CHAPTER 74 Disorders of the Joints
GENERAL CONSIDERATIONS
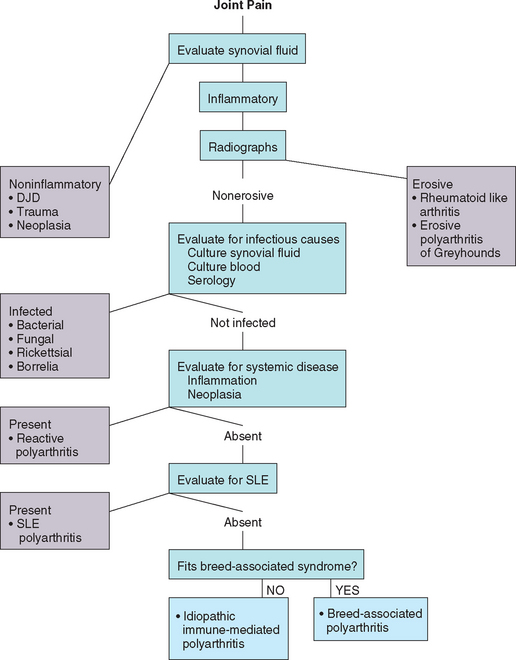
The diagnostic approach to dogs and cats with joint disease is discussed in detail in Chapter 74. Joint disorders are characterized as inflammatory or noninflammatory on the basis of synovial fluid analysis. The most common non-inflammatory joint disease is degenerative joint disease (DJD), with characteristic clinical and radiographic featuers. When synovial fluid is inflammatory, careful evaluation for an infectious cause should be performed. Noninfectious inflammatory polyarthritis is considered to be immune mediated. Animals with immune-mediated polyarthritis usually have primary idiopathic immune-mediated disease, but some have systemic lupus erythematosus (SLE), and others develop immune complex–mediated polyarthritis secondary to prolonged systemic antigenic stimulation (reactive polyarthritis; see Chapter 73). Most immune-mediated polyarthritis syndromes are nonerosive. Disorders causing radiographic evidence of bone destruction (erosive disease) are rare.
NONINFLAMMATORY JOINT DISEASE
DEGENERATIVE JOINT DISEASE
Etiology
Degenerative joint disease (DJD), or osteoarthritis, is a chronic, progressive disorder of joints that results in articular cartilage damage and degenerative and proliferative changes in the periarticular tissues. Joint instability, trauma, and developmental orthopedic diseases are the most commonly identified underlying causes. Although considered noninflammatory on the basis of synovial fluid cytology, inflammatory mediators are involved in the clinical manifestations and progression of DJD. It is estimated that approximately 20% of the adult canine population in North America is affected by DJD in at least one joint.
Clinical Features
The clinical signs of DJD are usually insidious in onset and confined to the musculoskeletal system, with no associated systemic signs. Lameness and stiffness may initially be prominent only after periods of overexertion and may worsen in cold and damp weather. Mildly affected dogs may “warm out” of their lameness with exercise. As DJD progresses, fibrosis and pain lead to decreased exercise tolerance; constant lameness; and, in severe cases, muscular atrophy. Either a single joint or multiple joints may be affected.
Diagnosis
DJD is usually diagnosed on the basis of history, physical examination findings, and characteristic radiographic features. Clinical examination may reveal pain in the affected joint or joints, decreased range of motion, crepitation on flexion and extension of the joint, and (perhaps) appreciable joint swelling. Radiographic changes characteristic of DJD include joint effusion, subchondral bone sclerosis, joint space narrowing, periarticular osteophyte formation, and bone remodeling (Fig. 74-1). A predisposing condition is often identified, such as trauma, rupture of supporting ligaments, poor conformation, or a congenital deformity. Animals with DJD do not exhibit the fever, leukocytosis, and depression commonly seen in animals with inflammatory joint disease.
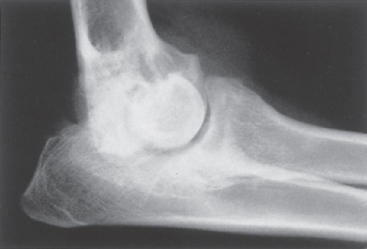
FIG 74-1 Close-up mediolateral radiograph of left elbow joint of a 14-month-old female German Shepherd Dog with severe degenerative changes secondary to a fragmented coronoid process.
Synovial fluid from a joint with DJD may be slightly less viscous than normal. The total nucleated cell count is normal or slightly increased, but it rarely exceeds 5000 cells/μl. Characteristically, mononuclear cells constitute at least 80% of the cells and neutrophils are rare (<10%). Acute joint injury or ligament rupture occasionally incites a more inflammatory response, with moderate increases in synovial fluid neutrophils for days to weeks following injury.
Treatment
The goals of treatment in dogs with DJD are to alleviate discomfort and prevent further degeneration. Surgical intervention may be necessary to stabilize the joint or correct a deformity and to relieve discomfort. Medical treatment is symptomatic and nonspecific. Weight reduction may decrease the stresses acting on the joint. Rest often helps to decrease the discomfort associated with acute exacerbations of disease. High-impact exercise, such as running and jumping, should be discouraged, whereas low-impact exercise done in moderation, such as swimming and leash walking, is recommended to maintain the animal’s strength and mobility. Other forms of physical therapy may include passive range of motion exercises, cold (acute) or heat (chronic) therapy, muscle and joint massage, ultrasound, and electrical stimulation. Dietary supplementation with omega-3 polyunsaturated fatty acids (PUFAs), eicosapentaenoic acid (EPA), and antioxidants (vitamin E, vitamin C, beta carotene, zinc and selenium) or feeding commercial “joint diets” containing these supplements may decrease the inflammation and pain of DJD.
Pharmacologic therapies may be used to decrease further degradation of the articular cartilage, inhibit the release of inflammatory mediators, and control pain. The nonsteroidal antiinflammatory drugs (NSAIDs) are often recommended because of their antiinflammatory and analgesic effects. The primary action of most NSAIDs is reversible inhibition of cyclooxygenase, preventing synthesis of the prostaglandins responsible for pain and inflammation. Selective inhibition of two forms of cyclooxygenase (COX-1 and COX-2) may explain some of the differences in efficacy and toxicity among the available NSAID agents. Preferential inhibition of COX-2 with relative sparing of COX-1 by an NSAID may be associated with improved control of inflammation and decreased potential for gastric irritation and ulceration or renal toxicity. Renal function should be assessed before prescribing any NSAID, after 7 days of treatment, and then at least every 6 months during chronic administration. Owners should also be instructed to monitor for inappetence, vomiting, or melena, which could indicate gastrointestinal toxicity. Because the clinical response to each NSAID varies between dogs, it is often advised to switch drugs to determine which one is most effective (Table 74-1). When switching from one NSAID to another, a washout period of at least 3 days without NSAID administration is recommended to prevent toxicity. In dogs that are intolerant of NSAIDs or those that require further analgesia, oral tramadol (2-5 mg/kg q8-12h) can provide relief.
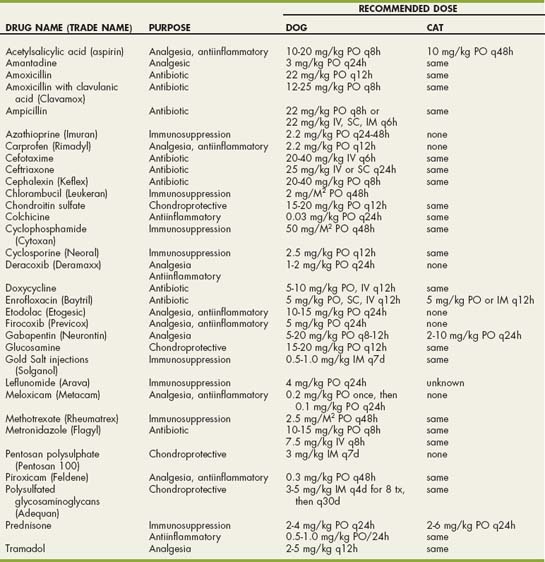
 TABLE 74-1 Dosages of Selected Drugs for the Treatment of Degenerative Joint Disease in Dogs
TABLE 74-1 Dosages of Selected Drugs for the Treatment of Degenerative Joint Disease in Dogs
| GENERIC NAME | DRUG NAME | DOSE |
|---|---|---|
| Nonsteroidal Antiinflammatory Drugs (NSAIDs) | ||
| Acetylsalicylic acid | (Aspirin) | 10-20 mg/kg PO q8-12h acid |
| Carprofen | (Rimadyl) | 2.2 mg/kg PO q12h |
| Deracoxib | (Deramaxx) | 1-2 mg/kg PO q24h |
| Etodolac | (Etogesic) | 10-15 mg/kg PO q24h |
| Firocoxib | (Previcox) | 5mg/kg PO q24h |
| Meloxicam | (Metacam) | 0.2 mg/kg PO once, then 0.1 mg/kg PO q24h |
| Piroxicam | (Feldene) | 0.3 mg/kg PO q48h |
| Disease-Modifying Chondroprotective Agents | ||
| Chondroitin sulfate | 15-20 mg/kg PO q12h | |
| Glucosamine | 15-20 mg/kg PO q12h | |
| Pentosan polysulphate | (Pentosan 100) | 3 mg/kg IM q7d |
| Polysulfated glycosaminoglycans | (Adequan) | 3-5 mg/kg IM q4d for 8 tx, then q30d |
| Analgesics | ||
| Tramadol | 2-5 mg/kg PO q8-12h | |
| Gabapentin | (Neurontin) | 5-20 mg/kg PO q8-12h |
PO, oral; IM, intramuscular.
Oral and injectable disease-modifying chondroprotective agents may improve cartilage biosynthetic activity, decrease synovial inflammation, and inhibit intraarticular degradative enzymes. Oral glucosamine and chondroitin sulfate can be administered separately or in combination. An orally administered combination of glucosamine HCl, chondroitin sulfate, and manganese ascorbate has also been recommended (Cosequin RS, 1 to 2 tablets q24h in cats or small dogs; Cosequin DS, 2 to 4 tablets q24h in large dogs; Nutramax Labs). Polysulfated glycosaminoglycans or pentosan polysulfate may be beneficial when administered intramuscularly (see Table 74-1). Hyaluronic acid is a nonsulfated glycosaminoglycan that can be administered as an intraarticular injection to improve synovial viscosity and decrease inflammation. To achieve the maximum theoretic benefit from all of these products, they should be administered before DJD has occurred. Therefore they may be indicated for the treatment of dogs that have sustained trauma or undergone surgery that is known to have damaged articular cartilage. Clinical trials are necessary to evaluate their efficacy.
INFECTIOUS INFLAMMATORY JOINT DISEASES
SEPTIC (BACTERIAL) ARTHRITIS
Etiology
Septic arthritis can result from a blood-borne infection or from direct inoculation of a joint. Bacterial infection of multiple joints suggests hematogenous spread of bacteria from a local site of infection. This is uncommon, except in neonates with omphalophlebitis, immunosuppressed animals, and dogs with preexisting polyarticular DJD. Monoarticular septic arthritis is much more common and usually follows direct inoculation of bacteria into a single joint as a result of surgery, a bite wound, foreign body penetration, or trauma. Staphylococcus spp., Streptococcus spp., and coliform organisms are most often incriminated in the dog, and Pasteurella spp. are most commonly identified in cats. Septic arthritis, regardless of the cause, is more common in dogs than cats, is most common in large-breed dogs, and more frequently affects males than females.
Clinical Features
Animals with septic polyarthritis are often systemically ill, febrile, and depressed. The affected joints are usually very painful, especially when manipulated, and may be palpably distended with synovial fluid. The periarticular soft tissues may be inflamed and edematous. Septic arthritis stemming from bacteremia usually involves one or a few of the large proximal joints.
Diagnosis
For septic arthritis to be diagnosed, bacteria must be identified in cytologic preparations of synovial fluid or cultured in synovial fluid, blood, or urine from an animal with appropriate clinical signs and inflammatory joint disease. Synovial fluid obtained by arthrocentesis is often yellow, cloudy, or bloody. The joint fluid is less viscous than normal as a result of the dilution and degradation of synovial mucin by bacterial hyaluronidase and the enzymes released from the inflammatory cells within the joint. Smears of the fluid should be made for the purpose of Gram’s staining and cytologic evaluation. Because it is common for synovial fluid from infected joints to clot rapidly, a portion of the fluid should be immediately placed in an anticoagulant (i.e., ethylenediaminetetraacetic acid [EDTA]) tube for future cytologic evaluation if an adequate sample is obtained. Cytologically, animals with septic arthritis show a marked increase in the number (40,000 to 280,000/μl) of nucleated cells in the synovial fluid, with neutrophils predominating (usually >90%). In very acute or severe cases it is common to see bacteria within the cells and the neutrophils may be toxic, ruptured, and degranulated. Organisms that do not cause rapid destruction of articular cartilage (i.e., streptococci, Mycoplasma) may not cause remarkable toxic or degenerative changes in synovial fluid neutrophils. In chronic infections bacteria may no longer be evident and the neutrophils may appear healthy.
Synovial fluid should be cultured for aerobic and anaerobic bacteria. A few drops of fluid should be removed from the joint and the smears stained for cytologic analysis. A larger sample should then be obtained from an affected joint for culture. Direct bacterial culture of the synovial fluid is positive in approximately half of all animals with septic arthritis; improved diagnostic yield may be obtained by inoculating synovial fluid into blood culture medium (9 : 1 ratio) and incubating it for 24 hours at 37° C before inoculation. Bacteria can also be recovered from cultures of synovial membrane biopsy, blood, or urine specimens.
Radiographic changes of the involved joints in septic arthritis may be minimal or nonspecific initially and limited to thickening of the joint capsule, widening of the joint space, and irregular thickening of periarticular soft tissues (Fig. 74-2). In chronic infections cartilage degeneration, periarticular new bone formation, a marked periosteal reaction, and subchondral bone lysis may be seen (Fig. 74-3).
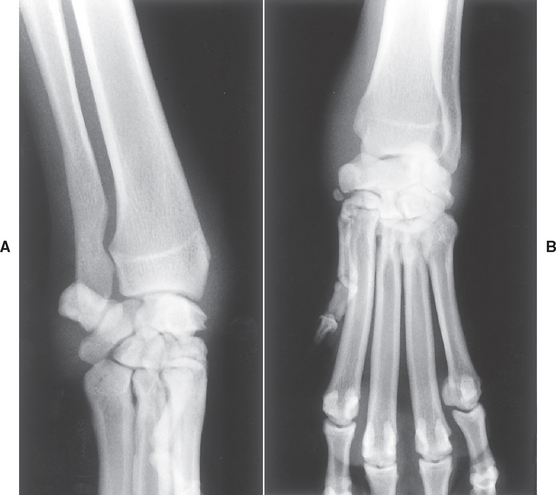
FIG 74-2 (A) Lateral and (B) dorsopalmar. Radiographs of the swollen left carpus of a 2-year-old Bullmastiff with a 1-week history of lameness caused by septic arthritis. Surgical exploration revealed two porcupine quills within the infected joint.
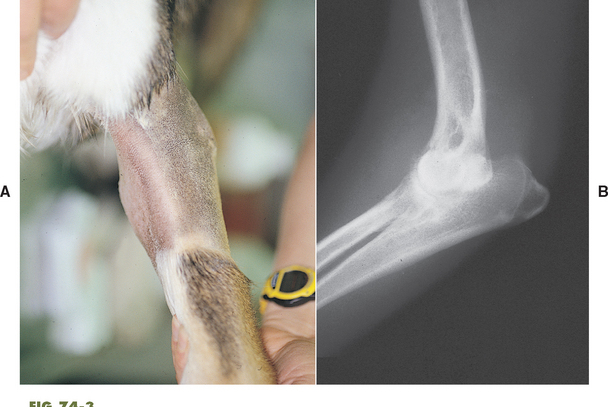
FIG 74-3 A, A Very swollen elbow in a Husky-cross dog with a 3-month history of a nonweight-bearing lameness not responding to antibiotics. B, Radiographs reveal marked swelling within the joint and diffuse periosteal proliferation. Synovial fluid showed septic inflammation, and surgical exploration revealed a single porcupine quill within the joint. The dog recovered completely.
If septic arthritis is suspected and the animal has no history of direct inoculation of the joint with bacteria, a septic site in the body should be sought. Radiography of the thorax, abdomen, and spine and cardiac and abdominal ultrasonography are especially helpful in identifying a focal site of infection. If possible, cultures of material from any suspected site of infection should be performed.
Treatment
The goals of therapy are to rapidly resolve the bacterial infection and remove intraarticular accumulations of enzymes and fibrin debris. Identifiable systemic sources of infection should also be eliminated. Antibiotics should be administered as soon as possible after all samples are collected in an animal suspected of having septic arthritis. Until culture results are available, a broad-spectrum, β-lactamaseresistant antibiotic such as a first-generation cephalosporin (e.g., cephalexin, 20 to 40 mg/kg q8h) or clavamox (Smith Kline-Beecham Animal Health; 12 to 25 mg/kg q8h) is indicated. Initially, the antibiotic can be administered parenterally, followed by long-term oral administration. Quinolones should be used if gram-negative organisms are suspected, and metronidazole should be added if anaerobic infection is suspected. Animals with acute septic arthritis can be treated conservatively initially with joint drainage and systemic antibiotics; however, if dramatic improvement is not seen within 3 days, surgery should be performed. Chronic infections, suspected intraarticular foreign bodies, postoperative joint infections, and infection in immature animals with open growth plates should all be treated with immediate surgical débridement and lavage. A minimum of 6 weeks of antibiotic therapy is administered, and cage rest is recommended to facilitate healing of articular cartilage.
MYCOPLASMA POLYARTHRITIS
Mycoplasma spp. are normal inhabitants of the upper respiratory and urogenital tracts of most species and are generally considered nonpathogenic. Systemic Mycoplasma infection may occasionally occur in debilitated or immunosuppressed animals, but the prevalence of Mycoplasma arthritis is low. Mycoplasma gatea and Mycoplasma felis are the two organisms that have been associated with polyarthritis and tenosynovitis in cats.
Mycoplasma polyarthritis results in a chronic polyarthritis indistinguishable from idiopathic immune-mediated, nonerosive polyarthritis. Clinical signs include lameness, joint pain, depression, and fever. Synovial fluid analysis reveals an increased nucleated cell count consisting predominantly of nondegenerate neutrophils. Routine aerobic and anaerobic cultures of joint fluid are negative because Mycoplasma organisms are deficient in cell walls and cannot revert to a parental state. Definitive diagnosis requires the isolation of organisms from synovial fluid cultured in special Mycoplasma medium. Idiopathic immune-mediated joint disease is very rare in cats, so empirical treatment with oral doxycycline (5 to 10 mg/kg q12h) for 3 weeks may be recommended in all cats with polyarthritis. Cats with polyarthritis should also be tested for feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV), and radiographs should be taken of the affected joints to look for erosive changes suggesting chronic progressive polyarthritis (see p. 1140).
BACTERIAL L FORM–ASSOCIATED ARTHRITIS
A rare syndrome of pyogenic subcutaneous (SC) abscesses with associated polyarthritis has been observed in cats. This syndrome appears to be infectious in nature and transmitted from one cat to another by bite wounds. No age or gender predilection exists. A bacterial L–form mutant bacteria that has lost its cell wall but can revert to its original form has been implicated. Affected cats have swollen, painful joints and fever. Fistulating SC wounds develop over the affected joints. Exudate from the joints or the SC abscesses contains degenerate and nondegenerate neutrophils and macrophages. Cultures for aerobic and anaerobic bacteria, Mycoplasma, and fungal organisms are all negative. Specific L-form media must be used to grow the organism. Radiographically, severely affected joints show extensive soft tissue swelling, periosteal proliferation, and destruction of articular cartilage and subchondral bone, resulting in subluxation and joint space collapse. Electron microscopic studies and antibiotic sensitivity testing can yield findings that help support a diagnosis of L-form bacterial infection. Rarely, cats are concurrently infected with FeLV or FIV. Treatment with doxycycline (5 mg/kg q12h) or chloramphenicol (10 to 15 mg/kg q12h) is effective, with improvement noted within 48 hours. Therapy should continue for 10 to 14 days.
RICKETTSIAL POLYARTHRITIS
Nonerosive polyarthritis has been recognized in association with several tick-borne rickettsial diseases, including Rocky Mountain spotted fever (RMSF) caused by Rickettsia rickettsii, canine monocytic ehrlichiosis caused by Ehrlichia canis, and canine granulocytic anaplasmosis (GA) caused by Anaplasma phagocytophilium (formerly Ehrlichia equi). The polyarthritis in these disorders may be related to immune complex deposition in the joints. Most infected dogs have other systemic signs of illness (see Chapter 96). Joint pain and effusion are noted, and increased numbers of nondegenerate neutrophils are identified in the joint fluid; occasionally, Ehrlichia or Anaplasma morulae can be identified in cytologic preparations of joint fluid. Fever and polyarthritis may be the only clinical abnormalities in dogs with ehrlichiosis and anaplasmosis, although hematologic abnormalities such as thrombocytopenia and anemia are common. Serologic testing for Ehrlichia canis and Anaplasma phagocytophilium is widely available, but positive results merely indicate prior exposure and do not necessarily indicate active infection.
Dogs with polyarthritis caused by RMSF are more likely to show a variety of clinical signs resulting from widespread vasculitis, including fever, petechiae, lymphadenopathy, neurologic signs, edema of the face or extremities, and pneumonitis. Hematologic abnormalities, including thrombocytopenia, are common. Diagnosis is made on the basis of the results of serologic testing and demonstration of a fourfold increase in serum IgG concentrations over 2 to 3 weeks (see Chapter 96).
Acute rickettsial infections causing polyarthritis are best treated with oral doxycycline (5 mg/kg q12h). Empirical antibiotic treatment is warranted in all dogs from endemic areas with confirmed polyarthritis, especially if there is concurrent thrombocytopenia or other evidence of rickettsial infection. Concurrent glucocorticoid therapy (prednisone, 0.5 to 2.0 mg/kg PO q24h) may be necessary in some dogs with confirmed rickettsial polyarthritis if antimicrobial therapy alone does not eliminate the fever, lameness, and joint swelling. Antibiotic treatment should continue for at least 3 weeks.
LYME DISEASE
Etiology
Infection by the tick-borne spirochete Borrelia burgdorferi (Bb) can cause illness (Lyme disease) in dogs. Ticks of the genus Ixodes transmit the spirochete, requiring at least 50 hours of tick attachment for transmission. Although serologic evidence of exposure is common in dogs throughout North America, most reports of canine Lyme disease have occurred in dogs from the northeastern and mid-Atlantic states, with Minnesota, Wisconsin, California, and Oregon accounting for most of the remaining cases.
Clinical Features
Most dogs bitten by ticks infected with Bb never develop clinical signs of illness. Experimentally infected healthy adult dogs remain asymptomatic, while 6- to 12-week-old puppies develop self-limiting, often recurrent polyarthritis. Acute polyarthritis is the most common form of Lyme borreliosis diagnosed in naturally infected dogs. Clinical features of Lyme polyarthritis include shifting leg lameness, joint swelling, fever, lymphadenopathy, and anorexia. Cytologic examination of synovial fluid reveals neutrophilic inflammation. Cardiac, renal, and neurologic manifestations (e.g., seizure, behavior change) have also been attributed to Bb infection in dogs. There are numerous reports of dogs with Bb antibody developing a unique progressive renal disorder characterized by immune-mediated glomerulonephritis, tubular necrosis, and lymphocytic-plasmacytic interstitial nephritis. This disorder is most common in Labrador and Golden Retrievers, resulting in uremia, proteinuria, peripheral edema, body cavity effusions, and death. Because of the high rate of seropositivity in endemic areas and the frequency of concurrent infection with other tick-borne diseases, it is difficult to determine how common Lyme disease is in clinical practice. The rate of veterinary diagnosis of canine Lyme polyarthritis certainly far exceeds its actual prevalence.
Diagnosis
Lyme disease should be suspected in dogs from endemic areas with fever, lameness, and anorexia. Synovial fluid analysis confirms polyarthritis. Attempts to culture Bb from the blood, urine, and synovial fluid of affected dogs are usually unsuccessful. Lyme disease polyarthritis should be diagnosed only if the animal has a history of recent potential exposure, the synovial fluid is confirmed to be inflammatory and sterile, serologic testing is positive, infection with other tick-borne diseases is eliminated, and a prompt and permanent response to appropriate antibiotic therapy is seen. The diagnosis can be supported by the identification of Borrelia organisms in biopsy specimens of tissues prepared using special stains and monoclonal antibodies.
Treatment
Antibiotics are the treatment of choice. Doxycycline (5 mg/kg, administered orally q12h), amoxicillin (22 mg/kg, administered orally q12h), ampicillin (22 mg/ kg, administered orally q8h), Clavamox (12.5 to 25 mg/kg, administered orally q8-12h), and Cephalexin (20 to 40 mg/kg, administered orally q8h) are all effective. Treatment during the acute stage of the disease should result in rapid clinical improvement (i.e., within 2 to 3 days). Treatment for at least 4 weeks is advised. Failure to recognize acute disease or the institution of inappropriate treatment can allow chronic disease to develop, including relapsing polyarthritis, glomerulonephritis, and cardiac abnormalities.
LEISHMANIASIS
Leishmaniasis is a chronic systemic disease caused by a protozoan parasite found mainly in Central and South America and in Africa, India, and the Mediterranean. In the United States Leishmania spp. are endemic in Ohio, Oklahoma, and Texas. Clinical abnormalities develop 3 months to 7 years after infection and typically consist of vague signs, including weight loss, lymphadenopathy, and splenomegaly. Hyperglobulinemia, hypoalbuminemia, and proteinuria are expected. Polyarthritis causing lameness and exercise intolerance is common. Many affected dogs will have erosive disease, with radiographic evidence of periarticular lysis and periosteal proliferation. Diagnosis is made when organisms are identified within macrophages in lymph node or splenic aspirates or in joint fluid (see Chapter 94).
FUNGAL ARTHRITIS
Fungal infection of the joints is very rare. When it does occur, it is usually as an extension of fungal osteomyelitis caused by Coccidioides immitis, Blastomyces dermatitidis, or Cryptococcus neoformans. More commonly a reactive, immunologically mediated, culture-negative polyarthritis occurs in dogs and cats with systemic fungal infections.
VIRAL ARTHRITIS
Calicivirus
Natural calicivirus infection and attenuated live calicivirus vaccination have been associated with the development of transient polyarthritis in 6- to 12-week-old kittens. Clinical signs include lameness, stiffness, and fever, which usually resolve spontaneously after 2 to 4 days (Fig. 74-4). Some kittens go on to develop overt calicivirus infection, with glossal and palatine vesicles or ulcers and signs of upper respiratory tract disease. Synovial fluid analysis reveals a mildly to greatly increased nucleated cell count, with small mononuclear cells and macrophages predominating, some of which contain phagocytosed neutrophils. Two specific strains of calicivirus have been implicated. Isolation of the virus from affected joints has been unrewarding, although the virus can be found in the oropharynx of some infected cats.
NONINFECTIOUS POLYARTHRITIS: NONEROSIVE
Noninfectious inflammatory joint diseases are very common in the dog and rare in the cat. These immune-mediated polyarthritis syndromes are routinely classified as being erosive or nonerosive on the basis of the presence or absence of radiographically evident joint destruction. Erosive disorders are very rare, consisting of fewer than 1% of canine polyarthritis cases. The nonerosive immune-mediated polyarthritis (IMPA) syndromes are all thought to be mediated through immune complex formation and deposition. Immune-mediated nonerosive polyarthritis occurs as a feature of systemic lupus erythematosus (SLE), secondary to antigenic stimulation from chronic infection, neoplasia, or drugs (i.e., reactive polyarthritis), or as an idiopathic syndrome. Breed-associated syndromes of polyarthritis or polyarthritis/meningitis or polyarthritis/myositis also exist and are thought to have a genetic basis.
SYSTEMIC LUPUS ERYTHEMATOSUS–INDUCED POLYARTHRITIS
SLE is a condition in which autoantibodies against tissue proteins and DNA result in circulating immune complexes that, when deposited in tissues, induce inflammation and organ damage (see Chapter 104). German Shepherd Dogs may be predisposed, but any breed of dog may be affected. SLE is most commonly diagnosed in dogs 2 to 4 years old. Criteria for SLE diagnosis vary between studies, but SLE is considered to be the cause of fewer than 20% of all cases of immune-mediated nonerosive polyarthritis in dogs. Although SLE is a relatively uncommon cause of polyarthritis in dogs compared with idiopathic immune-mediated polyarthritis, its effects on other organ systems can be devastating, which makes accurate diagnosis important.
Clinical Features
The clinical manifestations of SLE vary with the organ involved and include intermittent fevers, polyarthritis, glomerulonephritis, skin lesions, hemolytic anemia, immune-mediated thrombocytopenia, myositis, and polyneuritis. Polyarthritis is the most common manifestation, occurring in 70% to 90% of dogs diagnosed with SLE. Some affected dogs show no signs referable to their joint disease, and polyarthritis is detected when synovial fluid is examined as part of a workup for fever, inflammatory clinicopathologic tests, or polysystemic immune-medicated disease. More often, dogs with SLE polyarthritis show generalized stiffness, joint swelling, or a shifting leg lameness. SLE causes a sterile, nonerosive polyarthritis, with distal joints (i.e., hocks, carpi) usually more severely affected than proximal joints. Synovial fluid analysis reveals an increased white blood cell count (5000 to 350,000/ml) consisting primarily of nondegenerate neutrophils (>80%). In rare instances, lupus erythematosus (LE) cells are detected in the synovial fluid (see Fig. 73-9).
Diagnosis
SLE should be considered in any dog with non-infectious polyarthritis. A complete blood count (CBC), platelet count, biochemistry profile, urinalysis, urine protein : creatinine ratio determination, and careful physical examination should be performed to search for other manifestations of this disease. Laboratory tests that may aid in the diagnosis of SLE polyarthritis include the LE cell test (positive in 30% to 90% of cases) and the antinuclear antibody (ANA) test (positive in 55% to 90% of cases). An animal may be said to have SLE if one or more of these “specific” diagnostic tests (e.g., ANA, LE) are positive and the animal has two or more of the clinical abnormalities known to be associated with SLE (e.g., polyarthritis, glomerulonephritis, anemia, thrombocytopenia, dermatitis; see Chapter 104). When two or more of the common clinical syndromes are recognized but none of the serologic tests are positive, the dog is determined to have an SLE-like multisystemic immune-mediated disease.
Treatment
Treatment for SLE-associated polyarthritis is the same as that used for idiopathic, immune-mediated polyarthritis (Chapter 74). If the animal is clinically normal and synovial fluid is non-inflammatory after 6 months of therapy, it may be worthwhile to discontinue medications because long periods of drug-free remission can occur.
Prognosis
The prognosis is good from the standpoint of controlling the polyarthritis, but multisystemic involvement (particularly glomerulonephritis) may progress despite therapy, occasionally resulting in death.
REACTIVE POLYARTHRITIS
Reactive polyarthritis accounts for approximately 25% of all nonerosive immune-mediated polyarthritis cases. Reactive polyarthritis is most often seen in association with chronic bacterial, fungal, or rickettsial infections; neoplasia; or drug administration. Reactive polyarthritis has been documented in dogs with endocarditis, foreign body abscesses or granulomas, diskospondylitis, heartworm disease, pancreatitis, prostatitis, pyelonephritis, pneumonia, other chronic infections, and a variety of tumors (Fig. 74-5). Drugs that have been implicated in causing reactive polyarthritis include sulfadiazine-trimethoprim, phenobarbital, erythropoietin, penicillin, cephalexin, and routine vaccinations. Rarely, gastrointestinal disorders such as inflammatory bowel disease, salmonellosis, and chronic active hepatitis have also been associated with reactive polyarthritis.
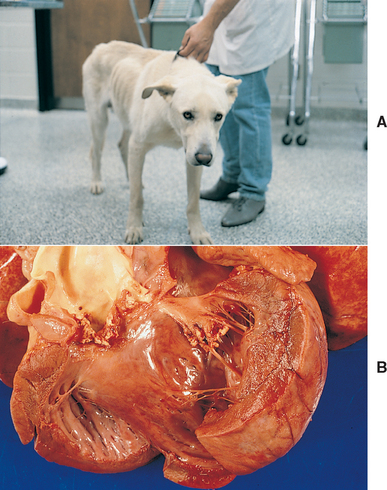
FIG 74-5 A 2-year-old German Shepherd Dog/Labrador Retriever cross with reactive polyarthritis (A). The dog was seen because of a 3-month history of shifting leg lameness and weight loss. There was joint swelling and pain and a grade IV/VI diastolic cardiac murmur. Synovial fluid was inflamed but sterile. A cardiac ultrasound study suggested infective endocarditis of the aortic valve, which was confirmed by postmortem evaluation (B).
Because many animals with reactive polyarthritis have vague or minimal clinical signs referable to their underlying disease, they will be presented for veterinary evaluation when their joint inflammation makes them reluctant to walk. Therefore it is important to perform a thorough physical examination of every animal with polyarthritis and to obtain a complete history regarding the administration of medications and the presence or absence of systemic signs. Once infectious causes of polyarthritis have been eliminated, screening tests (i.e., CBC, biochemical panel, urinalysis, thoracic and abdominal radiography, abdominal ultrasonography, culture of urine and blood, lymph node aspirates, cardiac ultrasonography) may be required to identify underlying chronic infections or neoplasia (Fig. 74-6).
Clinical signs in dogs with reactive polyarthritis typically include cyclic fevers, stiffness, and lameness. Synovial fluid analysis reveals an increase in the WBC count and the percentage of neutrophils in affected joints, but synovial fluid culture is negative. Even if the underlying inflammatory disease is infectious, the polyarthritis in these patients is caused by synovial deposition of circulating immune complexes, not by infection of the joints. Radiographically, the only finding is joint swelling.
Treatment must be directed at eliminating the underlying disease or antigenic stimulus using medications or surgery whenever possible. If this can be done, the polyarthritis usually resolves without additional therapy. Short-term, low-dose corticosteroid therapy (prednisone, 0.25 to 1.0 mg/kg q24h) or NSAID therapy may be warranted to control the synovitis in severe cases.
IDIOPATHIC, IMMUNE-MEDIATED, NONEROSIVE POLYARTHRITIS
Nonerosive, noninfectious polyarthritis in which a primary or underlying disease cannot be identified is referred to as idiopathic immune-mediated polyarthritis (IMPA). This disorder can be diagnosed only by ruling out the other causes of polyarthritis, but it is the most common form of polyarthritis diagnosed in dogs (Box 74-1). It is especially common in sporting and large breeds. Dogs of any age can be affected, but the incidence peaks at 2.5 to 4.5 years. Idiopathic, immune-mediated, nonerosive polyarthritis is uncommon in cats.
 BOX 74-1 Classification of Polyarthritis in Dogs
BOX 74-1 Classification of Polyarthritis in Dogs
Noninfectious, Nonerosive
Idiopathic, immune-mediated polyarthritis
Reactive polyarthritis (bacterial, fungal, parasitic, neoplastic, enterohepatic, drug reaction, vaccine induced)
Clinical Features
The clinical signs of idiopathic IMPA may include cyclic fevers, stiffness, and lameness. Multiple joints are usually involved, with the small distal joints (i.e., carpus, hock) affected most severely. Approximately 20% to 50% of all affected dogs may not have palpable joint effusion or localizable pain. Cervical pain and vertebral hypersensitivity are common complaints, reflecting either intervertebral facetal joint involvement or the presence of concurrent steroid-responsive meningitis-arteritis (see Chapter 69). Some dogs are evaluated because of a vague history of decreased appetite or because of fever of unknown origin.
Diagnosis
Idiopathic IMPA is diagnosed on the basis of the results of synovial fluid analysis, failure to identify an infectious cause, and the absence of evidence to support a diagnosis of SLE or reason to suspect reactive polyarthritis (Fig. 74-6). A CBC typically reveals neutrophilia, although some dogs have a normal CBC. Hyperglobulinemia and hypoalbuminemia are common, reflecting ongoing systemic inflammation. When radiographs are taken, the findings are normal or limited to joint and periarticular swelling with no bone or cartilage abnormalities. Synovial fluid is thin and may be turbid. Nucleated cell counts are increased (4000 to 370,000 cells/μl), and nondegenerate neutrophils predominate (usually >80%). In animals with less severe or fluctuating disease and animals that have received corticosteroids, there may be a lower synovial fluid white blood cell count and a lower percentage of neutrophils (15% to 80%). Blood, urine, and synovial fluid cultures are negative for bacteria and Mycoplasma spp.
Treatment
Glucocorticoids are the initial treatment of choice for dogs with idiopathic IMPA. Prednisone treatment alone results in remission in 50% of cases. Immunosuppressive doses are initially administered, and the dosage is gradually decreased every 3 to 4 weeks if the animal is clinically normal and the inflammation in the synovial fluid has subsided (Box 74-2). Synovial fluid should be monitored carefully during treatment and determined to be noninflammatory before each decrease in drug dose. If the joints are not inflamed, the drug doses may be slowly tapered. If a dog can be maintained on a low, alternate-day dose of prednisone (0.25 mg/kg q48h) for 2 months and the synovial fluid is not inflammatory, it should be possible to discontinue all therapy. Approximately 50% of affected dogs will need at least alternate-day low-dose prednisone therapy for the remainder of their lives. In dogs receiving a stable dose of medication, synovial fluid should be evaluated every 4 to 6 months.
 BOX 74-2 Treatment Recommendations for Idiopathic Immune-Mediated Polyarthritis
BOX 74-2 Treatment Recommendations for Idiopathic Immune-Mediated Polyarthritis
If clinical signs have resolved, the dose of prednisone is gradually tapered, evaluating clinical response and synovial fluid before each dose reduction.
If clinical signs of joint inflammation are present at any recheck, return to step 2 and add azathioprine (2 mg/kg/day) to treatment. Continue prednisone taper after signs resolve and synovial fluid is normal.
Azathioprine (Imuran; Burroughs Wellcome) should be administered to dogs with persistent inflammation of synovial fluid despite prednisone therapy and to dogs that cannot be tapered to a low dose of prednisone without relapse. Azathioprine may also be used from the beginning in dogs that do not tolerate prednisone therapy. Azathioprine (2.2 mg/kg) is administered once daily for 4 to 6 weeks and then only on alternate days if the animal is doing well clinically and the synovial fluid is no longer inflammatory. Some dogs will require lifelong azathioprine therapy. In most dogs azathioprine is well tolerated, with myelosuppression its major toxicity. A CBC and platelet count should be performed initially every 2 weeks and then every 6 to 8 weeks during therapy. Hepatic enzyme activities should also be monitored to facilitate the early detection of hepatotoxicity. Dogs treated with azathioprine and prednisone may also be at increased risk for developing pancreatitis.
Additional immunosuppressive agents are rarely necessary because idiopathic, nonerosive IMPA is easy to control in most patients. If the polyarthritis is refractory to treatment, the patient should be reevaluated for infectious disease, reactive polyarthritis, and erosive disease. When necessary, other immunosuppressive agents can be administered (Table 74-2). In addition to medical treatment, management should initially include restricted exercise, followed by regular gentle exercise and weight control. Chondroprotective agents, omega-3 fatty acids, and antioxidants may also prove beneficial. (See Chapter 103 for more information on immunosuppressive treatment.)
 TABLE 74-2 Drugs Used in the Treatment of Immune-Mediated Polyarthritis
TABLE 74-2 Drugs Used in the Treatment of Immune-Mediated Polyarthritis
| DRUG | DOSAGE |
|---|---|
| Prednisone | Variable |
| Azathioprine (Imuran, GlaxoSmithKline) | 2.2 mg/kg PO q24-48h |
| Cyclosporine (Neoral, Novartis) | 2.5 mg/kg PO q12 h |
| Target blood level 400 ng/ml | |
| Leflunomide (Arava, Aventis Pharma) | 4 mg/kg q24h |
| Target trough blood level 20 μg/ml | |
| Gold salt injections (Solganol, Shering) | 0.5-1.0 mg/kg weekly IM for 8 weeks, then monthly |
| Cyclophosphamide (Cytoxan, Bristol-Myers-Squibb | 50 mg/M2 PO q48h |
| Chlorambucil (Leukeran, GlaxoSmithKline) | 2 mg/M2 PO q48h |
| Methotrexate (Rheumatrex, Lederle) | 2.5 mg/M2 PO q48h |
PO, oral; IM, intramuscular.
Prognosis
The prognosis for animals with idiopathic, immunemediated, nonerosive polyarthritis is good. One dog in 50 is very difficult to treat and keep in remission. Dogs that require long-term (4 to 5 years) high-dose immunosuppressive drug therapy for this disorder may develop symptomatic DJD secondary to chronic low-grade synovial inflammation or the detrimental effects of corticosteroids on cartilage synthesis and repair.
BREED-SPECIFIC POLYARTHRITIS SYNDROMES
Immune-mediated polyarthritis has been shown to be a problem in a number of breeds. A heritable polyarthritis has been documented in Akitas younger than 1 year of age and sporadically in Newfoundlands and Weimaraners. Many of these dogs have a concurrent meningitis resembling the meningeal vasculitis syndromes seen in a few other breeds (see Chapter 69). ANA tests are negative in these animals, and generally they respond poorly to immunosuppressive therapy. In contrast, polyarthritis that accompanies meningeal vasculitis in some Boxers, Bernese Mountain dogs, German Shorthair Pointers, and Beagles often responds completely to immunosuppressive therapy.
Familial polyarthritis with concurrent myositis has been rarely reported in a few Spaniel breeds. Affected dogs are exercise intolerant and exhibit a crouched stance at rest. Widespread muscle atrophy is common, occasionally leading to muscle fibrosis, contracture, and reduced mobility. Muscle enzymes (CK, AST) may be increased. Response to therapy is often poor.
FAMILIAL CHINESE SHAR-PEI FEVER
A disorder characterized by recurrent fevers and periarticular swelling has been documented in the Shar-Pei and is known as “familial Shar-Pei fever” (FSF) or “Sharpei hock” syndrome. Growing pups or young adult dogs are initially affected by episodes of fever lasting 24 to 36 hours. Approximately 50% of affected dogs develop swelling of the tissues around the hock joint during the febrile episodes, and a few dogs develop polyarthritis. Affected dogs are at increased risk for systemic amyloidosis, leading to renal or hepatic failure. Renal amyloid deposition is primarily medullary, and not all dogs will exhibit proteinuria. Hyperglobulinemia and increased serum concentrations of the cytokine interleukin-6 are common. Glomerulonephritis, pyelonephritis, renal infarcts, and systemic thromboembolic disease may occur. This disorder is inherited as an autosomal trait. Treatment is symptomatic to control the fevers and inflammation. Oral administration of colchicine (0.03 mg/kg q24h) may decrease amyloid deposition.
LYMPHOPLASMACYTIC SYNOVITIS
Lymphoplasmacytic synovitis is present in some dogs with partial and complete tears of the cranial cruciate ligament, but the relationship between the immune-mediated response and the ligament rupture is uncertain. Partial tears or ruptures of the cruciate ligament commonly initiate an inflammatory reaction directed against the collagen of the ligament, resulting in mildly inflammatory synovial fluid and synovial fluid antibodies directed against type 1 and type 2 collagen. An alternative theory is that lymphoplasmacytic synovitis is a primary immune-mediated disorder that causes joint laxity and instability, eventually leading to rupture of the cranial cruciate ligament. Some investigators have estimated that perhaps as many as 10% to 25% of cruciate ruptures in dogs are caused by this immunologic disorder, but this is a controversial claim.
Dogs diagnosed with lymphoplasmacytic synovitis are the same dogs typically presented for cruciate ligament rupture, with Rottweilers, Newfoundlands, Staffordshire Bull Terriers, and Labrador Retrievers most commonly affected. Clinical signs are limited to acute or chronic lameness involving one or both stifles. Cruciate ligament rupture at the time of diagnosis may be partial or complete and not usually historically associated with trauma. Arthroscopy or magnetic resonance imaging (MRI) may be required to confirm the diagnosis of partial rupture. Affected animals are in good body condition and are not systemically ill; CBC is normal. Synovial fluid is thin and turbid, with an increased nucleated cell count (5000 to 20,000 cells/μl, but occasionally >200,000/μl). Lymphocytes and plasma cells predominate (60% to 90%) in the synovial fluid. Characteristic histopathologic changes in the synovial lining include lymphocytic and plasmacytic infiltration and villous hyperplasia. Biopsy of ligament and synovium should be performed at the time of surgical exploration and repair in all dogs with nontraumatic cruciate ligament ruptures. Surgical stabilization of the stifle and treatment with NSAIDs usually results in rapid resolution of clinical signs. Some dogs will have persistent effusion and discomfort that responds well to immunosuppressive treatment with prednisone and/or azathioprine, initiated a minimum of 3 days after NSAID therapy is discontinued.
NONINFECTIOUS POLYARTHRITIS: EROSIVE
CANINE RHEUMATOID-LIKE POLYARTHRITIS
A disorder resembling human rheumatoid arthritis (RA) rarely results in erosive polyarthritis and progressive joint destruction in dogs. Small and toy breeds are most commonly affected. The age of onset is variable (i.e., 9 months to 13 years), but most affected dogs are young or middle-aged. Initially, the disease is indistinguishable from idiopathic nonerosive polyarthritis, but the joints are destroyed over time (weeks to months), with distal joints most severely affected.
Etiology
The pathogenesis of canine RA-like polyarthritis is poorly understood. Antibodies directed against immunoglobulin G (i.e., rheumatoid factors [RF]) form and complex with IgG within the synovium. This results in complement activation and the chemotactic attraction of plasma cells, lymphocytes and neutrophils into the joint fluid. The synovial membrane thickens and develops a fibrous, vascular granulation tissue (pannus), which invades articular cartilage, tendons, ligaments, and subchondral bone. Proteolytic enzymes are released that erode the articular cartilage and the subchondral bone, leading to joint collapse and the radiographically visible “punched-out” subchondral bone lesions. Articular and periarticular inflammation and instability lead to joint subluxation and luxation, resulting in joint deformity.
Clinical Features
Affected dogs initially have signs indistinguishable from those of other forms of polyarthritis. A low-grade fever, depression, anorexia, and reluctance to exercise are common. Joint-related clinical signs such as joint pain and stiff gait are prominent. Signs may be sporadic initially, and stiffness is generally worse after rest and improves with mild exercise. The joints may appear normal or be swollen and painful. The joints most commonly affected are the carpi, hocks, and phalanges, although elbows, shoulders, and stifles can also be affected. As the disease progresses, clinical examination reveals crepitus, laxity, luxation, and deformity of affected joints (Fig. 74-7).
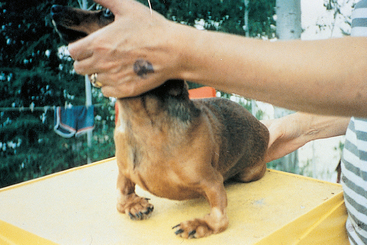
FIG 74-7 Complete collapse of both carpi resulting in luxation and severe distortion of the forelimbs in a Dachshund with rheumatoid arthritis (RA).
(Courtesy Dr. D. Haines, University of Saskatchewan.)
Radiographic features may be subtle at the time of initial diagnosis, with intracapsular swelling the only consistent finding. Later, characteristic changes consist of focal, irregular, radiolucent, cystlike areas of subchondral bone destruction (Fig. 74-8); joint space collapse; and joint subluxation and luxation. If RA is suspected, carpi and hocks should be radiographed bilaterally.
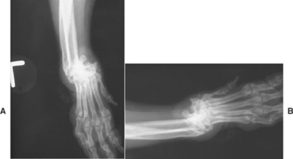
FIG 74-8 Radiographs of both carpal joints of a 9-year-old female Shih Tzu. Both carpi are severely deformed secondary to erosive rheumatoid-like polyarthritis. The intercarpal spaces have thinned laterally, and there are focal radiolucent cystlike areas of subchondral bone destruction and regional soft-tissue swelling. There is dislocation of the radius and ulna from the carpus bilaterally.
Diagnosis
RA-like polyarthritis should be suspected in any dog with erosive polyarthritis once infectious causes have been eliminated. The synovial fluid in affected joints is thin, cloudy, and hypercellular (6000 to 80,000 white blood cells/μl; mean, 30,000/μl). Neutrophils may be the predominant cell (20% to 95%; average 74%), or mononuclear cells may predominate. Culture of the synovial fluid is negative. Whenever possible, the synovial fluid should be collected during a period when the dog is most symptomatic because the cyclical nature of the disease occasionally makes diagnosis difficult.
Serologic tests for circulating RF are positive in 20% to 70% of affected dogs (see Chapter 73). Weak false-positive results are common in dogs with other systemic inflammatory diseases. Synovial biopsy may help to establish the diagnosis, revealing synovial thickening, hyperplasia, and proliferation with pannus formation. The pannus is composed primarily of proliferating activated synoviocytes, lymphocytes, plasma cells, macrophages, and neutrophils. Culture of the synovial biopsy is negative. RA is diagnosed on the basis of the typical clinical findings and radiographic features, characteristic synovial fluid features, a positive RF test result, and the typical histopathologic changes seen in a synovial biopsy specimen.
Treatment
Early treatment of RA is important to prevent irreversible changes and progressive disease. Medical treatment usually includes immunosuppressive drugs and chondroprotective agents. Initially, most dogs are treated with oral prednisone (2 to 4 mg/kg q24h for 14 days, then 1 to 2 mg/kg q24h for 14 days) and azathioprine (2.2 mg/kg q24h), administered as described for the treatment of refractory idiopathic, nonerosive polyarthritis. Oral chondroprotective agents (see Table 74-1) are routinely administered. Subjective improvement has also been observed in dogs receiving injectable chondroprotective agents (e.g., Adequan).
After 1 month of therapy, the dog is reexamined and synovial fluid is evaluated. If the fluid is non-inflammatory, the corticosteroid dose is decreased to 1 to 2 mg/kg orally every 48 hours and treatment with azathioprine is continued. If the fluid is still inflammatory, then daily administration of prednisone (1 to 2 mg/kg) and azathioprine (2.2 mg/kg) continues and oral methotrexate (Rheumatrex, Lederle; 2.5 mg/m2 q48h) may be added to the treatment regimen. Monthly evaluation of synovial fluid is recommended. If inflammation of the synovial fluid persists after 2 months, additional therapy such as Leflunomide (Arava; Aventis Pharma), a pyrimidine synthesis inhibitor, may be added to the treatment regimen (see Table 74-2). Leflunomide is administered at an initial dose of 4 mg/kg q24h, and the dose is adjusted to maintain a trough plasma level of 20 mg/ml (the usual maintenance dose is 0.5 mg/kg q24h). (See Chapter 103 for more information on immunosuppressive treatment.)
Some therapeutic success may be expected if treatment is initiated before joint damage is severe. In most cases, however, damage to the articular cartilage is severe before the diagnosis is made. Many dogs require additional therapy with analgesics such as tramadol to control joint discomfort. RA is a relentlessly progressive disorder, and even with appropriate therapy most dogs show deterioration with time. Surgical procedures can occasionally be used to improve joint stability and pain. Synovectomy, arthroplasty, joint replacement, and arthrodesis may decrease pain and improve function.
EROSIVE POLYARTHRITIS OF GREYHOUNDS
An erosive, immune-mediated polyarthritis occurs in Greyhounds from 3 to 30 months of age. This disorder is primarily seen in Australia and Britain. The proximal interphalangeal joints, carpi, hocks, elbows and stifles are most commonly affected. Clinical signs include generalized stiffness, joint pain or swelling, and a single or multiple limb lameness that may be intermittent. The synovial membrane is infiltrated with lymphocytes, and plasma cells and synovial fluid analysis reveals an increase in these same cells. There is extensive necrosis of deep articular cartilage zones, with relative sparing of the superficial surface cartilage. Therapy is as for refractory idiopathic, immune-mediated, nonerosive polyarthritis: administering prednisone, azathioprine, and chondroprotective agents. Response to treatment is variable.
FELINE CHRONIC PROGRESSIVE POLYARTHRITIS
An uncommon syndrome of erosive polyarthritis has been reported in cats. This disorder affects primarily intact and castrated male cats, and the onset of signs is usually between 1.5 and 4 years of age, although older cats are occasionally affected. The pathogenesis of the disorder is not well understood, but all affected cats are infected with FeSFV (feline syncytium-forming virus) and approximately 60% are infected with FeLV or FIV or both. Two clinical variants of this disorder affect cats: (1) a proliferative periosteal form and (2) a more severe, deforming erosive arthritis that resembles RA.
The periosteal proliferative form is most common and is characterized by the acute onset of fever, stiff gait, joint pain, lymphadenopathy, and edema of the skin and soft tissues overlying the joint. Synovial fluid analysis initially reveals inflammation with an increased white blood cell count, particularly neutrophils. As the disease becomes chronic, the proportion of lymphocytes and plasma cells increase. Initially, the radiographic changes are mild and include periarticular soft tissue swelling and mild periosteal proliferation. With time, the periosteal proliferation worsens and periarticular osteophytes, subchondral cysts, and collapse of the joint space may be noted.
The deforming type of chronic progressive polyarthritis is rare and has an insidious onset, with the slow development of lameness and stiffness. Deformation of the carpal and distal joints is common. Severe subchondral central and marginal erosions, luxations, and subluxations can be seen radiographically, which can lead to joint instability and deformities. Cytologic findings in synovial fluid are less remarkable than those in the periosteal proliferative form and consist of a mild to moderate increase in inflammatory cells (i.e., neutrophils, lymphocytes, macrophages).
Diagnosis
The diagnosis is based on the typical signalment, clinical signs, radiographic features, and results of synovial fluid analysis. Tests for FeSFV (when available) and FeLV may be positive. In addition, cultures of synovial fluid are negative, and no evidence of an underlying disorder causing a reactive polyarthritis is seen.
Treatment
Treatment with prednisone (4 to 6 mg/kg/day) may slow the progression of these diseases. If the cat shows clinical improvement after 2 weeks, the dose of prednisone can be decreased to 2 mg/kg daily. Long-term alternate-day prednisone therapy (2 mg/kg q48h) may be adequate in some cats. Combination therapy with chlorambucil (Leukeran; Burroughs Wellcome; 20 mg/m2, administered orally every 2 weeks) may aid in long-term control. Concurrent treatment with analgesics such as amantadine (3 mg/kg, administered orally q24h), amitryptyline (0.5-2.0 mg/kg, administered orally q24h), or gabapentin (2-10 mg/kg, administered orally q24h) may make affected cats more comfortable. Although many cats respond initially to therapy, the prognosis for adequate long-term control is poor, and most affected cats are euthanized.
Agut A, et al. Clinical and radiographic study of bone and joint lesions in 26 dogs with leishmaniasis. Vet Rec. 2003;153:648.
Bennett D. Immune-based erosive inflammatory joint disease of the dog: canine RA. Small Anim Pract. 1987;28:779.
Carro T. Polyarthritis in cats. Compend Cont Educ Pract Vet. 1994;16(1):57.
Clements DN, et al. Type I immune-mediated polyarthritis in dogs: 39 cases (1997-2002). J Am Vet Med Assoc. 2004;224(8):1323.
Clements DN, et al. Retrospective study of bacterial infective endocarditis in 31 dogs. J Small Anim Pract. 2005;46(4):171.
Crook T, McGowan C, Pead M. Effect of passive steretching on the range of motion of osteoarthritic joints in 10 Labrador Retrievers. Vet Rec. 2007;160:545.
Danielson F, Ekman S, Andersson M. Inflammatory response in dogs with spontaneous cranial cruciate ligament rupture. Vet Comp Orthop Traumatol. 2005;17:237.
Foley J, et al. Association between polyarthritis and thrombocytopenia and increased prevalence of vectorborne pathogens in Californian dogs. Vet Rec. 2007;160:159.
Greene CE, Straubinger RK. Borreliosis. In Greene CE, editor: Infectious diseases of the dog and cat, ed 3, Philadelphia: Elsevier, 2006.
Hanna FY. Disease modifying treatment for feline rheumatoid arthritis. Vet Comp Orthop Traumatol. 2005;18(2):94.
Jacques D, et al. A retrospective study of 40 dogs with polyarthritis. Vet Surg. 2002;31(5):428.
Littman MP, et al. ACVIM Small Animal Consensus statement on Lyme disease in dogs: diagnosis, treatment and prevention. J Vet Int Med. 2006;20:422.
Rondeau MP, et al. Suppurative, nonseptic polyarthropathy in dogs. J Vet Int Med. 2005;19:654.

 Drugs Used in Joint Disease
Drugs Used in Joint Disease