as such, are believed to be of greater benefit in improving control of glycemia. Most commercial high-fiber diets predominantly contain insoluble fiber, although diets containing mixtures of soluble and insoluble fiber are becoming available. The amount of fiber varies considerably among products, ranging from 3% to 25% of dry matter (normal diets contain less than 2% fiber on a dry matter basis). In general, diets containing 12% or more insoluble fiber or 8% or more of a mixture of soluble and insoluble fiber are most likely to be effective in improving glycemic control in diabetic dogs (Box 52-6).
 BOX 52-6 Recommendations for Dietary Treatment of Diabetes Mellitus in Dogs and Cats
BOX 52-6 Recommendations for Dietary Treatment of Diabetes Mellitus in Dogs and Cats
Correct obesity and maintain body weight in an acceptable range (see Chapter 54).
Maintain consistency in the timing and caloric content of the meals.
Minimize the impact of food on postprandial blood glucose concentrations.
| Veterinary Diets for Diabetic Dogs | Veterinary Diets for Diabetic Cats |
|---|---|
The dog’s susceptibility to the complications of high-fiber diets, its body weight and condition, and the presence of a concurrent disease (e.g., pancreatitis, renal failure) in which diet is an important aspect of therapy ultimately dictate which, if any, fiber diet is fed. Common clinical complications of diets high in insoluble fiber include excessive frequency of defecation, constipation and obstipation, hypoglycemia 1 to 2 weeks after the increase in fiber content of the diet, and refusal to eat the diet. Complications of soluble fiber–containing diets include soft-to-watery stools, excessive flatulence, hypoglycemia 1 to 2 weeks after the increase in fiber content of the diet, and refusal to eat the diet. If firm stools or constipation becomes a problem with diets that are high in insoluble fiber, a mixture of insoluble- and soluble-fiber diets can be fed or soluble fiber (e.g., psyllium, canned pumpkin) can be added to the diet to soften the stool. If soft or watery diarrhea or flatulence becomes a problem with soluble fiber–containing diets, an insoluble-fiber diet can be added and the quantity of the soluble-fiber diet decreased. If palatability is a problem initially, the animal can be gradually switched from its regular diet to a diet containing small amounts of fiber, after which diets containing more fiber are provided. Refusal to consume high-fiber diets months after their initiation is usually a result of boredom with the food. Periodic changes in the types of high-fiber diets and mixtures of diets have been helpful in alleviating this problem. Finally, high-fiber diets should not be fed to thin or emaciated diabetic dogs until control of glycemia is established and a normal body weight attained using a higher-calorie-dense, lower-fiber diet designed for maintenance.
EXERCISE
Exercise plays an important role in maintaining glycemic control in the diabetic dog by helping promote weight loss and eliminating the insulin resistance induced by obesity. Exercise also has a glucose-lowering effect by increasing the mobilization of insulin from its injection site, presumably resulting from increased blood and lymph flow, by increasing blood flow (and therefore insulin delivery) to exercising muscles, and by stimulating glucose transporters in muscle cells. The daily routine for diabetic dogs should include exercise, preferably at the same time each day. Strenuous and sporadic exercise can cause severe hypoglycemia and should be avoided. If unavoidable, the insulin dose should be decreased in dogs subjected to sporadic strenuous exercise on those days of anticipated increased exercise. The reduction in insulin dose required to prevent hypoglycemia is variable and determined by trial and error. Reducing the insulin dose by 50% initially is recommended with further adjustments based on the occurrence of symptomatic hypoglycemia and the severity of polyuria and polydipsia that develops during the ensuing 24 to 48 hours. In addition, clients must be aware of the signs of hypoglycemia and have a source of glucose readily available to give their dog should any of these signs develop.
IDENTIFICATION AND CONTROL OF CONCURRENT PROBLEMS
Concurrent disease and insulin-antagonistic drugs can interfere with tissue responsiveness to insulin, resulting in insulin resistance and poor control of the diabetes. Concurrent disease and insulin-antagonistic drugs typically cause insulin resistance by altering insulin metabolism (prereceptor problem), by decreasing the concentration or binding affinity of insulin receptors on the cell membrane (receptor problem), by interfering with the insulin receptor signaling cascade (postreceptor problem), or by a combination of these. Depending on the etiology, insulin resistance may be mild and easily overcome by increasing the dose of insulin (e.g., obesity); may be severe, causing sustained and marked hyperglycemia regardless of the type and dose of insulin administered (e.g., hyperadrenocorticism); or may fluctuate in severity over time (e.g., chronic pancreatitis; Box 52-7). Some causes of insulin resistance are readily apparent at the time diabetes is diagnosed, such as obesity and the administration of insulin-antagonistic drugs (e.g., glucocorticoids). Other causes of insulin resistance are not readily apparent and require an extensive diagnostic evaluation to be identified. In general, any concurrent inflammatory, infectious, hormonal, or neoplastic disorder can cause insulin resistance and interfere with the effectiveness of insulin therapy. Identification and treatment of concurrent disease play integral roles in the successful management of the dia betic dog. A thorough history, physical examination, and complete diagnostic evaluation are imperative in the newly diagnosed diabetic dog (see the section on diagnosis, p. 769).
PROTOCOL FOR IDENTIFYING INITIAL INSULIN REQUIREMENTS
Diabetic dogs require several days to equilibrate to changes in insulin dose or preparation. Therefore newly diagnosed diabetic dogs are typically hospitalized for no more than 24 to 48 hours to finish the diagnostic evaluation of the dog and begin insulin therapy. During hospitalization blood glucose concentrations are typically determined at the time insulin is administered and 3, 6, and 9 hours later. The intent is to identify hypoglycemia (i.e., blood glucose less than 80 mg/dl) in those dogs that are unusually sensitive to the actions of insulin. If hypoglycemia occurs, the insulin dose is decreased before sending the dog home. The insulin dose is not adjusted in those dogs that remain hyperglycemic during the first few days of insulin therapy. The objective during this first visit is not to establish perfect glycemic control before sending the dog home. Rather, the objective is to begin to reverse the metabolic derangements induced by the disease, allow the patient to equilibrate to the insulin and change in diet, teach the client how to administer insulin, and give the client a few days to become accustomed to treating the diabetic dog at home. Adjustments in insulin therapy are made on subsequent evaluations, once the client and pet have become accustomed to the treatment regimen.
Diabetic dogs are typically evaluated once weekly until an effective insulin treatment protocol is identified. Glycemic control is attained when clinical signs of diabetes have resolved; the pet is healthy and interactive in the home; its body weight is stable (unless the dog is undergoing weight loss to correct obesity); the client is satisfied with the progress of therapy; and, if possible, the blood glucose concentrations range between 100 and 250 mg/dl throughout the day. The client is informed at the time insulin therapy is initiated that it will take approximately 1 month to establish a satisfactory insulin treatment protocol, assuming unidentified insulin-antagonistic disease is not present. The goals of therapy are also explained to the client. During this month changes in insulin dose, type, and frequency of administration are common and should be anticipated by the client. At each evaluation the client’s subjective opinion of water intake, urine output, and overall health of the pet is discussed; a complete physical examination is performed; change in body weight noted; and serial blood glucose measurements obtained over an 8- to 12-hour period after insulin administration are assessed. Adjustments in insulin therapy are based on this information, the pet is sent home, and an appointment is scheduled for the next week to reevaluate the response to any change in therapy. If the dog remains poorly controlled, the dose of insulin is gradually increased by 1 to 5 U/injection (depending on the size of the dog) each week until control is attained. This gradual increase in dose helps prevent hypoglycemia and the Somogyi response. Control of glycemia can be established in most dogs using insulin doses in the range of 1.0 U of insulin/kg or less administered twice each day. If the insulin dose exceeds 1.5 U/kg/injection without adequate glycemic control, then further investigations to determine the reason for treatment failure are indicated (see the section on complications of insulin therapy, p. 779). If hypoglycemia is noted either clinically or biochemically at any time, the insulin dosage should be decreased and further adjustments in the insulin dose performed as needed to attain glycemic control.
Many factors affect the dog’s glycemic control from day to day, including variations in insulin administration and absorption, dietary indiscretions and caloric intake, amount of exercise, and variables that affect insulin responsiveness (e.g., stress, concurrent inflammation, infection). As a consequence, the insulin dosage required to maintain glycemic control typically changes with time. Initially, a fixed dose of insulin is administered at home and changes are made only after the client consults with the veterinarian. As the insulin dose range required to maintain glycemic control becomes apparent and as confidence is gained in the client’s ability to recognize signs of hypoglycemia and hyperglycemia, the client is eventually allowed to make slight adjustments in the insulin dose at home on the basis of clinical observations of the pet’s well-being. However, the client is instructed to stay within the agreed-upon insulin dose range. If the insulin dose is at the upper or lower end of the established range and the pet is still symptomatic, the client is instructed to call the veterinarian before making further adjustments in the insulin dose.
Techniques for Monitoring Diabetic Control
The basic objective of insulin therapy is to eliminate the clinical signs of diabetes mellitus while avoiding the common complications associated with the disease (see Box 52-5). Common complications in dogs include blindness caused by cataract formation, weight loss, hypoglycemia, recurring ketosis, and recurrence of polyuria and polydipsia. The devastating chronic complications of human diabetes (e.g., nephropathy, vasculopathy, coronary artery disease) require several decades to develop and are uncommon in diabetic dogs. As such, the need to establish nearly normal blood glucose concentrations is not necessary in diabetic dogs. Generally speaking, most clients are happy and most dogs are healthy and relatively asymptomatic if blood glucose concentrations are kept between 100 and 250 mg/dl.
HISTORY AND PHYSICAL EXAMINATION
The most important initial parameters for assessing control of glycemia are the client’s subjective opinion of severity of clinical signs and overall health of the pet, findings on physical examination, and stability of body weight. If the client is happy with results of treatment, the physical examination is supportive of good glycemic control, and the body weight is stable, the diabetic dog is usually adequately controlled. Measurement of serum fructosamine concentration can add further objective evidence for status of glycemic control (discussed in more detail later). Poor control of glycemia should be suspected and additional diagnostics or a change in insulin therapy considered if the client reports clinical signs suggestive of hyperglycemia or hypoglycemia, the physical examination identifies problems consistent with poor control of glycemia (e.g., thin appearance, poor haircoat), or the dog is losing weight.
SINGLE BLOOD GLUCOSE DETERMINATION
Measuring a single blood glucose concentration is helpful only if hypoglycemia is identified. Documenting hypoglycemia supports insulin overdosage and the need to decrease the insulin dose, especially if glycemic control is poor (see the discussion of the Somogyi response, p. 780). In contrast, documenting an increased blood glucose concentration does not, by itself, confirm poor control of glycemia. Stress or excitement can cause marked hyperglycemia, which does not reflect the dog’s responsiveness to insulin and can lead to the erroneous belief that the diabetic dog is poorly controlled. If a discrepancy exists between the history, physical examination findings, and blood glucose concentration or if the dog is fractious, aggressive, excited, or scared and the blood glucose concentration is known to be unreliable, measurement of serum fructosamine concentration should be done to further evaluate status of glycemic control. In addition, a single blood glucose concentration is not reliable for evaluating the effect of a given insulin type and dose in a poorly controlled diabetic dog (see the section on serial blood glucose curve).
SERUM FRUCTOSAMINE CONCENTRATION
Fructosamines are glycated proteins that result from an irreversible, nonenzymatic, insulin-independent binding of glucose to serum proteins. The extent of glycosylation of serum proteins is directly related to the blood glucose concentration; the higher the average blood glucose concentration during the preceding 2 to 3 weeks, the higher the serum fructosamine concentration, and vice versa. Serum fructosamine concentration is not affected by acute increases in the blood glucose concentration, as occurs with stress- or excitement-induced hyperglycemia, but can be affected by concurrent hypoalbuminemia (less than 2.5 g/dl), hyperlipidemia (triglycerides greater than 150 mg/dl), or hyperthyroidism (Table 52-3). Serum fructosamine concentrations can be measured during the routine evaluation of glycemic control performed every 3 to 6 months; to clarify the effect of stress or excitement on blood glucose concentrations; to clarify discrepancies between the history, physical examination findings, and serial blood glucose concentrations; and to assess the effectiveness of changes in insulin therapy.
 TABLE 52-3 Sample Handling, Methodology, and Normal Values for Serum Fructosamine Concentrations Measured in Our Laboratory
TABLE 52-3 Sample Handling, Methodology, and Normal Values for Serum Fructosamine Concentrations Measured in Our Laboratory
| FRUCTOSAMINE | |
|---|---|
| Blood sample | 1-2 ml; allow to clot, obtain serum |
| Sample handling | Freeze until assayed |
| Methodology | Automated colorimetric assay using nitroblue tetrazolium chloride |
| Factors affecting results | Hypoalbuminemia (decreased), hyperlipidemia (mild decrease—dogs), azotemia (mild decrease—dogs), hyperthyroidism (decreased—cats), storage at room temperature (decreased) |
| Normal range | 225 to 375 μmol/L (dogs) |
| 190 to 365 μmol/L (cats) | |
| Interpretation in Diabetic Dogs and Cats | |
| Excellent control | 350-400 μmol/L |
| Good control | 400-450 μmol/L |
| Fair control | 450-500 μmol/L |
| Poor control | >500 μmol/L |
| Prolonged hypoglycemia | <300 μmol/L |
Fructosamine is measured in serum, which should be frozen and shipped on cold packs overnight to the laboratory. Storage of serum at room temperature overnight can decrease serum fructosamine results by 10%. Each laboratory should furnish its own reference range. In our laboratory the normal reference range for serum fructosamine in dogs is 225 to 375 μmol/L; a range determined in healthy dogs with persistently normal blood glucose concentrations. Interpretation of serum fructosamine in a diabetic dog must take into consideration the fact that hyperglycemia is common, even in well-controlled diabetic dogs (see Table 52-3). Most clients are happy with the pet’s response to insulin treatment if serum fructosamine concentrations can be kept between 350 and 450 μmol/L. Values greater than 500 μmol/L suggest inadequate control of the diabetic state, and values greater than 600 μmol/L indicate serious lack of glycemic control. Serum fructosamine concentrations in the lower half of the normal reference range (i.e., less than 300 μmol/L) or below the normal reference range should raise concern for significant periods of hypoglycemia in the diabetic dog. Increased serum fructosamine concentrations (i.e.,>500 μmol/L) suggest poor control of glycemia and a need for insulin adjustments but do not identify the underlying problem.
URINE GLUCOSE MONITORING
Occasional monitoring of urine for glycosuria and ketonuria is helpful in diabetic dogs that have problems with recurring ketosis or hypoglycemia to identify ketonuria or persistent negative glycosuria, respectively. The client is instructed not to adjust daily insulin doses on the basis of morning urine glucose measurements, except to decrease the insulin dose in dogs with recurring hypoglycemia and persistent negative glycosuria. The vast majority of diabetic dogs develop complications because clients were misled by morning urine glucose concentrations. Persistent glycosuria throughout the day and night suggests inadequate control of the diabetic state and the need for a more complete evaluation of diabetic control using other techniques discussed in this section.
SERIAL BLOOD GLUCOSE CURVES
If an adjustment in insulin therapy is deemed necessary after review of the history, physical examination, changes in body weight, and serum fructosamine concentration, then a serial blood glucose curve should be generated to provide guidance in making the adjustment, unless blood glucose measurements are unreliable because of stress, aggression, or excitement. The serial blood glucose curve provides guidelines for making adjustments in insulin therapy. Evaluation of a serial blood glucose curve is mandatory during the initial regulation of the diabetic dog and is necessary in the dog in which clinical manifestations of hyperglycemia or hypoglycemia have developed. Reliance on history, physical examination, body weight, and serum fructosamine concentration to determine when a blood glucose curve is needed helps reduce the frequency with which blood glucose curves must be performed, thereby minimizing the animal’s aversion to these evaluations and improving the chances of obtaining meaningful results when a blood glucose curve is needed.
When a blood glucose curve is being generated, the insulin and feeding schedule used by the client should be maintained, the dog dropped off at the hospital early in the morning, and blood obtained every 1 to 2 hours throughout the day for glucose determination. It is more important to maintain the pet’s daily routine than to risk inaccurate blood glucose results caused by inappetence in the hospital or insulin administration at an unusual time (Fig. 52-2). If there are concerns regarding the client’s technique for administering insulin, the client can administer insulin (using his or her own insulin and syringe) in the hospital after the initial blood glucose is obtained or can demonstrate his or her technique using sterile saline after arriving to pick up the pet at the end of the day. The veterinarian or a veterinary technician should closely evaluate the entire insulin administration procedure. By measuring blood glucose concentration every 1 to 2 hours throughout the day, the clinician will be able to determine if the insulin is effective and identify the glucose nadir, time of peak insulin effect, duration of insulin effect, and severity of fluctuation in blood glucose concentrations in that particular dog. Determining the glucose nadir and the time of the glucose nadir in relation to the time of insulin administration is critical for assessing the duration of insulin effect. If the glucose nadir has not been identified by the time of the next insulin injection, the glucose curve should be continued, the scheduled insulin injection aborted, and the dog fed its evening meal (see the discussion of the prolonged duration of insulin effect, p. 781). Obtaining only 1 or 2 blood glucose concentrations has not been reliable for evaluating the effect of a given insulin dose (Fig. 52-3). Persistent poor control of the diabetic state often stems from misinterpretation of the effects of insulin that is based on assessment of only 1 or 2 blood glucose concentrations.
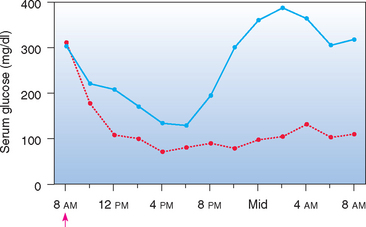
FIG 52-2 Mean blood glucose concentrations in eight diabetic dogs after the administration of NPH insulin (↑) and the feeding of equal-sized meals at 8 AM and 6 PM (blue line) or feeding them nothing (red line) during the 24 hours of blood sampling.
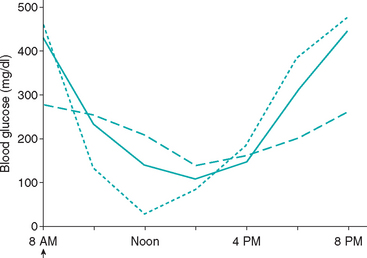
FIG 52-3 Blood glucose concentration curve in a Dachshund receiving 0.8 U of recombinant human lente insulin per kilogram of body weight twice a day (solid line), a Miniature Poodle receiving 0.6 U of recombinant human lente insulin per kilogram of body weight twice a day (dashed line), and a Terrier-mix receiving 1.1 U of recombinant human lente insulin per kilogram of body weight twice a day (dotted line). Insulin and food was given to each dog at 8 AM. Interpretation of the blood glucose curves suggest short duration of insulin effect in the Dachshund, insulin underdosing in the Miniature Poodle, and the Somogyi response in the Terrier-mix. The blood glucose concentrations were similar in all dogs at 2 PM and 4 PM; the glucose results at these times do not establish the diagnosis in any of the dogs.
Blood glucose concentrations are typically determined by a point-of-care glucose analyzer or hand-held portable blood glucose monitoring device. Commercially available portable blood glucose–monitoring devices provide blood glucose concentrations that are reasonably close to those obtained with reference methods, although results often overestimate or underestimate actual glucose values. Blood glucose values determined by most portable blood glucose monitoring devices are typically lower than actual glucose values determined by reference methods (Fig. 52-4). This may result in an incorrect diagnosis of hypoglycemia or the misperception that glycemic control is better than it actually is. Failure to consider this error could result in insulin underdosage and the potential for persistence of clinical signs despite apparently acceptable blood glucose results. One exception is the AlphaTRAK® by Abbott Laboratories. Accuracy of this portable glucometer is very good, but glucose values may be higher or lower than glucose values measured by benchtop methodologies on the same blood sample, forcing the veterinarian to accept the blood glucose concentration at face value.
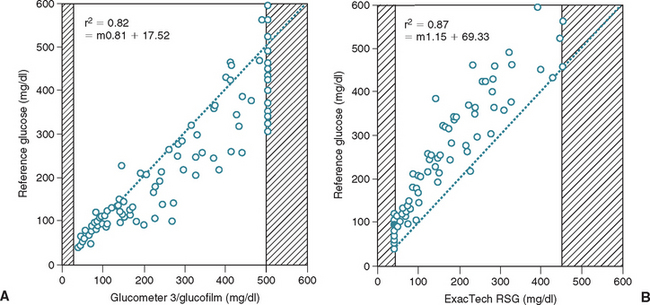
FIG 52-4 Scatter plots of blood glucose concentrations obtained with two portable blood-glucose meters versus concentrations obtained using a reference method. Data represent 110 blood samples from 34 dogs. Shaded areas represent concentrations greater than or less than the concentrations that can be detected by each meter. The dashed line represents the theoretical line of equality. Note that one glucose meter tends to read higher (A) and one glucose meter tends to read lower (B) than the reference concentration.
(From Cohn LA et al: Assessment of five portable blood glucose meters, a point-of-care analyzer, and color test strips for measuring blood glucose concentration in dogs, J Am Vet Med Assoc 216:198, 2000.)
Insulin therapy is adjusted according to interpretation of a single serial blood glucose curve, and the impact of the change is initially assessed by client perceptions of clinical response and change in serum fructosamine concentration. If problems persist, the blood glucose curve can be repeated. If possible, performing blood glucose curves on multiple, consecutive days should be avoided because it promotes stress-induced hyperglycemia. Information gained from a prior serial blood glucose curve should never be assumed to be reproducible on subsequent curves. Lack of consistency in the results of serial blood glucose curves is a source of frustration for many veterinarians. This lack of consistency is a direct reflection of all the variables that affect the blood glucose concentration in diabetics. Daily self-monitoring of blood glucose concentrations and adjustments in insulin dose are used in human diabetics to minimize the effect of these variables on control of glycemia. A similar approach for diabetic dogs and cats will undoubtedly become more common in the future, as home glucose monitoring techniques are refined. For now, initial assessment of control of glycemia is based on the client’s perception of the diabetic pet’s health combined with periodic examinations by the veterinarian. Serial blood glucose measurements are indicated if poor control of glycemia is suspected. The goal of serial blood glucose measurements is to obtain a glimpse of the actions of insulin in that diabetic animal and identify a possible reason that the diabetic dog is poorly controlled.
Protocol for Generating the Serial Blood Glucose Curve at Home
Hyperglycemia induced by stress, aggression, or excitement is the single biggest problem affecting accuracy of the serial blood glucose curve, especially in cats (Fig. 52-5). The biggest factors inducing stress-induced hyperglycemia are hospitalization and multiple venipunctures. An alternative to hospital-generated blood glucose curves is to have the client generate the blood glucose curve at home using the ear or lip prick technique and a portable home glucose-monitoring device that allows the client to touch the drop of blood on the ear or lip with the end of the glucose test strip. This technique is usually reserved for diabetic dogs in which the reliability of blood glucose results generated in the veterinary hospital is questionable. The reader is referred to p. 792 for more information on monitoring blood glucose concentrations at home.
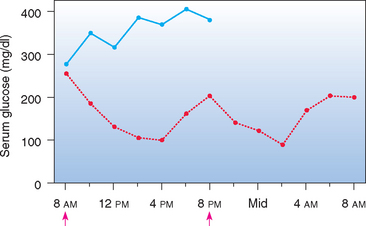
FIG 52-5 Blood glucose concentration curves in a fractious Terrier-mix. The same dose of NPH insulin was given for each curve. One glucose curve (blue line) was obtained with the dog in an agitated state requiring physical restraint each time a blood specimen was obtained; blood for the other glucose curve (red line) was obtained through a jugular catheter with minimal-to-no restraint and the dog in a quiet state. ↑, Insulin administration and food.
Interpreting the Serial Blood Glucose Curve
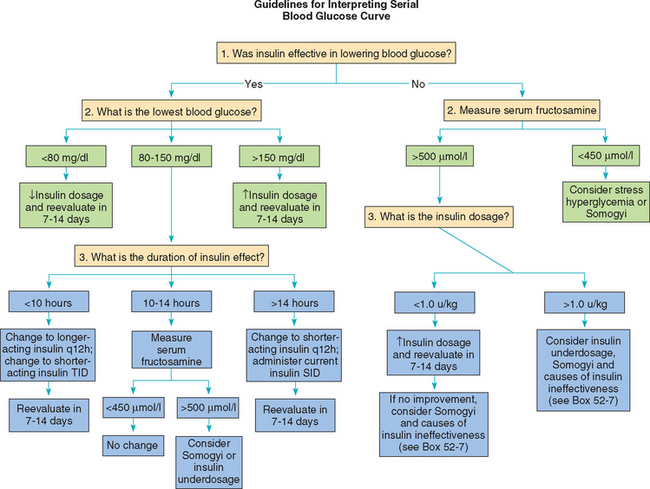
An overview of interpreting results of a serial blood glucose curve is provided in Fig. 52-6. The ideal goal is to maintain the blood glucose concentration between 100 mg/dl and 250 mg/dl throughout the day and night, although many diabetic dogs do well despite blood glucose concentrations consistently in the high 100′s to low 300′s. Typically, the highest blood glucose concentrations occur at the time of each insulin injection, but this does not always occur. If the blood glucose nadir is greater than 150 mg/dl, the insulin dose may need to be increased, and if the nadir is less than 80 mg/dl, the insulin dose should be decreased.
Duration of insulin effect can be assessed if the glucose nadir is greater than 80 mg/dl and there has not been a rapid decrease in the blood glucose concentration after insulin administration. Assessment of duration of insulin effect may not be valid when the blood glucose decreases to less than 80 mg/dl or decreases rapidly because of the potential induction of the Somogyi response, which can falsely decrease the apparent duration of insulin effect (see p. 780). A rough approximation of the duration of effect of insulin can be gained by examining the time of the glucose nadir. For most well-controlled diabetic dogs, the initial blood glucose concentration near the time of insulin administration is less than 300 mg/dl and the glucose nadir occurs 8 to 10 hours after injection of insulin. An initial blood glucose concentration greater than 300 mg/dl, combined with a glucose nadir occurring less than 8 hours after insulin administration and subsequent blood glucose concentrations exceeding 250 mg/dl, is supportive of short duration of insulin effect (see p. 781). A glucose nadir occurring 12 hours or longer after insulin administration is supportive of prolonged duration of insulin effect (see p. 781). Dogs may develop hypoglycemia or the Somogyi response if the duration of insulin effect is greater than 14 hours and the insulin is being administered twice a day (Fig. 52-7).
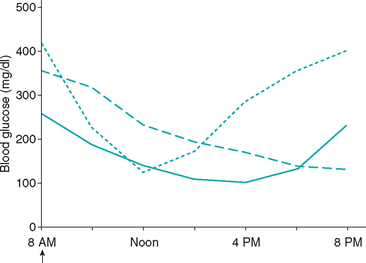
FIG 52-7 Blood glucose concentration curves obtained from three diabetic dogs treated with recombinant human lente insulin twice a day, illustrating a difference between dogs in the duration of insulin effect. The insulin is effective in lowering the blood glucose concentration in all dogs, and the blood glucose nadir is between 100 and 175 mg/dl for the dogs. However, the duration of insulin effect is approximately 12 hours (solid line) in one dog with good control of glycemia (ideal duration of effect), approximately 8 hours (dotted line) in one dog with persistently poor control of glycemia (short duration of effect), and greater than 12 hours (dashed line) in one dog with a history of good days and bad days of glycemic control (prolonged duration of effect)—a history suggestive of the Somogyi response (see Fig. 52-8).
Role of Serum Fructosamine in Aggressive, Excitable, or Stressed Dogs
Blood glucose curves are unreliable in aggressive, excitable, or stressed dogs because of problems related to stress-induced hyperglycemia. In these dogs the clinician must make an educated guess as to where the problem lies (e.g., wrong type of insulin, low dose), make an adjustment in therapy, and rely on changes in serum fructosamine to assess the benefit of the change in treatment. The reader is referred to p. 792 for more information on the use of serum fructosamine in diabetic pets with stress-induced hyperglycemia.
INSULIN THERAPY DURING SURGERY
Generally, surgery should be delayed in diabetic dogs until the animal’s clinical condition is stable and the diabetic state is controlled with insulin. The exception are those situations in which surgery is required to eliminate insulin resistance (e.g., ovariohysterectomy in a diestrus bitch) or to save the animal’s life. The surgery itself does not pose a greater risk in a stable diabetic animal than in a nondiabetic animal. The concern is the interplay between insulin therapy and the lack of food intake during the perioperative period. The stress of anesthesia and surgery also causes the release of diabetogenic hormones, which promote ketogenesis. Insulin must be administered during the perioperative period to prevent severe hyperglycemia and minimize ketone formation. To compensate for the lack of food intake and prevent hypoglycemia, the amount of insulin administered during the perioperative period is decreased and IV dextrose is administered when needed.
The following protocol is used during the perioperative period in dogs and cats undergoing surgery. The day before surgery the dog or cat is given its normal dose of insulin and fed as usual. Food is withheld after 10 PM. On the morning of the procedure the blood glucose concentration is measured before the dog or cat is given insulin. If the blood glucose concentration is less than 100 mg/dl, insulin is not given and an IV infusion of 2.5% to 5% dextrose is initiated. If the blood glucose concentration is between 100 and 200 mg/dl, one quarter of the animal’s usual morning dose of insulin is given and an IV infusion of dextrose is initiated. If the blood glucose concentration is more than 200 mg/dl, one half of the usual morning dose of insulin is given but the IV dextrose infusion is withheld until the blood glucose concentration is less than 150 mg/dl. In all three situations the blood glucose concentration is measured every 30 to 60 minutes during the surgical procedure. The goal is to maintain the blood glucose concentration between 150 and 250 mg/dl during the perioperative period. A 2.5% to 5% dextrose infusion is administered intravenously as needed to correct or prevent hypoglycemia. When the blood glucose concentration exceeds 300 mg/dl, the dextrose infusion should be discontinued and the blood glucose concentration evaluated 30 and 60 minutes later. If the blood glucose concentration remains greater than 300 mg/dl, regular crystalline insulin is administered intramuscularly at approximately 20% of the dose of long-acting insulin being used at home. Subsequent doses of regular crystalline insulin should be given no more frequently than every 4 hours, and the dose should be adjusted on the basis of the effect of the first insulin injection on the blood glucose concentration.
On the day after surgery the diabetic dog or cat can usually be returned to the routine schedule of insulin administration and feeding. An animal that is not eating can be maintained with IV dextrose infusions and regular crystalline insulin injections given subcutaneously every 6 to 8 hours. Once the animal is eating regularly, it can be returned to its normal insulin and feeding schedule.
COMPLICATIONS OF INSULIN THERAPY
Hypoglycemia
Hypoglycemia is a common complication of insulin therapy. Signs of hypoglycemia are most apt to occur after sudden large increases in the insulin dose, with excessive overlap of insulin action in dogs receiving insulin twice a day, after prolonged inappetence, during unusually strenuous exercise, following sudden improvement in concurrent insulin resistance, and in insulin-treated cats that have reverted to a non–insulin-dependent state (see p. 785). In these situations severe hypoglycemia may occur before glucose counterregulation (i.e., secretion of glucagon, epinephrine, cortisol, and growth hormone) is able to compensate for and reverse hypoglycemia. The occurrence and severity of clinical signs is dependent on the rate of blood glucose decline and the severity of hypoglycemia. In many diabetic dogs signs of hypoglycemia are not apparent to clients, and hypoglycemia is identified during evaluation of a serial blood glucose curve or suspected when a low serum fructosamine concentration is identified. Clinical signs and treatment of hypoglycemia are discussed on p. 765. If clinical signs of hypoglycemia have occurred, insulin therapy should be stopped until hyperglycemia and glycosuria recur. The adjustment in the insulin dose is somewhat arbitrary; as a general rule of thumb, the insulin dose initially should be decreased 25% to 50% and subsequent adjustments in the dose based on clinical response and results of blood glucose measurements. Failure of glycosuria to recur after a hypoglycemic episode suggests reversion to a non–insulin-dependent diabetic state or impaired glucose counterregulation.
Recurrence of Clinical Signs
Recurrence or persistence of clinical signs is perhaps the most common complication of insulin therapy in diabetic dogs. This is usually caused by problems with client technique in administering insulin; problems with insulin therapy relating to the insulin type, dose, species, or frequency of administration; or problems with responsiveness to insulin caused by concurrent inflammatory, infectious, neoplastic, or hormonal disorders (i.e., insulin resistance).
Problems with client administration and insulin activity.
Failure to administer an appropriate dose of biologically active insulin will result in recurrence or persistence of clinical signs. Common reasons include administration of biologically inactive insulin (e.g., outdated, overheated, previously frozen, destroyed by shaking the bottle), administration of diluted insulin, use of inappropriate insulin syringes for the concentration of insulin (e.g., U100 syringe with U40 insulin), or problems with insulin administration technique (e.g., failure to correctly read the insulin syringe, inappropriate injection technique). These problems are identified by evaluating the client’s insulin administration technique and by administering new, undiluted insulin and measuring several blood glucose concentrations throughout the day.
Problems with the insulin treatment regimen.
The most common problems with the insulin treatment regimen in the dog include insulin underdosage, insulin overdosage causing the Somogyi response, short duration of effect of lente or NPH insulin, and once-daily insulin administration. The insulin treatment regimen should be critically evaluated for possible problems in these areas and appropriate changes made in an attempt to improve insulin effectiveness, especially if the history and physical examination do not suggest a concurrent disorder causing insulin resistance.
Diluted insulin.
Diluted insulin should be replaced with full-strength insulin. In some dogs insufficient amounts of insulin are administered when diluted insulin is used, despite appropriate dilution and insulin administration techniques. These inadequacies are corrected when full-strength insulin is used.
Insulin underdosing.
Control of glycemia can be established in most dogs using less than 1.0 U of insulin/kg of body weight administered twice daily. An inadequate dose of insulin in conjunction with once-daily insulin therapy is a common cause for persistence of clinical signs. In general, insulin underdosing should be considered if the insulin dose is less than 1.0 U/kg and the animal is receiving insulin twice a day. If insulin underdosing is suspected, the dose of insulin should be gradually increased by 1 to 5 U/injection (depending on the size of the dog) per week. The effectiveness of the change in therapy should be evaluated by client perception of clinical response and measurement of serum fructosamine or serial blood glucose concentrations. Other causes for insulin ineffectiveness, most notably the Somogyi response, should be considered once the insulin dose exceeds 1.0 to 1.5 U/kg/injection, the insulin is being administered every 12 hours, and control of glycemia remains poor.
Insulin overdosing and the Somogyi response.
The Somogyi response results from a normal physiologic response to impending hypoglycemia induced by excessive insulin. When the blood glucose concentration declines to less than 65 mg/dl or when the blood glucose concentration decreases rapidly regardless of the glucose nadir, direct hypoglycemia-induced stimulation of hepatic glycogenolysis and secretion of diabetogenic hormones, most notably epinephrine and glucagon, increase the blood glucose concentration, minimize signs of hypoglycemia, and cause marked hyperglycemia within 12 hours of glucose counterregulation. The marked hyperglycemia that occurs after hypoglycemia is due, in part, to an inability of the diabetic dog to secrete sufficient endogenous insulin to dampen the rising blood glucose concentration. By the next morning the blood glucose concentration can be extremely elevated (greater than 400 mg/dl), and the morning urine glucose concentration is consistently 1 to 2 gm/dl as measured with urine glucose test strips. Unrecognized short duration of insulin effect, combined with insulin dose adjustments based on morning urine glucose concentrations, is historically the most common cause for the Somogyi response in dogs.
Clinical signs of hypoglycemia are typically mild or not recognized by the client; clinical signs caused by hyperglycemia tend to dominate the clinical picture. The insulin dose that induces the Somogyi response is variable and unpredictable. The Somogyi response is often suspected in poorly controlled diabetic dogs in which insulin dosage is approaching 2.2 U/kg body weight/injection but can also occur at insulin dosages less than 0.5 U/kg/injection. Toy and miniature breeds of dogs are especially susceptible to development of the Somogyi response with lower-than-expected doses of insulin.
The diagnosis of the Somogyi response requires demonstration of hypoglycemia (less than 80 mg/dl) followed by hyperglycemia (greater than 300 mg/dl) after insulin administration (Fig. 52-8). The Somogyi response should also be suspected when the blood glucose concentration decreases rapidly regardless of the glucose nadir (e.g., a drop from 400 to 100 mg/dl in 2 to 3 hours). If the duration of insulin effect is greater than 12 hours, hypoglycemia often occurs at night after the evening dose of insulin and the serum glucose concentration is typically greater than 300 mg/dl the next morning. Unfortunately, the diagnosis of the Somogyi response can be elusive, in part because of the effects of the diabetogenic hormones on blood glucose concentrations after an episode of glucose counterregulation. Secretion of diabetogenic hormones during the Somogyi response may induce insulin resistance, which can last 24 to 72 hours after the hypoglycemic episode (Fig. 52-9). If a serial blood glucose curve is obtained on the day glucose counterregulation occurs, hypoglycemia will be identified and the diagnosis established. However, if the serial blood glucose curve is obtained on a day when insulin resistance predominates, hypoglycemia will not be identified and the insulin dose may be incorrectly increased in response to the high blood glucose values. A cyclic history of one or two days of good glycemic control followed by several days of poor control should raise suspicion for insulin resistance caused by glucose counterregulation. Serum fructosamine concentrations are unpredictable but are usually increased (>500 μmol/L)—results that confirm poor glycemic control but do not identify the underlying cause.
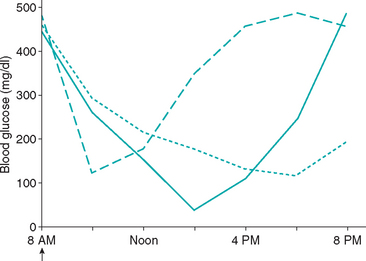
FIG 52-8 Blood glucose concentration curves obtained from three poorly controlled diabetic dogs treated with recombinant human lente insulin twice a day, illustrating the typical blood glucose curves suggestive of the Somogyi response. In one dog (solid line) the glucose nadir is less than 80 mg/dl and is followed by a rapid increase in the blood glucose concentration. In one dog (dashed line) a rapid decrease in the blood glucose concentration occurs within 2 hours of insulin administration and is followed by a rapid increase in the blood glucose concentration; the rapid decrease in blood glucose stimulates glucose counterregulation, despite maintaining the blood glucose nadir above 80 mg/dl. In one dog (dotted line) the blood glucose curve is not suggestive of the Somogyi response, per se. However, the insulin injection causes the blood glucose to decrease by approximately 300 mg/dl during the day, and the blood glucose concentration at the time of the evening insulin injection is considerably lower than the 8 AM blood glucose concentration. If a similar decrease in the blood glucose occurs with the evening insulin injection, hypoglycemia and the Somogyi response would occur at night and would explain the high blood glucose concentration in the morning and the poor control of the diabetic state.
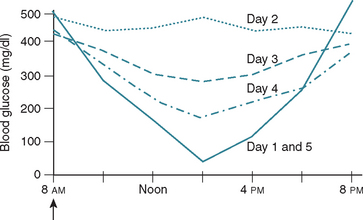
FIG 52-9 Schematic of the change in the results of blood glucose curves obtained on sequential days after induction of the Somogyi response to hypoglycemia induced by an overdose of insulin. Hypoglycemia and the Somogyi response occur on day 1. The secretion of diabetogenic hormones in response to the hypoglycemia causes insulin resistance and increased blood glucose concentrations on day 2. Insulin resistance gradually wanes over the ensuing couple of days (days 3 and 4), eventually resulting in hypoglycemia and the Somogyi response (day 5) as sensitivity to insulin returns to normal. The same dose of insulin is administered each day (arrow).
Establishing the diagnosis may require several days of hospitalization and serial blood glucose curves, an approach that eventually leads to problems with stress-induced hyperglycemia. An alternative, preferable approach is to arbitrarily reduce the insulin dose 1 to 5 units and have the client evaluate the dog’s clinical response over the ensuing 2 to 5 days. If clinical signs of diabetes worsen after a reduction in the insulin dose, another cause for the insulin ineffectiveness should be pursued. However, if the client reports no change or improvement in clinical signs, continued gradual reduction of the insulin dose should be pursued. Alternatively, glycemic regulation of the diabetic dog could be started over using an insulin dose of 0.25 U/kg given twice daily.
Short duration of insulin effect.
For most dogs, the duration of effect of lente and NPH insulin is 10 to 14 hours and twice-daily insulin administration is effective in controlling blood glucose concentrations. However, in some diabetic dogs the duration of effect of lente and NPH insulin is less than 10 hours, a duration that is too short to prevent periods of hyperglycemia and persistence of clinical signs (Fig. 52-10). A diagnosis of short duration of insulin effect is made by demonstrating an initial blood glucose concentration greater than 300 mg/dl combined with a glucose nadir above 80 mg/dl that occurs less than 8 hours after insulin administration and recurrence of hyperglycemia (greater than 250 mg/dl) within 10 hours of the insulin injection (see Fig. 52-7). Treatment involves changing to a longer-acting insulin (e.g., switching to insulin glargine; Fig. 52-11) or increasing the frequency of insulin administration (e.g., initiating therapy q8h). PZI insulin of beef/pork source should not be used in dogs because of potential problems with insulin antibodies (discussed later).
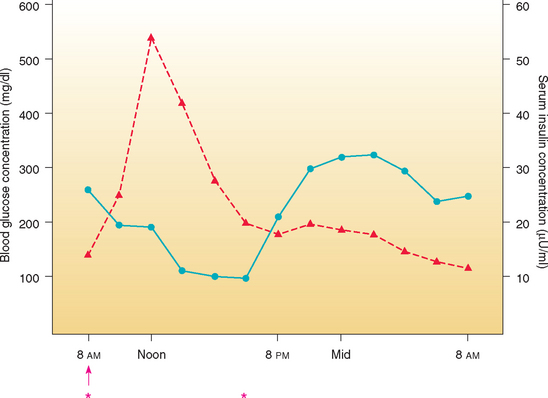
FIG 52-10 Mean blood glucose (blue line) and serum insulin (red line) concentrations in eight dogs with diabetes mellitus treated with a beef-pork source NPH insulin subcutaneously once daily. The duration of NPH effect is too short, resulting in prolonged periods of hyperglycemia beginning shortly after the evening meal. ↑, Insulin injection; *, equal-sized meals consumed.
Prolonged duration of insulin effect.
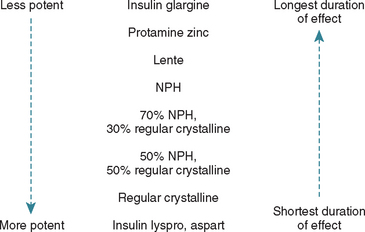
In some diabetic dogs the duration of effect of lente or NPH insulin is greater than 12 hours, and twice-daily insulin administration creates problems with hypoglycemia and the Somogyi response. In these dogs the glucose nadir after the morning administration of insulin typically occurs near or after the time of the evening insulin administration, and the morning blood glucose concentration is usually greater than 300 mg/dl (see Fig. 52-7). The effectiveness of insulin in lowering the blood glucose concentration is variable from day to day, presumably because of varying concentrations of diabetogenic hormones, the secretion of which was induced by prior hypoglycemia. Serum fructosamine concentrations are variable but usually greater than 500 μmol/L. An effective treatment depends, in part, on the duration of effect of the insulin. A 24-hour blood glucose curve should be generated after administration of insulin once in the morning and feeding the dog at the normal times of the day. This will allow the clinician to estimate the duration of effect of the insulin. If the duration of effect is less than 16 hours, a shorter-acting insulin given twice a day or a lower dose of the same insulin given in the evening, compared with the morning insulin dose, can be tried (see Fig. 52-11). If the duration of effect is 16 hours or longer, switching to a longer-acting insulin administered once a day or administering NPH or lente insulin in the morning and regular crystalline insulin at bedtime (i.e., 16 to 18 hours after the morning insulin injection) can be tried. When different types of insulin are used in the same 24-hour period, the goal is to have the combined duration of effect of the insulins equal 24 hours. Differences in potency of intermediate- and long-acting insulins versus regular crystalline insulin often necessitate use of different dosages for the morning and evening insulin injection; because regular crystalline insulin is more potent, less of it is required to get the same glycemic effect, compared with lente, NPH, PZI, and glargine insulin.
Inadequate insulin absorption.
Slow or inadequate absorption of ultralente insulin was a problem in dogs and cats, but ultralente insulin is no longer commercially available. A similar problem is uncommon in diabetic dogs treated with NPH or lente insulin. Impaired absorption of insulin may also occur as a result of thickening of the skin and inflammation of the subcutaneous tissues caused by chronic injection of insulin in the same area of the body. Rotation of the injection site will help prevent this problem.
Circulating insulin-binding antibodies.
Insulin antibodies result from repeated injections of a foreign protein (i.e., insulin). The structure and amino acid sequence of the injected insulin relative to the native endogenous insulin influence the development of insulin antibodies. Conformational insulin epitopes are believed to be more important in the development of insulin antibodies than differences in the linear subunits of the insulin molecule, per se. The more divergent the insulin molecule being administered from the species being treated, the greater the likelihood that significant amounts of insulin antibodies will be formed. Canine, porcine, and recombinant human insulin are similar, and development of insulin antibodies is uncommon in dogs treated with porcine or recombinant human insulin. In contrast, canine and beef insulin differ and serum insulin antibodies have been identified in 40% to 65% of dogs treated with beef/pork or beef insulin. The presence of serum insulin antibodies is often associated with erratic and poor diabetic control, frequent adjustments in the insulin dose to improve control, and occasional development of severe insulin resistance. Dogs treated with porcine or recombinant human insulin have more stable control of glycemia for extended periods of time compared with dogs treated with beef insulin. Although uncommon, insulin antibodies can develop in dogs treated with recombinant human insulin and should be suspected as the cause of poor glycemic control when another cause cannot be identified. Documentation of serum insulin antibodies should make use of assays that have been validated in diabetic dogs. A switch to porcine-source insulin, a switch to a purer form of insulin (i.e., regular crystalline insulin), or both should be considered if insulin antibodies are identified in a poorly controlled diabetic dog.
Allergic reactions to insulin.
Significant reactions to insulin occur in as many as 5% of human diabetics treated with insulin and include erythema, pruritus, induration, and lipoatrophy at the injection site. Allergic reactions to insulin have been poorly documented in diabetic dogs and cats. Pain on injection of insulin is usually caused by inappropriate injection technique, inappropriate site of injection, a reaction to the cold temperature of insulin stored in the refrigerator, or issues with behavior and not an adverse reaction to insulin, per se. Rarely, diabetic dogs and cats will develop focal subcutaneous edema and swelling at the site of insulin injection. Insulin allergy is suspected in these animals. Treatment includes switching to a less antigenic insulin and to a more purified insulin preparation (e.g., regular crystalline insulin). Systemic allergic reactions to insulin in dogs or cats have yet to be identified.
Concurrent disorders causing insulin resistance.
Insulin resistance is a condition in which a normal amount of insulin produces a subnormal biologic response. Insulin resistance may result from problems occurring before the interaction of insulin with its receptor, at the receptor, or at steps distal to the interaction of insulin and its receptor. No insulin dose clearly defines insulin resistance. For most diabetic dogs control of glycemia can usually be attained using 1.0 U or less of NPH or lente insulin per kilogram of body weight given twice daily. Insulin resistance should be suspected if control of glycemia is poor despite an insulin dosage in excess of 1.5 U/kg, when excessive amounts of insulin (i.e., insulin dosage>1.5 U/kg) are necessary to maintain the blood glucose concentration below 300 mg/dl, and when control of glycemia is erratic and insulin requirements are constantly changing in an attempt to maintain control of glycemia. Failure of the blood glucose concentration to decrease below 300 mg/dl during a serial blood glucose curve is suggestive of, but not definitive for, the presence of insulin resistance. An insulin resistance–type blood glucose curve can also result from stress-induced hyperglycemia, the Somogyi response, and other problems with insulin therapy, and a decrease in the blood glucose concentration below 300 mg/dl can occur with disorders causing relatively mild insulin resistance. Serum fructosamine concentrations are typically greater than 500 μmol/L in dogs with insulin resistance and can exceed 700 μmol/L if resistance is severe.
Many disorders can interfere with insulin action (see Box 52-7). The most common in diabetic dogs include diabetogenic drugs (i.e., glucocorticoids), severe obesity, hyperadrenocorticism, diestrus, chronic pancreatitis, renal insufficiency, oral and urinary tract infections, hyperlipidemia, and insulin antibodies in dogs treated with beef insulin. Obtaining a complete history and performing a thorough physical examination is the most important step in identifying these concurrent disorders. If the history and physical examination are unremarkable, a CBC, serum biochemical analysis, serum pancreatic lipase immunoreactivity, serum progesterone concentration (intact female dog), abdominal ultrasound, and urinalysis with bacterial culture should be obtained to further screen for concurrent illness. Additional tests will be dependent on results of the initial screening tests (Box 52-8).
CHRONIC COMPLICATIONS OF DIABETES MELLITUS
Complications resulting from diabetes or its treatment are common in diabetic dogs and include blindness and anterior uveitis resulting from cataract formation, hypoglycemia, chronic pancreatitis, recurring infections, poor glycemic control, and ketoacidosis (see Box 52-5). Many clients are hesitant to treat their newly diagnosed diabetic dog because of knowledge regarding chronic complications experienced in human diabetics and concern that a similar fate awaits their pet. However, clients should be assured that the devastating effects of human diabetes (e.g., nephropathy, vasculopathy, coronary artery disease) require 10 to 20 years or longer to develop and therefore are uncommon in diabetic dogs.
Cataracts
Cataract formation is the most common and one of the most important long-term complications of diabetes mellitus in the dog. A retrospective-cohort study on the development of cataracts in 132 diabetic dogs referred to a university referral hospital found cataract formation in 14% of dogs at the time diabetes was diagnosed and a time interval for 25%, 50%, 75%, and 80% of the study population to develop cataracts at 60, 170, 370, and 470 days, respectively (Beam et al., 1999). The pathogenesis of diabetic cataract formation is thought to be related to altered osmotic relationships in the lens induced by the accumulation of sorbitol and fructose, sugars that are potent hydrophilic agents and cause an influx of water into the lens, leading to swelling and rupture of the lens fibers and the development of cataracts. Cataract formation is an irreversible process once it begins, and it can occur quite rapidly. Diabetic dogs that are poorly controlled and have problems with wide fluctuations in the blood glucose concentration seem especially at risk for rapid development of cataracts. Blindness may be eliminated by removing the abnormal lens. Vision is restored in approximately 75% to 80% of diabetic dogs that undergo cataract removal. Factors that affect the success of surgery include the degree of glycemic control preceding surgery, presence of retinal disease, and presence of lens-induced uveitis. Acquired retinal degeneration affecting vision is more of a concern in older diabetic dogs than is diabetic retinopathy. Fortunately, acquired retinal degeneration is unlikely in an older diabetic dog with vision immediately before cataract formation. If available, electroretinography should be performed before surgery to evaluate retinal function.
Lens-Induced Uveitis
During embryogenesis the lens is formed within its own capsule, and its structural proteins are not exposed to the immune system. Therefore immune tolerance to the crystalline proteins does not develop. During cataract formation and reabsorption lens proteins are exposed to the local immune system, resulting in inflammation and uveitis. Uveitis that occurs in association with a reabsorbing, hypermature cataract may decrease the success of cataract surgery and must be controlled before surgery. The treatment of lens-induced uveitis focuses on decreasing the inflammation and preventing further intraocular damage. Topical ophthalmic corticosteroids are the most commonly used drug for the control of ocular inflammation. However, systemic absorption of topically applied corticosteroids may cause insulin resistance and interfere with glycemic control of the diabetic state, especially in toy and miniature breeds. An alternative is the topical administration of nonsteroidal antiinflammatory agents (e.g., 0.03% flurbiprofen) or cyclosporine.
Diabetic Neuropathy
Although a common complication in the diabetic cat (see p. 795), diabetic neuropathy is infrequently recognized in the diabetic dog. Subclinical neuropathy is probably more common than is severe neuropathy resulting in clinical signs. Clinical signs consistent with diabetic neuropathy are most commonly recognized in dogs that have been diabetic for a long time (i.e., 5 years or longer). Clinical signs and physical examination findings include weakness, knuckling, abnormal gait, muscle atrophy, depressed limb reflexes, and deficits in postural reaction testing. Diabetic neuropathy in the dog is primarily a distal polyneuropathy, characterized by segmental demyelination and remyelination and axonal degeneration and regeneration. There is no specific treatment for diabetic neuropathy besides meticulous metabolic control of the diabetic state.
Diabetic Nephropathy
Although diabetic nephropathy has occasionally been reported in the dog, its clinical recognition appears to be low. Histopathologic findings include membranous glomerulonephropathy, glomerular and tubular basement membrane thickening, an increase in the mesangial matrix material, the presence of subendothelial deposits, glomerular fibrosis, and glomerulosclerosis. The pathogenic mechanism of diabetic nephropathy is unknown. Clinical signs depend on the severity of glomerulosclerosis and the functional ability of the kidney to excrete metabolic wastes. Initially, diabetic nephropathy is manifested as proteinuria, primarily albuminuria. As glomerular changes progress, glomerular filtration becomes progressively impaired, resulting in the development of azotemia and eventually uremia. With severe fibrosis of the glomeruli, oliguric and then anuric renal failure develops. There is no specific treatment for diabetic nephropathy apart from meticulous metabolic control of the diabetic state, conservative medical management of the renal insufficiency, and control of systemic hypertension.
Systemic Hypertension
Diabetes mellitus and hypertension commonly co-exist in dogs. Struble et al. (1998) found the prevalence of hyperten sion to be 46% in 50 insulin-treated diabetic dogs, in which hypertension was defined as systolic, diastolic, or mean blood pressure greater than 160, 100, and 120 mm Hg, respectively. The development of hypertension was associated with the duration of diabetes and an increased albumin : creatinine ratio in the urine. Diastolic and mean blood pressure were higher in dogs with longer duration of disease. A correlation between control of glycemia and blood pressure was not identified. Treatment for hypertension should be initiated if the systolic blood pressure is consistently greater than 160 mm Hg.
Prognosis
The prognosis is dependent on the presence and reversibility of concurrent diseases, ease of regulation of the diabetic state with insulin, and client commitment toward treating the disease. The mean survival time in diabetic dogs is approximately 3 years from the time of diagnosis. This survival time is somewhat skewed because dogs are often 8 to 12 years old at the time of diagnosis and a relatively high mortality rate exists during the initial 6 months because of concurrent life-threatening or uncontrollable disease (e.g., ketoacidosis, acute pancreatitis, renal failure). Diabetic dogs that survive the initial 6 months can easily maintain a good quality of life for longer than 5 years with proper care by the clients, timely evaluations by the veterinarian, and good client-veterinarian communication.
DIABETES MELLITUS IN CATS
Etiology
Common histologic abnormalities in cats with diabetes mellitus include islet-specific amyloidosis, β-cell vacuolation and degeneration, and chronic pancreatitis. The cause of β-cell degeneration is not known. Other diabetic cats have a reduction in the number of pancreatic islets and/or insulin-containing β cells on immunohistochemical evaluation, suggesting additional mechanisms may be involved in the physiopathology of diabetes mellitus in cats. Although lymphocytic infiltration of islets, in conjunction with islet amyloidosis and vacuolation, has been described in diabetic cats, this histologic finding is very uncommon, and β cell and insulin autoantibodies have not been identified in newly diagnosed diabetic cats. The role of genetics remains to be determined.
Noninsulin-dependent type 2 diabetes may be identified in as many as 50% to 70% of newly diagnosed diabetic cats. Islet amyloidosis and insulin resistance are important factors in the development of noninsulin-dependent type 2 diabetes in cats. Islet-amyloid polypeptide (IAPP), or amylin, is the principal constituent of amyloid in adult cats with diabetes, is stored in β-cell secretory granules, and is co-secreted with insulin by the β cell. Stimulants of insulin secretion also stimulate the secretion of amylin. Chronic increased secretion of insulin and amylin, as occurs with obesity and other insulin-resistant states, results in aggregation and deposition of amylin in the islets as amyloid (Fig. 52-12). IAPP-derived amyloid fibrils are cytotoxic and associated with apoptotic cell death of islet cells. If deposition of amyloid is progressive, as occurs with a sustained demand for insulin secretion in response to persistent insulin resistance, islet cell destruction progresses and eventually leads to diabetes mellitus. The severity of islet amyloidosis and β cell destruction determines, in part, whether the diabetic cat has IDDM or NIDDM. Total destruction of the islets results in IDDM and the need for insulin treatment for the rest of the cat’s life. Partial destruction of the islets may or may not result in clinically evident diabetes, insulin treatment may or may not be required to control glycemia, and diabetes may or may not revert to a noninsulin-requiring state once treatment is initiated. If amyloid deposition is progressive, the cat will progress from subclinical diabetes to NIDDM and ultimately to IDDM. Current research regarding the etiopathogenesis of diabetes in the cat suggests that the difference between IDDM and NIDDM is primarily a difference in severity of loss of β cells and severity and reversibility of concurrent insulin resistance. Cats may have IDDM or NIDDM at the time diabetes is diagnosed, cats with NIDDM may progress to IDDM with time, cats with apparent IDDM may revert to a noninsulin requiring state after initiation of treatment, and cats may flip back and forth between IDDM and NIDDM as severity of insulin resistance and impairment of β cell function waxes and wanes.
FIG 52-12 A, Severe islet amyloidosis (straight arrow) in a cat with initial noninsulin-dependent diabetes mellitus (NIDDM) that progressed to insulin-dependent diabetes mellitus (IDDM). A pancreatic biopsy specimen was obtained while the animal was in the IDDM state. Residual β cells containing insulin (red arrows) are also present. (Immunoperoxidase stain, ×100.) B, Severe vacuolar degeneration of islet cells. Pancreatic tissue was evaluated at necropsy 28 months after diabetes was diagnosed and 20 months after cat progressed from NIDDM to IDDM, requiring insulin to control blood glucose concentrations. The cat died from metastatic exocrine pancreatic adenocarcinoma. (H&E, ×500.) C, Severe chronic pancreatitis with fibrosis in a diabetic cat with IDDM. The cat was euthanized because of persistent problems with lethargy, inappetence, and poorly controlled diabetes mellitus. (H&E, ×100.)
(A from Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
Approximately 20% of diabetic cats become transiently diabetic, usually within 4 to 6 weeks after the diagnosis of diabetes has been established and treatment has been initiated. In these cats hyperglycemia, glycosuria, and clinical signs of diabetes resolve, and insulin treatment can be discontinued. Some diabetic cats may never require insulin treatment once the initial bout of clinical diabetes mellitus has dissipated, whereas others become permanently insulin dependent weeks to months after the resolution of a prior diabetic state. Studies suggest that cats with transient diabetes mellitus are in a subclinical diabetic state that becomes clinical when the pancreas is stressed by exposure to a concurrent insulin-antagonistic drug or disease, most notably glucocorticoids, megestrol acetate, and chronic pancreatitis (Fig. 52-13). Unlike healthy cats, those with transient diabetes mellitus have a reduced population of β cells, dysfunctional β cells, or both, which impairs the ability of the pancreas to compensate for concurrent insulin resistance. An inadequate insulin response results in hyperglycemia. Persistent hyperglycemia can, in turn, cause hypoinsulinemia by suppressing function of remaining β cells and can induce insulin resistance by promoting downregulation of glucose transport systems and causing a defect in posttransport insulin action. This phenomenon is referred to as glucose toxicity. β cells have an impaired response to stimulation by insulin secretagogues, thereby mimicking IDDM. The effects of glucose toxicity are potentially reversible upon correction of the hyperglycemic state. The clinician makes a correct diagnosis of diabetes mellitus, insulin and treatment of insulin-antagonistic disorders improve hyperglycemia and insulin resistance, glucose toxicity and β cell function improve, insulin secretion returns, and an apparent IDDM state resolves. The future requirement for insulin treatment depends on the underlying abnormality in the islets. If the abnormality is progressive (e.g., amyloidosis), eventually enough β cells will be destroyed and IDDM will develop.
SIGNALMENT
Although diabetes mellitus may be diagnosed in cats of any age, most diabetic cats are more than 9 years old (mean 10 years) at the time of diagnosis. Diabetes mellitus occurs predominantly in neutered male cats; no apparent breed predisposition has been discovered, although Burmese cats may be overrepresented in Australia.
HISTORY
The history in virtually all diabetic cats includes polydipsia, polyuria, polyphagia, and weight loss. A common complaint of cat owners is the constant need to change the litter and an increase in the size of the litter clumps. Additional clinical signs include lethargy; decreased interaction with family members; lack of grooming behavior and development of a dry, lusterless, unkempt, or matted haircoat; and decreased jumping ability, rear limb weakness, or development of a plantigrade posture (Fig. 52-14). If the client does not notice clinical signs associated with uncomplicated diabetes, a dia betic cat may be at risk for developing DKA (see p. 796). The time sequence from the onset of initial clinical signs to the development of DKA is unpredictable.

FIG 52-14 A, Plantigrade posture in a cat with diabetes mellitus and exocrine pancreatic insufficiency. B, Resolution of hind limb weakness and plantigrade posture after improving glycemic control by adjusting insulin therapy and initiating pancreatic enzyme replacement therapy. C, Severe diabetic neuropathy in a cat with diabetes mellitus. Note the palmigrade and plantigrade posture. The more severe and the more chronic the neuropathy, the less likely the neuropathy will improve after improvement in diabetic control.
PHYSICAL EXAMINATION
Physical examination findings depend on the presence and severity of DKA and the nature of other concurrent disorders. The nonketotic diabetic cat has no classic physical examination findings. Many diabetic cats are obese but otherwise in good physical condition. Cats with prolonged untreated diabetes may have lost weight but are rarely emaciated unless concurrent disease (e.g., hyperthyroidism) is present. Newly diagnosed and poorly controlled diabetic cats often stop grooming and develop a dry, lusterless haircoat. Diabetes-induced hepatic lipidosis may cause hepatomegaly. Impaired ability to jump, weakness in the rear limbs, ataxia, or a plantigrade posture (i.e., the hocks touch the ground when the cat walks) may be evident if the cat has developed diabetic neuropathy. Distal muscles of the rear limbs may feel hard on digital palpation, and cats may object to palpation or manipulation of the rear limbs, presumably because of pain associated with the neuropathy. Additional abnormalities may be identified in the ketoacidotic diabetic cat (see p. 796).
Diagnosis
Establishing the diagnosis of diabetes mellitus is similar for cats and dogs and is based on identification of appropriate clinical signs, persistent hyperglycemia, and glycosuria (see p. 769). Transient, stress-induced hyperglycemia is a common problem in cats and can cause the blood glucose concentration to increase above 300 mg/dl. Unfortunately, stress is a subjective state that cannot be accurately measured, is not always easily recognized, and may evoke inconsistent responses among individual cats. Glycosuria usually does not develop in cats with transient stress–induced hyperglycemia but can be present if stress is prolonged (i.e., hours). For this reason, presence of appropriate clinical signs, persistent hyperglycemia, and glycosuria should always be documented when establishing a diagnosis of diabetes mellitus in cats. If the clinician is in doubt, the stressed cat can be sent home with instructions for the client to monitor the urine glucose concentration with the cat in the nonstressed home environment. Alternatively, a serum fructosamine concentration can be measured (see p. 774). Documenting an increase in the serum fructosamine concentration supports the presence of sustained hyperglycemia; however, a serum fructosamine concentration in the upper range of normal can occur in symptomatic diabetic cats if the diabetes developed shortly before presentation of the cat to the veterinarian.
Clinical signs develop when hyperglycemia causes glycosuria and are the same regardless of the functional status of pancreatic islets. Information used to establish the diagnosis of diabetes mellitus does not provide information on the status of pancreatic islet health, presence of glucose toxicity, ability of the cat to secrete insulin, or the severity and reversibility of concurrent insulin resistance. Unfortunately, measurements of baseline serum insulin concentration or serum insulin concentrations after administration of an insulin secretagogue have not been consistent aids in differentiating IDDM and NIDDM in the cat. Identification of a baseline serum insulin concentration greater than 15 μU/ml (reference range, 5 to 20 μU/ml) in a newly diagnosed, untreated diabetic cat supports the presence of functional β cells and partial destruction of the islets; however, low or undetectable serum insulin concentrations do not rule out partial β cell loss because of the suppressive effects of glucose toxicity on circulating insulin concentrations.
A thorough evaluation of the cat’s overall health is recommended once the diagnosis of diabetes mellitus has been established, for reasons discussed on p. 769. The minimal laboratory evaluation in any diabetic cat should include a CBC, serum biochemical panel, serum thyroxine concentration, and urinalysis with bacterial culture. If available, abdominal ultrasound should also be a routine part of the diagnostic evaluation because of the high prevalence of chronic pancreatitis in diabetic cats. Measurement of baseline serum insulin concentration or performance of an insulin secretory response test is not routinely done in cats because of problems encountered with glucose toxicity. Additional tests may be warranted after obtaining the history, performing the physical examination, or identifying ketoacidosis. See Box 52-4 for a list of potential clinical pathologic abnormalities.
Treatment
The significant incidence of NIDDM in cats raises interesting questions regarding the need for insulin treatment. Glycemic control can be maintained in some diabetic cats with dietary changes, oral hypoglycemic drugs, control of current diseases, discontinuation of insulin-antagonistic drugs, or a combination of these. The ultimate differentiation between IDDM and NIDDM is usually made retrospectively, after the clinician has had several weeks to assess the response of the cat to therapy and to determine the cat’s need for insulin. The initial treatment strategy is based on the severity of clinical signs and physical abnormalities, presence or absence of ketoacidosis, general health of the cat, and client wishes. For most newly diagnosed diabetic cats, treatment includes insulin, adjustments in diet, and correction or control of concurrent insulin resistance.
INITIAL INSULIN RECOMMENDATIONS FOR DIABETIC CATS
Diabetic cats are notoriously unpredictable in their response to exogenous insulin. No single type of insulin is routinely effective in maintaining control of glycemia, even with twice-daily administration. The initial insulin of choice ultimately is based on personal preferences and experiences. Commonly used insulin preparations for the long-term management of diabetic cats include human recombinant NPH, porcine lente, beef/pork PZI, and the insulin analog glargine (see the section on overview of insulin preparations, p. 769; see Fig. 52-11). All have potential problems in diabetic cats, primarily related to duration of insulin effect, not species of insulin and insulin antibody formation. Although lente and NPH insulin are consistently and rapidly absorbed after subcutaneous administration, the duration of effect of lente and especially NPH insulin can be considerably shorter than 12 hours, resulting in inadequate control of glycemia despite twice-daily administration (see Table 52-2). Although PZI is a longer-acting insulin, the timing of the glucose nadir is variable and occurs within 9 hours of PZI administration in the majority of treated diabetic cats. In one study PZI significantly improved control of glycemia in newly diagnosed diabetic cats and poorly controlled diabetic cats previously treated with ultralente or NPH insulin (Nelson et al., 2001). Comparison of efficacy between PZI and lente insulin has not been reported.
Insulin glargine is the longest-acting commercially available insulin for treatment of diabetes in humans and is currently a popular initial choice by veterinarians for the treatment of diabetes in cats. An unpublished study identified better glycemic control and a higher diabetes remission rate in newly diagnosed diabetic cats treated with glargine twice a day, compared with lente or PZI administered twice a day (Weaver and Rand, 2005). Another study found no difference in glycemic control in diabetic cats treated with glargine once a day versus diabetic cats treated with lente insulin twice a day, and a higher diabetes remission rate in diabetic cats treated with lente insulin (Weaver et al., 2006). In my experience, the duration of effect of glargine is quite variable, with the glucose nadir occurring as soon as 4 hours and as late as 20 hours after administration. Glargine works well when given once or twice a day in some diabetic cats and does not work very well in others. Problems are usually related to duration of effect (i.e., too short or too long).
Currently, my personal preference for the initial treatment of newly diagnosed diabetes in cats is PZI at an initial dose of 1 U/cat. Because the majority of diabetic cats require PZI insulin twice a day, I prefer to start with twice-daily insulin therapy while the insulin dose is low to prevent problems with hypoglycemia and the Somoygi response. I switch to lente insulin given twice a day if problems with prolonged duration of PZI effect develop and glycemic control cannot be maintained with once-daily PZI, and I switch to glargine given twice a day if problems with short duration of PZI effect develop. When using glargine for the treatment of newly diagnosed diabetic cats, I use an initial dose of 1 unit/cat administered once a day and switch to twice-daily therapy if subsequent blood glucose evaluations support a duration of effect of 12 hours or less. If PZI insulin becomes unavailable, I would use porcine lente insulin at an initial dose of 1 U/cat twice a day in the newly diagnosed diabetic cat.
DIET
The general principles for dietary therapy are listed in Box 52-6. Obesity, feeding practices, and content of the diet warrant discussion in diabetic cats. Obesity is common in diabetic cats and results from excessive caloric intake typically caused by free-choice feeding of dry cat food. Obesity causes reversible insulin resistance that resolves as obesity is corrected. Control of glycemia often improves, and some diabetic cats may revert to a subclinical diabetic state after weight reduction. Correction of obesity is difficult in cats because it requires restriction of daily caloric intake without a corresponding increase in caloric expenditure (i.e., exercise). Although there are several diets specifically formulated for weight reduction in cats, diets containing increased amounts of fiber and diets containing increased protein and decreased carbohydrate should be used in the obese diabetic cat for reasons discussed later. The reader is referred to Chapter 54 for more information on correction of obesity in cats.
The eating habits of cats vary considerably, from those cats that eat everything at the time it is offered to those that graze throughout the day and night. The primary goal of dietary therapy is to minimize the impact of a meal on postprandial blood glucose concentrations. Consuming the same amount of calories in multiple small amounts throughout a 12-hour period should have less impact than consuming the calories at a single large meal. Half of the cat’s total daily caloric intake should be offered at the time of each insulin injection and remain available to the cat to consume when it wishes. Attempts to force a grazing cat to eat the entire meal at one time usually fail and are not warranted as long as the cat has access to the food during the ensuing 12 hours. A similar approach is taken for diabetic dogs that are finicky eaters.
Cats are carnivores and, as such, have higher dietary protein requirements than omnivores such as humans and dogs. Hepatic glucokinase and hexokinase activity is lower in cats, compared with that for carnivores with omnivorous eating habits, and suggests that diabetic cats may be predisposed to developing higher postprandial blood glucose concentrations after consumption of diets containing a high carbohydrate load, and vice versa. Dietary studies in diabetic cats have documented improved control of glycemia with diets containing increased fiber content, increased protein and decreased carbohydrate content, and increased fat and decreased carbohydrate content plus treatment with the α-glucosidase inhibitor acarbose. The central theme in these dietary studies has been restriction of carbohydrate absorption by the gastrointestinal tract, either by inhibiting starch digestion (acarbose), inhibiting intestinal glucose absorption (fiber), or decreasing carbohydrate ingestion (low carbohydrate–containing diets). Intuitively, the most effective means to minimize gastrointestinal absorption of carbohydrates in the diabetic cat is to feed diets that contain minimal amounts of carbohydrate. Current recommendations include diets with high protein and low carbohydrate content and diets containing increased fiber and moderate carbohydrate content (see Box 52-6). Which diet will be most beneficial in improving control of glycemia in any given diabetic cat is unpredictable. The initial diet of choice is based on personal preference. Currently, I initially use diets containing high protein and low carbohydrate content, and if palatability, problems with renal insufficiency, or adverse effects become an issue or poor control of glycemia persists despite adjustments in insulin therapy, a switch to one of the fibercontaining diets should be considered. Diets containing high fat and low carbohydrate content (e.g., growth diets) are not recommended because of concerns related to the impact of high dietary fat content on obesity, hepatic lipidosis, chronic pancreatitis, and insulin resistance—the latter induced by increased circulating concentrations of nonesterified fatty acids, β-hydroxybutyric acid, and triglycerides.
IDENTIFICATION AND CONTROL OF CONCURRENT PROBLEMS
Identification and correction of concurrent disorders that cause insulin resistance and interfere with the success of insulin therapy is critical to the successful treatment of diabetes in cats. Examples include obesity; chronic pancreatitis and other chronic inflammatory diseases; infection; and insulin-resistant disease such as hyperthyroidism, hyperadrenocorticism, and acromegaly. In diabetic cats with partial loss of β cells correction of insulin resistance may result in reversion from an insulin-dependent to a non–insulin-dependent or subclinical diabetic state. An evaluation of the diabetic cat for concurrent problems is indicated at the time diabetes is diagnosed and whenever control of glycemia deteriorates in a previously well-controlled cat and should include a thorough history, physical examination, CBC, serum biochemistry panel, serum thyroxine concentration, urinalysis with culture, and (if available) abdominal ultrasound.
ORAL HYPOGLYCEMIC DRUGS
In the United States, five classes of oral hypoglycemic drugs are approved for the treatment of NIDDM in human beings: sulfonylureas, meglitinides, biguanides, thiazolidinediones, and α-glucosidase inhibitors. These drugs work by stimulating pancreatic insulin secretion (sulfonylureas, meglitinides), enhancing tissue sensitivity to insulin (biguanides, thiazolidinediones), or slowing postprandial intestinal glucose absorption (α-glucosidase inhibitors). Although controversial, chromium and vanadium are trace minerals that may also function as insulin sensitizers. Studies have documented the efficacy of sulfonylureas for treating diabetes in cats and α-glucosidase inhibitors for improving glycemic control in diabetic dogs. Insulin sensitizers as the sole therapeutic agent are of questionable benefit in diabetic dogs and cats because they require the presence of circulating insulin to be effective. Most diabetic cats subsequently shown to have NIDDM have low or nondetectable insulin concentrations at the time diabetes is diagnosed, in part because of the effects of concurrent glucose toxicity on circulating insulin concentrations.
Sulfonylureas
Sulfonylurea drugs (e.g., glipizide, glyburide) are the most commonly used oral hypoglycemic drugs for the treatment of diabetes mellitus in cats. Sulfonylureas stimulate insulin secretion by pancreatic β cells. Some endogenous pancreatic insulin secretory capacity must exist for sulfonylureas to be effective. Clinical response to glipizide and glyburide treatment in diabetic cats has been variable, ranging from excellent (i.e., blood glucose concentrations decreasing to less than 200 mg/dl) to partial response (i.e., clinical improvement but failure to resolve hyperglycemia) to no response. Presumably, the population of functioning β cells varies from none (severe IDDM) to near normal (mild NIDDM) in treated cats, resulting in a response range from none to excellent. Cats with a partial response to glipizide have some functioning β cells but not enough to decrease the blood glucose concentration to less than 200 mg/dl. These cats may have severe NIDDM or the early stages of IDDM. Glipizide treatment has been found effective in improving clinical signs and severity of hyperglycemia in approximately 20% of diabetic cats.
No consistent parameters have been identified that allow the clinician to prospectively determine which cats will respond to glipizide or glyburide therapy. Identifying a high preprandial serum insulin concentration or an increase in serum insulin concentration during an insulin secretagogue test supports the diagnosis of NIDDM, but failure to identify these changes does not rule out the potential for a beneficial response to glipizide or glyburide. Selection of diabetic cats for treatment with glipizide must rely heavily on the veterinarian’s assessment of the cat’s health, severity of clinical signs, presence or absence of ketoacidosis, other diabetic complications (e.g., peripheral neuropathy), and the client’s desires.
Glipizide (Glucotrol, Pfizer; 2.5 mg/cat administered q12h) and glyburide (Micronase, Pharmacia and Upjohn Company; 0.625 mg/cat q12h) are initially administered in conjunction with a meal to diabetic cats that are nonketotic and relatively healthy on physical examination (Fig. 52-15). Each cat is examined weekly during the first month of therapy. A history, complete physical examination, body weight, urine glucose/ ketone measurement, and blood glucose concentration are evaluated at each examination. If adverse reactions (Table 52-4) have not occurred after 2 weeks of treatment, the glipizide and glyburide dose is increased to 5.0 mg and 1.25 mg, respectively, q12h. Therapy is continued as long as the cat is stable. If euglycemia or hypoglycemia develops, the dose may be tapered down or discontinued and blood glucose concentrations reevaluated 1 week later to assess the need for the drug. If hyperglycemia recurs, the dose is increased or the sulfonylurea is reinitiated, with a reduction in dose in those cats previously developing hypoglycemia. Sulfonylurea treatment is discontinued and insulin therapy initiated if clinical signs continue to worsen, the cat becomes ill or develops ketoacidosis or peripheral neuropathy, blood glucose concentrations remain greater than 300 mg/dl after 1 to 2 months of therapy, or the client becomes dissatisfied with the treatment. In some cats sulfonylureas become ineffective weeks to months later, and exogenous insulin is ultimately required to control the diabetic state. Presumably, the progression to IDDM coincides with progressive loss of β cells, a loss that may be exacerbated by sulfonylurea treatment. Regardless, the primary value of sulfonylureas is an alternative palatable option (pills versus injections) for clients initially unwilling to consider insulin injections and contemplating euthanasia of their cat. During the ensuing weeks many of these clients become willing to try insulin injections if sulfonylurea therapy fails.
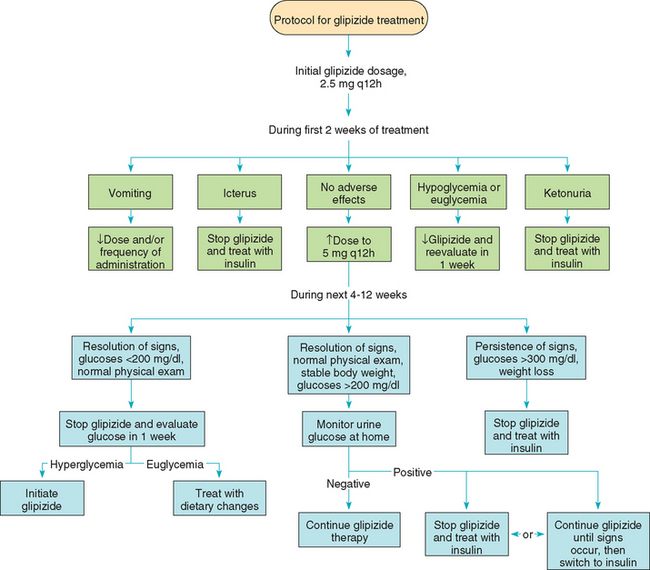
FIG 52-15 Algorithm for treating diabetic cats with the oral sulfonylurea drug, glipizide.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
 TABLE 52-4 Adverse Reactions to Glipizide Treatment in Diabetic Cats
TABLE 52-4 Adverse Reactions to Glipizide Treatment in Diabetic Cats
| ADVERSE REACTION | RECOMMENDATION |
|---|---|
| Vomiting within 1 hour of administration | Vomiting usually subsides after 2 to 5 days of glipizide therapy; decrease dose or frequency of administration if vomiting is severe; discontinue if vomiting persists >1 week |
| Increased serum hepatic enzyme activities | Continue treatment and monitor enzymes every 1 to 2 weeks initially; discontinue glipizide if cat becomes ill (lethargy, inappetence, vomiting) or the alanine transaminase activity exceeds 500 IU/L |
| Icterus | Discontinue glipizide treatment; reinstitute glipizide treatment at lower dose and frequency of administration once icterus resolves (usually within 2 weeks); discontinue treatment permanently if icterus recurs |
| Hypoglycemia | Discontinue glipizide treatment; recheck blood glucose concentration in 1 week; reinstitute glipizide therapy at lower dose or frequency of administration if hyperglycemia recurs |
Acarbose
Although the α-glucosidase inhibitor acarbose has been effective in improving glycemic control in diabetic dogs and cats, the drug is not commonly used because of cost and adverse effects. Diarrhea and weight loss as a result of carbohydrate malassimilation occur in approximately 35% of treated dogs. Feeding carbohydrate-restricted diets is recommended in lieu of acarbose treatment in diabetic cats.
IDENTIFYING INITIAL INSULIN REQUIREMENTS
The approaches to identifying insulin requirements in the newly diagnosed diabetic cat and dog are similar and discussed on p. 773. Most clients of diabetic cats are happy with the response to insulin treatment if the blood glucose concentrations range between 100 and 300 mg/dl throughout the day. Diabetic cats can have problems with hypoglycemia and the Somogyi response (see p. 780) at relatively small doses of insulin (1 to 2 U/injection). As such, the preference is to have the client administer a fixed dose of insulin once control of glycemia is attained and discourage clients from adjusting the insulin dose at home without first consulting their veterinarian.
Techniques for Monitoring Diabetic Control
The techniques for monitoring diabetic control are discussed on p. 774. One important factor that affects monitoring of diabetic cats is the propensity to develop stress-induced hyperglycemia caused by frequent visits to the veterinary hospital for blood samplings. Once stress-induced hyperglycemia develops, it is a perpetual problem and blood glucose measurements can no longer be considered accurate. Veterinarians must remain wary of stress hyperglycemia in diabetic cats and should take steps to prevent its development. Micromanaging diabetic cats is not recommended, and serial blood glucose curves should be done only when the clinician perceives a need to change insulin therapy. The determination of good versus poor control of glycemia should be based on the client’s subjective opinion of the presence and severity of clinical signs and the overall health of the pet, ability of the cat to jump, grooming behavior, findings on physical examination, and stability of body weight. Generation of a serial blood glucose curve should be reserved for newly diagnosed and poorly controlled diabetic cats.
Protocol for Generating the Serial Blood Glucose Curve at Home
An alternative to hospital-generated blood glucose curves is to have the client generate the blood glucose curve at home using the marginal ear vein prick technique in cats (the ear or lip prick technique in dogs) and a portable home blood glucose monitoring device that allows the client to touch the drop of blood on the ear with the end of the glucose test strip (Fig. 52-16). The marginal ear vein prick technique decreases the need for physical restraint during sample collection, thereby minimizing the cat’s discomfort and stress. Accuracy of blood glucose results are similar when blood for glucose determination is obtained by ear prick and venipuncture. However, blood glucose results obtained by portable blood glucose monitoring devices may overestimate or, more commonly, underestimate the actual blood glucose values obtained with reference methods. This inherent error must be considered when interpreting blood glucose results obtained by a portable home blood glucose monitoring device. Several Web sites explain in detail the marginal ear vein prick technique in layman’s terms and provide information on client experiences with the technique and with different portable home blood glucose meters. After diagnosing diabetes, the clinician should recommend a particular Web site and find out whether the client would be interested in monitoring blood glucose concentrations at home. The clinician should allow for ample time to teach the technique to clients who are willing to give it a try and provide advice regarding the proper way to perform a blood glucose curve (ideally, no more frequently than 1 day every 4 weeks) and how often to measure the blood glucose concentration on the day of the curve (typically, at the time of insulin administration and 3, 6, 9, and 12 hours later). Use of the ear prick technique in cats has produced excellent results. Stress is often significantly reduced, and accuracy of the blood glucose measurements improved. Problems with the marginal ear vein prick technique include overzealous clients who start monitoring blood glucose concentrations too frequently, insulin overdosing and the Somogyi response caused by clients who interpret blood glucose results and adjust the insulin dose independent of input from the veterinarian, difficulty obtaining blood from the ear vein, and cats who do not tolerate manipulation and pricking of the ear.
FIG 52-16 Ear prick technique for measuring blood glucose concentration. A, A hot washcloth is applied to the pinna for 2 to 3 minutes to increase circulation to the ear. B, A spot is identified on the periphery of the outer side of the pinna, a small coating of petrolatum jelly is applied, and the spot is pricked with the lancet device supplied with the portable blood glucose meter. Gauze should be placed between the pinna and the digit holding the pinna to prevent pricking the finger if the blade of the lancet accidentally passes through the pinna. Petrolatum jelly is applied to help the blood form into a ball on the pinna as it seeps from the site that is lanced. C, Digital pressure is applied in the area of the lanced skin to promote bleeding. The glucose test strip is touched to the drop of capillary blood that forms and is removed once enough blood has been drawn into the test strip to activate the meter.
Role of Serum Fructosamine in Stressed Diabetic Cats
The use of serum fructosamine concentrations for assessing control of glycemia is discussed on p. 777. Serum fructosamine concentrations are not affected by acute transient increases in blood glucose concentration. Unlike blood glucose measurements, evaluation of serum fructosamine concentration in fractious or stressed diabetic cats provides reliable objective information on the status of glycemic control during the previous 2 to 3 weeks. In fractious or stressed cats the clinician must make an educated guess as to where the problem lies (e.g., wrong type of insulin, low insulin dose), make an adjustment in therapy, and rely on changes in serum fructosamine to assess the benefit of the change in treatment. Serum fructosamine concentrations can be measured before and 2 to 3 weeks after changing insulin therapy to assess the effectiveness of the change. If changes in insulin therapy are appropriate, a decrease in serum fructosamine concentration should occur. If the serum fructosamine concentration is the same or has increased, the change was ineffective in improving glycemic control, another change in therapy based on an educated guess should be done, and the serum fructosamine measured again 2 to 3 weeks later.
INSULIN THERAPY DURING SURGERY
The approaches to managing the diabetic cat and dog during surgery are similar and are discussed on p. 778.
COMPLICATIONS OF INSULIN THERAPY
Complications of insulin therapy are similar for diabetic dogs and cats and are discussed on p. 779. The most common complications of insulin therapy in the diabetic cat are recurring hypoglycemia; insulin overdose, which causes the Somogyi response; incorrect assessment of glycemic control caused by stress-induced hyperglycemia; short duration of effect of NPH; lente and, less commonly, PZI and glargine insulin; prolonged duration of effect of PZI and glargine insulin; and insulin resistance caused by concurrent inflammatory and hormonal disorders, most notably chronic pancreatitis.
Stress Hyperglycemia
Transient hyperglycemia is a well-recognized problem in fractious, scared, or otherwise stressed cats. Hyperglycemia develops as a result of increased catecholamines and, in struggling cats, lactate concentrations. Blood glucose concentrations typically exceed 200 mg/dl in affected cats, and values in excess of 300 mg/dl are common. Stress hyperglycemia can significantly increase blood glucose concentrations in diabetic cats despite the administration of insulin, an effect that seriously compromises the clinician’s ability to accurately judge the effectiveness of the insulin injection. Frequent hospitalizations and venipunctures for monitoring blood glucose concentrations are the most common cause of stress hyperglycemia. Blood glucose concentrations can remain greater than 400 mg/dl throughout the day despite administration of insulin. Failure to recognize the effect of stress on blood glucose results may lead to the erroneous perception that the diabetic cat is poorly controlled. Insulin therapy is invariably adjusted, often by increasing the insulin dose, and another blood glucose curve recommended 1 to 2 weeks later. A vicious cycle ensues, which eventually culminates in the Somogyi response, clinically apparent hypoglycemia, or referral for evaluation of insulin resistance.
Failure to identify the presence of stress hyperglycemia and its impact on the interpretation of blood glucose measurements is one of the most important reasons that the status of glycemic control in diabetic cats is misinterpreted. Stress hyperglycemia should be suspected if the cat is visibly upset or aggressive or struggles during restraint and the venipuncture process. However, stress hyperglycemia can also be present in diabetic cats that are easily removed from the cage and do not resist the blood-sampling procedure. These cats are scared, but rather than become aggressive, they remain crouched in the back of the cage, often have dilated pupils, and usually are flaccid when handled. Stress hyperglycemia should also be suspected if a disparity exists between assessment of glycemic control based on results of the history, physical examination, and stability of body weight; assessment of glycemic control based on results of blood glucose measurements; or when the initial blood glucose concentration measured in the morning is in an acceptable range (i.e., 150 to 250 mg/dl) but subsequent blood glucose concentrations increase steadily throughout the day (Fig. 52-17). Once stress hyperglycemia develops, it is a perpetual problem and blood glucose measurements can no longer be considered accurate. If stress hyperglycemia is suspected, reliance on home monitoring of blood glucose or evaluation of sequential serum fructosamine concentrations (see p. 792) should be done, in addition to the history and physical examination findings.
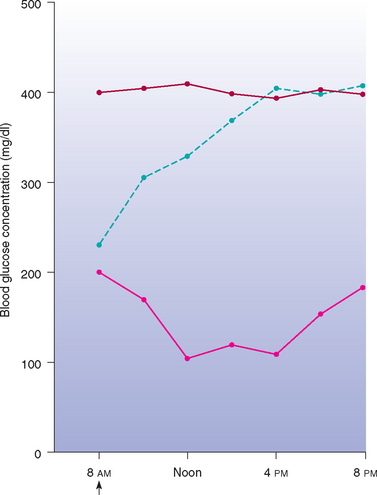
FIG 52-17 Blood glucose concentration curves in a 5.3-kg male cat receiving 2 U of recombinant human ultralente insulin (pink line) 2 weeks after the initiation of insulin therapy, 2 U of recombinant human ultralente insulin (blue line) 2 months later, and 6 U of recombinant human ultralente insulin (red line) 4 months later. The insulin dose had been gradually increased on the basis of the blood glucose concentration curves. The client reported minimal clinical signs regardless of the insulin dose; at the 4-month recheck the cat had maintained its body weight and results of the physical exanination were normal. The cat became progressively more fractious during each hospitalization, supporting the existence of stress-induced hyperglycemia as the reason for the discrepancy between the blood glucose values and other parameters used to evaluate glycemic control. ↑, Subcutaneous insulin injection and food.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
Hypoglycemia
Hypoglycemia, a common complication of insulin therapy, is discussed on p. 779. In diabetic cats symptomatic hypoglycemia is most apt to occur after sudden large increases in the insulin dose, after sudden improvement in concurrent insulin resistance, with excessive duration of insulin action in cats receiving insulin twice a day, after prolonged inappetence, and in insulin-treated cats that have reverted to a non–insulin-dependent state. In these situations severe hypoglycemia may occur before glucose counterregulation (i.e., secretion of glucagon, cortisol, epinephrine, growth hormone) is able to compensate for and reverse low blood glucose concentrations. The initial treatment approach for hypoglycemia is to discontinue insulin until hyperglycemia recurs and then reduce the ensuing insulin dose 25% to 50%. If hypoglycemia remains a reoccurring problem despite reductions in the insulin dose, excessive duration of insulin effect (see p. 781) or reversion to a noninsulin-dependent diabetic state should be considered. Reversion to a non–insulin-dependent diabetic state should be suspected if hypoglycemia remains a persistent problem despite administration of small doses of insulin (i.e., 1 U or less per injection) and administration of insulin once a day, if blood glucose concentrations are consistently below 150 mg/dl before insulin administration, if serum fructosamine concentration is less than 350 μmol/L, or if urine glucose test strips are consistently negative. Insulin therapy should be discontinued and periodic urine glucose testing should be performed in the home environment to identify recurrence of glycosuria.
Insulin Overdosing and the Somogyi Response
Insulin overdosing and the Somogyi response is discussed on p. 780. A similar phenomenon, characterized by wide fluctuations in blood glucose concentration after which there are several days of persistent hyperglycemia, is recognized clinically in diabetic cats. However, the exact role of the counterregulatory hormones remains to be clarified. Insulin overdose that induces the Somogyi response is one of the most common causes of poor glycemic control in diabetic cats. It can be induced with insulin doses of 1 to 2 U per injection and can result in cats receiving 10 to 15 U of insulin per injection as veterinarians react to the persistence of clinical signs and increased blood glucose and serum fructosamine concentrations. A cyclic history of 1 or 2 days of good glycemic control after which there are several days of poor control should raise suspicion for insulin overdosing and the Somogyi response. Arbitrarily decreasing the insulin dose and evaluating the clinical response over the ensuing 2 to 5 days is perhaps the best way to establish the diagnosis.
Insulin Underdosing
Insulin underdosing is discussed on p. 780. Control of glycemia can be established in most diabetic cats using 1 U or less of insulin/kg of body weight administered twice each day. In general, insulin underdosing should be considered if the insulin dose is less than 1 U/kg/injection and the cat is receiving insulin twice a day. If insulin underdosing is suspected, the dose of insulin should be gradually increased by 0.5 to 1 U/injection per week. The effectiveness of the change in therapy should be evaluated by client perception of clinical response and measurement of serum fructosamine or serial blood glucose concentrations. Other causes for poor glycemic control should be ruled out before an increase in the insulin dose above 1 U/kg/injection is considered.
Short Duration of Insulin Effect
Short duration of insulin effect is discussed on p. 781. Short duration of insulin effect is a common problem in diabetic cats despite twice-daily insulin administration. Short duration of effect is most common with NPH and lente insulin (see Table 52-2). A diagnosis of short duration of insulin effect is made by demonstrating an initial blood glucose concentration greater than 300 mg/dl combined with a glucose nadir above 80 mg/dl that occurs less than 8 hours after insulin administration and recurrence of hyperglycemia (greater than 250 mg/dl) within 10 hours of the insulin injection (see Fig. 52-7). Treatment involves changing to a longer-acting insulin preparation (i.e., PZI or glargine insulin).
Prolonged Duration of Insulin Effect
Prolonged duration of insulin effect is discussed on p. 781. In diabetic cats problems with prolonged duration of insulin effect are most common with twice-daily administration of PZI and glargine insulin.
Inadequate Insulin Absorption
Slow or inadequate absorption of subcutaneously deposited insulin was most commonly observed in diabetic cats receiving ultralente insulin, a long-acting basal insulin that had a slow onset and prolonged duration of effect. In affected cats the blood glucose concentration would decrease minimally, if at all, despite insulin doses of 8 to 12 U/cat. Ultralente insulin is no longer commercially available. A similar problem has not been reported for PZI or glargine insulin. Impaired and erratic absorption of insulin may occur as a result of thickening of the skin and inflammation of the subcutaneous tissues caused by chronic injection of insulin in the same area of the body. Rotation of the injection site helps prevent this problem.
Circulating Insulin-Binding Antibodies
Insulin-binding antibodies are discussed on p. 782. Feline and beef insulin are similar, and feline, human, and porcine insulin differ. Fortunately, insulin antibody formation is not common in diabetic cats treated with exogenous human insulin, despite differences between human and feline insulin. Studies identified an approximately equal frequency of positive serum insulin antibody titers in diabetic cats treated with beef insulin and recombinant human insulin. In my experience, antiinsulin antibody titers are weakly positive in most cats that develop insulin antibodies, prevalence of persistent titers is low, and presence of serum insulin antibodies do not appear to affect control of glycemia. Insulin resistance caused by insulin antibody formation appears to be uncommon. Switching from recombinant human or porcine source insulin to beef-/pork-source PZI may improve control of glycemia if insulin antibodies are the suspected cause for insulin ineffectiveness.
Concurrent Disorders Causing Insulin Resistance
Concurrent disorders causing insulin resistance is discussed on p. 783. The most common concurrent disorders interfering with insulin effectiveness in cats include severe obesity, chronic inflammation such as chronic pancreatitis and gingivitis, renal insufficiency, hyperthyroidism, acromegaly, and hyperadrenocorticism (see Box 52-7). Obtaining a complete history and performing a thorough physical examination are the most important steps in identifying these concurrent disorders. If the history and physical examination are unremarkable, a CBC, serum biochemical analysis, serum thyroxine concentration, urinalysis with bacterial culture, and (if available) abdominal ultrasound should be obtained to further screen for concurrent illness. Additional tests will depend on the results of the initial screening tests (see Box 52-8).
CHRONIC COMPLICATIONS OF DIABETES MELLITUS
Chronic complications of diabetes mellitus are discussed on p. 783. The most common complications in the diabetic cat are hypoglycemia; chronic pancreatitis; weight loss; poor grooming behavior causing a dry, lusterless, and unkempt haircoat; and peripheral neuropathy of the hind limbs, causing weakness, inability to jump, a plantigrade stance, and ataxia (see Box 52-5). Diabetic cats are also at risk for ketoacidosis.
Diabetic Neuropathy
Diabetic neuropathy is one of the most common chronic complications of diabetes in cats, with a prevalence of approximately 10%. Clinical signs of a co-existent neuropathy in the diabetic cat include weakness, impaired ability to jump, knuckling, a plantigrade posture with the cat’s hocks touching the ground when it walks (see Fig. 52-14), muscle atrophy, depressed limb reflexes, and deficits in postural reaction testing. Clinical signs may progress to include the thoracic limbs (palmigrade posture; see Fig 52-14). Abnormalities on electrophysiologic testing are consistent with demyelination at all levels of the motor and sensory peripheral nerves and include decreased motor and sensory nerve conduction velocities in pelvic and thoracic limbs and decreased muscle action potential amplitudes. Electromyographic abnormalities are usually absent and, when identified, are consistent with denervation. The most striking abnormality detected on histologic examination of nerve biopsies from affected cats is Schwann cell injury; axonal degeneration is identified in severely affected cats. The cause of diabetic neuropathy is not known. Currently, there is no specific therapy. Aggressive glucoregulation with insulin may improve nerve conduction and reverse the posterior weakness and plantigrade posture (see Fig. 52-14). However, the response to therapy is variable, and the risks of hypoglycemia increase with aggressive insulin treatment. Generally, the longer the neuropathy has been present and the more severe the neuropathy, the less likely it is that improving glycemic control will reverse the clinical signs of neuropathy.
Prognosis
Diabetic cats and dogs have a similar prognosis (see p. 785). The mean survival time in diabetic cats is approximately 3 years from time of diagnosis. However, this survival time is skewed because cats are usually 8 to 12 years old at the time of diagnosis, and a high mortality rate exists during the first 6 months because of concurrent life-threatening or uncontrollable disease (e.g., ketoacidosis, pancreatitis, renal failure). Diabetic cats that survive the first 6 months can easily live longer than 5 years with the disease.
DIABETIC KETOACIDOSIS
Etiology
The etiopathogenesis of DKA is complex and usually affected by concurrent clinical disorders. Virtually all dogs and cats with DKA have a relative or absolute deficiency of insulin. DKA develops in some diabetic dogs and cats even though they receive daily injections of insulin, and their circulating insulin concentrations may even be increased. The “relative” insulin deficiency in these animals is created by concurrent insulin resistance, which in turn is created by concurrent disorders such as pancreatitis, infection, or renal insufficiency. Increased circulating concentrations of diabetogenic hormones, most notably glucagon, accentuate insulin deficiency by promoting insulin resistance; stimulate lipolysis, leading to ketogenesis; and stimulate hepatic gluconeogenesis, which worsens hyperglycemia.
Insulin deficiency and insulin resistance, together with increased circulating concentrations of diabetogenic hormones, play a critical role in the stimulation of ketogenesis. For the synthesis of ketone bodies (i.e., acetoacetic acid, β-hydroxybutyric acid, acetone) to be enhanced, there must be two major alterations in intermediary metabolism: (1) enhanced mobilization of free fatty acids (FFAs) from triglycerides stored in adipose tissue and (2) a shift in hepatic metabolism from fat synthesis to fat oxidation and ketogenesis. Insulin is a powerful inhibitor of lipolysis and FFA oxidation. A relative or absolute deficiency of insulin allows lipolysis to increase, thus increasing the availability of FFAs to the liver and in turn promoting ketogenesis. As ketones continue to accumulate in the blood, the body’s buffering system becomes overwhelmed and metabolic acidosis develops. As ketones accumulate in the extracellular space, the amount eventually surpasses the renal tubular threshold for complete resorption and they spill into the urine, contributing to the osmotic diuresis caused by glycosuria and enhancing the excretion of solutes (e.g., sodium, potassium, magnesium). Insulin deficiency per se also contributes to the excessive renal losses of water and electrolytes. The result is an excessive loss of electrolytes and water, leading to volume contraction, an underperfusion of tissues, and the development of prerenal azotemia. The rise in the blood glucose concentration raises plasma osmolality, and the resulting osmotic diuresis further aggravates the rise in plasma osmolality by causing water losses in excess of salt loss. The increase in plasma osmolality causes water to shift out of cells, leading to cellular dehydration. The metabolic consequences of DKA, which include severe acidosis, hyperosmolality, obligatory osmotic diuresis, dehydration, and electrolyte derangements, eventually become life threatening.
Clinical Features
DKA is a serious complication of diabetes mellitus that occurs most commonly in dogs and cats with diabetes that has gone undiagnosed. Less commonly, DKA develops in an insulin-treated diabetic dog or cat that is receiving an inadequate dose of insulin, often occurring in conjunction with an infectious, inflammatory, or insulin-resistant hormonal disorder. Because of the close association between DKA and newly diagnosed diabetes mellitus, the signalment of DKA in dogs and cats is similar to that of nonketotic diabetics.
The history and physical examination findings are variable, in part because of the progressive nature of the disorder and the variable time between the onset of DKA and client recognition of a problem. Polyuria, polydipsia, polyphagia, and weight loss develop initially but are either unnoticed or considered insignificant by the client. Systemic signs of illness (e.g., lethargy, anorexia, vomiting) ensue as ketonemia and metabolic acidosis develop and worsen, with the severity of these signs directly related to the severity of the metabolic acidosis and the nature of concurrent disorders that are often present. The time interval from the onset of the initial clinical signs of diabetes to the development of systemic signs of DKA is unpredictable and ranges from a few days to longer than 6 months. Once ketoacidosis begins to develop, however, severe illness usually becomes evident within 7 days.
Common physical examination findings include dehydration, lethargy, weakness, tachypnea, vomiting, and sometimes a strong odor of acetone on the breath. Slow, deep breathing may be observed in animals with severe metabolic acidosis. Gastrointestinal tract signs such as vomiting and abdominal pain are common in animals with DKA, in part because of the common concurrent occurrence of pancreatitis. Other intraabdominal disorders should also be con sidered and diagnostic tests (e.g., abdominal ultrasound) performed to help identify the cause of the gastrointestinal signs.
Diagnosis
The diagnosis of diabetes mellitus is based on appropriate clinical signs, persistent fasting hyperglycemia, and glycosuria. Documenting ketonuria with reagent test strips that measure acetoacetic acid (KetoDiastix; Ames Division, Miles Laboratories) establishes the diagnosis of diabetic ketosis (DK), and documenting metabolic acidosis establishes the diagnosis of DKA. If ketonuria is not present but DKA is suspected, serum or urine can be tested for acetone using Acetest tablets (Ames Division, Miles Laboratories), serum can be tested for the presence of β-hydroxybutyrate using a benchtop chemistry analyzer, and plasma from heparinized hematocrit tubes can be used to test for the presence of acetoacetic acid using urine reagent strips used to document ketonuria. β-hydroxybutyrate and acetone are derived from acetoacetic acid, and commonly used urine reagent strips do not detect β-hydroxybutyrate and acetone. However, it is extremely uncommon for DKA to develop without an excess of acetoacetic acid.
Treatment of “Healthy” Dogs or Cats with Diabetic Ketosis or Diabetic Ketoacidosis
If systemic signs of illness are absent or mild, serious abnormalities are not readily identifiable on physical examination, and metabolic acidosis is mild (i.e., total venous CO2 or arterial bicarbonate concentration greater than 16 mEq/L), short-acting regular crystalline insulin can be administered subcutaneously three times daily until the ketonuria resolves. Fluid therapy and intensive care are usually not needed. The insulin dose should be adjusted on the basis of blood glucose concentrations. To minimize hypoglycemia, the dog or cat should be fed one third of its daily caloric intake at the time of each insulin injection. The blood glucose and urine ketone concentrations, as well as the animal’s clinical status, should be monitored. A decrease in the blood glucose concentration implies a decrease in ketone production. This, in combination with metabolism of ketones and loss of ketones in urine, will usually correct ketosis within 48 to 96 hours of initiating insulin therapy. Prolonged ketonuria is suggestive of a significant concurrent illness or inadequate blood insulin concentrations to suppress lipolysis and ketogenesis. Once the ketosis has resolved and the dog or cat is stable, eating, and drinking, insulin therapy may be initiated using longer-acting insulin preparations (see pp. 765 and 788).
Treatment of Sick Dogs or Cats with Diabetic Ketoacidosis
Aggressive therapy is called for if the dog or cat has systemic signs of illness (e.g., lethargy, anorexia, vomiting); physical examination reveals dehydration, depression, weakness, or a combination of these; or metabolic acidosis is severe (i.e., total venous CO2 or arterial bicarbonate concentration less than 12 mEq/L). The five goals of treatment of a severely ill ketoacidotic, diabetic pet are (1) to provide adequate amounts of insulin to suppress lipolysis, ketogenesis, and hepatic gluconeogenesis; (2) to restore water and electrolyte losses; (3) to correct acidosis; (4) to identify the factors precipitating the present illness; and (5) to provide a carbohydrate substrate (i.e., dextrose) when necessary to allow continued administration of insulin without causing hypoglycemia (Box 52-9). Proper therapy does not mean forcing a return to a normal state as rapidly as possible. Because osmotic and biochemical problems can arise as a result of overly aggressive therapy as well as from the disease itself, rapid changes in various vital parameters can be as harmful as, or more harmful than, no change. If all abnormal parameters can be slowly returned toward normal over a period of 24 to 48 hours, therapy is more likely to be successful.
 BOX 52-9 Initial Management of Dogs or Cats with Severe Diabetic Ketoacidosis
BOX 52-9 Initial Management of Dogs or Cats with Severe Diabetic Ketoacidosis
Fluid Therapy
Rate: 60 to 100 ml/kg q24h initially; adjust based on hydration status, urine output, persistence of fluid losses
Potassium supplement: based on serum K+ concentration (Table 55-1); if unknown, initially add KCl to provide 40 mEq of KCl per liter of fluids
Phosphate supplement: not indicated until serum phosphorus is less than 1.5 mg/dl, then 0.01 to 0.03 mmol phosphate/kg/hr in calcium-free intravenous fluids
Dextrose supplement: not indicated until blood glucose concentration is less than 250 mg/dl, then begin 5% dextrose infusion
Bicarbonate Therapy
Indication: administer if plasma bicarbonate concentration is less than 12 mEq/L or total venous CO2 concentration is less than12 mmol/L; if not known, do not administer unless animal is severely ill and then only once
Amount: mEq HCO3- = body weight (kg) × 0.4 × (12 - animal’s HCO3-) × 0.5; if animal’s HCO3- or total CO2 concentration is unknown, use 10 in place of (12 - animal’s HCO3-)
Administration: add to intravenous fluids and give over 6 hours; do not give as bolus infusion
Retreatment: only if plasma bicarbonate concentration remains less than 12 mEq/L after 6 hours of therapy
Administration Technique
Intermittent intramuscular technique: initial dose, 0.2 U/kg intramuscularly; then 0.1 U/kg intramuscularly hourly until blood glucose concentration is less than 250 mg/dl; then switch to regular insulin administered subcutaneously q6-8h.
Low-dose intravenous infusion technique: to prepare infusion, add 2.2 U/kg (dogs) or 1.1 U/kg (cats) of regular insulin to 250 ml of 0.9% saline; run 50 ml through the drip set and discard; then administer via infusion or syringe pump through a line separate from that used for fluid therapy at an initial rate of 10 ml/hour; adjust infusion rate according to hourly blood glucose measurements; switch to subcutaneous regular insulin q6-8h once blood glucose is less than 250 mg/dl or continue insulin infusion at a decreased rate to prevent hypoglycemia until the insulin preparation is exchanged for a longer-acting product.
Goal: gradual decline in blood glucose concentration, preferably around 75 mg/dl/hour until concentration is less than 250 mg/dl
Ancillary Therapy
Concurrent pancreatitis is common in diabetic ketoacidosis; nothing by mouth and aggressive fluid therapy usually indicated
Concurrent infections are common in diabetic ketoacidosis; use of broad-spectrum, parenteral antibiotics usually indicated
Additional therapy may be needed, depending on nature of concurrent disorders
Patient Monitoring
Blood glucose measurement q1-2h initially; adjust insulin therapy and begin dextrose infusion when decreases below 250 mg/dl
Hydration status, respiration, pulse q2-4h; adjust fluids accordingly
Serum electrolyte and total venous CO2 concentrations q6-12h; adjust fluid and bicarbonate therapy accordingly
Urine output, glycosuria, ketonuria q2-4h; adjust fluid therapy accordingly
Body weight, packed cell volume, temperature, and blood pressure daily
Critically important information for formulating the initial treatment protocol include hematocrit and total plasma protein concentration; serum glucose, albumin, creatinine, and urea nitrogen concentrations; serum electrolytes; venous total CO2 or arterial acid-base evaluation; and urine specific gravity. Abnormalities frequently associated with DKA are listed in Box 52-10. Once treatment for DKA is initiated, additional studies, such as a CBC, serum biochemistry panel, urinalysis, thoracic radiographs, and abdominal ultrasound, or diagnostic tests for pancreatitis, diestrus in the female dog, hyperthyroidism, and hyperadrenocorticism are usually warranted to identify underlying concurrent disorders (see Box 52-8).
FLUID THERAPY
Initiation of appropriate fluid therapy should be the first step in the treatment of DKA. Replacement of fluid deficiencies and maintenance of normal fluid balance are important to ensure adequate cardiac output, blood pressure, and blood flow to all tissues. Improvement of renal blood flow is especially critical. In addition to the general beneficial aspects of fluid therapy in any dehydrated animal, fluid therapy can correct the deficiency in total body sodium and potassium, dampen the potassium-lowering effect of insulin treatment, and lower the blood glucose concentration in diabetics, even in the absence of insulin administration. Unfortunately, fluid therapy alone does not suppress ketogenesis. For this reason, insulin is always required.
The type of parenteral fluid initially used will depend on the animal’s electrolyte status, blood glucose concentration, and osmolality. Most dogs and cats with DKA have severe deficits in total body sodium, regardless of the measured serum concentration. Unless serum electrolyte concentrations dictate otherwise, the initial IV fluid of choice is 0.9% sodium chloride with appropriate potassium supplementation (see Table 55-1 and Table 55-2). Most dogs and cats with severe DKA usually are sodium depleted and therefore not suffering from dramatic hyperosmolality. Additional replacement crystalloid solutions that could be used if physiologic (0.9%) saline was not available include Ringer’s solution, Ringer’s lactated solution, Plasma-Lyte 148® (Baxter Healthcare Corporation), and Normosol-R (Abbott Laboratories). Hypotonic fluids (e.g., 0.45% saline) are rarely indicated in dogs and cats with DKA, even when severe hyperosmolality is present. Hypotonic fluids do not provide adequate amounts of sodium to correct the sodium deficiency, restore normal fluid balance, or stabilize blood pressure. Rapid administration of hypotonic fluids can also cause a rapid decrease in the osmolality of extracellular fluid (ECF), which may result in cerebral edema, deterioration in mentation, and eventually coma. Hyperosmolality is best treated with isotonic fluids and the judicious administration of insulin. Fluid administration should be directed at gradually replacing hydration deficits over 24 hours while also supplying maintenance fluid needs and matching ongoing losses. Rapid replacement of fluids is rarely indicated unless the dog or cat is in shock. Once the animal is out of this critical phase, fluid replacement should be decreased in an effort to correct the fluid imbalance in a slow but steady manner. As a general rule of thumb, a fluid rate of 1.5 to 2 times maintenance (i.e., 60 to 100 ml/kg q24h) is typically chosen initially, with subsequent adjustments based on frequent assessment of hydration status, urine output, severity of azotemia, and persistence of vomiting and diarrhea.
Potassium Supplementation
Most dogs and cats with DKA initially have either normal or decreased serum potassium concentrations. During therapy for DKA the serum potassium concentration decreases because of rehydration (dilution), insulin-mediated cellular uptake of potassium (with glucose), continued urinary losses, and correction of acidemia (translocation of potassium into the intracellular fluid compartment; Fig. 52-18). Severe hypokalemia is the most common complication that devel ops during the initial 24 to 36 hours of treatment of DKA. Dogs and cats with hypokalemia require aggressive potassium replacement therapy to replace deficits and to prevent worsening, life-threatening hypokalemia after initiation of insulin therapy. The exception to potassium supplementation of fluids is hyperkalemia associated with oliguric renal failure. Potassium supplementation should initially be withheld in these dogs and cats until glomerular filtration is restored, urine production increases, and hyperkalemia is resolving.
FIG 52-18 Redistribution of extracellular fluid (ECF) and intracellular fluid (ICF) hydrogen, potassium, and phosphate ions in response to a decrease in ECF pH (i.e., acidosis), an increase in ECF glucose and osmolality, and the translocation of water from the ICF to the ECF compartment and subsequent correction of acidosis and the intracellular shift of glucose and electrolytes with insulin treatment. A, Normal ECF pH. B, ECF H+ concentration increases during acidosis, causing H+ to move into cells and down its concentration gradient. Increase in ECF glucose and osmolality causes extracellular shift of water, K+, and PO4+2. C, ECF H+ concentration decreases during correction of acidosis, causing H+ to move out of cells. Insulin administration and correction of acidemia cause an intracellular shift of glucose, K+ and PO4+2, decreasing ECF K+ and PO4+2 concentration.
(Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
Ideally, the amount of potassium required should be based on actual measurement of the serum potassium concentration. If an accurate measurement of serum potassium is not available, 40 mEq of potassium should initially be added to each liter of intravenous fluids. Normal saline solution does not contain potassium, and Ringer’s solution contains 4 mEq of potassium per liter; thus these fluids should be supplemented with 40 mEq and 36 mEq of potassium, respectively. Subsequent adjustments in potassium supplementation should be based on measurement of serum potassium, preferably every 6 to 8 hours until the dog or cat is stable and serum electrolytes are in the normal range.
Phosphate Supplementation
Most dogs and cats with DKA have either normal or decreased serum phosphorus concentrations on pretreatment testing. Within 24 hours of initiating treatment for DKA, serum phosphorus concentration can decline to severe levels (i.e., <1 mg/dl) as a result of the dilutional effects of fluid therapy, the intracellular shift of phosphorus following the initiation of insulin therapy, and continuing renal and gastrointestinal loss (see Fig. 52-18). Hypophosphatemia affects primarily the hematologic and neuromuscular systems in dogs and cats. Hemolytic anemia is the most common problem and can be life threatening if not recognized and treated. Weakness, ataxia, and seizures may also be observed. Severe hypophosphatemia may be clinically silent in many animals.
Phosphate therapy is indicated if clinical signs or hemolysis are identified or if the serum phosphorus concentration decreases to less than 1.5 mg/dl. Phosphate is supplemented by IV infusion. Potassium and sodium phosphate solutions contain 3 mmol of phosphate and either 4.4 mEq of potassium or 4 mEq of sodium per milliliter. The recommended dosage for phosphate supplementation is 0.01 to 0.03 mmol of phosphate per kilogram of body weight per hour, preferably administered in calcium-free IV fluids (e.g., 0.9% sodium chloride). In dogs and cats with severe hypophosphatemia it may be necessary to increase the dosage to 0.03 to 0.12 mmol/kg/hour. Because the dose of phosphate necessary to replete an animal and the animal’s response to therapy cannot be predicted, it is important to initially monitor the serum phosphorus concentration every 8 to 12 hours and adjust the phosphate infusion accordingly. Adverse effects from overzealous phosphate administration include iatrogenic hypocalcemia and its associated neuromuscular signs, hypernatremia, hypotension, and metastatic calcification. Serum total or (preferably) ionized calcium concentration should be measured at the same time as serum phosphorus concentration and the rate of phosphate infusion decreased if hypocalcemia is identified. Phosphorus supplementation is not indicated in dogs and cats with hypercalcemia, hyperphosphatemia, oliguria, or suspected tissue necrosis. If renal function is in question, phosphorus supplementation should not be done until the status of renal function and serum phosphorus concentration are known.
Magnesium Supplementation
Plasma total and ionized magnesium concentrations may be within or below the reference range at the time DKA is diagnosed in the dog or cat, often decrease during the initial treatment of DKA, and typically normalize without treatment as the DKA resolves. Clinical signs of hypomagnesemia do not usually occur until the serum total and ionized magnesium concentration is less than 1.0 and 0.5 mg/dl, respectively, and even at these low levels many dogs and cats remain asymptomatic. I do not routinely treat hypomagnesemia in dogs or cats with DKA unless problems with persistent lethargy, anorexia, weakness, or refractory hypokalemia or hypocalcemia are encountered after 24 to 48 hours of fluid and insulin therapy and another cause for the problem cannot be identified (see p. 780).
Bicarbonate Therapy
The clinical presentation of the dog or cat, in conjunction with the plasma bicarbonate or total venous CO2 concentration, should be used to determine the need for bicarbonate therapy. Bicarbonate supplementation is not recommended when plasma bicarbonate (or total venous CO2) is 12 mEq/L or greater, especially if the animal is alert. An alert dog or cat probably has a normal or near-normal pH in the cerebrospinal fluid (CSF). The acidosis in these animals is corrected through insulin and fluid therapy. Improvement in renal perfusion enhances urinary loss of ketoacids, and insulin therapy markedly diminishes the production of ketoacids. Acetoacetate and β-hydroxybutyrate are also metabolically usable anions, and 1 mEq of bicarbonate is generated from each 1 mEq of ketoacid metabolized.
When the plasma bicarbonate concentration is 11 mEq/L or less (total venous CO2 is below 12), bicarbonate therapy should be initiated. Many of these animals have severe depression that may be a result of concurrent severe central nervous system acidosis. Metabolic acidosis should be corrected slowly, thereby avoiding major alterations in the pH of the CSF. Only a portion of the bicarbonate deficit is given initially over a 6-hour period. The bicarbonate deficit (i.e., the milliequivalents of bicarbonate initially needed to correct acidosis to the critical level of 12 mEq/L over a period of 6 hours) is calculated by the following formula:
If the serum bicarbonate concentration is not known, the following formula should be used:
The difference between the animal’s serum bicarbonate concentration and the critical value of 12 mEq/L represents the treatable base deficit in DKA. If the animal’s serum bicarbonate concentration is not known, the number 10 should be used for the treatable base deficit. The factor 0.4 corrects for the ECF space in which bicarbonate is distributed (40% of body weight). The factor 0.5 provides one half of the required dose of bicarbonate in the IV infusion. This technique allows a conservative dose to be given over a 6-hour period. Bicarbonate should never be given by bolus infusion. After 6 hours of therapy the acid-base status should be reevaluated and a new dose calculated. Once the plasma bicarbonate level is greater than 12 mEq/L, further bicarbonate supplementation is not indicated.
INSULIN THERAPY
Insulin therapy is critical for the resolution of ketoacidosis. However, overzealous insulin treatment can cause severe hypokalemia, hypophosphatemia, and hypoglycemia during the first 24 hours of treatment—problems that can be minimized by appropriate fluid therapy, frequent monitoring of serum electrolytes and blood glucose concentrations, and modification of the initial insulin treatment protocol as indicated. Initiating appropriate fluid therapy should always be the first step in the treatment of DKA. Delaying insulin therapy for a minimum of 1 to 2 hours is recommended to allow the benefits of fluid therapy to begin to be realized before the glucose, potassium, and phosphorus-lowering effects of insulin therapy commence. Additional delays and decisions on the initial dosage of insulin administered are based on serum electrolyte results. If the serum potassium concentration is within the normal range after 2 hours of fluid therapy, insulin treatment should commence as described in the subsequent paragraphs. If hypokalemia persists, insulin therapy can be delayed an additional 1 to 2 hours to allow fluid therapy to replenish potassium, the initial insulin dose can be reduced to dampen the intracellular shift of potassium and phosphorus, or both can be done. However, insulin therapy should be started within 4 hours of initiating fluid therapy.
The amount of insulin needed by an individual animal is difficult to predict. Therefore an insulin preparation with a rapid onset of action and a brief duration of effect is ideal for making rapid adjustments in the dose and frequency of administration to meet the needs of that particular dog or cat. Rapid-acting regular crystalline insulin meets these criteria and is recommended for the treatment of DKA.
Insulin protocols for the treatment of DKA include the hourly intramuscular technique, the continuous low-dose IV infusion technique, and the intermittent intramuscular then subcutaneous technique. All three routes (IV, intramuscular, subcutaneous) of insulin administration are effective in decreasing blood glucose and ketone concentrations. Successful management of DKA is not dependent on the route of insulin administration. Rather, it is dependent on proper treatment of each disorder associated with DKA.
Intermittent Intramuscular Regimen
Dogs and cats with severe DKA should receive an initial regular crystalline insulin loading dose of 0.2 U/kg followed by 0.1 U/kg every hour thereafter. The insulin dose can be reduced by 25% to 50% for the first 2 to 3 injections if hypokalemia is a concern. The insulin should be administered into the muscles of the rear legs to ensure that the injections are penetrating muscle rather than fat or subcutaneous tissue. Diluting regular insulin 1 : 10 with sterile saline and using 0.3 ml U100 insulin syringes are helpful when small doses of insulin are required. The blood glucose concentration should be measured every hour using a point-of-care chemistry analyzer or portable blood glucose monitoring device and the insulin dosage adjusted accordingly. The goal of initial insulin therapy is to slowly lower the blood glucose concentration to the range of 200 to 250 mg/dl, preferably over a 6- to 10-hour period. An hourly decline of 50 mg/dl in the blood glucose concentration is ideal. This provides a steady moderate decline, with no major shifts in osmolality. A declining blood glucose concentration also ensures that lipolysis and the supply of FFAs for ketone production have been effectively turned off. Glucose concentrations, however, decrease much more rapidly than do ketone levels. In general, hyperglycemia is corrected within 12 hours, but ketosis may take 48 to 72 hours to resolve.
Once the initial hourly insulin therapy brings the blood glucose concentration near 250 mg/dl, hourly administration of regular insulin should be discontinued and regular insulin given every 4 to 6 hours intramuscularly or, if hydration status is good, every 6 to 8 hours subcutaneously. The initial dose is usually 0.1 to 0.3 U/kg, with subsequent adjustments based on blood glucose concentrations. In addition, at this point the IV infusion solution should have enough 50% dextrose added to create a 5% dextrose solution (100 ml of 50% dextrose added to each liter of fluids). The blood glucose concentration should be maintained between 150 and 300 mg/dl until the animal is stable and eating. Usually, a 5% dextrose solution is adequate in maintaining the desired blood glucose concentration. If the blood glucose concentration dips below 150 mg/dl or rises above 300 mg/dl, the insulin dose can be lowered or raised accordingly. Dextrose helps minimize problems with hypoglycemia and allows insulin to be administered on schedule. Delaying the administration of insulin delays correction of the ketoacidotic state.
Constant Low-Dose Insulin Infusion Technique
Constant IV infusion of regular crystalline insulin is also effective in decreasing blood glucose concentrations. To prepare the infusion, regular crystalline insulin (2.2 U/kg for dogs; 1.1 U/kg for cats) is added to 250 ml of 0.9% saline and initially administered at a rate of 10 ml/hour in a line separate from that used for fluid therapy. This provides an insulin infusion of 0.05 (cat) and 0.1 (dog) U/kg/hour, an infusion rate that has been shown to produce plasma insulin concentrations between 100 and 200 μU/ml in dogs. Because insulin adheres to glass and plastic surfaces, approximately 50 ml of the insulin-containing fluid should be run through the drip set before it is administered to the animal. The rate of insulin infusion can be reduced for the initial 2 to 3 hours if hypokalemia is a concern. Two separate catheters are recommended for treatment: a peripheral catheter for insulin administration and a central catheter for fluid administration and blood sampling. An infusion or syringe pump should be used to ensure a constant rate of insulin infusion.
Adjustments in the infusion rate are based on hourly measurements of blood glucose concentration; an hourly decline of 50 mg/dl in the blood glucose concentration is ideal. Once the blood glucose concentration approaches 250 mg/dl, the insulin infusion can be discontinued and regular insulin given every 4 to 6 hours intramuscularly or every 6 to 8 hours subcutaneously, as discussed for the hourly intramuscular protocol. Alternatively, the insulin infusion can be continued (at a decreased rate to prevent hypoglycemia) until the insulin preparation is exchanged for a longer-acting product. Dextrose should be added to the IV fluids once the blood glucose concentration approaches 250 mg/dl, as discussed in the section on hourly intramuscular insulin technique.
Intermittent Intramuscular/ Subcutaneous Technique
The intermittent intramuscular followed by intermittent subcutaneous insulin technique is less labor intensive than the other techniques for insulin administration, but the decrease in blood glucose can be rapid and the risk of hypoglycemia is greater. The initial regular crystalline insulin dose is 0.25 U/kg, administered intramuscularly. Subsequent intramuscular injections are repeated every 4 hours. Usually, insulin is administered intramuscularly only once or twice. Once the animal is rehydrated, the insulin is administered subcutaneously rather than intramuscularly every 6 to 8 hours. Subcutaneous administration is not recommended initially because of problems with insulin absorption from subcutaneous sites of deposition in a dehydrated dog or cat. The dosage of intramuscular or subcutaneous insulin is adjusted according to blood glucose concentrations, which initially should be measured hourly beginning with the first intramuscular injection. An hourly decline of 50 mg/dl in the blood glucose concentration is ideal. Subsequent insulin dosages should be decreased by 25% to 50% if this goal is exceeded. Dextrose should be added to the IV fluids once the blood glucose concentration approaches 250 mg/dl, as discussed in the section on hourly intramuscular insulin technique.
Initiating Longer-Acting Insulin
Longer-acting insulin (e.g., NPH, lente, PZI) should not be administered until the dog or cat is stable; eating; maintaining fluid balance without any IV infusions; and no longer acidotic, azotemic, or electrolyte-deficient. The initial dose of the longer-acting insulin is similar to the regular insulin dose being used just before switching to the longer-acting insulin. Subsequent adjustments in the longer-acting insulin dose should be based on clinical response and measurement of blood glucose concentrations, as described on p. 775.
CONCURRENT ILLNESS
Therapy for DKA frequently involves the management of concurrent, often serious illness. Common concurrent illnesses in dogs and cats with DKA include bacterial infection; pancreatitis; congestive heart failure; renal failure; cholangiohepatitis; and insulin-antagonistic disorders, most notably hyperadrenocorticism, hyperthyroidism, and diestrus. It may be necessary in such animals to modify the therapy for DKA (e.g., fluid therapy in animals with concurrent heart failure) or implement additional therapy (e.g., antibiotics), depending on the nature of the concurrent illness. Insulin therapy, however, should never be delayed or discontinued. Resolution of ketoacidosis can be achieved only through insulin therapy. If nothing is to be given per os, insulin therapy should be continued and the blood glucose concentration maintained with IV dextrose infusions. If a concurrent insulin-antagonistic disease is present, it may be necessary to eliminate the disease while the animal is still ill to improve insulin effectiveness and resolve the ketoacidosis.
COMPLICATIONS OF THERAPY FOR DIABETIC KETOACIDOSIS
Complications caused by therapy for DKA are common and include hypoglycemia, central nervous system signs secondary to cerebral edema, severe hypokalemia, severe hypernatremia and hyperchloremia, and hemolytic anemia resulting from hypophosphatemia. Complications usually result from overly aggressive treatment, inadequate monitoring of the animal’s condition, and failure to reevaluate biochemical parameters in a timely manner. DKA is a complex disorder that is associated with a high mortality rate if improperly managed. To minimize the risk of therapeutic complications and improve the chances of a successful response to therapy, all abnormal parameters should be slowly returned toward normal over a period of 24 to 48 hours, the physical and mental status of the animal must be evaluated frequently (at least three to four times daily), and biochemical parameters (e.g., blood glucose, serum electrolyte, blood gas values) must be evaluated in a timely fashion. During the initial 24 hours the blood glucose concentration should be measured every 1 to 2 hours and serum electrolyte and blood gas values measured every 6 to 8 hours, with modifications in fluid, insulin, and bicarbonate therapy made accordingly.
Prognosis
DKA remains one of the most difficult metabolic therapeutic challenges in veterinary medicine. Despite all precautions and diligent therapy, a fatal outcome is sometimes inevitable. Approximately 30% of cats and dogs with severe DKA die or are euthanized during the initial hospitalization. Death is usually the result of a severe underlying illness (e.g., oliguric renal failure, necrotizing pancreatitis), severe metabolic acidosis (i.e., arterial blood pH less than 7), or complications that develop during therapy (e.g., cerebral edema, hypokalemia). Nevertheless, if logical therapy is implemented and animals are monitored carefully, a positive outcome is attainable.
INSULIN-SECRETING BETA-CELL NEOPLASIA
Etiology
Functional tumors arising from the β cells of the pancreatic islets are malignant tumors that secrete insulin independent of the typically suppressive effects of hypoglycemia. β cell tumors, however, are not completely autonomous and respond to provocative stimuli such as an increase in blood glucose by secreting insulin, often in excessive amounts. Immunohistochemical analysis of β cell tumors has revealed a high incidence of multihormonal production, including pancreatic polypeptide, somatostatin, glucagon, serotonin, and gastrin. However, insulin has been identified as the most common product demonstrated within the neoplastic cells, and clinical signs are primarily those that result from insulin-induced hypoglycemia.
Insulin-secreting β cell tumors are uncommon in dogs and rare in cats. Virtually all β cell tumors in dogs are malignant, and most dogs have microscopic or grossly visible metastatic lesions at the time of surgery. The most common metastatic sites are the regional lymphatics and lymph nodes, liver, and peripancreatic mesentery. Pulmonary metastasis is uncommon and occurs late in the disease. In most dogs hypoglycemia recurs weeks to months after surgical excision of the tumor. The high prevalence of metastatic lesions at the time afflicted dogs are initially examined results, in part, from the typically protracted time it takes for clinical signs to develop and the interval between the time a client initially observes signs and seeks assistance from a veterinarian. Most dogs are symptomatic for 1 to 6 months before being brought to a veterinarian.
SIGNALMENT OF TREATMENT
β cell tumors typically occur in middle-aged or older dogs. The median age at the time of diagnosis of a β cell tumor in 97 dogs in our series was 10 years with an age range of 3 to 14 years. No sex-related predilection is seen. β cell tumors are most commonly diagnosed in large breeds of dogs such as the German Shepherd Dog, Labrador Retriever, and Golden Retriever. β cell tumors have been reported in Siamese and mixed-breed cats older than 10 years of age.
CLINICAL SIGNS
Clinical signs are caused by hypoglycemia and an increase in circulating catecholamine concentrations and include seizures, weakness, collapse, ataxia, muscle fasciculations, and bizarre behavior (Box 52-11). The severity of clinical signs depends on the duration and severity of hypoglycemia. Dogs with chronic hypoglycemia or with recurring episodes appear to tolerate low blood glucose concentrations (20 to 30 mg/dl) for prolonged periods without clinical signs, and only small additional changes in the blood glucose concentration are then required to produce symptomatic episodes. Fasting, excitement, exercise, and eating may trigger the development of clinical signs. Because of the compensatory counterregulatory mechanisms that are designed to increase the blood glucose concentration when hypoglycemia develops, clinical signs tend to be episodic and are generally observed for only a few seconds to minutes. If these counterregulatory mechanisms are inadequate, seizures occur as the blood glucose concentration continues to decrease. Seizures are often self-limiting, lasting from 30 seconds to 5 minutes, and may stimulate further catecholamine secretion and the activation of other counterregulatory mechanisms that increase the blood glucose concentration above critical levels.
PHYSICAL EXAMINATION
Physical examination findings in animals with β cell tumors are surprisingly unremarkable; dogs are usually free of visible or palpable abnormalities. Weakness and lethargy are the most common findings and are identified in approximately 40% and 20% of our cases, respectively. Collapsing episodes and seizures may occur during the examination but are uncommon. Weight gain is evident in some dogs and is probably a result of the potent anabolic effects of insulin.
Peripheral Neuropathy
Peripheral neuropathies have been observed in dogs with β cell tumors and may cause paraparesis to tetraparesis; facial paresis to paralysis; hyporeflexia to areflexia; hypotonia; and muscle atrophy of the appendicular, masticatory, and/or facial muscles. Sensory nerves may also be affected. Onset of clinical signs may be acute (i.e., days) or insidious (i.e., weeks to months). The pathogenesis of the polyneuropathy is not known. Proposed theories include metabolic derangements of the nerves induced by chronic and severe hypoglycemia or some other tumor-induced metabolic deficiency, an immune-mediated paraneoplastic syndrome resulting from shared antigens between tumor and nerves, or toxic factors produced by the tumor that deleteriously affect the nerves. Treatment is aimed at surgical removal of the β cell tumor. Prednisone therapy (initially 1 mg/kg q24h) may also improve clinical signs.
CLINICAL PATHOLOGY
Results of the CBC and urinalysis are usually normal. The only consistent abnormality identified in serum biochemistry profiles is hypoglycemia. The median initial blood glucose concentration in 97 of our dogs with a β cell tumor was 38 mg/dl, with a range of 15 to 78 mg/dl. Ninety percent of the dogs had a random blood glucose concentration less than 60 mg/dl. Dogs with β cell tumors occasionally have a blood glucose concentration of 60 to 80 mg/dl. Such a finding does not rule out hypoglycemia as a cause of episodic weakness or seizure activity. Fasting with hourly evaluations of the blood glucose concentration should be carried out in dogs with suspected hypoglycemia. The time required to induce hypoglycemia with fasting in dogs with a β cell tumor depends in part on the extent of disease at the time the dog is examined and ranges from a few hours to longer than 24 hours. The remainder of the serum biochemistry profile is usually normal. Hypoalbuminemia, hypophosphatemia, hypokalemia, and increased alkaline phosphatase and alanine aminotransferase activities may occur, but these findings are considered nonspecific and not helpful in arriving at a definite diagnosis. A correlation between increased liver enzyme activities and metastasis of β cell tumors to the liver has not been established.
Diagnosis
The diagnosis of a β cell tumor requires initial confirmation of hypoglycemia, followed by documentation of inappropriate insulin secretion and identification of a pancreatic mass using ultrasonography or laparotomy. Considering the potential differential diagnoses for hypoglycemia (see Box 52-2), a tentative diagnosis of a β cell tumor can often be made on the basis of the history, physical examination findings, and an absence of abnormalities other than hypoglycemia shown by routine blood tests. Abdominal ultrasonography can be used to identify a mass in the region of the pancreas and to look for evidence of potential metastatic disease in the liver and surrounding structures (Fig. 52-19). Because of the small size of most β cell tumors, abdominal ultrasonographic findings are often interpreted as normal, although a pancreatic mass or metastatic lesion can be found at surgery. A normal abdominal ultrasonographic finding does not rule out the diagnosis of a β cell tumor. Although computed tomographic imaging was better than ultrasonography or somatostatin receptor scintigraphy at identifying primary tumors, false-positive identification of metastatic sites was unacceptably high in one study (Robben et al., 2005). Thoracic radiographs are of minimal value in documenting metastatic disease, primarily because identifiable metastatic nodules in the lung occur late in the disease.
FIG 52-19 Ultrasonogram of the pancreas showing an islet β-cell tumor (arrow) (A) and an enlarged hepatic lymph node (arrows) (B) resulting from metastasis of the β-cell tumor to the liver in a 9-year-old Cocker Spaniel.
The diagnosis of a β cell tumor is established by evaluating the serum insulin concentration at a time when hypoglycemia is present. Hypoglycemia suppresses insulin secretion in normal animals, with the degree of suppression directly related to its severity. Hypoglycemia fails to have this same suppressive effect on insulin secretion if the insulin is synthesized and secreted from autonomous neoplastic cells because tumor cells that produce and secrete insulin are less responsive to hypoglycemia than are normal β cells. Invariably, the dog with a β cell tumor will have an inappropriate excess of insulin relative to that needed for a particular blood glucose concentration. Confidence in identifying an inappropriate excess of insulin depends on the severity of the hypoglycemia; the lower the blood glucose concentration, the more confident the clinician can be in identifying inappropriate hyperinsulinemia, especially when the serum insulin concentration falls in the normal range. If the blood glucose concentration is low and the insulin concentration is in the upper half of the normal range or increased, the animal has a relative or absolute excess of insulin that can best be explained by the presence of an insulin-secreting β cell tumor.
Most dogs with β cell neoplasia are persistently hypoglycemic. If the blood glucose concentration is less than 60 mg/dl (preferably less than 50 mg/dl), serum should be submitted to a commercial veterinary endocrine laboratory for determination of glucose and insulin concentrations. If the blood glucose concentration is greater than 60 mg/dl, fasting may be necessary to induce hypoglycemia. Blood glucose concentrations should be evaluated hourly during the fast and blood obtained for glucose and insulin determination when the blood glucose concentration decreases to less than 50 mg/dl. It is important to remember that blood glucose results obtained from portable home blood glucose– monitoring devices are often lower than results obtained using benchtop methodologies. A blood sample for submission to a commercial laboratory for glucose and insulin determinations should not be obtained until the blood glucose measured on these devices is less than 40 mg/dl. Once hypoglycemia has been induced, the dog can be fed several small meals over the next 1 to 3 hours to prevent a marked increase in the blood glucose concentration and a potential postprandial reactive hypoglycemia.
Serum insulin concentrations must be evaluated simultaneously in relation to the blood glucose concentration. The serum insulin and glucose concentrations in the healthy fasted dog are usually between 5 and 20 μU/ml and 70 and 110 mg/dl, respectively. Fnding a serum insulin concentration greater than 20 μU/ml in a dog with a corresponding blood glucose concentration less than 60 mg/dl (preferably less than 50 mg/dl) in combination with appropriate clinical signs and clinicopathologic findings strongly supports the diagnosis of a β cell tumor. A β cell tumor is also possible if the serum insulin concentration is in the high-normal range (10 to 20 μU/ml). Insulin values in the low-normal range (5 to 10 μU/ml) may be found in animals with other causes of hypoglycemia as well as a β cell tumor. Carefully reviewing the history, physical examination findings, and diagnostic tests results and, if necessary, repeating serum glucose and insulin measurements when hypoglycemia is more severe will usually identify the cause of the hypoglycemia. Any serum insulin concentration that is below the normal range (typically less than 5 μU/ml) is consistent with insulinopenia and does not indicate the presence of a β cell tumor. Similar guidelines are used for cats with a suspected β cell tumor.
OVERVIEW OF TREATMENT
Treatment options for a β cell tumor include surgical exploration, medical treatment for chronic hypoglycemia, or both. Surgery offers a chance to cure dogs with a resectable solitary mass. In dogs with nonresectable tumors or with obvious metastatic lesions, removal of as much abnormal tissue as possible frequently results in remission, or at least alleviation, of clinical signs and an improved response to medical therapy. Survival time is longer in dogs undergoing surgical exploration and tumor debulking followed by medical therapy, compared with dogs that receive only medical treatment. Despite these benefits, surgery remains a relatively aggressive mode of treatment, in part because of the high prevalence of metastatic disease, the older age of many dogs at the time β cell neoplasia is diagnosed, and the potential for postoperative pancreatitis. As a general rule, I am less inclined to recommend surgery in aged dogs (i.e., 12 years and older), dogs with metastatic disease identified by ultrasonography, and dogs with significant concurrent disease. (See Suggested Readings for detailed information on surgical techniques.)
PERIOPERATIVE MANAGEMENT OF DOGS UNDERGOING SURGERY
Until surgery is performed, the dog or cat with a β cell tumor must be protected from episodes of severe hypoglycemia. This can usually be accomplished through the frequent feeding of small meals and administration of glucocorticoids (Box 52-12). The IV administration of a balanced electrolyte solution containing 2.5% to 5% dextrose is important during the perioperative period. The goal of the dextrose infusion is to prevent clinical signs of hypoglycemia and maintain the blood glucose concentration at greater than 35 mg/dl, not to reestablish a normal blood glucose concentration.
If the dextrose infusion is ineffective in preventing severe hypoglycemia, a constant rate infusion of glucagon should be considered. Glucagon is a potent stimulant of hepatic gluconeogenesis and is effective in maintaining normal blood glucose concentrations in dogs with β cell neoplasia when administered by constant-rate infusion. Lyophilized glucagon USP (1 mg) is reconstituted with the diluent provided by the manufacturer (Eli Lilly), and the solution is added to 1 L of 0.9% saline, making a 1 μg/ml solution that can be administered by syringe pump. The initial dose is 5 to 10 ng/kg of body weight/minute. The dose is adjusted, as needed, to maintain the blood glucose concentration within the normal range. When discontinuing glucagon, the dose should be gradually decreased over 1 to 2 days.
POSTOPERATIVE COMPLICATIONS
The most common postoperative complications are pancreatitis, hyperglycemia, and hypoglycemia. The development of these complications is directly related to the expertise of the surgeon, the location of the tumor in the pancreas (i.e., peripheral lobe versus central region; Fig. 52-20), the presence or absence of functional metastatic lesions, and the adequacy of fluid therapy during the perioperative period. Severe pancreatitis occurs most commonly with attempts to remove tumors located in the central region of the pancreas, where the blood supply and pancreatic ducts are located. Tumors located in the central region of the pancreas should be considered inoperable because of the high prevalence of postoperative life-threatening pancreatitis despite appropriate treatment aimed at preventing its development, including aggressive fluid therapy, nothing by mouth for up to 72 hours after surgery, and appropriate dietary therapy during the ensuing week. The reader is referred to Chapter 40 for information on the treatment of pancreatitis.
FIG 52-20 Tumor location in 87 dogs with islet β-cell tumors.
(Adapted from Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
The development of transient diabetes mellitus after surgical removal of a β cell tumor is not an indication of cure. It is believed to result from inadequate insulin secretion by atrophied normal β cells. Removal of all, or most, of the neoplastic cells acutely deprives the animal of insulin. Until the atrophied normal cells regain their secretory abilities, the animal will be hypoinsulinemic and may require exogenous insulin injections to maintain euglycemia. Insulin therapy is initiated postoperatively only if hyperglycemia and glycosuria persist for longer than 2 or 3 days beyond the time that all dextrose-containing IV fluids have been discontinued. Initial insulin therapy should be conservative—that is, 0.25 U of NPH or lente insulin per kilogram of body weight given once daily. Subsequent adjustments in insulin therapy should be made according to clinical response and blood glucose determinations (see p. 774). The need for insulin treatment is usually transient, lasting from a few days to several months. Rarely, a dog will remain diabetic for more than a year. Client evaluation of the pet’s urine glucose level is helpful in identifying when insulin therapy is no longer needed. Failure to identify glucose in the urine in conjunction with the disappearance of polyuria and polydipsia is an indication to discontinue insulin therapy. If hyperglycemia and glycosuria recur, insulin therapy can be reinstituted but at a lower dose.
Dogs that remain hypoglycemic after surgical removal of a β cell tumor have functional metastatic lesions. The dextrose and/or glucagon infusion should be continued postoperatively until pancreatitis has resolved (if present); the dog is stable, eating, and drinking; and medical treatment for chronic hypoglycemia can be initiated (see Box 52-12).
MEDICAL TREATMENT FOR CHRONIC HYPOGLYCEMIA
Medical treatment for chronic hypoglycemia should be initiated if surgery is not performed or when clinical signs of hypoglycemia recur following surgery. The goals of medical treatment are to reduce the frequency and severity of clinical signs of hypoglycemia and prevent an acute hypoglycemic crisis, not to establish euglycemia, per se. Medical treatment is palliative and minimizes hypoglycemia by increasing the absorption of glucose from the intestinal tract (frequent feedings); increasing hepatic gluconeogenesis and glycogenolysis (glucocorticoids); or inhibiting the synthesis, secretion, or peripheral cellular actions of insulin (glucocorticoids, diazoxide, somatostatin; see Box 52-12).
Frequent Feedings
Frequent feedings provide a constant source of calories as a substrate for the excess insulin secreted by β cell tumors. Diets that are high in fat, complex carbohydrates, and fiber will delay gastric emptying and slow intestinal glucose absorption, helping to minimize the postprandial increase in the portal blood glucose concentration and the stimulation of insulin secretion by the tumor. Simple sugars are rapidly absorbed, have a potent stimulatory effect on insulin secretion by neoplastic β cells, and should be avoided. A combination of canned and dry dog food, fed in three to six small meals daily, is recommended. Daily caloric intake should be controlled because hyperinsulinemia promotes obesity. Exercise should be limited to short walks on a leash.
Glucocorticoid Therapy
Glucocorticoid therapy should be initiated when dietary manipulations are no longer effective in preventing clinical signs of hypoglycemia. Glucocorticoids antagonize the effects of insulin at the cellular level, stimulate hepatic glycogenolysis, and indirectly provide the necessary substrates for hepatic gluconeogenesis. Prednisone is most often used at an initial dose of 0.25 mg/kg q12h. Adjustments in the dose are based on clinical response. The dose of prednisone required to control clinical signs increases with time in response to growth of the tumor and its metastatic sites. Eventually, the adverse effects of prednisone, specifically polyuria and poly dipsia, become unacceptable to clients. When this occurs, the dose of prednisone should be reduced but not stopped and additional therapy considered.
Diazoxide Therapy
Diazoxide (Proglycem; Baker Norton Pharmaceuticals) is a benzothiadiazide diuretic that inhibits insulin secretion, stimulates hepatic gluconeogenesis and glycogenolysis, and inhibits tissue use of glucose. The net effect is hyperglycemia. Unfortunately, diazoxide is difficult to procure and is expensive. The initial dose is 5 mg/kg q12h. The dose is adjusted according to clinical response but should not exceed 60 mg/kg/day. The most common adverse reactions to diazoxide are anorexia and vomiting. Administering the drug with a meal or decreasing the dose, at least temporarily, is usually effective in controlling adverse gastrointestinal signs.
Somatostatin Therapy
Octreotide (Sandostatin; Novartis Pharmaceuticals) is an analog of somatostatin that inhibits the synthesis and secretion of insulin by normal and neoplastic β cells. The responsiveness of β cell tumors to the suppressive effects of octreotide depends on the presence of membrane receptors for somatostatin on the tumor cells. Octreotide at a dose of 10 to 40 μg/dog, administered subcutaneously two to three times a day, has alleviated hypoglycemia in approximately 40% to 50% of treated dogs. Adverse reactions have not been seen at these doses. Octreotide is not a viable option for most clients because of cost.
Streptozotocin Therapy
Streptozotocin is a naturally occurring nitrosourea that selectively destroys pancreatic β cells. The treatment protocol for β cell tumors in dogs involves a 0.9% saline diuresis for 7 hours with streptozotocin (500 mg/m2) administered over a 2-hour period beginning 3 hours after initiating the diuresis. Antiemetics are administered immediately after streptozotocin administration to minimize vomiting. Streptozotocin treatment is repeated every 3 weeks. The effectiveness of streptozotocin in improving hypoglycemia, controlling clinical signs, and prolonging survival time has been variable. Adverse reactions of streptozotocin treatment include vomiting, pancreatitis, diabetes mellitus, and renal failure. Renal failure is less likely when the drug is administered during fluid diuresis as described previously. (See Moore et al. [2002] in Suggested Readings for more information on the use of streptozotocin in treating β cell neoplasia in dogs.)
Prognosis
The long-term prognosis for β cell neoplasia is guarded to poor. Survival time is dependent, in part, on the willingness of the client to treat the disease. Tobin et al. (1999) reported a median survival time after diagnosis of only 74 days (range 8 to 508 days) in dogs treated medically, compared with 381 days (range 20 to 1758 days) in dogs that initially underwent surgery at a tertiary care center. The short survival time for dogs treated medically was because many clients opted for euthanasia when seizures recurred or signs of iatrogenic hyperadrenocorticism developed. The extent to which surgery can alter the prognosis depends on the clinical stage of the disease, most notably the extent of metastatic lesions. Approximately 10% to 15% of dogs undergoing surgery for a β cell tumor die or are euthanized at the time of or within 1 month of surgery because of metastatic disease causing postoperative hypoglycemia that is refractory to medical management or because of complications related to pancreatitis. An additional 20% to 25% of dogs die or are euthanized within 6 months of surgery because of recurrence of clinical hypoglycemia that is refractory to medical management. The remaining 60% to 70% live beyond 6 months postoperatively, many beyond 1 year after surgery, before uncontrollable hypoglycemia develops, resulting in death or necessitating euthanasia. Additional surgery to debulk metastatic lesions may improve the animal’s responsiveness to medical therapy and prolong the survival time in some dogs that become nonresponsive to medical treatment after the initial surgery.
GASTRIN-SECRETING NEOPLASIA
Gastrin-secreting tumors (gastrinomas) are functional malignant tumors usually located in the pancreas of dogs and cats. Sites of metastasis include the liver, regional lymph nodes, spleen, and mesentery. Clinical signs result from the consequences of excess gastric hydrochloric acid secretion in response to excess secretion of gastrin by the tumor.
Clinical Features
The most consistent clinical signs are chronic vomiting, weight loss, anorexia, and diarrhea in an older animal (Box 52-13). Gastric and duodenal ulcers and esophagitis are common and may cause hematemesis, hematochezia, melena, and regurgitation. Acidification of intestinal contents may inactivate pancreatic digestive enzymes, precipitate bile salts, interfere with formation of chylomicrons, and damage intestinal mucosal cells. Diarrhea with malabsorption and steatorrhea may develop as a consequence. Findings on physical examination include lethargy, fever, dehydration, abdominal pain, and shock if blood loss is severe or ulcers have perforated. Potential abnormalities identified on a CBC include a regenerative anemia, hypoproteinemia, and neutrophilic leukocytosis. Abnormalities in the serum biochemistry panel include hypoproteinemia, hypoalbuminemia, hypocalcemia, and mild increases in serum alanine aminotransferase and alkaline phosphatase activities. Hyponatremia, hypochloremia, hypokalemia, and metabolic alkalosis may develop in dogs and cats that vomit frequently. Hyperglycemia and hypoglycemia have been noted in a few cases. The urinalysis is usually unremarkable.
Abdominal radiographs are usually normal. If an ulcer has perforated through the serosal surface, radiographic signs consistent with peritonitis may be present. Contrast-enhanced radiographic studies may show gastric or duodenal ulcers; thickening of the gastric rugal folds, pyloric antrum, or intestine; and the rapid intestinal transit of barium. In an animal with concurrent severe esophagitis, secondary megaesophagus or aberrant, nonperistaltic esophageal motility may be identified fluoroscopically. Ultrasonographic evaluation of the abdomen may identify a pancreatic mass or its metastasis. However, gastrinomas vary tremendously in size and may not be detected with ultrasound.
Gastroduodenoscopy may reveal severe esophagitis and ulceration, especially near the cardia. Gastric rugal folds may be thickened. Gastric and duodenal hyperemia, erosions, or ulcerations are often visible. Histologic evaluation of esophageal, gastric, and duodenal biopsy specimens may be normal or may reveal variable degrees of inflammation consisting of infiltrates of lymphocytes, plasma cells and neutrophils, gastric mucosal hypertrophy, fibrosis, and loss of the mucosal barrier.
Diagnosis
Gastrinoma should be included among the differential diagnoses for any dog or cat with melena or hematemesis or in which severe gastric and duodenal ulceration is identified. Unless a pancreatic mass is identified by ultrasonography, most dogs and cats with gastrinoma will inadvertently be diagnosed with severe inflammatory bowel disease, gastroduodenal erosions, and ulcers, and they will be treated with inhibitors of gastric acid secretion, mucosal protectants, antibiotics, and changes in diet. The probability of a gastrinoma increases if ultrasonography reveals a pancreatic mass, the dog or cat does not respond to medical therapy directed at nonspecific inflammation and ulceration of the gastrointestinal tract, or clinical signs and gastrointestinal tract ulceration recur after antiulcer therapy is discontinued. A definitive diagnosis of gastrinoma requires histologic and immunocytochemical evaluation of a pancreatic mass excised at surgery. Finding increased baseline serum gastrin concentrations from blood obtained after an overnight fast increases the suspicion of gastrinoma. Additional differential diagnoses for increased serum gastrin concentration include gastric outflow tract obstruction, renal failure, short-bowel syndrome, chronic gastritis, hepatic disease, and animals receiving antacid therapy (e.g., H2-receptor antagonists, proton pump inhibitors). Baseline serum gastrin concentrations may vary, with occasional values in the reference range in animals with gastrinoma. Provocative testing (e.g., secretin stimulation test, calcium challenge test) may be considered in dogs strongly suspected of having gastrinoma but with normal baseline serum gastrin concentrations. Exploratory laparotomy should also be considered. (See Suggested Readings for more information on provocative testing).
Treatment
Treatment should be directed at surgical excision of the tumor and control of gastric acid hypersecretion. Gastrointestinal tract ulceration can usually be managed by reducing gastric hyperacidity through the administration of H2-receptor antagonists (e.g., ranitidine, famotidine), proton pump inhibitors (e.g., omeprazole), gastrointestinal tract protectants (e.g., sucralfate), or prostaglandin E1 analogs (e.g., misoprostol). (See Chapter 30 for more information on these gastrointestinal tract drugs.) Surgical resection of an ulcer may be required, especially if the ulcer has perforated the bowel. Surgical resection of the tumor is necessary to obtain a cure, although metastasis to the liver, regional lymph nodes, and mesentery is common. Even if metastatic disease is present, tumor debulking may enhance the success of medical therapy.
Prognosis
The long-term prognosis for gastrinoma is guarded to poor. Evidence of metastasis was present in 76% of reported dogs and cats at the time a gastrinoma was diagnosed. Reported survival time in dogs and cats treated surgically, medically, or both ranged from 1 week to 18 months (mean, 4.8 months). However, the short-term prognosis has improved with the advent of drugs that can reduce gastric hyperacidity (e.g., ranitidine, famotidine) and protect and promote healing of the ulcers (e.g., sucralfate, misoprostol).
Feldman EC, Nelson RW. Canine and feline endocrinology and reproduction, ed 3. St Louis: WB Saunders, 2004.
Fossum TW. Small animal surgery, ed 3. St Louis: Mosby, 2007.
Slatter D. Textbook of small animal surgery, ed 3. Philadelphia: WB Saunders, 2003.
Alt N, et al. Day-to-day variability of blood glucose concentration curves generated at home in cats with diabetes mellitus. J Am Vet Med Assoc. 2007;230:1011.
Beam S, et al. A retrospective-cohort study on the development of cataracts in dogs with diabetes mellitus: 200 cases. Vet Ophthalmol. 1999;2:169.
Bennett N, et al. Comparison of a low carbohydrate-low fiber diet and a moderate carbohydrate-high fiber diet in the management of feline diabetes mellitus. J Fel Med Surg. 2006;8:73.
Briggs C, et al. Reliability of history and physical examination findings for assessing control of glycemia in dogs with diabetes mellitus: 53 cases (1995–1998). J Am Vet Med Assoc. 2000;217:48.
Casella M, et al. Home-monitoring of blood glucose in cats with diabetes mellitus: evaluation over a 4-month period. J Fel Med Surg. 2004;7:163.
Cohn LA, et al. Assessment of five portable blood glucose meters, a point-of-care analyzer, and color test strips for measuring blood glucose concentration in dogs. J Am Vet Med Assoc. 2000;216:198.
Davison LJ, et al. Anti-insulin antibodies in dogs with naturally occurring diabetes mellitus. Vet Immunol Immunopath. 2003;91:53.
Feldman EC, et al. Intensive 50-week evaluation of glipizide administration in 50 cats with previously untreated diabetes mellitus. J Am Vet Med Assoc. 1997;210:772.
Frank G, et al. Use of a high-protein diet in the management of feline diabetes mellitus. Vet Therap. 2001;2:238.
Goossens M, et al. Response to insulin treatment and survival in diabetic cats: 104 cases (1985–1995). J Vet Intern Med. 1998;12:1.
Graham PA, et al. Influence of a high fibre diet on glycaemic control and quality of life in dogs with diabetes mellitus. J Small Anim Pract. 2003;43:67.
Guptill L, et al. Is canine diabetes on the increase? In: Recent advances in clinical management of diabetes mellitus. Dayton, Ohio: Iams Co; 1999:24.
Hess RS, et al. Effect of insulin dosage on glycemic response in dogs with diabetes mellitus: 221 cases (1993–1998). J Am Vet Med Assoc. 2000;216:217.
Hess RS, et al. Breed distribution of dogs with diabetes mellitus admitted to a tertiary care facility. J Am Vet Med Assoc. 2000;216:1414.
Monroe WE, et al. Efficacy and safety of a purified porcine insulin zinc suspension for managing diabetes mellitus in dogs. J Vet Intern Med. 2005;19:675.
Nelson RW, et al. Effect of dietary insoluble fiber on control of glycemia in dogs with naturally acquired diabetes mellitus. J Am Vet Med Assoc. 1998;212:380.
Nelson RW, et al. Transient clinical diabetes mellitus in cats: 10 cases (1989–1991). J Vet Intern Med. 1998;13:28.
Nelson RW, et al. Effect of dietary insoluble fiber on control of glycemia in cats with naturally acquired diabetes mellitus. J Am Vet Med Assoc. 2000;216:1082.
Nelson RW, et al. Efficacy of protamine zinc insulin for treatment of diabetes mellitus in cats. J Am Vet Med Assoc. 2001;218:38.
Struble AL, et al. Systemic hypertension and proteinuria in dogs with naturally occurring diabetes mellitus. J Am Vet Med Assoe. 1998;213:822.
Weaver KE, et al. Use of glargine and lente insulins in cats with diabetes mellitus. J Vet Intern Med. 2006;20:234.
Wess G, et al. Assessment of five portable blood glucose meters for use in cats. Am J Vet Res. 2000;61:1587.
Wess G, et al. Capillary blood sampling from the ear of dogs and cats and use of portable meters to measure glucose concentration. J Small Anim Pract. 2000;41:60.
Brady MA, et al. Evaluating the use of plasma hematocrit samples to detect ketones utilizing urine dipstick colorimetric methodology in diabetic dogs and cats. J Vet Emerg Crit Care. 2003;13:1.
Bruskiewicz KA, et al. Diabetic ketosis and ketoacidosis in cats: 42 cases (1980–1995). J Am Vet Med Assoc. 1997;211:188.
Duarte R, et al. Accuracy of serum β-hydroxybutyrate measurements for the diagnosis of diabetic ketoacidosis in 116 dogs. J Vet Intern Med. 2002;16:411.
Fincham SC, et al. Evaluation of plasma-ionized magnesium concentration in 122 dogs with diabetes mellitus: A retrospective study. J Vet Intern Med. 2004;18:612.
Hume DZ, et al. Outcome of dogs with diabetic ketoacidosis: 127 cases (1993-2003). J Vet Intern Med. 2006;20:547.
Norris CR, et al. Serum total and ionized magnesium concentrations and urinary fractional excretion of magnesium in cats with diabetes mellitus and diabetic ketoacidosis. J Am Vet Med Assoc. 1999;215:1455.
Insulin-Secreting Islet Cell Neoplasia
Fischer JR, et al. Glucagon constant-rate infusion: a novel strategy for the management of hyperinsulinemic-hypoglycemic crisis in the dog. J Am Anim Hosp Assoc. 2000;36:27.
Moore AS, et al. A diuresis protocol for administration of streptozotocin to dogs with pancreatic islet cell tumors. J Am Vet Med Assoc. 2002;221:811.
Polton GA, et al. Improved survival in a retrospective cohort of 28 dogs with insulinoma. J Sm Anim Pract. 2007;48:151.
Robben JH, et al. Comparison of ultrasonography, computed tomography, and single-photon emission computed tomography for the detection and localization of canine insulinoma. J Vet Intern Med. 2005;19:15.
Tobin RL, et al. Outcome of surgical versus medical treatment of dogs with beta-cell neoplasia: 39 cases (1990–1997). J Am Vet Med Assoc. 1999;215:226.