CHAPTER 37 Hepatobiliary Diseases in the Cat
GENERAL CONSIDERATIONS
The causes, clinical signs, and prognosis of hepatobiliary tract diseases in cats are very different from those of dogs. Causes of liver disease in cats, both primary and secondary, are outlined in Table 37-1. Cats typically have hepatobiliary disease or acute hepatic lipidosis, but chronic parenchymal disease is uncommon in this species; in addition, feline liver disease rarely progresses to cirrhosis, as is sometimes the case in dogs. The clinical signs of hepatobiliary disease in cats are generally nonspecific and similar to the signs of inflammatory bowel disease (IBD) and pancreatitis; the three conditions may co-exist, further confusing diagnosis. Hepatic lipidosis presents with more classical signs of liver disease, including jaundice and encephalopathy. The important differences between feline and canine hepatobiliary diseases are outlined in Table 37-2 and Fig. 37-1.
 TABLE 37-1 Clinically Relevant Hepatobiliary Diseases in Cats
TABLE 37-1 Clinically Relevant Hepatobiliary Diseases in Cats
| PRIMARY | SECONDARY |
|---|---|
| Common | |
| Idiopathic lipidosis | Secondary lipidosis |
| Neutrophilic cholangitis | Hyperthyroidism |
| Lymphocytic cholangitis | Pancreatitis |
| Diabetes mellitus | |
| Uncommon or Rare | |
| Congenital portosystemic shunt | Secondary neoplasia (less common than primary) |
| Extrahepatic bile duct obstruction | Biliary stasis associated with extrahepatic sepsis |
| Liver flukes (except in hunting cats in endemic areas) | Hepatic abscess |
| Primary neoplasia | |
| Infections (see Box 37-5) | |
| Drug- or toxin-induced hepatopathy | |
| Biliary cysts | |
| Sclerosing cholangitis/biliary sclerosis | |
| Hepatic amyloidosis | |
| Intrahepatic arteriovenous fistula |
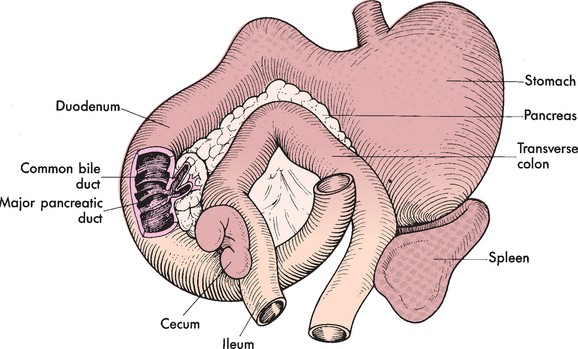
FIG 37-1 Anatomic relationship between pancreas, common bile duct, and duodenum i the cat.
(From Strombeck DR: Small animal gastroenterology, Davis, Calif, 1979, Stonegate Publishing.)
The feline hepatopathies in this chapter are described approximately in order of their frequency in clinical practice in the United States. Historically, hepatic lipidosis has been most common in the United States and cholangitis most common in Europe, but lipidosis is becoming increasingly common in Europe, and cholangitis is now commonly recognized in the United States.
HEPATIC LIPIDOSIS
PRIMARY HEPATIC LIPIDOSIS
Primary or idiopathic hepatic lipidosis usually affects obese cats and remains the most common hepatic disease of cats in North America; it is also now emerging as an increasingly common problem in Europe. It is effectively an acute hepatopathy with a massive accumulation of fat in hepatocytes leading to acute loss of hepatocyte function, which is reversible if the fat can be mobilized (Fig. 37-2). The reason for the differences in prevalence in different countries is unknown but intriguing. Some researchers suggest environmental differences (e.g., differences in outdoor/indoor lifestyle or feeding habits), genetic differences among cats, or both.
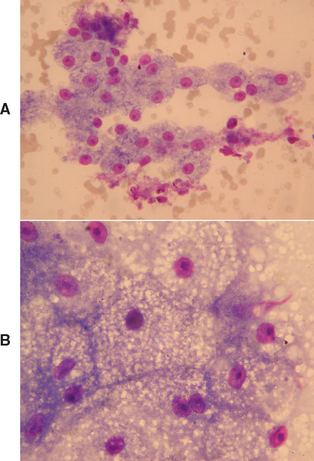
FIG 37-2 Cytology of (A) normal hepatocytes and (B) feline hepatocytes with hepatic lipidosis showing marked swelling of hepatocytes with lipid.
(A and B, Courtesy Elizabeth Villiers; B, From Hall EJ, Simpson JW, Williams, DA, editors: BSAVA manual of canine and feline gastroenterology, ed 2, Gloucestershire, United Kingdom, 2005, British Small Animal Veterinary Association.)
The pathogenesis of primary hepatic lipidosis remains incompletely understood, but it seems to involve a combination of excessive peripheral lipid mobilization to the liver, deficiency of dietary proteins and other nutrients that would usually allow fat metabolism and transport out of the liver, and concurrent primary disturbances in appetite. Excessive mobilization of peripheral fat occurs particularly during periods of anorexia or stress in previously overweight cats. Concurrently, anorexia results in deficiencies of dietary proteins and other nutrients; cats are particularly susceptible to these because of their high dietary requirements (see Table 37-2). Some of these nutrients are important in fat metabolism and mobilization, particularly methionine, carnitine, and taurine, so deficiencies in these nutrients are implicated as contributing to the pathogenesis of the disease. Methionine is an important precursor in the synthesis of an important hepatic antioxidant, glutathione, and hepatic concentrations of glutathione may decrease markedly in cats with hepatic lipidosis. Relative arginine deficiency will contribute to the resultant hepatic encephalopathy caused by decreased urea cycle activity. Concurrent primary appetite disturbances result in persistent and marked anorexia, which is likely due to disturbances in the complex neurohormonal control of appetite. Recent studies have suggested peripheral insulin resistance does not play a significant role in the disease.
SECONDARY HEPATIC LIPIDOSIS
Secondary hepatic lipidosis is also common in cats; its pathogenesis is similar to the primary disease but complicated by the more marked neuroendocrine responses to stress. Secondary lipidosis can therefore be seen in cats that are less obese than those presenting with the primary disease and even in cats with normal or thin body condition. Any anorexic cat with concurrent disease must therefore be regarded as at high risk of hepatic lipidosis, and appropriate feeding support should be instituted as rapidly as possible. Secondary lipidosis may occur in association with any disease causing anorexia in cats but has been most commonly recognized in cats with pancreatitis, diabetes mellitus (DM), other hepatic disorders, IBD, and neoplasia.
Clinical Features
Most affected cats are middle-aged, but they can be of any age or sex. There is no reported breed predilection. Cats with primary lipidosis are commonly obese, are housed indoors, and have experienced a stressful event (e.g., introduction of a new pet into the household, abrupt dietary change) or an illness that causes them to become anorexic and lose weight rapidly. The initiating event is not always known. Secondary lipidosis may affect cats of normal or thin body condition as well as obese animals, and the clinical signs are complicated by the signs of the concurrent disease. For example, the clinical signs of acute diabetic ketoacidosis are similar to those of developing hepatic lipidosis.
Clinical signs are typical of an acute (reversible) loss of hepatocyte function and of hepatocyte swelling with resultant intrahepatic cholestasis. Cats are usually jaundiced, and have intermittent vomiting and dehydration. They may also have diarrhea or constipation. There is usually palpable hepatomegally on physical examination. Hepatic encephalopathy, most often manifested as depression and ptyalism, is related to severe hepatocellular dysfunction and relative arginine deficiency, to which the anorexic cat is predisposed. Previously obese cats have extensive loss of muscle mass but maintain certain fat stores, such as those in the falciform ligament and inguinal region (Fig. 37-3).
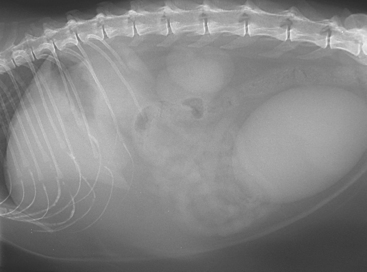
FIG 37-3 Lateral abdominal radiograph of a Domestic Short-haired cat with hepatic lipidosis secondary to prolonged fasting because of a diet change. Note maintenance of large falciform fat pad below the liver in spite of weight loss and loss of subcutaneous fat under the spine.
(Courtesy the diagnostic imaging department at the Queen’s Veterinary School Hospital, University of Cambridge.)
Diagnosis
The only truly definitive and reliable method of diagnosis and of identifying concurrent and causative conditions is histopathology of a wedge biopsy of liver obtained at laparotomy or laparoscopy or (less reliably) a Tru-Cut biopsy taken under ultrasound guidance. However, all of these procedures require a general anesthetic, and the majority of cats with hepatic lipidosis are too ill on presentation to be safely anesthetized. Therefore cytology of a fine needle aspirate (FNA) of the liver obtained either blindly or under ultrasonographic guidance in an awake or sedated cat can give a preliminary diagnosis; this will allow intensive management and tube feeding for a few days to stabilize the patient before anesthesia is considered for a more definitive diagnosis. Because coagulopathies are common in cats with lipidosis, a few days of therapy will help correct them before considering surgery. The clinician must be aware, however, that FNA cytology, although useful for emergency diagnosis and management, can be misleading in cats, and hepatic parenchymal disease can be misdiagnosed as lipidosis using this technique. In addition, concurrent diseases of the liver and other organs (including the pancreas and small intestine) will be overlooked without a laparoscopic or surgical biopsy. It is important to differentiate mild to moderate lipid accumulation in hepatocytes, which is common in sick and anorexic cats and causes no clinical problems, from clinically severe lipidosis on cytology (see Fig. 37-2).
FNAs can be taken under ultrasonographic guidance while the cat is being imaged or be obtained blindly if there is palpable hepatomegaly. The procedure is performed in a similar way to aspiration of a mass: The enlarged liver is palpated, and the abdominal wall overlying it is clipped and prepped. A 22-gauge needle is passed through the skin into the liver from ventrally on the left side, which prevents inadvertent puncturing of the gallbladder, and gentle suction is applied to a 5-ml syringe two or three times, before withdrawing and gently expressing the needle contents onto a slide (see Fig. 36-13). Analgesia is recommended for either procedure because puncture of the liver capsule is painful. Opiate partial agonists, such as buprenorphine, are a good choice; buprenorphine appears to be more effective than butorphanol as an analgesic in cats.
Clinically relevant hepatic lipidosis is usually easily recognizable on routine Giemsa or Diff-Quik staining of cytology samples or routine hematoxylin and eosin-stained histology samples (see Fig. 37-2). It is possible to use special staining procedures with Oil red O applied to snap-frozen biopsy samples to confirm that hepatocellular vacuolation is indeed lipid, but these procedures are not practical in a private practice setting. In addition, glycogen accumulation is uncommon in feline (as opposed to canine) hepatocytes.
Clinicopathologic findings reflect cholestasis and marked hepatocellular dysfunction. Hyperbilirubinemia is present in more than 95% of cases, and the activities of the hepatocellular enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are also markedly elevated in most cats. Alkaline phosphatase activity (ALP) is also markedly elevated in more than 80% of cases; this is particularly relevant in cats, in which this enzyme has a short half-life and no steroid induction (see Table 37-2). In cats with classical primary (idiopathic) lipidosis, a particular hallmark of the disease is an inappropriately low γ-glutamyl transferase (GGT) activity, which is only mildly increased in the face of marked increase in the activity/concentration of the other cholestatic markers (i.e., bilirubin and ALP). This is in contrast to cats with primary biliary tract disease in which both GGT and ALP activities are high. However, in cats with secondary lipidosis associated with an underlying primary hepatopathy or pancreatic disease, GGT activity may be high as well. Therefore finding a high GGT activity does not rule out hepatic lipidosis but should stimulate a search for an underlying cause. Blood urea (BUN) concentration is low in more than half of the cats with lipidosis, reflecting generalized hepatocyte dysfunction. Electrolyte abnormalities are relatively common and can contribute to mortality if not addressed. Up to a third of cats are hypokalemic, and hypophosphatemia has been reported in 17% of the cases; hypomagnesemia has also been reported in cats with lipidosis. Hypokalemia was a poor prognostic indicator in one study (Center et al., 1993). There is no value in measuring serum bile acids as an indication of hepatic function in these cats because they will be high as a result of the concurrent cholestasis. Fasting cholesterol and glucose concentrations may also be high, and sometimes hyperglycemia is so marked as to result in glucosuria. This is usually a stress/metabolic response and typically resolves after appropriate therapy. However, some cats may become diabetic as a result of an underlying disease process, or DM may be the cause of their lipidosis; therefore blood and urine glucose and ketones should be monitored carefully. The appearance of ketonuria in addition to glycosuria in a hyperglycemic cat is highly suggestive of overt DM.
Hemostatic abnormalities are common in cats with lipidosis, occurring in between 20% and 60% of the cases. They are more reliably detected with the PIVKA test (proteins invoked by vitamin K absence; Center et al., 2000), but PIVKA tests are not readily available to many practitioners, and overt prolongation of clotting times are also seen in some cases. Anemia is present in about a quarter of cats, and there is often an increase in Heinz bodies. Because lipidosis is non-inflammatory, a neutrophilia is not characteristic but may occur as a result of another underlying disease.
Radiographs show hepatomegaly in most cases, whereas abdominal effusion is uncommon (Fig. 37-3). Ultrasonography helps differentiate parenchymal from biliary tract disease and also allows assessment of other abdominal organs to look for underlying disease, particularly of the pancreas and intestine. Characteristically, the lipidotic liver appears hyperechoic, although this is not a specific finding and can also be seen in cats with other generalized parenchymal diseases, such as lymphoma or hepatic amyloidosis.
Additional diagnostic tests are performed to determine the presence of concurrent illnesses that could be causing protracted anorexia and secondary hepatic lipidosis. Tests are selected according to clues in the history, physical examination, and clinicopathologic and ultrasonographic evaluations. For example, serum feline specific pancreatic lipase immunoreactivity should be evaluated in cats suspected of having pancreatitis (see Chapter 40).
Treatment and Prognosis
Treatment recommendations for cats with hepatic lipidosis are outlined in Box 37-1. The single most important factor in reducing mortality is early and intensive feeding of a high-protein diet. In most cases, this requires some form of tube feeding. If the cat is very ill at presentation, a nasoesophageal tube can be placed for the first few days while the cat is stabilized (Box 37-2 and Fig. 37-4) and then an esophagostomy or gastrostomy tube may be placed for long-term feeding (see Box 37-2 and Fig. 37-5). Most cats need 4 to 6 weeks of tube feeding, but many cats can be sent home with a gastrostomy tube in place for home feeding once they have stabilized. A high-protein diet, such as those manufactured for feline intensive care patients, is ideal (e.g., Royal Canin feline concentration instant or Hills AD diet or “Fortol” liquid feed [Arnolds]). In some cats, however, a high-protein diet will worsen signs of encephalopathy during the first few days of therapy. Attempts should be made to control this by other methods, such as by feeding smaller amounts more frequently rather than by reducing the protein content of the diet. Concurrent pancreatitis does not alter the dietary recommendations; the current recommendations in cats with pancreatitis is to feed them as soon as possible and not to restrict fat (see Chapter 40).
 BOX 37-1 Outline of Treatment of Hepatic Lipidosis in Cats
BOX 37-1 Outline of Treatment of Hepatic Lipidosis in Cats
 BOX 37-2 Outline of Method of Placement of Feeding Tubes
BOX 37-2 Outline of Method of Placement of Feeding Tubes
Nasoesophageal Tube
For short-term nutritional support (<1 week) while stabilizing cat before placement of esophagostomy or gastrostomy tube.
Placement
Gastrostomy Tube
Indicated for longer-term nutritional support (>1-2 weeks). The tube must be in at least 5 to 7 days for surgical tubes and 14 to 21 days for endoscopically placed tubes to allow adhesions to form between stomach and body wall.
Advantages over nasoesophageal tube of longer-term support: can feed thicker food; better tolerated by animal, which is more likely to start eating with tube in place; easier to manage; could be managed by owner at home. However, it is necessary to use a general anaesthetic for placement.
Placement at laparotomy
Placement endoscopically
This is quicker and less invasive if you are not already doing a laparotomy, but it is necessary to use a fiberoptic endoscope. (However, it is possible to use gastrostomy introducers and do it blind, although there is a higher incidence of traumatic injuries with inexperienced operators, who can easily push the tube through visceral surface of stomach and damage or entrap the spleen. It is best to insufflate stomach first if doing it blind and attempt it only if taught by an experienced operator and practiced on cadavers first.) Several companies make PEG tube kits suitable for veterinary use.
Note on gastrostomy tube removal: Do not remove for at least 5 to 7 days (surgical) or 14 to 21 days (PEG tubes). Method of removal depends on tube placed. Always refer to the manufacturer’s instructions, and do not attempt simply to pull the tube out. Most manufactured tube kits for human use cannot be pulled out but have to be cut close to the body wall and the end retrieved from the stomach endoscopically. (The end can be left to pass through into the feces in medium- to large-breed dogs but not cats, in which it may act as a pyloric foreign body.) The Pezzar mushroom-tipped catheters placed surgically can be removed completely by using a stylet in the tube to flatten out the mushroom.
Experience with a trained operator is highly recommended before attempting surgical placement of a gastrostomy tube or blind placement of a gastrostomy tube.
Fluid and electrolyte abnormalities should also be addressed effectively in the first few days, and antiemetics should be used if necessary. Many cats require vitamin K therapy for coagulopathies [0.5 mg/kg of vitamin K1 (Phytomenadione) subcutaneously or intramuscularly q12h for 3 days]; clinicians should not place any central catheters or invasive feeding tubes until hemostasis is normal. There is the potential for serious and undetected bleeding around a central venous catheter in a cat with a coagulopathy. Antioxidant therapy is also indicated in cats with lipidosis because of the associated glutathione depletion in many cats; vitamin E and S-adenosylmethione supplementation should be considered. (S-adenosylmethionine: 20 mg/kg once a day given whole on an empty stomach, cats and dogs, or 100- to 400-mg total dose daily in cats. The ideal dose of vitamin E in a cat is unclear, but the authors use 100 IU daily.)
Prognosis for recovery in cats with hepatic lipidosis is reasonably good as long as feeding is rapidly and effectively instituted. Studies have reported between 55% and 80% survival in intensively fed cats, whereas mortality is very high without supportive feeding. One large study (Center et al., 1993) suggested that anemia, hypokalemia, and older age were poor prognostic indicators for survival and that cats with secondary hepatic lipidosis may do slightly worse than those with primary disease. However, the differences were not significant, which suggests that it is well worth treating cats with secondary lipidosis as aggressively as those with primary disease.
BILIARY TRACT DISEASE
Biliary tract diseases are the second most common disorders of the feline liver (see Table 37-1). This contrasts with dogs, in which parenchymal diseases are most common. As discussed in the previous section, cats also often have concurrent pancreatitis and/or intestinal disease; it has been proposed that this is a reflection of the anatomy of their pancreatic and bile ducts, which usually join before entering the proximal duodenum through a common outflow tract (see Fig. 37-1). It has been proposed that this increases the likelihood of intestinal contents being refluxed up both the pancreatic and bile ducts during vomiting. However, it is also possible that the disease associations reflect common causative agents or events independent of the anatomy in this species.
The nomenclature of biliary tract disease has recently been standardized by the World Small Animal Veterinary Association (WSAVA); its recommended categorizations of disease will be used here (Rothuizen et al., 2006; Table 37-3). A wide variety of alternative names have been used in the literature, sometimes blurring the categories and confusing comparisons between studies. It is to be hoped that a standardized nomenclature will aid in the search for causes and treatment of these diseases.
All disorders of the biliary tract in cats can present with very similar clinical signs, including lethargy, anorexia, and jaundice. Clinical, clinicopathologic, and diagnostic imaging findings do not allow differentiation of the types of disease; in most cases, cytology, culture of bile, and histopathology of the liver are necessary for accurate diagnosis and most effective treatment.
CHOLANGITIS
Cholangitis refers to inflammation of the biliary tract, which in some (but not all) cats may also extend to the surrounding hepatic parenchyma. It is more common in cats than in dogs, and it is typically divided into three categories, likely associated with different etiologies: neutrophilic cholangitis, lymphocytic cholangitis, and chronic cholangitis associated with liver fluke infestation.
Neutrophilic Cholangitis
Neutrophilic cholangitis is also known as suppurative cholangitis, exudative cholangitis/cholangiohepatitis, and acute cholangitis/cholangiohepatitis.
Pathogenesis and Etiology
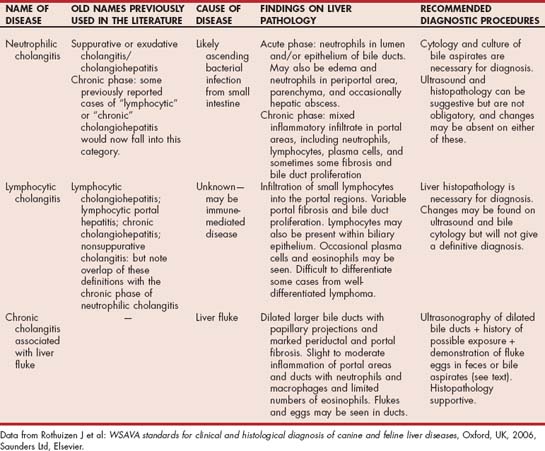
This process is believed to be due to an ascending bacterial infection originating in the small intestine. The most common organism isolated is Escherichia coli, although Streptococcus spp., Clostridium spp., and even occasionally Salmonella spp. may be involved. Concurrent pancreatic and intestinal disease are common (as outlined in the preceding sections). The result is a neutrophilic infiltrate in the lumen of the bile duct and often also invasion of the bile duct walls with neutrophils and edema and neutrophils within the portal areas (Fig. 37-6). Occasionally, an associated hepatic abscess may develop. Cholecystitis (inflammation of the gallbladder) may occur concurrently, or the two conditions may occur separately. A more chronic stage of neutrophilic cholangitis is also recognized; in these cases there is a mixed inflammatory infiltrate in the portal areas consisting of neutrophils, lymphocytes, and plasma cells. These cases are thought to represent more chronic, persistent infection of the biliary tract, but there is some overlap with cats with lymphocytic cholangitis according to some studies.
Clinical Features
Cats of all ages and breeds can be affected, but acute cholangitis is most often seen in young to middle-aged cats. It usually presents acutely (less than a month’s history), although the more chronic form may be present for longer. Cats typically have signs of biliary stasis and sepsis with lethargy, pyrexia, and jaundice.
Diagnosis
Clinicopathologic and imaging findings show overlap with the other diseases of the biliary tract, so a definitive diagnosis of neutrophilic cholangitis cannot be made simply from a characteristic history and clinicopathologic findings. However, cats with this acute disease tend to have higher segmented and band neutrophil counts, ALT activities, and total bilirubin concentrations than cats with lymphocytic cholangitis. They may have a coarse or nodular texture to the liver on ultrasonography and may develop dilated biliary tracts more chronically, but cats with the truly acute disease may have no dilation of the biliary tract on ultrasonography.
An accurate diagnosis of neutrophilic cholangitis caused by acute ascending infection requires cytology and culture of bile. Histopathology of the liver alone is not enough in this particular disease because in many cases the disease is confined to the biliary tract, and changes on liver pathology are mild and nonspecific. Samples of bile for bacterial culture can be taken carefully from the gallbladder during laparotomy or laparoscopy or under ultrasonographic guidance. There is a small but definite risk of bile leakage, particularly if the gallbladder wall is devitalized and/or there is increased pressure within it. In these cases it might be safer to obtain a sample at laparotomy rather than under ultrasonographic guidance. In the latter case a general anesthetic is strongly recommended to prevent patient movement while the needle is in the gallbladder, which greatly increases the risk of bile leakage. The needle should be placed in the gallbladder through the hepatic parenchyma further to reduce the risk of leakage. The cat should be monitored carefully for any leakage of bile after the procedure; any suspicion of leakage and bile peritonitis warrants surgery. Cytology of bile usually shows bacteria and neutrophils, and culture and sensitivity tests should be performed.
Treatment and Prognosis
Cats should be treated for 4 to 6 weeks with an appropriate antibiotic on the basis of the results of culture and sensitivity tests. Amoxycillin is a good initial choice at a dose of 15-20 mg/kg, PO q8h. Ursodeoxycholic acid may be given as an additional choleretic and antiinflammatory agent at a dose of 15 mg/kg, PO q24h, although there are no studies demonstrating their benefit in cats. Septic or extremely sick cats may require hospitalization for intravenous (IV) fluid and IV antibiotic administration during the initial stages of therapy. Careful attention should be paid to feeding anorexic cats to prevent the concurrent development of hepatic lipidosis; a high-protein diet designed for critical care use, as outlined in the lipidosis section, would be much more appropriate in these animals than a protein-restricted liver diet. The prognosis is generally good, and these cats usually recover completely provided they are treated early and appropriately. It is thought that the more chronic form of neutrophilic cholangitis may represent long-term persistence of a low-grade infection in untreated or only partially treated cats.
Lymphocytic Cholangitis
Lymphocytic cholangitis is also known as lymphocytic cholangiohepatitis, lymphocytic portal hepatitis, and nonsuppurative cholangitis.
Pathogenesis and Etiology
Lymphocytic cholangitis is a slowly progressive chronic disease characterized by infiltration of the portal areas of the liver with small lymphocytes. Occasionally, plasma cells and eosinophils may also be seen. There is often associated proliferation of bile ducts, and there may be portal fibrosis. It particularly affects the larger bile ducts, which may become irregularly distended with thickened walls but usually remain patent. In severe cases the main differential diagnosis on histology is lymphoma. The cause is unknown. An immune-mediated etiology has been suggested by some researchers, but the disease does not resolve with immunosuppressive medication. Other studies have suggested possible infectious etiologies, such as Helicobacter spp. or Bartonella spp. (Boomkens et al., 2004; Greiter-Wilke et al., 2006; Kordick et al., 1999), although more evidence is required before infectious organisms are confirmed as a cause. However, the use of immunosuppressive medication in these cases is subject to question.
Clinical Features
Cats with lymphocytic cholangitis are typically young to middle-aged, and Persians appear to be overrepresented. They tend to have a long history (months to years) of waxing and waning low-grade illness. Many become jaundiced, and they often lose weight and have intermittent anorexia and lethargy, but they are less likely to be pyrexic than cats with neutrophilic cholangitis. About a third of cats may also present with a high-protein ascites, reportedly most commonly in the United Kingdom. This makes differentiation from feline infectious peritonitis (FIP) important. Ultimately, the differentiation in these cats can be made only on histopathology.
Diagnosis
Diagnosis in these cases relies ultimately on hepatic histopathology, although ultrasonographic and clincopathologic findings can support a presumptive clinical diagnosis. Increases in liver enzyme activities are mild to moderate and tend to be less marked than in cats with neutrophilic cholangitis. Peripheral blood neutrophilia is less common than in cats with the acute disease but may be present. A particular feature of most cats with lymphocytic cholangitis is an increase in γ-globulin concentration, which again may cause confusion with FIP. Radiographic signs are nonspecific: There may be hepatomegaly (which is often due to enlargement of the larger bile ducts) and in some cases ascites (Fig. 37-7). Ultrasonography is more helpful and reveals dilation of the biliary tract in all cases (see Fig. 36-10). The common bile duct typically appears dilated, and there may be dilation of the gallbladder and “sludge” within it. The main differential diagnosis for these cats is extrahepatic biliary obstruction; the ultrasonographer should attempt to rule this out by carefully imaging the surrounding pancreas, small intestine, and mesentery.
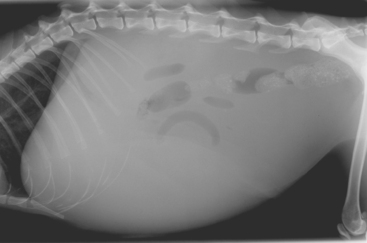
FIG 37-7 A lateral abdominal radiograph from a cat with lymphocytic cholangitis and associated ascites. The major differential diagnosis in this case would be feline infectious peritonitis.
(Courtesy the diagnostic imaging department, The Queen’s Veterinary School Hospital, University of Cambridge.)
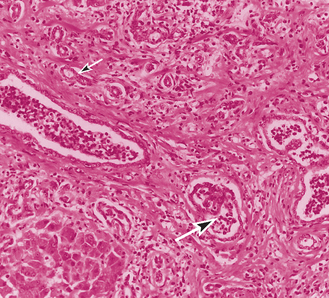
It is very important to evaluate a hemostasis profile before performing a liver biopsy in view of how commonly coagulation times are prolonged in cats with liver disease. Vitamin K should be given before biopsy (0.5 mg/kg of vitamin K1 SQ or IM q12h for 3 days) if there is any concern about clotting function; fresh frozen plasma should be available to manage postbiopsy bleeding if it occurs. Bile aspiration is not necessary unless the disease is more acute and there is a possibility of neutrophilic cholangitis. Histology is important to rule out FIP (see Chapter 97). The typical hepatic lesion in cats with FIP is a multifocal pyogranulomatous reaction with evidence of vasculitis or perivasculitis, which is quite distinct from the periportal lymphocytic infiltrate seen in cats with lymphocytic cholangitis (Fig. 37-8). Serology or PCR for Bartonella spp. might be considered, although the importance of this organism in the naturally occurring disease is unclear.
Treatment and Prognosis
Researchers disagree on the recommended therapy of this disease, which likely reflects our uncertainty about the etiology. A number of authors recommend immunosuppressive doses of corticosteroids. However, although these tend to ameliorate the acute flare-ups of the disease, they do not lead to resolution of signs, and the condition invariably recurs. Antibiotic therapy is wise, at least early in the treatment, until an infectious etiology has been ruled out. There is good logical reason to use ursodeoxycholic acid (15 mg/kg PO q24h) in these cats for its choleretic and antiinflammatory effect as well as its effect on modulating the bile acid pool and reducing toxic bile acids. Use of antioxidants such as S-adenosylmethionine (20 mg/kg or 200 to 400 mg total once a day on an empty stomach) and vitamin E (approximately 100 IU daily) is also logical because bile is a potent oxidizing toxin in the liver. However, none of these therapies has been critically evaluated in cats with lymphocytic cholangitis. Again, it is important to ensure that affected cats eat to prevent the development of concurrent hepatic lipidosis; as discussed in the preceding sections, a highly digestible, high-quality diet without protein restriction is indicated. A diet formulated for feline intestinal disease (such as Iam’s feline intestinal or Royal-Canin feline selected protein or Hills ID) might be the most appropriate because of the relatively high prevalence of concurrent inflammatory bowel disease. Tube feeding should be considered if necessary, as outlined in the section on hepatic lipidosis. Cats with more acute signs, particularly associated with concurrent intestinal and/or pancreatic disease, may require hospitalization and IV fluid therapy.
The prognosis for cure appears to be poor because the disease appears to wax and wane chronically in spite of treatment. However, few cats with lymphocytic cholangitis die as a result of their disease. This is likely because, unlike in dogs, the disease does not generally progress to end-stage cirrhosis.
Sclerosing Cholangitis
Sclerosing cholangitis, or biliary cirrhosis, involves an end-stage fibrotic liver, and is very uncommon in cats. The condition is characterized histologically by diffuse proliferative fibrosis of bile duct walls spreading to involve the hepatic lobules and disrupting their architecture and circulation. It is thought in most cases to represent an end stage of chronic biliary tract disease: usually complete obstruction or chronic severe liver fluke infestation (see the next section). It is very unusual for neutrophilic or lymphocytic cholangitis to progress to sclerosing cholangitis in cats. Affected cats present with typical clinical signs of chronic biliary tract disease, as outlined in the cholangitis and extrahepatic biliary tract obstruction sections. Affected cats may also develop chronic portal hypertension, with the resultant development of ascites, gastrointestinal ulceration, and/or acquired portosystemic shunts (PSS) and hepatic encephalopathy (see Chapter 39). Acquired PSSs are much less common in cats than in dogs, although they are occasionally recognized. Sclerosing cholangitis is diagnosed on hepatic biopsy; again, it is very important to evaluate hemostasis profiles before biopsy and to administer vitamin K (0.5 mg/kg SQ or IM q12h for up to 3 days) as necessary because vitamin K deficiency is common in cats with chronic biliary tract obstruction. It should be noted that cats with sclerosing cholangitis may have hepatomegaly on radiography, which is unexpected (cirrhosis usually results in a small liver in dogs). Presumably, this reflects the biliary tract dilation and florid peribiliary fibrosis in these cases. Treatment is supportive, with treatment of only the clinical signs associated with portal hypertension, as outlined in Chapter 39.
Etiology and Pathogenesis
Liver fluke infestation is regularly observed in cats from areas endemic for the family Opisthorchiidae (Platynosomum spp. and also occasionally Amphimerus pseudofelineus and Metametorchis intermedius). It is estimated that in Florida and Hawaii Platynosomum fastosum (the most common feline liver fluke) has prevalence of up to 70%; the clinical feline disease is referred to as “lizard poisoning.” The flukes require two intermediate hosts: water snails and lizards, amphibians, geckos, or fish, depending on the species. The cat is the final host and is infested by ingesting the metacercariae in the second intermediate host. The immature flukes migrate from the intestine to the liver via the bile ducts and become adult and patent by 8 to 10 weeks. Eggs can then be found in the feces (inconsistent) or bile aspirates (more reliable). The severity of associated disease seems to depend on the parasite load and on individual responses. Many cases are mild. In some cases the pancreas may also be affected. The clinical signs are caused by peribiliary inflammation and fibrosis in the liver, culminating, in severe cases, in effectively a posthepatic jaundice. The fluke takes 8 to 12 weeks from infestation to reach adulthood. In experimental infestations hepatic lesions are visible histologically from 3 weeks postinfestation. There is an initial distention of proximal bile ducts and a neutrophilic and eosinophilic inflammatory response, which progresses chronically to adenomatous hyperplasia of ducts and surrounding florid fibrosis. Eosinophils may be absent in the later stages of disease, and flukes and eggs may not be seen on histology.
Clinical Signs
Commonly, cats with low-grade infestations remain asymptomatic. However, heavy infestations can be associated with severe and often fatal disease (Haney et al., 2006; Xavier et al, 2007). In these cases clinical signs are typically those of posthepatic jaundice combined with inflammatory liver disease (e.g., jaundice, anorexia, depression, weight loss, and lethargy). Diarrhea and vomiting have been features of clinical cases but do not occur in experimental cases; affected cats may also have hepatomegaly and ascites.
Diagnosis
Diagnosis is made after a history of exposure (cats often have a history of hunting lizards) combined with finding the flukes or eggs in feces and bile. Supportive findings are high liver enzyme activities typical of cholestasis; ALT and AST activities and bilirubin concentration are particularly high, but ALP activity is surprisingly often only mildly elevated. Eosinophilia may be seen in severe cases but is inconsistent. Ultrasonography reveals changes typical of biliary tract disease, such as dilation of the bile ducts. In one case fluke infestation also caused acquired polycystic disease of the biliary system (Xavier et al., 2007).
Ova may be found in the feces using the formalin-ether sedimentation method (Box 37-3). However, shedding of eggs is sporadic; also, of course, eggs will not be present if the fluke infestation has resulted in a complete biliary obstruction. The most reliable way of demonstrating flukes and eggs is on bile aspirates.
 BOX 37-3 Formalin-Ether Sedimentation Technique for Detecting Platynosomum concinnum Ova in Feces
BOX 37-3 Formalin-Ether Sedimentation Technique for Detecting Platynosomum concinnum Ova in Feces
From Bielsa LM et al: Liver fl ukes (Platynosomum concinnum) in cats, J Am Anim Hosp Assoc 21:269, 1985.
CHOLECYSTITIS
Cholecystitis refers to inflammation of the gallbladder. Neutrophilic cholecystitis is frequently seen in cats but rarely in dogs. It may occur alone or in combination with neutrophilic cholangitis. Ultrasonographically, the gallbladder wall often appears thickened and sometimes irregular; there may be “sludging” of the bile and/or choleliths. Clinical signs, diagnosis, and treatment are very similar to those of neutrophilic cholangitis (see preceding section). Lymphocytic cholecystitis is also occasionally recognized.
BILIARY CYSTS
Most cystic lesions in the feline liver are of bile duct origin and may be congenital or acquired. Congenital cysts are usually multiple and often present as part of a polycystic disease of several organs, including the kidneys. The cystic contents are clear. Persian cats and Persian crosses are at increased risk. Cysts may be an incidental finding on imaging, particularly if they are small, but large cysts can cause clinical signs as a result of destruction of hepatic tissue and also compression of surrounding bile ducts resulting in signs of biliary tract obstruction (see next section). Treatment is not indicated if they are small and nonprogressive, but if they are large and causing problems, they may be treated surgically by removal or omentalization (Friend et al., 2001).
Acquired hepatic cysts may be single or multiple and may be small or very large. The contents may be clear, bloody, or bilious. They may occur secondary to trauma, inflammation, or neoplasia (including biliary cystadenomas) or in rare cases caused by liver flukes. Therapy depends on the cause, but surgical management may be necessary if they are large.
EXTRAHEPATIC BILE DUCT OBSTRUCTION
Pathogenesis and Etiology
Extrahepatic bile duct obstruction (EBDO) is a syndrome associated with several different underlying causes. Causes of EBDO may be categorized as extraluminal compressive or intraluminal obstructive lesions, but often diseases cause EBDO through a combination of these mechanisms (e.g., cholangitis may result in a combination of extraluminal compression by associated edema and inflammation and intraluminal obstruction by inspissated bile). Therefore it is more practically helpful to divide the causes into common and less common causes (Box 37-4). Several studies have shown inflammation of the small intestine, pancreas, biliary tract, or a combination of these (“triaditis”) to be the most common cause of EBDO in cats; neoplasia of the biliary tract or pancreas are the next most common cause. Choleliths are very uncommon in cats. Those reported in the literature are usually cholesterol or calcium salts or a mixture of these and are associated with cholangitis. They are variably radiodense depending on the amount of calcium in the stone, but they are easily visualized with ultrasonography of the biliary tract. Two out of the three cases of bilirubin choleliths reported in the literature were from Somali cats with pyruvate kinase deficiency, and it was assumed that they were secondary to hemolysis (Harvey et al., 2007). Therefore finding bilirubin choleliths in a cat should stimulate a search for underlying hemolytic disease.
 BOX 37-4 Causes of Extrahepatic Bile Duct Obstruction (EBDO) in Cats
BOX 37-4 Causes of Extrahepatic Bile Duct Obstruction (EBDO) in Cats
Note that sepsis distant to the liver can produce an associated biliary stasis, which may appear clinicopathologically to be very similar to EBDO. Note also that biliary tract rupture (usually traumatic) produces similar clinicopathological findings to EBDO.
Clinical Features
In case series of cats with EBDO, clinical signs, clinicopathologic findings, and survey radiographic findings were indistinguishable from those associated with other severe cholestatic hepatopathies; jaundice, anorexia, depression, vomiting, and hepatomegaly were the main presenting features. If biliary obstruction is complete, feces will be pale or acholic. There may be a cranial abdominal mass on palpation, because of either a very distended gallbladder or underlying neoplasia, but often abdominal palpation is normal (other than the hepatomegaly). Cats with EBDO are at particular risk of malabsorption of fat-soluble vitamins, including vitamin K, because of the lack of intestinal bile salts reducing fat digestion. This is compounded in many cases by the concurrent intestinal and/or pancreatic disease, which further reduces fat absorption. As discussed previously, it is very important in these cases to assess coagulation times before performing biopsies or surgery and to supplement vitamin K parenterally as necessary.
Diagnosis
Ultrasonography is the most useful diagnostic tool to differentiate EBDO from other biliary tract diseases in cats; sometimes, the cause of EBDO is determined. Clinicopatho logic findings are nonspecific; the high concentration/activities of hepatocellular and biliary enzymes, bilirubin, and cholesterol resulting from cholestasis are indistinguishable from those in cats with other severe cholestatic hepatopathies. Ultrasonography will usually reveal dilation of the gallbladder and the extrahepatic and intrahepatic biliary trees, although gallbladder dilation is not a consistent and essential finding. A search should then be conducted for a possible cause of obstruction by carefully examining the small intestine, liver, and pancreas for evidence of inflammation or neoplasia. Biliary tract rupture can present in a similar way and should be ruled out by identifying and analyzing any free abdominal fluid; cats with biliary rupture have a high concentration of bilirubin in the fluid. FNA of bile from the gallbladder under ultrasonographic guidance should be avoided or approached with great care if EBDO is suspected or confirmed because there is a high risk of leakage on account of the increased pressure. In these cats it is preferable to aspirate bile during surgery. It may be necessary to undertake an exploratory laparotomy to assess bile duct patency and the cause of the obstruction. Hemostatic function should be assessed first, and vitamin K therapy given as 0.5 mg/kg of vitamin K1 SQ or IM q12h for 3 days. The liver, pancreas, and small intestine should be carefully inspected and biopsied, as deemed necessary.
Treatment
Treatment depends on the underlying cause of the EBDO and whether the obstruction is complete or partial. Biliary tract surgery in the cat carries a high morbidity and mortality and should be undertaken only when necessary to relieve complete obstruction. The prognosis for partial obstructions is surprisingly good when using medical management, and surgery may not be necessary in all cases. Recent studies of EBDO in acute-on-chronic pancreatitis in humans suggest that medical management rather than surgery or stenting is the treatment of choice in most cases and that there are usually no long-term sequelae. Similar studies have not been reported in cats.
If the feces are not acholic and there is some evidence of bile flow into the duodenum, cats can be managed medically with a choleretic (ursodeoxycholic acid 15 mg/kg PO q24h) and an antioxidant such as S-adenosylmethionine (20 mg/kg or 200 to 400 mg daily on an empty stomach) to protect the hepatocytes against bile-induced oxidant damage. The underlying disorder should also be treated as outlined in the preceding section. However, if the cat does not improve after several days or signs of complete obstruction, such as acholic feces, develop, surgical intervention is indicated. If the cat requires cholecystoenterostomy, the prognosis is poor.
HEPATIC AMYLOIDOSIS
Etiology
Hepatic amyloidosis is an uncommon but apparently emerging cause of liver disease in cats. Historically, amyloidosis has been recognized most commonly as a familial disease in Siamese cats with both renal and hepatic involvement. Abyssinian cats also suffer from familial amyloidosis, but it is predominantly renal in this breed. However, more recently it has been reported sporadically in a number of breeds, including domestic short-haired cats with purely hepatic and no renal involvement (Beatty et al., 2002). The amyloid in both familial and sporadic cases is amyloid A (inflammatory), and in sporadic cases there is usually an underlying chronic inflammatory process in another organ (such as chronic gingivitis) thought to be the driving force for the formation of the inflammatory amyloid.
Clinical Signs and Diagnosis
Affected cats usually present with signs of anemia and hypotension related to rupture of the hepatic capsule and hemoabdomen. These cats are predisposed to hepatic rupture because the liver is enlarged and also rigid and therefore easily damaged with normal trauma such as knocking the abdomen when jumping. Affected cats usually exhibit lethargy, anorexia, pale mucous membranes, a bounding pulse, and a heart murmur secondary to the anemia but rarely any specific signs of liver disease. There may be hepatomegaly on abdominal palpation.
Diagnosis
Diagnosis relies on histopathology of a liver biopsy; although clinicopathologic and ultrasonograhic findings are supportive, it is important to rule out the major differential diagnoses of FIP, hepatic lipidosis, and hepatic lymphoma. The transient anemia resolves as blood is reabsorbed from the abdomen (autotranfusion). There are mild to marked increases in ALT activity and globulin concentration but rarely increases in ALP and GGT activities, which helps differentiate amyloidosis from biliary tract disease and lipidosis. On ultrasonography amyloidosis can resemble both lymphoma and lipidosis, with hepatomegaly and a generalized increase in hepatic parenchymal echogenicity or mixed hypo- and hyperechoic appearance (Beatty et al, 2002), but no dilation of the biliary tract. FNA cytology is not helpful because amyloid does not appear on the aspirate. Therefore hepatic biopsy, after careful evaluation of hemostasis profiles, is the recommended method of diagnosis.
Treatment and Prognosis
Treatment is supportive because there is no specific antiamyloid medication. Colchicine is of uncertain efficacy and is not indicated in cats because of its potential toxicity. Instead, the focus should be on reducing or eliminating the underlying inflammatory disorder driving the amyloid deposition, and supportive care with antioxidants and vitamin K supplementation as necessary (0.5 mg/kg SQ or IM every 7 to 20 days). Blood transfusions may be necessary in cats with acute hemoabdomen. The long-term prognosis is poor, and most cats die as a result of intraabdominal bleeding.
NEOPLASIA
Etiology
Primary liver tumors are uncommon in cats but are nevertheless more common than in dogs. Hepatic tumors are much less common in both species than they are in people, possibly because two of the predisposing factors for development of liver tumors (hepatitis virus infection and α-protease inhibitor deficiency) have not been recognized in small animals. Cirrhosis also predisposes to liver tumors in people but is rare in cats. Liver tumors represent 1% to 2.9% of all neoplasms in cats (Liptak, 2007) but up to 6.9% of the nonhematopoietic tumors. No predisposing factors have been identified. In contrast to dogs, benign tumors are more common than malignant tumors in cats; they may be an incidental finding during workup for other diseases. An unusual benign tumor occasionally found in cats is the myelolipoma, which has a suggested association with chronic hypoxia and hepatic involvement in diaphragmatic hernias. Biliary carcinomas are the most common malignant tumors in cats; this may mirror the high prevalence of biliary tract disease in this species. Trematodes are also a predisposing cause in humans and may be in some cats, but bile duct carcinomas also occur in cats outside the range of liver fluke infestations, so there are obviously other factors involved. Also in contrast to dogs, primary hepatobiliary tumors are more common than metastatic neoplasia in cats. Secondary tumors include particularly hematopoietic tumors, such as lymphoma and, less commonly, leukemias, histiocytic tumors, and mast cell tumors and metastases from other organs such as the pancreas, mammary glands, and gastrointestinal tract. Hemangiosarcomas in the liver may be primary or secondary, and sometimes the origin is difficult to ascertain if multiple organs are involved, although primary hepatic hemangiosarcomas appear to be more common in cats than in dogs.
The common feline primary liver tumors recognized and their behavior are outlined in Table 37-4.
 TABLE 37-4 Primary Liver Tumors in Cats
TABLE 37-4 Primary Liver Tumors in Cats
| TYPE OF TUMOR | BEHAVIOR |
|---|---|
| Recognized but less common than biliary tumors. Adenoma more common than carcinoma. | |
| Very rare but very aggressive | |
Note: Benign tumors are more common than malignant tumors in this species.
MR, Metastatic rate.
Clinical Features
Primary malignant liver tumors are usually seen in older cats (mean age 10 to 12 years). There is no obvious gender predisposition reported. The presenting clinical signs and clinicopathologic findings are indistinguishable from those in cats with other primary liver diseases. There may be lethargy, vomiting, weight loss, ascites, or jaundice. Some affected cats may have palpable hepatomegaly, ascites, or liver masses on abdominal palpation. However, at least 50% of cats with liver tumors are asymptomatic.
Diagnosis
Diagnosis relies on a combination of diagnostic imaging, cytology, and histology. A suspicion may be gained from the clinical findings, but given that more than half of affected animals have no clinical signs, the liver mass may be a serendipitous finding while the cat is being imaged for another reason. On clinical pathology high liver enzyme activity and bile acid concentration and mild anemia and neutrophilia are common but nonspecific findings. Jaundice is uncommon but can occur. Liver function is usually normal because the tumor must involve more than 70% of liver mass before resulting in a reduction in liver function. The exception to this is diffuse hematological malignancy (e.g., lymphoma), which can result in significant disturbance of hepatocyte function (including coagulopathies). The functional defects often resolve when the tumor is cytoreduced by chemotherapy.
Radiographs may show hepatomegaly; the liver may have an irregular border or focal enlargement of one lobe. There may be also involvement of other organs (e.g., lymphadenopathy in cats with lymphoma), and thoracic radiographs may reveal evidence of metastases. However, radiographs may also be normal. Some malignant hepatic tumors commonly metastasize to the peritoneum and local lymph nodes and less commonly to the lungs. As in other diseases of the liver, ultrasonography is more helpful in identifying a hepatic mass and also in evaluating for metastases; it also allows for FNA of the mass(es). Hepatic tumors can also be cystic, particularly cystadenocarcinomas. Cats, unlike dogs, rarely have benign nodular hyperplasia in the liver, so this is not a differential diagnosis for a hepatic mass. Diffuse hepatic tumors (e.g., lymphoma) may show a diffuse change in echogenicity, or the liver may appear normal on ultrasonography. Important differential diagnoses for diffuse hepatic tumors are FIP, lipidosis, and amyloidosis. A thorough abdominal ultrasonographic examination should be undertaken to search for evidence of metastases. It should be kept in mind that because benign tumors are more common than malignant tumors in cats, no animal should be euthanized on the basis of finding a hepatic mass with no evidence of metastases on ultrasonography.
A definitive diagnosis is usually obtained using cytology or histopathology. In some cases FNAs may be diagnostic, but in others they may be difficult to interpret, particularly in cats with benign hepatocellular tumors, in which the cells look indistinguishable from normal hepatocytes. Ultrasonography-guided Tru-Cut biopsies are usually diagnostic; alternatively, biopsies can be obtained during laparoscopy or laparotomy. In the case of an apparently single lesion, the clinician may elect to proceed straight to surgical removal and an “excisional” biopsy. Hemostasis profiles should be evaluated before performing a biopsy. It is unusual for the one-stage prothrombin time and activated partial thromboplastin time to be prolonged in cats with primary liver tumors, but they can be markedly prolonged in cats with diffuse hepatic infiltration with lymphoma or other diffuse secondary tumors (e.g., mast cell tumors). Biopsies should not be considered in these cases until clotting factors have been replenished with a fresh frozen plasma transfusion.
Treatment
Treatment of primary hepatic tumors relies on surgical removal if they are resectable. This is advisable even in cats with benign tumors, including biliary adenomas. Treatment of diffuse, nodular, or metastatic tumors may be difficult. Primary hepatic tumors generally have a poor response to chemotherapy. It has been suggested that this is because hepatocytes, both normal and transformed, have high expression of the multidrug resistance membrane-associated P-glycoprotein and also that hepatocytes are naturally high in detoxifying enzymes. Radiotherapy is not wise because normal liver tissue is very radiosensitive. For additional information, please see Chapters 80 (the section on lymphoma) and 82 (the section on mast cell tumors).
Prognosis
Prognosis of benign tumors is good after resection. Prognosis is very poor for cats with any type of malignant liver tumor; however, most cats with lymphoma of the liver respond to chemotherapy (see Chapter 80).
CONGENITAL PORTOSYSTEMIC SHUNTS
Etiology and Pathogenesis
PSSs are abnormal vascular communications between the portal and systemic circulation. They may be congenital or acquired secondary to portal hypertension. Those of the latter type are usually multiple vessels and are very rare in cats because they usually occur secondary to severe hepatic fibrosis and cirrhosis, both uncommon in cats. Acquired PSS secondary to a congenital hepatic arteriovenous (AV) fistula has been reported in a young cat, but this is very rare (McConnell et al., 2006). Most cases of PSS in cats are therefore congenital, but even these are recognized less commonly than in dogs. Congenital PSSs are usually single or at most double vessels and may be intrahepatic or extrahepatic in location. Cats may have either type of PSS (Lipscomb et al., 2007). Extrahepatic PSSs represent abnormal communcations between the portal vein or one of its contributors (left gastric, splenic, cranial, or caudal mesenteric or gastroduodenal veins) and the caudal vena cava or azygos vein. Intrahepatic PSSs may be left-sided, in which case they are believed to represent a persistence of the fetal ductus venosus after birth (patent ductus venosus, PDV; White and Burton, 2001), or they may be right-sided or centrally located in the liver, in which case they are believed to be anomalous vessels. The reason that congenital PSSs develop at all is unknown, although it is assumed that there may be genetic reasons and/or developmental problems in utero that resulted in abnormal development of the liver vasculature.
The pathophysiology of congenital PSS largely relates to the shunting of unfiltered blood directly into the systemic circulation, resulting in hyperammoniemia and hepatic encephalopathy (HE). The pathophysiology of HE is outlined in Chapter 35. The shunting vessel acts as a lowresistance pathway for some of the portal blood, bypassing the higher resistance intrahepatic portal vasculature. Portal pressure is therefore lower than normal in cats with congenital PSS, which is an important distinguishing feature from (rare) cases of acquired shunting, in which there is portal hypertension and therefore an increased portal pressure. Concurrent hepatic microvascular dysplasia or portal vein hypoplasia, which can confuse this differentiation, occurs in some dogs (see Chapter 38) but has not been reported in cats. Shunting may also allow bacteremia and potentially infections of hematogenous origin that may present as “pyrexia of unknown origin,” although this is rare. Additional effects of portal blood bypassing the liver are hepatic atrophy and a reduction in the metabolic activity of the liver, which contributes to inefficient use of dietary components, poor growth, and loss of lean body mass.
Liver atrophy (microhepatia) and changes in hepatic organelle function are partly due to changes in hepatic per fusion. The portal blood usually provides about 50% of the liver’s oxygen requirement, but this is obviously reduced in cats with PSS. Cats with PSS typically have arteriolar hyperplasia in an attempt to compensate for the reduced portal flow, but they often still have some degree of hepatic underperfusion. In addition, PSS results in reduced delivery of “hepatotrophic factors,” such as insulin, to the liver, which further contributes to hepatic atrophy.
Clinical Features
Persian and Himalayan cats have been reported to be at increased risk for congenital PSS in small case series, and another series noted that purebred cats in general were overrepresented; however, cats of any breed, including mixed-breed cats, can be affected. Both sexes appear to be equally at risk. There is no reported associated between breed and shunt types (unlike in dogs), although in one study 6 out of 13 cats with an intrahepatic PSS were Siamese (Lipscomb et al., 2007). Most cases present before 2 years of age; many are younger than 1 year old, but old cats with congenital PSSs are frequently recognized.
The typical clinical signs in cats with congenital PSS are gastrointestinal, urinary, or neurological (HE), although the latter tend to predominate in cats and, anecdotally, are often more severe than in dogs. Cats typically present with a history of waxing and waning neurological signs consistent with HE rather than a sudden acute HE crisis. The typical signs of HE are outlined in Box 35-1. Hypersalivation is a common sign of HE in cats, but it is rare in dogs. There is sometimes an association between HE and feeding, which may relate to glutamine metabolism by enterocytes releasing ammonia, although not all cats display these signs. Cats in acute crisis may present comatose or with seizures; cats appear to be more susceptible to seizures than dogs, both preoperatively and postoperatively. The reason for this is unknown, although it has been suggested that sudden changes in the concentrations of ammonia and other metabolites in the blood after surgery or sudden changes in medical management may destabilize neurotransmitters in cats. Drug intolerance is common, particularly prolonged recovery from routine anesthesia for spaying/neutering. Animals with PSS may also show intermittent vomiting and/or diarrhea. Urinary tract signs are due to cystitis associated with urate calculi and polyuria/polydipsia, but they are less common in cats than in dogs. Impaired urine-concentrating ability may reflect reduced renal-concentrating gradient secondary to low urea concentration and increased blood cortisol concentration secondary to reduced hepatic breakdown, although this has been demonstrated only in dogs thus far. Cats with congenital PSS also often (but not always) show signs of poor growth compared with their littermates (Fig. 37-9). There has been a reported high prevalence of copper-colored irises in cats with PSS (see Fig. 37-9), but this is not a consistent feature.
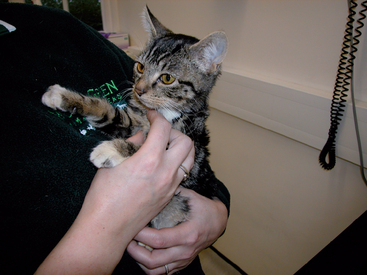
FIG 37-9 A 6-month-old kitten with a congenital portosystemic shunt, demonstrating very small size for its age and also copper-color irises, which are often noted in kittens with portosystemic shunts.
Because of the low portal pressure, ascites is not a feature in cats, which helps in distinguishing congenital PSS from the rare feline cases of acquired PSS, in which ascites is more common because of portal hypertension.
Diagnosis
A suspicion for congenital PSS can be gained from the history of recurrent neurological signs combined with high fasting and/or postprandial bile acid or ammonia concentrations. Care should be taken when performing traditional ammonia tolerance tests, which can precipitate severe HE. Postprandial ammonia or bile acid determinations are safer alternatives. Serum bile acid concentrations should be measured before and 2 hours after feeding. (see Box 36-1). If ammonia is measured instead, the postprandial sample should be taken 6 hours after feeding (Walker et al., 2001). Other typical (but not pathognomonic) clinicopathologic findings in some (but not all) cats include low serum urea concentration, mildly increased liver enzyme activities, and microcytosis. Notable differences from dogs are that decreases in total protein or albumin concentrations, hypoglycemia, and anemia are much less common in cats. Urine specific gravity is low in many dogs but occurs in fewer than 20% of affected cats. If fasting bile acid concentrations are very high, it is not necessary to obtain a postprandial sample, but the diagnostic sensitivity of doing both is higher than just measuring fasting concentrations. If biliary stasis (which also causes high bile acid concentrations) is ruled out and the cat does not have hepatic lipidosis (which causes hepatocellular failure and HE with increases in bile acid and ammonia concentration in many cases), it is likely that the cat has a congenital PSS because other causes of HE and high bile acid concentrations are uncommon in cats. Abdominal radiographs show a small liver in 50% of cases (Lamb et al., 1996). However, for definitive diagnosis the shunting vessel must be visualized.
Visualization of the shunting vessel is achieved by ultrasonography or portal venography (see Fig. 36-7, A and B) Transcolonic portal scintigraphy will also demonstrate portosystemic shunting, but it does not differentiate congenital from acquired shunting. A liver biopsy should be taken at the time of surgery or portovenography (after evaluation of hemostasis profiles) to rule out other or concurrent conditions. This shows histological features very similar to those in dogs and typical of portal venous hypoperfusion with loss of smaller portal veins, increased numbers of arterioles, hepatocellular atrophy with lipogranulomas, and sometimes periportal sinusoidal dilation but minimal inflammation.
Treatment
Treatment involves complete or partial ligation of the shunting vessel using one of several methods, including silk or cellophane or ameroid constrictors; a detailed explanation is beyond the scope of this book. The procedure is best reserved for referral centers, particularly in cats, which are more prone to complications than dogs. The postoperative mortality in cats appears to be higher than in dogs, which is often due to intractable severe neurological signs. Pretreatment with phenobarbital has been attempted, but too few cases have been reported to assess its value. Propofol infusions are often used for HE-associated seizures in dogs, but care must be taken in cats because of their susceptibility to Heinz body anemia when given propofol infusions.
Cats should be managed medically before and for a period of about 2 months after surgery while the portal vasculature and liver mass recover. This involves careful mild dietary protein restriction with additional antibiotics (usually amoxicillin, 15 to 20 mg/kg PO q8h) and sometimes also a soluble fiber source such as lactulose (2.5 to 5 ml, given PO q8h to effect). Some anecdotal data suggest that changes in medical management should be made more gradually in cats than in dogs to prevent the risk of seizures (e.g., change the diet first, then add antibiotics after a week or more, and then add the soluble fiber source later). Details of medical management of HE are described in Chapter 39. Cats do not tolerate marked dietary protein restriction because of their high obligate protein requirement (see Table 37-2). A diet manufactured for cats with liver disease (such as Hills LD) is appropriate, and, unlike in dogs, home-made diets based on dairy protein should be avoided in cats because dairy protein is relatively deficient in arginine, which is essential for the urea cycle; deficiency will further predispose to hyperammonemia. Medical management alone is effective in some dogs long term (see Chapter 38), but anecdotally, cats do not do as well with medical management of congenital PSS, probably because of their high obligate protein metabolism, which would make them more susceptible to hyperammonemia, regardless of the diet fed.
HEPATOBILIARY INFECTIONS
Several infectious organisms can infect the liver, either as a primary target or as part of a more generalized infection. These are listed in Box 37-5. In addition, neutrophilic cholangitis likely has a primary infectious cause in most cats (discussed in more detail in a previous section).
 BOX 37-5 Infectious Diseases with Hepatic Involvement in Cats
BOX 37-5 Infectious Diseases with Hepatic Involvement in Cats
Note also that neutrophilic cholangitis is often due to ascending bacterial infection from the gut. Bartonella spp. may be involved in the etiology of some cases of lymphocytic cholangitis.
Hepatic involvement is common in both the dry and effusive forms of FIP (see Chapter 97). Because cats with effusive FIP can present with the same signs as cats with lymphocytic cholangitis, it is an important differential diagnosis for this disease. A liver biopsy may be necessary to distinguish them; a diagnosis is occasionally made cytologically.
Disseminated toxoplasmosis is uncommon in cats, but when it occurs, the liver is usually involved with intracellular growth of Toxoplasma gondii during the active clinical disease, resulting in cell death. Effects of delayed hypersensitivity reactions and immune-complex vasculitis also contribute to clinical illness. Infection of the lungs, liver, and central nervous system (including the eyes) with trophozoites is most commonly responsible for clinical signs. As expected, high serum ALT activity and hyperbilirubinemia commensurate with the degree of hepatocellular necrosis are the typical serum biochemical findings in cats with liver involvement. Cholangiohepatitis resulting from infection of the biliary epithelium has been noted occasionally in experimental and spontaneously occurring cases of toxoplasmosis in cats. The distribution of affected tissues in disseminated histoplasmosis often includes the lung, eye, bone marrow, spleen, lymph node, skin, bone, and liver. Infection with Bartonella spp. can cause cholangitis in cats.
TOXIC HEPATOPATHY
Pathogenesis and Etiology
By definition, toxic hepatopathy refers to a hepatic injury directly attributable to exposure to environmental toxins or certain therapeutic agents. Any therapeutic agent could potentially be heptatotoxic as a result of an idiosyncratic reaction, but only a limited number of such drugs have been reported in cats (Box 37-6) in addition to reported environmental hepatotoxins. Cats are particularly sensitive to phenol toxicity because of their limited hepatic glucuronide transferase activity. A variety of essential oils used topically have been reported to be hepatotoxic in cats. Essential oils are absorbed rapidly, both orally and dermally, and are metabolized by the liver to glucuronide and glycine conjugates; it is believed that cats are more sensitive than dogs to their hepatotoxic effects (Means, 2002).
 BOX 37-6 Therapeutic Agents or Environmental Toxins that Can Cause Clinically Relevant Hepatic Toxicity in Cats
BOX 37-6 Therapeutic Agents or Environmental Toxins that Can Cause Clinically Relevant Hepatic Toxicity in Cats
Complete information that could support meaningful conclusions about the frequency, character, and substances that consistently cause hepatotoxicity in cats is not available. Clinicians therefore must rely on anecdotal reports, clinical observations, and data accumulated by central agencies such as the National Animal Poison Control Center in Urbana, Illinois (888-426-4435; $55 per case via credit card), and the U.S. Food and Drug Administration’s Center for Veterinary Medicine, in Washington, DC (the toll-free telephone number for reporting suspected adverse drug experiences is 1-888-FDA-VETS). In general, drug- or toxin-induced hepatic injury in cats is extremely uncommon, and most reactions are acute (occuring within 5 days of exposure). The character and severity of the toxic reaction depend on the characteristics of the substance, as well as the dose and the duration of exposure.
Three therapeutic agents have been reported to be hepatotoxic in certain cats: tetracycline (1 cat), diazepam (17 cats), and stanozolol (16 cats). Veterinarians have used these agents for years without known deleterious effects. For each drug, clinical and clinicopathologic signs of hepatotoxicosis developed within 13 days of daily oral administration at recommended dosages. The adverse hepatic reaction to tetracycline was serious but nonlethal, and the cat recovered completely after drug discontinuation and 6 weeks of supportive care (Kaufman et al., 1993). Histologic findings in the liver included centrilobular fibrosis, mild cholangiohepatitis, and mild lipid deposition in hepatocytes. In the cats that experienced diazepam-associated hepatic failure, the outcome was death in 16 of 17 despite intensive treatment. The oral dosages of diazepam that cats received, primarily for inappropriate urination, ranged from 1 mg every 24 hours to 2.5 mg every 12 hours. The histologic lesions in the liver were similar to those observed in the cat with tetracycline-associated hepatic injury but more severe: massive, predominantly centrilobular necrosis; suppurative cholangitis; and mild lipid vacuolation in some cats. Because of the severity of the lesions reported in cats apparently susceptible to diazepam-associated hepatic necrosis, serum liver enzyme activities should be evaluated during the window of days 3 to 5 of administration in cats given diazepam by mouth. Until there is more information that would improve understanding of this lethal and unpredictable hepatic reaction, use of other agents for control of behavior and elimination problems in cats is recommended. Cats that experienced an adverse reaction to stanozolol were healthy or had chronic renal failure (14 of 18 cats) or gingivitis/stomatitis (2 of 3 cats; Harkin et al., 2000). Serum ALT activity was markedly increased in most cats given 1 mg orally every 24 hours for several months or 4 mg orally every 24 hours (and 25 mg intramuscularly once) for 3 weeks; all but one survived after the drug was discontinued and intensive supportive care given. The histologic lesions were moderate to marked, diffuse centrilobular lipidosis and evidence of intrahepatic cholestasis (accumulation of bile and lipofuscin in hepatocytes and Kupffer cells).
The new microsomal triglyceride transfer protein (MTP) inhibitors marketed for weight loss in dogs are known to increase liver enzymes reversibly in that species but could result in clinically significant hepatic lipidosis in cats if used off-label in that species. This has not been reported yet because their use in cats is specifically contraindicated; however, clinicians should be aware of the risk.
The discriminatory eating habits of cats may account for the relatively uncommon occurrence of hepatotoxicity from ingested environmental toxins such as pesticides, household products, and other chemicals. It is certainly possible that many adverse hepatic reactions to drugs or toxic chemicals go unnoticed in cats because the first clinical signs of illness are vomiting and diarrhea, after which the medication is stopped. If the signs resolve, there usually is no further evaluation and the medication is not readministered to prove that the substance caused the reaction.
Diagnosis
Clinical evidence that suggests drug- or toxin-induced hepatic damage includes supportive history (e.g., known exposure); normal liver size to mild generalized tender hepatomegaly; laboratory test results consistent with acute liver injury (e.g., high serum ALT and/or AST activity, hyperbilirubinemia); and, if the exposure was nonlethal, recovery with discontinuation of the agent and specific or supportive care. There are no pathognomonic histologic changes in the liver, although necrosis with minimal inflammation and lipid accumulation are considered classic findings. In many cases all clinical and clinicopathologic markers of a toxic liver insult are present, but the inciting chemical cannot be identified. In the case of hepatotoxicity from therapeutic agents, idiosyncratic reactions can occur that are not dose related, although drug overdose is usually the reason for liver injury.
Treatment
In cats with suspected acute hepatotoxicity, the basic principles for treatment of toxicoses are applied: preventing further exposure and absorption, managing life-threatening cardiopulmonary and renal complications, hastening elimination of the substance, implementing specific therapy if possible, and providing supportive care. Because few hepatotoxins have specific antidotes, the success of recovery often relies on time and aggressive supportive care. More guidance on supportive treatment of acute toxic hepatopathy is provided in Box 38-4.
Acetaminophen is one of the few toxins with a specific antidote. Acetaminophen is particularly toxic to cats, in which the usual hepatic detoxification pathways of sulphation and glucuronidation are particularly limited. Acetaminophen is oxidized to a toxic metabolite that causes methemoglobinuria within hours of ingestion and Heinz body anemia, hemolysis, and liver failure within 2 to 7 days of ingestion. N-acetylcysteine is a specific antidote that binds the toxic metabolite and increases the glucuronidation process. It should be administered at a dose of 140 mg/kg intravenously or orally as a loading dose and then continued at 70 mg/kg q6h for a total of seven treatments or for up to 5 days. There is also evidence that additional S-adenosylmethionine (20 mg/kg or 200 to 400 mg total daily) is beneficial in cats with acetaminophen toxicity because it replenishes glutathione, which inactivates the toxic metabolite (Webb et al., 2003).
HEPATOBILIARY INVOLVEMENT IN CATS WITH SYSTEMIC DISEASE
Several feline systemic illnesses have hepatic manifestations that may be identified by physical, clinicopathologic, or radiographic examination, but the major clinical signs can be attributed to another disease (see Table 37-1). In such cases the hepatic lesion should recede with satisfactory treatment of the primary illness.
Metastatic neoplasia could be the underlying reason for abdominal enlargement resulting from hepatomegaly or, rarely, malignant abdominal effusion, although primary neoplasia is more common than metastatic neoplasia in the feline liver. Some of the signs of hyperthyroidism, especially occasional vomiting, diarrhea, and weight loss, can resemble those of primary hepatobiliary disease. Thyrotoxic cats commonly have high liver enzyme activities; more than 75% of affected cats have high serum AP activity (twofold to twelvefold), although in cats it is not known whether this is of liver or bone origin or, as is true for hyperthyroid human patients, both. More than 50% of hyperthyroid cats have high serum ALT or AST activity (twofold to tenfold). More than 90% of affected cats have high serum activity of at least one of the enzymes AP, ALT, and AST. Approximately 3% are hyperbilirubinemic. Histopathologic changes are minimal, and there appears to be little functional disturbance. It is thought that malnutrition, hepatocellular hypoxia, and the direct effects of thyroid hormone on liver cells are responsible for these liver-related abnormalities. Hepatomegaly associated with mild to moderate lipid deposition is a common physical examination finding in cats with diabetes mellitus; a small number of cats may also be icteric. Mild to moderate increases in liver-specific enzyme activities are typical. More severe clinicopathologic abnormalities might be expected in cats with more severe hepatic lipidosis. Hyperadrenocortism is rare in cats, and, unlike in dogs, obvious liver involvement is unusual. The liver is usually normal in size on radiographs, and it is unusual to identify high serum AP and ALT activities in hyperadrenocorticoid cats. Unlike dogs, cats do not possess a steroid-induced isoenzyme of ALP, and increased ALT, when recognized, is probably related to intercurrent diabetes mellitus.
Aronson LR, et al. Acetaminophen toxicosis in 17 cats. J Vet Emerg Crit Care. 1996;6:65.
Bacon NJ, et al. Extrahepatic biliary tract surgery in the cat: a case series and review. J Small Anim Pract. 2003;44:231.
Beatty JA, et al. Spontaneous hepatic rupture in six cats with systemic amyloidosis. J Small Anim Pract. 2002;43:355.
Brain PH, et al. Feline cholecystitis and acute neutrophilic cholangitis: clinical findings, bacterial isolates and response to treatment in six cases. J Feline Med Surg. 2006;8:91.
Broussard JD, et al. Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983 to 1993. J Am Vet Med Assoc. 1995;206:302.
Brown B, et al. Metabolic and hormonal alterations in cats with hepatic lipidosis. J Vet Intern Med. 2000;14:20.
Buote NJ, et al. Cholecystoenterostomy for treatment of extrahepatic biliary tract obstruction in cats: 22 cases (1994-2003). J Am Vet Med Assoc. 2006;228:1376.
Center SA. Feline hepatic lipidosis. Vet Clin N Am Small Anim Pract. 2005;35:224.
Center SA, et al. A retrospective study of 77 cats with severe hepatic lipidosis: 1975-1990. J Vet Intern Med. 1993;7:349.
Center SA, et al. Fulminant hepatic failure associated with oral administration of diazepam in 11 cats. J Am Vet Med Assoc. 1996;209:618.
Center SA, et al. Proteins invoked by vitamin K absence and clotting times in clinically ill cats. J Vet Intern Med. 2000;14:292.
Cole TL, et al. Diagnostic comparison of needle and wedge biopsy specimens of the liver in dogs and cats. J Am Vet Med Assoc. 2002;220:1483.
Crowe DT, et al. Esophagostomy tubes for feeding and decompression: clinical experience in 29 small animal patients. J Am Anim Hosp Assoc. 1997;33:393.
Friend EJ, et al. Omentalisation of congenital liver cysts in a cat. Vet Rec. 2001;149:275.
Greiter-Wilke A, et al. Association of Helicobacter with cholangiohepatitis in cats. J Vet Intern Med. 2006;20:822.
Haney DR, et al. Severe cholestatic liver disease secondary to liver fluke (Platynosomum concinnum) infection in three cats. J Am Anim Hosp Assoc. 2006;42:234.
Harkin KR, et al. Hepatotoxicity of stanozolol in cats. J Am Vet Med Assoc. 2000;217:681.
Harvey M, et al. Treatment and long-term follow-up of extrahepatic biliary obstruction with bilirubin cholelithiasis in a Somali cat with pyruvate kinase deficiency. J Feline Med Surg. 2007;4:424.
Havig M, et al. Outcome of ameroid constrictor occlusion of single congenital extrahepatic portosystemic shunts in cats: 12 cases (1993-2000). J Am Vet Med Assoc. 2002;220:337.
Hunt GB. Effect of breed on anatomy of portosystemic shunts resulting from congenital diseases in dogs and cats: a review of 242 cases. Aust Vet J. 2004;82:746.
Kaufman AC, et al. Increased alanine transaminase activity associated with tetracycline administration in a cat. J Am Vet Med Assoc. 1993;202:628.
Kordick DL, et al. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J Clin Microbiol. 1999;37:1536.
Lamb CR, et al. Ultrasonographic diagnosis of congenital portosystemic shunt in 14 cats. J Small Anim Pract. 1996;37:205.
Lipscomb VJ, et al. Complications and long-term outcomes of the ligation of congenital portosystemic shunts in 49 cats. Vet Rec. 2007;160:465.
Liptak JM. Hepatobiliary tumors. In Withrow SJ, Vail DM, editors: Withrow and MacEwen’s small animal clinical oncology, ed 4, St Louis: Saunders, 2007.
Mayhew PD, et al. Pathogenesis and outcome of extrahepatic biliary obstruction in cats. J Small Anim Pract. 2002;43:247.
McConnell JF, et al. Ultrasonographic diagnosis of unusual portal vascular abnormalities in two cats. J Small Anim Pract. 2006;47:338.
Means C. Selected herbal hazards. Vet Clin Small Anim. 2002;32:367.
Rothuizen J, et al. WSAVA standards for clinical and histological diagnosis of canine and feline liver diseases. Oxford, UK: Saunders Ltd Elsevier, 2006.
Savary-Bataille KC, et al. Percutaneous ultrasound-guided cholecystocentesis in healthy cats. J Vet Intern Med. 2003;17:298.
Walker MC, et al. Postprandial venous ammonia concentrations in the diagnosis of hepatobiliary disease in dogs. J Vet Intern Med. 2001;15:463.
Wang KY, et al. Accuracy of ultrasound-guided fine-needle aspiration of the liver and cytologic findings in dogs and cats: 97 cases (1990-2000). J Am Vet Med Assoc. 2004;224:75.
Webb CB, et al. S-adenosylmethionine (SAMe) in a feline acetaminophen model of oxidative injury. J Feline Med Surg. 2003;5:69.
Weiss DJ, et al. Relationship between feline inflammatory liver disease and inflammatory bowel disease, pancreatitis, and nephritis in cats. J Am Vet Med Assoc. 1996;209:1114.
White RN, et al. Anatomy of the patent ductus venosus in the cat. J Feline Med Surg. 2001;3:229.
Willard MD, et al. Fine-needle aspirate cytology suggesting hepatic lipidosis in four cats with infiltrative hepatic disease. J Feline Med Surg. 1999;1:215.
Xavier FG, et al. Cystic liver disease related to high Platynosomum fastosum infection in a domestic cat. J Feline Med Surg. 2007;9:51.