CHAPTER 73 Clinical Manifestations of and Diagnostic Tests for Joint Disorders
GENERAL CONSIDERATIONS
Disorders affecting the joints can be divided into two major categories: noninflammatory and inflammatory (Box 73-1). Noninflammatory joint diseases include developmental, degenerative, neoplastic, and traumatic processes. These disorders are discussed in greater detail in surgery textbooks such as those listed in Suggested Readings. Inflammatory joint diseases can be infectious or immune-mediated. When multiple joints are inflamed, polyarthritis is said to be present. Immune-mediated polyarthritis is further classified as erosive or nonerosive disease on the basis of physical examination findings and results of radiographs of affected joints.
Immune-mediated, nonerosive polyarthritis (IMPA) is the most common inflammatory joint disorder recognized in dogs. It results from immune-complex deposition within the synovium, causing a sterile synovitis. IMPA usually occurs as an idiopathic syndrome, but it may also be a feature of systemic lupus erythematosus (SLE) or secondary to antigenic stimulation (reactive polyarthritis) caused by chronic infection, neoplasia, or administration of drugs. In addition, a few breed-associated syndromes of polyarthritis, polyarthritis/meningitis, or polyarthritis/myositis are thought to have a genetic basis in dogs (see Chapter 74).
CLINICAL MANIFESTATIONS
Animals with joint disease are commonly presented with a history of lameness or gait abnormality. Traumatic or developmental disorders typically involve only one joint, with lameness consistently described in the same limb. When multiple joints are affected, a shifting-leg lameness may be reported. Animals with degenerative joint disease typically exhibit low-grade chronic discomfort that causes lameness and a reluctance to exercise without systemic signs of illness. The pain associated with polyarthritis is usually more severe, and affected animals may refuse to walk or may cry in pain when moved or touched (Fig. 73-1). Some animals with polyarthritis are not obviously lame but are presented with a vague history of decreased appetite, fever, weakness, stiffness, or exercise intolerance. Polyarthritis is one of the most common causes of cyclic fevers and nonspecific inflammation in dogs, and because many affected animals do not have obvious joint pain or detectable joint swelling, it is important to maintain a high index of suspicion for this disorder.
DIAGNOSTIC APPROACH
Animals with nonspecific pain, a stiff gait, reluctance to exercise, or fever of unknown origin should always receive a careful physical examination in an attempt to localize a region of pain or inflammation. Observation of the animal’s posture and gait and thorough manipulation and palpation of the spine and the muscles, bones, and joints of each limb are important. Palpation of the bones themselves will elicit pain in animals after trauma and in dogs affected by panosteitis, hypertrophic osteodystrophy, osteomyelitis, or bone neoplasia. Palpation of affected muscles will be painful in animals with myositis or strain/sprain injuries. Pain on palpation or manipulation of the neck could indicate a variety of spinal cord or vertebral abnormalities, intracranial disease, meningitis, or polyarthritis; inflammation of the intervertebral facetal joints can manifest as neck or back pain (see Box 69-1).
Some animals with joint disease experience obvious discomfort during joint manipulation. Flexing and extending a joint affected by degenerative or erosive disease commonly reveal a restricted range of motion and crepitation, suggesting articular wear, the presence of osteophytes, or other periarticular changes. The stability of the painful joint should be evaluated to assess the integrity of the supporting ligaments. Animals with nonerosive polyarthritis are less likely to have joints that are obviously abnormal on palpation, although joint swelling and pain on manipulation are common (Fig. 73-2). Approximately 30% of dogs with IMPA have no detectable joint swelling or pain, so normal palpation should not deter further diagnostic evaluation for polyarthritis.
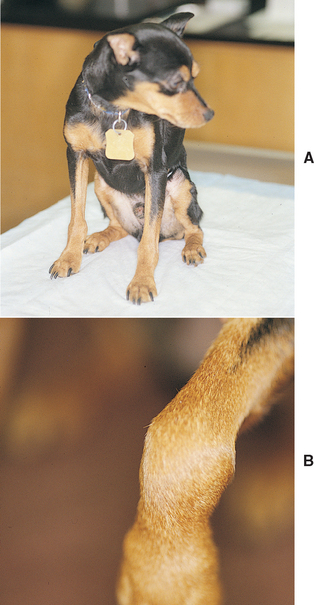
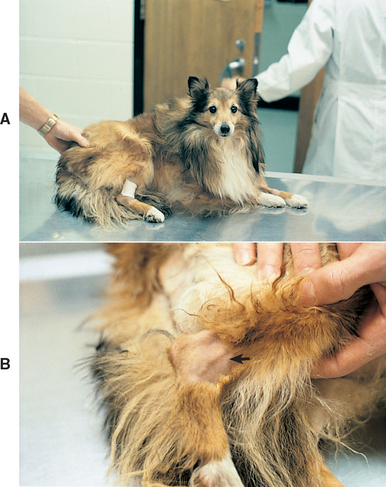
FIG 73-2 A, A 4-year-old Miniature Pinscher was referred for intermittent fever and depression during the previous year. All joints are palpably and visibly swollen, particularly the carpus (B).
Synovial fluid should be collected and evaluated from multiple joints in all dogs and cats with suspected polyarthritis and those with monoarticular disease accompanied by systemic or local signs of inflammation. Synovial fluid analysis is necessary to differentiate inflammatory from noninflammatory joint disease (see Box 73-1). When synovial fluid is inflammatory, the first step is to investigate and eliminate possible infectious diseases as differential diagnoses. Infectious agents causing arthritis include bacteria, Mycoplasma spp., bacterial L-forms, spirochetes, rickettsial agents, and fungi. Diagnostic tests may include a complete blood count (CBC); urinalysis; culture of urine, blood, and synovial fluid; and serology for tick-borne diseases. Thoracic radiographs and fungal serology may also be warranted. Once infectious causes of polyarthritis have been ruled out, immune-mediated conditions should be considered.
Noninfectious IMPA is common in dogs and uncommon in cats. Immune-mediated polyarthritis can occur as an idiopathic syndrome, as a feature of SLE, or secondary to systemic antigenic stimulation (reactive polyarthritis). In reactive polyarthritis the joints are not infected but articular deposition of immune complexes results in synovitis. Reactive polyarthritis has been reported in association with chronic bacterial or fungal infections, neoplasia, or the administration of drugs or vaccines. When the history does not reveal an inciting event, a battery of tests is required to look for systemic evidence of infection or neoplasia (e.g., CBC, thoracic and abdominal radiographs, ophthalmologic examination, bacterial culture of urine and blood, lymph node aspirates, cardiac ultrasonography, abdominal ultrasound) or SLE (e.g., CBC, platelet count, urine protein : creatinine ratio, antinuclear antibody [ANA] titer). Normal results on all of these tests warrant a diagnosis of idiopathic IMPA.
Because most dogs with IMPA have nonerosive disease, radiographs are not always performed during initial evaluation. If dogs with presumed IMPA do not respond quickly and completely to treatment or if joints are unstable or deformed on palpation, radiographs should be taken to evaluate for evidence of erosive disease affecting the articular surfaces, focal “punched out” lesions of lysis in subchondral bone, and proliferation and calcification of periarticular soft tissues. Erosive polyarthritis is an uncommon immune-mediated disorder in dogs, with some similarities to human rheumatoid arthritis. This disorder is characterized by progressive joint inflammation, destruction, and deformity. Serologic testing for rheumatoid factor and synovial membrane biopsy aid in the diagnosis of this rare disorder (see p. 1138).
Feline polyarthritis is uncommon. Infectious arthritis has been reported as resulting from bacteria, including bacterial L-forms and Mycoplasma spp., and calicivirus. Periosteal proliferative polyarthritis, an erosive polyarthritis syndrome, has been identified in male cats in association with feline leukemia virus and feline syncytium-forming virus infections. Noninfectious IMPA resulting from SLE also occurs occasionally in cats.
DIAGNOSTIC TESTS
MINIMUM DATABASE
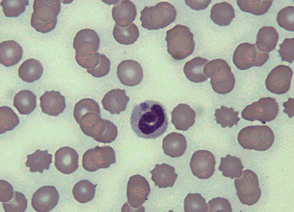
Evaluation of a minimum database consisting of a CBC, serum biochemistry profile, and urinalysis should be normal in animals with noninflammatory joint disease. In dogs and cats with polyarthritis it is common to identify a leukocytosis, hyperglobulinemia, and mild hypoalbuminemia. Many of the tick-borne pathogens causing polyarthritis also cause thrombocytopenia. Organisms may be identified within red or white blood cells in animals with some infectious causes of polyarthritis (Fig. 73-3). Proteinuria and hypoalbuminemia will be seen in dogs with concurrent glomerulonephritis. Cats with polyarthritis should always be tested for feline leukemia virus (FeLV) antigen and feline immunodeficiency virus (FIV) antibody. Normal clinical pathology does not rule out polyarthritis.
RADIOGRAPHY
Radiographs should routinely be taken during initial evaluation whenever only one joint is clinically affected or joint palpation reveals crepitation, instability, or a restricted range of motion. In dogs with presumed IMPA radiographs are recommended if the response to treatment is not as rapid and complete as expected. Radiographic abnormalities of the joints and periarticular region are expected in animals with degenerative joint disease (DJD), chronic septic arthritis, and immune-mediated erosive (rheumatoid-like) arthritis. Results of the physical examination usually help to identify which joints should be radiographically evaluated. Each joint evaluated requires two views (i.e., lateral and anterior/posterior). Radiographs from patients with infectious polyarthritis caused by rickettsial agents, Lyme disease, or viruses are similar to radiographs from patients with immunemediated nonerosive polyarthritis; typically, the only abnormalities seen are mild joint capsule distention and associated soft-tissue swelling.
Radiographs of the thorax and abdomen and abdominal ultrasound are often recommended in dogs and cats with polyarthritis to evaluate for underlying infectious or neoplastic disease. In addition, radiographs of the spine should be performed in dogs with concurrent polyarthritis and neck or back pain to screen for diskospondylitis as a cause for reactive polyarthritis.
Radiography is an important tool, but it is limited. Many of the bony changes seen with DJD and erosive immune disease are not apparent for weeks to months after the onset of signs. Although positive findings contribute a great deal to the diagnosis, negative findings should be interpreted with caution. Sequential radiographic studies may be warranted.
SYNOVIAL FLUID COLLECTION AND ANALYSIS
Synovial fluid collection and analysis is the most useful test for establishing a diagnosis in dogs and cats with joint disease. It is of greatest value in confirming that a specific joint is abnormal and in differentiating inflammatory from noninflammatory disease. Synovial fluid collection and analysis may also provide information regarding a specific diagnosis.
Collection Method
Arthrocentesis requires little in the way of expertise or equipment, involves minimal risk to the animal, is inexpensive to perform, and has a high diagnostic yield. In dogs and cats, although synovial fluid can sometimes be collected without sedation or anesthesia, light tranquilization or sedation is usually used to prevent the animal from moving during sample collection and thereby contaminating the sample. Immunologically mediated disease tends to be most prominent in the distal small joints, such as the hock and carpus. Whenever polyarthritis is suspected, synovial fluid should be analyzed from at least six joints, including both carpi, both hocks, and both stifles. Elbows and shoulders should be tapped in animals with poorly localized forelimb lameness. When they are swollen or painful, the smaller metacarpophalangeal and interphalangeal joints can also be sampled. Even if only one joint is clinically affected, synovial fluid should be analyzed from multiple joints.
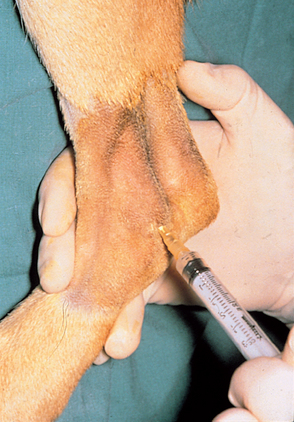
The hair should be clipped from the area and the skin washed as for surgery. Wearing sterile gloves is necessary if the area where the needle will be inserted is to be palpated. Arthrocentesis in dogs and cats typically requires a 25-gauge needle attached to a 3-ml syringe (Fig. 73-4). A 22-gauge, 1½-inch needle is used for the shoulder, elbow, and stifle joints of larger dogs. Large dogs may require a 3-inch spinal needle to enter the hip joint.
Landmarks for arthrocentesis vary according to personal preference, but some recommended approaches are outlined in Fig. 73-5. After aseptic preparation, the needle attached to the syringe is inserted into the joint. Once the tip of the needle is in the joint, gentle negative pressure is applied to the syringe. Only a very small amount of joint fluid (one to three drops) is needed for the critical determination of viscosity, estimated cell count, differential white blood cell (WBC) count, and culture. The negative pressure on the syringe is released before withdrawal of the needle through the skin. The appearance of blood should prompt immediate release of suction and withdrawal of the needle. Slides are made immediately (Fig. 73-6), with one drop of synovial fluid used for each slide.
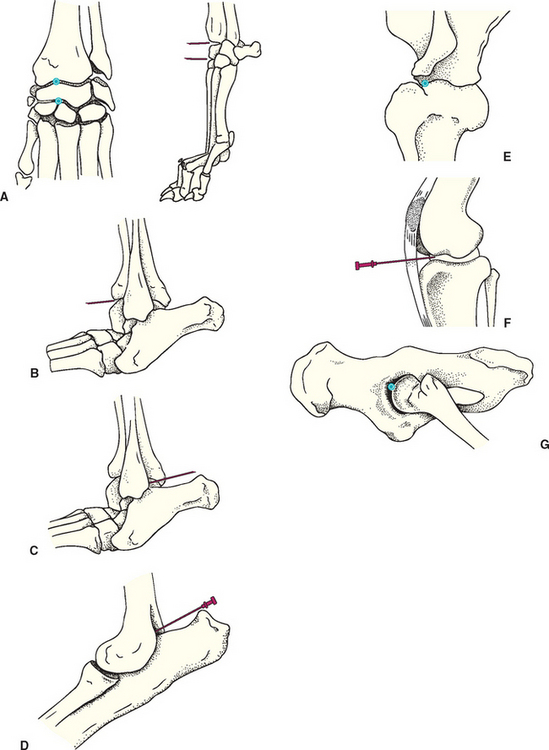
FIG 73-5 Recommended sites for arthrocentesis in the dog and cat. A, Carpus: Partially flex the joint. Palpate and enter the anteromedial aspect of the radiocarpal or carpometacarpal space. B, Hock: anterior approach. Palpate the space between the tibia and tibiotarsal bone on the anterolateral surface of the hock; insert the needle in the shallow, palpable space until bone is encountered and aspirate. C, Hock: lateral approach. Partially flex the joint, and insert the needle just caudal to the distal end of the lateral malleolus of the fibula, directing the needle medially and slightly cranially. D, Elbow: Insert the needle just medial to the lateral epicondyle of the humerus at the level of the dorsal edge of the olecrenon. Advance cranially parallel to the olecranon process while applying medial pressure on the shaft of the needle. E, Shoulder: lateral approach. With the joint held in partial flexion as if weight bearing, insert the needle just distal to the acromion process cranial to the glenohumeral ligament and direct the needle medially. F, Stifle: With the joint in partial flexion, insert the needle just lateral to the straight patellar ligament equidistant between the distal patella and the tibial tuberosity. Direct the needle slightly medially as it is inserted caudally toward the center of the joint. G, Coxofemoral: Support the limb parallel to the table as though the dog were standing. Insert a spinal needle straight in medially just dorsal to the greater trochanter until bone is encountered, then abduct and medially rotate the limb while advancing the needle ventrally and caudally.
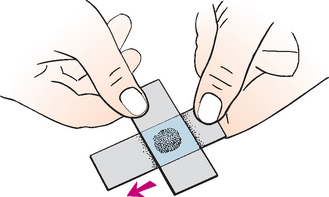
FIG 73-6 Preparing a smear of synovial fluid. A drop of fluid is placed onto a slide. A second slide is used to gently spread the fluid using a pull smear technique.
After the collection of samples for cytologic evaluation, a larger sample should be collected for culture and sensitivity. Selection of the most appropriate joint to culture is based on clinical findings or on the gross characteristics of the joint fluid. Aseptic preparation of the joint is repeated, and as much fluid as possible is obtained with gentle suction. This fluid can be either submitted for culture in a sterile tube or directly inoculated into enrichment media.
Analysis of Gross Appearance
Normal synovial fluid is clear and colorless. Cloudiness or turbidity is seen in any condition that causes red blood cells (RBCs) or WBCs to enter the joint in high numbers. Color change may be an indication of blood contamination or a pathologic condition. Hemorrhage from an earlier puncture attempt or an ongoing disease process typically results in a diffuse red discoloration of the synovial fluid, whereas blood from a traumatic tap is not usually homogeneously mixed with the joint fluid. A yellowish fluid (xanthochromia) may indicate previous hemorrhage into the joint and is occasionally seen in degenerative, traumatic, and inflammatory joint diseases.
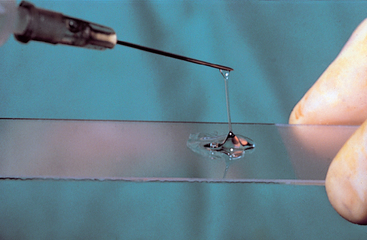
Normal synovial fluid is very viscous. It forms a long string when allowed to drop from the tip of a needle onto a slide (Fig. 73-7). A thin or watery consistency indicates that the synovial fluid is deficient in polymerized hyaluronic acid. This may occur after dilution by serum or through degradation by an intense intraarticular inflammatory reaction.
Analysis of Microscopic Appearance
Cytologic evaluation is the most important aspect of synovial fluid analysis. Usually, only a few drops of synovial fluid are collected, and estimates of cell numbers are made from a stained direct smear of the fluid. One drop of fluid can be placed on a slide and a second slide used to spread the fluid to make a thin smear (see Fig. 73-6). This smear should be air dried and then stained with Diff-Quik or Wrights-Giemsa stain. Because normal synovial fluid contains fewer than 3000 WBCs/μl, no more than three WBCs should be seen per high-dry power (40×) field on a stained smear. Experienced clinicians find simple microscopic scanning of a stained slide of synovial fluid sufficient to estimate cell numbers as normal, mildly increased, or greatly increased.
Normal synovial fluid contains a mixture of large and small mononuclear cells that frequently contain many vacuoles and granules. An occasional neutrophil may be observed, but these cells should represent less than 10% of the total. Blood contamination during synovial fluid collection will result in approximately 1 neutrophil for every 500 RBCs contaminating the fluid. The presence of platelets indicates recent intraarticular hemorrhage or significant blood contamination. Hemosiderin-laden macrophages and erythrophagia confirm prior hemorrhage.
Degenerative joint disease causes a slightly increased cell count (<6000 cells/μl) and an increased volume of synovial fluid, but almost all of the cells are mononuclear cells (Table 73-1). An increase in the number of neutrophils within a joint indicates inflammation of the synovial lining. The more inflamed the synovium, the greater is the concentration of WBCs in the synovial fluid, and the greater the percentage of neutrophils (Fig. 73-8).
 TABLE 73-1 Synovial Fluid Cytology in Common Joint Disorders
TABLE 73-1 Synovial Fluid Cytology in Common Joint Disorders
| WBC/μL | % PMN | |
|---|---|---|
| Normal | 200-3,000 | <10 |
| Degenerative | 1,000-6,000 | 0-12 |
| Traumatic | Variable | <25 |
| Septic | 40,000-280,000 | 90-99 |
| Immune-mediated disease | ||
| Nonerosive immune | 4,000-370,000 | 15-95 |
| Erosive arthritis (Rheumatoid-like) | 6,000-80,000 | 20-80 |
WBC, white blood cell; PMN, polymorphonuclear neutrophil leukocytes.
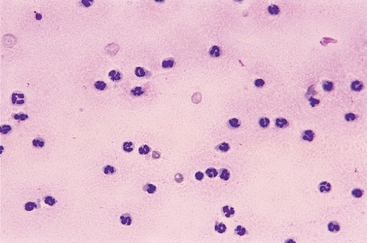
FIG 73-8 Synovial fluid with an increased nucleated cell count consisting primarily of neutrophils from an adult dog with idiopathic immune-mediated polyarthritis.
In addition to the actual or estimated WBC count and WBC differential, cytologic evaluation of the cells in the joint fluid is important. Neutrophils in the synovial fluid of dogs and cats with immune-mediated disease should have a normal appearance. In acute or severe cases of septic arthritis, it is common to see bacteria within the cells, and neutrophils in the joint may be toxic, ruptured, and degranulated. Organisms may occasionally be observed within the cells in the synovial fluid of animals with polyarthritis caused by rickettsial infections or Leishmania. In dogs with SLE-induced polyarthritis, lupus erythromatosus (LE) cells are in rare cases seen within the synovial fluid (Fig. 73-9).
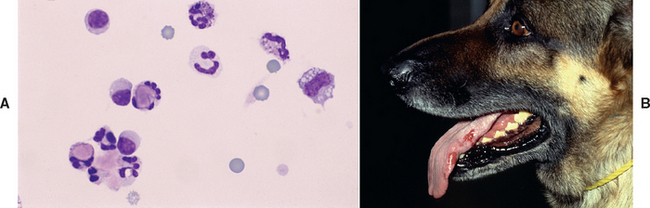
FIG 73-9 Synovial fluid from an adult German Shepherd Dog with polyarthritis. A, Some of the cells are lupus erythromatosus cells containing phagocytized, opsonized, amorphous nuclear material. Finding these lupus erythromatosus cells supports a diagnosis of systemic lupus erythromatosus. B, This dog also has proteinuria, tongue ulcers caused by vasculitis, and a positive antinuclear antibody test.
SYNOVIAL FLUID CULTURE
Bacteria are the most common cause of joint infection. Septic arthritis can often be diagnosed on the basis of the appearance of toxic changes within neutrophils and the identification of bacteria on stained smears of synovial fluid. Some organisms, such as Mycoplasma spp., do not, however, induce characteristic cytologic abnormalities. Any joint fluid with an increased nucleated cell count and a high percentage of neutrophils warrants a culture. Synovial fluid should be submitted for aerobic and anaerobic culture and for specific Mycoplasma spp. culture. Because direct bacterial culture of synovial fluid is positive in only approximately half of all cases of septic arthritis, failure to grow bacteria in synovial fluid does not rule out septic arthritis. The diagnostic yield can be greatly improved (85% to 100% positive) if infected synovial fluid is collected and inoculated into brothenrichment media (e.g., thioglycolate blood culture bottles), incubated for 24 hours, and then recultured. Microbiologic culture of blood, urine, and synovial membrane biopsy specimens should also be considered to improve chances of recovering the offending organism.
SYNOVIAL MEMBRANE BIOPSY
Performing synovial membrane biopsy can support a diagnosis already suspected on the basis of the history, physical examination, radiographic studies, and synovial fluid analysis. It may also be used to collect a sample for microbiologic culture in cases of suspected septic arthritis. Examination of the synovial membrane is especially valuable in the diagnosis of neoplasia and in the differentiation of infectious arthritis from the immune-mediated disorders. An undetermined cause of joint disease or ineffective routine therapy warrants synovial membrane analysis.
Synovial membrane biopsies may be obtained by needle biopsy or surgical arthrotomy. Surgical excision of a wedge of synovial membrane allows visualization of the entire joint and selection of a specific site from which to obtain the biopsy. Needle biopsy of the synovial membrane is quick and minimally traumatic, but samples are small and easily obtained only from the stifle joint. Techniques for both procedures are described in Suggested Readings.
IMMUNOLOGIC AND SEROLOGIC TESTS
Lyme Disease Titers
Infection with the spirochete Borrelia burgdorferi, the etiologic agent for Lyme disease, causes primary infectious synovitis as well as immunologically mediated synovitis resulting from immune complex deposition. Affected dogs develop an antibody response that can be detected using an indirect fluorescent antibody (IFA) test or an enzyme-linked immunosorbent assay (ELISA). Dogs with clinical signs of Lyme disease generally have high titers, but asymptomatic dogs in endemic areas may also have titers greater than 1 : 8000. Therefore a positive antibody titer merely indicates exposure to the organism and cannot be used to diagnose active disease. The varied, nonspecific clinical signs of Lyme arthritis warrant questioning of the significance of a positive titer. A diagnosis of Lyme disease polyarthritis must rely on a combination of the history (i.e., recent exposure to an area in which the disease is enzootic), clinical signs, elimination of other known causes of polyarthritis, serologic testing, and response to therapy (see p. 1132).
Rickettsial Titers
Serologic testing plays an important role in the diagnosis of Rocky Mountain spotted fever (RMSF), canine monocytc ehrlichiosis, canine granulocytic anaplasmosis, and bartonellosis (see Chapter 96 for more discussion of rickettsial diseases and Chapter 100 for more discussion of bartonellosis). Demonstration of a rising titer is necessary to make the diagnosis of acute RMSF, with a fourfold increase between acute and convalescent titers expected. Demonstration of antibody against Ehrlichia canis and Anaplasma phagocytophilium indicate prior exposure, with antibody levels remaining elevated for months after successful treatment.
Systemic Lupus Erythematosus
Tests used to help identify SLE include the LE cell test and the ANA test. The LE cell test requires identification of the LE cell, which is a neutrophil or other WBC that has phagocytized opsonized nuclear material. The cytoplasm of these cells is filled with amorphous purple material (see Fig. 73-9). The LE cell test reliability is laboratory dependent, requiring an experienced technician. The ANA test detects circulating antibodies to nuclear material. These antibodies are the most prominent of the autoantibodies associated with canine and feline SLE. The ANA test is a sensitive indicator for the diagnosis of SLE and is positive (>1 : 10) in 55% to 90% of SLE cases. The ANA is constant from day to day and is less steroid labile than the LE cell test. Unfortunately, a positive ANA test is not specific for SLE, and false-positive results may be seen in dogs and cats with many other systemic inflammatory or neoplastic diseases.
Rheumatoid Factor
The laboratory test for rheumatoid factor (RF) detects serum agglutinating antibody directed against the patient’s own IgG. A titer of 1 : 16 or higher is generally considered positive, and a titer of 1 : 8 is considered suspect and should be repeated. The reliability of the test increases with the severity and chronicity of the disease. The test is reported to be positive in 20% to 70% of dogs with erosive (rheumatoid-like) arthritis. Any disease associated with systemic inflammation and immune-complex generation and deposition can result in weak, false-positive results.
Bennett D. Immune-mediated and infective arthritis. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. Philadelphia: Elsevier Saunders, 2005.
Clements DN, et al. Type I immune-mediated polyarthritis in dogs: 39 cases (1997-2002). J Am Vet Med Assoc. 2004;224(8):1323.
Goldstein RE. Swollen joints and lameness. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. Philadelphia: Elsevier Saunders, 2005.
MacWilliams PS, Friedrichs KR. Laboratory evaluation and interpretation of synovial fluid. Vet Clin N Am Small Anim Pract. 2003;33(1):153.
Siegfried R, et al. Evaluation of different techniques for percutaneous needle biopsy of synovial membrane in the god. Vet Comp Orthop Traumatol. 2005;18(3):127.